Abstract
Study Design:
Systematic Review.
Objectives:
To review the literature surrounding the cost-effectiveness of implanting spinal cord stimulators for failed back surgery syndrome.
Methods:
A systematic review was conducted inclusive of all publications in the Medline database and Cochrane CENTRAL trials register within the last 10 years (English language only) assessing the cost-effectiveness of Spinal Cord Stimulator device implantation (SCSdi) in patients with previous lumbar fusion surgery.
Results:
The majority of reviewed publications that analyzed cost-effectiveness of SCSdi compared to conventional medical management (CMM) or re-operation in patients with failed back surgery syndrome (FBSS) showed an overall increase in direct medical costs; these increased costs were found in nearly all cases to be offset by significant improvements in patient quality of life. The cost required to achieve these increases in quality adjusted life years (QALY) falls well below $25 000/QALY, a conservative estimate of willingness to pay.
Conclusions:
The data suggest that SCSdi provides both superior outcomes and a lower incremental cost: effectiveness ratio (ICER) compared to CMM and/or re-operation in patients with FBSS. These findings are in spite of the fact that the majority of studies reviewed were agnostic to the type of device or innervation utilized in SCSdi. Newer devices utilizing burst or higher frequency stimulation have demonstrated their superiority over traditional SCSdi via randomized clinical trials and may provide lower ICERs.
Keywords: spinal cord stimulator, failed back surgery syndrome, cost effectiveness, quality of life, quality adjusted life years, cost benefit, lumbar interbody fusion, electric stimulation therapy, spinal cord, cost utility
Introduction
Low back pain (LBP) continues to burden patients and the health care system. Approximately 20% of adults currently have LBP1 and 90% will experience LBP at some point in their life.2 LBP is the most commonly cited reason for disability, lost work, and lost wages in industrialized nations.3,4 Comorbidities associated with chronic pain including depression, anxiety, and sleep disorders further add to this burden.5
Direct costs of LBP such as the cost of hospitalizations, surgeries, prescriptions, and physical therapy are estimated to be in the hundreds of billions of dollars per year in the US alone.6,7 The indirect costs such as lost wages for missing work, emotional impact of chronic pain and any treatment or aid sought to help it, retraining for new jobs that are more tolerable for the patient, and even healthcare allocation opportunity costs are more difficult to calculate.7
Lumbar fusions for degenerative spondylolisthesis and associated LBP have risen significantly over the past 20 years.8,9 As many as 40% of patients who receive lumbar fusions may continue to have unsatisfactory relief of their symptoms.10 Patients with chronic back pain after a fusion surgery are deemed to have failed back surgery syndrome (FBSS). For some patients with apparent FBSS, there may be imaging findings, such as pseudarthrosis, fractures, or instrumentation failure, that could favor revision surgical treatment to improve symptoms. Nonsurgical treatments for patients with FBSS can include conventional medical management (CMM) including use of analgesics, physical therapy, and cognitive behavioral therapy, among others.11 More interventional treatments for FBSS include medial nerve blocks, epidural injections, additional spinal fusion surgeries, or the implantation of a spinal cord stimulator (SCS).10 At least one high quality study found SCS device implantation (SCSdi) to provide superior patient satisfaction when compared to re-operation in patients with FBSS. Additionally, fewer patients in this study chose to crossover from their SCS device to a re-operation, further establishing this treatment as patient-centric in addition to efficacious.12 This study among others suggests SCSdi to be a safe and effective treatment option for patients with FBSS. However, it is important to also assess the costs of this procedure. Economic evaluation provides decision-making metrics such as incremental cost: effectiveness ratio (ICER) to evaluate against a common willingness-to-pay threshold. When the outcome of interest is quality-adjusted life years (QALY) instead of merely life years gained or specific clinical outcomes, the economic evaluation is called cost-utility analysis. The Cost-Utility and Cost-Effectiveness conveys the same meaning when the effectiveness is on utility measures such as QALY.
As such, direct and indirect ICER of this procedure versus CMM and/or re-operation has come in to question and requires further analysis. The aim of this manuscript was to review the cost-effectiveness of SCSdi compared to CMM and/or repeat/additional fusions in patients who continue to suffer from LBP after at least one lumbar fusion operation.
