Key Points
Question
Relative to their representation in underlying stroke populations, are women underenrolled in contemporary randomized clinical trials of acute stroke therapies?
Findings
In this meta-analysis of 115 acute stroke trials published in the last decade, relative to their representation in underlying stroke populations, women were underenrolled by 5.3 percentage points across all studies. The use of an upper age limit of 80 years as an exclusion criterion was associated with significantly less enrollment of women after multivariable adjustment.
Meaning
Per these findings, further efforts including changes to eligibility criteria are needed to ensure increased participation of women in acute stroke trials.
This meta-analysis assesses if women were underenrolled in contemporary randomized clinical trials of acute stroke therapies published in 9 major journals, after accounting for their representation in underlying stroke populations.
Abstract
Importance
The underenrollment of women in randomized clinical trials represents a threat to the validity of the evidence supporting clinical guidelines and potential disparities in access to novel treatments.
Objective
To determine whether women were underenrolled in contemporary randomized clinical trials of acute stroke therapies published in 9 major journals after accounting for their representation in underlying stroke populations.
Data Sources
MEDLINE was searched for acute stroke therapeutic trials published between January 1, 2010, and June 11, 2020.
Study Selection
Eligible articles reported the results of a phase 2 or 3 randomized clinical trial that enrolled patients with stroke and/or transient ischemic attack and examined a therapeutic intervention initiated within 1 month of onset.
Data Extraction
Data extraction was performed by 2 independent authors in duplicate. Individual trials were matched to estimates of the proportion of women in underlying stroke populations using the Global Burden of Disease database.
Main Outcomes and Measures
The primary outcome was the enrollment disparity difference (EDD), the absolute difference between the proportion of trial participants who were women and the proportion of strokes in the underlying disease populations that occurred in women. Random-effects meta-analyses of the EDD were performed, and multivariable metaregression was used to explore the associations of trial eligibility criteria with disparity estimates.
Results
The search returned 1529 results, and 115 trials (7.5%) met inclusion criteria. Of 121 105 randomized patients for whom sex was reported, 52 522 (43.4%) were women. The random-effects summary EDD was −0.053 (95% CI, −0.065 to −0.040), indicating that women were underenrolled by 5.3 percentage points. This disparity persisted across virtually all geographic regions, intervention types, and stroke types, apart from subarachnoid hemorrhage (0.117 [95% CI, 0.084 to 0.150]). When subarachnoid hemorrhage trials were excluded, the summary EDD was −0.067 (95% CI, −0.078 to −0.057). In the multivariable metaregression analysis, an upper age limit of 80 years as an eligibility criterion was associated with a 6–percentage point decrease in the enrollment of women.
Conclusions and Relevance
Further research is needed to understand the causes of the underenrollment of women in acute stroke trials. However, to maximize representation, investigators should avoid imposing age limits on enrollment.
Introduction
The inadequate enrollment of women in randomized clinical trials (RCTs) has been a long-standing issue in clinical medicine.1 Despite efforts to increase their representation, recent analyses provide evidence that disparities persist in the participation of women in clinical trials of various cardiovascular diseases, including stroke.2,3,4,5,6,7,8,9 In the past decade, significant progress in acute stroke therapy has been made, including treatment of large-vessel occlusions with mechanical thrombectomy,10,11,12 reduction in the risk of recurrent stroke with dual antiplatelet therapy,13,14 and advances in neuroprotection.15 However, given the well-documented importance of sex in the epidemiology and pathophysiology of stroke,16 the possible underenrollment of women in these and other stroke trials represents a threat to their generalizability and in turn to the validity of the evidence base with regards to the treatment of women. It also introduces the potential for unequal access to novel treatments.
Furthermore, previous studies of enrollment disparities in stroke trials were limited by inadequate consideration of the representation of women in the underlying stroke populations,2,3,4 which is likely to vary by geographic location and stroke type.17 These studies also did not investigate associations between disparity measures and eligibility criteria that are likely to affect the enrollment of women. Thus, the objectives of this study were to (1) determine, after accounting for the representation of women in the underlying disease populations, whether a sex disparity in enrollment exists in acute stroke RCTs published in major clinical journals in the last decade and (2) explore whether the magnitude of the sex disparity is associated with eligibility criteria that could differentially affect the participation of men and women.
Methods
Search and Eligibility Criteria
Working with an experienced medical librarian to develop the initial set of search terms, we searched MEDLINE (PubMed) for RCTs published between January 1, 2010, and June 11, 2020, using the following query: (Stroke OR “Stroke” [MeSH]) AND acute AND (“Randomized Controlled Trial” [Publication Type] OR “Randomized Controlled Trials as Topic” [MeSH] OR “Controlled Clinical Trial” [Publication Type] OR randomized [TIAB] OR randomized [TIAB] OR randomly [TIAB] OR Trial*). Because this strategy did not capture all hemorrhagic stroke trials, we conducted additional searches using the MeSH terms “cerebral hemorrhage” and “subarachnoid hemorrhage.” Eligible studies were those meeting the following criteria:
Final primary results of a phase 2 or 3 RCT design with a planned sample size of 100 patients or more. Cluster RCTs and noninferiority trials were included.
The trial population was patients with acute stroke of any type (ie, acute ischemic stroke [AIS], transient ischemic attack [TIA], intracerebral hemorrhage [ICH], or subarachnoid hemorrhage [SAH]) enrolled within 1 month of ictus.
Treatment (either pharmacological or nonpharmacological) compared with a control (placebo, usual care, or nonstandard comparator) was initiated within 1 month of stroke onset. Trials testing detection methods, educational interventions, or techniques for improvement of quality of care were excluded. Secondary prevention trials were included if treatment was initiated within 1 month of onset. For simplicity, they are also described as trials of acute stroke therapies.
Published in 1 of 9 major journals (BMJ, Circulation, JAMA, JAMA Neurology, Lancet, Lancet Neurology, NEJM, Neurology, and Stroke) in the last 10 years (January 2010-June 2020). Unpublished trials were not included.
The reference lists of all articles ultimately deemed eligible were also manually reviewed to identify any additional relevant RCTs (ie, snowballing). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines18 to report our findings. This meta-analysis was not registered.
Screening and Data Abstraction
Two authors (B.S. and J.P.) independently screened the title and abstract of each result and determined whether it was eligible for full-text review. For those studies that passed the initial screening, final determinations of eligibility were also done in duplicate based on the full-text article. All eligible trials then underwent duplicate, independent data abstraction. Discrepancies in study eligibility determinations or data abstractions were resolved by consensus and, when necessary, by consultation with the senior author (M.J.R.). The following characteristics were abstracted from eligible trials: the number of enrolled women; number of enrolled men; number of enrolling clinical sites; period of enrollment; geographic location of patient enrollment or clinical sites; enrollment numbers for each country or region; details of the experimental and control interventions (intervention type); primary end point; preplanned and final sample size; involvement of industry; mean or median age of participants; enrollment of different stroke types (frequencies); whether 1 or more women were represented in trial leadership; and eligibility criteria pertaining to age, stroke severity, prestroke disability, time from onset, qualification for specific medical treatments (ie, endovascular therapy [EVT] and intravenous thrombolysis [IVT]), and qualifying comorbidities (eg, hypertension, diabetes). These eligibility criteria were selected based on the assumption that they could potentially affect enrollment by sex. Because treatment efficacy was not under study, we did not assess risk of bias. More detailed variable definitions are provided in the eMethods in the Supplement.
To determine the representation of women in underlying stroke populations, the Global Burden of Disease (GBD) 2017 database (https://gbd2017.healthdata.org/gbd-search/), which provides data for individual countries, as well as administrative units within some nations, was queried.17 Individual trials were matched to GBD incidence data on the basis of their included stroke type, geographic area (the smallest area or region in which >80% of trial enrollment took place), and the middle year of the trial enrollment period. We abstracted the number of incident strokes in women and associated 95% uncertainty interval, as well as the number of incident strokes in men and associated 95% uncertainty interval.
Measure of Enrollment Disparity
To quantify the enrollment of women, we used a modified version of the enrollment disparity difference (EDD), a quantitative measure previously developed to characterize enrollment disparities in RCTs of lung cancer therapies.19 For each RCT, we first calculated the proportion of trial participants who were women (PPW). We then calculated the proportion of strokes occurring in women in the underlying stroke populations (PSW) using GBD data. We defined the EDD as the difference between the PPW and PSW (ie, EDD = PPW – PSW), such that a negative EDD value is indicative of an underenrollment of women (and a positive EDD value indicates overenrollment).
