Abstract
Background:
Evidence links fine particulate matter (PM2.5) to Alzheimer’s Disease (AD), but no community-based prospective cohort studies in older adults have evaluated the association between long-term exposure to PM2.5 and markers of AD neuropathology at autopsy.
Objective:
Using a well-established autopsy cohort and new spatiotemporal predictions of air pollution, we evaluated associations of 10-year PM2.5 exposure prior to death with Braak stage, Consortium to Establish a Registry for AD (CERAD) score, and combined AD neuropathologic change (ABC score).
Methods:
We used autopsy specimens (N=832) from the Adult Changes in Thought (ACT) study, with enrollment ongoing since 1994. We assigned long-term exposure at residential address based on two-week average concentrations from a newly developed spatiotemporal model. To account for potential selection bias, we conducted inverse probability weighting (IPW). Adjusting for covariates with tiered models, we performed ordinal regression for Braak and CERAD and logistic regression for dichotomized ABC score.
Results:
10-year average (SD) PM2.5 from death across the autopsy cohort was 8.2 (1.9) μg/m3. Average (SD) age at death was 89 (7) years. Each 1 μg/m3 increase in 10-year average PM2.5 prior to death was associated with a suggestive increase in the odds of worse neuropathology as indicated by CERAD score (OR: 1.35 (0.90, 1.90)) but a suggestive decreased odds of neuropathology as defined by the ABC score (OR: 0.79 (0.49, 1.19)). There was no association with Braak stage (OR: 0.99 (0.64, 1.47)).
Conclusions:
We report inconclusive associations between PM2.5 and AD neuropathology at autopsy among a cohort where 94% of individuals experienced 10-year exposures below the current EPA standard. Prior studies of AD risk factors and AD neuropathology are similarly inconclusive, suggesting alternative mechanistic pathways for disease or residual confounding.
Keywords: Alzheimer’s disease, dementia, air pollution, particulate matter, neuropathology, autopsy
INTRODUCTION
Neurodegenerative diseases, including Alzheimer’s Disease (AD) and related dementias (ADRD), pose a growing burden on our rapidly aging society [1, 2]. In 2016, dementia was the fifth leading cause of death around the world [3]. The most common cause of dementia is AD, which is characterized by the presence of extracellular β-amyloid1–42 (Aβ1–42) plaques and intraneuronal tau aggregations (neurofibrillary tangles (NFTs)), among other alterations, that disrupt cell-to-cell communication and transport and trigger pathologic inflammatory processes [4, 5].
Increasing evidence has linked air pollution, such as fine particulate matter (PM2.5), to neurodegeneration and ADRD [6–10]. Additionally, several studies have specifically documented aggregations of Aβ1–42, standardized AD stages, and other pathologic changes, in brain tissue from children, young adults, and canines exposed to high levels of air pollution [11–14]. Experimental laboratory studies also provide evidence of alterations in levels of AD and related molecular markers after exposure to air pollution and/or inhaled metals [15–17]. Current hypotheses suggest that these central nervous system (CNS) effects of PM2.5 and other air pollutants may be mediated through direct and/or indirect pathways leading to oxidative stress and inflammation [18–20].
Despite the growing links between air pollution and neurodegeneration as well as the plausible mechanisms to support these associations, no studies to date have evaluated the association between exposure to PM2.5 and AD neuropathology at death in older adults: the primary population affected by AD and one that might be particularly sensitive to the effects of PM2.5 due to decreased antioxidant defenses in the aging brain [21, 22]. Most of the human studies conducted to date have focused primarily on children and young adults and/or have been descriptive analyses [11–13, 23]. There is one recent cross-sectional study of adults with mild cognitive impairment or dementia that identified an association between elevated PM2.5 and amyloid-ß accumulation assessed via positron emission tomography (PET) scan [24].
To address this gap, we conducted a novel analysis designed to investigate the association between long-term PM2.5 exposure and markers of AD neuropathology at autopsy among participants in the Adult Changes in Thought (ACT) prospective community-based cohort study in Seattle, Washington, USA [25]. The results of this pathophysiological-based epidemiological investigation complement prior studies that have evaluated clinical outcomes in AD and expand upon our understanding of the neurotoxic effects of PM2.5.
METHODS
Study Design
This study was approved by the University of Washington Institutional Review Board. As previously described, the ACT study is a prospective community-based cohort study in Seattle, WA, USA. This cohort is comprised of an urban and suburban elderly (>65 years) population randomly sampled from a well-established health maintenance organization (HMO) (Group Health, now Kaiser Permanente of Washington) [25]. Enrollment of cognitively intact (defined as CASI (Cognitive Abilities Screening Instrument) score of > 85 or consensus diagnosis of “not demented” after comprehensive assessment) individuals began in 1994–96 (original cohort, n=2581) and has been expanded to maintain 2000 person-years at-risk per calendar year. Consent for autopsy is optional and is discussed at study enrollment and follow-up visits. When an individual does consent, it is confirmed by next-of-kin at the time of participant death, as required by Washington State law. As of the most recent ACT study data freeze in September 2018, 5546 participants have been enrolled, and 832 autopsies have been conducted.
Exposure Assessment
We assigned PM2.5 exposure uniquely to each participant based on residential addresses geocoded with ArcMap version 10.5 (Redlands, California) for individuals living within the Puget Sound modeling region covered by our spatiotemporal exposure prediction model. We obtained high quality participant address history from billing records starting in 1989; prior to that date, address data were available from various sources including Group Health/Kaiser Permanente administrative records. Updated addresses, due to participant change of residence, for example, were incorporated when possible. If participants moved out of the spatiotemporal modeling region during the course of our study, no exposure estimates were able to be generated following the move. Additional information on how gaps in address coverage were addressed are detailed in the Supplement.
We estimated annual average PM2.5 concentrations based on two-week average concentrations from a hierarchical spatiotemporal prediction model using land use regression (LUR) and geostatistical smoothing, similar to prior published work [26–28]. This new model was developed from PM2.5 monitoring data covering the years 1978–2019 across the Puget Sound region in Washington State, including 46 long-term (>2 years) regulatory monitors at 29 sites, 52 sites from research campaigns conducted in 1999–2001 and 2012, and 110 community and study participant home sites (2017–2019) using low-cost sensor measurements (with 5 sites co-located with regulatory monitors). See Supplemental Information for additional model details. For long-term averages at regulatory monitoring locations, the final model had a cross-validated R2 (R2CV) of 0.87 and a root mean square error (RMSE) of 1.29 μg/m3; at low-cost measurement sites, the corresponding values were R2CV = 0.78, RMSE = 0.89 μg/m3. Using this final model, we had the ability of predict long-term average PM2.5 at participant homes from 1978–2018; based on these data, we created different exposure averaging periods of interest for our analyses.
Outcome Assessment
Preservation and evaluation procedures for autopsied brain tissues have been described previously [29]. Briefly, neuritic plaque density in the cerebral cortex was assessed using the Consortium to Establish a Registry for AD (CERAD) score (none; sparse; moderate; frequent) [30]. NFT distribution was assessed by Braak staging (I-II; III-IV; V-VI) [31]. Combined AD pathology (plaques and NFTs) was assessed with an ABC score, in line with recent National Institute on Aging-Alzheimer’s Association (NIA-AA) recommendations [32, 33]. For tissue collected prior to these 2012 recommendations, the ABC score was simulated based on CERAD and Braak score. Because ‘intermediate’ or ‘high’ AD neuropathologic change is considered sufficient explanation for dementia [33], we converted ABC scores to a binary variable (“not/low” vs. “intermediate/high”) for our inferential analyses. The simulated score was used for all cases as the primary analysis for this endpoint to ensure consistency among participants given the substantial missingness in the raw scores.
