Abstract
To determine the differences in the characteristic volatile compounds between winemaking areas in the Xinjiang region, this study was conducted by sampling Cabernet Sauvignon grapes from four winemaking areas in Xinjiang, named Tianshanbeilu, Yili, Yanqi, and Hami. After undergoing the same alcoholic fermentation treatment, the wines from the four areas were subjected to GC–MS and sensory analysis. The results showed that fifty aromatic compounds (including higher alcohols, esters, acids, terpenes, aldehydes/ketones, et al.) were identified and quantified. Interestingly, the terpene and phenylalanine derivative contents of the wines from northern Xinjiang were higher than those from the south. Additionally, four vineyards highly contributed to the development of key volatile compounds in the Xinjiang region. Sensory analysis showed that the wines from northern Xinjiang were impressive with a flowery and fruity aroma and the wines from southern Xinjiang had a stronger wine body and astringency.
Keywords: Cabernet Sauvignon wine, Volatile compounds, GC–MS, Heatmap cluster, Random forest, Sensory analysis
Introduction
Wine is a popular alcoholic beverage that has been considered a healthy product in terms of its nutritional value, containing compounds such as resveratrol, melatonin and polysaccharides (Chen et al. 2019; Vitalini et al. 2011). Additionally, wine aroma has been regarded as an important feature of wine quality (Asproudi et al. 2016). Wine aroma chemistry has been meticulously studied over the past few decades, and several comprehensive reviews have reported more than 1300 volatile compounds in grapes and wines from different cultivars (Bonada et al. 2015). It has been reported that the consumer acceptability of a wine is generally determined by whether it contains a complex but well-balanced aromatic profile (Villamor and Ross 2013). Normally, wine aromatic compounds are derived from grape berries and the fermentation and ageing processes (Wu et al. 2017). As a result, wine aroma can be influenced by many factors, such as grape variety, viticulture, yeast metabolism and ageing conditions (Robinson et al. 2014). All of these factors will determine the complexity of the wine aroma. Abundant free forms of volatile compounds related to wine aroma are those that are developed during alcoholic fermentation, such as higher alcohols, esters, acids, aldehydes and ketones (Alessandrini et al. 2017; Chen et al. 2017). Many of these compounds are transformed by hydrolysis, esterification or acetylation reactions to a significant degree from the early stages of wine maturation, causing changes in the organoleptic perception of the wines (Sánchez-Palomo et al. 2010). A popular variety, Cabernet Sauvignon, originated from the Bordeaux region in France. It is characterized by stable quality and a wide adaptability for growth in different environments. Cabernet Sauvignon was first imported into China in 1892 and planed and has become the largest cultivated red wine variety in China (Tao and Zhang 2010). Currently, most studies have focused on the typical volatile compounds of the varietal aroma of Cabernet Sauvignon. However, the effects of winemaking areas on the wine aroma profile, specifically the identification of characteristic aromatic compounds attributable to the winemaking area, have been scarcely reported.
The concept of terroir, including the grape cultivar, considers the interactions with the climatic conditions, soils, cultural practises and indigenous microbes, all of which can influence grape and wine quality. Meteorological variables, including the macroclimate, mesoclimate and microclimate, play a key role in vine vegetative and productive characteristics that influence grape quality, directly affecting the biosynthesis of primary and secondary metabolites and their accumulation in the berry (Abbott et al. 1991). For instance, regarding the distinctive environment of ice wine making during late harvest, natural freeze–thaw cycles and desiccation can cause cellular degradation and compartmentalization and consequently influence the development of norisoprenoids and phenylalanine-derived volatiles in the off-vine Vidal blanc grape (Chen et al. 2019). The microbial diversity of the soil, grape leaves and grape fruits from three winemaking areas in Xinjiang, China, were analysed using high-throughput sequencing; herein, four wild strains of Saccharomyces cerevisiae can produce higher amounts of esters than commercial strains (Feng et al. 2019). Xinjiang Province has complex ecological geographical conditions and different climates in its northern and southern parts. It is one of the most important vine and wine regions in China since the soil, rainfall and temperature are suitable for vine cultivation (Ma et al. 2018). In recent years, the wine industry in Xinjiang Province has grown rapidly, with a total annual grape yield of 1.1 million tons on a total of 80,000 hm2 of vineyard. Four major winemaking areas, named Tianshanbeilu, Yili, Yanqi and Hami, have been developed and certified. As we know, a grape variety can perform differently if it was cultivated in various regions; hence, the wines could be remarkably different with obvious characteristics of regionalization.
In this study, head space-solid phase microextraction (HS-SPME) and gas chromatography-mass spectrometry (GC–MS) were jointly used to analyse the Cabernet Sauvignon wine samples, which were subjected to the same fermentation as the grapes from the Tianshanbeilu, Yili, Yanqi and Hami areas in Xinjiang Province. The results are expected to provide a basis for the identification of the key aromatic compounds of Cabernet Sauvignon wines from four winemaking areas in the Xinjiang region using a heatmap cluster and principal component analysis (PCA).
Materials and methods
Reagents and chemical standards
Analytical grade solvents, including sodium chloride, sodium hydroxide, citric acid, Folin and Ciocalteu’s phenol reagent and disodium hydrogen phosphate, were obtained from Beijing Chemical Works (Beijing, China). Phosphomolybdic acid, tannic acid, sodium carbonate, gallic acid monohydrate, sodium acetate trihydrate, rutin, sodium nitrite, and aluminium chloride were all purchased from China National Pharmaceutical Group Co., Ltd. (Beijing, China). GC grade solvents, including ethanol, methanol and dichloromethane, were supplied by Honeywell (Morris Township, NJ, U.S.). Water was obtained from a Milli-Q purification system (Millipore, North Ryde, NSW, Australia). The internal standard, 4-methyl-2-pentanol, was supplied by Sigma-Aldrich (St. Louis, MO, U.S.). C8-C40 n-alkanes were purchased from J&K Technology, China (Beijing, China).
