Abstract
Psychostimulants, including amphetamines and methylphenidate, are first-line pharmacotherapies for individuals with attention-deficit/hyperactivity disorder (ADHD). This review aims to educate physicians regarding differences in pharmacology and mechanisms of action between amphetamine and methylphenidate, thus enhancing physician understanding of psychostimulants and their use in managing individuals with ADHD who may have comorbid psychiatric conditions. A systematic literature review of PubMed was conducted in April 2017, focusing on cellular- and brain system–level effects of amphetamine and methylphenidate. The primary pharmacologic effect of both amphetamine and methylphenidate is to increase central dopamine and norepinephrine activity, which impacts executive and attentional function. Amphetamine actions include dopamine and norepinephrine transporter inhibition, vesicular monoamine transporter 2 (VMAT-2) inhibition, and monoamine oxidase activity inhibition. Methylphenidate actions include dopamine and norepinephrine transporter inhibition, agonist activity at the serotonin type 1A receptor, and redistribution of the VMAT-2. There is also evidence for interactions with glutamate and opioid systems. Clinical implications of these actions in individuals with ADHD with comorbid depression, anxiety, substance use disorder, and sleep disturbances are discussed.
Keywords: amphetamine, attention-deficit/hyperactivity disorder, methylphenidate, pharmacology
1.0. Introduction
Attention-deficit/hyperactivity disorder (ADHD) was initially identified in children (1) but is now understood to persist into adulthood in about two thirds of cases (2-4). In a 2007 meta-analysis that included more than 100 studies, the estimated worldwide prevalence of ADHD in individuals <18 years old was 5.29% (5). The estimated prevalence in adults was 4.4% in a national survey in the United States (6), 3.4% in a 10-nation survey (7), and 2.5% in a meta-regression analysis of 6 studies (8).
A large body of evidence suggests that multiple neurotransmitters and brain structures play a role in ADHD (9-11). Although a substantial amount of research has focused on dopamine (DA) and norepinephrine (NE), ADHD has also been linked to dysfunction in serotonin (5-hydroxytryptamine [5-HT]), acetylcholine (ACH), opioid, and glutamate (GLU) pathways (10, 12-15). The alterations in these neurotransmitter systems affect the function of brain structures that moderate executive function, working memory, emotional regulation, and reward processing (Figure 1) (11).
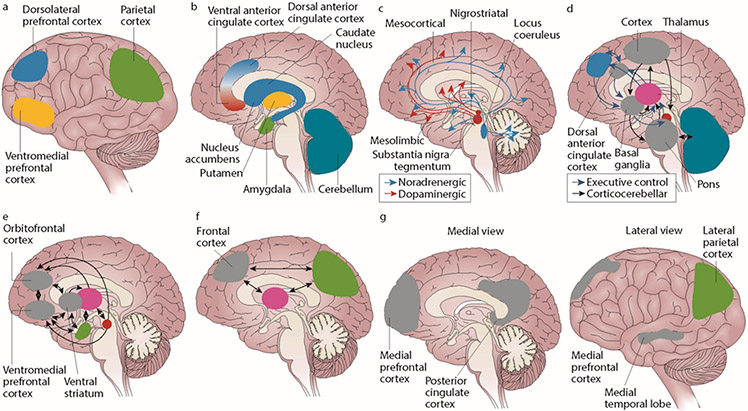
Figure 1. Brain Mechanisms in ADHD*.
(a) The cortical regions (lateral view) of the brain have a role in attention-deficit/hyperactivity disorder (ADHD). The dorsolateral prefrontal cortex is linked to working memory, the ventromedial prefrontal cortex to complex decision making and strategic planning, and the parietal cortex to orientation of attention. (b) ADHD involves the subcortical structures (medial view) of the brain. The ventral anterior cingulate cortex and the dorsal anterior cingulate cortex subserve affective and cognitive components of executive control. Together with the basal ganglia (comprising the nucleus accumbens, caudate nucleus, and putamen), they form the frontostriatal circuit. Neuroimaging studies show structural and functional abnormalities in all of these structures in patients with ADHD, extending into the amygdala and cerebellum. (c) Neurotransmitter circuits in the brain are involved in ADHD. The dopamine system plays an important part in planning and initiation of motor responses, activation, switching, reaction to novelty, and processing of reward. The noradrenergic system influences arousal modulation, signal-to-noise ratios in cortical areas, state-dependent cognitive processes, and cognitive preparation of urgent stimuli. (d) Executive control networks are affected in patients with ADHD. The executive control and cortico-cerebellar networks coordinate executive functioning (ie, planning, goal-directed behavior, inhibition, working memory, and the flexible adaptation to context). These networks are underactivated and have lower internal functional connectivity in individuals with ADHD compared with individuals without the disorder. (e) ADHD involves the reward network. The ventromedial prefrontal cortex, orbitofrontal cortex, and ventral striatum are at the center of the brain network that responds to anticipation and receipt of reward. Other structures involved are the thalamus, the amygdala, and the cell bodies of dopaminergic neurons in the substantia nigra, which, as indicated by the arrows, interact in a complex manner. Behavioral and neural responses to reward are abnormal in ADHD. (f) The alerting network is impaired in ADHD. The frontal and parietal cortical areas and the thalamus intensively interact in the alerting network (indicated by the arrows), which supports attentional functioning and is weaker in individuals with ADHD than in controls. (g) ADHD involves the default-mode network (DMN). The DMN consists of the medial prefrontal cortex and the posterior cingulate cortex (medial view) as well as the lateral parietal cortex and the medial temporal lobe (lateral view). DMN fluctuations are 180° out of phase with fluctuations in networks that become activated during externally oriented tasks, presumably reflecting competition between opposing processes for processing resources. Negative correlations between the DMN and the frontoparietal control network are weaker in patients with ADHD than in people who do not have the disorder.
*Reprinted with permission from Macmillan Publishers Ltd: [NAT REV DIS PRIMERS] (Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Nat Rev Dis Primers. 2015 Aug 6;1:15020), copyright 2015.
Individuals with ADHD are often diagnosed with additional psychiatric comorbidities, including anxiety, mood, substance use, sleep disturbances, and antisocial personality disorders (16-18). Importantly, the neurobiological substrates that mediate behaviors associated with ADHD share commonalities to some extent with those involved in these comorbid disorders (19-21). Genetic studies have also identified shared genetic risk factors between ADHD and associated comorbid disorders (22, 23). As such, comorbidities need to be taken into account when considering pharmacotherapy in an individual with ADHD.
Although stimulants (including amphetamine [AMP]–based and methylphenidate [MPH]–based agents) and nonstimulants (eg, atomoxetine, clonidine, and guanfacine) are approved for the treatment of ADHD (24), stimulants are considered first-line therapy in children, adolescents, and adults with ADHD because of their greater efficacy (25-30). AMP and MPH have been shown to exhibit comparable efficacy in 2 meta-analyses (31, 32), with other analyses reporting that AMP has moderately greater effects than MPH (33-35). The tolerability and safety profiles of AMP and MPH in terms of adverse events, treatment discontinuation, and cardiovascular effects are also generally comparable (36-38), although weight loss and insomnia have been reported to be more common with AMP than with MPH (32). Additional work is planned that will further compare the efficacy, tolerability, and safety profiles of different pharmacologic interventions in children, adolescents, and adults with ADHD (39).
Although increased synaptic availability of DA and NE is a key result of exposure to both AMP and MPH (40, 41), differences in the specific cellular mechanisms of action of AMP and MPH may influence their effects on the neurobiological substrates of ADHD and response to treatment in individuals with ADHD as well as their effects on common comorbidities, such as depression and anxiety.
The objective of this review is to educate physicians about the mechanisms of action of AMP and MPH and the implications of these actions on the management of ADHD and its comorbidities. To achieve this goal, a systematic review of the published literature was conducted to obtain articles describing the cellular- and brain system–level effects of AMP and MPH. The results of relevant studies are described and interpreted in the context of the treatment of ADHD and in light of the comorbidities associated with ADHD.
2.0. Methods
A systematic literature review of PubMed was conducted on April 24, 2017; no limits were included for publication year or the language of publication. The search consisted of titles and abstracts and used the following search string: (amphetamine [MESH term] OR methylphenidate [MESH term]) AND (cellular OR receptor binding OR neuroimaging OR FMRI OR SPECT OR PET OR positron emission tomography OR magnetic resonance OR tomography OR spectroscopy) AND (dopamine OR serotonin OR norepinephrine OR acetylcholine OR glutamate OR opioid OR opiate) NOT (methamphetamine OR MDMA OR ecstasy OR addiction OR abuse). The literature search included both animal and human studies.
Additional articles of interest were obtained via an assessment of the relevant articles obtained through the literature review and based on author knowledge. A publication was excluded if it did not specifically focus on the mechanism of action or pharmacologic effects of AMP or MPH or if it was an imaging study in individuals who were other than healthy (ie, studies in those with psychiatric disorders were not included).
3.0. Results
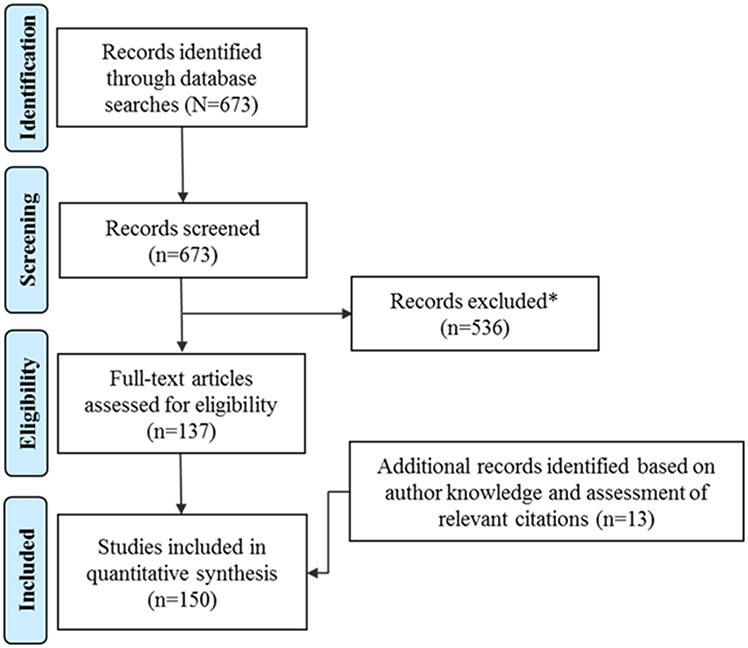
The literature search yielded 673 articles (Figure 2). Of these, 137 were considered relevant to the topic of this review. Additional articles (n=13) were identified based on author knowledge and assessment of the reference sections of relevant citations. The articles included in this review are summarized in Table 1 (42-191).
Figure 2. PRISMA Flow Diagram of the Literature Search.
*Publications were excluded if they did not focus on the mechanism of action or pharmacologic effects of amphetamine or methylphenidate or if the publications were imaging studies in individuals who were not healthy (ie, studies in those with psychiatric disorders were not included).
Table 1.
