Abstract
Angiotensin II (Ang II) has various cardiac effects and causes vasoconstriction. Ang II activates the type-1 angiotensin receptor—Gq/11 signaling pathway resulting in the release of 2-arachidonoylglycerol (2-AG). We aimed to investigate whether cardiac Ang II effects are modulated by 2-AG-release and to identify the role of type-1 cannabinoid receptors (CB1R) in these effects. Expression of CB1R in rat cardiac tissue was confirmed by immunohistochemistry. To characterize short-term Ang II effects, increasing concentrations of Ang II (10−9–10−7 M); whereas to assess tachyphylaxis, repeated infusions of Ang II (10−7 M) were administered to isolated Langendorff-perfused rat hearts. Ang II infusions caused a decrease in coronary flow and ventricular inotropy, which was more pronounced during the first administration. CB agonist 2-AG and WIN55,212-2 administration to the perfusate enhanced coronary flow. The flow-reducing effect of Ang II was moderated in the presence of CB1R blocker O2050 and diacylglycerol-lipase inhibitor Orlistat. Our findings indicate that Ang II-induced cardiac effects are modulated by simultaneous CB1R-activation, most likely due to 2-AG-release during Ang II signalling. In this combined effect, the response to 2-AG via cardiac CB1R may counteract the positive inotropic effect of Ang II, which may decrease metabolic demand and augment Ang II-induced coronary vasoconstriction.
Keywords: Angiotensin II, cardiac, endocannabinoid, CB1 cannabinoid receptor, myocardial function, coronary flow, vasoconstriction
1. Introduction
The renin-angiotensin-aldosterone system (RAAS) plays a key regulatory role in cardiovascular and salt-water homeostasis. Angiotensin II (Ang II) is the main effector molecule of the RAAS. Ang II is an octapeptide hormone produced by the angiotensin-converting enzyme. It plays a crucial role in numerous physiological and pathological processes involving aldosterone secretion, cell-proliferation, inflammation and atherosclerosis [1,2,3]. In the cardiovascular system, its peripheral vasoconstrictor and central pressor effects lead to the elevation of systemic blood pressure [3]. Direct short-term cardiac effects of Ang II include a decrease in coronary flow (CF) and controversial effects on contractility [4,5,6,7,8,9]. Moreover, Ang II produced by the locally activated RAAS has adverse effects on the remodelling process of the vascular and cardiac tissues in various pathophysiological conditions [3,10].
Ang II activates type-1 and type-2 angiotensin receptors (AT1R and AT2R), among which AT1R mediates its major short-term and long-term actions [2]. Ang II binding to AT1R causes AT1R interaction with heterotrimeric G proteins, including Gq/11, G12/13, and Gi [2,11]. Coupling to Gq/11 classically leads to phospholipase C stimulation and activation of downstream inositol-triphosphate and diacylglycerol dependent pathways [2]. Additionally, Ang II-mediated Gq/11 activation-dependent signalling can lead to endocannabinoid formation and release [12,13].
Endocannabinoids (i.e., endogenous cannabinoids, such as arachidonoyl ethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG) are produced by several cell types, and by acting on type-1 and type-2 cannabinoid receptors (CB1R and CB2R), they exert diverse biological effects [14,15,16,17,18,19] including tissue-specific local regulatory mechanisms [20]. In the cardiovascular system, negative inotropic, hypotensive and cardioprotective actions of cannabinoids have been previously reported [21,22,23,24,25,26,27], in which endothelium-derived nitric oxide and effects from perivascular nerves are involved [18,28].
We have previously reported that activation of AT1R can cause 2-AG-mediated paracrine transactivation of CB1R in cells expressing both AT1 and CB1 receptors. Ang II-induced CB1R activation was inhibited by diacylglycerol-lipase (DAGL) inhibitors, suggesting that diacylglycerol is converted to 2-AG by DAGL during the signalling of calcium-mobilizing hormones [29]. We also investigated this mechanism in the vascular tissue and found that the vasoconstrictor effect of Ang II was attenuated via Gq/11-mediated vascular endocannabinoid formation [15].
Cardiac AT1R activation has utmost relevance in heart (patho)physiology [3,30,31,32]; however, it is unknown whether concomitant 2-AG production and CB1R activation modulate these effects [29]. The main aim of this study was to reveal the participation of paracrine endocannabinoid mechanisms in Ang II signalling in the heart. For this purpose, we investigated how inhibition of 2-AG formation and CB1R activation modify the effects of Ang II administration in isolated Langendorff-perfused rat hearts. As short-term actions of both Ang II and 2-AG in the heart are complex and controversial [4,5,6,7,8,9], we first characterized the responses of the isolated rat heart to these mediators in our experimental setting.
2. Materials and Methods
2.1. Animals
The experiments were performed on isolated hearts of adult male Sprague-Dawley rats weighing 300–350 g. Animals were bred and housed in the animal facility at Semmelweis University, kept in a 12/12-h diurnal cycle with free access to water and standard rat chow. All applied procedures conform to the guidelines of the Hungarian Law of Animal Protection (28/1998) and were approved by the Government Office of Pest County (Permission number: PEI/001/820-2/2015 and PE/EA/1428-7/2018).
2.2. Langendorff Heart Preparation
Animals were anesthetised by intraperitoneal injection of 40 mg/kg pentobarbital (Euthasol 40%; Produlab Pharma BV, Raamsdonksveer, The Netherlands) before the excision of the heart. The isolated hearts were cannulated and perfused using a gravitational Langendorff apparatus (Experimetria Ltd., Budapest, Hungary) at constant, 70 mmHg pressure with a modified Krebs–Henseleit buffer. A detailed description of the perfusion and methodology of measuring hemodynamic parameters in a Langendorff-perfused heart preparation was given previously [33,34,35]. Briefly, left ventricular pressure (LVP) was recorded using a fluid-filled balloon catheter introduced into the ventricle and connected to a pressure gauge (Experimetria Ltd., Budapest, Hungary), whereas coronary flow (CF) was continuously monitored with a transit-time flow probe placed into the inflow line (Transonic 2-PXN flow probe, TS410-tubing flow module Transonic Systems Inc., Ithaca, NY, USA) [33,34,35].
Data acquisition and analysis were performed using the Haemosys software (Experimetria Ltd., Budapest, Hungary). Left ventricular developed pressure (LVDevP) was calculated as the difference between peak systolic and minimum diastolic pressures. The positive and negative maximum values of the first derivative of the LVP (+dLVP/dtmax, −dLVP/dtmax) were determined as indices of left ventricular contractile and lusitropic performance, respectively [33,34,35].
2.3. Experimental Protocol
After cannulation of the isolated heart, a 30-min equilibration period was allowed. Afterward, baseline data were recorded, and the respective substances or their vehicles were infused as described previously [33].
To describe the effects of Ang II on the CF and contractile function of isolated Langendorff-perfused rat hearts, we obtained concentration-response relationship curves by infusing Ang II (Sigma-Aldrich, Budapest, Hungary) dissolved in calcium-free Krebs–Henseleit buffer in increasing concentrations (10−9, 3 × 10−9, 10−8, 10−7 M). Each concentration was administered for 3 min.