Statistical Analysis
Accounting for Selection Bias
Selection bias is a challenge to the generalizability of the analysis [34, 35]. To be included in the autopsy dataset, participants had to pass through several stages of selection: enrollment, consent to autopsy, continuation in the study over the course of life, death, and next-of-kin consent to autopsy. To address this issue, we employed inverse probability weighting (IPW) [36, 37] to create stabilized weights for use in the inferential analyses. IPW creates a pseudo-population that allows us to model what would have happened if all individuals were included in the autopsy cohort.
Because our inferential analysis did not evaluate time-varying covariates and all aspects of selection are known at autopsy, we considered all stages of selection together in one model. Missing values of key covariates were replaced with the mode or mean for selection modeling. (Missingness was less than 7% for each of the covariates). We fit a logistic regression model to estimate the probability of inclusion in the autopsy subset, given a final set of covariates as determined by forward selection based on the following starting covariates obtained at baseline: ACT study cohort, age at baseline, birth cohort, sex, race, educational degree, neighborhood median household income, smoking status, alcohol use, regular exercise, APOE-ε4 status, body mass index (BMI), diabetes, cardiovascular disease, hypertension, multivitamin use, self-rated health, and challenges with instrumental activities of daily living (IADL). While non-linearity was explored for selected variables, such as age, we determined there was no added benefit of these alternatives. Based on the selected model, we computed stabilized weights [36, 38] using sex as the numerator. Stabilization is recommended for IPW because standard, non-stabilized weights could be very large (unstable) for observations with low probabilities [36]. Extreme weights were truncated at 10. The stabilized weights were included as the weight parameter in the inferential analyses.
Regression Analyses
We conducted ordinal logistic regression to evaluate the association between long-term exposure to PM2.5 and ordered Braak and CERAD stage. We conducted logistic regression to evaluate the association between long-term exposure to PM2.5 and dichotomized ABC score (none/low vs. intermediate/high). Importantly, we evaluated these outcomes in all individuals, regardless of clinical dementia diagnosis status. Because of the extended period of disease development in AD [39, 40], it is highly relevant to examine these neuropathologies in cognitively normal individuals. Furthermore, based on the cognitive reserve and brain reserve hypotheses, some individuals may appear cognitively normal despite significant AD-related neuropathology [41–45].
We addressed potential confounding with detailed covariate data based on baseline (enrollment) information unless otherwise noted below. The following key covariates and precision variables, with missingness filled in with mean or mode as described for the IPW modeling above, were selected in a tiered model approach: model 1 (M1) (crude/minimally adjusted model): sex [46–48], APOE genotype (defined as ≥1 ε4 allele vs. 0 ε4 alleles) [49–52], age at death [53, 54]; model 2 (M2) (a priori main model): M1 + year of death, educational degree [55, 56], neighborhood median household income [57]; model 3 (M3) (extended model): M2 + race [58–60], smoking pack years [61, 62], regular exercise [63, 64]; model 4 (M4) (extended + mediation model): M3 + body mass index (BMI) [65–67], diabetes [68–71], hypertension [72–74], and cardiovascular disease [75–77]. Strong temporal trends in our exposure data informed our decision to use calendar year of death to adjust for confounding by time. B-splines with three degrees of freedom were used to model age at death, year of death, and smoking pack years.
Our primary analyses focused on the association between 10-year average PM2.5 and the outcomes of interest, using the M2 covariates described above. We selected this exposure window because it was the longest averaging period for which we had high confidence in our exposure modeling and address history coverage for the cohort; this extended period mirrors the long disease development and progression in ADRD [39, 54]. In secondary analyses, we evaluated alternative exposure averaging periods (1-yr; 5-yr; 20-yr) as well as an exposure period incorporating a lag time (5-yr with 10-yr lag) given the extended timeline involved in the development of dementia pathologies.
In additional secondary analyses, we evaluated the potential for effect modification between APOE genotype status and PM2.5 exposure in the a priori model. If PM2.5 contributes to the development of AB plaques and NFTs through oxidative stress and neuroinflammation, then individuals with one or two copies of the ε4 allele may be more susceptible to the neurotoxic effects of PM2.5. We also evaluated the sensitivity of our results to the following cohort restrictions: never smokers; non-smoker at baseline; complete address history; and death after year 2000. We also conducted a sensitivity analysis using dichotomous rather than ordered categorical outcomes for Braak and CERAD.
The processes described above for both the selection and regression modeling were implemented on 1000 bootstrap samples drawn with replacement from the original 5546 person dataset. Point estimates were calculated by averaging the results of these replicate regression analyses, and confidence intervals were obtained from the 2.5th and 97.5th percentiles of the empirical bootstrap distribution.
All data analysis was performed using R version 4.0.0.
RESULTS
Descriptive statistics
Overall, population characteristics of the autopsy cohort were fairly similar to those of the full ACT cohort (Table 1). However, the autopsy cohort had fewer participants who were in the latest birth cohort (autopsy: 12%; full ACT: 38%); a higher proportion of participants who were white (autopsy: 94%; full ACT: 89%), a higher proportion with ≥1APOE-ε4 allele (autopsy: 27%; full ACT: 21%); a lower proportion who were obese (autopsy: 26%; full ACT: 31%), and a larger proportion of individuals with a dementia diagnosis (autopsy: 45%; full ACT: 23 %). The average (standard deviation (SD)) age at entry and age at death for individuals in the autopsy cohort was 77 (7) and 89 (7) years, respectively.
Table 1:
Population characteristics of the full cohort and autopsy cohort. Continuous variables presented as mean (standard deviation (SD)); categorical variables presented as n (%).
