Abstract
Myelin, multilayered lipid-rich membrane extensions formed by oligodendrocytes around neuronal axons, is essential for fast and efficient action potential propagation in the central nervous system. Initially thought to be a static and immutable process, myelination is now appreciated to be a dynamic process capable of responding to and modulating neuronal function throughout life. While the importance of this type of plasticity, called adaptive myelination, is now well-accepted, we are only beginning to understand the underlying cellular and molecular mechanisms by which neurons communicate experience-driven circuit activation to oligodendroglia and precisely how changes in oligodendrocytes and their myelin refine neuronal function. Here we review recent findings addressing this reciprocal relationship in which neurons alter oligodendroglial form and oligodendrocytes conversely modulate neuronal function.
Keywords: oligodendrocyte, myelin, experience, adaptive myelination, plasticity, neuronal activity, cell-cell interactions
In Brief
Adaptive myelination is a form of neuroplasticity in which external experience alters oligodendrocyte myelination. Pease-Raissi and Chan review mechanisms by which neurons communicate experiential input to alter oligodendrocytes, and how subsequent changes in oligodendrocytes and their myelin modulate neuronal function.
Introduction to myelin plasticity
A fundamental property of the central nervous system (CNS) is its ability to alter its structure and function in response to external life experience, thereby enabling learning and memory and sensory-based circuit refinement. However, the effects and effectors of CNS plasticity are not restricted to neurons alone. External experience also modulates non-neuronal cells such as glial cells in order to further fine-tune circuit function. An emerging field of neuroplasticity is that of experience-dependent regulation of oligodendrocytes, the myelin-forming glial cells of the CNS. Accumulating evidence has identified experience-driven oligodendrogenesis and myelination, called adaptive myelination, as a significant and lifelong form of CNS plasticity that can impact diverse aspects of neuronal function.
Oligodendrocytes are glial cells that produce myelin sheaths to facilitate rapid action potential propagation by reducing membrane capacitance and clustering voltage-gated ion channels into discrete domains, enabling saltatory conduction. A large part of the myelination process is intrinsic to the oligodendroglial cell, as oligodendroglia will differentiate and form myelin even in the complete absence of neuronal signals, as long as the structure to be myelinated is above a minimum threshold diameter (Bechler et al., 2015; Lee et al., 2012a; Mayoral et al., 2018). Despite this, not all suprathreshold axons are myelinated in the mature CNS. Instead, the presence of nonmyelinated, suprathreshold axons and of discontinuously myelinated axons (Hill et al., 2018; Tomassy et al., 2014) suggests the possible presence of environmental cues that refine the innate myelination program as well as a wide dynamic range for potential addition of new myelin and/or modification of preexisting myelin in adulthood. Indeed, numerous studies now show that myelin continues to increase and new oligodendrocytes continue to be generated throughout the life of an organism, even in nearly fully myelinated tracts (Young et al., 2013). Because oligodendrocytes are highly stable, with most surviving into and probably beyond old age in the mouse (Tripathi et al., 2017), it is likely that most adult-born oligodendrocytes serve to modulate existing patterns and degrees of myelination later in life (Hughes et al., 2018). Furthermore, many myelin sheaths exhibit longitudinal lengthening or shortening events even in adult animals (Bacmeister et al., 2020; Hill et al., 2018; Yang et al., 2020), suggesting a lifelong capacity for fine-tuning spatial parameters of preexisting or newly formed myelin sheaths.
Given the intimate relationship between axons and myelin, it has long been hypothesized that neurons would instruct their own myelination. Adaptive myelination is a change in any spatiotemporal parameter of myelin or oligodendrocytes in response to external experience, which can occur throughout life. As myelination occurs in the absence of non-cell autonomous cues, experience-dependent cues are not required for myelination but instead modulate the parameters of myelin established by the innate myelination program. Numerous studies using optogenetic, pharmacogenetic, electrical, and pharmacological manipulations to artificially drive neuronal activity have demonstrated neuronal influence on oligodendrocyte lineage progression and/or myelin (Barres and Raff, 1993; Gautier et al., 2015; Geraghty et al., 2019; Gibson et al., 2014; Hines et al., 2015; Li et al., 2010; Marisca et al., 2020; Mensch et al., 2015; Mitew et al., 2018; Ortiz et al., 2019; Stedehouder et al., 2018; Wake et al., 2010, 2014). Furthermore, studies manipulating experiential input demonstrate physiologically relevant changes in oligodendrocyte lineage progression and/or myelin. For example, paradigms involving enrichment or deprivation of sensory or social input, such as social isolation, whisker stimulation, or monocular visual deprivation, have been shown to variously alter proliferation, differentiation, survival, and/or myelination by oligodendrocyte lineage cells (Bacmeister et al., 2020; Etxeberria et al., 2016; Fukui et al., 1991; Gyllensten and Malmfors, 1963; Hughes et al., 2018; Kato et al., 2020; Liu et al., 2012; Makinodan et al., 2012; Osanai et al., 2018; Sinclair et al., 2017; Swire et al., 2019; Tauber et al., 1980; Yang et al., 2013; Yang et al., 2020; Zhao et al., 2011). The importance of experience-dependent myelination is particularly evidenced by studies showing that increased oligodendrogenesis evoked by motor, spatial, and fear conditioning learning paradigms is necessary for proper learning and memory (McKenzie et al., 2014; Pan et al., 2020; Steadman et al., 2020; Wang et al., 2020; Xiao et al., 2016; Xin and Chan, 2020; Zheng et al., 2019; Zhou et al., 2020). Altogether these studies convincingly demonstrate the capacity for experiential input and neuronal activity to shape oligodendroglia and myelin, but the precise cellular and molecular events underlying this plasticity are less well-understood.
In this review we will address recent advances on the causes and effects of adaptive myelination, breaking this process down into its individual steps with particular attention to the reciprocal modulation between neurons and oligodendroglia. First, we will discuss which aspects of oligodendrocytes and myelin are altered in adaptive myelination, then precisely how these changes alter neuronal function. We will then address how a neuron directly or indirectly induces adaptive myelination, and what downstream molecular mechanisms are engaged within oligodendrocyte lineage cells to enact this plasticity. Given the wide range of effects seen across neuronal subtypes, CNS regions, ages, and experimental paradigms, adaptive myelination appears to encompass multiple and temporally-evolving modes of experience-driven changes to oligodendroglial lineage cells, with equally diverse implications for modulating neuronal function.
How are oligodendrocytes altered in adaptive myelination?
Starting during development, oligodendrocytes terminally differentiate from proliferative oligodendrocyte precursor cells (OPCs) and form myelin. Each step in the myelination process, from OPC proliferation and oligodendrocyte generation to initiation, growth, and maintenance of myelin, represents an inflection point at which experience can modify oligodendroglial cells and thus directly or indirectly modulate any spatial parameter of myelin (Figure 1). Experiential input can therefore impact myelin in two non-exclusive ways: 1) a primary effect on progression of the oligodendrocyte lineage that secondarily affects myelin and/or 2) a primary effect on oligodendrocytes to directly alter myelin.
Figure 1. Experience- and activity-induced changes to oligodendrocyte lineage cells and myelin.
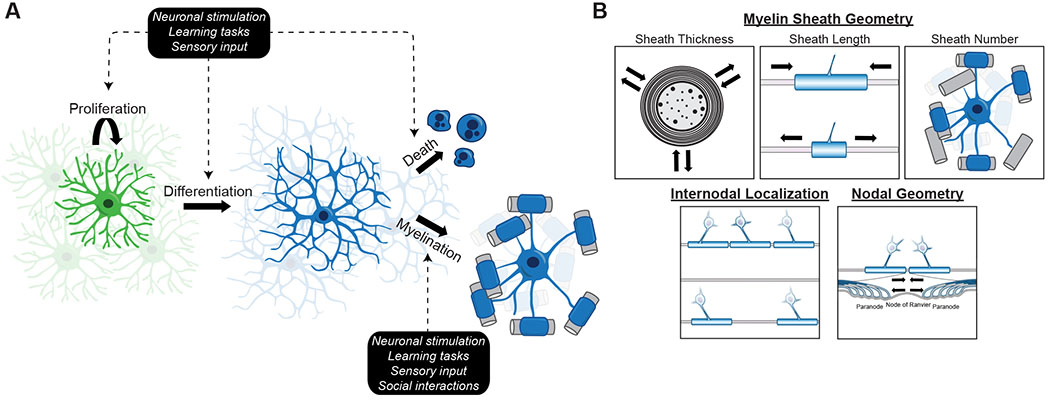
A) Enhanced or reduced experiential input or neuronal activity can directly alter any stage of oligodendrocyte lineage progression, including OPC (green) proliferation, OPC differentiation, or death of newly generated oligodendrocytes (blue). Experiential input or neuronal activity can also directly alter myelination by newly generated or preexisting oligodendrocytes by B) altering internodal localization, nodal geometry, or various aspects of myelin sheath geometry.
Experience-dependent regulation of oligodendrocyte lineage progression
Experience-dependent cues may directly regulate the number of new oligodendrocytes in order to indirectly induce changes in myelin. Multiple paradigms that directly manipulate neuronal activity have been shown to first induce an increase in proliferating OPCs and then a later increase in oligodendrocytes, including optogenetic stimulation of the mouse premotor cortex (Geraghty et al., 2019; Gibson et al., 2014), pharmacogenetic stimulation of the mouse somatosensory cortex (Mitew et al., 2018), and pharmacological stimulation of the developing zebrafish spinal cord (Marisca et al., 2020). Enhanced experiential input has also been shown to increase OPC proliferation and generation of new oligodendrocytes (Bacmeister et al., 2020; Hughes et al., 2018; McKenzie et al., 2014; Pan et al., 2020; Steadman et al., 2020; Xiao et al., 2016). However, impaired neuronal activity or sensory deprivation have been reported to have more variable effects on oligodendrocyte cell numbers (Barres and Raff, 1993; Etxeberria et al., 2016; Hill et al., 2014, 2018; Hines et al., 2015; Mayoral et al., 2018; Mensch et al., 2015).
A series of seminal studies by the Richardson lab was the first to demonstrate causality between experience-induced oligodendrogenesis and behavioral outcomes. A motor learning task where a mouse learns to run on a wheel with irregularly-spaced rungs resulted in first a rapid increase in new oligodendrocytes within several hours of training (Xiao et al., 2016), then an increase in OPC proliferation within several days of training, and finally a second wave of new oligodendrocytes generated from these proliferative OPCs after several weeks of training (McKenzie et al., 2014). Oligodendrocyte generation was necessary for motor learning, as genetically preventing new oligodendrocyte generation (Emery et al., 2009) impaired both early (Xiao et al., 2016) and late (McKenzie et al., 2014) phases of learning. Similarly, both spatial learning and contextual fear conditioning induce OPC proliferation, new oligodendrocyte formation, and increased myelination, and genetically blocking new oligodendrocyte formation impairs learning and memory in both paradigms (Pan et al., 2020; Steadman et al., 2020; Wang et al., 2020). Remarkably, enhancing oligodendrogenesis improves learning and memory in both paradigms (Pan et al., 2020; Wang et al., 2020). These studies demonstrate that oligodendrogenesis is both necessary and sufficient for behavioral outcomes of multiple complex experiential paradigms.
However, it does not appear to be the case that enhanced experiential input or elevated neuronal activity always increases the number of oligodendrocytes. The nuanced effect of altering experiential input is particularly evident in a study by Bacmeister et al (2020), where they used in vivo imaging to show that learning a forelimb motor task initially suppressed and then later increased the rate of oligodendrocyte generation. This suggests motor learning and therefore perhaps other types of experiential input can bidirectionally control the rate of oligodendrocyte generation, and that perhaps there is a physiological role for differentially altering oligodendrocyte cell numbers at different phases of learning.
