Abstract
Background
The introduction of point‐of‐care devices for the management of patients on oral anticoagulation allows self‐testing by the patient at home. Patients who self‐test can either adjust their medication according to a pre‐determined dose‐INR (international normalized ratio) schedule (self‐management), or they can call a clinic to be told the appropriate dose adjustment (self‐monitoring). Increasing evidence suggests self‐testing of oral anticoagulant therapy is equal to or better than standard monitoring. This is an updated version of the original review published in 2010.
Objectives
To evaluate the effects on thrombotic events, major haemorrhages, and all‐cause mortality of self‐monitoring or self‐management of oral anticoagulant therapy compared to standard monitoring.
Search methods
For this review update, we re‐ran the searches of the Cochrane Central Register of Controlled Trials (CENTRAL), 2015, Issue 6, the Cochrane Library, MEDLINE (Ovid, 1946 to June week 4 2015), Embase (Ovid, 1980 to 2015 week 27) on 1 July 2015. We checked bibliographies and contacted manufacturers and authors of relevant studies. We did not apply any language restrictions .
Selection criteria
Outcomes analysed were thromboembolic events, mortality, major haemorrhage, minor haemorrhage, tests in therapeutic range, frequency of testing, and feasibility of self‐monitoring and self‐management.
Data collection and analysis
Review authors independently extracted data and we used a fixed‐effect model with the Mantzel‐Haenzel method to calculate the pooled risk ratio (RR) and Peto’s method to verify the results for uncommon outcomes. We examined heterogeneity amongst studies with the Chi2 and I2 statistics and used GRADE methodology to assess the quality of evidence.
Main results
We identified 28 randomised trials including 8950 participants (newly incorporated in this update: 10 trials including 4227 participants). The overall quality of the evidence was generally low to moderate. Pooled estimates showed a reduction in thromboembolic events (RR 0.58, 95% CI 0.45 to 0.75; participants = 7594; studies = 18; moderate quality of evidence). Both, trials of self‐management or self‐monitoring showed reductions in thromboembolic events (RR 0.47, 95% CI 0.31 to 0.70; participants = 3497; studies = 11) and (RR 0.69, 95% CI 0.49 to 0.97; participants = 4097; studies = 7), respectively; the quality of evidence for both interventions was moderate. No reduction in all‐cause mortality was found (RR 0.85, 95% CI 0.71 to 1.01; participants = 6358; studies = 11; moderate quality of evidence). While self‐management caused a reduction in all‐cause mortality (RR 0.55, 95% CI 0.36 to 0.84; participants = 3058; studies = 8); self‐monitoring did not (RR 0.94, 95% CI 0.78 to 1.15; participants = 3300; studies = 3); the quality of evidence for both interventions was moderate. In 20 trials (8018 participants) self‐monitoring or self‐management did not reduce major haemorrhage (RR 0.95, 95% CI, 0.80 to 1.12; moderate quality of evidence). There was no significant difference found for minor haemorrhage (RR 0.97, 95% CI 0.67 to 1.41; participants = 5365; studies = 13). The quality of evidence was graded as low because of serious risk of bias and substantial heterogeneity (I2 = 82%).
Authors' conclusions
Participants who self‐monitor or self‐manage can improve the quality of their oral anticoagulation therapy. Thromboembolic events were reduced, for both those self‐monitoring or self‐managing oral anticoagulation therapy. A reduction in all‐cause mortality was observed in trials of self‐management but not in self‐monitoring, with no effects on major haemorrhage.
Plain language summary
Self‐monitoring and self‐management of oral anticoagulation therapy
Background
Point‐of‐care testing devices have made it possible for people on long‐term oral anticoagulation to monitor their blood clotting time, measured as the international normalized ratio (INR). Patients who self‐test can either adjust their medication dose according to a pre‐determined dose‐INR schedule (self‐management) or they can call a clinic to be told the appropriate dose adjustment (self‐monitoring). Several published studies and systematic reviews have suggested these methods of monitoring anticoagulation therapy may be equal to or better than standard monitoring by a physician.
Study characteristics
This is an update of the original review published in 2010. We performed a new search and found 10 new studies (with 4227 participants) to add to the original review, which changed some of the findings.
Main results
In total, we found 28 randomised trials including 8950 participants that compared self‐monitoring and self‐management with standard monitoring. The quality of the evidence was generally low to moderate. The combined results of the 28 trials showed a halving of thromboembolic events with self‐monitoring and self‐management and no reduction in the number of major bleeds. Self‐management had similar reductions in thromboembolic events and mortality to the overall benefit, with no effect on major bleeds. Self‐monitoring halved the number of major haemorrhages that occurred but did not significantly reduce the rates of thrombotic events or all‐cause mortality.
Conclusion
In conclusion, self‐monitoring or self‐management can improve the quality of oral anticoagulant therapy, leading to fewer thromboembolic events and lower mortality, without a reduction in the number of major bleeds. Self‐monitoring and self‐management are not feasible for all patients, which requires the identification and education of suitable patients.
Summary of findings
Background
Terminology
Point‐of‐care testing (POC): diagnostic testing performed in a clinic, home, or other site of patient care (rather than in standard reference laboratory)
Point‐of‐care device: portable monitor used by a healthcare provider (physician, nurse, or other) or patient to determine a clinical measure
Self‐monitoring: the trained participant uses point‐of‐care testing to perform the international normalized ratio (INR) test and inform his or her healthcare provider of the result. The physician or another healthcare provider adjusts the anticoagulant dose using the results obtained by the participant
Self‐management: trained participant uses point‐of‐care testing to perform the INR test, interpret the result, and adjust the dosage of anticoagulant accordingly (adapted from Brown 2007)
Description of the condition
Oral anticoagulation therapy has been shown to reduce thromboembolic events in atrial fibrillation, treatment of deep‐vein thrombosis, prosthetic heart valves, and acute myocardial infarction (Connolly 1991; Go 2003; SPAF 1996). Optimal anticoagulation with warfarin or other vitamin K antagonists like acenocoumarol or phenprocoumon could potentially prevent more than half of the strokes related to atrial fibrillation and heart valve replacements with a relatively low risk of major bleeding complications (Buckingham 2002; Hart 2007); however, much of this potential is still not obtained because of under and suboptimal use (Stafford 1998).
The number of patients receiving oral anticoagulant drugs has been increasing. Reasons include improvements in clinical outcomes, increasing common disease indications for their use (Manotti 2001), and improvements in anticoagulant safety (Ansell 2001). In 1994, 250,000 patients in the UK were receiving anticoagulant therapy (Baglin 1994); 10 years later this had increased to around 950,000 patients (Fitzmaurice 2005). Vitamin K antagonist (warfarin, acenocoumarol, or phenprocoumon) treatment usually requires regular monitoring of prothrombin time (PT) with dose‐adjustment by a specialised hospital service, primary care physician, registered nurse, nurse practitioner, or pharmacist (Hirsh 1998).
Numerous obstacles to the use of warfarin exist; including practical, patient, physician, and healthcare system‐related barriers. Due to the complex pharmacokinetics of warfarin, continuous monitoring and dose adjustments are required. Different values and preferences amongst physicians and patients about the relative importance of bleeding and thromboembolic events, non‐adherence to drug treatment, drug interactions, and increased costs of monitoring have significant roles to play in the management of anticoagulation therapy (Heneghan 2008).
Description of the intervention
Vitamin K antagonists belong to the drug class known as coumarins and produce their anticoagulant effect by interfering with the metabolism of vitamin K. There are various different types of coumarins but warfarin is the most prescribed. Warfarin has a high bioavailability (Breckenridge 1978), and is rapidly absorbed from the gastrointestinal tract, with maximal blood concentrations reached 90 minutes after oral administration. Warfarin has a half‐life of 36 to 42 hours; in the blood it is bound to plasma proteins (mainly albumin). It accumulates in the liver where the two isomers are metabolically transformed by different pathways (Ansell 2004). An anticoagulation effect generally occurs within 24 hours of treatment imitation, and peak effect for warfarin takes two to five days.
Another vitamin K antagonist is acenocoumarol, which has a similar action to warfarin but differs in some pharmacological properties (for example, it has a shorter half‐life Barcellona 1998). Phenprocoumon is another vitamin K antagonist that has traditionally been the oral anticoagulant of choice in Europe. It has similar actions to other vitamin K antagonists but has a half‐life of 144 hours. As a result of their pharmacokinetic properties, these agents interact with many other drugs and their blood levels are affected by vitamin K intake in the diet, changes in metabolism, and concomitant illnesses, which makes the levels difficult to control (Greenblatt 2005).
The pharmacodynamics of warfarin are subject to genetic and environmental variability (Hirsh 2001), such that there is considerable variation in the action of these drugs both between different individuals (inter‐individually) and within the same individual (intra‐individually). A 'therapeutic target range' has been established to deal with this variability and is expressed as the international normalized ratio (INR). This INR was established as a standard way of reporting the prothrombin time (PT). Furthermore, using the INR formula (INR = patient PT/mean normal PT) the ratio between patient PT and normal PT is calculated to the power of the ISI (International Sensitivity Index), which is the conversion factor for the used thromboplastin against the World Health Organization (WHO) standard.
The ‘therapeutic range’ for anticoagulants is narrow. INR values over 4.5 increase the risk of major bleeding and an INR less than 2 increases the risk of thromboembolism (Cannegieter 1995; Hylek 1996; Kearon 2003). The inter‐ and intra‐individual variability and the narrow target range generally requires frequent testing and appropriate adjustment of the drug dose. In addition, time within the therapeutic INR target range is highly dependent on the frequency of testing (Horstkotte 1998). Different values and preferences amongst patients and physicians have also been described with the former willing to accept a much higher risk of bleeding for an associated reduction in risk of stroke (Devereaux 2001).
An economic model analysed the cost of suboptimal oral anticoagulation and showed the following. If 50% of those not receiving warfarin prophylaxis had optimal anticoagulation, 19,380 emboli would be prevented and 1.1 billion US dollars could be saved. If 50% of those currently receiving warfarin as part of routine medical care had optimal anticoagulation, 9852 emboli would be prevented and 1.3 billion US dollars could be saved (Caro 2004).
How the intervention might work
Current models of oral anticoagulation management within the UK include the traditional hospital outpatient model and various forms of community‐based models, all requiring patient attendance at a clinic (Fitzmaurice 2002). In other countries, such as Canada, a primary care physician monitors the INR and adjusts the warfarin dose (Sunderji 2004).
The introduction of point‐of‐care devices allows the patient to self‐test at home with a drop of whole blood. Portable monitors for monitoring long‐term oral anticoagulation were introduced in the 1990s. Devices have proved to be reliable with regard to analytical accuracy, although INR measurements tend to be lower with the portable coagulometers compared to laboratory analysers (Christensen 2009; Poller 2006), and have proved to be reliable devices for monitoring INR when checked regularly (Barcellona 2009).
Generally, patients receive a structured educational programme given by the nurses or physicians responsible for their care. In addition, they receive training in self‐testing, instructions to prevent bleeding and thromboembolic complications, and are made aware of the effects of diet and medications. Patients who self‐test can either adjust their therapy according to a pre‐determined dose‐INR schedule (self‐management) or they can call a clinic to be told the appropriate dose adjustment (self‐monitoring).
Why it is important to do this review
In some countries, such as Germany, self‐monitoring and self‐management with portable monitors are established therapeutic methods. There are several available point‐of‐care devices and the most well known is the CoaguChek® monitor. Other available monitors are the ProTime® Microcoagulation System, INRatio® Monitor, Hemochron Junior Signature, and the TAS near‐patient test system. Potential advantages of self‐monitoring and self‐management include improved convenience for patients, better treatment adherence, more frequent monitoring, and fewer thromboembolic and haemorrhagic complications (Taborski 1999). Near‐patient testing devices have made self‐testing of anticoagulation therapy with vitamin K antagonists possible. Guidelines generally do not endorse self‐monitoring or self‐management (Fitzmaurice 2001), despite several authors of trials suggesting this approach may be equal to or better than standard monitoring (Anderson 1993; Cromheecke 2000; Sawicki 1999). A recent study suggested that self‐monitoring and self‐management are cost‐effective strategies for those receiving long‐term oral anticoagulation (Regier 2006). In addition, newer oral anticoagulants that do not require monitoring have not been established in heart valve patients (Eikelboom 2013) and are not suitable for all because of the numerous exclusion and individuals who cannot tolerate these drugs (DiNicolantonio 2012).
To establish the current strength of the available evidence, we updated our systematic review of the impact of patient self‐monitoring or self‐management on treatment with oral anticoagulation therapy.
Objectives
To evaluate the effects on thrombotic events, major haemorrhages, and all‐cause mortality of self‐monitoring or self‐management of oral anticoagulation compared with standard monitoring.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) assessing the therapeutic effectiveness and safety of self‐monitoring or self‐management of oral anticoagulation therapy.
Types of participants
All patients, adults and children, on long‐term anticoagulant therapy (treatment duration longer than two months), irrespective of the indication for treatment (for example, valve replacement, venous thromboembolism, atrial fibrillation).
Types of interventions
Self‐monitoring or self‐management of oral anticoagulation compared with:
control of and dosage by personal physician;
anticoagulation managed services (hospital anticoagulation service);
anticoagulation clinics (management conducted by registered nurses, nurse practitioners, or pharmacists using dosage‐adjustment protocols).
Types of outcome measures
Primary outcomes
Thromboembolic events
All‐cause mortality
Major haemorrhage (e.g. haemorrhage requiring hospital admission or transfusion)
Time in range, and proportion of measurements within the therapeutic range for each particular condition
Secondary outcomes
Minor haemorrhage (e.g. bleeding after minor trauma, nose bleed)
Frequency of testing
Feasibility of testing: participant factors (e.g. physical limitations), and non‐participant factors (e.g. inability to attend training)
Quality of life and general satisfaction with treatment
Search methods for identification of studies
Electronic searches
The searches for the initial review were run in November 2007 (Appendix 1). We re‐ran the searches on 27 November 2013 (Appendix 2). We updated the searches on 1 July 2015 (Appendix 2) with exception of CINAHL which was last searched on 27 November 2013 (an updated search of CINAHL was not mandatory):
Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 6, the Cochrane Library,
MEDLINE (Ovid, 1946 to June week 4 2015),
Embase (Ovid, 1980 to 2015 week 27), and
CINAHL (EBSCO, 1982 to November 2013).
We limited our searches to randomised controlled trials by using a maximally sensitive strategy (Dickersin 1994; Lefebvre 1996 in 2007 and Lefebvre 2011 in 2015).
Searching other resources
We also searched for ongoing trials on the UK National Research Register (webarchive.nationalarchives.gov.uk), Trials Central, Current Controlled Trials (www.controlled-trials.com/mrct/) (November 2013) and handsearched reference lists of all retrieved papers. We contacted Roche® Diagnostics (one manufacturer of PT and INR monitors) in order to identify further published and unpublished studies. There were no language restrictions.
Data collection and analysis
Data extraction
Two review authors (EAS and IJO) screened studies for inclusion and retrieved all potentially relevant studies. Three review authors (JM, PA, CH) independently extracted data on study population, intervention, pre‐specified outcomes, methodology, and quality from eligible trials. The authors were not blinded to any aspect of the studies (for example, journal type, authors' names, institution). We resolved disagreements by consensus. If needed, we sought additional information from trial authors. We used Cohen’s kappa to assess agreement between the two authors on the selection of articles for inclusion.
We extracted information on disease characteristics and training provided to the different groups. In the self‐management group, we extracted information on the actions participants subsequently undertook. We extracted the characteristics of the population studied, including the number of, and reasons for, participants not entering the trial (for example, refusal or exclusion). Additionally, we sought information on the reasons for discontinuation by participants allocated to the intervention.
In the case of cross‐over studies, the outcomes of interest are potentially confounded by the cross‐over and we only used data from the first part of the trial (before cross‐over).
Quality assessment
Three review authors (EAS, IJO, CH) independently extracted methodological information for the assessment of risk of bias. They used the following five components: method of randomisation, concealment of allocation, intention‐to‐treat, number of, and reasons for, participant losses to follow‐up, and blinding. We performed a sensitivity analyses for study quality by including only those studies with clear methods of randomisation and concealment of allocation (high‐quality studies). We also used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) (GRADE 2008) to assess the quality of the included studies.
Quantitative data synthesis
For the analysis we used Review Manager (RevMan) Version 5.3. For the statistical analysis we calculated risk ratios (RRs) and 95% confidence intervals (CIs) as summary statistics. We used a fixed‐effect model with the Mantzel‐Haenzel method to calculate the pooled odds ratio; and Peto’s method to verify the results in uncommon outcomes. We examined heterogeneity amongst studies with the Chi2 and I2 statistics (Higgins 2003). Where significant heterogeneity existed, we used the random‐effects model (DerSimonian 1986).
We examined publication bias by constructing a funnel plot of precision (SE of the log RR) against RR for the endpoints of major haemorrhage and thromboembolic episodes. We performed a sensitivity analysis by excluding studies with high risk of bias and pre‐specified subgroup analyses according to clinical indication (mechanical valve replacement or atrial fibrillation), and self‐monitoring or self‐management therapy. We performed a post‐hoc subgroup analysis according to who provided the control group care (specialist anticoagulation clinic, family physician). Meta‐regression in STATA tested any subgroup interaction on the outcomes. The ratio of the average test frequency per individual patient/year between intervention and control groups was calculated and linear regression was used to assess the association with study duration. Pooling of the mean percentage of tests in the therapeutic range was not possible; results were summarised using means and ranges. We tested subgroup interactions using meta‐regression (Intercooled STATA 9.1 for Windows).
To provide further insight into the adequacy of the total sample size across all trials, we calculated a posteriori the optimal information needed for our meta‐analysis (Pogue 1997). To determine this optimal information size, we assumed a 2% risk of thromboembolism (median control event rate from trials in the review) and a 50% RR reduction with a power of 95% and a two‐sided alpha = 0.01.
Results
Description of studies
Results of the search
The search for the initial review retrieved 463 citations, which included 18 relevant trials. The updated searches in November 2013 and July 2015 identified 5136 new citations and identified an additional 10 trials (with 4227 participants) for inclusion.
In total, we identified 5894 citations through database searching as well as one additional unpublished citation (Kaatz Unpublished). Of these, we excluded 758 duplicate records, leaving 5136 potentially relevant studies. A further 5067 citations were removed after being deemed irrelevant to our research question. We independently assessed 69 full‐text articles for eligibility. Of these, 22 articles were excluded and 20 articles were secondary publications of primary studies already included in the review (Figure 1).
1.
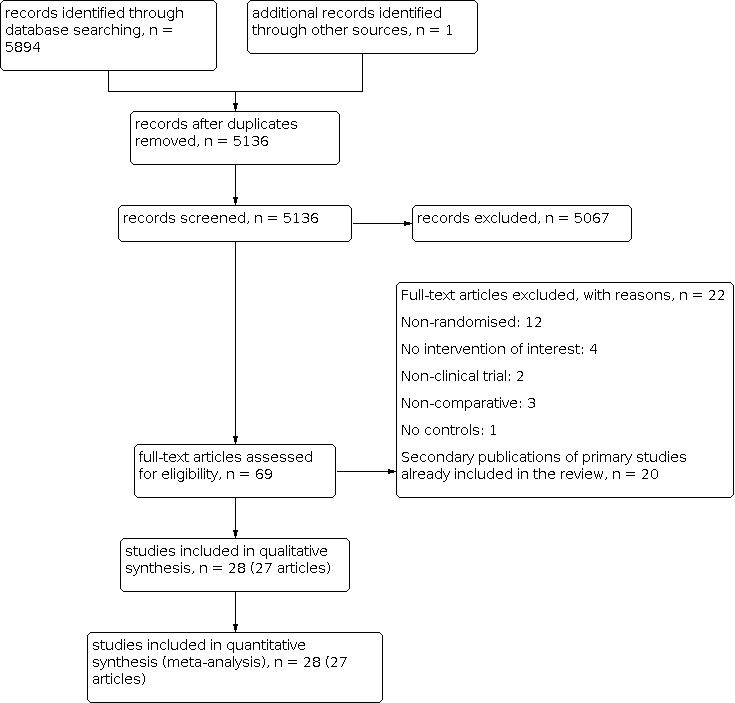
Flowchart showing the results of combined searches
Included studies
A total of 27 included publications provided data on 28 trials including 8950 participants (one publication, Gadisseur 2003 Self mge; Gadisseur 2003 Self monit, contained data on two trials that compared self‐monitoring or self‐management of oral anticoagulation with standard monitoring). Trials were published between 1989 and 2013 and were largely undertaken in Europe (five each in UK and Germany; three each in the Netherlands and Denmark; one in each of Ireland, France, Spain and Austria); seven were undertaken in United States and Canada; and one was conducted in Australia. In total, 4723 participants on long‐term anticoagulation were included in our analysis. Three trials (Cromheecke 2000; Grunau 2011; Ryan 2009) used a cross‐over design. The remaining 25 trials were parallel design; this included the unpublished study for which we were given access to the complete data by the authors (Kaatz Unpublished).
One trial (Gadisseur 2003 Self mge; Gadisseur 2003 Self monit) presented results on four groups. One group used self‐management therapy (Gadisseur 2003 Self mge), one used self‐monitoring therapy (Gadisseur 2003 Self monit). The two other arms with no self‐monitoring were combined (trained and untrained participants) to provide an overall control group and were then subdivided for the independent comparisons.
Six trials included only participants on life‐long anticoagulation therapy following mechanical valve insertion (Azarnoush 2009; Horstkotte 1998; Körtke 2001; Sidhu 2001; Soliman Hamad 2009; Thompson 2013); two trials included participants on long‐term anticoagulation for atrial fibrillation (Khan 2004; Voller 2005); 20 trials included participants on long‐term anticoagulation for any indication (Beyth 2000; Christensen 2006; Christensen 2011; Cromheecke 2000; Dignan 2013; Fitzmaurice 2002; Fitzmaurice 2005; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Gardiner 2005; Grunau 2011; Kaatz Unpublished; Menendez‐Jandula 2005; Rasmussen 2012; Ryan 2009; Sawicki 1999; Siebenhofer 2007; Sunderji 2004; Verret 2012; White 1989). In 15 trials the intervention group used self‐management (Christensen 2006; Cromheecke 2000; Dignan 2013; Fitzmaurice 2002; Fitzmaurice 2005; Grunau 2011; Körtke 2001; Menendez‐Jandula 2005; Sawicki 1999; Sidhu 2001; Siebenhofer 2007; Soliman Hamad 2009; Sunderji 2004; Verret 2012; Voller 2005) and 12 trials used self‐monitoring (Azarnoush 2009; Beyth 2000; Christensen 2011; Gardiner 2005; Horstkotte 1998; Kaatz Unpublished; Khan 2004; Matchar 2010; Rasmussen 2012; Ryan 2009; Thompson 2013; White 1989). One further study (Gadisseur 2003 Self mge; Gadisseur 2003 Self monit), reported information on both self‐management and self‐monitoring groups. Eleven trials used primary care for the control group (Beyth 2000; Fitzmaurice 2002; Grunau 2011; Horstkotte 1998; Körtke 2001; Rasmussen 2012; Sawicki 1999; Sidhu 2001; Sunderji 2004; Thompson 2013; Voller 2005); 13 studies used specialist anticoagulation clinics (Christensen 2011; Cromheecke 2000; Fitzmaurice 2005; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Gardiner 2005; Kaatz Unpublished; Khan 2004; Matchar 2010; Menendez‐Jandula 2005; Ryan 2009; Soliman Hamad 2009; Verret 2012; White 1989); and one trial (Azarnoush 2009) used a medical analysis laboratory. In the three remaining trials participants in the control group could use either primary care or specialist clinics (Christensen 2006; Dignan 2013; Siebenhofer 2007). Duration of studies varied from two months (White 1989) to more than 24 months (Körtke 2001; Matchar 2010); the mean duration was 12 months.
Analysis of publication bias using funnel plots of major haemorrhage and thromboembolic events showed no evidence of asymmetry (Figure 2, Figure 3).
2.