Studies of the Actions of AMP and MPH
| Preclinical studies | |
| Amphetamine | |
| al-Tikriti et al (42) | • AMP increased the washout rate of [123I]IBF (a D2 receptor antagonist) from the striatum of baboons, as measured using SPECT |
| Annamalai et al (43) | • AMP-stimulated downregulation of the NET was linked to the PKC-resistant T258/S259 structural motif and mediated by reduced plasma membrane insertion and enhanced endocytosis |
| Avelar et al (44) | • AMP increased electrically evoked DA levels, inhibited DA uptake, and upregulated DA vesicular release in rat striatum |
| Bjorklund et al (45) | • Adenosine A3 receptor knockout mice exhibited reduced locomotor responses to AMP, suggesting potential alterations in monoaminergic (DA, 5-HT, or NE) systems |
| Carson et al (46) | • AMP reduced [11C]raclopride (a D2 receptor antagonist) binding in the striatum of rhesus monkeys, as measured by PET |
| Castner et al (47) | • AMP reduced [123I]IBZM (a D2 receptor antagonist) binding in the striatum of rhesus monkeys, as measured by SPECT |
| Chen et al (48) | • AMP-induced increases in whole-brain relative cerebral blood volume were attenuated by electroacupuncture (electrical paw stimulation) in rats |
| Chen et al (49) | • Rapid AMP-induced increases in striatal surface DAT levels and DA release in mice were not observed in PKC-β knockout mice |
| Choe et al (50) | • AMP increased phosphorylation of CREB in rats; intrastriatal administration of PHCCC (an mGluR type 1 antagonist) and systemic administration of MPEP (an mGluR type 5 antagonist) attenuated this effect |
| Chou et al (51) | • AMP reduced [11C]FLB 457 (a D2 receptor antagonist) binding in the thalamus, cortex, and striatum of cynomolgus monkeys, as measured by PET |
| Covey et al (52) | • AMP increased DA release in rat striatum when administered with a short-duration electrical stimulus and decreased DA release when administered with a long-duration electrical stimulus |
| Dewey et al (53) | • AMP decreased [11C]raclopride (a D2 receptor antagonist) binding in the striatum of baboons, as measured by PET |
| Dixon et al (54) | • AMP caused widespread increases in fMRI BOLD signal intensity in subcortical structures containing rich DA innervation, with decreases in BOLD signal observed in the superficial layers of the cortex in rats |
| Drevets et al (55) | • AMP-induced reductions in [11C]raclopride (a D2 receptor antagonist) binding in baboons were greater in the anteroventral striatum than in the dorsal striatum, as measured by PET |
| Duttaroy et al (56) | • Chronic AMP exposure blocked naloxone-induced supersensitivity of μ- and δ-opioid receptors without altering naloxone-induced upregulation of these receptors in mice |
| Easton et al (57) | • AMP isomers increased DA release (basal and electrically stimulated) in the nucleus accumbens, medial entorhinal cortex, colliculi, hippocampal area CA1, and thalamic nuclei of rats |
| Easton et al (58) | • AMP inhibited accumulation of DA or NE in rat brain synaptosomes and vesicles, which was mitigated by inhibitors of the DAT and NET |
| Finnema et al (59) | • AMP reduced binding of [11C]ORM-13070 (an adrenergic α2c receptor antagonist) in the striatum of cynomolgus monkeys, as measured by PET, and increased rat striatal NE and DA concentrations |
| Floor and Meng (60) | • Low AMP concentrations increased DA release from rat brain synaptic vesicles independent of vesicular alkalinization; high-AMP-concentration DA release was reduced as a function of the proton gradient |
| Gallezot et al (61) | • AMP reduced [11C]raclopride (a D2 receptor antagonist) and [11C]PHNO (a D2/D3 receptor agonist) binding in the striatum, globus pallidus, and substantia nigra of rhesus monkeys, as measured by PET |
| Ginovart et al (62) | • Chronic AMP administration (14 days) was associated with an increased [11C]raclopride (a D2 receptor antagonist) binding affinity after 1 day of drug withdrawal and with binding after 7 and 14 days of withdrawal in cynomolgus monkeys, as measured by PET |
| Hartvig et al (63) | • AMP increased DA biosynthesis in the brain in nonhuman primates, as measured by PET |
| Helmeste and Seeman (64) | • Mice with high D2 receptor density in the striatum and olfactory tubercle exhibited hypolocomotion in response to low-dose AMP, whereas mice with low D2 receptor density were nonresponsive to AMP |
| Howlett and Nahorski (65) | • AMP exposure increased [3H]spiperone (a D2/D3 receptor antagonist) binding affinity and density after 4 days of exposure and decreased binding density after 20 days of exposure in rat striatum |
| Inderbitzin et al (66) | • AMP increased preprodynorphin mRNA expression and κ-opioid receptor binding in the basal ganglia of rats |
| Jedema et al (67) | • AMP increased DA release in the caudate and prefrontal cortex of rhesus macaques, as measured using microdialysis, with DA levels remaining elevated for a longer duration in the prefrontal cortex than in the caudate |
| Joyce et al (68) | • Administration of a combination of mixed amphetamine salts directly into the striatum of rats stimulated DA release |
| Kashiwagi et al (69) | • AMP increased relative cerebral blood volume in awake and anesthetized rats, with changes correlated to changes in striatal DA concentration |
| Konradi et al (70) | • AMP-induced activation of immediate early genes in dissociated rat striatal cultures was blocked by MK-801 (an NMDA receptor antagonist) |
| Kuczenski and Segal (71) | • AMP increased DA and 5-HT efflux in the caudate and NE efflux in the hippocampus of rats following systemic administration |
| Lahti and Tamminga (72) | • AMP increased D2 receptor occupancy in the cortex and caudate of rats, as measured by receptor autoradiography |
| Landau et al (73) | • AMP decreased binding of [11C]yohimbine (an adrenergic ɑ2 receptor antagonist) in the thalamus, cortex, and caudate of pigs, as measured by PET |
| Laruelle et al (74) | • AMP reduced [123I]IBZM and [123I]IBF (D2 receptor antagonists) in vervets (nonhuman primates), as measured by SPECT, with binding changes correlating with peak DA release as measured by microdialysis |
| Le Masurier et al (75) | • AMP-reduced [11C]raclopride (a D2 receptor antagonist) binding in the striatum of rats, as measured by PET, was attenuated by pretreatment with a tyrosine-free amino acid mixture |
| Lind et al (76) | • AMP decreased [11C]raclopride (a D2 receptor antagonist) binding in the caudate, putamen, and ventral striatum of pigs, as measured by PET |
| Liu et al (77) | • AMP increased cytosolic calcium concentrations and NE release in a dose-dependent and extracellular calcium-dependent manner in bovine adrenal chromaffin cells; nicotinic receptor antagonists suppressed these effects • AMP acted as a nicotinic receptor agonist to induce calcium increases and NE release in bovine adrenal chromaffin cells |
| May et al (78) | • AMP stimulated DA release in the caudate nucleus of rats, as measured by in vivo fast-scan cyclic voltammetry |
| Miller et al (79) | • AMP inhibited MAO type A activity |
| Mukherjee et al (80) | • AMP reduced [18F]fallypride (a D2 receptor antagonist) binding in the striatum of rats and monkeys, as measured by PET |
| Patrick et al (81) | • Stimulation of DA synthesis in rat brain striatal synaptosomes produced by the depolarizing agent veratridine markedly reduced AMP, suggesting AMP altered interactions between tyrosine hydroxylase and the synaptosomes regulating catecholamine formation |
| Pedersen et al (82) | • AMP-induced decreases in [11C]raclopride (a D2 receptor antagonist) binding in the dorsal and ventral striatum of rats were not altered by pretreatment with pargyline, as measured using PET |
| Preece et al (83) | • AMP reduced BOLD signal intensity in the nucleus accumbens and prefrontal cortex and increased signal intensity in the motor cortex of rats as measured by fMRI; the signal intensity changes in the nucleus accumbens and caudate (but not in the motor cortex) were attenuated by pretreatment with a tyrosine-free amino acid mixture |
| Price et al (84) | • After administration of AMP, a general increase in CBF that gradually declined toward baseline values was observed using a bolus injection PET method in baboons |
| Pum et al (85) | • AMP dose-dependently increased DA and 5-HT levels in the perirhinal, entorhinal, and prefrontal cortices |
| Quelch et al (86) | • AMP reduced [3H]carfentanil (a μ-opioid receptor antagonist) binding in rat total brain homogenate, as measured by subcellular fractionation, and in the superior colliculi, hypothalamus, and amygdala of rats, as measured by in vivo receptor binding |
| Ren et al (87) | • AMP increased DA release in the caudate/putamen and cAMP activity in the caudate/putamen, nucleus accumbens, and medial prefrontal cortex in rats |
| Riddle et al (88) | • Administration of low-dose AMP altered VMAT-2 distribution within nerve terminals selectively in monoaminergic neurons |
| Ritz and Kuhar (89) | • AMP exhibited high affinity for DA, NE, and 5-HT reuptake sites and for α2 adrenergic receptor sites, as measured using in vivo binding in rats |
| Robinson (90) | • AMP enantiomers (d and l) inhibited MAO type A and MAO type B activity in rat liver mitochondria |
| Saelens et al (91) | • AMP increased [3H]spiroperidol (a D2 receptor antagonist) binding in regions containing DA terminals (eg, the septum, nucleus accumbens, cortex, caudate-putamen) or dendrites (eg, ventral tegmental area, substantia nigra), as measured by in vivo receptor binding |
| Schwarz et al (92) | • AMP produced widespread increases in regional CBF in multiple brain region clusters that are involved in primary DA pathways in rats |
| Schwendt et al (93) | • AMP decreased RGS4 mRNA in the caudate-putamen and cortex and RGS4 protein levels in the caudate-putamen of rats but did not modify the effects of SCH 23390 or eticlopride (D2 receptor antagonists) on RGS4 mRNA or protein levels in these same brain regions |
| Schiffer et al (94) | • AMP elevated extracellular DA in the striatum but to a greater degree than MPH; reductions in [11C]raclopride (a D2 receptor antagonist) binding were similar with AMP and MPH |
| Seneca et al (95) | • AMP reduced binding of [11C]MNPA and [11C]raclopride (D2 receptor antagonists) in the striatum of cynomolgus monkeys, as measured by PET |
| Shaffer et al (96) | • AMP reduced striatal and increased medial prefrontal cortical protein expression of mGluR type 5 in rats |
| Skinbjerg et al (97) | • AMP-induced decreases in [18F]fallypride (a D2 receptor antagonist) binding were associated with receptor internalization, as assessed using PET in knockout mice lacking the ability to internalize D2 receptors |
| Smith et al (98) | • Melanin-concentrating hormone type 1 receptor knockout mice exhibited an increased locomotor response to AMP but did not exhibit altered DA, NE, or 5-HT release in the striatum in response to AMP |
| Sulzer et al (99) | • AMP produced a rapid release of DA from vesicles, resulting in redistribution to the cytosol |
| Sulzer and Rayport (100) | • AMP reduced the pH gradient in rat chromaffin cells, resulting in reduced DA uptake |
| Sun et al (101) | • AMP decreased binding of [3H]raclopride but not of [3H]spiperone (D2 receptor antagonists) in rat striatum |
| Tomic et al (102) | • AMP decreased [3H]SCH 23390 (a D1 receptor antagonist) binding in the caudate and nucleus accumbens, increased [3H]SCH 23390 binding in the substantia nigra, and decreased [3H]spiperone (a D2 receptor antagonist) binding in the striatum and nucleus accumbens in rats, as measured using autoradiography |
| Tomic and Joksimovic (103) | • Acute treatment with AMP reduced [3H]SCH 23390 (a D1 receptor antagonist) and [3H]spiperone (a D2 receptor antagonist) binding in the striatum and nucleus accumbens in rats, as measured by autoradiography |
| van Berckel et al (104) | • AMP-induced decrease in [3H]raclopride (a D2 receptor antagonist) binding in the striatum of baboons was enhanced by LY354740 (an mGluR type 2/3 receptor agonist), as measured by PET |
| Xiao and Becker (105) | • AMP-induced striatal DA release in rats was increased by catechol estrogens, as measured using in vivo microdialysis |
| Yin et al (106) | • AMP produced time-dependent changes in levels of GABA, 5-HT, and NE in the cerebellar vermis of mice |
| Young et al (107) | • AMP increased DA concentrations in the striatum, but not in the cortex or ventral tegmental area, in prairie voles |
| Yu et al (108) | • AMP enhanced the cellular response of cortical and hippocampal neurons to CHPG (an mGluR type 5 agonist) in rats |
| Methylphenidate | |
| Andrews and Lavin (109) | • Intracortical MPH increased cortical cell excitability in rats, an effect that was lost following catecholamine depletion and was mediated via stimulation of α2 adrenergic receptors but not D1 receptors |
| Bartl et al (110) | • MPH exerted diverse cellular effects including increased neurotransmitter levels, downregulated synaptic gene expression, and enhanced cell proliferation in rat pheochromocytoma cells |
| Ding et al (111) | • MPH reduced binding of [11C]dl-threo-MPH in the striatum of baboons, as measured by PET |
| Ding et al (112) | • MPH reduced binding of [11C]d-threo-MPH (but not [11C]l-threo-MPH) in the striatum of baboons, as measured by PET |
| Dresel et al (113) | • MPH reduced [99Tc]TRODAT-1 (a DAT/SERT ligand) binding to DAT in the striatum, but not to the SERT in the midbrain/hypothalamus in baboons, as measured by PET |
| Easton et al (58) | • MPH inhibited accumulation of DA or NE in rat brain synaptosomes and vesicles, with greater potency observed for synaptosomal inhibition |
| Federici et al (114) | • MPH reduced spontaneous firing of DA neurons, as measured by electrophysiologic recordings in rat brain slices, via blockade of the DAT |
| Gamo et al (115) | • MPH increased performance on a spatial working memory task in rhesus monkeys, an effect that was blocked by the α2 adrenergic antagonist idazoxan |
| Gatley et al (116) | • [11C]d-threo-MPH exhibited high affinity for the DAT that was insensitive to competition with endogenous DA in baboons, as measured by PET; the highest concentrations of [11C]d-threo-MPH binding in mouse brain were found in striatum |
| Gatley et al (117) | • MPH reduced [3H]cocaine (a DAT agonist) binding in the olfactory tubercle and striatum in mice, as measured by PET |
| Gatley et al (118) | • MPH exhibited higher affinities for the DAT and NET than for the serotonin transporter in rat brain |
| Kuczenski and Segal (71) | • MPH increased DA efflux in the caudate and NE efflux in the hippocampus of rats following systemic administration |
| Markowitz et al (119) | • MPH acted as an agonist at the 5-HT1A receptor in guinea pig ileum |
| Markowitz et al (120) | • MPH exhibited affinity at the NET and DAT and at 5-HT1A and 5-HT2B receptors |
| Michaelides et al (121) | • MPH differentially altered metabolism in the prefrontal cortex and cerebellar vermis as a function of DA D4 receptor functionality in mice |
| Nikolaus et al (122) | • MPH reduced [123I]FP-CIT (a DAT ligand) binding in rat striatum, as measured by SPECT |
| Nikolaus et al (123) | • MPH dose-dependently reduced [123I]FP-CIT (a DAT ligand) binding in rat striatum, as measured by SPECT |
| Nikolaus et al (124) | • MPH decreased [123I]IBZM (a D2 receptor antagonist) binding in the striatum of rats, as measured by SPECT |
| Riddle et al (88) | • Administration of low-dose MPH altered VMAT-2 distribution within nerve terminals selectively in monoaminergic neurons |
| Sandoval et al (125) | • MPH increased vesicular DA uptake and binding of VMAT-2 and altered VMAT-2 cellular distribution |
| Schiffer et al (94) | • MPH elevated extracellular DA in the striatum but to a lesser degree than AMP; reductions in [11C]raclopride (a D2 receptor antagonist) binding were similar with MPH and AMP |
| Somkuwar et al (126) | • MPH administered during the adolescent period in rats produced strain-dependent alterations in cortical DAT activity at adulthood |
| Volkow et al (127) | • MPH reduced [11C]raclopride (a D2 receptor antagonist) binding potential but not binding affinity in baboons, as measured by PET |
| Wall et al (128) | • MPH caused little or no change in DA, NE, or 5-HT efflux, although it blocked uptake mediated by both NET and DAT in transfected cell lines |
| Human neuroimaging studies | |
| Amphetamine | |
| Aalto et al (129) | • AMP did not alter [11C]FLB 457 (a D2/D3 receptor ligand) binding in the cortex of healthy adults, as measured by PET |
| Boileau et al (130) | • AMP decreased [11C]raclopride (a D2 receptor antagonist) binding in ventral striatum and putamen of healthy adults in response to conditioned stimuli, as measured by PET |
| Buckholtz et al (131) | • AMP-induced reductions in [18F]fallypride (a D2 receptor antagonist) binding in the striatum of healthy adults were associated with increased trait impulsivity |
| Cardenas et al (132) | • AMP produced decreases in [11C]raclopride (a D2 receptor antagonist) binding that were sustained for up to 6 hours in healthy adults, as measured using PET studies |
| Colasanti et al (133) | • AMP reduced [11C]carfentanil (a μ-opioid receptor antagonist) binding in the caudate, putamen, frontal cortex, thalamus, insula, and anterior cingulate cortex of healthy adult males, as measured by PET |
| Cropley et al (134) | • AMP reduced [18F]fallypride (a D2 receptor antagonist) binding in the substantia nigra, medial prefrontal and orbital cortices, and caudate of healthy adults, as measured by PET |
| Devous et al (135) | • AMP increased regional CBF to the prefrontal cortex, inferior orbital frontal cortex, ventral tegmentum, amygdala, and anterior thalamus, and decreased regional CBF to the motor and visual cortices, fusiform gyrus, and regions of the temporal lobe of healthy adults, as measured by SPECT |
| Drevets et al (136) | • AMP-induced reductions in [11C]raclopride (a D2 receptor antagonist) binding in the anteroventral striatum of healthy adults, as measured by PET, were negatively correlated with feelings of euphoria |
