Abstract
Background:
Fracture is a high-burden condition that accelerates unhealthful aging and represents a considerable economic burden. Adults with neurodevelopmental disabilities (NDDs) may be susceptible for fracture at younger ages compared to adults without NDDs; and yet, very little is known about the burden of fracture for these underserved populations. The purpose of this study was to determine the sex-stratified prevalence of all-cause fracture among adults with NDDs, as compared to adults without NDDs, and if comorbidity of NDDs is associated with greater risk of fracture.
Methods:
Data from 2016 were extracted from Optum Clinformatics® Data Mart (private insurance) and a random 20% sample from Medicare fee-for-service (public insurance). ICD-10-CM diagnosis codes were used to identify adults with NDDs, including intellectual disabilities, autism spectrum disorders, and cerebral palsy. Age-standardized prevalence of any fracture and fracture by anatomical location was compared between adults with and without NDDs, and then for adults with 1 NDD vs. 2 and 3 NDDs.
Results:
Adults with intellectual disabilities (n=69,456), autism spectrum disorders (n=21,844), and cerebral palsy (n=29,255) had a higher prevalence of any fracture compared to adults without NDDs (n=8.7 million). For women, it was 8.3%, 8.1%, and 8.5% vs. 3.5%, respectively. For men, it was 6.6%, 5.9%, and 6.7% vs. 3.0%, respectively. Women with NDDs had a higher prevalence of fracture of the head/neck, thoracic, lumbar/pelvis, upper extremities, and lower extremities compared to women without NDDs. A similar pattern was observed for men, except for no difference for lumbar/pelvis for all NDDs and thoracic for autism spectrum disorders. For women and men, increasing comorbidity of NDDs was associated with a higher prevalence of any fracture: 1 NDD (women, 7.7%; men, 5.7%); 2 NDDs (women, 9.4%; men, 7.2%); all 3 NDDs (women, 11.3%; men, 13.7%).
Conclusions:
Study findings suggest that adults with NDDs have an elevated prevalence of fracture compared to adults without NDDs, with the fracture risk being higher with greater numbers of comorbid NDD conditions for most anatomical locations. Our study findings indicate a need for earlier screening and preventive services for musculoskeletal frailty for adults with NDDs.
Keywords: Fracture, epidemiology, intellectual disabilities, autism spectrum disorders, cerebral palsy, neurodevelopmental disabilities
Introduction
Fractures are a major public health issue. In the U.S., there was an estimated 2 million fractures in adults >50 years of age in 2005, which totaled $17 billion in medical costs. These estimates are projected to increase annually, with the economic burden reaching up to $26 billion by 2025 [1]. In older adults, fractures are a major cause of functional disability [2], development of noncommunicable diseases [3], diminished quality of life [4], and early mortality [5]. However, fracture risk and post-fracture burden may disproportionately affect adults with pediatric-onset disabilities, due to barriers in achieving optimal skeletal health and preservation throughout the lifespan.
Neurodevelopmental disabilities (NDDs) refer to a group of congenital or acquired chronic conditions that originate in childhood, and consist of impairments in neurological functioning and/or processing. Three of the more common NDDs are intellectual disabilities, autism spectrum disorders, and cerebral palsy, which are often comorbid with one another. The etiology of NDDs can include genetic predisposition, brain lesions, and maternal/environmental exposures that negatively affect neurodevelopment [6–8]. The commonality linking all individuals with NDDs is the potential for restriction of activities of daily living and societal integration, which increases risk for developing health complications throughout the lifespan.
In childhood, individuals with NDDs have low levels of physical activity levels [9–11], excess body fat [9, 12–14], and an underdeveloped musculoskeletal system [9, 15–17], which collectively heightens susceptibility for low-energy fractures among these pediatric populations [18–21]. The transition into and throughout adulthood is accompanied by a profound elevation of musculoskeletal diseases [22, 23], which provides an additive burden to fracture risk throughout the adult years [21, 24, 25]. However, knowledge of fracture epidemiology and fracture-related burden facing adults with NDDs is lacking. Moreover, inferences from the current state of the literature are limited because the few studies that have examined fracture risk among adults with NDDs have had small sample sizes [25–27] or leveraged databases that are not representative of the entire population with CP or ASD [21, 24].
Previous fracture epidemiologic studies have led to important contributions to the literature informing clinically-relevant health-related policies to reduce fracture burden [28, 29]. However, these studies are typically focused on older adults from the general population. Many people with NDDs have an early development of noncommunicable disease [22] and early mortality [30, 31] compared to the general population, and are therefore likely to be underrepresented in fracture epidemiologic studies. Inadequate information about high-risk subpopulations could lead to missed opportunities for policy reform and improving clinical decision-making processes for healthcare management to optimize healthful aging. Importantly, microsimulation models have estimated that even modest improvements of 20% in identifying osteoporosis among elderly women- an at-risk population for fracture- would prevent 2.6 million fractures from 2018 to 2040, which would reduce cumulative fracture-related costs of nearly $42 billion over the same time period.
To adequately assess the potential for healthcare policy reform to reduce the burden of fracture for adults with NDDs, determining the risk for fracture among a national sample of adults with NDDs is necessary. Accordingly, the primary objective of this descriptive study sought to inform public health and clinical guidelines for fracture assessment by determining sex-stratified prevalence of fracture among adults with NDDs, as compared to adults without NDDs, using nationwide administrative claims data. We hypothesized that women and men with intellectual disabilities, autism spectrum disorders, and cerebral palsy would have higher prevalence of fracture compared to women and men without NDDs. The secondary objective sought to improve patient-centered care by determining if comorbidity of NDDs is associated with higher prevalence of fracture. We hypothesized that adults with 2 or more NDDs would have higher prevalence of fracture compared to adults with 1 NDD.
