Abstract
The triglyceride glucose (TyG) index, derived from a combination of fasting glucose and triglycerides, has been suggested as a useful marker for insulin resistance (IR), in addition to modified TyG indices that combine obesity parameters. This study investigated the association and utility of TyG and modified TyG indices for IR prediction in youth. Based on the Korea National Health and Nutritional Examination Survey, the data of 3728 youth aged 10–19 years were analyzed. Odds ratios (ORs) and 95% confidence intervals (CIs) of tertiles 2 and 3 for each parameter were calculated and compared with tertile 1 as a reference. To compare the parameters for identifying IR, receiver operating characteristic curves were plotted and the area under the curve (AUC) was calculated. The ORs and 95% CIs for insulin resistance (IR) progressively increased across tertiles of each parameter. Overall, all modified TyG indices presented higher ORs and AUC than the TyG index. The TyG-body mass index standard deviation score showed the largest AUC for IR detection in all subjects. In conclusion, TyG and modified TyG indices could be used as valuable markers for the prediction of IR in youth. Moreover, modified TyG indices had better diagnostic accuracy than the TyG index.
Keywords: triglycerides, glucose, insulin resistance, child, adolescent
1. Introduction
Insulin resistance (IR), characterized by an inadequate physiological response with insensitivity to insulin, is a major risk factor for metabolic syndrome and cardiovascular diseases (CVD) [1,2,3]. A systematic review revealed that the prevalence of metabolic syndrome was high, at 3.3% in children and 29.2% in obese children [4]. Similarly, a population-based study in Korea found that the prevalence of metabolic syndrome had increased from 4.0% in 1998 to 7.8% in 2007 [5]. Considering the increasing prevalence of metabolic syndrome in youth and association of metabolic syndrome with risk of type 2 diabetes and CVD, it is important to detect IR in children and adolescents [6].
For measuring IR, the glucose clamp technique is considered as the gold standard [7]. However, because of its complicated and invasive nature, this test is difficult to perform in youth [8]. Therefore, homeostasis model assessment of insulin resistance (HOMA-IR) index, calculated as the product of the fasting levels of glucose and insulin, is suggested as a robust marker for IR quantification [1,9,10]. However, insulin measurement is not a routine test in the clinical setting and has standardization problems [11]. Thus, various indices combining glucose levels and lipid parameters were suggested as predictors of IR [12,13]. Among them, the triglyceride glucose (TyG) index, derived from the combination of fasting glucose and triglycerides (TG), has been suggested as a useful marker for IR in adults [14].
In addition, modified TyG indices that combine obesity indices such as body mass index (BMI), waist circumference (WC), and waist-to-height ratio (WHtR) have been suggested because obesity is closely associated with IR [15,16]. Kim et al. [16] reported that such modified TyG indices could act as alternative markers for assessing IR. Lee et al. [13] reported that the TyG index combined with BMI or WC was superior to the TyG index alone in among U.S population. However, investigations into the association between IR and the TyG and modified TyG indices are extremely limited in youth.
Therefore, this study aimed to investigate the association of TyG and modified TyG indices with IR in youth through the analysis of the Korean National Health and Nutrition Examination Survey (KNHANES) data. The objectives of our study were (1) to compare the TyG and modified TyG indices as surrogate markers for predicting IR and (2) to determine valid cut-off values of the TyG and modified TyG indices for predicting IR.
2. Materials and Methods
2.1. Participants
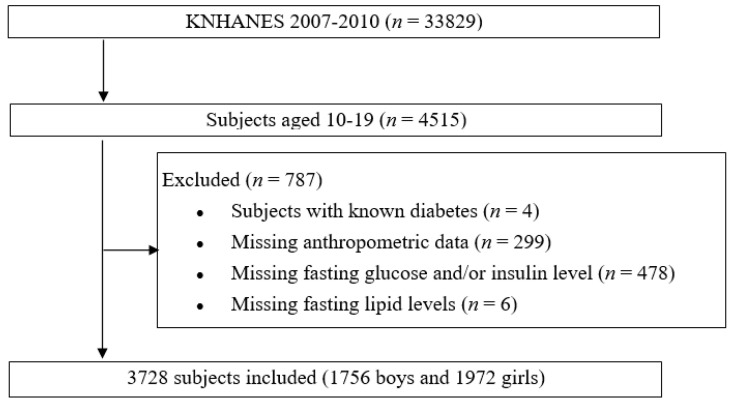
This study included the data acquired in the third and fourth KNHANES, conducted from 2007 to 2010. Figure 1 depicts the flowchart of study design and patient inclusion. KNHANES is a cross-sectional and nationally representative survey with a complex, stratified, multistage probability sampling of the Korean population. It is conducted annually by the Korea Centers for Disease Control and Prevention (KCDC) based on the National Health Promotion and consists of health surveys, examinations, and nutrition surveys. These data provide a variety of information about health status and behavior, socio-economic demographics, and laboratory tests. Sample weights were used to account for differential probabilities of selection and non-response and were included in the estimation process for all analyses. The weighted data were then adjusted to represent the sex- and age-specific Korean populations [17]. KNHANES is approved by the KCDC.
Figure 1.
Flowchart of the study population selection process. KNHANES, Korea National Health and Nutrition Examination Survey.
2.2. Study Variables
Data on age, sex, anthropometric measurement, plasma lipid levels, and insulin levels were collected. A portable stadiometer (range, 850–2060 mm; Seriter, Holtain Ltd., Crymych, UK) was used to the nearest 0.1 cm for height, and a calibrated balance beam scale (Giant 150N; HANA, Seoul, Korea) was used in the upright position to the nearest 0.1 kg for weight. BMI was calculated as weight (kg)/height squared (m2). The height, weight, and BMI were presented as standard deviation score (SDS) values on the basis of the 2017 Korean National Growth Charts [18]. Children were classified as normal weight (<85th percentile), overweight (85th–95th percentile), or obesity (≥95th percentile) according to their BMI. WC was measured midway between the costal margin and iliac crest at the end of a normal expiration, and WHtR was calculated as WC (cm)/height (cm). Central obesity was defined as WC >90th percentile using the Korean waist reference data [19].
2.3. Laboratory Analysis
Blood samples were collected from an antecubital vein after an 8-h fast, processed, and immediately refrigerated. The serum level of fasting glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and TG were measured using the Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan). Serum insulin was measured using the Wizard 1470 gamma counter (Perkin-Elmer, Turku, Finland).
Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula (1) [20]:
| LDL-C = TC − [HDL-C + (TG/5)] | (1) |
and non-HDL-C was calculated as TC − HDL-C [21]. The atherogenic index of plasma (AIP) was defined as log (TG/HDL-C) [22]. For quantification of IR, HOMA-IR was calculated as fasting insulin (mg/dL) × fasting glucose (mg/dL)/22.5. IR was defined as the HOMA-IR of >95th percentile for each sex and age using Korean HOMA-IR reference data [16]. TyG and modified TyG indices were defined and calculated as formula (2–6) [15,17]:
| TyG index = Ln [TG (mg/dL) × fasting glucose (mg/dL)/2] | (2) |
| TyG-BMI = TyG index × BMI | (3) |
| TyG-BMI SDS = TyG index × BMI SDS | (4) |
| TyG-WC = TyG index × WC | (5) |
| TyG-WHtR = TyG index × WHtR | (6) |
2.4. Statistical Analysis
The sampling weights were considered in all analyses to report representative estimates of the Korean children and adolescents. The data were analyzed using SAS, version 9.4 (SAS Inc., Cary, NC, USA), and R, version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/; accessed on 8 August 2020), for the complex survey design with clustering, stratification, and unequal weighting of the KNHANES sample. All continuous variables were expressed as weighted means with standard errors, and categorical variables were expressed as numbers and weighted percentages. The independent-samples t-test was used to compare continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Logistic regression analyses were performed to explain the relationship between IR as the dependent variable and various markers. ORs and 95% CIs of tertiles 2 and 3 for each parameter were calculated and compared with tertile 1 as a reference. The correlation of TyG and modified TyG indices with HOMA-IR was demonstrated using a scatter plot and adjusted line. Sensitivity and specificity were calculated as the markers’ optimal cut-off values based on Youden’s index. Receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC) was calculated to compare the relative diagnostic strengths of these parameters for identifying IR. The bootstrap method was used to perform pairwise comparisons between AUCs for the parameters. Significance was determined as p < 0.05.
3. Results
3.1. Baseline Characteristics of the Subjects
Table 1 shows the baseline characteristics of participants according to sex. The prevalence of IR was higher in males (13.19%) than in females (10.69%). WC, WHtR, glucose level, and proportion of subjects with central obesity or IR were higher in males than in females, while overall lipid levels were higher in females than in males. The TyG-BMI, TyG-WC, and TyG-WHtR indices were higher in males than in females, while the TyG index and TyG-BMI SDS were not significantly different between males and females.
Table 1.
Baseline characteristics of participants according to sex.
| Total (n = 3728) | Male (n = 1756) | Female (n = 1972) | p | |
|---|---|---|---|---|
| Age (years) | 14.56 (0.06) | 14.53 (0.07) | 14.60 (0.09) | 0.476 |
| Height (cm) | 161.79 (0.23) | 165.53 (0.33) | 157.52 (0.22) | <0.001 |
| Height SDS | 0.21 (0.02) | 0.24 (0.03) | 0.17 (0.03) | 0.063 |
| Weight (kg) | 54.96 (0.29) | 58.80 (0.42) | 50.56 (033) | <0.001 |
| Weight SDS | 0.03 (0.03) | 0.07 (0.04) | −0.02 (0.04) | 0.056 |
| BMI (kg/m2) | 20.73 (0.07) | 21.17 (0.10) | 20.23 (0.11) | <0.001 |
| BMI SDS | −0.09 (0.03) | −0.06 (0.04) | −0.13 (0.04) | 0.225 |
| WC (cm) | 70.11 (0.21) | 72.42 (0.29) | 67.48 (0.27) | <0.001 |
| WC > 90p | 16.45% (0.75) | 14.75% (0.98) | 18.40% (1.18) | 0.020 |
| WHtR | 0.43 (0.01) | 0.44 (0.02) | 0.43 (0.02) | <0.001 |
| Glucose (mg/dL) | 88.82 (0.19) | 89.32 (0.25) | 88.24 (0.20) | <0.001 |
| Insulin (µIU/mL) | 13.62 (0.18) | 13.53 (0.25) | 13.71 (0.23) | 0.574 |
| HOMA-IR | 3.04 (0.05) | 3.05 (0.08) | 3.03 (0.06) | 0.834 |
| IR * | 12.03% (0.68) | 13.19% (0.90) | 10.69% (0.93) | 0.044 |
| TC (mg/dL) | 158.25 (0.58) | 154.59 (0.81) | 162.44 (0.74) | <0.001 |
| LDL-C (mg/dL) | 91.23 (0.50) | 88.96 (0.69) | 93.82 (0.65) | <0.001 |
| Non-HDL-C (mg/dL) | 108.65 (0.56) | 106.35 (0.78) | 111.28 (0.73) | <0.001 |
| TG (mg/dL) | 88.55 (1.16) | 88.60 (1.62) | 88.50 (1.50) | 0.962 |
| HDL-C (mg/dL) | 49.61 (0.20) | 48.25 (0.25) | 51.16 (0.28) | <0.001 |
| AIP | 0.46 (0.01) | 0.48 (0.02) | 0.441 (0.02) | 0.083 |
| TyG index | 8.14 (0.01) | 8.14 (0.02) | 8.14 (0.01) | 0.650 |
| TyG-BMI | 169.17 (0.69) | 172.89 (0.99) | 164.91 (0.95) | <0.001 |
| TyG-BMI SDS | −0.58 (0.22) | −0.277 (0.30) | −0.931 (0.32) | 0.140 |
| TyG-WC | 571.93 (2.05) | 591.16 (2.93) | 549.94 (2.47) | <0.001 |
| TyG-WHtR | 3.54 (0.01) | 3.57 (0.02) | 3.49 (0.02) | 0.003 |
Values are presented as mean (standard error), and categorical data as percentages (standard error). * IR was defined as the HOMA-IR of >95th percentile for each sex and age. SDS, standard deviation score; BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; HOMA-IR, homeostasis model assessment of insulin resistance; IR, insulin resistance; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; AIP, atherogenic index of plasma; TyG, triglyceride glucose index.
3.2. ORs of TyG and Modified TyG Indices for Predicting IR
The ORs and 95% CIs for IR progressively increased across tertiles of each parameter (Table 2). All TyG and modified TyG indices exhibited significantly higher ORs and 95% CIs of tertile 3 (OR range 6.06–14.66) than that of tertile 1 (all p < 0.001) in the total subjects. In contrast, the lipid parameters exhibited ORs of tertile ranged as 0.43–4.25 compared with those of tertile 1 in the total subjects. Among the modified indices, TyG-WHtR presented the highest ORs and 95% CIs for IR in the total subjects (OR = 14.66) and males (OR = 21.59) while TyG-BMI SDS presented the highest ORs and 95% CIs in females (OR = 8.78). Overall, all modified TyG indices presented higher ORs and 95% CIs than TyG index and lipid profiles in the subjects.
