Abstract
Background and aims. Vitamin D is synthesized in the skin with the aid of ultraviolet-B radiation, playing a variety of roles in the body. Temporomandibular disorders (TMDs) are a group of pathological conditions involving the temporomandibular joints as well as the masticatory muscles and othersurrounding tissues. In the present narrative review, we investigated the potential role of vitamin D in the etiology of temporomandibular disorders in order todetermine whether the current knowledge supports 25-hidroxyvitamin D (25-OHD) supplementation in temporomandibular disorders associated with insufficient or deficient levels of vitamin D. Methods. A literature research was performed in PubMed, Scopus, Science Direct, and Google Scholar databases, and a total of 10 articles were included for analysis. Results.Among the observational studies published to date, investigating the role for vitamin D in the etiology of TMDs, six of them suggest that there is a connection between the two aspects. In this context, patients suffering from TMD, with deficient levels of vitamin D (<30 ng/mL), are most likely to benefit from supplementation, whereas individuals with vitamin D level >50ng/mL probably have little benefit from supplementation.Conclusion.Vitamin D might be a safe, simple, and potentially beneficial way to prevent TMDs or to reduce pain; however, more randomized and placebo-controlled trials are required before any firm conclusions can be drawn.
Keywords: vitamin D, 25-hidroxyvitamin D, temporomandibular disorders, osteoarthritis
1. Introduction
25-hidroxyvitamin D (25-OHD), also known as vitamin D, is a fat-soluble vitamin, synthesized in the skin (from a precursor), and obtained from dietary sources (e.g., oily fish, dietary supplements, or vitamin D-fortified foods). Among the causes of vitamin D deficiency, insufficient UVB exposure or decreased bioavailability are often mentioned, along with some medication, such as glucocorticoids, antiretroviral drugs, or anticonvulsants [1].
As the majority of human body tissues express vitamin D receptors [2], it is known that the lack of 25-OHD is implicated in a range of pathological conditions, including musculoskeletal disorders; metabolic, autoimmune, respiratory, and cardiovascular diseases; malignancies; psychiatric conditions; and chronic pain [1].
Temporomandibular disorders (TMDs) represent a group of pathological conditions involving the temporomandibular joints, the masticatory muscles, as well as the surrounding tissues and bone structures, or any combination of the structures mentioned above [3,4]. In 2014, an updated classification structure for TMDs was published by the International Research Diagnostic Criteria for Temporomandibular Dysfunction Consortium Network. As a result, four main categories of conditions were included, such as (a) temporomandibular joint disorders (intra-articular disorders), (b) mastication muscle disorders (extra-articular disorders), (c) headache (attributed to TMD), and (d) disorders of the associated structures [5] (Table 1).
Table 1.
Classification of intra-articular and extra-articular temporomandibular disorders.
|
INTRA-ARTICULAR
DISORDERS |
1. Joint Pain | Arthralgia/Arthritis/Osteoarthritis | ||
| 2. Joint disorders | Disk disorders/disk displacement |
with reduction with intermittent locking without reduction, with limited locking without reduction, without limited locking |
||
| Other hypomobility disorders |
Adhesions Adherence Ankylosis (fibrous/osseous) |
|||
| Hypermobility disorders | Dislocations (subluxation/luxation) | |||
| 3. Joint diseases | Systemic arthritis/condylysis/osteonecrosis | |||
| Degenerative joint disease/osteochondritis dissecans/ synovial chondromatosis/neoplasm | ||||
| 4. Congenital disorders | Aplasia/hypoplasia/hyperplasia | |||
|
EXTRA-ARTICULAR
DISORDERS |
1. Muscle pain | Myalgia/ tendonitis/ myositis/spasm |
Local myalgia/myofascial pain/ myofascial pain with referral |
|
| 2. Contracture | ||||
| 3. Hypertrophy | ||||
| 4. Neoplasm | ||||
| 5. Movement disorders | Orofacial dyskinesia/oromandibular dystonia | |||
| 6. Masticatory muscle pain due to systemic pain disorder | Fibromyalgia Widespread pain |
|||
Multiple factors are involved in the etiology of TMDs, including local and systemic conditions; additionally, biological, environmental, emotional, and cognitive factors are known to be triggers for this pathology [6]. Among the systemic diseases, rheumatoid arthritis, inflammatory conditions, ankylosing spondylitis, and immune diseases such as lupus are known to be implicated in TMDs [7].
The prevalence of TMDs among the general population is considered to be 5%; there are studies suggesting that approximately 5%–60% of the population experience at least one of the signs of TMDs [3,6]. Ryan et al. found in their research that the prevalence of TMDs peaks among people aged 25–45, and it seems that women are more affected than men. Among adolescents aged 14–18 years, TMDs have a prevalence of about 30% [7,8,9,10].
Over the last two decades, and especially during the last year [11,12,13,14], scientific interest in vitamin D has increased greatly. As the etiology and pathogenesis of TMDs arenot completely understood, the therapeutic options are not always successful. Therefore, understanding the etiology of temporomandibular joint disorders is important, as well as identifying and eliminating the potential pathogenic factors. The aims of this review areto examine the evidence to date regarding vitamin D and temporomandibular disorders and to investigate whether the current knowledge supports 25-OHD supplementation in temporomandibular disorders associated with insufficient or deficient levels of vitamin D.
