Abstract
A significant number of studies invoked diabetes as a risk factor for virus infections, but the issue remains controversial. We aimed to examine whether non-autoimmune diabetes mellitus enhances the risk of virus infections compared with the risk in healthy individuals without non-autoimmune diabetes mellitus. In this systematic review and meta-analysis, we assessed case-control and cohort studies on the association between non-autoimmune diabetes and viruses. We searched PubMed, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, and Web of Science with no language restriction, to identify articles published until February 15, 2021. The main outcome assessment was the risk of virus infection in individuals with non-autoimmune diabetes. We used a random-effects model to pool individual studies and assessed heterogeneity (I2) using the χ2 test on Cochrane’s Q statistic. This study is registered with PROSPERO, number CRD42019134142. Out of 3136 articles identified, we included 68 articles (90 studies, as the number of virus and or diabetes phenotype varied between included articles). The summary OR between non-autoimmune diabetes and virus infections risk were, 10.8(95% CI: 10.3–11.4; 1-study) for SARS-CoV-2; 3.6(95%CI: 2.7–4.9, I2 = 91.7%; 43-studies) for HCV; 2.7(95% CI: 1.3–5.4, I2 = 89.9%, 8-studies;) for HHV8; 2.1(95% CI: 1.7–2.5; 1-study) for H1N1 virus; 1.6(95% CI: 1.2–2.13, I2 = 98.3%, 27-studies) for HBV; 1.5(95% CI: 1.1–2.0; 1-study) for HSV1; 3.5(95% CI: 0.6–18.3 , I2 = 83.9%, 5-studies) for CMV; 2.9(95% CI: 1–8.7, 1-study) for TTV; 2.6(95% CI: 0.7–9.1, 1-study) for Parvovirus B19; 0.7(95% CI: 0.3–1.5 , 1-study) for coxsackie B virus; and 0.2(95% CI: 0–6.2; 1-study) for HGV. Our findings suggest that, non-autoimmune diabetes is associated with increased susceptibility to viruses especially SARS-CoV-2, HCV, HHV8, H1N1 virus, HBV and HSV1. Thus, these viruses deserve more attention from diabetes health-care providers, researchers, policy makers, and stakeholders for improved detection, overall proper management, and efficient control of viruses in people with non-autoimmune diabetes.
Subject terms: Endocrinology, Endocrine system and metabolic diseases, Diabetes, Type 2 diabetes
Introduction
Diabetes mellitus (DM) is one of the most chronic, costly and fast-growing health challenges. In 2019, 463 million adults were estimated to have DM, and this number is expected to rise to 700 million by 2045 worldwide1. There are three main categories of diabetes: type 1 diabetes (T1D), type 2 diabetes (T2D) and gestational diabetes (GDM). T1D results from the destruction of insulin-producing pancreatic islet beta cells and T2D is caused by insufficient insulin production from beta cells, coupled with impaired insulin action in target tissues such as muscle, adipose tissue, and liver (a condition termed insulin resistance)2. GDM is characterized by high blood glucose levels during pregnancy. The prevalence of T2D in particular has markedly increased over the last 50 years in parallel with obesity, accounting for 85–95% of all diagnosed individuals with diabetes. Epidemiological data predict an inexorable and unsustainable increase in global health expenditure attributable to T2D, so disease prevention should be given high priority.
For an effective prevention of T2D associated complications, it is necessary to study and understand the risk factors involved. Multifactorial disorder and several different mechanisms have been implicated in the progression of T2D. The likely etiology is a combination of factors, including age, genetic inheritance, environmental factors, lifestyle, as well as infections which constitute a risk factor for developing T2D3–10. There is growing evidence for an etiological interaction between infectious diseases and T2D, as well as for the bidirectional influence of clinical presentation, spread, and outcomes10, 11. Moreover, a recent investigation suggests an interaction between a virus and ketosis-prone diabetes (KPD)12.
Viral infections seem to be strongly associated with non-autoimmune diabetes as viruses and T2D may coexist in an individual13, 14. Several studies reported that viruses can promote the increased prevalence of T2D15; however, whether T2D can increase the prevalence of viral infection still need to be elucidated. The repercussions associated with their infectivity may trigger T2D complications such as hypoglycemia and ketoacidosis16. An understanding of the complex association between virus infection and non-autoimmune diabetes is necessary for the design and development of novel drug therapies and lifestyle guidelines aimed at the treatment and/or prevention of these life-threatening diseases.
In this study, we assessed whether non-autoimmune diabetes is associated with an increased risk of contracting viruses compared to individuals without non-autoimmune diabetes. Establishing such evidence and defining the most common viruses found in individuals with non-autoimmune diabetes may lead to new paths and concepts for developing novel and specific preventive action and pharmacological treatment approaches of non-autoimmune diabetes-associated complications due to viruses.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard (S1 Table) 17. The protocol was registered in the PROSPERO International prospective register of systematic reviews (registration number: CRD42019134142).
Types of studies
We considered case-control and cohort studies.
Participants
There is no specific filter for population. Individuals with non-autoimmune diabetes and without non-autoimmune diabetes (controls) were included.
Exposure
The exposure considered in this study is non-autoimmune diabetes diagnosed by a physician, or diagnosed based on the measured fasting plasma glucose, oral glucose tolerance test according to WHO criteria, or self-reported based on A1C haemoglobin.
Outcomes
The outcome was defined for any virus infection diagnosed with serological or molecular techniques.
Exclusion criteria
Case reports and secondary analyses, animal studies or studies reporting autoimmune type 1 diabetes outcomes only was excluded. Furthermore, we excluded letters, case reports and case series, review articles, editorials, commentaries, and cross-sectional studies.
Literature search
Systematic literature searches were conducted by a medical librarian at Albert Einstein College of Medicine in the electronic databases PubMed (1947 to February 15, 2021), Embase (1973 to February 15, 2021), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (Ovid) (1991 to February 15, 2021), and Web of Science (1985 to February 15, 2021). For all databases, both controlled vocabulary and text word searches were performed, using the combination of terms “diabetes”, “diabetes mellitus”, “non-autoimmune diabetes mellitus”, “type 2 diabetes mellitus”, “type II diabetes”, “T2D”, “T2DM”, “type 2 DM”, “non-insulin-dependent diabetes”, “NIDDM”, “ketosis-prone diabetes”, “fulminant diabetes”, “virus diseases” and “viral infection” (for the full search strategy see Appendix 1). The search was performed without any geographical limitation but limited to human studies (Appendix 1). Manual hand searches of references from retrieved articles, major journals in the field, and grey literature (e.g., abstracts from scientific proceedings) were also performed to identify any additional relevant articles possibly missed by online indexes.
Two investigators (ELY, CF) independently identified articles and sequentially screen their title and abstracts for eligibility. Then, full texts of articles deemed potentially eligible were retrieved. Further, these investigators independently assessed the eligibility for inclusion in the review based on the inclusion and exclusion criteria. Additional information from study authors was performed to resolve questions about eligibility. Any disagreements between the two investigators were resolved through discussion and by consulting a third review author (LD) if necessary.
Data extraction and management
Three investigators (CF, LD and SFKE) independently extracted data following the methods outlined in the Cochrane Handbook for systematic reviews of interventions. All the extracted data were cross-checked by a fourth investigator (ELY). All the disagreements were resolved through consensus. Data was collected on the first author name, year of publication, study design (case-control or cohort studies), geographical location (population where the study was performed), ethnicity, period of the study, number of the viruses screened, type of virus infection, the diagnostic technique used for the virus detection, diabetes phenotype, sample size (individuals with and without non-autoimmune diabetes), number of subjects infected (individuals with and without non-autoimmune diabetes), age and the gender (S2 Table). Any disagreement between the three investigators was resolved through discussion and by consulting a fourth investigator (ELY) if necessary.
Assessment of methodological quality and data reporting
Three investigators (CF, LD, and SFKE) independently assessed study methodological quality of the included studies. Furthermore, all assessments were independently reviewed by a fourth investigator (ELY) and the disagreements were resolved by a consensus. A score for quality and bias, modified from the Newcastle–Ottawa scale, was used to assess each study. The scale assesses three domains: selection (five points), comparability, (two points), and outcome (three points) for a total score of 10 points. Studies scoring 7–10, 3–6, and 0–3 points were identified as having a low, moderate and high risk of bias respectively (S3 Table). Score disagreements were resolved by consensus and a final agreed-upon rating was assigned to each study.
