Summary
Through mechanisms that remain poorly defined, defects in nucleocytoplasmic transport and accumulations of specific nuclear pore complex associated proteins have been reported in multiple neurodegenerative diseases including C9orf72 ALS/FTD. Using super resolution structured illumination microscopy, we have explored the mechanism by which nucleoporins are altered in nuclei isolated from C9orf72 induced pluripotent stem cell derived neurons (iPSNs). Of the twenty three nucleoporins evaluated, we observed a reduction in a subset of eight including key components of the nuclear pore complex scaffold and the transmembrane nucleoporin POM121. Reduction in POM121 appears to initiate a decrease in the expression of seven additional nucleoporins ultimately impacting the localization of Ran GTPase and subsequent cellular toxicity in C9orf72 iPSNs. Collectively, our data suggest that expression of expanded C9orf72 ALS/FTD repeat RNA alone impacts nuclear POM121 expression in the initiation of a pathological cascade affecting nucleoporin levels within neuronal nuclei and ultimately downstream neuronal survival.
eTOC Blurb
Coyne et al. demonstrate that G4C2 repeat RNA initiates a reduction of POM121 expression within C9orf72 neuronal nuclear pore complexes. Decreased nuclear POM121 impacts the expression of 7 additional nucleoporins resulting in altered nuclear pore composition. This combined nucleoporin reduction impacts the localization of nucleocytoplasmic transport proteins and neuronal survival.
Introduction
A GGGGCC (G4C2) hexanucleotide repeat expansion (HRE) in intron 1 of the C9orf72 gene is causative of familial and sporadic forms of the motor neuron disease Amyotrophic Lateral Sclerosis (ALS) and the second most common form of dementia, Frontotemporal dementia (FTD) (DeJesus-Hernandez et al., 2011; Renton et al., 2011). The HRE is bidirectionally transcribed to form sense (G4C2) and antisense (G2C4) RNA species that pathologically accumulate into nuclear RNA foci. Repeat associated non-ATG translation (RANT) of G4C2 and G2C4 RNA produces five dipeptide repeat (DPR) proteins [poly(GA), poly(GP), poly(GR), poly(PR), poly(PA)]. Together, these RNA species and DPR proteins are thought to contribute to disease through gain of toxicity mechanisms. In addition, the C9orf72 HRE may also lead to haploinsufficiency of C9ORF72 protein resulting in a mild cellular, but not clinical, loss of function phenotype (Gitler and Tsuiji, 2016; Taylor et al., 2016). Recently, alterations in an essential cellular process, nucleocytoplasmic transport (NCT), have been identified in models of C9orf72 ALS/FTD (Freibaum et al., 2015; Jovicic et al., 2015; Zhang et al., 2015). However, the molecular mechanisms underlying these disruptions and the normal biology governing NCT in human neurons remain largely unknown.
The mammalian nuclear pore complex (NPC) is a ~120 MDa protein complex consisting of multiple copies of approximately 30 nucleoporins (Nups) which collectively function to maintain NCT, genome organization, and gene expression. These Nups are found within modular subcomplexes that form the major architectural units of the NPC including the outer and inner ring complexes which form the core NPC scaffold. This scaffold is anchored to the nuclear envelope via transmembrane Nups. Ultimately, this structure anchors the cytoplasmic filaments, nuclear basket, and central transport channel. The central channel is comprised of intrinsically disordered proteins containing multiple FG-repeat motifs. These establish a size-selective diffusion barrier and transport channel for nuclear transport receptors (NTRs) and their bound cargo (Beck and Hurt, 2017; Li et al., 2016; Lin and Hoelz, 2019; Raices and D’Angelo, 2012). Additionally, a subset of Nups can also function outside of the NPC in regulating gene transcription (Pascual-Garcia and Capelson, 2019).
While small (<5 nm) macromolecules can passively diffuse through the NPC (Popken et al., 2015; Timney et al., 2016), the nuclear transport of signal-bearing macromolecules is mediated by NTRs (also called karyopherings, importins, and exportins). NTRs recognize import and export signals and transport cargo through the NPC by binding to FG-repeat sequences. The energy to fuel multiple transport cycles is provided by RanGTPase which binds to NTRs in its GTP-bound form to destablize import complexes and stablize export complexes. As a result, in order for bidirectional nuclear transport to occur, a chromatin-bound RanGEF (RCC1) ensures that RanGTP is maintained at high levels within the nucleus and RanGAP drives GTP hydrolysis in the cytoplasm (Melchior, 2001; Raices and D’Angelo, 2012). The relative steady-state localization of Ran between the nucleus and cytoplasm has previously been used as a metric for global alterations to NCT and cellular function in neurodegenerative disease models (Eftekharzadeh et al., 2018; Grima et al., 2017; Zhang et al., 2015).
Scaffold Nups extremely long-lived in non-dividing cells including neurons, many having half-lives measured in months to years. As a result, alterations in NPCs and NCT have been reported during aging (D’Angelo et al., 2009; Hetzer, 2010; Savas et al., 2012; Toyama et al., 2013). In age-related neurodegenerative diseases such as ALS, Huntington’s Disease (HD), and Alzheimer’s Disease (AD), disruptions in NCT appear to be exacerbated over time (Chou et al., 2018; Eftekharzadeh et al., 2018; Freibaum et al., 2015; Gasset-Rosa et al., 2017; Grima et al., 2017; Jovicic et al., 2015; Zhang et al., 2015). In addition, specific Nups have been reported as modifiers of C9orf72 mediated toxicity in the Drosophila eye (Freibaum et al., 2015). Furthermore, mislocalization of the NPC associated protein RanGAP1 and Nups has been reported in postmortem human tissue and multiple mouse models based on overexpression of the C9orf72 HRE or individual DPRs (Chew et al., 2019; Zhang et al., 2015; Zhang et al., 2018; Zhang et al., 2016; Zhang et al., 2019b). Collectively, these studies suggest that altered NCT is a primary pathological feature of ALS and FTD. In order to mechanistically understand how alterations to the NPC and NCT contribute to disease pathogenesis, a comprehensive evaluation of the precise deficits in the context of endogenous C9orf72 HRE expression is necessary.
Here, using an induced pluripotent stem cell (iPSC) derived neuron (iPSN) model of C9orf72 ALS and super resolution structured illumination microscopy (SIM), we show that the expression of a specific subset of eight Nups is decreased in human C9orf72 neuronal nuclei and NPCs. Mechanistically, genetic manipulation of candidate Nups reveals that the transmembrane Nup POM121 plays an integral role in maintaining the nuclear levels of these eight NPC components in iPSNs. Loss of POM121 in C9orf72 ALS/FTD is not mediated by DPRs or loss of C9ORF72 protein but occurs in the presence of pathologic G4C2 repeat RNA. These data provide direct evidence that pathologic repeat RNA initiates early disruptions to the NPC in C9orf72 mediated disease. Ultimately, alterations in NPC associated POM121 subsequently impacts the nuclear and NPC associated repertoire of an additional seven Nups and collectively these alterations affect the distribution of Ran GTPase and cellular toxicity.
Results
Characterization of C9orf72 pathology in an accelerated iPSC derived spinal neuron differentiation protocol
While the use of postmortem human tissue is valuable for identification of pathological hallmarks of disease, the study of neurodegenerative diseases using iPSCs provides an unparalleled opportunity to identify underlying molecular mechanisms of disease pathogenesis. Neurons derived from human iPSCs maintain endogenous expression of disease associated genes and proteins rather than relying on overexpression models. However, many previous iPSC differentiation protocols take months to generate spinal neurons leading to increased differentiation and batch variability (Sances et al., 2016; Zhang et al., 2019a). To model C9orf72 ALS with iPSCs, spinal neurons were differentiated using the direct induced motor neuron (diMNs) protocol (Fig. S1A). With this protocol, terminal differentiation and neuronal maturation begins at day 12 of differentiation with the addition of specific neuronal maturation growth factors (see Methods, Fig. S1A). Ultimately, a reproducible population of spinal neurons is generated by day 18 of differentiation of which about 30% are Islet-1 positive lower motor neurons (Fig. S1A–B). Importantly, C9orf72 ALS/FTD clinically and pathologically affects multiple different populations of spinal and cortical neurons across two clinically distinct but genetically overlapping neurodegenerative diseases (Cook and Petrucelli, 2019; Ferrari et al., 2011). As more than just Islet-1 positive lower motor neurons are affected in disease, the analysis of all neurons within iPSC derived spinal neuron cultures is essential for a comprehensive understanding of pathogenic mechanisms. As a result, we began by evaluating C9orf72 pathology in these mixed iPSN cultures. Similar to our previous publications showing iPSNs produce G4C2 repeat RNA which can infrequently accumulate into RNA foci (Donnelly et al., 2013), RT-qPCR experiments reveal an age dependent increase in both G4C2 and G2C4 repeat RNA from day 18 to day 32 of differentiation (Fig. S1C). Conversely, Poly(GP) DPR levels, as detected by immunoassay, remain constant over time for each individual iPSC line tested (Fig. S1D) consistent with our previous studies in human CSF showing variability in Poly(GP) levels amongst individual patients (Gendron et al., 2017). Furthermore, we find a slight reduction in C9orf72 RNA levels (Fig. S1E) and no change in C9ORF72 protein (lower band as determined by western blot on C9orf72−/− iPSC line (data not shown; Fig. S1F–G)) at day 32 of differentiation. Taken together, these data suggest that while our iPSN model of C9orf72 ALS recapitulates pathologic repeat RNA and DPR production, it does not result in haploinsufficiency of C9ORF72, consistent with variability in C9ORF72 protein levels seen in actual patients (Sareen et al., 2013; Waite et al., 2014).
A major pathologic hallmark of the large majority of ALS and FTD cases, including C9orf72, includes the cytoplasmic mislocalization and accompanying accumulation of the normally nuclear RNA binding protein TDP-43 in a subset of neurons at autopsy (Ling et al., 2013). Using immunostaining and confocal imaging, we show that TDP-43 does not mislocalize to the cytoplasm of C9orf72 iPSNs compared to controls (Fig. S1H–I) at day 32 of differentiation. In agreement with human biopsy and autopsy studies (Vatsavayai et al., 2016), this suggests that TDP-43 mislocalization may be a much later event in ALS pathogenesis. In contrast, while we see no overt difference in cell death at baseline, C9orf72 iPSNs are susceptible to glutamate induced excitotoxicity (Fig. S1J) similar to our previous reports using a much longer spinal neuron differentiation protocol (Zhang et al., 2015).
Specific nucleoporins are altered in C9orf72 iPSN nuclei in an age dependent manner
Previous reports have pathologically characterized cytoplasmic accumulations of a small number of Nups in artificial overexpression model systems and postmortem patient tissue (Chew et al., 2019; Zhang et al., 2015; Zhang et al., 2018; Zhang et al., 2016; Zhang et al., 2019b). However, none of these studies have truly evaluated the expression of Nups in actual nuclei and NPCs, an analysis critical for the understanding of C9orf72 HRE mediated disease pathogenesis.
To examine the nuclear levels of 23 individual human Nups in NeuN positive nuclei isolated from iPSNs we used super resolution structured illumination microscopy (SIM). SIM provides an estimated lateral imaging resolution of 100 nm which, unlike standard light microscopy, is sufficient to resolve individual NPCs (Lin and Hoelz, 2019; Maglione and Sigrist, 2013; Schermelleh et al., 2008). However, as human NPCs themselves are ~100 nm in diameter (Lin and Hoelz, 2019), SIM cannot resolve individual Nup molecules within the NPC octet structure. Nonetheless, SIM is routinely used with conventional immunofluorescent staining protocols and specific anti-Nup antibodies to quantify NPC number by counting resolvable “spots” and their intensity on the nuclear envelope (Maglione and Sigrist, 2013; Schermelleh et al., 2008; Thevathasan et al., 2019).
Following SIM, an automated image analysis pipeline was employed to determine the number of Nup spots per nucleus. For the majority of the immunostained Nups this approach was successful. However, there were a few anti-Nup antibodies that did not produce sufficient signal-to-noise ratios for individual spots to be confidently quantified. In cases where individual spots could not be resolved, we calculated the percent of the total nucleus volume occupied by the anti-Nup signal (see Methods for additional details). In addition to variable signal-to-noise ratios amongst individual Nups, we observed that there was variability in the number of spots detected and level of fluorescence amongst spots for individual Nups (Fig. 1A–C). Although this suggests that there may be a different number of Nup molecules per individual NPCs, we note that differential antibody affinities do not allow for a comparison of absolute Nup spot or volume numbers amongst each of the 23 Nups evaluated. Thus, all analyses were conducted on an individual Nup basis comparing C9orf72 to controls. Using this approach, we reproducibly found that 8 of the 23 Nups examined including the nuclear basket Nups Nup50 and TPR, the central channel Nup Nup98, all three transmembrane Nups GP210, NDC1, and POM121, and the Y complex outer ring Nups Nup107 and Nup133 are significantly reduced in C9orf72 iPSN nuclei at day 32 but not day 18 when compared to controls at the respective time point (Fig. 1). These data highlight the age dependence of specific Nup alterations in the maturation of pathogenic cascades in C9orf72 ALS.
Figure 1: Specific nucleoporins are altered in nuclei from C9orf72 iPSNs.
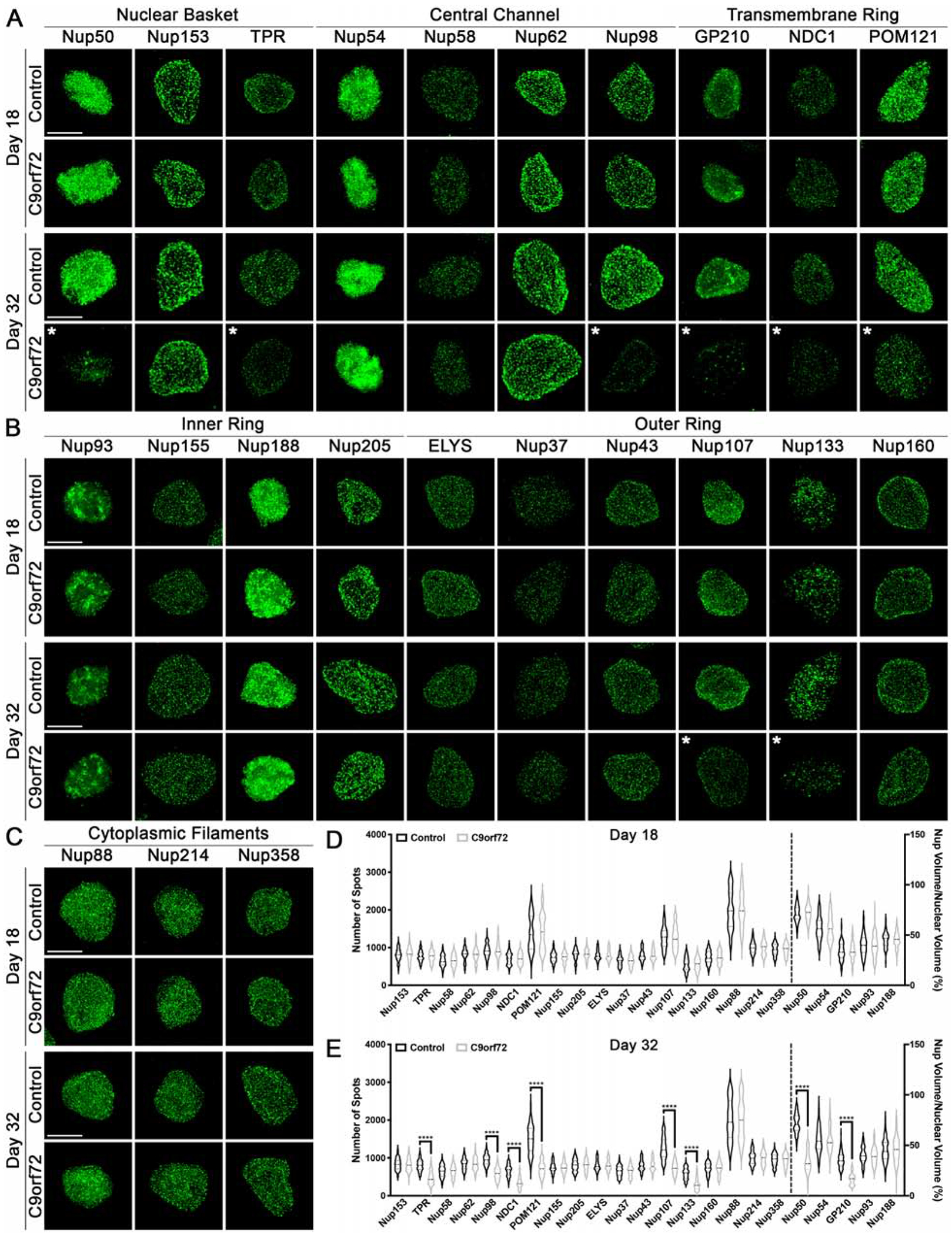
(A-C) Maximum intensity projections from SIM imaging of Nups in nuclei isolated from control and C9orf72 iPSNs. Genotype and time point as indicated on left, antibodies as indicated on top. (D-E) Quantification of Nup spots and volume. Time point as indicated on top. N = 8 control and 8 C9orf72 iPSC lines (including 1 isogenic pair), 50 NeuN+ nuclei per line/time point. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. Scale bar = 5 μm. * indicates significantly altered Nups.
