Abstract
Several vaccines for SARS-CoV-2 are expected to be available in Australia in 2021. Initial supply is limited and will require a judicious vaccination strategy until supply is unrestricted. If vaccines have efficacy as post-exposure prophylaxis (PEP) in contacts, this provides more policy options. We used a deterministic mathematical model of epidemic response with limited supply (age-targeted or ring vaccination) and mass vaccination for the State of New South Wales (NSW) in Australia. For targeted vaccination, the effectiveness of vaccinating health workers, young people and older adults was compared. For mass vaccination, we tested varying vaccine efficacy (VE) and distribution capacities. With a limited vaccine stockpile enough for 1 million people in NSW, if there is efficacy as PEP, the most efficient way to control COVID-19 will be ring vaccination, however at least 90% of contacts per case needs to be traced and vaccinated. Health worker vaccination is required for health system resilience. Age based strategies with restricted doses make minimal impact on the epidemic, but vaccinating older people prevents more deaths. Herd immunity can only be achieved with mass vaccination. With 90% VE against all infection, herd immunity can be achieved by vaccinating 66% of the population. A vaccine with less than 70% VE cannot achieve herd immunity and will result in ongoing risk of outbreaks. For mass vaccination, distributing at least 60,000 doses per day is required to achieve control. Slower rates of vaccination will result in the population living with COVID-19 longer, and higher cases and deaths.
Keywords: COVID-19, SARS-CoV-2, Vaccines, Epidemic, Australia
1. Introduction
Coronavirus disease 19 (COVID-19) has caused an unprecedented pandemic, with catastrophic health, social and economic impacts [1]. Without available drugs or vaccines, nations require ongoing or intermittent use of non-pharmaceutical approaches such as lockdowns, border closures and social distancing to control COVID-19 [2], [3]. In addition, case finding through increased testing and surveillance, with contact tracing and quarantine, are the mainstays of epidemic control [4]. While Australia has achieved good control over the pandemic through border closures, testing, contact tracing and hotel quarantine, the risk of intermittent outbreaks of COVID-19 will continue until an effective vaccine is available [3] and administered appropriately to a large enough proportion of the population. The societal impact of ongoing restrictions and border closure cannot be under-estimated.
There is a massive global effort to fast-track the development of COVID-19 vaccines [5], [6]. Currently, there are 48 COVID-19 vaccine candidates undergoing clinical evaluation, with 11 already in phase 3 clinical trials [7]. Data from phase 3 trials suggest that mRNA vaccines have over 94% efficacy against COVID-19 infection [8], [9]. The ChAdOx1 nCoV-19, the largest component of the planned Australian vaccine stockpile, has efficacy of 62% against symptomatic infection in the intended two-dose schedule [10]. On December 11 it was announced that the second largest component of the Australian stockpile had been withdrawn from further development [11]. A small proportion of the Australian vaccine plan includes the BNT162b2 mRNA vaccine, which has 95% efficacy against symptomatic infection [11], [12]. As more vaccine candidates become available through 2021, we will continue to see variation in efficacy and safety between them.
It is likely there will be initial vaccine shortages. Thus, an effective vaccination strategy will need to be developed to best utilise limited vaccine supply in the early phases of vaccine rollout [13], with a plan for expanded vaccination at a later stage.
Several COVID-19 vaccination strategies have been proposed, [13] such as prioritising healthcare and aged care workers and other frontline responders at high risk of disease transmission, and sociodemographic groups at significantly higher risk of severe disease, such as older adults or people with high risk chronic health conditions [13], [14]. However, prioritizing young people may impact transmission more, since vaccines are more effective in younger people, and transmission is highest in young adults [15]. Alternatively, a ring vaccination strategy could be utilized, which involves identifying the close contacts of a confirmed case and vaccinating them. This strategy was used effectively against Ebola, and also smallpox in settings where mass vaccination was not possible, despite efficacy being half that of primary prevention [16], [17], [18], [19]. Many vaccines including measles, hepatitis A and smallpox, are effective as post-exposure prophylaxis (PEP) and can be given to contacts during an outbreak, albeit with lower efficacy than primary prevention [20]. Whether COVID-19 vaccines will be effective as PEP is unknown as yet, but may well be given the long incubation period [21].
In Australia, the stated priority groups for early COVID-19 vaccination are: 1) individuals with increased risk of severe disease, such as older adults, Aboriginal and Torres Strait Islander people, and those with high risk chronic conditions; 2) individuals at higher risk of disease, such as health and aged care workers; and 3) individuals working in critical services [22]. Vaccination is being rolled out to frontline health workers, aged care residents and hotel quarantine workers first. The aim of this paper was to model the impact of various COVID-19 vaccine strategies under a limited supply scenario, as well as varied vaccine efficacy and speed of vaccination for mass vaccination, on COVID-19 case numbers and mortality in New South Wales, the most populous state of Australia.
2. Methods
A previously published age structured deterministic compartmental model for Covid-19 spread [23] was modified to test several possible vaccination strategies under limited or unlimited supply assumptions, as described in more detail below. We used the NSW population and age distribution from 2020 [24], stratified into 16 five-year age groups from 0 to 74 years old and the last age group comprising people 75 years and over.
