Abstract
Experimental studies have provided strong evidence that chronic inflammation triggered by the sub-endothelial accumulation of cholesterol-rich lipoproteins in arteries is essential in the initiation and progression of atherosclerosis. Recent clinical trials highlighting the efficacy of anti-inflammatory therapies in coronary patients have confirmed that this is also true in humans Monocytes/macrophages are central cells in the atherosclerotic process, but adaptive immunity, through B and T lymphocytes, as well as dendritic cells, also modulates the progression of the disease. Analysis of the role of different T cell subpopulations in murine models of atherosclerosis identified effector Th1 cells as proatherogenic, whereas regulatory T cells (Tregs) have been shown to protect against atherosclerosis. For these reasons, better understanding of how Tregs influence the atherosclerotic process is believed to provide novel Treg-targeted therapies to combat atherosclerosis. This review article summarizes current knowledge about the role of Tregs in atherosclerosis and discusses ways to enhance their function as novel immunomodulatory therapeutic approaches against cardiovascular disease.
Keywords: atherosclerosis, immunity, cytokines, T lymphocytes, regulatory T cells
1. Introduction
Atherosclerosis is the result of an inflammatory response triggered by the sub-endothelial accumulation of low-density lipoprotein (LDL) cholesterol and its subsequent oxidation in arteries. Until recently, this view was mainly based on experimental evidence or clinical association studies. Yet recent clinical trials highlighting the clinical benefit of inflammation-targeted therapy with anti-interleukin (IL)-1β neutralizing antibodies [1] or colchicine [2,3] for the prevention of recurrent events in patients recovering from an acute coronary syndrome (ACS), or in the stable phase of coronary artery disease, have provided a direct proof of concept that atherosclerosis is a chronic inflammatory disease, as claimed by R. Ross at the end of the last century [4]. Although monocytes/macrophages constitute the central cells responsible for the innate immune response and are mandatory for the formation of foam cells, adaptive immune responses, through T and B cells, as well as dendritic cells (DCs), have been shown to substantially regulate atherosclerosis in experimental models [5]. Conventional B2 cells have been shown to exert proatherogenic activities, whereas natural B1 cells producing anti-oxidized LDL (oxLDL) IgM display antiatherogenic properties [6]. Animal studies have implicated cluster of differentiation (CD)4+ T helper type 1 (Th1) cells as important contributors to the initiation of atherosclerosis, whereas the role of Th2 and Th17 cells remains debated [5]. Importantly, regulatory T cells (Tregs) expressing the IL-2 receptor α-chain (CD25) and the transcription factor FoxP3 have been shown to protect against atherosclerosis [7]. Our understanding that the imbalance between the suppressive activity of Tregs and activation of harmful Th1 responses contributes to atherosclerosis development has opened new opportunities for enhancing immune tolerance by stimulating Tregs in order to promote atheroprotective immunity. In this review, we discuss recent advances in our understanding of the roles of Tregs in atherosclerosis, and discusses ways to stimulate Treg-based immunomodulatory approaches as novel therapeutic strategies against the disease.
2. Role of T Cell Activation in Atherosclerosis
The presence of CD3+ T cells in both mouse [8] and human atherosclerotic plaques [9], and the pronounced expression of major histocompatibility complex (MHC) class II (HLA-DR) in human plaques [10] were the first evidence supporting a role for adaptive immunity in atherosclerosis. More recently, single-cell RNA sequencing (scRNA-seq) analysis confirmed the presence of both CD4+ and CD8+ T cells in mouse [11,12] and human [13] plaques. T lymphocytes are one of the earliest cells recruited in the atherosclerotic plaque [14]. More direct and convincing evidence for a role of adaptive immunity in atherosclerosis has been provided using experimental mouse models of atherosclerosis, especially Apolipoprotein E (Apoe)−/− or low-density lipoprotein receptor (Ldlr)−/− mice, in which human-like atherosclerotic lesions develop spontaneously or in response to a high-fat diet. Athero-prone mice crossed with T/B cell-deficient Rag1−/− (or Rag2−/−) or Scid/Scid mice display reduced atherosclerosis lesions when fed a chow diet [5]. In addition, purified CD4+ T cells supplementation into Scid/Scid/Apoe−/− mice reverses the beneficial effect of lymphocyte deficiency [15]. CD8+ T cells also contribute to atherosclerosis progression [16]. Depletion of CD8+ T cells with anti-CD8α or -CD8β antibodies in Apoe−/− [17] or Ldlr−/− mice [18] attenuated atherosclerosis by modulating monopoiesis in the bone marrow and reducing circulating monocyte levels in the blood.
The exact mechanism that orchestrates T cell immunity in atherosclerosis is complex, but accumulating evidence supports an antigen-specific-driven response. T cells derived from Apoe−/− mice exhibit a highly restricted T cell receptor (TCR)α/β repertoire [19]. Moreover, TCRα deficiency in Apoe−/− mice has been shown to protect against atherosclerosis [20]. In the same line, T cells from human atherosclerotic plaques respond to oxLDL in an HLA-DR-dependent manner, but not to naïve LDL [21], suggesting that oxLDL is an important antigen in atherosclerosis. In addition, the transfer of T cells pre-exposed to oxLDL into scid/scid/Apoe−/− mice accelerates atherosclerotic lesion development [15]. However, CD4+ T cells recognizing epitopes derived from unmodified apolipoprotein B100 (ApoB) has also been found, first in human ApoB100 transgenic mice [22], and more recently in the blood from subjects with and without atherosclerosis, by using an MHC class II-tetramer loaded with an ApoB epitope [23]. Of note, these ApoB100-autoreactive CD4+ T cells are mainly comprised FoxP3+ Tregs in healthy individuals, whereas in patients with subclinical atherosclerosis, they are comprised less of Tregs and more of T-bet+ Th1 and retinoic-acid receptor-related orphan receptor (ROR)-γt+ Th17 cells. Atherosclerosis-related antigens recognized by CD8+ T cells have not yet been identified [16].
Other autoantigens, such as heat-shock proteins (HSPs) 60/65, have been detected in atherosclerotic plaques [24,25], and the transfer of effector T cells from MRL-lpr mice, which develop an autoimmune disease resembling systemic lupus erythematosus (SLE), into Ldlr−/− mice has been shown to increase atherosclerosis [26]. SLE autoantigens are most often of nuclear origin, suggesting that antigens not specific to atherosclerosis can also be proatherogenic. Human epidemiological studies support these experimental data: the risk of cardiovascular disease (CVD) after adjustment to traditional cardiovascular risk factors is increased in patients with autoimmune disorders, including rheumatoid arthritis [27], SLE [28], or psoriasis [29]. More recently, a clinical trial conducted in cancer patients, showing higher risk for atherosclerotic cardiovascular events after treatment with immune checkpoint inhibitors, including anti-programmed death (PD)-1, anti-programmed death ligand (PDL)-1, and anti-cytotoxic T-lymphocyte antigen (CTLA)-4, that activate CD4+ and CD8+ T cells, has provided, for the first time, direct evidence in humans for a pro-atherogenic role of effector T cells in atherosclerosis [30]. Notably, a previous experimental study in Ldlr−/− mice had reported that deficiency in PDL1/2 enhanced atherosclerosis by activating T cells [31].
3. Th1/Th2/Th17 Subsets
Depending on co-stimulatory or -inhibitory signaling, and pro- or anti-inflammatory cytokine production in the micro environment, naïve CD4+ T cells differentiate into various effector or regulatory T cell subsets upon engagement of the TCR with the antigen–MHC-II complex on an antigen-presenting cell (APC).
3.1. Th1 Cells
Th1 polarization is driven by inflammatory cytokines, such as interferon (IFN)-γ and IL-12, which trigger two key lineage-defining transcription factors: T-box transcription factor (TBX)-21 (also referred to as T-bet) and signal transducer and activator of transcription (STAT-4). T-bet induces the optimal production of IFN-γ and expression of the high-affinity IL-12 receptor, while inhibiting the expression of Th2 cytokines, such as IL-4 and IL-5. Th1 cells are critical for immunity against intracellular pathogens, and play a major role in the development of several autoimmune and inflammatory diseases. Two decades of animal studies have yielded evidence that atherosclerosis is a Th1-driven disease. Th1 T cells are the most abundant T-cell subset in human atherosclerotic plaques, [32], secreting IFN-γ, tumor necrosis factor (TNF)-α, and IL-2. The hallmark Th1 cytokine, IFN-γ, exerts several pro-atherogenic actions, promoting both immune and vascular (endothelial and smooth muscle) cell activation [33].
