Abstract
Background
Asthma is an illness that commonly affects adults and children, and it serves as a common reason for children to attend emergency departments. An asthma exacerbation is characterised by acute or subacute worsening of shortness of breath, cough, wheezing, and chest tightness and may be triggered by viral respiratory infection, poor compliance with usual medication, a change in the weather, or exposure to allergens or irritants.
Most children with asthma have mild or moderate exacerbations and respond well to first‐line therapy (inhaled short‐acting beta‐agonists and systemic corticosteroids). However, the best treatment for the small proportion of seriously ill children who do not respond to first‐line therapy is not well understood. Currently, a large number of treatment options are available and there is wide variation in management.
Objectives
Main objective
‐ To summarise Cochrane Reviews with or without meta‐analyses of randomised controlled trials on the efficacy and safety of second‐line treatment for children with acute exacerbations of asthma (i.e. after first‐line treatments, titrated oxygen delivery, and administration of intermittent inhaled short‐acting beta2‐agonists and oral corticosteroids have been tried and have failed)
Secondary objectives
‐ To identify gaps in the current evidence base that will inform recommendations for future research and subsequent Cochrane Reviews
‐ To categorise information on reported outcome measures used in trials of escalation of treatment for acute exacerbations of asthma in children, and to make recommendations for development and reporting of standard outcomes in future trials and reviews
‐ To identify relevant randomised controlled trials that have been published since the date of publication of each included review
Methods
We included Cochrane Reviews assessing interventions for children with acute exacerbations of asthma. We searched the Cochrane Database of Systematic Reviews. The search is current to 28 December 2019. We also identified trials that were potentially eligible for, but were not currently included in, published reviews. We assessed the quality of included reviews using the ROBIS criteria (tool used to assess risk of bias in systematic reviews). We presented an evidence synthesis of data from reviews alongside an evidence map of clinical trials. Primary outcomes were length of stay, hospital admission, intensive care unit admission, and adverse effects. We summarised all findings in the text and reported data for each outcome in 'Additional tables'.
Main results
We identified 17 potentially eligible Cochrane Reviews but extracted data from, and rated the quality of, 13 reviews that reported results for children alone. We excluded four reviews as one did not include any randomised controlled trials (RCTs), one did not provide subgroup data for children, and the last two had been updated and replaced by subsequent reviews.
The 13 reviews included 67 trials; the number of trials in each review ranged from a single trial up to 27 trials. The vast majority of comparisons included between one and three trials, involving fewer than 100 participants. The total number of participants included in reviews ranged from 40 to 2630. All studies included children; 16 (24%) included children younger than two years of age. Most of the reviews reported search dates older than four years.
We have summarised the published evidence as outlined in Cochrane Reviews. Key findings, in terms of our primary outcomes, are that (1) intravenous magnesium sulfate was the only intervention shown to reduce hospital length of stay (high‐certainty evidence); (2) no evidence suggested that any intervention reduced the risk of intensive care admission (low‐ to very low‐certainty evidence); (3) the risk of hospital admission was reduced by the addition of inhaled anticholinergic agents to inhaled beta2‐agonists (moderate‐certainty evidence), the use of intravenous magnesium sulfate (high‐certainty evidence), and the use of inhaled heliox (low‐certainty evidence); (4) the addition of inhaled magnesium sulfate to usual bronchodilator therapy appears to reduce serious adverse events during hospital admission (moderate‐certainty evidence); (5) aminophylline increased vomiting compared to placebo (moderate‐certainty evidence) and increased nausea and nausea/vomiting compared to intravenous beta2‐agonists (low‐certainty evidence); and (6) the addition of anticholinergic therapy to short‐acting beta2‐agonists appeared to reduce the risk of nausea (high‐certainty evidence) and tremor (moderate‐certainty evidence) but not vomiting (low‐certainty evidence).
We considered 4 of the 13 reviews to be at high risk of bias based on the ROBIS framework. In all cases, this was due to concerns regarding identification and selection of studies. The certainty of evidence varied widely (by review and also by outcome) and ranged from very low to high.
Authors' conclusions
This overview provides the most up‐to‐date evidence on interventions for escalation of therapy for acute exacerbations of asthma in children from Cochrane Reviews of randomised controlled trials. A vast majority of comparisons involved between one and three trials and fewer than 100 participants, making it difficult to assess the balance between benefits and potential harms. Due to the lack of comparative studies between various treatment options, we are unable to make firm practice recommendations. Intravenous magnesium sulfate appears to reduce both hospital length of stay and the risk of hospital admission. Hospital admission is also reduced with the addition of inhaled anticholinergic agents to inhaled beta2‐agonists. However, further research is required to determine which patients are most likely to benefit from these therapies.
Due to the relatively rare incidence of acute severe paediatric asthma, multi‐centre research will be required to generate high‐quality evidence. A number of existing Cochrane Reviews should be updated, and we recommend that a new review be conducted on the use of high‐flow nasal oxygen therapy. Important priorities include development of an internationally agreed core outcome set for future trials in acute severe asthma exacerbations and determination of clinically important differences in these outcomes, which can then inform adequately powered future trials.
Plain language summary
Interventions for acute severe asthma attacks in children: an overview of Cochrane Reviews
Background
Asthma is a common childhood illness that is caused by narrowing of the small air passages in the lungs. This narrowing is due to swelling and inflammation and to muscles around the air passages becoming tighter. An acute asthma attack results in shortness of breath, cough, wheeze, and chest tightness.
When children have an asthma attack, the standard treatment is to give steroids to reduce inflammation and swelling (usually given by mouth) and inhaled medications to relax the muscles in the air passages (called "bronchodilators"). In this review, we call that standard treatment "first‐line" treatment. These medications are well understood to be the best treatments for use in the first instance.
Some children's asthma attacks do not improve with first‐line treatment, and more treatment is necessary ‐ usually at the emergency department or hospital; in this review, we call this 'second‐line' treatment. However, the best second‐line treatment for children who do not respond to first‐line treatment is poorly understood. Many treatment options are available, and what is done for children varies from hospital to hospital.
We wanted to look at existing Cochrane Reviews of second‐line treatments for children having asthma attacks. We hoped to be able to bring this information together in a useful document and to be able to present the evidence that would help the practitioner make the best treatment decision for each child having an asthma attack when inhaled bronchodilators and oral steroids have not helped with symptoms.
Review question
What is the effectiveness and safety of treatment options available for children with acute asthma who do not improve with standard first‐line treatment?
Study characteristics
We included 13 Cochrane Systematic Reviews on various treatment options, including inhaled medication, intravenous medications, and other therapies. This overview provides the most up‐to‐date evidence from systematic reviews with meta‐analyses of randomised controlled trials on acute severe asthma in children. This overview is current to December 2019.
Quality of the evidence
The quality of these reviews was assessed using a checklist, which helped us assess the risk of bias. Nine of the 13 reviews were considered to be high quality. Four reviews were considered at risk of bias due to concerns with the way studies were identified for inclusion in the reviews. Most of the reviews are out‐of‐date because they have not been updated since 2016. The quality of evidence for specific comparisons ranged from very low to high, with many results coming from small studies. It is difficult to be confident in making recommendations for clinical practice.
Key results
For children with acute severe asthma requiring additional treatment, we found that:
‐ intravenous magnesium sulfate (a bronchodilator given through a vein) appears to reduce the length of time spent in hospital;
‐ no evidence suggests that any treatment reduced the risk of being admitted to intensive care;
‐ some treatments appeared to reduce the risk of hospital admission. These included adding a second type of inhaled bronchodilator treatment (anticholinergic medication such as ipratropium bromide) to standard inhaled treatment (beta‐agonist such as salbutamol), giving intravenous magnesium sulfate, and breathing a mixture of helium and oxygen;
‐ serious adverse events may be reduced by inhaled magnesium sulfate;
‐ nausea and/or vomiting is more common with aminophylline (another bronchodilator medication given through a vein); and
‐ adding a second type of inhaled bronchodilator treatment (anticholinergic medication such as Ipratropium bromide) reduces the risk of nausea and tremor but not vomiting.
Recommendations for future research
One of the major problems with existing research is that a small number of patients is included in each study, likely because severe acute asthma in children is relatively uncommon. To work out whether or not a treatment is effective and/or to tell the difference between treatments, a research study must include enough patients receiving each treatment. Therefore, high‐quality research into severe acute asthma in children is likely to require studies that include a number of hospitals.
It is also important to be able to compare results across studies. To do this, researchers across the world should agree on a standard way of measuring results in studies of acute severe asthma in children.
Background
Description of the condition
Asthma is defined as "a chronic inflammatory disorder associated with variable airflow obstruction and bronchial hyper‐responsiveness" (Papadopoulos 2012). Clinical features include recurrent episodes of cough, shortness of breath, wheeze, and chest tightness (Papadopoulos 2012), which may be triggered by viral respiratory infection, poor compliance with usual medication, exercise, a change in the weather, or exposure to allergens or irritants (GINA 2019).
Airflow obstruction results primarily from episodic bronchoconstriction due to contraction of airway smooth muscle. However, other mechanisms also contribute, including mucosal oedema, inflammation, mucus hyper‐secretion, airway hyper‐responsiveness, and airway remodelling (NHLBI 2007).
The diagnosis and management of asthma are complicated in younger children, particularly those from birth to five years (Cave 2014). In this age group, viral‐induced wheezing is very common and has clinical features overlapping those of asthma but does not necessarily have the same longer‐term implications (Martinez 1995; Caudri 2009; Konstantinou 2013).
Care of a child with asthma requires long‐term management aimed at preventing recurrent exacerbations, as well as acute management of symptomatic exacerbations. Treatment for asthma addresses the underlying pathophysiological mechanisms of inflammation and bronchoconstriction.
An asthma exacerbation is defined as an "acute or subacute episode of progressively worsening shortness of breath, cough, wheezing, and chest tightness ‐ or some combination of these symptoms" (NHLBI 2007). First‐line therapy for management of acute exacerbations of asthma is well established and requires titrated oxygen delivery and administration of intermittent inhaled short‐acting beta2‐agonists (SABAs) and oral corticosteroids (OCSs) (NHLBI 2007; GINA 2019; National Asthma Council Australia 2019).
Description of the interventions
Most children with asthma have mild or moderate exacerbations and respond well to first‐line therapy (Powell 2003; Kelly 2004; Giordano 2012; O'Connor 2014). A minority of children with severe exacerbations are unresponsive to first‐line therapy and require escalation of (i.e. second‐line) treatment (O'Connor 2014; Biagini Myers 2015; Morris 2015). Many options are available for second‐line treatment, and available regimens show considerable variability amongst healthcare providers (Babl 2008; Lyttle 2015).
Second‐line treatment can be grouped into the following broad categories.
Additional inhaled bronchodilators, including continuous inhaled beta2‐agonists, anticholinergic medications such as ipratropium, and nebulised magnesium sulfate.
Parenteral bronchodilators, including selective beta2‐agonists such as salbutamol or terbutaline; adrenaline (epinephrine), an agonist at both α‐ and β‐receptors; magnesium sulfate; methylxanthines such as theophylline or aminophylline; and ketamine. Subcutaneous, intramuscular, and intravenous routes may be utilised, and intravenous treatment may be delivered as a single loading dose or as a continuous infusion.
Interventions to reduce the work of breathing, including inhalation of heliox (a mixture of helium and oxygen), administration of high‐flow humidified nasal oxygen therapy, or provision of non‐invasive ventilation with the use of continuous positive airway pressure (CPAP) or bi‐level positive airway pressure (BiPAP).
How the intervention might work
Bronchodilators
Relief of bronchoconstriction, a major therapeutic target in an acute exacerbation of asthma, is achieved by several pharmacological agents acting by various mechanisms. Inhaled short‐acting beta2‐agonists (SABAs), such as salbutamol and terbutaline, are effective, provide rapid onset of action, and are accepted as first‐line therapy for acute asthma exacerbations (Vezina 2014). In young children, administration using a spacer or a holding chamber is preferred over delivery via nebuliser (Ferguson 2006). In patients with severe exacerbations unresponsive to first‐line administration of an intermittent inhaled SABA, healthcare providers may wish to administer continuous inhaled SABA (often by nebuliser) to saturate all available respiratory tract beta2‐receptors and achieve maximum bronchodilation from this pathway (Kenyon 2014). If a patient requires oxygen, this can be provided via nasal prongs and SABA administered by spacer, or, alternatively, oxygen can be used to nebulise SABA.
Inhaled anticholinergic agents such as ipratropium bromide are thought to cause bronchodilation by relieving cholinergic bronchoconstriction and reducing mucosal oedema and airway secretions (Vezina 2014). Although not as effective as beta2‐agonists, it has been suggested that combining these medications may lead to greater bronchodilation than using either agent alone (Griffiths 2013).
Magnesium sulfate is an effective bronchodilator and is administered by nebuliser or by the intravenous route. The mode of action of magnesium sulfate is thought to be related to direct smooth muscle relaxation; however, additional mechanisms may be related to blocking calcium ion influx into smooth muscle cells, thus modulating mast cell histamine release, anti‐inflammatory properties, and cholinergic neural transmission (Powell 2012). Some evidence suggests that simultaneous administration of magnesium sulfate and a beta2‐agonist has an additive bronchodilator effect, perhaps owing to magnesium sulfate augmenting the beta‐receptor agonist response (Neame 2015).
In the setting of severe acute asthma, it has been suggested that inhaled beta2‐agonists may not reach their site of action through the airway owing to significant airflow obstruction, and that systemic (subcutaneous or intravenous) administration of bronchodilators may lead to a more rapid therapeutic response (Travers 2012a).
Adrenaline (epinephrine) is a potent beta‐agonist with bronchodilating effects similar to the more selective beta2‐agonists. Historically, parenteral adrenaline was a standard therapy for acute asthma (Rees 1967; Shim 1984); however, similar clinical efficacy and the less invasive nature of inhaled bronchodilators as reported by Naspitz 1987 have led clinicians to reserve this treatment as an option for severely ill patients who are unresponsive to inhaled therapy (Hon 2017).
Methylxanthines, such as theophylline and aminophylline, are used to treat patients with asthma. Bronchodilator effects may be due to inhibition of phosphodiesterase, leading to accumulation of cyclic adenosine monophosphate (cAMP) in smooth muscle cells, adenosine antagonism, and release of catecholamines (Neame 2015). Other actions are thought to include anti‐inflammatory and immunomodulatory effects (Neame 2015).
Ketamine is commonly used in the emergency department (ED) for procedural sedation, analgesia, and intubation, and it has many effects, including dissociative anaesthesia, analgesia, amnesia, and anxiolysis. It can also induce bronchodilation, possibly as a sympathomimetic effect or as a direct effect on bronchial smooth muscle (Jat 2012). Other potential effects include immunomodulation and inhibition of vagal outflow (Goyal 2013).
Interventions to reduce the work of breathing
Room air comprises nitrogen (79%) and oxygen (21%). Heliox (helium‐oxygen mixture) is produced when helium replaces nitrogen, leading to a less dense gas mixture. Theoretically, this may reduce turbulent airflow and airflow obstruction in patients with asthma. Heliox has also been used to deliver nebulised therapy, as it has been suggested that it may lead to improved transport of medication to the distal airways (Rehder 2017).
Non‐invasive respiratory support can be delivered via high‐flow nasal cannulae (HFNC), continuous positive airway pressure (CPAP), or bi‐level positive airway pressure (BiPAP). Patients with severe asthma often develop elevated intrinsic positive end‐expiratory pressure (PEEP). It is theorised that delivery of extrinsic positive pressure via face mask or nasal cannulae may overcome this intrinsic pressure, thereby reducing the work of breathing.
HFNC provide warmed, humidified gas delivered via nasal prongs at a flow rate that exceeds the patient's peak inspiratory flow rate. This results in washout of anatomic dead space and also provides some PEEP, although the PEEP delivered is less consistent than that provided by CPAP or BiPAP (Rehder 2017). High‐flow delivery is more comfortable and therefore is better tolerated with less requirement for sedation than other methods of non‐invasive respiratory support (Baudin 2017).
CPAP provides constant pressure throughout the respiratory cycle, and BiPAP provides variable pressure according to phases of the respiratory cycle, with higher pressure delivered during inspiration. Positive effects of CPAP and BiPAP include a direct bronchodilating effect, improved alveolar recruitment, improved airflow, re‐expansion of areas of collapse, reduced hyperinflation, and reduced work of breathing (Korang 2016).
Why it is important to do this overview
Clinical rationale
Asthma is a common reason for paediatric visits to the ED (Alpern 2006; Acworth 2009); it is one of the most common reasons for a child to be admitted to hospital after an ED visit (Weiss 2011). In the USA, the rate of paediatric ED visits for asthma increased by 13.3% between 2001 and 2010 (Nath 2015), and in the UK, it is estimated that a child is admitted to hospital every 20 minutes owing to an asthma attack (Asthma UK).
The care of children with asthma is based upon escalation of treatment in response to disease severity: mild disease receives less intensive treatment than severe disease. Broadly speaking, interventions take the form of inhaled bronchodilators, parenteral (intravenous or subcutaneous) pharmacotherapy, and mechanical efforts to reduce the work of breathing. With increasing 'level of treatment' come risks of increasing costs, patient discomfort, potential for complications, and requirement for monitoring and/or transfer to intensive care units. Some treatments ‐ particularly intravenous bronchodilators or assisted ventilation ‐ are given in higher‐acuity settings such as intensive care, and other treatments may be given in a standard ward environment.
Variation in the management of acute severe asthma in children is considerable and may be due to considerations around efficacy, safety, cost, clinical experience, and individual practitioner preference. A recent survey of emergency physicians in the UK and Ireland found that over half preferred salbutamol as first‐line intravenous treatment, 28% preferred magnesium sulfate, and 15% preferred aminophylline (Lyttle 2015). An earlier survey of paediatric emergency specialists in Australia and New Zealand found that aminophylline was used by 45%, intravenous magnesium sulfate by 55%, and intravenous salbutamol by 87% of respondents (Babl 2008). A recent prospective study of 24 EDs in the UK and Ireland found wide variation in the prevalence of intravenous treatment for acute paediatric asthma, ranging from 0% to 19.4% (Morris 2015).
With a large number of treatment options and wide variation in self‐reported and actual practice, it is important to have a single comprehensive and user‐friendly document that provides the best available evidence upon which to base clinical decisions. There is a need to present available evidence clearly to assist healthcare providers, patients, and other knowledge users.
An overview of reviews of acute asthma treatment in children attending EDs was published in 2015 (Castro‐Rodriguez 2015). The review authors excluded reviews of treatment on the ward or in the intensive care unit. As treatment for a seriously ill child often involves transfer to higher levels of care, restricting inclusion to only reviews evaluating treatment within the ED may have led to exclusion of potentially important studies.
The purpose of a Cochrane overview is to systematically summarise evidence from a range of Cochrane intervention reviews for a single health condition (Pollack 2018). This overview will document the efficacy of second‐line interventions from systematic reviews and will provide information about toxicity and adverse effects.
Potential additional benefits of this overview will include a clear foundation upon which further research can be based and an understanding of reported outcome measures, which may be used to assist in development of a set of core outcome measures for future clinical trials.
Methodological rationale
Currently, the Cochrane Airways Group has prepared approximately 50 published reviews on the effectiveness of various interventions for acute asthma. These include 43 reviews on pharmacotherapy and another seven reviews on non‐pharmacotherapy interventions. Given the large number of potentially relevant reviews and the likely heterogeneity in eligibility criteria and study outcomes, we have chosen to utilise an overview design rather than a network meta‐analysis as the first step in assessing the literature.
Objectives
Main objective
To summarise Cochrane Reviews with or without meta‐analyses of randomised controlled trials on the efficacy and safety of second‐line treatment for children with acute exacerbations of asthma (i.e. after first‐line treatments, titrated oxygen delivery, and administration of intermittent inhaled short‐acting beta2‐agonists and oral corticosteroids have been tried and have failed)
Secondary objectives
To identify gaps in the current evidence base that will inform recommendations for future research and subsequent Cochrane Reviews
To categorise information on reported outcome measures used in trials of escalation of treatment for acute exacerbations of asthma in children, and to make recommendations for development and reporting of standard outcomes in future trials and reviews
To identify relevant randomised controlled trials that have been published since the date of publication of each included review
Methods
Criteria for considering reviews for inclusion
Types of reviews
We included Cochrane systematic reviews on second‐line treatment of patients with acute asthma published in the Cochrane Database of Systematic Reviews (CDSR). We included Cochrane Reviews of randomised controlled trials (RCTs) and non‐randomised controlled clinical trials (CCTs).
Types of participants
We included systematic reviews of children with a physician‐diagnosed acute exacerbation of asthma. We defined a child as any person younger than 18 years of age. However, as the definition of 'child' could vary between systematic reviews, we planned to include any systematic review in which a population was described as children, and we recorded the ages included within each review. We also included systematic reviews of adults and children in which the summary data for children could be separated from the summary data for adults.
Types of interventions/comparisons
We included all treatments that may be considered second‐line therapy for acute exacerbations of asthma. We did not include Cochrane systematic reviews examining interventions including only corticosteroids or intermittent inhaled beta2‐agonists.
We planned to divide treatments into the following categories, consistent with steps in the escalation of therapy (inhaled treatment, parenteral treatment, and other interventions to reduce the work of breathing).
-
Inhaled bronchodilators.
Continuous nebulised inhaled beta2‐agonists.
Anticholinergic medications.
Magnesium sulfate.
-
Parenteral bronchodilators.
beta2‐agonists.
Adrenaline/epinephrine.
Magnesium sulfate.
Methylxanthines.
Ketamine.
-
Interventions to reduce the work of breathing.
Heliox.
High‐flow nasal cannulae.
Non‐invasive ventilation (CPAP or BiPAP).
Types of comparisons
We included systematic reviews with all possible comparisons, that is, versus placebo and/or versus another active comparator (ongoing first‐line treatment or an alternative intervention).
Types of outcome measures
Primary outcomes
Length of stay (duration of ED stay and duration of inpatient stay)
ED disposition (hospital admission/intensive care unit (ICU) admission/ED discharge)
Number of adverse events in each treatment group
Secondary outcomes
Symptom scores/clinical asthma scores (such as the Pulmonary Index (Becker 1984), the Clinical Asthma Score (Parkin 1996), the Pediatric Respiratory Assessment Measure (Ducharme 2008), and any other scores identified in the included systematic reviews)
Lung function (peak expiratory flow rate (PEFR), forced expiratory volume in one second (FEV1), and other measures identified in included systematic reviews)
Adverse events (vomiting, nausea, tremor, tachycardia, convulsions, and any other adverse events identified in included systematic reviews)
Vital signs (pulse, blood pressure, respiratory rate, and pulse oximetry)
Requirement for additional bronchodilator treatment
Requirement for respiratory support (intubation, non‐invasive ventilation)
Economic outcomes such as healthcare costs
We planned to report on the primary and secondary outcomes outlined above. However, we tabulated all outcomes identified in the overview to present a taxonomy of outcomes for future reviews on this topic.
Search methods for identification of reviews
We searched for systematic reviews in the Cochrane Library using the filter for reviews. We also searched for but did not identify any Cochrane Review protocols or titles for future inclusion. We used the search terms "asthma" and "respiratory sounds" (which included the medical subject heading (MeSH) term for "wheeze"). Our search strategy is detailed in Appendix 1. The search is current to 28 December 2019.
We included only the most recently published version of each systematic review. We did not include protocols and earlier versions of a review that have been superseded. We did not include systematic reviews from outside the Cochrane Library. If multiple reviews addressed the same question, we planned to examine them for unique content, and if none was found, we planned to include the most up‐to‐date review. If multiple systematic reviews addressed the same question and unique content was found in each, we planned to include them all and extract the unique data for each one.
To identify possibly relevant research papers that have been published since the date of publication for each included systematic review, we utilised the search strategy of each included systematic review. We supplemented this by cross‐checking this against current British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) guidelines (BTS/SIGN 2019), as well as the Global Initiative for Asthma (GINA) guidelines (GINA 2019), but we identified no additional reviews.
Data collection and analysis
Selection of reviews
We assessed in two stages the eligibility of identified Cochrane Reviews. Two independent overview authors (SC and AG) screened each title and abstract. We planned to use a third overview author to resolve discrepancies when the two first authors could not reach consensus; however, no discrepancies occurred. Two independent overview authors (SC and AG) assessed in full text all titles/abstracts and then selected reviews by consensus. Again, no discrepancies occurred between the two overview authors.
Data extraction and management
A pilot data extraction form was designed and piloted by several authors (SC, CL, AG). Two overview authors (SC and one other of SRD, CVP, or AG) independently extracted data using the finalised data extraction form. We planned to involve an independent third overview author to resolve disagreements; however, there were none requiring their involvement.
Data extracted (see Appendix 2) include the following.
Details of the included systematic reviews, including first author name, year of publication, number of included primary studies, eligibility criteria of included systematic reviews, numbers of included participants, and sample size of included RCTs.
Details of trial populations, including age and severity of asthma (including inclusion criteria and definition of exacerbation of asthma for each review, treatment before enrolment, and severity of asthma at enrolment).
