Abstract
Xenopus tadpoles are a unique model for regeneration in that they exhibit two distinct phases of age-specific regenerative competence. In Xenopus laevis, young tadpoles fully regenerate following major injuries such as tail transection, then transiently lose regenerative competence during the “refractory period” from stages 45–47. Regenerative competence is then regained in older tadpoles before being permanently lost during metamorphosis. Here we show that a similar refractory period exists in X. tropicalis. Notably, tadpoles lose regenerative competence gradually in X. tropicalis, with full regenerative competence lost at stage 47. We find that the refractory period coincides closely with depletion of maternal yolk stores and the onset of independent feeding, and so we hypothesized that it might be caused in part by nutrient stress. In support of this hypothesis, we find that cell proliferation declines throughout the tail as the refractory period approaches. When we block nutrient mobilization by inhibiting mTOR inhibitor signaling, we find that tadpole growth and regeneration are reduced, while yolk stores persist. Finally, we are able to restore regenerative competence and cell proliferation during the refractory period by abundantly feeding tadpoles. Our study argues that nutrient stress contributes to lack of regenerative competence and introduces the X. tropicalis refractory period as a valuable new model for interrogating how metabolic constraints inform regeneration.
Keywords: Xenopus, Xenopus tropicalis, tail regeneration, mTOR, vitellogenin, refractory period, nutrient availability, nutrient stress
Introduction
Animals have variable responses to large-scale injuries. Some are able to fully restore lost structures through regeneration, while others only complete wound closure followed by scarring. Organisms such as axolotls and zebrafish have robust regenerative potential and are able to restore limbs and organs throughout their lives (Erickson & Echeverri, 2018; Marques et al., 2019; Tanaka, 2016). Most mammals have relatively limited regenerative potential. Xenopus provides an ideal model to study variations in regenerative competency, as frogs of this genus are capable of regenerating their limbs and tail during the tadpole stage but gradually lose this potential during metamorphosis (Filoni & Bosco, 1981; Slack et al., 2008). This allows one to directly compare injury response between pro- and non-regenerative phases in a single model to understand how cellular and genetic contexts impact regeneration.
A notable phase of Xenopus laevis development is the refractory period, a window of development in which regenerative potential is transiently lost between Nieuwkoop and Faber stages 45 and 47 (Beck et al., 2003; Slack et al., 2004). While several factors have been identified that can ameliorate the refractory period and restore regeneration, the fundamental mechanisms underlying this reversible loss of regeneration are not understood. The refractory period coincides with peak utilization of maternal yolk stores in tadpoles around stage 45 (Selman & Pawsey, 1965). Further, feeding begins at stage 45 of development, following perforation of the buccopharyngeal membrane and opening of the mouth at stage 40–41 (Dickinson & Sive, 2006; McNamara et al., 2018; Tabler et al., 2014). The temporal correlation of this nutritional transition and the loss of regenerative competence raises the possibility that nutrient stress could contribute to the refractory period. Nutrient stress has been shown to restrict proliferation of neural progenitors and to cause developmental delays in Xenopus laevis (N. K. Love et al., 2014; McKeown et al., 2017; McKeown & Cline, 2019). In the case of neural progenitors, starvation-induced G2 cell cycle arrest was shown to be at least partially regulated by the mammalian target of rapamycin (mTOR) pathway and was mimicked by treatment with the mTOR inhibitor rapamycin (McKeown & Cline, 2019). The mTOR signaling pathway is known to link nutrient availability, proliferation, and tissue growth in organisms ranging from yeast to mammals (Sabatini, 2017). In states of nutrient sufficiency, mTOR promotes growth in response to molecular signals of nutrient surplus such as leptin and insulin (Chantranupong et al., 2015; Yuan et al., 2013).
Nutrient sensing pathways are also known to play key roles in regeneration. Leptin signaling in particular has been shown to be necessary for regeneration in zebrafish retina and mouse liver models, and promotes wound healing of the epidermis by promoting dermal proliferation (Leclercq et al., 2003; Tadokoro et al., 2015; Zhao et al., 2014). Work in Xenopus and zebrafish has implicated nutrient sensing and response pathways, specifically leptin, in appendage regeneration, although its functional role remains unclear. Expression of leptin and its receptor are highly upregulated after tail amputation, and the lepb enhancer is a reliable marker of regeneration in multiple zebrafish tissues (Aztekin et al., 2019; Anneke Dixie Kakebeen et al., 2020; Kang et al., 2016; N. R. Love et al., 2011). A role for carbohydrate metabolism, specifically glycolysis and the production of biosynthetic intermediates via the pentose phosphate pathway, has also been suggested but not yet rigorously tested in appendage regeneration (N. R. Love et al., 2014). mTOR signaling has been established as a regulator of regeneration in numerous models. Treatment with rapamycin to block mTOR inhibits proliferation during liver regeneration, and also curtails expansion of satellite cells that direct skeletal muscle regeneration (Espeillac et al., 2011; Ge et al., 2009). Further, ectopic activation of mTOR stimulates epidermal wound closure in mice and epidermal regeneration in Drosophila larvae, suggesting that this signal drives wound healing responses in these contexts (Kakanj et al., 2016; Squarize et al., 2010). By contrast, regeneration remains robust during starvation in planaria, which undergo morphallactic regeneration, and in this animal mTOR may have an antagonistic effect (Galliot & Ghila, 2010; González-Estévez, Felix, Rodríguez-Esteban, et al., 2012; González-Estévez, Felix, Smith, et al., 2012; Romero & Baguñà, 1991). Thus, while nutrient sensing and response pathways are implicated in regeneration, nutrient stress as a regenerative constraint has not been well characterized.
Here we address the link between nutrient availability and regeneration by first establishing the timeline of a regenerative refractory period in Xenopus tropicalis tadpoles. X. tropicalis are a valuable complementary model organism to X. laevis, due to their diploid genome and more rapid maturation (Harland & Grainger, 2011; Anneke D. Kakebeen & Wills, 2019). We find that X. tropicalis tadpoles are highly regenerative at stage 41, have reduced regeneration between stages 43 and 46, and minimal regenerative capacity at stage 47. We show that this loss of regenerative competency correlates with a decrease in proliferation and changes in distribution of maternally deposited yolk in the unamputated posterior tail. To determine if nutritional stress regulates regenerative competency, we inhibit mTOR signaling using rapamycin or torin and find reduced developmental growth and regeneration length in tadpoles, accompanied by changes in proliferation and yolk distribution. Finally, we ask if the refractory period is a result of nutritional scarcity by abundantly feeding tadpoles during this period and find that relieving nutrient stress restores regeneration and proliferation rates in the tail. These findings highlight nutrient availability as a potential contributor to regenerative refractory periods and suggest new connections between nutrient storage and the regulation of tissue growth in a regenerative context.
