Abstract
As an important platform molecule, atropisomeric QUINOL plays a crucial role in the development of chiral ligands and catalysts in asymmetric catalysis. However, efficient approaches towards QUINOL remain scarce, and the resulting high production costs greatly impede the related academic research as well as downstream industrial applications. Here we report a direct oxidative cross-coupling reaction between isoquinolines and 2-naphthols, providing a straightforward and scalable route to acquire the privileged QUINOL scaffolds in a metal-free manner. Moreover, a NHC-catalyzed kinetic resolution of QUINOL N-oxides with high selectivity factor is established to access two types of promising axially chiral Lewis base catalysts in optically pure forms. The utility of this methodology is further illustrated by facile transformations of the products into QUINAP, an iconic ligand in asymmetric catalysis.
Subject terms: Organocatalysis, Synthetic chemistry methodology
1-(Isoquinolin-1-yl)naphthalen-2-ol (QUINOL) is an atropisomeric heterobiaryl that serves as a platform for the synthesis of other biaryl ligands useful in asymmetric catalysis. Here, the authors report a straightforward oxidative cross-coupling reaction between isoquinolines and 2-naphthols to efficiently access the QUINOL scaffolds in a metal-free manner.
Introduction
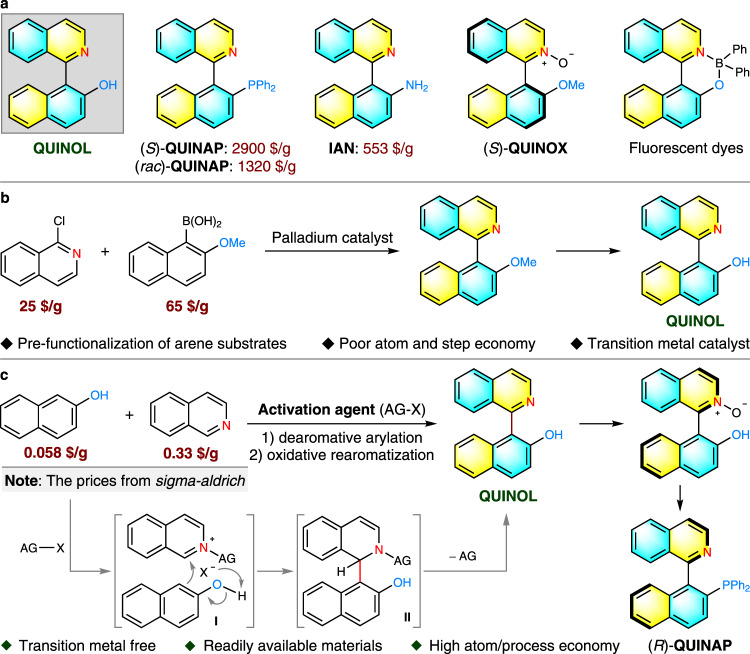
The capability to procure axially chiral biaryl skeletons with facility and efficiency has a major impact on advancing catalytic synthesis and material sciences owing to the widespread occurrence of these scaffolds in organocatalysts, ligands, and functional materials1–7. In particular, a streamlined access to a core framework from which modular installation of diversity is feasible for divergent construction of functional molecules, will greatly accelerate the development of these fields. 1-(Isoquinolin-1-yl)naphthalen-2-ol (QUINOL), a representative atropisomeric heterobiaryl, was described to function as reliable N–O chelating ligand in the controlled polymerization of cyclic esters8 while itself has been synthesized as a platform molecule to access other iconic ligands such as QUINAP9–13, QUINOX14,15 and IAN16,17. A novel organic dye derived from QUINOL has also shown potential as vesicle stains in confocal fluorescence microscopy imaging (Fig. 1a)18. In view of these apparent utilities, concise and practical preparation of QUINOL and its derivatives becomes imperative, which remains an unaddressed synthetic challenge. The venerable Suzuki–Miyaura coupling reaction in the transition metal catalysis domain provides a reliable entry19,20 but the necessity to pre-functionalize the coupling partners has elongated step counts, hampering overall efficiency which culminates in the high production costs (Fig. 1b). This is reflected in the prices of some commercially available ligands derived from QUINOL. As displayed in Fig. 1a, enantiopure (S)-QUINAP costs about 2900 USD/g and even the racemic analog is sold at 1320 USD/g. The cost factor coupled with a lack of modularity has immensely restricted the related academic research as well as downstream industrial applications. These factors collectively illuminate the practical appeal to devise a unified strategy that constructs QUINOLs concisely, particularly in a cost-effective and metal-free manner.
Fig. 1. The significance of QUINOL and its synthetic approaches.
a QUINOL and representative biaryl isoquinoline structures. b Current strategy: transition-metal-catalyzed cross-coupling. c Our strategy: metal-free oxidative cross-coupling.
