Abstract
Immune-related manifestations are increasingly recognized conditions in patients with COVID-19, with around 3,000 cases reported worldwide comprising more than 70 different systemic and organ-specific disorders. Although the inflammation caused by SARS-CoV-2 infection is predominantly centred on the respiratory system, some patients can develop an abnormal inflammatory reaction involving extrapulmonary tissues. The signs and symptoms associated with this excessive immune response are very diverse and can resemble some autoimmune or inflammatory diseases, with the clinical phenotype that is seemingly influenced by epidemiological factors such as age, sex or ethnicity. The severity of the manifestations is also very varied, ranging from benign and self-limiting features to life-threatening systemic syndromes. Little is known about the pathogenesis of these manifestations, and some tend to emerge within the first 2 weeks of SARS-CoV-2 infection, whereas others tend to appear in a late post-infectious stage or even in asymptomatic patients. As the body of evidence comprises predominantly case series and uncontrolled studies, diagnostic and therapeutic decision-making is unsurprisingly often based on the scarcely reported experience and expert opinion. Additional studies are required to learn about the mechanisms involved in the development of these manifestations and apply that knowledge to achieve early diagnosis and the most suitable therapy.
Subject terms: Connective tissue diseases, SARS-CoV-2
Immune-related disorders in patients with COVID-19 are increasingly being reported worldwide, with thousands of cases recorded of manifestations that can mimic a broad range of systemic and organ-specific inflammatory and autoimmune diseases.
Key points
COVID-19 can produce a systemic inflammatory reaction involving extra-pulmonary organs.
Immune-related manifestations are increasingly recognized conditions in patients with COVID-19.
~3,000 cases involving >70 different systemic and organ-specific immune-related disorders have been reported.
The clinical phenotype varies and seems to be influenced by age, sex and/or ethnicity.
The severity of immune-related manifestations of COVID-19 ranges from completely benign, self-limiting manifestations to systemic, life-threatening syndromes.
Some features tend to appear within the first 2 weeks of SARS-CoV-2 infection, and others emerge in a late post-infectious stage or even in asymptomatic patients.
Introduction
In January 2020, a novel coronavirus — severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) — was identified as the aetiological agent for a cluster of pneumonia cases initially detected in Wuhan City, China1. The disease caused by SARS-CoV-2, COVID-19, has a very wide clinical spectrum, ranging from asymptomatic cases (accounting for a substantial proportion of infections) to development of bilateral pneumonia that can progress to respiratory failure and, in some cases, multi-organ failure and death2. Some patients can develop a hyperinflammatory response caused by an excessive reaction to the virus, characterized by a highly impaired interferon type I response associated with a persistent blood viral load, with inflammation being partially driven by the transcription factor NF-κB with increased TNF and IL-6 production3. This exacerbated immune-related inflammatory response can promote the development of extrapulmonary features4, including immune-related manifestations that can mimic a wide variety of systemic and organ-specific inflammatory and autoimmune diseases (Box 1), as has been reported in other viral infections5. To date, reports of these manifestations have been scattered among hundreds of manuscripts. This Review compiles the current knowledge of immune-related manifestations associated with the COVID-19 (both systemic and organ-specific) and focuses principally on specific epidemiological, clinical and virological aspects that could help specialists to identify and manage patients presenting with these features.
Box 1 Immune-related disorders reported in patients with COVID-19.
Systemic immune-related manifestations
Multisystem inflammatory syndrome in children
Haemophagocytic syndromes or macrophage activation syndrome
- Vasculitis
- Kawasaki disease in children and adults
- Retinal vasculitis
- Cutaneous leukocytoclastic vasculitis
- IgA vasculitis
- Small and medium-sized vessel gastrointestinal vasculitis
- Diffuse alveolar haemorrhage
- Central nervous system vasculitis
Antiphospholipid antibodies
- Myositis
- Acute myalgia
- Rhabdomyolysis
- Autoimmune inflammatory myopathy
- Necrotizing autoimmune myopathy
- Arthritis
- Acute arthralgias
- Symmetric polyarthritis
- Asymmetric oligoarthritis
- Monoarthritis
- Psoriatic arthritis
- Other systemic autoimmune diseases
- Systemic lupus erythematosus-related symptoms
- Sicca symptoms and/or parotid enlargement
- Sarcoidosis
Organ-specific immune-related manifestations
- Cutaneous
- Chilblain lesions
- Erythema multiforme
- Livedo reticularis
- Retiform purpura
- Oral ulcers
- Erythema nodosum
- Periorbital erythema
- Generalized pustular figurate erythema
- Sweet syndrome
- Livedo racemose
- Haematological
- Immune thrombocytopenic purpura
- Thrombotic thrombocytopenic purpura
- Autoimmune haemolytic anaemia
- Evans syndrome
- Neurological
- Guillain–Barré syndrome
- Miller Fisher syndrome
- Meningoencephalitis
- Autoimmune encephalitis
- Acute disseminated encephalomyelitis
- Acute necrotizing encephalopathy
- Mild encephalitis or encephalopathy with reversible splenial lesion
- Longitudinal extensive transverse myelitis
- Neuromyelitis optica-like syndrome
- Transversal myelitis
- Polyneuritis cranialis
- Optic neuritis
- Plexopathy
- Myasthenia gravis
- Pulmonary
- Interstitial lung disease
- Post-viral organizing pneumonia
- Mediastinal lymphadenopathies
- Pleural effusion
- Cardiac
- Acute myocarditis
- Pericardial effusion
- Cardiac tamponade
- Renal
- Proximal tubular dysfunction
- Collapsing glomerulonephritis
- Focal segmental glomerulonephritis
- Minimal change disease
- Crescentic glomerulonephritis
- ANCA-associated renal vasculitis
- Membranous glomerulonephritis
- IgA glomerulonephritis
- Endocrine
- Clinical hyperthyroidism or thyrotoxicosis
- Subclinical hypothyroidism
- Adrenal haemorrhage
- Adrenal infarction
- Adrenal insufficiency
- Pancreatic
- Acute pancreatitis
- Ocular
- Uveitis
- Conjunctivitis
Immune-related systemic manifestations
Some patients with COVID-19 can develop a severe, acute virus-induced lung injury under the umbrella of acute respiratory distress syndrome (ARDS), a clinical syndrome characterized by acute lung inflammation and increased-permeability pulmonary oedema due to injury to the alveolar capillary barrier6. The hyperinflammatory phenotype of ARDS is characterized by elevated concentrations of pro-inflammatory cytokines, an increased incidence of shock and adverse clinical outcomes7,8. Although the mechanisms of COVID-19-induced ARDS are still being elucidated9, the term ‘cytokine storm’ has become synonymous with its pathophysiology, although some authors have suggested use of this term is misleading in the context of severe COVID-19 (ref.10). Even the term cytokine storm has been frequently interchanged with the term ‘cytokine release syndrome’ (CRS)10, which describes an immune-related dysregulation associated with the release of large amounts of cytokines that trigger systemic inflammation with multi-organ failure and high mortality rates. CRS is one of the most frequent serious adverse effects of chimeric antigen receptor T (CAR-T) cell therapies11 and is characterized by fever, tachycardia, tachypnoea and hypotension, the key symptoms that define systemic inflammatory response syndrome10. IL-6, a pro-inflammatory cytokine, is an important mediator of the acute inflammatory response in ARDS and CRS, and seems to also factor in severe COVID-19, contributing to elevated C-reactive protein concentrations, hypercoagulation and hyperferritinaemia12,13. However, serum IL-6 concentrations reported in patients with COVID-19 are substantially lower than those reported in patients with CRS, ARDS or sepsis10,14, or even influenza15. Why some patients with severe COVID-19 rapidly enter a state of multi-organ failure is unknown, but the pathophysiology of COVID-19-associated ARDS seems to be more complex than a simple overproduction of cytokines9.
The systemic phenotype related to the inflammatory reaction triggered by SARS-CoV-2 infection is very broad and can be reminiscent of that of some autoimmune or inflammatory diseases. In children, systemic involvement has a substantial overlap with Kawasaki disease, whereas in adults it seems to be closer to haemophagocytic lymphohistiocytosis (HLH), antiphospholipid syndrome (APS) or systemic vasculitis (Table 1, Fig. 1).
Table 1.
Summary of reported cases of systemic immune-related manifestations of COVID-19
| Characteristic | MIS-C | Haemophagocytic syndrome | Vasculitis in adults | Myositis | Arthritis |
|---|---|---|---|---|---|
| Number of cases reviewed | 717 | 20 | 19 | 24 | 6 |
| Sex ratio (male:female) | 4:3 | 7:3 | 1:1 | 7:1 | 5:0 |
| Mean age (years) | 8.5 | 66.0 | 55.4 | 52.4 | 54.3 |
| Age distribution (%) | |||||
| <18 years | 717 (100) | 0 (0) | 0/16 (0) | 2/16 (12.5) | 0/6 (0) |
| 18–50 years | 0 (0) | 1 (5) | 6/16 (37.5) | 5/16 (31.25) | 3/6 (50) |
| >50 years | 0 (0) | 19 (95) | 10/16 (62.5) | 9/16 (56.25) | 3/6 (50) |
| Geographical distribution (%) | |||||
| Europe | 368 (51.3) | 13 (65) | 15 (78.9) | 2 (8.3) | 3 (50) |
| North America | 330 (46) | 6 (30) | 3 (15.8) | 17 (70.8) | 0 (0) |
| Asia | 19 (2.6) | 0 (0) | 0 (0) | 2 (8.3) | 3 (50) |
| Other | 0 (0) | 1 (5) | 1 (5.3) | 3 (12.5) | 0 (0) |
| Virological results (%) | |||||
| PCR positive | 221/563 (39.3) | 20 (100) | 15 (78.9) | 24 (100) | 6 (100) |
| Serology positive, PCR negative | 223/563 (39.6) | 0 (0) | 2 (10.5) | 0 (0) | 0 (0) |
| Negative | 60/563 (10.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not tested using any technique | 59/563 (10.5) | 0 (0) | 2 (10.5) | 0 (0) | 0 (0) |
| Asymptomatic SARS-CoV-2 infection (%) | 184/207 (88.9) | ND | 2/18 (11) | 4/16 (25) | 0 (0) |
| Time to first symptom after COVID-19 onset (%) | |||||
| <7 days | NA | 0/2 (0) | 6/14 (42.8) | 8/12 (66.7) | 1/5 (20) |
| 7–14 days | NA | 2/2 (100) | 3/14 (21.4) | 1/12 (8.3) | 1/5 (20) |
| >14 days | NA | 0/2 (0) | 5/14 (35.6) | 3/12 (25) | 3/5 (60) |
| Intensive care (%) | 415/582 (71.3) | 19 (95) | 6 (31.6) | 8 (33.3) | 0 (0) |
| Death (%) | 11 (1.5) | 11/14 (78.6) | 3 (15.8) | 2 (8.3) | 0 (0) |
Summary of epidemiological profile, results of virological testing, clinical presentation and outcomes. Details of the selected studies are provided in the Supplementary Tables online. MIS-C, multisystem inflammatory syndrome in children; NA, not applicable; ND, not determined.
