Abstract
Arsenic stress causes rapid transcriptional responses in plants. However, transcriptional regulators of arsenic-induced gene expression in plants remain less well known. To date, forward genetic screens have proven limited for dissecting arsenic response mechanisms. We hypothesized that this may be due to the extensive genetic redundancy present in plant genomes. To overcome this limitation, we pursued a forward genetics screen for arsenite tolerance using a randomized library of plants expressing >2,000 artificial microRNAs (amiRNAs). This library was designed to knock-down diverse combinations of homologous gene family members within sub-clades of transcription factor and transporter gene families. We identified six transformant lines showing an altered response to arsenite in root growth assays. Further characterization of an amiRNA line targeting closely homologous CBF and ERF transcription factors show that the CBF1,2 and 3 transcription factors negatively regulate arsenite sensitivity. Furthermore, the ERF34 and ERF35 transcription factors are required for cadmium resistance. Generation of CRISPR lines, higher-order T-DNA mutants, and gene expression analyses, further support our findings. These ERF transcription factors differentially regulate arsenite sensitivity and cadmium tolerance.
Keywords: amiRNA, redundancy, ERF transcription factors, heavy metal, arsenic
Summary statement:
The transcription factors (TFs) mediating resistance to arsenic and cadmium (Cd) remain largely unknown. A redundancy- and lethality-circumventing amiRNA screen uncovers CBFs as negative regulators of arsenite sensitivity and the ERF34 & 35 TFs as positive regulators mediating Cd resistance.
Introduction
Arsenic (As) is a carcinogenic metalloid found widely in the environment (Clemens & Ma, 2016; Cooper et al., 2020). Arsenic toxicity decreases crop yields, and accumulation of arsenic in edible crop tissues can cause contamination of the food chain and lead to severe human health problems (Clemens, 2019; Rai, Lee, Zhang, Tsang, & Kim, 2019; Abedi & Mojiri, 2020; Palma-Lara et al., 2020). For example, rice grain can accumulate up to 2.24 mg/kg As and has become a dominant dietary exposure route to arsenic (As) (Gilbert-Diamond et al., 2011; Pan, Wu, Xue, & Hartley, 2014). Thus, understanding the molecular mechanisms of As uptake and accumulation in plants is an urgent priority for food security worldwide.
Recent studies have identified genes involved in the uptake, transport, and sequestration of arsenic in Arabidopsis, rice, and other plant species (Mendoza-Cozatl, Jobe, Hauser, & Schroeder, 2011; Abbas et al., 2018; Kumari, Rastogi, Shukla, Srivastava, & Yadav, 2018; Garbinski, Rosen, & Chen, 2019; Shri et al., 2019; Abedi & Mojiri, 2020). These include nutrient transporters that transport arsenite (As(III)) across plant membranes and can function in arsenic accumulation, including Nodulin 26-like Intrinsic Proteins (NIPs), Plasma membrane Intrinsic Proteins (PIPs), Natural Resistance-Associated Macrophage Protein OsNRAMP1, and Tonoplast Intrinsic Proteins (TIP) (Bienert et al., 2008; Isayenkov & Maathuis, 2008; Kamiya & Fujiwara, 2009; Kamiya et al., 2009; Mosa et al., 2012; Tiwari et al., 2014; Xu et al., 2015; Yang et al., 2015; Duan et al., 2016b; Lindsay & Maathuis, 2016). Furthermore, phosphate transporters (PHTs) play a role in arsenate (As(V)) uptake (Shin, Shin, Dewbre, & Harrison, 2004; Catarecha et al., 2007; Remy et al., 2012; LeBlanc, McKinney, Meagher, & Smith, 2013; Fontenot et al., 2015), and arsenate reductase enzymes are responsible for the reduction of As(V) to arsenite As(III) (Chao et al., 2014; Shi et al., 2016; Xu et al., 2017). The enzymes γ-glutamylcysteine synthetase (g-ECS), glutathione synthetase (GS), and phytochelatin synthase (PCS) are critical enzymes for As detoxification (Schmoger, Oven, & Grill, 2000; Li et al., 2004; Picault et al., 2006; Gasic & Korban, 2007; Guo, Dai, Xu, & Ma, 2008; Herschbach et al., 2010; Wojas, Clemens, Sklodowska, & Maria Antosiewicz, 2010; Hayashi et al., 2017). Additionally, the ATP Binding Cassette (ABC) transporters ABCC1 and ABCC2 transport As-GSH and As-PC complexes into vacuoles as a mechanism for arsenic accumulation (Song et al., 2010; Song et al., 2014; Hayashi et al., 2017). The inositol transporters (AtINT2 and AtINT4) in A. thaliana and OsNIP6;1 and OsNIP7;1 in rice play a role in arsenic transport into seeds (Duan et al., 2016a; Lindsay & Maathuis, 2017).
Moreover, several regulatory proteins involved in plant responses to arsenic have been identified. For example, glutaredoxins (Grx) regulate As(V) reduction (Verma et al., 2017), and the transcription factors WRKY6, WRKY45, and OsARM1 (Arsenite-Responsive Myb1) have been implicated in the regulation of arsenic transporters (Castrillo et al., 2013; Wang et al., 2014; Wang et al., 2017). The calcium-dependent protein kinase (CPK31) was reported to regulate AtNIP1;1 (Ji et al., 2017), and miR528 was found to be necessary for As(III) responses (Liu et al., 2015). Despite the above list of genes identified as being involved in arsenic responses in plants, many genes and mechanisms have yet to be determined.
Arsenic is known to cause large transcriptional responses in plants (Chakrabarty et al., 2009; Jobe et al., 2012; Castrillo et al., 2013; Srivastava, Srivastava, Sablok, Deshpande, & Suprasanna, 2015; Zvobgo et al., 2018; Huang et al., 2019). Research has suggested that transcriptional activators and transcriptional repressors play essential roles in arsenic-induced gene expression (Jobe et al., 2012; Castrillo et al., 2013; Wang et al., 2014; Wang et al., 2017). While WRKY transcription factors have been shown to regulate phosphate transporters that contribute to arsenic tolerance (Castrillo et al., 2013; Wang et al., 2014; Wang et al., 2017), additional transcription factors that mediate arsenic-induced gene expression and repressors remain unknown.
Forward genetic screens are powerful tools for identifying new genes. However, forward genetic screens have limitations due to the extensive over-lapping functions of closely related genes (“redundancy”) mediated by large gene families found in plant genomes (Arabidopsis Genome, 2000). Phenotypes linked to a single gene loss-of-function mutation have been found for less than 15% of the genes encoded in the Arabidopsis genome (Lloyd & Meinke, 2012; Cusack et al., 2020). A genome-wide analysis showed that approximately 75% of Arabidopsis genes are members of gene families (Hauser et al., 2013). Thus, partially overlapping gene functions, referred to as functional redundancies, have hampered the identification of new gene functions in forward genetic screens. In previous research, we computationally designed artificial microRNAs (amiRNAs) (Schwab, Ossowski, Riester, Warthmann, & Weigel, 2006), and synthesized 22,000 amiRNAs designed to combinatorially co-silence closely homologous gene clade members (Hauser et al., 2013). These amiRNAs were separated into 10 amiRNA libraries for genome-wide knockdown of homologous gene family members based on the predicted functional classes of the genes they target (Hauser et al., 2013; Hauser et al., 2019). Using these 22,000 amiRNAs, libraries were synthesized (Hauser et al., 2013) and used to transform Arabidopsis. To date, 14,000 T2 generation lines have been generated (Hauser et al., 2019). Recent studies have used this resource to discover new genes and determine their functions in plant hormone signaling and transport, illustrating the power of this system (Hauser et al., 2013; Zhang et al., 2018; Hauser et al., 2019; Takahashi et al., 2020).
To overcome the genetic redundancy limitations, we screened amiRNA seed libraries targeting two different gene classes (1: DNA and RNA binding proteins and 2: transporters and channels) for arsenic tolerance. Here, we report the identification of new transcription factors and transporters that play crucial roles in arsenic responses.
Material and Methods
Genetic screen for mutants with altered arsenite response
Wild type (Col-0) and individually isolated T2 lines from the amiRNA libraries (Hauser et al., 2013) were surface sterilized, stratified at 4°C for 48 hrs in the dark, and germinated under a 16 hrs light/ 8 hrs dark photoperiod.
For root length assays(Lee, Chen, & Schroeder, 2003; Jobe et al., 2012; Mendoza-Cozatl et al., 2014), 1/2 MS plates (1/2 MS, 0.5 g/L MES, 0.5% sucrose, 1% phytagel, adjusted with KOH to pH 5.5) were supplemented with/without 10 μM sodium arsenite and allowed to grow vertically for 14 days. The root lengths of seedlings were measured using Image J. The same protocol was performed on the next generation of (T3) seeds for candidate lines showing a phenotype.
Root length assays for erf34×35, CRISPRcbf1/2/3 were performed on minimal medium (5 mM KNO3, 2.5 mM H3PO4, 1 mM Ca(NO3)2, 2 mM MgSO4, 1 mM MES, adjusted with KOH to pH 5.5, 1% phytagel) without microelements supplied with and without 10 μM arsenite.
Identification of amiRNA sequences
Genomic DNA for candidate amiRNA lines were extracted(Kang, Cho, Yoon, & Eun, 1998) and used as templates, and the primers (primers pha2804f and pha3479r(Hauser et al., 2019), Supplemental Table 1) were used to amplify the PCR product. The purified PCR samples were sent for sequencing to identify the amiRNA present(Hauser et al., 2019). The genes targeted by the amiRNA could be putatively identified by using the “Target Search Function” on the WMD3 website (Ossowski, Fitz, Schwab, Riester, & Weigel). Several studies have demonstrated the accuracy of in-silico predictions of amiRNA target genes (Hauser et al., 2013; Zhang et al., 2018; Hauser et al., 2019; Takahashi et al., 2020).
Plasmid Construction
To test whether the phenotype of the amiRNA lines was caused by the knockdown of target genes, and to confirm that the phenotypes were not caused by the position of the amiRNA insertion, we generated retransformed lines using the genomic DNA of candidate amiRNA lines as a template. We named these lines Re-10-9. The primers RE-AMI-F and RE-AMI-R were used to amplify the original amiRNA and inserted it into pDONR221® using BP Clonase II® and then recombined into pFH0032 (Hauser et al., 2013; Hauser et al., 2019) using LR Clonase II® (Invitrogen, Carlsbad,CA, USA). (Supplemental Table 1).
We generated erf34×erf35 by crossing the T-DNA lines. Primers for genotyping were designed using the website (http://signal.salk.edu/tdnaprimers.2.html). A list of primers used in this study is provided in Supplemental Table 2.
We used CRISPR/Cas9 gene editing technology (Gao & Zhao, 2014; Gao et al., 2015; Gao, Chen, Dai, Zhang, & Zhao, 2016) to generate cbf1/2/3 knockout mutants. Guide RNA 1 and 2 were used to delete the CBF1/2/3 genes, which are tandemly arrayed on chromosome IV. The target sequence for target 1 was CCGATTACGAGCCTCAAGGCGG, and the sequence for target 2 was CCGGAACAGAGCCAAGATGCGT (PAM sites are underlined). The GT1-3 primers were used to isolate the homozygous lines for CRISPR cbf1/2/3. GT1 is the forward primer, GT2 and GT3 are reverse primers. The homozygous plants were isolated as: GT1+GT2 has band, and no band for GT1+GT3 (Supplemental Figure 3A and B).
Plant Transformation
Sequenced plasmids were transformed into Agrobacterium tumefacians strain GV3101 and pSoup was used as the helper plasmid for pFH0032. Arabidopsis thaliana was transformed as previously described using the floral dip method(Clough & Bent, 1998).
Total RNA isolation and quantitative RT-PCR
For RT-qPCR, plants were grown vertically on 1/2 MS media for 14 days and transfer to liquid treatment (1/2 MS, 0.5 g/L MES, 0.5% sucrose, pH 5.5–5.8 with/without 10 μM Arsenite) for 3 days. Leaves and roots were separated, and RNA was prepared using the Spectrum Plant Total RNA Kits (Sigma-Aldrich). cDNA was then prepared from RNA using Maxima H Minus First Strand cDNA Synthesis Kit (Thermo Scientific™) for RT-PCRs. Transcript abundance was then determined with SYBR® Green (Bio-Rad, MO, USA) using a Bio-Rad CFX96 Real-Time System with the following conditions: 95 °C for 5 min, then 50 cycles of 95°C for 15 s, 52°C for 15 s, 72°C for 1 min, then 72°C for 5 min. Primers used for qPCR(Versaw & Harrison, 2002; B. Guo et al., 2008; Remy et al., 2012; Lapis-Gaza, Jost, & Finnegan, 2014) are listed in Supplemental Table 3.