Funnel plot of comparison: 1 Major haemorrhage, outcome
3.

Funnel plot of comparison: 2 Thromboembolic events
Risk of bias in included studies
The reported risk of bias was generally low to moderate. The nature of the intervention made blinding of the allocation of intervention to the participants impossible, although blinding of study staff and outcome assessment was possible. We contacted nine authors of the 27 included trials for additional details of randomisation process, concealment of allocation, and blinding. The additional information provided generally raised our ratings of the quality of the trial, indicating that authors had met methodological criteria. We also obtained valuable validity information from the ACP Journal Club structured reviews on two occasions. ACP reviews contact study authors when needed and are a valuable source of additional information for validity issues.
After the addition of extra information supplied by authors, 11 trials were judged to be of high risk of bias (Azarnoush 2009; Christensen 2011; Gardiner 2005; Khan 2004; Matchar 2010; Rasmussen 2012; Sidhu 2001; Soliman Hamad 2009; Thompson 2013; Verret 2012; White 1989) and were removed in the sensitivity analysis. These 11 trials did not perform intention‐to‐treat analyses and randomisation and/or allocation concealment was unclear. Overall, the available evidence was judged to be moderate according to the GRADE Working Group grades of evidence (Table 1). This was due to flaws in study design; most commonly there was an absence of information about the allocation concealment procedure or blinding and the number of events was less than 300 for the primary outcomes (Characteristics of included studies). The overall risk of bias graph and summary table are shown in Figure 4 and Figure 5.
Summary of findings 1. Self‐monitoring or self‐management of oral anticoagulation vs. standard care.
| Self‐monitoring or self‐management of oral anticoagulation vs. standard care | |||||
|
Patient or population: Patients on long‐term anticoagulant therapy (treatment duration longer than two months) irrespective of the indication for treatment Settings: Primary care, specialist clinics (Europe, America, Canada) Intervention: Self‐monitoring or self‐management Comparison: Standard care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Self‐monitoring or self‐management | ||||
|
Thromboembolic events Follow‐up: 3 to 57 months |
Study population |
RR 0.58 (0.45 to 0.74) |
7594 (18 studies) |
⊕⊕⊕⊝ Moderate1 | |
| 35 per 1000 |
21 per 1000 (16 to 26) |
||||
| Moderate risk population | |||||
| 22 per 1000 |
12 per 1000 (10 to 16) |
||||
|
All‐cause mortality Follow‐up: 6 to 57 months |
Study population | RR 0.85 (0.71 to 1.01) | 6358 (11 studies) | ⊕⊕⊕⊝ Moderate1 | |
| 64 per 1000 | 54 per 1000 (45 to 64) | ||||
| Moderate risk population | |||||
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
|
Major haemorrhage Follow‐up: 4 to 57 months |
Study population |
RR 0.95 (0.80 to 1.12) |
8018 (20 studies) |
⊕⊕⊕⊝ Moderate1 | |
| 62 per 1000 |
59 per 1000 (50 to 69) |
||||
| Moderate risk population | |||||
| 18 per 1000 |
17 per 1000 (14 to 20) |
||||
|
Minor haemorrhage Follow‐up: 4 to 57 months |
Study population | RR 0.97 (0.67 to 1.41) | 5365 (13 studies) | ⊕⊕⊝⊝ Low2 | |
| 217 per 1000 | 210 per 1000 (145 to 306) | ||||
| Moderate risk population | |||||
| 45 per 1000 | 44 per 1000 (30 to 63) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded from high to moderate because of serious risk of bias.
2 Downgraded from high to low because of serious risk of bias and substantial heterogeneity.
4.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
5.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Randomisation and allocation concealment
Twenty‐one trials reported adequate information about the randomisation process (Christensen 2006; Christensen 2011; Cromheecke 2000; Dignan 2013; Fitzmaurice 2002; Fitzmaurice 2005; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Grunau 2011; Kaatz Unpublished; Khan 2004; Körtke 2001; Menendez‐Jandula 2005; Rasmussen 2012; Ryan 2009; Sawicki 1999; Sidhu 2001; Siebenhofer 2007; Sunderji 2004; Voller 2005; White 1989) and were therefore judged to be of low risk of bias in this domain.
However, the method of allocation concealment was generally not reported in the published papers. After contacting authors for additional information, 14 of the 28 trials had an appropriate method of concealment (Beyth 2000; Christensen 2006; Cromheecke 2000; Fitzmaurice 2002; Fitzmaurice 2005; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Kaatz Unpublished; Körtke 2001; Menendez‐Jandula 2005; Ryan 2009; Siebenhofer 2007; Sunderji 2004; Voller 2005). Four studies used both concealment of allocation and intention‐to‐treat (Christensen 2006; Fitzmaurice 2005; Menendez‐Jandula 2005; Siebenhofer 2007) (see Figure 4 and Figure 5).
Blinding
Participant blinding was not possible due to the nature of the intervention. Seven studies included information about blinding. Two trials blinded data collectors (Beyth 2000; Sawicki 1999), three blinded healthcare providers (Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Rasmussen 2012), and six trials blinded outcome assessors (Cromheecke 2000; Dignan 2013; Fitzmaurice 2005; Grunau 2011; Menendez‐Jandula 2005; Siebenhofer 2007) (see Figure 4 and Figure 5).
Follow‐up
Of those assigned to the intervention, 25% (range 0% to 57%) stopped self‐monitoring or self‐management by the end of the trial. Nine trials used an intention‐to‐treat analysis (Beyth 2000; Dignan 2013; Fitzmaurice 2005; Grunau 2011; Kaatz Unpublished; Matchar 2010; Menendez‐Jandula 2005; Sawicki 1999; Sunderji 2004). All included studies described appropriate participant follow‐up (see Figure 4 and Figure 5).
Financial support
Eight studies (Beyth 2000; Cromheecke 2000; Grunau 2011; Horstkotte 1998; Kaatz Unpublished; Körtke 2001; Soliman Hamad 2009; Thompson 2013) did not describe financial support. Six studies were supported by grants from professional associations or national agencies (Christensen 2006; Fitzmaurice 2005; Khan 2004; Matchar 2010; Sunderji 2004; White 1989). Twelve studies (Azarnoush 2009; Fitzmaurice 2002; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Gardiner 2005; Menendez‐Jandula 2005; Ryan 2009; Sawicki 1999; Sidhu 2001; Siebenhofer 2007; Verret 2012; Voller 2005) were part funded by an unrestricted research grant from industry or received the coagulometer and strips for utilisation during the study. One study was funded by a private hospital (Christensen 2011); and two by a combination of government and private agencies (Dignan 2013; Rasmussen 2012).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 2. Self‐monitoring of oral anticoagulation vs. standard care.
| Self‐monitoring of oral anticoagulation vs. standard care | |||||
|
Patient or population: Patients on long‐term anticoagulant therapy (treatment duration longer than two months) irrespective of the indication for treatment Settings: Primary care, specialist clinics (Europe, America, Canada) Intervention: Self‐monitoring Comparison: Standard care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Self‐monitoring | ||||
|
Thromboembolic events Follow‐up: 3 to 57 months |
Study population | RR 0.69 (0.49 to 0.97) | 4097 (7 studies) | ⊕⊕⊕⊝ Moderate2 | |
| 35 per 1000 | 24 per 1000 (17 to 34) | ||||
| Moderate risk population | |||||
| 34 per 1000 | 23 per 1000 (17 to 33) | ||||
|
All‐cause mortality Follow‐up: 6 to 57 months |
Study population | RR 0.94 (0.78 to 1.15) | 3300 (3 studies) | ⊕⊕⊕⊝ Moderate2 | |
| 90 per 1000 | 85 per 1000 (70 to 104) | ||||
| Moderate risk population | |||||
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
|
Major haemorrhage Follow‐up: 4 to 57 months |
Study population | RR 0.90 (0.74 to 1.09) | 4038 (7 studies) | ⊕⊕⊝⊝ Low1 | |
| 91 per 1000 | 82 per 1000 (67 to 99) | ||||
| Moderate risk population | |||||
| 49 per 1000 | 44 per 1000 (36 to 53) | ||||
|
Minor haemorrhage Follow‐up: 4 to 57 months |
Study population | RR 1.16 (0.95 to 1.42) | 3503 (6 studies) | ⊕⊕⊕⊝ Moderate2 | |
| 275 per 1000 | 319 per 1000 (259 to 391) | ||||
| Moderate risk population | |||||
| 188 per 1000 | 218 per 1000 (177 to 267) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded from high to low because of serious risk of bias and strong suspicion of publication bias.
2 Downgraded from high to moderate because of serious risk of bias.
Summary of findings 3. self‐management of oral anticoagulation vs. standard care.
| Self‐management of oral anticoagulation vs. standard care | |||||
|
Patient or population: Patients on long‐term anticoagulant therapy (treatment duration longer than two months) irrespective of the indication for treatment Settings: Primary care, specialist clinics (Europe, America, Canada) Intervention: Self‐management Comparison: Standard care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Self‐management | ||||
|
Thromboembolic events Follow‐up: 3 to 57 months |
Study population | RR 0.47 (0.31 to 0.70) | 3497 (11 studies) | ⊕⊕⊕⊝ Moderate2 | |
| 36 per 1000 | 17 per 1000 (12 to 25) | ||||
| Moderate risk population | |||||
| 16 per 1000 | 7 per 1000 (5 to 11) | ||||
|
All‐cause mortality Follow‐up: 6 to 57 months |
Study population | RR 0.55 (0.36 to 0.84) | 3058 (8 studies) | ⊕⊕⊕⊝ Moderate2 | |
| 33 per 1000 | 18 per 1000 (12 to 28) | ||||
| Moderate risk population | |||||
| 17 per 1000 | 9 per 1000 (6 to 14) | ||||
|
Major haemorrhage Follow‐up:4 to 57 months |
Study population | RR 1.08 (0.79 to 1.47) | 3980 (13 studies) | ⊕⊕⊝⊝ Low1 | |
| 33 per 1000 | 36 per 1000 (22 to 44) | ||||
| Moderate risk population | |||||
| 18 per 1000 | 19 per 1000 (14 to 26) | ||||
|
Minor haemorrhage Follow‐up: 4 to 57 months |
Study population | RR 0.91 (0.47 to 1.76) | 1862 (7 studies) | ⊕⊕⊝⊝ Low3 | |
| 137 per 1000 | 125 per 1000 (64 to 241) | ||||
| Moderate risk population | |||||
| 2 per 1000 | 2 per 1000 (1 to 4) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded from high to low because of serious risk of bias and imprecision of effect estimate.
2 Downgraded from high to moderate because of serious risk of bias.
3 Downgraded from high to low because of serious risk of bias and substantial heterogeneity.
Primary endpoints
Thromboembolic events
Twenty‐six trials reported thromboembolic outcomes; however, eight trials showed no events in either the intervention or control arm (Christensen 2006; Dignan 2013; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Gardiner 2005; Grunau 2011; Khan 2004; Verret 2012), and were therefore not included in the pooled analysis (chapter 16.9.3, Higgins 2011). Eighteen trials provided the information to calculate the overall effect size (Azarnoush 2009; Beyth 2000; Cromheecke 2000; Dignan 2013; Fitzmaurice 2005; Horstkotte 1998; Kaatz Unpublished; Körtke 2001; Matchar 2010; Menendez‐Jandula 2005; Ryan 2009; Sawicki 1999; Sidhu 2001; Siebenhofer 2007; Soliman Hamad 2009; Sunderji 2004; Voller 2005; White 1989).
Compared to standard therapy, self‐monitoring or self‐management reduced thromboembolic events (risk ratio (RR) 0.58, 95% confidence interval (CI) 0.45 to 0.75; participants = 7594; studies = 18) (Analysis 1.1). The overall quality of evidence was downgraded from high to moderate because of serious risk of bias (Table 1). The findings were not affected by the removal of the five trials deemed to have high risk of bias (RR 0.50, 95% CI 0.36 to 0.69; participants = 4558; studies = 13) (Analysis 1.3).
1.1. Analysis.

Comparison 1: Thromboembolic events, Outcome 1: Self‐monitoring and self‐management
1.3. Analysis.

Comparison 1: Thromboembolic events, Outcome 3: Events by Self‐management (sensitivity)
In those groups that self‐managed (Cromheecke 2000; Fitzmaurice 2005; Körtke 2001; Menendez‐Jandula 2005; Sawicki 1999; Soliman Hamad 2009; Sidhu 2001; Siebenhofer 2007; Sunderji 2004; Verret 2012; Voller 2005), the effect was larger (RR 0.47, 95% CI 0.31 to 0.70; participants = 3497; studies = 11; Table 3) than in the groups that self‐monitored (RR 0.69, 95% CI 0.49 to 0.97; participants = 4097; studies = 7); Table 2) (Azarnoush 2009; Beyth 2000; Horstkotte 1998; Kaatz Unpublished; Matchar 2010; Ryan 2009; White 1989). In either group, the quality of the evidence was downgraded from high to moderate because of serious risk of bias. However, the subgroup interaction was non‐significant (P = 0.66).
Compared to standard therapy, self‐monitoring or self‐management in patients with mechanical valves (Azarnoush 2009; Horstkotte 1998; Körtke 2001; Sidhu 2001; Soliman Hamad 2009; Thompson 2013) resulted in a significant effect on thromboembolic events (RR 0.53, 95% CI 0.32 to 0.90; participants = 1816; studies = 6) (Analysis 1.2). The post‐hoc subgroup analysis for specialised care (RR 0.63, 95% CI 0.44 to 0.90; participants = 4947; studies = 8) and family physician care (RR 0.56, 95% CI 0.38 to 0.84; participants = 2397; studies = 8) (Analysis 1.4) showed both to be significant (subgroup interaction P = 0.33).
1.2. Analysis.

Comparison 1: Thromboembolic events, Outcome 2: Events by Clinical Condition
1.4. Analysis.

Comparison 1: Thromboembolic events, Outcome 4: Events by specialty
All‐cause mortality
Twenty‐two trials reported information on mortality; 11 trials did not report any deaths in the intervention and control groups and are therefore excluded form the pooled analysis (Azarnoush 2009; Christensen 2006; Christensen 2011; Dignan 2013; Horstkotte 1998; Kaatz Unpublished; Khan 2004; Sunderji 2004; Verret 2012; Voller 2005; White 1989) (chapter 16.9.3, Higgins 2011). Eleven trials (Beyth 2000; Fitzmaurice 2002; Fitzmaurice 2005; Gardiner 2005; Körtke 2001; Matchar 2010; Menendez‐Jandula 2005; Sawicki 1999; Sidhu 2001; Siebenhofer 2007; Soliman Hamad 2009) reported all‐cause mortality events. No reduction in all‐cause mortality was found (RR 0.85, 95% CI 0.71 to 1.01; participants = 6358; studies = 11) (Analysis 2.1). The overall quality of evidence was downgraded from high to moderate because of serious risk of bias (Table 1). Removal of three studies deemed to have high risk of bias resulted in no difference of the effect (RR 0.85, 95% CI 0.71 to 1.02; participants = 6160; studies = 8) (Analysis 2.3). In three studies of participants with mechanical valves (Körtke 2001; Sidhu 2001; Soliman Hamad 2009) self‐monitoring or self‐management showed a significant reduction in mortality (RR 0.50, 95% CI 0.29 to 0.86; participants = 1295; studies = 3) (Analysis 2.2).
2.1. Analysis.

Comparison 2: All‐cause mortality, Outcome 1: Events by Self‐management
2.3. Analysis.

Comparison 2: All‐cause mortality, Outcome 3: Events by Self‐management (sensitivity)
2.2. Analysis.

Comparison 2: All‐cause mortality, Outcome 2: Events by Clinical Condition
Two studies (Khan 2004; Voller 2005) reported on participants with atrial fibrillation, no deaths were reported. A reduction in mortality occurred in participants who self‐managed (Fitzmaurice 2002; Fitzmaurice 2005; Körtke 2001; Menendez‐Jandula 2005; Sawicki 1999; Sidhu 2001; Siebenhofer 2007; Soliman Hamad 2009) compared to standard therapy (RR 0.55, 95% CI 0.36 to 0.84; participants = 3058; studies = 8) (Analysis 2.1). The overall quality of evidence was downgraded from high to moderate because of serious risk of bias (Table 3). No effect was found for the self‐monitoring only trials (Beyth 2000; Gardiner 2005; Matchar 2010) (RR 0.94, 95% CI 0.78 to 1.15; participants = 3300; studies = 3) (Analysis 2.1); The overall quality of evidence was downgraded from high to moderate because of serious risk of bias (Table 2). The subgroup interaction was significant (P = 0.02). The post‐hoc subgroup analysis for specialised care (RR 0.92, 95% CI 0.75 to 1.13; participants = 4387; studies = 5) and family physician care (RR 0.62, 95% CI 0.43 to 0.90; participants = 1776; studies = 5) (Analysis 2.4) showed only family physician care to be significant; however the subgroup interaction was not significant (P = 0.06).
2.4. Analysis.

Comparison 2: All‐cause mortality, Outcome 4: Events by specialty
Major haemorrhage
Twenty‐four trials reported major haemorrhage outcomes, four of which did not report any events (Christensen 2006; Cromheecke 2000; Gardiner 2005; White 1989). Twenty trials provided the information to calculate the overall effect size (Azarnoush 2009; Beyth 2000; Dignan 2013; Fitzmaurice 2002; Fitzmaurice 2005; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Horstkotte 1998; Kaatz Unpublished; Khan 2004; Körtke 2001; Matchar 2010; Menendez‐Jandula 2005; Sawicki 1999; Sidhu 2001; Siebenhofer 2007; Soliman Hamad 2009; Sunderji 2004; Verret 2012; Voller 2005). Compared to standard therapy, self‐monitoring or self‐management were associated with no reduction in major haemorrhage (RR 0.95, 95% CI 0.80 to 1.12; participants = 8018; studies = 20) (Analysis 3.1). The overall quality of evidence was downgraded from high to moderate because of serious risk of bias (Table 1). After removal of the six studies deemed to have high risk of bias, the result remained similar (RR 0.96, 95% CI 0.81 to 1.14; participants = 7337; studies = 14) (Analysis 3.3). In terms of clinical condition, four studies (Horstkotte 1998; Körtke 2001; Sidhu 2001; Soliman Hamad 2009) included participants with mechanical valves only and two studies (Khan 2004; Voller 2005) reported on participants with atrial fibrillation. No significant differences were found.The inability to distinguish between the two conditions in the remaining trials meant there was insufficient power to determine significance by clinical condition (Analysis 3.2).
3.1. Analysis.

Comparison 3: Major haemorrhage, Outcome 1: Events by Self‐management
3.3. Analysis.

Comparison 3: Major haemorrhage, Outcome 3: Events by Self‐management (sensitivity)
3.2. Analysis.

