Abstract
Invariant Natural Killer T (iNKT) cells are key players in the immunity to several pathogens; however, their involvement in the resistance to Paracoccidioides brasiliensis infection remains unknown. Using splenocytes from CD1d (CD1d−/−) and iNKT-deficient (Jα18−/−) mice, we found that iNKT cells are the innate source of IFN-γ after P. brasiliensis infection and are required to potentiate macrophage oxidative burst and control fungal growth. To determine whether iNKT cells contribute in vivo to host resistance against P. brasiliensis infection, we infected intratracheally wild-type and Jα18−/− C57BL/6 mouse strains with the virulent Pb18 isolate. iNKT cell deficiency impaired the airway acute inflammatory response, resulting in decreased airway neutrophilia and reduced IFN-γ, KC, and nitric oxide (NO) production. The deficient innate immune response of Jα18−/− mice to Pb18 infection resulted in increased fungal burden in the lungs and spleen. Besides, the activation of iNKT cells in vivo by administration of the exogenous iNKT ligand α-galactosylceramide (α-GalCer) improved host resistance to P. brasiliensis infection. Although the mechanisms responsible for this phenomenon remain to be clarified, α-GalCer treatment boosted the local inflammatory response and reduced pulmonary fungal burden. In conclusion, our study is the first evidence that iNKT cells are important for the protective immunity to P. brasiliensis infection and their activation by an exogenous ligand is sufficient to improve the host resistance to this fungal infection.
1. Introduction
Paracoccidioidomycosis (PCM) is caused by a fungus from the Paracoccidioides genus and is considered one of the highest causes of mortality among Brazilian systemic mycoses [1]. Clinical studies demonstrated a relationship between the characteristics of the immune response and disease severity [2]. In humans, a prominent Th1 response is associated with infection without disease, the chronic form of the disease with Th1/Th17 immunity, and the most severe manifestation, the acute or juvenile form, shows a prominent Th2/Th9 profile [1]. These data are supported by murine models of P. brasiliensis infection showing the association between the classical Th1 immune response, with high levels of IL-2 and IFN-γ, with resistance against the fungi [3, 4].
The lung is the primary site of fungal infection, where alveolar macrophages (MΦ) recognize Pathogen-Associated Molecular Patterns (PAMPs) through several Pattern Recognition Receptors (PRRs) [5]. Although P. brasiliensis proliferates in resident alveolar MΦ, these cells can inhibit fungal growth upon activation mediated by the synergistic action between lytic enzymes and metabolites from the oxidative burst that mediate fungal killing [6, 7]. In parallel, these cells potentiate the immunity against the fungi by secreting cytokines and chemokines that coordinate the influx and activation of other immune cells, such as neutrophils and T lymphocytes, to the site of infection [8]. In this scenario, Th1-biased lymphocytes increase the fungicidal ability of phagocytes, promoting resistance against the pathogen [9, 10]. Although IFN-γ drives the quality of the inflammatory response during the acute phase of P. brasiliensis infection, the source of the early IFN-γ production remains unclear. Because invariant Natural Killer T (iNKT) lymphocytes are poised for the rapid production of IFN-γ, we hypothesized that they are important players in the immunity to P. brasiliensis infection [11].
The iNKT cells are a subpopulation of unconventional T lymphocytes that due to an invariant T cell receptor (TCR) promptly respond to lipid antigens presented in the context of CD1d molecules [12, 13]. In addition to this unique specificity, they can rapidly secrete several cytokines and chemokines, acting as a bridge between innate and adaptive immunity [14, 15]. This ability confers an essential immune regulatory function to these cells that participate in diverse types of immune responses, including those against pathogens [16, 17]. Although a previous study described that iNKT cells from both healthy controls and cured PCM patients have the same ability to expand and produce cytokines, there are no data regarding their role in in vivo models of P. brasiliensis infection [18]. Therefore, we used the intratracheal model of P. brasiliensis infection with the virulent Pb18 strain, and wild-type (WT) and iNKT-deficient (Jα18−/−) C57BL-6 mice to determine the role of these cells in host resistance to P. brasiliensis [13, 19].
Our findings show that iNKT lymphocytes are the primary innate source of IFN-γ, and their deficiency leads to impaired airway inflammation and increased pulmonary fungal burden. Furthermore, the specific activation of iNKT cells by α-galactosylceramide (α-GalCer) in an ongoing disease increased host resistance against this fungal pathogen. Therefore, iNKT cells were shown to play an essential role in shaping the protective immunity against the fungi, and the treatment with specific iNKT agonists potentiates host resistance to PCM.
2. Materials and Methods
2.1. Animals
Isogenic male C57Bl/6 mice from wild-type (WT), CD1d−/−, and iNKT-deficient strain (Jα18−/−), aged 8–12 weeks, were obtained from the Animal Care Facility at the Federal University of São Paulo (UNIFESP). The C57Bl/6 Jα18−/− strain was a gift from Dr. Masaru Taniguchi at the RIKEN Research Center for Allergy and Immunology (Japan) [13]. All animals were housed in individual standard cages and had free access to food and water. All procedures were previously reviewed and approved by the internal ethics committee of Universidade de São Paulo (USP-180/2011) and Universidade Federal de São Paulo (UNIFESP–CEP 0372/12).
2.2. Fungus
The virulent isolate Pb18 from Paracoccidioides brasiliensis was used throughout the experiments outlined in this work [20]. Pb18 yeast cells were subcultivated every seven days in semisolid Fava-Netto culture medium at 37°C until use. The yeast cells were collected and washed with sterile phosphate-buffered saline (PBS, pH 7.2). Fungal viability was determined by the Janus Green B vital dye. All experimental procedures were carried out with fungal suspensions presenting viability between 90 and 95%.
