Abstract
In this work, we investigated the use of noninvasive, targeted transcutaneous electrical nerve stimulation (TENS) of peripheral nerves to provide sensory feedback to two amputees, one with targeted sensory reinnervation (TSR) and one without TSR. A major step in developing a closed-loop prosthesis is providing the sense of touch back to the amputee user. We investigated the effect of targeted nerve stimulation amplitude, pulse width, and frequency on stimulation perception. We discovered that both subjects were able to reliably detect stimulation patterns with pulses less than 1 ms. We utilized the psychophysical results to produce a subject specific stimulation pattern using a leaky integrate and fire (LIF) neuron model from force sensors on a prosthetic hand during a grasping task. For the first time, we show that TENS is able to provide graded sensory feedback at multiple sites in both TSR and non-TSR amputees while using behavioral results to tune a neuromorphic stimulation pattern driven by a force sensor output from a prosthetic hand.
I. Introduction
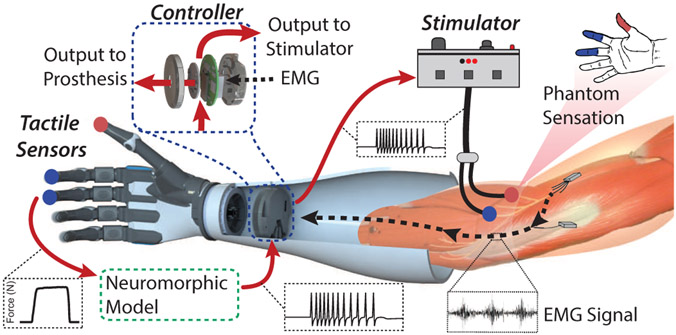
An overarching goal for upper limb prosthetic technology is a system with both forward motor control and sensory feedback through the use of nerve stimulation to elicit sensations in the missing hand of the user (Fig. 1). Touch sensation plays a vital role in our ability to explore texture, manipulate objects, or even use tools. The sensations we perceive from cutaneous afferents allow us to understand and infer seemingly complex details of an object such as shape, weight, or temperature [1]. Two major issues resulting from upper limb amputation are the loss of both motor control signals and sensory information. Significant developments with myoelectric (EMG) prosthesis control, specifically pattern recognition strategies, have shown promise in restoring intuitive hand movements to amputees [2]-[4]. There has been a great deal of recent progress in sensor development [5], [6] and local closed-loop tactile feedback strategies for improving prosthesis grasping [7]-[9], and even sensory feedback through direct nerve stimulation [10]-[12].
Figure 1.
Schematic of targeted transcutaneous electrical nerve stimulation on the residual limb of an amputee for sensory feedback to the phantom limb. Multiple peripheral nerve sites can be stimulated on an amputee to elicit activation of specific regions of the phantom hand. Sensor outputs from a prosthetic hand can be mapped to specific nerve stimulation sites and a neuromorphic tactile signal used to drive stimulation for closed-loop sensory feedback for amputees.
In addition to advances in prosthesis hardware and control methods, novel surgical methods have emerged, including targeted motor and sensory reinnervation [13]-[15]. In targeted reinnervation surgery, amputated efferent or afferent peripheral nerves that once innervated muscles or skin in the missing limb are rerouted to muscle or skin in the residual limb [13]. The muscle with the reinnervated peripheral nerves produces EMG signals that correspond to the original motor commands of the missing limb [13]. Targeted muscle reinnervation allows for more intuitive and higher dimensional motor control [13]; targeted sensory reinnervation can enable more intuitive noninvasive sensory feedback as cutaneous interactions with the reinnervated sites are perceived to localize to the missing limb [14]. In a previously reported case study, transcutaneous electrical nerve stimulation (TENS) of a targeted reinnervation site was used to activate regions of an amputee’s phantom hand [14]. In a recent advancement of the targeted sensory reinnervation (TSR) surgery, individual nerve fascicles of the median and ulnar nerves were routed to regions of an amputee’s residual limb away from motor regions to create more distinction and separation between activated sensory regions of the phantom hand [15]. In this work we attempt to understand the sensory perception created by using noninvasive TENS of the median and ulnar nerves in the residual limb of two transhumeral amputees. We investigate the use of TENS as a viable method for sensory feedback in 1) an amputee with TSR surgery and 2) an amputee with no TSR surgery through sensory mapping and psychophysics to determine stimulation thresholds. Finally, we build upon our previous work [7] to create a subject specific neuromorphic stimulation pattern, driven by the output from force sensors on a prosthetic hand, using a leaky and integrate fire (LIF) neuron model.
