Abstract
PBMT using 630 + 660 nm wavelengths transcutaneously at 7 cm above chest area irradiating lungs and heart regions of patients with acute, infectious respiratory syndrome alleviated their respiratory symptoms, mitigated pulmonary inflammation and hypoxia. PBMT could prevent more severe respiratory distress requiring emergency care and reduce the strain on healthcare. This case report's clinical experience can be the basis of future research evaluating oxygen saturation levels pre‐ and post‐PBMT.
Keywords: acute respiratory distress syndrome, coronavirus, dyspnoea, hypoxemia, oxygenation, photobiomodulation, red light, viral pandemic
PBMT using 630 + 660 nm wavelengths transcutaneously at 7 cm above chest area irradiating lungs and heart regions of patients with acute, infectious respiratory syndrome alleviated their respiratory symptoms, mitigated pulmonary inflammation and hypoxia. PBMT could prevent more severe respiratory distress requiring emergency care and reduce the strain on healthcare. This case report's clinical experience can be the basis of future research evaluating oxygen saturation levels pre‐ and post‐PBMT.

1. INTRODUCTION
A worldwide outbreak of severe acute respiratory syndrome emerged in 2019 and developed in a pandemic of COVID‐19. This respiratory βeta‐coronavirus is characterized by the severity of its complications, that is acute pulmonary inflammation, pneumonia, lung fibrosis and organ dysfunction, ultimately inducing fatality. Scientists worldwide have focused on finding medication and vaccine for fighting the viral disease, yet its high transmission and mutagenicity renders international efforts challenging. Drugs may also not be the only approach. Previous animal studies on pulmonary infectious diseases and a recent case report of a COVID‐19 patient with comorbidities demonstrated the mitigation of inflammatory processes addressed with Infrared Photobiomodulation therapy (IR‐PBMT). Here, we report the use of PBMT at different wavelengths and treatment parameters on two patients with acute infectious respiratory syndrome, who may represent early COVID‐19 patients in the United Kingdom and France. This case report's dissemination to the broader scientific community is of interest for PBMT was successfully applied according to clinical presentation, double‐blind to COVID‐19 diagnosis. Two females, mean age 60, manifested signs of acute respiratory infection at the end of 2019. According to the CARE checklist, we retrospectively collected data from patients and screening laboratories to ascertain epidemiological and symptomatologic aspects and reported clinical, laboratory, radiological, scanner and immunology findings. Both patients experienced at the onset of illness fever, burning feeling in the throat, throbbing cough, myalgia, severe fatigue, headache, loss of taste, dyspnoea and pneumonia signs. Symptoms aggravated into chest tightness and respiratory difficulties. One patient experienced diarrhoea and cutaneous manifestations. Patients received PBMT with non‐coherent red light (RL) 630 nm and 660 nm, significantly improving oxygenation, respiratory function and inflammatory syndrome, avoiding emergency care. This case report demonstrates the potential clinical value of PBMT in mitigating inflammation, improving respiratory distress and oxygenation at early stages of COVID‐19 and in other infectious lung diseases, preventing viral infections complications. It also expands our knowledge on RL and its positive effects on living organisms, oxyhaemoglobin, interfacial waters, cellular respiration and immunological processes, paving the way for further research.
The pneumonia outbreak in China at the end of 2019, spreading rapidly outside mainland, was defined by the World Health Organization as a severe acute respiratory infectious disease or SARS‐CoV‐2, officially declared a public health emergency on 30 January, 2020, a pandemic on 11 March, 2020. 1 It is a non‐segmented β‐coronavirus RNA‐enveloped in a fatty membrane, with two prevalent genotypes, L(∼70%) and S(∼30%). 2 Clinical features are characterized by the viral migration from upper to lower respiratory pathways, specifically to lung alveoli. The angiotensin‐converting enzyme 2 (ACE2) is acting as the cellular binding site, and functional receptor for SARS‐CoV‐2 and the transmembrane protease serine2 (TMPRSS2) serving the virus entering cells via endosomes, resulting in COVID‐19 3 Initial viral infection is by direct transmission person‐to‐person, via respiratory droplets, aerosols and surfaces. 4 The disease's demographics demonstrated gender equality in infection, 0.5‐3% mortality rate with prevalence in men, significantly increasing over 60, and in individuals with comorbidities. 5 Health complications include severe forms of potentially fatal pneumonia, respiratory failure requiring mechanical ventilation and admission to intensive care units, following an exacerbated immune response and cytokine storm. 6 Although most individuals remain asymptomatic, mean incubation time was defined between 2 and 14 days, making viral transmission still possible. 7
At the first signs of respiratory syndrome, the two cases of this report were addressed regarding their clinical presentation, not yet identified as SARS‐CoV‐2. The condition was unknown towards the end of 2019. PBMT was available at patients' location and brought a significant therapeutical action at different levels. Although treatment algorithms of viral infectious disease do not usually include PBMT, at least not as a first‐line intervention, the observations of this case report have a clinically significant value supporting the use of PBMT in the treatment of early stages of COVID‐19 disease. PBMT cannot prevent viral infection but has a viral defence role as a potent immunity and pulmonary inflammation modulator, improving lung function, mitigating potential fibrosis, lung injury and restoring homeostasis. Infrared (IR)‐PBMT on COVID‐19 was investigated in recent studies using pulsed low‐level laser 808 nm and 905 nm. 8 , 9 Here, we report the use of non‐coherent light‐emitting diodes (LED) 630 nm and 660 nm simultaneously with +15 nm spectral shift (Table 1).
