Abstract
Prostate cancer is responsible for over 30,000 US deaths annually, attributed largely to incurable metastatic disease. Here, we demonstrate that high levels of plectin are associated with localized and metastatic human prostate cancer when compared to benign prostate tissues. Knock-down of plectin inhibits prostate cancer cell growth and colony formation in vitro, and growth of prostate cancer xenografts in vivo. Plectin knock-down further impairs aggressive and invasive cellular behavior assessed by migration, invasion, and wound healing in vitro. Consistently, plectin knock-down cells have impaired metastatic colonization to distant sites including liver, lung, kidney, bone, and genitourinary system. Plectin knock-down inhibited number of metastases per organ, as well as decreased overall metastatic burden. To gain insights into the role of plectin in prostate cancer growth and metastasis, we performed proteomic analysis of prostate cancer plectin knock-down xenograft tissues. Gene set enrichment analysis shows an increase in levels of proteins involved with extracellular matrix and laminin interactions, and a decrease in levels of proteins regulating amino acid metabolism, cytoskeletal proteins, and cellular response to stress. Collectively these findings demonstrate that plectin is an important regulator of prostate cancer cell growth and metastasis.
Keywords: prostate cancer, metastasis, plectin, cellular organization
Introduction
Prostate cancer is the most common non-cutaneous malignancy affecting men worldwide 45. Approximately 20% of those with localized prostate cancer will develop regional and/or distant metastatic disease 5, 12. Disseminated disease accounts for the majority of prostate cancer related mortalities, as 5-year survival dramatically decreases from close to 100% to nearly 30% in these patients 2,4. Bone is the most common site of distant metastasis, followed by lung, lymph node, and liver, the most lethal metastatic site 5, 8. Treatment options to slow the spread of metastatic prostate cancer include androgen receptor (AR)-targeted therapies (abiraterone 9, 14, 36–38, enzalutamide 3, 13, 16, 35), taxane chemotherapeutics (docetaxel 24, cabazitaxel 10), immunotherapy (provenge 46), and metastasis-directed radiotherapy 4. However, metastatic prostate cancer remains lethal, thereby highlighting the crucial need to define the regulators of prostate cancer metastasis.
Plectin, a member of the plakin family of proteins, is a cytoskeletal linker that coordinates and maintains cytoskeletal scaffolding elements essential for cell division, growth and motility 55. Plectin can bind F-actin, as well as intermediate filaments including desmin, cytokeratin, and vimentin 42, 51. Plectin additionally mediates the cellular interaction with the extracellular matrix (ECM) through regulation of fibronectin polymerization, altering focal adhesions 7. An integral role of plectin in maintenance of cellular architecture has been characterized in fibroblasts, and epithelial, endothelial, skeletal, and stem cells 7, 30, 33, 51, 56, 57. In cancer, plectin modulates protein kinase C (PKC) signaling 28 and mitogen-activated protein (MAP) kinases involved in cellular stress responses and migration 29. Loss of plectin renders cells unable to invade the ECM 7. Plectin is overexpressed in pancreatic, head and neck, and ovarian cancers 2, 20. In pancreatic ductal adenocarcinoma, plectin is essential for exosome production and promotes cancer growth 44.
In this study, we demonstrate that high levels of plectin are observed across prostate cancer xenografts tissues, as well as in human localized and metastatic prostate cancer. In vitro and in vivo, knock-down of plectin leads to a decrease in prostate cancer cell and tumor growth, as well as migration, invasion, and metastatic colonization. Consistent with these findings, proteomic profiling of tumors with modulated plectin knock-down reveals that plectin regulates cytoskeletal rearrangement, interactions with the ECM, and selenoamino acid metabolism. Our study demonstrates that plectin is a critical scaffold in enabling prostate cancer growth and metastasis.
Methods
Animals and in vivo tumor growth.
All animal studies and procedures were approved and performed in accordance with Stanford Administrative Panel on Laboratory Animal Care (APLAC), IACUC, and the USAMRMC Animal Care and Use Review Office (ACURO). For the xenograft panel, cancer cells (1×106) were mixed with 100 μl of 100% Matrigel and implanted subcutaneously in rear flanks of 8-week old male NOD/SCID/IL-2Rγnull (NSG) mice. Tumor volumes were measured every three days (length x width x height)/2. Once the average tumor volumes of the control (shControl) tumors reached ~ 400 mm3, tumors were collected, fixed overnight in 10% buffered formalin at 4 °C, and subsequently processed in paraffin for histological analyses. DU145 shControl and PLEC-KD tumors (n=8 per condition) were grown for 39 days. Average tumor volumes were graphed ± SEM.
Tissue Microarrays (TMAs).
Construction of the TMAs from paraffin embedded prostate and prostate cancer tissues was previously described 18, 54. TMAs from two patient cohorts were collectively analyzed. One set of the TMAs contained cores (3 cores per sample) from patient-matched benign (benign adjacent to cancer) and localized prostate cancer tissues from 124 patients. One patient was excluded from the analysis due to lack of sufficient tissue. The second set of TMAs contained patient-matched tissue cores (2 cores per sample) from benign adjacent to cancer, prostate cancer, confirmed cancer negative lymph nodes, and confirmed lymph nodes positive for prostate cancer from 15 patients with metastatic prostate cancer. 4-micron sections from the TMA blocks were then used for IHC staining for plectin. Plectin intensity was scored as negative (0), low (1), intermediate (2) or high (3) and averaged across all patients. The average core intensity of staining was calculated and assigned as an average intensity of plectin staining in each sample.
Histology and Immunohistochemistry (IHC) staining.