| Mean (SD) / n(%) | Total | Autopsy |
|---|---|---|
| (n=5546) | (n=832) | |
| Baseline Age (Years) | 74 (6) | 77(7) |
| Age at Death (Years) | 87 (7) | 89 (7) |
| ACT Cohort | ||
| Original | 2581 (47%) | 512 (61%) |
| Expansion | 811 (15%) | 188 (23%) |
| Replacement | 2154 (39%) | 132 (16%) |
| Birth Cohort | ||
| 1890–1910 | 637 (11%) | 151 (18%) |
| 1915 | 783 (14%) | 206 (25%) |
| 1920 | 1049 (19%) | 220 (26%) |
| 1925 | 973 (18%) | 158 (19%) |
| 1930–1950 | 2104 (38%) | 97 (12%) |
| ≥1 APOE ε4 allele | 1179 (21%) | 224 (27%) |
| Female | 3228 (58%) | 480 (58%) |
| White | 4956 (89%) | 786 (94%) |
| Census Tract Median Household Income | ||
| <35,000 | 528 (10%) | 77 (9%) |
| 35,000–49,999 | 1709 (31%) | 262 (31%) |
| 50,000–74,999 | 2703 (49%) | 414 (50%) |
| >75,000 | 606 (11%) | 79 (9%) |
| Degree | ||
| None | 465 (8%) | 65 (8%) |
| GED/High School | 2089 (38%) | 356 (43%) |
| Bachelors | 1285 (23%) | 197 (24%) |
| Masters | 859 (15%) | 109 (13%) |
| Doctorate | 327 (6%) | 43 (5%) |
| Other | 521 (9%) | 62 (7%) |
| Smoking Status | ||
| Never | 2706 (49%) | 372 (45%) |
| Past | 2569 (46%) | 411 (49%) |
| Current | 271 (5%) | 49 (6%) |
| Regular Exercise | 3969 (72%) | 584 (70%) |
| Body Mass Index (BMI) | ||
| Underweight/Normal | 2014 (36%) | 327 (39%) |
| Overweight | 1836 (33%) | 285 (34%) |
| Obese | 1696 (31%) | 220 (26%) |
| Diabetes | 577 (10%) | 86 (10%) |
| Cardiovascular Disease | 492 (9%) | 92 (11%) |
| Hypertension | 2284 (41%) | 314 (38%) |
| Avg PM2.5 (μg/m3) for the 10-yrs Prior to Death1 | 9.0(2.4) | 8.2(1.9) |
| Braak stage | ||
| 0 | - | 24 (3%) |
| B1 | - | 204 (25%) |
| B2 | - | 291 (35%) |
| B3 | - | 304 (37%) |
| Missing | - | 9 (1%) |
| CERAD score | ||
| None | - | 187 (22%) |
| Sparse | - | 205 (25%) |
| Moderate | - | 199 (24%) |
| Frequent | - | 238 (29%) |
| Missing | - | 3 (0.4%) |
| ABC score (simulated) | ||
| None/Low | - | 414 (50%) |
| Interm/High | - | 412 (50%) |
| Missing | - | 6 (0.7%) |
| Dementia diagnosis | 1270 (23%) | 378 (45%) |
Calculated for those 2747 in the cohort who have already died and also have air pollution predictions (out of 2771 deaths)
Exposure coverage and quality information is provided in the Supplement. Mean (SD) 10-year average PM2.5 from death across the autopsy cohort was 8.2 (1.9) μg/m3. However, this overall summary masks an important temporal trend across time, as depicted in Figure 1, with 10-year average PM2.5 decreasing over time as expected based on secular trends in air pollution. This strong temporal trend informed our decision to use calendar year of death to adjust for confounding by time. Between-year variation (SD: 2.6) was much higher than within-year variation (SD: 0.4) in our dataset.
Figure 1:
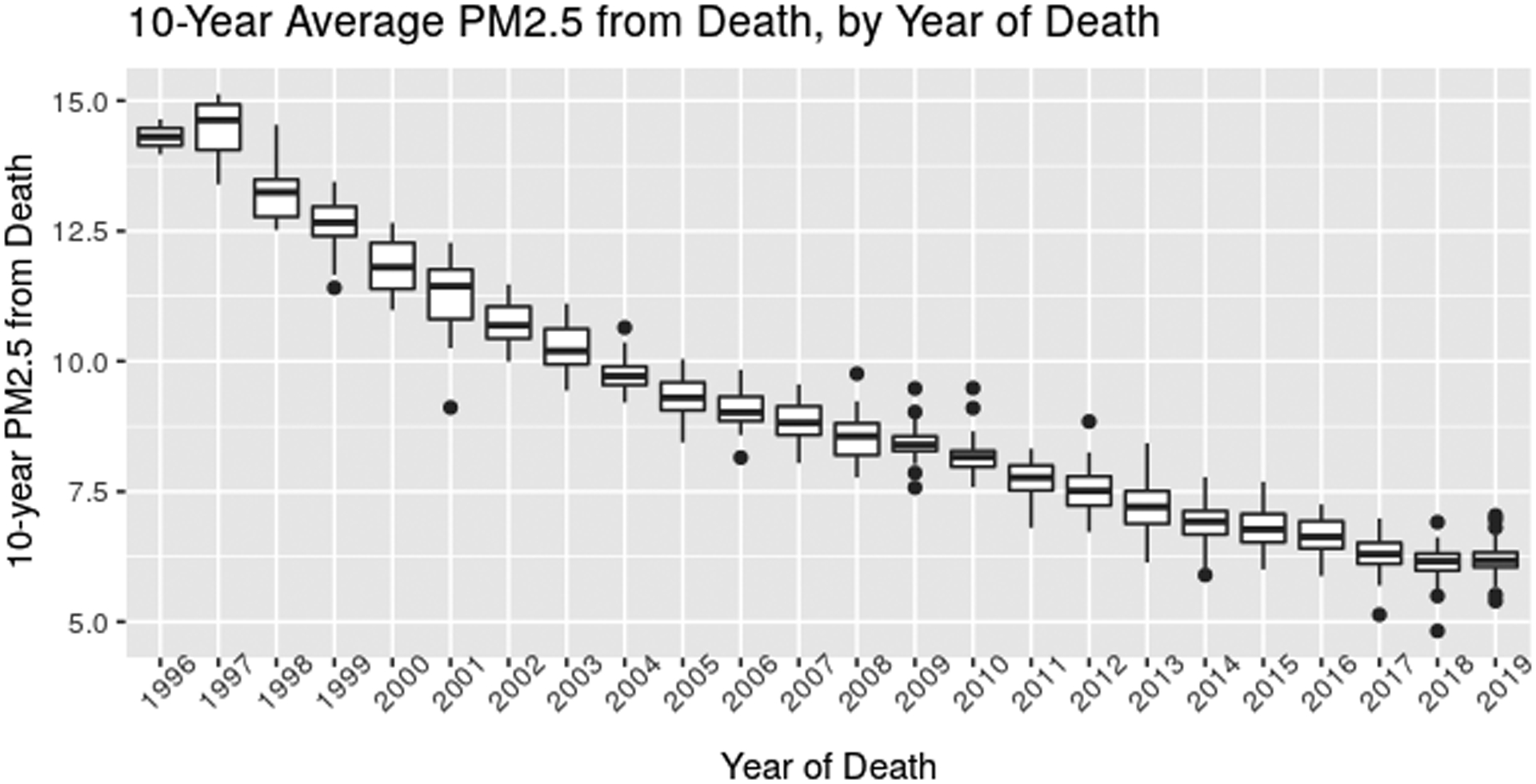
10-year average PM2.5 exposure by Year of Death in the Autopsy Cohort. In each boxplot, the middle line represents the median value; the edges of the box represent the 25th and 75th percentiles, and the whiskers extended up to 1.5 times the interquartile range (IQR). Points represent outlier observations outside this range.
With respect to the distribution of outcomes in the autopsy cohort, the most common findings were stage B3 Braak stage (37%) and frequent CERAD score (29%). There were equal proportions of individuals in the none/low and intermediate/high (simulated) ABC score groups (50%).
There were an average of 830 autopsies across 1000 bootstrapped samples of the full dataset. The only variable included in all selection models across these bootstrapped datasets was birth cohort. Other commonly selected variables included: ACT cohort, educational degree, race, APOE genotype, smoking status, self-rated health, challenges with IADL, and hypertension. After applying these selection models to the IP weight modeling process and prior to truncation, there were, on average, 6 individuals in each dataset with weights above 10. The mean of stabilized, truncated IP weights across all bootstrapped samples was 0.99, with a range of 0.25 to 10.
Regression Analyses
In our a priori primary analyses using IP-weighting, we estimated that each 1 μg/m3 increase in 10-year average PM2.5 prior to death was associated with a suggestive increase in the odds of worse brain pathology as indicated by higher CERAD score (OR: 1.35 (0.90, 1.90)) (Figure 2). However, there was no association with Braak score (OR: 0.99 (0.64, 1.47)), and PM2.5 was suggestively associated with less pathology as indicated by a lower odds of the simulated ABC score (OR: 0.79 (0.49, 1.19)). However, for all outcomes, the confidence intervals were consistent with a range of effects, including no association, and therefore we cannot draw strong conclusions.
