Significance
Cholesteryl-α-D-glucopyranoside (CGL) is a cell wall constituent of Helicobacter pylori. It is synthesized by cholesterol α-glucosyltransferase, which transfers glucose from UDP-glucose to a carbon atom at the third position of cholesterol with an α1,3-linkage. We previously discovered that αGlcNAc contained in gastric gland mucins serves as antibiotic against H. pylori by inhibiting CGL biosynthesis. Here, we reveal that cholestenone exhibits antibiotic activity against H. pylori including a clarithromycin-resistant H. pylori strain by suppressing CGL biosynthesis in vitro. Strikingly, oral administration of cholestenone alone successfully eradicated H. pylori infection in C57BL/6 mice. Given its safety, cholestenone therapy could be promising to eradicate H. pylori, including antibiotic-resistant strains.
Keywords: antimicrobial resistance, glycolipid, eradication, Helicobacter pylori
Abstract
Helicobacter pylori, a pathogen responsible for gastric cancer, contains a unique glycolipid, cholesteryl-α-D-glucopyranoside (CGL), in its cell wall. Moreover, O-glycans having α1,4-linked N-acetylglucosamine residues (αGlcNAc) are secreted from gland mucous cells of gastric mucosa. Previously, we demonstrated that CGL is critical for H. pylori survival and that αGlcNAc serves as antibiotic against H. pylori by inhibiting CGL biosynthesis. In this study, we tested whether a cholesterol analog, cholest-4-en 3-one (cholestenone), exhibits antibacterial activity against H. pylori in vitro and in vivo. When the H. pylori standard strain ATCC 43504 was cultured in the presence of cholestenone, microbial growth was significantly suppressed dose-dependently relative to microbes cultured with cholesterol, and cholestenone inhibitory effects were not altered by the presence of cholesterol. Morphologically, cholestenone-treated H. pylori exhibited coccoid forms. We obtained comparable results when we examined the clarithromycin-resistant H. pylori strain “2460.” We also show that biosynthesis of CGL and its derivatives cholesteryl-6-O-tetradecanoyl-α-D-glucopyranoside and cholesteryl-6-O-phosphatidyl-α-D-glucopyranoside in H. pylori is remarkably inhibited in cultures containing cholestenone. Lastly, we asked whether orally administered cholestenone eradicated H. pylori strain SS1 in C57BL/6 mice. Strikingly, mice fed a cholestenone-containing diet showed significant eradication of H. pylori from the gastric mucosa compared with mice fed a control diet. These results overall strongly suggest that cholestenone could serve as an oral medicine to treat patients infected with H. pylori, including antimicrobial-resistant strains.
Helicobacter pylori is a gram-negative microaerophilic pathogen that colonizes the human stomach in approximately half the world’s population. It is well established that H. pylori infection is closely associated with pathogenesis of chronic active gastritis, peptic ulcer, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma (1–4). Thus, in 1994, H. pylori was categorized as a Group I carcinogen by the World Health Organization’s International Agency for Research on Cancer. Accordingly, eradication therapy for H. pylori is expected to decrease the incidence of gastric cancer (5–7). In fact, eradication of the bacterium has been successfully achieved in ∼90% of infected patients using a combination of three drugs, namely, a proton pump inhibitor (PPI), clarithromycin, and amoxicillin (8, 9). However, successful eradication has been challenged by emergence of drug-resistant strains, in particular, clarithromycin-resistant H. pylori (10). Thus, development of new strategies as eradication therapy for H. pylori including drug-resistant strains is needed.
The cell wall of Helicobacter species, including H. pylori, characteristically contains unique glycolipid α-cholesteryl glucosides (αCGs), of which the major components are cholesteryl-α-D-glucopyranoside (CGL), cholesteryl-6-O-tetradecanoyl-α-D-glucopyranoside (CAG), and cholesteryl-6-O-phosphatidyl-α-D-glucopyranoside (CPG) (11). αCGs are synthesized by cholesterol α-glucosyltransferase (αCgT), which transfers glucose from UDP-glucose to a carbon atom at the third position of cholesterol with an α1,3-linkage (SI Appendix, Fig. S1A). On the other hand, gastric gland mucous cells secrete unique O-glycans having terminal α1,4-linked N-acetylglucosamine (αGlcNAc) attached to the scaffold protein MUC6. Previously, we revealed that αGlcNAc suppresses H. pylori growth by inhibiting αCgT activity, which forms CGL (12, 13). Because the H. pylori genome does not encode enzymes required for cholesterol biosynthesis, microbes require exogenous cholesterol to synthesize αCGs (14, 15).
Cholestenone is a cholesterol analog catabolized from cholesterol by intestinal bacteria, including human-derived Escherichia coli, Eubacterium, and Bacteroides sp. that replace the steroid 3β-hydroxyl group with a keto group (16–20). Because the hydroxyl group at the cholesterol third position is critical to form CGL, we hypothesized that cholestenone cannot serve as an αCgT substrate (SI Appendix, Fig. S1B) and thus that cholestenone treatment could inhibit H. pylori growth due to defective CGL biosynthesis.
In the present study, we examined growth capacity of H. pylori in vitro in the presence of cholesterol and analogs including cholestenone, β-sitosterol, and cholestanol (SI Appendix, Fig. S2). Our results clearly indicate that growth of H. pylori, including that of a clarithromycin-resistant strain, was significantly suppressed by cholestenone through inhibition of CGL biosynthesis. When cholestenone was orally administered to H. pylori-infected C57BL/6 mice, mice showed successful eradication of the microbe. Because cholestenone is safe, therapy using cholestenone could be a promising approach to eliminate H. pylori infection in humans, including infection with antibiotic-resistant strains.
Results
Incorporation of Cholesterol into H. pylori.