2.3. Peritoneal MΦ, Splenocytes, and In Vitro Culture
A sterile solution of 3% thioglycolate was injected in the peritoneal cavity, and four days later, peritoneal leukocytes were collected, and thioglycolate-elicited peritoneal MΦ were isolated by adherence (2 h at 37°C in 5% CO2) in plastic-bottom tissue-culture plates. Spleens were homogenized using the plunger end of a 3 mL syringe and a 70 μm strainer, erythrocytes were removed using ACK solution (0.15 M NH4Cl; 1 mM KHCO3; 0.1 mM Na2 EDTA), and cell viability was determined using trypan blue. After removing nonadherent cells, MΦ were cultivated alone or with P. brasiliensis yeasts in an MΦ: yeast ratio of 10 : 1 (2 h at 37°C in 5% CO2) to allow fungi adhesion and ingestion. Supernatants were removed, and cells were gently washed with PBS to remove any noningested or nonadherent yeast. After that, splenocytes from WT, CD1d−/− or Jα18−/− mice were added to the culture in an MΦ: splenocyte ratio of 1 : 10. After 48 h of coculture, supernatants were collected and analyzed for IFN-γ, IL-10, and NO production. After removing coculture supernatants, the wells were washed with distilled water to lyse the cells, and suspensions were collected in individual tubes. Cell homogenates were assayed for the presence of viable yeasts, as previously described [21].
2.4. Colony-Forming Units Assay
The number of viable Pb18 yeasts in cell cultures and infected organs was determined by counting the number of colony-forming units (CFU) as previously described [21].
2.5. Induction of Experimental PCM
Animals were deeply anesthetized and infected by intratracheal (i.t.) delivery of 1 x 106 viable Pb yeast cells. Animals were euthanized 72 hours postinfection to assess iNKT cells' role in the acute phase of infection. Depending on the objective of the experiment, euthanasia occurred forty-five days or eight weeks postinfection to determine the severity of Pb infection.
2.6. Bronchoalveolar Lavage Fluid (BAL)
To determine the content of inflammatory cells in the airways, mice were euthanatized, the trachea was surgically exposed, and 0.5 mL of cold PBS was injected with a plastic cannula to obtain the BAL. After BAL collection, the total number of cells was determined in the Neubauer chamber and samples were centrifuged for supernatant analysis. Cellular precipitated was suspended in PBS for cytocentrifugation (Shandon, USA or Fanem, Br), and cellular populations were analyzed upon slides staining with hematoxylin/eosin.
2.7. Cytokine and Chemokine Production
IFN-γ and KC levels in BAL were analyzed with a multiplex kit (Millipore, USA) following the manufacturer's recommendations. The IFN-γ levels in culture supernatants were quantified using ELISA (R&D Systems, USA).
2.8. Nitric Oxide Production
Nitric oxide production in BAL was assessed using Nitrate/Nitrite Colorimetric Assay (Cayman Chemicals, USA) according to the manufacturer's recommendations. In culture supernatants, NO production was quantified by nitrite accumulation in the supernatants using a standard Griess reaction [22].
2.9. Flow Cytometry Assay
To determine the inflammatory state of lung parenchyma, the organs were digested with a DNAse (1 mg/mL) and collagenase (2 mg/mL) solution (Invitrogen), homogenized, centrifuged in Percoll 35% (G&E, USA) solution, and stained for different surface markers (eBioscience, USA). The T lymphocyte population was analyzed according to the expression of CD3, CD4, CD8, and CD69. Myeloid-derived cells were analyzed according to the expression of GR1, CD11b, and MHC-II. All data concerning the FACS assays were analyzed using the FlowJo software (BD, USA), according to specific cell population characteristics. Further information about analysis strategy is presented in Supplementary Figures 1 and 2.
2.10. α-Galactosylceramide Treatment
At the week 4 after infection, mice were treated intravenously (i.v.) with 10 μg of the NKT cell agonist α-GalCer (KRN7000, Cayman Chemical Company, USA) and euthanized four weeks later to determine the fungal burden in the lung.
2.11. Statistical Analysis
For the comparison between two groups, we used a 2-tailed unpaired t-test, and for multiple comparisons, we used a two-way ANOVA followed by Turkey's or Bonferroni's multiple comparison test. All statistical analyses were performed using the GraphPad Prism 7 software (San Diego, CA).
3. Results
3.1. Invariant Natural Killer T Cells Are the Innate Source of IFN-γ in Response to Fungal Infection and Are Required to Control P. brasiliensis Growth
To determine the role of the iNKT cells in the immunity against P. brasiliensis, we tested the ability of naïve splenocytes from CD1d−/− mouse, deficient in diverse CD1d-restricted cells, or Jα18−/− mouse strain, which lacks only the CD1d-restrict iNKT cell subset, to respond to fungal infection [13, 23]. Thioglycolate-elicited peritoneal MΦ were exposed to Pb18 yeasts for 2 h, followed by coculture with 5 x 106 splenocytes from naive WT, CD1d−/−, or Jα18−/− mice. Two days later, culture supernatants were collected, and MΦ were lysed to determine the number of viable yeasts. Figure 1(a) demonstrates that the addition of naive WT splenocytes to MΦ culture increased the fungicidal activity of macrophages. In contrast, splenocytes from both CD1d−/− and Jα18−/− mice failed to increase MΦ killing capacity, indicating that the ability of naive splenocytes to control fungal growth depends on iNKT cells. In concordance, coculture with splenocytes from WT mice resulted in a higher NO production than those from CD1d−/− and Jα18−/− (Figure 1(b)). Further analysis revealed that splenocytes from both CD1d−/− and Jα18−/− failed to produce IFN-γ in response to fungal infection (Figure 1(c)). Therefore, these data show that among CD1d-restrict cells, the iNKT cell subset is the primary source of IFN-γ during P. brasiliensis infection and essential for early control of fungal growth.
Figure 1.