Figure 2: Association between 1 μg/m3 increase in 10-year average exposure to PM2.5 and AD neuropathology at autopsy.
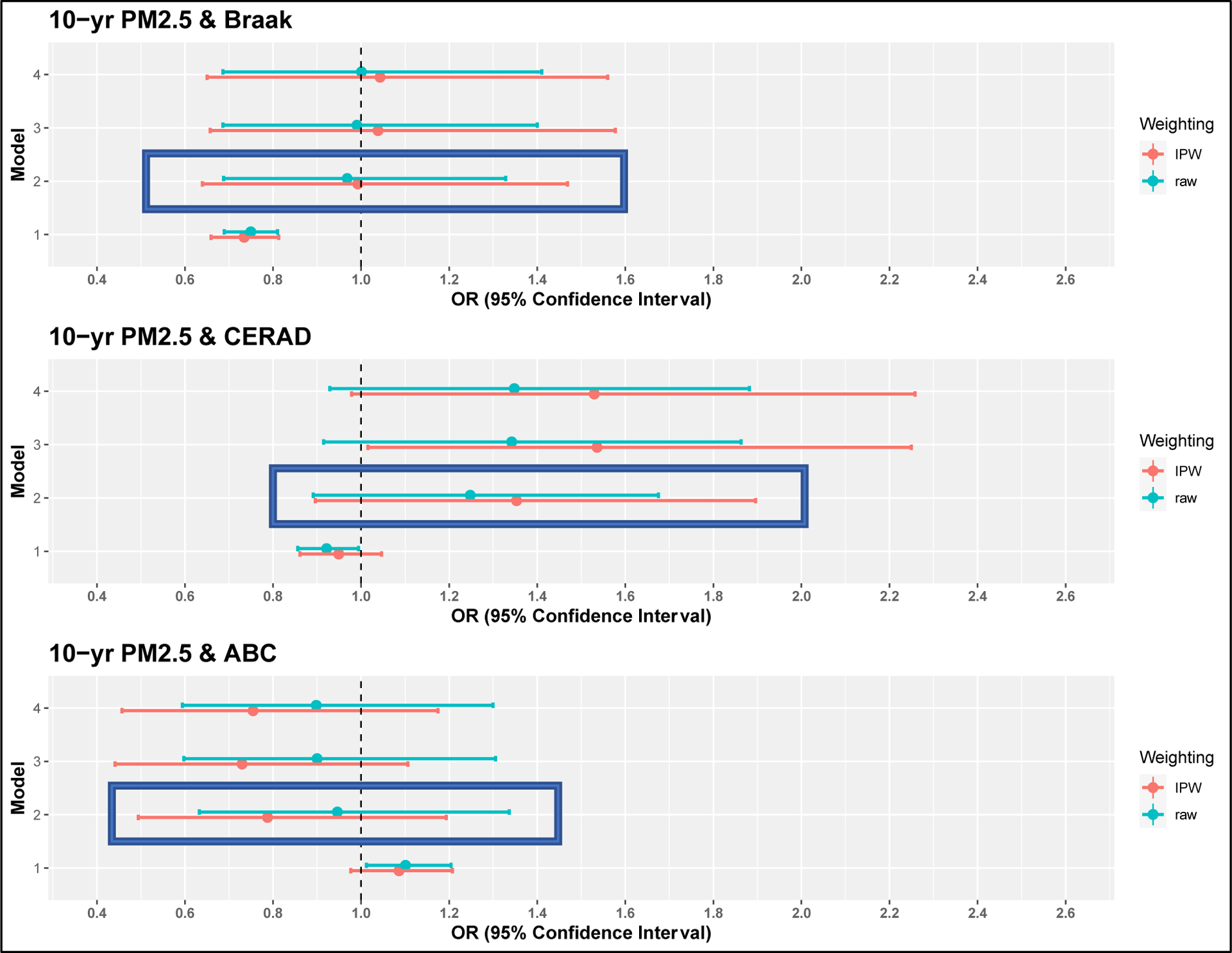
Box indicates a priori model. M1: sex, APOE-ε4 status, age at death; M2 (a priori): M1 + year of death, educational degree, neighborhood median household income; M3: M2 + race, smoking pack years, regular exercise; M4: M3 + BMI, diabetes, hypertension, and cardiovascular disease.
Overall, our results were robust to different modeling strategies. Results from crude models were attenuated while results from more richly adjusted models were similar to the primary models. Results from sensitivity analyses were similar to the primary analysis, though the estimate was larger and confidence intervals much wider when the population was restricted to never smokers for CERAD in particular (See Supplement Figure S5). Similarly, while there was some variation in the effect estimates across different exposure averaging periods, the ranges of the confidence intervals were overlapping with the primary analyses. (See Supplement Figures S6–8). Overall, IP-weighting showed associations that overlapped considerably with unweighted associations though the IP-weighted estimates had larger confidence intervals. There was no evidence of effect modification by APOE genotype (APOE interaction p-values: Braak = 0.97; CERAD = 0.09; ABC = 0.24).
DISCUSSION
Our study is the first to evaluate the association between PM2.5 and AD neuropathologies using autopsy samples in a community-based prospective cohort study comprised of older adults. While specific point estimates for the outcomes of interest suggest potential associations, the results were inconsistent across outcomes and none of the observed associations could be distinguished from no association. These findings were obtained from a population composed predominantly of individuals who self-identified as white, with an average age of nearly 90, and where 94% of the cohort had a 10-year average PM2.5 exposure below the current US annual standard of 12 μg/m3.
Prior in vivo and in vitro experimental studies suggest adverse effects of PM2.5 on AD neuropathologies. For example, 9-month PM2.5 exposure has been shown to cause early AD-related changes and increased expression of pro-inflammatory enzymes in mice [16]. Three-week and six-month exposure to diesel exhaust, a complex mixture of gases and particulates (including PM2.5), has been shown to stimulate and/or accelerate AD markers, including plaque formation, in both mice and rats [78, 79]. In vitro studies have demonstrated that PM2.5 leads to increased Aβ levels in ex vivo mouse hippocampal tissue [80] and that nano-size traffic-related PM leads to increases in both oxidative stress and Aβ production in mouse neuronal cells [81].
While there are currently no other prospective community-based cohort studies of air pollution and AD neuropathology in older adults at autopsy to our knowledge, previously published analyses that have documented aggregations of Aβ1–42, standardized AD stages, and other molecular changes such as those reflecting oxidative stress, in brain tissue from children, young and middle age adults, and canines exposed to high levels of air pollution [11–14, 23]. Yet, it is important to note that these prior data are based on populations living in Mexico City, which has a yearly average PM2.5 concentration of 25 μg/m3 [14]. By contrast, our study population experienced lower exposure concentrations: the overall mean (SD) across all years in our autopsy cohort was 8.2 (1.9) μg/m3, with a range of 4.8–15.1 μg/m3. The overall study mean is below the current U.S. Environmental Protection Agency (EPA) national ambient air quality standard for PM2.5 of 12 μg/m3, and 94% of the autopsy cohort individuals experienced a 10-year average PM2.5 below this level. This may partially explain our inconclusive results: between the lower exposure levels and the relatively small variation of exposure within year (SD: 0.4), we may not have had enough power to estimate effects of the low exposures experienced by our cohort. However, our findings of a suggestive association between PM2.5 and CERAD score results are aligned with a recently published cross-sectional study of adults with mild cognitive impairment and dementia. This study of over 18,000 adults experiencing exposures in a similar range to our study population identified associations between increased PM2.5 and Aβ accumulation assessed via PET scan [24].
