Abstract
Viruses are dependent on host factors at all parts of the infection cycle, such as translation, genome replication, encapsidation, and cell-to-cell and systemic movement. RNA viruses replicate their genome in compartments associated with the endoplasmic reticulum, chloroplasts, and mitochondria or peroxisome membranes. In contrast, DNA viruses replicate in the nucleus. Viral infection causes changes in plant gene expression and in the subcellular localization of some host proteins. These changes may support or inhibit virus accumulation and spread. Here, we review host proteins that change their subcellular localization in the presence of a plant virus. The most frequent change is the movement of host cytoplasmic proteins into the sites of virus replication through interactions with viral proteins, and the protein contributes to essential viral processes. In contrast, only a small number of studies document changes in the subcellular localization of proteins with antiviral activity. Understanding the changes in the subcellular localization of host proteins during plant virus infection provides novel insights into the mechanisms of plant–virus interactions and may help the identification of targets for designing genetic resistance to plant viruses.
Keywords: antiviral, colocalization, host factors, protein relocalization, proviral, replication proteins, TuMV
1. Introduction
The most abundant plant viruses have a genome that is a positive single-strand RNA (Group IV) or a negative single-strand RNA (Group V). Single-strand RNA viruses replicate in compartments or vesicles bound to membranes in the cytoplasm or in subcellular organelles [1]. Plant-infecting DNA viruses, on the other hand, are less numerous. Single-strand DNA (Group II) and reverse-transcribing DNA (Group VII) viruses replicate by forming a minichromosome in the nucleus [2].
Viral RNA is translated into proteins using the cellular machinery. Viral nucleic acids and proteins execute their functions in cooperation with host proteins, RNAs, or other factors such as membranes or lipids [3,4]. These components condition susceptibility, and their absence reduces virus accumulation or movement, and may turn a host into a nonhost. These factors encode loss-of-susceptibility genes, also named susceptibility genes [3,5]. Because the presence and activity of these host components are essential for the virus, the terms cellular factors with proviral activity or proviral host factors are often used in publications [3,6].
The establishment of a viral infection is genetically determined at two sequential phases. Initially, the absence of susceptibility genes results in the lack of infection or reduced virus replication and/or movement [3]. When a plant has the susceptibility genes needed for the initiation of infection, in a second phase, virus accumulation, spread, and disease severity are determined by the balance between plant defense and viral suppression of defense responses [7].
Viral infection induces changes in host gene expression [8,9] resulting in the upregulation of susceptibility genes [10] and activation or downregulation of antiviral defense responses [11,12], and may also lead to up- or downregulation of genes that have no effect on the virus [8,9]. Upregulation of antiviral genes indicates the activation of defense responses by multiple mechanisms including autophagy, RNA decay, or gene silencing [7,13,14]. Antiviral defense is mediated by host factors that target viral proteins or nucleic acids and antagonize key parts of virus replication and/or movement, reducing virus accumulation or limiting the spread of infection within the plant [7,15]. However, to protect themselves, viruses may downregulate expression, suppress activity, or induce degradation of antiviral defense components [16]. The molecular mechanisms and significance of changes in host gene expression during viral infection are still poorly understood.
Viruses divert host proteins from their natural roles to execute essential viral processes such as translation, virus replication, or movement [5,17,18]. Changes in activity are often associated with a change in the subcellular localization of the host protein. A protein is considered to relocalize when, in the presence of a virus, a fraction of the total protein accumulates in a new place in the cell. These changes have been detected and characterized by a combination of approaches such as yeast two hybrid, subcellular fractionation, bimolecular fluorescence complementation, immunofluorescence confocal microscopy, or co-precipitation [19,20,21].
In this review, we present an analysis of publications documenting changes in the subcellular localization of host proteins following viral infection. Results present a profile of the host proteins that change, the experimental approaches used to identify them, their natural and new locations, and their role in favor or against viruses. The profiles advance our understanding of the mechanisms that govern plant–virus interactions and establish the basis for the identification of novel host factors with antiviral activity or that condition virus susceptibility and that can be targeted to generate virus-resistant plants by genetic engineering.
2. Profile of Host Proteins
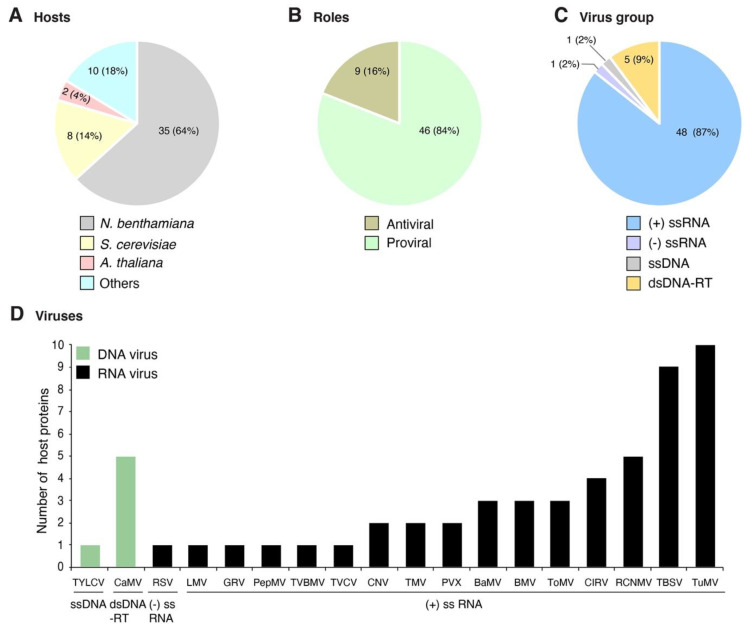
Plant viruses for which at least one host protein has been reported to change subcellular localization were grouped based on their genome organization. The site of genome replication, name of the replication protein, and movement form were compiled and used as a guide to interpret interaction with and recruitment of host proteins (Table 1). We classified the host proteins based on their natural subcellular localization in the absence of viral infection. Changes in subcellular localization were documented for 55 combinations of host protein and plant virus. After profiling features of these proteins and viruses, several general patterns emerged: (1) 45 of the 55 combinations were identified using model hosts (Arabidopsis thaliana, Nicotiana benthamiana, or Saccharomyces cerevisiae, Figure 1A); (2) the majority (48) were identified using model positive-strand RNA viruses (Figure 1C), particularly brome mosaic virus (BMV), tomato bushy stunt virus (TBSV), and turnip mosaic virus (TuMV) (Figure 1D); (3) in 46 of the 55 combinations, the host protein is beneficial to the virus; (4) host proteins with antiviral roles were less abundant (nine) (Figure 1B); and (5) the most frequent group (30 out of 55) was host proteins that moved from the cytoplasm to the sites of virus replication (Figure 2A and Table 2) through interactions with viral proteins (Table 2). These patterns are heavily influenced by the combination of experimental hosts and viruses used as model systems.
Table 1.
Site of replication and movement form of plant viruses for which at least one host protein has been reported to change subcellular localization.
| Virus | RdRp | Site of Replication | Intracellular Movement of the Replication Compartments |
Cell-To-Cell Movement Form |
Reference |
|---|---|---|---|---|---|
| Group II: Single-strand DNA | |||||
| TYLCV | Rep protein |
Nucleus | From nucleus to plasmodesmata | Minichromosome | [22] |
| Group IV: Single positive-strand RNA | |||||
| BaMV | 155 kDa | Chloroplast | From chloroplast to plasmodesmata | Virions or ribonucleoprotein particles | [23] |
| BMV | 2a | Endoplasmic reticulum | Non-motile | Virions or ribonucleoprotein particles | [24] |
| CIRV | p36 | Mitochondria | Non-motile | Ribonucleoprotein particles | [25] |
| CNV | p33 | Peroxisome | Non-motile | Ribonucleoprotein particles | [26] |
| GRV | RdRp | Cytoplasm | NA | Ribonucleoprotein particles | [27] |
| LMV | NIb | Endoplasmic reticulum | From ER to plasmodesmata | Replication vesicles | [28] |
| PepMV | 164 kDa | Cytoplasm (membrane association with ER is unclear) | From cytoplasm to plasmodesmata | Ribonucleoprotein particles | [29] |
| PVX | RdRp | Endoplasmic reticulum | NA | Virions or ribonucleoprotein particles | [30] |
| RCNMV | p27 and p88 | Endoplasmic reticulum | From ER to plasmodesmata | Virions | [31,32,33] |
| TVCV | RdRp | Endoplasmic reticulum | From ER to plasmodesmata | Virions or ribonucleoprotein particles | [34] |
| TBSV | p92pol | Peroxisomes | Non-motile | Ribonucleoprotein particles | [35,36] |
| TMV | RdRp | Endoplasmic reticulum | From ER to plasmodesmata | Replication complexes or ribonucleoprotein particles |
[37] |
| ToMV | 130K and 180K | Endoplasmic reticulum | From ER to plasmodesmata | Virions or ribonucleoprotein particles | [38] |
| TuMV | NIb | ER and chloroplasts | From ER to chloroplast and/or to Golgi apparatus and to plasmodesmata | Replication vesicles | [28] |
| TVBMV | NIb | Chloroplasts | From ER to chloroplast and/or to Golgi apparatus and to plasmodesmata | Replication vesicles | [39,40] |
| Group V: Single negative-strand RNA | |||||
| RSV | 337 kDa | Cytoplasm (membrane association is unknown) | From ER to Golgi to plasmodesmata | Virion–protein complexes | [41,42,43] |
| Group VII: Double-strand DNA-RT | |||||
| CaMV | Rep protein | Nucleus | From nucleus to ER and/or directly to plasmodesmata | Virions | [44] |
Viruses: bamboo mosaic virus (BaMV), brome mosaic virus (BMV), cauliflower mosaic virus (CaMV), carnation Italian ringspot virus (CIRV), cucumber necrosis virus (CNV), groundnut rosette virus (GRV), lettuce mosaic virus (LMV), pepino mosaic virus (PepMV), potato virus X (PVX), red clover necrotic mosaic virus (RCNMV), rice stripe virus (RSV), turnip vein-clearing virus (TVCV), tobacco mosaic virus (TMV), tomato bushy stunt virus (TBSV), tomato mosaic virus (ToMV), turnip mosaic virus (TuMV), tobacco vein banding mosaic virus (TVBMV), tomato yellow leaf curl virus (TYLCV). Viral proteins. NIb: nuclear inclusion protein b, the RNA-dependent RNA polymerase in potyviruses; RdRp: RNA-dependent RNA polymerase; NA: information not available; ER: endoplasmic reticulum.
Figure 1.
Profile of host proteins that change their subcellular localization during plant virus infection as reported in the literature. Fifty-five combinations of host protein-plant virus were documented in publications. (A) Number and proportion of proteins by host species. (B) Number and proportion of host proteins with antiviral role or beneficial to the virus. (C) Number and proportion of host proteins by virus group. (D) Number of host proteins by virus species. Viruses are grouped based on their genome organization.
Figure 2.
