Abstract
Background
Increased tissue cortisol availability has been implicated in abnormal glucose and fat metabolism in patients with obesity, metabolic syndrome, and type 2 diabetes (T2DM). Our objective was to evaluate whether blockade of glucocorticoid receptor (GR) with mifepristone ameliorates insulin resistance (IR) in overweight/obese subjects with glucose intolerance.
Methods
We conducted a randomized, double-blinded, placebo-controlled, crossover study in overweight/obese individuals (n = 16, 44% female) with prediabetes or mild T2DM but not clinical hypercortisolism. Mifepristone (50 mg every 6 h) or placebo was administered for 9 days, followed by crossover to the other treatment arm after a washout period of 6 to 8weeks. At baseline and following each treatment, oral glucose tolerance test (OGTT) and frequently sampled intravenous glucose tolerance test (FSIVGTT) were performed. Insulin sensitivity was measured using FSIVGTT [primary outcome: insulin sensitivity index (SI)] and OGTT [Matsuda index (MI) and oral glucose insulin sensitivity index (OGIS)]. Hepatic and adipose insulin resistance were assessed using hepatic insulin resistance index (HIRI), and adipose tissue insulin sensitivity index (Adipo-SI) and adipo-IR, derived from the FSIVGTT.
Results
Mifepristone administration did not alter whole-body glucose disposal indices of insulin sensitivity (SI, MI, and OGIS). GR blockade significantly improved Adipo-SI (61.7 ± 32.9 vs 42.8 ± 23.9; P = 0.002) and reduced adipo-IR (49.9 ± 45.9 vs 65.5 ± 43.8; P = 0.004), and HIRI (50.2 ± 38.7 vs 70.0 ± 44.3; P = 0.08). Mifepristone increased insulin clearance but did not affect insulin secretion or β-cell glucose sensitivity.
Conclusion
Short-term mifepristone administration improves adipose and hepatic insulin sensitivity among obese individuals with hyperglycemia without hypercortisolism.
Keywords: mifepristone, insulin sensitivity, glucose intolerance, adipose
Cortisol is an important regulator of metabolism that modulates the use of glucose and fat as fuels. Glucocorticoids (GC) acting through the GC receptor (GR) are known to impair insulin secretion (1-3), reduce insulin sensitivity (4-6), decrease glucose effectiveness (7) and increase hepatic glucose production and visceral adiposity (8-10). In Cushing’s syndrome, these adverse effects of excessive GCs underlie the development of glucose intolerance, type 2 diabetes mellitus (T2DM), dyslipidemia, hypertension, adiposity, and hepatic steatosis (4,9-11). Consistent with this notion, the GR antagonist mifepristone improves glucose tolerance and insulin sensitivity in patients with Cushing’s syndrome, and in patients with adrenal incidentalomas and dysregulated cortisol secretion (12-14).
The cluster of metabolic abnormalities seen in hypercortisolemic states also is present in a more prevalent condition, the metabolic syndrome (15). However, circulating cortisol levels are mostly within the normal reference range in individuals with central obesity, insulin resistance, and metabolic syndrome (16,17), leading to the speculation that increased local tissue exposure to cortisol or its active metabolites may play a role in the development of the metabolic syndrome. One mechanism for increased tissue exposure to cortisol is increased local regeneration of cortisol from cortisone by the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD1). Consistent with this hypothesis, transgenic mice that overexpress 11β-HSD1 in adipose tissue develop insulin-resistant diabetes (18). In obese humans, levels and activity of 11β-HSD1 in visceral adipose tissue are increased (19-22). Indeed, 11β-HSD1 inhibitors improve insulin sensitivity and glycemic control in patients with T2DM (23,24). These studies suggest that accentuated levels of GCs in peripheral tissues may play an important role in the pathogenesis of insulin resistance in obesity.
Despite the potential involvement of GCs in the pathogenesis of metabolic syndrome, it is not known whether GR blockade improves insulin sensitivity in these patients. In this pilot study, we hypothesized that reduction in cortisol action by mifepristone might improve insulin sensitivity in patients with prediabetes and T2DM. To test this hypothesis, we used a randomized, double-blinded, placebo-controlled, crossover study design to evaluate the effects of mifepristone (50 mg every 6 h) or placebo administration for 9 days on measures of insulin sensitivity in overweight/obese individuals with prediabetes or mild T2DM.
Materials and Methods
Trial design and primary outcome
This study used a prospective, randomized, double-blind, placebo-controlled, crossover design in which eligible subjects were randomized (in blocks of 6) to take either placebo or mifepristone (50 mg capsules every 6 h) for 9 days. This was followed by a 6- to 8-week washout period. Subjects were then crossed over to the other treatment arm for an additional 9 days. The primary outcome was a change in insulin sensitivity index based on the effect of insulin on glucose metabolism in the frequently sampled intravenous glucose tolerance test (FSIVGTT). The study was conducted at the National Institutes of Health (NIH) Clinical Center (Bethesda, MD, USA).
Rationale for the dose and duration of the intervention
We used a low total daily dose of mifepristone to avoid drug-induced adrenal insufficiency with activation of the hypothalamic-pituitary-adrenal axis. We gave mifepristone 4 times a day based on the hypothesis that this would result in a lower exposure of the corticotrope cells compared to the liver. We postulated that the first pass effect would provide a larger mifepristone “dose” to antagonize intrahepatic cortisol action before hepatic metabolism resulted in a lower circulating level and less antagonism of cortisol at the pituitary gland. We predicted that this might result in a lower adrenocorticotropin (ACTH) response (compared to a once-daily dose) and hence minimize cortisol increases that might have adverse effects on glucose metabolism. In this study, we sought to examine the short-term effects of mifepristone on various metabolic parameters. GR antagonist administration in fa/fa Zucker rats for 7 days reduced fasting plasma glucose levels and hepatic glucose production and increased peripheral glucose uptake (25). Likewise, GR antagonism normalizes blood glucose levels and improves dyslipidemia in ob/ob mice in 5 days (25). A single oral administration of RU 486 (600 mg) transiently lowered plasma glucose concentrations and hepatic glucose production in healthy men (n = 8) (26). Administration of mifepristone to healthy men (n = 8) on 2 consecutive days was associated with a reduction in fasting triglyceride and glucose levels (27). Based on these findings, we sought to examine the metabolic effects of oral mifepristone (50 mg every 6 h) for 9 days in this exploratory study.
Participants
Subjects eligible for the study included men and women 35 to 70 years of age who were overweight or obese [body mass index (BMI) 25-37 kg/m2] with prediabetes [defined as fasting glucose ≥100 mg/dL or a 2-h postprandial glucose value ≥140 mg/dL during an oral glucose tolerance test (OGTT)] or mild diabetes (defined as a A1C ≤ 7% and taking no medications or on a stable dose of metformin and no other hypoglycemic agents for ≥3 months before beginning the study).
Subjects with endogenous hypercortisolism due to Cushing’s syndrome or pseudo-Cushing’s syndrome, and those with exogenous hypercortisolism, were excluded by a detailed history [use of herbal supplements within 14 days of study drug initiation, use of GCs or megestrol within 6 months of study initiation, depression (evaluated by PHQ-9 with cut-off of ≥10), excessive alcohol use (>2 drinks/day for women and >3 drinks/day for men)], and a requirement for 2 normal midnight salivary cortisol levels. Additional exclusion criteria included pregnant and lactating women and subjects with: changes in metformin or other hypoglycemic agent dose/use within 3 months of study entry, uncontrolled hypertension (>180/110 mmHg) or any unstable medical conditions (eg, impaired cardiac function, severe respiratory insufficiency), liver function tests (alanine aminotransferase and aspartate aminotransferase) more than 3 times the upper normal limit, severe renal impairment (creatinine clearance <30 mL/min), evidence of human immunodeficiency virus or hepatitis C infection, history of hemorrhagic disorders or use of anticoagulants, history of endometrial cancer or hyperplasia, unexplained vaginal bleeding, or endometrial thickness greater than 6 mm, changes in lipid-lowering medication dose during the study or within 1 month of study entry, use of any medications or dietary supplements with strong inhibition of CYP3A4, use of estrogen-based hormone therapy, use of grapefruit juice within 14 days of study drug initiation, untreated thyroid dysfunction, moderate/severe anemia (hemoglobin <10 g/dL), blood donations totaling 500 mL or more within 1 month of study initiation, prolonged QTc interval, and subjects in a weight loss program or who were actively dieting. Postmenopausal women and women in the perimenopausal phase underwent a transvaginal ultrasound to evaluate endometrial thickness, and pelvic magnetic resonance imaging was performed if the transvaginal ultrasound did not provide an accurate reading of the endometrial thickness.