Altered differentiation from OPCs into oligodendrocytes is a primary route for controlling oligodendrocyte numbers during development and in adulthood, and so is thought to be the major cause of changes to oligodendrocyte numbers in many adaptive myelination studies. This is supported most directly by in vivo imaging studies that longitudinally follow the fate of individual OPCs, such as in zebrafish (Marisca et al., 2020) or mice (Bacmeister et al., 2020). However, it is intriguing that so many studies observe a change in proliferation as well as differentiation in response to altered neuronal signaling or experiential input, as proliferation (undergoing mitosis) and differentiation (becoming post-mitotic oligodendrocytes) are in fact opposing processes, and asymmetric division to generate one OPC and one oligodendrocyte accounts for only a small proportion of oligodendrocyte generation (Bacmeister et al., 2020; Hughes et al., 2013). How then does a change in proliferation relate to a change in differentiation? One possibility is that elevated proliferation precedes elevated differentiation, and that experience-induced increased proliferation itself either indirectly triggers differentiation of OPCs such as through increased spatial constraints (Calver et al., 1998; Mayoral and Chan, 2016; Rosenberg et al., 2008), or else differentiation is independently promoted by additional experience-dependent signals. Either of these could be the case in studies in which elevated OPC proliferation precedes elevated differentiation by several weeks and the newly generated oligodendrocytes were shown to be derived from OPCs that had recently undergone cell division (e.g. Gibson et al., 2014; Pan et al., 2020). An alternative but non-exclusive possibility is that elevated differentiation instead precedes elevated proliferation. In this case, depletion of OPCs by differentiation would trigger proliferation through homeostatic mechanisms, as has been shown to occur under basal conditions in the adult mammalian brain (Hughes et al., 2013). This appears to be the case in the initial phase of learning to run on a complex wheel task, where training initially causes rapid direct differentiation of OPCs into immature oligodendrocytes (Xiao et al., 2016). Application of methods to inducibly impair OPC proliferation without directly affecting differentiation could provide great insight into whether proliferation is necessary for experience-induced generation of new oligodendrocytes and subsequent behavioral outcomes.
In addition to altering differentiation, a possible alternative route to generating more oligodendrocytes is promoting survival or inhibiting death of immature, premyelinating oligodendrocytes. Several studies suggest that during both development and adulthood a certain percentage of early stage oligodendrocytes undergo apoptosis rather than maturing and forming myelin (Barres et al., 1992; Calver et al., 1998; Hughes et al., 2018; Raff et al., 1993; Sun et al., 2018; Trapp et al., 1997; Tripathi et al., 2017). Immature oligodendrocyte survival has been proposed to be reduced by somatosensory deprivation (Hill et al., 2014), by impaired neuron to OPC glutamatergic signaling (Kougioumtzidou et al., 2017), and during initial learning-mediated suppression of oligodendrocyte generation (Bacmeister et al., 2020). Further studies are required to conclusively determine whether experience and subsequent neuronal activity do in fact alter oligodendrocyte survival, in addition to controlling the rate of OPC differentiation, as a means of altering the number of mature myelinating oligodendrocytes.
Experience-dependent regulation of oligodendrocytes to directly shape myelin
Experience-dependent cues may also modulate myelination by directly acting on either newly formed or preexisting oligodendrocytes and regulating processes such as myelin sheath addition, removal, growth, or maintenance. Such direct manipulation of myelin has the potential for highly spatially restricted effects, such as altering myelination patterns along specific axons. We discuss here three major spatial parameters of myelin that could be altered in adaptive myelination: 1) internodal localization (which axons are myelinated and their pattern of myelination); 2) myelin sheath geometry, including sheath length, sheath thickness, and number of sheaths per oligodendrocyte; and 3) nodal geometry (Figure 1B).
It has long been hypothesized that neuronal activity may act as an attractive cue to promote myelination of relevant axons, thereby modulating internodal localization (Osso and Chan, 2017). Indeed, preferential formation of myelin sheaths on electrically active axons has been suggested by direct manipulation of neuronal activity (Hines et al., 2015; Mitew et al., 2018; Wake et al., 2014), though has not yet been demonstrated with physiological circuit manipulations by experiential input. For instance, pharmacogenetic stimulation of somatosensory neurons of mice, in addition to increasing OPC proliferation and differentiation, increases myelination of the active axons without affecting the overall number of myelinated axons, while genetic attenuation of somatosensory neuron activity reduces myelination of those axons (Mitew et al., 2018). However, a study using early postnatal monocular deprivation found that individual oligodendrocytes in the optic chiasm showed no bias in ensheathing neighboring axons from either the normal or monocularly deprived eyes (Osanai et al., 2018). Whether myelin selection of a particular axon is influenced by its activity status may therefore differ by CNS region. In a tract like the optic nerve where almost all axons are fully myelinated, it may be that the relative activity of axons is less instructive than in regions like the cortex and corpus callosum that maintain a large percentage of unmyelinated axons throughout life (Gyllensten and Malmfors, 1963; Sturrock, 1980). Importantly, the experience-dependent myelination of relevant axons need not happen in an all-or-none fashion. Not all myelinated axons are fully myelinated, evidenced by the diverse, discontinuous myelination patterns in grey matter (Hughes et al., 2018; Micheva et al., 2016; Tomassy et al., 2014). in vivo imaging further suggests myelin is continuously added to partially myelinated axons through adulthood, thus altering the pattern of myelination (Hill et al., 2018). It is still unclear why or how such patterns are established or modified. Is this pattern of myelination instructed or merely stochastic? It will be of great interest to determine whether experience can instruct myelin localization on a fine scale, helping to shape the precise pattern of myelination along an axon.
Experience can alter the geometric features of new myelin formed by newly generated oligodendrocytes, including the length, thickness, and number of myelin sheaths per oligodendrocyte. We also now know that experience can induce new myelin sheath formation by preexisting oligodendrocytes under specific contexts (Bacmeister et al., 2020; Yang et al., 2020) and that preexisting myelin is plastic and can be remodeled (Auer et al., 2018; Bacmeister et al., 2020; Hill et al., 2018; Hughes et al., 2018; Yang et al., 2020). In the developing zebrafish spinal cord, inhibiting neuronal vesicular release reduces the number and length of myelin internodes in a neuronal subtype-specific manner, indicating only some neuronal subtypes employ activity-dependent mechanisms to regulate their myelination (Hines et al., 2015; Koudelka et al., 2016; Mensch et al., 2015). These effects have been proposed to be mediated through regulation of myelin sheath growth rates and retraction (Hines et al., 2015), parameters recently demonstrated to vary during development by neuronal subtype (Nelson et al., 2020). After this early developmental period in which myelin sheath length is established (Auer et al., 2018), many myelin sheaths continue to undergo dynamic lengthening and shortening events in adulthood (Bacmeister et al., 2020; Hill et al., 2018; Yang et al., 2020), indicating potential plasticity of this myelin parameter. Some experiential paradigms also induce alterations in sheath length or longitudinal growth dynamics (Bacmeister et al., 2020; Etxeberria et al., 2016; Osanai et al., 2018; Yang et al., 2020), while others do not (Hughes et al., 2018; Swire et al., 2019). For example, somatosensory enrichment via chronic whisker stimulation had no effect on sheath dynamics (Hughes et al., 2018), while a motor learning task increased the number of dynamically lengthening or shortening myelin sheaths (Bacmeister et al., 2020). Visual deprivation in the adult mouse was also shown to alter myelin sheath length and growth dynamics in a neuronal subtype-specific manner (Yang et al., 2020), consistent with the subtype-specific effects of neuronal activity seen in zebrafish. Both the type of experiential input and the subtypes of neurons involved therefore appear to affect whether and how myelin sheath length is altered.
Several studies have also found that experience- and activity-dependent effects on internodal length were determined not just by the activity state of single axons but also by the activity state of neighboring axons (Etxeberria et al., 2016; Hines et al., 2015; Osanai et al., 2018). While sparsely inhibiting vesicular release by individual zebrafish spinal cord neurons results in shorter myelin sheaths on silenced axons, simultaneous global inhibition of neuronal activity eliminates this effect (Hines et al., 2015). This is consistent with the possibility that oligodendrocytes may sense and integrate comparative levels of neuronal activity and adjust myelin sheath lengths accordingly. In addition, in monocular deprivation of the mammalian visual system, myelin sheath length is reduced on deprived fibers, but sheath length on non-deprived fibers is influenced by the local abundance of axons from the deprived vs. non-deprived eyes (Etxeberria et al., 2016; Osanai et al., 2018), suggesting the presence of an experience-dependent cue that signals a local environmental change in oligodendrocyte morphology rather than specific localization of such a cue along deprived or non-deprived axons only.
Both optogenetic (Gibson et al., 2014) and pharmacogenetic (Mitew et al., 2018) stimulation in mice have been shown to increase myelin thickness. Interestingly, pharmacogenetic stimulation, which biased myelination toward stimulated axons, showed increased myelin thickness of those stimulated axons but also a very mild increase in myelin thickness of neighboring non-stimulated axons, indicating both some specificity and some diffusion of the effect (Mitew et al., 2018). In both types of stimulation these changes in myelin thickness were observed without an increase in the number of myelinated axons and in adult animals, suggesting the possibility that neuronal stimulation could reinitiate and promote radial growth of preexisting myelin sheaths, such as through reopening of cytoplasmic channels (Snaidero et al., 2014). While increased myelin thickness has not yet been observed in experiential enrichment paradigms, experiential deprivation has been shown to reduce myelin sheath thickness and sheath number per oligodendrocyte in models of juvenile (Liu et al., 2012; Makinodan et al., 2012; Swire et al., 2019) and adult (Liu et al., 2012) social deprivation, and to decrease myelin thickness in a model of mild adult auditory deprivation (Sinclair et al., 2017). These studies suggest the possibility that sensory deprivation could induce thinning of preexisting myelin sheaths in adult animals, such as by inducing removal of myelin wraps (Dutta et al., 2018) or inhibiting active myelin sheath maintenance mediated by continual synthesis of new proteins (Wu et al., 2020) or new lipids (Zhou et al., 2020). In the developing visual system, it was found that ablation of neuronal signaling by enucleation just prior to oligodendrogenesis and myelination onset did not impair myelination but instead resulted in thicker myelin in the deprived optic nerve, suggesting the possibility that neuronal signaling may act to restrict myelin overgrowth in some contexts (Mayoral et al., 2018). Together these studies demonstrate a capacity for modulation of myelin thickness by both experiential input and neuronal activity.
Finally, experience-mediated cues could regulate geometric parameters of the nodal gap between preexisting or newly formed myelin sheaths, which could potentially alter the number of nodal sodium channels. Nodal length varies widely across the CNS and has been predicted to impact conduction velocity (Arancibia-Cárcamo et al., 2017). There is some precedent for regulation of nodal geometry in gerbil auditory brainstem neurons, which have progressively shorter myelin sheaths and increased nodal diameters at more distal axonal regions. Simulations predict this configuration acts to facilitate rapid action potential propagation into the target region (Ford et al., 2015). Dynamic changes in internodal length observed throughout life (Bacmeister et al., 2020; Hill et al., 2018; Hughes et al., 2018) and in response to altered experiential input (Bacmeister et al., 2020) could also potentially alter nodal length. Future studies are required to directly test whether experiential input alters nodal geometry as a means of modulating neuronal function.
The evidence overwhelming supports the conclusion that experience and neuronal activity can sculpt myelin both by inducing generation of new oligodendrocytes—which could then be instructed by the same or additional experience-dependent cues to generate myelin with specific spatial dimensions and localization—as well as by refining myelin parameters of preexisting oligodendrocytes. As these conclusions are largely based on zebrafish and rodent studies, it will be of great interest to determine the relative contribution of newly generated vs. preexisting oligodendrocytes to adaptive myelination in humans, where adult oligodendrogenesis still occurs but appears more limited than in rodents (Yeung et al., 2014). In addition, at present we lack tools that can specifically prevent or promote experience-induced changes in myelination without also altering differentiation, and therefore cannot uncouple a need for oligodendrocyte differentiation and a need for myelin modifications. Such tools would represent a major advance that would enable investigation of precisely what aspects of adaptive myelination are necessary and sufficient for behavioral outcomes.
How does adaptive myelination fine-tune neuronal function?
An open question in the field is exactly how adaptive changes to oligodendrocytes and myelin alter neuronal function and enable behavioral outputs like sensory refinement and memory formation. Accumulating evidence suggests there may be many non-mutually exclusive ways by which experience-induced oligodendrogenesis and myelination modify neuronal function.