Schematic representation of changes in subcellular localization after viral infection. Representative host proteins and plant viruses that induce relocation in more than two proteins are illustrated. Host proteins are color-coded with spheres. Viruses are indicated by numbers. (A) Changes in host cytoplasmic proteins and (B) changes in host proteins naturally localized to organelles, and their movement in the presence of a virus.
Table 2.
Host proteins that participate in essential viral processes and that change subcellular localization during viral infection. Host proteins are organized based on their natural distribution in the absence of virus.
| Virus | Viral Protein or RNA | Host Protein | Host | Movement of Host Protein into |
Role | Initial Detection | Mechanism of Interaction | Experimental System for Detecting of New Localization Sites * |
Method of Observation: Time | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasmic proteins | ||||||||||
| CaMV | TAV | RISP | Arabidopsis thaliana | Inclusion bodies (cytoplasmic and nuclear) | Stimulates translation re-initiation | Yeast two hybrid | Protein–protein | Brassica rapa leaves | Immunofluorescence and confocal microscopy: 15 dpi | [45] |
| BaMV | 155 kDa and 3′ UTR | HSP90 | Nicotiana benthamiana | Chloroplast | Formation of replication compartments | Partially purified replicase | Protein–protein and RNA–protein | Saccharomyces cerevisiae and Escherichia coli | Yeast two hybrid, GST-pull down | [46] |
| 3′ UTR | NbGSTU4 | N. benthamiana | Chloroplast | Binds to the 3′ UTR and stimulates negative-strand RNA synthesis | Partially purified replicase | RNA–protein | E. coli | UV crosslink | [47] | |
| BMV | 1a | ESCRT- III | S. cerevisiae | Perinuclear ER | Formation of replication compartments | Yeast genetic analysis | Protein–protein | S. cerevisiae | Immunofluorescence and confocal microscopy: 48 h | [48] |
| 1a and 2b | LSM1 | S. cerevisiae | ER | Promotes viral RNA translation | Yeast mutagenesis | Protein–protein | S. cerevisiae | Immunofluorescence and confocal microscopy: 48 h | [49,50] | |
| CIRV | p36 | ESCRT-I | N. benthamiana | Mitochondria | Formation of replication compartments | Split ubiquitin assay | Protein–protein | S. cerevisiae | Immunofluorescence and confocal microscopy: 15-45 min | [51] |
| p36 | ORP |
N. benthamiana and S. cerevisiae |
Mitochondria and ER | Formation of replication compartments | Yeast two hybrid | Protein–protein | N. benthamiana leaves | BiFC: 48 h | [52] | |
| PepMV | p26 | Catalase 1 | Solanum lycopersicum | Cytoplasm and nucleus | Antagonist to antiviral response | Yeast two hybrid | Protein–protein | N. benthamiana leaves | BiFC, immunolabeling, and electron microscopy: 3–4 dpi | [53] |
| PVX | TGB12K | TIP | Nicotiana tabacum | Peripheral bodies | Regulates plasmodesmata opening | Yeast two hybrid | Protein–protein | N. benthamiana leaves | Confocal microscopy: 3 dpi | [54] |
| RCNMV | p27 | HSP70 | N. benthamiana | ER | Formation of replication compartments | Affinity purification | Protein–protein | N. benthamiana leaves | Confocal microscopy: 3 dpi | [21] |
| p27 | NbRACK1 | N. benthamiana | ER-derived aggregates | Increases ROS to benefit the virus | Co-immunoprecipitation | Protein–protein | N. benthamiana leaves | BiFC: 4 dpi | [55] | |
| RSV | 337 kDa | HSP20 | N. benthamiana and Oryza sativa | Nucleus | Antagonist to antiviral response | Yeast two hybrid | Protein–protein | N. benthamiana leaves | BiFC: 48 h | [56] |
| TBSV | p33 | eEF1A | S. cerevisiae | Peroxisomal membrane | Stabilization of p33 | Purified replicase proteomics | Protein–protein | S. cerevisiae | Co-purification | [35,57] |
| p33 | ESCRT-I | N. benthamiana | Peroxisomal membrane | Formation of replication compartments | Split ubiquitin assay | Protein–protein | S. cerevisiae | Confocal microscopy: 15–45 min | [58] | |
| p33 | GAPDH | N. benthamiana and S. cerevisiae | Peroxisomal membrane | Viral genomic RNA synthesis | Purified replicase proteomics | Indirect: mediated by p92pol | S. cerevisiae | Confocal microscopy: 16 h | [59] | |
| p33 and p92pol |
HSP70 | S. cerevisiae | Peroxisomal membrane | Formation of replication compartments | Reconstitution assay | Protein–protein | S. cerevisiae | Confocal microscopy: 16 and 24 h | [60,61] | |
| p33 | ORP | S. cerevisiae | Peroxisome and ER | Formation of replication compartments | Affinity purification | Protein–protein | S. cerevisiae and N. benthamiana leaves | BiFC: 2 dpi | [52] | |
| TMV | RdRp and3′ UTR | eEF1A | N. tabacum | Replication compartment | Formation of replication compartments and cell-to-cell movement | Pull-down assay | Protein–protein | N. tabacum | Immunoprecipitation: 4 dpi | [62] |
| TuMV | VPg | AtRH8 | Prunus persica and A. thaliana | Chloroplast membrane | Formation of replication compartments | Yeast two hybrid | Protein–protein | N. benthamiana leaves | BiFC: 2 and 10 dpi | [63] |
| 6K2 | AtRH9 | A. thaliana | Chloroplast membrane | Formation of replication compartments | Confocal microcopy | Protein–protein | N. benthamiana leaves | Confocal microscopy: 72 h | [64] | |
| VPg and NIb | eEF1A | A. thaliana | ER-derived replication compartments | Viral RNA translation, formation of replication compartments | Tandem affinity purification | Protein–protein | N. benthamiana leaves | Immunofluorescence and confocal microscopy: 4–5 dpi | [65] | |
| VPg | eIF(iso)4e | A. thaliana | ER and chloroplasts | Viral RNA translation, formation of replication compartments | Pull-down assay | Protein–protein | N. benthamiana leaves | Immunofluorescence and confocal microscopy: 2–4 dpi | [65] | |
| TuMV | NIb | HSP70 | A. thaliana | Nucleus and replication compartments in the ER | Formation of replication compartments, regulation of NIb activity | Tandem affinity purification | Indirect: mediated by RdRp | N. benthamiana leaves | Confocal microscopy: 2–4 dpi | [20] |
| VPg | PABP2 | Brassica perviridis | Nucleus and ER | Formation of replication compartments | Subcellular fractionation | Protein–protein | N. benthamiana leaves | Confocal microscopy: 4–5 dpi | [66] | |
| ToMV | 130K and 180K | eEF1A | N. tabacum | ER membranes | Viral RNA translation, formation of replication compartments | Subcellular fractionation | Protein–protein | Transgenic N. tabacum BY-2 protoplast | Affinity purification | [67] |
| 130K and 180K | HSP70 | N. tabacum | ER membranes | Formation of replication compartments | Subcellular fractionation | Protein–protein | Transgenic N. tabacum BY-2 protoplast | Affinity purification | [67] | |
| TYLCV | CP | HSP70 | S. lycopersicum | Cytoplasm and nucleus aggregates | Movement of virions | Subcellular fractionation | Protein–protein | S. lycopersicum leaves | Immunodetection and confocal microscopy: 28 or 49 dpi | [68] |
| Endosomal proteins | ||||||||||
| CIRV | p36 | RAB5-GTPase | A. thaliana | Mitochondria | Formation of replication compartments | Yeast two hybrid | Protein–protein | N. benthamiana leaves | BiFC: 2 dpi | [69] |
| CNV | p33 | RAB5-GTPase | A. thaliana | Peroxisome | Formation of replication compartments | Yeast two hybrid | Protein–protein | N. benthamiana leaves | BiFC: 2 dpi | [69] |
| TBSV | p33 | RAB5-GTPase | A. thaliana | Peroxisome | Formation of replication compartments | Yeast two hybrid | Protein–protein | N. benthamiana leaves | BiFC: 2 dpi | [69] |
| Endoplasmic reticulum proteins | ||||||||||
| BMV | 1a | RHP | S. cerevisiae | Perinuclear ER membrane | Formation of replication compartments | Immunoprecipitation | Protein–protein | S. cerevisiae | Co-Ip and confocal microscopy: 12 dpi | [70] |
| TuMV | 6K2 | SNARE -SYP71 | A. thaliana | Chloroplast | Fusion replication compartments in chloroplast | Confocal microscopy | Indirect: mediated by Vap27-1 | N. benthamiana leaves | Confocal microscopy: 48 h | [71] |
| 6K2 | RHD3 | A. thaliana | Replication compartments | Maturation of replication compartments | Yeast two hybrid | Protein–protein | N. tabacum leaves | Confocal microscopy: 7 dpi | [28] | |
| Golgi apparatus proteins | ||||||||||
| BaMV | NA | RABG3f | N. benthamiana | Replication compartments | Formation and movement of replication compartments | Immunofluorescence | Unknown | N. benthamiana leaves | Confocal microscopy: 5 dpi | [72] |
| CaMV | MP | µA-adaptin | A. thaliana | Plasma membrane | MP trafficking | GST pull-down | Protein–protein | Escherichia coli and A. thaliana | GST-pull down | [73] |
| RCNMV | p27 | ARF1 | N. benthamiana and N. tabacum | ER | Formation of replication compartments | Affinity purification | Protein–protein | N. tabacum protoplast | Confocal microscopy: 16 h | [74] |
| Plasma membrane proteins | ||||||||||
| CaMV | p6 | AtSRC2.2 | A. thaliana | Inclusion bodies (cytoplasmic and nuclear) |
Cell-to-cell movement | Yeast two hybrid | Protein–protein | N. benthamiana leaves | Co-immunoprecipitation and confocal microscopy: 3 dpi | [75] |
| RCNMV | p27 | RBOHB | N. benthamiana | Perinuclear ER-derived aggregates | ROS synthesis | Immunoprecipitation | Protein–protein | N. benthamiana leaves | Confocal microscopy andBiFC : 4 dpi |
[76] |
| TVBMV | P3N-PIPO and CI | DREPP | N. benthamiana | Plasmodesmata | Cell-to-cell movement | Yeast two hybrid | Protein–protein | N. benthamiana leaves | BiFC: 2 and 5 dpi | [40] |
| Plasma membrane proteins | ||||||||||
| TVCV | MP | SYTA | A. thaliana | Plasmodesmata | Alters plasmodesmata permeability | Confocal microscopy | Protein–protein | N. benthamiana leaves | Confocal microscopy and BiFC | [34] |
| TuMV | P3N-PIPO | PCaP1 | N. benthamiana | Plasmodesmata | Cell-to-cell movement | Yeast two hybrid | Protein–protein | N. benthamiana leaves | BiFC: 38 h | [77] |
| Nuclear proteins | ||||||||||
| GRV | ORF3 | Fibrillarin | N. benthamiana and A. thaliana | Cytoplasm | Systemic movement | Affinity purification and chromatography | Protein–protein | N. benthamiana leaves and E. coli | Far Western blotting | [27] |
| RCNMV | p27 | HSP90 | N. benthamiana | ER | Formation of replication compartments | Partially purified replicase | Protein–protein | N. benthamiana leaves | BiFC: 3 and 4 dpi | [21] |
| TMV | MP | NTH201 | N. tabacum | Cytoplasm and plasmodesmata | Enhances replication compartment formation | Confocal microscopy | Indirect | N. benthamiana leaves | Confocal microscopy: 24 h | [78] |
| TBSV | p19 | ALY | N. benthamiana and A. thaliana | Cytoplasm | Co-factor | Yeast two hybrid | Protein–protein | N. benthamiana leaves | Confocal microscopy: 3 dpi | [79] |
| Vacuolar proteins | ||||||||||
| ToMV | 130K and 180K | TOM1 TOM3 |
A. thaliana and N. tabacum | ER | Formation and anchoring of replication compartments | Membrane flotation | Protein–protein | S. cerevisiae and N. tabacum leaves | Yeast two hybrid and subcellular fractionation at 2 dpi | [80,81] |
* Experimental plants are wild type unless noted. BiFC: bimolecular fluorescence complementation; Co-Ip: co-immunoprecipitation; dpi: days post inoculation, agroinfiltration, or induction; NA: information not available.
