Abstract
In this study, a series of coumarin derivatives were synthesized and their inhibitory effects on the activity of mushroom tyrosinase were evaluated. As a result of measuring the inhibition of tyrosinase activity of these derivatives, the compounds 3e (1.05 μM), 3f (0.83 μM), 3h (0.85 μM), 3i (1.05 μM), and 3k (0.67 μM) of the geranyloxycoumarin derivatives were highly active at a concentration of 0.8%. The geranyloxycoumarin derivatives exhibited better activity than the hydroxycoumarin derivatives. Among the geranyloxycoumarin derivatives, compound 3k was two times more active than arbutin, a positive control, at a concentration of 0.4%. The above results suggest that geranyloxycoumarin derivatives have great potential for application as functional cosmetic ingredients with tyrosinase-inhibiting activity.
Keywords: geranyloxycoumarin, tyrosinase, coumarin, inhibitor, melanin
1. Introduction
Tyrosinase is known to be a multifunctional copper-containing enzyme from the oxidase superfamily, and it is the key protein involved in the biosynthesis of the large biological pigment, melanin [1]. Melanin is produced by melanocytes in the basal layer of the epidermis [2]. Tyrosinase promotes hydroxylation of L-Tyrosine to L-DOPA (L-3,4-dihydroxyphenylalanine) in the process of melanin biosynthesis, then promotes oxidation from L-DOPA to dopaquinone. Subsequently, dopaquinone forms melanin through several stages [3]. Melanin plays an important role in human skin, but excessive melanin formation caused by abnormal melanin production can cause pigmentation disorders, such as freckles, blemishes, and age spots [4]. These excessive melanin expressions can be suppressed by effective tyrosinase inhibitors.
Tyrosinase inhibitors include hydroquinone [5], arbutin [6], kojic acid [7], ascorbic acid [8], and ellagic acid [9]. Hydroquinone is potentially mutagenic in mammalian cells and is associated with several side effects, including contact dermatitis [10]. Arbutin is chemically unstable in its natural form and potentially toxic to the bone marrow [6]. Kojic acid is restricted to use in cosmetics [11] due to carcinogenicity and instability during storage. Ellagic acid is insoluble and has low bioavailability [9]. Ascorbic acid is heat-sensitive and easily decomposed [12]. Because of these problems, tyrosinase inhibitors previously used have limited use, or are only permitted to be used at low concentrations.
Coumarin (2H-chromen-2-one, benzo-α-pyrone) and its derivatives are widely distributed in plants and are especially abundant in the bark and leaves of plants [13,14]. In addition, coumarin is a fragrant chemical compound of benzopyrone, notably found in cinnamon extracts [15,16,17]. Coumarins have shown evidence of many biological activities. The biomedical activities of coumarin include anti-HIV [18], anti-inflammatory [19], anti-cancer [20], anti-diabetes [21], and whitening effects [22]. Despite the many pharmaceutical effects of coumarin, the skin-protective function of coumarin has not been actively studied. In this study, the discovery of whitening active ingredients is urgent, and, in order to prepare for demand, this study aimed to find raw materials to be used in functional cosmetics by synthesizing derivatives of coumarin known to have whitening effects as lead materials.
2. Results and Discussion
2.1. Synthesis
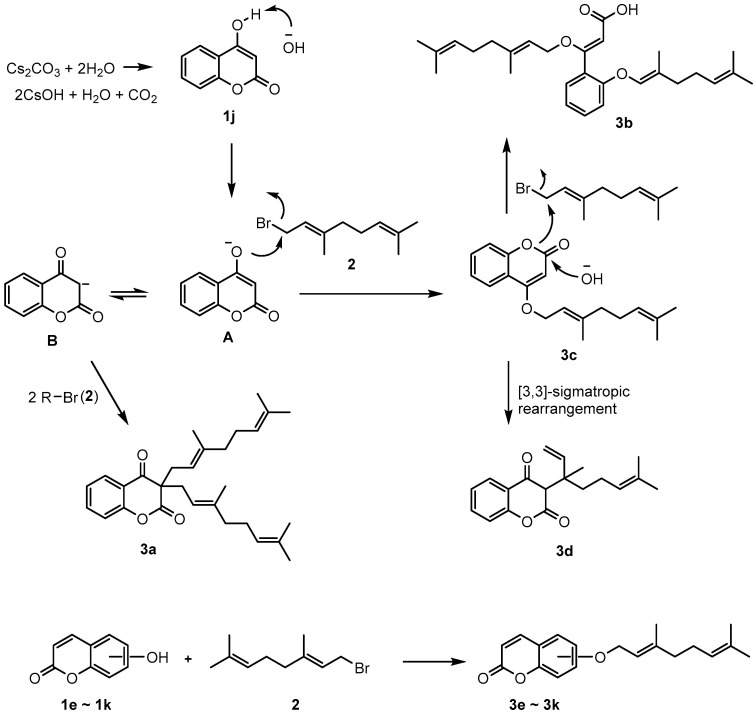
When there is an OH group at the 4-position of coumarin, geranyloxycoumarin derivatives are C-alkylated, with 18% O-alkylated coumarin 3c, when reacting with geranyl bromide. This is due to the base-induced keto-enol tautomerization. As shown in Figure 1, 3a was reacted with two equivalents of geranyl bromide (2) and keto form B to obtain an 11% yield. On the other hand, it is reported that 3d [23] is formed by the rearrangement of O-alkylated coumarin (3c). It was confirmed that the formation of 3d was generated through [3,3]-sigma tropic rearrangement by refluxing the isolated 3c under acetonitrile solvent. As a result of these, the reaction between 1j and excess geranyl bromide (2) gave the products 3a, 3b, and 3d. Additionally, in this reaction, the yield of 4-geranyloxycoumarin (3c) obtained using triethylamine (TEA) as a base was as low as 2%, and 3a, 3b, and 3d were not formed. However, under basic conditions, with K2CO3, CsOH, Cs2CO3, and Ag2CO3 as bases, geranyloxycoumarin derivative 3b was obtained in a 35% yield; it had an open coumarin ring structure due to moisture in the atmosphere (Figure 1).
