Abstract
Surgical site infection (SSI) following caesarean section is associated with increased morbidity, mortality, and significant health care costs. This study evaluated the epidemiological, clinical, and microbiological features of Acinetobacter spp. in women with SSIs who have undergone caesarean section at a referral hospital in the Brazilian Amazon region. This study included 69 women with post-caesarean SSI by Acinetobacter spp. admitted to the hospital between January 2012 and May 2015. The 69 Acinetobacter isolates were subjected to molecular species identification, antimicrobial susceptibility testing, detection of carbapenemase-encoding genes, and genotyping. The main complications of post-caesarean SSI by Acinetobacter were inadequate and prolonged antibiotic therapy, sepsis, prolonged hospitalization, and re-suture procedures. A. baumannii, A. nosocomialis and A. colistiniresistens species were identified among the isolates. Carbapenem resistance was associated with OXA-23-producing A. baumannii isolates and IMP-1-producing A. nosocomialis isolate. Patients with multidrug-resistant A. baumannii infection showed worse clinical courses. Dissemination of persistent epidemic clones was observed, and the main clonal complexes (CC) for A. baumannii were CC231 and CC236 (Oxford scheme) and CC1 and CC15 (Pasteur scheme). This is the first report of a long-term Acinetobacter spp. outbreak in women who underwent caesarean section at a Brazilian hospital. This study demonstrates the impact of multidrug resistance on the clinical course of post-caesarean infections.
Keywords: caesarean, surgical site infection, Acinetobacter, clonal complexes
1. Introduction
Among surgical site infections (SSIs), caesarean section is considered an important risk factor for postpartum infections due to uterine skin rupture, bladder catheterization, and contact with health care workers (HCWs) [1,2]. SSI following caesarean section is associated with increased morbidity and mortality, prolonged hospitalization, secondary infertility, and increased health care costs [3]. However, caesarean section is one of the most commonly performed surgical procedures worldwide, with reported frequencies ranging from 15% to 60% and associated postpartum bacterial infection rates of up to 25%, which is approximately 5–20 times higher than those of vaginal delivery [4,5].
Risk factors associated with post-caesarean SSI are classified into intrinsic (age and body mass index) and extrinsic (limited prenatal consultations, smoking, diabetes mellitus, obesity, hypertensive disorders, and anemia) factors [6]. During the intrapartum stage, other risk factors are also relevant, including emergency caesarean section, prolonged labor, premature rupture of membranes, and chorioamnionitis [7]. Moreover, complications generated by post-caesarean SSI include prolonged healing time after surgery due to several factors, e.g., wound dehiscence, prolonged hospital admission, prolonged use of antibiotics, possibility of re-admission and re-suture, sepsis and, in rare cases, mortality [1].
Several bacterial agents have been associated with post-caesarean SSI. Among Gram-positive bacteria, Staphylococcus aureus was responsible for 20–30% of hospital SSI. Other frequently isolated organisms are Gram-negative bacilli, including Pseudomonas aeruginosa, Klebsiella spp., and Escherichia coli [8]. Despite rarely being isolated from SSI postpartum patients, Acinetobacter species are considered to have major clinical relevance, especially A. baumannii, which causes bacteraemia, pneumonia, skin and soft tissue infections, and meningitis [9]. The World Health Organization (WHO) has classified this species as a critical-tier priority pathogen for antimicrobial development owing to its high resistance rates [10,11]. Additionally, other Acinetobacter species, such as A. nosocomialis and A. pittii, have been increasingly associated with hospital-acquired infections (HAIs) [9]. Despite the phenotypic similarities, differences between species of Acinetobacter have been described, mainly in relation to virulence factors and susceptibility profiles to antimicrobials [12].
Several molecular typing methods are applied to the study of molecular epidemiology, clonal relatedness studies, and assessments of transmission of bacterial pathogens. Semi-automated repetitive extragenic palindromic polymerase chain reaction (rep-PCR) on the DiversiLab® System, one of the most effective fingerprinting methods, has a discriminatory feature similar to pulsed-field gel electrophoresis (PFGE), the standard technique for A. baumannii typing [13]. Multilocus sequence typing (MLST) is a tool widely used for molecular epidemiological investigations, providing insights into the global population structure, genetic diversity, and high-risk clone identification [14].
The present study first describes the epidemiological, clinical, and microbiological aspects related with post-caesarean SSI at a hyperendemic setting for Acinetobacter spp. at a referral hospital in Belém, Amazon Region, Brazil.
2. Materials and Methods
2.1. Study Design and Clinical Data Collection
This was a single-center, retrospective cohort study, performed at a 484-bed tertiary-care teaching hospital for high-risk pregnant women and newborns, located in Belém, Amazon Region, Brazil. The present study included women who underwent caesarean delivery and were admitted to the obstetric ward and with a surgical wound culture positive for Acinetobacter spp., between January 2012 and May 2015. Demographic, epidemiological, and clinical data were obtained from patients’ medical records.
Surgical site infection was defined according to criteria designed by the Centers for Disease Control and Prevention [15]. This study was approved by the Institutional Ethics Committee of Evandro Chagas Institute (N° 2.999.939/05.Nov.2018) and the hospital under study (N° 3.126.011/30.Jan.2019). Written informed consent was obtained from all participants.