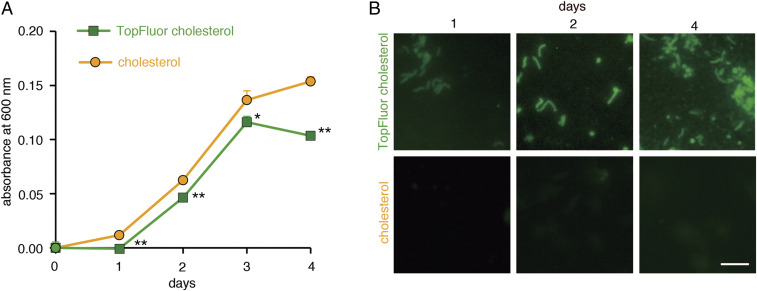
Because H. pylori cannot synthesize cholesterol, microbes require exogenous cholesterol to synthesize CGL required for bacterial growth (12). However, actually incorporated cholesterol into H. pylori had not been visualized. To visualize exogenous cholesterol incorporation into the cell wall, we grew microbes in cultivation broth containing the fluorocholesterol, TopFluor cholesterol, rather than cholesterol (SI Appendix, Fig. S3). H. pylori cultured with TopFluor cholesterol grew well, but the growth rate was significantly lower than that seen in the presence of cholesterol (Fig. 1A). Using fluorescent microscopy, we detected incorporation of TopFluor cholesterol into the H. pylori cell wall, and the relative number of H. pylori incorporating TopFluor cholesterol increased with days in culture (Fig. 1B). These results indicate that H. pylori incorporates exogenous fluorocholesterol.
Fig. 1.
Cholesterol incorporation into H. pylori. (A) Growth curves of H. pylori (ATCC 43504) in media containing cholesterol (circles) or the fluorocholesterol TopFluor cholesterol (squares). Assay was done in triplicate, and error bars indicate SE (*P < 0.05, **P < 0.01). (B) Fluorescence microscopy of H. pylori (ATCC 43504) cultured as indicated over 4 d (Scale bar, 5 μm).
Effects of Cholesterol Derivatives on H. pylori Growth.
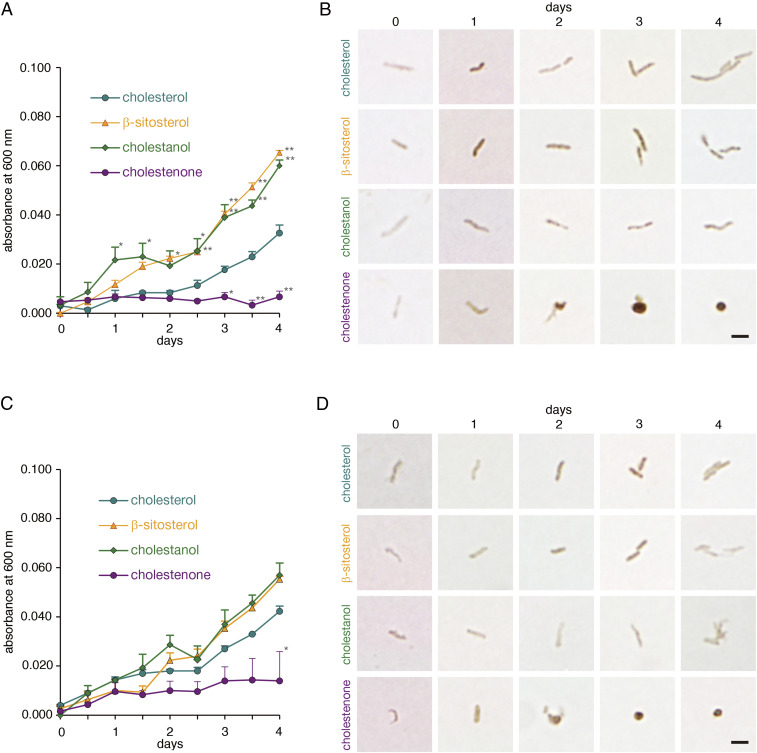
We hypothesized that a hydroxyl group at the third position of the cholesterol carbon atom was critical to form CGL, which is essential for H. pylori survival (SI Appendix, Fig. S1), as α-glucose is attached to this site. To test this hypothesis, we used three kinds of sterol derivatives, including cholestenone, cholestanol, and β-sitosterol, as supplements for H. pylori (ATCC 43504) culture. Among them, cholestenone has a keto group at the third position of carbon atom, while both cholestanol and β-sitosterol have a hydroxyl group at that position (SI Appendix, Fig. S2). As a positive control, we employed cholesterol as a supplement. Most rewardingly, H. pylori growth was markedly suppressed in the presence of cholestenone (Fig. 2A), and microbial shape changed from a normal spiral form to a coccoid form, a transformation evident by day 2 of culture (Fig. 2B). By contrast, H. pylori cultured with cholestanol or β-sitosterol grew as well as microbes grown in cholesterol (Fig. 2A). We observed similar effects on bacterial proliferation and morphology when we tested various cholesterol derivatives using the clinically isolated H. pylori strain “2460,” which is resistant to clarithromycin (Fig. 2 C and D). These results suggest that cholestenone inhibits H. pylori growth, most likely by blocking CGL biosynthesis.
Fig. 2.
Growth curves and morphology of H. pylori cultured with various sterols. (A) Growth curves of a standard H. pylori strain (ATCC 43504) cultured in medium supplemented with 150 μM indicated sterols. Each value represents the average of triplicate measurements, and error bars indicate SE. Statistical analysis was performed using cholesterol as a reference (*P < 0.05, **P < 0.01). (B) Morphology of H. pylori (ATCC 43504) cultured with indicated sterols over a 4 d culture period. Immunocytochemistry was performed using an anti-H. pylori antibody (Scale bar, 2 μm). (C) Growth curves of the clarithromycin-resistant strain H. pylori (“2460”) cultured in medium supplemented with 150 μM indicated sterols. Each value represents the average of triplicate measurements, and error bars indicate SE. Statistical analysis was performed using cholesterol as a reference (*P < 0.05). (D) Morphology of H. pylori (“2460”) cultured with indicated sterols over a 4 d culture period. Immunocytochemistry was performed using an anti-H. pylori antibody (Scale bar, 2 μm).