Setting (ED, hospital ward, ICU).
Types of interventions.
Dose, duration, and frequency of intervention administration.
Description of the comparison (placebo, regular doses of bronchodilators).
Description of outcome measures used, including our predefined primary and secondary outcomes and all other reported outcomes.
Timing of determination of outcome measures and duration of follow‐up.
Risk of bias assessments of RCTs included in the reviews.
For each predetermined primary and secondary outcome measure, and for all additional outcomes, numbers of participants in intervention and control groups; control event rate; effect estimates for the pooled risk ratio; odds ratio, hazard ratio, standardised mean difference, or absolute risk reduction and corresponding 95% confidence intervals (if not provided, we will calculate these, using the equations published in the Cochrane Handbook for Systematic Reviews of Interventions (Schüneman 2019)).
Quality assessment tools used (e.g. GRADE), along with the mean or median and the range of any reported quality scores.
If the included systematic reviews included all RCTs relevant to a particular outcome, we extracted summary data alone. We extracted only data from RCTs conducted exclusively among children, or for which authors of the systematic review had been able to retrieve data for children.
If we identified overlapping information across systematic reviews at the data extraction stage, we planned to extract data only from the most recently published review. We planned to acknowledge overlap among different reviews (overlapping trials), depict any potential overlap in tables, and discuss this limitation in the results. We identified no overlapping information.
If we identified discrepant data across systematic reviews, we planned to extract data from all included reviews and reconcile the discrepancies by contacting the authors of included reviews, retrieving primary studies from the included reviews, and searching relevant trial registries. We planned to discuss potential discrepancies in data in the Results section. We identified no discrepant data.
We planned to present the data in a series of summary tables.
Assessment of methodological quality of included reviews
Two overview authors independently assessed the risk of bias of included systematic reviews using the Risk of Bias in Systematic Reviews (ROBIS) tool (Whiting 2016). The ROBIS tool (see Appendix 3) consists of three phases: assessment of relevance of the systematic review to the study question, identification of potential concerns regarding the review process, and a judgement of risk of bias. We planned to report in a table assessment for individual ROBIS items or domains (along with the rationale for judgements for each assessment).
We defined a high‐quality systematic review with meta‐analysis as one that has low risk of bias judgements for the first three domains of the ROBIS tool, namely, specification of study eligibility (domain 1), methods used to identify and/or select studies (domain 2), and methods used to collect data and appraise studies (domain 3) (Whiting 2016). If more than one review on a specific topic was included, we planned to choose the review judged as having low risk of bias in all three ROBIS domains, as well as the review that most closely matched our overview PICO criteria. However, we found no overlapping reviews.
We planned to use the risk of bias assessment to conduct sensitivity analyses, but we did not exclude reviews on the basis of the risk of bias assessment.
We planned to present a summary of this information according to guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Quality of evidence in included reviews
Two overview authors independently evaluated the certainty of evidence on the basis of judgements made by the authors of the original Cochrane Reviews, if provided.
We assessed the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Balshem 2011).
First, we extracted the GRADE assessments for each systematic review for each independent outcome. We planned to first assess whether the domain judgements were consistent; if they were inconsistent, we planned to reconcile the inconsistency by comparing extracted data between reviews for missing or discrepant data, contacting the authors of the primary studies, or searching trial registries, if two or more systematic reviews reported GRADE assessments for the same outcome. We planned to choose the highest‐quality systematic review with meta‐analysis from which to extract effect estimates for our GRADE assessment of inconsistency and imprecision if we continued to note inconsistency in the reported GRADE domains (or none reported).
We planned to independently conduct this assessment by constructing 'Summary of findings' tables using GRADEpro software if the original review published no GRADE assessment (GradePro 2015), or if outcome data in our overview had been re‐analysed from a subset of primary studies within a review.
We based our assessment of the certainty of evidence in included reviews on data provided in the 'Characteristics of included studies', 'Risk of bias', and 'Summary of findings' tables provided in the included reviews, and we planned to present a summary of this information according to guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019)
Dealing with missing data
We planned to address data missing from an included systematic review or variation in information reported across reviews by retrieving and examining the full reports of RCTs included in the systematic reviews; contacting systematic review authors for missing information or clarification; searching systematic review protocols; and/or searching registries of systematic reviews or clinical trials for further information. If this occurred, we planned to include discussion on potential discrepancies with information provided in the original reviews.
Data synthesis
We planned to tabulate PICO (population, intervention, control, and outcome) elements at the review level. Results tables include effect estimates, 95% confidence intervals (CIs), and measures of heterogeneity/risk of bias, as appropriate.
We aimed to group data into the three broad groups described above: inhaled bronchodilators, parenteral bronchodilators, and interventions to reduce the work of breathing. We intended to compare all outcomes between inhaled bronchodilators (e.g. standard therapy/placebo versus continuous nebulised SABA versus inhaled magnesium), between parenteral bronchodilators (e.g. standard therapy/placebo versus aminophylline versus magnesium versus ketamine versus salbutamol versus terbutaline), and between interventions to reduce the work of breathing (standard therapy/placebo versus CPAP versus BiPAP versus heliox vs HFNC). We planned to extract effect estimates from the included systematic reviews, categorised by intervention and primary and secondary outcomes, and to present them in tables and figures.
We planned to structure narrative descriptions of effect estimates of the included reviews according to risk of bias and GRADE assessments.
We also planned to assess the impact of inclusion criteria (severity of asthma), treatment before enrolment (including type of first‐line intervention applied), and control treatment on the effects of interventions.
The choice of effect estimate for summary and tabulation depended on the outcomes reported in various reviews. We intended to standardise the outcomes reported if an outcome was expressed differently between reviews. We standardised to risk ratios (RRs) or odds ratios (ORs) for dichotomous outcomes. We standardised to mean differences (MDs) or standardised mean differences (SMDs) by using equations published in the Cochrane Handbook for Systematic Reviews of Interventions for continuous outcomes (Higgins 2019a).
We planned to discuss the limitations of currently available evidence with regards to heterogeneity of inclusion criteria for each review, consistency of effect size for each intervention, and consistent use of outcome measures. We planned to identify gaps in the current evidence base and to make recommendations for future research.
Assessment of non‐statistical heterogeneity
We planned to determine whether there is clinical heterogeneity between systematic reviews (i.e. differences in severity of asthma or differences in treatment administered before enrolment) by assessing the inclusion criteria of each systematic review. We also planned to assess clinical heterogeneity within each systematic review that will contribute to the certainty of evidence assessment of each review.
We planned to identify commonly used outcomes and to categorise them in a taxonomy by creating a list of all outcomes and discussing their categorisation among the review author group until consensus was reached. This taxonomy will inform recommendations for a core set of outcome measures, which may be applicable in future RCTs.
Subgroup analysis
Given the pathophysiological differences between preschoolers and older children, we intended to subgroup studies of children from birth to five years of age and children aged six to 18 years (or younger and older children as defined by review authors) and to provide separate summary tables within the overview.
Finally, we planned to group studies occurring in the ED/outpatient setting separately from those occurring in the inpatient setting (ward or intensive care unit).
We planned to extract summary event data for each treatment/placebo group from the included reviews for all subgroup analyses.
Sensitivity analysis
We planned to conduct sensitivity analysis based on the ROBIS assessment of systematic reviews by comparing results of all reviews against data derived only from reviews in which the ROBIS tool identified domains with a "high" level of concern (i.e. by excluding studies that have one or more domains in the ROBIS tool rated as causing a "high" level of concern).
Results
Results of the search
The search of the Cochrane Library (issue 3, 2018) identified 293 records for Cochrane reviews. After review of titles and abstracts, 276 records were excluded. We excluded four reviews for the following reasons (Figure 1 and Table 1): one review because it did not include any trials (Jones 2001); one review because it did not have any trials where the results for children were able to be separated from the results for adults (Manser 2001); one because it had been updated and replaced by separate reviews on adults and children (Rowe 2000), and one because it had been replaced by two newer reviews, both of which were also included in this review (Travers 2001).
1.
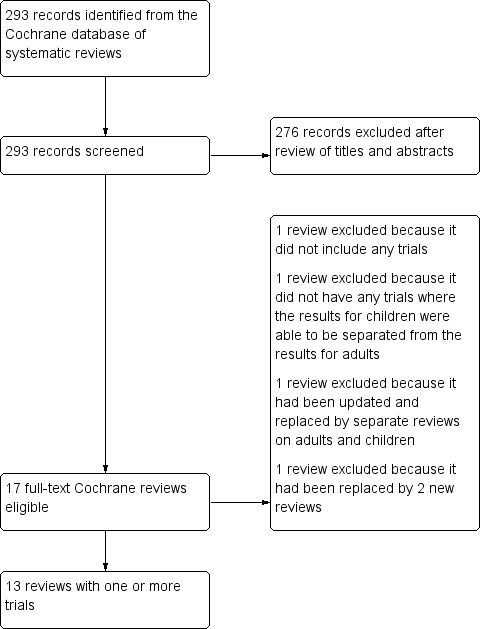
Flow diagram.
1. Excluded reviews.
| Reference | Title | Reason for exclusion |
| Rowe 2000 | Magnesium sulfate for treating exacerbations of acute asthma in the emergency department | Replaced by separate reviews in adults and children, Griffiths 2016 contains all relevant paediatric trials |
| Travers 2001 | Intravenous beta2‐agonists for acute asthma in the emergency department | Replaced by 2 new reviews: "Intravenous beta2‐agonists versus intravenous aminophylline for acute asthma" (Travers 2012b), and "Addition of intravenous beta2‐agonists for acute asthma" (Travers 2012a) |
| Manser 2001 | Corticosteroids for acute severe asthma in hospitalised patients | No studies where the results for children could be separated from the results for adults |
| Jones 2001 | Inhaled beta2‐agonists for asthma in mechanically ventilated patients | No trials were included in the review |
We included and extracted data from 13 reviews (Table 2). No ongoing reviews were identified. An updated search of the library on 28 December 2019 did not identify any new or updated reviews.
2. Review characteristics.
| Review ID | Date of last search | Total number of studies (total number of participants) | Number of studies involving children (number of children in review) | Population | Interventions | Comparison interventions | Outcomes for which data were reported | Review limitations |
| Inhaled treatment | ||||||||
|
Camargo 2003 Continuous vs intermittent beta‐agonists for acute asthma |
9 March 2011 | 8 (644) | 1 (70) | Participants presenting to an ED (or its equivalent) with acute asthma | Continuous (defined as 1 nebulisation every 15 minutes or > 4 nebulisations per hour) inhaled beta‐agonist therapy | Intermittent inhaled beta‐agonist therapy | ‐ PEFR ‐ Admission to hospital ‐ ED treatment time ‐ Respiratory therapist time ‐ Symptom scores ‐ Tremor ‐ Nausea/vomiting |
Limited paediatric data |
|
Griffiths 2013 Combined inhaled anticholinergics and short‐acting beta‐agonists for initial treatment of acute asthma in children |
18 April 2012 | 20 (2632) | 20 (2632) | Children aged 18 months to 18 years presenting to an ED with an acute exacerbation of asthma | Single or repeated doses of nebulised or inhaled short‐acting anticholinergics plus SABAs | Single or repeated doses of nebulised or inhaled placebo plus SABAs | ‐ Hospital admission ‐ FEV1 ‐ PEFR ‐ Respiratory resistance ‐ Clinical asthma score ‐ Need for repeat bronchodilator treatment ‐ Oxygen saturation measurements ‐ Need for corticosteroids in the ED prior to disposition ‐ Tremor ‐ Vomiting ‐ Nausea ‐ Relapse |
|
|
Vezina 2014 Inhaled anticholinergic and short‐acting beta2‐agonists vs short‐acting beta2‐agonists alone for children with acute asthma in hospital |
November 2013 | 4 (472) | 4 (472) | Children 1 to 18 years of age who were hospitalised for an acute asthma exacerbation | Nebulised or inhaled anticholinergics with SABA | Nebulised or inhaled SABA | ‐ Duration of hospital stay ‐ Admission to ICU ‐ Need for supplemental asthma therapy ‐ Time to SABA spaced at 4 hours or longer ‐ Asthma clinical scores ‐ Relapse within 72 hours of discharge from hospital ‐ Predicted PEFR ‐ Adverse health effects ‐ Overall withdrawals ‐ Withdrawals due to deterioration |
|
|
Knightly 2017 Inhaled magnesium sulfate in the treatment of acute asthma |
6 September 2017 | 25 (3301) | 8 (1247) | Patients with acute asthma | ‐ Inhaled MgSO4 ‐ Inhaled MgSO4 and inhaled beta2‐agonist ‐ Inhaled MgSO4 and inhaled beta2‐agonist and ipratropium |
‐ Inhaled beta2‐agonist ‐ Inhaled beta2‐agonist and placebo ‐ Inhaled beta2‐agonist and ipratropium and placebo |
‐ Clinical severity scores ‐ Admission at first presentation ‐ Admission to PICU/HDU or intubation ‐ Serious adverse events ‐ Any adverse event (during admission) ‐ Hypotension ‐ Flushing ‐ Pulmonary function (% predicted FEV1) ‐ PEF ‐ Admission to hospital |
|
| Parenteral treatment | ||||||||
|
Mitra 2005 Intravenous aminophylline for acute severe asthma in children over 2 years of age receiving inhaled bronchodilators |
February 2007 | 7 (380) | 7 (380) | Children aged between 2 and 17 years with acute severe asthma or status asthmaticus (acute, severe, refractory exacerbations) attending EDs, or in hospital wards or ICUs | Loading dose of IV aminophylline followed by maintenance infusion, intravenous aminophylline boluses, or oral theophylline | Placebo | ‐ FEV1 ‐ Peak flow ‐ Oxygenation (SaO2 or PaO2) ‐ Supplemental oxygen reduction ‐ Change in symptom scores ‐ Number of nebulisers required in 24 hours ‐ ICU admission rates ‐ Rates of patients mechanically ventilated in ICU ‐ Length of hospital stay ‐ Vomiting ‐ Headache ‐ Tremor ‐ Seizures ‐ Hypokalaemia ‐ Arrhythmias ‐ Blood pressure ‐ Magnitude of diuresis ‐ Death ‐ Withdrawal due to adverse health effects ‐ Withdrawal due to poor asthma control ‐ Withdrawal (any cause) |
|
|
Travers 2012a Addition of intravenous beta2‐agonists to inhaled beta2‐agonists for acute asthma |
September 2012 | 3 (102) | 2 (73) | Adult or paediatric patients with severe acute asthma presenting to an emergency department (or its equivalent) | IV selective or non‐selective beta1‐ and beta2‐agonists in addition to inhaled beta2‐agonists and existing standards of care | Inhaled beta2‐agonists and standard care alone | ‐ Length of stay ‐ Pulse rate ‐ Clinical failure |
Limited paediatric data |
|
Travers 2012b Intravenous beta2‐agonists vs intravenous aminophylline for acute asthma |
September 2012 | 11 (371) | 4 (168) | Adults and children with severe acute asthma presenting to an ED (or its equivalent) and patients admitted to hospital with acute severe asthma | IV beta2‐agonists and standard care | Intravenous aminophylline and standard asthma care | ‐ Length of stay ‐ Clinical failure ‐ CPK elevation ‐ CPK‐MB elevation ‐ Dysrhythmia ‐ Headache ‐ Hyperglycaemia ‐ Hypokalaemia ‐ Palpitations ‐ Tremor ‐ Nausea/vomiting ‐ Nausea ‐ Vomiting |
Limited paediatric data |
|
Griffiths 2016 Intravenous magnesium sulfate for treating children with acute asthma in the emergency department |
23 February 2016 | 5 (182) | 5 (182) | Children (18 months to 18 years) treated in the ED for acute asthma (all severities) | Any dose of IV MgSO4 | Placebo | ‐ Hospital admissions ‐ ED treatment time ‐ Return to ED within 48 hours ‐ Hospital length of stay |
Limited paediatric data, small studies |
|
Jat 2012 Ketamine for management of acute exacerbations of asthma |
4 September 2017 | 1 (68) | 1 (68) | Children (< 18 years) presenting with an acute asthma exacerbation who had not responded to standard therapy with aerosolised beta2‐agonist, with or without aerosolised anticholinergic drugs and oral or parenteral corticosteroid, for at least 1 hour | Ketamine (IM or IV) | Placebo | ‐ Reduction in pulmonary index score ‐ Disposition for enrolled patients after study enrolment ‐ Side effects |
Limited paediatric data |
| Interventions to reduce the work of breathing | ||||||||
|
Rodrigo 2006 Heliox for non‐intubated acute asthma patients |
25 August 2010 | 10 (529) | 3 (82) | Children or adult patients presenting to an ED or equivalent care settings for treatment of acute asthma | Inhaled heliox | Control (oxygen or air) | ‐ Pulmonary function ‐ Heart rate ‐ Oxygen saturations ‐ Dyspnoea or pulmonary index ‐ Hospital admissions |
Limited paediatric data |
|
Korang 2016 Non‐invasive positive‐pressure ventilation for acute asthma in children |
February 2016 | 2 (40) | 2 (40) | Children (aged < 18 years) hospitalised for an asthma attack (as defined by the trialists) | Any type of NPPV (including CPAP and BiPAP) as add‐on therapy to usual care | Usual care | ‐ Mortality ‐ Serious adverse events ‐ Asthma symptom score ‐ Pneumonia ‐ Non‐serious adverse events |
Limited paediatric data |
| Other interventions | ||||||||
|
Normansell 2018 Antibiotics for exacerbations of asthma |
17 October 2017 | 6 (670) | 3 (133) | Children and adults who presented to an ED, primary care, outpatient clinics, or inpatient wards with an asthma exacerbation | Intravenous or oral antibiotics, given at any dose and for any duration of treatment | Placebo or standard care | ‐ Adverse events ‐ Serious adverse events ‐ Length of hospital stay ‐ Peak expiratory flow |
Limited paediatric data |
|
Watts 2012 Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children |
February 2012 | 8 (1940) | 4 (470) | Children and adults with acute asthma presenting for acute medical care to an ED or equivalent setting | LTRA (oral or IV) and standard care (inhaled beta2‐agonists, systemic corticosteroids ± oxygen, ipratropium bromide) | Placebo and standard care (inhaled beta2‐agonists, systemic corticosteroids ± oxygen, ipratropium bromide) | ‐ Hospital admission ‐ Requirement for additional care at end of study ‐ Change in FEV1 ‐ Change in pulmonary index score ‐ Change in respiratory rate ‐ Withdrawals ‐ Relapse (within 7 days) |
|
ED: emergency department; PEFR: peak expiratory flow rate; FEV1: forced expiratory volume in one second; SABA: short‐acting beta‐agonist; ICU: intensive care unit; LTRA: leukotriene receptor antagonist; MgSO4: magnesium sulfate; PICU: paediatric intensive care unit; HDU: high‐dependency unit; PEF: peak expiratory flow; IV: intravenous; SaO2: saturation of oxygen; PaO2: arterial pressure of oxygen; CPK: creatine phosphokinase; CPK‐MB: creatine phosphokinase myocardial band; IM: intramuscular; NPPV: non‐invasive positive‐pressure ventilation; CPAP: continuous positive airway pressure; BiPAP: bilevel positive airway pressure.
We utilised the search strategy (in August 2019) of each included review to update the searches for new randomised controlled trials (RCTs). We identified 1,885 records, and after screening of titles and abstracts, we identified 31 new RCTs which may be eligible for inclusion in updated reviews (Table 3).
3. Evidence map.
| Intervention | Cochrane Review | Number of included studies involving children (number of participants) | Potential new studies based on review search strategy | Overview team recommendations for new Cochrane Reviews, or changes to existing reviews | Overview team suggested research priorities based on evidence presented in this evidence map |
| Inhaled treatment | |||||
| Continuous vs intermittent beta‐agonists | Camargo 2003 | 1 (70) | Rose 2011; Sabato 2011; Wilkinson 2018 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
| Combined inhaled anticholinergics and short‐acting beta‐agonists | Emergency department setting Griffiths 2013 |
20 (2632) | Supriyatno 2012; Sengul Gokalp 2013; Memon 2016 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
| Inpatient setting Vezina 2014 |
4 (472) | Wyatt 2015 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
|
| Inhaled magnesium sulfate | Knightly 2017 | 8 (1247) | Motamed 2017; Mustafa 2017 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
| Parenteral bronchodilators | |||||
| Intravenous aminophylline | Mitra 2005 | 7 (380) | Naao 2007; Chen 2008; D'Avila 2008; Singhi 2014; Tiwari 2016 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
| Addition of intravenous beta2‐agonists to inhaled beta2‐agonists | Travers 2012a | 2 (73) | Aubuchon 2012; Schneider 2012; House 2015 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
| Intravenous beta2‐agonists vs intravenous aminophylline | Travers 2012b | 4 (168) | Singhi 2014 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
| Intravenous magnesium sulfate | Griffiths 2016 | 5 (182) | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
||
| Ketamine | Jat 2012 | 1 (68) | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
||
| Interventions to reduce the work of breathing | |||||
| Heliox | Rodrigo 2006 | 3 (82) | Bigham 2010; Brandão 2011; Ortiz 2012; Morimoto 2018 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
| Non‐invasive positive‐pressure ventilation | Korang 2016 | 2 (40) | Navanandan 2017 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
| High‐flow nasal oxygen therapy | No review | N/A | New review needed | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
|
| Other interventions | |||||
| Antibiotics | Normansell 2018 | 3 (133) | Mandhane 2017 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
| Leukotriene receptor antagonists | Watts 2012 | 4 (470) | Adachi 2012; Matsuse 2012; Zubairi 2013; Kanchanateeraphong 2014; Magazine 2016; Chaudhury 2017; Magazine 2018; Wang 2018 | Update review | Compare effectiveness in preschool and older children Develop and use core outcome measures, consistent assessment of asthma severity at study entry, and consistent inclusion criteria in future trials |
Description of included reviews
A table of the main characteristics of the included reviews is presented in Table 2.
Study design
All 13 reviews included RCTs, with 74 comparisons conducted from 67 individual trials. A majority of trials were of parallel‐group design, and three were cross‐over studies. Each review included from 1 to 27 trials. Thirty‐eight per cent (n = 28) of the comparisons included 40 or fewer participants. The total number of participants included in reviews ranged from 40 in Korang 2016 to 2630 in Griffiths 2013; five reviews included more than 300 participants.
Included participants
Of the 67 RCTs included across the reviews, all included children. Twenty‐four per cent (n = 16) of the studies included children younger than two years of age, and three studies (4%) included children younger than one year of age.
Diagnosis of asthma in included reviews
Table 4 outlines details of asthma diagnosis and asthma severity in the included reviews. Inclusion criteria were varied and comprised acute asthma (six reviews), acute exacerbation of asthma (three reviews), asthma exacerbation (two reviews), and asthma attack (one review). One review did not provide a clear statement on inclusion criteria with regards to how diagnosis of asthma was defined (Camargo 2003). Three studies provided information regarding exclusion of other respiratory illnesses such as bronchiectasis and chronic obstructive pulmonary disease.
4. Details of asthma diagnosis and asthma severity in included reviews.
| Review | Asthma diagnosis | Asthma severity |
| Inhaled treatment | ||
| Camargo 2003 | No information provided | Patients presenting to an ED or its equivalent |
| Griffiths 2013 | Acute exacerbation of asthma | Patients presenting to an ED |
| Vezina 2014 | Acute asthma exacerbation | Hospitalised for an acute asthma exacerbation |
| Knightly 2017 | Acute asthma (excluded chronic or “stable” asthma). “We accepted any reasonable diagnosis of asthma, namely clinical and guideline‐based criteria” | No information provided |
| Parenteral treatment | ||
| Mitra 2005 | Acute severe asthma or status asthmaticus | No definition provided for severe Status asthmaticus defined as “acute, severe, refractory exacerbations” Patients were attending ED, hospital wards, or intensive care |
| Travers 2012a | Severe acute asthma | No definition provided for “severe”. Included patients presenting to an ED (or its equivalent) |
| Travers 2012b | Severe acute asthma | No definition provided for “severe”. Included patients presenting to an ED or admitted to hospital |
| Griffiths 2016 | Acute asthma | Patients treated in the ED with acute asthma (all severities) |
| Jat 2012 | Acute asthma exacerbation | Not responded to standard therapy with aerosolised beta2‐agonist, with or without aerosolised anticholinergic drugs and oral or parenteral corticosteroid, for at least 1 hour |
| Other interventions to reduce the work of breathing | ||
| Rodrigo 2006 | Clinical diagnosis asthma exacerbation (according to accepted criteria such as those published by the American Thoracic Society). Excluded patients with COPD | Presenting to an ED or equivalent care setting. Excluded patients requiring mechanical ventilation at presentation |
| Korang 2016 | Asthma attack (as defined by the trialists). Excluded children with pneumonia, aspiration, bronchiolitis, cystic fibrosis, or any ciliary dyskinetic syndrome | Children hospitalised for an asthma attack |
| Other interventions | ||
| Normansell 2018 | Asthma exacerbation. Excluded pneumonia, COPD, and bronchiectasis | Presented to the ED, primary care, outpatient clinics, or inpatient wards. Included both inpatients and outpatients |
| Watts 2012 | Acute asthma | Presenting for acute medical care to an ED or equivalent setting |
COPD: chronic obstructive pulmonary disease; ED: emergency department.