Results
Xenopus tropicalis tadpoles exhibit a graded refractory period for tail regeneration
Our first goal was to establish whether the timing and degree of a refractory period for regenerative competence in Xenopus tropicalis was similar to that of Xenopus laevis. To this end, we reared tadpoles at 22°C to stages 41–47 and assessed their total length, length from the anal opening (vent) to tail tip, regenerated tissue length, and regeneration index (Beck, 2012) at 24 hours post amputation (hpa), 72 hpa, and 7 days post amputation (dpa). Tadpole stages were assessed by morphological criteria, particularly the extent of gut coiling, abdominal iridophores, and facial morphology with stage 47 reached by 7 dpf at 22 °C (Figure 1, Supplemental figure 1). We find that at stage 41, the length of the uninjured tadpole is an average of 3.65+/−0.08 mm (Figure 1A, Y). Following amputation of the distal third of the tail, a strong regenerative response is initiated in which the regenerated tissue makes up more than 30% of the vent-to-tail tip length by 72 hpa, and then increasing slightly in proportion to the growing tadpole by 7 dpa (Figure 1Z, A’). We scored tail regeneration quality as previously described by binning tails into complete, robust, poor, or none groups (Beck, 2012), and find that 100% of tadpoles amputated at stage 41 regenerate either completely or robustly (Figure 1B’). As development progresses, tadpoles increase in length (Figure 1B–E, Y), reaching 6.72+/−0.41 mm in total length by stage 47. Among tadpoles with tails amputated at stages 43–46, we observe reduced regenerated tail tissue at 72 hpa, and a gradual decline in regeneration length as a percentage of vent-to-tail tip length at 7 dpa from stages 43 to 46 (Figure 1Z, A’). Although the regenerate is shorter, we find that quality of regeneration of tails amputated at stage 41–46 is largely complete or robust (Figure 1B’). In tadpoles with tails amputated at stage 47, we see a stronger decrease in regenerate length at 72 hpa and 7 dpa, and most tadpoles only regenerate partially or not at all (Figure 1Y).
Figure 1. A graded refractory period in Xenopus tropicalis shows reduced regenerative capacity at stages 43–46 and lost regenerative capacity at stage 47.

A-E, U) Brightfield images of X. tropicalis across a developmental timecourse. Animals were reared at 22°C. Nieuwkoop and Faber developmental stages as well as days post fertilization (dpf) are indicated. Scale bar is 1 mm. F-J, V) Brightfield images of the regenerating tail at 24 hours post amputation (hpa). Amputations were performed at stage 41 (F), 43 (G), 45 (H), 46 (I), 47 (J), or 49 (V) and removed the distal third of the tail measured from the vent. Scale bar is 0.1 mm. K-O, W) Brightfield images of the regenerating tail at 72 hpa. Amputations were performed at stage 41 (K), 43 (L), 45 (M), 46 (N), 47 (O) or 49 (W). Scale bar is 0.1 mm. P-T, X) Brightfield images of the regenerating tail at 7 days post amputation (dpa). Amputations were performed at stage 41 (P), 43 (Q), 45 (R), 46 (S), 47 (T) or 49 (X). Scale bar is 0.1 mm. Y) Box-and-whisker plot showing the length of the tadpole across a developmental timecourse. Z, A’) Box- and-whisker plots showing the length of the regenerated tail at 72 hpa (Z) or 7 dpa (A’), normalized to vent-to-tail tip length of the tadpole at that timepoint. B’) Regeneration quality index showing regeneration quality over development at 7 dpa. Regeneration scores are shown as complete, robust, poor, or none. The stage indicated represents the stage at amputation. For (Y-A’), the midline represents the median of all tadpoles measured, box size represents the interquartile range, and the lines represent the range. Outliers are indicated as points. Stage numbers above a condition indicated statistical significance (p<0.05) between the given group and those stages as determined by ANOVA (p=1.59×10−13) followed by Tukey’s post-test. For (Z) n ranges 3 – 10; for (A’) n range is 7 – 29; for (B’) n ranges 14 – 15. White arrows indicate amputation site.
We note that at 24 and 72 hpa the regenerative response of tadpoles at stage 41 is qualitatively different than at stages 43–46, with younger tadpoles forming a clear regeneration bud with better epidermal outgrowth of the fin than older tadpoles (Figure 1F–I, K–N). At stage 47, we see a further decrease with tadpoles at this stage mounting almost no regenerative response by 72 hpa, with the cut tail remaining blunt and the fin epidermis curling at the healed wound edge (Figure 1O). From stage 41 to stage 46, the rate of tadpole growth is slowing, which may partially account for the declining magnitude of regeneration (Supplemental Figure 1H). In Xenopus laevis, the refractory period typically ends by stage 48, the progression to which requires feeding. To determine whether X. tropicalis tadpoles also recover their regenerative response, we assayed regeneration at stages 48 and 49, and find that regeneration is greatly improved relative to stage 47 (Supplemental Figure 2, Figure 1V–X). We elected to assay regenerate length and score at stage 49 to avoid any possibility of staging ambiguity with stage 47. We find that regenerate length at 7dpa and regeneration scores are significantly improved at stage 49 relative to stage 47 (Figure 1 A’, B’). From these data, we conclude that a graded refractory period exists in X. tropicalis, in which regeneration is robust at stage 41, declines from stages 43–46 (which we hereafter term reduced regeneration), and is absent at stage 47 (which we hereafter term refractory).
Cell proliferation declines with the refractory period in uninjured tadpoles
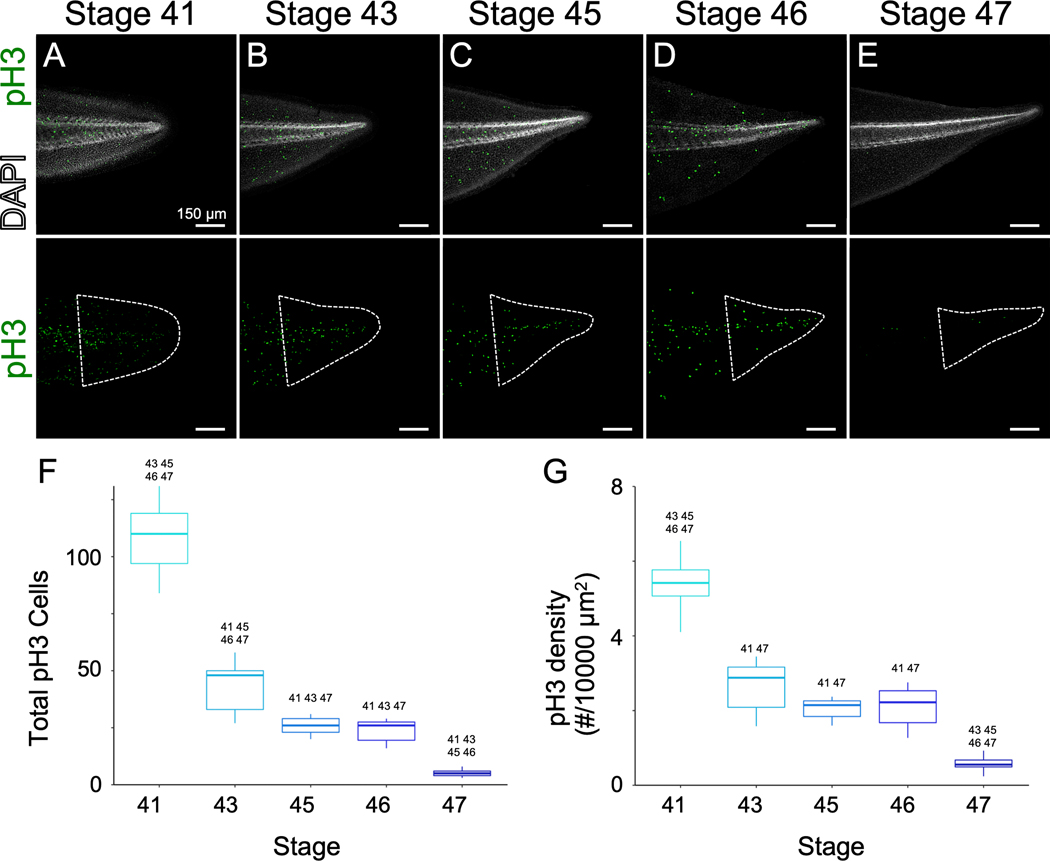
Having established that tail regeneration declines with developmental age, we next interrogated whether cell proliferation in the tail changed over the same developmental timecourse. We hypothesized that cells in the tadpole tail may undergo a decline in their steady-state proliferation rate as tadpoles approach the refractory period. To investigate proliferation in the tail, we used immunofluorescence to stain tadpoles for the mitotic histone variant phospho-histone H3 (pH3) from stage 41 to 47. We found that pH3+ cells are abundant throughout the tail at stage 41, decline at stages with reduced regeneration (stages 43–46), and are virtually absent at stage 47 (Figure 2A–F). To account for differences in tail size, we normalized the number of divisions to the area of the tail and found that there was a statistically significant decline in the area-normalized number of mitotic cells from stage 41 to stage 43, with a further decline from stage 46 to stage 47 (Figure 2G). We therefore conclude that the decline in regenerative competence from stage 41 to stage 47 is paralleled by a similarly graded decline in cell proliferation throughout the tail.