Comparison 3: Major haemorrhage, Outcome 2: Events by Clinical Condition
In those who self‐monitored (Azarnoush 2009; Beyth 2000; Gadisseur 2003 Self mge; Horstkotte 1998; Kaatz Unpublished; Khan 2004; Matchar 2010), there was no significant reduction in the number of events that occurred compared to standard therapy (RR 0.90, 95% CI 0.74 to 1.09; participants = 4038; studies = 7). The quality of the evidence was downgraded from high to low because of serious risk of bias and strong suspicion of publication bias (Table 2). Self‐management (Dignan 2013; Fitzmaurice 2002; Fitzmaurice 2005; Gadisseur 2003 Self monit; Körtke 2001; Menendez‐Jandula 2005; Sawicki 1999; Sidhu 2001; Siebenhofer 2007; Soliman Hamad 2009; Sunderji 2004; Verret 2012; Voller 2005) was comparable with standard therapy (RR 1.08, 95% CI 0.79 to 1.47; participants = 3980; studies = 13) (Analysis 3.1). The subgroup interaction for this outcome, between the two groups, was not significant (P = 0.32). The quality of the evidence was downgraded from high to low because of serious risk of bias and imprecision of effect estimate (Table 3). The post‐hoc subgroup analysis for specialised care (RR 0.98, 95% CI 0.80 to 1.19; participants = 5054; studies = 9) and family physician care (RR 0.93, 95% CI 0.66 to 1.30; participants = 2267; studies = 8) showed no effect (subgroup interaction P = 0.79).
Tests in range
Sixteen trials reported results of mean international normalized ratio (INR) within target range (Cromheecke 2000; Fitzmaurice 2002; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Grunau 2011; Horstkotte 1998; Kaatz Unpublished; Körtke 2001; Menendez‐Jandula 2005; Sawicki 1999; Sidhu 2001; Siebenhofer 2007; Soliman Hamad 2009; Sunderji 2004; Voller 2005; White 1989). All studies but one (Kaatz Unpublished), reported improvements in the self‐monitoring or self‐management groups; six were statistically significant (Horstkotte 1998; Körtke 2001; Menendez‐Jandula 2005; Sidhu 2001; Voller 2005; White 1989). Pooling of the mean percentage of tests in range was not possible as information was collected in two different ways: as the percentage of overall tests in range (Cromheecke 2000; Fitzmaurice 2002; Horstkotte 1998; Körtke 2001; Sawicki 1999; Sidhu 2001; Sunderji 2004; Voller 2005; White 1989), and the percentage of tests for each individual in range (Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Menendez‐Jandula 2005; Ryan 2009). Improvements ranged from 3% to 21%. Eighteen trials reported the percentage time within range (Azarnoush 2009; Beyth 2000; Christensen 2006; Christensen 2011; Fitzmaurice 2002; Fitzmaurice 2005; Gardiner 2005; Grunau 2011; Kaatz Unpublished; Khan 2004; Matchar 2010; Menendez‐Jandula 2005; Rasmussen 2012; Sidhu 2001; Siebenhofer 2007; Sunderji 2004; Thompson 2013; Verret 2012). Seven studies (Azarnoush 2009; Beyth 2000; Christensen 2011; Matchar 2010; Sidhu 2001; Siebenhofer 2007; Thompson 2013) reported a significant improvement in the time in therapeutic range in the self‐monitoring or self‐management groups (see additional tables, Table 4).
1. Tests in range.
| Mean INR within target range, % | TIme within range, % | |||||
| Source | P value | P value | ||||
| White 1989 | 68 | 87 | < 0.001 | ‐ | ‐ | ‐ |
| Horstkotte 1998 | 22.3 | 43.2 | < 0.001 | ‐ | ‐ | ‐ |
| Sawicki 1999 | 43.2 | 53 | 0.22 | ‐ | ‐ | ‐ |
| Beyth 2000 | ‐ | ‐ | ‐ | 32 | 56 | < 0.001 |
| Cromheecke 2000 | 49 | 55 | 0.06 | ‐ | ‐ | ‐ |
| Sidhu 2001 | 58 | 67.60 | < 0.0001 | 63.8 | 76.5 | < 0.0001 |
| Fitzmaurice 2002 | 66 (61‐71)* | 72 (65‐80)* | NS | 77 (67‐86)* | 74 (67‐81)* | NS |
| Gadisseur 2003 Self mge; Gadisseur 2003 Self monit | 61.3 | 65 | 0.14 | ‐ | ‐ | ‐ |
| Gardiner 2005 | ‐ | ‐ | ‐ | 64 (26) | 61 (20) | NS |
| Kaatz Unpublished | 54.2 | 64.6 | < 0.05 | 66.9 | 63.5 | 0.127 |
| Sunderji 2004 | 58.7 (5.8)** | 64.8 (5.9)** | 0.23 | 63.2 (5.8)** | 71.8 (5.5)** | 0.14 |
| Khan 2004 | ‐ | ‐ | ‐ | 70.4 (24.5)** | 71.1 (14.5)** | NS |
| Körtke 2001 | 60.5 | 78.3 | < 0.001 | ‐ | ‐ | ‐ |
| Voller 2005 | 58.5 (19.8)** | 67.8 (17.6)** | 0.0061 | ‐ | ‐ | ‐ |
| Menendez‐Jandula 2005 | 55.6 (19.6)** | 58.6% (14.3)** | 0.02 | 64.9 (19.9) | 64.3 (14.3) | 0.2 |
| Fitzmaurice 2005 | ‐ | ‐ | ‐ | 68 (65.2‐70.6) | 70 (68.1‐72.4) | NS |
| Christensen 2006, Denmark | ‐ | ‐ | ‐ | 68.9 (59.3‐78.2) | 78.7 (69.2‐81.0) | NS |
| Siebenhofer 2007, Austria† | 57.1 (40.4‐72.4) | 72.4 (53.5‐79.4) | < 0.001 | 66.5 (47.1‐81.5) | 75.4 (59.4‐85.0) | < 0.029 |
| Azarnoush 2009 | ‐ | ‐ | ‐ | 61.5 | 55.5 | 0.0343 |
| Christensen 2011 | ‐ | ‐ | ‐ | 79.9 | 72.7 | < 0.0001 |
| Grunau 2011 | 82.4 | 80.2 | 0.82 | 82.2 | 89.7 | 0.76 |
| Matchar 2010 | ‐ | ‐ | ‐ | 66.2 | 62.4 | < 0.001 |
| Rasmussen 2012 | ‐ | ‐ | ‐ | Intervention A 49 Intervention B 55 |
55 | NS |
| Soliman Hamad 2009 | 72.9 | 53.9 | 0.01 | ‐ | ‐ | ‐ |
| Thompson 2013 | ‐ | ‐ | ‐ | 52 | 45 | 0.05 |
| Verret 2012 | ‐ | ‐ | ‐ | 80 | 75.5 | 0.79 |
* 95% Confidence intervals
** Standard Deviations
† Used median not mean
The method used to estimate the time within therapeutic INR target range in 16 studies was linear interpolation (Beyth 2000; Christensen 2006; Fitzmaurice 2002; Fitzmaurice 2005; Gardiner 2005; Grunau 2011; Kaatz Unpublished; Khan 2004; Matchar 2010; Menendez‐Jandula 2005; Rasmussen 2012; Ryan 2009; Sidhu 2001; Siebenhofer 2007; Sunderji 2004; Verret 2012).
Secondary endpoints
Minor haemorrhage
Eighteen trials reported minor haemorrhage outcomes, with 13 reporting events (Azarnoush 2009; Cromheecke 2000; Fitzmaurice 2002; Fitzmaurice 2005; Gardiner 2005; Kaatz Unpublished; Khan 2004; Matchar 2010; Menendez‐Jandula 2005; Sawicki 1999; Sidhu 2001; Verret 2012; White 1989). Compared to standard therapy, self‐monitoring or self‐management there was no difference in minor haemorrhage (RR 0.97, 95% CI 0.67 to 1.41; participants = 5365; studies = 13) (Analysis 4.1), but results varied considerably (I2 = 82%). The overall quality of evidence was downgraded from high to low because of serious risk of bias and substantial heterogeneity (Table 1).
4.1. Analysis.