Interestingly, although individual Nups are localized in close proximity, we rarely observe colocalization between two Nups within the pairings we evaluated (Fig S2). Moreover, in single Z slices from our SIM analyses, we observe areas within iPSN nuclei that contain only one of the Nups in our double immunolabeling experiments (Fig. S2) consistent with previous reports of Nup heterogeneity within NPCs in a single nucleus in non-neuronal cellular systems (Kinoshita et al., 2012; Rajoo et al., 2018; Toyama et al., 2018). Nonetheless, in C9orf72 iPSN nuclei, we observe a reduction in multiple Nups within each neuronal nucleus (Fig. S3).
When available, a second antibody targeting an alternate region of Nup proteins was used to verify Nup reduction by SIM. For example, antibodies that recognize both the C- (Fig. 1A, E) and N-termini of POM121 (Fig. S4A–B) revealed a similar reduction in POM121 at day 32 of differentiation. Importantly, we observe a reduction of nuclear POM121 in Islet1 positive lower motor neurons at day 32, but not at day 18 (Fig. S4C–D) and in spinal neurons derived using a previously described iPSN differentiation protocol (Donnelly et al., 2013; Zhang et al., 2015) (Fig. S4E–F). Together, these data suggest that our observed alterations in specific Nups are reproducible in multiple neuronal subtypes affected in ALS pathogenesis and across multiple differentiation protocols.
While isolated nuclei preparations provide for more accurate reconstruction and increased resolution of Nup immunostaining, we observe a similar decrease in POM121, Nup133, and Nup50 nuclear intensity using standard confocal microscopy in intact iPSNs (Fig. S5A–D) suggesting that our results are not an artifact of SIM or nuclei isolation. Furthermore, we do not observe mislocalization of POM121, Nup133, or Nup50 from the nucleus to the cytoplasm in intact iPSNs (Fig. S5A–C). Moreover, we performed additional validation with western blots which quantitatively confirm a decrease in total POM121, Nup133, and Nup50 protein in isolated nuclei from C9orf72 iPSNs (Fig. S5E–F). Reduction in Nup protein is not a result of decreased steady state mRNA levels (Fig. S5G), altered mRNA stability as measured by actinomycin D chase (Fig. S6AߝJ), or decreased association with polyribosomes (Fig. S6K–T). In fact, for some Nups, the association of their mRNA with actively translating polyribosome fractions is increased in C9orf72 iPSNs (Fig. S6K–T) despite the reduced protein expression observed by multiple experimental methodologies. Collectively, these data suggest that nuclear levels of specific Nups are decreased at the level of protein expression and/or stability in C9orf72 iPSN nuclei without corresponding cytoplasmic mislocalization or accumulation. Although, reduced POM121 in C9orf72 iPSN nuclei is a reproducible and robust pathology across multiple iPSN lines, we note that there is variable decrease in POM121 amongst individual nuclei (Fig. S7) in accordance with our quantification represented by violin plots. In spite of this variable reduction in 8 specific Nups from the nucleus, we do not find alterations in the overall number or distribution of FG repeat containing Nups based on immunostaining with a general antibody (mAb414; 414) that recognizes 4 FG repeat containing Nups: Nup153, Nup62, Nup214, and Nup358/RanBP2 (Fig. S8A–C).
To globally evaluate NPC structure and distribution in control and C9orf72 iPSN nuclear envelopes at high resolution, we used direct surface imaging by field emission scanning electron microscopy (SEM). Here we found that that the overall number and distribution of NPCs is unchanged in C9orf72 iPSN nuclei (Fig. S8D). Furthermore, higher magnification imaging focusing on individual NPCs shows no overt change in NPC architecture in C9orf72 IPSN nuclei (Fig. S8D). Together, these data suggest that individual Nup molecules may be reduced from the nuclear envelope, presumably from pore octamers, without overall loss of NPCs.
C9orf72 iPSN nuclei are not leaky
Given our previous report that active NCT is disrupted in C9orf72 iPSNs (Zhang et al., 2015) and our data that 8 Nups are decreased in C9orf72 iPSN nuclei without overall effects on NPC distribution and structure (Fig. 1, Fig. S8D), we next wanted to determine if C9orf72 iPSN nuclei were passively leaky. Normally, some macromolecules can passively diffuse through the NPC in a manner dependent on their size (Popken et al., 2015; Timney et al., 2016). Accordingly, fluorescent dextrans of various sizes have been used to evaluate the passive permeability of NPCs (Zhu et al., 2016). Confocal imaging of digitonin permeabilized iPSNs revealed that C9orf72 nuclei are not passively leaky compared to controls (Fig. S9) suggesting that the overall passive permeability barrier of the NPC is intact despite the nuclear reduction in 8 specific Nups in C9orf72 iPSNs.
Nucleoporin alterations in postmortem C9orf72 patient motor cortex and thoracic spinal cord are identical to those in C9orf72 iPSNs
To determine whether our observed Nup pathology in C9orf72 iPSNs was reflective of real changes that occur in human patients, we next examined isolated nuclei from postmortem motor and occipital cortex from non-neurologic control and C9orf72 patients by SIM. Of the 13 Nups analyzed, Nup50, TPR, Nup98, NDC1, POM121, Nup107, and Nup133 but not Nup153, Nup54, Nup62, Nup188, Nup205, or Nup160 are decreased in NeuN positive nuclei from C9orf72 patient motor cortex (Fig. 2A–C) but not occipital cortex (Fig. 2A–B, D), a brain region unaffected in ALS. Similar to our results in postmortem motor cortex, the nuclear levels of 6 Nups (Nup50, TPR, NDC1, POM121, Nup107, and Nup133) are reduced in NeuN positive nuclei isolated from C9orf72 patient thoracic spinal cord compared to controls (Fig. S10). Together, these data in patient tissue mimic our results in iPSNs. Notably, staining of postmortem paraffin embedded motor cortex tissue sections with an antibody recognizing the C-terminus of POM121 also shows a decrease in nuclear POM121 intensity (Fig. S11), albeit without the resolution provided by SIM. Collectively, these data suggest that our iPSN model recapitulates human disease pathogenesis, identifies specific Nup alterations as a possible initiating pathologic event in C9orf72 ALS, and highlights the use of our iPSN model as a tool for studying the disease mechanisms underlying alterations in individual NPC components.
Figure 2. Specific nucleoporins are altered in nuclei from C9orf72 patient motor cortex.
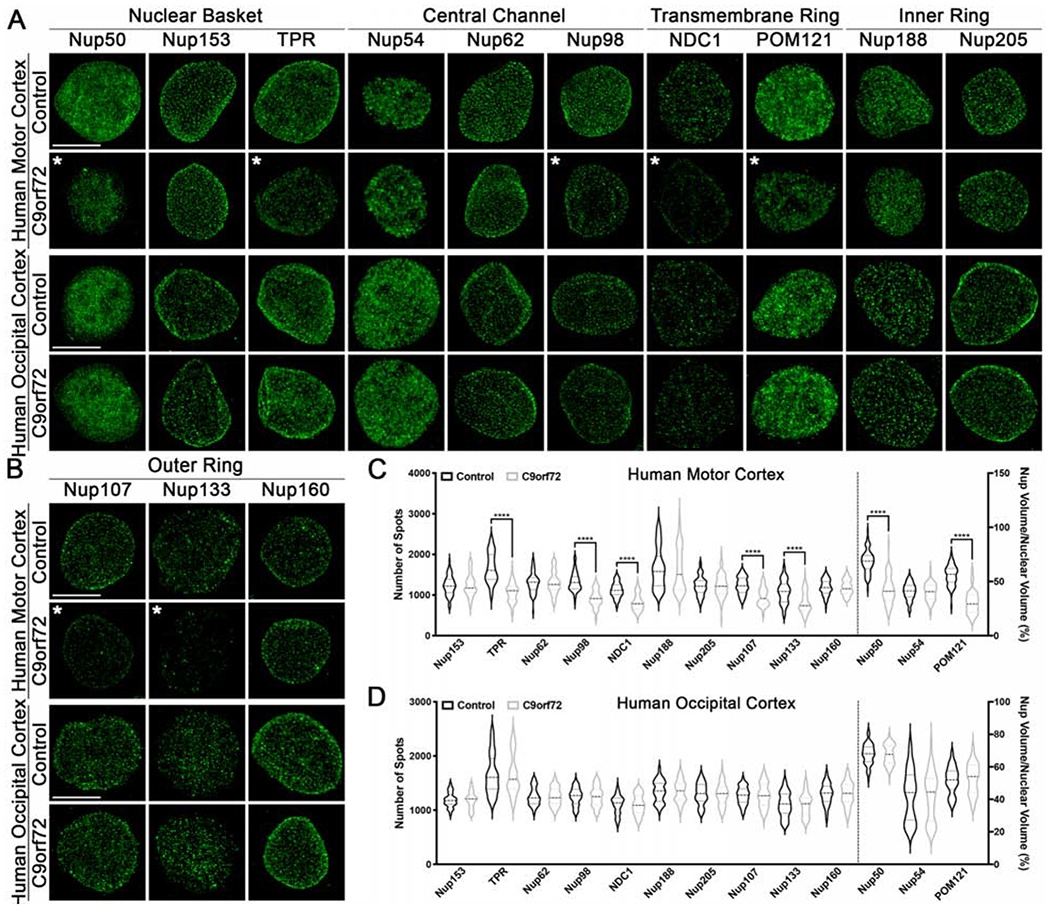
(A-B) Maximum intensity projections from SIM imaging of Nups in nuclei isolated from postmortem human brain tissue. Genotype and brain region as indicated on left, antibodies as indicated on top. (C-D) Quantification of Nup spots and volume. Brain region as indicated on top. N = 3 control and 3 C9orf72 cases, 50 NeuN+ nuclei per case. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. Scale bar = 5 μm. * indicates significantly altered Nups.
Reversibility of NPC injury: Treatment with C9orf72 sense repeat targeting ASO mitigates alterations in specific Nups and cellular localization of Ran GTPase
Following validation that antisense oligonucleotides (ASOs) targeting the intron upstream of the G4C2 repeat (sense strand) could mitigate G4C2 pathology in our accelerated iPSN system (Fig. S12) in a manner similar to our previous reports (Gendron et al., 2017), we next investigated whether ASO treatment of iPSNs can mitigate alterations in Nup levels within the nucleus. Compared to the scrambled control ASO, 3-week sense ASO treatment restored the 8 affected Nups Nup50, TPR, Nup98, GP210, NDC1, POM121, Nup107, and Nup133 within C9orf72 iPSN nuclei, but had no effect on the unaffected Nups Nup153, Nup54, Nup62, Nup93, Nup188, Nup205, and Nup160 (Fig. 3A–T). This suggests that observed Nup disruptions are a direct or indirect pathological consequence of the C9orf72 HRE.
Figure 3: Sense repeat RNA targeting ASOs mitigate alterations in specific Nups, restore the localization of Ran GTPase, and protect against cellular toxicity in C9orf72 iPSNs.

(A-E) Maximum intensity projections from SIM imaging of Nups in nuclei isolated from 5 μM sense strand targeting or scrambled ASO treated control and C9orf72 iPSNs. Treatment as indicated on left, genotype and antibodies as indicated on top. (F-T) Quantification of Nup spots and volume. N = 5 control and 5 C9orf72 iPSC lines, 50 NeuN+ nuclei per line/treatment. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. (U) Confocal imaging of 5 μM sense strand targeting or scrambled ASO treated control and C9orf72 iPSNs immunostained for Ran. Treatment as indicated on left, genotype and antibodies as indicated on top. (V) Quantification of nuclear to cytoplasmic ratio of Ran. N = 3 control and 3 C9orf72 iPSC lines, 30 cells per line. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. (W) Quantification of percent cell death following exposure to glutamate. N = 4 control and 4 C9orf72 iPSC lines, 10 frames per well. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. Scale bar = 5 μm (A-E), 10 μm (U). * indicates significantly altered Nups, ** indicates significantly restored Nups.
To determine whether the ASO mediated rescue of 8 specific Nups within neuronal nuclei could perhaps subsequently impact Ran GTPase localization, we performed immunostaining for endogenous Ran in iPSNs treated with scrambled or sense ASOs for 3 weeks. Similar to our findings with Nups, sense targeting ASOs restored localization of Ran to the nucleus compared to scrambled controls (Fig. 3U–V) supporting observations from our previous study which used different iPSC lines and a longer differentiation protocol (Zhang et al., 2015). As a proper Ran GTPase gradient across the nuclear envelope is critical for the maintenance of functional NCT, our data suggest that C9orf72 HRE targeting ASOs may act to restore the normal Nup levels in nuclei of C9orf72 iPSNs and in turn impact NCT. In addition, downstream of the biological effects on Nups and Ran localization, G4C2 targeting ASOs mitigated susceptibility to glutamate excitotoxicity in C9orf72 iPSNs (Fig. 3W, Fig. S13). Collectively, these data suggest that pathologic alterations in the nuclear repertoire of individual NPC components, Ran GTPase localization, and downstream deficits in cellular survival are biological consequences of C9orf72 repeat RNA and/or DPRs.
Overexpression of POM121 restores the nuclear expression of specific Nups, localization of Ran GTPase and fluorescent NCT reporters, and protects against cellular toxicity
Our data support a model in which only a subset of Nups are reduced from fully assembled NPCs as an underlying pathogenic event in C9orf72 mediated neurodegeneration. To begin to understand the relationship between specific Nups in neurons and in disease, we re-introduced GFP tagged Nups (Fig. S14A–B) and used SIM (see Methods for details regarding nuclei selection), to evaluate the localization and levels of individual NPC components. The re-introduction of Nups was conducted at a timepoint after their initial reduction from the nucleus (30 days, Fig. 1). While overexpression of Nup98, only restored nuclear levels Nup98 (Fig. 4A–T), overexpression of Nup133, restored nuclear levels of Nup50, TPR, Nup98, Nup107, and Nup133 but not GP210, NDC1, and POM121 in C9orf72 IPSNs (Fig. 4A–T). In contrast, overexpression of POM121 variably mitigated all alterations in affected Nups in C9orf72 iPSNs within about 2 weeks (Fig. 4A–T). Interestingly, Nup133 and POM121 overexpression does not alter the total number or distribution of FG repeat containing Nups (Fig. S14C–D).
Figure 4: POM121 overexpression restores the nuclear expression of specific Nups, localization of Ran GTPase and mitigates cellular toxicity.