Data analysis
Study-specific estimates were pooled through a DerSimonian and Laird random-effects meta-analysis model18. The strength of the association was measured with odd ratios with 95% confidence interval (95%CI). We conducted subgroup analyzes according to study design (case-control and cohort studies), ethnicity (African, European, American and Asian) and type of non-autoimmune diabetes (T2D, KPD and gestational diabetes). We examined the robustness of the results with sensitivity analyses including only studies with a low risk of bias. Heterogeneity was evaluated by the χ2 test on Cochrane’s Q statistic which is quantified by I2 values, assuming that I2 values of 25%, 50% and 75% represent low, medium and high heterogeneity respectively 19, 20. The value of H close to 1 is indicative of some homogeneity between studies. We detected the publication bias through a visual inspection of funnel graphics and the Egger test21 with p < 0.10 indicating significant publication bias. To take into account the publication bias identified in the global analyses, we performed adjusted analyses by the Trim-and-fill approach22. The p < 0.05 values were indicative of a significant difference. We performed all the above analyses with the R version 3.5.1 software23.
Results
Study selection and characteristics
Of 3136 articles identified, 2901 remained after the elimination of duplicates. After screening titles and abstracts, we found 2716 articles to be irrelevant and we excluded them. We assessed full texts of the remaining 185 papers for eligibility, of which 117 were excluded. Sixty-eight (68) articles were included in this review for a total of 90 studies analyzed as the number of studied viruses and or diabetes phenotypes varied in between each included article (Fig. S1).
Meta-analysis of the association between non-autoimmune diabetes mellitus and type-specific virus
We included 90 eligible studies10, 12, 24–89 in the meta-analysis for the measurement of the association between non-autoimmune diabetes and type-specific virus infection. Among these 90 studies, 82 (91.1%) were case-control and 8 (8.9%) were cohort studies (Table 1). The studies represented 4 ethnic groups globally: Africans (23.3%), Americans (10.0%), Asians (47.8%), and Europeans (18.9%) (Table 1). Sixty-three (70%) studies had a low risk of bias and 30 (30%) had a moderate risk of bias in their methodological quality. None had a high risk of bias (Table 1).
Table 1.
Characteristics of included studies.
| Characteristics | Number (N) | Percentage (%) |
|---|---|---|
| Risk of bias | ||
| Moderate | 27 | 30 |
| Low | 63 | 70 |
| Total | 90 | 100 |
| Study design | ||
| Case-control | 82 | 91.1 |
| Cohort | 8 | 8.9 |
| Total | 90 | 100 |
| Diagnostic techniques | ||
| Immunoassay | 52 | 57.8 |
| Molecular assay | 12 | 13.3 |
| Molecular assay + immunoassay | 21 | 23.3 |
| Unclear/Not reported | 5 | 5.6 |
| Total | 90 | 100 |
| Type of DM | ||
| T2D | 89 | 98.9 |
| KPD | 1 | 1.1 |
| Total | 90 | 100 |
| Virus type | ||
| CMV | 5 | 5.6 |
| SARS-CoV-2 | 1 | 1.1 |
| Coxsackie B virus | 1 | 1.1 |
| H1N1 Virus | 1 | 1.1 |
| HBV | 27 | 30.0 |
| HCV | 43 | 47.8 |
| HGV | 1 | 1.1 |
| HHV8 | 8 | 8.9 |
| HSV1 | 1 | 1.1 |
| Parvovirus B19 | 1 | 1.1 |
| TTV | 1 | 1.1 |
| Total | 90 | 100 |
| Ethnicity | ||
| Africans | 21 | 23.3 |
| Americans | 9 | 10.0 |
| Asians | 43 | 47.8 |
| European | 17 | 18.9 |
| Total | 90 | 100 |
A total of 11 viruses were reported: HCV (43 studies)10, 24–60, 82, 84, 86, 88, 89, HBV (27 studies)10, 30, 35, 36, 38, 41, 44, 48, 52, 53, 56, 59, 60, 66–74, 82, 84, 85, HHV8 (8 studies)12, 61–65, 81, CMV (5 studies)76–80, SARS-CoV-2 (1 study)83, H1N1 virus (1 study)87, HSV1 (1 study)75, TTV (1 study)37, Parvovirus B19 (1 study)79, coxsackie B (1 study)79 and HGV (1 study)37 (Table 1 ).
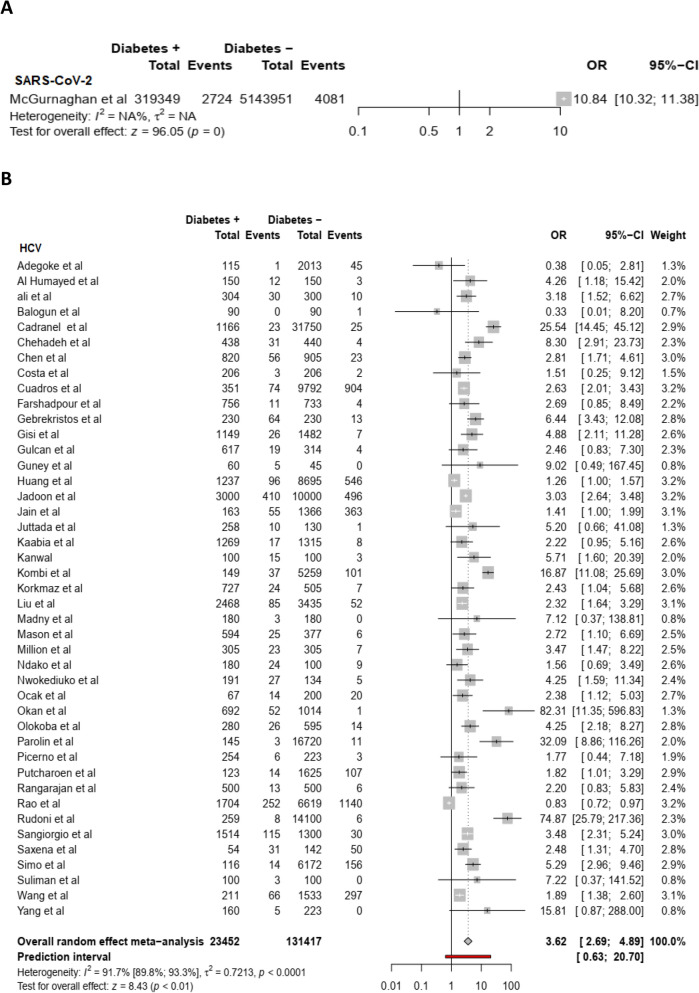
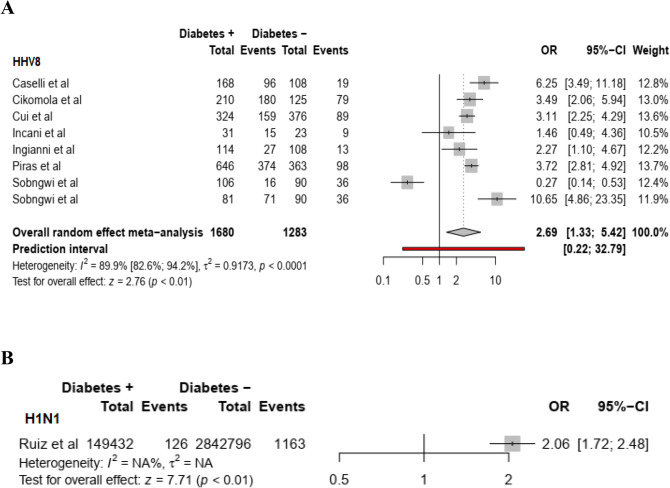
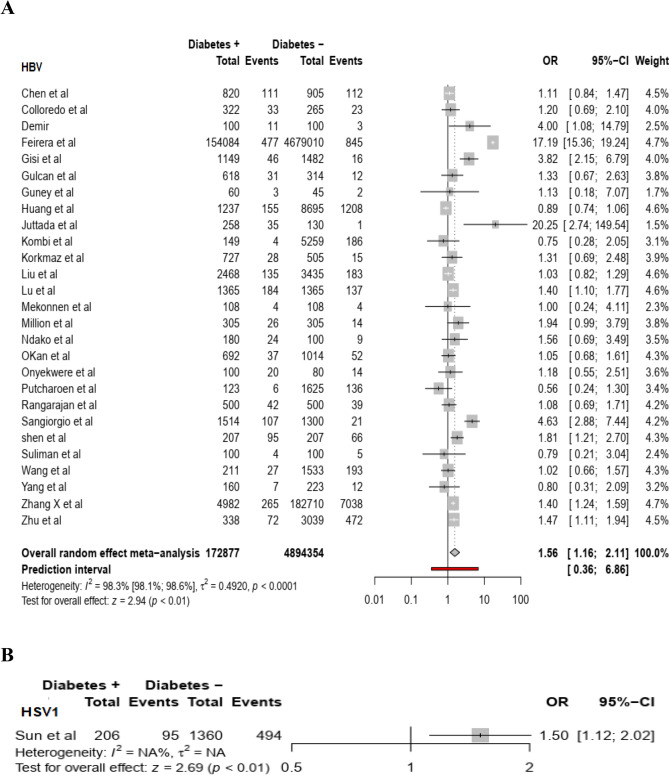
