Abstract
Neurodegenerative disease etiology is still unclear, but different contributing factors, such as lifestyle and genetic factors are involved. Altered components of the gut could play a key role in the gut-brain axis, which is a bidirectional system between the central nervous system and the enteric nervous system. Variations in the composition of the gut microbiota and its function between healthy people and patients have been reported for a variety of human disorders comprising metabolic, autoimmune, cancer, and, notably, neurodegenerative disorders. Diet can alter the microbiota composition, affecting the gut-brain axis function. Different nutraceutical interventions have been devoted to normalizing gut microbiome dysbiosis and to improving biological outcomes in neurological conditions, including the use of probiotics. Preclinical and clinical investigations discussed in this review strengthen the correlation between intestinal microbiota and brain and the concept that modifying the microbiome composition may improve brain neurochemistry, modulating different pathways. This review will discuss the potential use of probiotics for Parkinson’s disease prevention or treatment or as adjuvant therapy, confirming that gut microbiota modulation influences different pro-survival pathways. Future investigations in Parkinson’s disease should consider the role of the gut-brain axis and additional comprehension of the underlying mechanisms is extremely necessary.
Keywords: age-related diseases, brain, dysbiosis, gut-brain axis, microbiota, neurodegeneration, nutraceuticals, Parkinson's disease, probiotics, SLAB51
Introduction
Neurodegenerative disorders represent the most debilitating conditions, typically characterized by altered genes, elevated reactive oxygen species, mitochondrial damage, altered Ca2+ homeostasis, atypical proteins accumulation, persistent inflammatory condition or/and neuronal damage in a specific brain area (Castelli et al., 2019; Cenini et al., 2019). Enhanced oxidative stress and mild chronic inflammation are characteristic of various conditions, comprising neuronal diseases. Contrarily, Ca2+ homeostasis is crucial for several cell functions. The calcium gradient across the membranes is accurately controlled by different specific mechanisms. Any subtle alteration in this mechanism induces continuous intracellular and intra-organellar accumulation, which is related to neurodegenerative diseases (Alvarez et al., 2020). Mitochondrial dysfunction leads to deleterious pathways activation and impaired cellular energy production (Akbar et al., 2016). Neurodegenerative disorders trigger progressive loss of brain performances and overlapping clinical conditions. For example, the cognitive decline appears not only in Alzheimer’s disease (AD) but also in dementia with Lewy bodies, vascular and mixed dementia, and Parkinson’s disease (PD). Comparably, motor impairment occurs in PD, amyotrophic lateral sclerosis, and Huntington’s disease. The common risk factor in all these disorders is aging (Franceschi et al., 2018; Castelli et al., 2019). Since lifespan is longer, the incidence of these disorders is radically increased, with high social, economic impact (Gan et al., 2018; Castillo et al., 2019). Neurodegenerative diseases show common mechanisms and features, which are involved in a close network of relationships that underlie these diseases beginning and progression, thus supporting the importance in understanding these mechanisms underlying the neuronal damage, as common targets to design intervention approaches that can potentially delay the onset and/or prevent neurodegeneration (Gan et al., 2018; Hussain et al., 2018).
Neurodegenerative disease etiology is still unclear, but different contributing factors, such as lifestyle and genetic factors are involved (Topiwala et al., 2018; Popa-Wagner et al., 2020). Indeed, exposure to environmental factors and the resulting gene-environment interactions have been reported to exert a pivotal role in neurodegenerative disorders onset and progress (Maitre et al., 2018; Benakis et al., 2020). Extremely assorted and intricate populations of microorganisms including archaea, bacteria, viruses, and microeukaryotes inhabit the human body, and the gut characterizes the major reservoir of bacterial biomass (Gilbert et al., 2018). Variations in the composition of the gut microbiota and its function between healthy people and patients have been reported for a variety of human disorders comprising metabolic, autoimmune, cancer, and, notably, neurodegenerative disorders (Duvallet et al., 2017; Durack and Lynch, 2019; Lombardi et al., 2020).
Altered components of the gut could play a key role in the gut-brain axis, which is a bidirectional system between the central nervous system (CNS) and the enteric nervous system (ENS). Diet can alter the microbiota composition, affecting the gut-brain axis function (Figure 1) (Ambrosini et al., 2019; Santos et al., 2019). The gastrointestinal tract is connected to the brain via vagal and spinal afferent fibers, while the brain communicates to the gut through parasympathetic and sympathetic efferent fibers (Breit et al., 2018; Santos et al., 2019). Recent evidence suggests that a healthy gut significantly influences on neurodegeneration, although the anatomical distance between the two organs (Houser and Tansey, 2017; Ma et al., 2019). In particular, gut-brain axis dysfunction is related with metabolic syndromes (Agustí et al., 2018; Dabke et al., 2019; Grasset and Burcelin, 2019), psychiatric disorders, including autism, anxiety, and neurodegenerative disorders (Breit et al., 2018; Srikantha and Mohajeri, 2019). Thus, these disorders are related to an unhealthy gut, due to altered microbiota composition, which interrupts the communication between the brain and gut (Zhu et al., 2020). Different investigations reported that the microbiome composition regulates not only gut-brain axis communication but modulates also the immune response stimulating chemokines and cytokines release. Comparably, gut-brain axis communicates with intestinal cells and ENS and the CNS via metabolic and neuroendocrine pathways (Kim et al., 2018; Martin et al., 2018). Moreover, the gut microbiome may influence ENS function, when they locally release neurotransmitters, such as active catecholamines, fatty acid- or aminoacid-derivates in the intestinal lumen (Martin et al., 2018; Baj et al., 2019). ENS is also affected by bacterial metabolites, comprising propionic acid and acetic acid, which promote the sympathetic nervous system activation, with consequent impacts on memory and learning processes (Mohajeri et al., 2018).
Figure 1.
