ABSTRACT
Background: Posttraumatic stress disorder (PTSD) is associated with dysregulated neural, cortisol, and cardiac stress reactivity and recovery. This understanding is predominantly based on studies in adults applying emotional-cognitive and trauma-related stimuli inducing negative emotions or perceived threat. Despite large numbers of adolescents with PTSD, few studies are available on neurobiological stress reactivity in this population. Moreover, no previous studies investigated neural reactivity to social-evaluative stress.
Objective: To investigate functional brain connectivity, cortisol and cardiac reactivity to acute social-evaluative stress, and additional cortisol measures in trauma-exposed adolescents with and without high PTSD symptoms.
Method: A speech preparation task to induce acute social-evaluative stress elicited by anticipatory threat, was used in a subsample of the Amsterdam Born Child and their Development (ABCD) birth cohort, consisting of trauma-exposed adolescents with (n = 20) and without (n = 29) high PTSD symptoms. Psychophysiological interaction analyses were performed to assess group differences in functional connectivity of the hippocampus, mPFC and amygdala during social-evaluative stress and recovery, measured by fMRI. Additionally, perceived stress, heart rate and cortisol stress reactivity and recovery, cortisol awakening response and day curve were compared.
Results: The stressor evoked significant changes in heart rate and perceived stress, but not cortisol. The PTSD symptom and control groups differed in functional connectivity between the hippocampus and cerebellum, middle and inferior frontal gyrus, and the mPFC and inferior frontal gyrus during social-evaluative stress versus baseline. Mostly, the same patterns were found during recovery versus baseline. We observed no significant group differences in amygdala connectivity, and cortisol and cardiac measures.
Conclusions: Our findings suggest threat processing in response to social-evaluative stress is disrupted in adolescents with PTSD symptoms. Our findings are mainly but not entirely in line with findings in adults with PTSD, which denotes the importance to investigate adolescents with PTSD as a separate population.
KEYWORDS: PTSD, adolescent, social-evaluative stress, functional connectivity, hippocampus, mPFC, amygdala, cortisol reactivity, cardiac reactivity
HIGHLIGHTS
Adolescents with PTSD symptoms showed different functional brain connectivity for the hippocampus and medial prefrontal cortex during social-evaluative stress.
Amygdala connectivity was not different.
Functional connectivity findings seem mostly similar to adults with PTSD.
Short abstract
Antecedentes: El trastorno de estrés postraumático (TEPT) está asociado con la recuperación. Esta comprensión se basa predominantemente en estudios con adultos, que aplican estímulos emocional-cognitivos y relacionados con el trauma que inducen emociones negativas, o percepción de amenaza. A pesar del gran número de adolescentes con TEPT, hay pocos estudios disponibles sobre la reactividad neurobiológica al estrés en esta población. Además, ningún estudio previo ha investigado la reactividad neuronal al estrés socio-evaluativo.
Objetivos: Investigar la conectividad cerebral funcional, el cortisol y la reactividad cardíaca al estrés socio-evaluativo, y medidas adicionales de cortisol en adolescentes expuestos a trauma con y sin síntomas elevados de TEPT.
Método: Se utilizó una tarea de preparación de discurso para inducir un estrés socio-evaluativo agudo, provocado por la amenaza anticipatoria, en una submuestra del cohorte de nacimiento del Niño nacido en Amsterdam y su Desarrollo (Amsterdam Born Child and their Development, ABCD), que consta de adolescentes expuestos a traumas con (n = 20) y sin (n = 29) síntomas elevados de TEPT. Se realizaron análisis de interacción psicofisiológica para evaluar las diferencias de grupo en la conectividad funcional del hipocampo, mPFC y amígdala durante el estrés socio-evaluativo y la recuperación, medido por fMRI. Además, se compararon el estrés percibido, la frecuencia cardíaca y la reactividad y recuperación del estrés por cortisol, la respuesta del cortisol al despertar, y la curva diurna.
Resultados: El estresor provocó cambios significativos en la frecuencia cardíaca y el estrés percibido, pero no del cortisol. Los grupos con síntomas de TEPT y control difirieron en la conectividad funcional entre el hipocampo y el cerebelo, la circunvolución frontal media e inferior, y la mPFC y la circunvolución frontal inferior durante el estrés socio-evaluativo frente al valor inicial. En su mayoría, se encontraron los mismos patrones durante la recuperación frente a la línea de base. No observamos diferencias significativas entre grupos respecto de la conectividad de la amígdala, ni en las medidas de cortisol y cardíacas.
Conclusiones: Nuestros hallazgos sugieren que el procesamiento de amenazas en respuesta al estrés socio-evaluativo se encuentra alterado en adolescentes con síntomas de TEPT. Nuestros hallazgos están principalmente, pero no completamente, en línea con los hallazgos en adultos con TEPT, lo que denota la importancia de investigar a adolescentes con TEPT como una población aparte.
PALABRAS CLAVE: TEPT; adolescente; estrés socio-evaluativo; conectividad funcional; hipocampo, mPFC; amígdala; cortisol; reactividad; reactividad cardíaca
背景: 创伤后应激障碍(PTSD)与康复相关。这种理解主要基于对成年人进行会引起负面情绪或感知威胁的情绪认知和创伤相关刺激的研究。尽管患有PTSD的青少年众多,但有关此人群神经生物学应激反应的研究很少。此外,以前没有研究考查神经反应对社会评价应激的影响。
目的: 在有、无高PTSD症状的创伤暴露青少年中,探讨对社会评价应激的急性脑功能连接、皮质醇和心脏反应以及皮质醇的其他测量方式。
方法: 在阿姆斯特丹出生儿童及其发育(ABCD)出生队列子样本中使用了演讲准备任务,以诱发由预期威胁引起的急性社会评价应激,样本包括创伤暴露的20名有高PTSD症状青少年和29名无高PTSD症状青少年。进行心理生理学交互分析,以评估在社会评价应激和恢复过程中,由fMRI测量的海马、mPFC和杏仁核在功能连接方面的群体差异。此外,比较了感知压力、心率和皮质醇应激反应和恢复、皮质醇唤醒反应和日曲线。
结果: 应激源引起了HR和感知压力的显著变化,而皮质醇则没有。 PTSD症状组和对照组的社会评价应激与基线水平对比时,海马与小脑、额中回和额下回,mPFC和额下回之间的功能连接不同。通常,在恢复期间和基线之间发现了相同的模式。我们没有观察到杏仁核连接性、皮质醇和心脏测量方面的显著组间差异。
结论: 我们的发现表明,在患有PTSD症状的青少年中,社会评价应激的威胁加工受到了干扰。我们的发现主要但不完全与PTSD成人的发现一致,这表明把患有PTSD的青少年作为一个单独人群进行调查的重要性。
关键词: PTSD, 青少年, 社会评价应激, 功能连接, 海马, mPFC, 杏仁核, 皮质醇反应, 心脏反应
1. Introduction
Approximately 16% of youth who experience a traumatic event subsequently develops posttraumatic stress disorder (PTSD; Alisic et al., 2014), which is more than double the estimated lifetime prevalence of PTSD in trauma-exposed adults (De Vries & Olff, 2009). Evidence is mounting that PTSD is associated with, and potentially causally related to, dysregulated neural reactivity to a variety of stressors (Hayes, Hayes, & Mikedis, 2012; Koch et al., 2016; Patel, Spreng, Shin, & Girard, 2012) and recovery from the stressors (Dickie, Brunet, Akerib, & Armony, 2011). Despite the large numbers of adolescents affected by PTSD, most studies on neural stress reactivity in PTSD examined adults. As of yet, neural stress reactivity in adolescents with PTSD remains scarcely investigated.
Previous studies on neural stress reactivity in PTSD mainly focused on the functioning of specific brain regions of interest in response to various stimuli inducing negative emotions or feelings of threat, ultimately leading to a stress response. These stressors, for example, include emotional images to induce emotional distress and traumatic script-driven imagery to provoke PTSD symptoms. Meta-analyses of PTSD studies in adults previously demonstrated that activity and connectivity patterns between the hippocampus, medial prefrontal cortex (mPFC) and amygdala in response to these stressors were disrupted. Included studies used functional neuroimaging methods such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET) and single-photon emission computed tomography (SPECT; Hayes et al., 2012; Koch et al., 2016; Patel et al., 2012). Resting-state studies have also shown that activity and connectivity within and between these regions differ between adult PTSD patients and trauma- and non-trauma-exposed controls during baseline conditions (Koch et al., 2016). The few studies available on adolescents with PTSD showed similar brain regions to be dysregulated. For example, PTSD in young adolescence was associated with increased left parahippocampal gyrus activity in response to trauma-related stimuli compared to trauma-exposed controls (Yang, Wu, Hsu, & Ker, 2004) and decreased amygdala-mPFC connectivity in response to threatening images compared to non-traumatized healthy controls (Wolf & Herringa, 2016). Also, resting-state connectivity findings in adolescent PTSD largely corroborate findings reported in adult PTSD (Viard et al., 2019).
