Abstract
Significance.
Think Tank 2019 affirmed that the rate of infection associated with contact lenses has not changed in several decades. Also, there is a trend towards more serious infections associated with acanthamoeba and fungi. The growing use of contact lenses in children demands our attention with surveillance and case control studies.
Purpose.
The American Academy of Optometry (AAO) gathered researchers and key opinion leaders from around the world to discuss contact lens associated microbial keratitis at the 2019 AAO Annual Meeting.
Methods.
Experts presented within four sessions. Session 1 covered the epidemiology of microbial keratitis, pathogenesis of Pseudomonas aeruginosa, and the role of lens care systems and storage cases in corneal disease. Session 2 covered non-bacterial forms of keratitis in contact lens wearers. Session 3 covered future needs, challenges and research questions in relation to microbial keratitis in youth and myopia control, microbiome, antimicrobial surfaces, and genetic susceptibility. Session 4 covered compliance and communication imperatives.
Results.
The absolute rate of microbial keratitis has remained very consistent over 3 decades despite new technologies, and extended wear significantly increases the risk. Improved oxygen delivery afforded by silicone hydrogel lenses has not impacted the rates, and while the introduction of daily disposable lenses has minimized the risk for severe disease, there is no consistent evidence that they have altered the overall rate of microbial keratitis. Overnight orthokeratology lenses may increase risk for microbial keratitis, especially secondary to Acanthamoeba, in children. Compliance remains a concern and a significant risk factor for disease. New insights into host microbiome and genetic susceptibility may uncover new theories. More studies such as case-control designs suited for rare diseases, and registries are needed.
Conclusions.
The first annual AAO Think Tank acknowledged that the risk of microbial keratitis has not decreased over decades, despite innovation. Important questions and research directions remain.
In the past several decades, we have seen tremendous increases in technology in the areas of contact lens materials, design, and wearing modalities. Changes have occurred in contact lens affordability and access around the world. New research and regulatory approvals in the critical area of myopia control have the potential to further expand the reach of contact lenses into ever younger and broader audiences. However, questions remain regarding ways to improve the safety of contact lenses, particularly with regards to contact lens-related microbial keratitis. The first annual American Academy of Optometry Think Tank gathered several researchers and key opinion leaders around the world to tackle this topic at the 2019 Annual Meeting of the American Academy of Optometry. The Think Tank Organizing Committee (BC, JS, CS, LS-F) consisted of 2 leaders from the 2019 American Academy of Optometry (AAO) Board of Directors (President and Immediate Past Chair) and 2 leaders from the AAO Section on Cornea, Contact Lenses and Refractive Technologies (2019 Chair and Special Advisor). The Organizing Committee selected researchers and key opinion leaders based on their past contributions and on-going research to the Think Tank topic, “Microbial Keratitis”. Consideration was given to matching presenters to specific topic areas of interest where gaps in research exist such as identifying and minimizing risk, making a timely diagnosis with new technology, and employing management strategies for corneal infections in contact lens wearers in order to reduce morbidity. Fifteen invitations were extended with only two invitees declining their offer. The individuals who declined had conflicts and either sent their research associate to present their laboratory’s data or suggested that other Think Tank members could comprehensively cover their same topic.
SESSION 1- WHAT WE KNOW TODAY ABOUT CONTACT LENS-RELATED MICROBIAL KERATITIS
Panel: Fiona Stapleton, FCOptom, PhD; Abby Kroken, PhD, Oliver Schein, MD, MPH, MBA; and Carol Lakkis, BScOptom, PhD
The first session of the Think Tank brought together experts on the epidemiology of microbial keratitis, the pathogenesis of Pseudomonas aeruginosa, a pathogen commonly responsible for contact lens-related microbial keratitis, and the role of lens care systems and storage cases in corneal disease.
Epidemiology
Stapleton has directed or been critically involved in many of the seminal studies on rates and risks of microbial keratitis since the hallmark studies by Poggio and Schein, published in 1989.1, 2 Her conclusions mirror those of the earlier studies: the absolute rate of microbial keratitis has remained very consistent at 2-4/10,000 contact lens wearers per year over many decades and technologies, and extended wear or overnight wear increases the risk.3 She also noted that while the introduction of daily disposable lenses has minimized the risk for severe disease (i.e. a positive corneal culture or a corneal infiltrate and overlying epithelial defect with either central location, uveitis or significant pain),4 there is no consistent evidence that they have significantly altered the overall rate of microbial keratitis.3
Numerous studies have confirmed that the incidence of infection is highest for extended wear and lowest for daily wear, with occasional overnight use associated with rates somewhere in between (Figure 1).1, 3–10 Newer data shows that the improved oxygen delivery afforded by silicone hydrogel (SiH) lenses has not appreciably impacted microbial keratitis rates, particularly for extended wear.3, 11 Corneal rigid gas permeable lenses (RGP/GP) worn on a daily wear basis have been associated with a low risk of infection.3 There are limited data on overnight use of RGPs, such as with orthokeratology, but a retrospective analysis estimated a similar rate of microbial keratitis as seen with other overnight wear modalities, although the confidence intervals on the estimate were large (7.7/10,000 orthokeratology wearers per year; range 0.9 to 27.8 per 10,000).9 It has also been reported that using overnight orthokeratology RGP lenses constituted a significant risk for acanthamoeba, along with storing lenses in tap water and topping off solutions.12 Population based studies to establish risks associated with corneal infections in specialty RGP lens use are unavailable.
Figure 1.
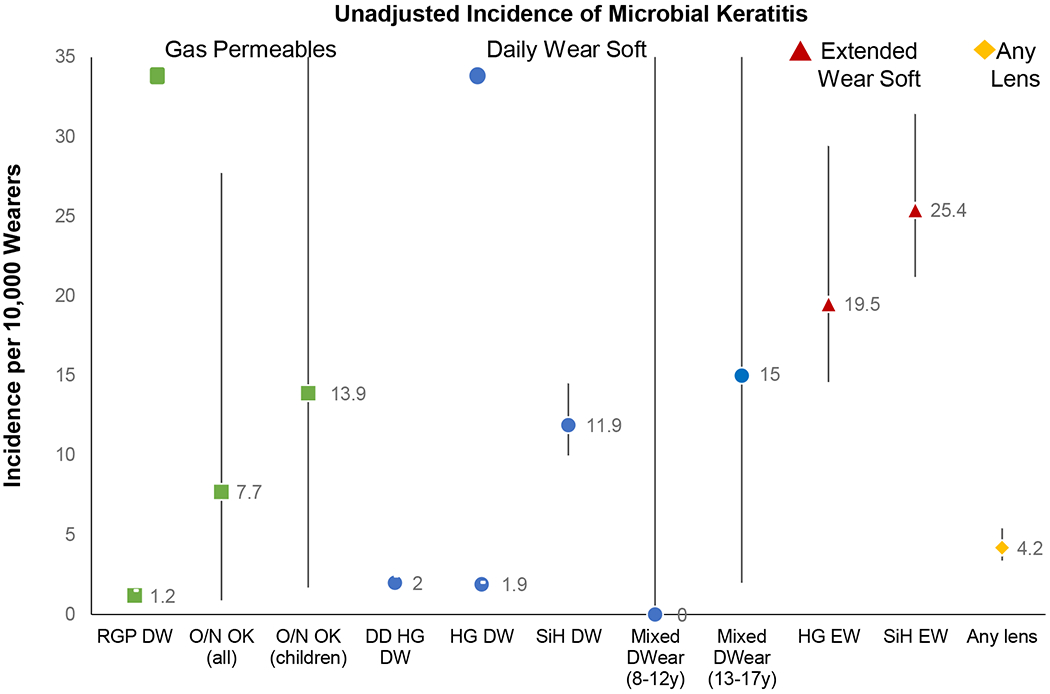
Unadjusted incidence of microbial keratitis reported by lens type and mode of wear. Incidence per 10,000 wearers with 95% confidence intervals. RGP = rigid gas permeable (corneal); HG = hydrogel; SiH = silicone hydrogel; Mixed = mixed hydrogel and silicone hydrogel; DD = daily disposable; DW = daily wear; O/N = overnight wear; EW = extended wear; OK = Orthokeratology. Data based on findings from these studies: Stapleton et al 2008,3 Chalmers et al 2011,8 Bullimore et al 2013,9 Bullimore 2017.10
A useful approach to understanding risks shared by Stapleton was the concept of dividing risk factors into categories13:
Consistent – corroborated by at least two well-controlled clinical studies
Probable – confirmed by one well-controlled study with supporting data from case reports, etc.
Inconclusive – limited to one study or conflicting information across studies
Further, as seen in Table 1, there are known risks for infection that are modifiable and non-modifiable. Over the past five years, younger adult age appears as a consistent risk factor for infection, with several studies showing increased risk in groups ranging from twenty years of age well into the thirties.14, 15 Other risks to note are the firmly established modifiable risk factors including contact with tap water and storage case hygiene.3–5,10,13 Importantly, a population attributable risk percentage model predicts that disease load in daily wear reusable lenses could be reduced by almost two thirds by attending to just TWO factors: storage case hygiene and storage case replacement.4
Table 1.
Approach to understanding risks related to microbial keratitis based on modifiability and certainty of evidence.
| Consistent | Probable | Inconclusive | |
|---|---|---|---|
| Non-modifiable | Gender | Shorter duration of lens wear | Dry eye |
| Younger adult age | Diabetes | Thyroid disease | |
| Season | |||
| Race | |||
| Myopia | |||
| Self-reported poor health | |||
| Socio-economic class | |||
| Prior red eye event | |||
| Modifiable | ANY overnight wear | Case replacement frequency | Lens age (Fusarium) |
| Increasing duration of overnight wear | MPDS vs H2O2 | Replacement frequency | |
| Contact with tap water | Swimming without goggles | Ophthalmologist fitting | |
| Case hygiene | Cosmetic/colored CL use | Lens material type (daily disposable) | |
| Solution type (Complete/ReNu MoistureLoc) | Degree of exposure in daily wear | Orthokeratology contact lens use | |
| Infrequent disinfection/topping off | Internet purchase | Use of lenses on holiday | |
| Overall compliance score | Solution type (Oxipol) | Failing to rub lenses on removal | |
| Not handwashing prior to handling | Group IV contact lenses (in combination with certain solution types) | ||
| Smoking | |||
| Lens other than RGP |
It is also important to distinguish the severe cases of microbial keratitis from those that have less potential for morbidity.(Figure 2) Data on severe cases strongly suggests that daily disposable lenses reduce the severity of disease.4, 16 Not surprisingly, daily disposable lenses, worn for pure daily wear (no overnight use), were associated with the lowest risk of severe infection.3,15 Severe disease was defined as central or large peripheral ulcers, or culture proven cases, or reduced best-corrected visual acuity. Daily disposable lenses proved to have a slightly lower culture proven rate of infection compared to other daily wear or extended wear microbial keratitis cases, and a significantly lower likelihood of environmental organisms as a cause. When looking solely at daily disposable lenses, overnight (non-compliant) wear, and not washing and drying hands are again impactful risk factors.16 In multivariable analyses, when controlling for other hygiene and compliance factors, material type (SiH or hydrogel) does not appear to be associated with an increased risk.3, 17 It has been well established, however, that the rate of infiltrative keratitis with SiH lenses is consistently higher than with hydrogels.18,19
Figure 2.
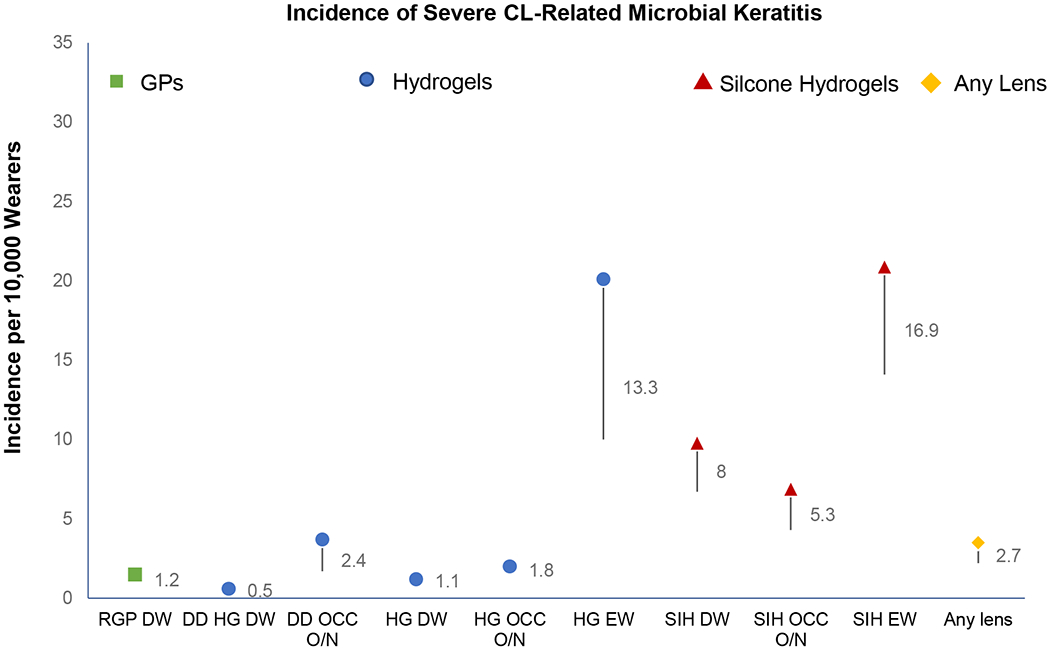
Incidence of severe microbial keratitis reported by lens type and mode of wear. Incidence per 10,000 wearers with 95% confidence intervals. RGP = rigid gas permeable (corneal); HG = hydrogel; SiH = silicone hydrogel; DD = daily disposable; DW = daily wear; O/N = overnight wear; EW = extended wear; Occ = occasional. Data based on findings from Stapleton et al 2008.3
Finally, in areas where colored/cosmetic lens use is prevalent, the rate of disease is disproportionately high, though there are no population based studies to develop incidence data.20–22 Of equal concern, is that despite the global focus on arresting myopia progression, the data on safety in children is scarce and variable.11,12
Pathogenicity of Pseudomonas aeruginosa
While recent years have seen a rise in keratitis due to environmental organisms such as Acanthamoeba and Fusarium, Pseudomonas aeruginosa remains the most common cause of serious infectious keratitis in contact lens wearers and is, therefore, an important area of study. Kroken provided an update on some of the newest research in the area of mechanisms of infection of the cornea by P. aeruginosa. A key finding was that individual P. aeruginosa bacteria behaved differently within a given cornea, suggesting that there are multiple virulence factors and some redundancy between them. Kroken discussed several related findings derived from observation of living P. aeruginosa in various environments, and more specifically, the intracellular lifestyle of P. aeruginosa.