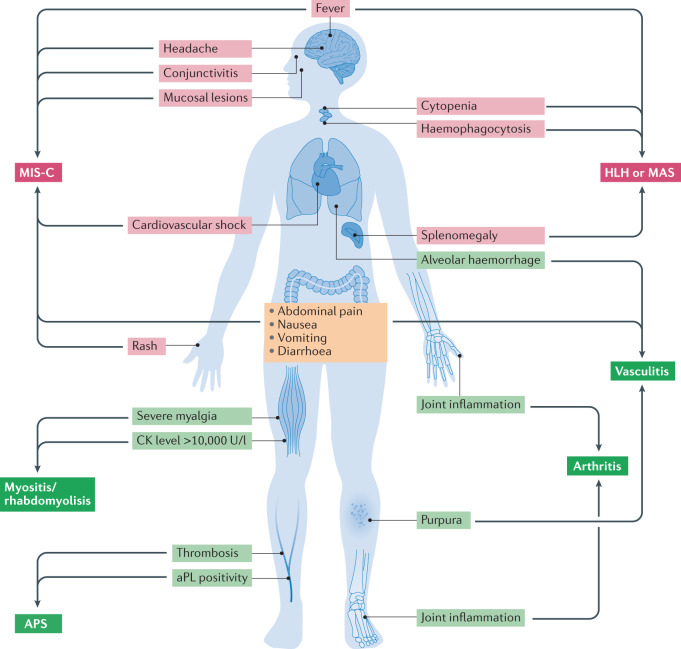
Fig. 1. Guiding signs and symptoms of suspected systemic immune-related disease in patients with COVID-19.
The two main systemic inflammatory syndromes associated with COVID-19, multisystem inflammatory syndrome in children (MIS-C) and haemophagocytic lymphohistiocytosis (HLH, including macrophage activation syndrome, MAS) are detailed at the top of the figure. The first sign prompting suspicion of these syndromes is persistent fever without a clear clinical source, together with multisystem organ involvement. The MIS-C phenotype includes Kawasaki disease-like features (conjunctivitis, red cracked lips, swollen hands and feet, and rash), coronary artery enlargement and/or aneurysms, gastrointestinal symptoms (abdominal pain, nausea, vomiting or diarrhoea) and neurological manifestations (headaches and meningitis). With respect to HLH and MAS, the cardinal features are enlarged lymphohaematopoietic organs (lymph nodes, spleen and/or liver) and severely abnormal values for multiple laboratory parameters suggesting involvement of multiple organs (such as severe cytopenia and liver and renal dysfunction). The main signs and symptoms of suspected systemic autoimmune and rheumatic diseases associated with COVID-19 are detailed at the bottom of the figure. Petechial and/or purpuric cutaneous lesions are the main signs prompting suspicion of vasculitis, and the addition of extracutaneous symptoms such as severe abdominal pain, haemoptysis or neurological features could indicate systemic vasculitis. In patients with thrombosis who have antiphospholipid (aPL) antibodies, fulfilment of the classification criteria for antiphospholipid syndrome (APS) should be ruled out. Severe myalgia in association with creatine kinase (CK) levels >10,000 U/l (concurrent with renal failure in some patients) are suggestive of myositis and/or rhabdomyolysis, whereas inflammation of several joints can follow different patterns including symmetric polyarthritis (resembling rheumatoid arthritis), oligoarticular arthritis with cutaneous lesions (resembling psoriatic arthritis) or axial involvement with enthesitis (resembling spondyloarthritis).
Multisystem inflammatory syndrome in children
In April 2020, a number of seriously ill children presented with systemic inflammatory response syndrome features resembling Kawasaki disease were reported in the UK, some of whom tested positive for SARS-CoV-2 infection, a condition that was termed paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection (PIMS-TS), also called multisystem inflammatory syndrome in children (MIS-C)16. Reports of more than 700 cases of MIS-C, including a large case series in the USA17, indicate that this syndrome mainly affects children who are aged >5 years and predominantly of non-white ethnicity, with a multisystemic presentation including gastrointestinal (92%), cardiovascular (80%), mucocutaneous (74%) and respiratory (70%) symptoms. The clinical presentation is severe; around half of the reported cases fulfilled the criteria for defined or incomplete Kawasaki disease and 73% required critical care, with an overall mortality rate of 1.7% (Supplementary Table 1). MIS-C has some notable differences from classic Kawasaki disease18–21, which predominantly affects children under the age of 5 years and most prominently in Asian populations, and has lower rates of admission to an intensive care unit (ICU) and death than does MIS-C. However, the systemic phenotype of MIS-C and the specific involvement of some organs, such as the coronary arteries, suggest a close link with Kawasaki disease22 and, in fact, the frequency of coronary aneurysms reported in the largest series of children diagnosed with MIS-C (8–23%)17,23–25 is quite similar to that reported in children with classic Kawasaki disease (6–17%)18,19. Most cases of MIS-C could be the result of a post-viral immune-related response. In non-Asian countries with large outbreaks of SARS-CoV-2 (such as France, Italy, Spain, UK and USA), most cases of MIS-C were reported in the late stages of the first wave of the pandemic17,26 (the reason why Asian countries such as China and Japan have not reported a similar number of MIS-C cases is unknown27) and were diagnosed a mean of 25–45 days after onset of SARS-CoV-2 infection17,28. Accordingly, the rate of SARS-CoV-2 infection confirmed by serological tests in children with MIS-C is ~90%, in contrast to only ~40% positivity using PCR tests (indicating an ongoing infection) (Supplementary Table 1). The development of a Kawasaki disease-like presentation in children presenting with severe COVID-19 cannot be considered unexpected29, in view of the important contribution of respiratory viral infections to the aetiopathogenesis of Kawasaki disease30. However, the question of whether MIS-C should be considered a discrete entity or could be an aetiologically driven subset of Kawasaki disease is still unresolved31, as the inflammatory response in MIS-C shares several features with Kawasaki disease but differs with respect to the T cell subsets involved, lack of IL-17A-mediated hyperinflammation and levels of biomarkers related to arterial damage32.
Cases of young adults with COVID-19 presenting with a classic phenotype of Kawasaki disease have also been reported33–36; these cases suggest that the Kawasaki disease phenotype presented by adults with COVID-19 follows the spectrum of classic Kawasaki disease, of which rare cases of adult-onset disease have been reported37 (Supplementary Table 2).
Haemophagocytic syndromes
Primary and secondary HLH are hyperferritinaemic hyperinflammatory syndromes that have a common terminal pathway but different pathogenetic roots, including viruses as one of the main external triggering factors38. The features of COVID-19 and the diagnostic criteria for HLH (high fever, splenomegaly, cytopenia, hypertriglyceridaemia, hyperferritinaemia, hypofibrinogenemia, high serum concentrations of soluble IL-2 receptor (also known as sCD25), low activity of natural killer cells and haemophagocytosis) overlap substantially39–47. However, the frequency of HLH in patients with COVID-19 is probably very small. Of 20 reported cases that were classified as probable HLH, only 20% fulfilled the required five diagnostic criteria for HLH (Supplementary Table 3). In addition, in those studies that used the H-score (a scoring system not validated in prospective studies for HLH diagnosis), only 0–10% of patients achieved the diagnostic cut-off for HLH41,48. Consequently, some authors suggest that these patients actually develop ARDS with some HLH features, rather than systemic macrophage activation (the hallmark of HLH)49,50. By contrast, other authors contend that all patients with severe COVID-19 should be investigated for underlying HLH51, considering that most of the reported cases lacked a full evaluation for HLH features (mostly the histopathological criteria and the measurement of natural killer cell function and sCD25 levels were not evaluated), and that macrophage activation syndrome has been reported in 25% of children presenting with MIS-C52,53. Probably, HLH related to COVID-19 is a condition with an incidence as rare as the virus-related HLH diagnosed in the pre-pandemic era38, but owing to the size of the pandemic worldwide reports are yielding a large number of cases. Considering the available evidence, a complete evaluation for possible HLH could be advised for adults with severe COVID-19 who develop cytopenia affecting at least two cell lineages in the peripheral blood (especially including thrombocytopenia), hyperferritinaemia (especially very high levels, that is, >2,000 ng/ml) and hypofibrinogenaemia, as well as in children presenting with life-threatening systemic COVID-19 (MIS-C).
Antiphospholipid syndrome
COVID-19 has been linked with coagulopathy and thrombosis, especially in patients who are severely ill admitted to an ICU54. However, SARS-CoV-2 itself does not seem to have intrinsic procoagulant effects, and the abnormal results of coagulation tests frequently detected in patients with COVID-19 seem to be mainly linked to the inflammatory systemic response1. However, an additional autoimmune hypothesis emerged when one study reported positivity for lupus anticoagulant in more than 90% of COVID-19 patients55. Since then, the number of studies reporting testing for antiphospholipid (aPL) antibodies in patients with COVID-19 has rapidly increased. Among 13 studies that overwhelmingly included patients with COVID-19 admitted to the ICU (Supplementary Table 4), lupus anticoagulant positivity was reported in more than half of the tested patients, although the rate of positive results ranged widely across the studies, from 3%56 to 91%55. Given the common use of heparins for thromboprophylaxis in patients with COVID-19 admitted to hospital, the potential interference of heparin with lupus anticoagulant analysis has been suggested40. In addition, lupus anticoagulant antibodies are heterogeneous and are detected in different contexts (including infections and inflammation) that enable the exposure of cell phospholipids that are normally not accessible to the immune system57; a positive result is not necessarily linked to the development of APS. With respect to anticardiolipin (aCL) antibodies, among patients with severe COVID-19, the positive rates are lower than those reported for lupus anticoagulant but also widely vary among studies (0–52% positive for IgG-aCL, 3–20% positive for IgM-aCL and 2–32% positive for IgA-aCL antibodies) (Supplementary Table 4), with some studies linking aPL antibody positivity with more severe COVID-19 (refs56,58). Therefore, aPL antibodies are frequently detected in patients with severe COVID-19, as has also been reported in patients with non-COVID-19 ARDS59. Owing to the high rates of aPL antibody positivity in patients with COVID-19, it seems rational to consider an autoimmune origin of the thrombosis under the umbrella of APS. Several factors must be considered with respect to a possible role of SARS-Cov-2 as a trigger of APS42,56,58,60–66 (Box 2). Although it cannot be discounted that some patients with COVID-19 could present with aPL antibody-related thrombosis, and could therefore be considered as having a COVID-19-related APS, the body of evidence we have reviewed does not support a central role for SARS-CoV-2 as a viral trigger of APS. Because almost all the studies reported to date have been carried out in patients who are severely ill (most with a long stay in ICU), pro-thrombotic factors other than aPL antibodies should always be carefully evaluated.
Box 2 Considering SARS-Cov-2 as a trigger of APS.
Results obtained from different studies42,56,58,60–65,182 indicate that a number of factors must be considered with respect to a possible role of SARS-CoV-2 in triggering antiphospholipid syndrome (APS).
Extent of association between antiphospholipid (aPL) antibodies and thrombosis:42,56,58,60,61
Using heterogeneous approaches, most studies have discarded a significant association between aPL antibody positivity and thrombosis in patients with COVID-19.
- Combined data from 56 patients admitted to intensive care units in two studies42,60 depicted a similar frequency of aPL antibody positivity in patients with or without thrombosis:
- 87% and 83% positive for any aPL antibodies
- 87% and 76% positive for lupus anticoagulant
- 47% and 44% positive for anti-cardiolipin (aCL) antibodies
- 0% and 22% positive for anti-β2 glycoprotein 1 (anti-β2GPI) antibodies
Persistence of aPL antibody positivity:56,60,61
In three studies, aPL antibodies were tested a second time 10–30 days after the first determination.
In more than 70% of cases, a previous positive result turned negative.
Measurement of aCL antibody titres:42,60
Only 24% of patients with COVID-19 included in two studies42,60 that detailed individual aCL antibody titres had high titres, and most did not develop thrombosis.