One of the most convergent retrosynthetic disconnections of QUINOL frameworks would trace back to cross-dehydrogenative coupling of isoquinolines and 2-naphthols under oxidative conditions. It is remarkable that related method has never been accomplished, albeit the atom- and step-economy, probably due to the great disparity in redox potential between the two participating reactants. As an exceptional workaround for the limitations encountered in traditional oxidative-coupling, nucleophilic C–H functionalization of electron-deficient N-heteroarenes founded on N-activation mode has been realized, which encompasses a dearomatizing nucleophilic addition and a sequential oxidative rearomatization. Transition metals and organic reagents with strong Lewis acidity, inherent electron-deficient property as well as Lewis basicity of azaarenes are the key control elements that have been exploited for the success of this reaction type21–30. For instance, ZnMe2 was utilized by Cho and Baik in a highly regioselective alkylation of N-heteroarenes with 1,1-diborylalkanes23, while Kanai and Kuninobu developed a site-selective perfluoroalkylation of pyridines and quinolines with perfluoroalkyltrimethylsilane using a sterically bulky borane as the activator24. In addition, the N-acyl activation mode in Reissert reaction has been demonstrated to be one of the most efficient protocols for direct C–H functionalization of six-membered heteroarenes by Fier25,26 and McNally’s group27–30. Building on these literature precedents, we envisaged the suitability of this activation mode to direct selective arylation of isoquinoline with 2-naphthol which will occur through a sequence of nucleophilic addition and oxidative re-aromatization to construct QUINOLs (Fig. 1c). The challenging aspect of this reaction design belongs to that of chemoselectivity; differentiation of multiple nucleophilic centers in 2-naphthol and several electrophilic sites in activated acyl isoquinolinium intermediate must be efficient to establish the correct point of attachment. Consequently, the pursuit of an appropriate activation system becomes essential to realize this transformation.
Here, we show our successful endeavor to overcome the above-mentioned challenges, opening up a practical synthetic pathway to structurally diversified QUINOLs by means of a metal-free oxidative cross-coupling reaction between isoquinoline and 2-naphthol. On further development of a chiral NHC-catalyzed kinetic resolution of QUINOL N-oxides, two types of axially chiral QUINOL N-oxides, which are promising Lewis base catalysts can be readily accessible in optically pure forms. Importantly, scalable synthesis and useful transformation have demonstrated the utility of this methodology.
Results
Reaction condition optimization
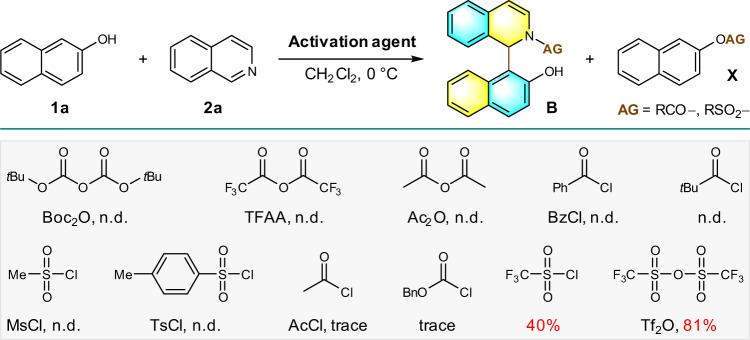
Initiating our investigations, the influence of the activation reagents on dearomative arylation step was examined in a model coupling reaction of 2-naphthol 1a and isoquinoline 2a (Fig. 2). In the excess of isoquinoline, the first attempt with Boc2O in CH2Cl2 at 0 °C only afforded the byproduct from the esterification of 2-naphthol. Evaluation of a panel of acyl chloride and anhydride including TFAA, Ac2O, BzCl, MsCl, TsCl, and pivaloyl chloride has resulted in similar reaction outcomes. The first hint of success came when a trace amount of target compound B was detected by LCMS in reactions that employed AcCl or benzyl chloroformate. Following investigations revealed that trifluoromethanesulfonyl chloride could impart major improvement in reaction efficiency to provide B in 40% isolated yield. Trifluoromethanesulfonic anhydride (Tf2O) proved to be the optimal activator for selective formation of the C–C bond under this set of conditions and delivered the desired product in 81% yield, along with 10% yield of esterification by-product.
Fig. 2. Initial screening of activation agents.
Conditions: acylation agent (0.5 mmol) and 2a (1.25 mmol) were stirred in CH2Cl2 (4 mL) for 0.5 h at 0 °C. The solution of 1a (0.50 mmol) in CH2Cl2 (4 mL) was then added and stirred for another 2 h. Isolated yield was provided.