We also assessed the effects of repeated Ang II treatments. For this purpose, we infused Ang II repetitively four times, each time for 3 min at a concentration of 10−7 M. Between the consecutive infusion periods, the hearts were allowed to equilibrate for 10 min. During this period, all measured parameters returned to control values.
The endocannabinoid 2-AG (Sigma-Aldrich, Budapest, Hungary) and the CB receptor agonist WIN55,212-2 (Sigma-Aldrich, Budapest, Hungary) were infused into isolated hearts for 5 min at a concentration of 10−6 M to assess the influence of CB receptor activation on CF and heart function. 2-AG administration was also repeated in the presence of the CB1R inhibitor O2050 (10−6 M Cayman Chemicals, Ann Arbor, MI, USA). After the first 2-AG infusion, the heart was allowed to equilibrate for 10 min. O2050 infusion was initiated 5 min before the second 2-AG infusion, then continuously coadministered during agonist treatment.
In order to assess the role of the CB1R and DAGL activation in mediating the effects of Ang II, Ang II was administered in the presence of the CB1R inhibitor O2050 (10−6 M Cayman Chemicals, Ann Arbor, MI, USA) and the DAGL inhibitor Orlistat (10−5 M) (tetrahydrolipstatin, Sigma-Aldrich, Budapest, Hungary) or their vehicles in separate experiments. In these protocols, after the first Ang II (10−7 M) infusion, Ang II (10−7 M) was repeatedly applied together with the antagonists. Each Ang II infusion lasted for 3 min, and the cardiac parameters were allowed to return to control values between the first and second infusion protocols.
2.4. Immunohistochemistry
Isolated hearts of 2 rats and 2 mice (CB1R knockout (−/−, CB1R-KO) and wild type (+/+, C57BL/6, Cnr1tm1zim) mice (25–30 g) which were kindly provided by Professor Andreas Zimmer, University of Bonn [36]), were fixed in 4% paraformaldehyde right after excision for 24 h and then placed in 10 and 20% sucrose solutions (15–18 h each) for cryoprotection. The tissues were quickly frozen on dry ice as described previously [37].
Cryostat sections (12-µm-thick cross-sections from the heart tissue) were mounted on Super Colorfrost slides (Thermo Fisher Scientific, Waltham, MA, USA). Sections were blocked with 1% bovine serum albumin (BSA) for 15 min. Endogenous peroxidase activity was eliminated using 3% H2O2 solution for 15 min; immunostaining was performed by applying CB1R primary antibody (1:500, Cayman Chemicals, Ann Arbor, MI, overnight). The reaction was developed using the standard ABC method (Vector Labs, Burlington, CA, USA). Diaminobenzidine was used for visualization.
2.5. Immunofluorescent Visualization of CB1 Receptors in Rat Cardiac Tissue
Two rat hearts fixed in 10% neutral-buffered formalin were dehydrated, embedded in paraffin, ~2 µm thick serial sections were cut, mounted on silanized glass slides, and kept in a thermostat at 65 °C for 1 h. Sections were dewaxed and rehydrated. For antigen retrieval, heating for 20 min in Tris-ethylenediaminetetraacetic acid (EDTA) buffer pH 9.0 (0.1 M Tris-base and 0.01 M EDTA) using an Avair electric pressure cooker (ELLA 6 LUX(D6K2A), Bitalon Ltd., Pécs, Hungary) followed by a 20 min cooling with an open lid was applied. Non-specific proteins were blocked in 3 % bovine serum albumin (BSA, #82-100-6, Millipore, Kankakee, IL, USA) diluted in 0.1 M Tris-buffered saline (TBS, pH 7.4) containing 0.01% sodium-azide for 30 min [38]. The sections were incubated with the primary antibodies (Anti-Troponin T (Cardiac Muscle) clone 9C2.1 (mouse), 1:50 dilution, Sigma-Aldrich; and CB1 Receptor Polyclonal Antibody (rabbit), 1:250 dilution, Cayman Chemicals, Ann Arbor, MI, USA) diluted in 1% BSA/TBS+TWEEN (TBST, pH 7.4) for 2 h. Afterward, Alexa 546 goat anti-mouse (Thermo Fisher Scientific, Waltham, MA, USA) and Alexa 488 goat anti-rabbit (Thermo Fisher Scientific, Waltham, MA, USA) secondary fluorescent antibodies (dilution 1:100) were used for 1h incubation. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fischer Scientific, Waltham, MA, USA; 1:100) for 10 min. Stained sections were covered with aqueous mounting medium (BrightMountPlus, ab103748, Abcam). Slides were imaged using an inverted microscope (Nikon Ti2) equipped with a 60× oil immersion objective (Plan Apo lambda, N.A. 1.4) plus a 1.5× intermediate magnification and a cooled sCMOS camera (Zyla 4.2, Andor Technology).
2.6. Statistical Analysis and Interpretation
To identify the effects of the time factor in experiments with a single treatment, one-way repeated measures ANOVA or the equivalent non-parametric test and Dunnett’s multiple comparison post-hoc test were used. In experiments with several treatment groups, two-way repeated measurement ANOVA was used. This was completed by Dunnett’s multiple comparison test to isolate which treatment groups differ from others. In O2050 + Ang II experiments, we supplemented this analysis with a comparison of data obtained at identical time points of the experiments using a t-probe. Maximal Ang II effects in Orlistat + Ang II experiments were compared with a t-test. P < 0.05 was accepted throughout as a level of significance. Results are expressed as mean ± SEM. Statistical analyses were performed using SigmaStat 3.5 (Systat Software Inc., San Jose, CA, USA).
3. Results
3.1. CB1 Receptors in Rat Cardiac Tissue
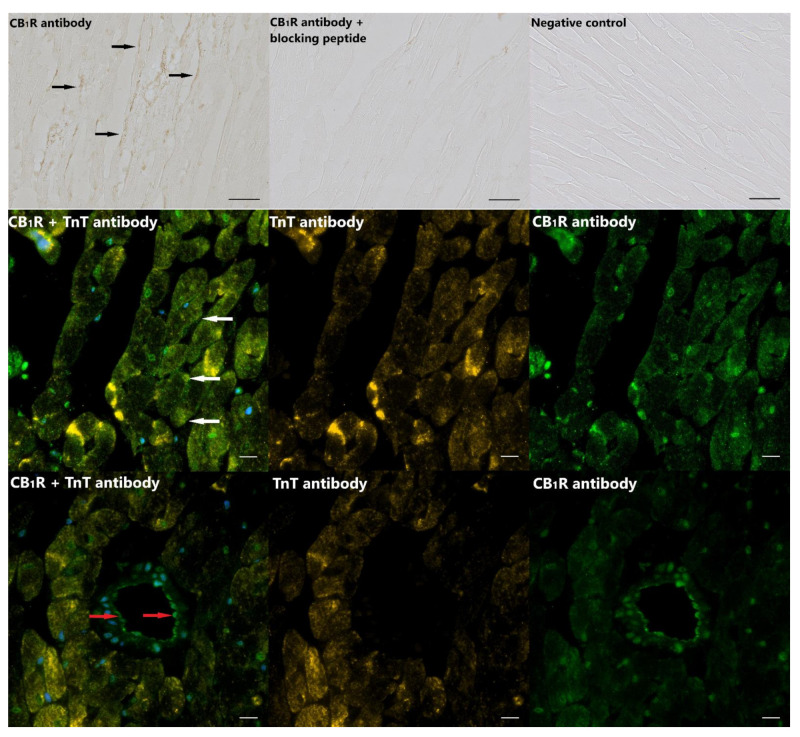
Assessment of CB1R staining by immunohistochemistry confirmed the expression of CB1 receptors in rat cardiac tissue (Figure 1, upper panel). Immunofluorescent visualization of CB1R in cardiac tissue sections revealed CB1R localization on the sarcolemma of cardiomyocytes (Figure 1, middle and lower panels). Moreover, CB1R staining was also detectable in the vascular wall in cross-sectional images of small vessels (Figure 1, lower panel). CB1R staining was also present in murine cardiac tissue but was not detectable in hearts of CB1R knockout mice (Supplementary Figure S1).