2. Method for Literature Search
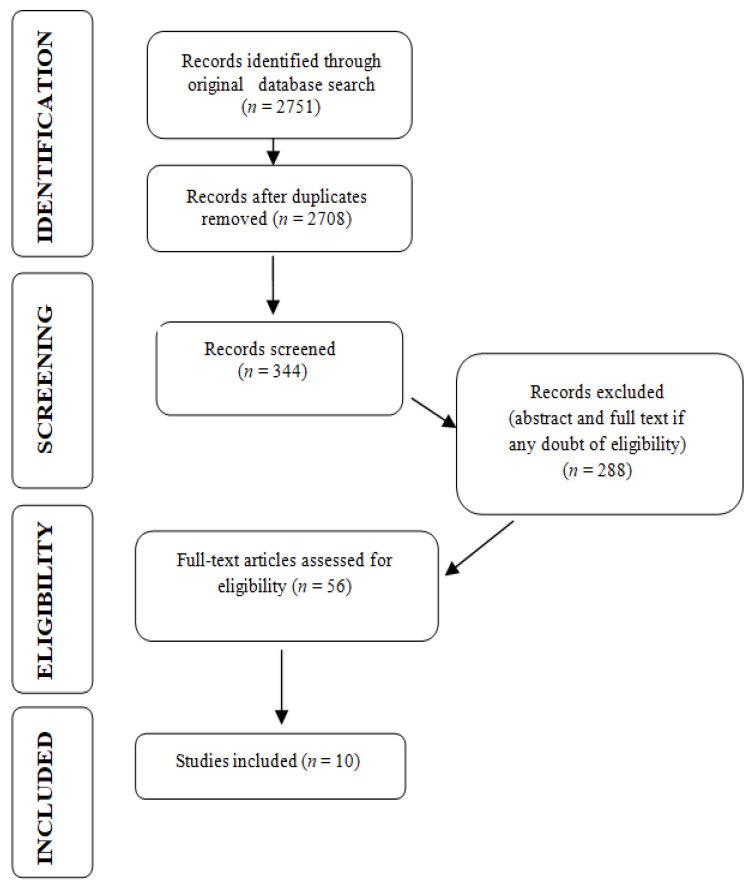
Serial literature searches for published studies of interest were performed between December 2020 and January 2021. PubMed, Scopus, Science Direct, and Google Scholar databases were searched using a combination of the following Medical Subject Headings (MeSH) terms:“vitamin D”, “temporomandibular disorders”, “masticatory muscles disorders”, “osteoarthritis”, and “bruxism”. Articles published between 2010 and 2021 were included. For this research, we considered the following to be eligible (all published in English language): clinical trials, randomized controlled trials, reviews, and systematic reviews.Exclusion criteria were non-human research, studies whereby outcomes of interest were not investigated, or articles written in languages other than English. A total of 2751 of records was identified, 43 duplicates were removed, 344 articles were screened, and 56 articles were assessed for eligibility. Studies investigating osteoarthritis localized on joints other than temporomandibular joints (TMJ) were not included in the narrative, and 10 studies were included in this review (Figure 1).
Figure 1.
PRISMA flow diagram for research stages.
3. Current Status of Knowledge
3.1. Definitions
The implications of vitamin D in temporomanidbular disorders can be evaluated after careful consideration and understanding of different types of disorders, physiology and genetic variances involved in this interaction.
3.1.1. Temporomandibular Disorders
Disk derangement disorders, osteoarthritis, autoimmune disorders, and myofascial pain disorder are some of the more common syndromes associated with temporomandibular disorders (Table 1). While musculoskeletal conditions are responsible for at least 50% of TMDs, osteoarthritis and disk displacement are the most frequent intra-articular disorders of temporomandibular joints (TMJs) [3,15,16].
Since pain is often the main symptom that determines the patients to seek medical aid, TMD diagnosis requires a thorough medical history and physical examination. This is because other conditions that mimic TMDs often challenge the clinician in establishing the differential diagnosis. Pathologic conditions such as dental lesions, oral abscess, or oral lesions, and conditions resulting from muscle overuse (e.g., bruxism, clenching, muscle spasm, and excessive chewing), trauma, maxillary sinusitis, salivary gland disorders, neuralgia, etc. are also associated with orofacial pain [3,16]. Therefore, in establishing a TMD diagnosis, other signs and symptoms should be emphasized—the limitation of mandibular movements and/or joint sounds. All the clinical findings associated with imaging studies (Panoramic X-rays, TMJ X-rays, CT scans, and MRI) and other complementary explorations (blood analysis and biochemistry of synovial fluid) lead to a specific diagnosis regarding the type of temporomandibular disorder [17].
Osteoarthritis represents a destructive process affecting temporomandibular joints [18]; however, it is considered that, at least in its initial phases, it has a non-inflammatory origin [19].The pathological mechanism behind this intra-articular TMD is defined by an articular cartilage degradation and abrasion, as well as a local thickening and remodeling of the underlying bone. Secondary inflammatory modifications are sometimes superimposed on top of these changes [19]. Among the clinical signs in the case of osteoarthritis, pain and reduced mobility of the jaw are the most frequent, regardless of the specific joint noises during mouth opening (crepitus) [15].
As the etiology of TMDs is multifactorial, their management protocol often involves a multidisciplinary approach. The initial aim of therapy should be resolving pain and dysfunction, while invasive treatments such as joint surgery should be reserved for non-responsive cases [3,20,21]. Among the conservative therapies, patient education and self-care, physical therapy, medication (non-inflammatory drugs and muscle relaxants), and the use of occlusal splints and occlusal adjustments are often indicated [3,14].
3.1.2. Physiology and Actions of Vitamin D
Vitamin D is synthesized in the skin with the aid of ultraviolet-B radiation and converted to the active form 1,25-dihydroxyvitamin D in two steps, which binds to the vitamin D receptor (VDR) [22]. A large number of genes are regulated by the activated VDR complex. Compared with1,25-dihydroxyvitamin D (which has a half-life of four hours), the pro-form 25-hydroxyvitamin D has a half-life of about three weeks, and it is also more stable than the former. As a result, 25-OHD is used to determine the vitamin D status [23,24].