Figure 1.
Reaction between hydroxycoumarin and alkenyl halide.
Except the coumarin with an OH group at the 4-position, which follows the reaction scheme shown in Figure 1, coumarins with OH groups at 3-, 5-, 6-, 7-, and 8-positions gave good yields of geranyloxycoumarin derivatives (3e–3l) when reacted with geranyl bromide under weakly basic conditions. As a result of the reaction between hydroxycoumarin (e.g., 6-hydroxycoumarin) and geranyl bromide in the presence of cesium carbonate and acetonitrile at room temperature, a general O-alkylated compound could be obtained in good yield. As shown in Figure 1, new coumarin derivatives were obtained by reacting various hydroxycoumarins and geranyl bromide under the given conditions.
The geranyloxycoumarin derivatives were synthesized by reacting a hydroxycoumarin with geranyl bromide in acetonitrile solvent at room temperature for 3 h in the presence of various bases (NaOH, K2CO3, triethylamine (TEA), CsOH, Cs2CO3, or Ag2CO3). Geranyloxy derivatives (3e–3l) were obtained in good yield.
2.2. Tyrosinase Inhibitory Activity
Tyrosinase is an enzyme that plays the most important role in the production of melanin from tyrosine when the skin is exposed to UV rays. Melanin is produced by enzymes, such as tyrosinase-related protein-1 (TRP-1) and dopachrome tautomerase (TRP-2), using tyrosine as a substrate from melanosomes in the melanocytes in the basal layer [23]. Melanin is produced by TRP-1 and TRP-2 as eumelanin, which appears black and brown, and pheomelanin, which appears yellow and red. Tyrosinase, a copper-containing metalloenzyme, is found in epidermal melanocytes, as well as the pigment epithelia of the retina, iris, and ciliary body of the eye [24]. Tyrosinase is one of the enzymes responsible for skin pigmentation in mammals. Melanin production can be inhibited by inhibiting the tyrosinase activity, thereby preventing the induction of melasma, freckles, and senile erythema [25].
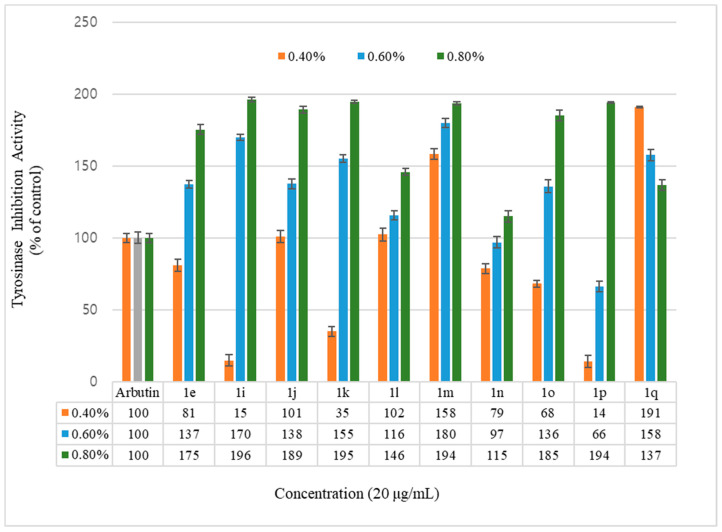
To evaluate the whitening effect of the coumarin derivatives, their ability to inhibit the tyrosinase activity was estimated. The coumarin derivatives were compared and evaluated by dividing them into the geranyloxycoumarin group (Table 1), containing the synthesized coumarin derivatives, and the hydroxycoumarin group (Table 2). After the compounds were treated at different concentrations of 0.4%, 0.6%, and 0.8%, the inhibitory ability of tyrosinase activity was calculated based on the following equation, and arbutin was used as a positive control. The results are shown in Figure 2 and Figure 3.
Table 1.
Synthesized geranyloxy coumarin compounds 3e–3l.
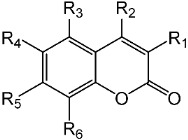
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | Yield (%) |
|---|---|---|---|---|---|---|---|
| 3e | Geranyloxy | H | H | H | H | H | 87 |
| 3f | Ph | H | H | H | Geranyloxy | H | 92 |
| 3g | H | CH3 | H | H | Geranyloxy | H | 91 |
| 3h | H | CF3 | H | H | Geranyloxy | H | 90 |
| 3i | H | H | H | H | Geranyloxy | H | 94 |
| 3j | H | CH3 | H | Cl | Geranyloxy | H | 90 |
| 3k | H | CH3 | H | Geranyloxy | Geranyloxy | H | 86 |
| 3l | H | CH3 | H | Geranyloxy | H | H | 89 |
Table 2.
Hydroxycoumarin derivatives with functional groups in several positions 1e–1q.

| Compound | R1 | R2 | R | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| 1e | OH | H | H | H | H | H |
| 1i | H | H | H | H | OH | H |
| 1j | H | CH3 | H | Cl | OH | H |
| 1k | H | CH3 | H | OH | OH | H |
| 1l | H | CH3 | H | OH | H | H |
| 1m | CN | H | H | H | OH | H |
| 1n | Cl | CH3 | H | H | OH | H |
| 1o | NO2 | OH | H | H | H | H |
| 1p | H | CH2COOH | H | H | OH | H |
| 1q | H | H | H | OH | H | H |
Figure 2.