Figure 2. Proliferation in the posterior of the tail declines over development.
A-E) Immunohistochemistry for pH3 in uninjured tails at stage 41 (A), 43 (B), 45 (C), 46 (D), 47 (E) counterstained with DAPI. Scale bar is 150 µm. F,G) Box-and-whisker plots showing total numbers of pH3 positive cells (F) or density of pH3 positive cells per 10,000 µm2 (G). For (F,G), the midline represents the median of all tadpoles assayed, box size represents the interquartile range, and the lines represent the range. Outliers are indicated as points. n ranges 7 – 9 per group. Stage numbers above a condition indicated statistical significance (p<0.05) between the given group and those stages as determined by ANOVA (p<2×10−16 for F) and G) followed by Tukey’s post-test. Area of quantification is indicated by dashed white outline.
We then assessed whether proliferation rates change in response to tail amputation by amputating at each stage from 41–47 and staining for pH3+ cells at 24 and 72 hpa (Supplemental Figure S3A–J). At all stages, there are more pH3+ cells at 72 hpa than 24 hpa. Interestingly, proliferation rates from tadpoles amputated at stage 41 are substantially higher than when amputated at all other stages at both timepoints (Supplemental Figure S3K,L). When we quantified the density of dividing cells in the regenerated tissue we found that there was not a significant change at 24 hpa and find an increase in pH3 density at 72 hpa (Supplemental Figure S4A, B). However, it is important to note that the variation in the area of regenerated tail tissue highly influences the resulting density, such that a small number of divisions in a poorly regenerated tail have more weight. Thus, in agreement with the graded refractory period we describe above, we find that overall proliferation declines from stages 43 to 46 when regeneration is reduced and further declines at the stage 47 refractory period, correlating with changes in regeneration outcome.
Maternal yolk stores decline during the refractory period and are redistributed following amputation.
Having observed that proliferation and regenerative competence both decline from stage 41 to stage 47, we next set out to test the hypothesis that these declines might be linked to nutrient availability. At stage 41, tadpoles are still relying on cell-autonomous stores of maternal yolk for nutrition (Dickinson & Sive, 2006; McNamara et al., 2018). Independent feeding begins at stage 45. We therefore predicted that maternal yolk stores would undergo exhaustion as tadpoles entered the period of reduced regeneration. To test this prediction, we blotted for the yolk protein vitellogenin in tail lysates across a developmental timecourse from stage 41 to 47 and found that yolk is substantially depleted from stages 41 to 43, after which it generally declines in abundance over time (Figure 3A). We then used immunofluorescence to identify where vitellogenin was localized in the tail. At stage 41, we find that vitellogenin is readily detectable throughout the tail, staining all tissues comparably (Figure 3B). From stages 43 to 46, vitellogenin staining in the fin declines revealing stores of vitellogenin in the somites, in agreement with previous reports that muscle cells are strongly labeled with vitellogenin (Figure 3C–E) (Jorgensen et al., 2009). At stage 47, while the overall amount of vitellogenin remains similar to stage 46 in western blot, the distribution of vitellogenin by IF becomes weak and diffuse, and is absent from the tail fin (Figure 3F). This graded decline of vitellogenin staining parallels the stepwise loss of regenerative competence and pH3 staining and is consistent with our central hypothesis that nutritive stress may contribute to loss of regenerative competence.
Figure 3. Yolk is redistributed during development and regeneration.
A) Western blot for vitellogenin and β-actin in uninjured tails at stage 41, 43, 45, 46, and 47. Immunohistochemistry for vitellogenin in uninjured tails at stage 41 (B), 43 (C), 45 (D), 46 (E), 47 (F) counterstained with DAPI. In G-O, amputation was performed at stage 41 (G,L), 43 (H,M), 45 (I,N), 46 (J,O), 47 (K,P) and tadpoles were collected at 24 hpa (G-K) or 72 hpa (L-P). White arrows indicate amputation site.
We next set out to test whether the additional stress of amputation altered the depletion of maternal yolk in the tadpole tail. We reasoned that wound healing and regeneration require accelerated cellular biosynthesis and may therefore be accompanied by redistribution or accelerated depletion of maternal yolk stores. Although we did not observe a clear decline in yolk, we found that amputation of the tail at all stages leads to formation of vitellogenin puncta throughout the tail at 24 hpa, but a decline in the background level of vitellogenin staining (Figure 3G–P).
Taken together we conclude that the distribution of maternal yolk is more abundant in the tail fin at stage 41 and declines quickly as regenerative competence is lost. While amputation does not appear to trigger a rapid decline in the overall abundance of maternal yolk, it does lead to a change in yolk distribution in which yolk accumulates in puncta throughout the tail.
Inhibition of mTOR activity reduces tadpole growth
Our next goal was to determine if nutrient sensing pathways impact growth and yolk utilization during development. We hypothesize that when nutrients are abundant, mTOR signaling will be active, triggering proliferation and tail growth. Therefore, if mTOR is inhibited we predict a decrease in growth, which may be accompanied by a slower decline in total yolk as these stores are no longer fully mobilized. We inhibited mTOR signaling using rapamycin or torin starting at stage 41 (Figure 4A) and assayed tadpole size, cell proliferation in the tail, and vitellogenin staining at stage 43 and 45 or 46. Compared to control DMSO-treated clutchmates, tadpoles treated with 10 µM rapamycin were shorter at both stage 43 and 46, though we note that the tadpoles were developing through NF stages at the same rate (Figure 4B–E, G). Treatment with rapamycin results in an accumulation of vitellogenin in western blot, suggesting, as expected, that yolk stores are mobilized less efficiently when mTOR is inhibited (Figure 4F). As rapamycin is known to play a role in cellular processes outside of mTOR regulation, we performed these assays in parallel with the ATP-competitive mTOR inhibitor, Torin1 (Chou et al., 2011; Thoreen et al., 2009). We find that treatment with 1 µM Torin1 results in decreased whole body length of tadpoles to a similar degree as rapamycin, suggesting that the effects we observe are due to regulation of mTOR (Figure 4H). Further, rapamycin-treated tadpoles had significantly lower densities of pH3+ cells on average at stage 43 than their DMSO counterparts, though by stage 45 the difference in division rates between groups was no longer significant (Figure 4I–N). The same was true of the total number of pH3+ cells (Supplemental Figure S4C). These data suggest that mTOR signaling is required for the full extent of Xenopus tropicalis tadpole growth and cell proliferation from stages 41 to 46, and for the consumption of yolk stores during this period.