Results
Genetic screen for mutants with altered arsenite response
In previous research, arsenite was found to decrease primary root growth, inhibit seedling development, and cause chlorosis (Liu, Zhang, Shan, & Zhu, 2005; Yoon, Lee, & An, 2015; Qian et al., 2018). We tested a range of arsenite concentrations on 1/2 MS media-containing plates to establish the concentration at which wild-type seedling root growth was decreased by 40%–60% compared to control plates. We chose a concentration of 10 μM arsenite for root length screening.
In total, ~2,000 T2 amiRNA expressing Arabidopsis transformant lines were screened. These amiRNA lines were screened individually in root growth assays. Seed transformed with amiRNA libraries targeting homologous genes that encode DNA and RNA binding proteins and transporter family proteins were screened (Hauser et al., 2013; Hauser et al., 2019). In the primary screen, 15 seeds per line were screened for differences in root growth compared to wild-type plants grown in parallel. Candidate lines showing an altered arsenite response were validated using the same phenotyping method in the next generation (T3). Isolated amiRNA lines with reproducible phenotypes were then analyzed for their putative target genes by sequencing and functional characterization. After screening these 2,000 amiRNA lines, six candidate amiRNA lines were identified with robust phenotypes. Their target genes and gene definitions are shown in Table 1 (e.g. Figure 1A–C and Supplemental Figure 1). In the present study, two amiRNA lines, amiRNA 4-138 and ami 10-9 were chosen for further characterization.
Table 1.
Candidate amiRNA lines with altered response to Arsenite and their target genes
| ID | PHENOTYPE | AMI SEQUENCE | TARGET GENES | GENE DESCRIPTION | VALIDATE D IN T3 | VALIDATED BY RETRANSFORM |
|---|---|---|---|---|---|---|
| 10-9 | More tolerant than WT | TATATAAGAAC CCGAACGCTC | AT5G43350 | Phosphate Transporter 1, PHT1, PHT1;1 | Yes | Yes |
| Root length | AT5G43370 | Phosphate Transporter 2, PHT2, PHT1;2 | ||||
| AT5G43360 | Phosphate Transporter 3, PHT3, PHT1;3 | |||||
| 4-138 | More tolerant than WT | TAACTTCTCAT CCGCACACCG | At2g44940 | Ethylene-response Transcription factor 34 (ERF34) | Yes | Yes |
| At3g60490 | Ethylene-response Transcription factor 35 (ERF35) | |||||
| Root length | At4g25480 | C-Rrepeat/DRE Binding Factor 3 (CBF3), DRE/CRT-Binding Protein 1C (DREB1A), | ||||
| At4g25470 | C-Rrepeat/DRE Binding Factor 2 (CBF2), DRE/CRT-Binding Protein 1b (DREB1B), Freezing Tolerance QTL 4 (FTQ4) | |||||
| At4g25490 | C-Rrepeat/DRE Binding Factor 1 (CBF1), DRE/CRT-Binding Protein 1c (DREB1C) | |||||
| At5g51990 | C-Rrepeat/DRE Binding Factor 4 (CBF4), DRE/CRT-Binding Protein 1d (DREB1D) | |||||
| 4-85 | More tolerant than WT | TATAAGAAATC TTGAGCACCT | AT1G10470 | Response Regulator 4 (ARR4), Induced By Cytokinin 7 (IBC7), Maternal Effect Embryo Arrest 7 (MEE7), | Yes | Yes |
| Root length | AT3G48100 | Response Regulator 5 (ARR5) | ||||
| AT5G62920 | Response Regulator 6 (ARR6) | |||||
| 4-89 | More tolerant than WT | TCTTTCCGAAA GGTCAAACTA | AT1G61660 | basic helix-loop-helix (bHLH) DNA-binding superfamily protein (ATBHLH112) | Yes | n.a |
| Root length | AT3G20640 | basic helix-loop-helix (bHLH) DNA-binding superfamily protein; | ||||
| AT2G31730 | basic helix-loop-helix (bHLH) DNA-binding superfamily protein; | |||||
| 4-82 | More tolerant than WT | TACTTTCCGGT GAGAGTGCGT | AT4G32890 | GATA Transcription Factor 9 (GATA9) | Yes | n.a |
| Root length | AT2G45050 | GATA Transcription Factor 2 (GATA2) | ||||
| AT4G36240 | GATA Transcription Factor 7 (GATA7) | |||||
| 4-184 | More tolerant than WT | ACGGGATTCA AGTCCGAAGCT | AT1G61990 | Mitochondrial Transcription Termination Factor Family Protein | Yes | n.a |
| Root length | AT1G62490 | Mitochondrial Transcription Termination Factor Family Protein | ||||
| AT1G61980 | Mitochondrial Transcription Termination Factor Family Protein | |||||
| AT1G61970 | Mitochondrial Transcription Termination Factor Family Protein | |||||
| AT1G62110 | Mitochondrial Transcription Termination Factor Family Protein | |||||
| AT1G47250 | 20S Proteasome Alpha Subunit 2 (PAF2) |
n.a: not applicable. Phenotypes have not been tested after amiRNA retransformation or via CRISPR or T-DNA gene knock outs.
Figure 1. amiRNA 4-138 targets ERF and CBF transcription factors, shows arsenite resistance, and phenocopies CRISPR lines targeting CBF1,2,3 genes but not erf34/35 double T-DNA mutants.
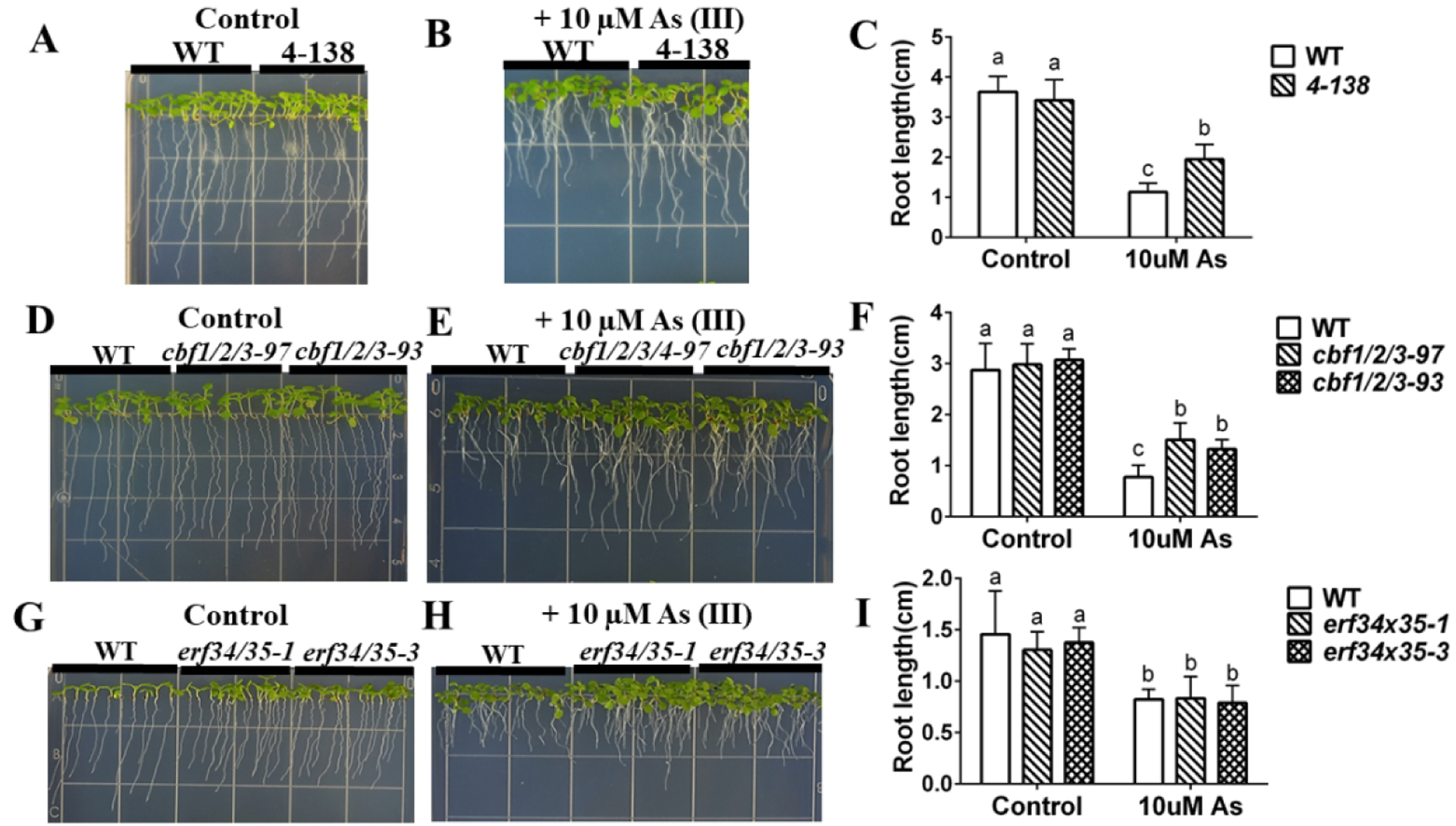
(A), (B) amiRNA 4-138 is more tolerant than WT to arsenite treatment: WT and amiRNA 4-138 lines were grown vertically on 1/2 MS with 0.5% sucrose plates without (control) or with 10 μM arsenite for 10 days; (C) Primary root lengths of amiRNA 4-138 seedlings were significantly longer than WT on arsenite-containing plates. (n=11 seedlings, means ± s.e.m.). (D), (E) CRISPR lines targeting CBF1/2/3 genes show arsenite resistance of root growth: CRISPR lines 97 and 93 targeting CBF1/2/3 were grown vertically on minimal media plates with 1% sucrose plates without (control) or with 10 μM arsenite for 7 days; (F) Primary root lengths of amiRNA 4-138 were significantly longer than WT on arsenite-containing plates. (n=11 seedlings, means ± s.e.m.). (H), (I) T-DNA double mutant in ERF34 and ERF35 genes showed similar root length to wild type under both control and arsenite treatment: WT and double mutant of erf34/35 line 1 and 3 were grown vertically on minimal plates with 1% sucrose plates without (control) or with 10 μM arsenite for 7 days. (I) Averaged primary root length (n=16 seedlings, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
amiRNA line 4-138 targets ERF and CBF transcription factors and is tolerant to arsenite
A mutant was isolated in the amiRNA screen showing an increased resistance to arsenite in root growth assays. Primary root lengths of amiRNA 4-138 seedlings were not different from WT on control plates but were significantly longer than WT on arsenite-containing plates (Figure 1A–C).
The isolated amiRNA 4-138 is predicted to target two different ERF transcription factor sub-families, specifically ERF 34, 35, and CBF 1, 2, 3, & 4. To determine which of these putative targets contributes to the arsenite-tolerant phenotype, we first screened single mutants and observed only weak or no phenotypes (see Supplemental figure 2A and C). Then double mutants were generated using T-DNA lines erf34-1 (SALK_020979C) and erf35-1 (SALK_111486C) (see Supplemental figure 4A to C). Furthermore, CRIPSR lines were created for cbf 1/2/3. Two independent cbf 1/2/3 CRISPR deletion alleles were isolated. The CRISPR lines targeting CBF1/2/3 did not show any visible phenotype under control conditions in plate growth assays (Figure 1D). However, both cbf1/2/3 CRISPR alleles showed arsenite resistance in seedling root growth assays (Figure 1D–F). In contrast, erf34/35 double mutants exhibited arsenite sensitivity similar to WT (Figure 1G–I). These data suggest that the arsenite-tolerant phenotype of amiRNA line 4-138 is caused by the knockdown of CBF 1, 2, 3 transcription factors, rather than the ERF 34 and 35 transcription factors.
CBF transcription factors and PHT transporters are down-regulated in response to arsenic treatment and are misregulated in the cbf1,2,3 mutant
The enhanced arsenic resistance of the cbf1/2/3 mutant alleles indicates that CBF transcription factors may function as negative regulators of arsenic resistance. To investigate whether the transcript levels of CBF1, CBF2, and CBF3 were affected by arsenite or arsenate stress, we measured the transcript levels of these transcription factors using qPCR. Interestingly, the transcript levels of these three genes decreased in seedlings exposed after 10 days of growth to 10 μM As(III) or 250 μM As (V) for 3 days (Figure 2A–C).