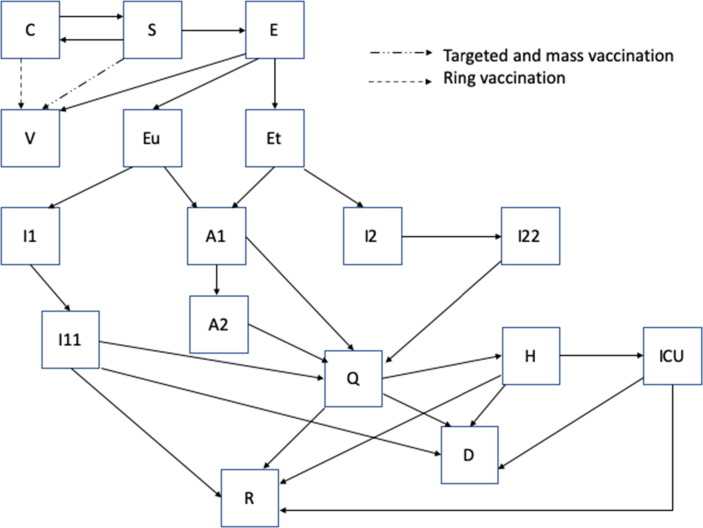
The model moves the population in 17 mutually exclusive compartments, each divided by the 17 age groups: susceptible (S), latent, not infectious (E), pre-symptomatic infectious and diagnosed (Et), pre-symptomatic infectious and undiagnosed (Eu), contacts traced (C) symptomatic, high infectiousness, diagnosed (I1) and undiagnosed (I2), asymptomatic, high infectiousness (A1), symptomatic, lower infectiousness, previous diagnosed (I11) and undiagnosed (I22), asymptomatic low infectiousness (A2), home isolated (Q), hospitalized (H), individuals admitted to intensive care (ICU) , recovered (R), successfully vaccinated (V) and COVID-19 deceased people (D) (Fig. 1 ). The duration of stay in each compartment determines the rate at which people move from one compartment to another for the epidemiological disease states. The duration of the latent period is assumed to be 5.2 days [25], of which the last two days before symptoms onset are considered infectious [26].
Fig. 1.
Model diagrams for targeted, mass and ring vaccination.
The transmission was distributed over the infectious period with 44% of transmissions occurring in the last two days of the pre-symptomatic state. We assume that the viral load is very high on the first day of symptoms and then decreases to a lower infectious level, spread over the following 6 days [26], so 36% of transmissions occur in the first day of symptoms and 20% in the following 6 days of symptoms [26]. Of the total people infected, 35% are considered asymptomatic and never develop symptoms, however we assumed that asymptomatic cases are as equally infectious as symptomatic ones [27], [28], [29]. In conjunction with vaccination, other disease control interventions such as case finding, contacts tracing, quarantine, isolation and hospital, ICU treatments were included, however we did not include additional measures such as universal masking or lockdowns, neither of which are in effect in NSW.
The force of infection that moves people from susceptible to infected is an age specific rate calculated as a combination of average age specific number of close contacts per day in Australia [30], the proportion of infectious people and the probability of infection per contact, which is estimated to reproduce an R0 of 2.5 [28]. Once infected, a person enters the latent compartment, and after 3.2 days will become infectious and pre-symptomatic for 2 days, where if traced will be quarantined with a 50% reduction in transmissions [31] and upon symptoms onset will take 1 day on average to get isolated with no more transmissions after isolation. However, if an infected person is not traced in latency, it will take 5 days to get isolated following symptom onset [32]. Hospitalizations and admission to ICU follow age-specific rates [33]. The model assumes that 80% of all close contacts are traced and quarantined for 14 days and 90% of the symptomatic cases are isolated after 5 days.
There are no data on COVID-19 vaccines as PEP, but early data from phase three trials indicate efficacy in excess of 90% as primary prevention of mRNA vaccines [8]. The vaccine efficacy (VE) determines the percentage of people fully protected, and all VE estimates in the model are against all infection (symptomatic and asymptomatic). VE against all infection is assumed to prevent transmission, as infection is prevented altogether. Both the BNT162b2 mRNA and ChAdOx1 nCoV-19 vaccines are used in Australia. Vaccine efficacy was set at 90% against all infection in the base case scenario if a person is susceptible, based on effectiveness estimates for the BNT162b2 mRNA vaccine against all infection of 92% from Israel [34]. A sensitivity analysis incorporating lower efficacy such as with the ChAdOx1 nCoV-19 (AZD1222) was done as described below [35]. Our model assumed people vaccinated as PEP has a 50% reduction in protection from vaccination (from 90% to 45%). Successful vaccination is considered to give immunity to the infection for at least 12 months. Duration of immunity beyond 12 months is not considered, as long term follow up data are not available yet to inform the modelling of waning immunity. Fig. 1 shows model diagrams, and in Table 1 are described all parameters used in the model, while the differential equations are described in the supplementary materials.
Table 1.
Model parameters used and their values.