Growth Inhibitory Effects of Cholestenone in Presence of Cholesterol.
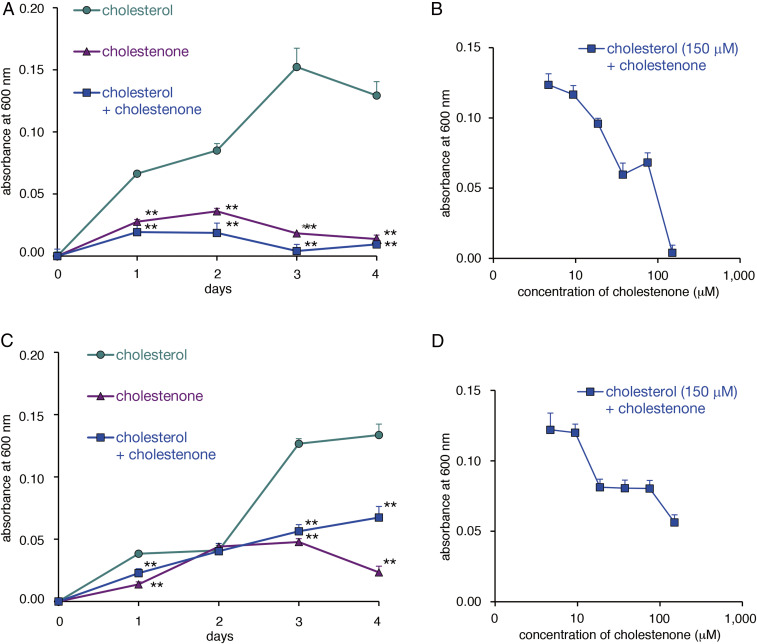
H. pylori attaches to surface mucous cells, which contain cholesterol (21). Thus, we asked whether cholestenone would inhibit growth of H. pylori growth cultured in medium containing an equal concentration of cholesterol. As shown in Fig. 3A, growth of the H. pylori standard strain ATCC 43504 was significantly inhibited by 150 μM cholestenone, either with or without the same concentration of cholesterol (Fig. 3A). Such inhibitory effects were also observed dose-dependently at day 4 of a 4 d culture at cholestenone concentrations ranging from 4.7 to 150 μM in the presence of 150 μM cholesterol (Fig. 3B). We then tested cholestenone antimicrobial effects using a clinically isolated H. pylori strain “2460” resistant to clarithromycin. Surprisingly, over a 4 d culture period, inclusion of 150 μM cholestenone in media suppressed microbial growth irrespective of the presence or absence of the same concentration of cholesterol, an effect that was significant except at day 2 of culture. (Fig. 3C). Moreover, in the presence of 150 μM cholesterol, inclusion of cholestenone in medium had an overall dose-dependent inhibitory effect on growth at concentrations ranging from 4.7 to 150 μM (Fig. 3D).
Fig. 3.
Effects of cholesterol and cholestenone on H. pylori growth. (A) Growth curves of a standard H. pylori strain (ATCC 43054) cultured in medium supplemented with either 150 μM cholesterol, 150 μM cholestenone, or both. Assay was done in triplicate, and error bars indicate SE. Statistical analysis was performed based on cholesterol values (**P < 0.01). (B) Growth curves of H. pylori ATCC 43054 strain cultured for 4 d in medium supplemented with 150 μM cholesterol plus concentrations of cholestenone ranging from 4.7 to 150 μM. All assays were done in triplicate, and error bars indicate SE. (C) Growth curves of a clarithromycin-resistant strain H. pylori “2460” cultured in medium supplemented with either 150 μM cholesterol, 150 μM cholestenone, or both. Assay was done in triplicate, and error bars indicate SE. Statistical analysis was performed based on cholesterol values (**P < 0.01). (D) Growth curves of the H. pylori “2460” strain cultured for 4 d in medium supplemented with 150 μM cholesterol plus concentrations of cholestenone ranging from 4.7 to 150 μM. All assays were done in triplicate, and error bars indicate SE.
H. pylori Cultured with Cholestenone Show Reduced CGL Expression.
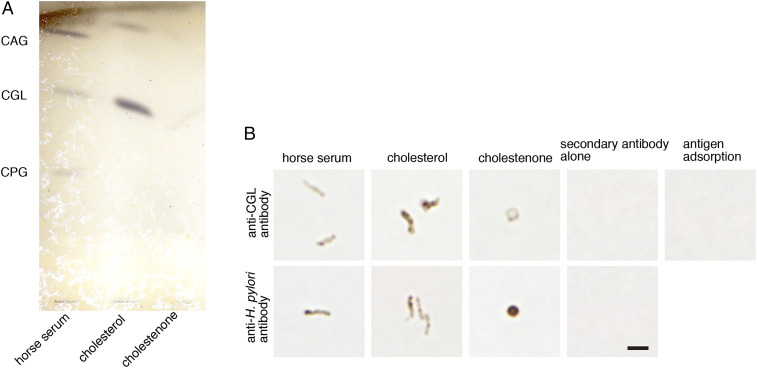
To determine whether cholestenone suppressed CGL biosynthesis, we analyzed lipid fractions extracted from H. pylori cultured 2 d with cholesterol or cholestenone by thin-layer chromatography. H. pylori (ATCC 43504) cultured with standard medium containing 10% inactivated horse serum exhibited three distinct bands representing CGL and its derivatives CAG and CPG (Fig. 4A). When H. pylori was cultured with cholesterol, we detected bands representing CGL and CAG. However, relative to levels seen in the presence of cholesterol, CGL and CAG levels were significantly decreased in H. pylori cultured with cholestenone (Fig. 4A). We also observed relatively reduced CGL expression in H. pylori cultured with cholestenone based on immunocytochemistry with a newly generated anti-CGL antibody, which we validated using an antigen adsorption test (Fig. 4B). Prior to that, immunocytochemistry with commercially available anti-H. pylori antibody revealed that H. pylori (ATCC 43504) cultured 4 d with inactivated horse serum or cholesterol exhibited the typical spiral form, whereas H. pylori cultured with cholestenone exhibited a coccoid form (Fig. 4B). When microbes in this analysis were immunostained with anti-CGL antibody, expression levels of CGL in coccoid forms of H. pylori cultured with cholestenone were reduced compared with spiral forms of the microbe cultured with cholesterol (Fig. 4B). These combined results strongly suggest that cholestenone suppresses CGL biosynthesis.