2.2. Bacterial Isolates and Antimicrobial Susceptibility Testing
Acinetobacter spp. isolates included in the study were obtained from surgical wound cultures of women with post-caesarean SSI. Preliminary identification was performed on the Vitek-2 System (bioMérieux, Marcy l’Etoile, France) in the hospital laboratory, and samples were sent to the Evandro Chagas Institute for further assays.
Antimicrobial susceptibility testing (AST) was performed on the Vitek-2 System (bioMérieux, Marcy l’Etoile, France), according to Clinical and Laboratory Standards Institute (CLSI) criteria [16]. Multidrug resistance (MDR) was defined as reduced or lack of susceptibility of an organism to three or more antimicrobial classes [17].
2.3. Acinetobacter Species Identification
Species identification was performed through rpoB partial sequencing, as described earlier [18]. Obtained sequences were compared to those available on the GenBank database using the BLAST search engine (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 30 December 2020).
2.4. Detection of Carbapenemase-Encoding Genes
All isolates non-susceptible to imipenem and/or meropenem were investigated for the presence of carbapenem-hydrolyzing class D β-lactamase (CHDL) (blaOXA-23-like, blaOXA-24/40-like, blaOXA-51-like, blaOXA-58-like and blaOXA143-like) and metallo-β-lactamase (blaNDM-like, blaIMP-like, blaVIM-like and blaSPM-like)-encoding genes by polymerase chain reaction (PCR) using primers as previously described [19,20].
2.5. Genotyping by rep-PCR and Multilocus Sequence Typing
Genetic relatedness of the isolates was assessed using semi-automated rep-PCR on the DiversiLab® System (bioMérieux) after following the manufacturer’s guidelines. Data were analyzed and interpreted using internet-based DiversiLab software (v. 3.4) with the Kullback-Leibler correlation coefficient. Strains presenting a similarity of ≥95% were considered clonally related [13]. Multilocus sequence typing (MLST) was performed on A. baumannii isolates that were not susceptible to carbapenems following protocols previously described by Bartual et al. 2005 [21] and Diancourt et al. 2010 [22] available at the PubMLST website (http://pubmlst.org/acinetobacter, accessed on 30 December 2020).
2.6. Statistical Analysis
Data were expressed as absolute and relative (percentage) frequencies for categorical variables or mean ± standard deviation (SD) for continuous variables. Non-parametric tests such as Fisher’s exact test and the G-test of independence were performed using the program BioEstat version 5.5 or GraphPad Prism version 8.00. p values ≤ 0.05 were considered indicative of statistical significance.
3. Results
3.1. Demographic and Clinical Data
From January 2012 to May 2015, a total of 69 women submitted to caesarean section at the study hospital acquired a surgical site infection by Acinetobacter spp. The mean age of the women was 25 years (12 to 46 years, SD: 7 years) and 100% of caesarean sections were emergency procedures. Clinical and sociodemographic data are presented in Table 1. During the gestational period, 51% (n = 35) of women had gynecological complications such as a urinary tract infection (UTI) and leukorrhea. Additionally, 19% (n = 13) of women had previously suffered between one and three miscarriages. Post-caesarean section infection by Acinetobacter spp. was more frequent in primiparous women (49% of cases, n = 34).
Table 1.
Patient characteristics and risk factors for developing post-caesarean section surgical site infection by Acinetobacter spp.
| N | % | |
|---|---|---|
| Gestational age (weeks) | ||
| Preterm (<37 weeks) | 25 | 36% |
| Full-term (37 to 41 weeks) | 36 | 52% |
| Post-term (>41 weeks) | 0 | 0% |
| N.I | 8 | 12% |
| Personal morbidities | ||
| Chronic noncommunicable diseases | 16 | 23% |
| Chronic communicable diseases | 3 | 4% |
| Infectious diseases | 4 | 6% |
| Anemia | 3 | 4% |
| No morbidities | 42 | 60% |
| N.I | 2 | 3% |
| Caesarean indication | ||
| CPD | 4 | 6% |
| Hypertensive diseases of pregnancy | 14 | 20% |
| PROM | 7 | 10% |
| Fetal distress | 14 | 20% |
| Fetal death | 2 | 3% |
| Sexually Transmitted Infections | 5 | 7% |
| Others | 19 | 28% |
| N.I | 4 | 6% |
| Sexually transmitted infections | ||
| Yes | 10 | 14% |
| No | 52 | 75% |
| N.I | 7 | 10% |
| Abortion | ||
| Yes | 13 | 19% |
| No | 55 | 80% |
| N.I | 1 | 1% |
| Gynecological complications | ||
| Yes | 35 | 51% |
| No | 28 | 41% |
| N.I | 6 | 9% |
| Age (years) | ||
| 12 a 19 | 20 | 29% |
| 20 a 35 | 45 | 65% |
| 36 a 46 | 4 | 6% |
| Marital status | ||
| Married | 6 | 9% |
| Single/unmarried | 12 | 17% |
| Stable union | 37 | 54% |
| N.I | 14 | 20% |
| Education level | ||
| Elementary and middle school | 27 | 40% |
| High school | 27 | 39% |
| University Education | 3 | 4% |
| N.I | 12 | 17% |
| Profession/occupation | ||
| Housewife | 30 | 43% |
| Student | 8 | 12% |
| Teacher | 2 | 3% |
| Autonomous | 2 | 3% |
| Others | 14 | 20% |
| N.I | 13 | 19% |
Abbreviations: CPD: Cephalopelvic disproportion, PROM: Premature rupture of membranes. Others: Amniotic Fluid Index, Restriction of Intrauterine Growth, Premature placental dislocation. Noncommunicable chronic diseases: systemic arterial hypertension, diabetes mellitus, asthma, epilepsy. Chronic communicable diseases: hepatitis B. Infectious diseases: malaria, leishmaniasis, pneumonia. N.I: No information.