| Parameter | Symbol | Value | Source |
|---|---|---|---|
| Basic reproduction number | R0 | 2.5 | [28] |
| Latent pre symptomatic period | 3.2 not infectious + 2 infectious = 5.2 | [25] | |
| Infectious period | 2 + 1 + 6 = 9 days of which 2 presymptomatic (44% transmissions), first day symptomatic with higher transmissions (36% transmissions) and following 6 days symptomatic with lower transmissions (20% transmissions) | [26], [28], [36], [37], [38], [39] | |
| Time to get isolated once symptomatic | 1 + 4 = 5 days | [32] | |
| Time in ICU | 5 days | ||
| Time in hospital | 15 days | ||
| Effectiveness of home quarantine | R0/2 | 50% reduction in the R0 | [31] |
| Duration of quarantine | 14 days | ||
| Proportion of asymptomatic or very mild infectious | 35%, we assumed 70% remain undiagnosed and 30% (adr) are diagnosed and isolated | [28], [40], [41] | |
| Asymptomatic diagnosed rate | adr | 30% | |
| Proportion of contacts traced | 80% | [42] | |
| Proportion of symptomatic people that get isolated after 5 days | 90% | [42] | |
| Age-specific case fatality rate (%) for the 16 age groups For the severe hospitalised cases For the cases that needed ICU |
|
0, 0, 0.2, 0.2, 0.2, 0.2, 0.2, 0.2, 0.4, 0.4, 1.3, 1.3, 3.6, 3.6, 8, 14.8 | [43] |
| Hospitalization rates | h | 0–4 years old 0.003 5–19 years old 0.001 20–49 years old 0.025 50–64 years old 0.074 65–74 years old 0.122 75 + years old 0.165 |
[44] |
| ICU rates from hospitalization | icu | 14.2% in the 54 to 79 years old and we used the age specific hospitalization rates to estimate the age distribution of the ICU rates, we get 0.0013 (0.13%) for 0–19 years old hospitalized 0.0337 (3.37%) for 20–49 and 0.142 (14.2%) for 50+ |
[45] |
To test the most effective vaccination distribution for outbreak control, we used a hypothetical epidemic in NSW with 100 symptomatic people and 250 untraced latent infected people as the starting conditions, so that we could test the effectiveness of different vaccination strategies in an epidemic scenario.
The vaccination strategies tested are:
-
1.
Limited vaccine supply (2 million doses for 1 million people) given to targeted age groups;
-
2.
Limited vaccine supply, ring vaccination (contact tracing and vaccination of contacts);
-
3.
Unlimited supply and mass vaccination assuming enough doses to vaccinate the entire NSW population.
In the limited supply scenario, we assumed a limited stock of only 2 million doses (for 1 million people in a two-dose schedule) for NSW as an estimate of initial supply. For mass vaccination we assumed a delivery capacity of 50,000–300,000 doses per day [46]. We explored 3 different scenarios with a limited vaccine supply:
-
•
Vaccine delivered in 8 days to 1 million young people (age group 10–29);
-
•
Vaccine delivered in 8 days (first dose) to 1 million older people (age group 65 + );
-
•
Vaccinate 125,000 HCWs with first dose and over the following 7 days 875,000 people aged 10–29.
The two dose schedule was assumed to be given with 21–28 days between dose one and two. [10], [12] The health care workers (HCWs) for NSW were estimated from the 2019 estimation State and Territory Statistics – 2019 [47] and were assumed to be 3 times more susceptible to infection with COVID-19 [48]. The number of medical practitioners, nurses, midwives, and pharmacists in NSW is 135,379 – we assumed 80–90% would be in the clinical workforce and require vaccination (125,000 vaccinations). As their average age is respectively 47, 44 and 41 years old [47], we distributed them through 8 age groups from 25 to 64 years old, following the age distribution of HCWs.
For ring vaccination we assumed varying proportions of vaccination of all the traced contacts − 70%, 80% and 90%. For use as PEP, we assumed a 50% reduction in efficacy (from 90% to 45%) when given to latent pre-symptomatic people.
For mass vaccination in NSW, assuming that the vaccine supply is available for the population, we tested the effect of speed of achieving high coverage by comparing the capacity to deliver 50,000, 75,000, 100,000, 125,000, and 300,000 doses per day to vaccinate the NSW population. We also varied the efficacy of the vaccine to determine the minimum vaccine efficacy to achieve herd immunity, and the epidemic scenario with low and high efficacy vaccines. We used the following formula to calculate the required vaccine coverage (Vc) for herd immunity assuming R0 = 2.5. [28] In the base case, vaccine efficacy (Ve) = 0.9 (90%).
To test the effect of varied efficacy, vaccine coverage was held constant at 70%, reflecting a feasible mass vaccination goal. We tested varied vaccine efficacy of 38%, 50%, 60%, 70%, 80%, 90% and 95%. These estimates were selected based on published upper efficacy of vaccines of 94–95% [9], [10], [12], and the remaining values to reflect a plausible efficacy range of other potential vaccines. The lower estimate of 38% is the efficacy against any laboratory confirmed infection (symptomatic or asymptomatic) calculated from ChAdOx1 nCoV-19 vaccine (AZD1222) published data, excluding the data from the Brazilian component of the study, because weekly testing for infection was not done in Brazil. [10]
Model outputs are the epidemic curve (cases), total number of cases and deaths and, for ring and mass vaccination, there is the added output of total number of vaccine doses used and days to vaccinate the entire susceptible population, respectively.
3. Results
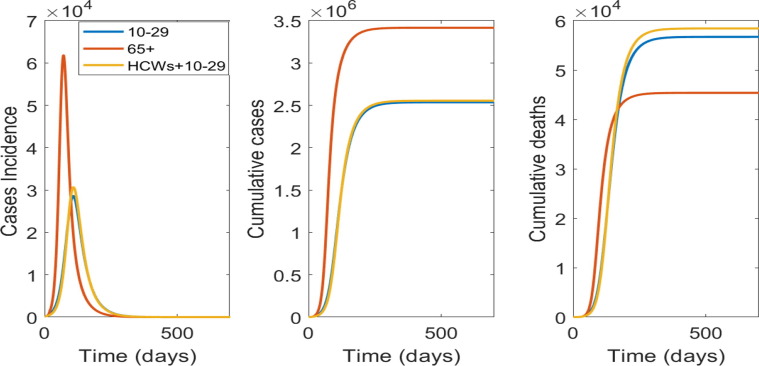
A restricted supply (for 1 million people) will not be enough to control the epidemic, with all scenarios resulting in a large number of cases and deaths after 500 days. Fig. 2 shows results for the choice of using the available doses in the age group 10–29 years old, vaccinating HCWs and then 10–29 years old, or vaccinating the 65 + years old. Targeting the younger age group will have more impact on reducing the number of cases, whilst vaccinating people 65 + will have more impact on deaths, as this age group is at much higher risk of death.