TABLE 1.
Treatment delivery parameters. Treatment parameters of PBMT in Case 1 and Case 2 using two wavelengths of red‐light (RL) 630 nm and 660 nm simultaneously. In Case 2, blue‐light (BL)‐PBMT was additionally applied on psoriasis patch in the back. A Light‐Emitting‐Diodes (LED) device was used for treatment delivery in both patients (Triwings LLS®) emitting 8 wavelengths in the same treatment including Infrared (IR) with stable, measurable output power along the session. We can see that an important area of 1000 cm2 at 7 cm distance from skin was treated at each session resulting in a high Blood Cumulated absorbing volume (V) assisting patients’ oxygenation. In the two columns far right treatment delivery parameters of noncoherent LED are put into perspective with low‐level‐laser parameters successfully used on a COVID‐19 patient 9
| Light‐emitting diodes | Low‐level laser | ||||
|---|---|---|---|---|---|
| Case 1 | Case 2 | ||||
| Wavelength | 630 nm + 660 nm | 630 nm + 660 nm | 465 nm | 808 nm | 905 nm |
| Spectral shift nm | ±15 nm | ±15 nm | ±15 nm | ±2 nm | ±2 nm |
| Emitting angle ° | 45 | 45 | 45 | n/a | n/a |
| Distance from the skin | 7 cm | 7 cm | 7 cm | 20 cm | 20 cm |
| Emission mode | CW | CW | CW | Pulsed 1500 Hz | Pulsed 1500 Hz |
| Pulse duration | n/a | n/a | n/a | 330 µS | 100 nS |
| Peak power | n/a | n/a | n/a | 3 W | 75 W × 3 |
| Average power | – | – | – | 1.5 W | 34 mW |
| Treated area at the same time |
900 cm2 at led contact 1000 cm2 at 7 cm |
900 cm2 at led contact 1000 cm2 at 7 cm |
900 cm2 at led contact 1000 cm2 at 7 cm |
2 × 250 cm2 500 cm2 at 20 cm |
|
| Power density mW/cm2 at LED contact | 80 mW | 80 mW | 80 mW | – | – |
| Power density mW/cm2 at 7 cm skin distance | 55 mW | 55 mW | 55 mW | – | – |
| Power density mW/cm2 at 20 cm skin distance | – | – | – | 1.5 W/20 cm2 = 75 mW/cm2 | 34 mW/20 cm2 = 1.7 mW/cm2 |
| Time per session | 15 min = 900 sec | 15 min = 900 sec | 15 min = 900 sec | 2 × 14 min = 28 min | |
| Total fluence J/cm2 per session of 15 min | 0.055 × 900 = 50 J/cm2 | 0.055 × 900 = 50 J/cm2 | 0.055 × 900 = 50 J/cm2 | – | – |
| Total fluence J/cm2 per session of 2 × 14 min | – | – | – | 7.2 J/cm2 | 114 mJ |
| Total energy delivered per session | 50 kJ on 1000 cm2 | 50 kJ on 1000 cm2 | 50 kJ on 1000 cm2 | 3.6 kJ on 500 cm2 | |
| Number of sessions per week | 3 | 2 | 2 | 4 per week | |
| Total number of sessions | 12 for resolution then 1/15 d for 9 months | 6 for resolution then 1/15 d for 6 months | 6 | 4 | |
| Mode of delivery of energy | 3 LED panels | 3 LED panels | 3 LED panels | Scanner | |
| V = Blood Cumulated absorbing volume on 1000 cm2 with total skin surface = 2 m2 per session, Blood flow 5 L/min | V = 1000 cm2/20 000 cm2 = 1/20 × 5 L/min × 15 min = V = 3.8 L | V = 1000 cm2/20 000 cm2 = 1/20 × 5 L/min × 15 min = V = 3.8 L | V = 1000 cm2/20 000 cm2 = 1/20 × 5 L/min × 15 min = V = 3.8 L | V = 500 cm2/20 000 cm2 = 1/40 × 5 L/min = V = 1.9 L | |
| HbO2 absorbance | Medium | Medium | Very strong | Medium | |
| Cytochrome oxidase a3 absorption | Strong | Strong | Medium | Low | |
2. CASE 1
Primary symptoms of possibly the first patient in France, a female aged 69, started on 29 November 2019, with body temperature reaching 40° for 4 days, headaches and intense burning sensation in the throat, nausea and general inebriation under no obvious factors. The patient had no notorious psycho‐social history. In 3 days, a rhinopharyngitis developed treated with aspirin and paracetamol. Symptoms disappeared for 1 week then deteriorated from 6 December 2019, with the resurgence of rhinopharyngitis, fever and dry, uncontrollable cough. Spaced coughing fits at the beginning became persistent in the following week. On 10 December biological findings showed neutrophils 74.5%, SR 17 mm/h, ferritin 67 µg/L CRP 6.3 mg/L. Two weeks later neutrophils were 81%, SR 47 mm/h and CRP 55.9 mg/L. A chest X‐ray performed on 26 December did not reveal a systemic evolution, neither pleural effusion to explain the symptomatology, except for a lateral‐vertebral nodule formation of 6 mm right‐hand side at face view.