Figure 2. CBF1, CBF2 and CBF3 are transcriptionally downregulated by arsenite and arsenate in the roots.
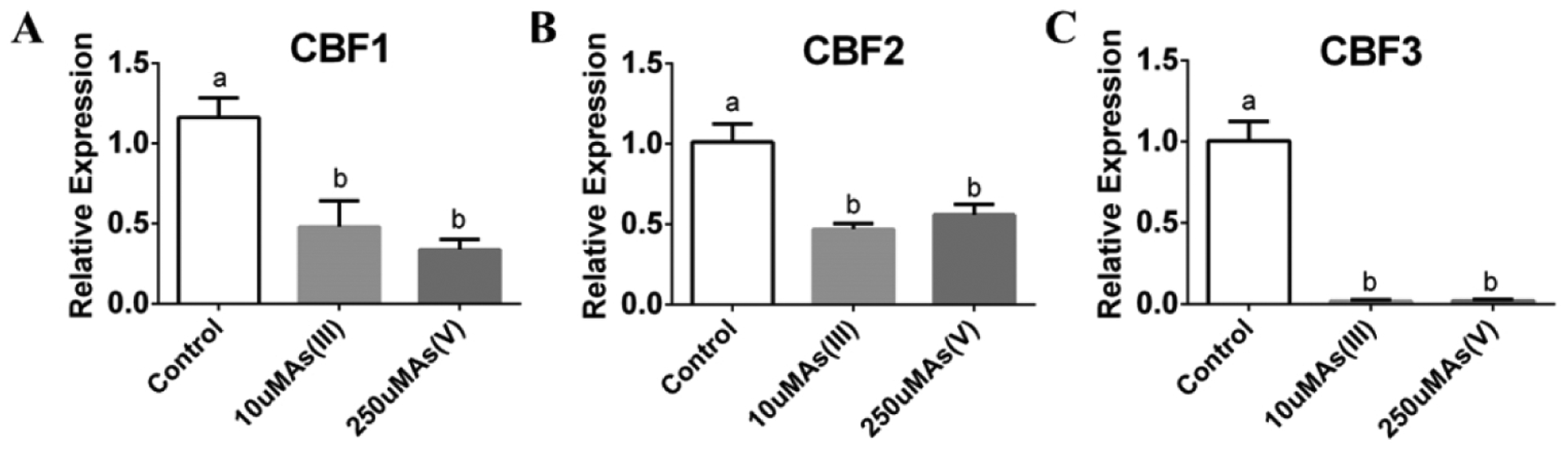
(A–C) Transcript levels of CBF1, 2, 3 under arsenite and arsenate treatment are significantly lower than control condition in roots of WT: WT plants were grown on 1/2 MS for 10 days and transferred to 1/2 MS plates with or without 10 μM sodium arsenite or 250 μM sodium arsenate for 3 days. Root tissues were harvested separately, followed by RNA extraction and cDNA synthesis, and the relative expression levels were detected by qRT-PCR. (n=3 samples, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
We examined possible DNA-binding targets of the CBF transcription factors. The Plant Cistrome Database (O’Malley et al., 2016) has used DNA affinity purification sequencing (DAP-seq) for resolving possible motifs and peaks for DNA binding domains of 529 recombinant Arabidopsis transcription factors. A list of genes putatively targeted by the CBF1-4 transcription factors was generated (O’Malley et al., 2016) (Supplemental data 2). Interestingly CBF1-4 binding was observed in the promoters of several phosphate transporters (PHT), including PHT1;1, PHT1;3, PHT1;6, PHT1;7, PHT1;8, PHT1;9, PHT3;3, PHT4;2, PHT2;1 PHT4;3 in these DAP-seq experiments (Table 2). Thus, we investigated whether these transporter genes might be targets of CBF1-4. Additionally, PHT1;1, PHT1;2 and PHT 1;3 were the target genes of the ami10-9 line that was isolated in our forward genetics screen, which unexpectedly showed an arsenite tolerant phenotype (Table 1), as described later.
Table 2.
Target phosphate transporters of CBF1, 2, 3 and 4
| TARGET GENES | GENE SYMBOL | GENE DESCRIPTION | |
|---|---|---|---|
| CBF1 | AT5G43340 | PHT1;6 | Phosphate Transporter 1;6 |
| AT5G43360 | PHT1;3 | Phosphate Transporter 1;3 | |
| AT1G20860 | PHT1;8 | Phosphate Transporter 1;8 | |
| AT1G76430 | PHT1;9 | phosphate transporter 1;9 | |
| AT2G17270 | PHT3;3 | phosphate transporter 3;3 | |
| AT2G38060 | PHT4;2 | phosphate transporter 4;2 | |
| AT3G26570 | PHT2;1 | phosphate transporter 2;1 | |
| AT3G46980 | PHT4;3 | phosphate transporter 4;3 | |
| CBF2 | AT1G76430 | PHT1;9 | phosphate transporter 1;9 |
| AT2G38060 | PHT4;2 | phosphate transporter 4;2 | |
| AT3G26570 | PHT2;1 | phosphate transporter 2;1 | |
| AT3G46980 | PHT4;3 | phosphate transporter 4;3 | |
| AT5G43360 | PHT1;3 | Phosphate Transporter 1;3 | |
| CBF3 | AT1G76430 | PHT1;9 | phosphate transporter 1;9 |
| AT3G46980 | PHT4;3 | phosphate transporter 4;3 | |
| AT3G26570 | PHT2;1 | phosphate transporter 2;1 | |
| CBF4 | AT1G20860 | PHT1;8 | Phosphate Transporter 1;8 |
| AT1G76430 | PHT1;9 | phosphate transporter 1;9 | |
| AT2G38060 | PHT4;2 | phosphate transporter 4;2 | |
| AT3G26570 | PHT2;1 | phosphate transporter 2;1 | |
| AT3G46980 | PHT4;3 | phosphate transporter 4;3 | |
| AT3G54700 | PHT1;7 | phosphate transporter 1;7 | |
| AT5G43350 | PHT1;1 | phosphate transporter 1;1 | |
| AT5G43360 | PHT1;3 | Phosphate Transporter 1;3 |
To determine whether transcript levels of PHT transporters are affected in the CRISPR cbf1/2/3 mutants, we measured the expression levels of the PHT transporter mRNAs. We found that the transcript levels of PHT1;1,1;2, 1;3 decreased significantly in wild-type Col-0 (WT) roots in response to arsenite treatment (Figure 3A–C). Furthermore, PHT 1;1, 1;2, and 1;3 transcript levels in the CRISPR cbf1/2/3 mutants were much lower in the roots under control conditions (Figure 3A–C). However, no differences between WT and CRISPR cbf1/2/3 mutants under arsenite treatment were observed, apparently because the transcripts were already expressed at low levels in arsenic-free controls (Figure 3A–C).
Figure 3. PHT transporter transcript levels are down-regulated in response to arsenite treatment and misregulated in roots of CRISPR cbf 1,2,3 mutant.
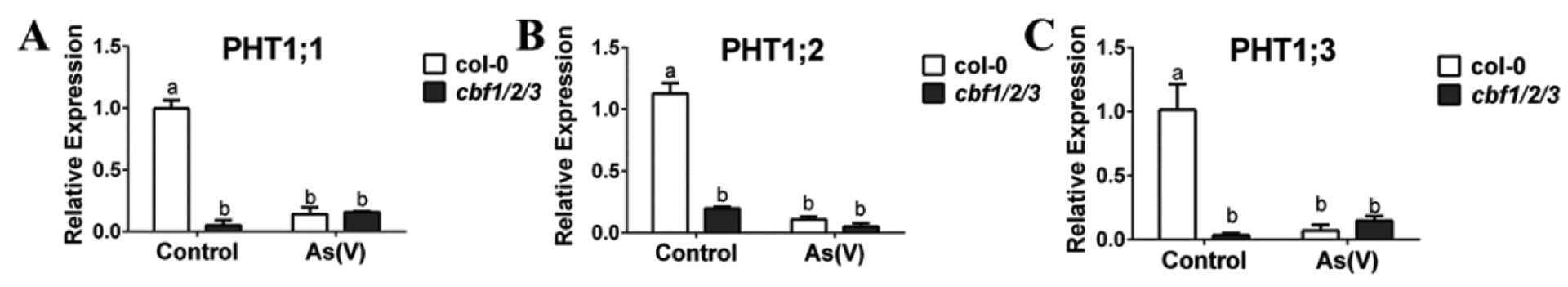
(A–C) PHT1;1; PHT1;2 and PHT1;3 are reduced significantly in roots by arsenite treatment, and PHT 1;1; PHT1;2 and PHT1;3 transcript levels in CRISPR cbf1/2/3 mutants are much lower in the roots under control conditions: WT and CRISPR cbf1/2/3 mutants were grown on 1/2 MS for 10 days and transferred to 1/2 MS plates with or without 10 μM arsenite for 3 days. Root tissues were harvested separately, followed by RNA extraction and cDNA synthesis, and the relative expression levels were detected by qRT-PCR. (n=3 samples, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
Because PHT transporters are known to transport phosphate and arsenate (Shin et al., 2004; Catarecha et al., 2007; Remy et al., 2012; LeBlanc et al., 2013; Fontenot et al., 2015), the transcript levels of PHTs were also measured under arsenate treatment. PHT 1;1, 1;2, 1;3 transcript levels were significantly decreased in wild-type roots in response to arsenate treatment (Figure 4A–C). In cbf1/2/3 triple mutants, the control non-stress transcript levels of these PHTs were decreased. These data suggest that CBF transcription factors play a role in upregulating several PHT transcripts under non-stress conditions.
Figure 4. PHT transporter transcript levels are down-regulated in response to arsenate treatment and misregulated in roots of CRISPR cbf 1,2,3 mutant.
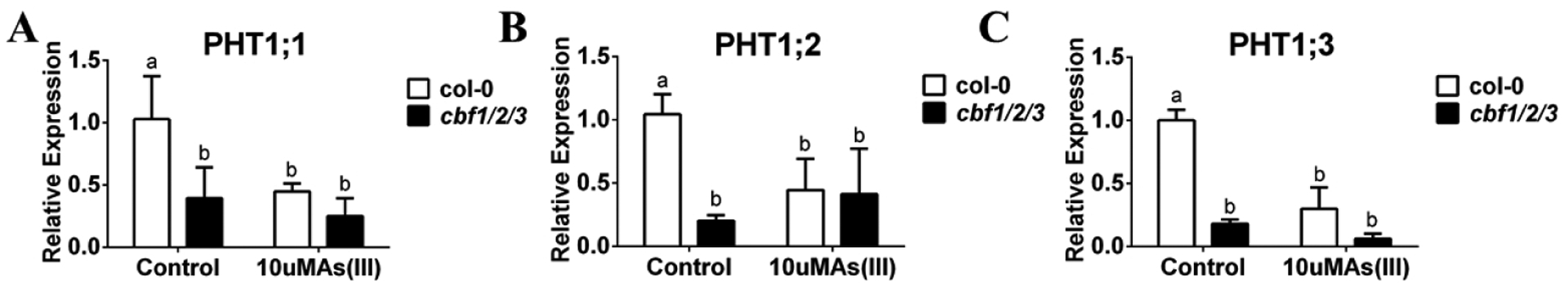
(A–C) PHT1;1; PHT1;2, PHT1;3 are reduced significantly in roots by arsenate treatment and PHT transcript levels in CRISPR cbf1/2/3 mutants are much lower in the roots under control conditions: WT and CRISPR cbf1/2/3 mutants were grown on 1/2 MS for 10 days and transferred to 1/2 MS plates with or without 250 μM sodium arsenite for 3 days. Root tissues were harvested separately, followed by RNA extraction and cDNA synthesis and the relative expression levels were detected by qRT-PCR. (n=3 samples, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
amiRNA 4-138 is sensitive to cadmium
Because arsenic and the toxic heavy metal cadmium elicit similar transcriptional responses (Abercrombie et al., 2008; Jobe et al., 2012; Shukla et al., 2018), we investigated whether amiRNA 4-138 also exhibits a cadmium-dependent phenotype. Interestingly, in contrast to As exposure (Figure 1A–C), seedlings of the amiRNA 4-138 line were more sensitive to cadmium compared to WT controls (Figure 5A–C). To determine which of the five predicted amiRNA targets contributes to the cadmium sensitivity, we evaluated root growth of the CRISPR cbf1/2/3 and erf34/35 mutant alleles in response to cadmium exposure. The two independent CRISPR alleles targeting CBF1/2/3 genes showed cadmium sensitive phenotypes that were only slightly more severe than WT controls (Figure 5D–F). Interestingly, however, the two erf34/35 double mutant alleles exhibited strong cadmium sensitivities compared to WT controls (Figure 5G–H). These data indicate that the ERF 34, 35 transcription factors and possibly in part the CBF 1, 2, 3 contribute to the cadmium sensitivity of the 4-138 amiRNA line.