Fig. 2.
Targeted vaccination strategies with a restricted supply for 1 million people: From left to right is the epidemic curve, the cumulative case and deaths numbers by targeted age group vaccinated.
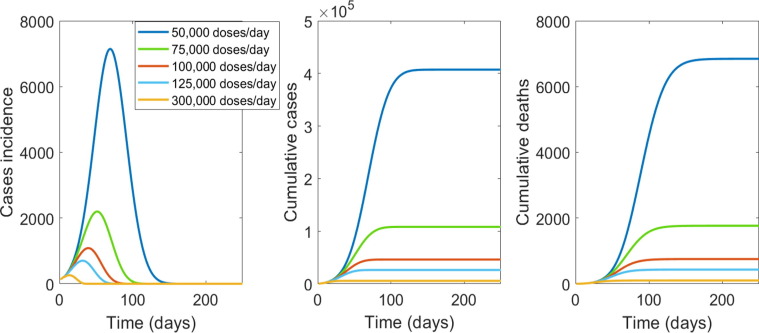
If enough vaccine is available to vaccinate the entire population of NSW, the important variables influencing the results will be vaccine efficacy and the speed of vaccination. In Fig. 3 we show results of varying vaccination capacity from 50,000, to 300,000 people per day. Vaccinating 50,000 people per day will take about 177 days to vaccinate the entire population with one dose, will result in a much longer and larger epidemic, compared to 125,000 or 300,000 people per day. However, if the health system has capacity to vaccinate at least 75,000 people per day the epidemic could be terminated rapidly (Fig. 3).
Fig. 3.
The impact of speed of mass vaccination with a vaccine of 90% efficacy. From left to right is the epidemic curve, the cumulative case and deaths numbers by number of number of people vaccinated per day in NSW.
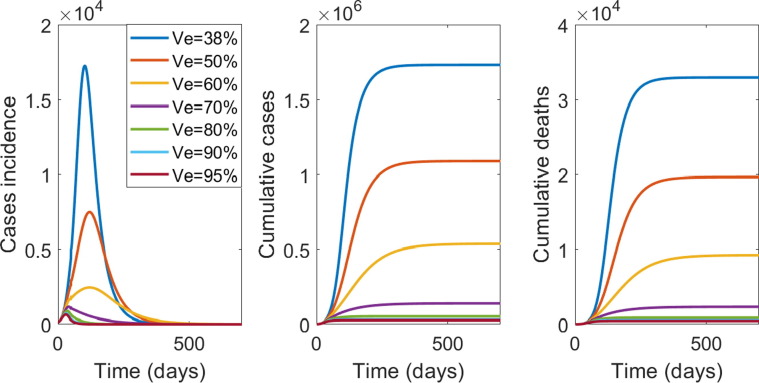
With a vaccine efficacy (VE) of 90% against all infection, vaccinating 66% of the total population (approximately 5.384 million people) will provide herd immunity and reduce the R0 to less than 1, while efficacy below 60% cannot achieve herd immunity. A minimum VE of 85% is required to achieve herd immunity at 70% population coverage. A VE of 80% will require 75% of the population to be vaccinated, and VE of 60% will require 100% of the population to be vaccinated to achieve herd immunity. Supplementary Table 1 shows the required vaccine coverage for varying VE, and that efficacy below 60% cannot achieve herd immunity.
Fig. 4 shows the effect of varied vaccine efficacy on the epidemic, with vaccine coverage of 70% (5.25 million people) with 125,000 doses delivered per day. When vaccine effectiveness drops to 70% the number of new cases a day (incidence) will not increase however will take up to one year to reduce the number of new cases per day to less than 50. At VE of 60% or less, transmission and the risk of outbreaks will continue, as the number of new cases keeps increasing for more than 3 months following the start of vaccination campaign.
Fig. 4.
From left to right is the epidemic curve, the cumulative case and deaths by vaccine efficacy (VE), with 125,000 people per day vaccinated until 70% of NSW population is vaccinated.
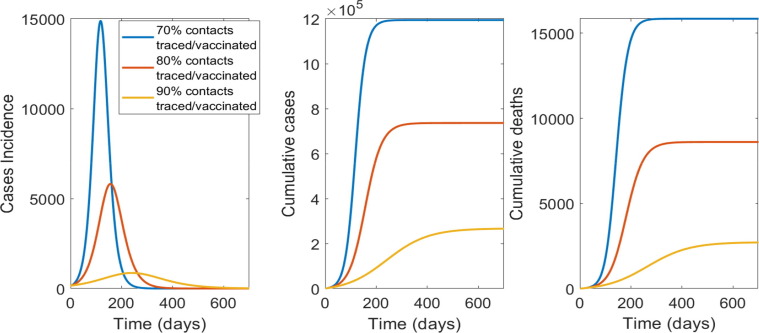
In Fig. 5 the results for ring vaccination with varying rates of contact tracing per day (70%, 80% or 90% of contacts of cases) are shown. The higher the proportion of contacts traced and vaccinated from the start, the lower the required vaccine doses. If only 70% of contacts are traced, the required vaccine doses are much higher and the epidemic impact much greater than 80 or 90% (Fig. 3).