Mushroom tyrosinase activity effects of hydroxycoumarin derivatives and reference. Results are expressed as means ± SEMs.
Figure 3.
Mushroom tyrosinase activity effects of geranyloxycoumarin derivatives and reference. Results are expressed as means ± SEMs.
Inhibition rate (%) = [(B − S)/B] 100
Here, B and S are the absorbances for the blank and samples. Arbutin was used as a reference standard inhibitor for comparison.
Inhibition of the tyrosinase activity was measured for the geranyloxycoumarin-derived compounds, 3e–3l, and the hydroxycoumarin-derived compounds, 1e–1q. The activity of the geranyloxy coumarin derivatives was found to be better than that of the hydroxycoumarin derivatives. The inhibitory activities of the eight geranyloxycoumarin derivatives at 0.4% concentration were similar to or better than that of arbutin, with the exception of compound 3f, and markedly superior at 0.6% and 0.8% concentrations. In particular, compounds 3e, 3f, 3h, 3i, and 3k among the geranyloxycoumarin derivatives, and compound 1i among the hydroxycoumarin derivatives, were 1.9 times more active than arbutin at 0.8% concentration, and compound 3k was two times more active than arbutin at a low concentration of 0.4%. The inhibitory effect of tyrosinase increased in a concentration-dependent manner, but the activities of compounds 3k and 1q were the highest at the lowest concentration of 0.4%. The activity tended to decrease with increasing concentration. The reason for the high tyrosinase-inhibitory activity of 3k is presumed to have played an important role due to the interaction between the two unsaturated geranyloxy groups bonded to the R4 and R5 positions and the tyrosinase. However, it is estimated that the decrease in tyrosinase-inhibitory activity of 6-hydroxycoumarin (1q) is because the 6-OH group of the coumarin skeleton interferes with the interaction between tyrosinase as the concentration increases.
3. Materials and Methods
3.1. Materials
In this study, geranyloxycoumarin derivatives were synthesized using substituted hydroxycoumarin (procured from TCI and AlfaAesar, Tokyo, Japan). Geranyl bromide (95%, Aldrich, St. Louis, MO, USA) was used as the alkenyl chain, and NaOH, triethylamine (Et3N), K2CO3, Cs2CO3, Ag2CO3, etc., were used as bases. Acetonitrile, ethyl acetate, dichloromethane, n-hexane, acetone, and ethanol were used as solvents. A nuclear magnetic resonance spectrometer (NMR spectrometer; BRUKER AVANCE 400 MHz, BRUKER, Karlsruhe, Germany) was used for analysis. CDCl3 and CD3OD containing tetramethylsilane (TMS), which is an internal standard, were used as analytical solvents. Infrared spectroscopy was performed on an FT/IR-4200 (JASCO, Tokyo, Japan) spectrophotometer, and KBr pellets were prepared to confirm the functional groups in the compound (see Supplementary Materials). In addition, the melting point was measured without calibrating the temperature. A thermometer was mounted under a paraffin oil container, and the open glass capillary method was used.
3.2. Synthesis
General method for the synthesis of geranyloxycoumarin derivative: 7-hydroxycoumarin (1.0 mmol) was added to a reaction mixture containing 25 mL of acetonitrile, cesium carbonate (1.1 mmol), and geranyl bromide (1.2 mmol, 95%) in a 50 mL double-neck round-bottom flask equipped with a thermometer, condenser, magnetic bar, and stirrer. The reaction mixture was stirred at room temperature for 3 h. After confirming the consumption of the starting material by thin-layer chromatography following 3 h of mixing, the reaction product was cooled at room temperature and filtered through a glass filter. After filtration, the solvent was removed using a rotary evaporator. Dichloromethane (20 mL) was added to the reaction mixture, and the mixture was stirred for 5 min. Following this, it was filtered and washed twice with 10 mL dichloromethane. The filtrate was concentrated using a rotary evaporator, and the residue was subjected to silica gel column chromatography using a mixed solvent of hexane/dichloromethane (1:1 volume ratio) to obtain geranyloxycoumarin derivatives. The analysis results of the synthesized coumarin derivatives are below.
3,3-bis((E)-3,7-dimethylocta-2,6-dien-1-yl)-chromane-2,4-dione (3a). Colorless liquid, Yield: 11%; IR (KBr, cm−1): ν 2966 (C-H), 2917 (C-H), 2854 (C-H), 1772 (C=O), 1689 (C=O), 1611, 1461, 1288 (C-O), 1142, 755; 1H-NMR (400 MHz, CDCl3): δ 1.47 (s, 6H, 2CH3), 1.55 (s, 6H, 2CH3), 1.59 (s, 6H, 2CH3), 1.72–1.84 (m, 8H, 2(-CH2CH2-)), 2.70–2.80 (m, 2H, -CH2-), 2.82–2.89 (m, 2H, -CH2-), 4.86–4.95 (m, 4H, 4(=CH)), 7.14–7.17 (m, 1H, H-6), 7.21–7.25 (m, 1H, H-5), 7.58–7.63 (m, 1H, H-8), 7.89–7.93 (m, 1H, H-7); 13C-NMR (100 MHz, CDCl3): δ 16.21 (CH3), 17.52 (CH3), 25.49 (CH3), 26.33 (CH), 37.62 (CH), 39.65 (CH), 62.24 (C), 116.89 (CH), 117.49 (C), 119.53 (CH), 123.78 (CH), 124.66 (CH), 126.55 (CH), 131.32 (C), 136.82 (C), 140.59 (C), 154.89 (C), 170.56 (C), 194.66 (C) ppm; MS m/z = 434 (M+); Anal. Calcd for C29H38O3: C, 80.14; H, 8.81, Found: C, 80.09; H, 8.76.