3.2. Th2 Cells
Th2 commitment is mainly induced by IL-6 and IL-13, produced by DCs, as well as OX40–OX40L interaction. Th2 cells secrete IL-4, IL-5, and IL-13. IL-4 activates STAT-6, which induces the expression of the Th2 master transcription factor, GATA-3, crucial for appropriate expression of the Th2-specific cytokines, IL-4 and IL-5. GATA-3 inhibits the production of IFN-γ and Th1 differentiation. The role of Th2 T cells in atherosclerosis remains controversial, and seems to depend on the stage of the disease, the site of the atherosclerotic lesion, the secreted Th2-specific cytokine, and the experimental model [34]. While the role of IL-4 in atherosclerosis remains unclear, other Th2-related cytokines, IL-5, IL-13, and IL-33 appear to exhibit anti-atherogenic properties. It is noteworthy that Th2 T cells share a common secretory profile with a subset of innate immune cells (ILCs), ILC-2, producing IL-5 and IL-13 but not IL-4. ILC-2 has been shown to be atheroprotective through IL-5 and IL-13 production [35].
3.3. Th17 Cells
Th17 commitment requires the nuclear receptors ROR-α and -γt, STAT-3, and runt-related transcription factor 1 (RUNX1). Th17 cells produce large amounts of IL-17A and to a lesser extent IL-17F, IL-22, and IL-23. In mice, TGF-β and IL-6 are required for Th17 differentiation, while IL1-β is instrumental in driving human Th17 differentiation. Next, IL-21 and IL-23 are required for Th17 proliferation and maintenance [36]. IL-6 activates the JAK/STAT-3 pathway, which is required for ROR-γt expression and function. Many experimental studies have shown that Th-specific cytokines and transcription pathways inhibit each other mutually. Th1 and Th2 cytokines, IFN-γ, and IL-4, negatively regulate Th17 differentiation, whereas IL-17 inhibits Th1 polarization, IFN-γ production, and T-bet expression [37]. Th17 cells provide protective immunity against extracellular bacteria and fungi by activating neutrophils with IL-17A and IL-17F. They also contribute to chronic inflammatory and autoimmune diseases, such as experimental autoimmune encephalomyelitis and rheumatoid arthritis [38]. Both IL-17 and IL-17-producing T cells have been detected in murine and human atherosclerotic lesions [39]. The role of IL-17 has been investigated in several mouse models of atherosclerosis, but results are controversial [39]. Interestingly, IL-17 can stimulate collagen synthesis by vascular smooth muscle cells, and increased levels of IL-17 in human carotid plaques are associated with characteristics of plaque stability [39,40]. In patients with ACS, low levels of IL-17 are associated with an increased risk of death and myocardial infarction [41]. In agreement with a pro-stabilizing role of IL-17, severe cardiovascular events have been recently reported in patients with a high cardiovascular risk after the initiation of treatment with ustekinumab (anti-IL-12/23p40 antibody), which targets the IL-17 pathway [42].
4. Treg Cells
Tregs constitute a specific subset of T cells, with several subtypes, including CD4+CD25+FoxP3+ Tregs, IL-10-secreting type 1 FoxP3− Treg (Tr1), TGF-β-secreting type 3 Th cells (Th3), and CD8+ Tregs.
4.1. Treg Ontogeny
To be efficient, the T cell immune response must be tightly regulated, in order to effectively respond to pathogens, prevent responses to self-antigens, and avoid mounting harmful responses to innocuous antigens. Cell-intrinsic mechanisms of tolerance in the thymus and periphery ensure the physical elimination of self-reactive T cells by clonal deletion or their functional inactivation by clonal anergy. In addition, cell-extrinsic mechanisms, mediated in part by specialized CD4+ T cells with immunosuppressive activity, Tregs, help regulate Th cell activity [43]. Naturally-occurring Tregs are generated in the thymus during T cell development. They comprise 5–10% of all peripheral CD4+ T cells. Treg lineage commitment essentially depends on TCR engagement by high avidity for self-peptide–MHC and costimulatory signals mediated by CD28, resulting in the upregulation of CD25. IL-2 or IL-15 are required for fully committed Tregs expressing the forkhead/winged helix transcription factor FoxP3, which is crucial for their development and function. Deficiency of FoxP3 in both humans and mice results in a lack of Tregs, and the development of severe systemic inflammatory diseases manifested by autoimmunity, colitis, and allergies [44]. The second route for the generation of FoxP3+ Tregs, termed induced Tregs (iTregs), is the differentiation of naïve CD4+ T cells in the periphery following TCR stimulation in the presence of IL-2 and TGF-β. Although iTregs compose only a small percentage of Tregs as a whole, this cell subpopulation is particularly enriched in certain tissues, such as the gut and maternal placenta, and crucially participates in the establishment of tolerance against commensal bacteria, foods, allergens, and the fetus in a pregnant mother [45]. In addition to the phenotypic and functional heterogeneity of Tregs, it has been suggested that Tregs can become unstable under certain inflammatory conditions and acquire a phenotype more characteristic of effector T cells, which promotes rather than suppresses inflammation [43].
4.2. Treg Suppressive Activity
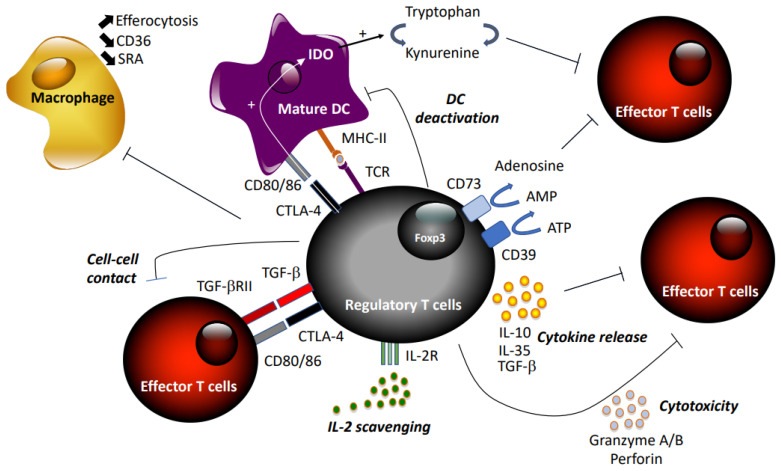
Tregs suppress excess immune responses against a diverse range of antigens, thereby preventing autoimmune diseases and maintaining self-tolerance. They are capable of blocking effector T cells at different stages, inhibiting naïve T cell proliferation, Th1/Th2/Th17 differentiation, and T cell activation [46] through direct interaction or inhibition of APCs (Figure 1). Tregs exert suppressive activity by three different mechanisms: cell–cell contact, local production of inhibitory cytokines, and local competition for growth factors [43]. First, Tregs may kill target T cells by direct receptor–ligand interaction, releasing suppressive factors like cyclic adenosine monophosphate (cAMP) [47], combined or not with suppressive cytokines, including TGF-β [48], via gap junctions. Tregs may also limit effector T cell activity indirectly via modulating APC through reverse signaling via Treg–CTLA-4 engagement of CD80/86 on DCs [49]. The immune-suppressive actions of Tregs may be transmitted through the secretion of the anti-inflammatory cytokines IL-10 [50], TGF-β, and IL-35 [51], or induced production by APCs. In addition, expression of CD73/CD39 by Tregs promotes the local production of adenosine to downregulate immune function [52]. Treg cells can also secrete granzyme A/B and perforin during Treg–effector cell interaction [53]. Finally, Tregs may compete with effector T cells and consume cytokine signaling via receptors that contain the common γ-chain (IL-2, IL-4, and IL-7) [54].
Figure 1.
Summary of suppressive mechanisms of regulatory T cells.
4.3. Tregs and Atheroprotection
The first evidence for a role of CD4+CD25+ Tregs was provided by studies in murine models of atherosclerosis, showing that Treg depletion obtained either by gene deletion (CD80/86, CD28, inducible T-cell costimulator (ICOS)) or following treatment with CD25-neutralizing antibodies exacerbated atherosclerosis [7,55]. Thereafter, evidence from animal and human studies confirmed that decreased Treg numbers or impaired immunosuppressive Treg function promoted atherosclerosis. The precise role of FoxP3+ Tregs was investigated in chimeric DEREG (depletion of regulatory T cells) crossed with Ldlr−/− mice, with specific ablation of FoxP3+ Tregs induced by diphtheria toxin injection. Depletion of FoxP3+ Tregs exacerbated atherosclerosis, but was also associated with higher plasma levels of atherogenic lipoprotein [56]. Conversely, in vivo supplementation with purified spleen CD4+CD25+ Tregs [7,57] or IL-10-producing Tr1-like Tregs [58] reduced atherosclerosis in Apoe−/− mice and induced a more stable plaque phenotype.
