Abstract
Nonalcoholic fatty liver disease (NAFLD) represents one of the most common chronic liver diseases in the world. It has been reported that epigallocatechin-3-gallate (EGCG) plays important biological and pharmacological roles in mammalian cells. Nevertheless, the mechanism underlying the beneficial effect of EGCG on the progression of NAFLD has not been fully elucidated. In the present study, the mechanisms of action of EGCG on the growth, apoptosis, and autophagy were examined using oleic acid- (OA-) treated liver cells and the high-fat diet- (HFD-) induced NAFLD mouse model. Administration of EGCG promoted the growth of OA-treated liver cells. EGCG could reduce mitochondrial-dependent apoptosis and increase autophagy possibly via the reactive oxygen species- (ROS-) mediated mitogen-activated protein kinase (MAPK) pathway in OA-treated liver cells. In line with in vitro findings, our in vivo study verified that treatment with EGCG attenuated HFD-induced NAFLD through reduction of apoptosis and promotion of autophagy. EGCG can alleviate HFD-induced NAFLD possibly by decreasing apoptosis and increasing autophagy via the ROS/MAPK pathway. EGCG may be a promising agent for the treatment of NAFLD.
1. Introduction
Tea, made from the leaves of Camellia sinensis, has long been considered a popular beverage worldwide [1–3]. Tea can be mainly classified into three types according to the manufacturing processes, including green tea (nonfermented), oolong tea (semifermented), and red and black teas (fermented) [4]. The functional constituents of tea can be attributable to the polyphenolic compounds, particularly catechins [1]. Four main catechins have been identified in green tea, such as epigallocatechin-3-gallate (EGCG), epigallocatechin, epicatechin-3-gallate, and epicatechin, with EGCG as the most active and abundant compound [3, 5]. These catechins have different hydroxyl groups on the B-ring with the presence/absence of a galloyl moiety [4]. EGCG exhibits strong binding to bioactive macromolecules, such as DNA and proteins via π-π stacking interaction, hydrogen bonding, and hydrophobic interaction [5, 6].
EGCG, a flavone-3-ol phenolic compound, has eight free hydroxyl groups [2], which might contribute to its diverse biological and pharmacological properties, such as antiamyloidogenic [7], chemopreventive [8], renoprotective [9], anticancer [10], antiaging [11], antiautoimmune [12], and antiviral [13] activities. Nonalcoholic fatty liver disease (NAFLD), a common chronic liver disease, has been considered one of the leading causes of end-stage liver disease, liver transplantation, and hepatocellular carcinoma [14–16]. The prevalence of NAFLD is growing in parallel with the global obesity epidemics, hypertension, type 2 diabetes, hyperlipidemia, and metabolic syndromes [15, 16]. It has been shown that EGCG could attenuate high-fat diet- (HFD-) induced NAFLD in rats and mice [17–19]. Nevertheless, the inhibitory effects and detailed mechanisms of EGCG in the initiation and progression of NAFLD need to be further investigated.
In this study, the mechanism of action of EGCG on the growth, apoptosis, and autophagy of oleic acid- (OA-) treated liver cells was elucidated. The HFD-induced NAFLD mouse model was further adopted to confirm the effect and mechanism of EGCG on NAFLD.
2. Materials and Methods
2.1. Cell Culture
Human liver cell lines L02 and QSG-7701 were purchased from Feiya Biological Technology Co., Ltd. (Yancheng, Jiangsu, China). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and 1% streptomycin/penicillin. Before each experiment, the cells were starved for 12 h in serum-free DMEM. Then, the cells were treated with the medium containing the 0.5 mM OA-bovine serum albumin (BSA; fatty acid-free, low endotoxin) complex (4 : 1, molar ratio), with or without a concentration of 50 μM EGCG for 24 h. The medium with only BSA was selected as the control [20].
2.2. Oil Red O (ORO) Staining
Cells were fixed in 4% paraformaldehyde for 15 min, incubated with isopropyl alcohol for 20 min, and stained with ORO solution for 20 min, followed by being counterstained with hematoxylin at room temperature. The staining intensity of ORO was measured by ImageJ software (National Institutes of Health, Bethesda, MD, USA) [21].
2.3. Cell Growth Assay
The 5-ethynyl-2′-deoxyuridine (EdU) experiment was carried out using economical kits (RiboBio, Guangzhou, China). The cell proliferation rate was calculated as the percentage of positive cells to total cells. In addition, the cell counting kit-8 (CCK-8) detection kits (Beyotime, Shanghai, China) were used to detect cell viability. Cell viability was expressed as a percentage to the control group [22].
2.4. Flow Cytometry Assay
Cells were incubated with propidium iodide (PI)/RNase A mixture for 20 min. A FACSVerse flow cytometer (BD, San Jose, CA, USA) was adopted to analyze the cell cycle. The apoptotic level was examined by Annexin V-FITC/PI assay kits (KeyGen, Nanjing, Jiangsu, China) and further analyzed using a FACSVerse flow cytometer.
2.5. Immunofluorescence Staining
The green fluorescent protein- (GFP-)-red fluorescent protein- (RFP-) microtubule-associated protein 1 light chain 3 (MAP1LC3/LC3) plasmid has been used to detect the autophagic level [23]. Then, the GFP-RFP-LC3 plasmid (Hanbio, Shanghai, China) was transfected into the cells. After 48 h of incubation, the cellular fluorescence was determined using an Eclipse Ti fluorescent microscope (Nikon, Melville, NY, USA). The autophagosomes (yellow dots) and autolysosomes (red dots) were calculated as the ratios of positive-stained cells to total cells [24].
2.6. Monodansylcadaverine (MDC) Staining
Morphologically, the formation of autophagic vacuoles in the cytoplasm is a typical characteristic of autophagy. MDC is a key marker for autophagic vacuoles [25]. Briefly, the liver cells were stained with 50 μmol/L MDC for 30 min at 37°C. Then, the cells were fixed with 5% paraformaldehyde and immediately observed under an Eclipse Ti fluorescent microscope (Nikon).
2.7. Measurement of Reactive Oxygen Species (ROS)
Cellular ROS levels were measured by 2′,7′-dichlorodihydrofluorescein diacetate (Beyotime).
2.8. Determination of Antioxidant Activity
The total superoxide dismutase (SOD) activity was determined using the kit with WST-8 (Beyotime). The catalase (CAT) activity was analyzed using the CAT assay kit (Beyotime). The glutathione peroxidase (GSH-Px) activity was detected by the GSH-Px assay kit with nicotinamide adenine dinucleotide phosphate (Beyotime).
2.9. Western Blot
Western blot assay was adopted to determine the expression levels of proteins. The primary antibodies, such as anti-cyclin D1/E1, anti-cyclin-dependent kinase (CDK) 2/4, anti-p21, anti-p27, anti-beclin-1, anti-P62, anti-LC3A/B, anti-extracellular signal-regulated protein kinase 1/2 (ERK1/2), anti-phospho- (p-) ERK1/2 (Thr202/Tyr204), anti-c-Jun N-terminal kinase (JNK), anti-p-JNK (Thr183/Tyr185), anti-p38, and anti-p-p38 (Thr180/Tyr182), and the horseradish peroxidase-conjugated secondary antibody were purchased from Cell Signaling Technology (CST, Danvers, MA, USA). Anti-B-cell lymphoma-2 (Bcl-2), anti-Bcl-2-associated X protein (Bax), anti-B-cell lymphoma-extra large (Bcl-xl), anti-Bcl-xl/Bcl-2-associated death promoter (Bad), anti-cleaved caspase-3/9, anti-cleaved poly-ADP-ribose polymerase (PARP), and anti-β-actin were obtained from Proteintech (Chicago, IL, USA). The bands were detected with a chemiluminescence system (Thermo, Rockford, IL, USA). Band intensities were analyzed by densitometry using ImageJ software.