Conductive properties
The prevailing hypothesis for the purpose of adaptive myelination is that it serves to modulate neuronal action potential conduction properties. This seems intuitive, given that enabling rapid and reproducible action potential conduction is a—if not the—major function of myelin, and that even small changes in myelin can have large impacts on conduction velocity. Multiple myelin parameters known to be modulated by experiential input or neuronal activity can alter conduction velocity. Myelination of previously unmyelinated regions would allow for dramatic increases in conduction velocity by facilitating saltatory conduction where there previously was none. However, more subtle changes in myelin structure can also alter conduction velocity. Thicker myelin (Chomiak and Hu, 2009; Rushton, 1951; Smith and Koles, 1970; Waxman and Bennett, 1972) and longer myelin internodes (Brill et al., 1977; Court et al., 2004; Huxley and Stämpeli, 1949; Simpson et al., 2013) support faster conduction velocity, up to a point. Longer and wider nodes have also been predicted to significantly accelerate conduction velocity, also up to a point. Notably, this predicted acceleration depends on the experimentally-supported postulate that nodal sodium channel density remains constant, meaning larger nodes would have more sodium channels (Arancibia-Cárcamo et al., 2017; Ford et al., 2015). This introduces the interesting possibility that conduction velocity could be shaped not only by altering nodal volume but also by directly regulating channel density. However, it is important to note that there is limited direct evidence that experience-driven changes to these internodal and nodal parameters alter conduction velocity, with most studies using modeling to predict the effect, and one study demonstrating a correlation between axonal conduction velocity and experience-dependent changes in both internodal length and oligodendrocyte numbers (Etxeberria et al., 2016).
In addition, myelin-mediated enhancement of conduction velocity is most often considered in the context of long-range projection neurons, but many short-range interneurons are also myelinated (Micheva et al., 2016; Stedehouder et al., 2017; Zonouzi et al., 2019), and pharmacogenetic stimulation of fast-spiking parvalbumin (PV) interneurons has been shown to increase their branching and myelination (Stedehouder et al., 2018). However, the comparative shortness of interneuron axons and their discontinuous, proximally-biased pattern of myelination (Micheva et al., 2016; Stedehouder et al., 2017) raises the questions of whether myelination significantly affects interneuron conduction properties and how changes in precise myelin patterning or geometric parameters play a role. A recent study found that genetic blockade of signaling from PV interneurons onto OPCs alters PV interneuron myelination and induces deficits in PV interneuron physiology, which could be due to the observed changes in myelination (Benamer et al., 2020). Altogether, modeling and experimental evidence supports a role for adaptive myelination in modulating conduction velocity, but further experimental studies are required to fully elucidate the contribution of experience-dependent changes in various myelin parameters to changes in neuronal signal propagation across multiple neuronal subtypes.
How would myelin-mediated alteration of conduction velocity facilitate functions like sensory refinement or motor learning? The nervous system is a complex array of interconnected neural circuits whose function requires precisely coordinated communication among these circuits. Synchronized arrival of inputs in a target region is important for signal summation and integration, permitting sufficient depolarization of postsynaptic neurons to trigger subsequent action potentials. In addition, synchronized neuronal activity enables generation of computationally meaningful patterns at multiple scales, ranging from spike trains of individual neurons to complex multi-circuit oscillatory behavior important for processes like perception, motor behavior, cognition, and learning and memory (Pajevic et al., 2013; Varela et al., 2001). Experience-dependent fine-tuning of conduction velocity could therefore serve to preferentially strengthen specific neurons or neuronal ensembles, establishing synchrony between or within circuits (Pajevic et al., 2013; Pan et al., 2020; Seidl et al., 2010; Steadman et al., 2020). Recent large-scale network modeling supports this idea (Noori et al., 2020). Action potentials from distant parts of the CNS can be adjusted to arrive simultaneously by precisely altering conduction velocity of one circuit relative to another. Such myelin-mediated temporal synchronization has been reported to be achieved between two axon tracts of considerably different lengths in the owl auditory system via differential internodal length and axon caliber (Seidl et al., 2010). Monocular deprivation, which results in shortened internodal lengths in the optic nerve, also results in slower compound action potential conduction with a widened range of conduction velocities, suggestive of impaired action potential synchrony within the optic nerve (Etxeberria et al., 2016). As axons can branch and form complex patterns of connectivity (Kalil and Dent, 2014), it is also possible that differential axonal branch myelination could sculpt neural connectivity and firing patterns in a highly nuanced manner (Seidl and Rubel, 2016). Adaptive myelin-mediated modulation of conduction velocity may also be bidirectional, either accelerating or decelerating signal propagation as needed to achieve temporal synchrony. Finally, it remains a significant gap in our knowledge how relevant neurons identify a need for altered conduction velocity. Are feedback or feedforward mechanisms involved, and is there overlap with mechanisms underlying other types of neuroplasticity? This remains poorly understood, even at a conceptual level.
Alongside modulation of conduction velocity via myelin-mediated insulation and voltage-gated channel clustering, oligodendrocytes may also influence circuit function by altering other action potential properties, such as firing frequency and reliability. Mice with hypomyelination were recently shown to exhibit deficits not only in conduction velocity but also in repetitive and precise action potential firing (Moore et al., 2020). These effects were proposed to be at least partly due to excess potassium flux leading to prolonged axonal hyperpolarization after action potential firing (Moore et al., 2020). Consistent with this idea, oligodendrocytes have been suggested to buffer extracellular potassium released during action potential propagation, thereby facilitating rapid repolarization and enabling high-frequency action potential firing (Larson et al., 2018). Oligodendrocyte-mediated potassium buffering may also act to modulate neuronal excitability. In a different study, oligodendrocytes with close apposition to neuronal cell bodies were found to exhibit action potential-induced inward potassium currents and to restrain neuronal action potential burst firing (Battefeld et al., 2016; Yamazaki et al., 2018). In line with this, mice whose oligodendrocytes have impaired potassium buffering exhibit spontaneous seizures (Larson et al., 2018). Oligodendrocytes may therefore impact circuit function by regulating neuronal excitability and conduction properties in multiple ways.
Axonal energetics and metabolism
In addition to altering axonal conduction properties, oligodendrocytes and myelin also play a role in axonal metabolism. The mammalian brain consumes a disproportionate amount of the body’s energy, with a majority of that energy consumption associated with signaling events (Laughlin 2001). Experience-induced changes to oligodendrocytes and myelin could respond to evolving energetic demands and adaptively support the function and integrity of specific axons or circuits. Traditionally, myelin-mediated saltatory conduction has been understood to conserve axonal energy by reducing ATP consumed from ion displacement during action potential firing (Ford et al., 2015; Harris and Attwell, 2012; Moore et al., 2020; Rushton, 1951; Saab et al., 2013). More recently, oligodendrocytes have also been suggested to play a more active role in axonal metabolism by supplying axons with lactate, a metabolite that can be converted into ATP within axons (Fünfschilling et al., 2012; Lappe-Siefke et al.; Lee et al., 2012b; Morrison et al., 2013; Saab et al., 2013, 2016). This role is important in supporting repetitive action potential firing (Moore et al., 2020) as well as maintaining axonal health (Lee et al., 2012b). Neuronal glutamate signaling has also been shown to enhance oligodendrocyte uptake of glucose, the metabolic precursor for lactate (Saab et al., 2016). Experience could therefore induce new metabolic support via myelination of previously unmyelinated axonal regions or could enhance axonal metabolic support from preexisting sheaths. However, it is still unclear to what degree oligodendrocyte-mediated lactate transfer contributes to behavioral outcomes of adaptive myelination. Future studies combining experiential paradigms with inducible ablation of oligodendrocyte lactate transport machinery (Bouçanova et al., 2020) would be informative. In addition, it is unknown whether differential geometric parameters of myelin affect transfer of such metabolites to axons and whether altered nodal volume can also influence axonal uptake of glucose directly from the extracellular environment.
Neuronal structural changes
During development, axons that become myelinated undergo stereotyped changes to their underlying cytoskeletal structure and intra-axonal trafficking. Neurofilament levels and phosphorylation increases, cytoskeletal-associated proteins are clustered into discrete domains relative to internodal and nodal localization, and axonal transport rates are altered (Pan and Chan, 2017). Myelination is thought to induce radial growth of axons, ostensibly through some of these cytoskeletal changes (Pan and Chan, 2017), which can impact axonal conduction velocity and other aspects of axonal functionality. Experience-induced axon myelination could induce similar changes in previously unmyelinated axons or axonal regions, or else more subtly alter axonal cytoskeletal structure or trafficking by changes in myelin patterning or geometric parameters.
Neuronal activity can also regulate axonal branching (Kalil and Dent, 2014). Myelination does not occur at or too close to branch sites (Stedehouder et al., 2017, 2018), and so selection of axonal target regions for myelination may be constrained by preexisting branch points. Alternatively or additionally, myelin itself could act as a physical barrier that obstructs available sites for further axonal branching. Some myelin-derived cues even actively limit axon branching and neurite outgrowth (Hu and Strittmatter, 2004; Tomita et al., 2007). Modifying the pattern or degree of myelination could therefore restrict or permit axonal arborization to help shape presynaptic connectivity, or else could occur in response to changes in axonal arborization. In support of these possibilities, pharmacogenetic stimulation of parvalbumin interneurons in adulthood has been shown to increase axonal arborization and myelination of distal axon branches (Stedehouder et al., 2018). Longitudinal imaging will be required to determine the sequence and likely causality of these events. Impairing signaling from these interneurons to OPCs did not affect interneuron arborization but did result in aberrant internodal localization and length with anomalous myelination of axonal branch points (Benamer et al 2020). Interneuron signaling may therefore prevent myelination at axonal branch points, which may be important for proper action potential conduction (Rama et al., 2018).
Oligodendrocytes and myelination have also been implicated in synapse formation or stabilization. Like axon branching sites, myelin internodes are also mutually exclusive of en passant synapses (synapses made directly from the axon shaft; Stedehouder et al 2017) and could act to restrict their formation. Oligodendrocytes have also been suggested to restrict dendritic branching and spine density (Zemmar et al., 2018). Conversely, delayed oligodendrogenesis and subsequent myelination induced by neonatal hypoxia is associated with a decreased density of cortical synapses, which is restored by enhancing oligodendrogenesis and myelination (Wang et al., 2018). Similarly, enhancing oligodendrogenesis and myelination increases synaptic density in aged mice (Wang et al., 2020). The mechanisms underlying potential myelin-mediated regulation of synapses are unknown, and experience-dependent changes in synapses have not yet been causally linked to experience- or activity-dependent changes in myelination.
Roles for oligodendrocytes beyond myelination
No study has yet determined whether behavioral changes induced by adaptive myelination are due to changes in myelin per se rather than due to additional functions of oligodendrocytes beyond myelination. This is due to a current technical inability to uncouple production of oligodendrocytes and formation of myelin. Current tools to study the importance of adaptive myelination in specific behaviors are limited to either increasing or preventing the generation of new oligodendrocytes. We have no way of allowing differentiation of new oligodendrocytes but preventing (or enhancing) myelination of those oligodendrocytes. In this sense, the term “adaptive myelination” may be a misnomer for some types of experience-driven changes to the oligodendrocyte lineage. For example, rapid differentiation into new oligodendrocytes is required during the first few hours of learning to run on a complex wheel (Xiao et al., 2016), a timeframe short enough that these new oligodendrocytes are unlikely to have formed compact myelin yet but during which they may participate in non-myelin-mediated functions that alter neuronal function. We do not yet know if many of the effects we attribute to myelin can instead (or in addition) be mediated directly by oligodendrocyte cell bodies or pre-myelinating processes, or even indirectly such as via oligodendrocyte-astrocyte gap junctions (Orthmann-Murphy et al., 2008). While some studies posit that oligodendrocyte-mediated transfer of lactate occurs through myelin itself (Lee et al., 2012b; Saab et al., 2016), this remains to be experimentally demonstrated. Similarly, oligodendrocyte-facilitated potassium buffering could be mediated independent of myelin, as the relevant potassium channel has been detected in oligodendrocyte cell bodies and proximal processes as well as in myelin (Larson et al., 2018; Schirmer et al., 2018). Oligodendrocytes have also been shown to support glutamatergic synaptic signaling by myelin-independent uptake and recycling of glutamate (Xin et al., 2019) and release of BDNF (Jang et al., 2019). Such myelin-independent functions could act in concert with myelin-dependent effects, thereby altering neuronal function in various ways and across a wide range of timescales, with important implications for different phases of experiential paradigms.