Figure 5. amiRNA 4-138 line and erf34/erf35 double mutant are sensitive to cadmium.
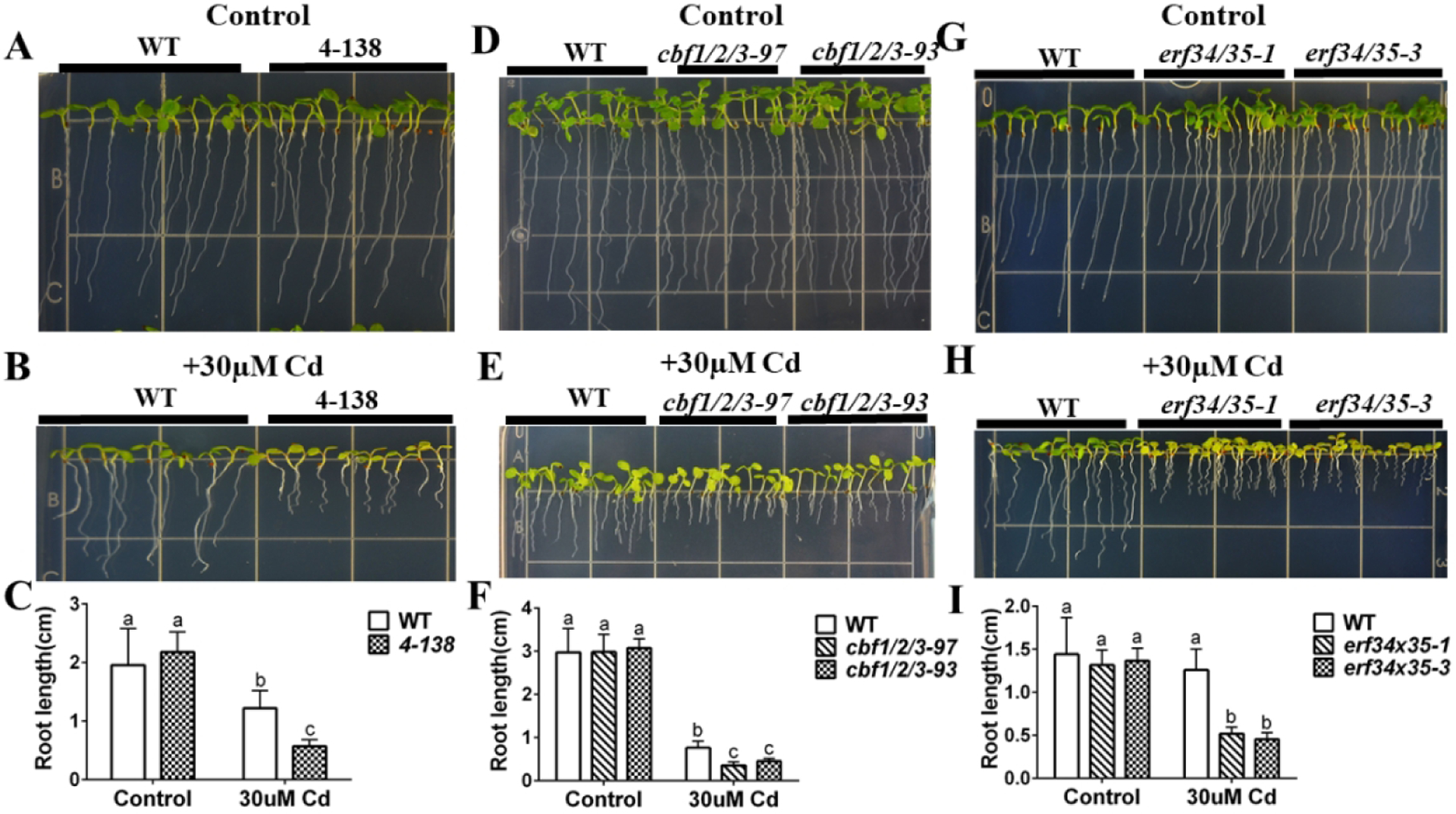
(A), (B) amiRNA line 4-138 shows enhanced cadmium sensitivity in root growth and yellowing of leaves compared to WT: WT and 4-138 amiRNA lines were grown vertically on minimal plates with 1% sucrose plates without (control) or with 30 μM cadmium for 7 days; (C) Primary root lengths of the amiRNA 4-138 line were significantly shorter than WT on cadmium-containing plates. (n=16 seedlings, means ± s.e.m.). (D), (E) CRISPR lines targeting CBF1/2/3 genes show cadmium sensitive phenotypes. WT and CRISPR lines targeting CBF1/2/3 genes were grown vertically on minimal plates with 1% sucrose plates without (control) or with 30 μM cadmium for 7 days; (F) Primary root length of CRISPR lines in CBF1/2/3 genes compared to WT on control and cadmium-containing plates. (n=11 seedlings, means ± s.e.m.). (G), (H) erf34/35 double mutant shows cadmium sensitive phenotype: WT and erf34/35-3 T-DNA double mutant were grown vertically on minimal plates with 1% sucrose plates without (control) or with 30 μM cadmium for 7 days; (I) Primary root length of erf34/35 compared to WT on control and cadmium-containing plates. (n=17 seedlings, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
Reduced NRT 1.8 transcript levels in erf34/35 in response to Cd
A list of candidate genes targeted by the ERF34 and ERF35 transcription factors were generated by searching The Plant Cistrome Database (O’Malley et al., 2016). The database was compared to genes significantly (p<0.05) induced more than 2-fold after 2 hours of exposure to 50 μM Cd2+ (Weber, Trampczynska, & Clemens, 2006). We examined possible DNA binding targets of ERF34 and ERF35, then analyzed which of these genes showed transcriptional regulation in response to cadmium exposure (Supplemental Figure 5). Based on the putative ERF34 and ERF35 targets from the Plant Cistrome Database, a list of candidate ERF34 and ERF35 targets also showing a transcriptional response to cadmium exposure was generated (Table 3). A previously identified nitrate transporter, NRT1.8, is a putative target of ERF34 and ERF35, based on analyses of the Plant Cistrome Database (O’Malley et al., 2016). Furthermore, the expression of NTR1.8 was induced 29-fold by cadmium treatment in WT (Weber et al., 2006) (Table 3). NRT1.8 belongs to the nitrate transporter (NRT1) family and functions in nitrate removal from the xylem sap (Li et al., 2010). NRT1.8 affects the content of Cd in the xylem sap and mediates tolerance to cadmium (Li et al., 2010).
Table 3.
Genes significantly (p<0.05) induced >2 folds after 2 hours of exposure to 50 μM Cd2+ in A. thaliana roots and targeted by EFR034 and ERF035.
| Gene ID | Gene Symbol | Gene description | Fold change | p-value |
|---|---|---|---|---|
| AT4G21680 | NRT1.8 | NITRATE TRANSPORTER 1.8 | 29 | 0.0406 |
| AT4G14680 | APS3 | Pseudouridine synthase/archaeosine transglycosylase-like family protein | 12.3 | 0.0401 |
| AT1G62300 | WRKY6 | WRKY family transcription factor | 5.6 | 0.0327 |
| AT4G01950 | GPAT3 | glycerol-3-phosphate acyltransferase 3 | 4.3 | 0.0367 |
| AT4G17500 | ERF-1 | ethylene responsive element binding factor 1 | 3.9 | 0.0483 |
| AT3G12580 | HSP70 | heat shock protein 70 | 3.4 | 0.0327 |
| AT1G08920 | ESL1 | ERD (early response to dehydration) six-like 1 | 3.3 | 0.0483 |
| AT2G32560 | AT2G32560 | F-box family protein | 3 | 0.0483 |
| AT1G78820 | AT1G78820 | D-mannose binding lectin protein with Apple-like carbohydrate-binding domain-containing protein | 2.9 | 0.04 |
| AT3G25230 | ROF1 | rotamase FKBP 1 | 2.7 | 0.0401 |
| AT4G26080 | ABI1 | Protein phosphatase 2C family protein | 2.6 | 0.0483 |
| AT3G47960 | GTR1 | Major facilitator superfamily protein | 2.5 | 0.0483 |
| AT2G18690 | AT2G18690 | transmembrane protein | 2.4 | 0.05 |
| AT2G41800 | AT2G41800 | imidazolonepropionase (Protein of unknown function, DUF642) | 2.4 | 0.0483 |
| AT2G21130 | AT2G21130 | Cyclophilin-like peptidyl-prolyl cis-trans isomerase family protein | 2.3 | 0.0418 |
| AT2G41410 | AT2G41410 | Calcium-binding EF-hand family protein | 2.3 | 0.0483 |
| AT5G16600 | MYB43 | myb domain protein 43 | 2.3 | 0.0483 |
| AT3G46130 | MYB48 | myb domain protein 48 | 2.2 | 0.0485 |
We examined cadmium-dependent NRT1.8 transcript levels to determine whether ERF34 and 35 affect NRT1.8 expression. NRT1.8 transcripts were detected at similar levels in both WT and the erf34/35 double mutant seedlings under control conditions (Figure 6). NRT1.8 was highly induced by Cd treatment in WT seedlings, consistent with previous findings (Weber et al., 2006; Li et al., 2010). However, cadmium did not significantly upregulate NRT1.8 expression in erf34/35 double mutant seedlings (Figure 6). The relatively low induction of NRT1.8 may contribute to the Cd sensitive phenotype of the erf34/35 double mutant.
Figure 6. ERF34 and ERF35 are required for NRT1;8 upregulation in response to cadmium.
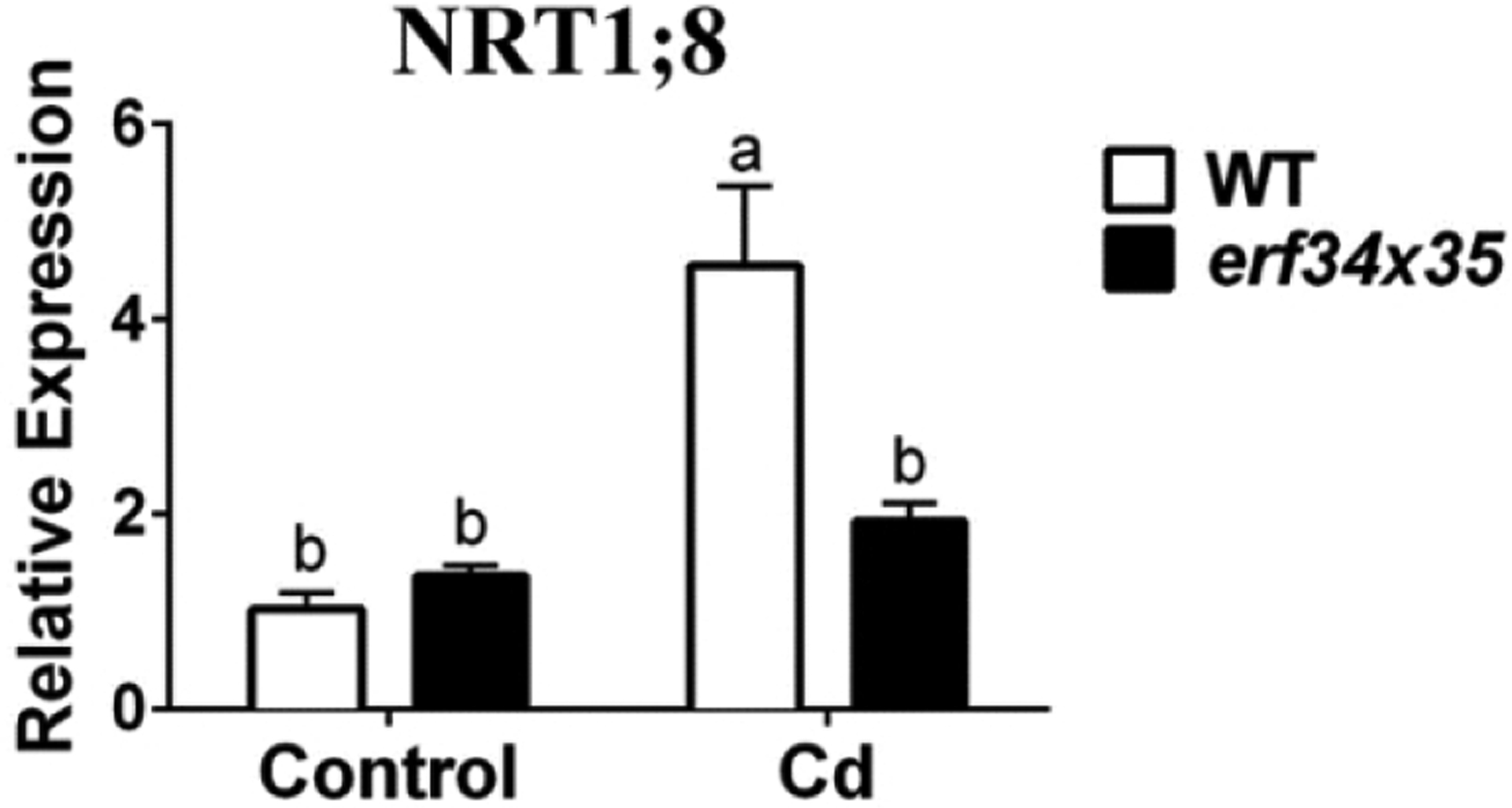
NRT1;8 transcript up-regulation is impaired in roots of erf34/35 double mutant: WT and erf34/35 double mutants were subjected to the same treatment as in Figure 5 (G) and (H) (n=3; p<0.0001);
ami10-9 is insensitive to arsenic and targets three phosphate transporters (PHTs)
In our forward genetics amiRNA screen for arsenic sensitivity, we also identified an amiRNA line 10-9 as showing arsenite resistance in root growth assays, that was similar to the amiRNA 4-138. In the absence of arsenic, seedlings from this line have a similar primary root length as WT plants (Figure 7A). However, this line showed a reduced sensitivity compared to WT seedlings when grown on media containing 10 μM arsenite. Primary root lengths of amiRNA line 10-9 seedlings were almost twice as long as WT plants when grown on medium containing 10 μM As(III) (Figure 7B). A similar As insensitive phenotype of amiRNA line 10-9 was found on media containing arsenate (Figure 8A–C).