2.10. Animals
The animal experiment was approved by the Committee of Medical Ethics and Welfare for Experimental Animals of Henan University School of Medicine (HUSOM-2017-208). C57BL/6J mice (8 weeks old, male), HFD (60% kcal as fat), and low-fat diet (LFD, 10% kcal as fat) were obtained from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All mice were maintained on a 12 h light/dark cycle and allowed access to food and water ad libitum. Mice were fed either HFD (n = 12) or LFD (n = 6) for a total of 14 weeks. After feeding for 10 weeks, HFD-fed mice were assigned to the HFD group (n = 6) and HFD+EGCG (50 mg/kg/day) group (n = 6). The mice were treated for an additional 4 weeks. Food/water intakes and body weights of the mice were measured. Then, the mice were killed and blood samples were collected. The liver, brown fat, and white fat were removed and weighed.
2.11. Biochemical Analysis
The concentrations of triglyceride (TG), total cholesterol (TC), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in the plasma were examined by an automated hematology analyzer (BC-6900, Mindray, Shenzhen, Guangdong, China). The contents of TG and TC in liver cells and tissues, as well as nonesterified fatty acid (NEFA) in liver tissues were detected by commercial enzyme-linked immunosorbent assay kits (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
2.12. Hematoxylin and Eosin (HE) Staining
Liver tissues were fixed in 10% neutral formalin, embedded in paraffin wax, sectioned at 4 μm, and then stained with HE.
2.13. Immunohistochemistry (IHC)
Liver samples were respectively stained with anti-Ki67 (CST), anti-beclin-1, and anti-cleaved caspase-3 antibodies. The proliferation index, autophagic index, and apoptotic index were determined by the ratios of positive cells to total cells.
2.14. Statistics
All data were presented as the mean ± standard error of the mean. Differences between the two groups were determined by the two-tailed Student's t-test and one-way analysis of variance using GraphPad Prism 6 software. P < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. EGCG Promotes the Growth of OA-Treated Liver Cells
As shown in Figures 1(a) and 1(b), OA induced the accumulation of lipid in OA-treated liver cells, as further evidenced by the increased levels of TC and TG (Figures 1(c) and 1(d)). Treatment with EGCG reduced the lipid level in OA-treated liver cells (Figures 1(a)–1(d)). The viability and proliferation of liver cells were decreased by OA; nevertheless, EGCG promoted the viability and proliferation of OA-treated liver cells (Figures 1(e)–1(g)). In addition, the results showed that OA triggered cell cycle arrest at the G1 phase and EGCG reversed the trend (Figures 2(a) and 2(b)). Many cell cycle-related proteins have been identified in mammals, such as cell cycle regulatory proteins, including cyclin D1/E1 and CDK2/4, as well as inhibitory cell cycle regulators, including p21 and p27 [26, 27]. Our data suggested that OA increased the expressions of cyclin D1, cyclin E1, CDK2, and CDK4 but downregulated the protein levels of p21 and p27; however, administration of EGCG exhibited reverse trends (Figures 2(c) and 2(d)). Taken together, the data indicate that EGCG can promote the growth of OA-treated liver cells through promoting G1 phase cell cycle progression.
Figure 1.
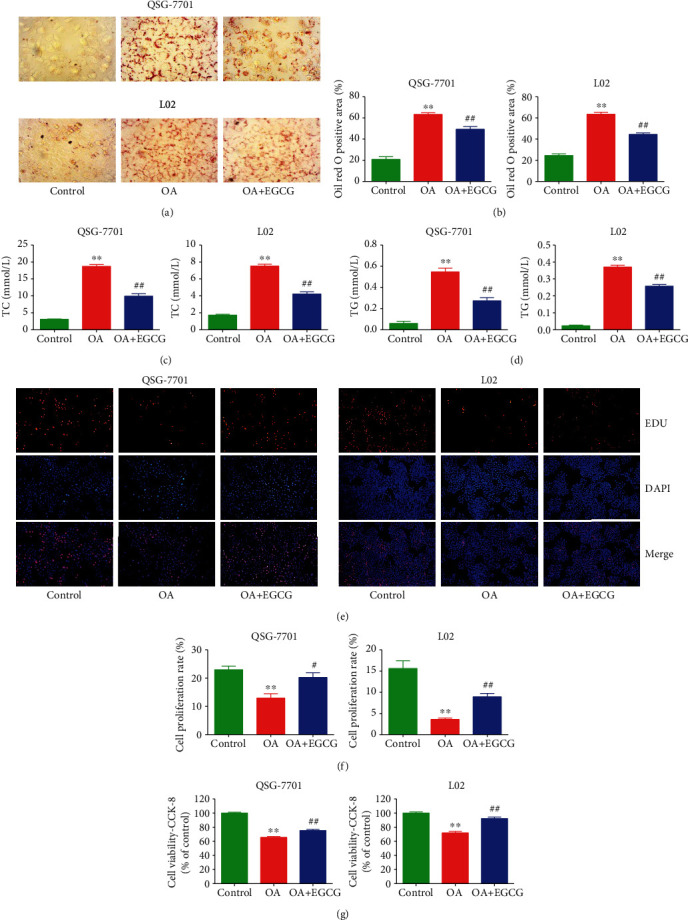
Effects of EGCG on lipid droplet formation and the growth of OA-treated QSG-7701 and L02 cells. (a) Representative photographs of ORO-stained QSG-7701 and L02 cells; original magnification ×400. (b) ORO-positive area was calculated. (c) The levels of TC were measured. (d) The levels of TG were measured. (e) DNA replication activities were examined by EdU assay; original magnification ×200. (f) The proliferation rate of each group was analyzed. (g) The percentages of viable cells were determined using CCK-8 assay, and the cell viability of control cells was normalized as 100%. Data are presented as mean ± SEM of three independent experiments; ∗∗P < 0.01 compared with the control group; #P < 0.05, ##P < 0.01 compared with the OA group.
Figure 2.