Fig. 4.
Cholestenone treatment reduces CGL expression in H. pylori. (A) Thin layer chromatogram of αCGs isolated from H. pylori (ATCC 43054) cultured with supplements including inactivated horse serum (as a control) or cholesterol or cholestenone, as indicated. (B) Morphology of H. pylori cultured with various supplements. H. pylori (ATCC 43054) was immunostained with anti-CGL antibody (Upper, first three panels) or anti-H. pylori antibodies (Lower, first three panels). Also shown is staining with secondary antibody (Histofine Simple Stain MAX-PO[R] Kit) alone (Upper and Lower, panel 4). Immunocytochemistry of H. pylori (ATCC 43504) incubated in Brucella broth was supplemented with 10% inactivated horse serum at 35 °C in 15% CO2 for 24 h with anti-CGL antibody preincubated with 0.75 μM of oxidized CGL as hapten is shown as antigen adsorption (Upper, panel 5) (Scale bar, 2 μm).
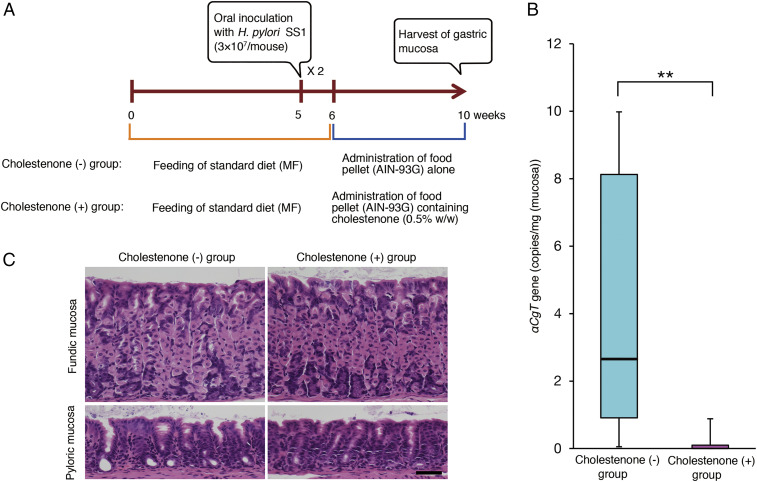
In Vivo Effects of Cholestenone Administration in H. pylori-Infected Mice.
To determine whether orally administered cholestenone would eliminate H. pylori in vivo, we orally infected 5-wk-old C57BL/6 mice (n = 54) with two doses of 3 × 107 H. pylori strain SS1 and then, starting a week later, fed them ad libitum with food pellets with or without 0.5% (wt/wt) cholestenone until 10 wk of age (Fig. 5A). At 10 wk of age, stomachs were removed from both cholestenone (+) and (−) groups. We then determined the number of H. pylori in stomach using qPCR of DNA harboring the H. pylori αCgT gene, which encodes cholesterol αCgT that catalyzes CGL biosynthesis (22–24). The copy number of αCgT ranged from 0 to 45.6 copies (median [25th to 75th percentile] = 2.65 [0.91 to 8.13]) per 1 mg gastric mucosa in the cholestenone (−) group (n = 24) and ranged from 0 to 9.1 copies (0.00 [0.00 to 0.10]) per the same weight of gastric mucosa in the cholestenone (+) group (n = 24) (Fig. 5B). Because αCgT is a single-copy gene in the microbe, we conclude that the number of H. pylori in the cholestenone (+) group was significantly lower than that in the cholestenone (−) group (P < 0.01). These results establish that 4 wk of dietary cholestenone administration successfully eradicates H. pylori infected in the gastric mucosa of mice. Histopathological examination of the gastric mucosa showed no obvious histopathological findings in all mice (n = 6), including three mice in the cholestenone (−) group and three mice in the cholestenone (+) group (Fig. 5C).
Fig. 5.
Effects of orally administered cholestenone on H. pylori-infected mice. (A) Treatment schedule used to test effects of cholestenone diet in C57BL/6 mice infected with H. pylori (SS1 strain). H. pylori was administrated orally a total of two times. (B) Copy numbers of the H. pylori αCgt gene present in 1 mg gastric mucosa. Boxes represent 25th to 75th percentile of copy numbers, and the horizontal line shows the median value. Whiskers represent 10th and 90th percentiles of copy numbers. Asterisk indicates a statistically significant difference (**P < 0.01). (C) Representative histopathology of the gastric mucosa of mice, with hematoxylin and eosin staining (Scale bar, 50 μm).