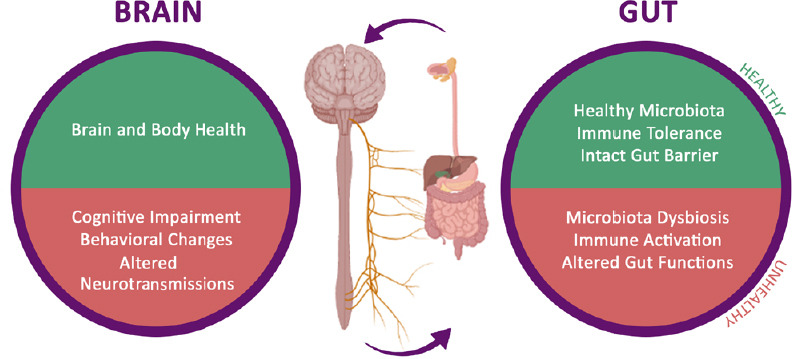
Gut-brain axis: how the microbiome affects brain health.
Altered components of the gut play a key role in the gut-brain axis, which is a bidirectional system between the central nervous system and the enteric nervous system.
This review will discuss the potential use of probiotics for Parkinson’s disease prevention or treatment or as adjuvant therapy, confirming that gut microbiota modulation influences different pro-survival pathways.
Microbiota Dysbiosis and Age-Related Disorders
Numerous studies have indicated that gut microbiota is critical for human health and it is crucial in the bidirectional communication between gut and brain. The early life experiences have a great impact on gut microbiota developing and on neurodevelopment and can potentially induce adverse mental health concerns in later life (Martin et al., 2018; Brett and de Weerth, 2019). Gut microbiome matures during life, but the variety and solidity of the microbiota declines with aging (Corey et al., 2019).
Gut microbiome composition is affected by dietary habits and the health condition of the individual (Singh et al., 2017). Further, during aging, individuals increase the use of medicines, the immune system weakens, malabsorption of nutrients occurs, accompanied by altered motility and digestive function, and increased oxidative stress. These factors impact on gut microbiome composition (Nagpal et al., 2018). In aging individuals, gut dysbiosis is concomitant with impaired cognitive and behavioral functions and decreased brain volume (Ticinesi et al., 2018), characteristics of age-related brain disorders, such as PD, AD. Recently, different studies emphasize the importance of preserving a healthy gut microbiome to maintain brain functions during aging (Singh et al., 2017).
Gut microbiome might be involved in the vulnerability associated with the aging process. Notably, the number of bifidobacteria declines with age, and the number of clostridia increases. During aging, pathogenic bacteria (i.e. Proteobacteria) increase at the expense of beneficial bacteria (Bifidobacterium species), probably inducing chronic low-grade inflammation (Vemuri et al., 2018; Rinninella et al., 2019). Mice treated with the supplement of Lactobacillus curvatus and plantarum, showed amelioration in age-dependent memory impairments via inhibition of nuclear factor-κB pathway (Jeong et al., 2015). In another interesting study, the effects of probiotics and prebiotics supplementation on middle-aged male rats were studied. The treatment ameliorated motor performances and increased butyrate levels which in turn, led to increased levels of neurotrophic factor, brain-derived growth factor (BDNF), parallel with a decrease in pro-inflammatory cytokines in the hippocampus (Romo-Araiza et al., 2018).
Altered microbiota is correlated with cognitive impairment and hepatic encephalopathy (Bajaj et al., 2016). The dysbiosis induced by antibiotics is associated with BDNF decrease, serotonin transporter dysfunction, thus leading to cognitive decline, including novel object recognition (Fröhlich et al., 2016). It has been demonstrated in vivo that probiotic formulation can improve cognitive tests by chronic restraint stress (Liang et al., 2015). Moreover, in rat hepatic encephalopathy model, characterized by hyperammonemia, the Lactobacillus helveticus administration induced improved spatial memory performances and anxiety-like signs (Luo et al., 2014). Further, Bifidobacterium administration was effective in enhanced non-spatial and spatial memory (Savignac et al., 2015). Enhanced gut permeability caused by microbial dysbiosis could indirectly or directly affect neurodegenerative disorders. Indeed, gut bacteria can release large quantities of lipopolysaccharide and amyloid, involved in the regulation of signaling pathways and the production of neuroinflammation in AD. Further, the altered gut microbiota is directly associated with other factors implicated in AD pathogenesis, including type 2 diabetes and obesity (Kim and Shin, 2018).
Conspicuous alterations in the gut microbiome of elderly patients affected by neurodegenerative disorders have also been detected (Singh et al., 2017; Cerovic et al., 2019; Zhu et al., 2020). It has been reported that indican, a product of tryptophan catabolism by gut microbiota, also known as a marker of fermentative dysbiosis and altered intestinal barrier integrity (Lombardi et al., 2020), is markedly elevated in PD patients (Gao et al., 2018).
In a cohort study in which 72 PD patients and 72 healthy subjects were enrolled, comparing the fecal microbiome, a significant decrease in Prevotellaceae in PD affected (about 78%) was reported. Prevotellaceae represent the main producers of mucin, a highly glycosylated protein which protects the epithelial wall against pathogens. PD patients also showed a strong increase in Enterobacteriaceae, which was positively associated with postural instability (Scheperjans et al., 2015). In another study, PD patients showed α-synuclein aggregation in their colon biopsies, concomitant with a strong decrease in anti-inflammatory butyrate-producing bacteria, and increased amount of proinflammatory bacteria species. Thus, proinflammatory dysbiosis appears in PD patients and may cause inflammation-induced misfolding of α-synuclein and the development of PD pathology (Houser and Tansey, 2017).