Figure 7. amiRNA 10-9 targets phosphate transporters and exhibits arsenite resistance. Arsenite resistance phenotype confirmed in amiRNA retransformation lines.
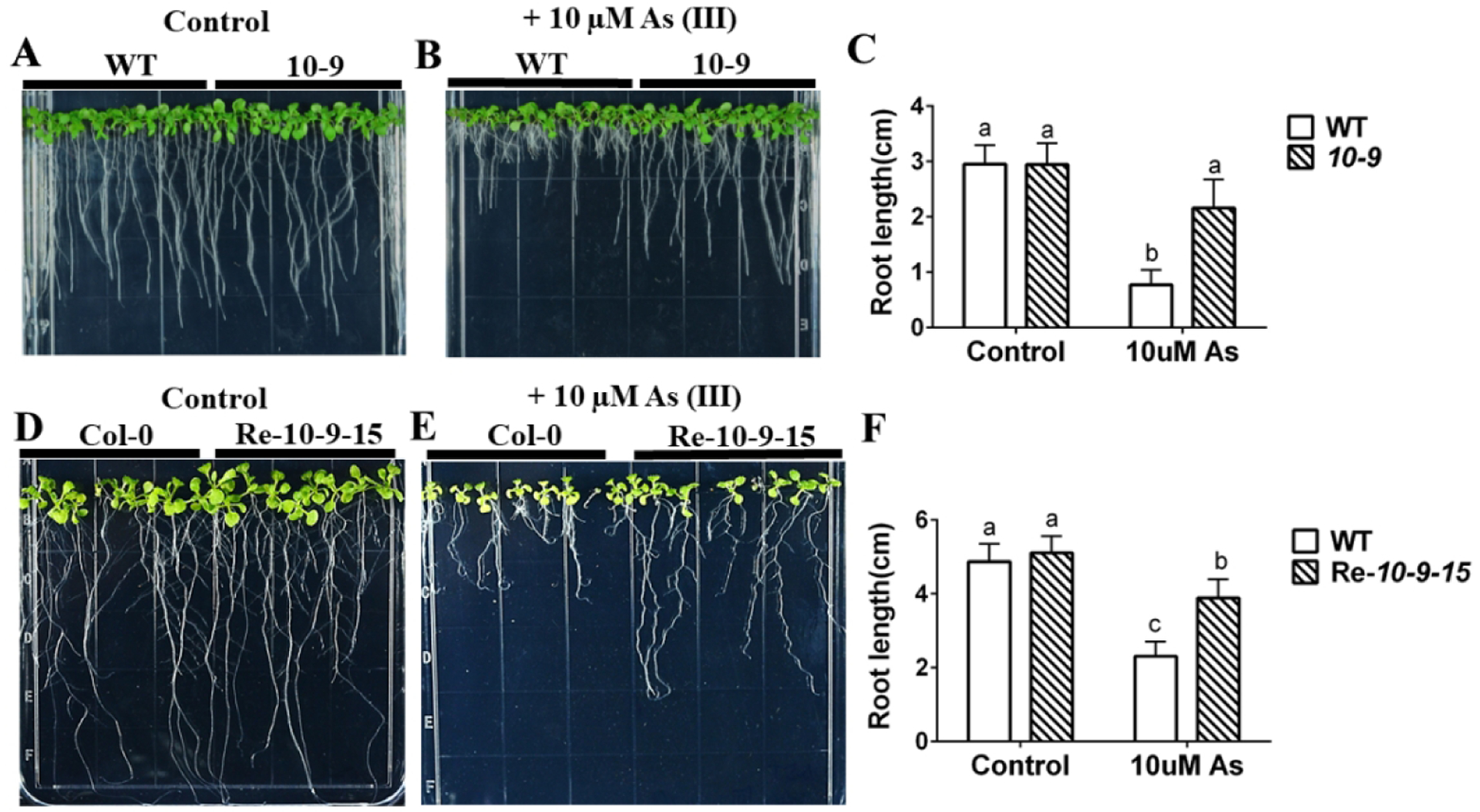
(A), (B) Root growth of amiRNA 10-9 is more tolerant than WT to arsenite treatment: WT and amiRNA 10-9 lines were grown vertically on 1/2 MS with 0.5% sucrose plates without (control) or with 10 μM arsenite for 10 days; (C) Primary root lengths of WT and amiRNA 10-9 were significantly longer than WT on arsenite plates and no different on control plates. (n=15 seedlings, means ± s.e.m.). (D), (E) amiRNA retransformation lines with the same amiRNA as the amiRNA 10-9 line are more tolerant than WT in root growth assays: WT and amiRNA 10-9 retransformation line 15 were germinated directly on 1/2 MS with 0.5% sucrose plates without (control) or with 10 μM arsenite for 14 days; (F) Primary root lengths of retransformation amiRNA Re-10-9-15 were significantly longer than WT on arsenite-containing plates and no clear difference was observed on control plates. (n=9 seedlings, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
Figure 8. amiRNA 10-9 predicted to target phosphate transporters causes arsenate resistance.
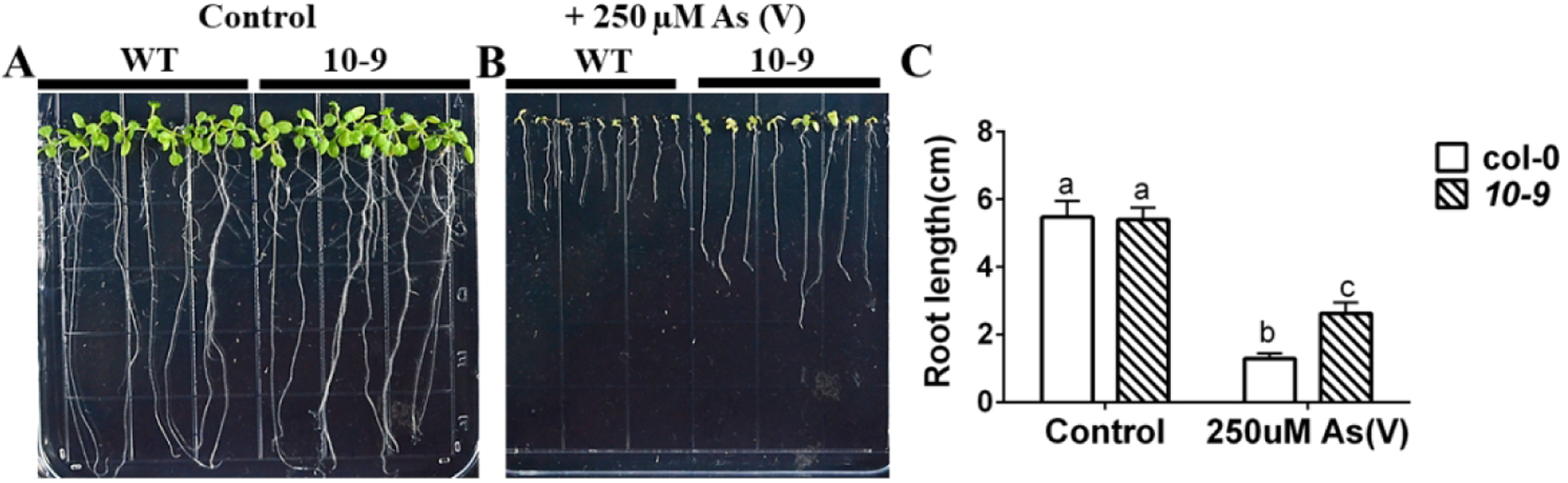
(A), (B) Root growth of amiRNA 10-9 is more tolerant than WT to arsenate treatment: WT and amiRNA 10-9 lines were grown vertically on 1/2 MS with 0.5% sucrose plates without (control) or with 250 μM sodium arsenate for 10 days; (C) Primary root lengths of WT and amiRNA 10-9 were significantly longer than WT on arsenate plates and no different on control plates. (n=15 seedlings, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
The amiRNA 10-9 line was isolated and sequenced and is predicted to target three high-affinity phosphate transporters (PHT1;1 AT5G43350, PHT1;2 AT5G43370 and PHT1;3 AT5G43360; amiRNA sequence listed in Table 1). Previous studies have shown that the PHT transporters AtPHT1;1, AtPHT1;4, AtPHT1;5; AtPHT1;7; 1;8; 1;9 function in both inorganic phosphate and arsenate (As(V)) transport in plants (Willsky & Malamy, 1980; Xu, Ma, & Nussinov, 2012). This is not surprising as the oxyanion chemical structure of As(V) is structurally analogous to that of phosphate (Willsky & Malamy, 1980). Interestingly however, the amiRNA line 10-9 also showed an enhanced sensitivity to arsenite (Figure 7A–C), which is not known to be a substrate of PHT phosphate transporters.
To determine whether the amiRNA 10-9 line phenotype is linked to the identified amiRNA, we amplified the original amiRNA sequence of amiRNA 10-9 and re-cloned and retransformed the amiRNA into Col-0 wild-type plants. The retransformed lines Re-10-9-15 showed a decreased sensitivity to As(III) compared to WT controls in 5 of 10 independent transformants (Figure 7D–F). Note that this frequency of phenotypes upon amiRNA retransformation is not low and may be ascribed to the fact that amiRNAs partially inhibit transcription or translation at intermediate levels and phenotypes depend on individual transformation events (Schwab et al., 2006; Hauser et al., 2019). Thus, using amiRNA’s to target gene families, we have obtained evidence suggesting an unexpected role for PHT transporters in arsenite tolerance.
Discussion
Forward genetic screens have enabled the identification of new genes and characterization of genetic pathways in Arabidopsis and other model organisms. While forward genetic screens continue to be powerful tools for unraveling biological processes, the presence of large gene families and many gene duplications with functional overlap in plant genomes, often buffers the effects of loss of function mutations leading to no observable phenotype in single-gene mutants (Lloyd & Meinke, 2012). Furthermore, higher-order loss of function mutations frequently lead to lethality, which also prevents the characterization of gene functions(Hauser et al., 2019). These pitfalls have hindered researchers’ ability to functionally characterize genes that belong to gene families (Lloyd & Meinke, 2012), which account for approximately 22,000 genes in Arabidopsis (Hauser et al., 2013).
To address these limitations, a library of artificial microRNAs (amiRNAs) was developed that targets diverse gene combinations mainly within sub-clades of multigene families to overcome genetic redundancy. This library has successfully enabled forward genetic screens to identify new genes involved in CO2 and ABA signaling as well as novel auxin transporters from very large gene families (Zhang et al., 2018; Hauser et al., 2019; Takahashi et al., 2020). Furthermore, because amiRNAs often cause transcriptional knockdown or translational inhibition rather than full loss of function, the amiRNA library has also enabled the identification of functionally redundant genes, for which double loss of function mutation causes lethality (Hauser et al., 2019). Together, these results demonstrate the power and utility of forward genetic screens using amiRNAs.