3. Cytoplasmic Host Proteins
Of the 55 host proteins that changed their localization during viral infection, 30 were cytoplasmic and moved to the sites of virus replication in the mitochondria, chloroplasts, endoplasmic reticulum (ER), peroxisomes, or nucleus and participated in essential processes such as the formation of the sites of virus replication, stimulation of RNA synthesis, or stability of the RNA-dependent RNA polymerase (Figure 2 and Table 2). Host proteins represented include heat shock proteins, translation factors, and proteins that mediate membrane topology (Table 2). Other proteins include GSTU4 (glutathione transferases), oxysterol-binding protein–related proteins (ORPs), catalase 1, endosomal sorting complexes required for transport (ESCRTs), and like Sm protein 1 (LSm1). Two antiviral proteins (NPR1 and 20S α5) moved from the cytoplasm to the nucleus or virus-induced aggregates. Their new localization was mediated by viral proteins and resulted in the loss of antiviral activity (Table 3). Cytoplasmic proteins with a new distribution in virus-infected cells are discussed below.
Table 3.
Host proteins with antiviral activity and that change subcellular localization in the presence of a plant virus. Host proteins are organized in blocks based on their natural distribution in the absence of virus.
| Virus | Viral Protein or RNA | Host Protein |
Host | Movement of Host Protein into | Role | Initial Detection Experiment | Mechanism of Interaction |
Experimental System for Detecting New Localization Sites * | Method of Observation: Time | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasmic proteins | ||||||||||
| CaMV | P6 | NBR1 | A. thaliana | Nucleus | Inhibits salicylic acid-dependent defense responses | Confocal microscopy | Enhanced jasmonic acid signaling | Transgenic A. thaliana expressing 35S:NPR1-GFP leaves | Confocal microscopy: 5 to 40 min | [82] |
| LMV | HC-Pro | 20S α5 | A. thaliana | HC-Pro aggregates | Reduces RNase activity on viral RNA | Subcellular fractionation | Protein–protein | Lactuca sativa leaves | BiFC: 4 dpi | [83,84] |
| PVX | CP, TGBp1, or TGBp2 | MPB2Cb | N. benthamiana | ER | Blocks formation of replication compartments | Yeast two hybrid | Protein–protein | N. benthamiana leaves | Confocal microscopy: 2 dpi | [85] |
| Nuclear proteins | ||||||||||
| CIRV | p36 and p95pol | RH30 | N. benthamiana and A. thaliana | Mitochondria | Blocks assembly of the sites of replication | Confocal microscopy | Protein–protein | N. benthamiana leaves | Confocal microscopy: 84 h | [86] |
| CNV | p33 and p92pol | RH30 | N. benthamiana and A. thaliana | Peroxisome | Blocks assembly of the sites of replication | Confocal microscopy | Protein–protein | N. benthamiana leaves | Confocal microscopy: 84 h | [86] |
| TBSV | p33 and p92pol | RH30 | N. benthamiana and A. thaliana | Peroxisome | Blocks assembly of the sites of replication | Confocal microscopy | Protein–protein | N. benthamiana leaves | Confocal microscopy: 84 h | [86] |
| TBSV | p19 | ALY1 ALY3 | A. thaliana | Cytoplasm | Unknown | Yeast two hybrid | Protein–protein | N. benthamiana leaves | Confocal microscopy: 3 dpi | [79] |
| Vacuolar proteins | ||||||||||
| CaMV | p4 | NBR1 | A. thaliana | Inclusion bodies | NBR1-dependent degradation of p4 | Yeast two hybrid | Protein–protein | N. benthamiana leaves | Confocal microscopy: 2 dpi | [82] |
| TuMV | HC-Pro | NBR1 | A. thaliana | Granule-like cytoplasmic structures | NBR1-dependent degradation of HC-Pro | Confocal microscopy | Protein–protein | Transgenic A. thaliana expressing NBR1-RFP leaves | Confocal microscopy on systemically infected leaves: 14 dpi | [87] |
* Experimental plants are wild type unless noted.
3.1. Heat Shock Proteins (HSPs)
HSPs are highly conserved in plants and animals. They are chaperones that protect other proteins from degradation and facilitate protein trafficking across membranes. HSPs have several physiological functions in plants, including protection from stress caused by heat, cold, light, heavy metals, salts, and ozone. HSPs are mainly located in the cytosolic part of the cell, and in some cases, in the nucleus, chloroplasts, or ER [88,89]. Several HSPs are chaperones of viral proteins, have essential roles in virus replication [20,21,56], and are recruited to the sites of virus replication (Table 2). Notable examples are discussed below.
HSP70 moves from the cytoplasm to the nucleus during tomato yellow leaf curl virus (TYLCV, single-strand DNA genome) infection [68], and to the ER compartments during TuMV (single positive-strand RNA genome) infection [20]. Downregulation of HSP70 results in the reduced accumulation of TYLCV genomic DNA in infected plants [68]. Both HSP70 and HSP90 localize mainly in the cytoplasm, and upon red clover necrotic mosaic virus (RCNMV, single positive-strand RNA genome) infection, HSP70 and HSP90 were detected in the ER (Table 2). Movement occurred through interactions with p27, a component of the virus replication compartments [31,74]. Without detectable physiological or developmental defects in the plants, the downregulation of HSP70 and HSP90 by virus-induced gene silencing prevented infection by RCNMV, confirming there are susceptibility genes [21,31]. Moreover, during infection with bamboo mosaic virus (BaMV, single positive-strand RNA genome), HSP90 enhances the formation of ribonucleoprotein complexes and facilitates their entry into the chloroplast, thus moving to the chloroplasts and playing an important role in BaMV replication [90]. HSP20 antagonizes the antiviral response by moving into the nucleus through interaction with the RNA-dependent RNA polymerase (RdRp) of rice stripe virus (RSV, single negative-strand RNA genome), blocking the recruitment of viral RNA to stress granules and, thus, blocking the degradation of viral RNA and enhancing viral RNA translation [56].
3.2. Endosomal Sorting Complexes Required for Transport (ESCRTs)
ESCRTs are peripheral membrane proteins in plant, mammalian, and yeast cells and play important roles in autophagy, sorting of ubiquitinated receptors, and in cytokinesis. They are also involved in the detachment of membrane vesicles, viral budding, and in the formation of the sites of virus replication [48,58,91]. ESCRT-I and ESCRT-III move from the cytoplasm to the sites of replication formed by TBSV (single positive-strand RNA genome) in the peroxisome and by BMV (single positive-strand RNA genome) in the perinuclear ER (Table 2) [18,48,58]. Their recruitment to the sites of virus replication is mediated by their interaction with TBSV replication protein p33 or BMV replication protein 1a (Table 2). ESCRT proteins are proposed participate in bending membranes to achieve the spherical shape of the compartments that function as sites of replication for both viruses [18,48,58]. Thus, the absence of ESCRT proteins in yeast cells, and their downregulation in plants, resulted in a reduction in the accumulation of BMV and TBSV, respectively [18,48,58].
3.3. Translation Factors
Viruses are dependent on the host machinery to translate their RNAs into proteins. Several viruses require eukaryote initiation factors Poly A binding protein2 (PABP2), eukaryote initiation factor eIF (iso)4e, eIF4e, and elongation factor eEF(1A). During viral infection, these proteins move from the cytoplasm to the sites of TuMV replication [5,45,92] (Table 2). Re-initiation supporting proteins (RISPs) move from the cytoplasm to trans-activator viroplasmin (TAV) aggregates [45,93] and are necessary for cauliflower mosaic virus (CaMV) infection [65,66]. Additionally, some translation factors contribute to the cell-to-cell movement and systemic spread of the virus [92]. The role of the initiation, elongation, and re-initiation factors in the sites of virus replication and in virus movement is unclear.
3.4. Asp-Glu-Ala-Asp (DEAD)-Box RNA Helicases (RHAs)
DEAD-box RNA helicases (RHAs) are a large family of RNA helicases involved in all steps of RNA metabolism. They are required for transcription, mRNA splicing and translation, RNA modification and transport, ribosome biogenesis, ribonucleoprotein complex assembly, and mRNA degradation [94]. RHAs are also involved in the response to biotic stress and in abiotic stress tolerance [95].
Several RHAs contribute to the translation and replication of viral RNA and change their localization upon viral infection [96,97]. The RNA helicases, AtRH8 and AtRH9, move from the cytoplasm to the sites of TuMV replication in the chloroplast in A. thaliana (Table 2). Migration into the chloroplast is mediated by viral proteins. AtRH8 interacts with virus-linked protein VPg [63], while AtRH9 interacts with potyvirus membrane protein 6K2 [64]. In mutant plants lacking either AtRH8 or AtRH9, accumulation of TuMV is reduced [63,64].
3.5. Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH)
GAPDH is a catalytic enzyme involved in glycolysis and mRNA binding. After infection with TBSV, GAPDH moves from the cytoplasm to the peroxisomal membrane and into the sites of TBSV replication. GAPDH is responsible for maintaining the ratio between positive- and negative-strand RNA genomes. Downregulation of GAPDH in N. benthamiana plants caused a fourfold reduction in the accumulation of TBSV and tobacco mosaic virus (TMV, single positive-strand RNA genome), pointing to a required role of this protein in both viruses [59]. In contrast, GAPDH has an antiviral role against BaMV and its satellite (satBaMV) as its downregulation in N. benthamiana plants resulted in an increase in BaMV and satBaMV RNA accumulation, while its overexpression reduced the accumulation of BaMV and satBaMV. The subcellular localization of GAPDH does not change during infection with BaMV or satBaMV [98].