Hematoxylin and eosin (H&E) and IHC staining were performed as previously described 17, 34. Briefly, formalin-fixed paraffin-embedded tissues were sliced (4-microns) and heated 1 hour at 65 °C. Clearify reagent was used for deparaffinization, followed by rehydration with 100, 95, and 70% ethanol. Antigen retrieval was performed in sodium citrate buffer (10 mM) pH 6.0 at 95 °C for 20 minutes in a steamer, followed by 3% hydrogen peroxide for 5 minutes. Blocking, and subsequent primary antibody dilution were performed in 2.5% goat serum for mouse antibodies (Vector Laboratories) and incubated in a humidity chamber overnight at 4 °C. Slides were washed in 1X PBS and incubated with secondary mouse HRP antibody (Vector Laboratories) for 1 hour, developed using 1:50 DAB reagent (DAKO), and counter-stained with hematoxylin. Slides were then dehydrated, mounted with cover slips, and imaged at 10X and 63X for xenografts, and 5X and 40X for TMAs. Anti-plectin primary monoclonal antibody (Santa Cruz Biotechnology, sc-33649) was used at 1:250 dilution. Anti-clusterin primary monoclonal antibody (Santa Cruz Biotechnology, sc-166907) was used at 1:100 dilution.
Western Blotting.
8–16% gradient acrylamide gels were acquired from Thermo Fisher Scientific and the western blotting was performed as previously described 48. Protein levels were quantified using a BCA assay. Samples were then diluted in 4X Laemelli Buffer containing DTT and boiled at 95 °C for 5 minutes. Each well was loaded with 40 μg of protein. Transfer was performed onto a nitrocellulose membrane. The membrane was blocked in 5% milk in Tris-buffered saline (TBS) for one hour and incubated with primary antibodies overnight in TBS containing 0.1% Tween-20. Secondary antibody (Thermo Fischer Scientific, anti-mouse-HRP) was applied for one hour after washing, then developed using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific) chemiluminescence on an IVIS Lumina imaging system. Antibodies: Anti-plectin monoclonal antibody (Abcam ab32528, 1:500 dilution), anti-GAPDH monoclonal antibody (sc-47724, 1:2000 dilution) and secondary goat anti-mouse HRP (PI31432, 1:5000 dilution).
Cell Lines and Culture.
LNCaP, 22RV1, PC3, DU145 and NCI-H660 cells were obtained from the American Type Culture Collection (ATCC), and C4–2 cells were a gift from Dr. Owen Witte (University of California, Los Angeles). ARCaP were purchased from Novicure Biotechnology. Cell lines were authenticated through the Stanford Functional Genomics Facility (SFGF) based on Short Tandem Repeat (STR) profiling and routinely checked for mycoplasma using MycoAlert Mycoplasma Detection kit (Lonza). LNCaP, 22RV1, PC3, DU145, and ARCaP cells were cultured in 10 cm plates in RPMI 1640 media (Thermo Fisher Scientific), supplemented with 10% FBS, 1% Penicillin/Streptomycin and 1% L-Glutamine, and incubated at 37 oC with 5% CO2. Warmed Trypsin/EDTA (0.25%) was used for dissociation. NCI-H660 (H660) were grown in suspension in T-25 flasks in RPMI 1640 medium supplemented with 5% fetal bovine serum, 0.005 mg/ml insulin, 0.01 mg/ml transferrin, 30 nM sodium selenite, 10 nM hydrocortisone, 10 nM beta-estradiol, and 4 mM L-glutamine.
DNA Constructs.
FUCRW lentiviral vector (ubiquitin promoter driven gene expression; CMV promoter driven RFP reporter expression) was a gift from Dr. Owen Witte. pHIV-Luc-ZsGreen was a gift from Dr. Bryan Welm (University of Utah, Addgene plasmid #39196; http://n2t.net/addgene:39196; RRID: Addgene_39196). These lentiviral vectors were used for transduction of fluorescent and bioluminescent tags into DU145 cells for metastatic tracking. To generate plectin knock-down cell lines, we used a control scramble shRNA vector, pLKO.1 (a gift from David Sabatini, Massachusetts Institute of Technology, Addgene plasmid #1864; http://n2t.net/addgene: 1864; RRID: Addgene_1864)39 and two plectin shRNAs from Millipore Sigma: TRCN0000082814 and TRCN0000082816.
Generation of PLEC-KD Cells.
A control scramble shRNA pLKO.1 lentiviral vector was obtained from Addgene (gift from David Sabatini, Addgene plasmid #1864; http://n2t.net/addgene: 1864; RRID: Addgene_1864)(CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG). pLKO.1 shRNA lentiviral vectors containing two different shRNAs against plectin (PLEC-KDs) were purchased from Millipore Sigma: TRCN0000082814 (CCGGGCACCAGTCCATCGAAGAATTCTCGAGAATTCTTCGATGGACTGGTGCTTTTTG) and TRCN0000082816 (CCGGCGATGAGGAGATGAACGAGATCTCGAGATCTCGTTCATCTCCTCATCGTTTTTG). The lentiviral vectors were transfected with viral packaging and envelope plasmids by calcium phosphate transfection into 293T cells to generate viral particles. Virus was filtered through 0.45-micron filters, ultracentrifuged and flash frozen in aliquots. 2×105 DU145 or C4–2 cells were transduced with generated lentivirus with polybrene (10 μg/ml). shControl or PLEC-KD cells were selected with 500 ng/mL puromycin for 9 days, and knock-down effect was confirmed by western blot. Subsequently, selected cells were transduced with lentivirus expressing red fluorescent protein (RFP) and luciferase. RFP and luciferase expression were confirmed 72 hours post infection by IVIS Lumina II from Caliper Life Sciences to determine bioluminescence and fluorescence, respectively.
Cell Proliferation.
5×102 DU145 or C4–2 cells were plated per well on 96-well plates. Cells were plated in five wells per timepoint for each condition. The experiments were performed in triplicate. At given timepoints: Day 1, Day 3, Day 5, and Day 7, wells were stained with 1X Calcein AM (Thermo Fisher Scientific), then incubated at 37 oC in a 5% CO2 incubator for 20 minutes. Wells were then imaged using a Celigo Imaging Cytometer (Nexcelom Bioscience) with green fluorescence, and quantified using ImageJ 41. Percentage of well confluence area was graphed as mean at each time point ± SD.
Colony Formation Assays.