Study approval
The study was approved by the Institutional Review Board of the National Institute of Child Health and Human Development, and all procedures were performed as per the institutional guidelines. Eligible participants provided written informed consent to participate in protocol 11-CH-0208 (Clinicaltrials.gov; NCT01419535).
Sample size
Patient recruitment and assessment for eligibility are shown in Figure 1. Initially, 63 subjects were screened for eligibility. Forty-four subjects failed the screening, and 19 subjects underwent randomization. One subject dropped out while 2 others had malfunctioning intravenous access resulting in incomplete data. Though the protocol listed a sample size goal of 17 subjects, the final analysis was performed on these 16 subjects. Beyond this, there were no changes to the protocol methodology after trial commencement. A predetermined interim analysis was conducted to evaluate the effect of the mifepristone dose (50 mg/6 h) on the hypothalamic-pituitary-adrenal axis. The study statistician was unblinded to the first 6 subjects (1 randomization block) to evaluate whether the 24-h urinary free cortisol (UFC) levels following mifepristone treatment were equal to or more than twice the upper limit of the reference range in 4 or more of the 6 subjects, which might have a negative effect on glucose metabolism. In that scenario, mifepristone dose would be decreased to 25 mg every 6 h (100 mg/day). However, UFC values remained below the threshold, and no dose adjustment was required. Insulin sensitivity as measured by the FSIVGTT was the primary outcome of the study, but insufficient data were available due to hemolysis or inability to obtain a sample in 4 subjects undergoing FSIVGTT.
Figure 1.
Diagram of patient flow from screening for eligibility, noting reasons for exclusions, and dropouts or loss of data after initiation of the study.
Randomization, blinding, and study drug management
Mifepristone was obtained through an active Investigational New Drug Application under a Cooperative Research and Development Agreement partnership with Laboratoire HRA-Pharma (Paris, France). Mifepristone and inert look-alike placebo were packaged into 50 mg capsules by the NIH Clinical Center Pharmaceutical Development Service (PDS) according to GMP guidelines. The PDS randomized subjects into initial treatment arms in blocks of 6 and dispensed the study agents. The study statistician was unblinded to the treatment arm of the first 6 subjects for the interim analysis. Other investigators remained unaware of treatment randomization throughout the entire study.
Clinical trial design/treatment arms
Subjects underwent baseline screening before being randomized into one of 2 treatment arms, placebo or mifepristone treatment. Eligible subjects were admitted to the NIH Clinical Center on the day prior to baseline (pre-treatment) testing. To reduce potential variability associated with estrogen in premenopausal women, testing was performed in the early follicular phase (days 1 to 7 of menstrual cycle). Baseline height and weight were measured after an overnight fast. Subjects received an isocaloric regular diet during the inpatient part of the study. Baseline testing took place over 3 inpatient days at the NIH Clinical Center, during which fasting blood was drawn, 24-h urine was collected, 24-h serial blood draws (every hour) were obtained, and patients received both FSIVGTT and a 75-g OGTT. Subsequent measurements included fasting dehydroepiandrosterone sulfate (DHEAS), androstenedione, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, total cholesterol, insulin, glucose, testosterone (men only), UFC, and hourly plasma ACTH and serum cortisol.
Subjects were then randomized to receive placebo or mifepristone for 9 days; of which the first 6 days the subjects returned home, and the final 3 days subjects underwent the same metabolic testing as during the baseline period. All subjects were instructed to take one capsule every 6 h during the 9 days; for those taking mifepristone; this resulted in a 200 mg/day dose. Following the completion of drug testing, subjects entered a washout period (6-8 weeks), after which they crossed over to the other treatment arm, serving as their own controls for the study drug treatments.
All subjects were instructed to record adverse events and report them to study personnel; women were asked to keep a record of any abnormal menstrual bleeding. Subjects were required to be weight stable for the 2 weeks prior to study entry and not to start any new diet/exercise programs during the study. During the inpatient stay, subjects were maintained on an isocaloric diet, and those on metformin at the beginning of the study were maintained on the same dose throughout. For safety monitoring, subjects returned approximately 1 week (day 19) and 3 weeks (day 33) after the discontinuation of the study drug for both treatment arms. During this safety visit, serum electrolytes, renal and liver function, and complete blood count were measured, and any treatment-related abnormalities/adverse events were evaluated and treated as needed. The schedule of study procedures is shown in Figure 2.
Figure 2.
Schedule of activities during the study, showing days of baseline and posttreatment testing and crossover design.
Frequently sampled intravenous glucose tolerance test
Insulin sensitivity index (SI) was determined from the minimal model using data from the insulin-modified FSIVGTT as previously described (version 6.02; MinMOD Millenium, Los Angeles, CA, USA) (28). Briefly, after an overnight fast, on day 2 of baseline and after 7 days of mifepristone/placebo treatment, 2 intravenous catheters were placed, 1 in each arm near or in the antecubital veins. A 50% dextrose (0.3 gm/kg) bolus was infused intravenously over 1 min at timepoint 0 min, and an insulin (0.03 units/kg) bolus was infused at the 20-min timepoint. Serial blood draws were obtained at −10, −1, 0, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150, and 180 min for measurement of glucose, insulin, and free fatty acids (FFA).
Oral glucose tolerance test
On day 3 of baseline and after 8 days of mifepristone/placebo administration, subjects underwent a 75-g OGTT. Serial blood draws for measurement of glucose, insulin, and c-peptide were obtained at −5, 0, 30, 60, 90, 120, 150, and 180 min.
Surrogate measures of insulin sensitivity and β-cell function
The Matsuda index and the oral glucose insulin sensitivity index (OGIS), the surrogate indices of whole-body insulin sensitivity were derived from plasma glucose and insulin concentrations during the OGTT as previously described (29). Hepatic insulin resistance index (HIRI) was calculated as the product of the area under the curves (AUCs) of glucose and insulin for the first 30 min of the OGTT (29). The adipose tissue insulin resistance index (Adipo-IR), a surrogate measure for fasting adipose-tissue insulin resistance, was calculated as the product of fasting insulin and fasting FFA (30). A dynamic surrogate index of adipose tissue sensitivity index (Adipo-SI) was assessed by calculating insulin’s ability to suppress FFA levels during an FSIVGTT (31,32). The Adipo-SI was calculated as ratio of the slope of the linear decrease in natural log transformed FFA [Ln (FFA) slope] during the first 90 min of the FSIVGTT and the AUC of insulin during that 90-min period (AUCInsulin 0-90 min). Mathematical modeling of C-peptide concentrations during OGTT was used to estimate insulin secretory rates (ISR) and calculate β-cell responsivity parameters [basal (ΦB), first-phase (Φ1), second-phase (Φ2), and total (ΦTOT)] as previously described (33,34). Assuming an absence of hepatic C-peptide extraction, postprandial insulin clearance was calculated as the ratio of mean insulin secretion and insulin concentration (35).
Assays
All measurements were made by the NIH Department of Laboratory Medicine. Serum androstenedione and DHEAS and UFC were analyzed by high-performance liquid chromatography-tandem mass spectrometry with intra- and inter-assay coefficients of variation of 4% to 9.5%. Plasma ACTH and serum cortisol and testosterone were analyzed by chemiluminescent immunoassay on Siemens Immulite 2000XPi analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) according to the manufacturer’s instructions. Reference ranges are shown on Table 2.
Table 2.
Clinical and metabolic parameters and hormone levels after placebo and mifepristone administration
| Placebo | Mifepristone | P value | |
|---|---|---|---|
| Weight (kg) | 97.7 ± 16.3 | 97.4 ± 15.8 | 0.590 |
| Body mass index (kg/m2) | 32.4 ± 4.3 | 32.2 ± 3.9 | 0.390 |
| Systolic blood pressure (mmHg) | 121 ± 12 | 130 ± 13 | 0.009 |
| Diastolic blood pressure (mmHg) | 71 ± 10 | 77 ± 9 | 0.010 |
| Total cholesterol (mg/dL) | 177.3 ± 38.7 | 163.6 ± 41.9 | 0.004 |
| HDL cholesterol (mg/dL) | 44.9 ± 8.8 | 41.3 ± 11.9 | 0.010 |
| LDL cholesterol (mg/dL) | 101.9 ± 31.1 | 97.1 ± 34.6 | 0.10 |
| Triglycerides (mg/dL) | 151.9 ± 79.3 | 125.6 ± 65.6 | 0.002 |
| Total testosterone (ng/dL), male subjects [RR 262-1593] | 355.2 ± 124.1 | 235.2 ± 87.5 | 0.010 |
| Androstenedione (ng/dL) [RR men: 26-125; RR women 17-175] | 66.7 ± 41.7 | 109.9 ± 64.7 | 0.007 |
| DHEA sulfate (µg/mL) [RR men 0.80-5.6; RR women 0.35-4.3] | 0.82 ± 0.45 | 1.04 ± 0.64 | 0.004 |
| Urinary free cortisola (µg/24 h) [RR 5-45] | 25.3 ± 30.2 | 74.1 ± 75.3 | <0.001 |
Post-treatment values shown are unadjusted means ± SD. Posttreatment values were adjusted for baseline value and treatment group when used in mixed-model regression. P-values indicate significance for comparisons between mifepristone and placebo.
a Urine free cortisol was measured by liquid chromatography, tandem mass spectrometry; the reference range is 5 to 45 ug/24 h.