Severity of asthma in included reviews
Of the 13 reviews, three referred to "severe" asthma exacerbations, but none provided information regarding how this was defined. One review described treatment duration and medication use prior to inclusion (Jat 2012). Another review excluded patients who required mechanical ventilation at presentation (Rodrigo 2006).
Interventions in included reviews
We mapped the Cochrane Reviews onto the framework of interventions specified in our protocol in Table 2, covering three broad classes of therapy: inhaled treatment, parenteral treatment, and other interventions to reduce the work of breathing (Craig 2018). Two reviews were not able to be classified within our prespecified subgroups.
Inhaled treatment
We identified four reviews on inhaled treatment.
One compared continuous nebulised versus intermittent nebulised beta‐agonists (Camargo 2003).
Two compared inhaled beta2‐agonists versus a combination of inhaled beta2‐agonists and inhaled anticholinergic agents. One of these was restricted to children hospitalised with asthma (Vezina 2014), and the other examined initial treatment in the ED (Griffiths 2013).
One assessed the use of inhaled magnesium sulfate (Knightly 2017).
Parenteral treatment
We identified five reviews on parenteral treatment, of which:
one assessed the use of intravenous aminophylline for children older than two years of age who were administered inhaled bronchodilators (Mitra 2005);
one assessed the addition of intravenous beta2‐agonists to inhaled beta2‐agonists (Travers 2012a);
one compared the use of intravenous beta2‐agonists to intravenous aminophylline (Travers 2012b);
one assessed the use of intravenous magnesium (Griffiths 2016); and
one assessed the use of intramuscular or intravenous ketamine (Jat 2012).
Interventions to reduce the work of breathing
We identified two reviews on interventions to reduce the work of breathing, of which:
one assessed the use of heliox for non‐intubated asthma patients (Rodrigo 2006); and
one assessed the use of non‐invasive positive‐pressure ventilation (Korang 2016).
Other interventions
We identified two additional reviews that did not fit into our prespecified classifications.
One compared the use of antibiotics (oral or parenteral) to no antibiotics for asthma (Normansell 2018).
One assessed the use of leukotriene receptor antagonists in addition to usual care for acute asthma (Watts 2012).
Methodological quality of included reviews
Risk of bias assessment of systematic reviews
We assessed the risk of bias of included reviews by using the ROBIS tool (Table 4; ROBIS assessment available in Appendix 1). All of the included reviews were at low risk of bias with regard to study eligibility criteria. Four reviews had high risk of bias with regard to identification and selection of studies. In all cases, this was due to a single author selecting studies (Mitra 2005; Griffiths 2013; Vezina 2014; Knightly 2017). One review had an unclear risk of bias for data collection and study appraisal, as no information was provided regarding who extracted the data or whether a data collection form was used (Mitra 2005). All reviews were rated as having low risk of bias regarding synthesis and findings.
Overall, we considered four of the thirteen included reviews (31%) to be at high risk of bias. In all cases, this was due to concerns regarding identification and selection of studies.
Only five of the thirteen reviews (38%) had conducted a literature search later than 1 January 2016.
Risk of bias assessment in included RCTs
The reviews used various tools to assess risk of bias of included RCTs (Table 5). Three reviews assessed the quality of trials using the Jadad Scale (an old quality scale with a maximum score of 5 points, in which a higher score suggests lower risk of bias) (Jadad 1996). Of these, one review included a single study with a score of 2/5 (Camargo 2003); another review included three RCTs, with two trials scored 3/5 and one 4/5 (Rodrigo 2006); and one review included seven RCTs, with three scored as 4/5 and four as 5/5 (Mitra 2005).
5. ROBIS assessment of included reviews.
| Review | Domain 1: study eligibility criteria | Domain 2: identification and selection of studies | Domain 3: data collection and study appraisal | Domain 4: synthesis and findings | Risk of bias in the review |
| Inhaled treatment | |||||
| Camargo 2003 | Low concern | Low concern | Low concern | Low concern | Low risk |
| Griffiths 2013 | Low concern | High concern | Low concern | Low concern | High risk |
| Vezina 2014 | Low concern | High concern | Low concern | Low concern | High risk |
| Knightly 2017 | Low concern | High concern | Low concern | Low concern | High risk |
| Parenteral treatment | |||||
| Mitra 2005 | Low concern | High concern | Unclear concern | Low concern | High risk |
| Travers 2012a | Low concern | Low concern | Low concern | Low concern | Low risk |
| Travers 2012b | Low concern | Low concern | Low concern | Low concern | Low risk |
| Griffiths 2016 | Low concern | Low concern | Low concern | Low concern | Low risk |
| Jat 2012 | Low concern | Low concern | Low concern | Low concern | Low risk |
| Other interventions to reduce the work of breathing | |||||
| Rodrigo 2006 | Low concern | Low concern | Low concern | Low concern | Low risk |
| Korang 2016 | Low concern | Low concern | Low concern | Low concern | Low risk |
| Other interventions | |||||
| Normansell 2018 | Low concern | Low concern | Low concern | Low concern | Low risk |
| Watts 2012 | Low concern | Low concern | Low concern | Low concern | Low risk |
Complete details of all components of the ROBIS assessment are presented in Appendix 4.
The other ten reviews used the Cochrane risk of bias tool for methodological quality assessments. Most assessments included a combination of low and unclear risk of bias. The review on antibiotics included three RCTs; each of these studies was rated as having high risk for two of the seven risk of bias domains: one RCT for performance bias and detection bias, and two RCTs for attrition bias and reporting bias (Normansell 2018). Four other reviews included at least one RCT with high risk of bias on at least one item, including selective reporting (2/8 RCTs in Knightly 2017), allocation concealment (2/7 RCTs in Mitra 2005), incomplete outcome data (1/2 RCTs in Korang 2016), and other bias (1/5 RCTs in Griffiths 2016).
Certainty of evidence assessments in included reviews
Eight reviews contained a Grading of Recommendations, Assessment, Development and Evaluation (GRADE) 'Summary of findings' table, which we have summarised in Table 6. One review judged the outcomes reported in the 'Summary of findings' table to be high certainty (Griffiths 2013), another moderate certainty (Vezina 2014), one low certainty (Griffiths 2016), and one very low certainty (Korang 2016). The other four reviews contained a mixture of ratings of certainty, including two reviews with a combination of moderate and low certainty (Travers 2012a; Travers 2012b); one review ranging from very low to moderate certainty (Normansell 2018); and one review ranging from very low to high certainty (Knightly 2017).
6. Certainty of evidence in the included reviews.
| Review | Number of studies where results for children are available and included in this overview | Summary of findings | Methodological quality assessment tool | Risk of bias assessment (from review authors) |
| Inhaled treatment | ||||
| Camargo 2003 | 1 | No | Jadad score | Jadad score: 2/5 Unclear sequence generation; no allocation concealment |
| Griffiths 2013 | 27 | Yes. High‐certainty evidence | Assessed according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions | Random sequence generation: low risk in 17/27 Allocation concealment: low risk in 21/27 Blinding: low risk in 23/27 Incomplete outcome data: low risk in 23/27 Selective reporting: low risk in 20/27 Other bias: low risk in 23/27 |
| Vezina 2014 | 4 | Yes. Moderate‐certainty evidence for all comparisons | Cochrane ‘Risk of bias’ tool | Random sequence generation: low risk in 2/4 Allocation concealment: low risk in 2/4 Blinding of participants and personnel: Low risk in 4/4 Blinding of outcome assessment: low risk in 4/4 Incomplete outcome data: low risk in 4/4 Selective reporting: low risk in 2/4 Other bias: low risk in 4/4 |
| Knightly 2017 | 8 | Yes. Certainty ranges from very low to low to moderate to high | Cochrane ‘Risk of bias’ tool | Random sequence generation: low risk in 4/8 Allocation concealment: low risk in 4/8 Blinding of participants and personnel: low risk in 5/8 Blinding of outcome assessment: low risk in 5/8 Incomplete outcome data: low risk in 5/8 Selective reporting: low risk in 5/8; high risk in 2/8 |
| Parenteral treatment | ||||
| Mitra 2005 | 7 | No | Jadad score | Jadad score: 4/5 (3 studies); 5/5 (4 studies) Adequate sequence generation: low risk in 2/7 Allocation concealment: low risk in 3/7; high risk in 2/7 Blinding: low risk in 7/7 |
| Travers 2012a | 2 | Yes. Moderate‐ and low‐certainty evidence | Assessed according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions | Random sequence generation: low risk in 2/2 Allocation concealment: low risk in 2/2 Blinding of participants and personnel: low risk in 2/2 Blinding of outcome assessment: low risk in 2/2 Incomplete outcome data: low risk in 2/2 Selective reporting: unclear risk in 2/2 |
| Travers 2012b | 4 | Yes. Most scores of moderate certainty. Low certainty for heart rate at 60 minutes | Cochrane ‘Risk of bias’ tool | Random sequence generation: low risk in 2/4 Allocation concealment: low risk in 2/4 Blinding of participants and personnel: low risk in 3/4 Blinding of outcome assessment: low risk in 3/4 Incomplete outcome data: unclear risk in 4/4 Selective reporting: unclear risk in 4/4 |
| Griffiths 2016 | 5 | Yes. Low certainty for all outcomes | Cochrane ‘Risk of bias’ tool | Random sequence generation: low risk in 2/5 Allocation concealment: low risk in 2/5 Blinding of participants and personnel: low risk in 5/5 Blinding of outcome assessment: low risk in 4/5 Incomplete outcome data: low risk in 2/5 Selective reporting: low risk in 3/5 Other bias: low risk in 4/5; high risk in 1/5 |
| Jat 2012 | 1 | No. | Cochrane ‘Risk of bias’ tool | Random sequence generation: low risk Allocation concealment: low risk Blinding of participants and personnel: unclear risk Blinding of outcome assessment: low risk Incomplete outcome data: low risk Selective reporting: unclear risk Other bias: unclear risk |
| Other interventions to reduce the work of breathing | ||||
| Rodrigo 2006 | 3 | No. | Jadad score | Jadad score: 4/5 (1 study); 3/5 (2 studies) Allocation concealment: unclear risk in 3/3 |
| Korang 2016 | 2 | Yes. Very low certainty for all outcomes | Cochrane ‘Risk of bias’ tool | Random sequence generation: unclear risk in 2/2 Allocation concealment: unclear risk in 2/2 Blinding of participants and personnel: low risk in 2/2 Blinding of outcome assessment: unclear risk in 2/2 Incomplete outcome data: high risk in 1/21/2; low risk in 1/2 Selective reporting: low risk in 1/2 Other bias: low risk in 2/2 |
| Other interventions | ||||
| Normansell 2018 | 3 | Yes. Moderate, low, and very low certainty. | Assessed according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions | Random sequence generation: low risk in 1/3 Allocation concealment: low risk in 1/3 Blinding of participants and personnel: high risk in 1/3; low risk in 2/3 Blinding of outcome assessment: high risk in 1/3 Incomplete outcome data: high risk in 2/3; low risk in 1/3 Selective reporting: high risk in 2/3 Other bias: low risk in 2/3 |
| Watts 2012 | 4 | No. | Assessed according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions | Random sequence generation: low risk in 3/4 Allocation concealment: low risk in 3/4 Blinding of participants and personnel: low risk in 3/4 Blinding of outcome assessment: low risk in 1/4 Incomplete outcome data: low risk in 4/4 |
We rated the certainty of evidence from the included systematic reviews with meta‐analysis using GRADE methods. The certainty of evidence varied widely (by review and also by outcome) and ranged from very low to high (Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19; Table 20).
7. Length of stay measures.
| Intervention/Comparison | Outcome | Results: treatment effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence |
| ED treatment time | |||||
| Continuous vs intermittent nebulisation: moderate to severe (Camargo 2003) | ED treatment time (units not specified) | MD ‐1.00 (‐13.50 to 11.50) | 70 (1) | Low | Certainty downgraded due to serious imprecision and serious risk of bias of the single study: unclear sequence generation, no allocation concealment (single‐blind study) |
| IV magnesium sulfate (Griffiths 2016) | ED treatment time (minutes) | MD 5.00 (‐24.40 to 34.40) | 27 (1) | Moderate | Certainty downgraded due to serious imprecision |
| Hospital length of stay | |||||
| Antibiotics vs placebo (Normansell 2018) | Length of hospital stay (days) | MD ‐0.10 (‐0.53 to 0.33) | 43 (1) | Very low | Certainty downgraded due to serious imprecision, risk of bias in single study (before good reporting standards introduced: 6 participants excluded but unclear from which arm they were excluded), indirectness (all children with status asthmaticus and study conducted before current asthma management had been introduced (e.g. they all received IV adrenaline) |
| Addition of IV SABA to inhaled SABA (Travers 2012a) | PICU length of stay (hours) | MD ‐12.95 (‐38.74 to 12.84) | 46 (1) | Moderate | Certainty downgraded due to serious imprecision |
| Inhaled anticholinergics + SABA vs SABA alone for children hospitalised with asthma (Vezina 2014) | Duration of hospital stay (hours) | MD ‐0.28 (‐5.07 to 4.52) | 327 (3) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision |
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) | Length of hospital stay (hours): all patients | MD ‐2.1 (‐9.45 to 5.25) | 231 (3) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision |
| Length of hospital stay (hours): submaximal inhaled beta2‐agonists (< 45 mg/kg/h) | MD 6.00 (‐20.49 to 32.49) | 26 (1) | Very low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and very serious imprecision | |
| Length of hospital stay (hours): maximised inhaled beta2‐agonists (≥ 45 mg/kg/h) | MD 4.10 (‐13.73 to 21.93) | 42 (1) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision | |
| Length of hospital stay (hours): maximised inhaled beta2‐agonists (≥ 45 mg/kg/h) and anticholinergics | MD ‐4.32 (‐12.79 to 4.15) | 163 (1) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision | |
| IV SABA vs intravenous aminophylline for acute asthma (Travers 2012b) | Length of stay (hours): all patients (positive values favour aminophylline) | MD 23.19 (‐2.40 to 48.77) | 73 (2) | Low | Certainty downgraded due to very serious imprecision |
| Length of stay (hours): paediatric (non‐PICU) patients (positive values favour aminophylline) | MD 28.10 (‐2.60 to 58.80) | 44 (1) | Low | Certainty downgraded due to very serious imprecision | |
| Length of stay (hours): PICU patients (positive values favour aminophylline) |
MD 12.00 (‐34.31 to 58.31) | 29 (1) | Low | Certainty downgraded due to very serious imprecision | |
| IV magnesium sulfate (Griffiths 2016) | Hospital length of stay (hours) | MD ‐5.30 (‐9.46 to ‐1.14) | 47 (1) | High | |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; ED: emergency department; IV: intravenous; MD: mean difference; PICU: paediatric intensive care unit: SABA: short‐acting beta2‐agonist; SMD: standardised mean difference.
8. Hospital admission.
| Intervention/Comparison | Population | Illustrative comparative risks (95% CI) | Relative effect: risk ratio (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| Inhaled treatment | |||||||
| Continuous vs intermittent nebulisation: moderate to severe (Camargo 2003) | Moderate to severe | 667 per 1000 | 460 per 1000 (220 to 974) | 0.69 (0.33 to 1.46) | 22 (1) | Very low | Certainty downgraded due to very serious imprecision; serious risk of bias of single study; unclear sequence generation; no allocation concealment (single‐blind study) |
| Less severe | 86 per 1000 | 58 per 1000 (11 to 322) | 0.67 (0.12 to 3.75) | 70 (1) | Very low | Certainty downgraded due to very serious imprecision; serious risk of bias of single study; unclear sequence generation; no allocation concealment (single‐blind study) | |
| Anticholinergic and SABA vs SABA alone (Griffiths 2013) | All studies | 232 per 1000 | 169 per 1000 (146 to 197) | 0.73 (0.63 to 0.85) | 2497 (19) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) |
| Single‐dose protocol | 176 per 1000 | 148 per 1000 (98 to 222) | 0.84 (0.56 to 1.26) | 419 (3) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| Multiple fixed‐dose protocol | 252 per 1000 | 181 per 1000 (154 to 212) | 0.72 (0.61 – 0.84) | 1998 (15) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| Multiple flexible‐dose protocol | 0 | 0 (not estimable) | Not estimable | 80 (1) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and single study with high risk of bias (consecutive assignment rather than randomised) | |
| Severe asthma | 298 per 1000 | 217 per 1000 (182 to 259) | 0.73 (0.61 to 0.87) | 1188 (8) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| Moderate to severe asthma | 269 per 1000 | 161 per 1000 (110 to 239) | 0.60 (0.41 to 0.89) | 371 (4) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| Moderate asthma | 145 per 1000 | 112 per 1000 (71 to 177) | 0.77 (0.49 to 1.22) | 463 (3) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| Mild to moderate asthma | 140 per 1000 | 122 per 1000 (73 to 206) | 0.87 (0.52 to 1.47) | 358 (2) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| Mild asthma | 70 per 1000 | 100 per 1000 (29 to 336) | 1.43 (0.42 to 4.79) | 117 (1) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision | |
| Highest tertile control group event rate | 487 per 1000 | 331 per 1000 (273 to 399) | 0.68 (0.56 to 0.82) | 669 (6) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| Middle tertile control group event rate | 241 per 1000 | 181 per 1000 (137 to 239) | 0.75 (0.57 to 0.99) | 780 (6) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| Lowest tertile control group event rate | 80 per 1000 | 73 per 1000 (47 to 114) | 0.91 (0.59 to 1.42) | 968 (6) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| Background corticosteroids | 317 per 1000 | 225 per 1000 (187 to 273) | 0.71 (0.59 to 0.86) | 1043 (8) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| No background corticosteroids | 297 per 1000 | 199 per 1000 (140 to 279) | 0.67 (0.47 to 0.94) | 353 (6) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| Corticosteroids administered variably (at physician discretion) | 214 per 1000 | 165 per 1000 (116 to 235) | 0.77 (0.54 to 1.10) | 511 (3) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| Background corticosteroids not reported | 91 per 1000 | 77 per 1000 (44 to 139) | 0.85 (0.48 to 1.53) | 500 (1) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision | |
| Inhaled magnesium sulfate (Knightly 2017) |
MgSO4 + SABA + Ipratropium/Placebo + SABA + Ipratropium (Admission at first presentation) | 957 per 1000 | 919 per 1000 (880 to 967) | 0.96 (0.92 to 1.01) | 508 (1) | Moderate | Certainty downgraded due to risk of bias in review (single author decided on trial inclusion) |
| MgSO4 + SABA vs Placebo + SABA (Knightly 2017) |
86 per 1000 | 98 per 1000 (38 to 257) | 1.14 (0.44 to 2.98) | 162 (2) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author decided on trial inclusion), and because a majority of patients were from a study judged to be at risk of reporting bias | |
| Parenteral treatment | |||||||
| Intravenous magnesium sulfate (Griffiths 2016) | Intravenous MgSO4/Placebo | 767 per 1000 | 537 per 1000 (414 to 698) | 0.70 (0.54 to 0.91) | 115 (3) | High | |
| Intravenous ketamine vs placebo (Jat 2012) | Ketamine/Placebo | 829 per 1000 | 788 per 1000 (622 to 995) | 0.95 (0.75 to 1.20) | 68 (1) | Moderate | Certainty downgraded due to single small study – risk of serious imprecision |
| Other interventions to reduce the work of breathing | |||||||
| Heliox vs placebo for non‐intubated asthma patients (Rodrigo 2006) | Heliox vs placebo | 750 per 1000 | 517 per 1000 (360 to 742) | 0.69 (0.48 to 0.99) | 71 (2) | Low | Risk of serious imprecision. SR shows asymmetrical funnel plot, suggesting publication bias |
| Other interventions | |||||||
| Leukotriene receptor antagonists in addition to usual care (Watts 2012) | Oral LTRA/Control | 62 per 1000 | 53 per 1000 (13 to 218) | 0.86 (0.12 to 3.52) | 194 (3) | Low | Certainty downgraded due to very serious imprecision |
| Intravenous LTRA/Control | 252 per 1000 | 199 per 1000 (129 to 310) | 0.79 (0.51 to 1.23) | 276 (1) | Moderate | Certainty downgraded due to serious imprecision | |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; LTRA: leukotriene receptor antagonist; MgSO4: magnesium sulfate; SABA: short‐acting beta2‐agonist.
9. Intensive care unit admission.
| Intervention/Comparison | Outcome | Illustrative comparative risks (95% CI) | Relative effect: risk ratio (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| Inhaled anticholinergics + SABA vs SABA alone for children hospitalised with asthma (Vezina 2014) | Admission to the ICU | 0 per 1000 | 0 (not estimable) | Not estimable | 210 (1) | Low | Certainty downgraded due to very serious imprecision |
| MgSO4 + SABA + Ipratropium/Placebo + SABA + Ipratropium (Knightly 2017) | HDU/ICU admission | 59 per 1000 | 87 per 1000 (47 to 165) | 1.48 (0.79 to 2.79) | 505 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author decided on trial inclusion) |
| Intravenous aminophylline + beta2‐agonists + systemic steroids vs placebo + beta2‐agonists + systemic steroids (Mitra 2005) | ICU admission rates | 500 per 1000 | 370 per 1000 (260 to 530) | 0.74 (0.52 to 1.06) | 163 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; HDU: high‐dependency unit; ICU: intensive care unit; LTRA: leukotriene receptor antagonist; MgSO4: magnesium sulfate; SABA: short‐acting beta2‐agonist.