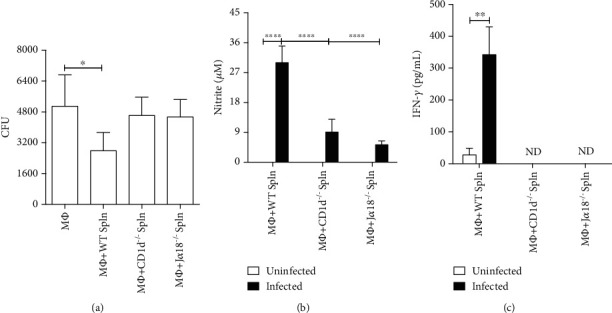
The invariant Natural Killer T cells drive P. brasiliensis killing by macrophages and respond for the innate source of IFN-γ upon fungal infection. Thioglycolate-elicited peritoneal MΦ from C57Bl/6 mice were infected with Pb18 yeast (1 : 10) for 2 h. After washing, splenocytes (Spln) from WT, CD1d−/−, or Jα18−/− C57BL/6 mouse strains were added to the MΦ culture (10 : 1) for 48 h. After this period, supernatant was collected, and adherent cells were lysed with distilled water for fungal recovery. (a) Number of viable cell yeast obtained by colony-forming units assay (CFU). (b) NO levels in culture supernatants. (c) IFN-γ levels in the culture supernatants (ND = not detected). Data represent the mean ± SD of quintuplicate samples from 1 of 2 independent experiments (a, b) and of 1 independent experiment (c). ∗p < 0.05; ∗∗p < 0.005; ∗∗∗∗p < 0.0001.
3.2. Deficiency in iNKT Cells Impairs the Acute Airway Inflammation upon P. brasiliensis Infection
To determine the contribution of iNKT cells to the innate immune response against P. brasiliensis infection, we infected WT or Jα18−/− C57Bl/6 mice by intratracheal (i.t.) route with 1 x 106 living Pb18 yeast cells, and the BAL was collected 72 h later. Jα18−/− mice presented a lower number of cells in the BAL compared to WT animals, which reflected an impaired airway neutrophilia (Figures 2(a) and 2(b), respectively). In accordance, the absence of iNKT cells resulted in lower levels of proinflammatory cytokines and chemokines, such as IFN-γ and KC in the airways (Figures 2(c) and 2(d), respectively). Moreover, NO levels in BAL were lower in the Jα18−/− animals than in the WT group (Figure 2(e)). Therefore, these data demonstrate that iNKT cells play an important role in the acute phase of the inflammatory response following pulmonary Pb18 infection.
Figure 2.
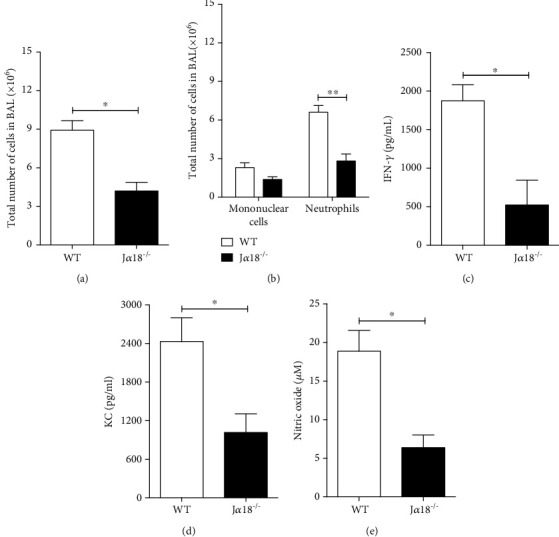
Deficiency in invariant Natural Killer T lymphocytes impairs the acute inflammatory response against P. brasiliensis infection. WT and Jα18−/− C57BL/6 mouse strains were infected with 1 x 106 Pb18 yeasts, and 72 h later, the BAL content was analyzed for: (a) total number of cells; (b) presence of mononuclear cells and neutrophils; BAL levels of (c) IFN-γ, (d) KC, and (e) NO. Data represent the mean ± SD from 1 of 2 independent experiments (n = 4 − 5/group). ∗p < 0.05; ∗∗p < 0.005.
3.3. iNKT Cells Drive Early CD8 T Cell Activation and Influence the Presence of Distinct GR1+CD11b+ Cellular Population in the Lungs of Infected Mice
The flow cytometric analysis of lung parenchyma did not reveal any significant difference in the frequency of CD4 and CD8 T lymphocytes between WT and Jα18−/− mice (Figure 3(a)). However, the frequency of CD8+CD69+ T cells in the Jα18−/− was lower than observed in WT group (Figure 3(b)). Because CD69 molecule represents an early activation marker, this result indicates that iNKT cells are required for the early activation of CD8+ T cells [24]. No significant difference was observed regarding CD69 expression in the CD4+ subset.
Figure 3.
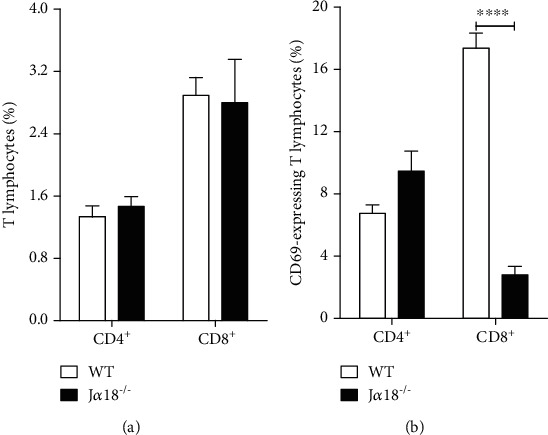
Deficiency in invariant Natural Killer T cells impairs early CD8 T lymphocyte activation during P. brasiliensis infection. WT and Jα18−/− C57BL/6 mouse strains were infected with 1 x 106 Pb18 yeasts, and 72 h later, the lung parenchyma was analyzed for: (a) frequency of CD4+ and CD8+ T lymphocytes and (b) frequency of CD69-expressing CD4 and CD8 T cells. Data represent the mean ± SD from 1 of 2 independent experiments (n = 5/group). ∗∗∗∗p < 0.0001.