Statistical analysis and sample size estimation
A change in insulin sensitivity as measured by FSIVGTT was prospectively designated as the primary endpoint for this exploratory study. All other comparisons were considered secondary. Studies in rodent models of insulin resistance (36-38) and in patients with Cushing syndrome (14) have suggested that mifepristone improves insulin sensitivity. However, data on the effects of mifepristone on insulin sensitivity are lacking in this study population. Consequently, our pilot study was intended to determine the treatment effect size and SD for mifepristone’s effects on change in insulin sensitivity, to allow for adequate powering of a larger future study. Prior studies have reported that a sample size of n = 18 was sufficient to detect a 25% difference in SI between placebo and mifepristone at a power of 80% and a type I error of 5% (39,40). Thus, in this pilot study, we chose a sample size of n = 19 subjects.
Continuous variables were described as mean and SD, and categorical data were represented as proportions and frequencies. All continuous data were examined for normality and homogeneity of variances and were either log transformed as appropriate or assessed using nonparametric tests. Between and within treatment comparisons were performed using mixed models for a crossover design that also considers sequence effect, if any. Treatment-by-period interaction was tested for primary outcome. Two-sided P-values were utilized for all analyses. Repeated measures were analyzed by repeated measures analysis of variance including treatment group, time, and treatment*time interaction as factors. Post-hoc pairwise comparisons were performed by Bonferroni’s test. The level of statistical significance was set a priori at <0.05, and the P-values were not adjusted for multiple comparisons. Data were analyzed with JMP version 13.0 (SAS Institute, Cary, NC, USA) and GraphPad Prism 7 (GraphPad Software Inc, La Jolla, CA, USA).
Results
Baseline characteristics of study subjects
The baseline characteristics of the 16 subjects who completed the study are shown in Table 1. The mean age of the participants was 55.5 years, and 44% of the subjects were female. Participants were overweight or obese with a mean BMI of 32.2 kg/m2 (range, 25.7-37.5 kg/m2). Sixty-three percent of the cohort (n = 10) were prediabetic [impaired fasting glucose, 13% (n = 2); impaired glucose tolerance, 31% (n = 5); and both impaired fasting glucose and impaired glucose tolerance, 19% (n = 3)], and 37% (n = 6) were diagnosed with T2DM. The average A1C (±SD) at baseline of the entire, prediabetic, and diabetic groups was 5.98 ± 0.57%, 5.88 ± 0.43%, and 6.26 ± 0.59%, respectively. Two of the diabetic patients were on stable doses of metformin. The mean systolic and diastolic blood pressures (BP) were 121 ± 17 and 69 ± 10 mmHg, respectively. Sixty-three percent of the participants (n = 10) were on anti-hypertensive agents, and 31% (n = 5) were on statins. Thirty-eight percent of the participants had low levels of HDL cholesterol [<40 mg/dL (men) or <50 mg/dL (women)] or high triglyceride levels (>150 mg/dL).
Table 1.
Clinical and metabolic characteristics at baseline
| Characteristics | Result (n = 16) |
|---|---|
| Age (years) | 55.5 ± 7.9 |
| Sex (F/M) | 7/9 |
| Weight (kg) | 97.1 ± 15.6 |
| Body-mass index (kg/m2) | 32.2 ± 3.7 |
| Systolic blood pressure (mmHg) | 121 ± 17 |
| Diastolic blood pressure (mmHg) | 69 ± 10 |
| Total cholesterol (mg/dL) | 181.1 ± 52.4 |
| HDL cholesterol (mg/dL) | 44.7 ± 9.6 |
| LDL cholesterol (mg/dL) | 107.8 ± 39.3 |
| Triglycerides (mg/dL) | 142.6 ± 83.8 |
| Free fatty acids (mmol/L) | 0.613 ± 0.195 |
| Fasting glucose (mg/dL) | 102.7 ± 11.6 |
| Fasting insulin (pmol/L) | 138.9 ± 89.0 |
| A1C (%) | 5.98 ± 0.60 |
| HOMA-IR | 5.16 ± 3.49 |
| QUICKI | 0.310 ± 0.025 |
| OGIS (mL/min/m2) | 283.9 ± 66.3 |
| Matsuda index | 2.15 ± 1.05 |
| SI (min−1·μU·mL−1) | 1.06 ± 0.61 |
| Urinary free cortisola (µg/24 h) | 22.9 ± 17.2 |
Data are presented as mean ± SD.
Abbreviations: HOMA-IR, homeostatic model assessment of insulin resistance; OGIS, oral glucose insulin sensitivity; QUICKI, quantitative insulin sensitivity check index; SI, insulin sensitivity index derived from the insulin-modified frequently sampled intravenous glucose tolerance test.
aUrine free cortisol was measured by liquid chromatography, tandem mass spectrometry; the reference range is 5 to 45 μg/24 h.
Surrogate measures of insulin sensitivity indices based on glucose and insulin levels during fasting [quantitative insulin sensitivity check index (QUICKI) and homeostatic model assessment of insulin resistance (HOMA-IR)] and glucose tolerance tests (OGIS and Matsuda index from OGTT and SI derived from the FSIVGTT) are reported in Table 1. HOMA-IR > 2.5, QUICKI ≤ 0.331, OGIS ≤ 344, Matsuda ≤ 2.5, or SI ≤ 2.1 has been used to define insulin resistance (41-44). Based on the mean values of HOMA-IR, QUICKI, OGIS, Matsuda index, and SI, subjects in our cohort were insulin resistant (Table 1). The mean 24-h UFC values were within normal limits (22.9 ± 17.2 µg/24 h; normal: 5-45 µg/24 h). Therefore, the final study population represented the inclusion criteria set for the trial.
Effects of mifepristone treatment on circulating levels of cortisol and ACTH levels and 24-h urinary free cortisol
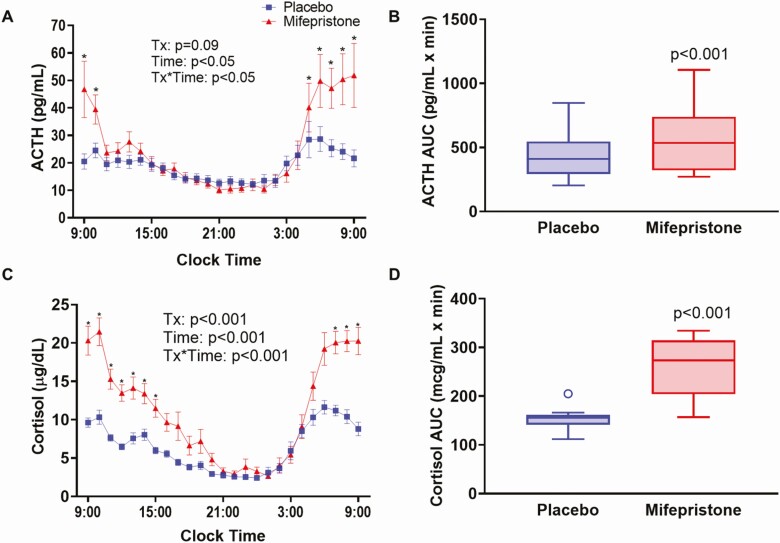
By antagonizing central GRs, mifepristone blocks the negative feedback effects of cortisol leading to elevations in ACTH and cortisol, especially during the early morning (45). As expected, mean ACTH and cortisol levels were higher during mifepristone than placebo administration specifically during the morning (Fig. 3). During mifepristone treatment, ACTH levels were significantly higher from 5 am to 10 am, while increased cortisol began at 7 am and remained significantly elevated until 3 pm when compared with placebo (Fig. 3A and 3C). The AUC measurements for ACTH and cortisol were significantly higher after mifepristone treatment (574.5 ± 260.8 vs 448.7 ± 193.5 pg/mL.min for ACTH; P < 0.001, and 255.4 ± 61.9 vs 151.1 ± 21.1µg/mL.min for cortisol; P < 0.001) (Fig. 3B and 3D).
Figure 3.