The main comorbidities in the clinical histories of the 69 women were systemic arterial hypertension (14%) and asthma (4%). Underlying diseases observed in the family history of patients included hypertension (26%; n = 18) and diabetes mellitus (22%; n = 15). Of the 69 women, nine (13%) were smokers and 13 (19%) alcoholics.
SSI was detected, on average, on the tenth day (interquartile range: 7 and 14 days) after the caesarean section procedure, and in 45% (n = 31) of the cases, the patients were readmitted to the hospital due to symptoms of post-caesarean infection.
3.2. Confirmation of Acinetobacter Species, Antimicrobial Susceptibility Testing, and Carbapenemase Gene Detection
Molecular identification of Acinetobacter spp. revealed three species associated with SSI in the 69 women: A. baumannii (71%, n = 49), A. nosocomialis (28%, n = 19), and A. colistiniresistens (1%, n = 1). Among the isolates of A. baumannii, 61% (30/49) showed resistance to carbapenems and were positive for the blaOXA-23-like and blaOXA-51-like genes; these isolates were also resistant to all β-lactams tested, ciprofloxacin, and gentamicin. Amikacin, tigecycline, and colistin were the most effective antimicrobial agents against carbapenem-resistant A. baumannii in vitro (Table 2). Significant differences in antimicrobial resistance rates were observed between A. baumannii and A. non-baumannii isolates (Table 2). Among A. non-baumannii isolates, only one A. nosocomialis isolate was resistant to carbapenems and harbored the blaIMP-1 gene. This isolate showed high minimal inhibitory concentration (MIC) values in response to the β-lactams tested but was susceptible to ciprofloxacin, aminoglycosides, tigecycline and colistin. A. colistiniresistens, showed resistance to colistin, an intrinsic characteristic of the species, and susceptibility to all other tested antimicrobials.
Table 2.
Antimicrobial susceptibility profile for A. baumannii and non-A. baumannii groups.
| Antimicrobial | A. baumannii (N = 49) | Non-A. baumannii (N = 20) | p-Value * | ||
|---|---|---|---|---|---|
| S | NS | S | NS | ||
| SAM | 18 | 31 | 19 | 1 | <0.0001 † |
| TZP | 17 | 32 | 17 | 3 | 0.0002 † |
| CTX | 1 | 48 | 3 | 17 | 0.0702 |
| CAZ | 15 | 34 | 19 | 1 | <0.0001 † |
| FEP | 15 | 34 | 19 | 1 | <0.0001 † |
| IMP | 19 | 30 | 19 | 1 | <0.0001 † |
| MEM | 19 | 30 | 19 | 1 | <0.0001 † |
| GEN | 17 | 32 | 20 | 0 | <0.0001 † |
| AMK | 33 | 16 | 20 | 0 | 0.0033 † |
| CIP | 15 | 34 | 20 | 0 | <0.0001 † |
| TIG | 49 | 0 | 20 | 0 | >0.9999 |
| COL | 48 | 1 | 20 | 0 | >0.9999 |
* Fisher Exact test. † Statistically significant. Abbreviations: S: susceptible; NS: non-susceptible; SAM: ampicillin-sulbactam; TZP: piperacillin/tazobactam; CTX: cefotaxime; CAZ: ceftazidime; FEP: cefepime; IMP: imipenem; MEM: meropenem; GEN: gentamicin; AMK: amikacin; CIP: ciprofloxacin; TIG: tigecycline; COL: colistin.
3.3. Complications of Post-Caesarean SSI by Susceptible and Carbapenem-Resistant Acinetobacter spp.
To assess the impact of carbapenem resistance in the clinical evolution of cases, patients were divided into two groups: SSI by (I) carbapenem-susceptible Acinetobacter and (II) carbapenem-resistant Acinetobacter. Patients in the carbapenem-resistant group (n = 31) demonstrated a more significant association with complications and prolonged post-caesarean SSI evolution than the carbapenem-susceptible group (n = 38) (Table 3). Post-caesarean SSI due to carbapenem-resistant Acinetobacter was also associated with a prolonged hospital stay (6 to 80 days) and an average discharge of 28 days.
Table 3.