10. Adverse effects.
| Intervention/Comparison | Outcome | Illustrative comparative risks (95% CI) | Relative effect: risk ratio (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| All adverse events | |||||||
| Antibiotics vs placebo (Normansell 2018) | All adverse events | 125 per 1000 | 100 per 1000 (19 to 541) | 0.80 (0.15 to 4.33) | 44 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in single study (possible attrition bias and reporting bias) |
| Inhaled anticholinergics + SABA vs SABA alone for children hospitalised with asthma (Vezina 2014) | Adverse health effects | 0 | 0 (not estimable) | Not estimable | 290 (2) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision |
| MgSO4 + SABA + Ipratropium vs Placebo + SABA + Ipratropium (Knightly 2017) | Any adverse event (during admission) | 204 per 1000 | 186 per 1000 (131 to 265) | 0.91 (0.64 to 1.30) | 507 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author decided on trial inclusion) |
| MgSO4 + SABA vs Placebo + SABA (Knightly 2017) | Any adverse events | 11 per 1000 | 5 per 1000 (0 to 57) | 0.46 (0.04 to 4.98) | 365 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author decided on trial inclusion) |
| Serious adverse events | |||||||
| Antibiotics vs placebo (Normansell 2018) | Serious adverse events | 0 | 0 (not estimable) | Not estimable | 40 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in single study (possible performance bias and detection bias) |
| MgSO4 + SABA + Ipratropium vs Placebo + SABA + Ipratropium (Knightly 2017) | Serious adverse events (during admission) | 47 per 1000 | 12 per 1000 (3 to 42) | 0.25 (0.07 to 0.89) | 507 (1) | Moderate | Certainty downgraded due to risk of bias in review (single author decided on trial inclusion) |
| MgSO4 + SABA vs Placebo + SABA (Knightly 2017) | Serious adverse events | 0 per 1000 | 0 (not estimable) | Not estimable | 62 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author decided on trial inclusion) |
| Non‐invasive positive‐pressure ventilation (Korang 2016) | Serious adverse events | 0 per 1000 | 0 (not estimable) | Not estimable | 35 (2) | Very low | Certainty downgraded due to risk of bias and very serious imprecision |
| Death | |||||||
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) | Death | 0 per 1000 | 0 (not estimable) | Not estimable | 326 (6) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) |
| Non‐invasive positive‐pressure ventilation (Korang 2016) | Mortality | 0 per 1000 | 0 (not estimable) | Not estimable | 16 (1) | Very low | Certainty downgraded due to risk of bias in included study and very serious imprecision |
| Tremor | |||||||
| Continuous vs intermittent nebulisation: moderate to severe (Camargo 2003) | Tremor | 257 per 1000 | 144 per 1000 (54 to 383) | 0.56 (0.21 to 1.49) | 70 (1) | Low | Certainty downgraded due to serious imprecision and serious risk of bias of the single study; unclear sequence generation; no allocation concealment (single‐blind study) |
| Anticholinergic and SABA vs SABA alone (Griffiths 2013) | Tremor | 202 per 1000 | 139 per 1000 (103 to 188) | 0.69 (0.51 to 0.93) | 524 (9) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) |
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) | Tremor: all patients | 263 per 1000 | 355 per 1000 (231 to 545) | 1.35 (0.88 to 2.07) | 192 (2) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) |
| Tremor: mean theophylline levels < 15 mg/L | 385 per 1000 | 500 per 1000 (216 to 1000) | 1.30 (0.56 to 3.02) | 29 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Tremor: mean theophylline levels ≥ 15 mg/L | 244 per 1000 | 334 per 1000 (205 to 544) | 1.37 (0.84 to 2.23) | 163 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| IV SABA vs IV aminophylline for acute asthma (Travers 2012b) | Tremor | 375 per 1000 with SABA | 694 (334 to 1000) with aminophylline | 1.85 (0.89 to 3.83) | 29 (1) | Low | Certainty downgraded due to very serious imprecision |
| Nausea and/or vomiting | |||||||
| Continuous vs intermittent nebulisation: moderate to severe (Camargo 2003) | Nausea/Vomiting | 57 per 1000 | 11 per 1000 (0 to 230) | 0.20 (0.01 to 4.02) | 70 (1) | Very low | Certainty downgraded due to very serious imprecision and serious risk of bias of the single study; unclear sequence generation; no allocation concealment (single‐blind study) |
| Anticholinergic and SABA vs SABA alone (Griffiths 2013) | Vomiting | 36 per 1000 | 32 per 1000 (18 to 56) | 0.88 (0.49 to 1.56) | 1230 (8) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision |
| Nausea | 107 per 1000 | 64 per 1000 (41 to 102) | 0.60 (0.38 to 0.95) | 757 (7) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations). | |
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) | Vomiting: all patients | 84 per 1000 | 311 per 1000 (181 to 534) | 3.69 (2.15 to 6.33) | 305 (5) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) |
| Vomiting: mean theophylline levels < 15 mg/L (mcg/mL) | 69 per 1000 | 189 per 1000 (81 to 443) | 2.73 (1.17 to 6.39) | 142 (4) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) | |
| Vomiting: mean theophylline levels ≥ 15 mg/L (mcg/mL) | 98 per 1000 | 433 per 1000 (214 to 874) | 4.43 (2.19 to 8.95) | 163 (1) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) | |
| IV SABA vs IV aminophylline for acute asthma (Travers 2012b) | Nausea/Vomiting | 0 per 1000 with SABA | Not estimable due to rate of 0 in control group | 19.00 (1.15 to 313.64) | 66 (1) | Low | Certainty downgraded due to very serious imprecision |
| Nausea | 313 per 1000 with SABA | 694 per 1000 (307 to 1000) with aminophylline | 2.22 (0.98 to 4.99) | 29 (1) | Low | Certainty downgraded due to very serious imprecision | |
| Vomiting | 438 per 1000 with SABA | 692 (359 to 1000) with aminophylline | 1.58 (0.82 to 3.07) | 29 (1) | Low | Certainty downgraded due to very serious imprecision | |
| Cardiovascular adverse effects | |||||||
| MgSO4 + SABA + Ipratropium /[SD2] Placebo + SABA + Ipratropium (Knightly 2017) | Hypotension | 8 per 1000 | 4 per 1000 (1 to 44) | 0.51 (0.05 to 5.54) | 507 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author decided on trial inclusion) |
| Flushing | 12 per 1000 | 8 per 1000 (2 to 27) | 0.67 (0.11 to 4.00) | 507 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author decided on trial inclusion) | |
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) | Arrhythmias | 26 per 1000 | 11 per 1000 (1 to 234) | 0.40 (0.02 to 9.12) | 75 (2) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) |
| IV SABA vs IV aminophylline for acute asthma (Travers 2012b) | Dysrhythmia | 188 per 1000 with SABA | 32 per 1000 (2 to 578) with aminophylline | 0.17 (0.01 to 3.08) | 29 (1) | Low | Certainty downgraded due to very serious imprecision |
| Palpitations | 0 per 1000 | 0 (not estimable) | Not estimable | 29 (1) | Low | Certainty downgraded due to very serious imprecision | |
| Neurological adverse effects | |||||||
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) | Headache: all patients | 124 per 1000 | 159 per 1000 (87 to 289) | 1.28 (0.70 to 2.33) | 238 (3) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) |
| Headache: mean theophylline levels < 15 mg/L (mcg/mL) | 0 per 1000 | Not estimable due to rate of 0 in control group | 5.48 (0.67 to 44.61) | 75 (2) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Headache: mean theophylline levels ≥ 15 mg/L (mcg/mL) | 183 per 1000 | 185 per 1000 (97 to 353) | 1.01 (0.53 to 1.93) | 163 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| IV SABA vs IV aminophylline for acute asthma (Travers 2012b) | Headache | 188 per 1000 with SABA | 231 per 1000 (57 to 959) with aminophylline | 1.23 (0.30 to 5.11) | 29 (1) | Low | Certainty downgraded due to very serious imprecision |
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) | Seizures: all patients | 7 per 1000 | 7 per 1000 (0 to 116) | 1.01 (0.06 to 15.91) | 274 (4) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) |
| Seizures: mean theophylline levels < 15 mg/L (mcg/mL) | 0 per 1000 | 0 (not estimable) | Not estimable | 88 (2) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Seizures: mean theophylline levels ≥ 15 mg/L (mcg/mL) | 11 per 1000 | 11 per 1000 (1 to 171) | 1.01 (0.06 to 15.91) | 186 (2) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Pneumonia | |||||||
| Non‐invasive positive‐pressure ventilation (Korang 2016) | Pneumonia | 0 per 1000 | 0 (not estimable) | Not estimable | 19 (1) | Very low | |
| Laboratory results | |||||||
| IV SABA vs IV aminophylline for acute asthma (Travers 2012b) | CPK elevation | 250 per 1000 with SABA | 385 (130 to 1000) with aminophylline | 1.54 (0.52 to 4.59) | 29 (1) | Low | Certainty downgraded due to very serious imprecision |
| CPK‐MB elevation | 63 per 1000 with SABA | 154 per 1000 (110 to 1000) with aminophylline | 2.46 (0.25 to 24.21) | 29 (1) | Low | Certainty downgraded due to very serious imprecision | |
| Hyperglycaemia | 1000 per 1000 with SABA | 920 per 1000 (750 to 1000) with aminophylline | 0.92 (0.75 to 1.12) | 29 (1) | High | ||
| Hypokalaemia | 163 per 1000 with SABA | 169 per 1000 (90 to 353) with aminophylline | 1.04 (0.48 to 2.25) | 95 (2) | Low | Certainty downgraded due to very serious imprecision | |
CI: confidence interval; CPK: creatine phosphokinase; CPK‐MB: creatine phosphokinase myocardial band; GRADE: Grading of Recommendations Assessment, Development and Evaluation; MgSO4: magnesium sulfate; SABA: short acting beta2‐agonist.
11. Symptom scores.
| Intervention/Comparison | Outcome | Results: treatment effect (95% CI) unless otherwise stated | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence |
| Continuous vs intermittent nebulisation (Camargo 2003) | Symptom scores | SMD 0.66 (0.18 to 1.14) | 70 (1) | Low | Certainty downgraded due to serious imprecision and serious risk of bias of the single study; unclear sequence generation; no allocation concealment (single‐blind study) |
| Anticholinergic and SABA vs SABA alone (Griffiths 2013) | Change in clinical score at 120 minutes (± 30 minutes) | SMD ‐0.23 (‐0.42 to ‐0.04) | 934 (3) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) |
| Oral LTRA vs control (Watts 2012) | Change in pulmonary index score (final assessment) | MD ‐1.20 (‐1.37 to ‐1.03) | 50 (1) | High | |
| Heliox vs placebo for non‐intubated asthma patients (Rodrigo 2006) | Dyspnoea or pulmonary index | MD ‐0.51 (‐1.14 to 0.11) | 93 (3) | Low | Risk of serious imprecision. SR shows asymmetrical funnel plot, suggesting publication bias |
| Inhaled anticholinergics + SABA vs SABA alone for children hospitalised with asthma (Vezina 2014) | Asthma clinical scores 8 to 36 hours after initial treatment | SMD 0.02 (‐0.34 to 0.38) | 117 (2) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision |
| Inhaled magnesium sulfate (Knightly 2017) | Yung asthma severity score at 60 minutes | MD ‐0.23 (‐0.48 to 0.02) | 472 (1) | Moderate | Certainty downgraded due to risk of bias in review (single author decided on trial inclusion) |
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) |
Change in symptom scores 6 to 8 hours after enrolment (all patients) | SMD ‐0.42 (‐0.70 to ‐0.13) | 215 (3) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) |
| Change in symptom scores 6 to 8 hours after enrolment: submaximal inhaled beta‐2 agonists (< 45 mg/kg/h) | SMD ‐0.31 (‐0.94 to 0.32) | 39 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in symptom scores 6 to 8 hours after enrolment: maximised inhaled beta‐2 agonists (≥ 45 mg/kg/h) | Not estimable | 21 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in symptom scores 6 to 8 hours after enrolment: maximised inhaled beta‐2 agonists (≥ 45 mg/kg/h) and anticholinergics | SMD ‐0.45 (‐0.77 to ‐0.13) | 155 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in symptom scores 12 to 18 hours after enrolment: submaximal inhaled beta‐2 agonists (< 45 mg/kg/h) | SMD ‐0.45 (‐1.09 to 0.19) | 39 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in symptom scores 12 to 18 hours after enrolment: maximised inhaled beta‐2 agonists (≥ 45 mg/kg/h) | Not estimable | 21 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in symptom scores 24 hours after enrolment (all patients) | SMD ‐0.13 (‐0.52 to 0.25) | 127 (4) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in symptom scores 24 hours after enrolment: submaximal inhaled beta‐2 agonists (< 45 mg/kg/h) | Not estimable | 21 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in symptom scores 24 hours after enrolment: maximised inhaled beta‐2 agonists (≥ 45 mg/kg/h) and anticholinergics | SMD ‐0.13 (‐0.52 to 0.25) | 127 (4) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| IV ketamine vs placebo (Jat 2012) | Pulmonary Index Score | MD 0.40 (‐1.21 to 0.41) | 68 (1) | Moderate | Certainty downgraded due to serious imprecision |
| Non‐invasive positive‐pressure ventilation (Korang 2016) | Asthma symptom score in the acute phase | MD ‐2.50 (‐4.70 to ‐0.30) | 19 (1) | Moderate | Certainty downgraded due to risk of bias in included study |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; LTRA: leukotriene receptor antagonist; MD: mean difference; mg/kg: milligram per kilogram; SABA: short‐acting beta2‐agonist; SMD: standardised mean difference; SR: systematic review.
12. Lung function.
| Intervention/Comparison | Outcome | Results: treatment effect (95% CI) unless otherwise stated | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence |
| Continuous vs intermittent nebulisation: moderate to severe (Camargo 2003) | PEFR values (end of study) | SMD 0.25 (‐0.22 to 0.72) | 70 (1) | Low | Certainty downgraded due to serious imprecision and serious risk of bias of the single study; unclear sequence generation; no allocation concealment (single‐blind study) |
| Anticholinergic and SABA vs SABA alone: variable corticosteroids (at physicians’ discretion) (Griffiths 2013) | Change from baseline in %predicted FEV1, 60 minutes after last dose of inhaled bronchodilator | MD 10.08 (6.24 to 13.92) | 402 (5) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) |
| Change from baseline in %predicted FEV1, 120 minutes after last dose of inhaled bronchodilator | MD 6.87 (1.17 to 12.56) | 117 (2) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| % Change in FEV1 or PEFR at 60 minutes after last inhaled bronchodilator (± 15 minutes) | SMD 0.57 (0.25 to 0.88) | 166 (4) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| % Change in FEV1 or PEFR at 120 minutes after last inhaled bronchodilator (± 30 minutes) | SMD 0.12 (‐0.15 to 0.39) | 219 (4) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| % Change in respiratory resistance at 60 minutes after inhaled bronchodilator (± 15 minutes) – all studies | MD 0.02 (‐0.02 to 0.07) | 294 (2) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| % Change in respiratory resistance at 60 minutes after inhaled bronchodilator (± 15 minutes) – with corticosteroids given within previous 60 minutes | MD ‐0.02 (‐0.13 to 0.09) | 70 (1) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| % Change in respiratory resistance at 60 minutes after inhaled bronchodilator (± 15 minutes) – no corticosteroids | MD 0.03 (‐0.02 to 0.08) | 224 (1) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| % Change in respiratory resistance at 120 minutes after inhaled bronchodilator (± 30 minutes) – all studies | MD ‐0.01 (‐0.09 to 0.07) | 108 (2) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| % Change in respiratory resistance at 120 minutes after inhaled bronchodilator (± 30 minutes) – with corticosteroids given within previous 120 minutes | MD 0.02 (‐0.12 to 0.16) | 47 (1) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| % Change in respiratory resistance at 120 minutes after inhaled bronchodilator (± 30 minutes) – no corticosteroids | MD ‐0.02 (‐0.12 to 0.08) | 61 (1) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision | |
| Antibiotics vs placebo (Normansell 2018) | PEFR (L/min) | MD 38.80 (‐11.19 to 88.79) | 40 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in single study (possible performance bias and detection bias) |
| Oral LTRA vs control (Watts 2012) | Change in FEV1 (predicted) | MD ‐3.10 (‐12.70 to 6.50) | 26 (1) | Moderate | Risk of serious imprecision |
| IV LTRA vs control (Watts 2012) | Change in FEV1 (litres) | MD 0.01 (‐0.06 to 0.08) | 276 (1) | High | |
| Heliox vs placebo for non‐intubated asthma patients (Rodrigo 2006) | Pulmonary function | SMD 0.32 (‐0.52 to 1.16) | 22 (1) | Low | Risk of serious imprecision. SR shows asymmetrical funnel plot, suggesting publication bias |
| Inhaled anticholinergics + SABA vs SABA alone for children hospitalised with asthma (Vezina 2014) | Percentages of predicted PEFR at 8 to 36 hours after initial treatment | MD ‐1.60 (‐17.20 to 14.00) | 20 (1) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision |
| MgSO4 + SABA vs Placebo + SABA (Knightly 2017) |
Pulmonary function %predicted FEV1 | MD 8.10 (‐3.03 to 19.23) | 62 (1) | Low | Certainty downgraded due to serious imprecision (single small study) and risk of bias in review (single author decided on trial inclusion) |
| Pulmonary function PEF L/min | MD 11.90 (‐6.86 to 30.66) | 80 (1) | Low | Certainty downgraded due to serious imprecision (single small study) and risk of bias in review (single author decided on trial inclusion) | |
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) | Change in % predicted FEV1 within 4 hours of enrolment: submaximal inhaled SABA (< 45 mg/kg/h) | Not estimable | 2 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) |
| Change in % predicted FEV1 within 4 hours of enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) | MD 2.08 (‐9.04 to 13.20) | 21 (1) | Low | Certainty downgraded due to serious imprecision (single small study) and risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 6 to 8 hours after enrolment (all patients) | MD 8.37 (0.82 to 15.92) | 65 (3) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 6 to 8 hours after enrolment: submaximal inhaled SABA (< 45 mg/kg/h) | Not estimable | 2 (1) | Very low | Certainty downgraded due to very serious imprecision (single small study) and risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 6 to 8 hours after enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) | MD 5.45 (‐6.33 to 17.23) | 21 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 6 to 8 hours after enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) and anticholinergics | MD 10.4 (0.57 to 20.23) | 42 (1) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 12 to 18 hours after enrolment (all patients) | MD 8.15 (1.04 to 15.27) | 57 (3) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 12 to 18 hours after enrolment: submaximal inhaled SABA (< 45 mg/kg/h) | Not estimable | 2 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 12 to 18 hours after enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) | MD 5.74 (‐6.14 to 17.62) | 20 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 12 to 18 hours after enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) and anticholinergics | MD 9.50 (0.62 to 18.38) | 35 (1) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 24 hours after enrolment (all patients) | MD 8.87 (1.25 to 16.5) | 62 (3) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 24 hours after enrolment: submaximal inhaled SABA (< 45 mg/kg/h) | Not estimable | 2 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 24 hours after enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) | MD 7.36 (‐7.61 to 22.33) | 39 (1) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author reviewed each abstract) | |
| Change in % predicted FEV1 24 hours after enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) and anticholinergics | MD 9.40 (0.54 to 18.26) | 62 (3) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) | |
| Change in PEF at 6 to 8 hours after enrolment (all patients) | SMD 0.92 (0.32 to 1.52) | 50 (2) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision | |
| Change in PEF at 6 to 8 hours after enrolment: submaximal inhaled SABA (< 45 mg/kg/h) | SMD 0.51 (‐0.96 to 1.98) | 8 (1) | Very low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and very serious imprecision | |
| Change in PEF at 6 to 8 hours after enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) and anticholinergics | SMD 1.00 (0.35 to 1.66) | 42 (1) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision | |
| Change in PEF at 12 to 18 hours after enrolment (all patients) | SMD 0.75 (0.25 to 1.26) | 67 (3) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision | |
| Change in PEF at 12 to 18 hours after enrolment: submaximal inhaled SABA (< 45 mg/kg/h) | SMD 0.71 (‐0.01 to 1.48) | 32 (2) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision | |
| Change in PEF at 12 to 18 hours after enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) and anticholinergics | SMD 0.77 (0.08 to 1.47) | 35 (1) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision | |
| Change in PEF at 24 hours after enrolment (all patients) | SMD 0.39 (‐0.51 to 1.30) | 70 (3) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision | |
| Change in PEF at 24 hours after enrolment: submaximal inhaled SABA (< 45 mg/kg/h) | SMD 0.33 (‐1.39 to 2.04) | 31 (2) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision | |
| Change in PEF at 24 hours after enrolment: maximised inhaled SABA (≥ 45 mg/kg/h) and anticholinergics | SMD 0.66 (0.01 to 1.31) | 39 (1) | Low | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) and serious imprecision |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; FEV1: forced expiratory volume in one second; LTRA: leukotriene receptor antagonist; MD: mean difference; mg/kg: milligram per kilogram; PEF: peak expiratory flow; PEFR: peak expiratory flow rate; SABA: short‐acting beta2-agonist; SMD: standardised mean difference.
13. Vital signs: dichotomous outcomes.
| Intervention/Comparison | Outcome | Illustrative comparative risks (95% CI) | Relative effect: risk ratio (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| Anticholinergic and SABA vs SABA alone (Griffiths 2013) | Oxygen saturations < 95% at 60 minutes (± 15 minutes) | 365 per 1000 | 267 per 1000 (210 to 354) | 0.73 (0.55 to 0.97) | 416 (2) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) |
| Oxygen saturations < 95% at 120 minutes (± 30 minutes) | 367 per 1000 | 404 per 1000 (279 to 583) | 1.10 (0.76 to 1.59) | 185 (2) | Very low | Certainty downgraded due to serious inconsistency, serious imprecision, and serious risk of bias in review (single author selected possible citations) | |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; SABA: short‐acting beta2‐agonist.
14. Vital signs: continuous outcomes.
| Intervention/Comparison | Outcome | Results: treatment effect (95% CI) unless otherwise stated | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence |
| Addition of IV SABA to inhaled SABA (Travers 2012a) | Pulse rate (beats/min) at 2 hours | MD 10.00 (‐1.07 to 21.07) | 29 (1) | Moderate | Certainty downgraded due to serious imprecision |
| Oral LTRA vs control (Watts 2012) | Change in respiratory rate (breaths/min) (final assessment) | MD ‐4.60 (‐6.84 to ‐2.36) | 50 (1) | High | |
| Heliox vs placebo for non‐intubated asthma patients (Rodrigo 2006) | Heart rate (beats/min) | MD 0.0 (‐13.80 to 13.80) | 22 (1) | Low | Serious imprecision. SR shows asymmetrical funnel plot, suggesting publication bias |
| SaO2 | MD 0.0 (‐2.51 to 2.51) | 22 (1) | Low | Serious imprecision. SR shows asymmetrical funnel plot, suggesting publication bias | |
| IV aminophylline + SABA + systemic steroids vs. placebo + SABA + systemic steroids (Mitra 2005) |
Blood pressure | Not estimable | 2 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; LTRA: leukotriene receptor antagonist; MD: mean difference; SABA: short‐acting beta2‐agonist; SaO2: oxygen saturation.
15. Rates of mechanical ventilation in the intensive care unit.
| Intervention/Comparison | Outcome | Illustrative comparative risks (95% CI) | Relative effect: risk ratio (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| IV aminophylline + SABA + systemic steroids vs Placebo + SABA + systemic steroids (Mitra 2005) | Rates of patients mechanically ventilated in ICU | 61 per 1000 | 6 per 1000 (1 to 100) | 0.09 (0.01 to 1.64) | 163 (1) | Very low | Certainty downgraded due to very serious imprecision and risk of bias in review (single author reviewed each abstract) |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; ICU: intensive care unit; SABA: short‐acting beta2‐agonist.
16. Clinical failure.
| Intervention/Comparison | Outcome | Illustrative comparative risks (95% CI) | Relative effect: risk ratio (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| Addition of IV SABA to inhaled SABA (Travers 2012a) | Clinical failure | 933 per 1000 | 354 per 1000 (177 to 728) | 0.38 (0.19 to 0.78) | 29 (1) | Moderate | One point deducted as data contributed by only 1 study |
| IV SABA vs IV aminophylline for acute asthma (Travers 2012b) | Clinical failure | 303 per 1000 with SABA | 303 per 1000 (145 to 630) with aminophylline | 1.00 (0.48 to 2.08) | 66 (1) | Low | Certainty downgraded due to very serious imprecision |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; IV: Intravenous; SABA: short‐acting beta2‐agonist.
17. Relapse/Return to the emergency department.
| Intervention/Comparison | Outcome | Illustrative comparative risks (95% CI) | Relative effect: risk ratio (95% CI) | Number of participants (studies) | Certaintyof the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| Anticholinergic and SABA vs SABA alone (Griffiths 2013) | Relapse | 45 per 1000 | 48 per 1000 (31 to 76) | 1.07 (0.68 to 1.68) | 1389 (10) | Low | Certainty downgraded due to serious imprecision and risk of bias in review (single author selected possible citations) |
| Oral LTRA vs control (Watts 2012) | Relapse within 7 days | 83 per 1000 | 32 per 1000 (1 to 727) | 0.39 (0.02 to 8.73) | 22 (1) | Low | Certainty downgraded due to very serious imprecision |
| Inhaled anticholinergics + SABA vs SABA alone for children hospitalised with asthma (Vezina 2014) | Relapse within 72 hours of discharge from hospital | 0 per 1000 | 0 (not estimable) | Not estimable | 80 (1) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision |
| IV magnesium sulfate (Griffiths 2016) | Return to ED within 48 hours | 22 per 1000 | 9 per 1000 (1 to 211) | 0.41 (0.02 to 9.71) | 85 (2) | Low | Certainty downgraded due to very serious imprecision |
CI: confidence interval; ED: emergency department; GRADE: Grading of Recommendations Assessment, Development and Evaluation; LTRA: leukotriene receptor antagonist; SABA: short acting beta2‐agonist.
18. Withdrawals.
| Intervention/Comparison | Outcome | Illustrative comparative risks (95% CI) | Relative effect: risk ratio (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| Oral LTRA vs control (Watts 2012) | Withdrawals | 28 per 1000 | 28 per 1000 (4 to 178) | 1.00 (0.16 to 6.34) | 143 (2) | Low | Downgraded due to very serious imprecision |
| IV LTRA vs control (Watts 2012) | Withdrawals | 31 per 1000 | 35 per 1000 (10 to 126) | 1.13 (0.31 to 4.12) | 276 (1) | Low | Downgraded due to very serious imprecision |
| Inhaled anticholinergics + SABA vs SABA alone for children hospitalised with asthma (Vezina 2014) | Overall withdrawals | 122 per 1000 | 76 per 1000 (38 to 154) | 0.62 (0.31 to 1.26) | 294 (2) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision |
| Withdrawals due to deterioration | 19 per 1000 | 39 per 1000 (7 to 206) | 2.04 (0.38 to 10.89) | 210 (1) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision | |
| IV aminophylline + SABA + systemic steroids vs placebo + SABA + systemic steroids (Mitra 2005) | Withdrawals due to adverse health effects: all patients | 11 per 1000 | 16 per 1000 (9 to 75) | 1.48 (0.32 to 6.85) | 370 (7) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision |
| Withdrawals due to adverse health effects: mean theophylline levels < 15 mg/L | 22 per 1000 | 32 per 1000 (7 to 148) | 1.48 (0.32 to 6.85) | 186 (5) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision | |
| Withdrawals due to adverse health effects: mean theophylline levels ≥ 15 mg/L | 0 per 1000 | 0 (not estimable) | Not estimable | 184 (2) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision | |
| Withdrawals due to poor asthma control: all patients | 16 per 1000 | 11 per 1000 (2 to 63) | 0.70 (0.13 to 3.90) | 370 (7) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision | |
| Withdrawals due to poor asthma control: mean theophylline levels < 15 mg/L | 32 per 1000 | 22 per 1000 (4 to 126) | 0.70 (0.13 to 3.90) | 186 (5) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision | |
| Withdrawals due to poor asthma control: mean theophylline levels ≥ 15 mg/L | 0 per 1000 | 0 (not estimable) | Not estimable | 184 (2) | Very low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and very serious imprecision | |
| Withdrawals due to any cause | 38 per 1000 | 63 per 1000 (26 to 154) | 1.65 (0.67 to 4.07) | 371 (7) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; LTRA: leukotriene receptor antagonist; mg/L: milligrams per litre; SABA: short acting beta2‐agonist.