5×102 DU145 or C4–2 cells were plated per well on six-well plates in triplicate. Cells were cultured for 12 days, with media changed every fourth day. On Day 12, colonies were fixed with ice-cold 100% methanol for twenty minutes and then stained with 0.1% crystal violet for 20 minutes at room temperature. Plates were then washed in a room temperature water bath for ten minutes and air dried overnight. Colony formation was quantified as percentage of area coverage per well. Experiments shown are representative of three replicates, ± SD.
Migration and Invasion.
Before plating, all transwell chambers were incubated at 37 oC with 5% CO2 for 30 minutes with unsupplemented medium to acclimate. Cells were then trypsinized, washed, and switched to serum-free medium. For the migration assays, 5×104 cells DU145 (shControl and PLEC-KD) and C4–2 (shControl, PLEC-KD) were plated in unsupplemented RPMI medium in the upper chambers of 24-well transwell inserts (Transwell™ Permeable Polyester Membrane Inserts from Corning Inc.) with 8 μm-pore size membranes. For invasion assays, 1×105 cells were plated in unsupplemented RPMI medium in 24-well Matrigel-coated Boyden chamber transwell inserts (Corning Inc.) with 8 μm-pore size membranes. FBS-supplemented medium was added to the bottom of each transwell. For migration and invasion, chambers were incubated for 20 hours, fixed with ice cold 100% methanol for 20 minutes, and then stained with 0.1% Crystal Violet for 20 minutes at room temperature. Wells were then washed in a room temperature water bath for 10 minutes and air dried overnight. Wells were imaged on a stereomicroscope at 80X, three images per well and two wells per condition. Cell number represents the average of three well images in duplicate ± SD. Experiments were performed in triplicate. Representative images are presented.
Wound Healing.
3×105 DU145 shControl or PLEC-KD cells were plated onto 24-well plates in triplicate and incubated 24 hours at 37 oC with 5% CO2 overnight to achieve 100% confluence of adherent cells. Using a 200 μL pipette tip, a linear scratch was made, running the length of each well. The plate was imaged using a Celigo Imaging Cytometer at timepoints 0, 24, and 48 hours. Each image was quantified using ImageJ. The non-confluent portion of each well was calculated as a mean percentage area for three wells and graphed ± SD. The experiment is representative of three experiments.
Metastasis Model.
shControl or PLEC-KD DU145 (1×105 each) cells transduced with luciferase and RFP were injected intracardially into left ventricles of NSG mice to evaluate in vivo metastatic colonization. Whole body bioluminescence was measured at Day 0 and at end point, Day 29. Mice were injected with 5 mg/kg D-Luciferin (Perkin Elmer), and after 5 minutes were imaged on a LAGO imager using Aura imaging software (Spectral Instruments Imaging) at Stanford Preclinical Imaging Facility at Porter Drive. After Day 29 imaging, mice were sacrificed, and the lungs, liver, hind limbs, genitourinary tract (GU), and kidneys were harvested and fixed in 10% formalin overnight. Intact tissues were imaged on Leica stereomicroscope under brightfield and RFP filters for metastatic site identification. After imaging, tissues were processed as previously described for histological analysis.
Statistics.
Student’s two-tailed T-test and one-way ANOVA were used as indicated in the figure legends.
LC-MS/MS.
Label free proteomic analysis was performed as previously described 15, 17. Briefly, flash-frozen DU145 shControl and PLEC-KD xenograft tissues (n=2 per condition) were homogenized in 1 ml lysis buffer containing 12.5 mM Tris pH 8.0 (Fisher Scientific), 0.5 mM EDTA (EMD Inc.), 7.5 M urea (Sigma Aldrich), and 1X protease inhibitor (Sigma Aldrich), followed by sonication and centrifugation. Fifty micrograms of protein per sample, quantified by Micro BCA protein assay (Thermo Fisher Scientific) was used for LC/MS analysis. Proteins were reduced with 5 μL of 200 mM Tris(2-carboxyethyl)phosphine (TCEP) (Sigma Aldrich) and incubated at room temperature for 1.5 hours. The free thiol groups were alkylated by adding 7.5 μL of 200 mM iodoacetamide (Acros Organics), incubated for 45 minutes, room temperature in the dark. Samples were digested at 37 oC overnight with 2 μg sequencing grade modified trypsin enzyme (Promega). Tryptic peptides were dried on a speed vacuum and reconstituted in 100 μl 0.1% formic acid (Fisher Scientific) in HPLC grade water (Fisher Scientific) for LC-MS analysis. Reconstituted tryptic peptides (5 μg) were loaded onto a C18 trap column (Thermo Fisher Scientific) coupled to a Dionex Ultimate Rapid Separation Liquid Chromatography system (Thermo Fisher Scientific), at a rate of 5 μl/min for 10 minutes. Reverse-phased liquid chromatography was used to separate tryptic peptides on 25 cm C18 analytical column (New Objective), packed with Magic C18 AQ resin (Michrom Bioresources). Eluting peptides were analyzed by a LTQ-Orbitrap Elite mass spectrometer (Thermo Fisher Scientific). Peptides were ionized at 1.8 kV nano-ESI source voltage. The HPLC flow rate was set to 0.6 μL/min throughout the gradient. Mobile phase A consisted of 0.1% formic acid in water and mobile phase B consisted of 0.1% formic acid in acetonitrile. Mobile phase B was held at 2% B for 10 minutes with a gradual increase to 35% B for the next 100 minutes, followed by a rapid increase to 85% B over 7 minutes, and ending with a 5-minute hold with 2% mobile phase B for column equilibration. Each biological replicate was analyzed in triplicate with three blanks between samples. The top ten most abundant ions per MS1 scan were selected for collision-induced dissociation (CID) with a collision energy of 35 eV and mass resolution to 60,000. The FT AGC target was set at 1e6 and the scan range was set from 400–1800 m/z). The MS2 AGC target was set at 3e4 and dynamic exclusion was enabled for 30 seconds.
Statistical Analysis of Proteomics Data.