Fig. 5.
The impact of contact tracing and ring vaccination on epidemic control for a vaccine with 45% efficacy as post-exposure prophylaxis: From left to right is the epidemic curve, the cumulative case and deaths numbers by percentage of contacts traced and vaccinated.
Table 2 shows all numerical results for the different scenario tested.
Table 2.
Total cases and deaths for targeted and mass vaccination scenarios, and total cases, deaths and vaccination used for ring vaccination scenarios.
| Targeted vaccination: age groups | Total cases | Total deaths | |
|---|---|---|---|
| 1) 10–29 | 2,536,040 | 56,708 | |
| 2) 65+ | 3,415,521 | 45,379 | |
| 3) HCWs + 10–29 | 2,554,442 | 58,409 | |
| Mass vaccination: people vaccinated per day (total 5,250,000 doses, 70% coverage) | Total cases | Total deaths | |
| 50,000 | 407,198 | 6,845 | |
| 75,000 | 108,233 | 1,766 | |
| 100,000 | 46,073 | 752 | |
| 125,000 | 26,197 | 431 | |
| 300,000 | 5,507 | 100 | |
| Contacts traced/vaccinated | Total doses used | Total cases | Total deaths |
| 70% | 2,494,500 | 1,194,140 | 15,850 |
| 80% | 1,849,300 | 736,254 | 8,608 |
| 90% | 794,720 | 266,164 | 2,707 |
In conclusion, with a limited vaccine stockpile the most efficient way to delivery vaccine doses during an epidemic will be ring vaccination, assuming a 45% efficacy when used as PEP, and at least 90% of contacts per case traced and vaccinated. If supply of vaccine is adequate to cover the entire NSW population, mass vaccination with a high efficacy vaccine and rapid uptake (at least 75,000 people vaccinated per day) will be the only strategy able to achieve herd immunity and prevent community transmission.
4. Discussion
We showed that herd immunity through vaccination is the only means to prevent sustained community transmission and requires mass vaccination of 66% of the population vaccinated with a vaccine of 90% efficacy against all infection. Vaccines of lower efficacy require higher vaccination coverage, but a VE below 70% against all infection may be inadequate for herd immunity. Mass vaccination with a high efficacy vaccine represents the best strategy for economic recovery, as herd immunity through effective vaccination will provide the best prospect for lifting of societal restrictions. Early results from Israel, which used the BNT162b2 mRNA vaccine are very promising, showing efficacy of 92% against asymptomatic infection, suggesting herd immunity is feasible [34]. Data from Scotland on use of the ChAdOx1 nCoV-19 show reduction in hospitalization, but do not report on asymptomatic infection [49]. Elimination cannot be achieved and sustained without vaccination [50].
We show that for a population of 7.5 million people, if faced with an initial restriction in vaccine supply, sustained epidemic control cannot be achieved by vaccination alone, and other non-pharmaceutical interventions will need to continue to mitigate the risk of outbreaks [2]. If COVID-19 vaccines have efficacy as PEP, contact tracing and ring vaccination is the best way to achieve epidemic control, but the majority of the population will remain non-immune and susceptible to outbreaks. Recurrent breaches in hotel quarantine [51] and associated with cruise ships have resulted in community transmission, and without herd immunity, this risk will continue and will hamper economic recovery [52].
During the period of initial restricted supply, health and aged care workers should be the highest priority, as protecting health workers and other first responders is essential for health system resilience in a pandemic. During a large second wave in the state of Victoria, over 3500 health and aged care workers became infected [53], and we demonstrated ongoing risk to health workers, even during periods of low community incidence [48]. After vaccinating health and aged care workers, the evidence supports vaccination of older adults. Age is the strongest predictor of morbidity and mortality, far more than any chronic disease [54], so protecting older adults will prevent the most deaths from COVID-19. Including younger people in the prioritisation group can reduce transmission and the overall infection rates, although this benefit is negated by the higher mortality rates in older people. Those findings are supported from a preprint study, which explored the most effective age-group to prioritize for vaccination in order to reduce morbidity and mortality from Covid-19 [55].
If COVID-19 vaccines are effective as PEP, this will be the best approach for epidemic control using a limited vaccine supply and requires capacity for high levels of contact tracing. If community transmission of COVID-19 remains low, complete contact tracing and ring vaccination will be a highly cost-effective, dose-sparing strategy. Whilst there are no data yet on the efficacy of COVID-19 vaccines as PEP, we showed that if a 50% reduction of efficacy is seen with these vaccines, that ring vaccination can be an effective strategy, and may be a better use of a restricted supply initially, if rates of contact tracing are high. Many vaccines have efficacy as post-exposure prophylaxis in outbreak settings, including measles, hepatitis A, varicella zoster and smallpox, with an efficacy of 50–90% of that for primary prevention [20]. This is a research gap for COVID-19 vaccines, which needs to be addressed. If a high (>80%) percentage of contacts cannot be traced (such as when an epidemic is very large and the scale of contact tracing is unmanageable, as occurred in the second wave in the State of Victoria [56], mass vaccination becomes more urgent.