Methods
Data sources
Data were extracted from administrative claims data from the year 2016. Optum Clinformatics® Data Mart Database (OptumInsight™, Eden Prairie, MN, USA) provided information for privately insured beneficiaries that had commercial or Medicare Advantage plans. A random 20% sample of the Medicare fee-for-service database from the Centers for Medicare and Medicaid Services provided information for publicly insured beneficiaries. Since data are de-identified, the University IRB approved this study as non-regulated.
Sample selection
Beneficiaries that were 18 years of age or older, had 12 months of continuous enrollment in at least one plan in Optum or Medicare, and had at least one instance of billable services covered by their insurance plan in 2016 were initially included for analysis. We excluded Medicare beneficiaries covered by HMO plans because of incomplete claims. Beneficiaries that had unknown or missing data for sex were excluded (n=991 from Optum; no missing data for Medicare).
International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) codes were used to identify all medical conditions. A single claim in any position for intellectual disabilities (F70–73, F78, F79), autism spectrum disorders (F84), and cerebral palsy (G80) was used to identify these beneficiaries. The single claim-based definition has shown to be accurate at identifying pediatric-onset conditions using administrative claims data, with sensitivity of 99% and positive predictive value of 79% [32]. To identify beneficiaries without NDDs, we extracted claims from the Optum data source only. Using Optum to extract claims for the group without NDDs was performed to enhance the representativeness of our sample of adults without NDDs, as enrollment criteria for Medicare among individuals under 65 years of age requires permanent disability, such as end-stage renal disease.
Fracture
The primary outcome measure was any fracture due to any cause. We identified fracture as the first fracture in 2016 using a single claim in any position, as previously described for adults with cerebral palsy [24]. A single claim has been shown to be an accurate claims-based algorithm for fracture with positive predictive values up to 97% [33]. The secondary outcome measure was fracture due to any cause based on anatomical location, including the head/neck, thoracic, lumbar/pelvic, upper extremity, and lower extremity regions. The ICD-10 codes used to identify fracture are presented in Table 1.
Table 1.
Diagnostic codes for fracture using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) codes.
| Fracture | ICD-10 codes |
|---|---|
| Osteoporosis with current pathological fracture | M80 family |
| Fracture of skull and facial bones | S02 family |
| Fracture of the cervical vertebra and other parts of neck | S12 family |
| Fracture of the rib(s), sternum, and thoracic spine | S22 family |
| Fracture of the lumbar spine and pelvis | S32 family |
| Fracture of the shoulder (clavicle, scapula) and humerus, forearm (all parts of ulna and radius), wrist and hand | S42 family, S52 family, S62 family |
| Fracture of the femur, lower leg, including ankle, foot and toes | S72 family, S82 family, S92 family |
Statistical analysis
First, we examined unadjusted prevalence of any fracture stratified by sex for each NDD group and for adults without NDDs. Age was categorized into the following groups to reflect different stages of the adult lifespan [22–24]: 18–30, 31–40, 41–50, 51–60, 61–70, and >70 years. Then, we examined unadjusted prevalence for any fracture for each NDD group stratified by sex and insurance coverage (Optum, Medicare) to determine if there were differences between the insurance-based cohorts.
Second, we performed direct age-standardization [34] for all fracture outcomes for each NDD group (combined sample of Optum and Medicare) and for adults without NDDs. The 2016 U.S. adult population was used as a standard population. The U.S. Census Bureau released a table on age (5-year age brackets) and sex composition in the U.S. for 2016 [35]. In order to make use of the population table in 5-year age groups, it was assumed that age was evenly distributed within the 15–19 year age bracket. Therefore, since 6.8% of U.S. males were 15–19 years old, it was assumed that 2.72% males were 18–19 years old (6.8% x (2/5)). A similar approach was performed for females.
Finally, we performed direct age-standardization for all fracture outcomes for adults with NDDs that had 1, 2, and all 3 NDDs, and for the unique NDD comorbid combinations.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Descriptive characteristics for adults without NDDs (n=8.7 million) and for adults with intellectual disabilities (n=69,456), autism spectrum disorders (n=21,844), and cerebral palsy (n=29,255) are presented in Table 2. Adults with autism spectrum disorders had a higher prevalence of men and 18–30 year olds than the other NDD groups and adults without NDDs. In general, adults with NDDs had younger age compared to adults without NDDs.
Table 2.
Descriptive characteristics for adults with and without neurodevelopmental disabilities (NDDs).
| Without NDDs (n=8,717,829) | Intellectual disabilities (n=69,456) | Autism spectrum disorders (n=21,844) | Cerebral palsy (n=29,255) | |
|---|---|---|---|---|
| % | % | % | % | |
| Age, mean (SD) | 55.2 (18.6) | 51.5 (15.1) | 38.3 (15.5) | 50.8 (16.3) |
| 18–30 years | 12.0 | 10.6 | 41.3 | 13.1 |
| 31–40 years | 13.1 | 14.8 | 20.0 | 16.4 |
| 41–50 years | 14.5 | 19.4 | 14.8 | 18.7 |
| 51–60 years | 17.0 | 26.5 | 13.2 | 22.0 |
| 61–70 years | 18.9 | 18.6 | 7.3 | 17.9 |
| >70 years | 24.2 | 10.1 | 3.3 | 11.8 |
| sex | ||||
| Women | 55.3 | 44.5 | 27.1 | 48.1 |
| Men | 44.7 | 55.5 | 72.9 | 51.9 |
SD, standard deviation.