Based on random effect model, individuals with non-autoimmune diabetes were at a higher risk of acquiring SARS-CoV-2 (summary OR = 10.8 (95% CI: 10.3–11.4), HCV (summary OR = 3.6 (95% CI:2.5–4.9, I2 = 0.91.7%), HHV8 (summary OR = 2.7 (95%CI:1.3–5.4; I2 = 89.9), H1N1 virus (summary OR = 2.1(95% CI: 1.7–2.5), HBV (summary OR = 1.6 (95% CI: 1.2–2.1, I2 = 98.3%), and HSV1 (summary OR = 1.5 95% CI: 1.12–2.02) infection compared to individuals without non-autoimmune diabetes [(Fig. 1A,B for COVID-19 and HCV), (Fig. 2A,B for HHV8 and H1N1), (Fig. 3A,B for HBV and HSV1) respectively]. The funnel plot suggests publication bias for HCV infection (S2 Fig.) whereas there was no publication bias for HHV8 (S3 Fig.) and HBV (S4 Fig.) infection. These results were confirmed by the Egger test (Table 2). Furthermore, the OR in studies with a low risk of bias was not different from the overall OR. When the Trim and Fill method was performed, the OR for HCV infection was attenuated but remained statistically significant (OR = 1.9 (95% CI: 1.3–2.8). Substantial heterogeneity was present overall and within all subgroups (Table 2).
Figure 1.
Meta-analysis of the association between SARS-CoV-2 (A), HCV (B) and non-autoimmune diabetes mellitus.
Figure 2.
Meta-analysis of the association between HHV8 (A), H1N1 virus (B) and non-autoimmune diabetes mellitus.
Figure 3.
Meta-analysis of the association between HBV (A), HSV1 (B) and non-autoimmune diabetes mellitus.
Table 2.
Summary statistic of the association between non-autoimmune diabetes mellitus and type-specific virus.
| Virus | OR (95%CI) | 95% Prediction interval | N Studies | N Cases | N Controls | H (95%CI) | I2 (95%CI) | P Heterogeneity | P Egger test |
|---|---|---|---|---|---|---|---|---|---|
| SARS-COV-2 | |||||||||
| Overall | 10.8 [10.3–11.4] | NA | 1 | 319,349 | 5,143,951 | NA | NA | 1 | NA |
| Low risk of bias | 10.8 [10.3–11.4] | NA | 1 | 319,349 | 5,143,951 | NA | NA | 1 | NA |
| HCV | |||||||||
| Overall | 3.6 [2.7–4.9] | [0.6–20.7] | 43 | 23,452 | 131,417 | 3.5 [3.1–3.9] | 91.7 [89.8–93.3] | < 0.001 | 0.001 |
| Low risk of bias | 4.5 [3–6.6] | [0.6–32.4] | 30 | 20,362 | 112,544 | 4.1 [3.7–4.6] | 94.1 [92.5–95.3] | < 0.001 | 0.001 |
| Trim-and-fill adjusted analysis | 1.9 [1.3–2.8] | [0.1–30.5] | 59 | NA | NA | 3.8 [3.5–4.1] | 93.2 [91.9–94.3] | < 0.001 | 0.645 |
| HHV8 | |||||||||
| Overall | 2.7 [1.3–5.4] | [0.2–32.8] | 8 | 1680 | 1283 | 3.2 [2.4–4.2] | 89.9 [82.6–94.2] | < 0.001 | 0.46 |
| Low risk of bias | 2.8 [1.1–6.9] | [0.1–74.6] | 6 | 1439 | 1135 | 3.7 [2.7–4.9] | 92.6 [86.6–95.9] | < 0.001 | 0.564 |
| H1N1 virus | |||||||||
| Overall | 2.1 [1.7–2.5] | NA | 1 | 149,432 | 2,842,796 | NA | NA | 1 | NA |
| Low risk of bias | 2.1 [1.7–2.5] | NA | 1 | 149,432 | 2,842,796 | NA | NA | 1 | NA |
| HBV | |||||||||
| Overall | 1.6 [1.2–2.1] | [0.4–6.9] | 27 | 172,877 | 4,894,354 | 7.8 [7.2–8.4] | 98.3 [98.1–98.6] | < 0.001 | 0.887 |
| Low risk of bias | 1.7 [1.2–2.4] | [0.3–8.4] | 20 | 170,684 | 4,889,141 | 9 [8.2–9.8] | 98.8 [98.5–99] | < 0.001 | 0.749 |
| HSV1 | |||||||||
| Overall | 1.5 [1.1–2] | NA | 1 | 206 | 1360 | NA | NA | 1 | NA |
| CMV | |||||||||
| Overall | 3.3 [0.6–18.3] | [0–1524.7] | 5 | 208 | 179 | 2.5 [1.7–3.7] | 83.9 [63.7–92.9] | < 0.001 | 0.286 |
| Low risk of bias | 6.4 [0.5–77.7] | [0.41–48.4] | 3 | 81 | 105 | 3 [1.8–5] | 89.1 [70.3–96] | < 0.001 | 0.468 |
| TTV | |||||||||
| Overall | 2.9 [1–8.7] | NA | 1 | 60 | 45 | NA | NA | 1 | NA |
| Low risk of bias | 2.9 [1–8.7] | NA | 1 | 60 | 45 | NA | NA | 1 | NA |
| Parvovirus B19 | |||||||||
| Overall | 2.6 [0.7–9.1] | NA | 1 | 83 | 30 | NA | NA | 1 | NA |
| Coxsackie B virus | |||||||||
| Overall | 0.7 [0.3–1.5] | NA | 1 | 83 | 30 | NA | NA | 1 | NA |
| HGV | |||||||||
| Overall | 0.2 [0–6.2] | NA | 1 | 60 | 45 | NA | NA | 1 | NA |
| Low risk of bias | 0.2 [0–6.2] | NA | 1 | 60 | 45 | NA | NA | 1 | NA |
CMV Cytomegalovirus; SARS-CoV-2 Severe Acute Respiratory Syndrome Coronavirus 2; HBV Hepatitis B Virus; HCV Hepatitis C Virus; HGV Hepatitis G Virus; HHV8 Human Herpes Virus 8; HSV1 Herpes Simplex Virus 1; TTV Transfusion Transmitted Virus.
