Abstract
Introduction
Prostate cancer (PCa) is the most common cancer in Canadian men. Current models of survivorship care are no longer adequate to address the chronic and complex survivorship needs of patients today. Virtual care models for cancer survivorship have recently been associated with comparable clinical outcomes and lower costs to traditional follow-up care, with patients favouring off-site and on-demand visits. Building on their viability, our research group conceived the Ned Clinic—a virtual PCa survivorship model that provides patients with access to lab results, collects patient-reported outcomes, alerts clinicians to emerging issues, and promotes patient self-care. Despite the promise of the Ned Clinic, the model remains limited by its dependence on oncology specialists, lack of an autonomous triage algorithm, and has only been implemented among PCa survivors living in Ontario.
Methods and analysis
Our programme of research comprises two main research objectives: (1) to evaluate the process and cost of implementing and sustaining five nurse-led virtual PCa survivorship clinics in three provinces across Canada and identify barriers and facilitators to implementation success and (2) to assess the impact of these virtual clinics on implementation and effectiveness outcomes of enrolled PCa survivors. The design phase will involve developing an autonomous triage algorithm and redesigning the Ned Clinic towards a nurse-led service model. Site-specific implementation plans will be developed to deploy a localised nurse-led virtual clinic at each centre. Effectiveness will be evaluated using a historical control study comparing the survivorship outcomes of 300 PCa survivors enrolled in the Ned Clinic with 300 PCa survivors receiving traditional follow-up care.
Ethics and dissemination
Appropriate site-specific ethics approval will be secured prior to each research phase. Knowledge translation efforts will include diffusion, dissemination, and application approaches to ensure that knowledge is translated to both academic and lay audiences.
Keywords: oncology, adult oncology, prostate disease, health informatics, telemedicine
Strengths and limitations of this study.
This large multicentre historically controlled study will improve on an existing virtual care model for prostate cancer (PCa) survivors in Canada, with the aim of making the model more efficient, scalable, and accessible to patients.
Redesigning the Ned Clinic towards a nurse-led model of care and assessing the appropriateness of different oncology nursing roles is expected to offload overburdened oncology specialists while maintaining effective survivorship care.
The pragmatic multisite design of this study will help validate the geographical generalisability of the Ned Clinic.
All components of the virtual care model for PCa survivorship are informed by consistent and continuous engagement with patient partners who are core members of the study team.
The use of a historical control introduces threats to internal validity and limits the ability to control for variables that can impact survivor needs, quality of life, and psychological well-being.
Background
Prostate cancer (PCa) is the most common cancer in Canadian men. Early diagnosis and effective treatment have increased PCa 5-year survival rates in Canada to 95%, with the number of cancer survivors increasing correspondingly.1 2 However, cancer survivors are often left with myriad functional impairments after treatment, as well as psychosocial and mental health challenges that diminish their quality of life.3 4 Conventional approaches to post-treatment cancer follow-up care require patients to attend in-person visits with oncologists at pre-specified intervals.5 These fixed protocols may be challenging for patients to maintain to given the necessary travel, time off work and costs of attendance. Moreover, they can be poorly suited to address patient needs in a timely manner, as they were not intended to manage needs that arise between routine visits.6 7 Further, the scope of conventional specialist visits is mostly, if not entirely, focused on assessing the risk of recurrence. Despite best intentions, the current model of care has limited capacity and time to provide comprehensive follow-up that meets endorsed survivorship practice guidelines.8–11 Combined, these systemic issues suggest that current models of survivorship care were established in a different era of cancer survival and are no longer adequate to address the chronic and complex survivorship needs of patients today.
In recent years, the pursuit of virtual care in cancer follow-up has garnered interest but has not been implemented or studied widely.12 Virtual cancer care models exploit technological innovation to deliver integrated, stratified, and tailored survivorship care to patients who are at low risk of recurrence.13 Recent studies of genitourinary telemedicine clinics have demonstrated that the remote delivery of urologic care is safe, cost-effective, and yields high patient satisfaction.14 Similarly, a recent large-scale evaluation of a PCa remote surveillance programme implemented in four treatment centres in the UK showed comparable clinical outcomes and lower costs to traditional follow-up care, with patients favouring off-site and on-demand visits.15 16 Specialist follow-up virtual visits delivered through video and email have been explored by our research team and found to be acceptable for a subgroup of survivors with non-recurrence-related needs.17
The Ned Clinic
Building on the viability of previous virtual care models, our research group received funding to design, develop, and implement three virtual PCa clinics across Ontario (Toronto, Mississauga, Niagara).18 Each of these virtual clinics employs a digital health platform called Ned (“No Evidence of Disease”), which was developed by a consortium of patients, researchers, and clinicians at the University Health Network and Trillium Health Partners in Ontario, Canada to support PCa survivors throughout their survivorship journey.19–21 Ned aims to support sustainable survivorship care through streamlining shared access to health data and informing real-time clinical decisions. The platform provides patients with access to individual-level prostate-specific antigen (PSA) values directly from the province’s Ontario Laboratory Information System to their personal smartphone. Ned comprises a web app for patients to submit patient-reported outcomes (PROs) and view PSA results, as well as a clinician web app and dashboard to visualise PSA kinetics, flag concerning lab results based on patient treatment history, review PROs, and release results. Interim results from a formative evaluation of the Ned platform conducted with 50 patients (mean enrolment of 21 months) indicate that the technology is safe and acceptable to patients, with no adverse events recorded and a mean compliance rate of 85% to the monthly PROs.