To quantify the imprecision of the EDD estimates, we computed the standard error (SE) of the EDD by estimating the SEs of the PPW and PSW and performing the following calculation:
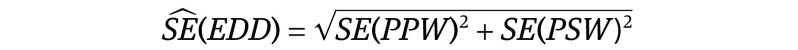 . .
|
For the PPW, we estimated the SE using the standard formula for the SE of a proportion, where n is the number of patients enrolled in the trial:
|
|
However, for the PSW, the number of men and women with stroke were estimates, which brought additional uncertainty. To account for this, we used the number of men and women with stroke and the associated 95% uncertainty intervals reported in the GBD database to fit γ distributions for each region-specific, stroke-specific, and time-specific estimate. We subsequently drew 100 000 samples from each of the γ distributions, computed the corresponding PSW, and used the PSWs from these samples to estimate the SE.
Statistical Analysis
We conducted a random-effects meta-analysis of the EDDs of individual trials to summarize the overall sex disparity among trials of acute stroke. A random-effects model, rather than assuming a single true estimate of the outcome, calculates the mean from what is assumed to be a distribution of estimates20 and is appropriate for quantifying enrollment disparities, which are likely to vary substantially between trials because of variability in the composition of underlying clinical populations and specification of inclusion and exclusion criteria. Heterogeneity was quantified with the use of the I2 statistic.21 Subgroup analyses were performed for the following trial characteristics: geographic region (Americas [mostly North America], Asia Pacific, Europe, or multiregion), stroke type (AIS or TIA, ICH, mixed ischemic and hemorrhagic strokes, or SAH), sample size (<250 patients, 250-750 patients, or >750 patients), industry involvement, intervention type (EVT; IVT; secondary prevention, if initiated within 30 days of onset; surgery; or other), and representation of 1 or more women in a leadership role. Temporal trends in trial enrollment were examined by fitting a metaregression model of the EDDs, with year of publication as the independent variable.
To investigate the association of various trial eligibility criteria with the enrollment of women, we conducted a random-effects multivariable metaregression of study-specific EDD estimates using the Knapp-Hartung modification to variance estimation.22 We assessed a prespecified list of eligibility criteria as potential covariates to minimize the probability of a type 1 error, as recommended by Thompson and Higgins.23 The following eligibility criteria were considered independent variables: the highest permitted age of participants, the highest permitted prestroke-modified Rankin Scale score, longest permitted time from stroke onset to a clinically important event (eg, enrollment, treatment, arrival), eligible range of stroke severity (ie, both mild and severe strokes, only mild strokes, or only severe strokes included), a requirement for IVT eligibility, a requirement for EVT eligibility, and a requirement for presentation with hypertension (the most common qualifying comorbidity). More detailed definitions of these variables are provided in the eMethods in the Supplement. Eligibility criteria that were associated with the EDD at P < .25 were selected for inclusion in the multivariable metaregression model. To ensure parsimony, independent variables that were not significant (ie, P > .05) and had only a minimal influence on other covariates were dropped from the model. No adjustment was made for multiple comparisons. We used the metan package in Stata version 16.1 (StataCorp) to perform the meta-analysis and the metareg package to conduct the metaregression.
Sensitivity Analyses
To verify that our conclusions were not dependent on the use of GBD data, we conducted a sensitivity analysis using an alternative source of data to estimate the PSW. We matched individual trials with greater than 80% case enrollment in a single country to published incidence studies that were conducted in the same country within 10 years of the trial enrollment period. Trials with largely multinational enrollment were therefore excluded from this analysis. We performed a random-effects meta-analysis on trials with which an incidence study could be matched and compared the pooled EDD using GBD data to the pooled EDD using published incidence data. Since several previous studies reporting on sex differences in trial enrollment calculated the participation-to-prevalence ratio,3,5,9 we also conducted a sensitivity analysis of all trials using the participation-to-prevalence ratio instead of the EDD. Methods for calculating this ratio are described in the eMethods of the Supplement. The analytic code and data used in the analysis are publicly available at the Open Science Framework (https://osf.io/fht2u/).
Results
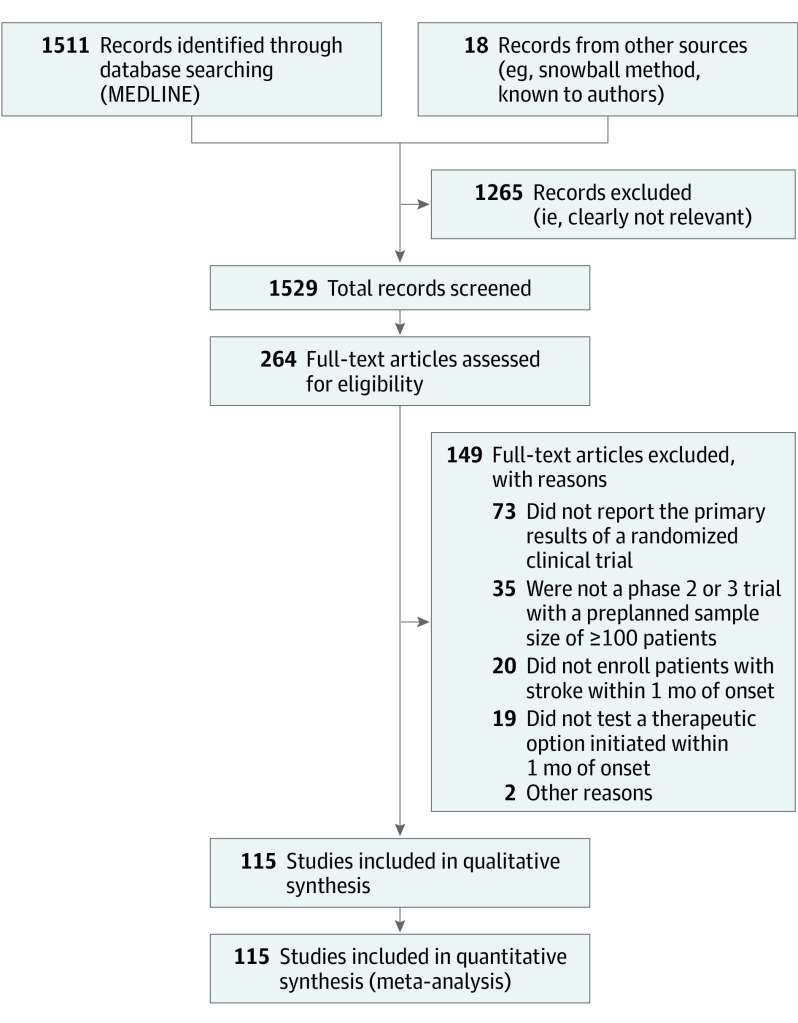
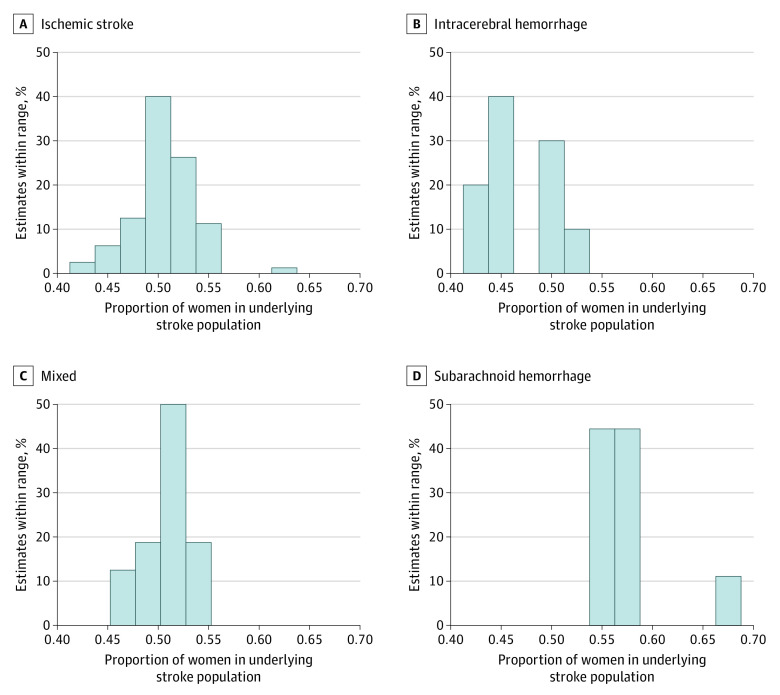
Of 1529 total search results, 115 (7.5%)11,12,13,14,15,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133 were deemed eligible and included in the meta-analysis (Figure 1). The characteristics of these 115 trials are presented in Table 1. Overall, the trials provided data on the sex of 121 105 randomized patients with acute stroke or TIA, 52 522 of whom were women (43.4%). The PPW ranged from 22.4% to 79.0% (median, 45.0% [interquartile range (IQR), 39.0%-50.1%]). There was also large variation in the GBD-based PSW estimates that were matched to the 115 individual stroke trials; PSW estimates ranged from 41.4% (China; 2014; ICH) to 67.2% (Japan; 2011; SAH) with a median of 50.8% (IQR, 49.1%-53.3%).17 The distribution of PSWs for each stroke type is presented in Figure 2.