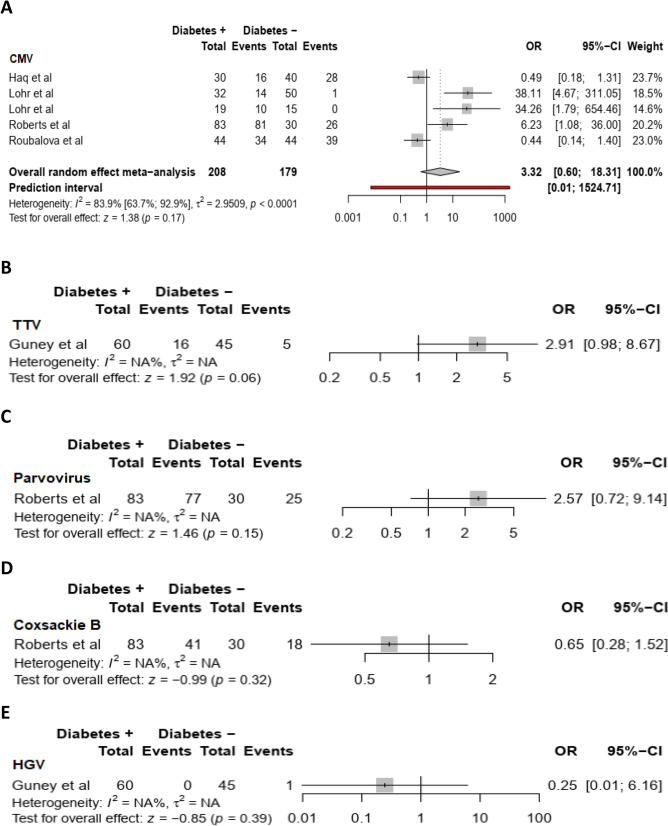
We found the risk for non-autoimmune diabetes individuals to acquire CMV (summary OR = 3.3 (95%CI:0.6–18.3, I2 = 83.9%) (Fig. 4A), TTV (summary OR = 2.9(95% CI: 0.98–8.67) (Fig. 4B), parvovirus B19 (summary OR = 2.6(95% CI: 0.72–9.14) (Fig. 4C), coxsackie B virus (summary OR = 0.7 95% CI: 0.3–1.5) (Fig. 4D) and HGV (summary OR: 0.2 95% CI: 0–6.2) (Fig. 4E) infections to be non-statistically significant compared to healthy individuals without non-autoimmune diabetes.
Figure 4.
Meta-analysis of the association between CMV (A), TTV (B), Parvovirus B19 (C), Coxsackie B virus (D), HGV (E) and non-autoimmune diabetes mellitus.
For CMV infection, the funnel plot did not suggest publication bias (S5 Fig.), and this result was confirmed by the Egger test. The OR in studies with low risk of bias (summary OR = 6.495% CI: 0.5–77.7) was different from the overall OR and substantial heterogeneity was present overall and within all subgroups (Table 2).
Subgroup analysis
With respect to ethnicity, the risk of HCV, HHV8 and HBV infection in individuals with non-autoimmune diabetes was higher in the European population than in other ethnic groups. According to type of non-autoimmune diabetes, the HHV8 infection risk was higher in KPD individuals (summary OR = 10.7 [95% CI: 4.9–23.3]) compared to T2D individuals (summary OR = 2.2 [95% CI: 1.1—4.5]; I2 = 90%). For diagnostic techniques used to detect virus infection, the risk of CMV and HCV infection in individuals with non-autoimmune diabetes was statistically different according to diagnostic techniques (immunoassay alone, molecular assay alone or molecular assay and immunoassay) (S4 Table).
Discussion
This systematic review and meta-analysis aimed to examine the association between non-autoimmune diabetes and the risk of acquiring virus infection compared to the general populations without non-autoimmune diabetes. In this study, we included 90 studies which assessed and compared the risk of virus infection in individuals with or without non-autoimmune diabetes. The results of this meta-analysis indicate an increased risk of SARS-CoV-2, HCV, HHV8, H1N1 virus, HBV, and HSV1 infection, which is statistically significant amongst individuals with non-autoimmune diabetes when compared to individuals without non-autoimmune diabetes.
Strikingly, we found in this study a 3.6-fold higher risk for individuals with non-autoimmune diabetes to acquire HCV infection. These results are consistent with a meta-analysis performed by Guo et al. in 2013 who reported an approximated 3.5-fold increase in HCV infection risk in individuals with T2D90. Indeed, HCV infection seems to be strongly associated with non-autoimmune diabetes. However, the direction of this association is not fully elucidated. A meta-analysis performed in 2012 by Naing et al. reported an excessive T2D risk in HCV-infected individuals91. More specifically, they found an about 1.7-fold increase in T2D risk in HCV infected individuals compared with non-infected individuals91. White et al. in 2009 in a meta-analysis demonstrated an approximately 1.7-fold significant increase in non-autoimmune diabetes risk with HCV infection15. Fabiani et al. in 2018 showed that HCV infection is associated with an increased risk of T2DM independently from the severity of the associated liver disease, in hepatitis C virus infection (CHC) and cirrhotic HCV individuals. As expected T2DM risk is higher in cirrhotic HCV patients than CHC, and the prevalence of HCV infection in T2DM patients is higher than in individuals without non-autoimmune diabetes 92. Thus, the question that arises is: does HCV precede non-autoimmune diabetes or vice-versa? Lim et al. in 2019 demonstrated that chronic hepatitis C patients have profound subcutaneous adipose tissue insulin resistance in comparison with BMI-matched controls and viral eradication improves global, hepatic and adipose tissue insulin sensitivity93, suggesting that HCV probably precedes non-autoimmune diabetes.
In the current meta-analysis, a 1.6- fold increase in HBV infection risk in individuals with non-autoimmune diabetes was reported. Cai et al. performed a meta-analysis in 2015 and found that the OR for the prevalence of diabetes mellitus was 1.33 (95% CI, 1.09–1.62; p = 0.005) between the individuals with and without HBV infection94. Two recent meta-analyses also reported that the summary OR of the risk of T2DM for hepatitis B cirrhosis patients was 1.99 (95% CI, 1.08–3.65) and 1.76 (95% CI: 1.44–2.14) when compared with the non-HBV individuals95, 96. The lower risk of HBV infection in individuals with non-autoimmune diabetes mellitus compared to HCV could be explained partly by the fact that Hepatitis B has been controlled in most countries, through active HBV vaccination programs. Also, the occurrence of chronic HBV and its complications in these countries is very low.
We report in this meta-analysis a 10.8-fold, 2.7-fold, 2.1-fold and 1.5-fold increase in SARS-CoV-2, HHV8, H1N1 virus and HSV1 infection risk respectively when individuals with and without non-autoimmune diabetes were compared. However, the association between non-autoimmune diabetes and these viruses was limited by the lower number of studies eligible for our study and/or the small sample size of the included studies. Though little is known about non-autoimmune diabetes been a risk factor for SARS-CoV-2infection, several studies have indicated COVID-19 severity in patients with diabetes through, increase in ACE-2 and furin expression, impaired T-cell function and increased interleukin-697. Similarly, about a decade ago, diabetes mellitus has been associated with increased severity and hospitalization with H1N1 virus infection 98, 99. HHV8 infection can induce an inflammatory state through the activation of reactive oxygen species and the production of acute-phase proteins which can have a fundamental role in metabolic modification and can lead to the onset of KPD100. It may also influence the pathogenesis of non-autoimmune diabetes either by cytokine secretion or by direct infection of the pancreatic β cell, or both12. Nguewa et al. found that symptomatic HHV-8 infection does not appear to be associated with decreased insulin sensitivity in individuals with non-autoimmune diabetes 101. However, Lontchi-Yimagou et al. in 2018 found that the positivity of HHV8 DNA in individuals with non-autoimmune diabetes was associated with low insulin secretion 102.
In this meta-analysis, we found a low and statistically not significant risk for individuals with non-autoimmune diabetes to acquire TTV, Parvovirus B19, Coxsackie B and HGV infection. Very few studies in the literature assessed the association between non-autoimmune diabetes and these viruses. Nevertheless, some studies report an association between viruses, (in particular enteroviruses) and type 1 diabetes 103–106. In a meta-analysis, Yeung et al., in 2011, reported a significant association between enteroviruses and type 1 diabetes-related autoimmunity and clinical type 1 diabetes 103.
In subgroup analysis according to geographic area, we reported a high risk of HCV, HBV and HHV8 in the European population with non-autoimmune diabetes mellitus. However, this disparity can be due to the lack of epidemiological studies assessing the risk of these virus infections in individuals with non-autoimmune diabetes in other areas, mainly in the African region where the prevalence of non-autoimmune diabetes and virus infection increased drastically during the last decade. For this reason, it is urgent to perform robust epidemiological studies in this region to fill this gap and develop approaches to break the bridge between non-communicable diseases and infectious diseases.