The cardiomediastinal silhouette was regular. A pulmonologist first diagnosed asthma and recommended a chest scanner. The patient had no family history of lung disease. From 25 December, cough is not receding, body temperature rises intermittently at 40° and again appears an overall and disturbing inebriation state. The patient sleeps in a semi‐sitting position at this stage, is concerned about not speaking without coughing, describing significant breath shortness and difficulties in walking. A new chest X‐ray is performed, and a chest scanner is scheduled. On 30 December, 2019, the chest scanner concluded into bilateral bronchial syndrome without systemic condensates, posterior respiratory disorder demonstrating bilateral declive with right‐side prevalence. At the time of screenings, the scientific community did not know the pulmonary signs of COVID‐19 infectious respiratory disease. In late February, when radiologists were better informed on the novel coronavirus manifestations, a second reading of the chest scanner revealed pulmonary involvement with interstitial abnormalities at lung basis identified as clinical signs of early fibrosis, without bronchiectasis signs, compatible to bilateral peripheral superficial abnormalities seen in COVID‐19, with a production of ground‐glass opacities predominantly distributed in peripheral septa. Detection was better at ultrasound than X‐rays screening that has a low sensitivity for these opacities.
The patient recovered from 6 January, 2020 but experienced major asthenia until the end of February 2020, with weight and sense of taste loss. Respiratory functional exploration was conducted on 15 February, 2020, demonstrating neat improvement of vital pulmonary function. A thorax‐abdomen‐pelvic control scanner was performed on 4 June, 2020, follow‐up suspicion of lung fibrosis formation and recurrent abdominal right‐sided pain. No mediastinal, hilar or axillary adenopathy was found at thoracic level, neither pleural nor pericardial effusion nor pulmonary embolism. The parenchymatous integrity was preserved, and no suspicious macronodule was detected. Compared to the thorax scanner's findings from 30 December, 2019, the ground‐glass images and the thickening of bronchial walls had receded, only some discrete declive ventilation disorders remaining. At abdominal‐pelvic level, liver (except for an infra‐centimetric cyst), sub‐hepatic veins, portal vein trunk, gallbladder, bile and pancreatic ducts, adrenal glands, kidneys (except for an infra‐centimetric cyst), spleen and bladder showed integrity. The screening found minimal calcified plaques of the bladder but no adenopathy nor fluid or air effusion. The only noticeable abnormality was a thickening of the ascending colon walls and angle, without peridigestive fatty tissue inflammation but a retro‐colic liquid sign in a slightly diverticular region, without abscess nor palpable mass. No suspicious lytic or condescending lesion at bony structure was seen, concluding previous thoracic findings' regression. Right colitis of undetermined aetiology remained. The patient had COVID‐19 antibodies serology performed on 20 May, 2020, 5 months after the first symptoms, which showed negative IgG and suspicious interpretation of IgM, both close to the detection limit. Second serologic testing was performed on 9 June, 2020, also negative, despite the clinical conclusions. A fibro‐colonoscopy was scheduled at the end of June 2020 and did not detect any anomaly. All medical images and laboratory tests are available.
2.1. Treatment
The patient received pharmacological intervention with amoxicillin 1000 mg, 3 times daily for 10 days, budesonide 0.5 mg nebulizer suspension, sinus‐nasal irrigation (Respimer), and RL‐PBMT 630 nm and 660 nm transcutaneously, 3 sessions of 15 mn weekly, irradiance 55 mW/cm2, fluence 50 J/cm2 on the presternal region 7 cm above the skin, with a LED device emitting the two wavelengths simultaneously (TriWings LLS® Biophoton). PBMT treatment parameters are detailed in Table 1. In the evidence of low or null efficacy of amoxicillin therapy, the intervention was changed to ceftriaxone (Rocephin) 1 g/3.5 mL, 1 intramuscular injection daily for 10 days. The antibiotherapy being efficient only on fever while cough remained tenacious, it was whanged for azithromycin, 2 tablets of 250 mg on day 1 then, 250 mg daily for 4 days. The patient also received pneumococcal vaccination (Pneumovax) a month later. Patient's assessment described PBMT as the only intervention to significantly and immediately relieve chest tightness and breath shortness. Therefore, PBMT was continued in February 2020, weekly at the same parameters.
