Abstract
Imaging plays a key role in the diagnosis and characterization of thyroid diseases, and the information provided by imaging studies is essential for management planning. A referral guideline for imaging studies may help physicians make reasonable decisions and minimize the number of unnecessary examinations. The Korean Society of Thyroid Radiology (KSThR) developed imaging guidelines for thyroid nodules and differentiated thyroid cancer using an adaptation process through a collaboration between the National Evidence-based Healthcare Collaborating Agency and the working group of KSThR, which is composed of radiologists specializing in thyroid imaging. When evidence is either insufficient or equivocal, expert opinion may supplement the available evidence for recommending imaging. Therefore, we suggest rating the appropriateness of imaging for specific clinical situations in this guideline.
Keywords: Thyroid, Thyroid malignancy, Thyroid nodule, Thyroid recurrent cancers, Guideline
INTRODUCTION
Thyroid nodules are a common finding in clinical practice. Approximately 5–15% of thyroid nodules are malignant [1], and the incidence of thyroid cancer is increasing due to various factors.
Various imaging techniques and procedures have been developed for the differential diagnosis of thyroid nodules. These imaging techniques include ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI). When a biopsy reveals thyroid cancer, imaging plays several roles according to the histologic subtype of the malignancy. Differentiated thyroid carcinoma (DTC) arising from thyroid follicular epithelial cells accounts for the majority of thyroid cancers. Papillary cancer accounts for approximately 85% of DTC cases, while follicular cancers account for approximately 12% [2]. In DTC, imaging plays an important role in preoperative staging, operative planning, and surveillance after treatment and in cases of suspected recurrence.
In the 1990s, the American College of Radiology (ACR) recognized the need to define national guidelines for the use of imaging technologies to assist physicians in making decisions. These guidelines are known as the ACR appropriateness criteria (ACR AC). A few studies have been published, including those on the management of incidental thyroid nodules detected on imaging [3] and the ACR AC for thyroid diseases [4].
The number of imaging procedures performed in Korea is estimated to be high, given the country's size and population [5]. However, this number may even be underestimated because many privately-funded examinations are not included [6]. Due to the highly commercial nature of these privately-funded examinations, quality control is required to prevent abuse. In Korea, the rate of imaging of the thyroid is predicted to increase because of its technological progress and the expansion of the insurance system. In the field of radiology, imaging examinations should abide by the principle of justification to protect patients from medical radiation exposure. According to this principle, the benefits must outweigh the potential harm in all circumstances in which patients are exposed, and clinicians should perform only necessary examinations. Apart from the specific medical situation of Korea, it is crucial to define generalizable indications for thyroid imaging to facilitate improvements in effective patient care. The development committee of the Korean Society of Thyroid Radiology (KSThR) has arrived at a consensus that a generalizable and precise AC is currently lacking and that there is a need for comprehensive AC guidelines from various international societies that could also provide detailed recommendations to maximize effectiveness in individual imaging examinations. Therefore, the purpose of this work is to provide a current and comprehensive evidence-based clinical guideline for the evaluation of thyroid nodules and DTC in specific clinical situations.
METHODOLOGY
The guidelines were developed through a collaboration between the KSThR and the National Evidence-based Healthcare Collaborating Agency (NECA) [7]. The scope of the guidelines is presented in Table 1.
Table 1. Scope of the Guidelines.
| Category | Content |
|---|---|
| Disease/condition(s) | Benign thyroid nodule, recurrent thyroid cancer, follicular neoplasm, and primary thyroid cancer |
| Guideline category | Diagnosis |
| Clinical specialty | Thyroid specialists (radiologists, endocrinologists, surgeons, nuclear medicine physicians, cytopathologists, family practice physicians, and other thyroid specialists) |
| Guideline objective(s) | To evaluate the appropriate use of imaging methods for diagnosing thyroid tumors and recurrent cancers |
| Target population | Patients with thyroid tumors |
| Patient-specific conditions and patient preferences that may influence the choice of diagnostic tools are considered before and after surgery | |
| Diagnosis | US including Doppler US |
| US-guided biopsy | |
| CT | |
| MRI | |
| Major outcomes considered | Utility of imaging modalities for diagnosing thyroid tumors and imaging analysis after surgery |
CT = computed tomography, MRI = magnetic resonance imaging, US = ultrasonography
The KSThR has made efforts to provide scientific evidence for the imaging-based management of nodular thyroid disease and thyroid cancers. The KSThR has published clinical guidelines for diagnostic imaging and interventional management of thyroid nodules since 2011, including US diagnosis and imaging-based management [8,9], fine-needle aspiration (FNA) [10], core needle biopsy [11,12], radiofrequency [13,14], and ethanol ablation therapy [15]. The NECA is a national agency that provides evidence-based information about medical devices, medicines, and health technology obtained through objective and reliable analyses. The KSThR launched a committee comprising 15 members appointed by the KSThR and the NECA to develop clinical practice guidelines for thyroid nodules and DTC. The committee included medical imaging specialists, research methodology specialists, and clinical guideline specialists to coordinate the overall planning and research methodology. The committee established an adaptation methodology for Korean clinical imaging guidelines (K-CIG) for guideline development [7]. The methodology, identifying information, and availability are summarized in Tables 2 and 3.
Table 2. Methodology.
| Category | Content |
|---|---|
| Methods used to collect/select the evidence | Searches of electronic databases, including Ovid-MEDLINE, EMBASE, KoreaMed, and G–I–N |
| Literature search procedure | The Medline literature search was based on keywords provided, and validated by methodology specialists and the main authors |
| Methods used to formulate the recommendations | Expert consensus |
| Cost analysis | The cost of imaging tools varies by country. Based on specific conditions including insurance, cost is considered for decisions based on the current evidence |
| Method of guideline validation | Internal peer review was performed by members of the Korean Society of Thyroid Radiology, after making a draft available for 1 month, on the website of the Korean Society of Thyroid Radiology (htpp://www.thyroidimaging.kr) |
Table 3. Identifying Information and Availability.
| Category | Content |
|---|---|
| Date released | 2021 |
| Guideline developer(s) | Korean Society of Radiology and Korean Society of Thyroid Radiology |
| Source(s) of funding | Korean Society of Radiology and Korean Society of Thyroid Radiology, Republic of Korea |
| Guideline committee | Committee on Guidelines and Task Force Team of Korean Society of Thyroid Radiology and member of National Evidence-based Healthcare Collaborating Agency |
| Composition of the group that authored the guidelines: Ji-hoon Kim, MD, PhD; Jung Hwan Baek, MD, PhD; Ji Ye Lee, MD; Hye Shin Ahn, MD; Yoon Jung Choi, MD; Eun Ju Ha, MD, PhD; So Lyung Jung, MD, PhD; Young Hen Lee, MD, PhD; Jeong Seon Park, MD, PhD; Jung Hee Shin, MD, PhD; Jin Yong Sung, MD; Jung Hyun Yoon, MD, PhD; Yoo Jin Lee, MD, PhD; Miyoung Choi, PhD; Dong Gyu Na, MD, PhD | |
| Financial disclosures/conflicts of interest | None of the members of the Guideline Committee has a financial disclosure or conflict of interest except Jung Hwan Baek. He has been a consultant of two radiofrequency companies, STARmed and RF Medical, since 2017 |
| Guideline status | This is the current release of the guideline |
| Guideline availability | Electronic copy on the website of the Korean Society of Thyroid Radiology (http://www.thyroidimaging.kr) |
| Previous guidelines | Not available |
Defining Clinical Situations
Clinical situations selected by the working group were reviewed by the development committee and a consensus group composed of clinical experts. Four clinical situations were identified.
1) Evaluation of known or suspected thyroid nodules, initial imaging.
2) Preoperative evaluation of DTC.
3) Early imaging after surgery for DTC.
4) Suspected recurrence of DTC.
Radiation Level
The relative radiation level (RRL) in the ACR AC was established according to the effective dose measured in mSv. The final RRL used in the K-CIG was determined after reviewing the ACR AC, as well as the recent literature and irradiation dose study results from Korea. Imaging examinations and radiation doses are indicated using symbols, which are described in the K-CIG (Supplementary Table 1) [7].
Search for Guidelines
A systematic search for previously-established guidelines was performed using international databases published up to October 2019, including Ovid-MEDLINE, Ovid-EMBASE, and National Guideline Clearinghouse, as well as major domestic databases.