Each LC-MS data file was searched using Byonic 2.11.0 (Protein Metrics) against both human (2017; 20,484 entries) and mouse (2017; 17,191 entries) Swiss-Prot databases in two separate searches. Parameters included trypsin digestion with a maximum of two missed cleavages and precursor mass tolerance of 10 ppm. Fixed cysteine carbamidomethylation and variable methionine oxidation and asparagine deamination were also specified. Peptide identifications were filtered to remove >1% false discovery rate (FDR). Peptides that were identified in both human and mouse searches were removed for a conservative analysis of only human proteins. Analysis was performed, for each of the two biological replicates, with three injections per experimental condition (shControl and PLEC-KD). Quantitative values for all identified peptides were extracted from MS1 spectra using methodology from the MSnbase package 15, and abundance changes were analyzed using the Generic Integration Algorithm. Calculation of statistical weight was performed at the spectrum level using the WSPP model 27. Final statistical analysis was performed using Perseus 52, considering only proteins having an FDR calculated based on permutation less than 1%, and fold-change greater than 1.5. These proteins were analyzed by String network analysis (String-db.org), and enriched pathways were identified.
Gene Set Enrichment Analysis (GSEA) was performed by an overrepresentation test of all proteins increased or decreased with a p-value <0.05 using PANTHER26 with the protein annotations from Reactome (version 54 released 2019–12-22). The whole human genome was used as a reference and a Fisher’s Exact test was applied to detect enriched pathways with FDR<0.01 and more than four proteins. A pre-ranked list of proteins belonging to significant pathways detected with the GSEA algorithm was generated from this analysis50.
Results
High levels of plectin are associated with localized prostate cancer and lymph node metastasis.
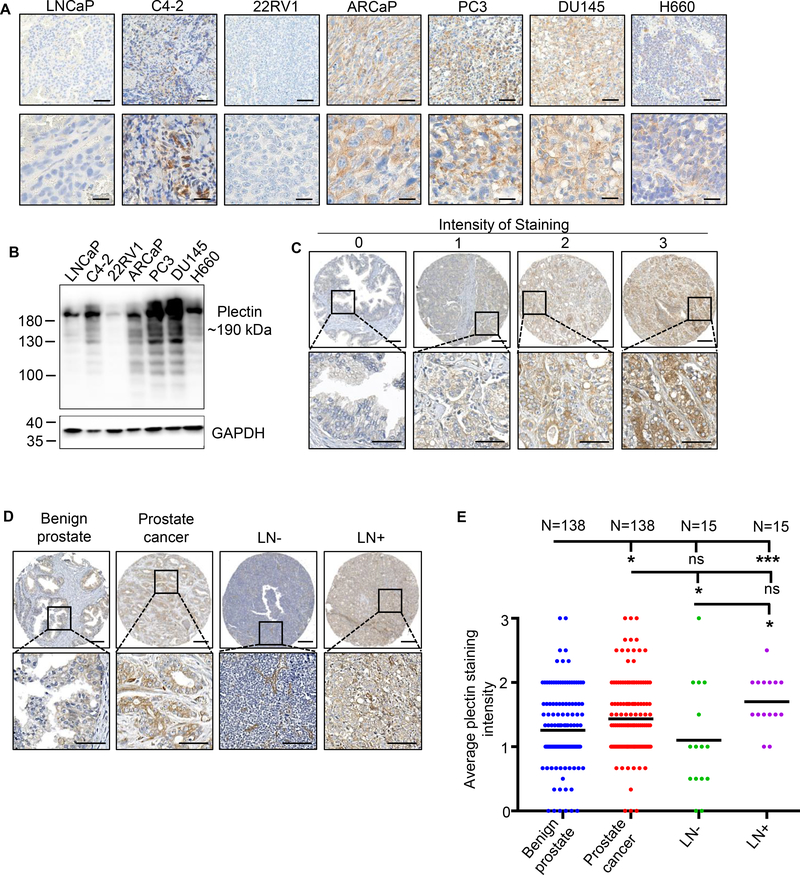
We first assessed the protein levels of plectin across multiple prostate cancer xenografts and cell lines and found that plectin is highly expressed in majority of prostate cancer cell lines and xenograft tissues (Figure 1A, B). We further evaluated levels of plectin in tissue samples from 124 prostate cancer patients with localized prostate cancer who underwent radical prostatectomy and an additional cohort of 15 patients with regional lymph node metastasis. Stained cores were scored based on plectin intensity, from 0 (negative) to 3 (high) intensity of staining (Figure 1C). Plectin protein levels are higher in cancerous tissues when compared to patient-matched benign prostate tissues adjacent to cancer (Figures 1D, E). Likewise, the average plectin staining intensity is significantly higher in lymph node metastasis when compared to the benign prostate and cancer-free lymph node tissues (Figure 1D, E). These results demonstrate that high levels of plectin are associated with localized prostate cancer and regional lymph node metastasis.
Figure 1. Plectin levels are increased in human prostate cancer.
(A) IHC staining for plectin in human prostate cancer cell xenografts, depicting variable levels across prostate cancer cell xenograft tissues. Scale bars represent 200 (top) and 25 (bottom) microns. (B) Western Blot validating expression levels of plectin from multiple cell lines. GAPDH was used as the loading control. (C) IHC of plectin on tissues microarrays (TMAs) composed of benign (n of patients=138), prostate cancer (n of patients=138), lymph node with no metastasis (LN-) (n of patients=15), and lymph node with metastasis (LN+) (n of patients=15) tissues. Plectin staining intensity was scored from 0 to 3 (0 is negative, 1 is low, 2 is weak positive, and 3 is strong positive). Representative images are shown per score. Scale bars represent 200 (top) and 50 (bottom) microns. (D) IHC staining of plectin on benign, prostate cancer, lymph node with no metastasis (LN-) and lymph node with metastasis (LN+) tissue samples. Representative cores were imaged to depict staining of plectin. Scale bars represent 200 (top) and 50 (bottom) microns. (E) Plectin average core intensity of staining in benign (n of patients=138), prostate cancer (n of patients=138), lymph node with no metastasis (LN-) (n of patients=15), and lymph node with metastasis (LN+) (n of patients=15) tissue samples. Black bars indicate mean. * = P<0.05, *** = P<0.005, ns=no significance determined by one-way ANOVA. .