Arsenic exposure has been shown to induce a robust transcriptional response in Arabidopsis (Abercrombie et al., 2008; Jobe et al., 2012; Shukla et al., 2018). However, relatively few transcriptional regulators of arsenic-induced gene expression have been identified (Castrillo et al., 2013; Wang et al., 2014; Wang et al., 2017), likely due to extensive genetic redundancy in transcription factor families. Similarly, we hypothesized that additional transporters that affect arsenite sensitivity might exist, given the large family sizes and substrate overlap in plant transporters (Hauser et al., 2013). Thus, the genetic screen performed in the present study used amiRNA’s targeting DNA- and RNA-binding proteins and transporters and channels in Arabidopsis (Hauser et al., 2013). Notably, eight additional amiRNA libraries are available targeting other classes of proteins, including a library of over 4,000 amiRNAs targeting gene combinations within clades of gene families of presently unpredictable (“unknown”) function (Hauser et al., 2013; Hauser et al., 2019), suggesting that future screens using this platform are likely to uncover additional new genes that affect arsenic sensitivity.
In the present study, we identified the amiRNA 4-138 line as an As(III) insensitive line in root growth assays using a forward genetic screen. The observed phenotypes were found using two different media and in two laboratories (see Methods), indicating robustness of these mutant phenotypes under the imposed conditions. This amiRNA line targets the CBF1,2,3 and ERF34 and ERF35 transcription factors. We found that the As(III) insensitive phenotype is due to the tandem repeat genes CBF1,2,3. Note that CBF4 may also contribute to this phenotype, but a cbf quadruple mutant may be lethal and was not investigated here. The CBF1,2 and 3 genes are located in tandem on chromosome 4, and therefore CRISPR-mediated deletion of these genes, shows that they are not essential for seedling survival (Figure 1D).
By analyzing DAP-seq data sets for candidate gene promoters to which CBF1,2 & 3 bind (O’Malley et al., 2016), we identified 2214 genes potentially targeted by CBF1,CBF2, CBF3 and CBF4 (Supplemental Figure 6A and B). DAP-seq data suggest CBFs bind to many PHT transporter promoter regions. Further analyses showed that PHT transcript levels are decreased in cbf1/2/3 triple mutants under non-arsenic stress control conditions. The present findings suggest that CBF1,2,3 are positive regulators of PHT transporters. Additional in-vivo studies would allow further characterization of the underlying mechanisms.
Surprisingly, follow-up experiments with the arsenite tolerant amiRNA 4-138 line found this line to be cadmium sensitive. The contrasting root elongation phenotypes in response to arsenic (tolerant) and cadmium (sensitive) are unexpected as both cadmium and arsenite detoxification require thiolate peptides, in particular phytochelatins (Schmoger et al., 2000; Gong, Lee, & Schroeder, 2003; Aborode et al., 2016). As discussed above, the arsenite tolerant phenotype is due to the disruption of CBF genes. In contrast, characterization of the erf34/35 double mutant suggests that the ERF transcription factors are the main contributors to the cadmium sensitivity phenotype of amiRNA 4-138.
The nitrate transporter NRT1.8 mRNA is one of the most strongly cadmium-induced transcripts based on microarray studies (Weber et al., 2006; Li et al., 2010). Furthermore, ERF34 and ERF35 transcription factors were found to bind the promoter of the NRT1.8 transporter in in-vitro binding assays (Table 3) (Weber et al., 2006; O’Malley et al., 2016). NRT1.8 is a plasma membrane transporter expressed predominantly in xylem parenchyma cells within the vasculature (Li et al., 2010). NRT1.8 transcripts were strongly upregulated by cadmium stress in roots in the present study and a previous study (Li et al., 2010). Thus, the cadmium sensitive phenotype observed in erf34/35 may be partly due to the misregulation of NRT1.8. The nrt1.8 knock out mutant accumulates cadmium in shoots and has a similar root sensitivity to cadmium (Li et al., 2010) as the erf34/35 double mutant (Figure 5). However, additional experiments are needed to confirm this hypothesis, as additional CBF/ERF targets are also likely involved.
Arsenate (As(V) can be transported by inorganic phosphate (Pi) transporters due to its chemical similarity to phosphate. Plasma membrane-localized Pi transporters are known to be responsible for arsenate uptake. In Arabidopsis thaliana, the As(V) transport capabilities of AtPHT1; 1, AtPHT1; 4, AtPHT1; 5, AtPHT1; 7, AtPHT1; 8, AtPHT1; 9 have been well characterized (Shin et al., 2004; Catarecha et al., 2007; Remy et al., 2012; LeBlanc et al., 2013; Fontenot et al., 2015). Decreased expression of these PHT1 genes results in tolerance to As(V) (Shin et al., 2004; Catarecha et al., 2007; Nagarajan et al., 2011; LeBlanc et al., 2013; Luan et al., 2018). As cbf1/2/3 triple CRISPR mutant seedlings showed a strong down-regulation of the three major phosphate uptake transporter transcripts, AtPHT1;1, AtPHT1;2, AtPHT1;3 (Figures 3 and 4), cbf1/2/3 triple mutant seedlings would be predicted to also show a reduced As(V) sensitivity, consistent with previous studies of mutants in these phosphate transporters (Shin et al., 2004; Catarecha et al., 2007; Nagarajan et al., 2011; LeBlanc et al., 2013; Luan et al., 2018). However, the involvement of PHT transporters in As(III) responses has not been previously established. In our study, amiRNA 10-9 seedlings showed reduced sensitivity to As(III) and As(V) in root elongation assays (Figures 1 & 7). AmiRNA 10-9 targets AtPHT1; 1, AtPHT1; 2, AtPHT1; 3, which share a high level of similarity, have overlapping expression patterns, and are reported to all contribute to Pi uptake (Ayadi et al., 2015). We have further found that both As(V) and As(III) downregulate the transcript levels of AtPHT1; 1, AtPHT1; 2, and AtPHT1; 3 (Figures 3 & 4). Furthermore, PHT1 was shown to transport Pi and As(V) (Shin et al., 2004; Catarecha et al., 2007; Nussaume et al., 2011; Remy et al., 2012; LeBlanc et al., 2013; Fontenot et al., 2015). WRKY transcription factors, namely WRKY6, WRKY42, and WRKY45, were shown to interact with the PHT1;1 promoter and mediate PHT1;1 expression (Castrillo et al., 2013; Wang et al., 2014; Su et al., 2015). WRKY6 and WRKY 42 were also shown to affect As(V) uptake into plants (Castrillo et al., 2013; Su et al., 2015), but As(III) sensitivity or transport were not analyzed in these mutants. Thus, the mechanism of As(III) resistance in the amiRNA 10-9 line is an unexpected interesting finding that requires further characterization.
In the present study, we pursued screening of amiRNA seed libraries that target diverse combinations of related DNA binding proteins and transporter proteins. Several putative amiRNA target genes, in addition to those characterized here were isolated. For example, we identified transcriptional regulators such as ethylene-response transcription factors, basic helix-loop-helix (bHLH) transcription factors, mitochondrial transcription termination factors, and ABCG transporters that warrant further investigation (Table 1).
In summary, the transcription factors that induce and repress gene expression in response to arsenic and cadmium remain to a large degree unknown. In the present study, a forward genetics screen of amiRNA lines that target combinatorial close homologs of DNA and RNA-binding proteins found that the CBF1, 2, and 3 transcription factors mediate negative regulation of arsenite and arsenate sensitivity and that the ERF34 and ERF35 transcription factors function in mediating cadmium resistance. Further studies of these transcription factors could identify the cadmium- and arsenic-induced transcriptional control network.
Supplementary Material
Supplemental Figure 1. amiRNA 4–85 targets ARR transcription factors, has long roots when grown on arsenite, and the phenotype was confirmed in amiRNA retransformation lines. (A), (B) amiRNA line 4–85 was more tolerant than WT under arsenite treatment: WT and 4–85 amiRNA lines were grown vertically on 1/2 MS with 0.5% sucrose plates without (control) or with 10 μM arsenite for 10 days; (C) Primary root lengths of amiRNA 4–85 compared to WT on control and arsenite-containing plates and on control plates. (n=15 seedlings, means ± s.e.m.). (D), (E) Seedlings of amiRNA retransformation line 4-85-10 are more tolerant than WT in root growth assays: WT and amiRNA retransformation line Re-4-85-10 were germinated directly on 1/2 MS with 0.5% sucrose plates without (control) or with 10 μM arsenite for about 14 days; (F) Primary root length of amiRNA Re-4-85-10 compared to WT on control and arsenite-containing plates. (n=11 seedlings, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
Supplemental Figure 2. erf34 and erf35 single mutant shows weak or no phenotype under cadmium and arsenite treatment. (A) erf34 and erf35 has similar root lengths on control plates. (B) erf35 has shorter roots compared to WT when Cd was added to the medium, but erf34 shows no different when compared to WT; (C) Both erf34 and erf35 single mutants have no phenotype on arsenite containing plates: WT erf34 and erf35 single mutants were grown vertically on minimal plates with 1% sucrose plates without (control) or with 30 μM cadmium or with 10 μM sodium arsenite for 7 days; (D) (n=15 seedlings, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
Supplemental Figure 3. CRISPR/Cas9 gene editing technology to generate CRISPR cbf1/2/3 knockout mutants (A) Cutting sites and primers for CRISPR cbf1/2/3; (B) Genotyping results for isolating homozygous for CRISPR cbf1/2/3.
Supplemental Figure 4. Genotyping results for isolating homozygous for erf34×erf35 knockout mutants (A) Genotyping results for erf34 gene; (B) Genotyping results for erf35 gene; (C) Cartoon showing the positions of the primers for erf34 and erf35 genoptying.
Supplemental Figure 5. Venn diagram of genes targeted by ERF34, ERF35 and Genes significantly up regulated and downregulated after 2 hours of exposure to 50 μM Cd2+ in A. thaliana roots.
Supplemental Figure 6. Venn diagram and enriched ontology clusters of genes targeted by CBF1, 2, 3 and 4. (A) Venn diagrame; (B) Enriched ontology clusters of genes targed by CBF1, 2, 3, 4 in the same time.
Supplemental Table 1. Primers used for amiRNA sequencing and cloning
Supplemental Table 2. Salk lines and primers for genotyping
Supplemental Table 3. Primers used for RTq-PCR
Acknowledgments
This research was funded by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P42ES010337 (JIS) and in part by the National Natural Science Foundation of China (31770283) (SX).
Funding information: National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P42ES010337; National Natural Science Foundation of China (31770283).
Footnotes
Conflict of Interest
The authors declare no conflict of interest. All co-authors agree with the contents of the manuscript, and there is no financial interest to report.
