Abstract
Although information processing and storage in the brain is thought to be primarily orchestrated by synaptic plasticity, other neural mechanisms such as intrinsic plasticity are available. While a number of recent studies have described the plasticity of intrinsic excitability in several types of neurons, the significance of non-synaptic mechanisms in memory and learning remains elusive. After reviewing plasticity of intrinsic excitation in relation to learning and homeostatic mechanisms, we focus on the intrinsic properties of a class of basal-ganglia projecting song system neurons in zebra finch, how these related to each bird’s unique learned song, how these properties change over development, and how they are maintained dynamically to rapidly change in response to auditory feedback perturbations. We place these results in the broader theme of learning and changes in intrinsic properties, emphasizing the computational implications of this form of plasticity, which are distinct from synaptic plasticity. The results suggest that exploring reciprocal interactions between intrinsic and network properties will be a fruitful avenue for understanding mechanisms of birdsong learning.
Keywords: songbirds, zebra finch, intrinsic properties, intrinsic excitation, development, auditory feedback
Characterizing activity-dependent changes in neuronal circuitry is central to developing a “mesoscopic” view of species-specific, organismal level learning and memory phenomena. Changes in functional network structure are driven by the demands for adaptive responses to an animal’s experience while being constrained by the basic circuitry (patterns of connections) reflective of the animal’s evolutionary and developmental history. One simplifying assumption is that appropriate network behavior requires that every cellular and synaptic property that underlies those behaviors be tightly regulated (Grashow, Brookings, and Marder, 2010). This may be true in some instances, but even small networks may have many solutions for the same output (Bucher, Prinz, and Marder, 2005; Drion, O’Leary, and Marder, 2015; Hamood and Marder, 2014; O’Leary, Williams, Caplan, and Marder, 2013; O’Leary, Williams, Franci, and Marder, 2014). And for more complex tasks each individual may express a unique solution, which may be realized as individual specific variation at the level of neural networks. A second simplification is the traditional emphasis on activity-dependent changes in synaptic strength as a mechanism for information storage (Abbott and Nelson, 2000; Malenka and Nicoll, 1999). Modification of synaptic transmission through the paradigm of long-term potentiation (LTP) is considered a leading mechanism for information storage and learning. Synapses enhanced via LTP have greater probability of eliciting action potentials in the postsynaptic neuron. LTP lasts long enough to set the baseline for stable memories and learning paradigms, can be input-specific thereby allowing selective memory storage, and can be reversed by long-term depression (LTD). Nevertheless, learning can result from single experiences while LTP usually requires repetitive stimulation, and a mechanism describing synaptic changes does not address coordination across populations of synapses. These are a few of the many challenges not addressed by the LTP paradigm.
As has emerged in studies of multiple systems, the cocktail of neuronal transmembrane ion channels and the details of how neurons integrate their synaptic inputs to generate action potentials also are plastic. Moreover, neurons can display a wide variety of different intrinsic membrane properties that depend on the number, kind, and distribution of voltage-gated and ligand-gated ion channels. Isolating a neuron from the network in which it is embedded, typically through the use of pharmacological agents, allows direct evaluation of the electrophysiological properties of the neuron intrinsic to that cell. Some neurons are silent when isolated, others fire single action potentials tonically, and still others fire bursts of action potentials (Marder and Thirumalai, 2002). In addition, the history of activity of the neuron itself adjusts a cell’s intrinsic properties (IP) leading to long lasting changes. These form candidate mechanisms contributing to learning and memory. Marder et al. (1996) proposed that memory in networks results from a constant interaction between external, synaptic input and a cell’s IP. Assessment of IP is commonly used to describe and classify neurons in a wide variety of preparations (Alaburda, Russo, MacAulay, and Hounsgaard, 2005; Cudmore and Turrigiano, 2004; Dougherty, Sawchuk, and Hochman, 2005; Helmstaedter, Sakmann, and Feldmeyer, 2009; Idoux, Serafin, Fort, Vidal, Beraneck, Vibert, Mühlethaler, and Moore, 2006; Prescott and De Koninck, 2002; Sautois, Soffe, Li, and Roberts, 2007), with the idea that these electrophysiological measures are directly predictive of a neuron’s activity and the information it relays to the circuit it resides in (Gittis and du Lac, 2007; Otte, Hasenstaub, and Callaway, 2010). For example, short term memory tasks mediated by bistable neurons which exhibit plateau properties enabled them to switch states while storing information about the specific input (Canavier, Baxter, Clark, and Byrne, 1994; Kiehn, 1991; Marder, 1991; Weimann, Marder, Evans, and Calabrese, 1993). State transitions are typically driven by synaptic input but can also be controlled by neuromodulation (Lechner, Baxter, Clark, and Byrne, 1996). Conductances with slow kinetics also can contribute to short-term memory formation. Modeling studies of the potassium (Kv 1.3) conductance revealed that its slow recovery from inactivation resulted in a heightened response to a depolarizing current pulse following a long depolarization (Marom and Abbott, 1994). Modeling studies of single cell homeostatic regulation of neuronal activity indicated that this could arise through plasticity of maximal conductances of the neurons (LeMasson, Marder, and Abbott, 1993). Recent observations that the voltage output of a neuron feeds back to regulate expression of channel mRNA presents a mechanism for homeostasis consistent with the modeling results (Santin and Schulz, 2019).
From these and a host of other studies, it has become clear that plasticity of IP has a significant impact on the dynamics of a neural circuit and can also serve as an essential mechanism for information storage (Golowasch, Abbott, and Marder, 1999a; Golowasch, Casey, Abbott, and Marder, 1999b; Marder, 1998; Marder and Prinz, 2002). It follows that learned information is represented by both the synaptic inputs that transmit information to neurons and by the response threshold and ion channel characteristics of the neurons, which regulate their contributions to and participation in different functional circuits. Our review paper focuses on regulation of intrinsic excitation (IE) as a mechanism contributing to learning, memory and information processing. Changes in IE – an electrophysiological response, are those electrophysiological changes that arise from changes in IP, which encompasses a broader suite of properties intrinsic to the cell. We focus on the role of regulation of ion channels that give rise to spiking activity.
The studies cited above are centered on work examining homeostatic regulation of rhythmogenesis in the spiny lobster stomatogastric ganglion (STG). Changes in the internal milieu are ultimately what drive homeostatic regulation of neuronal IP. If similar changes arise from the external environment (perhaps conveyed via synaptic activity), then this may drive similar somatic changes in intrinsic plasticity. This motivates the search for learning–induced intrinsic plasticity, and the rules associating such plasticity to animal behavior. We develop these themes in what follows.
1-. Learning-specific changes in intrinsic membrane excitability
Many of the same experimental manipulations and learning paradigms that induce plasticity at the level of synapses can also induce plasticity in IE in a number of vertebrate and invertebrate systems. Thus, plasticity of intrinsic membrane properties of neurons has been studied for decades as a possible mechanism for learning (Daoudal and Debanne, 2003; Frick and Johnston, 2005; Hansel, Linden, and D’Angelo, 2001; Marder et al., 1996; Mozzachiodi and Byrne, 2010; Zhang and Linden, 2003).
Extensive evidence suggests that IE can be regulated by activity (Daoudal and Debanne, 2003; Santin and Schulz, 2019; Zhang and Linden, 2003). More recently, evidence is emerging in a number of systems linking IE to learning (this issue, (Debanne and Russier, 2019; Dunn and Kaczorowski, 2019; Johansson, 2019), and see below). For example, using intracellular recordings from L5 pyramidal neurons in the motor cortex of anesthetized rats, IE properties of these neurons showed long-term changes after intracellularly conditioning them via repetitive stimulation of depolarizing current pulses (Paz, Mahon, Tiret, Genet, Delord, and Charpier, 2009). Intracellular conditioning of L5 pyramidal neurons of the barrel cortex in anesthetized rats caused intrinsic potentiation or depression in synaptic currents (Mahon and Charpier, 2012). These results were seen also in non-anesthetized (awake) decerebrate rats receiving stimulation to the parallel fiber (PF) in Purkinje neurons. This caused increases in Purkinje neuron spontaneous firing, a process mediated by the small-conductance, calcium-dependent K+ (SK) current (Belmeguenai, Hosy, Bengtsson, Pedroarena, Piochon, Teuling, He, Ohtsuki, De Jeu, Elgersma, De Zeeuw, Jorntell, and Hansel, 2010). Similarly, eyeblink conditioning of rabbits increased the excitability of Purkinje neurons when recorded intracellularly, seen in ex vivo recordings (Schreurs, Gusev, Tomsic, Alkon, and Shi, 1998) and then later in in vitro recordings (Titley, Watkins, Lin, Weiss, McCarthy, Disterhoft, and Hansel, 2020), the latter study implicating downregulation of SK currents. Another set of eyeblink conditioning experiments showed similar increases in the firing patterns of neurons when recorded intracellularly: motor cortex neurons of awake cats (Aou, Woody, and Birt, 1992), and pyramidal neurons of CA1 hippocampal rabbit brain slices after the conditioning (Disterhoft, Coulter, and Alkon, 1986; Matthews and Disterhoft, 2009; McKay, Oh, Galvez, Burgdorf, Kroes, Weiss, Adelman, Moskal, and Disterhoft, 2012; Thompson, Moyer, and Disterhoft, 1996), a mechanism mediated by SK or calcium-activated slow AHP K+ currents. Moreover, when rats were exposed to fear conditioning, pyramidal neurons in CA1 hippocampal slices showed intrinsic potentiation or depression (McKay, Matthews, Oliveira, and Disterhoft, 2009). These observations are not the results of artifacts of the in vitro recording conditions because experiments to control for any unspecified variation that might emerge in the IP of the recorded neurons (like rundown, artificial cerebro-spinal fluid or intracellular solution changes, etc…) had been performed. Together, these findings suggest that intrinsic plasticity exists under physiological conditions and that it contributes to mechanisms of learning.
The accessibility of neurons in a brain slice preparation facilitates quantification of physiological properties that influence IE, but also greatly facilitates manipulation of individual ion currents via pharmacology. This is important because in many examples, ion currents emerge as the maestros of the IE show. For example, across different cells and paradigms, IE changes are mediated by alterations in the A-type K+ channels (Frick, Magee, and Johnston, 2004; Schreurs et al., 1998; Titley, Brunel, and Hansel, 2017), BK channels (Nelson, Gittis, and du Lac, 2005), SK channels (Belmeguenai et al., 2010; Grasselli, He, Wan, Adelman, Ohtsuki, and Hansel, 2016; Lin, Lujan, Watanabe, Adelman, and Maylie, 2008; Sourdet, Russier, Daoudal, Ankri, and Debanne, 2003), as well as hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels (Brager and Johnston, 2007; Fan, Fricker, Brager, Chen, Lu, Chitwood, and Johnston, 2005; Nolan, Malleret, Lee, Gibbs, Dudman, Santoro, Yin, Thompson, Siegelbaum, Kandel, and Morozov, 2003). SK channel modulation has been particularly well studied. SK channel downregulation amplifies spine calcium transients and enhances LTP in pyramidal cells (Hammond, Bond, Strassmaier, Ngo-Anh, Adelman, Maylie, and Stackman, 2006; Ngo-Anh, Bloodgood, Lin, Sabatini, Maylie, and Adelman, 2005; Stackman, Hammond, Linardatos, Gerlach, Maylie, Adelman, and Tzounopoulos, 2002). SK channel deactivation is slow, which is reflected in the SK channel participation in the medium-late component of the after-hyperpolarizing (AHP) currents (ImAHP; (Bond, Herson, Strassmaier, Hammond, Stackman, Maylie, and Adelman, 2004)). In cerebellar Purkinje cells, this feature enables SK channel plasticity to not only facilitate action potential generation per se (one spike versus no spike) but also to promote spike burst firing (burst versus one spike or no spike) (Ohtsuki and Hansel, 2018), which might well be the relevant parameter for integration of neurons into active ensembles (Titley et al., 2017). Tetanization applied to ascending Purkinje fibers in brain slices showed remarkable increase (~2 fold) in the firing frequency of Purkinje neurons in mice that had their SK channels knocked out (Fig 1), showing the significant role this ion channel plays in orchestrating the dynamics of these cells in their network (Grasselli et al., 2016). One interesting trend is that in most studies only one specific current is implicated in changes in IE. Yet changes to IP are likely to be coordinated across many ion channels, so it is interesting to investigate if and how such coordination is achieved (see below).