Figure 1.
Type-1 cannabinoid receptor (CB1R) expression and localization in rat cardiac tissue. Upper panel: Immunohistochemical localization of the CB1R protein in cardiac tissue of rats (black arrows). No staining was detected in the absence of the primary antibody (negative control), and a robust decrease in immunostaining was observed when the CB1R primary antibody was applied together with blocking peptide; scale bar: 20 µm. Middle and lower panels: Immunofluorescent staining of CB1R (green) and cardiac Troponin T (TnT, orange) revealed CB1R localization on the sarcolemma of cardiomyocytes (white arrows). CB1R staining was also detectable in the wall of vessels (red arrows); scale bar: 10 µm.
3.2. The Effects of Ang II Infusion on Isolated Rat Hearts
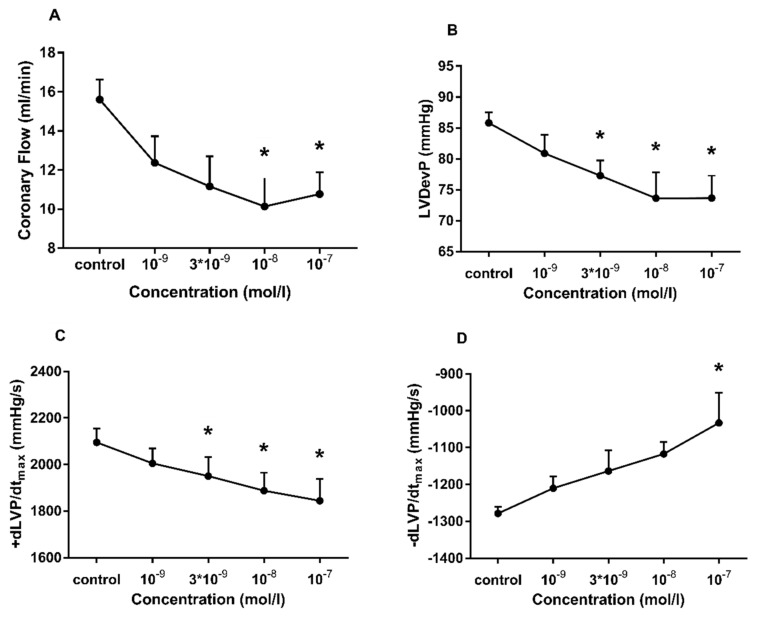
To characterize the effect of Ang II on CF and contractile function, we carried out concentration-response experiments on isolated Langendorff-perfused hearts. Administration of Ang II resulted in a concentration-dependent decrease in CF (Figure 2A). Along with CF deprivation, deterioration of left ventricular contractile performance could be observed, which is evidenced by the declining inotropic (LVDevP, +dP/dtmax) (Figure 2B,C) and lusitropic (−dP/dtmax) function (Figure 2D). It is notable that the relative decreases in inotropic and lusitropic function were moderate compared to the relative decline in CF (CF: 31 ± 12%, LVDevP: 14 ± 9%, +dLVP/dtmax: 12 ± 8%, −dLVP/dtmax: 19 ± 13% at a concentration of 10−7 M).
Figure 2.
Concentration-dependent effects of angiotensin II (Ang II) on coronary flow (A), left ventricular developed pressure (LVDevP) (B), +dLVP/dtmax (C) and −dLVP/dtmax (D) of isolated rat hearts. In these experiments, Ang II was applied in the range of 10−9 to 10−7 M. The increasing concentrations of Ang II were infused into the perfusion line consecutively, each for 3 min. Maximal effects measured during the 3-min infusion periods are presented. Mean ± SEM; n = 4; * p < 0.05 vs. control (pre-infusion value); one-way repeated measurement ANOVA or ANOVA on ranks and Dunnett’s post-hoc test.
3.3. Effects of Repetitive Ang II Infusion on Coronary Flow and Contractile Function
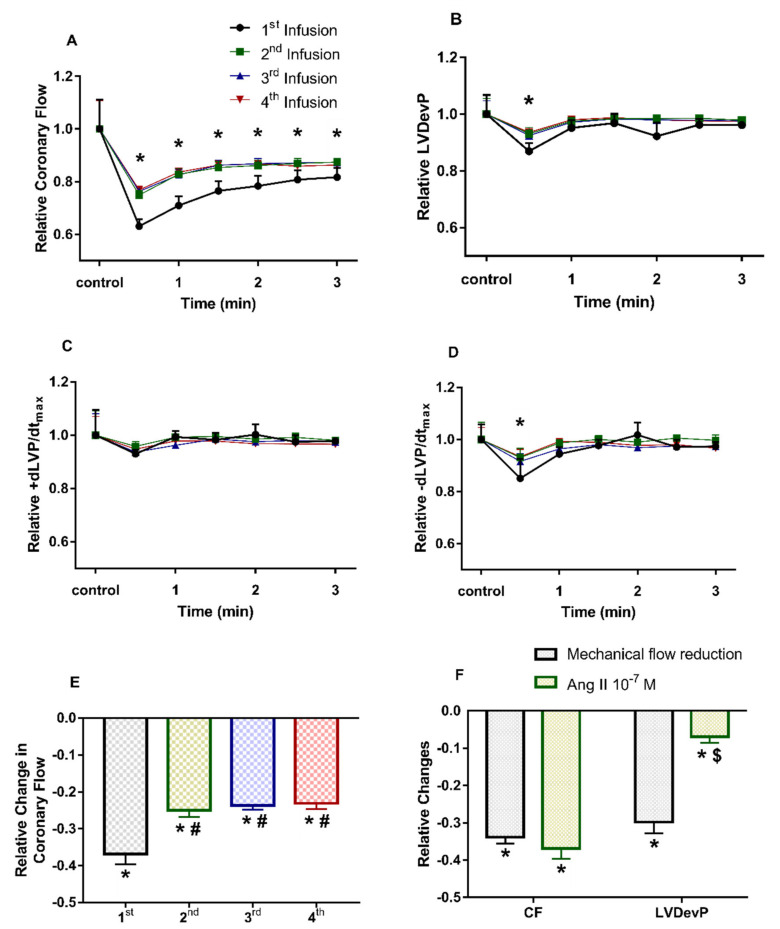
To assess the kinetics of Ang II effects on isolated hearts and to judge the impact of tachyphylaxis on the interpretation of our findings, we infused Ang II (10−7 M) repetitively four times. The first administration of Ang II resulted in a more pronounced decline in CF than the subsequent interventions. However, from the second infusion on, the consecutive Ang II effects did not differ from each other significantly (Figure 3A–D).