Next to neural stress reactivity, endocrine and physiological stress regulation and reactivity have been found to differ between trauma-exposed individuals with and without PTSD as well. Findings on acute cortisol reactivity and its recovery in response to psychological stressors in adults with PTSD have been inconsistent thus far, demonstrating both increased and blunted responses (Elzinga, Schmahl, Vermetten, Van Dyck, & Bremner, 2003; De Kloet et al., 2006; Zorn et al., 2017). Results from pharmacological challenge studies administering synthetic stress hormones, such as dexamethasone, are more consistent in finding PTSD-related enhanced suppression of cortisol secretion (Schumacher et al., 2019). Only two studies thus far focussed on cortisol stress reactivity in adolescents, and results were unequivocal. In one study, adolescents with (partial) PTSD showed a blunted cortisol response to trauma-related stimuli (Zandvoort et al., 2019). In the other study, female adolescents with PTSD symptoms showed blunted cortisol responses to a psychosocial stressor, whereas male adolescents with PTSD symptoms showed increased cortisol responses (Zimmerman et al., 2020). Meta-analyses in adults demonstrated lower levels of cortisol in the morning and across the day in individuals with PTSD compared to trauma-exposed and non-exposed controls (Morris, Compas, & Garber, 2012; Schumacher et al., 2019). Also, cortisol awakening responses (CAR) are likely attenuated in adults with PTSD compared to healthy controls (De Kloet et al., 2007; Wessa, Rohleder, Kirschbaum, & Flor, 2006). The results available on basal cortisol in adolescent PTSD are mostly in line with studies in adults with PTSD. Adolescents with PTSD symptoms showed lower baseline and morning cortisol levels than healthy controls (Feldman, Vengrober, Eidelman-Rothman, & Zagoory-Sharon, 2013; King, Mandansky, King, Fletcher, & Brewer, 2001; Pan, Wang, Wu, Wen, & Liu, 2018). Also, the CAR was shown to be flattened in adolescents with PTSD or PTSD symptoms (Keeshin, Strawn, Out, Granger, & Putnam, 2014). However, in contrast to what was shown in adult PTSD, studies on diurnal cortisol showed an elevated day curve in young adolescents with PTSD symptoms (Carrion et al., 2002; De Bellis et al., 1999). These changes in endocrine output are thought to result from increased sensitivity of glucocorticoid receptors within the Hypothalamic Pituitary Adrenal (HPA) axis, which causes increased negative feedback by cortisol (Olff & Van Zuiden, 2017). This dysfunction of the HPA axis is thought to underlie PTSD-related changes in hippocampus, amygdala and mPFC functioning and several symptoms, such as intrusions and hyperarousal (De Kloet, Joels, & Holsboer, 2005; Herringa, 2017), although the observed correlation between endocrine and neural measures is generally only moderate (Van Zuiden et al., 2019).
Another measure of physiological stress regulation and reactivity that has been studied in PTSD more rarely, is cardiovascular activity regulated by the autonomic nervous system. While adult PTSD has been associated with elevated heart rate in response to trauma-related stimuli in a meta-analysis (Pole, 2007), adolescents with PTSD symptoms showed no difference in heart rate reactivity to a social stressor or trauma-related stimuli compared to controls (Jones-Alexander, Blanchard, & Hickling, 2005; MacMillan et al., 2009).
In sum, it is still not certain if neural, cortisol and cardiac stress reactivity is dysregulated in adolescent PTSD and whether this is similar to adult PTSD (Leenarts, Diehle, Doreleijers, Jansma, & Lindauer, 2013). The brain undergoes extensive changes during development (Herringa, 2017; Heyn et al., 2019; Lupien, McEwen, Gunnar, & Heim, 2009; Weems, Russell, Neill, & McCurdy, 2019), and thus findings from adult populations may not be directly generalizable to adolescent populations. Therefore, the aim of the present study was to investigate neurobiological stress reactivity in adolescents with PTSD symptoms. Specifically, we investigated differences in fMRI functional brain connectivity (using psychophysiological interaction analyses (PPI)), cortisol, and cardiac reactivity to a social-evaluative stress task between trauma-exposed adolescents with high PTSD symptom levels compared to trauma-exposed controls with no to mild PTSD symptoms.
To the best of our knowledge, neural reactivity to social-evaluative stress in PTSD has not been investigated yet, in either adults or adolescents. Nevertheless, as PTSD is associated with problems in social functioning (McLean, Rosenbach, Capaldi, & Foa, 2013; Schnurr, Lunney, Bovin, & Marx, 2009), it is of interest to investigate whether neural reactivity to this type of stressor is also dysregulated in individuals with PTSD, as was found for other types of stressors. Social-evaluative stressors are potent activators of central and peripheral stress systems (Dickerson & Kemeny, 2004; Ginty, Kraynak, Kuan, & Gianaros, 2019; Wager et al., 2009a). Furthermore, in both healthy adult and adolescent populations, involved brain regions and networks largely overlap between reactivity to and recovery from social-evaluative stressors and other types of stressors (Hermans, Henckens, Joëls, & Fernández, 2014; Liu et al., 2012; Van Oort et al., 2017), specifically for the hippocampus, mPFC, and amygdala. Therefore, integrating previous findings on neurobiological mechanisms implicated in adults and adolescents with PTSD, combined with the available literature on neural regions involved in reactivity to social-evaluative stress in healthy populations, we expected differential functional connectivity involving the hippocampus, mPFC and amygdala, reduced cortisol reactivity but no differential heart rate reactivity in response to the social-evaluative stress task, as well as lower CAR and cortisol day curves in adolescents with high PTSD symptom levels compared to trauma-exposed controls.
2. Methods
2.1. Participants
In 2017, the ABCD-Early Life Stress and Obesity (ELSO) study was initiated; with the primary aim to investigate the potential mechanisms underlying the association between early life stress and childhood obesity. For the ABCD-ELSO study, a subsample of the Amsterdam Born Children and their Development (ABCD) cohort was invited to participate. The study design of this prospective population-based multiethnic birth cohort has been described in detail previously (Van Eijsden, Vrijkotte, Gemke, & Van der Wal, 2011).
Participants for the ABCD-ELSO study were selected based on criteria for four distinct groups – this information was collected 2 years earlier during the fourth follow-up assessment of the cohort in 2015; (1) children whose mother had high Body Mass Index (BMI; BMI>26.6 kg/m2 (p50, i.e. 50th percentile of the study population)) pre-pregnancy; (2) children whose mother had high anxiety symptoms (State-Trait Anxiety Inventory scores >37 (p50)) during pregnancy; (3) trauma-exposed children with high PTSD symptom levels (PTSD symptom group); (4) a trauma-exposed healthy control group with no to mild PTSD symptom levels (TC). N = 315 participants met criteria for one of these groups and were invited to participate. The subsample was invited by mail and a total of n = 120 by then adolescent participants accepted the invitation (overall response rate: 38%; with a mean CRIES score for participants who accepted the invitation of 12.45 (SD 12.78) versus 12.30 (12.55) for those who declined the invitation, p = .732). For the present study, we specifically focused on two groups from the ABCD-ELSO subsample; the PTSD symptom and TC groups, a total of N = 54 participants. Results within the other groups will be reported elsewhere. High PTSD symptoms were classified by a cut-off total score of ≥24 (p90) on the 13-item Children Revised Impact of Event Scale (CRIES-13; Perrin, Meiser-Stedman, & Smith, 2005) at the previous assessment at age 11–12. The CRIES-13 was filled out based on the experienced life event with the highest perceived impact (i.e. index trauma) and subscale scores for symptom clusters Intrusion, Avoidance and Arousal as well as a total score were calculated. Children in the TC group had no or very mild PTSD symptoms (maximum CRIES total scores: 7 (p50)). Participants were excluded in case of claustrophobia, non-MRI compatible implants or braces, and severe neurological disorders. For female participants, hormonal contraception use was not an exclusion criterion, but upon finalizing the study, it appeared that only n = 2 (3.9%) used hormonal contraception. As sensitivity analyses indicated that removing these participants from the analyses greatly influenced the neuroimaging results, it was decided to exclude these participants (see supplementary Table S6-S7). The final sample consisted of N = 49 participants (PTSD symptom: n = 20; TC: n = 29). Approval for this study was obtained from the local medical ethical committee, and the study was conducted following the Helsinki Declaration. All participants and their parents gave written informed consent prior to any study procedures.
2.2. Experimental session and fMRI task design
Enrolled participants were mailed questionnaires and a set of five Salivettes for saliva collection prior to a clinic visit. All participants were asked to abstain from eating and drinking (except for water) 1.5 h before their visit. Upon arrival, participants handed in their collected Salivettes and filled-out questionnaires. They received further instructions about the study procedure and MRI equipment and had the opportunity to ask questions. The scanning session consisted of a T1-weighted anatomical image, followed by a food-related Go/No-Go-task, a food reward- and anticipation-related Milkshake task, a 3D T2-weighted anatomical image, and finally a 9-min long social-evaluative stress task (Stress) to study acute neural, cardiac and cortisol stress reactivity in a laboratory setting (Kirschbaum, Pirke, & Hellhammer, 1993; Wager et al., 2009a). We used a variation on the speech task from the Trier Social Stress Test eliciting anticipatory threat, which has been shown to generate acute stress responses on a neural, psychological, cardiac and endocrine level (Wager et al., 2009b). For a timeline of the used stress task, consisting of a Baseline, Instruction, Stress and Recovery phase, see Figure 1. For a detailed description of the stress task, see Supplemental S1.
Figure 1.
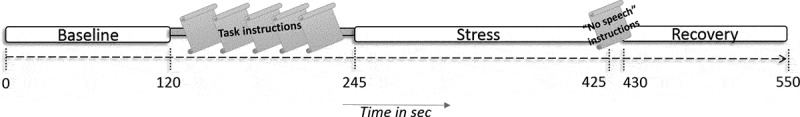
Overview of the timeline of the used stress task
2.3. Psychological measures
To measure perceived stress participants were repeatedly asked to indicate how stressed they were during the experimental phases Baseline, Stress and Recovery. Possible answers ranged from 1 (‘low perceived stress’) to 7 (‘high perceived stress’). The Baseline item was administered before scanning, for Stress directly after scanning and for Recovery at the end of the experimental session. Additionally, perceived impact of the stress task was measured directly after scanning with a questionnaire consisting of four items; Difficulty, Involvement, Expected performance and Control over the task, scored using a 7-point Likert Scale.
General perceived stress experienced during the previous two weeks was measured using the 14-item Perceived Stress Questionnaire (PSQ14; Cohen, Kamarck, & Mermelstein, 1983) on a 5-point Likert scale ranging from 0 (‘Never’) to 4 (‘Very often’). Total scores (ranging from 0–56) were calculated, with higher scores indicating higher general perceived stress. To measure socio-emotional behaviour and problems, the Strengths and Difficulties Questionnaire (SDQ; Van Widenfelt, Goedhart, Treffers, & Goodman, 2003) was administered on a 3-point Likert scale ranging from 0 (‘Not true’) to 2 (‘Definitely true’). This self-report questionnaire measures five subscales; Emotional symptoms, Conduct problems, Hyperactivity/Inattention, Peer problems, and Prosocial behaviour. Total- and subscores were calculated representing psychosocial problems and behaviours, with higher scores indicating more socio-emotional problems and poorer psychosocial behaviour. Eating behaviour was measured using the self-report 33-item Dutch Eating Behaviour Questionnaire (DEBQ; Van Strien, Frijters, Bergers, & Defares, 1986) on a 5-point Likert scale ranging from 0 (‘Never’) till 4 (‘Very Often’). Scores on the three subscales Emotional Eating, Restrained Eating and External Eating were calculated, with higher scores indicating more problems in eating behaviour. The PSQ14, SDQ and DEBQ were filled out at home prior to the experimental session. Sleep problems were measured using an adjusted version of the Children’s Report of Sleep Patterns (CRSP; Meltzer et al., 2013), filled out during the clinic visit. Mean total scores were calculated of 6 items following a 3-point Likert scale to represent sleep problems.