Twenty-four-hour profile of serum ACTH and cortisol in study participants after receiving either placebo or mifepristone. Serial time course of plasma ACTH (A) and cortisol (C) every hour from 9:00 am one day to 9:00 am the following day. Data shown are mean ± SEM. P-values for the effects of treatment group, time, and the treatment*time interaction were obtained with repeated-measures analysis of variance with post-hoc Bonferroni’s test. Tukey box-and-whisker plots of ACTH (B) and cortisol (D) area under the curve for the 24-h period. Top and bottom box limits represent the 75th and 25th percentile, respectively; midlines represent the median; and top and bottom whiskers represent the 75th percentile plus 1.5 of the interquartile range and the 25th percentile minus 1.5 interquartile range, respectively. Symbols represent any values that lie outside the range of the whiskers. Posttreatment values were adjusted for baseline value and treatment group using mixed-model regression. P-values indicate significance for comparisons between mifepristone and placebo.
In the entire cohort, mifepristone significantly increased 24-h UFC compared with placebo (74.1 ± 75.3 vs 25.3 ± 30.2 µg/24 h, P < 0.001; Table 2). These stimulatory effects of mifepristone were not uniform, with posttreatment mean UFC above normal limits in 8 subjects (120.4 ± 83.9 µg/24 h, range 48.4-317.7 µg/24 h, ULN 45 µg/24 h) and within the normal range in 8 subjects (27.7 ± 13.7 µg/24 h). Early morning (4-8 am) ACTH levels are very sensitive to low-dose mifepristone treatment (45). In the mifepristone treatment arm, early am (4-8 am) AUC of serum ACTH levels were not different in the subjects with higher 24-h UFC compared to the subjects with normal 24-h UFC (185.6 ± 120.2 vs 159.6 ± 97.8 pg/mL.min for ACTH; P = 0.65).
Plasma DHEAS and androstenedione levels were significantly higher after mifepristone therapy when compared with placebo (Table 2). While DHEAS levels remained within normal limits, 3 women had elevated androstenedione values that remained less than 2-fold normal. Overall, these results suggest that in our study, administration of mifepristone (50 mg every 6 h) uniformly increased circulating ACTH and cortisol levels on the seventh and eighth day as a compensatory response to GR blockade.
Effect of mifepristone treatment on body weight, BP, and serum lipids
We compared body weight, BP, and serum lipid concentrations in each study subject at baseline and after 7- to 9-day administration of mifepristone or placebo. There were no significant differences in body weight or BMI changes between the treatment arms (Table 2). BP was higher after mifepristone treatment for both systolic (130 ± 13 vs 121 ± 12 mmHg, P = 0.009) and diastolic values (77 ± 9.2 vs 71 ± 9.5 mmHg, P = 0.01) (Table 2). Mifepristone treatment was associated with significantly lower serum total cholesterol, HDL cholesterol, and triglycerides (Table 2). The mean plasma LDL levels were lower after mifepristone treatment, but the difference did not reach statistical significance. Serum potassium was lower, albeit within normal limits following mifepristone administration (3.9 ± 0.2 vs 4.2 ± 0.3mmol/L, P = 0.003).
Effect of mifepristone on plasma glucose, serum insulin, and indices of insulin sensitivity
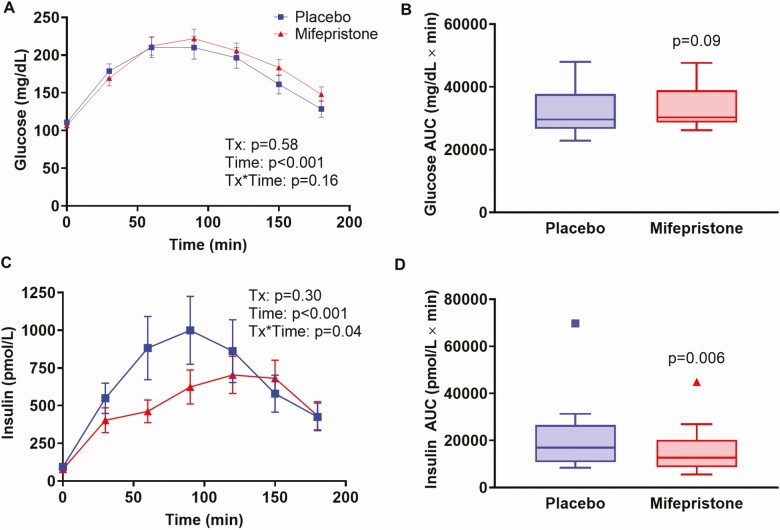
Mifepristone treatment resulted in significant lowering of fasting plasma glucose (100.4 ± 15.3 vs. 107.8 ± 15.7mg/dL in placebo group; P < 0.001) and insulin (95.7 ± 76.1 vs 142.8 ± 102.2pmol/L; P < 0.001) (Table 3; Figs. 4 and 5). Consequently, mifepristone significantly reduced HOMA-IR and improved QUICKI when compared with placebo (Table 3). The time course of glucose and insulin concentrations and the AUC for glucose during the OGTT were not significantly different between the treatment groups (Fig. 4A-4C). However, the AUC for insulin was significantly lower (by ~25%) after mifepristone treatment compared to placebo (Fig. 4D).
Table 3.
Effects of placebo and mifepristone administration on metabolic parameters and measures of insulin sensitivity/resistance
| Placebo | Mifepristone | P value | |
|---|---|---|---|
| Fasting glucose (mg/dL) | 107.8 ± 15.7 | 100.4 ± 15.3 | <0.001 |
| Fasting insulin (pmol/L) | 142.8 ± 102.2 | 95.6 ± 76.1 | <0.001 |
| HOMA-IR | 5.78 ± 4.92 | 3.58 ± 3.27 | <0.001 |
| QUICKI | 0.310 ± 0.029 | 0.332 ± 0.034 | <0.001 |
| SI (min−1·μU·mL−1) | 1.41 ± 0.87 | 1.49 ± 1.17 | 0.60 |
| OGIS (mL/min/m2) | 308.9 ± 72.9 | 305.0 ± 48.9 | 0.92 |
| Matsuda index | 2.52 ± 1.96 | 2.67 ± 1.03 | 0.16 |
| Hepatic insulin resistance index (HIRI) | 70.0 ± 44.3 | 50.2 ± 38.7 | 0.08 |
| Adipose-tissue insulin resistance index (Adipo-IR) | 65.5 ± 43.8 | 49.9 ± 45.9 | 0.004 |
| Adipose-tissue insulin sensitivity index (Adipo-Si) | 42.8 ± 23.9 | 61.7 ± 32.9 | 0.002 |
Posttreatment values shown are unadjusted means ± SD. Post-treatment values were adjusted for baseline value and treatment group when used in mixed-model regression. P-values indicate significance for comparisons between mifepristone and placebo.
Abbreviations: HOMA-IR, homeostatic model assessment of insulin resistance; OGIS, oral glucose insulin sensitivity; QUICKI, quantitative insulin sensitivity check index; RR, assay reference range; SI, insulin sensitivity index derived from the insulin-modified frequently sampled intravenous glucose tolerance test.
Figure 4.
Time profiles of plasma glucose and insulin following an OGTT in study participants after receiving either placebo or mifepristone. Time course of plasma glucose (A) and insulin (C) during OGTT. Data shown are mean ± SEM. P-values for the effects of treatment group, time, and the treatment*time interaction were obtained with repeated-measures analysis of variance with post-hoc Bonferroni’s test. Tukey box-and-whisker plots of glucose (B) and insulin (D) area under the curve for the duration of 3-h OGTT. Top and bottom box limits represent the 75th and 25th percentile, respectively; midlines represent the median; and top and bottom whiskers represent the 75th percentile plus 1.5 of the interquartile range and the 25th percentile minus 1.5 interquartile range, respectively. Symbols represent any values that lie outside the range of the whiskers. Posttreatment values were adjusted for baseline value and treatment group using mixed-model regression. P-values indicate significance for comparisons between mifepristone and placebo.
