Abstract
Numerous surveillance pathways sculpt eukaryotic transcriptomes by degrading unneeded, defective and potentially harmful noncoding RNAs (ncRNAs). Because aberrant and excess ncRNAs are largely degraded by exoribonucleases, a key characteristic of these RNAs is an accessible, protein-free 5’ or 3’ end. Most exoribonucleases function with co-factors that recognize ncRNAs with accessible 5’ or 3’ ends and/or increase the availability of these ends. Noncoding RNA surveillance pathways were first described in budding yeast, and there are now high-resolution structures of many components of the yeast pathways and significant mechanistic understanding as to how they function. Studies in human cells are revealing the ways in which these pathways both resemble and differ from their yeast counterparts, and are also uncovering numerous pathways that lack equivalents in budding yeast. In this review, we describe both the well-studied pathways uncovered in yeast and the new concepts that are emerging from studies in mammalian cells. We also discuss the ways in which surveillance pathways compete with chaperone proteins that transiently protect nascent ncRNA ends from exoribonucleases, with partner proteins that sequester these ends within RNPs, and with end modification pathways that protect the ends of some ncRNAs from nucleases.
Graphical Abstract
1. INTRODUCTION
The pathways that recognize and degrade excess and defective noncoding RNAs (ncRNAs) play critical roles in sculpting the transcriptomes of eukaryotic cells. By mass, the most abundant transcripts at steady state are classical ncRNAs such as ribosomal RNAs (rRNAs), transfer RNAs (tRNAs), spliceosomal small nuclear RNAs (snRNAs) and small nucleolar RNAs (snoRNAs)1–3. These ncRNAs must fold into complex structures and assemble with proteins, and sometimes with other RNAs, to form functional ribonucleoproteins (RNPs). In addition, since many of these ncRNAs are made as 5’ and/or 3’ extended precursors, they must undergo an intricate series of processing events to generate the mature ncRNAs. Defective ncRNAs can arise from genetic mutations, transcriptional errors, misfolding, processing mistakes and the failure to assemble with partner proteins and RNAs to become functional RNPs. Just as cells have evolved numerous quality control pathways to identify defective mRNAs and proteins and target them for degradation4–7, cells require surveillance pathways to recognize and rid themselves of aberrant and excess ncRNAs and ncRNPs. The contributions of ncRNA surveillance pathways in shaping transcriptomes is significant, as approximately half of all newly synthesized pre-tRNAs in yeast fail to become mature tRNAs and are instead degraded8.
In addition to the roles of surveillance pathways in ensuring quality control of classical ncRNAs, the use of tiling arrays and high-throughput sequencing to interrogate eukaryotic transcriptomes has resulted in the realization that ncRNA surveillance pathways have wide-ranging functions in regulating genomic output, influencing gene expression and ensuring genomic integrity. All examined genomes are extensively transcribed, with numerous transcripts derived from intergenic regions that were previously believed to be silent9–16. This “pervasive transcription”, which is largely due to the inherent bidirectionality of promoters11,14,16–18, produces a tremendous diversity of RNAs, most of which do not encode proteins. These newly discovered ncRNAs are often far less stable than classical ncRNAs, since many are only detected when specific ribonucleases are depleted or absent11,12,14–16. Failure to degrade some of these ncRNAs can alter expression of adjacent genes19–21 and can result in formation of RNA-DNA hybrids (R-loops) that promote DNA breaks22–25. Thus, surveillance pathways that target these potentially harmful ncRNAs for decay are of critical importance.
The focus of this review is primarily on ncRNA surveillance pathways in yeast and mammalian cells. Although ncRNA surveillance occurs in all kingdoms of life, in eukaryotes these pathways have been studied most extensively in the budding yeast Saccharomyces cerevisiae. Recent studies have begun to reveal the extent to which these pathways are conserved in mammalian cells and have identified additional mechanisms that lack budding yeast counterparts. Moreover, the finding that mutations in several components of surveillance pathways are associated with specific human diseases26,27 allows these pathways to be correlated with disease phenotypes, an important step in understanding disease etiology.
2. PRINCIPLES OF NONCODING RNA SURVEILLANCE
Although ncRNA surveillance pathways are active in all organisms, they have been studied most extensively in the bacterium Escherichia coli, the budding yeast S. cerevisiae and mammalian cells. From these studies, as well as findings in other model organisms, several principles have emerged. The first and most obvious principle is that ribonucleases are major players. Most characterized surveillance pathways target defective RNAs to exoribonucleases28–31, which degrade RNAs from either the 5’ end (5’ to 3’ exoribonucleases) or the 3’ end (3’ to 5’ exoribonucleases). Endoribonucleases, which cleave within the RNA body, also participate8,32–34, often by cleaving within highly structured ncRNAs to generate additional ends for exoribonucleases. Illustrating the importance of both types of activities, a major decay pathway in eukaryotic cells involves the RNA exosome, a multiprotein complex in which a key nuclease, Dis3 (also called Rrp44), is both a 3’ to 5’ exoribonuclease and an endoribonuclease35–37 (Figure 1).
Figure 1.

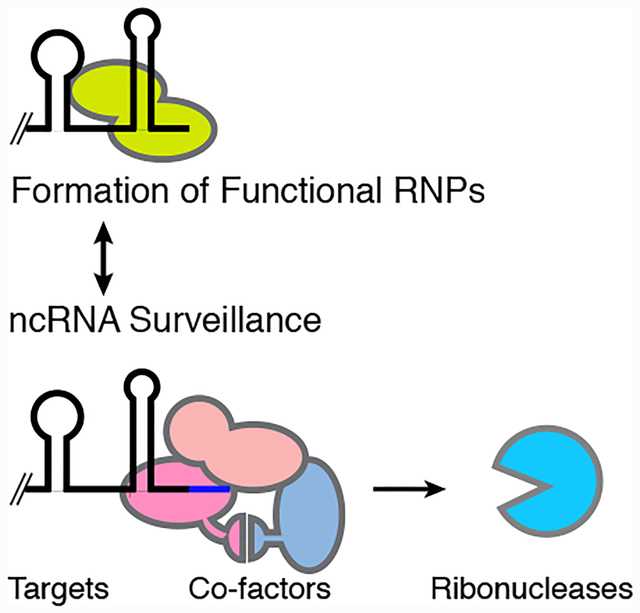
Components of noncoding RNA surveillance pathways. Noncoding RNA surveillance pathways occur in competition with formation of functional RNPs. Some targets of these pathways are shown in the left panel. The middle panel describes ways in which cofactors assist in recognizing aberrant ncRNAs and assisting in their degradation. The right panel lists the major ribonucleases that carry out degradation of aberrant ncRNAs.
A second principle is that although exoribonucleases are key effectors in ncRNA surveillance, they rarely function on their own. To initiate decay, most exoribonucleases require that the RNA substrate contain a protein-free single-stranded end. Thus, exoribonucleases often function with co-factors that recognize ncRNAs with accessible 5’ or 3’ ends and/or increase the availability of these ends (Figure 1). For example, degradation of mutant pre-tRNAs, in both bacteria and budding yeast, involves addition of a short polyA tail to the 3’ end of the aberrant tRNA, followed by degradation by 3’ to 5’ exoribonuclease(s)28,30,38. Additionally, because many exoribonucleases have difficulty progressing through structured substrates, they often function with helicases or other proteins that assist RNA unwinding10,38–43.
Third, since exoribonucleases require a single-stranded protein-free end to initiate degradation, the accessibility of these ends is a major determinant of whether a ncRNA will be targeted for decay. The 5’ ends of newly made RNA polymerase II and III transcripts are usually protected from decay by the presence of caps (RNA polymerase II transcripts) or 5’ triphosphates (RNA polymerase III transcripts). The 3’ ends of both RNA polymerase II and III-transcribed ncRNAs are protected from exoribonucleases by bound partner proteins44–47, by forming double-stranded stems or triple-helical structures48–50, or in the case of mature tRNAs, by CCA addition and aminoacylation51. Thus, aberrant or excess ncRNAs can be recognized by their failure to bind proteins or fold into the structures that normally protect these ends.
Fourth, since the accessibility of ncRNA ends is a critical factor in determining whether an RNA will be degraded, ncRNA surveillance often occurs in competition with chaperone proteins that transiently protect nascent ncRNA ends from exoribonucleases46,52–57, with the binding of proteins that sequester these ends within stable noncoding RNPs44,47,54,57–59, and with end modification pathways that protect the ends of some ncRNAs from degradation60–63.
Finally, surveillance pathways are often functionally overlapping, with backup pathways that degrade ncRNAs that escape degradation by other pathways29,31,64. Consequently, it is often necessary to deplete or inactivate multiple pathways to detect accumulation of aberrant ncRNAs.
3. THE RNA EXOSOME
The RNA exosome, a multiprotein nuclease complex, is a prominent contributor to ncRNA surveillance in all examined eukaryotic cells. The role of the exosome in ncRNA surveillance has been most extensively characterized in budding yeast, where its targets include excess and defective pre-tRNAs8,30,33,46,65, pre-snoRNAs and pre-snRNAs that are misprocessed and/or have not assembled with core proteins47,66, truncated forms of 5S rRNA and the signal recognition particle (SRP) RNA46,57,67, aberrant pre-rRNA processing intermediates68,69 and cryptic unstable transcripts (CUTs) that originate from bidirectional promoters10,11,14,16. Although the role of the RNA exosome in ncRNA surveillance in mammalian cells is less well-defined, many of the same ncRNAs have been shown to be exosome targets70–73 (Table 1).
Table 1.
Examples of noncoding RNAs and their described surveillance pathways
| Budding Yeast | |||||
|---|---|---|---|---|---|
| Noncoding RNAs | Defects | Nuclease(s) | Cofactors | Other components | References |
| Mature 28S rRNAs | Nonfunctional in protein synthesis | Rtt101, Mms1 | 267,271,272 | ||
| Structurally unstable tRNAs | Rat1, Xrn1 | Unknown | 210,219–221 | ||
| pre-snoRNAs | Nuclear exosome | Nrd1-Nab3, TRAMP | 8,33,45,47,65,143 | ||
| XRN1 | Dcp1, Dcp2 | 58 | |||
| U6 snRNA | Nuclear exosome | TRAMP | 8,33,67 | ||
| 5S rRNAs | Truncated | Nuclear exosome | Nrd1-Nab3, TRAMP | 33,65,67,143 | |
| lncRNAs | Many without known binding proteins | Rat1 | Dcp1 | 19 | |
| SRP RNA | Truncated | Nuclear exosome | TRAMP | 33,46,57 | |
| CUTs | No known binding proteins | Nuclear exosome | Nrd1-Nab3, TRAMP, Rrp47, MPP6 | 8,10,11,33,105,110,122,124,140,141 | |
| XUTs | No known binding proteins | Xrn1 | Dcp1, Dcp2 | NMD often important | 15,225–227,229–231 |
| telomeric repeat transcripts | No known binding proteins | Rat1 | Rai1 | 22 | |
| Mammals | |||||
| Aberrant precursors, excised spacers | XRN2 | NKRF | 126,249,250 | ||
| PROMPTs | No known binding proteins | Nuclear exosome | NEXT complex | 70,72,73,85,150,151,160 | |
| Enhancer RNAs | No known binding proteins | Nuclear exosome | NEXT complex | 73,151,331 | |
| Weak acceptor stems | CCA-adding enzyme | CCACCA addition | 203 | ||
| DIS3L2 | TUT4, TUT7 | 64 | |||
| other lncRNAs | Unknown | NMD often important | 231,235,238,239 | ||
| Mature hTR RNA | Unknown | Nucleolar exosome | DGCR8 | 168 | |
| DIS3L2 | TUT4, TUT7 | 189,191 | |||
| DXO | 218,254 | ||||
| DIS3L2 | TUT4/TUT7 | 189 | |||
| Pre-let-7 miRNA | miRNA harmful to stem cells | DIS3L2 | TUT4/TUT7 | 183,185–188 | |
| Transcripts from Alu elements | Unknown | DICER1 | Unknown | 32,278 | |
3.1. The RNA exosome in budding yeast
The exosome was first identified in S. cerevisiae as a complex of 3’ to 5’ exoribonucleases associated with Rrp4, (Ribosomal RNA processing 4), a protein required for 3’ processing of 5.8S rRNA74–76. This complex is essential in yeast and highly conserved, as it is widespread in eukaryotes and Archaea75,77,78 and shares structural similarities with two bacterial 3’ to 5’ exoribonucleases, RNase PH and polynucleotide phosphorylase (PNPase)75,79,80.
In yeast, as in all studied eukaryotes, the “core” exosome consists of a ring formed by six RNase PH domain-containing subunits (Rrp41, Rrp42, Rrp43, Rrp45, Rrp46 and Mtr3) capped by a ring of 3 proteins harboring RNA-binding S1 and KH domains (Rrp4 and Rrp40) or only a S1 domain (Csl4)81. Together, these subunits form a barrel-like structure with a central channel that can accommodate single-stranded, but not double-stranded RNA. Although degradation takes place within the central channel of the archaeal exosome80,82,83, the RNase PH domains of the orthologous eukaryotic subunits contain inactivating mutations, making the central channel catalytically inactive81,84. Thus, in eukaryotes, RNA degradation is carried out by exosome-associated ribonucleases that differ based upon their subcellular location84–86. For these organisms, passage through the RNA-binding ring and the central channel could contribute to the unwinding of structured substrates during degradation42 (Figure 2).
Figure 2.

The nuclear RNA exosome. (A) Structure of a complex consisting of the nine-subunit exosome core, Rrp6, Dis3, Rrp47 and single-stranded RNA87 (PDB 5C0W). In this structure, RNA directly accesses Dis3. (B) Structure of a complex consisting of the nine-subunit core, Dis3, a fragment of Rrp6 and RNA consisting of a 5’ stem-loop and a 31 nt single-stranded overhang88 (PDB 4IFD). In this structure, RNA accesses Dis3 via the channel. The RNA within the channel was not fully visualized88. (C) Structure of a complex consisting of the nine-subunit core, Dis3, Rrp6, the exosome-binding domain of MPP6 and an RNA engineered to have two 3’ ends. One 3’ end enters the Rrp6 active site, while the other uses the direct access path to the Dis3 exoribonuclease89 (PDB 5VZJ). (D-F) Cartoons of RNA paths to the exosome, configured similarly to the view in Figure 2A, left panel. (D) Path by which a structured RNA substrate can directly access the Dis3 exoribonuclease. (E) Path by which an RNA substrate passes through the central channel to reach the Dis3 exoribonuclease. The conformation of Dis3 differs between the direct access and channel-dependent paths42,88,90–92. (F) RNA path to Rrp6.
In yeast, all exosomes contain Dis3, a multi-domain protein that is both an endoribonuclease and a processive hydrolytic 3’ to 5’ exoribonuclease resembling bacterial RNase II and RNase R35–37,81,84,93. Dis3 consists of an N-terminal PIN domain harboring endonuclease activity, followed by the two cold-shock domains, RNB domain and S1 domain91 that are characteristic of RNase II/R family members35–37,93. Dis3 binds at the bottom of the barrel, largely through interactions between its PIN domain and Rrp41, Rrp43 and 4542,88,90,94 (Figure 2A). Structural studies have revealed that Dis3 can adopt two conformations that allow substrates to access the exonuclease activity by distinct paths42,88,90–92. In one path, RNAs bypass the channel to directly access the exonuclease active site42,91,92 (Figures 2A and 2D). In the other path, single-stranded RNA is channeled through the central cavity to the Dis3 exoribonuclease catalytic center88,91,92 (Figures 2B and 2E). Less is known as to how RNA substrates access the Dis3 endonuclease. In all structures to date, the endonuclease active site faces the solvent42,87,88,91,92,94. As a circular RNA can be degraded by the intact exosome42, at least some substrates can directly access this activity. Consistent with direct access to the endonuclease, single-particle electron microscopy of exosomes bound to streptavidin-conjugated RNAs detected a population of exosomes with the RNA near the PIN domain91. However, experiments in which mutations were used to occlude the channel support a model in which passage through the channel is required to access endonuclease activity95,96.
Most ncRNA surveillance involving the exosome occurs within nuclei. The nuclear form of the exosome contains an additional exoribonuclease, the distributive hydrolytic Rrp6, a member of the RNase D family76,81,97,98. Rrp6 is a multi-domain protein, with an N-terminal domain that binds its cofactor Rrp47 (see below), an EXO domain shared with other members of the DEDD nuclease superfamily99, a HDRC (helicase and RNase D C-terminal) domain100, and a C-terminal domain. Both the EXO and HDRC domains are required for exoribonuclease activity. Rrp6 binds at the top of the exosome (Figure 2), with its EXO domain contacting the cap proteins Rrp4 and Rrp40, the HRDC domain contacting Rrp4, and its C-terminal domain contacting a conserved surface formed by Csl4 and the RNase PH-like subunits Mtr3 and Rrp4387,88,101. Structural and biochemical studies revealed that substrates bind the S1/KH domains of the cap proteins before entering the Rrp6 active site (Figures 2C and 2F)94,95,101.
3.2. Role of the yeast nuclear exosome nucleases in ncRNA surveillance
The ways in which the various catalytic activities of the nuclear exosome contribute to ncRNA surveillance has been parsed out by combining in vivo RNA:protein crosslinking with examination of the RNAs that accumulate in mutant strains. One caveat to these studies is that mutations affecting one nuclease could indirectly affect the other, since in vitro, RNA binding by Rrp6 stimulates Dis3 activity and a mutation that disrupts the exoribonuclease active site of Dis3 inhibits Rrp6 activity95,102. These experiments revealed significant overlap between Rrp6 and Dis3 targets, as well as between the exonuclease and endonuclease activities of Dis3. For example, CUTs accumulate when either Dis3 or Rrp6 is mutated, and accumulate to higher levels when both Dis3 and Rrp6 contain mutations8. Although mutating the Dis3 endonuclease domain had little effect, CUTs accumulate more strongly when both the endonuclease and Dis3 exonuclease domains are mutated than when strains carry only a mutation in the exonuclease8. Consistent with these findings, CUTs crosslink to both domains of Dis3 and to Rrp633.
Some targets appear to be preferentially degraded by one or the other nuclease. Both snRNA and snoRNA precursors and the mature RNAs appear more dependent on Rrp6, as they accumulate more strongly when Rrp6 is mutated than when both catalytic domains of Dis3 are mutated8 and are more enriched in Rrp6 immunoprecipitates33. In contrast, pre-tRNAs, U6 snRNA and truncated forms of 5S and SRP RNAs are largely Dis3 targets8,33. In support of a role for endonucleolytic cleavage of highly structured RNAs, both the endonucleolytic and exonucleolytic activities are important for pre-tRNA and U6 degradation8,33. Remarkably, U6 snRNAs and mature tRNAs increase 2 to 3-fold when both the endonucleolytic and exonucleolytic domains of Dis3 endonuclease are mutated8,33, while both U4 and U5 snRNAs show similar increases when Rrp6 is deleted66. Thus, more than half the transcripts encoding some nascent ncRNAs are normally degraded.
Several studies have addressed the relative roles of the direct access and channel routes to Dis3. Since an RNA substrate must have a ~31 to 33 single-stranded tail to reach Dis3 via the channel42, substrates that lack such tails are good candidates for the direct access path. There is now evidence that some structured ncRNAs are degraded via this pathway. Two well-characterized exosome substrates, a hypomodified pre-tRNAiMet 30and a truncated 5S rRNA67, accumulate in yeast containing mutations predicted to prevent Dis3 from forming the direct access conformation103. Moreover, in vivo crosslinking revealed that association of U6 snRNA, RNase P RNA and many pre-tRNAs with Dis3 requires the S1 domain, which is predicted to be important for binding RNAs that approach Dis3 via the direct access path, but not for binding RNAs that thread through the channel104.
3.3. Cofactors of the yeast nuclear exosome
3.3.1. Rrp47.
Because Rrp47 initially co-purified with the nuclear exosome in substoichiometric amounts, it has been considered an exosome co-factor105. However, recent functional and structural analyses indicate that Rrp47 is an integral subunit of the nuclear exosome. Rrp47 binds to the N-terminus of Rrp6 and is required for all known Rrp6 activities, including degradation of CUTs and aberrant rRNA processing intermediates105. One role of Rrp47 is to stabilize Rrp6, since Rrp6 is unstable in cells lacking Rrp47 when yeast cells are grown in minimal media106. In addition, experiments with reconstituted exosomes have revealed that Rrp6 activity is enhanced in the presence of Rrp4789. Consistent with roles in stabilizing Rrp6 and increasing its activity, the defects in ncRNA surveillance and RNA processing that are detected in yeast lacking Rrp47 can be ameliorated by overexpressing Rrp6106.
Structural analyses have revealed that the N-termini of Rrp6 and Rrp47 interact to form a binding surface that allows the Mtr4 RNA helicase, a component of TRAMP and other co-factor complexes, to associate with the exosome107. Thus, rather than functioning as a traditional co-factor to recruit RNA substrates and assist in their decay, Rrp47 appears to be a structural component that stabilizes Rrp6 and assists in recruiting co-factors to the exosome. Human cells contain an Rrp47 ortholog, called C1D, that associates with the Rrp6 ortholog EXOSC10/hRRP6 and whose localization to nucleoli also depends on EXOSC10/hRRP6108.
3.3.2. Mpp6.
MPP6 was first identified as an exosome cofactor in human cells, where it co-purified with the exosome, localized to nucleoli, bound RNA in vitro and was found to be important for 5.8S rRNA maturation109. Yeast strains lacking the ortholog Mpp6 resemble rrp47Δ and rrp6Δ strains, in that they accumulate CUTs, intergenic transcripts and aberrant rRNA processing intermediates110. Consistent with functional redundancy, strains lacking Mpp6 and either Rrp47 or Rrp6 are inviable110. Because of both the synthetic lethality with Rrp6 and the fact that the RNAs that accumulate in mpp6Δ strains are also Dis3 targets8,110, it was proposed that Mpp6 targets these RNAs for degradation by Dis3111.