This study provides an insight into the question of whether individuals with non-autoimmune diabetes have an increased susceptibility to virus infections. Additionally, the results of this meta-analysis may have important clinical implications; given the demonstrated increased risk of some virus infection in individuals with non-autoimmune diabetes, it would be important to screen for those virus infections in individuals with non-autoimmune diabetes.
However, the main limitation of our meta-analysis was the substantial heterogeneity among studies which could be explained partially by the variation in the sample size of the primary studies. Moreover, differentiating T1D from T2D at mid-adulthood is a challenge raising the possible misdiagnosis of T1D in the included studies107. Indeed, Atkinson et al. in 2014, reported that about 50% of T1D are misdiagnosed as T2D108. Furthermore, due to the few numbers of some studies assessing the association between virus type infection and non-autoimmune diabetes, we were unable to perform subgroup analysis for those studies. Finally, the heterogeneity in the results could also be explained by the use of different techniques (including, ELISA, PCR, immunofluorescence, RT-qPCR, rapid immunochromatography) to detect the viruses in the included studies.
Viruses have been appealing to explain the increasing prevalence of diabetes, seasonal variation in onset 109, and enhanced susceptibility of trans-migratory populations 110. Indeed, viruses in the context of genetic associations may trigger the development of diabetes following several mechanisms including: direct destruction of pancreatic beta cells, increasing inflammation, increase insulin requirement, increasing insulin resistance, molecular mimicry, increased processing and presentation of autoantigens during infection. Some viruses may directly induce diabetes, while others have an indirect action on the development of diabetes.
The potential mechanisms of non-autoimmune diabetes favoring viral infection include; a hyperglycemic environment that increases the virulence of some pathogens; lower production of interleukins in response to infection; reduced chemotaxis and phagocytic activity, immobilization of polymorphonuclear leukocytes; glycosuria, gastrointestinal and urinary dysmotility. Further studies are required to determine on one hand, whether the risk of virus infection could be prevented or reversed with the improvement of glycemic control in individuals with non-autoimmune diabetes mellitus and on the other hand, whether the eradication of virus infection with appropriate therapy can reduce or abolish the onset of diabetes in these individuals.
Conclusion
This study suggests that non-autoimmune diabetes mellitus is associated with increased susceptibility to viruses especially SARS-CoV-2, HCV, HHV8, H1N1, HBV and HSV1. Thus, these virus infections deserve more attention from diabetes health-care providers, researchers, policy makers, and stakeholders for improved detection, overall proper management, and efficient control of viruses in people with non-autoimmune diabetes mellitus. This systematic review and meta-analysis also suggest the need for more extensive studies that include larger populations. There is sufficient empirical evidence to justify greater research emphasis on the interaction between viruses and non-autoimmune diabetes.
Data availability
Data are available as supplementary material.
Supplementary Information
Acknowledgements
We are grateful to the participants and the researchers of the primary studies identified for this analysis. We extend our thanks to Vanessa Lambou Fopa for her assistance with the literature collection. This work was supported by a grant from the FFRD (Fondation Francophone pour la Recherche sur le Diabète).
Author contributions
E.L.Y., C.F., J.L.N., S.P.C., J.C.M., J.F.G., and E.S. conceived the study and designed the protocol. E.L.Y., C.F., A.L.D.Z. and S.K. conceived the literature search. E.L.Y., C.F., A.L.D.Z. and S.F.K.E. selected the studies. E.L.Y., C.F., A.L.D.Z. and S.F.K.E. extracted the relevant information. S.K. analyzed data. E.L.Y., S.K., and C.F. synthesized and interpreted the data. E.L.Y. and C.F. wrote the first draft of the paper. E.L.Y., C.F., S.K., A.L.D.Z., S.F.K.E., J.L.N., S.P.C., J.C.M., J.F.G. and E.S. critically revised successive drafts of the paper and approved its final version. E.S. supervised the overall work and is the guarantor of the review.
Funding
This work was supported by a grant from FFRD (Fondation Francophone pour la Recherche sur le Diabète). The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eric Lontchi-Yimagou, Email: lontchifrenzy@yahoo.fr.
Eugene Sobngwi, Email: sobngwieugene@yahoo.fr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88598-6.
References
- 1.International Diabetes Federation and IDF Diabetes Atlas, 9th edn. Brussels, Belgium: International Diabetes Federation,. 2019.
- 2.Rojas J, Bermudez V, Palmar J, Martinez MS, Olivar LC, Nava M, et al. Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J. Diabetes Res. 2018;2018:9601801. doi: 10.1155/2018/9601801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunachie S, Chamnan P. The double burden of diabetes and global infection in low and middle-income countries. Trans. R. Soc. Trop. Med. Hyg. 2019;113(2):56–64. doi: 10.1093/trstmh/try124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 5.Toniolo A, Cassani G, Puggioni A, Rossi A, Colombo A, Onodera T, et al. The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev. Med. Microbiol. 2019;30(1):1–17. doi: 10.1097/MRM.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N. Engl. J. Med. 1999;341(25):1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 7.Dryden M, Baguneid M, Eckmann C, Corman S, Stephens J, Solem C, et al. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin. Microbiol. Infect. 2015;21(Suppl 2):S27–32. doi: 10.1016/j.cmi.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. infections in patients with diabetes mellitus. J. Clin. Med. 2019;8(1):1. doi: 10.3390/jcm8010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remais JV, Zeng G, Li G, Tian L, Engelgau MM. Convergence of non-communicable and infectious diseases in low- and middle-income countries. Int. J. Epidemiol. 2013;42(1):221–227. doi: 10.1093/ije/dys135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korkmaz H, Kesli R, Pamuk BO, Ipekci SH, Terzi Y, Kebapcilar L. Assessment of evidence for positive association and seroprevalence of hepatitis B and C in diabetic patients in a developing country. J. Investig. Med. 2015;63(2):251–257. doi: 10.1097/JIM.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 11.Peer N. The converging burdens of infectious and non-communicable diseases in rural-to-urban migrant Sub-Saharan African populations: a focus on HIV/AIDS, tuberculosis and cardio-metabolic diseases. Trop. Dis. Travel Med. Vaccines. 2015;1:6. doi: 10.1186/s40794-015-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobngwi E, Choukem SP, Agbalika F, Blondeau B, Fetita LS, Lebbe C, et al. Ketosis-prone type 2 diabetes mellitus and human herpesvirus 8 infection in sub-saharan africans. JAMA. 2008;299(23):2770–2776. doi: 10.1001/jama.299.23.2770. [DOI] [PubMed] [Google Scholar]
- 13.Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002;36(5 Suppl 1):S1–2. doi: 10.1053/jhep.2002.36992. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Ashour W, Twells L, Valcour J, Randell A, Donnan J, Howse P, et al. The association between diabetes mellitus and incident infections: a systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res. Care. 2017;5(1):e000336. doi: 10.1136/bmjdrc-2016-000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J. Hepatol. 2008;49(5):831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J. Endocrinol. Metab. 2012;16(Suppl 1):S27–36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J. Epidemiol. Commun. Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 21.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R, A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2017.