(E)-3-(((E)-3,7-dimethylocta-2,6-dien-1-yl)oxy)-3-(2-(((E)-3,7-dimethylocta-2,6-dien-1-yl)oxy)phenyl) acrylic acid (3b). Colorless liquid, Yield: 35%; IR (KBr, cm−1): ν 3328 (bs OH), 3012 (Aromatic C-H), 2994 (aliphatic C-H), 1712 (C=O), 1112 (C-O), 928, 806; 1H-NMR (400 MHz, CDCl3): δ 1.54 (s, 6H, 2CH3), 1.58 (s, 6H, 2CH3), 1.62 (s, 6H, 2CH3), 1.85–2.01 (m, 8H, 2(-CH2CH2-)), 2.25–2.32 (m, 2H, -CH2-), 2.41–2.48 (m, 2H, -CH2-), 3.47–3.54 (m, 1H, =CH), 4.98–5.03 (m, 2H, -OCH2-), 5.06–5.11 (m, 2H, -OCH2-), 6.86–6.90 (m, 1H, H-3), 6.95–6.98 (m, 1H, H-5), 7.42–7.46 (m, 1H, H-4), 7.77 (dd, J = 1.8 Hz, 8.2Hz, 1H, H-2), 12.66 (s, OH, D2O exch.); 13C-NMR (100 MHz, CDCl3): δ 16.10 (CH3), 17.67 (CH3), 25.65 (CH3), 26.54 (CH2), 30.60 (CH2), 39.74 (CH2), 46.39(CH2), 118.56 (C), 118.68(CH), 119.65 (C), 121.10(CH), 124.11 (CH), 130.24 (C), 131.45 (C), 136.20 (C), 137.59 (C), 162.93 (C), 210.28 (C) ppm; MS m/z = 452 (M+); Anal. Calcd for C29H40O4: C, 76.95; H, 8.91, Found: C, 76.93; H, 8.91.
(E)-4-(3,7-dimethylocta-2,6-dienyloxy)-2H-chromen-2-one (3c). White powder, Yield: 18%, m.p. 47–48 °C; IR (KBr, cm−1): ν 2923 (C-H), 1718 (C=O), 1620, 1371, 1235 (C-O), 1182 (C-O), 1104 (C-O), 923, 817, 764, 751, 500; 1H-NMR (400 MHz, CDCl3): δ 1.62 (s, 3H, CH3), 1.69 (s, 3H, CH3), 1.77 (s, 3H, CH3), 2.08–2.18 (m, 4H, -CH2CH2-), 4.71 (d, J = 6.7 Hz, 2H, -CH2-), 5.08–5.12 (m, 1H, =CH), 5.49–5.53 (m, 1H, =CH), 5.69 (s, 1H, H-3), 7.25–7.33 (m, H6 and H-8), 7.53–7.57 (m, 1H, H-7), 7.84 (dd, J = 2.2Hz, 5.8Hz, 1H, H-5); 13C-NMR (100 MHz, CDCl3): δ 16.82 (CH3), 17.75 (CH3), 25.70 (CH3), 26.19 (CH), 39.50 (CH), 66.27 (CH), 90.64 (CH), 115.92 (C), 116.75 (CH), 117.06 (CH), 123.19 (CH), 123.46 (CH), 123.83 (CH), 132.13 (CH), 132.31 (C), 143.74 (C), 153.35 (C), 163.15 (C), 165.61 (C) ppm; MS m/z = 298 (M+); Anal. Calcd for C19H22O3: C, 76.48; H, 7.43, Found: C, 76.49; H, 7.40.
(E)-3-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-2H-chromen-2-one (3e). White solid, Yield: 87%; m.p. 72–73 °C; IR (KBr, cm−1): ν 3086 (Aromatic C-H), 3052 (Aromatic C-H), 2975 (Aliphatic C-H), 2917 (Aliphatic C-H), 2885 (Aliphatic C-H), 1745 (Carbonyl (ester -C=O)), 1638 (C=C bond), 1585, 1503, 1468, 1446, 1413, 1390, 1331, 1319, 1225, 1218, 1164, 1122, 996, 950, 938, 900, 864, 790, 761, 603; 1H-NMR (400 MHz, CDCl3): δ 1.57 (s, 3H, CH3), 1.62 (s, 3H, CH3), 1.73 (s, 3H, CH3), 2.03–2.13 (m, 4H, -CH2CH2-), 4.61 (d, J = 8.0 Hz, 2H, -CH2-), 5.02–5.06 (m, 1H, =CH), 5.46–5.51 (m, 1H, =CH), 6.79 (s, 1H, H-4), 7.20–7.28 (m, 2H, H6 and H-8), 7.32–7.37 (m, 2H, H-5 and H-7); 13C-NMR (100 MHz, CDCl3): δ 16.82 (CH3), 17.72 (CH3), 25.66 (CH3), 26.17 (CH), 39.50 (CH), 66.24 (CH), 113.69 (CH), 116.29 (CH), 117.96 (C), 119.82(CH), 123.59 (C), 124.64(CH), 126.37 (CH), 128.33 (CH), 132.00 (CH), 142.51 (C), 143.69 (C), 149.53 (C), 157.77 (C) ppm; MS m/z = 298 (M+); Anal. Calcd for C19H22O3: C, 76.48; H, 7.43, Found: C, 76.44; H, 7.42.