Notably, recent studies have identified roles for Mpp6 in recruiting the Mtr4 helicase to the exosome and in stimulating Rrp6 activity89,112. Structures of either the nine-subunit core exosome112 or the Rrp6- and Dis3-containing exosome89 complexed with the minimal Mpp6 domain needed to bind the exosome and stimulate Rrp6 activity revealed that Mpp6 binds the Rrp40 exosome core protein and is positioned near Rrp6, contacting the S1 and KH domains of the Rrp40 cap protein (Figures 2C and 2F)89,112. Biochemical assays showed that the presence of Mpp6 was sufficient to recruit Mtr4 to exosomes lacking Rrp47, and that maximum Mtr4 binding occurred when both Rrp47 and Mpp6 were present89. A role for Mpp6 in recruiting Mtr4 is consistent with findings that human MPP6 and the Mtr4 ortholog SKIV2L2/hMTR4 associate in vitro108. Thus, another explanation for the finding that strains lacking Mpp6 and Rrp47 are inviable110 is that Rrp47 and Mpp6 function redundantly to recruit Mtr4 to the nuclear exosome.
3.3.3. The Mtr4 helicase and its associated adaptors.
The Mtr4 RNA helicase is a central player in both the maturation and surveillance functions of the nuclear exosome. Mtr4 is a superfamily (SF) 2 helicase that is a member of the Ski2-like group of DExH helicases113. Its closest relative, Ski2, is required for all known functions of the cytoplasmic exosome. Structural analyses revealed that Mtr4 contains a poorly structured N-terminus and a DExH helicase core with a prominent “arch domain” rising from the helicase core114,115. The N-terminus of Mtr4 binds the N-termini of Rrp6 and Rrp47, tethering Mtr4 to the exosome near the channel entrance107, where it likely contributes to unwinding structured RNAs116. The arch domain was shown recently to function as a docking site for two adaptor proteins, Utp18 and Nop53, that target the exosome to specific substrates117 (Figure 3A).
Figure 3.
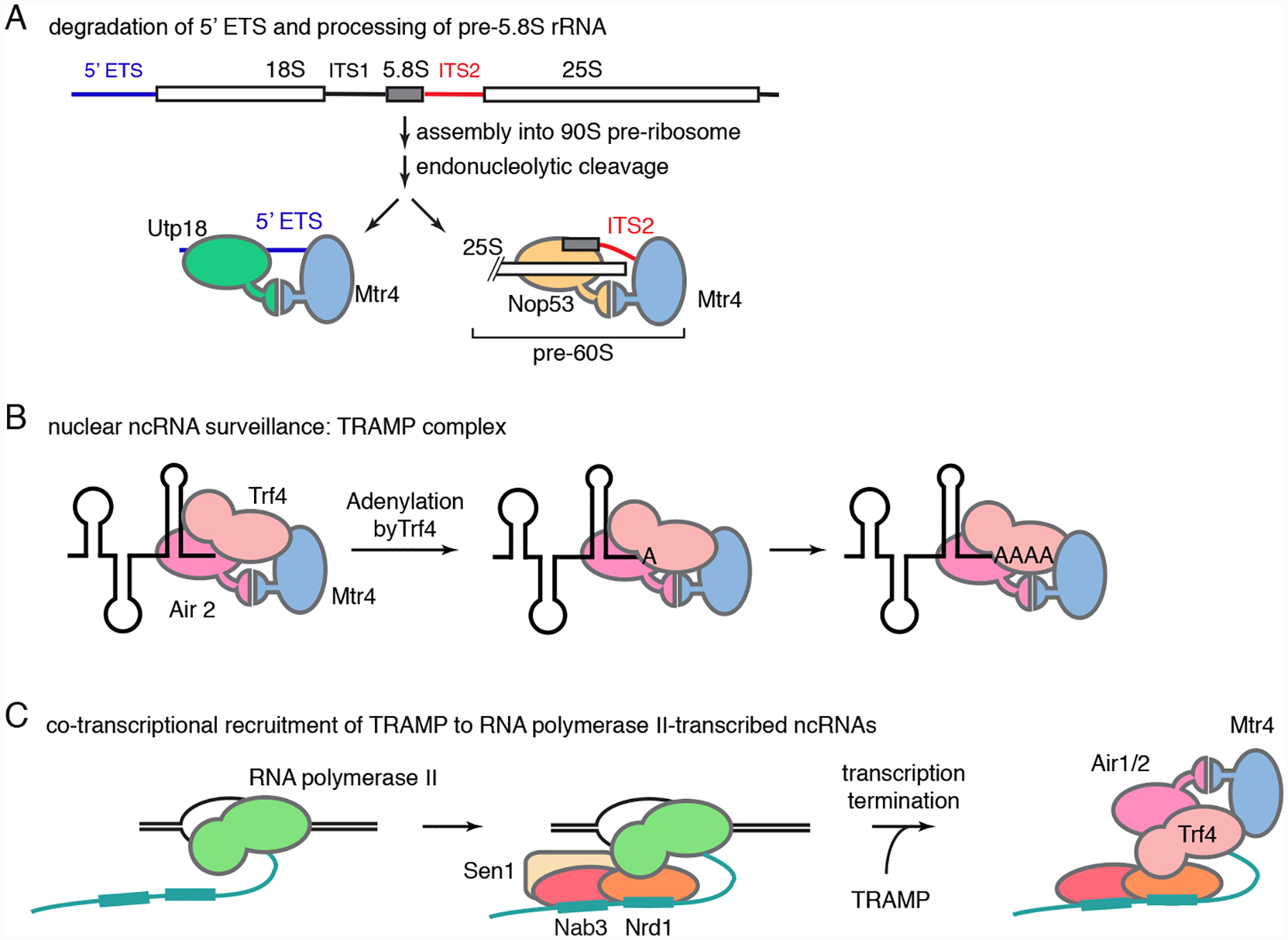
Recruitment of the nuclear exosome to its targets in budding yeast. (A) Following assembly of the pre-rRNA with proteins and snoRNAs to form the 90S pre-ribosome, and endonucleolytic cleavage to release the 5’ external transcribed spacer (5’ ETS), the Utp18 adaptor protein (left) binds the cleaved spacer and also binds the Mtr4 arch domain, targeting the 5’ ETS to the nuclear exosome for degradation117. After cleavage within ITS1 to separate pre-40S from pre-60S ribosomes, and cleavage within ITS2 to separate pre-25S rRNA from pre-5.8S rRNA, Nop53 interacts with pre-60S ribosomal subunits (right) and also with the Mtr4 arch, an interaction required for the exosome to mature the 5.8S rRNA 3’ end117. (B) TRAMP, which consists of the Trf4 oligo(A) polymerase, the Air2 RNA-binding protein, and the Mtr4 helicase, adds a short A tail to RNAs destined for degradation and recruits the exosome. Both Trf4 and Air2 contact Mtr4. Air2 interacts with both the Mtr4 helicase domain and the arch domain, with the contacts to the Mtr4 arch resembling those of Nop53 and Utp18118,119. (C) For RNA polymerase II transcripts, the Nrd1-Nab3 heterodimer of the Nrd1-Nab3-Sen1 complex recognizes short motifs on the nascent ncRNA. Nrd1 also interacts with the C-terminal domain of the large subunit of the polymerase. Following release of the polymerase, Nrd1 interacts with Trf4 to recruit TRAMP and the exosome to the nascent ncRNA120–123.
Utp18 associates with 90S pre-ribosomes and is important for degradation of the 5’ external transcribed spacer, while Nop53 binds pre-60S ribosomal subunits and is required for 5.8S rRNA processing117. Remarkably, both Utp19 and Nop53 use a similar short sequence motif (AIM, for arch interaction motif) to interact with Mtr4117. These data reveal that Mtr4 acts as a scaffold to link the exosome to adaptor proteins and their bound RNAs. Additional examples of this role are described below.
3.3.3.1. The TRAMP complex.
The TRAMP (Trf4-Air2-Mtr4 polyadenylation) complex is a major contributor to ncRNA surveillance in budding yeast. TRAMP, which adds a short A tail to ncRNAs destined for degradation10,38,41, was first identified because mutations in its Trf4 catalytic subunit prevent degradation of a hypomodified form of tRNAiMet 30. Other ncRNAs targeted by TRAMP include aberrant 23S rRNA-processing intermediates38, unspliced and/or unprocessed pre-tRNAs46,65, truncated 5S rRNAs and SRP RNAs46,67, snoRNAs that fail to assemble with core proteins47, U6 snRNA33,67, antisense ncRNAs124,125 and CUTs10. TRAMP consists of a non-canonical oligo(A) polymerase (Trf4), an RNA-binding subunit (the zinc knuckle protein Air2) and the RNA helicase Mtr4 (Figure 3B). The tails added by TRAMP, which average 3–5 A residues65,126, are too short to be bound by the poly(A) binding protein and thus provide an accessible single-stranded end for Mtr4 binding and subsequent exoribonuclease degradation. Additionally, through interactions between the Mtr4 N-terminus and Rrp6/Rrp47, Mtr4 recruits TRAMP-bound RNAs to the nuclear exosome107. TRAMP is primarily nucleoplasmic, and a structurally related complex, TRAMP5 (Trf5-Air1-Mtr4), targets nucleolar RNAs for degradation by the nuclear exosome125,127–129.
Structural and biochemical studies have begun to reveal both the architecture of TRAMP and the way in which it recognizes RNA targets. Air2 contains unstructured N- and C-termini that bracket five zinc knuckles, elements which interact with single-stranded RNA in retrovirus nucleocapsid proteins130. The Air2 N-terminus interacts with the helicase core and arch of Mtr4, the fourth and fifth zinc knuckles interact with Trf4, while the remaining zinc knuckles are implicated in RNA binding118,131–134. Interestingly, the Air2 contacts with the Mtr4 arch resemble those of Utp18 and Nop53, suggesting that these adaptor proteins may compete with Air2 for Mtr4 binding118,119. The N-terminus of Trf4 also interacts with the Mtr4 helicase core, with Trf4/Air2 positioned such that RNA could thread from Mtr4 to the Trf4 active site118.
3.3.3.2. Recruitment of TRAMP to ncRNA targets.
In yeast, targeting of TRAMP and the nuclear exosome to RNA polymerase II-transcribed ncRNAs, such as CUTs and pre-snoRNAs, occurs co-transcriptionally. Coupling is mediated by the Nrd1-Nab3-Sen1 complex, which is recruited to the transcribing polymerase. The Nrd1-Nab3 heterodimer recognizes short sequence motifs on nascent ncRNAs65,120,121 and interacts both with TRAMP and with the carboxyl-terminal domain (CTD) of the large subunit of RNA polymerase II122 (Figure 3C). Because the form of the CTD recognized is phosphorylated on Serine 5 of the heptad repeat (Tyr1-Ser2-Pro3-Thr4-Ser5-Pro6-Ser7)122,135, a modification that peaks early in elongation136, Nrd1-Nab3-Sen1 binds preferentially to short transcripts. Binding of the Sen1 helicase to the nascent transcript, together with ATP hydrolysis, results in transcription termination and release of the nascent ncRNA137.
Remarkably, the association between the Nrd1-Nab3-Sen1 complex and TRAMP involves interactions between the CTD-interacting domain of Nrd1 and a motif in the Trf4 C-terminus that mimics a Ser5-phosphorylated heptad repeat123. Thus, following dissociation of the CTD from Nrd1, the Nrd1-Nab3 complex uses similar interactions between Nrd1 and Trf4 to recruits TRAMP to the newly terminated RNAs. Moreover, since both Nrd1 and Nab3 also interact directly with Rrp6123,138,139, they may also contribute to recruiting the exosome.
Although binding of the TRAMP/exosome complex to newly transcribed CUTs results in degradation of these ncRNAs140,141, binding to newly transcribed snoRNAs can result in either degradation of the entire transcript or exonucleolytic removal of the 3’ extension to form mature snoRNAs47,138,142. For snoRNAs, those precursors that successfully assemble with their protein partners may be protected against further degradation following removal of the 3’ extension, while those pre-snoRNAs that fail to assemble into snoRNPs will be degraded47. In the case of CUTs, the lack of specific binding partners may result in their complete degradation.
Interestingly, purified recombinant Mpp6 and a peptide spanning the Trf4 C-terminus were found to compete with each other for binding to Nrd1111. Consistent with a model in which Mpp6 and Trf4 bind to similar sites on Nrd1, binding of Mpp6 to Nrd1 requires a sequence near the Mpp6 C-terminus that strongly resembles the Trf4 sequence that interacts with Nrd1111. These findings support a model in which Nrd1 can hand nascent RNA polymerase II transcripts to either Trf4 or Mpp6111. Whether the choice of exosome cofactors is stochastic, or whether features of the nascent ncRNAs and/or other co-factors contribute, remains to be determined.
There is now evidence that Nrd1 and Nab3 can target some RNA polymerase I and III transcripts for polyadenylation by TRAMP and degradation by the nuclear exosome65,143,144. In UV crosslinking studies, Nrd1 and Nab3 were found to crosslink to many RNA polymerase III transcripts, including 5S rRNA, pre-RNase P RNA, and pre-tRNAs65,143. Many of these RNAs contained non-templated A tails added by TRAMP. Consistent with a model in which Nab1-Nab3 binds post-transcriptionally to some RNA polymerase III transcripts and targets them to TRAMP, depletion of either Nab1 or Nab3 resulted in reduced levels of polyadenylated pre-RNase P RNAs and accumulation of unspliced pre-tRNAs65. In support of a role for Nrd1-Nab3 in also targeting defective rRNAs for decay, aberrant polyadenylated pre-rRNAs accumulate in yeast containing mutations in Rrp6, Nrd1 or Nab3144. For RNA polymerase I transcripts, Nrd1-Nab3 binding may be co-transcriptional and mediated by interactions between Nrd1 and sequences in the C-terminus of Spt5, a transcription elongation factor144.
How does TRAMP identify other substrates, such as already terminated RNA polymerase III transcripts and/or truncated ncRNAs, for targeting to the nuclear exosome? One major determinant is an accessible 3’ single-stranded end. In vitro, TRAMP only adenylates substrates containing a 3’ overhang of at least one nucleotide126,131. Consistent with a requirement for a single-stranded 3’ end, TRAMP competes with La, a protein that binds the 3’ end of all newly made RNA polymerase III transcripts, for binding to a hypomodified pre-tRNAiMet 53,55 (Figure 4).
Figure 4.
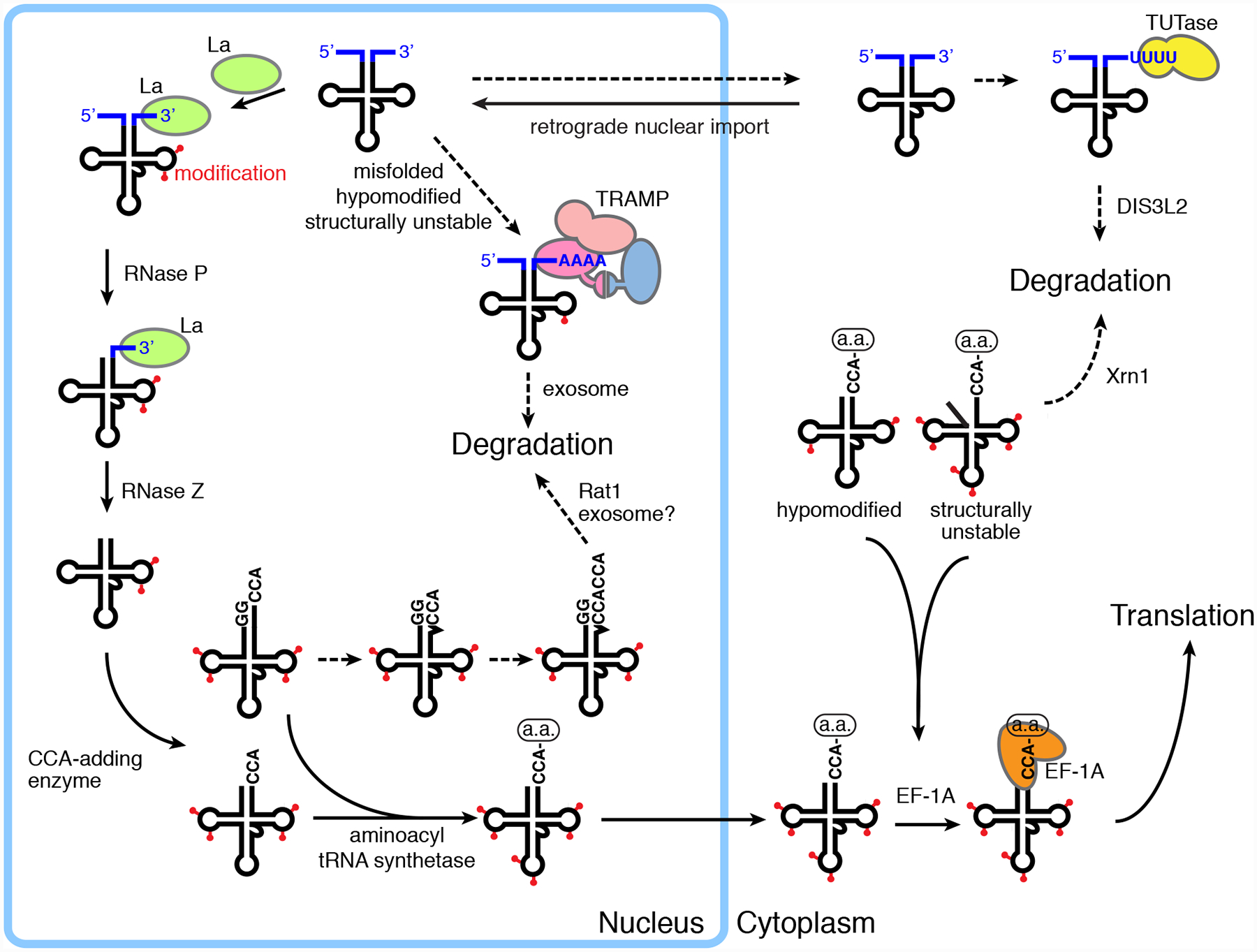
tRNA biogenesis competes with tRNA surveillance pathways. The major steps in tRNA biogenesis are depicted with solid arrows. Some tRNAs also contain intervening sequences that are removed by splicing, an event that takes place in the nucleus in mammals and the cytoplasm in yeast. Steps in which misfolded, unprocessed, unstable tRNAs accumulate and/or are targeted for degradation are indicated with dashed arrows. While most depicted steps occur in both yeast and mammals, TUTases and DIS3L2 are not present in budding yeast.
However, TRAMP must also recognize other determinants, since in vitro, both TRAMP and a Trf4-Air2 subcomplex preferentially polyadenylate the misfolded forms of several tRNAs compared with their correctly folded counterparts41,131. As the mutant tRNAs contain alterations that disrupt conserved stems and/or tertiary contacts needed to form the canonical three-dimensional structure, the mutant tRNAs may be less compact and more likely than wild-type tRNAs to have single-stranded RNA available for binding by the Air2 zinc knuucles30,41,131. In this case, nascent pre-RNAs and other ncRNAs that contain mutations that cause them to misfold and/or fail to assemble with their correct proteins would be preferentially targeted by TRAMP.
Although most studies of TRAMP have been carried out in budding yeast, studies in fission yeast revealed that one role of TRAMP is to protect abundant ncRNAs, such as rRNAs and tRNAs, from entering the RNA interference pathway and competing with bona fide substrates of the Dicer1 endoribonuclease for cleavage145. Although in S. cerevisiae, a yeast lacking an RNA interference (RNAi) pathway, cells lacking both Trf4 and Trf5 are inviable146, Schizosaccharomyces pombe (S. pombe) cells lacking the single Trf4/Trf5 ortholog Cid14 are viable147, but have reduced siRNAs derived from heterochomatic regions and defects in heterochromatic silencing148. Sequencing of Argonaute-associated small ncRNAs in the cid14Δ cells revealed a highly skewed population, with large increases in short RNAs derived from rRNAs and tRNAs145. Thus, TRAMP may function in cells with a functional RNAi pathway to protect the integrity of this pathway.
3.4. The nuclear exosome in human cells
Although the human exosome resembles its yeast counterpart in overall structural organization, it can contain up to three distinct catalytic subunits that vary based on their subcellular location. EXOSC10/hRRP6, the homolog of yeast Rrp6, is predominantly nuclear, with strong enrichment in nucleoli76,85. DIS3 is also largely nuclear, but is excluded from nucleoli85,86. Human cells also contain a third exosome-associated 3’ to 5’ exonuclease, cytoplasmic DIS3L (DIS3-like), which is structurally similar to DIS3 but contains mutations in the PIN domain that inactivate the endoribonuclease86,149. These findings have led to a model in which the human exosome exists in multiple forms: a nucleolar form that contains only EXOSC10/hRRP6, a nuclear form that contains both EXOSC10/hRRP6 and DIS3, and cytoplasmic forms that contain either DIS3 or DIS3L and may also contain EXOSC10/hRRP685,86.
As in yeast, there is considerable overlap in the substrates of the various catalytic subunits (Table 1). For example, a major target of the human exosome are PROMPTs (promoter upstream transcripts), which resemble yeast CUTs in being capped and polyadenylated ncRNAs derived from bidirectional transcription upstream of canonical promoters70,150. Although the levels of specific PROMPTS increase 30 to 50-fold when the exosome is inactivated by depleting the core EXOSC3/hRRP40 subunit, only minor increases in these RNAs are seen when a single catalytic subunit is depleted85. Instead, simultaneous depletion of EXOSC10/hRRP6 and DIS3 is required to detect robust (15 to 30-fold) increases in PROMPTs, and depleting all three nucleases is required to raise PROMPT levels to that seen when a core subunit is depleted85. In support of functional redundancy, levels of the DIS3L exoribonuclease increase when EXOSC10/hRRP6 is depleted, and EXOSC10/hRRP6 increases when DIS3L or DIS3 is depleted85.