Discussion
Here, we report that cholestenone suppresses H. pylori growth via a mechanism distinct from that of clarithromycin or amoxicillin, which are generally used for H. pylori eradication. Specifically, clarithromycin inhibits peptide translation, and amoxicillin inhibits peptidoglycan biosynthesis in the cell wall (25). Hirai et al. reported that H. pylori exhibits the unique glycolipids CGL, CAG, and CPG, which make up about 25% of total lipid (11). H. pylori can be grown in liquid cultures containing Columbia broth, Brain Heart infusion broth, or Brucella broth, all enriched with inactivated horse serum (26). H. pylori incorporates exogenous cholesterol contained in all of these media to form CGL as a cell wall component, and CGL is critical for H. pylori survival (27). Previously, we found that H. pylori can grow well with exogenous cholesterol but poorly in serum-free medium (12). Despite the fact that H. pylori requires cholesterol to construct its cell membrane (13), H. pylori does not possess a cholesterol biosynthetic pathway (14, 15, 28, 29). Here, we supplemented culture medium with fluorescent cholesterol and found it incorporated into H. pylori (Fig. 1B). We also demonstrated that cholestenone inhibited biosynthesis of CGL and its derivatives CAG and CPG (Fig. 4A). Cholestenone inhibition of H. pylori growth was also associated with the coccoid form of the microbe (Fig. 2B), a morphological transformation also seen in αCgT-deficient H. pylori (30). It is well known that like spiral forms, coccoid forms of H. pylori are viable, but unlike spiral forms, coccoid forms are nonculturable and relatively less virulent (31). Notably, Eaton et al. revealed that coccoid forms of H. pylori cannot colonize gnotobiotic piglets, unlike spiral forms (32). These results combined together strongly suggest that H. pylori cultured with cholestenone is nonvirulent and loses infectivity.
The present study demonstrated that cholestenone displayed remarkable antibiotic activity against H. pylori, even in the presence of cholesterol (Fig. 3). We previously reported that αCgT acts in an ordered Bi–Bi manner (i.e., UDP-Glucose binds to catalytic site of αCgT, and then cholesterol binds to another substrate binding site of the enzyme, thus forming CGL) (33). In the same study, we also demonstrated that CGL inhibits αCgT in presence of cholesterol in a mixed-type manner. Because of structural similarity between CGL and cholestenone (SI Appendix, Fig. S1), it is most possible that cholestenone inhibited αCgT in a mixed-type manner. Further study will be needed to address this problem.
Catabolic transformation of cholesterol to cholestenone requires oxidation of the 3β-hydroxyl group, which is catalyzed by two different enzymes: cholesterol oxidase (ChOx) or 3β-hydroxysteroid dehydrogenase (3β-HsD) (18–20). ChOx and 3β-HsD have been isolated from bacteria such as Bacteroides sp., Bacillus spp., Mycobacterium sp., Rhodococcus spp., Nocardia sp., Arthrobacter sp., and Pseudomonas sp. Some strains of E. coli, Eubacterium, and Bacteroides isolated from humans produce cholestenone (16, 17), and Bacteroides is a major microbe in human gut microbiota (34). However, little is known about why cholesterol is converted to cholestenone in microorganisms, except that in Myobacterium tuberculosis, cholestenone may suppress the host immune responses (18). Importantly, cholestenone has few toxicological effects. In fact, Suzuki et al. demonstrated favorable effects of cholestenone on lipid metabolism, such as body weight control in mice, and reported that mice fed cholestenone are healthy without clinical abnormalities (35). Furthermore, there is no evidence that cholestenone is a mutagen (36).
We revealed that cholestenone alone administered orally to H. pylori-infected mice had an eradication effect (Fig. 5B). As yet, no drug has been reported to have eradicative effects in vivo on H. pylori infection when given as a single agent. In this study, no attempt was made to detect cholestenone in the stomach of mice. However, since cholestenone is resistant to acid (37), it is thought to act stably against H. pylori in the stomach. For the clinical application of cholestenone, studies on dosage, administration period, and safety are needed. In the event that clinical use in humans is approved in the future, administration of cholestenone is expected to be as effective as antibiotics in eradicating H. pylori.
Strikingly, H. pylori growth suppression by cholestenone was also effective against a clinically isolated drug-resistant H. pylori strain. First-line triple therapy including amoxicillin, clarithromycin, and a PPI administered one week is used to treat H. pylori infection (38). However, this treatment fails in 17.2% of patients, mainly due to clarithromycin resistance (39). Thus, metronidazole is employed instead of clarithromycin as second-line triple therapy (40). However, 26.7% of those patients fail due to metronidazole resistance (39). In addition, H. pylori showing multidrug resistance is increasing worldwide (41–43), and emergence of antibiotic-resistant H. pylori strains has led to reduced success with traditional treatments (44). Vivas et al. have proposed that if no new drug is developed to treat pathogenic bacteria by 2050, there will be no effective antibiotic available (45). Thus, new antibiotics having mechanisms distinct from traditional agents are required for H. pylori eradication. Our findings indicate that cholestenone is a potent candidate as an antibiotic for H. pylori including clarithromycin-resistant strain by suppressing CGL.
Materials and Methods
Bacterial Strains.
A standard strain of H. pylori ATCC 43504 (46) and a mouse-adapted strain H. pylori SS1 (47) were used as described (12, 13, 48). Also used was the drug-resistant H. pylori “2460”, which was clinically isolated at Shinshu University Hospital, Matsumoto, Japan and stored at −80 °C in Department of Laboratory Medicine in the same hospital.
Bacterial Growth Assay.
Microbes (ATCC 43504, H. pylori “2460”, and H. pylori SS1) were preincubated in Brucella broth supplemented with 10% inactivated horse serum at 35 °C in 15% CO2 for 24 h, as described previously (49). After preincubation, H. pylori were subsequently cultured in 96-well plates in Mueller Hinton broth supplemented with various concentrations of cholesterol and analogs including fluorocholesterol (23-(dipyrrometheneboron difluoride)-24-norcholesterol; TopFluor Cholesterol), cholestenone, β-sitosterol, and cholestanol. Supplement concentrations were adjusted to 150 μM, 75 μM, 38 μM, 19 μM, 9 μM, and 4.7 μM by twofold serial dilutions. Microbes were cultured 4 d in 15% CO2 at 35 °C. Bacterial growth was measured based on OD at 600 nm (OD600), and the absorbance of Mueller Hinton broth alone without microbes was measured at each time point as background. Horse serum was purchased from GIBCO/Life Technologies. Brucella broth was purchased from Becton Dickinson. Mueller Hinton broth was purchased from Eiken Chemical. Cholesterol, cholestenone, and β-sitosterol were purchased from Wako Pure Chemical. Cholestanol was purchased from Toronto Research Chemicals. TopFluor Cholesterol was purchased from Avanti Polar Lipids.