Previously, their group demonstrated that P. aeruginosa exit after the invasion of a cell. The capacity to cross epithelial cell multilayers in vivo required a type of surface-associated movement called twitching motility.23, 24 Twitching is conferred by type IV pili (T4P), composed of PilA protein, and is accomplished through the extension (dependent on PilB) and retraction (dependent on PilT) of T4P by ATPases that antagonistically polymerize and depolymerize PilA.25
Another promising area of work has been the ability to look at how bacterial populations change when they are in contact with the ocular surface. As noted previously, bacteria require functioning T4P to move around in host tissue. Li and coworkers,24 showed that DMBT1, an abundant mucosal fluid glycoprotein, enabled tear fluid to inhibit P. aeruginosa twitching motility, even though T4P was expressed. These findings contribute to our understanding of mucosal fluid protection against infection, and suggest that DMBT1, or its derivatives, have potential as novel anti-virulence agents that protect against infection.
In new work by Nieto et al,26 live wide-field fluorescent imaging combined with quantitative image analysis was used to explore how twitching contributes to epithelial cell egress. They found that twitching defective/lacking T4P mutants could invade epithelial cells, where they replicated forming intracellular aggregates but were defective in their capacity for cell egress. The wild-type bacteria slowly disseminated throughout the cytoplasm in a pattern consistent with twitching motility, while rapidly replicating. The authors concluded that intracellular motility is driven primarily by the T4P twitching function. In a publication related to this work,27 Kroken established that P. aeruginosa entered epithelial cells, with subsequent intracellular survival and replication occurring in both the cytosol and bleb niches, and that its entry was not restricted by the strain type nor epithelial cell type.
She also shared unpublished work showing that bacteria that make the secretion system needed for virulence (T3SS) only appeared to migrate about halfway through the corneal epithelium, but other T3SS-negative bacteria existed down to the basal lamina, suggesting a concept of sharing the burden of making virulence factors within a population. In short, bacteria not only adapt to the ocular surface through gene regulation but within a population, they also diversify in their activities to cause disease.
Furthermore, Kroken shared some research that looked at the host response to P. aeruginosa invasion using a live mouse model.28 Compared to the healthy mouse cornea, the contact lens wearing cornea contained moving cells in the stroma. Neutrophils were identified as a portion of this population through staining of fixed corneas. Of note is that no visible corneal opacification was discernible after a week of extended wear. However, when a lens with an established bacterial biofilm was placed on the eye for a period approaching 3 weeks, confocal microscopy revealed a slightly distorted epithelium and a complete remodeling of the stroma. Here the morphology of the keratocytes was missing, replaced by motile neutrophils, yet only moderate haze was visible on slit lamp examination. This work yielded a new model of the pathogenesis of contact lens-related keratitis.29(Figure 3)
Figure 3.
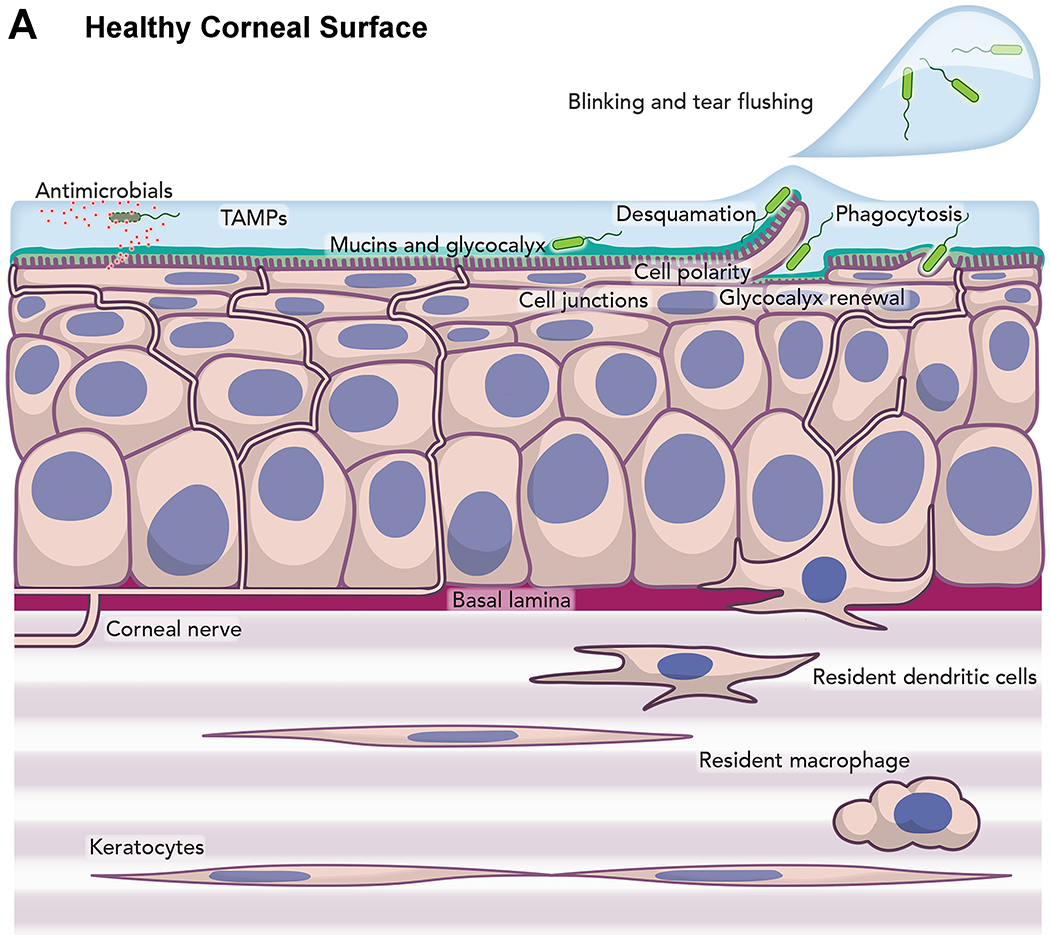
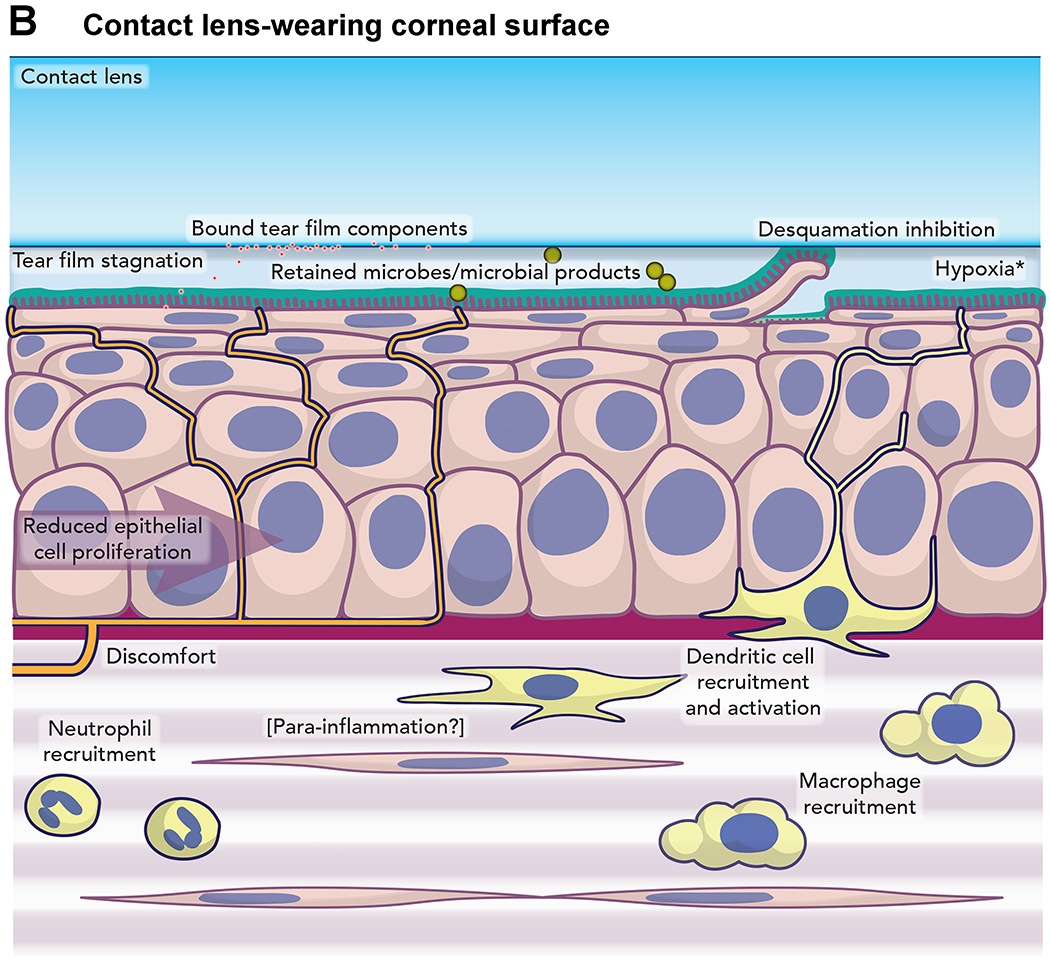
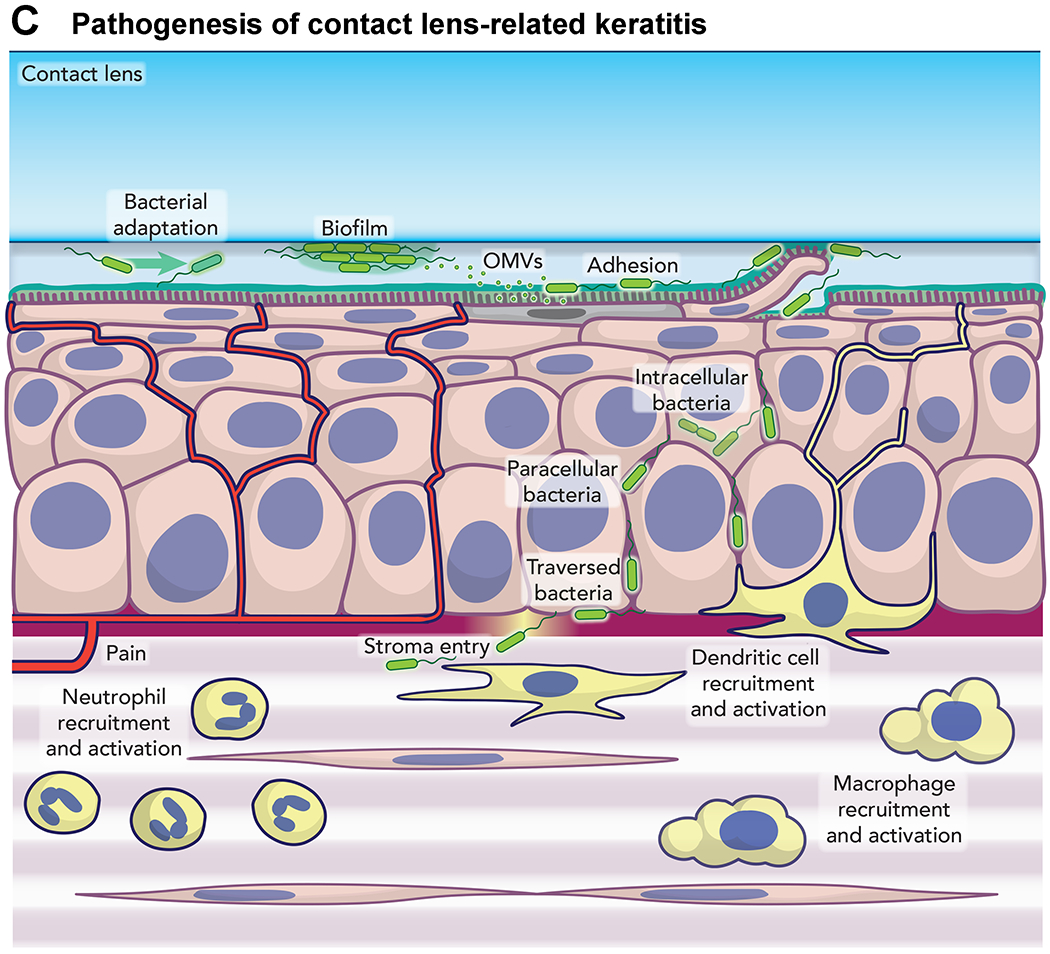
Model of mechanism of infection of cornea by Pseudomonas aeruginosa.29
This work yields some potentially promising avenues for a better understanding of both bacterial virulence factors and the host response to the bacteria. If we cannot keep the bacteria out of the cornea, can we at least limit its virulence? What are the implications of bacterial population biostability on anti-virulence strategies? Is the para-inflammatory environment after extended wear protective, or does it enable susceptibility to P. aeruginosa?
The Role of Care Solutions
Across the world, approximately 35% of contact lens wearers are in daily disposable contact lenses, either hydrogel (12%) or silicone hydrogel (22%), and this proportion continues to grow as affordability and parameter availability improve in the various markets.30 However, that means that 65% of wearers still require care solutions and cases, both of which are identified as risk factors for contact lens microbial keratitis. Lakkis shared some research on the relationship between solutions and microbial keratitis.