Figure 1:
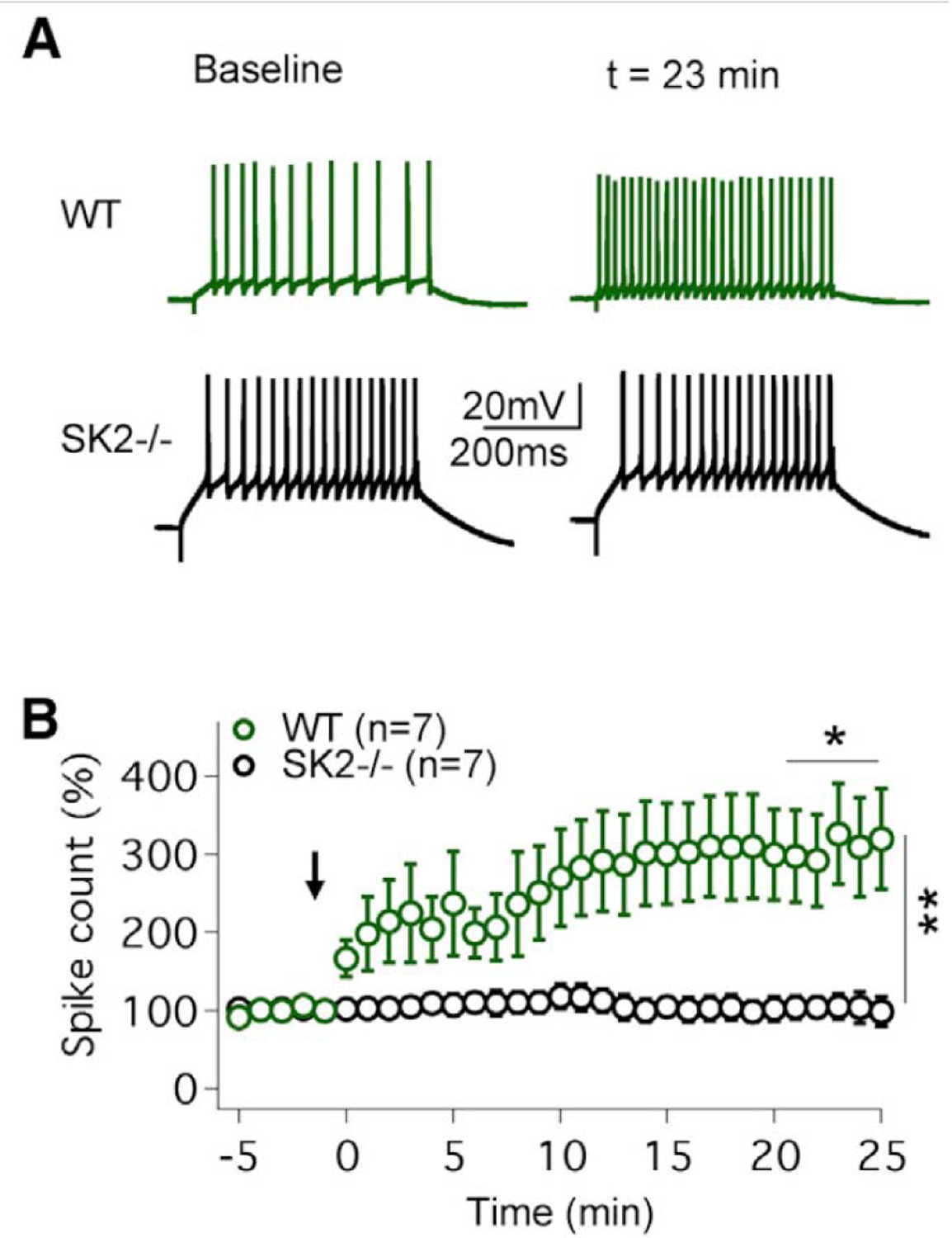
Intrinsic plasticity is absent from SK2−/− mice. (A) Top traces: in wild-type (WT) mice an increase in the spike count is triggered by a 5 Hz injection of depolarizing currents. Bottom traces: no excitability change is seen in SK2−/−. Purkinje cells. (B) Time graph showing that intrinsic plasticity is triggered in WT (n = 7), but not in SK2−/− Purkinje cells. The arrow indicates the time point of tetanization. Taken from Fig. 1, Grasselli et al., 2016, under the CC BY-NC-ND license.
As well as channels such as SK and BK that are triggered by internal calcium concentration, IE can also be mediated by changes in voltage-dependent and ligand-dependent ion conductances. For example, pharmacological manipulation of non SK/BK ion channels alters IE (Bekkers and Delaney, 2001). PKA is also known to phosphorylate and downregulate K+ channels, causing an increase in IE (Hoffman and Johnston, 1998). Similar to synaptic plasticity, intrinsic excitability changes may result from brain-derived neurotrophic factor (BDNF) signaling (Desai, Rutherford, and Turrigiano, 1999; Graves, Moore, Spruston, Tryba, and Kaczorowski, 2016), and is not mediated by changes in neurotransmitter receptors but results from modifications of voltage- or ligand-dependent ion channels including potassium (K+) channels. Moreover, long-term increases in excitability can be triggered by both metabotropic (Sourdet et al., 2003) and inhibitory (Nelson, Krispel, Sekirnjak, and du Lac, 2003) receptor activation. It has been also shown that long-lasting potentiation can be induced in layer 5 neocortical pyramidal neurons after short periods of AP firing, which doesn’t require synaptic activation and which simply required calcium influx and activation of PKA (Cudmore and Turrigiano, 2004). Thus, there is a panoply of ion channel mechanisms that can give rise to neuronal plasticity beyond synaptic plasticity.
The above results were observed using in vitro preparations, but plasticity of IP is also observed in vivo. Such studies usefully extend our understanding of intrinsic plasticity by connecting it to behavior while also helping to address the otherwise implacable concerns of professional skeptics that the results of all these studies arise from artifacts of the in vitro recording conditions. For example, the enhanced excitability subsequent to eyeblink conditioning described above for a brain slice preparation has also been observed in intracellular recordings from the motor cortex of awake cats (Aou et al., 1992). Overexpressing CREB or dnKCNQ2 in mice causes increases in the firing of principal neurons in the lateral amygdala, which contributes to cell-specific CREB fear memory allocation during subsequent training (Yiu, Mercaldo, Yan, Richards, Rashid, Hsiang, Pressey, Mahadevan, Tran, Kushner, Woodin, Frankland, and Josselyn, 2014). Classical conditioning of visual responses in Hermissenda is modulated by IE of photoreceptors (Alkon, 1984a; b; Matzel and Gandhi, 2000). Water-maze learning (Oh, Kuo, Wu, Sametsky, and Disterhoft, 2003) causes long-lasting potentiation in IE in hippocampal CA1 pyramidal neurons. Operant conditioning has similar effects on pyramidal neurons in the olfactory (piriform) cortex (Saar and Barkai, 2003; Saar, Grossman, and Barkai, 1998; 2002). These studies show that intrinsic plasticity is an activity-dependent mechanism that is observed in vivo in diverse cell types. They also show that intrinsic plasticity differs from homeostatic plasticity (Turrigiano, 2011) because, in the majority of recordings, neuronal activation causes a further increase in spike firing. Taken together, these studies indicate that learning paradigms could modify IE and that IE contributes to mechanisms of learning and memory.
Changes in IE are not restricted to particular neurons but can be induced in cultured cortical neurons and in variety of invertebrate and vertebrate systems. IE works to increase the characteristic firing in hippocampal and neocortical pyramidal cells (Frick et al., 2004; Mozzachiodi and Byrne, 2010; Zhang and Linden, 2003). IE also works in a homeostatic fashion to restore cellular firing properties to their baseline behavior (Desai et al., 1999; Thoby-Brisson and Simmers, 1998; Turrigiano, Abbott, and Marder, 1994; Turrigiano, LeMasson, and Marder, 1995). In the cerebellar system, intrinsic plasticity has been found in granule cells (Armano, Rossi, Taglietti, and D’Angelo, 2000) and Purkinje cells (Belmeguenai et al., 2010; Schreurs et al., 1998), in neurons of the cerebellar nuclei (Aizenman and Linden, 2000) and vestibular nuclei (Nelson et al., 2005). Presynaptic excitability can be modulated on long and short time scales via synaptic drives (Ganguly, Kiss, and Poo, 2000; Nick and Ribera, 2000), and postsynaptic activity can rapidly modulate the IE of deep cerebellar nuclei neurons (Aizenman and Linden, 2000).
The extensive results show that intrinsic plasticity is an activity-dependent phenomenon that can be observed both in vivo and in vitro, suggest that IE may often act in concert with synaptic plasticity, and demonstrate a significant role of IP in some forms of learning. Indeed, various studies showed that pharmacological intervention that alters the regulation of specific ion channels prevents proper learning. For example, BK channel blockade (Matthews and Disterhoft, 2009) or SK channel activation (McKay et al., 2012) impairs trace eyeblink conditioning, suggesting that availability of both these channel types is necessary eyeblink associative learning. Similarly, mice with Purkinje cell calcineurin PP2B knockout had impaired adaptation of VOR and conditioning of eyeblink response (Schonewille, Belmeguenai, Koekkoek, Houtman, Boele, van Beugen, Gao, Badura, Ohtsuki, Amerika, Hosy, Hoebeek, Elgersma, Hansel, and De Zeeuw, 2010). It remains unresolved what governs when IE acts in coordination with synaptic plasticity and when it acts independent of synaptic plasticity. It is likely that interactions between synaptic and intrinsic plasticity varies across systems and forms of learning, rendering a mechanistic understanding of learning essential for assigning roles to different types of plasticity. Clearly, however, IE contributes to coordinating activity across neural networks. This suggests that the hegemony of synaptic plasticity explanations for learning mechanisms provides for an incomplete viewpoint.
2-. Intrinsic excitability and homeostasis
The activity levels of individual neurons and networks are homeostatically regulated to maintain physiologically appropriate and stable network performance in the face of growth, channel turnover, and perturbations by modification of either synaptic or intrinsic parameters or both (Davis, 2006; Dickman and Davis, 2009; Frank, Pielage, and Davis, 2009; Golowasch et al., 1999b; Günay and Prinz, 2010; Haedo and Golowasch, 2006; LeMasson et al., 1993; Liu, Golowasch, Marder, and Abbott, 1998; Luther, Robie, Yarotsky, Reina, Marder, and Golowasch, 2003; O’Leary, van Rossum, and Wyllie, 2010; Olypher and Prinz, 2010; Paradis, Sweeney, and Davis, 2001; Pratt and Aizenman, 2007; Rich and Wenner, 2007; Soto-Treviño, Thoroughman, Marder, and Abbott, 2001; Turrigiano, 2007; Turrigiano et al., 1994; Turrigiano, 2008; Turrigiano and Nelson, 2000; 2004; Wilhelm and Wenner, 2008; Zhang and Golowasch, 2007; Zhang, Khorkova, Rodriguez, and Golowasch, 2009). Implicit in the concept of homeostasis is that there is a target or desired phenotype or activity pattern and a sensor to detect deviations from that target level. These error signals are then used as feedback to trigger alterations of one or more processes to move the system back towards its target activity pattern (Grashow et al., 2010). In principle, two very different classes of mechanisms could result in maintenance of a neuron’s intrinsic membrane properties: (1) a sensor that evaluates a neuron’s firing could provide negative feedback control of the synthesis, insertion, or degradation of ion channels to regulate homeostatically the final activity pattern of the neuron, or (2) the expression or posttranslational processing or modification of several ion channels with “counteracting” influences on activity could be coupled independently of directly assessing activity. Both explanations allow for a balance between the mechanisms that allow for plasticity with those that maintain stability (O’Leary and Marder, 2016; O’Leary et al., 2010; O’Leary et al., 2014).
How precisely must neurons constrain the values of their many membrane conductances so that they can function correctly in the networks in which they are found? How different are neurons of the same class between animals or within a given animal? Work in small networks has shown that similar circuit and neuronal performance can be produced with highly variable sets of synaptic and intrinsic conductances (Goaillard, Taylor, Schulz, and Marder, 2009; Prinz, Bucher, and Marder, 2004). Similar behavior of single neurons can also result from substantially different sets of values of the maximal conductances of their voltage dependent currents (Achard and De Schutter, 2006; Goldman, Golowasch, Marder, and Abbott, 2001; Golowasch, Goldman, Abbott, and Marder, 2002; Grashow et al., 2010; Schulz, Goaillard, and Marder, 2006; Schulz, Goaillard, and Marder, 2007; Sobie, 2009; Swensen and Bean, 2005; Taylor, Goaillard, and Marder, 2009; Tobin and Calabrese, 2006). For example, overexpression of the transient outward current (IA) in lobster stomatogastric ganglion (STG) neurons produced little change in neuronal firing because it triggered compensatory changes in the hyperpolarization-activated inward current (Ih) (MacLean, Zhang, Goeritz, Casey, Oliva, Guckenheimer, and Harris-Warrick, 2005; MacLean, Zhang, Johnson, and Harris-Warrick, 2003). Consistent with these data are results that show strong correlations between the expression of the mRNA encoding Ih and IA in crab STG neurons (Goaillard et al., 2009; Schulz et al., 2006; Schulz et al., 2007). Moreover, theoretical work has argued that similar activity patterns, both at the single neuron level and at the network level, can arise from different combinations of correlated and compensating membrane and synaptic currents. For example, model single-spike bursters with nearly identical voltage trajectories can have maximal conductance ratios of sodium (Na+) to potassium (K+) that vary more than 40-fold (Golowasch et al., 2002). Model networks with very similar triphasic motor patterns can arise from combinations of underlying conductances in the component neurons and their synaptic strengths that vary 20- to 30-fold (Prinz et al., 2004). A biologically plausible model of gene regulation shows how neurons can use a single physiological variable - [intracellular Ca2+] - to robustly control their activity and develop specific electrophysiological properties that enable function at the circuit level (O’Leary et al., 2014). Thus, the intrinsic excitability of single neurons are subject to slow homeostatic regulation that can stabilize neuronal function despite ongoing turnover of channels and receptors (Aizenman, Huang, and Linden, 2003; Davis and Bezprozvanny, 2001; Davis and Goodman, 1998; Desai et al., 1999; Golowasch et al., 1999a; Golowasch et al., 1999b; LeMasson et al., 1993; Liu et al., 1998; MacLean et al., 2003; Turrigiano et al., 1995; Zhang and Linden, 2003). Furthermore, different combinations of conductance densities of voltage-dependent currents and synaptic strengths in networks carrying out the same function in different animals.