Comparison 4: Minor haemorrhage, Outcome 1: Events by Self‐management
There was no reduction in minor haemorrhage with self‐management (RR 0.91, 95% CI 0.47 to 1.76; participants = 1862; studies = 7), or with self‐monitoring (RR 1.16, 95% CI 0.95 to 1.42; participants = 3503; studies = 6) (Analysis 4.1). The quality of the evidence for self‐monitoring was downgraded from high to moderate because of serious risk of bias (Table 2). The quality of evidence for self‐management was downgraded from high to low because of serious risk of bias and high heterogeneity (82%) (Table 3). Only one trial (Menendez‐Jandula 2005) showed a significant effect on minor haemorrhage with self‐management (RR 0.41, 95% CI 0.31 to 0.54).
Frequency of testing
Fourteen studies reported on the total number of tests performed throughout the study (Fitzmaurice 2002; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Horstkotte 1998; Körtke 2001; Menendez‐Jandula 2005; Sidhu 2001; Siebenhofer 2007; Soliman Hamad 2009; Sunderji 2004; Thompson 2013; Verret 2012; Voller 2005; White 1989). Maximum test frequency occurred in the study with the shortest duration (White 1989). The ratio of tests in the self‐monitoring or self‐management groups compared to the control groups ranged from 1.00 to 4.98; this ratio increased with duration of study (test for linear trend P < 0.002). Due to inadequate data, we were unable to rate the quality of the evidence.
Feasibility of testing
A population of 11,738 was sampled in 15 trials (Beyth 2000; Christensen 2006; Fitzmaurice 2002; Fitzmaurice 2005; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Gardiner 2005; Khan 2004; Kaatz Unpublished; Körtke 2001; Menendez‐Jandula 2005; Sawicki 1999; Sidhu 2001; Sunderji 2004; Siebenhofer 2007). Of that population, 7974 were either excluded or decided not to take part. The average proportion of people that could not (or would not) take part in the trials was 68% (range 31% to 88%). In trials which included older populations (Beyth 2000; Fitzmaurice 2005), the exclusion rates were much higher. Of the participants assigned to the intervention 24.9% (range 0% to 57.3%) were unable to complete self‐monitoring or self‐management. The main reasons for the dropouts were: problems with the device, physical limitations preventing self‐testing and problems with attending the training assessments or failing the assessment. Due to inadequate data, we were unable to rate the quality of the evidence.
Other outcomes
Thirteen studies evaluated quality of life outcomes. These included ease of use (Gardiner 2005), anxiety caused by testing (Kaatz Unpublished), beliefs specific to warfarin (Khan 2004), and quality of life (Cromheecke 2000; Fitzmaurice 2002; Fitzmaurice 2005; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Grunau 2011; Khan 2004; Kaatz Unpublished; Matchar 2010; Sawicki 1999; Soliman Hamad 2009; Verret 2012 ). Khan 2004 evaluated health status and quality of life using a validated tool, the 36‐item United Kingdom Short Form Health Survey (UKSF‐36) and the European Quality of Life questionnaire (Euroqol). Fitzmaurice 2002 used the individual quality of life (SEIQoL) tool for estimating quality of life and reported on results of participant interviews (Fitzmaurice 2005). Grunau 2011 used a treatment‐related satisfaction survey measuring five categories; Matchar 2010 used the Health Utilities Index Mark; Soliman Hamad 2009 used the SF‐36v2 questionnaire; and Verret 2012 used a validated questionnaire including 32 statements covering five socio‐psychological topics: general treatment satisfaction, self‐efficacy, daily hassles, psychological distress, and strained social network. Four trials (Cromheecke 2000; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Sawicki 1999) used a questionnaire designed by Sawicki on patients' feelings towards anticoagulation therapy. Six studies (Cromheecke 2000; Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Matchar 2010; Sawicki 1999; Verret 2012) showed a significant difference in treatment satisfaction. In addition, one study (Gadisseur 2004) (one of the articles from Gadisseur 2003 Self mge; Gadisseur 2003 Self monit) reported quality of life outcomes showing greater treatment satisfaction in the self‐monitoring group compared to the self‐management group. One study (Matchar 2010), reported that there were no adverse events resulting from physical interaction with the testing device. Due to inadequate data, we were unable to rate the quality of the evidence for quality of life and satisfaction.
Optimal information size
The calculated optimal information size needed for a reliable and conclusive treatment effect was 5150 in each arm. This assumed a 2% thromboembolic event rate in the control group, a 50% RR reduction, a power of 95%, and a two‐sided alpha = 0.01. The current meta‐analysis has approximately 4000 in each arm, which would give a 78% power using the same assumptions.
One of the main trials included in the meta‐analysis showed a clear absence of correlation between the benefits observed and the degree of control (Menendez‐Jandula 2005). We therefore questioned the influence of this study by performing a post hoc sensitivity analysis that removed the trial; the beneficial effects observed for all the major outcomes remained similar.
Discussion
To our knowledge the present review is the most comprehensive review to date. We identified 28 randomised controlled trials (RCTs) trials (8950 participants). Self‐monitoring or self‐management of oral anticoagulation leads to a significant 50% reduction in thromboembolism but no reduction in all‐cause mortality. However, trials of self‐management led to a significant reduction in all‐cause mortality. Self‐management did not reduce major haemorrhages nor did self‐monitoring.
Quality of the evidence
The GRADE approach was employed to interpret result findings and the GRADE profiler (GRADEPRO) allowed us to import data from Review Manager to create 'Summary of findings' tables. The overall quality of the evidence for the effect of self‐monitoring or self‐management of oral anticoagulation on major haemorrhage was moderate; the quality was downgraded because of serious risk of bias across the studies. The quality of the evidence for trials of self‐management was downgraded from high to low because of serious risk of bias and imprecision of effect estimate (i.e. large confidence intervals). The quality of the evidence for trials of self‐monitoring was downgraded from high to low because of serious risk of bias across the studies and strong suspicion of publication bias.
The overall quality of the evidence for thromboembolic events was moderate; downgraded because of serious risk of bias across the included studies. The quality of the evidence for trials of either self‐monitoring or self‐management were downgraded from high to moderate because of serious risk of bias. The overall quality of the evidence for mortality was moderate; downgraded because of serious risk of bias across the included studies. The quality of the evidence for trials of either self‐monitoring or self‐management were downgraded from high to moderate because of serious risk of bias.
The overall quality of the evidence for minor haemorrhage of the evidence was low; downgraded because of serious risk of bias and substantial heterogeneity in the meta‐analysis result. The quality of the evidence for trials of self‐management was downgraded from high to low because of serious risk of bias and substantial heterogeneity. The quality of the evidence for trials of self‐monitoring was downgraded from high to moderate because of serious risk of bias across the included studies. Due to inadequate data, we were unable to rate the quality of the evidence for the following outcomes: (i) Frequency of testing; (ii) Feasibility of testing; and (iii) Quality of life and satisfaction.
Comparison with other reviews
This systematic review provides information additional to a substantial body of evidence from previously published reviews of self‐monitoring or self‐management of oral anticoagulation (Bazian 2005; Bloomfield 2011Christensen 2007; Connock 2007; de Solà‐Morales 2005; Heneghan 2006a; Ødegaard 2004; Siebenhofer 2004) and a meta‐analysis of individual patient data (Heneghan 2012).
The main results of this review are consistent with previous reviews. The Christensen 2007 review of 10 trials showed that self‐management was associated with a reduced risk of mortality and major complications with increased time being spent within the therapeutic INR target range. A 2004 review of eight trials (Ødegaard 2004) identified a significant reduction in major clinical events and a 2004 review of four trials (Siebenhofer 2004) concluded that patient self‐management is safe and can improve the quality of anticoagulation control. A 2005 review of 12 trials (seven RCTs and five quasi‐experimental trials) (de Solà‐Morales 2005), reported no difference between participants undertaking self‐management and those receiving usual care in the time spent in the therapeutic range and in the incidence of adverse effects. Bazian's review (which was less comprehensive) also did not show a difference between self‐management and routine care (Bazian 2005). In the Bloomfield 2011 review, patients assigned to self‐monitoring or self‐management had significantly lower total mortality, lower risk for major thromboembolism, and no increased risk in major haemorrhage.
An individual patient data meta‐analysis (Heneghan 2012), which included 11 trials with data for 6417 participants and 12,800 person‐years of follow‐up, reported a significant reduction in thromboembolic events in the self‐monitoring group (Hazard Ratio (HR) 0.51; 95% CI 0.31 to 0.85), but not for major haemorrhagic events (HR 0.88, 95% CI, 0.74 to 1.06) or mortality (HR 0.82, 95% CI 0.62 to 1.09). In this review patients, younger than 55 years showed marked reductions in thrombotic events (HR 0.33, 95% CI 0.17 to 0.66), as did patients with mechanical heart valve (HR 0.52, 95% CI, 0.35 to 0.77). The greater reduction in mortality with self‐management compared with self‐monitoring observed in this review might be related to the higher frequency of thromboembolic events seen in the latter group. Also, reduced mortality might be affected by increased patient empowerment, whereby self‐management influences other aspects of a patient's lifestyle (i.e. adherence to treatments) as they take on more of a locus of control for their condition.
A 2015 Health Technology Assesment (HTA) systematic review (Sharma 2015) on the clinical effectiveness and cost‐effectiveness of point‐of‐care tests of people receiving long‐term vitamin K antagonist therapy reported that self‐monitoring significantly prevented thromboembolic events and reduced all‐cause mortality in people with artificial heart valves, and similarly to this current review, reported greater reductions in thromboembolic events and all‐cause in those self‐managing. In addition, the review reported net UK health and social care costs, which over a 10‐year period were equivalent to standard monitoring costs.
Intrinsic limitations to self‐monitoring and self‐management include the reluctance of individuals to participate in self‐management and the extensive training required to do so. Self‐monitoring is not feasible for up to half of the patients requiring anticoagulation. Factors influencing patient participation within trials included problems with the device; physical limitations; attending training sessions; or failing the assessment. An additional problem with adoption in clinical practice will be the relatively high cost of the test strips. The reliability of self‐testing devices can affect test results; however, available devices give INR results which are comparable with those obtained in laboratory testing (Ansell 2005). Self‐monitoring and self‐management are also associated with a rate of testing that is higher than with usual care. In effect self‐managed warfarin dosing is analogous to self‐adjusted insulin dosing according to a pre‐specified sliding scale (Ansell 1996). Such self‐managed treatment has been practiced for years by people with diabetes (Ansell 1996), and the use of self‐monitoring or self‐management offers independence and freedom to travel for selected patients.
Our review has some potential limitations. First, our search was comprehensive, making serious publication bias less likely, but it remains a concern. Therefore, the results may represent an overestimate of the true effect of treatment. Second, variability in the quality of care in the control groups can affect the rate of testing and hence the benefit and safety of standard anticoagulation monitoring. Specialist programmes may improve outcomes by the same mechanism as self‐monitoring or self‐management, that is improving the time in therapeutic range and lessening the frequency of adverse outcomes. However, our post hoc subgroup analysis did not verify this effect. A further modifying factor is education and training. The two trials in which patients consented to participate and received education alone had better results than did those patients allocated to routine care (Gadisseur 2003 Self mge; Gadisseur 2003 Self monit; Khan 2004). Third, for all the major outcomes of this review, limitations in the published reports led to an absence of information about the allocation concealment procedure or blinding. However, several authors were successfully contacted and the additional information that they provided generally raised the assessed quality of the trials. This finding is in agreement with recent empirical evidence suggesting that authors fail to report concealment of randomisation and blinding (Devereaux 2004). Finally, for all the major outcomes there was a low numbers of events. Overestimates are likely in trials with fewer than 500 events and large overestimates of the effects are more likely in trials with fewer than 200 events. (Bassler 2010)
Self‐monitoring or self‐management are likely to prevent thromboembolism to a greater extent than standard monitoring. The mechanism of effect is probably through increasing the number of INR values in range and therefore the longer time that patients are in the therapeutic range. Despite the limitations outlined above the apparent beneficial effects are large, and even smaller true underlying effects would probably justify widespread use of self‐monitoring and self‐management of oral anticoagulation in suitable candidates.
Authors' conclusions
Implications for practice.
Self‐monitoring or self‐management by patients can improve the quality of oral anticoagulation therapy compared to standard monitoring. Patients spend more time within the therapeutic range resulting in decreases in thromboembolic events with no increase in harms. Self‐monitoring or self‐management is potentially not feasible for half of the patients requiring anticoagulation. In addition, National Institute for Health and Care Excellence (NICE) guidance in the UK (NICE diagnostics guidance [DG14]) for atrial fibrillation and heart valve disease currently recommends both the CoaguChek XS and InRatio2 PT/INR meters for patients if they are able and suitably trained. NICE also recommends not routinely offering self‐monitoring to patients who have had a deep vein thrombosis or a pulmonary embolism (NICE Venous thromboembolic diseases (CG144). The British Committee for Standards in Haematology guidance (Jennings 2014) highlights the need for motivated patients to demonstrate competency and to be trained. Patients should also be reviewed at least every six months with documentation of their results and dosing, with external quality assessment to be undertaken at least every six months. The guidance also recommends that an INR > 8·0 (if confirmed on a repeat sample) requires a venous sample to be analysed in a hospital laboratory and that medical advice is sought.
Implications for research.
For the results to be generalisable to the population at large, there is a need for population‐based studies that collect data on adverse event rates, time in range, and factors that impinge on successful self‐monitoring and self‐management (Nagler 2014). The nature of this intervention lends itself to a registry to guarantee its safety and effectiveness in clinical practice. Future studies should set out to understand why people decide to use self‐management (or not) and should incorporate consumer knowledge about self‐management, triggers to seek care, self‐efficacy or self‐confidence to self‐manage, and perceived or actual support. Further studies should explore components of the intervention that affect the feasibility of self‐monitoring and self‐management and identify means to improve uptake and effectiveness. In addition, given the low rates of adverse events in trials of self‐monitoring, studies comparing its use to newer oral anticoagulants, which do not require monitoring, are warranted.
Feedback
New Feedback,
Summary
Date of Submission: 20th October 2011 Name: Dominique Roberfroid Email Address: dominique.roberfroid@kce.fgov.be Personal Description: Occupation epidemiologist Feedback: Revisiting the 'unbelievable' protective effects of Patient Self‐Management with POC Anticoagulation?
Dear Authors, You conclude that the Point of Care approach (POC) associated with Patient Self Management (PSM) reduces all‐cause mortality by 45% (RR 0.55, 95% CI 0.36 to 0.84) over a follow‐up period of 2 years, in comparison to patients with a laboratory‐based monitoring of coagulation parameters. However, we believe that such statement is not supported by available evidence. To date, fourteen studies on POC+PSM reported all‐cause mortality as an outcome. However, the results are estimable in only 7 of them. On the basis of these 7 studies, the all cause mortality rate would decrease by 45% (95%CI: 16%‐64%) for patients using POC+PSM (Figure 9 of your review). Similar results were reported by other authors [1]. As displayed in the forest plot (figure 9 of your review), one specific study is very influential in the analysis of POC+PSM, the one by Koertke et al. [2], which is the long‐term follow‐up of another study by the same authors [3]. That study bears a 59.0% weight in the meta‐analysis POC+PSM. Thus the quality of that study determines the strength or the weakness of the analysis. Strikingly, that study presents a number of important flaws:
1. The randomization procedure is unclear and allocation concealment is not described.
2. Only 930 patients over the 1,155 patients (80.5%) in the initial trial 4 participated in the follow‐ up study (442 in the control group and 488 in the PSM group). How the patient selection was done is not described. We don’t know how much the 930 follow‐up patients differed from the 1,155 patients of the initial study. There are indirect clues of selection bias. In the 2001 paper, no differences at baseline between groups were reported (although the results were not displayed). In contradiction, in the follow‐up study, important and clinically relevant differences between groups at baseline were obvious (table 1 in [2)]. Patients in the control group were significantly in worse health condition at baseline than PSM patients: 5.9% were in NYHA functional class vs. 2.0% of PSM patients (p=0.04), and 10.2 % had undergone a double valve replacement vs. 5.3% of the PSM patients (p=0.01). Authors of the paper acknowledged that a double valve replacement bears a higher mortality risk. Age difference between groups at baseline is not reported in the paper, although being also a determinant of lethality. As the patient selection is not described in the follow‐up study, we cannot conclude if such differences were due to selection bias or differential lost to follow‐up, but this question is of secondary interest.
3. The authors reported 236 deaths over the follow‐up period (94 in PSM patients, 142 in controls). However, when these numbers are subtracted from the initial numbers of participants, 300 and 394 participants should remain at months 120 in the control and PSM group, respectively. In figure 2 of the paper by Koertke et al. [2], these numbers are 260 and 305. So, 40 were lost to follow‐up in the control group and 89 in the PSM group. Such difference could explain some or most of the mortality difference between groups. However, the authors did not discuss the fact nor provide a description of these lost to follow‐ups, in particular if they presented differential characteristics between groups. Authors laconically reported that “122 patients were either noncompliant within the first two years of the study or switched over from INR self‐management to INR measurement by a GP and vice‐versa during follow‐up (p27 in [2]). On the basis of these elements, the study can be rated low quality. Christensen et al. also categorized the study by Koertke et al. in?lower quality trials? [1]. We argue that when a study presents such potential flaws and bears a dominant weight in the meta‐analysis, a sensitivity analysis should be performed for a more realistic appraisal of evidence, and results of such sensitivity analysis should be fully discussed. Not surprisingly in this case, when the study by Koerkte et al. is removed from the meta‐analysis, there is no more statistically significant evidence that POC+PSM confers an improved survival to patients (RR=0.72; 95%CI: 0.42‐1.24). Christensen et al. also reached a non significant RR=0.49 (95%CI: 0.21‐1.14) when analysing only high quality trials [1].
Also noteworthy, Koerkte et al. reported 4 baseline factors associated to an excess mortality in their study: age; being a control; atrial fibrillation; and other than aortic surgery. When adjusting for these factors in a multivariate analysis, the “protective effect” of PSM fell to 23% (95%: 0%‐42%), a much lower value than the one used in previous meta‐analyzes [1, 4, 5]. I would appreciate receiving your thoughts on my comments. Best regards, Dominique Roberfroid, MD, MSc, MPhil
Belgian Health Care Knowledge Centre (https://kce.fgov.be/)
Bibliography 1. Christensen TD, Johnsen SP, Hjortdal VE, Hasenkam JM. Self‐management of oral anticoagulant therapy: a systematic review and meta‐analysis. Int J Cardiol. 2007;118(1):54‐61. 2. Koertke H, Zittermann A, Wagner O, Koerfer R. Self‐management of oral anticoagulation therapy improves long‐term survival in patients with mechanical heart valve replacement. Ann Thorac Surg. 2007;83(1):24‐9. 3. Kortke H, Minami K, Breymann T, Seifert D, Baraktaris A, Wagner O, et al. [INR self‐management after mechanical heart valve replacement: ESCAT (Early Self‐Controlled Anticoagulation Trial)]. Z Kardiol. 2001;90 Suppl 6:118‐24. 4. Connock M, Stevens C, Fry‐Smith A, Jowett S, Fitzmaurice D, Moore D, et al. Clinical effectiveness and cost‐effectiveness of different models of managing long‐term oral anticoagulation therapy: a systematic review and economic modelling. Health Technol Assess. 2007;11(38):iii‐iv, ix‐66. 5. Heneghan C, Alonso‐Coello P, Garcia‐Alamino JM, Perera R, Meats E, Glasziou P. Self‐monitoring of oral anticoagulation: a systematic review and meta‐analysis. Lancet. 2006;367(9508):404‐11.
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
In response to comments by Dominique Roberfroid submitted 2nd April 2012:
Dear Dominique,
In terms of mortality we report a reduction in all‐cause mortality of 36% (RR 0.64, 95% CI 0.46 to 0.89) and as stated for self‐management of 45% (RR 0.55, 95% CI 0.36 to 0.84) over a follow‐up period of 2 years.[1]
It is correct that the study by Koertke et al [2] provides a substantial amount of data to the mortality analysis for self‐management. In terms of the GRADE of the paper we therefore judged the evidence to be of moderate quality around the reported effects, particularly due to an absence of information on allocation concealment and also because there was a few number of events. However, in terms of study quality we confirmed in a subsequent publication, [3] and with direct communication with the authors for this Cochrane review that randomization and allocation concealment were clear in this study.
In terms of the baseline difference in this trial these imbalances could have led to differences in the outcomes. We stated in the discussion that the 36% reduction in mortality from all causes was largely influenced by one study. In addition we applied the logic of early stopping of randomized controlled trials to determine whether our meta‐analysis could be considered definitive. It is not, the calculated optimal information size needed to reliably detect a plausible treatment effect is 2,300 patients per group for thromboembolic events alone.
Further to the publication of this review a large RCT was published in the US.[4] We have analysed this in our subsequent publication ‘Self‐monitoring of oral anticoagulation: systematic review and meta‐analysis of individual patient data’ [3] including individual patient data from 11 trials of 6,417 participants including the Koertke data.
This allowed us to verify the trial methods as well as undertake time‐to‐event outcomes analysed with hazard ratios (HR) with 5 years of follow‐up data, which take into account the number of people randomized and timing of events, and the time until last follow‐up for each patient not experiencing an event. In this study [3] we reported a significant reduction in thromboembolic events in the self‐monitoring group (HR 0.51; 95% CI 0.31 to 0.85) but a non‐significant reduction in death for the self‐monitoring group (HR 0·82, 95% CI 0.62 to 1.09) and a non‐significant effect in terms of death for the self‐management group alone (HR 0.75, 95% CI, to 0.42 to 1.33).
Therefore, the protective effect is lower than previously estimated in our previous review.[1] Reasons for this could include lower quality trial methodology but could also results as improvements in the control group care occur over time. The increase in the number of trials and participants allows us to improve the confidence around our estimated and we will incorporate this additional data into our updated review.
References
[1] Garcia‐Alamino JM, Ward AM, Alonso‐Coello P, Perera R, Bankhead C, Fitzmaurice D, Heneghan CJ Self-monitoring and self-management of oral anticoagulation. Cochrane Database Syst Rev. 2010 Apr 14;(4):CD003839. Review.
[2] Koertke H, Zittermann A, Wagner O, Koerfer R. Self‐management of oral anticoagulation therapy improves long‐term survival in patients with mechanical heart valve replacement. Ann Thorac Surg. 2007;83(1):24‐9.
[3] Heneghan C, Ward A, Perera R, et al. Self‐monitoring of oral anticoagulation: systematic review and meta‐analysis of individual patient data. Lancet 2012;379(9813):322‐34.
[4] Matchar DB, Jacobson A, Dolor R, et al. Effect of home testing of international normalized ratio on clinical events. N Engl J Med 2010;363(17):1608‐20.
Contributors
Heneghan CJ, Garcia‐Alamino JM, Ward AM and Perera R
What's new
| Date | Event | Description |
|---|---|---|
| 10 March 2021 | Amended | The authors know of one additional study for inclusion (DOI: 10.4040/jkan.2015.45.4.554) based on a search up to 11 November 2019 but the new information is unlikely to change the review findings. The conclusions of this Cochrane review are therefore still considered up to date. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 4, 2010
| Date | Event | Description |
|---|---|---|
| 1 June 2016 | New search has been performed | The search was updated in July 2015. Ten new trials were found for inclusion. |
| 1 June 2016 | New citation required and conclusions have changed | The original review reported a reduction in mortality with self‐management or self‐monitoring; in this update, with the addition of the 10 new trials (adding 4227 participants) this risk reduction was no longer statistically significant; however, after the removal of low‐quality studies, the original finding of a reduction in all‐cause mortality was found. Secondly, the original review found that trials of self‐monitoring alone did not result in a statistically significant reduction in thromboembolic events; the new finding is a statistically significant reduction. Thirdly, in the original review, self‐monitoring significantly reduced major haemorrhage; in this update, self‐monitoring did not reduce major haemorrhage, but removal of studies with low quality resulted in a statistically significant reduction. |
| 27 March 2012 | Amended | Feedback added |
| 7 March 2012 | Amended | Add information about external funding sources. |
| 25 May 2010 | Amended | Amended Figure 4 ‐ GRADE table. |
Appendices
Appendix 1. Search strategies 2007
CENTRAL,The Cochrane Library (Spanish version platform)
#1. ANTICOAGULANTS*:TA #2. ANTICOAGULANT*:TA #3. ANTI‐COAGULANT*:TA #4. WARFARIN*:TA #5. (VITAMIN ANTAGONIST*):TA #6. VITAMIN‐K*:TA #7. COUMARINS*:TA #8. COUMARIN*:TA #9. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10. SELF‐CARE:TA #11. SELF‐ADMINISTRATION:TA #12. CONSUMER‐PARTICIPATION:TA #13. CONSUMER‐PARTICIPATION*:TA #14. (PATIENT CENTRED):TA #15. (PATIENT CENTERED):TA #16. (PATIENT* PARTICIPAT*):TA #17. SELF*:TA #18. HOME*:TA #19. COAGUCHECK:TA #20. COAGUCHEK:TA #21. (PROTHROMBIN MONITOR*):TA #22. COAGULOMETER*:TA #23. CONSUMER*:TA #24. (PATIENT* MONITOR*):TA #25. (PATIENT* MANAGE*):TA #26. (PATIENT* MEASUR*):TA #27. (PATIENT* TEST*):TA #28. (PATIENT* ADJUST*):TA #29. #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 #30. #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 #31. #29 OR #30 #32. #9 AND #31
MEDLINE on PubMed
#1. "Anticoagulants"[Mesh] #2. Anticoagulant* #3. Anti‐coagulant* #4. warfarin* #5. vitamin antagonist* #6. "Vitamin K"[Mesh] #7. "Coumarins"[Mesh] #8. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 #9. "Self Care"[Mesh] #10. "Self Administration"[Mesh] #11. "Consumer Participation"[Mesh] #12. patient centred #13. patient centered #14. patient* participat* #15. self* #16. home* #17. coagucheck #18. coaguchek #19. prothrombin monitor* #20. coagulometer* #21. consumer* #22. patient* monitor* #23. patient* manage* #24. patient* measur* #25. patient* test* #26. patient* adjust* #27. #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 #28. #8 AND #27
EMBASE
#1. fondaparinux/ or low molecular weight heparin/ or hirudin/ or enoxaparin/ or heparin/ or new drug/ or warfarin/ or ximelagatran/ or anticoagulant agent/ or acetylsalicylic acid/ #2. anticoagulant protein/ or lupus anticoagulant/ or anticoagulant agent/ or coumarin anticoagulant/ or anticoagulant therapy/ or tick anticoagulant peptide/ #3. rivaroxaban/ or acetylsalicylic acid/ or bleeding/ or antithrombocytic agent/ or warfarin/ or dabigatran etexilate/ or heparin/ or anticoagulation/ or anticoagulant agent/ or anticoagulant therapy/ #4. anticoagulation/ or bleeding/ or stroke/ or anticoagulant agent/ or thromboembolism/ or herbaceous agent/ or acetylsalicylic acid/ or heart atrium fibrillation/ or warfarin/ or phytomenadione/ #5. (vitamin adj antagonist*).mp. #6. antivitamin K/ or phytomenadione/ or menadione/ or oral drug administration/ or warfarin/ or anticoagulant agent/ or vitamin K deficiency/ or bleeding/ or vitamin K group/ or brain hemorrhage/ #7. coumarin/ or plant extract/ or phytochemistry/ or osthole/ or drug isolation/ or cytotoxic agent/ or unclassified drug/ or drug synthesis/ or coumarin derivative/ or drug identification/ #8. coumarin 7 hydroxylase/ or coumarin/ or coumarin derivative/ or coumarin anticoagulant/ #9. (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8).mp. #10. self care/ #11. drug self administration/ #12. disability/ or health care policy/ or medical research/ or drug dependence/ or health care planning/ or mental disease/ or mental health service/ or health care system/ or consumer/ or health care delivery/ #13. (patient adj centred).mp. #14. (patient adj centered).mp. #15. (patient* adj participat*).mp. #16. self monitoring/ or self control/ or self care/ or patient self‐determination act/ or self examination/ or self medication/ or self injection/ or self‐directed learning/ or self evaluation/ #17. home diagnostic test/ or home monitoring/ or home/ or home delivery/ or home care/ or home safety/ #18. warfarin/ or anticoagulant therapy/ or international normalized ratio/ or acenocoumarol/ or prothrombin time/ or thromboembolism/ or prothrombin complex/ or vitamin K group/ or anticoagulant agent/ or anticoagulation/ #19. thrombosis/ or anticoagulation/ or device/ or anticoagulant therapy/ or international normalized ratio/ or coumarin anticoagulant/ or anticoagulant agent/ or international standard unit/ or prothrombin time/ or warfarin/ #20. (prothrombin adj monitor*).mp. #21. international standard unit/ or blood clotting/ or warfarin/ or thromboembolism/ or blood clotting test/ or laboratory test/ or anticoagulant agent/ or thromboplastin/ or prothrombin time/ or coagulometer/ #22. consumer advocacy/ or consumer/ or consumer health information/ or consumer attitude/ #23. (patient* adj monitor*).mp. #24. (patient* adj manage*).mp. #25. (patient* adj measur*).mp. #26. (patient* adj test*).mp. #27. (patient* adj adjust*).mp. #28. (#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19).mp. #29. (#20 or #21 or #22 or #23 or #24 or #25 or #26 or #27).mp. #30. (#28 or #29).mp. #31. (#9 and #30).mp.
CINAHL (EBSCO host)
#1. anticoagulants* #2. anticoagulant* #3. anti‐coagulant* #4. warfarin* #5. vitamin NEAR antagonist* #6. vitamin‐k* #7. coumarins* #8. coumarin* #9. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10. self‐care #11. self‐administration #12. consumer‐participation* #13. patient NEAR centred #14. patient NEAR centered #15. patient* NEAR participat* #16. self* #17. home* #18. coaguchek #19. prothrombin NEAR monitor* #20. coagulometer* #21. consumer* #22. patient* NEAR monitor* #23. patient* NEAR manage* #24. patient* NEAR measur* #25. patient* NEAR test* #26. patient* NEAR adjust* #27. #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 #28. #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 #29. #27 OR #28
Appendix 2. Search strategies 2013
CENTRAL
#1MeSH descriptor: [Anticoagulants] explode all trees #2MeSH descriptor: [Coumarins] explode all trees #3anticoagulant* #4anti‐coagulant* #5warfarin* #6vitamin antagonist* #7vitamin‐K* #8coumarin* #9#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10MeSH descriptor: [Self Care] explode all trees #11MeSH descriptor: [Consumer Participation] explode all trees #12self near/3 admin* #13self near/3 car* #14consumer near/3 participat* #15patient near/3 participat* #16patient next cent* #17self* #18home* #19coagucheck* #20coaguchek* #21prothrombin next monitor* #22coagulometer* #23consumer* #24patient* near/2 monitor* #25patient* near/2 manag* #26patient* near/2 measur* #27patient* near/2 test* #28patient* near/2 adjust* #29#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 #30#9 and #29 from 2007 to 2013
MEDLINE OVID
1. exp Anticoagulants/ 2. anticoagulant*.tw. 3. anti‐coagulant*.tw. 4. (thrombin adj2 inhibit*).tw. 5. antithrombin*.tw. 6. Warfarin/ 7. warfarin*.tw. 8. coumadin*.tw. 9. (vitamin adj2 antagonist*).tw. 10. Coumarins/ 11. vitamin k.tw. 12. or/1‐11 13. exp Self Care/ 14. exp Consumer Participation/ 15. (patient adj2 cent*).tw. 16. (patient* adj2 participat*).tw. 17. self*.tw. 18. home*.tw. 19. coagucheck.tw. 20. coaguchek.tw. 21. prothrombin monitor*.tw. 22. coagulometer*.tw. 23. consumer*.tw. 24. (patient* adj2 monitor*).tw. 25. (patient* adj2 manage*).tw. 26. (patient* adj2 measur*).tw. 27. (patient* adj2 test*).tw. 28. (patient* adj2 adjust*).tw. 29. or/13‐28 30. 12 and 29 31. randomized controlled trial.pt. 32. controlled clinical trial.pt. 33. randomized.ab. 34. placebo.ab. 35. drug therapy.fs. 36. randomly.ab. 37. trial.ab. 38. groups.ab. 39. 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 40. exp animals/ not humans.sh. 41. 39 not 40 42. 30 and 41 43. (200711* or 200712* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013*).ed. 44. 42 and 43
EMBASE OVID
1. exp anticoagulant agent/ 2. anticoagulant protein/ 3. lupus anticoagulant/ 4. anticoagulant therapy/ 5. tick anticoagulant peptide/ 6. fondaparinux/ 7. low molecular weight heparin/ 8. hirudin/ 9. enoxaparin/ 10. heparin/ 11. warfarin/ 12. ximelagatran/ 13. acetylsalicylic acid/ 14. rivaroxaban/ 15. antithrombocytic agent/ 16. dabigatran etexilate/ 17. anticoagulation/ 18. exp thromboembolism/ 19. herbaceous agent/ 20. phytomenadione/ 21. exp vitamin K group/ 22. vitamin K deficiency/ 23. coumarin/ 24. osthole/ 25. coumarin derivative/ 26. coumarin 7 hydroxylase/ 27. (vitamin adj2 antagonist*).tw. 28. anticoagulant*.tw. 29. anti‐coagulant*.tw. 30. (thrombin adj2 inhibit*).tw. 31. antithrombin*.tw. 32. warfarin*.tw. 33. coumadin*.tw. 34. vitamin k.tw. 35. or/1‐34 36. exp self care/ 37. drug self administration/ 38. (patient adj2 cent*).tw. 39. (patient* adj2 participat*).tw. 40. self monitoring/ 41. home monitoring/ 42. self*.tw. 43. home*.tw. 44. coagucheck.tw. 45. coaguchek.tw. 46. prothrombin monitor*.tw. 47. coagulometer*.tw. 48. consumer*.tw. 49. (patient* adj2 monitor*).tw. 50. (patient* adj2 manage*).tw. 51. (patient* adj2 measur*).tw. 52. (patient* adj2 test*).tw. 53. (patient* adj2 adjust*).tw. 54. or/36‐53 55. 35 and 54 56. random$.tw. 57. factorial$.tw. 58. crossover$.tw. 59. cross over$.tw. 60. cross‐over$.tw. 61. placebo$.tw. 62. (doubl$ adj blind$).tw. 63. (singl$ adj blind$).tw. 64. assign$.tw. 65. allocat$.tw. 66. volunteer$.tw. 67. crossover procedure/ 68. double blind procedure/ 69. randomized controlled trial/ 70. single blind procedure/ 71. 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 72. (animal/ or nonhuman/) not human/ 73. 71 not 72 74. 55 and 73 75. (20074* or 20075* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013*).em. 76. 74 and 75
CINAHL
S22S20 AND S21 S21EM 2007‐2013 S20S7 AND S19 S19S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 S18(patient* N2 adjust*) S17(patient* N2 test*) S16(patient* N2 measur*) S15(patient* N2 manage*) S14(patient* N2 monitor*) S13"prothrombin monitor*" S12self* or home* or coagucheck or coaguchek or coagulometer* or consumer* S11(patient* N2 participat*) S10(patient N2 cent*) S9(MH "Consumer Participation") S8(MH "Self Care+") S7S1 OR S2 OR S3 OR S4 OR S5 OR S6 S6vitamin N2 k S5(MH "Warfarin") S4(vitamin N2 antagonist*) S3(thrombin N2 inhibit*) S2anticoagulant* or anti‐coagulant* or antithrombin* or warfarin* or coumadin* S1(MH "Anticoagulants+")
Data and analyses
Comparison 1. Thromboembolic events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Self‐monitoring and self‐management | 18 | 7594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 1.1.1 Self‐management | 11 | 3497 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.31, 0.70] |
| 1.1.2 Self‐monitoring | 7 | 4097 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.49, 0.97] |
| 1.2 Events by Clinical Condition | 17 | 4809 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.71] |
| 1.2.1 Mechanical Valve | 6 | 1816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.32, 0.90] |
| 1.2.2 Atrial Fibrillation | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 1.2.3 Any indication | 10 | 2791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.34, 0.77] |
| 1.3 Events by Self‐management (sensitivity) | 13 | 4558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.36, 0.69] |
| 1.3.1 Self‐management | 9 | 3618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.30, 0.66] |
| 1.3.2 Self‐monitoring | 4 | 940 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.36, 1.09] |
| 1.4 Events by specialty | 16 | 7344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.46, 0.78] |
| 1.4.1 Specialised Care | 8 | 4947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.90] |
| 1.4.2 Primary Care | 8 | 2397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.38, 0.84] |
Comparison 2. All‐cause mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Events by Self‐management | 11 | 6358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.71, 1.01] |
| 2.1.1 Self‐management | 8 | 3058 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.36, 0.84] |
| 2.1.2 Self‐monitoring | 3 | 3300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.15] |
| 2.2 Events by Clinical Condition | 13 | 6645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.71, 1.01] |
| 2.2.1 Mechanical Valve | 3 | 1295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.86] |
| 2.2.2 Atrial Fibrillation | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.2.3 Any indication | 8 | 5063 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.10] |
| 2.3 Events by Self‐management (sensitivity) | 8 | 6160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.71, 1.02] |
| 2.3.1 Self‐management | 6 | 2918 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.88] |
| 2.3.2 Self‐monitoring | 2 | 3242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.77, 1.14] |
| 2.4 Events by specialty | 10 | 6163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.00] |
| 2.4.1 Specialised Care | 5 | 4387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.75, 1.13] |
| 2.4.2 Primary Care | 5 | 1776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.43, 0.90] |
Comparison 3. Major haemorrhage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Events by Self‐management | 20 | 8018 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.12] |
| 3.1.1 Self‐management | 13 | 3980 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.79, 1.47] |
| 3.1.2 Self‐monitoring | 7 | 4038 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 3.2 Events by Clinical Condition | 16 | 4594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.17] |
| 3.2.1 Mechanical Valve | 4 | 1445 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.75, 1.65] |
| 3.2.2 Atrial Fibrillation | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.31, 27.47] |
| 3.2.3 Any indication | 10 | 2862 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.41, 0.97] |
| 3.3 Events by Self‐management (sensitivity) | 14 | 7337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.14] |
| 3.3.1 Self‐management | 10 | 3726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.76, 1.44] |
| 3.3.2 Self‐monitoring | 4 | 3611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.76, 1.14] |
| 3.4 Events by specialty | 17 | 7321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.14] |
| 3.4.1 Specialised Care | 9 | 5054 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.19] |
| 3.4.2 Primary Care | 8 | 2267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.30] |
3.4. Analysis.