19. Other measures: continuous outcomes.
| Intervention/Comparison | Outcome | Results: treatment effect (95% CI) unless otherwise stated | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence |
| Continuous vs intermittent nebulisation: moderate to severe (Camargo 2003) | Respiratory therapist time | MD ‐22.00 (‐26.82 to ‐17.18) | 70 (1) | Moderate | Certainty downgraded due to serious risk of bias of the single study; unclear sequence generation; no allocation concealment (single‐blind study) |
| Inhaled anticholinergics + SABA vs SABA alone for children hospitalised with asthma (Vezina 2014) | Time to SABA spaced at 4 hours or longer (hours) | MD ‐2.17 (‐7.01 to 2.66) | 290 (2) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision |
| IV aminophylline + SABA + systemic steroids vs Placebo + SABA + systemic steroids (Mitra 2005) | Number of nebulisers required in 24 hours | MD 0.15 (‐0.52 to 0.83) | 69 (1) | Moderate | Certainty downgraded due to risk of bias in review (single author reviewed each abstract) |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; MD: mean difference; SABA: short‐acting beta2‐agonist.
20. Other measures: dichotomous outcomes.
| Intervention/Comparison | Outcome | Illustrative comparative risks (95% CI) | Relative effect: risk ratio (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments: overview authors' assessment of the certainty of evidence | |
| Assumed risk | Corresponding risk | ||||||
| With comparator | With intervention | ||||||
| Anticholinergic and SABA vs SABA alone (Griffiths 2013) | Need for repeat bronchodilator treatment after standard protocol prior to disposition | 539 per 1000 | 469 per 1000 (426 to 523) | 0.87 (0.79 to 0.97) | 1074 (9) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) |
| Need for corticosteroids in ED prior to disposition | 516 per 1000 | 459 per 1000 (377 to 557) | 0.89 (0.73 to 1.08) | 378 (2) | Moderate | Certainty downgraded due to risk of bias in review (single author selected possible citations) | |
| Oral LTRA vs control (Watts 2012) | Requirement for additional care at end of study | 77 per 1000 | 42 per 1000 (4 to 431) | 0.54 (0.05 to 5.60) | 50 (1) | Low | Certainty downgraded due to very serious imprecision |
| Inhaled anticholinergics + SABA vs SABA alone for children hospitalised with asthma (Vezina 2014) | Need for supplemental asthma therapy | 86 per 1000 | 66 per 1000 (35 to 122) | 0.77 (0.41 to 1.42) | 465 (4) | Low | Certainty downgraded due to risk of bias in review (single author selected possible citations) and serious imprecision |
| IV ketamine vs placebo (Jat 2012) | Worsened and required other adjuvant therapy | 29 per 1000 | 61 per 1000 (6 to 638) | 2.12 (0.2 to 22.31) | 68 (1) | Low | Certainty downgraded due to very serious imprecision |
CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; ED: emergency department; LTRA: leukotriene receptor antagonist; SABA: short‐acting beta2‐agonist.
Effect of interventions
Outcomes varied considerably between systematic reviews, and similar outcomes were measured via different methods at different points in time. A categorised list of all outcomes is presented in Appendix 4. A series of tables is presented, including data for each outcome from the included systematic reviews (Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19; Table 20). These tables present odds or risk ratios and also absolute event rates per thousand in each group. We summarise below information from reviews that contribute data to each outcome, including the certainty of evidence assessment we performed on each included systematic review with meta‐analysis. Comparisons are between treatment and placebo unless otherwise stated.
Common themes in recommendations for future research (Appendix 5) from the included reviews were the need for adequately powered and methodologically sound RCTs, an agreed core outcome set for acute asthma in children, reliable assessment of baseline severity and response to treatment, and the need to perform subgroup analyses in preschool and school‐aged children, and for varying degrees of asthma severity.
There was no overlap between reviews. We contacted no review authors for further information. We obtained data only from the included Cochrane Reviews; we did not obtain data from individual trial reports.
Primary outcome: length of stay
Two reviews included ED treatment time as an outcome measure, and six reviews included hospital length of stay (Table 7). Comparisons ranged from single RCTs with as few as 29 participants to three RCTs with a total of 327 participants.
Inhaled treatment
Two reviews reported low‐certainty evidence for the effects of inhaled treatment on length of stay. The review comparing continuous versus intermittent nebulisation showed an unclear difference in ED treatment time between the two groups (mean difference (MD) ‐1.00 hours; 95% confidence interval (CI) ‐13.50 to 11.50 hours; 70 participants; 1 trial) (Camargo 2003).
A review examining the effects of adding inhaled anticholinergics to short acting beta2‐agonists (SABAs) found an unclear difference in hospital length of stay (MD ‐0.28 hours; 95% CI ‐5.07 to 4.52 hours; 327 participants; 3 trials) (Vezina 2014).
Parenteral treatment
Four reviews examined the effects of intravenous medications on hospital length of stay. No clear difference was demonstrated when intravenous beta2‐agonists were added to inhaled beta2‐agonists (moderate‐certainty evidence; MD ‐12.95 hours; 95% CI ‐38.74 to 12.84; 46 participants; 1 trial) (Travers 2012a); when intravenous aminophylline was used (low‐certainty evidence; MD ‐2.1 hours, 95% CI ‐9.45 to 5.25; 231 participants; 3 trials) (Mitra 2005); or when intravenous beta2‐agonists were compared to intravenous aminophylline (low‐certainty evidence; MD 23.19 hours; 95% CI ‐2.40 to 48.77; 44 participants; 1 trial) (Travers 2012b).
The review on intravenous magnesium sulfate showed high‐certainty evidence of a reduction in hospital length of stay (MD ‐5.3 hours, 95% CI ‐9.46 to ‐1.14 hours; 47 participants; 1 trial) and moderate‐certainty evidence of an unclear effect on ED treatment time compared to placebo (MD 5.00 minutes, 95% CI ‐24.40 to 34.40; 27 participants; 1 trial) (Griffiths 2016).
Interventions to reduce the work of breathing
Neither review on interventions to reduce the work of breathing included length of stay as an outcome measure.
Other interventions
One review reported very low‐certainty evidence for the effects of antibiotics versus placebo on hospital length of stay and found an unclear difference (MD ‐0.10, 95% CI ‐0.53 to 0.33; 43 participants; 1 trial) (Normansell 2018).
Primary outcome: hospital admission
Seven reviews included hospital admission as an outcome measure (Table 8). Comparisons ranged from planned subgroup analyses of a single RCT with 22 participants to a review including 19 RCTs and 2497 participants.
Inhaled treatment
Very low‐certainty evidence suggested an unclear difference in the rate of hospital admissions for continuous nebulised versus intermittent nebulised beta2‐agonists for both moderate to severe asthma (RR 0.69, 95% CI 0.33 to 1.46; 22 participants; 1 trial) and less severe asthma (RR 0.67, 95% CI 0.12 to 3.75; 70 participants; 1 trial) (Camargo 2003).
This review conducted a number of subgroup analyses for this outcome and identified a reduction in the risk of hospital admission for those with more severe asthma, along with an unclear effect in children with milder asthma (Table 8). A multiple fixed‐dose protocol of inhaled anticholinergic agents resulted in reduced risk of hospital admission compared with SABA alone (RR 0.72, 95% CI 0.61 to 0.84; 1998 participants; 15 trials), but a single‐dose regimen did not have a clear effect on the risk of hospital admission (RR 0.84, 95% CI 0.56 to 1.26; 419 participants; 3 trials).
When RCTs were grouped by co‐intervention with corticosteroids, a reduction in risk was noted when corticosteroids were given (RR 0.71, 95% CI 0.59 to 0.86; 1043 participants; 8 trials), and in RCTs in which corticosteroids were not administered (RR 0.67, 95% CI 0.47 to 0.94; 353 participants; 6 trials). However, in RCTs in which corticosteroids were administered at physician discretion, the reduction in risk was non‐significant (RR 0.77, 95% CI 0.54 to 1.10; 511 participants; 3 trials).
Inhaled magnesium sulfate had an unclear effect on hospital admission when administered with SABAs (very low‐certainty evidence; RR 1.14, 95% CI 0.44 to 2.98; 162 participants; 2 trials) or with a combination of inhaled anticholinergics and SABAs (moderate‐certainty evidence; RR 0.96, 95% CI 0.92 to 1.01; 508 participants; 1 trial) (Knightly 2017).
Parenteral treatment
The review on intravenous magnesium sulfate yielded high‐certainty evidence of a reduction in the risk of hospital admission compared to placebo (RR 0.70, 95% CI 0.54 to 0.91; 115 participants; 3 trials) (Griffiths 2016).
The review on intravenous ketamine showed moderate‐certainty evidence of an unclear effect (RR 0.95, 95% CI 0.75 to 1.20; 68 participants; 1 trial) (Jat 2012).
Interventions to reduce the work of breathing
Low‐certainty evidence suggests that the use of heliox led to a small reduction in hospital admission compared to placebo (RR 0.69, 95% CI 0.48 to 0.99; 71 participants; 2 trials) (Rodrigo 2006).
Other interventions
The use of leukotriene receptor antagonists (LTRA) in addition to usual care had an unclear effect on hospital admission for either oral LTRA (RR 0.86, 95% CI 0.12 to 3.52; 194 participants; 3 trials) or intravenous LTRA (RR 0.79, 95% CI 0.51 to 1.23; 276 participants; 1 trial) (Watts 2012).
Primary outcome: intensive care unit admission
Three reviews included intensive care admission as an outcome measure (Table 9). Each review included a single trial. Comparisons ranged from a trial with 163 participants to a trial with 508 participants.
Inhaled treatment
Results show no intensive care unit admissions for treatment or control participants in the review on inhaled anticholinergics added to SABA versus SABA alone (Vezina 2014).
Very low‐certainty evidence suggests that the addition of inhaled magnesium sulfate to a combination of ipratropium and a SABA had an unclear effect on the rate of high dependency/intensive care unit admission (RR 1.48, 95% CI 0.79 to 2.79; 505 participants; 1 trial) (Knightly 2017).
Parenteral treatment
The addition of intravenous aminophylline to inhaled SABA and systemic corticosteroids had an unclear effect on intensive care unit admission (RR 0.74, 95% CI 0.52 to 1.06; 163 participants; 1 trial) (Mitra 2005).
Interventions to reduce the work of breathing
No reviews reported intensive care unit admission as an outcome.
Other intervention
No reviews reported intensive care unit admission as an outcome.
Primary outcome: adverse events
Table 10 presents our findings related to adverse events (a primary outcome for this overview) and secondary outcomes related to specific adverse events (tremor, nausea and vomiting, and other adverse events).
Four reviews presented a total of eight comparisons related to adverse events. Of these comparisons, half were related to any adverse event, and the other half examined serious adverse events. Comparisons ranged from a review including 35 participants within two RCTs to a single RCT of 507 participants.
Inhaled treatment
Risk for adverse events was not estimable, as none occurred in the review on inhaled anticholinergics combined with SABA versus SABA alone (290 participants; 2 trials) (Vezina 2014).
Very low‐certainty evidence suggests an unclear difference in the risk for either any adverse events or serious adverse events when inhaled magnesium sulfate and SABA were compared to inhaled SABA alone (Knightly 2017). When inhaled magnesium sulfate was added to a combination of SABA and inhaled anticholinergic, a single trial of 507 participants provided low‐certainty evidence for an unclear effect on the risk of any adverse event (RR 0.91, 95% CI 0.64 to 1.30), and moderate‐certainty evidence for reduced risk of serious adverse events (RR 0.25, 95% CI 0.07 to 0.89).
Parenteral treatment
No reviews of parenteral treatment provided overall estimates of adverse events or serious adverse events. Information related to specific adverse events is provided under secondary outcomes (below).
Interventions to reduce the work of breathing
Risk for adverse events was not estimable, as none occurred in the review on non‐invasive positive‐pressure ventilation (35 participants; 2 trials) (Korang 2016).
Other intervention
Very low‐certainty evidence from the review on antibiotics versus placebo suggests an unclear difference in the risk for all adverse events (RR 0.80, 95% CI 0.15 to 4.33; 44 participants; 1 trial) (Normansell 2018). Review authors were unable to estimate a risk for serious adverse events, as no such events occurred in the 40 included participants.
Secondary outcome: death/mortality
Two reviews, one on intravenous aminophylline ‐ Mitra 2005 ‐ and another on non‐invasive positive‐pressure ventilation ‐ Korang 2016, presented comparisons related to death or mortality (Table 10). No events were reported in either review, both of which were rated as providing very low‐certainty evidence, and we were unable to estimate a relative risk for death/mortality.
Secondary outcome: adverse events (tremor)
Four reviews presented a total of six comparisons related to tremor (Table 10). Comparisons ranged from 29 participants in a single RCT to a total of 524 participants in nine RCTs.
Inhaled treatment
The review on continuous nebulised versus intermittent nebulised beta2‐agonists identified a single RCT showing low‐certainty evidence suggesting an unclear effect on tremor (RR 0.56, 95% CI 0.21 to 1.49; 70 participants) (Camargo 2003).
The review on anticholinergic agents added to SABAs for initial treatment of asthma showed moderate‐certainty evidence for a reduction in the risk of tremor when use of anticholinergic medication was compared to use of SABAs alone (RR 0.69, 95% CI 0.51 to 0.93; 524 participants; 9 trials).
Parenteral treatment
A review assessing the effectiveness of intravenous aminophylline presented very low‐certainty evidence of an unclear effect on tremor between treatment groups (RR 1.35, RR 0.88 to 2.07; 192 participants; 2 trials) (Mitra 2005). Subgroup analysis by mean theophylline levels demonstrated similar results.
The review comparing intravenous aminophylline to intravenous beta2‐agonists presented low‐certainty evidence suggesting an unclear difference between treatment groups (RR 1.85, 95% CI 0.89 to 3.83; 29 participants; 1 trial) (Travers 2012b).
Interventions to reduce the work of breathing
No reviews reported tremor as an outcome.
Other intervention
No reviews reported tremor as an outcome.
Secondary outcome: adverse events (nausea and/or vomiting)
Four reviews presented a total of nine comparisons related to nausea and/or vomiting (Table 10). Comparisons ranged from 29 participants within a single RCT to a total of 1330 participants in eight RCTs.
Inhaled treatment
The review on continuous nebulised versus intermittent nebulised beta2‐agonists identified a single RCT showing very low‐certainty evidence for an unclear effect on nausea/vomiting (RR 0.20, 95% CI 0.01 to 4.02; 70 participants) (Camargo 2003).
The review on anticholinergic agents added to SABAs for initial treatment of asthma presented separate results for nausea and vomiting (Griffiths 2013). The addition of anticholinergic treatment did not affect the incidence of vomiting (RR 0.88, 95% CI 0.49 to 1.56; 1230 participants; 8 trials; low‐certainty evidence), although the risk of nausea was reduced (RR 0.60, 95% CI 0.38 to 0.95; 757 participants; 7 trials; high‐certainty evidence).
Parenteral treatment
The review assessing effectiveness of intravenous aminophylline presented data on vomiting for all patients, as well as subgroup analysis according to mean theophylline levels (Mitra 2005). All comparisons yielded moderate‐certainty evidence of increased risk of vomiting for participants randomised to theophylline (RR 3.69, 95% CI 2.15 to 6.33; 305 participants; 5 trials), regardless of whether the mean theophylline level was < 15 mg/L (RR 2.73, 95% CI 1.17 to 6.39) or ≥ 15 mg/L (RR 4.43, 95% CI 2.19 to 8.95; 163 participants; 1 trial).
The review comparing intravenous aminophylline to intravenous beta2‐agonists presented low‐certainty evidence suggesting that aminophylline administration led to increased risk for nausea (RR 2.22, 95% CI 0.98 to 4.99; 29 participants; 1 trial) and nausea/vomiting (RR 19.00, 95% CI 1.15 to 313.64; 66 participants; 1 trial) (Travers 2012b). The risk for vomiting alone was not significantly different (RR 1.58, 95% CI 0.82 to 3.07; 29 participants; 1 trial).
Interventions to reduce the work of breathing
No reviews reported nausea/vomiting as an outcome.
Other intervention
No reviews reported nausea/vomiting as an outcome.
Secondary outcome: cardiovascular adverse events (hypotension, flushing, palpitations, arrhythmia)
Three reviews presented a total of five comparisons related to cardiovascular adverse events, including hypotension, flushing, palpitations, dysrhythmia, and arrhythmia (Table 10). Comparisons ranged from 29 participants from a single RCT to 507 participants from a single RCT.
Inhaled treatment
The review comparing inhaled magnesium sulfate and SABA to inhaled SABA alone (507 participants; 1 trial) provided low‐certainty evidence suggesting an unclear effect on hypotension (RR 0.51, 95% CI 0.05 to 5.54; 507 participants; 1 trial) or flushing (RR 0.67, 95% CI 0.11 to 4.00) (Knightly 2017).
Parenteral treatment
The review assessing the effectiveness of aminophylline in addition to SABA and systemic steroids presented very low‐certainty evidence suggesting an unclear effect on arrhythmia compared to SABA and systemic steroids alone (RR 0.40, 95% CI 0.02 to 9.12; 75 participants; 2 trials) (Mitra 2005).
The review comparing intravenous beta2‐agonists to intravenous aminophylline presented low‐certainty evidence suggesting an unclear effect on dysrhythmia (RR 0.17, 95% CI 0.01 to 3.08; 29 participants; 1 trial) (Travers 2012b). This review was unable to provide an estimate for the risk of palpitations, as none were identified in either treatment group in the single trial (29 participants) included.
Interventions to reduce the work of breathing
No reviews reported cardiovascular adverse events as an outcome.
Other intervention
No reviews reported cardiovascular adverse events as an outcome.
Secondary outcome: neurological adverse events (headache and seizures)
Two reviews presented data on seizures and/or headaches (Table 10). Comparisons ranged from 29 participants from a single RCT to 274 participants from four RCTs.
Inhaled treatment
No reviews reported neurological adverse events as an outcome.
Parenteral treatment: headache
The review assessing the effectiveness of aminophylline in addition to SABA and systemic steroids presented very low‐certainty evidence suggesting an unclear effect on headache compared to SABA and systemic steroids alone (RR 1.28, 95% CI 0.70 to 2.33; 238 participants; 3 trials) (Mitra 2005). Subgroup analysis according to mean theophylline levels provided similar results.
The review comparing intravenous beta2‐agonists to intravenous aminophylline presented low‐certainty evidence suggesting an unclear effect on headache (RR 1.23, 95% CI 0.30 to 5.11; 29 participants; 1 trial) (Travers 2012b).
Parenteral treatment: seizures
The review assessing the effectiveness of aminophylline in addition to SABA and systemic steroids presented very low‐certainty evidence suggesting an unclear effect on seizures compared to SABA and systemic steroids alone (RR 1.01, 95% CI 0.06 to 15.91; 274 participants; 4 trials) (Mitra 2005). Subgroup analysis according to mean theophylline levels was not estimable in those with a mean theophylline level < 15 mg/L due to no events in either treatment group, and the analysis of those with mean theophylline levels ≥ 15 mg/L provided similar results to the overall comparison.
Interventions to reduce the work of breathing
No reviews reported neurological adverse events as an outcome.
Other intervention
No reviews reported neurological adverse events as an outcome.
Secondary outcome: adverse events (pneumonia)
A single review on the use of non‐invasive positive‐pressure ventilation presented information on the risk for pneumonia (Table 10).
Inhaled treatment
No reviews reported pneumonia as an outcome.
Parenteral treatment
No reviews reported pneumonia as an outcome.
Interventions to reduce the work of breathing
A review on the addition of non‐invasive positive‐pressure ventilation was unable to estimate the risk of pneumonia because no events occurred in either treatment group (19 participants; 1 trial) (Korang 2016).
Other intervention
No reviews reported pneumonia as an outcome.
Secondary outcome: changes in laboratory results
One review comparing intravenous beta2‐agonists versus intravenous aminophylline presented four comparisons related to laboratory results (Table 10) (Travers 2012b). Three comparisons were from a single RCT involving 29 participants, and the other comparison involved 95 participants from two RCTs.
Inhaled treatment
No reviews reported changes in laboratory results as an outcome.
Parenteral treatment
The review comparing intravenous beta2‐agonists to intravenous aminophylline presented low‐certainty evidence suggesting an unclear effect on creatine phosphokinase (CPK) elevation (RR 1.54, 95% CI 0.52 to 4.59; 29 participants; 1 trial), CPK‐myocardial band (CPK‐MB) elevation (RR 2.46, 95% CI 0.25 to 24.21; 29 participants; 1 trial), and hypokalaemia (RR 1.04, 95% CI 0.48 to 2.25; 95 participants; 2 trials) (Travers 2012b). This review presented high‐certainty evidence showing an unclear difference in hyperglycaemia (RR 0.92, 95% CI 0.75 to 1.12; 29 participants; 1 trial).
Interventions to reduce the work of breathing
No reviews reported changes in laboratory results as an outcome.
Other intervention
No reviews reported changes in laboratory results as an outcome.
Secondary outcome: symptom scores/clinical asthma scores
Nine reviews presented a total of 17 comparisons related to symptom scores/clinical asthma scores (Table 11). Comparisons ranged from 19 participants within a single RCT to 934 participants in three RCTs.
Inhaled treatment
The review on continuous nebulised versus intermittent nebulised beta2‐agonists identified a single RCT showing low‐certainty evidence suggesting the benefit of continuous therapy (standardised mean difference (SMD) 0.66, 95% CI 0.18 to 1.14; 70 participants; 1 trial) (Camargo 2003).
The review on anticholinergic agents added to SABAs for initial treatment of asthma identified three RCTs showing moderate‐certainty evidence for a small benefit when anticholinergic agents were added compared to SABAs alone (SMD ‐0.23, 95% CI ‐0.42 to ‐0.04; 934 participants; 3 trials) (Griffiths 2013). However anticholinergic agents added to SABAs in children admitted to hospital with asthma showed low‐certainty evidence of no change in asthma scores compared to SABA treatment alone (SMD 0.02, 95% CI ‐0.34 to 0.38; 117 participants; 2 trials) (Vezina 2014).
The addition of inhaled magnesium to SABA and ipratropium yielded moderate‐certainty evidence of an unclear effect on the Yung asthma severity score at 60 minutes compared to SABA and ipratropium alone (MD ‐0.23, 95% CI ‐0.48 to 0.02; 472 participants; 1 trial) (Knightly 2017).
Parenteral treatment
The review assessing the effectiveness of intravenous aminophylline presented data on asthma scores at 6 to 8 hours, at 12 to 18 hours, and at 24 hours after study enrolment (very low‐ to low‐certainty evidence) (Mitra 2005). Subgroup analysis was also performed according to the dosing of inhaled beta2‐agonists (submaximal and maximal doses), and whether or not anticholinergic medications were also administered. Addition of aminophylline reduced symptom scores overall at 6 to 8 hours after enrolment (SMD ‐0.42, 95% CI ‐0.70 to ‐0.13; 215 participants; 3 trials); all subgroups suggested a similar direction of effect, although this was statistically significant only in the subgroup that included combined maximised inhaled beta2‐agonist and anticholinergic treatment (SMD ‐0.45, 95% CI ‐0.77 to ‐0.13; 155 participants; 1 trial). Very low‐ and low‐certainty evidence suggests no effect of aminophylline at 12 to 18 hours after enrolment (SMD ‐0.45, 95% CI ‐1.09 to 0.19; 39 participants; 1 trial) nor at 24 hours after enrolment (SMD ‐0.13, 95% CI ‐0.52 to 0.25; 127 participants; 1 trial).
The review assessing use of intravenous ketamine provided moderate‐certainty evidence that ketamine had an unclear effect on the Pulmonary Index score (MD 0.40, 95% CI ‐1.21 to 0.41; 68 participants; 1 trial) (Jat 2012).
Interventions to reduce the work of breathing
Low‐certainty evidence suggests that the use of heliox had no clear effect on asthma symptom scores (MD ‐0.51, 95% CI ‐1.14 to 0.11; 93 participants; 3 trials) (Rodrigo 2006).
The use of non‐invasive positive‐pressure ventilation resulted in moderate‐certainty evidence of a reduction in asthma symptom score in the acute phase (MD ‐2.50, 95% CI ‐4.70 to ‐0.30; 19 participants; 1 trial) (Korang 2016).
Other interventions
The review on the use of leukotriene receptor antagonists (LTRAs) provided high‐certainty evidence of a change in the Pulmonary Index score at final assessment (MD ‐1.20, 95% CI ‐1.37 to ‐1.03; 50 participants; 1 trial) for those administered an oral LTRA (Watts 2012).
Secondary outcome: lung function
Eight reviews presented a total of 41 comparisons related to a variety of lung function tests including peak expiratory flow rate (PEFR), change from baseline in forced expiratory volume in 1 second (FEV1), change in respiratory resistance, and pulmonary function at various time points after enrolment (Table 12). Comparisons ranged from two participants within a single RCT to 402 participants in five RCTs.