Production of the CGL Polyclonal Antibody.
CGL was purified from glycolipid fractions of the standard strain of H. pylori (ATCC43504) separated on a silica gel thin-layer chromatography plate using chloroform–methanol–water (70:30:5) solvent. A carboxy group was added to CGL by oxidization with KMnO4, followed by keyhole limpet hemocyanin (KLH) conjugation via amine coupling. In brief, 10 mg KLH was dissolved in 2.5 mL of 0.1 M 2-(N-morpholino) ethanesulfonic acid (MES, pH 6.0). An aliquot (5 mL) of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide 8 mg) and 12 mg of N-hydroxysuccinimide dissolved in MES buffer (pH 6.0) was added to oxidized CGL and mixed for 15 min at room temperature (RT). Then, 2.5 mL KLH was added and the mixture allowed to react 2 h at RT. The oxidized CGL–KLH conjugate was dialyzed over 24 h in 1 L × 4 of 0.15 M saline solution at 4 °C. Subsequently, oxidized CGL–KLH was subcutaneously injected into two New Zealand White rabbits (SLC, Shizuoka, Japan) at weekly intervals three times, followed by additional injections given at 2 wk intervals, and animals were bled at 14 d after injection. Antisera were used without purification. The protocol for animal experiments was approved by the Animal Care Committee of Shinshu University and conducted in accordance with guidelines for use of laboratory animals at the same university (no. 200022).
Immunocytochemistry.
A total 2 μL culture media of H. pylori (ATCC 43504 and “2460” strains) used for the bacterial growth assay (as described above) was obtained at each culture day, placed on Micro Slides Demarcation glass (Muto Pure Chemical), air dried, and heat fixed using a flame. Slides were incubated with anti-H. pylori antibody (DakoCytomation) or the anti-CGL antibody described above for 1 h at room temperature, anti-rabbit immunoglobulins conjugated with horseradish peroxidase (HRP)-labeled polymer. A Histofine Simple Stain MAX-PO(R) Kit (Nichirei Biosciences, Tokyo, Japan) served as the secondary antibody. HRP activity was visualized using 3,3′-diaminobenzidine (Dojindo) with H2O2. As negative controls, primary antibodies were omitted from the immunocytochemistry procedure for H. pylori (ATCC 43504) incubated in Brucella broth supplemented with 10% inactivated horse serum at 35 °C in 15% CO2 for 24 h; no specific staining was observed (Fig. 4B). In addition, anti-CGL antibody adsorbed with 0.75 μM of oxidized CGL as hapten was used for the same culture conditions of H. pylori (ATCC 43504), and no specific staining was noted (Fig. 4B).
Incorporation of Fluorescently Labeled Cholesterol.
To verify that H. pylori growth requires exogenous cholesterol, H. pylori were cultured with fluorescent cholesterol (SI Appendix, Fig. S3). Specifically, after preincubation, bacteria were cultured in 96-well microtiter plates in Mueller Hinton broth supplemented with 150 μM of TopFluor cholesterol purchased from Avanti Polar Lipids. Cholesterol alone, purchased from Sigma-Aldrich, served as a positive control. Bacterial growth was measured as above. Incorporation of fluorescent cholesterol was observed by fluorescence microscopy at days 1, 2, and 4.
Preparation of Cholestenone/Cholesterol Mixtures.
After preincubation, H. pylori (ATCC 43504 and “2460”) were cultured in 96-well plates in Mueller Hinton broth supplemented with cholesterol, cholestenone, or both. Concentrations of cholesterol or cholestenone alone were 150 μM, 75 μM, 38 μM, 19 μM, 9 μM, and 4.7 μM. In analysis of mixtures, cholesterol concentrations were held constant at 150 μM, while cholestenone concentrations were 150 μM, 75 μM, 38 μM, 19 μM, 9 μM, and 4.7 μM. As controls, bacteria were also cultured in Mueller Hinton broth supplemented with 150 μM cholesterol. Bacterial growth was measured as above.
Thin-Layer Chromatography.
Thin-layer chromatography was performed to evaluate αCG synthesis. After preincubation, H. pylori (ATCC 43504) were cultured 2 d in Mueller Hinton broth supplemented with 150 μM cholestenone or 150 μM cholesterol as a control. As a standard culture, H. pylori were also cultured in Brucella broth supplemented with 10% inactivated horse serum. After centrifugation, cell pellets were washed in saline. A total 5 mL chloroform:methanol (2:1) were added to pellets, and total lipids were extracted for 1 h at room temperature. Extracts were filtered, and 1 mL water was added, followed by removal of the aqueous layer. The remaining layer was dried under nitrogen and redissolved in chloroform:methanol (2:1). Purified lipids were separated on silica gel plates (Whatman, Maidstone, England), activated with a solvent of chloroform–methanol–water (16:8:1), and analyzed using orcinol sulfate.
Mice.
C57BL/6J mice were purchased from Charles River Japan (Kanagawa, Japan) and maintained in autoclaved cages under specific pathogen-free conditions at the Animal Facility of Shinshu University, Matsumoto, Japan. Mice were bred until 10 wk of age. The protocol for animal experiments was approved by the Animal Care Committee of Shinshu University and conducted in accordance with guidelines for use of laboratory animals at the same university (no. 300022).
Cholestenone Diet.
A 0.5% cholestenone mix based on AIN-93G and AIN-93G control diets used in the present study were purchased from Oriental Yeast. Specifically, chemically synthesized cholestenone was purchased from Wako Pure Chemical (catalog number 32-3190), and the purity measured by high-performance liquid chromatography was 98.9% (lot number DSH8741). Then, cholestenone diet was prepared by uniformly mixing cholestenone to 0.5% in AIN-93G at Oriental Yeast. Both cholestenone and AIN-93G control diets sterilized by γ-ray irradiation were stored at 4 °C until the time of use.