Plectin knock-down inhibits prostate cancer cell growth in vitro and in vivo.
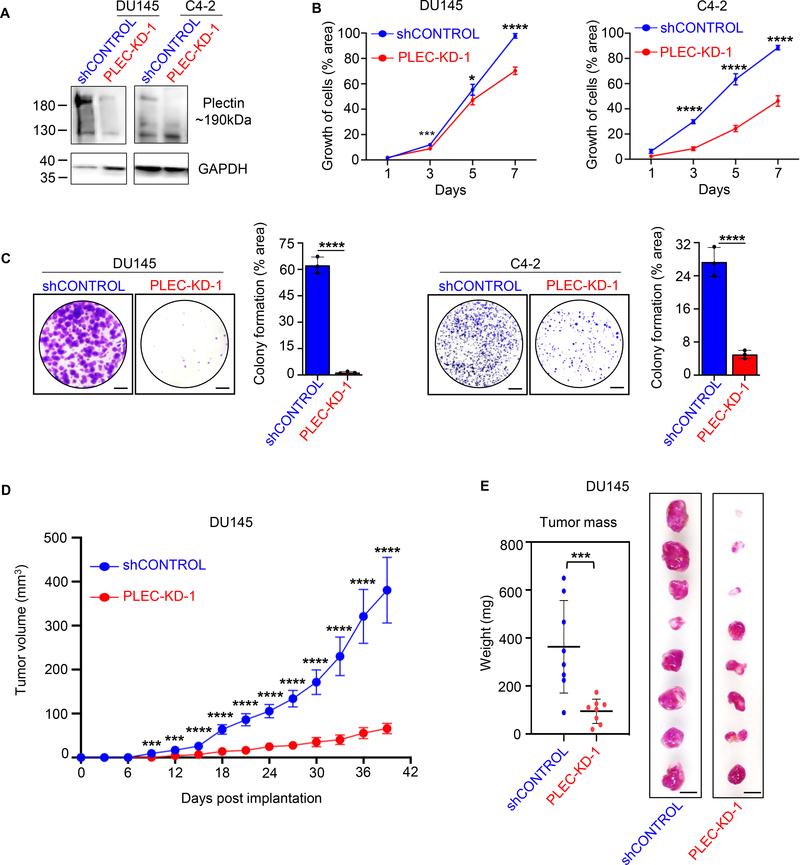
To determine the role of plectin in tumorigenesis, we generated stable plectin knock-down DU145 and C4–2 cell lines by transducing with lentivirus carrying short hairpin RNA (shRNA) targeting plectin, red fluorescent protein (RFP) and luciferase (PLEC-KD). Knock-down of plectin was validated via western blot (Figure 2A). In clonogenic assays, knock-down of plectin in DU145 and C4–2 prostate cancer cells leads to a significant decrease in cell proliferation (Figure 2B and Supplementary Figure S1A). Similarly, knock-down of plectin significantly reduced colony formation rates of DU145 and C4–2 cells (Figure 2C and Supplementary Figure S1B). The average colony formation measured by percent area of the colonies in the DU145 control cells was ~ 43–60% and 25% in C4–2 control cells when compared to 5–25% in DU145 and 5% in C4–2 cells with plectin knock-down (Figure 2C and Supplementary Figure S1B).
Figure 2. Plectin knock-down inhibits prostate cancer cell growth in vitro and in vivo.
(A) Plectin protein levels were assessed by Western Blot in DU145 and C4–2 parental cells transfected with guide shRNA (shControl), or shPlectin clone 1 (PLEC-KD-1) following antibiotic selection. GAPDH was used as loading control. Plectin full length expected size is ~280 kDa and plectin isoforms below 280 kDa. (B) Cell proliferation of DU145 (left) or C4–2 (right) shControl or PLEC-KD-1 cells with live cell imaging of 5 × 102 plated cells. Wells were imaged at 1, 3, 5, and 7 days after plating, with Calcein-AM fluorescent dye. Cells quantified as percentage of well covered from 5 averaged wells per time point. Error bars represent standard deviation (SD). (C) Colony formation assays of DU145 shControl and DU145 PLEC-KD-1 (left) and of C4–2 shControl and C4–2 PLEC-KD-1 (right). Cells were fixed and stained after 12 days, and quantified by percentage area covered by colonies across average of three wells using ImageJ. Scale bars represent 1 cm. Error bars represent SD. (D) Growth of DU145 shControl and PLEC-KD-1 subcutaneously xenografted tumors in intact male NSG mice (n=8). Tumor volume was measured every three days with calipers and calculated as (length x width x height)/2 and plotted. Error bars represent standard error of the mean (SEM). (E) Tumor mass of DU145 shControl and PLEC-KD-1 subcutaneous xenografts after harvest. Error bars represent SD. Scale bar=1 cm. * = P<0.05, *** = P<0.005, **** = P<0.001 determined by one-way ANOVA (in panels B and D) or two-tailed Student’s t-test (in panels C and E)
To determine effects of plectin knock-down on prostate cancer growth in vivo, we implanted PLEC-KD and shControl DU145 cells subcutaneously into the flanks of immunodeficient NOD SCID gamma mice (NSG). Knock-down of plectin significantly decreased tumor growth rates when compared to the shControl cells (Figure 2D), and PLEC-KD tumor weights are significantly lower than their shControl counterparts (n=8 tumors per group) (Figure 2E). These results demonstrate that plectin knock-down significantly delays prostate cancer growth in vitro and in vivo.
Proteomic analysis identifies the effect of plectin knock-down on cell motility impairment.