The results presented above relate intrinsic plasticity with homeostatic regulation. Since different ion currents are regulated by different mechanisms (sensitivity to voltage, ligands, or intracellular Ca2+) and the response dynamics of each channel differs, changes in the magnitude of one current can be homeostatically compensated for only partially by changes in the magnitude of other ion currents. Yet both for single cells and for the network, in the results reported above, the homeostatic response is adequate to maintain normal neuronal and network activity. This implies that there are many suboptimal solutions, as modeling studies have determined. Defining what is an adequate solution for the network, however, depends on the criteria used and thus adopting more stringent criteria results in fewer solutions (Prinz et al., 2004). Furthermore, this work has mostly been conducted in lobster STG where the size of the network is small, and the precision and complexity of the behavior is relatively low. In what follows we extend these ideas to studies in the bird song system, where the numbers of cells and the complexity and precision of behavior are much higher. Still, ultimately as an adult bird sings it too is using feedback to regulate its vocal output towards a previously acquired target, the song the bird has learned. We examine how IP regulation contributes to this behavior.
3-. Intrinsic plasticity in the song control system
The above examples arise from effects on IP following experimental manipulation of an animal’s behavior using conditioning paradigms or from experimental manipulation of upregulation or downregulation of specific ion currents. Such approaches provide for a powerful methodology to bring strong experimental control over a broad range of behaviors. A complementary approach to the use of operant conditioning is to manipulate feedback related to a given species’ adaptations that arise from its evolutionary history, which is the neuroethological approach.
Birdsong learning is a well-established model for studying the neural representations of vocal learning. Song learning is regulated by auditory feedback, essential for song development in juveniles (Konishi, 1965; 1978) and song maintenance in adults (Nordeen and Nordeen, 1992). The acquisition and production of birdsong occurs through a set of forebrain nuclei that form a well-characterized network, known as the “song system” (Bolhuis, Okanoya, and Scharff, 2010; Ikeda, Trusel, and Roberts, 2020; Margoliash and Schmidt, 2010). Across species, there is beautiful and compelling biological variation in all aspects of developmental and adult song learning, the features of song production and degree of sexual dimorphism, and how song contributes to social interaction. For reasons of technical advantage zebra finches are a preferred target species in electrophysiological studies and thus the description that follows reflects this species bias. Song in zebra finches is a complex behavior that unfolds in a stereotypic fashion over time. In order to learn to sing, juvenile zebra finches require access to adult song during a critical period early in life, optimally through interaction with their father. This is called the sensory learning phase. Starting somewhat later in development but overlapping with the latter stages of sensory learning, zebra finches engage in rehearsals of their own song using auditory feedback to match it to the memorized template. This is referred to as the sensorimotor learning phase. Many individuals ultimately make excellent copies of the father’s song, but there is significant variation across individuals in song copying accuracy (Mooney and Spiro, 1997). Once the song is mastered, a zebra finch normally retains it for the remainder of its life.
One premotor forebrain area in particular, nucleus HVC (used as a proper name), has been extensively studied in controlling the temporal structure of the bird’s song (Amador, Perl, Mindlin, and Margoliash, 2013; Hahnloser, Kozhevnikov, and Fee, 2002; Kozhevnikov and Fee, 2007; Long, Jin, and Fee, 2010; Lynch, Okubo, Hanuschkin, Hahnloser, and Fee, 2016; McCasland, 1987; Picardo, Merel, Katlowitz, Vallentin, Okobi, Benezra, Clary, Pnevmatikakis, Paninski, and Long, 2016; Vu, Mazurek, and Kuo, 1994; Yu and Margoliash, 1996). Different classes of HVC projection neurons project to different nuclei and exhibit different functional and cellular properties (Daou, Ross, Johnson, Hyson, and Bertram, 2013; Kubota and Taniguchi, 1998; Mooney, 2000; Mooney and Prather, 2005). Each class of projection neurons can be subdivided into at least two different morphological classes (Fortune and Margoliash, 1995; Nixdorf, Davis, and DeVoogd, 1989). The HVCRA neurons project via a caudal route to the robust nucleus of the arcopallium (RA) (Fortune and Margoliash, 1995; Vicario, 1991), however there are at least two and possibly more different functional classes (Daliparthi, Tachibana, Cooper, Hahnloser, Kojima, Sober, and Roberts, 2019; Kozhevnikov and Fee, 2007) and morphological classes (Fortune and Margoliash, 1995). The HVCX neurons send primary axons to the basal ganglia component of the song system known as Area X. For HVCX as well there are at least two and possibly more functional classes (Kozhevnikov and Fee, 2007; Rajan, 2018) and morphological classes (Fortune and Margoliash, 1995). To date, the functional and morphological subclasses of HVC projection neurons have yet to be linked. Recently, an additional class of HVC projection neurons that targets both to RA and Area X has been suggested (Kornfeld, Benezra, Narayanan, Svara, Egger, Oberlaender, Denk, and Long, 2017). There is also a small and intriguing class of neurons projecting to the presumptive auditory structure Avalanche (Akutagawa and Konishi, 2010; Roberts, Hisey, Tanaka, Kearney, Chattree, Yang, Shah, and Mooney, 2017). Beyond this complexity of 5–6 classes of projection neurons there are numerous types of neurons that project locally (interneurons). There are at least three different classes of HVCINT based on calcium binding protein reactivity (Wild, Williams, Howie, and Mooney, 2005), and physiological evidence to support three classes of HVCINT (A. Daou, unpublished data). The HVCINT innervate the projecting neurons and during singing show dips in tonic activity closely in synchrony with the phasic bursts of projection neurons (Amador et al., 2013; Kosche, Vallentin, and Long, 2015). Finally, HVC activity in zebra finches is sensitive to behavioral state (Shea and Margoliash, 2010) including singing, relative arousal outside of singing, and neuromodulatory tone (Schmidt and Konishi, 1998; Shea and Margoliash, 2010), and fluctuates depending on sleep (Schmidt and Konishi, 1998). We attempt to capture some of this complexity in Figure 2.
Figure 2: Placing intrinsic properties of HVC neurons in the context of the song system and singing behavior.
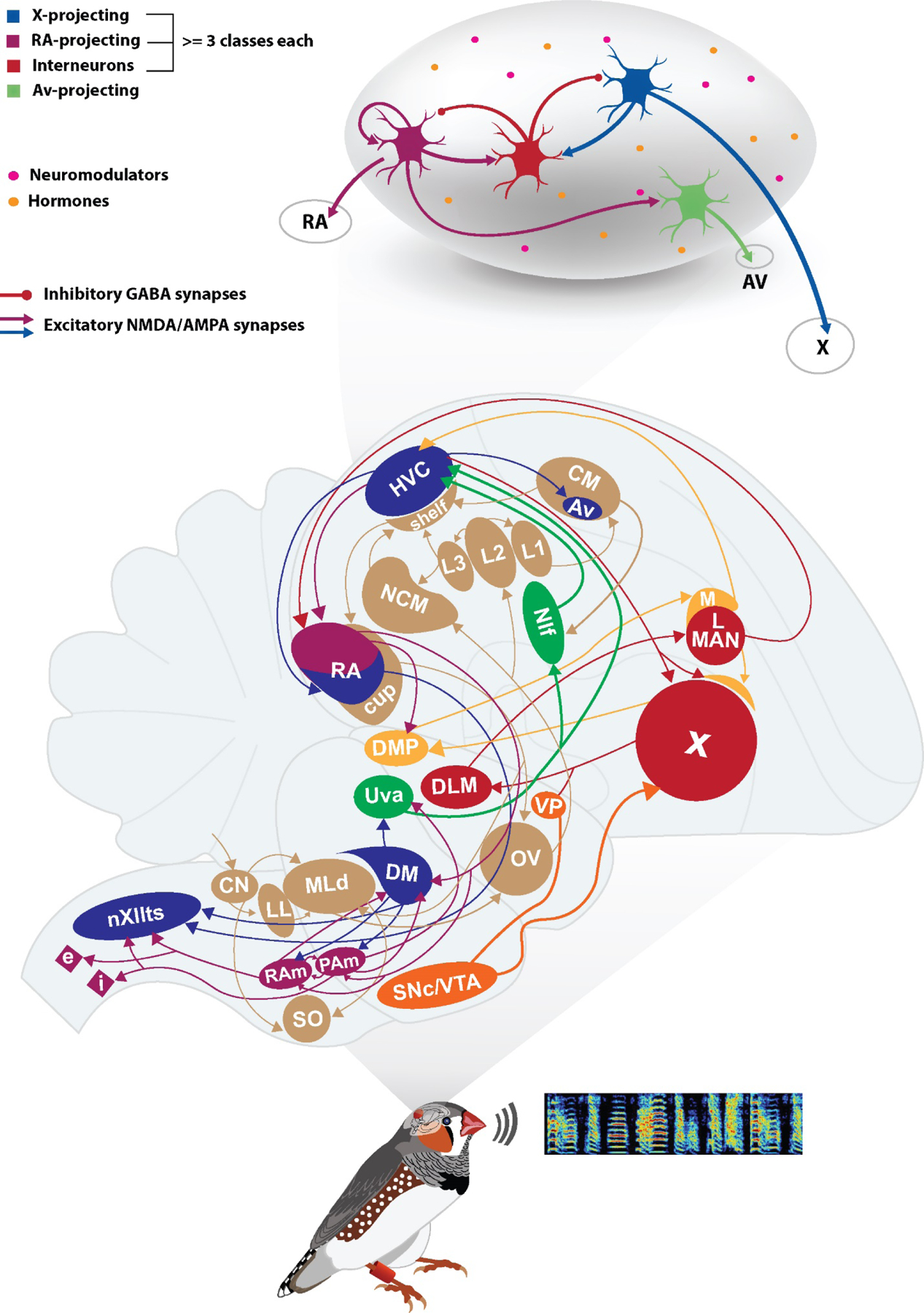
Schematic of a male zebra finch along with a sample spectrogram of a song showing frequency of sound versus time are shown in the lower panel. Reasonably complete song system and auditory system pathways are shown in the middle panel (the cross-hemisphere connections and pathways to SNc/VTA and VP are not illustrated). The vocal motor pathway (VMP, blue color) contains circuits that directly pattern song output. Incoming sensory information is processed by HVC and NIf (green color), and HVC and RA shape motor sequences that project out to the peripheral vocal organs, the syrinx and respiratory muscles, via the hindbrain nucleus nXIIts and brainstem respiratory nuclei RAm and PAm (purple color). In particular, the dorsal part of RA (purple) sends its afferent axons to nuclei DMP, DM, RAm and PAm, while the ventral part of RA (blue) send its axons down to nXIIts. The anterior forebrain loop (AFP, red color) pathway contains circuits that are important for song learning and song variability. HVC sends projections to a basal ganglia loop (striatal-thalamic-cortical-striatal) which has an important output projection to the song motor pathway at nucleus RA. The auditory pathway (light brown color) contains circuits that process sounds, including feedback during singing. Auditory signals enter the brain at the cochlear nucleus (CN) eventually reaching forebrain nuclei such as NCM and CM. Two nuclei that are known to be connected to the song system but are not well understood are medial MAN, which sends its output to HVC, and VTA (orange color). VTA sends dopaminergic inputs to area X. The upper panel shows a schematic of HVC’s internal circuitry. HVC includes multiple types of HVCX neurons that project to area X, HVCRA neurons that projects to nucleus RA, HVC interneurons, and a very sparse population of HVCAv that projects to nucleus Avalanche. The nucleus is bathed with a wide set of hormones and neuromodulators (colored circles). X-projecting and RA-projecting neurons are known to excite interneurons via NMDA and AMPA synapses, while interneurons in their turn inhibit both classes of projecting neurons via GABA synapses. Moreover, HVCRA neurons send excitatory projections onto HVCAV neurons. See Ashmore et al. 2005 for nomenclature and further discussion.