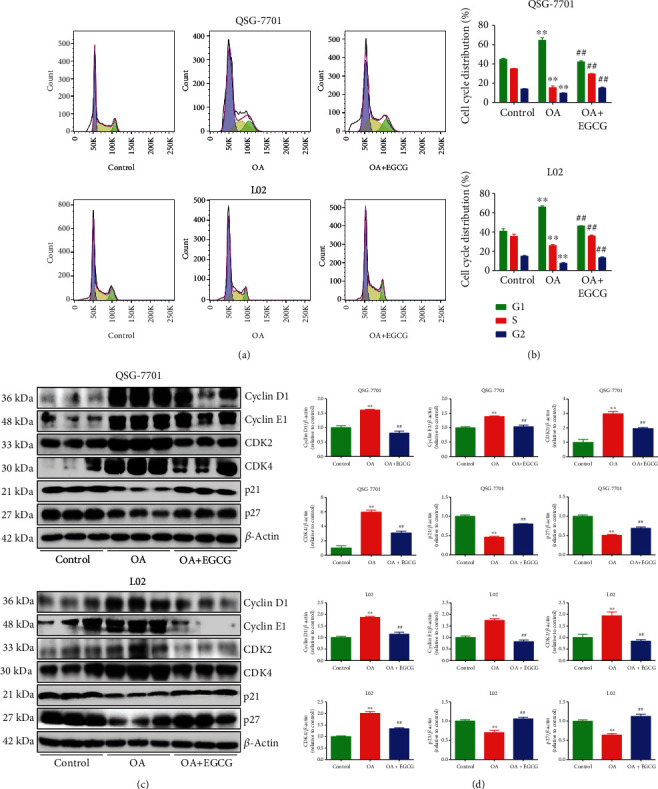
Effects of EGCG on cell cycle progression of OA-treated QSG-7701 and L02 cells. (a) Flow cytometry assay was used to determine cell cycle distribution. (b) Cell cycle distribution was analyzed. (c) Western blot analysis for the expression levels of cyclin D1, cyclin E1, CDK2, CDK4, p21, and p27 in each group. β-Actin was used as the loading control. (d) The densitometry analysis of each factor was performed in each group, normalized to the corresponding β-actin level. Data are presented as mean ± SEM of three independent experiments; ∗∗P < 0.01 compared with the control group; ##P < 0.01 compared with the OA group.
3.2. EGCG Decreases Apoptosis in OA-Treated Liver Cells
As shown in Figures 3(a) and 3(b), the data suggested that OA increased the early and late apoptotic cell populations, whereas EGCG decreased the early and late apoptosis in OA-treated liver cells. The ratios of Bax/Bcl-2 and Bad/Bcl-xl are regarded as key factors in regulating apoptosis. Increased ratios of Bax/Bcl-2 and Bad/Bcl-xl are key phenomena in mitochondrial-dependent apoptosis in mammals [28, 29]. Furthermore, cleaved caspase-3/9 could induce apoptosis through the mitochondrial-mediated pathway [30]. PARP, a nuclear enzyme involved in DNA repair, is an important target for caspases during apoptosis [31]. The data showed that OA increased both the Bax/Bcl-2 and Bad/Bcl-xl ratios and the expression levels of cleaved caspase-3/9 and cleaved PARP, which were reversed by the administration of EGCG (Figures 3(c) and 3(d)). The results suggest that OA can induce mitochondrial-dependent apoptosis in liver cells and EGCG could reduce the apoptotic levels in OA-treated liver cells.
Figure 3.
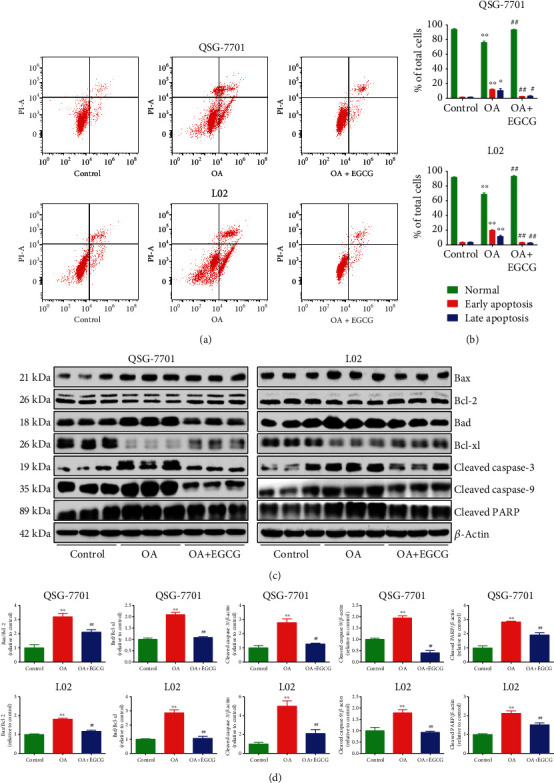
Effects of EGCG on the apoptosis of OA-treated QSG-7701 and L02 cells. (a) Flow cytometry assay was used to determine the apoptotic level. (b) The results of flow cytometry were analyzed. (c) Western blot analysis for the expression levels of Bax, Bcl-2, Bad, Bcl-xl, cleaved caspase-3/9, and cleaved PARP in each group. β-Actin was used as the loading control. (d) The densitometry analysis of each factor was performed in each group, normalized to the corresponding β-actin level. Data are presented as mean ± SEM of three independent experiments; ∗P < 0.05, ∗∗P < 0.01 compared with the control group; #P < 0.05, ##P < 0.01 compared with the OA group.
3.3. EGCG Increases Autophagy in OA-Treated Liver Cells
Autophagy is responsible for the degradation of intracellular protein aggregates, invasive pathogens, and damaged organelles and therefore is essential in maintaining cellular homeostasis and responding to stress conditions [32, 33]. A crucial step in autophagy is the conversion of LC3 from the nonlipidated form (LC3-I) to the lipid-conjugated form (LC3-II) [33, 34]. Autophagic turnover could be molecularly monitored using a GFP-conjugated LC3 and the conversion of LC3-I to LC3-II [35]. In the present study, the GFP-RFP-LC3 plasmid was transfected into liver cells and further detected by fluorescence microscopy. Treatment with OA decreased the numbers of free red dots (autolysosomes) and yellow dots (autophagosomes), whereas administration of EGCG showed the opposite effects (Figures 4(a) and 4(b)). A similar trend was observed in MDC staining (Figures 4(c) and 4(d)). Apart from LC3, beclin-1 and P62 have also been considered specific markers of autophagy [33, 36]. The expression levels of beclin-1 and LC3 in the OA group were lower than those in the control group, but the protein levels of these two factors were higher in the OA+EGCG group than in the OA group. Furthermore, the expression level of P62 exhibited a reverse trend (Figures 4(e) and 4(f)). These results together suggest that the autophagic level is downregulated in OA-treated liver cells and treatment with EGCG could upregulate the autophagy machinery.
Figure 4.
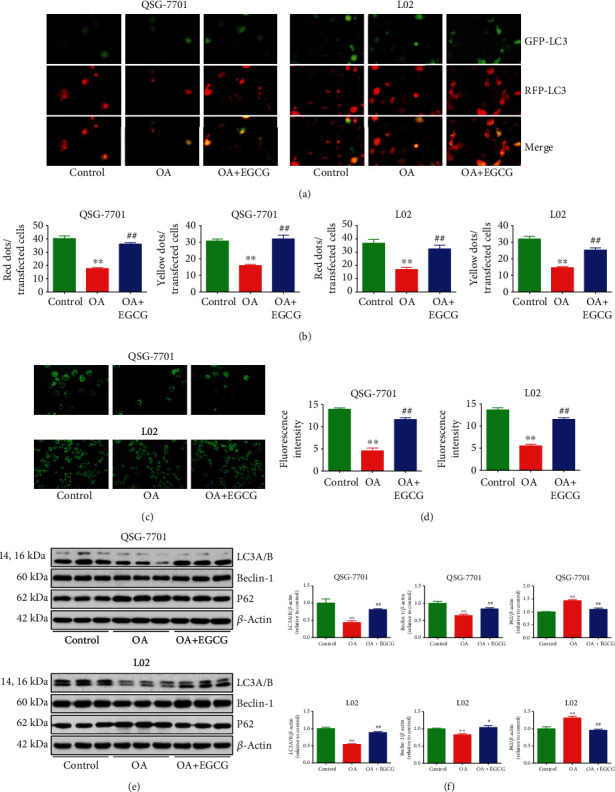
Effects of EGCG on the autophagy of OA-treated QSG-7701 and L02 cells. (a) GFP-RFP-LC3-transfected QSG-7701 and L02 cells were examined by fluorescence microscopy; original magnification ×1000. (b) The ratios of red and yellow dots to transfected cells were calculated. (c) Representative photographs of MDC staining. (d) The fluorescence intensity was analyzed. (e) Western blot analysis for the expression levels of LC3A/B, beclin-1, and P62 in each group. β-Actin was used as the loading control. (f) The densitometry analysis of each factor was performed in each group, normalized to the corresponding β-actin level. Data are presented as mean ± SEM of three independent experiments; ∗∗P < 0.01 compared with the control group; #P < 0.05, ##P < 0.01 compared with the OA group.