Comparison 3: Major haemorrhage, Outcome 4: Events by specialty
Comparison 4. Minor haemorrhage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Events by Self‐management | 13 | 5365 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.67, 1.41] |
| 4.1.1 Self‐management | 7 | 1862 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.47, 1.76] |
| 4.1.2 Self‐monitoring | 6 | 3503 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.95, 1.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azarnoush 2009.
| Study characteristics | ||
| Methods | Single‐centre, randomised, parallel‐group controlled trial. | |
| Participants | 206 adult patients who had undergone valve replacement with a mechanical prosthesis, with or without myocardial revascularisation. | |
| Interventions |
Self‐monitoring vs standard monitoring Randomised to standard monitoring of INR at a laboratory including at least one monthly assay at a medical analysis laboratory (n = 103), or self‐testing using either the CoaguChek® system (n = 55) or the INRatio® system (n = 48). Self‐testing was performed weekly, and in addition once monthly INR measurements were carried out at the laboratory on the same day as the self‐measurement. Only the results of the monthly tests for each group were compared. Education relating to VKA therapy was provided, the same to all participants in all allocation groups. The target INR and target range were determined for each participant on the basis of the type of surgery, and according to their risk factors for thromboembolic disease (target ranges were between 2 and 3.5 INR). Aspirin was prescribed to some participants according to risk factors. |
|
| Outcomes | Mean time within target range. Clinical adverse events, and serious bleeding. | |
| Trial identification | Clinicaltrials.gov NCT00925197 | |
| Study duration | 49.0 ± 10.3 weeks | |
| Oral anticoagulant used | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated to have been done but no methods reported. |
| Allocation concealment (selection bias) | Unclear risk | Possibly adequate or not used. |
| Intention to treat analysis | High risk | ITT not performed. |
| Reporting of losses of follow‐up | Low risk | 7% of participants were lost to follow‐up. |
| Blinding | Unclear risk | Blinding not reported. |
Beyth 2000.
| Study characteristics | ||
| Methods | Multicentre, randomised controlled trial. | |
| Participants | 325 hospitalised patients aged 65+ years (mean age 75 years) commencing warfarin therapy of at least 10 days duration. The study was based in several university hospitals (Cleveland, Ohio, USA). Exclusions included: warfarin therapy within previous 6 months; admission from nursing home; too ill to give informed consent. |
|
| Interventions |
Self‐monitoring vs usual care The intervention group (n = 163) used home self‐testing using Coumatrak Protime Test System® to self‐monitor prothrombin time. 1‐hour education session, patients phoned results to coach who made recommendations on dosage adjustment. The conventional management group (n = 162) received medical care including management, dosing and medical information managed by primary care physician as per usual care. Randomisation was stratified according to baseline risk for major bleeding. |
|
| Outcomes | Primary outcome: first major bleeding event during the 6‐month intervention period. Secondary outcomes: death; recurrent venous thromboembolism at 6 months; major bleeding after 6 months; percentage time INR within target range. |
|
| Trial identification | ||
| Study duration | 6 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | One‐to‐one training, lasting 30‐60 minutes. Participants instructed to check prothrombin 3 times in the first week after hospital discharge and weekly in the first month, and monthly thereafter depending on the results. 100% up at 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated to have been done but no method reported. |
| Allocation concealment (selection bias) | Low risk | Clearly adequate concealment. |
| Intention to treat analysis | Low risk | ITT analysis was performed. |
| Reporting of losses of follow‐up | Unclear risk | < 20% losses to follow‐up. |
| Blinding | Low risk | Blinded data collectors. |
Christensen 2006.
| Study characteristics | ||
| Methods | Single centre, open‐label, randomised controlled trial | |
| Participants | 100 ambulatory patients aged > 18 years (mean age 63 years in intervention group, 69 years in control group) receiving oral anticoagulation therapy for > 8 months. The study was based in the Center of Self‐managed Oral Anticoagulation (Denmark). Exclusion criteria included: previous self‐management. |
|
| Interventions |
Self‐management vs usual care Randomisation to a) self‐management (n = 50), in which participants were trained to self‐monitor using a Coaguchek® coagulometer to measure INR once a week and also to adjust their anticoagulant dosage accordingly. b) usual care (n = 50), in which conventional management included at least monthly INR testing at a hospital or physician centre and dosage adjusted by the physician. In both groups an additional INR analysis was performed monthly and the participant contacted if INR was < 1.5 or > 4.5. After 6 months intervention, the control group began training to self‐manage their anticoagulation therapy. |
|
| Outcomes | Primary outcome: composite of the variance of the monthly INR test plus negative score points for death, major complications, or study discontinuation. Secondary outcomes: variance of INR values in the control sample; time within therapeutic INR range. |
|
| Trial identification | ||
| Study duration | 6 months | |
| Oral anticoagulant used | Warfarin or phenprocoumon. | |
| Notes | Training took place over 6 months, during which time the participant gradually assumed self‐management of oral anticoagulation monitoring and dosing. Assessment of ability to self‐manage was made at the end of 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation was not reported, contact with author led to information on appropriate method of concealment |
| Intention to treat analysis | High risk | ITT analysis was not used |
| Reporting of losses of follow‐up | Low risk | 4% of participants were lost to follow‐up during the relevant period of this trial; reasons were not reported |
| Blinding | High risk | The trial was open‐label to participants and study staff |
Christensen 2011.
| Study characteristics | ||
| Methods | Single centre, randomised, parallel‐group, controlled trial. | |
| Participants | Adult participants on lifelong anticoagulation therapy receiving treatment for at least the previous 6 months, able to use the internet and demonstrate ability to use the CoaguChek XS system after a 1‐hour training session. | |
| Interventions |
Self‐monitoring vs usual care Randomised to one of three groups: A) self‐monitoring by measurement of INR once/week at home and reporting the value via a computer system to the anticoagulation clinic B) self‐monitoring by measurement of INR twice/week at home and reporting the values via a computer system to the anticoagulation clinic C) continuing regular visits to the anticoagulation clinic |
|
| Outcomes | Primary outcome: Time in treatment range appropriate to the individual participant, as measured by the Rosendaal method. Secondary outcomes: number of INR measurements < 1.5 or > 5.0; number of adverse clinical events. |
|
| Trial identification | ||
| Study duration | 12 months | |
| Oral anticoagulant used | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Intention to treat analysis | High risk | ITT analysis was not performed |
| Reporting of losses of follow‐up | Low risk | 17 participants (12.1%) withdrew; reasons reported |
| Blinding | High risk | The trial was not blinded to participants and study staff |
Cromheecke 2000.
| Study characteristics | ||
| Methods | Single centre, randomised, controlled cross‐over trial. | |
| Participants | Participants were 50 consecutive outpatients who were receiving long‐term anticoagulation (mean age 42 years). The study was based in the departments of cardiology and internal medicine of the Academic Medical Centre (Amsterdam, The Netherlands). | |
| Interventions |
Self‐management vs usual care The intervention group used home self‐testing using Coaguchek® to self‐monitor prothrombin time and self‐dosing testing performed once a week. The conventional management was done by the anticoagulation clinic. INR testing was also performed in all participants at 1‐2 week intervals by the central laboratory; these results were not made available to participants or managing physicians. After three months patients crossed over the alternative management strategy. |
|
| Outcomes | Primary outcome: no. of INR measurements within 0.5 of therapeutic target INR. Secondary outcomes: Percentage time within target INR range; no. participants within target range for 0%‐100% of the time; no. participants who achieved better control of anticoagulation. |
|
| Trial identification | ||
| Study duration | 3 months (followed by cross‐over to alternative intervention group, for a further 3 months. The outcomes used here are those at the end of the first 3 months) | |
| Oral anticoagulant used | Phenprocoumon (35% of participants) or acenocoumarol (65% of participants) | |
| Notes | All patients were educated and trained to self‐manage anticoagulation during a structured educational program of two 2 hours sessions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of the randomisation sequence was not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes concealed the allocation. |
| Intention to treat analysis | High risk | ITT analysis was not performed |
| Reporting of losses of follow‐up | Low risk | 0% lost to follow‐up |
| Blinding | Low risk | Blinded outcome assessors |
Dignan 2013.
| Study characteristics | ||
| Methods | Multicentre, randomised, controlled trial | |
| Participants | Patients at least 18 years of age who were receiving warfarin for at least 3 months for either atrial fibrillation or for one or more mechanical heart valves. Patients needed to have a stable INR within the therapeutic range for the 2 weeks before enrolment, without maintenance dose adjustments above 2 mg per day, so that an individual algorithm could be developed. The study was conducted at Liverpool, Royal Prince Alfred, and Strathfield Private Hospitals, all in Australia | |
| Interventions | The intervention group was trained to perform home INR testing and warfarin dosing using a validated ColourChart algorithm. Patients checked their INR at least once a week, and more frequently if required by the algorithm. Patients were instructed to call the study nurse to discuss maintenance dose adjustment if the INR was less than 1.6, greater than 4.5, or out‐of‐range for more than 4 tests. The usual‐care group was also given instructions on how to complete a black‐and‐white chart similar to the ColourChart to record their clinical INR test results but without the algorithm instructions. For 12 months, all patients had monthly outcome INRs measured at a central accredited laboratory. |
|
| Outcomes | Primary outcome was the proportion of out‐of‐range INRs. Secondary endpoints included: 1. the number of times outcome INR results occurred in extreme ranges (≥ 4.5, < 1.5); and 2. rates of serious adverse events related to bleeding or thrombosis. Subsidiary (tertiary) endpoints were: 1. the average deviation from the middle of each individual's INR target range; 2. the mean outcome INR, by treatment group allocation; and 3. time to the first INR reading in an extreme range. Serious adverse events were classified as embolism, thrombosis, moderate bleeding (requiring medical evaluation or treatment, minor and nuisance bleeding excluded), severe, life‐threatening, or fatal bleeding, and other events, and were adjudicated by a blinded assessor as to nature and cause. |
|
| Trial identification | ACTRN12606000019505 | |
| Study duration | 12 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central phone‐based randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Intention to treat analysis | Low risk | ITT analysis conducted |
| Reporting of losses of follow‐up | Low risk | Reasons for loss‐to‐follow‐up reported |
| Blinding | Low risk | Outcome assessors blinded |
Fitzmaurice 2002.
| Study characteristics | ||
| Methods | Multicentre, randomised controlled trial. | |
| Participants | Participants were ambulatory adults aged > 18 years attending an anticoagulation clinic, receiving anticoagulation therapy for > 6 months, judged as capable of self‐management, and with satisfactory INR control (n = 56). The study was based in six general practices that used the Birmingham model of anticoagulation management (West Midlands, UK). |
|
| Interventions |
Self‐management vs usual care Participants were randomised to: a) self‐management (n = 30): self‐testing using Coaguchek® device and self‐adjustment of dosing. Testing was performed every 2 weeks or after 1 week following dosage adjustment. b) conventional management (n = 26) in practice clinics. |
|
| Outcomes | Primary and secondary outcomes were not specified. Data were reported for: percentage time in INR range; adverse events; frequency of testing; costs; quality of life. |
|
| Trial identification | ||
| Study duration | 6 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | Participants in the intervention group attended two 1‐2 hour duration educational workshops on anticoagulation self‐management. Workshops were based within individual practices, were organised by research staff and attended by practice staff. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated coding |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation was not reported, contact with author led to information on appropriate method of concealment |
| Intention to treat analysis | High risk | ITT analysis was not performed |
| Reporting of losses of follow‐up | Low risk | 7/56 (12.5%) of participants were lost to follow‐up; reasons reported |
| Blinding | High risk | Participants and study staff were not blinded to the intervention |
Fitzmaurice 2005.
| Study characteristics | ||
| Methods | Multicentre, randomised controlled trial. | |
| Participants | Participants (n = 617) were adults aged > 18 years receiving long‐term anticoagulation therapy at primary care centres within the Midlands Research Consortium (UK). | |
| Interventions |
Self‐management vs usual care Participants were randomised to: a) self‐management (n = 337) comprising home self‐testing using Coaguchek® managed anticoagulation for 12 months, testing INR very two weeks (one week after a dose change) and self‐adjusting dosage according to a dosing schedule. b) usual care, comprising anticoagulation management in hospital or practice based anticoagulant clinics (n = 280). |
|
| Outcomes | Primary outcome: % time within therapeutic INR range. Secondary outcomes: adverse events, serious bleeding rates, serious thrombosis rates. |
|
| Trial identification | ISRCTN 19313375 | |
| Study duration | 12 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | Intervention participants attended two training sessions. Trained anticoagulation nurses gave participants training at the practice. After the training, participants considered capable of doing self‐management were given home testing equipment Coaguchek® managed anticoagulation for 12 months, testing INR very two weeks (one week after a dose change). Adjusted dosage by using a laminated dosing schedule. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Variable block random allocation |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation |
| Intention to treat analysis | Low risk | ITT analysis was performed |
| Reporting of losses of follow‐up | Unclear risk | Inadequate report or > 20% losses (41.5% losses to follow‐up) |
| Blinding | Low risk | Blinded outcome assessors |
Gadisseur 2003 Self mge.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. | |
| Participants | Participants (n = 320) were adults aged 18 to 75 (mean 57) years having received anticoagulation therapy for > 3 months, requiring long‐term anticoagulation therapy, and attending one of two anticoagulation clinics (The Netherlands). Exclusion criteria included: antiphospholipid syndrome; life‐threatening illness; < 1 year life expectancy; diminished understanding or physical limitations preventing participation. |
|
| Interventions |
Self‐monitoring or self‐management vs usual care Participants were randomised to one of four groups, using a 2‐step partial Zelen design. Group A (n = 52): self‐testing using Coagucheck® monitoring device. Group B (n = 47): self‐testing using Coagucheck® and self‐dosing. Group C (n = 60): received education alone and routine care. Group D (n = 161): received only routine care. Group D did not provide informed consent for randomisation into the study and were unaware of study participation. For the purposes of this review, the results of the trial are presented as Gadisseur 2003 self‐management, which is group B versus group D, and separately as Gadisseur self‐monitoring, which is group A versus group D. |
|
| Outcomes | Primary and secondary outcomes were not specified. Data were reported for: no. INR tests within target range; time spent within target range; thromboembolism or haemorrhage; ability to self‐manage by adjusting anticoagulation dosage estimated by as the inverse of the no. of dosage corrections made by physicians. | |
| Trial identification | ||
| Study duration | 6.5 months | |
| Oral anticoagulant used | Phenprocoumon (70% participants) or acenocoumarol (30% participants) | |
| Notes | Groups A, B and C received the same training (three sessions of 90‐120 min). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation was not reported, contact with author led to information on appropriate method of concealment |
| Intention to treat analysis | High risk | ITT analysis was not performed |
| Reporting of losses of follow‐up | High risk | Loss to follow‐up was not reported |
| Blinding | Low risk | Participants were not blinded to the intervention (except group D who were unaware of trial participation). Healthcare providers were blinded to the intervention. |
Gadisseur 2003 Self monit.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. Participants (n = 320) were adults aged 18 to 75 (mean 57) years having received anticoagulation therapy for > 3 months, requiring long‐term anticoagulation therapy, and attending one of two anticoagulation clinics (The Netherlands). Exclusion criteria included: antiphospholipid syndrome; life threatening illness; < 1 year life expectancy; diminished understanding or physical limitations preventing participation. |
|
| Participants | The study enrolled 320 participants. Mean age 57 years who were receiving long‐term anticoagulation. The study was based in two Dutch anticoagulation clinics. | |
| Interventions |
Self‐monitoring or self‐management vs usual care Participants were randomised to one of four groups, using a 2‐step partial Zelen design. Group A (n = 52): self‐testing using Coagucheck® monitoring device. Group B (n = 47): self‐testing using Coagucheck® and self‐dosing. Group C (n = 60): received education alone and routine care. Group D (n = 161): received only routine care. Group D did not provide informed consent for randomisation into the study and were unaware of study participation. For the purposes of this review, the results of the trial are presented as Gadisseur 2003 self‐management, which is group B versus group D, and separately as Gadisseur self‐monitoring, which is group A versus group D. |
|
| Outcomes | Primary and secondary outcomes were not specified. Data were reported for: no. INR tests within target range; time spent within target range; thromboembolism or haemorrhage | |
| Trial identification | ||
| Study duration | 6.5 months | |
| Oral anticoagulant used | Phenprocoumon (70% participants) or acenocoumarol (30% participants) | |
| Notes | Groups A, B and C received the same training (three sessions of 90‐120 min). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation was not reported, contact with author led to information on appropriate method of concealment |
| Intention to treat analysis | High risk | ITT analysis was not performed |
| Reporting of losses of follow‐up | High risk | Loss to follow‐up was not reported |
| Blinding | Low risk | Participants were not blinded to the intervention (except group D who were unaware of trial participation). Health care providers were blinded to the intervention |
Gardiner 2005.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. | |
| Participants | Participants (n = 84) were adults aged > 18 (mean 58) years who had received anticoagulation therapy for > 8 months and had a record of good compliance. The study was based in an anticoagulation clinic in University Hospital (London, UK). |
|
| Interventions |
Self‐monitoring vs usual care Participants were randomised to: a) self‐testing (n = 44) using the Coagucheck® monitoring device once per week b) control (n = 40), receiving usual care by visiting the hospital anticoagulation clinic for testing every 4 weeks. |
|
| Outcomes | Primary and secondary outcomes were not specified. Data were reported for: Major and minor bleeding, thromboembolism and mortality, percentage of time within target range, and acceptability. |
|
| Trial identification | ||
| Study duration | 6 months | |
| Oral anticoagulant used | Not reported | |
| Notes | The intervention group attended two training sessions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation was not reported |
| Intention to treat analysis | High risk | ITT analysis was not performed |
| Reporting of losses of follow‐up | Low risk | 15/84 (17.9%) of participants were lost to follow‐up; reasons were reported |
| Blinding | High risk | Participants and study staff were not blinded to the intervention |
Grunau 2011.
| Study characteristics | ||
| Methods | Single centre, open‐label randomised, cross,over trial, feasibility study. | |
| Participants | Participants (n = 11) were adults aged > 18 (mean 73) years receiving warfarin therapy for > 3 months from a private family practice in British Columbia (Canada), and competent to use drug‐adjustment nomograms. Exclusions included: severe psychiatric disease; serious language barrier, poor physical acuity; primary care physician judgement of unsuitability. |
|
| Interventions |