CD8+CD25+ Tregs with atheroprotective functions have been found in atherosclerotic plaques of Apoe−/− mice fed a high-fat diet [59]. Another subset of CD8+ Tregs that recognize self-peptides associated with Qa-1, the mouse ortholog of the human class Ib MHC HLA-E, has been identified in murine models of systemic autoimmune disease [60]. These CD8+ Tregs have also been shown to be potent modulators of atherosclerosis in Apoe−/− mice through follicular helper T (Tfh) function in tertiary lymphoid organs in the aorta [61].
Analysis of the time course of Treg infiltration in atherosclerotic lesions indicates that Tregs accumulate in the aorta of Apoe−/− mice after initiation of a high-fat diet, but their numbers decline over time under sustained hypercholesterolemia, whereas effector T cell numbers and atherosclerotic lesion size increase over the same period of time [62]. A recent study shows that under conditions of sustained hypercholesterolemia, a population of IFNγ-producing Tregs, so-called Th1/Tregs, is generated within the aorta and secondary lymphoid organs of aged Apoe−/− mice [63], which might account for the decline in Treg numbers under a prolonged high-fat diet. A similar population of Th1/Treg expressing CCR5 has also been reported in Apoe−/− mice on atherogenic diet for a long period of time (12–20 weeks) [64], supporting that sustained hypercholesterolemia promote plasticity in Tregs [65]. Similarly, during atherosclerosis development in western diet-fed Apoe−/− mice, it has been shown by tracking Treg lineage that a fraction of Treg cells switches their phenotype into pro-atherogenic Tfh cells [66]. Interestingly, ApoAI treatment was able to reduce this switch by maintaining CD25 expression on Treg cells. Altogether, these experimental studies suggest that the initial CD4+ T cell response in atherosclerotic plaque is more oriented toward a Treg phenotype, but may switch overtime toward a pro-atherogenic effector T cell phenotype, likely due to the inflammatory and hypercholesterolemic microenvironment. Sustained hypercholesterolemia likely leads to intracellular accumulation of cholesterol and affects lipid rafts on the Treg membrane, in which CD25 is present, promoting Th1/Th17-like Treg polarization [67].
In human atherosclerotic plaques, Tregs are barely found during all stages of development, with less than 5% of infiltrating T cells being positive for FoxP3 [68]. Description of the immune cell landscape of advanced human atherosclerotic plaques by scRNA-seq technology has confirmed the presence of activated CD8+ and CD4+ Th1 lymphocytes, but identified only a small Treg cluster, based on the expression of FoxP3, CD25, and CTLA-4 [69]. Treg numbers have been shown to be lower in vulnerable than in stable plaques [70], but to be elevated in the thrombus attached to ruptured plaques [71]. Another level of complexity to the role of Tregs in atherosclerosis is that, in contrast to mice, FoxP3 is found in humans in several isoforms, including FoxP3Δ2 lacking exon 2, the dominant form in activated Tregs. Interestingly, the expression of FoxP3Δ2 was decreased in unstable carotid atherosclerotic plaques from patients with ischemic symptoms, compared with stable plaques from asymptomatic patients [72]. In clinical studies, reduced numbers of circulating Tregs have been reported in patients with ACS compared to patients with stable coronary artery disease (CAD) or normal coronary arteries [73,74]. Treg-suppressive functions may also be compromised in patients with ACS, with both FoxP3 and CTLA-4 expression being decreased, as well as ex vivo immune suppressive capacities [73]. Similarly, in a large prospective cohort study, increased risk for myocardial infarction was associated with low levels of circulating Tregs [75]. However, more recently, in two population-based cohorts, no association was found between CD4+CD25+CD127− Tregs in peripheral blood and future myocardial infarction or stable CAD [76].
4.4. Mechanisms of Atheroprotection by Tregs
4.4.1. Secretion of Anti-Inflammatory Cytokines
The mechanisms whereby Tregs protect against atherosclerosis are multiple (Figure 1). The main cytokines produced by Tregs, IL-10 and TGF-β, have strong anti-atherogenic activities in murine models. Gene deletion, or inhibition of IL-10 or TGF-β with neutralizing antibodies, aggravates atherosclerosis in mice and exacerbates effector Th1 and Th2 responses [77,78,79]. However, given that both TGF-β and IL-10 are not exclusively produced by Tregs, but can also be released by macrophages, these studies did not provide evidence of causality between Tregs and protection against atherosclerosis. Yet, overexpression of IL-10 by T cells reduced atherosclerosis in Ldlr−/− mice [80], and inactivation of TGF-β signaling specifically in T cells exaggerated atherosclerosis [81,82], providing evidence for an important role of these cytokines in Treg-dependent atheroprotection.
4.4.2. Modulation of Immune Cell Functions
Tregs prevent T cell polarization into Th1 and Th17 subtypes, and limit their pathogenic activities. They can also modulate macrophage functions. They inhibit the proinflammatory properties of macrophages and shift macrophage differentiation toward an anti-inflammatory phenotype [83,84]. Foam cell formation is reduced when macrophages are co-cultured with Tregs, and CD36 and SRA expression are significantly down-regulated [83]. Tregs also enhance macrophage efferocytosis by secreting IL-13 and subsequent IL-10 production in macrophages [85]. Endothelial cell activation and leukocyte recruitment can also be regulated by Tregs, independent of their immunosuppressive activities on T cells [62]. Monocyte recruitment into atherosclerotic lesions is impeded through the inhibition of MCP1 expression in DCs and macrophages [86].
4.4.3. Tolerogenic DCs
DCs are the most potent APCs, with the unique property of inducing the maturation and differentiation of naïve CD4+ T cells by antigen processing and MHC II presentation, expressing membrane-bound costimulatory molecules, and secreting cytokines [87]. Immature tolerogenic DCs, expressing low levels of costimulatory molecules, promote tolerance by inducing T cell death and apoptosis, or deviation of immune response [88]. Activated or mature DCs can also change to a tolerogenic phenotype in response to anti-inflammatory signals, such as TGF-β or IL-10. Chemokines expressed by DCs have an important effect on the recruitment and development of Tregs: DCs expressing CCL-17 limit Treg expansion and promote atherosclerosis [89]. Inhibitory co-receptors also play important roles in the regulation of immune responses. Hematopoietic deficiency in ICOS or PD-1—inhibitory receptors of the TNF superfamily expressed by T cells—reduces Treg suppressive functions and survival and enhances atherosclerosis [31,55]. The crucial role of DCs in the regulation of adaptive immune responses has also been shown by vaccination strategies against atherosclerosis using DCs. Intravenous administration of DCs pulsed with oxLDL in Ldlr−/− mice attenuated atherosclerosis and stabilized plaque phenotype [90]. Also, injection of DCs pulsed with ApoB-100-derived peptides and IL-10 to induce a tolerogenic phenotype in human ApoB-100-transgenic Ldlr−/− mice has led to diminished atherosclerosis, as well as attenuated systemic and local inflammatory responses [91].
Indoleamine 2,3-dioxygenase (IDO), a rate-limiting enzyme involved in the catabolism of tryptophan, can promote peripheral immune tolerance by inhibiting T cell activation and proliferation [92]. IDO-expressing mature DCs can stimulate proliferation of Foxp3+ Tregs [93]. Conversely, CTLA-4-expressing Tregs have been shown to activate IDO in DCs, which contributes to the maintenance of DC tolerogenic phenotype [94]. Interestingly, injection of DCs rendered tolerogenic by TGFβ2 treatment into hyperlipidemic Ldlr−/− mice promoted the expansion of FoxP3+ Tregs, and was associated increased IDO expression and reduced atherosclerosis [95].
4.4.4. Metabolism
Finally, a large body of evidence indicates that the intracellular metabolism of Tregs controls their suppressive function, stability, and expansion capacity. Further studies are required to better understand how intracellular metabolism might be involved in the atheroprotective effects of Tregs [67].
5. Strategies to Promote Atheroprotective T Cell Immunity
A prior study by our group in the late 90s showing that the administration of ovalbumin-specific Tr1 cells, together with their cognate antigen, reduced atherosclerosis in Apoe−/− mice was the first demonstration that manipulation of Tregs is a promising anti-atherosclerotic strategy. Subsequently, other strategies were developed in experimental models of atherosclerosis, based on antigen-specific or -non-specific approaches to promote Treg activation, expansion, survival, or suppressive functions, and protect against atherosclerosis (Table 1).
Table 1.