Clinical complications developed by women with post-caesarean surgical site infection by carbapenem-susceptible or -resistant Acinetobacter spp.
| Complications | Carbapenem Resistant (N = 31) n (%) |
Carbapenem Susceptible (N = 38) n (%) |
p-Value * |
|---|---|---|---|
| Re-suture time (hours) | |||
| >1 | 2 (6%) | 2 (5%) | 1.0000 |
| <1 | 25 (81%) | 30 (79%) | |
| Number of re-sutures | |||
| 1 | 17 (55%) | 33 a (87%) | 0.0007 † |
| 2 | 12 a (39%) | 2 (5%) | |
| Other surgeries | |||
| Yes | 12 a (39%) | 4 (11%) | 0.0091 † |
| No | 19 (61%) | 34 a (89%) | |
| Prolonged wound healing (days) | |||
| 1–20 | 9 b (29%) | 25 a (66%) | 0.0044 † |
| 21–40 | 18 a (58%) | 10 b (26%) | |
| 41–62 | 2 (6%) | 0 | |
| Culture (days) | |||
| 4–20 | 15 (48%) | 32 a (84%) | 0.0003 † |
| 21–40 | 9 a (29%) | 2 b (5%) | |
| 41–62 | 5 a (16%) | 0 b | |
| Sepsis | |||
| Yes | 4 a (13%) | 0 | 0.0364 † |
| No | 27 (87%) | 38 a (100%) | |
| Wound dehiscence | |||
| Yes | 23 (74%) | 30 (79%) | 0.7760 |
| Not | 8 (26%) | 8 (21%) | |
| Hospitalization (days) | |||
| 6–30 | 13 (42%) | 29 a (76%) | 0.0060 † |
| >30 | 18 a (58%) | 9 (24%) | |
| Antibiotic use (days) | |||
| 3–11 | 0 b | 8 a (21%) | 0.0003 † |
| 12–36 | 21 (68%) | 28 (74%) | |
| > | 10 a (32%) | 2 b (5%) | |
| Antibiotic therapy | |||
| Adequate | 2 (6%) | 35 a (92%) | <0.0001 † |
| Inadequate | 29 a (94%) | 3 (8%) | |
| Nº of antimicrobials used in the therapy | |||
| 1–3 | 8 b (25.8%) | 16 (42.1%) | |
| 4–6 | 19 (61.3%) | 22 (57.9%) | 0.0271 † |
| >6 | 4 (12.9%) | 0 b |
* Fisher’s Exact Test or G-Test of Independence (Chi-square Residue Analysis), as appropriate. † Statistically significant. a Frequency higher than expected at random. b Frequency lower than expected at random.
It is worth noting that 93% (64/69) of all women with post-caesarean SSI by Acinetobacter were submitted to at least one re-suture procedure. In addition, 16 (23%) underwent other surgical procedures, due to the severity of the infection, including total hysterectomy (n = 4), exploratory laparotomy (n = 4), debridement (n = 2), hematoma drainage (n = 6), vessel ligation (n = 2), and graft insertion (n = 1). Four cases of SSI by carbapenem-resistant A. baumannii evolved to septicemia and three of these patients underwent other surgical procedures, such as exploratory laparotomy and total hysterectomy with a longer duration of hospital stay, ranging from 39 to 80 days (mean 32 days, SD: 16 days).
In relation to the antimicrobial therapy used for the treatment of SSI by Acinetobacter, the most used empirical therapies were the combination of penicillin, gentamicin, and metronidazole (39%), followed by ceftriaxone (23%) and oxacillin (16%). For carbapenem-resistant isolates in 94% of cases, the empirical therapy was inadequate. After the antimicrobial susceptibility testing results (adjusted therapy), the most used antimicrobials were ciprofloxacin (21.7%), amikacin (14.5%), and polymyxin B (5.8%).
3.4. rep-PCR and MLST Genotyping
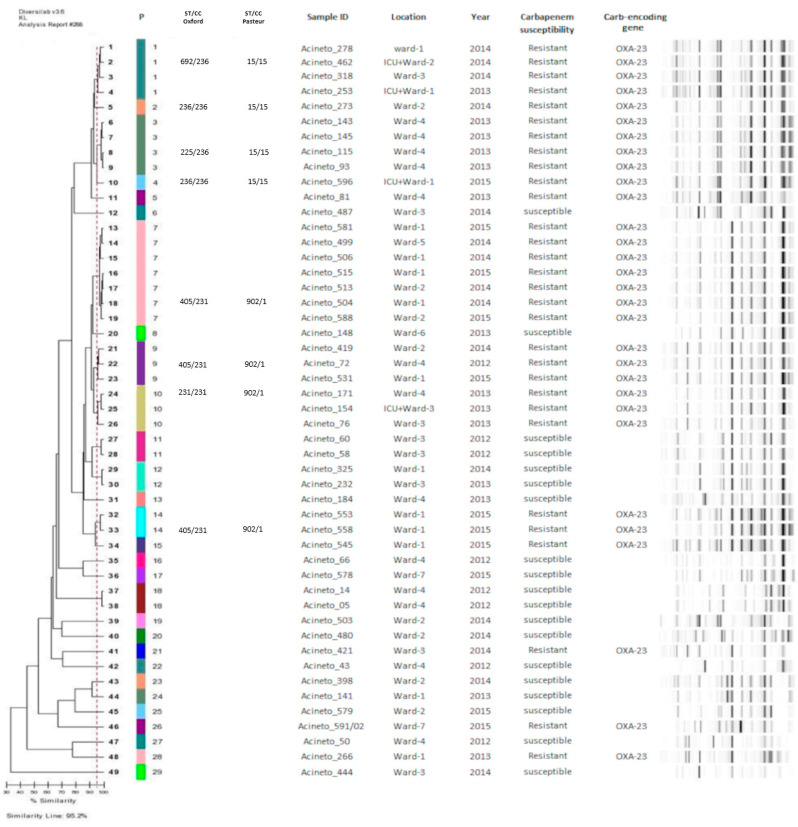
All A. baumannii (n = 49) and A. nosocomialis (n = 19) isolates were genotyped by rep-PCR. A. baumannii isolates revealed 29 distinct genetic patterns (P), of which nine clusters were genetically related isolates (similarity > 95%) and 20 had unique profiles.