Self‐management vs usual care Participants were randomised to: a) self‐management (n = 6): written instructions on how to adjust dosage of anticoagulant, including a dose‐adjustment nomogram. Participants were asked to contact the study centre by phone/in person if they were having difficulty with the self‐management or if their INR value > 5. b) usual care (n = 5): physician anticoagulation management at the clinic. Measurement of INR among all participants was done by community laboratories and the results communicated by mail or in person. Participants were followed for 4.5 months, then allocation was reversed. |
|
| Outcomes | Primary outcome: proportion of INR values in therapeutic range. Secondary outcomes: number of days in therapeutic range. Feasibility end points included proportion of eligible participants consenting, preferred management strategy of participants at the end of the study, a treatment‐related satisfaction survey and additional office visits and phone calls pertaining to anticoagulation. Safety endpoints were bleeding or thromboembolic events. |
|
| Trial identification | Clinicaltrials.gov NCT00925028 | |
| Study duration | 9 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | Feasibility study: only 11 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Intention to treat analysis | Low risk | Outcomes were analysed according to ITT |
| Reporting of losses of follow‐up | Low risk | There were no losses to follow‐up. |
| Blinding | Low risk | Participants were not blinded; clinic staff were unaware of treatment allocation. |
Horstkotte 1998.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. | |
| Participants | Participants (n = 150) were adults who had received aortic or mitral valve Implantation, presenting consecutively at a single medical centre, Free University of Berlin (Germany). | |
| Interventions |
Self‐monitoring vs usual care a) self‐monitoring using Coagucheck device to measure INR twice weekly b) usual care: INR testing and dosage adjustment by home physician All participants had outpatient evaluation once every 3 months. |
|
| Outcomes | Major haemorrhage, thromboembolic events, mortality. | |
| Trial identification | ||
| Study duration | Not reported | |
| Oral anticoagulant used | Not reported | |
| Notes | It is unclear whether participants were adjusting their own dose of anticoagulation therapy (self‐management) in addition to self‐monitoring. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of the randomisation sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Possibly adequate or not used. |
| Intention to treat analysis | High risk | ITT analysis was not performed |
| Reporting of losses of follow‐up | Low risk | 3/150 (2%) lost to follow‐up. |
| Blinding | High risk | Participants were not blinded to the intervention. Blinding of study staff and physicians was not reported. |
Kaatz Unpublished.
| Study characteristics | ||
| Methods | Multicentre, randomised controlled trial. | |
| Participants | Participants (n = 201) were individuals receiving long‐term anticoagulation therapy. The study was based in three anticoagulation clinics. | |
| Interventions |
Self‐monitoring vs usual care Participants were randomised to: a) self‐testing (n = 101) using the Coagucheck® monitoring device. b) usual care (n = 100) comprising point‐of‐care testing at the anticoagulation clinics. |
|
| Outcomes | Major and minor bleeding; thromboembolic events; percentage of time within the therapeutic range; participant convenience, satisfaction and worry. | |
| Trial identification | ||
| Study duration | Not reported | |
| Oral anticoagulant used | Warfarin | |
| Notes | Participants were trained by the anticoagulation clinic research nurse to use Coagucheck®. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated using variable block sizes and stratification. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Intention to treat analysis | Low risk | ITT analysis was performed |
| Reporting of losses of follow‐up | Low risk | No participants were lost to follow‐up. |
| Blinding | High risk | Participants were not blinded to the intervention. It is unclear whether study or medical staff were blinded to the intervention. |
Khan 2004.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. | |
| Participants | Participants (n = 125) were adults aged > 65 (mean 73) years with atrial fibrillation receiving oral anticoagulation for > 12 months previously for atrial fibrillation. Exclusion criteria included: inability to use the Coagucheck system due to general frailty, poor hearing or eyesight, impairment of hand function, dementia, residence in care home. The study was based in a university based anticoagulation service (Newcastle, UK). |
|
| Interventions |
Self‐monitoring vs usual care Participants were randomised to: a) Group A (n = 44) used home weekly self‐testing using the Coagucheck® monitoring device. Weekly dosage adjustment was advised by telephone by the study co‐ordinator. b) Group B (n = 41) received education alone and clinical care. c) Group C (n = 40) received usual care. All study participants attended the anticoagulation clinic for INR measurement at intervals determined by the stability of their INR and with dosage changes determined through a computerised dosage program. |
|
| Outcomes | Primary and secondary outcomes were not specified. Data were reported for: Percentage of time within target range; major and minor bleeding; thromboembolism; death; number of dosage changes; quality of life. |
|
| Trial identification | ||
| Study duration | 6 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | Groups A and B received one training session (2 hours) attended in groups of 2‐3 people. Sessions were based on educational materials and led by a doctor, 4.8% of participants were lost to follow‐up. Control group C participants were unaware of their participation in the study. Adverse events were not monitored in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table, computer‐generated program |
| Allocation concealment (selection bias) | Unclear risk | Possibly adequate or not used |
| Intention to treat analysis | High risk | ITT analysis was not performed |
| Reporting of losses of follow‐up | Low risk | 6/85 (7.1%) lost to follow‐up; reasons reported |
| Blinding | Unclear risk | Participants in groups A and B were not blinded to the allocation; participants in group C were unaware of study participation. It is not reported whether study staff were blinded to the interventions. |
Körtke 2001.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. | |
| Participants | Participant (n = 600) were adults (mean age 63 years) receiving life long‐term oral anticoagulation after mechanical heart valve replacement. The study was based in Ruhr University (Germany). |
|
| Interventions |
Self‐management vs usual care Participants were randomised to: a) self‐management (n = 305): self‐testing using Coagucheck® system, and self‐adjustment of dosage. In addition, monthly INR measurements were reviewed by the anticoagulation clinic. b) control (n = 295): outpatient cardiologic check up and coagulation controls every 6 months. It is unclear if these participants adjusted their anticoagulation dosage themselves. |
|
| Outcomes | Primary and secondary outcomes were not specified. Data were reported for: Percentage of INR within therapeutic range; haemorrhagic events; thromboembolic events. |
|
| Trial identification | ESCAT | |
| Study duration | ≤ 51 months. | |
| Oral anticoagulant used | Phenprocoumon | |
| Notes | No details about training. It is unclear if participants in the control group adjusted their own anticoagulation dosages or whether this was done by clinic or study staff. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated using a standard randomisation list |
| Allocation concealment (selection bias) | Low risk | Opaque randomisation envelopes were used |
| Intention to treat analysis | High risk | ITT analysis was not performed. |
| Reporting of losses of follow‐up | Unclear risk | 90/600 (15%) of participants were lost to follow‐up; reasons were not reported |
| Blinding | Unclear risk | Blinding of participants, study staff or medical staff was not reported. |
Matchar 2010.
| Study characteristics | ||
| Methods | Multi‐centre, randomised, parallel‐group, controlled trial | |
| Participants | Participants (n = 2,992) were adults with atrial fibrillation, a mechanical heart valve, or both, requiring long‐term warfarin therapy and competent in self‐testing | |
| Interventions |
Self‐monitoring vs usual care Randomisation was within strata of anticoagulation duration (< 3 or ≥ 3 months) and indication for warfarin (atrial fibrillation with or without mechanical heart valve) within each site. Participants were randomised to a) self‐testing (n = 1465): participants measured their INR once a week and recorded the results via an interactive voice‐response reporting system with web‐based local monitoring. If the participant reported a measurement outside the assigned INR range or reported having been hospitalised, the system directed the participant to contact study staff. b) usual care (n = 1457): approximately once monthly clinic testing of INR |
|
| Outcomes | Primary outcome: time to a first major event (stroke, major bleeding episode, or death). Secondary outcomes: time within the INR target range, participant satisfaction with anticoagulation, quality of life, utilisation and costs of services. |
|
| Trial identification | Clinicaltrials.gov NCT00032591 | |
| Study duration | 2.0 to 4.75 years, giving 8730 participant‐years of follow‐up. | |
| Oral anticoagulant used | Warfarin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated to have been done but sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not specified |
| Intention to treat analysis | Low risk | Analysis was based on ITT |
| Reporting of losses of follow‐up | Low risk | Loss to follow‐up was 1% |
| Blinding | High risk | Participants and study staff were not blinded to allocation. |
Menendez‐Jandula 2005.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. | |
| Participants | Participants (n=737) were ambulatory adults aged > 18 (mean 66) years, receiving long‐term anticoagulation therapy for > before the study for at least 3 months. The study was based in a University Hospital (Barcelona, Spain). |
|
| Interventions |
Self‐management vs usual care Participants were randomised to: a) self‐management (n = 368): home self‐testing using the Coagucheck® ,self‐adjustment of dosage of oral anticoagulant, and self‐determination of time to next INR test. b) usual care (n = 369): visited the hospital for every four weeks to have their INR checked. |
|
| Outcomes | Primary outcomes: percentage of INR values within target range; time within target range. Secondary outcomes: major thromboembolic or haemorrhagic complications; minor bleeding; death. |
|
| Trial identification | ACOA | |
| Study duration | Up to 17 months | |
| Oral anticoagulant used | Warfarin or acenocoumarol | |
| Notes | Training: two 2‐hour sessions in consecutive days run by a trained nurse. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised telephone randomisation. |
| Allocation concealment (selection bias) | Low risk | The sequence of randomisation was concealed until the participant was assigned to a group. |
| Intention to treat analysis | Low risk | ITT analysis was used |
| Reporting of losses of follow‐up | Unclear risk | 11.9% of participants were lost to follow‐up; reasons not reported. |
| Blinding | Low risk | Blinded outcome assessors. |
Rasmussen 2012.
| Study characteristics | ||
| Methods | Single‐centre, parallel‐group, randomised controlled study. | |
| Participants | Participants were individuals requiring oral anticoagulation therapy (n = 54). | |
| Interventions |
Self‐monitoring vs usual care Participants were randomised to self‐testing with computer aided decision making, two different algorithms (computer algorithm group A n = 19, computer algorithm group B n = 18), or to usual care (monitoring and treatment by physicians) (n = 17). |
|
| Outcomes | Time to therapeutic range, time in therapeutic range, INR. | |
| Trial identification | ||
| Study duration | Mean 28 week follow‐up | |
| Oral anticoagulant used | Warfarin | |
| Notes | Authors reported that there were insufficient data to provide valid measurements of thromboembolic events and severe bleeding. in this study it is difficult to estimate the individual effects of self‐testing and of computer dosage calculation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by computer. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Intention to treat analysis | Unclear risk | ITT not reported. |
| Reporting of losses of follow‐up | Unclear risk | Losses to follow‐up were not reported. |
| Blinding | Low risk | Study investigators were blinded to computer algorithm group A vs group B allocation; statisticians were blinded to allocation. |
Ryan 2009.
| Study characteristics | ||
| Methods | Single centre, randomised controlled cross‐over study | |
| Participants | Participants were individuals receiving ongoing warfarin therapy for > 2 months and who had internet access, and were able to use a home INR meter (n = 162). | |
| Interventions |
Self‐monitoring vs usual care Participants were randomised to supervised self‐testing or to usual care (conventional clinic management) for 6 months; subsequently the allocation was reversed for a further 6 months. In the self‐testing group, participants initially self‐tested INR twice weekly. Once the INR was therapeutic for 2‐3 consecutive readings, the interval between tests was increased to a maximum of every 2 weeks. Participants accessed a web‐based system to enter signs and symptoms and INR and receive instant automated guidance on dose and testing; if INR deviation was serious the participant was asked to take a bolus dose of warfarin (< 1.5) or hold their warfarin (> 5.0) and/or to log in later the same day for additional instructions. If a participant reported a symptom suggestive of a bleed or an embolus they were told to seek immediate medical advice. The research pharmacist accessed the caregiver interface of the program at least once daily to review participant problems. Any participant who failed to test their INR or log in to the program as scheduled was contacted by telephone the same day. All new dosage recommendations were reviewed and adjusted if necessary. All extreme INRs (<1.5 or >5.0) were discussed by the research pharmacist with a consultant haematologist. Participants attended the anticoagulation clinic every 2 months to give a venous blood sample for laboratory analysis of INR, which was compared with the self‐tested value. The comparison group received conventional clinic management. |
|
| Outcomes | Time to therapeutic range, proportion of participants within therapeutic range, testing frequency, number of extreme INRs recorded, participant satisfaction, thrombotic events, bleeding. | |
| Trial identification | ||
| Study duration | 12 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Allocation implemented via sealed envelopes |
| Intention to treat analysis | High risk | ITT was not performed. |
| Reporting of losses of follow‐up | Low risk | 30 participants (19%) withdrew; reasons reported |
| Blinding | High risk | Participants and study staff were not blind to the intervention allocation. It is not reported whether data analysts were blind to allocation. |
Sawicki 1999.
| Study characteristics | ||
| Methods | Multicentre, partially blinded, randomised study. | |
| Participants | Participants (n = 179) were individuals (mean age 55 years), receiving long term oral anticoagulation. The study was based in 5 referral centres (Germany). | |
| Interventions |
Self‐management vs usual care Participants were randomised to a) self‐management (n = 90): home self‐testing and self‐dosing using a Coagucheck® monitor, measuring INR 1‐2 times per week and adjusting their anticoagulant according to their INR values. Participants recorded INR values routinely, recorded the results and anticoagulation dosages in their logbook. b) usual care (n = 89): conventional management via twice monthly clinic visits for INR testing with dosage adjustment advised by the general practitioner. |
|
| Outcomes | Primary and secondary outcomes were not specified. Data were reported for: squared INR deviation from the mean of the individual INR target range; percentage participants with INR within target range at 3 months and at 6 months; major bleeding; minor bleeding; thromboembolic events; treatment satisfaction. | |
| Trial identification | ||
| Study duration | 6 months | |
| Oral anticoagulant used | Phenprocoumon | |
| Notes | Participants randomised to the self‐management group received a structured educational program comprising three consecutive weekly teaching sessions of 60‐90 minutes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program. |
| Allocation concealment (selection bias) | Unclear risk | Possibly adequate or not used. |
| Intention to treat analysis | Low risk | ITT analysis was used |
| Reporting of losses of follow‐up | Low risk | 14/179 (7.8%) of participants were lost to follow‐up; reasons not reported |
| Blinding | Low risk | Participants were not blinded to the intervention; data collectors were blinded. |
Sidhu 2001.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. | |
| Participants | Participants (n = 100) adults aged < 85 years (mean age 61 years) who had undergone a heart valve operation and had been prescribed life‐long anticoagulation. | |
| Interventions |
Self‐management vs usual care Participants were randomised to a) self‐management (n = 51): home self‐testing using the Coagucheck® and self‐dosing. INR testing performed once a week, participants were encouraged to perform more frequent INR measurements if they were necessary. They adjusted their anticoagulant dosage according to a protocol. Participants recorded the results of their INR measurements in a standard book. b) usual care (n = 49) : conventional management by hospital anticoagulant clinic or family physician care. |
|
| Outcomes | Primary and secondary outcomes were not specified. Data were reported for: Time within therapeutic range; minor bleeding events; major bleeding events; minor thromboembolic events; major thromboembolic events; death. |
|
| Trial identification | ||
| Study duration | 24 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | For participants in the self‐management group, training comprised two 3‐hour sessions (in groups of 2‐5 participants). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple random number generator program |
| Allocation concealment (selection bias) | Unclear risk | Possibly adequate or not used |
| Intention to treat analysis | High risk | ITT analysis was not used |
| Reporting of losses of follow‐up | High risk | 33.3% of participants were lost to follow‐up in intervention group and 2% in the conventional management group |
| Blinding | Unclear risk | Participants were not blinded to the intervention. It was not reported if study or medical staff were blinded to the intervention. |
Siebenhofer 2007.
| Study characteristics | ||
| Methods | Multicentre, randomised controlled trial. | |
| Participants | Participants (n = 195) were adults aged > 60 (mean 69) years, with an indication for long‐term oral anticoagulation. Exclusion criteria included: previous participation in an anticoagulation self‐management program; severe cognitive or terminal illness. The study was based in 3 departments specialising in the treatment of participants receiving long‐term oral anticoagulation therapy (Austria). |
|
| Interventions |
Self‐management vs usual care Participants were randomised to a) self‐management (n = 99): home self‐testing using the Coagucheck® and self‐dosing. INR testing performed once a week, adjusting anticoagulant dosage accordingly. Participants were asked to contact the training centre in case of difficulties. b) usual care (n = 96): anticoagulant dosage adjusted by usual attending physicians in general practice or at a hospital based specialised anticoagulation clinic. |
|
| Outcomes | Primary outcome: composite of all thromboembolic events requiring hospitalisation and all major bleeding complications. Secondary outcomes: frequency and duration of hospitalisation; mortality; recurrence of stroke; numbers of INR values above 4.5 or lower than 1.7; treatment‐related quality of life; cost‐effectiveness. |
|
| Trial identification | ||
| Study duration | 12 months | |
| Oral anticoagulant used | Phenprocoumon (90% participants), acenocoumarol (10% participants) | |
| Notes | Participants assigned to the self‐management group participated in four consecutive weekly instruction sessions of 90 to 120 minutes each, in groups of three to six participants. Participants assigned to the control group participated in a single 90‐miute session including basic theoretical information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based system. |
| Allocation concealment (selection bias) | Low risk | Allocation was done by a central statistical office by fax and without awareness of participant data. The sequence of randomisation was concealed until the participant was assigned to a group. |
| Intention to treat analysis | Unclear risk | ITT analysis was used for primary outcome; per protocol analyses used for other outcomes |
| Reporting of losses of follow‐up | Low risk | 19/195 (9.7%) participants lost to follow‐up; reasons reported |
| Blinding | Low risk | Blinded outcome assessors. |
Soliman Hamad 2009.
| Study characteristics | ||
| Methods | Single centre, parallel‐group, randomised controlled study. | |
| Participants | Participants were individuals who had undergone elective mechanical aortic valve replacement and who were computer competent (n=58) | |
| Interventions |
Self‐management vs usual care Participants were randomised to self‐management using CoaguChek devices (n = 29) or usual care (conventional clinic care) (n = 29). In the intervention group, participants received training and self‐tested under the supervision of hospital ward staff until discharge, after which time participants self‐tested and notified INR dose via the study website. For 4 weeks advice was given from the clinic; subsequently the participant's data were evaluated by the clinic every 3 months. In the comparison group, participants received conventional care by the thrombosis clinic. At one year all participants completed a quality of life questionnaire and were evaluated by a study physician. |
|
| Outcomes | Primary endpoints were the total number of INR values within the target range as well as the quality of life measurements (SF‐36v2)W one‐year postoperatively, mortality, postoperative complications up to one year. | |
| Trial identification | ||