Whether synthesized endogenously in the skin after sun exposure or ingested in the diet, vitamin D (25-OHD) plays a role in a wide range of processes in the body.
Vitamin D is required for a healthy musculoskeletal system, as it plays an important role in calcium and phosphorus metabolism—not only by increasing their intestinal absorption and regulating the parathyroid hormone (PTH) production, but also by inducing the pre-osteoclasts to become mature osteoclasts [25,26,27].
Moreover, vitamin D is also known to regulate ~3% of the human genome through vitamin D receptor (VDR), which forms a heterodimer with the retinoid X receptor (RXR) [22,28]. Several types of tissue in the human body (such as pancreatic islets, prostate, macrophages, malignant, and immune cells) are capable of producing local 1,25-dihydroxyvitamin D as a result of possessing 1-α-hydroxylase [29,30]. This aspect suggests the role of vitamin D in preventing cancers, in affecting endocrine systems and insulin release, as well as in other metabolic processes.
Controversies still exist regarding the optimal serum levels of 25-OHD; however, over the last decade, an overall agreement was reached that circulating 25-OHD values below 30ng/mL are an indication of low vitamin D status [22]. Ideally, in deficient cases, vitamin D supplementation should be made according to the vitamin D serum levels (Table 2).
Table 2.
Definition of vitamin D deficiencies and recommended supplementation with cholecalciferol.
| Serum Vitamin D Levels | <5 ng/mL | 5–15 ng/mL | 16–30 ng/mL |
|---|---|---|---|
| Definition | Severe vitamin D deficiency | Mild vitamin D deficiency | Vitamin D insufficiency |
| Dose of cholecalciferol | 8000 IU/day orally or enterally for 4 weeks, followed by 4000 IU/day | 4000 IU/day orally or enterally for 12 weeks | 2000 IU/day |
3.1.3. Vitamin D and Genetic Variances
A large number of genes is regulated by the activated VDR complex, and since the VDR gene polymorphisms have been investigated, several associations have been evaluated with a wide variety of diseases, including musculoskeletal conditions, osteoarthritis, diabetes, malignant tumors, cardiovascular diseases, viral infections, tuberculosis, oral diseases, urinary stone, and autoimmune diseases [31,32].
It is known that the VDR gene is situated on chromosome 12 (12q12-q14). It has numerous single nucleotide polymorphisms (SNPs) (gene polymorphisms sites) and expresses a ligand-activated transcription factor [33,34]. Some of these SNPs play critical roles in the modification of 1,25-dihydroxyvitamin D uptake [17] and can promote vitamin D function and metabolism [33]. As both the non-coding and coding regions of VDR’s sequence have several biallelic polymorphic sites, they can influence the rate gene transcription, the quality of the resulting protein, or the stability of mRNA. Therefore, it is considered that predisposition or disease severity might be linked to these sites [35].
The VDR gene contains over 100 restriction polymorphic sites, the most well-known of which are ApaI (rs7975232), TaqI (rs731236), BsmI (rs1544410), and FokI (rs1544410) [36]. Several studies have investigated the links between the VDR polymorphism and cartilaginous tissue diseases (such as osteoarthritis of knee, hip, or inter-vertebral disks, lumbar disks, etc.), and a large majority of them revealed an association between them.
In 2018, Yilmaz et al. investigated whether VDR polymorphism also presents susceptibility to internal derangements in temporomandibular disorders. Performing a clinical study on 119 patients, the authors could not reveal any association between VDR TaqI and ApaI gene polymorphisms and intra-articular temporomandibular disorders [32].
In 2020, Yildiz et al. published a research investigating the relationship between vitamin D and a Bsml variant with TMD, specifically, disk displacements with reduction (DDR). The case–control study included 104 Turkish patients diagnosed with DDR and 102 healthy Turkish individuals, investigating a Bsml variant of VDR. The authors concluded that the Bsml variant of the VDR gene, as well as the low levels of vitamin D, plays a role in the etiology and pathogenesis of disk displacements with reduction [37].
3.2. Vitamin D and Temporomandibular Disorders
It has been shown that vitamin D plays a significant role in the musculoskeletal and cardiovascular systems, as well as in the control of calcium and phosphate metabolism and the maintenance of sufficient blood levels of these minerals [38,39]; it seems that this nutrient is also related to cartilage regeneration in people with osteoarthritis (OA), although the mechanism is still unclear [40]. In the context of investigating a possible association between vitamin D and temporomandibular disorders, 10 articles were included in this literature review (Table 3).
Table 3.