We showed that for mass vaccination, a slow trickle approach will leave us living with COVID-19 longer with all the associated societal and economic implications - rapid speed of vaccination will be the best strategy to reduce morbidity and mortality. The logistics of rapid delivery is key, and we show that slower uptake will result in more cases and deaths and a longer epidemic risk. Rapid vaccine uptake requires concerted planning for vaccination infrastructure, including training more accredited vaccinators, planning mass vaccination clinics, and testing the capacity to deliver 300,000 or more vaccine doses a day, which provides the best outcomes. We assumed in the base case scenario for mass vaccination that the health system will be able to deliver 125,000 doses per day, which is similar to the distribution capacity estimated in Victoria of about 117,000 doses per day [44]. We showed that the speed of Covid-19 vaccine uptake is critical to rapid epidemic control, however the logistics of vaccine distribution is complex [57], particularly for a country such Australia, where an estimated 29% of the population lives in rural and remote areas [58]. Therefore, development of detailed logistical plans and tools to support increased capacity to vaccinate, as well as effective vaccine transport, storage, and continuous cold-chain monitoring is required.
No country has attempted mass vaccination since the eradication of smallpox. Currently in Australia, vaccination is delivered mainly in primary care. Under the National Immunisation Program about 5 million doses are delivered across the country each year in the infant, adolescent and adult vaccination programs. Mass vaccination requires this to be scaled up by five times. Nurse vaccinators can add to the primary care capacity of general practitioners but require accredited training to be able to vaccinate. In addition, pharmacists can vaccinate for influenza in Australia and may be an additional source of accredited vaccinators but giving a new vaccine with uncertain safety profile for rare adverse events would be best done in general practice or in public vaccination clinics staffed by medical and nursing personnel. Modelling of GP capacity to rapidly vaccinate 70–80% of the population, and the impact on other medical care, is needed, and logistic support and resourcing for GPS to expand their capacity to achieve this will be required. This includes cold chain infrastructure for vaccines that require deep freezing [12]. Decisions need to be made on public vaccination clinics, staffing, training of more nurse vaccinators and other measures to increase vaccination capacity.
Australia does not have a goal of herd immunity through mass vaccination at this stage, which means uptake will not likely be adequate for herd immunity. A vaccine with 90% VE requires 66% coverage, which is feasible, even without children being vaccinated. At this stage, because of lack of phase three clinical trial data in children, initial vaccine roll out will be restricted to adults, who comprise 80% of the population. This also makes age-based targeted vaccination only feasible for older adults. However, adult vaccination is more challenging than infant vaccination, as adults are a mobile population – for people over 65 years, about 70% receive influenza vaccine annually. [59] Achieving whole-of-life vaccination rates above 70% may be challenging, and impossible while COVID-19 vaccines remain unlicensed in children, who comprise 20% of the population. This is another impetus for choosing a vaccine with at least 90% VE, as the required coverage for herd immunity (66%) is far more feasible, even if children are not vaccinated.
Vaccine hesitancy may also affect the ability to achieve rapid, high uptake and must also be addressed well in advance of vaccination programs to ensure that programmatic competency to achieve high uptake rapidly will not be met by unexpected social or behavioral obstacles. Australia has very high rates of vaccination for National Immunization Program vaccines [47], especially with the use of financial disincentives, and has a more accepting culture of public health interventions, so large scale vaccine refusal is not expected. We also identified that vaccine hesitancy about COVID-19 vaccines mostly does not reflect fixed objection to vaccination, and that there is scope and need for health promotion and positive messaging to ensure high vaccine uptake [60].
The main limitation of this study is the uncertainty of the parameters used and the assumptions. The model assumes that symptomatic and asymptomatic infected people have the same infectiousness, based on several published studies that show the proportion of asymptomatic cases and the same viral load in both types of infection [27], [28], [29]. If symptomatic infection is more transmissible, our model may overestimate the impact of a COVID-19 outbreak. However, symptomatic people may reduce their contacts with others, while asymptomatic people will continue with normal behaviors, which may result in similar rates of transmission. We used a contact matrix estimated for Australia, based on pre-COVID mixing patterns, however reduced contacts are considered in the model when people are latent or traced. As this study focuses on the relative differences between vaccine scenarios in an unmitigated epidemic, we did not consider social distancing or lockdown. Further the exact performance and characteristics of the different vaccines vary and there no data as yet around vaccines as PEP. The ability of vaccines to prevent transmission (by preventing any infection, symptomatic or asymptomatic) is uncertain, but data from Israel, which conducted a rapid mass vaccination campaign using the Pfizer vaccine, do show a reduction in transmission [61].
In conclusion, restricted vaccine supply can protect first responders but will not have an impact on epidemic control, unless the vaccine has some efficacy as PEP. If vaccines are effective as PEP, then vaccinating health workers and ring vaccination would be the preferred use of a restricted supply. This remains a gap in research, and we recommend that clinical trials urgently be conducted to study the efficacy of COVID-19 vaccines as PEP given to close contacts of cases. When vaccine supply is available for mass vaccination, the quicker a high uptake can be achieved, the greater the impact on epidemic control, morbidity, and mortality, but herd immunity cannot be achieved with vaccines of less than 60% efficacy. The VE of the ChAdOx1 nCoV-19 vaccine, the largest component of the Australian planned stockpile, is less than 40% against any laboratory confirmed infection (symptomatic or asymptomatic) based on interim analysis. [10] The use of high efficacy vaccines provides the only prospect of achieving herd immunity against SARS-COV-2, and such vaccines should be procured if herd immunity is a goal. There are no data on serial dosing with two different vaccines, which is a possible scenario if Australia starts with a lower efficacy vaccine. This is another reason to ensure adequate supply of high efficacy vaccines. The logistics of mass vaccination, including cold chain, delivery, capacity to vaccinate and addressing vaccine hesitancy should be a high priority for planning and resourcing.