II. Methods & Experiments
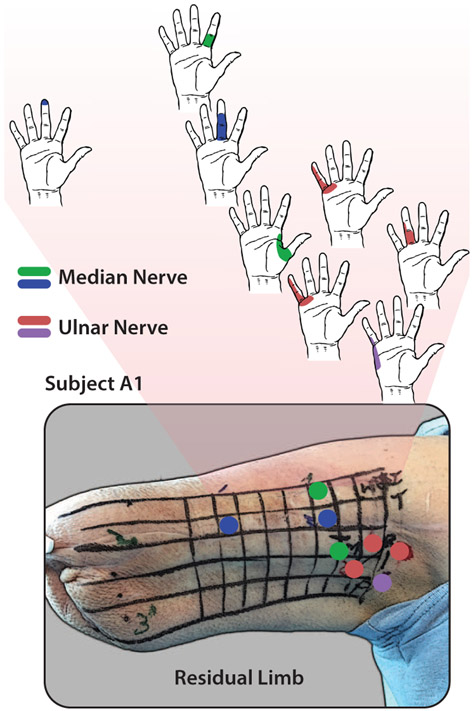
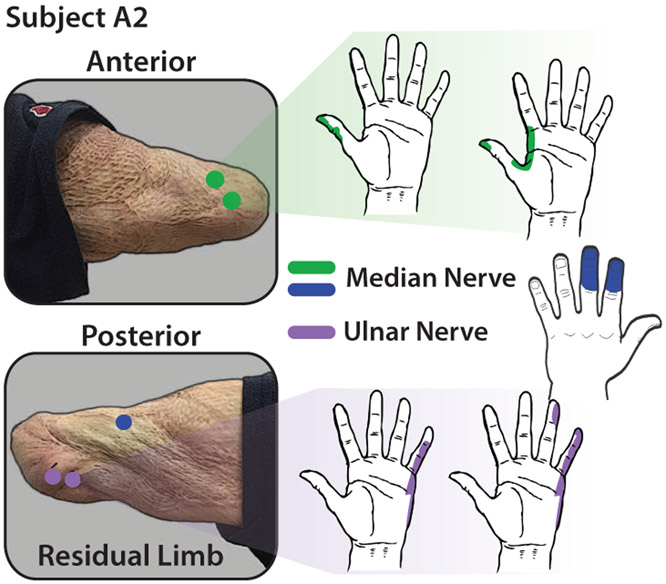
Two transhumeral amputee subjects participated in this study. One subject (A1) had undergone targeted sensory and muscle reinnervation surgery 1.5 years prior to the study and the other subject (A2) was amputated (5 years prior) without any targeted reinnervation. In both cases, amputation was a result of severe sepsis. Both users have operated myoelectric prostheses but neither had undergone electrical stimulation for sensory feedback. Characteristics of each subject are shown in Table I. To understand the mapping between peripheral nerves in the residual limb and activation in the phantom hand of each subject, we used an Ag-AgCl probe with a 2 mm tip to electrically stimulate regions of the residual limb. The anterior region, which was the target of the TSR surgery, of the residual limb was scanned with the stimulating probe for subject A1 and both anterior and posterior sides of subject A2’s residual limb were scanned. An outline of a hand was used for each subject to indicate which regions were activated during electrical stimulation. Figure 2 shows the stimulation sites and activation regions in the phantom hand for subject A1 and Fig. 3 shows the mapping for subject A2. For both subjects, the median and ulnar nerves were targeted for stimulation. In general, the subjects reported sensations of tingling and occasional pressure in the activated regions on the phantom hand.
Table I.
Subject characteristics
| Subject | A1 | A2 |
|---|---|---|
| Gender | Female | Male |
| Age | 43 | 29 |
| Amputation Side | Right | Left |
| Amputation Level | Transhumeral | Transhumeral |
| Amputation Type | TSR | No TSR |
| Time Since Amputation | 1.5 yr | 5 yr |
Figure 2.
Phantom hand activation mapping for stimulation of the median and ulnar nerve areas for subject A1. Targeted reinnervation of median and ulnar nerves to the anterior part of the residual limb provides distinct mapping to various parts of the phantom hand.