Overview of the articles investigating an association between different types of temporomandibular disorders and low level of vitamin D.
| First Author and Year | Type of Study | Sample Size | Results |
|---|---|---|---|
| Yilmaz, A. D., 2018 [32] | Clinical Trial | 119 subjects | No association between the VDR TaqI and ApaI gene polymorphisms and intra-articular TMDs. |
| Yildiz, S., 2020 [37] | Case–control Study | 206 subjects | Bsml variant of VDR gene, as well as low levels of vitamin D, plays a role in the etiology and pathogenesis of intra-articular TMDs (disk displacement with reduction). |
| Madani, A., 2019 [41] | Case–control study | 80 subjects | No significant association between vitamin D serum concentration levels and TMDs. |
| Yilmaz, F., 2020 [42] | Case–control study | 146 subjects | High prevalence of TMDs among chronic hemodialysis patients. |
| Khanna, S., 2017 [44] | Case–control study | 100 subjects | Vitamin D serum level had a significant impact on the TMJ pain and discomfort. |
| Jagur, O., 2011 [46] | Case–control study | 95 subjects | TMJ radiographic changes and teeth loss seem to be related to the low level of bone mineral density and vitamin D serum levels. |
| Demir, C. Y., 2019 [47] | Clinical trial | 100 subjects | Vitamin D status was similar between patients with TMDs and control group; increased parathyroid hormone levels in response to vitamin D deficiency were significantly higher in patients with TMDs. |
| Staniszewski, K., [48] | Controlled Cross-Sectional Study | 120 subjects | Serum analyses should not be used as a biomarker of TMDs. |
| Ahmed, H. S. [49] | Clinical trial | 45 subjects | Vitamin D levels were significantly lower in TMD patients with RA. |
| Alkhatatbeh, M. J., [51] | Case–control study | 100 subjects | Vitamin D levels were significantly lower in subjects with sleep bruxism. |
VDR—vitamin D receptor; TMDs—temporomandibular disorders; TMJ—temporomandibular joint; RA—rheumatoid arthritis.
In 2019, Madani et al. published a study that aimed to identify whether among the multiple factors involved in calcium metabolism, vitamin D is associated with temporomandibular disorders [41]. A total of 51 patients with different types of TMDs were included in their study, who were compared with 29 healthy subjects. Blood sample analysis revealed no significant association between the calcium-metabolism-related factors (such as serum phosphate, alkaline phosphate, parathyroid hormone (PTH), and vitamin D concentrations) and temporomandibular disorders.
Another study, published by Yilmaz et al. in 2019 [42], suggests a prevalence of TMDs (especially the muscular disorders) among chronic kidney disease (CKD) patients (~41.5%); however, due to the limitations of their study, the authors could not establish a cause and effect relationship between TMD and hemodialysis patients. These results are significant for future studies on vitamin D, which is considered to be deficient in CKD patients [22,43] and may play a role in the evolution of temporomandibular disorders on hemodialysis patients by inducing inflammatory cytokine production.
An exploratory study, published in 2017 by Khanna et al., investigated the influence of vitamin D on the temporomandibular joints and the daily activities of a population in Mumbai. The research revealed an association between TMJ pain and discomfort, affecting also the daily activities of the subjects’ daily activities [44]. Although those results confirm similar findings published earlier by Shen et al. and Jagur et al. [45,46], future studies (which might investigate the influence of vitamin D on TMJ health post-supplementation) should be carried out in order to establish a link between the two aspects.
Those results were also confirmed by Yilmaz and Ersoz in 2018 [47], whose research investigated the biochemical changes in patients diagnosed with TMDs. The serum levels of vitamin D, parathyroid hormone, calcitonin, calcium, phosphorus, and magnesium were investigated in a group of TMDs patients, compared with a control group. A total of 100 subjects were included in this prospective study, with a total of 50 patients diagnosed with TMDs and intra-articular pathologies. The results revealed no significant difference inthe serum levels of vitamin D, calcium, phosphorus, and magnesium between the two groups; however, the levels of parathyroid hormone in response to vitamin D deficiency weresignificantly higherin the study groups (TMDs subjects) compared to the control group. The authors concluded that, in patients with TMDs, the vitamin D serum level should be investigated, and a correction should be performed in case of a deficiency.
Different results were obtained by Staniszewski et al. [48]. In their research, published in 2019, a serum analysis was performed on a group of patients with temporomandibular disorders compared with a group of healthy subjects. They investigated several common variables (such as blood cell count, TSH, FT4, PTH, creatinine, sodium, potassium, calcium, albumin, and vitamin D.). The authors concluded that there was no clear evidence of systemic disease or malnutrition in the TMD group. Although the results might implythat serum analysis should not be used as a biomarker in the case of temporomandibular disorders, the investigation also revealed the fact that the administration of high doses of vitamin supplements in a group of TMD patients contributed to elevated serum levels in some variables.
As inflammatory arthritis (especially rheumatoid arthritis) is also known to have an affinity to temporomandibular joints, Ahmed H. S. published a study in 2017, investigating the serum levels of some variables (including vitamin D, calcium, and interleukin-1) in 45 TMD patients suffering from rheumatoid arthritis. Comparing the results with a group of healthy individuals, the research revealed that the vitamin D levels were significantly lower in TMD patients. The results also showed a significant increase in serum total alkaline phosphatase as bone marker and interleukin-1 in TMD patients. Their research also revealed that vitamin D might exert anti-inflammatory effects by diminishing the release of pro-inflammatory cytokines, as well as by inhibiting T-cell response [49].
Several oral habits and parafunctions are considered to be involved in the etiology of temporomandibular disorders. Bruxism is a rhythmic masticatory muscle activity, characterized by teeth grinding and clenching, which can occur during sleep or while awake [50].In 2021, Alkhatatbeh et al. published a case–control study, investigating whether there is an association among the low intake of dietary calcium, low levels of vitamin D, headache, and psychological symptoms (such as anxiety and depression) and sleep bruxism. A total of 100 subjects were enrolled in this observational study, and the results showed that, compared with the control groups, the subjects with sleep bruxism had higher anxiety and depression rates, and their vitamin D levels were significantly lower [51].