3. CASE 2
The second patient, a female aged 53, attended in mid‐December 2019 an international congress gathering having exhibitors and attendees from China. On 24 December, 2019, 2 weeks after returning to the United Kingdom, symptoms onset started with intense sore throat and headache evolving in 2 days into a severe dry cough, extreme fatigue and body temperature at 40° for 4 days. The patient's primary complaint was the intensity of chest tightness and breathlessness unexperienced before. She was also concerned by myalgia, asthenia and for having difficulties in walking, eating and speaking. The patient medical history did not reveal any salient family or occupational event or predisposal to allergies that could explain her respiratory signs. The patient did not take any regular medication. From 30 December, 2019, symptoms evolved into a paroxysmal cough, increasing in frequency and severity with more oppressive chest tightness, lung weakness, and trouble breathing, yet no rhinitis signs. An episode of sharp abdominal pain and diarrhoea lasted 3 days. The patient reported mainly being concerned by her breath shortness, describing congestion in the cardiac area. The patient also reported the appearance of two psoriasis patches on the right‐hand side of her back, as described in a study on COVID‐19 cutaneous manifestations. 10 Although the patient experienced symptoms compatible with SARS‐CoV‐2 infection, specifically concerning fever, cough, chest tightness and shortness of breath, in a serologic screening conducted on 12 June, 2020, 6 months later, IgG was negative, IgM inconclusive. The patient gradually improved from 7 January, 2020, yet reported asthenia, muscle fatigue and intermittent abdominal pain throughout the year. Laboratory immunology tests are available.
3.1. Treatment
No pharmacological intervention neither antibiotherapy was administered. The patient spontaneously quarantined for 2 weeks receiving at home 2‐3 times per week RL‐PBMT. The patient described the intervention's outcome, immediately and significantly improved the dyspnoea symptoms, shortness of breath, chest oppression, pain and lack of oxygen, with a delayed effect the following days. PBMT was continued at same settings twice weekly for 2 weeks until the beginning of remission from 7 January, 2020. Psoriasis patches were treated with PBMT too. Treatment parameters and delivery are reported in Table 1.
4. UPDATE
Both patients' evolution reports persistent overall fatigue lasting 1‐year post‐disease, with remaining neurologic signs and digestive symptoms like throbbing pain in the abdomen. However, laboratory findings returned with negative serologic antibodies. This clinical experience opens the question of whether antibodies do not decay gradually in certain patients to the undetectable range and whether they protect from reinfection and long‐term immunity with non‐short‐lived neutralizing antibodies. 11 Serologic testing might be optimized if stratified according to disease severity and distance from the disease at the time of testing.
This may also have important implications in vaccine design and in the role of cell‐innate and cell‐adaptive immunity when more evidence is building for a cell‐mediated rather than antibody‐mediated immunity. 12
Case 2 persistent abdominal pain was investigated with ultrasound 3.5 MHz and Doppler recording of the supramesocolic floor on 9 January, 2021. The screening revealed a hypertrophic, slightly dysmorphic liver with thickening of the caudate lobe at 23 mm, while parenchymal echotexture did not present any suspicious focal mass, neither was seen any anomaly of the biliary ducts or gall bladder. Pancreatic, renal and splenic echotexture and the permeability of vascular axes were normal. Both patients reporting repetitive abdominal pain, with no previous medical history, the possibility that a particular hepatic sequel of COVID‐19 disease occurs, with micro‐embolisms and multi‐system inflammation or multiple organ dysfunction, is to be considered. Recent research supported by the Russian Federation described SARS‐CoV‐2 as an autoimmune virus, triggering potentially pathogenic autoantibodies. 13
5. DISCUSSION
Although all mechanisms of action are not yet understood, photobiological responses in PBMT result from photon energy absorption by a photoacceptor, converting light into signals acting as biological stimuli (Figure 1). Mitochondrial cytochrome‐c‐oxidase (CCO), intracellular waters, calcium ion channels and, more recently, epidermal opsins are identified as primary chromophores to light. By influencing key cellular pathways, they upregulate adenosine triphosphate (ATP) and nitric oxide production, downregulate reactive oxygen species (ROS) and activate light‐sensitive ion channels increasing intracellular calcium concentrations. Recent findings have demonstrated that the activity of PBMT, separate from the effects of anti‐inflammatory drugs, lies in the “mitigation” of the critical components in inflammatory processes and in the modulation of the individual's immune response by restoring normal immuno‐dynamics. 14 , 15 The current coronavirus infection provokes the immune system's dysregulation, resulting in the overproduction and release by macrophages of proinflammatory cytokines, chemokines and ferritin. In this early and acute inflammatory response, the massive infiltration of lungs by macrophages is described as a “cytokine storm”. 6 Studies have suggested that the interstitial macrophages, residents of the lung parenchyma, are involved in the pathophysiology of SARS‐CoV‐2 and possibly responsible for the COVID‐19‐associated acute respiratory distress syndrome (ARDS). 16
FIGURE 1.