5. Data statement**a
This research is a mathematical model. All data used in the model were from published papers listed in the reference list.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CR MacIntyre has been on an advisory board for COVID-19 vaccine for Seqirus and consulted for Astra Zeneca Australia on COVID-19 vaccines. She has been on advisory boards for influenza, RSV and pneumococcal vaccines in the last 3 years for Seqirus, Sanofi, Pfizer and Janssen. The other authors have no conflicts to declare.
Acknowledgements
Chandini Raina MacIntyre is supported by a NHMRC Principal Research Fellowship, grant number 1137582.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.04.042.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/. (Accessed: 2nd December 2020).
- 2.MacIntyre C.R. Case isolation, contact tracing, and physical distancing are pillars of COVID-19 pandemic control, not optional choices. Lancet Infect Dis. 2020;20:1105–1106. doi: 10.1016/S1473-3099(20)30512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science (80-.). 2020;368:860–8. [DOI] [PMC free article] [PubMed]
- 4.Kucharski A.J., et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brüssow H. Efforts towards a <scp>COVID</scp> -19 vaccine. Environ Microbiol. 2020;22:4071–4084. doi: 10.1111/1462-2920.15225. [DOI] [PubMed] [Google Scholar]
- 6.Florian K. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 7.Draft landscape of COVID-19 candidate vaccines. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. (Accessed: 2nd December 2020).
- 8.Evan C. What Pfizer’s landmark COVID vaccine results mean for the pandemic. Nature. 2020 doi: 10.1038/d41586-020-03166-8. [DOI] [PubMed] [Google Scholar]
- 9.Evan C. COVID vaccine excitement builds as Moderna reports third positive result. Nature. 2020 doi: 10.1038/d41586-020-03248-7. [DOI] [PubMed] [Google Scholar]
- 10.Voysey M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (London, England) 0, (2020). [DOI] [PMC free article] [PubMed]
- 11.Normile D. Development of unique Australian COVID-19 vaccine halted. Science 80- 2020 doi: 10.1126/science.abg1208. [DOI] [Google Scholar]
- 12.Polack F.P., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;NEJMoa2034577 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO SAGE Roadmap For Prioritizing Uses Of COVID-19 Vaccines In The Context Of Limited Supply. Available at: https://www.who.int/publications/m/item/who-sage-roadmap-for-prioritizing-uses-of-covid-19-vaccines-in-the-context-of-limited-supply. (Accessed: 2nd December 2020).
- 14.Schmidt H., Pathak P., Sönmez T., Ünver M.U. Covid-19: How to prioritize worse-off populations in allocating safe and effective vaccines. BMJ. 2020;371 doi: 10.1136/bmj.m3795. [DOI] [PubMed] [Google Scholar]
- 15.Giubilini A., Savulescu J., Wilkinson D. COVID-19 vaccine: vaccinate the young to protect the old? J Law Biosci. 2020;7:1–13. doi: 10.1093/jlb/lsaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strassburg M.A. The global eradication of smallpox. AJIC Am J Infect Control. 1982;10:53–59. doi: 10.1016/0196-6553(82)90003-7. [DOI] [PubMed] [Google Scholar]
- 17.MacIntyre CR, Costantino V, Mohanty B, Nand D, Kunasekaran MP, Heslop D. Epidemic size, duration and vaccine stockpiling following a large-scale attack with smallpox. Global Biosecurity. 2019;1(1):74–81. http://doi.org/10.31646/gbio.13.
- 18.Kucharski A.J., et al. Effectiveness of ring vaccination as control strategy for Ebola virus disease. Emerg Infect Dis. 2016;22:105–108. doi: 10.3201/eid2201.151410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells C., et al. Harnessing Case Isolation and Ring Vaccination to Control Ebola. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher T., Lipsitch M. Postexposure Effects of Vaccines on Infectious Diseases. Epidemiol Rev. 2019;41:13–27. doi: 10.1093/epirev/mxz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wassie G.T., Azene A.G., Bantie G.M., Dessie G., Aragaw A.M. Incubation Period of Severe Acute Respiratory Syndrome Novel Coronavirus 2 that Causes Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Curr Therapeut Res - Clin Exper. 2020;93 doi: 10.1016/j.curtheres.2020.100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COVID-19 vaccination prioritisation | Australian Government Department of Health. Available at: https://www.health.gov.au/news/health-alerts/novel-coronavirus-2019-ncov-health-alert/vaccines-and-treatments/covid-19-vaccination-prioritisation. (Accessed: 2nd December 2020).
- 23.Costantino V., Heslop D.J., MacIntyre C.R. The effectiveness of full and partial travel bans against COVID-19 spread in Australia for travellers from China during and after the epidemic peak in China. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ABS. National, state and territory population, March 2020 | Australian Bureau of Statistics. (2020). Available at: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release. (Accessed: 15th October 2020).
- 25.Li Q., et al. N. Engl. J; Med: 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 27.Bai Lingsheng, Wei Tao, Tian Fei, Jin Dong-Yan, Chen Lijuan, Wang Meiyun YY. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA Published; 2020. [DOI] [PMC free article] [PubMed]
- 28.COVID-19 Pandemic Planning Scenarios | CDC. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. (Accessed: 29th June 2020).