Inhaled treatment
The review on continuous nebulised versus intermittent nebulised beta2‐agonists identified a single RCT showing low‐certainty evidence of an unclear effect on PEFR values at the end of the RCT (SMD 0.25, 95% CI ‐0.22 to 0.72; 70 participants; 1 trial) (Camargo 2003).
The review on anticholinergic agents added to SABAs for initial treatment of asthma presented a number of subgroup comparisons (Griffiths 2013). Moderate‐certainty evidence showed a change from baseline in percentage of predicted FEV1 at 60 minutes (MD 10.08, 95% CI 6.24 to 13.92; 402 participants; 5 trials) and at 120 minutes (MD 6.87, 95% CI 1.17 to 12.56; 117 participants; 2 trials) after the last dose of inhaled bronchodilator. The review also presented low‐certainty evidence suggesting a difference in percentage change in FEV1 or PEFR at 60 minutes after the last dose of inhaled bronchodilator (SMD 0.57, 95% CI 0.25 to 0.88; 166 participants; 4 trials). An unclear effect on the percentage change in respiratory resistance from baseline measured at 60 minutes or 120 minutes after the last inhaled bronchodilator was noted, regardless of the relative timing of corticosteroids.
When anticholinergic agents were tested in children admitted to hospital with asthma (Vezina 2014), low‐certainty evidence suggested an unclear effect on percentages of predicted PEFR at 8 to 36 hours after identification of initial treatment (MD ‐1.60, 95% CI ‐17.20 to 14.00; 20 participants; 1 trial).
Inhaled magnesium did not appear to significantly alter the percentage of predicted FEV1 (MD 8.10, 95% CI ‐3.03 to 19.23; 62 participants; 1 trial; low‐certainty evidence) or peak expiratory flow rates (MD 11.90, 95% CI ‐6.86 to 30.66; 80 participants; 1 trial; low‐certainty evidence) (Knightly 2017).
Parenteral treatment
The review assessing the effectiveness of intravenous aminophylline presented 23 comparisons relating to lung function tests: 14 relating to FEV1 and 9 to PEFR (Mitra 2005).
At 6 to 8 hours after enrolment, moderate‐certainty evidence showed improvement in the percentage of predicted FEV1 for all patients (MD 8.37, 95% CI 0.82 to 15.92; 65 participants; 3 trials). Similar findings were seen at 12 to 18 hours and at 24 hours after enrolment.
Changes in PEF were also assessed at 6 to 8 hours after enrolment, at 12 to 18 hours after enrolment, and at 24 hours after enrolment. At 6 to 8 hours after enrolment, low‐certainty evidence suggested a change in PEFR for all patients (SMD 0.92, 95% CI 0.32 to 1.52; 50 participants; 2 trials). Similar findings were seen at 12 to 18 hours. At 24 hours, low‐certainty evidence suggested a change when SABAs were combined with inhaled anticholinergic medication (SMD 0.66, 95% CI 0.01 to 1.31; 39 participants; 1 trial).
Interventions to reduce the work of breathing
Low‐certainty evidence suggests that use of heliox had an unclear effect on pulmonary function (SMD 0.32, 95% CI ‐0.52 to 1.16; 22 patients; 1 trial) (Rodrigo 2006).
Other intervention
A review on the use of LTRAs resulted in high‐certainty evidence of no significant change in FEV1 for intravenous LTRA therapy (MD 0.01, 95% CI ‐0.06 to 0.08; 276 participants; 1 trial) and moderate‐certainty evidence showing an unclear effect on FEV1 for oral LTRA therapy (MD ‐3.10, 95% CI ‐12.70 to 6.50; 26 participants; 1 trial) (Watts 2012).
Low‐certainty evidence from the review on antibiotics versus placebo suggested an unclear effect on PEFR (MD 38.80, 95% CI ‐11.19 to 88.79; 40 participants; 1 trial) (Normansell 2018).
Secondary outcome: vital signs
Five reviews presented seven comparisons related to vital signs (Table 13; Table 14). Comparisons ranged from two participants in a single RCT to 416 participants in two RCTs.
Inhaled treatment
The review on anticholinergic agents added to SABAs for initial treatment of asthma presented moderate‐certainty evidence showing lower risk of oxygen saturation < 95% at 60 minutes (± 15 minutes) after study commencement (RR 0.73, 95% CI 0.55 to 0.97; 416 participants; 2 trials) and very low‐certainty evidence suggesting an unclear effect on the proportion of participants with oxygen saturation < 95% at 120 minutes (± 15 minutes) after study commencement (RR 1.10, 95% CI 0.76 to 1.59; 185 participants; 2 trials) (Griffiths 2013).
Parenteral treatment
The review comparing the addition of intravenous beta2‐agonists to inhaled beta2‐agonists presented moderate‐certainty evidence showing an unclear effect on pulse rate at two hours (MD 10.00, 95% CI ‐1.07 to 21.07; 29 participants; 1 trial) (Travers 2012a).
Interventions to reduce the work of breathing
Low‐certainty evidence suggests that the use of heliox had no effect on heart rate (MD 0.0, 95% CI ‐13.80 to 13.80) nor on oxygen saturation (MD 0.0, 95% CI ‐2.51 to 2.51) compared to placebo (22 participants; 1 trial) (Rodrigo 2006).
Other intervention
The review on the use of LTRAs yielded high‐certainty evidence showing a reduction in the respiratory rate at final assessment (MD ‐4.60, 95% CI ‐6.84 to ‐2.36; 50 participants; 1 trial) for those administered an oral LTRA (Watts 2012).
Secondary outcome: mechanical ventilation in the intensive care unit
A single review assessed the rates of patients receiving mechanical ventilation in the intensive care unit (Table 15).
Inhaled treatment
No reviews reported mechanical ventilation in the intensive care unit as an outcome.
Parenteral treatment
The review assessing the effectiveness of aminophylline presented very low‐certainty evidence suggesting an unclear effect on rates of patients mechanically ventilated in the intensive care unit (RR 0.09, 95% CI 0.01 to 1.64; 163 participants; 1 trial) (Mitra 2005).
Interventions to reduce the work of breathing
No reviews reported mechanical ventilation in the intensive care unit as an outcome.
Other intervention
No reviews reported mechanical ventilation in the intensive care unit as an outcome.
Secondary outcome: clinical failure
Clinical failure was presented for two reviews, both for parenteral treatment (Table 16). There were no comparisons for inhaled treatment, interventions to reduce the work of breathing, or other interventions.
Inhaled treatment
No reviews reported clinical failure as an outcome.
Parenteral treatment
The review comparing intravenous beta2‐agonists to intravenous aminophylline presented low‐certainty evidence suggesting an unclear effect on clinical failure (RR 1.00, 95% CI 0.48 to 2.08; 66 participants; 1 trial) (Travers 2012b).
The review comparing the addition of intravenous beta2‐agonists to inhaled beta2‐agonists presented moderate‐certainty evidence of a reduction in clinical failure with the addition of intravenous beta2‐agonists (RR 0.38, 95% CI 0.19 to 0.78; 29 participants; 1 trial) (Travers 2012a).
Interventions to reduce the work of breathing
No reviews reported clinical failure as an outcome.
Other intervention
No reviews reported clinical failure as an outcome.
Secondary outcome: relapse/return to ED
Four reviews each presented a single comparison related to relapse/return to the ED, although this was defined differently for each review (Table 17). Comparisons ranged from 22 participants in a single RCT to 1389 participants in ten RCTs.
Inhaled treatment
The addition of anticholinergic agents to inhaled beta2‐agonists yielded low‐certainty evidence suggesting an unclear effect on the risk of relapse (RR 1.07, 95% CI 0.68 to 1.68; 1389 participants; 10 trials) (Griffiths 2013).
The review on anticholinergic agents for children admitted to hospital was unable to estimate the risk of relapse within 72 hours of discharge from hospital because no events occurred in either treatment group (80 participants; 1 trial) (Vezina 2014).
Parenteral treatment
The review on intravenous magnesium sulfate demonstrated low‐certainty evidence suggesting an unclear effect on risk of return to the ED within 48 hours (RR 0.41, 95% CI 0.02 to 9.71; 85 participants; 2 trials) (Griffiths 2016) .
Interventions to reduce the work of breathing
No reviews reported relapse/return to ED as an outcome.
Other intervention
A review on the use of LTRAs presented low‐certainty evidence suggesting an unclear effect on relapse within seven days (RR 0.39, 95% CI 0.02 to 8.73; 22 participants; 1 trial) when oral LTRAs were compared to control (Watts 2012).
Secondary outcome: withdrawals
Four reviews presented a total of 1 comparisons related to treatment withdrawals (Table 18). Comparisons ranged from 143 participants in two RCTs to 371 participants in seven RCTs. No data were presented for interventions to reduce the work of breathing.
Inhaled treatment
The review on anticholinergic agents in children admitted to hospital presented low‐certainty evidence suggesting an unclear effect on overall withdrawals (RR 0.62, 95% CI 0.31 to 1.26; 294 participants; 2 trials) and very low‐certainty evidence suggesting an unclear effect on withdrawals due to deterioration (RR 2.04, 95% CI 0.38 to 10.89; 210 participants; 1 trial) (Vezina 2014).
Parenteral treatment
The review assessing effectiveness of intravenous aminophylline presented seven comparisons related to withdrawals (Mitra 2005). Moderate‐certainty evidence showed an unclear effect on withdrawals due to any cause (RR 1.65, 95% CI 0.67 to 4.07; 371 participants; 7 trials). Very low‐certainty evidence was provided for all other comparisons, including withdrawals due to adverse health effects and withdrawals due to poor asthma control (both overall and subgrouped by mean theophylline levels).
Interventions to reduce the work of breathing
No reviews reported withdrawals as an outcome.
Other intervention
The review on the use of LTRAs presented low‐certainty evidence suggesting an unclear effect on withdrawals for both oral LTRA and control (RR 1.00, 95% CI 0.16 to 6.34; 143 participants; 2 trials) and intravenous LTRA versus control (RR 1.13, 95% CI 0.31 to 4.12; 276 participants; 1 trial) (Watts 2012).
Secondary outcome: other measures of treatment effect
Six reviews presented a total of eight comparisons related to other diverse measures of treatment effect, including three with continuous outcomes (Table 19) and five with dichotomous outcomes (Table 20). Comparisons ranged from 27 participants in a single RCT to 1074 participants in nine RCTs.
Inhaled treatment
The review on anticholinergic agents added to SABAs for initial treatment of asthma identified nine studies showing moderate‐certainty evidence for a small benefit of the addition of anticholinergic agents in terms of the need for repeat bronchodilator treatment after standard protocol prior to disposition (RR 0.87, 95% CI 0.79 to 0.97; 1074 participants; 9 trials) and an unclear effect on the need for corticosteroids in the ED prior to disposition (RR 0.89, 95% CI 0.73 to 1.08; 378 participants; 2 trials) (Griffiths 2013).
The review on anticholinergic agents for children admitted to hospital presented low‐certainty evidence suggesting an unclear effect on the need for supplemental asthma therapy (RR 0.77, 95% CI 0.41 to 1.42; 465 participants; 4 trials) and an unclear effect on the time to SABA spaced at four hours or longer (MD ‐2.17, 95% CI ‐7.01 to 2.66; 290 participants; 2 trials) (Vezina 2014).
The review on continuous nebulisation of SABA compared to intermittent nebulisation of SABA demonstrated moderate‐certainty evidence showing a reduction in respiratory therapist time (MD ‐22.00, 95% CI ‐26.82 to ‐17.18; 70 participants; 1 trial) favouring continuous nebulisation (Camargo 2003).
Parenteral treatment
The addition of intravenous aminophylline to inhaled SABA and systemic corticosteroids demonstrated moderate‐certainty evidence showing an unclear effect on the number of nebulisers required in 24 hours (MD 0.15, 95% CI ‐0.52 to 0.83; 69 participants; 1 trial) (Mitra 2005).
The review on intravenous ketamine yielded low‐certainty evidence suggesting an unclear effect on the rate of participants worsening and requiring other adjuvant therapy (RR 2.12, 95% CI 0.2 to 22.31; 68 participants; 1 trial) (Jat 2012).
Interventions to reduce the work of breathing
No reviews reported other measures of treatment effect as an outcome.
Other interventions
The review on the use of LTRAs presented low‐certainty evidence suggesting an unclear effect on the requirement for additional care at the end of the RCT for oral LTRA versus control (RR 0.54, 95% CI 0.05 to 5.60; 50 participants; 1 trial) (Watts 2012).
Discussion
Summary of main results
In this overview, we examined evidence from published Cochrane Reviews on interventions for escalation of therapy for acute exacerbations of asthma in children. We synthesised the results of published Cochrane Reviews, and we identified significant gaps in a number of clinically important questions. We identified further randomised controlled trials (RCTs) that may be eligible for inclusion in review updates.
The primary outcomes of our review were length of stay, emergency department (ED) disposition, and adverse events.
Eight of the 13 included reviews provided data on ED or hospital length of stay; the only intervention shown to have a beneficial effect was intravenous magnesium sulfate (reduced hospital length of stay). Neither continuous versus intermittent nebulised short‐acting beta2‐agonists (SABAs) nor the addition of intravenous magnesium sulfate was shown to reduce ED treatment time. Moderate‐certainty evidence showed an unclear effect on hospital length of stay with the addition of intravenous beta‐agonists. All other comparisons yielded evidence of low or very low certainty, and fewer than 100 participants were included in all but one comparison; none demonstrated a clear difference in hospital length of stay.
Less than half of the included reviews had hospital admission as an included outcome; only three reviews provided information on intensive care unit admission, and none demonstrated a reduction in the risk of intensive care unit admission for any therapy.
The risk of hospital admission was reduced by the addition of inhaled anticholinergic agents (particularly in a multiple fixed‐dose protocol) to inhaled beta2‐agonists and by the use of intravenous magnesium sulfate. We found low‐certainty evidence for a small beneficial effect on the rate of hospital admission for inhaled heliox and no demonstrable effect on hospital admission for intravenous ketamine or leukotriene receptor antagonists (LTRAs).
Only 4 of the 13 included reviews provided information on overall adverse events. Apart from evidence of moderate certainty showing a reduction in overall serious adverse events during hospital admission when inhaled magnesium sulfate was added to usual bronchodilator therapy, there was low‐ or very low‐certainty evidence suggesting an unclear effect on adverse events between treatment groups.
Nausea and vomiting were more frequent with aminophylline. The addition of anticholinergic therapy to SABAs appeared to reduce the risk of nausea and tremor but not vomiting. Continuous nebulised SABAs were not associated with higher risk of tremor than intermittent dosing.
Secondary outcomes were variably reported in trials included in the reviews. Studies assessing spirometry and symptom scores used different measures, often applied at different times after initiation of study treatment.
Inhaled anticholinergics appeared to have a beneficial effect on symptom scores in ED treatment, but not after children had been admitted to hospital. Continuous nebulised SABAs, heliox, non‐invasive positive‐pressure ventilation, and oral LTRAs appeared to slightly improve respiratory scores, and inhaled magnesium and intravenous ketamine did not alter asthma scores. Aminophylline reduced symptom scores at six to eight hours, but this effect was not sustained.
Spirometric measures varied between studies, including peak expiratory flow rate (PEFR), forced expiratory volume in one second (FEV1), and changes in respiratory resistance. These measures were recorded at different times in different studies and were presented in various ways including percentage of predicted values and/or change from baseline. Effects of specific therapies appeared to be conflicting and inconclusive, or effects were no different from controls.
Many other comparisons reporting no significant evidence of benefit were based on single small trials or unpooled combinations of small trials. Due to lack of clear guidelines on minimal clinically significant differences, the clinical impact of statistically significant differences between comparison groups was unclear for many outcomes.
Overall completeness and applicability of evidence
This overview summarises published Cochrane Reviews of all RCTs examining interventions for escalation of therapy for acute exacerbations of asthma in children.
Key findings include the need to update a number of reviews, clinical heterogeneity between RCTs and reviews, and lack of consistent outcome measures between reviews. Clinical heterogeneity was evident with regard to study setting (inpatient units, emergency departments, and intensive care units), definition and assessment of asthma severity at the point of study entry, and inclusion criteria. The difference in response to treatment for young children with viral induced wheeze and asthma is a topic of increasing interest – future RCTs and reviews should specifically address this.
Outcome measures were inconsistent, and different measures were used at different times between RCTs and between reviews.
Given the wide range of treatment options, the heterogeneity of existing evidence, and gaps in the current literature, it is not surprising that practice varies between clinicians. In the absence of a robust evidence base, guidelines and clinical treatment will continue to be based upon other considerations including clinician experience, cost, adverse effects, and established local practices.
A further limitation of the included reviews is that only 3 of the 13 reviews had a date of last search in 2017 or later.
Quality of the evidence
Overall, 4 of the 13 included reviews were considered to be at high risk of bias. In all cases, this was due to concerns regarding identification and selection of studies.
The reviews used various tools to assess the risk of bias of included trials. Most assessments included a combination of low and unclear risk of bias.
Our assessment of the certainty of evidence varied widely (by review and also by outcome) and ranged from very low to high.
Potential biases in the overview process
We conducted the overview according to the published protocol, and we have highlighted any differences between our published protocol and the overview.
We identified potentially eligible reviews by applying the published search terms for each review, screening all titles and abstracts, and adding them to the evidence map (Table 6). It is possible that this list is incomplete, and that further comprehensive searches for each systematic review or update would identify additional eligible RCTs.
We included only Cochrane Reviews; there may be other systematic reviews on interventions for escalation of therapy for acute exacerbations of asthma in children published outside of the Cochrane Library, but we are unable to comment on that. Results and outcomes reported in non‐Cochrane Reviews may have showed different results.
Of greatest concern, the overview results are limited to the internal and external validity of the included reviews and trials. For example, there was a degree of heterogeneity between the various review authors in their application of assessment of the certainty of evidence using the GRADE classification, with similar appearing results rated differently by various review groups.
Agreements and disagreements with other studies or reviews
A 2015 overview of systematic reviews of acute asthma treatment in childhood included 28 systematic reviews on all aspects of acute asthma treatment in children (Castro‐Rodriguez 2015). The paper included reviews that we excluded, in particular, those related to established treatments such as use of short‐acting bronchodilators and systemic or inhaled corticosteroids. In addition, reviews were included if they included children or a mixed population (adults and children); our review extracted only data for children alone.
Castro‐Rodriguez 2015 reached similar conclusions compared to our overview: (1) the addition of ipratropium in children with moderate to severe exacerbations reduced hospital admission and improved clinical asthma score; (2) heliox‐driven nebulisers reduced exacerbation severity compared to oxygen‐driven nebulisers; and (3) intravenous magnesium sulfate reduced hospital admissions and improved lung function (Castro‐Rodriguez 2015).
Given the inclusion of systematic reviews addressing both mixed populations and children alone, it is unclear how readily results of the Castro‐Rodriguez 2015 overview may be extrapolated to a paediatric population (i.e. problem with indirectness).
A recently published review on the efficacy of macrolide antibiotics for children with an acute exacerbation of asthma/wheeze found no difference in hospitalisation between those given macrolides and those who received placebo (Pincheira 2020). The review (3 studies; 334 children) found more rapid resolution of symptoms in two of the three included studies, but review authors were unable to perform a meta‐analysis. Single studies reported reductions in symptom severity, reduced use of salbutamol, and improved lung function. Our overview includes a Cochrane Review on various antibiotics for the treatment of acute asthma (Normansell 2018); however, the more recent review differs from this by choice of outcome measure and choice of antibiotics.
Authors' conclusions
Implications for practice.
This overview provides the most up‐to‐date evidence on interventions for escalation of therapy for acute exacerbations of asthma in children from systematic reviews of RCTs. Four of the 13 included reviews were rated as being at high risk of bias. A vast majority of the 74 comparisons reported involved between one and three RCTs, with fewer than 100 participants.
The key findings of this overview, in terms of our primary outcomes are that:
intravenous magnesium sulfate was the only intervention shown to reduce hospital length of stay (high‐certainty evidence);
no intervention was demonstrated to reduce the risk of intensive care admission (low‐ to very low‐certainty evidence);
risk of hospital admission was reduced by the addition of inhaled anticholinergic agents to inhaled beta2‐agonists (moderate‐certainty evidence), the use of intravenous magnesium sulfate (high‐certainty evidence), and the use of inhaled heliox (low‐certainty evidence);
the addition of inhaled magnesium sulfate to usual bronchodilator therapy appears to reduce serious adverse events during hospital admission (moderate‐certainty evidence);
aminophylline increases vomiting compared to placebo (moderate‐certainty evidence) and increases nausea and nausea/vomiting compared to intravenous beta2‐agonists (low‐certainty evidence); and
the addition of anticholinergic therapy to SABAs appears to reduce the risk of nausea (high‐certainty evidence) and tremor (moderate‐certainty evidence) but not vomiting (low‐certainty evidence).
Comparative studies of the various treatment options are few; additional studies would enable us to draw firmer conclusions about which treatments are most effective. Further research is required to determine which patients are most likely to benefit from these therapies.
Implications for research.
Due to the relatively rare incidence of acute severe paediatric asthma, multi‐centre research on a national or international scale will be required to ensure that high‐quality evidence is generated.
Several of the included Cochrane Reviews need to be urgently updated. Further, we recommend that a new review into the use of high‐flow nasal oxygen therapy should be conducted.
The GRADE assessment between reviews was heterogeneous. We recommend that consistent methods of applying this within the review group should be developed.
More research should be conducted with preschool children separately, or results should be reported separately in studies with a combined population of younger and older children/adolescents.
Clinical heterogeneity with regard to study setting (inpatient units, emergency departments, and intensive care units) should be addressed by using reliable and reproducible assessments of asthma severity at the point of study entry, enabling consistent inclusion criteria. Reproducible bedside assessment of asthma severity – particularly in young children who are unable to perform reliable spirometry ‐ will also prove useful in determining response to treatment.
Currently, outcome measures are inconsistently used, and different measures are used at different times between studies and between reviews. The development of a core outcome set – with input from patients and families, clinicians, and researchers ‐ for future trials on acute severe asthma exacerbations in children is an international priority (Craig 2020); we believe this will allow for better comparisons between treatments in the future.
Once a set of core outcome measures is developed, agreement on clinically important differences will inform adequately powered studies able to determine which of the many available treatments is most effective for this group of children.
History
Protocol first published: Issue 3, 2018 Review first published: Issue 8, 2020
Acknowledgements
We thank Liz Stovold, Information Specialist, Cochrane Airways Group, for advice on search strategies for this overview. We also thank Yao Xu, Research Assistant, Monash University Department of Paediatrics, for assistance in conducting updated literature searches for each of the included reviews.
The review authors and the Airways Editorial Team,are grateful to the following peer reviewers for their time and comments.
Hebatullah M Abdulazeem (consumer), Germany.
Lt. Col. Dr George N Konstantinou, 424 General Military Training Hospital, Greece.
SK Kabra, India.
James Y Paton, University of Glasgow, UK.
Kenneth A Macleod, Royal Hospital for Sick Children Edinburgh, UK.
The project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Search strategy for Cochrane Database of Systematic Reviews
#1 MeSH descriptor: [Asthma] explode all trees
#2 MeSH descriptor: [Respiratory Sounds] this term only
#3 asthma*:ti,ab,kw
#4 #1 or #2 or #3
Appendix 2. Data collection tool
Details of the review
First author name
Year of publication
Number of included primary studies
Countries and years of original studies
Eligibility criteria of included studies
Numbers of included participants
Sample size of included RCTs
Details of included RCTs
Participant characteristics
Age
Severity of asthma
Definition of exacerbation of asthma for each RCT
Treatment before enrolment
Setting
Emergency department
Hospital ward
Intensive care unit
Types of interventions
Name of medication/intervention
Dose of medication/intervention
Duration of treatment
Frequency of intervention administration
Description of the comparative treatment (placebo, regular doses of bronchodilators)
Description of outcome measures used
For each outcome measure
Number of participants in intervention group
Number of participants in control group
Intervention event rate
Control event rate
Effect estimates for pooled results (risk ratio, odds ratio, hazard ratio, standardised mean difference, or absolute risk reduction and corresponding 95% confidence intervals)
-
Details of statistical tests for heterogeneity
Chi² test
I² test
Predefined primary outcome measures
-
Length of stay
Emergency department length of stay
Hospital length of stay
-
Emergency department disposition
Hospital admission
ICU admission
ED discharge
-
Adverse events
Vomiting
Nausea
Tremor
Tachycardia
Arrhythmia
Convulsion
Other (specify)
Predefined secondary outcome measures
-
Symptom scores/clinical asthma scores
Name of score
Definition/reference
Time of recording of outcome measure
Interpretation of score result (cut‐off)
-
Lung function tests
Examples: peak expiratory flow rate, forced expiratory volume in 1 second, and other measures
Name of test
Definition/reference
Time of recording of outcome measure
Interpretation of test result (cut‐off)
-
Vital signs
Examples: pulse, blood pressure, respiratory rate, pulse oximetry
Name of vital sign
Definition/reference
Time of recording of vital signs
Interpretation of vital signs results (cut‐off)
-
Requirement for additional bronchodilator treatment
Name of outcome measure
Definition/reference
Time of recording of outcome
Interpretation
-
Requirement for respiratory support
Intubation
Time of recording of outcome
-
Non‐invasive ventilation
Name of outcome measure (CPAP, BiPap, etc.)