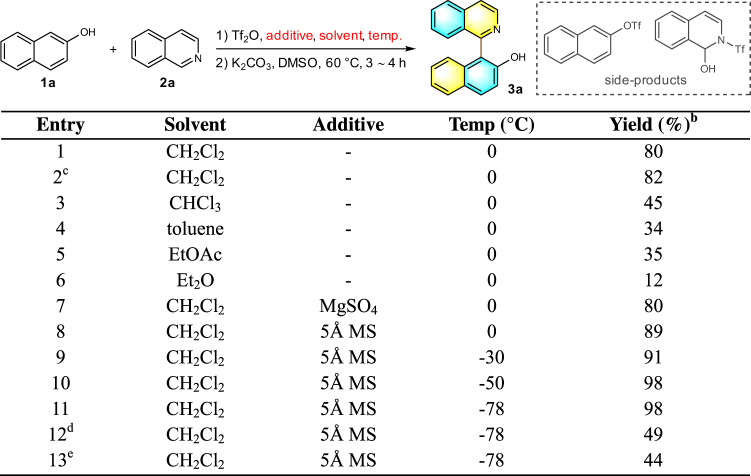
After a simple workup, intermediate B could be readily converted to the target QUINOL 3a in nearly quantitative yield by treatment with K2CO3 in DMSO at 60 °C for 3 h (Fig. 3, entry 1). The identity of 3a was confirmed by single-crystal X-ray diffraction analysis (CCDC 2005905). This achievement encouraged us to investigate other reaction parameters of the first arylation step to further enhance the overall effectiveness of this cross-coupling reaction. An increased loading of 2-naphthol (Fig. 3, entry 2) failed to suppress the side reactions, whereas switching the reaction medium to chloroform, toluene, ethyl acetate or diethyl ether did not improve the outcomes as well (Fig. 3, entries 3–6). Given H2O adduct was identified in LCMS during the initial optimization process, we reckoned that the moisture in the reaction system could be detrimental to the productive reaction pathway. As such, a series of additives known to sequester moisture were included. The addition of Mg2SO4 did not bring about any positive effect in terms of product yield (Fig. 3, entry 7) while 5 Å molecular sieve successfully obstructed the O-acylation thus raised the product yield to 89% (Fig. 3, entry 8). Interestingly, competitive esterification of 2-naphthol was inhibited altogether when at lower reaction temperatures (Fig. 3, entries 9–11) with quantitative yield being secured at −50 or −78 °C. The introduction of an exogenous base such as Et3N or NaHCO3 has eroded the chemoselectivity instead (Fig. 3, entries 12 and 13).
Fig. 3. Optimization of the conditionsa.
aReaction conditions: To a Schlenk tube was added additive (0 or 250 mg), 2a (1.25 mmol), CH2Cl2 (4 mL) and Tf2O (0.5 mmol) successively. After stirring at specified temperature for 0.5 h, a solution of 1a (0.5 mmol) in CH2Cl2 (4 mL) was added. The reaction mixture slowly increased to room temperature, and then continued to stir for 2 h. bIsolated yield. c1a (0.55 mmol) was used. d2a (0.5 mmol) and Et3N (1.5 eq.) were used. e2a (0.5 mmol) and NaHCO3 (1.5 eq.) were used.
Substrate scope
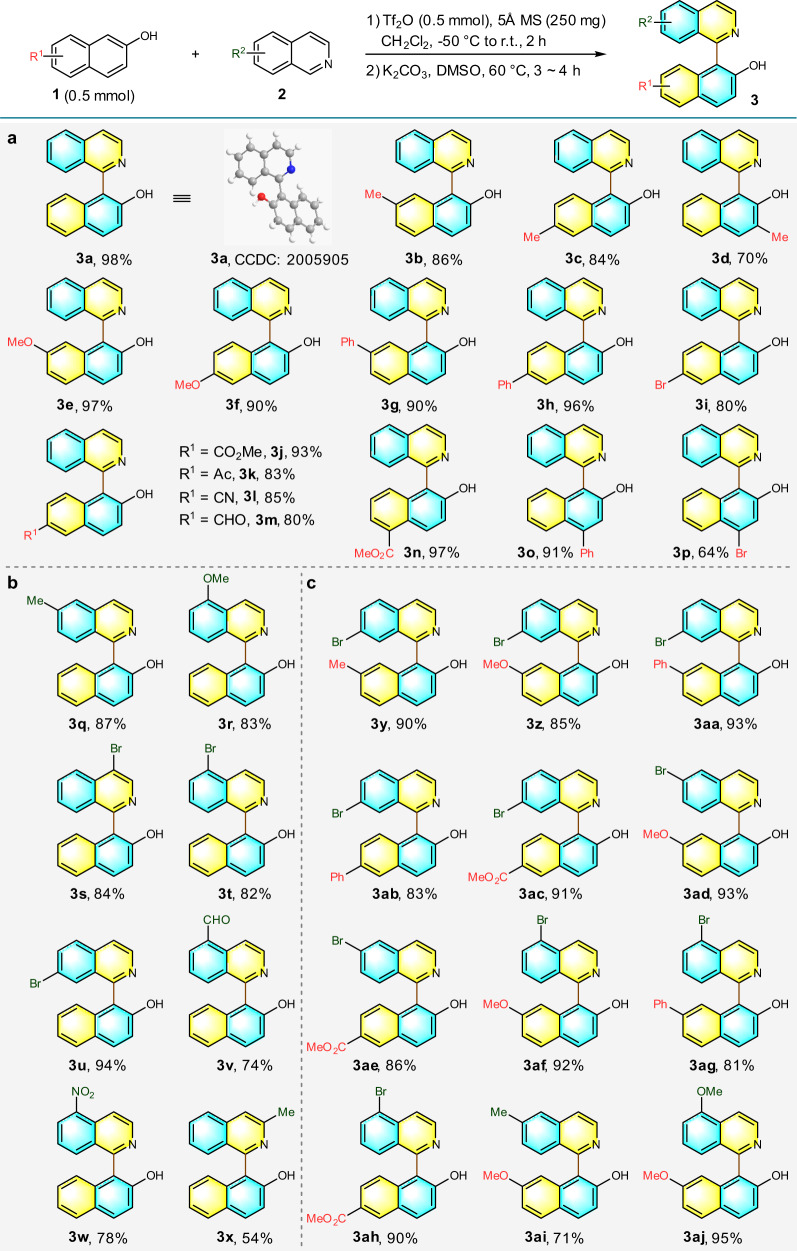
Having identified the optimal reaction parameters for the model coupling, we turned to probe the generality of this set of conditions. As shown in Fig. 4, a wide range of substrates with different substituents attached in varied patterns were well tolerated to form target products in high to excellent yields without chromatographic purification of the intermediates. Firstly, electronic variations on 2-naphthol substrates posed limited influence on the reactivity. Substrates with electron-donating and electron-withdrawing groups furnished the corresponding QUINOLs (3b–3p) in more than 80% yields within similar reaction duration, except 3d and 3p. The slight decrease in the yields of 3k, 3l and 3m may be ascribed to the poor solubility of reacting substrates. Notably, the tolerance of ester, cyano, aldehyde, and acetyl groups signified the potential chemical manipulations to introduce other useful functionalities onto product scaffold (Fig. 4b). Next, the conditions were studied