Data Availability Statement
Data sets described in the manuscript have been included in the Supplemental materials
References
- Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, … Natasha. (2018). Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int J Environ Res Public Health, 15(1). doi: 10.3390/ijerph15010059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedi T, & Mojiri A (2020). Arsenic Uptake and Accumulation Mechanisms in Rice Species. Plants (Basel), 9(2). doi: 10.3390/plants9020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie JM, Halfhill MD, Ranjan P, Rao MR, Saxton AM, Yuan JS, & Stewart CN Jr. (2008). Transcriptional responses of Arabidopsis thaliana plants to As (V) stress. BMC Plant Biol, 8, 87. doi: 10.1186/1471-2229-8-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aborode FA, Raab A, Voigt M, Costa LM, Krupp EM, & Feldmann J (2016). The importance of glutathione and phytochelatins on the selenite and arsenate detoxification in Arabidopsis thaliana. J Environ Sci (China), 49, 150–161. doi: 10.1016/j.jes.2016.08.009 [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome I (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature, 408(6814), 796–815. doi: 10.1038/35048692 [DOI] [PubMed] [Google Scholar]
- Ayadi A, David P, Arrighi JF, Chiarenza S, Thibaud MC, Nussaume L, & Marin E (2015). Reducing the genetic redundancy of Arabidopsis PHOSPHATE TRANSPORTER1 transporters to study phosphate uptake and signaling. Plant Physiol, 167(4), 1511–1526. doi: 10.1104/pp.114.252338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert GP, Thorsen M, Schussler MD, Nilsson HR, Wagner A, Tamas MJ, & Jahn TP (2008). A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol, 6, 26. doi: 10.1186/1741-7007-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo G, Sanchez-Bermejo E, de Lorenzo L, Crevillen P, Fraile-Escanciano A, Tc M, … Leyva A (2013). WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell, 25(8), 2944–2957. doi: 10.1105/tpc.113.114009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarecha P, Segura MD, Franco-Zorrilla JM, Garcia-Ponce B, Lanza M, Solano R, … Leyva A (2007). A mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell, 19(3), 1123–1133. doi: 10.1105/tpc.106.041871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty D, Trivedi PK, Misra P, Tiwari M, Shri M, Shukla D, … Tuli R (2009). Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere, 74(5), 688–702. doi: 10.1016/j.chemosphere.2008.09.082 [DOI] [PubMed] [Google Scholar]
- Chao DY, Chen Y, Chen J, Shi S, Chen Z, Wang C, … Salt DE (2014). Genome-wide association mapping identifies a new arsenate reductase enzyme critical for limiting arsenic accumulation in plants. PLoS Biol, 12(12), e1002009. doi: 10.1371/journal.pbio.1002009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S (2019). Safer food through plant science: reducing toxic element accumulation in crops. J Exp Bot, 70(20), 5537–5557. doi: 10.1093/jxb/erz366 [DOI] [PubMed] [Google Scholar]
- Clemens S, & Ma JF (2016). Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu Rev Plant Biol, 67, 489–512. doi: 10.1146/annurev-arplant-043015-112301 [DOI] [PubMed] [Google Scholar]
- Clough SJ, & Bent AF (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J, 16(6), 735–743. doi: 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Cooper AM, Felix D, Alcantara F, Zaslavsky I, Work A, Watson PL, … Schroeder JI (2020). Monitoring and mitigation of toxic heavy metals and arsenic accumulation in food crops: A case study of an urban community garden. Plant Direct, 4(1), e00198. doi: 10.1002/pld3.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack SA, Wang P, Moore BM, Meng F, Conner JK, Krysan PJ, … Shiu S-H (2020). Genome-wide predictions of genetic redundancy in Arabidopsis thaliana. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan GL, Hu Y, Schneider S, McDermott J, Chen J, Sauer N, … Zhu YG (2016a). Inositol transporters AtINT2 and AtINT4 regulate arsenic accumulation in Arabidopsis seeds. Nature Plants, 2(1). doi:Artn 15202 10.1038/Nplants.2015.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan GL, Hu Y, Schneider S, McDermott J, Chen J, Sauer N, … Zhu YG (2016b). Inositol transporters AtINT2 and AtINT4 regulate arsenic accumulation in Arabidopsis seeds. Nat Plants, 2(1), 15202. doi: 10.1038/nplants.2015.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot EB, Ditusa SF, Kato N, Olivier DM, Dale R, Lin WY, … Smith AP (2015). Increased phosphate transport of Arabidopsis thaliana Pht1;1 by site-directed mutagenesis of tyrosine 312 may be attributed to the disruption of homomeric interactions. Plant Cell Environ, 38(10), 2012–2022. doi: 10.1111/pce.12522 [DOI] [PubMed] [Google Scholar]
- Gao X, Chen J, Dai X, Zhang D, & Zhao Y (2016). An Effective Strategy for Reliably Isolating Heritable and Cas9-Free Arabidopsis Mutants Generated by CRISPR/Cas9-Mediated Genome Editing. Plant Physiol, 171(3), 1794–1800. doi: 10.1104/pp.16.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, & Zhao Y (2014). Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol, 56(4), 343–349. doi: 10.1111/jipb.12152 [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, & Zhao Y (2015). Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci U S A, 112(7), 2275–2280. doi: 10.1073/pnas.1500365112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbinski LD, Rosen BP, & Chen J (2019). Pathways of arsenic uptake and efflux. Environ Int, 126, 585–597. doi: 10.1016/j.envint.2019.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic K, & Korban SS (2007). Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Molecular Biology, 64(4), 361–369. doi: 10.1007/s11103-007-9158-7 [DOI] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, … Karagas MR (2011). Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci U S A, 108(51), 20656–20660. doi: 10.1073/pnas.1109127108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JM, Lee DA, & Schroeder JI (2003). Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci U S A, 100(17), 10118–10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Jin Y, Wussler C, Blancaflor EB, Motes CM, & Versaw WK (2008). Functional analysis of the Arabidopsis PHT4 family of intracellular phosphate transporters. New Phytol, 177(4), 889–898. doi: 10.1111/j.1469-8137.2007.02331.x [DOI] [PubMed] [Google Scholar]
- Guo J, Dai X, Xu W, & Ma M (2008). Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere, 72(7), 1020–1026. doi: 10.1016/j.chemosphere.2008.04.018 [DOI] [PubMed] [Google Scholar]
- Hauser F, Chen W, Deinlein U, Chang K, Ossowski S, Fitz J, … Schroeder JI (2013). A genomic-scale artificial microRNA library as a tool to investigate the functionally redundant gene space in Arabidopsis. Plant Cell, 25(8), 2848–2863. doi: 10.1105/tpc.113.112805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser F, Ceciliato PHO, Lin YC, Guo D, Gregerson JD, Abbasi N, … Schroeder JI (2019). A seed resource for screening functionally redundant genes and isolation of new mutants impaired in CO2 and ABA responses. J Exp Bot, 70(2), 641–651. doi: 10.1093/jxb/ery363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Kuramata M, Abe T, Takagi H, Ozawa K, & Ishikawa S (2017). Phytochelatin synthase OsPCS1 plays a crucial role in reducing arsenic levels in rice grains. Plant Journal, 91(5), 840–848. doi: 10.1111/tpj.13612 [DOI] [PubMed] [Google Scholar]
- Herschbach C, Rizzini L, Mult S, Hartmann T, Busch F, Peuke AD, … Ensminger I (2010). Over-expression of bacterial gamma-glutamylcysteine synthetase (GSH1) in plastids affects photosynthesis, growth and sulphur metabolism in poplar (Populus tremula × Populus alba) dependent on the resulting gamma-glutamylcysteine and glutathione levels. Plant Cell Environ, 33(7), 1138–1151. doi: 10.1111/j.1365-3040.2010.02135.x [DOI] [PubMed] [Google Scholar]
- Huang Y, Chen H, Reinfelder JR, Liang X, Sun C, Liu C, … Yi J (2019). A transcriptomic (RNA-seq) analysis of genes responsive to both cadmium and arsenic stress in rice root. Sci Total Environ, 666, 445–460. doi: 10.1016/j.scitotenv.2019.02.281 [DOI] [PubMed] [Google Scholar]
- Isayenkov SV, & Maathuis FJ (2008). The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Lett, 582(11), 1625–1628. doi: 10.1016/j.febslet.2008.04.022 [DOI] [PubMed] [Google Scholar]
- Ji R, Zhou L, Liu J, Wang Y, Yang L, Zheng Q, … Lan W (2017). Calcium-dependent protein kinase CPK31 interacts with arsenic transporter AtNIP1;1 and regulates arsenite uptake in Arabidopsis thaliana. PLoS One, 12(3), e0173681. doi: 10.1371/journal.pone.0173681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe TO, Sung DY, Akmakjian G, Pham A, Komives EA, Mendoza-Cozatl DG, & Schroeder JI (2012). Feedback inhibition by thiols outranks glutathione depletion: a luciferase-based screen reveals glutathione-deficient gamma-ECS and glutathione synthetase mutants impaired in cadmium-induced sulfate assimilation. Plant J, 70(5), 783–795. doi: 10.1111/j.1365-313X.2012.04924.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, & Fujiwara T (2009). Arabidopsis NIP1;1 transports antimonite and determines antimonite sensitivity. Plant Cell Physiol, 50(11), 1977–1981. doi: 10.1093/pcp/pcp130 [DOI] [PubMed] [Google Scholar]
- Kamiya T, Tanaka M, Mitani N, Ma JF, Maeshima M, & Fujiwara T (2009). NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J Biol Chem, 284(4), 2114–2120. doi: 10.1074/jbc.M806881200 [DOI] [PubMed] [Google Scholar]
- Kang HW, Cho YG, Yoon UH, & Eun MY (1998). A Rapid DNA Extraction Method for RFLP and PCR Analysis from a Single Dry Seed. Plant Molecular Biology Reporter, 16(1), 90–90. doi: 10.1023/A:1007418606098 [DOI] [Google Scholar]
- Kumari P, Rastogi A, Shukla A, Srivastava S, & Yadav S (2018). Prospects of genetic engineering utilizing potential genes for regulating arsenic accumulation in plants. Chemosphere, 211, 397–406. doi: 10.1016/j.chemosphere.2018.07.152 [DOI] [PubMed] [Google Scholar]
- Lapis-Gaza HR, Jost R, & Finnegan PM (2014). Arabidopsis PHOSPHATE TRANSPORTER1 genes PHT1;8 and PHT1;9 are involved in root-to-shoot translocation of orthophosphate. BMC Plant Biol, 14, 334. doi: 10.1186/s12870-014-0334-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc MS, McKinney EC, Meagher RB, & Smith AP (2013). Hijacking membrane transporters for arsenic phytoextraction. J Biotechnol, 163(1), 1–9. doi: 10.1016/j.jbiotec.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Chen A, & Schroeder JI (2003). ars1, an Arabidopsis mutant exhibiting increased tolerance to arsenate and increased phosphate uptake. Plant J, 35(5), 637–646. doi: 10.1046/j.1365-313x.2003.01835.x [DOI] [PubMed] [Google Scholar]
- Li JY, Fu YL, Pike SM, Bao J, Tian W, Zhang Y, … Gong JM (2010). The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell, 22(5), 1633–1646. doi: 10.1105/tpc.110.075242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dhankher OP, Carreira L, Lee D, Chen A, Schroeder JI, … Meagher RB (2004). Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol, 45(12), 1787–1797. doi: 10.1093/pcp/pch202 [DOI] [PubMed] [Google Scholar]
- Lindsay ER, & Maathuis FJ (2016). Arabidopsis thaliana NIP7;1 is involved in tissue arsenic distribution and tolerance in response to arsenate. FEBS Lett, 590(6), 779–786. doi: 10.1002/1873-3468.12103 [DOI] [PubMed] [Google Scholar]
- Lindsay ER, & Maathuis FJM (2017). New Molecular Mechanisms to Reduce Arsenic in Crops. Trends in Plant Science, 22(12), 1016–1026. doi: 10.1016/j.tplants.2017.09.015 [DOI] [PubMed] [Google Scholar]
- Liu QP, Hu HC, Zhu LY, Li RC, Feng Y, Zhang LQ, … Zhang HM (2015). Involvement of miR528 in the Regulation of Arsenite Tolerance in Rice (Oryza sativa L.). Journal of Agricultural and Food Chemistry, 63(40), 8849–8861. doi: 10.1021/acs.jafc.5b04191 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang S, Shan X, & Zhu YG (2005). Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere, 61(2), 293–301. doi: 10.1016/j.chemosphere.2005.01.088 [DOI] [PubMed] [Google Scholar]
- Lloyd J, & Meinke D (2012). A comprehensive dataset of genes with a loss-of-function mutant phenotype in Arabidopsis. Plant Physiol, 158(3), 1115–1129. doi: 10.1104/pp.111.192393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan M, Liu J, Liu Y, Han X, Sun G, Lan W, & Luan S (2018). Vacuolar Phosphate Transporter 1 (VPT1) Affects Arsenate Tolerance by Regulating Phosphate Homeostasis in Arabidopsis. Plant Cell Physiol, 59(7), 1345–1352. doi: 10.1093/pcp/pcy025 [DOI] [PubMed] [Google Scholar]
- Mendoza-Cozatl DG, Jobe TO, Hauser F, & Schroeder JI (2011). Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr Opin Plant Biol, 14(5), 554–562. doi: 10.1016/j.pbi.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cozatl DG, Xie Q, Akmakjian GZ, Jobe TO, Patel A, Stacey MG, … Schroeder JI (2014). OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of cadmium in seeds. Mol Plant, 7(9), 1455–1469. doi: 10.1093/mp/ssu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosa KA, Kumar K, Chhikara S, McDermott J, Liu Z, Musante C, … Dhankher OP (2012). Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res, 21(6), 1265–1277. doi: 10.1007/s11248-012-9600-8 [DOI] [PubMed] [Google Scholar]
- Nagarajan VK, Jain A, Poling MD, Lewis AJ, Raghothama KG, & Smith AP (2011). Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol, 156(3), 1149–1163. doi: 10.1104/pp.111.174805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussaume L, Kanno S, Javot H, Marin E, Pochon N, Ayadi A, … Thibaud MC (2011). Phosphate Import in Plants: Focus on the PHT1 Transporters. Front Plant Sci, 2, 83. doi: 10.3389/fpls.2011.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley RC, Huang SC, Song L, Lewsey MG, Bartlett A, Nery JR, … Ecker JR (2016). Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell, 166(6), 1598. doi: 10.1016/j.cell.2016.08.063 [DOI] [PubMed] [Google Scholar]
- Ossowski S, Fitz J, Schwab R, Riester M, & Weigel D personal communication.