Since its initial development, Ned has moved from a standalone product to an integrated service: the Ned Clinic. Once enrolled in the clinic, patients are monitored remotely by their specialist, who conducts asynchronous virtual follow-up reviews of their lab results, PROs, and medical history using the Ned clinician dashboard and either (1) generates a care plan and schedules a follow-up review in accordance with their surveillance protocol if their results and outcomes fall within an acceptable range or (2) requests a video or phone call to address emerging needs. Following each virtual interaction, specialists can generate a clinic note that is both logged on the Ned platform and the site-specific electronic medical records (EMR) system, as well as a patient report and updated care plan that patients can access on their Ned app. Figure 1 presents Ned Clinic screenshots.
Figure 1.
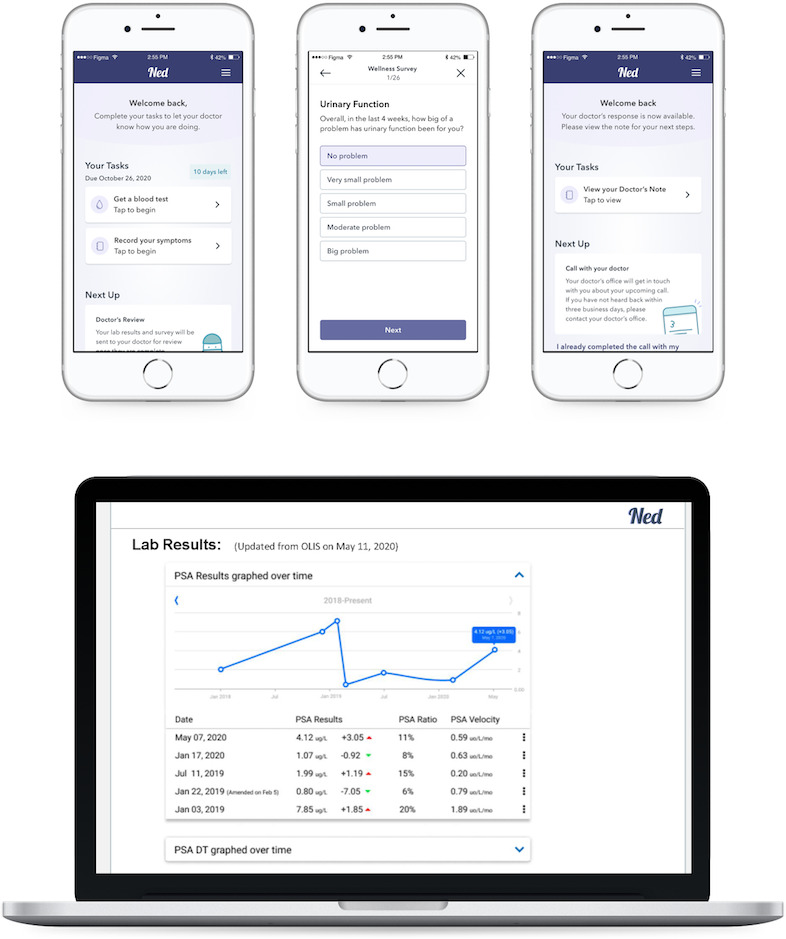
Ned Clinic patient and provider application screenshots. PSA, prostate-specific antigen; DT, doubling time; OLIS, Ontario Laboratories Information System.
Despite the promise of the Ned Clinic, there remains a pressing need to further advance the model of virtual survivorship care. First, the current model remains overly dependent on the involvement of oncology specialists. The worsening physician shortage and increasing number of specialist referrals have led to long waiting lists in Canada, with the average time between referral and specialist consultation estimated at 10 weeks.22 These factors leave specialists with demanding schedules and limited availability to address subacute patient needs. There is mounting evidence to support the effectiveness of nurse-led interventions in cancer survivorship care,23–25 as well as the numerous benefits likely to result from better integration of nursing roles in survivorship services.26–29 These findings motivate the expansion of the Ned Clinic to support the provision of nurse-led survivorship care in between specialist follow-up visits. An additional limitation of the current Ned Clinic is its lack of an autonomous triage algorithm that will be necessary to manage a large roster of PCa survivors. Algorithms have been previously used to guide PCa detection and treatment30–32; however, they have not yet been developed for the survivorship context. The operationalisation of a triaging algorithm within the existing Ned Clinic will help providers to care for those patients with the greatest needs and refer patients to tailored patient self-management strategies to optimally address symptoms. Lastly, the Ned Clinic is limited by the fact that it has only been implemented among PCa survivors living in Ontario. Implementation in new jurisdictions across Canada would help validate the generalisability of the service and allow a greater number of cancer survivors to benefit from the Ned Clinic.
Objectives
Our programme of research comprises two main research objectives: (1) to evaluate the process and cost of implementing and sustaining five nurse-led virtual PCa survivorship clinics in three provinces across Canada and identify barriers and facilitators to implementation success, and (2) to assess the impact of these virtual clinics on implementation and effectiveness outcomes of enrolled PCa survivors.
Our primary research question is as follows: in a Canadian population of adult men with PCa who are in the post-treatment follow-up phase of the survivorship journey, will a patient-centred nurse-led virtual survivorship clinic be adopted, accepted, cost-effective, and lead to improvements in unmet survivorship needs, cancer-specific quality of life, psychological well-being, and satisfaction with care?