Investigators have used the ACT cohort to evaluate the effect of other exposures on these standardized categorical scores and stages for AD neuropathology, providing another point of comparison for this research. For example, heavy anticholinergic use – which is linked to increased risk of dementia [82] - was associated with a suggestive increase in CERAD score (1.22 (0.81, 1.88)) and a suggestive decrease in Braak stage (0.89 (0.47, 1.66)), but both of these confidence intervals were overlapping with the null. High glucose exposure in the five years prior to death was not associated with elevated Braak stage (RR: 1.06 (0.53, 2.04)) or CERAD score (RR: 1.01 (0.67, 1.51))[83]. Our effect estimates are in a similar range to these findings from studies also investigating the impact of known dementia risk factors on AD neuropathology. These results could suggest that the elevated dementia risk from PM2.5 (or the other risk factors investigated in prior ACT studies) are mediated through mechanisms other than formation of tau tangles and beta-amyloid plaques.
Another relevant comparison may be found in autopsy studies of smoking and AD neuropathology. In an analysis using a smaller sample from the ACT cohort (N=238), Tsuang et al. reported that compared to moderate smokers (5–50 pack years), never/low smokers (0–5 pack years) had a suggestive elevated risk of higher ABC score (IP-weighted RR: 1.16 (0.55, 2.81)), while heavy smokers (>50 pack years) had a suggestive decreased risk (IP-weighted RR: 0.65 (0.17, 2.25)) though both estimates had large confidence intervals consistent with a range of effects [84]. In an analysis using the Honolulu-Asia Aging Study with light smokers (0–26.7 pack-years) as the reference category, medium (26.7–40.5 pack-years) and heavy (40.5–55.5 pack-years) smokers were more likely to have higher counts of neocortical neuritic plaques (count ratios (95% CI); medium: 2.12 (1.17, 3.86), heavy: 2.09 (1.14, 3.84)), though this association was attenuated in very heavy smokers (>55.5–156 pack-years) (1.25 (0.60, 2.58)). Associations between smoking intensity and hippocampal NFTs, neocortical NFTs, and hippocampal neuritic plaques, respectively, were mixed, with some effect estimates suggesting adverse effects while other suggesting protective effects; overall, confidence intervals for most of these estimates were overlapping with the null [85]. Most other published studies report null or protective effects of smoking and AD neuropathology [61, 86–88].
A likely reason for the inconclusive and potentially counterintuitive results across all of these smoking studies as well as our study is that these exposures are associated with premature mortality [89], yet age is associated with neuropathology [90]. Therefore, we cannot rule out the possibility that our results are biased due to the fact that age at death is behaving like a quasi-mediator in the association between PM2.5 and AD neuropathology. This can also be perceived as a form of selection bias, where individuals who die earlier from higher intensity exposures would exhibit less severe neuropathology because they died younger than other individuals. While most studies of smoking and AD neuropathology– as well as this current analysis of PM2.5 – have included age at death as a covariate, simple adjustment for this mediator may still lead to bias if there are unmeasured confounders that impact both age at death and the outcome of interest. Advancements in biostatistical methods, including by incorporating tools from the causal inference literature, are needed to address this potential bias and identify alternative estimands that better address the scientific questions at hand.
We did not observe evidence of effect modification by APOE genotype. Some prior studies of air pollution and cognitive decline or dementia risk suggests that effects of air pollution exposure may be more pronounced people with ≥1 ε4 allele [11, 12, 91–95], though other studies do not support effect modification by APOE genotype [96]. Future studies in the area of air pollution and ADRD should continue to evaluate the potential for interaction by APOE genotype.
The central, novel contribution of this work is the evaluation of the association between PM2.5 and AD neuropathological stages in a community-based cohort rather than clinical AD diagnosis alone, as has been the focus of prior cohort studies [6–8, 10]. AD neuropathology and AD dementia diagnosis may not be aligned in many individuals [97]. Due to the extended period of disease development in AD, plaques and tangles manifest prior to detectable symptoms [39, 40, 98, 99]. In fact, studies suggest that approximately 20–40% of cognitively normal elderly individuals have significant amyloid plaques [100–104], though this statistic varies by age and APOE genotype [103]. Furthermore, based on the cognitive reserve and brain reserve hypotheses, some individuals may appear cognitively normal (ie: receive no clinical AD diagnosis) despite significant AD-related neuropathology [41–45]. Therefore, a study that directly evaluates AD neuropathology provides different information than one relying on incidence data alone.
To evaluate neuropathology, we used standardized, well-accepted stage and score classifications for AD, which allows for comparison to other published studies. Yet, using continuous measures could provide more precision to answer this scientific question. Future analyses could use quantitative measures of neuropathology, such as histelide [105] or Luminex-based [106] approaches.
A major strength of this work is that we utilized a newly developed spatiotemporal exposure prediction model, specifically for the Puget Sound, that provided estimates of residence-based PM2.5 for 40 years (1978–2018). This provides extensive exposure history with which to examine our research question. We complemented this exposure assessment data with detailed address histories available through Group Health/Kaiser Permanente of Washington records, with nearly complete histories since 1989 for the entire cohort and reasonably good coverage prior to 1989. Overall, we were able to estimate 10-year average PM2.5 exposures using known address history for 98% of the individuals in the autopsy dataset across the entire study period. Evaluating a long exposure period is crucial for this research question, given the extended period of disease development in ADRD [39]. We acknowledge that our primary time window of 10 years, while an unprecedently long exposure period for this type of analysis, may not capture the etiologically relevant exposure window for AD. In fact, the specific window of vulnerability for AD is still unknown. Our sensitivity analysis investigating a 20-year period provides information on even more extended exposures and suggests similar results to our primary analysis. Future studies leveraging new technologies may be able to capture lifetime exposures to air pollution to better understand the effects of exposures across the lifecourse on the development of AD neuropathologies.
Yet, there were also challenges in utilizing an extended exposure history. For example, there were limited monitoring sites across the region during the early years; therefore, the spatial contrasts in our model rely heavily on information from more recent years. This issue is especially important to consider given that by adjusting for year of death in our a priori and extended models, we are essentially eliminating the larger between-year temporal contrasts and relying entirely on the smaller within-year spatial contrasts for the inferential analyses: the spatial contrasts during earlier periods may have more bias. Additionally, measurement error concerns aside, these low within-year spatial exposure contrasts likely explain the fairly wide confidence intervals for our results.
Another challenge for our study – as in all cohort studies of elderly populations – is selection bias, which occurs with differential enrollment or attrition of study participants. Overall, the ACT study has an exceptional Completeness of Follow-up Index (95.6%) [107], which minimizes our concern with bias due to selective attrition in general. However, differential enrollment into the autopsy cohort, specifically, could still be an issue. An important strength of this work is that we utilized IP-weighting in our inferential models to minimize the impact of this selection bias. In the end, we observed minimal impact of this weighting across our analyses.