2.4. Biological measures
2.4.1. Cortisol
For saliva sampling, participants received Salivettes with a biocompatible synthetic swab at home (Sarstedt, Germany). Participants were asked to place the Salivette in their mouth for approximately one minute and lightly chew on the swab to absorb enough saliva, transfer it back into the tube and store it in the freezer until the day of the clinic visit. Sampling was instructed to take place on a weekend day (Stalder et al., 2016) according to a fixed schedule; directly after awakening, 30 and 45 minutes after awakening for CAR assessment (Pruessner et al., 1997) and additionally at 12:00 and 20:00 for assessment of the cortisol day curve. Participants were asked to abstain from food 30 minutes before sampling, abstain from dairy drinks one h before sampling, and not consume any products that contain caffeine on the collection day. During the clinic visit, saliva samples were collected right before scanning and 8, 14, and 23 minutes respectively after the social stressor started, for assessment of acute cortisol stress reactivity. The large majority (85.64%) of assessments took place between 12:00pm and 18:00pm. The earliest assessment started at 10:09 and the latest at 14:30. Time of the first cortisol assessment did not differ between groups (mean(SD) PTSD symptom: 12:22:36 (1:29:34); TC: 11:42:25 (1:33:46); T48 = −1.49, p = .143). All Salivette samples were centrifuged, pipetted, encoded and frozen (−80°C) before being sent to the Biological Psychology Laboratory in Dresden, Germany, for analysis. Samples were analysed using a luminescence immunoassay (IBL International, Germany). Intra- and inter-assay variations are <4.5% and <4.3% respectively.
Cortisol output for CAR and acute cortisol stress reactivity (using all available samples from the clinic visit) were studied by calculating the ‘Area under the Curve with respect to the Ground’ (AUCg) and ‘with respect to the Increase’ (AUCi) following guidelines by Pruessner, Kirschbaum, Meinlschmid, and Hellhammer (2003). In addition, the ‘Maximum increase with respect to baseline’ (MaxInc) was computed. For the cortisol day curve, AUCg (using the first, fourth and fifth sample; Pruessner et al. (2003)) and MaxInc (using all five samples) were calculated. All calculations were corrected for reported sampling time, and in case of missing sample values, imputation was applied a missing value by the mean of non-missing adjacent sample cases.
2.4.2. Heart rate
Heart rate (HR) was monitored continuously during fMRI acquisition using a wireless MR-compatible HR peripheral pulse monitor attached to the left index finger with a sampling rate of 500 Hz. R-wave peaks were identified using Matlab (version R2016a, The Mathworks, Inc., USA). Individual outlier R-wave peaks were identified and removed from the data. Mean HR was averaged across 1-min intervals (meanHR/min) using the remaining R-waves. All data was smoothed before selecting the corresponding meanHR/min peaks for every task phase separately (Baseline, Instruction, Stress, Recovery), and were time-locked to the functional scans. Finally, meanHR/min was averaged per phase. Due to technical failure, n = 2 participants were excluded from HR analyses.
2.5. fMRI data analyses
2.5.1. fMRI data acquisition
Scans were acquired using a 3 T Philips Ingenia MRI system (Philips, Best, The Netherlands) with a 32-channel head coil. To obtain functional images, a single-shot echo-planar imaging (EPI) sequence sensitive to blood oxygenation level-dependent contrast was used, and an additional EPI scan with opposite phase polarity was acquired for distortion correction with the following scan parameters: TR/TE = 730/30 ms; flip angle = 55°; multiband factor = 4; SENSE = 1.8; field of view = 240 × 240 × 132 mm; voxel size = 2.5 × 2.5 × 2.75 mm; 48 slices. A T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence was used for anatomical referencing with the following scan parameters: TR/TE = 7/3 ms; flip angle = 9°; field of view = 240 × 180 × 256 mm; voxel size = 1 mm isotropic; 180 slices. For every participant the last seven volumes were removed because they were obtained beyond the task duration.
2.5.2. Preprocessing and first-level analysis
Preprocessing was performed using FMRIPREP v1.2.3 (Esteban et al., 2019) and included motion correction, distortion correction, registration, and normalization to MNI space, smoothing and ICA-AROMA (for details see Supplemental S2). Framewise Displacement (FD) was calculated to add as a covariate to second-level analyses. Subsequently, a high pass filter (256s) was applied using FSL and grand-mean scaling was performed.
Seed-to-voxel functional connectivity was calculated using psychophysiological interaction (PPI) analyses in FSL FEAT (www.fmrib.ox.ac.uk/fsl; Woolrich, Ripley, Brady, & Smith, 2001). Time-courses were extracted for each region of interest (ROI) based on previously identified relevant regions concerning stress reactivity in PTSD: 1) hippocampus, 2) mPFC, and 3) amygdala (Koch et al., 2016; Shin, Rauch, & Pitman, 2006). For anatomical definition details, see Supplement S3. In the first-level model, the contrasts for Stress vs. Baseline and Recovery vs. Baseline were used as psychological regressors of interest. In addition, for every ROI, individual time-courses were used as physiological regressors of interest. Time-series functional connectivity analyses were carried out using FILM with local autocorrelation correction (Woolrich et al., 2001).
2.5.3. Higher-level analysis
Due to excessive motion, n = 10 (PTSD symptom group n = 3; TC n = 7) participants were excluded from the second level analyses (FD > 0.3; Achterberg & Van der Meulen, 2019). First-level PPI maps were fed into higher-level analysis in FEAT to assess between and within-group differences. Additionally, demeaned FD (Power et al., 2014) and demeaned age values (Morris et al., 2012) were included as covariates. A 25% thresholded MNI avg152T1 grey matter mask was applied to Z statistic images before thresholding. First Z-statistic images were thresholded using clusters determined by Z > 3.1 and a corrected cluster significance threshold of P = 0.05 at the single-subject and group level (Worsley, 2001). Additionally, a False Discovery Rate (FDR) threshold of 0.01 was applied for between- and within-group results on the observed significant clusters within all test runs for ROIs and contrasts, to further control for multiple comparisons (Benjamini & Hochberg, 1995).
2.6. Statistical analyses
Independent samples T-tests, or in case of non-normally distributed data Mann-Whitney U tests, were performed to assess group differences in demographics, psychological characteristics, and questionnaire mean item, scale and delta scores. For categorical variables, Pearson Chi-square tests and Fisher’s exact tests, or in case of >2 categories per variable Fisher-Freeman-Halton exact tests, were performed.
Furthermore, Linear Mixed Models (LMM) with Restricted Maximum Likelihood (REML) were performed to determine group differences in cortisol, HR levels, and perceived stress across the experimental session, whilst controlling for sex and age. For details on these analyses, see Supplement S4.
One-way analyses of covariance (ANCOVAs) were conducted for cortisol AUCg, AUCi, and MaxInc, including sex and age as covariates. Missing values and outliers (Z ≥ 3.29) were excluded from the respective analyses.
Exploratory Pearson Correlations between cortisol AUCg, AUCi and MaxInc and questionnaire scores on sleep problems were performed. To expore potential effects of eating behaviour on our significant functional connectivity clusters, we extracted Functional Connectivity values (FC values; extracted from ROIs of our significant functional connectivity clusters using the CONN toolbox version 18b) of the significant clusters. One-way ANCOVAs were performed including questionnaire scores on eating behaviour that significantly differed between groups as covariate.
For all analyses, following significant main or interaction effects, post hoc T-tests with Bonferroni correction (α = 5%) for multiple comparisons were performed.
3. Results
3.1. Demographics and psychological measures
By definition, groups differed significantly on PTSD symptom total score, which was reported at age 11–12 during the fourth follow-up assessment of the cohort in 2015 (Table 1). Current PTSD symptom scores were not measured during this substudy. Compared to the TC group, the PTSD symptom group reported significantly more types of traumatic life events and more general perceived stress, sleep problems, psychosocial problems and behaviours on the subscales Emotional symptoms, Peer problems, and Total Difficulties, and Emotional Eating problems.
Table 1.