Figure 3.
Phantom hand activation mapping for stimulation of identified median and ulnar nerve areas for subject A2. Although targeted reinnervation was not performed on this subject at the time of amputation, natural reinnervation occurred in the residual limb but with less order than in the case of subject A1.
A. Perception Experiments
To better understand how noninvasive peripheral nerve stimulation can be utilized for sensory feedback in prosthetic limbs we performed psychophysical experiments to determine detection thresholds by varying pulse width and frequency of the stimulation pattern. In this study, a monophasic, single channel, isolated constant current stimulator (DS3, Digitimer, England) was used. A 5 mm disposable Ag-AgCl electrode was placed on the skin over a region corresponding to a specific activation area of the phantom hand. For subject A1 the middle finger (median nerve) of the phantom hand was stimulated and the pinky finger (ulnar nerve) was stimulated for subject A2. These regions were chosen because they caused very distinct and comfortable activation of the phantom hand for the subjects. Unless otherwise noted, an electrical stimulation amplitude of 1.0 mA was used for all experiments.
To understand the sensory perception of the nerve stimulation we performed two experiments. 1) Stimulation detection to determine the minimum levels of detectable stimulation for the targeted activation sites and 2) discrete vs continuous frequency detection to determine the levels of stimulation that result in the sensation of a continuous perception in the targeted activation sites. The subject was seated in front of a computer monitor that displayed a visual cue when stimulation was on. For each experiment, the targeted nerve site was stimulated for 2 s. For the first experiment the subject verbally indicated if he/she felt the stimulation. The stimulation frequency and amplitude were held constant while the pulse width was modulated. For the second experiment the subject verbally indicated if he/she perceived the stimulation as being discrete or continuous. The stimulation amplitude and pulse width were held constant while the frequency was modulated. Every stimulation pattern was randomized and presented at least 5 times for each experiment. Psychometric functions were fit to the data using a sigmoid link function:
| (1) |
where α is the detection threshold and β is the discrimination sensitivity. Both parameters were found using the curve fitting toolbox in MATLAB (MathWorks, USA).
B. Neuromorphic Sensor Model
As a demonstration, we implemented a leaky integrate and fire neuron model on customized prosthesis hardware (Infinite Biomedical Technologies, USA) to create a subject specific neuromorphic spiking output from previously developed force sensors on a bebionic3 prosthetic hand (Steeper, UK) [5]. Subject A2 performed a grasping task with the prosthetic hand where he picked up, held, and released an object. The prosthesis was controlled using subject A2’s EMG signals. The pulse width and frequency of the neuromorphic output was based on the stimulation detection thresholds for subject A2. In this demonstration, the neuromorphic tactile signal was used as feedback to the prosthesis controller but not the subject to demonstrate feasibility. The neuromorphic model is given by
| (2) |
where I(t) is the model input current from the prosthesis grip force, v(t) represents the neuron’s membrane potential at time t, and τm is the membrane time constant. R is the membrane resistance. When the neuron potential reaches a spiking threshold, vth, it is reset instantaneously to a lower value, vr. The refractory conductance of the neuron is given by g(t) and Ek is the reversal potential for the model’s spike rate adaption. The use of this model is discussed in more detail in our previous work [7]. The pulse width and maximum frequency of the neuromorphic tactile signal is tuned to subject A2’s stimulation perceptions. The prosthesis grip force is mapped to frequency because subject A2 reported that he perceived increase in stimulation intensity with increasing frequency. For more details on this model and its parameters see [7]. All experiments were approved by the Johns Hopkins Medicine Institutional Review Boards and the subjects provided informed consent before participating, and all data was collected and analyzed using MATLAB.