Figure 1. PRISMA Flow Diagram Showing the Number of Studies Excluded in Each Step of the Systematic Review Process.
Table 1. Number of Randomized Clinical Trials and Enrollment of Women by Various Trial Characteristics (N = 115 Trials).
| Trial characteristica | No. of trials (%) | No. of women/total enrollment (%) |
|---|---|---|
| Region | ||
| North and South America | 23 (20.0) | 6400/13 974 (45.8) |
| Asia Pacific | 22 (19.1) | 5715/15 314 (37.3) |
| Europe | 48 (41.7) | 20 646/44 612 (46.3) |
| Multiregion | 22 (19.1) | 19 761/47 205 (41.9) |
| Stroke type | ||
| Acute ischemic stroke or transient ischemic attack | 80 (69.6) | 28 044/66 530 (42.2) |
| Intracerebral hemorrhage | 10 (8.7) | 3479/8675 (40.1) |
| Mixed | 16 (13.9) | 17 754/41 182 (43.1) |
| Subarachnoid hemorrhage | 9 (7.8) | 3245/4718 (68.8) |
| No. of patients in sample | ||
| <250 | 42 (36.5) | 2858/6149 (46.5) |
| 250-750 | 40 (34.8) | 7860/17 503 (44.9) |
| >750 | 33 (28.7) | 41 804/97 453 (42.9) |
| Industry involvement | ||
| Yes | 65 (56.5) | 26 127/58 662 (44.5) |
| No | 50 (43.5) | 26 395/62 443 (42.3) |
| Year of publication | ||
| 2010-2012 | 20 (17.4) | 7732/14 739 (52.5) |
| 2013-2014 | 21 (18.3) | 9206/22 312 (41.3) |
| 2015-2016 | 25 (21.7) | 13 977/32 976 (42.4) |
| 2017-2018 | 26 (22.6) | 15 102/35 691 (42.3) |
| 2019-2020 | 23 (20.0) | 6505/15 387 (42.3) |
| Intervention type | ||
| Endovascular therapy | 19 (16.5) | 2305/4823 (47.8) |
| Intravenous thrombolysis | 19 (16.5) | 5543/12 541 (44.2) |
| Secondary prevention | 11 (9.6) | 11 529/28 956 (39.8) |
| Surgery | 5 (4.3) | 895/1918 (46.7) |
| Other | 61 (53.0) | 32 250/72 867 (45.3) |
| ≥1 Women in a trial leadership role | ||
| Yes | 26 (22.6) | 11 635/25 423 (45.8) |
| No | 89 (77.4) | 40 887/95 682 (42.7) |
Definitions of trial characteristics are provided in the eMethods in the Supplement.
Figure 2. Distribution of the Proportion of Women in the Underlying Stroke Populations .
One hundred fifteen estimates were included from the Global Burden of Disease database. Data for each stroke type are presented separately.
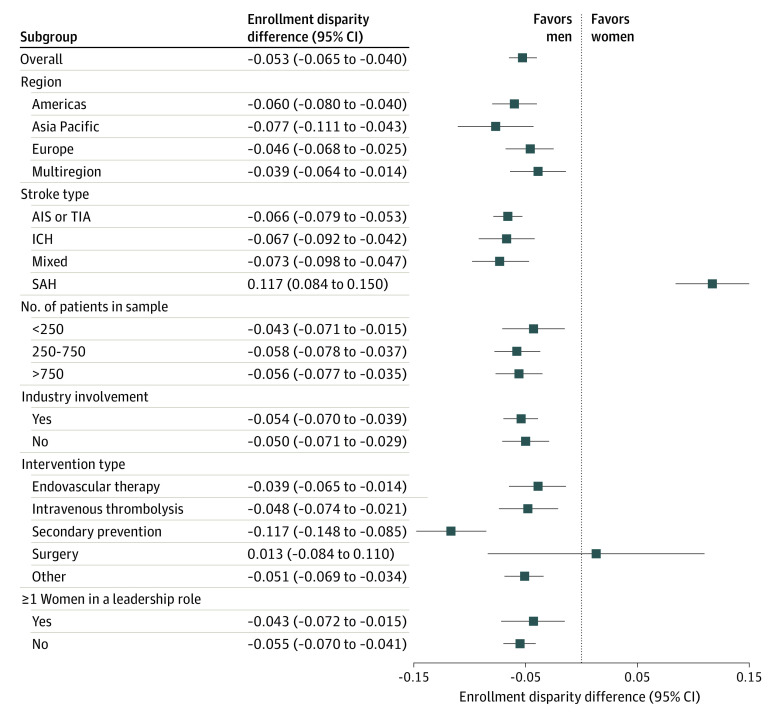
The random-effects pooled EDD of the 115 trials was −0.053 (95% CI, −0.065 to −0.040), indicating that women were underenrolled by an absolute difference of 5.3 percentage points relative to their representation in the underlying stroke populations. However, there was substantial variability between trials in the participation of women (I2, 84.4%). Results of the subgroup analyses are presented in Figure 3. Stroke type was identified as a significant source of heterogeneity: the summary EDD for trials enrolling SAHs was 0.117 (95% CI, 0.084-0.150); this highly positive EDD estimate indicates that women were considerably overenrolled in these 9 studies. Conversely, among the remaining 106 trials, women were underenrolled by approximately 6.7 percentage points relative to the underlying stroke populations (summary EDD, −0.067 [95% CI, −0.078 to −0.057]) (eFigure 1 in the Supplement). The largest sex disparity was observed among trials of secondary prevention therapies,13,14,24,45,46,61,72,73,125,130,132 which showed a large, negative EDD value (summary EDD, −0.117 [95% CI, −0.148 to −0.085]). When temporal trends were investigated using a univariable metaregression model, there was a statistically significant decrease in the representation of women over time (a decrease of 0.005 in the EDD per year [95% CI, −0.010 to 0.000 per year]; P = .05) (eFigure 2 in the Supplement). However, this trend was likely the result of the publication of most of the SAH trials,93,99,101,114,116,117,126,133 which had a significantly higher mean participation of women, in 2015 or earlier. The association of time with the EDD was greatly attenuated and became nonsignificant after adjustment for stroke type and after excluding the 9 SAH trials47,93,99,101,114,116,117,126,133 from the analysis (eFigure 3 in the Supplement).
Figure 3. Forest Plot With the Random-Effects Pooled Enrollment Disparity Differences for All Trials and Trial Subgroups .
One hundred fifteen trials were included. AIS indicates acute ischemic stroke; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage; TIA, transient ischemic attack.
In the main sensitivity analysis (eAppendix in the Supplement), 49 of the 115 trials were matched to a published incidence study from a single country. Among these 49 studies,11,12,13,14,25,27,28,30,31,35,38,40,43,44,45,46,48,50,52,55,62,65,66,67,68,71,74,75,78,84,88,89,91,96,99,100,101,105,106,107,108,111,112,114,118,119,121,124,131 the pooled, random-effects EDD when the representation of women in the underlying stroke populations (PSW) was based on GBD data was −0.056 (95% CI, −0.077 to −0.035). When estimates of the PSW were calculated using the published incidence studies, the disparity was slightly larger (summary EDD, −0.072 [95% CI, −0.088 to −0.055]), and women were no longer overrepresented in SAH trials. Further information on the results of this sensitivity analysis is provided in eFigures 4 and 5 in the Supplement. When the disparity was quantified as a ratio, the summary participation to prevalence ratio for all 115 trials was 0.89 (95% CI, 0.87-0.92) (eFigure 6 in the Supplement).
When we examined the association of eligibility criteria with the EDD using metaregression, we were only able to include the 80 trials11,12,13,14,15,24,25,26,27,28,29,30,31,32,33,34,36,37,40,41,42,44,45,46,48,49,50,51,53,55,56,58,59,61,62,63,64,67,71,72,73,74,75,77,78,79,80,82,84,85,86,87,89,90,94,95,96,97,98,103,104,105,107,108,109,111,113,115,118,119,120,121,123,124,125,128,129,130,132 enrolling patients with AIS and TIA, because there was insufficient variation in the eligibility criteria among trials of other stroke types (ie, ICH, SAH, and mixed).35,38,39,43,47,52,54,57,60,65,66,68,70,76,81,83,88,91,92,93,99,100,101,102,106,110,112,114,116,117,122,126,127,131,133 Only the imposition of an upper age limit and requirements for IVT and EVT eligibility were significantly associated with the EDD in the final model, but prestroke disability and limits on stroke severity were retained because they had an influence on the magnitude of the other independent variables (Table 2). The imposition of an upper age limit of 80 years or younger was associated with a 6–percentage point decrease in the enrollment of women, while requirements for IVT eligibility and EVT eligibility were associated with 3–percentage point increases in the enrollment of women after adjustment.