3.4. EGCG Suppresses the ROS/Mitogen-Activated Protein Kinase (MAPK) Pathway in OA-Treated Liver Cells
ROS such as hydroxyl radical, hydrogen peroxide, and superoxide anion are normally generated as by-products of aerobic metabolism [37, 38]. ROS can be scavenged by the antioxidant defense system that mainly includes GSH-Px, SOD, and CAT [37–39]. Compared with the control group, the ROS levels were increased, but GSH-Px, SOD, and CAT activities were downregulated in the OA group, which can be reversed by the administration of EGCG (Figures 5(a) and 5(b)). The results suggest that EGCG can reduce OA-induced oxidative stress in liver cells. It has been shown that ROS can activate the MAPK pathway and attenuation of ROS by ROS scavengers could deactivate MAPK signaling [40, 41]. As shown in Figures 5(c) and 5(d), OA reduced the expression of p-ERK1/2 but increased the levels of p-JNK and p-p38, while administration of EGCG exhibited reverse effects on the kinases. The data suggest that EGCG may suppress the ROS/MAPK pathway in OA-treated liver cells.
Figure 5.
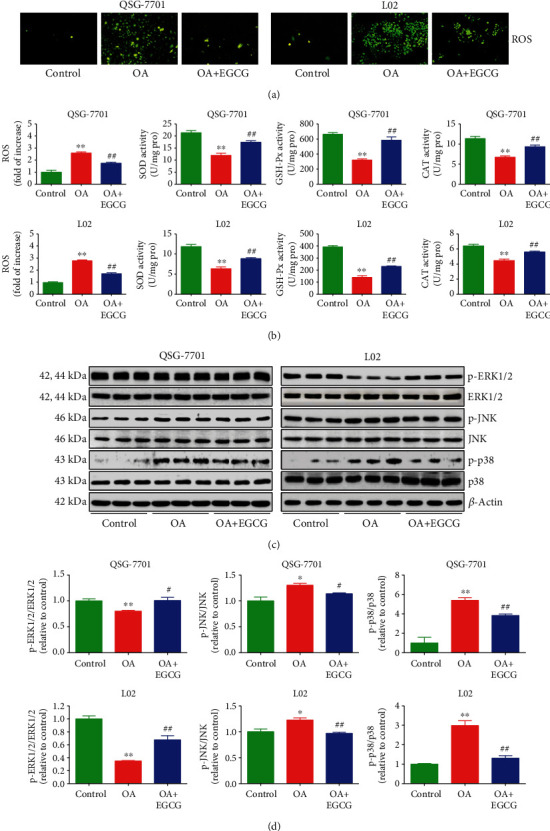
Effects of EGCG on the ROS/MAPK signaling pathway in OA-treated QSG-7701 and L02 cells. (a) The intracellular ROS production was detected using the fluorescent probe DCF-DA (shown in green; original magnification, ×400). (b) The intracellular ROS production and the activities of SOD, GSH-Px, and CAT were measured. (c) The protein expressions of ERK1/2, p-ERK1/2, JNK, p-JNK, p38, and p-p38 were analyzed by Western blot. β-Actin was used as the loading control. (d) The densitometry analysis of each factor was performed in each group, normalized to the corresponding β-actin level. Data are presented as mean ± SEM of three independent experiments; ∗P < 0.05, ∗∗P < 0.01 compared with the control group; #P < 0.05, ##P < 0.01 compared with the OA group.
3.5. EGCG Attenuates HFD-Induced NAFLD in Mice
Compared with the mice fed with LFD for 10 weeks, HFD-fed mice exhibited increased body weight, indicating that an alimentary obesity model had been successfully established. In addition, compared to the LFD group, HFD-fed mice showed decreased food and water intakes, as well as increased weights of liver, white fat, and brown fat. Treatment with EGCG reversed these changes except for the food intake (Figures 6(a)–6(h)). Furthermore, HFD-fed mice exhibited increased levels of TC, TG, ALT, and AST when compared to the LFD group, which could be reversed by the treatment with EGCG (Figures 6(i)–6(l)). Moreover, HFD-fed mice showed upregulated levels of TC, TG, and NEFA in the liver when compared with the LFD group, which were reduced by the administration of EGCG (Figures 6(m)–6(o)). Compared to the LFD group, the HFD group exhibited a higher apoptotic index, as well as a lower proliferation index and autophagic index, which could be reversed by the administration of EGCG (Figures 7(a)–7(d)). These results indicate that EGCG can attenuate HFD-induced NAFLD in mice.
Figure 6.
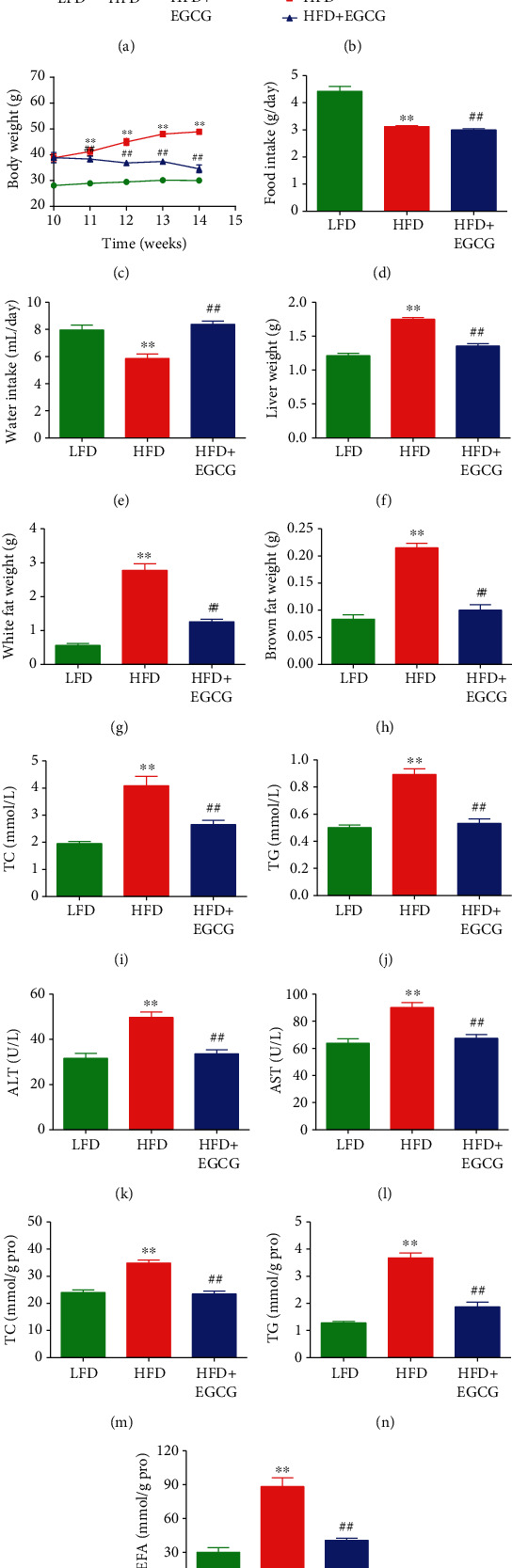
Effects of EGCG on HFD-induced NAFLD in mice. (a) Representative photographs of mice in each group. (b, c) The body weights of mice were measured. (d, e) Food intake and water intake were determined. (f–h) The liver weight, white fat weight, and brown fat weight were calculated. (i–l) The levels of TC, TG, ALT, and AST in the plasma of mice were detected. (m–o) The expression levels of TC, TG, and NEFA in the liver of mice were detected. Data are presented as mean ± SEM (n = 6). ∗P < 0.05, ∗∗P < 0.01 compared with the control group; ##P < 0.01 compared with the OA group.