Selection of Searched Guidelines
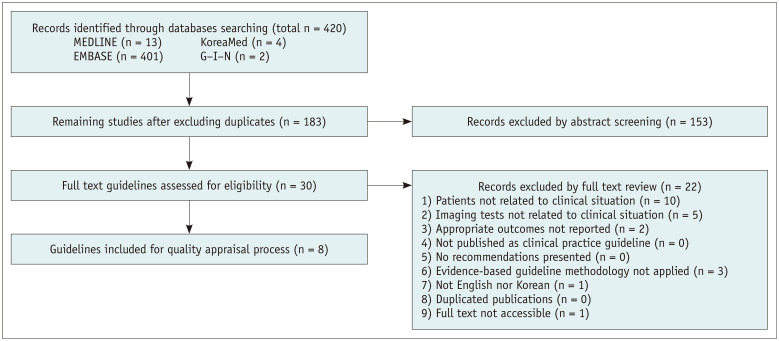
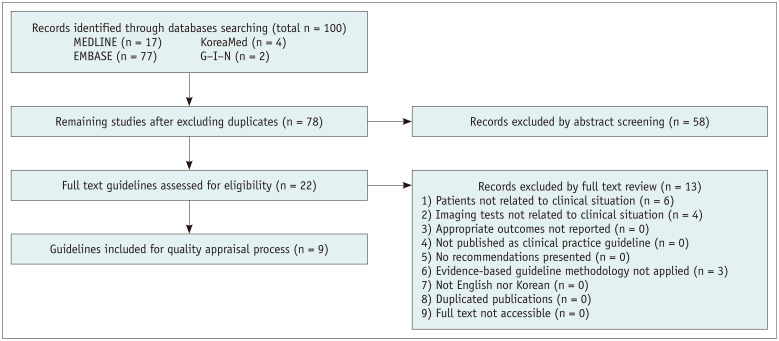
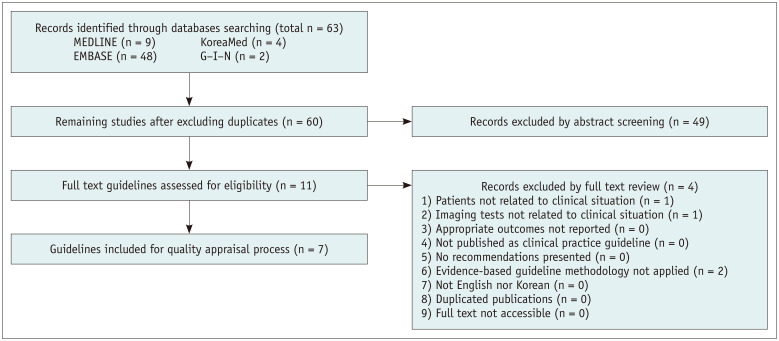
After identifying previously-established guidelines addressing clinical situations 1, 2, 3, and 4, guideline selection was performed independently based on predefined inclusion/exclusion criteria. After excluding duplicates, screening titles, and abstracts, the full texts of the remaining articles were read, and eight, nine, seven, and seven articles were selected for clinical situations 1, 2, 3, and 4, respectively (Figs. 1, 2, 3).
Fig. 1. Flow diagram of guideline selection (clinical situation 1).
Fig. 2. Flow diagram of guideline selection (clinical situation 2).
Fig. 3. Flow diagram of guideline selection (clinical situations 3 and 4).
Quality Appraisal of the Guidelines
Quality appraisal of the selected guidelines was performed by two members of the development committee (with 7 years of experience in thyroid imaging, and an expert in clinical guideline development) using the Korean Appraisal of Guidelines for Research and Evaluation II tool [16]. Guidelines with a score of < 50 in the “Rigour of Development” domain were not recommended by the development committee.
Domestic guidelines that were developed by experts were selected despite a low score [7]. Eight, seven, five, and five guidelines were finally selected for clinical situations 1, 2, 3, and 4, respectively.
Grading the Level of Evidence
The members of the development committee reviewed the literature supporting the recommendations of the selected guidelines. The level of evidence of each literature citation was graded according to the evidence level criteria of the K-CIG [7]. The details of the level of evidence grading and assessment of recommendations are described in Table 4. The evidence used is summarized in Supplementary Table 2.
Table 4. Criteria for Evidence Level.
| Criteria for level of evidence of selected literature | |
| Level | Content |
| 1 | Research satisfying all of the following three criteria: |
| Criteria 1. Good reference standard | |
| Criteria 2. Consecutive patients' study | |
| Criteria 3. Blind interpretation | |
| Systematic review of level 1 | |
| Randomized controlled trial or cross-sectional cohort study that compares index test with comparators | |
| 2 | Research satisfying the following two criteria: |
| Criteria 1. Good reference standard | |
| Criteria 2. Consecutive patient study or blind interpretation | |
| Systematic review of level 2 | |
| Observational studies that compare index test with comparators | |
| 3 | Without consistently applied reference standards |
| 4 | Case-control study |
| Poor or non-independent reference standard | |
| 5 | Expert opinion |
| Definition of overall evidence level for each clinical scenario | |
| Overall Evidence level | Definition |
| High (I) | Results are from appropriately designed experiments with low risk of bias |
| Moderate (II) | Results are from appropriately designed experiments with intermediate risk of bias |
| Low (III) | Results are from inappropriately designed experiments, or risk of bias is high |
| Very low (IV) | Results are from inappropriately designed experiments, or risk of bias is high |
Definition of Appropriateness
The concept of appropriateness, as applied to health care, balances the risks and benefits of a treatment, test, or procedure in the context of available resources for an individual patient with specific characteristics (Table 5) [17].
Table 5. Definition of Appropriateness.
| Grading | Definition | Level of Evidence |
|---|---|---|
| Usually appropriate | The imaging procedure is indicated in the specified clinical scenarios when benefits generally outweigh risks; an effective option for individual care plans, although not always necessary depending on physician judgment and patient-specific preferences | High or moderate |
| May be appropriate | The imaging procedure may be indicated in the specified clinical scenarios as an alternative to imaging procedures with a more favorable risk–benefit ratio, or the risk–benefit ratio for patients is equivocal; effectiveness for individual care must be determined by a patient's physician in consultation with the patient on the basis of additional clinical variables and judgment along with patient preferences | High or moderate |
| May be appropriate, disagreement | The individual suggestions are dispersed | Low |
| Usually not appropriate | The imaging procedure is unlikely to be indicated in the specified clinical scenario with lack of clear benefit/risk advantage; rarely an effective option for individual care plans; exceptions should have documentation of the clinical reasons for proceeding with this care option | High or moderate |
Document Drafting and Finalizing the Recommendations
After a discussion and comparison of the recommendations and the evidence in the literature for the selected guidelines, draft recommendations were prepared by the guideline committee. Quality of evidence, clinical benefits and harms, acceptability and applicability, and radiation dose were used for the drafting of guidelines using the methodology described in the RAND Corporation/University of California Los Angeles Appropriateness Method User Manual [18].
Panels composed of experts in thyroid radiology and methodology reviewed the evidence and discussed the recommendations. For each clinical situation, feedback and suggestions for revisions were obtained from panel members via e-mail and face-to-face meetings. All suggestions were regularly reviewed by all panel members through tracked changes and online meetings. The drafted document was revised until no further revisions were requested by any panel member. Thus, a general consensus was reached regarding the recommendations and the manuscript. The evidence level and net benefits were the main factors determining the recommendations. Appropriateness was discussed further to consider the clinical applicability of the guidelines and their impact on patient outcomes. After a draft of the guidelines was finalized, an internal peer review was performed by members of the KSThR. The draft was made available for a month on the KSThR website (http://www.thyroidimaging.kr). For clinical situations and imaging modalities with a low level of evidence, the recommendations were often based on expert opinion.
RECOMMENDATIONS
Clinical Situation 1: Evaluation of Known or Suspected Thyroid Nodules, Initial Imaging
1. Neck US is usually appropriate for the evaluation of suspected thyroid nodules.
2. Neck US with US-guided biopsy of thyroid nodules is usually appropriate for the evaluation of thyroid nodules.
3. Neck CT or MRI with or without intravenous (IV) contrast is usually not appropriate for initial imaging of known or suspected thyroid nodules. Neck CT or MRI with IV contrast can be additionally performed when a nodule is suspicious for intrathoracic extension or advanced thyroid cancer.
Clinical and Imaging Rationale
For patients with clinically-suspected thyroid nodules, imaging should be performed to determine the origin of the mass and to evaluate associated changes in the thyroid gland and neck. After reviewing the guidelines for the diagnosis and treatment of thyroid nodules, the final eight guidelines were selected for clinical situation 1 [2,4,19,20,21,22,23]. Table 6 shows the recommendation matrix of the existing guidelines for clinical situation 1.
Table 6. Recommendation Matrix of Existing Guidelines (Clinical Situation 1: Known or Suspected Thyroid Nodule, Initial Imaging).
| Source Guidelines | AGREE II | Recommendation | Grading of Recommendation |
|---|---|---|---|
| 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer | 72 | Thyroid sonography with survey of cervical lymph nodes should be performed in all patients with known or suspected thyroid nodules | Strong recommendation, high-quality evidence |
| AACE/AME/ETA Medical Guidelines for Clinical Practice for Diagnosis and Management of Thyroid Nodules | 72 | US evaluation is recommended for patients who are at risk for thyroid malignancy, have palpable thyroid nodules or goiter, or have neck lymphadenopathy suggestive of a malignant lesion MRI and CT are not recommended for routine thyroid nodule evaluation | Grade A; BEL 2 |
| British Thyroid Association Guidelines for the Management of Thyroid Cancer | 84 | US is extremely sensitive examination for thyroid nodules. It can be specific for diagnosis of thyroid carcinoma (particularly papillary carcinoma), and aids decision making about which nodules to perform FNA | Good practice point |
| 1) All patients being investigated for possible thyroid cancer should undergo US of neck in secondary care by appropriate, competent practitioner | |||
| MRI or CT scanning is indicated when the limits of the goiter cannot be determined clinically or for fixed tumors or in patients with hemoptysis | Not available | ||
| Gadolinium-enhanced MRI may provide useful information without compromising subsequent radioiodine uptake by any remaining thyroid tissue | |||
| US Diagnosis and Imaging-based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations | 50 | Among modern imaging modalities, high-resolution US is most sensitive diagnostic modality for detection of thyroid nodules, and it is necessary to perform US for nodules found after palpation | Not available |
| ACR Appropriateness Criteria® Thyroid Disease | 69 | US thyroid; usually appropriate | Limited |
| CT neck with IV contrast; may be appropriate | Limited | ||
| CT neck without IV contrast; may be appropriate | |||
| MRI neck without and with IV contrast; usually not appropriate | Expert consensus | ||
| MRI neck without IV contrast; usually not appropriate | |||
| Primary Imaging Test and Appropriate Biopsy Methods for Thyroid Nodules: Guidelines by Korean Society of Radiology and National Evidence-based Healthcare Collaborating Agency | We recommend neck US for the diagnosis of thyroid nodules detected by imaging other than US or in patients with suspected thyroid nodules | Recommendation grade A, evidence level II | |
| NCCN Clinical Practice Guidelines in Oncology, Thyroid Carcinoma, Version 1. 2019 | 75 | For thyroid nodules known or suspected on clinical or imaging findings, US is recommended | 2A |
| Revised Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Thyroid Cancer | 33 | Thyroid sonography with survey of cervical lymph nodes should be performed in all patients with known or suspected thyroid nodules | 1 |
AACE/AME/ETA = American Association of Clinical Endocrinologists/Italian Association of Clinical Endocrinologists/European Thyroid Association, ACR = American College of Radiology, CT = computed tomography, FNA = fine-needle aspiration, IV = intravenous, MRI = magnetic resonance imaging, NCCN = National Comprehensive Cancer Network, US = ultrasonography
Neck US
The eight guidelines recommend neck US as a primary diagnostic modality when a thyroid nodule is suspected [2,4,19,20,21,22,23]. US is a highly sensitive method for the detection of thyroid nodules and can be used to determine the size and morphological features of the nodule, to determine nodal involvement [24,25,26,27,28,29], and to determine the need for FNA [30,31]. Morphologic features and nodule size were used to guide the decision to perform biopsy under various risk stratification criteria [9,19,21,23,32,33]. US-guided biopsy or follow-up should be performed according to the findings of initial US scans together with other clinical and laboratory findings [27].