Most patients with COVID-19 have low-to-moderate aCL antibody titres that are not related to thrombosis.
Detection of multiple aPL antibodies:42,56,60,61,66
The frequency of double and triple positivity (for lupus anticoagulant, aCL antibodies and/or anti-β2GPI antibodies) in patients with COVID-19 was low (24% and 4%, respectively).
Patients with COVID-19 and triple positivity rarely developed thrombosis.
Systemic vasculitis
Emerging evidence seems to support a potential link between SARS-Cov-2 and systemic vasculitis, including the Kawasaki disease phenotype reported in a considerable percentage of children with MIS-C, the increasing number of reported cases of vasculitis in adults and the post-mortem descriptions of vasculitis involving various organs67. In adults, among 15 patients with COVID-19 and vasculitis, cases involved organ-specific involvement of the skin (n = 9), central nervous system (n = 3), the lungs (n = 2) and the gastrointestinal tract (n = 1), mainly appearing 2 weeks after the first symptoms of SARS-CoV-2 infection. Histopathological studies confirmed vasculitis in the skin (mainly classified as leukocytoclastic vasculitis) and gastrointestinal tissue (infiltration of small and medium-sized vessels) (Supplementary Table 2).
Vasculitis other than that within the spectrum of Kawasaki disease has been rarely reported in children in association with SARS-CoV-2 infection, and included cutaneous vasculitis68, retinal vasculitis69 and a possible central nervous system vasculitis70; these children often lack any previous symptoms suggestive of COVID-19 and had negative results of PCR tests but positive IgG serology.
Myositis
Several clinical findings and laboratory abnormalities suggest that a substantial percentage of patients with COVID-19 can have muscular inflammation. Myalgia was reported in approximately 24% of patients included in several large COVID-19 case series (although with a wide range of frequencies, ranging from 1% to 74%), whereas the frequency of raised creatine kinase (CK) levels is ~11% (Supplementary Table 5); myalgias can remain as active, chronic symptoms in ~10–20% of cases of acute COVID-19 (refs71,72). Only two studies have estimated the frequency of myositis in hospitalized patients with COVID-19, finding myositis in 3–11% of patients73,74, whereas the frequency of rhabdomyolysis is reportedly considerably lower (0.2–1.1%)73,75. In these studies, almost all patients with suspected myositic involvement did not undergo myositis-specific tests (such as electromyography, imaging and histopathological studies). Isolated reports of patients with COVID-19 presenting with myositis and/or rhabdomyolysis indicate that most cases occurred in adult males presenting with myalgia (in some cases severe) appearing mainly during the first week of COVID-19, with CK levels being higher than 10,000 U/l in most cases (Supplementary Table 6). The pathogenesis of immune-related muscular damage in COVID-19 is probably multifactorial, and could involve factors linked to critical illness and long ICU admissions, such as critical illness myopathy and superimposed steroid myopathy73. In other patients, a direct cytolytic viral effect or damage related to hypercytokinaemia could be involved in the development of an immune-related muscular damage within the spectrum of necrotizing autoimmune myopathy74, especially in patients presenting with an acute onset of severe muscle weakness with increased inflammatory markers and very high CK levels (in the thousands)76. For these patients, a specific muscle-centred diagnostic approach is highly recommended.
Arthritis
Joint pain with no notable evidence of inflammation upon physical examination of the involved joints was reported in ~36% of patients with COVID-19 (refs71,77) (Supplementary Table 5). Although it is not currently known whether COVID-19 will cause an increase in new-onset chronic pain for the population at large, several factors linked to the pandemic (such as psychological distress, epidemiological and socioeconomic factors, poor sleep and reduced physical activity) could be involved in the development of chronic widespread pain78.
In contrast to non-inflammatory joint pain, arthritis has been reported in isolated cases (affecting predominantly men with a mean age of 54 years), including a wide variety of articular presentations (for example, symmetric polyarthritis, monoarthritis, enthesitis or psoriatic arthritis), and mainly appearing after COVID-19 has resolved (Supplementary Table 7).
Systemic autoimmune diseases
COVID-19 patients can present with several systemic lupus erythematosus (SLE)-related features, including cytopenia (lymphopenia, thrombocytopenia or haemolytic anaemia), arthralgia, serositis, chilblain lesions or aPL antibodies. So far, only one case of probable SLE triggered by SARS-CoV-2 has been reported, which developed in a previously healthy 18-year-old woman who fulfilled the 2019 ACR–EULAR classification criteria for SLE79.
Sicca symptoms are not usually recorded in COVID-19 clinical studies, and only one small study reported that ~25% of patients mentioned sicca syndrome71. Another important feature related to Sjögren syndrome, parotid enlargement, has been reported in 20 patients, who were mostly young people (<30 years old) and were more often women than men (Supplementary Table 8).
Although mediastinal lymphadenopathy has been reported in 3–5% of patients with COVID-19 (refs80,81), only one case of probable sarcoidosis has been reported82.
Immune-related organ-specific manifestations
In contrast to the above-mentioned systemic presentations that can involve multiple organs, some patients with COVID-19 present with immune-related manifestations involving a single organ, which can mimic a wide range of organ-specific autoimmune diseases (Table 2, Fig. 2).
Table 2.
Summary of reported cases of organ-specific immune-related manifestations of COVID-19
| Characteristic | Chilblains | Erythema multiforme | ITP | Haemolytic anaemiaa | GBS | Encephalitis | Myelitis | Myocarditis | Pericardial tamponade | Glomerulonephritis | Thyroiditis | Pancreatitis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases reviewed | 1,333 | 17 | 39 | 19 | 73 | 58 | 8 | 25 | 25 | 36 | 85 | 30 |
| Sex ratio (male:female) | 1:1 | 1:1 | 1:1 | 4:3 | 5:2 | 1:1 | 3:1 | 3:2 | 4:3 | 2:1 | 2:3 | 1:2 |
| Mean age (years) | 21.0 | 33.3 | 58.7 | 55.4 | 56.35 | 55.0 | 45.2 | 49.1 | 62.85 | 57.03 | 52.4 | 43.83 |
| Age distribution (%) | ||||||||||||
| <18 years | 9/17 (52.9) | 9 (52.9) | 1/38 (2.6) | 2 (10.5) | 3/66 (4.5) | 1/57 (1.8) | 0 (0) | 1 (4) | 0 (0) | 0/34 (0) | 0 (0) | 2 (12.5) |
| 18–50 years | 8/17 (47.1) | 2 (11.8) | 8/38 (21.1) | 4 (21.1) | 15/66 (22.7) | 15/57 (26.3) | 4 (50) | 12 (48) | 2 (28.6) | 12/34 (35.3) | 9 (60) | 8 (50) |
| >50 years | 0/17 (0) | 6 (35.3) | 29/38 (76.3) | 13 (68.4) | 48/66 (72.7) | 41/57 (71.9) | 4 (50) | 12 (48) | 5 (71.4) | 22/34 (64.7) | 6 (40) | 6 (37.5) |
| Geographical distribution | ||||||||||||
| Europe | 1015 (76.1) | 14 (82.4) | 32 (82.1) | 13 (68.4) | 55 (75.3) | 42 (72.4) | 4 (50) | 11 (44) | 4 (57.1) | 7 (19.4) | 55 (64.7) | 7 (23.3) |
| North America | 318 (23.9) | 0 (0) | 3 (7.6) | 5 (26.3) | 9 (12.3) | 5 (8.6) | 2 (25) | 10 (40) | 3 (42.9) | 27 (75) | 0 (0) | 19 (63.3) |
| Asia | 0 (0) | 1 (5.8) | 4 (10.3) | 1 (5.3) | 7 (9.6) | 11 (19) | 2 (25) | 4 (16) | 0 (0) | 2 (5.6) | 30 (35.3) | 4 (13.3) |
| Other | 0 (0) | 2 (11.8) | 0 (0) | 0 (0) | 2 (2.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Virological results | ||||||||||||
| PCR positive (%) | 43/1,262 (3.4) | 9 (52.9) | 36 (92.3) | 19 (100) | 56 (76.7) | 41 (85.4) | 6 (75) | 24 (96) | 7 (100) | 32 (88.9) | 83 (97.6) | 29 (96.7) |
| Serology positive, PCR negative (%) | 28/1,262 (2.2) | 1 (5.9) | 0 (0) | 0 (0) | 6 (8.2) | 1 (2.1) | 2 (25) | 1 (4) | 0 (0) | 4 (11.1) | 2 (2.4) | 1 (3.3) |
| Negative (%) | 528/1,262 (41.8) | 4 (23.5) | 3 (7.7) | 0 (0) | 7 (9.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not tested using any technique (%) | 663/1,262 (52.5) | 3 (17.6) | 0 (0) | 0 (0) | 4 (5.5) | 6 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Asymptomatic COVID-19 (%) | 675/1,095 (61.6) | 1 (5.9) | 5 (12.8) | 1 (5.3) | 3 (4.1) | 8/48 (16.7) | 0 (0) | 1 (4) | 0 (0) | 5/13 (38.5) | 0 (0) | 0 (0) |
| Time to first symptom after COVID-19 onset (%) | ||||||||||||
| <7 days | 12/31 (38.7) | 1/3 (33.3) | 7/38 (18.4) | 3/8 (37.5) | 6/59 (10.2) | 27/47 (61.4) | 3/6 (50) | 8/15 (53.3) | 3/5 (60) | 4/8 (50) | 0/7 (0) | 9/16 (56.3) |
| 7–14 days | 2/31 (6.5) | 1/3 (33.3) | 12/38 (31.6) | 2/8 (25) | 22/59 (37.3) | 7/47 (14.9) | 3/6 (50) | 4/15 (26.7) | 1/5 (20) | 2/8 (25) | 1/7 (14.3) | 6/16 (37.5) |
| >14 days | 17/31 (54.8) | 1/3 (33.3) | 19/38 (50) | 3/8 (37.5) | 31/59 (52.5) | 13/47 (27.7) | 0/6 (0) | 3/15 (20) | 1/5 (20) | 2/8 (25) | 6/7 (85.7) | 1/16 (6.2) |
| Intensive care | 0 (0) | 1 (5.9) | 2 (5.1) | 3 (15.8) | 20 (27.4) | 20 (34.5) | 0 (0) | 17/21 (81) | 7 (100) | 0 (0) | 0 (0) | 11 (36.7) |
| Death | 0 (0) | 1 (5.9) | 2 (5.1) | 1 (5.3) | 2 (2.7) | 5 (8.6) | 0 (0) | 6/21 (28.6) | 2 (28.6) | 4 (11.1) | 0 (0) | 3 (10) |
Summary of epidemiological profile, results of virological testing, clinical presentation and outcomes; details of the selected studies are provided in the Supplementary Tables online. GBS, Guillain–Barré syndrome; ITP, immune thrombocytopenia. aIncluding Evans syndrome.