Table 2.
Odds ratio for insulin resistance according to tertiles of each parameter.
| Total | Male | Female | ||||
|---|---|---|---|---|---|---|
| OR (95% Cl) | p | OR (95% Cl) | p | OR (95% Cl) | p | |
| TC (mg/dL) | ||||||
| T2 | 1.10 (0.82–1.49) | 0.133 | 1.38 (0.90–2.10) | 0.515 | 0.73 (0.46–1.14) | 0.066 |
| T3 | 1.75 (1.30–2.36) | <0.001 | 2.36 (1.61–3.47) | <0.001 | 1.09 (0.72–1.65) | 0.175 |
| LDL-C (mg/dL) | ||||||
| T2 | 0.83 (0.62–1.11) | 0.010 | 1.16 (0.79–1.71) | 0.317 | 0.61 (0.39–0.93) | 0.018 |
| T3 | 1.34 (1.00–1.80) | 0.003 | 1.88 (1.26–2.78) | 0.001 | 0.94 (0.62–1.44) | 0.329 |
| Non-HDL-C (mg/dL) | ||||||
| T2 | 1.02 (0.75–1.40) | 0.004 | 1.12 (0.74–1.68) | 0.005 | 0.73 (0.44–1.19) | 0.027 |
| T3 | 2.26 (1.66–3.08) | <0.001 | 3.28 (2.19–4.89) | <0.001 | 1.34 (0.89–2.14) | 0.012 |
| TG (mg/dL) | ||||||
| T2 | 1.96 (1.35–2.84) | 0.706 | 2.65 (1.63–4.30) | 0.925 | 1.28 (0.76–2.14) | 0.340 |
| T3 | 4.25 (2.99–6.05) | <0.001 | 6.78 (4.23–10.88) | <0.001 | 2.45 (1.56–3.86) | <0.001 |
| HDL-C (mg/dL) | ||||||
| T2 | 0.61 (0.47–0.79) | 0.544 | 0.63 (0.45–0.89) | 0.764 | 0.38 (0.25–0.58) | 0.004 |
| T3 | 0.43 (0.32–0.59) | <0.001 | 0.44 (0.29–0.67) | 0.003 | 0.45 (0.30–0.68) | 0.114 |
| AIP | ||||||
| T2 | 1.82 (1.26–2.63) | 0.565 | 2.66 (1.65–4.27) | 0.693 | 1.27 (0.77–2.30) | 0.385 |
| T3 | 3.90 (2.78–5.46) | <0.001 | 6.14 (3.88–9.72) | <0.001 | 2.33 (1.48–3.66) | <0.001 |
| TyG index | ||||||
| T2 | 2.77 (1.84–4.16) | 0.427 | 3.28 (1.92–5.56) | 0.596 | 1.90 (1.09–3.30) | 0.857 |
| T3 | 6.06 (4.13–8.89) | <0.001 | 8.76 (5.228–14.667) | <0.001 | 3.34 (2.05–5.45) | <0.001 |
| TyG-BMI | ||||||
| T2 | 2.21 (1.35–3.62) | 0.013 | 2.75 (1.32–5.71) | 0.048 | 2.02 (1.08–3.80) | 0.240 |
| T3 | 11.43 (7.11–18.36) | <0.001 | 20.59 (10.45–40.56) | <0.001 | 7.17 (4.10–12.55) | <0.001 |
| TyG-BMI SDS | ||||||
| T2 | 3.53 (2.03–6.16) | 0.831 | 3.438 (1.661–7.119) | 0.330 | 3.580 (1.771–7.235) | 0.430 |
| T3 | 13.50 (7.84–23.25) | <0.001 | 19.05 (9.43–38.50) | <0.001 | 8.78 (4.58–16.83) | <0.001 |
| TyG-WC | ||||||
| T2 | 1.732 (1.109–2.707) | <0.001 | 2.476 (1.285–4.772) | 0.023 | 1.92 (1.08–3.40) | 0.286 |
| T3 | 9.10 (5.92–13.99) | <0.001 | 17.44 (9.38–32.42) | <0.001 | 5.90 (3.47–10.05) | <0.001 |
| TyG-WHtR | ||||||
| T2 | 2.45 (1.42–4.22) | 0.018 | 2.72 (1.27–5.79) | 0.048 | 1.93 (1.03–3.63) | 0.090 |
| T3 | 14.66 (8.88–24.21) | <0.001 | 21.59 (11.15–41.80) | <0.001 | 8.25 (4.62–14.73) | <0.001 |
ORs and 95% CIs of tertiles 2–3 for each parameter were calculated and compared with those of tertile 1 as a reference. OR, odds ratio; CI, confidence interval; T, tertile; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; AIP, atherogenic index of plasma; TyG, triglyceride glucose index; BMI, body mass index; SDS, standard deviation score; WC, waist circumference; WHtR, waist-to-height ratio.