| Garrett et al (137) | • AMP increased fMRI-based BOLD signal variability in healthy adults, with the effects being more pronounced in older adults (60–70 years old) than younger adults (20–30 years old) |
| Guterstam et al (138) | • AMP had no effect on [11C]carfentanil (a μ-opioid receptor antagonist) binding in the striatum, cortex, amygdala, or hippocampus of healthy adult males, as measured by PET |
| Hariri et al (139) | • AMP potentiated responses of the right amygdala to angry and fearful facial expressions of healthy adults, as measured by fMRI, without producing changes in performance of an emotional recognition task or a sensorimotor control task |
| Kegeles et al (140) | • AMP decreased [123I]IBZM (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by SPECT |
| Knutson et al (141) | • AMP exerted an equalizing influence on activity in the ventral striatum by enhancing tonic activity over phasic activity during anticipation of positive and negative incentives in healthy adults, as measured by fMRI |
| Laruelle et al (142) | • AMP decreased [123I]IBZM (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by SPECT |
| Laruelle and Innis (143) | • AMP decreased [123I]IBZM (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by SPECT |
| Leyton et al (144) | • AMP-decreased [11C]raclopride (a D2 receptor antagonist) binding in the ventral striatum of healthy adults, as measured by PET, was correlated with increases in drug wanting and novelty seeking |
| Leyton et al (145) | • AMP-induced decreases in [11C]raclopride (a D2 receptor antagonist) binding in the ventral striatum of healthy adults, as measured by PET, were attenuated by acute depletion of phenylalanine and tyrosine |
| Martinez et al (146) | • AMP decreased [11C]raclopride (a D2 receptor antagonist) binding in healthy adults, as measured by PET, with larger reductions observed in the limbic (ventral) and the sensorimotor (postcommissural) striatal regions than associative (caudate and precommissural) striatal regions |
| Mick et al (147) | • AMP reduced [11C]carfentanil (a μ-opioid receptor antagonist) binding in the caudate, putamen, frontal lobe, thalamus, nucleus accumbens, insula, amygdala, and anterior cingulate cortex of healthy adults, as measured by PET |
| Narendran et al (148) | • AMP decreased [11C]FLB 457 (a D2/D3 receptor ligand) and [11C]fallypride (a D2 receptor antagonist) binding in the medial temporal lobe, anterior cingulate cortex, dorsolateral and medial prefrontal cortices, and parietal cortex of healthy adults, as measured by PET |
| Narendran et al (149) | • AMP decreased [11C]NPA (a D2/D3 receptor agonist) and [11C]raclopride (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by PET, with reductions of [11C]NPA binding being greater than those of [11C]raclopride |
| Oswald et al (150) | • AMP-induced reductions in [11C]raclopride (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by PET, were correlated with increased cortisol release and positive subjective ratings of AMP |
| Oswald et al (151) | • AMP decreased [11C]raclopride (a D2 receptor antagonist) binding in the striatum of healthy adults, with lower binding in the right dorsal caudate being associated with lower winnings in the Iowa Gambling Task |
| Riccardi et al (152) | • AMP reduced [18F]fallypride (a D2 receptor antagonist) binding in healthy adults, as measured by PET, with the reductions across brain regions differing as a function of gender |
| Riccardi et al (153) | • AMP reduced [18F]fallypride (a D2 receptor antagonist) binding in the striatum, substantia nigra, amygdala, temporal cortex, and thalamus of healthy adults, as measured by PET |
| Riccardi et al (154) | • AMP reduced [18F]fallypride (a D2 receptor antagonist) binding in healthy adults, as measured by PET, with the reductions in selected baring regions being correlated with Stroop test scores, Stroop test interference, and affective state |
| Rose et al (155) | • AMP increased CBF in the cerebellum, brainstem, temporal lobe, striatum, and prefrontal and parietal cortices of healthy adults, as measured by MRI |
| Schouw et al (156) | • AMP-induced increases in regional CBF in the striatum, anterior cingulate cortex, thalamus, and cerebellum of healthy adults, as measured by MRI, were not correlated with reductions in [123I]IBZM receptor binding in the striatum, as measured by SPECT |
| Shotbolt et al (157) | • AMP induced reductions in [11C]–(+)-PHNO (a D2/D3 receptor agonist) binding in the ventral pallidum, ventral striatum, thalamus, and putamen of healthy adults, as measured by PET, which were greater than the reductions in [11C]raclopride (a D2 receptor antagonist) binding |
| Silverstone et al (158) | • AMP increased myo-inositol and phosphomonoester concentrations in the temporal lobe of healthy adults, as measured by MRS |
| Slifstein et al (159) | • AMP reduced [18F]fallypride (a D2 receptor antagonist) binding in the striatum, globus pallidus, midbrain, hippocampus, and amygdala of healthy adults, as measured by PET |
| Vollenweider et al (160) | • AMP increased regional cerebral glucose metabolism in the anterior and posterior cingulate cortex, caudate nucleus, putamen, and thalamus of healthy adults, as measured by PET with [18F]-FDG |
| Wand et al (161) | • AMP-induced reductions in [11C]raclopride (a D2 receptor antagonist) binding in striatum of healthy adults, as measured PET, were associated with stress-induced cortisol levels |
| Willeit et al (162) | • AMP reduced [11C]–(+)-PHNO (a D2/D3 receptor agonist) binding in the striatum but not in the globus pallidus of healthy adults, as measured by PET |
| Wolkin et al (163) | • AMP decreased regional cerebral glucose metabolism in the frontal cortex, temporal cortex, and striatum of healthy adults, as measured by PET |
| Woodward et al (164) | • AMP-induced reductions in [18F]fallypride (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by PET, were positively correlated with overall schizotypal traits |
| Methylphenidate | |
| Booij et al (165) | • MPH reduced [123I]IBZM (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by SPECT |
| Clatworthy et al (166) | • MPH reduced [11C]raclopride (a D2 receptor antagonist) binding in the putamen, ventral striatum, and postcommissural caudate of healthy adults, as measured by PET, with reductions in the postcommissural caudate being negatively correlated with reversal learning performance |
| Costa et al (167) | • MPH increased neuronal activation in the putamen of healthy adults as measured by fMRI, during a go/no-go task when a response inhibition error occurred but not when a response was successfully inhibited |
| Ding et al (112) | • MPH reduced binding of [11C]d-threo-MPH to a greater degree than [11C]l-threo-MPH in the striatum of healthy adults, as measured by PET |
| Hannestad et al (168) | • MPH dose-dependently reduced [11C]MRB (a NET ligand) binding across the brain (eg, locus coeruleus, raphe nucleus, hypothalamus, thalamus) of healthy adults, as measured using PET |
| Kasparbauer et al (169) | • MPH increased BOLD signal during successful go/no-go trials in healthy adult carriers of the SLC6A3 9R allele of the DAT gene but a decrease in 10/10 allele homozygotes, as measured by fMRI |
| Montgomery et al (170) | • MPH reduced [11C]FLB 457 (a D2/D3 receptor ligand) binding in the frontal cortex, anterior cingulate cortex, temporal cortex, and thalamus of healthy adults, as measured by PET |
| Moeller et al (171) | • MPH improved Stroop color-word task performance and concurrently reduced dorsal anterior cingulate cortex responses of healthy adults, as measured by fMRI |
| Mueller et al (172) | • MPH increased connectivity strength between the dorsal attention network and thalamus, with the left and right frontoparietal networks and the executive control networks also showing increased connectivity to sensory-motor and visual cortex regions, and decreased connectivity to cortical and subcortical components of cortico-striato-thalamo-cortical circuits in healthy adults, as measured by fMRI |
| Ramaekers et al (173) | • MPH reduced functional connectivity between the nucleus accumbens and the basal ganglia, medial prefrontal cortex, and temporal cortex without changing functional connectivity between the medial dorsal nucleus and the limbic circuit, as measured by fMRI |
| Ramasubbu et al (174) | • MPH decreased oxy-hemoglobin levels, an index of decreased neural activation, in the right lateral prefrontal cortex of healthy adults, as measured by fNIRS |
| Schabram et al (175) | • MPH decreased DA turnover in the caudate and putamen of healthy adult females, as measured by PET using [18F]FDOPA |
| Schweitzer et al (176) | • MPH improved Paced Auditory Serial Addition Test performance, reduced regional CBF in the prefrontal cortex, and increased regional CBF in the right thalamus and precentral gyrus of healthy adults, as measured by PET |
| Schlosser et al (177) | • Processing of uncertain information was associated with higher activation of the parietal association cortex and posterior cingulate cortex after placebo relative to MPH, but with higher left and right parahippocampal and cerebellar activation after MPH relative to placebo, as measured by fMRI |
| Spencer et al (178) | • Both short-acting and long-acting MPH formulations decreased [11C]altropane (a DAT ligand) binding in the striatum of healthy adults, as measured by PET |
| Spencer et al (179) | • Plasma MPH concentrations were correlated with [11C]altropane (a DAT ligand) binding in the striatum of healthy adults, as measured by PET |
| Tomasi et al (180) | • MPH increased the activation of the parietal and prefrontal cortices and increased the deactivation of the insula and posterior cingulate cortex in healthy adults, as measured by fMRI during visual attention and working memory tasks |
| Udo de Haes et al (181) | • MPH reduced [11C]raclopride (a D2 receptor antagonist) binding in healthy adults, as measured by PET, with binding changes in the dorsal striatum correlating with MPH-induced increases in euphoria |
| Volkow et al (182) | • MPH decreased [11C]raclopride (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by PET |
| Volkow et al (183) | • MPH decreased [11C]d-threo-MPH binding in the striatum of healthy adults, as measured by PET |
| Volkow et al (184) | • MPH produced variable changes in brain metabolism in healthy adults, as measured by PET using [18F]FDG, but produced consistent increases in cerebellar metabolism and decreases in the basal ganglia |
| Volkow et al (185) | • MPH dose-dependently reduced [11C]cocaine (a DAT ligand) binding in the striatum of healthy adults, as measured by PET, with effects observed at therapeutic doses used for ADHD |
| Volkow et al (186) | • MPH dose-dependently reduced [11C]cocaine (a DAT ligand) binding in the striatum of healthy adults, as measured by PET, with self-reports of MPH-induced “high” and “rush” being significantly correlated with [11C]cocaine binding |
| Volkow et al (187) | • MPH reduced [11C]raclopride (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by PET |
| Volkow et al (188) | • MPH reduced [11C]cocaine (a DAT ligand) binding in the striatum of healthy adults, as measured by PET |
| Volkow et al (189) | • A review of PET studies suggested that mechanism of action of MPH was related to binding of MPH to the DAT levels sufficient to increase signal-to-noise ratios and to increase the salience of stimuli, with variability in MPH response being related to differences in DAT level and DA release across individuals |
| Volkow et al (190) | • MPH reduced [11C]raclopride (a D2 receptor antagonist) binding in the striatum of healthy adults in a context-dependent manner, as measured by PET, with reductions observed when a remunerated mathematical task was performed but not when scenery cards were passively viewed |
| Wang et al (191) | • MPH reduced [11C]raclopride (a D2 receptor antagonist) binding in the striatum of healthy adults, as measured by PET, with changes being reproducible in individuals at 1- to 2-week intervals |
5-HT=5-hydroxytryptamine (serotonin); ADHD=attention-deficit/hyperactivity disorder; AMP=amphetamine; BOLD=blood-oxygen-level dependent; cAMP=cyclic adenosine monophosphate; CBF=cerebral blood flow; CHPG=2-chloro-5-hydroxyphenylglycine; CREB=cAMP-responsive element binding protein; DA=dopamine; DAT=dopamine transporter; FDG=2-deoxy-2-fluoro-D-glucose; FDOPA=fluorodopamine; fMRI=functional magnetic resonance imaging; fNIRS=functional near infrared spectroscopy; FP-CIT=N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)-nortropane; GABA=gamma-aminobutyric acid; GLU=glutamate; IBF=iodobenzofuran; IBZM=iodobenzamide; MAO=monoamine oxidase; mGluR=metabotropic glutamate receptor; MNPA=(R)-2-CH3O-N-n-propylnorapomorphine; MPEP=2-methyl-6-(phenylethynyl)pyridine hydrochloride; MPH=methylphenidate; MRB=methylreboxetine; MRI=magnetic resonance imaging; mRNA=messenger ribonucleic acid; MRS=magnetic resonance spectroscopy; NE=norepinephrine; NET=norepinephrine transporter; NMDA=N-methyl-D-aspartate; NPA=2-methoxy-N-propylnorapomorphine; PET=positron emission tomography; PHCCC=N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide; PHNO=(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol; PKC=protein kinase C; RGS4=regulator of G-protein signaling 4; SERT=serotonin transporter; SPECT=single-photon emission computed tomography; TRODAT=([2-[2-[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3,2,1]oct-2-yl]methyl](2-mercaptoethyl)amino]ethyl-amino-ethan-etio-lato-(3-)-oxo-[1R-(exo-exo)]; VMAT-2=vesicular monoamine transporter
3.1. Preclinical Studies
3.1.1. Amphetamine
The main mechanism of action of AMP is to increase synaptic extracellular DA and NE levels (44, 52, 59, 60, 67, 68, 71, 78, 80, 85, 87, 94, 105, 107, 128). This effect is mediated by inhibition of DA transporters (DAT) and NE transporters (NET) (44, 52, 58), which reduces the reuptake of these molecules from the synapse. In wild-type mice, AMP initially increases surface trafficking of DAT and DA uptake, but continued AMP exposure results in decreased surface expression of the DAT and decreases in DA uptake (49). In a dose-dependent and region-specific manner, AMP also increases vesicular DA release via inhibition of the vesicular monoamine transporter 2 (VMAT-2), which releases DA from vesicular storage, and the concomitant release of cytosolic DA via reverse transport by the DAT (58, 88, 99). Furthermore, AMP inhibits monoamine oxidase (MAO) activity (79, 90), which decreases cytosolic monoamine breakdown. A wide array of studies using positron emission tomography (PET) or single-photon emission computed tomography (SPECT) have demonstrated that AMP produced reductions in the binding potential of ligands for DA receptors (42, 46, 47, 51, 53, 55, 61, 62, 65, 74-76, 80, 82, 91, 94, 95, 101-104) and NE receptors (59, 73), which is an indirect indicator of increased competition for binding sites resulting from increased extracellular DA or NE. The striatum, which contains most of the DATs in the brain (192, 193), appears to be a principal site of action of AMP (44, 194), but direct effects in the cortex and the ventral tegmental area have also been reported (85, 87, 195). The effects of AMP extend to and are modulated by other neurotransmitter systems (50, 56, 66, 70, 77, 85, 86, 89, 96, 98, 106, 108), including ACH, 5-HT, opioid, and GLU, either directly through enhanced release from presynaptic terminals or via downstream effects.
In other studies, AMP has been shown to produce changes at a more global level. In studies of cerebral blood flow (CBF), AMP increased whole brain CBF in rats and baboons (48, 69, 84, 92), with changes in CBF being correlated with striatal DA concentration (69). In a functional magnetic resonance imaging (fMRI) study in rats, AMP caused widespread increases in blood-oxygen-level–dependent (BOLD) signal intensity in subcortical structures with rich DA innervation and with decreases in BOLD signal in the superficial layers of the cortex (54). In another fMRI study in rats, AMP reduced BOLD signal intensity in the nucleus accumbens and prefrontal cortex and increased signal intensity in the motor cortex of rats, with signal intensity changes in the nucleus accumbens and caudate (but not in the motor cortex) being attenuated by pretreatment with a tyrosine-free amino acid mixture (83).
3.1.2. Methylphenidate
The direct effects of MPH include inhibition of the DAT and NET (113, 114, 118, 120, 123, 128), an affinity for and agonist activity at the 5-HT1A receptor (119, 120), and redistribution of VMAT-2 (88, 125). As a consequence of these interactions, MPH elevates extracellular DA and NE levels (58, 71, 94, 107). The enhanced efflux of DA and NE associated with MPH exposure results in increased availability of DA and NE to bind to their respective transporters (ie, the DAT or NET) or to DA or NE receptors, as evidenced by reductions in ligand binding in PET and SPECT studies (112, 113, 117, 122-124, 127).
Although increases in extracellular levels of striatal DA in rats measured using microdialysis are less pronounced with MPH than with AMP, both compounds have been shown to exhibit similar magnitude of effects with regard to reductions in DA binding potential as measured by PET in rodents and nonhuman primates (94). Multiple studies have demonstrated that MPH also directly interacts with adrenergic receptors (109, 115, 119, 120). Through activation of α2 adrenergic receptors, MPH has been demonstrated to stimulate cortical excitability (109). Further evidence for the interaction of MPH with α2 adrenergic receptors comes from data indicating that the procognitive effects of MPH in a working memory task are blocked by the α2 adrenergic antagonist idazoxan (115). The effects of MPH on α2 adrenergic receptors are notable given that two α2 adrenergic receptor agonist drugs (extended-release forms of guanfacine and clonidine) are indicated for the treatment of ADHD (196).