Methods
Eligibility Criteria
To be included in our analysis, the reviewed manuscript must provide novel data surrounding the cost-effectiveness of SCSdi in FBSS patients; the manuscript’s abstract or title had to contain language suggestive of (1): implantation of SCS devices in FBSS patients, (2): either direct or indirect costs of the patients studied, and (3): a cohort of patients who received CMM and/or re-operation. We also reviewed and included any proposed trials that would meet these criteria when published. We excluded any publications that were replies or comments to other manuscripts. Manuscripts that did not provide novel data surrounding our aims, but discussed previously published data in reviews was included in qualitative analyses but not in our quantitative analyses.
Information Sources
In April 2020, an electronic search of the Medline database and Cochrane CENTRAL trials register was performed to identify studies in the English language published in the past 10 years.
Search Strategy
We utilized the following phrases combined with “and” or “or” Boolean operators: “electrical stimulation,” “spinal cord stimulation,” “electric stimulation therapy,” “spinal cord stimulator,” “dorsal column stimulation,” “dorsal column stimulator,” “spinal fusion,” “lumbar vertebrae,” “low back pain,” “failed back surgery syndrome,” “back pain,” “fbss,” “cost effectiveness,” “cost-benefit analysis, cost-utility analysis, quality of life, and QALY.”
Study Selection
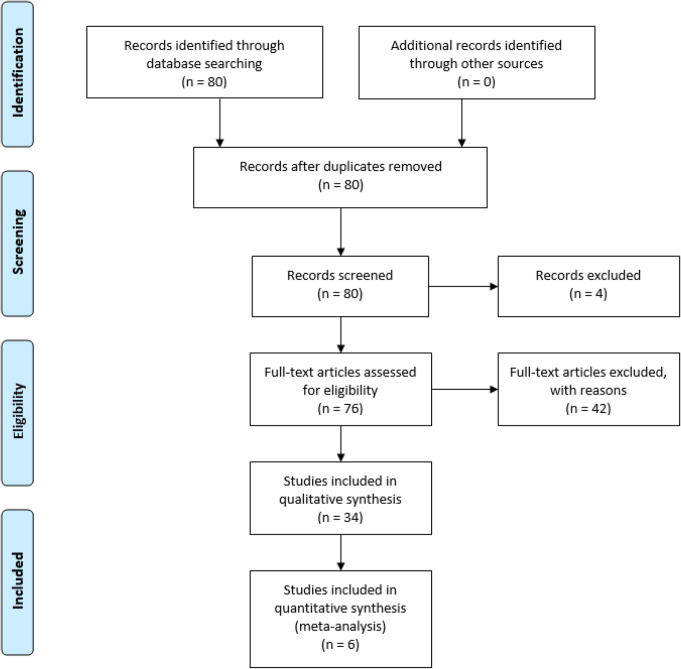
Four independent reviewers screened titles and abstracts from which we selected full-length articles. Reference texts within these full-length articles were also considered. We analyzed all 80 resulting publications for relevancy to our aims. Review of the title and abstract of these 80 studies resulted in 34 studies that met inclusion criteria and lacked the exclusion criterion. Four studies were not reviewed based on our exclusion criteria. The remaining 76 studies failed to meet inclusion criteria. Figure 1 depicts a flow chart summarizing our search methodology and findings.
Figure 1.
Flow diagram of search methodology and results.
Data Collection
We extracted the following items from each article: author, year, sample characteristics including sample size, objective patient outcomes, and length of follow-up. We extracted reported costs and quality of life improvements and broke them down by direct and indirect costs when available.
Risk of Bias Within Individual Studies
We assessed all reviewed studies for the utilization of randomization and blinding in their studies. We additionally reviewed the rates of inclusion/exclusion in each study and the percentage of patients who completed the study after selection in any prospective analyses. We utilized the ROBINS-I assessment tool for determination of likelihood of bias within each study.13 A risk of bias across the studies was not performed due to the variable nature of exchange rates of the currency utilized in each study. As such, we felt comparing effects and confidence intervals of the results to be inappropriate.
Summary Measures
We made and reported comparisons between the difference in means between the groups within each study. No financial comparisons between studies were made due to the difference in currency used for each study and variable exchange rates before, during, and after the time of study.