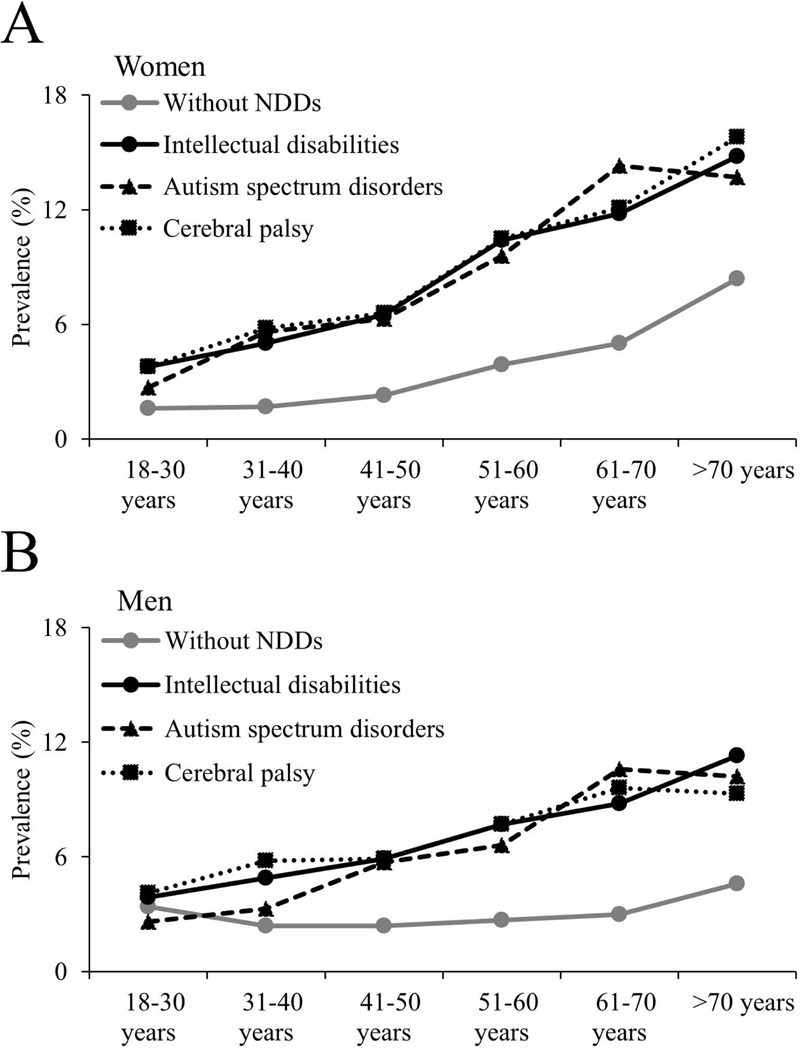
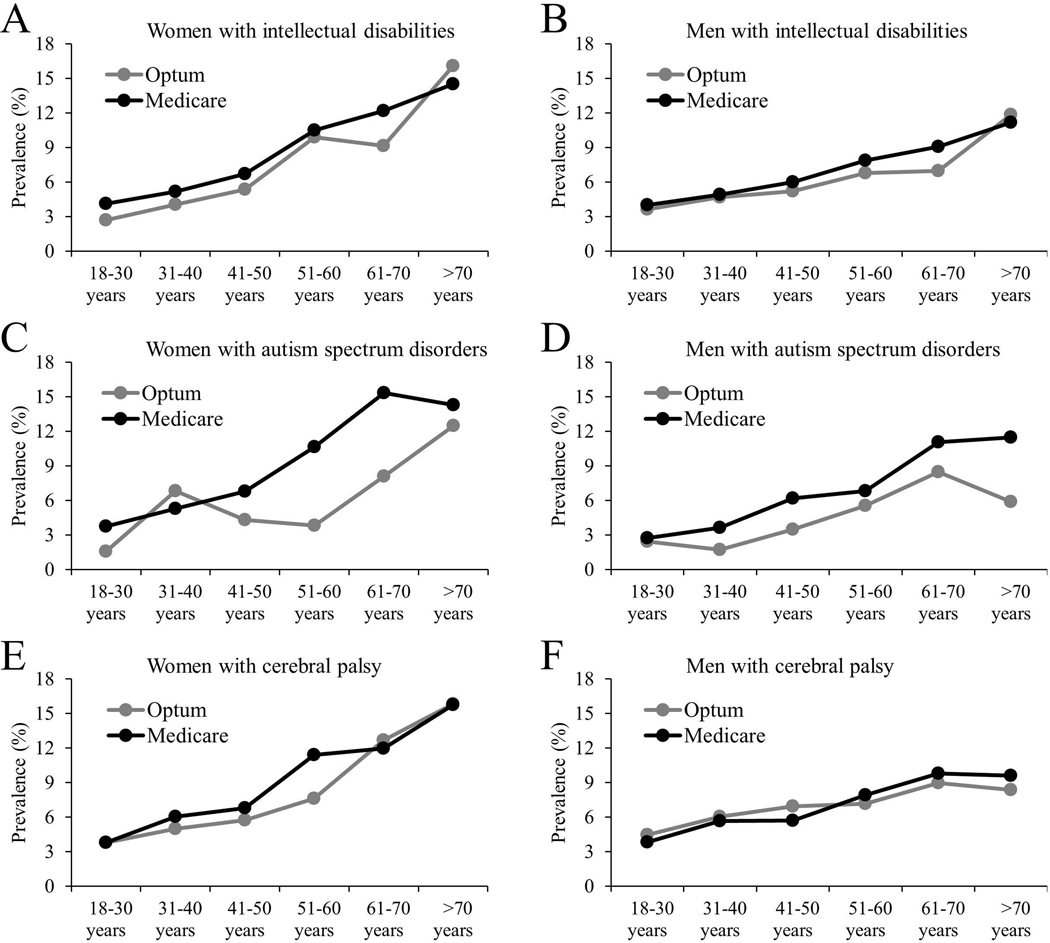
Unadjusted prevalence of any fracture across age groups is presented in Fig. 1A for women and Fig. 1B for men. Women with NDDs had a higher prevalence of any fracture across all age groups, while men with NDDs had a higher prevalence of any fracture across all age groups, except for the 18–30 year age group. There was no apparent difference in prevalence of any fracture among the NDD groups for women or men. When stratified by insurance coverage (Figure 2), there was no apparent different in prevalence of any fracture based on insurance coverage, except for the higher prevalence among publically insured women (51–60 and 61–70 year age groups) and men (>70 year age group) with autism spectrum disorders.