Simultaneous to our assessment of this primary research question, we aim to answer the following secondary research questions:
How should the nurse-led service of virtual follow-up for PCa be designed and implemented to facilitate improved survivorship care and self-management?
What implementation strategies are effective for integrating Ned Clinics into varying clinical contexts and workflows to promote acceptance, adoption, and sustainability?
What is the most appropriate nursing role to fulfil all Ned Nurse responsibilities?
What are the challenges and opportunities to funding virtual services within the framework of the Canadian healthcare system from a policy perspective?
Methods and analysis
Setting
The uro-oncology programmes of five Canadian university-affiliated cancer centres will be enrolled in this research: (1) Queen Elizabeth II Health Sciences Centre in Halifax, Nova Scotia, (2) Walker Family Cancer Centre in Niagara, Ontario, (3) Carlo Fidani Cancer Centre in Mississauga, Ontario, (4) Princess Margaret Cancer Centre in Toronto, Ontario and (5) Prostate Cancer Centre in Calgary, Alberta; representing the Atlantic, Central and Prairie regions of Canada.
Methodological approach
This research will involve an initial design refinement phase followed by an implementation-effectiveness phase. The initial design phase will be guided by both user-centred design and service design principles.33 34 The latter phase will adhere to the implementation-effectiveness hybrid type 2 methodology for conducting implementation studies.35 This multimethod study will be complemented by an embedded historical control study comparing the survivorship outcomes of 300 men enrolled in the Ned Clinic with 300 men receiving traditional follow-up care. Our research methods draw from foundational work by Frankland et al on a supported self-management and remote surveillance programme for PCa follow-up care.15 16
Design and development of Ned algorithm
We will engage in a systematic and phased approach to design, build, validate, and refine a patient-centred PCa survivorship management algorithm. Previous decision algorithm development initiatives pursued by our research team have adhered to this approach and yielded a successful output.36–38 As a first step in the design phase, we recently conducted a scoping review of existing virtual care models for cancer survivorship.39 Simultaneous to this work, we have started to review current data inputs to the Ned platform with the goal of establishing clinical cut-offs for the algorithm. This formative work will inform the creation of a draft decision tree used to algorithmically triage patients in the Ned Clinic.
On completion of this work, we will convene a half-day virtual consensus meeting to validate the proposed symptom inputs and consequent alert and advice outputs. Fifteen knowledge experts will be recruited based on their clinical and research expertise in PCa survivorship to attend the meeting. We will use the nominal group technique to develop consensus on decision nodes and pathways40; this will involve two facilitated rounds of voting on various components of the algorithm, with consensus achieved when 75% of participants endorse a given answer.
Following this consensus meeting, expert responses will be used to develop a prototype algorithm for validation and refinement by 10 patients and 10 providers through semi-structured interviews. Providers will be asked to review the algorithm and provide feedback on the appropriateness of suggested alerts. Patients will be asked to validate decision pathways that lead to direct intervention from a nurse versus those that can be resolved through supported self-management strategies, as well as to comment on the format and tone of the symptom inputs and advice outputs.
Completion of all 20 interviews will result in the identification of core survivorship symptom inputs from patients, valid rules for alert generation, and appropriate survivorship self-management advice to embed in the Ned platform.
Design and development of the Nurse Ned service model
In addition to the design of the Ned Clinic triaging algorithm, the service will also undergo a redesign to become a nurse-led service model. This redesign will involve three main phases (Immersion, Design, Launch) and will be guided by the service design approach—a phased and human-centred design process used to create services that deeply consider the needs and experiences of end users.33 41
The initial immersion phase will involve qualitative research (eg, shadowing, interviews) to uncover the lived experience of nurses at all five centres. In the design phase, codesign workshops will be conducted with representative stakeholders (eg, patients, caregivers, nurses) to explore different concepts and approaches to creating a nurse-led service model.
Based on the results of these workshops, design prototypes will be created and will undergo rigorous usability testing. The product will then be committed to code and undergo quality assurance. The service model will also be finalised and documented as a service blueprint to share the insights and details of the nurse role with those involved in this care pathway.41 42
In the launch phase, we will identify three oncology nurses to assume the Ned Nurse role. Two of the nurses will work in advanced practice roles including a nurse practitioner for the Ontario sites and a clinical nurse specialist in Calgary. The third will be a specialised oncology nurse working in a non-advanced role in Halifax. Inclusion of the three types of specialised oncology roles at an advanced and non-advanced level of practice will permit evaluation to determine the most appropriate role for the Ned Nurse.
Once appointed, our Ned Nurses will (1) map all local services that are potentially accessible to survivors, (2) contact identified services to ensure availability and establish referral pathways, and (3) prepare administrative documents and processes in anticipation of the virtual clinic launch.