Results
Adapted from: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed100009714
Tables 1 and 2 provides a brief summary of the manuscripts that provided novel data surrounding the cost-effectiveness of one or more types of SCS compared to CMM and/or re-operation in patients with FBSS published from 2010-2020. The 6 manuscripts reviewed that provided novel information regarding the cost-effectiveness of SCSdi in FBSS patients were all retrospective analyses with one study having an additional prospective arm. These studies were all non-randomized and no blinding was utilized.
Table 1.
Summary of Findings in Novel, Relevant Studies Published in the Last 10 Years.
| Authors | Design | No. pts. | F/U | Pt. outcomes | CUA |
|---|---|---|---|---|---|
| Farber et al15 | Multicenter, retrospective | 122, 827 | 108 mo. | N/A | Data was purely cost related; 40% overall lower costs at 108 mo. compared to CMM |
| Zucco et al16 | Multicenter, observational, ambispective | 80 | 24 mo. | 50% increase in EQ-5D-3 L; 33% decrease in ODI | Total initial cost increase; €/QALY break even w/i 24 mo. |
| Annemans et al17 | Multicenter, retrospective | N/A | 180 mo. | All 3 forms of SCS provided significant improvements in QALYs over CMM | Total initial cost increase w/ any SCS device; only direct costs assessed |
| Kumar and Rizvi18 | Single center, retrospective | 335 | 240 mo. | SCS provided significant improvements in QALYs over CMM | Total cost increase at 240 mo.; $CDN/QALY break even w/i 18 months |
| Hollingworth et al19 | Single center, retrospective | 158 | 24 mo. | <10% of patients in any treatment group achieved pain relief | Total cost increase w/ SCS at 24 mo. of at least $29 000 compared to usual care |
| Taylor et al20 | Model-derived retrospective analysis | N/A | 180 mo. | N/A | Total cost increase at 180 mo. w/ SCS; break even w/i 18 mo. compared to CMM; w/i 12 mo. compared to re-op |
No. Pts. = number of patients; F/U = mean follow-up time; Pt. Outcomes = patient outcomes; CUA = Cost-utility analysis; HF10-SCS = 10 kHz High Frequency Spinal Cord Stimulation.
Table 2.
Cost Utility Analysis in Novel, Relevant Studies Published in the Last 10 Years.
| Cost/intervention/yr | QALYs/intervention/yr | Incremental | |||
|---|---|---|---|---|---|
| Authors | CMM | SCS | CMM | SCS | Cost/QALY |
| Farber et al15 | $10 103.9 | $9611 | N/P | N/P | N/P |
| Zucco et al16 | €6567 | €13 216 | N/P | 0.173* | €3222 |
| Annemans et al17 | €5374 | €5761 | 0.221 | 0.343 | €3153 |
| Kumar and Rizvi18 | $CDN 7676 |
$CDN 8322 |
0.173 | 0.242 | $CDN 9293 |
| Hollingworth et al19 | $19 151 | $18 195 | N/P | N/P | $USD 335 000 |
| Taylor et al20 | €5466 | €5934 | 0.271 | 0.354 | €5622 |
N/P = not provided in published work.
Five of the 6 studies demonstrated similar findings with respect to cost: on average, patients who underwent SCSdi incurred higher total medical costs to their insurer or national health service compared to patients who underwent CMM or re-operation. The bulk of this cost was within the first 12 months and was largely attributed to the cost of the device. One study by Farber et al, however, found conflicting results.15
Of the 5 studies listed in Table 1 that performed some form of a cost-effectiveness analysis, 4 found SCSdi to provide significantly lower ICER metrics.16,17,18,20 The break-even point where the difference in total costs was met by the savings per QALY was consistently found between 18-24 months across these studies. The single reviewed study that found SCS to be cost-ineffective had < 10% of patients who achieved satisfactory pain relief in any study arm, including CMM.19 This finding is much lower than the previously reported findings of 50-80% success with SCS devices. As such, it’s difficult to determine the applicability of the findings within this study.