Further, in PD patients microbiota, reduced levels of fecal short-chain fatty acids were described, which may generate alteration in the ENS, reducing intestinal motility in PD patients (Koh et al., 2016). During aging, reduced microbiota-related metabolic capability, including lower short-chain fatty acids levels (acetate, propionate, and butyrate), may also be correlated to age-related disorders, such as cognitive impairment, altered bowel transit, hypertension, diabetes, arthritis, and vitamin D deficiency (Nagpal et al., 2018). PD patients showed significant reductions in butyrate and histone deacetylase able to increase glial cell line-derived neurotrophic factor and BDNF, thus protect dopaminergic neurons from degeneration (Sharma and Taliyan, 2015; Cantu-Jungles et al., 2019; Srivastav et al., 2019). To restore or ameliorate the microbiome composition, which results impaired especially during aging, dietary interventions (using nutraceutical approaches, i.e. fiber-rich foods or indigestible-carbohydrates that help short-chain fatty acids production, prebiotics, probiotics, and polyphenol use) or therapeutic methodologies (fecal transplant) are relevant. Indeed, a fecal transplant from healthy donors to PD patients ameliorated non-motor but also motor symptoms (Flameling and Rijkers, 2018; Van Laar et al., 2019). Notably, germ-free α-synuclein overexpressing mice preserved greater physical ability compared to wild-type animals, confirming that the microbiota is crucial in the development of motor symptoms in PD. α-Synuclein animals with a complex microbiome composition showed motor impairment as germ-free PD animals, even if with a late-onset (12 weeks after). Further, the microbiota of the α-synuclein animal model stimulated α-synuclein-dependent microglia activation in the affected brain area, intensifying the neuroinflammation, and aggravating the disease condition (Sampson et al., 2016). Moreover, transplanting feces samples from PD patients to germ-free mice promoted α-synuclein-induced physical impairment, comparing to microbiota transplants from healthy human donors. These findings demonstrated that gut microbiome is related to PD motor impairment and that microbiome dysbiosis represents a risk factor for PD (Sampson et al., 2016; Ma et al., 2019).
Regarding AD, different bacterial species exacerbate the amyloid b plaques, such as Mycobacterium spp., Salmonella spp., E. coli., Streptococcus spp. and Staphylococcus aureus (Boon Wong et al., 2018). In AD patients enhanced quantities of Gram-negative bacteria parallel to mucosal disruption in response to this dysbiosis were reported (Boon Wong et al., 2018; Cerovic et al., 2019). In germ-free mice, the hypothalamic-pituitary-adrenal response was substantially elevated compared to normal gut microbiome animals. The restoration of a healthy gut microbiome at an early stage was able to partially revert the hypothalamic-pituitary-adrenal response to stress. Interestingly, in the hippocampus and cortex of germ-free mice low BDNF levels were found, neurotrophin crucial for synaptic plasticity and neuronal survival (Sudo et al., 2004). Further, the absence of healthy microbiota impacts on central nervous system development and behavior. The germ-free condition affects memory, learning, physical activity, and anxiety but also led to impaired hippocampal development, altered BBB permeability, and hormone levels (Sampson et al., 2016).
The bacteriophage components of the microbiome should be included in microbiome dysbiosis (Gogokhia et al., 2019). Bacteriophages are viral parasites of bacteria and are important regulators of host-microbiome interactions but can also impact human health by involving on intestinal inflammatory processes (Gogokhia et al., 2019) and possibly causing α-synuclein misfolding (Tetz et al., 2018). Early signs of PD in the gut are altered gut permeability and dopamine production, concomitant with a reduction of Lactococcus bacteria (Houser and Tansey, 2017; Tetz et al., 2018; Darby et al., 2019). An alternative antimicrobial strategy is represented by phage therapy, which through manipulating the microbiome could contribute to counteracting PD (Tetz et al., 2018).
The Influence of Gut Microbiota, Gut-Brain Axis and Nutraceutical Interventions in Neurodegenerative Diseases
Gut microbiome composition is influenced by early childhood experiences, stress, age, use of drugs, and, especially, long-term dietary habits (Figure 2). High consumption of refined sugar and animal fat is correlated with inflammation and neurodegeneration (d’Angelo et al., 2019). Diet rich in protein and fat induce elevated levels of Bacteroides, while a diet rich in high fiber stimulates Prevotella enterotypes. Western, high-energy diets alter the microbiome profile increasing the Firmicutes population (Rinninella et al., 2019). A high fructose diet was reported to induce hippocampal neuroinflammation, neuronal loss, and gliosis. Further, upon this diet, these mice had altered gut microbiome composition (dysbiosis), decreased fecal short-chain fatty acid, weakened intestinal epithelial barrier, and increased serum endotoxin levels (Li et al., 2019). Prebiotics are indigestible food ingredients that selectively promote the growth and activities of helpful microorganisms, such as Bifidobacterium and Lactobacillus (Davani-Davari et al., 2019).
Figure 2.
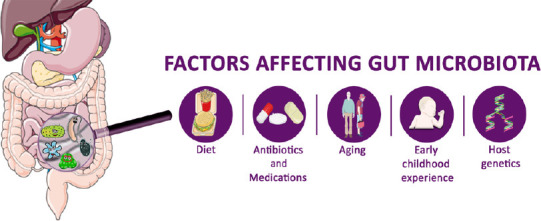
Factors affecting gut microbiota.
Several factors influence the gut microbiota composition, including diet, use of medication and/or antibiotics, aging, early childhood experiences and host genetics.
Other factors that influence gut microbiome are represented by antibiotics use, infection, and can be detrimental to the host. Indeed, even short-term antibiotic treatment can induce long-term dysbiosis, with disease exacerbation (Burrello et al., 2018).
Increasing data reported that gut microbiota, participating in the physiology and pathology of cellular organisms, is implicated in health and disease (Wang et al., 2017). Inherited gut microbiome changes with diet habits and environmental signals (Gomez de Aguero et al., 2016; Koh et al., 2016). Gut microflora is also influenced by the immune system and dysbiosis is due to immune signaling that occurs during cancer, inflammatory bowel disease, and autoimmune disease (Lazar et al., 2018).
The development and maturation of the human CNS are controlled by extrinsic and intrinsic components. Different investigations reported a correlation between CNS neurochemistry and physiology and gut microbiota, as demonstrated in animals treated with broad-spectrum antibiotics and in germ-free animals (GF) (Ma et al., 2019). GF mice showed a strong alteration in N-methyl-D-aspartate, 5-hydroxytryptamine, and BDNF, which trigger neurological impairment in memory, cognition, learning, and emotional behaviors (Maqsood and Stone, 2016). As mentioned above, neuropathology is related to gut microbiome dysbiosis, but it is also implicated in homeostasis and development of CNS (Tremlett et al., 2017).
Dietary habits of the individual represent the main issue, influencing the gut-brain axis and, thus gut microbiota. Gut microbiome influences the pathogenesis of numerous disorders outside the gastrointestinal tract due to its tight correlation with inflammation, nutrition, endocrine, neural and immune systems (Kho and Lal, 2018).