Neck CT and Neck MRI
There is insufficient evidence supporting the ability of CT and MRI to differentiate thyroid cancers from benign nodules unless there is evidence of either local tumor invasion or metastasis [3,34]; however, these methods may be helpful for determining the extent of goiter with intrathoracic or retropharyngeal extension. They may be used as additional modalities if there is a suspicion of advanced thyroid cancer (aerodigestive tract or recurrent laryngeal nerve invasion, lymph node [LN] metastasis) [4,19,23]. In patients with advanced disease, IV contrast agents should be administered to optimize imaging. CT can define the degree of tracheal compression more effectively than US. Although MRI is an alternative to CT, CT is preferred because it is associated with a lower risk of respiratory motion artifacts, shorter scan time, and a higher resolution. Therefore, CT is more effective for evaluating LNs in the entire neck. However, the preferred modality for a suspected thyroid nodule is US [35].
Clinical Situation 2: Preoperative Evaluation of DTC
1. Neck US is usually appropriate for the preoperative evaluation of DTC.
2. US-guided biopsy and/or washout thyroglobulin (Tg) for neck LNs are usually appropriate for the preoperative evaluation of DTC.
3. Neck CT or MRI with IV contrast may be appropriate as an adjunct to US for the preoperative evaluation of DTC with clinical suspicion of advanced disease.
4. Chest CT with IV contrast may be appropriate to evaluate the lung parenchyma or the mediastinum in patients with clinical suspicion of advanced disease.
Clinical and Imaging Rationale
The basic goals of initial therapy for patients with DTC are to remove the primary tumor, treat the disease that has extended beyond the thyroid capsule, and treat clinically significant LN metastases [21]. The completeness of surgical resection is an important determinant of the outcome, whereas residual metastatic LNs represent the most common site of persistent disease or recurrence [36,37,38]. Preoperative staging and the risk of recurrence determine the need for radioiodine ablation (RAI) therapy after thyroidectomy, as well as the protocol for postoperative surveillance [21]. Therefore, accurate preoperative staging is important for surgical planning and predicting tumor recurrence. After reviewing the guidelines for the diagnosis and treatment of thyroid nodules, seven guidelines were selected for clinical situation 2 [2,4,9,19,21,23,39,40]. Table 7 shows the recommendation matrix of the existing guidelines for clinical situation 2.
Table 7. Recommendation Matrix of Existing Guidelines (Clinical Situation 2: Preoperative Evaluation of Thyroid Cancer).
| Source Guidelines | AGREE II | Recommendation | Grading of Recommendation |
|---|---|---|---|
| 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer | 72 | Preoperative neck US for cervical (central and especially lateral neck compartments) lymph nodes is recommended for all patients undergoing thyroidectomy for malignant or suspicious for malignancy cytologic or molecular findings | Strong recommendation, high-quality evidence |
| Preoperative use of cross-sectional imaging studies (CT, MRI) with IV contrast is recommended as an adjunct to US for patients with clinical suspicion for advanced disease, including invasive primary tumor, or clinically apparent multiple or bulky lymph node involvement | Strong recommendation, low-quality evidence | ||
| AACE/AME/ETA Medical Guidelines for Clinical Practice for Diagnosis and Management of Thyroid Nodules | 72 | US examination of the neck, FNA biopsy of any concomitant suspicious nodule or lymph node, and vocal cord assessment with laryngoscopy are recommended before surgery | Grade A; BEL 2 |
| In the case of suspicious US features, confirm the metastatic nature of a lymph node with measurement of Tg or calcitonin in the washout of the FNA needle | BEL 2, GRADE A | ||
| Consider the use of MRI, CT, and/or 18FDG PET/CT in selected cases with aggressive features for more accurate preoperative staging | BEL 3, GRADE B | ||
| British Thyroid Association Guidelines for the Management of Thyroid Cancer | 84 | In patients with thyroid cancer assessment of extra-thyroidal extension and lymph node disease in the central and lateral neck compartments should be undertaken pre-operatively | Good practice point |
| A combination of US and CT/MRI imaging is advised depending upon local expertise | Good practice point | ||
| Lymph nodes that are equivocal/suspicious on US should be assessed by FNAC. Measurement of Tg in aspirate washout fluid may aid the diagnosis of lymph node metastasis | 4, D | ||
| US Diagnosis and Imaging-based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations | 50 | US is the established primary imaging modality for the assessment of the lymph nodes in the patients with thyroid nodules and proven thyroid cancers | Not available |
| Contrast enhanced CT has a complementary role in the preoperative assessment of the extent of the primary tumors and nodal metastases | Not available | ||
| We recommend preoperative contrast enhanced CT for the patients with a suspected invasive primary tumor or cervical lymph node metastasis | |||
| ACR Appropriateness Criteria® Thyroid Disease | 69 | US thyroid; usually appropriate | Strong |
| CT neck with IV contrast; may be appropriate | Strong | ||
| CT neck without IV contrast; usually not appropriate | |||
| MRI neck without IV contrast; may be appropriate | Expert consensus | ||
| MRI without and with IV contrast; may be appropriate | |||
| Revised Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Thyroid Cancer | 33 | Preoperative neck US for cervical (central and especially lateral neck compartments) lymph nodes is recommended for all patients undergoing thyroidectomy for malignant or suspicious for malignancy cytologic or molecular findings | Not available |
| Preoperative use of CT, MRI with IV contrast is recommended as an adjunct to US for patients with clinical suspicion for advanced disease, including invasive primary tumor, or clinically apparent multiple or bulky lymph node involvement | |||
| NCCN Clinical Practice Guidelines in Oncology, Thyroid Carcinoma, Version 1. 2019 | 75 | Perform thyroid and neck US (including central and lateral compartments), if not previously done | 2A |
| Perform CT/MRI with contrast for fixed, bulky, or substernal lesions | 2A |
AACE/AME/ETA = American Association of Clinical Endocrinologists/Italian Association of Clinical Endocrinologists/European Thyroid Association, ACR = American College of Radiology, CT = computed tomography, FNA = fine-needle aspiration, FNAC = FNA cytology, IV = intravenous, MRI = magnetic resonance imaging, NCCN = National Comprehensive Cancer Network, PET = positron emission tomography, Tg = thyroglobulin, US = ultrasonography, 18FDG = 18-fluorodeoxyglucose
Neck US
The seven guidelines recommended neck US for the preoperative evaluation of DTC [2,4,9,19,21,23,39]. Preoperative US should be performed in patients with thyroid cancer to evaluate the site and size of the primary tumor, contralateral lobe, gross extrathyroidal extension (ETE), and LN metastasis in the neck [23,41,42,43,44,45,46,47,48,49]. US is accurate for predicting ETE and detecting cancer in the contralateral lobe [47,50,51,52,53,54,55,56]. US has excellent resolution and shows good diagnostic accuracy for detecting cervical LN metastasis [42,43,44,45,46,47,48,49], especially for LNs in the lateral neck [57]. US-guided biopsy should be performed to confirm malignancy in suspicious cervical LNs (≥ 3–5 mm) and indeterminate LNs (> 5 mm; short diameter) detected by US in cases in which biopsy may change the surgical management [9]. The addition of FNA-Tg washout to the evaluation of cervical LNs might be appropriate in selected patients; however, the interpretation of the results may be difficult in patients with intact thyroid glands [58,59,60,61,62,63,64,65].