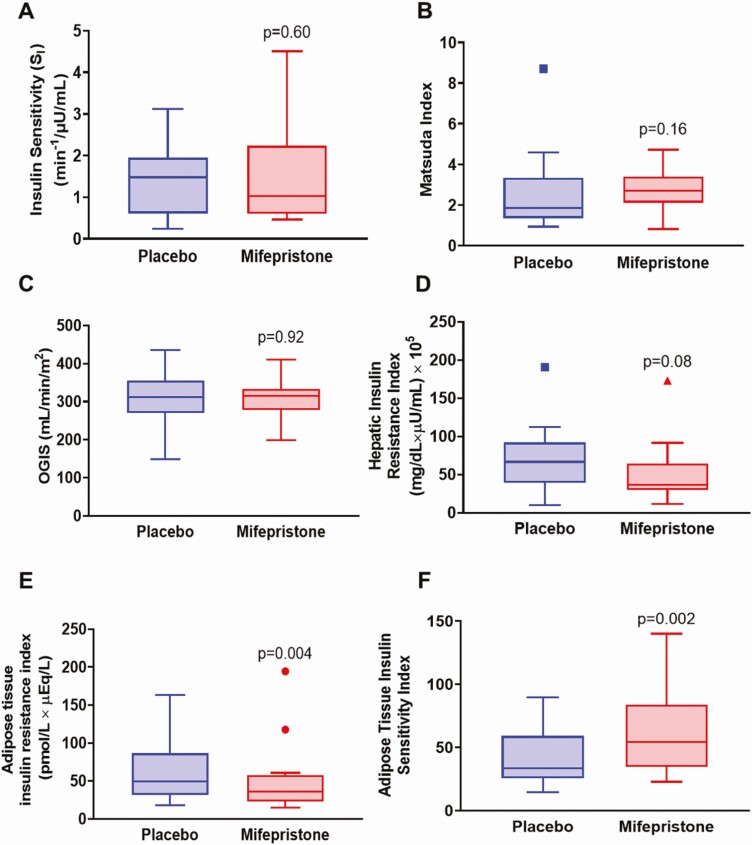
Figure 5.
Effects of placebo and mifepristone on indices of insulin sensitivity. Tukey box-and-whisker plots of (A) SI, insulin sensitivity index derived from the insulin-modified frequently sampled intravenous glucose tolerance test, (B) Matsuda index, (C) oral glucose insulin sensitivity index (OGIS), (D) hepatic insulin resistance index (HIRI), (E) adipose tissue insulin resistance index (Adipo-IR), and (F) adipose tissue insulin sensitivity index (Adipo-SI). Top and bottom box limits represent the 75th and 25th percentile, respectively; midlines represent the median; and top and bottom whiskers represent the 75th percentile plus 1.5 of the interquartile range and the 25th percentile minus 1.5 interquartile range, respectively. Symbols represent any values that lie outside the range of the whiskers. Posttreatment values were adjusted for baseline value and treatment group using mixed-model regression. P-values indicate significance for comparisons between mifepristone and placebo.
The primary endpoint, SI as measured by FSIVGTT was not significantly different between the treatment arms (1.49 ± 1.17 vs 1.41 ± 0.87 min−1·μU·mL−1, P = 0.60) (Fig. 5A). Overall insulin sensitivity indices derived from OGTT, the OGIS and Matsuda index, were not significantly different between placebo and mifepristone (Fig. 5B and 5C). HIRI was lower during mifepristone administration, but this did not reach statistical significance (P = 0.08) (Fig. 5D). Mifepristone administration was associated with a significant attenuation in Adipo-IR and an increase in Adipo-SI (Fig. 5E and 5F).
Effect of mifepristone on β-cell function
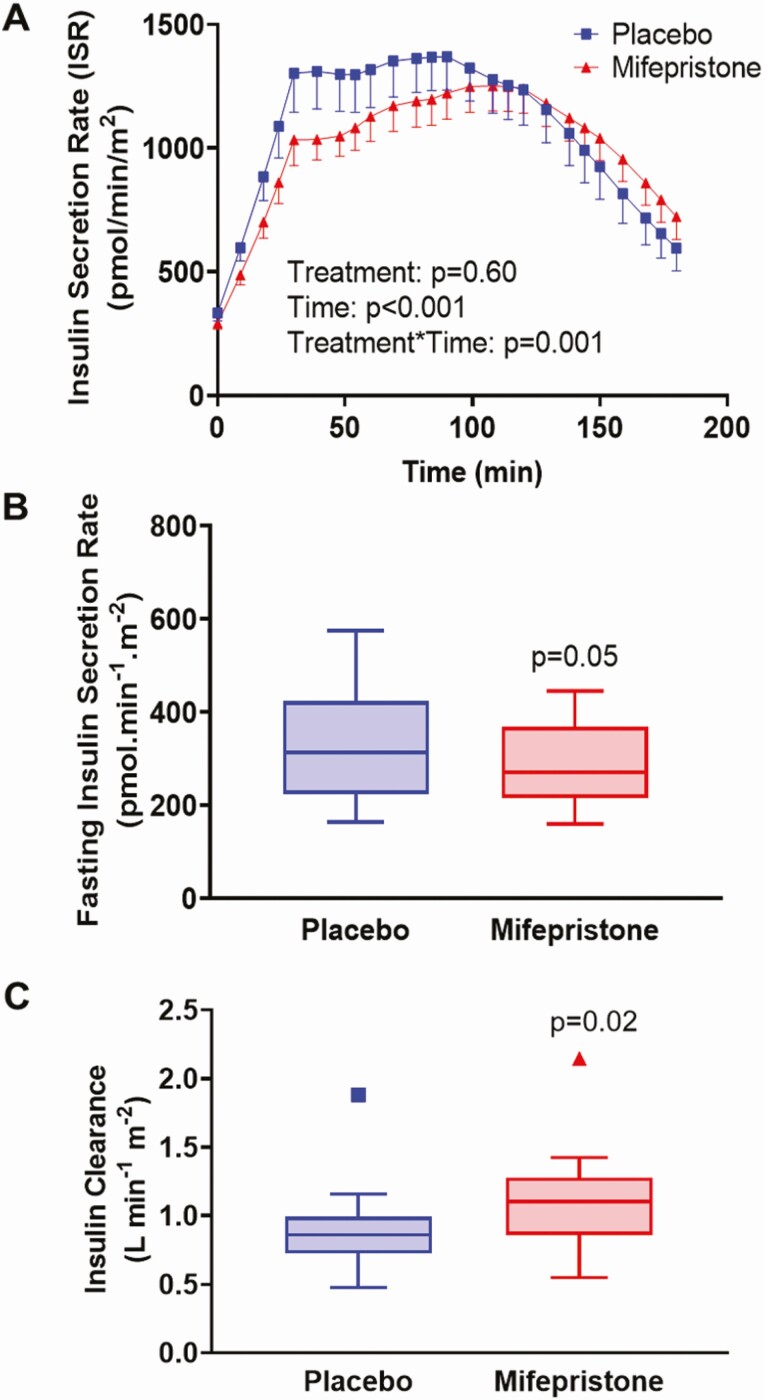
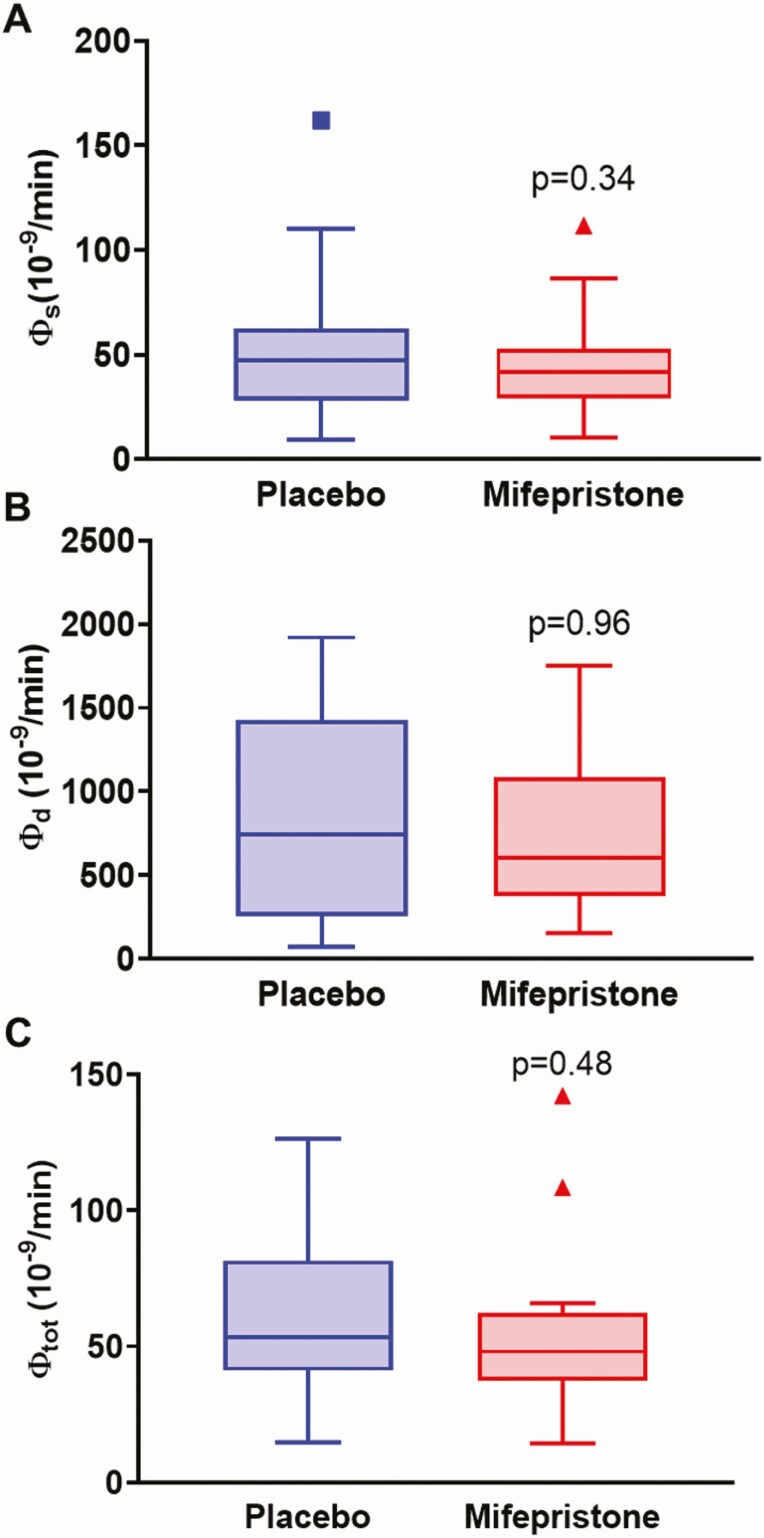
Insulin secretory rates were derived by mathematical modeling of C-peptide and glucose concentrations during an OGTT (Fig. 6). ISR during OGTT (Fig. 6A) and fasting ISR were similar between the 2 groups, although there was a tendency toward a decreased fasting ISR in the mifepristone group (292.1 ± 93.8 vs 333.9 ± 129.9 pmol.min-1.m−2, P = 0.05, Fig. 6B). Insulin clearance during OGTT was higher during mifepristone therapy than placebo (1.11 ± 0.37 vs 0.89 ± 0.32L min−1 m−2, P = 0.02; Fig. 6C). β-cell sensitivity parameters derived from the modeling included basal (Φ B), first phase (Φ 1), second phase (Φ 2), and total (Φ TOT). The static, dynamic, and total β-cell responsivity indices derived from OGTT were similar in the mifepristone and placebo groups (Fig. 7A-7C).