Strategies developed in animals to expand athero-protective regulatory T cells. Apoe, Apolipoprotein e; CD: Cluster of Differentiation; DC, dendritic cells; FoxP3, Forkhead/winged helix transcription factor 3; G-CSF, Granulocyte- Colony-Stimulating Factor; HSP, Heat Shock Protein; IFN-γ: Interferon-γ; IL, InterLeukin; LAP, Latency-associated peptide; LDLr, Low Density Lipoprotein receptor; MDA-LDL, Malondialdehyde-LDL; TGF-β, Transforming growth factor- β; Th, T Helper; TNF-α, Tumor Necrosis Factor- α; Treg, regulatory T cell.
| Antigen | Route of Delivery | Species | Immune Effects | References | |
|---|---|---|---|---|---|
| Antigen-specific induction | MDA-LDL Copper-oxidized LDL |
Subcutaneous + Freund’s adjuvant |
Rabbit | Increased auto-antibodies titers | [97,98] |
| apoB-derived peptides | Subcutaneous | Apoe−/− mice | Increased Foxp3+ Tregs Decreased Th1 and Th2 signature |
[101] | |
| apoB-derived peptides | Subcutaneous + Freund’s adjuvant |
Apoe−/− mice | Increased Foxp3+ CCR5+Tregs Increased IL-10 production |
[100] | |
| Ox- and MDA-LDL | Oral route | Ldlr−/− mice | Increased Foxp3+Tregs Increased TGF-β production |
[102] | |
| apoB-derived peptide (aBp210) | Subcutaneous + Alum |
Apoe−/− mice | Increased Foxp3+ CD25+ Tregs Decreased Th1 and Th2 signature |
[104] | |
| ApoB100 | Loaded on DC stimulated by IL-10 |
HuB100(tg) X Ldlr−/− mice |
Decreased T effector proliferation Decreased Th1 signature |
[91] | |
| HSP 60 | Oral route | Ldlr−/− mice | Increased Foxp3+ CD25+Tregs Increased IL-10, TGF-β production |
[103] | |
| ApoB100 peptide + HSP60 peptide + Chlamydophila pneumoniae peptide |
Subcutaneous + Alum |
Apobtm2Sgy
Ldlr tm1Her J mice |
Increased Foxp3+ CD4+ T cells Increased IL-10 and TGF-β production Decreased IFN-γ and TNF-α production |
[105] | |
| Non-antigen specific induction | Anti-CD3 alone or combined with IL-2 | Intravenous | Ldlr−/− mice | Increased Foxp3+Tregs Decreased IFN-γ and TNF-α production |
[106] |
| Anti-CD3 | Oral route | Apoe−/− mice | Increased Foxp3+Tregs Decreased Th1 and Th2 signature Increased TGF-β production |
[107] | |
| G-CSF | Subcutaneous | Apoe−/− mice | Increased CD4+ CD25+Tregs | [108] | |
| vitamin D3 | Oral route | Apoe−/− mice | Increased Foxp3+Tregs Decreased CD80+CD86+ DC Increased IL-10 production |
[109] | |
| FTY720 | Oral route | Apoe−/− mice | Increased LAP+Foxp3+ cells Decreased Th1 signature Increased TGF-β production |
[110] | |
| Nucleoprotein of measles virus | Intraperitoneal | Apoe−/− mice | Decreased T effector proliferation Decreased Th1 signature Increased IL-10 production |
[111] | |
| Low IL-2 | Intravenous | Apoe−/− mice | Increased Foxp3+ CD25+ Tregs | [112] | |
| Intraperitoneal | Ldlr−/− mice | Increased Foxp3+ CD25+ Tregs Decreased Th1 and Th2 signature |
[113] | ||
| Intraperitoneal | Apoe−/− mice | Increased Foxp3+ CD25+ Tregs Decreased Th1, Th2, Th17 signature |
[114] |
5.1. Antigen-Specific Induction of Tregs
The induction of antigen-specific immunological tolerance has the potential to inhibit harmful immune responses to self- or allogeneic antigens, while preserving the integrity of the remaining immune system. Different strategies of immunization with LDL, oxLDL, or ApoB-related peptides have proved beneficial in experimental models of atherosclerosis [96]. Unexpected observations of reduced atherosclerosis in hypercholesterolemic rabbits immunized with oxLDL were the first indications that it could be possible to use a “vaccine” approach to treat atherosclerosis [97,98]. Subsequent studies have identified certain peptide sequences in Apo-B-100 as major antigens responsible for the immunization-induced, pro-atherosclerotic response [99,100]. Subcutaneous administration of apoB-derived peptides in Apoe−/− mice induces antigen-specific Treg expansion, inhibits effector T cell responses, and decreases atherosclerosis [101]. Oral treatment with oxLDL promotes the expansion of Tregs in secondary lymphoid organs and inhibits atherosclerosis in Ldlr−/− mice [102,103]. Atherosclerosis is reduced by the adoptive transfer of tolerogenic DCs pulsed with human ApoB-100 and IL-10 in transgenic Ldlr−/− mice expressing human ApoB [91]. Notably, depletion of Tregs prevents in Apoe−/− mice the atheroprotective effect of immunization with ApoB-peptides [104]. Interestingly, immunization of Ldlr−/− mice with multi-antigenic epitopes from ApoB100, hHSP60, and Chlamydophila pneumoniae, with alum as adjuvant, was more effective in inhibiting atherosclerosis than single- or bi-epitope vaccine, associated with a stronger specific Treg response [105]. Altogether, these preclinical studies provide evidence that an antigen-specific Treg response can be induced by immunization to protect against atherosclerosis (Table 1).
5.2. Non-Antigen-Specific Induction of Tregs
5.2.1. Anti-CD3
Following preclinical and clinical studies showing that treatment with anti-CD3 monoclonal antibodies can restore immune tolerance in type 1 diabetes [115], this strategy was applied in experimental atherosclerosis. Anti-CD3 antibody treatment reduced atherosclerosis in Ldlr−/− [106] and Apoe−/− mice [107], and enhanced the expression of Foxp3 in spleen cells [106]. Oral administration of anti-CD3 also protected against atherosclerosis, as a result of the selective expansion of Tregs expressing latent associated protein (LAP), the amino-terminal domain of the TGF-β precursor peptide [116]. In line with these findings, in vivo neutralization of TGF-β prevented the atheroprotective effects of anti-CD3 antibodies [117].
5.2.2. G-CSF
The growth factor granulocyte-colony-stimulating factor (G-CSF), often used during cancer treatments to correct chemotherapy-induced neutropenia, has been shown to modulate T cell and DC functions. Interestingly, treatment with G-CSF inhibits atherosclerosis in Apoe−/− mice, increases the number of Tregs in lymph nodes, spleen, and atherosclerotic lesions, and enhances their suppressive function in vitro [108].
5.2.3. Vitamin D3
Based on studies showing that calcitriol, the active form of vitamin D3, is a potent immunomodulator, its effect was evaluated in atherosclerosis. Oral treatment with calcitriol reduced atherosclerotic lesions in Apoe−/− mice via the induction of Tregs and tolerogenic DCs [109].
5.2.4. FTY720
Similarly, the sphingosine-1-phosphate receptor agonist FTY720 (Fingolimod), which increases the functional activity of Tregs, inhibits atherosclerosis in Apoe−/− when orally administered at low doses by the expansion of Tregs, reduces effector T cell responses, and increases TGF-β expression [110].
5.2.5. mTOR
Another way to regulate Treg differentiation and function is through mammalian target of rapamycin (mTOR) signaling. Rapamycin, an inhibitor of mTOR, has potent immunosuppressive properties, induces Treg expansion, and depletes effector T cells [118]. Recently, it has been shown in Ldlr−/− mice that increased intracellular cholesterol in Tregs due to T cell–specific deficiency in ABCG1 leads to mTOR inhibition, which promotes Treg development and results in protection against atherosclerosis [119].
5.2.6. Measles Virus
The nucleoprotein of the measles virus has been shown to block DC activation [120]. Treatment of Apoe−/− mice with this viral nucleoprotein markedly reduces atherosclerosis and induces a Tr1-type regulatory immune response characterized by enhanced IL-10 but reduced IFN-γ and IL-4 production [111].
5.2.7. Low-Dose IL-2
IL-2 is essential for Treg homeostasis in vivo [38]. IL-2 combined with the IL-2-specific monoclonal antibody (JES6-1) has been shown to specifically expand FoxP3+ Tregs in mice [121]. Notably, in clinical studies, low-dose IL-2 has increased circulating Treg levels in patients with chronic graft-versus-host disease, with no effect on effector T cell levels [122]. A substantial proportion of treated patients showed significant clinical improvement. In patients with autoimmune vasculitis induced by hepatitis C virus, treatment with was also able to improve clinical symptoms and increased the percentage of circulating FoxP3+ Tregs [123]. A similar beneficial effect of low-dose IL-2 treatment was observed in patients with type 1 diabetes [124]. In Apoe−/− mice, local delivery of IL-2 to atherosclerotic lesions [112] or treatment with IL-2/anti-IL-2 mAb immunocomplexes [113,114] lead to a reduction in atherosclerosis due to Treg expansion. These findings in experimental atherosclerosis are being translated into clinical applications, with the first ongoing double-blind, placebo-controlled phase I/II clinical trial, LILACS, aiming to assess the safety and efficacy of low-dose IL-2 in patients with stable ischemic heart disease and in patients with ACS [125].