With respect to long-term cost savings, the data suggest 6,000 to 10 000 dollars in cost-savings per QALY to insurers and national health services when comparing SCSdi to CMM and/or re-operation. Further, the overall medical costs of SCSdi was consistently found to be below $25 000/QALY. In the study with the largest sample size and longest follow-up, reviewing the records of over 120 000 patients over 9 years, there was a reported decrease of over 50% in overall medical costs across the 9-year window for patients who underwent SCSdi compared to those managed with CMM and/or re-operations.15 The drawback to this finding is a lack of pain relief effectiveness reporting; the manuscript did not directly report intervention QALYs for these patients. Their analysis instead relied on previous reports and extrapolations of pain relief effectiveness from them.
All studies reviewed were retrospective with only one study having a prospective arm. As such, there was no randomization and with it a serious risk of bias judgements due to uncontrolled confounders in the pre-intervention portion of these studies. The studies, however, did utilize the same time frame for patients with limited exclusion criteria. The intervention groups in all novel studies reviewed were clearly defined and non-interchangeable. The novel studies reviewed had few excluded patients reviewed with none reporting exclusions due to missing data. Taken together, we believe there was a low risk of bias post-intervention.
Discussion
SCS Technology and Cost-Effectiveness
The field of SCS devices has grown rapidly in the 40 years since its initial utilization. An estimated 30 000 to 50 000 devices are now implanted annually across the world.21 Naturally, the technology powering these devices continues to evolve and improve. Most notably, the types of delivery system used and the frequency and tonicity of the stimulation provided by the device are under heavy development. The use of a more novel paddle design and configuration has shown superior outcomes compared to traditional electrode size and placement.22,23 This is more than likely simply due to having a wider spread of effect and with it a higher chance of providing stimulation to the pathological area(s) of the spinal cord.
Additional technological improvements include the use of SCS devices that provide stimulation at much higher frequencies (10 000 vs. 50-100 Hz). A recent randomized trial demonstrated that not only do patients prefer the higher frequency SCS devices’ lack of paresthesia compared to traditional stimulation devices, the higher frequency devices also provide superior and more durable pain relief.24 A different stimulation method that also seemingly improves upon traditional stimulation methods provides SCS in a burst pattern rather than tonic stimulation.25 A randomized study has also shown this stimulation pattern to be preferable to patients as well as able to provide better pain relief.26 The burst stimulation method is more novel than the high-frequency method. As such, studies assessing its efficacy at time points greater than a year remain unpublished. Further research needs to be conducted to clarify if the burst method provides a non-inferior durability of pain relief that the high-frequency method shows. Literature that examined the cost-effectiveness of these more novel devices was not found. It is reasonable to hypothesize that these devices will provide even greater QALY/cost to patients and insurers than their predecessors.
Further, a parallel improvement in SCS cost-effectiveness would result from prolonging the battery life of non-rechargeable devices. As it currently stands, the published literature that compared the cost-effectiveness of non-rechargeable and rechargeable devices showed a slight benefit to rechargeable devices. This is largely due to having fewer replacements over the patient’s lifetime and the associated surgical costs. The industry standard device longevity for non-rechargeable devices is ∼4.5 years.27 If a non-rechargeable device does not require replacement until after 4.5 years from initial implantation, it becomes more economical to utilize compared to the rechargeable models, given the initial device costs are similar. As such, if the cost of non-rechargeable devices could be maintained while simultaneously improving battery life, this would further improve cost-effectiveness of SCS devices.
Improving SCS Cost-Effectiveness With Refined Patient Selection
An alternative method to improving the cost effectiveness of SCS devices is further refining patient selection. Several studies have analyzed this; however, most of them utilize rather small sample sizes. Combining the findings from these studies, an ideal responder would not use tobacco,28 be of normal weight,29 and be free of psychiatric comorbidities other than anxiety.30-32 The data surrounding which age group might better respond to SCS for LBP is mixed. One study suggested that younger patients fared worse29 while another suggested older patients have inferior responses.31 One possible explanation for this disparity might be the finding that the efficacy of SCS decreases as latency between pain onset and treatment or the number of prior interventions increases.33 As it also relates to patient selection, North et al found that patients who failed SCSdi and crossed over to re-operation failed to achieve adequate pain relief.12 This cross-over resulted in inferior outcomes for patients of lesser pain-relief achieved and lower patient satisfaction, both coming at higher costs as well; a patient who did not respond to SCS and underwent subsequent re-operation ended up costing more than double the average patient who just had re-operation and over 5 times the amount of a patient just receiving SCSdi.