Figure 1.
Unadjusted prevalence of any fracture at any location for women (A) and men (B) with and without neurodevelopmental disabilities (NDDs).
Figure 2.
Unadjusted prevalence of any fracture at any location for: (A) women with intellectual disabilities (Optum, n=4,604; Medicare, n=26,323); (B) men with intellectual disabilities (Optum, n=5,347; Medicare, n=33,182); (C) women with autism spectrum disorders (Optum, n=1,736; Medicare, n=4,180); (D) men with autism spectrum disorders (Optum, n=4,893; Medicare, n=11,035); (E) women with cerebral palsy (Optum, n=3,615; Medicare, n=10,455); and (F) men with cerebral palsy (Optum, n=3,733; Medicare, n=11,452).
Age-standardized prevalence of all fracture outcomes for adults with and without NDDs is presented in Table 3. The prevalence of any fracture was higher for women and men with intellectual disabilities (women, 8.3%; men, 6.6%), autism spectrum disorders (women, 8.1%; men, 5.9%), and cerebral palsy (women, 8.5%; men, 6.7%) compared to women and men without NDDs (women, 3.5%; men, 3.0%). The prevalence of fracture across all anatomical regions were higher for women with intellectual disabilities, autism spectrum disorders, and cerebral palsy compared to women without NDDs: head/neck region (0.7–1.1% vs. 0.2%); thoracic region (0.7–1.0% vs. 0.4%); lumbar/pelvis region (0.7–0.9% vs. 0.4%); upper extremities (2.4–2.6% vs. 1.1%); and lower extremities (3.9–4.7% vs. 1.7%). A similar pattern was observed for men, except for the similar prevalence of lumbar/pelvis region for all NDD groups (0.6% vs. 0.5%) and thoracic region for autism spectrum disorders (0.6% vs. 0.5%).
Table 3.
Age-standardized prevalence of fracture for adults with and without neurodevelopmental disabilities (NDDs).
| Without NDDs (n=8,717,829) | Intellectual disabilities (n=69,456) | Autism spectrum disorders (n=21,844) | Cerebral palsy (n=29,255) | |
|---|---|---|---|---|
| Women | % | % | % | % |
| Any fracture | 3.5 | 8.3 | 8.1 | 8.5 |
| Head/neck region | 0.2 | 1.0 | 1.1 | 0.7 |
| Thoracic region | 0.4 | 0.7 | 0.9 | 1.0 |
| Lumbar/pelvis region | 0.4 | 0.7 | 0.8 | 0.9 |
| Upper extremities | 1.1 | 2.6 | 2.5 | 2.4 |
| Lower extremities | 1.7 | 4.2 | 3.9 | 4.7 |
| Men | % | % | % | % |
| Any fracture | 3.0 | 6.6 | 5.9 | 6.7 |
| Head/neck region | 0.3 | 1.2 | 1.2 | 1.0 |
| Thoracic region | 0.5 | 0.9 | 0.6 | 0.9 |
| Lumbar/pelvis region | 0.5 | 0.6 | 0.6 | 0.6 |
| Upper extremities | 1.2 | 2.1 | 1.8 | 1.8 |
| Lower extremities | 1.1 | 2.8 | 2.4 | 3.4 |
Age-standardized prevalence of fracture measures for adults with single and comorbid NDDs are presented in Table 4. Of the sample with NDDs, 87,163 adults had 1 NDD, 15,553 had 2 NDDs (cerebral palsy and autism spectrum disorders, 2.3%; cerebral palsy and intellectual disabilities, 54.0%; intellectual disabilities and autism spectrum disorders, 43.7%), and 762 had all 3 NDDs. Prevalence of any fracture increased with the number of comorbid NDDs. For women with 1 NDD, 2 NDDs, and 3 NDDs, prevalence of any fracture was 7.7%, 9.4%, and 11.3%, respectively. For men, it was 5.7%, 7.2%, and 13.7%. For both women and men with increasing number of NDDs, there was an increasing trend for fracture prevalence of the head/neck, upper extremities, and lower extremities, and decreasing trend for fracture prevalence of the lumbar/pelvis. Further, for women and men with intellectual disabilities only, autism spectrum disorders only, and cerebral palsy only, the age-standardized prevalence of fracture was higher compared to women and men without NDDs. Women and men with autism spectrum disorders only tended to have lower age-standardized prevalence of any fracture and fracture of the lower extremities relative to the other single NDD conditions.
Table 4.