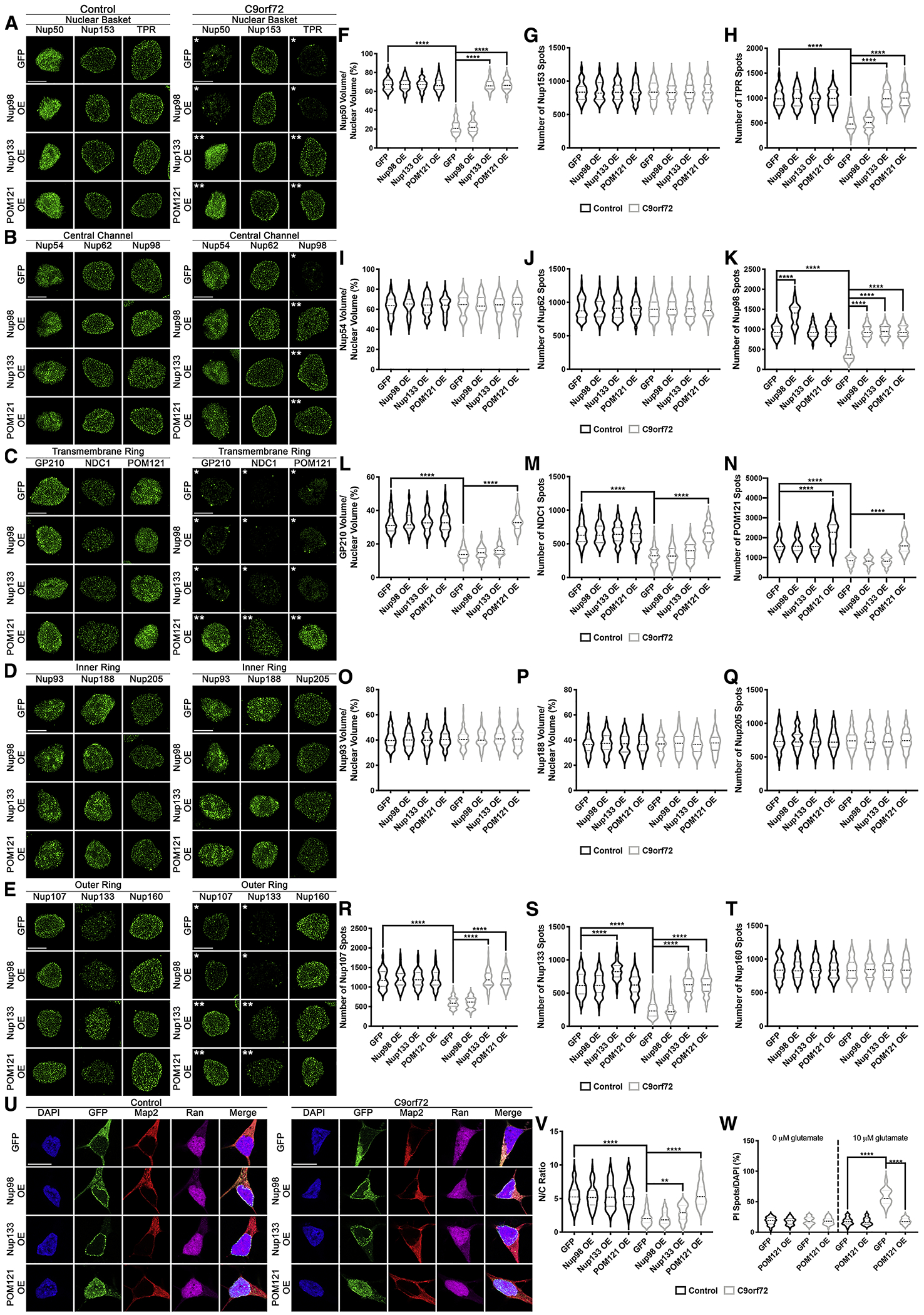
(A-E) Maximum intensity projections from SIM imaging of Nups in nuclei isolated from control and C9orf72 iPSNs overexpressing GFP tagged Nup98, Nup133, or POM121. Overexpression as indicated on left, genotype and antibodies as indicated on top. (F-T) Quantification of Nup spots and volume. N = 4 control and 4 C9orf72 iPSC lines, 50 GFP+ nuclei per line/overexpression. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. (U) Confocal imaging of control and C9orf72 iPSNs overexpressing GFP tagged Nup98, Nup133, or POM121 immunostained for Ran. Overexpression as indicated on left, genotype and antibodies as indicated on top. (V) Quantification of nuclear to cytoplasmic ratio of Ran. N = 4 control and 4 C9orf72 iPSC lines, at least 50 cells per line/overexpression. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. ** p < 0.01, **** p < 0.0001. (W) Quantification of percent cell death following exposure to glutamate. N = 4 control and 4 C9orf72 iPSC lines, 10 frames per well. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. Scale bar = 5 μm (A-E), 10 μm (U). * indicates significantly altered Nups, ** indicates significantly restored Nups.
In contrast to POM121 overexpression, neither a soluble variant of POM121 (sPOM121) which lacks the transmembrane domain and does not localize to the NPC (Franks et al., 2016), nor overexpression of GP210 or NDC1 were able to restore nuclear expression of candidate Nups (Fig. S15, Fig. S16). Interestingly, GP210, NDC1, and sPOM121 overexpression all partially restored the nuclear expression of Nup98 (Fig. S15A,C, Fig. S16A,D) suggesting that Nup98 is highly sensitive to a variety of Nup manipulations. Together, these data suggest that NPC associated POM121 is necessary for the re-establishment of proper nuclear and NPC expression of specific Nups in C9orf72 iPSNs.
In addition to the rescue of nuclear levels of Nups, POM121 overexpression resulted in the relocalization of Ran from the cytoplasm to the nucleus in C9orf72 iPSNs (Fig. 4U–V). To evaluate whether POM121 overexpression could additionally mitigate defects in the localization of a fluorescent NCT reporter construct, we employed the previously characterized lentiviral NLS-tdTomato-NES (S-tdTomato) reporter (Zhang et al., 2015). In this paradigm (Fig. S17A), while S-tdTomato is typically predominately nuclear given the strong NLS signal, by 3 days post transduction its localization shifts to the cytoplasm in C9orf72 iPSNs (Fig. S17B–D) indicative of potential disruptions in NCT. However, by 10 days post transduction, overexpression of POM121 restores the nuclear localization of the S-tdTomato reporter (Fig. S17B–E) in C9orf72 iPSNs. Moreover, using glutamate induced cell death assays we found that POM121 overexpression was sufficient to suppress glutamate induced excitotoxicity and promoted cellular survival in C9orf72 iPSNs (Fig. 4W, Fig. S18). Collectively, these data suggest that POM121 overexpression can mitigate alterations in the nuclear expression of specific Nups, the combination of which positively impacts nuclear Ran GTPase and S-tdTomato reporter localization and ultimately mitigates downstream cellular toxicity.
Knockdown of POM121 alone in wildtype neurons recapitulates disease phenotypes
Given the variable yet robust phenotypic rescue provided by POM121 overexpression, we hypothesized that reduction in nuclear POM121 may be an initiating event in C9orf72 mediated disease. Due to the long half-life of many scaffold Nups in neurons (Savas et al., 2012; Toyama et al., 2013), we employed the Trim Away method (Clift et al., 2017) to rapidly degrade endogenous Nup133, POM121, NDC1, or GAPDH protein in control iPSNs (Fig. S19). While knockdown of Nup133 resulted in subsequent nuclear reduction of Nup50, TPR, Nup107, and Nup133 (Fig. 5A–P), knockdown of POM121 recapitulated the nuclear decrease in Nup50, TPR, GP210, NDC1, POM121, Nup107, and Nup133 (Fig. 5A–P) observed in C9orf72 iPSNs (Fig. 1), human motor cortex (Fig. 2), and thoracic spinal cord (Fig. S10) within 48 hours. Notably, this effect on the levels of Nups in iPSN nuclei was specific to the transmembrane Nup POM121 as Trim21 mediated reduction of the transmembrane Nup NDC1 had no effect on Nups other than itself (Fig. 5A–P). Interestingly, neither Nup133, NDC1, nor POM121 knockdown resulted in disruptions in nuclear associated Nup98 (Fig. 5A–P). In combination with our overexpression data, this suggests that Nup98 reduction may be the result of an independent mechanism. Importantly, knockdown of GAPDH, a non-NPC associated control protein, had no effect on nuclear expression of any of the 15 Nups analyzed (Fig. 5A–P).
Figure 5: Reduction in POM121 in wildtype iPSNs recapitulates C9orf72 mediated alterations in specific Nups and Ran GTPase and increases susceptibility to glutamate induced excitotoxicity.
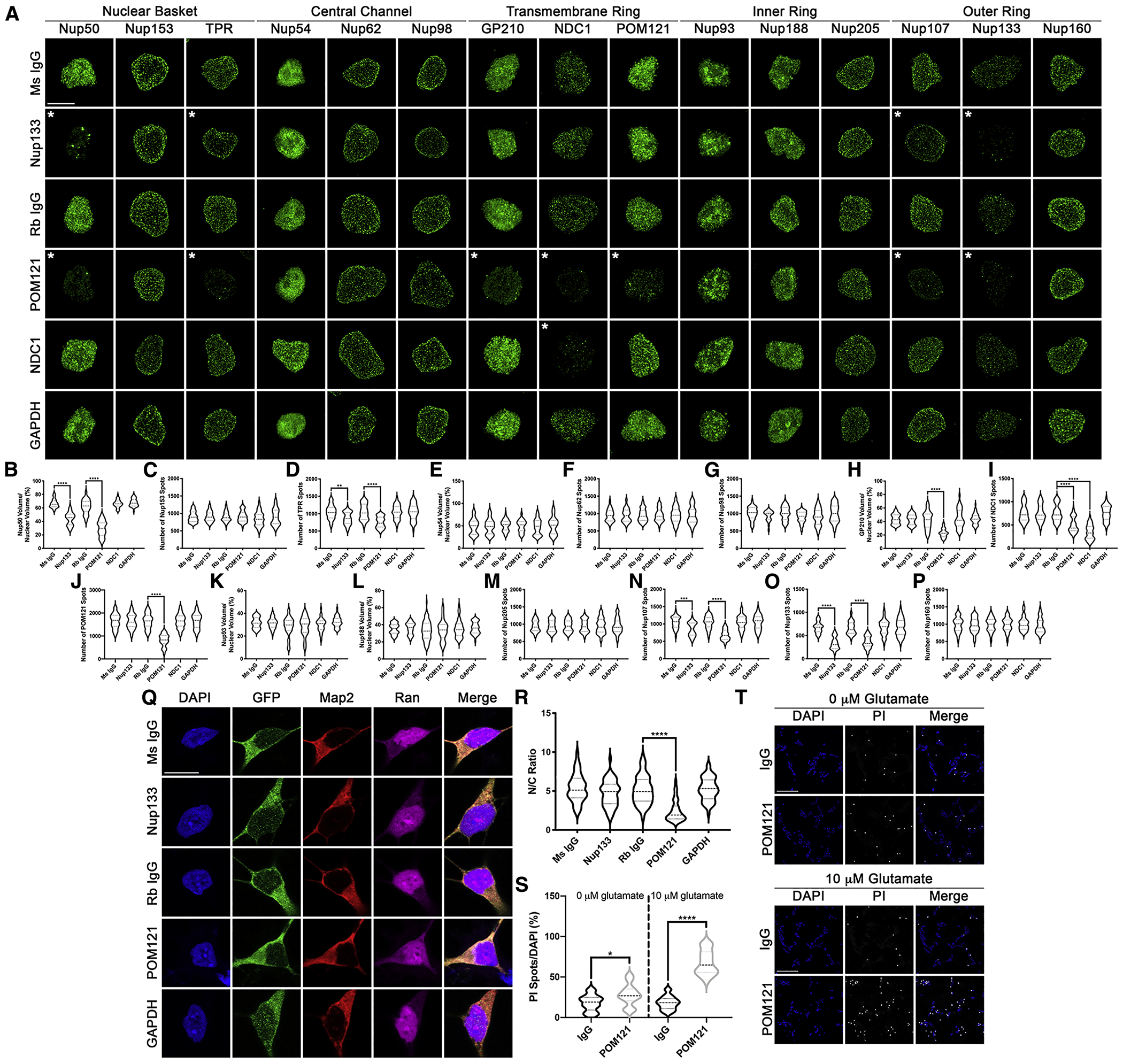
(A-P) Maximum intensity projections from SIM imaging (A) and quantification (B-P) of Nup spots and volume in nuclei isolated from wildtype iPSNs following knockdown of Nup133, POM121, NDC1, or GAPDH. Antibody used for Trim21 GFP mediated knockdown as indicated on left, antibodies as indicated on top. N = 3 wildtype iPSC lines, 50 GFP+ nuclei per line/knockdown. One-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. ** p < 0.01, *** p < 0.001, **** p < 0.0001. (Q) Confocal imaging of control iPSNs following Trim21 GFP knockdown of Nup133, POM121, or GAPDH immunostained for Ran. Antibody used for Trim21 knockdown as indicated on left, antibodies as indicated on top. (R) Quantification of nuclear to cytoplasmic ratio of Ran. N = 3 wildtype iPSC lines, at least 50 cells per line/knockdown. One-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. (S) Quantification of percent cell death following exposure to glutamate. N = 3 control iPSC lines, 10 frames per well. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. (T) Confocal imaging of cell death in control and C9orf72 iPSNs as measured by propidium iodide (PI) incorporation. Antibody used for Trim Away as indicated on left, glutamate concentration and stain as indicated on top. Scale bar = 5 μm (A), 10 μm (Q), 100 μm (T). * indicates significantly altered Nups.
In addition to the nuclear reduction of specific NPC components, knockdown of POM121, but not Nup133 or GAPDH, resulted in Ran GTPase mislocalization from the nucleus to the cytoplasm (Fig. 5Q–R). Furthermore, Trim21 mediated knockdown of POM121 increased the susceptibility of wildtype neurons to glutamate induced excitotoxicity (Fig. 5S–T). Together, these data suggest that nuclear decrease in POM121 can initiate a pathological cascade reminiscent of C9orf72 mediated disease alterations including disruption of NPC components and downstream effects on Ran GTPase localization and cellular sensitivity to stressors.
Loss of C9ORF72 and DPRs do not contribute to alterations in the POM121
It has been proposed that the C9orf72 HRE exerts toxicity through the combination of three pathological phenomena including haploinsufficiency of C9ORF72 protein, accumulation of G4C2 and G2C4 repeat RNA, and production of toxic DPRs (Balendra and Isaacs, 2018; Cook and Petrucelli, 2019; Gitler and Tsuiji, 2016). Although we do not observe a reduction in C9ORF72 protein in our iPSN model (Fig. S1F–G), we first used a C9orf72 knock out (C9orf72−/−) iPSC line to investigate whether complete loss of C9ORF72 protein affected the nuclear expression of individual Nups. Using SIM, we found that none of the 22 Nups assessed were altered in nuclei isolated from C9orf72−/− iPSNs compared to controls (Fig. 6A–B) suggesting that loss of C9ORF72 protein does not play a role in Nup alterations in C9orf72 ALS.
Figure 6: Loss of C9ORF72 protein or DPRs do not alter the nuclear expression of POM121.

(A) Maximum intensity projections from SIM imaging of Nups in nuclei isolated from control and C9orf72−/− iPSNs. Genotype as indicated on left, antibodies as indicated on top. (B) Quantification of Nup spots and volume. N = 1 control and 1 C9orf72 null line, differentiations conducted in triplicate, 50 NeuN+ nuclei per line/differentiation. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. (C) SIM imaging of POM121 in nuclei isolated from wildtype iPSNs overexpressing GFP tagged Poly(GA), Poly(GR), and Poly(PR) DPRs. Overexpression as indicated on left, antibodies and time points as indicated on top. (D-E) Quantification of percent total nuclear volume occupied by POM121 2 days (D) and 7 days (E) after overexpression of DPRs. N = 3 wildtype iPSC lines, 50 GFP+ nuclei per line/overexpression. One-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. Scale bar = 5 μm.
Multiple studies have reported that artificial overexpression of DPRs produced by the C9orf72 HRE can result in disruptions in NCT potentially via cytoplasmic mislocalization and aggregation of a subset of NPC associated proteins and Nups (Chew et al., 2019; Shi et al., 2017; Zhang et al., 2018; Zhang et al., 2016; Zhang et al., 2019b). While we do not observe this pathology in iPSNs (Fig. S5C) or postmortem human tissue (Fig. S11), we nonetheless used SIM to evaluate whether overexpression of individual DPRs in wildtype iPSNs affected nuclear expression of POM121. While both Poly(GR) and Poly(PR) strongly accumulate in and/or around the nucleus, nuclear localization of Poly(GA) was rarely observed (Fig. 6C). However, neither Poly(GA), Poly(GR), nor Poly(PR) affected the expression or distribution of POM121 within nuclei after 48 hours (Fig. 6C–D) or one week (Fig. 6C,E) of overexpression. These data indicate that like loss of C9ORF72 protein, DPRs are not responsible for the initiation of POM121 reduction from human neuronal nuclei during C9orf72 disease pathogenesis.
Expression of G4C2 repeat RNA results in reduced POM121 within nuclei in iPSNs
To test whether pathological G4C2 repeat RNA was responsible for the decrease POM121, we first treated control and C9orf72 iPSNs with G4C2 targeting ASOs for only 5 days beginning at day 30 of differentiation. After 5 day treatment with ASO, we observed a large reduction in G4C2 repeat RNA (Fig. 7A) but not the Poly(GP) DPR (Fig. 7B) indicating that this short ASO treatment paradigm can selectively reduce pathologic repeat RNA but not DPRs consistent with our previous reports that it takes about 3 weeks to reduce Poly(GP) levels in iPSNs (Gendron et al., 2017). Interestingly, using SIM we found that this 5 day ASO treatment mitigated the reduced nuclear expression of POM121 in C9orf72 IPSN nuclei compared to untreated and scrambled ASO controls (Fig. 7C–D).
Figure 7. Expression of pathologic G4C2 repeat RNA initiates the nuclear reduction in POM121.