Sampling and fermentation
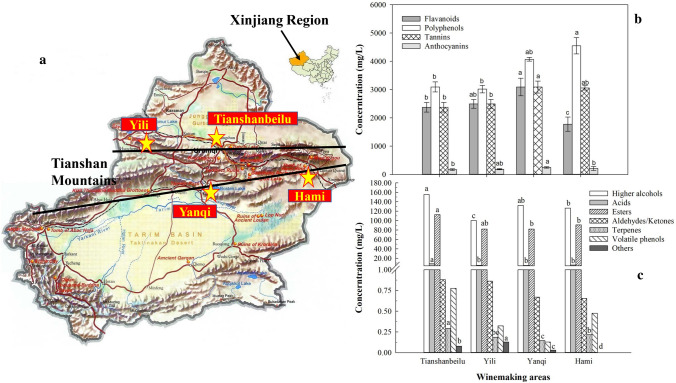
Cabernet Sauvignon vines were planted in eighteen commercial vineyards located in the northern part (Tianshanbeilu and Yili winemaking areas) and the southern part (Yanqi and Hami winemaking areas) of Xinjiang Province. In terms of the practicality and representativeness of sampling, we selected six vineyards in the Tianshanbeilu area, four vineyards in the Yili area, six vineyards in the Yanqi area and two vineyards in the Hami area (Fig. 1a, map details are provided by www.sinomaps.com). Regular fungicide was applied to control powdery mildew and Botrytis during the growing season. During the harvest seasons of 2017 and 2018 vintages, sampling was conducted from the selected vines (5 or 6 years old), which were all grown on a trellis system. All grapes were harvested at the end of September, which was approximately the end of the maturation period (between 23 and 25°Brix). After harvest, the grape berries were immediately transported to the laboratory within a day as soon as sampling was completed.
Fig. 1.
Analysis of phenolic indexes and content of volatile compounds from Cabernet Sauvignon wines from four winemaking areas in the Xinjiang region. a The locations of the four winemaking areas; 6 and 4 commercial vineyards were selected for sampling from the Tianshanbeilu and Yili areas in northern Xinjiang, respectively. Moreover, 6 and 2 commercial vineyards were selected for sampling from the Yanqi and Hami areas in southern Xinjiang, respectively. b The concentrations of polyphenols, flavonoids, tannins and anthocyanins of the wines from the four winemaking areas. c The concentrations of seven major types of volatile compounds of the wines from the four winemaking areas. Different letters on each bar show the ANOVA results at p < 0.05
Fermentation was carried out under the same conditions for all samples. Specifically, a Cabernet Sauvignon sample from each vineyard was pumped into a 60 L stainless steel fermentation tank after mechanical destemming and crushing (Zeta-5 destemmer, Lanbosi Technology Co. Ltd., Beijing, China). Prior to alcoholic fermentation, 60 mg/L sulphur dioxide was mixed with the must. The maceration process occurred at 8–10 °C for 3 days, and during the maceration period, the must was pumped every 8 h. Subsequently, the activated commercial yeast Zymaflore® FX10 (Laffort, Bordeaux, France) (0.2 g/L) and 3 g/L Tanin VR Color (Laffort, Bordeaux, France), which used for colour stabilisation, were homogeneously mingled with must after the maceration to initiate alcoholic fermentation (AF) at 22–25 °C. During alcoholic fermentation, the must was pumped over the same time interval as the maceration process, and the wine density was monitored. AF was totally accomplished in approximately 15 days (the reducing sugar was below 4 g/L). Then, the wine samples were stored at − 20 °C prior to further analysis. Each fermentation was performed in duplicate.
Determination of physicochemical parameters
Each wine sample was centrifuged (10,000 rpm at 4 °C for 10 min) for physicochemical analysis. Total sugar, alcohol, pH, total acidity, dry extracts and titratable acidity were analysed according to the National Standard of the People’s Republic of China (GB/T 15038-2006). Values of pH were determined using a PB-10 pH Basic+ meter (Sartorius, Gottingen, Germany).
Determination of phenolic indexes
A MACY UV-1700 UV–Visible spectrophotometer (MACY Instrument, Shanghai, China) was used to determine the phenolic indexes. Samples were analysed in a quartz cuvette with a 1 mm path length in the range of 380–900 nm. The absorbance at 420 nm, 520 nm, and 620 nm was measured, and then the colour intensity was calculated as the sum of the absorbance values at the three wavelengths, while the hue was calculated as the ratio of the absorbance between 420 and 520 nm (Chen et al. 2018). Four phenolic indexes were determined for the evaluation of wine quality. Total polyphenols were analysed based on the Folin–Ciocalteu index. The total flavonoid content was analysed based on the method of Marinova et al. (2007). Total tannin content was carried out by the acid hydrolysis methods from Ribereaugayon et al. (2006). Total anthocyanin content measurements were carried out based on the methods of Boulton (2001).
HS-SPME
Volatile compounds were determined by headspace–solid phase micro-extraction (HS-SPME). The procedure was performed following the specific method of previous reports with minor modifications (Chen et al. 2019; Lan et al. 2016). Five millilitres of the centrifuged wine was added to 10 μL of the internal standard 4-methyl-2-pentanol (2800 μg/L) and 1 g of sodium chloride in a 15 mL vial. The mixture was tightly capped with a polytetrafluoroethylene (PTFE) silicon septum containing a magnetic stirrer. Subsequently, the sample was equilibrated at 40 °C for 30 min with continuous stirring. A pre-treated SPME fibre (PDMS/CAR/DVB, 50/30 μm, Supelco, Bellefonte, PA, USA) was inserted into the headspace and extracted at 40 °C for 30 min with continuous heating and agitation.
Gas chromatography–mass spectrometry (GC–MS) analysis
An Agilent 6890 N GC equipped with an Agilent 5973 mass spectrometer with a 30 m × 0.25 mm × 0.25 μm DP-WAX capillary column (J&W Scientific, Folsom, CA) was used to separate and identify the volatile compounds. The carrier gas used was pure helium (> 99.999%) at a flow rate of 1 mL/min. The SPME extracts were injected in the splitless mode. Operating conditions were set as follows: injector temperature, 250 °C; thermal analysis time, 8 min. The temperature program was from 60 °C (holding 1 min) to 220 °C by increasing the temperature at 3 °C/min and then held at 220 °C for 2 min. Mass spectrometry was performed with 70 eV electron ionization (EI). The ion source and quadrupole were set to 230 °C and 150 °C, respectively. The mass detector was operated with full scan mode (m/z 30–350). Triple extractions were performed for each sample.
Data analysis was conducted using ChemStation Software (Agilent Technologies, Inc.). Volatile compounds were identified by comparing the mass spectra of the standard in the NIST 11 library and retention indices (RI), which were calculated by the Automated Mass Spectral Deconvolution and Identification System (AMDIS). Quantification was carried out from the total ion current peak areas according to the internal standard method (4-methyl-2-pentanol). The response factor of the standard volatile compounds to the internal standard was experimentally obtained and applied to correct the peak area of each analyte. For compounds lacking reference standards, the response factors of standards with similar chemical structures were used.
Sensory analysis
Sensory analysis was performed according to the method of Santiago Benito (Chen et al. 2018). Previously, trained panellists (10 females and 10 males) were employed from the College of Food Science & Pharmacy, Xinjiang Agricultural University. Experimental Cabernet Sauvignon wines were evaluated by blindfolded tasting. Before the tasting session, the studied parameters were established by consensus. Wines were evaluated in a randomized order in an air-conditioned (24 °C) tasting room. Twenty millilitres of each wine was served at 14 °C for tasting; more wine was provided when required by any of the panellists. The panellists evaluated the wines based on eleven attributes with an unstructured scale from 0 (absent) to 5 (very intense), representing the intensity of each attribute. Additionally, the panellists were free to write comments for each sample.