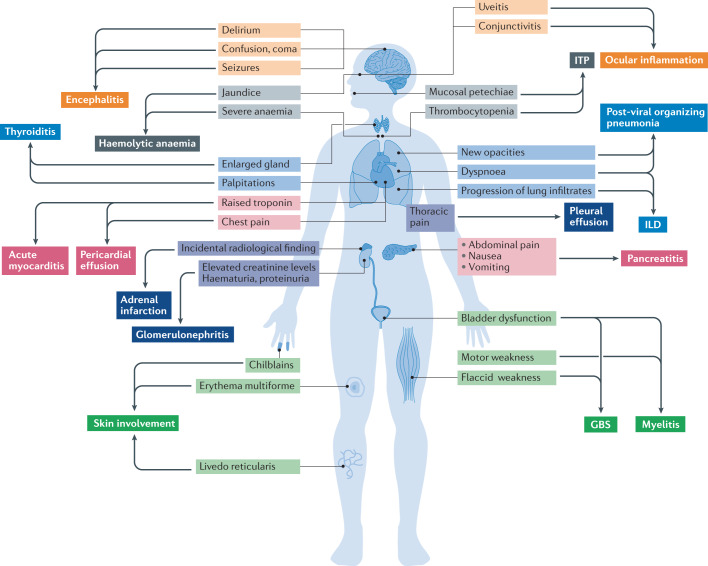
Fig. 2. Guiding signs and symptoms of suspected organ-specific immune-related diseases in patients with COVID-19.
The list of clinical symptoms is long, including important features such as dyspnoea (suggestive of interstitial lung disease (ILD) or organizing pneumonia), chest pain (myocarditis, pleuritis and pericarditis), severe acute upper abdominal pain with nausea and vomiting (acute pancreatitis) and neurological features such as confusion, seizures (encephalitis) or weakness with bladder dysfunction (myelitis and Guillain–Barré syndrome (GBS)). Examination is crucial when organ-specific immune-related disease is suspected in patients with COVID-19, paying special attention to eye redness (conjunctivitis and uveitis), jaundice (haemolytic anaemia), cutaneous lesions such as petechiae (immune thrombocytopenia (ITP)) or painful red inflammation on the hands or feet (chilblains), and glandular enlargement in the neck (thyroiditis). Simple laboratory tests such as haemography, biochemical analyses (measuring troponin, pancreatic enzymes, parameters of haemolysis, creatine kinase, haematuria and proteinuria) and determination of thyroid hormone levels could have an important role in diagnosis.
Cutaneous involvement
Symptoms of cutaneous involvement can affect 0.2% to 5% of patients with COVID-19 (ref.83), including maculopapular eruptions, urticarial lesions, chilblains and livedoid/necrotic lesions84. For maculopapular and urticarial lesions, a predominant drug-induced aetiology is suggested85, whereas immune-related mechanisms could be postulated for other cutaneous lesions.
The term chilblains (also referred to as pernio) describes a rare inflammatory condition affecting the extremities after exposure to cold, which can cause painful or itchy erythematous or violaceous lesions86. An association between chilblains and COVID-19 was initially supported because most reported cases of chilblains in southern Europe occurred during the first peak of the pandemic, and because in one of the largest case series cutaneous lesions appeared after infection onset in two-thirds of patients with symptoms of COVID-19 (ref.87). The patient profile derived from more than 1,300 cases of chilblains included in selected studies indicates a clear predominance of young people, with half of the studies reporting only children under the age of 18 years, and the other half including patients with a mean age ranging from 22 to 32 years. However, only 6% of these reported cases had confirmed COVID-19 (although it should be noted that testing for the virus was not performed in nearly half of the cases), supporting a weak link between chilblains and COVID-19 (Supplementary Table 9). Potentially, lifestyle changes related to lockdown lead to more inactivity for long periods and this inactivity could contribute to triggering chilblains, especially in predisposed patients (that is, those with a previous history of perniosis, Raynaud syndrome or β-blocker treatment)88. Reports of the presence of viral particles in biopsy-obtained skin from children with chilblains and negative results of PCR testing for SARS-CoV-2 might support the need for histopathological studies to confirm a causal relationship between SARS-CoV-2 and these skin lesions89.
Erythema multiforme is an inflammatory dermatological condition that has been overwhelmingly linked to infectious agents and, less frequently, to drugs90. Reports of cases of erythema multiforme in patients with COVID-19 reveal a clearly differentiated age-dependent pattern. Most cases reported in children were associated with chilblain lesions or Kawasaki disease91,92 and had negative PCR results for SARS-CoV-2, whereas the use of drugs was noted in all reported cases in adults (hydroxychloroquine in all, in most in combination with azithromycin, antivirals and/or antibiotics) (Supplementary Table 10).
Other immune-related skin manifestations have been reported in patients with COVID-19, including livedoid and/or acrocyanotic lesions84,93–96, retiform purpura97–99, oral ulcers84,100, erythema nodosum101,102, periorbital erythema103, generalized pustular figurate erythema (in all reported cases, appearing in patients who were being treated with hydroxychloroquine)94,104,105, drug reaction with eosinophilia and systemic symptoms94,106 and Sweet syndrome107.
Haematological involvement
Lymphopenia is a prominent feature of COVID-19, not only because of its high frequency (around half of COVID-19 cases) but also because of its relevance to prognosis (it has been linked with the development of ARDS, a need for intensive care and poor survival)108–110, whereas thrombocytopenia and anaemia have been reported in 24% and 59% of COVID-19 cases, respectively (Supplementary Table 5). Cytopenia is overwhelmingly asymptomatic, and symptomatic autoimmune cases (such as thrombocytopenic purpura or haemolytic anaemia) have been infrequently reported in patients with COVID-19 (Supplementary Table 11).
Patients with COVID‐19 can present with symptomatic thrombocytopenia, including immune thrombocytopenic purpura (ITP) and thrombotic thrombocytopenic purpura. COVID-19-related ITP predominantly affects people older than 50 years (~75%) presenting with a platelet count below 10,000 per mm3 (~80%), with the ITP symptoms (purpura and mucosal bleeding) appearing at least 2 weeks after onset of COVID-19 symptoms in nearly half the cases. In two of the three patients with COVID-19 presenting with thrombotic thrombocytopenic purpura, infection was confirmed by positive IgG serology, suggesting a delayed immune-related response. Autoimmune haemolytic anaemia (AIHA; presenting as either warm or cold haemolysis) is also diagnosed predominantly in people older than 50 years (~70%) presenting with a haemoglobin lower than 8 g/l (74%), with the AIHA symptoms (mainly asthenia and jaundice) appearing during the first/second week of COVID-19 (Supplementary Table 11).
Neurological involvement
The neurological manifestations caused by SARS-CoV-2 are diverse and have been related to neuroinvasion or neurotropic damage (including encephalopathy, encephalitis and cerebrovascular pathologies) or to neuroinflammatory damage (Guillain–Barré syndrome (GBS) or acute myelitis)111,112.
Encephalitis is inflammation of the brain parenchyma, clinical evidence of which includes cerebrospinal fluid pleocytosis, neuroimaging results or focal abnormalities on electroencephalogram112. Reported cases of encephalitis in patients with COVID-19 reveal a similar extent of involvement among women and men, with a mean age at diagnosis of 55 years (including cases in patients ranging from 11 to 84 years old) (Supplementary Table 12). In one-third of cases, neurological symptoms started at least 2 weeks after COVID-19 onset. Although several cases were classified as non-specific viral encephalitis or meningoencephalitis, some specific clinical entities were identified in other patients, including autoimmune encephalitis associated with anti-NMDA receptor autoantibodies, acute disseminated encephalomyelitis, acute necrotizing encephalopathy and mild encephalitis/encephalopathy with a reversible splenial lesion (Supplementary Table 12). The pathogenesis of COVID-19-associated encephalitis is unknown, although one study has suggested that patients with COVID-19 can develop neurological manifestations that share notable similarities with those of CAR-T cell-related encephalopathy, involving different pathophysiological mechanisms including CRS, endothelial activation, blood–brain barrier dysfunction and immune-related damage113.
GBS is a typical post-infectious disorder, with more than two-thirds of patients reporting symptoms of respiratory or digestive tract infections within the 6 weeks prior to GBS onset114. Therefore, that SARS-CoV-2 could be a potential new viral trigger of GBS is not unexpected, but the frequency of COVID-19-related GBS is unknown, with only one large study estimating a frequency of GBS of ~0.1% among hospitalized patients with COVID-19 (ref.73). So far, almost all cases of GBS related to SARS-CoV-2 have been reported as isolated cases and in one small series; these cases mainly affected men aged >50 years (90% of cases) and were diagnosed at least 2 weeks after the onset of COVID-19 respiratory symptoms in two-thirds of reviewed cases. The clinical presentation and severity of GBS in these cases was similar to that in non-COVID-19 GBS; the electrodiagnostic pattern was classified as demyelinating in most cases (although other phenotypic variants, such as Miller Fisher syndrome and acute motor and sensory axonal neuropathy, have also been reported), serum anti-ganglioside antibodies were absent in most patients tested and cerebrospinal fluid, when assessed, was negative for SARS-CoV-2 (refs115,116) (Supplementary Table 13).
Several cases of myelitis have been reported in patients with COVID-19, mainly in men and with two discrete age peaks, one at ~30 years old and the other at ~60–70 years old (Supplementary Table 14); additional reports of other immune-related neurological manifestations include cranial neuropathies and optic neuritis73,117–120, plexopathy121 or myasthenia gravis122.
Pulmonary involvement
Among studies of COVID-19 pneumonia to date, few have evaluated the long-term natural history of pulmonary damage, and they often have a short follow-up period (~1 month after starting COVID-19 symptoms). These studies have reported that a substantial percentage of patients have abnormal pulmonary findings, including abnormal pulmonary function test (PFT) results in 54% of patients and abnormal CT imaging studies in 40–94%123–126. One small study has reported that PFT results remain abnormal in ~25% of patients evaluated 3 months after diagnosis of COVID-19 (ref.127), suggesting the development of a post-pneumonia interstitial lung disease. Some studies have reported individual cases of patients who developed severe, bilateral pulmonary fibrosis after COVID-19 (refs128–130). Owing to the large number of patients affected by severe COVID-19 pneumonia, long-term respiratory complications can be expected and could cause substantial population morbidity131.
Although several post-mortem studies have suggested diffuse alveolar damage as the predominant pathological lung damage caused by SARS-Cov-2, other studies suggest a more heterogeneous pathological scenario, including a predominant pattern suggestive of organizing pneumonia in some patients47,132. A late development of new respiratory symptoms and opacities (>2 weeks after the first symptoms of COVID-19), especially if these features were not detected in previous CT studies, could suggest the late development of organizing pneumonia, as has been reported in influenza infection133.
Pleural involvement has also been linked to COVID-19, with an estimated frequency of 27% for pleural thickening and 5–6% for pleural effusion80,81. Some patients can have symptoms of pleurisy as the initial manifestation of COVID-19 (ref.134) and others might develop pleural effusion even though it was absent in the initial examination135.
Cardiovascular involvement
In patients with COVID-19, development of myocardial damage is indicated by abnormal laboratory parameters, cardiac imaging studies, and in vivo and post-mortem histopathological data. Around 40–80% of patients with COVID-19 can have raised troponin-I levels136,137, cardiac MRI identified cardiac involvement in 78%137, and several studies have reported myocardial interstitial infiltration by mononuclear cells and lymphocytic infiltration138 with evidence of active viral replication139,140; a myocyte-specific upregulation of ACE2 has been suggested as a putative pathogenic mechanism for SARS-CoV-2-associated viral myocarditis141. Acute myocarditis is often categorized into the histologically defined entities of lymphocytic, eosinophilic and giant cell myocarditis and sarcoid heart disease142. To date, acute myocarditis related to COVID-19 has been overwhelmingly described as lymphocytic and rarely as eosinophilic, in contrast to SARS-CoV-2-associated myocarditis, which did not exhibit lymphocytic infiltration143,144. Reports of cases of acute myocarditis in patients with COVID-19 show that a wide range of ages are involved (from 17 to 79 years), more frequently affecting men than women, with the main symptoms (thoracic pain and dyspnoea) being presented mainly during the first 2 weeks of COVID-19, although several cases have been described some weeks after the infection is resolved. Only around half cases had a confirmatory MRI study (the remaining underwent only cardiac ultrasonography) and most required ICU admission, with a mortality rate of ~30% (Supplementary Table 15).