We acknowledge that our cohort was comprised of mostly self-identified white, middle class individuals with relatively low rates of co-morbidities; therefore, our results may have limited generalizability. Lack of diversity is a well-recognized problem in ADRD research [108]. Future studies should aim to enroll and follow individuals from more diverse populations. Nevertheless, the ACT study is one of the few true community-based cohort studies of older adults, and the generalizability of results has been excellent to date [25, 109].
In summary, we report suggestive but inconclusive results regarding the association between long-term PM2.5 and AD neuropathology. Our results are similar to other studies of known AD risk factors on AD neuropathology [83, 110]. Given the potential bias resulting from mediation by age at death, a future analysis that more appropriately accounts for this factor may provide more accurate effect estimates. Further work is needed in this area, including development of appropriate statistical methods, given the growing evidence of the association between long-term exposure to PM2.5 and clinical AD [7].
Supplementary Material
ACKNOWLEDGEMENTS:
RMS was supported by NIEHS F31ES030972–02, NIEHS T32ES015459, NIA T32AG052354, the University of Washington Retirement Association Aging Fellowship, and the Seattle Chapter of the Achievement Rewards for College Scientists (ARCS) Foundation. GL, MC, JDK, EBL, PKC, and LS were supported by NIEHS and NIA R01ES026187. EBL and PKC were supported by U01 AG006781. Additional sources of support for study maintenance and data collection include NIEHS ES026187, NIA AG05136, R01 AG056711 and U01 NS091272.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
The authors would like to thank Cooper Schumacher (previously at the University of Washington) for his work in developing the fine particulate matter exposure model; Brian High, Thomas Lumley, Alex Kritchevsky, Eric Morenz, and Charles Lanfear for their programming and data analysis support; Roy Pardee for his data management; Adam Szpiro for his helpful conversations in designing the analysis; autopsy technicians and the neuropathology core coordinators for their work in analyzing the samples; and study participants, staff, and family members for their contributions to this research.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE STATEMENT:
SAD has served as a consultant to the Health Effects Institute (HEI) on matters related to air pollution.
REFERENCES:
- [1].Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M (2016) World Alzheimer report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future. [Google Scholar]
- [2].Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N Dementia prevention, intervention, and care. Lancet 390, 2673–2734. [DOI] [PubMed] [Google Scholar]
- [3].Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, Aichour MTE, Akinyemi RO, Alahdab F, Asgedom SW (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Selkoe DJ (1991) The molecular pathology of Alzheimer’s disease. Neuron 6, 487–498. [DOI] [PubMed] [Google Scholar]
- [5].Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological Alterations in Alzheimer Disease. Cold Spring Harbor Persp in Med: 1, a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen H, Kwong JC, Copes R, Hystad P, van Donkelaar A, Tu K, Brook JR, Goldberg MS, Martin RV, Murray BJ, Wilton AS, Kopp A, Burnett RT (2017) Exposure to ambient air pollution and the incidence of dementia: A population-based cohort study. Environ Int 108, 271–277. [DOI] [PubMed] [Google Scholar]
- [7].Power MC, Adar SD, Yanosky JD, Weuve J (2016) Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology 56, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oudin A, Forsberg B, Adolfsson AN, Lind N, Modig L, Nordin M, Nordin S, Adolfsson R, Nilsson L-G (2016) Traffic-Related Air Pollution and Dementia Incidence in Northern Sweden: A Longitudinal Study. Environ Health Perspect 124, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, Hystad P, Martin RV, Murray BJ, Jessiman B, Wilton AS, Kopp A, Burnett RT (2017) Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 389, 718–726. [DOI] [PubMed] [Google Scholar]
- [10].Yuchi W, Sbihi H, Davies H, Tamburic L, Brauer M (2020) Road proximity, air pollution, noise, green space and neurologic disease incidence: a population-based cohort study. Environ Health 19, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, Dragustinovis I, Franco-Lira M, Aragon-Flores M, Solt AC (2004) Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol 32, 650–658. [DOI] [PubMed] [Google Scholar]
- [12].Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Stone I, Garcia R, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, Reed W (2008) Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 36, 289–310. [DOI] [PubMed] [Google Scholar]
- [13].Calderon-Garciduenas L, Franco-Lira M, Henriquez-Roldan C, Osnaya N, Gonzalez-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Herritt L, Brooks D, Keefe S (2010) Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol 62, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, Gomez-Garza G, Barragan-Mejia G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR (2008) Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn 68, 117–127. [DOI] [PubMed] [Google Scholar]
- [15].Durga M, Devasena T, Rajasekar A (2015) Determination of LC50 and sub-chronic neurotoxicity of diesel exhaust nanoparticles. Environ Toxicol Pharmacol 40, 615–625. [DOI] [PubMed] [Google Scholar]
- [16].Bhatt DP, Puig KL, Gorr MW, Wold LE, Combs CK (2015) A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS One 10, e0127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim SH, Knight EM, Saunders EL, Cuevas AK, Popovech M, Chen L-C, Gandy S (2012) Rapid doubling of Alzheimer’s amyloid-β40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000Research 1, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Block ML, Calderon-Garciduenas L (2009) Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jayaraj RL, Rodriguez EA, Wang Y, Block ML (2017) Outdoor Ambient Air Pollution and Neurodegenerative Diseases: the Neuroinflammation Hypothesis. Curr Environ Health Rep 4, 166–179. [DOI] [PubMed] [Google Scholar]
- [20].Heusinkveld HJ, Wahle T, Campbell A, Westerink RHS, Tran L, Johnston H, Stone V, Cassee FR, Schins RPF (2016) Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 56, 94–106. [DOI] [PubMed] [Google Scholar]
- [21].Galasko D, Montine TJ (2010) Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomarkers Med 4, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Golden TR, Hinerfeld DA, Melov S (2002) Oxidative stress and aging: beyond correlation. Aging Cell 1, 117–123. [DOI] [PubMed] [Google Scholar]
- [23].Calderón-Garcidueñas L, Gónzalez-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Mukherjee PS, Kulesza RJ, Torres-Jardón R, Ávila-Ramírez J, Villarreal-Ríos R (2018) Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤ 40 years of age. Environ Res 164, 475–487. [DOI] [PubMed] [Google Scholar]
- [24].Iaccarino L, La Joie R, Lesman-Segev OH, Lee E, Hanna L, Allen IE, Hillner BE, Siegel BA, Whitmer RA, Carrillo MC, Gatsonis C, Rabinovici GD (2020) Association Between Ambient Air Pollution and Amyloid Positron Emission Tomography Positivity in Older Adults With Cognitive Impairment. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB (2002) Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 59, 1737–1746. [DOI] [PubMed] [Google Scholar]
- [26].Keller JP, Olives C, Kim S-Y, Sheppard L, Sampson PD, Szpiro AA, Oron AP, Lindström J, Vedal S, Kaufman JD (2015) A Unified Spatiotemporal Modeling Approach for Predicting Concentrations of Multiple Air Pollutants in the Multi-Ethnic Study of Atherosclerosis and Air Pollution. Environ Health Perspect 123, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sampson PD, Szpiro AA, Sheppard L, Lindström J, Kaufman JD (2011) Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmos Environ 45, 6593–6606. [Google Scholar]