- 24.Adegoke OA, Kolawole BA, Ikem RT, Adediran A, Aboderin AO, Salawu A. Seroprevalence of hepatitis C virus infection in Nigerians with type 2 diabetes mellitus. Niger. J. Clin. Pract. 2008;11(3):199–201. [PubMed] [Google Scholar]
- 25.Al Humayed SM, Mahfouz AA, Awadalla NJ, Alsabaani AA. Hepatitis C virus infection at primary healthcare level in Abha City, Southwestern Saudi Arabia: Is type 2 diabetes mellitus an associated factor? Int. J. Environ. Res. Public Health. 2018;15(11):1. doi: 10.3390/ijerph15112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali S, Abera S, Mihret A, Abebe T. Association of hepatitis C virus infection with type II diabetes in ethiopia: a hospital-based case-control study. Interdiscip. Perspect. Infect. Dis. 2012;2012:354656. doi: 10.1155/2012/354656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balogun WO, Adeleye JO, Akinlade KS, Kuti M, Otegbayo JA. Low prevalence of hepatitis-C viral seropositivity among patients with type-2 diabetes mellitus in a tertiary hospital. J. Natl Med. Assoc. 2006;98(11):1805–1808. [PMC free article] [PubMed] [Google Scholar]
- 28.Cadranel JF, Di Martino V, Lambrey G, Mourlhon C, Nalet B, Anciaux ML, et al. Prevalence of hepatitis C infection and risk factors in hospitalized diabetic patients: results of a cross-sectional study. Eur J Gastroenterol Hepatol. 2008;20(9):829–836. doi: 10.1097/MEG.0b013e3282fc73a1. [DOI] [PubMed] [Google Scholar]
- 29.Chehadeh W, Kurien SS, Abdella N, Ben-Nakhi A, Al-Arouj M, Almuaili T, et al. Hepatitis C virus infection in a population with high incidence of type 2 diabetes: impact on diabetes complications. J. Infect. Public Health. 2011;4(4):200–206. doi: 10.1016/j.jiph.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Chen HF, Li CY, Chen P, See TT, Lee HY. Seroprevalence of hepatitis B and C in type 2 diabetic patients. J. Chin. Med. Assoc. 2006;69(4):146–152. doi: 10.1016/S1726-4901(09)70195-9. [DOI] [PubMed] [Google Scholar]
- 31.Costa LM, Mussi AD, Brianeze MR, Souto FJ. Hepatitis C as a risk factor for diabetes type 2: lack of evidence in a hospital in central-west Brazil. Braz. J. Infect. Dis. 2008;12(1):24–26. doi: 10.1590/S1413-86702008000100007. [DOI] [PubMed] [Google Scholar]
- 32.Cuadros DF, Miller FD, Nagelkerke N, Abu-Raddad LJ. Association between HCV infection and diabetes type 2 in Egypt: is it time to split up? Ann. Epidemiol. 2015;25(12):918–923. doi: 10.1016/j.annepidem.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Farshadpour F, Taherkhani R, Ravanbod MR, Eghbali SS. Prevalence and genotype distribution of hepatitis C virus infection among patients with type 2 diabetes mellitus. Med. Princ. Pract. 2018;27(4):308–316. doi: 10.1159/000488985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gebrekristos G, Teweldemedhin M, Hagos L, Gebrewahid T, Gidey B, Gebreyesus H. Hepatitis C virus infections and associated risk factors in patients with diabetes mellitus; case control study in North West Tigray, Ethiopia. BMC Res. Notes. 2018;11(1):873. doi: 10.1186/s13104-018-3983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gisi K, Cetinkaya A, Ozkaya M, Kantarceken B, Gisi G, Koroglu S. Hepatitis B and C seroprevalence in patients with diabetes mellitus and its relationship with microvascular complications. Przeglad Gastroenterologiczny. 2017;12(2):105–110. doi: 10.5114/pg.2016.64748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gulcan A, Gulcan E, Toker A, Bulut I, Akcan Y. Evaluation of risk factors and seroprevalence of hepatitis B and C in diabetic patients in Kutahya, Turkey. . J. Investig. Med. 2008;56(6):858–863. doi: 10.2310/JIM.0b013e3181788d28. [DOI] [PubMed] [Google Scholar]
- 37.Guney C, Kadayifci A, Savas MC, Uygun A, Balkan A, Kubar A. Frequency of hepatitis G virus and transfusion-transmitted virus infection in type II diabetes mellitus. Int. J. Clin. Pract. 2005;59(2):206–209. doi: 10.1111/j.1742-1241.2004.00288.x. [DOI] [PubMed] [Google Scholar]
- 38.Huang JF, Dai CY, Hwang SJ, Ho CK, Hsiao PJ, Hsieh MY, et al. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am. J. Gastroenterol. 2007;102(6):1237–1243. doi: 10.1111/j.1572-0241.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 39.Jadoon NA, Shahzad MA, Yaqoob R, Hussain M, Ali N. Seroprevalence of hepatitis C in type 2 diabetes: evidence for a positive association. Virol. J. 2010;7:1. doi: 10.1186/1743-422X-7-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain MK, Aragaki C, Fischbach L, Gibson S, Arora R, May L, et al. Hepatitis C is associated with type 2 diabetes mellitus in HIV-infected persons without traditional risk factors. HIV Med. 2007;8(8):491–497. doi: 10.1111/j.1468-1293.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 41.Juttada U, Smina TP, Kumpatla S, Viswanathan V. Seroprevalence and risk factors associated with HBV and HCV infection among subjects with type 2 diabetes from South India. Diabetes Res. Clin. Pract. 2019;153:133–137. doi: 10.1016/j.diabres.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Kaabia N, Ben Jazia E, Slim I, Fodha I, Hachfi W, Gaha R, et al. Association of hepatitis C virus infection and diabetes in central Tunisia. World J. Gastroenterol. 2009;15(22):2778–2781. doi: 10.3748/wjg.15.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanwal N, Nasir B, Abrar MA, Kaukab I, Nawaz A, Nisar N, et al. Prevalence of hepatitis C in diabetic patients: a prospective study. Acta Pol. Pharm. 2016;73(3):771–775. [PubMed] [Google Scholar]
- 44.Kombi PK, Agasa SB, Mukonkole JPM, Bome LB, Bokele CA, Tshilumba CK. Seroprevalence of hepatitis B and C virus infections among diabetic patients in Kisangani (North-eastern Democratic Republic of Congo) Pan. Afr. Med. J. 2018;31:160. doi: 10.11604/pamj.2018.31.160.17176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason AL, Lau JY, Hoang N, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 46.Nwokediuko SC, Oli JM. Hepatitis C virus infection in Nigerians with diabetes mellitus. Niger. J. Clin. Pract. 2008;11(2):94–99. [PubMed] [Google Scholar]
- 47.Ocak S, Duran N, Kaya H, Emir I. Seroprevalence of hepatitis C in patients with type 2 diabetes mellitus and non-diabetic on haemodialysis. Int. J. Clin. Pract. 2006;60(6):670–674. doi: 10.1111/j.1368-5031.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 48.Okan V, Araz M, Aktaran S, Karsligil T, Meram I, Bayraktaroglu Z, et al. Increased frequency of HCV but not HBV infection in type 2 diabetic patients in Turkey. Int. J. Clin. Pract. 2002;56(3):175–177. [PubMed] [Google Scholar]
- 49.Olokoba AB, Badung LH, Abdulrahman MB, Salawu FK, Danburam A, Aderibigbe S, et al. Hepatitis C virus infection in Nigerians with diabetes mellitus . Am. J. Sci. Ind. Res. 2010;1(2):135–138. [Google Scholar]
- 50.Parolin MB, Rea R, Vargas RM, de Almeida AC, Baldanzi GR, Lopes RW. Prevalence of hepatitis C infection in patients with type 2 diabetes mellitus. Arq. Gastroenterol. 2006;43:77–80. doi: 10.1590/S0004-28032006000200003. [DOI] [PubMed] [Google Scholar]
- 51.Picerno I, Di Pietro A, Spataro P, Di Benedetto A, Romano G, Scoglio ME. Is diabetes mellitus a risk factor for HCV infection? Ann Ig. 2002;14(6):473–477. [PubMed] [Google Scholar]
- 52.Putcharoen O, Wattanachanya L, Sophonphan J, Siwamogsatham S, Sapsirisavat V, Gatechompol S, et al. New-onset diabetes in HIV-treated adults: predictors, long-term renal and cardiovascular outcomes. AIDS. 2017;31(11):1535–1543. doi: 10.1097/QAD.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 53.Rangarajan J, Somanathan GC, Ramesh R, Varghese F, Jayachandran S, Vijayarajan K. A study of seroprevalence of hepatitis B and C in type 2 Diabetes Patients in A Tertiary Care Teaching Institute in South India. Int. J. Contemp. Med. Res. 2016;3(7):2111–2114. [Google Scholar]