- 29.He D., et al. The relative transmissibility of asymptomatic COVID-19 infections among close contacts. Int J Infect Dis. 2020;94:145–147. doi: 10.1016/j.ijid.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prem K., Cook A.R., Jit M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput Biol. 2017;13 doi: 10.1371/journal.pcbi.1005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S., et al. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: A data-driven analysis. Int J Infect Dis. 2020;93:201–204. doi: 10.1016/j.ijid.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson S., et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dagan N., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;NEJMoa2101765 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voysey M., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y, Xie B, et al. C. D. Asymptomatic Novel Coronavirus Pneumonia Patient Outside Wuhan: The Value of CT Images in the Course of the Disease. Clin Imaging 2020;63:7–9. [DOI] [PMC free article] [PubMed]
- 37.Rothe M, Sothmann P, et al. C. S. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020. http://doi.org/10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed]
- 38.Zou L., et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020 doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du Z., et al. Serial Interval of COVID-19 among Publicly Reported Confirmed Cases. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2606.200357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizumoto K., Chowell G. Transmission potential of the novel coronavirus (COVID-19) onboard the diamond Princess Cruises Ship, 2020. Infect Dis Model. 2020;5:264–270. doi: 10.1016/j.idm.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estimating the asymptomatic ratio of 2019… | Oxford Martin School. Available at: https://www.oxfordmartin.ox.ac.uk/publications/estimating-the-asymptomatic-ratio-of-2019-novel-coronavirus-onboard-the-princess-cruises-ship/. (Accessed: 24th March 2020).
- 42.MacIntyre Valentina, Kunasekaram, Mohana Priya, CRC. Health system capacity in Sydney, Australia in the event of a biological attack with smallpox. PLoS One 2019;14. [DOI] [PMC free article] [PubMed]
- 43.Team, T. N. C. P. E. R. E. Vital Surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020. China CDC Wkly. 2020;2:113–22. [PMC free article] [PubMed]
- 44.Garg S., et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson S., et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mass Vaccination Clinics during an Influenza Pandemic Policy manual, Infectious Diseases Population Health-Communicable Diseases. (2018).
- 47.Health Workforce Data | Summary Statistics. Available at: https://hwd.health.gov.au/summary.html#part-3. (Accessed: 15th October 2020).
- 48.Quigley A.L., Stone H., Nguyen P.Y., Chughtai A.A., MacIntyre C.R. Estimating the Burden of COVID-19 on the Australian Healthcare Workers and Health System. Int J Nurs Stud. 2020;103811 doi: 10.1016/j.ijnurstu.2020.103811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torjesen I. Covid-19: First doses of vaccines in Scotland led to a substantial fall in hospital admissions. BMJ. 2021;372 doi: 10.1136/bmj.n523. [DOI] [PubMed] [Google Scholar]
- 50.Heywood A.E., Macintyre C.R. Elimination of COVID-19: what would it look like and is it possible? Lancet Infect Dis. 2020;20:1005–1007. doi: 10.1016/S1473-3099(20)30633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blakely T., et al. The probability of the 6-week lockdown in Victoria (commencing 9 July 2020) achieving elimination of community transmission of SARS-CoV-2. Med J Aust. 2020;213:349–351.e1. doi: 10.5694/mja2.50786. [DOI] [PubMed] [Google Scholar]
- 52.Quigley A.L., Yen Nguyen P., Stone H., Lim S., Raina MacIntyre C. Cruise Ship Travel and the Spread of COVID-19 – Australia as a Case Study. Int Travel Med Cent Iran. 2021;9:10–18. [Google Scholar]
- 53.Department of Health and Human Services Victoria | Victorian healthcare worker (clinical and non-clinical) coronavirus (COVID-19) data. Available at: https://www.dhhs.vic.gov.au/victorian-healthcare-worker-covid-19-data. (Accessed: 16th December 2020).
- 54.Docherty A.B., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foy BH. et al. Comparing COVID-19 vaccine allocation strategies in India: a mathematical modelling study. medRxiv 2020.11.22.20236091, 2020. http://doi.org/10.1101/2020.11.22.20236091. [DOI] [PMC free article] [PubMed]
- 56.Parliament of Victoria - Inquiry into the Victorian Government’s COVID‐19 Contact Tracing System and Testing Regime. Available at: https://www.parliament.vic.gov.au/1005-lsic-lc/inquiry-into-the-victorian-government-s-covid-19-contact-tracing-system-and-testing-regime. (Accessed: 16th December 2020).
- 57.We modelled how a COVID vaccine roll-out would work. Here’s what we found. Available at: https://theconversation.com/we-modelled-how-a-covid-vaccine-roll-out-would-work-heres-what-we-found-150544. (Accessed: 2nd December 2020).
- 58.Aihw. 5.2 Rural and remote populations.
- 59.Trent M.J., Zhang E.J., Chughtai A.A., MacIntyre C.R. Parental opinions towards the “No Jab, No Pay” policy in Australia. Vaccine. 2019;37:5250–5256. doi: 10.1016/j.vaccine.2019.07.066. [DOI] [PubMed] [Google Scholar]
- 60.Mallory Trent, Holly Seale, Abrar Chinghtai, Salmon Daniel M.C.R. and Australia. Unpublished; 2020. Trust in government, intention to vaccinate and COVID-19 vaccine hesitancy: a comparative survey in the United States, United Kingdom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamin D, Gerlic M. The rise of SARS-CoV-2 variant B.1.1.7 in Israel intensifies the role of surveillance and vaccination in elderly. medRxiv 2021.02.16.21251819 (2021). http://doi.org/10.1101/2021.02.16.21251819.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.