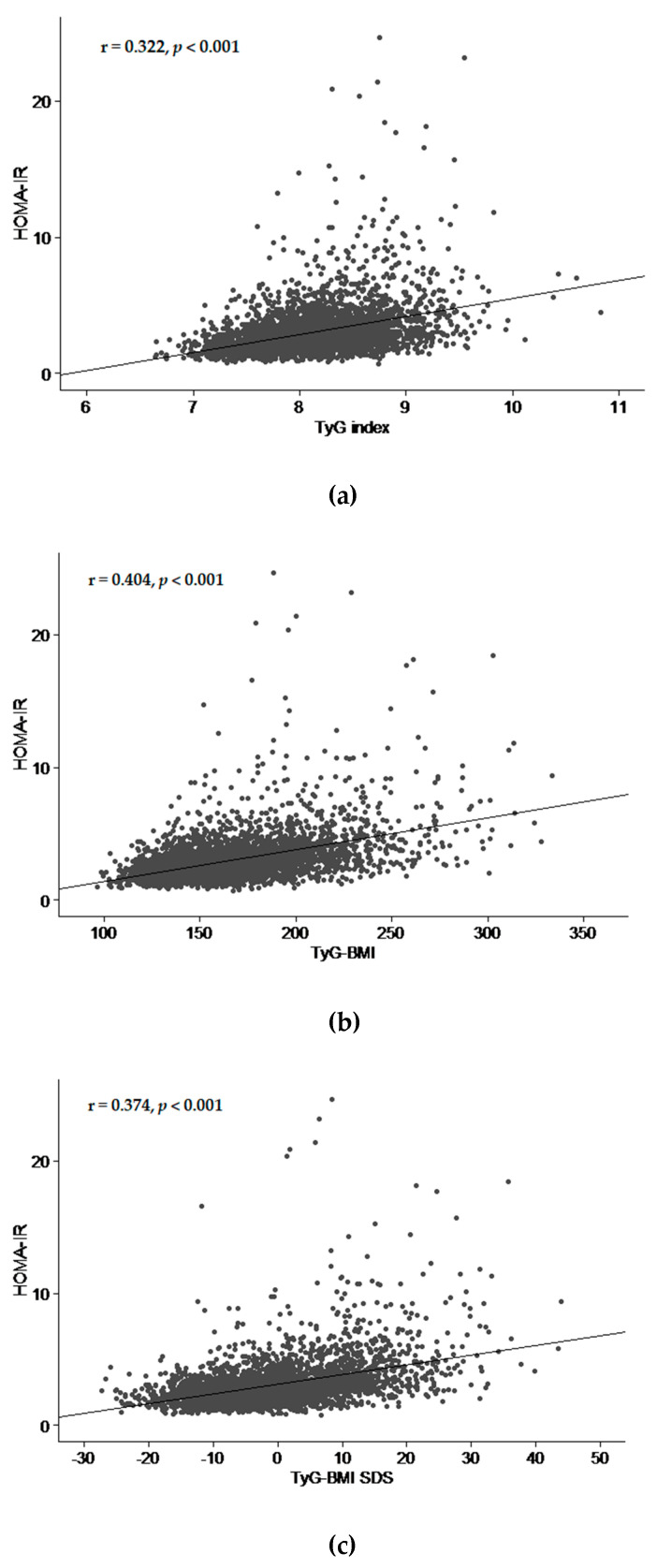
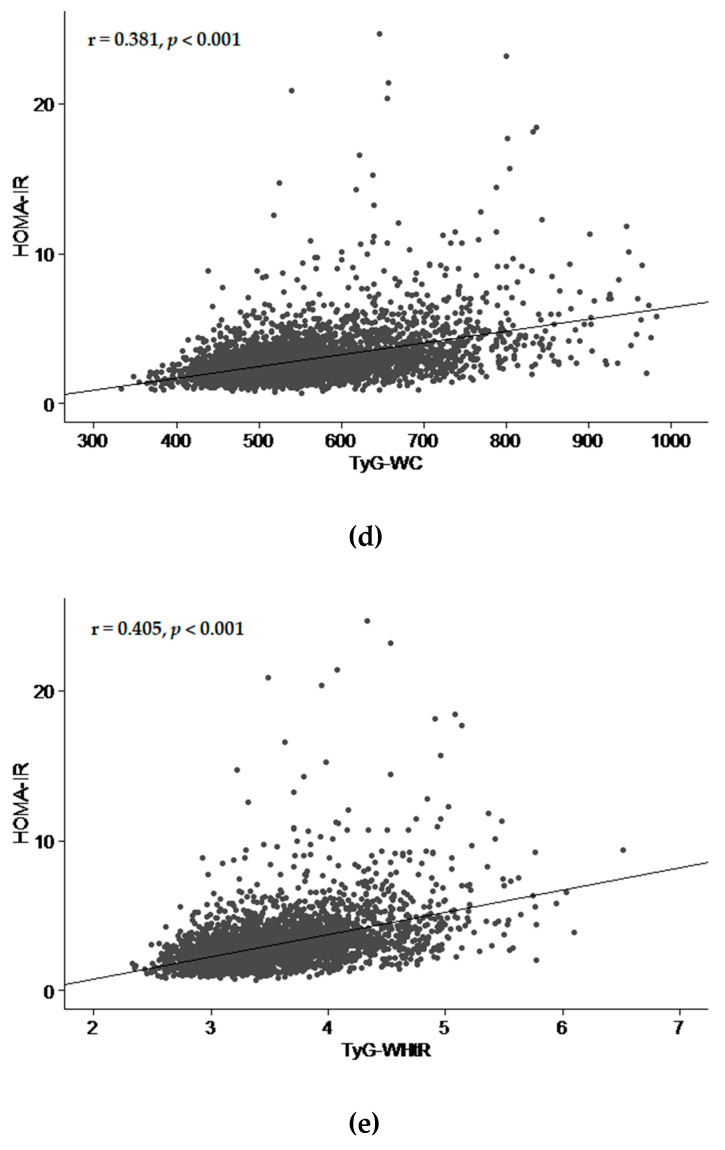
3.3. Correlation of TyG and Modified TyG Indices with HOMA-IR
In scatter plot and fitted line of TyG and modified TyG indices with HOMA-IR, HOMA-IR tended to increase with increasing TyG and modifed TyG indices (all p < 0.001) (Figure 2). Among the indices, coefficient of correlation was highest in TyG-WHtR (r = 0.405, p < 0.001).
Figure 2.
Scatter plot and fitted line of HOMA-IR and TyG index (a), TyG-BMI, (b), TyG-BMI SDS (c), TyG-WC (d), and TyG-WHtR (e) in all subjects. HOMA-IR, homeostasis model assessment of insulin resistance; TyG index, triglyceride glucose index; BMI, body mass index; SDS, standard deviation score; WC, waist circumference; WHtR, waist-to-height ratio.