Participant characteristics
| PTSD symptom group (n = 20) | TC (n = 29) | Statistics | |
|---|---|---|---|
| Boys (n (%)) | 12 (60.0%) | 14 (48.3%) | X2 = .65, df = 1, p = .419 |
| Age (years) | 14.25 (0.44) | 14.79 (0.41) | U = 132.50, p < .001* |
| Puberty stage1 | 3.21 (0.71) | 3.34 (0.81) | T46 = 0.59, p = .561 |
| Index trauma (n (%)) Death Emotional Trauma Medical Trauma Physical Trauma Accidents Other |
5 (25.0%) 5 (25.0%) 2 (10.0%) 1 (5.0%) 1 (5.0%) 6 (30.0%) |
13 (44.8%) - 8 (27.6%) 3 (10.3%) 1 (3.4%) 4 (13.8%) |
|
| Total trauma exposure (nr of experienced event types) | 5.65 (3.69) | 2.86 (1.81) | U = 140.50, p = .002* |
| PTSD symptom severity (total CRIES score)2 Intrusion Avoidance Arousal |
32.30 (5.98) 8.20 (5.36) 11.85 (3.90) 11.95 (4.72) |
2.34 (2.08) 0.34 (0.61) 0.69 (1.20) 1.31 (1.95) |
T22.19 = −21.54, p < .001* U = 563.00, p < .001* U = 579.50, p < .001* T46.45 = −11.71, p < .001* |
|
General functioning General perceived stress3 Sleep problems4 Socio-emotional behaviour and problems5 Emotional symptoms Conduct problems Hyperactivity/Inattention Peer problems Prosocial Behaviour Total Difficulties Eating behaviour and problems6 Emotional eating Restrained eating External eating |
25.33 (4.77) 0.95 (0.39) 3.56 (2.77) 1.11 (1.13) 4.17 (2.07) 1.22 (1.17) 8.56 (1.15) 10.06 (4.49) 14.72 (9.77) 8.94 (6.94) 19.83 (4.27) |
20.50 (4.35) 0.68 (0.37) 1.43 (1.29) 0.89 (0.96) 2.82 (2.55) 0.50 (0.92) 8.89 (1.37) 5.64 (3.71) 8.21 (7.60) 5.68 (6.12) 20.29 (5.75) |
T44 = −3.54, p = .001* T47 = −2.41, p = .020* U = 142.00, p = .011* U = 225.50, p = .524 U = 169.00, p = .059 U = 162.00, p = .023* U = 195.50, p = .185 U = 113.00, p = .002* T44 = −2.53, p = .015* U = 325.50, p = .097 T44 = 0.29, p = .776 |
| Ethnicity (n (%)) Western European Non-Western European |
19 (95.0%) 1 (5.0) |
29 (100%) - |
p = .408 |
| Hand preference (n right-handed (n (%))) | 17 (85.0%) | 29 (100%) | p = .062 |
Scores are displayed as mean (SD) or n (%). 1Categorical stage of puberty according to Petersen, Crockett, Richards, and Boxer (1988), PTSD symptom group n = 19; TC n = 29; 2Children Revised Impact of Event Scale (CRIES-13) total score based on index trauma, PTSD symptom group n = 20; TC n = 29; 3Perceived Stress Questionnaire (PSQ14), PTSD symptom group n = 18; TC n = 28; 4Children’s Report of Sleep Patterns (CRSP), PTSD symptom group n = 20; TC n = 29; 5Strengths and Difficulties Questionnaire (SDQ), PTSD symptom group n = 18; TC n = 28, 6Dutch Eating Behaviour Questionnaire (DEBQ), PTSD symptom group n = 18; TC n = 28, *p < .05
3.2. Acute stress reactivity
3.2.1. Functional connectivity
We observed significantly increased functional connectivity for Stress vs. Baseline between the right hippocampus and right cerebellum in the PTSD symptom group compared to TC (Figure 2(a), Table 2). For TC compared to the PTSD symptom group, significantly increased functional connectivity was found for Stress vs. Baseline between the right hippocampus and left middle frontal gyrus (MFG, Figure 2(b)); between the left hippocampus and left inferior frontal gyrus (pars opercularis; IFG) and right MFG (Figure 2(c)); and between the mPFC and left IFG (pars opercularis, Figure 2(d)). At a more liberal threshold for FDR correction of p = .05, for Stress vs. Baseline significantly increased functional connectivity was found between the mPFC and left insula in the PTSD symptom group compared to TC. Additionally, in TC compared to the PTSD symptom group, significantly increased connectivity was found between the mPFC and an additional cluster in the left IFG (pars opercularis; see Supplementary Table S1).
Figure 2.
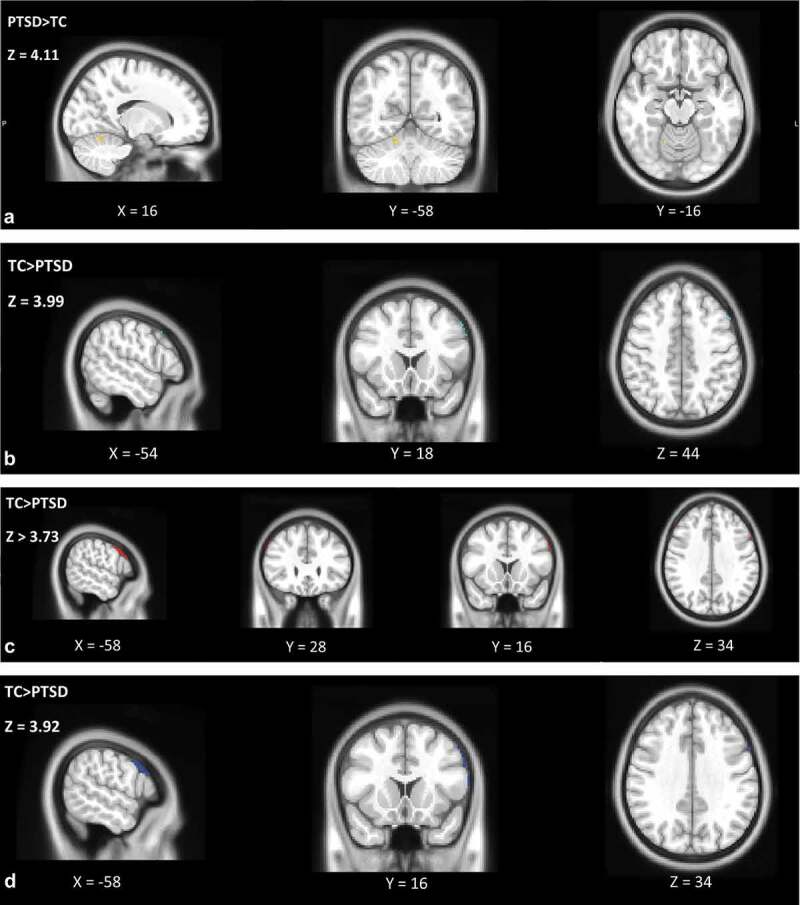
Representations of the significant increased functional connectivity clusters between adolescents with high PTSD symptoms vs. trauma-exposed controls for (a). the right hippocampus with the right cerebellum during Stress vs. Baseline for PTSD symptom group>TC group; in yellow, (b). the right hippocampus and left MFG during Recovery vs. Baseline for the TC>PTSD symptom group; in light blue, (c). the left hippocampus and left IFG and left hippocampus and right MFG during Recovery vs. Baseline for the TC>PTSD symptom group; in red, and (d). the mPFC and left IFG during Recovery vs. Baseline for the TC>PTSD symptom group; in dark blue. Significance level was defined as cluster p-values <.05 after FDR correction (α = 0.01), PTSD symptom group n = 17, TC n = 22
Table 2.
Between-group cluster list of task effects for PTSD symptom group > TC and TC > PTSD symptom group
| Stress vs. Baseline | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTSD symptom group vs. TC |
TC vs. PTSD symptom group |
|||||||||||||
| Region | X | Y | Z | Z-score | Cluster size | p | Region | X | Y | Z | Z-score | Cluster size | p | |
| R Hippocampus | R Cerebellum | 16 | −58 | −16 | 4.11 | 28 | < .001 | L Middle Frontal Gyrus | −54 | 18 | 44 | 3.99 | 20 | .002 |
| L Hippocampus | L Inferior Frontal Gyrus (pars opercularis) | −58 | 16 | 34 | 3.85 | 31 | < .001 | |||||||
| R Middle Frontal Gyrus | 54 | 28 | 38 | 3.73 | 18 | .004 | ||||||||
| R Amygdala | ||||||||||||||
| L Amygdala | ||||||||||||||
| mPFC |
|
|
|
|
|
|
|
L Inferior Frontal Gyrus (pars opercularis) |
−58 |
16 |
34 |
3.92 |
42 |
< .001 |
| Recovery vs. Baseline | ||||||||||||||
| PTSD symptom group vs. TC |
TC vs. PTSD symptom group |
|||||||||||||
| |
Region |
X |
Y |
Z |
Z-score |
Cluster size |
p |
Region |
X |
Y |
Z |
Z-score |
Cluster size |
p |
| R Hippocampus | L Inferior Frontal Gyrus (pars opercularis) | −58 | 16 | 34 | 3.80 | 22 | .001 | |||||||
| L Hippocampus | L Inferior Frontal Gyrus (pars opercularis) | −58 | 16 | 34 | 3.85 | 37 | < .001 | |||||||
| R Middle Frontal Gyrus | 54 | 28 | 38 | 3.68 | 24 | < .001 | ||||||||
| R Amygdala | ||||||||||||||
| L Amygdala | ||||||||||||||
| mPFC | L Inferior Frontal Gyrus (pars opercularis) | −58 | 16 | 34 | 3.75 | 28 | < .001 | |||||||
Significant results after correcting for multiple testing by FDR correction (α = 0.01) according to Benjamini and Hochberg (1995). Regions were identified using the Harvard-Oxford Cortical and Subcortical Structural Atlases or if probability of the labels was low (<15%) using the MNI Structural Atlas. PTSD symptom group n = 17, TC n = 22.
For Recovery vs. Baseline significantly increased functional connectivity was found between the left hippocampus and left IFG (pars opercularis), and right MFG; between the right hippocampus and left IFG (pars opercularis); and between the mPFC and left IFG (pars opercularis) in TC compared to the PTSD symptom group. At an FDR correction of p = .05 we found significantly increased connectivity between the left hippocampus and two extra clusters in the left IFG (pars opercularis and pars triangularis) in TC compared to the PTSD symptom group (see Supplementary Table S1).
Significant effects of the task on functional connectivity during Stress vs. Baseline and Recovery vs. Baseline in TC and PTSD symptom groups separately are summarized in Supplementary Table S2.
Additionally, our exploratory analyses showed no significant covariate effect of Emotional Eating problems on FC values.
3.2.2. Cortisol reactivity
No significant group difference were found in any of the cortisol stress reactivity measures (AUCg (F(1,40) = 0.63, p = .433, η2 = 0.02), AUCi (F(1,39) = 0.22, p = .640, η2 = 0.01), and MaxInc (F(1,39) = 0.19, p = .667, η2 = 0.01)). For both groups, cortisol levels did not change significantly throughout the experimental session (group (F(42.11) = <0.01, p = .973), time (F(58.45) = 2.41, p = .076), group*time (F(58.45) = 0.14, p = .937); Figure 3(a), Supplementary Table S3).
Figure 3.