Lakkis reiterated data shared by Stapleton regarding the known association between contamination of storage cases when certain contact lens solutions are used, and microbial keratitis. Of note is that case contamination can occur even with proper compliance to wear and care instructions. She also underscored the concern around the use of tap water by contact lens wearers. She reinforced that multiple factors contribute to the development of contamination: the inherent disinfection efficacy of the care system, microbial resistance, and the formation of biofilms.
Though all contact lens disinfecting systems must pass regulatory scrutiny before marketing, there have been several solutions associated with infections under certain conditions, including chlorine,31 heat,32 certain formulations containing PHMB4, 14 and the chlorine disinfectant in oxipol.33 Behavioral issues add to risks with case replacement4 and handwashing16 being two of the most relevant and modifiable factors.
Two outbreaks of note that were associated with solution inadequacies involved the environmental pathogens Fusarium solani and Acanthamoeba. During the Fusarium outbreak, it was discovered that the efficacy of the ReNu MoistureLoc formulation decreased with solution evaporation34 and the formulation showed instability at higher temperatures.35 Topping off was also a factor that contributed to the failure of the system.34 Acanthamoeba infections with Complete MoisturePlus formulation were investigated. The combination of potassium chloride buffer and propylene glycol36 promoted encystment of Acanthamoeba. Additionally, risky behaviors including solution reuse and topping off, lack of lens rubbing, and showering with contact lenses37 were contributing factors.
Regardless of the pathogen, contamination of storage cases occurs in up to 80% of cases sampled.38–41 Frequent case replacement is essential, though single isolated colonies can be seen as early as 7 days of use of a new storage case, with microcolonies seen at 14 days and mature biofilms and heavy contamination by 30 days.42 When looking at risk factors for storage case contamination, reuse of solution is a major component, with 100% of cases contaminated when solutions are reused.41 Other key risks include male gender,41 bottle tip contamination,41 not rinsing the case, failure to handwash,40 and use of tap water to rinse.39
The literature surrounding contact lens care solutions and storage cases is clear: elimination of solutions and cases by using a daily disposable modality can reduce the risk of more severe infection but not eliminate the risk. Since affordability and availability issues remain, looking at enhanced antimicrobial activity is an option. Here it would be wise to focus on Gram-negative bacteria, P. aeruginosa in particular, and technologies that inhibit virulence and adherence, as mentioned by Kroken. Antimicrobial cases or antifouling technologies for lenses and storage cases are areas that require more research. Compliance and education could play a role, particularly if focused on high-risk behaviors, but as shown in many fields, this represents a very daunting task.
A near term fix might be new and improved standards for testing and approving solutions that more closely represent real-world conditions, such as using clinical isolates, and testing in the presence of nutrients. Addressing the impact of biofilm, non-compliant behaviors, and more attention to the environmental organisms may reduce the incidence of disease. However, with increases in daily disposable prescribing worldwide, significant investment in new solution disinfection technologies is unlikely.
Summary and Unanswered Questions
As summarized by Schein, we know that the risk of microbial keratitis with contact lens wear has remained remarkably consistent at 2-4/10,000 patients per year over many decades and technologies, that daily disposable modalities minimize the risk of severe disease, and extended wear, or overnight wear of any kind significantly increases the risk. We know that storage case contamination, lens care solutions, and wearer behaviors can influence the risk of infection, particularly for the rarer, non-bacterial conditions discussed in more detail in session 2, such as Acanthamoeba and Fusarium keratitis. However, a worldwide increase in the use of daily disposables has not lowered the rate of infections. There is still much we do not know. As we move to more widespread prescribing to children, what types of research is required? Are regulatory bodies doing enough to ensure expedient approval of lenses and solutions that will address the known risk factors? These and other questions posed in the other sessions should help guide focus and funding for future research endeavors.
SESSION 2- TRENDS IN NON-BACTERIAL CONTACT LENS RELATED INFECTIONS
Panel: Eduardo Alfonso, MD; Elmer Tu, MD; Deborah Jacobs, MD, MSc; and Nicole Carnt, BOptom, PhD
This session dealt with non-bacterial forms of keratitis in contact lens wearers. These infections are found infrequently and are more difficult to diagnose on the first presentation. Despite increased research and new information on contact lens related microbial keratitis, important questions remain. The important questions include: what are the most effective treatment options for these rare infections and how we can minimize risk to lens wearers. Furthermore, little is known of the role of gut microbiome, changes that take place in the ocular microbiome before and during infection, and why corticosteroids are harmful in the early management of both fungal and Acanthamoeba keratitis.
Fungal Keratitis
Alfonso stated that empiric antibiotic therapy cannot adequately treat the increasing number of non-bacterial corneal ulcers. Empiric treatment is problematic because of the increasing presence of non-bacterial organisms. He addressed the problem of fungal keratitis in contact lens wearers and stressed that future studies should investigate maintaining and improving the quality and function of the tear film during contact lens wear. Early diagnosis of fungal infections is the key to better outcomes and remains elusive. In the future, artificial intelligence might aid in an early diagnosis, since clinical information misleads us about 55% of the time. Endogenous (hands, eyes, throat) and exogenous (contact lens paraphernalia, water, air) sources have been implicated and are evident in fungal keratitis.
Compared to bacterial infections of the cornea, which are usually caught quickly, the diagnosis of non-bacterial events is often delayed as they are more challenging to diagnose. Fungal keratitis may present with similar characteristics to other forms of infectious keratitis, but does not generally respond to empiric antibiotic therapy. A thorough scraping, biopsy, and culturing of lens paraphernalia assists in the diagnosis of fungal keratitis. Confocal microscopy (Figure 4) has excellent specificity (93%) and sensitivity (89%) in making a differential diagnosis when a lens wearer presents with a suspicious infiltrate(s).43 It is exceedingly difficult to grow fungi from a small infiltrate. Therefore, patients should be referred to a center that has confocal imaging when a definitive diagnosis is in question.
Figure 4.
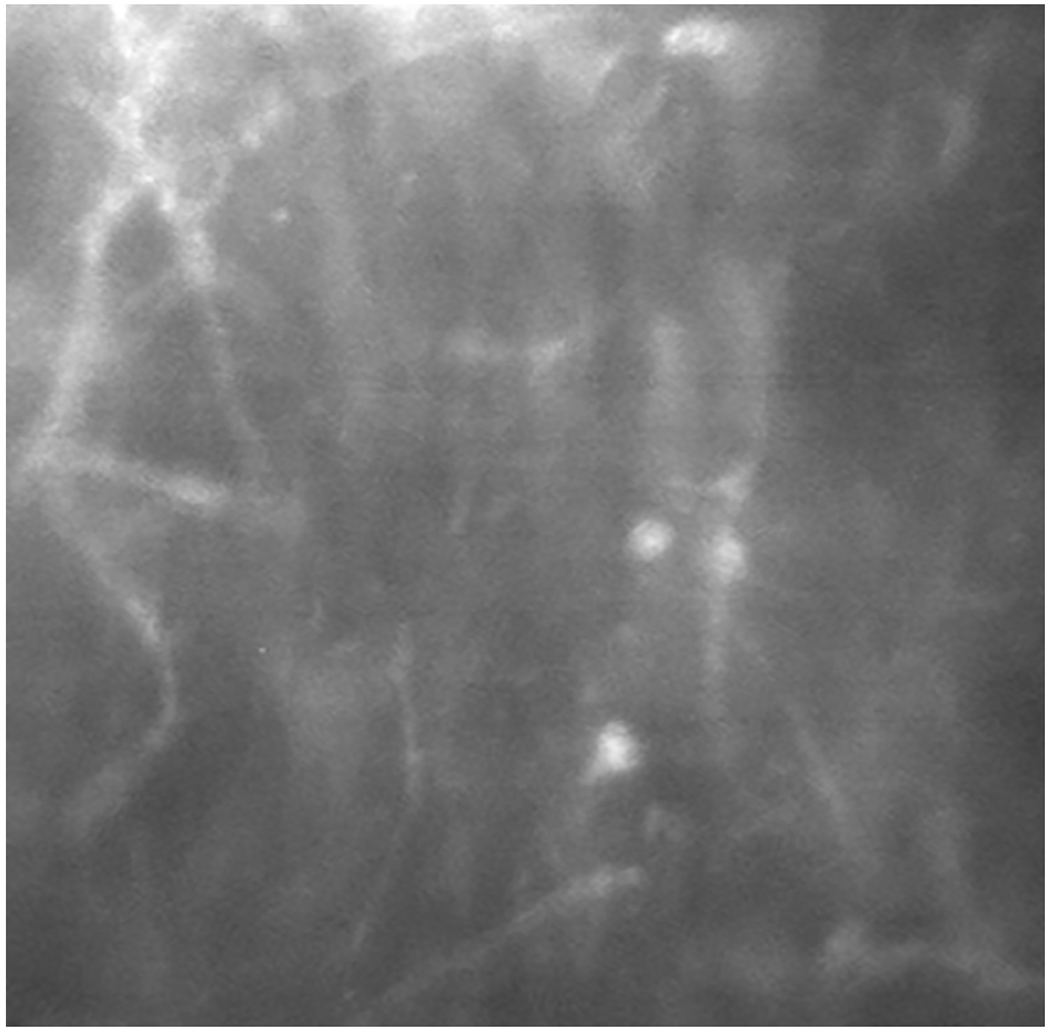
Confocal Microscopy of Filamentous Elements in a Patient with Fungal Keratitis
Molecular diagnosis, looking at a transcribed region of the organism (target encoding), can give us valuable information on the important characteristics of fungal organisms and aids in developing sensitivity profiles of the organism by isolating mycotoxins.44–46 Research conducted at Bascom Palmer Eye Institute, University of Miami, using multiplex polymerase chain reaction (PCR) identification, identified significant mycotoxin presence in fungal keratitis.47 When FUM1 mycotoxin positive F. solani was detected by PCR, the patient had a worse acuity outcome and required penetrating keratoplasty more often. Thus the genetic footprint of the mycotoxins, will likely determine the outcome.48 Overall, anti-fungal agents have poor penetration into the cornea.45, 46 Corneal scrapings remove epithelium and necrotic material and allow for better topical anti-fungal drop penetration. Providers who treat these infections use multiple agents in an attempt to impart a cure. Unfortunately, there are few topical anti-fungal agents. Recent fungal treatment studies (Mycotic Ulcer Study I and II) have shown natamycin, the only Food and Drug Administration approved anti-fungal agent, to be the most effective agent for filamentous fungi infections.45 Oral voriconazole did not demonstrate a significant benefit in treating filamentous fungal keratitis.46
The use of topical corticosteroids is contraindicated in early fungal disease. It is speculated that this is a result of the enhancement of virulence of the microbes due to uncontrolled fungal growth accompanied by a reduced host immune response. Fungal corneal infections, in particular Fusarium, can be more destructive than bacteria, and can quickly ravage the whole eye.49 Although infrequent, endophthalmitis is more commonly seen with this fungal infection than with any bacteria, and enucleations are an unfortunate possibility.
Recent reports have cited the use of photodynamic therapy with rose bengal, similar to corneal cross-linking, as a treatment for fungal keratitis. The mechanism is the inhibition of the growth of microbes. The results for fungal keratitis are mixed. This treatment is likely most effective when treating anterior stromal disease.50,51
In summary, the number of non-bacterial keratitis events in contact lens wearers has increased over the past few decades.52 Target encoding and mycotoxin profiling is an exciting new research area that should improve outcomes. It is impossible to predict “the perfect storm” in the microbial world. Therefore active surveillance is needed.
Protozoan Infections
Tu addressed protozoan infections, specifically Acanthamoeba, and highlighted the epidemiology of disease transmission from lens to patient. Protozoans are a class of organisms that may become corneal parasites. Unlike many corneal parasites, Acanthamoeba do not require a host to survive. The most common parasitic infections of the cornea are microsporidia (although these are now generally classified as fungi or as a closely related group) and Acanthamoeba. Both present, initially, with a non-specific keratitis (sub-acute, chronic), which makes them difficult to recognize on examination.
Acanthamoeba, a free-living protozoan, was described as a potential eye pathogen in 1973 and subsequently with modest seasonal variations (more prevalent in warmer temperatures).53 Generally, early detection and treatment is strongly associated with a more favorable outcome, but individual patients show a varied clinical course and a wide range of symptoms. Surprisingly, some patients present with very little pain; whereas others have excruciating pain. Several masquerade syndromes are commonly confused with this disease including dry eye, fungal, microsporidia and most commonly herpes simplex pseudo-dendritic epithelial or stromal keratitis. A peculiar ground glass appearance of the epithelium seen in early Acanthamoeba infections can easily resemble dry eye corneal epitheliopathy, but in Acanthamoeba keratitis, the granular appearance will usually stop a millimeter or two from the limbus (unlike dry eye disease).
It is critical to understand the risk factors associated with Acanthamoeba keratitis. Most Acanthamoeba corneal infections occur in contact lens wearers (85-100%). Seven to eleven percent of cases are bilateral either at presentation or sequentially. Rates of infection in the United Kingdom were 21.1/1,000,000 for soft lens wearers, and 17.5/1,000,000 for RGP lens wearers.54 There have been reports of an alarmingly high proportion of Acanthamoeba keratitis in orthokeratology-related infections (33%).55
Diagnosis is often elusive. Unfortunately, there is a relatively low yield on standard culture media (30–50%).56 Molecular diagnostic techniques (high sensitivity, high specificity) can be difficult to validate in a rare disease where Acanthamoeba commonly colonizes contact lens paraphernalia without causing disease.39 A multimodal approach to diagnosis including clinical, microbiologic, confocal imaging (high sensitivity, high specificity) and molecular techniques is the best, most accurate approach. Regardless, clinicians should always suspect Acanthamoeba keratitis in any contact lens-related infection responding poorly or inappropriately to empiric antibacterial therapy.