III. Results
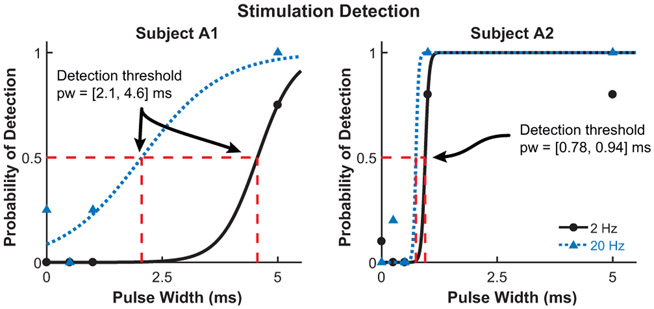
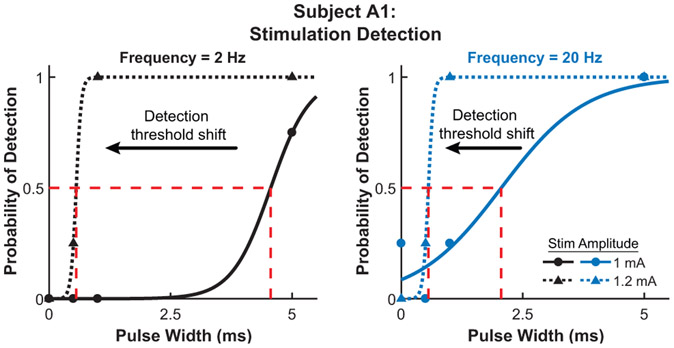
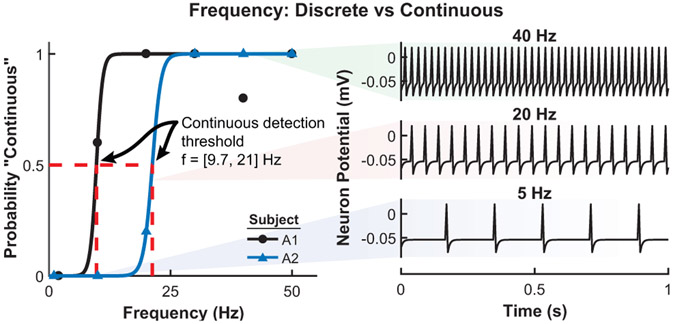
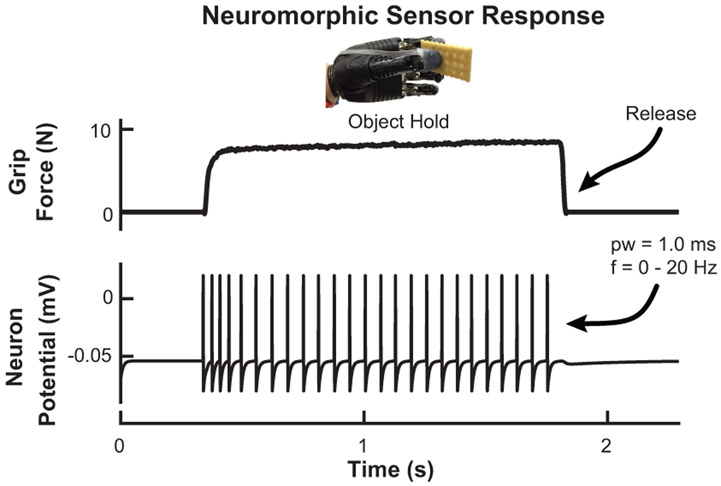
The results from the stimulation detection experiment for both subjects are shown in Fig. 4. The detection threshold is defined as the pulse width where the probability of feeling the stimulation is 0.5. Subject A1 has a detection threshold of 2.1 ms and 4.6 ms for stimulation with an amplitude of 1.0 mA and frequency of 20 Hz and 2 Hz, respectively. Subject A2 has a detection threshold of 0.78 ms and 0.94 ms for a stimulation frequency of 20 Hz and 2 Hz, respectively. Figure 5 shows the shift in detection threshold for subject A1 when the stimulation amplitude is increased from 1.0 mA to 1.2 mA. The increased stimulation amplitude causes a decrease (leftward shift) in the detection threshold. The results from the second experiment, with a corresponding neuromorphic tactile response, are shown in Fig. 6. The frequency of the stimulation is modulated while the pulse width is held constant at 5 ms, which is well above the stimulation detection threshold for both subjects. Subject A1 has a threshold for perceiving a stimulation as continuous at 9.7 Hz while the threshold for subject A2 is 21 Hz. The neuromorphic tactile signal from the prosthesis grasping task is shown in Fig. 7. Based on the psychophysical results for subject A2, each spike from the neuromorphic tactile sensing output has a pulse width of 1 ms and the maximum spiking frequency is mapped to 20 Hz, which corresponds to a grip force of 20 N.
Figure 4.