| Study duration | 12 months | |
| Oral anticoagulant used | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated to have been done but sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Intention to treat analysis | Unclear risk | ITT was not reported |
| Reporting of losses of follow‐up | Unclear risk | Losses to follow‐up unclear |
| Blinding | Unclear risk | Blinding was not reported |
Sunderji 2004.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. | |
| Participants | Participants (n = 139) were adults aged > 18 (mean 60) years, receiving warfarin for at least one month before randomisation and requiring anticoagulation for at least the subsequent year, and competent to manage their own anticoagulation therapy. Exclusion criteria included: known hypercoagulable disorder, mental incompetence, a language barrier or an inability to attend training sessions. Based in a tertiary care setting or by referral as an outpatient at the University of British Colombia (Canada). |
|
| Interventions |
Self‐management vs usual care Participants were randomised to: a) self‐management (n = 69): home self‐testing using Protime micro coagulation system and self‐dosing determining the appropriate dose of oral anticoagulant and the time of the next INR test using a nomogram, recording INR results and warfarin doses in a pocket calendar. b) usual care (n = 70): conventional care by primary care physician. |
|
| Outcomes | Primary outcomes: proportion of INRs within target range; error rate in warfarin dosage adjustments. Secondary outcomes: concordance between self‐monitored values and laboratory INR measures; patient satisfaction; major thromboembolic events; major bleeding events. |
|
| Trial identification | ||
| Study duration | Up to 8 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | Participants in the self‐management group were trained by a pharmacist in a 2‐3 session, then required at a second pharmacist appointment to demonstrate competency in self‐testing and self‐dosing. In a first 2‐yo 3‐hour visit participants received education from a pharmacist. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code. |
| Allocation concealment (selection bias) | Low risk | Randomisation code concealed. |
| Intention to treat analysis | Low risk | ITT analysis was used. |
| Reporting of losses of follow‐up | Low risk | 10% of participants were lost to follow‐up; reasons reported |
| Blinding | Unclear risk | Participants were not blinded to intervention; it was not reported if study or medical staff were blinded to the intervention |
Thompson 2013.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial | |
| Participants | 200 participants who had received mechanical heart valve prostheses at Mayo Clinic (Rochester, Minnesota, USA) | |
| Interventions | Participants were randomised to a) self‐testing; once the participant was discharged, dosage decisions using the self‐tested INR values were made by the primary care physician. or b) usual care. | |
| Outcomes | Primary and secondary outcomes were not specified. Reported outcomes included mean percentage of INR tests within therapeutic range; adverse events within 90 days of hospital discharge; time required to obtain INR result. | |
| Trial identification | ClinicalTrials.gov identifier NCT00703963 | |
| Study duration | 3 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of randomisation sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Intention to treat analysis | High risk | ITT analysis not performed |
| Reporting of losses of follow‐up | Low risk | 21 participants withdrew (10.5%); reasons reported |
| Blinding | High risk | Participants and study staff were not blind to the intervention allocation |
Verret 2012.
| Study characteristics | ||
| Methods | Randomised, controlled, open‐label trial | |
| Participants | Participants were adults aged 18–75 years receiving long term warfarin at a specialised anticoagulation clinic (n = 114). | |
| Interventions |
Self‐management vs usual care Randomised to pharmacist‐led warfarin patient self‐management program (n = 58) or conventional anticoagulation clinic care. All participants attended an educational session on anticoagulation provided by a pharmacist. Participants randomised to the self‐management group also received training to use the CoaguChek XS device and a self‐management dosing algorithm. Participants monitored their INR weekly and adjusted their dose of warfarin according to a written algorithm. Participants recorded their INR results and dose adjustments on a voicemail, reviewed by pharmacists. The participant was contacted by a pharmacist if a contact was missed. If an INR fell outside the algorithm limits (1.5 or 4.5 for a target INR of 2.0–3.0, or 2.2 or 5.0 for a target INR of 2.5–3.5), the dose was adjusted by the pharmacist. Each week participants completed a pre‐INR questionnaire to identify and inform the pharmacist of new factors that could affect their INR e.g. the addition of prescription or non‐prescription drugs, changes in diet. Participants in the self‐management group kept a record of their INR, daily doses of warfarin, and adverse events. Participants in the control group (n = 56) received standard anticoagulation clinic care. All participants attended clinic at 4 months to check INR and complete quality of life questionnaires. |
|
| Outcomes | Primary outcome: anticoagulation‐related quality of life. Secondary outcomes: time in therapeutic range, time in extended therapeutic range, anticoagulation knowledge. |
|
| Trial identification | Clinicaltrials.gov NCT01033279 | |
| Study duration | 4 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated using permuted random blocks |
| Allocation concealment (selection bias) | High risk | Not reported |
| Intention to treat analysis | Unclear risk | ITT not reported |
| Reporting of losses of follow‐up | Low risk | No participants were lost to follow‐up |
| Blinding | High risk | The study was open‐label |
Voller 2005.
| Study characteristics | ||
| Methods | Multicentre, randomised controlled trial. | |
| Participants | The study enrolled 202 participants, mean age 64 years, with permanent non‐valvular atrial fibrillation in long term anticoagulation. The study was based in 33 centres (Germany). | |
| Interventions |
Self‐management vs usual care Self‐testing using the Coagucheck® monitor and self‐adjusted dosing (regimen not reported). Usual care by family doctors (regime not reported). |
|
| Outcomes | Percentage of INR within therapeutic range. Days within range. Complications. | |
| Trial identification | ||
| Study duration | Up to 19 months | |
| Oral anticoagulant used | Not reported | |
| Notes | Stopped early trial due to low number of events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Allocation concealed |
| Intention to treat analysis | High risk | ITT not performed |
| Reporting of losses of follow‐up | Unclear risk | 19.8% dropout. |
| Blinding | Unclear risk | Blinding not reported |
White 1989.
| Study characteristics | ||
| Methods | Single centre, randomised controlled trial. | |
| Participants | Participants were outpatients discharge from a university hospital or community hospital, who started on warfarin for the first time, who demonstrated ability to self‐monitor, and who had not yet achieved stable INR whilst in hospital. Home monitor mean age 50 ± 14 years. Anticoagulation clinic group 49 ± 16 years. |
|
| Interventions |
Self‐monitoring vs usual care Participants were randomised to: a) self‐monitoring (n = 26): using the home monitor Coumatrak to measure INR, dosage adjustments advised by hospital physician, with a telephone contact support for self‐testing. b) usual care (n = 24): conventional management by registered nurse specialists at an anticoagulation clinic. |
|
| Outcomes | Primary outcome: percentage time within target INR range. Secondary outcomes: percentage time above therapeutic range; percentage time below therapeutic range; major thromboembolic or haemorrhagic events; differences between self‐monitoring measurements and laboratory measurements. |
|
| Trial identification | ||
| Study duration | 2 months | |
| Oral anticoagulant used | Warfarin | |
| Notes | Participants were trained to use the INR monitor and were required to demonstrate competence to be eligible for randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Possibly adequate or not used. |
| Intention to treat analysis | High risk | ITT analysis was not used. |
| Reporting of losses of follow‐up | Low risk | 4.1% of participants in intervention group and 11.5% of participants in control group lost to follow‐up. |
| Blinding | Unclear risk | Participants were not blinded to the intervention. It was not reported if study or medical staff were blinded to the intervention. |
INR: international normalized ratio ITT: intention‐to‐treat no. = number VKA = vitamin K antagonists
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bauman 2010 | No relevant comparison group: the trial compared self‐monitoring with self‐management |
| Bussey 2010 | Non‐randomised trial. |
| Bussey 2011 | Non‐randomised trial. |
| Christensen 2001 | Non‐randomised trial. |
| Christensen 2003 | Non‐randomised trial. |
| Clarkesmith 2013 | Intervention was educational and did not comprise self‐monitoring or self‐management. |
| Gallagher 2015 | Secondary report of a trial already included in the review |
| Hambleton 2003 | Not a clinical trial. |
| Hasenkam 1997 | Non‐randomised trial. |
| Hasenkam 1998 | Non‐randomised trial. |
| Heidinger 2000 | Non‐randomised trial. |
| Horstkotte 2004 | Non‐comparative study. |
| Lafata 2000 | Non‐randomised trial. |
| Laurence 2008 | No intervention of interest evaluated |
| Leger 2004 | Non‐comparative study. |
| Levi 2001 | Non‐comparative study. |
| Piso 2002 | Non‐randomised, comparative study. |
| Rosengart 2002 | Not a clinical trial. |
| Schmidtke 2001 | Non‐randomised trial. |
| Staresinic 2006 | Self‐monitoring/self‐management not included in the intervention |
| Sunderji 2005 | No intervention of interest evaluated. |
| Watzke 2000 | Non‐randomised study. |
Differences between protocol and review
Changes were introduced between protocol and review stage to increase the scope and quality of the review. The title changed from "Self management for oral anticoagulation" to "Self‐monitoring and self‐management of oral anticoagulation". The authors' list changed from Garcia‐Alamino JM, Martin JLR, Subirana M, Gich I to Garcia‐Alamino JM, Ward AM, Alonso‐Coello P, Perera R, Bankhead C, Fitzmaurice D, Heneghan C. The type of studies changed from "Randomised controlled trials assessing the therapeutic efficacy and safety of self‐management" to "Randomised controlled trials assessing the therapeutic effectiveness and safety of self‐monitoring or self‐management of oral anticoagulation". In the 'Types of outcome measures' mortality was added as an outcome. The quality assessment of the studies in the review now includes assessment of the evidence with the GRADE system.
Contributions of authors
CH: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
CH, IO, ES, KM: checked and analysed the data.
CH, IO, ES, KM, JMG‐A, RP, PA‐C, AW, and CB, DF: contributed to the manuscript.
RP, CH and IO: Statistical analysis
All authors approved the final manuscript.
Sources of support
Internal sources
-
NIHR School of Primary Care Research, UK
The University Department of Primary Health Care is part of the NIHR School of Primary Care Research which provides financial support for senior staff who contributed to this paper.
-
NIHR HTA, UK
The HTA has provided financial support as part of a Monitoring in Chronic diseases project grant for the analysis and write‐up of this review.
Hospital de la Santa Creu i Sant Pau, Spain
External sources
-
Instituto de Salud Carlos III, European Regional Development Fund (PI07/90406), Other
This work was partially funded by the Instituto de Salud Carlos III, co‐financed by the European Regional Development Fund (PI07/90406).
Declarations of interest
CJH: CH has received financial support from the National Institute of Health Research (NIHR) School of Primary Care Research in the UK for this update and is also part funded by the NIHR Oxford Diagnostic Evidence Co‐operative (NIHR DEC). The opinions are those of the authors and not the Department of Health.
JMGA: None known.
EAS: None known.
AMW: None known.
RP: I am an investigator in the cohort study looking at estimating how successful self‐monitoring is (in terms of control levels) in individuals that attempt self‐monitoring (non‐RCT setting). The results of this study would not be included in this Cochrane SR but could inform its discussion.
CB: None known.
PAC: None known.
DF: The institution I work for, The Univeristy of Birmingham, has received educational grants from Roche Diagnsotics UK to deliver on‐going educational activities in the area of oral anticoagulation management.
KRM: None known.
IJO: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Azarnoush 2009 {published data only}
- Azarnoush K, Camilleri L, Aublet-Cuvelier B, Geoffroy E, Dauphin C, Dubray C, et al. Results of the first randomized French study evaluating self-testing of the International Normalized Ratio. Journal of Heart Valve Disease 2011;20(5):518-25. [PubMed] [Google Scholar]
- Dauphin C, Legault B, Jaffeux P, Motreff P, Azarnoush K, Joly H, et al. Comparison of INR stability between self-monitoring and standard laboratory method: preliminary results of a prospective study in 67 mechanical heart valve patients. Archives of Cardiovascular Diseases 2008;101(11-12):753-61. [DOI] [PubMed] [Google Scholar]
Beyth 2000 {published data only}
- Beyth RJ, Quinn L, Landefeld CS. A multicomponent intervention to prevent major bleeding complications in older patients receiving warfarin. A randomized, controlled trial. Annals of Internal Medicine 2000;133(9):687-95. [DOI] [PubMed] [Google Scholar]
Christensen 2006 {published data only}
- Christensen TD, Maegaard M, Sørensen HT, Hjortdal VE, Hasenkam JM. Self-management versus conventional management of oral anticoagulant therapy: A randomized, controlled trial. European Journal of Internal Medicine 2006;17(4):260-6. [DOI] [PubMed] [Google Scholar]
Christensen 2011 {published data only}
- Christensen H, Lauterlein JJ, Sørensen PD, Petersen ERB, Madsen JS, Brandslund I. Home management of oral anticoagulation via telemedicine versus conventional hospital-based treatment. Telemedicine and e-Health 2011;17(3):169-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cromheecke 2000 {published data only}
- Cromheecke ME, Levi M, Colly LP, Mol BJ, Prins MH, Hutten BA, et al. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomised cross-over comparison. Lancet 2000;356(9224):97-102. [DOI] [PubMed] [Google Scholar]
Dignan 2013 {published data only}
- Dignan R, Keech AC, Gebski VJ, Mann KP, Hughes CF, Warfarin SMART Investigators. Is home warfarin self-management effective? Results of the randomised Self-Management of Anticoagulation Research Trial. International Journal of Cardiology 2013;168(6):5378-84. [DOI] [PubMed] [Google Scholar]
Fitzmaurice 2002 {published data only}
- Fitzmaurice DA, Murray ET, Gee KM, Allan TF, Hobbs FD. A randomised controlled trial of patient self management of oral anticoagulation treatment compared with primary care management. Journal of Clinical Pathology 2002;55(11):845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahon D, Murray ET, Murray K, Holder RL, Fitzmaurice DA. Does self-management of oral anticoagulation therapy improve quality of life and anxiety? Family Practice 2011;28(2):134-40. [DOI] [PubMed] [Google Scholar]
Fitzmaurice 2005 {published data only}
- Fitzmaurice DA, Murray ET, McCahon D, Holder R, Raftery JP, Hussain S, et al. Self management of oral anticoagulation: randomised trial. BMJ 2005;331(7524):1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahon D, Fitzmaurice DA, Murray ET, Fuller CJ, Hobbs RF, Allan TF, et al. SMART: self-management of anticoagulation, a randomised trial. BMC Family Practice 2003;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Fitzmaurice D, McCahon D, Fuller C, Sandhur H. Training for patients in a randomised controlled trial of self management of warfarin treatment. BMJ 2004;328(7437):437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadisseur 2003 Self mge {published data only}
- Gadisseur AP, Breukink-Engbers WG, Meer FJ, den Besselaar AM, Sturk A, Rosendaal FR. Comparison of the quality of oral anticoagulant therapy through patient self-management and management by specialized anticoagulation clinics in the Netherlands: a randomized clinical trial. Archives of Internal Medicine 2003;163(21):2639-46. [DOI] [PubMed] [Google Scholar]
- Gadisseur AP, Kaptein AA, Breukink-Engbers WG, Meer FJ, Rosendaal FR. Patient self-management of oral anticoagulant care vs. management by specialized anticoagulation clinics: positive effects on quality of life. Journal of Thrombosis and Haemostasis 2004;2(4):584-91. [DOI] [PubMed] [Google Scholar]
Gadisseur 2003 Self monit {published data only}
- Gadisseur AP, Breukink-Engbers WG, Meer FJ, den Besselaar AM, Sturk A, Rosendaal FR. Comparison of the quality of oral anticoagulant therapy through patient self-management and management by specialized anticoagulation clinics in the Netherlands: a randomized clinical trial. Archives of Internal Medicine 2003;163(21):2639-46. [DOI] [PubMed] [Google Scholar]
- Gadisseur AP, Kaptein AA, Breukink-Engbers WG, Meer FJ, Rosendaal FR. Patient self-management of oral anticoagulant care vs. management by specialized anticoagulation clinics: positive effects on quality of life. Journal of Thrombosis and Haemostasis 2004;2(4):584-91. [DOI] [PubMed] [Google Scholar]
Gardiner 2005 {published data only}
- Gardiner C, Williams K, Longair I, Mackie IJ, Machin SJ, Cohen H. A randomised control trial of patient self-management of oral anticoagulation compared with patient self-testing.. British Journal of Haematolgy 2006;132(5):598-603. [DOI] [PubMed] [Google Scholar]
- Gardiner C, Williams K, Mackie IJ, Machin SJ, Cohen H. Patient self-testing is a reliable and acceptable alternative to laboratory INR monitoring. British Journal of Haematology 2005;128(2):242-7. [DOI] [PubMed] [Google Scholar]
Grunau 2011 {published data only}
- Grunau BE, Wiens MO, Harder KK. Patient self-management of warfarin therapy: pragmatic feasibility study in Canadian primary care. Canadian Family Physician Medecin de Famille Canadien 2011;57(8):e292-8. [PMC free article] [PubMed] [Google Scholar]
Horstkotte 1998 {published data only}
- Horstkotte D, Piper C, Wiemer M. Optimal frequency of patient monitoring and intensity of oral anticoagulation therapy in valvular heart disease. Journal of Thrombosis and Thrombolysis 1998;5 Suppl 1(3):19-24. [DOI] [PubMed] [Google Scholar]
Kaatz Unpublished {unpublished data only}
- Kaatz S, Elston-Lafata J, Gooldy S. Anticoagulation therapy home and office monitoring evaluation study. Journal of Thrombosis and Thrombolysis 2001;12:111. [Google Scholar]
Khan 2004 {published data only}
- Khan TI, Kamali F, Kesteven P, Avery P, Wynne H. The value of education and self-monitoring in the management of warfarin therapy in older patients with unstable control of anticoagulation. British Journal of Haematology 2004;126(4):557-64. [DOI] [PubMed] [Google Scholar]
Körtke 2001 {published data only}
- Eitz T, Schenk S, Fritzsche D, Bairaktaris A, Wagner O, Koertke H, et al. International normalized ratio self-management lowers the risk of thromboembolic events after prosthetic heart valve replacement. Annals of Thoracic Surgery 2008;85(3):949-54. [DOI] [PubMed] [Google Scholar]
- Körtke H, Körfer R. International normalized ratio self-management after mechanical heart valve replacement: is an early start advantageous? Annals of Thoracic Surgery 2001;72(1):44-8. [DOI] [PubMed] [Google Scholar]
- Körtke H, Minami K, Breymann T, Seifert D, Baraktaris A, Wagner O, et al. INR self-management after mechanical heart valve replacement: ESCAT (Early Self-Controlled Anticoagulation Trial). Zeitschrift fur Kardiologie 2001;90 Suppl:6118-24. [DOI] [PubMed] [Google Scholar]
- Koertke H, Minami K, Bairaktaris A, Wagner O, Koerfer R. INR self-management following mechanical heart valve replacement. Journal of Thrombosis and Thrombolysis 2000;9 Suppl 1:41-5. [DOI] [PubMed] [Google Scholar]
- Koertke H, Minami K, Boethig D, Breymann TH, Seifert D, Wagner O, et al. INR self-management permits lower anticoagulation levels after mechanical heart valve replacement. Circulation 2003;108(10 Suppl 1):II-75-II-78. [DOI] [PubMed] [Google Scholar]
- Koertke H, Zittermann A, Minami K, Tenderich G, Wagner O, El-Arousy M, et al. Low-dose international normalized ratio self-management: a promising tool to achieve low complication rates after mechanical heart valve replacement. Annals of Thoracic Surgery 2005;79(6):1909-14. [DOI] [PubMed] [Google Scholar]
- Koertke H, Zittermann A, Tenderich G, Wagner O, El-Arousy M, Krian A, et al. Low-dose oral anticoagulation in patients with mechanical heart valve prostheses: final report from the early self-management anticoagulation trial II. European Heart Journal 2007;28(20):2479-84. [DOI] [PubMed] [Google Scholar]
- Koertke H, Zittermann A, Wagner O, Ennker J, Saggau W, Sack FU, et al. Efficacy and safety of very low-dose self-management of oral anticoagulation in patients with mechanical heart valve replacement. Annals of Thoracic Surgery 2010;90(5):1487-93. [DOI] [PubMed] [Google Scholar]
- Koertke H, Zittermann A, Wagner O, Koerfer R. Self-management of oral anticoagulation therapy improves long-term survival in patients with mechanical heart valve replacement. Annals of Thoracic Surgery 2007;83(1):24-9. [DOI] [PubMed] [Google Scholar]
Matchar 2010 {published and unpublished data}
- Matchar DB, Dolor R, Jacobson A, Love S, Edson R, Uyeda L. More frequent self-testing of prothrombin time results in improved time in target range. Circulation 2012;126:21 Suppl. [Google Scholar]
- Matchar DB, Jacobson A, Dolor R, Edson R, Uyeda L, Phibbs CS, et al, THINRS Executive Committee and Site Investigators. Effect of home testing of international normalized ratio on clinical events. New England Journal of Medicine 2010;363(17):1608-20. [DOI] [PubMed] [Google Scholar]
Menendez‐Jandula 2005 {published data only}
- Menéndez-Jándula B, Souto JC, Oliver A, Montserrat I, Quintana M, Gich I, et al. Comparing self-management of oral anticoagulant therapy with clinic management: a randomized trial. Annals of Internal Medicine 2005;142(1):1-10. [DOI] [PubMed] [Google Scholar]
Rasmussen 2012 {published data only}