Among the nine clusters, the most prevalent were P7 (seven isolates), P3 (five isolates), and P1 (four isolates). The P7 clonal group was formed by OXA-23-producing A. baumannii and was previously detected at the hospital in 2014 and 2015, circulating in three different obstetric wards and was associated with ST405/CC231. In addition, P1, P9, P10 clonal groups of OXA-23-producing A. baumannii also showed dissemination between different obstetric wards (Figure 1).
Figure 1.
Dendrogram of A. baumannii isolates analysed in the study. Abbreviations: P: Patterns (≥95% similarity), ST: sequence type, CC: clonal complexes. Sample ID: Identification of A. baumannii isolates obtained from surgical site of caesarean section patients. Location: Obstetric wards in which women remained after caesarean section surgery.
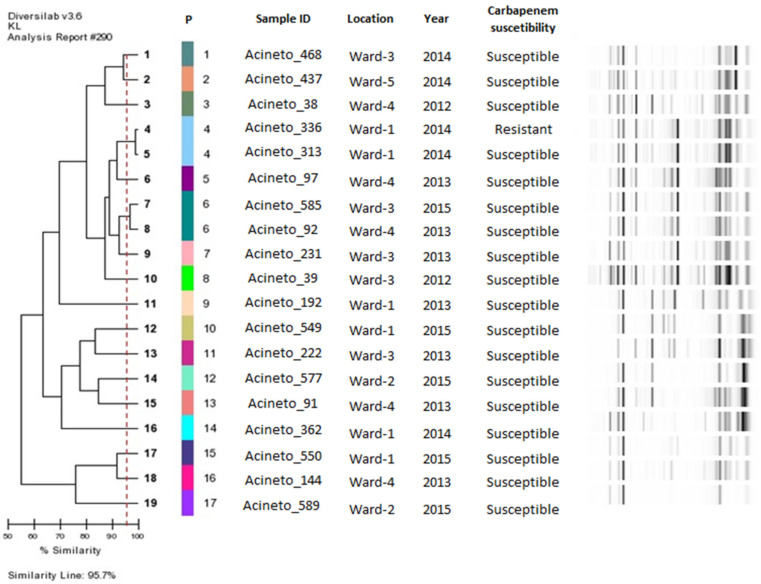
A wide diversity of clonal patterns was observed among the 19 isolates of A. nosocomialis. Seventeen distinct genetic patterns were identified, among which two clusters of two isolates each existed. Clonal dissemination between the different wards was identified only in the P6 group (Figure 2).
Figure 2.
Dendrogram of A. nosocomialis isolates analysed in the study. Abbreviations: P: Patterns (number of genetic patterns identified by DiversiLab System analysis, formed with ≥95% similarity). Sample ID: Identification of A. nosocomialis isolates obtained from surgical site of caesarean section patients. Location: Obstetric wards in which women remained after caesarean section surgery.
Analysis of representative isolates from different clusters of A. baumannii by MLST revealed that the isolates belonged to epidemic clones, including the ST225, ST236, ST692 (CC236); ST231, and ST405 (CC231), according to the Oxford scheme, and ST902 (CC1) and ST15 (CC15), according to the Pasteur scheme (Figure 1).
4. Discussion
SSI is one of the most common complications after caesarean delivery, occurring in approximately 10% of cases. Post-caesarean section SSI is a major cause of prolonged hospital stays and increasing healthcare costs. In Brazil, more than 1 million caesarean deliveries are performed annually, with SSI rates of 1.42% (2018). However, this number likely reflects underreported cases, since this type of infection only became mandatory to report in 2014 [23].
The most commonly reported pathogens in post-caesarean SSI include Staphylococcus aureus, Streptococcus spp., and members of the Enterobacteriaceae family [24]. Acinetobacter spp. are not commonly reported as a causative agent post-caesarean SSI. Herein, we report the occurrence of 69 cases of post-caesarean SSI related to three distinct species of Acinetobacter and the impact of multidrug resistance on the clinical evolution of patients.
All patients who developed SSI of Acinetobacter spp. underwent an emergency caesarean section procedure, which is a known risk factor for developing SSI [5]. The main indications for performing caesarean section in our study were fetal suffering, premature rupture of membranes, and hypertensive diseases of pregnancy. This was expected because the hospital under study is a reference hospital for high-risk pregnancies. It is also known that premature rupture to the membrane contributes to the colonization of the microbiota from the lower genital tract in the amniotic fluid, leading to wound and peritoneal cavity contamination [25].
Diagnosis of a UTI during pregnancy is another important risk factor for SSI, which was observed in 51% (n = 35) of women in this study. Women diagnosed with UTIs during pregnancy are three times more likely to develop post-caesarean SSI [26]. Therefore, strict surveillance measures must be taken during prenatal care to diagnose UTIs and offer treatment.