Statistics
All statistical analyses were performed using SPSS 19.0 software (IBM SPSS Inc., Chicago). For each wine sample, the group differences were identified with ANOVA based on Duncan’s multiple range test at p < 0.05. Two factors (areas and vintages) could simultaneously influence the volatile profile of the Cabernet Sauvignon wines. Therefore, a heatmap of cluster analysis (CA) with Ward’s method and PCA were jointly applied for a comprehensive analysis of the variances and observations. The data analysis portion of CA and PCA was carried out using MetaboAnalyst 3.0 (www.metaboanalyst.ca).
Results and discussion
Analysis of the physicochemical parameters of the Cabernet Sauvignon wines
Prior to AF, grape quality and maturation should be evaluated by many factors, such as the acidity, sugar content, and tannin, anthocyanin, and polyphenol contents. However, the grape variety, terroir, and viticulture conditions can result in differences in the chemical compounds of the grape berry. Based on the literature and wine standards, wine quality should be given an overall evaluation of its physicochemical parameters, polyphenols profile, which includes tannins, anthocyanin and non-anthocyanin phenols, aroma, and sensory analysis. The balance of content and variety of these compounds can interact with participation from microbes and generate a series of chemical reactions during the fermentation and ageing processes, finally developing a unique aroma profile.
In terms of physicochemical parameters (Table 1), in general, the influences from the winemaking area could be ignorable since few differences between some parameters of the wines were found according to the ANOVA results, such as the hue, volatile acidity, alcohol degree, total acidity and pH. Temperature could affect grape shooting, leaf growth, blooms, and the percent of fruit set. In particular, the evolution of wine aroma depends on grape quality, which can be influenced by the annual accumulative temperature and precipitation to a great extent (Keller 2015). A comparatively high accumulative temperature (4326–5574 °C) and low precipitation (< 100 mm) could positively affect the accumulation of sugar content in the southern part of the Xinjiang region (Yanqi and Hami areas), which is characterized by a hot and arid climate (Blättel et al. 2009). This directly resulted in colour density (13.59) and dry extracts (38.08 g/L) of Hami wine that were significantly higher than the Cabernet Sauvignon wines from the northern part of Xinjiang region, namely, the wines from the Tianshanbeilu and Yili areas. However, the highest content of residual sugar (2.29 g/L) was found in Yili wine, which was similar to the wine of the Tianshanbeilu area (2.1 g/L). Both wines from the northern part of Xinjiang contained a lower alcohol degree than the wines from the Hami and Yanqi areas, which are located in the southern part of Xinjiang. Regarding yeast metabolism during alcoholic fermentation, understandably, this could be related to the higher levels of residual sugar in the wines of northern Xinjiang than those of the south.
Table 1.
Analysis of physicochemical parameters of Cabernet Sauvignon wines of four winemaking areas after AF
| Wine areas | Color intensity | Hue | Volatile acidity (g/L) | Alcohol (%vol) | Dry extracts (g/L) | Residual sugar (g/L) | Total acidity (g/L) | pH |
|---|---|---|---|---|---|---|---|---|
| Tianshanbeilu | 9.52 ± 2.66b | 0.84 ± 0.08 | 0.44 ± 0.05 | 12.88 ± 0.76 | 31.09 ± 2.02c | 2.1 ± 0.45ab | 5.23 ± 0.45 | 3.81 ± 0.23 |
| Yili | 9.17 ± 1.67b | 0.77 ± 0.05 | 0.4 ± 0.06 | 12.81 ± 0.67 | 32.56 ± 4.11ac | 2.29 ± 0.4a | 5.75 ± 0.52 | 3.77 ± 0.17 |
| Yanqi | 12.67 ± 1.17ab | 0.81 ± 0.05 | 0.45 ± 0.04 | 13.49 ± 0.73 | 36.41 ± 1.98ab | 1.56 ± 0.12b | 5.26 ± 0.27 | 3.82 ± 0.02 |
| Hami | 13.59 ± 1.61a | 0.83 ± 0.12 | 0.5 ± 0.03 | 13.76 ± 0.33 | 38.08 ± 1.03a | 1.75 ± 0.23ab | 5.36 ± 0.25 | 3.82 ± 0.01 |
In the same column, letters indicate the significant differences of four producing areas which were conducted with Duncan’s multiple range test at p < 0.05
Analysis of phenolic indexes and content of volatile compounds in Cabernet Sauvignon wines from four winemaking areas
The relationship between terrestrial ecosystems and climate is an important research field in geographical and plant science, especially because the relationship between vegetation and climate differences has attracted more interest for grapevine and wine research in recent years. Xinjiang Province is located in the hinterland of Eurasia, northwest China, and geographically divided into northern and southern parts by the Tianshan Mountains, which is the largest mountain range in the arid world area (Cai et al. 2019). The climate between the Tianshan Mountains is different; in particular, the annual precipitation (200–500 mm) is concentrated on the northern slope of the Tianshan Mountains. Therefore, the climate of the northern part of Xinjiang Province is warm-humid, and the southern part is hot-arid (Chen et al. 2005). To investigate the influence of the winemaking areas on the quality of Cabernet Sauvignon wines, we selected six and four commercial vineyards for grape sampling from Tianshanbeilu and Yili winemaking areas, respectively, located in the northern part of the Xinjiang region. Moreover, in the southern part, six and two qualified vineyards were also selected from the Yanqi and Hami areas, respectively. Geographic information of Xinjiang Providence and the specific locations of the sampling areas are shown in Fig. 1a.
The profile and content of phenolic compounds are important factors for the assessment of wine quality. In general, phenolic compounds can be classified mainly as flavonoids (flavonols, anthocyanins and flavan-3-ols) and non-flavonoids (phenolic acids and stilbenes). Among them, oligomers and polymers of flavan-3-ols are called proanthocyanidins or condensed tannins (Perez-Jiménez et al. 2019). These compounds largely contribute to wine colour, astringency and bitterness. In this work (Fig. 1b), the total content of polyphenols from the wines from the southern part of Xinjiang was higher than that from the north. The wines of two areas, Yanqi and Hami, had total polyphenol contents of 4548.20 mg/L and 4017.45 mg/L, respectively. This is due to the hot-arid climate and abundant sun exposure (2550–3500 h annually) in the southern part of Xinjiang, which contributed to the accumulation of phenolic compounds (Keller 2010). Moreover, Anthocyanins are phenolic molecules composed of a glycosylated flavylium ion, and monoglucoside anthocyanins are present in the largest amounts. Wine colour is mainly dependent on the development and composition of anthocyanins. The results showed that the change in anthocyanins was similar to that in polyphenols, namely, the total anthocyanin content in the wines of southern Xinjiang was 24.9% higher than that in the north (Tianshanbeilu and Yili area). According to Table 1, the colour intensity results are also related to this phenomenon. In addition, the wine from the Yanqi area contains the highest amount of tannin in the Xinjiang region; moreover, the wines from southern Xinjiang contained 19.3% more tannins than the north as well.