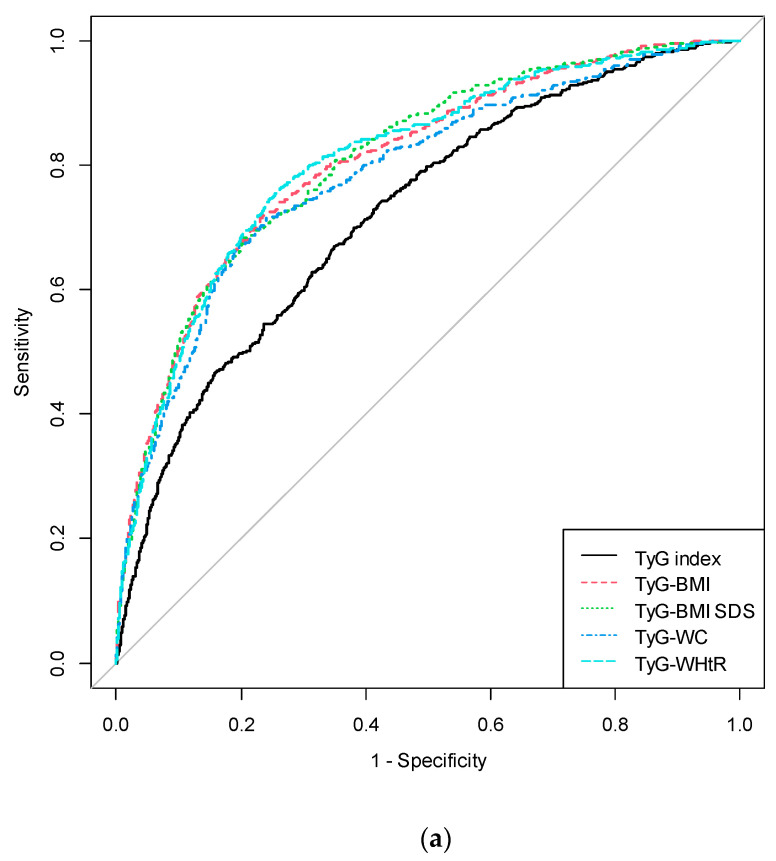
3.4. Cut-off Values and AUC of the TyG and Modified TyG Indices for Predicting IR
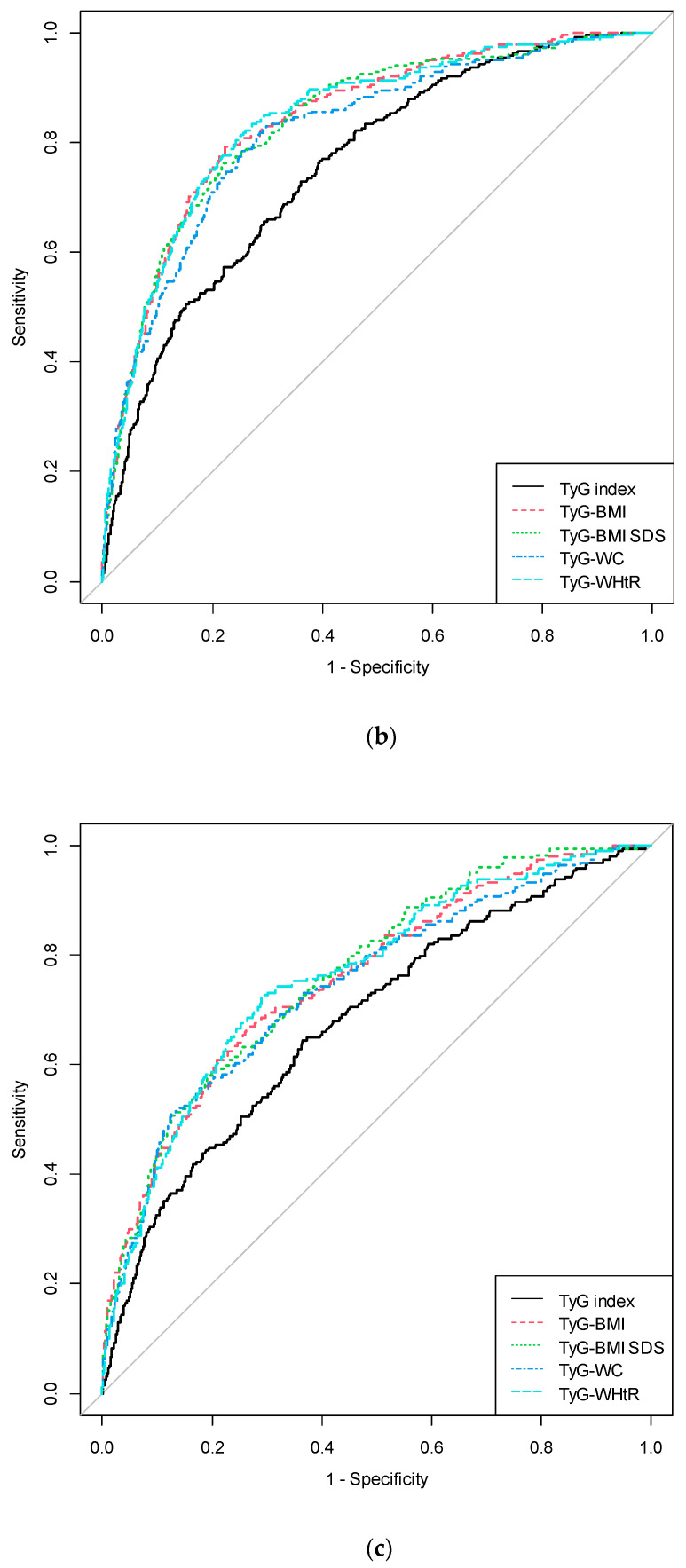
The results of ROC curve analyses and AUCs with the corresponding 95% CIs for TyG and modified TyG indices are shown in Table 3 and Figure 3. AUC ranged from 0.723 to 0.810 in total subjects. All TyG and modified TyG indices predicted IR significantly (all p < 0.001). In total subjects, the cut-off values for IR prediction were 8.261, 178.957, 5.105, 599.800, and 3.696 in TyG index, TyG-BMI, TyG-BMI SDS, TyG-WC, and TyG-WHtR, respectively (all p < 0.001). TyG-BMI SDS showed the largest AUC for IR detection with 0.810 in total subjects and 0.766 in females (p < 0.001), respectively. In males, TyG-BMI and TyG-WHtR showed the largest AUC with 0.842 for IR detection (p < 0.001). Overall, all modified TyG indices—TyG-BMI, TyG-BMI SDS, TyG-WC, and TyG-WHtR—presented significantly higher AUC and 95% CIs than the TyG index (Table A1). Among the modified TyG indices, TyG-BMI and TyG-BMI SDS presented significantly higher AUC and 95% CIs than TyG-WC in the total subjects. In addition, TyG-WHtR presented significantly higher AUC and 95% CIs than TyG-WC overall (total subjects, p < 0.001; males, p = 0.002; females, p = 0.034).
Table 3.
Cut-off values and areas under the receiver operating characteristic curves for each parameter for predicting insulin resistance.