- [28].Szpiro AA, Sampson PD, Sheppard L, Lumley T, Adar SD, Kaufman JD (2010) Predicting intra-urban variation in air pollution concentrations with complex spatio-temporal dependencies. Environmetrics 21, 606–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ (2007) Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 62, 406–413. [DOI] [PubMed] [Google Scholar]
- [30].Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41, 479–486. [DOI] [PubMed] [Google Scholar]
- [31].Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [32].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tsuang D, Simpson KL, Li G, Barnhart RL, Edland SD, Bowen J, McCormick W, Teri L, Nochlin D, Larson EB, Thompson ML, Leverenz JB (2005) Evaluation of selection bias in an incident-based dementia autopsy case series. Alzheimer Dis Assoc Disord 19, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haneuse S, Schildcrout J, Crane P, Sonnen J, Breitner J, Larson E (2009) Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology 32, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cole SR, Hernán MA (2008) Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hernan MA, Hernandez-Diaz S, Robins JM (2004) A structural approach to selection bias. Epidemiology 15, 615–625. [DOI] [PubMed] [Google Scholar]
- [38].Hernan MA RJ (2020) Causal Inference: What If., Chapman & Hall/CRC, Boca Raton. [Google Scholar]
- [39].Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Braak H, Del Tredici K (2015) The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138, 2814–2833. [DOI] [PubMed] [Google Scholar]
- [41].Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8, 448–460. [PubMed] [Google Scholar]
- [42].Satz P (1993) Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology 7, 273. [Google Scholar]
- [43].Katzman R, Aronson M, Fuld P, Kawas C, Brown T, Morgenstern H, Frishman W, Gidez L, Eder H, Ooi WL (1989) Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol 25, 317–324. [DOI] [PubMed] [Google Scholar]
- [44].Katzman R (1993) Education and the prevalence of dementia and Alzheimer’s disease. Neurology. [DOI] [PubMed] [Google Scholar]
- [45].Stern Y (2006) Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord 20, S69–S74. [DOI] [PubMed] [Google Scholar]
- [46].Ghebremedhin E, Schultz C, Thal DR, Rüb U, Ohm TG, Braak E, Braak H (2001) Gender and age modify the association between APOE and AD-related neuropathology. Neurology 56, 1696–1701. [DOI] [PubMed] [Google Scholar]
- [47].Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA (2005) Sex differences in the clinical manifestations of alzheimer disease pathology. Arch Gen Psychiatry 62, 685–691. [DOI] [PubMed] [Google Scholar]
- [48].Clougherty JE (2010) A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect 118, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ (2009) Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 106, 6820–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD (1993) Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 90, 9649–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Postupna N, Keene CD, Crane PK, Gonzalez-Cuyar LF, Sonnen JA, Hewitt J, Rice S, Howard K, Montine KS, Larson EB, Montine TJ (2015) Cerebral Cortical Aβ 42 and PHF-τ in 325 Consecutive Brain Autopsies Stratified by Diagnosis, Location, and APOE. J Neuropathol Exp Neurol 74, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J (2004) Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology 62, 1977–1983. [DOI] [PubMed] [Google Scholar]
- [53].Pope III CA, Coleman N, Pond ZA, Burnett RT (2019) Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res, 108924. [DOI] [PubMed] [Google Scholar]
- [54].Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70, 960–969. [DOI] [PubMed] [Google Scholar]
- [55].O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, Wilkinson P, Fletcher T, Cifuentes L, Schwartz J (2003) Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect 111, 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Koepsell TD, Kurland BF, Harel O, Johnson EA, Zhou XH, Kukull WA (2008) Education, cognitive function, and severity of neuropathology in Alzheimer disease. Neurology 70, 1732–1739. [DOI] [PubMed] [Google Scholar]
- [57].Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O’Neill MS, Sheppard L, Kaufman JD (2013) Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 121, 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Perlin SA, Wong D, Sexton K (2001) Residential proximity to industrial sources of air pollution: interrelationships among race, poverty, and age. J Air Waste Manag Assoc 51, 406–421. [DOI] [PubMed] [Google Scholar]
- [59].Jones MR, Diez-Roux AV, Hajat A, Kershaw KN, O’Neill MS, Guallar E, Post WS, Kaufman JD, Navas-Acien A (2014) Race/ethnicity, residential segregation, and exposure to ambient air pollution: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Public Health 104, 2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schlesinger D, Grinberg LT, Alba JG, Naslavsky MS, Licinio L, Farfel JM, Suemoto CK, de Lucena Ferretti RE, Leite REP, de Andrade MP, dos Santos ACF, Brentani H, Pasqualucci CA, Nitrini R, Jacob-Filho W, Zatz M (2011) African ancestry protects against Alzheimer’s disease-related neuropathology. Mol Psychiatry 18, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Court JA, Johnson M, Religa D, Keverne J, Kalaria R, Jaros E, McKeith IG, Perry R, Naslund J, Perry EK (2005) Attenuation of Aβ deposition in the entorhinal cortex of normal elderly individuals associated with tobacco smoking. Neuropathol Appl Neurobiol 31, 522–535. [DOI] [PubMed] [Google Scholar]
- [62].Moreno-Gonzalez I, Estrada LD, Sanchez-Mejias E, Soto C (2013) Smoking exacerbates amyloid pathology in a mouse model of Alzheimer’s disease. Nat Commun 4, 1–10. [DOI] [PubMed] [Google Scholar]
- [63].Rovio S, Kåreholt I, Helkala E-L, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M (2005) Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol 4, 705–711. [DOI] [PubMed] [Google Scholar]
- [64].Blondell SJ, Hammersley-Mather R, Veerman JL (2014) Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 14, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA (2006) Body mass index in older persons is associated with Alzheimer disease pathology. Neurology 67, 1949–1954. [DOI] [PubMed] [Google Scholar]
- [66].Hsu DC, Mormino EC, Schultz AP, Amariglio RE, Donovan NJ, Rentz DM, Johnson KA, Sperling RA, Marshall GA (2016) Lower late-life body-mass index is associated with higher cortical amyloid burden in clinically normal elderly. J Alzeimers Dis 53, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gu Y, Scarmeas N, Cosentino S, Brandt J, Albert M, Blacker D, Dubois B, Stern Y (2014) Change in body mass index before and after Alzheimer’s disease onset. Curr Alzheimer Res 11, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T (2010) Insulin resistance is associated with the pathology of Alzheimer disease. Neurology 75, 764–770. [DOI] [PubMed] [Google Scholar]
- [69].Pruzin JJ, Nelson PT, Abner EL, Arvanitakis Z (2018) Review: Relationship of type 2 diabetes to human brain pathology. Neuropathol Appl Neurobiol 44, 347–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Beeri MS, Silverman JM, Davis KL, Marin D, Grossman HZ, Schmeidler J, Purohit DP, Perl DP, Davidson M, Mohs RC (2005) Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol A Biol Sci 60, 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rajagopalan S, Brook RD (2012) Air Pollution and Type 2 Diabetes: Mechanistic Insights. Diabetes 61, 3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Auchincloss AH, Diez Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, Goff DC, Kaufman JD, O’Neill MS (2008) Associations between Recent Exposure to Ambient Fine Particulate Matter and Blood Pressure in the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 116, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Kopp A (2013) Spatial association between ambient fine particulate matter and incident hypertension. Circulation, 129, 562–569 [DOI] [PubMed] [Google Scholar]