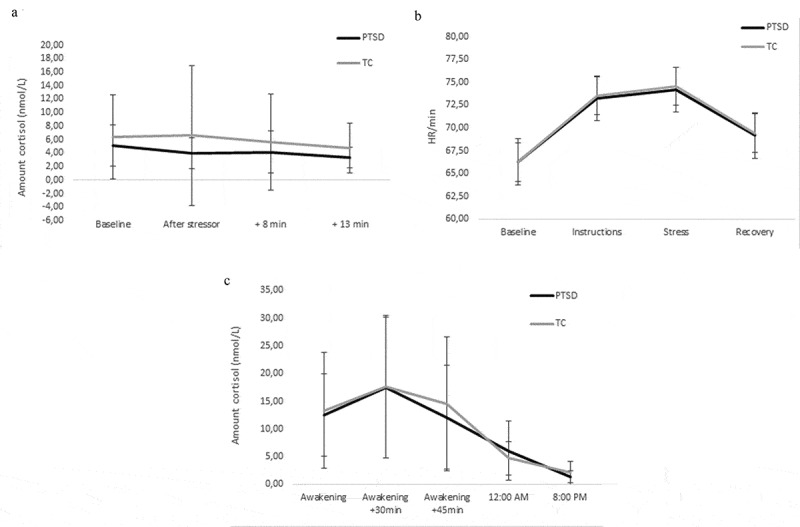
(a). Cortisol acute stress reactivity. Displayed as means of cortisol levels across the experimental session. PTSD symptom group – Baseline n = 19, After stressor n = 19, +8:00 n = 19, +13:00 n = 19; TC – Baseline n = 29, After stressor n = 29, +8:00 n = 29 + 13:00 n = 28, Error bars represent SDs. (b). Heart rate reactivity. Displayed as estimated marginal means of heart rates per minute during the task. PTSD symptom group n = 20, TC n = 27, Error bars represent SEs. (c). CAR and cortisol day curve. PTSD symptom group – CAR AUCg n = 13, AUCi n = 13, MaxInc n = 14, cortisol day curve AUCg n = 13; TC – CAR AUCg n = 21, AUCi n = 21, MaxInc n = 27, cortisol day curve AUCg n = 19. There were no effects of group on cortisol acute stress reactivity, heart rate reactivity, CAR or cortisol day curve. Error bars represent SDs
3.2.3. Heart rate reactivity
HR significantly changed throughout the experimental session (time (F(101.38) = 42.65, p = <.001; Figure 3(b)); Supplementary Table S4). Post-hoc comparisons showed HR significantly increased after baseline, peaking during Stress, and significantly decreased again afterwards. There were no significant effects of group (F(42.89) < 0.01, p = .949) and group*time (F(101.38) = 0.08, p = .971) on HR.
3.2.4. Subjective perceived stress
Perceived stress significantly changed throughout the experimental session (F(79.60) = 25.73, p = <.001; Figure 4; Supplementary Table S5). Post-hoc comparisons showed perceived stress increased after Baseline, peaking during Stress, and decreasing again during Recovery, with least perceived stress during Recovery. There were no significant effects of group (F(38.06) = 0.33, p = .571) and group*time (F(79.60) = 0.02, p = .985). Subjective impact of the stressor did not differ between groups (Table 3).
Figure 4.
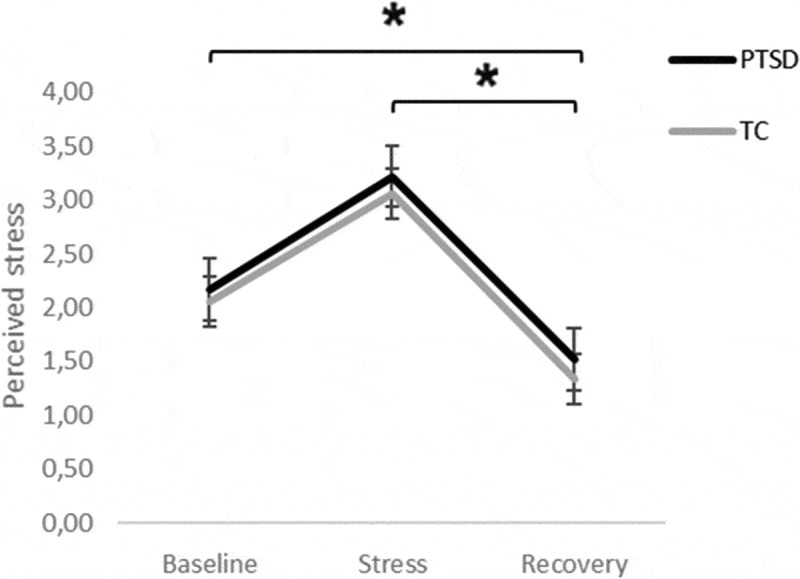
Perceived subjective levels for all participants during Baseline, Stress, and Recovery on perceived stress. Displayed as estimated marginal means perceived subjective levels across the experimental session PTSD symptom group n = 20; TC n = 29, *p < .05, Error bars represent SEs
Table 3.
Perceived impact of the stress task
| Item | PTSD symptomgroup (n = 20) | TC (n = 29) | Statistics |
|---|---|---|---|
| Difficulty | 3.40 (1.76) | 3.14 (1.33) | T47 = −0.59, p = .555 |
| Involvement | 3.50 (1.99) | 4.31 (1.31) | T30.29 = −1.60, p = .120 |
| Expected performance | 3.80 (1.44) | 4.34 (1.20) | T47 = −1.44, p = .157 |
| Control over the task | 4.70 (1.08) | 5.10 (1.18) | T47 = −1.22, p = .229 |
Scores are displayed as mean (SD). PTSD symptom group n = 20; TC n = 29.
3.3. CAR and cortisol day curve
There were no significant group differences for any of the CAR measures (AUCg (F(1,30) = 0.04, p = .840, η2 < 0.01), AUCi (F(1,30) = 0.09, p = .766, η2 < 0.01), MaxInc (F(1,37) = 0.05, p = .822, η2 < 0.01); Figure 3(c)). Additionally, for cortisol day curves no significant group difference in AUCg was observed (F(1,28) = 4.01, p = .055, η2 = 0.13; Figure 3(c)).
Sleep problems correlated moderately but non-significantly with CAR AUCg (r = .509, p = .075) and cortisol day curve AUCg (r = .441, p = .132) within the PTSD symptom group.
4. Discussion
To our best knowledge, this is the first study that examined functional brain connectivity in response to social-evaluative stress in trauma-exposed adolescents with and without high PTSD symptoms. Adolescents with high PTSD symptoms showed differential functional brain connectivity between the hippocampus and the cerebellum, MFG and IFG; and between the mPFC and IFG during acute social-evaluative stress compared to trauma-exposed controls. Mostly, the same patterns were observed during recovery. Contrary to our hypothesis, no group difference was found in functional brain connectivity for the amygdala during acute social-evaluative stress or recovery. We also investigated cortisol and cardiac reactivity and did not find an association between the presence of PTSD symptoms and cortisol reactivity, HR reactivity, CAR and cortisol day curve.
A neuroimaging study in adolescent PTSD demonstrated differential activity patterns in similar regions as dysregulated functional connectivity was observed in our study: Yang et al. (2004) found increased activity in hippocampal and cerebellar regions during trauma-related imagery compared to trauma-exposed controls. However, they did not examine functional connectivity between these regions. Moreover, a resting-state fMRI study in adults also demonstrated increased functional connectivity between the hippocampus and the cerebellum in PTSD patients compared to non-trauma-exposed healthy controls (Rabellino, Densmore, Théberge, McKinnon, & Lanius, 2018). The authors interpreted this to be associated with ongoing scanning of the environment for potential threat and, at the same time, storing this information and comparing it to past memories. It seems reasonable to expect this altered threat processing, i.e. increased attention to potential threat within the environment, to be similarly dyregulated during stressful conditions, as observed in our study during social-evaluative stress. Our finding of increased functional connectivity between the bilateral hippocampus and the bilateral MFG in response to stress in trauma-exposed controls compared to adolescents with high PTSD symptoms also suggests dysregulated threat processing under social-evaluative stress. Previously, Bremner et al. (2003a) proposed that both the hippocampus – essential in learning and memory processes (Squire & Zola-Morgan, 1991) – and MFG are involved in a dysfunctional emotional memory network in PTSD, associated explicitly with hypervigilance and impairments in inhibitory control of the fear response. In line with this model, O’Doherty et al. (2017) hypothesized that the reduction they found in MFG volumes could be related to PTSD-related hypervigilance, due to possible impairments in inhibitory modulation of the HPA axis and of fear responses. Additionally, a magnetoencephalography study by Popescu et al. (2019) found that MFG activity during a working memory task was associated with false positive memories in adults with PTSD as well. This was thought to be related to hypervigilance and to reflect persistent retrieval of previously consolidated memory traces that are irrelevant or no longer relevant. Altogether, the differential connectivity that we observed in adolescents with high PTSD symptoms probably relates to difficulties in adequately focusing on and performing the stress task, without getting disrupted by potential threatening stimuli in the environment that attract attention, due to difficulties in separating irrelevant from relevant information.
We also observed increased functional connectivity between both the left hippocampus and mPFC and the left IFG – pars opercularis (also referred to as Broca’s area) in response to stress in trauma-exposed controls compared to adolescents with high PTSD symptoms. The IFG is pivotal for multiple internal speech processes (Grèzes & Decety, 2001; Koski et al., 2002) and controlling motoric processes for a fight-or-flight response in a situation of stress because of threat (Kogler et al., 2015). As the IFG is also important for reconstructing semantic representations to be able to describe personal experiences (Rauch et al., 1996). Hull (2002) considered IFG activity disruptions in PTSD consistent with difficulties in restructuring traumatic experiences and memories. This reasoning was supported by Lindauer et al. (2004) reporting decreased activity in both the IFG and medial frontal cortex in police officers with PTSD in response to individualized trauma scripts compared to trauma-exposed controls. Thus, our findings could indicate that effective communication for the hippocampus and mPFC with the IFG is required in the presence of social-evaluative stress to prepare and practice the assigned speech internally, next to representing their personalized speech, whilst controlling a motoric stress response.