Different nutraceutical interventions were devoted to normalizing gut microbiome dysbiosis and to improve biological outcome in different pathological conditions. These nutraceutical approaches include probiotics, Ginko biloba, green tea, α-lipoic acid, vitamin A, biotin, and curcumin. Nutraceuticals are defined as “food or food product that provides medical or health benefits including the prevention and treatment of diseases” (Abd El-Salam and El-Shibiny, 2017).
Indeed, neuro-nutraceuticals represent active compounds, derived from plants or food products that influence CNS functions. Neuro-nutraceuticals could include amino acids, minerals, and vitamins with positive effects on health and disease conditions. Brain aging and neurodegenerative diseases are characterized by redox metals homeostasis, oxidative stress, and inflammation, thus antioxidant and anti-inflammatory molecules can represent a valid strategy for numerous brain diseases, including PD, AD, depression, and dementia (Castelli et al., 2018).
Preclinical studies showed that nutraceutical administrations (including probiotics) can diminish anxiety and depression and reestablish brain chemistry. Indeed, the impairment in germ-free animals was ameliorated upon short-chain fatty acids (Koh et al., 2016). Interestingly, in aged rats, probiotic formulation VSL#3 (now named Visbiome in U.S. and Vivomixx in Europe) was able to ameliorate gut microbiome composition and brain performances, through dampening neuroinflammation and stimulating BDNF and the protein synapsin, involved in neural plasticity (Distrutti et al., 2013).
The gut microbiome is fundamental also for nutraceuticals bioavailability, which concomitantly helps to counteract neuronal and cell aging under normal circumstances (Rogers et al., 2016). Notably, blueberries (rich in polyphenols) ameliorated spatial memory and motor performances in aged animals (Spencer, 2010), increased neural stem cell proliferation, and insulin-like growth factor-1 level, the key modulator of hippocampal neurogenesis (Shukitt-Hale et al., 2015).
Animals treated with Omega-3 polyunsaturated acids (n-3 PUFA) showed improved synaptic plasticity, diminished oxidative stress and neuroinflammation, and inhibited microglial activation (Corsi et al., 2015; Joffre et al., 2019). Some evidence in animal models suggested that long-term consumption of fish oil (rich in n-3 PUFA) may predispose the brain to lipid oxidation. Plant nutraceuticals, i.e. phytosterols esters can lower cholesterol levels, inhibit oxidative stress, and ameliorate cognitive performances in aged rats (Morris Water Maze tests). Notably, phytosterols improved cholinergic activity, decreasing acetylcholinesterase activity, increasing choline acetyltransferase, and restoring acetylcholine levels (van Kessel et al., 2019). Overall, preclinical investigations suggest that nutraceuticals can improve brain functions during aging or neurodegeneration, taking benefits from antioxidant, anti-inflammatory, and neuroprotective properties of enriched diet.
Probiotic bacteria not only modulate host immune responses but also create a healthy gut environment through the balancing of the intestinal microflora. Ingestion of probiotics may restore the composition of the gut microflora to a state more favorable for beneficial microorganisms (Boon Wong et al., 2018; Romo-Araiza et al., 2018). Probiotics recently have attracted attention in the context of brain function and health because they serve to reestablish gut microflora toward a beneficial state, which could affect gut-brain axis (Wang et al., 2016).
Probiotics: New Hope for Parkinson’s Disease
Emerging evidence suggests that lifestyle factors can contribute to PD pathology. Nutraceutical intervention, in particular the use of probiotics, may provide opportunities to complement the traditional PD therapies.
Preclinical or clinical evidence on the beneficial activities exerted by probiotics in PD is still limited. Probiotics may represent a strong tool to restore gut dysbiosis occurring in PD, ameliorating gastrointestinal function, reducing ENS neuroinflammation, and reducing gut leakiness. The first clinical trial, dated back to 2011, revealed that fermented milk enriched in Lactobacillus casei Shirota improved chronic constipation in PD patients, decreasing abdominal pain and swelling (Cassani et al., 2011). A more recent clinical trial demonstrated that a probiotic formulation (administered in tablet) was able to improve insulin resistance and sensitivity and to ameliorate the motor score (Tamtaji et al., 2019). Another recent clinical study is based on the administration of a liquid probiotic formulation, named Symprove (K-1803), able to reach the lower gut in an active form (Gazerani, 2019).
Bacillus subtilis probiotic inhibited α-synuclein aggregation and cleared pre-formed aggregates in an established Caenorhabditis elegans model of synucleinopathy (Goya et al., 2020).
Probiotic formulations improves the CNS activity through the modulation of inflammation and positive interactions with the commensal gut microbiota (Wang et al., 2016). PD patients’ microbiota is rich in pro-inflammatory cytokines due to enhanced intestinal permeability to endotoxins (lipopolysaccharide). Bacterial amyloids may also support a pro-inflammatory environment in the gut (Miraglia and Colla, 2019).
A recent in vitro study reported that probiotics reduced oxidative stress, pro-inflammatory cytokines, and counteracted pathogenic bacterial overgrowth in PD patients. This study was performed in peripheral blood mononuclear cells isolated from PD patients testing different probiotic microorganisms belonging to the Lactobacillus and Bifidobacterium species (Magistrelli et al., 2019).
Specific probiotic strains could counteract pathogens and produce tyrosine decarboxylase (van Kessel et al., 2019). This enzyme converts levodopa to dopamine in the gut, even in the presence of a competitive substrate. Indeed, levodopa levels are reduced by the high presence of tyrosine decarboxylase in PD individuals (Nicola et al., 2016).
Notably, another research group showed that long-term probiotic administration (six bacterial strains) exerted neuroprotective e?ects on dopaminergic neurons and was able to counteract motor impairments in a genetic PD mouse model (Hsieh et al., 2020).
Probiotic formulations may dampen the inflammation through cytokines production (Nowak et al., 2019), and decrease the oxidative stress through a reduction in reactive oxygen species (Gazerani, 2019). This aspect is of high interest since PD progression is accelerated in the presence of infections (Su et al., 2018). It has been demonstrated that a probiotic formulation VSL#3 can control the expression of different genes in the brain cortex of aging animals, dampening the inflammation and improving neuronal performances (Distrutti et al., 2014).