Stimulation detection for subjects A1 (left) and A2 (right). Multiple stimulation pulse widths (pw) and frequencies were used for each subject. From the fitted psychometric functions, the detection threshold for subject A1 is 2.1 ms and 4.6 ms for a stimulation frequency of 2 Hz and 20 Hz, respectively. The detection threshold for subject A2 is 0.78 ms and 0.94 ms for a stimulation frequency of 2 Hz and 20 Hz, respectively. The coefficient of determination, R2, is > 0.91 for every psychometric function fit.
Figure 5.
Detection threshold shift due to varying stimulation amplitude for subject A1 at a frequency of 2 Hz (left) and 20 Hz (right). Slight increase in stimulation amplitude from 1 mA to 1.2 mA for subject A1 causes a leftward shift in the detection threshold. The higher amplitude lowers the detection threshold to 0.56 ms for both 2 Hz and 20 Hz stimulation. Every psychometric function has an R2 > 0.91.
Figure 6.
Perception of discrete or continuous stimulation for subjects A1 and A2 (left) with corresponding prosthesis neuromorphic output (right). The stimulation amplitude for subject A1 is 1.0 mA and 1.4 mA for subject A2. The frequency (f) threshold for perceiving a stimulation as continuous is 9.7 Hz for subject A1 and 21 Hz for subject A2. The fitted psychometric functions have an R2 > 0.90. The neuromorphic tactile response represents points along the psychometric function at a particular frequency.
Figure 7.
Neuromorphic tactile signal during the prosthesis grasping task. The LIF neuron model produces spikes, using stimulation and frequency detection thresholds for subject A2 to determine the pulse width (1 ms) and frequency range (0 - 20 Hz). This spiking output form the prosthesis sensors can then be used as feedback on the residual limb to create the closed-loop prosthesis.
IV. Discussion
This work presents a unique look at how noninvasive TENS can be used to provide sensory feedback to both TSR and non-TSR transhumeral amputees. Targeted TENS provides distinct sensory activation in the phantom limb (Figs. 2 and 3) for both subjects. The TSR subject (A1) seemed to have more localized regions of phantom hand activation compared to the non-TSR subject (A2). This is likely due to the intentional placement of peripheral nerve fascicles in the anterior portion of subject A1’s residual limb. For subject A2, median and ulnar nerve sites naturally reinnervated the skin but without any external guidance, causing less structure or ordering of the nerve sites. However, this does not mean that a non-TSR amputee is not a good candidate for targeted TENS for sensory feedback.
The minimum level of stimulation needed for reliable detection shown in Fig. 4 offers valuable insight to designing a fully closed-loop system. The frequency of the stimulation seems to influence the detection threshold if the stimulation amplitude is low enough. Results in Fig. 5 show that both psychometric functions shift leftward (reduces the detection threshold) to 0.56 ms with slightly increased amplitude. For subject A2 (Fig. 4) it appears that the amplitude used was high enough for the detection thresholds to converge, a phenomenon seen for subject A1 after the stimulation amplitude was increased. Another important value is the threshold for perceiving a stimulation pattern as a discrete or continuous activation (Fig. 6). This is crucial for noninvasive electrical stimulation due to the low-pass filtering effects of skin and soft tissue on the residual limb. This threshold is 9.7 Hz and 21 Hz for subjects A1 (TSR) and A2, respectively. This threshold can be influenced by a variety of factors such as electrode placement, depth of nerve site in the skin, or skin conductance. The neuromorphic tactile response that would correspond to the psychometric function is also shown in Fig. 6. The psychometric function shows how the neuromorphic response from the prosthesis would be perceived by the user. The neurmorphic sensor model combined with prosthesis hardware gives subject specific stimulation patterns during object grasping to enable more advanced feedback (Fig. 7).
V. Conclusion
We showed how results from TENS on amputees can be utilized to tune a real-time neuromorphic output based on the grip force of a prosthetic hand. By understanding the detection thresholds for subject A2, we successfully implemented the LIF neuron model on prosthesis hardware to create a spiking output that is specific for the user (Fig. 7), which in this case is defined by the minimum stimulation pulse width (1 ms) and frequency range needed for discrete stimulation pulse detection (0 - 20 Hz). Our goal is to further understand how noninvasive methods can be used for providing sensory feedback and how we can combine this with existing prosthesis sensors and hardware to create a fully closed-loop prosthetic system, as shown in Fig. 1.