Implementing the Ned Clinic
Methods
The implementation of five Ned Clinics across Canada will follow the four phases defined in the Quality Implementation Framework.43
Phase 1 comprises the previously described service design activities that will minimise the disruption to each clinic site and identify site characteristics that may have implications for implementation success. Phase 2 will initiate the formation of implementation teams (ITs) that will be accountable for ensuring that effective service adaptations and evidence-based implementation methods are used to implement each Ned Clinic as intended.44 These ITs will be made up of nominated site personnel who are critical to the success of the project, human factors specialists, and project management personnel. Phase 3 will involve mobilising ITs to train Ned Clinic staff and operationalise workflows. ITs will work to create an implementation plan tailored to the specific context and culture of each clinic. Implementation progress will be monitored through regular videoconferences with each IT, core team audits, review of technical support tickets, site visits, and remote technical support. Within this structured approach, we will also embed cycles of quality improvement to adapt the intervention and uptake strategies where needed. Phase 4 will consist of aggregating the data from all participating sites, harmonising data sets, and conducting analyses. We will assess sustainability by revisiting each site 3 months after the completion of the active implementation phase. Through discussions with ITs, we will also ascertain how they have continued to run the virtual clinics and what has happened since the end of the active phase of the study.45
Data collection
Semi-structured interviews will be conducted at study end with a maximum of five virtual clinic staff (eg, clinical leads, managerial staff) from each of the five study sites. Interviews with clinic staff will be conducted in person or by telephone and will focus on identifying factors that helped or hindered virtual clinic deployment. Interviews will also be conducted with a maximum of 12 men at each of the five study sites and will include both men enrolled in Ned Clinic (eight per site) and men in the historical control group (four per site) for a total of 60 interviews. We will purposefully select a representative sample of men who vary by age, treatment history, time since treatment, and adherence to clinic tasks. Interviews with patients will focus on how men experience post-treatment follow-up care, their interactions with virtual clinic staff, and their assessment of clinic services.
Implementation outcome measures
The implementation of the Ned Clinics into routine oncological practice will be evaluated using Proctor et al’s framework of implementation outcomes.46 We will measure eight framework-guided outcomes using a combination of quantitative, qualitative, and observational research methods. Table 1 presents our full list of implementation outcomes alongside their data collection strategy and frequency of administration. The identification of barriers and facilitators to implementation will be accomplished through semi-structured interviews structured and analysed using the Consolidated Framework for Implementation Research (CFIR).47 The appropriateness of the three nursing roles will be examined in various dimensions. Role autonomy and interprofessional collaboration will be assessed through documentation of interactions with other providers, patient referrals to other providers, and the number of consultations with oncologists. Consultation with patients, nurses and oncologists will help identify the nursing expertise necessary for the Ned Nurse role, patient/nurse satisfaction with the virtual care model, and implementation issues specifically associated with a particular nursing role.
Table 1.
Ned Clinic implementation outcomes
| Outcome | Measure |
| Acceptability the perception among implementation stakeholders that the Ned Clinic is agreeable, palatable, or satisfactory | Post-implementation interviews with clinic staff (n=25) and patients (n=40) across all five study sites |
| Adoption the intention, initial decision, or action to try or employ the Ned Clinic | |
| Appropriateness the perceived fit, relevance, or compatibility of the Ned Clinic for a given practice, provider, or patient; and/or perceived fit of the clinic to address fragmented survivorship care | |
| Sustainability the extent to which the Ned Clinic is maintained or institutionalised within a service settings’ ongoing, stable operations | |
| Cost the cost impact of implementing the Ned Clinic | Clinic administrative logs |
| Feasibility the extent to which the Ned Clinic can be successfully used or carried out within a given setting | Monthly videoconferences with Implementation Teams Quarterly audit and review of technical support tickets Annual observational site visits Log data analytics Clinic administrative logs |
| Fidelity the degree to which the Ned Clinic was implemented as it was prescribed in the original protocol or as it was intended by the clinic developers | Monthly videoconferences with implementation teams Quarterly audit and review of technical support tickets Annual observational site visits Log data analytics Clinic administrative logs Checklist of fidelity to implementation service design |
| Penetration the integration of the Ned Clinic within a service setting and its subsystems; also referred to as reach | The absolute number, proportion, and representativeness of PCa patients using virtualsed clinic services from the total population of PCa patients within each cancer centre |
Ned, no evidence of disease; PCa, prostate cancer.
Data analysis