Improving SCS Cost-Effectiveness With More Encompassing Data Analysis
Often overlooked when comparing the cost of SCSdi to decompression and fusion surgeries are the indirect costs to patients. A particularly relevant indirect cost with respect to patients with LBP are side effects and unintended consequences from opioid use which may be difficult to fully estimate before reoperation. Estimates of the burden of opioid use in post-lumbar fusion patients suggest upward of 30 000 dollars in medical cost differences between patients who uses opioids in the short term after a surgery compared to those who use opioids for more than a year after surgery.34-37 The data surrounding the use of opioids after neuromodulation suggests either no difference or overall less opioid usage at 1 year post SCSdi compared to lumbar decompressions and fusions.38,39
The field of SCS device implantation currently has 5 randomized controlled trials comparing the safety and efficacy of SCS device implantation compared to CMM and/or re-operation of the lumbar spine.12,24,40-42 While these studies all demonstrate the superior efficacy and safety profiles of SCSdi compared to both CMM and re-operation as far out as 120 months, they do not assess cost-effectiveness. It is clear that additional research will be needed to further delineate SCS effectiveness, patient-selection, and cost-effectiveness. Several multicenter randomized controlled trials have been proposed including the ESTIMET43 and EVIDENCE44 studies; their data remain unpublished. The execution and evaluation of results from these studies will help guide care for patients.
Limitations
This analysis relies on primarily retrospective, non-randomized data. The likelihood of confounders between patients who elect for SCSdi compared to those who do not is nearly guaranteed. As such, the presented data need to be considered thoughtfully. This review also lacks data from studies that are sure to alter the landscape either negatively or positively for SCSdi as an alternative for CMM. As these studies finish and are published, the conclusions made by this review may be made obsolete by technology. The efficacy, direct costs, or indirect costs are likely to be impacted by more manufacturers designing devices, improved technology, continued surgical experience, and compete against improvements to CMM as well.
Strengths
The primary strength of this analysis is its direct and narrow question. The number of manuscripts published that attempt to answer our proposed question of the cost-utility of SCSdi compared to CMM in FBSS patients is small. Additionally, the data found and presented in the studies were attained in a very uniform fashion, across a span of nearly a decade, and minimized bias when possible.
Conclusions
The literature suggests SCSdi is likely more expensive in the short term when compared to CMM or re-operation for patients with FBSS. This initial expense is likely negated by the improvements in quality of life SCSdi provides when compared to CMM or re-operation. The literature reported the cost/QALY for SCSdi to be lower than even the conservative estimate of $25 000/QALY for an insurer’s willingness to pay. The break-even point for the initial up-front costs seems to be ∼24 months; one large analysis suggested there is more than a 50% reduction in overall medical cost-savings with SCSdi compared to CMM and/or re-operation.
Using the GRADE approach,45 we believe the studies reviewed provide a moderate quality of evidence. We believe the strong likelihood of confounders being present greatly reduces the quality. However, this is partially offset by the striking consistency and large magnitude of effect on lower ICERs found across FBSS patients receiving SCS devices across different settings and time frames compared to FBSS patients who received CMM or secondary operations.
With the proposed large scale, multicenter, randomized controlled trials currently ongoing, it is likely we will see much more robust and applicable cost-effectiveness analyses that have greatly diminished if not absent confounders published in the coming years. These studies are also likely to include and differentiate the more novel high-frequency and burst devices as well as provide more encompassing costs of SCSdi compared to alternative treatments.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Smith reports personal fees from Zimmer Biomet, personal fees from Nuvasive, personal fees from Cerapedics, personal fees from Carlsmed, personal fees from Stryker, grants and personal fees from DePuy Synthes/ISSG, other from Alphatec, grants from NREF, grants from AO, outside the submitted work. This supplement was supported by a grant from AO Spine North America.