4. Vitamin D and Pain Management
The International Association for the Study of Pain describes pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or describes in terms of such damage” [49,52]. There is a multitude of published studies investigating the relationship between vitamin D and different types of pain; especially for patients suffering from osteoarthritis, including TMD osteoarthritis, pain is a common symptom. In the Cochrane review, published in 2015, Straube et al. concluded that, based on the current evidence, for chronic painful conditions in adults, vitamin D supplementation might not be beneficial in terms of pain relief.The authors also mention that, in the clinical context, deficient levels of vitamin D should be corrected by treatment even thougha significant reduction of pain intensity (at least 50% reduction) should not be expected [1].
Headache is also an important symptom for patients suffering from temporomandibular disorders. According to the third edition of the International Classification of Headache Disorders (ICHD-3), the headache attributed to temporomandibular disorders is diagnosed in association with painful pathological conditions of TMJs and mastication muscles. Usually, this type of headache is most prominent in the temporalis, masseter muscle, and preauricular area. When the temporomandibular disorder is uncertain, tension type headache is a differential diagnosis (caused by the tenderness of pericranial muscles) [53].
In a literature review published in 2020, Nowaczewska et al. investigated the role of vitamin D in primary headaches [54], emphasizing the strong correlation between vitamin D deficiency and chronic musculoskeletal pain; patients with tension type headache seem to have low levels of vitamin D, and there is also evidence that patients with chronic headache have lower melatonin levels, with decreased nocturnal serum melatonin levels during cluster periods [55]. As vitamin D acts like a neuroprotective agent (it is involved in regulating the production of several neurotrophic factors, activates different enzymes in brain, and influences the neurotransmitters), Nowaczewska et al. concluded that there is important evidence in establishing a connection between the serum levels of 25-OHD and migraines but also with other types of headaches through different mechanisms.
Similar conclusions have also been drawn by Wu et al. in a systematic review published in 2018, emphasizing an association between the low serum levels of vitamin D and, in the case of arthritis, muscle pain and chronic widespread pain [56].
Several studies published to date have investigated the relationship between vitamin D serum levels and other types of pain. The results of a clinical study performed on 175 patients with knee osteoarthritis, published in 2017 by Manoy et al, revealed that supplementation with 40,000 IU of vitamin D2 (ergocalciferol) for 6 months improved grip strength and physical performance, although it did not improve knee extensions. The results also showed that vitamin D supplementation reduced oxidative protein damage, reduced pain, and improved the quality of life. Nevertheless, the authors conclude that it remains unclear whether vitamin D supplementation influences musculoskeletal pain or not [57].
In 2019, Park published a literature narrative review, which focused on the potential link between vitamin D and osteoarthritis (OA) by analyzing different types of data, including experimental or in vitro studies. Based on the research, Park concluded that vitamin D may have a preventative effect on joint pain, although clinical observations showed that it has little effect on cartilage volume loss or radiologic OA initiation. The effect of vitamin D may also differ according to the disease state [58].
In 2020, Jin et al. published a systematic review investigating the effects of vitamin D supplementation on pain and physical function in patients with knee osteoarthritis. Based on their analysis, the authors conclude that despite the fact vitamin D supplementation may improve pain and function in patients with knee osteoarthritis, the evidence from randomized clinical trials (RCTs) was controversial, because not all the RCTs measured the potential moderators of interest, or, not all of them were measured in the same way. Nevertheless, the results published by Jin et al. can provide critical evidence to help clinicians make subgroup-specific treatment decisions in order to increase therapeutic efficacy [59].
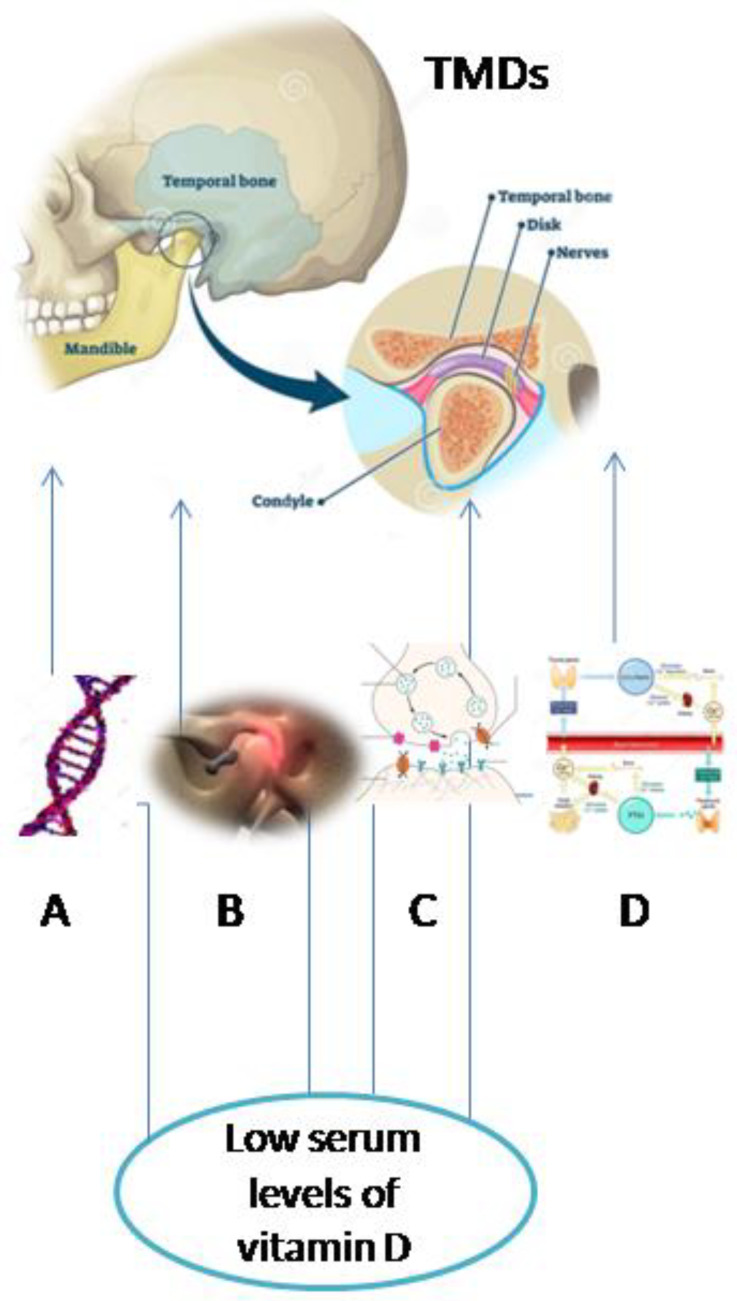
Overall, this research revealed the different directions in which temporomandibular disorders might be associated with low levels of vitamin D. This is because vitamin D influences the calcium and PTH metabolism, has anti-inflammatory functions by diminishing the release of pro-inflammatory cytokines as well as by inhibiting T-cell response, and acts like a neuroprotective agent. Additionally, the Bsml variant of the VDR gene has been associated with temporomandibular disorders, specifically, disk displacements with reduction (Figure 2).