How does a neuron trigger adaptive myelination?
A major focus in the field is the identity of cues by which neurons induce adaptive myelination. However, it must first be considered exactly how experience induces neurons to produce these cues. The prevalent view of adaptive myelination is that experience depolarizes relevant neurons, causing them to fire action potentials, perhaps in a specific pattern, and that these action potentials induce release of signaling molecules that alter oligodendrocyte lineage progression and/or myelination. This has largely been addressed in systems where neuronal activity, vesicular release, or activation of downstream oligodendroglial receptors are directly stimulated or silenced, or in simple circuit processing like visually-evoked activation of the optic nerve. It is less clear how exactly neuronal activity triggers adaptive myelination in complex physiological manipulations that recruit multiple neural circuits, like motor learning or social deprivation. Combining experiential paradigms with paradigms that directly manipulate neuronal activity in specific neuronal subsets would greatly advance our understanding of the role of neuronal activity in adaptive myelination and address many outstanding questions. For instance, which neurons or neuronal ensembles must be activated, and what kinds of computationally relevant firing patterns are involved? Different patterns of electrical activity evoke distinctive responses in oligodendrocyte lineage cells, with only some patterns appearing to induce adaptive myelination (Gibson et al., 2014; Nagy et al., 2017; Ortiz et al., 2019). Changes in experience- or activity-dependent oligodendrogenesis and myelination are also typically observed specifically in relevant brain areas, indicating a potential circuit-level specificity of the effects. We must also question a key presumption about adaptive myelination: is it necessarily always the case that adaptive myelination is initiated by stimulation (or suppression) of action potential conduction? For example, can adaptive myelination also be initially triggered by changes in subthreshold neuronal activity, which can be synchronized among neurons (Engel et al., 2001; Lampl et al., 1999) and can alter neuronal intracellular signaling, synaptic communication, and firing properties (Alle and Geiger, 2006; Eilers et al., 1995; Ludwar et al., 2020; Shu et al., 2006; Soldado-Magraner et al., 2020)? In addition, the initial trigger for adaptive myelination (e.g. the first neurons that fire action potentials) may not directly induce adaptive myelination, but could instead activate, inhibit, or otherwise modulate downstream neurons or even downstream non-neuronal cells that then present an adaptive myelination cue to oligodendrocyte lineage cells. It follows then that an adaptive myelination cue need not necessarily be released by the neuron whose myelination is meant to be altered, although that does appear to be likely in cases where axon selection itself is biased toward active axons (Mitew et al., 2018). Cue-releasing neurons could even intentionally prevent their own myelination, allowing such neurons to recurrently modulate myelination in an immutable fashion that is unaltered by previous experiential input.
With these questions in mind, we discuss here several putative neuronal cues that have been proposed to stimulate adaptive myelination by acting on OPCs or oligodendrocytes through the classical conception of activity-induced neuronal secretion, as well as explore several alternative biophysical and non-neuronal adaptive myelination cues (Figure 2).
Figure 2. Putative adaptive myelination cues.
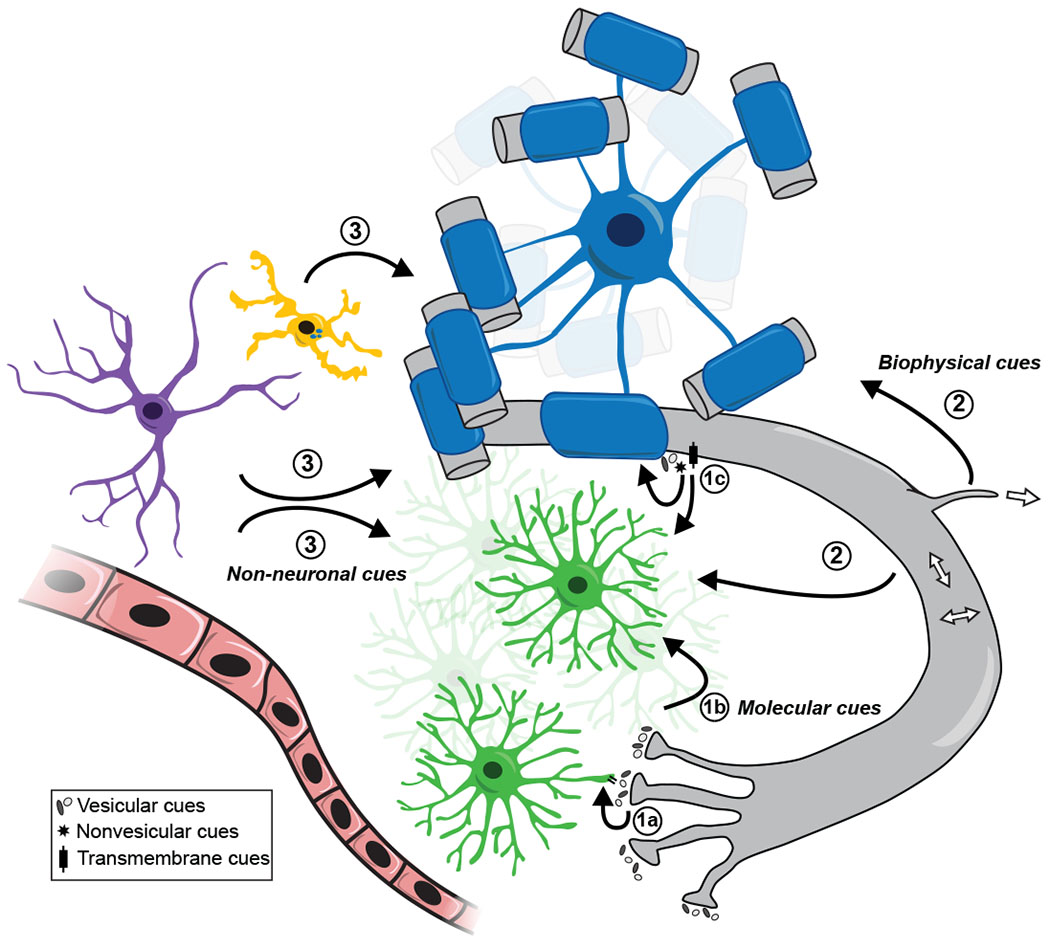
Experiential input could alter OPCs (green) or oligodendrocytes (blue) via 1) neuronal molecular cues, including secretion of vesicular cues like glutamate, GABA, or BDNF at neuron-OPC synapses (1a), spillover of such cues from synapses acting on extrasynaptic oligodendroglial receptors (1b), or extrasynaptic secretion or expression of vesicular, nonvesicular, or transmembrane cues (1c); 2) biophysical cues, such as axonal radial or lateral growth or axonal branching; 3) non-neuronal cues such as from astrocytes (purple) or endothelial cells (pink), or myelin phagocytosis by microglia (yellow).
Amino acid neurotransmitters
Bergles and colleagues made the remarkable discovery twenty years ago that OPCs, unlike all other non-neuronal cells, receive direct synaptic input from neurons (Bergles et al., 2000). Indeed, neurons form both glutamatergic and GABAergic synapses onto OPCs in multiple brain regions and throughout life (Bergles et al., 2000, 2010; Lin and Bergles, 2003; Lin et al., 2005; Spitzer et al., 2019; Tanaka et al., 2009; Ziskin et al., 2007), providing a potential route by which OPCs can listen in on and respond to changes in neuronal activity with high spatiotemporal resolution. Monosynaptic tracing reveals that OPCs receive synaptic input from functionally interrelated regions across the brain, but that this synaptic connectivity itself is not affected by somatosensory deprivation in early adulthood (Mount et al., 2019). However, more extensive studies with different ages and different experimental paradigms are required to fully exclude an experience- or neuronal activity-dependent effect on neuron-OPC synaptic connectivity.
Given the prevalence of OPC-neuron synapses, it seems intuitive that neuronal vesicles would contain cues capable of inducing adaptive myelination. In support of this, neuronal vesicular release modulates myelination in the developing zebrafish spinal cord (Hines et al., 2015; Koudelka et al., 2016; Mensch et al., 2015). Interestingly, vesicle-modulated myelination is only observed in some neuronal subtypes, while myelination of other neuronal subtypes is independent of their vesicular release, indicating possible diversity of vesicle contents or diversity of axonal expression of other regulatory cues (Hines et al., 2015; Koudelka et al., 2016). The potential adaptive myelination cues contained within these vesicles is a matter of intense investigation, with the small molecule neurotransmitters glutamate and GABA being the most well-studied potential cues. OPCs express both metabotropic and ionotropic glutamate receptors (Bergles et al., 2000; Haberlandt et al., 2011; Káradóttir et al., 2005; Spitzer et al., 2019; Zhang et al., 2014; Zonouzi et al., 2011), with most studies focused on the role of AMPA receptors and NMDA receptors. Release of vesicular glutamate from unmyelinated callosal axons was shown to depolarize OPCs primarily through AMPA receptors (Ziskin et al., 2007). However, while glutamate alters OPC proliferation and differentiation in vitro (Dutta et al., 2006; Gallo et al., 1996), limited effects have been seen in animals with impaired glutamatergic signaling to OPCs. Mice whose oligodendrocyte lineage cells lack NMDA receptor signaling exhibit no deficits (De Biase et al., 2011; Guo et al., 2012), while those whose oligodendrocyte lineage cells lack AMPA receptor signaling exhibit only modest deficits in oligodendroglial survival and myelination (Kougioumtzidou et al., 2017). In addition, mice with genetic blockade of glutamatergic signaling in the optic nerve do not have impaired proliferation, differentiation, or overall degree of myelination, exhibiting instead an increased number of oligodendrocytes and shortened internodes (Etxeberria et al., 2016). While ionotropic glutamatergic signaling may therefore play only a minor role in oligodendroglial dynamics under basal developmental conditions, there could be specific circumstances under which this type of signaling is more impactful (Lundgaard et al., 2013), such as in remyelination (Gautier et al., 2015; Lundgaard et al., 2013) or when challenged with experiential paradigms. AMPA receptor and NMDA receptor expression also differ by brain region and by age (Spitzer et al., 2019) which may underlie some of the differences seen between different glutamatergic signaling-deficient mice and could result in distinct effects from different types of experiential input.
OPCs also express GABA receptors (Balia et al., 2015; Lin and Bergles, 2004; Orduz et al., 2015; Passlick et al., 2013), and GABA has been shown to depolarize OPCs and to decrease oligodendrocyte number and myelination in vitro (Hamilton et al., 2017). However, mice whose OPCs lack synaptically-localized GABA-A receptors (Passlick et al., 2013) exhibit no deficits in proliferation or differentiation, though they do have a mild adult-onset decrease in OPC numbers (Balia et al., 2017). While initial reports showed the degree of interneuron myelination was also unaffected by loss of this GABA-A receptor mediated signaling (Balia et al., 2017), more detailed studies now show PV interneuron physiology and internodal localization and geometry is perturbed, which results in deficits in interneuron signaling and impaired sensory behavior (Benamer et al., 2020). Future studies will be required to determine precise causality among events and whether this phenotype represents a persistent deficit or a developmental delay. Given these subtle but potentially impactful changes in myelin, as well as the possibility that the effect of neurotransmission to OPCs may be context-dependent, it will be important in future to further combine experiential and neuronal activity paradigms with mice deficient in different types of glutamatergic and GABAergic signaling to assess the relative contribution of these cues to observed adaptive myelination changes and behaviorally relevant outcomes.