ORCID iD: Jesse J. McClure, PharmD, PhD  https://orcid.org/0000-0002-5689-4731
https://orcid.org/0000-0002-5689-4731
References
- 1. Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt CO, Raspe H, Pfingsten M, et al. Back pain in the German adult population: prevalence, severity, and sociodemographic correlates in a multiregional survey. Spine. 2007;32(18):2005–2011. [DOI] [PubMed] [Google Scholar]
- 3. Straus BN. Chronic pain of spinal origin: the costs of intervention. Spine. 2002;27(22):2614–2619; discussion 2620. [DOI] [PubMed] [Google Scholar]
- 4. Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–974. [DOI] [PubMed] [Google Scholar]
- 5. Taylor RS, Taylor RJ. The economic impact of failed back surgery syndrome. Br J Pain. 2012;6(4):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21–24. [DOI] [PubMed] [Google Scholar]
- 7. Mekhail N, Wentzel DL, Freeman R, Quadri H. Counting the costs: case management implications of spinal cord stimulation treatment for failed back surgery syndrome. Prof Case Manage. 2011;16(1):27–36. [DOI] [PubMed] [Google Scholar]
- 8. Kepler CK, Vaccaro AR, Hilibrand AS, et al. National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine. 2014;39(19):1584–1589. [DOI] [PubMed] [Google Scholar]
- 9. Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012;37(1):67–76. [DOI] [PubMed] [Google Scholar]
- 10. Chan CW, Peng P. Failed back surgery syndrome. Pain Med. 2011;12(4):577–606. [DOI] [PubMed] [Google Scholar]
- 11. Daniell JR, Osti OL. Failed back surgery syndrome: a review article. Asian Spine J. 2018;12(2):372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106; discussion 106-107. [DOI] [PubMed] [Google Scholar]
- 13. Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farber SH, Han JL, Elsamadicy AA, et al. Long-term cost utility of spinal cord stimulation in patients with failed back surgery syndrome. Pain Physician. 2017;20(6):E797–e805. [PMC free article] [PubMed] [Google Scholar]
- 16. Zucco F, Ciampichini R, Lavano A, et al. Cost-effectiveness and cost-utility analysis of spinal cord stimulation in patients with failed back surgery syndrome: results from the PRECISE study. Neuromodulation. 2015;18(4):266–276; discussion 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Annemans L, Van Buyten JP, Smith T, Al-Kaisy A. Cost effectiveness of a novel 10 kHz high-frequency spinal cord stimulation system in patients with failed back surgery syndrome (FBSS). J Long Term Eff Med Implants. 2014;24(2-3):173–183. [DOI] [PubMed] [Google Scholar]
- 18. Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013;14(11):1631–1649. [DOI] [PubMed] [Google Scholar]
- 19. Hollingworth W, Turner JA, Welton NJ, et al. Costs and cost-effectiveness of spinal cord stimulation (SCS) for failed back surgery syndrome: an observational study in a workers’ compensation population. Spine. 2011;36(24):2076–2083. [DOI] [PubMed] [Google Scholar]
- 20. Taylor RS, Ryan J, O’Donnell R, et al. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain. 2010;26(6):463–469. [DOI] [PubMed] [Google Scholar]
- 21. Duy PQ, Anderson WS. Two surgeries do not always make a right: spinal cord stimulation for failed back surgery syndrome. Yale J Biol Med. 2018;91(3):323–331. [PMC free article] [PubMed] [Google Scholar]
- 22. Babu R, Hazzard MA, Huang KT, et al. Outcomes of percutaneous and paddle lead implantation for spinal cord stimulation: a comparative analysis of complications, reoperation rates, and health-care costs. Neuromodulation. 2013;16(5):418–426; discussion 426-417. [DOI] [PubMed] [Google Scholar]
- 23. Kinfe TM, Quack F, Wille C, et al. Paddle versus cylindrical leads for percutaneous implantation in spinal cord stimulation for failed back surgery syndrome: a single-center trial. J Neurol Surg A Cent Eur Neurosurg. 2014;75(6):467–473. [DOI] [PubMed] [Google Scholar]
- 24. Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79(5):667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Ridder D, Plazier M, Kamerling N, et al. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013;80(5):642–649.e641. [DOI] [PubMed] [Google Scholar]
- 26. Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56–66. [DOI] [PubMed] [Google Scholar]