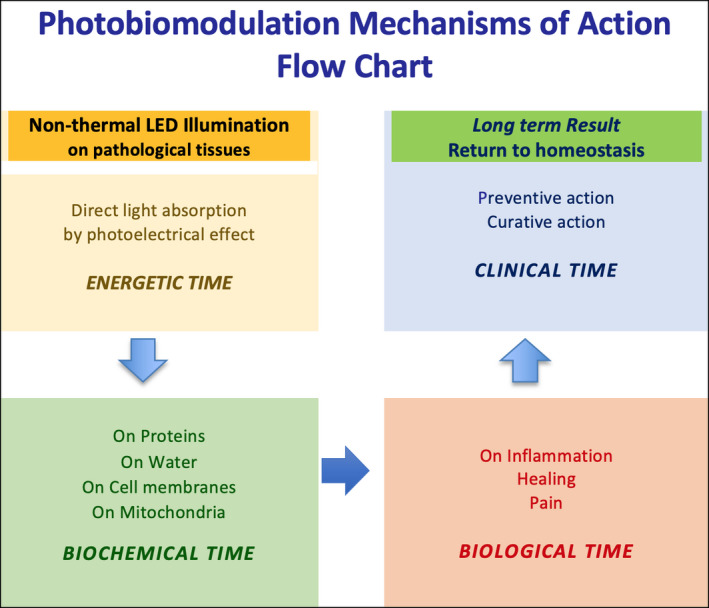
Schematic representation of PBMT mechanisms of action
The use of phototherapy in infectious lung diseases was first recorded in sanatoriums, where patients were daily exposed to sunlight and open air. 17 , 18 Several studies have evaluated the efficacy of PBMT on ARDS in animal models, demonstrating a significant decrease in the neutrophilic influx and TNFα levels in bronchoalveolar lavage fluid involved in ARDS pathogenesis. 19 The downregulation of proinflammatory cytokines and the decrease in collagen deposits and pro‐fibrotic secretions in lungs were demonstrated in mice receiving PBMT (660 ± 20 nm, irradiance 33.3 mW/cm2, fluence 5 J/cm2). 20 Airflow inflammation in chronic obstructive pulmonary disease (COPD) and pulmonary oedema were also reduced in murine models with a diode laser (660 ± 20 nm, irradiance 30 mW, fluence 3 J/cm2). 21 Several murine experiments demonstrated the anti‐inflammatory and antimicrobial activity of visible light on bacteria and secondary bacterial reinfection and strong viricidal action on certain enveloped and non‐enveloped viruses. 22 , 23 The anti‐inflammatory and anti‐fibrotic activity of RL‐NIR PBMT is also evidenced in acute lung injury provoked by sepsis. 14 , 24
PBMT has also demonstrated a biphasic dose‐response described through the Arndt Shultz curve: low power of light having stimulative and high power inhibitory effects resulting in ROS decrease activating NF‐kB transcription factors according to cells' redox state. 15 , 25 , 26 In a recent case report, IR‐PBMT at peak powers 808 nm and 905 nm has enabled the cytokine storm's management in a COVID‐19 patient. 12 In the pathophysiology of COVID‐19, a non‐invasive therapeutic modality able to decrease the IL‐1β, IL‐6, IL‐8, TNFα and MCP‐1 proinflammatory cytokines would reduce the fatality rate of the disease. 27 , 28 The complications of an overreacting immune system, particularly in populations at risk with underlying comorbidities, may also lead to critical complications, as vascular hyperpermeability and multi‐organ injuries, abnormal coagulation parameters and micro‐thrombosis having been described in hospitalized patients. 29
PBMT is here described using two wavelengths, 630 nm and 660 nm, from a dual RL emitter (Triwings LS®, Biophoton) with a spectral shift of +15 nm, both wavelengths remaining in the visible spectrum, yet the second approaching the near‐infrared (NIR) range. Although RL is primarily known for its wound‐healing activity, this clinical experience reports RL action on mitigating the excess of inflammation rather than suppressing it and opens our reflection on fundaments of cellular respiration where RL has a positive action on patients' oxygenation. Effectively, RL is absorbed by oxyhaemoglobin (HbO2), whereas IR wavelengths 800 nm and above, with a deeper penetration influencing cytokines, are not strongly absorbed by HbO2. 12 Clinical observation may evidence the changes that occur upon oxygenation and deoxygenation of blood even at a macroscopic level, the oxygen‐rich blood being red‐coloured by iron binding to oxygen. Previous studies have evidenced the effects of RL absorption, by HbO2 identifying four major actions: the augmentation of oxygen transportation, the normalization of iron and oxygen homeostasis, the improvement of erythrocyte deformability reducing blood viscosity, the repolarization and repair of erythrocyte membrane from free radicals' damages, reducing the risk of micro‐embolisms. The clinical experience here reported applied RL‐PBMT 7 cm above patients' sternum, irradiating lungs, heart and chest blood vessels, the importance of skin vasculature favouring RL HbO2 absorption. Additionally, mitochondrial CCO absorption of RL induces a significant cells' re‐energization and ATP augmentation, which simultaneously with erythrocytes' membrane repolarization improves oxygenation and micro‐embolisms dislocation via electrostatic repulsion. 30 , 31 , 32 , 33
Considering the amount and velocity of circulating blood in an adult at rest, determined by size, weight and the total cross section of the studied area, the cardiac output (heart rate × stroke volume) has a normal range value between 4 to 6 L/min. PBMT at the sternum area would treat in 1 minute almost the whole blood volume passing through the heart (Table 1). A neat increase of 1.6 times in local tissue oxygen tension was previously described with HeNe laser (633 nm, 1 mW, 10 mn irradiation), without production of singlet oxygen in the process of molecular oxygen photodissociation from haemoglobin. 34 A parallel can be drawn with the findings of a study that evaluated in vitro the effects of RL 635 nm on cellular function in hypoxia and reoxygenation conditions, concluding enhanced mitochondrial activity when ATP levels were challenged: weaker initial ATP inducing higher stimulative effects. 35 , 36 Recent investigations from the French National Institute for Medical Research (INSERM) have identified at electron microscopy 3.7 million cell‐free functional mitochondria per ml of plasma in human blood acting as cellular respiration organelles and potential diagnostic biomarkers. 37 An earlier study discussed the correspondence between platelet mitochondrial respiration and impaired ATP levels in depressed individuals, describing erythrocytes illumination with RL as a possible systemic therapy in depression. 36 , 38 RL‐PBMT has a significant impact on the current pandemic; by treating blood circulating in all organs, boosting erythrocytes, enhancing ATP and oxygen saturation levels, it may avoid the requirement for mechanical ventilation. RL‐PBMT may also potentialize the effects of pharmacological treatments, reduce their use and improve mental health too. 39