Synaptic communication is specific to OPCs as glutamatergic synaptic connections are lost when OPCs differentiate into oligodendrocytes (Biase et al., 2010; Etxeberria et al., 2010; Kukley et al., 2010), and GABAergic synaptic input is dramatically reduced after the second postnatal week of life in rodents (Vélez-Fort et al., 2010). Though neurons classically release vesicles at synapses, vesicular contents can spill-over from the synaptic cleft and vesicles can also be released extrasynaptically along the axon. It is therefore also possible that neuronal vesicular release can activate extrasynaptic GABAergic (Maldonado et al., 2011; Passlick et al., 2013; Vélez-Fort et al., 2010) or glutamatergic (Micu et al., 2016; Wake et al., 2015) receptors on OPCs, or even directly affect oligodendrocytes. In support of this latter idea, NMDA receptors have also been shown to be expressed by oligodendrocytes and localized to myelin (Káradóttir et al., 2005; Micu et al., 2006; Saab et al., 2016; Salter and Fern, 2005). Activation of oligodendrocyte NMDA receptors upregulates oligodendrocyte glucose uptake, which has been proposed to alter their metabolic support of axons (Saab et al., 2016). Several studies are consistent with the possibility that neuronal vesicles could act directly on oligodendrocytes to regulate sheath formation or retraction, with vesicular release capable of occurring under sites of oligodendroglial ensheathment (Doyle et al., 2018; Hines et al., 2015; Hughes and Appel, 2019; Mensch et al., 2015). The adaptive myelination cues contained within these vesicles have yet to be identified and could include neurotransmitters like glutamate and GABA and/or other molecules known to be released in neuronal vesicles.
Other neuronal molecular cues
While GABA and glutamate are the major neurotransmitters implicated in adaptive myelination, other neurotransmitters or neuromodulators may play a role. Neurotransmitters in particular may be ideal for rapid, spatially-restricted communication to oligodendrocyte lineage cells, while neuromodulators like neuropeptides could be particularly well-suited to induce broad effects on oligodendrocyte lineage cells. ATP and its metabolite adenosine have both been proposed as adaptive myelination cues in vitro, with the former thought to indirectly affect oligodendroglia via astrocytes and the latter thought to act directly on OPCs to promote differentiation (Ishibashi et al., 2006; Stevens et al., 2002). OPC differentiation is also potently inhibited by activation of the M1 muscarinic acetylcholine receptor (Mei et al., 2014, 2016), though a role for acetylcholine in experience-driven myelination has not been described.
Vesicular release of the neurotrophin BDNF has also been suggested to act as an adaptive myelination cue. BDNF signaling has been shown in numerous in vitro studies to alter OPC proliferation and differentiation and to promote developmental myelination (Fletcher et al., 2018). BDNF has also been suggested to sensitize the myelination process to NMDA receptor modulation in neuron-oligodendroglia cocultures (Lundgaard et al., 2013). Mice lacking the BDNF receptor TrkB in oligodendrocytes do not have altered oligodendrocyte numbers but instead exhibit a developmental delay in myelin thickness, evidencing a direct albeit temporary in vivo effect of BDNF on oligodendrocytes (Wong et al., 2013). These mutant mice also have TrkB ablated in a small population of OPCs and exhibit an increase in OPC numbers, proposed to be due to increased proliferation (Wong et al., 2013). BDNF has also been directly implicated in adaptive myelination induced by neuronal stimulation, where its effects partially recapitulate these developmental effects. In work from the Monje lab, inducible conditional knockout in juvenile mice of TrkB in OPCs or of activity-regulated BDNF in stimulated neurons did not alter basal OPC proliferation, but prevented increased OPC proliferation, increased oligodendrocyte generation, and increased myelin thickness induced by optogenetic stimulation (Geraghty et al., 2019). These findings indicate BDNF may serve as a cue that communicates neuronal activation to OPCs and/or oligodendrocytes. Further investigations of the underlying mechanisms will be particularly important to resolve how a single cue can induce both proliferation and differentiation. It will also be important to determine if this role for BDNF is conserved in experiential paradigms, how generalizable this role is to different brain regions (Du et al., 2003), and how BDNF may interact with other adaptive myelination cues like glutamate in vivo (Lundgaard et al., 2013).
Neuronal activity has been shown to induce numerous changes in neurons, including broad transcriptional and translational changes (Choe and Cho, 2020; Flavell and Greenberg, 2008; Yap and Greenberg, 2018), providing many other candidates to induce adaptive myelination. For example, as with synaptic transmission, transmembrane proteins could induce effects with high spatial specificity, particularly if their expression is restricted to the structure that is to be myelinated (or not myelinated). Neuronal expression of the transmembrane protein neuregulin-1 has been proposed as an adaptive myelination cue underlying hypomyelination in social deprivation (Makinodan et al., 2012). Beyond transmembrane proteins, neurons could also utilize many other types of molecular cues to induce adaptive myelination, such as secreted proteins like growth factors or neuropeptides, or secreted non-protein cues like sugars, lipids, or other metabolites. In addition, while it is canonically thought neurons would present cues to induce adaptive myelination, it is also plausible that experience could remove cues inhibitory to myelination (see Osso and Chan, 2017). It is also possible that certain signals in the microenvironment could “prime” oligodendrocyte lineage cells to be receptive to experience-dependent cues. This could be the case for OPCs in the zebrafish spinal cord, where the local environment of the OPC cell body was shown to influence its likelihood to differentiate, morphology, calcium signaling, and propensity to proliferate upon neuronal stimulation (Marisca et al., 2020). In further support of this idea, ion channel expression and activity in mouse OPCs differs across brain regions and ages and can be influenced by local molecular cues (Lundgaard et al., 2013; Spitzer et al., 2019). Interactions among cues, both experience-dependent and -independent, may therefore add further complexity to how experience modulates oligodendroglia and myelin.
Biophysical cues
Axonal longitudinal growth (Polleux and Snider, 2010), branching (Kalil and Dent, 2014; Stedehouder et al., 2018; Yamada et al., 2010), and radial growth (Chéreau et al., 2017; Seidl et al., 2010) can be altered by experiential input and/or neuronal activity, providing new substrates for myelination. For example, activation of PV interneurons causes longitudinal growth and branching of these axons and their subsequent novel myelination (Stedehouder et al., 2018). In addition, as myelination does not occur on axons below a minimum axon diameter (Lee et al., 2012a; Mayoral et al., 2018), axonal radial growth of a subthreshold diameter axon to a suprathreshold diameter could permit and perhaps trigger myelination of that previously unmyelinated axon. The state of axonal growth itself could also be sensed by oligodendroglial cells and stimulate myelination or myelin remodeling. Oligodendroglia are innately sensitive to the size of the fibers they ensheathe. While the lack of myelination of subthreshold diameter fibers could be due to biophysical limitations of membrane curvature, oligodendroglia also exhibit a preference for larger caliber fibers, even if the smaller caliber fibers are suprathreshold. Larger caliber axons are the first to become myelinated in vivo (Schwab and Schnell, 1989). in vitro, oligodendrocytes and OPCs preferentially ensheathe larger fibers in cultures containing mixed, suprathreshold diameter polystyrene nanofibers (Lee et al., 2012a), and oligodendrocytes make longer sheaths on larger caliber nanofibers (Bechler et al., 2015). These results suggest oligodendroglia may utilize as yet unidentified mechanisms, perhaps such as curvature-sensing proteins (McMahon and Boucrot, 2015) or mechanosensitive channels (Segel et al., 2019), to detect absolute size of targets and could therefore also possibly detect whether and how fast a target is actively growing. In this way, axonal radial growth could then instruct radial growth of new myelin sheaths or possibly reinitiate radial growth of preexisting sheaths (Jeffries et al., 2016; Snaidero et al., 2014) such that an appropriate number of myelin wraps relative to axon caliber is maintained. Consistent with this idea, concomitant growth in axon caliber has been proposed to drive remyelination with normal myelin sheath thickness and length in zebrafish, compared to the typical thin and short myelin sheaths observed in most demyelination models (Karttunen et al., 2017).
Non-neuronal cues and effects
Neuronal activity and experiential input are well known to affect many non-neuronal cell types in the CNS (e.g. Attwell et al., 2010; Hasel et al., 2017; Hughes and Appel, 2020; Li et al., 2012; Liu et al., 2019; Rosskothen-Kuhl et al., 2018; Tremblay et al., 2010). For instance, neuronal activity intricately controls brain blood flow by acting on vascular and vasculature-associated cells (Attwell et al., 2010), and experiential input modulates neurovasculature organization during development (Kole, 2015; Lacoste et al., 2014). Inadequate oxygen supply during development also impairs OPC differentiation (Yuen et al., 2014). Can acute or chronic changes in blood and oxygen supply to the CNS or other vascular signaling also participate in adaptive myelination? A recent study suggested juvenile sensory deprivation alters vascular cell expression of the vasoconstrictive peptide endothelin to alter myelin sheath number (Swire et al., 2019). Astrocytes have also been implicated in indirect control of adaptive myelination, where an in vitro study found that neuronal activity-induced secretion of the cytokine LIF from astrocytes increases myelination (Ishibashi et al., 2006). Additional studies using mice with vascular- or astrocyte-specific ablation of these cues are required to determine the in vivo relevance of these mechanisms.
Microglia have also recently been demonstrated to play a role in adaptive myelination. While some myelin sheaths are retracted in an oligodendrocyte-autonomous manner during development (Auer et al., 2018; Baraban et al., 2018; Hines et al., 2015; Hughes and Appel, 2020), other sheaths are phagocytosed by microglia (Hughes and Appel, 2020). Both autonomous sheath retraction (Hines et al., 2015) and myelin phagocytosis (Hughes and Appel, 2020) are increased by blocking neuronal vesicular release. This suggests neuronal signaling controls myelin sheath removal by both direct and indirect mechanisms, though whether these mechanisms are truly independent and how a sheath is selected for phagocytosis are still unclear. The involvement of non-neuronal cells in adaptive myelination is therefore not limited to molecular cues, and crosstalk may exist with neuronal mechanisms previously thought to act only through direct regulation of oligodendroglia.
What downstream mechanisms enact adaptive myelination?
There is still much unknown about adaptive myelination cues, and even more unknown about the intracellular mechanisms engaged within OPCs and oligodendrocytes by these cues. However, experience-dependent cues are likely to impact many of the same downstream intracellular processes engaged during non-experience-dependent oligodendrogenesis and myelination. Much can be learned, therefore, by examining known molecular mechanisms that regulate proliferation, differentiation, and myelination during development. Such developmental mechanisms have been thoroughly reviewed elsewhere (e.g. Chang et al., 2016; Elbaz and Popko 2019; Gaesser and Fyffe-Maricich 2016; Paez and Lyons, 2020), and we highlight here several of these mechanisms in the context of adaptive myelination.
Intracellular signaling cascades like the PI3K/AKT pathway and the ERK1/2 pathway are important in regulating developmental myelination and can be activated by numerous extracellular signals, including growth factors like BDNF. However, the role of these signaling pathways in adaptive myelination has not been well-studied. Enhancing PI3K/AKT signaling during development induces hypermyelination, and driving pathway activation during adulthood reverts myelin ultrastructure to a more immature state and increases myelin thickness, suggesting reinitiation of myelin growth (Flores et al., 2008; Goebbels et al., 2010; Snaidero et al., 2014). Similarly, ERK1/2 signaling promotes developmental myelination, and driving pathway activation in preexisting oligodendrocytes in adulthood increases myelin thickness (Fyffe-Maricich et al., 2011, 2013; Ishii et al., 2012, 2016; Jeffries et al., 2016). Interestingly, elevated ERK1/2 signaling in adult oligodendrocytes enhanced conditional fear learning but not motor learning or novel object recognition learning, suggesting effects of oligodendroglial ERK1/2 signaling influence some behaviors and not others (Jeffries et al., 2016). Both PI3K/AKT and ERK1/2 signaling impinge on cellular mechanisms governing cell survival, growth, proliferation, and metabolism, and they are both capable of acting through downstream activation of mTOR signaling, a central regulator of protein and lipid synthesis that is also known to positively regulate differentiation and myelination (Figlia et al., 2017). Whether and how these intracellular pathways act as direct effectors of adaptive myelination will require further investigation, such as subjecting mice with enhanced or deficient PI3K/AKT, ERK1/2, or mTOR signaling in oligodendroglia to different experiential paradigms.