Definition/reference
Time of recording of outcome
Interpretation
-
Economic outcomes/healthcare costs
Definition/reference
Time of recording of outcome
Interpretation
-
Additional outcome measures
Name of outcome measure
Definition/reference
Time of recording of outcome
Interpretation
Risk of bias assessments of RCTs included in the reviews
Quality assessment tools used (e.g. GRADE), along with the mean or median and range of any reported quality scores
Conclusions of each review
Review recommendations for further research
If the included systematic review includes all studies relevant to a particular outcome, we will extract summary data alone. If data are required to be extracted from a subgroup of studies (i.e. only children), then we will extract study‐level data from all RCTs included in the review. These data will include numerical primary study results and risk of bias data
Appendix 3. ROBIS tool
The ROBIS tool to assess risk of bias in systematic reviews consists of the following assessment criteria.
Phase 1. Assessing relevance (optional)
For intervention reviews, assessment of patients/populations; interventions; comparators; and outcomes.
Phase 2. Identifying concerns with the review process
Domain 1. Study eligibility criteria
Did the review adhere to predefined objectives and eligibility criteria?
Were the eligibility criteria appropriate for the review question?
Were eligibility criteria unambiguous?
Were all restrictions in eligibility criteria based on study characteristics appropriate?
Were any restrictions in eligibility criteria based on sources of information appropriate?
Domain 2. Identification and selection of studies
Did the search include an appropriate range of databases/electronic sources for published and unpublished reports?
Were methods additional to database searching used to identify relevant reports?
Were the terms and structure of the search strategy likely to retrieve as many eligible studies as possible?
Were restrictions based on date, publication format, or language appropriate?
Were efforts made to minimise error in selection of studies?
Domain 3. Data collection and study appraisal
Were efforts made to minimise errors in data collection?
Were sufficient study characteristics available for both review authors and readers to be able to interpret the results?
Were all relevant study results collected for use in the synthesis?
Was risk of bias (or methodological quality) formally assessed by appropriate criteria?
Were efforts made to minimise error in risk of bias assessment?
Domain 4. Synthesis and findings
Did the synthesis include all studies that it should?
Were all predefined analyses reported or departures explained?
Was the synthesis appropriate given the nature and similarity of research questions, study designs, and outcomes across included studies?
Was between‐study variation (heterogeneity) minimal or addressed in the synthesis?
Were the findings robust (e.g. as demonstrated through funnel plot or sensitivity analyses)?
Were biases in primary studies minimal or addressed in the synthesis?
We will rate each criterion as Y = Yes, PY = Probably yes, PN = Probably no, N = No, NI = No information.
We will then interpret each domain as having 'low', 'high', or 'unclear' concerns for bias.
Phase 3. Judging the risk of bias
Concerns from each domain are summarised.
We will then determine whether the conclusions are supported by the evidence presented.
Did interpretation of the findings address all concerns identified in domains one through four?
Was the relevance of identified studies to the review's research question appropriately considered?
Did the review authors avoid emphasising results on the basis of their statistical significance?
Risk of bias in the review? LOW/HIGH/UNCLEAR
Appendix 4. Categorised list of outcomes
| Outcome category | Outcome | Specific outcome measure used | Reviews |
| Length of stay | Emergency department length of stay | ED treatment time (units not specified) | Camargo 2003 |
| ED treatment time (minutes) | Griffiths 2016 | ||
| Hospital length of stay | Length of hospital stay (days) | Normansell 2018 | |
| Length of hospital stay (hours) | Mitra 2005; Travers 2012b; Vezina 2014; Griffiths 2016 | ||
| PICU length of stay | PICU length of stay (hours) | Travers 2012a | |
| Hospital admission | Hospital admission rate | Hospital admission rate | Camargo 2003; Rodrigo 2006; Jat 2012; Watts 2012; Griffiths 2013; Griffiths 2016; Knightly 2017 |
| Intensive care unit admission | Intensive care unit admission | HDU/ICU admission rates | Mitra 2005; Vezina 2014; Knightly 2017 |
| Adverse effects | All adverse effects | All adverse events | Normansell 2018 |
| Adverse health effects | Vezina 2014 | ||
| Any adverse event (during admission) | Knightly 2017 | ||
| Any adverse events | Knightly 2017 | ||
| Serious adverse events | Serious adverse events | Korang 2016; Knightly 2017; Normansell 2018 | |
| Serious adverse events (during admission) | Knightly 2017 | ||
| Death | Death | Mitra 2005 | |
| Mortality | Korang 2016 | ||
| Tremor | Tremor | Camargo 2003; Mitra 2005; Travers 2012b; Griffiths 2013 | |
| Nausea/vomiting | Nausea/vomiting | Camargo 2003; Travers 2012b | |
| Vomiting | Mitra 2005; Travers 2012b; Griffiths 2013 | ||
| Nausea | Travers 2012b; Griffiths 2013 | ||
| Cardiovascular adverse effects | Hypotension | Knightly 2017 | |
| Flushing | Knightly 2017 | ||
| Arrhythmia | Mitra 2005 | ||
| Dysrhythmia | Travers 2012b | ||
| Palpitations | Travers 2012b | ||
| Neurological adverse effects | Headache | Mitra 2005; Travers 2012b | |
| Seizures | Mitra 2005 | ||
| Pneumonia | Pneumonia | Korang 2016 | |
| Abnormal laboratory results | CPK elevation | Travers 2012b | |
| CPK‐MB elevation | Travers 2012b | ||
| Hyperglycaemia | Travers 2012b | ||
| Hypokalaemia | Travers 2012b | ||
| Symptom scores | Symptom scores | Symptom scores | Camargo 2003 |
| Change in clinical score at 120 minutes (± 30 minutes) | Griffiths 2013 | ||
| Change in pulmonary index score (final assessment) | Watts 2012 | ||
| Dyspnoea or pulmonary index | Rodrigo 2006 | ||
| Asthma clinical scores 8 to 36 hours after initial treatment | Vezina 2014 | ||
| Yung asthma severity score at 60 minutes | Knightly 2017 | ||
| Change in symptom scores 6 to 8 hours after enrolment | Mitra 2005 | ||
| Change in symptom scores 12 to 18 hours after enrolment | Mitra 2005 | ||
| Change in symptom scores 24 hours after enrolment | Mitra 2005 | ||
| Pulmonary index score | Jat 2012 | ||
| Asthma symptom score in the acute phase | Korang 2016 | ||
| Lung function | Peak expiratory flow | PEFR values (end of study) | Camargo 2003 |
| Peak expiratory flow (L/min) | Normansell 2018 | ||
| Percentages of predicted PEFR at 8 to 36 hours after initial treatment | Vezina 2014 | ||
| Pulmonary function PEF L/min | Knightly 2017 | ||
| Change in PEF 6 to 8 hours after enrolment | Mitra 2005 | ||
| Change in PEF 12 to 18 hours after enrolment | Mitra 2005 | ||
| Change in PEF 24 hours after enrolment | Mitra 2005 | ||
| Pulmonary function | Pulmonary function | Rodrigo 2006 | |
| Forced expiratory volume – one second | Change in %predicted FEV1 within 4 hours of enrolment | Mitra 2005 | |
| Change in %predicted FEV1 6 to 8 hours after enrolment | Mitra 2005 | ||
| Change in %predicted FEV1 12 to 18 hours after enrolment | Mitra 2005 | ||
| Change in %predicted FEV1 24 hours after enrolment | Mitra 2005 | ||
| Pulmonary function %predicted FEV1 | Knightly 2017 | ||
| Change in FEV1 (predicted) | Watts 2012 | ||
| Change in FEV1 (litres) | Watts 2012 | ||
| Change from baseline in %predicted FEV1, 60 minutes after last dose of inhaled bronchodilator | Griffiths 2013 | ||
| Change from baseline in %predicted FEV1, 120 minutes after last dose of inhaled bronchodilator | Griffiths 2013 | ||
| FEV1 or PEFR | %Change in FEV1 or PEFR at 60 minutes after last inhaled bronchodilator (± 15 minutes) | Griffiths 2013 | |
| %Change in FEV1 or PEFR at 120 minutes after last inhaled bronchodilator (± 30 minutes) | Griffiths 2013 | ||
| Respiratory resistance | %Change in respiratory resistance at 60 minutes after inhaled bronchodilator (± 15 minutes) | Griffiths 2013 | |
| %Change in respiratory resistance at 120 minutes after inhaled bronchodilator (± 30 minutes) | Griffiths 2013 | ||
| Vital signs | Oxygen saturations | Oxygen saturations < 95% at 60 minutes (± 15 minutes) | Griffiths 2013 |
| Oxygen saturations < 95% at 120 minutes (± 30 minutes) | Griffiths 2013 | ||
| SaO2 | Rodrigo 2006 | ||
| Pulse rate | Pulse rate (beats/min) at 2 hours | Travers 2012a | |
| Respiratory rate | Change in respiratory rate (breaths/min) (final assessment) | Watts 2012 | |
| Blood pressure | Blood pressure | Mitra 2005 | |
| Mechanical ventilation | Mechanical ventilation | Rates of patients mechanically ventilated in ICU | Mitra 2005 |
| Clinical failure | Clinical failure | Clinical failure | Travers 2012a; Travers 2012b |
| Relapse | Relapse/return to hospital | Relapse | Griffiths 2013 |
| Relapse within 7 days | Watts 2012 | ||
| Relapse within 72 hours of discharge from hospital | Vezina 2014 | ||
| Return to ED within 48 hours | Griffiths 2016 | ||
| Withdrawals | Withdrawals | Withdrawals | Watts 2012 |
| Overall withdrawals | Vezina 2014 | ||
| Withdrawals due to deterioration | Vezina 2014 | ||
| Withdrawals due to adverse health effects | Mitra 2005 | ||
| Withdrawals due to poor asthma control | Mitra 2005 | ||
| Treatment intensity | Respiratory therapist time | Respiratory therapist time | Camargo 2003 |
| Frequency of nebuliser treatment | Time to SABA spaced at 4 hours or longer (hours) | Vezina 2014 | |
| Number of nebulisers required in 24 hours | Mitra 2005 | ||
| Need for additional treatment | Need for further treatment after study medication | Need for repeat bronchodilator treatment after standard protocol prior to disposition | Griffiths 2013 |
| Need for corticosteroids in ED prior to disposition | Griffiths 2013 | ||
| Requirement for additional care at end of study | Watts 2012 | ||
| Need for supplemental asthma therapy | Vezina 2014 | ||
| Worsened and required other adjuvant therapy | Jat 2012 |
Footnotes
CPK: creatine phosphokinase; CPK‐MB: creatine phosphokinase myocardial band; ED: emergency department; FEV1: forced expiratory volume – one second; HDU: high‐dependency unit; ICU: intensive care unit; PEF: peak expiratory flow; PEFR: peak expiratory flow rate; PICU: paediatric intensive care unit; SABA: short‐acting beta2‐agonist.
Appendix 5. Conclusions and recommendations for future research from included reviews
| Intervention | Cochrane Review | Implications for practice | Implications for research |
| Additional inhaled bronchodilators | |||
| Continuous nebulised vs intermittent nebulised beta‐agonists | Camargo 2003 | Continuous SABA appears to be useful in severe acute asthma to reduce hospitalisation and improve lung function Continuous SABA appears to be safe and well tolerated |
Future trials should ‐ Assess optimal dose for continuous SABA ‐ Clearly define severity according to lung function and response to initial SABA therapy ‐ Include standard evidence‐based asthma therapy ‐ Concentrate on well‐defined outcomes, including criteria for admission/discharge, systematic reporting of lung function ‐ Clearly describe their methods |
| Combined inhaled anticholinergics and short‐acting beta‐agonists | Emergency department setting Griffiths 2013 |
Emergency department use of inhaled anticholinergics and SABA, when compared to SABA alone, results in ‐ Lower risk of hospital admission ‐ Greater improvement in lung function ‐ Less risk of nausea and tremor The combination of inhaled anticholinergics and SABA is likely to be more useful in moderate to severe exacerbations of asthma |
Future trials should ‐ Address severity of asthma at study entry, child's age, intensity/flexibility of therapy, dose of anticholinergics, and delivery device (nebuliser vs inhaler) ‐ Reliably define severity of asthma at study entry, with consideration of the use of clinical scores ‐ Include administration of systemic corticosteroids ‐ Include sensitive and reliable endpoints, such as number of bronchodilator inhalations, oxygenation, hospital length of stay, duration of need for intensive bronchodilator treatment, time to full recovery ‐ Assess effects in children with impending respiratory failure |
| Inpatient setting Vezina 2014 |
In children hospitalised for an acute asthma exacerbation, there is no evidence of benefit when nebulised anticholinergics were added to SABA | Future trials should ‐ Be of high methodological quality ‐ Compare different intensities of anticholinergic treatment ‐ Allow subgroup analyses based on age group (preschool vs school‐aged) and severity of asthma on admission ‐ Report changes from baseline in severity ‐ Systematically document adverse health effects and reasons for withdrawals ‐ Assess the efficacy of anticholinergics in children with impending respiratory failure |
|
| Inhaled magnesium sulfate | Knightly 2017 | Nebulised MgSO4 may result in modest additional benefits when added to inhaled SABA and anticholinergics, but there remains substantial uncertainty Nebulised MgSO4 does not appear to result in an increase in serious adverse events It is unclear whether nebulised MgSO4 is more effective in particular subgroups |
An agreement on the core outcomes for studies in acute asthma is needed, including physiological, cost, and those relevant to patients Future trials should ‐ Compare IV and inhaled MgSO4 ‐ Compare inhaled MgSO4 to placebo in patients not responding to standard maximal treatment ‐ Include economic evaluations of treatment options |
| Parenteral bronchodilators | |||
| Addition of intravenous beta2‐agonists to inhaled beta2‐agonists | Travers 2012a | Current evidence is insufficient to provide recommendations regarding the addition of IV SABA to inhaled SABA | Future trials should ‐ Be adequately powered and of high quality ‐ Assess outcomes when IV SABA is given in addition to maximal standard therapy (inhaled SABA + inhaled anticholinergics + systemic glucocorticoids) ‐ Consider evaluating subcutaneous routes for SABA administration ‐ Completely report clinically relevant outcomes ‐ Completely report pulmonary function tests ‐ Include outcomes important to patients ‐ Standardise reporting of asthma severity scores, adverse reactions, and side effects |
| Intravenous aminophylline | Mitra 2005 | In children with severe acute asthma unresponsive to maximised bronchodilation, supplemental oxygen, and systemic steroids, aminophylline improves lung function and symptoms within 6 to 8 hours of treatment Evidence is insufficient to confirm whether these benefits translate to more important outcomes |
Larger paediatric studies are needed Future studies should ‐ Compare aminophylline with placebo as add‐on therapy to maximised inhaled SABA/anticholinergics and early IV glucocorticoids ‐ Be sufficiently powered to examine the rate of intubation and mechanical ventilation in children ‐ Measure and report changes in lung function, symptoms, saturation, oxygen requirement, ICU admission rates, intubation rates, and side effects at various points in time (e.g. 4, 6, 12, and 24 hours) |
| Intravenous magnesium sulfate | Griffiths 2016 | IV MgSO4 may reduce the need for hospital admission in children presenting to the ED with moderate to severe exacerbations of asthma Evidence is limited due to the number and size of studies |
Widespread use of internationally agreed core outcome sets would facilitate future meta‐analyses Future studies should ‐ Classify treatment by severity to power studies adequately to detect subgroup differences ‐ Use pragmatic markers of severity ‐ Use practical outcome measures not dependent on spirometry. |
| Intravenous beta2‐agonists vs intravenous aminophylline | Travers 2012b | There was no consistent evidence favouring either IV SABA or IV aminophylline for patients with acute asthma | Future studies should ‐ Assess outcomes when IV SABA or IV aminophylline is given in addition to standard evidence‐based therapy for acute severe asthma (inhaled SABA + inhaled anticholinergic + systemic glucocorticoids) ‐ Assess baseline asthma management ‐ Be adequately powered ‐ Completely and systematically report pulmonary function test data ‐ Include measures important to patients ‐ Standardise reporting of asthma symptoms, asthma severity scores, and adverse reactions/side effects ‐ Utilise well‐defined outcomes |
| Ketamine | Jat 2012 | A single study on non‐intubated children did not show significant benefit for ketamine in acute asthma There were no studies in ventilated children |
Future studies should ‐ Be sufficiently powered and of high methodological quality ‐ Use objective outcome measures of clinical importance ‐ Explore different doses of ketamine ‐ Assess the role of ketamine in children requiring mechanical ventilation due to severe asthma |
| Interventions to reduce the work of breathing | |||
| Heliox | Rodrigo 2006 | Current evidence does not suggest a clear benefit for the administration of heliox to all ED patients with acute asthma Heliox may be useful in those with highest severity of acute asthma; however, other evidence‐based treatments should take precedence |
Future trials should ‐ Clearly define severity – ideally based on pulmonary function results and response to initial SABA therapy ‐ Assess the effects of heliox in very young children ‐ Assess the effects of heliox based on prior inhaled steroid use ‐ Utilise well‐defined outcomes, including criteria for discharge and reporting of lung function test data ‐ Clearly describe their methods |
| Non‐invasive positive‐pressure ventilation | Korang 2016 | Evidence regarding effects of NPPV in children with acute asthma is insufficient NPPV can be considered as an add‐on therapy to standard care, but its use is controversial and additional research is required |
High‐quality RCTs are needed Future trials should ‐ Be adequately powered ‐ Have low risk of bias ‐ Consider the use of blinding (i.e. sham NPPV) ‐ Assess all‐cause mortality, serious adverse events, asthma symptom scores, and quality of life |
| Other interventions | |||
| Antibiotics | Normansell 2018 | Limited evidence suggests that antibiotics given at the time of an asthma exacerbation may improve symptoms and PEFR at follow‐up compared with standard care or placebo Findings are inconsistent and confidence in effect estimates is low Antibiotics should not routinely be used for acute exacerbations of asthma |
Carefully weigh up the benefits of future research in cohorts for whom antibiotics are not currently recommended against the harms of antibiotic overuse Core outcome sets, including patient important outcomes, should be used Future trials should ‐ Provide details of asthma severity and presenting symptoms ‐ Stratify by inflammatory marker measurement (e.g. CRP) ‐ Pay careful attention to adverse event data |
| Leukotriene receptor antagonists | Watts 2012 | Evidence does not support routine use of LTRAs in acute asthma | Additional high‐quality studies are needed Future trials should ‐ Address safety of higher than standard doses of LTRAs and IV use of LTRAs ‐ Assess the benefit of additional LTRAs in those already taking long‐term LTRAs ‐ Assess health economic and clinical outcomes ‐ Assess the effect of commencing LTRAs early on exacerbation of asthma ‐ Assess response to LTRAs in children with different phenotypes of acute wheeze |
Footnotes
CRP: C‐reactive protein; ED: emergency department; IV: intravenous; LTRAs: leukotriene receptor antagonists; MgSO4: magnesium sulfate; NPPV: non‐invasive positive‐pressure ventilation; PEFR: peak expiratory flow rate; RCT: randomised controlled trial; SABA: short‐active beta2‐agonists.
Appendix 6. ROBIS assessment of included reviews
| QUALITY ASSESSMENT | Carmargo 2003 | Griffiths 2013 | Normansell 2018 | Travers 2012a | Watts 2012 | Rodrigo 2006 | Vezina 2014 | Knightly 2017 | Mitra 2005 | Travers 2012b | Griffiths 2016 | Jat 2012 | Korang 2016 |
| Domain 1. Study eligibility criteria | |||||||||||||
| • Did the review adhere to predefined objectives and eligibility criteria? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were the eligibility criteria appropriate for the review question? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were eligibility criteria unambiguous? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | PY | Yes | PY | Yes |
| • Were all restrictions in eligibility criteria based on study characteristics appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | PY | Yes | Yes | Yes | Yes | Yes |
| • Were any restrictions in eligibility criteria based on sources of information appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| DOMAIN 1 ‐ OVERALL | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern |
| Domain 2. Identification and selection of studies | |||||||||||||
| • Did the search include an appropriate range of databases/electronic sources for published and unpublished reports? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were methods additional to database searching used to identify relevant reports? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were the terms and structure of the search strategy make it likely to retrieve as many eligible studies as possible? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were restrictions based on date, publication format, or language appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were efforts made to minimise error in selection of studies? | Yes | Noa | Yes | Yes | Yes | Yes | Nob | Noc | Nod | Yes | Yes | Yes | Yes |
| DOMAIN 2 ‐ OVERALL | Low concern | High concern | Low concern | Low concern | Low concern | Low concern | High concern | High concern | High concern | Low concern | Low concern | Low concern | Low concern |
| Domain 3. Data collection and study appraisal | |||||||||||||
| • Were efforts made to minimise errors in data collection? | PY | PY | Yes | PY | Yes | PY | PY | Yes | NIe | Yes | Yes | Yes | Yes |
| • Were sufficient study characteristics available for both review authors and readers to be able to interpret the results? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were all relevant study results collected for use in the synthesis? | Yes | PY | PY | Yes | PY | PY | PY | Yes | PY | PY | Yes | Yes | PY |
| • Was risk of bias (or methodological quality) formally assessed by appropriate criteria? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were efforts made to minimise error in risk of bias assessment? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| DOMAIN 3 ‐ OVERALL | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Unclear concern | Low concern | Low concern | Low concern | Low concern |
| Domain 4. Synthesis and findings | |||||||||||||
| • Did the synthesis include all studies that it should? | Yes | PY | Yes | Yes | Yes | Yes | Yes | PY | Yes | PY | PY | Yes | Yes |
| • Were all predefined analyses reported or departures explained? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Was the synthesis appropriate given the nature and similarity of research questions, study designs, and outcomes across included studies? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Was between‐study variation (heterogeneity) minimal or addressed in the synthesis? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Were the findings robust (e.g. as demonstrated through funnel plot or sensitivity analyses)? | Yes | Yes | Yes | No | No | No | No | Yes | No | No | No | No | No |
| • Were biases in primary studies minimal or addressed in the synthesis? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| DOMAIN 4 ‐ OVERALL | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern | Low concern |
| Final assessment of bias | |||||||||||||
| • Did interpretation of the findings address all concerns identified in domains one through four? | Yes | No | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes |
| • Was the relevance of identified studies to the review's research question appropriately considered? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| • Did the review authors avoid emphasising results on the basis of their statistical significance? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| RISK OF BIAS IN THE REVIEW | LOW RISK | HIGH RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | HIGH RISK | HIGH RISK | HIGH RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK |
Abbreviations: NI: no information; PY: probably yes.
Footnotes
a"One review author examined each new citation and classified it as clearly included, possibly included, or clearly not an RCT".
bOne review author independently reviewed each abstract and annotated each as (1) an RCT; (2) clearly not an RCT; or (3) unclear.
cAnother author independently decided on trial inclusion using predetermined eligibility criteria.
dEach abstract was reviewed by one reviewer and was annotated as (1) an RCT, (2) a possible RCT, (3) clearly not an RCT.
eNo information was provided on who extracted data or whether a form was used.
Differences between protocol and review
We were unable to present data on the use of high‐flow nasal cannulae, as no systematic review on its use in asthma was identified.
We did not extract from each review individual trial data related to either the year each trial included in each review was conducted or the country in which the research occurred.
We did not summarise the range of I² variation, as this was accounted for in the GRADE assessment of the certainty of evidence.
We did not map systematic reviews to their included RCTs as we presented summary statistics for each intervention rather than data from individual RCTs.
Searching for potentially eligible studies to update each included review was restricted to titles and abstracts; we did not obtain the full text of potentially eligible papers, nor did we extract data for inclusion in the GRADE assessment of certainty of evidence.
We did not use any figures to present data.
We did not present a single 'Summary of findings' table, as the results are summarised in relevant additional tables within the text.
Contributions of authors
SC – screening search, extracting data, conducting ROBIS classification, drafting tables and results, drafting discussion, co‐ordinating team and process, editing review sections drafted by others.
SRD – contributing clinical expertise to team discussions, extracting data, conducting ROBIS classification, editing review sections drafted by others.
CVEP ‐ contributing clinical expertise to team discussions, extracting data, conducting ROBIS classification, editing review sections drafted by others.
AG ‐ screening search, extracting data, conducting ROBIS classification, editing review sections drafted by others.
FEB ‐ contributing clinical expertise to team discussions, extracting data, conducting ROBIS classification, editing review sections drafted by others.
CL – contributing methodological overview of review expertise to team discussions, providing critical comments on data extraction tool and piloting extraction, drafting the protocol and review drafts, editing review sections drafted by others.
Contributions of editorial team
Chris Cates (Co‐ordinating Editor) edited the protocol; advised on methodology; approved the protocol prior to publication.
Katharine Pike (Contact Editor) edited the review; advised on methodology, interpretation, and content.