Figure 2.
Possible pathways of interconnection between temporomandibular disorders and low levels of vitamin D (A—VDR gene polymorphism, B—inflammatory processes, C—neuroprotective function, and D—calcium and parathyroid hormone (PTH) metabolism).
5. Conclusions
There are few observational studies showing an association between vitamin D deficiency and intra-articular temporomandibular disorders. There are several randomized controlled trials revealing a positive effect of vitamin D supplementation on pain management and on the quality of life for patients suffering from osteoarthritis. To date, there are no studies investigating the possible positive effects of vitamin D on pain management or on the prevention of temporomandibular disorders.
As clinical studies published to date are limited in their duration to study the role of vitamin D on TMDs prevention, vitamin D deficiency might be investigated and corrected. Although the evidence is weak for making general recommendations for vitamin D deficiency in cases of temporomandibular disorders, patients with vitamin D level <30nmol/L could benefit from supplementation. Therefore, vitamin D supplements might be indicated as an independent therapy for deficient patients with temporomandibular disorders.
Further well-designed randomized controlled trials are needed in order to confirm the relationship between vitamin D levels and the different types of temporomandibular disorders, or whether normal levels of vitamin D might prevent these disorders.
Author Contributions
Conceptualization, A.K. and A.L.; methodology, A.K.; software, M.N.; validation, S.B. (Smaranda Buduru), S.B (Silvia Balhuc) and A.L.; resources, M.N.; writing—original draft preparation, A.K.; writing—review and editing, A.L.; visualization, M.N.; supervision, S.B. (Smaranda Buduru); project administration, S.B. (Silvia Balhuc). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Straube S., Derry S., Straube C., Moore R.A. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst. Rev. 2015;5:CD007771. doi: 10.1002/14651858.CD007771.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Gauer R.L., Semidey M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Physician. 2015;91:378–386. [PubMed] [Google Scholar]
- 4.De Rossi S.S., Greenberg M.S., Liu F., Steinkeler A. Temporomandibular disorders: Evaluation and management. Med. Clin. N. Am. 2014;98:1353–1384. doi: 10.1016/j.mcna.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P., List T., Svensson P., Gonzalez Y., Lobbezoo F., et al. International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan J., Akhter R., Hassan N., Hilton G., Wickham J., Ibaragi S. Epidemiology of Temporomandibular Disorder in the General Population: A Systematic Review. Adv. Dent. Oral Health. 2019;10:555787. [Google Scholar]
- 7.Paduano S., Bucci R., Rongo R., Silva R., Michelotti A. Prevalence of temporomandibular disorders and oral parafunctions in adolescents from public schools in Southern Italy. Cranio. 2020;38:370–375. doi: 10.1080/08869634.2018.1556893. [DOI] [PubMed] [Google Scholar]
- 8.Lövgren A., Häggman-Henrikson B., Visscher C.M., Lobbezoo F., Marklund S., Wänman A. Temporomandibular pain and jaw dysfunction at different ages covering the lifespan—A population based study. Eur. J. Pain. 2016;20:532–540. doi: 10.1002/ejp.755. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khotani A., Naimi-Akbar A., Albadawi E., Ernberg M., Hedenberg-Magnusson B., Christidis N. Prevalence of diagnosed temporomandibular disorders among Saudi Arabian children and adolescents. J. Headache Pain. 2016;17:41. doi: 10.1186/s10194-016-0642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graue A.M., Jokstad A., Assmus J., Skeie M.S. Prevalence among adolescents in Bergen, Western Norway, of temporomandibular disorders according to the DC/TMD criteria and examination protocol. Acta Odontol. Scand. 2016;74:449–455. doi: 10.1080/00016357.2016.1191086. [DOI] [PubMed] [Google Scholar]
- 11.Zemb P., Bergman P., Camargo C.A., Jr., Cavalier E., Cormier C., Courbebaisse M., Hollis B., Joulia F., Minisola S., Pilz S., et al. Vitamin D deficiency and the COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2020;22:133–134. doi: 10.1016/j.jgar.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health. 2020;13:1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilezikian J.P., Bikle D., Hewison M., Lazaretti-Castro M., Formenti A.M., Gupta A., Madhavan M.V., Nair N., Babalyan V., Hutchings N., et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183:R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machon V., Hirjak D., Lukas J. Therapy of the osteoarthritis of the temporomandibular joint. J. Cranio-maxillofac. Surg. 2011;39:127–130. doi: 10.1016/j.jcms.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Poveda Roda R., Bagan J.V., Díaz Fernández J.M., Hernández Bazán S., Jiménez Soriano Y. Review of temporomandibular joint pathology. Part I: Classification, epidemiology and risk factors. Med. Oral Patol. Oral Cir. Bucal. 2007;12:E292–E298. [PubMed] [Google Scholar]