The course of disease is generally non-linear with a variable response worsened by poor access to effective topical medications. There is always a guarded prognosis with deep stromal inflammation, ring infiltrates (late sign), and extra-corneal inflammation. Standard medical therapy includes: (1) mechanical debridement (debulks organism load and aids in diagnostic confirmation), (2) combination topical agents (propamidine and biguanide/chlorhexidine 0.02% q 1 h for the first month) tapered according to response, (3) oral agents as needed, and (4) elimination or reduction of corticosteroid use at the time of diagnosis. Benzalkonium chloride (BAK) containing compounds may be helpful. Laboratory studies demonstrate that BAK-containing compounds have similar effectiveness as 3% hydrogen peroxide for some strains.57 Alkylphosphocholine (miltefosine), FDA-approved for both leishmaniasis and Acanthamoeba, and dosed at 50mg BID-TID has recently been shown to have efficacy against Acanthamoeba keratitis and systemic infections.58
The role of topical and oral corticosteroids during treatment is controversial and unnecessary in most cases. These agents are reserved for uveitis, extra-corneal inflammation, indolent ulcers, and severe pain after at least two weeks of biguanide treatment. Supplemental or alternative use of systemic NSAIDs is advised, whenever possible, and can be helpful for pain. Topical NSAIDs should be used sparingly over concerns for ocular surface toxicity and potential for corneal melts.
Another concern is treatment resistance and an apparent increase in treatment failures. A recent article from the Wills Eye Institute examined the increasing treatment times and poorer outcomes of recent cases that are also being experienced around the world.59 Environmental shifts may be influencing these outbreaks that center around natural disasters. Treatment failures are often a result of drug resistance, compliance problems, and polymicrobial infections, along with other inflammatory and infectious sequelae. A significant cause of inadequate response to seemingly appropriate therapy for Acanthamoeba keratitis is co-infection with other organisms. Commonly encountered co-infecting microbes are members of the “viridians” streptococci presenting as an infectious crystalline keratopathy. For recalcitrant Acanthamoeba infections, an increase in both frequency and concentration in biguanide agents (0.02% to 0.06%) can beconsidered along with a second line systemic medication such as miltefosine. The cost of this anti-amebic medication (miltefosine), and others, can present a barrier to use for many patients. Surgical management primarily involves corneal transplantation, preferably after medical control of the infection and cross-linking for very select cases of superficial disease.
In summary, Acanthamoeba is the best-characterized form of parasitic infection in the eye. Contact lens wear is the primary risk factor in Acanthamoeba keratitis and a possibility in any contact lens related infection. Early recognition improves prognosis significantly. Treatment is generally successful, although there is some evidence that more resistant cases are occurring. Surgical options remain viable treatments in medically recalcitrant cases.
Summary and Unanswered Questions
As summarized by Carnt, the presentations on non-bacterial keratitis highlighted the need for high-level imaging, as we have seen in diagnostic advances in the retina and optic nerve. Artificial intelligence will likely play a significant role in the management of non-bacterial infections of the cornea very soon. Molecular diagnosis for fungal and protozoan infection is sensitive and works well in combination with confocal microscopy.
Acanthamoeba keratitis continues to be a concern in orthokeratology patients (most are children). The majority of cases used water in their care sequence.12, 60, 61 A 2016 CDC multi-state investigation showed a large percentage (nearly 25%) of Acanthamoeba infections among gas permeable lens wearers using an orthokeratology program.12 Active surveillance is crucial to monitor these dreaded conditions in contact lens wearing populations. It is the only way to adequately detect any increase in prevalence and gauge severity over time. We look forward to novel treatment options for these infections that occur in contact lens wearers.
SESSION 3- FUTURE NEEDS AND RESEARCH QUESTIONS
Panel: Robin Chalmers, OD; Loretta Szczotka-Flynn, OD, PhD; Charlotte Joslin, OD, PhD; Mark Willcox, PhD; Eric Pearlman, PhD; and Carol Lakkis, BScOptom, PhD
Youth and Contact Lenses
The third session of the Think Tank addressed future needs, challenges and research questions. Chalmers reviewed some of her work with youth and contact lenses. These results brought the conversation back to the questions posed in the first session around compliance and the risks for children wearing contact lenses for myopia control. In the past, the primary use of contact lenses in young children was for medical conditions such as amblyopia and aphakia. It is intuitive that the parents of these children understood the medical necessity of the lenses and the care and vigilance required. The difference now is that, with the use of contact lenses for myopia control, the eyes of many more children will be exposed to the risks and benefits of contact lens use.
Under myopia control protocols, children as young as six years old are considered for contact lens fitting, in order to achieve the best efficacy early in the development of their myopia. Several groups62,63 have now published the success and risks of their myopia control clinical trials. However, most of these studies have included relatively small sample sizes of approximately 200 subjects per study, which is insufficient to study rare safety outcomes. Therefore, the risk of microbial keratitis in this age group is unknown. There are growing concerns in this age group. Specifically, one study suggested that contact lenses were the most commonly used medical device among children presenting for urgent care.64 However, since the study could not estimate the prevalence of contact lens use among children in general, if they are the most commonly used medical device in the pediatric population, the study does not demonstrate added risk. Additionally, another study65 concluded that the incidence of ulcerative keratitis in their entire population in Northern California, including children, was higher than previously reported.
Understandably, the FDA has special provisions for devices that carry an indication for pediatric use. Specifically, Section 522 of the Federal Food, Drug and Cosmetic Act allows the FDA to mandate post-market surveillance in any product used in a pediatric population. Such surveillance will ensure that our understanding of the risks of contact lens wear in children will grow. In one such study, Bullimore9 reported two microbial keratitis cases in children wearing orthokeratology lenses. The estimated incidence in children was 13.9 per 10,000 patient-years (95% CI = 1.7 to 50.4) while there were no cases in adults (0 per 10,000 patient-years (95% CI 0 to 31.7).
A survey of fitting practices of contact lenses for myopia control in children, between 2011 through 2018,66 found a wide variation by country, with some countries having negligible fitting and a few countries such as the Netherlands, Canada, and Australia doing much more. The greatest increase in types of lenses used for myopia control were RGP lenses, presumably used for overnight orthokeratology. With the conclusion of studies and release of soft multifocal lenses for myopia control,67 there will likely be a large increase in soft lens use for this purpose. The CLAY retrospective chart review study was conducted with sufficient sample size to detect the rate of infiltrates, including microbial keratitis, across younger age groups wearing soft lenses.8 This study observed 3,549 patients and 14,305 visits. In the 8-12-year-old age group, there was a very low rate of corneal infiltrative events (CIEs) (< 2%) and no microbial keratitis. A study utilizing the MiSight lens on 144 children,67 reported no cases of serious ocular adverse events, and four asymptomatic CIEs over three years. Additionally, among 202 children and teens in the TEMPO Registry of daily disposable lenses, no CIEs were reported.68
To minimize microbial keratitis in children, we expect that daily disposable lenses will be prescribed since daily disposable lenses demonstrated a decreased risk of severe, though not overall, microbial keratitis in adults.3 Additionally, we should emphasize contact lens hygiene at each patient visit, including no use of tap water. We must also stress the importance of parental involvement. The persistent gap in knowledge is the incidence of microbial keratitis in daily disposable wearers in all age groups. This knowledge is essential for the future, as children are now being fitted with myopia control contact lenses. We also do not know how effective our repeated teaching and training are on long-term compliance.
Microbial Keratitis Risk in Myopia Control
Joslin discussed the risk of microbial keratitis with myopia control procedures. A recent publication in Ophthalmology summarized the use of orthokeratology in slowing myopia progression, but added: “safety remains a concern because of the risk of potentially blinding microbial keratitis from contact lens wear.”69 The problem remains that there is very little data on rates or risk factors for microbial keratitis in children using overnight orthokeratology lenses or daily wear multifocal soft contact lenses. Of note, a recent case series of 47 minors with Acanthamoeba keratitis identified a high proportion of cases among minors with orthokeratology use (n=6, 13%), which exceeds the expected proportion of orthokeratology cases based on orthokeratology market penetrance (1%), suggesting a potential increased risk of AK in orthokeratology users compared to users of other lens modality types.70 In general, the relatively small prospective studies performed to date are not adequate to address these problems, and different study designs are required to determine both rate and risk factors when rare diseases such as microbial keratitis are studied.
Case control studies may prove to be the best approach to this disease as this type of design is the most efficient way to study rare diseases. They require much smaller study samples than a cohort study, avoid logistical challenges of prospective follow-up, allow intensive evaluation of exposures of cases and controls, and if properly performed (e.g., appropriate sampling) provide information that mirrors a cohort study, with considerably less cost and time. An adequately designed case-control study may allow us to efficiently understand the modifiable behavioral risk factors for microbial keratitis in children in considerably less time than a cohort study. Such case-control studies that recruit based upon disease status, as opposed to exposure, have been significantly underutilized, given the rare disease status of microbial keratitis. Additionally, a disease registry would be beneficial.
Microbiome in Relation to Microbial Keratitis
Willcox presented on the influence of the ocular and systemic microbiome on predisposition to microbial keratitis. The term microbiome refers to the combined genetic material of the microbes at a particular site. While this type of research can identify, in theory, any microbe in an environment, as it does not require them to be amenable to growth in the laboratory, DNA does not determine whether the organisms are alive, dead, transient, or static. Furthermore, as DNA can be found almost anywhere as a common contaminant, these studies require excellent environmental and reagent controls. The biogeography of the human ocular microbiota, compared to animals, demonstrates that the skin above the lid has the most microbiota (and hence microbiome) compared to other sites. The conjunctival surface had the second largest amount, followed by the lid margin, and lastly, tissue samples had very low levels.71 Therefore, all the sites differ in the types of bacteria they harbor (Figure 5), with the conjunctival tissue having the most diverse microbiome.
Figure 5.
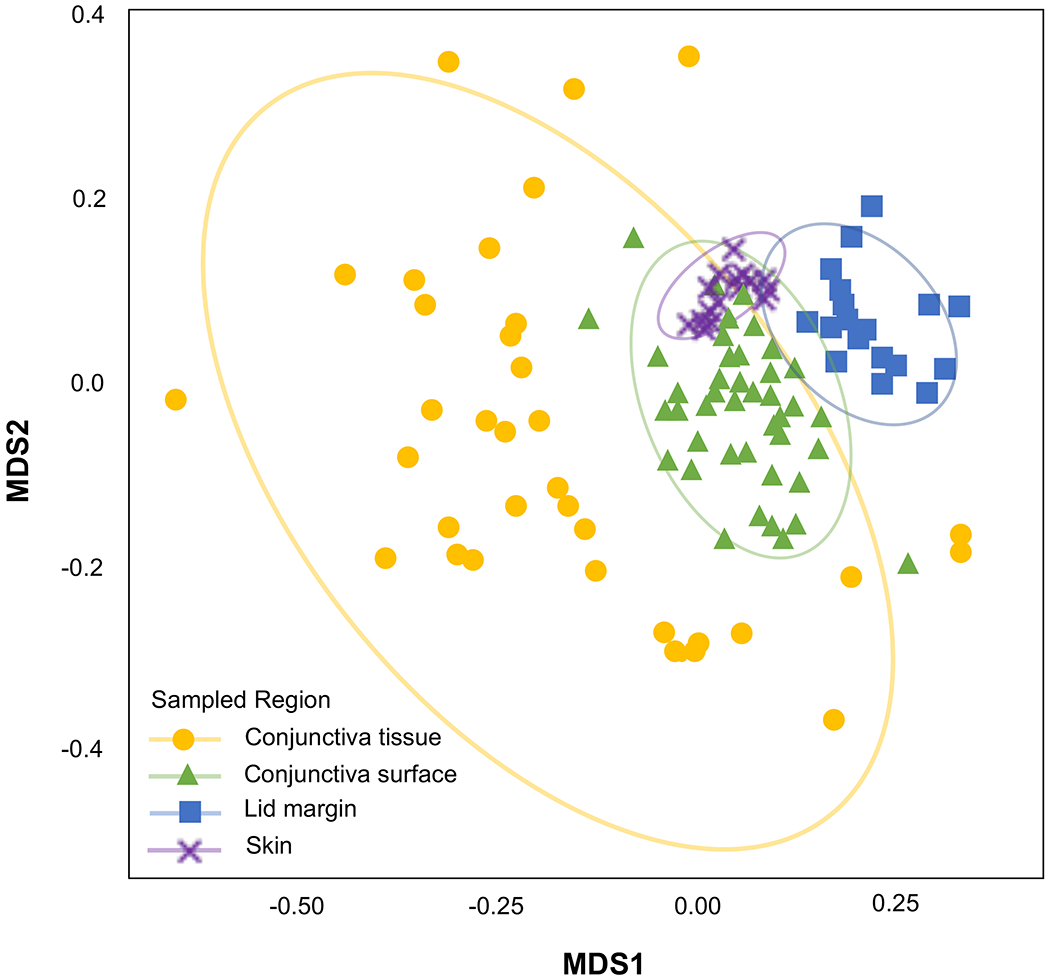
Biogeography of the ocular microbiome (Courtesy of Dr Jerome Ozkan, School of Optometry and Vision Science, University of New South Wales). Conjunctiva_tissue = limbal conjunctiva removed from fellow eye of patients undergoing pterygium surgery; Conjunctiva_surface = swabs of the bulbar conjunctiva of humans; Lid_margin = swabs of the lid margin of humans; skin = swabs of the skin on the upper eyelid of humans. MDS = multidimensional scaling which is used to display the positions of the OTUs in two dimensions; this gives information regarding the similarities of OTUs between and within mammalian species.