Neck CT and Neck MRI
Seven guidelines recommend the preoperative use of cross-sectional imaging studies of the neck (CT or MRI) with IV contrast as an adjunct to US in cases with clinical suspicion of advanced disease, including invasive primary tumors or clinically apparent multiple bulky LNs [2,4,9,19,21,23,39]. Recent studies indicate that the diagnostic performance of the combination of CT with US is higher than that of US alone for detecting cervical LN metastasis [66,67,68]. CT may be used for the evaluation of suspicious metastatic neck LNs detected by US. CT or MRI can detect LN metastasis in the retropharyngeal or superior mediastinal compartments that may be missed by US [66]. Pre-contrast CT or MRI scans could be helpful in detecting calcified metastatic LNs or ectopic thyroid tissue [69]; however imaging studies with IV contrast injection are mandatory to assess LN metastases with strong or heterogeneous contrast enhancement as well as cystic changes [4]. A recent meta-analysis indicated that the protocol used is an important factor determining the diagnostic accuracy of neck CT and suggested that arterial phase scans and thin reconstruction slices might be helpful for improving sensitivity [70]. Neck MRI could be comparable to CT for evaluating invisible sites on US and for detecting hyperenhancing lesions after IV contrast injection. MRI has a superior soft-tissue resolution, and the gadolinium-based contrast medium does not interfere with future radioiodine administration. A recent meta-analysis reported that CT and MRI have a high pooled specificity of 87% (95% confidence interval [CI], 90–95%) and 85% (95% CI, 63–95%), respectively, for detecting metastatic LNs [70,71], although the performances of MRI and CT were not directly compared. However, because macroscopic metastatic lesions from thyroid cancer could be small (> 3 mm), and up to 61% of LN metastases have a diameter of < 10 mm [72], conventional MRI techniques may have a lower sensitivity than CT for detecting such small metastatic LNs. Moreover, MRI might be less sensitive than CT in detecting calcified metastatic LNs. Therefore, KSThR recommends neck CT as the primary cross-sectional imaging modality for detecting LN metastasis in DTC [9].
Invasive DTC occurs in 10–15% of patients [21]. For these patients, contrast-enhanced cross-sectional imaging could be instrumental in accurately delineating the extent of aerodigestive tract invasion and vascular involvement [4,21]. MRI and CT have similar degrees of accuracy in predicting local invasion of the esophagus, trachea, and recurrent laryngeal nerve [73,74,75,76]. The scan range of CT or MRI should extend from the skull base to the superior mediastinum of the aortic arch [4,40] to accurately define the inferior border of the disease and to evaluate anatomic variations that could significantly influence surgical planning, such as an aberrant right subclavian artery [77].
Chest CT
Chest CT is useful for defining the inferior border of the disease and for determining the extent of mediastinal involvement in cases with significant caudal spread. In addition, it could be useful for detecting macroscopic pulmonary metastases in high-risk patients [21]. CT findings may influence patient management by indicating the need for sternotomy and/or tracheal resection/reconstruction. The administration of IV contrast is important for differentiating metastatic LNs from nonspecific mediastinal LNs, which is an important information for preoperative surgical mapping. Moreover, since the presence of lung metastasis is related to the survival rate [78,79], preoperative chest CT may be recommended before treatment only in patients with clinicopathologic risk factors [80,81].
Clinical Situation 3: Early Imaging after Surgery for DTC
1. Neck US is appropriate for the postoperative evaluation of DTC after definitive treatment.
2. Neck CT with IV contrast may be appropriate for early imaging after definitive treatment of DTC. It may be considered in patients with a high risk of persistent disease when the response to therapy cannot be accurately evaluated by US and serum Tg.
Clinical and Imaging Rationale
After thyroidectomy, the American Thyroid Association (ATA) risk stratification system is recommended to predict the risk of disease recurrence and/or persistence [21]. ATA recommends the evaluation of postoperative disease status using serum Tg measurement and neck US. Imaging studies performed in the early post-treatment period can help determine the presence of residual tumor/remnant thyroid tissue and can provide baseline information, which may be helpful for further surveillance. Moreover, early imaging studies are useful to determine the need for RAI ablation based on the results of initial risk stratification and postoperative disease status. According to the test results in the early follow-up period, the response to therapy can be classified into four categories: excellent response, biochemical response, structural incomplete response, and indeterminate response. The application of response-to-therapy assessments can affect patient management [21,82]. Five guidelines were selected for clinical situation 3. Table 8 shows the recommendation matrix of the existing guidelines for clinical situation 3.
Table 8. Recommendation Matrix of Existing Guidelines (Clinical Situation 3: Early Imaging after Surgery of DTC).
| Source Guidelines | AGREE II | Recommendation | Grading of Recommendation |
|---|---|---|---|
| 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer | 72 | Following surgery, cervical US to evaluate the thyroid bed and central and lateral cervical nodal compartments should be performed at 6–12 months and then periodically, depending on the patient's risk for recurrent disease and Tg status | Strong recommendation, moderate-quality evidence |
| British Thyroid Association Guidelines for the Management of Thyroid Cancer | 84 | A stimulated Tg and neck US should be performed in preference to a diagnostic 131I WBS between 9 and 12 months from RRA | Grade C; BEL 2 |
| ACR Appropriateness Criteria® Thyroid Disease | 69 | US thyroid; usually appropriate | Limited |
| Neck CT without IV contrast; may be appropriate | Expert opinion | ||
| Neck CT with IV contrast; usually appropriate | |||
| Neck CT without and with IV contrast; usually not appropriate | |||
| Neck MRI without IV contrast; may be appropriate | Expert opinion | ||
| Neck MRI without and with IV contrast; usually appropriate | |||
| Revised Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Thyroid Cancer | 33 | Cervical US to evaluate the thyroid bed and central and lateral cervical nodal compartments should be performed at 6–12 months following surgery, and then periodically, depending on the patient's risk for recurrent disease and Tg status | Not available |
| NCCN Clinical Practice Guidelines in Oncology, Thyroid Carcinoma, Version 1. 2019 | 75 | Physical examination, US, TSH, and Tg measurement + anti-Tg antibodies at 6 and 12 months, and then annually if disease-free | 2A |
| For patients with known or suspected distant metastasis at presentation, appropriate cross-sectional imaging (CT or MRI with contrast) of known metastatic foci is recommended at 6–12 weeks post-thyroidectomy | 2A |
ACR = American College of Radiology, CT = computed tomography, DTC = differentiated thyroid carcinoma, IV = intravenous, MRI = magnetic resonance imaging, NCCN = National Comprehensive Cancer Network, RRA = radioiodine remnant ablation, Tg = thyroglobulin, TSH = Thyroid stimulating Hormone, US = ultrasonography, WBS = whole body scan
Neck US
The five selected guidelines recommend US of the thyroid bed and neck as the first-line imaging study for DTC after initial therapy in patients who undergo total thyroidectomy or lobectomy [2,9,21,23,39]. Neck US should be performed at 6–12 months after surgery to evaluate the thyroid bed and the central and lateral neck regions [21]. US is useful for detecting and characterizing abnormalities in the neck and for determining the extent of residual tumors [43,44,83,84,85,86]. US could be helpful even in the initial phases, when the Tg assay results may be difficult to interpret [41]. In the early phase (after surgery or at the time of ablation therapy), neck US should be considered in cases lacking detailed preoperative US data to detect the persistence of any LN metastases or when obvious activity is detected outside the thyroid bed on a post-ablation scan, or if pre-ablation therapy Tg values are unexpectedly high [87,88]. After lobectomy, US is the principal monitoring tool because the serum Tg level is of limited value [87,89]. After the initial US examination, periodic US scans should be performed according to the risk of recurrence and serum Tg levels [21]. In low-risk patients, periodic US may not be necessary [90].
Neck CT and MRI
CT or MRI is usually not recommended as a first-line imaging tool after definitive treatment of DTC. Two of the selected guidelines mentioned the use of cross-sectional imaging as a second-line imaging modality after treatment of DTC in the following special situations [2,4,21]: 1) in patients with a high risk of persistent disease, and 2) in cases with elevated serum Tg levels or Tg antibody levels with negative findings on neck US. In these cases, CT with IV contrast injection is necessary to detect LN metastases and aerodigestive tract invasion. Although MRI may have the same role as CT in the evaluation of residual tumors, it has potential disadvantages for the detection of small enhancing lesions in the thyroid bed and other areas of the neck. Please refer to the ‘Harms and benefits’ for the general advantages and disadvantages of CT and MRI.
Clinical Situation 4: Suspected Recurrence of DTC
1. Neck US is usually appropriate when recurrence of DTC is suspected.
2. US-guided biopsy and/or washout Tg for neck LNs are usually appropriate when recurrence of DTC is suspected on imaging.
3. Neck CT or MRI with IV contrast may be appropriate as a second-line imaging modality in cases of widely-distributed recurrent disease, when aerodigestive tract invasion is suspected, or in cases of discrepancy between serum Tg and US results.
4. Chest CT with or without IV contrast may be appropriate in high-risk patients with elevated serum Tg or rising anti-Tg antibodies.
Clinical and Imaging Rationale
Recurrence of DTC may be symptomatic; however, many patients are asymptomatic and present with persistently elevated serum Tg levels or rising anti-Tg antibody levels. In these patients, neck US serves as a primary imaging tool to detect, classify, and localize potentially abnormal lesions in the neck. For any structural lesion in the neck detected by US, decisions regarding further management (active surveillance, revision surgery, local ablation therapy, or RAI) should be made according to the location of the lesion and individual clinical factors [2,21,39]. Imaging plays an important role in delineating the extent and location of the disease before additional treatment. Cross-sectional imaging of the neck and other body parts (especially the lung and mediastinum) may complement US findings when neck US fails to localize the recurrent tumor. After imaging, the response to initial therapy in these patients can be categorized as elevated serum Tg with structural disease (structural incomplete response) or elevated serum Tg with no detectable abnormality on imaging (biochemical incomplete response) [21]. Five guidelines were selected for clinical situation 4 [2,4,21,23,39]. Table 9 shows the recommendation matrix of the existing guidelines for clinical situation 4.