Further analysis revealed the presence of distinct GR1+CD11b+SSChigh and GR1+CD11b+SSClow cellular populations between WT and Jα18−/− animals (Figure 4). We first found that the GR1+CD11b+SSChigh, but not the GR1+CD11b+SSClow, subset was significantly increased in the Jα18−/− group versus WT animals (Figures 4(a) and 4(d), respectively). These cellular populations have been described as suppressor or effector myeloid cells according to their functional state [25]. Therefore, to better characterize their immunological status, we first determined the surface expression of the MHC-II molecule [26]. In the WT group, the frequency of MHC-II-expressing cells and its surface expression was higher in both GR1+CD11b+SSChigh and GR1+CD11b+SSClow cells indicating a proinflammatory profile (Figures 4(b), 4(c), 4(e), and 4(f), respectively) [26]. This notion is corroborated by a higher surface expression of CD11b molecule, especially within the GR1+CD11b+SSClow subset (Figures 4(g) and 4(h)) [27].
Figure 4.
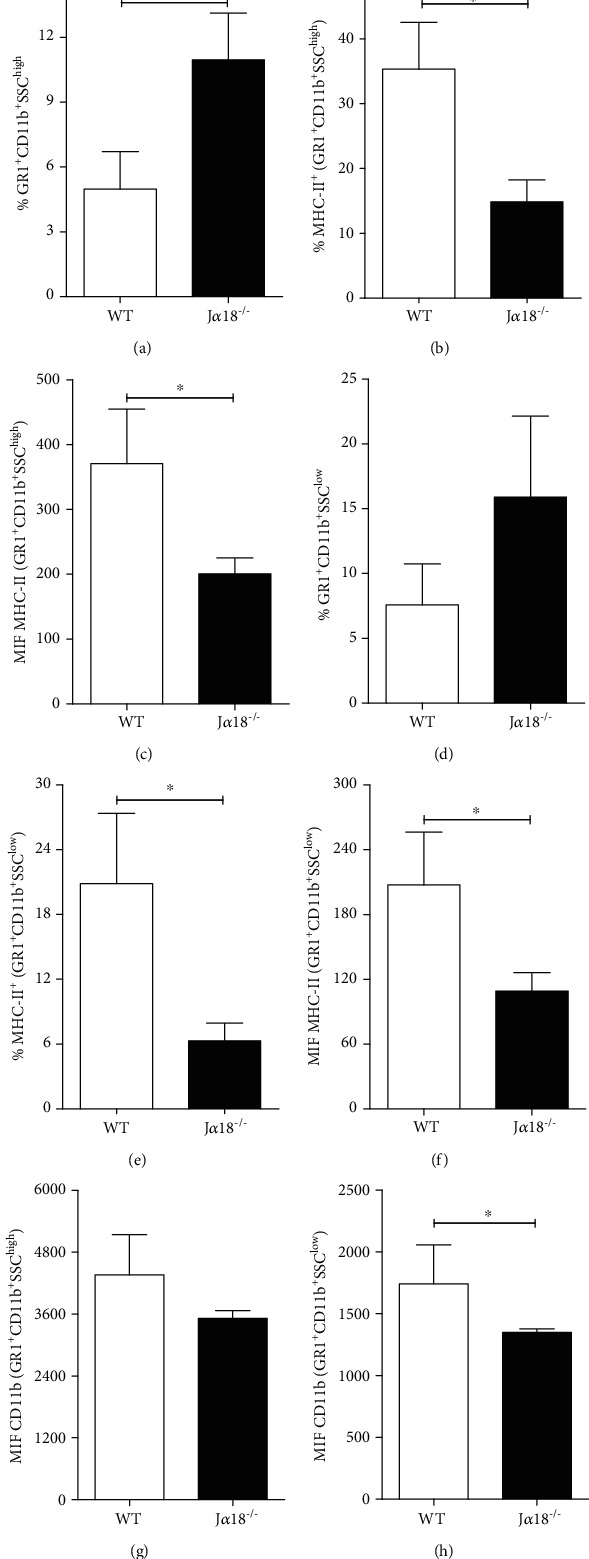
iNKT cells deficiency is associated with the accumulation of suppressor myeloid-derived cells in the lungs of P. brasiliensis infected mice. WT and Jα18−/− C57BL/6 mouse strains were infected with 1 x 106 Pb18 yeasts, and 72 h later, the lung parenchyma was analyzed for: Frequency of (a) GR1+CD11b+SCChigh and (b) GR1+CD11b+SCClow myeloid-derived cells. Frequency of MHC-II-expressing cells within (b) GR1+CD11b+SCChigh and (e) GR1+CD11b+SCClow subsets and MHC-II surface expression level (c, f, respectively). Expression levels of CD11b molecules within the (g) GR1+CD11b+SCChigh and (h) GR1+CD11b+SCClow myeloid-derived cells subsets. Data represent the mean ± SD from 1 of 2 independent experiments (n = 5/group). ∗p < 0.05.
3.4. Deficiency in iNKT Cells Impairs Host Resistance against P. brasiliensis Infection
Because iNKT deficiency impaired the early immune response against Pb18, we next determined their influence on a late phase of fungal infection. To this end, WT and Jα18−/− mice were infected with 1 x 106 living Pb yeast cells, and 45 days later, the lungs, spleen, and liver were analyzed to determine fungal loads. Figures 5(a) and 5(b) demonstrate that Jα18−/− mice presented a higher number of viable fungal cells in the lungs and spleen than WT animals. No differences in CFUs were detected in the livers (Figure 5(c)). These data indicate that the absence of iNKT cells increases the susceptibility of iNKT-deficient mice to Pb18 infection and support the idea that iNKT cells are important to control fungal growth at the site of infection and its dissemination to other organ.
Figure 5.