Acknowledgment
This research was supported in part by Space@Hopkins as well as the grant R44HD072668 from the National Institutes of Health. Nitish Thakor is co-founder of Infinite Biomedical Technologies. His efforts and conflict of interest have been declared with and is managed by Johns Hopkins University. The authors would like to thank the subjects who devoted their time to this study.
References
- [1].Lumpkin E and Caterina M, “Mechanisms of sensory transduction in the skin,” Nature, vol. 445, no. 7130, pp. 858–865, 2007. [DOI] [PubMed] [Google Scholar]
- [2].Farina D, Vujaklija I, Sartori M, Kapelner T, Negro F, Jiang N, Bergmeister K, Andalib A, Principe J, and Aszmann OC, “Man/machine interface based on the discharge timings of spinal motor neurons after targeted muscle reinnervation,” Nat. Biomed. Eng, vol. 1, p. 0025, 2017. [Google Scholar]
- [3].Betthauser J, Hunt C, Osborn L, Masters M, Lévay G, Kaliki R, and Thakor N, ‘Limb position tolerant pattern recognition for myoelectric prosthesis control with adaptive sparse representations from extreme learning,” IEEE Trans. Biomed. Eng, 2017. doi: 10.1109/TBME.2017.2719400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Powell M, Kaliki R, and Thakor N, “User training for pattern recognition-based myoelectric prostheses: Improving phantom limb movement consistency and distinguishability,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 22, no. 3, pp. 522–532, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Osborn L, Lee WW, Kaliki R, and Thakor N, “Tactile feedback in upper limb prosthetic devices using flexible textile force sensors,” in Conf. IEEE Biomed. Robot. Biomechatronics, 2014, pp. 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Osborn L, Nguyen H, Betthauser J, Kaliki R, and Thakor N, “Biologically inspired multi-layered synthetic skin for tactile feedback in prosthetic limbs,” in Conf. IEEE Eng. Med. Biol., 2016, pp. 4622–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Osborn L, Nguyen H, Kaliki R, and Thakor N, “Prosthesis grip force modulation using neuromorphic tactile sensing,” in Myoelec. Controls Symp, 2017, pp. 188–191. [Google Scholar]
- [8].Osborn L, Kaliki R, Soares A, and Thakor N, “Neuromimetic event-based detection for closed-loop tactile feedback control of upper limb prostheses,” IEEE Trans. Haptics, vol. 9, no. 2, pp. 196–206, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Engeberg E and Meek S, “Adaptive sliding mode control for prosthetic hands to simultaneously prevent slip and minimize deformation of grasped objects,” IEEE/ASME Trans. Mechatronics, vol. 18, no. 1, pp. 376–385, 2013. [Google Scholar]
- [10].Graczyk E, Schiefer M, Saal H, Delhaye B, Bensmaia S, and Tyler D, “The neural basis of perceived intensity in natural and artificial touch,” Sci. Transl. Med, vol. 8, no. 362, p. 362ra142, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oddo C, Raspopovic S, Artoni F, Mazzoni A, Spigler G, Petrini F, Giambattistelli F, Vecchio F, Miraglia F, Zollo L, Pino G, Camboni D, Carrozza M, Guglielmelli E, Rossini P, Faraguna U, and Micera S, “Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans,” eLife, vol. 5, pp. e09148, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schiefer M, Tan D, Sidek S, and Tyler D, “Sensory feedback by peripheral nerve stimulation improves task performance in individuals with upper limb loss using a myoelectric prosthesis,” J. Neural Eng, vol. 13, no. 1, pp. 016001, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kuiken T, Marasco P, Lock B, Harden R, and Dewald J, “Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation,” Proc. Natl. Acad. Sci, vol. 104, no. 50, pp. 20061–20066, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kuiken T, Miller L, Lipschutz R, Lock B, Stubblefield K, Marasco P, Zhou P, and Dumanian G, “Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study,” Lancet, vol. 369, no. 9559, pp. 371–80, 2007. [DOI] [PubMed] [Google Scholar]
- [15].Hebert J, Olson J, Morhart M, Dawson M, Marasco P, Kuiken T, and Chan K, “Novel targeted sensory reinnervation technique to restore functional hand sensation after transhumeral amputation,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 22, no. 4, pp. 765–773, 2014. [DOI] [PubMed] [Google Scholar]