3.5. MTR4-containing complexes in human cells
3.5.1. TRAMP.
Human cells contain likely orthologs of all TRAMP subunits; however, the extent to which these proteins form a complex analogous to yeast TRAMP remains under investigation. Although a stable ternary complex has not been purified from mammalian cells, the Trf4 ortholog PAPD5/hTRF4–2 and the Mtr4 ortholog SKIV2L2/hMTR4 are detected in immunoprecipitates when an epitope-tagged version of the Air1/2 ortholog ZCCHC7 is overexpressed in human cells72,132. Moreover, as in yeast, where Trf4 and Mtr4 have reduced half-lives in air2 mutant strains, the levels of ZCCHC7 decrease when either PAPD5/hTRF4–2 or SKIV2L2/hMTR4 are depleted with siRNAs72. To date, the best documented function for the putative human TRAMP is the polyadenylation and degradation of prematurely terminated rRNA precursors71,72 (Figure 5A).
Figure 5.
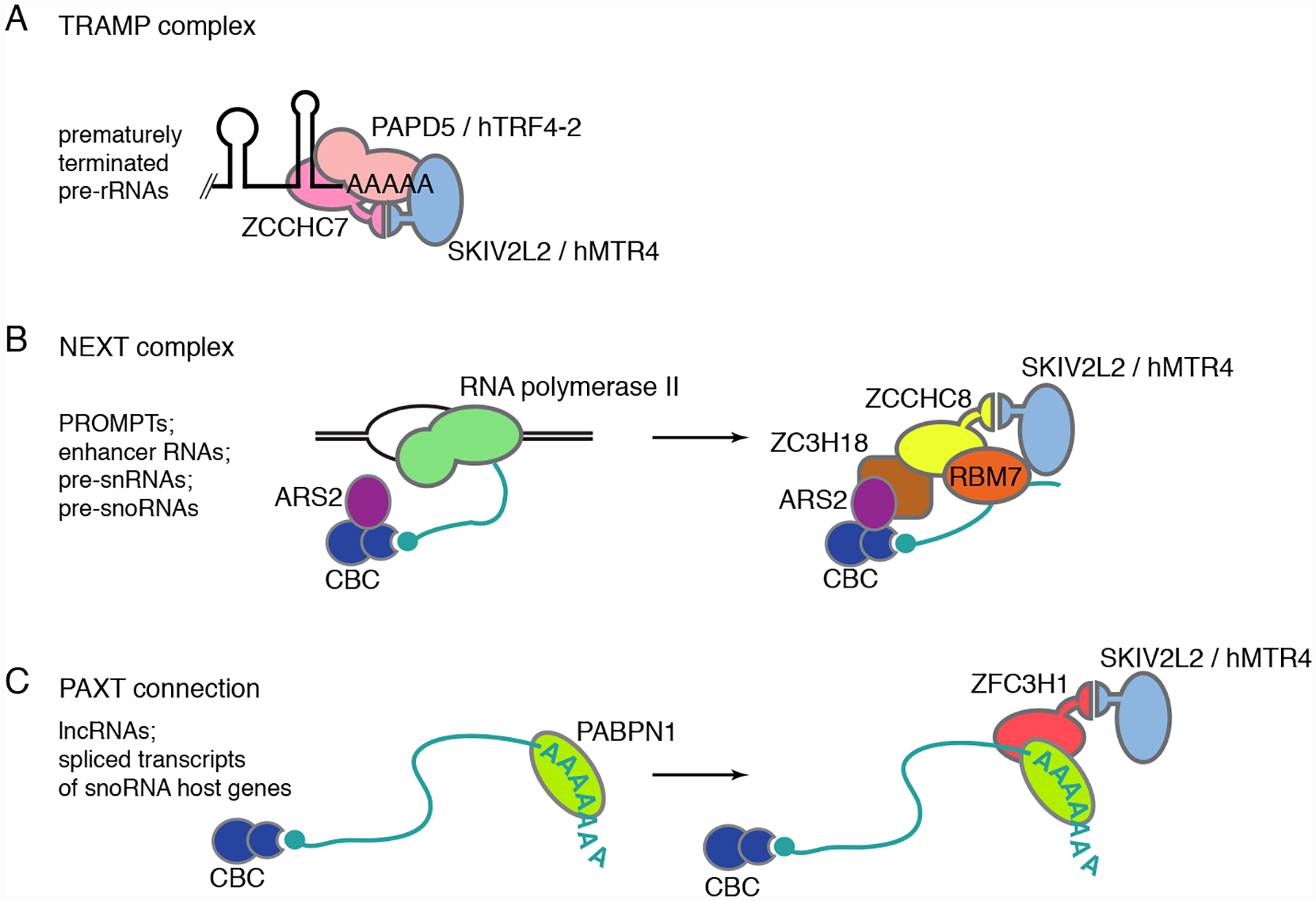
Mtr4 helicase-containing complexes in human cells. (A) A complex resembling yeast TRAMP targets prematurely terminated ribosomal precursors for degradation by the nucleolar exosome71,72. (B) The NEXT complex recognizes short capped RNA polymerase II transcripts, such as PROMPTs, enhancer RNAs and precursors to snoRNAs and snRNAs, during transcription and targets them for degradation by the nuclear exosome72,151. (C) Following polyadenylation of longer RNA polymerase II transcripts by the canonical poly(A) polymerases PAPα and PAPγ, interaction of PAXT with the nascent ncRNAs targets them for degradation by the nuclear exosome152,153. A related complex, consisting of ZFC3H1and SKIV2L2/hMTR4 but lacking PABPN1, has also been described154.
These transcripts accumulate when cells are treated with low concentrations of the transcription inhibitor actinomycin D and can also be detected in untreated cells71. Consistent with polyadenylation by a TRAMP polymerase and subsequent degradation by the nuclear exosome, depletion of either PAPD5/hTRF4–2 or ZCCHC7 reduces levels of the poly(A)-tailed rRNAs, while depleting SKIV2L2/hMTR4 or EXOSC10/hRRP6 results in increased levels of these RNAs71,72. Another possible substrate for a TRAMP-like complex is the RNA component of telomerase, called hTR. Those hTR RNAs that fail to assemble with their core protein dyskerin (DKC1) are degraded, in part through polyadenylation by PAPD5/hTRF4–2 and degradation by the EXOSC10/hRRP6-containing exosome59,155,156.
There is also evidence that PAPD5/hTRF4–2 can function independently of the Air1/2 ortholog ZCCHC7. Although yeast Trf4 and Trf5 lack RNA-binding modules and are inactive in polyadenylation without their Air1/2 partners41, PAPD5/hTRF4–2 contains a C-terminal RNA-binding domain and is active in polyadenylation in the absence of other proteins157. Also, in contrast to yeast, where Trf4 and Air2 co-localize to nuclei, ZCCHC7 is nucleolar, while PAPD5/hTRF4–2 and SKIV2L2/hMTR4 are both nucleolar and nucleoplasmic72. Since the only catalytic subunit of the nucleolar exosome is EXOSC10/hRRP6, the putative human TRAMP could target RNAs to this form of the exosome, while PAPD5/hTRF4–2 and SKIV2L2/hMTR4 may have additional substrates in the nucleoplasm. In this regard, it will be interesting to determine if ZCCHC7 is required for polyadenylation of unassembled forms of hTR RNA.
3.5.2. The NEXT complex.
In addition to its role in a putative TRAMP, SKIV2L2/hMTR4 is a component of other exosome adaptor complexes in human cells. Co-immunoprecipitation experiments, coupled with high-resolution mass spectrometry, revealed that SKIV2L2/hMTR4 associates with the zinc knuckle protein ZCCHC8 and the putative RNA-binding protein RBM7 to form the Nuclear Exosome Targeting (NEXT) complex72 (Figure 5B). RBM7 carries out RNA recognition, showing some preference for polyuridines151,158. ZCCHC8 functions as a scaffold, with separate binding sites for RBM7 and hMTR4, while hMTR4 likely contacts the exosome159. Unlike SKIV2L2/hMTR4, which is both nucleoplasmic and nucleolar and nucleoplasm, ZCCHC8 and RBM7 are exclusively nucleoplasmic72. The RNAs bound by NEXT are largely short newly synthesized RNA polymerase II transcripts, such as PROMPTs, enhancer RNAs and 3’ extended small nuclear and small nucleolar RNAs72,151,160. These ncRNA targets, together with the nucleoplasmic localization of ZCCHC8 and RBM7, support a model in which NEXT targets these ncRNAs for degradation by the nucleoplasmic form of the exosome.
Recruitment of NEXT to its ncRNA targets occurs early in their biogenesis, with binding partly dependent on interactions with the cap-binding complex (CBC)151,160 (Figure 5B). The CBC, which is composed of the CBP20 and CBP80 proteins, associates with the ARS2 protein to promote 3’ end processing of cap-proximal short transcripts, such as PROMPTs and pre-snRNAs161. Association of the CBC-ARS2 complex with NEXT is bridged by the zinc finger domain–containing protein ZC3H18, which interacts via protein-protein interactions with the ZCCHC8 component of NEXT162. As both cap formation and CBC binding occurs when ~20 nt of RNA have been synthesized, this coupling allows NEXT to be loaded onto nascent RNAs early in their biogenesis160. Upon premature termination of RNA polymerase II transcripts to form PROMPTs, NEXT is positioned to target these ncRNAs for degradation by the exosome.
3.5.3. The PAXT connection.
In addition to NEXT, whose targets are largely short unprocessed cap-containing ncRNAs, human cells contain a SKIV2L2/hMTR4-containing adaptor that recognizes longer processed RNA polymerase II transcripts and targets them to the nuclear exosome. In this pathway, called the PAXT [poly(A) tail exosome targeting] connection, poly(A) tailed ncRNAs are recognized by the nuclear poly(A)-binding protein PABPN1152,153,163. PABPN1 associates with the zinc finger protein ZFC3H1 through interactions that are partly RNase resistant, while ZFC3H1 binds SKIV2L2/hMTR4153 (Figure 5C). Thus, as in NEXT, a zinc finger protein (in this case, ZFC3H1) acts as a scaffold to bring a RNA binding protein and SKIV2L2/hMTR4 into proximity. Since the interaction between ZFC3H1 and PABPN1 partly depends on RNA153, ZFC3H1 may also contribute to RNA recognition.
Although the targets of NEXT and PAXT show considerable overlap, the RNAs that accumulate when PAXT components are depleted are, on average, longer than those detected when NEXT components are depleted. Prominent targets of PAXT include lncRNAs such as NEAT1 and TUG1, spliced transcripts of noncoding snoRNA host genes and prematurely terminated RNAs152–154,163. These and other PAXT targets also differ from the RNAs targeted by NEXT in that they are more likely to be polyadenylated153. Polyadenylation is required for recognition by PAXT, as PAPBN1 binding and exosome degradation of these lncRNA targets is reduced when cells are treated with cordycepin, an adenosine analog that inhibits poly(A) polymerase152,162,163. Polyadenylation is likely carried out by the canonical poly(A) polymerases PAPα and PAPγ, as the tails are longer than those added by TRAMP and co-depletion of PAPα and PAPγ results in accumulation of many of the same lncRNAs152. As a result, this degradation pathway has been named “PABPN1 and PAPα/γ-mediated decay” or PPD152.
How might PABPN1 and other components of the PAXT connection select ncRNAs for degradation? A possible clue comes from studies of the roles of polyadenylation and PABPN1 in the degradation of mRNAs lacking introns152, a class of mRNAs that are exported more slowly than their intron-containing counterparts164. In these studies, it was proposed that polyadenylated RNAs that are poorly exported to the cytoplasm are targets for PABPN1-mediated decay152. Consistent with this hypothesis, a β-globin mRNA reporter lacking introns undergoes PABPN1-mediated decay, while its intron-containing counterpart is exported efficiently and is stable165. In support of the idea that nuclear ncRNAs with an accessible poly(A) tail are targeted for decay by PABPN1, several viral and cellular ncRNAs that accumulate to high levels in the nucleus possess short poly(A) tails that are sequestered within triple helical structures, rendering the tails inaccessible to PABPN1 and the exosome49,50,166.
Interestingly, a second complex, consisting of SKIV2L2/hMTR4 and ZF3CH1, but lacking PABPN1, was described recently154. These authors showed that when either SKIV2L2/hMTR4 or ZF3CH1 are depleted, many target RNAs are exported to the cytoplasm and are found in polysome fractions154. Depletion of SKIV2L2/hMTR4 or ZF3CH1 was also associated with a shift of bona fide mature mRNAs to lighter polysome fractions, a decrease in the overall population of heavy polysomes and a reduction in overall translation154. Since many of the normally unstable lncRNAs that reach the cytoplasm when either SKIV2L2/hMTR4 or ZF3CH1 is depleted are likely to contain short open reading frames, these lncRNAs may compete with bona fide mRNAs for ribosome binding, resulting in decreased protein synthesis. The relationship of this complex, called “polysome protector complex”154, to PAXT remains to be investigated.
3.5.4. Other nuclear exosome adaptors in human cells.
Human cells contain additional adaptors that, similar to yeast Utp18 and Nop53, target specific ncRNAs to the nuclear exosome. One such adaptor is the DiGeorge syndrome critical region gene 8 (DGCR8) protein, a double-stranded RNA-binding protein best known for its role is assisting the RNase III endoribonuclease DROSHA in cleaving primary miRNAs (pri-miRNAs) to produce ~70 nt stem-loop containing pre-miRNAs167. In a role that is separate from this function, DGCR8 binds directly to mature human snoRNAs and to the telomerase RNA (hTR), and also associates with the EXOSC10/hRRP6-containing form of the exosome168. Consistent with a role as an exosome adaptor, DGCR8 is required for the association of mature snoRNAs and hTR with EXOSC10/hRRP6168. As the levels of these ncRNAs increase when DGCR8 or EXOSC10/hRRP6 is depleted, DGCR8 likely targets these RNAs for exosome degradation168. Similar experiments have implicated DGCR8 and the exosome in the degradation of ncRNAs called long intervening noncoding RNAs (lincRNAs) that initiate from their own promoters and do not overlap exons of protein-coding genes169. The mechanism by which DGCR8 identifies ncRNAs for exosome degradation has not been determined. However, given the specificity of DGCR8 for RNA hairpins170, coupled with the requirement of EXOSC10/hRRP6 for a single-stranded 3’ end, one possibility is that the ncRNAs targeted by DGCR8 for degradation by the nucleolar exosome contain protein-free RNA hairpins adjacent to single-stranded 3’ ends.
3.6. Role of the cytoplasmic exosome in ncRNA surveillance
Although the cytoplasmic exosome largely functions in mRNA degradation171, this form of the exosome functions in at least one ncRNA surveillance pathway in yeast, called nonfunctional rRNA decay (Section 7.2), and may also participate in the decay of excess newly synthesized ncRNAs that undergo nuclear export and degradation in the mammalian cytoplasm64,172. In yeast, the cytoplasmic exosome functions with the Ski complex, a heterotetramer consisting of the Mtr4-related helicase Ski2, two copies of the β-propeller protein Ski8, and the tetratricopeptide retreat protein Ski3173–175. The tetrameric Ski complex, which unwinds RNA and funnels the resulting single-stranded RNA into the exosome channel175, is tethered to the exosome via the Ski7 protein176. Biochemical and structural studies have shown that Ski7 binds to the exosome via a domain near its N-terminus92,176,177. This Ski7 domain wraps around the exosome, contacting the same surface formed by Csl4, Mtr3 and Rrp43 that is bound by the Rrp6 C-terminus in structures of the nuclear exosome92,177.
Although the mechanisms by which the Ski proteins and the cytoplasmic exosome contribute to ncRNA surveillance are largely unknown, the Ski complex was recently demonstrated to bind ribosomes that have stalled on mRNAs lacking stop codons178. The Ski complex binds directly to the stalled 40S ribosomal subunits, with interactions both to the 18S rRNA and to ribosomal proteins178. Conformational changes that occur upon Ski complex binding position the mRNA such that the 3’ end can enter the Ski2 helicase channel178. The functions of the cytoplasmic exosome have been less studied in human cells; however, humans possess orthologs of all four SKI proteins177,179,180 and the human Ski2 ortholog SKIV2L has been demonstrated to associate with 40S ribosomal subunits181.
4. URIDYLATION FOLLOWED BY DIS3L2 DEGRADATION
Although in budding yeast, degradation of most newly made ncRNAs occurs in nuclei182, recent studies in other species have revealed pathways in which these ncRNAs are exported to the cytoplasm for degradation. A prominent pathway in many organisms involves uridylation by terminal U transferases and degradation by DIS3L2, a 3’ to 5’ processive exoribonuclease, related in structure to DIS3 and DIS3L, that preferentially degrades uridylated RNAs183–185 (Table 1 and Figure 6).
Figure 6.
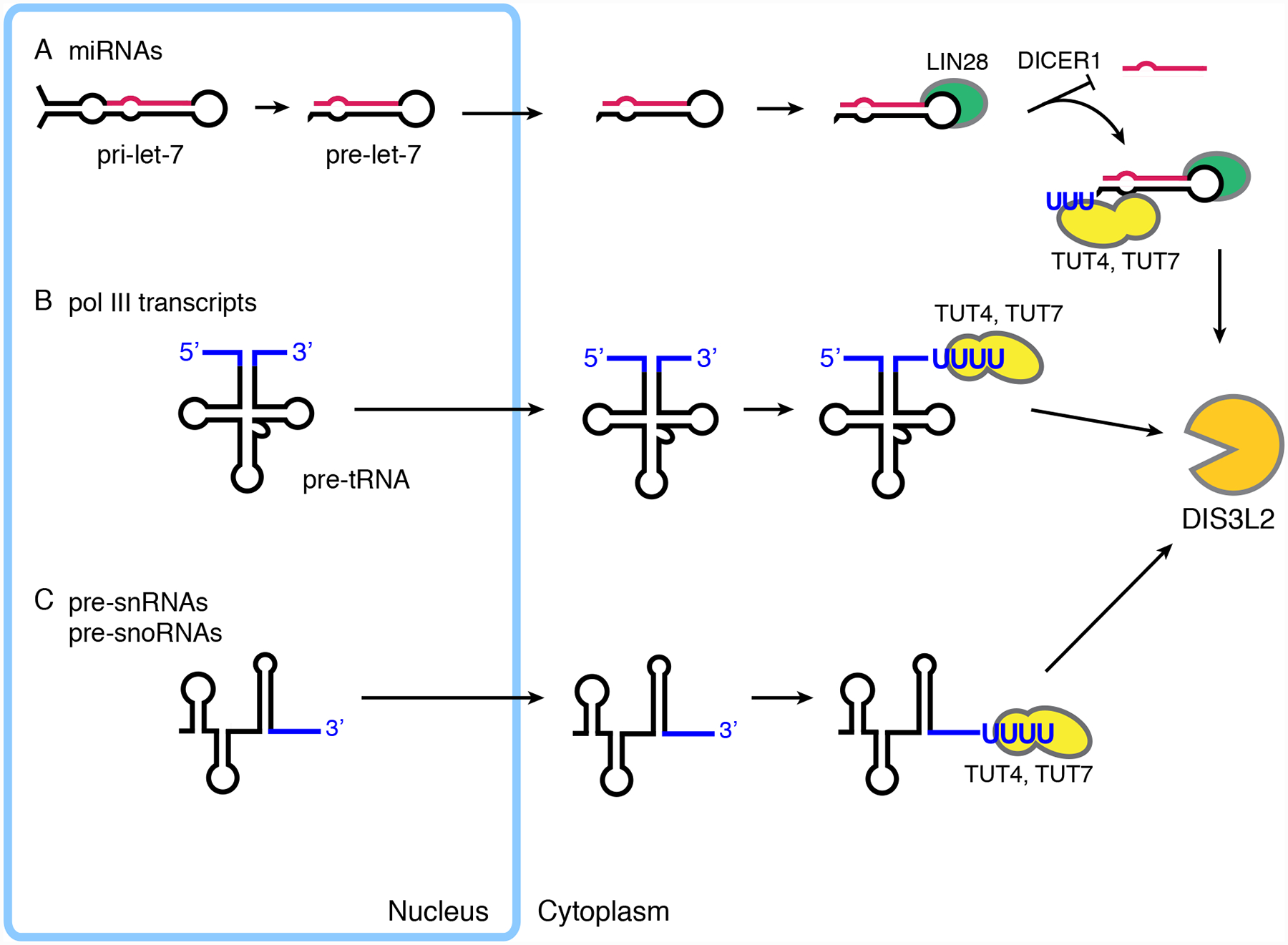
Newly synthesized ncRNAs can be exported to the cytoplasm, uridylated and degraded by DIS3L2. (A) In mouse and human embryonic stem cells, pre-let-7 miRNA is exported to the cytoplasm and bound by LIN28, which blocks cleavage by DICER1 and recruits TUT4 and TUT7 to the pre-miRNA. After uridylation, DIS3L2 degrades the pre-miRNA183,185–188. (B) Unprocessed forms of many RNA polymerase transcripts, including pre-tRNAs and 3’ extended forms of 7SL RNA, are exported to the cytoplasm, uridylated by TUT4 and TUT7 and degraded by DIS3L264,189–191. (C) 3’ extended pre-snRNAs and pre-snoRNAs can also be exported to the cytoplasm, uridylated by TUT4 and TUT7, and degraded by DIS3L2189–191.