A second major and interrelated intracellular mechanism known to regulate oligodendrocyte lineage cells and myelination is calcium signaling. OPCs and oligodendrocytes express many different calcium-permeable channels and can also be induced to release calcium from intracellular stores (Paez and Lyons 2020). Many putative adaptive myelination cues can alter intracellular calcium flux, including extracellular factors like BDNF, GABA, glutamate, or adenosine, by acting on receptor tyrosine kinases, ion channels, or G-protein-coupled receptors (Amaral and Pozzo-Miller, 2007; Káradüttir and Attwell, 2007; McCutchen et al., 2002; Segal and Greenberg, 1996; Stevens et al., 2002; Tanaka et al., 2009). Calcium flux can modulate various intracellular signaling cascades, along with myriad other cellular processes such as cytoskeletal dynamics, transcription, and translation. To add further complexity, precise amplitude, duration, and localization of calcium signaling evokes differential cellular responses.
We are only beginning to understand some of the downstream cellular functions recruited by distinct signatures of calcium signaling in oligodendroglia and how such signaling may cause adaptive myelination. Calcium signaling in OPCs has been shown to mediate activity-dependent increases in OPC proliferation in the zebrafish spinal cord (Marisca et al., 2020). Calcium signaling has also been implicated in regulating myelin sheath growth and retraction. In zebrafish, high-amplitude, long-duration calcium transients within individual myelin sheaths tend to precede sheath retraction. Such sheath retractions also appear to be mediated via the calcium-dependent protease calpain within oligodendrocytes (Baraban et al., 2018), possibly even locally within myelin sheaths in a manner reminiscent of calpain-mediated dendritic or axonal pruning (Kanamori et al., 2015; Pease and Segal, 2014). It will be intriguing to determine if such calcium signatures are also seen in sheaths phagocytosed by microglia (Hughes and Appel, 2020). In contrast, later, low-amplitude, short-duration calcium transients within individual myelin sheaths correlates with increased rate of sheath growth, and this growth rate is reduced by calcium buffering (Baraban et al., 2018; Krasnow et al., 2018). Interestingly, pharmacologically suppressing neuronal activity reduced this intrasheath calcium activity in only half the sheaths (Krasnow et al., 2018), suggesting there are both activity-dependent and activity-independent calcium transients within myelin. In support of this, calcium flux in growing myelin sheaths in the mouse cortex has been shown to occur independently of neuronal activity (Battefeld et al., 2019). Altogether these studies suggest intracellular calcium signaling, alongside or in addition to intracellular signaling cascades, is likely to mediate at least some aspects of adaptive myelination. Future investigations will be required to determine the precise calcium signatures and downstream pathways that underlie different experience-induced changes to oligodendrocytes and myelination and their subsequent behavioral consequences.
Moving toward a holistic view of adaptive myelination
Altogether the studies described here show that experience and neuronal activity can sculpt myelin by inducing new oligodendrocyte formation and by refining myelin parameters of novel or preexisting oligodendrocytes using perhaps a multitude of different cues. But it is clear we are still in the preliminary stages of understanding this form of neuroplasticity. Experiential input evokes complex and temporally evolving responses across the CNS, and a snapshot of the state of OPCs, oligodendrocytes, or myelin likely misses changes occurring in other regions, at other times, or in other parameters. A holistic view of behaviorally relevant changes in oligodendrocytes and myelin driven by experience to reciprocally alter neuronal function will require simultaneous examination of multiple aspects of myelin and the oligodendrocyte lineage (proliferation, differentiation, death) across time, at various spatial scales (gross, single-cellular, and ultrastructural), and in multiple CNS regions. A better understanding of downstream intracellular mechanisms and additional signaling events that lead to such changes would be exceedingly powerful in the pursuit to understand adaptive myelination, as they could potentially lead to development of tools through which we could directly induce or inhibit specific adaptive myelination changes and perhaps demonstrate causality of changes to neuronal function. In addition, physiological changes induced by experiential input are likely to be much more complex than those induced by artificial manipulation of neuronal activity, and so combining both types of manipulation is a necessary next step to understand the contribution of neuronal activity to experience-driven adaptive myelination and its behavioral outcomes.
Acknowledgements
We thank members of the Chan laboratory for insightful and helpful discussions and advice. This work was supported by the NIH/National Institute of Neurological Disorders and Stroke (R01NS097428 and R01NS095889), the Adelson Medical Research Foundation (ANDP grant A130141), and the Rachleff family endowment to J.R.C., and by the NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development (F32HD098829 to S.E.P.-R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Alle H, and Geiger JRP (2006). Combined Analog and Action Potential Coding in Hippocampal Mossy Fibers. Science 311, 1290–1293. [DOI] [PubMed] [Google Scholar]
- Amaral MD, and Pozzo-Miller L (2007). BDNF Induces Calcium Elevations Associated With IBDNF, a Nonselective Cationic Current Mediated by TRPC Channels. J Neurophysiol 98, 2476–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia-Cárcamo LI, Ford MC, Cossell L, Ishida K, Tohyama K, and Attwell D (2017). Node of Ranvier length as a potential regulator of myelinated axon conduction speed. ELife 6, e23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, and Newman EA (2010). Glial and neuronal control of brain blood flow. Nature 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer F, Vagionitis S, and Czopka T (2018). Evidence for Myelin Sheath Remodeling in the CNS Revealed by In Vivo Imaging. Curr. Biol 28, 549–559.e3. [DOI] [PubMed] [Google Scholar]
- Bacmeister CM, Barr HJ, McClain CR, Thornton MA, Nettles D, Welle CG, and Hughes EG (2020). Motor learning promotes remyelination via new and surviving oligodendrocytes. Nature Neuroscience 23, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balia M, Vélez-Fort M, Passlick S, Schäfer C, Audinat E, Steinhäuser C, Seifert G, and Angulo MC (2015). Postnatal Down-Regulation of the GABAA Receptor γ2 Subunit in Neocortical NG2 Cells Accompanies Synaptic-to-Extrasynaptic Switch in the GABAergic Transmission Mode. Cerebral Cortex 25, 1114–23. [DOI] [PubMed] [Google Scholar]
- Balia M, Benamer N, and Angulo MCC (2017). A specific GABAergic synapse onto oligodendrocyte precursors does not regulate cortical oligodendrogenesis. Glia 65, 1821–1832. [DOI] [PubMed] [Google Scholar]
- Baraban M, Koudelka S, and Lyons DA (2018). Ca 2+ activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci 21, 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, and Raff MC (1993). Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HSR, Burne JF, Voyvodic JT, Richardson WD, and Raff MC (1992). Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70, 31–46. [DOI] [PubMed] [Google Scholar]
- Battefeld A, Klooster J, and Kole MHP (2016). Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nature Communications 7, 11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battefeld A, Popovic MA, Vries S.I. de, and Kole M (2019). High-Frequency Microdomain Ca2+ Transients and Waves during Early Myelin Internode Remodeling. Cell Reports 26, 182–191.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechler ME, Byrne L, and Ffrench-Constant C (2015). CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Current Biology: CB 25, 2411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamer N, Vidal M, Balia M, and Angulo MC (2020). Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits. Nature Communications 11, 5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JDB, Somogyi P, and Jahr CE (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jabs R, and Steinhäuser C (2010). Neuron-glia synapses in the brain. Brain Research Reviews 63, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biase LMD, Nishiyama A, and Bergles DE (2010). Excitability and Synaptic Communication within the Oligodendrocyte Lineage. The Journal of Neuroscience 30, 3600–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouçanova F, Pollmeier G, Sandor K, Urbina CM, Nijssen J, Médard J-J, Bartesaghi L, Pellerin L, Svensson CI, Hedlund E, et al. (2020). Disrupted function of lactate transporter MCT1, but not MCT4, in Schwann cells affects the maintenance of motor end-plate innervation. Glia n/a. [DOI] [PubMed] [Google Scholar]
- Brill MH, Waxman SG, Moore JW, and Joyner RW (1977). Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. Journal of Neurology, Neurosurgery & Psychiatry 40, 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, and Richardson WD (1998). Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron 20, 869–882. [DOI] [PubMed] [Google Scholar]
- Chang K-JJ, Redmond SA, and Chan JR (2016). Remodeling myelination: implications for mechanisms of neural plasticity. Nat. Neurosci 19, 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéreau R, Saraceno EG, Angibaud J, Cattaert D, and Nägerl VU (2017). Superresolution imaging reveals activity-dependent plasticity of axon morphology linked to changes in action potential conduction velocity. Proceedings of the National Academy of Sciences 114, 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe HK, and Cho J (2020). Comprehensive Genome-Wide Approaches to Activity-Dependent Translational Control in Neurons. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomiak T, and Hu B (2009). What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Sherman DL, Pratt T, Garry EM, Ribchester RR, Cottrell DF, Fleetwood-Walker SM, and Brophy PJ (2004). Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature 431, 191–195. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, and Bergles DE (2011). NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J. Neurosci 31, 12650–12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle S, Hansen DB, Vella J, Bond P, Harper G, Zammit C, Valentino M, and Fern R (2018). Vesicular glutamate release from central axons contributes to myelin damage. Nature Communications 9, 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Lee LN, Lercher LD, and Dreyfus CF (2003). Regionally Specific Effects of BDNF on Oligodendrocytes. Developmental Neuroscience 25, 116–126. [DOI] [PubMed] [Google Scholar]
- Dutta DJ, Woo DH, Lee PR, Pajevic S, Bukalo O, Huffman WC, Wake H, Basser PJ, SheikhBahaei S, Lazarevic V, et al. (2018). Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proceedings of the National Academy of Sciences 115, 201811013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, et al. (2006). Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Annals of Neurology 59, 478–489. [DOI] [PubMed] [Google Scholar]
- Eilers J, Augustine GJ, and Konnerth A (1995). Subthreshold synaptic Ca 2+ signalling in fine dendrites and spines of cerebellar Purkinje neurons. Nature 373, 155–158. [DOI] [PubMed] [Google Scholar]
- Elbaz B, and Popko B (2019). Molecular Control of Oligodendrocyte Development. Trends in Neurosciences 42, 263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, and Barres BA (2009). Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138, 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, and Singer W (2001). Dynamic predictions: Oscillations and synchrony in top–down processing. Nature Reviews Neuroscience 2, 704–716. [DOI] [PubMed] [Google Scholar]
- Etxeberria A, Mangin J-M, Aguirre A, and Gallo V (2010). Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nature Neuroscience 13, 287–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, Redmond SA, Ullian EM, and Chan JR (2016). Dynamic Modulation of Myelination in Response to Visual Stimuli Alters Optic Nerve Conduction Velocity. J. Neurosci 36, 6937–6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlia G, Norrmén C, Pereira JA, Gerber D, and Suter U (2017). Dual function of the PI3K-Akt-mTORC1 axis in myelination of the peripheral nervous system. Elife 6, e29241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, and Greenberg ME (2008). Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31, 563–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JL, Murray SS, and Xiao J (2018). Brain-Derived Neurotrophic Factor in Central Nervous System Myelination: A New Mechanism to Promote Myelin Plasticity and Repair. International Journal of Molecular Sciences 19, 4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick H, Yin X, Kidd G, Avila RL, Kirschner DA, and Macklin WB (2008). Constitutively active Akt induces enhanced myelination in the CNS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, Pecka M, Attwell D, and Grothe B (2015). Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nature Communications 6, 8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y, Hayasaka S, Bedi KS, Ozaki HS, and Takeuchi Y (1991). Quantitative study of the development of the optic nerve in rats reared in the dark during early postnatal life. Journal of Anatomy 174, 37–47. [PMC free article] [PubMed] [Google Scholar]
- Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, et al. (2012). Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Karlo JC, Landreth GE, and Miller RH (2011). The ERK2 Mitogen-Activated Protein Kinase Regulates the Timing of Oligodendrocyte Differentiation. The Journal of Neuroscience 31, 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Schott A, Karl M, Krasno J, and Miller RH (2013). Signaling through ERK1/2 controls myelin thickness during myelin repair in the adult central nervous system. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 33, 18402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser JM, and Fyffe-Maricich SL (2016). Intracellular signaling pathway regulation of myelination and remyelination in the CNS. 283, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou J, McBain C, Wright P, Knutson P, and Armstrong R (1996). Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. Journal of Neuroscience 16, 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier HOO, Evans KA, Volbracht K, James R, Sitnikov S, Lundgaard I, James F, Cristina L-P, Reynolds R, Franklin RJ, et al. (2015). Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat Commun 6, 8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Paşca SP, et al. (2019). Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron 103, 250–265.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero J, et al. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Möbius W, Liu X, Corinna L-S, et al. (2010). Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci 30, 8953–8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ko EM, Delgado M, Horiuchi M, Soulika A, Miers L, Burns T, Itoh T, Shen H, et al. (2012). Disruption of NMDA Receptors in Oligodendroglial Lineage Cells Does Not Alter Their Susceptibility to Experimental Autoimmune Encephalomyelitis or Their Normal Development. The Journal of Neuroscience 32, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten L, and Malmfors T (1963). Myelinization of the optic nerve and its dependence on visual function–a quantitative investigation in mice. Journal of Embryology and Experimental Morphology 11, 255–66. [PubMed] [Google Scholar]
- Haberlandt C, Derouiche A, Wyczynski A, Haseleu J, Pohle J, Karram K, Trotter J, Seifert G, Frotscher M, Steinhéuser C, et al. (2011). Gray Matter NG2 Cells Display Multiple Ca2+-Signaling Pathways and Highly Motile Processes. PLOS ONE 6, e17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Clarke LE, IL A-C, Kougioumtzidou E, Matthey M, Káradóttir R, Whiteley L, Bergersen LH, Richardson WD, and Attwell D (2017). Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia 65, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, and Attwell D (2012). The Energetics of CNS White Matter. The Journal of Neuroscience 32, 356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasel P, Dando O, Jiwaji Z, Baxter P, Todd AC, Heron S, Márkus NM, McQueen J, Hampton DW, Torvell M, et al. (2017). Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nature Communications 8, 15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Goncalves CM, Grutzendler J, and Nishiyama A (2014). Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nature Neuroscience 17, 1518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Li AM, and Grutzendler J (2018). Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci 21, 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, and Appel B (2015). Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci 18, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, and Strittmatter SM (2004). Regulating axon growth within the postnatal central nervous system. Seminars in Perinatology 28, 371–378. [DOI] [PubMed] [Google Scholar]
- Hughes AN, and Appel B (2019). Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nature Communications 10, 4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AN, and Appel B (2020). Microglia phagocytose myelin sheaths to modify developmental myelination. Nature Neuroscience 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, and Bergles DE (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci 16, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Orthmann-Murphy JE, Langseth AJ, and Bergles DE (2018). Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF, and Stämpeli R (1949). Evidence for saltatory conduction in peripheral myelinated nerve fibres. The Journal of Physiology 108, 315–339. [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, and Fields RD (2006). Astrocytes Promote Myelination in Response to Electrical Impulses. Neuron 49, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Sharyl L,F-M, Furusho M, Miller RH, and Bansal R (2012). ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci 32, 8855–8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Dupree JL, and Bansal R (2016). Strength of ERK1/2 MAPK Activation Determines Its Effect on Myelin and Axonal Integrity in the Adult CNS. J. Neurosci 36, 6471–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Gould E, Xu J, Kim EJ, and Kim JH (2019). Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem. ELife 8, e42156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries MA, Urbanek K, Torres L, Wendell SG, Rubio ME, and Sharyl L,F-M (2016). ERK1/2 Activation in Preexisting Oligodendrocytes of Adult Mice Drives New Myelin Synthesis and Enhanced CNS Function. J. Neurosci 36, 9186–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K, and Dent EW (2014). Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nature Reviews Neuroscience 15, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori T, Yoshino J, Yasunaga K, Dairyo Y, and Emoto K (2015). Local endocytosis triggers dendritic thinning and pruning in Drosophila sensory neurons. Nature Communications 6, 6515. [DOI] [PubMed] [Google Scholar]
- Káradüttir R, and Attwell D (2007). Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience 145, 1426–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradüttir R, Cavelier P, Bergersen LH, and Attwell D (2005). NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karttunen MJ, Czopka T, Goedhart M, Early JJ, and Lyons DA (2017). Regeneration of myelin sheaths of normal length and thickness in the zebrafish CNS correlates with growth of axons in caliber. PLoS ONE 12, e0178058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato D, Wake H, Lee PR, Tachibana Y, Ono R, Sugio S, Tsuji Y, Tanaka YH, Tanaka YR, Masamizu Y, et al. (2020). Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 68, 193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole K (2015). Experience-dependent plasticity of neurovascularization. Journal of Neurophysiology 114, 2077–2079. [DOI] [PubMed] [Google Scholar]
- Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, and Lyons DA (2016). Individual Neuronal Subtypes Exhibit Diversity in CNS Myelination Mediated by Synaptic Vesicle Release. Current Biology 26, 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougioumtzidou E, Shimizu T, Hamilton NB, Tohyama K, Sprengel R, Monyer H, Attwell D, and Richardson WD (2017). Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife 6, e28080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow AM, Ford MC, Valdivia LE, Wilson SW, and Attwell D (2018). Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat. Neurosci 21, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Nishiyama A, and Dietrich D (2010). The Fate of Synaptic Input to NG2 Glial Cells: Neurons Specifically Downregulate Transmitter Release onto Differentiating Oligodendroglial Cells. The Journal of Neuroscience 30, 8320–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Comin CH, Ben-Zvi A, Kaeser PS, Xu X, Costa L. da F., and Gu C (2014). Sensory-Related Neural Activity Regulates the Structure of Vascular Networks in the Cerebral Cortex. Neuron 83, 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl I, Reichova I, and Ferster D (1999). Synchronous Membrane Potential Fluctuations in Neurons of the Cat Visual Cortex. Neuron 22, 361–374. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, and Nave K-A Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. 33, 366. [DOI] [PubMed] [Google Scholar]
- Larson VA, Mironova Y, Vanderpool KG, Waisman A, Rash JE, Agarwal A, and Bergles DE (2018). Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. Elife 7, e34829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SB, 2001. Energy as a constraint on the coding and processing of sensory information. Curr. Opin. Neurobiol 11, 475–480. [DOI] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong S, Mellon SH, Tuck SJ, Feng Z-QQ, Corey JM, and Chan JR (2012a). A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang P-W, et al. (2012b). Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Brus-Ramer M, Martin JH, and McDonald JW (2010). Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neuroscience Letters 479, 128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Du X, Liu C, Wen Z, and Du J (2012). Reciprocal Regulation between Resting Microglial Dynamics and Neuronal Activity In Vivo. Developmental Cell 23, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Lin S, and Bergles DE (2003). Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nature Neuroscience 7, 24–32. [DOI] [PubMed] [Google Scholar]
- Lin SC, and Bergles DE (2004). Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci 7, 24–32. [DOI] [PubMed] [Google Scholar]
- Lin S-CC, Huck JH, Roberts J, Macklin WB, Somogyi P, and Bergles DE (2005). Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron 46, 773–785. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, et al. (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature Neuroscience 15, 1621–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YU, Ying Y, Li Y, Eyo UB, Chen T, Zheng J, Umpierre AD, Zhu J, Bosco DB, Dong H, et al. (2019). Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nature Neuroscience 22, 1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwar B.Ch., Weiss KR, and Cropper EC (2020). Background calcium induced by subthreshold depolarization modifies homosynaptic facilitation at a synapse in Aplysia. Scientific Reports 10, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HOO, Franklin RJ, and Attwell D, et al. (2013). Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, and Corfas G (2012). A Critical Period for Social Experience-Dependent Oligodendrocyte Maturation and Myelination. Science 337, 1357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado PP, Vález-Fort M, and Angulo M (2011). Is neuronal communication with NG2 cells synaptic or extrasynaptic? Journal of Anatomy 219, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marisca R, Hoche T, Agirre E, Hoodless LJ, Barkey W, Auer F, Castelo-Branco G, and Czopka T (2020). Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nature Neuroscience 23, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral SR, and Chan JR (2016). The environment rules: spatiotemporal regulation of oligodendrocyte differentiation. Curr. Opin. Neurobiol 39, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral SR, Etxeberria A, Shen Y-AA, and Chan JR (2018). Initiation of CNS Myelination in the Optic Nerve Is Dependent on Axon Caliber. Cell Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchen ME, Bramham CR, and Pozzo-Miller LD (2002). Modulation of neuronal calcium signaling by neurotrophic factors. Int J Dev Neurosci 20, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, Faria J.P. [ISP-CHECK]de, Emery B, Tohyama K, and Richardson WD (2014). Motor skill learning requires active central myelination. Science (New York, N.Y.) 346, 318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, and Boucrot E (2015). Membrane curvature at a glance. Journal of Cell Science 128, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Fancy SP, Shen Y-AA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, et al. (2014). Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med 20, 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Klaus L-H, Shen Y-AA, Rankin KA, Stebbins KJ, Lorrain DS, Pekarek K, A Sagan S, Xiao L, Teuscher C, et al. (2016). Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife 5, e18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, Manira A, and Lyons DA (2015). Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nature Neuroscience 18, 628–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, and Bock DD (2016). A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife 5, e15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, et al. (2006). NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992. [DOI] [PubMed] [Google Scholar]
- Micu I, Plemel JR, Lachance C, Proft J, Jansen AJ, Cummins K, Minnen J van, and Stys PK (2016). The molecular physiology of the axo-myelinic synapse. Experimental Neurology 276, 41–50. [DOI] [PubMed] [Google Scholar]
- Mitew S, Gobius I, Fenlon LR, Stuart J,M, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, et al. (2018). Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat Commun 9, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Meschkat M, Ruhwedel T, Trevisiol A, Tzvetanova ID, Battefeld A, Kusch K, Kole MHP, Strenzke N, Möbius W, et al. (2020). A role of oligodendrocytes in information processing. Nature Communications 11, 5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BM, Lee Y, and Rothstein JD (2013). Oligodendroglia: metabolic supporters of axons. Trends Cell Biol. 23, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount CW, Yalçin B, Cunliffe-Koehler K, Sundaresh S, and Monje M (2019). Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. ELife 8, e49291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B, Hovhannisyan A, Barzan R, Chen T-J, and Kukley M (2017). Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biology 15, e2001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HN, Treichel AJ, Eggum EN, Martell MR, Kaiser AJ, Trudel AG, Gronseth JR, Maas ST, Bergen S, and Hines JH (2020). Individual neuronal subtypes control initial myelin sheath growth and stabilization. Neural Development 15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori R, Park D, Griffiths JD, Bells S, Frankland PW, Mabbott D, and Lefebvre J (2020). Activity-dependent myelination: A glial mechanism of oscillatory self-organization in large-scale brain networks. Proceedings of the National Academy of Sciences 117, 13227–13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orduz D, Maldonado PP, Balia M, Vélez-Fort M, Sars V. [ISP-CHECK]de, Yanagawa Y, Emiliani V, and Angulo MC (2015). Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex. ELife 4, e06953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Abrams CK, and Scherer SS (2008). Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci 35, 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz FC, Habermacher C, Graciarena M, Houry P-Y, Nishiyama A, Oumesmar BN, and Angulo MC (2019). Neuronal activity in vivo enhances functional myelin repair. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai Y, Shimizu T, Mori T, Hatanaka N, Kimori Y, Kobayashi K, Koyama S, Yoshimura Y, Nambu A, and Ikenaka K (2018). Length of myelin internodes of individual oligodendrocytes is controlled by microenvironment influenced by normal and input-deprived axonal activities in sensory deprived mouse models. Glia 66, 2514–2525. [DOI] [PubMed] [Google Scholar]