with respect to variations on isoquinoline where all selected substrates generally furnished the expected products 3q–3x in high efficiency (Fig. 4b). It could be discerned that the coupling efficacy of electron-poor isoquinolines (3v and 3w) was lower compared to the more electron-rich analogs (3q and 3r) or those carrying halide moiety (3s–3u). Moreover, the 3-position substituent of isoquinoline posed a negative effect on the transformation (3x). To enrich the structural diversity of QUINOL framework, a wide spectrum of di-substituted QUINOL derivatives were synthesized smoothly using the same set of conditions (3y–3aj). It is worth pointing out that the ability to introduce substituents at both 7,7′-positions of QUINOL backbone provides the flexibility to adjust the dihedral angle of the biaryl motifs, which is a strong asset in the design and fine-tuning of ligands or organocatalysts. More importantly, bromo substituent which was commonly incompatible with conventional palladium-catalyzed Suzuki-Miyaura cross-couplings was left unscathed on both coupling partners under our metal-free reaction conditions (3i, 3s–3u, and 3y–3ah). The orthogonality of the present method to classic cross-coupling chemistry would permit downstream functionalization and further diversification of QUINOL scaffolds via these robust coupling protocols.
Fig. 4. Substrate scope of the oxidative cross-coupling of 2-naphthols and isoquinolines.
a Scope of 2-naphthalenol. b Scope of isoquinoline. c Modification of both coupling partners.
The untapped utilitarian potential of these structurally diverse QUINOL analogs in asymmetric catalysis provides a strong incentive to resolve the racemates using kinetic resolution. Initial efforts to isolate the individual enantiomers by preparative chiral HPLC revealed the low configurational stability of the stereogenic axis in these scaffolds. We sought to continue the endeavor through synthesizing the more stable QUINOL N-oxide derivatives, which could be easily achieved from the oxidation of prepared QUINOLs with DMDO. Several relevant works have served as important precedents for our approach. The preparation of axially chiral QUINOL scaffolds via kinetic resolution is pioneered by You and Gu in 2014, where Pd(II)-catalyzed direct C–H iodination forged axially chiral isoquinoline N-oxides with moderate enantioselectivity31. A biocatalytic dynamic kinetic resolution enabled by configurational lability of precursors was then prominently disclosed by Clayden and Turner32. Diversely, Matsubara and Asano attained a highly enantioselective aromatic electrophilic bromination of 1-(3-hydroxyphenyl)isoquinoline 2-oxides to construct axially chiral isoquinoline N-oxides by using a bifunctional catalyst33. Recognizing the structural characteristic of our QUINOL N-oxide products, the contributions from Zhao34 and Wang35 on enantioselective kinetic resolution of NOBIN analogs via chiral N-heterocyclic carbene (NHC) catalyzed acylation reactions inspired our formulation of an analogous strategy to obtain the axially chiral QUINOL derivatives in enantioenriched forms.