Figure 3.
Effects of repetitive angiotensin II (Ang II) infusion on coronary flow (A), left ventricular developed pressure (LVDevP) (B), +dLVP/dtmax (C) and −dLVP/dtmax (D) of isolated rat hearts. Maximal decrease in coronary flow during repetitive infusion periods (E). Decrease in LVDevP when the coronary flow is reduced either mechanically by compressing the perfusion line (grey) or by the vasoactive effect of Ang II (green) (F). Ang II (10−7 M) was administered repetitively to the perfusate of isolated rat hearts (A–E). Each Ang II infusion period lasted for 3 min and was followed by a 10-min wash-out period. Mean ± SEM; n = 4; * p < 0.05 vs. control (pre-infusion value) in each group; one-way repeated measurement ANOVA and Dunnett’s post-hoc test; # p < 0.05 vs. first infusion period; one-way ANOVA and Dunnett’s post-hoc test; $ p < 0.05 vs. relative decrease in coronary flow, paired t-test.
As to the kinetics of Ang II effects, it can be observed that at each administration, the decline in CF reached its maximum level in 1 min. This decrease in CF was associated with a moderate decline in LVDevP and lusitropic performance in the first minute of the Ang II administration (Figure 3B,D). However, later during the infusion period LVDevP and −dP/dtmax values gradually rose and approached control values in spite of the reduced CF. This might be attributed to the direct inotropic effects of Ang II, because a decrease in CF elicited by compression of the perfusion line entails a decrease of the same magnitude in LVDevP (Figure 3F).
3.4. Effects of CB Receptor Agonists and CB1R Antagonist O2050 on Coronary Flow and Contractile Function
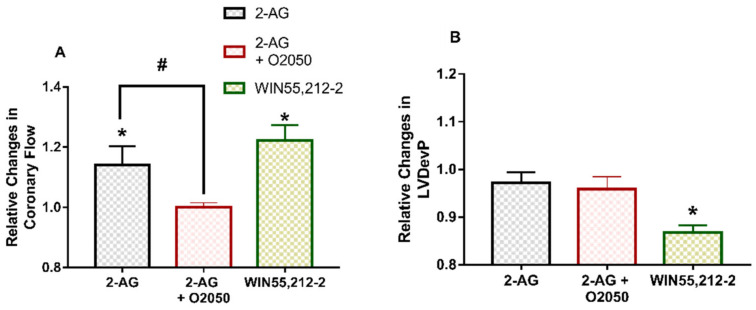
In separate experiments, we also assessed the effects of 2-AG and synthetic CB receptor agonist WIN55,212-2. Both CB receptor agonists enhanced CF (Figure 4A). 2-AG administration had no significant effect on contractile performance; however, WIN55,212-2 produced a marked decline in LVDevP (Figure 4B). 2-AG was also applied in the presence of CB1R neutral antagonist O2050. The CF-increasing effect of 2-AG was prevented by O2050 (Figure 4A). Repeated infusions of 10−6 M 2-AG proved to have identical effects in separate experiments (data not shown). We also tested the effects of O2050 infusion alone, and it did not have any significant effect on the cardiac parameters studied (data not shown).
Figure 4.
Peak effects of 2-arachydonoyl-glycerol (2-AG) and synthetic CB receptor agonist WIN55,212-2 on coronary flow (A) and left ventricular developed pressure (LVDevP) (B) of isolated rat hearts. 2-AG (10−6 M) and WIN55,212-2 (10−6 M) were administered to the perfusate for 5 min. After a 10-min wash-out period, the 2-AG infusion was repeated in the presence of CB1R antagonist O2050 (10−6 M). Data are presented as relative values compared to pre-infusion control data. Mean ± SEM; n = 9 (2-AG and 2-AG + O2050 experiments) and n = 5 (WIN55,212-2 experiments); * p < 0.05 vs. pre-infusion value; # p < 0.05 vs. 2-AG peak effect, paired t-test.
3.5. The Influence of CB1R Antagonist O2050 and DAGL Inhibitor Orlistat on Ang II Effects
To test how 2-AG-release during AT1R—Gq/11 signalling may modify the primary Ang II responses in the heart via potential transactivation of CB1 receptors, Ang II was also infused in the presence of CB1R antagonist O2050 (Figure 5) and DAGL inhibitor Orlistat (Figure 6).
Figure 5.
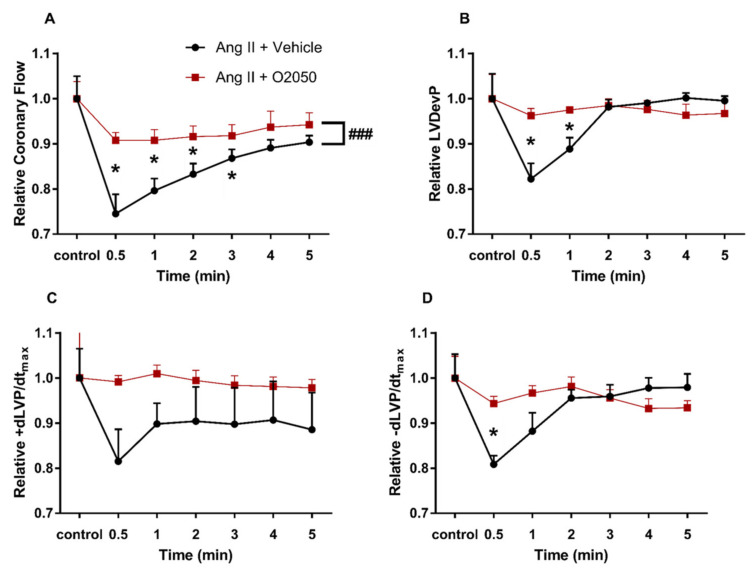
Influence of CB1R inhibitor O2050 on the effects of angiotensin II (Ang II) on coronary flow (A), left ventricular developed pressure (LVDevP) (B), +dLVP/dtmax (C) and −dLVP/dtmax (D) of isolated rat hearts. Ang II (10−7 M) was administered repetitively to the perfusate. The presented data were recorded during the second infusion period, when Ang II was infused either in the presence of CB1R inhibitor O2050 (10−6 M) or its vehicle and are expressed as relative values compared to pre-infusion control data. Mean ± SEM; n = 7 & 8; * p < 0.05 vs. control (pre-infusion value); ### p < 0.001 vs. vehicle; two-way repeated measurement ANOVA and Dunnett’s post-hoc test.
Figure 6.