Figure 7.
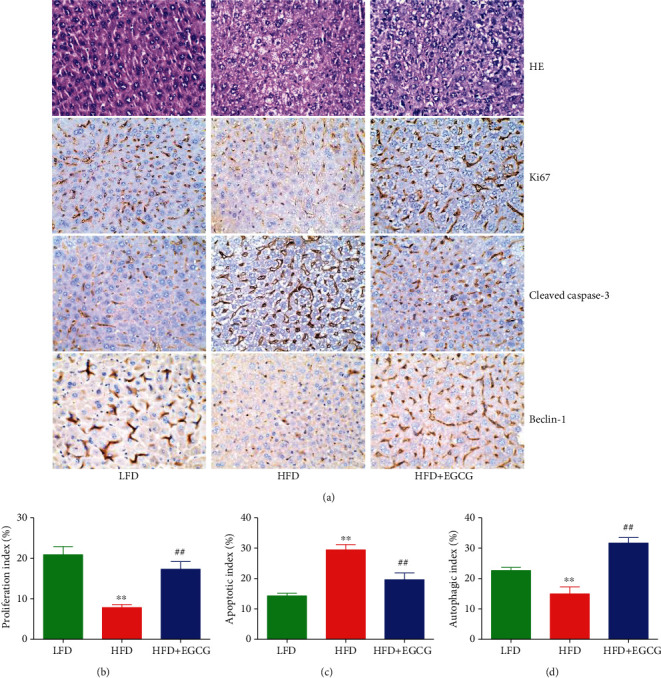
Effects of EGCG on the proliferation, apoptosis, and autophagy in liver tissues of the NAFLD mice. (a) Representative photographs of HE, Ki67, cleaved caspase-3, and beclin-1 staining in the liver of mice; original magnification ×400. (b–d) The proliferation index, apoptotic index, and autophagic index were calculated. Data are presented as mean ± SEM (n = 3). ∗∗P < 0.01 compared with the control group; ##P < 0.01 compared with the OA group.
4. Discussion
NAFLD, the most common chronic liver disease, leads to end-stage liver disease, liver transplantation, and hepatocellular carcinoma [14–16]. It has been shown that EGCG plays important biological and pharmacological roles in mammals. Nevertheless, the effect and mechanism of EGCG in the process of NAFLD are largely unknown. Human normal liver cells QSG-7701 and L02 have been widely adopted to investigate the mechanism of action of novel drugs and donors [42, 43]. OA, a monounsaturated fatty acid, has been successfully used in the establishment of the NAFLD model [44]. In this study, QSG-7701 and L02 cells were adopted to examine the effects of EGCG on NAFLD induced by OA in vitro. A recent study has revealed that epoxy stearic acid, a type of oxidative product from OA, can induce cytotoxicity and G0/G1 phase cell cycle arrest in HepG2 cells [45]. Our data indicated that OA decreased the viability and proliferation of liver cells and induced G1 phase cell cycle arrest. The changes could be reversed by the administration of EGCG. These data together indicate that EGCG acts as an effector molecule in promoting the growth of OA-treated liver cells.
Apoptosis is a conserved cell death pathway which can play key roles in the maintenance of organismal homeostasis and normal eukaryotic development [46, 47]. Two main apoptotic pathways have been identified in mammals: the mitochondrial-mediated intrinsic pathway and the death receptor-mediated extrinsic pathway [48]. Bcl-2 family proteins are involved in the regulation of apoptosis, such as Bax, Bad, Bcl-2, and Bcl-xl [49]. Many apoptotic stimuli can activate caspases, and PARP is activated by cleaved caspase-3, leading to the occurrence of apoptosis [31, 38, 49]. It has been reported that OA could induce apoptosis by increasing the levels of Bax and PARP but decreasing the level of Bcl-2 in HepG2 cells [50]. Another study shows that OA can promote the expressions of both cleaved caspase-3 and PARP1 [51]. Similarly, our data indicated that OA induced the early and late apoptosis, as well as increased the ratios of both Bax/Bcl-2 and Bad/Bcl-xl and the expressions of cleaved caspase-3/9 and cleaved PARP in liver cells. EGCG significantly reduced the apoptotic levels in the OA group. The data suggest that the apoptotic levels are increased in OA-treated liver cells and treatment with EGCG could reduce apoptosis.
Autophagy, an evolutionarily conserved catabolic pathway, serves to deliver cytoplasmic materials to lysosomes for recycling and degradation, leading to macromolecular synthesis and energy production [36, 52]. Autophagy is activated by many environmental factors, including cytokines, hormones, and nutrients [53]. Recent studies have indicated that autophagy is impaired in lipid-overloaded hepatocytes and in the liver from the NAFLD murine model and NAFLD patients [54–56]. In line with the above studies, we observed that the autophagic levels were decreased in OA-treated liver cells. Another study has reported that EGCG can increase the autophagic level by increasing lysosomal acidification and stimulating autophagic flux in liver cells and the mouse liver [57]. Our data showed that EGCG could increase autophagy in OA-treated liver cells, indicating that autophagic activation can serve as a potential therapeutic target for NAFLD.