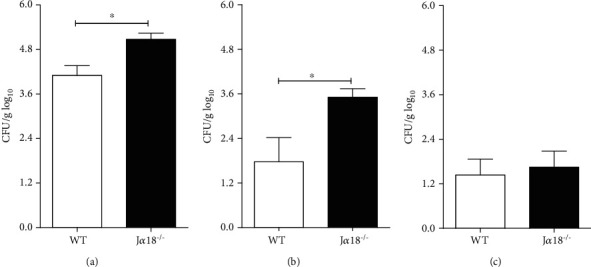
Invariant Natural Killer T cells restrain P. brasiliensis growth and dissemination. WT and Jα18−/− C57BL/6 mouse strains were infected with 1 x 106 Pb18 yeast, and 45 days later, the fungal loads were determined in the lungs (a), spleen (b), and liver (c). Data represent the mean ± SD from 1 of 2 independent experiments (n = 5/group). ∗p < 0.05.
3.5. Treatment of Mice with an iNKT Cells Agonist (α-GalCer) Confers Resistance to P. brasiliensis Infection
It is well described that activation of iNKT cells by exogenous ligands, especially α-GalCer, modulates different immune responses [28]. Thus, C57Bl/6 WT mice were treated with 10 μg of α-GalCer at week four after infection to test its effect on the host resistance to P. brasiliensis infection. Briefly, four weeks after i.t. infection with 1 x 106 living yeasts, infected mice received one single i.v. injection of 10 μg of α-GalCer, and four weeks later, the influx of leukocytes to the airways and pulmonary fungal loads was determined.
The α-GalCer treatment resulted in a more intense inflammatory reaction against P. brasiliensis inoculation. The total number of cells and number of neutrophils were higher in the BAL of α-GalCer-treated mice compared to nontreated mice (Figures 6(a) and 6(b), respectively). This increased inflammatory response was concomitant with lower fungal loads in the lungs of α-GalCer-treated mice (Figure 6(c)). Therefore, these data further indicate the protective role of iNKT cells and suggest that iNKT activation by specific agonist can be used as a possible therapeutic tool in pulmonary paracoccidioidomycosis.
Figure 6.
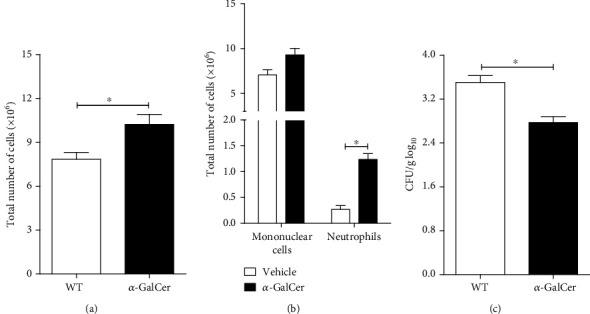
Treatment of P. brasiliensis infected mice with α-GalCer, an iNKT specific agonist, enhances host resistance to P. brasiliensis infection. WT C57BL/6 mice were infected with 1 x 106 Pb18 yeasts and treated four weeks later with 10 μg of α-GalCer by the i.v. route. At week 8 after infection, the number of inflammatory cells in the BAL and the number of viable yeasts in the lung parenchyma of α-GalCer-treated and untreated mice were determined. (a) Total number of inflammatory cells in BAL. (b) Mononuclear and neutrophil cell counts in the airways. (c) Fungal loads in lung parenchyma. Data represent the mean ± SD from 1 independent experiment (n = 5/group). ∗p < 0.05.
4. Discussion
Paracoccidioides brasiliensis is an opportunistic fungus, which causes occasional, self-limited, infections in the majority of immunocompetent individuals. However, systemic spreading with severe clinical manifestations has been correlated with intrinsic genetic characteristics of hosts, such as primary immunodeficiencies and individual lifestyle such as smoking, alcohol abuse, malnutrition, and treatment with immunosuppressive drugs [29, 30]. Despite the extensive literature about the importance of innate immunity in the acute host response to infection and fungal control, the role of iNKT cells remains unknown [31]. To determine the impact of invariant Natural Killer T cells in P. brasiliensis infection, we used animals that lack the CD1d molecule, which are deficient in diverse CD1d-restricted cells, or the Jα281 TCR gene segment (Jα18−/−), which results in the specific depletion of the iNKT phenotype [13, 23].
In response to the contact with Pb-infected MΦ, naive splenocytes from WT mice produced high levels of IFN-γ, induced high levels of NO, and controlled fungal growth. These findings corroborate the notion that in vitro control of fungal growth by macrophages correlates with the ability of splenocytes to produce IFN-γ [32]. In contrast, splenic cells from CD1d−/− mice failed to produce IFN-γ, stimulate NO production, and control P. brasiliensis growth. Therefore, we could demonstrate that CD1d-restricted cells are responsible for the splenocytes IFN-γ production in response to P. brasiliensis infection and exerted an essential role for the early contention of fungal growth.
Several studies demonstrated that CD1d-restricted T cells comprise distinct cell subpopulations [23]. Thus, to demonstrate that it was specifically the iNKT cell subset, the one that exerted a regulatory role in pulmonary PCM, we used the Jα18−/− mice strain, which lacks these cells due to the deletion of the Jα281 gene segment [13]. Similar to CD1d−/− splenocytes, naive cells from Jα18−/− mice failed to produce IFN-γ, induce NO production, and control fungal growth. Because no IFN-γ production was detected in the presence of naive splenocytes from both CD1d−/− or Jα18−/− mice, it is conceivable to assume that the iNKT cells are responsible for the IFN-γ secretion and the control of fungal growth by P. brasiliensis infected MΦ.