The role of HVC in song learning and production has been extensively modeled (Abarbanel, Gibb, Mindlin, Rabinovich, and Talathi, 2004; Abarbanel, Talathi, Mindlin, Rabinovich, and Gibb, 2004; Amador et al., 2013; Bertram, Daou, Hyson, Johnson, and Wu, 2014; Daou et al., 2013; Drew and Abbott, 2003; Gibb, Gentner, and Abarbanel, 2009a; b; Jin, 2009; Jin, Ramazanoglu, and Seung, 2007; Katahira, Okanoya, and Okada, 2007; Li and Greenside, 2006; Long et al., 2010; Mooney and Prather, 2005; Troyer and Doupe, 2000) and this remains fruitful territory for competing hypotheses (Amador et al., 2013; Lynch et al., 2016; Picardo et al., 2016). HVC is of course embedded in a complex system of pathways that may give rise to numerous sources of feedback to regulate activity during singing (Figure 2). Modeling this activity requires more than the commonly used simplified models of HVC that recognize only three classes of HVC neurons (HVCRA, HVCX, HVCINT) ignoring the complexity of circuitry and functional properties. This arises at least in part from the limited knowledge of HVC circuitry and functional circuitry on which to base detailed models (Kornfeld et al., 2017; Kosche et al., 2015; Lewicki and Konishi, 1995; Mooney, 2000; Mooney and Prather, 2005; Shea, Koch, Baleckaitis, Ramirez, and Margoliash, 2010)
A related limitation is that the biophysical properties of HVC neurons are largely undetermined. Understanding the components of the ion currents of HVC neurons and their contributions to spike generation, in the context of circuitry, should permit for more detailed and biologically realistic models of HVC activity. A number of studies have characterized the electrophysiological properties of the different classes of HVC neurons based on current injection (Daou et al., 2013; Dutar, Vu, and Perkel, 1998; Kubota and Saito, 1991; Kubota and Taniguchi, 1998; Mooney, 2000; Schmidt and Perkel, 1998; Shea et al., 2010). These properties include the resting membrane potential, input resistance, sag, spike duration, spike threshold, AHP amplitude, and AHP time to peak. For example, HVCX neurons exhibit fast and time-dependent inward rectification where a sag appears in response to hyperpolarizing current pulses. To directly examine how ion currents interact to control the cell-specific properties of HVC neurons’ action potentials, a recent study combined pharmacological and electrophysiological approaches (Daou et al., 2013). As well as the fast sodium (INa) and potassium (IK) currents, Daou et al. 2013 also identified a hyperpolarization-activated inward current (Ih) and a low-threshold T-type Ca2+ current (ICa-T) in the HVCX and HVCINT neurons; a Ca2+-activated K+ current (ISK) and an A-type K+ current (IA) in the HVCRA and HVCX neurons, as well as highlighting a possible role for the Na+-dependent K+ current (IKNa) in the HVCX neurons (Figure 3). An important contribution of that study was to develop extended Hodgkin-Huxley models of the different classes of neurons, showing how to model the dynamics of the neurons (as recorded in the in vitro preparation) based on their complement of ion currents.
Figure 3:
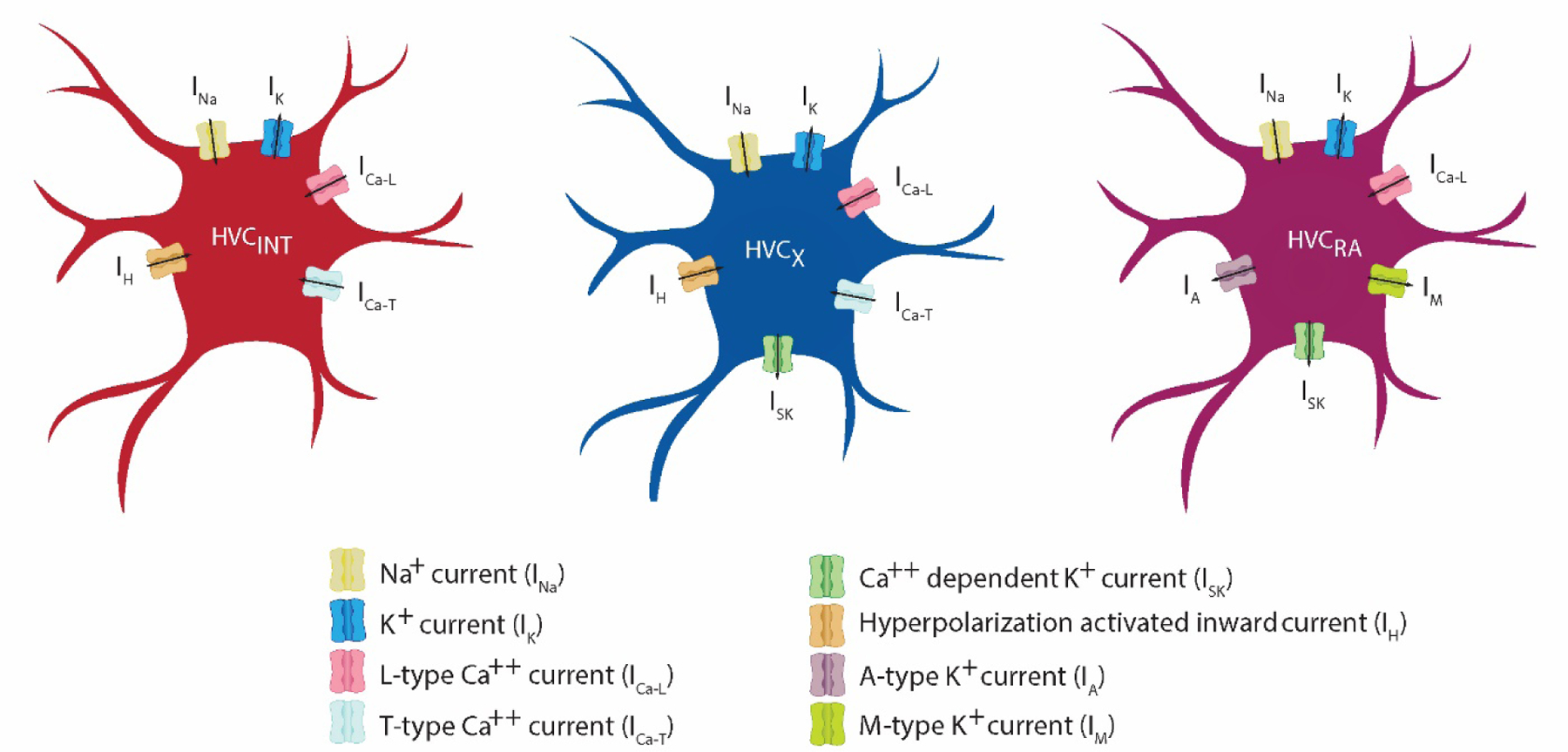
Schematic models depicting the ion currents present in each of 3 HVC model neurons based on the pharmacological identifications conducted in Daou et al (2013) as well as unpublished observations (particularly the expression of M-type K+ current in HVCRA neurons). Similar ion conductances across the 3 classes of HVC neurons are coded with the same colors. This shows indirectly the contributions of the various ion currents to shaping the firing properties of HVC neurons.
Recently we have extended this work. Using intracellular whole-cell current clamp recordings from HVCX neurons in brain slice preparations of adult zebra finches, Daou and Margoliash (2020) unveiled a direct correlation between IE properties and learning in the songbird system. In response to depolarizing current pulses, they observed that the spike trains of HVCX neurons of a given bird all tended to have similar onsets, numbers of spikes, and timing of spikes, whereas these features varied from bird to bird (Fig. 4a). The activity that HVCX neurons transmit to their network was relatively uniform within each bird yet varied considerably from bird to bird. In addition to the “timing features” that showed homogeneity within a single bird and heterogeneity across birds, the spike waveforms/morphology of HVCX neurons also tended to conform to a common shape for a given bird (Fig. 4b), while spike morphology varied from bird to bird (Daou and Margoliash, 2020). These features were retained in slices bathed with a cocktail of synaptic blockers, demonstrating they are intrinsic to the cells. The similarity of IE properties of HVCX within each bird is unanticipated given the degree of variation that these neurons exhibit during singing. For those HVCX that are active during singing, a given neuron reliably emits a given number of short bursts of spikes at precise moments during the song motif, but this varies between 1–4 spike bursts for a given neuron (Kozhevnikov and Fee, 2007). This suggest that the IE properties of HVCX are regulated at the level of individual spike bursts or that there are common features of song shared across all the spike bursts.
Figure 4:
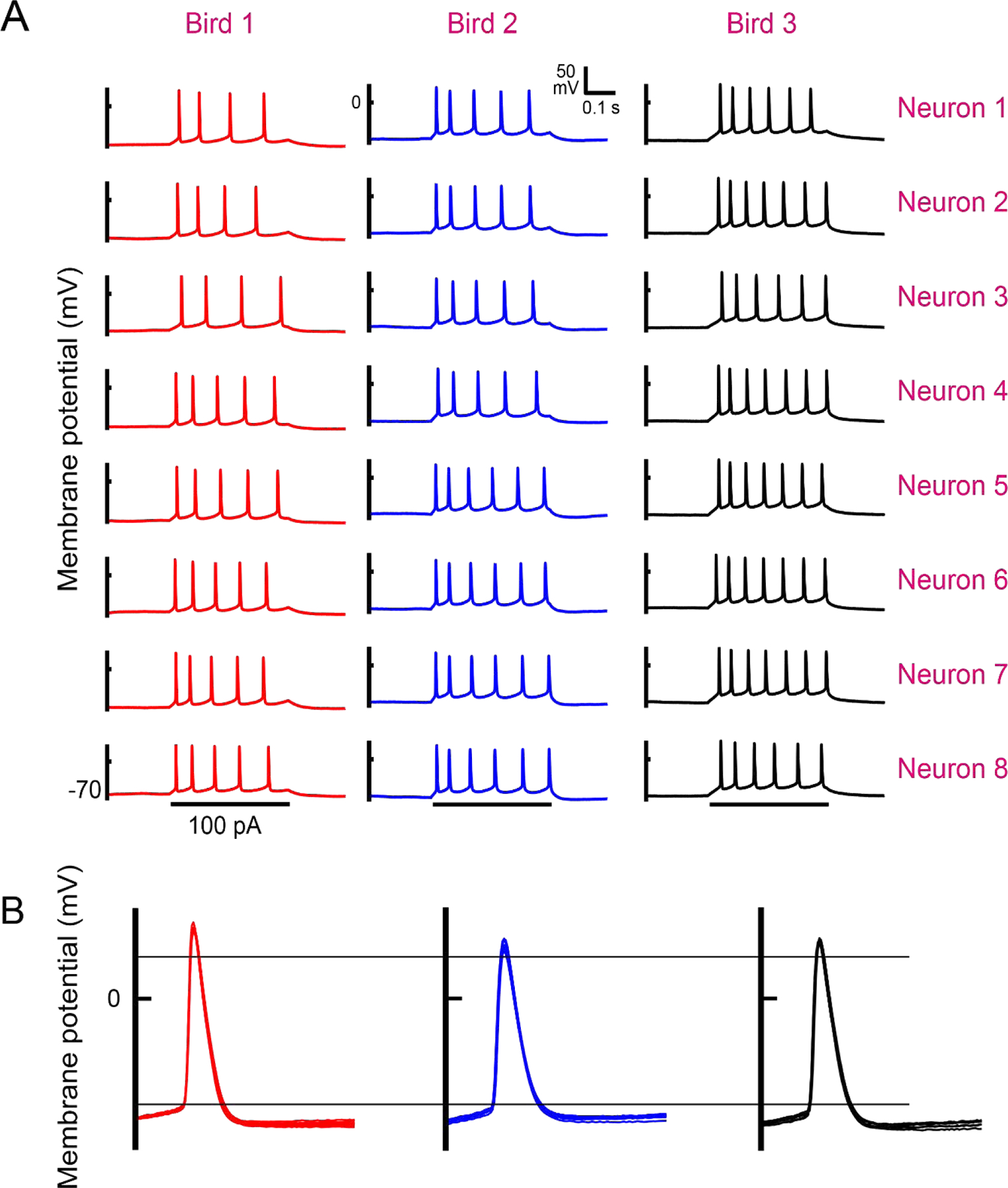
Individual zebra finches exhibit uniform HVCX IP within animal and distinct properties across animals. (A) Eight neurons were recorded in vitro in each of the three birds shown. While there is some variation in the number of spikes elicited from different neurons within a given birds, nevertheless note the striking within-bird homogeneity and across-birds heterogeneity of the spiking patterns (responses to 100 pA, 200 ms depolarizing current pulses). The neurons from a given bird were recorded in experiments ranging over 4–6 hours. (B) The waveforms from the first spikes from the traces in (A) from each of the eight neurons are collapsed on top of each other for each of the three birds. Two fiducial lines have been added to facilitate comparisons. Note there are consistent if subtle differences in the spike height (Bird 1 > Bird 3 > Bird 2), spike threshold (Bird 1 > Bird 2 > Bird 3), and many others (see text). Adopted and extended from (Daou and Margoliash, 2020).
To explore the relation between HVCX dynamics and spike morphology, Daou and Margoliash (2020) modeled the magnitude of five principal ion currents (INa, IK, ISK, ICaT, and Ih) that these neurons express(Daou et al., 2013). As anticipated by the organization observed for the recording traces (raw data), the magnitudes of the modeled currents were similar for cells from each animal and there were large differences between animals. This was observed for the conductances of all the modeled ion channels not just a subset covaried from bird to bird, as has also been reported for STG studies (Golowasch et al., 1999b). Importantly, the differences in the intrinsic ion currents of HVCX in different zebra finches were related to differences in the acoustic features of the birds’ songs (Daou & Margoliash, 2020). This result predicts that birds singing the same song should have overlapping ion current magnitudes, which was confirmed by examining HVCX spike morphology, spike trains, and predicted ion current magnitudes in pairs of adult siblings who learned good copies of their tutor’s song. This and other observations suggest that the IP of HVCX in adult zebra finches is principally driven by learning mechanisms, not genetics.