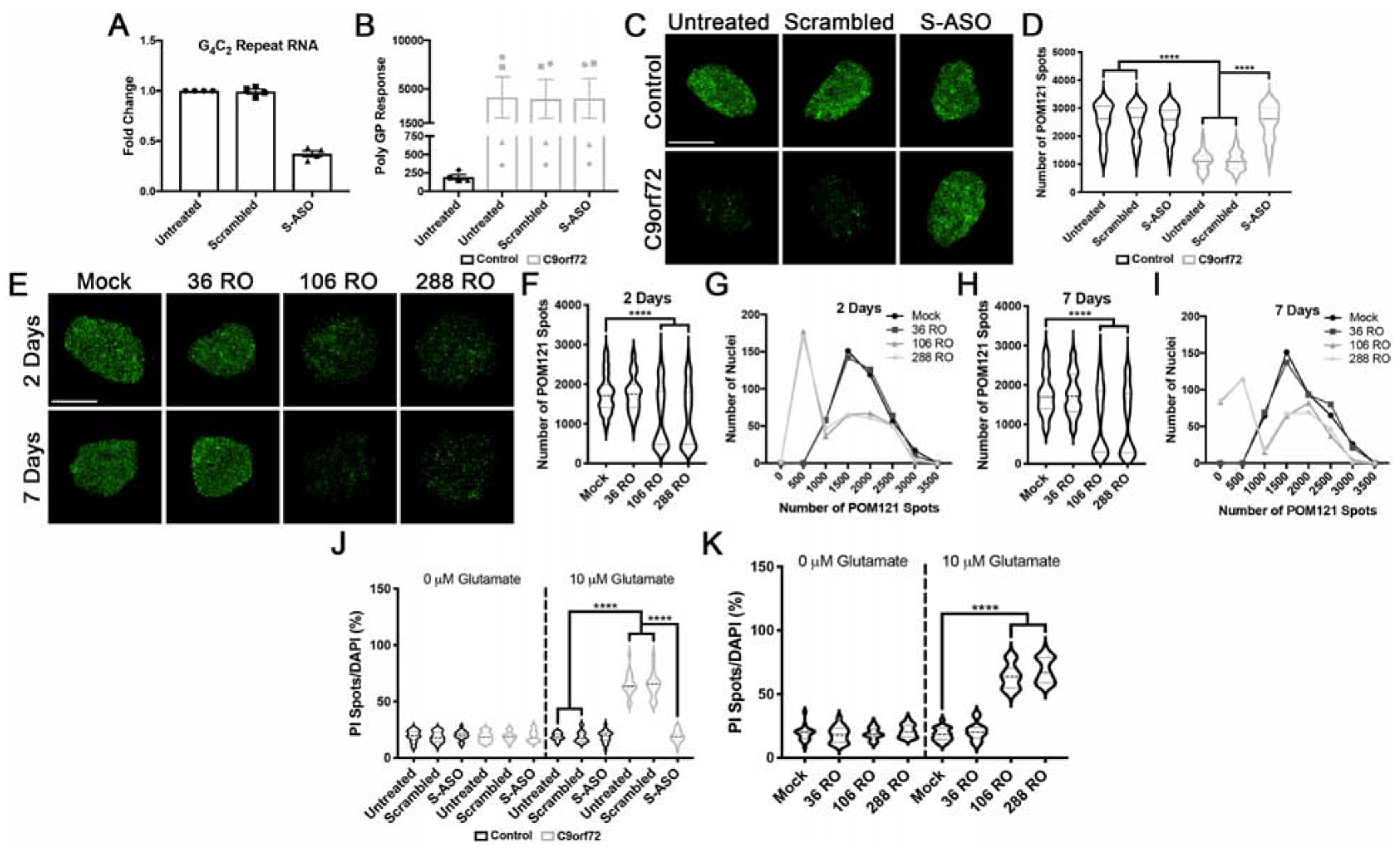
(A-B) RT-qPCR for G4C2 repeat RNA (A) and MSD Elisa for Poly(GP) DPR levels (B) in C9orf72 iPSNs following 5 days of treatment with G4C2 targeting ASO. (C) SIM imaging for POM121 in nuclei isolated from control and C9orf72 iPSNs. Genotype as indicated on left, treatment as indicated on top. (D) Quantification of percent total nuclear volume occupied by POM121 following 5 days of treatment with G4C2 targeting ASO. N = 4 control and 4 C9orf72 IPSC lines, 50 NeuN+ nuclei per line/treatment. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. (E) Maximum intensity projections from SIM imaging of POM121 in nuclei isolated from wildtype iPSNs overexpressing 36, 106, or 288 G4C2 RNA repeats. Time point as indicated on left, overexpression as indicated on top. (F-I) Quantification and histogram distributions of POM121 spots 2 days (F-G) and 7 days (H-I) after overexpression of G4C2 repeat RNA. N = 4 wildtype iPSC lines, 100 NeuN+ nuclei per line/overexpression. One-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. (J) Quantification of percent cell death following exposure to glutamate in iPSNs treated with ASOs for 5 days. N = 4 control and 4 C9orf72 iPSC lines, 10 frames per well. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. (K) Quantification of percent cell death following exposure to glutamate in wildtype iPSNs overexpressing G4C2 repeat RNA. N = 4 control iPSC lines, 10 frames per well. Two-way ANOVA with Tukey’s multiple comparison test was used to calculate statistical significance. **** p < 0.0001. Scale bar = 5 μm.
As an additional assessment to confirm the effects of G4C2 repeat RNA on nuclear expression of POM121, we transfected wildtype iPSNs with stop codon optimized constructs that produce G4C2 repeat RNA and generate pathologic RNA foci, but not DPRs, in immortalized cell lines (Mizielinska et al., 2014) and iPSNs (Fig. S20). Notably, RNA foci were only produced in ~15% of iPSNs (Fig. S20A–B). This is consistent with prior iPSN studies (Donnelly et al., 2013) and human pathology (DeJesus-Hernandez et al., 2017) where only a subset of neurons contain foci despite the notion that every patient neuron expresses the C9orf72 HRE. Using SIM, we found that overexpression of 106 or 288, but not 36, G4C2 RNA repeats in wildtype iPSNs resulted in a variable reduction in the nuclear expression of POM121 after 2 and 7 days of G4C2 repeat RNA expression (Fig. 7E–I). Importantly, overexpression of CGG and CTG repeat RNAs (which cause Fragile X and DM1 muscular dystrophy, respectively) for 7 days in iPSNs did not induce a decrease in POM121 within the nucleus (Fig. S21). Together, these data suggest that reduced nuclear expression of POM121 in spinal neurons is a specific effect of pathological G4C2 repeat RNA.
To assess the contribution of G4C2 repeat RNA itself to cell death, we performed glutamate toxicity assays. While treatment of C9orf72 iPSNs with sense targeting ASOs for 5 days mitigated susceptibility to glutamate induced excitotoxicity (Fig. 7J, Fig. S22A), overexpression of 106 or 288 RNA repeats in wildtype iPSNs variably but significantly increased susceptibility to glutamate induced excitotoxicity (Fig. 7K, Fig. S22B), mirroring the effects on POM121. Collectively, these data suggest that via direct or indirect mechanisms, G4C2 repeat RNA, but not DPRs or loss of C9ORF72 protein, initiates a pathological cascade impacting nuclear expression of POM121 and subsequent cellular toxicity in human neurons.
Discussion
Multiple studies have identified nuclear or cytoplasmic mislocalization and accumulation of some NPC associated proteins in various model systems (Chew et al., 2019; Eftekharzadeh et al., 2018; Hutten and Dormann, 2019; Zhang et al., 2015; Zhang et al., 2018; Zhang et al., 2016; Zhang et al., 2019b). However, many of these reports simply provide an overview of Nup pathology and have focused on a handful of individual Nups or NCT associated proteins. To date, few studies have linked cytoplasmic Nup accumulations to alterations within the nucleus, nuclear envelope, and NPC itself. Thus, a comprehensive evaluation of the expression and localization of individual Nups within the nucleus and NPCs is lacking. Moreover, the mechanisms by which Nup alterations arise in human CNS cell types remain elusive.
Nucleoporin Alterations in C9orf72 ALS/FTD
To evaluate the nuclear distribution and expression of the majority of individual human Nups, we harnessed the power and resolution of SIM. Here we studied >1,000,000 nuclei and comprehensively assessed the actual nuclear repertoire of NPC components in nuclei from iPSNs as well as in postmortem human nervous system tissue from patients harboring the C9orf72 HRE. We found that 8 of the 23 Nups analyzed are reliably and reproducibly, albeit variably, reduced within human neuronal nuclei in a large number of different C9orf72 patient iPSN lines (Fig. 1) and postmortem human motor cortex (Fig. 2) and spinal cord (Fig. S10) without corresponding cytoplasmic mislocalization (Fig. S5, Fig. S11).
Notably, the stable NPC structure is formed early in cellular development and some of its individual Nup components are extremely long lived having half-lives measured in years (Savas et al., 2012; Toyama et al., 2013). The evaluation of Nups at multiple time points in the lifespan of our iPSNs reveals that initial nuclear expression of individual Nups is not disrupted by the C9orf72 HRE, but there is an age-related decrease in specific Nups from the nucleus (Fig. 1). Although expression profiles and synaptic physiology of iPSNs more closely resemble those of young and immature fetal neurons (Ho et al., 2016), the age dependence (day 18 to day 32) of our observed alterations in specific Nups highlights the ability of our iPSN system to capture the maturation of early pathogenic cascades in C9orf72 mediated neurodegeneration. Importantly, our iPSNs are not dying in culture without the addition of exogenous stressors and therefore the alterations in NPC components are not merely reflective of cell death cascades.
Although all 3 Nups of the transmembrane ring (GP210, NDC1, POM121) show decreased nuclear expression, the remainder of the disrupted Nups span across multiple domains of the NPC including the nuclear basket (Nup50, TPR), central channel (Nup98), and outer ring (Nup107, Nup133). Collectively, these Nups are involved in both maintaining the structural integrity and the function of the NPC, as well as NPC independent nuclear functions (Lin and Hoelz, 2019; Raices and D’Angelo, 2012). As a result, this suggests that the combined C9orf72 mediated alterations in individual NPC components themselves are likely to impact multiple cellular processes likely including both NCT and gene expression.
Interestingly, there have been reports that the composition of NPCs varies amongst cell types and that each NPC within a single nucleus in a given cell can vary as individual Nups are replaced (Kinoshita et al., 2012; Rajoo et al., 2018; Toyama et al., 2018). This phenomenon of NPC heterogeneity within individual nuclei may explain differences in Nup staining patterns. Indeed, in accordance with this hypothesis, using SIM we observe heterogeneity of Nup immunostaining within human iPSN nuclei (Fig. S2). Importantly, primary antibodies for this study were selected on the basis of use in prior publications and/or knockdown validation as indicated on manufacturer website (see Tables S5–6 for additional information). However, the possibility remains that some Nup signals are moderately non-specific or the result of partial cross reactivity with other nuclear proteins. Nevertheless, these caveats do not undermine the consistent, although variable, observation that a subset of Nups are reproducibly reduced within human C9orf72 neuronal nuclei in vitro and in vivo as evaluated by multiple experimental methods and antibodies across multiple patient tissue samples and iPSC lines (see Tables S1–2, S6).
Recently, it has been shown that the stoichiometry of Nups within NPCs varies across cell types (Ori et al., 2013; Rajoo et al., 2018). Due to the limitations of SIM, future experiments will be needed to determine the exact stoichiometry of individual Nups within human neurons and NPCs. However, using SEM we show that the overall distribution and architecture of NPCs in the nuclear envelope of C9orf72 iPSNs remains intact (Fig. S8D) despite an average reduction of 50% for 8 individual Nups (Fig. 1E). Although arranged into a highly organized octet structure, the NPC itself can be comprised of multiple copies (8, 16, 32 etc.) of each individual Nup (Hampoelz et al., 2019; Kim et al., 2018; Lin and Hoelz, 2019). While SIM provides for increased resolution in detecting individual spots within isolated nuclei, it remains an open question as to whether these spots represent single or multiple Nup molecules within an NPC. As a result, we propose a scenario whereby a variable number of individual Nup molecules may be missing in each NPC without causing the collapse of the whole structure (see Fig. S7B). Additionally, we cannot rule out the possibility that some Nup spots detected by SIM are not associated with the NPC. Specific Nups have been shown to localize within the nucleus for NPC independent functions in gene expression and cell division (Raices and D’Angelo, 2012). Although human neurons are no longer dividing, it is possible that a subset of Nup spots detected by SIM are not associated with the NPC itself. The methods to reliably detect reduction of a selected Nup from an individual NPC in human iPSNs remain unclear at this time.
Nuclear Reduction of POM121 as an Initiating Pathological Event in C9orf72 ALS/FTD
As opposed to maintenance pathways, the coordinated assembly of the NPC during mitosis and interphase in dividing cells and yeast is more frequently studied (Lin and Hoelz, 2019; Otsuka and Ellenberg, 2018). To begin to investigate Nup relationships and NPC maintenance in human neurons, we overexpressed candidate Nups from multiple domains of the NPC. Using two independent GFP tagged POM121 overexpression plasmids we show that re-introduction of this transmembrane Nup variably re-established the nuclear levels of individual Nups (Fig 4A–T, Fig S15, Table S7) in C9orf72 iPSNs. Consistent with previous reports indicating that POM121 insertion into the nuclear envelope is a critical initiating event in NPC assembly during interphase (Antonin et al., 2005; Doucet et al., 2010; Funakoshi et al., 2011; Talamas and Hetzer, 2011), our data suggest that in human neurons, POM121 is critical for mediating the nuclear levels of individual Nups.
Together, our overexpression and knockdown experiments suggest that nuclear reduction of the transmembrane Nup POM121 is an initiating event in specific Nup alterations in human C9orf72 neurodegeneration. Reduced POM121 alters the human neuronal nuclear repertoire of Nups and it is likely the combinatorial effect of these changes that impacts the subcellular distribution of Ran GTPase. Among other possibilities, disruption of the Ran localization may be the result of alterations to Nups directly involved in NCT and/or a result of potential disruptions in gene transcription that may ultimately impact NCT. Future studies are necessary to define the impact of individual Nup alterations on neuronal gene expression and specific aspects of functional NCT including those involving NTRs and cargo loading/unloading. Collectively, the combined loss of 8 specific Nups and mislocalization of Ran GTPase are likely to impact multiple cellular pathways, the combination of which renders human neurons susceptible to stressor mediated cell death.
G4C2 Repeat RNA Mediated Reduction of POM121 and Initiation of Nuclear Pore Complex Deficits in Human Neurons
It has previously been shown that Nups can modify C9orf72 toxicity in Drosophila photoreceptors (Freibaum et al., 2015) and has been proposed that pathologic DPRs produced by the C9orf72 HRE can interact with Nups to disrupt NCT (Lee et al., 2016; Shi et al., 2017). Although in the current study overexpression of DPRs does not negatively impact POM121, the possibility remains that DPRs may directly impact the localization of other NPC components (e.g. Nup98, NPC associated proteins). Furthermore, it is possible that DPRs may directly contribute to Nup independent alterations in functional NCT potentially via pathologic associations with NTRs which have previously been identified as strong DPR interactors (Lee et al., 2016). Indeed, recent work from our lab shows that arginine rich DPRs Poly(GR) and Poly(PR) can directly interfere with importin beta function in non-neuronal systems (Hayes et al., 2020). As a result, DPR dependent effects on the NCT machinery itself may further compound alterations in NCT that arise as a result of compromised nuclear expression of Nups. This could suggest a two-hit model for disruptions in NCT in C9orf72 ALS/FTD.
We provide multiple lines of evidence suggesting that expression of G4C2 repeat RNA initiates the deficit in nuclear Nup levels (Fig. 7, Fig. S21, Table S9). Notably, these results differ from previous studies in dividing cells or fly models overexpressing only G4C2 repeat RNA where no overt toxicity or degeneration was observed (Mizielinska et al., 2014). In the context of our current study in human spinal neurons, we note that flies do not have a gene encoding a POM121 orthologue and thus, would not be susceptible to this RNA meditated pathologic event. Moreover, we have been unable to observe POM121 reduction in BAC C9orf72 and AAV-(G4C2)149 mouse models (unpublished data). However, we note that human and mouse POM121 only share ~60% protein sequence homology which may not be sufficient for this toxic G4C2 repeat RNA mediated event to occur. Interestingly, nucleofection of even very low amounts of G2C4 repeat RNA only plasmid constructs (antisense strand; generously provided by A. Isaacs) resulted in complete lethality of iPSNs within 24 hours (unpublished observations). Future studies are warranted to explore the mechanisms underlying this robust G2C4 repeat RNA mediated neuronal toxicity.