The known microbiota of the ocular surface, such as Corynebacteria spp. and Staphylococcus spp., were detected on the skin, the lid margin tissue, and the conjunctival surface.72 Interestingly, there is a group of gram-negative bacteria found almost exclusively in the conjunctival tissue of humans that we believe are persistent members of our normal ocular microbiome. For example, a Pseudomonas species is found in human conjunctival tissue. (Note: this Pseudomonas is not Pseudomonas aeruginosa). These bacteria can be visualized in the ocular tissue using fluorescence in situ hybridization.73
There is a microbiome “fingerprint” for individuals. Some people have more than one type of microbe in their tissue, some people seem to have none, and some people predominantly have, one, for example the Pseudomonas species. At this point we have no knowledge of what these differences might result in – could it be that the differences predict problems during contact lens wear or development of dry eye symptoms? More research is needed in this area. During contact lens wear, the common genera Corynebacteria and Staphylococcus decrease while Pseudomonas increases with contact lens wear.74, 75 Future research should determine whether changes in the levels of common or sporadic genera are more likely to lead to adverse events with contact lens wear.
The ocular microbiota is likely involved in defense. Studies in germ-free animals show that they are more susceptible to Pseudomonas keratitis.76 Thus, the resident microbiota have a protective effect. Introducing a possible commensal organism has an immune effect in mice. These treated animals, when challenged with Candida or Pseudomonas, demonstrate an increase in T cell signaling.77 From a disease standpoint, there is evidence of fairly strong relationships between changes in the gut microbiota and various forms of ocular disease.78 Removing gut commensals increases the number of bacteria and clinical scores in a Pseudomonas keratitis mouse model.77 Animal models demonstrate that the gut microbiome alters the ability to produce secretory IgA in the eye.79 This demonstrates the concept of the common mucosal immune system.
Similar to the bacterial microbiome, there is an ocular surface fungiome, that is, a microbiome composed of fungi,80 although its stability and viability have yet to be proven. The ocular fungiome is altered during fungal keratitis as well.81 Also, changes to the ocular bacteriome have been observed during fungal keratitis.82 Although concomitant antimicrobial therapy was not considered, there was dysbiosis in patients with fungal keratitis, with fewer Corynebacteria and Staphylococcus but more Pseudomonas, Serratia, and others.81 Lastly, there is some evidence of dysbiosis of the conjunctival microbiome in people who have experienced a contact lens induced CIE. Specifically, studies suggest that after experiencing a CIE, some patients may have had more Neisseria on their conjunctiva.83
In summary, there is a stable specific microbiome on the ocular surface with regional differences that are person-specific. We have yet to determine what effect this has on ocular surface susceptibility to infections or CIEs with contact lens wear. It is extremely important to account for possible contamination from the swabs or buffers etc. that are used during microbiome research as DNA can be present almost anywhere as a simple contaminant. Future research of the effects of the ocular microbiome on susceptibility to infection or pathogenesis of dry eye should use suitable animals models that as nearly as possible replicate the normal human ocular microbiome. There is emerging evidence of the ocular microbiome being important in defense, and some evidence suggests that dysbiosis of the microbiome is associated with keratitis.
Antimicrobial Surfaces
Lakkis resumed with a presentation about antimicrobial surfaces and why the contact lens industry is not using them. Several antimicrobial devices are in use in the healthcare environment, including stents and catheters. One of the features of an antimicrobial system is the need for a variety of different mechanisms by which it is active, so that resistance does not develop. For example, some of the technologies presently available either kill microbes on the surface or in the vicinity with different mechanisms, or inhibit microbial adhesion, growth, or replication. From a regulatory (FDA) perspective, such devices are considered combination products and regulatory review is based upon the primary mode of action. However, policies and regulations from both the device and drug or biologic sides may apply, which makes it a much more complicated path for approval.
An ideal anti-microbial contact lens device would include a broad spectrum of activity, a long shelf life (e.g.> 5 years), retention of activity when bound or incorporated in a contact lens or case, compatibility and retention of activity when exposed to lens care solutions and tear film deposits, and low toxicity at the ocular surface and systemically. The device should not promote resistance development in microbes nor impact the normal microbiota. Ideally, a compound should not be released into the ocular environment, or should only be triggered to release when it is needed. Lastly, and practically, lens parameters and performance, including comfort and vision, must not be impacted, and the product must be autoclavable, affordable and scalable.
In the contact lens field, there has been a variety of antimicrobial strategies investigated. The two technologies with the most information include silver (and silver salt) and cationic peptides. There are different methods of incorporating these anti-microbials, including infusion, adsorption, covalent binding, and impregnation. Silver is a broad-spectrum anti-microbial with multiple mechanisms of action that are concentration-dependent.84 In low concentrations, it is bacteriostatic, while at higher concentrations, it can be bactericidal. Some bacteria such Citrobacter freundii, Proteus mirabilis, Enterobacter cloacae, and Klebsiella pneumoniae are inherently resistant to silver. Although silver is broad-spectrum, some bacteria can develop resistance although this resistance does not appear to be widespread. Additionally, as we have learned from people who have used colloidal silver eye drops, long-term use of high concentrations can lead to irreversible graying of the conjunctiva (argyrosis) and deposits within the cornea, which would be a negative side effect.85 However, the amount of silver used in lenses or lens cases is very much less than amounts that have been shown to cause these side effects, even if lenses are used daily for a long period.
There have been several studies investigating silver iodide-infused soft contact lenses to better understand their ability to reduce microbial adhesion and their impact on clinical performance.86–88 In vitro, there was about a 2-log reduction (which corresponds to about a 99% reduction) in bacterial adherence, in addition to delayed Fusarium hyphael invasion.88 Silver salt-infused galyfilcon A lenses in a daily wear clinical study performed similarly to normal galyfilcon A lenses, with no significant differences in comfort, acuity, and ocular health.87 The test lenses were compatible with multipurpose contact lens solutions.86 There were no significant differences between test and control eyes in the incidence of positive cultures, culture classification grades, levels or types of microorganisms isolated from the conjunctiva of study participants.86 A 60 patient 6-night extended wear pilot study in India of these lenses found a 50% reduction in CIEs in the anti-microbial lens wearing eyes compared to historical data in other silicone hydrogels.[Lakkis and Lorenz, personal communication] What remains unknown are the long-term adverse event rates and exposure effects from such silver technology on the ocular environment.
The other application for silver has been in contact lens cases. In vitro, good efficacy of silver cases against bacteria and in particular gram-negative bacteria has been demonstrated.89–91 As seen in Table 2, in clinical trials, overall contamination rates are not necessarily reduced with the use of silver cases, but there is a significant reduction in gram-negative contamination rates as well as the numbers of microbes contaminating lenses.92, 93 Therefore, silver cases do show potential in terms of antimicrobial activity and reducing contamination by pathogens. However, studies have not been sufficiently powered to show evidence for lessening of adverse events rates with such anti-microbial storage cases.89,92,93
TABLE 2.
In-vitro silver lens cases decrease contamination & biofilm formation (cells represent percentages of cases with microbial contamination unless otherwise indicated).
| In vivo studies | MicroBlock (CIBA Vision) | Control | P-value |
|---|---|---|---|
| Contamination rate | |||
| Lakkis & Lakkola 2006* | 90% | 100% | NS |
| Dantam et al 201288 | 71% | 82% | NS |
| Gram-positive contamination | |||
| Lakkis & Lakkola 2006* | 81% | 83% | NS |
| Dantam et al 2012- cocci88 | 64% | ~45% | NS |
| Dantam et al 2012- bacilli88 | 33% | 53% | P = .03 |
| Gram-negative contamination | |||
| Lakkis & Lakkola 2006* | 26% | 46% | P < 0.05 |
| Dantam et al 201288 | 11% | 25% | P = .04 |
| Contamination levels | |||
| Lakkis & Lakkola 2006* | 19% Mod/Heavy | 43% Mod/Heavy | P < .05 |
| Dantam et al 201288 | 1.7 log CFU per well | 4.1 log CFU per well | P < .005 |
Lakkis C, et al. CLAE 2006;29;BCLA Abstract 205.
The antimicrobial synthetic peptide, melimine has been investigated as an antimicrobial contact lens coating. When covalently attached to hydrogel contact lenses, it reduces bacterial adhesion by 2–3 log10 and gives 1-log10 reduction of Acanthamoeba and Fusarium adhesion.94 In animal model studies, Contact Lens Acute Red Eye (CLARE), Contact Lens Peripheral Ulcers (CLPU), and microbial keratitis were reduced.95, 96 However, when they tested these lenses in a one-day clinical study, the lenses did retain antimicrobial activity but the corneas exhibited increased corneal staining. It was hypothesized that this might have been due to an amino acid interaction with cell membranes.97 A shorter version of the peptide, with the hydrophobic amino acids removed, was synthesized that gave 1–2 log10 reductions in bacterial adhesion and no corneal staining after one week of daily wear.98 They recently completed a three-month extended wear study in India and showed that they had a very low CIE rate in both test and control eyes. Also there was less gram-positive bacteria cultured from melimine lens wearing eyes.99
In the patent literature, there is another naturally occurring antimicrobial peptide produced by Streptomyces spp, epsilon poly-L-lysine (e-PLL, εPL or PεL), a common food preservative and classified as “generally regarded as safe” by regulatory authorities. The patent holders have identified e-PLL as an antimicrobial that may be used in lens packing solutions. Soaking contact lenses in a solution of e-PLL produced > 2 log10 reductions of P. aeruginosa, S. aureus and S. marcescens binding to lenses.100, 101 In a handling study there was 1.0–1.7 log10 reduction of bacterial contamination of soft contact lenses but this was reduced to reductions of 0.7–0.9 log10 in the presence of an artificial tear fluid.102
To summarize, there are multiple reasons why antimicrobial surfaces are not yet commonly used in the contact lens field. There are high costs and complexities in getting a combination product approved from the regulatory standpoint. Currently, there are no established efficacy targets regarding bacterial reductions in adhesion or reductions in adverse event incidence. Large-scale clinical and post-market surveillance studies are needed to demonstrate reductions in CIE incidence rates, with good short-and long-term ocular and systemic safety data and evidence of no impact on normal ocular microbiota. The product should have no impact on resistance and cross-resistance to avoid the same situation which occurred with triclosan,103 an antimicrobial used in many products including toothpaste, furniture, kids’ toys, and hand soap. Lastly, we need better technologies and animal models for testing the impact on contact lens-related microbial keratitis. If the microbial keratitis rate can be reduced to an acceptably low level with daily disposable lenses – lower than we have experienced in the past 40 years with reusable lenses – then the need for anti-microbial lenses is significantly diminished. The TEMPO registry68 already demonstrated incredibly low rates of CIEs with daily disposable lenses and microbial keratitis epidemiology studies have also shown that certain daily disposable lens materials have lower rates of microbial keratitis compared to others.16, 17 The severity of microbial keratitis is reduced in daily disposable lens wearers.3, 17 Therefore, it might make more sense to put more effort into determining the best daily disposable lens materials to avoid microbial keratitis. However, we must not forget the potential need for antimicrobial lenses when rigid lenses are required in orthokeratology, myopia control, and corneal disease and when soft lenses must be worn overnight.
Genetic Susceptibility to microbial keratitis
Szczotka-Flynn discussed the emerging role of host genetics in susceptibility to contact lens-related microbial keratitis infections. With more than 125 million people worldwide wearing contact lenses, it is quite surprising that, even though most worn lenses and used storage cases harbor microbial organisms, microbial keratitis is still a relatively rare event. We believe that contact lens-associated microbial keratitis requires microbial exposure, subsequent attachment, and infection to the cornea. Thus, although bacterial contamination may be necessary, it is not sufficient to cause microbial keratitis in all circumstances. Other factors must be involved that escalate the initial steps to infection. Genetic predisposition could affect corneal integrity and immune responses, thus influencing how the eye will respond when confronted with microbial challenges. Additionally, the microbiome may be genetically determined and further impact which patients proceed to microbial keratitis.
There is a plethora of evidence on the high rate of substantial bioburden recovered from worn contact lenses and storage cases, including isolation of known microbial keratitis pathogens. In a series of studies encompassing > 700 patients wearing lenses for daily or extended wear, approximately 1/3 of lenses worn on normal eyes, without complications, harbored substantial bioburden, and about 10% of the organisms were highly pathogenic species.104–106 Additionally, at any one point in time, about 50% of used storage cases are heavily contaminated,107 and 46% of patients present with contaminated cases repeatedly when assessed over time.105 Nevertheless, among these hundreds of patients that were exposed, the majority of whom were using lenses for extended wear, only 1 of 700 experienced microbial keratitis.108 This apparent resistance provides opportunities for unique study designs to understand the genetic basis of microbial keratitis.
There is evidence that genetic predisposition plays a role in other infectious diseases. For example, Toll-like receptor polymorphisms have been identified in pyelonephritis, tuberculosis, and multiple other gram-positive and gram-negative infections.109–113 Large scale genomic approaches have uncovered genetic susceptibility to infection in HIV, Hepatitis C, and typhoid fever.114–116 Of particular interest are resistance genes. For example, among 128 South African families, all presumed to be exposed to M. tuberculosis, 40% had no reaction to the PPD delayed hypersensitivity test. This resistance was controlled by a major locus on chromosome 11p14.117 In P. aeruginosa infection protein variants have been discovered that are associated with such infection in cystic fibrosis.118
In murine models of corneal infection, the absence of certain inflammatory mediators leads to increased severity of infection, and these mediators are genetically determined. IL-6 gene knockout mice exposed to both P. aeruginosa and S. aureus have increased bacterial load and inflammation in corneal tissue.119, 120 Similarly, Toll-like receptor (TLR) 4 and 5 signaling, as well as IL-1 alpha and beta production, are required for protection from P. aeruginosa corneal infection.121,122
Evidence for genetic predisposition in microbial keratitis is building in humans. Variants in the genes for IL-6, IL-10, β-defensin and IL-17F123, 124 have been associated with both susceptibility and severity to contact lens-related microbial keratitis. Specifically, haplotypes of the anti-inflammatory Th2-associated cytokine IL-10 were associated with protection and reduction of severity of bacterial keratitis.125 However, no IL-10 SNP was associated with keratitis in another study.126 SNPs in the promoter region of pro-inflammatory IL-6 cytokine are associated with susceptibility to contact lens-related microbial keratitis with a dose-response effect.123 Lastly, genetic variation in the β-defensin gene DEFB1 and IL-17A exhibit tendencies toward increased susceptibility or severity of CL-microbial keratitis.124,126
The host immune response to infection in Acanthamoeba keratitis,127,128 especially the most severe form of the disease with inflammatory complications, ring-like infiltrates, and scleritis is being investigated. Although there are predictors for this severe stage, including older age, corticosteroids pre-diagnosis and treatment, and treatment for Herpes simplex keratitis before diagnosis, they do not explain all the risk. However, when genetic factors were considered from 105 Acanthamoeba patients, 40 of which had the severe inflammatory reactions,129 there was a significant effect of a mutation in the gene for IL-8 that remained after adjusting for age, sex, steroids, and HSK misdiagnosis and steroids pretreatment.