It has been reported the effect of an innovative probiotic formulation SLAB51 (commercially sold as Sivomixx) in exerting beneficial effects on cognitive performances. Indeed, transgenic 3xTg-AD mice upon SLAB51 presented partial restoration of autophagy and the ubiquitin-proteasome system, concomitant with an improvement in cognitive impairment due to reduced accumulation of amyloid plaques and brain injury. This novel formulation reduced plasma inflammatory cytokines and gut metabolic hormones, therapeutic targets in neurodegeneration. Overall, in this study Bonfili and collaborators demonstrated that SLAB51, modulating the microbiota, influenced neuroprotective pathways, counteracting the progression of AD (Bonfili et al., 2017). Further, the same research group demonstrated that SLAB51 formulation was able to significantly decrease oxidative stress in AD mice brain, by stimulating sirtuin 1-dependent mechanisms, thus this formulation could represent a potential adjuvant in AD treatment (Bonfili et al., 2018).
Recently, it has been demonstrated that this novel probiotic formulation SLAB51 can counteract 6-hydroxydopamine-induced detrimental effects both in in vitro and in vivo models of PD. In particular, SLAB51 exerted anti-inflammatory activities, restored pro-survival and neuroprotective pathways, protected dopaminergic neurons, and ameliorated behavioral impairments. These findings propose this probiotic mixture as a promising candidate for PD prevention or treatment or as adjuvant therapy, confirming that gut microbiota modulation influences different pro-survival pathways, delaying PD progression (Castelli et al., 2020).
Overall, these data indicated that probiotics supplement can represent a valid treatment able to ameliorate brain and gut functions in PD (a summary schematic image is reported in Figure 3).
Figure 3.
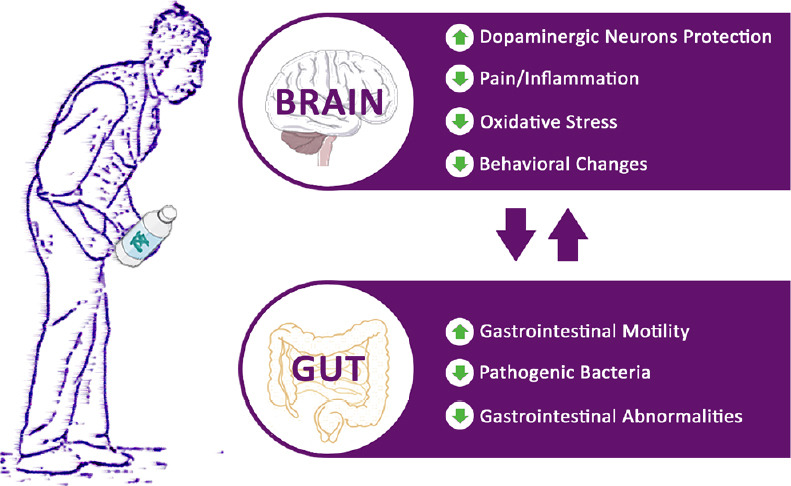
Effects of probiotics supplement in Parkinson’s disease.
Probiotics treatment is able to ameliorate brain and gut functions in Parkinson’s disease.
Conclusion
PD is a frequent neurodegenerative disorder, characterized by motor and non-motor symptoms, comprising abnormalities in the gut function, which may occur before the motor sign. From a molecular point of view, PD underlying mechanisms include increased oxidative stress and inflammation (Castelli et al., 2019). To date, the available therapies can help alleviate PD-associated symptoms, but there is no cure to control the onset and progression of this disorder.
A growing body of evidence indicated that probiotics administration influenced positively on CNS disease, modifying the gut microbiota, via the gut-brain axis, mediating different pathways, such as neural, hormonal, immune, inflammatory, and antioxidant signaling (Wang et al., 2016; Boon Wong et al., 2018; Gazerani, 2019). In particular, a healthy intestinal microbiota reduced the risk of developing different pathologies, such as neurological and neurodegenerative disorders, including PD (Wang et al., 2017). This new understanding of PD pathogenesis has intensified the study for new therapeutic approaches and the detection of early biomarkers. Among these therapeutic approaches, relevant importance is given to nutraceutical interventions, in particular the use of probiotics. Preclinical and clinical investigations discussed in this review strengthen the correlation between intestinal microbiota and brain, and the concept that modifying the microbiome composition may improve brain neurochemistry, modulating different pathways. Future investigations in PD should consider the role of gut-brain axis and additional comprehension on the underlying mechanisms is extremely necessary.
Further studies are necessary regarding the potential therapeutic effect of probiotics in maintaining protein and oxidative homeostasis in ENS. Another point to be considered is whether continual exposure to probiotic supplement may induce to long-term colonization of gut microbiome in PD patients, or if microbiota would revert to its original composition once the treatment is stopped.
To clarify the potential of probiotics for these debilitating disorders, further development and characterization of the biochemical impacts of the probiotic supplement on people affected by neurodegenerative disorders need to be examined. Moreover, it is of crucial importance to identify the most appropriate probiotic as adjuvant treatment for neurodegenerative diseases, including PD, basing also on the specific gut microbiome picture of a single patient, to formulate a personalized therapy.
Furthermore, since the properties of a probiotic strain are crucial for the efficacy of the treatment, maintenance of specific characteristics and relative efficacy should be verified. Indeed, increasing evidence indicated that production and manufacturing procedures may strongly influence the quality and safety of probiotic (Trinchieri et al., 2017; Palumbo et al., 2019; Plaza-Diaz et al., 2019), thus imposing particular attention on choosing the formulation to be administered.
Finally, to use probiotics for the prevention and treatment of these disorders, greater investment in clinical trials is necessary.