A study137 in 100 patients evaluated a mean of 2 months after confirmed COVID-19 diagnosis showed that raised levels of high-sensitivity troponin were detected in 76% and that cardiac MRI showed cardiac involvement in 78%, including evidence of active myocardial inflammation in 60%. In comparison with healthy volunteers, the patients who had recovered from COVID-19 had lower left ventricular ejection fractions and higher left ventricular volumes; moreover, 32% manifested myocardial late gadolinium enhancement and 22% had pericardial involvement. The clinical relevance of these findings remains unclear, although the findings demonstrating chronic inflammation and left ventricular dysfunction a couple of months after the clinical onset of COVID-19 could represent an increased risk of developing new-onset heart failure and other cardiovascular complications145.
Pericardial effusion has been reported in ~5% of patients with COVID-19 (ref.81), and it seems that patients with suspected myocarditis could have a higher rate of pericardial effusion (22–75%)137,146. Cardiac tamponade was reported in 11 (1%) of 1,216 patients with available echocardiographic findings147, and several additional cases have been reported, mainly diagnosed in the first 7–10 days of COVID-19 illness (Supplementary Table 16).
Renal involvement
COVID-19 has been associated with both tubular and glomerular renal damage. Proximal tubule dysfunction has been reported in a subset of patients with COVID-19 presenting with low-molecular-weight proteinuria, neutral aminoaciduria and defective handling of uric acid148. With respect to glomerular disease, several studies have reported patients with biopsy-proven glomerulonephritis presenting with acute renal failure (in some cases accompanied by haematuria and/or nephrotic syndrome), with a clear differentiation in profile between podocytopathies and other types of glomerulonephritis (Supplementary Table 17). Most renal pathology in patients with COVID-19 falls within the spectrum of podocytopathies (with most classified as collapsing glomerulonephritis, and some as focal segmental glomerulosclerosis or minimal change disease), primarily affecting men of African ancestry carrying high-risk APOL1 genotypes. The next most frequently reported type of glomerulonephritis after podocytopathies in patients with COVID-19 is pauci-immune crescentic glomerulonephritis associated with autoantibodies, which in all cases but one affected women. Other types of glomerulonephritis have been also reported, including membranous and IgA glomerulonephritis. In some patients, acute renal disease appeared more than 2 weeks after onset of COVID-19 symptoms, showing negative PCR results and positive serological tests. Patients with COVID-19 presenting with glomerulonephritis have a poor prognosis, and more than half of the reported cases required dialysis (most even after being discharged from the hospital) (Supplementary Table 17).
Endocrine involvement
Studies have reported abnormal thyroid function potentially related to SARS-CoV-2 infection. A retrospective study in patients hospitalized with COVID-19 found thyrotoxicosis in 20% and hypothyroidism in 5%149, whereas another study found low concentrations of thyroid-stimulating hormone in 56% of patients150. To date, all reported cases of COVID-19-associated thyroid dysfunction are overwhelmingly consistent with overt hyperthyroidism (defined as low levels of thyroid-stimulating hormone plus high levels of free T4), often presenting with clinical symptoms of thyrotoxicosis and enlarged painful thyroid gland in physical and ultrasonography examinations; cases presenting with subclinical hypothyroidism are rare. From a pathogenic point of view, some findings seem to suggest that thyroid dysfunction could be a transient phenomenon related to the hyperinflammatory biological scenario (correlating with increased concentrations of IL-6 and infection severity, with abnormal values reverting after infection recovery). Most patients who were tested for anti-thyroid antibodies had negative results. Adrenal involvement can include acute adrenal infarction (as an incidental CT finding in one quarter of patients), adrenal haemorrhages and micro-infarctions and, rarely, adrenal insufficiency47,151,152 (Supplementary Table 18).
Pancreatic involvement
Several patients with COVID-19 presenting with abdominal pain and elevated concentrations of pancreatic enzymes have been diagnosed with acute pancreatitis, most frequently women (Supplementary Table 19). The clinical and epidemiological scenario is wide, and includes involvement of children and older people, patients presenting without clinical symptoms, post-mortem studies, family cases, or patients with underlying predisposing factors. Compared with patients without COVID-19, patients with COVID-19 presenting with acute pancreatitis showed a similar epidemiological profile but a worse bedside index for severity in acute pancreatitis (BISAP) score, a higher frequency of persistent organ failure and a worse survival rate153 (Supplementary Table 19). Several pathogenic mechanisms have been suggested to explain the putative association between acute pancreatitis and COVID-19, including direct viral damage to pancreatic cells, endothelial damage and ischaemic and/or thrombotic mechanisms154.
Ocular involvement
Some inflammatory ocular diseases have been diagnosed in patients with COVID-19, including one reported case of bilateral anterior uveitis155 and conjunctivitis, which has been reported in more than 50 adult patients, mostly from Asian countries156.
Probing the underlying pathogenesis
An increasing number of studies are reporting about collateral manifestations related to an excessive response of the immune system against SARS-CoV-2, breaking the natural self-tolerance maintained by the immune system, as has been previously described in other acute and chronic viral infections157–160 or that has been related to the administration of biologic drugs161,162. Immune-related manifestations related to COVID-19 were initially described in hospitalized patients, especially in those with severe disease, but have also been described in patients with an already resolved infection or even in asymptomatic patients. The clinical phenotype seems to be modified by epidemiological factors such as age (manifestations clearly differentiated between children and adults)163, sex (myositis, arthritis, GBS, myelitis and glomerulonephritis are reported mainly in men, whereas thyroiditis and pancreatitis occur more frequently in women) or ethnicity (although the role of socioeconomic factors must always be assessed)164,165 (Tables 1 and 2), and some pathogenic mechanisms could be specifically involved in certain epidemiological subsets of patients. For instance, in comparison with adults, children with COVID-19 predominantly generate IgG antibodies specific for the SARS-CoV-2 spike protein, targeting mainly the S2 subunit, but not for the nucleocapsid protein, a virus-related pathogenic mechanism that could help to explain the differentiated phenotype reported in children and adults166,167. The severity of the manifestations of COVID-19 is also very wide, ranging from completely benign and self-limiting manifestations (for example, perniosis) to systemic syndromes (such as MIS-C or HLH) that can lead to the need for intensive care and potentially to death. Multidisciplinary management of patients with COVID-19 is mandatory, including experts in the corresponding systemic and organ-specific autoimmune diseases, and should always follow a holistic diagnostic approach owing to the large variety of multisystem symptoms that patients with COVID-19 might present (Figs 2 and 3). In the absence of specific data about the therapeutic management of immune-related COVID-19 manifestations, following an approach similar to that used in the corresponding non-COVID-19 diseases could be a reasonable option, as has been suggested for neuroinflammatory COVID-19 with the use of corticosteroids, intravenous immunoglobulin and plasma exchange121,168.
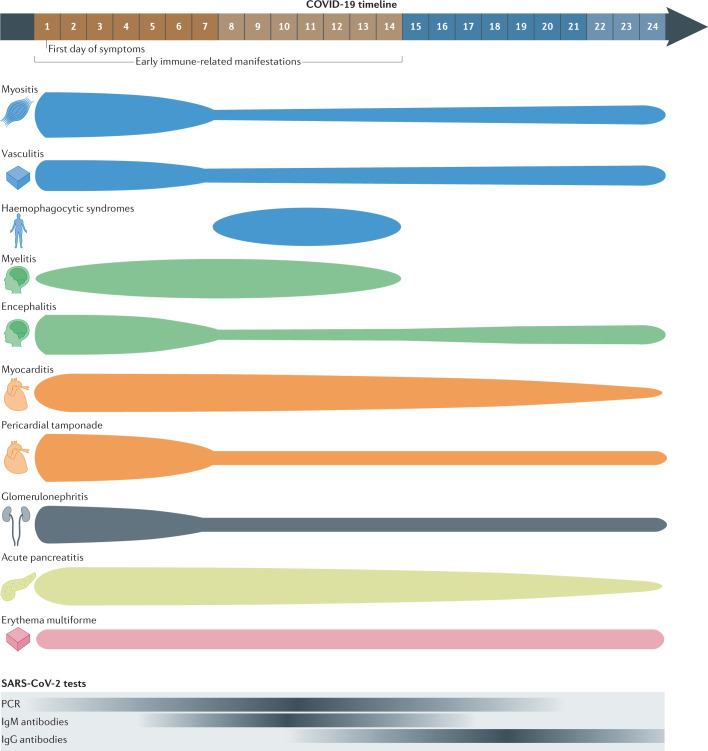
Fig. 3. Immune-related manifestations predominantly diagnosed during the first 2 weeks of COVID-19 (early features).
This figure illustrates the distribution of reported cases of immune-related manifestations predominantly diagnosed within 2 weeks of the onset of symptoms of acute COVID-19, as summarized in Table 3. The thickness of each segment corresponds to the proportion of cases reported in each time period (within the first 7 days of onset, between 8 and 14 days after, and 15 or more days after; the last period includes cases diagnosed in patients with asymptomatic infection). The bottom of the figure illustrates the representative positivity rate of the main microbiological tests from the first day of symptomatic infection; the intensity of colour corresponds to a higher rate of positive test results.
Little is known about the pathogenesis of these manifestations169, although involvement of specific responses by the acquired immune system seems to be of little consequence if we consider that serum autoantibodies (one of the main pathogenic hypotheses of autoimmune diseases) are absent in most patients tested for them. Therefore, the use of terms such as ‘immune-related’ or ‘immune-mediated’ could be more appropriate than the term ‘autoimmune’ to refer to these manifestations, as has been proposed for terminology of immune-related manifestations associated with checkpoint inhibitors161. Studies centred on investigating the role of interferon-related pathways in COVID-19, an important mechanism also involved in the pathogenesis of several autoimmune diseases170, have reported the presence of autoantibodies against type I interferon or inborn errors of type I interferon immunity171,172 in patients with severe COVID-19. In the literature we reviewed, there seems to be a certain pattern of occurrence of many immune-related manifestations in relation to the onset of SARS-CoV-2 infection: some features tend to appear in the first 2 weeks of infection (Fig. 3), whereas others tend to emerge in a late post-infectious stage or even in asymptomatic patients (Fig. 4). This differentiated temporal distribution along the different stages of SARS-CoV-2 infection could suggest the involvement of different aetiopathogenic mechanisms triggered by a common aetiological agent173,174, with some features being predominantly linked to early viral immune responses and other features with subsequent inflammatory responses emerging once the virus has been eliminated.