Figure 4. Rapamycin treatment inhibits growth, proliferation, and yolk distribution over development.
A) Schematic of treatment and collection strategy. Tadpoles are treated with either DMSO (B,D,K,M), 10 µM Rapamycin (C,E,L,N) or 1 µM Torin1 before collection at indicted stages. B-E) Brightfield images of tadpoles at stage 43 (C,D) and stage 46 (E,F) following DMSO or Rapamycin treatment. Scale bar is 0.5 mm. F) Western blot for vitellogenin and β-actin at stages 43 and 46, of either DMSO or Rapamycin treated tadpoles. G) Box-and-whisker plot showing the length of tadpoles over development following DMSO or Rapamycin treatment. H) Box-and-whisker plot showing the length of tadpoles at stage 46 following either DMSO or Torin1 treatment. I) Box-and-whisker plot showing the density of pH3+ cells across groups. J-N) Immunohistochemistry for pH3 and vitellogenin in uninjured, untreated stage 41 (J) or treated (K-N) tadpoles at indicated stages with DAPI counterstain. Scale bar is 150 µm. For (G-I), the midline represents the median of all tadpoles assayed, box size represents the interquartile range, and the lines represent the range. For (G-I), statistical significance between DMSO and Rapamycin or Torin1 groups is determined with a two-sided t-test (*p<0.05, **p<0.01, ***p<0.001). For (G) n ranges 7 – 13 per group; for (H) n ranges 18 – 19 per group: for (I) n ranges 5 – 10 per group.
Inhibition of mTOR activity reduces tail regeneration
After finding that mTOR signaling is required for full tadpole growth during development, we asked if it was necessary for tissue growth during regeneration. To this end, we amputated tails at stage 41 and then reared tadpoles in either DMSO, 10 µM rapamycin, or 1 µM Torin1 and assayed regeneration at 24 and 72 hpa (Figure 5A). We find that at 24 hpa there is not a significant difference between the 2 groups, but by 72 hpa mTOR inhibited tails regenerate significantly less tissue than DMSO controls (Figure 5B–C, Supplemental Figure S4G). The regeneration scores are comparable between DMSO and rapamycin treatments, indicating that while growth is impaired, quality of regeneration is not (Figure 5D). In agreement with a reduced regenerate size, rapamycin-treated tails have significantly fewer dividing cells in the regenerating tissue at 24hpa and 72hpa, suggesting that mTOR is necessary to promote regeneration by activating proliferation (Figure 5E–I, Supplemental Figure S4D–F). We therefore infer that the decline in proliferation beginning by 24hpa may result in the reduced regenerate length observed by 72hpa. These data indicate that regeneration is reduced when nutrient sensing via mTOR is lost and suggest that the ability to detect sufficient nutrients may be an important part of the tadpole’s ability to execute a regenerative response following amputation.
Figure 5. Rapamycin treatment reduces regeneration and proliferation while disrupting regeneration induced yolk mobilization.
A) Schematic of treatment and collection strategy. Tadpoles are amputated then treated with either DMSO (F,H), 10 µM Rapamycin (G,I), or 1 µM Torin1 before collection at indicted timepoints. B) Box- and-whisker plot showing the length of the regenerated tail normalized to vent-to-tail tip length of the tadpole at 72 hpa following DMSO or Rapamycin treatment. C) Box-and-whisker plot showing the length of the regenerated tail normalized to vent-to-tail tip length of the tadpole at 72 hpa following DMSO or Torin1 treatment. D) Regeneration quality index showing regeneration quality of DMSO vs Rapamycin treated tadpoles at 72 hpa. Regeneration scores are shown as complete, robust, poor, or none. E) Box- and-whisker plot showing total numbers of pH3 positive cells at 72 hpa. F-I) Immunohistochemistry for pH3 and vitellogenin at 24 hpa (F,G) or 72 hpa (H,I) after treatment with DMSO or 10 µM Rapamycin with DAPI counterstain. Scale bar is 150 µm. For (B,C, E), the midline represents the median of all tadpoles assayed, box size represents the interquartile range, and the lines represent the range. For (F-H), statistical significance between DMSO and Rapamycin groups is determined with a two-sided t-test (*p<0.05, **p<0.01,***p<0.001, ****p<0.0001). For (B) n ranges 8 −10; for (C) n ranges 18 – 19; for (D) n ranges 6 – 8; for (E) n ranges 9 – 10. White arrows indicate amputation site. Note autofluorescence of axial tissue in 5G was consistent across samples in this treatment group.
Proliferation and regeneration are promoted by feeding during the refractory period
Having observed that yolk stores decline as tadpoles approach the refractory period, we asked if lack of nutrient availability impacts regenerative outcomes. If nutritive stress contributes to the refractory period, then we predict that generously feeding tadpoles could promote regeneration once feeding behaviors begin. To test this, we provided sera micron (SM) to tadpoles starting at stage 46 (Figure 6A). This feeding scheme did not result in substantial changes in overall developmental rate relative to unfed clutchmates at the stages assayed (Figure 6A, C–D, Supplemental Figure S5A). We compared normalized regeneration length at 7 days post amputation (dpa) and found that fed tadpoles regenerate much more successfully than unfed tadpoles, both in length of tissue as a proportion of vent-to-tail tip length and in regeneration quality (Figure 6B–K). We then examined proliferation rates by quantifying pH3+ cells and found that proliferation rates were increased in the fed condition relative to the unfed in both uninjured and regenerative contexts. In the uninjured context, we found that proliferation rate increased in the fed condition by 9 dpf, 72 hours after the start of feeding (Figure 6 L–Q, T, Supplemental Figure S4H). In the regenerative context, we found that the absolute number of dividing cells in the regenerated tissue of fed tadpoles is higher than in unfed tadpoles, which have little to no division following injury (Figure 6, compare R to S, U, Supplemental Figure S4I).
Figure 6. Proliferation and regeneration are promoted by feeding during the refractory period.
A) Schematic of feeding and regeneration assay. Unfed group are provided no food for the duration of the experiment. Fed group are fed daily starting at stage 46 (24 hours before amputation for regeneration assays). B) Box-and-whisker plot showing regeneration length normalized to vent-to-amputation plane length. C-J) Brightfield images of tadpoles under indicated conditions. For regeneration assays, tadpoles were collected uninjured at stage 47 (C, D), 72 hpa (E,F), 96 hpa (G, H) or 7 dpa (I,J). C-J) Brightfield images of tadpoles under indicated conditions. Scale bars are 1 mm or 0.15 mm. K) Regeneration quality index showing regeneration quality of unfed and fed tadpoles at 7 dpa. Regeneration scores are shown as complete, robust, poor, or none. L-S) Immunohistochemistry for pH3 of uninjured tails at 7 dpf (L,M), 8 dpf (N,O), and 9 dpf (P,Q) under indicated unfed or fed conditions with DAPI counterstain. R,S) Immunohistochemistry for pH3 of 7 dpa tails under unfed (R) or fed (S) conditions with DAPI counterstain. Scale bar is 150 µm. T) Box-and -whisker plot showing density of pH3 positive cells in uninjured tails following feeding protocol at 7, 8, and 9 dpf. U) Box- and -whisker plot showing number of pH3 positive cells 7 dpa. For (B, T- U), statistical significance between unfed and fed groups is determined with a two-sided t-test (*p<0.05, **p<0.01, ns ‘not significant’). For (B-K), n ranges 11–12; for (L-T), n ranges 3 – 9; for (U), n is 3 per condition.