It has been shown that low concentrations of intracellular ROS are necessary for many physiological roles including signal transduction and cell proliferation. Nevertheless, ROS overproduction can induce oxidative stress and cellular redox imbalance, thus ultimately affecting many cellular functions [41, 58]. Our data indicated that OA increased ROS levels and decreased the activities of GSH-Px, SOD, and CAT, which were consistent with the results of a previous study [59]. The effects were significantly reversed by the treatment with EGCG. In mammalian cells, three major types of MAP kinases are present: ERK, p38, and JNK, which are associated with EGCG interaction in the MAPK pathway [60, 61]. MAPK cascades play key roles in the progression of NAFLD, and elevation of ROS activates the MAPK pathway [38, 41, 62]. It has been revealed that intraperitoneal administration of EGCG (5 mg/kg) for 14 days inhibits phosphorylation of ERK, JNK, and p38 in animals with artificial unilateral ureteral obstruction [63]. Another study indicates that MAPK and hypoxia-inducible factor-1α are decreased after the treatment with EGCG, suggesting that EGCG could suppress MAPK-related oxidative stress [64]. A recent study has shown that EGCG can increase the expression levels of antioxidant enzymes, reverse the increase of ROS production, and regulate mitochondrial-involved autophagy [65]. Furthermore, EGCG could prevent αTC1-6 cells from H2O2-induced ROS production via the activation of Akt signaling and suppression of the p38 and JNK pathway [66]. In this study, OA upregulated the expression levels of p-JNK and p-p38 but downregulated the levels of p-ERK1/2. However, administration of EGCG remarkably reversed the levels of the proteins. Furthermore, the ROS/MAPK pathway is an important signaling cascade which can mediate the processes of apoptosis and autophagy in mammalian cells [67, 68]. In sum, the data suggest that EGCG can reduce apoptosis and induce autophagy possibly through the ROS/MAPK pathway in OA-treated liver cells.
In this study, a mouse model of HFD-induced NAFLD was used to imitate unhealthy dietary habits. Our results suggested that HFD feeding induced obvious increases in body weight, liver weight, white fat weight, and brown fat weight, as well as the levels of TC, TG, ALT, and AST in the plasma of mice, indicating the successful establishment of the NAFLD model. It has been reported that NAFLD patients tend to have higher TC and TG levels [69]. EGCG markedly decreased the levels of TG and TC in both the plasma and the liver. Furthermore, ALT and AST are important indicators of liver damage in NAFLD [70]. Administration of EGCG significantly alleviated liver damage in HFD-fed mice by reducing ALT and AST levels. The flux of NEFA can be delivered to hepatocytes for TG synthesis, resulting in the development of NAFLD [71]. Treatment with EGCG decreased the level of NEFA in the liver of HFD-fed mice. Moreover, EGCG can increase the proliferation and autophagy but decrease the apoptosis in the liver of HFD-fed mice. The data together indicate that EGCG might alleviate HFD-induced NAFLD by inhibiting apoptosis and promoting autophagy.
In summary, our data suggest that EGCG can alleviate HFD-induced NAFLD through inhibition of apoptosis and promotion of autophagy possibly via the ROS/MAPK pathway. EGCG could be developed as an effective agent for the treatment of NAFLD.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (Nos. 81802718, U1504817, and 81870591), the Foundation of Science & Technology Department of Henan Province, China (Nos. 202102310480, 182102310335, and 192102310151), the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038), and the Science Foundation for Young Talents of Henan University College of Medicine, China (No. 2019013).
Abbreviations
- EGCG:
Epigallocatechin-3-gallate
- NAFLD:
Nonalcoholic fatty liver disease
- HFD:
High-fat diet
- OA:
Oleic acid
- DMEM:
Dulbecco's modified Eagle's medium
- FBS:
Fetal bovine serum
- BSA:
Bovine serum albumin
- ORO:
Oil red O
- EdU:
5-Ethynyl-2′-deoxyuridine
- CCK-8:
Cell counting kit-8
- PI:
Propidium iodide
- GFP:
Green fluorescent protein
- RFP:
Red fluorescent protein
- MAP1LC3/LC3:
Microtubule-associated protein 1 light chain 3
- MDC:
Monodansylcadaverine
- ROS:
Reactive oxygen species
- SOD:
Superoxide dismutase
- CAT:
Catalase
- GSH-Px:
Glutathione peroxidase
- CDK:
Cyclin-dependent kinase
- ERK1/2:
Extracellular signal-regulated protein kinase 1/2
- p:
Phospho
- JNK:
Jun N-terminal kinase
- CST:
Cell Signaling Technology
- Bcl-2:
B-cell lymphoma-2
- Bax:
Bcl-2-associated X protein
- Bcl-xl:
B-cell lymphoma-extra large
- Bad:
Bcl-xl/Bcl-2-associated death promoter
- PARP:
Poly-ADP-ribose polymerase
- LFD:
Low-fat diet
- TG:
Triglyceride
- TC:
Total cholesterol
- ALT:
Alanine aminotransferase
- AST:
Aspartate aminotransferase
- NEFA:
Nonesterified fatty acid
- HE:
Hematoxylin and eosin
- IHC:
Immunohistochemistry
- MAPK:
Mitogen-activated protein kinase.
Contributor Information
Zhongwen Xie, Email: zhongwenxie@ahau.edu.cn.
Ailing Ji, Email: ailingji@163.com.
Yanzhang Li, Email: yanzhang206@163.com.
Data Availability
All data generated or analyzed in this study are included in this published article.
Ethical Approval
The animal experiment was approved by the Committee of Medical Ethics and Welfare for Experimental Animals of Henan University School of Medicine (HUSOM-2017-208).
Conflicts of Interest
The authors declare that there is no conflict of interests.
Authors' Contributions
D. W., Z. X., A. J., and Y. L. conceived and designed the experiments; Z. L., Y. W., Q. Z., J. L., and P. Z. performed the experiments and prepared the figures; and D. W. wrote the main manuscript text. All authors reviewed and approved the manuscript.