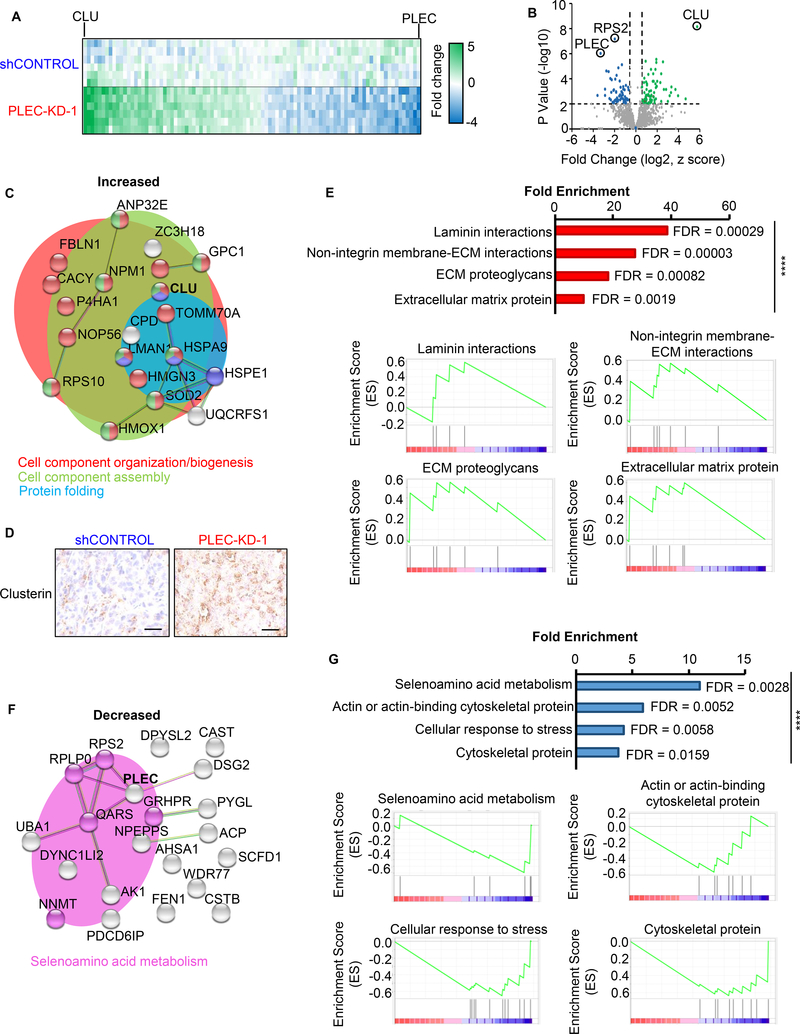
To gain additional biological insights into the role of plectin in prostate cancer growth, we performed LC-MS/MS proteomic analysis of tumor tissues from DU145 shControl and PLEC-KD xenografts. Proteins with a p-value <0.01 and a fold change of greater than 1.5 were selected for network and functional analysis (Figure 3A, B) using STRING (http://string-db.org/). Proteins with increased levels in plectin knock-down samples were related to cell component organization, assembly, and protein folding (Figure 3C). Clusterin (CLU), the protein which showed the highest significance and increase in levels upon plectin knock-down, was further validated by IHC (Figure 3D). Gene set enrichment analysis (GSEA) of proteins with increased levels further implicated laminin interactions, extracellular matrix (ECM) proteins, membrane-ECM interactions, and ECM proteoglycans (Figure 3E). Proteins with decreased levels upon plectin knock-down showed enrichment in selenoamino acid metabolism by String (Figure 3F) and GSEA, along with cytoskeletal proteins, actin-binding cytoskeletal proteins, and cellular response to stress (Figure 3G).
Figure 3. Proteomic analysis identifies the effects of plectin knock-down on cellular organization.
DU145 shControl or PLEC-KD-1 tumors (n=2) from Figure 2D were homogenized and lysed for proteomic analysis. (A) Liquid chromatography tandem mass spectrometry (LC-MS/MS) was performed, with triplicate analysis. The heat map indicates protein levels from −4 to +5-fold change. Green and blue indicate proteins with increased and decreased levels in plectin knock-down tumor samples respectively. (B) Volcano plot of fold changes vs. p-value. Proteins with ≥1.5-fold change, P<0.01, and a FDR of <1% are colored. (C) Functional protein association networks for proteins with increased levels in PLEC-KD tumor tissues were analyzed using STRING (https://string-db.org/). Line thickness indicates the strength of data to support correlation between each node. Enriched reactomes are indicated by color: red=cell component organization/biogenesis; green=cell component assembly; and blue=protein folding. (D) IHC of DU145 xenografts for clusterin (gene CLU), most significant protein from (C). Scale bar represents 25 microns. (E) Gene set enrichment analysis (GSEA) for increased proteins from PLEC-KD tumor tissue, P<0.05 with overrepresentation analysis. Reactome pathways are graphed by fold enrichment with indicated false discovery rates (FDR). Enrichment scores for each pathway graphed below. (F) Functional protein association networks for proteins decreased in PLEC-KD-1 tumors using STRING. Pink coded proteins indicate selenoamino acid metabolism. (G) GSEA of decreased proteins. (Top) Fold enrichment reactome pathways. (Bottom) Enrichment score graphed per pathway. **** = P<0.001.
Plectin knock-down inhibits prostate cancer cell migration and invasion ability in vitro.