During recovery, we observed mainly the same differences in functional connectivity patterns between the groups with and without high PTSD symptoms as observed in response to the social-evaluative stress task, i.e. differential functional connectivity between the left hippocampus and left IFG, left hippocampus and right MFG, and mPFC and left IFG. Rauch, Shin, and Phelps (2006) proposed a functional network in adult PTSD containing hippocampal and mPFC regions that may mediate PTSD-related deficits in threat-related fear extinction and associated attentional processes. They provided foundational reasoning for the necessity of proper functioning of these regions, not only for adequate functioning during acutely stressful or threatening situations, but also in its immediate aftermath during recovery, which seems to be dysregulated in PTSD as also observed here.
Based on evidence from adults demonstrating the amygdala to be a key player in PTSD (Koch et al., 2016; Rauch et al., 2006), with amygdala functioning being linked to specific threat-sensitive behaviours related to PTSD symptoms such as threat detection and fear conditioning (Öhman, 2005), we expected to find differential amygdala connectivity patterns in response to stress in our adolescent sample as well. However, amygdala connectivity during and after the social-evaluative stress task did not significantly differ between adolescents with and without high PTSD symptoms. Previous studies on amygdala functional connectivity during a cognitive-emotional task in adolescents with PTSD have been inconsistent (for an overview see Weems et al., 2019). Puberty has also been found to be a critical period for changes in amygdala functioning in anxious adolescents (Ferri, Bress, Eaton, & Proudfit, 2014), who likely also deal with comparable dysregulated fear processing. Previous studies that did find amygdala connectivity alterations in adolescent PTSD included participants with more comprehensive ranges of pubertal stages (Cisler, Steele, Smitherman, Lenow, & Kilts, 2013; Wolf & Herringa, 2016; Cisler, Privratsky, Smitherman, Herringa, & Kilts, 2018, in Weems et al., 2019). However, the age (14–15 years) and pubertal stage (mean pubertal stage 3.28: indicating mid pubertal stage) of our participants is quite similar to the previous studies, not finding dysregulated amygdala functional connectivity. This could suggest a developmental element underlying these differential findings and that the amygdala is not yet or differentially associated with PTSD symptoms during these pubertal stages.
Another alternative explanation for our amygdala null-result may be the type of stressor we used to induce social-evaluative stress. Social-evaluative stress was previously found to be able to induce stressor-evoked connectivity changes in the amygdala in healthy females (Ginty et al., 2019), possibly due to its role in autonomic changes such as HR in response to multiple types of stressors, which we also demonstrated in our participants. Nevertheless, it remains to be investigated how comparable the various paradigms of stress studies are, as social-evaluative stress may not provoke different amygdala responses between those affected and not affected by PTSD in the manner of trauma-related and emotional stimuli, because the latter induce traumatic memory recall.
We found no differences between adolescents with and without high PTSD symptoms in cortisol and cardiac reactivity to the social-evaluative stress task, and in the CAR and the cortisol day curve. Our findings may again be explained from a developmental perspective, as developmental timing of trauma was a significant moderator of morning and afternoon/evening cortisol levels in an extensive meta-analysis on cortisol in PTSD compared to trauma-exposed and non-trauma-exposed controls (Morris et al., 2012). Additionally, it has been demonstrated that mid-puberty may be a recalibration window for HPA axis functioning in which cortisol-related impairments could potentially reset (Gunnar, DePasquale, Reid, Donzella, & Miller, 2019). On the other hand, there are quite a few studies on cortisol and cardiac activity that, similar to our results, also found no differences between adults (Bremner et al., 2003b; Veazey, Blanchard, Hickling, & Buckley, 2004) or adolescents (Jones-Alexander et al., 2005; Lipschitz et al., 2003) with and without PTSD, suggesting that other factors than developmental stage also may be at play, such as time since index trauma (Morris et al., 2012).
The main limitation to our study is that participants for the current study were selected based on their reported trauma exposure and PTSD symptom severity at age 11–12, without repeated assessment at the time of the completion of the stress protocol, approximately 2 years later. Although the participants in the PTSD symptom group on average reported more current PTSD-related behavioural problems, such as increased perceived stress, sleep problems and socio-emotional problem behaviours, than the trauma-exposed controls, we do not know whether their previously reported PTSD symptoms were still present (i.e. chronic) or had already remitted. This hampers the interpretation of our current findings. We cannot conclude whether the observed findings were associated with chronicity of PTSD symptoms or potentially were still present despite remitted symptoms. Additionally, we do not know whether the observed neuroimaging findings may actually reflect relatively stable vulnerability factors for development of PTSD symptoms upon trauma exposure. This is important as it has become increasingly clear that the course of PTSD symptoms upon their onset is heterogeneous (Santiago et al., 2013) and neurobiological correlates of PTSD may differ depending on the exact stage of the symptoms (McFarlane, Lawrence-Wood, Van Hooff, Malhi, & Yehuda, 2017).
There were also some other limitations to our study. We had to exclude 20% of the participants due to excessive movement in the scanner, leading to reduced and relatively small sample sizes, limiting our statistical power and possibly resulting in not observing additional between-group differences in neurobiological stress reactivity. This may have been particularly relevant for our null-finding regarding amygdala connectivity, as our within-group results on functional connectivity do indicate some differences despite the absence of significant group differences: amygdala connectivity – with predominantly frontal regions – seemed to be present to a greater extent in trauma-exposed controls than in the group with PTSD symptoms. Additionally, both sex and hormonal contraception use have been increasingly associated with brain structure and functioning, including memory, emotion regulation, and behavioural, endocrine and physiological stress reactivity (Garza & Jovanovic, 2017; Haag et al., 2019; Pletzer et al., 2010). However, given our sample size, we were unable to investigate whether sex potentially moderated the associations between neural, cortisol and cardiac stress reactivity and PTSD symptoms. Also, as only two female participants used hormonal contraception, we could also not investigate this potential effect in more detail. Another limitation is the cross-sectional design of our study, which restricted us in investigating potential developmental changes and drawing conclusions on the causality of our findings. Thus, longitudinal studies are imperative to follow-up on these outstanding issues. Also, the stress task we applied in the MR scanner may also be considered a limitation. The social-evaluative stress task did not result in increased cortisol levels, possibly indicating that the acute stressor was not very powerful. However, we did observe an effect of the stressor on perceived stress, cardiac reactivity and functional connectivity in both groups. The absence of the cortisol response may be due to high baseline levels induced by anticipatory anxiety and distress for the MRI experiment. Also, some may consider the 2-minute time length of the Stress phase of the social-evaluative stress task too short to exhibit an accurate stress response for PPI analyses. Although our experimental design was previously demonstrated to induce a robust neural and cardiac stress response (Ginty et al., 2019; Wager et al., 2009b, 2009a), Noack, Nolte, Nieratschker, Habel, and Derntl (2019) stated that social-evaluative threat (SET) has not yet been sufficiently proven to be a well-suited and robust stressor paradigm for the MR scanner – despite its promising purpose – so therefore we recommend using a more extensively validated task for future PTSD-related research. Additionally, home saliva sampling was performed on one day instead of multiple days and on a weekend day, which could have caused variability of the CAR and diurnal cortisol profiles (Stalder et al., 2016). No information on bedtimes nor awakening times was collected, so we were not able to control for this in our cortisol analyses. During the scanning session the tasks prior to the stress task were both food-related. Since comorbidity between eating disorders and PTSD has been reported (Reyes-Rodriguez et al., 2011) and our participants with PTSD symptoms reported more problems in emotional eating behaviour, our adolescents with PTSD symptoms may have been emotionally aroused by the previous food-related tasks, ultimately probing the neural stress system differently and influencing our results. However, our exploratory analyses showed that eating problems did not influence our functional connectivity findings and therefore we can likely exclude this as a potential substantial confounder.
A significant strength of our study is that we focussed on adolescents with PTSD symptoms, which is a scarcely studied population. Also, our control group consisted of trauma-exposed controls instead of non-exposed controls or controls with unspecified exposure, which allows us to cautiously conclude that the observed group differences do not result from trauma exposure in general.
In conclusion, this is the first neuroimaging study on social-evaluative stress reactivity in adolescent PTSD. We found that the presence of PTSD symptoms was associated with differential functional connectivity of the hippocampus, mPFC, cerebellum, IFG and MFG during acute social-evaluative stress, and mostly the same patterns during recovery. Together, our findings indicated that neural threat processing in response to social-evaluative stress appears to be disrupted in adolescents with PTSD symptoms. Our findings are mainly but not entirely in line with functional connectivity findings in adults with PTSD. This denotes the importance of investigating adolescents with PTSD as a specific population, instead of generalizing findings from adult research. It also highlights that treatment guidelines in adolescents with PTSD symptoms ideally should be based on research uncovering dysregulated neurobiological mechanisms in adolescents with PTSD symptoms. This could eventually provide more specialized and targeted treatment possibilities, ultimately improving treatment effectiveness for adolescents with PTSD symptoms. We strongly suggest that future studies focusing on neurobiological stress reactivity and recovery in adolescent PTSD adopt a longitudinal perspective, capturing potential developmental changes.
Supplementary Material
Acknowledgments
The authors thank all participating children for their time and involvement.
Funding Statement
This work was financially supported by the Amsterdam Brain and Cognition Centre (ABC, University of Amsterdam), Nutricia Research Foundation [Grant number 2017-09], a Veni grant from ZonMw awarded to dr. M. van Zuiden, the Netherlands organization for Health Research and Development [Grant number 91617037] and a Young Investigator grant from Brain and Behaviour Research Foundation [Grant number #24928].
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, CEH. The data are not publicly available due to their containment of information that could compromise the privacy of research participants.
Supplemental Material
Supplemental data for this article can be accessed here.