Figure 6.
Effects of placebo and mifepristone on insulin secretory rates and insulin clearance. Time course of insulin secretory rate (ISR) during OGTT after treatment with placebo and mifepristone (A). Data shown are mean ± SEM. P values for the effects of treatment group, time, and treatment*time interaction were obtained with repeated-measures analysis of variance with post-hoc Bonferroni’s test. Tukey box-and-whisker plots of (B) fasting ISR and (C) insulin clearance. Top and bottom box limits represent the 75th and 25th percentile, respectively; midlines represent the median; and top and bottom whiskers represent the 75th percentile plus 1.5 of the interquartile range and the 25th percentile minus 1.5 interquartile range, respectively. Symbols represent any values that lie outside the range of the whiskers. Posttreatment values were adjusted for baseline value and treatment group using mixed-model regression. P-values indicate significance for comparisons between mifepristone and placebo.
Figure 7.
Effects of placebo and mifepristone on β-cell responsivity indices. Tukey box-and-whisker plots of (A) Φ Static, (B) Φ Dynamic, and (C) Φ Total β-cell responsivity indices derived from OGTT. Top and bottom box limits represent the 75th and 25th percentile, respectively; midlines represent the median; and top and bottom whiskers represent the 75th percentile plus 1.5 of the interquartile range and the 25th percentile minus 1.5 interquartile range, respectively. Symbols represent any values that lie outside the range of the whiskers. Posttreatment values were adjusted for baseline value and treatment group using mixed-model regression. P-values indicate significance for comparisons between mifepristone and placebo.
Adverse effects associated with mifepristone and placebo administration
There were no serious adverse events reported by subjects or detected by investigators. The safety laboratory parameters remained within normal limits except for 2 subjects with increased alanine transferase values before and during mifepristone, 1 patient before and during placebo and another patient after placebo administration. Abnormal values were less than 1-fold the upper limit of normal, 40 U/L, ranging from 41 to 47 U/L.
Discussion
This was an exploratory study of the effects of mifepristone on insulin sensitivity in overweight and obese individuals with prediabetes or mild T2DM. We demonstrated for the first time in such a population, that short-term GR antagonism as evidenced by elevated ACTH and cortisol had variable effects on insulin sensitivity: a significant improvement at the adipose tissue, a tendency to improve at the liver, and no significant difference in the primary outcome, peripheral insulin sensitivity. These salutary changes were accompanied by significant decreases in AUC for insulin concentrations during an OGTT, with lower fasting glucose and insulin levels and an increase in insulin clearance. However, at the end of a 7- to 8-day exposure to mifepristone, there was no change in body weight, β-cell glucose responsivity, or insulin secretion following an oral glucose load. These findings shed light on the interplay between GCs and the metabolic syndrome and suggest a potentially novel adjunctive treatment for T2DM.
What might be the molecular mechanism(s) by which mifepristone can improve insulin sensitivity in adipose tissue (and potentially in the liver)? As detailed next, many components of insulin action are adversely affected by GCs. We speculate that mifepristone may decrease hepatic 11β-HSD1 activity and limit intracellular cortisol regeneration and also reduce cortisol action by directly blocking GR-mediated transcription. Adipose tissue lipolysis and hepatic glucose production are more sensitive to insulin than skeletal muscle glucose uptake (46). In our study, mifepristone administration significantly improved adipocyte sensitivity as judged by decreased Adipo-IR, a surrogate measure of adipose tissue insulin resistance, and increased Adipo-SI, a measure of insulin-mediated suppression of FFA during FSIVGTT. In obesity, increased adipose tissue expression of 11β-HSD1 may increase local concentrations of cortisol by approximately 15-fold (18-22). Increased intracellular GC concentration enhances basal lipolysis by increasing the activity and expression of lipolytic enzymes such as adipose triglyceride lipase and hormone-sensitive lipase. GCs also counteract the insulin-mediated suppression of FFA by decreasing phosphodiesterase 3B and upregulating the 3′,5′-cyclic adenosine 5′-monophosphates and protein kinase A pathway that leads to phosphorylation and activation of hormone-sensitive lipase (47,48). Mifepristone reduces basal and stimulated lipolysis in rodents and in in vitro studies (49,50). In a randomized, double-blinded, placebo-controlled crossover study using stable isotopes, Macfarlane et al administered metyrapone (an inhibitor of cortisol synthesis) and mifepristone at 11 pm and 8:00 am to decrease cortisol production and block its action in 14 men with T2DM (51). Compared to placebo, the mifepristone/metyrapone combination augmented insulin-mediated suppression of FFA concentrations and the rate of appearance of glycerol during a euglycemic hyperinsulinemic clamp following the second dose (51). Our findings of improved adipose tissue sensitivity are consistent with these studies.
We found that HIRI tended (P = 0.08) to be lower following short-term mifepristone administration, suggesting a potential for improvement in hepatic insulin sensitivity. Indeed, in the study by Macfarlane et al, the mifepristone and metyrapone combination decreased hepatic glucose production and HIRI (51). Improvement in hyperglycemia with mifepristone treatment of ob/ob mice and db/db mice was associated with significant reductions in hepatic expression of gluconeogenic enzymes, phosphoenolpyruvate carboxykinase, and glucose 6-phosphatase (52,53), which are induced by GCs.
In this study, mifepristone significantly decreased fasting insulin and glucose concentrations and HOMA-IR and increased QUICKI. Mifepristone significantly reduced the insulin AUC during OGTT compared to placebo, without changes in the glucose AUC, showing that less insulin was needed during mifepristone exposure to maintain plasma glucose levels similar to those seen with placebo. Similar results have been reported with higher doses and longer duration of mifepristone. Patients with adrenal nodules and autonomous cortisol secretion who received chronic mifepristone (300 or 400 mg/day) had reduced insulin AUC during OGTT and decreased HOMA-IR after 1 to 6 months (54,55). HOMA-IR also decreased modestly in postmenopausal women after 6 weeks of mifepristone administration (600 mg once daily) compared with placebo (56).