In summary, the evidence to date in the genetics of contact lens associated microbial keratitis points to the need for appropriate clearance of pathogens, which is critically dependent on host immunity. This immunity is rooted in the genetic variation of the host. The process is likely similar to other infectious diseases where only a fraction of exposed individuals develop clinical disease, suggesting the importance of the resistance genotype. Studies are now underway funded by the National Eye Institute in the United States (U.S.) to explore the genetic differences between cases and presumed “exposed” at-risk contact lens wearers with no evidence of disease.
Summary and Unanswered Questions
Pearlman summarized some of the presentations in this session surrounding neutrophils, as they are critically important in microbial keratitis inflammation. Neutrophils are important in Pseudomonas and Streptococcus pneumoniae corneal ulcers, and are among the first white blood cells to mobilize to the site of infection. Neutrophils can be involved in the inflammatory response which produces some of the pathology associated with microbial keratitis, yet are critical for killing infecting microbes. Understanding events in neutrophil recruitment and activation may identify potential new targets for both anti-inflammatory and antimicrobial therapy. TLRs are important for susceptibility to infection. Corneal epithelial cells make or express TLRs and respond to specific TLR receptor ligands. TLRs are present on corneal epithelial cells. Binding their ligands leads to activation and release of pro-inflammatory cytokines, including the chemokine IL-8, which leads to the recruitment of neutrophils. Neutrophils also express TLRs, which become activated, leading to the killing of microbes but also tissue damage that is associated with corneal opacity.
SESSION 4- COMPLIANCE & COMMUNICATION IMPERATIVES
Panel: Robin Chalmers, OD, Loretta Szczotka-Flynn, OD, PhD, Deborah S. Jacobs MD, MSc and Sarah Collier, MPH
In the last session, the Think Tank looked at compliance. Of special interest were the accomplishments of the Centers for Disease Control and Prevention (CDC) over the last number of years. We were fortunate to hear from Sarah Collier, MPH, from CDC’s Waterborne Disease Prevention Branch in this session. The CDC Waterborne Disease Prevention Branch investigated the 2007 multistate outbreak of Acanthamoeba keratitis.130 This outbreak resulted in the recall of the AMO Complete Moisture Plus solution. After the recall, when the number of cases remained elevated above the pre-outbreak baseline,131 another multistate investigation was launched in 2011. The 2011 investigation did not identify a single contact lens care product, but did identify some hygiene-related risk factors.132 The most significant hygiene-related risk factor was topping off lens solution.
CDC has since partnered with the Contact Lens Institute to work on spreading the word about healthy contact lens habits. When the program was established, one of the first things accomplished was developing web content on healthy contact lens wear and care. The website (https://www.cdc.gov/contactlenses/) now has 47-pages including evidence-based recommendations and multiple health communications resources. CDC’s main health promotion push comes during Contact Lens Health Week, which is held annually during the third week in August, and usually involves a report in the Morbidity and Mortality Weekly Report (MMWR). Past MMWR reports have included an estimate of the burden of keratitis in the U.S. and national surveys estimating the number of contact lens wearers in the U.S. and their risk behaviors.133–137 These campaigns include widely disseminated health promotion materials using social media, podcasts, newsletters, and other platforms. Outside of their primary observance week, they also disseminate messages at Halloween regarding decorative contact lenses. A third effort occurs in March, in time for most university spring breaks, with messages that focus on behaviors that are more likely to happen while traveling, including sleeping in lenses or forgetting a backup pair of lenses or glasses.
Collier then addressed the effectiveness of compliance campaigns. Health promotion campaigns can be quite effective. For example, the “truth” campaign (https://www.thetruth.com/) was an anti-smoking campaign that focused on preventing teenagers from starting smoking. The cost-benefit analysis demonstrated a campaign cost of $324 million but a savings of $1.9 billion in health care costs.138 This is an example of an economically successful health promotion campaign. Campaigns can be more successful if they are grounded in the theories of behavior change, e.g. the Health Belief Model, which considers the likelihood of engaging in a health-promoting behavior based on several modifying variables.139 Another theoretical model is the Stages of Change Model, which focuses on individual decision-making.140 These models have been incorporated into survey questions asked of contact lens wearers, i.e. respondents are asked about their awareness of the CDC’s messages and their perception of their risk for contact lens-related eye infection.
The CDC Contact Lens Health program regularly quantifies the reach and engagement of their campaigns. As part of Contact Lens Health Week in 2019, CDC subject matter experts took part in a Reddit “Ask Me Anything,” which is a community of forums where people can post questions and interact anonymously. A common query (among hundreds of questions) was, “How bad is it really to wear contacts longer than I’m supposed to?” The forum was an opportunity to promote healthy contact lens wear and care messages. The chat was viewed over 60,000 times. The CDC Contact Lens Health program regularly assesses how many people have heard healthy contact lens wear and care messages using nationally representative surveys. Between 2016 and 2018, awareness increased from 20% to 30%. However, because there is a constant stream of new contact lens wearers, many of whom are young, contact lens education requires continuous effort.
The CDC Contact Lens Health program has many free printable, downloadable materials on their website, including posters and tear-off pads that are free to order at https://wwwn.cdc.gov/pubs/CDCInfoOnDemand.aspx.
Summary and Unanswered Questions
Jacobs summarized the key think tank questions and brought up some opportunities for impact. What can clinicians do today to reduce the burden of microbial keratitis? Her suggestions were to counsel against extended wear, prescribe daily disposables, eliminate all tap water, and dispose of storage cases regularly. Lastly, we need to advocate for enforcement to prevent unregulated sale of lenses.
Jacobs commented on the many barriers in these areas. For example, patients have preference for extended wear, because no solutions or cases are required, there’s no mess, no fuss, and they want to wake up with good vision. In these scenarios, information campaigns and changing the habits of prescribers can help. She also reiterated that microbial keratitis severity could be reduced to the lowest possible level if patients were refit and truly compliant with daily disposal. Infections will not be eliminated, because of endogenous pathogens. It is noteworthy that the U.S. market has a relatively low rate of daily disposable prescribing. We need to understand the barriers to change. Are daily disposable lenses the preferred option for new patients? Why not? Why aren’t practitioners converting more patients? If the issue is cost, the manufacturers and sellers could be pressured to lower pricing, and the professional organizations can be involved in changing the habits of prescribers.
Summary and Unanswered Questions for the Entire Think Tank
Jacobs then led the final discussion on future research questions. Case-control studies are needed to answer many of the questions brought up in this Think Tank. Most importantly, the group felt we need them soon, especially in children, to guide us on myopia control. However, the issue is not merely determining odds ratios for risk of infection. One also needs to have a better understanding of absolute risk no matter how difficult that is to get.
Additionally, data on effectiveness of long-term myopia control with orthokeratology or myopia control with soft multi-focal contact lenses is crucial because parents must know the potential benefit before they assume the risk. The group suggested creating a registry database. For example, a central data collection site for populations fitted with overnight orthokeratology or soft multifocal lenses, would allow industry, the FDA, and researchers to look retrospectively and assess risk. There are examples in other medical specialties, like psychiatry, where the entire industry has committed to making all of their clinical data available in one database so that any researcher can use it to test hypotheses or look at mechanisms. A registry or database is something to push for as a profession, especially with the questions around safety of contact lenses for myopia control in children. The group also discussed compliance campaigns, explicitly targeting direct-to-consumer campaigns as the CDC is doing. The social media platforms can be matched to the target audience working with health communicators and graphic artists. The messaging should be vetted by researchers to assure it is evidence based and tested in contact lens wearers to assure it is meaningful. Additionally, in collaborative work between the CDC and CLAY Study teams,141 it was found that the behaviors of contact lens wearers who bought contact lenses directly from their optometrist’s office were no different to those in wearers who bought on the internet. This should encourage practitioners to emphasize retraining and contact lens risk education at every follow-up visit.
In conclusion, collectively the Think Tank participants agreed that complacency must not hold us back. We should not just accept the status quo. We should not subscribe to the mentality that if we cannot fix it easily, then we will simply acknowledge the rate of infection and eventually lose track of it. We understand that the risk of microbial keratitis, of overnight wear, and of Acanthamoeba keratitis has not decreased over decades, despite innovation. For these reasons, the profession, federal agencies, and industry need to move forward in funding studies to solve these problems. We need to push harder against the barriers to change.
ACKNOWLEDGMENTS
Presented at the 2019 Annual Meeting of the American Academy of Optometry, Orlando, Florida.
REFERENCES
- 1.Poggio E, Glynn R, Schein O, et al. The Incidence of Ulcerative Keratitis among Users of Daily-Wear and Extended-Wear Soft Contact Lenses. N Engl J Med 1989;321:779–83 [DOI] [PubMed] [Google Scholar]
- 2.Schein O, Glynn R, Poggio E, et al. The Relative Risk of Ulcerative Keratitis among Users of Daily-Wear and Extended-Wear Soft Contact Lenses. A Case-Control Study. Microbial Keratitis Study Group. N Engl J Med 1989;321:773–8. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F, Keay L, Edwards K, et al. The Incidence of Contact Lens-Related Microbial Keratitis in Australia. Ophthalmology 2008;115:1655–62. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton F, Edwards K, Keay L, et al. Risk Factors for Moderate and Severe Microbial Keratitis in Daily Wear Contact Lens Users. Ophthalmology 2012;119:1516–21. [DOI] [PubMed] [Google Scholar]
- 5.Lam D, Houang E, Fan D, et al. Incidence and Risk Factors for Microbial Keratitis in Hong Kong: Comparison with Europe and North America. Eye 2002;16:604–18. [DOI] [PubMed] [Google Scholar]
- 6.Cheng K, Leung S, Hoekman W, et al. Incidence of Contact-Lens-Associated Microbial Keratitis and Its Related Morbidity. Lancet 1999;354:181–5. [DOI] [PubMed] [Google Scholar]
- 7.Seal D, Kirknews C, Bennett H, Peterson M. Population-based Cohort Study of Microbial Keratitis in Scotland: Incidence and Features. Cont Lens Anterior Eye 1999;22:49–57. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers R, Wagner H, Mitchell G, et al. Age and Other Risk Factors for Corneal Infiltrative and Inflammatory Events in Young Soft Contact Lens Wearers from the Contact Lens Assessment in Youth (CLAY) Study. Invest Ophthalmol Vis Sci 2011;52:6690–6. [DOI] [PubMed] [Google Scholar]
- 9.Bullimore M, Sinnott L, Jones-Jordan L. The Risk of Microbial Keratitis with Overnight Corneal Reshaping Lenses. Optom Vis Sci 2013;90:937–44. [DOI] [PubMed] [Google Scholar]
- 10.Bullimore M The Safety of Soft Contact Lenses in Children. Optom Vis Sci 2017;94:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schein O, McNally J, Katz J, et al. The Incidence of Microbial Keratitis among Wearers of a 30-Day Silicone Hydrogel Extended-Wear Contact Lens. Ophthalmology 2005;112:2172–9. [DOI] [PubMed] [Google Scholar]
- 12.Cope J, Collier S, Schein O, et al. Acanthamoeba Keratitis among Rigid Gas Permeable Contact Lens Wearers, United States, 2005-2011. Ophthalmology 2016:1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf 2017;15:334–65. [DOI] [PubMed] [Google Scholar]
- 14.Lim C, Carnt N, Farook M, et al. Risk Factors for Contact Lens-related Microbial Keratitis in Singapore. Eye (Lond) 2015:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards K, Keay L, Naduvilath T, et al. Characteristics of and Risk Factors for Contact Lens-related Microbial Keratitis in a Tertiary Referral Hospital. Eye 2009;23:153–60. [DOI] [PubMed] [Google Scholar]
- 16.Stapleton F, Naduvilath T, Keay L, et al. Risk Factors and Causative Organisms in Microbial Keratitis in Daily Disposable Contact Lens Wear. PLoS One 2017;12:e0181343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dart J, Radford C, Minassian D, et al. Risk Factors for Microbial Keratitis with Contemporary Contact Lenses: A Case-control Study. Ophthalmology 2008;115:1647–54. [DOI] [PubMed] [Google Scholar]
- 18.Szczotka-Flynn L, Diaz M. Risk of Corneal Inflammatory Events with Silicone Hydrogel and Low Dk Hydrogel Extended Contact Lens Wear: A Meta-Analysis. Optom Vis Sci 2007;84:247–56. [DOI] [PubMed] [Google Scholar]
- 19.Chalmers R, Keay L, McNally J, Kern J. Multicenter Case-Control Study of the Role of Lens Materials and Care Products on the Development of Corneal Infiltrates. Optom Vis Sci 2012;89:316–25. [DOI] [PubMed] [Google Scholar]
- 20.Sauer A, Bourcier T. Microbial Keratitis as a Foreseeable Complication of Cosmetic Contact Lenses: A Prospective Study. Acta Ophthalmol 2011;89:e439–42. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Song J, Hyon J, et al. A Survey of Contact Lens-Related Complications in Korea: The Korean Contact Lens Study Society. J Korean Ophthalmol Soc 2014;55:20–31. [Google Scholar]
- 22.Lim C, Stapleton F, Mehta J. A Review of Cosmetic Contact Lens Infections. Eye 2019;33:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alarcon I, Evans D, Fleiszig S. The Role of Twitching Motility in Pseudomonas Aeruginosa Exit from and Translocation of Corneal Epithelial Cells. Invest Ophthalmol Vis Sci 2009:2237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Metruccio ME, Evans DJ, Fleiszig SJ. Mucosal Fluid Glycoprotein DMBT1 Suppresses Twitching Motility and Virulence of the Opportunistic Pathogen Pseudomonas aeruginosa. PLoS Pathog 2017;13:e1006392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattick J Type IV Pili and Twitching Motility. Annu Rev Microbiol 2002;56:289–314. [DOI] [PubMed] [Google Scholar]