References
- Achterberg, M., & Van der Meulen, M. (2019). Genetic and environmental influences on MRI scan quantity and quality. Developmental Cognitive Neuroscience, 38, 38. doi: 10.1016/j.dcn.2019.100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisic, E., Zalta, A. K., Van Wesel, F., Larsen, S. E., Hafstad, G. S., Hassanpour, K., & Smid, G. E. (2014). Rates of post-traumatic stress disorder in trauma-exposed children and adolescents: Meta-analysis. British Journal of Psychiatry, 204(5), 335–16. doi: 10.1192/bjp.bp.113.131227. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bremner, J. D., Vythilingam, M., Vermetten, E., Adil, J., Khan, S., Nazeer, A., … Charney, D. S. (2003b). Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology, 28(6), 733–750. doi: 10.1016/S0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Bremner, J. D., Vythilingam, M., Vermetten, E., Southwick, S. M., McGlashan, T., Staib, L. H., & Charney, D. S. (2003a). Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological Psychiatry, 53(10), 879–889. doi: 10.1016/S0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Carrion, V. G., Weems, C. F., Ray, R. D., Glaser, B., Hessl, D., & Reiss, A. L. (2002). Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry, 51(7), 575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Cisler, J. M., Privratsky, A., Smitherman, S., Herringa, R. J., & Kilts, C. D. (2018). Large-scale brain organization during facial emotion processing as a function of early life trauma among adolescent girls. Neuroimage: Clinical, 17, 778–785. doi: 10.1016/j.nicl.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler, J. M., Steele, J. S., Smitherman, S., Lenow, J. K., & Kilts, C. D. (2013). Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: A network-level analysis among adolescent girls. Psychiatry Research: Neuroimaging, 214(3), 3. doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- De Bellis, M. D., Baum, A. S., Birmaher, B., Keshavan, M. S., Eccard, C. H., Boring, A. M., & Ryan, N. D. (1999). Developmental traumatology part I: Biological stress Systems. Society of Biological Psychiatry, 45(10), 1259–1270. [DOI] [PubMed] [Google Scholar]
- De Kloet, C. S., Vermetten, E., Heijnen, C. J., Geuze, E., Lentjes, E. G., & Westenberg, H. G. M. (2007). Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology, 32(3), 215–226. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- De Kloet, E. R., Joels, M., & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6(6), 463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Vries, G.-J., & Olff, M. (2009). The lifetime prevalence of traumatic events and posttraumatic stress disorder in the Netherlands. Journal of Traumatic Stress, 22(4), 259–267. doi: 10.1002/jts.20429. [DOI] [PubMed] [Google Scholar]
- Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickie, E. W., Brunet, A., Akerib, V., & Armony, J. L. (2011). Neural correlates of recovery from post-traumatic stress disorder: A longitudinal fMRI investigation of memory encoding. Neuropsychologia, 49(7), 1771–1778. doi: 10.1016/j.neuropsychologia.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Elzinga, B. M., Schmahl, C. G., Vermetten, E., Van Dyck, R., & Bremner, J. D. (2003). Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology, 28(9), 1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Esteban, O., Markiewicz, C. J., Blair, R. W., Moodie, C. A., Isik, A. I., Erramuzpe, A., & Gorgolewski, K. J. (2019). FMRIPrep: A robust preprocessing pipeline for functional MRI. Nature Methods, 16(1) bioRxiv, 111–116. doi: 10.1101/306951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, R., Vengrober, A., Eidelman-Rothman, M., & Zagoory-Sharon, O. (2013). Stress reactivity in war-exposed young children with and without posttraumatic stress disorder: Relations to maternal stress hormones, parenting, and child emotionality and regulation. Development and Psychopathology, 25(4pt1), 943–955. doi: 10.1017/S0954579413000291. [DOI] [PubMed] [Google Scholar]
- Ferri, J., Bress, J. N., Eaton, N. R., & Proudfit, G. H. (2014). The impact of puberty and social anxiety on amygdala activation to faces in adolescence. Developmental Neuroscience, 36(3–4), 239–249. doi: 10.1159/000363736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza, K., & Jovanovic, T. (2017). Impact of gender on child and adolescent PTSD. Current Psychiatry Reports, 19(11), 87. doi: 10.1007/s11920-017-0830-6. [DOI] [PubMed] [Google Scholar]
- Ginty, A. T., Kraynak, T. E., Kuan, D. C., & Gianaros, P. J. (2019). Ventromedial prefrontal cortex connectivity during and after psychological stress in women. Psychophysiology, 56(11), 11. doi: 10.1111/psyp.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes, J., & Decety, J. (2001). Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Human Brain Mapping, 12(1), 1–19. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar, M. R., DePasquale, C. E., Reid, B. M., Donzella, B., & Miller, B. S. (2019). Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proceedings of the National Academy of Sciences, 116(48), 23984–23988. doi: 10.1073/pnas.1909699116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag, K., Fraser, A., Hiller, R., Seedat, S., Zimmerman, A., & Halligan, S. L. (2019). The emergence of sex differences in PTSD symptoms across development: Evidence from the ALSPAC cohort. Psychological Medicine, 50(10), 1755–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, J. P., Hayes, S. M., & Mikedis, A. M. (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2(1), 1–13. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans, E. J., Henckens, M. J. A. G., Joëls, M., & Fernández, G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neurosciences, 37(6), 304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Herringa, R. J. (2017). Trauma, PTSD, and the developing brain. Current Psychiatry Reports, 19(10), 69. doi: 10.1007/s11920-017-0825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn, S. A., Keding, T. J., Ross, M. C., Cisler, J. M., Mumford, J. A., & Herringa, R. J. (2019). Abnormal prefrontal development in pediatric posttraumatic stress disorder: A longitudinal structural and functional magnetic resonance imaging study. Biol Psychiatry Cog Neurosci Neuroimaging, 4(2), 171. doi: 10.1016/j.bpsc.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull, A. M. (2002). Neuroimaging findings in post-traumatic stress disorder. British Journal of Psychiatry, 181, 102–110. doi: 10.1192/bjp.181.2.102. [DOI] [PubMed] [Google Scholar]
- Jones-Alexander, J., Blanchard, E. B., & Hickling, E. J. (2005). Psychophysiological assessment of youthful motor vehicle accident survivors. Applied Psychophysiology and Biofeedback, 30(2), 115–123. doi: 10.1007/s10484-005-4307-8. [DOI] [PubMed] [Google Scholar]
- Keeshin, B. R., Strawn, J. R., Out, D., Granger, D. A., & Putnam, F. W. (2014). Cortisol awakening response in adolescents with acute sexual abuse related posttraumatic stress disorder. Depression and Anxiety, 31(2), 107–114. doi: 10.1002/da.22154. [DOI] [PubMed] [Google Scholar]
- King, J. A., Mandansky, D., King, S., Fletcher, K. E., & Brewer, J. (2001). Early sexual abuse and low cortisol. Psychiatry and Clinical Neurosciences, 55(1), 71–74. doi: 10.1046/j.1440-1819.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum, C., Pirke, K.-M., & Hellhammer, D. H. (1993). The ‘trier social stress test’ – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kloet, C. S., Vermetten, E., Geuze, E., Kavelaars, A., Heijnen, C. J., & Westenberg, H. G. M. (2006). Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Research, 40(6), 550–567. [DOI] [PubMed] [Google Scholar]
- Koch, S. B. J., Van Zuiden, M., Nawijn, L., Frijling, J. L., Veltman, D. J., & Olff, M. (2016). Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depression and Anxiety, 33(7), 592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Kogler, L., Müller, V. I., Chang, A., Eickhoff, S. B., Fox, P. T., Gur, R. C., & Derntl, B. (2015). Psychosocial versus physiological stress — Meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage, 119, 235–251. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski, L., Wohlschlager, A., Bekkering, H., Woods, R. P., Dubeau, M. C., Mazziotta, J. C., & Iacoboni, M. (2002). Modulation of motor and premotor activity during imitation of target-directed actions. Cerebral Cortex, 12(8), 847–855. doi: 10.1093/cercor/12.8.847. [DOI] [PubMed] [Google Scholar]
- Leenarts, L. E. W., Diehle, J., Doreleijers, T. A. H., Jansma, E. P., & Lindauer, R. J. L. (2013). Evidence-based treatments for children with trauma-related psychopathology as a result of childhood maltreatment: A systematic review. European Child & Adolescent Psychiatry, 22(5), 269–283. doi: 10.1007/s00787-012-0367-5. [DOI] [PubMed] [Google Scholar]
- Lindauer, R. J. L., Booij, J., Habraken, J. B. A., Uylings, H. B. M., Olff, M., Carlier, I. V. E., … Gersons, B. P. R. (2004). Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biological Psychiatry, 56(11), 853–861. doi: 10.1016/j.biopsych.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lipschitz, D. S., Rasmusson, A. M., Yehuda, R., Wang, S., Anyan, W., Gueoguieva, R., & Southwick, S. M. (2003). Salivary cortisol responses to dexamethasone in adolescents with posttraumatic stress disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 42(11), 1310–1317. doi: 10.1097/01.chi.0000084832.67701.0d. [DOI] [PubMed] [Google Scholar]
- Liu, J., Chaplin, T. M., Wang, F., Sinha, R., Mayes, L. C., & Blumberg, H. P. (2012). Stress reactivity and corticolimbic response to emotional faces in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 51(3), 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience, 10(6), 434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacMillan, H. L., Georgiades, K., Duku, E. K., Shea, A., Steiner, M., Niec, A., & Schmidt, L. A. (2009). Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the youth mood project. Biological Psychiatry, 66(1), 62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane, A. C., Lawrence-Wood, E., Van Hooff, M., Malhi, G. S., & Yehuda, R. (2017). The need to take a staging approach to the biological mechanisms of PTSD and its treatment. Current Psychiatry Reports, 19(2), 10. doi: 10.1007/s11920-017-0761-2. [DOI] [PubMed] [Google Scholar]