Kinetic resolution of QUINOL N-oxides
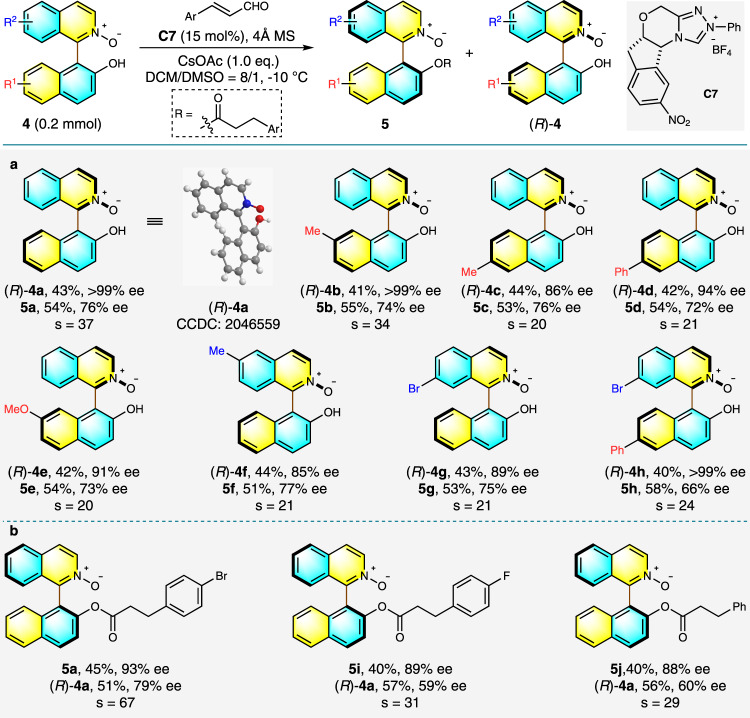
An interrogation of different NHC precatalysts for this kinetic resolution process has demonstrated the sensitivity of enantioselectivity outcome towards the identity of aryl group on the triazolium moiety where sterically hindered ones were found unfavorable. C7 which possesses a nitro group on the other aryl group, was subsequently identified as the lead precatalyst. The poor solubility of QUINOL N-oxide in most common solvents put an additional constraint on stereochemical control. Eventually, this was effectively addressed through employing a binary mixture of CH2Cl2 and DMSO (for more detailed reaction optimization of the kinetic resolution, see Supplementary Table 3). We also investigated the generality of this NHC-catalyzed kinetic resolution strategy (Fig. 5) and found it to be pleasingly broad. Most racemic QUINOL N-oxides could be stereochemically enriched to 85->99% ee and isolated in 40–44% yield. Particularly noteworthy is that the acylation products 5 and the remaining (R)-4 could be obtained with high yields and good ee values simply by adjusting the loading of aldehydes. Under the standard conditions (0.65 equiv of aldehyde), (R)-4a was produced in 43% yield with >99% ee while the acylation product 5a was afforded in 45% yield and 93% ee using 0.5 equiv. of aldehyde (Fig. 5). The absolute configuration of (R)-4a was determined by X-ray crystallographic analysis (CCDC 2046559), and those of other QUINOL N-oxides displayed in Fig. 5 were assigned by analogy.
Fig. 5. Substrate scope of NHC-catalyzed kinetic resolution of QUINOL N-oxides.
a Ar = 4-Br-Ph (0.65 eq. aldehyde). b with 0.5 eq. aldehydes.
Preparative synthesis and transformations
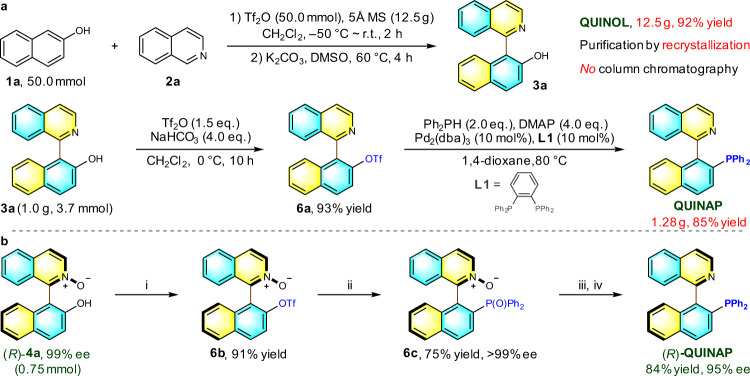
The practicality of the methodology in terms of scalability was first testified with a decagram-scale synthesis of QUINOL product performed under the optimal reaction conditions (Fig. 6a). QUINOL 3a was effortlessly generated in 92% yield and 12.5 gram when the standard reaction was performed on a 50 mmol scale. The procedural simplicity (i.e., post-reaction treatment and rapid recrystallization of product) holds promises for industrial-scale production. The streamlined synthesis of QUINAP from QUINOL 3a was also scalable and an overall yield of 79% was obtained over two steps. Subsequently, optically active (R)-QUINAP was efficiently synthesized in four steps using enantiopure (R)-QUINOL N-oxide as the starting material with routine transformations (Fig. 6b).
Fig. 6. Preparative synthesis and transformations.
a Gram-scale synthesis of (rac)-QUINOL and (rac)-QUINAP. b Synthesis of (R)-QUINAP from (R)-QUINOL N-oxide. Reaction conditions: i Tf2O (1.05 eq.), DMAP (2.0 eq.), CH2Cl2, r.t., 10 h; ii diphenylphosphine oxide (2.0 eq.), Pd(OAc)2 (12 mol%), L1 (24 mol%), DIPEA (5.5 eq.), DMSO, 110 °C, 24 h; iii bis(pinacolato)diboron (1.0 eq.), diglyme, 70 °C, 12 h; iv HSiCl3 (10.0 eq.), DIPEA (40.0 eq.), toluene, 70 °C, 4 h.