Early evidence for this pathway came from the discovery that let-7 miRNA levels were regulated post-transcriptionally in mouse and human embryonic stem cells. Specifically, while the primary transcript encoding let-7 miRNA (pri-let-7 miRNA) was abundant, the mature let-7 miRNA was undetectable192,193. In these cells, after cleavage of pri-let-7 miRNA by the DROSHA endoribonuclease, the resulting pre-let-7 miRNA is exported to the cytoplasm and bound by LIN28, an RNA-binding protein that blocks processing and recruits two terminal uridyltransferases, TUT4 and TUT7186–188. These enzymes are members of the same family of non-canonical poly(A) polymerases as yeast Trf4 and Trf5194,195. Following uridylation, DIS3L2 degrades the pre-let-7 miRNA183–185 (Figure 6A).
A second line of evidence came from studies of RNA packaging by retroviruses. Although retroviruses such as Moloney murine leukemia virus (MLV) and the human immunodeficiency virus HIV-1 assemble in the cytoplasm, numerous newly synthesized ncRNAs, including precursors to tRNAs, small nuclear RNAs and small nucleolar RNAs, are highly enriched in virions64,196,197. Several of these ncRNAs, such as the 7SL RNA component of the signal recognition particle and cytoplasmic vault and Y RNAs, appeared to be packaged shortly after synthesis, before assembling with their usual protein partners64,198,199. Consistent with cytoplasmic recruitment, encapsidation of both pre-tRNAs and U6 snRNA by MLV was reduced when the nuclear export receptor Exportin-5 was depleted64. Remarkably, uridylated forms of unprocessed tRNAs and U6 snRNA accumulated in cells and virions when DIS3L2 was depleted, either by itself or together with the exosome64. Thus, retroviruses package some cellular ncRNAs from a surveillance pathway in which newly synthesized ncRNAs undergo nuclear export, poly(U) addition and degradation by DIS3L264.
Subsequent studies have established that a wide range of newly made ncRNAs undergo cytoplasmic uridylation and degradation by DIS3L2. Experiments in which the RNAs bound to catalytically inactive DIS3L2 mutants were immunoprecipitated from human, mouse and Drosophila melanogaster cells revealed numerous ncRNAs, including RNase MRP, 5S rRNA, 7SL RNA, pre-tRNAs, snoRNAs, snRNAs, Y RNAs, vault RNAs and pre-miRNAs189,190,200. Consistent with newly made ncRNAs, many of these RNAs contained 3’ extensions. The 3’ extended RNAs included RNAs normally made as precursors (such as pre-tRNAs, pre-snRNAs, and pre-snoRNAs; Figures 6B and 6C) as well as ncRNAs, such as 7SL RNA, in which the 3’ trailer was generated by failure to terminate at the normal RNA polymerase III termination site189,190,200. Many of these RNAs also contained short (less than 26 nt) U-tails189,190,200. Consistent with a decay pathway, the uridylated forms of these RNAs increased when DIS3L2 was depleted or absent189–191,200. Experiments in which TUT4 and TUT7 were depleted revealed that at least some uridylation was due to these enzymes190,191.
How conserved is ncRNA surveillance mediated by TUTases and DIS3L2? Although TUTases and DIS3L2 are absent in budding yeast, they are present in most eukaryotes194,195,201, and DIS3L2 contributes to mRNA decay in both fission yeast and plants201,202. Thus, the roles of TUTases and DIS3L2 in degrading newly synthesized aberrant ncRNAs may be widespread.
5. OTHER PATHWAYS THAT MAY INVOLVE 3’ TO 5’ EXORIBONUCLEASES.
5.1. CCACCA addition targets some tRNAs and tRNA-like ncRNAs for decay
The CCA-adding enzyme, which adds CCA to tRNA 3’ ends, assists in identifying structurally unstable tRNAs and tRNA-like ncRNAs and marking them for degradation (Figure 4). Specifically, this enzyme adds CCACCA, rather than the usual CCA, to the 3’ ends of certain tRNAs and tRNA-like ncRNAs with weak acceptor stems203. Structural and biochemical studies revealed that, following CCA addition, nucleotide binding to the enzyme active site results in a conformational change that produces torque on the bound tRNA, leading to release of tRNAs with stable acceptor stems204. For tRNAs with weak acceptor stems, the torque causes the stems to refold while the CCA-containing tRNA remains bound to the enzyme. For tRNAs that begin with 5’ GG, the two newly added Cs can basepair with the two Gs, allowing another round of CCA addition204. Although the enzymes that degrade the CCACCA end in vivo have not been identified, the CCACCA tail enhances degradation of tRNAs by 3’ to 5’ exoribonucleases in vitro203.
6. SURVEILLANCE PATHWAYS MEDIATED BY 5’ to 3’ EXORIBONUCLEASES
The major 5’ to 3’ exoribonucleases involved in ncRNA surveillance in eukaryotes are members of the XRN family205. Because these highly processive enzymes preferentially degrade single-stranded RNAs containing 5’-terminal monophosphates206, their substrates must undergo removal of either the 5’ triphosphate (RNA polymerase I and III transcripts) or the 5’ cap (RNA polymerase II transcripts) prior to degradation. In all studied organisms, distinct XRN family members carry out degradation in the nucleus and cytoplasm205. For example, budding yeast Xrn1 is largely cytoplasmic207 while the XRN2 ortholog Rat1 is nuclear208. Despite their disparate locations, these two enzymes are functionally similar, since expression of Rat1 in the cytoplasm is sufficient to alleviate the growth phenotypes of xrn1Δ strains, and targeting of Xrn1 to the nucleus complements the temperature-sensitivity of a rat1–1 mutant strain208.
6.1. ncRNA surveillance mediated by yeast Rat1
Noncoding RNAs that are targets of Rat1-mediated surveillance in S. cerevisiae include aberrant and excess pre-rRNA processing intermediates31, rRNA precursors in misassembled pre-ribosomes209, antisense and intergenic lncRNAs19, telomeric repeat-containing ncRNAs22, some hypomodified and/or structurally compromised tRNAs210, and the noncoding transcripts that remain associated with RNA polymerase II following 3’ end cleavage of the nascent pre-mRNA211,212 (Table 1). Consistent with the requirement of Rat1 for a 5’ monophosphate, all these substrates undergo either decapping (in the case of lncRNAs19) or endonucleolytic cleavage (e.g., pre-rRNA processing intermediates and mature tRNAs31,209,210) to generate this end. Indeed, for some yeast lncRNAs, degradation is regulated at the level of decapping19.
In both budding and fission yeast, Rat1 co-purifies with the Rai1 protein, which stabilizes Rat1 exonuclease activity and increases the ability of Rat1 to degrade structured RNAs213,214. S. pombe Rai1, but not S. cerevisiae Rai1, also possesses pyrophosphohydrolase activity, which allows it to convert 5’ triphosphates to 5’ monophosphates214,215. Moreover, all known fungal Rai1 enzymes possess a decapping endonuclease activity that allows them to remove the entire unmethylated cap (GpppN) from mRNAs215,216. (This activity is distinct from that of classical cytoplasmic decapping enzymes, such as yeast Dcp2, which cleave the m7GpppN cap to form m7Gpp and 5’ phosphate containing mRNA217.) The Rai1 decapping endonuclease activity is important for degrading immature mRNAs when S. cerevisiae is subjected to nutritional stress218; however, a role has not been reported in ncRNA decay. Nonetheless, the decapping endonuclease activity could potentially assist Rat1 in degrading nascent RNA polymerase II transcribed ncRNAs, such as CUTs, telomeric repeat containing RNAs and pre-snRNAs and pre-snoRNAs. Similarly, for species in which Rai1 is also a pyrophosphohydrolase, this activity could potentially convert newly made RNA polymerase III transcripts into Rat1 substrates.
6.2. Xrn1 and Rat1 monitor tRNA integrity
A pathway that monitors the integrity of many mature tRNAs is rapid tRNA decay (RTD). Described primarily in budding yeast, RTD degrades mature tRNAs that contain mutations that destabilize structure and/or lack nucleotide modifications important for structural stability219–221. Degradation is carried out largely by Rat1 and Xrn1210, consistent with the fact that mature tRNAs contain 5’ monophosphates, making them substrates for these exonucleases.
How are mature tRNAs recognized as aberrant? Comprehensive mutagenesis of a nonsense suppressor tRNA revealed that mutations throughout the tRNA can result in RTD221. These data, together with experiments studying other tRNAs, are largely consistent with a model in which mutations that increase accessibility of the 5’ end to exoribonucleases confer sensitivity to RTD220,221. In support of models in which ncRNA surveillance occurs in kinetic competition with ncRNA biogenesis222, RTD occurs in competition with binding of proteins such as tRNA synthetases and EF1A to the mature tRNAs223,224 (Figure 4).
As CCACCA has been detected at the 3’ end of several tRNAs that are RTD targets203, the CCA adding enzyme may also contribute to recognition of RTD substrates. Experiments in which tRNA degradation was studied in vitro revealed that, although the CCACCA tail enhanced degradation by 3’ to 5’ exoribonucleases, degradation was more efficient in the presence of Xrn1203. Thus, it was proposed that the CCACCA tail may allow 3’ to 5’ exoribonucleases to initiate decay, while Xrn1 and Rat1 are important for efficient degradation of the tRNA body203.
6.3. Xrn1 functions with nonsense-mediated decay to degrade some lncRNAs
Some unstable lncRNAs resemble mRNAs in that they are transcribed by RNA polymerase II, polyadenylated and exported to the cytoplasm, where they undergo decapping and degradation by Xrn115,225–227. This pathway has been best characterized in yeast, where intergenic and antisense ncRNAs that are Xrn1 targets are called XUTs (Xrn1-sensitive unstable transcripts)15. Although most XUTs do not encode functional proteins, degradation of many of these RNAs by Xrn1 requires nonsense mediated decay (NMD), a translation-dependent surveillance pathway that targets mRNAs containing premature stop codons for degradation228. Most XUTS are stabilized in yeast deleted for one or more of the NMD factors UPF1, UPF2, and UPF3225,227,229–231. Consistent with NMD, these XUTs contain one or more small open reading frames (ORFs) near the 5’ end of the RNA that are bound by ribosomes in ribosome-profiling experiments229,231. These lncRNAs also contain long ribosome-free 3’ UTRs229–231, consistent with findings that long 3’ UTRs contribute to targeting yeast and mammalian mRNAs for NMD232,233.
Some vertebrate lncRNAs may similarly be targeted for decay by NMD. As in yeast, many annotated zebrafish and mammalian lncRNAs are both ribosome-associated and contain long ribosome-free 3’ UTRs231,234–237. Moreover, many ribosome-bound lncRNAs increase in levels when UPF1 is depleted or when translation inhibitors that block NMD are present231,235,238,239. However, while XUTs are defined by their upregulation upon Xrn1 depletion15, the enzymes that carry out cytoplasmic degradation of mammalian lncRNAs are largely undescribed. In addition, the finding that many lncRNAs are ribosome-bound in both yeast and human cells raises the question of whether the RNAs are truly noncoding. Attempts to determine if the only role of the small ORFs is to target the lncRNA for NMD, or whether some ORFs encode proteins with additional functions, have largely focused on ORF size and conservation231,234,237,240.
6.4. Roles of human XRN2 in ncRNA surveillance
Some ncRNAs that are targets of Rat1 in budding yeast are also degraded by its human ortholog XRN2, including aberrant pre-rRNAs241 and the transcripts attached to RNA polymerase II following pre-mRNA 3’ cleavage212,242. XRN2 is also important for degrading some endogenous retrovirus transcripts243, and for the production of Transcription Start Site (TSS) RNAs, which are short RNAs protected by stalled RNA polymerase II from degradation244.
As in yeast, XRN2 functions with co-factors. These partner proteins, which are unrelated to Rai1, contain a conserved domain called the XRN2 binding domain (XTBD)245,246. This domain was first described in the nematode Caenorhabditis elegans, where the XTBD-containing protein PAXT-1 (Partner of XRN Two) stabilizes correctly folded XRN2245,246. Humans contain three proteins with predicted XTBD domains, two of which have been characterized functionally245–250. Rather than stabilizing XRN2, both proteins influence its subnuclear location and substrate targeting. One protein, the nucleolar NF-κB repressing factor (NKRF), is required for localization of XRN2 to nucleoli and for its roles in maturing pre-rRNAs and degrading aberrant pre-rRNAs249,250. NKRF acts as an adapter, as it also binds directly to pre-rRNAs and is required for association of XRN2 with pre-ribosomes250. NKRF also regulates XRN2 localization, since both NKRF and XRN2 redistribute to the nucleoplasm during heat stress, with new synthesis of NKRF required for XRN2 to return to nucleoli249. Interestingly, overexpression of a second XTBD-containing protein, nucleoplasmic CDKN2AIP/CARF, reduces the amount of XRN2 in nucleoli247. Thus, CARF and NKRF may act in opposing ways to regulate the distribution of XRN2 between the nucleoplasm and nucleoli.
6.5. The DXO/Rai1 family of de-NADding enzymes regulates levels of some mature snoRNAs and scaRNAs in human cells
A newly discovered class of 5’ to 3’ exoribonucleases that possess decapping activity are members of the DXO/Rai family. The founding member, S. cerevisiae Dxo1, is related in sequence to Rai1, the binding partner of Rat1. Similar to Rai1, Dxo1 exhibits decapping endonuclease activity on unmethylated capped RNAs251. Dxo1 differs from Rai1 in that it lacks pyrophosphohydrolase activity and is capable of decapping RNAs with m7GpppN caps, albeit with lower efficiency than RNAs with unmethylated caps251. Moreover, while Rai1 functions in the nucleus, Dxo1 is both nuclear and cytoplasmic251. Finally, Dxo1, but not Rai1, possesses distributive 5’ to 3’ exoribonuclease activity251.
Human cells contain a single ortholog of Dxo1 and Rai1, called DXO1. DXO1 possesses all three catalytic activities exhibited collectively by yeast Rai1 and Dxo1: pyrophosphohydrolase, decapping endonuclease and distributive 5’ to 3’ exoribonuclease activities218. DXO1 can also remove a 5’ nicotinamide adenine dinucleotide (NAD+) cap, a modification that occurs in some bacterial, yeast and human mRNAs252–254 and also in some snoRNAs and small Cajal body RNAs (scaRNAs)254. Upon DXO1 depletion, unprocessed mRNAs with unmethylated, immature caps accumulate, as do mRNAs and mature snoRNAs and scaRNAs with NAD+ caps218,254. All the affected snoRNAs and scaRNAs, similar to most mammalian snoRNAs and scaRNAs, are encoded within pre-mRNA introns and are matured from the excised debranched intron following splicing of the host pre-mRNA254. The finding that some mature snoRNAs and scaRNAs contain NAD+ caps implies the existence of a pathway that adds these caps post-transcriptionally to already mature snoRNAs, targeting them for DXO1 degradation. S. pombe Rai1 and Kluveromyces lactis Dxo1 can also remove NAD+ caps from RNA in vitro; however, the cellular substrates of these enzymes were not identified254.
6.6. Other ncRNA surveillance pathways that may involve 5’ to 3’ exoribonucleases
6.6.1. DUSP11-dependent ncRNA degradation.
The triphosphate that is the initial 5’ end of all RNA polymerase III transcripts acts as a barrier to digestion by XRN1, the major cytoplasmic exonuclease255. For some ncRNAs, such as tRNAs, the 5’ triphosphate-containing leader sequence is removed during maturation. However, for most polymerase III transcripts, including 5S rRNA, 7SL, Y RNAs, and vault RNAs, the mature ncRNAs retain the triphosphate. Recently, DUSP11 (dual specificity phosphatase 11), which removes the γ and β phosphates from triphosphate-containing transcripts256, was shown to act on vault ncRNAs and Alu transcripts in vivo257. As both vault and Alu RNAs increased by ~two-fold when DUSP11 was depleted from HEK293T cells257, these findings support a model in which, after removal of the 5’ triphosphate by DUSP11, these RNAs are degraded by a 5’ to 3’ exoribonuclease. DUSP11 is both nuclear and cytoplasmic258,259, making the subcellular location of this pathway unclear.
7. ADDITIONAL MECHANISMS OF NONCODING RNA SURVEILLANCE.
7.1. Retrograde tRNA nuclear import
Another mechanism by which defective tRNAs can be targeted for degradation and/or repair is retrograde nuclear import. In this pathway, cytosolic tRNAs undergo nuclear import, followed by re-export to the cytoplasm260,261. Although the full extent to which this pathway contributes to tRNA biogenesis remains under investigation, retrograde nuclear import is required for modification of at least one tRNA262.
In budding yeast, retrograde nuclear import is important for removing non-functional tRNAs from the cytoplasm263 (Figure 4). All eukaryotic tRNAs are synthesized as precursors with 5’ leader and 3’ trailer sequences that must be removed by processing. Afterwards, CCA is added to the 3’ end. Although the major tRNA export receptor in yeast, Los1/Exportin-t, preferentially binds end-matured, CCA-containing pre-tRNAs264, a small fraction of tRNAs with 5’ and 3’ extensions are exported to the cytoplasm263. The resulting pre-tRNAs cannot function in protein synthesis, since tRNAs with immature 3’ ends cannot undergo aminoacylation. As these aberrant tRNAs accumulate in the cytoplasm when nuclear re-import is blocked263, their normal fate may be to undergo nuclear re-import, followed by 3’ end maturation or degradation.
The retrograde tRNA nuclear import pathway also operates in mammalian cells265; however, the extent to which this pathway contributes to tRNA quality control in these cells is unclear. Although pre-tRNAs containing 5’ and 3’ extensions access the cytoplasm of mammalian cells, at least some of these aberrant pre-tRNAs undergo uridylation by TUTases and degradation by DIS3L2, a pathway that is absent in budding yeast64,189. Moreover, some misfolded pre-tRNAs that reach the cytoplasm undergo cleavage by the DICER1 endonuclease266, an enzyme that is also absent in S. cerevisiae. Thus, mammalian cells have additional pathways to prevent non-functional tRNAs from accumulating in the cytoplasm.
7.2. Nonfunctional rRNA decay
In addition to degradation of aberrant pre-rRNAs by the TRAMP/exosome pathway38,127–129 and degradation of pre-rRNAs in misassembled pre-60S ribosomes by Rat131,209, mature non-functional rRNAs are subject to surveillance. In this pathway, called nonfunctional rRNA decay (NRD), yeast 18S and 28S rRNAs containing point mutations that rendered them inactive in protein synthesis were found to be unstable267. Surveillance occurred after the rRNAs were assembled into their respective ribosomal subunits267. Degradation of the defective 40S subunits requires translation and involves the proteins Dom34 and Hbs1268, which rescue stalled ribosomes by promoting their dissociation into individual subunits269,270. Although the nucleases involved in degradation of the defective 18S rRNAs have not been fully identified, both Xrn1 and Ski7, which tethers the Ski complex to the cytoplasmic exosome175,176, are important for decay268. In contrast, degradation of the defective 60S ribosomal subunits requires the ubiquitination of 60S ribosomal subunits by a ubiquitin E3 ligase complex containing Rtt101 and its partner protein Mms1271. Following degradation of one or more ubiquitinated proteins by the proteasome, degradation of the defective 28S rRNA is carried out by unknown RNase(s)272.
Although NRD was uncovered by studying yeast containing rRNA mutations, this pathway may be important in wild-type cells when ribosomes are damaged by environmental stress. When yeast are treated with H2O2, many ribosomal proteins undergo oxidation and/or crosslinking to RNA273. Interestingly, strains lacking the Ski8 component of the Ski complex are hypersensitive to H2O2 stress274. Moreover, although yeast containing single deletions in Ski7, Dom34 or Hbs1 are similar to wild-type strains in their H2O2 sensitivity, yeast lacking both Ski7 and Dom34 or Ski7 and Hbs1 are hypersensitive274. Levels of ubiquitinated ribosomal proteins also increase upon H2O2 treatment, although this ubiquitination does not require Rtt101272.
Although 40S subunits containing mutations that render them inactive in translation have not been assayed in mammalian cells, a similar pathway likely exists to release nonfunctional ribosomes from mRNAs and target them for degradation. The human Dom34 and Hbs1 homologs PELO and HBS1L are important for recycling ribosomes that accumulate in 3’ untranslated regions during differentiation of a leukemic cell line to erythroid lineages275. Additionally, the mouse Dom34 homolog Pelota functions with the HBS1-related protein GTPBP2 to release ribosomes that stall when a particular tRNA is limiting276.
7.3. Noncoding RNA surveillance by components of the RNA interference machinery
In addition to their roles in RNA interference, components of the miRNA processing machinery contribute to degrading some structured ncRNAs. DGCR8 and DROSHA, which together form the Microprocessor that cleaves pri-miRNAs to pre-miRNAs167, cleave transcripts of Long Interspersed repetitive element 1 (LINE-1) retrotransposons to reduce their levels and activity in human cells277. DICER1, which is best known for its role in cleaving pre-miRNAs to release mature miRNAs, also cleaves other ncRNAs in human cells and C. elegans to reduce their abundance32,278. The ncRNAs whose levels are modulated by DICER1 include specific tRNAs, vault RNAs, Y RNAs and transcripts from the Alu family of repetitive elements278.
Remarkably, reduced levels of DICER1 in human retinal pigment epithelium are reported to cause a form of age-related macular degeneration known as geographic atrophy (GA)32. Specifically, decreased DICER1 is associated with accumulation of Alu transcripts in the retinal pigment epithelial cells of patients with GA32. These transcripts contribute to retinal pigment epithelial cell death, since introduction of oligonucleotides that target Alu sequences into DICER1-depleted retinal pigment epithelial cells increases cell viability32. As purified DICER1 cleaves Alu RNAs in vitro32, the data support a model in which DICER1, by cleaving Alu transcripts, prevents their accumulation in these cells.