Audiorecordings of each interview will be transcribed verbatim. Two members of our research team will follow the framework method of thematic analysis and coding based on CFIR constructs.48 The multiple participating sites also allow for the comparison of barriers and facilitators between sites, which will lead to more generalisable knowledge. CFIR constructs at each deployment site will be assigned a valence (−2 to –1, 0, +1, +2) to quantify their positive, negative, or neutral impact on implementation success. Valences will be compared with identify how CFIR constructs differ between sites with low and high implementation success. Valence ratings will be determined through a deliberate consensus process among the research team members involved in the thematic analysis.47
Effectiveness of the Ned Clinic
Methods
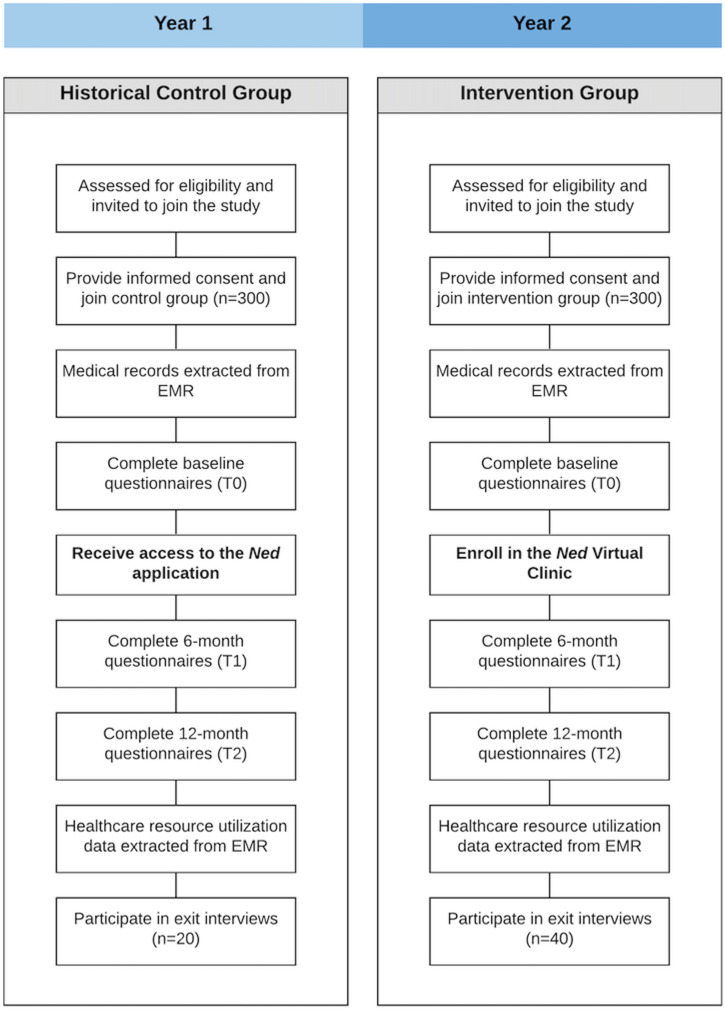
The effect of the Ned Clinics on patient outcomes will be assessed by comparing the clinical outcomes of 300 men enrolled across the five virtual clinics with the outcomes of a pre-implementation cohort of 300 men, using a repeated measures design. The evaluation will use a pragmatic approach in a real clinical setting, allowing for flexibility in terms of how virtual clinic implementation and delivery may impact outcomes.15 49 Figure 2 presents the effectiveness study flow diagram.
Figure 2.
Effectiveness study flow diagram. EMR, electronic medical records.
Comparator group
Across our five study sites, we will recruit a historical control group of 300 men from the cohort of PCa survivors receiving specialist-led follow-up care during the 1-year study period immediately prior to the introduction of the Ned Clinic. Men in the control group will receive specialist-led follow-up care for the duration of this study. To control for exposure and time spent engaging with health technology, as well as the behaviour of reflecting on outcomes and submitting monthly in-app surveys, they will also receive access to the current Ned patient-facing app. Through the app, they will be able to view PSA results, log PROs monthly, and review symptoms in context; however, they will not receive virtual care from the Ned Nurse.
Eligibility criteria
Men are eligible to participate in this research if they meet the following criteria: (1) 18 years of age or older, (2) completed curative-intent treatment, (3) receiving standard PCa post-treatment follow-up care, (4) no evidence of disease at the time of enrolment, (5) low risk of recurrence as determined by their treatment specialist, and (6) adequate English language ability (or a caregiver willing to provide translation) to complete study activities as determined by the site research assistant (RA) in charge of enrollment. If amenable, both patients and their caregivers will be enrolled in this research.
Data collection
Data will be collected from patients at baseline study entry (T0), 6 months post-baseline (T1), and 12 months post-baseline (T2). Once baseline data collection is completed, intervention group patients will be enrolled into the Ned Clinic. RAs will access site-specific EMR systems to extract clinical and treatment data (eg, cancer stage, grade, date of diagnosis, and treatment received). They will administer all outcome measures through the secure Research Electronic Data Capture (REDCap) site hosted at the Princess Margaret Cancer Centre. Patients will be prompted by research staff to log into REDCap at all three time points to complete study questionnaires.
Effectiveness outcome measures
This evaluation aims to compare the nurse-led Ned Clinic with specialist-led follow-up care across a series of outcomes that reflect the anticipated impact of virtual clinic services. Measures of unmet needs, health status, treatment side effects, cancer-specific quality of life, overall quality of life, psychological well-being, and health behaviours will be collected at three time points throughout the study (ie, T0, T1, T2). Additionally, demographic and clinical characteristics will be collected at study start, and satisfaction with follow-up care at study end. The primary effectiveness outcome for this evaluation is unmet need, assessed using the Cancer Survivors’ Unmet Needs (CaSUN) measure.50 Unmet need has been identified as an important patient-centred indicator of effective virtual survivorship programmes.16 The CaSUN assesses unmet needs across information, patient care, psychosocial, physical, and sexual domains. The 35-item scale has good acceptability, internal consistency (Cronbach’s α=0.96), construct validity, and has been used in numerous PCa survivorship trials and observational studies.51–53 Table 2 presents our full list of effectiveness outcomes alongside their data collection strategy and frequency of administration.
Table 2.
Ned Clinic effectiveness outcomes
| Outcome | Measure | Time |
| Unmet need (primary outcome) |
Cancer Survivors’ Unmet Needs50 | T0*, T1†, T2‡ |
| Health status | EQ-5D-5L57 | T0, T1, T2 |
| Prostate cancer health-related quality of life | Expanded Prostate Cancer Index Composite Short Form-2658 | T0, T1, T2 |
| Overall health-related quality of life | Functional Assessment of Cancer Therapy-Prostate59 | T0, T1, T2 |
| Psychological well-being | General Health Questionnaire-1260 | T0, T1, T2 |
| Activation to self-manage | Patient Activation Measure-1361 | T0, T2 |
| Satisfaction with care | 11 questions regarding experience and acceptability of follow-up care16 | T0, T2 |
| Health behaviours | Questions to assess health practices (eg, smoking, fitness, alcohol consumption) | T0, T2 |
| Demographic and clinical characteristics | Electronic medical record and survey data collection will be used to capture the following data points: clinic site, age, ethnicity, education, employment, marital status, living arrangement, caregiver availability, technology use, time since diagnosis, time since treatment completion, treatment type, cancer stage and grade, comorbidities, healthcare resource utilisation | T0 |
*T0: baseline (ie, at study start; immediately after the provision of informed consent).