3.2. Human Neuroimaging Studies
3.2.1. Amphetamine
Reductions in ligand binding in PET (130-132, 134, 136, 144-146, 148-154, 157, 159, 161, 162, 164) and SPECT (140, 142, 143, 156) studies in healthy humans indicate that AMP increases DA release across multiple brain regions, including the dorsal and ventral striatum, substantia nigra, and regions of the cortex. AMP also has been shown to alter regional CBF to areas of the brain with DA innervation, including the striatum, anterior cingulate cortex, prefrontal and parietal cortex, inferior orbital cortex, thalamus, cerebellum, and amygdala (135, 155, 156, 160, 163). The effects of AMP on regional CBF appear to be dependent on the dose, with lower doses decreasing rates of blood flow in the frontal and temporal cortices and in the striatum (163) and higher doses increasing blood flow in the anterior cingulate cortex, caudate nucleus, putamen, and thalamus (160). In fMRI studies in healthy adults, AMP increased BOLD signal variability (137) and exerted an “equalizing” effect on ventral striatum activity during incentive processing (141). In addition, AMP was shown to strengthen amygdalar responses during the processing of angry and fearful facial expressions (139).
Changes in neuronal activity have been shown to correlate with various behavioral traits (131, 136, 144, 164). In PET studies, changes in the binding potential of [11C]raclopride (a D2 receptor antagonist) in regions of the ventral striatum of healthy adults associated with AMP binding have been reported to be negatively correlated with changes in AMP-associated euphoria (136) and with increases in drug wanting and novelty seeking (144).
3.2.2. Methylphenidate
Methylphenidate has also been shown to increase striatal DA availability, as measured by reductions in ligand binding potential in PET studies (165, 166, 170, 178, 179, 181, 182, 187, 190, 191), with evidence to indicate that this effect is related to binding to the DAT (185, 186, 188). MPH-induced reductions in striatal [11C]raclopride binding were associated with MPH-induced changes in euphoria and anxiety and were correlated to age (181, 182). In addition, NE systems have been implicated as key targets for MPH, with MPH dose-dependently blocking the NET in the thalamus and other NET-rich regions; the estimated occupancy of the NET at therapeutic doses of 0.35 to 0.55 mg/kg MPH is 70% to 80% (168).
Assessments of functional activity using fMRI have provided evidence for the widespread functional effects of MPH (167, 171-173, 177, 180). Using fMRI, it has been shown that MPH increases activation of the parietal and prefrontal cortices and increases deactivation of the insula and posterior cingulate cortex during visual attention and working memory tasks (180). Another fMRI study reported MPH-induced activation in the putamen during a go/no-go task when a response inhibition error occurred but not when a response was successfully inhibited (167), suggesting that the effects of MPH are context dependent. Furthermore, MPH exposure altered connectivity strength across various cortical and subcortical networks (172) and shifted brain activation under conditions of uncertainty to higher levels of activation in left and right parahippocampal regions and cerebellar regions (177). Lastly, MPH-associated decreases in task-related errors on the Stroop color-word task were associated with concurrent decreases in anterior cingulate cortex activity (171). MPH has also been shown to reduce regional CBF in the prefrontal cortex and increase regional CBF in the thalamus and precentral gyrus (176). In another study that used functional near-infrared spectroscopy, MPH-associated improvements in the performance of a working memory task corresponded with decreased oxy-hemoglobin levels in the right lateral prefrontal cortex, which is a surrogate for decreased neural activation (174).
4.0. Discussion
Although their mechanisms of action differ, the primary central nervous system effects of AMP and MPH within the brain include increased catecholamine availability in striatal and cortical regions, as evidenced in preclinical (44, 52, 58-60, 67, 68, 71, 78, 80, 85, 87, 94, 105, 107, 128) and human (130-132, 134, 136, 140, 142-146, 148-154, 156, 157, 159, 161, 162, 164-166, 170, 178, 179, 181, 182, 187, 190, 191) studies. These increases in DA and NE availability affect corticostriatal systems that subserve behaviors related to cognition and executive function (171, 177, 180), risky decision making (151), emotional responsivity (139), and the regulation of reward processes (197). Importantly, ADHD has been associated with structural and functional alterations in regions of the brain where AMP and MPH have been shown to alter DA and NE activity.
4.1. Structural Alterations in ADHD
A meta-analysis of imaging data from individuals with ADHD across all age groups revealed altered white matter integrity in diverse brain areas, including the striatum and the frontal, temporal, and parietal lobes (198). In a meta-analysis of imaging data from children and adults (199), global gray matter volume was significantly smaller in those with ADHD, especially in basal ganglia structures integral to executive function. In adults with ADHD, reduced gray matter volume in the caudate and parts of the dorsolateral prefrontal cortex, inferior parietal lobe, anterior cingulate cortex, putamen, and cerebellum were observed; increased volume was noted in other parts of the dorsolateral prefrontal cortex and inferior parietal lobe (200). A meta-analysis of 1713 persons with ADHD and 1529 controls found volumetric reductions in the accumbens, amygdala, caudate, hippocampus, and putamen (201).
In a meta-analysis of 9 PET and SPECT studies, which included 169 patients with ADHD and 173 healthy controls, it was reported that striatal DAT density in patients with ADHD was 14% higher than in healthy controls (202). Meta-regression analysis further revealed that previous exposure to ADHD medication influenced striatal DAT density, with lower DAT density being associated with a lack of medication exposure (202). As the correlation between medication exposure and striatal DAT density accounted for 48% of the variance across studies (202), it was suggested that the higher striatal DAT density in individuals with ADHD was a neuroadaptive response to stimulant exposure.
4.2. Functional Alterations in ADHD
A meta-analysis of imaging data focusing specifically on timing function, which is important for impulsiveness in ADHD, showed consistent deficits in the left inferior prefrontal, parietal, and cerebellar regions of individuals with ADHD (203). A meta-analysis of 24 task-related fMRI studies coupled with functional decoding based on the BrainMap database reported hypoactivation in the left putamen, inferior frontal gyrus, temporal pole, and right caudate of individuals with ADHD (204). When examining these deficits in regard to the BrainMap database, it was suggested that individuals with ADHD may exhibit deficits in the cognitive aspects of music, perception and audition, speech and language, and executive function (204).
In high-functioning, drug-naive young adults with ADHD, resting-state fMRIs revealed altered connectivity in the orbitofrontal-temporal-occipital and frontal-amygdala-occipital networks (relating to inattentive and hyperactive/impulsive symptoms, respectively) compared with matched controls; these abnormalities were not related to developmental delays, impaired cognition, or use of pharmacotherapy (205). Imaging studies have also reported hypoactivity in the prefrontal cortex and weak connections to other brain regions in individuals with ADHD (206). A review of progress in neuroimaging indicated that the initial focus on frontostriatal dysfunction has given way to a broader understanding of the complex interactions of various regions of the brain in which alterations may contribute to ADHD symptoms across the life span (207).
A substantial body of literature has examined the role of DA systems in the neurobiology of ADHD and ADHD-related symptoms and behaviors. Among treatment-naive adolescents with ADHD, DAT density shows a significant inverse relationship with blood flow in the cingulate cortex, the frontal and temporal lobes, and the cerebellum, brain regions which are involved in modulating attention (208). In a PET study of treatment-naive men with ADHD and men without ADHD, more pronounced reductions in AMP-induced reductions in striatal [11C]raclopride binding were associated with worse response inhibition, and those with ADHD had the highest-magnitude reductions and AMP-induced reductions in striatal [11C]raclopride binding (209). Two SPECT studies and 1 PET study showed that adults with ADHD had higher DAT concentrations than adults without ADHD (210-212), with the SPECT studies further reporting that MPH reduced DAT availability in adults with ADHD (210, 211). Furthermore, studies have shown significant correlations between global clinical improvement in ADHD symptoms following MPH treatment and striatal DAT availability (213, 214), and the MPH-induced increases in DA availability in the ventral striatum are associated with improved ADHD symptomology in adults with ADHD (215). In another study, in adults with ADHD, long-term MPH treatment increased striatal DAT availability (216). In another PET study in adults with ADHD, decreased DAT and D2/D3 receptor availability in the nucleus accumbens and midbrain compared with individuals without ADHD was reported, and reduced availability of DAT and D2/D3 receptor availability was significantly correlated with lower indices of motivation only in those with ADHD (217). Also, a PET study in young male adults with ADHD has also reported dysfunctional DA metabolism in the putamen, amygdala, and dorsal midbrain relative to healthy controls regardless of treatment status (naive vs previously treated with MPH) and that a history of MPH treatment resulted in a down-regulation of DA turnover (218). Despite the neuroimaging evidence that supports a role for the DAT in adult ADHD, a systematic literature review examining the pharmacogenetics of adult ADHD found that only 1 of 5 identified studies reported finding a polymorphism at the DAT gene associated with ADHD (219).
Studies of NET availability have not consistently reported altered NET availability in individuals with ADHD (220, 221), but one study reported genotype-dependent increases in NET binding in the thalamus and cerebellum of adults with ADHD compared with controls; this effect was largely due to the effect of NET gene polymorphisms on NET binding potential (220). Another study reported no NET differences across multiple brain regions, including the hippocampus, thalamus, and midbrain, in individuals with ADHD compared with controls (221).
Beyond the changes observed in DA and NE systems in individuals with ADHD, there is evidence that GLU, 5-HT, ACH, and opioid systems play a role in ADHD. Studies examining neurometabolism using proton MRIs have reported glutamatergic deficits in the frontal cortical and striatal regions in individuals with ADHD that may be related to cognitive control and symptom severity (12, 222, 223). Regarding the 5-HT systems, it has been reported that increased methylation of the 5-HT transporter is associated with worse clinical presentation and reduced cortical thickness in children with ADHD (224). In adults, significant differences in 5-HT transporter interregional correlations between the precuneus and hippocampus have been reported in adults with ADHD compared with controls without ADHD (225). Functionally, it has been reported that decreased levels of activation are observed in the precuneus of adolescents with ADHD compared with individuals without ADHD; however, there is a significantly greater increase in the activation of the precuneus following the administration of fluoxetine in adolescents with ADHD compared with controls (226). At present, there are no published neuroimaging studies of endogenous opioid or ACH systems in individuals with ADHD. However, altered function in both systems has been implicated in ADHD (13, 14).
4.3. Co-occurring Psychiatric Conditions and ADHD
Attention-deficit/hyperactivity disorder is often associated with comorbid psychiatric disorders (16-18), such as anxiety and mood disorders. Importantly, the same brain regions and neurotransmitter systems that underlie ADHD are also implicated in the psychiatric disorders that are frequently comorbid with ADHD (19-21). Thus, it is important to understand how ADHD therapies might influence psychiatric comorbidities.
4.3.1. Anxiety disorders
In healthy human volunteers, AMP has been reported to potentiate amygdalar activity in response to the processing of angry and fearful facial expressions (139). These data provide a potential neurobiologic basis for the anxiogenic effects of AMP (139). However, it has been theorized that stimulant-associated augmentation of serotonergic drive could ameliorate the comorbid anxiety associated with ADHD (227). In practice, the effects of stimulants on anxiety can be complex, with acute administration of MPH reducing anxiety in adults and chronic treatment during early life increasing anxiety during adulthood (228).
4.3.2. Depressive disorders
Psychostimulants have been used in the treatment of major depressive disorder (MDD) since the early 1950s, when the use of MPH was first examined for MDD (229). The rationale for examining the potential utility of psychostimulants in depressive disorders is based on preclinical and clinical evidence implicating DA in depressive symptomatology (230). However, the rapid onset of action of psychostimulants suggests the mechanisms by which they may influence depressive symptoms is likely to differ from that of antidepressants (231).
Based on the published literature, the effects of psychostimulants on depressive symptoms appear equivocal. Although one meta-analysis published in 2008 based on 3 short-term trials reported statistically significant improvements favoring monotherapy with psychostimulants compared with placebo for depressive symptoms (232), a systematic review of augmentation therapy for MDD published in 2009 based on MPH (2 studies) and modafinil (2 studies) reported that neither augmentation strategy was clinically superior to antidepressant monotherapy in reducing depressive symptoms (233). In addition, 2 large phase 3 studies and a phase 2 study of lisdexamfetamine dimesylate augmentation in adults with MDD and inadequate response to antidepressant therapy failed to meet their primary efficacy endpoint versus placebo (234, 235). The lack of treatment effect in these recently published studies, taken together with inconsistent findings based on meta-analyses and systematic reviews, suggests that psychostimulants are ineffective in treating the undifferentiated symptoms of depression. Psychostimulants have also been examined in other mood disorders, including treatment-resistant depression, bipolar depression, and depression associated with specific medical conditions (236). For bipolar depression, there is some evidence supporting the efficacy of psychostimulant augmentation, but the quality and quantity of studies does not allow for a strong evidence-based recommendation for their use to be put forth (236).
Despite inconclusive evidence regarding the efficacy of psychostimulants in treating depressive symptoms, the continued publication of review articles on this topic (231, 237, 238) demonstrates continued interest in this area of research. These review articles hypothesize that the lack of consistent clinical efficacy of psychostimulant augmentation could be due to poorly defined psychopathology and that psychostimulant effects may be more pronounced in selected symptom domains (237, 238) or that the effects of psychostimulants are short-lasting (231). It has also been speculated that the use of clinician-rated scales (rather than patient-rated scales) in some studies (234, 235) may not have adequately captured the effects of psychostimulant treatment.
It should also be noted that the use of stimulants as augmentation agents in combination with tricyclic antidepressants and MAO inhibitors is controversial, with issues concerning the possible development of an adrenergic crisis, the emergence of serotonin syndrome, or a hypertensive crisis being raised (239). In fact, both MPH-based agents and AMP-based agents are contraindicated in individuals taking an MAO inhibitor (currently or within the preceding 2 weeks) because a hypertensive crisis may result (240).
4.3.3. Substance use disorders
The neurobiology of reward and addiction and the key role of mesolimbic DA systems have been described in great detail (241, 242). Although associations have been made between ADHD and substance abuse, their relationship is complex. Several reviews have emphasized that substance use disorders can be comorbid with ADHD (16, 17). For example, in a study of 208 adults diagnosed with ADHD and treated with psychostimulants as youths, the relative risk of having a diagnosis of substance use disorder or alcohol abuse, respectively, compared with the general population was 7.7 or 5.2 (243). Furthermore, a 2012 review noted that there was evidence for increased rates of substance abuse in individuals with ADHD treated with psychostimulants (244). Given the known abuse liability of psychostimulants and data indicating that psychostimulant medications are associated with misuse and diversion (245, 246), it is not surprising that psychostimulant medications approved for use in ADHD are schedule II medications with black box warnings for potential drug dependence (247). Some treatment guidelines suggest that nonstimulant alternatives be considered as therapies for ADHD when issues related to abuse and dependence are a concern (25, 28, 248).
However, multiple studies have provided evidence that psychostimulant treatment in individuals with substance use disorders and ADHD is not associated with a significant worsening of substance abuse (249, 250). In a study that examined the efficacy of extended-release mixed AMP salts in the treatment of ADHD symptoms and cocaine abuse in 126 cocaine-dependent adults with ADHD (250), significantly greater reductions in ADHD symptoms and higher abstinence from cocaine use were observed with extended-release mixed AMP salts than placebo. In another study, osmotic-controlled release oral delivery system (OROS) MPH did not produce significantly greater reductions in ADHD symptoms than placebo in 24 AMP-dependent adults with newly diagnosed ADHD. However, OROS MPH treatment also was not associated with evidence of increased AMP abuse, as measured by self-reported days of AMP use or craving for AMP, time to relapse, or cumulative abstinence duration (249).