Additionally, 50 volatile compounds, including higher alcohols, esters, acids, terpenes, aldehydes, ketones, volatile phenols and others, were detected and are presented in Table 2 (Chen et al. 2017; Duarte et al. 2010; Selli et al. 2009, 2012; Zepka et al. 2014). In this work, 19 types of higher alcohols were identified, and the range of the total content of higher alcohols was 100–160 mg/L. Based on a previous study, higher alcohols could positively contribute to the fruity and floral aroma of wines if the total content is lower than 300 mg/L, while above 400 mg/L, higher alcohols could inhibit the release of other aromatic compounds and affect the composition of wine aroma (Chen et al. 2017). Interestingly, the Cabernet Sauvignon wines of the Tianshanbeilu area showed the highest amount of higher alcohols (155.48 mg/L), esters (112.63 mg/L), acids (8.46 mg/L), aldehydes/ketones (0.88 mg/L), terpenes (0.29 mg/L) and volatile phenols (0.77 mg/L) among the four winemaking areas in the Xinjiang region. Terpenes play an important role in the varietal aroma and largely contribute to the fruity and floral aroma of wines since the thresholds of terpenes are usually very low, as the threshold of linalool is 25 μg/L and that of geraniol is 20 μg/L (Yuan and Qian 2016; Zhang et al. 2016). According to our results, β-damascenone, linalool and citronellol were the major terpenes from the Xinjiang region. However, the wines of the Yanqi area contained the lowest concentration of terpenes (0.14 mg/L). It should be noted that wines in the Yanqi area contained high amounts of tannins, while phenolic compounds can influence wine aroma since they can interact with different types of aromatic molecules, changing the volatility of the aromatic molecules and modifying aroma release (Pozo-Bayón and Reineccius 2009). A previous study also indicated that the yeast material exchange with must could be affected and that yeast growth kinetics were probably reduced by grape tannins during AF (Mekoue Nguela et al. 2015). Therefore, the synthesis of glucosidase by yeasts was limited, and the development of free terpenes was consequently reduced. In addition, the wines from the Tianshanbeilu area contained the highest content of 2,4-DTBP (0.78 mg/L), which was the major volatile phenol in the Xinjiang region.
Table 2.
Identification and qualification of volatile compounds of Cabernet Sauvignon wines of four winemaking areas after AF
| No | Name | CAS | RIa | Method of IDb | Descriptionsc | Thresholds (mg/L)d |
|---|---|---|---|---|---|---|
| Higher alcohols | ||||||
| 1 | 1-Propanol | 71-23-8 | 1029.1 | S,MS,RI | Alcohol | 50 |
| 2 | 2-Methyl-1-propanol | 78-83-1 | 1097.3 | MS,RI | Fuel oil | 75 |
| 3 | 1-Butanol | 71-36-3 | 1152.3 | S,MS,RI | Alcohol | 150 |
| 4 | Isoamyl alcohol | 123-51-3 | 1208.7 | S,MS,RI | Bitter almond | 7 |
| 5 | Isohexanol | 626-89-1 | 1310.5 | MS,RI | ||
| 6 | 4-Penten-2-ol | 625-31-0 | 1315.9 | MS,RI | ||
| 7 | 3-Methyl-1-pentanol | 589-35-5 | 1323.3 | S,MS,RI | Apple, cocoa bean | |
| 8 | 1-Hexanol | 111-27-3 | 1348.8 | S,MS,RI | Green grass | 5.2 |
| 9 | 3-Hexen-1-ol | 544-12-7 | 1360.7 | MS,RI | Green grass | 0.4 |
| 10 | Neopentyl alcohol | 75-84-3 | 1480.4 | MS,RI | ||
| 11 | 2-Nonanol | 628-99-9 | 1516.1 | S,MS,RI | Rose, fruity | |
| 12 | (R,R)-2,3-Butanediol | 24347-58-8 | 1539.4 | MS,RI | ||
| 13 | 1-Octanol | 111-87-5 | 1556 | S,MS,RI | Citrus, herbs | 0.9 |
| 14 | 2,3-Butanediol | 513-85-9 | 1576.5 | MS,RI | Rubber | 120 |
| 15 | Methionol | 505-10-2 | 1875.9 | MS,RI | Raw potato, garlic | 1 |
| 16 | Benzyl alcohol | 100-51-6 | 2405.6 | S,MS,RI | Almond | 200 |
| 17 | Phenylethyl alcohol | 60-12-8 | 2517.2 | S,MS,RI | Muscat, peach | 7.5 |
| 18 | 1-Nonanol | 143-08-8 | 1684.9 | MS,RI | Grass | 0.015 |
| 19 | 1-Decanol | 112-30-1 | 2011.6 | MS,RI | Flora nerolia | 0.4 |
| Acids | ||||||
| 1 | Acetic acid | 64-19-7 | 1453 | MS,RI | Vinegar | 200 |
| 2 | 2-Methyl butyric acid | 116-53-0 | 1710.8 | MS,RI | 0.05 | |
| 3 | Octanoic acid | 124-07-2 | 2946.5 | S,MS,RI | Cheese, spoiled | 15 |
| 4 | n-Decanoic acid | 334-48-5 | 3542.6 | S,MS,RI | Unpleasant fats | 8 |
| 5 | Hexanoic acid | 142-62-1 | 2293.3 | S,MS,RI | Cat urine, sweat odor | 8.8 |
| Esters | ||||||
| 1 | Ethyl acetate | 141-78-6 | 891.9 | S,MS,RI | Fruity | 17 |
| 2 | Propyl formate | 110-74-7 | 1109.4 | MS,RI | ||
| 3 | Isoamyl acetate | 123-92-2 | 1122.6 | MS,RI | Babana | 0.2 |
| 4 | Ethyl caproate | 123-66-0 | 1233 | S,MS,RI | Green apple, fennel, strawberry | 0.014 |
| 5 | Ethyl glycolate | 623-50-7 | 1271 | MS,RI | ||
| 6 | Ethyl L(-)-lactate | 687-47-8 | 1342.9 | S,MS,RI | Milk, raspberry, coconut | 14 |
| 7 | Ethyl caprylate | 106-32-1 | 1435.4 | S,MS,RI | Fennel, sweet | 0.25 |
| 8 | Isoamyl lactate | 19329-89-6 | 1572.2 | MS,RI | ||
| 9 | Ethyl-2-furoate | 614-99-3 | 1642.6 | MS,RI | ||
| 10 | Ethyl caprate | 110-38-3 | 1658.8 | S,MS,RI | Apple, floral, wax | 0.2 |
| 11 | Diethyl succinate | 123-25-1 | 1739.7 | S,MS,RI | Melon, grass | 200 |
| 12 | Ethyl-9-decenoate | 1782 | MS,RI | |||
| 13 | Methyl salicylate | 119-36-8 | 2092.8 | MS,RI | Ilex | 0.1 |
| 14 | Ethyl phenylacetate | 101-97-3 | 2118.1 | S,MS,RI | Honey, syrup | |
| 15 | Butyl succinate | 2132.2 | MS,RI | |||
| 16 | Phenethyl acetate | 103-45-7 | 2216.1 | S,MS,RI | Fruity | 0.65 |
| 17 | Ethyl isopentyl succinate | 28024-16-0 | 2476.6 | MS,RI | ||
| Aldehydes/ketones | ||||||