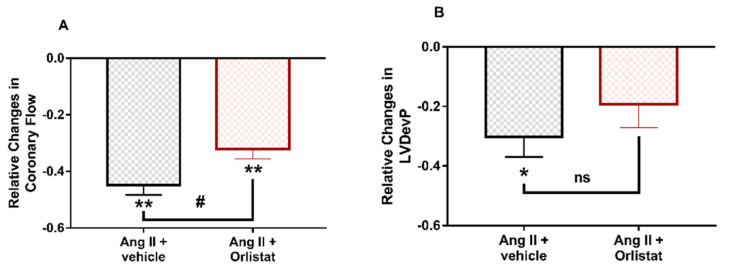
Influence of diacylglycerol-lipase (DAGL) inhibitor Orlistat on the peak effects of angiotensin II (Ang II) on coronary flow (A) and left ventricular developed pressure (LVDevP) (B) of isolated rat hearts. Ang II (10−7 M) was administered repetitively to the perfusate. The presented data were recorded during the second infusion period when Ang II was infused either in the presence of DAGL inhibitor Orlistat (10−5 M) or its vehicle and are expressed as relative values compared to pre-infusion control data. Mean ± SEM; n = 4 & 6; * p < 0.05 and ** p < 0.01 vs. control (pre-infusion value) paired t-test; # p < 0.05 and ns: non-significant vs. Ang II +vehicle; t-test.
The presence of O2050 in the perfusate substantially altered the effects of Ang II. The deep decline in CF was attenuated by O2050 (Figure 5A). In addition, the temporary decrease in inotropic and lusitropic function, which paralleled the decrease in CF during the first 90 s of the ‘Ang II + vehicle’ infusion was completely abolished when CB1 receptors were blocked by O2050 (Figure 5B–D).
Inhibition of DAGL, the enzyme which is supposed to produce 2-AG from DAG during Gq/11-coupled signalling of Ang II, moderated the Ang II-induced peak reduction in coronary flow (Figure 6A) and the significant decrease in LVDevP was not observable (Figure 6B).
4. Discussion
The main finding of this study is that in an isolated rat heart preparation with intact circulation, the cardiac responses to Ang II are mediated in part by CB1 receptor activation, most probably through 2-AG release during angiotensin receptor type-1—Gq/11 signalling. In our experiments, Ang II induced a concentration-dependent decrease in CF, which was associated with a moderate decrease in cardiac function. These effects were effectively blocked by CB1 receptor and DAGL inhibition. Since cardiac CB1 receptors were also detected by immunohistochemistry in our experimental setting, this observation proves a functional vascular/hemodynamic effect of Ang II, which is partially exerted via coronary/cardiac CB1 receptors. In addition to this observation, direct administration of 2-AG, an endocannabinoid and CB1R agonist WIN55,212-2 into the perfusate induced a significant increase in CF, indicating that the endocannabinoid system and CB1 receptors play a relevant role in the regulation of cardiac tissue perfusion.
4.1. Effects of Endocannabinoids on Cardiac Function
Endocannabinoids, such as arachidonoyl ethanolamide (anandamide, AEA), 2-AG and 2-arachidonoylglyceryl ether [18,20] are known to have diverse cardiovascular effects, which are attributed mostly to CB1 cannabinoid receptors [13,15,23,28,39,40]. Cannabinoids and their synthetic analogues cause vasodilation and hypotension by acting on vascular CB1Rs [23,39,41,42].
Besides their vascular expression, CB1Rs have also been detected in the cardiac tissue by several research groups [43,44] and also by us (Figure 1, and Supplementary Figure S1). Cannabinoid actions in the heart are the result of an interplay between coronary and cardiomyocyte effects. AEA and 2-AG have been shown in various experimental settings to cause coronary vasodilation, increase in coronary blood flow and decrease in cardiac contractility [22,43,45,46,47,48]. In Langendorff hearts, AEA has been reported to cause vasodilation and to decrease LVDevP, effects that are attributed mainly to CB1 receptors [49]. Similarly, in vasopressin-pretreated Langendorff hearts, the selective CB1 receptor agonist AEA concentration-dependently increased CF [22]. Similar effects were observed with metabolically stable endocannabinoid derivatives, R-methanandamide and noladin ether as well. Moreover, in this study, AEA and 2-AG were detected in the cardiac tissue [22]. In a previous study performed on isolated coronary vessels, we also observed vasodilation of precontracted vessels in response to CB1R agonist WIN55,212, an effect that was blocked by CB1R antagonists AM251 and O2050 [50].
In agreement with literature data, we also found that 2-AG and WIN55,212-2 infusion (Figure 4A) significantly increased CF. The inhibition of 2-AG-induced CF response by O2050 indicates the functional presence of CB1R on coronary vessels and suggests that CB1 receptors have a significant role in the control of coronary perfusion. Notably, we reported CB1Rs on rat coronary vessels in a previous study [50]. The negative inotropic response of the heart to cannabinoids reported by other research groups [40,43,49] was observed only during WIN55,212-2 administration in our study, and did not develop when 2-AG was applied (Figure 4B). This discrepancy can be explained by the fast metabolism of 2-AG, which may not allow a build-up of 2-AG concentration in the close environment of cardiomyocytes which is high enough to elicit the negative inotropy when intracoronary application is used. On the other hand, we need to take into account that the observed 2-AG-induced rise in CF evokes a consequential positive effect on contractility, which can mask its direct negative inotropic action.
4.2. Role of Angiotensin II-Induced Endocannabinoid Release in Cardiac Function
We have previously reported that Ang II via AT1 receptors induces the release of 2-AG in the vascular tissue, which decreases Ang II-induced vasoconstriction [13,15]. Signalling-induced endocannabinoid release via activation of G protein-coupled receptors (GPCRs) and downstream phospholipase C-activation-mediated mechanisms have been detected first in the neural tissue serving as retrograde synaptic inhibition [20]. Endocannabinoid release has also been detected by bioluminescence resonance energy transfer method in isolated cell systems in response to calcium-signal-generating GPCR agonists, such as Ang II via AT1 receptors, and acetylcholine via muscarinic receptors [12,51]. GPCR agonist-induced release of 2-AG has also been reported in the vascular tissue previously [47,52]. Since CB1 receptors have also been detected in the cardiac tissue, we can postulate that Ang II-induced 2-AG release may also have relevance in the heart.
In our study, Ang II concentration-dependently decreased coronary perfusion flow with a moderate negative inotropic and lusitropic effect in Langendorff rat hearts (Figure 2). This is in agreement with the findings of van Esch et al. [4], but seems to contradict other studies which reported positive inotropy at higher Ang II-concentrations [5] and in isolated cardiomyocytes [7,8]. However, in our experimental setting, the negative functional effects on the heart are suggested to be an indirect consequence of decreased coronary perfusion. In our system, interventions, which decrease CF without having a direct influence on cardiac cells (e.g., mechanical compression of the inflow line (Figure 3F), or vasoconstrictors with mere vascular effects [33]), normally elicit a decrease of the same magnitude in contractile function. In the current study, CF decreased and remained lower than control throughout the Ang II administration (Figure 3A); however, this was accompanied by only a moderate and transient decrease in contractile performance (Figure 3B–D). This may suggest that a positive inotropic effect was present but could only mitigate the detrimental functional effects of the simultaneous strong coronary vasoconstriction.
The multiple administrations of Ang II into the coronary perfusion resulted in desensitization, in which the effect of the 2nd administration was diminished compared to the first one but did not differ from the further ones (Figure 3). Therefore, we applied Ang II pretreatment to prevent tachyphylaxis and used the 2nd Ang II infusion to assess the presence of CB1 receptor transactivation. We suggest that this setting is the most compliant to the in vivo situation. Tachyphylaxis induced by Ang II has also been excluded similarly by others [53].