4.3.4. Sleep disturbances
The neurobiologic substrates of sleep are diverse and distributed throughout the brain, with monoaminergic systems playing an important role in wakefulness (21). Substantial literature exists regarding the sleep disturbances associated with ADHD, which include insomnia, disordered sleep, difficulty falling asleep, sleep apnea, daytime somnolence, and increased nocturnal motor activity (see (18, 251-253) for reviews). Evidence suggests that impaired and/or disordered sleep is present in individuals not being treated with psychostimulants (18). For example, in a study of the effects of MPH on sleep in children with ADHD, parents reported that approximately 10% of study participants had sleep problems before starting their medication (254). However, in regard to the reported effects of psychostimulants on sleep in individuals with ADHD, there are some discrepancies. In a meta-analysis of 9 articles, the use of psychostimulant medication was associated with longer sleep latency, worse sleep efficiency, and shorter sleep duration (255). A review of the safety and tolerability of ADHD medications noted that insomnia was one of the most commonly reported adverse events associated with psychostimulant treatment (36). In contrast, some studies have shown that psychostimulants have no significant negative impact on sleep (254, 256, 257). A post hoc analysis of the effects of lisdexamfetamine or SHP465 mixed amphetamine salts in adults with ADHD demonstrated that the proportions of participants exhibiting a worsening of sleep during treatment, as measured by the Pittsburgh Sleep Quality Index, did not differ from that of placebo (257). Discrepancies in the effects of stimulants on sleep in individuals with ADHD might be attributable to various factors, including sleep quality prior to treatment, the stimulant formulation, the length of treatment, and the method of sleep assessment (251, 254, 255). For example, in a study of MPH in children with ADHD, 23% of participants without preexisting sleep problems developed sleep problems while taking MPH, whereas 68.5% of those with preexisting sleep problems no longer experienced sleep problems after taking MPH (254).
4.4. Other Safety Concerns
In addition to considering the potential effect of AMP and MPH in individuals with other comorbid psychiatric disorders, the peripheral effects of AMP and MPH related to their pharmacology need to be considered, particularly in regard to their effects on cardiovascular function. Increased heart rate and blood pressure are among the most frequent treatment-emergent adverse events reported with psychostimulant treatment (36, 37); increases in pulse and blood pressure are also frequently reported (36, 37, 258). There is also a safety concern related to the potential for adverse cardiovascular outcomes, including ischemic attacks, myocardial infarction, and stroke (259). In a self-controlled case series analysis, treatment with MPH was associated with an increased overall risk of arrhythmia and with an increased risk of myocardial infarction from 1 week to 2 months after treatment initiation in children and adolescents with ADHD (260). Another study reported that the risk for an emergency department visit for cardiac-related reasons among youths did not differ between those being treated with AMP versus MPH (261). However, these findings should be considered in light of data from 2 large studies that reported no significant increase in risk for cardiovascular events in current users of ADHD medications compared with nonusers (262, 263).
The neurotransmitter systems responsible for stimulant-associated adverse events and safety concerns are in large part related to stimulation of peripheral NE activity (36, 259). Based on these concerns, package inserts for stimulants include black box warnings regarding the potential for serious adverse cardiovascular events (247). Assessments of the risks and benefits of stimulant therapy for ADHD should be made on an individual basis, and individuals on psychostimulant treatment should be monitored.
Another issue of potential concern is the possible neuroinflammatory effects of psychostimulants. In rats, MPH administration has been reported to produce neuroinflammation and oxidative stress in the hippocampus and cerebral cortex, as measured by the inflammatory markers tumor necrosis factor α and interleukin 1β (264, 265). In a systematic review of 14 studies (266), no study assessed the relationship between psychostimulant treatment and neuroinflammation so the relevance of preclinical models of neuroinflammation to psychostimulant treatment in individuals with ADHD is unknown. The same review did find evidence suggesting a role for inflammation in the pathogenesis of ADHD (266), which is consistent with a meta-analysis finding elevated levels of oxidative stress in patients diagnosed with ADHD (267). Oxidative stress has also been implicated in the lower brain volumes seen in ADHD patients (268).
5.0. Conclusions
Based on the published literature, the primary pharmacologic effects of both AMP and MPH are related to increased central DA and NE activity in brain regions that include the cortex and striatum. These regions are involved in the regulation of executive and attentional function (11). In ADHD, dysfunction in the DA and NE systems, which are critical to proper cortical and striatal function, likely account for some of the pathophysiology of ADHD (10, 11, 206). Although it is a limitation of the review that the only database searched was PubMed, it is unlikely that important studies were not captured.
It has been speculated that the moderately greater efficacy of AMP-based agents compared with MPH-based agents in ADHD may be related to differences in their molecular actions (33), but to date there is no conclusive clinical evidence to support this speculation. Furthermore, there is no conclusive clinical evidence supporting a prospective choice for an AMP-based agent over as MPH-based agent (or vice versa) based on the mechanisms of action of these drug classes. As such, the current understanding of differences in the mechanisms of action of AMP and MPH has not led to clinical guidelines regarding their use in specific patient populations. Furthermore, it is possible that differences in the pharmacologic profile between AMP and MPH, in combination with the complexities associated with the etiology of ADHD (11), contribute to individual differences in treatment response to AMP-based agents or MPH-based agents in individuals with ADHD. Interactions among these factors might explain why some patients have a differential response to these drugs.
When contemplating pharmacotherapy for ADHD, in addition to taking into account the potential for adverse cardiovascular outcomes (259), the presence of comorbid psychiatric disorders should be considered. Multiple psychiatric comorbidities, including depression and anxiety (16, 17), are thought to be mediated in part by shared neurobiological pathways that are also implicated in the pathophysiology of ADHD (19, 20). As such, the effect of psychostimulant treatment on the symptoms of these disorders and the potential interactions with medications used to treat these disorders need to be considered.
Highlights.
This review discusses amphetamine (AMP) and methylphenidate (MPH) pharmacology.
AMP and MPH increase corticostriatal catecholamine availability in different ways.
Catecholamine alterations occur in attention-deficit/hyperactivity disorder (ADHD).
Differential mechanisms of AMP and MPH may influence individual treatment response.
Considering stimulant effects is vital when treating ADHD with comorbidities.
Acknowledgments
Shire Development LLC (Lexington, MA) provided funding to Complete Healthcare Communications, LLC (CHC; West Chester, PA), an ICON plc company, for support in writing and editing this manuscript. Under the direction of the author, writing assistance was provided by Madhura Mehta, PhD, and Craig Slawecki, PhD, employees of CHC. Editorial assistance in the form of proofreading, copyediting, and fact checking was also provided by CHC. The author exercised full control over the content throughout the development and had final approval of the manuscript for submission.
Dr. Faraone is supported by the K. G. Jebsen Centre for Research on Neuropsychiatric Disorders, University of Bergen, Bergen, Norway, the European Union’s Seventh Framework Programme for research, technological development, and demonstration under grant agreement no 602805, the European Union’s Horizon 2020 research and innovation programme under grant agreement No 667302, and NIMH grants 5R01MH101519 and U01 MH109536-01.
Footnotes
Disclosures
In the past year, Dr. Faraone received income, potential income, travel expenses, continuing education support, and/or research support from Otsuka, Lundbeck, Kenpharma, Rhodes, Arbor, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA, Sunovion, Genomind, and NeuroLifeSciences. With his institution, he holds US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD.
References
- 1.Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd GW, et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am J Psychiatry. 1994;151(11):1673–85. [DOI] [PubMed] [Google Scholar]
- 2.Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111(2):279–89. [PubMed] [Google Scholar]
- 3.Weiss G, Hechtman L, Milroy T, Perlman T. Psychiatric status of hyperactives as adults: a controlled prospective 15-year follow-up of 63 hyperactive children. J Am Acad Child Psychiatry. 1985;24(2):211–20. [DOI] [PubMed] [Google Scholar]
- 4.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–65. [DOI] [PubMed] [Google Scholar]
- 5.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry Suppl. 2007;190:402–9. [DOI] [PubMed] [Google Scholar]
- 8.Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204–11. [DOI] [PubMed] [Google Scholar]
- 9.Purper-Ouakil D, Ramoz N, Lepagnol-Bestel AM, Gorwood P, Simonneau M. Neurobiology of attention deficit/hyperactivity disorder. Pediatr Res. 2011;69(5 Pt 2):69R–76R. [DOI] [PubMed] [Google Scholar]
- 10.Cortese S The neurobiology and genetics of attention-deficit/hyperactivity disorder (ADHD): what every clinician should know. Eur J Paediatr Neurol. 2012;16(5):422–33. [DOI] [PubMed] [Google Scholar]
- 11.Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. [DOI] [PubMed] [Google Scholar]
- 12.Maltezos S, Horder J, Coghlan S, Skirrow C, O'Gorman R, Lavender TJ, et al. Glutamate/glutamine and neuronal integrity in adults with ADHD: a proton MRS study. Transl Psychiatry. 2014;4:e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum K, Chen AL, Braverman ER, Comings DE, Chen TJ, Arcuri V, et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr Dis Treat. 2008;4(5):893–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter AS, Schaubhut G, Shipman M. Targeting the nicotinic cholinergic system to treat attention-deficit/hyperactivity disorder: rationale and progress to date. CNS Drugs. 2014;28(12):1103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elia J, Glessner JT, Wang K, Takahashi N, Shtir CJ, Hadley D, et al. Genome-wide copy number variation study associates metabotropic glutamate receptor gene networks with attention deficit hyperactivity disorder. Nat Genet. 2011;44(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao AR, Findling RL. Comorbidities in adult attention-deficit/hyperactivity disorder: a practical guide to diagnosis in primary care. Postgrad Med. 2014;126(5):42–51. [DOI] [PubMed] [Google Scholar]
- 17.Kooij JJ, Huss M, Asherson P, Akehurst R, Beusterien K, French A, et al. Distinguishing comorbidity and successful management of adult ADHD. J Atten Disord. 2012;16(5 Suppl):3S–19S. [DOI] [PubMed] [Google Scholar]
- 18.Konofal E, Lecendreux M, Cortese S. Sleep and ADHD. Sleep Med. 2010;11(7):652–8. [DOI] [PubMed] [Google Scholar]
- 19.Farb DH, Ratner MH. Targeting the modulation of neural circuitry for the treatment of anxiety disorders. Pharmacol Rev. 2014;66(4):1002–32. [DOI] [PubMed] [Google Scholar]
- 20.Sternat T, Katzman MA. Neurobiology of hedonic tone: the relationship between treatment-resistant depression, attention-deficit hyperactivity disorder, and substance abuse. Neuropsychiatr Dis Treat. 2016;12:2149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz MD, Kilduff TS. The neurobiology of sleep and wakefulness. Psychiatr Clin North Am. 2015;38(4):615–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharp SI, McQuillin A, Marks M, Hunt SP, Stanford SC, Lydall GJ, et al. Genetic association of the tachykinin receptor 1 TACR1 gene in bipolar disorder, attention deficit hyperactivity disorder, and the alcohol dependence syndrome. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(4):373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey CE, Agrawal A, Bucholz KK, Hartz SM, Lynskey MT, Nelson EC, et al. Associations between polygenic risk for psychiatric disorders and substance involvement. Front Genet. 2016;7:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas M, Rostain A, Prevatt F. ADHD diagnosis and treatment in college students and young adults. Adolesc Med State Art Rev. 2013;24(3):659–79. [PubMed] [Google Scholar]
- 25.Atkinson M, Hollis C. NICE guideline: attention deficit hyperactivity disorder. Arch Dis Child Educ Pract Ed. 2010;95(1):24–7. [DOI] [PubMed] [Google Scholar]
- 26.Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management, Wolraich M, Brown L, Brown RT, DuPaul G, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rostain AL. Attention-deficit/hyperactivity disorder in adults: evidence-based recommendations for management. Postgrad Med. 2008;120(3):27–38. [DOI] [PubMed] [Google Scholar]
- 28.Bolea-Alamanac B, Nutt DJ, Adamou M, Asherson P, Bazire S, Coghill D, et al. Evidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: update on recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(3):179–203. [DOI] [PubMed] [Google Scholar]
- 29.Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugue M, Carpentier PJ, et al. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry. 2010;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA). Canadian ADHD Practice Guidelines, Third Edition. Toronto, ON, Canada: CADDRA, 2011. [Google Scholar]
- 31.Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71:754–63. [DOI] [PubMed] [Google Scholar]
- 32.Catala-Lopez F, Hutton B, Nunez-Beltran A, Page MJ, Ridao M, Macias Saint-Gerons D, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta-analyses of randomised trials. PLoS ONE. 2017;12(7):e0180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19(4):353–64. [DOI] [PubMed] [Google Scholar]
- 34.Joseph A, Ayyagari R, Xie M, Cai S, Xie J, Huss M, et al. Comparative efficacy and safety of attention-deficit/hyperactivity disorder pharmacotherapies, including guanfacine extended release: a mixed treatment comparison. Eur Child Adolesc Psychiatry. 2017;26(8):875–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuhec M, Munda B, Svab V, Locatelli I. Comparative efficacy and acceptability of atomoxetine, lisdexamfetamine, bupropion and methylphenidate in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis with focus on bupropion. J Affect Disord. 2015;178:149–59. [DOI] [PubMed] [Google Scholar]