| 1 | Acetoin | 513-86-0 | 1291.6 | MS,RI | Cream, fats | 150 |
| 2 | 2,6,8-Trimethyl-4-nonanone | 123-18-2 | 1395 | MS,RI | ||
| 3 | Furfural | 98-01-1 | 1470.6 | MS,RI | Caramel, wood, toast | 14.1 |
| 4 | 5-Methyl furfural | 620-02-0 | 1581.9 | MS,RI | Caramel, liquorice | |
| Terpenoids | ||||||
| 1 | β-Damascenone | 23726-93-4 | 2228.3 | MS,RI | Bark, sweet apple | 0.00005 |
| 2 | Linalool | 78-70-6 | 1547 | S,MS,RI | Marshmallow, lily | 0.1 |
| 3 | Citronellol | 106-22-9 | 2027.4 | S,MS,RI | Rose, grass, lilac | 0.1 |
| Volatile phenols | ||||||
| 1 | 2,4-DTBP | 96-76-4 | 3651.2 | MS,RI | Carbonate | 0.2 |
| Alkanes | ||||||
| 1 | Dimethyl hexane | 584-94-1 | 2344.6 | MS,RI | ||
aRetention indices in DB-wax column
bMethod of identification: S, by comparison of mass spectrum and retention time with those of standard compounds; MS, by comparison of mass spectrum with those included in the NIST 11.0
cOdor descriptions are mainly gathered from following literatures: Chen et al. (2016), Selli et al. (2009, 2012), Duarte et al. (2010)
dThresholds are mainly obtained from those literature which applied water as the matrix: Selli et al. (2009, 2012), Duarte et al. (2010), Zepka et al. (2014), Chen et al. (2016)
Heatmap cluster and random forest to analyse the characteristic volatile compounds of Cabernet Sauvignon wines in the Xinjiang region
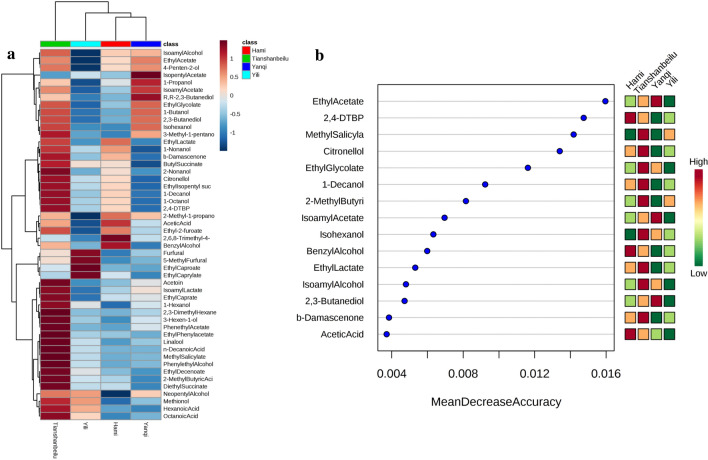
Heatmap is a visualization method that can be used to analyse data distribution, control data quality and integrity, and cluster the sample and related experimental data (Liu et al. 2015). In this work, the relationship between the four winemaking areas in Xinjiang Province and the volatile compounds of Cabernet Sauvignon wines were classified and analysed with the heatmap cluster method (Fig. 2a). Regarding the cluster of winemaking areas, three major clusters (Tianshanbeilu, Yili, Yanqi and Hami) were established; herein, Tianshanbeilu was regarded as an individual sample because of its high correlation with many higher alcohols, esters and terpenes. On the other hand, the Yanqi and Hami areas were clustered as one group according to the similarity of their volatile component composition. It is obvious that volatile compounds of the wines of southern Xinjiang were similar to each other but different from the wines of the north. Especially for the Tianshanbeilu area, the complexity of positively correlated data was the highest in the Xinjiang region. For instance, higher alcohols (2-nonanol, 3-hexen-1-ol, 1-hexanol), acids (n-decanoic acid, 2-methyl butyric acid, octanoic acid), esters (isoamylactate, ethylcaprate, methylsalycylate, ethyldecenoate, diethylsuccinate), terpenes (β-damascenone, linalool, citronellol), phenylalanine derivatives (phenethyl acetate, ethyl phenylacetate, phenylethyl alcohol), aldehydes/ketones (acetoin, 2,3-dimethyl hexane), and a volatile phenol (2,4-DTBP) were all significantly correlated to the wines of the Tianshanbeilu area. Terpenes and phenylalanine derivatives are characterized by a fruity and floral aroma and can enhance the sensory experience. Moreover, furfural and 5-methyl furfural were positively corrected to the wines of the Yili area. These compounds were described to have a caramel and roasted aroma (Table 2). In terms of the wines of southern Xinjiang, 2,6,8-trimethyl-4-nonanone and isoamyl lactate were highly positively correlated with the Hami and Yanqi areas, respectively.
Fig. 2.
Analysis of characteristic volatile compounds of Cabernet Sauvignon wines from the Xinjiang region. a Heatmap cluster of analysis of the relationship between the winemaking area and the related volatile compounds (distance measured using Euclidean and clustering algorithm using Ward’s D). b Random forest analysis of the importance of characteristic volatile compounds of each winemaking area. The features were ranked by the mean decrease in classification accuracy when they were permuted
Random forest is a supervised learning algorithm that is suitable for high dimensional data analysis. It also provides other useful information, such as out-of-bag error, variable importance measures, and outlier measures. To reduce the number of important variables and highlight the importance of key volatile compounds for each winemaking area, a random forest was created as a supplement and validation for the heatmap cluster. The results showed that 9 volatile compounds (methyl salicylate, citronellol, ethyl glycolate, 1-decanol, 2-methyl butyric acid, isohexanol, ethyl lactate, isoamyl alcohol, and β-damascenone) were regarded as key compounds in the aroma composition of the wines from the Tianshanbeilu area. For the wines of southern Xinjiang, 3 volatile compounds (2,4-DTBP, benzyl alcohol, acetic acid) showed high importance in the Hami area. In addition, ethyl acetate, isoamyl acetate and 2,3-butanediol were important in the wines from the Yanqi area. Above all, the heatmap cluster and random forest were jointly used to select key volatile compounds of the Xinjiang region. As a result, the Tianshanbeilu winemaking area could be regarded as a representative area since it largely contributed to the aromatic characteristics of the wines of northern Xinjiang. Meanwhile, the Yanqi and Hami areas showed different aromatic profiles and characteristic volatile compounds. Both areas jointly contributed to the wine aroma characteristics of southern Xinjiang.