SI is a composite measure of peripheral and hepatic insulin sensitivity that is assessed by minimal modeling of FSIVGTT data (46). In our study, mifepristone administration for 8 days did not significantly affect SI or whole-body insulin sensitivity indices derived from an OGTT. Likewise, 1-day acute administration of mifepristone and metyrapone did not affect insulin-mediated peripheral glucose disposal as measured by the euglycemic hyperinsulinemic clamp technique (51). Consistent with these findings, administration of carbenoxolone, a nonselective 11β-HSD1 and 2 inhibitor for 7 days to patients with T2DM did not significantly change peripheral insulin sensitivity (57). It is possible that a longer duration of mifepristone treatment may increase peripheral insulin sensitivity. Although GCs are known to acutely impair glucose-stimulated insulin secretion in human β-cells (2,58,59), mifepristone did not alter either ISR during an oral glucose load or β-cell sensitivity parameters. This suggests that short-term GR antagonism does not significantly change β-cell function in overweight/obese individuals.
Mifepristone had significant effects on lipids and BP. Plasma total cholesterol, triglycerides, and HDL cholesterol levels decreased significantly with mifepristone administration, as has been observed in other studies. However, mifepristone also causes a net increase in the per particle cholesterol efflux capacity of HDL, which is a protective factor against cardiovascular disease (56). Therefore, HDL reductions by mifepristone therapy might not necessarily confer an increased cardiovascular risk. BP increased during mifepristone administration, with a mean systolic BP increase of 9 mmHg and a mean diastolic BP increase of 6 mmHg. This may be due to cortisol effects via the mineralocorticoid receptor to increase responsivity of the vasculature to catecholamines (60). Nonetheless, the mean BP values in our subjects remained within the pre-hypertensive range for systolic BP and within normotensive range for diastolic BP. Concurrent to the changes in BP, plasma potassium levels were lower following mifepristone suggesting a likely effect of cortisol on the renal mineralocorticoid receptors.
As expected, mifepristone treatment increased plasma ACTH and cortisol levels. However, the increments in the plasma ACTH and cortisol occurred only during the early morning hours, with normal values during the later hours of the day and a lag in normalization of cortisol levels by 5 h after ACTH (10:00 am for ACTH vs 3 pm for cortisol) (Fig. 3). Bertagna et al had similar findings in 4 healthy men in whom ACTH and cortisol increased 6 to 10 h after administration of 400 mg of mifepristone at 2:00 am, but not at 4:00 am (61). These results suggest that the uniform elevations in early morning ACTH concentrations reflect effective GC blockade by mifepristone. The 24-h UFC increased during mifepristone treatment but remained normal in 8 subjects, likely reflecting the normal values of cortisol during much of the day. Other hormones normally stimulated by ACTH, DHEAS, and androstenedione also increased but generally remained normal.
There are several strengths to our study. The crossover design reduced the potential for interperson variability and increased our ability to detect treatment differences in this small sample. Also, we allowed for a wash-out period of 6 to 8 weeks to minimize carryover effects of treatment. Efforts were made to maintain study subjects on isocaloric diets with a stable exercise routine and without any new medications to minimize influences on period effects.
Our study had some limitations. The small study population might not have allowed us to detect other significant influences of mifepristone on metabolic parameters. As the duration of mifepristone treatment was short (8 days), we could not evaluate effects of long-term mifepristone exposure on glycemic control, weight, and adverse events. Moreover, we did not assess insulin sensitivity with the gold standard technique, the hyperinsulinemic euglycemic clamp.
In conclusion, short-term mifepristone therapy improves adipose tissue and likely hepatic insulin sensitivity in overweight and obese individuals with prediabetes or mild T2DM, thus highlighting the pivotal role of GR and its potential as a novel therapeutic target in insulin-resistant states. A randomized controlled trial consisting of a larger cohort and with longer duration of follow-up is recommended to investigate the possible long-term effects of mifepristone treatment on glucose and lipid metabolism.
Acknowledgments
We thank the nurses on the NIH CRC Metabolic Unit for their assistance with the serial tests and patients.
Financial Support: This work was supported by the Intramural program of the National Institute of Diabetes, Digestive and Kidney Diseases, NIH (grant number ZIA DK075121-04) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The study was also supported by a Cooperative Research and Development Agreement (CRADA) (grant number #02241) between the National Institutes of Health (NIH) and Laboratoire HRA-Pharma in Paris, France.
Clinical Trial Information : ClinicalTrials.gov Identifier: NCT01419535
Author Contributions: SG, SGr, and RM analyzed and interpreted data and wrote, reviewed, and approved the manuscript. STS contributed to the study design, conducted the study, acquired and analyzed data, and contributed to manuscript writing. RMc contributed to patient recruitment and management, data collection, data interpretation, and drafting the manuscript. LKN designed and conducted the clinical study, acquired and analyzed data, and wrote the manuscript. LKN had full access to all data in the study and takes responsibility for the integrity of data and the accuracy of the data analysis.
Additional Information
Disclosure Summary: This research study was conducted under a Clinical Research and Development Agreement (CRADA) between the National Institutes of Health (NIH) and Laboratoire HRA-Pharma in Paris, France. LKN is a co-inventor on a provisional patent application related to this work; all other authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Hollingdal M, Juhl CB, Dall R, et al. . Glucocorticoid induced insulin resistance impairs basal but not glucose entrained high-frequency insulin pulsatility in humans. Diabetologia. 2002;45(1):49-55. [DOI] [PubMed] [Google Scholar]
- 2. Delaunay F, Khan A, Cintra A, et al. . Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 1997;100(8):2094-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hansen KB, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. Reduced glucose tolerance and insulin resistance induced by steroid treatment, relative physical inactivity, and high-calorie diet impairs the incretin effect in healthy subjects. J Clin Endocrinol Metab. 2010;95(7):3309-3317. [DOI] [PubMed] [Google Scholar]
- 4. Scaroni C, Zilio M, Foti M, Boscaro M. Glucose metabolism abnormalities in cushing syndrome: from molecular basis to clinical management. Endocr Rev. 2017;38(3):189-219. [DOI] [PubMed] [Google Scholar]
- 5. Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982;54(1):131-138. [DOI] [PubMed] [Google Scholar]
- 6. Qi D, Rodrigues B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J Physiol Endocrinol Metab. 2007;292(3):E654-E667. [DOI] [PubMed] [Google Scholar]
- 7. Nielsen MF, Caumo A, Chandramouli V, et al. . Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercortisolemia in healthy subjects. Am J Physiol Endocrinol Metab. 2004;286(1): E102-E110. [DOI] [PubMed] [Google Scholar]
- 8. Abad V, Chrousos GP, Reynolds JC, et al. . Glucocorticoid excess during adolescence leads to a major persistent deficit in bone mass and an increase in central body fat. J Bone Miner Res. 2001;16(10):1879-1885. [DOI] [PubMed] [Google Scholar]
- 9. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913-927. [DOI] [PubMed] [Google Scholar]
- 10. Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7): 611-629. [DOI] [PubMed] [Google Scholar]
- 11. Rockall AG, Sohaib SA, Evans D, et al. . Hepatic steatosis in Cushing’s syndrome: a radiological assessment using computed tomography. Eur J Endocrinol. 2003;149(6): 543-548. [DOI] [PubMed] [Google Scholar]
- 12. Nieman LK, Chrousos GP, Kellner C, et al. . Successful treatment of Cushing’s syndrome with the glucocorticoid antagonist RU 486. J Clin Endocrinol Metab. 1985;61(3):536-540. [DOI] [PubMed] [Google Scholar]
- 13. Wallia A, Colleran K, Purnell JQ, Gross C, Molitch ME. Improvement in insulin sensitivity during mifepristone treatment of Cushing syndrome: early and late effects. Diabetes Care. 2013;36(9):e147-e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C; SEISMIC Study Investigators . Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97(6):2039-2049. [DOI] [PubMed] [Google Scholar]
- 15. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1-12. [DOI] [PubMed] [Google Scholar]
- 16. Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94(8):2692-2701. [DOI] [PubMed] [Google Scholar]
- 17. Pasquali R, Vicennati V, Cacciari M, Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann N Y Acad Sci. 2006;1083:111-128. [DOI] [PubMed] [Google Scholar]
- 18. Masuzaki H, Paterson J, Shinyama H, et al. . A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294(5549):2166-2170. [DOI] [PubMed] [Google Scholar]