6. Conclusions
The three positive clinical trials in the last years, CANTOS [1], ColCot [2], and LoDoCo2 [3], have provided exciting evidence that targeting inflammation reduces the risk of another cardiovascular event in patients who had a prior heart attack. The anti-inflammatory agents used in these trials, anti-IL-1β and Colchicine, act on the innate immune system. Yet there is a large body of evidence accumulated from animal and human studies to indicate that adaptive immune responses also contribute to the initiation and progression of atherosclerosis, and that Tregs are crucial atheroprotective modulators. Strategies that boost the development of Tregs by using biologicals or induce their antigen-specific generation permit the specific blockade of the deleterious effects of self-reactive immune destruction while maintaining the ability of the immune system to clear non-self-antigens. Several prototype atherosclerosis vaccines have demonstrated beneficial effects in experimental animal models by inducing Treg-dependent tolerance against LDL-associated antigens. In this respect, they resemble the immunization strategies developed for the prevention of other autoimmune diseases. However, translating these strategies from the bench to the bedside for the treatment of atherosclerosis is still challenging, and represents a stimulating endeavor, which ultimately may improve cardiac outcomes in patients with CVD.
Another exciting approach for treatment of atherosclerosis is Treg therapy, based on a phenomenon known as “infectious tolerance” [126]. The principle is that the anti-inflammatory and tolerogenic effects of Tregs promote the differentiation of recipient T cells into new Tregs, even after the transferred Tregs are eliminated. Adoptive cell therapy with Tregs is a promising therapeutic strategy to treat autoimmune diseases, because sustained tolerance and suppression of inflammation is ultimately mediated by the recipient’s own immune system [127]. In humans, Treg therapy was pioneered in hematopoietic stem cell transplantation [128,129], and has subsequently been shown to be safe in a number of transplant- and autoimmune-related diseases [130]. New phase II trials are ongoing to treat autoimmune diseases and prevent graft rejection [131]. Preclinical studies have shown that the adoptive transfer of Tr1-like Tregs reduces experimental atherosclerosis [58]. The development of similar Treg-based therapeutic strategies in humans could be a promising, attractive approach to combat atherothrombotic diseases.
Abbreviations
| ABCG1 | ATP-binding cassette G1 |
| ACS | Acute coronary syndrome |
| APC | Antigen-presenting cell |
| APOE | Apolipoprotein E |
| CCL-17 | Chemokine (C-C motif) ligand-17 |
| CD | Cluster of differentiation |
| CTLA-4 | Cytotoxic T-lymphocyte antigen 4 |
| CVD | Cardiovascular disease |
| DC | Dendritic cell |
| FoxP3 | Forkhead/winged helix transcription factor |
| G-CSF | Granulocyte-colony-stimulating factor |
| HSP | Heat shock protein |
| ICOS | Inducible T cell co-stimulator |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN-γ | Interferon- γ |
| IL | Interleukin |
| ILC | Innate lymphoid cell |
| LDLr | Low-density lipoprotein receptor |
| LPS | Lipopolysaccharide |
| MCP1 | Monocyte chemoattractant protein 1 |
| MDA-LDL | Malondialdehyde–LDL |
| MHC | Major histocompatibility complex |
| mTOR | Mammalian target of rapamycin |
| oxLDL | Oxidized low-density lipoprotein |
| PD-1 | Programmed death-1 |
| PDL-1 | Programmed death ligand-1 |
| RAG | Recombination activating gene |
| ROR | Retinoic-acid receptor-related orphan receptor |
| RUNX1 | Runt-related transcription factor 1 |
| SCID | Severe combined immunodeficiency |
| SLE | Systemic lupus erythematosus |
| SRA | Scavenger receptor class A |
| STAT | Signal transducer and activator of transcription |
| T-bet | T-box expressed in T cell |
| TBX | T-box transcription factor |
| TCR | T cell receptor |
| TGF-β | Transforming growth factor-β |
| TLO | Tertiary lymphoid organ |
| TNF-α | Tumor necrosis factor-α |
| Treg | Regulatory T cell |
Author Contributions
Review concept and design, all authors. Drafting of the manuscript, all authors. Critical revision of manuscript, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
H.A.-O., and A.T. are supported by Institut National de la Santé et de la Recherche Médicale (INSERM). J.R.L. is supported by the ANR ERA-CVD program (JTC2018 MEMORY).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 2.Tardif J.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P., Pinto F.J., Ibrahim R., Gamra H., Kiwan G.S., et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 3.Nidorf S.M., Fiolet A.T.L., Mosterd A., Eikelboom J.W., Schut A., Opstal T.S.J., The S.H.K., Xu X.F., Ireland M.A., Lenderink T., et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis-an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Ait-Oufella H., Sage A.P., Mallat Z., Tedgui A. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ. Res. 2014;114:1640–1660. doi: 10.1161/CIRCRESAHA.114.302761. [DOI] [PubMed] [Google Scholar]
- 6.Sage A.P., Tsiantoulas D., Binder C.J., Mallat Z. The role of B cells in atherosclerosis. Nat. Rev. Cardiol. 2019;16:180–196. doi: 10.1038/s41569-018-0106-9. [DOI] [PubMed] [Google Scholar]
- 7.Ait-Oufella H., Salomon B.L., Potteaux S., Robertson A.K., Gourdy P., Zoll J., Merval R., Esposito B., Cohen J.L., Fisson S., et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 8.Roselaar S.E., Kakkanathu P.X., Daugherty A. Lymphocyte populations in atherosclerotic lesions of ApoE -/- and LDL receptor -/- mice—Decreasing density with disease progression. Arterioscler. Thromb. Vasc. Biol. 1996;16:1013–1018. doi: 10.1161/01.ATV.16.8.1013. [DOI] [PubMed] [Google Scholar]
- 9.Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Atherosclerosis. 1986;6:131–138. doi: 10.1161/01.ATV.6.2.131. [DOI] [PubMed] [Google Scholar]
- 10.Jonasson L., Holm J., Skalli O., Gabbiani G., Hansson G.K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J. Clin. Investig. 1985;76:125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkels H., Ehinger E., Vassallo M., Buscher K., Dinh H.Q., Kobiyama K., Hamers A.A.J., Cochain C., Vafadarnejad E., Saliba A.E., et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ. Res. 2018;122:1675–1688. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zernecke A., Winkels H., Cochain C., Williams J.W., Wolf D., Soehnlein O., Robbins C.S., Monaco C., Park I., McNamara C.A., et al. Meta-Analysis of Leukocyte Diversity in Atherosclerotic Mouse Aortas. Circ. Res. 2020;127:402–426. doi: 10.1161/CIRCRESAHA.120.316903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez D.M., Rahman A.H., Fernandez N.F., Chudnovskiy A., Amir E.D., Amadori L., Khan N.S., Wong C.K., Shamailova R., Hill C.A., et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019;25:1576–1588. doi: 10.1038/s41591-019-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimokama T., Haraoka S., Watanabe T. Immunohistochemical and ultrastructural demonstration of the lymphocyte-macrophage interaction in human aortic intima. Mod. Pathol. 1991;4:101–107. [PubMed] [Google Scholar]
- 15.Zhou X., Nicoletti A., Elhage R., Hansson G.K. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.CIR.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 16.Schafer S., Zernecke A. CD8(+) T Cells in Atherosclerosis. Cells. 2020;10:37. doi: 10.3390/cells10010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyaw T., Winship A., Tay C., Kanellakis P., Hosseini H., Cao A., Li P., Tipping P., Bobik A., Toh B.H. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation. 2013;127:1028–1039. doi: 10.1161/CIRCULATIONAHA.112.001347. [DOI] [PubMed] [Google Scholar]
- 18.Cochain C., Koch M., Chaudhari S.M., Busch M., Pelisek J., Boon L., Zernecke A. CD8+ T Cells Regulate Monopoiesis and Circulating Ly6C-high Monocyte Levels in Atherosclerosis in Mice. Circ. Res. 2015;117:244–253. doi: 10.1161/CIRCRESAHA.117.304611. [DOI] [PubMed] [Google Scholar]
- 19.Paulsson G., Zhou X., Tornquist E., Hansson G.K. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2000;20:10–17. doi: 10.1161/01.ATV.20.1.10. [DOI] [PubMed] [Google Scholar]