3.6. Glutathione Transferase U4 (GSTU4)
GSTU4 belongs to the family of plant glutathione transferases (GSTs), which catalyze the reduction of hydroperoxides formed during oxidative stress, participate in ultraviolet-inducible cell signaling pathways, and in the regulation of apoptosis [99]. During infection with BaMV, GSTU4 is upregulated, interacts with the 3′ untranslated region (UTR) in BaMV genomic RNA, moves from the cytoplasm to the sites of virus replication in the chloroplasts, and enhances the synthesis of negative-strand RNA. Downregulation of GSTU4 caused a reduction in the accumulation of BaMV and potato virus X (PVX, single positive-strand RNA genome) but not in the accumulation of cucumber mosaic virus (CMV, single positive-strand RNA genome) or TMV [47]. In soybean, GSTU10-10 is induced in response to the systemic infection of the plants by soybean mosaic virus (SMV, single positive-strand RNA genome), but no subcellular localization data were reported [100]. Thus, GSTs may have a role in virus susceptibility, mediated by their ability to reduce oxidative stress, which supports viral replication.
3.7. Other Cytoplasmic Proteins
Other cytoplasmic proteins with altered localization during viral infection include oxysterol-binding protein–related proteins (ORPs), a yeast RNA-binding protein (LSm1), receptor for activated C kinase 1 (RACK1), and a proteasome protein (20S α5). ORPs are lipid transfer proteins that participate in vesicular trafficking, lipid metabolism and signaling, non-vesicular sterol transfer, directional sterol transport, and other processes [101]. ORPs facilitate the redistribution of sterols and enhance membrane folding during the formation of the replication sites in the peroxisomes by TBSV, and in the mitochondrial membrane by carnation Italian ringspot virus (CIRV, single positive-strand RNA genome) (Table 2). Deletion of ORPs lowers the efficiency of the viral replicase assembly and activity and resulted in a major reduction in virus accumulation [52].
LSm1 is a yeast protein related to the core small nuclear ribonucleoprotein particle (snRNP). LSm1 is involved in BMV RNA de-capping and translation. LSm1 protein is recruited from the cytoplasm to the ER by replication protein 1a and conditions susceptibility to BMV [50].
RACK1 is a cytoplasmic protein involved in the regulation of several plant processes including development, hormone response, and environmental stress response [102]. It is also involved in the innate immune response against fungal and bacterial pathogens [103,104,105]. During RCNMV infection of N. benthamiana, RACK1 interacts with viral replication protein p27 and moves from the cytoplasm to the sites of replication in the ER (Table 2). RACK1 is essential for the p27-mediated induction of reactive oxygen species (ROS) bursts that enhances virus replication. Downregulation of RACK1 resulted in the reduced accumulation of RCNMV RNA [55].
The 20S α5 is a subunit of the ubiquitin–proteasome system with RNAse activity and is involved in the degradation of viral RNA [83,84]. After infection with lettuce mosaic virus (LMV, single positive-strand RNA genome), the 20S α5 colocalizes with HC-Pro in cytoplasmic aggregates (Table 3). Interaction with HC-Pro and movement into cytoplasmic aggregates blocks the RNAse activity of 20S α5, protecting the virus from degradation. Consistent with its antiviral role, downregulation of 20S α5 enhances LMV accumulation [84].
4. Endosomal Proteins
Rab GTPases are central regulators of vesicle budding, movement, and fusion [106]. Endosomal protein Rab5-small guanosine triphosphatase (RAB5-GTPase) regulates endosome biogenesis and homotypic and heterotypic fusions [107]. During infection with TBSV and CNV (Table 2), RAB5-GTPase moves to the sites of virus replication in the peroxisomes, or in the mitochondria during CIRV infection [69]. RAB5-GTPase increases the amount of endosomal phospholipid phosphatidylethanolamine needed to form the sites of virus replication and to establish robust virus replication [69].
5. Endoplasmic Reticulum Proteins
Several RNA viruses form their sites of replication in endoplasmic reticulum (ER)-bound membranes [108], and several ER proteins move into the sites of virus replication formed in other subcellular organelles (Figure 2B). Representative groups are discussed below (Table 2).
5.1. Soluble N-Ethyl Maleimide Sensitive Factor Adaptor Protein Receptors (SNAREs)
SNAREs belong to the syntaxin family of proteins that mediate membrane fusion between transport replication compartments and their target membranes [109]. The potyviral 6K2 protein induces the formation of ER-derived complexes that subsequently translocate to the chloroplasts, where potyviral replication occurs [71]. A. thaliana SYP71 is a SNARE protein located both in the ER and in the plasma membrane [110], is essential for TuMV replication, and contributes to the movement of replication compartments from the ER to the chloroplast (Table 2). Downregulation of SYP71 inhibits TuMV infection [71].
5.2. Reticulon Homology Domain Proteins (RHPs)
RHPs are a family of membrane-shaping proteins that induce and stabilize positively curved peripheral ER membranes and are involved in apoptosis, cell division, and intracellular trafficking [111]. RHPs normally localize to the peripheral ER. During BMV infection, they interact with replication protein 1a, move to the perinuclear ER, and are incorporated into BMV sites of replication. RHPs are essential for the formation of BMV replication compartments. Mutant plants lacking RHPs have a reduced number of replication compartments and an 80% of reduction in viral replication compared to the wild-type plants [70].
5.3. Root Hair Defective 3 (RHD3)
RHD3 is a member of the dynamin-like atlastin GTPase family of proteins. It plays a vital role in generating of the interconnected tubular ER network, is required for Golgi distribution and motility in plant cells, and is essential for plant development [112]. RHD3 is essential for the formation, maturation, and intracellular movement of TuMV replication compartments. Interaction with TuMV 6K2 protein moves RHD3 from the ER to the TuMV replication compartments (Table 2). In mutant plants lacking RHD3, replication and systemic movement of TuMV are reduced [28].
6. Golgi Apparatus Proteins
Rab guanosine triphosphatase 3f (RABG3f) belongs to the family of Rab GTPases that regulate the intracellular trafficking between organelles [113]. RABG3f is located in the Golgi apparatus and moves to the chloroplast during BaMV infection (Table 2), participates in the formation of the sites of replication, and is required for the efficient infection of in N. benthamiana by BaMV. Downregulation of NbRABG3f reduces the accumulation of BaMV [72].
ADP-ribosylation factor (ARF1) 1 is a Golgi body GTPase [114]. During infection with RCNMV, ARF1 interacts with viral protein p27 and is moved into the sites of virus replication on the ER membrane (Table 2). Inhibition of ARF1 activity causes a reduction in RCNMV RNA accumulation [74].
7. Plasma Membrane Proteins
Viruses move from cell to cell through plasmodesmata using specialized viral movement proteins and host proteins [115]. Potyviral replication compartments are transported to the plasmodesmata throughout the ER–Golgi secretory pathway and the actomyosin motility system [40]. The phosphatidylinositol phosphates Ca-binding protein (PCaP1) and the developmentally regulated protein (DREPP) are plasma membrane proteins that mediate the cell-to-cell movement of TuMV and tobacco vein banding mosaic virus (TVBMV, single positive-strand RNA genome) respectively, by interacting with P3N-PIPO and CI (cylindric inclusion) proteins (Table 2). The interaction between PCaP1 or DREPP and P3N-PIPO or CI and their relocalization to the plasmodesmata are required for efficient virus replication and local and systemic movement [40,77]. CaMV (single-strand DNA genome) moves through the plasmodesmata as a virion. Cytoplasmic and nuclear inclusion bodies necessary for virion assembly are formed by viral protein P6. AtSRC2.2, a C2 calcium-dependent membrane-targeting protein, is part of the inclusion bodies and has been implicated in CaMV movement [75].
The respiratory burst oxidase homolog (RBOHB) is a plasma membrane protein in the family of plant NADPH oxidases and plays an essential role in ROS production and signaling [116]. During infection of N. benthamiana by RCNMV, RBOHB moves to the perinuclear ER-bound sites of virus replication (Table 2) through interactions with replication protein p27 and is required for robust viral RNA replication [19]. In contrast, during infection with TMV, RBOHB-induced ROS burst has an antiviral effect, with no protein relocalization reported [117,118].
8. Nuclear Proteins
DNA viruses replicate in the nucleus [2]. RNA viruses do not enter the nucleus. However, some RNA viruses need nuclear host factors to replicate or move. Fibrillarin is a major nucleolar protein that forms part of Cajal bodies, is a core component of small nucleolar ribonucleoprotein particles, and is required for rRNA processing. Similar to all other umbraviruses, groundnut rosette virus (GRV, single positive-strand RNA genome) does not encode for a coat protein and does not form virions. The lack of a coat protein is compensated by open reading frame 3 (ORF3), and GRV moves as ribonucleoprotein particles. In infected plants, ORF3 cycles from the cytoplasm to the nucleus passing through Cajal bodies and back into the cytoplasm. This movement is dependent on interactions between ORF3 and fibrillarin and is required for the formation of ribonucleoprotein particles. Downregulation of fibrillarin through virus-induced gene silencing in N. benthamiana did not affect virus replication or cell-to-cell movement. However, in the absence of fibrillarin ORF3 remained in the Cajal bodies, failed to fuse with the nucleolus, and prevented systemic movement of GRV [27].
Allies of LEF-1 and AML-1 (ALY proteins) belong to a family of nuclear polypeptides involved in mRNA export from the nucleus to the cytosol, in the regulation of plant immunity, in controlling the aperture of stomata, and plant growth and development [119].
During infection by TBSV, two A. thaliana ALYs (AtALY2 and AtALY4) and an N. benthamiana ALY protein (NbALY617) move from the nucleus to the cytoplasm (Table 2) by interacting with P19, a strong silencing suppressor of cytoplasmic distribution that interferes with antiviral gene silencing [79]. The biological significance of this relocation and interaction remains to be determined. In contrast, A. thaliana proteins AtALY1 and AtALY3 and N. benthamiana proteins NbALY615 and NbALY1693 inhibit the silencing suppression activity of P19 (Table 3). This effect is mediated by the sequestration of P19 in the nucleus by nuclear ALY proteins via an RNA-binding motif [120].
Nuclear DEAD-box RNA helicase RH30 interacts with TBSV replication proteins p33 and p92pol and moves from the nucleus to TBSV sites of replication in the peroxisome (Table 3). RH30 inhibits the formation of replication compartments, the recruitment of genomic positive-strand RNA into replication, and RNA synthesis. The antiviral effect has been shown against TBSV, CNV, CIRV, RCNMV, and TMV [86].
9. Vacuolar Proteins
Tobamovirus multiplication 1 (TOM1) and TOM3 (Table 2) are vacuolar membrane proteins required for the replication of tobamoviruses. Both interact with the helicase-like domain of replication proteins 130K and 180K in tomato mosaic virus (ToMV, single positive-strand RNA genome) and participate in the formation and anchoring of replication compartments to the ER [80,81].