Although, the reduction in nuclear POM121 levels is specific to the expression of G4C2 repeat RNA in spinal neurons (Fig. 7, Fig. S21, Table S9), it still remains possible that CGG and/or CTG repeat RNAs can elicit similar deficits when expressed in their disease specific neuronal subtype. In addition, we do not observe a decreased in the nuclear levels of POM121 in human iPSC derived astrocyte nuclei or oligodendrocyte nuclei from postmortem motor cortex (Fig. S23A–D). Moreover, overexpression of G4C2 repeat RNA in human astrocytes and HEK293T cells has no effect on the nuclear expression of POM121 (Fig. S23E–H). Together, these data support a role for the cell-type specific nature of G4C2 repeat RNA in the initiation of pathogenic Nup disruption specifically in disease relevant human neuronal subtypes. Given that G4C2 repeat RNA foci are rarely observed in iPSNs and patient tissue (DeJesus-Hernandez et al., 2017; Donnelly et al., 2013), future experiments are necessary to determine if reduced nuclear POM121 is the result of direct interactions between POM121 and soluble or foci accumulated G4C2 or G2C4 RNA. While we have repeatedly attempted to perform FISH/IF to address the possibility of an interaction between RNA foci and POM121, these experiments are technically challenging in iPSN systems and were unrevealing. Alternatively, it is equally possible that indirect interactions via intermediate proteins and/or RNAs may contribute to G4C2 repeat RNA mediated POM121 reduction. Our previous work did not identify POM121 as a direct interactor of G4C2 repeat RNA (Zhang et al., 2015), suggesting that C9orf72 HRE mediated effects on the nuclear expression of POM121 could be due to indirect interactions between G4C2 repeat RNA and POM121 and may involve currently unidentified proteins and/or RNAs.
Collectively, our data support a role for G4C2 repeat RNA in early pathogenic disruptions in ALS. Furthermore, our data highlight the importance of POM121 in the initiating events leading to overall disruptions in nuclear Nup levels, Ran GTPase localization, and downstream impaired cellular survival in the pathogenesis of C9orf72 ALS/FTD within human neurons.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jeffrey D Rothstein (jrothstein@jhmi.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate or analyze datasets or code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
iPSC Neuron Differentiation
Peripheral blood mononuclear cell (PBMC)-derived iPSC lines from C9orf72-ALS patients and non-neurological controls were obtained from the Cedars-Sinai Answer ALS repository (see Table S1 for demographic information). Fibroblast derived isogenic iPSC lines were a kind gift from Kevin Talbot (Ababneh et al., 2020). The C9orf72 null line was a gift from Justin Ichida. While Nup alterations were reproducible across all C9orf72 iPSC lines evaluated, the influence of sex was not investigated in this study. iPSCs were maintained in MTeSR according to standard Cedars Sinai protocols and differentiated into spinal neurons according to the direct induced motor neurons (diMNs) protocol (Fig. S1A), which generates a mixed population consisting of 20-30% islet-1 positive motor neurons. Briefly, iPSC colonies were maintained on Matrigel coated 10 cm dishes for three weeks before passaging for differentiation. Once iPSC colonies reached 30-40% confluence (about 4-5 days after passaging), stage 1 media consisting of 47.5% IMDM (Gibco), 47.5% F12 (Gibco), 1% NEAA (Gibco), 1% Pen/Strep (Gibco), 2% B27 (Gibco), 1% N2 (Gibco), 0.2 μM LDN193189 (Stemgent), 10 μM SB431542 (StemCell Technologies), and 3 μM CHIR99021 (Sigma Aldrich) was added and exchanged daily until day 6. On day 6 of differentiation, cells were incubated in StemPro Accutase (Gibco) for 5 minutes at 37°C. Cells were collected from plates and centrifuged at 500 × g for 1.5 minutes. Cells were plated at 1 × 10Λ6 cells per well of a 6 well plate or 5 × 10Λ6 cells per T25 flask in stage 2 media consisting of 47.5% IMDM (Gibco), 47.5% F12 (Gibco), 1% NEAA (Gibco), 1% Pen/Strep (Gibco), 2% B27 (Gibco), 1% N2 (Gibco), 0.2 μM LDN193189 (Stemgent), 10 μM SB431542 (StemCell Technologies), 3 μM CHIR99021 (Sigma Aldrich), 0.1 μM all-trans RA (Sigma Aldrich), and 1 μM SAG (Cayman Chemicals). Media was exchanged daily until day 12. For the majority of experiments, on day 12 of differentiation, cells were switched to stage 3 media consisting of 47.5% IMDM (Gibco), 47.5% F12 (Gibco), 1% NEAA (Gibco), 1% Pen/Strep (Gibco), 2% B27 (Gibco), 1% N2 (Gibco), 0.1 μM Compound E (Millipore), 2.5 μM DAPT (Sigma Aldrich), 0.1 μM db-cAMP (Millipore), 0.5 μM all-trans RA (Sigma Aldrich), 0.1 μM SAG (Cayman Chemicals), 200 ng/mL Ascorbic Acid (Sigma Aldrich), 10 ng/mL BDNF (PeproTech), 10 ng/mL GDNF (PeproTech). For all whole cell imaging (TDP-43, Ran), cells were trypsinized and plated in 24 well optical bottom plates (Cellvis) at a density of 250,000 cells per well. Stage 3 media was exchanged every 3 days for the duration of the experiment. All cells were maintained at 37°C with 5% CO2 until day 18, 32, or 46 of differentiation as indicated by each experiment. iPSCs and iPSNs routinely tested negative for mycoplasma.
Human Tissue Immunofluorescence
Postmortem paraffin embedded motor cortex (See Table S2 for demographic information) was rehydrated with xylene 3X 5 mins, 100% ethanol 2X 5 mins, 90% ethanol 5 mins, 70% ethanol, dH2O 3X 5 mins. Antigen retrieval was performed using Tissue-Tek antigen retrieval solution (IHC World) for 1 hour in a steamer. Slides were cooled for 10 mins, washed 3X 5 mins with dH2O followed by 2X 5 mins in 1X PBS. Tissue was permeabilized with 0.4% Triton X-100 in 1X PBS for 10 mins with gentle agitation on a shaker. Slides were washed 3X 5 mins with 1X PBS and blocked in DAKO protein-free serum block (DAKO) overnight in a humidified chamber at 4°C. Tissue was incubated in primary antibody diluted in DAKO antibody diluent reagent with background reducing components (DAKO) for 12 hours at room temperature with gentle agitation on a shaker followed by 2 days at 4°C (See Table S5 for antibody information). Slides were washed 3X 5 mins in 1X PBS and incubated in secondary antibody diluted in DAKO antibody diluent reagent with background reducing components (DAKO) for 1 hour at room temperature with gentle agitation on a shaker (See Table S5 for antibody information). Slides were washed 3X 5 mins in 1X PBS, rinsed briefly with autofluorescence eliminator reagent (Millipore), washed 5X 5 mins in 1X PBS, stained with Hoescht diluted 1:1000 in 1X PBS for 20 mins, and washed 3X 5 mins in 1X PBS. Slides were coverslipped using Prolong Gold Antifade Reagent with DAPI and nuclei from Map2 positive Layer V neurons were imaged with a Zeiss Axioimager Z2 fluorescent microscope equipped with an apotome2 module. POM121 nuclear intensity was quantified using FIJI. While POM121 alterations were reproducible across all C9orf72 ALS patients, the influence of sex was not investigated in this study. Images presented are maximum intensity projections generated in Zeiss Zen Blue 2.3.
METHODS DETAILS
ASO Treatment of iPSC Derived Neurons
Scrambled ASO (676630): CCTATAGGACTATCCAGGAA and G4C2 ASO (619251): CAGGCTGCGGTTGTTTCCCT were provided by Ionis Pharmaceuticals. For 5 day ASO treatment, 5 μM scrambled or sense targeting ASO was added to the culture media on day 30. Media was exchanged and ASO replaced on day 32 and experiments were conducted on day 35 of differentiation.
RT-qPCR
Samples were harvested in 1× DPBS with calcium and magnesium, then spun down in a microcentrifuge and the DPBS was aspirated. RLT Buffer (500 μL) was added to samples and RNA was isolated with an RNeasy kit (QIAGEN). RNA concentrations were determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). For C9orf72 detection, 1 μg RNA was used for cDNA synthesis using gene specific primers and a Superscript IV First-Strand cDNA Synthesis System (Thermo Fisher Scientific). RT-qPCR reactions were carried out using TaqMan Gene Expression Master Mix (TaqMan) and an Applied Biosystems Step One Plus Real Time PCR Machine (Applied Biosystems) using previously described primer/probe sets (see Table S3 for sequences) (Lagier-Tourenne et al., 2013). Levels of C9orf72 transcripts were normalized to GAPDH. For Nup mRNA detection, 1 μg RNA was used for cDNA synthesis using random hexamers and a Superscript IV First-Strand cDNA Synthesis System (Thermo Fisher Scientific). RT-qPCR reactions were carried out using TaqMan Gene Expression Master Mix (TaqMan) and an Applied Biosystems Step One Plus Real Time PCR Machine (Applied Biosystems) using TaqMan Gene Expression Assays (see Table S4) Levels of Nup transcripts were normalized to GAPDH.
Actinomycin D Treatment
On day 32 of differentiation, control and C9orf72 iPSNs were incubated with 10 μg/mL Actinomycin D. Samples were harvested following 0.5, 1, 2, 3.5, and 5 hour incubation with Actinomycin D. RNA was isolated using standard TRIzol-LS (Thermo Fisher Scientific) phenol-chloroform extraction and 1 μg RNA was used for cDNA synthesis using a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific). RT-qPCR was performed as described above. Nup mRNA was normalized to RPS18.
Polyribosome Fractionation
On day 32 of differentiation, iPSNs were incubated with 100 μg/mL cycloheximide for 5 minutes. iPSNs were subsequently washed with ice cold 1X DPBS containing 100 100 μg/mL cycloheximide and harvested with a cell scraper. iPSNs were pelleted by centrifugation at 500 × g for 5 min and washed with ice cold 1X DPBS supplemented with 100 μg/mL cycloheximide. Following pelleting by centrifugation, DPBS was aspirated and iPSNs were lysed in 400 μL ice cold polysome lysis buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 1X protease inhibitor cocktail set III, EDTA-free (Millipore), 100 μg/mL cycloheximide, 1 mM DTT, 25 U/mL TurboDNase, 20 U/mL RNase inhibitor (RNaseOUT, Thermo Fisher Scientific) by trituration through a 27-gauge needle 6-8 times. Lysates were snap frozen in liquid nitrogen and stored at −80°C until use. Lysates were clarified by centrifugation at 15,000 × g at 4 °C for 5 min. 50 μl lysate was reserved for inputs and 350 μl lysate used for fractionation. For fractionation, a10-50% (w/v) sucrose gradient was prepared in a polysome buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 5mM MgCl2, 1X protease inhibitor cocktail, cycloheximide 100 μg/ml, 1 mM DTT, and 20 U/ml RNaseOUT. 350 μl lysate were loaded on the sucrose gradient and centrifuged at 35,000 × g with a Beckman SW41 rotor at 4 °C for 3 h. Fractions were collected from the top and UV absorbance monitored using a Gradient Station (BioCamp) equipped with ECONO UV monitor (BioRad). 500 μl fraction were collected using a FC203B (Gilson) fraction collector. RNA was isolated using TRIzol-LS (Thermo Fisher Scientific) and standard phenol-chloroform extraction. cDNA synthesis was performed using a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific). RT-qPCR was performed as described above. Nup mRNA in each fraction was normalized to 18S rRNA.
Meso Scale Discovery (MSD) ELISA
All reagents were purchased from MSD unless otherwise noted. 96-well small spot streptavidin coated plates were blocked overnight at 4° in 1% blocker A in PBS. Wells were washed 3X in PBS-0.05% Tween (PBST) and then coated with 0.35 μg/mL biotinylated rabbit anti-GP antibody (Cheng et al., 2019) for 1 hour at room temperature. Following 3 washes with PBST, cell lysates were added to wells in duplicate. A standard curve of GP8 peptide was included on each plate. Plates were incubated for 2 hours at room temperature, wells were washed three times with PBST, and incubated with 0.25 μg/mL sulfo-tagged detection antibody (Cheng et al., 2019) for 1 hour. Wells were washed 3X with PBST and 150 μL read buffer was added.
Samples were immediately imaged using MESO QuickPlex SQ 120. Specificity was verified using lysates of HEK cells overexpressing GFP-tagged dipeptide repeat proteins. Linearity across range of interest assessed by serial dilution.
Western Blots
Samples were harvested in 1× PBS, then spun down in a microcentrifuge and the PBS was aspirated. Samples were resuspended in RIPA buffer (Millipore Sigma) containing 1× protease inhibitor cocktail (Roche). Samples were spun at 12,000g for 15 minutes to remove debris, and the supernatant was transferred to a new tube. 6× Laemmli buffer (12% SDS, 50% glycerol, 3% Tris-HCl pH 7.0, 10% 2-mercaptoethanol in dH2O, bromophenol blue to color) was added to a final 1× concentration. Equal protein (by mass for quantifiable samples, by volume for unquantifiable samples) was loaded into 4-20% acrylamide gels and run until the dye front reached the bottom. Protein was transferred onto nitrocellulose membrane (Bio-Rad). Following transfer, blots were blocked for 1 hour in 5% milk in 1x TBS-Triton (0.1%). Blots were probed with primary antibody (see Table S5) overnight (approximately 16 hours) at 4 degrees. Blots were washed four times in 1× TBST for 10 minutes each, probed with secondary antibody for 1 hour at room temperature, and washed another four times in 1× TBST for 10 minutes each. ECL substrate (Millipore Sigma, Thermo Fisher Scientific) was applied for 30 seconds, then images were taken using the GE Healthcare ImageQuant LAS 4000. Images were quantified using FIJI. For nuclei westerns, nucleoporins were normalized to total protein levels using the BLOT-FastStain Kit (G-Biosciences). For Trim Away westerns, GAPDH was used for normalization.
Nuclei Isolation and Super Resolution Structured Illumination Microscopy
Nuclei were isolated using Nuclei Pure Prep Nuclei Isolation Kit (Sigma Aldrich) using a 1.85 M sucrose gradient to enrich for neuronal nuclei. A 1.8 M gradient was used for astrocyte and HEK293T nuclei and a 1.9 M gradient was used for oligodendrocyte nuclei. For preparation of iPSN, astrocyte, or HEK293T lysates, media was aspirated from each well and cells were briefly rinsed with 1X PBS. iPSNs were scraped in lysis buffer using a cell scraper, transferred to a 50 mL conical tube, and vortexed. For preparation of postmortem human brain lysates, 75-100 mg of fresh frozen tissue was homogenized in lysis buffer using a dounce homogenizer. Sucrose gradients were assembled and centrifuged following the Nuclei Pure Prep Nuclei Isolation Kit protocol (Sigma Aldrich). Samples were centrifuged at 15,600 rpm and 4°C using a Swi32T swinging bucket rotor and Beckman ultracentrifuge (Beckman Coulter) for 45 minutes. Following isolation, nuclei were resuspended in 1 mL of Nuclei Storage Buffer and 25-100 μL was spun onto slides coated with 1 mg/mL collagen (Advanced Biomatrix) using a CytoSpin 4 Centrifuge (Thermo Fisher Scientific). Nuclei were fixed in 4% PFA for 5 minutes, washed with 1X PBS 3X 10 minutes, permeabilized with 1X PBST containing 0.1% Triton X-100 for 15 minutes, blocked in 10% normal goat serum diluted in 1X PBS for 1 hour, and incubated in primary antibody overnight at 4°C (See Table S5 for antibody information). The next day, nuclei were washed with 1X PBS 3X 10 minutes, incubated in secondary antibody (See Table S5 for antibody information) for 1 hour at room temperature, and washed in 1X PBS 3X 10 minutes. Nuclei were coverslipped using Prolong Gold Antifade Reagent (Invitrogen) and 18 mm × 18 mm 1.5 high tolerance coverslips (MatTek). NeuN or GFP positive nuclei were identified looking through microscope eyepieces and a single z slice image (~110 nm thick) was acquired with widefield imaging on a Zeiss ELYRA S1. Only the Nup channel(s) subsequently imaged by super resolution structured illumination microscopy (SIM) with 5 grid rotations and optimal z sectioning using a Zeiss ELYRA S1. SIM images were constructed using default parameters with Zeiss Zen Black 2.3 SP1 software as per Zeiss recommendation. Each image acquired contained an individual NeuN or GFP positive nucleus to allow for the most accurate processing and 3D reconstruction. All images for a given Nup were imaged and acquired with identical parameters. Nucleoporin spots and volume quantification was automated using Imaris version 9.2.0 (Bitplane) and the 3D suite in FIJI 1.52i and 1.52p as previously described (Eftekharzadeh et al., 2018). Briefly, nucleoporin spots were counted using automated spot detection in which a Bayesian classifier which takes into account features such as volume, average intensity, and contrast was applied to detect and segment individual spots. The total number of nucleoporin spots was determined using a 3D-rendering of super resolution SIM images where z-slices were taken through the entire nucleus. When individual nucleoporin spots could not be resolved due to limits of resolution of immunofluorescent SIM, the percent total nuclear volume occupied by the nucleoporin was calculated. X and Y axis length was measured in the center z-slice for each nucleus and Z axis length was estimated by multiplying z-slice thickness by the number of z slices imaged for each nucleus. These measurements were used to calculate total nuclear volume. For volume of the nucleoporin, automatic thresholding was applied and 3D suite in FIJI was used to determine the volume of the thresholded area for each nucleus. Nucleoporin volume was divided by total nuclear volume to yield a percentage of the total nuclear volume occupied by a given nucleoporin. In total across all experiments, over 1,000,000 nuclei were imaged and analyzed. Images presented are 3D maximum intensity projections generated in Zeiss Zen Black 2.3 SP1. With the exception Figure 6C, nucleoporin images were faux colored green for contrast and display purposes.