7.4. Trafficking of unneeded ncRNAs into extracellular vesicles.
There is now evidence that some ncRNAs packaged within the small membrane-bound vesicles known as exosomes (no relation to the nuclease complex described above) consist of ncRNAs that have not complexed with their partner proteins. Similar to the cellular ncRNAs packaged by retroviruses64,196,197, these vesicles are enriched in SRP RNA, Y RNAs and repeat-derived RNAs and also contain ncRNAs that are normally confined to nuclei, such as snoRNAs and snRNAs279–281. As is the case for the ncRNAs within retroviral virions64,198, most protein partners of these ncRNAs are not detected within the vesicles. Since exosomes and retroviruses assemble using the same cellular machinery (ESCRT, the endosomal-sorting complex required for transport)282, they could potentially recruit their constituent ncRNAs from the same surveillance pathways. Although most attention has focused on the roles of extracellular vesicle ncRNAs in cell:cell communication282, packaging of excess ncRNAs into vesicles could serve to rid cells of unneeded ncRNAs. Consistent with this possibility, both circular RNAs (circRNAs) and specific noncoding RNAs were found to be enriched in extracellular vesicles, compared to their concentration in cells281,283,284. Since circRNAs cannot be degraded by exoribonucleases, it was suggested that packaging into extracellular vesicles may be a mechanism of removing these ncRNAs from cells284.
7.5. Noncoding RNA surveillance mediated by the Ro60 autoantigen
In some animal cell nuclei, the Ro 60 kDa (Ro60) protein is found complexed with misfolded variant pre-5S rRNAs and U2 snRNAs285–287. Structural studies have revealed that Ro60 folds to form a monomeric ring that binds the single-stranded 3’ ends of these RNAs in its central cavity and adjacent helices on its surface288,289. Because binding of Ro60 to misfolded ncRNAs is not strongly sequence-specific, it has been proposed that Ro60 scavenges RNAs that fail to bind their specific protein partners289. In all cells that have been examined, Ro60 also binds and stabilizes Y RNAs287,290,291. Although both the fate of the misfolded ncRNAs (re-folding vs. degradation) and the roles of Y RNAs are not well-understood in animal cells, a bacterial Ro60 is tethered by Y RNA to the ring-shaped 3’ to 5’ exoribonuclease PNPase43. In this bacterial RNA degradation machine, Ro60 is positioned such that RNA substrates can thread through the Ro60 ring into the PNPase cavity for degradation. Moreover, the presence of Ro60 and Y RNA increases the ability of PNPase to degrade structured RNAs43. However, Ro60 has not been described to associate with nuclease(s) in metazoans, making it unclear if Ro60 functions similarly in these cells.
8. COMPETITION WITH NONCODING RNA BIOGENESIS PATHWAYS
For all surveillance pathways involving classical ncRNAs, the cofactors that target defective ncRNAs for degradation and the enzymes that degrade these ncRNAs compete with the normal biogenesis pathways for access to the ncRNAs. Some examples are described below. In addition, Figure 4 depicts some ways in which components involved in normal tRNA biogenesis compete with components that recognize and degrade the aberrant and non-functional tRNAs.
8.1. Competition with chaperone proteins that bind nascent ncRNAs
The La autoantigen functions as a chaperone for many newly synthesized ncRNAs. This ~50 kDa phosphoprotein binds the 3’ end of all newly made RNA polymerase III transcripts, including pre-tRNAs292, pre-5S rRNAs292, pre-U6 snRNAs293, 7SL RNA294, Y RNAs295, vault RNAs296 and Alu transcripts297. La binds all these ncRNAs because its high affinity binding site is the UUUOH that is the initial 3’ end of all RNA polymerase III transcripts298. In budding yeast, La also binds processing intermediates of spliceosomal snRNAs and snoRNAs that terminate with UUUOH44,45,299. Because most of these ncRNAs undergo subsequent 3’ maturation, La binding is usually transient, such that La is not part of the mature ncRNP. Binding by La protects the 3’ ends of nascent ncRNAs from 3’ to 5’ exoribonucleases44,45,52,57,300,301, and can assist their folding and assembly into RNPs44,266,302,303. For pre-tRNAs, La can also influence the mechanism by which the 3’ leader sequence is matured, as La binding favors endonucleolytic removal of some pre-tRNA 3’ trailers by RNase Z46,52,304. In cells lacking La, the 3’ end of these tRNAs is matured by exoribonucleases46,52.
Binding by La protects multiple nascent ncRNAs from surveillance pathways. Consistent with competition between binding of La to ncRNA 3’ ends and targeting of the RNAs for degradation, the levels of some ncRNAs are reduced in yeast and Trypanosoma brucei cells lacking La55,305. In human cells, binding of La to specific pre-tRNAs prevents their folding into alternative structures that are cleaved by DICER to generate Argonaute 2-bound small RNAs266. The roles of La have been best studied in budding yeast, where La becomes essential when yeast contain mutations that disrupt tRNA structure52,302, eliminate nucleotide modifications important for stabilizing correctly folded tRNAs53,55,306 or impair assembly of La-bound snRNAs into snRNPs44,54,307. In many of the mutant strains, La binding allows the newly synthesized ncRNA to escape surveillance, enabling the ncRNA to undergo end maturation and/or assembly into functional RNPs52,54,55,302. Consistent with competition between La binding and targeting of the nascent ncRNAs for degradation, overexpression of La in yeast results in increased levels of a hypomodified tRNA that is normally targeted for decay by the TRAMP/exosome pathway53. Similarly, La overexpression results in increased U6 snRNA levels in yeast containing a mutation in a core U6 protein54 and increased levels of the 7SL RNA subunit of the signal recognition particle in cells deleted for SRP core proteins57. La overexpression also results in decreased levels of aberrant tRNA processing intermediates that are normally TRAMP targets46.
8.2. Competition with ncRNP assembly
Many ncRNAs, including U1, U4 and U6 snRNAs44,54, snoRNAs308–310, the 7SL RNA component of the signal recognition particle311, telomerase RNA312, Y RNAs290 and vault RNAs313 are present at reduced levels in cells when their core proteins are mutated or absent. For those unassembled ncRNAs whose decay pathways have been elucidated, surveillance pathways have been found to be responsible. For example, unassembled forms of the yeast U1 snRNA are degraded both by the TRAMP/nuclear exosome pathway and by decapping and Xrn1 degradation58, degradation of unassembled yeast 7SL RNAs requires TRAMP and the nuclear exosome57, and degradation of unassembled forms of U1 snRNA and telomerase RNA in human cells involves the nuclear exosome as well as decapping and Xrn1 decay58,59,172. Consistent with a model in which ncRNP assembly competes with surveillance, depletion of EXOSC10/hRRP6 and the decapping enzyme DCP2 restores telomerase activity in human cells depleted of dyskerin, a protein that stabilizes hTR RNA but is not required for activity59. Similarly, human cells depleted of the survival of motor neuron (SMN) protein, which functions in a complex that assists loading of the Sm core proteins onto the spliceosomal U snRNAs, have reduced levels of these snRNAs and defects in pre-mRNA splicing58,172. As co-depletion of the decapping enzyme Dcp2 partially restores U snRNA levels and ameliorates the splicing defects58, snRNP assembly occurs in competition with decapping of the unassembled RNPs.
8.3. Competition with enzymes that mature ncRNA 3’ ends
Some ncRNAs acquire 3’ end modifications that protect the RNAs from surveillance. One such ncRNA is the U6 snRNA, a component of the spliceosome. After transcription by RNA polymerase III and La binding54,293, U6 assembles with a heptameric ring complex comprised of the Lsm2-Lsm8 proteins54,314–316, undergoes 3’ uridylation by a U6-specific TUTase293,317, followed by 3’ trimming by the Usb1/Mpn1 exoribonuclease, which leaves a terminal cyclic 2’,3’ phosphate60,61,318. This 3’ end modification is highly conserved, as U6 snRNAs from many metazoans, plants and fission yeast all terminate with cyclic 2’,3’ phosphate61,318. In budding yeast, an organism that lacks TUTases194, the Usb1/Mpn1 exoribonuclease trims gene-encoded terminal uridylates, and most U6 terminates with a 3’ or 2’ monophosphate318. These 3’ end modifications prevent both La rebinding and adenylation by TRAMP, which like other poly(A) polymerases, requires that substrates contain 3’-OH41. USB1/MPNI is disrupted in patients with the rare autosomal recessive disease poikiloderma with neutropenia, and in patient-derived cell lines, U6 is less stable and contains non-templated oligo(A) tails61–63. Consistent with competition between U6 end modification by Usb1/Mpn1 and targeting for decay, U6 is less stable in budding and fission yeast depleted of Usb1/Mpn160,61,63. Other examples of end modifications that protect ncRNAs from surveillance include CCA addition and aminoacylation of tRNAs51 and the 3’ methylation that stabilizes germ-line specific ncRNAs called piRNAs319.
9. Conclusions and Perspectives
It is now clear that cells possess myriad ways to recognize and target aberrant ncRNAs for degradation. It is likely that most of the ribonucleases involved have been identified, and many of their adaptors are also known. High resolution structures exist for the most prominent nucleases involved in ncRNA surveillance, such as the RNA exosome, members of the XRN family, DIS3L2 and endoribonucleases such as DICER1. Much progress has also been made in identifying the nuclease cofactors that recognize defective ncRNAs; however less is known as to how they select their specific targets. For example, although there is good evidence that a protein-free accessible end is critical for recognition by both non-canonical poly(A) polymerases and exoribonucleases, it remains unclear whether other features of RNA structure, such as protein-free helices or single-stranded regions adjacent to the RNA end, contribute to recognition of specific ncRNAs by adaptor proteins. Since structural and biochemical investigations of most prominent nuclease cofactors, such as the TRAMP and NEXT complexes, are well underway, it should not be long before these studies, together with in vivo analyses, identify the exact requirements for binding.
Some surveillance targets, such as the defective mature rRNAs that are subject to NRD, are targeted for degradation as RNPs, yet the ways in which the protein components are handled and removed from the ncRNAs are largely unaddressed. For example, although protein components of inactive 60S ribosomal subunits undergo ubiquitination and degradation by the proteasome271,272, it is not known how the protein and rRNA degradation are coordinated, or if dedicated helicases exist that expose the rRNA for degradation. Moreover, although the defective rRNAs that assemble into inactive ribosomes267 are the clearest example of an RNP that must be dismantled for degradation, there may be other nonfunctional ncRNPs, such as snoRNPs and spliceosomal snRNPs, for which protein and RNA decay need to be coordinated. Moreover, although many ncRNAs that are targeted for surveillance are not bound by their normal protein partners57–59, it is unknown if other protein(s) are bound to these RNAs.
Other areas that are only beginning to be addressed are the ways in which damaged ncRNAs are targeted for degradation and how ncRNA repair systems interface with decay pathways. For example, ultraviolet light, reactive oxygen species, alkylating agents and chemotherapeutic agents such as cisplatin and mitomycin C can all damage RNA320,321, yet the full range of how these agents impact ncRNA function and how cells recognize and target the damaged RNAs for degradation remains poorly understood. Moreover, the widespread occurrence of RNA ligases322, coupled with evidence that they can repair damaged RNAs323, suggests that RNA repair systems may compete with degradation systems to handle ncRNAs rendered inactive by endonucleolytic cleavage.
Lastly, a major challenge is to elucidate the ways in which failure to eliminate aberrant and excess ncRNAs impacts human disease. This is particularly challenging in mammalian cells, since the abundance of surveillance pathways in these cells results in numerous redundancies in the ways in which ncRNAs can be degraded. Moreover, the multitude of mRNAs and ncRNAs targeted by most surveillance pathways vastly increases the difficulties in elucidating how a specific mutation results in human disease. Yet, the fact that recessive mutations in the exosome core subunits EXOSC3/hRRP40324 and EXOSC8/hRRP43325, the NEXT component RBM7326 and the DIS3L2 exoribonuclease327 all result in child-onset diseases with high mortality make deciphering the disease mechanisms a priority. Also, the finding that mutations that inactivate DIS3 are a common secondary event in the progression of multiple myeloma328 makes it important to understand how dysregulation of this exosome catalytic subunit contributes to cancer. The ongoing efforts to characterize large numbers of patient tumor transcriptomes329, coupled with the appropriate bioinformatics, may make it possible to formulate hypotheses that can be tested in model organisms and in patient-derived cell lines. Moreover, given reports that inappropriate activation of cytoplasmic innate immune sensors by endogenous ncRNAs contributes to both autoimmunity330 and cancer280, identifying compounds that boost ncRNA surveillance pathways could be a therapeutic strategy.
ACKNOWLEDGMENTS
The Wolin laboratory is supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, the Center for Cancer Research.
Biographies
Cedric Belair obtained his B.S. in Cell Biology and Physiology and his Ph.D. in Microbiology/Immunology from the University of Bordeaux, France. His doctoral project, performed under the supervision of Dr. Fabien Darfeuille, was to study the role of microRNAs in the response of gastric epithelial cells to infection by the bacterium Helicobacter pylori. In 2011, he became a postdoctoral associate in the laboratory of Dr. Sandra Wolin at Yale University in New Haven, CT, where he investigated the role of RNA surveillance pathways, most notably the RNA exosome, in human embryonic stem cell biology. He is currently a research fellow in the laboratory of Dr. Sandra Wolin at the National Cancer Institute in Frederick, Maryland, where he continues to investigate RNA surveillance pathways.
Soyeong Sim received both her B.S. in Chemistry and her Ph.D. in Biochemistry from the Korea Advanced Institute of Science and Technology, where she studied bacterial RNA processing under the direction of Dr. Younghoon Lee. She joined the group of Dr. Sandra Wolin at the Yale School of Medicine in New Haven, CT in 2003, where she was supported by a James Hudson Brown-Alexander Brown Coxe postdoctoral fellowship and by a postdoctoral fellowship from the Arthritis Foundation. In 2008, she became an associate research scientist in the same group. Currently, she is a staff scientist in the group of Dr. Sandra Wolin at the National Cancer Institute in Frederick, Maryland. Her research focuses on the function of ncRNAs and RNA binding proteins.
Sandra L. Wolin is Chief of the RNA Biology Laboratory at the National Cancer Institute in Frederick Maryland and heads the Center for Cancer Research’s RNA Initiative. She received her undergraduate degree in Biochemical Sciences from Princeton University, her M.D. from the Yale School of Medicine, and her Ph.D. degree in Molecular Biophysics and Biochemistry from Yale University, where she worked under the supervision of Dr. Joan Steitz. Her postdoctoral studies were carried out with Dr. Marc Kirschner and Dr. Peter Walter in the Department of Biochemistry and Biophysics at the University of California, San Francisco. She joined the faculty of Yale University in 1991, where she rose to become Professor of Cell Biology and Director of the Yale Center for RNA Science and Medicine. In 2017, she moved to the National Cancer Institute in Frederick, Maryland, to lead the newly established RNA Biology Laboratory.
REFERENCES
- (1).Hannon GJ; Rivas FV; Murchison EP; Steitz JA The expanding universe of noncoding RNAs. Cold Spring Harb. Symp. Quant. Biol 2006, 71, 551–564. [DOI] [PubMed] [Google Scholar]
- (2).Hogg JR; Collins K Structured non-coding RNAs and the RNP Renaissance. Curr. Opin. Chem. Biol 2008, 12, 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cech TR; Steitz JA The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [DOI] [PubMed] [Google Scholar]
- (4).Isken O; Maquat LE Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 2007, 21, 1833–1856. [DOI] [PubMed] [Google Scholar]
- (5).Shyu AB; Wilkinson MF; van Hoof A Messenger RNA regulation: to translate or to degrade. EMBO J. 2008, 27, 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ravid T; Hochstrasser M Diversity of degradation signals in the ubiquitin-proteasome system. Nat. Rev. Mol. Cell. Biol 2008, 9, 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Balch WE; Morimoto RI; Dillin A; Kelly JW Adapting proteostasis for disease intervention. Science 2008, 319, 916–919. [DOI] [PubMed] [Google Scholar]
- (8).Gudipati RK; Xu Z; Lebreton A; Seraphin B; Steinmetz LM; Jacquier A; Libri D Extensive degradation of RNA precursors by the exosome in wild-type cells. Mol. Cell 2012, 48, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bertone P; Stolc V; Royce TE; Rozowsky JS; Urban AE; Zhu X; Rinn JL; Tongprasit W; Samanta M; Weissman S et al. Global identification of human transcribed sequences with genome tiling arrays. Science 2004, 306, 2242–2246. [DOI] [PubMed] [Google Scholar]
- (10).Wyers F; Rougemaille M; Badis G; Rousselle J-C; Dufour M-E; Boulay J; Regnault B; Devaux F; Namane A; Seraphin B et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 2005, 121, 725–737. [DOI] [PubMed] [Google Scholar]
- (11).Davis CA; Ares M Jr. Accumulation of unstable promoter-associated transcripts upon loss of the nuclear exosome subunit Rrp6p in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 3262–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Chekanova JA; Gregory BD; Reverdatto SV; Chen H; Kumar R; Hooker T; Yazaki J; Li P; Skiba N; Peng Q et al. Genome-wide high-resolution mapping of exosome substrates reveals hidden features in the Arabidopsis transcriptome. Cell 2007, 131, 1340–1353. [DOI] [PubMed] [Google Scholar]
- (13).Kapranov P; Cheng J; Dike S; Nix DA; Duttagupta R; Willingham AT; Stadler PF; Hertel J; Hackermuller J; Hofacker IL et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 2007, 316, 1484–1488. [DOI] [PubMed] [Google Scholar]
- (14).Neil H; Malabat C; d’Aubenton-Carafa Y; Xu Z; Steinmetz LM; Jacquier A Widespread bidirectional promoters are the major source of cryptic transcripts in yeast. Nature 2009, 457, 1038–1042. [DOI] [PubMed] [Google Scholar]
- (15).van Dijk EL; Chen CL; d’Aubenton-Carafa Y; Gourvennec S; Kwapisz M; Roche V; Bertrand C; Silvain M; Legoix-Ne P; Loeillet S et al. XUTs are a class of Xrn1-sensitive antisense regulatory non-coding RNA in yeast. Nature 2011, 475, 114–117. [DOI] [PubMed] [Google Scholar]
- (16).Xu Z; Wei W; Gagneur J; Perocchi F; Clauder-Munster S; Camblong J; Guffanti E; Stutz F; Huber W; Steinmetz LM Bidirectional promoters generate pervasive transcription in yeast. Nature 2009, 457, 1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Core LJ; Waterfall JJ; Lis JT Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 2008, 322, 1845–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Seila AC; Calabrese JM; Levine SS; Yeo GW; Rahl PB; Flynn RA; Young RA; Sharp PA Divergent transcription from active promoters. Science 2008, 322, 1849–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Geisler S; Lojek L; Khalil AM; Baker KE; Coller J Decapping of long noncoding RNAs regulates inducible genes. Mol. Cell 2012, 45, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Houseley J; Rubbi L; Grunstein M; Tollervey D; Vogelauer M A ncRNA modulates histone modification and mRNA induction in the yeast GAL gene cluster. Mol. Cell 2008, 32, 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Camblong J; Iglesias N; Fickentscher C; Dieppois G; Stutz F Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation in S. cerevisiae. Cell 2007, 131, 706–717. [DOI] [PubMed] [Google Scholar]
- (22).Luke B; Panza A; Redon S; Iglesias N; Li Z; Lingner J The Rat1p 5′ to 3′ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol. Cell 2008, 32, 465–477. [DOI] [PubMed] [Google Scholar]
- (23).Wahba L; Amon JD; Koshland D; Vuica-Ross M RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol. Cell 2011, 44, 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lim J; Giri PK; Kazadi D; Laffleur B; Zhang W; Grinstein V; Pefanis E; Brown LM; Ladewig E; Martin O et al. Nuclear proximity of Mtr4 to RNA exosome restricts DNA mutational asymmetry. Cell 2017, 169, 523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Pefanis E; Wang J; Rothschild G; Lim J; Kazadi D; Sun J; Federation A; Chao J; Elliott O; Liu ZP et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell 2015, 161, 774–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Fabre A; Badens C Human Mendelian diseases related to abnormalities of the RNA exosome or its cofactors. Intractable Rare Dis. Res 2014, 3, 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Pashler AL; Towler BP; Jones CI; Newbury SF The roles of the exoribonucleases DIS3L2 and XRN1 in human disease. Biochem. Soc. Trans 2016, 44, 1377–1384. [DOI] [PubMed] [Google Scholar]