- 36.Duong S, Chung K, Wigal SB. Metabolic, toxicological, and safety considerations for drugs used to treat ADHD. Expert Opin Drug Metab Toxicol. 2012;8(5):543–52. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941–55. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Raga J, Ferreros A, Knecht C, de Alvaro R, Carabal E. Attention-deficit hyperactivity disorder medication use: factors involved in prescribing, safety aspects and outcomes. Thera Advances Drug Saf. 2017;8(3):87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortese S, Adamo N, Mohr-Jensen C, Hayes AJ, Bhatti S, Carucci S, et al. Comparative efficacy and tolerability of pharmacological interventions for attention-deficit/hyperactivity disorder in children, adolescents and adults: protocol for a systematic review and network meta-analysis. BMJ Open. 2017;7(1):e013967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.dela Pena I, Gevorkiana R, Shi WX. Psychostimulants affect dopamine transmission through both dopamine transporter-dependent and independent mechanisms. Eur J Pharmacol. 2015;764:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu J, Reith ME. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets. 2008;7(5):393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.al-Tikriti MS, Baldwin RM, Zea-Ponce Y, Sybirska E, Zoghbi SS, Laruelle M, et al. Comparison of three high affinity SPECT radiotracers for the dopamine D2 receptor. Nucl Med Biol. 1994;21(2):179–88. [DOI] [PubMed] [Google Scholar]
- 43.Annamalai B, Mannangatti P, Arapulisamy O, Ramamoorthy S, Jayanthi LD. Involvement of threonine 258 and serine 259 motif in amphetamine-induced norepinephrine transporter endocytosis. J Neurochem. 2010;115(1):23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avelar AJ, Juliano SA, Garris PA. Amphetamine augments vesicular dopamine release in the dorsal and ventral striatum through different mechanisms. J Neurochem. 2013;125(3):373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjorklund O, Halldner-Henriksson L, Yang J, Eriksson TM, Jacobson MA, Dare E, et al. Decreased behavioral activation following caffeine, amphetamine and darkness in A3 adenosine receptor knock-out mice. Physiol Behav. 2008;95(5):668–76. [DOI] [PubMed] [Google Scholar]
- 46.Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, et al. Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab. 1997;17(4):437–47. [DOI] [PubMed] [Google Scholar]
- 47.Castner SA, al-Tikriti MS, Baldwin RM, Seibyl JP, Innis RB, Goldman-Rakic PS. Behavioral changes and [123I]IBZM equilibrium SPECT measurement of amphetamine-induced dopamine release in rhesus monkeys exposed to subchronic amphetamine. Neuropsychopharmacology. 2000;22(1):4–13. [DOI] [PubMed] [Google Scholar]
- 48.Chen YI, Wang FN, Nelson AJ, Xu H, Kim Y, Rosen BR, et al. Electrical stimulation modulates the amphetamine-induced hemodynamic changes: an fMRI study to compare the effect of stimulating locations and frequencies on rats. Neurosci Lett. 2008;444(2):117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R, Furman CA, Zhang M, Kim MN, Gereau RW, Leitges M, et al. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther. 2009;328(3):912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choe ES, Chung KT, Mao L, Wang JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology. 2002;27(4):565–75. [DOI] [PubMed] [Google Scholar]
- 51.Chou YH, Halldin C, Farde L. Effect of amphetamine on extrastriatal D2 dopamine receptor binding in the primate brain: a PET study. Synapse. 2000;38(2):138–43. [DOI] [PubMed] [Google Scholar]
- 52.Covey DP, Juliano SA, Garris PA. Amphetamine elicits opposing actions on readily releasable and reserve pools for dopamine. PLoS One. 2013;8(5):e60763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993;13(4):350–6. [DOI] [PubMed] [Google Scholar]
- 54.Dixon AL, Prior M, Morris PM, Shah YB, Joseph MH, Young AM. Dopamine antagonist modulation of amphetamine response as detected using pharmacological MRI. Neuropharmacology. 2005;48(2):236–45. [DOI] [PubMed] [Google Scholar]
- 55.Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, et al. PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology. 1999;21(6):694–709. [DOI] [PubMed] [Google Scholar]
- 56.Duttaroy A, Billings B, Candido J, Yoburn BC. Chronic d-amphetamine inhibits opioid receptor antagonist-induced supersensitivity. Eur J Pharmacol. 1992;221(2-3):211–5. [DOI] [PubMed] [Google Scholar]
- 57.Easton N, Marshall F, Fone KC, Marsden CA. Differential effects of the D- and L-isomers of amphetamine on pharmacological MRI BOLD contrast in the rat. Psychopharmacology (Berl). 2007;193(1):11–30. [DOI] [PubMed] [Google Scholar]
- 58.Easton N, Steward C, Marshall F, Fone K, Marsden C. Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharmacology. 2007;52(2):405–14. [DOI] [PubMed] [Google Scholar]
- 59.Finnema SJ, Hughes ZA, Haaparanta-Solin M, Stepanov V, Nakao R, Varnas K, et al. Amphetamine decreases alpha2C-adrenoceptor binding of [11C]ORM-13070: a PET study in the primate brain. Int J Neuropsychopharmacol. 2015;18(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Floor E, Meng L. Amphetamine releases dopamine from synaptic vesicles by dual mechanisms. Neurosci Lett. 1996;215(1):53–6. [DOI] [PubMed] [Google Scholar]
- 61.Gallezot JD, Kloczynski T, Weinzimmer D, Labaree D, Zheng MQ, Lim K, et al. Imaging nicotine- and amphetamine-induced dopamine release in rhesus monkeys with [(11)C]PHNO vs [(11)C]raclopride PET. Neuropsychopharmacology. 2014;39(4):866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ginovart N, Farde L, Halldin C, Swahn CG. Changes in striatal D2-receptor density following chronic treatment with amphetamine as assessed with PET in nonhuman primates. Synapse. 1999;31(2):154–62. [DOI] [PubMed] [Google Scholar]
- 63.Hartvig P, Torstenson R, Tedroff J, Watanabe Y, Fasth KJ, Bjurling P, et al. Amphetamine effects on dopamine release and synthesis rate studied in the rhesus monkey brain by positron emission tomography. J Neural Transm (Vienna). 1997;104(4-5):329–39. [DOI] [PubMed] [Google Scholar]
- 64.Helmeste DM, Seeman P. Amphetamine-induced hypolocomotion in mice with more brain D2 dopamine receptors. Psychiatry Res. 1982;7(3):351–9. [DOI] [PubMed] [Google Scholar]
- 65.Howlett DR, Nahorski SR. Acute and chronic amphetamine treatments modulate striatal dopamine receptor binding sites. Brain Res. 1979;161(1):173–8. [DOI] [PubMed] [Google Scholar]
- 66.Inderbitzin S, Lauber ME, Schlumpf M, Lichtensteiger W. Amphetamine-induced preprodynorphin mRNA expression and kappa-opioid receptor binding in basal ganglia of adult rats after prenatal exposure to diazepam. Brain Res Dev Brain Res. 1997;98(1):114–24. [DOI] [PubMed] [Google Scholar]
- 67.Jedema HP, Narendran R, Bradberry CW. Amphetamine-induced release of dopamine in primate prefrontal cortex and striatum: striking differences in magnitude and timecourse. J Neurochem. 2014;130(4):490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joyce BM, Glaser PE, Gerhardt GA. Adderall produces increased striatal dopamine release and a prolonged time course compared to amphetamine isomers. Psychopharmacology (Berl). 2007;191(3):669–77. [DOI] [PubMed] [Google Scholar]
- 69.Kashiwagi Y, Rokugawa T, Yamada T, Obata A, Watabe H, Yoshioka Y, et al. Pharmacological MRI response to a selective dopamine transporter inhibitor, GBR12909, in awake and anesthetized rats. Synapse. 2015;69(4):203–12. [DOI] [PubMed] [Google Scholar]
- 70.Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16(13):4231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68(5):2032–7. [DOI] [PubMed] [Google Scholar]
- 72.Lahti RA, Tamminga CA. Effect of amphetamine, alpha-methyl-p-tyrosine (alpha-MPT) and antipsychotic agents on dopamine D2-type receptor occupancy in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(7):1277–83. [DOI] [PubMed] [Google Scholar]
- 73.Landau AM, Doudet DJ, Jakobsen S. Amphetamine challenge decreases yohimbine binding to alpha2 adrenoceptors in Landrace pig brain. Psychopharmacology (Berl). 2012;222(1):155–63. [DOI] [PubMed] [Google Scholar]
- 74.Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25(1):1–14. [DOI] [PubMed] [Google Scholar]
- 75.Le Masurier M, Houston G, Cowen P, Grasby P, Sharp T, Hume S. Tyrosine-free amino acid mixture attenuates amphetamine-induced displacement of [11C]raclopride in striatum in vivo: a rat PET study. Synapse. 2004;51(2):151–7. [DOI] [PubMed] [Google Scholar]
- 76.Lind NM, Olsen AK, Moustgaard A, Jensen SB, Jakobsen S, Hansen AK, et al. Mapping the amphetamine-evoked dopamine release in the brain of the Gottingen minipig. Brain Res Bull. 2005;65(1):1–9. [DOI] [PubMed] [Google Scholar]
- 77.Liu PS, Liaw CT, Lin MK, Shin SH, Kao LS, Lin LF. Amphetamine enhances Ca2+ entry and catecholamine release via nicotinic receptor activation in bovine adrenal chromaffin cells. Eur J Pharmacol. 2003;460(1):9–17. [DOI] [PubMed] [Google Scholar]
- 78.May LJ, Kuhr WG, Wightman RM. Differentiation of dopamine overflow and uptake processes in the extracellular fluid of the rat caudate nucleus with fast-scan in vivo voltammetry. J Neurochem. 1988;51(4):1060–9. [DOI] [PubMed] [Google Scholar]
- 79.Miller HH, Shore PA, Clarke DE. In vivo monoamine oxidase inhibition by d-amphetamine. Biochem Pharmacol. 1980;29(10):1347–54. [DOI] [PubMed] [Google Scholar]
- 80.Mukherjee J, Yang ZY, Lew R, Brown T, Kronmal S, Cooper MD, et al. Evaluation of d-amphetamine effects on the binding of dopamine D-2 receptor radioligand, 18F-fallypride in nonhuman primates using positron emission tomography. Synapse. 1997;27(1):1–13. [DOI] [PubMed] [Google Scholar]
- 81.Patrick RL, Berkowitz AL, Regenstein AC. Effects of in vivo amphetamine administration on dopamine synthesis regulation in rat brain striatal synaptosomes. J Pharmacol Exp Ther. 1981;217(3):686–91. [PubMed] [Google Scholar]
- 82.Pedersen K, Simonsen M, Ostergaard SD, Munk OL, Rosa-Neto P, Olsen AK, et al. Mapping the amphetamine-evoked changes in [11C]raclopride binding in living rat using small animal PET: modulation by MAO-inhibition. Neuroimage. 2007;35(1):38–46. [DOI] [PubMed] [Google Scholar]
- 83.Preece MA, Sibson NR, Raley JM, Blamire A, Styles P, Sharp T. Region-specific effects of a tyrosine-free amino acid mixture on amphetamine-induced changes in BOLD fMRI signal in the rat brain. Synapse. 2007;61(11):925–32. [DOI] [PubMed] [Google Scholar]
- 84.Price JC, Drevets WC, Ruszkiewicz J, Greer PJ, Villemagne VL, Xu L, et al. Sequential H(2)(15)O PET studies in baboons: before and after amphetamine. J Nucl Med. 2002;43(8):1090–100. [PubMed] [Google Scholar]
- 85.Pum M, Carey RJ, Huston JP, Muller CP. Dissociating effects of cocaine and d-amphetamine on dopamine and serotonin in the perirhinal, entorhinal, and prefrontal cortex of freely moving rats. Psychopharmacology (Berl). 2007;193(3):375–90. [DOI] [PubMed] [Google Scholar]
- 86.Quelch DR, Katsouri L, Nutt DJ, Parker CA, Tyacke RJ. Imaging endogenous opioid peptide release with [11C]carfentanil and [3H]diprenorphine: influence of agonist-induced internalization. J Cereb Blood Flow Metab. 2014;34(10):1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren J, Xu H, Choi JK, Jenkins BG, Chen YI. Dopaminergic response to graded dopamine concentration elicited by four amphetamine doses. Synapse. 2009;63(9):764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Riddle EL, Hanson GR, Fleckenstein AE. Therapeutic doses of amphetamine and methylphenidate selectively redistribute the vesicular monoamine transporter-2. Eur J Pharmacol. 2007;571(1):25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248(3):1010–7. [PubMed] [Google Scholar]
- 90.Robinson JB. Stereoselectivity and isoenzyme selectivity of monoamine oxidase inhibitors. Enantiomers of amphetamine, N-methylamphetamine and deprenyl. Biochem Pharmacol. 1985;34(23):4105–8. [DOI] [PubMed] [Google Scholar]
- 91.Saelens JK, Simke JP, Neale SE, Weeks BJ, Selwyn M. Effects of haloperidol and d-amphetamine on in vivo 3H-spiroperiodol binding in the rat forebrain. Arch Int Pharmacodyn Ther. 1980;246(1):98–107. [PubMed] [Google Scholar]
- 92.Schwarz AJ, Gozzi A, Reese T, Bifone A. Functional connectivity in the pharmacologically activated brain: resolving networks of correlated responses to d-amphetamine. Magn Reson Med. 2007;57(4):704–13. [DOI] [PubMed] [Google Scholar]
- 93.Schwendt M, Gold SJ, McGinty JF. Acute amphetamine down-regulates RGS4 mRNA and protein expression in rat forebrain: distinct roles of D1 and D2 dopamine receptors. J Neurochem. 2006;96(6):1606–15. [DOI] [PubMed] [Google Scholar]
- 94.Schiffer WK, Volkow ND, Fowler JS, Alexoff DL, Logan J, Dewey SL. Therapeutic doses of amphetamine or methylphenidate differentially increase synaptic and extracellular dopamine. Synapse. 2006;59(4):243–51. [DOI] [PubMed] [Google Scholar]
- 95.Seneca N, Finnema SJ, Farde L, Gulyas B, Wikstrom HV, Halldin C, et al. Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: a comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse. 2006;59(5):260–9. [DOI] [PubMed] [Google Scholar]
- 96.Shaffer C, Guo ML, Fibuch EE, Mao LM, Wang JQ. Regulation of group I metabotropic glutamate receptor expression in the rat striatum and prefrontal cortex in response to amphetamine in vivo. Brain Res. 2010;1326:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skinbjerg M, Liow JS, Seneca N, Hong J, Lu S, Thorsell A, et al. D2 dopamine receptor internalization prolongs the decrease of radioligand binding after amphetamine: a PET study in a receptor internalization-deficient mouse model. Neuroimage. 2010;50(4):1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith DG, Tzavara ET, Shaw J, Luecke S, Wade M, Davis R, et al. Mesolimbic dopamine super-sensitivity in melanin-concentrating hormone-1 receptor-deficient mice. J Neurosci. 2005;25(4):914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15(5 Pt 2):4102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5(6):797–808. [DOI] [PubMed] [Google Scholar]
- 101.Sun W, Ginovart N, Ko F, Seeman P, Kapur S. In vivo evidence for dopamine-mediated internalization of D2-receptors after amphetamine: differential findings with [3H]raclopride versus [3H]spiperone. Mol Pharmacol. 2003;63(2):456–62. [DOI] [PubMed] [Google Scholar]
- 102.Tomic M, Vukosavic S, Joksimovic J. Acute amphetamine and/or phencyclidine effects on the dopamine receptor specific binding in the rat brain. Eur Neuropsychopharmacol. 1997;7(4):295–301. [DOI] [PubMed] [Google Scholar]
- 103.Tomic M, Joksimovic J. Psychotomimetics moderately affect dopamine receptor binding in the rat brain. Neurochem Int. 2000;36(2):137–42. [DOI] [PubMed] [Google Scholar]
- 104.van Berckel BN, Kegeles LS, Waterhouse R, Guo N, Hwang DR, Huang Y, et al. Modulation of amphetamine-induced dopamine release by group II metabotropic glutamate receptor agonist LY354740 in non-human primates studied with positron emission tomography. Neuropsychopharmacology. 2006;31(5):967–77. [DOI] [PubMed] [Google Scholar]
- 105.Xiao L, Becker JB. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse. 1998;29(4):379–91. [DOI] [PubMed] [Google Scholar]
- 106.Yin HS, Lai CC, Tien TW, Han SK, Pu XL. Differential changes in cerebellar transmitter content and expression of calcium binding proteins and transcription factors in mouse administered with amphetamine. Neurochem Int. 2010;57(3):288–96. [DOI] [PubMed] [Google Scholar]
- 107.Young KA, Liu Y, Gobrogge KL, Dietz DM, Wang H, Kabbaj M, et al. Amphetamine alters behavior and mesocorticolimbic dopamine receptor expression in the monogamous female prairie vole. Brain Res. 2011;1367:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu MF, Lin WW, Li LT, Yin HS. Activation of metabotropic glutamate receptor 5 is associated with effect of amphetamine on brain neurons. Synapse. 2003;50(4):334–44. [DOI] [PubMed] [Google Scholar]
- 109.Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31(3):594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bartl J, Link P, Schlosser C, Gerlach M, Schmitt A, Walitza S, et al. Effects of methylphenidate: the cellular point of view. Atten Defic Hyperact Disord. 2010;2(4):225–32. [DOI] [PubMed] [Google Scholar]
- 111.Ding YS, Fowler JS, Volkow ND, Gatley SJ, Logan J, Dewey SL, et al. Pharmacokinetics and in vivo specificity of [11C]dl-threo-methylphenidate for the presynaptic dopaminergic neuron. Synapse. 1994;18(2):152–60. [DOI] [PubMed] [Google Scholar]