References
- 1.Wang D., Wei Y., Wang T., et al. Melatonin attenuates (-)-epigallocatehin-3-gallate-triggered hepatotoxicity without compromising its downregulation of hepatic gluconeogenic and lipogenic genes in mice. Journal of Pineal Research. 2015;59(4):497–507. doi: 10.1111/jpi.12281. [DOI] [PubMed] [Google Scholar]
- 2.Gan R. Y., Li H. B., Sui Z. Q., Corke H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): an updated review. Critical Reviews in Food Science and Nutrition. 2018;58(6):924–941. doi: 10.1080/10408398.2016.1231168. [DOI] [PubMed] [Google Scholar]
- 3.Kim H. S., Quon M. J., Kim J. A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biology. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinmann J., Buer J., Pietschmann T., Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. British Journal of Pharmacology. 2013;168(5):1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang K., Chung J. E., Gao S. J., Yongvongsoontorn N., Kurisawa M. Highly augmented drug loading and stability of micellar nanocomplexes composed of doxorubicin and poly(ethylene glycol)-green tea catechin conjugate for cancer therapy. Advanced Materials. 2018;30(14, article e1706963) doi: 10.1002/adma.201706963. [DOI] [PubMed] [Google Scholar]
- 6.Liang K., Bae K. H., Lee F., et al. Self-assembled ternary complexes stabilized with hyaluronic acid-green tea catechin conjugates for targeted gene delivery. Journal of Controlled Release. 2016;226:205–216. doi: 10.1016/j.jconrel.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Hyung S. J., DeToma A. S., Brender J. R., et al. Insights into antiamyloidogenic properties of the green tea extract (-)-epigallocatechin-3-gallate toward metal-associated amyloid-β species. Proceedings of the National Academy of Sciences. 2013;110(10):3743–3748. doi: 10.1073/pnas.1220326110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chikara S., Nagaprashantha L. D., Singhal J., Horne D., Awasthi S., Singhal S. S. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Letters. 2018;413:122–134. doi: 10.1016/j.canlet.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Kanlaya R., Thongboonkerd V. Protective effects of epigallocatechin-3-gallate from green tea in various kidney diseases. Advances in Nutrition. 2019;10(1):112–121. doi: 10.1093/advances/nmy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung J. E., Tan S., Gao S. J., et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nature Nanotechnology. 2014;9(11):907–912. doi: 10.1038/nnano.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong L. G., Chen Y. J., Tong J. W., Gong Y. S., Huang J. A., Liu Z. H. Epigallocatechin-3-gallate promotes healthy lifespan through mitohormesis during early-to-mid adulthood in Caenorhabditis elegans. Redox Biology. 2018;14:305–315. doi: 10.1016/j.redox.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z. S., Cai H., Xue W., et al. G3BP1 promotes DNA binding and activation of cGAS. Nature Immunology. 2019;20(1):18–28. doi: 10.1038/s41590-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calland N., Albecka A., Belouzard S., et al. (-)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology. 2012;55(3):720–729. doi: 10.1002/hep.24803. [DOI] [PubMed] [Google Scholar]
- 14.Targher G., Byrne C. D. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nature Reviews. Nephrology. 2017;13(5):297–310. doi: 10.1038/nrneph.2017.16. [DOI] [PubMed] [Google Scholar]
- 15.Vilar-Gomez E., Calzadilla-Bertot L., Wai-Sun Wong V., et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443–457.e17. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Younossi Z. M. Non-alcoholic fatty liver disease - a global public health perspective. Journal of Hepatology. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 17.Kuzu N., Bahcecioglu I. H., Dagli A. F., Ozercan I. H., Ustündag B., Sahin K. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. Journal of Gastroenterology and Hepatology. 2008;23(8):e465–e470. doi: 10.1111/j.1440-1746.2007.05052.x. [DOI] [PubMed] [Google Scholar]
- 18.Gan L., Meng Z. J., Xiong R. B., et al. Green tea polyphenol epigallocatechin-3-gallate ameliorates insulin resistance in non-alcoholic fatty liver disease mice. Acta Pharmacologica Sinica. 2015;36(5):597–605. doi: 10.1038/aps.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Y., Sun X., Chen Y., Deng Y., Qian K. Epigallocatechin gallate attenuated non-alcoholic steatohepatitis induced by methionine- and choline-deficient diet. European Journal of Pharmacology. 2015;761:405–412. doi: 10.1016/j.ejphar.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Vidyashankar S., Sandeep Varma R., Patki P. S. Quercetin ameliorate insulin resistance and up-regulates cellular antioxidants during oleic acid induced hepatic steatosis in HepG2 cells. Toxicology In Vitro. 2013;27(2):945–953. doi: 10.1016/j.tiv.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Qiu T., Pei P., Yao X., et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death & Disease. 2018;9(10):p. 946. doi: 10.1038/s41419-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu D., Li J., Zhang Q., et al. Exogenous hydrogen sulfide regulates the growth of human thyroid carcinoma cells. Oxidative Medicine and Cellular Longevity. 2019;2019(6927298):18. doi: 10.1155/2019/6927298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai Y., Lin P., Feng Z., et al. TNFAIP3-DEPTOR complex regulates inflammasome secretion through autophagy in ankylosing spondylitis monocytes. Autophagy. 2018;14(9):1629–1643. doi: 10.1080/15548627.2018.1458804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Nie H., Zhao X., Qin Y., Gong X. Bicyclol induces cell cycle arrest and autophagy in HepG2 human hepatocellular carcinoma cells through the PI3K/AKT and Ras/Raf/MEK/ERK pathways. BMC Cancer. 2016;16(1):p. 742. doi: 10.1186/s12885-016-2767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan Y., Ding D., Zhang N., et al. TNF-α induces autophagy through ERK1/2 pathway to regulate apoptosis in neonatal necrotizing enterocolitis model cells IEC-6. Cell Cycle. 2018;17(11):1390–1402. doi: 10.1080/15384101.2018.1482150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y., Li S., Sun L., et al. Fungal immunomodulatory protein from Nectria haematococca suppresses growth of human lung adenocarcinoma by inhibiting the PI3K/Akt pathway. International Journal of Molecular Sciences. 2018;19(11):p. 3429. doi: 10.3390/ijms19113429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Q., Eun J. W., Lee K., et al. Barrier to autointegration factor 1, procollagen-lysine, 2-oxoglutarate 5-dioxygenase 3, and splicing factor 3b subunit 4 as early-stage cancer decision markers and drivers of hepatocellular carcinoma. Hepatology. 2018;67(4):1360–1377. doi: 10.1002/hep.29606. [DOI] [PubMed] [Google Scholar]
- 28.Pitchakarn P., Suzuki S., Ogawa K., et al. Induction of G1 arrest and apoptosis in androgen-dependent human prostate cancer by Kuguacin J, a triterpenoid from Momordica charantia leaf. Cancer Letters. 2011;306(2):142–150. doi: 10.1016/j.canlet.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 29.Chen M., Wang X., Zha D., et al. Apigenin potentiates TRAIL therapy of non-small cell lung cancer _via_ upregulating DR4/DR5 expression in a p53-dependent manner. Scientific Reports. 2016;6(1, article 35468) doi: 10.1038/srep35468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Man S. M., Kanneganti T. D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nature Reviews. Immunology. 2016;16(1):7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu D., Tian W., Li J., et al. Peptide P11 suppresses the growth of human thyroid carcinoma by inhibiting the PI3K/AKT/mTOR signaling pathway. Molecular Biology Reports. 2019;46(3):2665–2678. doi: 10.1007/s11033-019-04698-7. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Zhu R., Chen K., et al. Potent and specific Atg8-targeting autophagy inhibitory peptides from giant ankyrins. Nature Chemical Biology. 2018;14(8):778–787. doi: 10.1038/s41589-018-0082-8. [DOI] [PubMed] [Google Scholar]