Cholestenone Administration to H. pylori-Infected Mice.
In total, 54 5-wk-old male mice were given a total of two oral doses of H. pylori SS1 every other day. Mice were fed the standard diet (MF, Oriental Yeast) for one week after infection, and then the diet was changed to the base diet AIN-93G for 27 mice (cholestenone [−] group) or the cholestenone-containing diet for the other 27 (cholestenone [+] group). Four weeks later, mice in both groups were killed by cervical dislocation, and stomachs were removed for quantitation of H. pylori (n = 48) and histological examination (n = 6).
qPCR.
Stomachs from 48 mice (24 in the cholestenone [−] group and 24 in the cholestenone [+] group) were opened along the greater curvature. The gastric mucosa was scraped off with a glass slide, transferred to a sterile 1.5 mL tube, and the mucosal weight was measured (average 57 mg each). The gastric mucosa was gently homogenized, followed by extraction of genomic DNA using a High Pure PCR preparation Kit (Roche Diagnostics GmbH), according to the manufacturer’s protocol. qPCR using SYBR Green I technology was performed using the 7300 Real-Time PCR System (Applied Biosystems) with the FastStart Universal SYBR Green Master (ROX) (Roche Diagnostics GmbH) as detection chemistry. The upstream and downstream primers designed to amplify H. pylori αCgT DNA for PCR were 5′-GGGCCT-GATGAGAAAAAAATC-3′ and 5′-GTCGCGCTTAAAGGGCTATT-3′, respectively (50). Because the αCgT gene is unique to H. pylori and present as one copy per cell (14, 22, 23, 28), this assay estimates the number of H. pylori in mouse stomach. The master mix for each PCR run was prepared as follows: 2x Fast Start Universal SYBR Green Master (ROX), 300 nM each primer, 4.0 μL extracted genomic DNA, and then DNase-free water to a final volume of 50 μL. The amplification program included an initial denaturation step at 95 °C for 10 min, followed by 60 cycles with denaturation at 95 °C for 15 s, and annealing and elongation in one step at 60 °C for 1 min. At the end, the dissociation stage was performed as follows: 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. Using a standard curve for αCgT gene in genomic DNA of H. pylori as described before (50, 51), copy number of αCgT gene was determined for each sample.
Histology.
Stomachs from six mice (three in the cholestenone [−] group and three in the cholestenone [+] group) were opened along the greater curvature and fixed in 10% buffered formalin for 24 h at 4 °C. Each stomach was cut longitudinally into four pieces of equal width and collectively embedded in paraffin. Serial 3 μm sections were prepared from tissue blocks and subjected to hematoxylin and eosin staining.
Statistics.
Statistical analysis was carried out using ystat2013 software (Igaku Tosho Shuppan). Significance was evaluated by Student's t test, Dunnett’s test, or Mann–Whitney U test. All parametric data are presented as means ± SE, and nonparametric data are presented as percentile. A value of P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Elise Lamar for editing the manuscript and Dr. Eriko Kasuga for providing H. pylori “2460” strain. This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Sciences, Nos. 16K08708 and 18K08419 (M.K.) and19H03441 (J.N.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2016469118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Parsonnet J., Helicobacter pylori. Infect. Dis. Clin. North Am. 12, 185–197 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Marshall B., Helicobacter pylori: 20 years on. Clin. Med. (Lond.) 2, 147–152 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suerbaum S., Michetti P., Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Peek R. M. Jr, Crabtree J. E., Helicobacter infection and gastric neoplasia. J. Pathol. 208, 233–248 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Uemura N., et al., Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345, 784–789 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Fukase K.et al.; Japan Gast Study Group , Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet 372, 392–397 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Rugge M., et al., Gastric cancer as preventable disease. Clin. Gastroenterol. Hepatol. 15, 1833–1843 (2017). [DOI] [PubMed] [Google Scholar]
- 8.O’Connor A., Gisbert J. P., McNamara D., O’Morain C., Treatment of Helicobacter pylori infection 2010. Helicobacter 15, 46–52 (2010). [DOI] [PubMed] [Google Scholar]
- 9.O’Morain N. R., Dore M. P., O’Connor A. J. P., Gisbert J. P., O’Morain C. A., Treatment of Helicobacter pylori infection in 2018. Helicobacter 23, e12519 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Luther J., et al., Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: Systematic review and meta-analysis of efficacy and tolerability. Am. J. Gastroenterol. 105, 65–73 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Hirai Y., et al., Unique cholesteryl glucosides in Helicobacter pylori: Composition and structural analysis. J. Bacteriol. 177, 5327–5333 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakubo M., et al., Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305, 1003–1006 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Hoshino H., et al., Membrane-associated activation of cholesterol α-glucosyltransferase, an enzyme responsible for biosynthesis of cholesteryl-α-D-glucopyranoside in Helicobacter pylori critical for its survival. J. Histochem. Cytochem. 59, 98–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomb J. F., et al., The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388, 539–547 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Marais A., Mendz G. L., Hazell S. L., Mégraud F., Metabolism and genetics of Helicobacter pylori: The genome era. Microbiol. Mol. Biol. Rev. 63, 642–674 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdonald I. A., Bokkenheuser V. D., Winter J., McLernon A. M., Mosbach E. H., Degradation of steroids in the human gut. J. Lipid Res. 24, 675–700 (1983). [PubMed] [Google Scholar]