- (28).Li Z; Reimers S; Pandit S; Deutscher MP RNA quality control: degradation of defective transfer RNA. EMBO J. 2002, 21, 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Cheng ZF; Deutscher MP Quality control of ribosomal RNA mediated by polynucleotide phosphorylase and RNase R. Proc. Natl. Acad. Sci. U. S. A 2003, 100, 6388–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kadaba S; Krueger A; Trice T; Krecic AM; Hinnebusch AG; Anderson J Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 2004, 18, 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Fang F; Phillips S; Butler JS Rat1p and Rai1p function with the nuclear exosome in the processing and degradation of rRNA precursors. RNA 2005, 11, 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kaneko H; Dridi S; Tarallo V; Gelfand BD; Fowler BJ; Cho WG; Kleinman ME; Ponicsan SL; Hauswirth WW; Chiodo VA et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 2011, 471, 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Schneider C; Kudla G; Wlotzka W; Tuck A; Tollervey D Transcriptome-wide analysis of exosome targets. Mol. Cell 2012, 48, 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Sulthana S; Basturea GN; Deutscher MP Elucidation of pathways of ribosomal RNA degradation: an essential role for RNase E. RNA 2016, 22, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lebreton A; Tomecki R; Dziembowski A; Seraphin B Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature 2008, 456, 993–996. [DOI] [PubMed] [Google Scholar]
- (36).Schaeffer D; Tsanova B; Barbas A; Reis FP; Dastidar EG; Sanchez-Rotunno M; Arraiano CM; van Hoof A The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat. Struct. Mol. Biol 2009, 16, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Schneider C; Leung E; Brown J; Tollervey D The N-terminal PIN domain of the exosome subunit Rrp44 harbors endonuclease activity and tethers Rrp44 to the yeast core exosome. Nucleic Acids Res. 2009, 37, 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).LaCava J; Houseley J; Saveanu C; Petfalski E; Thompson E; Jacquier A; Tollervey D RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 2005, 121, 713–724. [DOI] [PubMed] [Google Scholar]
- (39).Py B; Higgins CF; Krisch HM; Carpousis AJ A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature 1996, 381, 169–172. [DOI] [PubMed] [Google Scholar]
- (40).de la Cruz J; Kressler D; Tollervey D; Linder P Dob1p (Mtr4p) is a putative ATP-dependent RNA helicase required for the 3′ end formation of 5.8S rRNA in Saccharomyces cerevisiae. EMBO J. 1998, 17, 1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Vanacova S; Wolf J; Martin G; Blank D; Dettwiler S; Friedlein A; Langen H; Keith G; Keller W A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 2005, 3, e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Bonneau F; Basquin J; Ebert J; Lorentzen E; Conti E The yeast exosome functions as a macromolecular cage to channel RNA substrates for degradation. Cell 2009, 139, 547–559. [DOI] [PubMed] [Google Scholar]
- (43).Chen X; Taylor DW; Fowler CC; Galan JE; Wang HW; Wolin SL An RNA degradation machine sculpted by Ro autoantigen and noncoding RNA. Cell 2013, 153, 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Xue D; Rubinson DA; Pannone BK; Yoo CJ; Wolin SL U snRNP assembly in yeast involves the La protein. EMBO J. 2000, 19, 1650–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kufel J; Allmang C; Chanfreau G; Petfalski E; Lafontaine DL; Tollervey D Precursors to the U3 small nucleolar RNA lack small nucleolar RNP proteins but are stabilized by La binding. Mol. Cell. Biol 2000, 20, 5415–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Copela LA; Fernandez CF; Sherrer RL; Wolin SL Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation. RNA 2008, 14, 1214–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Grzechnik P; Kufel J Polyadenylation linked to transcription termination directs the processing of snoRNA precursors in yeast. Mol. Cell 2008, 32, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Li Z; Pandit S; Deutscher MP 3′ exoribonucleolytic trimming is a common feature of the maturation of small, stable RNAs in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A 1998, 95, 2856–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Brown JA; Valenstein ML; Yario TA; Tycowski KT; Steitz JA Formation of triple-helical structures by the 3′-end sequences of MALAT1 and MENbeta noncoding RNAs. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 19202–19207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Wilusz JE; JnBaptiste CK; Lu LY; Kuhn CD; Joshua-Tor L; Sharp PA A triple helix stabilizes the 3′ ends of long noncoding RNAs that lack poly(A) tails. Genes Dev. 2012, 26, 2392–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Dutta T; Malhotra A; Deutscher MP How a CCA sequence protects mature tRNAs and tRNA precursors from action of the processing enzyme RNase BN/RNase Z. J. Biol. Chem 2013, 288, 30636–30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Yoo CJ; Wolin SL The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell 1997, 89, 393–402. [DOI] [PubMed] [Google Scholar]
- (53).Anderson J; Phan L; Cuesta R; Carlson BA; Pak M; Asano K; Bjork GR; Tamame M; Hinnebusch AG The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998, 12, 3650–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Pannone B; Xue D; Wolin S A role for the yeast La protein in U6 snRNP assembly: evidence that the La protein is a molecular chaperone for RNA polymerase III transcripts. EMBO J. 1998, 17, 7442–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Calvo O; Cuesta R; Anderson J; Gutierrez N; Garcia-Barrio MT; Hinnebusch AG; Tamame M GCD14p, a repressor of GCN4 translation, cooperates with Gcd10p and Lhp1p in the maturation of initiator methionyl-tRNA in Saccharomyces cerevisiae. Mol. Cell. Biol 1999, 19, 4167–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Kucera NJ; Hodsdon ME; Wolin SL An intrinsically disordered C terminus allows the La protein to assist the biogenesis of diverse noncoding RNA precursors. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Leung E; Schneider C; Yan F; Mohi-El-Din H; Kudla G; Tuck A; Wlotzka W; Doronina VA; Bartley R; Watkins NJ et al. Integrity of SRP RNA is ensured by La and the nuclear RNA quality control machinery. Nucleic Acids Res. 2014, 42, 10698–10710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Shukla S; Parker R Quality control of assembly-defective U1 snRNAs by decapping and 5′-to-3′ exonucleolytic digestion. Proc. Natl. Acad. Sci. U. S. A 2014, 111, E3277–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Shukla S; Schmidt JC; Goldfarb KC; Cech TR; Parker R Inhibition of telomerase RNA decay rescues telomerase deficiency caused by dyskerin or PARN defects. Nat. Struct. Mol. Biol 2016, 23, 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Mroczek S; Krwawicz J; Kutner J; Lazniewski M; Kucinski I; Ginalski K; Dziembowski A C16orf57, a gene mutated in poikiloderma with neutropenia, encodes a putative phosphodiesterase responsible for the U6 snRNA 3′ end modification. Genes Dev. 2012, 26, 1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Shchepachev V; Wischnewski H; Missiaglia E; Soneson C; Azzalin CM Mpn1, mutated in poikiloderma with neutropenia protein 1, is a conserved 3′-to-5′ RNA exonuclease processing U6 small nuclear RNA. Cell Rep. 2012, 2, 855–865. [DOI] [PubMed] [Google Scholar]
- (62).Hilcenko C; Simpson PJ; Finch AJ; Bowler FR; Churcher MJ; Jin L; Packman LC; Shlien A; Campbell P; Kirwan M et al. Aberrant 3′ oligoadenylation of spliceosomal U6 small nuclear RNA in poikiloderma with neutropenia. Blood 2013, 121, 1028–1038. [DOI] [PubMed] [Google Scholar]
- (63).Shchepachev V; Wischnewski H; Soneson C; Arnold AW; Azzalin CM Human Mpn1 promotes post-transcriptional processing and stability of U6atac. FEBS Lett. 2015, 589, 2417–2423. [DOI] [PubMed] [Google Scholar]
- (64).Eckwahl MJ; Sim S; Smith D; Telesnitsky A; Wolin SL A retrovirus packages nascent host noncoding RNAs from a novel surveillance pathway. Genes Dev. 2015, 29, 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Wlotzka W; Kudla G; Granneman S; Tollervey D The nuclear RNA polymerase II surveillance system targets polymerase III transcripts. EMBO J. 2011, 30, 1790–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Allmang C; Kufel J; Chanfreau G; Mitchell P; Petfalski E; Tollervey D Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 1999, 18, 5399–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Kadaba S; Wang X; Anderson JT Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA 2006, 12, 508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Zanchin NI; Goldfarb DS The exosome subunit Rrp43p is required for the efficient maturation of 5.8S, 18S and 25S rRNA. Nucleic Acids Res. 1999, 27, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Allmang C; Mitchell P; Petfalski E; Tollervey D Degradation of ribosomal RNA precursors by the exosome. Nucleic Acids Res. 2000, 28, 1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Preker P; Nielsen J; Kammler S; Lykke-Andersen S; Christensen MS; Mapendano CK; Scheirup MH; Jensen TH RNA exosome depletion reveals transcription upstream of active human promoters. Science 2008, 322, 1851–1854. [DOI] [PubMed] [Google Scholar]
- (71).Shcherbik N; Wang M; Lapik YR; Srivastava L; Pestov DG Polyadenylation and degradation of incomplete RNA polymerase I transcripts in mammalian cells. EMBO Rep. 2010, 11, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Lubas M; Christensen MS; Kristiansen MS; Domanski M; Falkenby LG; Lykke-Andersen S; Andersen JS; Dziembowski A; Jensen TH Interaction profiling identifies the human nuclear exosome targeting complex. Mol. Cell 2011, 43, 624–637. [DOI] [PubMed] [Google Scholar]
- (73).Szczepinska T; Kalisiak K; Tomecki R; Labno A; Borowski LS; Kulinski TM; Adamska D; Kosinska J; Dziembowski A DIS3 shapes the RNA polymerase II transcriptome in humans by degrading a variety of unwanted transcripts. Genome Res. 2015, 25, 1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Mitchell P; Petfalski E; Tollervey D The 3′ end of yeast 5.8S rRNA is generated by an exonuclease processing mechanism. Genes Dev. 1996, 10, 502–513. [DOI] [PubMed] [Google Scholar]
- (75).Mitchell P; Petfalski E; Shevchenko A; Mann M; Tollervey D The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′-->5′ exoribonucleases. Cell 1997, 91, 457–466. [DOI] [PubMed] [Google Scholar]
- (76).Allmang C; Petfalski E; Podtelejnikov A; Mann M; Tollervey D; Mitchell P The yeast exosome and human PM-Scl are related complexes of 3′ --> 5′ exonucleases. Genes Dev. 1999, 13, 2148–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Koonin EV; Wolf YI; Aravind L Prediction of the archaeal exosome and its connections with the proteasome and the translation and transcription machineries by a comparative-genomic approach. Genome Res. 2001, 11, 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Evguenieva-Hackenberg E; Walter P; Hochleitner E; Lottspeich F; Klug G An exosome-like complex in Sulfolobus solfataricus. EMBO Rep. 2003, 4, 889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Mian IS Comparative sequence analysis of ribonucleases HII, III, II PH and D. Nucleic Acids Res. 1997, 25, 3187–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Lorentzen E; Walter P; Fribourg S; Evguenieva-Hackenberg E; Klug G; Conti E The archaeal exosome core is a hexameric ring structure with three catalytic subunits. Nat. Struct. Mol. Biol 2005, 12, 575–581. [DOI] [PubMed] [Google Scholar]
- (81).Liu Q; Greimann JC; Lima CD Reconstitution, activities, and structure of the eukaryotic RNA exosome. Cell 2006, 127, 1223–1237. [DOI] [PubMed] [Google Scholar]
- (82).Lorentzen E; Conti E Structural basis of 3′ end RNA recognition and exoribonucleolytic cleavage by an exosome RNase PH core. Mol. Cell 2005, 20, 473–481. [DOI] [PubMed] [Google Scholar]
- (83).Buttner K; Wenig K; Hopfner KP Structural framework for the mechanism of archaeal exosomes in RNA processing. Mol. Cell 2005, 20, 461–471. [DOI] [PubMed] [Google Scholar]
- (84).Dziembowski A; Lorentzen E; Conti E; Seraphin B A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat. Struct. Mol. Biol 2007, 15–22. [DOI] [PubMed] [Google Scholar]
- (85).Tomecki R; Kristiansen MS; Lykke-Andersen S; Chlebowski A; Larsen KM; Szczesny RJ; Drazkowska K; Pastula A; Andersen JS; Stepien PP et al. The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J. 2010, 29, 2342–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Staals RH; Bronkhorst AW; Schilders G; Slomovic S; Schuster G; Heck AJ; Raijmakers R; Pruijn GJ Dis3-like 1: a novel exoribonuclease associated with the human exosome. EMBO J. 2010, 29, 2358–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Makino DL; Schuch B; Stegmann E; Baumgartner M; Basquin C; Conti E RNA degradation paths in a 12-subunit nuclear exosome complex. Nature 2015, 524, 54–58. [DOI] [PubMed] [Google Scholar]
- (88).Makino DL; Baumgartner M; Conti E Crystal structure of an RNA-bound 11-subunit eukaryotic exosome complex. Nature 2013, 495, 70–75. [DOI] [PubMed] [Google Scholar]
- (89).Wasmuth EV; Zinder JC; Zattas D; Das M; Lima CD Structure and reconstitution of yeast MPP6-nuclear exosome complexes reveals that Mpp6 stimulates RNA decay and recruits the Mtr4 helicase. Elife 2017, 6, e29062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Wang HW; Wang J; Ding F; Callahan K; Bratkowski MA; Butler JS; Nogales E; Ke A Architecture of the yeast Rrp44 exosome complex suggests routes of RNA recruitment for 3′ end processing. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 16844–16849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Liu JJ; Bratkowski MA; Liu X; Niu CY; Ke A; Wang HW Visualization of distinct substrate-recruitment pathways in the yeast exosome by EM. Nat. Struct. Mol. Biol 2014, 21, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Liu JJ; Niu CY; Wu Y; Tan D; Wang Y; Ye MD; Liu Y; Zhao W; Zhou K; Liu QS et al. CryoEM structure of yeast cytoplasmic exosome complex. Cell Res. 2016, 26, 822–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Lorentzen E; Basquin J; Tomecki R; Dziembowski A; Conti E Structure of the active subunit of the yeast exosome core, Rrp44: diverse modes of substrate recruitment in the RNase II nuclease family. Mol. Cell 2008, 29, 717–728. [DOI] [PubMed] [Google Scholar]
- (94).Zinder JC; Wasmuth EV; Lima CD Nuclear RNA exosome at 3.1 A reveals substrate specificities, RNA paths, and allosteric inhibition of Rrp44/Dis3. Mol. Cell 2016, 64, 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Wasmuth EV; Lima CD Exo- and endoribonucleolytic activities of yeast cytoplasmic and nuclear RNA exosomes are dependent on the noncatalytic core and central channel. Mol. Cell 2012, 48, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Drazkowska K; Tomecki R; Stodus K; Kowalska K; Czarnocki-Cieciura M; Dziembowski A The RNA exosome complex central channel controls both exonuclease and endonuclease Dis3 activities in vivo and in vitro. Nucleic Acids Res. 2013, 41, 3845–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Briggs MW; Burkard KT; Butler JS Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8 S rRNA 3′ end formation. J. Biol. Chem 1998, 273, 13255–13263. [DOI] [PubMed] [Google Scholar]
- (98).Burkard KT; Butler JS A nuclear 3′ to 5′ exonuclease involved in mRNA degradation interacts with poly(A) polymerase and the hnRNA protein Npl3p. Mol. Cell Biol 2000, 20, 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Zuo Y; Deutscher MP Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res. 2001, 29, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Morozov V; Mushegian AR; Koonin EV; Bork P A putative nucleic acid-binding domain in Bloom’s and Werner’s syndrome helicases. Trends Biochem. Sci 1997, 22, 417–418. [DOI] [PubMed] [Google Scholar]
- (101).Wasmuth EV; Januszyk K; Lima CD Structure of an Rrp6-RNA exosome complex bound to poly(A) RNA. Nature 2014, 511, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Wasmuth EV; Lima CD The Rrp6 C-terminal domain binds RNA and activates the nuclear RNA exosome. Nucleic Acids Res. 2017, 45, 846–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Han J; van Hoof A The RNA exosome channeling and direct access conformations have distinct in vivo functions. Cell Rep. 2016, 16, 3348–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Delan-Forino C; Schneider C; Tollervey D Transcriptome-wide analysis of alternative routes for RNA substrates into the exosome complex. PLoS Genet. 2017, 13, e1006699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Mitchell P; Petfalski E; Houalla R; Podtelejnikov A; Mann M; Tollervey D Rrp47p is an exosome-associated protein required for the 3′ processing of stable RNAs. Mol. Cell. Biol 2003, 23, 6982–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Feigenbutz M; Garland W; Turner M; Mitchell P The exosome cofactor Rrp47 is critical for the stability and normal expression of its associated exoribonuclease Rrp6 in Saccharomyces cerevisiae. PLoS One 2013, 8, e80752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Schuch B; Feigenbutz M; Makino DL; Falk S; Basquin C; Mitchell P; Conti E The exosome-binding factors Rrp6 and Rrp47 form a composite surface for recruiting the Mtr4 helicase. EMBO J. 2014, 33, 2829–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Schilders G; van Dijk E; Pruijn GJM C1D and hMtr4p associate with the human exosome subunit PM/Scl-100 and are involved in pre-rRNA processing. Nucleic Acids Res. 2007, 35, 2564–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Schilders G; Raijmakers R; Raats JMH; Pruijn GJM MPP6 is an exosome-associated RNA-binding protein involved in 5.8S rRNA maturation. Nucleic Acids Res. 2005, 33, 6795–6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Milligan L; Decourty L; Saveanu C; Rappsilber J; Ceulemans H; Jacquier A; Tollervey D A yeast exosome cofactor, Mpp6, functions in RNA surveillance and in the degradation of noncoding RNA transcripts. Mol. Cell. Biol 2008, 28, 5446–5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Kim K; Heo DH; Kim I; Suh JY; Kim M Exosome cofactors connect transcription termination to RNA processing by guiding terminated transcripts to the appropriate exonuclease within the nuclear exosome. J. Biol. Chem 2016, 291, 13229–13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Falk S; Bonneau F; Ebert J; Kogel A; Conti E Mpp6 incorporation in the nuclear exosome contributes to RNA channeling through the Mtr4 helicase. Cell Rep. 2017, 20, 2279–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Hardwick SW; Luisi BF Rarely at rest: RNA helicases and their busy contributions to RNA degradation, regulation and quality control. RNA Biol. 2013, 10, 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Jackson RN; Klauer AA; Hintze BJ; Robinson H; van Hoof A; Johnson SJ The crystal structure of Mtr4 reveals a novel arch domain required for rRNA processing. EMBO J. 2010, 29, 2205–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Weir JR; Bonneau F; Hentschel J; Conti E Structural analysis reveals the characteristic features of Mtr4, a DExH helicase involved in nuclear RNA processing and surveillance. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 12139–12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Wang X; Jia H; Jankowsky E; Anderson JT Degradation of hypomodified tRNA(iMet) in vivo involves RNA-dependent ATPase activity of the DExH helicase Mtr4p. RNA 2008, 14, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Thoms M; Thomson E; Bassler J; Gnadig M; Griesel S; Hurt E The exosome Is recruited to RNA substrates through specific adaptor proteins. Cell 2015, 162, 1029–1038. [DOI] [PubMed] [Google Scholar]
- (118).Falk S; Weir JR; Hentschel J; Reichelt P; Bonneau F; Conti E The molecular architecture of the TRAMP complex reveals the organization and interplay of its two catalytic activities. Mol. Cell 2014, 55, 856–867. [DOI] [PubMed] [Google Scholar]
- (119).Losh JS; van Hoof A Gateway arch to the RNA exosome. Cell 2015, 162, 940–941. [DOI] [PubMed] [Google Scholar]
- (120).Carroll KL; Ghirlando R; Ames JM; Corden JL Interaction of yeast RNA-binding proteins Nrd1 and Nab3 with RNA polymerase II terminator elements. RNA 2007, 13, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Carroll KL; Pradhan DA; Granek JA; Clarke ND; Corden JL Identification of cis elements directing termination of yeast nonpolyadenylated snoRNA transcripts. Mol. Cell. Biol 2004, 24, 6241–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Vasiljeva L; Kim M; Mutschler H; Buratowski S; Meinhart A The Nrd1-Nab3-Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat. Struct. Mol. Biol 2008, 15, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Tudek A; Porrua O; Kabzinski T; Lidschreiber M; Kubicek K; Fortova A; Lacroute F; Vanacova S; Cramer P; Stefl R et al. Molecular basis for coordinating transcription termination with noncoding RNA degradation. Mol. Cell 2014, 55, 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Houseley J; Kotovic K; El Hage A; Tollervey D Trf4 targets ncRNAs from telomeric and rDNA spacer regions and functions in rDNA copy number control. EMBO J. 2007, 26, 4996–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).San Paolo S; Vanacova S; Schenk L; Scherrer T; Blank D; Keller W; Gerber AP Distinct roles of non-canonical poly(A) polymerases in RNA metabolism. PLoS Genet. 2009, 5, e1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Jia H; Wang X; Liu F; Guenther UP; Srinivasan S; Anderson JT; Jankowsky E The RNA helicase Mtr4p modulates polyadenylation in the TRAMP complex. Cell 2011, 145, 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Houseley J; Tollervey D Yeast Trf5p is a nuclear poly(A) polymerase. EMBO Rep. 2006, 7, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Dez C; Houseley J; Tollervey D Surveillance of nuclear-restricted pre-ribosomes within a subnucleolar region of Saccharomyces cerevisiae. EMBO J. 2006, 25, 1534–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Wery M; Ruidant S; Schillewaert S; Lepore N; Lafontaine DL The nuclear poly(A) polymerase and exosome cofactor Trf5 is recruited cotranscriptionally to nucleolar surveillance. RNA 2009, 15, 406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).D’Souza V; Summers MF How retroviruses select their genomes. Nat. Rev. Microbiol 2005, 3, 643–655. [DOI] [PubMed] [Google Scholar]