Furthermore, the stronger absorption of R‐NIR light by water is also to be considered in the positive outcomes of this case report. Studies have demonstrated the influence of 633 nm and 670 nm at 4 mW on interfacial water layers (IWL), density and tension between bound water/free water as a fine‐tuning of volume expansion and viscosity reduction supporting ATP synthesis and cell proliferation. 40 , 41 , 42
When IWL viscosity is increased at ROS production, it affects the speed of mitochondrial rotary motors. RL‐NIR PBMT demonstrated biostimulative effects at this level, improving stressed cells' metabolism by reducing IWL viscosity. A concept of photonic water emerges from this report, which would be of interest in further research, as well as the relationship between bound water and tissue stiffness. A higher tissue stiffness and permeability have been associated with severe forms of COVID‐19, the adhesion of viral particles being increased on stiffer cell surfaces, similar to platelets on hardened arterial wall surfaces. 43 We may also consider the role of RL‐PBMT in improving water transport and catabolic evacuation by releasing bound water, facilitating alveolar fluid clearance in pulmonary oedema, hence supporting self‐repair mechanisms and faster return to homeostasis. The role of aquaporins (AQPs) water channels across cell membranes is also important, particularly in lungs, with their isoforms AQP1, AQP3, AQP4 AQP5. 44 All these aspects have a clear clinical value in COVID‐19.
The simultaneous use of two RL might have played a substantial role in mitigating the inflammatory processes, preventing lung fibrosis, improving lymphocytic balance through an immuno‐modulative action, described in previous studies. 45 , 46 , 47 Beyond the anti‐inflammatory action, this case report's strength is that it provides a significant therapeutical key for exploring RL‐PBMT capacity to enhance oxygen fixation on erythrocytes to restore oxygen delivery at local and global levels. It also supports rationale for the understanding that fatality in the COVID‐19 is undoubtedly linked to an exacerbated immune response, deleterious inflammation and consequent organ damages rather than to a pathogen. Therefore, RL‐PBMT by influencing IWL may assist fluid clearance and possibly pulmonary surfactant homeostasis, where the regulation of secretion and degradation processes is essential before patients cross the line of respiratory distress, especially when refractory to invasive oxygen supplementation.
Limitations of this case report are the absence of early PCR tests in 2019. However, clinical signs were conclusive to COVID‐19 once diagnostic knowledge was acquired during the pandemic. 48
6. CONCLUSION
Photobiomodulation therapy cannot prevent viral infection, but it demonstrates strong clinical evidence in supporting the management of infective respiratory diseases in patients of all ages, improving oxygenation and modulating their inflammatory response, both responsible for the aggravation of their condition. From these clinical experiences, RL‐PBMT was beneficial overall, no complication did occur during the support protocol, and we recommend the use of RL‐PBMT at the early stages of respiratory and inflammatory symptoms. Given the pandemic's magnitude and the increase in critical complications with age, 49 RL‐PBMT is a promising, cost‐effective, in‐clinic or home care modality potentially reducing life‐threatening aggravations and alleviating the burden on public health. We may also consider PBM as a prophylactic therapy supporting the native immune system to fight viruses and bacteria and produce antibodies.
Future clinical trials may investigate further the absorption of RL by HbO2 and cells' membranes and their role in securing oxygen transportation. Pulse oximetry measurements pre‐ and post‐PBMT could test the hypothesis that the alleviation of respiratory symptoms and breathing comfort patients experience do correspond to the saturation of their oxygen levels. This would have relevant implications in clinical practice to treat pathologies related to impaired micro‐circulation and oxygen supply. Beyond the impact of PBMT on biological processes, the informational light it delivers acts on the field and not the mere pathology, which also has a clinical significance for neurologic sequelae and fatigue post‐COVID‐19.
7. PATIENT'S PERSPECTIVE
Case 1 was alerted by unrestrainable cough, breathlessness, devastating fatigue, loss of appetite and taste sense. She feared the chest tightness and reported her thorax freed from oppression after PBMT. The second patient, particularly isolated, worried about fatal shortness of breath or heart disorder, whereas she never suffered from respiratory illness before. She reported a feeling of airway oedema and chest tightness clearing with PBMT as if catching back her breath and recovering some elasticity and dynamics in the lungs. Both patients continued RL‐PBMT on the sternum 9 months post‐disease, describing the intervention to support their recovery from long‐term fatigue.
CONFLICT OF INTEREST
None declared.