- 27. Hornberger J, Kumar K, Verhulst E, et al. Rechargeable spinal cord stimulation versus non-rechargeable system for patients with failed back surgery syndrome: a cost-consequences analysis. Clin J Pain. 2008;24(3):244–252. [DOI] [PubMed] [Google Scholar]
- 28. De La Cruz P, Fama C, Roth S, et al. Predictors of spinal cord stimulation success. Neuromodulation. 2015;18(7):599–602; discussion 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bir SC, Konar S, Maiti T, et al. Neuromodulation in intractable pain management: outcomes and predictors of revisions of spinal cord stimulators. Neurosurg Focus. 2016;40(5): E4. [DOI] [PubMed] [Google Scholar]
- 30. Fama CA, Chen N, Prusik J, et al. The Use of preoperative psychological evaluations to predict spinal cord stimulation success: our experience and a review of the literature. Neuromodulation. 2016;19(4):429–436. [DOI] [PubMed] [Google Scholar]
- 31. Burchiel KJ, Anderson VC, Wilson BJ, et al. Prognostic factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery. 1995;36(6):1101–1110; discussion 1110-1101. [DOI] [PubMed] [Google Scholar]
- 32. Tamai K, Buser Z, Wang C, et al. The primary diagnosis and the coexisting anxiety disorders have no impact on the additional surgical procedure after spinal cord stimulators implantation: an analysis of 11,029 patients. J Clin Neurosci. 2018;47:208–213. [DOI] [PubMed] [Google Scholar]
- 33. North R, Shipley J, Prager J, et al. Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med. 2007;8(suppl 4):S200–275. [DOI] [PubMed] [Google Scholar]
- 34. Anderson JT, Haas AR, Percy R, et al. Chronic opioid therapy after lumbar fusion surgery for degenerative disc disease in a workers’ compensation setting. Spine. 2015;40(22):1775–1784. [DOI] [PubMed] [Google Scholar]
- 35. Anastassopoulos KP, Chow W, Tapia CI, et al. Economic study on the impact of side effects in patients taking oxycodone controlled-release for noncancer pain. J Manag Care Pharm. 2012;18(8):615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin BI, Turner JA, Mirza SK, et al. Trends in health care expenditures, utilization, and health status among US adults with spine problems, 1997-2006. Spine. 2009;34(19):2077–2084. [DOI] [PubMed] [Google Scholar]
- 37. Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine. 2014;39(7):556–563. [DOI] [PubMed] [Google Scholar]
- 38. Burchiel KJ, Anderson VC, Brown FD, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine. 1996;21(23):2786–2794. [DOI] [PubMed] [Google Scholar]
- 39. Sanders RA, Moeschler SM, Gazelka HM, et al. Patient outcomes and spinal cord stimulation: a retrospective case series evaluating patient satisfaction, pain scores, and opioid requirements. Pain Pract. 2016;16(7):899–904. [DOI] [PubMed] [Google Scholar]
- 40. Kumar K, Nath R, Wyant GM. Treatment of chronic pain by epidural spinal cord stimulation: a 10-year experience. Journal of neurosurgery. 1991;75(3):402–407. [DOI] [PubMed] [Google Scholar]
- 41. Rigoard P, Basu S, Desai M, et al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain. 2019;160(6):1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eldabe SS, Taylor RS, Goossens S, et al. A Randomized controlled trial of subcutaneous nerve stimulation for back pain due to failed back surgery syndrome: the SubQStim study. Neuromodulation. 2019;22(5):519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roulaud M, Durand-Zaleski I, Ingrand P, et al. Multicolumn spinal cord stimulation for significant low back pain in failed back surgery syndrome: design of a national, multicentre, randomized, controlled health economics trial (ESTIMET Study). Neurochirurgie. 2015;61(suppl 1):S109–116. [DOI] [PubMed] [Google Scholar]
- 44. North RB, Kumar K, Wallace MS, et al. Spinal cord stimulation versus re-operation in patients with failed back surgery syndrome: an international multicenter randomized controlled trial (EVIDENCE study). Neuromodulation. 2011;14(4):330–335; discussion 335-336. [DOI] [PubMed] [Google Scholar]
- 45. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924. [DOI] [PMC free article] [PubMed] [Google Scholar]