Our earlier data suggested that nutrient sensing via mTOR is necessary for regeneration, and so we asked if tadpoles needed to be fed prior to amputation or if supplementing food at the time of injury was sufficient to rescue regeneration. Tadpoles that were not fed until amputation did not regenerate, whereas those that were fed 24 or 48 hours before injury regenerated robustly (Supplemental Figure S5B–C). This suggests surplus nutrient sensing at or prior to the time of injury is critical to regenerative response and that there is a lag between feeding and ability to activate a regenerative program. Collectively, these data show that feeding is sufficient to rescue regeneration and proliferation in the refractory period of Xenopus tropicalis and suggest that access to nutrients through feeding can rescue the deficits caused by yolk depletion.
DISCUSSSION
The refractory period in Xenopus tropicalis is graded
The mid-tadpole stage refractory period of X. laevis has been fruitfully exploited to identify factors that can limit or increase regenerative competence. For example, stimulation of the BMP or Notch signaling pathways has been shown to partially rescue regeneration in the refractory period, suggesting these signals are pro-regenerative (Slack et al., 2004). Immunosuppression with a range of inhibitor-κB kinase inhibitors is also sufficient to rescue regeneration, implicating immune cell activation in this process (Fukazawa et al., 2009). Stabilization of the stress-induced transcriptional regulator hypoxia inducible factor 1α (Hif1α) by DMOG has also been shown to partially restore tail regeneration, suggesting that proper integration of stress signaling could play a role in regenerative competency (Ferreira et al., 2018). Despite these insights into pro-regenerative cues, relatively little is known about the underlying cause of the refractory period, or whether it is a common feature in anurans. Our findings confirm that a similar refractory period exists in the diploid X. tropicalis. However, there are clear differences in the onset and progression of the refractory period between the two species. Whereas in X. laevis the refractory period begins abruptly with full loss of regeneration at stage 45 (Beck et al., 2003), in X. tropicalis we first observe a decline in regeneration length at stage 43. Modest, but statistically significant loss of regenerative response persists through stage 46, and then becomes much more pronounced at stage 47 with a loss of all axial and fin regeneration. As in X. laevis, the refractory period is reversible in X. tropicalis, and if tadpoles are fed beginning by stage 47, regenerative capability is fully restored by stage 49.
Nutrient availability is constrained during the refractory period
The contribution of nutrition to regeneration has been considered in multiple contexts. Here we find regeneration is reduced beginning at stage 43, which precedes the tadpole’s ability to feed independently, and persists through the early stages of independent feeding. During this period, we find that maternal yolk stores decline, reflected in reduced vitellogenin in the tail, and although the tadpole has not fully stopped growing, rates of cell proliferation in the tail decline relative to their stage 41 levels. During this period, the introduction of a major wound such as a tail amputation would be expected to place an even greater demand on the tadpole’s limited nutritive stores, both to replace lost tissue as well as restore homeostasis and maintain overall growth. We find that yolk distribution is altered in response to injury, becoming distributed and punctate. By stage 47, when tails are refractory to regeneration, unfed tadpoles have virtually no cell proliferation in the tail and substantially reduced yolk stores.
The transition to independent feeding is a unique point in the tadpole life cycle, and the relationship between nutritive stress and regenerative competence may well differ during later regenerative stages when independent feeding has been ongoing for days or weeks. Indeed, early experiments by T.H. Morgan in older Rana tadpoles suggested that regeneration could proceed even during starvation (Morgan, 1901a). It is striking that feeding has not been previously linked to the refractory period in X. laevis. We consider two possibilities for this discrepancy. One is that feeding is simply not sufficient to restore regeneration in X. laevis, in contrast to X. tropicalis. X. tropicalis differ from X. laevis in many respects that may impact nutrient availability and mobilization, including their preferred rearing temperature and size (McNamara et al., 2018), and this may be another point of difference. The other is that the amount and timing of feeding must be specific in order to restore regeneration. We find that X. tropicalis tadpoles that are not fed until amputation do not regenerate, suggesting that the timing is critical. The possibility that nutrient stress contributes to the refractory period represents an opportunity to consider nutrient sources and their mobilization in other regenerative contexts, as well as in non-regenerative species. It is not yet clear what specific nutrient pathways are required for regeneration. The strong transcriptional upregulation of leptin after injury (Aztekin et al., 2019; Crespi & Denver, 2006; Kakebeen et al., 2020; N. R. Love et al., 2011) suggests that this may be one of the major pathways contributing to nutrient dependence and its interpretation in regeneration, although there is not yet conclusive functional evidence that any specific leptin gene product or receptor is required for appendage regeneration.
The mTOR pathway is required for normal growth and regeneration
The mTOR kinases serve a multitude of roles in various cells, which include cell growth, proliferation, cell motility, cell survival, autophagy, and regulation of transcription and translation (Giguère, 2018; Sabatini, 2017). Many of these processes must be under active regulation as regeneration proceeds in order for the final size and cell type distribution of the regenerated structure to be restored. It has been shown previously that mTOR is required for several of these processes, including proliferation, in the regeneration of zebrafish caudal fin (Hirose et al., 2014). We find that pharmacological inhibition of mTOR with rapamycin and Torin1 limits tadpole growth and regeneration, but this observation does not discriminate between which of these specific functions is most critical. Similarly, mTOR can be activated by several signaling mechanisms. One mechanism is through leptin signaling, which indicates nutrient abundance, and activates mTOR to permit growth (Haissaguerre et al., 2014; Hu et al., 2016). In mice where growth has been restricted by limited nutrition, endogenous leptin production precedes re-initiation of growth hormone production and tibial length during catch-up growth, effects which can be mimicked by leptin injection (Carro et al., 1997; Gat-Yablonski et al., 2008; Gat-Yablonski & Phillip, 2015). Conversely, inhibition of mTOR by rapamycin treatment mimics nutrient scarcity, restricting growth and proliferation (Fingar & Blenis, 2004). In regeneration, we predict that mTOR signaling is required for growth and proliferation required to regrow the tail, and inhibition of mTOR cues the tadpole that there are not enough nutrients available for the full execution of this process, similar to the natural nutrient scarcity encountered as the tadpole approaches the refractory period. While we expect that these reductions are due to mTORs role in nutrient sensing, we also must consider that decreased growth and regeneration could result from the changes to the pleiotropic roles of mTOR listed above. Interestingly, our finding that yolk is not fully depleted during the reduced regenerative period of stages 43 – 46 suggests that this cue may not be binary but rather graded to account for available, albeit respectively limited, remaining nutritional stores. As the tadpole begins to rebuild nutrient stores through independent feeding, we hypothesize that this restriction is relieved, and the capacity for regeneration is restored until metamorphosis. Consistent with this, we find that feeding must be initiated prior to tail amputation, and regeneration fails if food is first provided simultaneously with tail amputation, suggesting that nutrient reserves have not recovered enough to enable regeneration.