Emma Dennett (Managing Editor) co‐ordinated the editorial process; advised on interpretation and content; edited the review.
Emma Jackson (Assistant Managing Editor) conducted peer review; edited the review, particularly the reference sections of the protocol and review.
Elizabeth Stovold (Information Specialist) reviewed the search strategy and the search methods section.
Sources of support
Internal sources
-
Monash Health, Australia
AG's salary is paid by Monash Health
SC's salary is partly funded by Monash Health
-
Monash University, Australia
SC's salary is partly funded by Department of Paediatrics, Monash University
-
Auckland District Health Board, New Zealand
SRD's salary is paid in part by Auckland District Health Board
-
University of Auckland, New Zealand
SRD's salary is paid in part by the University of Auckland
-
Sidra Medicine, Qatar
CVP's salary is paid by Sidra Health
-
Royal Children's Hospital Foundation, Melbourne, Australia
FEB's time was funded in part by a grant from the Royal Children's Hospital Foundation, Melbourne, Australia
-
Murdoch Children's Research Institute, Australia
FEB's time was funded in part by a Melbourne Children's Clinician Scientist Fellowship, administered by the Murdoch Children's Research Institute, Melbourne, Australia
-
University of British Columbia, Canada
CL's salary is paid by the Therapeutics Initiative, University of British Columbia
External sources
-
Australasian College for Emergency Medicine Foundation, Australia
SC's time is funded in part by the Al Spilman Early Career Researcher Grant from the ACEM Foundation
-
National Health and Medical Research Council, Canberra, Australia, Australia
FEB's time is funded in part by Practitioner Fellowship GNT1124466 SRD, FEB, SC, and AG are members of the Paediatric Research in Emergency Departments International Collaborative (PREDICT) Network, which is funded by a Centre of Research Excellence grant (GNT1058560).
-
Cure Kids New Zealand, New Zealand
SRD's time is funded in part by Cure Kids New Zealand
Declarations of interest
SC: none known.
SRD: none known.
CP is a review author on an included review (Knightly 2017).
AG: none known.
FEB: none known.
CL: no conflicts of interest.
New
References
References to included reviews
Camargo 2003
- Camargo CA Jr, Spooner CH, Rowe BH. Continuous versus intermittent beta-agonists in the treatment of acute asthma. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No: CD001115. [DOI: 10.1002/14651858.CD001115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 2013
- Griffiths B, Ducharme FM. Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD000060. [DOI: 10.1002/14651858.CD000060.pub2] [DOI] [PubMed] [Google Scholar]
Griffiths 2016
- Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD011050. [DOI: 10.1002/14651858.CD011050.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jat 2012
- Jat KR, Chawla D. Ketamine for management of acute exacerbations of asthma in children. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD009293. [DOI: 10.1002/14651858.CD009293.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knightly 2017
- Knightly R, Milan SJ, Hughes R, Knopp-Sihota JA, Rowe BH, Normansell R, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD003898. [DOI: 10.1002/14651858.CD003898.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Korang 2016
- Korang SK, Feinberg J, Wetterslev J, Jakobsen JC. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD012067. [DOI: 10.1002/14651858.CD012067.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitra 2005
- Mitra A, Bassler D, Goodman K, Lasserson TJ, Ducharme FM. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD001276. [DOI: 10.1002/14651858.CD001276.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Normansell 2018
- Normansell R, Sayer B, Waterson S, Dennett EJ, Del Forno M, Dunleavy A. Antibiotics for exacerbations of asthma. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD002741. [DOI: 10.1002/14651858.CD002741.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodrigo 2006
- Rodrigo G, Pollack C, Rodrigo C, Rowe BH. Heliox for nonintubated acute asthma patients. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD002884. [DOI: 10.1002/14651858.CD002884.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Travers 2012a
- Travers AH, Milan SJ, Jones AP, Camargo CA Jr, Rowe BH. Addition of intravenous beta2-agonists to inhaled beta2-agonists for acute asthma. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD010179. [DOI: 10.1002/14651858.CD010179] [DOI] [PMC free article] [PubMed] [Google Scholar]
Travers 2012b
- Travers AH, Jones AP, Camargo CA Jr, Milan SJ, Rowe BH. Intravenous beta(2)-agonists versus intravenous aminophylline for acute asthma. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD010256. [DOI: 10.1002/14651858.CD010256] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vezina 2014
- Vezina K, Chauhan BF, Ducharme FM. Inhaled anticholinergics and short-acting beta2-agonists versus short-acting beta2-agonists alone for children with acute asthma in hospital. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD010283. [DOI: 10.1002/14651858.CD010283.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watts 2012
- Watts K, Chavasse RJPG. Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD006100. [DOI: 10.1002/14651858.CD006100.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to excluded reviews
Jones 2001
- Jones A, Rowe B, Peters J, Camargo C, Hammarquist C, Rowe B. Inhaled beta-agonists for asthma in mechanically ventilated patients. Cochrane Database of Systematic Reviews 2001, Issue 4. Art. No: CD001493. [DOI: 10.1002/14651858.CD001493] [DOI] [PubMed] [Google Scholar]
Manser 2001
- Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database of Systematic Reviews 2001, Issue 1. Art. No: CD001740. [DOI: 10.1002/14651858.CD001740] [DOI] [PubMed] [Google Scholar]
Rowe 2000
- Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CAJr. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2000, Issue 1. Art. No: CD001490. [DOI: 10.1002/14651858.CD001490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Travers 2001
- Travers A, Jones AP, Kelly K, Barker SJ, Camargo CA, Rowe BH. Intravenous beta2-agonists for acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2001, Issue 2. Art. No: CD002988. [DOI: 10.1002/14651858.CD002988] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Acworth 2009
- Acworth J, Babl F, Borland M, Ngo P, Krieser D, Schutz J, et al. Patterns of presentation to the Australian and New Zealand Paediatric Emergency Research Network. Emergency Medicine Australasia 2009;21(1):59-66. [DOI] [PubMed] [Google Scholar]
Adachi 2012
- Adachi M, Taniguchi H, Tohda Y, Sano Y, Ishine T, Smugar SS, et al. The efficacy and tolerability of intravenous montelukast in acute asthma exacerbations in Japanese patients. Journal of Asthma 2012;49(6):649-56. [DOI] [PubMed] [Google Scholar]
Alpern 2006
- Alpern ER, Stanley RM, Gorelick MH, Donaldson A, Knight S, Teach SJ, et al. Epidemiology of a pediatric emergency medicine research network: the PECARN core data project. Pediatric Emergency Care 2006;22(10):689-99. [DOI] [PubMed] [Google Scholar]
Asthma UK
- Asthma UK. Asthma facts and statistics. www.asthma.org.uk/about/media/facts-and-statistics/ (accessed 3 August 2017).
Aubuchon 2012
- Aubuchon K, Matsuda K, House L, Ferguson I, Schneider J, Lewis L. Increased serum albuterol concentrations may be associated with elevations of serum lactate in subjects with acute asthma exacerbations. Academic Emergency Medicine 2012;19:S312‐3. [Google Scholar]
Babl 2008
- Babl FE, Sheriff N, Borland M, Acworth J, Neutze J, Krieser D, et al. Paediatric acute asthma management in Australia and New Zealand: practice patterns in the context of clinical practice guidelines. Archives of Disease in Childhood 2008;93(4):307-12. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Baudin 2017
- Baudin F, Buisson A, Vanel B, Massenavette B, Pouyau R, Javouhey E. Nasal high flow in management of children with status asthmaticus: a retrospective observational study. Annals of Intensive Care 2017;7(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 1984
- Becker AB, Nelson NA, Simons FE. The pulmonary index. Assessment of a clinical score for asthma. American Journal of Diseases of Children 1984;138(6):574-6. [DOI] [PubMed] [Google Scholar]
Biagini Myers 2015
- Biagini Myers JM, Simmons JM, Kercsmar CM, Martin LJ, Pilipenko VV, Austin SR, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio Pediatric Asthma Repository. Pediatrics 2015;135(2):271-9. [DOI] [PubMed] [Google Scholar]
Bigham 2010
- Bigham MT, Jacobs BR, Monaco MA, Brilli RJ, Wells D, Conway EM, et al. Helium/oxygen-driven albuterol nebulization in the management of children with status asthmaticus: a randomized, placebo-controlled trial. Pediatric Critical Care Medicine 2010;11(3):356-61. [PubMed] [Google Scholar]
Brandão 2011
- Brandão DC, Britto MC, Pessoa MF, Sá Rafaela B, Alcoforado L, Matos LO, et al. Heliox and forward-leaning posture improve the efficacy of nebulized bronchodilator in acute asthma: a randomized trial. Respiratory Care 2011;56(7):947-52. [DOI] [PubMed] [Google Scholar]
BTS/SIGN 2019
- SIGN158 - British guideline on the management of asthma: a national clinical guideline. First published 2003. Revised edition published July 2019. www.brit-thoracic.org.uk/document-library/guidelines/asthma/btssign-guideline-for-the-management-of-asthma-2019/ (accessed prior to 3 March 2020).
Castro‐Rodriguez 2015
- Castro-Rodriguez JA, Rodrigo JG, Rodríguez-Martínez CE. Principal findings of systematic reviews of acute asthma treatment in childhood. Journal of Asthma 2015;52(10):1038-45. [DOI] [PubMed] [Google Scholar]
Caudri 2009
- Caudri D, Wijga A, Schipper CM, Hoekstra M, Postma DS, Koppelman GH, et al. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. Journal of Allergy and Clinical Immunology 2009;124(5):903-10.e1-7. [DOI] [PubMed] [Google Scholar]
Cave 2014
- Cave AJ, Atkinson LL. Asthma in preschool children: a review of the diagnostic challenges. Journal of the American Board of Family Medicine 2014;27(4):538-48. [DOI] [PubMed] [Google Scholar]
Chaudhury 2017
- Chaudhury A, Gaude GS, Hattiholi J. Effects of oral montelukast on airway function in acute asthma: a randomized trial. Lung India 2017;34(4):349-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2008
- Chen Z-G, Li M, Chen H, Chen Y-F, Chen F-H, Ji J-Zhi. Efficacy of pulmicort suspension plus salbutamol and ipratropium bromide for management of acute asthma exacerbation in children: a comparative study. Journal of Southern Medical University 2008;28(3):470-2. [PubMed] [Google Scholar]
Craig 2018
- Craig SS, Dalziel SR, Powell CVE, Graudins A, Babl FE, Lunny C. Interventions for escalation of therapy for acute exacerbations of asthma in children: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD012977. [DOI: 10.1002/14651858.CD012977] [DOI] [PMC free article] [PubMed] [Google Scholar]
Craig 2020
- Craig S, Babl FE, Dalziel SR, Gray C, Powell C, Al Ansari K, et al. Acute severe paediatric asthma: study protocol for the development of a core outcome set, a Pediatric Emergency Reserarch Networks (PERN) study. Trials 2020;21(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Avila 2008
- D'Avila RS, Piva JP, Marostica PJ, Amantea SL. Early administration of two intravenous bolus of aminophylline added to the standard treatment of children with acute asthma. Respiratory Medicine 2008;102(1):156-61. [DOI] [PubMed] [Google Scholar]
Ducharme 2008
- Ducharme FM, Chalut D, Plotnick L, Savdie C, Kudirka D, Zhang X, et al. The pediatric respiratory assessment measure: a valid clinical score for assessing acute asthma severity from toddlers to teenagers. Journal of Pediatrics 2008;152(4):476-80, 480.e1. [DOI] [PubMed] [Google Scholar]
Ferguson 2006
- Ferguson C, Gidwani S. Best evidence topic reports. Delivery of bronchodilators in acute asthma in children. Emergency Medicine Journal 2006;23(6):471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2019
- Global Initiative for Asthma. 2019 GINA Report, Global Strategy for Asthma Management and Prevention 2019. https://ginasthma.org/gina-reports/ (accessed prior to 3 March 2020).
Giordano 2012
- Giordano K, Rodriguez E, Green N, Armani M, Richards J, Shaffer TH, et al. Pulmonary function tests in emergency department pediatric patients with acute wheezing/asthma exacerbation. Pulmonary Medicine 2012;2012:724139. [DOI: 10.1155/2012/724139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goyal 2013
- Goyal S, Agrawal A. Ketamine in status asthmaticus: a review. Indian Journal of Critical Care Medicine 2013;17(3):154-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
GradePro 2015 [Computer program]
- GRADEpro GDT. Version accessed prior to 3 March 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2019
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019a
- Higgins JPT, Li T, Deeks JJ. Chapter 6. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hon 2017
- Hon KL, Leung AK. Management of status asthmaticus in children. Recent Patents on Inflammation and Allergy Drug Discovery 2017;11(1):12-21. [DOI] [PubMed] [Google Scholar]
House 2015
- House SL, Matsuda K, O'Brien G, Makhay M, Iwaki Y, Ferguson I, et al. Efficacy of a new intravenous β2-adrenergic agonist (bedoradrine, MN-221) for patients with an acute exacerbation of asthma. Respiratory Medicine 2015;109(10):1268-73. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
Kanchanateeraphong 2014
- Kanchanateeraphong S, Phongsamart G, Dangsuwan T, Vangveeravong M. Study of montelukast for the treatment of acute and post viral-induced wheezing. Journal of Allergy and Clinical Immunology 2014;133(2 Suppl 1):AB190. [Google Scholar]
Kelly 2004
- Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respiratory Medicine 2004;98(8):777-81. [DOI] [PubMed] [Google Scholar]
Kenyon 2014
- Kenyon CC, Fieldston ES, Luan X, Keren R, Zorc JJ. Safety and effectiveness of continuous aerosolized albuterol in the non-intensive care setting. Pediatrics 2014;134(4):e976-82. [DOI] [PubMed] [Google Scholar]
Konstantinou 2013
- Konstantinou GN, Xepapadaki P, Manousakis E, Makrinioti H, Kouloufakou-Gratsia K, Saxoni-Papageorgiou P, et al. Assessment of airflow limitation, airway inflammation, and symptoms during virus-induced wheezing episodes in 4- to 6-year-old children. Journal of Allergy and Clinical Immunology 2013;131(1):87-93.e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyttle 2015
- Lyttle MD, O'Sullivan R, Doull I, Hartshorn S, Morris I, Powell CV. Variation in treatment of acute childhood wheeze in emergency departments of the United Kingdom and Ireland: an international survey of clinician practice. Archives of Disease in Childhood 2015;100(2):121-5. [DOI] [PubMed] [Google Scholar]
Magazine 2016
- Magazine R, Shahul HA, Chogtu B, Kamath A. Comparison of oral montelukast with oral zileuton in acute asthma: a randomized, double-blind, placebo-controlled study. Lung India 2016;33(3):281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magazine 2018
- Magazine R, Surendra VU, Chogtu B. Comparison of oral montelukast with oral ozagrel in acute asthma: a randomized, double-blind, placebo-controlled study. Lung India 2018;35(1):16-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandhane 2017
- Mandhane PJ, Paredes Zambrano de Silbernagel P, Aung YN, Williamson J, Lee Bonita E, Spier S, et al. Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: a placebo-controlled randomized trial. PloS One 2017;12(8):e0182411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 1995
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. New England Journal of Medicine 1995;332(3):133-8. [DOI] [PubMed] [Google Scholar]
Matsuse 2012
- Matsuse H, Fukahori S, Tsuchida T, Kawano T, Tomari S, Matsuo N, et al. Effects of a short course of pranlukast combined with systemic corticosteroid on acute asthma exacerbation induced by upper respiratory tract infection. Journal of Asthma 2012;49(6):637-41. [DOI] [PubMed] [Google Scholar]
Memon 2016
- Memon BN, Parkash AT, Ahmed KKM, Gowa Murtaza A, Bai C. Response to nebulized salbutamol versus combination with ipratropium bromide in children with acute severe asthma. Journal of Pakiston Medical Association 2016;66(3):243-6. [PubMed] [Google Scholar]
Morimoto 2018
- Morimoto K, Shimizu N, Saito O, Kodani T, Iki Y, Nagai Y, et al. The impact of heliox on obstructive airway diseases with mechanical ventilation. A pilot study. Pediatric Critical Care Medicine 2018;19(6 Suppl 1):46. [Google Scholar]
Morris 2015
- Morris I, Lyttle MD, O'Sullivan R, Sargant N, Doull IJ, Powell CV. Which intravenous bronchodilators are being administered to children presenting with acute severe wheeze in the UK and Ireland? Thorax 2015;70(1):88-91. [DOI] [PubMed] [Google Scholar]
Motamed 2017
- Motamed H, Verki MM, Pooladzade M, Majidi A. Efficacy evaluation of nebulized magnesium, as an additional complementary treatment, in clinical and peak flow metric improvements of acute asthma attack: a randomized double-blinded clinical trial. Jundishapur Journal of Natural Pharmaceutical Products 2017;12:4. [Google Scholar]
Mustafa 2017
- Mustafa J, Iqbal SMJ, Azhar IA, Sultan MA. Nebulized magnesium sulphate as an adjunct therapy in the management of children presenting with acute exacerbation of asthma. Pakistan Journal of Medical and Health Sciences 2018;12(2):554‐5. [Google Scholar]
Naao 2007
- Naao M, Katsunuma T, Kim KC, Fujisawa T. Efficacy and safety of intravenous aminophylline in children with acute exacerbation of asthma: a multicenter randomized trial. Journal of Allergy and Clinical Immunology 2007;119(1 Suppl 2):8. [Google Scholar]
Naspitz 1987
- Naspitz CK, Sole D, Wandalsen N. Treatment of acute attacks of bronchial asthma. A comparative study of epinephrine (subcutaneous) and fenoterol (inhalation). Annals of Allergy 1987;59(1):21-4. [PubMed] [Google Scholar]
Nath 2015
- Nath JB, Hsia RY. Children's emergency department use for asthma, 2001-2010. Academic Pediatrics 2015;15(2):225-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
National Asthma Council Australia 2019
- National Asthma Council Australia. Australian Asthma Handbook, Version 2.0. National Asthma Council Australia, Melbourne, 2019. https://www.asthmahandbook.org.au/about (accessed prior to 3 March 2020).
Navanandan 2017
- Navanandan Ni, Federico M, Mistry RD. Positive expiratory pressure for the treatment of acute asthma exacerbations: a randomized controlled trial. Journal of Pediatrics 2017;185:149-54.e2. [DOI] [PubMed] [Google Scholar]
Neame 2015
- Neame M, Aragon O, Fernandes RM, Sinha I. Salbutamol or aminophylline for acute severe asthma: how to choose which one, when and why? Archives of Disease in Childhood. Education and Practice Edition 2015;100(4):215-22. [DOI] [PubMed] [Google Scholar]
NHLBI 2007
- National Heart, Lung, and Blood Institute (NHLBI). National Asthma Education and Prevention Program Expert Panel Report 3: guidelines for the diagnosis and management of asthma 2007. www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/full-report (accessed 3 August 2017).
O'Connor 2014
- O'Connor MG, Saville BR, Hartert TV, Arnold DH. Treatment variability of asthma exacerbations in a pediatric emergency department using a severity-based management protocol. Clinical Pediatrics 2014;53(13):1288-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ortiz 2012
- Ortiz JA, Fonseca JS, Salas-Segura DA. Use of heliox in moderate and severe asthma attacks in the emergency service Rafael Angel Calderon Guardia Hospital. A prospective study. Revista del Instituto Nacional de Enfermedades Respiratorias 2012;71(1):21-8. [Google Scholar]
Papadopoulos 2012
- Papadopoulos NG, Arakawa H, Carlsen KH, Custovic A, Gern J, Lemanske R, et al. International consensus on (ICON) pediatric asthma. Allergy 2012;67(8):976-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parkin 1996
- Parkin PC, Macarthur C, Saunders NR, Diamond SA, Winders PM. Development of a clinical asthma score for use in hospitalized children between 1 and 5 years of age. Journal of Clinical Epidemiology 1996;49(8):821-5. [DOI] [PubMed] [Google Scholar]
Pincheira 2020
- Pincheira MA, Bacharier LB, Castro-Rodriguez JA. Efficacy of macrolides on acute asthma or wheezing exacerbations in children with recurrent wheezing: a systematic review and meta-analysis. Pediatric Drugs 2020;22:217-28. [DOI] [PubMed] [Google Scholar]
Pollack 2018
- Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V. Overviews of reviews. Draft version (8 October 2018) for inclusion. in: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch V (editors). Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane. Available from www.training.cochrane.org/handbook.
Powell 2003
- Powell CV, Kelly AM, Kerr D. Lack of agreement in classification of the severity of acute asthma between emergency physician assessment and classification using the National Asthma Council Australia guidelines (1998). Emergency Medicine 2003;15(1):49-53. [DOI] [PubMed] [Google Scholar]
Powell 2012
- Powell C, Dwan K, Milan SJ, Beasley R, Hughes R, Knopp-Sihota JA, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD003898. [DOI: 10.1002/14651858.CD003898.pub5] [DOI] [PubMed] [Google Scholar]
Rees 1967
- Rees HA, Millar JS, Donald KW. Adrenaline in bronchial asthma. Lancet 1967;2(7527):1164-7. [DOI] [PubMed] [Google Scholar]
Rehder 2017
- Rehder KJ. Adjunct therapies for refractory status asthmaticus in children. Respiratory Care 2017;62(6):849-65. [DOI] [PubMed] [Google Scholar]
Rose 2011
- Rose J, Cancelliere S, Matye P, O'Riordan M. Comparing a breath-actuated vs a conventional continuous-output nebulizer in treating acute asthma in a pediatric emergency department: an ongoing randomized controlled trial. Academic Emergency Medicine 2011;18(5):S83-4. [Google Scholar]
Sabato 2011
- Sabato K, Ward P, Hawk W, Gildengorin V, Asselin JM. Randomized controlled trial of a breath-actuated nebulizer in pediatric asthma patients in the emergency department. Respiratory Care 2011;56(6):761-70. [DOI] [PubMed] [Google Scholar]
Schneider 2012
- Schneider J, Matsuda K, House SL, Ferguson I, Aubuchon K, Lewis L. Dyspnea scores may be a better predictor of hospital admissions than FEV1 for patients with acute asthma exacerbations. Academic Emergency Medicine 2012;19(s1):S312. [Google Scholar]
Schüneman 2019
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15, Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sengul Gokalp 2013
- Sengul Gokalp A, Bicer S, Siraneci R. Efficiacy of nebulized ipratropium bromide in the treatment of acute asthma in children: randomised, double blind, placebo-controlled trial. Nobel Medicus 2013;9(1):67-75. [Google Scholar]
Shim 1984
- Shim C. Adrenergic agonists and bronchodilator aerosol therapy in asthma. Clinics in Chest Medicine 1984;5(4):659-68. [PubMed] [Google Scholar]
Singhi 2014
- Singhi S, Grover S, Bansal A, Chopra K. Randomised comparison of intravenous magnesium sulphate, terbutaline and aminophylline for children with acute severe asthma. Acta Paediatrica 2014;103(12):1301-6. [DOI] [PubMed] [Google Scholar]
Supriyatno 2012
- Supriyatno B, Harumdini M, Indawati W. The efficacy of adding ipratropium bromide to salbutamol nebulization for mild acute asthma attack in children. Paediatric Respiratory Reviews 2012;13:S78. [Google Scholar]
Tiwari 2016
- Tiwari A, Guglani V, Jat KR. Ketamine versus aminophylline for acute asthma in children: a randomized, controlled trial. Annals of Thoracic Medicine 2016;11(4):283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2018
- Wang X, Zhou J, Zhao X, Yi X. Montelukast treatment of acute asthma exacerbations in children aged 2 to 5 years: a randomized, double-blind, placebo-controlled trial. Pediatric Emergency Care 2018;34(3):160-4. [DOI] [PubMed] [Google Scholar]
Weiss 2011
- Weiss AJ, Wier LM, Stocks C, Blanchard J. Overview of emergency department visits in the United States, 2011. HCUP Statistical Brief #174. June 2014. Rockville, MD: Agency for Healthcare Research and Quality. www.hcup-us.ahrq.gov/reports/statbriefs/sb174-Emergency-Department-Visits-Overview.pdf (accessed 2 November 2017). [PubMed]
Whiting 2016
- Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. Journal of Clinical Epidemiology 2016;69:225-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 2018
- Wilkinson M, King B, Iyer S, Higginbotham E, Wallace A, Hovinga C, et al. Comparison of a rapid albuterol pathway with a standard pathway for the treatment of children with a moderate to severe asthma exacerbation in the emergency department. Journal of Asthma 2018;55(3):244-51. [DOI] [PubMed] [Google Scholar]
Wyatt 2015
- Wyatt EL, Borland ML, Doyle SK, Geelhoed GC. Metered-dose inhaler ipratropium bromide in moderate acute asthma in children: a single-blinded randomised controlled trial. Journal of Paediatrics and Child Health 2015;51(2):192-8. [DOI] [PubMed] [Google Scholar]
Zubairi 2013
- Zubairi AB, Salahuddin N, Khawaja A, Awan S, Shah AA, Haque AS, et al. A randomized, double-blind, placebo-controlled trial of oral montelukast in acute asthma exacerbation. BMC Pulmonary Medicine 2013;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]