Footnotes
C-Editors: Zhao M, Wang L; T-Editor: Jia Y
Conflicts of interest: There is no conflict of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Abd El-Salam MH, El-Shibiny S. Preparation, properties, and uses of enzymatic milk protein hydrolysates. Crit Rev Food Sci Nutr. 2017;57:1119–1132. doi: 10.1080/10408398.2014.899200. [DOI] [PubMed] [Google Scholar]
- 2.Agustí A, García-Pardo MP, López-Almela I, Campillo I, Maes M, Romaní-Pérez M, Sanz Y. Interplay between the gut-brain axis, obesity and cognitive function. Front Neurosci. 2018;12:155. doi: 10.3389/fnins.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbar M, Essa MM, Daradkeh G, Abdelmegeed MA, Choi Y, Mahmood L, Song BJ. Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res. 2016;1637:34–55. doi: 10.1016/j.brainres.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez J, Alvarez-Illera P, García-Casas P, Fonteriz RI, Montero M. The role of Ca2+ signaling in aging and neurodegeneration: insights from Caenorhabditis elegans models. Cells. 2020;9:204. doi: 10.3390/cells9010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosini YM, Borcherding D, Kanthasamy A, Kim HJ, Willette AA, Jergens A, Allenspach K, Mochel JP. The gut-brain axis in neurodegenerative diseases and relevance of the canine model: a review. Front Aging Neurosci. 2019;11:130. doi: 10.3389/fnagi.2019.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baj A, Moro E, Bistoletti M, Orlandi V, Crema F, Giaroni C. Glutamatergic signaling along the microbiota-gut-brain axis. Int J Mol Sci. 2019;20:1482. doi: 10.3390/ijms20061482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj JS, Ahluwalia V, Steinberg JL, Hobgood S, Boling PA, Godschalk M, Habib S, White MB, Fagan A, Gavis EA, Ganapathy D, Hylemon PB, Stewart KE, Keradman R, Liu EJ, Wang J, Gillevet PM, Sikaroodi M, Moeller FG, Wade JB. Elderly patients have an altered gut-brain axis regardless of the presence of cirrhosis. Sci Rep. 2016;6:38481. doi: 10.1038/srep38481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benakis C, Martin-Gallausiaux C, Trezzi J-P, Melton P, Liesz A, Wilmes P. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr Opin Neurobiol. 2020;61:1–9. doi: 10.1016/j.conb.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, Fiorini D, Boarelli MC, Rossi G, Eleuteri AM. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7:2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonfili L, Cecarini V, Cuccioloni M, Angeletti M, Berardi S, Scarpona S, Rossi G, Eleuteri AM. SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an ad mouse model. Mol Neurobiol. 2018;55:7987–8000. doi: 10.1007/s12035-018-0973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boon Wong C, Kobayashi Y, Xiao J. Probiotics for preventing cognitive impairment in Alzheimer's disease. In: Evrensel A, Önen Ünsalver B, editors. Gut Microbiota - Brain Axis. London, UK: IntechOpen; 2018. pp. 85–104. [Google Scholar]
- 12.Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brett BE, de Weerth C. The microbiota-gut-brain axis: a promising avenue to foster healthy developmental outcomes. Dev Psychobiol. 2019;61:772–782. doi: 10.1002/dev.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrello C, Garavaglia F, Cribiù FM, Ercoli G, Bosari S, Caprioli F, Facciotti F. Short-term oral antibiotics treatment promotes inflammatory activation of colonic invariant natural killer T and conventional CD4+ T cells. Front Med (Lausanne) 2018;5:21. doi: 10.3389/fmed.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantu-Jungles TM, Rasmussen HE, Hamaker BR. Potential of prebiotic butyrogenic fibers in Parkinson’s disease. Front Neurol. 2019;10:663. doi: 10.3389/fneur.2019.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassani E, Privitera G, Pezzoli G, Pusani C, Madio C, Iorio L, Barichella M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol. 2011;57:117–121. [PubMed] [Google Scholar]
- 17.Castelli V, Benedetti E, Antonosante A, Catanesi M, Pitari G, Ippoliti R, Cimini A, d’Angelo M. Neuronal cells rearrangement during aging and neurodegenerative disease: metabolism, oxidative stress and organelles dynamic. Front Mol Neurosci. 2019;12:132. doi: 10.3389/fnmol.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castelli V, d’Angelo M, Lombardi F, Alfonsetti M, Antonosante A, Catanesi M, Benedetti E, Palumbo P, Cifone MG, Giordano A, Desideri G, Cimini A. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging. 2020;12:4641–4659. doi: 10.18632/aging.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castelli V, Palumbo P, d’Angelo M, Moorthy NK, Antonosante A, Catanesi M, Lombardi F, Iannotta D, Cinque B, Benedetti E, Ippoliti R, Cifone MG, Cimini A. Probiotic DSF counteracts chemotherapy induced neuropathic pain. Oncotarget. 2018;9:27998–28008. doi: 10.18632/oncotarget.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castillo X, Castro-Obregón S, Gutiérrez-Becker B, Gutiérrez-Ospina G, Karalis N, Khalil AA, Lopez-Noguerola JS, Rodríguez LL, Martínez-Martínez E, Perez-Cruz C, Pérez-Velázquez J, Piña AL, Rubio K, García HPS, Syeda T, Vanoye-Carlo A, Villringer A, Winek K, Zille M. Re-thinking the etiological framework of neurodegeneration. Front Neurosci. 2019;13:728. doi: 10.3389/fnins.2019.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cenini G, Lloret A, Cascella R. Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view. Oxid Med Cell Longev. 2019;2019:1–18. doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerovic M, Forloni G, Balducci C. Neuroinflammation and the gut microbiota: possible alternative therapeutic targets to counteract Alzheimer’s disease. Front Aging Neurosci. 2019;11:284. doi: 10.3389/fnagi.2019.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corey S, Kvederis L, Kingsbury C, Bonsack B R, Sanberg P, Castelli V, Lee JY, Borlongan CV. Gut microbiome: lactation, childbirth, lung dysbiosis, animal modeling, stem cell treatment, and CNS disorders CNS. Neurol Disord Drug Targets. 2019;18:687–694. doi: 10.2174/1871527318666191021145252. [DOI] [PubMed] [Google Scholar]
- 24.Corsi L, Momo Dongmo B, Avallone R. Supplementation of omega 3 fatty acids improves oxidative stress in activated BV2 microglial cell line. Int J Food Sci Nutr. 2015;66:293–299. doi: 10.3109/09637486.2014.986073. [DOI] [PubMed] [Google Scholar]