Controlled experiments and proposed reaction processes
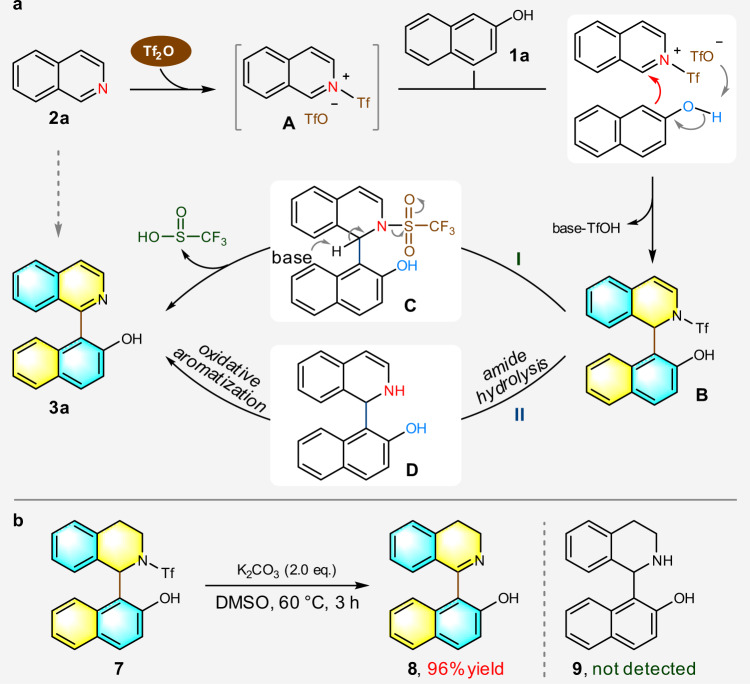
Guided by our continuous understanding of the construction of axially chiral compounds with 2-naphthol as the nucleophile36, a plausible mechanistic pathway was rationalized as outlined in Fig. 7. This coupling reaction is initiated when Tf2O activates the isoquinoline 2a in the form acyl isoquinolinium A. The nucleophilic 2-naphthol 1a then adds to the iminium carbon of activated species A selectively from C1 position, as simultaneously promoted by the proton abstraction from hydroxyl group by the proximal triflate anion; tautomerization at naphthol ring then gives rise to dearomatized intermediate B. Such intermolecular synergistic activation-addition events may play a crucial role to overcome the otherwise challenging chemoselectivity issue resulting from competitive nucleophilic and electrophilic sites present on these reacting partners. Finally, an oxidative aromatization process delivers the target product QUINOL 3a along with the removal of Tf group. Noteworthily, there are two possible pathways for the last step, namely, base-promoted redox process (Fig. 7a, pathway I) or amide hydrolysis-aromatization-driven oxidation cascade process (Fig. 7a, pathway II). To obtain more insights into this step, a control experiment was designed. In light of the powerful driving force for aromatization in intermediate D, it should be infeasible to capture such highly active species. Therefore, the hydrogenation product 7 of intermediate B was prepared as the starting material for mechanistic investigation. As displayed in Fig. 7b, the treatment of 7 under the standard conditions provided product 8 in 96% yield with no formation of product 9. This lent indirect evidence to rule out the possibility of pathway II and indicated that Tf2O is not only an activator of isoquinoline but also an oxidant for the oxidative aromatization process.
Fig. 7. Proposed reaction mechanism and control experiment.
a Proposed reaction pathway. b Control experiment.
Discussion
In conclusion, a metal-free oxidative cross-coupling reaction between isoquinoline and 2-naphthol has been discovered to deliver structurally diversified QUINOLs. This protocol features desirable blend of high yield, exclusive chemoselectivity, robust functional group tolerance, and step economy for the construction of privileged QUINOL derivatives. Moreover, the low cost of raw materials, ease of purification, and decagram-scale preparation portend the adaptability to process settings. For illustration, a streamlined and large-scale synthesis of QUINAP from QUINOL product was demonstrated which presents superiority in terms of production cost. Following a chiral NHC-catalyzed kinetic resolution, two types of axially chiral QUINOL N-oxides which are promising Lewis base catalysts were readily accessible in enantioenriched forms. Derivatization into (R)-QUINAP was also accomplished with standard chemical transformations, thus providing a practical method to access these important frameworks.
Methods
Procedure for the enantioselective synthesis of 3
To a dried Schlenk tube was added 5 Å molecular sieve (250 mg), isoquinoline 2 (1.25 mmol), and CH2Cl2 (4 mL) under argon atmosphere. After the solution was cooled to −50 °C, Tf2O (0.5 mmol) was added through a microsyringe and stirred for half an hour until white solid salts appeared. After adding the solution of 2-naphthol 1 (0.5 mmol) in CH2Cl2 (4 mL), the reaction was slowly raised to room temperature and then continued to stir for 2 h. The molecular sieve was removed by filtration, and the resulting filtrate was washed successively with 3 M HCl, saturated sodium bicarbonate, and brine, and then dried over anhydrous Na2SO4. After evaporating the solvent under reduced pressure, the resulting intermediate B was dissolved in 4 mL of DMSO, and K2CO3 (138 mg, 1.0 mmol) was then added. The mixture was stirred at 60 °C for about 3–4 h until the intermediate was consumed completely. The reaction system was adjusted to weakly acidic with 3 M HCl, and then to weakly basic with saturated sodium bicarbonate. The mixture was diluted with 20 mL of water, and extracted with EA (20 mL × 3). The combined organic phase was washed with brine (20 mL × 2), dried over anhydrous Na2SO4, and concentrated to afford the crude product, which was purified by column chromatography eluted with PE/EA (10/1 to 3/1) to afford the pure product.
Procedure for the kinetic resolution of 4
Under argon atmosphere, substrate 4 (0.2 mmol), cinnamaldehyde (0.13 mmol, 0.65 eq.), C7 (13 mg, 0.03 mmol), activated 4 Å MS (400 mg), solvent (18 mL, CH2Cl2/DMSO = 8/1), and CsOAc (0.2 mmol, 1.0 eq.) were added sequentially to a dried Schlenk flask. The mixture was stirred at −10 °C until the aldehyde was completely consumed (monitored by TLC). After removing the molecular sieve by filtration, the obtained filtrate was diluted with 30 mL of water, and then extracted with DCM (30 mL × 3). The combined organic phase was washed with 30 mL brine, dried over anhydrous Na2SO4, and concentrated to afford crude products, which were purified by column chromatography eluted with DCM/EA (20/1 to 1/1) to afford the pure products 5 and (R)-4.