| Cut-off | Sensitivity | Specificity | AUC | 95% Cl | p | |
|---|---|---|---|---|---|---|
| Total | ||||||
| TyG index | 8.261 | 66.449 | 65.555 | 0.723 | (0.699–0.748) | <0.001 |
| TyG-BMI | 178.957 | 72.331 | 76.201 | 0.807 | (0.785–0.829) | <0.001 |
| TyG-BMI SDS | 5.105 | 68.445 | 79.450 | 0.810 | (0.788–0.831) | <0.001 |
| TyG-WC | 599.800 | 71.678 | 75.834 | 0.786 | (0.763–0.810) | <0.001 |
| TyG-WHtR | 3.696 | 74.728 | 75.436 | 0.809 | (0.788–0.831) | <0.001 |
| Male | ||||||
| TyG index | 8.175 | 76.604 | 60.457 | 0.756 | (0.726–0.786) | <0.001 |
| TyG-BMI | 184.058 | 79.245 | 77.680 | 0.842 | (0.817–0.867) | <0.001 |
| TyG-BMI SDS | 5.137 | 76.190 | 78.274 | 0.838 | (0.811–0.865) | <0.001 |
| TyG-WC | 600.847 | 83.019 | 70.299 | 0.820 | (0.792–0.848) | <0.001 |
| TyG-WHtR | 3.719 | 81.132 | 75.220 | 0.842 | (0.817–0.868) | <0.001 |
| Female | ||||||
| TyG index | 8.260 | 64.433 | 63.508 | 0.680 | (0.639–0.721) | <0.001 |
| TyG-BMI | 172.931 | 67.010 | 73.367 | 0.757 | (0.720–0.794) | <0.001 |
| TyG-BMI SDS | 4.883 | 58.101 | 80.623 | 0.766 | (0.730–0.802) | <0.001 |
| TyG-WC | 616.650 | 51.031 | 87.452 | 0.744 | (0.705–0.783) | <0.001 |
| TyG-WHtR | 3.588 | 72.680 | 70.551 | 0.761 | (0.725–0.797) | <0.001 |
AUC, area under the curve; TyG, triglyceride glucose index; BMI, body mass index; SDS, standard deviation score; WC, waist circumference; WHtR, waist-to-height ratio.
Figure 3.
Receiver operating characteristic curve for each parameter in the prediction of insulin resistance. (a) ROC curve of the prediction of insulin resistance in all subjects. (b) ROC curve of the prediction of insulin resistance in males. (c) ROC curve of the prediction of insulin resistance in females. ROC: receiver operating characteristic; TyG index, triglyceride glucose index; BMI, body mass index; SDS, standard deviation score; WC, waist circumference; WHtR, waist-to-height ratio.
4. Discussion
This study shows that the TyG and modified TyG indices can be important predictors for IR in youth. The ORs and 95% CIs for IR progressively increased across tertiles of each parameter, and the TyG and modified TyG indices predicted IR significantly in the ROC curve analysis. Overall, modified TyG indices presented higher ORs and 95% CIs for predicting IR than the TyG index and lipid profiles. Among all indices, TyG-WHtR showed the strongest association with HOMA-IR, and TyG-BMI SDS was the most powerful predictor for IR in total subjects. In addition, TyG-WHtR was superior to TyG-WC for predicting IR in the present study.
IR plays an important role in type 2 diabetes, metabolic syndrome, and CVD [23]. Therefore, early detection of IR in people at risk for future CVD is important. Among the detection methods, TyG and modified TyG indices have been proposed as reliable markers in adults [16,24,25]. A population-based cross-sectional study suggested that the TyG index is useful for IR prediction in adults [26]. Another longitudinal study reported that the TyG index predicts type 2 diabetes in middle-aged and older adults [27]. However, elaborate studies that investigated the relationship between the TyG and modified TyG indices in youth are extremely limited.
Several studies have validated the relationship between the TyG index and IR. First, hypertriglyceridemia may increase hepatic glucose output with the increased transport of free fatty acids to the liver, making it one of the important risk factors for type 2 diabetes [28,29]. Research has shown that TG elevation can induce IR through the impairment of muscle glucose metabolism [30]. Second, insulin accelerates adipocyte TG stores by promoting TG synthesis and inhibiting lipolysis as well as promoting the maturation of adipocytes [28]. In addition, insulin stimulates lipoprotein lipase activity, thus increasing the uptake of fatty acids from circulating lipoproteins.
Obesity is strongly associated with IR [16]. Therefore, a combination of the TyG index and obesity parameters may predict IR better than the TyG index alone. Because BMI is a simple and widely used indicator of obesity and other metabolic risks, TyG-BMI can be a useful predictive marker [25]. Lim et al. [16] reported that TyG-BMI was superior to other modified TyG indices for predicting IR in adults. In children, obesity is defined as a BMI of ≥95th percentile of sex- and age-specific references; thus, BMI SDS should be used more commonly than BMI itself [31]. Therefore, we suggested a new parameter, TyG-BMI SDS, to explain obesity better than TyG-BMI in youth. It exhibited the largest AUC for the prediction of IR among all parameters in total subjects and females.
Measures of central obesity, such as WC and WHtR, have been suggested as better indices than BMI because central obesity is closely associated with fat distribution but not BMI [32]. A meta-analysis revealed that measures of central obesity predict cardiovascular risk factors, including diabetes, hypertension, and dyslipidemia better than BMI [33]. A systematic review reported that combining BMI with measures of central obesity is superior to using BMI alone to assess the mortality risk of patients with coronary artery disease [34]. Among the measures of central obesity, WC does not directly reflect the difference in height, and age-dependent WC cutoffs are required [35]. However, WHtR has been reported to outperform WC and BMI in predicting metabolic syndrome and cardiovascular risks accounting for height, especially in Asians [36]. Overweight children with a higher WHtR are more likely to have higher cardiometabolic risk factors [32,37]. Thus, we analyzed both TyG-WHtR and TyG-WC and found TyG-WHtR to be superior to TyG-WC in predicting IR in youth. In contrast, TyG-WC was superior to TyG-WHtR in an adult study [16].
High-carbohydrate diet increases insulin level, which raises TG synthesis and glucose level [28,29,38]. Thus, response to dietary carbohydrate restriction might provide an operational definition of metabolic syndrome [38]. Carbohydrate restriction is considered as effective nutritional therapy for metabolic syndrome [39,40,41]. Thus, the ability of carbohydrate restriction and ketogenic diets to control the markers of the metabolic syndrome has been suggested [42,43]. Our study investigated association of the TyG and modified TyG indices with IR in the population with cross-sectional data. Further studies investigating improvement in the TyG and modified TyG indices after dietary carbohydrate restriction and ketogenic diet could make these parameters even more robust.
This study has some limitations. First, this was a cross-sectional study exclusive to Korean youth, thus limiting the generalizability of our findings. Second, associated factors, such as pubertal status, diet, and physical activity, were not considered. Third, lean and fat body mass were not considered in this study. Despite these limitations, the present study assessed the TyG and modified TyG indices as markers of IR across a large number of children and adolescents. In addition, we proposed a new parameter, TyG-BMI SDS, as one of the important predictors of IR in youth.
5. Conclusions
This study indicates that the TyG and modified TyG indices could serve as valuable predictors of IR in youth. Moreover, combinations of obesity parameters with the TyG index—including the new parameter, TyG-BMI SDS—have better diagnostic accuracy than the TyG index. The TyG and modified TyG indices are simple and cost-effective markers of IR. Thus, these markers are useful for the assessment of cardiometabolic risk factors.
Appendix A
Table A1.