Immunofluorescent Staining and Confocal Imaging
On day 12 of differentiation, iPSNs were plated in 24 well optical bottom plates (Cellvis). iPSNs were fixed in 4% PFA for 15 minutes, washed with 1X PBS 3X 10 minutes, permeabilized with 1X PBST containing 0.3% Triton X-100 for 15 minutes, blocked in 10% normal goat serum diluted in 1X PBS for 1 hour, and incubated in primary antibody for 2 hours at room temperature (See Table S5 for antibody information). iPSNs were then washed with 1X PBS 3X 10 minutes, incubated in secondary antibody (See Table S5 for antibody information) for 1 hour at room temperature, washed in 1X PBS 2X 10 minutes, incubated with Hoescht diluted 1:1000 in 1X PBS for 10 minutes, and washed in 1X PBS 2X 10 minutes. iPSNs were mounted using Prolong Gold Antifade Reagent with DAPI. iPSNs were imaged using a Zeiss LSM 800 confocal microscope and nuclear intensity and N/C ratios were calculated with FIJI as previously described (Zhang et al., 2015). Images presented are maximum intensity projections generated in Zeiss Zen Blue 2.3.
Plasmids and Nucleofection for Nup, G4C2 repeat RNA, and DPR Overexpression
iPSNs were dissociated with accutase and transfected in suspension using the Lonza nucleofection system (program DC104) and a Lonza P3 Primary Cell 4D Nucleofector Kit (Lonza). GFP tagged POM121, Nup133, NDC1, GP210, and Nup98 plasmids were obtained from Origene Technologies. Additional GFP tagged POM121 and sPOM121 plasmids (Franks et al., 2016) were a kind gift from Martin Hetzer. GFP control plasmids were obtained from Origene Technologies, Addgene, and Lonza. GFP tagged Poly(GA)50, Poly(GR)50, and Poly(PR)50 DPR plasmids (Wen et al., 2014) were a kind gift from Davide Trotti and (G4C2)n RNA only plasmids (Mizielinska et al., 2014) were a kind gift from Adrian Isaacs. The (CGG)99 plasmid was obtained from Addgene and the (CTG)202 plasmid (Nutter et al., 2019; Osborne and Thornton, 2008) was a kind gift from Maurice Swanson. For Nup overexpression experiments, 5 × 106 iPSNs were nucleofected with 4 μg of DNA on day 30 of differentiation. Expression was observed at day 31 and iPSNs were assayed at day 46 of differentiation. For DPR overexpression experiments, 5 × 106 iPSNs were nucleofected with 4 μg or 1 μg of DNA on day 18 and assayed at day 20 or 25 of differentiation respectively. For RNA only experiments, 5 × 106 iPSNs were nucleofected with 4 μg or 1 μg of DNA on day 18 and assayed at day 20 or 25 of differentiation respectively. For CGG and CTG overexpression experiments, 5 × 106 iPSNs were nucleofected with 1 μg of DNA on day 18 and assayed at day 25 of differentiation. Following nucleofection of all overexpression plasmids, media was exchanged every 48 hours until iPSNs were assayed unless otherwise noted.
Knockdown of Nups by Trim Away
Trim21 mediated knockdown of Nups was modified from a previously described protocol (Clift et al., 2017). POM121 (Thermo Fisher Scientific), Nup133 (Santa Cruz Biotechnology), NDC1 (Novus Biologicals), and GAPDH (Cell Signaling) antibodies were dialyzed using Slide-A-Lyzer MINI Dialysis Device, 20K MWCO (Thermo Fisher Scientific). Following dialysis, antibodies were concentrated using Amicon Ultra-0.5 Centrifugal Filter Unit with Ultracel-100 membrane (Millipore). Antibody concentration was determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific) as described (Clift et al., 2017). On day 18 of differentiation, 5 × 106 iPSNs were nucleofected with 5 μg of antibody and 4 μg Trim21 GFP plasmid DNA (Origene Technolgies) with Lonza P3 Primary Cell 4D Nucleofector Kit (Lonza) using program DC154. The next day, Trim21 GFP expression was observed and iPSNs were assayed on day 20 of differentiation.
NLS-tdTomato-NES (S-tdTomato) Nucleocytoplasmic Transport Assay
On day 32 of differentiation, control and C9orf72 iPSNs were nucleofected using the Lonza nucleofection system (program DC104) and a Lonza P3 Primary Cell 4D Nucleofector Kit (Lonza) as described above. One day later, on day 33 of differentiation, iPSNs were transduced with NLS-tdTomato-NES (S-tdTomato) lenti virus as previously described (Zhang et al., 2015). On days 34, 36, and 43 of differentiation (1, 3, and 10 or 2, 5, and 11 days post S-tdTomato transduction or POM121 OE respectively), iPSNs were fixed with 4% PFA for 15 mins, washed 3X 10 mins with 1X PBS and then incubated with Hoescht diluted 1:1000 in 1X PBS for 10 minutes, and washed in 1X PBS 2X 10 minutes. iPSNs were then immediately imaged using a Zeiss LSM 800 confocal microscope and the N/C ratio of S-tdTomato was calculated with FIJI as previously described (Zhang et al., 2015).
Glutamate Toxicity
On day 12 of differentiation, iPSNs were plated in 24 well optical bottom plates (Cellvis) at a density of 250,000 neurons per well. Neurons were rinsed with 1X PBS and fed with fresh stage 3 media daily remove dead cells and debris prior to incubation with glutamate. For POM121 overexpression experiments, iPSNs were split using accutase to dissociate cells and help promote single cell suspension, transfected as described above, and plated into optical bottom plates on day 30 of differentiation. For these experiments, iPSNs were washed with 1X PBS/fed daily from day 32-46 before the toxicity experiments were performed. Daily washes were performed to remove cells that died as a result of passage and nucleofection stress thus ensuring that on the day of the experiment, we were only imaging PI incorporation in cells that died as a result of glutamate exposure. As a result of accumulated death over the 2 week period post-nucleofection, we note that the cells remaining on the day of the toxicity experiment are only a subset of the initial population dissociated and nucleofected. For a schematic representation of this experimental paradigm please see the flow chart (Fig. S24) contained within the experimental procedures. For 5 day ASO treatment experiments, iPSNs were treated with 5 μM ASO on day 30 and 32 during which time they were washed with 1X PBS. For G4C2 Repeat RNA only experiments, iPSNs were split and transfected with 1 μg of DNA on day 18 as described above. iPSNs were washed/fed daily from day 19-25 before the toxicity experiments were performed. On the day of the experiment (day 46 for POM121 OE, day 35 for 5 Day ASO, day 25 for G4C2 Repeat RNA only), iPSNs were washed with 1X PBS to remove any remaining debris and dead cells. Media was replaced with ACSF (Tocris) containing 0, 1, 10, or 100 μM glutamate (Sigma Aldrich). iPSNs were incubated at 37°C with 5% CO2 for 4 hours. After 3.5 hours, one drop of NucBlue Live ReadyProbes (Thermo Fisher Scientific) and 1 μM propidium iodide (Thermo Fisher Scientific) and returned to the incubator for 30 mins. iPSNs were imaged in an environmentally controlled chamber with a Zeiss LSM 800 confocal microscope. 10 images were taken and analyzed per well. PI and DAPI spots were counted using FIJI. For POM121 overexpression experiments, following live imaging, PI and NucBlue were washed out of neurons and iPSNs were subsequently immunostained for GFP and Map2 as described above and imaged with a Zeiss LSM 800 confocal microscope.
Scanning Electron Microscopy
Previously published protocols (Fichtman et al., 2019; Fichtman et al., 2014) were modified to expose iPSN nuclei for high resolution imaging. Briefly, day 32 control and C9orf72 iPSNs were frozen in growth medium supplemented with 10% DMSO, shipped on dry ice, and stored under liquid nitrogen until use. Thawed cells were pelleted, resuspended in mildly hypotonic buffer in the absence of detergents, and gently passed through a 21-gauge needle. The specimens were then centrifuged onto the surface of 5 × 5 mm2 silicon chips (Ted Pella), fixed, and further processed for electron microscopy as previously described (Fichtman et al., 2019; Fichtman et al., 2014), including controlled dehydration through a graded series of ethanol solutions and critical-point drying on a K850 apparatus (Quorum Technologies). The specimens were then coated with a ~1 nm thick layer of iridium using a Q150T turbo-pumped sputter coater (Quorum Technologies) and imaged on a Merlin field emission scanning electron microscope (Zeiss) equipped with a secondary electron in-lens detector.
Dextran Exclusion
On day 32 of differentiation, iPSNs were rinsed briefly with 1X PBS to remove remaining traces of media. iPSNs were permeabilized in permeabilization buffer (10 mM Tris pH 7.5, 10% OptiPrep, ultrapure water) containing 200 μg/mL digitonin on ice for 5 mins. iPSNs were then rinsed with ice cold transport buffer (4 mM HEPES-KOH pH 7.5, 22 mM KOAc, 0.4 mM Mg(OAc)2, 1 mM NaOAc, 0.1 mM EGTA, 50 mM sucrose) 2X 5 mins. Permeabilized iPSNs were incubated with 0.6 mg/mL fluorescent dextrans diluted in transport buffer for 25 mins at room temperature protected from light. During this time, one drop of NucBlue was added to each well. Following incubation with dextrans and NucBlue, iPSNs were imaged using a Zeiss LSM 800 confocal microscope and a 40X objective. Nuclear intensity of fluorescent dextrans was determined with FIJI.
RNA FISH
Following one week of (G4C2)n repeat RNA overexpression, iPSNs were rinsed briefly in 1X PBS and fixed in 4% paraformaldehyde in 1X PBS for 15 minutes at room temperature. iPSNs were then rinsed 3X 5 mins with 1X PBS, permeabilized with 1X PBS containing 0.3% Triton X-100 for 15 mins at room temperature, and subsequently washed 3X 5 mins with 1X PBS. iPSNs were then equilibrated in 1X SSC for 10 mins at room temperature. For RNase A treatment, 40 μg/mL RNase A (Thermo Fisher Scientific) in 1X SSC was added to corresponding wells and iPSNs were incubated at 37°C for 45 mins. RNase treated iPSNs were washed briefly 2X with 1X SSC and then equilibrated in 1X SSC for 10 mins at room temperature. iPSNs were then equilibrated in 50% formamide in 2X SSC for 10 mins at 60°C and then hybridized with hybridization buffer (40% formamide, 2 mg/mL heat shock inactivated BSA (Sigma Aldrich), 1 mM ribonucleoside-vanadyl complex (New England BioLabs), 10 mM NaPO4, 1X SSC) and probe mixture (10 μg salmon sperm DNA (Thermo Fisher Scientific), 10 μg E.coli tRNA (Millipore), 80% formamide, 0.2 μM custom LNA probe) for 1 hour at 60°C. Custom 5’ DIG labeled LNA probes (Qiagen) are as follows: G4C2 probe: CCCCGGCCCCGGCCCC lot number 302094012, scrambled probe: GTGTAACACGTCTATACGCCCA lot number 302495047. iPSNs were then washed 1X 20 mins with 50% formamide in 2X SSC at 65°C, 1X 15 mins with fresh 50% formamide in 2X SSC at 65°C, 1X 10 mins with 1X SSC in 40% formamide at 60°C, 3X briefly with 1X SSC at room temperature, 2X 5 mins with 1X SSC at room temperature, 1X 5 mins with TBS (50 mM Tris pH 7.5, 15 mM NaCl), 1X 5 mins with Tris Glycine (0.75% glycine, 0.2 M Tris pH 7.5), post-fixed with 3% paraformaldehyde in 1X PBS, and briefly rinsed 3X with 1X PBS. iPSNs were blocked with 1% normal goat serum and 5% heat shock inactivated BSA in TBS for 1 hour at room temperature and then incubated in 1:500 Mouse Anti-DIG (Jackson Immunoresearch) diluted in immunofluorescence buffer (2% heat shock inactivated BSA, TBS) overnight at 4°C. iPSNs were then washed 3X 5 mins with immunofluorescence buffer at room temperature and incubated with secondary antibody (1:500 Goat Anti-Mouse Alexa 568) diluted in immunofluorescence buffer for 30 mins at room temperature before undergoing a series of washes: 1X 5 mins TBS, 1X 5 mins Tris Glycine, 1X 5 mins PBS MgCl2 (2 mM MgCl2, 1X PBS), 1X 5 mins PBS, 1X 5 mins PBS with 1:1000 Hoescht, 1X 5 mins PBS. iPSNs were mounted with prolong gold antifade reagent with DAPI and imaged using a Zeiss LSM 800 confocal microscope and 63X objective. All solutions were made using RNase free water.
Evaluation of POM121 in Astroglia, Oligodendroglia, and HEK293T Cells
iPSC derived astrocytes were generated as previously described (Zhang et al., 2015). Primary human astrocytes (ScienCell) were cultured according to manufacturer instructions. HEK293T cells were obtained from ATCC and maintained in DMEM containing Pen/Strep and 10% FBS. Primary human astrocytes were nucleofected with G4C2 repeat RNA only constructs as described above. For overexpression of G4C2 repeat RNA only constructs in HEK293T cells, Lipofectamine 3000 (Thermo Fisher Scientific) was used according to manufacturer protocol. Nuclei from all cell types were isolated using the Nuclei PURE Prep Nuclei Isolation Kit (Sigma Aldrich) and imaged by SIM as described above. Postmortem oligodendrocyte nuclei were isolated from postmortem motor cortex and selected on the basis of Olig2 positive immunoreactivity.