Table 9. Recommendation Matrix of Existing Guidelines (Clinical Situation 4: Suspected Recurrence of DTC).
| Source Guidelines | AGREE II | Recommendation | Grading of Recommendation |
|---|---|---|---|
| 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer | 72 | Following surgery, cervical US to evaluate the thyroid bed and central and lateral cervical nodal compartments should be performed at 6–12 months and then periodically, depending on the patient's risk for recurrent disease and Tg status | Strong recommendation, moderate-quality evidence |
| If a positive result on US would change management, ultrasonographically suspicious lymph nodes > 8–10 mm in the smallest diameter should be biopsied for cytology with Tg measurement in the needle washout fluid | Strong recommendation, low-quality evidence | ||
| Cross-sectional imaging of the neck and upper chest (CT, MRI) with IV contrast should be considered 1) in the setting of bulky and widely distributed recurrent nodal disease where US may not completely delineate disease; 2) in the assessment of possible invasive recurrent disease where potential aerodigestive tract invasion requires complete assessment; or 3) when neck US is felt to be inadequately visualizing possible neck nodal disease (high Tg, negative neck US) | Strong recommendation, moderate-quality evidence | ||
| CT imaging of the chest without IV contrast (imaging pulmonary parenchyma) or with IV contrast (to include the mediastinum) should be considered in high risk DTC patients with elevated serum Tg (generally > 10 ng/mL) or rising Tg antibodies with or without negative RAI imaging | Strong recommendation, moderate-quality evidence | ||
| Imaging of other organs including MRI brain, MR skeletal survey, and/or CT or MRI of the abdomen should be considered in high-risk DTC patients with elevated serum Tg (generally > 10 ng/mL) and negative neck and chest imaging who have symptoms referable to those organs or who are being prepared for TSH-stimulated RAI therapy (withdrawal or rhTSH) and may be at risk for complications of tumor swelling | Strong recommendation, low-quality evidence | ||
| British Thyroid Association Guidelines for the Management of Thyroid Cancer | 84 | Neck US should assess the thyroid bed for residual thyroid tissue as well as assessing the cervical lymph nodes for signs of metastatic disease. US-guided FNAC should be carried out when metastatic disease is suspected | 4, D |
| ACR Appropriateness Criteria® Thyroid Disease | 69 | US of the thyroid; usually appropriate | Limited |
| Neck CT without IV contrast; may be appropriate | Strong | ||
| Neck CT with IV contrast; usually appropriate | |||
| Neck CT with or without IV contrast; usually not appropriate | |||
| Neck MRI without IV contrast; may be appropriate | Expert consensus | ||
| Neck MRI with or without IV contrast; usually appropriate | |||
| Chest CT without IV contrast; may be appropriate | Limited | ||
| Chest CT with IV contrast; may be appropriate | |||
| Chest CT with or without IV contrast; usually not appropriate | |||
| Revised Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Thyroid Cancer | 33 | Cervical US to evaluate the thyroid bed and central and lateral cervical nodal compartments should be performed at 6–12 months following surgery, and then periodically, depending on the patient's risk for recurrent disease and Tg status | Not available |
| CT, MRI of the neck and upper chest with IV contrast should be considered 1) in the setting of bulky and widely distributed recurrent nodal disease where US may not completely delineate disease; 2) in the assessment of possible invasive recurrent disease where potential aerodigestive tract invasion requires complete assessment; or 3) when neck US is felt to be inadequately visualizing possible neck nodal disease (high Tg, negative neck US) | Not available | ||
| CT of the chest without IV contrast (imaging pulmonary parenchyma) or with IV contrast (to include the mediastinum) should be considered in high risk DTC patients with elevated serum Tg (generally > 10 ng/mL) or rising Tg antibodies with or without negative RAI imaging | |||
| NCCN Clinical Practice Guidelines in Oncology, Thyroid Carcinoma, Version 1. 2019 | 75 | Periodic neck US or other imaging (e.g., CT or MRI with contrast, chest X-ray, PET/CT) as clinically appropriate, may be considered in cases of clinical suggestion of recurrent disease | 2A |
ACR = American College of Radiology, CT = computed tomography, DTC = differentiated thyroid carcinoma, IV = intravenous, MRI = magnetic resonance imaging, NCCN = National Comprehensive Cancer Network, PET = positron emission tomography, RAI = radioactive Iodine, rhTSH = recombinant human thyrotropin, Tg = thyroglobulin, TSH = Thyroid stimulating Hormone, US = ultrasonography
Neck US
Five guidelines recommended neck US as the primary imaging modality for suspected recurrence of DTC [2,4,21,23,39]. US can detect and characterize recurrent tumors in the neck [43,44,83,84,85,86], and US scans should include both the thyroidectomy bed and cervical LNs [87]. US-guided biopsy should be performed to confirm malignancy in cases of suspicious or indeterminate cervical LNs (≥ 8–10 mm; short diameter on US or CT/MRI) detected by US [9]. Biopsy should be performed if the results are necessary to determine the appropriate therapeutic intervention. The addition of FNA-Tg washout to cytology increases the sensitivity of the evaluation of cervical LNs (58–65).
Neck CT or MRI
Four guidelines recommended neck CT or MRI as an adjunct to US in cases of recurrent DTC. Cross-sectional imaging of the neck with IV contrast should be considered in the following cases: 1) in the setting of bulky and widely-distributed recurrent nodal disease where US may not completely delineate the extent of disease; 2) in patients with suspected recurrent disease requiring assessment of possible aerodigestive tract invasion; or 3) when neck US is inadequate for visualizing possible neck nodal disease (high Tg, negative neck US) [21]. Imaging studies should be performed with IV contrast to detect small metastatic tumors with hyperenhancement and cystic changes. Neck CT or MRI with IV contrast complements neck US for the detection of additional metastasis in the central and lateral neck regions [68,91,92]. CT or MRI is beneficial in regions that cannot be visualized by US as well as for the assessment of invasive recurrent disease in the aerodigestive tract.
Contrast-enhanced CT of the neck is the most frequently recommended first-line cross-sectional imaging modality for detecting LN metastases. MRI is also recommended for imaging of the neck and mediastinum, and its diagnostic performance is comparable to that of CT for the detection of cervical LN metastases [71]. However, its disadvantages (refer to the ‘Harms and benefits’) may limit its sensitivity for the detection of small metastatic lesions. Before therapeutic intervention (revision surgery or image-guided interventions), target lesions should be accurately defined using anatomic studies, including US or cross-sectional imaging, to complement RAI imaging and to allow for adequate preoperative mapping and definitive surgical localization.
Chest CT
Four guidelines recommend the use of chest CT in high-risk DTC patients with elevated serum Tg (> 10 ng/mL) or rising anti-Tg antibodies with or without negative RAI imaging. Chest CT is favored because it can detect small lung metastases [21]. Although chest CT with IV contrast is preferred, chest CT without IV contrast may also be helpful for detecting lung metastases when the iodinated contrast agent is contraindicated. One guideline [21] recommended imaging of other organs, including brain MRI, MR skeletal survey, and/or CT or MRI of the abdomen in high-risk DTC patients with elevated serum Tg (> 10 ng/mL) and with negative neck and chest imaging results.
CONSIDERATIONS FOR RECOMMENDATIONS
Harms and Benefits
Clinical Situation 1
US is a sensitive method for the detection and diagnosis of thyroid nodules. There is no risk of radiation exposure, and it can evaluate changes in the thyroid parenchyma and examine LNs. Although the combination of US features can help differentiate benign from malignant thyroid nodules, some features of benign and malignant nodules overlap. This may lead to unnecessary biopsy of benign nodules, which can increase medical expenditures and complications.
The use of CT as an initial imaging test for the diagnosis of thyroid nodules is associated with a relatively high risk of radiation exposure. MRI does not have a risk of radiation exposure; however, MRI requires a longer scan time than US and CT, and it is associated with high medical costs. CT and MRI are not effective for differentiating between benign and malignant thyroid nodules. Given that CT and MRI do not have the spatial resolution to identify suspicious features on US, they should not be used as the primary imaging modality in patients with suspicious thyroid nodules.
Clinical Situations 2, 3, and 4
US is the modality of choice for the preoperative and postoperative evaluation of DTC to assess ETE and cervical LN metastasis without the risk of exposure to ionizing radiation. However, US is an operator-dependent technique, and the evaluation of cervical LNs tends to be affected by the radiologist's experience. Furthermore, it is difficult to evaluate the retropharyngeal, retrosternal areas and the mediastinum using US alone [66]. CT should be considered as an adjunct to US for the detection of deeply-located or locally-invasive lesions involving the aerodigestive tract. Therefore, despite the risk of radiation exposure, CT has advantages over US when expert radiologists are not available or in cases in which invasive tumors are suspected. IV injection of iodinated contrast media during CT is necessary for the detection of LN metastasis or recurrence in the neck, which shows strong or heterogeneous enhancement. The iodinated contrast media used in contrast-enhanced CT may delay RAI therapy because it alters RAI uptake for months [93]. However, the use of iodinated contrast media is not contraindicated for DTC, as suggested by recent data on iodine retention [4,9,21]. These studies demonstrated that delayed RAI therapy is not necessary for patients who undergo preoperative contrast-enhanced CT and that the iodine content of the body is not an important determinant of thyroid ablation [94,95,96]. CT scans without IV contrast may be useful for detecting metastatic LNs with macrocalcifications or ectopic thyroid tissues; however, its value as an isolated imaging study has not been investigated. Therefore, a protocol including both pre- and post-contrast scans is preferred for evaluating LN metastases in patients with DTC.
MRI of the neck and mediastinum has the same role as CT for evaluating sites with limited detection on US. It provides superior soft-tissue contrast and does not require the injection of iodinated contrast media. However, MRI has disadvantages such as a longer scan time and possible motion artifacts in the lower neck associated with respiration or swallowing, and it is less sensitive than CT for the detection of small cervical LNs or small lung nodules because of its lower spatial resolution. Recent advances in MR image quality and faster MRI acquisition may overcome these limitations; however, these advances remain to be implemented in patients with DTC. Lastly, MRI is associated with more medical expenditure.