- 112.Ding YS, Fowler JS, Volkow ND, Dewey SL, Wang GJ, Logan J, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacology (Berl). 1997;131(1):71–8. [DOI] [PubMed] [Google Scholar]
- 113.Dresel SH, Kung MP, Huang X, Plossl K, Hou C, Shiue CY, et al. In vivo imaging of serotonin transporters with [99mTc]TRODAT-1 in nonhuman primates. Eur J Nucl Med. 1999;26(4):342–7. [DOI] [PubMed] [Google Scholar]
- 114.Federici M, Geracitano R, Bernardi G, Mercuri NB. Actions of methylphenidate on dopaminergic neurons of the ventral midbrain. Biol Psychiatry. 2005;57(4):361–5. [DOI] [PubMed] [Google Scholar]
- 115.Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49(10):1011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gatley SJ, Ding YS, Volkow ND, Chen R, Sugano Y, Fowler JS. Binding of d-threo-[11C]methylphenidate to the dopamine transporter in vivo: insensitivity to synaptic dopamine. Eur J Pharmacol. 1995;281(2):141–9. [DOI] [PubMed] [Google Scholar]
- 117.Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, et al. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology (Berl). 1999;146(1):93–100. [DOI] [PubMed] [Google Scholar]
- 118.Gatley SJ, Pan D, Chen R, Chaturvedi G, Ding YS. Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sci. 1996;58(12):231–9. [DOI] [PubMed] [Google Scholar]
- 119.Markowitz JS, DeVane CL, Ramamoorthy S, Zhu HJ. The psychostimulant d-threo-(R,R)-methylphenidate binds as an agonist to the 5HT(1A) receptor. Pharmazie. 2009;64(2):123–5. [PubMed] [Google Scholar]
- 120.Markowitz JS, DeVane CL, Pestreich LK, Patrick KS, Muniz R. A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study. J Child Adolesc Psychopharmacol. 2006;16(6):687–98. [DOI] [PubMed] [Google Scholar]
- 121.Michaelides M, Pascau J, Gispert JD, Delis F, Grandy DK, Wang GJ, et al. Dopamine D4 receptors modulate brain metabolic activity in the prefrontal cortex and cerebellum at rest and in response to methylphenidate. Eur J Neurosci. 2010;32(4):668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nikolaus S, Wirrwar A, Antke C, Arkian S, Schramm N, Muller HW, et al. Quantitation of dopamine transporter blockade by methylphenidate: first in vivo investigation using [123I]FP-CIT and a dedicated small animal SPECT. Eur J Nucl Med Mol Imaging. 2005;32(3):308–13. [DOI] [PubMed] [Google Scholar]
- 123.Nikolaus S, Antke C, Beu M, Kley K, Larisch R, Wirrwar A, et al. In-vivo quantification of dose-dependent dopamine transporter blockade in the rat striatum with small animal SPECT. Nucl Med Commun. 2007;28(3):207–13. [DOI] [PubMed] [Google Scholar]
- 124.Nikolaus S, Antke C, Beu M, Kley K, Wirrwar A, Huston JP, et al. Binding of [123I]iodobenzamide to the rat D2 receptor after challenge with various doses of methylphenidate: an in vivo imaging study with dedicated small animal SPECT. Eur J Nucl Med Mol Imaging. 2011;38(4):694–701. [DOI] [PubMed] [Google Scholar]
- 125.Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J Neurosci. 2002;22(19):8705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Somkuwar SS, Darna M, Kantak KM, Dwoskin LP. Adolescence methylphenidate treatment in a rodent model of attention deficit/hyperactivity disorder: dopamine transporter function and cellular distribution in adulthood. Biochem Pharmacol. 2013;86(2):309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Volkow ND, Fowler JS, Gatley SJ, Dewey SL, Wang GJ, Logan J, et al. Comparable changes in synaptic dopamine induced by methylphenidate and by cocaine in the baboon brain. Synapse. 1999;31(1):59–66. [DOI] [PubMed] [Google Scholar]
- 128.Wall SC, Gu H, Rudnick G. Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux. Mol Pharmacol. 1995;47(3):544–50. [PubMed] [Google Scholar]
- 129.Aalto S, Hirvonen J, Kaasinen V, Hagelberg N, Kajander J, Nagren K, et al. The effects of d-amphetamine on extrastriatal dopamine D2/D3 receptors: a randomized, double-blind, placebo-controlled PET study with [11C]FLB 457 in healthy subjects. Eur J Nucl Med Mol Imaging. 2009;36(3):475–83. [DOI] [PubMed] [Google Scholar]
- 130.Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, et al. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27(15):3998–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329(5991):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cardenas L, Houle S, Kapur S, Busto UE. Oral D-amphetamine causes prolonged displacement of [11C]raclopride as measured by PET. Synapse. 2004;51(1):27–31. [DOI] [PubMed] [Google Scholar]
- 133.Colasanti A, Searle GE, Long CJ, Hill SP, Reiley RR, Quelch D, et al. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol Psychiatry. 2012;72(5):371–7. [DOI] [PubMed] [Google Scholar]
- 134.Cropley VL, Innis RB, Nathan PJ, Brown AK, Sangare JL, Lerner A, et al. Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse. 2008;62(6):399–408. [DOI] [PubMed] [Google Scholar]
- 135.Devous MD Sr., Trivedi MH, Rush AJ. Regional cerebral blood flow response to oral amphetamine challenge in healthy volunteers. J Nucl Med. 2001;42(4):535–42. [PubMed] [Google Scholar]
- 136.Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49(2):81–96. [DOI] [PubMed] [Google Scholar]
- 137.Garrett DD, Nagel IE, Preuschhof C, Burzynska AZ, Marchner J, Wiegert S, et al. Amphetamine modulates brain signal variability and working memory in younger and older adults. Proc Natl Acad Sci U S A. 2015;112(24):7593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guterstam J, Jayaram-Lindstrom N, Cervenka S, Frost JJ, Farde L, Halldin C, et al. Effects of amphetamine on the human brain opioid system-a positron emission tomography study. Int J Neuropsychopharmacol. 2013;16(4):763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036–40. [DOI] [PubMed] [Google Scholar]
- 140.Kegeles LS, Zea-Ponce Y, Abi-Dargham A, Rodenhiser J, Wang T, Weiss R, et al. Stability of [123I]IBZM SPECT measurement of amphetamine-induced striatal dopamine release in humans. Synapse. 1999;31(4):302–8. [DOI] [PubMed] [Google Scholar]
- 141.Knutson B, Bjork JM, Fong GW, Hommer D, Mattay VS, Weinberger DR. Amphetamine modulates human incentive processing. Neuron. 2004;43(2):261–9. [DOI] [PubMed] [Google Scholar]
- 142.Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, et al. SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med. 1995;36(7):1182–90. [PubMed] [Google Scholar]
- 143.Laruelle M, Innis RB. Images in neuroscience. SPECT imaging of synaptic dopamine. Am J Psychiatry. 1996;153(10):1249. [DOI] [PubMed] [Google Scholar]
- 144.Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27(6):1027–35. [DOI] [PubMed] [Google Scholar]
- 145.Leyton M, Dagher A, Boileau I, Casey K, Baker GB, Diksic M, et al. Decreasing amphetamine-induced dopamine release by acute phenylalanine/tyrosine depletion: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2004;29(2):427–32. [DOI] [PubMed] [Google Scholar]
- 146.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23(3):285–300. [DOI] [PubMed] [Google Scholar]
- 147.Mick I, Myers J, Stokes PR, Erritzoe D, Colasanti A, Bowden-Jones H, et al. Amphetamine induced endogenous opioid release in the human brain detected with [11C]carfentanil PET: replication in an independent cohort. Int J Neuropsychopharmacol. 2014;17(12):2069–74. [DOI] [PubMed] [Google Scholar]
- 148.Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63(6):447–61. [DOI] [PubMed] [Google Scholar]
- 149.Narendran R, Mason NS, Laymon CM, Lopresti BJ, Velasquez ND, May MA, et al. A comparative evaluation of the dopamine D(2/3) agonist radiotracer [11C](−)-N-propylnorapomorphine and antagonist [11C]raclopride to measure amphetamine-induced dopamine release in the human striatum. J Pharmacol Exp Ther. 2010;333(2):533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30(4):821–32. [DOI] [PubMed] [Google Scholar]
- 151.Oswald LM, Wand GS, Wong DF, Brown CH, Kuwabara H, Brasic JR. Risky decision-making and ventral striatal dopamine responses to amphetamine: a positron emission tomography [(11)C]raclopride study in healthy adults. Neuroimage. 2015;113:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, et al. Sex differences in amphetamine-induced displacement of [18F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006;163(9):1639–41. [DOI] [PubMed] [Google Scholar]
- 153.Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology. 2006;31(5):1016–26. [DOI] [PubMed] [Google Scholar]
- 154.Riccardi P, Park S, Anderson S, Doop M, Ansari MS, Schmidt D, et al. Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [18F]fallypride. Synapse. 2011;65(2):99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rose SE, Janke AL, Strudwick MW, McMahon KL, Chalk JB, Snyder P, et al. Assessment of dynamic susceptibility contrast cerebral blood flow response to amphetamine challenge: a human pharmacological magnetic resonance imaging study at 1.5 and 4 T. Magn Reson Med. 2006;55(1):9–15. [DOI] [PubMed] [Google Scholar]
- 156.Schouw ML, Kaag AM, Caan MW, Heijtel DF, Majoie CB, Nederveen AJ, et al. Mapping the hemodynamic response in human subjects to a dopaminergic challenge with dextroamphetamine using ASL-based pharmacological MRI. Neuroimage. 2013;72:1–9. [DOI] [PubMed] [Google Scholar]
- 157.Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, et al. Within-subject comparison of [11C]-(+)-PHNO and [11C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32(1):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Silverstone PH, O'Donnell T, Ulrich M, Asghar S, Hanstock CC. Dextro-amphetamine increases phosphoinositol cycle activity in volunteers: an MRS study. Hum Psychopharmacol. 2002;17(8):425–9. [DOI] [PubMed] [Google Scholar]
- 159.Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [18F] fallypride. Synapse. 2010;64(5):350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vollenweider FX, Maguire RP, Leenders KL, Mathys K, Angst J. Effects of high amphetamine dose on mood and cerebral glucose metabolism in normal volunteers using positron emission tomography (PET). Psychiatry Res. 1998;83(3):149–62. [DOI] [PubMed] [Google Scholar]
- 161.Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, et al. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32(11):2310–20. [DOI] [PubMed] [Google Scholar]
- 162.Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, et al. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: a [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33(2):279–89. [DOI] [PubMed] [Google Scholar]
- 163.Wolkin A, Angrist B, Wolf A, Brodie J, Wolkin B, Jaeger J, et al. Effects of amphetamine on local cerebral metabolism in normal and schizophrenic subjects as determined by positron emission tomography. Psychopharmacology (Berl). 1987;92(2):241–6. [DOI] [PubMed] [Google Scholar]
- 164.Woodward ND, Cowan RL, Park S, Ansari MS, Baldwin RM, Li R, et al. Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am J Psychiatry. 2011;168(4):418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Booij J, Korn P, Linszen DH, van Royen EA. Assessment of endogenous dopamine release by methylphenidate challenge using iodine-123 iodobenzamide single-photon emission tomography. Eur J Nucl Med. 1997;24(6):674–7. [DOI] [PubMed] [Google Scholar]
- 166.Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29(15):4690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Costa A, Riedel M, Pogarell O, Menzel-Zelnitschek F, Schwarz M, Reiser M, et al. Methylphenidate effects on neural activity during response inhibition in healthy humans. Cereb Cortex. 2013;23(5):1179–89. [DOI] [PubMed] [Google Scholar]
- 168.Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, van Dyck CH, et al. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 2010;68(9):854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kasparbauer AM, Rujescu D, Riedel M, Pogarell O, Costa A, Meindl T, et al. Methylphenidate effects on brain activity as a function of SLC6A3 genotype and striatal dopamine transporter availability. Neuropsychopharmacology. 2015;40(3):736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Montgomery AJ, Asselin MC, Farde L, Grasby PM. Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C]FLB 457 PET. J Cereb Blood Flow Metab. 2007;27(2):369–77. [DOI] [PubMed] [Google Scholar]
- 171.Moeller SJ, Honorio J, Tomasi D, Parvaz MA, Woicik PA, Volkow ND, et al. Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cereb Cortex. 2014;24(3):643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mueller S, Costa A, Keeser D, Pogarell O, Berman A, Coates U, et al. The effects of methylphenidate on whole brain intrinsic functional connectivity. Hum Brain Mapp. 2014;35(11):5379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ramaekers JG, Evers EA, Theunissen EL, Kuypers KP, Goulas A, Stiers P. Methylphenidate reduces functional connectivity of nucleus accumbens in brain reward circuit. Psychopharmacology (Berl). 2013;229(2):219–26. [DOI] [PubMed] [Google Scholar]
- 174.Ramasubbu R, Singh H, Zhu H, Dunn JF. Methylphenidate-mediated reduction in prefrontal hemodynamic responses to working memory task: a functional near-infrared spectroscopy study. Hum Psychopharmacol. 2012;27(6):615–21. [DOI] [PubMed] [Google Scholar]
- 175.Schabram I, Henkel K, Mohammadkhani Shali S, Dietrich C, Schmaljohann J, Winz O, et al. Acute and sustained effects of methylphenidate on cognition and presynaptic dopamine metabolism: an [18F]FDOPA PET study. J Neurosci. 2014;34(44):14769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schweitzer JB, Lee DO, Hanford RB, Zink CF, Ely TD, Tagamets MA, et al. Effect of methylphenidate on executive functioning in adults with attention-deficit/hyperactivity disorder: normalization of behavior but not related brain activity. Biol Psychiatry. 2004;56(8):597–606. [DOI] [PubMed] [Google Scholar]
- 177.Schlosser RG, Nenadic I, Wagner G, Zysset S, Koch K, Sauer H. Dopaminergic modulation of brain systems subserving decision making under uncertainty: a study with fMRI and methylphenidate challenge. Synapse. 2009;63(5):429–42. [DOI] [PubMed] [Google Scholar]
- 178.Spencer TJ, Biederman J, Ciccone PE, Madras BK, Dougherty DD, Bonab AA, et al. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry. 2006;163(3):387–95. [DOI] [PubMed] [Google Scholar]
- 179.Spencer TJ, Bonab AA, Dougherty DD, Martin J, McDonnell T, Fischman AJ. A PET study examining pharmacokinetics and dopamine transporter occupancy of two long-acting formulations of methylphenidate in adults. Int J Mol Med. 2010;25(2):261–5. [PubMed] [Google Scholar]
- 180.Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, et al. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 2011;54(4):3101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Udo de Haes JI, Kortekaas R, Van Waarde A, Maguire RP, Pruim J, den Boer JA. Assessment of methylphenidate-induced changes in binding of continuously infused [11C]raclopride in healthy human subjects: correlation with subjective effects. Psychopharmacology (Berl). 2005;183(3):322–30. [DOI] [PubMed] [Google Scholar]
- 182.Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16(4):255–62. [DOI] [PubMed] [Google Scholar]
- 183.Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J, et al. Relationship between psychostimulant-induced "high" and dopamine transporter occupancy. Proc Natl Acad Sci U S A. 1996;93(19):10388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Volkow ND, Wang GJ, Fowler JS, Logan J, Angrist B, Hitzemann R, et al. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154(1):50–5. [DOI] [PubMed] [Google Scholar]
- 185.Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325–31. [DOI] [PubMed] [Google Scholar]
- 186.Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of "high". J Pharmacol Exp Ther. 1999;288(1):14–20. [PubMed] [Google Scholar]
- 187.Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):Rc121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43(3):181–7. [DOI] [PubMed] [Google Scholar]