QUANTIFICATION AND STATISTICAL ANALYSIS
All data analysis was conducted with Imaris version 8.3.1 or FIJI as described in individual experimental sections above. Analysis was either completely automated or blinded to eliminate bias. All statistical analyses were performed using GraphPad Prism version 7 and 8 (GraphPad). Statistical analyses were performed with regards to individual iPSC lines or patients such that the average of all nuclei or cells evaluated per iPSC line or patient represents N = 1 with total N per experiment and group as indicated in figure legends. Student’s t-test, One-way ANOVA with Tukey’s multiple comparison test, or Two-way ANOVA with Tukey’s multiple comparison test was used as described in figure legends. * p < 0.05, ** p < 0.005, *** p < 0.0005, **** p < 0.0001. Violin plots are used to display the full spread and variability of large data sets (>10 data points). As opposed to bar graphs with individual data points representing the average value for each iPSC line analyzed (e.g. the exact graphical representation of our statistical analyses descried above), violin plots were chosen to provide full transparency with regard to the true variability of each individual nucleus/cell. Center dotted line indicates median value. Two additional dotted lines indicate the the 25th and 75th percentiles. For smaller data sets, bar graphs displaying individual data points shown where error bars represent +/− SEM.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit Anti-Nup50 | Abcam | Cat#ab137092 |
| Rabbit Anti-Nup153 | Abcam | Cat#ab84872; RRID: AB_1859766 |
| Mouse Anti-TPR | Santa Cruz Biotechnology | Cat#sc-271565; RRID: AB_10649043 |
| Rabbit Anti-Nup54 | Abcam | Cat#ab220890 |
| Mouse Anti-NUPL1 | Abcam | Cat#ab69954; RRID: AB_1269611 |
| Rat Anti-Nup62 | Millipore | Cat#MABE1043 |
| Rat Anti-Nup98 | Abcam | Cat#ab50610; RRID: AB_881769 |
| Rabbit Anti-GP210 | Abcam | Cat#ab15601; RRID: AB_2236461 |
| Rabbit Anti-TMEM48 | Thermo Fisher Scientific | Cat#PA5-67130; RRID: AB_2664610 |
| Rabbit Anti-TMEM48 | Novus Biologicals | Cat#NBP1-91604; RRID: AB_11042869 |
| Rabbit Anti-TMEM48 | Novus Biologicals | Cat#NBP1-91603; RRID: AB_11030742 |
| Rabbit Anti-POM121 | Thermo Fisher Scientific | Cat#PA5-36498; RRID: AB_2553555 |
| Rabbit Anti-POM121 | Novus Biologicals | Cat#NBP2-19890 |
| Mouse Anti-Nup93 | Santa Cruz Biotechnology | Cat#sc-374399; RRID: AB_10990113 |
| Rabbit Anti-Nup155 | Abcam | Cat#ab199528 |
| Rabbit Anti-Nup188 | Thermo Fisher Scientific | Cat#PA5-63984; RRID: AB_2644894 |
| Mouse Anti-Nup205 | Santa Cruz Biotechnology | Cat#sc-377047 |
| Rabbit Anti-AHCTF1 | Thermo Fisher Scientific | Cat#PA5-56812; RRID: AB_2637780 |
| Rabbit Anti-Nup37 | Thermo Fisher Scientific | Cat#PA5-66007; RRID: AB_2664134 |
| Rabbit Anti-Nup43 | Abcam | Cat#ab69447; RRID: AB_1269608 |
| Mouse Anti-Nup107 | Thermo Fisher Scientific | Cat#MA110031; RRID: AB_2154446 |
| Mouse Anti-Nup133 | Santa Cruz Biotechnology | Cat#sc-376699; RRID: AB_11149388 |
| Rabbit Anti-Nup133 | Abcam | Cat#ab155990 |
| Rabbit Anti-Nup160 | Abcam | Cat#ab74147; RRID: AB_1524055 |
| Rabbit Anti-Nup88 | Abcam | Cat#ab79785; RRID: AB_2042496 |
| Rabbit Anti-Nup214 | Bethyl Laboratories | Cat#A300-716A; RRID: AB_533409 |
| Mouse Anti-RANBP2 | Santa Cruz Biotechnology | Cat#sc-74518; RRID: AB_2176784 |
| Mouse Anti-414 | Abcam | Cat#ab50008; RRID: AB_881761 |
| Chicken Anti-NeuN | Millipore | Cat#ABN91; RRID: AB_11205760 |
| Chicken Anti-GFP | Millipore | Cat#AB16901; RRID: AB_90890 |
| Mouse Anti-TurboGFP | Origene Technologies | Cat#TA150041; RRID: AB_2622256 |
| Rabbit Anti-TurboGFP | Thermo Fisher Scientific | Cat#PA5-22688; RRID: AB_2540616 |
| Rabbit Anti-TDP-43 | ProteinTech | Cat#10782-2-AP; RRID: AB_615042 |
| Guinea Pig Anti-Map2 | Synaptic Systems | Cat#188004; RRID: AB_2138181 |
| Mouse Anti-Ran | BD Biosciences | Cat#610341; RRID: AB_397731 |
| Mouse Anti-SMI32 | Covance | Cat#SMI-32P; RRID: AB_2314912 |
| Mouse Anti-Tuj1 | Sigma Aldrich | Cat#MAB1637; RRID: AB_2210524 |
| Rat Anti-NKX6.1 | DSHB | Cat#F55A12; RRID: AB_532379 |
| Goat Anti-Islet1 | R&D Systems | Cat#AF1837; RRID: AB_2126324 |
| Goat Anti-Olig2 | R&D Systems | Cat#AF2418; RRID: AB_2157554 |
| Rabbit Anti-C9ORF72 | ProteinTech | Cat#22637-1-AP; RRID: AB_10953528 |
| Mouse Anti-GAPDH | Life Technologies | Cat#AM4300; RRID: AB_2536381 |
| Rabbit Anti-Alpha Tubulin | Cell Signaling | Cat#2125; RRID: AB_2619646 |
| Mouse Anti-DIG | Jackson Immunoresearch | Cat#200-002-156; RRID: AB_2339005 |
| Goat Anti-Mouse Alexa 488 | Invitrogen | Cat#A11029; RRID: AB_138404 |
| Goat Anti-Rabbit Alexa 488 | Invitrogen | Cat#A11034; RRID: AB_2576217 |
| Goat Anti-Rat Alexa 488 | Invitrogen | Cat#A11006; RRID: AB_2534074 |
| Goat Anti-Chicken Alexa 488 | Invitrogen | Cat#A11039; RRID: AB_142924 |
| Goat Anti-Mouse Alexa 568 | Invitrogen | Cat#A11031; RRID: AB_144696 |
| Goat Anti-Rabbit Alexa 568 | Invitrogen | Cat#A11036; RRID: AB_143011 |
| Goat Anti-Rat Alexa 568 | Invitrogen | Cat#A11077; RRID: AB_141874 |
| Goat Anti-Guinea Pig Alexa 568 | Invitrogen | Cat#A11075; RRID: AB_141954 |
| Goat Anti-Mouse Alexa 647 | Invitrogen | Cat#A21236; RRID: AB_141725 |
| Goat Anti-Rabbit Alexa 647 | Invitrogen | Cat#A21245; RRID: AB_141775 |
| Goat Anti-Rat Alexa 647 | Invitrogen | Cat#A21247; RRID: AB_141778 |
| Goat Anti-Chicken Alexa 647 | Invitrogen | Cat#A21449; RRID: AB_1500594 |
| Goat Anti-Guinea Pig Alexa 647 | Invitrogen | Cat#A21450; RRID: AB_141882 |
| Donkey Anti-Mouse Alexa 488 | Invitrogen | Cat#A21202; RRID: AB_141607 |
| Donkey Anti-Rat Alexa 488 | Invitrogen | Cat#A21208; RRID: AB_141709 |
| Donkey Anti-Mouse Alexa 568 | Invitrogen | Cat#A10037; RRID: AB_2534013 |
| Donkey Anti-Goat Alexa 568 | Invitrogen | Cat#A11057; RRID: AB_142581 |
| Donkey Anti-Rabbit IgG HRP | Thermo Fisher Scientific | Cat#45-000-682 |
| Horse Anti-Mouse IgG HRP | Cell Signaling | Cat#7076S; RRID: AB_330924 |
| Mouse IgG Isotype Control | Thermo Fisher Scientific | Cat#02-610-0; RRID: AB_2532935 |
| Rabbit IgG Isotype Control | Thermo Fisher Scientific | Cat#02-610-2; RRID: AB_2532938 |
| Biological Samples | ||
| Non-neurological control and C9orf72 postmortem tissue | Target ALS Human Postmortem Tissue Core | N/A |
| C9orf72 postmortem tissue | Emory University ADRC Tissue Bank | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| LDN193189 | Stemgent | Cat#04-0074-02 |
| SB431542 | STEMCELL Technologies | Cat#72234 |
| CHIR99021 | Sigma-Aldrich | Cat#SML1046 |
| Retinoic Acid | Sigma-Aldrich | Cat#R2625 |
| SAG | Cayman Chemicals | Cat#11914 |
| Compound E | Millipore | Cat#565790 |
| DAPT | Sigma-Aldrich | Cat#D5942 |
| db-cAMP | Millipore | Cat#28745 |
| Ascorbic Acid | Sigma-Aldrich | Cat#A4544 |
| BDNF | PeproTech | Cat#450-02 |
| GDNF | PeproTech | Cat#450-10 |
| L-glutamic acid | Sigma Aldrich | Cat#G1251 |
| Propidium Iodide | Thermo Fisher Scientific | Cat#P3566 |
| Dextran, Texas Red, 70,000 MW | Thermo Fisher Scientific | Cat#D1864 |
| Fluorescein isothiocyanate dextran, 500,000 MW | Sigma-Aldrich | Cat#FD500S |
| DTT | Sigma-Aldrich | Cat#D0632 |
| Actinomycin D | Sigma-Aldrich | Cat#A9415 |
| Cycloheximide | Sigma-Aldrich | Cat#C7698 |
| Protease Inhibitor Cocktail Set III, EDTA-free | Millipore | Cat#539134 |
| Complete Mini EDTA-free Protease Inhibitor Cocktail | Roche | Cat#11836170001 |
| TurboDNase | Thermo Fisher Scientific | Cat#AM2238 |
| RNaseOUT | Thermo Fisher Scientific | Cat#10777019 |
| RNase A | Thermo Fisher Scientific | Cat#EN0531 |
| Bovine Serum Albumin Fraction V | Sigma Aldrich | Cat#3117057001 |
| Ribonucleoside-Vanadyl Complex | New England BioLabs | Cat#S1402S |
| Salmon Sperm DNA | Thermo Fisher Scientific | Cat#15-632-011 |
| E.coli tRNA | Millipore | Cat#10109541001 |
| Critical Commercial Assays | ||
| Autofluorescence Eliminator Reagent | Millipore | Cat#2160 |
| P3 Primary Cell 4D Nucleofector Kit | Lonza | Cat#V4XP-3024 |
| Nuclei PURE Prep Nuclei Isolation Kit | Sigma-Aldrich | Cat#NUC201 |
| Streptavidin Coated Plates | Meso Scale Discovery | Cat#L45SA-2 |
| SuperSignal West Pico PLUS ECL Kit | Thermo Fisher Scientific | Cat#34580 |
| Immonilon ECL Ultra Western HRP Substrate | Millipore | Cat#WBULS0100 |
| BLOT-FASTSTAIN | G Biosciences | Cat#786-34 |
| RNeasy Mini Kit | Qiagen | Cat#74104 |
| Ambion TRIzol Reagent | Thermo Fisher Scientific | Cat#15-596-018 |
| SuperScript IV First-Strand Synthesis System | Thermo Fisher Scientific | Cat#18091050 |
| High-Capacity cDNA Reverse Transcription Kit | Thermo Fisher Scientific | Cat#4368814 |
| TaqMan Fast Advanced Master Mix | Thermo Fisher Scientific | Cat#44-449-64 |
| Experimental Models: Cell Lines | ||
| Human iPSC: EDi036-A | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0594ictr-lbcnxx/ |
| Human iPSC: EDi037-A | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0702ictr-lbcnxx/ |
| Human iPSC: EDi029-A | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0188ictr-lbcnxx/ |
| Human iPSC: EDi034-A | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0465ictr-lbcnxx/ |
| Human iPSC: EDi022-A | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0617ictr-lbcnxx/ |
| Human iPSC: EDi044-A | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs1185ictr-lbcnxx/ |
| Human iPSC: CS1ATZ | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs1atzictr-nxx/ |
| Human iPSC: CS8PAA | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs8paaictr-nxx/ |
| Human iPSC: EDi043-A | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs1050ictr-lbcnxx/ |
| Human iPSC: CS0201 | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0201ictr-nxx/ |
| Human iPSC: CS0002 | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0002ictr-nxx/ |
| Human iPSC: CS9XH7 | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs9xh7ictr-nxx/ |
| Human iPSC: CS0BUU | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0buuials-nxx/ |
| Human iPSC: CS7VCZ | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs7vczials-nxx/ |
| Human iPSC: CS0LPK | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0lpkials-nxx/ |
| Human iPSC: CS6ZLD | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs6zldials-nxx/ |
| Human iPSC: CS8KT3 | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs8kt3ials-nxx/ |
| Human iPSC: CS2YNL | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs2ynlials-nxx/ |
| Human iPSC: CS0NKC | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs0nkcials-nxx/ |
| Human iPSC: CS6CLW | Cedars-Sinai Induced Pluripotent Stem Cell Core | https://biomanufacturing.cedars-sinai.org/product/cs6clwials-nxx/ |
| Human iPSC: 59-1 | Ababneh et al., 2019 | N/A |
| Human iPSC: OXC9-02-02 | Dafinca et al., 2016 | N/A |
| Human iPSC: 3231 | Justin Ichida | N/A |
| Human iPSC: 3-11 | Justin Ichida | N/A |
| Primary Human Astrocytes | ScienCell | Cat#1800-5 |
| HEK293T Cells | ATCC | Cat#CRL-11268; RRID: CVCL_1926 |
| Oligonucleotides | ||
| C9orf72 and Scrambled ASOs | Ionis Pharmaceutcials | N/A |
| C9orf72 Primers | Lagier-Tourenne et al., 2013; This Paper (Table S2) | N/A |
| DIG G4C2 FISH probe | Qiagen | N/A |
| DIG scrambled FISH probe | Qiagen | N/A |
| Nup50 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs00855432_g1 |
| TPR TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs00162918_m1 |
| Nup98 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs00180522_m1 |
| GP210 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs00227779_m1 |
| NDC1 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs00917927_m1 |
| POM121 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs00208021_m1 |
| Nup107 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs00220703_m1 |
| Nup133 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs00217272_m1 |
| Nup62 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs02621445_s1 |
| Nup205 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs01046411_m1 |
| GAPDH TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs02786624_g1 |
| RPS18 TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs01375212_g1 |
| 18S rRNA TaqMan Gene Expression Assay | Thermo Fisher Scientific | Cat#Hs99999901_s1 |
| Recombinant DNA | ||
| Plasmid: pCMV6-AC-GFP-POM121 | Origene Technologies | Cat#RG235113 |
| Plasmid: pCMV6-AC-GFP-Nup133 | Origene Technologies | Cat#RG204410 |
| Plasmid: pCMV6-AC-GFP-Nup98 | Origene Technologies | Cat#RG213664 |
| Plasmid: pCMV6-AC-GFP-GP210 | Origene Technologies | Cat#RG213658 |
| Plasmid: pCMV6-AC-GFP-NDC1 | Origene Technologies | Cat#RG200141 |
| Plasmid: pEGFP-POM121 | Eilenberg Lab; Doucet et al., 2010; Talamas and Hetzer, 2011; Franks et al., 2016 | N/A |
| Plasmid: pQXCIB-sPOM121 | Franks et al., 2016 | N/A |
| Plasmid: pCMV6-AC-GFP-Trim21 | Origene Technologies | Cat#RG202088 |
| Plasmid: pLenti-NLS-tdTomato-NES | Zhang et al., 2015 | RRID: Addgene_112579 |
| Plasmid: pcDNA3.1-EGFP-(GA)50 | Wen et al., 2014 | N/A |
| Plasmid: pcDNA3.1-EGFP-(GR)50 | Wen et al., 2014 | N/A |
| Plasmid: pcDNA3.1-EGFP-(PR)50 | Wen et al., 2014 | N/A |
| Plasmid: pcDNA3.1+ 36RO | Mizielinska et al., 2014 | N/A |
| Plasmid: pcDNA3.1+ 106RO | Mizielinska et al., 2014 | N/A |
| Plasmid: pcDNA3.1+ 288 RO | Mizielinska et al., 2014 | N/A |
| Plasmid: 5’ UTR CGG 99x FMR1-EGFP | Addgene | Cat#63091; RRID: Addgene_63091 |
| Plasmid: pCTG202 | Nutter et al., 2019; Osborne and Thornton, 2008 | N/A |
| Plasmid: pCMV6-AC-GFP | Origene Technologies | Cat#PS100010 |
| Plasmid: mEGFP-N1 | Addgene | Cat#54767; RRID: Addgene_54767 |
| Plasmid: pmaxGFP | Lonza | Cat#V4XP-3024 |
| Software and Algorithms | ||
| FIJI | NIH | https://fiji.sc; RRID: SCR_002285 |
| Imaris | Bitplane | http://www.bitplane.com/; RRID: SCR_007370 |
| GraphPad Prism 7 | GraphPad Software | https://www.graphpad.com/; RRID: SCR_002798 |
| GraphPad Prism 8 | GraphPad Software | https://www.graphpad.com/; RRID: SCR_002798 |
| Zen 2 Pro | Carl Zeiss | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html; RRID: SCR_013672 |
| Zen 2.1 | Carl Zeiss | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html; RRID: SCR_013672 |
| Zen 2.3 SP1 | Carl Zeiss | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html; RRID: SCR_018163 |
| Other | ||
| MTeSR | STEMCELL Technologies | Cat#85850 |
| IMDM | Life Technologies | Cat#12440053 |
| F12 | Life Technologies | Cat#11765062 |
| DMEM | Life Technologies | Cat#11995073 |
| Penicillin Streptomycin | Gibco | Cat#15140122 |
| NEAA | Life Technologies | Cat#11140-050 |
| B27 | Gibco | Cat#17504044 |
| N2 | Life Technologies | Cat#17502048 |
| Versene | Thermo Fisher Scientific | Cat#15040066 |
| ReLeSR | STEMCELL Technologies | Cat#05872 |
| EZ Pass Tool | Life Technologies | Cat#23181-010 |
| StemPro Accutase | Gibco | Cat#A11105-01 |
| Trypsin-EDTA | Life Technologies | Cat#25300054 |
| Matrigel | Corning | Cat#354230 |
| PureCol Type I Collagen Solution | Advanced BioMatrix | Cat#5005 |
| ACSF | Tocris | Cat#3525 |
| Normal Goat Serum | Vector Labs | Cat#S-1000; RRID: AB_2336615 |
| Donkey Serum | Sigma-Aldrich | Cat#D9663; RRID: AB_2810235 |
| ProLong Gold Antifade Reagent | Invitrogen | Cat#P36930 |
| ProLong Gold Antifade Reagent with DAPI | Life Technologies | Cat#P36931 |
| Hoescht | Thermo Fisher Scientific | Cat#H3570 |
| DAKO Protein Block Serum-Free | Dako | Cat#X0909 |
| Antibody Diluent with Background-Reducing Components | Dako | Cat#S302283-2 |
| OptiPrep Density Gradient Medium | Sigma-Aldrich | Cat#D1556 |
| RIPA | Sigma Aldrich | Cat#R0278 |
| NucBlue Live ReadyProbes | Thermo Fisher Scientific | Cat#R37605 |
| Slide-A-Lyzer MINI Dialysis Device, 20K MWCO | Thermo Fisher Scientific | Cat#88402 |
| Amicon Ultra-0.5 Centrifugal Filter Unit | Millipore | Cat#UFC510024 |
| NanoDrop 1000 Spectrophotometer | Thermo Fisher Scientific | N/A |
| Applied Biosystems Step One Plus Real Time PCR Machine | Thermo Fisher Scientific | Cat#4376600 |
| ImageQuant LAS 4000 | GE Healthcare | N/A |
| MESO QuickPlex SQ 120 | Meso Scale Discovery | N/A |
| CytoSpin 4 | Thermo Fisher Scientific | Cat#A78300003 |
| High Tolerance Coverslips | MatTek | Cat#PCS-170-1818 |
| Zeiss ELYRA S1 | Carl Zeiss | N/A |
| Zeiss LSM 800 with Airyscan | Carl Zeiss | RRID: SCR_015963 |
| Zeiss Apotome | Carl Zeiss | N/A |
Highlights.