Post-caesarean infections have been reported to increase maternal morbidity and mortality. Puerperal sepsis is the third most common cause of maternal mortality, accounting for 10.7% of maternal deaths worldwide [26]. In our study, 13% of patients with SSI evolved to septicaemia and were transferred from the obstetric ward to the ICU due to clinical complications. However, no deaths were observed. All cases of sepsis occurred in patients with multidrug-resistant A. baumannii infection.
The cases of SSI occurred, on average, 10 days after the caesarean section. In 45% of cases, the patients were readmitted with signs and symptoms of infection in the surgical wound, a fact that may be associated with the lack post-discharge surveillance. Since SSI may take several days to become apparent and the average length of the post-operative stay in the hospital following caesarean delivery has gradually reduced to three days or less, methods that assure active post-discharge surveillance are an important prerequisite for the effective surveillance of SSI following caesarean delivery [27].
The spread of broad antimicrobial resistance strains of Acinetobacter is posing a challenge to infections treatment, leading to high morbidity, mortality, and longer hospital stays. This scenario of multidrug-resistance has also led to an increase in the antimicrobial therapy based on the combination of two of more antibiotics for treatment of multidrug-resistant A. baumannii infections [11]. In the present study, most of the patients received prolonged and inadequate antibiotic therapy, which was associated with carbapenem-resistant isolates. In addition, both empirical and adjusted antibiotic therapy was based on the traditional strategy of combining antibiotics from different classes; however, only after antimicrobial susceptibility testing of the correct antibiotic was therapy applied, which was mainly based on ciprofloxacin, amikacin, and polymyxin B. Finally, our data demonstrates the importance of AST and encouraging teams of professionals to gain prior knowledge of hospital bacterial resistance when prescribing antibiotics for treatment and prophylaxis of SSI [28,29].
This study demonstrated an association between septicemia and other complications, such as the need for further surgery, extended hospital stay (6 to 80 days), and OXA-23-producing A. baumannii infection, with the epidemic clonal complexes CC236 (ST692, ST225 and ST236) and CC231 (ST231 and ST405) (Oxford scheme), and ST902 (CC1) and ST15 (CC15) (Pasteur scheme), demonstrating the severity of these specific infections. In Brazil, the high levels of carbapenem-resistance have been associated with the spread of OXA-23-producing A. baumannii strains, particularly those included in the clonal complexes CC 104, CC 109, and CC113 (Oxford scheme) [30]. Although not among the most prevalent, epidemic CC15 and CC1 (Pasteur scheme) have been reported in other Brazilian hospitals to be associated with cases of bloodstream infection and meningitis [31]. Moreover, CC15 (Pasteur scheme) was the second most prevalent complex in Buenos Aires and Rosario, Argentina, in a study conducted by Stietz and collaborators [32]. Finally, genotyping revealed the permanence of different epidemic clones of A. baumannii over the years of the study as well as their spread between different obstetric wards of the hospital, highlighting the ability of these clones to spread and remain in the hospital over time.
Despite previous reports linking A. nosocomialis isolates to susceptibility to several antimicrobial classes, this species has emerged as an important pathogen worldwide owing to its increasing prevalence in nosocomial infections and capacity to acquire various mechanisms of antimicrobial resistance [33]. In our study, 28% of SSI cases were related to this species. We emphasize that these isolates were mistakenly identified as A. baumannii at the hospital using a semi-automated identification method, reinforcing the need for accurate species identification of Acinetobacter genus. An IMP-1-producing A. nosocomialis strain was identified, and to the best of our knowledge, this is the first report of this pathogen in a post-caesarean section SSI. This reinforces the clinical relevance of this emerging pathogen and highlights the need for further studies on virulence factors related to this species and others of the so-called Acinetobacter baumannii-A. calcoaceticus complex. One A. colistiniresistens strain was detected among our population. This species is rarely associated with HAIs and is associated with the peculiar feature of intrinsic resistance to colistin [34].
5. Conclusions
In conclusion, the present study firstly describes the clinical, epidemiological, and microbiological aspects related to post-caesarean SSI at a hyperendemic setting for Acinetobacter spp. in the Brazilian Amazon Region. Patients with carbapenem-resistant A. baumannii infection were associated with more complications, prolonged clinical evolution, and epidemic clonal lineages. Therefore, the implementation of effective infection control measures during caesarean section surgery and active post-discharge surveillance is essential in order to provide a safe postpartum experience. These measures will effectively reduce healthcare costs and help to alleviate the emotional and physical burden that caesarean section SSIs cause.
Acknowledgments
We would like to thank the Santa Casa de Misericórdia do Para Hospital for providing access to the medical records of the patients involved in this study and for the Acinetobacter strains.
Author Contributions
Conceptualization, D.M.B. and K.V.B.L.; validation, D.M.B., Y.C.R., and K.V.B.L.; formal analysis, I.P.F.; investigation, B.G.C. and Y.C.R.; resources, D.M.B. and K.V.B.L.; writing—original draft preparation, B.G.C., D.M.B., and K.V.B.L.; writing—review and editing, B.G.C., D.M.B., I.P.F., Y.C.R., and K.V.B.L.; visualization, B.G.C., D.M.B., and I.P.F.; supervision, D.M.B. and K.V.B.L.; project administration, D.M.B. and K.V.B.L.; funding acquisition, D.M.B. and K.V.B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health of Brazil (Instituto Evandro Chagas, Secretaria de Vigilância em Saúde, Ministério da Saúde) and Fundação de Amparo à Pesquisa do Pará/Universidade do Estado do Pará (FAPESPA/UEPA) [Cooperation grant Nº004/2019].