- Palma-Lara I, Martinez-Castillo M, Quintana-Perez JC, Arellano-Mendoza MG, Tamay-Cach F, Valenzuela-Limon OL, … Hernandez-Zavala A (2020). Arsenic exposure: A public health problem leading to several cancers. Regul Toxicol Pharmacol, 110, 104539. doi: 10.1016/j.yrtph.2019.104539 [DOI] [PubMed] [Google Scholar]
- Pan W, Wu C, Xue S, & Hartley W (2014). Arsenic dynamics in the rhizosphere and its sequestration on rice roots as affected by root oxidation. J Environ Sci (China), 26(4), 892–899. doi: 10.1016/S1001-0742(13)60483-0 [DOI] [PubMed] [Google Scholar]
- Picault N, Cazale AC, Beyly A, Cuine S, Carrier P, Luu DT, … Peltier G (2006). Chloroplast targeting of phytochelatin synthase in Arabidopsis: effects on heavy metal tolerance and accumulation. Biochimie, 88(11), 1743–1750. doi: 10.1016/j.biochi.2006.04.016 [DOI] [PubMed] [Google Scholar]
- Qian L, Qi S, Cao F, Zhang J, Zhao F, Li C, & Wang C (2018). Toxic effects of boscalid on the growth, photosynthesis, antioxidant system and metabolism of Chlorella vulgaris. Environ Pollut, 242(Pt A), 171–181. doi: 10.1016/j.envpol.2018.06.055 [DOI] [PubMed] [Google Scholar]
- Rai PK, Lee SS, Zhang M, Tsang YF, & Kim KH (2019). Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ Int, 125, 365–385. doi: 10.1016/j.envint.2019.01.067 [DOI] [PubMed] [Google Scholar]
- Remy E, Cabrito TR, Batista RA, Teixeira MC, Sa-Correia I, & Duque P (2012). The Pht1;9 and Pht1;8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytol, 195(2), 356–371. doi: 10.1111/j.1469-8137.2012.04167.x [DOI] [PubMed] [Google Scholar]
- Schmoger ME, Oven M, & Grill E (2000). Detoxification of arsenic by phytochelatins in plants. Plant Physiol, 122(3), 793–801. doi: 10.1104/pp.122.3.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, & Weigel D (2006). Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell, 18(5), 1121–1133. doi: 10.1105/tpc.105.039834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Wang T, Chen Z, Tang Z, Wu Z, Salt DE, … Zhao FJ (2016). OsHAC1;1 and OsHAC1;2 Function as Arsenate Reductases and Regulate Arsenic Accumulation. Plant Physiol, 172(3), 1708–1719. doi: 10.1104/pp.16.01332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Shin HS, Dewbre GR, & Harrison MJ (2004). Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J, 39(4), 629–642. doi: 10.1111/j.1365-313X.2004.02161.x [DOI] [PubMed] [Google Scholar]
- Shri M, Singh PK, Kidwai M, Gautam N, Dubey S, Verma G, & Chakrabarty D (2019). Recent advances in arsenic metabolism in plants: current status, challenges and highlighted biotechnological intervention to reduce grain arsenic in rice. Metallomics, 11(3), 519–532. doi: 10.1039/c8mt00320c [DOI] [PubMed] [Google Scholar]
- Shukla T, Khare R, Kumar S, Lakhwani D, Sharma D, Asif MH, & Trivedi PK (2018). Differential transcriptome modulation leads to variation in arsenic stress response in Arabidopsis thaliana accessions. J Hazard Mater, 351, 1–10. doi: 10.1016/j.jhazmat.2018.02.031 [DOI] [PubMed] [Google Scholar]
- Song WY, Yamaki T, Yamaji N, Ko D, Jung KH, Fujii-Kashino M, … Ma JF (2014). A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proceedings of the National Academy of Sciences of the United States of America, 111(44), 15699–15704. doi: 10.1073/pnas.1414968111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WY, Park J, Mendoza-Cozatl DG, Suter-Grotemeyer M, Shim D, Hortensteiner S, … Martinoia E (2010). Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proceedings of the National Academy of Sciences of the United States of America, 107(49), 21187–21192. doi: 10.1073/pnas.1013964107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Srivastava AK, Sablok G, Deshpande TU, & Suprasanna P (2015). Transcriptomics profiling of Indian mustard (Brassica juncea) under arsenate stress identifies key candidate genes and regulatory pathways. Front Plant Sci, 6, 646. doi: 10.3389/fpls.2015.00646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, Xu Q, Zhang FC, Chen Y, Li LQ, Wu WH, & Chen YF (2015). WRKY42 modulates phosphate homeostasis through regulating phosphate translocation and acquisition in Arabidopsis. Plant Physiol, 167(4), 1579–1591. doi: 10.1104/pp.114.253799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Zhang J, Hsu PK, Ceciliato PHO, Zhang L, Dubeaux G, … Schroeder JI (2020). MAP3Kinase-dependent SnRK2-kinase activation is required for abscisic acid signal transduction and rapid osmotic stress response. Nat Commun, 11(1), 12. doi: 10.1038/s41467-019-13875-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, & Trivedi PK (2014). Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ, 37(1), 140–152. doi: 10.1111/pce.12138 [DOI] [PubMed] [Google Scholar]
- Verma PK, Verma S, Pande V, Mallick S, Tripathi RD, Dhankher OP, & Chakrabarty D (2017). Overexpression of Rice Glutaredoxin OsGrx_C7 and OsGrx_C2.1 Reduces Intracellular Arsenic Accumulation and Increases Tolerance in Arabidopsis thaliana (vol 7, 740, 2016). Frontiers in Plant Science, 8. doi:ARTN 1884 10.3389/fpls.2017.01884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versaw WK, & Harrison MJ (2002). A chloroplast phosphate transporter, PHT2;1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell, 14(8), 1751–1766. doi: 10.1105/tpc.002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FZ, Chen MX, Yu LJ, Xie LJ, Yuan LB, Qi H, … Chen QF (2017). OsARM1, an R2R3 MYB Transcription Factor, Is Involved in Regulation of the Response to Arsenic Stress in Rice. Front Plant Sci, 8, 1868. doi: 10.3389/fpls.2017.01868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu Q, Kong YH, Chen Y, Duan JY, Wu WH, & Chen YF (2014). Arabidopsis WRKY45 transcription factor activates PHOSPHATE TRANSPORTER1;1 expression in response to phosphate starvation. Plant Physiol, 164(4), 2020–2029. doi: 10.1104/pp.113.235077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Trampczynska A, & Clemens S (2006). Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd(2+)-hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ, 29(5), 950–963. doi: 10.1111/j.1365-3040.2005.01479.x [DOI] [PubMed] [Google Scholar]
- Willsky GR, & Malamy MH (1980). Effect of arsenate on inorganic phosphate transport in Escherichia coli. J Bacteriol, 144(1), 366–374. doi: 10.1128/JB.144.1.366-374.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojas S, Clemens S, Sklodowska A, & Maria Antosiewicz D (2010). Arsenic response of AtPCS1- and CePCS-expressing plants - effects of external As(V) concentration on As-accumulation pattern and NPT metabolism. J Plant Physiol, 167(3), 169–175. doi: 10.1016/j.jplph.2009.07.017 [DOI] [PubMed] [Google Scholar]
- Xu JM, Shi SL, Wang L, Tang Z, Lv TT, Zhu XL, … Wu ZC (2017). OsHAC4 is critical for arsenate tolerance and regulates arsenic accumulation in rice. New Phytologist, 215(3), 1090–1101. doi: 10.1111/nph.14572 [DOI] [PubMed] [Google Scholar]
- Xu W, Dai W, Yan H, Li S, Shen H, Chen Y, … Ma M (2015). Arabidopsis NIP3;1 Plays an Important Role in Arsenic Uptake and Root-to-Shoot Translocation under Arsenite Stress Conditions. Mol Plant, 8(5), 722–733. doi: 10.1016/j.molp.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Xu Y, Ma B, & Nussinov R (2012). Structural and functional consequences of phosphate-arsenate substitutions in selected nucleotides: DNA, RNA, and ATP. J Phys Chem B, 116(16), 4801–4811. doi: 10.1021/jp300307u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Menz J, Haussermann I, Benz M, Fujiwara T, & Ludewig U (2015). High and Low Affinity Urea Root Uptake: Involvement of NIP5;1. Plant Cell Physiol, 56(8), 1588–1597. doi: 10.1093/pcp/pcv067 [DOI] [PubMed] [Google Scholar]
- Yoon Y, Lee WM, & An YJ (2015). Phytotoxicity of arsenic compounds on crop plant seedlings. Environ Sci Pollut Res Int, 22(14), 11047–11056. doi: 10.1007/s11356-015-4317-x [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nasser V, Pisanty O, Omary M, Wulff N, Di Donato M, … Shani E (2018). A transportome-scale amiRNA-based screen identifies redundant roles of Arabidopsis ABCB6 and ABCB20 in auxin transport. Nat Commun, 9(1), 4204. doi: 10.1038/s41467-018-06410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvobgo G, Sagonda T, Lwalaba JLW, Mapodzeke JM, Muhammad N, Chen G, … Zhang G (2018). Transcriptomic comparison of two barley genotypes differing in arsenic tolerance exposed to arsenate and phosphate treatments. Plant Physiol Biochem, 130, 589–603. doi: 10.1016/j.plaphy.2018.08.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. amiRNA 4–85 targets ARR transcription factors, has long roots when grown on arsenite, and the phenotype was confirmed in amiRNA retransformation lines. (A), (B) amiRNA line 4–85 was more tolerant than WT under arsenite treatment: WT and 4–85 amiRNA lines were grown vertically on 1/2 MS with 0.5% sucrose plates without (control) or with 10 μM arsenite for 10 days; (C) Primary root lengths of amiRNA 4–85 compared to WT on control and arsenite-containing plates and on control plates. (n=15 seedlings, means ± s.e.m.). (D), (E) Seedlings of amiRNA retransformation line 4-85-10 are more tolerant than WT in root growth assays: WT and amiRNA retransformation line Re-4-85-10 were germinated directly on 1/2 MS with 0.5% sucrose plates without (control) or with 10 μM arsenite for about 14 days; (F) Primary root length of amiRNA Re-4-85-10 compared to WT on control and arsenite-containing plates. (n=11 seedlings, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
Supplemental Figure 2. erf34 and erf35 single mutant shows weak or no phenotype under cadmium and arsenite treatment. (A) erf34 and erf35 has similar root lengths on control plates. (B) erf35 has shorter roots compared to WT when Cd was added to the medium, but erf34 shows no different when compared to WT; (C) Both erf34 and erf35 single mutants have no phenotype on arsenite containing plates: WT erf34 and erf35 single mutants were grown vertically on minimal plates with 1% sucrose plates without (control) or with 30 μM cadmium or with 10 μM sodium arsenite for 7 days; (D) (n=15 seedlings, means ± s.e.m.). Letters at the top of columns are grouped based on two-way ANOVA and Tukey’s multiple comparisons test, P < 0.05).
Supplemental Figure 3. CRISPR/Cas9 gene editing technology to generate CRISPR cbf1/2/3 knockout mutants (A) Cutting sites and primers for CRISPR cbf1/2/3; (B) Genotyping results for isolating homozygous for CRISPR cbf1/2/3.
Supplemental Figure 4. Genotyping results for isolating homozygous for erf34×erf35 knockout mutants (A) Genotyping results for erf34 gene; (B) Genotyping results for erf35 gene; (C) Cartoon showing the positions of the primers for erf34 and erf35 genoptying.
Supplemental Figure 5. Venn diagram of genes targeted by ERF34, ERF35 and Genes significantly up regulated and downregulated after 2 hours of exposure to 50 μM Cd2+ in A. thaliana roots.
Supplemental Figure 6. Venn diagram and enriched ontology clusters of genes targeted by CBF1, 2, 3 and 4. (A) Venn diagrame; (B) Enriched ontology clusters of genes targed by CBF1, 2, 3, 4 in the same time.
Supplemental Table 1. Primers used for amiRNA sequencing and cloning
Supplemental Table 2. Salk lines and primers for genotyping
Supplemental Table 3. Primers used for RTq-PCR