- 25.d’Angelo M, Castelli V, Tupone MG, Catanesi M, Antonosante A, Dominguez-Benot R, Ippoliti R, Cimini AM, Benedetti E. Lifestyle and food habits impact on chronic diseases: roles of PPARs. Int J Mol Sci. 2019;20:5422. doi: 10.3390/ijms20215422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darby TM, Owens JA, Saeedi BJ, Luo L, Matthews JD, Robinson BS, Naudin CR, Jones RM. Lactococcus Lactis Subsp. cremoris is an efficacious beneficial bacterium that limits tissue injury in the intestine i. Science. 2019;12:356–367. doi: 10.1016/j.isci.2019.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 Protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One. 2013;8:e63893. doi: 10.1371/journal.pone.0063893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Distrutti E, O’Reilly J-A, McDonald C, Cipriani S, Renga B, Lynch MA, Fiorucci S. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS One. 2014;9:e106503. doi: 10.1371/journal.pone.0106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20–40. doi: 10.1084/jem.20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duvallet C, Gibbons SM, Gurry T, Irizarry RA, Alm EJ. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat Commun. 2017;8:1784. doi: 10.1038/s41467-017-01973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flameling IA, Rijkers GT. Fecal microbiota transplants as a treatment option for Parkinson's disease. In: Evrensel A, Önen Ünsalver B, editors. Gut Microbiota - Brain Axis. London, UK: IntechOpen; 2018. pp. 71–83. [Google Scholar]
- 34.Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, Monti D, Capri M, Salvioli S. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne) 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B, Zinser E, Bordag N, Magnes C, Fröhlich E, Kashofer K, Gorkiewicz G, Holzer P. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan L, Cookson MR, Petrucelli L, La Spada AR. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci. 2018;21:1300–1309. doi: 10.1038/s41593-018-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazerani Probiotics for Parkinson’s disease. Int J Mol Sci. 2019;20:4121. doi: 10.3390/ijms20174121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24:392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gogokhia L, Buhrke K, Bell R, Hoffman B, Brown DG, Hanke-Gogokhia C, Ajami NJ, Wong MC, Ghazaryan A, Valentine JF, Porter N, Martens E, O’Connell R, Jacob V, Scherl E, Crawford C, Stephens WZ, Casjens SR, Longman RS, Round JL. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe. 2019;25:285–299e8. doi: 10.1016/j.chom.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 42.Goya ME, Xue F, Sampedro-Torres-Quevedo C, Arnaouteli S, Riquelme-Dominguez L, Romanowski A, Brydon J, Ball KL, Stanley-Wall NR, Doitsidou M. Probiotic bacillus subtilis protects against α-synuclein aggregation in C. elegans. Cell Rep. 2020;30:367–380e7. doi: 10.1016/j.celrep.2019.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grasset E, Burcelin R. The gut microbiota to the brain axis in the metabolic control. Rev Endocr Metab Disord. 2019;20:427–438. doi: 10.1007/s11154-019-09511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis. NPJ Parkinsons Dis. 2017;3:3. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsieh TH, Kuo CW, Hsieh KH, Shieh MJ, Peng CW, Chen YC, Chang YL, Huang YZ, Chen CC, Chang PK, Chen KY, Chen HY. Probiotics alleviate the progressive deterioration of motor functions in a mouse model of Parkinson’s disease. Brain Sci. 2020;10:206. doi: 10.3390/brainsci10040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hussain R, Zubair H, Pursell S, Shahab M. Neurodegenerative diseases: regenerative mechanisms and novel therapeutic approaches. Brain Sci. 2018;8:177. doi: 10.3390/brainsci8090177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong JJ, Kim KA, Ahn YT, Sim JH, Woo JY, Huh CS, Kim DH. Probiotic mixture KF attenuates age-dependent memory deficit and lipidemia in Fischer 344 rats. J Microbiol Biotechnol. 2015;25:1532–1536. doi: 10.4014/jmb.1505.05002. [DOI] [PubMed] [Google Scholar]
- 48.Joffre C, Rey C, Layé S. N-3 polyunsaturated fatty acids and the resolution of neuroinflammation. Front Pharmacol. 2019;10:1022. doi: 10.3389/fphar.2019.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kho ZY, Lal SK. The human gut microbiome – a potential controller of wellness and disease. Front Microbiol. 2018;9:1835. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim N, Yun M, Oh YJ, Choi HJ. Mind-altering with the gut: modulation of the gut-brain axis with probiotics. J Microbiol. 2018;56:172–182. doi: 10.1007/s12275-018-8032-4. [DOI] [PubMed] [Google Scholar]
- 51.Kim YK, Shin C. The microbiota-gut-brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr Neuropharmacol. 2018;16:559–573. doi: 10.2174/1570159X15666170915141036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 53.Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L, Chifiriuc MC. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li JM, Yu R, Zhang LP, Wen SY, Wang SJ, Zhang XY, Xu Q, Kong LD. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: a benefit of short-chain fatty acids. Microbiome. 2019;7:98. doi: 10.1186/s40168-019-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 56.Lombardi F, Fiasca F, Minelli M, Maio D, Mattei A, Vergallo I, Cifone MG, Cinque B, Minelli M. The effects of low-nickel diet combined with oral administration of selected probiotics on patients with systemic nickel allergy syndrome (SNAS) and Gut Dysbiosis. Nutrients. 2020;12:1040. doi: 10.3390/nu12041040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo J, Wang T, Liang S, Hu X, Li W, Jin F. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci China Life Sci. 2014;57:327–335. doi: 10.1007/s11427-014-4615-4. [DOI] [PubMed] [Google Scholar]
- 58.Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation. 2019;16:53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magistrelli L, Amoruso A, Mogna L, Graziano T, Cantello R, Pane M, Comi C. Probiotics may have beneficial effects in Parkinson’s disease: in vitro evidence. Front Immunol. 2019;10:969. doi: 10.3389/fimmu.2019.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maitre L, de Bont J, Casas M, Robinson O, Aasvang GM, Agier L, Andrušaitytė S, Ballester F, Basagaña X, Borràs E, Brochot C, Bustamante M, Carracedo A, de Castro M, Dedele A, Donaire-Gonzalez D, Estivill X, Evandt J, Fossati S, Giorgis-Allemand L, et al. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open. 2018;8:e021311. doi: 10.1136/bmjopen-2017-021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maqsood R, Stone TW. The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochem Res. 2016;41:2819–2835. doi: 10.1007/s11064-016-2039-1. [DOI] [PubMed] [Google Scholar]