Acceptability and Applicability
Currently, US, CT, and MRI are available in most hospitals in Korea. An evaluation of domestic acceptability and the applicability of guidelines indicated that neck US, CT, and MRI could be used for the evaluation of thyroid nodules and for the pre- and postoperative evaluation of DTC. The cost of preoperative US, CT, and MRI might be reasonable because the individual becomes eligible for insurance coverage in cases in which thyroid cancer is diagnosed through biopsy in South Korea.
SUMMARY OF RECOMMENDATIONS
The summary of recommendations and evidence levels are described in Table 10.
Table 10. Summary of Recommendations and Evidence.
| Clinical Scenario | Modality | Appropriateness Category | Evidence Level | RRL |
|---|---|---|---|---|
| Known or suspected thyroid nodule, initial imaging | Neck US | Usually appropriate | I | 0 |
| Neck US with US-guided biopsy of thyroid nodule | Usually appropriate | I | 0 | |
| Neck CT without IV contrast | Usually not appropriate | II |  |
|
| Neck CT with IV contrast | Usually not appropriate | II |  |
|
| Neck CT with and without IV contrast | Usually not appropriate | II |  |
|
| Neck MRI without IV contrast | Usually not appropriate | IV | 0 | |
| Neck MRI with and without IV contrast | Usually not appropriate | IV | 0 | |
| Preoperative evaluation of thyroid cancer | Neck US | Usually appropriate | I | 0 |
| US-guided biopsy and/or washout Tg for neck lymph node | Usually appropriate | I | 0 | |
| Neck CT without IV contrast | Usually not appropriate | II |  |
|
| Neck CT with IV contrast | May be appropriate | II |  |
|
| Neck CT with and without IV contrast | May be appropriate | II |  |
|
| Neck MRI without IV contrast | Usually not appropriate | II | 0 | |
| Neck MRI with and without IV contrast | May be appropriate | II | 0 | |
| Chest CT without IV contrast | Usually not appropriate | IV |  |
|
| Chest CT with IV contrast | May be appropriate | IV |  |
|
| Chest CT with and without IV contrast | Usually not appropriate | IV |  |
|
| Early imaging after surgery of DTC | Neck US | Usually appropriate | II | 0 |
| Neck CT without IV contrast | Usually not appropriate | IV |  |
|
| Neck CT with IV contrast | May be appropriate | IV |  |
|
| Neck CT with and without IV contrast | May be appropriate | IV |  |
|
| Neck MRI without IV contrast | Usually not appropriate | IV | 0 | |
| Neck MRI with and without IV contrast | Usually not appropriate | IV | 0 | |
| Suspected recurrence of DTC | Neck US | Usually appropriate | II | 0 |
| US-guided biopsy and/or washout Tg | Usually appropriate | II | 0 | |
| Neck CT without IV contrast | Usually not appropriate | II |  |
|
| Neck CT with IV contrast | May be appropriate | II |  |
|
| Neck CT with and without IV contrast | May be appropriate | II |  |
|
| Neck MRI without IV contrast | Usually not appropriate | II | 0 | |
| Neck MRI with and without IV contrast | May be appropriate | II | 0 | |
| Chest CT without IV contrast | May be appropriate | II |  |
|
| Chest CT with IV contrast | May be appropriate | II |  |
|
| Chest CT with and without IV contrast | Usually not appropriate | II |  |
CT = computed tomography, DTC = differentiated thyroid carcinoma, IV = intravenous, MRI = magnetic resonance imaging, RRL = relative radiation level, Tg = thyroglobulin, US = ultrasonography
Footnotes
This study was supported by a grant from the 2019 Clinical Practice Guideline Research Fund by the Korean Society of Radiology & Korean Society of Thyroid Radiology, and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea.
Conflicts of Interest: None of the members of the Guideline Committee has a financial disclosure or conflict of interest except Jung Hwan Baek. He has been a consultant of two radiofrequency companies, STARmed and RF Medical, since 2017.
- Conceptualization: Ji Ye Lee, Jung Hwan Baek.
- Data curation: Miyoung Choi, Ji Ye Lee.
- Formal analysis: all authors.
- Funding acquisition: Jung Hwan Baek, Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology.
- Investigation: Ji Ye Lee, Jung Hwan Baek, Miyoung Choi.
- Methodology: Jung Hwan Baek, Eun Ju Ha, Miyoung Choi, Ji Ye Lee.
- Project administration: Jung Hwan Baek.
- Resources: Ji Ye Lee, Jung Hwan Baek, Eun Ju Ha, Miyoung Choi, Dong Gyu Na.
- Supervision: all authors.
- Validation: Ji Ye Lee, Jung Hwan Baek, Dong Gyu Na.
- Visualization: Ji Ye Lee, Miyoung Choi.
- Writing—original draft: Ji Ye Lee, Jung Hwan Baek.
- Writing—review & editing: Ji Ye Lee, Jung Hwan Baek, Eun Ju Ha, Jin Yong Sung, Jung Hee Shin, Ji-hoon Kim, Min Kyoung Lee, So Lyung Jung, Young Hen Lee, Hye Shin Ahn, Jung Hyun Yoon, Yoon Jung Choi, Jeong Seon Park, Yoo Jin Lee, Dong Gyu Na.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.3348/kjr.2020.0578.
Relative radiation levels in Korean clinical imaging
Evidence table
References
- 1.Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, thyroid carcinoma. Nccn.org Web site. [Accessed January 22, 2020]. https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.
- 3.Hoang JK, Langer JE, Middleton WD, Wu CC, Hammers LW, Cronan JJ, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol. 2015;12:143–150. doi: 10.1016/j.jacr.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel on Neurological Imaging. Oldan JD, Mandel SJ, Policeni B, Agarwal V, Burns J, et al. ACR Appropriateness Criteria® thyroid disease. J Am Coll Radiol. 2019;16:S300–S314. doi: 10.1016/j.jacr.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”--screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 6.Do KH, Jung SE. Current status of medical radiation exposure in Korea - recent efforts to develop a radiation exposure control system focussed on justification and optimisation. Ann ICRP. 2016;45:113–121. doi: 10.1177/0146645316637783. [DOI] [PubMed] [Google Scholar]
- 7.Choi SJ, Jeong WK, Jo AJ, Choi JA, Kim MJ, Lee M, et al. Methodology for developing evidence-based clinical imaging guidelines: joint recommendations by Korean Society of Radiology and National Evidence-Based Healthcare Collaborating Agency. Korean J Radiol. 2017;18:208–216. doi: 10.3348/kjr.2017.18.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12:1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016;17:370–395. doi: 10.3348/kjr.2016.17.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH Korean Society of Thyroid Radiology (KSThR); Korean Society of Radiology. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2015;16:391–401. doi: 10.3348/kjr.2015.16.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek JH, Na DG, Lee JH, Jung SL, Kim JH, Sung JY, et al. Core needle biopsy of thyroid nodules: consensus statement and recommendations. Ultrasonography. 2013;32:95–102. [Google Scholar]
- 12.Na DG, Baek JH, Jung SL, Kim JH, Sung JY, Kim KS, et al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017;18:217–237. doi: 10.3348/kjr.2017.18.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632–655. doi: 10.3348/kjr.2018.19.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–125. doi: 10.3348/kjr.2012.13.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn SY, Shin JH, Na DG, Ha EJ, Ahn HS, Lim HK, et al. Ethanol ablation of the thyroid nodules: 2018 consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2019;20:609–620. doi: 10.3348/kjr.2018.0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steering Committee for Clinical Practice Guideline. Korean appraisal of guidelines for research & evaluation II. Agreetrust.org Web site. [Accessed May 15, 2019]. http://www.agreetrust.org/wp-content/uploads/2013/06/AGREE_II_Korean.pdf.
- 17.American College of Radiology. ACR Appropriateness Criteria® rating round information. Acr.org Web site. [Accessed May 14, 2019]. https://www.acr.org/-/media/ACR/Files/Appropriateness-Criteria/RatingRoundInfo.pdf.