Autophagy is a conserved mechanism of protein degradation involved in cell homeostasis. Autophagy may benefit the virus or have antiviral roles [121,122]. Selective autophagy substrate, NBR1, is a cargo receptor protein that suppresses TuMV and CaMV infection by targeting the silencing suppressor HC-Pro and the structural capsid protein P4, respectively. To create an environment favorable to replication and movement, TuMV antagonizes NBR1-dependent autophagy by a mechanism that is dependent on the viral proteins VPg and 6K2 (Table 3). NBR1 is normally found in the autophagosome, cytoplasm, and vacuoles. During TuMV infection, NBR1 moves to virus replication compartments [123]. NBR1 mediates the degradation of TuMV HC-Pro. In turn, TuMV VPg and 6K2 proteins counteract NBR1 antiviral effects [87]. However, the role of NBR1 in plant–TuMV interactions is more complex. During TuMV infection of Brassica napus, large amounts of secondary siRNAs are formed from the NBR1 mRNA, which in turn direct cleavage and downregulation of NBR1 mRNA, actin depolymerization factor (ADF), and other transcripts [124].
10. Conclusions
Host proteins needed by the virus may be redirected to subcellular compartments where they contribute to essential viral processes, such forming sites of viral replication, RNA synthesis, or virus movement (Table 2 and Figure 2). In the opposite direction, host proteins with antiviral roles may be forced to move away from their natural subcellular localization. Changes in subcellular localization of host proteins are mediated by direct or indirect interactions with viral proteins or RNA (Table 2). However, it is not clear whether accumulation in a new location results from the movement of existing host proteins or from newly synthesized ones.
Changes in the subcellular localization of host proteins have been identified and characterized mainly using experimental hosts N. benthamiana, A. thaliana, and S. cerevisiae (Figure 1A). The most frequent change is cytoplasmic proteins redirected to virus replication compartments formed in the ER, peroxisome, chloroplast, mitochondria, and nucleus (Figure 1 and Figure 2). In contrast, the number of studies documenting changes in subcellular localization of antiviral proteins is significantly (fivefold) lower (Figure 1B and Table 3). These antiviral proteins block the formation of replication compartments or degrade viral proteins. Subcellular relocalization was needed to perform antiviral activities. Interestingly, in some cases, antiviral activity is inhibited by virus-induced changes in localization (Table 3).
Mutational inactivation or downregulation of proteins that participate in essential viral processes results in lack of infection or reduction of viral RNA accumulation [5,46]. However, genetic changes in the host may cause developmental abnormalities, reduced fitness, or altered physiology, masking the real effect or indirectly affecting virus replication or movement. HSP70, RABG3f, GSTU4, RACK1, GAPDH, AtRH8, and AtRH9 were downregulated by silencing or mutationally inactivated. The source paper explicitly indicated that plants did not show detectable physiological or severe developmental defects compared to the wild-type plants. RACK1-knockdown plants had narrow leaves, categorized as a minor physiological defect [55]. Other publications did not provide information on the phenotype of mutant plants.
The dependence of viruses on host resources at all steps of the infection process puts evolutionary pressure both on the virus and on the host. Virus–host co-evolution might favor interactions that increase both host and virus fitness rather than decreasing fitness of either the host or the virus. Accordingly, it might be to the benefit of viruses to interact with existing processes at their natural location in a non-interfering manner rather than through mechanisms that recruit host proteins away from their natural sites and normal functions, which may potentially lead to disease. All documented cases of changes in subcellular localization (Table 2 and Table 3) come from viruses that are pathogenic. Currently, no information is available on the changes, or lack thereof, of host proteins for virus–plant combinations in which the interaction does not lead to disease.
Recruitment of cytoplasmic proteins to the sites of virus replication in membrane-bound compartments through mechanisms that include direct protein–protein interactions between viral and host proteins and indirectly through membranes, other proteins, or RNA (Table 2, Figure 2) imposes selection pressure on viral proteins to maintain the ability to interact with host proteins. Viral proteins are usually multifunctional. In addition to interacting with other viral proteins or RNA, efficient interaction with host proteins must be maintained. The host might be genetically uniform or diverse. These constraints exert selection pressure on viral proteins to maintain functionality and be able to interact with the cognate host proteins. These observations suggest that interactions between viral and host proteins that are essential at any part of the infection process are also important determinants of virus evolution and host adaptation. Viral proteins might respond to selection pressure by developing a perfect sequence and structure that is functional and able to interact with host proteins that are genetically diverse. Alternatively, viral proteins might respond to selection pressure by incorporating mutationally robust areas coding for structurally flexible domains capable of interacting with host proteins that are genetically diverse. Viruses might be generalists with a wide host range or specialists with a narrow host range. Through co-evolution, in response to selection pressure from the virus, plants might generate diversity in their alleles and/or by incorporating redundant alleles.
This model is illustrated by translation initiation factors and potyviruses. The translation initiation factors, eEF1A and eIF(iso)4e, participate in TuMV viral RNA translation and formation of replication compartments through interactions with VPg [5,125]. Although eIF(iso)4e is necessary for potyvirus infection, it is dispensable for normal plant growth and development [5]. In addition to interacting with eIF(iso)4e, VPg must recognize and interact with TuMV genomic RNA, the RNA-dependent RNA polymerase, and protein 6K1 [5,125]. In connection with its multifunctionality and ability to interact with multiple partners, potyviral VPg is structurally disordered and mutationally robust [126]. Translation initiation factors eIF4E are a multigene family. Ten allelic variants of eIF4 were detected in natural pepper populations with varying levels of susceptibility to potyviruses [127]. Accessions resistant to potyvirus infection encoded mutations in eIF4 that disrupted interaction with VPg [127], consistent with the essential role of both VPg and eIF4 in potyvirus–plant interactions and with the model that plants respond to selection pressure by accumulating diversity to buffer the effect of viral infection.
Changes in subcellular localization of host proteins documented to date and summarized here represent bonafide events associated with basic mechanisms of plant–virus interactions. However, these patterns are heavily influenced by the combinations of model hosts and viruses used to develop model experimental systems to identify host factors that affect virus replication (Figure 1A). A large fraction of the host proteins documented were identified using only three viruses BMV, TBSV, and TuMV (single positive-strand RNA, Figure 1D). Reasons for this bias mainly include the availability of infectious clones; well-characterized experimental systems to study virus replication, gene expression, and function; and genetically tractable heterologous hosts in combination with well-developed biochemical approaches for protein expression, purification, and localization [28,128,129]. Overexpression of viral proteins in non-natural hosts is a powerful tool to identify potential host proteins that play important roles during infection. It is possible that some of the resulting relocalization may be an artifact of overexpression of individual viral proteins in a heterologous system. However, genetic analyses, virus-induced gene silencing, chemical treatments, or a combination of approaches have been used to validate the requirement of particular host proteins, ruling out the possibility of an experimental artifact [21,28,68]. Furthermore, basic information about factors required for viral infection identified in heterologous hosts has been used to engineer resistance in crops. Based on the observation that eukaryote translation initiation factors are susceptibility genes to potyviruses [5], through interaction with potyviral VPg [28], CRISPR-Cas9 was used in tomato to engineer resistance to pepper mottle virus by editing eIF4E1 [130].
New approaches are needed for the genome-wide identification of factors required for the virus or with antiviral activities in natural hosts. Although these approaches are likely to be developed using model experimental systems, it would be of immense benefit to implement them in staple crops. Alternatively, or in addition, these approaches could be directed to particular diseases to which natural resistance is not readily available, such as maize lethal necrosis [131].
Acknowledgments
We are grateful to Katherine LaTourrette for critical reading of the manuscript.
Author Contributions
H.G.-R. conceptualized the review. H.G.-R. and R.R.-P. analyzed the data and made tables and figures. H.G.-R., R.R.-P. and K.E.M. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Institutes of Health (NIH) grant R01GM120108 to H.G.-R. and by the Nebraska Agricultural Experiment Station with funding from the Hatch Act (accession number 1007272) through the United States Department of Agriculture (USDA) National Institute of Food and Agriculture. Open access costs were provided by the same grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Den Boon J.A., Diaz A., Ahlquist P. Cytoplasmic viral replication complexes. Cell Host Microbe. 2010;8:77–85. doi: 10.1016/j.chom.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceniceros-Ojeda E.A., Rodriguez-Negrete E.A., Rivera-Bustamante R.F. Two populations of viral minichromosomes are present in a geminivirus-infected plant showing symptom remission (recovery) J. Virol. 2016;90:3828–3838. doi: 10.1128/JVI.02385-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Ruiz H. Susceptibility genes to plant viruses. Viruses. 2018;10:484. doi: 10.3390/v10090484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanov K.I., Eskelin K., Lohmus A., Makinen K. Molecular and cellular mechanisms underlying potyvirus infection. J. Gen. Virol. 2014;95:1415–1429. doi: 10.1099/vir.0.064220-0. [DOI] [PubMed] [Google Scholar]
- 5.Lellis A.D., Kasschau K.D., Whitham S.A., Carrington J.C. Loss-of-susceptibility mutants of arabidopsis thaliana reveal an essential role for eif(iso)4e during potyvirus infection. Curr. Biol. 2002;12:1046–1051. doi: 10.1016/S0960-9822(02)00898-9. [DOI] [PubMed] [Google Scholar]