(E)-7-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-3-phenyl-2H-chromen-2-one (3f). White solid, Yield: 92%; m.p. 104–105 °C; IR (KBr, cm−1): ν 3054 (Aromatic C-H), 3036 (Aromatic C-H), 2965 (Aliphatic C-H), 2909 (Aliphatic C-H), 2851 (Aliphatic C-H), 1707 (Carbonyl (ester -C=O)), 1606 (C=C bond), 1503, 1450, 1443, 1429, 1365, 1272, 11220, 1178, 1123, 1105, 1012, 990, 941, 827, 784, 691, 630; 1H-NMR(400 MHz, CDCl3): 1H-NMR (400 MHz, CDCl3): δ 1.59 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.75 (s, 3H, CH3), 2.05–2.15 (m, 4H, -CH2CH2-), 4.60 (d, J = 8.0 Hz, 2H, -CH2-), 5.05–5.09 (m, 1H, =CH), 5.44–5.48 (m, 1H, =CH), 6.84–6.87 (m, 2H, H-6 and H-8), 7.34–7.44 (m, 4H), 7.65–7.68 (m, 2H), 7.74 (s, 1H, H-4); 13C-NMR (100 MHz, CDCl3): δ 16.76 (CH3), 17.69 (CH3), 25.64 (CH3), 26.20 (CH2), 39.48 (CH2), 65.47 (CH2), 101.12 (CH), 113.19 (CH), 113.39 (CH), 118.38 (CH), 123.58 (C), 124.63 (CH), 128.36 (CH), 128.39 (CH), 128.74 (CH), 131.94 (C), 135.03 (C), 140.06 (C), 142.34 (CH), 155.23 (C), 160.95 (C), 161.88 (C) ppm; MS m/z = 374 (M+); Anal. Calcd for C25H26O3: C, 80.18; H, 7.00, Found: C, 80.16; H, 6.99.
(E)-7-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-4-methyl-2H-chromen-2-one (3g). White solid, Yield: 91%; m.p. 54–55 °C; IR(KBr, cm−1): ν 3078 (Aromatic C-H), 3028 (Aromatic C-H), 2964 (Aliphatic C-H), 2917 (Aliphatic C-H), 2856 (Aliphatic C-H), 1726 (Carbonyl (ester -C=O)), 1617 (C=C bond), 1508, 1441, 1420,1390, 1345, 1278, 1257, 1199, 1154, 1134, 1070, 992, 982, 843, 825; 1H-NMR (400 MHz, CDCl3): δ 1.57 (s, 3H, CH3), 1.63 (s, 3H, CH3), 1.73 (s, 3H, CH3), 2.03–2.13 (m, 4H, -CH2CH2-), 2.37 (d, J = 1.1 Hz, 3H, CH3), 4.57 (d, J = 6.5 Hz, 2H, -CH2-), 5.03–5.07 (m, 1H, =CH), 5.42–5.46 (m, 1H, =CH), 6.10 (q, J = 1.2 Hz, 2.4 Hz, 1H, H-3), 6.79 (d, J = 2.5 Hz, 8.8 Hz, 1H, H-8), 6.84 (d, J = 2.5 Hz, 1H, H-6), 7.46 (dd, J = 2.6 Hz, 8.8 Hz, 1H, H-5); 13C-NMR (100 MHz, CDCl3): δ 16.79 (CH3), 17.73 (CH3), 18.70 (CH3), 25.68 (CH3), 26.24 (CH2), 39.53 (CH2), 65.44 (CH2), 101.59 (CH), 111.85 (CH), 112.94 (C), 113.47 (CH), 118.45 (CH), 123.63 (CH), 125.46 (CH), 131.97 (C), 142.35 (C), 152.61 (C), 155.25 (C), 161.41 (C), 161.95 (C) ppm; MS m/z = 312 (M+); Anal. Calcd for C20H24O3: C, 76.89; H, 7.74, Found: C, 76.88; H, 7.72.
(E)-7-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-4-(trifluoromethyl)-2H-chromen-2-one (3h). White solid, Yield: 90%; m.p. 62–63 °C; IR (KBr, cm−1): ν 3077 (Aromatic C-H), 3030 (Aromatic C-H), 2974 (Aliphatic C-H), 2914 (Aliphatic C-H), 2894 (Aliphatic C-H), 1730 (Carbonyl (ester -C=O)), 1609 (C=C bond), 1556, 1516, 1452, 1427, 1400, 1351, 1275, 1215, 1192, 1166, 1137, 1014, 999, 958, 872, 818, 781, 715, 649, 626; 1H-NMR (400 MHz, CDCl3): δ 1.57 (s, 3H, CH3), 1.62 (s, 3H, CH3), 1.73 (s, 3H, CH3), 2.03–2.13 (m, 4H, -CH2CH2-), 4.59 (d, J = 4.0 Hz, -OCH2-), 5.01–5.06 (m, 1H, =CH), 5.40–5.45 (m, 1H, =CH), 6.57 (s, 1H, H-3), 6.83 (d, J = 2.5 Hz, 1H, H-8), 6.88 (dd, J = 2.5 Hz, 9.0 Hz, 1H, H-6), 7.56–7.59 (m, 1H, H-5); 13C-NMR (100 MHz, CDCl3): δ 16.79 (CH3), 17.70 (CH3), 25.65 (CH3), 26.20 (CH2), 39.50 (CH2), 65.67 (CH2), 102.13 (CH), 106.88 (CH), 112.04 (q, 3JCF = 5.7 Hz), 113.98 (C), 120.25(CH), 123.00 (CH), 123.54(CH), 126.24 (q, 3JCF = 2.0 Hz, CH), 132.02 (C), 141.61 (q, 2JCF = 32.7 Hz, CCF3), 142.78 (C), 156.31 (C), 159.49 (C), 162.86 (C) ppm; MS m/z = 366 (M+); Anal. Calcd for C20H21F3O3: C, 65.57; H, 5.78, Found: C, 65.54; H, 5.76.