- 18.Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The RAND/UCLA appropriateness method user's manual (No. RAND/MR-1269-DG-XII/RE) Santa Monica, CA: Rand Corp; 2001. [Google Scholar]
- 19.Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update. Endocr Pract. 2016;22:622–639. doi: 10.4158/EP161208.GL. [DOI] [PubMed] [Google Scholar]
- 20.Ha EJ, Lim HK, Yoon JH, Baek JH, Do KH, Choi M, et al. Primary imaging test and appropriate biopsy methods for thyroid nodules: guidelines by Korean Society of Radiology and National Evidence-Based Healthcare Collaborating Agency. Korean J Radiol. 2018;19:623–631. doi: 10.3348/kjr.2018.19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ, et al. 2016 revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016;9:59–126. [Google Scholar]
- 23.Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81 Suppl 1:1–122. doi: 10.1111/cen.12515. [DOI] [PubMed] [Google Scholar]
- 24.Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:1253–1263. doi: 10.1210/jc.2013-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith-Bindman R, Lebda P, Feldstein VA, Sellami D, Goldstein RB, Brasic N, et al. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: results of a population-based study. JAMA Intern Med. 2013;173:1788–1796. doi: 10.1001/jamainternmed.2013.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solbiati L, Osti V, Cova L, Tonolini M. Ultrasound of thyroid, parathyroid glands and neck lymph nodes. Eur Radiol. 2001;11:2411–2424. doi: 10.1007/s00330-001-1163-7. [DOI] [PubMed] [Google Scholar]
- 27.Chiofalo MG, Signoriello S, Fulciniti F, Avenia N, Ristagno S, Lombardi CP, et al. Predictivity of clinical, laboratory and imaging findings in diagnostic definition of palpable thyroid nodules. A multicenter prospective study. Endocrine. 2018;61:43–50. doi: 10.1007/s12020-018-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YH, Kim DW, In HS, Park JS, Kim SH, Eom JW, et al. Differentiation between benign and malignant solid thyroid nodules using an US classification system. Korean J Radiol. 2011;12:559–567. doi: 10.3348/kjr.2011.12.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandel SJ. Diagnostic use of ultrasonography in patients with nodular thyroid disease. Endocr Pract. 2004;10:246–252. doi: 10.4158/EP.10.3.246. [DOI] [PubMed] [Google Scholar]
- 30.Cesur M, Corapcioglu D, Bulut S, Gursoy A, Yilmaz AE, Erdogan N, et al. Comparison of palpation-guided fine-needle aspiration biopsy to ultrasound-guided fine-needle aspiration biopsy in the evaluation of thyroid nodules. Thyroid. 2006;16:555–561. doi: 10.1089/thy.2006.16.555. [DOI] [PubMed] [Google Scholar]
- 31.Hambly NM, Gonen M, Gerst SR, Li D, Jia X, Mironov S, et al. Implementation of evidence-based guidelines for thyroid nodule biopsy: a model for establishment of practice standards. AJR Am J Roentgenol. 2011;196:655–660. doi: 10.2214/AJR.10.4577. [DOI] [PubMed] [Google Scholar]
- 32.Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6:225–237. doi: 10.1159/000478927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 34.Shetty SK, Maher MM, Hahn PF, Halpern EF, Aquino SL. Significance of incidental thyroid lesions detected on CT: correlation among CT, sonography, and pathology. AJR Am J Roentgenol. 2006;187:1349–1356. doi: 10.2214/AJR.05.0468. [DOI] [PubMed] [Google Scholar]
- 35.Hoang JK, Branstetter BF, 4th, Gafton AR, Lee WK, Glastonbury CM. Imaging of thyroid carcinoma with CT and MRI: approaches to common scenarios. Cancer Imaging. 2013;13:128–139. doi: 10.1102/1470-7330.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah MD, Hall FT, Eski SJ, Witterick IJ, Walfish PG, Freeman JL. Clinical course of thyroid carcinoma after neck dissection. Laryngoscope. 2003;113:2102–2107. doi: 10.1097/00005537-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Wang TS, Dubner S, Sznyter LA, Heller KS. Incidence of metastatic well-differentiated thyroid cancer in cervical lymph nodes. Arch Otolaryngol Head Neck Surg. 2004;130:110–113. doi: 10.1001/archotol.130.1.110. [DOI] [PubMed] [Google Scholar]
- 38.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. discussion 1057-1058. [PubMed] [Google Scholar]
- 39.Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ, et al. 2016 revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016;9:59–126. [Google Scholar]
- 40.Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, et al. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid. 2015;25:3–14. doi: 10.1089/thy.2014.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacini F, Basolo F, Bellantone R, Boni G, Cannizzaro MA, De Palma M, et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J Endocrinol Invest. 2018;41:849–876. doi: 10.1007/s40618-018-0884-2. [DOI] [PubMed] [Google Scholar]
- 42.Eun NL, Son EJ, Kim JA, Gweon HM, Kang JH, Youk JH. Comparison of the diagnostic performances of ultrasonography, CT and fine needle aspiration cytology for the prediction of lymph node metastasis in patients with lymph node dissection of papillary thyroid carcinoma: a retrospective cohort study. Int J Surg. 2018;51:145–150. doi: 10.1016/j.ijsu.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Jiang HJ, Wu CW, Chiang FY, Chiou HC, Chen IJ, Hsiao PJ. Reliable sonographic features for nodal thyroglobulin to diagnose recurrent lymph node metastasis from papillary thyroid carcinoma. Clin Otolaryngol. 2018 Mar; doi: 10.1111/coa.13103. [Epub] [DOI] [PubMed] [Google Scholar]
- 44.Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3590–3594. doi: 10.1210/jc.2007-0444. [DOI] [PubMed] [Google Scholar]
- 45.O'Connell K, Yen TW, Quiroz F, Evans DB, Wang TS. The utility of routine preoperative cervical ultrasonography in patients undergoing thyroidectomy for differentiated thyroid cancer. Surgery. 2013;154:697–701. doi: 10.1016/j.surg.2013.06.040. discussion 701-703. [DOI] [PubMed] [Google Scholar]
- 46.Rosário PW, de Faria S, Bicalho L, Alves MF, Borges MA, Purisch S, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005;24:1385–1389. doi: 10.7863/jum.2005.24.10.1385. [DOI] [PubMed] [Google Scholar]
- 47.Shimamoto K, Satake H, Sawaki A, Ishigaki T, Funahashi H, Imai T. Preoperative staging of thyroid papillary carcinoma with ultrasonography. Eur J Radiol. 1998;29:4–10. doi: 10.1016/s0720-048x(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 48.Solorzano CC, Carneiro DM, Ramirez M, Lee TM, Irvin GL., 3rd Surgeon-performed ultrasound in the management of thyroid malignancy. Am Surg. 2004;70:576–580. discussion 580-582. [PubMed] [Google Scholar]
- 49.Stulak JM, Grant CS, Farley DR, Thompson GB, van Heerden JA, Hay ID, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489–494. doi: 10.1001/archsurg.141.5.489. discussion 494-496. [DOI] [PubMed] [Google Scholar]
- 50.Lee CY, Kim SJ, Ko KR, Chung KW, Lee JH. Predictive factors for extrathyroidal extension of papillary thyroid carcinoma based on preoperative sonography. J Ultrasound Med. 2014;33:231–238. doi: 10.7863/ultra.33.2.231. [DOI] [PubMed] [Google Scholar]
- 51.Kwak JY, Kim EK, Youk JH, Kim MJ, Son EJ, Choi SH, et al. Extrathyroid extension of well-differentiated papillary thyroid microcarcinoma on US. Thyroid. 2008;18:609–614. doi: 10.1089/thy.2007.0345. [DOI] [PubMed] [Google Scholar]
- 52.Choi JS, Chung WY, Kwak JY, Moon HJ, Kim MJ, Kim EK. Staging of papillary thyroid carcinoma with ultrasonography: performance in a large series. Ann Surg Oncol. 2011;18:3572–3578. doi: 10.1245/s10434-011-1783-3. [DOI] [PubMed] [Google Scholar]
- 53.Park JS, Son KR, Na DG, Kim E, Kim S. Performance of preoperative sonographic staging of papillary thyroid carcinoma based on the sixth edition of the AJCC/UICC TNM classification system. AJR Am J Roentgenol. 2009;192:66–72. doi: 10.2214/AJR.07.3731. [DOI] [PubMed] [Google Scholar]
- 54.Moon SJ, Kim DW, Kim SJ, Ha TK, Park HK, Jung SJ. Ultrasound assessment of degrees of extrathyroidal extension in papillary thyroid microcarcinoma. Endocr Pract. 2014;20:1037–1043. doi: 10.4158/EP14016.OR. [DOI] [PubMed] [Google Scholar]
- 55.Kim SS, Lee BJ, Lee JC, Kim SJ, Lee SH, Jeon YK, et al. Preoperative ultrasonographic tumor characteristics as a predictive factor of tumor stage in papillary thyroid carcinoma. Head Neck. 2011;33:1719–1726. doi: 10.1002/hed.21658. [DOI] [PubMed] [Google Scholar]
- 56.Kuo EJ, Thi WJ, Zheng F, Zanocco KA, Livhits MJ, Yeh MW. Individualizing surgery in papillary thyroid carcinoma based on a detailed sonographic assessment of extrathyroidal extension. Thyroid. 2017;27:1544–1549. doi: 10.1089/thy.2017.0457. [DOI] [PubMed] [Google Scholar]
- 57.Zhao H, Li H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: diagnosis of central and lateral compartment nodal metastases. Eur J Radiol. 2019;112:14–21. doi: 10.1016/j.ejrad.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab. 2006;91:1364–1369. doi: 10.1210/jc.2005-1705. [DOI] [PubMed] [Google Scholar]
- 59.Chung J, Kim EK, Lim H, Son EJ, Yoon JH, Youk JH, et al. Optimal indication of thyroglobulin measurement in fine-needle aspiration for detecting lateral metastatic lymph nodes in patients with papillary thyroid carcinoma. Head Neck. 2014;36:795–801. doi: 10.1002/hed.23371. [DOI] [PubMed] [Google Scholar]
- 60.Frasoldati A, Toschi E, Zini M, Flora M, Caroggio A, Dotti C, et al. Role of thyroglobulin measurement in fine-needle aspiration biopsies of cervical lymph nodes in patients with differentiated thyroid cancer. Thyroid. 1999;9:105–111. doi: 10.1089/thy.1999.9.105. [DOI] [PubMed] [Google Scholar]