- 54.Rao GA, Pandya PK. Statin therapy improves sustained virologic response among diabetic patients with chronic hepatitis C. Gastroenterology. 2011;140(1):144–152. doi: 10.1053/j.gastro.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 55.Rudoni S, Petit JM, Bour JB, Aho LS, Castaneda A, Vaillant G, et al. HCV infection and diabetes mellitus: influence of the use of finger stick devices on nosocomial transmission. Diabetes Metab. 1999;25(6):502–505. [PubMed] [Google Scholar]
- 56.Sangiorgio L, Attardo T, Gangemi R, Rubino C, Barone M, Lunetta M. Increased frequency of HCV and HBV infection in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2000;48(2):147–151. doi: 10.1016/S0168-8227(99)00135-7. [DOI] [PubMed] [Google Scholar]
- 57.Saxena AK, Panhotra BR. The susceptibility of patients with type-2 diabetes to hepatitis C virus infection during long-term haemodialysis. Swiss. Med. Wkly. 2003;133(45–46):611–618. doi: 10.4414/smw.2003.10402. [DOI] [PubMed] [Google Scholar]
- 58.Simo R, Hernandez C, Genesca J, Jardi R, Mesa J. High prevalence of hepatitis C virus infection in diabetic patients. Diabetes Care. 1996;19(9):998–1000. doi: 10.2337/diacare.19.9.998. [DOI] [PubMed] [Google Scholar]
- 59.Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Community-based study of hepatitis C virus infection and type 2 diabetes: an association affected by age and hepatitis severity status. Am. J. Epidemiol. 2003;158(12):1154–1160. doi: 10.1093/aje/kwg259. [DOI] [PubMed] [Google Scholar]
- 60.Yang SQ, Chen HS, Jiang D, Wei L, Ji LN, Wang Y. Relationship between chronic hepatitis C and type II diabetes mellitus. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2003;17(1):46–49. [PubMed] [Google Scholar]
- 61.Caselli E, Rizzo R, Ingianni A, Contini P, Pompei R, Di Luca D. High prevalence of HHV8 infection and specific killer cell immunoglobulin-like receptors allotypes in Sardinian patients with type 2 diabetes mellitus. J Med Virol. 2014;86(10):1745–1751. doi: 10.1002/jmv.23771. [DOI] [PubMed] [Google Scholar]
- 62.Cikomola JC, Vandepoele K, Katchunga PB, Kishabongo AS, Padalko EY, Speeckaert MM, et al. The association between fructosamine-3 kinase 900C/G polymorphism, transferrin polymorphism and human herpesvirus-8 infection in diabetics living in South Kivu. Acta Trop. 2016;163:14–19. doi: 10.1016/j.actatropica.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Cui M, Fang Q, Zheng J, Shu Z, Chen Y, Fan Y, et al. Kaposi's sarcoma-associated herpesvirus seropositivity is associated with type 2 diabetes mellitus: a case-control study in Xinjiang, China. . Int J Infect Dis. 2019;80:73–79. doi: 10.1016/j.ijid.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Ingianni F, Carta A, Reina M, Manai A, Desogus R, Pompei, Prevalence of HHV8 in type 2 diabetes mellitus patients. Am. J. Infect. Dis. 2007;3(3):123–128. doi: 10.3844/ajidsp.2007.123.127. [DOI] [Google Scholar]
- 65.Piras E, Madeddu MA, Palmieri G, Angius F, Contini P, Pompei R, et al. High prevalence of human herpesvirus 8 infection in diabetes type 2 patients and detection of a new virus subtype. Adv. Exp. Med. Biol. 2017;973:41–51. doi: 10.1007/5584_2016_73. [DOI] [PubMed] [Google Scholar]
- 66.Colloredo Mels G, Bettale G, Bellati G, Guanziroli M, Tiraboschi L, Angeli G, et al. Role of hepatitis B virus infection in chronic liver disease of diabetic patients: a case-control study. Acta Diabetol. Lat. 1986;23(1):29–34. doi: 10.1007/BF02581351. [DOI] [PubMed] [Google Scholar]
- 67.Demir M, Serin E, Gokturk S, Ozturk NA, Kulaksizoglu S, Ylmaz U. The prevalence of occult hepatitis B virus infection in type 2 diabetes mellitus patients. Eur. J. Gastroenterol. Hepatol. 2008;20(7):668–673. doi: 10.1097/MEG.0b013e3282f55e1e. [DOI] [PubMed] [Google Scholar]
- 68.Ferreira GLC, Marano C, De Moerlooze L, Guignard A, Feng Y, El Hahi Y, et al. Incidence and prevalence of hepatitis B in patients with diabetes mellitus in the UK: A population-based cohort study using the UK Clinical Practice Research Datalink. J. Viral Hepatitis. 2018;25(5):571–580. doi: 10.1111/jvh.12841. [DOI] [PubMed] [Google Scholar]
- 69.Lu J, Hou X, Tu H, Tang Z, Xiang Y, Bao Y, et al. Chronic hepatitis B virus infection status is more prevalent in patients with type 2 diabetes. J. Diabetes Investig. 2017;8(4):619–625. doi: 10.1111/jdi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mekonnen D, Gebre-Selassie S, Fantaw S, Hunegnaw A, Mihret A. Prevalence of hepatitis B virus in patients with diabetes mellitus: a comparative cross sectional study at Woldiya General Hospital, Ethiopia. . Pan. Afr. Med. J. 2014;17:40. doi: 10.11604/pamj.2014.17.40.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onyekwere CA, Anomneze EE, Wali SS. Prevalence of serological markers of chronic hepatitis B virus infection in diabetics in the Lagos University Teaching Hospital, Lagos. . Niger. Postgrad. Med. J. 2002;9(3):129–133. [PubMed] [Google Scholar]
- 72.Shen Y, Zhang J, Cai H, Shao JG, Zhang YY, Liu YM, et al. Identifying patients with chronic hepatitis B at high risk of type 2 diabetes mellitus: a cross-sectional study with pair-matched controls. BMC Gastroenterol. 2015;15:32. doi: 10.1186/s12876-015-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Zhu X, Ji Y, Li H, Hou F, Xiao C, et al. Increased risk of hepatitis B virus infection amongst individuals with diabetes mellitus. Biosci. Rep. 2019;39(3):1. doi: 10.1042/BSR20181715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu HJ, Wang YY, Yu LG, Xu YF, Zhou H, Ding Y, et al. Serological and molecular analysis on the relationships between type 2 diabetes mellitus and hepatitis B virus infection. J. Infect. Dev. Ctries. 2016;10(8):837–844. doi: 10.3855/jidc.7656. [DOI] [PubMed] [Google Scholar]
- 75.Sun YH, Pei WD, Wu YJ, Yang YJ. An association of Herpes simplex virus type 1 infection with type 2 diabetes. Diabetes Care. 2005;28(2):435–436. doi: 10.2337/diacare.28.2.435. [DOI] [PubMed] [Google Scholar]
- 76.Haq K, Fulop T, Tedder G, Gentleman B, Garneau H, Meneilly GS, et al. Cytomegalovirus seropositivity predicts a decline in the T cell but not the antibody response to influenza in vaccinated older adults independent of type 2 diabetes status. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72(9):1163–1170. doi: 10.1093/gerona/glw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lohr JM, Oldstone MB. Detection of cytomegalovirus nucleic acid sequences in pancreas in type 2 diabetes. Lancet. 1990;336(8716):644–648. doi: 10.1016/0140-6736(90)92145-8. [DOI] [PubMed] [Google Scholar]
- 78.Lohr M, Bergstrome B, Maekawa R, Oldstone MBA, Kloppel G. Human cytomegalovirus in the pancreas of patients with type-2 diabetes - is there a relation to clinical-features, messenger-rna and protein expression of insulin, somatostatin, and MHC class-II. Virchows Archiv. Pathol. Anat. Histopathol. 1992;421(5):371–378. doi: 10.1007/BF01606908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roberts BW, Cech I. Association of type 2 diabetes mellitus and seroprevalence for cytomegalovirus. South Med. J. 2005;98(7):686–692. doi: 10.1097/01.SMJ.0000163310.12516.2D. [DOI] [PubMed] [Google Scholar]