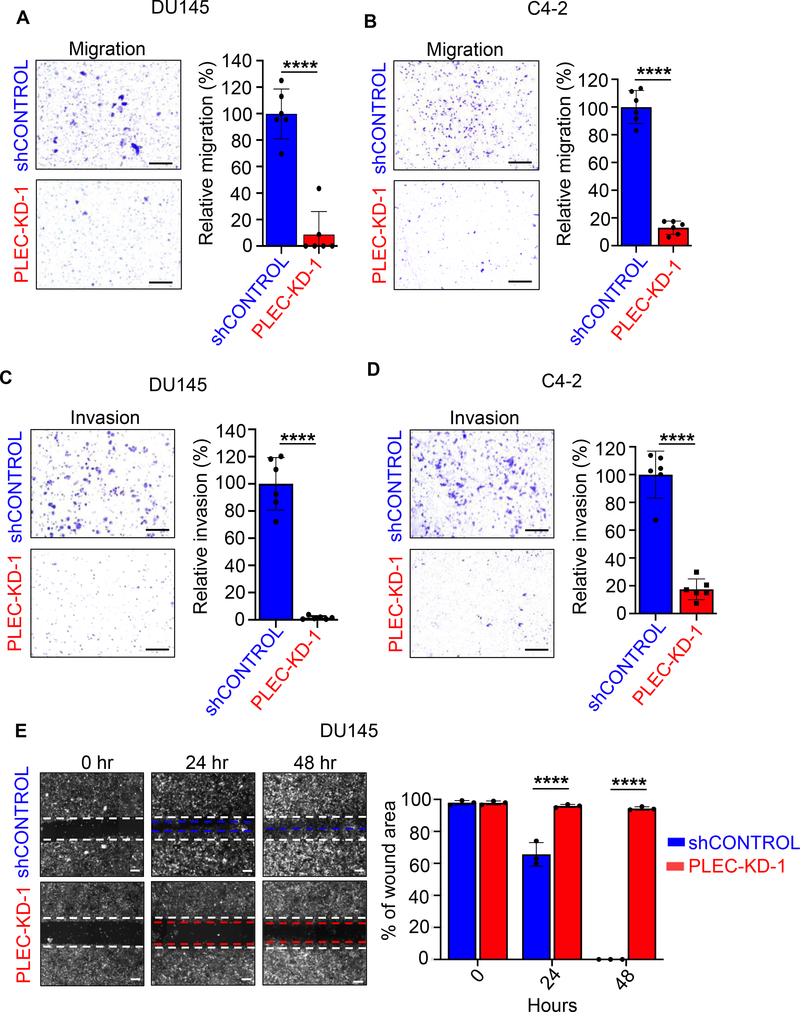
As plectin is a critical regulator for cell cytoarchitecture and motility, we next measured the effects of plectin knock-down on prostate cancer invasion and migration. In Boyden chamber transwell assays, DU145 and C4–2 cells with decreased plectin (PLEC-KD) demonstrated a significant decrease in migration potential compared to those of shControl (Figures 4A, B and Supplementary Figure S2A). DU145 PLEC-KD and C4–2 PLEC-KD cells also significantly reduced invasion in comparison to their respective shControl cells in transwell assays (Figures 4C, D and Supplementary Figure S2B). Migration and invasion experiments were performed 20 hours post plating prior to cell doubling time and effects on cell proliferation. Likewise, in wound healing assays, DU145 shControl cells were highly motile, with 100% wound closure at 48 hours, while PLEC-KD cells had significantly impaired motility (Figure 4E and Supplementary Figure S2C). These results demonstrate the essential role of plectin in prostate cancer cell invasion and migration.
Figure 4. Plectin knock-down inhibits migration and invasion of prostate cancer cells in vitro.
(A, B) Transwell chamber assays were performed to measure migration 20-hour post plating of (A) DU145 or (B) C4–2 shControl and PLEC-KD-1 cells. Two transwells per condition were plated, and three images per well were counted and averaged to determine relative migration (%) compared to shControl. One of three independent experiments is shown. Scale bars equal 100 microns. (C, D) Matrigel coated Boyden chamber assays to measure invasion after 20 hours incubation of (C) DU145, or (D) C4–2 shControl and PLEC-KD-1 cells. Experiments were performed in duplicate and three images per well were used to quantify stained cells that had migrated. The average number of cells is graphed as relative invasion (%) compared to shControl. One of t independent experiments is shown. Scale bars equal 100 microns. (E) Wound healing assays of DU145 shControl and DU145 PLEC-KD-1 cells. Experiments were performed in triplicate. The average of the wound area in three wells, assessed in ImageJ, at 0 hr was assigned 100% and used to quantify changes in wound area over time. Dotted white line indicates 0 hr, and blue (shControl) or red (PLEC-KD1) indicate area of cell movement at 48 hours. Representative images are shown. Scale bar is 100 microns. Error bars in Figure 4 represent SD. **** = P<0.001 determined by two-tailed Student’s t-test.
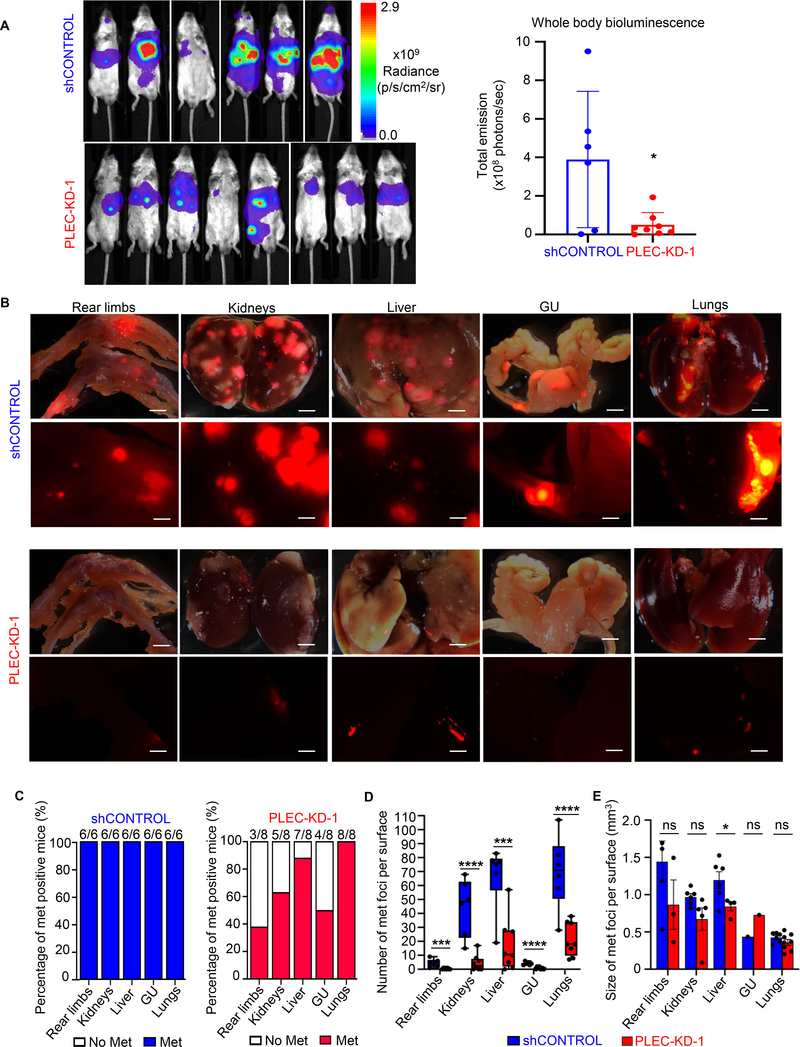
Plectin knock-down impairs metastatic colonization in vivo.