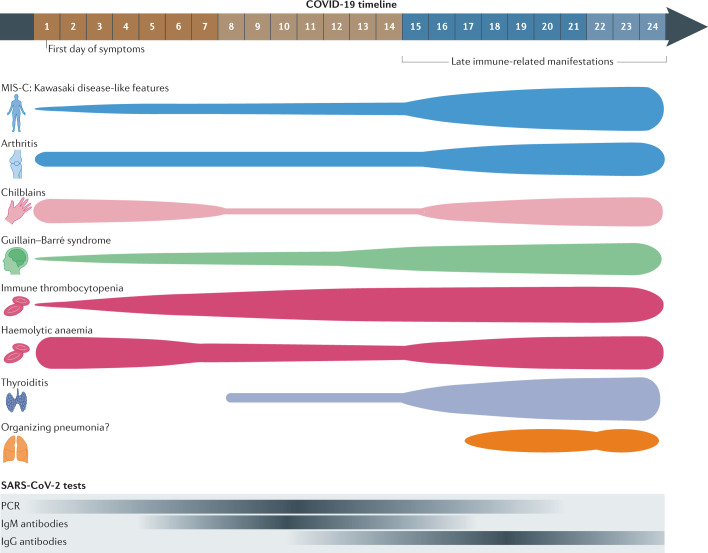
Fig. 4. Immune-related manifestations predominantly diagnosed after the first 2 weeks of COVID-19 (late features).
The top part of the figure illustrates the distribution of reported cases of immune-related manifestations predominantly diagnosed more than 2 weeks after the onset of symptoms of acute COVID-19, as summarized in Table 3. The thickness of each segment corresponds to the proportion of cases reported within each time period (the first 7 days, between 8 and 14 days after onset, and 15 or more days after onset; the last period includes cases reported in patients with asymptomatic SARS-CoV-2 infection). The bottom part of the figure illustrates the representative positivity rate of the main microbiological tests from the first day of symptomatic infection; the intensity of colour corresponds to a higher rate of positive test results. MIS-C, multisystem inflammatory syndrome in children.
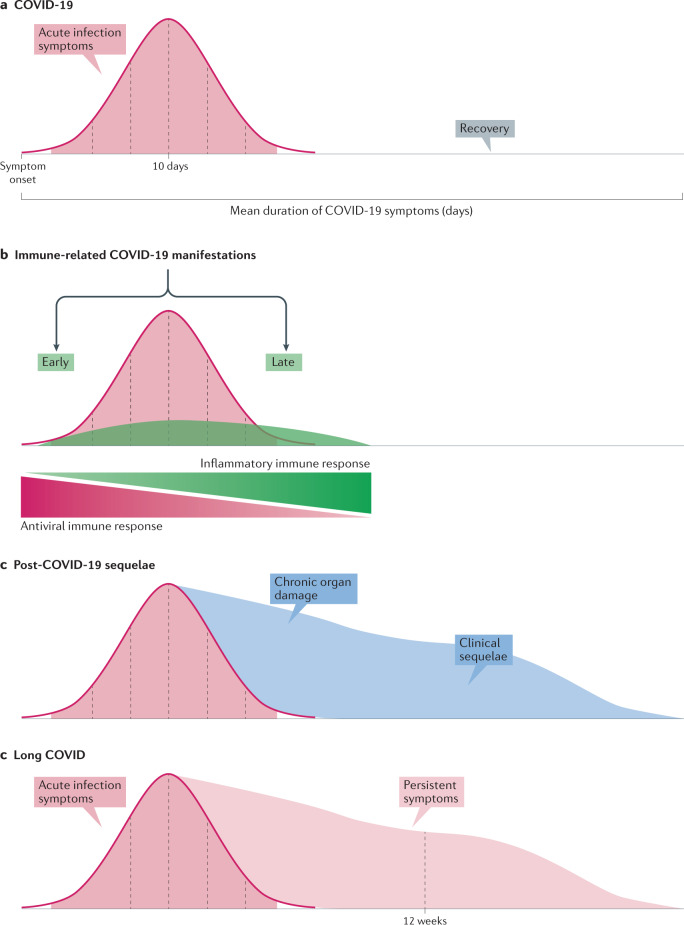
Immune-related manifestations of COVID-19 should be distinguished from other clinical scenarios with a different pathogenic basis (Fig. 5). In patients with severe COVID-19, some vital internal organs can be severely damaged by the inflammatory process. In a considerable percentage of patients with COVID-19 pneumonia, results of pulmonary function evaluations can remain abnormal several weeks after acute infection, including abnormal PFT results in 54% of patients and abnormal CT imaging studies in 40–94%123–126. Several cases of pulmonary fibrosis have also been reported128–130, suggesting that long-term respiratory complications could cause substantial morbidity in the population131,175. Other studies have reported chronic cardiac inflammation and left ventricular dysfunction a couple of months after the clinical onset of COVID-19, findings that could increase the risk of developing new-onset heart failure and other cardiovascular complications145. Another clinical scenario that has been scarcely explored is the persistence over time of acute symptoms of COVID-19, such as fatigue, pain, chills, anosmia, dysgeusia and headaches (referred to as ‘long COVID’)71,72,127,137,176–180 (Table 3). Some patients also develop a syndrome that can resemble myalgic encephalomyelitis/chronic fatigue syndrome or fibromyalgia181. To date, no study has demonstrated that immune-related mechanisms could be involved in the pathogenesis of these symptoms144.
Fig. 5. Time-dependent clinical scenarios in patients with symptomatic SARS-CoV-2 infection.
a | Acute infection. The mean duration of symptoms in patients with symptomatic SARS-CoV-2 infection has been reported as ~11 days76,180, and as long as 13–28 days in patients with COVID-19 requiring hospitalization74,123,181–187. Complete recovery is reported in >85% of patients 4 weeks after the onset of symptoms180. b | Immune-related manifestations of COVID-19. Most immune-related COVID-19 manifestations are diagnosed during the first 4–6 weeks after symptom onset. Some immune-related manifestations tend to appear during the first 2 weeks of infection (early immune-related features of COVID-19), whereas others tend to emerge in a late post-infectious stage or even in asymptomatic patients (late immune-related features of COVID-19). c | Post-COVID-19 sequelae. Symptoms related to organ-specific sequelae caused by the viral infection — affecting internal organs such as the lungs (interstitial lung disease in patients with severe pneumonia), the heart (chronic heart failure in patients with myocarditis) or the kidneys (chronic renal failure in patients with glomerulonephritis) — can emerge after resolution of the acute infection. d | Long COVID. One or more of the symptoms related to the acute viral infection, such as fatigue, pain, chills, anosmia, dysgeusia or headaches (as the most frequently reported), can persist for more than 12 weeks, a situation often referred to as ‘long COVID’. These symptoms can affect any bodily system, are not explained by an alternative diagnosis and, in some patients, may follow a relapsing–remitting pattern, possibly fluctuating and changing over time188.
Table 3.
Persistent symptoms and organ-specific sequelae reported in patients with COVID-19
| Features | Weeks after first symptom of acute COVID-19 | ||
|---|---|---|---|
| 4 weeksa | 8 weeks | 12 weeks | |
| General features71,72,127,176 | |||
| Fever | 4% | 0% | – |
| Chills | 5% | – | – |
| Fatigue | 35% | 53% | 16% |
| Musculoskeletal features71,72,176 | |||
| Arthralgia | 10–15% | 16–27% | – |
| Myalgia | – | 6% | – |
| Myalgia, headache and/or fatigue | 36% | 21% | – |
| Pulmonary features71,72,127,176–178 | |||
| Dyspnoea | 11–27% | 8–43% | 14% |
| Chest pain | 20% | 22% | – |
| Cough | 43% | 18% | 2% |
| Sputum production | – | 8% | 2% |
| Abnormal pulmonary function testsb | 47–54% | – | 25% |
| Ear, nose and throat features71,72,127,176 | |||
| Rhinitis and/or congestion | 28% | 15% | – |
| Sore throat | 15% | 7% | – |
| Anosmia | 23% | 17% | – |
| Dysgeusia | 24% | 10% | – |
| Anosmia and/or ageusia | 28% | 23% | 4% |
| Cardiovascular features137,179,180 | |||
| Raised troponin levels | – | 78% | – |
| Imaging myocardial inflammation | – | 60% | – |
| Imaging pericardial enhancement | – | 22% | – |
| Late myocardial gadolinium enhancement | – | 32% | – |
| Post-discharge thrombosis | 0.5–2.5% | – | – |
| Gastrointestinal features71,72,127,176 | |||
| Abdominal pain | 15% | – | – |
| Nausea | 10% | – | – |
| Vomiting | 4% | – | – |
| Diarrhoea | – | 3% | – |
| Diarrhoea or vomiting | 17% | 11% | 31% |
| Lack of appetite | – | 8% | – |
| Weight loss >5% | 16% | 17% | – |
| Neurological features71,127,176 | |||
| Headache | 14% | 9% | 18% |
| Confusion | 21% | – | – |
| Vertigo | – | 6% | – |
| Other features71 | |||
| Sicca syndrome | – | 16% | – |
| Red eyes | – | 10% | – |
aTenforde et al.176 evaluated features at 2–3 weeks. bAt least one abnormal parameter.
The novelty of these manifestations and the large number of different specialties involved make it very difficult at present to have consensus on a diagnostic definition for most of them, and two different approaches have been followed to date. The first has been to propose a new syndrome, as has been done with the MIS-C in children, to separate it from other known diseases with which this syndrome has notable similarities (in the case of MIS-C, Kawasaki disease). The second has been to include SARS-CoV-2 within the multi-aetiological spectrum often reported in patients affected by the classical syndrome or disease, considering that patients with COVID-19 might have a different clinical phenotype but under the umbrella of the same syndrome (such as HLH, vasculitis, autoimmune cytopenia, GBS or glomerulonephritis).
As knowledge of COVID-19 pathogenesis remains limited, there remain many more doubts than certainties, such as knowing why immune-related manifestations affect only certain people with COVID-19, why the phenotype is so different in children from that in adults, why some manifestations emerge especially in the acute infection phase and others when the infection is overcome, why some manifestations vary greatly with respect to geography and/or ethnicity of the affected patients, why a substantial proportion of patients remain symptomatic several months after being exposed to the virus, or why the number of reported cases of each immune-related manifestation of COVID-19 is clearly imbalanced, given that only two manifestations (MIS-C and chilblains being precisely those manifestations that particularly affect children and young people) make up two-thirds of the total reported cases.
Conclusions
Immune-related manifestations are increasingly recognized in patients with COVID-19, with a protean clinical presentation affecting a wide range of organ systems in both children and adults. The body of evidence consists predominantly of case series and uncontrolled studies that had reported ~3,000 cases worldwide as of August 2020, including more than 70 different systemic and organ-specific disorders. Unsurprisingly, therefore, diagnostic and therapeutic decision-making are often based on scarce clinical experience and expert opinion. Without being able to offer solid conclusions and plausible aetiopathogenic explanations, the main objective of this Review is to awaken the interest of the scientific community in this emerging group of manifestations and thus facilitate the development of studies specifically devoted to investigating the pathogenic mechanisms that could help enable the early detection and adequate management of immune-related manifestations of COVID-19.
Supplementary information
Author contributions
M.R.-C. and P. B.-Z. researched data for the article. All authors made a substantial contribution to discussion of the content, writing and review/editing of the manuscript before submission.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Rheumatology thanks J. Bayry, R. Giacomelli, who co-reviewed with O. Berardicurti, and L. Quartuccio for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
A search for original articles published without date limitation was performed in PubMed in August 2020. Search terms included “SARS-CoV-2”, “COVID-19” and the individual immune-related disorders that have been reported in patients with COVID-19 and that are detailed in Box 1.
Supplementary information
The online version contains supplementary material available at 10.1038/s41584-021-00608-z.