(E)-7-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-2H-chromen-2-one (3i). White solid, Yield: 94%; m.p. 68–69 °C; IR (KBr, cm−1): ν 3082 (Aromatic C-H), 3053 (Aromatic C-H), 2972 (Aliphatic C-H), 2896 (Aliphatic C-H), 2879 (Aliphatic C-H), 2849 (Aliphatic C-H), 2833 (Aliphatic C-H), 1728 (Carbonyl (ester -C=O)), 1611 (C=C bond), 1507, 1452, 1430, 1403, 1369, 1348, 1280, 1234, 1201, 1165, 1126, 1103, 1022, 990, 889, 852, 830, 776, 760; 1H-NMR (400 MHz, CDCl3): δ 1.60 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.75 (s, 3H, CH3), 2.06–2.15 (m, 4H, -CH2CH2-), 4.59 (d, J = 6.7 Hz, 2H, -CH2-), 5.05–5.09 (m, 1H, =CH), 5.44–5.48 (m, 1H, =CH), 6.24 (d, J = 9.5 Hz, 1H, H-3), 6.81 (d, J = 2.5 Hz, 1H, H-6), 6.84 (dd, J = 2.4 Hz, 8.4 Hz, 1H, H-8), 7.36 (d, J = 8.6 Hz, 1H, H-5), 7.63 (d, J = 9.5 Hz, 1H, H-4); 13C-NMR (100 MHz, CDCl3): δ 16.37 (CH3), 17.31 (CH3), 25.26 (CH3), 25.82 (CH2), 39.11 (CH2), 65.08 (CH2), 101.18 (CH), 112.01 (CH), 112.56 (C), 112.84 (CH), 117.99 (CH), 123.20 (CH), 128.26 (CH), 131.57 (C), 141.98 (C), 143.04 (CH), 155.47 (C), 160.90 (C), 161.74 (C) ppm; MS m/z = 298 (M+); Anal. Calcd for C19H22O3: C, 76.48; H, 7.43, Found: C, 76.45; H, 7.41.
(E)-6-chloro-7-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-4-methyl-2H-chromen-2-one (3j). White solid, Yield: 90%; m.p. 95–96 °C; IR (KBr, cm−1): ν 3078 (Aromatic C-H), 3003 (Aromatic C-H), 2965 (Aliphatic C-H), 2916 (Aliphatic C-H), 2856 (Aliphatic C-H), 2854 (Aliphatic C-H), 1728 (Carbonyl (ester -C=O)), 1609 (C=C bond), 1494, 1414, 1388, 1378, 1320, 1274, 1205, 1157, 1083, 1047, 982, 883, 829; 1H-NMR (400 MHz, CDCl3): δ 1.59 (s, 3H, CH3), 1.65 (s, 3H, CH3), 1.77 (s, 3H, CH3), 2.06–2.15 (m, 4H, -CH2CH2-), 2.38 (d, J = 1.2 Hz, 3H, CH3), 4.69 (d, J = 6.4Hz, 2H, -CH2-), 5.04–5.08 (m, 1H, =CH), 5.44–5.48 (m, 1H, =CH), 6.16 (dd, J = 1.4 Hz, 2.6 Hz, 1H, H-3), 6.83 (s, 1H), 7.56 (s, 1H); 13C-NMR (100 MHz, CDCl3): δ 16.89 (CH3), 17.73 (CH3), 18.65 (CH3), 25.65 (CH3), 26.19 (CH2), 39.50 (CH2), 66.63 (CH2), 101.57 (CH), 112.74 (CH), 113.65 (C), 118.03 (CH), 119.34 (C), 123.54(CH), 125.32 (CH), 132.01 (C), 142.66 (C), 151.70 (C), 153.52 (C), 156.93 (C), 160.79 (C) ppm; MS m/z = 346 (M+); Anal. Calcd for C20H23ClO3: C, 69.26; H, 6.68, Found: C, 69.24; H, 6.65.
6-(((E)-3,7-dimethylocta-2,6-dien-1-yl)oxy)-7-(((Z)-3,7-dimethylocta-2,6-dien-1-yl)oxy)-4-methyl-2H-chromen-2-one (3k). White solid, Yield: 86%; m.p. 63–64 °C; IR (KBr, cm−1): ν 3065 (Aromatic C-H), 2937 (Aliphatic C-H), 2917 (Aliphatic C-H), 2854 (Aliphatic C-H), 1708 (Carbonyl (ester -C=O)), 1612 (C=C bond), 1562, 1520, 1430, 1384, 1280, 1231, 1164, 984, 822; 1H-NMR (400 MHz, CDCl3): δ 1.59 (s, 6H, 2CH3), 1.64 (s, 6H, 2CH3), 1.77 (s, 6H, 2CH3), 2.06–2.15 (m, 8H, 2(-CH2CH2-)), 2.37 (d, J = 1.2 Hz, 3H, CH3), 4.67 (d, J = 6.4 Hz, 4H, 2(-CH2-)), 5.04–5.08 (m, 2H, 2(=CH)), 5.44–5.48 (m, 2H, 2(=CH)), 6.15 (dd, J = 1.4 Hz, 2.64 Hz, 1H, H-3), 6.83 (s, 1H, H-5), 7.55 (s, 1H, H-8); 13C-NMR (100 MHz, CDCl3): δ 17.04 (CH3), 17.17 (CH3), 18.01 (CH3), 19.14 (CH3), 25.95 (CH3), 26.52 (CH3), 26.59 (CH3), 39.81 (CH2), 39.86 (CH2), 66.63 (CH2), 67.16 (CH2), 101.75 (CH), 108.76 (CH), 112.37 (C), 112.65 (CH), 119.06 (CH), 119.92 (C), 123.96 (CH), 132.22 (C), 141.50 (C), 141.91 (C), 145.76 (C), 149.76 (C), 152.70 (C), 153.07 (C), 161.95 (C) ppm; MS m/z = 346 (M+); Anal. Calcd for C20H23ClO3: C, 69.26; H, 6.68, Found: C, 69.24; H, 6.65.