In our experiments, we found that CB1 receptor blockade by O2050 (Figure 5) abolished, and inhibition of DAGL attenuated (Figure 6) Ang II-induced CF reduction and cardiac effects. This seems to contradict previous studies on isolated coronary arteries and also on the aorta, which reported augmented vasoconstriction in response to Ang II when CB1R inhibitor O2050 was applied [15,50]. However, it is important to note that the Langendorff-perfused heart is a more complex system in which the applied pharmacons act on the cardiac tissue and on the coronary vessels as well, influencing CF and cardiac function in a complex manner. In the intact heart, CF is primarily controlled by the oxygen demand of cardiac work. This regulatory phenomenon often overwrites the direct vascular effects of vasoactive agents and may explain why we observed a CF effect that is opposite to the one expected on the basis of isolated vessel studies. This phenomenon can be most probably explained by the altered cardiac effects of Ang II upon CB1R inhibition. Specifically, we propose that O2050 blocks the CB1R-induced negative inotropic effect, which allows the Ang II-induced positive inotropy to develop (which is otherwise masked by flow deprivation). This increases the overall oxygen demand and thus the CF, and overweighs the CF-reducing effect of the loss of direct vasodilator 2-AG action.
Although we did not observe negative inotropy upon 2-AG administration to the coronary perfusate, it was evident when WIN55,212-2 was applied (Figure 4A). The lack of 2-AG effect may be caused by its fast metabolism, which may prevent the build-up of a concentration in the cardiac tissue upon intracoronary application, which is sufficiently high to exert this effect. However, we may assume that when 2-AG is released from cardiomyocytes during Ang II signalling, negative inotropy develops as a paracrine/autocrine effect.
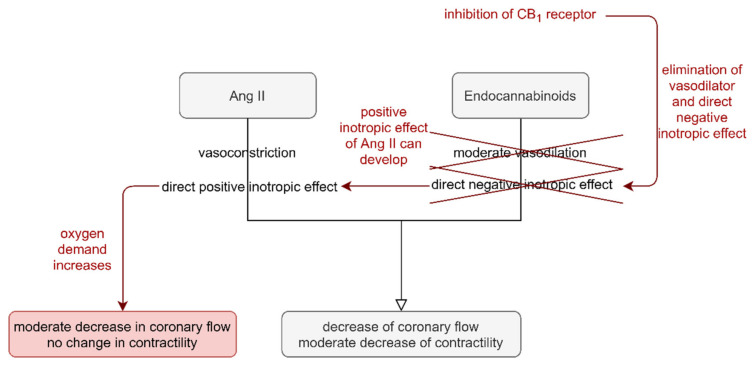
In summary, the diminished CF-reducing effect of Ang II during DAGL blockade and CB1R inhibition can be attributed to the enhanced cardiac oxygen demand, which is evoked by increased cardiac contractility. This overrides Ang II-induced vasoconstriction. Thus, our observation suggests that the acute effects of Ang II on isolated Langendorff hearts are modulated by PLC-signalling-induced endocannabinoid release via CB1R stimulation. We propose that the short-term cardiac effects of Ang II are exhibited as a delicate balance of the simultaneous cardiac and vascular effects of Ang II and 2-AG (Figure 7).
Figure 7.
Postulated mechanism of the role of angiotensin II-induced (Ang II) 2-arachidonoylgycerol (2-AG) release in Ang II-induced short-term cardiac effects. Ang II causes vasoconstriction of the coronaries and has positive inotropic effects on cardiomyocytes. On the other hand, 2-AG release during Ang II signalling causes moderate vasodilation and has negative inotropic effects. The summation of these opposing effects manifests in diminished coronary flow (CF) accompanied by a moderate decrease in contractility in the Langendorff-perfused rat heart (black arrows). Inhibition of CB1 receptor, and 2-AG generation eliminates the counteraction of 2-AG on the positive inotropic effect of Ang II. The unopposed positive inotropic effect of Ang II enhances cardiac oxygen demand, which counterregulates the vasoconstrictor effect of Ang II. As a result, the flow-reducing effect of Ang II is moderated, and the opposing effects of diminished CF and increased contractility cancel each other out, leaving the contractile function unaltered (red arrows).
5. Conclusions
In this study, we report that the short-term effects of Ang II in an isolated heart preparation with intact circulation are modulated by DAGL activation and CB1 receptor transactivation, indicating a significant role of Ang II-signalling-induced release of the endocannabinoid 2-AG. We propose that the short-term cardiac effects of Ang II are exhibited as the summation of the simultaneous cardiac and vascular effects of Ang II and 2-AG.
Our study has proved that short-term Ang II effects are partially mediated by CB1 receptor activation. Whether this holds for long-term effects of Ang II, when the local renin-angiotensin system is upregulated in the heart under pathological conditions, and whether these potential effects are detrimental or beneficial needs further investigation.
Acknowledgments
The authors express their special thanks to Margit Nagy, Éva Balogh, Ildikó Oravecz and Ilona Oláh for their expert assistance, to Erzsébet Fejes for critically reading the manuscript and also to Balázs Czakó for his valuable help in data editing.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/10/4/724/s1, Figure S1: CB1 receptor (CB1R) immunostaining in wild type and CB1R knockout murine cardiac tissue. Figure S2: Concentration-dependent effects of 2-arachidonoylglycerol (2AG) on coronary flow and left developed ventricular pressure (LVDevP) of isolated rat hearts.
Author Contributions
Conceptualization, M.S., Z.M. and L.H.; methodology, Z.M., M.S., T.I.; validation, Z.M., D.W., and Z.B.; formal analysis, Z.M., M.S., Z.L.; investigation, B.B., Z.L., Z.M., Z.E.T., M.S.; resources, L.H., T.I., G.L.N., G.D., Z.B.; data curation, Z.L., D.W.; writing—original draft preparation, M.S., Z.M., D.W.; writing—review and editing, Z.B., G.L.N., G.D., L.H.; visualization, B.B, D.W.; supervision, Z.M., M.S.; project administration, L.H., M.S.; funding acquisition, L.H., M.S., Z.M., Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Hungarian National Research, Development and Innovation Fund (NKFI-OTKA K-116954, K-112964, K-115422, K-125174 and NVKP_16-1-2016-0039) as well as by the Higher Education Institutional Excellence Program of the Ministry for Innovation and Technology in Hungary, within the framework of the Molecular Biology thematic program of the Semmelweis University, as well as by the Higher Education Institutional Excellence Program (FIKP) of the Ministry for Innovation and Technology in Hungary, within the framework of the Neurology thematic program of the Semmelweis University and supported by the EFOP-3.6.3-VEKOP-16-2017-00009 grant.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Hungarian Law of Animal Protection (28/1998) and were approved by the Government Office of Pest County (Permission number: PEI/001/820-2/2015 and PE/EA/1428-7/2018).