- 26.Nieto V, Kroken A, Grosser M, et al. Type IV Pili Can Mediate Bacterial Motility within Epithelial Cells. mBio 2019;20:DOI 10.1128/mBio.02880-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroken A, Chen C, Evans D, et al. The Impact of ExoS on Pseudomonas aeruginosa Internalization by Epithelial Cells Is Independent of FleQ and Correlates with Bistability of Type Three Secretion System Gene Expression. mBio 2018;9:DOI 10.1128/mBio.00668-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metruccio M, Wan S, Horneman H, et al. A Novel Murine Model for Contact Lens Wear Reveals Clandestine Il-1r Dependent Corneal Parainflammation and Susceptibility to Microbial Keratitis Upon Inoculation with Pseudomonas Aeruginosa. Ocul Surf 2019;17:119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleiszig SMJ, Kroken AR, Nieto V, et al. Contact Lens-Related Corneal Infection: Intrinsic Resistance and Its Compromise. Progress in Retinal and Eye Research 2020;76:DOI 10.1016/j.preteyeres.2019.100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan P, Woods C, Tranoudis J. International Contact Lens Prescribing in 2019. Contact Lens Spectrum 2019;35:26–32. [Google Scholar]
- 31.Radford C, Bacon A, Dart J, Minassian D. Risk Factors for Acanthamoeba Keratitis in Contact Lens Users: A Case-Control Study. BMJ 1995;310:1567–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stapleton F, Dart J, Minassian D. Risk Factors with Contact Lens Related Suppurative Keratitis. CLAO J 1993;19:204–10. [PubMed] [Google Scholar]
- 33.Carnt N, Hoffman J, Verma S, et al. Acanthamoeba Keratitis: Confirmation of the UK Outbreak and a Prospective Case-Control Study Identifying Contributing Risk Factors. Br J Ophthalmol 2018;102:1621–8. [DOI] [PubMed] [Google Scholar]
- 34.Levy B, Heiler D, Norton S. Report on Testing from an Investigation of Fusarium Keratitis in Contact Lens Wearers. Eye Contact Lens 2006;32:256–61. [DOI] [PubMed] [Google Scholar]
- 35.Bullock J, Warwar R, Elder B, Northern W. Temperature Instability of Renu with Moistureloc: A New Theory to Explain the Worldwide Fusarium Keratitis Epidemic of 2004-2006. Arch Ophthalmol 2008;126:1493–8. [DOI] [PubMed] [Google Scholar]
- 36.Kilvington S, Heaselgrave W, Lally J, et al. Encystment of Acanthamoeba During Incubation in Multipurpose Contact Lens Disinfectant Solutions and Experimental Formulations. Eye Contact Lens 2008;34:133–9. [DOI] [PubMed] [Google Scholar]
- 37.Joslin C, Ey T, Shoff M, et al. The Association of Contact Lens Solution Use and Acanthamoeba Keratitis. Am J Ophthalmol 2007;144:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stehr-Green J, Bailey T, Brandt F, et al. Acanthamoeba Keratitis in Soft Contact Lens Wearers: A Case-Control Study. JAMA 1987;258:57–60. [PubMed] [Google Scholar]
- 39.Larkin D, Kilvington S, Easty D. Contamination of Contact Lens Storage Cases by Acanthamoeba and Bacteria. Br J Ophthalmol 1990:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilia D, Lazon de la Jara P, Zhu H, et al. The Effect of Compliance on Contact Lens Case Contamination. Optom Vis Sci 2014;91:262–71. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Willcox M, Stapleton F. The Effect of Contact Lens Hygiene Behavior on Lens Case Contamination. Optom Vis Sci 2015;92:167–74. [DOI] [PubMed] [Google Scholar]
- 42.Lakkis C, Anastasopoulos F, Terry C, Borazjani R. Time Course of the Development of Contact Cens Case and Contact Lens Contamination Invest Ophthalmol Vis Sci 2009;50:6352. [Google Scholar]
- 43.Vaddavalli P, Garg P, Sharma S, et al. Role of Confocal Microscopy in the Diagnosis of Fungal and Acanthamoeba Keratitis. Ophthalmology 2011;118:29–35. [DOI] [PubMed] [Google Scholar]
- 44.Alfonso E Genotypic Identification of Fusarium Species from Ocular Sources Comparison to Morphological Classification and Anti-Fungal Sensitivity Testing. Trans Am Ophthalmol Soc 2008;106:227–31. [PMC free article] [PubMed] [Google Scholar]
- 45.Prajna N, Krishnan T, Mascarenhas J. The Mycotic Ulcer Treatment Trial: A Randomized Trial Comparing Natamycin vs. Voriconazole. JAMA Ophthalmol 2013;131:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prajna N, Krishnan T, Rajaraman R, Patel S. Effect of Oral Voriconazole on Fungal Keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II): A Randomized Clinical Trial. JAMA Ophthalmol 2016;134:1365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anutarapongpan O, Maestre-Mesa J, Alfonso E, et al. Multiplex PCR Screening of Mycotoxin Genes from Ocular Isolates of Fusarium species. Cornea 2018;37:1042–6. [DOI] [PubMed] [Google Scholar]
- 48.Ibrahum Y, Boase D, Cree I. Factors Affecting the Epidemiology of Acanthamoeba Keratitis. Ophthalmol Epidemiology 2007;14:53–60. [DOI] [PubMed] [Google Scholar]
- 49.Dursun D, Fernandez V, Miller D, Alfonso EC. Advanced Fusarium Keratitis Progressing to Endophthalmitis. Cornea 2003;22:300–3. [DOI] [PubMed] [Google Scholar]
- 50.Naranjo A, Arboleda A, Martinez JD, et al. Rose Bengal Photodynamic Antimicrobial Therapy for Patients with Progressive Infectious Keratitis: A Pilot Clinical Study. Am J Ophthalmol 2019;208:387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amescua G, Arboleda A, Nikpoor N, et al. Rose Bengal Photodynamic Antimicrobial Therapy: A Novel Treatment for Resistant Fusarium Keratitis. Cornea 2017;36:1141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gower EW, Keay LJ, Oechsler RA, et al. Trends in Fungal Keratitis in the United States, 2001 to 2007. Ophthalmology 2010;117:2263–7. [DOI] [PubMed] [Google Scholar]
- 53.Nagington J, Watson P, Playfair T, et al. Amoebic Infection of the Eye. Lancet 1974;304:1537–40. [DOI] [PubMed] [Google Scholar]
- 54.Radford C, Minassian D, Dart J. Acanthamoeba Keratitis in England and Wales: Incidence, Outcome, and Risk Factors. Br J Ophthalmol 2002;86:536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watt K, Swarbrick H. Trends in Microbial Keratitis Associated with Orthokeratology. Eye Contact Lens 2007;33:373–7. [DOI] [PubMed] [Google Scholar]
- 56.Peretz A, Geffen Y, Socea S, et al. Comparison of Fluorescence Microscopy and Different Growth Media Culture Methods for Acanthamoeba Keratitis Diagnosis. Am J Trop Med Hyg 2015;93:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tu E, Shoff M, Gao W, et al. Effect of Low Concentrations of Benzalkonium Chloride on Acanthamoeba Survival and Its Potential Impact on Empirical Therapy of Infectious Keratitis. JAMA Ophthalmol 2013;13:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walochnik J, Duchêne M, Seifert K, et al. Cytotoxic Activities of Alkylphosphocholines against Clinical Isolates of Acanthamoeba spp. Antimicrob Agents Chemother 2002;46:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roozbahani M, Hammersmith K, Rapuano C, et al. Acanthamoeba Keratitis: Are Recent Cases More Severe? Cornea 2018;37:1381–7. [DOI] [PubMed] [Google Scholar]
- 60.Lee J, Hahn T, Oum B, et al. Acanthamoeba Keratitis Related to Orthokeratology. Int Ophthalmol 2007;27:45–9. [DOI] [PubMed] [Google Scholar]
- 61.Li W, Wang Z, Qu J, et al. Acanthamoeba Keratitis Related to Contact Lens Use in a Tertiary Hospital in China. BMC Ophthalmol 2019;19:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walline JJ, Gaume Giannoni A, Sinnott L, et al. A Randomized Trial of Soft Multifocal Contact Lenses for Myopia Control: Baseline Data and Methods. Optom Vis Sci 2017;94:856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sankaridurg P, Chen X, Naduvilath T, et al. Adverse Events during 2 Years of Daily Wear of Silicone Hydrogels in Children. Optom Vis Sci 2013;90:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C, Hefflin B, Cope J, et al. Emergency Department Visits for Medical Device-Associated Adverse Events among Children. Pediatrics 2010;126:247–59. [DOI] [PubMed] [Google Scholar]
- 65.Jeng B, Gritz D, Kumar A, et al. Epidemiology of Ulcerative Keratitis in Northern California. Arch Ophthalmol 2010;128:1022–8. [DOI] [PubMed] [Google Scholar]
- 66.Efron N, Morgan P, Woods C, et al. International Survey of Contact Lens Fitting for Myopia Control in Children. Cont Lens Anterior Eye 2020;43:4–8. [DOI] [PubMed] [Google Scholar]
- 67.Chamberlain P, Peixoto-de-Matos S, Logan N, et al. A 3-Year Randomized Clinical Trial of Misight Lenses for Myopia Control. Optom Vis Sci 2019;96:556–67. [DOI] [PubMed] [Google Scholar]
- 68.Chalmers R, Hickson-Curran S, Keay L, et al. Rates of Adverse Events with Hydrogel and Silicone Hydrogel Daily Disposable Lenses in a Large Postmarket Surveillance Registry: The Tempo Registry Invest Ophthalmol Vis Sci 2015;56:654–63. [DOI] [PubMed] [Google Scholar]
- 69.VanderVeen D, Kraker R, Pineles S, et al. Use of Orthokeratology for the Prevention of Myopic Progression in Children: A Report by the American Academy of Ophthalmology. Ophthalmology 2019;126:623–36. [DOI] [PubMed] [Google Scholar]
- 70.Scanzera AC, Tu EY, Joslin CE. Acanthamoeba Keratitis in Minors with Orthokeratology (OK) Lens Use: A Case Series. Eye Contact Lens 2020;July 7:DOI 10.1097/ICL.0000000000000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ozkan J, Willcox M, Wemheuer B, et al. Biogeography of the Human Ocular Microbiota Ocul Surf 2019;17:111–8. [DOI] [PubMed] [Google Scholar]
- 72.Ozkan J, Nielsen S, Diez-Vives C, et al. The Temporal Stability and Composition of the Ocular Surface Microbiome. Sci Reps 2017;7:9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ozkan J, Coroneo M, Willcox M, et al. Identification and Visualization of a Distinct Microbiome in Ocular Surface Conjunctival Tissue. Invest Ophthalmol Vis Sci 2018;59:4268–76. [DOI] [PubMed] [Google Scholar]
- 74.Shin H, Price K, Albert L, et al. Changes in the Eye Microbiota Associated with Contact Lens Wearing. mBio 2016;7:e00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H, Zhao F, Hutchinson D, et al. Conjunctival Microbiome Changes Associated with Soft Contact Lens and Orthokeratology Lens Wearing. Invest Ophthalmol Vis Sci 2017;58:128–36. [DOI] [PubMed] [Google Scholar]
- 76.Kugadas A, Christiansen SH, Sankaranarayanan S, et al. Impact of Microbiota on Resistance to Ocular Pseudomonas aeruginosa-induced Keratitis. PLoS Pathogens 2016;12:e1005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.St Leger AJ, Desai JV, Drummond RA, et al. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal Gammadelta T Cells. Immunity 2017;47:148–58 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalyana Chakravarthy S, Jayasudha R, Ranjith K, et al. Alterations in the Gut Bacterial Microbiome in Fungal Keratitis Patients. PLoS One 2018;13:e0199640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kugadas A, Wright Q, Geddes-McAlister J, Gadjeva M. Role of Microbiota in Strengthening Ocular Mucosal Barrier Function through Secretory IGA. Invest Ophthalmol Vis Sci 2017;58:4593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shivaji S, Jayasudha R, Prashanthi G, et al. The Human Ocular Surface Fungal Microbiome. Invest Ophthalmol Vis Sci 2019;60:451–9. [DOI] [PubMed] [Google Scholar]
- 81.Prashanthi G, Jayasudha R, Chakravarthy S, et al. Alterations in the Ocular Surface Fungal Microbiome in Fungal Keratitis Patients. Microorganisms 2019;7:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ge C, Wei C, Yang B, et al. Conjunctival Microbiome Changes Associated with Fungal Keratitis: Metagenomic Analysis. . Int J Ophthalmol 2019;12:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chao C, Akileswaran L, JN CB, et al. Potential Role of Ocular Microbiome, Host Genotype, Tear Cytokines, and Environmental Factors in Corneal Infiltrative Events in Contact Lens Wearers. Invest Ophthalmol Vis Sci 2018;59:5752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Slawson R, Lee H, Trevors J. Bacterial Interactions with Silver. Biol Met 1990;3:151–4. [DOI] [PubMed] [Google Scholar]
- 85.Gallardo M, Randleman J, Price K, et al. Ocular Argyrosis after Long-term Self-Application of Eyelash Tint. Am J Ophthalmol 2006;141:198–200. [DOI] [PubMed] [Google Scholar]
- 86.Lakkis C, Anastasopoulos F, Slater J, May L. The Effect of Silver-infused Silicone Hydrogel Contact Lenses on the Ocular Biota During Daily Wear. Cont Lens Anterior Eye 2011;34(Suppl. 1):S10. [Google Scholar]
- 87.Lakkis C, Anastasopoulos F, Slater J, Pall B. Clinical Performance of Silver Salt-infused Silicone Hydrogel Contact Lenses over Six Months of Daily Wear. Optom Vis Sci 2011;88:E-Abstract 110432. [Google Scholar]
- 88.May L, Williams C, Zhang S, Ahearn D. A Silicone Hydrogel Lenses Infused with Silver Iodide Delay or Inhibit in-Vitro Surface Colonixation by Bacteria and Fungi Associated with Adverse Ocular Events. Invest Ophthalmol Vis Sci 2011;52:E. [Google Scholar]
- 89.Amos C, George M. Clinical and Laboratory Testing of a Silver Impregnated Lens Case. Cont Lens Ant Eye 2006;29:247–55. [DOI] [PubMed] [Google Scholar]
- 90.Vermeltfoort P, Hooymans J, Busscher H, van der Mei H. Bacterial Transmission from Lens Storage Cases to Contact Lenses—Effects of Lens Care Solutions and Silver Impregnation of Cases. J Biomed Mater Res (B) 2008;87:237–43. [DOI] [PubMed] [Google Scholar]
- 91.Dantam J, Zhu H, Stapleton F. Biocidal Efficacy of Silver-Impregnated Contact Lens Storage Cases in Vitro. Invest Ophthalmol Vis Sci 2011;52:51–7. [DOI] [PubMed] [Google Scholar]
- 92.Lakkis C, Lakkola S. Antibacterial Lens Case Efficacy During Silicone Hydrogel Daily Wear. Cont Lens Anterior Eye 2006;29:205. [Google Scholar]
- 93.Dantam J, Zhu H, Willcox M, et al. In Vivo Assessment of Antimicrobial Efficacy of Silver-Impregnated Contact Lens Storage Cases. Invest Ophthalmol Vis Sci 2012;53:1641–8. [DOI] [PubMed] [Google Scholar]
- 94.Dutta B, Cole N, Kumar N, Willcox M. Broad Spectrum Antimicrobial Activity of Melimine Covalently Bound to Contact Lenses. Invest Ophthalmol Vis Sci 2013;54:175–82. [DOI] [PubMed] [Google Scholar]
- 95.Cole N, Hume E, Vijay A, et al. In-vivo Performance of Melimine as an Antimicrobial Coating for Contact Lenses in Models of CLARE and CLPU. Invest Ophthalmol Vis Sci 2010;51:390–5. [DOI] [PubMed] [Google Scholar]
- 96.Dutta D, Vijay A, Kumar N, Willcox M. Melimine-coated Antimicrobial Contact Lenses Reduce Microbial Keratitis in an Animal Model. Invest Ophthalmol Vis Sci 2016;57:5616–24. [DOI] [PubMed] [Google Scholar]
- 97.Dutta D, Ozkan J, Willcox M. Biocompatibility of Antimicrobial Melimine Lenses: Rabbit and Human Studies. Optom Vis Sci 2014;91:570–81. [DOI] [PubMed] [Google Scholar]
- 98.Dutta D, Zhao T, Cheah K, et al. Activity of a Melimine Derived Peptide Mel4 against Stenotrophomonas, Delftia, Elizabethkingia, Burkholderia and Biocompatibility as a Contact Lens Coating. Cont Lens Anterior Eye 2017;40:175–83. [DOI] [PubMed] [Google Scholar]
- 99.Dutta D, Parthasarathi K, Konda N, et al. Contact Lens-Induced Corneal Infiltrative Events During Extended Melimine Antimicrobial Contact Lens Macl Wear Clinical Trial. Optom Vis Sci 2018;95:E-abstract 180029. [Google Scholar]
- 100.Morris CA, Luk A, Maltseva I, et al. Antimicrobial Ophthalmic Medical Devices. United States Patent: US 20140178327 A1. June 26, 2014.