†T1: 6 months post-baseline.
‡T2: 12 months post-baseline.
Sample size calculation
The sample size for this effectiveness evaluation is calculated to achieve at least 90% power in two-sided tests to detect a moderate statistical effect size of 0.3 or larger in the total CaSUN score at 12 months. This effect size is derived from a recent large-scale evaluation of a nurse-led remote patient monitoring programme for PCa survivors that demonstrated significant improvements in the CaSUN.16 It translates to a meaningful clinical change in strength and total number of unmet needs. Based on a calculated sample size requirement of 470 participants (ie, 235 per group) and an estimated attrition rate of 20%, we aim to recruit 120 participants (ie, 60 per group) at each of our five study sites, for a total of 600 participants (ie, 300 per group).
Data analysis
Descriptive analyses will first be conducted on baseline demographic and clinical characteristics to compare differences between study groups. We will conduct regression analyses for each of our outcome measures at 6-month and 12-month time points separately and with control for relevant baseline covariates (eg, study site, time since treatment completion, comorbidities, demographics). A mixed analysis of variance will be conducted to compare differences in outcomes at 6 and 12 months between participants enrolled in the Ned Clinic and those receiving specialist-led follow-up care in the active control group. Lastly, we will perform statistical analyses to ascertain subgroups for which virtual care is effective or undesirable. Exploratory regression analyses will also be conducted to model subgroup effects.
Patient and public involvement
We have engaged patients throughout the conceptualisation of this research.54 Our patient partners and their support group peers at Prostate Cancer Support (PCS)55 validated the rationale for pursuing this research and directed research aims to address the gaps that they perceived to be of highest priority. Moving forward, we will continue to engage patients in the research process through (1) establishing a Patient Council that will receive biannual progress reports and provide guidance regarding any necessary action; (2) attending PCS support group meetings to present research progress and seek feedback; and (3) validating design artefacts and development features and functionality with patient partners on an ad hoc basis. We aim to promote mutual respect and minimise perceived power differentials between patient partners and researchers; thus, patient partners will receive financial compensation for their involvement in research activities.
Ethics and dissemination
Ethics considerations
Ethics approval for this research programme was granted by the Clinical Trials Ontario Research Ethics Boards (CTO ID#3238). The design of the Ned algorithm and Nurse Ned service model was completed in January 2021. The historical control study is scheduled to start in Summer 2021.
Dissemination plan
Our knowledge translation efforts will include diffusion, dissemination, and application approaches to ensure that knowledge is translated to both academic and lay audiences.56 As such, we will disseminate knowledge through (1) publications in open-access journals; (2) presentations at conferences across numerous knowledge areas; (3) articles in cancer centre and university newsletters; (4) social media posts to highlight completion of research deliverables; (5) a summit for all study sites to share and contrast experiences in implementing their respective virtual clinics and (6) a 3 min video featuring patients and providers who express interest in sharing their experiences with the Ned Clinic.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the time and effort contributed to this research by the patients, providers and healthcare administrators who were involved in our formative studies. We also recognise the Ned product team (project managers, analysts, designers, and software developers) at the Centre for Global eHealth Innovation for their commitment to delivering a digital health intervention for prostate cancer survivors and their circle of care.
Footnotes
Twitter: @DrQPham
Contributors: QP conceived the rationale and designed the methods for this research with contributions from AB, JLB, DB-L, AHF and JAC. QP and JH drafted the manuscript and AB, JLB, IB, DB-L, AHF, AF, GG, RH, RR and JAC read, revised and approved the final version.
Funding: This research is supported by a Cancer Survivorship Team Grant (201909CAS) from the Canadian Cancer Society and the Canadian Institutes of Health Research.