PCA to analyse the contribution of the vineyards and key volatile compounds of Cabernet Sauvignon wines in the Xinjiang region
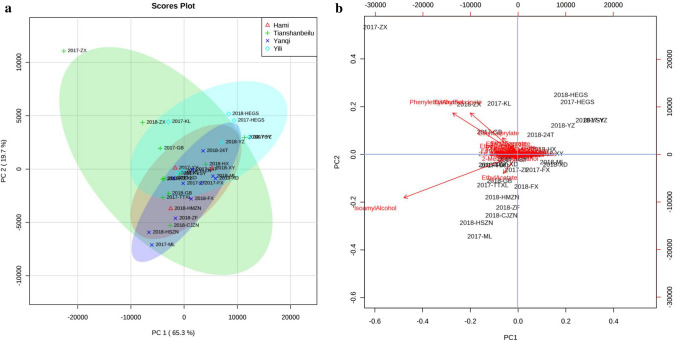
The correlation of sampling vineyards and related key volatile compounds was analysed using PCA. The results showed that the contribution rate of PC 1 was 65.3% and the contribution rate of PC 2 was 19.7%. The PCA score plot (Fig. 3a) indicated the contribution of the sampling vineyards of each winemaking area. Sampling vineyards in vintages 2017 and 2018 were selected as important factors that were regarded as typical and significantly contributed to the PCA. According to the distribution of each vineyard, Tianshanbeilu possessed the largest distribution area of the four winemaking areas. This meant that the Tianshanbeilu area provided the highest contribution to the PCA. In contrast, the Hami area provided the lowest contribution, which was most likely due to the fewest samples collected. In addition, the distribution areas of Yanqi and Hami overlapped on a large scale. This showed the high similarity of volatile compound composition between the two areas. In terms of the position of each vineyard, Zhongxinguoan (ZX) from 2017 and 2018 in the Tianshanbeilu area were located outside the range of the distribution of the other three winemaking areas. This result indicated that ZX vineyard was an important contributor and could be a characteristic variable in the Tianshanbeilu area. Similarly, both Huoerguosi (HEGS) and Yizhu (YZ) vineyards were regarded as characteristic of the Yili area. Therefore, the characteristic vineyards of northern Xinjiang were ZX, HEGS and YZ. Nevertheless, except for the Milan (ML) vineyard of 2017 located separately from other areas, the concentration of vineyards in southern Xinjiang was too high to select the major contributors of the volatile composition.
Fig. 3.
Principal component analysis to analyse the contributions of the vineyards and key volatile compounds of Cabernet Sauvignon wines in the Xinjiang region. a Score plots of the contribution of different sampling vineyards to the winemaking areas. b Biplot of PCA to analyse the influence of key volatile compounds on the sampling vineyards
The relationships of the characteristic vineyards and the involved key volatile compounds are reflected in the PCA biplot (Fig. 3b). Accordingly, diethyl succinate (melon and grass aroma) and phenylethyl alcohol (Muscat, peach) are located close to ZX vineyard. As mentioned above, the same results have been shown with the heatmap cluster analysis (Fig. 2a), which indicated that these compounds were characteristic of the Tianshanbeilu area but were also highly related to the ZX vineyard. In other words, these compounds have been confirmed as key aromatic compounds of the ZX vineyard and northern Xinjiang. Additionally, isoamyl alcohol, which has been described as a bitter almond aroma, was located in the third quadrant of PC 2 and close to the ML and HSZN vineyards, which belong to the Yanqi area. The heatmap confirmed that isoamyl alcohol was highly related to ML and can be a key aromatic compound in the Yanqi area. Nevertheless, ethyl caprylate and ethyl acetate showed a high correlation with the 2017 and 2018 vintages of the Gebi (GB) vineyard, which is in the Tianshanbeilu area (Fig. 3, 4).
Fig. 4.
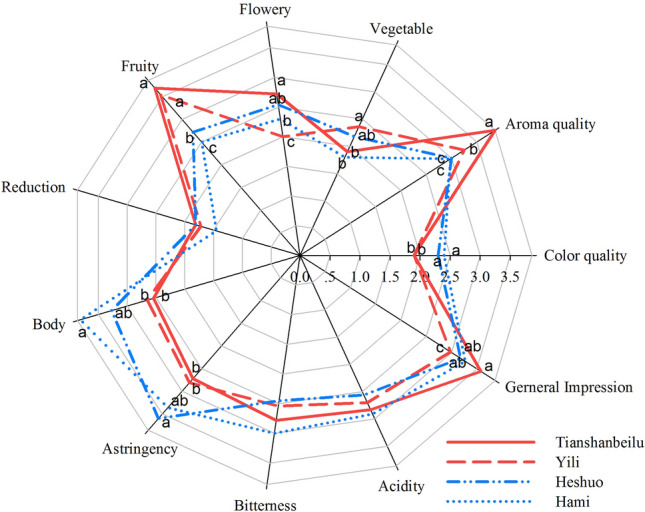
Sensory analysis radar plot of Cabernet Sauvignon wines from four winemaking areas in the Xinjiang region. Lines with red colour highlight the areas in northern Xinjiang, whereas lines with blue colour highlight the areas in southern Xinjiang. The benchmark for evaluation ranged from zero to five. Different letters show the results of ANOVA at p < 0.05
Sensory analysis of Cabernet Sauvignon wines in the Xinjiang region
Sensory analysis was performed by 20 panellists. Based on their feedback, as expected, the wines of the Tianshanbeilu area received the highest scores on general impression and aromatic quality in the Xinjiang region. However, the colour quality of the wines of northern Xinjiang scored lower than those of the south. This was probably due to the influence of the anthocyanin content, which is illustrated in Fig. 1b. In terms of the flowery and fruity aroma, the wines of northern Xinjiang performed better than the south since a higher content of terpenes (Fig. 1c) could positively influence the sensory experience. Nevertheless, flowery and fruity aroma could potentially enhance the sweetness though taste–aroma interactions (Noble 1996). Interestingly, the wines of the Yili area performed impressively on vegetable aroma. This could be related to a comparatively high content of ethyl caproate and ethyl caprylate (fennel aroma), which showed a high correlation with Yili area according to the result of the heatmap cluster (Fig. 2a). It can be understandable that the wines of southern Xinjiang had better performance on wine body and astringency, which is highly related to the content of polyphenols and tannins (Fig. 1b). Although the wines of the Tianshanbeilu area had the best general impression, a lower level of astringency and wine body could affect the balance of the overall sensory experience, especially for Cabernet Sauvignon wines.