The relation of HVCX IPs to the bird’s song raises an interesting comparison with results from STG studies. Homologous STG neurons tend to have very similar IPs within a given individual, which varies among individuals (Schulz et al., 2006). Our result for HVCX extends the principle from small numbers of STG neurons to the approximately 20,000 HVCX per HVC. Moreover, in modeling studies of the STG pyloric rhythm, numerous combinations of synaptic strengths and IPs could give rise to similar network activity in the three-cell model that was evaluated. The numbers of successful combinations remained very large even as the criterion for similarity of network activity was made more stringent, suggesting that different individual animals might utilize different cellular solutions to find the same network solution (Prinz et al., 2004). The birdsong result is complementary. As suggested by studies of numerous species where HVC auditory responses are selective for the individual bird’s song (Margoliash (1986), Margoliash et al. (1994)), and by the precise auditory/motor integration observed in the few species studied to date (e.g. Prather, Peters, Nowicki, & Mooney (2010)), the network properties of HVC are variable from bird to bird. (This remains unresolved regarding activity during singing in zebra finches; compare Amador et al., 2013 with Lynch et al. 2016 and Picardo et al. 2016.) The contrasting results from birds singing different songs and from siblings singing the same songs suggests that each unique song hence each unique individual bird, excepting siblings, is expressed as a unique cellular (IP) solution. The differences between the STG and HVC results likely arise from the influence of learning mechanisms in the songbirds, and the highly precise singing of zebra finches. Learning influences song development of all oscine passerine birds studied to date, so future studies examining the IP distribution within HVC of species with more variable songs than zebra finches would help to give insight into these comparative questions.
Relating the IP of the HVCX to learned features of song should help to constrain models of HVC functional organization especially with regard to the temporal structure of song. The hyperpolarization-activated ion current (Ih) is important for orchestrating the timing of central pattern generators. The unique nature of Ih, i.e., an inward current activated on hyperpolarization beyond resting potential, makes it particularly useful in rhythmogenesis (Angstadt and Calabrese, 1989; Golowasch, Buchholtz, Epstein, and Marder, 1992; Thoby-Brisson, Telgkamp, and Ramirez, 2000), and slow oscillations such as observed in thalamus (Budde, Biella, Munsch, and Pape, 1997; McCormick and Pape, 1990; Munsch and Pape, 1999; Pape, 1996). Related, the Ca2+ dependent K+ current contributes to rebound firing of HVC neurons (Daou et al., 2013), and is known in other systems as an ion current for burst generation (Deschenes, Roy, and Steriade, 1982; Fraser and MacVicar, 1991; Friedman and Gutnick, 1987; Gerber, Greene, and McCarley, 1989; Huguenard, 1996; Llinas and Jahnsen, 1982; Llinas and Yarom, 1981a; b; Mulle, Madariaga, and Deschenes, 1986; Wilcox, Gutnick, and Christoph, 1988). During singing a prominent feature of HVC projection neurons (including HVCX) is their bursting (Hahnloser et al., 2002; Kozhevnikov and Fee, 2007). Thus, it may be useful to consider the activity of HVCX not as time keepers (Lynch et al., 2016) but as rhythm generators, a subtle but conceptually important difference. The latter perspective is consistent with the extensive observations linking HVC activity to learned features of song, where songbird siblings who learned the same song from their tutor end up exhibiting the same IP of their HVCx neurons. We note that to date it remains speculation whether the IP organization that Daou and Margoliash (2020) observed for the HVCX, which project to the basal ganglia, will also obtain for the HVCRA, which project to the motor cortex analog RA (n. robustus arcopallium). It has been observed that all classes of HVC neurons showed IP changes over the time course of development (Ross, Flores, Bertram, Johnson, and Hyson, 2017) (see below), suggesting that in adults similar results for HVCX and HVCRA might obtain. If so, this might suggest that HVCRA, emitting one burst per song motif, should be viewed as a degenerate case of a rhythm generator.
The results of Daou and Margoliash (2020) indicate that the tight regulation of IE properties in the songbird system is not contingent upon a single ion current. In other systems the most common mechanisms for IP regulation reported involve a decrease/increase in various types of outward potassium currents following operant training, typically one current per experimental manipulation. At least for zebra finch HVCX, multiple ion currents are involved in regulating the IE properties in a lawful manner, and similarly many ion currents are involved in regulating STG neurons. One possible explanation for this difference is purely an issue of experimental design. In most systems it is experimentally convenient to focus on just one ion current at a time, and the modeling to consider all in context is not commonly attempted. Alternatively, it may be that changes induced by natural behavior (such as homeostasis in STG and learning in songbirds) take advantage of the full complement of intracellular machinery available to induce those changes, whereas operantly induced changes may powerfully induce artificial conditions on nervous system functional organization. Stated otherwise, evolution is an even more powerful shaper of behavior than is operant conditioning, and animals are better at inducing coordinated plastic changes in brain and behavior than are scientists. It is a worthwhile endeavor to try to bridge this gap in experimental, computational, and theoretical approaches in future studies.
4-. Developmental modification and adult regulation of intrinsic plasticity
Over what time scales are HVCX IP regulated? The above considerations as well as prior results (Ross et al., 2017; Ross, Flores, Bertram, Johnson, Wu, and Hyson, 2019) suggest that HVCX should vary over the period of song development. In our study we observed systematic differences between the IP of juvenile and adult HVCX neurons (Daou and Margoliash, 2020). As compared to adults, two features characterized the juvenile neurons. First, neurons from any given juvenile tended to be quite variable. It was particularly compelling that a pair of juvenile siblings failed to show the near-identical properties we had seen in adult siblings. The variability in the juvenile neuronal properties can be seen as reflecting the variability in juvenile plastic singing behavior. For example, our results were collected in 58 – 62 days post hatch zebra finches by which time the birds may be expressing multiple distinct structures in their singing structure (Tchernichovski, Mitra, Lints, and Nottebohm, 2001) albeit not crystalized song. Perhaps different juvenile neurons are reflecting different tendencies in the plastic singing. Second, the variation of neurons from all the juveniles was confined to a small region of the total space occupied by adult birds. While this was a limited data set (four juveniles) provides preliminary evidence suggesting that development of IP in zebra finches follows two processes: reduction in variation among neurons and change in IP properties towards the final adult target.
Prior studies have focused on recording from multiple HVC neuron types, necessarily reducing the yield of recordings from any particular class of HVC neurons. Without large samples sizes of HVCX per animal it is not possible to evaluate the homogeneity of IP observed by Daou and Margoliash (2020). For example, a recent study (Ross et al., 2017) observed differences comparing juvenile and adult HVC neurons. Here, focusing only on their subset of HVCX neurons, Ross et al. (2017) found that during subsong (early in sensorimotor development), HVCX neurons showed no sag response (primarily controlled by the H-current) and no rebound spiking (primarily controlled by T-type Ca++ current) in response to hyperpolarizing current pulses. By the time the birds reached plastic song (later in sensorimotor development), HVCX neurons showed an increased variation in the magnitude of their sag response such that some neurons show little to no sag response and some showed adult-like levels, but still no rebound spiking. Associated modeling studies attributed the changes in spike morphology to ion channel dynamics, in particular to changes in the H- and T-type Ca++ current magnitudes, where the H-conductance appears to be larger for juveniles and the T-type Ca++ conductance appears smaller for juveniles than in adults. Daou and Margoliash (2020) extended these results, observing developmental changes in the magnitudes of fast sodium, potassium, and SK current as well as for H- and T-type Ca++ currents.
In a subsequent study, Ross et al. (2019) investigated whether the observed changes result from learning or are experience-independent developmental changes. They showed that the intrinsic physiology of HVC neurons change as a function of tutor exposure, thereby showing that the intrinsic plasticity seen across development is learning dependent. Their results suggested that 10 days of tutor exposure (from 30 to 40 days post hatch) is sufficient to produce levels of physiological changes similar to those observed in normally raised juveniles. Ross et al. (2019) also showed that tutor deprivation resulted in juvenile HVC neurons showing an adult-like phenotype not present in tutor-exposed juveniles, a result they characterized as counterintuitive. Larger samples of neurons per tutor-deprived animal may help resolve this issue (see (Daou & Margoliash, 2020). Moreover, varying the amount of tutor exposure had a direct effect on the IP of HVC projection neurons, where increased exposure led to larger physiological changes. Ross et al. (2019) found that due to tutor exposure, HVCX neurons exhibited a decrease in their sag ratio and rebound depolarization, characterized by absence of rebound action potentials when the neurons were given negative current pulses. To quantify this further, they fitted manually their biological traces to a biophysical model based on Daou et al (2013). For HVCX neurons, the modelling indicated that the primary effect of tutor exposure was suppressing the Ih and T-type Ca2+ currents.
Maintaining precision of behavior demands adaptive responses to continuous ontogenetic variation in an organism’s biomechanical properties. For zebra finches, this is reflected in the need for feedback to precisely maintain adult song (Nordeen and Nordeen, 1992) in an adaptive fashion (Brainard and Doupe, 2000a; b). Given the variation in IP we had observed in adult animals, this suggested a role of auditory feedback in regulating HVCx IP that is first expressed during juvenile song learning but also continues into adulthood. To explore this hypothesis, Daou and Margoliash (2020) subjected 7 adult zebra finches to continuous delayed auditory feedback (cDAF) during singing. The cDAF manipulation consisted of implanting a small piezoelectric accelerometer attached to the skull, permitting the continuous recording of uncontaminated songs under high amplitude altered feedback. Each accelerometer recording was then used to provide feedback signals, delivered to the bird through a fixed speaker, delayed by a 100 ms. This process is known to induce speech dysfluencies in humans and rapid acoustic alterations in the songs of zebra finches (Fukushima and Margoliash, 2015). A compelling result was that the HVCX of the cDAF adult birds with ≤ 1 day exposure showed enormous variation in IP compared to control adult birds, providing direct evidence that IP in the HVCX of songbirds are dynamically regulated in adult animals in a fashion closely tied to ongoing behavior. While the time course of these changes remains to be characterized, they occur very quickly. In an animal with only 4 hrs exposure to cDAF (the briefest exposure attempted), the spike waveform shapes and the modeled ion current magnitudes had already begun to change. Fitting the data from all 7 animals, the predicted intersect with IP values from normal adults predicted that IP changes would begin within several seconds of singing. This remains to be experimentally confirmed, but it points to the possibility that variation in neuronal IP is an important signal in regulating network dynamics on the time scale of behavioral learning.
Optogenetic and electrical stimulation methods to disrupt the activity of HVC neurons in juvenile zebra finches as they listened to the song of a tutor (Roberts, Gobes, Murugan, Ölveczky, and Mooney, 2012) impaired the quality of song imitation, indicating that the activity patterns of neural activity in this premotor area during auditory experience is critical to subsequent vocal motor learning. Furthermore, blocking NMDA receptors in HVC during tutoring blocked spine enlargement and also impaired vocal imitation of the tutor song, suggesting an important role the NMDA receptors play in strengthening the synapses of HVC to encode the tutor song experience. The findings by Roberts et al. (2012) support the idea that synapses in HVC are important sites for the encoding experience of the tutor song during sensorimotor learning. Prior studies in singing birds show that HVC premotor neurons fire precise bursts of action potentials that are tightly linked to the temporal organization of song (Hahnloser et al., 2002; Long and Fee, 2008), raising the possibility that the same neural machinery that controls the song’s temporal organization in adults is also employed to encode the temporal features of the song model early in juvenile life. Whether these rapid changes in the HVC connectivity observed are due to purely synaptic mechanisms is not clear; however combined with the results of Daou and Margoliash (2020) where cDAF exposure rapidly alters the IP of HVC neurons in vitro (which subsequently have an effect on the HVC circuit dynamics), it is likely that both intrinsic and synaptic mechanisms are being altered in a rapid fashion at the onset of any/specific behavioral or electrophysiological manipulation that targets the HVC circuitry. It is also not clear whether intrinsic changes are a precursor to synaptic changes or vice versa, but given the faster time dynamics that IP exhibits, it is possible that IP changes are a prerequisite for synaptic changes.
Examining changes in dendritic spines and weakening of synaptic input following deafening, Tschida and Mooney (2012) also reported changes in the intrinsic excitability of HVCX neurons post-deafening. They recorded from HVCX neurons in brains slices prepared at one week post deafening, when structural and functional synaptic changes due to deafening were evident. Their analysis revealed that HVCX neurons from deafened birds exhibited several significant changes associated with increased excitability, including increased action potential frequency in response to current injection, decreased action potential duration, decreased after-hyperpolarization time-to-peak and increased resting membrane potential. Moreover, to check whether the changes in intrinsic excitability that were evident in the brain slice recordings could potentially translate changes in synaptic strength to changes in action potential output, they also recorded intracellularly from HVCX neurons in anesthetized male zebra finches and showed that these neurons exhibited a significant decrease in inter-spike intervals (ISIs) and increased mean spontaneous action potential firing rates. Taken together, these results show that the synapses onto and the action potential output of HVCX neurons are sensitive to activity-dependent changes due to experimental manipulations like deafening. Since the onset of behavioral changes following deafening was slow (over days), the design of those studies could not address the relative timing of changes in intrinsic and synaptic properties. This conceptually important issue requires more attention (see also Daou & Margoliash, 2020).