Age-standardized prevalence of fracture for adults with single or comorbid neurodevelopmental disabilities (NDDs).
| Any | Head or neck | Thoracic | Lumbar or pelvis | Upper extremities | Lower extremities | ||
|---|---|---|---|---|---|---|---|
| Women | n | % | % | % | % | % | % |
| 1 NDD | 37,665 | 7.7 | 0.8 | 0.8 | 0.8 | 2.2 | 4.0 |
| ID | 24,597 | 7.8 | 0.9 | 0.7 | 0.7 | 2.5 | 4.0 |
| ASD | 3,451 | 5.9 | 0.6 | 0.7 | 0.7 | 1.9 | 2.7 |
| CP | 9,617 | 7.9 | 0.6 | 1.0 | 1.0 | 2.0 | 4.3 |
| 2 NDDs | 6,177 | 9.4 | 1.0 | 0.7 | 0.6 | 2.9 | 5.1 |
| ID+CP | 4,010 | 9.6 | 0.8 | 0.6 | 0.5 | 3.3 | 5.3 |
| ID+ASD | 2,022 | 9.4 | 1.4 | 0.8 | 1.0 | 3.2 | 4.9 |
| CP+ASD | 145 | 12.9 | 2.9 | 1.9 | 0.9 | 2.8 | 4.3 |
| 3 NDDs | 298 | 11.3 | 2.6 | 1.5 | 0.3 | 4.2 | 5.6 |
| Men | % | % | % | % | % | % | |
| 1 NDD | 49,498 | 5.7 | 1.0 | 0.9 | 0.6 | 1.8 | 2.5 |
| ID | 28,905 | 6.2 | 1.1 | 0.9 | 0.6 | 2.0 | 2.6 |
| ASD | 10,477 | 4.3 | 0.8 | 0.7 | 0.7 | 1.3 | 1.7 |
| CP | 10,116 | 6.1 | 0.9 | 1.0 | 0.6 | 1.6 | 3.2 |
| 2 NDDs | 9,376 | 7.2 | 1.3 | 0.7 | 0.4 | 2.1 | 3.2 |
| ID+CP | 4,389 | 7.2 | 1.2 | 0.8 | 0.3 | 2.0 | 3.4 |
| ID+ASD | 4,771 | 8.1 | 1.7 | 0.7 | 0.5 | 2.1 | 3.8 |
| CP+ASD | 216 | 3.1 | 0.0 | 0.0 | 0.3 | 1.4 | 1.7 |
| 3 NDDs | 464 | 13.7 | 2.3 | 0.0 | 0.4 | 4.6 | 7.4 |
ID, intellectual disabilities; ASD, autism spectrum disorders; CP, cerebral palsy.
Discussion
The primary finding of this study is that women and men with NDDs have a higher prevalence of fracture compared to adults without NDDs. Further, the prevalence of any fracture outcomes did not differ substantially among the NDD groups. The secondary finding of this investigation was that increasing comorbidity of intellectual disabilities, autism spectrum disorders, and cerebral palsy was associated with even higher prevalence of fracture for most anatomical locations. Taken together, study findings highlight the need to improve healthcare management strategies to enable early detection of musculoskeletal fragility and fracture risk among adults with NDDs, regardless of the presence of motor impairment, and especially when NDDs are comorbid. Early detection could allow for earlier rehabilitation efforts, such as screening, exercise interventions including strength training, and pharmacological interventions. These fracture prevention strategies have been shown to minimize fracture incidence or mitigate the burden of fracture for adults without NDDs at risk for fracture [36–38], and can lead to substantial medical and societal cost savings long term [29].
In the current study, we found that adults with NDDs, regardless of the type of NDD, had a higher prevalence of all fracture outcomes compared to adults without NDDs. This is an important finding, because the burden of fracture on health, function, and survival outcomes is greater for individuals that have poor overall health status prior to sustaining a fracture [2]. Our previous research has documented that adults with NDDs have an elevated prevalence of health disparities at younger ages, compared to adults without NDDs, evidenced by early development of noncommunicable diseases and multimorbidity [22, 23, 39, 40]. Fractures may therefore incur a more devastating burden on health-related outcomes among adults with NDDs. Future research is needed to determine how fracture contributes to the unhealthful aging process among adults with NDDs, which may be a novel target for intervention.
Interestingly, there were no substantial differences in the prevalence of any of the fracture outcomes among the NDD groups. Cerebral palsy represents a heterogeneous group of movement disorders, ranging from mild (independent ambulation) to severe (dependent wheelchair user) motor impairment. Children with cerebral palsy have low levels of physical activity, which correlates with the severity of motor impairment [9, 41]. The elevated rates of physical inactivity among children with cerebral palsy, which further decreases throughout adulthood [42], represents a potentially potent mechanism for increased fracture risk for this adult population [24]. While physical activity and/or fitness levels have been shown to be lower among children with autism spectrum disorders [10] and intellectual disabilities [11] compared to typically developing children, these NDD groups are not necessarily associated with the extent of motor impairment and resultant decrease in mechanical loading seen in cerebral palsy. Future studies are needed to determine factors associated with fracture risk that may be unique to these NDD populations.
Although, it is important to note that there was a higher proportion of adults with autism spectrum disorders in the 18–30 year age group. This may be due to under-diagnosis of the condition in the past, given the increasing trend in autism spectrum disorders [43] that may be in response to better attention, awareness, and clinical assessment, as well as changes in diagnostic criteria. While we accounted for the younger age by analyzing age-standardized prevalence estimates, the under-diagnosis at older ages may bias outcomes to be more conservative estimates of the true fracture prevalence for adults with autism spectrum disorders, since older age is a strong predictor of fracture. This may affect interpretations drawn from the breakdown of fracture prevalence by unique comorbid combinations. Moreover, this may explain why we saw differences at some age groups for women and men with autism spectrum disorders based on insurance coverage. Further research is needed to identify how the unique comorbidities of NDDs contributes to risk of fracture, as this knowledge will enhance patient-centered clinical care.