- 6.Hyodo K., Okuno T. Pathogenesis mediated by proviral host factors involved in translation and replication of plant positive-strand rna viruses. Curr. Opin. Virol. 2016;17:11–18. doi: 10.1016/j.coviro.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Ruiz H. Host factors against plant viruses. Mol. Plant. Pathol. 2019;20:1588–1601. doi: 10.1111/mpp.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Z., Yeakley J.M., Garcia E.W., Holdridge J.D., Fan J.B., Whitham S.A. Salicylic acid-dependent expression of host genes in compatible arabidopsis-virus interactions. Plant Physiol. 2005;137:1147–1159. doi: 10.1104/pp.104.056028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ascencio-Ibanez J.T., Sozzani R., Lee T.J., Chu T.M., Wolfinger R.D., Cella R., Hanley-Bowdoin L. Global analysis of arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol. 2008;148:436–454. doi: 10.1104/pp.108.121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S., Jiang G., Wu J., Liu Y., Qian Y., Zhou X. Characterization of a novel polerovirus infecting maize in china. Viruses. 2016;8:120. doi: 10.3390/v8050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Z., Fan B., Chen C., Chen Z. An important role of an inducible rna-dependent rna polymerase in plant antiviral defense. Proc. Natl. Acad. Sci. USA. 2001;98:6516–6521. doi: 10.1073/pnas.111440998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy M., Edelbaum O., Sela I. Tobacco mosaic virus regulates the expression of its own resistance gene n. Plant Physiol. 2004;135:2392–2397. doi: 10.1104/pp.104.044859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgyan J., Havelda Z. Viral suppressors of rna silencing. Trends Plant. Sci. 2011;16:265–272. doi: 10.1016/j.tplants.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 14.De Ronde D., Butterbach P., Kormelink R. Dominant resistance against plant viruses. Front. Plant Sci. 2014;5:1–17. doi: 10.3389/fpls.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F., Wang A. Rna decay is an antiviral defense in plants that is counteracted by viral rna silencing suppressors. PLoS Pathog. 2018;14:e1007228. doi: 10.1371/journal.ppat.1007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng X., Wang A. The potyvirus silencing suppressor protein vpg mediates degradation of sgs3 via ubiquitination and autophagy pathways. J. Virol. 2017;91 doi: 10.1128/JVI.01478-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollari M., De S., Wang A., Mäkinen K. The potyviral silencing suppressor hcpro recruits and employs host argonaute1 in pro-viral functions. PLoS Pathog. 2020;16:e1008965. doi: 10.1371/journal.ppat.1008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovalev N., de Castro I.F., Pogany J., Barajas D., Pathak K., Risco C., Nagy P.D. Role of viral rna and co-opted cellular escrt-i and escrt-iii factors in formation of tombusvirus spherules harboring the tombusvirus replicase. J. Virol. 2016;90:3611–3626. doi: 10.1128/JVI.02775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang Y.T., McCartney A.W., Gidda S.K., Mullen R.T. Localization of the carnation italian ringspot virus replication protein p36 to the mitochondrial outer membrane is mediated by an internal targeting signal and the tom complex. BMC Cell Biol. 2008;9:54. doi: 10.1186/1471-2121-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dufresne P.J., Thivierge K., Cotton S., Beauchemin C., Ide C., Ubalijoro E., Laliberte J.F., Fortin M.G. Heat shock 70 protein interaction with turnip mosaic virus rna-dependent rna polymerase within virus-induced membrane vesicles. Virology. 2008;374:217–227. doi: 10.1016/j.virol.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Mine A., Hyodo K., Tajima Y., Kusumanegara K., Taniguchi T., Kaido M., Mise K., Taniguchi H., Okuno T. Differential roles of hsp70 and hsp90 in the assembly of the replicase complex of a positive-strand rna plant virus. J. Virol. 2012;86:12091–12104. doi: 10.1128/JVI.01659-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y., Rojas M.R., Park M.R., Seo Y.S., Lucas W.J., Gilbertson R.L. Histone h3 interacts and colocalizes with the nuclear shuttle protein and the movement protein of a geminivirus. J. Virol. 2011;85:11821–11832. doi: 10.1128/JVI.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou Y.-L., Hung Y.-J., Tseng Y.-H., Hsu H.-T., Yang J.-Y., Wung C.-H., Lin N.-S., Meng M., Hsu Y.-H., Chang B.-Y. The stable association of virion with the triple-gene-block protein 3-based complex of bamboo mosaic virus. PLoS Pathog. 2013;9:e1003405. doi: 10.1371/journal.ppat.1003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okinaka Y., Mise K., Suzuki E., Okuno T., Furusawa I. The c terminus of brome mosaic virus coat protein controls viral cell-to-cell and long-distance movement. J. Virol. 2001;75:5385–5390. doi: 10.1128/JVI.75.11.5385-5390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalmay T., Rubino L., Burgyan J., Kollar A., Russo M. Functional analysis of cymbidium ringspot virus genome. Virology. 1993;194:697–704. doi: 10.1006/viro.1993.1310. [DOI] [PubMed] [Google Scholar]
- 26.Xiang Y., Kakani K., Reade R., Hui E., Rochon D. A 38-amino-acid sequence encompassing the arm domain of the cucumber necrosis virus coat protein functions as a chloroplast transit peptide in infected plants. J. Virol. 2006;80:7952–7964. doi: 10.1128/JVI.00153-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S.H., Macfarlane S., Kalinina N.O., Rakitina D.V., Ryabov E.V., Gillespie T., Haupt S., Brown J.W., Taliansky M. Interaction of a plant virus-encoded protein with the major nucleolar protein fibrillarin is required for systemic virus infection. Proc. Natl. Acad. Sci. USA. 2007;104:11115–11120. doi: 10.1073/pnas.0704632104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Movahed N., Sun J., Vali H., Laliberte J.F., Zheng H. A host er fusogen is recruited by turnip mosaic virus for maturation of viral replication vesicles. Plant Physiol. 2019;179:507–518. doi: 10.1104/pp.18.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathioudakis M.M., Khechmar S., Owen C.A., Medina V., Ben Mansour K., Tomaszewska W., Spanos T., Sarris P.F., Livieratos I.C. A thioredoxin domain-containing protein interacts with pepino mosaic virus triple gene block protein 1. Int. J. Mol. Sci. 2018;19:3747. doi: 10.3390/ijms19123747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verchot-Lubicz J., Torrance L., Solovyev A.G., Morozov S.Y., Jackson A.O., Gilmer D. Varied movement strategies employed by triple gene block-encoding viruses. Mol. Plant Microbe Interact. 2010;23:1231–1247. doi: 10.1094/MPMI-04-10-0086. [DOI] [PubMed] [Google Scholar]
- 31.Turner K.A., Sit T.L., Callaway A.S., Allen N.S., Lommel S.A. Red clover necrotic mosaic virus replication proteins accumulate at the endoplasmic reticulum. Virology. 2004;320:276–290. doi: 10.1016/j.virol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Kusumanegara K., Mine A., Hyodo K., Kaido M., Mise K., Okuno T. Identification of domains in p27 auxiliary replicase protein essential for its association with the endoplasmic reticulum membranes in red clover necrotic mosaic virus. Virology. 2012;433:131–141. doi: 10.1016/j.virol.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Kaido M., Tsuno Y., Mise K., Okuno T. Endoplasmic reticulum targeting of the red clover necrotic mosaic virus movement protein is associated with the replication of viral rna1 but not that of rna2. Virology. 2009;395:232–242. doi: 10.1016/j.virol.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Levy A., Zheng J.Y., Lazarowitz S.G. Synaptotagmin syta forms er-plasma membrane junctions that are recruited to plasmodesmata for plant virus movement. Curr. Biol. 2015;25:2018–2025. doi: 10.1016/j.cub.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCartney A.W., Greenwood J.S., Fabian M.R., White K.A., Mullen R.T. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell. 2005;17:3513–3531. doi: 10.1105/tpc.105.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholthof H.B., Scholthof K.B., Kikkert M., Jackson A.O. Tomato bushy stunt virus spread is regulated by two nested genes that function in cell-to-cell movement and host-dependent systemic invasion. Virology. 1995;213:425–438. doi: 10.1006/viro.1995.0015. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami S., Watanabe Y., Beachy R.N. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc. Natl. Acad. Sci. USA. 2004;101:6291–6296. doi: 10.1073/pnas.0401221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C., Liu Y., Sun X., Qian W., Zhang D., Qiu B. Characterization of a specific interaction between ip-l, a tobacco protein localized in the thylakoid membranes, and tomato mosaic virus coat protein. Biochem. Biophys. Res. Commun. 2008;374:253–257. doi: 10.1016/j.bbrc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Geng C., Yan Z.Y., Cheng D.J., Liu J., Tian Y.P., Zhu C.X., Wang H.Y., Li X.D. Tobacco vein banding mosaic virus 6k2 protein hijacks nbpsbo1 for virus replication. Sci. Rep. 2017;7:43455. doi: 10.1038/srep43455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geng C., Cong Q.Q., Li X.D., Mou A.L., Gao R., Liu J.L., Tian Y.P. Developmentally regulated plasma membrane protein of nicotiana benthamiana contributes to potyvirus movement and transports to plasmodesmata via the early secretory pathway and the actomyosin system. Plant Physiol. 2015;167:394–410. doi: 10.1104/pp.114.252734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiraguri A., Netsu O., Sasaki N., Nyunoya H., Sasaya T. Recent progress in research on cell-to-cell movement of rice viruses. Front. Microbiol. 2014;5:210. doi: 10.3389/fmicb.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan Z., Chen H., Chen Q., Omura T., Xie L., Wu Z., Wei T. The early secretory pathway and an actin-myosin viii motility system are required for plasmodesmatal localization of the nsvc4 protein of rice stripe virus. Virus Res. 2011;159:62–68. doi: 10.1016/j.virusres.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Cho W.K., Lian S., Kim S.M., Park S.H., Kim K.H. Current insights into research on rice stripe virus. Plant Pathol. J. 2013;29:223–233. doi: 10.5423/PPJ.RW.10.2012.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harries P.A., Palanichelvam K., Yu W.C., Schoelz J.E., Nelson R.S. The cauliflower mosaic virus protein p6 forms motile inclusions that traffic along actin microfilaments and stabilize microtubules. Plant Physiol. 2009;149:1005–1016. doi: 10.1104/pp.108.131755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiébeauld O., Schepetilnikov M., Park H.-S., Geldreich A., Kobayashi K., Keller M., Hohn T., Ryabova L.A. A new plant protein interacts with eif3 and 60s to enhance virus-activated translation re-initiation. EMBO J. 2009;28:3171–3184. doi: 10.1038/emboj.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y.W., Hu C.C., Liou M.R., Chang B.Y., Tsai C.H., Meng M., Lin N.S., Hsu Y.H. Hsp90 interacts specifically with viral rna and differentially regulates replication initiation of bamboo mosaic virus and associated satellite rna. PLoS Pathog. 2012;8:e1002726. doi: 10.1371/journal.ppat.1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen I.H., Chiu M.H., Cheng S.F., Hsu Y.H., Tsai C.H. The glutathione transferase of nicotiana benthamiana nbgstu4 plays a role in regulating the early replication of bamboo mosaic virus. New Phytol. 2013;199:749–757. doi: 10.1111/nph.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz A., Zhang J., Ollwerther A., Wang X., Ahlquist P. Host escrt proteins are required for bromovirus rna replication compartment assembly and function. PLoS Pathog. 