References
- 1.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Hadjadj J, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 5.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware LB. Physiological and biological heterogeneity in COVID-19-associated acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:1163–1165. doi: 10.1016/S2213-2600(20)30369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr. Opin. Crit. Care. 2019;25:12–20. doi: 10.1097/MCC.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha P, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir. Med. 2020;8:1209–1218. doi: 10.1016/S2213-2600(20)30366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha P, Matthay MA, Calfee CS. Is a ‘Cytokine Storm’ relevant to COVID-19? JAMA Intern. Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 11.England JT, et al. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev. 2021;45:100707. doi: 10.1016/j.blre.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElvaney OJ, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am. J. Respir. Crit. Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Valle DM, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leisman DE, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mudd PA, et al. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci. Adv. 2020;6:eabe3024. doi: 10.1126/sciadv.abe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez OM, Bridges ND, Goldmuntz E, Pascual V. The immune roadmap for understanding multi-system inflammatory syndrome in children: opportunities and challenges. Nat. Med. 2020;26:1819–1824. doi: 10.1038/s41591-020-1140-9. [DOI] [PubMed] [Google Scholar]
- 17.Feldstein LR, et al. Multisystem inflammatory syndrome in US children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman KG, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J. Am. Heart Assoc. 2016;5:e003289. doi: 10.1161/JAHA.116.003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son MBF, et al. Predicting coronary artery aneurysms in Kawasaki disease at a North American center: an assessment of baseline z scores. J. Am. Heart Assoc. 2017;6:e005378. doi: 10.1161/JAHA.116.005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominguez SR, et al. Kawasaki disease in a pediatric intensive care unit: a case-control study. Pediatrics. 2008;122:e786–e790. doi: 10.1542/peds.2008-1275. [DOI] [PubMed] [Google Scholar]
- 21.Chang L-Y, et al. Epidemiologic features of Kawasaki disease in Taiwan, 1996–2002. Pediatrics. 2004;114:e678–e682. doi: 10.1542/peds.2004-0726. [DOI] [PubMed] [Google Scholar]
- 22.Valverde I, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2020;143:21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 23.Whittaker E, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dufort EM, et al. Multisystem inflammatory syndrome in children in New York state. N. Engl. J. Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies P, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc. Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouldali N, et al. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc. Health. 2020;4:662–668. doi: 10.1016/S2352-4642(20)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kam K-Q, Ong JSM, Lee JH. Kawasaki disease in the COVID-19 era: a distinct clinical phenotype? Lancet Child Adolesc. Health. 2020;4:642–643. doi: 10.1016/S2352-4642(20)30207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toubiana J, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeung RS, Ferguson PJ. Is multisystem inflammatory syndrome in children on the Kawasaki syndrome spectrum? J. Clin. Invest. 2020;130:5681–5684. doi: 10.1172/JCI141718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turnier JL, et al. Concurrent respiratory viruses and Kawasaki disease. Pediatrics. 2015;136:e609–e614. doi: 10.1542/peds.2015-0950. [DOI] [PubMed] [Google Scholar]
- 31.Jiang L, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Consiglio CR, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokolovsky S, Soni P, Hoffman T, Kahn P, Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am. J. Emerg. Med. 2021;39:253.e1–253.e2. doi: 10.1016/j.ajem.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cogan E, et al. Multisystem inflammatory syndrome with complete Kawasaki disease features associated with SARS-CoV-2 infection in a young adult. A case report. Front. Med. 2020;7:428. doi: 10.3389/fmed.2020.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaigany S, et al. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet. 2020;396:e8–e10. doi: 10.1016/S0140-6736(20)31526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chérif MY, et al. Coronavirus disease 2019-related Kawasaki-like disease in an adult: a case report. JAAD Case Rep. 2020;6:780–782. doi: 10.1016/j.jdcr.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraison J-B, et al. Kawasaki disease in adults: observations in France and literature review. Autoimmun. Rev. 2016;15:242–249. doi: 10.1016/j.autrev.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 39.Henter J-I, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 40.Tang N. Response to ‘Lupus anticoagulant is frequent in patients with Covid-19’ (JTH-2020-00483) J. Thromb. Haemost. 2020;18:2065–2066. doi: 10.1111/jth.14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood H, et al. Secondary HLH is uncommon in severe COVID-19. Br. J. Haematol. 2020;190:e283–e285. doi: 10.1111/bjh.16934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pineton de Chambrun M, et al. High frequency of antiphospholipid antibodies in critically ill COVID-19 patients: a link with hypercoagulability? J. Intern. Med. 2021;289:422–424. doi: 10.1111/joim.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alrubayyi A. NK cells in COVID-19: protectors or opponents? Nat. Rev. Immunol. 2020;20:520. doi: 10.1038/s41577-020-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Y, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care. 2020;8:e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng Y, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J. Hematol. Oncol. 2020;13:75. doi: 10.1186/s13045-020-00907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prieto-Pérez L, et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod. Pathol. 2020;33:2139–2146. doi: 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanley B, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loscocco GG, et al. The HScore for secondary hemophagocytic lymphohistiocytosis, calculated without a marrow biopsy, is consistently low in patients with COVID-19. Int. J. Lab. Hematol. 2020;42:e270–e273. doi: 10.1111/ijlh.13310. [DOI] [PubMed] [Google Scholar]
- 49.Loscocco GG. Secondary hemophagocytic lymphohistiocytosis, HScore and COVID-19. Int. J. Hematol. 2020;112:125–126. doi: 10.1007/s12185-020-02895-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leverenz DL, Tarrant TK. Is the HScore useful in COVID-19? Lancet. 2020;395:e83. doi: 10.1016/S0140-6736(20)31057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verdoni L, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belot A, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet. Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowles L, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N. Engl. J. Med. 2020;383:288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao M, et al. Brief report: anti-phospholipid antibodies in critically ill patients with coronavirus disease 2019 (COVID-19) Arthritis Rheumatol. 2020;23:998–1008. [Google Scholar]
- 57.Helms J, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bertin D, et al. Anti-cardiolipin IgG autoantibody level is an independent risk factor of COVID-19 severity. Arthritis Rheumatol. 2020;72:1953–1955. doi: 10.1002/art.41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masi P, et al. Systemic inflammatory response syndrome is a major contributor to COVID-19-associated coagulopathy: insights from a prospective, single-center cohort study. Circulation. 2020;142:611–614. doi: 10.1161/CIRCULATIONAHA.120.048925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Devreese KMJ, Linskens EA, Benoit D, Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J. Thromb. Haemost. 2020;18:2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siguret V, et al. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thrombosis Res. 2020;195:74–76. doi: 10.1016/j.thromres.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turiel M, et al. Thrombotic risk factors in primary antiphospholipid syndrome: a 5-year prospective study. Stroke. 2005;36:1490–1494. doi: 10.1161/01.STR.0000170645.40562.09. [DOI] [PubMed] [Google Scholar]
- 63.Neville C, et al. Thromboembolic risk in patients with high titre anticardiolipin and multiple antiphospholipid antibodies. Thromb. Haemost. 2003;90:108–115. [PMC free article] [PubMed] [Google Scholar]
- 64.Chayoua W, et al. Identification of high thrombotic risk triple-positive antiphospholipid syndrome patients is dependent on anti-cardiolipin and anti-β2glycoprotein I antibody detection assays. J. Thromb. Haemost. 2018;16:2016–2023. doi: 10.1111/jth.14261. [DOI] [PubMed] [Google Scholar]
- 65.Pengo V, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J. Thromb. Haemost. 2010;8:237–242. doi: 10.1111/j.1538-7836.2009.03674.x. [DOI] [PubMed] [Google Scholar]
- 66.Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemost. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fox SE, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papa A, Salzano AM, Di Dato MT, Varrassi G. Images in practice: painful cutaneous vasculitis in a SARS-CoV-2 IgG-positive child. Pain Ther. 2020;9:805–807. doi: 10.1007/s40122-020-00174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quintana-Castanedo L, et al. Concurrent chilblains and retinal vasculitis in a child with COVID-19. J. Eur. Acad. Dermatol. Venereol. 2020;34:e764–e766. doi: 10.1111/jdv.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Regev T, et al. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS-COV-2 infection. Pediatr. Infect. Dis. J. 2020;39:e206–e207. doi: 10.1097/INF.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 71.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carvalho-Schneider C, et al. Follow-up of adults with non-critical COVID-19 two months after symptoms’ onset. Clin. Microbiol. Infect. 2020;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romero-Sánchez CM, et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mao L, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan W-J, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalakas MC. Guillain-Barré syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:e781. doi: 10.1212/NXI.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lechien JR, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clauw DJ, Häuser W, Cohen SP, Fitzcharles M-A. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. 2020;161:1694–1697. doi: 10.1097/j.pain.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mantovani Cardoso E, Hundal J, Feterman D, Magaldi J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin. Rheumatol. 2020;39:2811–2815. doi: 10.1007/s10067-020-05310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu J, et al. CT imaging features of 4121 patients with COVID-19: a meta-analysis. J. Med. Virol. 2020;92:891–902. doi: 10.1002/jmv.25910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J. Am. Coll. Radiol. 2020;17:701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Behbahani S, et al. Sarcoid-like reaction in a patient recovering from coronavirus disease 19 pneumonia. JAAD Case Rep. 2020;6:915–917. doi: 10.1016/j.jdcr.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matar S, et al. Cutaneous manifestations in SARS-CoV-2 infection (COVID-19): a French experience and a systematic review of the literature. J. Eur. Acad. Dermatol. Venereol. 2020;34:e686–e689. doi: 10.1111/jdv.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galván Casas C, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Català A, et al. Maculopapular eruptions associated to COVID-19: a subanalysis of the COVID-Piel study. Dermatol. Ther. 2020;33:e14170. doi: 10.1111/dth.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum. Pathol. 1997;28:478–484. doi: 10.1016/s0046-8177(97)90038-1. [DOI] [PubMed] [Google Scholar]
- 87.Freeman EE, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J. Am. Acad. Dermatol. 2020;83:486–492. doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herman A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998–1003. doi: 10.1001/jamadermatol.2020.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colmenero I, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br. J. Dermatol. 2020;183:729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am. Fam. Physician. 2019;100:82–88. [PubMed] [Google Scholar]