To determine the in vivo influence of iNKT cells on host response to P. brasiliensis, we took advantage of the murine model of intratracheal fungal infection. The BAL analysis revealed that during the early phase of infection, the absence of iNKT cells impaired the migration of inflammatory cells to the airways, as determined by reduced airway neutrophilia. In parallel to this finding, iNKT cell deficiency also impaired KC production, a chemokine extensively described as a homing factor for neutrophils [33]. Although the mechanisms that control KC and neutrophilic inflammation are complex, it has been described that during P. brasiliensis infection, IFN-γ mediates diverse chemokines production, including KC [34]. Thus, it is pertinent to suppose that the lower levels of KC found in the BAL of Jα18−/−-infected mice result from impaired IFN-γ production in the absence of iNKT cells. Although iNKT cells are rapid IFN-γ producing cells, neutrophils also seem to contribute for IFN-γ production during P. brasiliensis infection [35]. Thus, the reduced IFN-γ levels in Jα18−/− mice during the acute responses may result from the absence of iNKT cells and the consequent impairment of neutrophil recruitment. The reduced ability to produce IFN-γ also impacted the secretion of fungicide, or fungistatic metabolites, such as nitric oxide (NO) [36].
The lung parenchyma analysis confirmed the in vivo inability of Jα18−/− mice to mount a protective inflammatory response. During the acute phase of the infection, iNKT deficiency was associated with a lower frequency of CD69-expressing CD8+, but not CD4+, T cells indicating their suboptimal activation. Although further experiments are required to better explore the relationship between iNKT and CD8+ cells during the acute phase of P. brasiliensis infection, the cross-talk between iNKT cells and CD8+ T lymphocytes is a phenomenon well recognized. The generation of short-lived effector cells in peripheral lymphoid organs depends on an initial short-lived interaction (0-6 h) followed by a prolonged-lasting contact (12-24 h) [37]. Also, IFN-γ production by iNKT cells is essential for the physiological induction of specific CD8 effector T cells [38]. Because the control of fungal loads has been associated with the CD8+ T lymphocyte subset, it is possible to propose that iNKT cells play an important role in generating effector CD8T+ cells during the beginning of P. brasiliensis infection [39, 40]. Furthermore, iNKT cell deficiency was also associated with the accumulation of myeloid-derived suppressor- (MDSC-) like cells. Although the interaction between iNKT lymphocytes and MDSC during infection remains unclear, there is evidence that activated iNKT cells can inhibit the immunosuppressive activity of MDSC [41, 42]. Myeloid cells expressing concomitantly the GR-1 and the CD11b markers represent a group of diverse polymorphonuclear (SSChigh) and monocytic (SSClow) suppressor or effector cells [25, 26, 43]. Although a clear distinction between the suppressor or effector subsets requires very specific assays, the high expression of MHC-II and CD11b molecules may be used to distinguish between these cellular subsets [26, 27]. In this context, we found that during P. brasiliensis infection, the frequency of GR-1+CD11b+ cells expressing low MHC-II and CD11b levels increased in the Jα18−/− animals, suggesting the appearance of the suppressive subset. Thus, it is reasonable to hypothesize that the early activation of iNKT cells in response to P. brasiliensis modulates the appearance of MDSC in the pulmonary environment.
The results discussed above support the idea that the absence of iNKT lymphocytes leads to an immunocompromised microenvironment that impairs host resistance against P. brasiliensis infection. Indeed, the importance of iNKT cells in the immunity against P. brasiliensis is evident when comparing fungal burdens between WT and Jα18−/− mice. iNKT cell deficiency resulted in higher fungal loads in both lungs and spleen compared to the WT mice, indicating that these cells are key players in the control of fungus dissemination.
Finally, a previous study demonstrated that a single high dose of α-GalCer improved the outcome of Mycobacterium tuberculosis infection [44]. Thus, we next addressed the impact of α-GalCer administration on P. brasiliensis-infected mice. Animals treated with α-GalCer four weeks after fungal infection exhibited a significant reduction in the pulmonary fungal loads at week 8 after infection. Although the mechanisms involved in this phenomenon remain an object of study, fungal growth control was parallel to a more robust airway inflammatory response.
In conclusion, this study provides the first evidence of the contribution of iNKT cells to host resistance against P. brasiliensis infection and that their activation by exogenous agonists could be considered a therapeutic tool to improve immunity to infection.
Acknowledgments
This study was supported by research grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (ACK: 2012/04692-1 and 2016/02189-1; VLGC: 2011/51258-2; and 2016/23189-0), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance code 001. ACK, DSR, FVL and VLGC have personal scientific grants from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq).
Data Availability
Data is available under reasonable request.
Conflicts of Interest
Although ACK is a member of the academic editorial board from the Journal of Immunology Research, he declares no conflict of interest. All the other authors declare that there is no conflict of interest.
Supplementary Materials
Supplemental Figure 1: analysis of CD4 and CD8 T lymphocytes in lung parenchyma. After exclusion of dead cells, the CD3+ cells were analyzed for CD4 or CD8 expression and the frequency of CD69-expressing cells was determined within these populations. Supplemental Figure 2: analysis of GR-1+CD11b+ subsets in lung parenchyma. After exclusion of dead cells, the GR-1+ cells, within the CD11b+ subset, were analyzed according to SSC size (high or low). Next, both subsets were analyzed for MHC-II and CD11b expression.