- 33.Galluzzi L., Baehrecke E. H., Ballabio A., et al. Molecular definitions of autophagy and related processes. The EMBO Journal. 2017;36(13):1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lépine S., Allegood J. C., Park M., Dent P., Milstien S., Spiegel S. Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death and Differentiation. 2011;18(2):350–361. doi: 10.1038/cdd.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klionsky D. J., Abdelmohsen K., Abe A., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D. Y., Hong Y., Chen Y. G., et al. PEST-containing nuclear protein regulates cell proliferation, migration, and invasion in lung adenocarcinoma. Oncogene. 2019;8(3):p. 22. doi: 10.1038/s41389-019-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nuvoli B., Camera E., Mastrofrancesco A., Briganti S., Galati R. Modulation of reactive oxygen species via ERK and STAT3 dependent signalling are involved in the response of mesothelioma cells to exemestane. Free Radical Biology & Medicine. 2018;115:266–277. doi: 10.1016/j.freeradbiomed.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Zhang G. Y., Lu D., Duan S. F., et al. Hydrogen sulfide alleviates lipopolysaccharide-induced diaphragm dysfunction in rats by reducing apoptosis and inflammation through ROS/MAPK and TLR4/NF-κB signaling pathways. Oxidative Medicine and Cellular Longevity. 2018;2018(9647809):15. doi: 10.1155/2018/9647809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C. Y., Hao H. S., Zhao Y. H., et al. Melatonin improves the fertilization capacity of sex-sorted bull sperm by inhibiting apoptosis and increasing fertilization capacitation via MT1. International Journal of Molecular Sciences. 2019;20(16):p. 3921. doi: 10.3390/ijms20163921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z., Ren Z., Chen S., et al. ROS generation and JNK activation contribute to 4-methoxy-TEMPO-induced cytotoxicity, autophagy, and DNA damage in HepG2 cells. Archives of Toxicology. 2018;92(2):717–728. doi: 10.1007/s00204-017-2084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu D., Luo N., Wang L., et al. Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κB signaling pathways. Scientific Reports. 2017;7(1):p. 455. doi: 10.1038/s41598-017-00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z., Wang J., Guo C., Fan X. MicroRNA-21 mediates epithelial-mesenchymal transition of human hepatocytes via PTEN/Akt pathway. Biomedicine & Pharmacotherapy. 2015;69:24–28. doi: 10.1016/j.biopha.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 43.Wu D., Li M., Tian W., et al. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Scientific Reports. 2017;7(1):p. 5134. doi: 10.1038/s41598-017-05457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu Q., Zhang S., Chen M., et al. Cherry anthocyanins regulate NAFLD by promoting autophagy pathway. Oxidative Medicine and Cellular Longevity. 2019;2019(4825949):16. doi: 10.1155/2019/4825949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y., Cheng Y., Li J., Wang Y., Liu Y. Epoxy stearic acid, an oxidative product derived from oleic acid, induces cytotoxicity, oxidative stress, and apoptosis in HepG2 cells. Journal of Agricultural and Food Chemistry. 2018;66(20):5237–5246. doi: 10.1021/acs.jafc.8b01954. [DOI] [PubMed] [Google Scholar]
- 46.Singh R., Letai A., Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nature Reviews. Molecular Cell Biology. 2019;20(3):175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu D., Si W., Wang M., Lv S., Ji A., Li Y. Hydrogen sulfide in cancer: friend or foe? Nitric Oxide. 2015;50:38–45. doi: 10.1016/j.niox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Wu D., Gao Y., Qi Y., Chen L., Ma Y., Li Y. Peptide-based cancer therapy: opportunity and challenge. Cancer Letters. 2014;351(1):13–22. doi: 10.1016/j.canlet.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Dong Q., Yang B., Han J. G., et al. A novel hydrogen sulfide-releasing donor, HA-ADT, suppresses the growth of human breast cancer cells through inhibiting the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways. Cancer Letters. 2019;455:60–72. doi: 10.1016/j.canlet.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 50.Pang L., Liu K., Liu D., et al. Differential effects of reticulophagy and mitophagy on nonalcoholic fatty liver disease. Cell Death & Disease. 2018;9(2):p. 90. doi: 10.1038/s41419-017-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel T. P., Rawal K., Soni S., Gupta S. Swertiamarin ameliorates oleic acid induced lipid accumulation and oxidative stress by attenuating gluconeogenesis and lipogenesis in hepatic steatosis. Biomedicine & Pharmacotherapy. 2016;83:785–791. doi: 10.1016/j.biopha.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 52.Wu D., Wang H., Teng T., Duan S., Ji A., Li Y. Hydrogen sulfide and autophagy: a double edged sword. Pharmacological Research. 2018;131:120–127. doi: 10.1016/j.phrs.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Clarke A. J., Simon A. K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nature Reviews. Immunology. 2019;19(3):170–183. doi: 10.1038/s41577-018-0095-2. [DOI] [PubMed] [Google Scholar]
- 54.González-Rodríguez A., Mayoral R., Agra N., et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death & Disease. 2014;5(4, article e1179) doi: 10.1038/cddis.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L., Yao Z., Ji G. Herbal extracts and natural products in alleviating non-alcoholic fatty liver disease via activating autophagy. Frontiers in Pharmacology. 2018;9:p. 1459. doi: 10.3389/fphar.2018.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueno T., Komatsu M. Autophagy in the liver: functions in health and disease. Nature Reviews. Gastroenterology & Hepatology. 2017;14(3):170–184. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J., Farah B. L., Sinha R. A., et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS One. 2014;9(1, article e87161) doi: 10.1371/journal.pone.0087161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui H., Wu S., Shang Y., et al. Pleurotus nebrodensis polysaccharide (PN50G) evokes A549 cell apoptosis by the ROS/AMPK/PI3K/AKT/mTOR pathway to suppress tumor growth. Food & Function. 2016;7(3):1616–1627. doi: 10.1039/C6FO00027D. [DOI] [PubMed] [Google Scholar]
- 59.Su H., Li Y., Hu D., et al. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free Radical Biology & Medicine. 2018;126:269–286. doi: 10.1016/j.freeradbiomed.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 60.Arthur J. S., Ley S. C. Mitogen-activated protein kinases in innate immunity. Nature Reviews Immunology. 2013;13(9):679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 61.Sharifi-Rad M., Pezzani R., Redaelli M., et al. Preclinical pharmacological activities of epigallocatechin-3-gallate in signaling pathways: an update on cancer. Molecules. 2020;25(3):p. 467. doi: 10.3390/molecules25030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen X., Guo H., Xu J., Wang J. Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. Journal of Cellular Physiology. 2019;234(10):18169–18179. doi: 10.1002/jcp.28450. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Liu N., Bian X., et al. Epigallocatechin-3-gallate reduces tubular cell apoptosis in mice with ureteral obstruction. The Journal of Surgical Research. 2015;197(1):145–154. doi: 10.1016/j.jss.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 64.Chen J., Xu J., Li J., et al. Epigallocatechin-3-gallate attenuates lipopolysaccharide-induced mastitis in rats via suppressing MAPK mediated inflammatory responses and oxidative stress. International Immunopharmacology. 2015;26(1):147–152. doi: 10.1016/j.intimp.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 65.Casanova E., Salvadó J., Crescenti A., Gibert-Ramos A. Epigallocatechin gallate modulates muscle homeostasis in type 2 diabetes and obesity by targeting energetic and redox pathways: a narrative review. International Journal of Molecular Sciences. 2019;20(3):p. 532. doi: 10.3390/ijms20030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao T., Zhang X., Yang D., et al. Antioxidant effects of epigallocatechin-3-gallate on the aTC1-6 pancreatic alpha cell line. Biochemical and Biophysical Research Communications. 2018;495(1):693–699. doi: 10.1016/j.bbrc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Wang K., Chen B., Yin T., et al. N-Methylparoxetine blocked autophagic flux and induced apoptosis by activating ROS-MAPK pathway in non-small cell lung cancer cells. International Journal of Molecular Sciences. 2019;20(14):p. 3415. doi: 10.3390/ijms20143415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y., Fan D. Ginsenoside Rg5 induces G2/M phase arrest, apoptosis and autophagy via regulating ROS-mediated MAPK pathways against human gastric cancer. Biochemical Pharmacology. 2019;168:285–304. doi: 10.1016/j.bcp.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 69.Zheng X., Gong L., Luo R., et al. Serum uric acid and non-alcoholic fatty liver disease in non-obesity Chinese adults. Lipids in Health and Disease. 2017;16(1):p. 202. doi: 10.1186/s12944-017-0531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lazo M., Rubin J., Clark J. M., et al. The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease. Journal of Hepatology. 2015;62(4):841–847. doi: 10.1016/j.jhep.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeong H. S., Kim K. H., Lee I. S., et al. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomedicine & Pharmacotherapy. 2017;88:625–634. doi: 10.1016/j.biopha.2017.01.114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are included in this published article.