Table 2. Results of the Random-Effects Multivariable Metaregression Analysis of the Enrollment Disparity Difference From Trials Enrolling Acute Ischemic Stroke and/or Transient Ischemic Attack (N = 80 studies).
| Eligibility criterion | No. of trials | β (95% CI) | |
|---|---|---|---|
| Unadjusteda | Adjusted | ||
| Highest permitted age of participants | |||
| ≥90 y or no upper age limit | 50 | 0 [Reference] | 0 [Reference] |
| >80-<90 y | 20 | 0.025 (−0.006 to 0.056) | 0.012 (−0.018 to 0.041) |
| ≤80 yb | 10 | −0.033 (−0.074 to 0.007) | −0.061 (−0.099 to −0.023) |
| Highest permitted score on prestroke-modified Rankin Scale | |||
| 1-Point increase | 80 | −0.009 (−0.017 to −0.001) | −0.006 (−0.013 to 0.002) |
| Requirement for intravenous thrombolysis eligibility | |||
| No | 51 | 0 [Reference] | 0 [Reference] |
| Yesb | 29 | 0.028 (0 to 0.055) | 0.029 (0.005 to 0.054) |
| Requirement for endovascular therapy eligibility | |||
| No | 56 | 0 [Reference] | 0 [Reference] |
| Yesb | 24 | 0.037 (0.008 to 0.066) | 0.029 (0.001 to 0.058) |
| Permitted severity of strokec | |||
| Mild and severe | 57 | 0 [Reference] | 0 [Reference] |
| Only mild | 5 | −0.038 (−0.086 to 0.011) | −0.011 (−0.055 to 0.034) |
| Only severe | 18 | 0.036 (0.004 to 0.069) | 0.031 (−0.002 to 0.064) |
Negative estimates indicate the eligibility criterion is associated with a lower representation of women, while positive estimates indicate it is associated with a greater representation of women. Independent variables are trial eligibility criteria.
Significantly associated with the representation of women after adjustment. The P value for of an upper age limit less than or equal to 80 years was .002; for intravenous thrombolysis eligibility, .02; for endovascular therapy eligibility, .04.
We considered mild strokes as those resulting in an National Institutes of Health Stroke Scale score of 7 or less or a Glasgow Coma Scale score of 13 or more. More detailed definitions of mild and severe strokes are provided in the eMethods in the Supplement.
Discussion
In this analysis of 115 acute stroke trials published between 2010 and 2020 in 9 highly cited medical journals, we found that women were underenrolled by approximately 5 percentage points relative to their representation in underlying disease populations. This finding is especially concerning given that women, on average, experience slightly more incident events than men in the underlying stroke populations, and it indicates that greater efforts are needed to increase their enrollment. The systematic underrepresentation of women in acute stroke trials also raises questions about the representativeness and generalizability of trial results to women. Furthermore, we note that a statistically significant disparity against women was seen in virtually all sensitivity and subgroup analyses. The largest disparity was observed among trials of secondary prevention therapies; while there are likely several factors that contribute to this, the lower representation of women may be partially explained by the exclusion of patients with indications of a stroke of cardioembolic origin from many of the antiplatelet-based secondary prevention trials. Stroke of cardioembolic origin is more common in women,134 and this exclusion likely has a disproportionate effect on them.
In the metaregression analysis, we found that the imposition of an upper age limit on eligibility of 80 years or younger was associated with a 6–percentage point decrease in the representation of women after adjustment. The older age of women at first stroke is a well-documented phenomenon; in a 2009 meta-analysis134 of 59 stroke incidence studies, women were a mean of 4.3 years older than men at the time of their first event. Consequently, upper age limits placed on trial enrollment exclude more women. An illustration of this phenomenon is provided by an analysis of patients with AIS in a large German registry, which showed that an upper age limit of 80 years in a hypothetical trial would exclude 19% of men but 44% of women.135 These findings suggest that an upper age limit on trial enrollment must be medically necessary to be justified. When an upper age limit is being considered, trial investigators should first contemplate whether age is serving as a proxy for some other age-associated characteristic, such as frailty, fall risk, or bleeding risk. In such cases, it may be possible to replace the age exclusion with an exclusion based on a valid risk assessment tool.136
Somewhat surprisingly, we also found that enrollment requirements for IVT and EVT eligibility were associated with a higher representation of women. Our finding with respect to IVT may be reflective of the facts that women are more likely to have a severe stroke137 and mild severity is a common reason for nontreatment with thrombolytic therapy.138 Regarding EVT eligibility, in an analysis of a German administrative database with more than 1 million observations, women were more likely to receive mechanical thrombectomy,139 which aligns with the findings of our metaregression.
We should note, however, that our metaregression model was unable to explain two-thirds of the between-trial variance. This indicates that factors apart from eligibility criteria affect the participation of women in these trials and contribute to their underrepresentation. One factor that may adversely affect the enrollment of women is their willingness to participate. Although not always a consistent finding,140 there is evidence that women are less likely to consent to inclusion in stroke trials than men,141,142 which may be the consequence of a higher perceived risk of trial participation and greater risk aversion among women.143,144 There may also be other factors operating in the prescreening phase that differentially affect the probability of a male or female patient with stroke being screened.3,9 These factors could reflect either a bias in trial design, such as the inclusion of clinical sites that see mostly male patients (such as US Veterans Health Administration facilities) or perhaps differences in the likelihood of hospitalization following stroke. For example, a prior analysis of stroke registry data indicates that elderly women who live in nursing homes are less likely to be admitted to acute care hospitals.145 Ultimately, more high-quality data are needed from screening logs to determine whether women are less likely to be approached for participation in the context of acute stroke trials.
Limitations
There are several limitations to this study. To begin, our finding that SAH trials overenrolled women by a significant margin should be approached with caution, since we found that this estimate was affected by the source of data used to generate the PSW (eFigures 4 and 5 in the Supplement). Subarachnoid hemorrhage is a relatively rare disease, and a well-designed population-based incidence study in Oxfordshire, United Kingdom, registered only 20 incident events during a 3-year period.146 Consequently, estimates of the PSW in the underlying SAH populations are likely to be imprecise. Second, conclusions drawn from the metaregression model are limited by the potential for residual confounding at either the trial or patient level because of the ecological nature of the analysis.23 Although our findings in conjunction with previous work provide a strong justification to eliminate upper age limits on enrollment, analyses of individual patient screening and recruitment data are needed to fully elucidate the role of these and other factors in the underenrollment of women. Unfortunately, such analyses may not be insightful in practice because of a lack of standardization of screening procedures across clinical sites.147 Our ability to classify trial eligibility criteria, such as stroke severity, was also quite limited and affected by the lack of an accepted definition for mild or severe strokes. Lastly, we only included trials published in 9 major clinical journals and excluded those with a planned sample size of less than 100 patients, which may limit the generalizability of our findings to other acute stroke trials. However, major clinical trials affecting acute stroke care are most likely to be published in these 9 journals.
Conclusions
In summary, we found that women were underenrolled in acute stroke trials published in the last decade by 5 percentage points relative to their representation in underlying stroke populations. This disparity was present for every stroke type except trials enrolling SAHs, which showed an overenrollment of women. We also found that the presence of an upper age limit of 80 years on eligibility was significantly associated with a lower representation of women. Thus, authors imposing an upper age limit as an eligibility criterion should provide a clear rationale for doing so. Further study is needed to monitor the magnitude of sex disparities in clinical trial enrollment, determine what modifications to trial design can feasibly be made to increase the participation of women, and understand the patient-level factors that may contribute to women being less likely to enroll. It will also be important to determine the clinical relevance and impact of the observed enrollment disparities on the validity, representativeness, and generalizability of trial data.
eMethods. Variable Definitions and Sensitivity Analyses
eFigure 1. Forest plot with the random effects, pooled enrolled enrollment disparity difference (EDD) for all trials (n=106) and for trial subgroups when subarachnoid hemorrhage trials were excluded.
eFigure 2. Temporal trend by publication date in the representation of women in randomized controlled trials of acute stroke with subarachnoid hemorrhage trials included (n=115).
eFigure 3. Temporal trend by publication date in the representation of women in randomized controlled trials of acute stroke with subarachnoid hemorrhage trials dropped (n=106).
eAppendix. Sensitivity Analyses.
eFigure 4. Sensitivity analysis using Global Burden of Disease data to account for the representation of women in the underlying stroke populations.
eFigure 5. Sensitivity analysis using published incidence data to account for the representation of women in the underlying stroke populations.
eFigure 6. Forest plot with the random effects, pooled participation to prevalence ratio (PPR) for all trials and for trial subgroups, estimated as part of a sensitivity analysis (n=115 trials).
eReferences.