References
- 1.Shikanai-Yasuda M. A., Mendes R. P., Colombo A. L., et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Revista da Sociedade Brasileira de Medicina Tropical. 2017;50(5):715–740. doi: 10.1590/0037-8682-0230-2017. [DOI] [PubMed] [Google Scholar]
- 2.Benard G., Romano C. C., Cacere C. R., Juvenale M., Mendes-Giannini M. J., Duarte A. J. Imbalance of IL-2, IFN-γ and IL-10 secretion in the immunosuppression associated with human paracoccidioidomycosis. Cytokine. 2001;13(4):248–252. doi: 10.1006/cyto.2000.0824. [DOI] [PubMed] [Google Scholar]
- 3.Kashino S. S., Fazioli R. A., Cafalli-Favati C., et al. Resistance to Paracoccidioides brasiliensis infection is linked to a preferential Th1 immune response, whereas susceptibility is associated with absence of IFN-gamma production. Journal of Interferon & Cytokine Research. 2000;20(1):89–97. doi: 10.1089/107999000312766. [DOI] [PubMed] [Google Scholar]
- 4.Almeida S. R., Lopes J. D. The low efficiency of dendritic cells and macrophages from mice susceptible to Paracoccidioides brasiliensis in inducing a Th1 response. Brazilian Journal of Medical and Biological Research. 2001;34(4):529–537. doi: 10.1590/S0100-879X2001000400014. [DOI] [PubMed] [Google Scholar]
- 5.Calich V. L., Pina A., Felonato M., Bernardino S., Costa T. A., Loures F. V. Toll-like receptors and fungal infections: the role of TLR2, TLR4 and MyD88 in paracoccidioidomycosis. FEMS immunology and Medical Microbiology. 2008;53(1):1–7. doi: 10.1111/j.1574-695X.2008.00378.x. [DOI] [PubMed] [Google Scholar]
- 6.Moreira A. P., Dias-Melicio L. A., Peracoli M. T., Calvi S. A., Victoriano de Campos Soares A. M. Killing of Paracoccidioides brasiliensis yeast cells by IFN-γ and TNF-α activated murine peritoneal macrophages: evidence of H2O2 and NO effector mechanisms. Mycopathologia. 2008;166(1):17–23. doi: 10.1007/s11046-007-9046-3. [DOI] [PubMed] [Google Scholar]
- 7.Lopera D., Aristizabal B. H., Restrepo A., Cano L. E., Gonzalez A. Lysozyme plays a dual role against the dimorphic fungus Paracoccidioides brasiliensis. Revista do Instituto de Medicina Tropical de Sao Paulo. 2008;50(3):169–175. doi: 10.1590/S0036-46652008000300008. [DOI] [PubMed] [Google Scholar]
- 8.Calich V. L., da Costa T. A., Felonato M., et al. Innate immunity to Paracoccidioides brasiliensis infection. Mycopathologia. 2008;165(4-5):223–236. doi: 10.1007/s11046-007-9048-1. [DOI] [PubMed] [Google Scholar]
- 9.Brummer E., Hanson L. H., Stevens D. A. Gamma-interferon activation of macrophages for killing of Paracoccidioides brasiliensis and evidence for nonoxidative mechanisms. International Journal of Immunopharmacology. 1988;10(8):945–952. doi: 10.1016/0192-0561(88)90041-0. [DOI] [PubMed] [Google Scholar]
- 10.Kurita N., Biswas S. K., Oarada M., Sano A., Nishimura K., Miyaji M. Fungistatic and fungicidal activities of murine polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Medical Mycology. 1999;37(1):19–24. doi: 10.1080/02681219980000031. [DOI] [PubMed] [Google Scholar]
- 11.Stetson D. B., Mohrs M., Reinhardt R. L., et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. Journal of Experimental Medicine. 2003;198(7):1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawano T., Cui J., Koezuka Y., et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 13.Cui J., Shin T., Kawano T., et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda J. L., Mallevaey T., Scott-Browne J., Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Current Opinion in Immunology. 2008;20(3):358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klibi J., Amable L., Benlagha K. A focus on NKT cell subset characterization and developmental stages. Immunology and Cell Biology. 2020;98(7):p. 607. doi: 10.1111/imcb.12378. [DOI] [PubMed] [Google Scholar]
- 16.McKee S. J., Mattarollo S. R., Leggatt G. R. Immunosuppressive roles of natural killer T (NKT) cells in the skin. Journal of Leukocyte Biology. 2014;96(1):49–54. doi: 10.1189/jlb.4RU0114-001R. [DOI] [PubMed] [Google Scholar]
- 17.Arora P., Foster E. L., Porcelli S. A. CD1d and natural killer T cells in immunity to Mycobacterium tuberculosis. Advances in Experimental Medicine and Biology. 2013;783:199–223. doi: 10.1007/978-1-4614-6111-1_11. [DOI] [PubMed] [Google Scholar]
- 18.Batista V. G., Moreira-Teixeira L., Leite-de-Moraes M. C., Benard G. Analysis of invariant natural killer T cells in human paracoccidioidomycosis. Mycopathologia. 2011;172(5):357–363. doi: 10.1007/s11046-011-9451-5. [DOI] [PubMed] [Google Scholar]
- 19.Defaveri J., Rezkallah-Iwasso M. T., de Franco M. F. Experimental pulmonary paracoccidioidomycosis in mice: morphology and correlation of lesions with humoral and cellular immune response. Mycopathologia. 1982;77(1):3–11. doi: 10.1007/BF00588649. [DOI] [PubMed] [Google Scholar]
- 20.Kashino S. S., Calich V. L., Burger E., Singer-Vermes L. M. In vivo and in vitro characteristics of six Paracoccidioides brasiliensis strains. Mycopathologia. 1985;92(3):173–178. doi: 10.1007/BF00437630. [DOI] [PubMed] [Google Scholar]
- 21.Singer-Vermes L. M., Ciavaglia M. C., Kashino S. S., Burger E., Calich V. L. The source of the growth-promoting factor(s) affects the plating efficiency of Paracoccidioides brasiliensis. Journal of Medical and Veterinary Mycology. 1992;30(3):261–264. doi: 10.1080/02681219280000331. [DOI] [PubMed] [Google Scholar]
- 22.Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. Journal of Immunology. 1988;141(7):2407–2412. [PubMed] [Google Scholar]
- 23.Pellicci D. G., Uldrich A. P. Unappreciated diversity within the pool of CD1d-restricted T cells. Seminars in Cell & Developmental Biology. 2018;84:42–47. doi: 10.1016/j.semcdb.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Testi R., Phillips J. H., Lanier L. L. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. Journal of Immunology. 1989;142(6):1854–1860. [PubMed] [Google Scholar]