Pelletier‐Aouizerate M, Zivic Y. Early cases of acute infectious respiratory syndrome treated with photobiomodulation, diagnosis and intervention: Two case reports. Clin Case Rep. 2021;9:2429–2437. 10.1002/ccr3.4058
REFERENCES
- 1. World Health organisation (WHO) . Coronavirus disease (COVID‐19) outbreak, WHO website. https://www.who.int/health‐topics/coronavirus#tab=tab_1. Accepted 15 March 2020.
- 2. Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS‐CoV‐2. Natl Sci Rev. 2020;7(6):1012‐1023. 10.1093/nsr/nwaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271.e8‐280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS‐CoV‐2 as Compared with SARS‐CoV‐1. N Engl J Med. 2020;382(16):1564‐1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259‐260. 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID‐19. J Infect. 2020;80(6):607‐613. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577‐582. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mokmeli S, Vetrici M. Low level laser therapy as a modality to attenuate cytokine storm at multiple levels, enhance recovery, and reduce the use of ventilators in COVID‐19. Can J Respir Ther. 2020;23(56):25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sigman SA, Mokmeli S, Vetrici MA. Adjunct low level laser therapy (LLLT) in a morbidly obese patient with severe COVID‐19 pneumonia: a case report. Can J Respir Ther. 2020;56:52‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galván Casas C, Catala A, Hernandez GC, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26:1200‐1204. 10.1038/s41591-020-09656 [DOI] [PubMed] [Google Scholar]
- 12. Guihot A, Litvinova E, Autran B, Debré P, Vieillard V. Cell‐mediated immune responses to COVID‐19 infection. Front Immunol. 2020;11:p1662. 10.3389/fimmu.2020.01662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halpert G, Shoenfeld Y. SARS‐CoV‐2, the autoimmune virus. Autoimmun Rev. 2020;19(12):102695. 10.1016/j.autrev.2020.102695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamblin MR. Mechanisms and applications of the anti‐inflammatory effects of photobiomodulation. AIMS Biophysics. 2020;4(3):337‐361. 10.3934/biophy.2017.3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amaroli A, Ferrando S, Benedicenti S. Photobiomodulation affects key cellular pathways of all life‐forms: considerations on old and new laser light targets and the calcium issue. Photochem Photobiol. 2019;95:455‐459. 10.1111/php.13032 [DOI] [PubMed] [Google Scholar]
- 16. Lalueza A, Ayuso B, Arrieta E, et al. Elevation of serum ferritin levels for predicting a poor outcome in hospitalised patients with influenza infection. Clin Microbiol Infect. 2020;26(11):1557.e9‐1557.e15. 10.1016/j.cmi.2020.02.018 [DOI] [PubMed] [Google Scholar]
- 17. Grzybowski A, Pietrzak K. From patient to discoverer—Niels Ryberg Finsen (1860–1904)—the founder of phototherapy in dermatology. Clin Dermatol. 2013;30(4):451‐455. 10.1016/j.clindermatol.2011.11.019 [DOI] [PubMed] [Google Scholar]
- 18. Hobday RA. The open‐air factor and infection control. J Hosp Infect. 2019;103(1):e23‐e24. 10.1016/j.jhin.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Lima FM, Moreira LM, Villaverde AB, Albertini R, Castro‐Faria‐Neto HC, Aimbire F. Low‐level laser therapy (LLLT) acts as cAMP‐elevating agent in acute respiratory distress syndrome. Lasers Med Sci. 2011;26(3):389‐400. 10.1007/s10103-010-0874-x [DOI] [PubMed] [Google Scholar]
- 20. Brochetti RA, Leal MP, Rodrigues R, et al. Photobiomodulation therapy improves both inflammatory and fibrotic parameters in experimental model of lung fibrosis in mice. Lasers Med Sci. 2017;32:1825‐1834. 10.1007/s10103-017-2281-z [DOI] [PubMed] [Google Scholar]
- 21. da Cunha Moraes G, et al. Low‐level laser therapy reduces lung inflammation in an experimental model of chronic obstructive pulmonary disease involving P2X7 receptor. Oxid Med Cell Longev. 2018;2018:6798238. 10.1155/2018/6798238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richardson TB, Porter CD. Inactivation of murine leukemia virus by exposure to visible light. Virology. 2005;341:321‐329. [DOI] [PubMed] [Google Scholar]
- 23. Enwemeka CS, Bumah VV, Masson‐Meyers DS. Light as a potential treatment for pandemic coronavirus infections: a perspective. J Photochem Photobiol B. 2020;207:111891. 10.1016/j.jphotobiol.2020.111891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Costa SG, Barioni ÉD, Ignácio A, et al. Beneficial effects of red light‐emitting diode treatment in experimental model of acute lung injury induced by sepsis. Sci Rep. 2017;7(1):12670. 10.1038/s41598-017-13117-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho D, Kraeva E, Wun T, Isseroff RR, Jagdeo J. A single‐blind, dose escalation, phase I study of high‐fluence light‐emitting diode‐red light (LED‐RL) on human skin: study protocol for a randomised controlled trial. Trials. 2016;17:385. 10.1186/s13063-016-1518-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamblin MR. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol. 2018;94(2):199‐212. 10.1111/php.12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Brito AA, da Silveira EC, Rigonato‐Oliveira NC, et al. Low‐level laser therapy attenuates lung inflammation and airway remodeling in a murine model of idiopathic pulmonary fibrosis: relevance to cytokines secretion from lung structural cells. J Photochem Photobiol B. 2020;203:111731. 10.1016/j.jphotobiol.2019.111731 [DOI] [PubMed] [Google Scholar]