Our central hypothesis that nutrient levels constrain regenerative competence makes several predictions that remain to be tested. One such prediction is that levels of active mTORC1 will fall as the tadpole enters the refractory period and increase as it exits the refractory period. It will also be interesting to interrogate the functions of nutrient sensing pathways such as leptin, insulin and ghrelin in the tail during regeneration. Exogenous leptin has been shown to increase growth and cell proliferation in the X. laevis limb (Crespi & Denver, 2006) and brain (Bender et al., 2017). In zebrafish. the leptin b enhancer is a robust reporter of regeneration activation (Kang et al., 2016), but a functional role for leptin b has not been shown. Several other signals, including insulin, can also activate mTOR, and may serve as the instructive signals in regeneration, though it is also possible that these signals regulate nutrient consumption independently. Further inhibition of upstream or parallel nutrient sensing pathways might yield more robust decreases in growth and regenerative potential (Yuan et al., 2013). The precise relationships between nutrient hormone levels, growth, and regenerative potential therefore remain to be articulated.
MATERIALS AND METHODS
X. tropicalis husbandry and use
Use of Xenopus tropicalis was carried out under the approval and oversight of the IACUC committee at UW, an AALAC-accredited institution, under animal protocol 4374–01. Ovulation of adult X. tropicalis and generation of embryos by natural matings were performed according to published methods (Khokha et al., 2002; Sive et al., 2000). Embryos were reared as described in (Khokha et al., 2002). Staging was assessed by the Nieuwkoop and Faber staging series (Nieuwkoop & Faber, 1994). Tadpoles were only fed for experiments in Figure 6 or if reared beyond stage 47. For experiments in Figure 6, sera micron (SM) was used at 0.5g/mL in 1/9th Modified Ringers solution (MR). 0.39µL SM/tadpole was added directly to 25mL MR daily. 100% of media was cycled daily for both fed and unfed groups. For rearing tadpoles beyond stage 47, we followed standard feeding protocols (McNamara et al., 2018).
X. tropicalis amputation assay
Tadpoles were anesthetized with 0.05% ms-222 in 1/9x MR and tested for response to touch prior to amputation surgery. Once fully anesthetized, a sterilized scalpel was used to amputate the posterior third of the tail. Amputated tadpoles were removed from anesthetic media within 10 minutes of amputation into new 1/9x MR. Tadpoles were left to regenerate at a density of no more than 3 tadpoles to 1 mL of media.
Pharmacological inhibition
Rapamycin (Sigma-Aldrich R0395) was resuspended to a 10 mM stock in DMSO as a vehicle and Torin1 (Millipore 475991–10MG) was resuspended to a 1 mM stock in DMSO. Uninjured and injured tadpoles were reared with 0.1% DMSO, 10 µM rapamycin, or 1 µM Torin1 diluted in 1/9x MR until collection at 24 or 72 hours following treatment.
Immunohistochemistry
X. tropicalis tadpoles were fixed for 1 hour in 1x MEM with 3.7% formaldehyde at room temperature. Tadpoles were permeabilized by washing 3X 20 minutes in PBS + 0.01% Triton x-100 (PBT). Tadpoles were blocked for 1 hour at room temperature in 10% CAS-block (Invitrogen #00–8120) in PBT. Then tadpoles were incubated in primary antibody [1:1000 mouse anti-Histone H3 (phospho S10), Abcam 14955; 1:500 rabbit anti-Vitellogenin (gifted by Marc Kirschner; Jorgensen et al., 2009)] diluted in 100% CAS-block overnight at 4°C. Tadpoles were then washed 3X 10 minutes at room temperature in PBT and blocked for 30 minutes in 10% CAS-block in PBT. Secondary antibody (goat anti-mouse 488, ThermoFisher A11001; goat anti-rabbit 594, Invitrogen A-11012) were diluted 1:500 in 100% CAS-block and incubated for 2 hours at room temperature. Tadpoles were then washed 3X 10 minutes in PBT followed by a 10 minute incubation in 1:2000 DAPI (Sigma D9542) before being washed with 1xPBS for 10 more minutes. Isolated tails were mounted on slides in ProLong Gold (ThermoFisher P36930). Images were acquired using a Leica DM 5500 B microscope using a 10X objective and processed using FIJI image analysis software (Schindelin et al., 2012).
Western Blotting
To isolate proteins, 5 tails amputated just posterior to the vent were collected in 100µL of lysis buffer (in 1/9x MR, 50mM Tris pH 7.6, 150mM NaCl, 10mM EDTA, 1% TritonX-100). Samples were homogenized on ice and centrifuged for 20 minutes at 14000 rpm at 4°C to remove insoluble fraction and protein lysate was isolated. Protein concentration was quantified with a Pierce BCA assay (Thermo Scientific 23227). Samples were denatured at RT for 10 minutes by adding 1/5 volume of 5x cracking buffer (0.3125 M Tris HCl pH 6.8. 12.5% SDS, 25% betamercaptoethanol, and 25% sucrose).Samples were loaded with equal input into a Bolt 4–14% Bis-Tris Gel (Thermo Scientific NW04120BOX) and run in MOPS buffer (prepared according to manufacturer instructions) for 32 minutes at 200V. Transfer to nitrocellulose membrane was performed in transfer buffer (in dH2O, 20% methanol, 25 mM Tris, 192 mM glycine) for 50 minutes at 100V. Membrane was washed 3X 15 minutes in 1xTBST (150 mM NaCl, 10 mM Tris pH 8.0, 0.1% Tween20) then for 1 hour in 4% milk in TBST. Membranes were incubated in primary antibodies (1:1000 rabbit anti-Vitellogenin, 1:10000, mouse anti-b-actin Santa Cruz Biotech sc-47778) were incubated in 4% milk in TBST O/N at 4°C, washed for 3X 10 minutes, then incubated in secondary (1:10000 anti-mouse DyLight 680 Fisher PI35518, 1:10000 anti-rabbit DyLight 800 Fisher PISA510036) for 40 minutes at RT in the dark. After 3X 15 minute washes, membranes were imaged on a LI-COR Imagining System (LI-COR Biosciences).
Tadpole size and regeneration length measurement
Stereoscope imaging was performed on a Leica M205 FA with a color camera. Fixed tadpoles were imaged in PBS on a 1% agarose pads and measurements were recorded using the LAS software.
Cell Proliferation Analysis
Proliferation was analyzed by quantifying pH3 positive cells in the posterior 500 µm or 750 µm of uninjured tails or in tissue posterior to the amputation place during regeneration using FIJI’s Cell Counter plugin. pH3 positive cells were quantified by density (number of cells/10,000 µm2 of tissue) and by absolute number of pH3 positive cells in the defined tissue region. Size of tissue was determined by tracing the outline of the tail and determining the area with FIJI. pH3 positive cells were counted in the outlined region and density was determined by dividing cell count by the total area.
Plotting and statistical analysis
Boxplots were generated using the R package ggplot2 (Wickham, 2009). Length and pH3 measurements between stages and regeneration conditions were compared using ANOVA and post hoc Tukey HSD to identify differences between groups. Pairwise comparisons of DMSO/Rapamycin and feeding experiments were performed using a two-sided t-test. Statistical analysis was performed in R (R Core Team, 2020).