- 17.Gérard P., Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens 3, 14–24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreit J., Microbial catabolism of sterols: Focus on the enzymes that transform the sterol 3β-hydroxy-5-en into 3-keto-4-en. FEMS Microbiol. Lett. 364, fnx007 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Giorgi V., Menéndez P., García-Carnelli C., Microbial transformation of cholesterol: Reactions and practical aspects-an update. World J. Microbiol. Biotechnol. 35, 131 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Mahato S. B., Garai S., Advances in microbial steroid biotransformation. Steroids 62, 332–345 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Hidaka E., et al., Helicobacter pylori and two ultrastructurally distinct layers of gastric mucous cell mucins in the surface mucous gel layer. Gut 49, 474–480 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebrun A. H., et al., Cloning of a cholesterol-α-glucosyltransferase from Helicobacter pylori. J. Biol. Chem. 281, 27765–27772 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Lee H., et al., Expression cloning of cholesterol α-glucosyltransferase, a unique enzyme that can be inhibited by natural antibiotic gastric mucin O-glycans, from Helicobacter pylori. Biochem. Biophys. Res. Commun. 349, 1235–1241 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Kawakubo M., et al., Cholesterol-α-glucosyltransferase gene is present in most Helicobacter species including gastric non-Helicobacter pylori helicobacters obtained from Japanese patients. Helicobacter 23, e12449 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Mégraud F., Resistance of Helicobacter pylori to antibiotics. Aliment. Pharmacol. Ther. 11, 43–53 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Whitmire J. M., Merrell D. S., Successful culture techniques for Helicobacter species: General culture techniques for Helicobacter pylori. Methods Mol. Biol. 921, 17–27 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Qaria M. A., et al., Roles of cholesteryl-α-glucoside transferase and cholesteryl glucosides in maintenance of Helicobacter pylori morphology, cell wall integrity, and resistance to antibiotics. mBio 9, e01523-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alm R. A., et al., Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397, 176–180 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Binh T. T., Suzuki R., Kwon D. H., Yamaoka Y., Complete genome sequence of a metronidazole-resistant Helicobacter pylori strain. Genome Announc. 3, e00051-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito Y., et al., Helicobacter pylori cholesteryl α-glucosides contribute to its pathogenicity and immune response by natural killer T cells. PLoS One 8, e78191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen L. P., Rasmussen L., Helicobacter pylori-coccoid forms and biofilm formation. FEMS Immunol. Med. Microbiol. 56, 112–115 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Eaton K. A., Catrenich C. E., Makin K. M., Krakowka S., Virulence of coccoid and bacillary forms of Helicobacter pylori in gnotobiotic piglets. J. Infect. Dis. 171, 459–462 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Lee H., et al., Alpha1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol alpha-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology 18, 549–558 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekirov I., Russell S. L., Antunes L. C., Finlay B. B., Gut microbiota in health and disease. Physiol. Rev. 90, 859–904 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K., Shimizu T., Nakata T., The cholesterol metabolite cholest-4-en-3-one and its 3-oxo derivatives suppress body weight gain, body fat accumulation and serum lipid concentration in mice. Bioorg. Med. Chem. Lett. 8, 2133–2138 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Wolfreys A. M., Hepburn P. A., Safety evaluation of phytosterol esters. Part 7. Assessment of mutagenic activity of phytosterols, phytosterol esters and the cholesterol derivative, 4-cholesten-3-one. Food Chem. Toxicol. 40, 461–470 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Rushdi A. I., Ritter G., Grimalt J. O., Simoneit B. R. T., Hydrous pyrolysis of cholesterol under various conditions. Org. Geochem. 34, 799–812 (2003). [Google Scholar]
- 38.Misiewicz J. J.et al.; Lansoprazole Helicobacter Study Group , One week triple therapy for Helicobacter pylori: A multicentre comparative study. Gut 41, 735–739 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Francesco V., et al., Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointestin. Liver Dis. 19, 409–414 (2010). [PubMed] [Google Scholar]
- 40.Murakami K., et al., Efficacy of triple therapy comprising rabeprazole, amoxicillin and metronidazole for second-line Helicobacter pylori eradication in Japan, and the influence of metronidazole resistance. Aliment. Pharmacol. Ther. 17, 119–123 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Rezaeimanesh N., Farzi N., Pirmanesh S., Emami S., Yadegar A., Management of multi-drug resistant Helicobacter pylori infection by supplementary, complementary and alternative medicine; A review. Gastroenterol. Hepatol. Bed Bench 10, S8–S14 (2017). [PMC free article] [PubMed] [Google Scholar]
- 42.Tacconelli E.et al.; WHO Pathogens Priority List Working Group , Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Boyanova L., Hadzhiyski P., Kandilarov N., Markovska R., Mitov I., Multidrug resistance in Helicobacter pylori: Current state and future directions. Expert Rev. Clin. Pharmacol. 12, 909–915 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Fallone C. A., et al., The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 151, 51–69.e14 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Vivas R., Barbosa A. A. T., Dolabela S. S., Jain S., Multidrug-resistant bacteria and alternative methods to control them: An overview. Microb. Drug Resist. 25, 890–908 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Goodwin C. S., et al., Transfer of Campylobacter pylori and Campylobacter mustelae to Helicobacter gen. nov. as Helicobacter pylori comb. nov. and Helicobacter mustelae comb. nov., respectively. Int. J. Syst. Bacteriol. 39, 397–405 (1989). [Google Scholar]
- 47.Lee A., et al., A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. Gastroenterology 112, 1386–1397 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Benoit B. N., et al., Role of ASC in the mouse model of Helicobacter pylori infection. J. Histochem. Cytochem. 57, 327–338 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawakubo M., Ito Y., Fukuda M., Nakayama J., “Helicobacter pylori” in Glycoscience: Biology and Medicine, Taniguchi N., Endo T., Hart G., Seeberger P., Wong C. H., Eds. (Springer, Japan, Tokyo, 2015), pp. 723–729. [Google Scholar]
- 50.Kawakubo M., et al., Cloning of Helicobacter suis cholesterol α-glucosyltransferase and production of an antibody capable of detecting it in formalin-fixed, paraffin-embedded gastric tissue sections. Histochem. Cell Biol. 148, 463–471 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Providenti M. A., O’Brien J. M., Ewing R. J., Paterson E. S., Smith M. L., The copy-number of plasmids and other genetic elements can be determined by SYBR-Green-based quantitative real-time PCR. J. Microbiol. Methods 65, 476–487 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.