Heightened fracture susceptibility is due to many complex and multifactorial components [44], such as diet, activity, environmental barriers, comorbidities, medications, and hypogonadism [27]. The burden of musculoskeletal fragility specific to adults with NDDs is a long-established pathological process. Children with NDDs have an underdeveloped musculoskeletal system, even among those without known physical impairments [9, 15–17]. Children with NDDs also have higher total body [12, 14, 45] and abdominal [13] fat accretion, as well as greater musculoskeletal fat infiltration [9] compared to typically developing children, which may impede musculoskeletal development and fracture-resistance independent of low mechanical loading [46]. The poorly developed and metabolically unhealthy musculoskeletal system throughout growth and development increases risk for poor musculoskeletal health in adulthood.
We have previously reported that young adults (18–30 years) with cerebral palsy have an elevated prevalence of musculoskeletal diseases, which is up to ten times more prevalent compared to young adults without cerebral palsy [22]. Of the musculoskeletal diseases, osteoporosis is a particularly strong predictor of fracture. The prevalence of osteoporosis among young adults with cerebral palsy is 8% [22], which is similar to the general population of adults ≥50 years of age (10%) [47]. Moreover, the prevalence of osteoporosis becomes more pronounced with age, reaching up to 26% among adults with cerebral palsy >50 years of age [23]. While little is known about prevalent osteoporosis for adults with cerebral palsy, even less is known for other adult NDD conditions [48, 49]. Nevertheless, study findings suggest that musculoskeletal fragility among adults with NDDs continues to get worse throughout the adult lifespan.
There are limitations to this study that must be discussed. First, administrative claims data can be subject to inaccurate coding. Second, we used a single claim to define each of the NDDs and fracture measures. Previous validation studies have shown that at least 2 claims for a medical condition tends to improve accurate identification of that medical condition [32, 50]. However, accurately identifying medical conditions depends on the number of years for the study period [51] and the condition examined [32, 51–53]. A single claim-based definition for identifying pediatric-onset disabilities and fracture performs well compared to other conditions, with positive predictive values of ~80% [32] and up to 97% [33], respectively. Third, it is not possible to determine the severity of NDDs in regards to NDD-specific classifications (e.g., gross motor function classification system), or to reliably determine cause of fracture. Fourth, this study did not examine if fracture was due to low or high trauma, which has important implications for prevention and treatment strategies. Adults with autism spectrum disorders have behavioral issues which may lead to differential fracture risk compared to other NDD groups. Future studies are needed to determine cause and mechanism of fracture for these NDD groups.
Conclusion
Our study findings suggest that adults with NDDs have an elevated prevalence of fracture compared to adults without NDDs, regardless of the type of NDD. Further, greater comorbidity of these NDDs is associated with greater likelihood of fracture for most anatomical locations. This information should serve to inform clinicians treating their patients with NDDs to incorporate musculoskeletal frailty assessment as part of their routine clinical care. Future research is needed to determine fracture mechanisms, as well as post-fracture morbidity and mortality burdens among adults with NDDs, as this information could assist in the planning and execution of tailored interventions to reduce the burden of fracture for these underserved adult populations.
Acknowledgements
Funding: This work was supported by the University of Michigan Office of Health Equity and Inclusion Diversity Fund (Dr. Whitney).
Role of funder: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- NDD
neurodevelopmental disabilities
Footnotes
Declarations of interest: none.
References
- [1].Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A, Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025, J Bone Miner Res 22(3) (2007) 465–75. [DOI] [PubMed] [Google Scholar]
- [2].Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM, Survival and functional outcomes after hip fracture among nursing home residents, JAMA Intern Med 174(8) (2014) 1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Veronese N, Stubbs B, Crepaldi G, Solmi M, Cooper C, Harvey NC, Reginster JY, Rizzoli R, Civitelli R, Schofield P, Maggi S, Lamb SE, Relationship Between Low Bone Mineral Density and Fractures With Incident Cardiovascular Disease: A Systematic Review and Meta-Analysis, J Bone Miner Res 32(5) (2017) 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Harvey-Kelly KF, Kanakaris NK, Obakponovwe O, West RM, Giannoudis PV, Quality of life and sexual function after traumatic pelvic fracture, J Orthop Trauma 28(1) (2014) 28–35. [DOI] [PubMed] [Google Scholar]
- [5].Kumar A, Rahman M, Trivedi AN, Resnik L, Gozalo P, Mor V, Comparing post-acute rehabilitation use, length of stay, and outcomes experienced by Medicare fee-for-service and Medicare Advantage beneficiaries with hip fracture in the United States: A secondary analysis of administrative data, PLoS Med 15(6) (2018) e1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wimalasundera N, Stevenson VL, Cerebral palsy, Pract Neurol 16(3) (2016) 184–94. [DOI] [PubMed] [Google Scholar]