- 101.Maltseva I, Morris CA, Khong K, Luk A. Protection of Contact Lenses from Microbial Contamination Caused by Handling. United States Patent: US 20150366311 A1. June 25, 2015
- 102.Maltseva I, Morris CA, Luk A. The Effect of a Novel Contact Lens Blister Pack Solution on Bacterial Contamination of Various Soft Contact Lens Materials During Lens Handling. Optom Vis Sci 2017;94:E-abstract 17537. [Google Scholar]
- 103.Schweizer H Triclosan: A Widely Used Biocide and Its Link to Antibiotics. FEMS Microbiol Lett 2001;202:1–7. [DOI] [PubMed] [Google Scholar]
- 104.Szczotka-Flynn L, Jacobs M, Bajaksouzian S, Rimm A. Risk Factors for Contact Lens Contamination During Continuous Wear. Optom Vis Sci 2009;86:1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ying Y, Jacobs M, Bajaksouzian S, et al. Risk Factors for Microbial Bioburden During Daily Wear of Silicone Hydrogel Contact Lenses. Eye Contact Lens 2014;40:148–56. [DOI] [PubMed] [Google Scholar]
- 106.Szczotka-Flynn L, Jiang Y, Stiegemeier M, et al. Mucin Balls Influence Corneal Infiltrative Events; a Clinical Trial. Optom Vis Sci 2017;94:448–57. [DOI] [PubMed] [Google Scholar]
- 107.Szczotka-Flynn L, Pearlman E, Ghannoum M. Microbial Contamination of Contact Lenses, Lens Care Solutions, and Their Accessories: A Literature Review. Eye Cont Lens 2010;36:116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szczotka-Flynn L, Jiang Y, Raghupathy S, et al. Corneal Inflammatory Events with Daily Silicone Hydrogel Lens Wear. Optom Vis Sci 2014;91:3–12. [DOI] [PubMed] [Google Scholar]
- 109.Agnese DM, Calvano JE, Hahm SJ, et al. Human Toll-Like Receptor 4 Mutations but Not Cd14 Polymorphisms Are Associated with an Increased Risk of Gram-Negative Infections. J Infect Dis 2002;186:1522–5. [DOI] [PubMed] [Google Scholar]
- 110.Hawn T, Scholes D, Li S, et al. Toll-Like Receptor Polymorphisms and Susceptibility to Urinary Tract Infections in Adult Women. PLoS One 2009;4:e5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Skevaki C, Pararas M, Kostelidou K, et al. Single Nucleotide Polymorphisms of Toll-Like Receptors and Susceptibility to Infectious Diseases. Clin Exp Immunol 2015;180:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sutherland AM, Walley KR, Russell JA. Polymorphisms in Cd14, Mannose-Binding Lectin, and Toll-Like Receptor-2 Are Associated with Increased Prevalence of Infection in Critically Ill Adults. Crit Care Med 2005;33:638–44. [DOI] [PubMed] [Google Scholar]
- 113.van Tong H, Velavan TP, Thye T, Meyer CG. Human Genetic Factors in Tuberculosis: An Update. Tropical Medicine & International Health 2017;22:1063–71. [DOI] [PubMed] [Google Scholar]
- 114.Pereyra F, Jia X, McLaren PJ, et al. The Major Genetic Determinants of HIV-1 Control Affect HLA Class I Peptide Presentation. Science 2010;330:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ge D, Fellay J, Thompson A, et al. Genetic Variation in IL28B Predicts Hepatitis C Treatment-Induced Viral Clearance. Nature 2009;461:399–401. [DOI] [PubMed] [Google Scholar]
- 116.Alvarez M, Glover L, Luo P, et al. Human Genetic Variation in VAC14 Regulates Salmonella Invasion and Typhoid Fever through Modulation of Cholesterol. Proc Natl Acad Sci U S A 2017;114:E7746–E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kinnear C, Hoal E, Schurz H, et al. The Role of Human Host Genetics in Tuberculosis Resistance. Expert Rev Respir Med 2017;11:721–37. [DOI] [PubMed] [Google Scholar]
- 118.Emond MJ, Louie T, Emerson J, et al. Exome Sequencing of Extreme Phenotypes Identifies Dctn4 as a Modifier of Chronic Pseudomonas aeruginosa Infection in Cystic Fibrosis. Nat Genet 2012;44:886–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cole N, Bao S, Stapleton F, et al. Pseudomonas aeruginosa Keratitis in Il-6-Deficient Mice. Int Arch Allergy Immunol 2003;130:165–72. [DOI] [PubMed] [Google Scholar]
- 120.Hume E, Cole N, Garthwaite L, et al. A Protective Role for Il-6 in Staphylococcal Microbial Keratitis. Invest Ophthalmol Vis Sci 2006;47:4926–30. [DOI] [PubMed] [Google Scholar]
- 121.Hazlett L Corneal Response to Pseudomonas aeruginosa Infection. Prog Retin Eye Res 2004;23:1–30. [DOI] [PubMed] [Google Scholar]
- 122.Berk R, Hazlett L. Further Studies on the Genetic Control of Murine Corneal Response to Pseudomonas aeruginosa. Rev Infect Dis 1983;5 Suppl:S936–40. [DOI] [PubMed] [Google Scholar]
- 123.Carnt N, Willcox M, Hau S, et al. Association of Single Nucleotide Polymorphisms of Interleukins-1beta, −6, and −12b with Contact Lens Keratitis Susceptibility and Severity. Ophthalmology 2012;119:1320–7. [DOI] [PubMed] [Google Scholar]
- 124.Carnt N, Willcox M, Hau S, et al. Immune Defense Single Nucleotide Polymorphisms and Recruitment Strategies Associated with Contact Lens Keratitis. Ophthalmology 2012;119:1997–2002. [DOI] [PubMed] [Google Scholar]
- 125.Keijser S, Kurreeman F, de Keizer R, et al. Il-10 Promotor Haplotypes Associated with Susceptibility to and Severity of Bacterial Corneal Ulcers. Exp Eye Res 2009;88:1124–8. [DOI] [PubMed] [Google Scholar]
- 126.Carnt N, Cipriani VV, Stapleton F, et al. Association Study of Single Nucleotide Polymorphisms in Il-10 and Il-17 Genes with the Severity of Microbial Keratitis. Cont Lens Anterior Eye 2019;42:658–61. [DOI] [PubMed] [Google Scholar]
- 127.Carnt NA, Subedi D, Connor S, Kilvington S. The Relationship between Environmental Sources and the Susceptibility of Acanthamoeba Keratitis in the United Kingdom. PLoS One 2020;15:e0229681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stapleton F, Carnt N. Contact Lens-related Microbial Keratitis: How Have Epidemiology and Genetics Helped Us with Pathogenesis and Prophylaxis. Eye (Lond) 2012;26:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carnt NA, Pang I, Burdon KP, et al. The Association of Interleukin 8 Gene SNP with Severe Inflammatory Complications in Contact Lens Wearers Experiencing Acanthamoeba Keratitis. Invest Ophthalmol Vis Sci 2020;61:E-Abstract 3998. [Google Scholar]
- 130.Verani J, Lorick S, Yoder J, et al. National Outbreak of Acanthamoeba Keratitis Associated with Use of a Contact Lens Solution, United States. Emerg Infect Dis 2009;15:1236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoder J, Verani J, Heidman N, et al. Acanthamoeba Keratitis: The Persistence of Cases Following a Multistate Outbreak. Ophthalmic Epidemiol 2012;19:221–5. [DOI] [PubMed] [Google Scholar]
- 132.Brown A, Ross J, Jones D, et al. Risk Factors for Acanthamoeba Keratitis-a Multistate Case-Control Study, 2008–2011. Eye Contact Lens 2018;44(Suppl.):S173–S8. [DOI] [PubMed] [Google Scholar]
- 133.Konne NM, Collier SA, Spangler J, Cope JR. Healthy Contact Lens Behaviors Communicated by Eye Care Providers and Recalled by Patients - United States, 2018. MMWR Morb Mortal Wkly Rep 2019;68:693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cope J, Collier S, Nethercut H, et al. Risk Behaviors for Contact Lens–Related Eye Infections among Adults and Adolescents — United States, 2016. MMWR Morb Mortal Wkly Rep 2017;66:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cope J, Collier S, Srinivasan K, et al. Contact Lens-Related Corneal Infections -United States, 2005–2015. MMWR Morb Mortal Wkly Rep 2016;65:817–21. [DOI] [PubMed] [Google Scholar]
- 136.Cope J, Collier S, Rao M, et al. Contact Lens Wearer Demographics and Risk Behaviors for Contact Lens-Related Eye Infections--United States, 2014. MMWR Morb Mortal Wkly Rep 2015;64:865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Collier S, Gronostaj M, MacGurn A, et al. Estimated Burden of Keratitis--United States, 2010. MMWR Morb Mortal Wkly Rep 2014;63:1027–30. [PMC free article] [PubMed] [Google Scholar]
- 138.Holtgrave D, Wunderink K, Vallone D, Healton C. Cost-Utility Analysis of the National Truth Campaign to Prevent Youth Smoking. Am J Prev Med 2009;36:385–8. [DOI] [PubMed] [Google Scholar]
- 139.Janz N, Becker M. The Health Belief Model: A Decade Later. Health Educ Q 1984;11:1–47. [DOI] [PubMed] [Google Scholar]
- 140.Prochaska J, DiClemente C. Stages and Processes of Self-Change of Smoking: Toward an Integrative Model of Change. J Consult Clin Psychol 1983;51:390–5. [DOI] [PubMed] [Google Scholar]
- 141.Chalmers R, Wagner H, Kinoshita B, et al. Is Purchasing Lenses from the Prescriber Associated with Better Habits among Soft Contact Lens Wearers? Cont Lens Anterior Eye 2016;39:435–41. [DOI] [PubMed] [Google Scholar]