- 61.Grani G, Fumarola A. Thyroglobulin in lymph node fine-needle aspiration washout: a systematic review and meta-analysis of diagnostic accuracy. J Clin Endocrinol Metab. 2014;99:1970–1982. doi: 10.1210/jc.2014-1098. [DOI] [PubMed] [Google Scholar]
- 62.Moon JH, Kim YI, Lim JA, Choi HS, Cho SW, Kim KW, et al. Thyroglobulin in washout fluid from lymph node fine-needle aspiration biopsy in papillary thyroid cancer: large-scale validation of the cutoff value to determine malignancy and evaluation of discrepant results. J Clin Endocrinol Metab. 2013;98:1061–1068. doi: 10.1210/jc.2012-3291. [DOI] [PubMed] [Google Scholar]
- 63.Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, et al. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab. 1992;74:1401–1404. doi: 10.1210/jcem.74.6.1592886. [DOI] [PubMed] [Google Scholar]
- 64.Pak K, Suh S, Hong H, Cheon GJ, Hahn SK, Kang KW, et al. Diagnostic values of thyroglobulin measurement in fine-needle aspiration of lymph nodes in patients with thyroid cancer. Endocrine. 2015;49:70–77. doi: 10.1007/s12020-014-0410-z. [DOI] [PubMed] [Google Scholar]
- 65.Snozek CL, Chambers EP, Reading CC, Sebo TJ, Sistrunk JW, Singh RJ, et al. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab. 2007;92:4278–4281. doi: 10.1210/jc.2007-1075. [DOI] [PubMed] [Google Scholar]
- 66.Lee Y, Kim JH, Baek JH, Jung SL, Park SW, Kim J, et al. Value of CT added to ultrasonography for the diagnosis of lymph node metastasis in patients with thyroid cancer. Head Neck. 2018;40:2137–2148. doi: 10.1002/hed.25202. [DOI] [PubMed] [Google Scholar]
- 67.Suh CH, Baek JH, Choi YJ, Lee JH. Performance of CT in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid cancer: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38:154–161. doi: 10.3174/ajnr.A4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lesnik D, Cunnane ME, Zurakowski D, Acar GO, Ecevit C, Mace A, et al. Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck. 2014;36:191–202. doi: 10.1002/hed.23277. [DOI] [PubMed] [Google Scholar]
- 69.Hoang JK, Vanka J, Ludwig BJ, Glastonbury CM. Evaluation of cervical lymph nodes in head and neck cancer with CT and MRI: tips, traps, and a systematic approach. AJR Am J Roentgenol. 2013;200:W17–W25. doi: 10.2214/AJR.12.8960. [DOI] [PubMed] [Google Scholar]
- 70.Cho SJ, Suh CH, Baek JH, Chung SR, Choi YJ, Lee JH. Diagnostic performance of CT in detection of metastatic cervical lymph nodes in patients with thyroid cancer: a systematic review and meta-analysis. Eur Radiol. 2019;29:4635–4647. doi: 10.1007/s00330-019-06036-8. [DOI] [PubMed] [Google Scholar]
- 71.Cho SJ, Suh CH, Baek JH, Chung SR, Choi YJ, Lee JH. Diagnostic performance of MRI to detect metastatic cervical lymph nodes in patients with thyroid cancer: a systematic review and meta-analysis. Clin Radiol. 2020;75:562.e1–562.e10. doi: 10.1016/j.crad.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 72.Choi JY, Choi YS, Park YH, Kim JH. Experience and analysis of level VII cervical lymph node metastases in patients with papillary thyroid carcinoma. J Korean Surg Soc. 2011;80:307–312. doi: 10.4174/jkss.2011.80.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seo YL, Yoon DY, Lim KJ, Cha JH, Yun EJ, Choi CS, et al. Locally advanced thyroid cancer: can CT help in prediction of extrathyroidal invasion to adjacent structures? AJR Am J Roentgenol. 2010;195:W240–W244. doi: 10.2214/AJR.09.3965. [DOI] [PubMed] [Google Scholar]
- 74.Takashima S, Takayama F, Wang J, Kobayashi S, Kadoya M. Using MR imaging to predict invasion of the recurrent laryngeal nerve by thyroid carcinoma. AJR Am J Roentgenol. 2003;180:837–842. doi: 10.2214/ajr.180.3.1800837. [DOI] [PubMed] [Google Scholar]
- 75.Wang J, Takashima S, Matsushita T, Takayama F, Kobayashi T, Kadoya M. Esophageal invasion by thyroid carcinomas: prediction using magnetic resonance imaging. J Comput Assist Tomogr. 2003;27:18–25. doi: 10.1097/00004728-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Wang JC, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Tracheal invasion by thyroid carcinoma: prediction using MR imaging. AJR Am J Roentgenol. 2001;177:929–936. doi: 10.2214/ajr.177.4.1770929. [DOI] [PubMed] [Google Scholar]
- 77.Lee YS, Son EJ, Chang HS, Chung WY, Nam KH, Park CS. Computed tomography is useful for preoperative identification of nonrecurrent laryngeal nerve in thyroid cancer patients. Otolaryngol Head Neck Surg. 2011;145:204–207. doi: 10.1177/0194599811406670. [DOI] [PubMed] [Google Scholar]
- 78.Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf) 2005;63:87–93. doi: 10.1111/j.1365-2265.2005.02304.x. [DOI] [PubMed] [Google Scholar]
- 79.Leite AK, Kulcsar MA, de Godoi Cavalheiro B, de Mello ES, Alves VA, Cernea CR, et al. Death related to pulmonary metastasis in patients with differentiated thyroid cancer. Endocr Pract. 2017;23:72–78. doi: 10.4158/EP161431.OR. [DOI] [PubMed] [Google Scholar]
- 80.Vuong HG, Duong UNP, Pham TQ, Tran HM, Oishi N, Mochizuki K, et al. Clinicopathological risk factors for distant metastasis in differentiated thyroid carcinoma: a meta-analysis. World J Surg. 2018;42:1005–1017. doi: 10.1007/s00268-017-4206-1. [DOI] [PubMed] [Google Scholar]
- 81.Lee YS, Lim YS, Lee JC, Wang SG, Kim IJ, Son SM, et al. Clinical implications of bilateral lateral cervical lymph node metastasis in papillary thyroid cancer: a risk factor for lung metastasis. Ann Surg Oncol. 2011;18:3486–3492. [Google Scholar]
- 82.Momesso DP, Tuttle RM. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am. 2014;43:401–421. doi: 10.1016/j.ecl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 83.Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–3673. doi: 10.1210/jc.2002-021925. [DOI] [PubMed] [Google Scholar]
- 84.Torlontano M, Crocetti U, Augello G, D'Aloiso L, Bonfitto N, Varraso A, et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab. 2006;91:60–63. doi: 10.1210/jc.2005-1185. [DOI] [PubMed] [Google Scholar]
- 85.Lee JI, Chung YJ, Cho BY, Chong S, Seok JW, Park SJ. Postoperative-stimulated serum thyroglobulin measured at the time of 131I ablation is useful for the prediction of disease status in patients with differentiated thyroid carcinoma. Surgery. 2013;153:828–835. doi: 10.1016/j.surg.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 86.Lepoutre-Lussey C, Maddah D, Golmard JL, Russ G, Tissier F, Trésallet C, et al. Post-operative neck ultrasound and risk stratification in differentiated thyroid cancer patients with initial lymph node involvement. Eur J Endocrinol. 2014;170:837–846. doi: 10.1530/EJE-13-0888. [DOI] [PubMed] [Google Scholar]
- 87.Leenhardt L, Erdogan MF, Hegedus L, Mandel SJ, Paschke R, Rago T, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013;2:147–159. doi: 10.1159/000354537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nascimento C, Borget I, Al Ghuzlan A, Deandreis D, Chami L, Travagli JP, et al. Persistent disease and recurrence in differentiated thyroid cancer patients with undetectable postoperative stimulated thyroglobulin level. Endocr Relat Cancer. 2011;18:R29–R40. doi: 10.1677/ERC-10-0292. [DOI] [PubMed] [Google Scholar]
- 89.Schlumberger M, Pacini F, Wiersinga WM, Toft A, Smit JW, Sanchez Franco F, et al. Follow-up and management of differentiated thyroid carcinoma: a European perspective in clinical practice. Eur J Endocrinol. 2004;151:539–548. doi: 10.1530/eje.0.1510539. [DOI] [PubMed] [Google Scholar]
- 90.Grani G, Ramundo V, Falcone R, Lamartina L, Montesano T, Biffoni M, et al. Thyroid cancer patients with no evidence of disease: the need for repeat neck ultrasound. J Clin Endocrinol Metab. 2019;104:4981–4989. doi: 10.1210/jc.2019-00962. [DOI] [PubMed] [Google Scholar]
- 91.Ahn JE, Lee JH, Yi JS, Shong YK, Hong SJ, Lee DH, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg. 2008;32:1552–1558. doi: 10.1007/s00268-008-9588-7. [DOI] [PubMed] [Google Scholar]
- 92.Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol. 2009;193:871–878. doi: 10.2214/AJR.09.2386. [DOI] [PubMed] [Google Scholar]
- 93.Spate V, Morris J, Nichols T, Baskett C, Mason M, Horsman T, et al. Longitudinal study of iodine in toenails following IV administration of an iodine-containing contrast agent. J Radioanal Nucl Chem. 1998;236:71–77. [Google Scholar]
- 94.Sohn SY, Choi JH, Kim NK, Joung JY, Cho YY, Park SM, et al. The impact of iodinated contrast agent administered during preoperative computed tomography scan on body iodine pool in patients with differentiated thyroid cancer preparing for radioactive iodine treatment. Thyroid. 2014;24:872–877. doi: 10.1089/thy.2013.0238. [DOI] [PubMed] [Google Scholar]
- 95.Mishra A, Pradhan PK, Gambhir S, Sabaretnam M, Gupta A, Babu S. Preoperative contrast-enhanced computerized tomography should not delay radioiodine ablation in differentiated thyroid carcinoma patients. J Surg Res. 2015;193:731–737. doi: 10.1016/j.jss.2014.07.065. [DOI] [PubMed] [Google Scholar]
- 96.Tala Jury HP, Castagna MG, Fioravanti C, Cipri C, Brianzoni E, Pacini F. Lack of association between urinary iodine excretion and successful thyroid ablation in thyroid cancer patients. J Clin Endocrinol Metab. 2010;95:230–237. doi: 10.1210/jc.2009-1624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative radiation levels in Korean clinical imaging
Evidence table