- Rasmussen RS, Corell P, Madsen P, Overgaard K. Effects of computer-assisted oral anticoagulant therapy. Thrombosis Journal 2012;10(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2009 {published data only}
- Ryan F, Byrne S, O'Shea S. Randomized controlled trial of supervised patient self-testing of warfarin therapy using an internet-based expert system. Journal of Thrombosis and Haemostasis : JTH 2007;7(8):1284-90. [DOI] [PubMed] [Google Scholar]
Sawicki 1999 {published data only}
- Sawicki PT, Glaser B, Kleespies C, Stubbe J, Schmitz N, Kaiser T, et al. Long-term results of patients' self-management of oral anticoagulation. Journal of Clinical & Basic Cardiology 2003;6(1-4):59-62. [DOI] [PubMed] [Google Scholar]
- Sawicki PT. A structured teaching and self-management program for patients receiving oral anticoagulation: a randomized controlled trial. Working Group for the Study of Patient Self-Management of Oral Anticoagulation. JAMA 1999;281(2):145-50. [DOI] [PubMed] [Google Scholar]
Sidhu 2001 {published data only}
- Sidhu P, O'Kane HO. Self-managed anticoagulation: results from a two-year prospective randomized trial with heart valve patients. Annals of Thoracic Surgery 2001;72(5):1523-7. [DOI] [PubMed] [Google Scholar]
Siebenhofer 2007 {published data only}
- Siebenhofer A, Hemkens LG, Rakovac I, Spat S, Didjurgeit U, SPOG 60+ Study Group. Self-management of oral anticoagulation in elderly patients - effects on treatment-related quality of life. Thrombosis Research 2012;130:e60-e6. [DOI] [PubMed] [Google Scholar]
- Siebenhofer A, Rakovac I, Kleespies C, Piso B, Didjurgeit U. Self-management of oral anticoagulation in the elderly: rationale, design, baselines and oral anticoagulation control after one year of follow-up. A randomized controlled trial. Thrombosis and Haemostasis 2007;97(3):408-16. [PubMed] [Google Scholar]
- Siebenhofer A, Rakovac I, Kleespies C, Piso B, Didjurgeit U. Self-management of oral anticoagulation reduces major outcomes in the elderly. A randomized controlled trial. Thrombosis & Haemostasis 2008;100(6):1089-98. [PubMed] [Google Scholar]
Soliman Hamad 2009 {published data only}
- Soliman Hamad MA, Eekelen E, Agt T, Straten AH. Self-management program improves anticoagulation control and quality of life: a prospective randomized study. European Journal of Cardiothoracic Surgery 2009;35(2):265-9. [DOI] [PubMed] [Google Scholar]
Sunderji 2004 {published data only}
- Sunderji R, Campbell L, Shalansky K, Fung A, Carter C, Gin K. Outpatient self-management of warfarin therapy: A pilot study. Pharmacotherapy 1999;19(6):787-93. [DOI] [PubMed] [Google Scholar]
- Sunderji R, Gin K, Shalansky K, Carter C, Chambers K, Davies C, et al. A randomized trial of patient self-managed versus physician-managed oral anticoagulation. Canadian Journal of Cardiology 2004;20(11):1117-23. [PubMed] [Google Scholar]
Thompson 2013 {published data only}
- Thompson JL, Burkhart HM, Daly RC, Dearani JA, Joyce LD, Suri RM, et al. Anticoagulation early after mechanical valve replacement: improved management with patient self-testing. Journal of Thoracic and Cardiovascular Surgery 2013;146:599-604. [DOI] [PubMed] [Google Scholar]
Verret 2012 {published data only}
- Verret L, Couturier J, Rozon A, Saudrais-Janecek S, St-Onge A, Nguyen A, et al. Impact of a pharmacist-led warfarin self-management program on quality of life and anticoagulation control: a randomized trial. Pharmacotherapy 2012;32(10):871-9. [DOI] [PubMed] [Google Scholar]
Voller 2005 {published data only}
- Völler H, Glatz J, Taborski U, Bernardo A, Dovifat C, Heidinger K. Self-management of oral anticoagulation in nonvalvular atrial fibrillation (SMAAF study). Zeitschrift fur Kardiologie 2005;94(3):182-6. [DOI] [PubMed] [Google Scholar]
White 1989 {published data only}
- White RH, McCurdy SA, Marensdorff H, Woodruff DE Jr, Leftgoff L. Home prothrombin time monitoring after the initiation of warfarin therapy. A randomized, prospective study. Annals of Internal Medicine 1989;111(9):730-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bauman 2010 {published data only}
- Bauman ME, Black K, Bauman ML, Bruce AA, Kuhle S, Bajzar L, et al. EMPoWarMENT: Edmonton pediatric warfarin self-management pilot study in children with primarily cardiac disease. Thrombosis Research 2010;126(2):e110-5. [DOI] [PubMed] [Google Scholar]
Bussey 2010 {published data only}
- Bussey HI Jr, Frei C, Walker MB, Bussey-Smith K. Superior oral anticoagulation management with self testing and automated management; An interim analysis. In: Pharmacotherapy. Vol. 30. 2010:396e.
Bussey 2011 {published data only}
- Walker MB, Bussey HI. Use of an interactive online information service to improve patient care in the field of anticoagulation and antithrombotic therapy. Journal of Thrombosis and Thrombolysis 2011;31:371:A8. [Google Scholar]
Christensen 2001 {published data only}
- Christensen TD, Attermann J, Pilegaard HK, Andersen NT, Maegaard M, Hasenkam JM. Self-management of oral anticoagulant therapy for mechanical heart valve patients. Scandinavian. Cardiovascular Journal 2001;35(2):107-13. [DOI] [PubMed] [Google Scholar]
Christensen 2003 {published data only}
- Christensen TD, Andersen NT, Attermann J, Hjortdal VE, Maegaard M, Hasenkam JM. Mechanical heart valve patients can manage oral anticoagulant therapy themselves. European Journal of Cardio-Thoracic Surgery 2003;23(3):292-8. [DOI] [PubMed] [Google Scholar]
Clarkesmith 2013 {published data only}
- Clarkesmith DE, Pattison HM, Lip GYH, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PLOS One 2013;8(9):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 2015 {published data only}
- Gallagher J, Mc Carthy S, Woods N, Ryan F, O' Shea S, Byrne S. Economic evaluation of a randomized controlled trial of pharmacist-supervized patient self-testing of warfarin therapy. Journal of Clinical Pharmacy and Therapeutics 2015;40(1):14-9. [DOI] [PubMed] [Google Scholar]
Hambleton 2003 {published data only}
- Hambleton J. Home monitoring of anticoagulation. Journal of Thrombosis and Thrombolysis 2003;16(1-2):39-42. [DOI] [PubMed] [Google Scholar]
Hasenkam 1997 {published data only}
- Hasenkam JM, Knudsen L, Kimose HH, Gronnesby H, Attermann J, Andersen NT, et al. Practicability of patient self-testing of oral anticoagulant therapy by the international normalized ratio (INR) using a portable whole blood monitor. A pilot investigation. Thrombosis Research 1997;85(1):77-82. [DOI] [PubMed] [Google Scholar]
Hasenkam 1998 {published data only}
- Hasenkam JM, Kimose HH, Gronnesby H, Andersen NT, Halborg J, Attermann J, et al. Self management of peroral anticoagulant therapy in patients with artificial heart valves. Ugeskrift for Laeger 1998;160(47):6811-5. [PubMed] [Google Scholar]
Heidinger 2000 {published data only}
- Heidinger KS, Bernardo A, Taborski U, Muller-Berghaus G. Clinical outcome of self-management of oral anticoagulation in patients with atrial fibrillation or deep vein thrombosis. Thrombosis Research 2000;98(4):287-93. [DOI] [PubMed] [Google Scholar]
Horstkotte 2004 {published data only}
- Horstkotte D, Piper C. Improvement of oral anticoagulation therapy by INR self-management. Journal of Heart Valve Disease 2004;13(3):335-8. [PubMed] [Google Scholar]
Lafata 2000 {published data only}
- Lafata JE, Martin SA, Kaatz S, Ward RE. Anticoagulation clinics and patient self-testing for patients on chronic warfarin therapy: A cost-effectiveness analysis. Journal of Thrombosis and Thrombolysis 2000;9 Suppl 1:13-9. [DOI] [PubMed] [Google Scholar]
Laurence 2008 {published data only}
- Laurence C, Gialamas A, Yelland L, Bubner T, Ryan P, Willson K, et al. A pragmatic cluster randomised controlled trial to evaluate the safety, clinical effectiveness, cost effectiveness and satisfaction with point of care testing in a general practice setting - rationale, design and baseline characteristics. Trials 2008;9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leger 2004 {published data only}
- Leger S, Allenet B, Calop J, Bosson JL. Therapeutic education of patients receiving anticoagulants for thromboembolic venous disease: Description of the Educ'AVK program. Journal des Maladies Vasculaires 2004;29(3):145-51. [DOI] [PubMed] [Google Scholar]
Levi 2001 {published data only}
- Levi M, Bruin TA, Meer FJ, Cromheecke ME, Mol BA. Self-monitoring and self dosing of oral anticoagulant therapy with vitamin K antagonists. Tijdschrift voor Geneeskunde 2001;145(48):2313-7. [PubMed] [Google Scholar]
Piso 2002 {published data only}
- Piso B, Jimenz-Boj E, Krinninger B, Watzke HH. The quality of oral anticoagulation before, during and after a period of patient self-management. Thrombosis Research 2002;106(2):101-4. [DOI] [PubMed] [Google Scholar]
Rosengart 2002 {published data only}
- Rosengart TK. Anticoagulation self-testing after heart valve replacement. Journal of Heart Valve Disease 2002;11 Suppl:61-5. [PubMed] [Google Scholar]
Schmidtke 2001 {published data only}
- Schmidtke C, Huppe M, Berndt S, Notzold A, Sievers HH. Quality of life after aortic valve replacement - Anticoagulation self-management or conventional therapy in mechanical prostheses versus pulmonary autograft. Zeitschrift fur Kardiologie 2001;90(11):860-6. [DOI] [PubMed] [Google Scholar]
Staresinic 2006 {published data only}
- Staresinic AG, Sorkness CA, Goodman BM, Pigarelli DW. Comparison of outcomes using 2 delivery models of anticoagulation care. Archives of Internal Medicine 2006;166(9):997-1006. [DOI] [PubMed] [Google Scholar]
Sunderji 2005 {published data only}
- Sunderji R, Gin K, Shalansky K, Carter C, Chambers K, Davies C, et al. Clinical impact of point-of-care vs laboratory measurement of anticoagulation. American Journal of Clinical Pathology 2005;123(2):184-8. [PubMed] [Google Scholar]
Watzke 2000 {published data only}
- Watzke HH, Forberg E, Svolba G, Jimenez-Boj E, Krinninger B. A prospective controlled trial comparing weekly self-testing and self-dosing with the standard management of patients on stable oral anticoagulation. Thrombosis and Haemostasis 2000;84(5):931-2. [PubMed] [Google Scholar]
Additional references
Anderson 1993
- Anderson DR, Harrison L, Hirsh J. Evaluation of a portable prothrombin time monitor for home use by patients who require long-term oral anticoagulant therapy. Archives of Internal Medicine 1993;153:1441-7. [PubMed] [Google Scholar]
Ansell 1996
- Ansell JE, Hughes R. Evolving models of warfarin management: anticoagulation clinics, patient self-monitoring, and patient self-management. American Heart Journal 1996;132(5):1095-100. [DOI] [PubMed] [Google Scholar]
Ansell 2001
- Ansell J, Hirsh J, Dalen J, Bussey H, Anderson D, Poller L, et al. Managing oral anticoagulant therapy. Chest 2001;119 Suppl(1):22-38. [DOI] [PubMed] [Google Scholar]
Ansell 2004
- Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonist. Chest 2004;126:204-33. [DOI] [PubMed] [Google Scholar]
Ansell 2005
- Ansell J, Jacobson A, Levy J, Völler H, Hasenkam JM, International Self-Monitoring Association for Oral Anticoagulation. Guidelines for implementation of patient self-testing and patient self-management of oral anticoagulation. International consensus guidelines prepared by International Self-Monitoring Association for Oral Anticoagulation. International Journal of Cardiology 2005;99(1):37-45. [DOI] [PubMed] [Google Scholar]
Baglin 1994
- Baglin T. Decentralization of anticoagulant control. Clinical and Laboratory Haematology 1994;16:327-9. [Google Scholar]
Barcellona 1998
- Barcellona D, Vannini ML, Fenu L, Balestrieri C, Marongiu F. Warfarin or acenocoumarol: which is better in the management of oral anticoagulants? Thrombosis and Haemostostasis 1998;80(6):899-902. [PubMed] [Google Scholar]
Barcellona 2009
- Barcellona D, Fenu L, Cornacchini S, Marongiu F. Point-of-care (POCT) prothrombin time monitors: is a periodical control of their performance useful? Thromb Res. 2009;123:775-9. [DOI] [PubMed] [Google Scholar]
Bassler 2010
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis.. JAMA 2010;303(12):1180-7. [DOI] [PubMed] [Google Scholar]
Bazian 2005
- Bazian Ltd. Self-management of oral anticoagulation. Evidence-Based Healthcare and Public Health October 2005;9(5):334-40. [Google Scholar]
Bloomfield 2011
- Bloomfield HE, Krause A, Greer N, Taylor BC, MacDonald R, Rutks I, et al. Meta-analysis: effect of patient self-testing and self-management of long-term anticoagulation on major clinical outcomes. Annals of Internal Medicine 2011;154(7):472-82. [DOI] [PubMed] [Google Scholar]
Breckenridge 1978
- Breckenridge AM. Oral anticoagulant drugs: pharmacokinetic aspects. Seminars in Hematology 1978;15:19-26. [PubMed] [Google Scholar]
Brown 2007
- Brown A, Wells P, Jaffey J, McGahan L, Poon M-C, Cimon K, et al. Point-of-care monitoring devices for long-term oral anticoagulation therapy: clinical and cost effectiveness. Canadian Agency for Drugs and Technologies in Health 2007. [www.cadth.ca]
Buckingham 2002
- Buckingham TA, Hatala R. Anticoagulants for atrial fibrillation: why is the treatment rate so low? Clinical Cardiology 2002;25(10):447-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cannegieter 1995
- Cannegieter SC, Rosendaal FR, Wintzen AR, Meer FJ, Vandenbroucke JP, Briët E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. New England Journal of Medicine 1995;333(1):11-7. [DOI] [PubMed] [Google Scholar]
Caro 2004
- Caro JJ. An economic model of stroke in atrial fibrillation: the cost of suboptimal oral anticoagulation. American Journal of Managed Care 2004;10(14 Suppl):451-8. [PubMed] [Google Scholar]
Christensen 2007
- Christensen TD, Johnsen SP, Hjortdal VE, Hasenkam JM. Self-management of oral anticoagulant therapy: a systematic review and meta-analysis. International Journal of Cardiology 2007;118(1):54-61. [DOI] [PubMed] [Google Scholar]
Christensen 2009
- Christensen TD, Larsen TB, Jensen C, Maegaard M, Sørensen B. International normalised ratio (INR) measured on the CoaguChek S and XS compared with the laboratory for determination of precision and accuracy. Thrombosis and Haemostasis 2009;101(3):563-9. [DOI] [PubMed] [Google Scholar]
Connock 2007
- Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al. Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anticoagulation therapy: a systematic review and economic modelling. Health Technology Assessment 2007;11(38):ix-66. [DOI] [PubMed] [Google Scholar]
Connolly 1991
- Connolly SJ, Laupacis A, Gent M, Roberts RS, Cairns JA, Joyner C. Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. Journal of the American College of Cardiology 1991;18(2):349-55. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
de Solà‐Morales 2005
- Solà-Morales Serra O, Elorza Ricart JM. Portable coagulometers: a systematic review of the evidence on self-management of oral anticoagulant treatment. Medicina Clinica (Barc) 2005;124(9):321-5. [DOI] [PubMed] [Google Scholar]
Devereaux 2001
- Devereaux PJ, Anderson DR, Gardner MJ, Putnam W, Flowerdew GJ, Brownell BF, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Devereaux 2004
- Devereaux PJ, Choi PT, El-Dika S, Bhandari M, Montori VM, Schünemann HJ, et al. An observational study found that authors of randomized controlled trials frequently use concealment of randomization and blinding, despite the failure to report these methods. Journal of Clinical Epidemiology 2004;57(12):1232-6. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
DiNicolantonio 2012
- DiNicolantonio JJ. Dabigatran or warfarin for the prevention of stroke in atrial fibrillation? A closer look at the RE-LY trial.. Expert Opinion in Pharmacotherapy 2012;13(8):1101-11. [DOI] [PubMed] [Google Scholar]
Eikelboom 2013
- Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ et al, RE-ALIGN Investigators. Dabigatran versus Warfarin in Patients with Mechanical Heart Valves. New England Journal of Medicine 2013;369(13):1206-14. [DOI] [PubMed] [Google Scholar]
Fitzmaurice 2001
- Fitzmaurice DA, Machin SJ, for The Bristish Society of Haematology Task Force for Haemostasis and Thrombosis. Recommendations for patients undertaking self management of oral anticoagulation. BMJ 2001;323:985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadisseur 2004
- Gadisseur AP, Kaptein AA, Breukink-Engbers WG, Meer FJ, Rosendaal FR. Patient self-management of oral anticoagulant care vs. management by specialized anticoagulation clinics: positive effects on quality of life. Journal of Thrombosis and Haemostasis 2004;2(4):584-91. [DOI] [PubMed] [Google Scholar]
Go 2003
- Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003;290(20):2685-92. [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, for the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenblatt 2005
- Greenblatt DJ, Moltke LL. Interaction of warfarin with drugs, natural substances, and foods. Journal of Clinical Pharmacology 2005;45:127-32. [DOI] [PubMed] [Google Scholar]
Hart 2007
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of Internal Medicine 2007;146:857-67. [DOI] [PubMed] [Google Scholar]
Heneghan 2006a
- Heneghan C, Alonso-Coello P, Garcia-Alamino JM, Perera R, Meats E, Glasziou P. Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet 2006;367(9508):404-11. [DOI] [PubMed] [Google Scholar]
Heneghan 2008
- Heneghan C, Perera R. Oral anticoagulation therapy. In: Glasziou P, Irwig L, Aronson JK, editors(s). Evidence Based Medical Monitoring. 1 edition. Oxford: Blackwells, 2008:229-244. [Google Scholar]
Heneghan 2012
- Heneghan C, Ward A, Perera R, Bankhead C, Fuller A, Stevens R, et al. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet 2012;379(9813):322-34. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
Hirsh 1998
- Hirsh J, Dalen JE, Anderson DR, Poller L, Bussey H, Ansell J, et al. Oral anticoagulation mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 1998;114 Suppl:445-69. [DOI] [PubMed] [Google Scholar]
Hirsh 2001
- Hirsh J, Dalen JE, Anderson DR, Poller L, Bussey H, Ansell J, et al. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001;119 Suppl 1:8-21. [DOI] [PubMed] [Google Scholar]
Hylek 1996
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. New England Journal of Medicine 1996;335(8):540-6. [DOI] [PubMed] [Google Scholar]
Jennings 2014
- Jennings I, Kitchen D, Keeling D, Fitzmaurice D, Heneghan C, BCSH Committee. Patient self-testing and self-management of oral anticoagulation with vitamin K antagonists: guidance from the British Committee for Standards in Haematology. British Journal of Haematology 2014;167(5):600-7. [DOI: doi: 10.1111/bjh.13070] [DOI] [PubMed] [Google Scholar]
Kearon 2003
- Kearon C, Ginsberg JS, Kovacs MJ, Anderson DR, Wells P, Julian JA, et al, Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. New England Journal of Medicine 2003;349(7):631-9. [DOI] [PubMed] [Google Scholar]
Lefebvre 1996
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Paper presented at the Fourth International Cochrane Colloquium, 20-24 Oct 1996; Adelaide. Australia 1996.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
Manotti 2001
- Manotti C, Moia M, Palareti G, Pengo V, Ria L, Dettori AG. Effect of computer-aided management on the quality of treatment in anticoagulated patients: a prospective, randomized, multicenter trial of APROAT (Automated Program for Oral Anticoagulant Treatment). Haematologica 2001;86:1060-70. [PubMed] [Google Scholar]
Nagler 2014
- Nagler M, Bachmann LM, Schmid P, Raddatz Müller P, Wuillemin WA. Patient self-management of oral anticoagulation with vitamin K antagonists in everyday practice: efficacy and safety in a nationwide long-term prospective cohort study. PLoS one 2014;9(4):e95761. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE diagnostics guidance [DG14]
- NICE. Atrial fibrillation and heart valve disease: self‑monitoring coagulation status using point‑of‑care coagulometers (the CoaguChek XS system and the INRatio2 PT/INR monitor). NICE diagnostics guidance [DG14] September 2014. [www.nice.org.uk/guidance/dg14]
NICE Venous thromboembolic diseases (CG144)
- NICE. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. NICE June 2012. [www.nice.org.uk/guidance/CG144] [PubMed]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta-analysis. Controlled Clinical Trials 1997;18(6):580-93. [DOI] [PubMed] [Google Scholar]
Poller 2006
- Poller L, Keown M, Ibrahim SA, Meer FJ, den Besselaar AM, Tripodi A, et al, European Concerted Action on Thrombosis. Quality assessment of CoaguChek point-of-care prothrombin time monitors: comparison of the European community-approved procedure and conventional external quality assessment. Clinical Chemistry 2006;52(10):1843-7. [DOI] [PubMed] [Google Scholar]
Regier 2006
- Regier DA, Sunderji R, Lynd LD, Gin K, Marra CA. Cost-effectiveness of self-managed versus physician-managed oral anticoagulation therapy. CMAJ: Canadian Medical Jurnal Association 2006;174(13):1847-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2015
- Sharma P, Scotland G, Cruickshank M, Tassie E, Fraser C, Burton C, et al. The clinical effectiveness and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation system) for the self-monitoring of the coagulation status of people receiving long-term vitamin K antagonist therapy, compared with standard UK practice: systematic review and economic evaluation. Health Technology Assessment 2015;19(48). [DOI: ] [DOI] [PMC free article] [PubMed]
Siebenhofer 2004
- Siebenhofer A, Berghold A, Sawicki PT. Systematic review of studies of self-management of oral anticoagulation. Thrombosis and Haemostasis 2004;91(2):225-32. [DOI] [PubMed] [Google Scholar]
SPAF 1996
- Stroke Prevention in Atrial Fibrillation Investigators. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996;348(9028):633-8. [PubMed] [Google Scholar]
Stafford 1998
- Stafford RS, Singer DE. Recent national patterns of warfarin use in atrial fibrillation. Circulation 1998;97(13):1231-3. [DOI] [PubMed] [Google Scholar]
Taborski 1999
- Taborski U, Wittstamm FJ, Bernardo A. Cost-effectiveness of self-managed anticoagulant therapy in Germany. Seminars in Thrombosis and Hemostasis 1999;25(1):103-7. [DOI] [PubMed] [Google Scholar]
Ødegaard 2004
- Ødegaard KJ. Self-management in anticoagulation--a meta-analysis. Tidsskrift for den Norske Laegeforening 2004;124(22):2900-3. [PubMed] [Google Scholar]
References to other published versions of this review
Cochrane 2010
- Garcia-Alamino JM, Ward AM, Alonso-Coello P, Perera R, Bankhead C, Fitzmaurice D, et al. Self-monitoring and self-management of oral anticoagulation. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No: CD003839. [DOI: 10.1002/14651858.CD003839.pub2] [DOI] [PubMed] [Google Scholar]
Heneghan 2006b
- Heneghan C, Alonso-Coello P, Garcia-Alamino JM, Perera R, Meats E, Glasziou P. Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet 2006;367(9508):404-11. [DOI] [PubMed] [Google Scholar]