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Evandro Chagas Institute (Nº 2.999.939/05.Nov.2018) and the Fundação Santa Casa de Misericórdia do Pará (hospital under study) (Nº 3.126.011/30.Jan.2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data is presented in the present article.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Delamou A., Camara B.S., Sidibé S., Camara A., Dioubaté N., El Ayadi A.M., Tayler-Smith K., Beavogui A.H., Baldé M.D., Zachariah R. Trends of and factors associated with cesarean section related surgical site infections in Guinea. J. Public Health Afr. 2019;10:31–34. doi: 10.4081/jphia.2019.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dillen J., Zwart J., Schutte J. Van Roosmalen, J. Maternal sepsis: Epidemiology, etiology and outcome. Curr. Opin. Infect. Dis. 2010;23:249–254. doi: 10.1097/QCO.0b013e328339257c. [DOI] [PubMed] [Google Scholar]
- 3.Lapinsky S.E. Obstetric Infections. Crit. Care Clin. 2013;29:509–520. doi: 10.1016/j.ccc.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Molina G., Weiser T.G., Lipsitz S.R., Esquivel M.M., Uribe-Leitz T., Azad T., Shah N., Semrau K., Berry W.R., Gawande A.A., et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. J. Am. Med. Assoc. 2015;314:2263–2270. doi: 10.1001/jama.2015.15553. [DOI] [PubMed] [Google Scholar]
- 5.Sway A., Nthumba P., Solomkin J., Tarchini G., Gibbs R., Ren Y., Wanyoro A. Burden of surgical site infection following cesarean section in sub-Saharan Africa: A narrative review. Int. J. Womens Health. 2019;11:309–318. doi: 10.2147/IJWH.S182362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeed K.B., Corcoran P., O’Riordan M., Greene R.A. Risk factors for surgical site infection after cesarean delivery: A case-control study. Am. J. Infect. Control. 2018;47:164–169. doi: 10.1016/j.ajic.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Killian C.A., Graffunder E.M., Vinciguerra T.J., Venezia R.A. Risk Factors for Surgical-Site Infections Following Cesarean Section. Infect. Control Hosp. Epidemiol. 2001;22:613–617. doi: 10.1086/501831. [DOI] [PubMed] [Google Scholar]
- 8.Agboeze J., Onoh R.C., Umeora O.U.J., Paul O. Ezeonu, Chukwuemeka Ukaegbe, Azubike, K. Onyebuchi CE, Ndukwe, E. Microbiological pattern of postcesarean wound infection at Federal Teaching Hospital, Abakaliki. Afr. J. Med. Health Sci. 2013;12:97–102. [Google Scholar]
- 9.Yang Y., Lee Y., Tsai W., Kuo S., Sun J., Yang C., Chen T., Lin J., Fung C., Chang F. Comparison between bacteremia caused by carbapenem resistant Acinetobacter baumannii and Acinetobacter nosocomialis. BMC Infect. Dis. 2013;13:311. doi: 10.1186/1471-2334-13-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 11.Durante-Mangoni E., Utili R., Zarrilli R. Combination therapy in severe Acinetobacter baumannii infections: An update on the evidence to date. Future Microbiol. 2014;9:773–789. doi: 10.2217/fmb.14.34. [DOI] [PubMed] [Google Scholar]
- 12.Cosgaya C., Ratia C., Marí-Almirall M., Rubio L., Higgins P.G., Seifert H., Roca I., Vila J. In vitro and in vivo Virulence Potential of the Emergent Species of the Acinetobacter baumannii (Ab) Group. Front. Microbiol. 2019;10:2429. doi: 10.3389/fmicb.2019.02429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vos D., Pirnay J.P., Bilocq F., Jennes S., Verbeken G., Rose T., Keersebilck E., Bosmans P., Pieters T., Hing M., et al. Molecular epidemiology and clinical impact of acinetobacter calcoaceticus-baumannii complex in a belgian burn wound center. PLoS ONE. 2016;11:e0156237. doi: 10.1371/journal.pone.0156237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammerum A.M., Hansen F., Skov M.N., Stegger M., Andersen P.S., Holm A., Jakobsen L., Justesen U.S. Investigation of a possible outbreak of carbapenem-resistant Acinetobacter baumannii in Odense, Denmark using PFGE, MLST and whole-genome-based SNPs. J. Antimicrob. Chemother. 2015;70:1965–1968. doi: 10.1093/jac/dkv072. [DOI] [PubMed] [Google Scholar]
- 15.Berriós-Torres S.I., Umscheid C.A., Bratzler D.W., Leas B., Stone E.C., Kelz R.R., Reinke C.E., Morgan S., Solomkin J.S., Mazuski J.E., et al. Centers for disease control and prevention guideline for the prevention of surgical site infection. JAMA Surg. 2017;152:784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 16.Patel J.B., Weinstein M., Eliopoulos G., Jenkins S., Lewis J., Limbago B. M100 Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Vol. 8. United State, Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. [DOI] [Google Scholar]