- (131).Hamill S; Wolin SL; Reinisch KM Structure and function of the polymerase core of TRAMP, a RNA surveillance complex. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 15045–15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Fasken MB; Leung SW; Banerjee A; Kodani MO; Chavez R; Bowman EA; Purohit MK; Rubinson ME; Rubinson EH; Corbett AH Air1 zinc knuckles 4 and 5 and a conserved IWRXY motif are critical for the function and integrity of the Trf4/5-Air1/2-Mtr4 polyadenylation (TRAMP) RNA quality control complex. J. Biol. Chem 2011, 286, 37429–37445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Holub P; Lalakova J; Cerna H; Pasulka J; Sarazova M; Hrazdilova K; Arce MS; Hobor F; Stefl R; Vanacova S Air2p is critical for the assembly and RNA-binding of the TRAMP complex and the KOW domain of Mtr4p is crucial for exosome activation. Nucleic Acids Res. 2012, 40, 5679–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Losh JS; King AK; Bakelar J; Taylor L; Loomis J; Rosenzweig JA; Johnson SJ; van Hoof A Interaction between the RNA-dependent ATPase and poly(A) polymerase subunits of the TRAMP complex is mediated by short peptides and important for snoRNA processing. Nucleic Acids Res. 2015, 43, 1848–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Kubicek K; Cerna H; Holub P; Pasulka J; Hrossova D; Loehr F; Hofr C; Vanacova S; Stefl R Serine phosphorylation and proline isomerization in RNAP II CTD control recruitment of Nrd1. Genes Dev. 2012, 26, 1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Komarnitsky P; Cho EJ; Buratowski S Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 2000, 14, 2452–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Porrua O; Libri D A bacterial-like mechanism for transcription termination by the Sen1p helicase in budding yeast. Nat. Struct. Mol. Biol 2013, 20, 884–891. [DOI] [PubMed] [Google Scholar]
- (138).Vasiljeva L; Buratowski S Nrd1 interacts with the nuclear exosome for 3′ processing of RNA polymerase II transcripts. Mol. Cell 2006, 21, 239–248. [DOI] [PubMed] [Google Scholar]
- (139).Fasken MB; Laribee RN; Corbett AH Nab3 facilitates the function of the TRAMP complex in RNA processing via recruitment of Rrp6 independent of Nrd1. PLoS Genet. 2015, 11, e1005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Thiebaut M; Kisseleva-Romanova E; Rougemaille M; Boulay J; Libri D Transcription termination and nuclear degradation of cryptic unstable transcripts: a role for the nrd1-nab3 pathway in genome surveillance. Mol. Cell 2006, 23, 853–864. [DOI] [PubMed] [Google Scholar]
- (141).Arigo JT; Eyler DE; Carroll KL; Corden JL Termination of cryptic unstable transcripts is directed by yeast RNA-binding proteins Nrd1 and Nab3. Mol. Cell 2006, 23, 841–851. [DOI] [PubMed] [Google Scholar]
- (142).Egecioglu DE; Henras AK; Chanfreau GF Contributions of Trf4p- and Trf5p-dependent polyadenylation to the processing and degradative functions of the yeast nuclear exosome. RNA 2006, 12, 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Jamonnak N; Creamer TJ; Darby MM; Schaughency P; Wheelan SJ; Corden JL Yeast Nrd1, Nab3, and Sen1 transcriptome-wide binding maps suggest multiple roles in post-transcriptional RNA processing. RNA 2011, 17, 2011–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Lepore N; Lafontaine DL A functional interface at the rDNA connects rRNA synthesis, pre-rRNA processing and nucleolar surveillance in budding yeast. PLoS One 2011, 6, e24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Buhler M; Spies N; Bartel DP; Moazed D TRAMP-mediated RNA surveillance prevents spurious entry of RNAs into the Schizosaccharomyces pombe siRNA pathway. Nat. Struct. Mol. Biol 2008, 15, 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Castano IB; Heath-Paliuso S; Sadoff BU; Fitzhugh DJ; Christman MF A novel family of TRF (DNA topoisomerase I-related function) genes required for proper nuclear segregation. Nucl. Acids Res 1996, 24, 2404–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Win TZ; Draper S; Read RL; Pearce J; Norbury CJ; Wang SW Requirement of fission yeast Cid14 in polyadenylation of rRNAs. Mol. Cell. Biol 2006, 26, 1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Buhler M; Haas W; Gygi SP; Moazed D RNAi-dependent and -independent RNA turnover mechanisms contribute to heterochromatic gene silencing. Cell 2007, 129, 707–721. [DOI] [PubMed] [Google Scholar]
- (149).Chlebowski A; Tomecki R; Lopez ME; Seraphin B; Dziembowski A Catalytic properties of the eukaryotic exosome. Adv. Exp. Med. Biol 2011, 702, 63–78. [DOI] [PubMed] [Google Scholar]
- (150).Preker P; Almvig K; Christensen MS; Valen E; Mapendano CK; Sandelin A; Jensen TH PROMoter uPstream Transcripts share characteristics with mRNAs and are produced upstream of all three major types of mammalian promoters. Nucleic Acids Res. 2011, 39, 7179–7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Lubas M; Andersen PR; Schein A; Dziembowski A; Kudla G; Jensen TH The human nuclear exosome targeting complex is loaded onto newly synthesized RNA to direct early ribonucleolysis. Cell Rep. 2015, 10, 178–192. [DOI] [PubMed] [Google Scholar]
- (152).Bresson SM; Hunter OV; Hunter AC; Conrad NK Canonical poly(A) polymerase activity promotes the decay of a wide variety of mammalian nuclear RNAs. PLoS Genet. 2015, 11, e1005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Meola N; Domanski M; Karadoulama E; Chen Y; Gentil C; Pultz D; Vitting-Seerup K; Lykke-Andersen S; Andersen JS; Sandelin A et al. Identification of a nuclear exosome decay pathway for processed transcripts. Mol. Cell 2016, 64, 520–533. [DOI] [PubMed] [Google Scholar]
- (154).Ogami K; Richard P; Chen Y; Hoque M; Li W; Moresco JJ; Yates JR 3rd; Tian B; Manley JL An Mtr4/ZFC3H1 complex facilitates turnover of unstable nuclear RNAs to prevent their cytoplasmic transport and global translational repression. Genes Dev. 2017, 31, 1257–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Nguyen D; Grenier St-Sauveur V; Bergeron D; Dupuis-Sandoval F; Scott MS; Bachand F A polyadenylation-dependent 3′ end maturation pathway Is required for the synthesis of the human telomerase RNA. Cell Rep. 2015, 13, 2244–2257. [DOI] [PubMed] [Google Scholar]
- (156).Tseng CK; Wang HF; Burns AM; Schroeder MR; Gaspari M; Baumann P Human telomerase RNA processing and quality control. Cell Rep. 2015, 13, 2232–2243. [DOI] [PubMed] [Google Scholar]
- (157).Rammelt C; Bilen B; Zavolan M; Keller W PAPD5, a noncanonical poly(A) polymerase with an unusual RNA-binding motif. RNA 2011, 17, 1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Hrossova D; Sikorsky T; Potesil D; Bartosovic M; Pasulka J; Zdrahal Z; Stefl R; Vanacova S RBM7 subunit of the NEXT complex binds U-rich sequences and targets 3′-end extended forms of snRNAs. Nucleic Acids Res. 2015, 43, 4236–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Falk S; Finogenova K; Melko M; Benda C; Lykke-Andersen S; Jensen TH; Conti E Structure of the RBM7-ZCCHC8 core of the NEXT complex reveals connections to splicing factors. Nat. Commun 2016, 7, 13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Andersen PR; Domanski M; Kristiansen MS; Storvall H; Ntini E; Verheggen C; Schein A; Bunkenborg J; Poser I; Hallais M et al. The human cap-binding complex is functionally connected to the nuclear RNA exosome. Nat. Struct. Mol. Biol 2013, 20, 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Hallais M; Pontvianne F; Andersen PR; Clerici M; Lener D; Benbahouche Nel H; Gostan T; Vandermoere F; Robert MC; Cusack S et al. CBC-ARS2 stimulates 3′-end maturation of multiple RNA families and favors cap-proximal processing. Nat. Struct. Mol. Biol 2013, 20, 1358–1366. [DOI] [PubMed] [Google Scholar]
- (162).Giacometti S; Benbahouche NEH; Domanski M; Robert MC; Meola N; Lubas M; Bukenborg J; Andersen JS; Schulze WM; Verheggen C et al. Mutually exclusive CBC-containing complexes contribute to RNA fate. Cell Rep. 2017, 18, 2635–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Beaulieu YB; Kleinman CL; Landry-Voyer AM; Majewski J; Bachand F Polyadenylation-dependent control of long noncoding RNA expression by the poly(A)-binding protein nuclear 1. PLoS Genet. 2012, 8, e1003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Valencia P; Dias AP; Reed R Splicing promotes rapid and efficient mRNA export in mammalian cells. Proc. Natl. Acad. Sci. USA 2008, 105, 3386–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Bresson SM; Conrad NK The human nuclear poly(A)-binding protein promotes RNA hyperadenylation and decay. PLoS Genet. 2013, 9, e1003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Mitton-Fry RM; DeGregorio SJ; Wang J; Steitz TA; Steitz JA Poly(A) tail recognition by a viral RNA element through assembly of a triple helix. Science 2010, 330, 1244–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Gregory RI; Yan KP; Amuthan G; Chendrimada T; Doratotaj B; Cooch N; Shiekhattar R The Microprocessor complex mediates the genesis of microRNAs. Nature 2004, 432, 235–240. [DOI] [PubMed] [Google Scholar]
- (168).Macias S; Cordiner RA; Gautier P; Plass M; Caceres JF DGCR8 acts as an adaptor for the exosome complex to degrade double-stranded structured RNAs. Mol. Cell 2015, 60, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Schlackow M; Nojima T; Gomes T; Dhir A; Carmo-Fonseca M; Proudfoot NJ Distinctive patterns of transcription and RNA processing for human lincRNAs. Mol. Cell 2017, 65, 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Quick-Cleveland J; Jacob JP; Weitz SH; Shoffner G; Senturia R; Guo F The DGCR8 RNA-binding heme domain recognizes primary microRNAs by clamping the hairpin. Cell Rep. 2014, 7, 1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Schaeffer D; Clark A; Klauer AA; Tsanova B; van Hoof A Functions of the cytoplasmic exosome. Adv. Exp. Med. Biol 2011, 702, 79–90. [DOI] [PubMed] [Google Scholar]
- (172).Prusty AB; Meduri R; Prusty BK; Vanselow J; Schlosser A; Fischer U Impaired spliceosomal UsnRNP assembly leads to Sm mRNA down-regulation and Sm protein degradation. J. Cell Biol 2017, 216, 2391–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Anderson JS; Parker RP The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 1998, 17, 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Brown JT; Bai X; Johnson AW The yeast antiviral proteins Ski2p, Ski3p, and Ski8p exist as a complex in vivo. RNA 2000, 6, 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Halbach F; Reichelt P; Rode M; Conti E The yeast Ski complex: crystal structure and RNA channeling to the exosome complex. Cell 2013, 154, 814–826. [DOI] [PubMed] [Google Scholar]
- (176).Araki Y; Takahashi S; Kobayashi T; Kajiho H; Hoshino S; Katada T Ski7p G protein interacts with the exosome and the Ski complex for 3′-to-5′ mRNA decay in yeast. EMBO J. 2001, 20, 4684–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Kowalinski E; Kogel A; Ebert J; Reichelt P; Stegmann E; Habermann B; Conti E Structure of a cytoplasmic 11-subunit RNA exosome complex. Mol. Cell 2016, 63, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Schmidt C; Kowalinski E; Shanmuganathan V; Defenouillere Q; Braunger K; Heuer A; Pech M; Namane A; Berninghausen O; Fromont-Racine M et al. The cryo-EM structure of a ribosome-Ski2-Ski3-Ski8 helicase complex. Science 2016, 354, 1431–1433. [DOI] [PubMed] [Google Scholar]
- (179).Zhu B; Mandal SS; Pham AD; Zheng Y; Erdjument-Bromage H; Batra SK; Tempst P; Reinberg D The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev. 2005, 19, 1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Kalisiak K; Kulinski TM; Tomecki R; Cysewski D; Pietras Z; Chlebowski A; Kowalska K; Dziembowski A A short splicing isoform of HBS1L links the cytoplasmic exosome and SKI complexes in humans. Nucleic Acids Res. 2017, 45, 2068–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Qu X; Yang Z; Zhang S; Shen L; Dangel AW; Hughes JH; Redman KL; Wu LC; Yu CY The human DEVH-box protein Ski2w from the HLA is localized in nucleoli and ribosomes. Nucleic Acids Res. 1998, 26, 4068–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Porrua O; Libri D RNA quality control in the nucleus: the Angels’ share of RNA. Biochim. Biophys. Acta 2013, 1829, 604–611. [DOI] [PubMed] [Google Scholar]
- (183).Chang HM; Triboulet R; Thornton JE; Gregory RI A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature 2013, 497, 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Faehnle CR; Walleshauser J; Joshua-Tor L Mechanism of Dis3l2 substrate recognition in the Lin28-let-7 pathway. Nature 2014, 514, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Ustianenko D; Hrossova D; Potesil D; Chalupnikova K; Hrazdilova K; Pachernik J; Cetkovska K; Uldrijan S; Zdrahal Z; Vanacova S Mammalian DIS3L2 exoribonuclease targets the uridylated precursors of let-7 miRNAs. RNA 2013, 19, 1632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Heo I; Joo C; Cho J; Ha M; Han J; Kim VN Lin28 mediates the terminal uridylation of let-7 precursor microRNA. Mol. Cell 2008, 32, 276–284. [DOI] [PubMed] [Google Scholar]
- (187).Heo I; Joo C; Kim YK; Ha M; Yoon MJ; Cho J; Yeom KH; Han J; Kim VN TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 2009, 138, 696–708. [DOI] [PubMed] [Google Scholar]
- (188).Hagan JP; Piskounova E; Gregory RI Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat. Struct. Mol. Biol 2009, 16, 1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Ustianenko D; Pasulka J; Feketova Z; Bednarik L; Zigackova D; Fortova A; Zavolan M; Vanacova S TUT-DIS3L2 is a mammalian surveillance pathway for aberrant structured non-coding RNAs. EMBO J. 2016, 35, 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Pirouz M; Du P; Munafo M; Gregory RI Dis3l2-mediated decay Is a quality control pathway for noncoding RNAs. Cell Rep. 2016, 16, 1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Labno A; Warkocki Z; Kulinski T; Krawczyk PS; Bijata K; Tomecki R; Dziembowski A Perlman syndrome nuclease DIS3L2 controls cytoplasmic non-coding RNAs and provides surveillance pathway for maturing snRNAs. Nucleic Acids Res. 2016, 44, 10437–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Suh MR; Lee Y; Kim JY; Kim SK; Moon SH; Lee JY; Cha KY; Chung HM; Yoon HS; Moon SY et al. Human embryonic stem cells express a unique set of microRNAs. Dev. Biol 2004, 270, 488–498. [DOI] [PubMed] [Google Scholar]
- (193).Thomson JM; Newman M; Parker JS; Morin-Kensicki EM; Wright T; Hammond SM Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006, 20, 2202–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Kwak JE; Wickens M A family of poly(U) polymerases. RNA 2007, 13, 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Rissland OS; Mikulasova A; Norbury CJ Efficient RNA polyuridylation by noncanonical poly(A) polymerases. Mol. Cell. Biol 2007, 27, 3612–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Onafuwa-Nuga AA; King SR; Telesnitsky A Nonrandom packaging of host RNAs in Moloney murine leukemia virus. J. Virol 2005, 79, 13528–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Eckwahl MJ; Arnion H; Kharytonchyk S; Zang T; Bieniasz PD; Telesnitsky A; Wolin SL Analysis of the human immunodeficiency virus-1 RNA packageome. RNA 2016, 22, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Onafuwa-Nuga AA; Telesnitsky A; King SR 7SL RNA, but not the 54-kd signal recognition particle protein, is an abundant component of both infectious HIV-1 and minimal virus-like particles. RNA 2006, 12, 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Garcia EL; Onafuwa-Nuga A; Sim S; King SR; Wolin SL; Telesnitsky A Packaging of host mY RNAs by murine leukemia virus may occur early in Y RNA biogenesis. J. Virol 2009, 83, 12526–12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Reimao-Pinto MM; Manzenreither RA; Burkard TR; Sledz P; Jinek M; Mechtler K; Ameres SL Molecular basis for cytoplasmic RNA surveillance by uridylation-triggered decay in Drosophila. EMBO J. 2016, 35, 2417–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Malecki M; Viegas SC; Carneiro T; Golik P; Dressaire C; Ferreira MG; Arraiano CM The exoribonuclease Dis3L2 defines a novel eukaryotic RNA degradation pathway. EMBO J. 2013, 32, 1842–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Zhang W; Murphy C; Sieburth LE Conserved RNaseII domain protein functions in cytoplasmic mRNA decay and suppresses Arabidopsis decapping mutant phenotypes. Proc. Natl. Acad. Sci. USA 2010, 107, 15981–15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Wilusz JE; Whipple JM; Phizicky EM; Sharp PA tRNAs marked with CCACCA are targeted for degradation. Science 2011, 334, 817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Kuhn CD; Wilusz JE; Zheng Y; Beal PA; Joshua-Tor L On-enzyme refolding permits small RNA and tRNA surveillance by the CCA-adding enzyme. Cell 2015, 160, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (205).Nagarajan VK; Jones CI; Newbury SF; Green PJ XRN 5′-->3′ exoribonucleases: structure, mechanisms and functions. Biochim. Biophys. Acta 2013, 1829, 590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Stevens A; Poole TL 5′-exonuclease-2 of Saccharomyces cerevisiae. Purification and features of ribonuclease activity with comparison to 5′-exonuclease-1. J. Biol. Chem 1995, 270, 16063–16069. [DOI] [PubMed] [Google Scholar]
- (207).Heyer WD; Johnson AW; Reinhart U; Kolodner RD Regulation and intracellular localization of Saccharomyces cerevisiae strand exchange protein 1 (Sep1/Xrn1/Kem1), a multifunctional exonuclease. Mol. Cell. Biol 1995, 15, 2728–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Johnson AW Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol. Cell. Biol 1997, 17, 6122–6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Sahasranaman A; Dembowski J; Strahler J; Andrews P; Maddock J; Woolford JL Jr. Assembly of Saccharomyces cerevisiae 60S ribosomal subunits: role of factors required for 27S pre-rRNA processing. EMBO J. 2011, 30, 4020–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Chernyakov I; Whipple JM; Kotelawala L; Grayhack EJ; Phizicky EM Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′−3′ exonucleases Rat1 and Xrn1. Genes Dev. 2008, 22, 1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Kim M; Krogan NJ; Vasiljeva L; Rando OJ; Nedea E; Greenblatt JF; Buratowski S The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 2004, 432, 517–522. [DOI] [PubMed] [Google Scholar]
- (212).West S; Gromak N; Proudfoot NJ Human 5′ --> 3′ exonuclease Xrn2 promotes transcription termination at co-transcriptional cleavage sites. Nature 2004, 432, 522–525. [DOI] [PubMed] [Google Scholar]
- (213).Xue Y; Bai X; Lee I; Kallstrom G; Ho J; Brown J; Stevens A; Johnson AW Saccharomyces cerevisiae RAI1 (YGL246c) is homologous to human DOM3Z and encodes a protein that binds the nuclear exoribonuclease Rat1p. Mol. Cell. Biol 2000, 20, 4006–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Xiang S; Cooper-Morgan A; Jiao X; Kiledjian M; Manley JL; Tong L Structure and function of the 5′-->3′ exoribonuclease Rat1 and its activating partner Rai1. Nature 2009, 458, 784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Wang VY; Jiao X; Kiledjian M; Tong L Structural and biochemical studies of the distinct activity profiles of Rai1 enzymes. Nucleic Acids Res. 2015, 43, 6596–6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).Jiao X; Xiang S; Oh C; Martin CE; Tong L; Kiledjian M Identification of a quality-control mechanism for mRNA 5′-end capping. Nature 2010, 467, 608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (217).Steiger M; Carr-Schmid A; Schwartz DC; Kiledjian M; Parker R Analysis of recombinant yeast decapping enzyme. RNA 2003, 9, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (218).Jiao X; Chang JH; Kilic T; Tong L; Kiledjian M A mammalian pre-mRNA 5′ end capping quality control mechanism and an unexpected link of capping to pre-mRNA processing. Mol. Cell 2013, 50, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Alexandrov A; Chernyakov I; Gu W; Hiley SL; Hughes TR; Grayhack EJ; Phizicky EM Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell 2006, 21, 87–96. [DOI] [PubMed] [Google Scholar]
- (220).Whipple JM; Lane EA; Chernyakov I; D’Silva S; Phizicky EM The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev. 2011, 25, 1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Guy MP; Young DL; Payea MJ; Zhang X; Kon Y; Dean KM; Grayhack EJ; Mathews DH; Fields S; Phizicky EM Identification of the determinants of tRNA function and susceptibility to rapid tRNA decay by high-throughput in vivo analysis. Genes Dev. 2014, 28, 1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Doma MK; Parker R RNA quality control in eukaryotes. Cell 2007, 131, 660–668. [DOI] [PubMed] [Google Scholar]