Conclusion
In this work, key aromatic compounds of Cabernet Sauvignon wines of four major winemaking areas in Xinjiang were identified and analysed. In general, the wines of the Tianshanbeilu area contained higher amounts of free terpenes, which resulted from the decreased influence from the low tannin content on the release of glucosidase by yeast during AF. In addition, the Tianshanbeilu area was also found to be suitable for the accumulation of higher alcohols, esters, acids, aldehydes/ketones and volatile phenols. It is due to the climate of the northern foothills of the Tianshan Mountains. Compared with other production areas, the climate of the northern foothills of the Tianshan Mountains is cooler, which is more conducive to the accumulation of acid and terpene aromas(Cabrita et al. 2007). However, it should be noticed that the terpenes differences of the Xinjiang production area mainly resulted from the changes of content rather than the terpenes types. By analysis of the heatmap cluster and random forest results, terpenes and phenylalanine derivatives were selected as the characteristic compounds in the Tianshanbeilu area. Similarly, furfural and 5-methyl furfural were highly correlated with the wines of the Yili area. Meanwhile, the Yanqi and Hami areas jointly contributed to the wine aroma characteristics of southern Xinjiang. Moreover, the ZX, HEGS, and YZ vineyards were regarded as characteristic of northern Xinjiang vineyards, and ML was a characteristic southern Xinjiang vineyard. According to the sensory analysis, a better score was given to the wines of northern Xinjiang regarding their flowery and fruity aroma, while the wines of southern Xinjiang had a stronger wine body and greater astringency. Hot climate and less precipitation in southern Xinjiang area result in high accumulations of polyphenols and tannins. A similar study was found by Leeuwen et al (Leeuwen et al. 2004) showed that water stress have a significant impact on maturity of grapes, especially in the early stages of growth, moderate water shortage can accelerate the accumulation of sugar content, decomposition of malic acid and the synthesis of polyphenols.
Acknowledgements
This work was supported by the Xinjiang Uygur Autonomous Region Key Project of Science and Technology (2017A01001-2). We are thankful for the support of the experimental facilities and manuscript proofreading by Prof. Jingming Li and Ph.D. Kai Chen from China Agricultural University.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbott NA, Coombe BG, Williams PJ. The contribution of hydrolyzed flavor precursors to quality differences in Shiraz juice and wines: an investigation by sensory descriptive analysis. Am J Enol Vitic. 1991;42(3):167–174. [Google Scholar]
- Alessandrini M, Gaiotti F, Belfiore N, Matarese F, D'Onofrio C, Tomasi D. Influence of vineyard altitude on Glera grape ripening (Vitis vinifera L.): effects on aroma evolution and wine sensory profile. J Sci Food Agric. 2017;97(9):2695–2705. doi: 10.1002/jsfa.8093. [DOI] [PubMed] [Google Scholar]
- Asproudi A, Petrozziello M, Cavalletto S, Guidoni S. Grape aroma precursors in cv. Nebbiolo as affected by vine microclimate. Food Chem. 2016;211:947–956. doi: 10.1016/j.foodchem.2016.05.070. [DOI] [PubMed] [Google Scholar]
- Blättel V, Wirth K, Claus H, Schlott B, Pfeiffer P, König H. A lytic enzyme cocktail from Streptomyces sp. B578 for the control of lactic and acetic acid bacteria in wine. Appl Microbiol Biotechnol. 2009;83(5):839–848. doi: 10.1007/s00253-009-1926-7. [DOI] [PubMed] [Google Scholar]
- Bonada M, Jeffery DW, Petrie PR, Moran MA, Sadras VO. Impact of elevated temperature and water deficit on the chemical and sensory profiles of Barossa Shiraz grapes and wines. Aust J Grape Wine Res. 2015;21(2):240–253. doi: 10.1111/ajgw.12142. [DOI] [Google Scholar]
- Boulton R. The copigmentation of anthocyanins and its role in the color of red wine: a critical review. Am J Enol Vitic. 2001;52(2):67–87. [Google Scholar]
- Cabrita MJO, Freitas AMC, Laureano O. Aroma compounds in varietal wines from Alentejo, Portugal. J Food Compos Anal. 2007;20(5):375–390. doi: 10.1016/j.jfca.2006.12.006. [DOI] [Google Scholar]
- Chen K, Escott C, Loira I. The effects of pre-fermentative addition of oenological tannins on wine components and sensorial qualities of red wine. Molecules. 2016;21(11):1445–1451. doi: 10.3390/molecules21111445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai P, Hamdi R, Luo G, He H, Zhang M, Termonia P, De Maeyer P. Agriculture intensification increases summer precipitation in Tianshan Mountains, China. Atmos Res. 2019;227:140–146. doi: 10.1016/j.atmosres.2019.05.005. [DOI] [Google Scholar]
- Chen X, Luo G, Xia J, Zhou K, Lou S, Ye M. Ecological response to the climate change on the northern slope of the Tianshan Mountains in Xinjiang. Sci China Ser D Earth Sci. 2005;48(6):765–777. doi: 10.1360/04yd0050. [DOI] [Google Scholar]
- Chen K, Han S-Y, Li M, Sheng W-J. Use of lysozyme and oligomeric proanthocyanidin to reduce sulfur dioxide and the evolution of volatile compounds in italian riesling ice wine during aging process. J Food Process Preserv. 2017;41(1):e12755. doi: 10.1111/jfpp.12755. [DOI] [Google Scholar]
- Chen K, Escott C, Loira I, del Fresno JM, Morata A, Tesfaye W, Calderon F, Suárez-Lepe JA, Han S, Benito S. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018;69:51–63. doi: 10.1016/j.fm.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Chen K, Wen J, Ma L, Wen H, Li J. Dynamic changes in norisoprenoids and phenylalanine-derived volatiles in off-vine Vidal blanc grape during late harvest. Food Chem. 2019;289:645–656. doi: 10.1016/j.foodchem.2019.03.101. [DOI] [PubMed] [Google Scholar]
- Duarte WF, Dias DR, Oliveira JM, Teixeira JA, de Almeida e Silva JB, Schwan RF. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT Food Sci Technol. 2010;43(10):1564–1572. doi: 10.1016/j.lwt.2010.03.010. [DOI] [Google Scholar]