Comparison of areas under receiver operating curves among each parameters for predicting insulin resistance.
| TyG index | TyG-BMI | TyG-BMI SDS | TyG-WC | TyG-WHtR | |
|---|---|---|---|---|---|
| Total | |||||
| TyG index | reference | <0.001 | <0.001 | <0.001 | <0.001 |
| TyG-BMI | <0.001 | reference | 0.617 | <0.001 | 0.739 |
| TyG-BMI SDS | <0.001 | 0.617 | reference | 0.011 | >0.999 |
| TyG-WC | <0.001 | <0.001 | 0.011 | reference | <0.001 |
| TyG-WHtR | <0.001 | 0.739 | >0.999 | <0.001 | reference |
| Male | |||||
| TyG index | reference | <0.001 | <0.001 | <0.001 | <0.001 |
| TyG-BMI | <0.001 | reference | 0.505 | <0.001 | >0.999 |
| TyG-BMI SDS | <0.001 | 0.505 | reference | 0.046 | 0.617 |
| TyG-WC | <0.001 | <0.001 | 0.046 | reference | 0.002 |
| TyG-WHtR | <0.001 | >0.999 | 0.617 | 0.002 | reference |
| Female | |||||
| TyG index | reference | <0.001 | <0.001 | <0.001 | <0.001 |
| TyG-BMI | <0.001 | reference | 0.413 | 0.149 | 0.739 |
| TyG-BMI SDS | <0.001 | 0.413 | reference | 0.143 | 0.721 |
| TyG-WC | <0.001 | 0.149 | 0.143 | reference | 0.034 |
| TyG-WHtR | <0.001 | 0.739 | 0.721 | 0.034 | reference |
Bootstrap method was used to perform pairwise comparisons between AUCs for the parameters. Values are presented as p values. TyG, triglyceride glucose index; BMI, body mass index; SDS, standard deviation score; WC, waist circumference; WHtR, waist-to-height ratio; AUC, area under the curve.
Author Contributions
Conceptualization: K.S. and H.S.L., and H.W.C.; methodology: K.S., G.P., H.S.L., and H.W.C.; software: K.S., Y.C., and J.S.O.; validation: H.W.C.; formal analysis: K.S., G.P., H.S.L, H.S.C., and J.S.; investigation: K.S. and A.K.; resources: K.S.; data curation: K.S.; writing—original draft preparation: K.S. and H.W.C.; writing—review and editing: H.S.L and H.W.C.; visualization: K.S and G.P.; supervision: H.-S.K. and H.W.C.; project administration: K.S. and H.W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Yonsei University Gangnam Severance Hospital (IRB, 3-2020-0122).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moon S., Park J.-S., Ahn Y. The Cut-off Values of Triglycerides and Glucose Index for Metabolic Syndrome in American and Korean Adolescents. J. Korean Med. Sci. 2017;32:427–433. doi: 10.3346/jkms.2017.32.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park K., Ahn C.W., Lee S.B., Kang S., Nam J.S., Lee B.K., Kim J.H., Park J.S. Elevated TyG Index Predicts Progression of Coronary Artery Calcification. Diabetes Care. 2019;42:1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 3.Castorani V., Polidori N., Giannini C., Blasetti A., Chiarelli F. Insulin resistance and type 2 diabetes in children. Ann. Pediatr. Endocrinol. Metab. 2020;25:217–226. doi: 10.6065/apem.2040090.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friend A., Craig L., Turner S. The Prevalence of Metabolic Syndrome in Children: A Systematic Review of the Literature. Metab. Syndr. Relat. Disord. 2013;11:71–80. doi: 10.1089/met.2012.0122. [DOI] [PubMed] [Google Scholar]
- 5.Lim S., Jang H.C., Park K.S., Cho S.I., Lee M.-G., Joung H., Mozumdar A., Liguori G. Changes in Metabolic Syndrome in American and Korean Youth, 1997-2008. Pediatrics. 2012;131:e214–e222. doi: 10.1542/peds.2012-0761. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hamad D., Raman V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017;6:397–407. doi: 10.21037/tp.2017.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A DeFronzo R., Tobin J.D., Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. Metab. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 8.Du T., Yuan G., Zhang M., Zhou X., Sun X., Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the tri-glycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S.H., Ahn M.B., Choi Y.J., Kim S.K., Kim S.H., Cho W.K., Cho K.S., Suh B.-K., Jung M.H. Comparison of different criteria for the definition of insulin resistance and its relationship to metabolic risk in children and adolescents. Ann. Pediatr. Endocrinol. Metab. 2020;25:227–233. doi: 10.6065/apem.2040002.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brar P.C. Update on the current modalities used to screen high risk youth for prediabetes and/or type 2 diabetes mellitus. Ann. Pediatr. Endocrinol. Metab. 2019;24:71–77. doi: 10.6065/apem.2019.24.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller W.G., Thienpont L.M., Van Uytfanghe K., Clark P.M., Lindstedt P., Nilsson G., Steffes M.W. Toward Standardization of Insulin Immunoassays. Clin. Chem. 2009;55:1011–1018. doi: 10.1373/clinchem.2008.118380. [DOI] [PubMed] [Google Scholar]
- 12.Katsa M.E., Ioannidis A., Sachlas A., Dimopoulos I., Chatzipanagiotou S., Gil A.P.R. The roles of triglyceride/high-density lipoprotein cholesterol ratio and uric acid as predisposing factors for metabolic syndrome in healthy children. Ann. Pediatr. Endocrinol. Metab. 2019;24:172–179. doi: 10.6065/apem.2019.24.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J., Kim B., Kim W., Ahn C., Choi H.Y., Kim J.G., Kim J., Shin H., Kang J.G., Moon S. Lipid indices as simple and clinically useful surrogate markers for insulin resistance in the U.S. population. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-021-82053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrero-Romero F., Simental-Mendía L.E., González-Ortiz M., Martínez-Abundis E., Ramos-Zavala M.G., Hernández-González S.O., Jacques-Camarena O., Rodríguez-Morán M. The Product of Triglycerides and Glucose, a Simple Measure of Insulin Sensitivity. Comparison with the Euglycemic-Hyperinsulinemic Clamp. J. Clin. Endocrinol. Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 15.Zheng S., Shi S., Ren X., Han T., Li Y., Chen Y., Liu W., Hou P.C., Hu Y. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: Cross-sectional and prospective cohort study. J. Transl. Med. 2016;14:260. doi: 10.1186/s12967-016-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J., Kim J., Koo S.H., Kwon G.C. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS ONE. 2019;14:e0212963. doi: 10.1371/journal.pone.0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kweon S., Kim Y., Jang M.-J., Kim Y., Kim K., Choi S., Chun C., Khang Y.-H., Oh K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES) Int. J. Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J.H., Yun S., Hwang S.-S., Shim J.O., Chae H.W., Lee Y.J., Lee J.H., Kim S.C., Lim D., Yang S.W., et al. The 2017 Korean National Growth Charts for children and adolescents: Development, improvement, and prospects. Korean J. Pediatr. 2018;61:135–149. doi: 10.3345/kjp.2018.61.5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon J.S., Lee S.Y., Nam C.M., Choi J.-M., Choe B.-K., Seo J.-W., Oh K., Jang M.-J., Hwang S.-S., Yoo M.H., et al. 2007 Korean National Growth Charts: Review of developmental process and an outlook. Korean J. Pediatr. 2008;51 doi: 10.3345/kjp.2008.51.1.1. [DOI] [Google Scholar]