References
- 1.Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health (Larchmt). 2011;20(3):315-320. doi: 10.1089/jwh.2010.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carcel C, Woodward M, Balicki G, et al. Trends in recruitment of women and reporting of sex differences in large-scale published randomized controlled trials in stroke. Int J Stroke. 2019;14(9):931-938. doi: 10.1177/1747493019851292 [DOI] [PubMed] [Google Scholar]
- 3.Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL. Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation. 2020;141(7):540-548. doi: 10.1161/CIRCULATIONAHA.119.043594 [DOI] [PubMed] [Google Scholar]
- 4.Burke JF, Brown DL, Lisabeth LD, Sanchez BN, Morgenstern LB. Enrollment of women and minorities in NINDS trials. Neurology. 2011;76(4):354-360. doi: 10.1212/WNL.0b013e3182088260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MS, Shahid I, Siddiqi TJ, et al. Ten-year trends in enrollment of women and minorities in pivotal trials supporting recent US Food and Drug Administration approval of novel cardiometabolic drugs. J Am Heart Assoc. 2020;9(11):e015594. doi: 10.1161/JAHA.119.015594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen QD, Peters E, Wassef A, Desmarais P, Rémillard-Labrosse D, Tremblay-Gravel M. Evolution of age and female representation in the most-cited randomized controlled trials of cardiology of the last 20 years. Circ Cardiovasc Qual Outcomes. 2018;11(6):e004713. doi: 10.1161/CIRCOUTCOMES.118.004713 [DOI] [PubMed] [Google Scholar]
- 7.Tahhan AS, Vaduganathan M, Greene SJ, et al. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol. 2018;3(10):1011-1019. doi: 10.1001/jamacardio.2018.2559 [DOI] [PubMed] [Google Scholar]
- 8.Tahhan AS, Vaduganathan M, Greene SJ, et al. Enrollment of older patients, women, and racial/ethnic minority groups in contemporary acute coronary syndrome clinical trials: a systematic review. JAMA Cardiol. 2020;5(6):714-722. doi: 10.1001/jamacardio.2020.0359 [DOI] [PubMed] [Google Scholar]
- 9.Scott PE, Unger EF, Jenkins MR, et al. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J Am Coll Cardiol. 2018;71(18):1960-1969. doi: 10.1016/j.jacc.2018.02.070 [DOI] [PubMed] [Google Scholar]
- 10.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 11.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 12.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston SC, Easton JD, Farrant M, et al. ; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators . Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215-225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wang Y, Zhao X, et al. ; CHANCE Investigators . Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11-19.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23803136&dopt=Abstract doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 15.Hill MD, Goyal M, Menon BK, et al. ; ESCAPE-NA1 Investigators . Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395(10227):878-887. doi: 10.1016/S0140-6736(20)30258-0 [DOI] [PubMed] [Google Scholar]
- 16.Bushnell CD, Chaturvedi S, Gage KR, et al. Sex differences in stroke: Challenges and opportunities. J Cereb Blood Flow Metab. 2018;38(12):2179-2191. doi: 10.1177/0271678X18793324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James SL, Abate D, Abate KH, et al. ; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang HH, Wang X, Stinchcombe TE, et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol. 2016;34(33):3992-3999. doi: 10.1200/JCO.2016.67.7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693-2710. doi: 10.1002/sim.1482 [DOI] [PubMed] [Google Scholar]
- 23.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559-1573. doi: 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 24.Butcher KS, Ng K, Sheridan P, et al. Dabigatran treatment of acute noncardioembolic ischemic stroke. Stroke. 2020;51(4):1190-1198. doi: 10.1161/STROKEAHA.119.027569 [DOI] [PubMed] [Google Scholar]
- 25.Campbell BCV, Mitchell PJ, Churilov L, et al. ; EXTEND-IA TNK Part 2 investigators . Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK Part 2 randomized clinical trial. JAMA. 2020;323(13):1257-1265. doi: 10.1001/jama.2020.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chabriat H, Bassetti CL, Marx U, et al. ; RESTORE BRAIN study investigators . Safety and efficacy of GABAA α5 antagonist S44819 in patients with ischaemic stroke: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2020;19(3):226-233. doi: 10.1016/S1474-4422(20)30004-1 [DOI] [PubMed] [Google Scholar]
- 27.Koga M, Yamamoto H, Inoue M, et al. ; THAWS Trial Investigators . Thrombolysis with alteplase at 0.6 mg/kg for stroke with unknown time of onset: a randomized controlled trial. Stroke. 2020;51(5):1530-1538. doi: 10.1161/STROKEAHA.119.028127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Dai Q, Ye R, et al. ; BEST Trial Investigators . Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19(2):115-122. doi: 10.1016/S1474-4422(19)30395-3 [DOI] [PubMed] [Google Scholar]
- 29.Martins SO, Mont’Alverne F, Rebello LC, et al. ; RESILIENT Investigators . Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. 2020;382(24):2316-2326. doi: 10.1056/NEJMoa2000120 [DOI] [PubMed] [Google Scholar]
- 30.Pico F, Lapergue B, Ferrigno M, et al. Effect of in-hospital remote ischemic perconditioning on brain infarction growth and clinical outcomes in patients with acute ischemic stroke: the RESCUE BRAIN randomized clinical trial. JAMA Neurol. 2020;77(6):725-734. doi: 10.1001/jamaneurol.2020.0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P, Zhang Y, Zhang L, et al. ; DIRECT-MT Investigators . Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi: 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 32.Alexandrov AV, Köhrmann M, Soinne L, et al. ; CLOTBUST-ER Trial Investigators . Safety and efficacy of sonothrombolysis for acute ischaemic stroke: a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Neurol. 2019;18(4):338-347. doi: 10.1016/S1474-4422(19)30026-2 [DOI] [PubMed] [Google Scholar]
- 33.Anderson CS, Huang Y, Lindley RI, et al. ; ENCHANTED Investigators and Coordinators . Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet. 2019;393(10174):877-888. doi: 10.1016/S0140-6736(19)30038-8 [DOI] [PubMed] [Google Scholar]
- 34.Bang OY, Chung JW, Kim SK, et al. Therapeutic-induced hypertension in patients with noncardioembolic acute stroke. Neurology. 2019;93(21):e1955-e1963. doi: 10.1212/WNL.0000000000008520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bath PM, Scutt P, Anderson CS, et al. ; RIGHT-2 Investigators . Prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): an ambulance-based, randomised, sham-controlled, blinded, phase 3 trial. Lancet. 2019;393(10175):1009-1020. doi: 10.1016/S0140-6736(19)30194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bornstein NM, Saver JL, Diener HC, et al. ; ImpACT-24B investigators . An injectable implant to stimulate the sphenopalatine ganglion for treatment of acute ischaemic stroke up to 24 h from onset (ImpACT-24B): an international, randomised, double-blind, sham-controlled, pivotal trial. Lancet. 2019;394(10194):219-229. doi: 10.1016/S0140-6736(19)31192-4 [DOI] [PubMed] [Google Scholar]
- 37.Bornstein NM, Saver JL, Diener H-C, et al. ; ImpACT-24A Investigators . Sphenopalatine ganglion stimulation to augment cerebral blood flow: a randomized, sham-controlled trial. Stroke. 2019;50(8):2108-2117. doi: 10.1161/STROKEAHA.118.024582 [DOI] [PubMed] [Google Scholar]
- 38.Dennis M, Forbes J, Graham C, et al. ; FOCUS Trial Collaboration . Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. Lancet. 2019;393(10168):265-274. doi: 10.1016/S0140-6736(18)32823-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanley DF, Thompson RE, Rosenblum M, et al. ; MISTIE III Investigators . Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021-1032. doi: 10.1016/S0140-6736(19)30195-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston KC, Bruno A, Pauls Q, et al. ; Neurological Emergencies Treatment Trials Network and the SHINE Trial Investigators . Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA. 2019;322(4):326-335. doi: 10.1001/jama.2019.9346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma H, Campbell BCV, Parsons MW, et al. ; EXTEND Investigators . Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-1803. doi: 10.1056/NEJMoa1813046 [DOI] [PubMed] [Google Scholar]