Supplementary Material
Highlights.
X. tropicalis has a graded refractory period in which tail regeneration is lost in a stepwise manner.
Loss of regenerative capacity in the refractory period correlates with a decline in cell proliferation and yolk abundance.
mTOR inhibition reduces tadpole growth and regenerative capacity.
Feeding during the natural refractory period restores regenerative competence.
Acknowledgements
We thank members of the Wills lab for thoughtful discussion throughout the course of this work. The vitellogenin antibody was a gift from Marc Kirschner’s group. We acknowledge Xenbase (www.xenbase.org) for staging and gene expression resources consulted throughout this work. This work was supported by R01NS199024 from NINDS to AEW and National Science Foundation Graduate Research Fellowship under Grant No. DGE-1762114 to JHP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Aztekin C, Hiscock TW, Marioni JC, Gurdon JB, Simons BD, & Jullien J. (2019). Identification of a regeneration-organizing cell in the Xenopus tail. Science, 364(6441), 653–658. 10.1126/science.aav9996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CW (2012). Studying Regeneration in Xenopus. In Hoppler S & Vize PD (Eds.), Xenopus Protocols (Vol. 917, pp. 525–539). Humana Press. 10.1007/978-1-61779-992-1_30 [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, & Slack JMW (2003). Molecular Pathways Needed for Regeneration of Spinal Cord and Muscle in a Vertebrate. Developmental Cell, 5(3), 429–439. 10.1016/S1534-5807(03)00233-8 [DOI] [PubMed] [Google Scholar]
- Bender MC, Sifuentes CJ, & Denver RJ (2017). Leptin Induces Mitosis and Activates the Canonical Wnt/β-Catenin Signaling Pathway in Neurogenic Regions of Xenopus Tadpole Brain. Frontiers in Endocrinology, 8, 99. 10.3389/fendo.2017.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Señaris R, Considine RV, Casanueva FF, & Dieguez C. (1997). Regulation of in vivo growth hormone secretion by leptin. Endocrinology, 138(5), 2203–2206. 10.1210/endo.138.5.5238 [DOI] [PubMed] [Google Scholar]
- Chantranupong L, Wolfson RL, & Sabatini DM (2015). Nutrient-Sensing Mechanisms across Evolution. Cell, 161(1), 67–83. 10.1016/j.cell.2015.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y-Y, Gao J-I, Chang S-F, Chang P-Y, & Lu S-C (2011). Rapamycin inhibits lipopolysaccharide induction of granulocyte-colony stimulating factor and inducible nitric oxide synthase expression in macrophages by reducing the levels of octamer-binding factor-2: Rapamycin inhibits LPS-induced G-CSF and iNOS expression. FEBS Journal, 278(1), 85–96. 10.1111/j.1742-4658.2010.07929.x [DOI] [PubMed] [Google Scholar]
- Crespi EJ, & Denver RJ (2006). Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proceedings of the National Academy of Sciences of the United States of America, 103(26), 10092–10097. 10.1073/pnas.0507519103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson AJG, & Sive H. (2006). Development of the primary mouth in Xenopus laevis. Developmental Biology, 295(2), 700–713. 10.1016/j.ydbio.2006.03.054 [DOI] [PubMed] [Google Scholar]
- Erickson JR, & Echeverri K. (2018). Learning from regeneration research organisms: The circuitous road to scar free wound healing. Developmental Biology, 433(2), 144–154. 10.1016/j.ydbio.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeillac C, Mitchell C, Celton-Morizur S, Chauvin C, Koka V, Gillet C, Albrecht JH, Desdouets C, & Pende M. (2011). S6 kinase 1 is required for rapamycin-sensitive liver proliferation after mouse hepatectomy. The Journal of Clinical Investigation, 121(7), 2821–2832. 10.1172/JCI44203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F, Raghunathan V, Luxardi G, Zhu K, & Zhao M. (2018). Early redox activities modulate Xenopus tail regeneration. Nature Communications, 9(1), 4296. 10.1038/s41467-018-06614-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoni S, & Bosco L. (1981). Comparative analysis of the regenerative capacity of caudal spinal cord in larvae of serveral Anuran amphibian species. Acta Embryologiae Et Morphologiae Experimentalis (“Halocynthia” Association”), 2(3), 199–226. [PubMed] [Google Scholar]
- Fingar DC, & Blenis J. (2004). Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene, 23(18), 3151–3171. 10.1038/sj.onc.1207542 [DOI] [PubMed] [Google Scholar]
- Fukazawa T, Naora Y, Kunieda T, & Kubo T. (2009). Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development, 136(14), 2323–2327. 10.1242/dev.033985 [DOI] [PubMed] [Google Scholar]
- Galliot B, & Ghila L. (2010). Cell plasticity in homeostasis and regeneration. Molecular Reproduction and Development, 77(10), 837–855. 10.1002/mrd.21206 [DOI] [PubMed] [Google Scholar]
- Gat-Yablonski G, & Phillip M. (2015). Nutritionally-induced catch-up growth. Nutrients, 7(1), 517–551. 10.3390/nu7010517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat-Yablonski G, Shtaif B, Abraham E, & Phillip M. (2008). Nutrition-induced catch-up growth at the growth plate. Journal of Pediatric Endocrinology & Metabolism: JPEM, 21(9), 879–893. 10.1515/jpem.2008.21.9.879 [DOI] [PubMed] [Google Scholar]
- Ge Y, Wu A-L, Warnes C, Liu J, Zhang C, Kawasome H, Terada N, Boppart MD, Schoenherr CJ, & Chen J. (2009). MTOR regulates skeletal muscle regeneration in vivo through kinase-dependent and kinase-independent mechanisms. American Journal of Physiology - Cell Physiology, 297(6), C1434–C1444. 10.1152/ajpcell.00248.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V. (2018). Canonical signaling and nuclear activity of mTOR-a teamwork effort to regulate metabolism and cell growth. The FEBS Journal, 285(9), 1572–1588. 10.1111/febs.14384 [DOI] [PubMed] [Google Scholar]
- González-Estévez C, Felix DA, Rodríguez-Esteban G, & Aboobaker AA (2012). Decreased neoblast progeny and increased cell death during starvation-induced planarian degrowth. The International Journal of Developmental Biology, 56(1–3), 83–91. 10.1387/ijdb.113452cg [DOI] [PubMed] [Google Scholar]
- González-Estévez C, Felix DA, Smith MD, Paps J, Morley SJ, James V, Sharp TV, & Aboobaker AA (2012). SMG-1 and mTORC1 act antagonistically to regulate response to injury and growth in planarians. PLoS Genetics, 8(3), e1002619. 10.1371/journal.pgen.1002619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haissaguerre M, Saucisse N, & Cota D. (2014). Influence of mTOR in energy and metabolic homeostasis. Molecular and Cellular Endocrinology, 397(1–2), 67–77. 10.1016/j.mce.2014.07.015 [DOI] [PubMed] [Google Scholar]