- 17.Poveda Roda R., Díaz Fernández J.M., Hernández Bazán S., Jiménez Soriano Y., Margaix M., Sarrión G. A review of temporomandibular joint disease (TMJD). Part II: Clinical and radiological semiology. Morbidity processes. Med. Oral Patol. Oral Cir. Bucal. 2008;13:E102–E109. [PubMed] [Google Scholar]
- 18.Okeson J.P., de Leeuw R. Differential diagnosis of temporomandibular disorders and other orofacial pain disorders. Dent. Clin. N. Am. 2011;55:105–120. doi: 10.1016/j.cden.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka E., Detamore M.S., Mercuri L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann R.G., Kotchen J.M., Kotchen T.A., Cowley T., Dasgupta M., Cowley A.W., Jr. Temporomandibular disorders and associated clinical comorbidities. Clin. J. Pain. 2011;27:268–274. doi: 10.1097/AJP.0b013e31820215f5. [DOI] [PubMed] [Google Scholar]
- 21.Batacchi Z., Robinson-Cohen C., Hoofnagle A.N., Isakova T., Kestenbaum B., Martin K.J., Wolf M.S., de Boer I.H. Effects of Vitamin D2 Supplementation on Vitamin D3 Metabolism in Health and CKD. Clin. J. Am. Soc. Nephrol. 2017;12:1498–1506. doi: 10.2215/CJN.00530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chau Y.Y., Kumar J. Vitamin D in chronic kidney disease. Indian J. Pediatr. 2012;79:1062–1068. doi: 10.1007/s12098-012-0765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helde-Frankling M., Björkhem-Bergman L. Vitamin D in Pain Management. Int. J. Mol. Sci. 2017;18:2170. doi: 10.3390/ijms18102170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holick M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocrmetabdisord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 26.Brown A.J. Vitamin D analogs for secondary hyperparathyroidism: What does the future hold? J. Steroid Biochem. Mol. Biol. 2007;103:578–583. doi: 10.1016/j.jsbmb.2006.12.089. [DOI] [PubMed] [Google Scholar]
- 27.Cranney A., Horsley T., O’Donnell S., Weiler H., Puil L., Ooi D., Atkinson S., Ward L., Moher D., Hanley D., et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep. Technol. Assess. (Full Rep.) 2007;158:1–235. [PMC free article] [PubMed] [Google Scholar]
- 28.Bouillon R., Carmeliet G., Verlinden L., van Etten E., Verstuyf A., Luderer H.F., Lieben L., Mathieu C., Demay M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008;88:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 30.Andress D.L. Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69:33–43. doi: 10.1038/sj.ki.5000045. [DOI] [PubMed] [Google Scholar]
- 31.Bonato L.L., Quinelato V., Borojevic R., Vieira A.R., Modesto A., Granjeiro J.M., Tesch R., Casado P.L. Haplotypes of the RANK and OPG genes are associated with chronic arthralgia in individuals with and without temporomandibular disorders. Int. J. Oral Maxillofac. Surg. 2017;46:1121–1129. doi: 10.1016/j.ijom.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz A.D., Yazicioglu D., TüzünerÖncül A.M., Yilmaz E., Ereş G. Vitamin D receptor gene polymorphisms (Apa1 and Taq1) in temporomandibular joint internal derangement/osteoarthritis in a group of Turkish patients. Mol. Biol. Rep. 2018;45:1839–1848. doi: 10.1007/s11033-018-4330-5. [DOI] [PubMed] [Google Scholar]
- 33.Zilahi E., Chen J.Q., Papp G., Szántó A., Zeher M. Lack of association of vitamin D receptor gene polymorphisms/haplotypes in Sjögren’s syndrome. Clin. Rheumatol. 2015;34:247–253. doi: 10.1007/s10067-014-2639-6. [DOI] [PubMed] [Google Scholar]
- 34.Monticielo O.A., de Mattos Teixeira T., Chies J.A.B., Brenol J.C.T., Xavier R.M. Vitamin D and polymorphisms of VDR gene in patients with systemic lupus erythematosus. Clin. Rheumatol. 2012;31:1411–1421. doi: 10.1007/s10067-012-2021-5. [DOI] [PubMed] [Google Scholar]
- 35.Bid H.K., Mishra D.K., Mittal R.D. Vitamin-D receptor (VDR) gene (Fok-I, Taq-I and Apa-I) polymorphisms in healthy individuals from north Indian population. Asian Pac. J. Cancer Prev. 2005;6:147–152. [PubMed] [Google Scholar]
- 36.Uitterlinden A.G., Fang Y., Van Meurs J.B., Pols H.A., Van Leeuwen J.P. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Yildiz S., Tumer M.K., Yigit S., Nursal A.F., Rustemoglu A., Balel Y. Relationship of Vitamin D and Bsml variant with Temporomandibular Diseases in Turkish population. Br. J. Oral Maxillofacsurg. 2020 doi: 10.1016/j.bjoms.2020.08.101. [DOI] [PubMed] [Google Scholar]
- 38.Zittermann A. The Biphasic Effect of Vitamin D on the Musculoskeletal and Cardiovascular System. Int. J. Endocrinol. 2017;2017:3206240. doi: 10.1155/2017/3206240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koundourakis N.E., Avgoustinaki P.D., Malliaraki N., Margioris A.N. Muscular effects of vitamin D in young athletes and non-athletes and in the elderly. Hormones. 2016;15:471–488. doi: 10.14310/horm.2002.1705. [DOI] [PubMed] [Google Scholar]