Data Availability Statement
The data presented in this study are openly available in Mendeley Data at https://data.mendeley.com/drafts/7vy77nx4yj, accessed on 25 January 2021.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lavoie J.L., Sigmund C.D. Minireview: Overview of the Renin-Angiotensin System—An Endocrine and Paracrine System. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 2.Kaschina E., Unger T. Angiotensin AT1/AT2 receptors: Regulation, signalling and function. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 3.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 4.Van Esch J.H., Gembardt F., Sterner-Kock A., Heringer-Walther S., Le T.H., Lassner D., Stijnen T., Coffman T.M., Schultheiss H.-P., Danser A.J., et al. Cardiac phenotype and angiotensin II levels in AT1a, AT1b, and AT2 receptor single, double, and triple knockouts. Cardiovasc. Res. 2010;86:401–409. doi: 10.1093/cvr/cvq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pörsti I., Hecker M., Bassenge E., Busse R. Dual action of angiotensin II on coronary resistance in the isolated perfused rabbit heart. Naunyn Schmiedeberg Arch. Pharmacol. 1993;348:650–658. doi: 10.1007/BF00167243. [DOI] [PubMed] [Google Scholar]
- 6.Booz G.W., Baker K.M. Actions of Angiotensin II on Isolated Cardiac Myocytes. Hear. Fail. Rev. 1998;3:125–130. doi: 10.1023/A:1009788013090. [DOI] [Google Scholar]
- 7.Mattiazzi A. Positive inotropic effect of Angiotensin II. Increases in intracellular Ca2+ or changes in myofilament Ca2+ responsiveness? J. Pharmacol. Toxicol. Methods. 1997;37:205–214. doi: 10.1016/S1056-8719(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 8.Barry W.H., Matsui H., Bridge J.H.B., Spitzer K.W. Excitation-Contraction Coupling in Ventricular Myocytes: Effects of Angiotensin II. In: Metzler J.B., editor. Tissue Engineering. Volume 382. Springer; Boston, MA, USA: 1995. pp. 31–39. [DOI] [PubMed] [Google Scholar]
- 9.Lefroy D.C., Crake T., Del Monte F., Vescovo G., Libera L.D., Harding S., Poole-Wilson P.A. Angiotensin II and contraction of isolated myocytes from human, guinea pig, and infarcted rat hearts. Am. J. Physiol. Content. 1996;270:H2060–H2069. doi: 10.1152/ajpheart.1996.270.6.H2060. [DOI] [PubMed] [Google Scholar]
- 10.Paul M., Mehr A.P., Kreutz R. Physiology of Local Renin-Angiotensin Systems. Physiol. Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 11.Hunyady L., Catt K.J. Pleiotropic AT1 Receptor Signaling Pathways Mediating Physiological and Pathogenic Actions of Angiotensin II. Mol. Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 12.Turu G., Várnai P., Gyombolai P., Szidonya L., Offertaler L., Bagdy G., Kunos G., Hunyady L. Paracrine Transactivation of the CB1 Cannabinoid Receptor by AT1 Angiotensin and Other Gq/11 Protein-coupled Receptors. J. Biol. Chem. 2009;284:16914–16921. doi: 10.1074/jbc.M109.003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szekeres M., Nádasy G.L., Turu G., Soltész-Katona E., Tóth Z.E., Balla A., Catt K.J., Hunyady L. Angiotensin II Induces Vascular Endocannabinoid Release, Which Attenuates Its Vasoconstrictor Effect via CB1 Cannabinoid Receptors. J. Biol. Chem. 2012;287:31540–31550. doi: 10.1074/jbc.M112.346296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benyó Z., Ruisanchez É., Leszl-Ishiguro M., Sándor P., Pacher P. Endocannabinoids in cerebrovascular regulation. Am. J. Physiol. Circ. Physiol. 2016;310:H785–H801. doi: 10.1152/ajpheart.00571.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szekeres M., Nadasy G.L., Turu G., Soltész-Katona E., Benyó Z., Offermanns S., Ruisanchez É., Szabó E., Takats Z., Batkai S., et al. Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol. Cell. Endocrinol. 2015;403:46–56. doi: 10.1016/j.mce.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Cajanus K., Holmström E.J., Wessman M., Anttila V., Kaunisto M.A., Kalso E. Effect of endocannabinoid degradation on pain. Pain. 2016;157:361–369. doi: 10.1097/j.pain.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 17.Cedernaes J., Fanelli F., Fazzini A., Pagotto U., Broman J.-E., Vogel H., Dickson S.L., Schiöth H.B., Benedict C. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology. 2016;74:258–268. doi: 10.1016/j.psyneuen.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Pacher P., Bátkai S., Kunos G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillard C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology. 2018;43:155–172. doi: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freund T.F., Katona I., Piomelli D. Role of Endogenous Cannabinoids in Synaptic Signaling. Physiol. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 21.Wagner J.A., Abesser M., Harvey-White J., Ertl G. 2-Arachidonylglycerol Acting on CB1 Cannabinoid Receptors Mediates Delayed Cardioprotection Induced by Nitric Oxide in Rat Isolated Hearts. J. Cardiovasc. Pharmacol. 2006;47:650–655. doi: 10.1097/01.fjc.0000211752.08949.eb. [DOI] [PubMed] [Google Scholar]
- 22.Wagner J.A., Abesser M., Karcher J., Laser M., Kunos G. Coronary Vasodilator Effects of Endogenous Cannabinoids in Vasopressin-Preconstricted Unpaced Rat Isolated Hearts. J. Cardiovasc. Pharmacol. 2005;46:348–355. doi: 10.1097/01.fjc.0000175437.87283.f2. [DOI] [PubMed] [Google Scholar]
- 23.Stanley C., O′Sullivan S.E. Vascular targets for cannabinoids: Animal and human studies. Br. J. Pharmacol. 2014;171:1361–1378. doi: 10.1111/bph.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iring A., Ruisanchez E., Leszl-Ishiguro M., Horváth B., Benko R., Lacza Z., Járai Z., Sandor P., Di Marzo V., Pacher P., et al. Role of Endocannabinoids and Cannabinoid-1 Receptors in Cerebrocortical Blood Flow Regulation. PLoS ONE. 2013;8:e53390. doi: 10.1371/journal.pone.0053390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiley C.R. Endocannabinoids and the Heart. J. Cardiovasc. Pharmacol. 2009;53:267–276. doi: 10.1097/FJC.0b013e318192671d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhopadhyay S., Chapnick B.M., Howlett A.C. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am. J. Physiol. Circ. Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- 27.Bondarenko A.I. Endothelial atypical cannabinoid receptor: Do we have enough evidence? Br. J. Pharmacol. 2014;171:5573–5588. doi: 10.1111/bph.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lípez-Miranda V., Herradón E., Martín M.I. Vasorelaxation caused by cannabinoids: Mechanisms in different vascular beds. Curr. Vasc. Pharmacol. 2008;6:335–346. doi: 10.2174/157016108785909706. [DOI] [PubMed] [Google Scholar]