- 20.Roberts W.C. The Friedewald-Levy-Fredrickson formula for calculating low-density lipoprotein cholesterol, the basis for lipid-lowering therapy. Am. J. Cardiol. 1988;62:345–346. doi: 10.1016/0002-9149(88)90248-2. [DOI] [PubMed] [Google Scholar]
- 21.Cui Y., Blumenthal R.S., Flaws J.A., Whiteman M.K., Langenberg P., Bachorik P.S., Bush T.L. Non-high-density lipoprotein cho-lesterol level as a predictor of cardiovascular disease mortality. Arch. Int. Med. 2001;161:1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 22.Dobiásová M., Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin. Biochem. 2001;34:583–588. doi: 10.1016/S0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 23.Yi K.H., Hwang J.S., Kim E.Y., Lee S.H., Kim D.H., Lim J.S. Prevalence of insulin resistance and cardiometabolic risk in Korean children and adolescents: A population-based study. Diabetes Res. Clin. Pract. 2014;103:106–113. doi: 10.1016/j.diabres.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Simental-Mendía L.E., Rodríguez-Morán M., Guerrero-Romero F. The Product of Fasting Glucose and Triglycerides As Surrogate for Identifying Insulin Resistance in Apparently Healthy Subjects. Metab. Syndr. Relat. Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 25.Er L.-K., Wu S., Chou H.-H., Hsu L.-A., Teng M.-S., Sun Y.-C., Ko Y.-L. Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS ONE. 2016;11:e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrero-Romero F., Villalobos-Molina R., Jiménez-Flores J.R., Simental-Mendia L.E., Méndez-Cruz R., Murguía-Romero M., Rodríguez-Morán M. Fasting Triglycerides and Glucose Index as a Diagnostic Test for Insulin Resistance in Young Adults. Arch. Med. Res. 2016;47:382–387. doi: 10.1016/j.arcmed.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Park B., Lee H.S., Lee Y.-J. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: A 12-year longitudinal study of the Korean Genome and Epidemiology Study cohort. Transl. Res. 2021;228:42–51. doi: 10.1016/j.trsl.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Kahn B.B., Flier J.S. Obesity and insulin resistance. J. Clin. Investig. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirosh A., Shai I., Bitzur R., Kochba I., Tekes-Manova D., Israeli E., Shochat T., Rudich A. Changes in Triglyceride Levels Over Time and Risk of Type 2 Diabetes in Young Men. Diabetes Care. 2008;31:2032–2037. doi: 10.2337/dc08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley D.E., Goodpaster B.H. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24:933–941. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 31.Styne D.M., Arslanian S.A., Connor E.L., Farooqi I.S., Murad M.H., Silverstein J.H., Yanovski J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017;102:709–757. doi: 10.1210/jc.2016-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung I.H., Park S., Park M.J., Yoo E.-G. Waist-to-Height Ratio as an Index for Cardiometabolic Risk in Adolescents: Results from the 1998-2008 KNHANES. Yonsei Med. J. 2016;57:658–663. doi: 10.3349/ymj.2016.57.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee C.M.Y., Huxley R.R., Wildman R.P., Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J. Clin. Epidemiol. 2008;61:646–653. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Coutinho T., Goel K., Corrêa de Sá D., Carter R.E., Hodge D.O., Kragelund C., Kanaya A.M., Zeller M., Park J.S., Kober L., et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: Role of "normal weight central obesity". J. Am. Coll. Cardiol. 2013;61:553–560. doi: 10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 35.Zimmet P., Magliano D., Matsuzawa Y., Alberti G., Shaw J. The Metabolic Syndrome: A Global Public Health Problem and A New Definition. J. Atheroscler. Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 36.Yang H., Xin Z., Feng J.-P., Yang J.-K. Waist-to-height ratio is better than body mass index and waist circumference as a screening criterion for metabolic syndrome in Han Chinese adults. Medicine. 2017;96:e8192. doi: 10.1097/MD.0000000000008192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoury M., Manlhiot C., McCrindle B.W. Role of the Waist/Height Ratio in the Cardiometabolic Risk Assessment of Children Classified by Body Mass Index. J. Am. Coll. Cardiol. 2013;62:742–751. doi: 10.1016/j.jacc.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Volek J.S., Feinman R.D. Carbohydrate restriction improves the features of Metabolic Syndrome. Metabolic Syndrome may be defined by the response to carbohydrate restriction. Nutr. Metab. 2005;2:31. doi: 10.1186/1743-7075-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinman R.D., Pogozelski W.K., Astrup A., Bernstein R.K., Fine E.J., Westman E.C., Accurso A., Frassetto L., Gower B.A., McFarlane S.I., et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition. 2015;31:1–13. doi: 10.1016/j.nut.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Hallberg S.J., McKenzie A.L., Williams P.T., Bhanpuri N.H., Peters A.L., Campbell W.W., Hazbun T.L., Volk B.M., McCarter J.P., Phin-ney S.D., et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018;9:583–612. doi: 10.1007/s13300-018-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Athinarayanan S.J., Adams R.N., Hallberg S.J., McKenzie A.L., Bhanpuri N.H., Campbell W.W., Volek J.S., Phinney S.D., McCarter J.P. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-randomized Clinical Trial. Front. Endocrinol. 2019;10:348. doi: 10.3389/fendo.2019.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volek J.S., Fernandez M.L., Feinman R.D., Phinney S.D. Dietary carbohydrate restriction induces a unique metabolic state posi-tively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res. 2008;47:307–318. doi: 10.1016/j.plipres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Hyde P.N., Sapper T.N., Crabtree C.D., LaFountain R.A., Bowling M.L., Buga A., Fell B., McSwiney F.T., Dickerson R.M., Miller V.J., et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight. 2019;4:4. doi: 10.1172/jci.insight.128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.