- 17.Magiorakos A., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 18.La S.B., Gundi V.A.K.B., Khamis A., Raoult D. Sequencing of the rpoB Gene and Flanking Spacers for Molecular Identification of Acinetobacter Species. J. Clin. Microbiol. 2006;44:827–832. doi: 10.1128/JCM.44.3.827-832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodford N., Ellington M.J., Coelho J.M., Turton J.F., Ward M.E., Brown S., Amyes S.G., Livermore D.M. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents. 2006;27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Mendes R.E., Castanheira M., Toleman M.A., Sader H.S., Jones R.N., Walsh T.R. Characterization of an integron carrying blaIMF-1 and a new aminoglycoside resistance gene, aac(6′)-31, and its dissemination among genetically unrelated clinical isolates in a Brazilian Hospital. Antimicrob. Agents Chemother. 2007;51:2611–2614. doi: 10.1128/AAC.00838-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartual S.G., Seifert H., Hippler C., Luzon M.A., Wisplinghoff H., Rodríguez-Valera F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 2005;43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diancourt L., Passet V., Nemec A., Dijkshoorn L., Brisse S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE. 2010;5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ANVISA—Agência Nacional de Vigilância Sanitária Boletim de Segurança do Paciente e Qualidade em Serviços de Saúde no 16: Avaliação dos indicadores nacionais das Infecções Relacionadas à Assistência à Saúde (IRAS) e Resistência microbiana do ano de 2016. Anvisa. 2016;16:1–12. [Google Scholar]
- 24.Bebell L.M., Ngonzi J., Bazira J., Fajardo Y., Boatin A.A., Siedner M.J., Bassett I.V., Nyehangane D., Nanjebe D., Jacquemyn Y., et al. Antimicrobial-resistant infections among postpartum women at a Ugandan referral hospital. PLoS ONE. 2017;12:e0175456. doi: 10.1371/journal.pone.0175456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelraheim A.R., Gomaa K., Ibrahim E.M., Mohammed M.M., Khalifa E.M., Youssef A.M., Abdelhakeem A.K., Hassan H., Abdelghany A., El Gelany S. Intra-abdominal infection (IAI) following cesarean section: A retrospective study in a tertiary referral hospital in Egypt. BMC Pregnancy Childbirth. 2019;19:1. doi: 10.1186/s12884-019-2394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller A.E., Morgan C., Vyankandondera J. Causes of puerperal and neonatal sepsis in resource-constrained settings and advocacy for an integrated community-based postnatal approach. Int. J. Gynecol. Obstet. 2013;123:10–15. doi: 10.1016/j.ijgo.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Wilson J., Wloch C., Saei A., McDougall C., Harrington P., Charlett A., Lamagni T., Elgohari S., Sheridan E. Inter-hospital comparison of rates of surgical site infection following caesarean section delivery: Evaluation of a multicentre surveillance study. J. Hosp. Infect. 2013;84:44–51. doi: 10.1016/j.jhin.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Warde E., Davies E., Ward A. Control of a multidrug-resistant Acinetobacter baumannii outbreak. Br. J. Nurs. (Mark. Allen Publ.) 2019;28:242–248. doi: 10.12968/bjon.2019.28.4.242. [DOI] [PubMed] [Google Scholar]
- 29.Saeed K.B., Corcoran P., Greene R.A. Incisional surgical site infection following cesarean section: A national retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;240:256–260. doi: 10.1016/j.ejogrb.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Clímaco E.C., de Pitondo-Silva A.O.M.L., Oliveira M.G., Medeiros M., Lincopan N., Darini A.L.D.C. Clonal complexes 104, 109 and 113 playing a major role in the dissemination of OXA-carbapenemase-producing Acinetobacter baumannii in Southeast Brazil. Infect. Genet. Evol. 2013;19:127–133. doi: 10.1016/j.meegid.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Coelho-Souza T., Reis J.N., Martins N., Martins I.S., Menezes A.O., Reis M.G., Silva N.O., Dias R.C.S., Riley L.W., Moreira B.M. Longitudinal surveillance for meningitis by Acinetobacter in a large urban setting in Brazil. Clin. Microbiol. Infect. 2013;19:E241–E244. doi: 10.1111/1469-0691.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stietz M.S., Ramírez M.S., Vilacoba E., Merkier A.K., Limansky A.S., Centrón D., Catalano M. Acinetobacter baumannii extensively drug resistant lineages in Buenos Aires hospitals differ from the international clones I-III. Infect. Genet. Evol. 2013;14:294–301. doi: 10.1016/j.meegid.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Knight D.B., Rudin S.D., Bonomo R.A., Rather P.N. Acinetobacter nosocomialis: Defining the role of efflux pumps in resistance to antimicrobial therapy, surface motility, and biofilm formation. Front. Microbiol. 2018;9:1–6. doi: 10.3389/fmicb.2018.01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemec A., Radolfova-Krizova L., Maixnerova M., Sedo O. Acinetobacter colistiniresistens sp. Nov. (formerly genomic species 13 sensu Bouvet and Jeanjean and genomic species 14 sensu Tjernberg and Ursing), isolated from human infections and characterized by intrinsic resistance to polymyxins. Int. J. Syst. Evol. Microbiol. 2017;67:2134–2141. doi: 10.1099/ijsem.0.001903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data is presented in the present article.