- Osso LA, and Chan JR (2017). Architecting the myelin landscape. Curr. Opin. Neurobiol 47, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez PM, Lyons DA, 2020. Calcium Signaling in the Oligodendrocyte Lineage: Regulators and Consequences. Annu. Rev. Neurosci 43, 163–186. [DOI] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, and Fields RD (2013). Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, and Chan JR (2017). Regulation and dysregulation of axon infrastructure by myelinating glia. The Journal of Cell Biology 216, 3903–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Mayoral SR, Choi HS, Chan JR, and Kheirbek MA (2020). Preservation of a remote fear memory requires new myelin formation. Nature Neuroscience 23, 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passlick S, Grauer M, Schäfer C, Jabs R, Seifert G, and Steinhäuser C (2013). Expression of the γ2-Subunit Distinguishes Synaptic and Extrasynaptic GABAA Receptors in NG2 Cells of the Hippocampus. The Journal of Neuroscience 33, 12030–12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease SE, and Segal RA (2014). Preserve and protect: maintaining axons within functional circuits. Trends in Neurosciences 37, 572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, and Snider W (2010). Initiating and Growing an Axon. Cold Spring Harbor Perspectives in Biology 2, a001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, and Jacobson MD (1993). Programmed cell death and the control of cell survival: lessons from the nervous system. Science 262, 695–700. [DOI] [PubMed] [Google Scholar]
- Rama S, Zbili M, and Debanne D (2018). Signal propagation along the axon. Current Opinion in Neurobiology 51, 37–44. [DOI] [PubMed] [Google Scholar]
- Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, and Chan JR (2008). The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl. Acad. Sci. U.S.A 105, 14662–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosskothen-Kuhl N, Hildebrandt H, Birkenhäger R, and Illing R-B (2018). Astrocyte Hypertrophy and Microglia Activation in the Rat Auditory Midbrain Is Induced by Electrical Intracochlear Stimulation. Frontiers in Cellular Neuroscience 12, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton WAH (1951). A theory of the effects of fibre size in medullated nerve. The Journal of Physiology 115, 101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab AS, Tzvetanova ID, and Nave K-AA (2013). The role of myelin and oligodendrocytes in axonal energy metabolism. Curr. Opin. Neurobiol 23, 1065–1072. [DOI] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Möbius W, Goetze B, Jahn HM, Huang W, et al. (2016). Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 91, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, and Fern R (2005). NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 438, 1167–1171. [DOI] [PubMed] [Google Scholar]
- Schirmer L, Möbius W, Zhao C, Cruz-Herranz A, Haim L, Cordano C, Shiow LR, Kelley KW, Sadowski B, Timmons G, et al. (2018). Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity. ELife 7, e36428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, and Schnell L (1989). Region-specific appearance of myelin constituents in the developing rat spinal cord. Journal of Neurocytology 18, 161–169. [DOI] [PubMed] [Google Scholar]
- Segal RA, and Greenberg ME (1996). Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19, 463–489. [DOI] [PubMed] [Google Scholar]
- Segel M, Neumann B, Hill MF, Weber IP, Viscomi C, Zhao C, Young A, Agley CC, Thompson AJ, Gonzalez GA, et al. (2019). Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, and Rubel EW (2016). Systematic and differential myelination of axon collaterals in the mammalian auditory brainstem. Glia 64, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW, and Harris DM (2010). Mechanisms for Adjusting Interaural Time Differences to Achieve Binaural Coincidence Detection. The Journal of Neuroscience 30, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, and McCormick DA (2006). Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature 441, 761–765. [DOI] [PubMed] [Google Scholar]
- Simpson AH, Gillingwater TH, Anderson H, Cottrell D, Sherman DL, Ribchester RR, and Brophy PJ (2013). Effect of Limb Lengthening on Internodal Length and Conduction Velocity of Peripheral Nerve. The Journal of Neuroscience 33, 4536–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JL, Fischl MJ, Alexandrova O, Heβ M, Grothe B, Leibold C, and Conny K-S (2017). Sound-Evoked Activity Influences Myelination of Brainstem Axons in the Trapezoid Body. J. Neurosci 37, 8239–8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, and Koles Z (1970). Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. American Journal of Physiology-Legacy Content 219, 1256–1258. [DOI] [PubMed] [Google Scholar]
- Snaidero N, Möbius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, et al. (2014). Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldado-Magraner S, Brandalise F, Honnuraiah S, Pfeiffer M, Moulinier M, Gerber U, and Douglas R (2020). Conditioning by subthreshold synaptic input changes the intrinsic firing pattern of CA3 hippocampal neurons. Journal of Neurophysiology 123, 90–106. [DOI] [PubMed] [Google Scholar]
- Spitzer SO, Sitnikov S, Kamen Y, Evans KA, Kronenberg-Versteeg D, Dietmann S, Faria O. [ISP-CHECK]de, Agathou S, and Káradóttir RT (2019). Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 101, 459–471.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, and Frankland PW (2020). Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 105, 150–164.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedehouder J, Couey J, Brizee D, Hosseini B, Slotman J, Dirven CMFM, Shpak G, Houtsmuller A, and Kushner S (2017). Fast-spiking Parvalbumin Interneurons are Frequently Myelinated in the Cerebral Cortex of Mice and Humans. Cereb. Cortex 27, 5001–5013. [DOI] [PubMed] [Google Scholar]
- Stedehouder J, Brizee D, Shpak G, and Kushner SA (2018). Activity-Dependent Myelination of Parvalbumin Interneurons Mediated by Axonal Morphological Plasticity. J. Neurosci 38, 3631–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, and Fields R (2002). Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock R (1980). Myelination of the mouse corpus callosum. Neuropathology and Applied Neurobiology. [DOI] [PubMed] [Google Scholar]
- Sun LO, Mulinyawe SB, Collins HY, Ibrahim A, Li Q, Simon DJ, Tessier-Lavigne M, and Barres BA (2018). Spatiotemporal Control of CNS Myelination by Oligodendrocyte Programmed Cell Death through the TFEB-PUMA Axis. Cell 175, 1811–1826.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swire M, Kotelevtsev Y, Webb DJ, Lyons DA, and ffrench-Constant C (2019). Endothelin signalling mediates experience-dependent myelination in the CNS. ELife 8, e49493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tozuka Y, Takata T, Shimazu N, Matsumura N, Ohta A, and Hisatsune T (2009). Excitatory GABAergic Activation of Cortical Dividing Glial Cells. Cereb Cortex 19, 2181–2195. [DOI] [PubMed] [Google Scholar]
- Tauber H, Waehneldt TV, and Neuhoff V (1980). Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neuroscience Letters 16, 235–238. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen H-HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung H, Lichtman JW, and Arlotta P (2014). Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, Kubo T, Matsuda K, Yano K, Tohyama M, and Hosokawa K (2007). Myelin-associated glycoprotein reduces axonal branching and enhances functional recovery after sciatic nerve transection in rats. Glia 55, 1498–1507. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nishiyama A, Cheng D, and Macklin W (1997). Differentiation and Death of Premyelinating Oligodendrocytes in Developing Rodent Brain. Journal of Cell Biology 137, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M-È, Lowery RL, and Majewska AK (2010). Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RB, Jackiewicz M, Ian A,M, Kougioumtzidou E, Grist M, and Richardson WD (2017). Remarkable Stability of Myelinating Oligodendrocytes in Mice. Cell Rep 21, 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux J-P, Rodriguez E, and Martinerie J (2001). The brainweb: Phase synchronization and large-scale integration. Nature Reviews Neuroscience 2, 229–239. [DOI] [PubMed] [Google Scholar]
- Vélez-Fort M, Maldonado PP, Butt AM, Audinat E, and Angulo MC (2010). Postnatal Switch from Synaptic to Extrasynaptic Transmission between Interneurons and NG2 Cells. The Journal of Neuroscience 30, 6921–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee P, and Fields DR (2010). Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, and Fields R (2014). Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nature Communications 6, 7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MCC, and Fields R (2015). Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun 6, 7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Yang Y-J, Yang N, Chen X-J, Huang N-X, Zhang J, Wu Y, Liu Z, Gao X, Li T, et al. (2018). Enhancing oligodendrocyte myelination rescues synaptic loss and improves functional recovery after chronic hypoxia. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Ren S-YY, Chen J-FF, Liu K, Li R-XX, Li Z-FF, Hu B, Niu J-QQ, Xiao L, Chan JR, et al. (2020). Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nature Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S, and Bennett M (1972). Relative Conduction Velocities of Small Myelinated and Non-myelinated Fibres in the Central Nervous System. Nature New Biology 238, 217–219. [DOI] [PubMed] [Google Scholar]
- Wong AW, Xiao J, Kemper D, Kilpatrick TJ, and Murray SS (2013). Oligodendroglial Expression of TrkB Independently Regulates Myelination and Progenitor Cell Proliferation. The Journal of Neuroscience 33, 4947–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Stone S, Nave K-A, and Lin W (2020). The integrated UPR and ERAD in oligodendrocytes maintains myelin thickness in adults by regulating myelin protein translation. The Journal of Neuroscience JN–RM-0604-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Ohayon D, Ian A,M, Alexander S-W, Wright JL, Fudge AD, Emery B, Li H, and Richardson WD (2016). Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci 19, 1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, and Chan JR (2020). Myelin plasticity: sculpting circuits in learning and memory. Nature Reviews Neuroscience 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Mironova YA, Shen H, Marino RAM, Waisman A, Lamers WH, Bergles DE, and Bonci A (2019). Oligodendrocytes Support Neuronal Glutamatergic Transmission via Expression of Glutamine Synthetase. Cell Reports 27, 2262–2271.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Uesaka N, Hayano Y, Tabata T, Kano M, and Yamamoto N (2010). Role of pre- and postsynaptic activity in thalamocortical axon branching. Proceedings of the National Academy of Sciences 107, 7562–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Hozumi Y, Kaneko K, and Fujii S (2018). Modulatory Effects of Perineuronal Oligodendrocytes on Neuronal Activity in the Rat Hippocampus. Neurochemical Research 43, 27–40. [DOI] [PubMed] [Google Scholar]
- Yang S, Li C, Qiu X, Zhang L, Lu W, Chen L, Zhao YY, Shi XY, Huang CX, Cheng GH, et al. (2013). Effects of an enriched environment on myelin sheaths in the white matter of rats during normal aging: A stereological study. Neuroscience 234, 13–21. [DOI] [PubMed] [Google Scholar]
- Yang SM, Michel K, Jokhi V, Nedivi E, and Arlotta P (2020). Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap E-L, and Greenberg ME (2018). Activity-Regulated Transcription: Bridging the Gap between Neural Activity and Behavior. Neuron 100, 330–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MSY, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, et al. (2014). Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774. [DOI] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn S-JJ, Cossell L, Attwell D, Tohyama K, and Richardson WD (2013). Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SPJ, Zahed H, Maltepe E, and Rowitch DH (2014). Oligodendrocyte-Encoded HIF Function Couples Postnatal Myelination and White Matter Angiogenesis. Cell 158, 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmar A, Chen C-C, Weinmann O, Kast B, Vajda F, Bozeman J, Isaad N, Zuo Y, and Schwab ME (2018). Oligodendrocyte- and Neuron-Specific Nogo-A Restrict Dendritic Branching and Spine Density in the Adult Mouse Motor Cortex. Cerebral Cortex (New York, N.Y. : 1991) 28, 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, Sean O, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y-Y, Shi X-Y, Qiu X, Zhang L, Lu W, Yang S, Li C, Cheng G-H, Yang Z-W, and Tang Y (2011). Enriched environment increases the total number of CNPase positive cells in the corpus callosum of middle-aged rats. Acta Neurobiologiae Experimentalis 71, 322–30. [DOI] [PubMed] [Google Scholar]
- Zheng J, Sun X, Ma C, Li B, and Luo F (2019). Voluntary wheel running promotes myelination in the motor cortex through Wnt signaling in mice. Molecular Brain 12, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, He C, Ren J, Dai C, Stevens SR, Wang Q, Zamler D, Shingu T, Yuan L, Chandregowda CR, et al. (2020). Mature myelin maintenance requires Qki to coactivate PPARβ-RXRα–mediated lipid metabolism. Journal of Clinical Investigation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, and Bergles DE (2007). Vesicular release of glutamate from unmyelinated axons in white matter. Nature Neuroscience 10, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonouzi M, Renzi M, Farrant M, and Cull-Candy SG (2011). Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells. Nature Neuroscience 14, 1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonouzi M, Berger D, Jokhi V, Kedaigle A, Lichtman J, and Arlotta P (2019). Individual Oligodendrocytes Show Bias for Inhibitory Axons in the Neocortex. Cell Reports 27, 2799–2808.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