- 20.Elhage R., Gourdy P., Brouchet L., Jawien J., Fouque M.J., Fievet C., Huc X., Barreira Y., Couloumiers J.C., Arnal J.F., et al. Deleting TCR alpha beta+ or CD4+ T lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein E-deficient mice. Am. J. Pathol. 2004;165:2013–2018. doi: 10.1016/S0002-9440(10)63252-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stemme S., Faber B., Holm J., Wiklund O., Witztum J.L., Hansson G.K. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermansson A., Ketelhuth D.F., Strodthoff D., Wurm M., Hansson E.M., Nicoletti A., Paulsson-Berne G., Hansson G.K. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T., Kobiyama K., Winkels H., Tse K., Miller J., Vassallo M., Wolf D., Ryden C., Orecchioni M., Dileepan T., et al. Regulatory CD4(+) T Cells Recognize Major Histocompatibility Complex Class II Molecule-Restricted Peptide Epitopes of Apolipoprotein B. Circulation. 2018;138:1130–1143. doi: 10.1161/CIRCULATIONAHA.117.031420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Q., Willeit J., Marosi M., Kleindienst R., Oberhollenzer F., Kiechl S., Stulnig T., Luef G., Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–259. doi: 10.1016/0140-6736(93)92613-X. [DOI] [PubMed] [Google Scholar]
- 25.Almanzar G., Ollinger R., Leuenberger J., Onestingel E., Rantner B., Zehm S., Cardini B., van der Zee R., Grundtman C., Wick G. Autoreactive HSP60 epitope-specific T-cells in early human atherosclerotic lesions. J. Autoimmun. 2012;39:441–450. doi: 10.1016/j.jaut.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelm A.J., Rhoads J.P., Wade N.S., Major A.S. Dysregulated CD4+ T cells from SLE-susceptible mice are sufficient to accelerate atherosclerosis in LDLr-/- mice. Ann. Rheum. Dis. 2015;74:778–785. doi: 10.1136/annrheumdis-2013-203759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roman M.J., Moeller E., Davis A., Paget S.A., Crow M.K., Lockshin M.D., Sammaritano L., Devereux R.B., Schwartz J.E., Levine D.M., et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann. Intern. Med. 2006;144:249–256. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- 28.Roman M.J., Shanker B.A., Davis A., Lockshin M.D., Sammaritano L., Simantov R., Crow M.K., Schwartz J.E., Paget S.A., Devereux R.B., et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 29.Gelfand J.M., Neimann A.L., Shin D.B., Wang X., Margolis D.J., Troxel A.B. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 30.Drobni Z.D., Alvi R.M., Taron J., Zafar A., Murphy S.P., Rambarat P.K., Mosarla R.C., Lee C., Zlotoff D.A., Raghu V.K., et al. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotsman I., Grabie N., Dacosta R., Sukhova G., Sharpe A., Lichtman A.H. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J. Clin. Investig. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frostegard J., Ulfgren A.K., Nyberg P., Hedin U., Swedenborg J., Andersson U., Hansson G.K. Cytokine expression in advanced human atherosclerotic plaques: Dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/S0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 33.Tedgui A., Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ait-Oufella H., Taleb S., Mallat Z., Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 35.Newland S.A., Mohanta S., Clement M., Taleb S., Walker J.A., Nus M., Sage A.P., Yin C., Hu D., Kitt L.L., et al. Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nat. Commun. 2017;8:15781. doi: 10.1038/ncomms15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuniga L.A., Jain R., Haines C., Cua D.J. Th17 cell development: From the cradle to the grave. Immunol. Rev. 2013;252:78–88. doi: 10.1111/imr.12036. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor W., Jr., Zenewicz L.A., Flavell R.A. The dual nature of T(H)17 cells: Shifting the focus to function. Nat. Immunol. 2010;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 38.Littman D.R., Rudensky A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Taleb S., Tedgui A., Mallat Z. IL-17 and Th17 cells in atherosclerosis: Subtle and contextual roles. Arterioscler. Thromb. Vasc. Biol. 2015;35:258–264. doi: 10.1161/ATVBAHA.114.303567. [DOI] [PubMed] [Google Scholar]
- 40.Gistera A., Robertson A.K., Andersson J., Ketelhuth D.F., Ovchinnikova O., Nilsson S.K., Lundberg A.M., Li M.O., Flavell R.A., Hansson G.K. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci. Transl. Med. 2013;5:196ra100. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- 41.Simon T., Taleb S., Danchin N., Laurans L., Rousseau B., Cattan S., Montely J.M., Dubourg O., Tedgui A., Kotti S., et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J. 2013;34:570–577. doi: 10.1093/eurheartj/ehs263. [DOI] [PubMed] [Google Scholar]
- 42.Poizeau F., Nowak E., Kerbrat S., Le Nautout B., Droitcourt C., Drici M.D., Sbidian E., Guillot B., Bachelez H., Ait-Oufella H., et al. Association Between Early Severe Cardiovascular Events and the Initiation of Treatment With the Anti-Interleukin 12/23p40 Antibody Ustekinumab. JAMA Derm. 2020;156:1208–1215. doi: 10.1001/jamadermatol.2020.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakaguchi S., Mikami N., Wing J.B., Tanaka A., Ichiyama K., Ohkura N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020;38:541–566. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 44.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 45.Kanamori M., Nakatsukasa H., Okada M., Lu Q., Yoshimura A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol. 2016;37:803–811. doi: 10.1016/j.it.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Sojka D.K., Huang Y.H., Fowell D.J. Mechanisms of regulatory T-cell suppression—A diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bopp T., Becker C., Klein M., Klein-Hessling S., Palmetshofer A., Serfling E., Heib V., Becker M., Kubach J., Schmitt S., et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura K., Kitani A., Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asseman C., Mauze S., Leach M.W., Coffman R.L., Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collison L.W., Workman C.J., Kuo T.T., Boyd K., Wang Y., Vignali K.M., Cross R., Sehy D., Blumberg R.S., Vignali D.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 52.Deaglio S., Dwyer K.M., Gao W., Friedman D., Usheva A., Erat A., Chen J.F., Enjyoji K., Linden J., Oukka M., et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grossman W.J., Verbsky J.W., Barchet W., Colonna M., Atkinson J.P., Ley T.J. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Pandiyan P., Zheng L., Ishihara S., Reed J., Lenardo M.J. CD4(+)CD25(+)Foxp3(+) regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4(+) T cells. Nat. Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 55.Gotsman I., Grabie N., Gupta R., Dacosta R., MacConmara M., Lederer J., Sukhova G., Witztum J.L., Sharpe A.H., Lichtman A.H. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 56.Klingenberg R., Gerdes N., Badeau R.M., Gistera A., Strodthoff D., Ketelhuth D.F., Lundberg A.M., Rudling M., Nilsson S.K., Olivecrona G., et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Investig. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng J., Zhang Z., Kong W., Liu B., Xu Q., Wang X. Regulatory T cells ameliorate hyperhomocysteinaemia-accelerated atherosclerosis in apoE-/- mice. Cardiovasc. Res. 2009;84:155–163. doi: 10.1093/cvr/cvp182. [DOI] [PubMed] [Google Scholar]
- 58.Mallat Z., Gojova A., Brun V., Esposito B., Fournier N., Cottrez F., Tedgui A., Groux H. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2003;108:1232–1237. doi: 10.1161/01.CIR.0000089083.61317.A1. [DOI] [PubMed] [Google Scholar]
- 59.Zhou J., Dimayuga P.C., Zhao X., Yano J., Lio W.M., Trinidad P., Honjo T., Cercek B., Shah P.K., Chyu K.Y. CD8(+)CD25(+) T cells reduce atherosclerosis in apoE(-/-) mice. Biochem. Biophys. Res. Commun. 2014;443:864–870. doi: 10.1016/j.bbrc.2013.12.057. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa H., Wang L., Cantor H., Kim H.J. New Insights Into the Biology of CD8 Regulatory T Cells. Adv. Immunol. 2018;140:1–20. doi: 10.1016/bs.ai.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Clement M., Guedj K., Andreata F., Morvan M., Bey L., Khallou-Laschet J., Gaston A.T., Delbosc S., Alsac J.M., Bruneval P., et al. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation. 2015;131:560–570. doi: 10.1161/CIRCULATIONAHA.114.010988. [DOI] [PubMed] [Google Scholar]