- [7].Villamor E, Tedroff K, Peterson M, Johansson S, Neovius M, Petersson G, Cnattingius S, Association Between Maternal Body Mass Index in Early Pregnancy and Incidence of Cerebral Palsy, JAMA 317(9) (2017) 925–936. [DOI] [PubMed] [Google Scholar]
- [8].Lai MC, Lombardo MV, Baron-Cohen S, Autism, Lancet 383(9920) (2014) 896–910. [DOI] [PubMed] [Google Scholar]
- [9].Whitney DG, Singh H, Miller F, Barbe MF, Slade JM, Pohlig RT, Modlesky CM, Cortical bone deficit and fat infiltration of bone marrow and skeletal muscle in ambulatory children with mild spastic cerebral palsy, Bone 94 (2017) 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tyler K, MacDonald M, Menear K, Physical activity and physical fitness of school-aged children and youth with autism spectrum disorders, Autism Res Treat 2014 (2014) 312163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hinckson EA, Curtis A, Measuring physical activity in children and youth living with intellectual disabilities: a systematic review, Res Dev Disabil 34(1) (2013) 72–86. [DOI] [PubMed] [Google Scholar]
- [12].Whitney DG, Miller F, Pohlig RT, Modlesky CM, BMI does not capture the high fat mass index and low fat-free mass index in children with cerebral palsy and proposed statistical models that improve this accuracy, Int J Obes (Lond) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Whitney DG, Singh H, Zhang C, Miller F, Modlesky CM, Greater Visceral Fat but No Difference in Measures of Total Body Fat in Ambulatory Children With Spastic Cerebral Palsy Compared to Typically Developing Children, J Clin Densitom (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Corvey K, Menear KS, Preskitt J, Goldfarb S, Menachemi N, Obesity, Physical Activity and Sedentary Behaviors in Children with an Autism Spectrum Disorder, Matern Child Health J 20(2) (2016) 466–76. [DOI] [PubMed] [Google Scholar]
- [15].Neumeyer AM, Cano Sokoloff N, McDonnell E, Macklin EA, McDougle CJ, Misra M, Bone Accrual in Males with Autism Spectrum Disorder, J Pediatr 181 (2017) 195–201 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].da Silva VZ, de Franca Barros J, de Azevedo M, de Godoy JR, Arena R, Cipriano G Jr., Bone mineral density and respiratory muscle strength in male individuals with mental retardation (with and without Down Syndrome), Res Dev Disabil 31(6) (2010) 1585–9. [DOI] [PubMed] [Google Scholar]
- [17].Modlesky CM, Whitney DG, Singh H, Barbe MF, Kirby JT, Miller F, Underdevelopment of trabecular bone microarchitecture in the distal femur of nonambulatory children with cerebral palsy becomes more pronounced with distance from the growth plate, Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 26(2) (2015) 505–12. [DOI] [PubMed] [Google Scholar]
- [18].Presedo A, Dabney KW, Miller F, Fractures in patients with cerebral palsy, Journal of pediatric orthopedics 27(2) (2007) 147–53. [DOI] [PubMed] [Google Scholar]
- [19].Uddenfeldt Wort U, Nordmark E, Wagner P, Duppe H, Westbom L, Fractures in children with cerebral palsy: a total population study, Dev Med Child Neurol 55(9) (2013) 821–6. [DOI] [PubMed] [Google Scholar]
- [20].Leet AI, Shirley ED, Barker C, Launay F, Sponseller PD, Treatment of femur fractures in children with cerebral palsy, J Child Orthop 3(4) (2009) 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Neumeyer AM, O’Rourke JA, Massa A, Lee H, Lawson EA, McDougle CJ, Misra M, Brief report: bone fractures in children and adults with autism spectrum disorders, J Autism Dev Disord 45(3) (2015) 881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Whitney DG, Hurvitz EA, Ryan JM, Devlin MJ, Caird MS, French ZP, Ellenberg EC, Peterson MD, Noncommunicable disease and multimorbidity in young adults with cerebral palsy, Clin Epidemiol 10 (2018) 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Whitney DG, Hurvitz EA, Devlin MJ, Caird MS, French ZP, Ellenberg EC, Peterson MD, Age trajectories of musculoskeletal morbidities in adults with cerebral palsy, Bone 114 (2018) 285–291. [DOI] [PubMed] [Google Scholar]
- [24].Whitney DG, Alford AI, Devlin MJ, Caird MS, Hurvitz EA, Peterson MD, Adults with cerebral palsy have higher prevalence of fracture compared to adults without cerebral palsy independent of osteoporosis and cardiometabolic diseases, J Bone Miner Res (2019). [DOI] [PubMed] [Google Scholar]
- [25].Schrager S, Kloss C, Ju AW, Prevalence of fractures in women with intellectual disabilities: a chart review, J Intellect Disabil Res 51(Pt 4) (2007) 253–9. [DOI] [PubMed] [Google Scholar]
- [26].Mouridsen SE, Rich B, Isager T, Fractures in individuals with and without a history of infantile autism. A Danish register study based on hospital discharge diagnoses, J Autism Dev Disord 42(4) (2012) 619–24. [DOI] [PubMed] [Google Scholar]
- [27].Trinh A, Wong P, Fahey MC, Brown J, Churchyard A, Strauss BJ, Ebeling PR, Fuller PJ, Milat F, Musculoskeletal and Endocrine Health in Adults With Cerebral Palsy: New Opportunities for Intervention, J Clin Endocrinol Metab 101(3) (2016) 1190–7. [DOI] [PubMed] [Google Scholar]
- [28].Force USPST, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr., Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Pignone M, Silverstein M, Simon MA, Tseng CW, Wong JB, Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement, JAMA 319(24) (2018) 2521–2531. [DOI] [PubMed] [Google Scholar]