- 25.Obregon-Henao A., Henao-Tamayo M., Orme I. M., Ordway D. J. Gr1(int)CD11b+ myeloid-derived suppressor cells in Mycobacterium tuberculosis infection. PLoS One. 2013;8(11, article e80669) doi: 10.1371/journal.pone.0080669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makarenkova V. P., Bansal V., Matta B. M., Perez L. A., Ochoa J. B. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. Journal of Immunology. 2006;176(4):2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 27.Duan M., Steinfort D. P., Smallwood D., et al. CD11b immunophenotyping identifies inflammatory profiles in the mouse and human lungs. Mucosal Immunology. 2016;9(2):550–563. doi: 10.1038/mi.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiefner A., Fujio M., Wu D., Wong C. H., Wilson I. A. Structural evaluation of potent NKT cell agonists: implications for design of novel stimulatory ligands. Journal of Molecular Biology. 2009;394(1):71–82. doi: 10.1016/j.jmb.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez R. New trends in paracoccidioidomycosis epidemiology. Journal of Fungi. 2017;3(1):p. 1. doi: 10.3390/jof3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Restrepo A., Benard G., de Castro C. C., Agudelo C. A., Tobon A. M. Pulmonary paracoccidioidomycosis. Seminars in Respiratory and Critical Care Medicine. 2008;29(2):182–197. doi: 10.1055/s-2008-1063857. [DOI] [PubMed] [Google Scholar]
- 31.Cezar-Dos-Santos F., Assolini J. P., Okuyama N. C. M., Viana K. F., de Oliveira K. B., Itano E. N. Unraveling the susceptibility of paracoccidioidomycosis: insights towards the pathogen-immune interplay and immunogenetics. Infection, Genetics and Evolution. 2020;86:p. 104586. doi: 10.1016/j.meegid.2020.104586. [DOI] [PubMed] [Google Scholar]
- 32.Brummer E., Hanson L. H., Restrepo A., Stevens D. A. In vivo and in vitro activation of pulmonary macrophages by IFN-gamma for enhanced killing of Paracoccidioides brasiliensis or Blastomyces dermatitidis. Journal of Immunology. 1988;140(8):2786–2789. [PubMed] [Google Scholar]
- 33.Kobayashi Y. Neutrophil infiltration and chemokines. Critical Reviews in Immunology. 2006;26(4):307–316. doi: 10.1615/CritRevImmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- 34.Souto J. T., Aliberti J. C., Campanelli A. P., et al. Chemokine production and leukocyte recruitment to the lungs of Paracoccidioides brasiliensis-infected mice is modulated by interferon-γ. The American Journal of Pathology. 2003;163(2):583–590. doi: 10.1016/S0002-9440(10)63686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pino-Tamayo P. A., Puerta-Arias J. D., Lopera D., Uran-Jimenez M. E., Gonzalez A. Depletion of neutrophils exacerbates the early inflammatory immune response in lungs of mice infected with Paracoccidioides brasiliensis. Mediators of Inflammation. 2016;2016:17. doi: 10.1155/2016/3183285.3183285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez A., de Gregori W., Velez D., Restrepo A., Cano L. E. Nitric oxide participation in the fungicidal mechanism of gamma interferon-activated murine macrophages against Paracoccidioides brasiliensis conidia. Infection and Immunity. 2000;68(5):2546–2552. doi: 10.1128/IAI.68.5.2546-2552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valente M., Dolen Y., van Dinther E., et al. Cross-talk between iNKT cells and CD8 T cells in the spleen requires the IL-4/CCL17 axis for the generation of short-lived effector cells. Proceedings of the National Academy of Sciences of the united States of America. 2019;116(51):25816–25827. doi: 10.1073/pnas.1913491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattarollo S. R., Yong M., Tan L., Frazer I. H., Leggatt G. R. Secretion of IFN-gamma but not IL-17 by CD1d-restricted NKT cells enhances rejection of skin grafts expressing epithelial cell-derived antigen. The Journal of Immunology. 2010;184(10):5663–5669. doi: 10.4049/jimmunol.0903730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiarella A. P., Arruda C., Pina A., Costa T. A., Ferreira R. C., Calich V. L. The relative importance of CD4+ and CD8+T cells in immunity to pulmonary paracoccidioidomycosis. Microbes and Infection. 2007;9(9):1078–1088. doi: 10.1016/j.micinf.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Paget C., Ivanov S., Fontaine J., et al. Potential role of invariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia. Journal of Immunology. 2011;186(10):5590–5602. doi: 10.4049/jimmunol.1002348. [DOI] [PubMed] [Google Scholar]
- 41.Ko H. J., Lee J. M., Kim Y. J., Kim Y. S., Lee K. A., Kang C. Y. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. Journal of Immunology. 2009;182(4):1818–1828. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- 42.Wu D., Shi Y., Wang C., et al. Activated NKT cells facilitated functional switch of myeloid-derived suppressor cells at inflammation sites in fulminant hepatitis mice. Immunobiology. 2017;222(2):440–449. doi: 10.1016/j.imbio.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Damuzzo V., Pinton L., Desantis G., et al. Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry Part B: Clinical Cytometry. 2015;88(2):77–91. doi: 10.1002/cyto.b.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sada-Ovalle I., Skold M., Tian T., Besra G. S., Behar S. M. Alpha-galactosylceramide as a therapeutic agent for pulmonary Mycobacterium tuberculosis infection. American Journal of Respiratory and Critical Care Medicine. 2010;182(6):841–847. doi: 10.1164/rccm.200912-1921OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: analysis of CD4 and CD8 T lymphocytes in lung parenchyma. After exclusion of dead cells, the CD3+ cells were analyzed for CD4 or CD8 expression and the frequency of CD69-expressing cells was determined within these populations. Supplemental Figure 2: analysis of GR-1+CD11b+ subsets in lung parenchyma. After exclusion of dead cells, the GR-1+ cells, within the CD11b+ subset, were analyzed according to SSC size (high or low). Next, both subsets were analyzed for MHC-II and CD11b expression.
Data Availability Statement
Data is available under reasonable request.