(E)-6-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-4-methyl-2H-chromen-2-one (3l). White solid, Yield: 89%; m.p. 57–58 °C; IR (KBr, cm−1): ν 3040 (Aromatic C-H), 2965 (aliphatic C-H), 2925 (aliphatic C-H), 2884 (aliphatic C-H), 1712(C=O), 1673, 1571, 1493, 1428, 1386, 1275, 1238 (C-O), 1167, 990, 926, 838; 1H-NMR (400 MHz, CDCl3): δ 1.60 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.77 (s, 3H, CH3), 2.06–2.17 (m, 4H, -CH2CH2-), 2.41 (d, J = 1.2 Hz, 3H, CH3), 4.59 (d, J = 6.6 Hz, 2H, -CH2-), 5.06–5.10 (m, 1H, =CH), 5.47–5.51 (m, 1H, =CH), 6.30 (q, J = 1.4 Hz, 2.6 Hz, 1H, H-3), 7.04 (d, J = 2.9 Hz, 1H, H-5), 7.13 (dd, J = 2.9 Hz, 9.0 Hz, 1H, H-7), 7.27 (d, J = 9.0 Hz, 1H, H-8); 13C-NMR (100 MHz, CDCl3): δ 16.74 (CH3), 17.72 (CH3), 18.74 (CH3), 25.68 (CH3), 26.27 (CH2), 39.55 (CH2), 65.56 (CH2), 108.82 (CH), 115.44 (CH), 117.89 (CH), 119.00 (CH), 119.32 (C), 120.43 (CH), 123.64 (CH), 131.96 (C), 141.96 (C), 147.84 (C), 152.02 (C), 155.20 (C), 161.03 (C) ppm; MS m/z = 312 (M+); Anal. Calcd for C20H24O3: C, 76.89; H, 7.74, Found: C, 76.86; H, 7.75.
3.3. Tyrosinase Inhibition Assay
During melanin synthesis, tyrosinase oxidizes tyrosine to DOPA, which is converted to DOPAquinone. The product of this reaction, DOPAquinone, has an absorbance maximum at 490 nm. The method for the oxidation of L-DOPA by tyrosinase, established by Fling et al. (1963), was followed [19]. Sodium phosphate buffer (50 mM, pH 7.2), 0.24 mM L-DOPA, inhibitor, and 53.3 units/mL mushroom tyrosinase (Sigma) were adjusted to 400 µL, and the change in absorbance at 490 nm was measured at room temperature for 10 min. The amount of DOPAchrome (ε = 3700 M−1·cm−1) produced was calculated based on the increase in enzyme absorbance. Arbutin was used as a positive control. All the chemicals and reagents were purchased from Sigma.
3.4. Statistical Analysis
The values are expressed as mean ± standard error (n = 3) and the biological significance p < 0.05 was determined by Student’s t-test.
4. Conclusions
In this study, geranyloxycoumarin derivatives were effectively synthesized using coumarin, which affects a number of physiological activities, as a lead compound. Various geranyloxycoumarin derivatives were obtained in high yields. Inhibition of the tyrosinase activity by various derivatives suggested that the geranyloxycoumarin derivatives exhibited better activity than the hydroxycoumarin derivatives. Among the geranyloxycoumarin derivatives, compound 3k was two times more active than arbutin, a positive control, at a concentration of 0.4%.
The above results suggest that geranyloxycoumarin derivatives have great potential for application as functional cosmetic ingredients with tyrosinase-inhibiting activity.