- [29].Lewiecki ME, Ortendahl JD, Vanderpuye-Orgle J, Grauer A, Arellano J, J. L, Harmon AL, M.S B., Singer AJ, Healthcare policy changes in osteoporosis can improve outcomes and reduce costs in the US, Journal of Bone and Mineral Research (2019) In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Himmelmann K, Sundh V, Survival with cerebral palsy over five decades in western Sweden, Developmental medicine and child neurology 57(8) (2015) 762–7. [DOI] [PubMed] [Google Scholar]
- [31].Hemming K, Hutton JL, Pharoah PO, Long-term survival for a cohort of adults with cerebral palsy, Developmental medicine and child neurology 48(2) (2006) 90–5. [DOI] [PubMed] [Google Scholar]
- [32].Reeves S, Garcia E, Kleyn M, Housey M, Stottlemyer R, Lyon-Callo S, Dombkowski KJ, Identifying sickle cell disease cases using administrative claims, Acad Pediatr 14(5 Suppl) (2014) S61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Narongroeknawin P, Patkar NM, Shakoory B, Jain A, Curtis JR, Delzell E, Lander PH, Lopez-Ben RR, Pitt MJ, Safford MM, Volgas DA, Saag KG, Validation of diagnostic codes for subtrochanteric, diaphyseal, and atypical femoral fractures using administrative claims data, J Clin Densitom 15(1) (2012) 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Age Standardization and Population Counts, National Center for Health Statistics (2014), 2014. [Google Scholar]
- [35].Age and Sex Composition in the United States: 2016, United States Census Bureau (2018), 2018. [Google Scholar]
- [36].Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Sone T, Nakano T, Ito M, Matsui S, Yoneda T, Takami H, Watanabe K, Osakabe T, Shiraki M, Fukunaga M, Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT), J Clin Endocrinol Metab 99(7) (2014) 2599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Viswanathan M, Reddy S, Berkman N, Cullen K, Middleton JC, Nicholson WK, Kahwati LC, Screening to Prevent Osteoporotic Fractures: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force, JAMA 319(24) (2018) 2532–2551. [DOI] [PubMed] [Google Scholar]
- [38].Singh NA, Quine S, Clemson LM, Williams EJ, Williamson DA, Stavrinos TM, Grady JN, Perry TJ, Lloyd BD, Smith EU, Singh MA, Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial, J Am Med Dir Assoc 13(1) (2012) 24–30. [DOI] [PubMed] [Google Scholar]
- [39].Cremer N, Hurvitz EA, Peterson MD, Multimorbidity in Middle-Aged Adults with Cerebral Palsy, Am J Med 130(6) (2017) 744 e9–744 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Peterson MD, Kamdar N, Hurvitz EA, Age-related trends in cardiometabolic disease among adults with cerebral palsy, Dev Med Child Neurol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Johnson DL, Miller F, Subramanian P, Modlesky CM, Adipose tissue infiltration of skeletal muscle in children with cerebral palsy, The Journal of pediatrics 154(5) (2009) 715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Day SM, Wu YW, Strauss DJ, Shavelle RM, Reynolds RJ, Change in ambulatory ability of adolescents and young adults with cerebral palsy, Developmental medicine and child neurology 49(9) (2007) 647–53. [DOI] [PubMed] [Google Scholar]
- [43].Van Naarden Braun K, Christensen D, Doernberg N, Schieve L, Rice C, Wiggins L, Schendel D, Yeargin-Allsopp M, Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan atlanta, 1991–2010, PLoS One 10(4) (2015) e0124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Veronese N, Maggi S, Epidemiology and social costs of hip fracture, Injury 49(8) (2018) 1458–1460. [DOI] [PubMed] [Google Scholar]
- [45].Rimmer JH, Yamaki K, Lowry BM, Wang E, Vogel LC, Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities, Journal of intellectual disability research : JIDR 54(9) (2010) 787–94. [DOI] [PubMed] [Google Scholar]
- [46].Whitney DG, Peterson MD, Devlin MJ, Caird MS, Hurvitz EA, Modlesky CM, Bone marrow fat physiology in relation to skeletal metabolism and cardiometabolic disease risk in children with cerebral palsy, Am J Phys Med Rehabil (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B, The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine, J Bone Miner Res 29(11) (2014) 2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Burke EA, McCallion P, Carroll R, Walsh JB, McCarron M, An exploration of the bone health of older adults with an intellectual disability in Ireland, J Intellect Disabil Res 61(2) (2017) 99–114. [DOI] [PubMed] [Google Scholar]
- [49].Cashin A, Buckley T, Trollor JN, Lennox N, A scoping review of what is known of the physical health of adults with autism spectrum disorder, J Intellect Disabil 22(1) (2018) 96–108. [DOI] [PubMed] [Google Scholar]
- [50].Kerr EA, McGlynn EA, Van Vorst KA, Wickstrom SL, Measuring antidepressant prescribing practice in a health care system using administrative data: implications for quality measurement and improvement, Jt Comm J Qual Improv 26(4) (2000) 203–16. [DOI] [PubMed] [Google Scholar]
- [51].Leslie WD, Lix LM, Yogendran MS, Validation of a case definition for osteoporosis disease surveillance, Osteoporos Int 22(1) (2011) 37–46. [DOI] [PubMed] [Google Scholar]
- [52].Doktorchik C, Patten S, Eastwood C, Peng M, Chen G, Beck CA, Jette N, Williamson T, Quan H, Validation of a case definition for depression in administrative data against primary chart data as a reference standard, BMC Psychiatry 19(1) (2019) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Noyes K, Liu H, Lyness JM, Friedman B, Medicare beneficiaries with depression: comparing diagnoses in claims data with the results of screening, Psychiatr Serv 62(10) (2011) 1159–66. [DOI] [PubMed] [Google Scholar]