Expression of eight nucleoporins is reduced in human C9orf72 neuronal nuclei
Reduction in POM121 impacts nuclear pore complex composition
Nucleoporin alterations diminish nucleocytoplasmic transport and neuronal survival
G4C2 repeat RNA initiates the pathogenic cascades leading to decreased nuclear POM121
Acknowledgements
We thank the ALS patients and their families for essential contributions to this research and the Target ALS Human Postmortem Tissue Core and Jonathan Glass (Emory University; ADRC NIH P50 AG025688-11REV) for providing postmortem human tissue. We also thank Martin Hetzer, Adrian Isaacs, Davide Trotti, and Maurice Swanson for kindly gifting POM121, G4C2 RNA Only, DPR, and (CTG)202 plasmids respectively. Expert technical assistance for iPSC maintenance was provided by Xiaopei Tang and Weibo Zhou. We thank Patrick Lusk for helpful comments in the editing of this manuscript. This work was supported by the ALSA Milton Safenowitz Postdoctoral Fellowship (ANC), along with funding to JDR from NIH-NINDS (RF1AG062171, P01NS099114, R01NS094239), The Robert Packard Center for ALS Research Answer ALS Program, ALS Finding a Cure, ALS Association, Muscular Dystrophy Association, and the Chan Zuckerberg Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interests
The authors declare no competing financial interests.
References
- Ababneh NA, Scaber J, Flynn R, Douglas A, Barbagallo P, Candalija A, Turner MR, Sims D, Dafinca R, Cowley SA, et al. (2020). Correction of amyotrophic lateral sclerosis related phenotypes in induced pluripotent stem cell-derived motor neurons carrying a hexanucleotide expansion mutation in C9orf72 by CRISPR/Cas9 genome editing using homology-directed repair. Human molecular genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W, Franz C, Haselmann U, Antony C, and Mattaj IW (2005). The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Molecular cell 17, 83–92. [DOI] [PubMed] [Google Scholar]
- Balendra R, and Isaacs AM (2018). C9orf72-mediated ALS and FTD: multiple pathways to disease. Nature reviews Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, and Hurt E (2017). The nuclear pore complex: understanding its function through structural insight. Nature reviews Molecular cell biology 18, 73–89. [DOI] [PubMed] [Google Scholar]
- Cheng W, Wang S, Zhang Z, Morgens DW, Hayes LR, Lee S, Portz B, Xie Y, Nguyen BV, Haney MS, et al. (2019). CRISPR-Cas9 Screens Identify the RNA Helicase DDX3X as a Repressor of C9ORF72 (GGGGCC)n Repeat-Associated Non-AUG Translation. Neuron 104, 885–898.e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew J, Cook C, Gendron TF, Jansen-West K, Del Rosso G, Daughrity LM, Castanedes-Casey M, Kurti A, Stankowski JN, Disney MD, et al. (2019). Aberrant deposition of stress granule-resident proteins linked to C9orf72-associated TDP-43 proteinopathy. Molecular neurodegeneration 14, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CC, Zhang Y, Umoh ME, Vaughan SW, Lorenzini I, Liu F, Sayegh M, Donlin-Asp PG, Chen YH, Duong DM, et al. (2018). TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nature neuroscience 21, 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift D, McEwan WA, Labzin LI, Konieczny V, Mogessie B, James LC, and Schuh M (2017). A Method for the Acute and Rapid Degradation of Endogenous Proteins. Cell 171, 1692–1706.e1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, and Petrucelli L (2019). Genetic Convergence Brings Clarity to the Enigmatic Red Line in ALS. Neuron 101, 1057–1069. [DOI] [PubMed] [Google Scholar]
- D’Angelo MA, Raices M, Panowski SH, and Hetzer MW (2009). Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 136, 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Finch NA, Wang X, Gendron TF, Bieniek KF, Heckman MG, Vasilevich A, Murray ME, Rousseau L, Weesner R, et al. (2017). In-depth clinicopathological examination of RNA foci in a large cohort of C9ORF72 expansion carriers. Acta neuropathologica 134, 255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. (2011). Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly CJ, Zhang PW, Pham JT, Haeusler AR, Mistry NA, Vidensky S, Daley EL, Poth EM, Hoover B, Fines DM, et al. (2013). RnA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80, 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet CM, Talamas JA, and Hetzer MW (2010). Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell 141, 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekharzadeh B, Daigle JG, Kapinos LE, Coyne A, Schiantarelli J, Carlomagno Y, Cook C, Miller SJ, Dujardin S, Amaral AS, et al. (2018). Tau Protein Disrupts Nucleocytoplasmic Transport in Alzheimer’s Disease. Neuron 99, 925–940.e927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R, Kapogiannis D, Huey ED, and Momeni P (2011). FTD and ALS: a tale of two diseases. Current Alzheimer research 8, 273–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtman B, Harel T, Biran N, Zagairy F, Applegate CD, Salzberg Y, Gilboa T, Salah S, Shaag A, Simanovsky N, et al. (2019). Pathogenic Variants in NUP214 Cause “Plugged” Nuclear Pore Channels and Acute Febrile Encephalopathy. American journal of human genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtman B, Shaulov L, and Harel A (2014). Imaging metazoan nuclear pore complexes by field emission scanning electron microscopy. Methods in cell biology 122, 41–58. [DOI] [PubMed] [Google Scholar]
- Franks TM, Benner C, Narvaiza I, Marchetto MC, Young JM, Malik HS, Gage FH, and Hetzer MW (2016). Evolution of a transcriptional regulator from a transmembrane nucleoporin. Genes & development 30, 1155–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freibaum BD, Lu Y, Lopez-Gonzalez R, Kim NC, Almeida S, Lee KH, Badders N, Valentine M, Miller BL, Wong PC, et al. (2015). GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 525, 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T, Clever M, Watanabe A, and Imamoto N (2011). Localization of Pom121 to the inner nuclear membrane is required for an early step of interphase nuclear pore complex assembly. Molecular biology of the cell 22, 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasset-Rosa F, Chillon-Marinas C, Goginashvili A, Atwal RS, Artates JW, Tabet R, Wheeler VC, Bang AG, Cleveland DW, and Lagier-Tourenne C (2017). Polyglutamine-Expanded Huntingtin Exacerbates Age-Related Disruption of Nuclear Integrity and Nucleocytoplasmic Transport. Neuron 94, 48–57.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron TF, Chew J, Stankowski JN, Hayes LR, Zhang YJ, Prudencio M, Carlomagno Y, Daughrity LM, Jansen-West K, Perkerson EA, et al. (2017). Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Science translational medicine 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, and Tsuiji H (2016). There has been an awakening: Emerging mechanisms of C9orf72 mutations in FTD/ALS. Brain research 1647, 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J, Geater C, Morozko E, Stocksdale J, Glatzer JC, et al. (2017). Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron 94, 93–107.e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampoelz B, Andres-Pons A, Kastritis P, and Beck M (2019). Structure and Assembly of the Nuclear Pore Complex. Annual review of biophysics. [DOI] [PubMed] [Google Scholar]
- Hayes LR, Duan L, Bowen K, Kalab P, and Rothstein JD (2020). C9orf72 arginine-rich dipeptide repeat proteins disrupt karyopherin-mediated nuclear import. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer MW (2010). The role of the nuclear pore complex in aging of post-mitotic cells. Aging 2, 74–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R, Sances S, Gowing G, Amoroso MW, O’Rourke JG, Sahabian A, Wichterle H, Baloh RH, Sareen D, and Svendsen CN (2016). ALS disrupts spinal motor neuron maturation and aging pathways within gene co-expression networks. Nature neuroscience 19, 1256–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten S, and Dormann D (2019). Nucleocytoplasmic transport defects in neurodegeneration - Cause or consequence? Seminars in cell & developmental biology. [DOI] [PubMed] [Google Scholar]
- Jovicic A, Mertens J, Boeynaems S, Bogaert E, Chai N, Yamada SB, Paul JW 3rd, Sun S, Herdy JR, Bieri G, et al. (2015). Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nature neuroscience 18, 1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Fernandez-Martinez J, Nudelman I, Shi Y, Zhang W, Raveh B, Herricks T, Slaughter BD, Hogan JA, Upla P, et al. (2018). Integrative structure and functional anatomy of a nuclear pore complex. Nature 555, 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita Y, Kalir T, Dottino P, and Kohtz DS (2012). Nuclear distributions of NUP62 and NUP214 suggest architectural diversity and spatial patterning among nuclear pore complexes. PloS one 7, e36137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Baughn M, Rigo F, Sun S, Liu P, Li HR, Jiang J, Watt AT, Chun S, Katz M, et al. (2013). Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proceedings of the National Academy of Sciences of the United States of America 110, E4530–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Zhang P, Kim HJ, Mitrea DM, Sarkar M, Freibaum BD, Cika J, Coughlin M, Messing J, Molliex A, et al. (2016). C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles. Cell 167, 774–788.e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Goryaynov A, and Yang W (2016). The selective permeability barrier in the nuclear pore complex. Nucleus (Austin, Tex) 7, 430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DH, and Hoelz A (2019). The Structure of the Nuclear Pore Complex (An Update). Annual review of biochemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SC, Polymenidou M, and Cleveland DW (2013). Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79, 416–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione M, and Sigrist SJ (2013). Seeing the forest tree by tree: super-resolution light microscopy meets the neurosciences. Nature neuroscience 16, 790–797. [DOI] [PubMed] [Google Scholar]
- Melchior F (2001). Ran GTPase cycle: oOne mechanism -- two functions. Current biology : CB 11, R257–260. [DOI] [PubMed] [Google Scholar]
- Mizielinska S, Gronke S, Niccoli T, Ridler CE, Clayton EL, Devoy A, Moens T, Norona FE, Woollacott IOC, Pietrzyk J, et al. (2014). C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science (New York, NY) 345, 1192–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter CA, Bubenik JL, Oliveira R, Ivankovic F, Sznajder Ł,J, Kidd BM, Pinto BS, Otero BA, Carter HA, Vitriol EA, et al. (2019). Cell-type-specific dysregulation of RNA alternative splicing in short tandem repeat mouse knockin models of myotonic dystrophy. Genes & development 33, 1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori A, Banterle N, Iskar M, Andres-Pons A, Escher C, Khanh Bui H, Sparks L, Solis-Mezarino V, Rinner O, Bork P, et al. (2013). Cell type-specific nuclear pores: a case in point for context-dependent stoichiometry of molecular machines. Molecular systems biology 9, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne RJ, and Thornton CA (2008). Cell-free cloning of highly expanded CTG repeats by amplification of dimerized expanded repeats. Nucleic acids research 36, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka S, and Ellenberg J (2018). Mechanisms of nuclear pore complex assembly - two different ways of building one molecular machine. FEBS letters 592, 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Garcia P, and Capelson M (2019). Nuclear pores in genome architecture and enhancer function. Current opinion in cell biology 58, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popken P, Ghavami A, Onck PR, Poolman B, and Veenhoff LM (2015). Size-dependent leak of soluble and membrane proteins through the yeast nuclear pore complex. Molecular biology of the cell 26, 1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raices M, and D’Angelo MA (2012). Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nature reviews Molecular cell biology 13, 687–699. [DOI] [PubMed] [Google Scholar]
- Rajoo S, Vallotton P, Onischenko E, and Weis K (2018). Stoichiometry and compositional plasticity of the yeast nuclear pore complex revealed by quantitative fluorescence microscopy. Proceedings of the National Academy of Sciences of the United States of America 115, E3969–e3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, et al. (2011). A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sances S, Bruijn LI, Chandran S, Eggan K, Ho R, Klim JR, Livesey MR, Lowry E, Macklis JD, Rushton D, et al. (2016). Modeling ALS with motor neurons derived from human induced pluripotent stem cells. Nature neuroscience 19, 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, O’Rourke JG, Meera P, Muhammad AK, Grant S, Simpkinson M, Bell S, Carmona S, Ornelas L, Sahabian A, et al. (2013). Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Science translational medicine 5, 208ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas JN, Toyama BH, Xu T, Yates JR 3rd, and Hetzer MW (2012). Extremely long-lived nuclear pore proteins in the rat brain. Science (New York, NY) 335, 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso MC, Agard DA, Gustafsson MG, et al. (2008). Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science (New York, NY) 320, 1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi KY, Mori E, Nizami ZF, Lin Y, Kato M, Xiang S, Wu LC, Ding M, Yu Y, Gall JG, et al. (2017). Toxic PRn poly-dipeptides encoded by the C9orf72 repeat expansion block nuclear import and export. Proceedings of the National Academy of Sciences of the United States of America 114, E1111–e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamas JA, and Hetzer MW (2011). POM121 and Sun1 play a role in early steps of interphase NPC assembly. The Journal of cell biology 194, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Brown RH Jr., and Cleveland DW (2016). Decoding ALS: from genes to mechanism. Nature 539, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan JV, Kahnwald M, Cieslinski K, Hoess P, Peneti SK, Reitberger M, Heid D, Kasuba KC, Hoerner SJ, Li Y, et al. (2019). Nuclear pores as versatile reference standards for quantitative superresolution microscopy. Nature methods 16, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timney BL, Raveh B, Mironska R, Trivedi JM, Kim SJ, Russel D, Wente SR, Sali A, and Rout MP (2016). Simple rules for passive diffusion through the nuclear pore complex. The Journal of cell biology 215, 57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama BH, Arrojo EDR, Lev-Ram V, Ramachandra R, Deerinck TJ, Lechene C, Ellisman MH, and Hetzer MW (2018). Visualization of long-lived proteins reveals age mosaicism within nuclei of postmitotic cells. The Journal of cell biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR, and Hetzer MW (2013). Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154, 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsavayai SC, Yoon SJ, Gardner RC, Gendron TF, Vargas JN, Trujillo A, Pribadi M, Phillips JJ, Gaus SE, Hixson JD, et al. (2016). Timing and significance of pathological features in C9orf72 expansion-associated frontotemporal dementia. Brain : a journal of neurology 139, 3202–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite AJ, Baumer D, East S, Neal J, Morris HR, Ansorge O, and Blake DJ (2014). Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiology of aging 35, 1779.e1775–1779.e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Tan W, Westergard T, Krishnamurthy K, Markandaiah SS, Shi Y, Lin S, Shneider NA, Monaghan J, Pandey UB, et al. (2014). Antisense proline-arginine RAN dipeptides linked to C9ORF72-ALS/FTD form toxic nuclear aggregates that initiate in vitro and in vivo neuronal death. Neuron 84, 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, Daley EL, Miller SJ, Cunningham KM, Vidensky S, et al. (2015). The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 525, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hu D, Shang Y, and Qi X (2019a). Using induced pluripotent stem cell neuronal models to study neurodegenerative diseases. Biochimica et biophysica acta Molecular basis of disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Gendron TF, Ebbert MTW, O’Raw AD, Yue M, Jansen-West K, Zhang X, Prudencio M, Chew J, Cook CN, et al. (2018). Poly(GR) impairs protein translation and stress granule dynamics in C9orf72-associated frontotemporal dementia and amyotrophic lateral sclerosis. Nature medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Gendron TF, Grima JC, Sasaguri H, Jansen-West K, Xu YF, Katzman RB, Gass J, Murray ME, Shinohara M, et al. (2016). C9ORF72 poly(GA) aggregates sequester and impair HR23 and nucleocytoplasmic transport proteins. Nature neuroscience 19, 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Guo L, Gonzales PK, Gendron TF, Wu Y, Jansen-West K, O’Raw AD, Pickles SR, Prudencio M, Carlomagno Y, et al. (2019b). Heterochromatin anomalies and double-stranded RNA accumulation underlie C9orf72 poly(PR) toxicity. Science (New York, NY) 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu TW, Madden Z, Yuzwa SA, Murray K, Cecioni S, Zachara N, and Vocadlo DJ (2016). Post-translational O-GlcNAcylation is essential for nuclear pore integrity and maintenance of the pore selectivity filter. Journal of molecular cell biology 8, 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate or analyze datasets or code.