2015;11:e1004742. doi: 10.1371/journal.ppat.1004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jungfleisch J., Chowdhury A., Alves-Rodrigues I., Tharun S., Diez J. The lsm1-7-pat1 complex promotes viral rna translation and replication by differential mechanisms. RNA. 2015;21:1469–1479. doi: 10.1261/rna.052209.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diez J., Ishikawa M., Kaido M., Ahlquist P. Identification and characterization of a host protein required for efficient template selection in viral rna replication. Proc. Natl. Acad. Sci. USA. 2000;97:3913–3918. doi: 10.1073/pnas.080072997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson L.G., Clendening E.A., Sheen H., Gidda S.K., White K.A., Mullen R.T. A unique n-terminal sequence in the carnation italian ringspot virus p36 replicase-associated protein interacts with the host cell escrt-i component vps23. J. Virol. 2014;88:6329–6344. doi: 10.1128/JVI.03840-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barajas D., Xu K., de Castro Martin I.F., Sasvari Z., Brandizzi F., Risco C., Nagy P.D. Co-opted oxysterol-binding orp and vap proteins channel sterols to rna virus replication sites via membrane contact sites. PLoS Pathog. 2014;10:e1004388. doi: 10.1371/journal.ppat.1004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathioudakis M., Rita S.L.V., Canto T., Medina V., Mossialos D., Makris Antonios M., Livieratos I. Pepino mosaic virus triple gene block protein 1 (tgbp1) interacts with and increases tomato catalase 1 activity to enhance virus accumulation. Mol. Plant Pathol. 2013;14:589–601. doi: 10.1111/mpp.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fridborg I., Grainger J., Page A., Coleman M., Findlay K., Angell S. Tip, a novel host factor linking callose degradation with the cell-to-cell movement of potato virus x. Mol. Plant Microbe Interact. 2003;16:132–140. doi: 10.1094/MPMI.2003.16.2.132. [DOI] [PubMed] [Google Scholar]
- 55.Hyodo K., Suzuki N., Okuno T. Hijacking a host scaffold protein, rack1, for replication of a plant rna virus. New Phytol. 2019;221:935–945. doi: 10.1111/nph.15412. [DOI] [PubMed] [Google Scholar]
- 56.Li J., Xiang C.Y., Yang J., Chen J.P., Zhang H.M. Interaction of hsp20 with a viral rdrp changes its sub-cellular localization and distribution pattern in plants. Sci. Rep. 2015;5:14016. doi: 10.1038/srep14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z., Pogany J., Tupman S., Esposito A.M., Kinzy T.G., Nagy P.D. Translation elongation factor 1a facilitates the assembly of the tombusvirus replicase and stimulates minus-strand synthesis. PLoS Pathog. 2010;6:e1001175. doi: 10.1371/journal.ppat.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barajas D., Jiang Y., Nagy P.D. A unique role for the host escrt proteins in replication of tomato bushy stunt virus. PLoS Pathog. 2009;5:e1000705. doi: 10.1371/journal.ppat.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R.Y., Nagy P.D. Tomato bushy stunt virus co-opts the rna-binding function of a host metabolic enzyme for viral genomic rna synthesis. Cell Host Microbe. 2008;3:178–187. doi: 10.1016/j.chom.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Pogany J., Stork J., Li Z., Nagy P.D. In vitro assembly of the tomato bushy stunt virus replicase requires the host heat shock protein 70. Proc. Natl. Acad. Sci. USA. 2008;105:19956–19961. doi: 10.1073/pnas.0810851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang R.Y.-L., Stork J., Nagy P.D. A key role for heat shock protein 70 in the localization and insertion of tombusvirus replication proteins to intracellular membranes. J. Virol. 2009;83:3276–3287. doi: 10.1128/JVI.02313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamaji Y., Kobayashi T., Hamada K., Sakurai K., Yoshii A., Suzuki M., Namba S., Hibi T. In vivo interaction between tobacco mosaic virus rna-dependent rna polymerase and host translation elongation factor 1a. Virology. 2006;347:100–108. doi: 10.1016/j.virol.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 63.Huang T.S., Wei T., Laliberte J., Wang A. A host rna helicase-like protein, atrh8, interacts with the potyviral genome-linked protein, vpg, associates with the virus accumulation complex, and is essential for infection. Plant Physiol. 2010;152:255–266. doi: 10.1104/pp.109.147983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y., Xiong R., Bernards M., Wang A. Recruitment of arabidopsis rna helicase atrh9 to the viral replication complex by viral replicase to promote turnip mosaic virus replication. Sci. Rep. 2016;6:30297. doi: 10.1038/srep30297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thivierge K., Cotton S., Dufresne P.J., Mathieu I., Beauchemin C., Ide C., Fortin M.G., Laliberte J.F. Eukaryotic elongation factor 1a interacts with turnip mosaic virus rna-dependent rna polymerase and vpg-pro in virus-induced vesicles. Virology. 2008;377:216–225. doi: 10.1016/j.virol.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 66.Beauchemin C., Laliberte J.F. The poly(a) binding protein is internalized in virus-induced vesicles or redistributed to the nucleolus during turnip mosaic virus infection. J. Virol. 2007;81:10905–10913. doi: 10.1128/JVI.01243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishikiori M., Dohi K., Mori M., Meshi T., Naito S., Ishikawa M. Membrane-bound tomato mosaic virus replication proteins participate in rna synthesis and are associated with host proteins in a pattern distinct from those that are not membrane bound. J. Virol. 2006;80:8459–8468. doi: 10.1128/JVI.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gorovits R., Moshe A., Ghanim M., Czosnek H. Recruitment of the host plant heat shock protein 70 by tomato yellow leaf curl virus coat protein is required for virus infection. PLoS ONE. 2013;8:e70280. doi: 10.1371/journal.pone.0070280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu K., Nagy P.D. Enrichment of phosphatidylethanolamine in viral replication compartments via co-opting the endosomal rab5 small gtpase by a positive-strand rna virus. PLoS Biol. 2016;14:e2000128. doi: 10.1371/journal.pbio.2000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diaz A., Wang X., Ahlquist P. Membrane-shaping host reticulon proteins play crucial roles in viral rna replication compartment formation and function. Proc. Natl. Acad. Sci. USA. 2010;107:16291–16296. doi: 10.1073/pnas.1011105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei T., Zhang C., Hou X., Sanfaçon H., Wang A. The snare protein syp71 is essential for turnip mosaic virus infection by mediating fusion of virus-induced vesicles with chloroplasts. PLoS Pathog. 2013;9:e1003378. doi: 10.1371/journal.ppat.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Y.P., Jhuo J.H., Tsai M.S., Tsai C.H., Chen H.C., Lin N.S., Hsu Y.H., Cheng C.P. Nbrabg3f, a member of rab gtpase, is involved in bamboo mosaic virus infection in nicotiana benthamiana. Mol. Plant Pathol. 2016;17:714–726. doi: 10.1111/mpp.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carluccio A.V., Zicca S., Stavolone L. Hitching a ride on vesicles: Cauliflower mosaic virus movement protein trafficking in the endomembrane system. Plant Physiol. 2014;164:1261. doi: 10.1104/pp.113.234534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hyodo K., Mine A., Taniguchi T., Kaido M., Mise K., Taniguchi H., Okuno T. Adp ribosylation factor 1 plays an essential role in the replication of a plant rna virus. J. Virol. 2013;87:163–176. doi: 10.1128/JVI.02383-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez A., Angel C.A., Lutz L., Leisner S.M., Nelson R.S., Schoelz J.E. Association of the p6 protein of cauliflower mosaic virus with plasmodesmata and plasmodesmal proteins. Plant Physiol. 2014;166:1345–1358. doi: 10.1104/pp.114.249250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hyodo K., Hashimoto K., Kuchitsu K., Suzuki N., Okuno T. Harnessing host ros-generating machinery for the robust genome replication of a plant rna virus. Proc. Natl. Acad. Sci. USA. 2017;114:E1282–E1290. doi: 10.1073/pnas.1610212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vijayapalani P., Maeshima M., Nagasaki-Takekuchi N., Miller W.A. Interaction of the trans-frame potyvirus protein p3n-pipo with host protein pcap1 facilitates potyvirus movement. PLoS Pathog. 2012;8:e1002639. doi: 10.1371/journal.ppat.1002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshii A., Shimizu T., Yoshida A., Hamada K., Sakurai K., Yamaji Y., Suzuki M., Namba S., Hibi T. Nth201, a novel class ii knotted1-like protein, facilitates the cell-to-cell movement of tobacco mosaic virus in tobacco. Mol. Plant Microbe Interact. 2008;21:586–596. doi: 10.1094/MPMI-21-5-0586. [DOI] [PubMed] [Google Scholar]
- 79.Uhrig J.F., Canto T., Marshall D., MacFarlane S.A. Relocalization of nuclear aly proteins to the cytoplasm by the tomato bushy stunt virus p19 pathogenicity protein. Plant Physiol. 2004;135:2411–2423. doi: 10.1104/pp.104.046086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagiwara-Komoda Y., Hirai K., Mochizuki A., Nishiguchi M., Meshi T., Ishikawa M. Overexpression of a host factor tom1 inhibits tomato mosaic virus propagation and suppression of rna silencing. Virology. 2008;376:132–139. doi: 10.1016/j.virol.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 81.Yamanaka T., Imai T., Satoh R., Kawashima A., Takahashi M., Tomita K., Kubota K., Meshi T., Naito S., Ishikawa M. Complete inhibition of tobamovirus multiplication by simultaneous mutations in two homologous host genes. J. Virol. 2002;76:2491–2497. doi: 10.1128/jvi.76.5.2491-2497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hafren A., Macia J.L., Love A.J., Milner J.J., Drucker M., Hofius D. Selective autophagy limits cauliflower mosaic virus infection by nbr1-mediated targeting of viral capsid protein and particles. Proc. Natl. Acad. Sci. USA. 2017;114:E2026–E2035. doi: 10.1073/pnas.1610687114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ballut L., Drucker M., Pugniere M., Cambon F., Blanc S., Roquet F., Candresse T., Schmid H.P., Nicolas P., Gall O.L., et al. Hcpro, a multifunctional protein encoded by a plant rna virus, targets the 20s proteasome and affects its enzymic activities. J. Gen. Virol. 2005;86:2595–2603. doi: 10.1099/vir.0.81107-0. [DOI] [PubMed] [Google Scholar]