- [74].Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ (2000) Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS☆. Neurobiol Aging 21, 57–62. [DOI] [PubMed] [Google Scholar]
- [75].Beeri MS, Rapp M, Silverman JM, Schmeidler J, Grossman HT, Fallon JT, Purohit DP, Perl DP, Siddiqui A, Lesser G, Rosendorff C, Haroutunian V (2006) Coronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriers. Neurology 66, 1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, Pandya Y, Esh C, Connor DJ, Sabbagh M (2007) Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta neuropathol 113, 13–21. [DOI] [PubMed] [Google Scholar]
- [77].Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12, 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hullmann M, Albrecht C, van Berlo D, Gerlofs-Nijland ME, Wahle T, Boots AW, Krutmann J, Cassee FR, Bayer TA, Schins RPF (2017) Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer’s disease. Part Fibre Toxicol 14, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Levesque S, Surace MJ, McDonald J, Block ML (2011) Air pollution & the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation 8, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jang S, Kim EW, Zhang Y, Lee J, Cho SY, Ha J, Kim H, Kim E (2018) Particulate matter increases beta-amyloid and activated glial cells in hippocampal tissues of transgenic Alzheimer’s mouse: Involvement of PARP-1. Biochem Biophys Res Commun 500, 333–338. [DOI] [PubMed] [Google Scholar]
- [81].Cacciottolo M, Morgan TE, Saffari AA, Shirmohammadi F, Forman HJ, Sioutas C, Finch CE (2020) Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radic Biol Med 147, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, Yu O, Crane PK, Larson EB (2015) Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 175, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Crane PK, Walker RL, Sonnen J, Gibbons LE, Melrose R, Hassenstab J, Keene CD, Postupna N, Montine TJ, Larson EB (2016) Glucose levels during life and neuropathologic findings at autopsy among people never treated for diabetes. Neurobiol Aging 48, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Tsuang D, Larson EB, Li G, Shofer JB, Montine KS, Thompson ML, Sonnen JA, Crane PK, Leverenz JB, Montine TJ (2010) Association between lifetime cigarette smoking and Lewy body accumulation. Brain Pathol 20, 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tyas SL, White LR, Petrovitch H, Webster Ross G, Foley DJ, Heimovitz HK, Launer LJ (2003) Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol Aging 24, 589–596. [DOI] [PubMed] [Google Scholar]
- [86].Sabbagh MN, Tyas SL, Emery SC, Hansen LA, Alford MF, Reid RT, Tiraboschi P, Thal LJ (2005) Smoking affects the phenotype of Alzheimer disease. Neurology 64, 1301–1303. [DOI] [PubMed] [Google Scholar]
- [87].Ulrich J, Johannson-Locher G, Seiler WO, Stahelin HB (1997) Does smoking protect from Alzheimer’s disease? Alzheimer-type changes in 301 unselected brains from patients with known smoking history. Acta Neuropathol 94, 450–454. [DOI] [PubMed] [Google Scholar]
- [88].Hellström-Lindahl E, Mousavi M, Ravid R, Nordberg A (2004) Reduced levels of Aβ 40 and Aβ 42 in brains of smoking controls and Alzheimer’s patients. Neurobiol Dis 15, 351–360. [DOI] [PubMed] [Google Scholar]
- [89].Ezzati M, Lopez AD (2003) Estimates of global mortality attributable to smoking in 2000. Lancet 362, 847–852. [DOI] [PubMed] [Google Scholar]
- [90].Keller JN (2006) Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing Res Rev 5, 1–13. [DOI] [PubMed] [Google Scholar]
- [91].Schikowski T, Vossoughi M, Vierkötter A, Schulte T, Teichert T, Sugiri D, Fehsel K, Tzivian L, Bae I-s, Ranft U, Hoffmann B, Probst-Hensch N, Herder C, Krämer U, Luckhaus C (2015) Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ Res 142, 10–16. [DOI] [PubMed] [Google Scholar]
- [92].Calderón-Garcidueñas L, Mora-Tiscareño A, Franco-Lira M, Zhu H, Lu Z, Solorio E, Torres-Jardón R, D’Angiulli A (2015) Decreases in short term memory, IQ, and altered brain metabolic ratios in urban apolipoprotein ε4 children exposed to air pollution. J Alzheimers Dis 45, 757–770. [DOI] [PubMed] [Google Scholar]
- [93].Calderon-Garciduenas L, Kavanaugh M, Block M, D’Angiulli A, Delgado-Chavez R, Torres-Jardon R, Gonzalez-Maciel A, Reynoso-Robles R, Osnaya N, Villarreal-Calderon R, Guo R, Hua Z, Zhu H, Perry G, Diaz P (2012) Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis 28, 93–107. [DOI] [PubMed] [Google Scholar]
- [94].Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J, Serre ML, Vizuete W, Sioutas C, Morgan TE, Gatz M, Chui HC, Shumaker SA, Resnick SM, Espeland MA, Finch CE, Chen JC (2017) Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 7, e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Calderon-Garciduenas L, Jewells V, Galaz-Montoya C, van Zundert B, Perez-Calatayud A, Ascencio-Ferrel E, Valencia-Salazar G, Sandoval-Cano M, Carlos E, Solorio E, Acuna-Ayala H, Torres-Jardon R, D’Angiulli A (2016) Interactive and additive influences of Gender, BMI and Apolipoprotein 4 on cognition in children chronically exposed to high concentrations of PM2.5 and ozone. APOE 4 females are at highest risk in Mexico City. Environ Res 150, 411–422. [DOI] [PubMed] [Google Scholar]
- [96].Wu YC, Lin YC, Yu HL, Chen JH, Chen TF, Sun Y, Wen LL, Yip PK, Chu YM, Chen YC (2015) Association between air pollutants and dementia risk in the elderly. Alzheimers Dement 1, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jack CR (2020) Preclinical Alzheimer’s disease: a valid concept. Lancet Neurol 19, 31. [DOI] [PubMed] [Google Scholar]
- [98].Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, Grant EA, Berg L (1996) Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46, 707–719. [DOI] [PubMed] [Google Scholar]
- [99].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM (1998) Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol 57, 1168–1174. [DOI] [PubMed] [Google Scholar]
- [102].Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45, 358–368. [DOI] [PubMed] [Google Scholar]
- [103].Jansen WJ, Ossenkoppele R, Knol DL, et al. (2015) Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 313, 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42, 1681–1688. [DOI] [PubMed] [Google Scholar]
- [105].Postupna N, Rose SE, Bird TD, Gonzalez-Cuyar LF, Sonnen JA, Larson EB, Keene CD, Montine TJ (2012) Novel antibody capture assay for paraffin-embedded tissue detects wide-ranging amyloid beta and paired helical filament-tau accumulation in cognitively normal older adults. Brain Pathol 22, 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Keene CD, Wilson AM, Kilgore MD, Bruner LT, Postupna NO, Darvas M (2019) Luminex-based quantification of Alzheimer’s disease neuropathologic change in formalin-fixed post-mortem human brain tissue. Labo Investig 99, 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Clark TG, Altman DG, De Stavola BL (2002) Quantification of the completeness of follow-up. Lancet 359, 1309–1310. [DOI] [PubMed] [Google Scholar]
- [108].Gilmore-Bykovskyi AL, Jin Y, Gleason C, Flowers-Benton S, Block LM, Dilworth-Anderson P, Barnes LL, Shah MN, Zuelsdorff M (2019) Recruitment and retention of underrepresented populations in Alzheimer’s disease research: A systematic review. Alzheimers Dement (N Y) 5, 751–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE (2013) Glucose levels and risk of dementia. N Engl J Med 369, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Gray SL, Anderson ML, Hanlon JT, Dublin S, Walker RL, Hubbard RA, Yu O, Montine TJ, Crane PK, Sonnen JA, Keene CD, Larson EB (2018) Exposure to Strong Anticholinergic Medications and Dementia-Related Neuropathology in a Community-Based Autopsy Cohort. J Alzheimers Dis 65, 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