- Harland RM, & Grainger RM (2011). Xenopus research: Metamorphosed by genetics and genomics. Trends in Genetics: TIG, 27(12), 507–515. 10.1016/j.tig.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Shiomi T, Hozumi S, & Kikuchi Y. (2014). Mechanistic target of rapamycin complex 1 signaling regulates cell proliferation, cell survival, and differentiation in regenerating zebrafish fins. BMC Developmental Biology, 14(1), 42. 10.1186/s12861-014-0042-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Xu Y, & Liu F. (2016). Hypothalamic roles of mTOR complex I: Integration of nutrient and hormone signals to regulate energy homeostasis. American Journal of Physiology. Endocrinology and Metabolism, 310(11), E994–E1002. 10.1152/ajpendo.00121.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P, Steen JAJ, Steen H, & Kirschner MW (2009). The mechanism and pattern of yolk consumption provide insight into embryonic nutrition in Xenopus. Development, 136(9), 1539–1548. 10.1242/dev.032425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakanj P, Moussian B, Grönke S, Bustos V, Eming SA, Partridge L, & Leptin M. (2016). Insulin and TOR signal in parallel through FOXO and S6K to promote epithelial wound healing. Nature Communications, 7(1), 1–16. 10.1038/ncomms12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakebeen Anneke D., & Wills AE. (2019). More Than Just a Bandage: Closing the Gap Between Injury and Appendage Regeneration. Frontiers in Physiology, 10. 10.3389/fphys.2019.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakebeen Anneke Dixie, Chitsazan AD, Williams MC, Saunders LM, & Wills AE (2020). Chromatin accessibility dynamics and single cell RNA-Seq reveal new regulators of regeneration in neural progenitors. ELife, 9. 10.7554/eLife.52648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Hu J, Karra R, Dickson AL, Tornini VA, Nachtrab G, Gemberling M, Goldman JA, Black BL, & Poss KD (2016). Modulation of tissue repair by regeneration enhancer elements. Nature, 532(7598), 201–206. 10.1038/nature17644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokha MK, Chung C, Bustamante EL, Gaw LWK, Trott KA, Yeh J, Lim N, Lin JCY, Taverner N, Amaya E, Papalopulu N, Smith JC, Zorn AM, Harland RM, & Grammer TC (2002). Techniques and probes for the study of Xenopus tropicalis development. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 225(4), 499–510. 10.1002/dvdy.10184 [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Field J, & Farrell GC (2003). Leptin-specific mechanisms for impaired liver regeneration in ob/ob mice after toxic injury. Gastroenterology, 124(5), 1451–1464. 10.1016/S0016-5085(03)00270-1 [DOI] [PubMed] [Google Scholar]
- Love NK, Keshavan N, Lewis R, Harris WA, & Agathocleous M. (2014). A nutrient-sensitive restriction point is active during retinal progenitor cell differentiation. Development, 141(3), 697–706. 10.1242/dev.103978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love NR, Chen Y, Bonev B, Gilchrist MJ, Fairclough L, Lea R, Mohun TJ, Paredes R, Zeef LA, & Amaya E. (2011). Genome-wide analysis of gene expression during Xenopus tropicalis tadpole tail regeneration. BMC Developmental Biology, 11(1), 70. 10.1186/1471-213X-11-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love NR, Ziegler M, Chen Y, & Amaya E. (2014). Carbohydrate metabolism during vertebrate appendage regeneration: What is its role? How is it regulated?: A postulation that regenerating vertebrate appendages facilitate glycolytic and pentose phosphate pathways to fuel macromolecule biosynthesis. BioEssays, 36(1), 27–33. 10.1002/bies.201300110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques IJ, Lupi E, & Mercader N. (2019). Model systems for regeneration: Zebrafish. Development, 146(18). 10.1242/dev.167692 [DOI] [PubMed] [Google Scholar]
- McKeown CR, & Cline HT (2019). Nutrient restriction causes reversible G2 arrest in Xenopus neural progenitors. Development, 146(20). 10.1242/dev.178871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown CR, Thompson CK, & Cline, Hollis. T. (2017). Reversible developmental stasis in response to nutrient availability in the Xenopus laevis central nervous system. The Journal of Experimental Biology, 220(3), 358–368. 10.1242/jeb.151043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara S, Wlizla M, & Horb ME (2018). Husbandry, general care, and transportation of Xenopus laevis and Xenopus tropicalis. Methods in Molecular Biology (Clifton, N.J.), 1865, 1–17. 10.1007/978-1-4939-8784-9_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TH(1901a). Regeneration (Vol. 3). The Macmillan Company. [Google Scholar]
- Nieuwkoop PD, & Faber J. (1994). Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. Garland Pub. [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.r-project.org/ [Google Scholar]
- Romero R, & Baguñà J. (1991). Quantitative cellular analysis of growth and reproduction in freshwater planarians (Turbellaria; Tricladida). I. A cellular description of the intact organism. Invertebrate Reproduction & Development, 19(2), 157–165. 10.1080/07924259.1991.9672170 [DOI] [Google Scholar]
- Sabatini DM (2017). Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proceedings of the National Academy of Sciences of the United States of America, 114(45), 11818–11825. 10.1073/pnas.1716173114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, & Cardona A. (2012). Fiji: An open-source platform for biological-image analysis. Nature Methods, 9(7), 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman GG, & Pawsey GJ (1965). The utilization of yolk platelets by tissues of Xenopus embryos studied by a safranin staining method. Development, 14(2), 191–212. [PubMed] [Google Scholar]
- Sive HL, Grainger RM, & Harland RM (2000). Early Development of Xenopus Laevis: A Laboratory Manual. CSHL Press. [Google Scholar]
- Slack JMW, Beck CW, Gargioli C, & Christen B. (2004). Cellular and molecular mechanisms of regeneration in Xenopus. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 359(1445), 745–751. 10.1098/rstb.2004.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JMW, Lin G, & Chen Y. (2008). Molecular and Cellular Basis of Regeneration and Tissue Repair: The Xenopus tadpole: a new model for regeneration research. Cellular and Molecular Life Sciences, 65(1), 54–63. 10.1007/s00018-007-7431-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarize CH, Castilho RM, Bugge TH, & Gutkind JS (2010). Accelerated wound healing by mTOR activation in genetically defined mouse models. PloS One, 5(5), e10643. 10.1371/journal.pone.0010643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler JM, Bolger TG, Wallingford J, & Liu KJ (2014). Hedgehog activity controls opening of the primary mouth. Developmental Biology, 396(1), 1–7. 10.1016/j.ydbio.2014.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S, Ide S, Tokuyama R, Umeki H, Tatehara S, Kataoka S, & Satomura K. (2015). Leptin promotes wound healing in the skin. PloS One, 10(3), e0121242. 10.1371/journal.pone.0121242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka EM (2016). The Molecular and Cellular Choreography of Appendage Regeneration. Cell, 165(7), 1598–1608. 10.1016/j.cell.2016.05.038 [DOI] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, & Gray NS (2009). An ATP-competitive Mammalian Target of Rapamycin Inhibitor Reveals Rapamycin-resistant Functions of mTORC1. Journal of Biological Chemistry, 284(12), 8023–8032. 10.1074/jbc.M900301200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag. 10.1007/978-0-387-98141-3 [DOI] [Google Scholar]
- Yuan H-X, Xiong Y, & Guan K-L (2013). Nutrient Sensing, Metabolism, and Cell Growth Control. Molecular Cell, 49(3), 379–387. 10.1016/j.molcel.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X-F, Wan J, Powell C, Ramachandran R, Myers MG, & Goldman D. (2014). Leptin and IL-6 Family Cytokines Synergize to Stimulate Müller Glia Reprogramming and Retina Regeneration. Cell Reports, 9(1), 272–284. 10.1016/j.celrep.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.