- 42.Neugebauer H, Schneider H, Bösel J, et al. Outcomes of hypothermia in addition to decompressive hemicraniectomy in treatment of malignant middle cerebral artery stroke: a randomized clinical trial. JAMA Neurol. 2019;76(5):571-579. doi: 10.1001/jamaneurol.2018.4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selim M, Foster LD, Moy CS, et al. ; i-DEF Investigators . Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18(5):428-438. doi: 10.1016/S1474-4422(19)30069-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turk AS III, Siddiqui A, Fifi JT, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet. 2019;393(10175):998-1008. doi: 10.1016/S0140-6736(19)30297-1 [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Chen W, Lin Y, et al. ; PRINCE Protocol Steering Group . Ticagrelor plus aspirin versus clopidogrel plus aspirin for platelet reactivity in patients with minor stroke or transient ischaemic attack: open label, blinded endpoint, randomised controlled phase II trial. BMJ. 2019;365:l2211. doi: 10.1136/bmj.l2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bath PM, Woodhouse LJ, Appleton JP, et al. ; TARDIS Investigators . Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391(10123):850-859. doi: 10.1016/S0140-6736(17)32849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gathier CS, van den Bergh WM, van der Jagt M, et al. ; HIMALAIA Study Group . Induced hypertension for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a randomized clinical trial. Stroke. 2018;49(1):76-83. doi: 10.1161/STROKEAHA.117.017956 [DOI] [PubMed] [Google Scholar]
- 48.Khatri P, Kleindorfer DO, Devlin T, et al. ; PRISMS Investigators . Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA. 2018;320(2):156-166. doi: 10.1001/jama.2018.8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraglund KL, Mortensen JK, Damsbo AG, et al. Neuroregeneration and vascular protection by citalopram in acute ischemic stroke (TALOS). Stroke. 2018;49(11):2568-2576. doi: 10.1161/STROKEAHA.117.020067 [DOI] [PubMed] [Google Scholar]
- 50.Nogueira RG, Frei D, Kirmani JF, et al. ; Penumbra Separator 3D Investigators . Safety and efficacy of a 3-dimensional stent retriever with aspiration-based thrombectomy vs aspiration-based thrombectomy alone in acute ischemic stroke intervention: a randomized clinical trial. JAMA Neurol. 2018;75(3):304-311. doi: 10.1001/jamaneurol.2017.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simonsen CZ, Yoo AJ, Sørensen LH, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75(4):470-477. doi: 10.1001/jamaneurol.2017.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sprigg N, Flaherty K, Appleton JP, et al. ; TICH-2 Investigators . Tranexamic acid for hyperacute primary intracerebral haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391(10135):2107-2115. doi: 10.1016/S0140-6736(18)31033-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomalla G, Simonsen CZ, Boutitie F, et al. ; WAKE-UP Investigators . MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611-622. doi: 10.1056/NEJMoa1804355 [DOI] [PubMed] [Google Scholar]
- 54.Anderson CS, Arima H, Lavados P, et al. ; HeadPoST Investigators and Coordinators . Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med. 2017;376(25):2437-2447. doi: 10.1056/NEJMoa1615715 [DOI] [PubMed] [Google Scholar]
- 55.Aoki J, Kimura K, Morita N, et al. ; YAMATO Study Investigators . YAMATO study (tissue-type plasminogen activator and edaravone combination therapy). Stroke. 2017;48(3):712-719. doi: 10.1161/STROKEAHA.116.015042 [DOI] [PubMed] [Google Scholar]
- 56.Barreto AD, Ford GA, Shen L, et al. ; ARTSS-2 Investigators . Randomized, multicenter trial of ARTSS-2 (argatroban with recombinant tissue plasminogen activator for acute stroke). Stroke. 2017;48(6):1608-1616. doi: 10.1161/STROKEAHA.117.016720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Ridder IR, den Hertog HM, van Gemert HMA, et al. ; Trial Organization . PAIS 2 (paracetamol [Acetaminophen] in stroke 2): results of a randomized, double-blind placebo-controlled clinical trial. Stroke. 2017;48(4):977-982. doi: 10.1161/STROKEAHA.116.015957 [DOI] [PubMed] [Google Scholar]
- 58.Elkins J, Veltkamp R, Montaner J, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16(3):217-226. doi: 10.1016/S1474-4422(16)30357-X [DOI] [PubMed] [Google Scholar]
- 59.Guekht A, Skoog I, Edmundson S, Zakharov V, Korczyn AD. ARTEMIDA Trial (A Randomized Trial of Efficacy, 12 Months International Double-Blind Actovegin): a randomized controlled trial to assess the efficacy of actovegin in poststroke cognitive impairment. Stroke. 2017;48(5):1262-1270. doi: 10.1161/STROKEAHA.116.014321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanley DF, Lane K, McBee N, et al. ; CLEAR III Investigators . Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603-611. doi: 10.1016/S0140-6736(16)32410-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong KS, Kwon SU, Lee SH, et al. ; Phase 2 Exploratory Clinical Study to Assess the Effects of Xarelto (Rivaroxaban) Versus Warfarin on Ischemia, Bleeding, and Hospital Stay in Acute Cerebral Infarction Patients With Non-valvular Atrial Fibrillation (Triple AXEL) Study Group . Rivaroxaban vs warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: a randomized clinical trial. JAMA Neurol. 2017;74(10):1206-1215. doi: 10.1001/jamaneurol.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lapergue B, Blanc R, Gory B, et al. ; ASTER Trial Investigators . Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017;318(5):443-452. doi: 10.1001/jama.2017.9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781-788. doi: 10.1016/S1474-4422(17)30253-3 [DOI] [PubMed] [Google Scholar]
- 64.Nacu A, Kvistad CE, Naess H, et al. NOR-SASS (Norwegian Sonothrombolysis in Acute Stroke Study): randomized controlled contrast-enhanced sonothrombolysis in an unselected acute ischemic stroke population. Stroke. 2017;48(2):335-341. doi: 10.1161/STROKEAHA.116.014644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roffe C, Nevatte T, Sim J, et al. ; Stroke Oxygen Study Investigators and the Stroke OxygenStudy Collaborative Group . Effect of routine low-dose oxygen supplementation on death and disability in adults with acute stroke: the Stroke Oxygen Study randomized clinical trial. JAMA. 2017;318(12):1125-1135. doi: 10.1001/jama.2017.11463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yelnik AP, Quintaine V, Andriantsifanetra C, et al. ; AMOBES Group . AMOBES (Active Mobility Very Early After Stroke): a randomized controlled trial. Stroke. 2017;48(2):400-405. doi: 10.1161/STROKEAHA.116.014803 [DOI] [PubMed] [Google Scholar]
- 67.Yoshimura S, Uchida K, Daimon T, Takashima R, Kimura K, Morimoto T; ASSORT Trial Investigator . Randomized controlled trial of early versus delayed statin therapy in patients with acute ischemic stroke: ASSORT trial (Administration of Statin on Acute Ischemic Stroke Patient). Stroke. 2017;48(11):3057-3063. doi: 10.1161/STROKEAHA.117.017623 [DOI] [PubMed] [Google Scholar]
- 68.Zheng J, Li H, Lin S, et al. Perioperative antihypertensive treatment in patients with spontaneous intracerebral hemorrhage. Stroke. 2017;48(1):216-218. doi: 10.1161/STROKEAHA.116.014285 [DOI] [PubMed] [Google Scholar]
- 69.Anderson CS, Robinson T, Lindley RI, et al. ; ENCHANTED Investigators and Coordinators . Low-Dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016;374(24):2313-2323. doi: 10.1056/NEJMoa1515510 [DOI] [PubMed] [Google Scholar]
- 70.Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. ; PATCH Investigators . Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387(10038):2605-2613. doi: 10.1016/S0140-6736(16)30392-0 [DOI] [PubMed] [Google Scholar]
- 71.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 72.Hong KS, Lee SH, Kim EG, et al. ; COMPRESS Investigators . Recurrent ischemic lesions after acute atherothrombotic stroke: clopidogrel plus aspirin versus aspirin alone. Stroke. 2016;47(9):2323-2330. doi: 10.1161/STROKEAHA.115.012293 [DOI] [PubMed] [Google Scholar]
- 73.Johnston SC, Amarenco P, Albers GW, et al. ; SOCRATES Steering Committee and Investigators . Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375(1):35-43. doi: 10.1056/NEJMoa1603060 [DOI] [PubMed] [Google Scholar]
- 74.Lyden P, Hemmen T, Grotta J, et al. ; Collaborators . Results of the ICTuS 2 trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke. 2016;47(12):2888-2895. doi: 10.1161/STROKEAHA.116.014200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mocco J, Zaidat OO, von Kummer R, et al. ; THERAPY Trial Investigators* . Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47(9):2331-2338. doi: 10.1161/STROKEAHA.116.013372 [DOI] [PubMed] [Google Scholar]