- 62.Maganto-Garcia E., Tarrio M.L., Grabie N., Bu D.X., Lichtman A.H. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–195. doi: 10.1161/CIRCULATIONAHA.110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butcher M.J., Filipowicz A.R., Waseem T.C., McGary C.M., Crow K.J., Magilnick N., Boldin M., Lundberg P.S., Galkina E.V. Atherosclerosis-Driven Treg Plasticity Results in Formation of a Dysfunctional Subset of Plastic IFNgamma+ Th1/Tregs. Circ. Res. 2016;119:1190–1203. doi: 10.1161/CIRCRESAHA.116.309764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J., McArdle S., Gholami A., Kimura T., Wolf D., Gerhardt T., Miller J., Weber C., Ley K. CCR5+T-bet+FoxP3+ Effector CD4 T Cells Drive Atherosclerosis. Circ. Res. 2016;118:1540–1552. doi: 10.1161/CIRCRESAHA.116.308648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ali A.J., Makings J., Ley K. Regulatory T Cell Stability and Plasticity in Atherosclerosis. Cells. 2020;9:2665. doi: 10.3390/cells9122665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaddis D.E., Padgett L.E., Wu R., McSkimming C., Romines V., Taylor A.M., McNamara C.A., Kronenberg M., Crotty S., Thomas M.J., et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun. 2018;9:1095. doi: 10.1038/s41467-018-03493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baardman J., Lutgens E. Regulatory T Cell Metabolism in Atherosclerosis. Metabolites. 2020;10:279. doi: 10.3390/metabo10070279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Boer O.J., van der Meer J.J., Teeling P., van der Loos C.M., van der Wal A.C. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS ONE. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Depuydt M.A.C., Prange K.H.M., Slenders L., Ord T., Elbersen D., Boltjes A., de Jager S.C.A., Asselbergs F.W., de Borst G.J., Aavik E., et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ. Res. 2020;127:1437–1455. doi: 10.1161/CIRCRESAHA.120.316770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dietel B., Cicha I., Voskens C.J., Verhoeven E., Achenbach S., Garlichs C.D. Decreased numbers of regulatory T cells are associated with human atherosclerotic lesion vulnerability and inversely correlate with infiltrated mature dendritic cells. Atherosclerosis. 2013;230:92–99. doi: 10.1016/j.atherosclerosis.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Klingenberg R., Brokopp C.E., Grives A., Courtier A., Jaguszewski M., Pasqual N., Vlaskou Badra E., Lewandowski A., Gaemperli O., Hoerstrup S.P., et al. Clonal restriction and predominance of regulatory T cells in coronary thrombi of patients with acute coronary syndromes. Eur. Heart J. 2015;36:1041–1048. doi: 10.1093/eurheartj/eht543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joly A.L., Seitz C., Liu S., Kuznetsov N.V., Gertow K., Westerberg L.S., Paulsson-Berne G., Hansson G.K., Andersson J. Alternative Splicing of FOXP3 Controls Regulatory T Cell Effector Functions and Is Associated With Human Atherosclerotic Plaque Stability. Circ. Res. 2018;122:1385–1394. doi: 10.1161/CIRCRESAHA.117.312340. [DOI] [PubMed] [Google Scholar]
- 73.Mor A., Luboshits G., Planer D., Keren G., George J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur. Heart J. 2006;27:2530–2537. doi: 10.1093/eurheartj/ehl222. [DOI] [PubMed] [Google Scholar]
- 74.Potekhina A.V., Pylaeva E., Provatorov S., Ruleva N., Masenko V., Noeva E., Krasnikova T., Arefieva T. Treg/Th17 balance in stable CAD patients with different stages of coronary atherosclerosis. Atherosclerosis. 2015;238:17–21. doi: 10.1016/j.atherosclerosis.2014.10.088. [DOI] [PubMed] [Google Scholar]
- 75.Wigren M., Bjorkbacka H., Andersson L., Ljungcrantz I., Fredrikson G.N., Persson M., Bryngelsson C., Hedblad B., Nilsson J. Low levels of circulating CD4+FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler. Thromb. Vasc. Biol. 2012;32:2000–2004. doi: 10.1161/ATVBAHA.112.251579. [DOI] [PubMed] [Google Scholar]
- 76.Olson N.C., Sitlani C.M., Doyle M.F., Huber S.A., Landay A.L., Tracy R.P., Psaty B.M., Delaney J.A. Innate and adaptive immune cell subsets as risk factors for coronary heart disease in two population-based cohorts. Atherosclerosis. 2020;300:47–53. doi: 10.1016/j.atherosclerosis.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mallat Z., Besnard S., Duriez M., Deleuze V., Emmanuel F., Bureau M.F., Soubrier F., Esposito B., Duez H., Fievet C., et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999;85:e17–e24. doi: 10.1161/01.RES.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 78.Caligiuri G., Rudling M., Ollivier V., Jacob M.P., Michel J.B., Hansson G.K., Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol. Med. 2003;9:10–17. doi: 10.1007/BF03402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Potteaux S., Esposito B., Van Oostrom O., Brun V., Ardouin P., Groux H., Tedgui A., Mallat Z. Leukocyte-Derived Interleukin 10 Is Required for Protection Against Atherosclerosis in Low-Density Lipoprotein Receptor Knockout Mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 80.Pinderski L.J., Fischbein M.P., Subbanagounder G., Fishbein M.C., Kubo N., Cheroutre H., Curtiss L.K., Berliner J.A., Boisvert W.A. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 2002;90:1064–1071. doi: 10.1161/01.RES.0000018941.10726.FA. [DOI] [PubMed] [Google Scholar]
- 81.Robertson A.K., Rudling M., Zhou X., Gorelik L., Flavell R.A., Hansson G.K. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J. Clin. Investig. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gojova A., Brun V., Esposito B., Cottrez F., Gourdy P., Ardouin P., Tedgui A., Mallat Z., Groux H. Specific abrogation of transforming growth factor-beta signaling in T cells alters atherosclerotic lesion size and composition in mice. Blood. 2003;102:4052–4058. doi: 10.1182/blood-2003-05-1729. [DOI] [PubMed] [Google Scholar]
- 83.Lin J., Li M., Wang Z., He S., Ma X., Li D. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J. Lipid Res. 2010;51:1208–1217. doi: 10.1194/jlr.D000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tiemessen M.M., Jagger A.L., Evans H.G., van Herwijnen M.J., John S., Taams L.S. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Proto J.D., Doran A.C., Gusarova G., Yurdagul A., Jr., Sozen E., Subramanian M., Islam M.N., Rymond C.C., Du J., Hook J., et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity. 2018;49:666–677 e666. doi: 10.1016/j.immuni.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Subramanian M., Thorp E., Hansson G.K., Tabas I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Investig. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 88.Bakdash G., Sittig S.P., van Dijk T., Figdor C.G., de Vries I.J. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front. Immunol. 2013;4:53. doi: 10.3389/fimmu.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weber C., Meiler S., Doring Y., Koch M., Drechsler M., Megens R.T., Rowinska Z., Bidzhekov K., Fecher C., Ribechini E., et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Investig. 2011;121:2898–2910. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Habets K.L., van Puijvelde G.H., van Duivenvoorde L.M., van Wanrooij E.J., de Vos P., Tervaert J.W., van Berkel T.J., Toes R.E., Kuiper J. Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in LDL receptor-deficient mice. Cardiovasc. Res. 2010;85:622–630. doi: 10.1093/cvr/cvp338. [DOI] [PubMed] [Google Scholar]
- 91.Hermansson A., Johansson D.K., Ketelhuth D.F., Andersson J., Zhou X., Hansson G.K. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–1091. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 92.Mellor A.L., Lemos H., Huang L. Indoleamine 2,3-Dioxygenase and Tolerance: Where Are We Now? Front. Immunol. 2017;8:1360. doi: 10.3389/fimmu.2017.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung D.J., Rossi M., Romano E., Ghith J., Yuan J., Munn D.H., Young J.W. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fallarino F., Grohmann U., Hwang K.W., Orabona C., Vacca C., Bianchi R., Belladonna M.L., Fioretti M.C., Alegre M.L., Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 95.Forteza M.J., Polyzos K.A., Baumgartner R., Suur B.E., Mussbacher M., Johansson D.K., Hermansson A., Hansson G.K., Ketelhuth D.F.J. Activation of the Regulatory T-Cell/Indoleamine 2,3-Dioxygenase Axis Reduces Vascular Inflammation and Atherosclerosis in Hyperlipidemic Mice. Front. Immunol. 2018;9:950. doi: 10.3389/fimmu.2018.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nettersheim F.S., De Vore L., Winkels H. Vaccination in Atherosclerosis. Cells. 2020;9:2560. doi: 10.3390/cells9122560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Palinski W., Miller E., Witztum J.L. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc. Natl. Acad. Sci. USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ameli S., Hultgardhnilsson A., Regnstrom J., Calara F., Yano J., Cercek B., Shah P.K., Nilsson J. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler. Thromb. Vasc. Biol. 1996;16:1074–1079. doi: 10.1161/01.ATV.16.8.1074. [DOI] [PubMed] [Google Scholar]
- 99.Fredrikson G.N., Hedblad B., Berglund G., Alm R., Ares M., Cercek B., Chyu K.Y., Shah P.K., Nilsson J. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2003;23:872–878. doi: 10.1161/01.ATV.0000067935.02679.B0. [DOI] [PubMed] [Google Scholar]