Competing interests: All authors are involved in the design and development of the Ned prostate cancer survivorship virtual care model described in the manuscript. QP, AB, AHF, AF, RH and JAC own intellectual property rights to the Ned Clinic intervention and are entitled to personally benefit from any commercial use of the intellectual property.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Canadian Cancer Society . Survival statistics for prostate cancer. Available: https://www.cancer.ca/en/cancer-information/cancer-type/prostate/prognosis-and-survival/survival-statistics/?region=on
- 2.Canadian Cancer Society . Survival statistics for breast cancer. Available: https://www.cancer.ca/en/cancer-information/cancer-type/breast/prognosis-and-survival/survival-statistics/?region=on
- 3.Shapiro CL. Cancer survivorship. N Engl J Med 2018;379:2438–50. 10.1056/NEJMra1712502 [DOI] [PubMed] [Google Scholar]
- 4.Matthew A, Lutzky-Cohen N, Jamnicky L, et al. The prostate cancer rehabilitation clinic: a biopsychosocial clinic for sexual dysfunction after radical prostatectomy. Curr Oncol 2018;25:393–402. 10.3747/co.25.4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canadian Cancer Society . Follow-up after treatment for prostate cancer. Available: https://www.cancer.ca/en/cancer-information/cancer-type/prostate/treatment/follow-up/?region=on
- 6.O'Brien R, Rose PW, Campbell C, et al. Experiences of follow-up after treatment in patients with prostate cancer: a qualitative study. BJU Int 2010;106:998–1003. 10.1111/j.1464-410X.2010.09292.x [DOI] [PubMed] [Google Scholar]
- 7.Howell D, Hack TF, Oliver TK, et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv 2012;6:359–71. 10.1007/s11764-012-0232-z [DOI] [PubMed] [Google Scholar]
- 8.Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of clinical oncology clinical practice guideline endorsement. J Clin Oncol 2015;33:1078–85. 10.1200/JCO.2014.60.2557 [DOI] [PubMed] [Google Scholar]
- 9.Skolarus TA, Wolf AMD, Erb NL, et al. American cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin 2014;64:225–49. 10.3322/caac.21234 [DOI] [PubMed] [Google Scholar]
- 10.Cancer Journey Survivorship Expert Panel, Howell D, Hack TF, et al. Survivorship services for adult cancer populations: a pan-Canadian guideline. Curr Oncol 2011;18:265–81. 10.3747/co.v18i6.956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthew A. Follow-up care and psychosocial needs of survivors of prostate cancer., 2015. Available: http://ocp.cancercare.on.ca/common/pages/UserFile.aspx?fileId=342321
- 12.Bender JL, Yue RYK, To MJ, et al. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res 2013;15:e287. 10.2196/jmir.2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickinson R, Hall S, Sinclair JE, et al. Using technology to deliver cancer follow-up: a systematic review. BMC Cancer 2014;14:311. 10.1186/1471-2407-14-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu S, Boxer R, Madison P, et al. Veterans Affairs telemedicine: bringing urologic care to remote clinics. Urology 2015;86:255–61. 10.1016/j.urology.2015.04.038 [DOI] [PubMed] [Google Scholar]
- 15.Frankland J, Brodie H, Cooke D, et al. Follow-up care after treatment for prostate cancer: protocol for an evaluation of a nurse-led supported self-management and remote surveillance programme. BMC Cancer 2017;17:656. 10.1186/s12885-017-3643-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankland J, Brodie H, Cooke D, et al. Follow-up care after treatment for prostate cancer: evaluation of a supported self-management and remote surveillance programme. BMC Cancer 2019;19:368. 10.1186/s12885-019-5561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwan JYY, Croke J, Panzarella T, et al. Personalizing post-treatment cancer care: a cross-sectional survey of the needs and preferences of well survivors of breast cancer. Curr Oncol 2019;26:138–46. 10.3747/co.26.4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Results of the March 2018 innovation grants competition. Available: https://www.cancer.ca/en/research/funding-results/recent-competition-results/march-2018-innov-results/
- 19.Pham Q, Cafazzo JA, Feifer A. Adoption, acceptability, and effectiveness of a mobile health APP for personalized prostate cancer survivorship care: protocol for a realist case study of the Ned APP. JMIR Res Protoc 2017;6:e197. 10.2196/resprot.8051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innovation E. Ned APP: a fresh take on clinical trial protocol. eHealth innovation @ UHN. eHealth innovation @ UHN, 2017. Available: https://ehealthinnovation.org/ned-app-fresh-take-clinical-trial-protocol/
- 21.Grant helps eHealth innovation at UHN team take prostate cancer survivor APP to next level. Available: https://www.uhn.ca/corporate/News/Pages/Grant_helps_eHealth_Innovation_at_UHN_team_take_prostate_cancer_survivor_app_to_next_level.aspx
- 22.Bacchus B, Mackenzie M. Waiting your turn: wait times for health care in Canada, 2019 report. Fraser Institute, 2019. [Google Scholar]
- 23.Truant TLO, Varcoe C, Gotay CC, et al. Toward equitably high-quality cancer survivorship care. Can Oncol Nurs J 2019;29:156–62. 10.5737/23688076293156162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuominen L, Stolt M, Meretoja R, et al. Effectiveness of nursing interventions among patients with cancer: an overview of systematic reviews. J Clin Nurs 2019;28:2401–19. 10.1111/jocn.14762 [DOI] [PubMed] [Google Scholar]
- 25.Cancer Care Ontario . Models of care for cancer survivorship, 2016. Available: https://www.cancercareontario.ca/en/content/models-care-cancer-survivorship
- 26.Donald F, Kilpatrick K, Reid K, et al. A systematic review of the cost-effectiveness of nurse practitioners and clinical nurse specialists: what is the quality of the evidence? Nurs Res Pract 2014;2014:896587 10.1155/2014/896587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryant-Lukosius D, Cosby R, Bakker D, et al. Effective use of advanced practice nurses in the delivery of adult cancer services in Ontario, 2015. Available: http://ocp.cancercare.on.ca/common/pages/UserFile.aspx?fileId=340704
- 28.Marshall DA, Donald F, Lacny S, et al. Assessing the quality of economic evaluations of clinical nurse specialists and nurse practitioners: a systematic review of cost-effectiveness. NursingPlus Open 2015;1:11–17. 10.1016/j.npls.2015.07.001 [DOI] [Google Scholar]
- 29.Bryant-Lukosius D, Dicenso A. A framework for the introduction and evaluation of advanced practice nursing roles. J Adv Nurs 2004;48:530–40. 10.1111/j.1365-2648.2004.03235.x [DOI] [PubMed] [Google Scholar]
- 30.Davison BJ, Szafron M, Gutwin C, et al. Using a web-based decision support intervention to facilitate patient-physician communication at prostate cancer treatment discussions. Can Oncol Nurs J 2014;24:241–7. 10.5737/1181912x244241247 [DOI] [Google Scholar]
- 31.Thurtle D, Rossi SH, Berry B, et al. Models predicting survival to guide treatment decision-making in newly diagnosed primary non-metastatic prostate cancer: a systematic review. BMJ Open 2019;9:e029149. 10.1136/bmjopen-2019-029149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palos GR, Gilmore KR, Lewis-Patterson PA, et al. Cancer survivorship algorithms as clinical tools for primary care providers. JCO 2016;34:100. 10.1200/jco.2016.34.3_suppl.100 [DOI] [Google Scholar]
- 33.Freire K, Sangiorgi D. Service design and healthcare innovation: from consumption to co-production to co-creation 2010.