- (223).Dewe JM; Whipple JM; Chernyakov I; Jaramillo LN; Phizicky EM The yeast rapid tRNA decay pathway competes with elongation factor 1A for substrate tRNAs and acts on tRNAs lacking one or more of several modifications. RNA 2012, 18, 1886–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Turowski TW; Karkusiewicz I; Kowal J; Boguta M Maf1-mediated repression of RNA polymerase III transcription inhibits tRNA degradation via RTD pathway. RNA 2012, 18, 1823–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (225).Thompson DM; Parker R Cytoplasmic decay of intergenic transcripts in Saccharomyces cerevisiae. Mol. Cell. Biol 2007, 27, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Berretta J; Pinskaya M; Morillon A A cryptic unstable transcript mediates transcriptional trans-silencing of the Ty1 retrotransposon in S. cerevisiae. Genes Dev. 2008, 22, 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (227).Marquardt S; Hazelbaker DZ; Buratowski S Distinct RNA degradation pathways and 3′ extensions of yeast non-coding RNA species. Transcription 2011, 2, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).He F; Jacobson A Nonsense-mediated mRNA decay: Degradation of defective transcripts Is only part of the story. Annu. Rev. Genet 2015, 49, 339–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (229).Wery M; Descrimes M; Vogt N; Dallongeville AS; Gautheret D; Morillon A Nonsense-mediated decay restricts lncRNA levels in yeast unless blocked by double-stranded RNA structure. Mol. Cell 2016, 61, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Malabat C; Feuerbach F; Ma L; Saveanu C; Jacquier A Quality control of transcription start site selection by nonsense-mediated-mRNA decay. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (231).Smith JE; Alvarez-Dominguez JR; Kline N; Huynh NJ; Geisler S; Hu W; Coller J; Baker KE Translation of small open reading frames within unannotated RNA transcripts in Saccharomyces cerevisiae. Cell Rep. 2014, 7, 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (232).Muhlrad D; Parker R Aberrant mRNAs with extended 3′ UTRs are substrates for rapid degradation by mRNA surveillance. RNA 1999, 5, 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Singh G; Rebbapragada I; Lykke-Andersen J A competition between stimulators and antagonists of Upf complex recruitment governs human nonsense-mediated mRNA decay. PLoS Biol. 2008, 6, e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (234).Chew GL; Pauli A; Rinn JL; Regev A; Schier AF; Valen E Ribosome profiling reveals resemblance between long non-coding RNAs and 5′ leaders of coding RNAs. Development 2013, 140, 2828–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Hurt JA; Robertson AD; Burge CB Global analyses of UPF1 binding and function reveal expanded scope of nonsense-mediated mRNA decay. Genome Res. 2013, 23, 1636–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (236).Guttman M; Russell P; Ingolia NT; Weissman JS; Lander ES Ribosome profiling provides evidence that large noncoding RNAs do not encode proteins. Cell 2013, 154, 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (237).Ji Z; Song R; Regev A; Struhl K Many lncRNAs, 5′UTRs, and pseudogenes are translated and some are likely to express functional proteins. Elife 2015, 4, e08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (238).Tani H; Torimura M; Akimitsu N The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells. PLoS One 2013, 8, e55684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (239).Carlevaro-Fita J; Rahim A; Guigo R; Vardy LA; Johnson R Cytoplasmic long noncoding RNAs are frequently bound to and degraded at ribosomes in human cells. RNA 2016, 22, 867–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (240).Cabrera-Quio LE; Herberg S; Pauli A Decoding sORF translation - from small proteins to gene regulation. RNA Biol. 2016, 13, 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (241).Wang M; Pestov DG 5′-end surveillance by Xrn2 acts as a shared mechanism for mammalian pre-rRNA maturation and decay. Nucleic Acids Res. 2011, 39, 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Ballarino M; Pagano F; Girardi E; Morlando M; Cacchiarelli D; Marchioni M; Proudfoot NJ; Bozzoni I Coupled RNA processing and transcription of intergenic primary microRNAs. Mol. Cell. Biol 2009, 29, 5632–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (243).Kammler S; Lykke-Andersen S; Jensen TH The RNA exosome component hRrp6 is a target for 5-fluorouracil in human cells. Mol. Cancer Res 2008, 6, 990–995. [DOI] [PubMed] [Google Scholar]
- (244).Valen E; Preker P; Andersen PR; Zhao X; Chen Y; Ender C; Dueck A; Meister G; Sandelin A; Jensen TH Biogenic mechanisms and utilization of small RNAs derived from human protein-coding genes. Nat. Struct. Mol. Biol 2011, 18, 1075–1082. [DOI] [PubMed] [Google Scholar]
- (245).Miki TS; Richter H; Ruegger S; Grosshans H PAXT-1 promotes XRN2 activity by stabilizing it through a conserved domain. Mol. Cell 2014, 53, 351–360. [DOI] [PubMed] [Google Scholar]
- (246).Richter H; Katic I; Gut H; Grosshans H Structural basis and function of XRN2 binding by XTB domains. Nat. Struct. Mol. Biol 2016, 23, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (247).Sato S; Ishikawa H; Yoshikawa H; Izumikawa K; Simpson RJ; Takahashi N Collaborator of alternative reading frame protein (CARF) regulates early processing of pre-ribosomal RNA by retaining XRN2 (5′−3′ exoribonuclease) in the nucleoplasm. Nucleic Acids Res. 2015, 43, 10397–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (248).Rother S; Bartels M; Schweda AT; Resch K; Pallua N; Nourbakhsh M NF-kappaB-repressing factor phosphorylation regulates transcription elongation via its interactions with 5′-->3′ exoribonuclease 2 and negative elongation factor. FASEB J. 2016, 30, 174–185. [DOI] [PubMed] [Google Scholar]
- (249).Coccia M; Rossi A; Riccio A; Trotta E; Santoro MG Human NF-kappaB repressing factor acts as a stress-regulated switch for ribosomal RNA processing and nucleolar homeostasis surveillance. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (250).Memet I; Doebele C; Sloan KE; Bohnsack MT The G-patch protein NF-kappaB-repressing factor mediates the recruitment of the exonuclease XRN2 and activation of the RNA helicase DHX15 in human ribosome biogenesis. Nucleic Acids Res. 2017, 45, 5359–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (251).Chang JH; Jiao X; Chiba K; Oh C; Martin CE; Kiledjian M; Tong L Dxo1 is a new type of eukaryotic enzyme with both decapping and 5′−3′ exoribonuclease activity. Nat. Struct. Mol. Biol 2012, 19, 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (252).Cahova H; Winz ML; Hofer K; Nubel G; Jaschke A NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 2015, 519, 374–377. [DOI] [PubMed] [Google Scholar]
- (253).Walters RW; Matheny T; Mizoue LS; Rao BS; Muhlrad D; Parker R Identification of NAD+ capped mRNAs in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (254).Jiao X; Doamekpor SK; Bird JG; Nickels BE; Tong L; Hart RP; Kiledjian M 5′ end nicotinamide adenine dinucleotide cap in human cells promotes RNA decay through DXO-mediated deNADding. Cell 2017, 168, 1015–1027 e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (255).Poole TL; Stevens A Comparison of features of the RNase activity of 5′-exonuclease-1 and 5′-exonuclease-2 of Saccharomyces cerevisiae. Nucleic Acids Symp. Ser 1995, 79–81. [PubMed] [Google Scholar]
- (256).Deshpande T; Takagi T; Hao L; Buratowski S; Charbonneau H Human PIR1 of the protein-tyrosine phosphatase superfamily has RNA 5′-triphosphatase and diphosphatase activities. J Biol. Chem 1999, 274, 16590–16594. [DOI] [PubMed] [Google Scholar]
- (257).Burke JM; Kincaid RP; Nottingham RM; Lambowitz AM; Sullivan CS DUSP11 activity on triphosphorylated transcripts promotes Argonaute association with noncanonical viral microRNAs and regulates steady-state levels of cellular noncoding RNAs. Genes Dev. 2016, 30, 2076–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Yuan Y; Li DM; Sun H PIR1, a novel phosphatase that exhibits high affinity to RNA. ribonucleoprotein complexes. J. Biol. Chem 1998, 273, 20347–20353. [DOI] [PubMed] [Google Scholar]
- (259).Burke JM; Sullivan CS DUSP11 - An RNA phosphatase that regulates host and viral non-coding RNAs in mammalian cells. RNA Biol. 2017, DOI: 10.1080/15476286.2017.1306169, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (260).Takano A; Endo T; Yoshihisa T tRNA actively shuttles between the nucleus and cytosol in yeast. Science 2005, 309, 140–142. [DOI] [PubMed] [Google Scholar]
- (261).Shaheen HH; Hopper AK Retrograde movement of tRNAs from the cytoplasm to the nucleus in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A 2005, 102, 11290–11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Ohira T; Suzuki T Retrograde nuclear import of tRNA precursors is required for modified base biogenesis in yeast. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 10502–10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (263).Kramer EB; Hopper AK Retrograde transfer RNA nuclear import provides a new level of tRNA quality control in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2013, 110, 21042–21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (264).Arts GJ; Kuersten S; Romby P; Ehresmann B; Mattaj IW The role of exportin-t in selective nuclear export of mature tRNAs. EMBO J. 1998, 17, 7430–7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (265).Shaheen HH; Horetsky RL; Kimball SR; Murthi A; Jefferson LS; Hopper AK Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 8845–8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (266).Hasler D; Lehmann G; Murakawa Y; Klironomos F; Jakob L; Grasser FA; Rajewsky N; Landthaler M; Meister G The lupus autoantigen La prevents mis-channeling of tRNA fragments into the human microRNA pathway. Mol. Cell 2016, 63, 110–124. [DOI] [PubMed] [Google Scholar]
- (267).LaRiviere FJ; Cole SE; Ferullo DJ; Moore MJ A late-acting quality control process for mature eukaryotic rRNAs. Mol. Cell 2006, 24, 619–626. [DOI] [PubMed] [Google Scholar]
- (268).Cole SE; LaRiviere FJ; Merrikh CN; Moore MJ A convergence of rRNA and mRNA quality control pathways revealed by mechanistic analysis of nonfunctional rRNA decay. Mol. Cell 2009, 34, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (269).Shoemaker CJ; Eyler DE; Green R Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science 2010, 330, 369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (270).Tsuboi T; Kuroha K; Kudo K; Makino S; Inoue E; Kashima I; Inada T Dom34:hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3′ end of aberrant mRNA. Mol. Cell 2012, 46, 518–529. [DOI] [PubMed] [Google Scholar]
- (271).Fujii K; Kitabatake M; Sakata T; Miyata A; Ohno M A role for ubiquitin in the clearance of nonfunctional rRNAs. Genes Dev. 2009, 23, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Fujii K; Kitabatake M; Sakata T; Ohno M 40S subunit dissociation and proteasome-dependent RNA degradation in nonfunctional 25S rRNA decay. EMBO J. 2012, 31, 2579–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (273).Mirzaei H; Regnier F Protein-RNA cross-linking in the ribosomes of yeast under oxidative stress. J. Proteome Res 2006, 5, 3249–3259. [DOI] [PubMed] [Google Scholar]
- (274).Jamar NH; Kritsiligkou P; Grant CM The non-stop decay mRNA surveillance pathway is required for oxidative stress tolerance. Nucleic Acids Res. 2017, 45, 6881–6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (275).Mills EW; Wangen J; Green R; Ingolia NT Dynamic regulation of a ribosome rescue pathway in erythroid cells and platelets. Cell Rep. 2016, 17, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Ishimura R; Nagy G; Dotu I; Zhou H; Yang XL; Schimmel P; Senju S; Nishimura Y; Chuang JH; Ackerman SL RNA function. Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration. Science 2014, 345, 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Heras SR; Macias S; Plass M; Fernandez N; Cano D; Eyras E; Garcia-Perez JL; Caceres JF The Microprocessor controls the activity of mammalian retrotransposons. Nat. Struct. Mol. Biol 2013, 20, 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (278).Rybak-Wolf A; Jens M; Murakawa Y; Herzog M; Landthaler M; Rajewsky N A variety of dicer substrates in human and C. elegans. Cell 2014, 159, 1153–1167. [DOI] [PubMed] [Google Scholar]
- (279).Nolte-’t Hoen EN; Buermans HP; Waasdorp M; Stoorvogel W; Wauben MH; t Hoen PA Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012, 40, 9272–9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (280).Nabet BY; Qi Y; Shabason JE; Wu TJ; Yoon T; Kim BC; Benci JL; DeMichele AM; Tchou J; Marcotrigiano J et al. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 2017, 170, 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (281).Shurtleff MJ; Yao J; Qin Y; Nottingham RM; Temoche-Diaz MM; Schekman R; Lambowitz AM A broad role for YBX1 in defining the small non-coding RNA composition of exosomes. Proc. Natl. Acad. Sci. U. S. A 2017, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Colombo M; Raposo G; Thery C Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol 2014, 30, 255–289. [DOI] [PubMed] [Google Scholar]
- (283).Li Y; Zheng Q; Bao C; Li S; Guo W; Zhao J; Chen D; Gu J; He X; Huang S Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (284).Lasda E; Parker R Circular RNAs co-precipitate with extracellular vesicles: a possible mechanism for circRNA clearance. PLoS One 2016, 11, e0148407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (285).O’Brien CA; Wolin SL A possible role for the 60 kd Ro autoantigen in a discard pathway for defective 5S ribosomal RNA precursors. Genes Dev. 1994, 8, 2891–2903. [DOI] [PubMed] [Google Scholar]
- (286).Shi H; O’Brien CA; Van Horn DJ; Wolin SL A misfolded form of 5S rRNA is associated with the Ro and La autoantigens. RNA 1996, 2, 769–784. [PMC free article] [PubMed] [Google Scholar]
- (287).Chen X; Smith JD; Shi H; Yang DD; Flavell RA; Wolin SL The Ro autoantigen binds misfolded U2 small nuclear RNAs and assists mammalian cell survival after UV irradiation. Curr. Biol 2003, 13, 2206–2211. [DOI] [PubMed] [Google Scholar]
- (288).Stein AJ; Fuchs G; Fu C; Wolin SL; Reinisch KM Structural insights into RNA quality control: the Ro autoantigen binds misfolded RNAs via its central cavity. Cell 2005, 121, 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (289).Fuchs G; Stein AJ; Fu C; Reinisch KM; Wolin SL Structural and biochemical basis for misfolded RNA recognition by the Ro protein. Nat. Struct. Mol. Biol 2006, 13, 1002–1009. [DOI] [PubMed] [Google Scholar]
- (290).Labbe JC; Hekimi S; Rokeach LA The levels of the RoRNP-associated Y RNA are dependent upon the presence of ROP-1, the Caenorhabditis elegans Ro60 protein. Genetics 1999, 151, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (291).Chen X; Wurtmann EJ; Van Batavia J; Zybailov B; Washburn MP; Wolin SL An ortholog of the Ro autoantigen functions in 23S rRNA maturation in D. radiodurans. Genes Dev 2007, 21, 1328–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (292).Rinke J; Steitz JA Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell 1982, 29, 149–159. [DOI] [PubMed] [Google Scholar]
- (293).Rinke J; Steitz JA Association of the lupus antigen La with a subset of U6 snRNA molecules. Nucleic Acids Res. 1985, 13, 2617–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (294).Chambers JC; Kurilla MG; Keene JD Association between the 7S RNA and the lupus La protein varies among cell types. J. Biol. Chem 1983, 258, 11438–11441. [PubMed] [Google Scholar]
- (295).Hendrick JP; Wolin SL; Rinke J; Lerner MR; Steitz JA Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol. Cell. Biol 1981, 1, 1138–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (296).Kickhoefer VA; Poderycki MJ; Chan EK; Rome LH The La RNA-binding protein interacts with the vault RNA and is a vault-associated protein. J. Biol. Chem 2002, 277, 41282–41286. [DOI] [PubMed] [Google Scholar]
- (297).Shen CK; Maniatis T The organization, structure, and in vitro transcription of Alu family RNA polymerase III transcription units in the human alpha-like globin gene cluster: precipitation of in vitro transcripts by lupus anti-La antibodies. J. Mol. Appl. Genet 1982, 1, 343–360. [PubMed] [Google Scholar]
- (298).Stefano JE Purified lupus antigen La recognizes an oligouridylate stretch common to the 3′ termini of RNA polymerase III transcripts. Cell 1984, 36, 145–154. [DOI] [PubMed] [Google Scholar]
- (299).Inada M; Guthrie C Identification of Lhp1p-associated RNAs by microarray analysis in Saccharomyces cerevisiae reveals association with coding and noncoding RNAs. Proc. Natl. Acad. Sci. U. S. A 2004, 101, 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (300).Fan H; Goodier JL; Chamberlain JR; Engelke DR; Maraia RJ 5′ processing of tRNA precursors can be modulated by the human La antigen phosphoprotein. Mol. Cell. Biol 1998, 18, 3201–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (301).Lin-Marq N; Clarkson SG Efficient synthesis, termination and release of RNA polymerase III transcripts in Xenopus extracts depleted of La protein. EMBO J. 1998, 17, 2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (302).Chakshusmathi G; Kim SD; Rubinson DA; Wolin SL A La protein requirement for efficient pre-tRNA folding. EMBO J. 2003, 22, 6562–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (303).Huang Y; Bayfield MA; Intine RV; Maraia RJ Separate RNA-binding surfaces on the multifunctional La protein mediate distinguishable activities in tRNA maturation. Nat. Struct. Mol. Biol 2006, 13, 611–618. [DOI] [PubMed] [Google Scholar]
- (304).Skowronek E; Grzechnik P; Spath B; Marchfelder A; Kufel J tRNA 3′ processing in yeast involves tRNase Z, Rex1, and Rrp6. RNA 2014, 20, 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (305).Arhin GK; Shen S; Perez IF; Tschudi C; Ullu E Downregulation of the essential Trypanosoma brucei La protein affects accumulation of elongator methionyl-tRNA. Molec. Biochem. Parasitol 2005, 144, 104–108. [DOI] [PubMed] [Google Scholar]
- (306).Copela L; Chakshusmathi G; Sherrer R; Wolin S The La protein functions redundantly with tRNA modification enzymes to ensure tRNA structural stability. RNA 2006, 12, 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (307).Pannone BK; Kim SD; Noe DA; Wolin SL Multiple functional interactions between components of the Lsm2-Lsm8 complex, U6 snRNA, and the yeast La protein. Genetics 2001, 158, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (308).Lafontaine DL; Bousquet-Antonelli C; Henry Y; Caizergues-Ferrer M; Tollervey D The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes Dev. 1998, 12, 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (309).Henras A; Henry Y; Bousquet-Antonelli C; Noaillac-Depeyre J; Gelugne JP; Caizergues-Ferrer M Nhp2p and Nop10p are essential for the function of H/ACA snoRNPs. EMBO J. 1998, 17, 7078–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (310).Watkins NJ; Gottschalk A; Neubauer G; Kastner B; Fabrizio P; Mann M; Luhrmann R Cbf5p, a potential pseudouridine synthase, and Nhp2p, a putative RNA-binding protein, are present together with Gar1p in all H BOX/ACA-motif snoRNPs and constitute a common bipartite structure. RNA 1998, 4, 1549–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (311).Brown JD; Hann BC; Medzihradszky KF; Niwa M; Burlingame AL; Walter P Subunits of the Saccharomyces cerevisiae signal recognition particle required for its functional expression. EMBO J. 1994, 13, 4390–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (312).Mitchell JR; Wood E; Collins K A telomerase component is defective in the human disease dyskeratosis congenita. Nature 1999, 402, 551–555. [DOI] [PubMed] [Google Scholar]
- (313).Kickhoefer VA; Liu Y; Kong LB; Snow BE; Stewart PL; Harrington L; Rome LH The Telomerase/vault-associated protein TEP1 is required for vault RNA stability and its association with the vault particle. J. Cell Biol 2001, 152, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (314).Mayes AE; Verdone L; Legrain P; Beggs JD Characterization of Sm-like proteins in yeast and their association with U6 snRNA. EMBO J. 1999, 18, 4321–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (315).Salgado-Garrido J; Bragado-Nilsson E; Kandels-Lewis S; B S. r. Sm and Sm-like proteins assemble in two related complexes of deep evolutionary origin. EMBO J. 1999, 18, 3451–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (316).Achsel T; Brahms H; Kastner B; Bachi A; Wilm M; Luhrmann R A doughnut-shaped heteromer of human Sm-like proteins binds to the 3′-end of U6 snRNA, thereby facilitating U4/U6 duplex formation in vitro. EMBO J. 1999, 18, 5789–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (317).Trippe R; Sandrock B; Benecke BJ A highly specific terminal uridylyl transferase modifies the 3′-end of U6 small nuclear RNA. Nucleic Acids Res. 1998, 26, 3119–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (318).Lund E; Dahlberg JE Cyclic 2′,3′ phosphates and nontemplated nucleotides at the 3′ end of spliceosomal U6 small nuclear RNA’s. Science 1992, 255, 327–330. [DOI] [PubMed] [Google Scholar]
- (319).Kamminga LM; Luteijn MJ; den Broeder MJ; Redl S; Kaaij LJ; Roovers EF; Ladurner P; Berezikov E; Ketting RF Hen1 is required for oocyte development and piRNA stability in zebrafish. EMBO J. 2010, 29, 3688–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (320).Wurtmann EJ; Wolin SL RNA under attack: cellular handling of RNA damage. Crit. Rev. Biochem. Mol. Biol 2009, 44, 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (321).Simms CL; Zaher HS Quality control of chemically damaged RNA. Cell. Mol. Life Sci 2016, 73, 3639–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (322).Burroughs AM; Aravind L RNA damage in biological conflicts and the diversity of responding RNA repair systems. Nucleic Acids Res. 2016, 44, 8525–8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (323).Nandakumar J; Schwer B; Schaffrath R; Shuman S RNA repair: an antidote to cytotoxic eukaryal RNA damage. Mol. Cell 2008, 31, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (324).Wan J; Yourshaw M; Mamsa H; Rudnik-Schoneborn S; Menezes MP; Hong JE; Leong DW; Senderek J; Salman MS; Chitayat D et al. Mutations in the RNA exosome component gene EXOSC3 cause pontocerebellar hypoplasia and spinal motor neuron degeneration. Nat. Genet 2012, 44, 704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).Boczonadi V; Muller JS; Pyle A; Munkley J; Dor T; Quartararo J; Ferrero I; Karcagi V; Giunta M; Polvikoski T et al. EXOSC8 mutations alter mRNA metabolism and cause hypomyelination with spinal muscular atrophy and cerebellar hypoplasia. Nat Commun 2014, 5, 4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (326).Giunta M; Edvardson S; Xu Y; Schuelke M; Gomez-Duran A; Boczonadi V; Elpeleg O; Muller JS; Horvath R Altered RNA metabolism due to a homozygous RBM7 mutation in a patient with spinal motor neuropathy. Hum Mol Genet 2016, 25, 2985–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Astuti D; Morris MR; Cooper WN; Staals RH; Wake NC; Fews GA; Gill H; Gentle D; Shuib S; Ricketts CJ et al. Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nat Genet 2012, 44, 277–284. [DOI] [PubMed] [Google Scholar]
- (328).Chapman MA; Lawrence MS; Keats JJ; Cibulskis K; Sougnez C; Schinzel AC; Harview CL; Brunet JP; Ahmann GJ; Adli M et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011, 471, 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (329).Cancer Genome Atlas Research Network; Weinstein JN; Collisson EA; Mills GB; Shaw KR; Ozenberger BA; Ellrott K; Shmulevich I; Sander C; Stuart JM The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet 2013, 45, 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Crampton SP; Bolland S Spontaneous activation of RNA-sensing pathways in autoimmune disease. Curr. Opin. Immunol 2013, 25, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (331).Andersson R; Gebhard C; Miguel-Escalada I; Hoof I; Bornholdt J; Boyd M; Chen Y; Zhao X; Schmidl C; Suzuki T et al. An atlas of active enhancers across human cell types and tissues. Nature 2014, 507, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]