- 40.Garfinkel R.J., Dilisio M.F., Agrawal D.K. Vitamin D and Its Effects on Articular Cartilage and Osteoarthritis. Orthop. J. Sports Med. 2017;5:2325967117711376. doi: 10.1177/2325967117711376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madani A., Shamsian S.A., Layegh P., Abrisham S.M., Ravaghi A., TayaraniNajjaran N. Are certain factors involved in calcium metabolism associated with temporomandibular disorders? Cranio. 2019;31:1–7. doi: 10.1080/08869634.2019.1596054. [DOI] [PubMed] [Google Scholar]
- 42.Yılmaz F., GünenYılmaz S., Sözel H., Bora F., Yılmaz A.B. The prevalence of temporomandibular disorders in chronic hemodialysis patients: A cross-sectional study. Cranio. 2020;20:1–9. doi: 10.1080/08869634.2020.1727170. [DOI] [PubMed] [Google Scholar]
- 43.Zand L., Kumar R. The Use of Vitamin D Metabolites and Analogues in the Treatment of Chronic Kidney Disease. Endocrinol. Metab. Clin. N. Am. 2017;46:983–1007. doi: 10.1016/j.ecl.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khanna S., Parulekar N.R., Dhaimade P.A. The Influence of Vitamin D on the Temporomandibular Joint and the Activities of Daily Living. J. Clin. Diagn. Res. 2017;11:ZC31–ZC34. doi: 10.7860/JCDR/2017/30885.10895. [DOI] [Google Scholar]
- 45.Shen M., Luo Y., Niu Y., Chen L., Yuan X., Goltzman D., Chen N., Miao D. 1,25(OH)2D deficiency induces temporomandibular joint osteoarthritis via secretion of senescence-associated inflammatory cytokines. Bone. 2013;55:400–409. doi: 10.1016/j.bone.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Jagur O., Kull M., Leibur E., Kallikorm R., Loorits D., Lember M., Voog-Oras U. Relationship between radiographic changes in the temporomandibular joint and bone mineral density: A population based study. Stomatologija. 2011;13:42–48. [PubMed] [Google Scholar]
- 47.Demir C.Y., Ersoz M.E. Biochemical changes associated with temporomandibular disorders. J. Int. Med. Res. 2019;47:765–771. doi: 10.1177/0300060518811009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staniszewski K., Lygre H., Berge T., Rosén A. Serum Analysis in Patients with Temporomandibular Disorders: A Controlled Cross-Sectional Study in Norway. Pain Res. Manag. 2019;2019:1360725. doi: 10.1155/2019/1360725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed H.S. Biochemical Changes Related with Temporomandibular Joint Disorders and Inflammatory Arthritis. Biomed. Pharmacol. J. 2017;10:2085–2090. doi: 10.13005/bpj/1331. [DOI] [Google Scholar]
- 50.Beddis H., Pemberton M., Davies S. Sleep bruxism: An overview for clinicians. Br. Dent. J. 2018;225:497–501. doi: 10.1038/sj.bdj.2018.757. [DOI] [PubMed] [Google Scholar]
- 51.Alkhatatbeh M.J., Hmoud Z.L., Razzak K.K.A., Alem E.M. Self-reported sleep bruxism is associated with vitamin D deficiency and low dietary calcium intake: A case-control study. BMC Oral Health. 2021;21:1–10. doi: 10.1186/s12903-020-01349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.International Association for the Study of Pain IASP Taxonomy. [(accessed on 27 January 2021)];1994 Available online: http://www.iasp-pain.org/Taxonomy#Pain.
- 53.Arnold M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 54.Nowaczewska M., Wiciński M., Osiński S., Kaźmierczak H. The Role of Vitamin D in Primary Headache–from Potential Mechanism to Treatment. Nutrients. 2020;12:243. doi: 10.3390/nu12010243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sohn J.H., Chu M.K., Park K.Y., Ahn H.Y., Cho S.J. Vitamin D deficiency in patients with cluster headache: A preliminary study. J. Headache Pain. 2018;19:54. doi: 10.1186/s10194-018-0886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Z., Malihi Z., Stewart A.W., Lawes C.M., Scragg R. The association between vitamin D concentration and pain: A systematic review and meta-analysis. Public Health Nutr. 2018;21:2022–2037. doi: 10.1017/S1368980018000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manoy P., Yuktanandana P., Tanavalee A., Anomasiri W., Ngarmukos S., Tanpowpong T., Honsawek S. Vitamin D Supplementation Improves Quality of Life and Physical Performance in Osteoarthritis Patients. Nutrients. 2017;9:799. doi: 10.3390/nu9080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park C.Y. Vitamin D in the Prevention and Treatment of Osteoarthritis: From Clinical Interventions to Cellular Evidence. Nutrients. 2019;11:243. doi: 10.3390/nu11020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin X., Antony B., Wang X., Persson M.S., McAlindon T., Arden N.K., Srivastava S., Srivastava R., Van Middelkoop M., Bierma-Zeinstra S.M., et al. Effect of vitamin D supplementation on pain and physical function in patients with knee osteoarthritis (OA): An OA Trial Bank protocol for a systematic review and individual patient data (IPD) meta-analysis. BMJ Open. 2020;10:e035302. doi: 10.1136/bmjopen-2019-035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.