Supplementary information
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (Nos. 21702092, 21772081, 21825105, and 21901105), Guangdong Provincial Key Laboratory of Catalysis (No. 2020B121201002), Guangdong Innovative Program (2019BT02Y335), Shenzhen Special Funds (JCYJ20190812112603598, JCYJ20180305123508258, and JCYJ20170412151701379), Shenzhen Nobel Prize Scientists Laboratory Project (C17213101), and SUSTech Special Fund for the Construction of High-Level Universities (No. G02216402).
Author contributions
P.-Y.J. and K.-F.F. performed experiments and prepared the supplementary information. S.-H.X. helped with characterizing some new compounds. B.T. and S.L. conceived and directed the project and B.T., S.L., and S.-H.X. wrote the paper.
Data availability
The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2005905, CCDC 2046559. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/ data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Peng-Ying Jiang, Kai-Fang Fan.
Contributor Information
Shaoyu Li, Email: lisy@sustech.edu.cn.
Bin Tan, Email: tanb@sustech.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-22621-2.
References
- 1.Cepanec, I. Synthesis of Biaryls (2004).
- 2.Ackermann, L. Modern Arylation Methods (2009).
- 3.Zhou, Q.-L. Privileged Chiral Ligands and Catalysts (2011).
- 4.Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002;102:1359–1469. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 5.Bringmann G, et al. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 2005;44:5384–5427. doi: 10.1002/anie.200462661. [DOI] [PubMed] [Google Scholar]
- 6.Surry DS, Buchwald SL. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 2008;47:6338–6361. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J-S, Cheng S-W, Cheng Y-J, Hsu C-S. Donor-acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 2015;44:1113–1154. doi: 10.1039/C4CS00250D. [DOI] [PubMed] [Google Scholar]
- 8.Howard RH, et al. Synthesis and structures of complexes with axially chiral isoquinolinyl-naphtholate ligands. Dalton Trans. 2009;2009:8667–8682. doi: 10.1039/b907982c. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Li X, Schreiber SL. Catalytic asymmetric [3+2] cycloaddition of azomethine ylides. Development of a versatile stepwise, three-component reaction for diversity-oriented synthesis. J. Am. Chem. Soc. 2003;125:10174–10175. doi: 10.1021/ja036558z. [DOI] [PubMed] [Google Scholar]
- 10.Gommermann N, Koradin C, Polborn K, Knochel P. Enantioselective, copper(I)-catalyzed three-component reaction for the preparation of propargylamines. Angew. Chem. Int. Ed. 2003;42:5763–5766. doi: 10.1002/anie.200352578. [DOI] [PubMed] [Google Scholar]
- 11.Miura T, Yamauchi M, Kosaka A, Murakami M. Nickel-catalyzed regio- and enantioselective annulation reactions of 1,2,3,4-benzothiatriazine-1,1(2H)-dioxides with allenes. Angew. Chem. Int. Ed. 2010;49:4955–4957. doi: 10.1002/anie.201001918. [DOI] [PubMed] [Google Scholar]
- 12.Lim AD, Codelli JA, Reisman SE. Catalytic asymmetric synthesis of highly substituted pyrrolizidines. Chem. Sci. 2013;4:650–654. doi: 10.1039/C2SC21617E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rokade BV, Guiry PJ. Axially chiral P,N-ligands: some recent twists and turns. ACS Catal. 2018;8:624–643. doi: 10.1021/acscatal.7b03759. [DOI] [Google Scholar]
- 14.Malkov AV, Dufková L, Farrugia L, Kočovský P. Quinox, a quinoline-type N-oxide, as organocatalyst in the asymmetric allylation of aromatic aldehydes with allyltrichlorosilanes: the role of arene-arene interactions. Angew. Chem. Int. Ed. 2003;42:3674–3677. doi: 10.1002/anie.200351737. [DOI] [PubMed] [Google Scholar]
- 15.Malkov AV, et al. On the mechanism of asymmetric allylation of aldehydes with allyltrichlorosilanes catalyzed by QUINOX, a chiral isoquinoline N-oxide. J. Am. Chem. Soc. 2008;130:5341–5348. doi: 10.1021/ja711338q. [DOI] [PubMed] [Google Scholar]
- 16.Cortright SB, Johnston JN. IAN-amines: direct entry to a chiral C2-symmetric zirconium(IV) β-diketimine complex. Angew. Chem. Int. Ed. 2002;41:345–348. doi: 10.1002/1521-3773(20020118)41:2<345::AID-ANIE345>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Cortright SB, Huffman JC, Yoder RA, Coalter JN, Johnston JN. IAN amines: chiral C2-symmetric zirconium(IV) complexes from readily modified axially chiral C1-symmetric β-diketimines. Organometallics. 2004;23:2238–2250. doi: 10.1021/om049897p. [DOI] [Google Scholar]