- 19. Morton NM. Obesity and corticosteroids: 11beta-hydroxysteroid type 1 as a cause and therapeutic target in metabolic disease. Mol Cell Endocrinol. 2010;316(2):154-164. [DOI] [PubMed] [Google Scholar]
- 20. Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production rates, free cortisol, and 11beta-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab. 2009;296(2):E351-E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kannisto K, Pietiläinen KH, Ehrenborg E, et al. . Overexpression of 11beta-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J Clin Endocrinol Metab. 2004;89(9):4414-4421. [DOI] [PubMed] [Google Scholar]
- 22. Wake DJ, Rask E, Livingstone DE, Söderberg S, Olsson T, Walker BR. Local and systemic impact of transcriptional up-regulation of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue in human obesity. J Clin Endocrinol Metab. 2003;88(8):3983-3988. [DOI] [PubMed] [Google Scholar]
- 23. Tomlinson JW, Stewart PM. Modulation of glucocorticoid action and the treatment of type-2 diabetes. Best Pract Res Clin Endocrinol Metab. 2007;21(4):607-619. [DOI] [PubMed] [Google Scholar]
- 24. Rosenstock J, Banarer S, Fonseca VA, et al. ; INCB13739-202 Principal Investigators . The 11-beta-hydroxysteroid dehydrogenase type 1 inhibitor INCB13739 improves hyperglycemia in patients with type 2 diabetes inadequately controlled by metformin monotherapy. Diabetes Care. 2010;33(7):1516-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobson PB, von Geldern TW, Ohman L, et al. . Hepatic glucocorticoid receptor antagonism is sufficient to reduce elevated hepatic glucose output and improve glucose control in animal models of type 2 diabetes. J Pharmacol Exp Ther. 2005;314(1):191-200. [DOI] [PubMed] [Google Scholar]
- 26. Garrel DR, Moussali R, De Oliveira A, Lesiège D, Larivière F. RU 486 prevents the acute effects of cortisol on glucose and leucine metabolism. J Clin Endocrinol Metab. 1995;80(2):379-385. [DOI] [PubMed] [Google Scholar]
- 27. Ottosson M, Mårin P, Karason K, Elander A, Björntorp P. Blockade of the glucocorticoid receptor with RU 486: effects in vitro and in vivo on human adipose tissue lipoprotein lipase activity. Obes Res. 1995;3(3):233-240. [DOI] [PubMed] [Google Scholar]
- 28. Fosam A, Sikder S, Abel BS, et al. . Reduced insulin clearance and insulin-degrading enzyme activity contribute to hyperinsulinemia in African Americans. J Clin Endocrinol Metab. 2020;105(4):e1835-e1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30(1):89-94. [DOI] [PubMed] [Google Scholar]
- 30. Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2017;102(4):1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carpentier A, Patterson BW, Leung N, Lewis GF. Sensitivity to acute insulin-mediated suppression of plasma free fatty acids is not a determinant of fasting VLDL triglyceride secretion in healthy humans. Diabetes. 2002;51(6):1867-1875. [DOI] [PubMed] [Google Scholar]
- 32. Trottier A, Battista MC, Geller DH, et al. . Adipose tissue insulin resistance in peripubertal girls with first-degree family history of polycystic ovary syndrome. Fertil Steril. 2012;98(6):1627-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toffolo G, Cefalu WT, Cobelli C. Beta-cell function during insulin-modified intravenous glucose tolerance test successfully assessed by the C-peptide minimal model. Metabolism. 1999;48(9):1162-1166. [DOI] [PubMed] [Google Scholar]
- 34. Cobelli C, Toffolo GM, Dalla Man C, et al. . Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293(1):E1-E15. [DOI] [PubMed] [Google Scholar]
- 35. Trico D, Natali A, Arslanian S, Mari A, Ferrannini E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight. 2018;3(24):e124912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kusunoki M, Cooney GJ, Hara T, Storlien LH. Amelioration of high-fat feeding-induced insulin resistance in skeletal muscle with the antiglucocorticoid RU486. Diabetes. 1995;44(6):718-720. [DOI] [PubMed] [Google Scholar]
- 37. Gettys TW, Watson PM, Taylor IL, Collins S. RU-486 (Mifepristone) ameliorates diabetes but does not correct deficient beta-adrenergic signalling in adipocytes from mature C57BL/6J-ob/ob mice. Int J Obes Relat Metab Disord. 1997;21(10):865-873. [DOI] [PubMed] [Google Scholar]
- 38. Liu Y, Nakagawa Y, Wang Y, et al. . Increased glucocorticoid receptor and 11{beta}-hydroxysteroid dehydrogenase type 1 expression in hepatocytes may contribute to the phenotype of type 2 diabetes in db/db mice. Diabetes. 2005;54(1):32-40. [DOI] [PubMed] [Google Scholar]
- 39. Sunehag AL, Treuth MS, Toffolo G, et al. . Glucose production, gluconeogenesis, and insulin sensitivity in children and adolescents: an evaluation of their reproducibility. Pediatr Res. 2001;50(1):115-123. [DOI] [PubMed] [Google Scholar]
- 40. Hermans MP, Levy JC, Morris RJ, Turner RC. Comparison of insulin sensitivity tests across a range of glucose tolerance from normal to diabetes. Diabetologia. 1999;42(6):678-687. [DOI] [PubMed] [Google Scholar]
- 41. Owei I, Umekwe N, Provo C, Wan J, Dagogo-Jack S. Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res Care. 2017;5(1):e000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kernan WN, Inzucchi SE, Viscoli CM, et al. . Pioglitazone improves insulin sensitivity among nondiabetic patients with a recent transient ischemic attack or ischemic stroke. Stroke. 2003;34(6):1431-1436. [DOI] [PubMed] [Google Scholar]
- 43. Antoniolli LP, Nedel BL, Pazinato TC, de Andrade Mesquita L, Gerchman F. Accuracy of insulin resistance indices for metabolic syndrome: a cross-sectional study in adults. Diabetol Metab Syndr. 2018;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26(12):3320-3325. [DOI] [PubMed] [Google Scholar]
- 45. Gaillard RC, Riondel A, Muller AF, Herrmann W, Baulieu EE. RU 486: a steroid with antiglucocorticosteroid activity that only disinhibits the human pituitary-adrenal system at a specific time of day. Proc Natl Acad Sci U S A. 1984;81(12):3879-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15-E26. [DOI] [PubMed] [Google Scholar]
- 47. Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60(11):1500-1510. [DOI] [PubMed] [Google Scholar]
- 48. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gray NE, Lam LN, Yang K, Zhou AY, Koliwad S, Wang JC. Angiopoietin-like 4 (Angptl4) protein is a physiological mediator of intracellular lipolysis in murine adipocytes. J Biol Chem. 2012;287(11):8444-8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu C, He J, Jiang H, et al. . Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol. 2009;23(8):1161-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Macfarlane DP, Raubenheimer PJ, Preston T, et al. . Effects of acute glucocorticoid blockade on metabolic dysfunction in patients with type 2 diabetes with and without fatty liver. Am J Physiol Gastrointest Liver Physiol. 2014;307(7):G760-G768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taylor AI, Frizzell N, McKillop AM, Flatt PR, Gault VA. Effect of RU486 on hepatic and adipocyte gene expression improves diabetes control in obesity-type 2 diabetes. Horm Metab Res. 2009;41(12):899-904. [DOI] [PubMed] [Google Scholar]
- 53. Wang Y, Nakagawa Y, Liu L, et al. . Tissue-specific dysregulation of hexose-6-phosphate dehydrogenase and glucose-6-phosphate transporter production in db/db mice as a model of type 2 diabetes. Diabetologia. 2011;54(2):440-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Debono M, Chadarevian R, Eastell R, Ross RJ, Newell-Price J. Mifepristone reduces insulin resistance in patient volunteers with adrenal incidentalomas that secrete low levels of cortisol: a pilot study. PLoS One. 2013;8(4):e60984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Belokovskaya R, Ravikumar A, Arumugam D, et al. . Mifepristone treatment for mild autonomous cortisol secretion due to adrenal adenomas: A Pilot Study. Endocr Pract. 2019;25(8): 846-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Page ST, Krauss RM, Gross C, et al. . Impact of mifepristone, a glucocorticoid/progesterone antagonist, on HDL cholesterol, HDL particle concentration, and HDL function. J Clin Endocrinol Metab. 2012;97(5):1598-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Andrews RC, Rooyackers O, Walker BR. Effects of the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone on insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab. 2003;88(1):285-291. [DOI] [PubMed] [Google Scholar]
- 58. Fine NHF, Doig CL, Elhassan YS, et al. . Glucocorticoids reprogram β-cell signaling to preserve insulin secretion. Diabetes. 2018;67(2):278-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Raalte DH, Nofrate V, Bunck MC, et al. . Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. Eur J Endocrinol. 2010;162(4):729-735. [DOI] [PubMed] [Google Scholar]
- 60. Walker BR, Connacher AA, Webb DJ, Edwards CR. Glucocorticoids and blood pressure: a role for the cortisol/cortisone shuttle in the control of vascular tone in man. Clin Sci (Lond). 1992;83(2):171-178. [DOI] [PubMed] [Google Scholar]
- 61. Bertagna X, Bertagna C, Luton JP, Husson JM, Girard F. The new steroid analog RU 486 inhibits glucocorticoid action in man. J Clin Endocrinol Metab. 1984;59(1):25-28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.