- 91.Le Cleach L, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on PCR and serology testing. Br. J. Dermatol. 2020;183:866–874. doi: 10.1111/bjd.19377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruggiero G, et al. Reply to: ‘Characterization of acute acro-ischemic lesions in non-hospitalized patients: a case series of 132 patients during the COVID-19 outbreak’. J. Am. Acad. Dermatol. 2020;83:e237–e239. doi: 10.1016/j.jaad.2020.05.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warkentin TE, Makris M, Jay RM, Kelton JG. A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am. J. Med. 2008;121:632–636. doi: 10.1016/j.amjmed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 94.Suarez-Valle A, et al. Comment on ‘Generalized pustular figurate erythema: a newly delineated severe cutaneous drug reaction linked with hydroxychloroquine’: report of a COVID-19 patient with particular findings. Dermatol. Ther. 2020;33:e13852. doi: 10.1111/dth.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, et al. [Clinical and coagulation characteristics in 7 patients with critical COVID-2019 pneumonia and acro-ischemia] Zhonghua Xue Ye Xue Za Zhi. 2020;41:302–307. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verheyden M, Grosber M, Gutermuth J, Velkeniers B. Relapsing symmetric livedo reticularis in a patient with COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020;34:e684–e686. doi: 10.1111/jdv.16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bosch-Amate X, et al. Retiform purpura as a dermatological sign of coronavirus disease 2019 (COVID-19) coagulopathy. J. Eur. Acad. Dermatol. Venereol. 2020;34:e548–e549. doi: 10.1111/jdv.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Freeman EE, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J. Am. Acad. Dermatol. 2020;83:1118–1129. doi: 10.1016/j.jaad.2020.06.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Magro C, et al. The differing pathophysiologies that underlie COVID-19 associated perniosis and thrombotic retiform purpura: a case series. Br. J. Dermatol. 2020;184:141–150. doi: 10.1111/bjd.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2021;27(Suppl. 3):710–712. doi: 10.1111/odi.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ordieres-Ortega L, et al. Atypical erythema nodosum in a patient with COVID-19 pneumonia. Dermatol. Ther. 2020;33:e13658. doi: 10.1111/dth.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suter P, Mooser B. & Pham Huu Thien, H. P. Erythema nodosum as a cutaneous manifestation of COVID-19 infection. BMJ Case Rep. 2020;13:e236613. doi: 10.1136/bcr-2020-236613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of Covid-19. JAAD Case Reports. 2020;6:996–998. doi: 10.1016/j.jdcr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robustelli Test E, et al. Acute generalized exanthematous pustulosis with erythema multiforme-like lesions induced by hydroxychloroquine in a woman with coronavirus disease 2019 (COVID-19) J. Eur. Acad. Dermatol. Venereol. 2020;34:e457–e459. doi: 10.1111/jdv.16613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz RA, Janniger CK. Generalized pustular figurate erythema: a newly delineated severe cutaneous drug reaction linked with hydroxychloroquine. Dermatol. Ther. 2020;33:e13380. doi: 10.1111/dth.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sernicola A, et al. ‘Toxic erythema’ and eosinophilia associated with tocilizumab therapy in a COVID-19 patient. J. Eur. Acad. Dermatol. Venereol. 2020;34:e368–e370. doi: 10.1111/jdv.16620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taşkın B, et al. Coronavirus 19 presenting with atypical Sweet’s syndrome. J. Eur. Acad. Dermatol. Venereol. 2020;34:e534–e535. doi: 10.1111/jdv.16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan L, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal. Transduct. Target. Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Terpos E, et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liao D, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet. Haematol. 2020;7:e671–e678. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yachou Y, El Idrissi A, Belapasov V, Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020;41:2657–2669. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ellul MA, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perrin P, et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 2021;28:248–258. doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 115.Caress JB, et al. COVID-19-associated Guillain-Barre Syndrome: the early pandemic experience. Muscle Nerve. 2020;62:485–491. doi: 10.1002/mus.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J. Neurol. 2021;268:1133–1170. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gutiérrez-Ortiz C, et al. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 118.Manganotti P, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J. Med. Virol. 2021;93:766–774. doi: 10.1002/jmv.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guilmot A, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2021;268:751–757. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Malayala SV, Raza A. A case of COVID-19-induced vestibular neuritis. Cureus. 2020;12:e8918. doi: 10.7759/cureus.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paterson RW, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Restivo DA, Centonze D, Alesina A, Marchese-Ragona R. Myasthenia Gravis associated with SARS-CoV-2 infection. Ann. Intern. Med. 2020;173:1027–1028. doi: 10.7326/L20-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu D, et al. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir. Res. 2020;21:125. doi: 10.1186/s12931-020-01385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan F, et al. Different computed tomography patterns of Coronavirus Disease 2019 (COVID-19) between survivors and non-survivors. Sci. Rep. 2020;10:11336. doi: 10.1038/s41598-020-68057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296:E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu Q, et al. Early CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur. J. Radiol. 2020;128:109017. doi: 10.1016/j.ejrad.2020.109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao Y-M, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Combet M, Pavot A, Savale L, Humbert M, Monnet X. Rapid onset honeycombing fibrosis in spontaneously breathing patient with Covid-19. Eur. Respir. J. 2020;56:2001808. doi: 10.1183/13993003.01808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen J-Y, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin. Med. J. 2020;133:1390–1396. doi: 10.1097/CM9.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grillo F, Barisione E, Ball L, Mastracci L, Fiocca R. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet. Infect. Dis. 2020;21:e72. doi: 10.1016/S1473-3099(20)30582-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fraser E. Long term respiratory complications of covid-19. BMJ. 2020;370:m3001. doi: 10.1136/bmj.m3001. [DOI] [PubMed] [Google Scholar]
- 132.Flikweert AW, et al. Late histopathologic characteristics of critically ill COVID-19 patients: Different phenotypes without evidence of invasive aspergillosis, a case series. J. Crit. Care. 2020;59:149–155. doi: 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu H, Li J, Chen M, Su J. Glucocorticoid treatment of suspected organizing pneumonia after H7N9 infection: a case report. Medicine. 2019;98:e16839. doi: 10.1097/MD.0000000000016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oleynick C. Symptoms of Pleurisy as the initial presentation of COVID-19. Am. J. Case Rep. 2020;21:e925775. doi: 10.12659/AJCR.925775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guan CS, et al. Imaging features of coronavirus disease 2019 (COVID-19): evaluation on thin-section CT. Acad. Radiol. 2020;27:609–613. doi: 10.1016/j.acra.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lala A, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J. Am. Coll. Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Puntmann VO, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rali AS, Ranka S, Shah Z, Sauer AJ. Mechanisms of myocardial injury in coronavirus disease 2019. Card. Fail. Rev. 2020;6:e15. doi: 10.15420/cfr.2020.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lindner D, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dolhnikoff M, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc. Health. 2020;4:790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tucker NR, et al. Myocyte-specific upregulation of ACE2 in cardiovascular disease: implications for SARS-CoV-2-mediated myocarditis. Circulation. 2020;142:708–710. doi: 10.1161/CIRCULATIONAHA.120.047911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cooper LTJ. Myocarditis. N. Engl. J. Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tschöpe C, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat. Rev. Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Topol EJ. COVID-19 can affect the heart. Science. 2020;370:408–409. doi: 10.1126/science.abe2813. [DOI] [PubMed] [Google Scholar]
- 145.Yancy CW, Fonarow GC. Coronavirus disease 2019 (COVID-19) and the heart-is heart failure the next chapter? JAMA Cardiol. 2020;5:1216–1217. doi: 10.1001/jamacardio.2020.3575. [DOI] [PubMed] [Google Scholar]
- 146.Esposito A, et al. Cardiac magnetic resonance characterization of myocarditis-like acute cardiac syndrome in COVID-19. JACC Cardiovasc. Imaging. 2020;13:2462–2465. doi: 10.1016/j.jcmg.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dweck MR, et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Werion A, et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98:1296–1307. doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lania A, et al. Thyrotoxicosis in patients with Covid-19: the Thyrcov Study. Eur. J. Endocrinol. 2020;183:381–387. doi: 10.1530/EJE-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid. 2021;31:8–11. doi: 10.1089/thy.2020.0363. [DOI] [PubMed] [Google Scholar]
- 151.Leyendecker P, et al. Acute adrenal infarction as an incidental CT finding and a potential prognosis factor in severe SARS-CoV-2 infection: a retrospective cohort analysis on 219 patients. Eur. Radiol. 2020;31:895–900. doi: 10.1007/s00330-020-07226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heidarpour M, Vakhshoori M, Abbasi S, Shafie D, Rezaei N. Adrenal insufficiency in coronavirus disease 2019: a case report. J. Med. Case Rep. 2020;14:134. doi: 10.1186/s13256-020-02461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dirweesh A, et al. Clinical outcomes of acute pancreatitis in patients with coronavirus disese 2019. Gastroenterology. 2020;159:1972–1974. doi: 10.1053/j.gastro.2020.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.de-Madaria E, Capurso G. COVID-19 and acute pancreatitis: examining the causality. Nat. Rev. Gastroenterol. Hepatol. 2021;18:3–4. doi: 10.1038/s41575-020-00389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bettach E, Zadok D, Weill Y, Brosh K, Hanhart J. Bilateral anterior uveitis as a part of a multisystem inflammatory syndrome secondary to COVID-19 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.26229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ozturker ZK. Conjunctivitis as sole symptom of COVID-19: a case report and review of literature. Eur. J. Ophthalmol. 2021;93:139–140. doi: 10.1177/1120672120946287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.von Herrath MG, Oldstone MB. Virus-induced autoimmune disease. Curr. Opin. Immunol. 1996;8:878–885. doi: 10.1016/S0952-7915(96)80019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Crum-Cianflone NF. Immune reconstitution inflammatory syndromes: what’s new? AIDS Read. 2006;16:199–206. [PubMed] [Google Scholar]
- 159.Smatti MK, et al. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ramos-Casals M, et al. Evidence-based recommendations on the management of extrahepatic manifestations of chronic hepatitis C virus infection. J. Hepatol. 2017;66:1282–1299. doi: 10.1016/j.jhep.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 161.Ramos-Casals M, et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers. 2020;6:38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Perez-De-Lis M, et al. Autoimmune diseases induced by biological agents. a review of 12,731 cases (BIOGEAS Registry) Expert. Opin. Drug Saf. 2017;16:1255–1271. doi: 10.1080/14740338.2017.1372421. [DOI] [PubMed] [Google Scholar]
- 163.Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr. Infect. Dis. J. 2020;39:469–477. doi: 10.1097/INF.0000000000002700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chin-Hong P, Alexander KM, Haynes N, Albert MA. Pulling at the heart: COVID-19, race/ethnicity and ongoing disparities. Nat. Rev. Cardiol. 2020;17:533–535. doi: 10.1038/s41569-020-0416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Inamdar S, et al. Prevalence, risk factors, and outcomes of hospitalized patients with COVID-19 presenting as acute pancreatitis. Gastroenterology. 2020;159:2226–2228.e2. doi: 10.1053/j.gastro.2020.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weisberg SP, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ng KW, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370:1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cao A, et al. Severe COVID-19-related encephalitis can respond to immunotherapy. Brain. 2020;143:e102. doi: 10.1093/brain/awaa337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cavounidis A, Alderson J, Quastel M. Multisystem inflammatory syndrome in children: getting to the heart of the matter. Nat. Rev. Immunol. 2020;20:520. doi: 10.1038/s41577-020-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bastard P, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang Q, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Diorio C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Giamarellos-Bourboulis EJ, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Raghu G, Wilson KC. COVID-19 interstitial pneumonia: monitoring the clinical course in survivors. Lancet Respir. Med. 2020;8:839–842. doi: 10.1016/S2213-2600(20)30349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tenforde MW, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a Multistate Health Care Systems Network — United States, March–June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Frija-Masson J, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur. Respir. J. 2020;56:2001754. doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mo X, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Patell R, et al. Post-discharge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roberts LN, et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136:1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Williams FMK, Muirhead N, Pariante C. Covid-19 and chronic fatigue. BMJ. 2020;370:m2922. doi: 10.1136/bmj.m2922. [DOI] [PubMed] [Google Scholar]
- 182.Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19: response to reply. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sudre CH, et al. Symptom clusters in Covid19: a potential clinical prediction tool from the COVID symptom study app. Preprint at. medRxiv. 2020 doi: 10.1101/2020.06.12.20129056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pan F, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gatto M, et al. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin. Exp. Rheumatol. 2020;38:754–759. [PubMed] [Google Scholar]
- 186.Beigel JH, et al. Remdesivir for the treatment of Covid-19 — preliminary report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 187.Liu Z, et al. Association between initial chest CT or clinical features and clinical course in patients with coronavirus disease 2019 pneumonia. Korean J. Radiol. 2020;21:736–745. doi: 10.3348/kjr.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.NICE. COVID-19 rapid guideline: managing the long-term effects of COVID-19. https://www.nice.org.uk/guidance/ng188 (2021) [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.