- 80.Roubalova K, Broz J, Hruba D, Hyblova M, Kraml P. Prevalence of active infection with Chlamydia pneumoniae and human cytomegalovirus in patients with type II diabetes mellitus. Folia Microbiol. (Praha) 2007;52(3):287–290. doi: 10.1007/BF02931311. [DOI] [PubMed] [Google Scholar]
- 81.Incani A, Marras L, Serreli G, Ingianni A, Pompei R, Deiana M, et al. Human Herpesvirus 8 infection may contribute to oxidative stress in diabetes type 2 patients. BMC Res. Notes. 2020;13(1):75. doi: 10.1186/s13104-020-4935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y, Ye S, Xiao X, Zhou T, Yang S, Wang G, et al. Association of diabetes mellitus with hepatitis B and hepatitis C virus infection: evidence from an epidemiological study. Infect. Drug. Resist. 2019;12:2875–2883. doi: 10.2147/IDR.S218536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McGurnaghan SJ, Weir A, Bishop J, Kennedy S, Blackbourn LAK, McAllister DA, et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Million Y, Teklu T, Alemu S, Ferede A, Belachew T, Desta K. Hepatitis B and hepatitis C viral infections and associated factors among patients with diabetes visiting gondar referral teaching hospital, Northwest Ethiopia: a comparative cross-sectional study. J. Hepatocell Carcinoma. 2019;6:143–150. doi: 10.2147/JHC.S222609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ndako JA, Nwankiti OO, Olorundare JO, Ojo SKS, Okolie CE, Olatinsu O, et al. Studies on the serological markers for hepatitis B virus infection among type 2 diabetic patients. J. Clin. Lab. Anal. 2021;35(1):e23464. doi: 10.1002/jcla.23464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ndako JA, Owolabi AO, Olisa JA, Akinwumi JA, Dojumo VT, Olatinsu O, et al. Studies on the prevalence of Hepatitis C virus infection in diabetic patients attending a tertiary health-care facility South-west Nigeria. BMC Infect. Dis. 2020;20(1):664. doi: 10.1186/s12879-020-05388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruiz PLD, Bakken IJ, Håberg SE, Tapia G, Hauge SH, Birkeland KI, et al. Higher frequency of hospitalization but lower relative mortality for pandemic influenza in people with type 2 diabetes. J Intern Med. 2020;287(1):78–86. doi: 10.1111/joim.12984. [DOI] [PubMed] [Google Scholar]
- 88.Madny AG, Adam AA. Seroprevalence of Hepatitis C Virus among type 2 diabetes mellitus patients in Blue Nile state, Sudan. . Am. J. Res. Commun. 2014;2(12):1. [Google Scholar]
- 89.Suliman MI. Relationship between chronic hepatitis C and type-2 diabetes mellitus in Bhawalpurian patients. Profession. Med. J. Q. 2004;11(3):315–319. [Google Scholar]
- 90.Guo X, Jin M, Yang M, Liu K, Li JW. Type 2 diabetes mellitus and the risk of hepatitis C virus infection: a systematic review. Sci. Rep. 2013;3:2981. doi: 10.1038/srep02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J. Gastroenterol. 2012;18(14):1642–1651. doi: 10.3748/wjg.v18.i14.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fabiani S, Fallahi P, Ferrari SM, Miccoli M, Antonelli A. Hepatitis C virus infection and development of type 2 diabetes mellitus: Systematic review and meta-analysis of the literature. Rev. Endocr. Metab. Disord. 2018;19(4):405–420. doi: 10.1007/s11154-017-9440-1. [DOI] [PubMed] [Google Scholar]
- 93.Lim TR, Hazlehurst JM, Oprescu AI, Armstrong MJ, Abdullah SF, Davies NP, et al. Hepatitis C virus infection is associated with hepatic and adipose tissue insulin resistance that improves after viral cure. Clin. Endocrinol. (Oxf) 2019;90(3):440–448. doi: 10.1111/cen.13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai C, Zeng J, Wu H, Shi R, Wei M, Gao Y, et al. Association between hepatitis B virus infection and diabetes mellitus: a meta-analysis. Exp. Ther. Med. 2015;10(2):693–698. doi: 10.3892/etm.2015.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang J, Shen Y, Cai H, Liu YM, Qin G. Hepatitis B virus infection status and risk of type 2 diabetes mellitus: a meta-analysis. Hepatol. Res. 2015;45(11):1100–1109. doi: 10.1111/hepr.12481. [DOI] [PubMed] [Google Scholar]
- 96.Shen Y, Zhang S, Wang X, Wang Y, Zhang J, Qin G, et al. Comparison of type 2 diabetes mellitus incidence in different phases of hepatitis B virus infection: a meta-analysis. Liver Int. 2017;37(10):1451–1460. doi: 10.1111/liv.13275. [DOI] [PubMed] [Google Scholar]
- 97.Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gilca R, De Serres G, Boulianne N, Ouhoummane N, Papenburg J, Douville-Fradet M, et al. Risk factors for hospitalization and severe outcomes of 2009 pandemic H1N1 influenza in Quebec, Canada. . Influenza Other Respir Viruses. 2011;5(4):247–255. doi: 10.1111/j.1750-2659.2011.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allard R, Leclerc P, Tremblay C, Tannenbaum TN. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care. 2010;33(7):1491–1493. doi: 10.2337/dc09-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pompei R. The role of human herpesvirus 8 in diabetes mellitus type 2: state of the art and a medical hypothesis. Adv Exp Med Biol. 2016;901:37–45. doi: 10.1007/5584_2015_5014. [DOI] [PubMed] [Google Scholar]
- 101.Nguewa JL, Lontchi-Yimagou E, Agbelika F, AitDjoudi M, Boudou P, Choukem S, et al. Relationship between HHV8 infection markers and insulin sensitivity in ketosis-prone diabetes. Diabetes Metab. 2017;43(1):79–82. doi: 10.1016/j.diabet.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 102.Lontchi-Yimagou E, Legoff J, Nguewa JL, Boudou P, Balti EV, Noubiap JJ, et al. Human herpesvirus 8 infection DNA positivity is associated with low insulin secretion: a case-control study in a sub-Saharan African population with diabetes. J. Diabetes. 2018;10(11):866–873. doi: 10.1111/1753-0407.12777. [DOI] [PubMed] [Google Scholar]
- 103.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Allen DW, Kim KW, Rawlinson WD, Craig ME. Maternal virus infections in pregnancy and type 1 diabetes in their offspring: Systematic review and meta-analysis of observational studies. Rev. Med. Virol. 2018;28(3):e1974. doi: 10.1002/rmv.1974. [DOI] [PubMed] [Google Scholar]
- 105.Maha MM, Ali MA, Abdel-Rehim SE, Abu-Shady EA, El-Naggar BM, Maha YZ. The role of coxsackieviruses infection in the children of insulin dependent diabetes mellitus. J. Egypt. Public Health Assoc. 2003;78(3–4):305–318. [PubMed] [Google Scholar]
- 106.Salminen KK, Vuorinen T, Oikarinen S, Helminen M, Simell S, Knip M, et al. Isolation of enterovirus strains from children with preclinical Type 1 diabetes. Diabet Med. 2004;21(2):156–164. doi: 10.1111/j.1464-5491.2004.01097.x. [DOI] [PubMed] [Google Scholar]
- 107.Thomas NJ, Lynam AL, Hill AV, Weedon MN, Shields BM, Oram RA, et al. Type 1 diabetes defined by severe insulin deficiency occurs after 30 years of age and is commonly treated as type 2 diabetes. Diabetologia. 2019;62(7):1167–1172. doi: 10.1007/s00125-019-4863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lontchi-Yimagou E, Tsalefac M, Tapinmene LM, Noubiap JJ, Balti EV, Nguewa JL, et al. Seasonality in diabetes in Yaounde, Cameroon: a relation with precipitation and temperature. BMC Public Health. 2016;16:470. doi: 10.1186/s12889-016-3090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yasuda H, Nagata M, Moriyama H, Kobayashi H, Akisaki T, Ueda H, et al. Development of fulminant Type 1 diabetes with thrombocytopenia after influenza vaccination: a case report. Diabet Med. 2012;29(1):88–89. doi: 10.1111/j.1464-5491.2011.03391.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as supplementary material.