- 28. Nejatifard M, Asefi S, Jamali R, Hamblin MR, Fekrazad R. Probable positive effects of the photobiomodulation as an adjunctive treatment in COVID‐19: a systematic review. Cytokine. 2021;137:155312. 10.1016/j.cyto.2020.155312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Hemost. 2020;18(4):844‐847. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mi XQ, Chen JY, Liang ZJ, Zhou LW. In vitro effects of helium‐neon laser irradiation on human blood: blood viscosity and deformability of erythrocytes. Photomed Laser Surg. 2004;22(6):477‐482. 10.1089/pho.2004.22.477 [DOI] [PubMed] [Google Scholar]
- 31. Cui Y, Guo Z, Zhao Y, et al. Reactive effect of low intensity He‐Ne laser upon damaged ultrastructure of human erythrocyte membrane in Fenton system by atomic force microscopy. Acta Biochim Biophys Sin. 2017;39(7):484‐489. 10.1111/j.1745-7270.2007.00309.x [DOI] [PubMed] [Google Scholar]
- 32. Gisbrecht A, Mamilov S. Experimental study of the laser‐induced oxyhemoglobin photodissociation in cutaneous blood vessels. Acta Medica Bulgarica. 2015;42(2):42‐48. 10.1515/amb-2015-0017 [DOI] [Google Scholar]
- 33. Zhu R, Avsievich T, Bykov A, Popov A, Meglinski I. Influence of pulsed he‐ne laser irradiation on the red blood cell interaction studied by optical tweezers. Micromachines. 2019;10(12):853. 10.3390/mi10120853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asimov MM, Korolevich AN, Konstantinova EÉ. Kinetics of oxygenation of skin tissue exposed to low‐intensity laser radiation. J Appl Spectrosc. 2007;74:133‐139. 10.1007/s10812-007-0020-0 [DOI] [Google Scholar]
- 35. Chaudary S, Karner L, Weidinger A, et al. In vitro effects of 635 nm photobiomodulation under hypoxia/reoxygenation culture conditions. J Photochem Photobiol B. 2020;209:111935. [DOI] [PubMed] [Google Scholar]
- 36. Sommer AP, Trelles MA. Light pumping energy into blood mitochondria: a new trend against depression? Photomed Laser Surg. 2014;32(2):59‐60. 10.1089/pho.2014.9866 [DOI] [PubMed] [Google Scholar]
- 37. Al Amir Dache Z, Otandault A, Tanos R, et al. Blood contains circulating cell free respiratory competent mitochondria. FASEB J. 2020;34:3616‐3630. 10.1096/fj.201901917RR [DOI] [PubMed] [Google Scholar]
- 38. Hroudová J, Fišar Z, Kitzlerová E, Zvěřová M, Raboch J. Mitochondrial respiration in blood platelets of depressive patients. Mitochondrion. 2013;13(6):795‐800. 10.1016/j.mito.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 39. Glazewski JB. Low energy laser therapy as quantum medicine. Laser Ther. 2000;12:39n‐42. [Google Scholar]
- 40. Sommer AP. Mitochondrial cytochrome c oxidase is not the primary acceptor for near infrared light‐it is mitochondrial bound water: the principles of low‐level light therapy. Ann Transl Med. 2019;7(Suppl 1):S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sommer A, Haddad MF, Fecht HJ. Light effect on water viscosity: implication for ATP biosynthesis. Sci Rep. 2015;5:12029. 10.1038/srep12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sommer AP, Zhu D, Mester AR, Försterling HD. Pulsed laser light forces cancer cells to absorb anticancer drugs–the role of water in nanomedicine. Artif Cells Blood Substit Immobil Biotechnol. 2011;39(3):169‐173. 10.3109/10731199.2010.516262 [DOI] [PubMed] [Google Scholar]
- 43. Kerch G. Role of changes in state of bound water and tissue stiffness in development of age‐related diseases. Polymers. 2020;12(6):1362. 10.3390/polym12061362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mariajoseph‐Antony LF, Kannan A, Panneerselvam A, Loganathan C, Anbarasu K, Prahalathan C. Could aquaporin modulators be employed as prospective drugs for COVID‐19 related pulmonary comorbidity? Med Hypotheses. 2020;143:110201. 10.1016/j.mehy.2020.110201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Front Immunol. 2020;11:827. 10.3389/fimmu.2020.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Z, John Wherry E. T‐cell responses in patients with COVID‐19. Nat Rev Immunol. 2020;20:529‐536. 10.1038/s41577-020-0402-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mehani S. Immunomodulatory effects of two different physical therapy modalities in patients with chronic obstructive pulmonary disease. J Phys Ther Sci. 2017;29(9):1527‐1533. 10.1589/jpts.29.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clark A, Jit M, Warren‐Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID‐19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003‐e1017. 10.1016/S2214-109X(20)30264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