- 18.Pais VF, et al. Red-emitting tetracoordinate organoboron chelates: synthesis, photophysical properties, and fluorescence microscopy. J. Org. Chem. 2016;81:9605–9611. doi: 10.1021/acs.joc.6b01569. [DOI] [PubMed] [Google Scholar]
- 19.Alcock NW, Brown JM, Hulmes DI. Synthesis and resolution of l-(2-diphenylphosphino-l-naphthyl)isoquinoline; a P-N chelating ligand for asymmetric catalysis. Tetrahedron. 1993;4:743–756. doi: 10.1016/S0957-4166(00)80183-4. [DOI] [Google Scholar]
- 20.Lim CW, et al. Practical preparation and resolution of 1-(2’-diphenylphosphino-1’-naphthyl)isoquinoline: a useful ligand for catalytic asymmetric synthesis. Org. Proc. Res. Dev. 2003;7:379–384. doi: 10.1021/op034007n. [DOI] [Google Scholar]
- 21.Murakami K, Yamada S, Kaneda T, Itami K. C-H functionalization of azines. Chem. Rev. 2017;117:9302–9332. doi: 10.1021/acs.chemrev.7b00021. [DOI] [PubMed] [Google Scholar]
- 22.Fier PS, Hartwig JF. Selective C-H fluorination of pyridines and diazines inspired by a classic amination reaction. Science. 2013;342:956–960. doi: 10.1126/science.1243759. [DOI] [PubMed] [Google Scholar]
- 23.Jo W, et al. ZnMe2‑mediated, direct alkylation of electron-deficient N‑heteroarenes with 1,1-diborylalkanes: scope and mechanism. J. Am. Chem. Soc. 2020;142:13235–13245. doi: 10.1021/jacs.0c06827. [DOI] [PubMed] [Google Scholar]
- 24.Nagase M, Kuninobu Y, Kanai M. 4-position-selective C−H perfluoroalkylation and perfluoroarylation of six-membered heteroaromatic compounds. J. Am. Chem. Soc. 2016;138:6103–6106. doi: 10.1021/jacs.6b01753. [DOI] [PubMed] [Google Scholar]
- 25.Fier PS. A bifunctional reagent designed for the mild, nucleophilic functionalization of pyridines. J. Am. Chem. Soc. 2017;139:9499–9502. doi: 10.1021/jacs.7b05414. [DOI] [PubMed] [Google Scholar]
- 26.Fier PS, Kim S, Cohen RD. A multifunctional reagent designed for the site-selective amination of pyridines. J. Am. Chem. Soc. 2020;142:8614–8618. doi: 10.1021/jacs.0c03537. [DOI] [PubMed] [Google Scholar]
- 27.Hilton MC, Dolewski RD, McNally A. Selective functionalization of pyridines via heterocyclic phosphonium salts. J. Am. Chem. Soc. 2016;138:13806–13809. doi: 10.1021/jacs.6b08662. [DOI] [PubMed] [Google Scholar]
- 28.Dolewski RD, Fricke PJ, McNally A. Site-selective switching strategies to functionalize polyazines. J. Am. Chem. Soc. 2018;140:8020–8026. doi: 10.1021/jacs.8b04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy JN, Alegre-Requena JV, Liu R, Paton RS, McNally A. Selective halogenation of pyridines using designed phosphine reagents. J. Am. Chem. Soc. 2020;142:11295–11305. doi: 10.1021/jacs.0c04674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilton MC, et al. Heterobiaryl synthesis by contractive C–C coupling via P(V) intermediates. Science. 2018;362:799–804. doi: 10.1126/science.aas8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao D-W, Gu Q, You S-L. Pd(II)-catalyzed intermolecular direct C−H bond iodination: an efficient approach toward the synthesis of axially chiral compounds via kinetic resolution. ACS Catal. 2014;4:2741–2745. doi: 10.1021/cs500813z. [DOI] [Google Scholar]
- 32.Staniland S, et al. Biocatalytic dynamic kinetic resolution for the synthesis of atropisomeric biaryl N-oxide lewis base. Catal. Angew. Chem. Int. Ed. 2016;55:10755–10759. doi: 10.1002/anie.201605486. [DOI] [PubMed] [Google Scholar]
- 33.Miyaji R, Asano K, Matsubara S. Bifunctional organocatalysts for the enantioselective synthesis of axially chiral isoquinoline N‑oxides. J. Am. Chem. Soc. 2015;137:6766–6769. doi: 10.1021/jacs.5b04151. [DOI] [PubMed] [Google Scholar]
- 34.Lu S, Poh SB, Zhao Y. Kinetic resolution of 1,1’-biaryl-2,2’-diols and amino alcohols through NHC-catalyzed atroposelective acylation. Angew. Chem. Int. Ed. 2014;53:11041–11045. doi: 10.1002/anie.201406192. [DOI] [PubMed] [Google Scholar]
- 35.Yang G, Guo D, Meng D, Wang J. NHC-catalyzed atropoenantioselective synthesis of axially chiral biaryl amino alcohols via a cooperative strategy. Nat. Commun. 2019;10:3062–3067. doi: 10.1038/s41467-019-10878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y-B, Tan B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 2018;51:534–547. doi: 10.1021/acs.accounts.7b00602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2005905, CCDC 2046559. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/ data_request/cif.