- 76.Qureshi AI, Palesch YY, Barsan WG, et al. ; ATACH-2 Trial Investigators and the Neurological Emergency Treatment Trials Network . Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375(11):1033-1043. doi: 10.1056/NEJMoa1603460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schönenberger S, Uhlmann L, Hacke W, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316(19):1986-1996. doi: 10.1001/jama.2016.16623 [DOI] [PubMed] [Google Scholar]
- 78.Sheth KN, Elm JJ, Molyneaux BJ, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15(11):1160-1169. doi: 10.1016/S1474-4422(16)30196-X [DOI] [PubMed] [Google Scholar]
- 79.von Kummer R, Mori E, Truelsen T, et al. ; DIAS-4 Investigators . Desmoteplase 3 to 9 hours after major artery occlusion stroke: the DIAS-4 trial (efficacy and safety study of desmoteplase to treat acute ischemic stroke). Stroke. 2016;47(12):2880-2887. doi: 10.1161/STROKEAHA.116.013715 [DOI] [PubMed] [Google Scholar]
- 80.Albers GW, von Kummer R, Truelsen T, et al. ; DIAS-3 Investigators . Safety and efficacy of desmoteplase given 3-9 h after ischaemic stroke in patients with occlusion or high-grade stenosis in major cerebral arteries (DIAS-3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Neurol. 2015;14(6):575-584. doi: 10.1016/S1474-4422(15)00047-2 [DOI] [PubMed] [Google Scholar]
- 81.Bath PMW, Woodhouse L, Scutt P, et al. ; ENOS Trial Investigators . Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2015;385(9968):617-628. doi: 10.1016/S0140-6736(14)61121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 83.Bernhardt J, Langhorne P, Lindley RI, et al. ; AVERT Trial Collaboration group . Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. 2015;386(9988):46-55. doi: 10.1016/S0140-6736(15)60690-0 [DOI] [PubMed] [Google Scholar]
- 84.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 85.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 86.Huang X, Cheripelli BK, Lloyd SM, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. 2015;14(4):368-376. doi: 10.1016/S1474-4422(15)70017-7 [DOI] [PubMed] [Google Scholar]
- 87.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 88.Kalra L, Irshad S, Hodsoll J, et al. ; STROKE-INF Investigators . Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet. 2015;386(10006):1835-1844. doi: 10.1016/S0140-6736(15)00126-9 [DOI] [PubMed] [Google Scholar]
- 89.Nighoghossian N, Berthezène Y, Mechtouff L, et al. Cyclosporine in acute ischemic stroke. Neurology. 2015;84(22):2216-2223. doi: 10.1212/WNL.0000000000001639 [DOI] [PubMed] [Google Scholar]
- 90.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 91.Saver JL, Starkman S, Eckstein M, et al. ; FAST-MAG Investigators and Coordinators . Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med. 2015;372(6):528-536. doi: 10.1056/NEJMoa1408827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Westendorp WF, Vermeij JD, Zock E, et al. ; PASS investigators . The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet. 2015;385(9977):1519-1526. doi: 10.1016/S0140-6736(14)62456-9 [DOI] [PubMed] [Google Scholar]
- 93.Wong GK, Chan DY, Siu DY, et al. ; HDS-SAH Investigators . High-dose simvastatin for aneurysmal subarachnoid hemorrhage: multicenter randomized controlled double-blinded clinical trial. Stroke. 2015;46(2):382-388. doi: 10.1161/STROKEAHA.114.007006 [DOI] [PubMed] [Google Scholar]
- 94.Chamorro A, Amaro S, Castellanos M, et al. ; URICO-ICTUS Investigators . Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13(5):453-460. doi: 10.1016/S1474-4422(14)70054-7 [DOI] [PubMed] [Google Scholar]
- 95.Hacke W, Schellinger PD, Albers GW, et al. ; NEST 3 Committees and Investigators . Transcranial laser therapy in acute stroke treatment: results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke. 2014;45(11):3187-3193. doi: 10.1161/STROKEAHA.114.005795 [DOI] [PubMed] [Google Scholar]
- 96.He J, Zhang Y, Xu T, et al. ; CATIS Investigators . Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311(5):479-489. doi: 10.1001/jama.2013.282543 [DOI] [PubMed] [Google Scholar]
- 97.Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45(1):159-167. doi: 10.1161/STROKEAHA.113.001346 [DOI] [PubMed] [Google Scholar]
- 98.Jüttler E, Unterberg A, Woitzik J, et al. ; DESTINY II Investigators . Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014;370(12):1091-1100. doi: 10.1056/NEJMoa1311367 [DOI] [PubMed] [Google Scholar]
- 99.Kirkpatrick PJ, Turner CL, Smith C, Hutchinson PJ, Murray GD; STASH Collaborators . Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol. 2014;13(7):666-675. doi: 10.1016/S1474-4422(14)70084-5 [DOI] [PubMed] [Google Scholar]
- 100.Liu N, Cadilhac DA, Andrew NE, et al. Randomized controlled trial of early rehabilitation after intracerebral hemorrhage stroke: difference in outcomes within 6 months of stroke. Stroke. 2014;45(12):3502-3507. doi: 10.1161/STROKEAHA.114.005661 [DOI] [PubMed] [Google Scholar]
- 101.Mutoh T, Kazumata K, Terasaka S, Taki Y, Suzuki A, Ishikawa T. Early intensive versus minimally invasive approach to postoperative hemodynamic management after subarachnoid hemorrhage. Stroke. 2014;45(5):1280-1284. doi: 10.1161/STROKEAHA.114.004739 [DOI] [PubMed] [Google Scholar]
- 102.Anderson CS, Heeley E, Huang Y, et al. ; INTERACT2 Investigators . Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355-2365. doi: 10.1056/NEJMoa1214609 [DOI] [PubMed] [Google Scholar]
- 103.Broderick JP, Palesch YY, Demchuk AM, et al. ; Interventional Management of Stroke (IMS) III Investigators . Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893-903. doi: 10.1056/NEJMoa1214300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen CL, Young SH, Gan HH, et al. ; CHIMES Study Investigators . Chinese medicine neuroaid efficacy on stroke recovery: a double-blind, placebo-controlled, randomized study. Stroke. 2013;44(8):2093-2100. doi: 10.1161/STROKEAHA.113.002055 [DOI] [PubMed] [Google Scholar]
- 105.Ciccone A, Valvassori L, Nichelatti M, et al. ; SYNTHESIS Expansion Investigators . Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(10):904-913. doi: 10.1056/NEJMoa1213701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dennis M, Sandercock P, Reid J, Graham C, Forbes J, Murray G; CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration . Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8 [DOI] [PubMed] [Google Scholar]
- 107.Ginsberg MD, Palesch YY, Hill MD, et al. ; ALIAS and Neurological Emergencies Treatment Trials (NETT) Investigators . High-dose albumin treatment for acute ischaemic stroke (ALIAS) part 2: a randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013;12(11):1049-1058. doi: 10.1016/S1474-4422(13)70223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kidwell CS, Jahan R, Gornbein J, et al. ; MR RESCUE Investigators . A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368(10):914-923. doi: 10.1056/NEJMoa1212793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lees KR, Bornstein N, Diener HC, Gorelick PB, Rosenberg G, Shuaib A; MACSI Investigators . Results of membrane-activated chelator stroke intervention randomized trial of DP-b99 in acute ischemic stroke. Stroke. 2013;44(3):580-584. doi: 10.1161/STROKEAHA.111.000013 [DOI] [PubMed] [Google Scholar]
- 110.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM; STICH II Investigators . Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397-408. doi: 10.1016/S0140-6736(13)60986-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pancioli AM, Adeoye O, Schmit PA, et al. ; CLEAR-ER Investigators . Combined approach to lysis utilizing eptifibatide and recombinant tissue plasminogen activator in acute ischemic stroke-enhanced regimen stroke trial. Stroke. 2013;44(9):2381-2387. doi: 10.1161/STROKEAHA.113.001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pandian JD, Kaur P, Arora R, et al. Shoulder taping reduces injury and pain in stroke patients: randomized controlled trial. Neurology. 2013;80(6):528-532. doi: 10.1212/WNL.0b013e318281550e [DOI] [PubMed] [Google Scholar]
- 113.Ringelstein EB, Thijs V, Norrving B, et al. ; AXIS 2 Investigators . Granulocyte colony-stimulating factor in patients with acute ischemic stroke: results of the AX200 for Ischemic Stroke trial. Stroke. 2013;44(10):2681-2687. doi: 10.1161/STROKEAHA.113.001531 [DOI] [PubMed] [Google Scholar]
- 114.Al-Tamimi YZ, Bhargava D, Feltbower RG, et al. Lumbar drainage of cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: a prospective, randomized, controlled trial (LUMAS). Stroke. 2012;43(3):677-682. doi: 10.1161/STROKEAHA.111.625731 [DOI] [PubMed] [Google Scholar]