- 62.Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miraglia F, Colla E. Microbiome, Parkinson’s disease and molecular mimicry. Cells. 2019;8:222. doi: 10.3390/cells8030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohajeri MH, La Fata G, Steinert RE, Weber P. Relationship between the gut microbiome and brain function. Nutrition Reviews. 2018;76:481–496. doi: 10.1093/nutrit/nuy009. [DOI] [PubMed] [Google Scholar]
- 65.Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, Yadav H. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4:267–285. doi: 10.3233/NHA-170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicola S, Amoruso A, Deidda F, Pane M, Allesina S, Mogna L, Del Piano M, Mogna G. Searching for the perfect homeostasis: five strains of bifidobacterium longum from centenarians have a similar behavior in the production of cytokines. J Clin Gastroenterol. 2016;50:S126–S130. doi: 10.1097/MCG.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 67.Nowak A, Paliwoda A, Błasiak J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: a review of mechanisms and therapeutic perspectives. Crit Rev Food Sci Nutr. 2019;59:3456–3467. doi: 10.1080/10408398.2018.1494539. [DOI] [PubMed] [Google Scholar]
- 68.Palumbo P, Lombardi F, Cifone MG, Cinque B. The epithelial barrier model shows that the properties of VSL#3 depend from where it is manufactured. Endocr Metab Immune Disord Drug Targets. 2019;19:199–206. doi: 10.2174/1871530318666181022164505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. 2019;10:S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Popa-Wagner A, Dumitrascu DI, Capitanescu B, Petcu EB, Surugiu R, Fang WH, Dumbrava DA. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen Res. 2020;15:394–400. doi: 10.4103/1673-5374.266045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the healthy gut microbiota composition. A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romo-Araiza A, Gutiérrez-Salmeán G, Galván EJ, Hernández-Frausto M, Herrera-López G, Romo-Parra H, García-Contreras V, Fernández-Presas AM, Jasso-Chávez R, Borlongan CV, Ibarra A. Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front Aging Neurosci. 2018;10:416. doi: 10.3389/fnagi.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santos SF, de Oliveira HL, Yamada ES, Neves BC, Pereira A. The gut and Parkinson’s disease—a bidirectional pathway. Front Neurol. 2019;10:574. doi: 10.3389/fneur.2019.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 77.Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 78.Sharma S, Taliyan R. Targeting histone deacetylases: a novel approach in Parkinson’s disease. Parkinsons Dis. 2015;2015:303294. doi: 10.1155/2015/303294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shukitt-Hale B, Bielinski DF, Lau FC, Willis LM, Carey AN, Joseph JA. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br J Nutr. 2015;114:1542–1549. doi: 10.1017/S0007114515003451. [DOI] [PubMed] [Google Scholar]
- 80.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, Bhutani T, Liao W. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spencer JPE. The impact of fruit flavonoids on memory and cognition. Br J Nutr. 2010;104:S40–S47. doi: 10.1017/S0007114510003934. [DOI] [PubMed] [Google Scholar]
- 82.Srikantha P, Mohajeri MH. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int J Mol Sci. 2019;20:2115. doi: 10.3390/ijms20092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Srivastav S, Neupane S, Bhurtel S, Katila N, Maharjan S, Choi H, Hong JT, Choi DY. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J Nutr Biochem. 2019;69:73–86. doi: 10.1016/j.jnutbio.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 84.Su CM, Kung CT, Chen FC, Cheng HH, Hsiao SY, Lai YR, Huang CC, Tsai NW, Lu CH. Manifestations and outcomes of patients with parkinson’s disease and serious infection in the emergency department. Biomed Res Int. 2018;2018:6014896. doi: 10.1155/2018/6014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice: commensal microbiota and stress response. The Journal of Physiology. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, Oryan S, Mafi A, Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 87.Tetz G, Brown SM, Hao Y, Tetz V. Parkinson’s disease and bacteriophages as its overlooked contributors. Sci Rep. 2018;8:10812. doi: 10.1038/s41598-018-29173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;13:1497–1511. doi: 10.2147/CIA.S139163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Topiwala H, Terrera GM, Stirland L, Saunderson K, Russ TC, Dozier MF, Ritchie CW. Lifestyle and neurodegeneration in midlife as expressed on functional magnetic resonance imaging: a systematic review. Alzheimers Dement (N Y) 2018;4:182–194. doi: 10.1016/j.trci.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E. The gut microbiome in human neurological disease: A review: Gut Microbiome. Ann Neurol. 2017;81:369–382. doi: 10.1002/ana.24901. [DOI] [PubMed] [Google Scholar]
- 91.Trinchieri V, Laghi L, Vitali B, Parolin C, Giusti I, Capobianco D, Mastromarino P, De Simone C. Efficacy and safety of a multistrain probiotic formulation depends from manufacturing. Front Immunol. 2017;8:1474. doi: 10.3389/fimmu.2017.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Kessel SP, Frye AK, El-Gendy AO, Castejon M, Keshavarzian A, van Dijk G, El Aidy S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun. 2019;10:310. doi: 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Laar T, Boertien JM, Herranz AH. Faecal transplantation, pro- and prebiotics in Parkinson’s disease: hope or hype. J Parkinsons Dis. 2019;9:S371–S379. doi: 10.3233/JPD-191802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vemuri R, Gundamaraju R, Shastri MD, Shukla SD, Kalpurath K, Ball M, Tristram S, Shankar EM, Ahuja K, Eri R. Gut microbial changes, interactions, and their implications on human lifecycle: an ageing perspective. Biomed Res Int. 2018;2018:4178607. doi: 10.1155/2018/4178607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang B, Yao M, Lv L, Ling Z, Li L. The human microbiota in health and disease. Engineering. 2017;3:71–82. [Google Scholar]
- 96.Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil. 2016;22:589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu S, Jiang Y, Xu K, Cui M, Ye W, Zhao G, Jin L, Chen X. The progress of gut microbiome research related to brain disorders. J Neuroinflammation. 2020;17:25. doi: 10.1186/s12974-020-1705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