Supplementary Materials
The following are available online.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khan S.B., Khan M.T.H., Jang E.S., Akhtar K., Seo J., Han H. Tyrosinase inhibitory effect of benzoic acid derivatives and their structure-activity relationships. J. Enzyme Inhib. Med. Chem. 2010;25:812–817. doi: 10.3109/14756366.2010.482529. [DOI] [PubMed] [Google Scholar]
- 2.Ohguchi K., Tanaka T., Ito T., Iinuma M., Matsumoto K., Akao Y., Nozawa Y. Inhibitory effects of resveratrol derivatives from dipterocarpaceae plants on tyrosinase activity. Biosci. Biotechnol. Biochem. 2003;67:1587–1589. doi: 10.1271/bbb.67.1587. [DOI] [PubMed] [Google Scholar]
- 3.Pillaiyar T., Manickam M., Namasivayam V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017;32:403–425. doi: 10.1080/14756366.2016.1256882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briganti S., Camera E., Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16:101–110. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 5.Arndt K.A., Fitzpatrick T.B. Topical Use of Hydroquinone as a Depigmenting Agent. JAMA J. Am. Med. Assoc. 1965;194:965–967. doi: 10.1001/jama.1965.03090220021006. [DOI] [PubMed] [Google Scholar]
- 6.Zhou H., Kepa J.K., Siegel D., Miura S., Hiraki Y., Ross D. Benzene metabolite hydroquinone up-regulates chondromodulin-I and inhibits tube formation in human bone marrow endothelial cells. Mol. Pharmacol. 2009;76:579–587. doi: 10.1124/mol.109.057323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabanes J., Chazarra S., Carmona F.G. Kojic acid, a Cosmetic Skin Whitening Agent, is a Slow-binding Inhibitor of Catecholase Activity of Tyrosinase. J. Pharm. Pharmacol. 1994;46:982. doi: 10.1111/j.2042-7158.1994.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 8.Iwahori A., Hirota Y., Sampe R. NII-Electronic Library Service. Chem. Pharm. Bull. 1970;43:2091. [Google Scholar]
- 9.Shimogaki H., Tanaka Y., Tamai H., Masuda M. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int. J. Cosmetic Sci. 2000;22:291–303. doi: 10.1046/j.1467-2494.2000.00023.x. [DOI] [PubMed] [Google Scholar]
- 10.Curto E.V., Kwong C., Hermersdörfer H., Glatt H., Santis C., Virador V., Hearing V.J., Dooley T.P. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999;57:663–672. doi: 10.1016/S0006-2952(98)00340-2. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto N., Onodera H., Mitsumori K., Tamura T., Maruyama S., Ito A. Changes in thyroid function during development of thyroid hyperplasia induced by kojic acid in F344 rats. Carcinogenesis. 1999;20:1567–1572. doi: 10.1093/carcin/20.8.1567. [DOI] [PubMed] [Google Scholar]
- 12.Spínola V., Mendes B., Câmara J.S., Castilho P.C. Effect of time and temperature on vitamin C stability in horticultural extracts. UHPLC-PDA vs. iodometric titration as analytical methods. LWT Food Sci. Technol. 2013;50:489–495. doi: 10.1016/j.lwt.2012.08.020. [DOI] [Google Scholar]
- 13.Akak C.M., Djama C.M., Nkengfack A.E., Tu P.F., Lei L. Di New coumarin glycosides from the leaves of Diospyros crassiflora (Hiern) Fitoterapia. 2010;81:873–877. doi: 10.1016/j.fitote.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Ngo N.T.N., Nguyen V.T., Van Vo H., Vang O., Duus F., Ho T.-D.H., Pham H.D., Nguyen L.-H.D. Cytotoxic Coumarins from the Bark of Mammea siamensis. Chem. Pharm. Bull. 2010;58:1487–1491. doi: 10.1248/cpb.58.1487. [DOI] [PubMed] [Google Scholar]
- 15.Blahová J., Nka Svobodová Z. Assessment of Coumarin Levels in Ground Cinnamon Available in the Czech Retail Market. Sci. World J. 2012 doi: 10.1100/2012/263851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woehrlin F., Fry H., Abraham K. Quantification of Flavoring Constituents in Cinnamon: High Variation of Coumarin in Cassia Bark from the German Retail Market and in Authentic Samples from Indonesia. J. Agric. Food Chem. 2010;58:10568–10575. doi: 10.1021/jf102112p. [DOI] [PubMed] [Google Scholar]
- 17.Rychlik M. Quantification of Free Coumarin and Its Liberation from Glucosylated Precursors by Stable Isotope Dilution Assays Based on Liquid Chromatography-Tandem Mass Spectrometric Detection. J. Agric. Food Chem. 2008;56:796–801. doi: 10.1021/jf0728348. [DOI] [PubMed] [Google Scholar]
- 18.Ong E.B.B., Watanabe N., Saito A., Futamura Y., Abd El Galil K.H., Koito A., Najimudin N., Osada H. Vipirinin, a coumarin-based HIV-1 Vpr inhibitor, interacts with a hydrophobic region of Vpr. J. Biol. Chem. 2011;286:14049–14056. doi: 10.1074/jbc.M110.185397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan R., Gao X.H., Li Y., Xia Y.F., Dai Y. Anti-arthritic effect of scopoletin, a coumarin compound occurring in Erycibe obtusifolia Benth stems, is associated with decreased angiogenesis in synovium. Fundam. Clin. Pharmacol. 2010;24:477–490. doi: 10.1111/j.1472-8206.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharyya S.S., Paul S., Mandal S.K., Banerjee A., Boujedaini N., Khuda-Bukhsh A.R. A synthetic coumarin (4-Methyl-7 hydroxy coumarin) has anti-cancer potentials against DMBA-induced skin cancer in mice. Eur. J. Pharmacol. 2009;614:128–136. doi: 10.1016/j.ejphar.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Bucolo C., Ward K.W., Mazzon E., Cuzzocrea S., Drago F. Protective effects of a coumarin derivative in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2009;50:3846–3852. doi: 10.1167/iovs.08-3328. [DOI] [PubMed] [Google Scholar]
- 22.Liu J., Wu F., Chen L., Zhao L., Zhao Z., Wang M., Lei S. Biological evaluation of coumarin derivatives as mushroom tyrosinase inhibitors. Food Chem. 2012;135:2872–2878. doi: 10.1016/j.foodchem.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 23.Cravotto G., Nano G.M., Palmisano G., Tagliapietra S. 4-Hydroxycoumarin and Related Systems: Sitoselectivity of the Mitsunobu Reaction with Prenyl Alcohols. Heterocycles. 2003;60:1351–1358. doi: 10.3987/COM-03-9737. [DOI] [Google Scholar]
- 24.Wang N., Hebert D.N. Tyrosinase maturation through the mammalian secretory pathway: Bringing color to life. Pigment Cell Res. 2006;19:3–18. doi: 10.1111/j.1600-0749.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 25.Jeon M.-J., Kim M.-H., Jang H.-J., Lee S.-W., Kim J.-H., Kim H.-S., Lee S.-H. Whitening Effect of Hizikia fusiformis Ethanol Extract and Its Fractions. J. Life Sci. 2012;22:889–896. doi: 10.5352/JLS.2012.22.7.889. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.