- 115.Dávalos A, Alvarez-Sabín J, Castillo J, et al. ; International Citicoline Trial on acUte Stroke (ICTUS) trial investigators . Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet. 2012;380(9839):349-357. doi: 10.1016/S0140-6736(12)60813-7 [DOI] [PubMed] [Google Scholar]
- 116.Macdonald RL, Higashida RT, Keller E, et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke. 2012;43(6):1463-1469. doi: 10.1161/STROKEAHA.111.648980 [DOI] [PubMed] [Google Scholar]
- 117.Dorhout Mees SM, Algra A, Vandertop WP, et al. ; MASH-2 Study Group . Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. Lancet. 2012;380(9836):44-49. Correction published in Lancet. 2012;380(9858)1994. doi: 10.1016/S0140-6736(12)60724-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nogueira RG, Lutsep HL, Gupta R, et al. ; TREVO 2 Trialists . Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380(9849):1231-1240. doi: 10.1016/S0140-6736(12)61299-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosso C, Corvol JC, Pires C, et al. Intensive versus subcutaneous insulin in patients with hyperacute stroke: results from the randomized INSULINFARCT trial. Stroke. 2012;43(9):2343-2349. doi: 10.1161/STROKEAHA.112.657122 [DOI] [PubMed] [Google Scholar]
- 120.Sandercock P, Wardlaw JM, Lindley RI, et al. ; IST-3 collaborative group . The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379(9834):2352-2363. doi: 10.1016/S0140-6736(12)60768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saver JL, Jahan R, Levy EI, et al. ; SWIFT Trialists . Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241-1249. doi: 10.1016/S0140-6736(12)61384-1 [DOI] [PubMed] [Google Scholar]
- 122.Sundseth A, Thommessen B, Rønning OM. Outcome after mobilization within 24 hours of acute stroke: a randomized controlled trial. Stroke. 2012;43(9):2389-2394. doi: 10.1161/STROKEAHA.111.646687 [DOI] [PubMed] [Google Scholar]
- 123.Zinkstok SM, Roos YB; ARTIS investigators . Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380(9843):731-737. doi: 10.1016/S0140-6736(12)60949-0 [DOI] [PubMed] [Google Scholar]
- 124.Chollet F, Tardy J, Albucher JF, et al. Fluoxetine for Motor Recovery After Acute Ischaemic Stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10(2):123-130. doi: 10.1016/S1474-4422(10)70314-8 [DOI] [PubMed] [Google Scholar]
- 125.Kwon SU, Hong KS, Kang DW, et al. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke. 2011;42(10):2883-2890. doi: 10.1161/STROKEAHA.110.609370 [DOI] [PubMed] [Google Scholar]
- 126.Macdonald RL, Higashida RT, Keller E, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011;10(7):618-625. doi: 10.1016/S1474-4422(11)70108-9 [DOI] [PubMed] [Google Scholar]
- 127.Sandset EC, Bath PM, Boysen G, et al. ; SCAST Study Group . The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377(9767):741-750. doi: 10.1016/S0140-6736(11)60104-9 [DOI] [PubMed] [Google Scholar]
- 128.Shuaib A, Bornstein NM, Diener HC, et al. ; SENTIS Trial Investigators . Partial aortic occlusion for cerebral perfusion augmentation: safety and efficacy of NeuroFlo in Acute Ischemic Stroke trial. Stroke. 2011;42(6):1680-1690. doi: 10.1161/STROKEAHA.110.609933 [DOI] [PubMed] [Google Scholar]
- 129.Siebler M, Hennerici MG, Schneider D, et al. Safety of tirofiban in acute ischemic stroke: the SaTIS trial. Stroke. 2011;42(9):2388-2392. doi: 10.1161/STROKEAHA.110.599662 [DOI] [PubMed] [Google Scholar]
- 130.Dengler R, Diener HC, Schwartz A, et al. ; EARLY Investigators . Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9(2):159-166. doi: 10.1016/S1474-4422(09)70361-8 [DOI] [PubMed] [Google Scholar]
- 131.Robinson TG, Potter JF, Ford GA, et al. ; COSSACS Investigators . Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol. 2010;9(8):767-775. doi: 10.1016/S1474-4422(10)70163-0 [DOI] [PubMed] [Google Scholar]
- 132.Wong KS, Chen C, Fu J, et al. ; CLAIR study investigators . Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9(5):489-497. doi: 10.1016/S1474-4422(10)70060-0 [DOI] [PubMed] [Google Scholar]
- 133.Wong GK, Poon WS, Chan MT, et al. ; IMASH Investigators . Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke. 2010;41(5):921-926. doi: 10.1161/STROKEAHA.109.571125 [DOI] [PubMed] [Google Scholar]
- 134.Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082-1090. doi: 10.1161/STROKEAHA.108.540781 [DOI] [PubMed] [Google Scholar]
- 135.Foerch C, Czapowski D, Misselwitz B, Steinmetz H, Neumann-Haefelin T; Arbeitsgruppe Schlaganfall Hessen (ASH) . Gender imbalances induced by age limits in stroke trials. Neuroepidemiology. 2010;35(3):226-230. doi: 10.1159/000319457 [DOI] [PubMed] [Google Scholar]
- 136.Carcel C, Reeves M. Under-enrollment of women in stroke clinical trials: what are the causes and what should be done about it? Stroke. 2021;52(2):452-457. doi: 10.1161/STROKEAHA.120.033227 [DOI] [PubMed] [Google Scholar]
- 137.Phan HT, Reeves MJ, Blizzard CL, et al. Sex differences in severity of stroke in the INSTRUCT study: a meta-analysis of individual participant data. J Am Heart Assoc. 2019;8(1):e010235. doi: 10.1161/JAHA.118.010235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Messé SR, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? results from a national registry. Neurology. 2016;87(15):1565-1574. doi: 10.1212/WNL.0000000000003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weber R, Krogias C, Eyding J, et al. Age and sex differences in ischemic stroke treatment in a nationwide analysis of 1.11 million hospitalized cases. Stroke. 2019;50(12):3494-3502. doi: 10.1161/STROKEAHA.119.026723 [DOI] [PubMed] [Google Scholar]
- 140.Kasner SE, Del Giudice A, Rosenberg S, et al. Who will participate in acute stroke trials? Neurology. 2009;72(19):1682-1688. doi: 10.1212/WNL.0b013e3181a55fbe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Neill ZR, Deptuck HM, Quong L, et al. Who says “no” to participating in stroke clinical trials and why: an observational study from the Vancouver Stroke Program. Trials. 2019;20(1):313. doi: 10.1186/s13063-019-3434-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bernhardt J, Raffelt A, Churilov L, et al. ; AVERT Trialists’ Collaboration . Exploring threats to generalisability in a large international rehabilitation trial (AVERT). BMJ Open. 2015;5(8):e008378. doi: 10.1136/bmjopen-2015-008378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ding EL, Powe NR, Manson JE, Sherber NS, Braunstein JB. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med. 2007;167(9):905-912. doi: 10.1001/archinte.167.9.905 [DOI] [PubMed] [Google Scholar]
- 144.Kapral MK, Devon J, Winter AL, Wang J, Peters A, Bondy SJ. Gender differences in stroke care decision-making. Med Care. 2006;44(1):70-80. doi: 10.1097/01.mlr.0000188911.83349.a8 [DOI] [PubMed] [Google Scholar]
- 145.Hallström B, Jönsson AC, Nerbrand C, Petersen B, Norrving B, Lindgren A. Lund Stroke Register: hospitalization pattern and yield of different screening methods for first-ever stroke. Acta Neurol Scand. 2007;115(1):49-54. doi: 10.1111/j.1600-0404.2006.00738.x [DOI] [PubMed] [Google Scholar]
- 146.Rothwell PM, Coull AJ, Silver LE, et al. ; Oxford Vascular Study . Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366(9499):1773-1783.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16298214&dopt=Abstract doi: 10.1016/S0140-6736(05)67702-1 [DOI] [PubMed] [Google Scholar]
- 147.Elm JJ, Palesch Y, Easton JD, et al. Screen failure data in clinical trials: are screening logs worth it? Clin Trials. 2014;11(4):467-472. doi: 10.1177/1740774514538706 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Variable Definitions and Sensitivity Analyses
eFigure 1. Forest plot with the random effects, pooled enrolled enrollment disparity difference (EDD) for all trials (n=106) and for trial subgroups when subarachnoid hemorrhage trials were excluded.
eFigure 2. Temporal trend by publication date in the representation of women in randomized controlled trials of acute stroke with subarachnoid hemorrhage trials included (n=115).
eFigure 3. Temporal trend by publication date in the representation of women in randomized controlled trials of acute stroke with subarachnoid hemorrhage trials dropped (n=106).
eAppendix. Sensitivity Analyses.
eFigure 4. Sensitivity analysis using Global Burden of Disease data to account for the representation of women in the underlying stroke populations.
eFigure 5. Sensitivity analysis using published incidence data to account for the representation of women in the underlying stroke populations.
eFigure 6. Forest plot with the random effects, pooled participation to prevalence ratio (PPR) for all trials and for trial subgroups, estimated as part of a sensitivity analysis (n=115 trials).
eReferences.