An important conclusion of Ross et al. (2017; 2019) is that HVC IP change over the time course of song development, even extending to the early phases when birds are first exposed to tutor song models. One important distinction that has yet to be resolved is whether the changes seen early in song development result directly from tutor song exposure or whether they result from the motor act of singing or feedback induce by singing following tutor song exposure. Resolving this distinction may help resolve if HVC activity contributes to tutor song representation (Adret, Meliza, and Margoliash, 2012). A prior study of RA neurons found that extracellular activity changed overnight in a tutor song specific manner following first exposure to the tutor song, and these changes could be suppressed as long as a bird was prevented from hearing feedback from his own singing (Shank and Margoliash, 2009). Given the rapid changes observed upon tutor song exposure, active mechanisms for feedback suppression will be necessary to resolve this issue.
5-. An ion current basis for learning and memory
The weight of these studies suggest that IP can be regulated in the context of learning, and that regulation of IP may contribute to learning mechanisms in a fashion distinct from synaptic plasticity. How are changes in IP expressed at the network level, and how does this manifest as novel or additional mechanisms related to learning and memory? And what does it mean for ion currents to be correlated with behavior (Daou & Margoliash, 2020)? Changes in IP can occur in single dendritic compartments (Losonczy, Makara, and Magee, 2008), but here we limit consideration to changes in IP that occur proximal to dendrites (i.e. at the soma or axon hillock). For example, Daou and Margoliash (2020) observed that spike threshold was regulated across individual birds, which is likely to arise from changes in channel density near the spike initiation zone. IP changes at the level of the soma or the axon initial segment should alter the neuron’s total output. The efficacy of all the neuron’s synaptic inputs will be affected, not just a limited set of the total synapses that contribute to activity in the postsynaptic neuron. Thus, somatic regulation of a neuron’s IP will change the neuron’s firing properties and its degree of integration into the network that it is embedded in. This modifies classical descriptions of network integration that emphasized synaptic plasticity (Singer, 1995). Recent studies demonstrate muscarinic cholinergic receptors drive changes in SK currents in L2/3 pyramidal neurons, a possible mechanism linking behavioral state (attention) with the engram (Gill and Hansel, 2020).
Another distinction contrasting intrinsic and synaptic modification is the time course of the changes. A longstanding issue with LTP as a learning correlate has been that LTP requires prolonged and repetitive stimulation for its induction and thus its role as a general cellular correlate for learning remains in doubt (Gallistel and Balsam, 2014; Gallistel and Matzel, 2013). In slice preparations, LTP induction usually requires stimulation in the range of minutes, although there are types of LTP that can be triggered using activation protocols that last 15–20 s (Frick et al., 2004; Salin, Malenka, and Nicoll, 1996). Intrinsic plasticity however can be evoked with very short activation periods. For example, Purkinje cell IE regulation results from synaptic or somatic injection of depolarizing currents with activation periods as short as 3 s (Belmeguenai et al., 2010; Ohtsuki, Piochon, Adelman, and Hansel, 2012). While there may be numerous explanations for these differences in the duration of the activation period, nevertheless the onus remains to explain how LTP mechanisms can contribute to learning in a time scale appropriate to observed behavioral changes. In contrast, IE regulation had been shown to be triggered by single experiences in fear conditioning behavioral experiments in rats, even after a single trial (McKay et al., 2009). Moreover, tetanization protocols conducted on dentate gyrus granule neurons shows that intrinsic plasticity is much more readily induced after mild conditioning than synaptic plasticity that requires much longer and stronger stimuli (Lopez-Rojas et al. (2016), and see above for zebra finches). Thus, intrinsic excitability can be seen as an independent and fast mechanism in learning and memory induction helping to regulate the synaptic penetrance factor of neurons. In contrast, although synaptic plasticity has attracted legions of studies equivalently widespread and compelling results have yet to obtain.
The rapid changes observed for IP and their presumptive somatic origin have significant computational implications. In typical artificial neural networks, nodes in the network (model neurons) are implemented as summing a set of inputs which then pass through a nonlinearity to produce an output. Learning in the network involves algorithms to modify the strength of inputs (“adjusting synaptic weights”), but the form of the nonlinearity is typically not modified. To capture the phenomenon that IP change during learning, in artificial neural networks the nonlinearity itself should be adjusted during learning. Whether such networks are stable, how this might affect the speed and capacity of learning, etc. remains unknown. But these considerations emphasize the unique computational contribution from somatic changes in IE, perhaps as a signal amplifier to boost the response amplitude and lower the spike threshold. A second point is that IP plasticity may occur very rapidly, as or more rapidly than synaptic plasticity. This suggests a learning algorithm as follows: behavioral/network changes in activity levels drive changes in synaptic input, resulting in modification of IP that eventually feedback back through the network to modify synaptic efficacy (i.e. synaptic plasticity). In this new conceptualization, changes in IP lead changes in synapses. This is attractive in that synaptic plasticity still acts as the locus of long–term structural changes that encode learning and memory but without the computational and time–dependent problems that emerge from a purely synaptic view of learning and memory. Perhaps this perspective helps to unify the cellular and circuit views of the engram (Han, Kushner, Yiu, Hsiang, Buch, Waisman, Bontempi, Neve, Frankland, and Josselyn, 2009; Zhou, Won, Karlsson, Zhou, Rogerson, Balaji, Neve, Poirazi, and Silva, 2009).
Finally, the results presented by Daou and Margoliash (2020) motivate comparative studies to assess generality in other species. Apart from the well-known example of birdsong, other birds (e.g. parrots) and some mammals (including cetaceans, some bats, and probably others) also generate complex acoustic sequences that are learned. Occasionally, such as with birdsong, there is insight into the adaptive role of these vocalizations (especially for social behaviors). Perhaps by studying the IP of equivalent basal-ganglia projecting neurons in these species, we might be able to unveil important insights generalizing a relation between ion current expression and behavior.
Acknowledgements:
Supported in part by the National Institutes of Health R56NS094831 and UF1NS115821.
References
- Abarbanel HD, Gibb L, Mindlin GB, Rabinovich MI, & Talathi S (2004). Spike timing and synaptic plasticity in the premotor pathway of birdsong. Biol Cybern, 91, 159–167. [DOI] [PubMed] [Google Scholar]
- Abarbanel HD, Talathi SS, Mindlin G, Rabinovich M, & Gibb L (2004). Dynamical model of birdsong maintenance and control. Phys Rev E Stat Nonlin Soft Matter Phys, 70, 051911. [DOI] [PubMed] [Google Scholar]
- Abbott LF, & Nelson SB (2000). Synaptic plasticity: taming the beast. Nat Neurosci, 3 Suppl, 1178–1183. [DOI] [PubMed] [Google Scholar]
- Achard P, & De Schutter E (2006). Complex parameter landscape for a complex neuron model. PLoS Comput Biol, 2, e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adret P, Meliza CD, & Margoliash D (2012). Song tutoring in presinging zebra finch juveniles biases a small population of higher-order song-selective neurons toward the tutor song. J Neurophysiol, 108, 1977–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman CD, Huang EJ, & Linden DJ (2003). Morphological correlates of intrinsic electrical excitability in neurons of the deep cerebellar nuclei. J Neurophysiol, 89, 1738–1747. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, & Linden DJ (2000). Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci, 3, 109–111. [DOI] [PubMed] [Google Scholar]
- Akutagawa E, & Konishi M (2010). New brain pathways found in the vocal control system of a songbird. J Comp Neurol, 518, 3086–3100. [DOI] [PubMed] [Google Scholar]
- Alaburda A, Russo R, MacAulay N, & Hounsgaard J (2005). Periodic high-conductance states in spinal neurons during scratch-like network activity in adult turtles. J Neurosci, 25, 6316–6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon DL (1984a). Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science, 226, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Alkon DL (1984b). Changes of membrane currents during learning. J Exp Biol, 112, 95–112. [DOI] [PubMed] [Google Scholar]
- Amador A, Perl YS, Mindlin GB, & Margoliash D (2013). Elemental gesture dynamics are encoded by song premotor cortical neurons. Nature, 495, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angstadt JD, & Calabrese RL (1989). A hyperpolarization-activated inward current in heart interneurons of the medicinal leech. J Neurosci, 9, 2846–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aou S, Woody CD, & Birt D (1992). Increases in excitability of neurons of the motor cortex of cats after rapid acquisition of eye blink conditioning. J Neurosci, 12, 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armano S, Rossi P, Taglietti V, & D’Angelo E (2000). Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J Neurosci, 20, 5208–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, & Delaney AJ (2001). Modulation of excitability by alpha-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons. J Neurosci, 21, 6553–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Hosy E, Bengtsson F, Pedroarena CM, Piochon C, Teuling E, He Q, Ohtsuki G, De Jeu MT, Elgersma Y, De Zeeuw CI, Jorntell H, & Hansel C (2010). Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci, 30, 13630–13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram R, Daou A, Hyson RL, Johnson F, & Wu W (2014). Two neural streams, one voice: pathways for theme and variation in the songbird brain. Neuroscience, 277, 806–817. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, & Scharff C (2010). Twitter evolution: converging mechanisms in birdsong and human speech. Nat Rev Neurosci, 11, 747–759. [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, & Adelman JP (2004). Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci, 24, 5301–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, & Johnston D (2007). Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci, 27, 13926–13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, & Doupe AJ (2000a). Auditory feedback in learning and maintenance of vocal behaviour. Nat Rev Neurosci, 1, 31–40. [DOI] [PubMed] [Google Scholar]
- Brainard MS, & Doupe AJ (2000b). Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature, 404, 762–766. [DOI] [PubMed] [Google Scholar]
- Bucher D, Prinz AA, & Marder E (2005). Animal-to-animal variability in motor pattern production in adults and during growth. J Neurosci, 25, 1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Biella G, Munsch T, & Pape HC (1997). Lack of regulation by intracellular Ca2+ of the hyperpolarization-activated cation current in rat thalamic neurones. J Physiol, 503 (Pt 1), 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavier CC, Baxter DA, Clark JW, & Byrne JH (1994). Multiple modes of activity in a model neuron suggest a novel mechanism for the effects of neuromodulators. J Neurophysiol, 72, 872–882. [DOI] [PubMed] [Google Scholar]
- Cudmore RH, & Turrigiano GG (2004). Long-term potentiation of intrinsic excitability in LV visual cortical neurons. J Neurophysiol, 92, 341–348. [DOI] [PubMed] [Google Scholar]
- Daliparthi VK, Tachibana RO, Cooper BG, Hahnloser RH, Kojima S, Sober SJ, & Roberts TF (2019). Transitioning between preparatory and precisely sequenced neuronal activity in production of a skilled behavior. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou A, & Margoliash M (2020). Intrinsic neuronal properties represent song and error in zebra finch vocal learning. Nature Communications. [DOI] [PMC free article] [PubMed]
- Daou A, Ross MT, Johnson F, Hyson RL, & Bertram R (2013). Electrophysiological characterization and computational models of HVC neurons in the zebra finch. J Neurophysiol, 110, 1227–1245. [DOI] [PubMed] [Google Scholar]
- Daoudal G, & Debanne D (2003). Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem, 10, 456–465. [DOI] [PubMed] [Google Scholar]
- Davis GW (2006). Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci, 29, 307–323. [DOI] [PubMed] [Google Scholar]
- Davis GW, & Bezprozvanny I (2001). Maintaining the stability of neural function: a homeostatic hypothesis. Annu Rev Physiol, 63, 847–869. [DOI] [PubMed] [Google Scholar]
- Davis GW, & Goodman CS (1998). Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr Opin Neurobiol, 8, 149–156. [DOI] [PubMed] [Google Scholar]
- Debanne D, & Russier M (2019). The contribution of ion channels in input-output plasticity. Neurobiol Learn Mem, 166, 107095. [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, & Turrigiano GG (1999). BDNF regulates the intrinsic excitability of cortical neurons. Learn Mem, 6, 284–291. [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Roy JP, & Steriade M (1982). Thalamic bursting mechanism: an inward slow current revealed by membrane hyperpolarization. Brain Res, 239, 289–293. [DOI] [PubMed] [Google Scholar]
- Dickman DK, & Davis GW (2009). The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science, 326, 1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Coulter DA, & Alkon DL (1986). Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc Natl Acad Sci U S A, 83, 2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KJ, Sawchuk MA, & Hochman S (2005). Properties of mouse spinal lamina I GABAergic interneurons. J Neurophysiol, 94, 3221–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PJ, & Abbott LF (2003). Model of song selectivity and sequence generation in area HVc of the songbird. J Neurophysiol, 89, 2697–2706. [DOI] [PubMed] [Google Scholar]
- Drion G, O’Leary T, & Marder E (2015). Ion channel degeneracy enables robust and tunable neuronal firing rates. Proc Natl Acad Sci U S A, 112, E5361–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AR, & Kaczorowski CC (2019). Regulation of intrinsic excitability: Roles for learning and memory, aging and Alzheimer’s disease, and genetic diversity. Neurobiol Learn Mem, 164, 107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Vu HM, & Perkel DJ (1998). Multiple cell types distinguished by physiological, pharmacological, and anatomic properties in nucleus HVc of the adult zebra finch. J Neurophysiol, 80, 1828–1838. [DOI] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, & Johnston D (2005). Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h). Nat Neurosci, 8, 1542–1551. [DOI] [PubMed] [Google Scholar]