- 84.Dielen A.S., Sassaki F.T., Walter J., Michon T., Menard G., Pagny G., Krause-Sakate R., Ide G.M., Badaoui S., Le Gall O., et al. The 20s proteasome alpha5 subunit of arabidopsis thaliana carries an rnase activity and interacts in planta with the lettuce mosaic potyvirus hcpro protein. Mol. Plant Pathol. 2011;12:137–150. doi: 10.1111/j.1364-3703.2010.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cho S.Y., Cho W.K., Choi H.S., Kim K.H. Cis-acting element (sl1) of potato virus x controls viral movement by interacting with the nbmpb2cb and viral proteins. Virology. 2012;427:166–176. doi: 10.1016/j.virol.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Wu C.Y., Nagy P.D. Blocking tombusvirus replication through the antiviral functions of ddx17-like rh30 dead-box helicase. PLoS Pathog. 2019;15:e1007771. doi: 10.1371/journal.ppat.1007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hafren A., Ustun S., Hochmuth A., Svenning S., Johansen T., Hofius D. Turnip mosaic virus counteracts selective autophagy of the viral silencing suppressor hcpro. Plant Physiol. 2018;176:649–662. doi: 10.1104/pp.17.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baltazar B.M., Espinoza L.C., Banda A.E., de la Fuente Martinez J.M., Tiznado J.A.G., Garcia J.G., Gutierrez M.A., Rodriguez J.L.G., Diaz O.H., Horak M.J., et al. Pollen-mediated gene flow in maize: Implications for isolation requirements and coexistence in mexico, the center of origin of maize. PLoS ONE. 2015;10:e0131549. doi: 10.1371/journal.pone.0131549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Timperio A., Egidi M., Zolla L. Proteomics applied on plant abiotic stresses: Role of heat shock proteins (hsp) Elsevier. 2008;71:391–411. doi: 10.1016/j.jprot.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y.P., Chen I.H., Tsai C.H. Host factors in the infection cycle of bamboo mosaic virus. Front. Microbiol. 2017;8:437. doi: 10.3389/fmicb.2017.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wollert T., Hurley J. Molecular mechanism of multivesicular body biogenesis by escrt complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Contreras-Paredes C.A., Silva-Rosales L., Daros J.A., Alejandri-Ramirez N.D., Dinkova T.D. The absence of eukaryotic initiation factor eif(iso)4e affects the systemic spread of a tobacco etch virus isolate in arabidopsis thaliana. Mol. Plant Microbe Interact. 2013;26:461–470. doi: 10.1094/MPMI-09-12-0225-R. [DOI] [PubMed] [Google Scholar]
- 93.Morris D.R., Geballe A.P. Upstream open reading frames as regulators of mrna translation. Mol. Cell. Biol. 2000;20:8635–8642. doi: 10.1128/MCB.20.23.8635-8642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cordin O., Banroques J., Tanner N.K., Linder P. The dead-box protein family of rna helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 95.Kim J.S., Kim K.A., Oh R.T., Park C.M., Kang H. Functional characterization of dead-box rna helicases in arabidopsis thaliana under abiotic stress conditions. Plant. Cell Physiol. 2008;49:1563–1571. doi: 10.1093/pcp/pcn125. [DOI] [PubMed] [Google Scholar]
- 96.Noueiry A.O., Chen J., Ahlquist P. A mutant allele of essential, general translation initiation factor ded1 selectively inhibits translation of a viral mrna. Proc. Natl. Acad. Sci. USA. 2000;97:12985–12990. doi: 10.1073/pnas.240460897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang Y., Serviene E., Gal J., Panavas T., Nagy P.D. Identification of essential host factors affecting tombusvirus rna replication based on the yeast tet promoters hughes collection. J. Virol. 2006;80:7394–7404. doi: 10.1128/JVI.02686-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prasanth K.R., Huang Y.W., Liou M.R., Wang R.Y., Hu C.C., Tsai C.H., Meng M., Lin N.S., Hsu Y.H. Glyceraldehyde 3-phosphate dehydrogenase negatively regulates the replication of bamboo mosaic virus and its associated satellite rna. J. Virol. 2011;85:8829–8840. doi: 10.1128/JVI.00556-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dixon D., Lapthorn A., Edwards R. Protein family review: Plant glutathione transferases. Genome Biol. 2002;3:1–10. doi: 10.1186/gb-2002-3-3-reviews3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skopelitou K., Muleta A.W., Papageorgiou A.C., Chronopoulou E., Labrou N.E. Catalytic features and crystal structure of a tau class glutathione transferase from glycine max specifically upregulated in response to soybean mosaic virus infections. Biochim. Biophys. Acta. 2015;1854:166–177. doi: 10.1016/j.bbapap.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Weber-Boyvat M., Zhong W., Yan D., Olkkonen V.M. Oxysterol-binding proteins: Functions in cell regulation beyond lipid metabolism. Biochem. Pharmacol. 2013;86:89–95. doi: 10.1016/j.bcp.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 102.Islas-Flores T., Rahman A., Ullah H., Villanueva M.A. The receptor for activated c kinase in plant signaling: Tale of a promiscuous little molecule. Front. Plant Sci. 2015;6:1090. doi: 10.3389/fpls.2015.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakashima A., Chen L., Thao N.P., Fujiwara M., Wong H.L., Kuwano M., Umemura K., Shirasu K., Kawasaki T., Shimamoto K. Rack1 functions in rice innate immunity by interacting with the rac1 immune complex. Plant Cell. 2008;20:2265–2279. doi: 10.1105/tpc.107.054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng Z., Li J.F., Niu Y., Zhang X.C., Woody O.Z., Xiong Y., Djonovic S., Millet Y., Bush J., McConkey B.J., et al. Pathogen-secreted proteases activate a novel plant immune pathway. Nature. 2015;521:213–216. doi: 10.1038/nature14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adams D.R., Ron D., Kiely P.A. Rack1, a multifaceted scaffolding protein: Structure and function. Cell Commun. Signal. 2011;9:22. doi: 10.1186/1478-811X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Otegui M.S., Spitzer C. Endosomal functions in plants. Traffic. 2008;9:1589–1598. doi: 10.1111/j.1600-0854.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- 107.Lundquist E. Small GTPases. WormBook. 2006:1–18. doi: 10.1895/wormbook.1.67.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Romero-Brey I., Bartenschlager R. Endoplasmic reticulum: The favorite intracellular niche for viral replication and assembly. Viruses. 2016;8:160. doi: 10.3390/v8060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanderfoot A., Kovaleva V., Bassham D., Raikhel N. Interactions between syntaxins identify at least five snare complexes within the golgi/prevacuolar system of the arabidopsis cell. Mol. Biol. Cell. 2001;12:3733–3743. doi: 10.1091/mbc.12.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suwastika N., Uemura T., Shiina T., Sato M., Takeyasu K. Suwastika in, uemura t, shiina t, sato mh, takeyasu. Cell Struct. Funct. 2008;33:185–192. doi: 10.1247/csf.08024. [DOI] [PubMed] [Google Scholar]
- 111.Nziengui H., Schoefs B. Functions of reticulons in plants: What we can learn from animals and yeasts. Cell Mol. Life Sci. 2009;66:584–595. doi: 10.1007/s00018-008-8373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen J., Stefano G., Brandizzi F., Zheng H. Arabidopsis rhd3 mediates the generation of the tubular er network and is required for golgi distribution and motility in plant cells. J. Cell Sci. 2011;124:2241–2252. doi: 10.1242/jcs.084624. [DOI] [PubMed] [Google Scholar]
- 113.Cheng S.-F., Huang Y.-P., Wu Z.-R., Hu C.-C., Hsu Y.-H., Tsai C.-H. Identification of differentially expressed genes induced by bamboo mosaic virus infection in nicotiana benthamianaby cdna-amplified fragment length polymorphism. BMC Plant Biol. 2010;10:286. doi: 10.1186/1471-2229-10-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Memon A.R. The role of adp-ribosylation factor and sar1 in vesicular trafficking in plants. Biochim. Biophys. Acta. 2004;1664:9–30. doi: 10.1016/j.bbamem.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 115.Harries P., Ding B. Cellular factors in plant virus movement: At the leading edge of macromolecular trafficking in plants. Virology. 2011;411:237–243. doi: 10.1016/j.virol.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki N., Miller G., Morales J., Shulaev V., Torres M.A., Mittler R. Respiratory burst oxidases: The engines of ros signaling. Curr. Opin. Plant Biol. 2011;14:691–699. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 117.Deng X.G., Zhu T., Zou L.J., Han X.Y., Zhou X., Xi D.H., Zhang D.W., Lin H.H. Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in nicotiana benthamiana. Plant J. 2016;85:478–493. doi: 10.1111/tpj.13120. [DOI] [PubMed] [Google Scholar]
- 118.Deng X.-G., Zhu T., Peng X.-J., Xi D.-H., Guo H., Yin Y., Zhang D.-W., Lin H.-H. Role of brassinosteroid signaling in modulating tobacco mosaic virus resistance in nicotiana benthamiana. Sci. Rep. 2016;6:20579. doi: 10.1038/srep20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pfaff C., Ehrnsberger H., Flores-Tornero M., Sorensen B., Schubert T., Längst G., Griesenbeck L., Sprunck S., Grasser M., Grasser K.D. Aly rna-binding proteins are required for nucleocytosolic mrna transport and modulate plant growth and development. Plant Physiol. 2018;177:2226–2240. doi: 10.1104/pp.18.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Canto T., Uhrig J.F., Swanson M., Wright K.M., MacFarlane S.A. Translocation of tomato bushy stunt virus p19 protein into the nucleus by aly proteins compromises its silencing suppressor activity. J. Virol. 2006;80:9064–9072. doi: 10.1128/JVI.00953-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clavel M., Michaeli S., Genschik P. Autophagy: A double-edged sword to fight plant viruses. Trends Plant Sci. 2017;22:646–648. doi: 10.1016/j.tplants.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 122.Haxim Y., Ismayil A., Jia Q., Wang Y., Zheng X., Chen T., Qian L., Liu N., Wang Y., Han S., et al. Autophagy functions as an antiviral mechanism against geminiviruses in plants. eLife. 2017;6:e23897. doi: 10.7554/eLife.23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hafren A., Lohmus A., Makinen K. Formation of potato virus a-induced rna granules and viral translation are interrelated processes required for optimal virus accumulation. PLoS Pathog. 2015;11:e1005314. doi: 10.1371/journal.ppat.1005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pitzalis N., Amari K., Graindorge S., Pflieger D., Donaire L., Wassenegger M., Llave C., Heinlein M. Turnip mosaic virus in oilseed rape activates networks of srna-mediated interactions between viral and host genomes. Commun. Biol. 2020;3:702. doi: 10.1038/s42003-020-01425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beauchemin C., Boutet N., Laliberte J.F. Visualization of the interaction between the precursors of vpg, the viral protein linked to the genome of turnip mosaic virus, and the translation eukaryotic initiation factor iso 4e in planta. J. Virol. 2007;81:775–782. doi: 10.1128/JVI.01277-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nigam D., LaTourrette K., Souza P.F.N., Garcia-Ruiz H. Genome-wide variation in potyviruses. Front. Plant Sci. 2019;10:1439. doi: 10.3389/fpls.2019.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Charron C., Nicolaï M., Gallois J.L., Robaglia C., Moury B., Palloix A., Caranta C. Natural variation and functional analyses provide evidence for co-evolution between plant eif4e and potyviral vpg. Plant J. 2008;54:56–68. doi: 10.1111/j.1365-313X.2008.03407.x. [DOI] [PubMed] [Google Scholar]
- 128.Ahlquist P., Noueiry A.O., Lee W.M., Kushner D.B., Dye B.T. Host factors in positive-strand rna virus genome replication. J. Virol. 2003;77:8181–8186. doi: 10.1128/JVI.77.15.8181-8186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Panavas T., Serviene E., Brasher J., Nagy P.D. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of rna viruses. Proc. Natl. Acad. Sci. USA. 2005;102:7326–7331. doi: 10.1073/pnas.0502604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoon Y.J., Venkatesh J., Lee J.H., Kim J., Lee H.E., Kim D.S., Kang B.C. Genome editing of eif4e1 in tomato confers resistance to pepper mottle virus. Front. Plant Sci. 2020;11:1098. doi: 10.3389/fpls.2020.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mahuku G., Lockhart B.E., Wanjala B., Jones M.W., Kimunye J.N., Stewart L.R., Cassone B.J., Sevgan S., Nyasani J.O., Kusia E., et al. Maize lethal necrosis (mln), an emerging threat to maize-based food security in sub-saharan africa. Phytopathology. 2015;105:956–965. doi: 10.1094/PHYTO-12-14-0367-FI. [DOI] [PubMed] [Google Scholar]