- 34.McCurdie T, Taneva S, Casselman M, et al. mHealth consumer apps: the case for user-centered design. Biomed Instrum Technol 2012;:49–56. 10.2345/0899-8205-46.s2.49 [DOI] [PubMed] [Google Scholar]
- 35.Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–26. 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jibb LA, Stevens BJ, Nathan PC, et al. A smartphone-based pain management APP for adolescents with cancer: establishing system requirements and a pain care algorithm based on literature review, interviews, and consensus. JMIR Res Protoc 2014;3:e15. 10.2196/resprot.3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seto E, Leonard KJ, Cafazzo JA, et al. Developing healthcare rule-based expert systems: case study of a heart failure telemonitoring system. Int J Med Inform 2012;81:556–65. 10.1016/j.ijmedinf.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 38.Stinson JN, Lalloo C, Harris L, et al. iCanCope with Pain™: User-centred design of a web- and mobile-based self-management program for youth with chronic pain based on identified health care needs. Pain Res Manag 2014;19:257–65. 10.1155/2014/935278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham Q, Hearn J, Gao B, et al. Virtual care models for cancer survivorship. NPJ Digit Med 2020;3:113. 10.1038/s41746-020-00321-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw J, Jamieson T, Agarwal P, et al. Virtual care policy recommendations for patient-centred primary care: findings of a consensus policy dialogue using a nominal group technique. J Telemed Telecare 2018;24:608–15. 10.1177/1357633X17730444 [DOI] [PubMed] [Google Scholar]
- 41.O’Reilly Media . This is service design doing, 2018. [Google Scholar]
- 42.Nielsen Norman Group . Service blueprints. definition. Available: https://www.nngroup.com/articles/service-blueprints-definition/
- 43.Meyers DC, Durlak JA, Wandersman A. The quality implementation framework: a synthesis of critical steps in the implementation process. Am J Community Psychol 2012;50:462–80. 10.1007/s10464-012-9522-x [DOI] [PubMed] [Google Scholar]
- 44.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci 2015;10:21. 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urquhart R, Kendell C, Cornelissen E, et al. Identifying determinants of intervention sustainability in cancer survivorship care. J Glob Oncol 2018;4:95s. 10.1200/jgo.18.28200 [DOI] [Google Scholar]
- 46.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13:117. 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Treweek S, Zwarenstein M. Making trials matter: pragmatic and explanatory trials and the problem of applicability. Trials 2009;10:37. 10.1186/1745-6215-10-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodgkinson K, Butow P, Hunt GE, et al. The development and evaluation of a measure to assess cancer survivors’ unmet supportive care needs: the CaSUN (cancer survivors' unmet needs measure). Psychooncology 2007;16:796–804. 10.1002/pon.1137 [DOI] [PubMed] [Google Scholar]
- 51.Emery JD, Jefford M, King M, et al. ProCare trial: a phase II randomized controlled trial of shared care for follow-up of men with prostate cancer. BJU Int 2017;119:381–9. 10.1111/bju.13593 [DOI] [PubMed] [Google Scholar]
- 52.Papadakos J, Quartey NK, Catton CN, et al. The prevalence and nature of unmet survivorship needs of prostate cancer patients. Int J Radiat Oncol Biol Phys 2018;102:e746. 10.1016/j.ijrobp.2018.07.1992 [DOI] [Google Scholar]
- 53.Mazariego CG, Juraskova I, Campbell R, et al. Long-term unmet supportive care needs of prostate cancer survivors: 15-year follow-up from the NSW prostate cancer care and outcomes study. Support Care Cancer 2020;28:5511–20. 10.1007/s00520-020-05389-x [DOI] [PubMed] [Google Scholar]
- 54.Canadian Institutes of Health Research . Strategy for patient-oriented research - Patient engagement framework. Available: https://cihr-irsc.gc.ca/e/48413.html
- 55.Prostate Cancer Canada Network - Toronto . Available: https://pccntoronto.ca/
- 56.Government of Canada & Canadian Institutes of Health Research . Knowledge translation in health care: moving from evidence to practice - CIHR, 2010. Available: https://cihr-irsc.gc.ca/e/40618.html
- 57.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szymanski KM, Wei JT, Dunn RL, et al. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology 2010;76:1245–50. 10.1016/j.urology.2010.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997;50:920–8. 10.1016/S0090-4295(97)00459-7 [DOI] [PubMed] [Google Scholar]
- 60.Goldberg DP, Blackwell B. Psychiatric illness in general practice. A detailed study using a new method of case identification. Br Med J 1970;1:439–43. 10.1136/bmj.2.5707.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40:1918–30. 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.