- 29.Turu G., Simon A., Gyombolai P., Szidonya L., Bagdy G., Lenkei Z., Hunyady L. The Role of Diacylglycerol Lipase in Constitutive and Angiotensin AT1 Receptor-stimulated Cannabinoid CB1 Receptor Activity. J. Biol. Chem. 2007;282:7753–7757. doi: 10.1074/jbc.C600318200. [DOI] [PubMed] [Google Scholar]
- 30.Dell′Italia L.J. Translational Success Stories: Angiotensin Receptor 1 Antagonists in Heart Failure. Circ. Res. 2011;109:437–452. doi: 10.1161/CIRCRESAHA.110.238550. [DOI] [PubMed] [Google Scholar]
- 31.Ishii H., Kobayashi M., Kurebayashi N., Yoshikawa D., Suzuki S., Ichimiya S., Kanashiro M., Sone T., Tsuboi H., Amano T., et al. Impact of Angiotensin II Receptor Blocker Therapy (Olmesartan or Valsartan) on Coronary Atherosclerotic Plaque Volume Measured by Intravascular Ultrasound in Patients With Stable Angina Pectoris. Am. J. Cardiol. 2013;112:363–368. doi: 10.1016/j.amjcard.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Heeneman S., Sluimer J.C., Daemen M.J.A.P. Angiotensin-Converting Enzyme and Vascular Remodeling. Circ. Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- 33.Wafa D., Koch N., Kovács J., Kerék M., Proia R.L., Tigyi G.J., Benyó Z., Miklós Z. Opposing Roles of S1P3 Receptors in Myocardial Function. Cells. 2020;9:1770. doi: 10.3390/cells9081770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemecsei P., Miklós Z., Bíró T., Marincsák R., Tóth B.I., Komlodi-Pasztor E., Barnucz E., Mirk É., Van Der Vusse G.J., Ligeti L., et al. Hearts of surviving MLP-KO mice show transient changes of intracellular calcium handling. Mol. Cell. Biochem. 2010;342:251–260. doi: 10.1007/s11010-010-0492-8. [DOI] [PubMed] [Google Scholar]
- 35.Miklós Z., Kemecsei P., Biro T., Marincsak R., Toth B.I., Buijs J., Benis E., Drozgyik A., Ivanics T. Early cardiac dysfunction is rescued by upregulation of SERCA2a pump activity in a rat model of metabolic syndrome. Acta Physiol. 2012;205:381–393. doi: 10.1111/j.1748-1716.2012.02420.x. [DOI] [PubMed] [Google Scholar]
- 36.Zimmer A., Zimmer A.M., Hohmann A.G., Herkenham M., Bonner T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tóth Z.E., Mezey É. Simultaneous Visualization of Multiple Antigens with Tyramide Signal Amplification using Antibodies from the same Species. J. Histochem. Cytochem. 2007;55:545–554. doi: 10.1369/jhc.6A7134.2007. [DOI] [PubMed] [Google Scholar]
- 38.Krenacs T., Meggyeshazi N., Forika G., Kiss E., Hamar P., Szekely T., Vancsik T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int. J. Mol. Sci. 2020;21:6270. doi: 10.3390/ijms21176270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bátkai S., Járai Z., Wagner J.A., Goparaju S.K., Varga K., Liu J., Wang L., Mirshahi F., Khanolkar A.D., Makriyannis A., et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat. Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- 40.Bátkai S., Pacher P., Osei-Hyiaman D., Radaeva S., Liu J., Harvey-White J., Offertáler L., Mackie K., Rudd M.A., Bukoski R.D., et al. Endocannabinoids Acting at Cannabinoid-1 Receptors Regulate Cardiovascular Function in Hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Randall M.D., Kendall D.A., O′Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br. J. Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dannert M., Alsasua A., Herradón E., Martin M., López-Miranda V. Vasorelaxant effect of Win 55,212-2 in rat aorta: New mechanisms involved. Vasc. Pharmacol. 2007;46:16–23. doi: 10.1016/j.vph.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Bonz A., Laser M., Küllmer S., Kniesch S., Babin-Ebell J., Popp V., Ertl G., Wagner J.A. Cannabinoids Acting on CB1 Receptors Decrease Contractile Performance in Human Atrial Muscle. J. Cardiovasc. Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 44.Mukhopadhyay P., Bátkai S., Rajesh M., Czifra N., Harvey-White J., Haskó G., Zsengeller Z., Gerard N.P., Liaudet L., Kunos G., et al. Pharmacological Inhibition of CB1Cannabinoid Receptor Protects Against Doxorubicin-Induced Cardiotoxicity. J. Am. Coll. Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Högestätt E.D., Zygmunt P. Cardiovascular pharmacology of anandamide. Prostaglandins Leukot. Essent. Fat. Acids. 2002;66:343–351. doi: 10.1054/plef.2001.0346. [DOI] [PubMed] [Google Scholar]
- 46.Lépicier P., Bouchard J.-F., Lagneux C., Lamontagne D. Endocannabinoids protect the rat isolated heart against ischaemia. Br. J. Pharmacol. 2003;139:805–815. doi: 10.1038/sj.bjp.0705313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gauthier K.M., Baewer D.V., Hittner S., Hillard C.J., Nithipatikom K., Reddy D.S., Falck J.R., Campbell W.B. Endothelium-derived 2-arachidonylglycerol: An intermediate in vasodilatory eicosanoid release in bovine coronary arteries. Am. J. Physiol. Circ. Physiol. 2005;288:H1344–H1351. doi: 10.1152/ajpheart.00537.2004. [DOI] [PubMed] [Google Scholar]
- 48.Gorbunov A.S., Maslov L.N., Tsibulnikov S.Y., Khaliulin I.G., Tsepokina A.V., Khutornaya M.V., Kutikhin A.G. CB-Receptor Agonist HU-210 Mimics the Postconditioning Phenomenon of Isolated Heart. Bull. Exp. Biol. Med. 2016;162:27–29. doi: 10.1007/s10517-016-3536-6. [DOI] [PubMed] [Google Scholar]
- 49.Ford W.R., A Honan S., White R., Hiley C.R. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br. J. Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szekeres M., Nádasy G.L., Soltész-Katona E., Hunyady L. Control of myogenic tone and agonist induced contraction of intramural coronary resistance arterioles by cannabinoid type 1 receptors and endocannabinoids. Prostaglandins Other Lipid Mediat. 2018;134:77–83. doi: 10.1016/j.prostaglandins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Gyombolai P., Pap D., Turu G., Catt K.J., Bagdy G., Hunyady L. Regulation of endocannabinoid release by G proteins: A paracrine mechanism of G protein-coupled receptor action. Mol. Cell. Endocrinol. 2012;353:29–36. doi: 10.1016/j.mce.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mechoulam R., Fride E., Ben-Shabat S., Meiri U., Horowitz M. Carbachol, an acetylcholine receptor agonist, enhances production in rat aorta of 2-arachidonoyl glycerol, a hypotensive endocannabinoid. Eur. J. Pharmacol. 1998;362:R1–R3. doi: 10.1016/S0014-2999(98)00777-8. [DOI] [PubMed] [Google Scholar]
- 53.França L.P., Pacheco N.A., Correa S.A., Han S.W., Nakaie C.R., Paiva A.C.M., Shimuta S.I. Angiotensin II-mediated cellular responses: A role for the 3’-untranslated region of the angiotensin AT1 receptor. Eur. J. Pharmacol. 2003;476:25–30. doi: 10.1016/S0014-2999(03)02172-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are openly available in Mendeley Data at https://data.mendeley.com/drafts/7vy77nx4yj, accessed on 25 January 2021.