- Fortune ES, & Margoliash D (1995). Parallel pathways and convergence onto HVc and adjacent neostriatum of adult zebra finches (Taeniopygia guttata). J Comp Neurol, 360, 413–441. [DOI] [PubMed] [Google Scholar]
- Frank CA, Pielage J, & Davis GW (2009). A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron, 61, 556–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DD, & MacVicar BA (1991). Low-threshold transient calcium current in rat hippocampal lacunosum-moleculare interneurons: kinetics and modulation by neurotransmitters. J Neurosci, 11, 2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, & Johnston D (2005). Plasticity of dendritic excitability. J Neurobiol, 64, 100–115. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, & Johnston D (2004). LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci, 7, 126–135. [DOI] [PubMed] [Google Scholar]
- Friedman A, & Gutnick MJ (1987). Low-threshold calcium electrogenesis in neocortical neurons. Neurosci Lett, 81, 117–122. [DOI] [PubMed] [Google Scholar]
- Fukushima M, & Margoliash D (2015). The effects of delayed auditory feedback revealed by bone conduction microphone in adult zebra finches. Sci Rep, 5, 8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR, & Balsam PD (2014). Time to rethink the neural mechanisms of learning and memory. Neurobiol Learn Mem, 108, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR, & Matzel LD (2013). The neuroscience of learning: beyond the Hebbian synapse. Annu Rev Psychol, 64, 169–200. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Kiss L, & Poo M (2000). Enhancement of presynaptic neuronal excitability by correlated presynaptic and postsynaptic spiking. Nat Neurosci, 3, 1018–1026. [DOI] [PubMed] [Google Scholar]
- Gerber U, Greene RW, & McCarley RW (1989). Repetitive firing properties of medial pontine reticular formation neurones of the rat recorded in vitro. J Physiol, 410, 533–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb L, Gentner TQ, & Abarbanel HD (2009a). Brain stem feedback in a computational model of birdsong sequencing. J Neurophysiol, 102, 1763–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb L, Gentner TQ, & Abarbanel HD (2009b). Inhibition and recurrent excitation in a computational model of sparse bursting in song nucleus HVC. J Neurophysiol, 102, 1748–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DF, & Hansel C (2020). Muscarinic Modulation of SK2-Type K(+) Channels Promotes Intrinsic Plasticity in L2/3 Pyramidal Neurons of the Mouse Primary Somatosensory Cortex. eNeuro, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, & du Lac S (2007). Firing properties of GABAergic versus non-GABAergic vestibular nucleus neurons conferred by a differential balance of potassium currents. J Neurophysiol, 97, 3986–3996. [DOI] [PubMed] [Google Scholar]
- Goaillard JM, Taylor AL, Schulz DJ, & Marder E (2009). Functional consequences of animal-to-animal variation in circuit parameters. Nat Neurosci, 12, 1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MS, Golowasch J, Marder E, & Abbott LF (2001). Global structure, robustness, and modulation of neuronal models. J Neurosci, 21, 5229–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Abbott LF, & Marder E (1999a). Activity-dependent regulation of potassium currents in an identified neuron of the stomatogastric ganglion of the crab Cancer borealis. J Neurosci, 19, Rc33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Buchholtz F, Epstein IR, & Marder E (1992). Contribution of individual ionic currents to activity of a model stomatogastric ganglion neuron. J Neurophysiol, 67, 341–349. [DOI] [PubMed] [Google Scholar]
- Golowasch J, Casey M, Abbott LF, & Marder E (1999b). Network stability from activity-dependent regulation of neuronal conductances. Neural Comput, 11, 1079–1096. [DOI] [PubMed] [Google Scholar]
- Golowasch J, Goldman MS, Abbott LF, & Marder E (2002). Failure of averaging in the construction of a conductance-based neuron model. J Neurophysiol, 87, 1129–1131. [DOI] [PubMed] [Google Scholar]
- Grashow R, Brookings T, & Marder E (2010). Compensation for variable intrinsic neuronal excitability by circuit-synaptic interactions. J Neurosci, 30, 9145–9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G, He Q, Wan V, Adelman JP, Ohtsuki G, & Hansel C (2016). Activity-Dependent Plasticity of Spike Pauses in Cerebellar Purkinje Cells. Cell Rep, 14, 2546–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AR, Moore SJ, Spruston N, Tryba AK, & Kaczorowski CC (2016). Brain-derived neurotrophic factor differentially modulates excitability of two classes of hippocampal output neurons. J Neurophysiol, 116, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günay C, & Prinz AA (2010). Model calcium sensors for network homeostasis: sensor and readout parameter analysis from a database of model neuronal networks. J Neurosci, 30, 1686–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedo RJ, & Golowasch J (2006). Ionic mechanism underlying recovery of rhythmic activity in adult isolated neurons. J Neurophysiol, 96, 1860–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, & Fee MS (2002). An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature, 419, 65–70. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, & Stackman RW (2006). Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci, 26, 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood AW, & Marder E (2014). Animal-to-Animal Variability in Neuromodulation and Circuit Function. Cold Spring Harb Symp Quant Biol, 79, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, & Josselyn SA (2009). Selective erasure of a fear memory. Science, 323, 1492–1496. [DOI] [PubMed] [Google Scholar]
- Hansel C, Linden DJ, & D’Angelo E (2001). Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci, 4, 467–475. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Sakmann B, & Feldmeyer D (2009). L2/3 interneuron groups defined by multiparameter analysis of axonal projection, dendritic geometry, and electrical excitability. Cereb Cortex, 19, 951–962. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, & Johnston D (1998). Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci, 18, 3521–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR (1996). Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol, 58, 329–348. [DOI] [PubMed] [Google Scholar]
- Idoux E, Serafin M, Fort P, Vidal PP, Beraneck M, Vibert N, Mühlethaler M, & Moore LE (2006). Oscillatory and intrinsic membrane properties of guinea pig nucleus prepositus hypoglossi neurons in vitro. J Neurophysiol, 96, 175–196. [DOI] [PubMed] [Google Scholar]
- Ikeda MZ, Trusel M, & Roberts TF (2020). Memory circuits for vocal imitation. Curr Opin Neurobiol, 60, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DZ (2009). Generating variable birdsong syllable sequences with branching chain networks in avian premotor nucleus HVC. Phys Rev E, 80, 051902. [DOI] [PubMed] [Google Scholar]
- Jin DZ, Ramazanoglu FM, & Seung HS (2007). Intrinsic bursting enhances the robustness of a neural network model of sequence generation by avian brain area HVC. J Comput Neurosci, 23, 283–299. [DOI] [PubMed] [Google Scholar]
- Johansson F (2019). Intrinsic memory of temporal intervals in cerebellar Purkinje cells. Neurobiol Learn Mem, 166, 107103. [DOI] [PubMed] [Google Scholar]
- Katahira K, Okanoya K, & Okada M (2007). A neural network model for generating complex birdsong syntax. Biol Cybern, 97, 441–448. [DOI] [PubMed] [Google Scholar]
- Kiehn O (1991). Plateau potentials and active integration in the ‘final common pathway’ for motor behaviour. Trends Neurosci, 14, 68–73. [DOI] [PubMed] [Google Scholar]
- Konishi M (1965). The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol, 22, 770–783. [PubMed] [Google Scholar]
- Konishi M (1978). Auditory environment and vocal development in birds. Perception and Experience, 105–118.
- Kornfeld J, Benezra SE, Narayanan RT, Svara F, Egger R, Oberlaender M, Denk W, & Long MA (2017). EM connectomics reveals axonal target variation in a sequence-generating network. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosche G, Vallentin D, & Long MA (2015). Interplay of inhibition and excitation shapes a premotor neural sequence. J Neurosci, 35, 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikov AA, & Fee MS (2007). Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol, 97, 4271–4283. [DOI] [PubMed] [Google Scholar]
- Kubota M, & Saito N (1991). Sodium- and calcium-dependent conductances of neurones in the zebra finch hyperstriatum ventrale pars caudale in vitro. J Physiol, 440, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M, & Taniguchi I (1998). Electrophysiological characteristics of classes of neuron in the HVc of the zebra finch. J Neurophysiol, 80, 914–923. [DOI] [PubMed] [Google Scholar]
- Lechner HA, Baxter DA, Clark JW, & Byrne JH (1996). Bistability and its regulation by serotonin in the endogenously bursting neuron R15 in Aplysia. J Neurophysiol, 75, 957–962. [DOI] [PubMed] [Google Scholar]
- LeMasson G, Marder E, & Abbott LF (1993). Activity-dependent regulation of conductances in model neurons. Science, 259, 1915–1917. [DOI] [PubMed] [Google Scholar]
- Lewicki MS, & Konishi M (1995). Mechanisms underlying the sensitivity of songbird forebrain neurons to temporal order. Proc Natl Acad Sci U S A, 92, 5582–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, & Greenside H (2006). Stable propagation of a burst through a one-dimensional homogeneous excitatory chain model of songbird nucleus HVC. Phys Rev E Stat Nonlin Soft Matter Phys, 74, 011918. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, & Maylie J (2008). SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci, 11, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Golowasch J, Marder E, & Abbott LF (1998). A model neuron with activity-dependent conductances regulated by multiple calcium sensors. J Neurosci, 18, 2309–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, & Jahnsen H (1982). Electrophysiology of mammalian thalamic neurones in vitro. Nature, 297, 406–408. [DOI] [PubMed] [Google Scholar]
- Llinas R, & Yarom Y (1981a). Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol, 315, 549–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, & Yarom Y (1981b). Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol, 315, 569–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, & Fee MS (2008). Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature, 456, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Jin DZ, & Fee MS (2010). Support for a synaptic chain model of neuronal sequence generation. Nature, 468, 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rojas J, Heine M, & Kreutz MR (2016). Plasticity of intrinsic excitability in mature granule cells of the dentate gyrus. Sci Rep, 6, 21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, & Magee JC (2008). Compartmentalized dendritic plasticity and input feature storage in neurons. Nature, 452, 436–441. [DOI] [PubMed] [Google Scholar]
- Luther JA, Robie AA, Yarotsky J, Reina C, Marder E, & Golowasch J (2003). Episodic bouts of activity accompany recovery of rhythmic output by a neuromodulator- and activity-deprived adult neural network. J Neurophysiol, 90, 2720–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GF, Okubo TS, Hanuschkin A, Hahnloser RH, & Fee MS (2016). Rhythmic Continuous-Time Coding in the Songbird Analog of Vocal Motor Cortex. Neuron, 90, 877–892. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Zhang Y, Goeritz ML, Casey R, Oliva R, Guckenheimer J, & Harris-Warrick RM (2005). Activity-independent coregulation of IA and Ih in rhythmically active neurons. J Neurophysiol, 94, 3601–3617. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Zhang Y, Johnson BR, & Harris-Warrick RM (2003). Activity-independent homeostasis in rhythmically active neurons. Neuron, 37, 109–120. [DOI] [PubMed] [Google Scholar]
- Mahon S, & Charpier S (2012). Bidirectional plasticity of intrinsic excitability controls sensory inputs efficiency in layer 5 barrel cortex neurons in vivo. J Neurosci, 32, 11377–11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, & Nicoll RA (1999). Long-term potentiation--a decade of progress? Science, 285, 1870–1874. [DOI] [PubMed] [Google Scholar]
- Marder E (1991). Modifiability of pattern generation. Curr Opin Neurobiol, 1, 571–576. [DOI] [PubMed] [Google Scholar]
- Marder E (1998). From biophysics to models of network function. Annu Rev Neurosci, 21, 25–45. [DOI] [PubMed] [Google Scholar]
- Marder E, Abbott LF, Turrigiano GG, Liu Z, & Golowasch J (1996). Memory from the dynamics of intrinsic membrane currents. Proc Natl Acad Sci U S A, 93, 13481–13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, & Prinz AA (2002). Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays, 24, 1145–1154. [DOI] [PubMed] [Google Scholar]
- Marder E, & Thirumalai V (2002). Cellular, synaptic and network effects of neuromodulation. Neural Netw, 15, 479–493. [DOI] [PubMed] [Google Scholar]
- Margoliash D (1986). Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J Neurosci, 6, 1643–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D, Fortune ES, Sutter ML, Yu AC, Wren-Hardin BD, & Dave A (1994). Distributed representation in the song system of oscines: evolutionary implications and functional consequences. Brain Behav Evol, 44, 247–264. [DOI] [PubMed] [Google Scholar]
- Margoliash D, & Schmidt MF (2010). Sleep, off-line processing, and vocal learning. Brain Lang, 115, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marom S, & Abbott LF (1994). Modeling state-dependent inactivation of membrane currents. Biophys J, 67, 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, & Disterhoft JF (2009). Blocking the BK channel impedes acquisition of trace eyeblink conditioning. Learn Mem, 16, 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, & Gandhi CC (2000). The tractable contribution of synapses and their component molecules to individual differences in learning. Behav Brain Res, 110, 53–66. [DOI] [PubMed] [Google Scholar]
- McCasland JS (1987). Neuronal control of bird song production. J Neurosci, 7, 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, & Pape HC (1990). Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol, 431, 291–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, & Disterhoft JF (2009). Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J Neurophysiol, 102, 2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Oh MM, Galvez R, Burgdorf J, Kroes RA, Weiss C, Adelman JP, Moskal JR, & Disterhoft JF (2012). Increasing SK2 channel activity impairs associative learning. J Neurophysiol, 108, 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R (2000). Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J Neurosci, 20, 5420–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, & Prather JF (2005). The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J Neurosci, 25, 1952–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, & Spiro JE (1997). Bird song: of tone and tempo in the telencephalon. Curr Biol, 7, R289–291. [DOI] [PubMed] [Google Scholar]