- McLean, C. P., Rosenbach, S. B., Capaldi, S., & Foa, E. B. (2013). Social and academic functioning in adolescents with child sexual abuse-related PTSD. Child Abuse & Neglect, 37(9), 675–678. doi: 10.1016/j.chiabu.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer, L. J., Avis, K. T., Biggs, S., Reynolds, A. C., Crabtree, V. M., & Bevans, K. B. (2013). The children’s report of sleep patterns (CRSP): A self-report measure of sleep for school-aged children. Journal of Clinical Sleep Medicine, 9(3), 235–245. doi: 10.5664/jcsm.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, M. C., Compas, B. E., & Garber, J. (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review, 32(4), 301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack, H., Nolte, L., Nieratschker, V., Habel, U., & Derntl, B. (2019). Imaging stress: An overview of stress induction methods in the MR scanner. Journal of Neural Transmission, 126(9), 1187–1202. doi: 10.1007/s00702-018-01965-y. [DOI] [PubMed] [Google Scholar]
- O’Doherty, D. C. M., Tickell, A., Ryder, W., Chan, C., Hermens, D. F., Bennett, M. R., & Lagopoulos, J. (2017). Frontal and subcortical grey matter reductions in PTSD. Psychiatry Research: Neuroimaging, 266, 1–9. doi: 10.1016/j.pscychresns.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Öhman, A. (2005). The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology, 30(10), 053–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Olff, M., & Van Zuiden, M. (2017). Neuroendocrine and neuroimmune markers in PTSD: Pre-, peri- and post-trauma glucocorticoid and inflammatory dysregulation. Current Opinion in Psychology, 6, 132–137. doi: 10.1016/j.copsyc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Pan, X., Wang, Z., Wu, X., Wen, S. W., & Liu, A. (2018). Salivary cortisol in post-traumatic stress disorder: A systematic review and meta-analysis. BMC Psychiatry, 18(1), 324. doi: 10.1186/s12888-018-1910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R., Spreng, R. N., Shin, L. M. F., & Girard, T. A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 36(9), 2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Perrin, S., Meiser-Stedman, R., & Smith, P. (2005). The children’s revised impact of event scale (CRIES): Validity as a screening instrument for PTSD. Behavioural and Cognitive Psychotherapy, 33(4), 487–498. doi: 10.1017/S1352465805002419. [DOI] [Google Scholar]
- Petersen, A. C., Crockett, L., Richards, M., & Boxer, A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pletzer, B., Kronbichler, M., Aichhorn, M., Bergmann, J., Ladurner, G., & Kerschbaum, H. H. (2010). Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Research, 1348, 55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Pole, N. (2007). The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin, 133(5), 725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Popescu, M., Popescu, E., DeGraba, T. J., Fernandez-Fidalgo, D. J., Riedy, G., & Hughes, J. D. (2019). Post-traumatic stress disorder is associated with altered modulation of prefrontal alpha band oscillations during working memory. Clinical Neurophysiology, 130(10), 1869–1881. doi: 10.1016/j.clinph.2019.06.227. [DOI] [PubMed] [Google Scholar]
- Power, J. D., Mitra, A., Laumann, T. O., Snyder, A. Z., Schlaggar, L., & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84(Supplement C), 320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner, J. C., Wolf, O. T., Hellhammer, D. H., Buske-Kirschbaum, A., Von Auer, K., Jobst, S., … Kirschbaum, C. (1997). Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences, 61(26), 2539–2549. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rabellino, D., Densmore, M., Théberge, J., McKinnon, M. C., & Lanius, R. A. (2018). The cerebellum after trauma: Resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Human Brain Mapping, 39(8), 3354–3374. doi: 10.1002/hbm.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch, S. L., Van der Kolk, B. A., Fisler, R. E., Alpert, N. M., Orr, S. P., Savage, C. R., ... Pitman, R. K. (1996). A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry, 53(5), 380–387 doi: 10.1016/j.biopsych.2006.06.004.. [DOI] [PubMed] [Google Scholar]
- Rauch, S. L., Shin, L. M., & Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—Past, present, and future. Biological Psychiatry, 60(4), 376–382. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- Reyes-Rodriguez, M. L., Von Holle, A., Ulman, T. F., Thornton, L. M., Klump, K. L., Brandt, H., & Bulik, C. M. (2011). Posttraumatic stress disorder in anorexia nervosa. Psychosomatic Medicine, 73(6), 491–497. doi: 10.1097/PSY.0b013e31822232bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago, P. N., Ursano, R. J., Gray, C. L., Pynoos, R. S., Spiegel, D., Lewis-Fernandez, R., … Coyne, J. (2013). A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: Intentional and non-intentional traumatic events. PLoS One, 8(4), 4. doi: 10.1371/journal.pone.0059236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr, P. P., Lunney, C. A., Bovin, M. J., & Marx, B. P. (2009). Posttraumatic stress disorder and quality of life: Extension of findings to veterans of the wars in Iraq and Afghanistan. Clinical Psychology Review, 29(8), 727–735. doi: 10.1016/j.cpr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Schumacher, S., Niemeyer, H., Engel, S., Cwik, J. C., Laufer, S., Klusmann, H., & Knaevelsrud, C. (2019). HPA axis regulation in posttraumatic stress disorder: A meta-analysis focusing on potential moderators. Neuroscience & Biobehavioral Reviews, 100, 35–57. doi: 10.1016/j.neubiorev.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Shin, L. M., Rauch, S. L., & Pitman, R. K. (2006). Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences, 1071, 67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Squire, L. R., & Zola-Morgan, S. (1991). The medial temporal lobe memory system. Science, 253(5026), 5026. [DOI] [PubMed] [Google Scholar]
- Stalder, T., Kirschbaum, C., Kudielka, B. M., Adam, E. K., Pruessner, J. C., Wüst, S., … Clow, A. (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Van Eijsden, M., Vrijkotte, T. G., Gemke, R. J., & Van der Wal, M. F. (2011). Cohort profile: The amsterdam born children and their development (ABCD) study. International Journal of Epidemiology, 40(5), 1176–1186. doi: 10.1093/ije/dyq128. [DOI] [PubMed] [Google Scholar]
- Van Oort, J., Tendolkar, I., Hermans, E. J., Mulders, P. C., Beckmann, C. F., Schene, A. H., … van Eijndhoven, P. F. (2017). How the brain connects in response to acute stress: A review at the human brain systems level. Neuroscience & Biobehavioral Reviews, 83, 281–297. doi: 10.1016/j.neubiorev.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Van Strien, T., Frijters, J. E. R., Bergers, G. P. A., & Defares, P. B. (1986). The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders, 100(2), 295–315. [Google Scholar]
- Van Widenfelt, B. M., Goedhart, A. W., Treffers, P. D., & Goodman, R. (2003). Dutch version of the Strengths and Difficulties Questionnaire (SDQ). European Child & Adolescent Psychiatry, 12(6), 281–289. doi: 10.1007/s00787-003-0341-3. [DOI] [PubMed] [Google Scholar]
- Van Zuiden, M., Savas, M., Koch, S. B. J., Nawijn, L., Staufenbiel, S. M., Frijling, J. L., … Olff, M. (2019). Associations among hair cortisol concentrations, posttraumatic stress disorder status, and amygdala reactivity to negative affective stimuli in female police officers. Journal of Traumatic Stress, 32(2), 238–248. doi: 10.1002/jts.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey, C. H., Blanchard, E. B., Hickling, E. J., & Buckley, T. C. (2004). Physiological responsiveness of motor vehicle accident survivors with chronic posttraumatic stress disorder. Applied Psychophysiology and Biofeedback, 29(1), 51–62. doi: 10.1023/B:APBI.0000017863.35714.a1. [DOI] [PubMed] [Google Scholar]
- Viard, A., Mutlu, J., Chanraud, S., Guenolé, F., Egler, P., Gérardin, P., … Guillery-Girard, B. (2019). Altered default mode network connectivity in adolescents with post-traumatic stress disorder.. NeuroImage. Clinical, 22. doi: 10.1016/j.nicl.2019.101731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D., Van Ast, V. A., Hughes, B. L., Davidson, M. L., Lindquist, M. A., & Ochsner, K. N. (2009b). Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage, 47(3), 836–851. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D., Waugh, C. E., Lindquist, M., Noll, D. C., Fredrickson, B. L., & Taylor, S. F. (2009a). Brain mediators of cardiovascular responses to social threat, part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage, 47(3), 821–835. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems, C. F., Russell, J. D., Neill, E. L., & McCurdy, B. H. (2019). Annual research review: Pediatric posttraumatic stress disorder from a neurodevelopmental network perspective. Journal of Child Psychology and Psychiatry, 60(4), 395–408. doi: 10.1111/jcpp.12996. [DOI] [PubMed] [Google Scholar]
- Wessa, M., Rohleder, N., Kirschbaum, C., & Flor, H. (2006). Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology, 31(2), 209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wolf, R. C., & Herringa, R. J. (2016). Prefrontal–amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology, 41(3), 822–831. doi: 10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich, M. W., Ripley, B. D., Brady, J. M., & Smith, S. M. (2001). Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage, 14(6), 1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley, K. J. (2001). Statistical analysis of activation images. In Jezzard P., Matthews P. M., & Smith S. M. (Eds.), Ch 14 in functional MRI: An introduction to methods (pp. 251–270). Oxford, UK: OUP. [Google Scholar]
- Yang, P., Wu, M., Hsu, C., & Ker, J. (2004). Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: A functional MRI study. Neuroscience Letters, 370(1), 13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Zandvoort, J. B., Ensinks, J. B. M., Op den Kelder, R., Wessel, A. M. A., Lok, A., & Lindauer, R. J. L. (2019). Pretreatment cortisol predicts trauma-focused psychotherapy response in youth with (partial) posttraumatic stress disorder. Psychoneuroendocrinology, 109. doi: 10.1016/j.psyneuen.2019.104380. [DOI] [PubMed] [Google Scholar]
- Zimmerman, A., Halligan, S., Skeen, S., Morgan, B., Fraser, A., Fearon, P., & Tomlinson, M. (2020). PTSD symptoms and cortisol stress reactivity in adolescence: Findings from a high adversity cohort in South Africa. Psychoneuroendocrinology, 121, 104846. (in press). doi: 10.1016/j.psyneuen.2020.104846. [DOI] [PubMed] [Google Scholar]
- Zorn, J. V., Schür, R. R., Boks, M. P., Kahn, R. S., Joëls, M., & Vinkers, C. H. (2017). Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology, 77, 25–36. doi: 10.1016/j.psyneuen.2016.11.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, CEH. The data are not publicly available due to their containment of information that could compromise the privacy of research participants.