- Feng L, Jia H, Wang J, Qin Y, Liu Y, Song Y. Selection of indigenous Saccharomyces cerevisiae strains for winemaking in Northwest China. Am J Enol Vitic. 2019;70(2):115–126. doi: 10.5344/ajev.2018.18035. [DOI] [Google Scholar]
- Keller M. Chapter 7—Environmental constraints and stress physiology. In: Keller M, editor. The science of grapevines. San Diego: Academic Press; 2010. pp. 227–310. [Google Scholar]
- Keller M. Chapter 3—water relations and nutrient uptake. In: Keller M, editor. The science of grapevines. 2. San Diego: Academic Press; 2015. pp. 101–124. [Google Scholar]
- Lan Y-B, Qian X, Yang Z-J, Xiang X-F, Yang W-X, Liu T, Zhu B-Q, Pan Q-H, Duan C-Q. Striking changes in volatile profiles at sub-zero temperatures during over-ripening of ‘Beibinghong’ grapes in Northeastern China. Food Chem. 2016;212:172–182. doi: 10.1016/j.foodchem.2016.05.143. [DOI] [PubMed] [Google Scholar]
- Leeuwen C, Friant P, Chone X, Tregoat O, Koundouras S, Dubourdieu D. Influence of climate, soil, and cultivar on terroir. Am J Enol Vitic. 2004;55(3):207–217. [Google Scholar]
- Liu B, Xu X-Q, Cai J, Lan Y-B, Zhu B-Q, Wang J. The free and enzyme-released volatile compounds of distinctive Vitis amurensis var. Zuoshanyi grapes in China. Eur Food Res Technol. 2015;240(5):985–997. doi: 10.1007/s00217-014-2403-9. [DOI] [Google Scholar]
- Ma W, Wu Y, Wei Y, Zou W, Yan Y, Xue J, Tian G, Wang L, Wang W, Pan H. Microbial diversity analysis of vineyards in the Xinjiang region using high-throughput sequencing. J Inst Brew. 2018;124(3):276–283. doi: 10.1002/jib.501. [DOI] [Google Scholar]
- Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Jm DI, Klein M. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell. 2007;19(6):2023–2038. doi: 10.1105/tpc.106.046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekoue Nguela J, Vernhet A, Sieczkowski N, Brillouet J-M. Interactions of condensed tannins with Saccharomyces cerevisiae yeast cells and cell walls: tannin location by microscopy. J Agric Food Chem. 2015;63(34):7539–7545. doi: 10.1021/acs.jafc.5b02241. [DOI] [PubMed] [Google Scholar]
- Noble AC. Taste-aroma interactions. Trends Food Sci Technol. 1996;7(12):439–444. doi: 10.1016/S0924-2244(96)10044-3. [DOI] [Google Scholar]
- Perez-Jiménez M, Chaya C, Pozo-Bayón MÁ. Individual differences and effect of phenolic compounds in the immediate and prolonged in-mouth aroma release and retronasal aroma intensity during wine tasting. Food Chem. 2019;285:147–155. doi: 10.1016/j.foodchem.2019.01.152. [DOI] [PubMed] [Google Scholar]
- Pozo-Bayón MÁ, Reineccius G. Interactions between wine matrix macro-components and aroma compounds. In: Moreno-Arribas MV, Polo MC, editors. Wine chemistry and biochemistry. New York: Springer; 2009. pp. 417–435. [Google Scholar]
- Ribereaugayon P, Glories Y, Maujean A, Dubourdieu D, Ribéreaugayon P, Glories Y, Maujean A, Dubourdieu D. Handbook of enology: volume 2. The chemistry of wine stabilization and treatments. New York: Wiley; 2006. pp. 109–139. [Google Scholar]
- Robinson AL, Boss PK, Solomon PS, Trengove RD, Heymann H, Ebeler SE. Origins of grape and wine aroma. Part 2. Chemical and sensory analysis. Am J Enol Vitic. 2014;65(1):25–42. doi: 10.5344/ajev.2013.13106. [DOI] [Google Scholar]
- Sánchez-Palomo E, Gómez García-Carpintero E, Alonso-Villegas R, González-Viñas MA. Characterization of aroma compounds of Verdejo white wines from the La Mancha region by odour activity values. Flavour Fragr J. 2010;25(6):456–462. doi: 10.1002/ffj.2005. [DOI] [Google Scholar]
- Selli S, Prost C, Serot T. Odour-active and off-odour components in rainbow trout (Oncorhynchus mykiss) extracts obtained by microwave assisted distillation–solvent extraction. Food Chem. 2009;114(1):317–322. doi: 10.1016/j.foodchem.2008.09.038. [DOI] [Google Scholar]
- Selli S, Gubbuk H, Kafkas E, Gunes E. Comparison of aroma compounds in Dwarf Cavendish banana (Musa spp. AAA) grown from open-field and protected cultivation area. Sci Hortic. 2012;141:76–82. doi: 10.1016/j.scienta.2012.04.008. [DOI] [Google Scholar]
- Tao Y, Zhang L. Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China) LWT Food Sci Technol. 2010;43(10):1550–1556. doi: 10.1016/j.lwt.2010.06.003. [DOI] [Google Scholar]
- Villamor RR, Ross CF. Wine matrix compounds affect perception of wine aromas. Annu Rev Food Sci Technol. 2013;4(1):1–20. doi: 10.1146/annurev-food-030212-182707. [DOI] [PubMed] [Google Scholar]
- Vitalini S, Gardana C, Zanzotto A, Fico G, Faoro F, Simonetti P, Iriti M. From vineyard to glass: agrochemicals enhance the melatonin and total polyphenol contents and antiradical activity of red wines. J Pineal Res. 2011;51(3):278–285. doi: 10.1111/j.1600-079X.2011.00887.x. [DOI] [PubMed] [Google Scholar]
- Wu YY, Xing K, Zhang XX, Wang H, Wang Y, Wang F, Li JM. Influence of freeze concentration technique on aromatic and phenolic compounds, color attributes, and sensory properties of Cabernet Sauvignon wine. Molecules. 2017;22(6):899. doi: 10.3390/molecules22060899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Qian MC. Development of C13-norisoprenoids, carotenoids and other volatile compounds in Vitis vinifera L. Cv. Pinot noir grapes. Food Chem. 2016;192:633–641. doi: 10.1016/j.foodchem.2015.07.050. [DOI] [PubMed] [Google Scholar]
- Zepka LQ, Garruti DS, Sampaio KL, Mercadante AZ, Da Silva MAAP. Aroma compounds derived from the thermal degradation of carotenoids in a cashew apple juice model. Food Res Int. 2014;56:108–114. doi: 10.1016/j.foodres.2013.12.015. [DOI] [Google Scholar]
- Zhang P, Fuentes S, Siebert T, Krstic M, Herderich M, Barlow EWR, Howell K. Terpene evolution during the development of Vitis vinifera L. cv. Shiraz grapes. Food Chem. 2016;204:463–474. doi: 10.1016/j.foodchem.2016.02.125. [DOI] [PubMed] [Google Scholar]