- 100.Kimura T., Tse K., McArdle S., Gerhardt T., Miller J., Mikulski Z., Sidney J., Sette A., Wolf D., Ley K. Atheroprotective vaccination with MHC-II-restricted ApoB peptides induces peritoneal IL-10-producing CD4 T cells. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H781–H790. doi: 10.1152/ajpheart.00798.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herbin O., Ait-Oufella H., Yu W., Fredrikson G.N., Aubier B., Perez N., Barateau V., Nilsson J., Tedgui A., Mallat Z. Regulatory T-cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:605–612. doi: 10.1161/ATVBAHA.111.242800. [DOI] [PubMed] [Google Scholar]
- 102.van Puijvelde G.H., Hauer A.D., de Vos P., van den Heuvel R., van Herwijnen M.J., van der Zee R., van Eden W., van Berkel T.J., Kuiper J. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- 103.Van Puijvelde G.H., van Es T., van Wanrooij E.J., Habets K.L., de Vos P., van der Zee R., van Eden W., van Berkel T.J., Kuiper J. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- 104.Wigren M., Kolbus D., Duner P., Ljungcrantz I., Soderberg I., Bjorkbacka H., Fredrikson G.N., Nilsson J. Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J. Intern. Med. 2011;269:546–556. doi: 10.1111/j.1365-2796.2010.02311.x. [DOI] [PubMed] [Google Scholar]
- 105.Lu X., Xia M., Endresz V., Faludi I., Szabo A., Gonczol E., Mundkur L., Chen D., Kakkar V. Impact of multiple antigenic epitopes from ApoB100, hHSP60 and Chlamydophila pneumoniae on atherosclerotic lesion development in Apob(tm2Sgy)Ldlr(tm1Her)J mice. Atherosclerosis. 2012;225:56–68. doi: 10.1016/j.atherosclerosis.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 106.Steffens S., Burger F., Pelli G., Dean Y., Elson G., Kosco-Vilbois M., Chatenoud L., Mach F. Short-term treatment with anti-CD3 antibody reduces the development and progression of atherosclerosis in mice. Circulation. 2006;114:1977–1984. doi: 10.1161/CIRCULATIONAHA.106.627430. [DOI] [PubMed] [Google Scholar]
- 107.Kasahara K., Sasaki N., Yamashita T., Kita T., Yodoi K., Sasaki Y., Takeda M., Hirata K. CD3 antibody and IL-2 complex combination therapy inhibits atherosclerosis by augmenting a regulatory immune response. J. Am. Heart Assoc. 2014;3:e000719. doi: 10.1161/JAHA.113.000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uchiyama R., Hasegawa H., Kameda Y., Ueda K., Kobayashi Y., Komuro I., Takano H. Role of regulatory T cells in atheroprotective effects of granulocyte colony-stimulating factor. J. Mol. Cell Cardiol. 2012;52:1038–1047. doi: 10.1016/j.yjmcc.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 109.Takeda M., Yamashita T., Sasaki N., Nakajima K., Kita T., Shinohara M., Ishida T., Hirata K. Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler. Thromb. Vasc. Biol. 2010;30:2495–2503. doi: 10.1161/ATVBAHA.110.215459. [DOI] [PubMed] [Google Scholar]
- 110.Huang K., Li S.Q., Wang W.J., Liu L.S., Jiang Y.G., Feng P.N., Wang Y.Q., Wang S.M. Oral FTY720 administration induces immune tolerance and inhibits early development of atherosclerosis in apolipoprotein E-deficient mice. Int. J. Immunopathol. Pharm. 2012;25:397–406. doi: 10.1177/039463201202500209. [DOI] [PubMed] [Google Scholar]
- 111.Ait-Oufella H., Horvat B., Kerdiles Y., Herbin O., Gourdy P., Khallou-Laschet J., Merval R., Esposito B., Tedgui A., Mallat Z. Measles virus nucleoprotein induces a regulatory immune response and reduces atherosclerosis in mice. Circulation. 2007;116:1707–1713. doi: 10.1161/CIRCULATIONAHA.107.699470. [DOI] [PubMed] [Google Scholar]
- 112.Dietrich T., Hucko T., Schneemann C., Neumann M., Menrad A., Willuda J., Atrott K., Stibenz D., Fleck E., Graf K., et al. Local delivery of IL-2 reduces atherosclerosis via expansion of regulatory T cells. Atherosclerosis. 2012;220:329–336. doi: 10.1016/j.atherosclerosis.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 113.Foks A.C., Frodermann V., ter Borg M., Habets K.L., Bot I., Zhao Y., van Eck M., van Berkel T.J., Kuiper J., van Puijvelde G.H. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi: 10.1016/j.atherosclerosis.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 114.Dinh T.N., Kyaw T.S., Kanellakis P., To K., Tipping P., Toh B.H., Bobik A., Agrotis A. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation. 2012;126:1256–1266. doi: 10.1161/CIRCULATIONAHA.112.099044. [DOI] [PubMed] [Google Scholar]
- 115.Belghith M., Bluestone J.A., Barriot S., Megret J., Bach J.F., Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 116.Sasaki N., Yamashita T., Takeda M., Shinohara M., Nakajima K., Tawa H., Usui T., Hirata K. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation. 2009;120:1996–2005. doi: 10.1161/CIRCULATIONAHA.109.863431. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X., Reddy J., Ochi H., Frenkel D., Kuchroo V.K., Weiner H.L. Recovery from experimental allergic encephalomyelitis is TGF-beta dependent and associated with increases in CD4+LAP+ and CD4+CD25+ T cells. Int. Immunol. 2006;18:495–503. doi: 10.1093/intimm/dxh390. [DOI] [PubMed] [Google Scholar]
- 118.Huang H., Long L., Zhou P., Chapman N.M., Chi H. mTOR signaling at the crossroads of environmental signals and T-cell fate decisions. Immunol. Rev. 2020;295:15–38. doi: 10.1111/imr.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheng H.Y., Gaddis D.E., Wu R., McSkimming C., Haynes L.D., Taylor A.M., McNamara C.A., Sorci-Thomas M., Hedrick C.C. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J. Clin. Investig. 2016;126:3236–3246. doi: 10.1172/JCI83136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marie J.C., Kehren J., Trescol-Biemont M.C., Evlashev A., Valentin H., Walzer T., Tedone R., Loveland B., Nicolas J.F., Rabourdin-Combe C., et al. Mechanism of measles virus-induced suppression of inflammatory immune responses. Immunity. 2001;14:69–79. doi: 10.1016/S1074-7613(01)00090-5. [DOI] [PubMed] [Google Scholar]
- 121.Webster K.E., Walters S., Kohler R.E., Mrkvan T., Boyman O., Surh C.D., Grey S.T., Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: Induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koreth J., Matsuoka K., Kim H.T., McDonough S.M., Bindra B., Alyea E.P., 3rd, Armand P., Cutler C., Ho V.T., Treister N.S., et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Saadoun D., Rosenzwajg M., Joly F., Six A., Carrat F., Thibault V., Sene D., Cacoub P., Klatzmann D. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 124.Hartemann A., Bensimon G., Payan C.A., Jacqueminet S., Bourron O., Nicolas N., Fonfrede M., Rosenzwajg M., Bernard C., Klatzmann D. Low-dose interleukin 2 in patients with type 1 diabetes: A phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 125.Zhao T.X., Kostapanos M., Griffiths C., Arbon E.L., Hubsch A., Kaloyirou F., Helmy J., Hoole S.P., Rudd J.H.F., Wood G., et al. Low-dose interleukin-2 in patients with stable ischaemic heart disease and acute coronary syndromes (LILACS): Protocol and study rationale for a randomised, double-blind, placebo-controlled, phase I/II clinical trial. BMJ Open. 2018;8:e022452. doi: 10.1136/bmjopen-2018-022452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Waldmann H., Adams E., Fairchild P., Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol. Rev. 2006;212:301–313. doi: 10.1111/j.0105-2896.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 127.Sullivan J.A., AlAdra D.P., Olson B.M., McNeel D.G., Burlingham W.J. Infectious Tolerance as Seen With 2020 Vision: The Role of IL-35 and Extracellular Vesicles. Front. Immunol. 2020;11:1867. doi: 10.3389/fimmu.2020.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Di Ianni M., Falzetti F., Carotti A., Terenzi A., Castellino F., Bonifacio E., Del Papa B., Zei T., Ostini R.I., Cecchini D., et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 129.Brunstein C.G., Miller J.S., McKenna D.H., Hippen K.L., DeFor T.E., Sumstad D., Curtsinger J., Verneris M.R., MacMillan M.L., Levine B.L., et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: Kinetics, toxicity profile, and clinical effect. Blood. 2016;127:1044–1051. doi: 10.1182/blood-2015-06-653667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferreira L.M.R., Muller Y.D., Bluestone J.A., Tang Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 2019;18:749–769. doi: 10.1038/s41573-019-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romano M., Fanelli G., Albany C.J., Giganti G., Lombardi G. Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity. Front. Immunol. 2019;10:43. doi: 10.3389/fimmu.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.