- Mozzachiodi R, & Byrne JH (2010). More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci, 33, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Madariaga A, & Deschenes M (1986). Morphology and electrophysiological properties of reticularis thalami neurons in cat: in vivo study of a thalamic pacemaker. J Neurosci, 6, 2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch T, & Pape HC (1999). Modulation of the hyperpolarization-activated cation current of rat thalamic relay neurones by intracellular pH. J Physiol, 519 Pt 2, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, & du Lac S (2005). Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron, 46, 623–631. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, & du Lac S (2003). Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron, 40, 609–620. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, & Adelman JP (2005). SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci, 8, 642–649. [DOI] [PubMed] [Google Scholar]
- Nick TA, & Ribera AB (2000). Synaptic activity modulates presynaptic excitability. Nat Neurosci, 3, 142–149. [DOI] [PubMed] [Google Scholar]
- Nixdorf BE, Davis SS, & DeVoogd TJ (1989). Morphology of Golgi-impregnated neurons in hyperstriatum ventralis, pars caudalis in adult male and female canaries. J Comp Neurol, 284, 337–349. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER, & Morozov A (2003). The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell, 115, 551–564. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, & Nordeen EJ (1992). Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol, 57, 58–66. [DOI] [PubMed] [Google Scholar]
- O’Leary T, & Marder E (2016). Temperature-Robust Neural Function from Activity-Dependent Ion Channel Regulation. Curr Biol, 26, 2935–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary T, van Rossum MC, & Wyllie DJ (2010). Homeostasis of intrinsic excitability in hippocampal neurones: dynamics and mechanism of the response to chronic depolarization. J Physiol, 588, 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary T, Williams AH, Caplan JS, & Marder E (2013). Correlations in ion channel expression emerge from homeostatic tuning rules. Proc Natl Acad Sci U S A, 110, E2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary T, Williams AH, Franci A, & Marder E (2014). Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron, 82, 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, & Disterhoft JF (2003). Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol, 90, 2171–2179. [DOI] [PubMed] [Google Scholar]
- Ohtsuki G, & Hansel C (2018). Synaptic Potential and Plasticity of an SK2 Channel Gate Regulate Spike Burst Activity in Cerebellar Purkinje Cells. iScience, 1, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki G, Piochon C, Adelman JP, & Hansel C (2012). SK2 channel modulation contributes to compartment-specific dendritic plasticity in cerebellar Purkinje cells. Neuron, 75, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olypher AV, & Prinz AA (2010). Geometry and dynamics of activity-dependent homeostatic regulation in neurons. J Comput Neurosci, 28, 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte S, Hasenstaub A, & Callaway EM (2010). Cell type-specific control of neuronal responsiveness by gamma-band oscillatory inhibition. J Neurosci, 30, 2150–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC (1996). Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol, 58, 299–327. [DOI] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, & Davis GW (2001). Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron, 30, 737–749. [DOI] [PubMed] [Google Scholar]
- Paz JT, Mahon S, Tiret P, Genet S, Delord B, & Charpier S (2009). Multiple forms of activity-dependent intrinsic plasticity in layer V cortical neurones in vivo. J Physiol, 587, 3189–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardo MA, Merel J, Katlowitz KA, Vallentin D, Okobi DE, Benezra SE, Clary RC, Pnevmatikakis EA, Paninski L, & Long MA (2016). Population-Level Representation of a Temporal Sequence Underlying Song Production in the Zebra Finch. Neuron, 90, 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, & Mooney R (2010). Persistent representation of juvenile experience in the adult songbird brain. J Neurosci, 30, 10586–10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, & Aizenman CD (2007). Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci, 27, 8268–8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, & De Koninck Y (2002). Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol, 539, 817–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz AA, Bucher D, & Marder E (2004). Similar network activity from disparate circuit parameters. Nat Neurosci, 7, 1345–1352. [DOI] [PubMed] [Google Scholar]
- Rajan R (2018). Pre-Bout Neural Activity Changes in Premotor Nucleus HVC Correlate with Successful Initiation of Learned Song Sequence. J Neurosci, 38, 5925–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, & Wenner P (2007). Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci, 30, 119–125. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Gobes SM, Murugan M, Ölveczky BP, & Mooney R (2012). Motor circuits are required to encode a sensory model for imitative learning. Nat Neurosci, 15, 1454–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Hisey E, Tanaka M, Kearney MG, Chattree G, Yang CF, Shah NM, & Mooney R (2017). Identification of a motor-to-auditory pathway important for vocal learning. Nat Neurosci, 20, 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MT, Flores D, Bertram R, Johnson F, & Hyson RL (2017). Neuronal Intrinsic Physiology Changes During Development of a Learned Behavior. eNeuro, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MT, Flores D, Bertram R, Johnson F, Wu W, & Hyson RL (2019). Experience-Dependent Intrinsic Plasticity During Auditory Learning. J Neurosci, 39, 1206–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, & Barkai E (2003). Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci, 17, 2727–2734. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, & Barkai E (1998). Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci, 10, 1518–1523. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, & Barkai E (2002). Learning-induced enhancement of postsynaptic potentials in pyramidal neurons. J Neurophysiol, 87, 2358–2363. [DOI] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, & Nicoll RA (1996). Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron, 16, 797–803. [DOI] [PubMed] [Google Scholar]
- Santin JM, & Schulz DJ (2019). Membrane Voltage Is a Direct Feedback Signal That Influences Correlated Ion Channel Expression in Neurons. Curr Biol, 29, 1683–1688.e1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautois B, Soffe SR, Li WC, & Roberts A (2007). Role of type-specific neuron properties in a spinal cord motor network. J Comput Neurosci, 23, 59–77. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, & Konishi M (1998). Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci, 1, 513–518. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, & Perkel DJ (1998). Slow synaptic inhibition in nucleus HVc of the adult zebra finch. J Neurosci, 18, 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, Gao Z, Badura A, Ohtsuki G, Amerika WE, Hosy E, Hoebeek FE, Elgersma Y, Hansel C, & De Zeeuw CI (2010). Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron, 67, 618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, & Shi T (1998). Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci, 18, 5498–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, & Marder E (2006). Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci, 9, 356–362. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, & Marder EE (2007). Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci U S A, 104, 13187–13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank SS, & Margoliash D (2009). Sleep and sensorimotor integration during early vocal learning in a songbird. Nature, 458, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Koch H, Baleckaitis D, Ramirez JM, & Margoliash D (2010). Neuron-specific cholinergic modulation of a forebrain song control nucleus. J Neurophysiol, 103, 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, & Margoliash D (2010). Behavioral state-dependent reconfiguration of song-related network activity and cholinergic systems. J Chem Neuroanat, 39, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W (1995). Development and plasticity of cortical processing architectures. Science, 270, 758–764. [DOI] [PubMed] [Google Scholar]
- Sobie EA (2009). Parameter sensitivity analysis in electrophysiological models using multivariable regression. Biophys J, 96, 1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Treviño C, Thoroughman KA, Marder E, & Abbott LF (2001). Activity-dependent modification of inhibitory synapses in models of rhythmic neural networks. Nat Neurosci, 4, 297–303. [DOI] [PubMed] [Google Scholar]
- Sourdet V, Russier M, Daoudal G, Ankri N, & Debanne D (2003). Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J Neurosci, 23, 10238–10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, & Tzounopoulos T (2002). Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci, 22, 10163–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, & Bean BP (2005). Robustness of burst firing in dissociated purkinje neurons with acute or long-term reductions in sodium conductance. J Neurosci, 25, 3509–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AL, Goaillard JM, & Marder E (2009). How multiple conductances determine electrophysiological properties in a multicompartment model. J Neurosci, 29, 5573–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, & Nottebohm F (2001). Dynamics of the vocal imitation process: how a zebra finch learns its song. Science, 291, 2564–2569. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, & Simmers J (1998). Neuromodulatory inputs maintain expression of a lobster motor pattern-generating network in a modulation-dependent state: evidence from long-term decentralization in vitro. J Neurosci, 18, 2212–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Telgkamp P, & Ramirez JM (2000). The role of the hyperpolarization-activated current in modulating rhythmic activity in the isolated respiratory network of mice. J Neurosci, 20, 2994–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR Jr., & Disterhoft JF (1996). Trace eyeblink conditioning in rabbits demonstrates heterogeneity of learning ability both between and within age groups. Neurobiol Aging, 17, 619–629. [DOI] [PubMed] [Google Scholar]
- Titley HK, Brunel N, & Hansel C (2017). Toward a Neurocentric View of Learning. Neuron, 95, 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titley HK, Watkins GV, Lin C, Weiss C, McCarthy M, Disterhoft JF, & Hansel C (2020). Intrinsic Excitability Increase in Cerebellar Purkinje Cells after Delay Eye-Blink Conditioning in Mice. J Neurosci, 40, 2038–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin AE, & Calabrese RL (2006). Endogenous and half-center bursting in morphologically inspired models of leech heart interneurons. J Neurophysiol, 96, 2089–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer TW, & Doupe AJ (2000). An associational model of birdsong sensorimotor learning II. Temporal hierarchies and the learning of song sequence. J Neurophysiol, 84, 1224–1239. [DOI] [PubMed] [Google Scholar]
- Tschida KA, & Mooney R (2012). Deafening drives cell-type-specific changes to dendritic spines in a sensorimotor nucleus important to learned vocalizations. Neuron, 73, 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G (2007). Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol, 17, 318–324. [DOI] [PubMed] [Google Scholar]
- Turrigiano G (2011). Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci, 34, 89–103. [DOI] [PubMed] [Google Scholar]
- Turrigiano G, Abbott LF, & Marder E (1994). Activity-dependent changes in the intrinsic properties of cultured neurons. Science, 264, 974–977. [DOI] [PubMed] [Google Scholar]
- Turrigiano G, LeMasson G, & Marder E (1995). Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. J Neurosci, 15, 3640–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG (2008). The self-tuning neuron: synaptic scaling of excitatory synapses. Cell, 135, 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, & Nelson SB (2000). Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol, 10, 358–364. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, & Nelson SB (2004). Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci, 5, 97–107. [DOI] [PubMed] [Google Scholar]
- Vicario DS (1991). Organization of the zebra finch song control system: II. Functional organization of outputs from nucleus robustus archistriatalis. J Comp Neurol, 309, 486–494. [DOI] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, & Kuo YC (1994). Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci, 14, 6924–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann JM, Marder E, Evans B, & Calabrese RL (1993). The effects of SDRNFLRFamide and TNRNFLRFamide on the motor patterns of the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol, 181, 1–26. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Gutnick MJ, & Christoph GR (1988). Electrophysiological properties of neurons in the lateral habenula nucleus: an in vitro study. J Neurophysiol, 59, 212–225. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Howie GJ, & Mooney R (2005). Calcium-binding proteins define interneurons in HVC of the zebra finch (Taeniopygia guttata). J Comp Neurol, 483, 76–90. [DOI] [PubMed] [Google Scholar]
- Wilhelm JC, & Wenner P (2008). GABAA transmission is a critical step in the process of triggering homeostatic increases in quantal amplitude. Proc Natl Acad Sci U S A, 105, 11412–11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA, Woodin MA, Frankland PW, & Josselyn SA (2014). Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron, 83, 722–735. [DOI] [PubMed] [Google Scholar]
- Yu AC, & Margoliash D (1996). Temporal hierarchical control of singing in birds. Science, 273, 1871–1875. [DOI] [PubMed] [Google Scholar]
- Zhang W, & Linden DJ (2003). The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci, 4, 885–900. [DOI] [PubMed] [Google Scholar]
- Zhang Y, & Golowasch J (2007). Modeling Recovery of Rhythmic Activity: Hypothesis for the role of a calcium pump. Neurocomputing, 70, 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Khorkova O, Rodriguez R, & Golowasch J (2009). Activity and neuromodulatory input contribute to the recovery of rhythmic output after decentralization in a central pattern generator. J Neurophysiol, 101, 372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, & Silva AJ (2009). CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci, 12, 1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]