- 189.Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6 suppl 1:S31–43. [DOI] [PubMed] [Google Scholar]
- 190.Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, et al. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry. 2004;161(7):1173–80. [DOI] [PubMed] [Google Scholar]
- 191.Wang GJ, Volkow ND, Fowler JS, Logan J, Pappas NR, Wong CT, et al. Reproducibility of repeated measures of endogenous dopamine competition with [11C]raclopride in the human brain in response to methylphenidate. J Nucl Med. 1999;40(8):1285–91. [PubMed] [Google Scholar]
- 192.Volkow ND, Fowler JS, Gatley SJ, Logan J, Wang GJ, Ding YS, et al. PET evaluation of the dopamine system of the human brain. J Nucl Med. 1996;37(7):1242–56. [PubMed] [Google Scholar]
- 193.Fischman AJ, Babich JW, Elmaleh DR, Barrow SA, Meltzer P, Hanson RN, et al. SPECT imaging of dopamine transporter sites in normal and MPTP-treated rhesus monkeys. J Nucl Med. 1997;38(1):144–50. [PubMed] [Google Scholar]
- 194.Kilbourn MR, Domino EF. Increased in vivo [11C]raclopride binding to brain dopamine receptors in amphetamine-treated rats. Eur J Pharmacol. 2011;654(3):254–7. [DOI] [PubMed] [Google Scholar]
- 195.Schwarz AJ, Gozzi A, Reese T, Heidbreder CA, Bifone A. Pharmacological modulation of functional connectivity: the correlation structure underlying the phMRI response to d-amphetamine modified by selective dopamine D3 receptor antagonist SB277011A. Magn Reson Imaging. 2007;25(6):811–20. [DOI] [PubMed] [Google Scholar]
- 196.Jain R, Katic A. Current and investigational medication delivery systems for treating attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2016;18(4). [DOI] [PubMed] [Google Scholar]
- 197.Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18(1):7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2012;36(4):1093–106. [DOI] [PubMed] [Google Scholar]
- 199.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168(11):1154–63. [DOI] [PubMed] [Google Scholar]
- 200.Seidman LJ, Biederman J, Liang L, Valera EM, Monuteaux MC, Brown A, et al. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol Psychiatry. 2011;69(9):857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LS, et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 2017;4(4):310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry. 2012;169(3):264–72. [DOI] [PubMed] [Google Scholar]
- 203.Hart H, Radua J, Mataix-Cols D, Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD). Neurosci Biobehav Rev. 2012;36(10):2248–56. [DOI] [PubMed] [Google Scholar]
- 204.Cortese S, Castellanos FX, Eickhoff CR, D'Acunto G, Masi G, Fox PT, et al. Functional Decoding and Meta-analytic Connectivity Modeling in Adult Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2016;80(12):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cocchi L, Bramati IE, Zalesky A, Furukawa E, Fontenelle LF, Moll J, et al. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J Neurosci. 2012;32(49):17753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23 (suppl 1):33–41. [DOI] [PubMed] [Google Scholar]
- 207.Cortese S, Castellanos FX. Neuroimaging of attention-deficit/hyperactivity disorder: current neuroscience-informed perspectives for clinicians. Curr Psychiatry Rep. 2012;14(5):568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.da Silva N Jr., Szobot CM, Anselmi CE, Jackowski AP, Chi SM, Hoexter MQ, et al. Attention deficit/hyperactivity disorder: is there a correlation between dopamine transporter density and cerebral blood flow? Clin Nucl Med. 2011;36(8):656–60. [DOI] [PubMed] [Google Scholar]
- 209.Cherkasova MV, Faridi N, Casey KF, O'Driscoll GA, Hechtman L, Joober R, et al. Amphetamine-induced dopamine release and neurocognitive function in treatment-naive adults with ADHD. Neuropsychopharmacology. 2014;39(6):1498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dresel S, Krause J, Krause KH, LaFougere C, Brinkbaumer K, Kung HF, et al. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27(10):1518–24. [DOI] [PubMed] [Google Scholar]
- 211.Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. 2000;285(2):107–10. [DOI] [PubMed] [Google Scholar]
- 212.Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, et al. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62(9):1059–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Krause J, la Fougere C, Krause KH, Ackenheil M, Dresel SH. Influence of striatal dopamine transporter availability on the response to methylphenidate in adult patients with ADHD. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):428–31. [DOI] [PubMed] [Google Scholar]
- 214.la Fougere C, Krause J, Krause KH, Josef Gildehaus F, Hacker M, Koch W, et al. Value of 99mTc-TRODAT-1 SPECT to predict clinical response to methylphenidate treatment in adults with attention deficit hyperactivity disorder. Nucl Med Commun. 2006;27(9):733–7. [DOI] [PubMed] [Google Scholar]
- 215.Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32(3):841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang GJ, Volkow ND, Wigal T, Kollins SH, Newcorn JH, Telang F, et al. Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS One. 2013;8(5):e63023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16(11):1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ludolph AG, Kassubek J, Schmeck K, et al. Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: a 3,4-dihdroxy-6-[18F]fluorophenyl-l-alanine PET study. Neuroimage. 2008;41:718–27. [DOI] [PubMed] [Google Scholar]
- 219.Contini V, Rovaris DL, Victor MM, Grevet EH, Rohde LA, Bau CH. Pharmacogenetics of response to methylphenidate in adult patients with attention-deficit/hyperactivity disorder (ADHD): a systematic review. Eur Neuropsychopharmacol. 2013;23(6):555–60. [DOI] [PubMed] [Google Scholar]
- 220.Sigurdardottir HL, Kranz GS, Rami-Mark C, James GM, Vanicek T, Gryglewski G, et al. Effects of norepinephrine transporter gene variants on NET binding in ADHD and healthy controls investigated by PET. Hum Brain Mapp. 2016;37(3):884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vanicek T, Spies M, Rami-Mark C, Savli M, Hoflich A, Kranz GS, et al. The norepinephrine transporter in attention-deficit/hyperactivity disorder investigated with positron emission tomography. JAMA Psychiatry. 2014;71(12):1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dramsdahl M, Ersland L, Plessen KJ, Haavik J, Hugdahl K, Specht K. Adults with attention-deficit/hyperactivity disorder - a brain magnetic resonance spectroscopy study. Front Psychiatry. 2011;2:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Arcos-Burgos M, Londono AC, Pineda DA, Lopera F, Palacio JD, Arbelaez A, et al. Analysis of brain metabolism by proton magnetic resonance spectroscopy (1H-MRS) in attention-deficit/hyperactivity disorder suggests a generalized differential ontogenic pattern from controls. Atten Defic Hyperact Disord. 2012;4(4):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Park S, Lee JM, Kim JW, Cho DY, Yun HJ, Han DH, et al. Associations between serotonin transporter gene (SLC6A4) methylation and clinical characteristics and cortical thickness in children with ADHD. Psychol Med. 2015;45(14):3009–17. [DOI] [PubMed] [Google Scholar]
- 225.Vanicek T, Kutzelnigg A, Philippe C, Sigurdardottir HL, James GM, Hahn A, et al. Altered interregional molecular associations of the serotonin transporter in attention deficit/hyperactivity disorder assessed with PET. Hum Brain Mapp. 2017;38(2):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chantiluke K, Barrett N, Giampietro V, Brammer M, Simmons A, Murphy DG, et al. Inverse effect of fluoxetine on medial prefrontal cortex activation during reward reversal in ADHD and autism. Cereb Cortex. 2015;25(7):1757–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present--a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sanchez-Perez AM, Garcia-Aviles A, Albert Gasco H, Sanjuan J, Olucha-Bordonau FE. [Effects of methylphenidate on anxiety]. Rev Neurol. 2012;55(8):499–506. [PubMed] [Google Scholar]
- 229.Robin AA, Wiseberg S. A controlled trial of methyl phenidate (ritalin) in the treatment of depressive states. J Neurol Neurosurg Psychiatry. 1958;21(1):55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Malhi GS, Byrow Y, Bassett D, Boyce P, Hopwood M, Lyndon W, et al. Stimulants for depression: on the up and up? Aust N Z J Psychiatry. 2016;50(3):203–7. [DOI] [PubMed] [Google Scholar]
- 232.Candy M, Jones L, Williams R, Tookman A, King M. Psychostimulants for depression. Cochrane Database Syst Rev. 2008(2):CD006722. [DOI] [PubMed] [Google Scholar]
- 233.Fleurence R, Williamson R, Jing Y, Kim E, Tran QV, Pikalov AS, et al. A systematic review of augmentation strategies for patients with major depressive disorder. Psychopharmacol Bull. 2009;42(3):57–90. [PubMed] [Google Scholar]
- 234.Richards C, McIntyre RS, Weisler R, Sambunaris A, Brawman-Mintzer O, Gao J, et al. Lisdexamfetamine dimesylate augmentation for adults with major depressive disorder and inadequate response to antidepressant monotherapy: results from 2 phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Affect Disord. 2016;206:151–60. [DOI] [PubMed] [Google Scholar]
- 235.Richards C, Iosifescu DV, Mago R, Sarkis E, Reynolds J, Geibel B, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of lisdexamfetamine dimesylate augmentation for major depressive disorder in adults with inadequate response to antidepressant therapy. J Clin Psychopharmacol. 2017;31(9):1190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dell'Osso B, Ketter TA. Use of adjunctive stimulants in adult bipolar depression. Int J Neuropsychopharmacol. 2013;16(1):55–68. [DOI] [PubMed] [Google Scholar]
- 237.McIntyre RS, Lee Y, Zhou AJ, Rosenblat JD, Peters EM, Lam RW, et al. The efficacy of psychostimulants in major depressive episodes: a systematic review and meta-analysis. J Clin Psychopharmacol.37(4):412–8. [DOI] [PubMed] [Google Scholar]
- 238.Hegerl U, Hensch T. Why do stimulants not work in typical depression? Aust N Z J Psychiatry. 2017;51(1):20–2. [DOI] [PubMed] [Google Scholar]
- 239.Stotz G, Woggon B, Angst J. Psychostimulants in the therapy of treatment-resistant depression: review of the literature and findings from a retrospective study in 65 depressed patients. Dialogues Clin Neurosci. 1999;1(3):165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Thomas SJ, Shin M, McInnis MG, Bostwick JR. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433–49. [DOI] [PubMed] [Google Scholar]
- 241.Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162(4):712–25. [DOI] [PubMed] [Google Scholar]
- 242.Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(suppl 1):23–30. [DOI] [PubMed] [Google Scholar]
- 243.Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood - a naturalistic long-term follow-up study. Addict Behav. 2014;39(1):325–8. [DOI] [PubMed] [Google Scholar]
- 244.Nelson A, Galon P. Exploring the relationship among ADHD, stimulants, and substance abuse. J Child Adolesc Psychiatr Nurs. 2012;25(3):113–8. [DOI] [PubMed] [Google Scholar]
- 245.Rabiner DL, Anastopoulos AD, Costello EJ, Hoyle RH, McCabe SE, Swartzwelder HS. The misuse and diversion of prescribed ADHD medications by college students. J Atten Disord. 2009;13(2):144–53. [DOI] [PubMed] [Google Scholar]
- 246.Garnier LM, Arria AM, Caldeira KM, Vincent KB, O'Grady KE, Wish ED. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71(3):262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Panagiotou OA, Contopoulos-Ioannidis DG, Papanikolaou PN, Ntzani EE, Ioannidis JP. Different black box warning labeling for same-class drugs. J Gen Intern Med. 2011;26(6):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pliszka S, AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921. [DOI] [PubMed] [Google Scholar]
- 249.Konstenius M, Jayaram-Lindstrom N, Beck O, Franck J. Sustained release methylphenidate for the treatment of ADHD in amphetamine abusers: a pilot study. Drug Alcohol Depend. 2010;108(1-2):130–3. [DOI] [PubMed] [Google Scholar]
- 250.Levin FR, Mariani JJ, Specker S, Mooney M, Mahony A, Brooks DJ, et al. Extended-Release Mixed Amphetamine Salts vs Placebo for Comorbid Adult Attention-Deficit/Hyperactivity Disorder and Cocaine Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2015;72(6):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cohen-Zion M, Ancoli-Israel S. Sleep in children with attention-deficit hyperactivity disorder (ADHD): a review of naturalistic and stimulant intervention studies. Sleep Med Rev. 2004;8(5):379–402. [DOI] [PubMed] [Google Scholar]
- 252.Lecendreux M, Cortese S. Sleep problems associated with ADHD: a review of current therapeutic options and recommendations for the future. Expert Rev Neurother. 2007;7(12):1799–806. [DOI] [PubMed] [Google Scholar]
- 253.Snitselaar MA, Smits MG, van der Heijden KB, Spijker J. Sleep and circadian rhythmicity in adult ADHD and the effect of stimulants. J Atten Disord. 2017;21(1):14–26. [DOI] [PubMed] [Google Scholar]
- 254.Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2016;37(5):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kidwell KM, Van Dyk TR, Lundahl A, Nelson TD. Stimulant medications and sleep for youth with ADHD: a meta-analysis. Pediatrics. 2015;136(6):1144–53. [DOI] [PubMed] [Google Scholar]
- 256.Owens J, Weiss M, Nordbrock E, Mattingly G, Wigal S, Greenhill LL, et al. Effect of Aptensio XR (methylphenidate HCl extended-release) capsules on sleep in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2016;26(10):873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Surman CB, Roth T. Impact of stimulant pharmacotherapy on sleep quality: post hoc analyses of 2 large, double-blind, randomized, placebo-controlled trials. J Clin Psychiatry. 2011;72(7):903–8. [DOI] [PubMed] [Google Scholar]
- 258.Santosh PJ, Sattar S, Canagaratnam M. Efficacy and tolerability of pharmacotherapies for attention-deficit hyperactivity disorder in adults. CNS Drugs. 2011;25(9):737–63. [DOI] [PubMed] [Google Scholar]
- 259.Westover AN, Halm EA. Do prescription stimulants increase the risk of adverse cardiovascular events?: A systematic review. BMC Cardiovasc Disord. 2012;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shin JY, Roughead EE, Park BJ, Pratt NL. Cardiovascular safety of methylphenidate among children and young people with attention-deficit/hyperactivity disorder (ADHD): nationwide self controlled case series study. BMJ. 2016;353:i2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Winterstein AG, Gerhard T, Shuster J, Saidi A. Cardiac safety of methylphenidate versus amphetamine salts in the treatment of ADHD. Pediatrics. 2009;124(1):e75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Motaghinejad M, Motevalian M, Shabab B, Fatima S. Effects of acute doses of methylphenidate on inflammation and oxidative stress in isolated hippocampus and cerebral cortex of adult rats. J Neural Transm (Vienna). 2017;124(1):121–31. [DOI] [PubMed] [Google Scholar]
- 265.Motaghinejad M, Motevalian M, Shabab B. Effects of chronic treatment with methylphenidate on oxidative stress and inflammation in hippocampus of adult rats. Neurosci Lett. 2016;619:106–13. [DOI] [PubMed] [Google Scholar]
- 266.Anand D, Colpo GD, Zeni G, Zeni CP, Teixeira AL. Attention-Deficit/Hyperactivity Disorder and inflammation: what does current knowledge tell us? A systematic review. Front Psychiatry. 2017;8:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Joseph N, Zhang-James Y, Perl A, Faraone SV. Oxidative stress and ADHD: a meta-analysis. J Atten Disord. 2015;19(11):915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hess JL, Akutagava-Martins GC, Patak JD, Glatt SJ, Faraone SV. Why is there selective subcortical vulnerability in ADHD? Clues from postmortem brain gene expression data. Mol Psychiatry. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]