To test the effect of plectin on prostate cancer metastasis in vivo, we intracardially injected DU145-RFP-Luciferase shControl or DU145-RFP-Luciferase PLEC-KD cells. Whole body bioluminescence imaging (BLI) and BLI quantification were performed immediately post-injection and at the endpoint of 29 days to reflect metastatic burden. At this time, PLEC-KD animals had substantially less metastatic signal, read as radiance (photons/second) than their shControl counterparts (Figure 5A, B). Organs with evidence of metastasis were excised, including the femurs (rear limbs), liver, kidneys, lungs, and genitourinary system (GU). PLEC-KD mice showed marked decreases in the instance, number, and size of metastatic nodules in individual organs (Figure 5B–E). While almost all shControl mice had metastasis (six out of six mice positive for metastases in rear limb, kidney, liver, GU and lung), fewer PLEC-KD mice had metastasis including: only 3/8 for femurs, 5/8 for kidneys, 7/8 for liver, 4/8 for GU and 8/8 for lungs (Figure 5C). Despite lungs maintaining 100% metastatic rate in both groups, the number of metastases were significantly reduced (Figure 5D, E). Liver tumors were significantly larger in shControl mice than they were in PLEC-KD mice (Figure 5E). These results demonstrate that knock-down of plectin significantly impairs metastatic colonization of prostate cancer cells.
Figure 5. Plectin knock-down impairs prostate cancer homing to distant sites in vivo.
(A) DU145 shControl and DU145 PLEC-KD-1 cells (1×105) expressing luciferase and RFP were injected into the left ventricle of male NSG mice (n=6 and n=8) and mice were observed for 29 days. Whole body bioluminescence intensity (BLI) is shown as total emission (photons/second) and quantified at Day 29 in right panel. Error bars represent SD. (B) Ex vivo detection of RFP signal in respective organs from DU145 shControl and DU145 PLEC-KD-1 mice. RFP signal overlaid with darkfield image, where scale bar represents 2 mm (top per condition). RFP signal shown with scale bar representing 800 microns (bottom per condition). (C) Bar graph depicting the percentage of total mice with any visible metastases from ex vivo RFP imaging across respective organ types from DU145 shControl and DU145 PLEC-KD-1 groups. (D) Box and whisker plot of quantification of visible, metastatic foci per respective organ surface between DU145 shControl, and DU145 PLEC-KD-1 groups. (E) Bar graph depicting the average diameter of all visible foci on respective organ surface across DU145 shControl and DU145 PLEC-KD-1 groups, measured in ImageJ. Error bars represent SEM. * = P<0.05, *** = P<0.005, **** = P<0.001, ns = no significance, determined by two-tailed Student’s t-test.
Discussion
Plectin plays a key role in maintenance of intermediate filaments, adherens and tight junctions, and facilitates adhesion of epithelial cells to the ECM, as well as formation of invadopodia 23, 25, 47, 51. Plectin overexpression has also been associated with multiple cancer types including pancreas, head and neck, ovarian, and lung cancer 2, 20, 33. Plectin expression is linked to poor disease prognosis in pancreatic, as well as in lung cancer, where it has been identified as a biomarker for lung cancer stem cells 33, 44. Yet, the role of plectin in prostate cancer tumor growth and metastasis, and the prevalence of plectin expression in prostate cancer patient cohorts have not been previously elucidated. Here, we demonstrate that protein levels of plectin are elevated in localized prostate cancer as well as lymph node metastasis when compared to benign prostate tissues. Our findings demonstrate that high levels of plectin are associated with prostate cancer in patient samples, and further implicate plectin in prostate cancer growth and metastasis.
Modulation of plectin by knock-down in cell growth and migration models of prostate cancer results in deficiencies in growth and motility. Furthermore, knock-down of plectin leads to a significant decrease in tumor growth and metastatic colonization. Consistent with the role of plectin in prostate cancer growth and metastasis, proteomic profiling of tumors with plectin knock-down reveals significant alterations involving extracellular matrix proteins and interactions, cell organization, and protein folding (Figure 3). One possibility for why plectin deficient cells experience impaired growth and motility may be through clusterin. Clusterin, a glycoprotein which acts as small heat shock protein 19, has several alternatively spliced isoforms dictating its functionality 31, 43, 53, 59. The cleaved nuclear clusterin, observed here as increased by IHC (Figure 3), is key in reinforcing mechanically stressed cells 1, and induces cell cycle arrest 21. Elevated expression of nuclear clusterin in prostate cancer cells has been shown to induce apoptosis 40. To our aggressive prostate cancer models, loss of plectin appears to be a stress inducing event, which may be the cause of nuclear clusterin induction, and may lead to impairments in growth and motility,
In addition to decreased actin and cytoskeletal proteins, we identify a significant reduction in selenoamino acid metabolism upon downregulation of plectin. Bioavailability of non-essential amino acids such as asparagine and glutamine correlates with metastatic relapse in multiple cancer types 22, 32. Among the downregulated proteins involved with the selenoamino acid metabolism reactome pathway include NNMT, QARS, RPS2, RPLP0, and GRHPR. Nicotinamide N-methyltransferase (NNMT) is associated with prostate cancer over benign prostatic hyperplasia 58. Plectin knock-down reduces QARS, or glutamine tRNA ligase (GlnRS), which is important for anti-apoptotic signaling and of particular importance as glutamine is one of the amino acids most associated with fueling metastasis 11, 49.
Another study by Burch et al. performed proteomic analysis of PC3 prostate cancer cells compared against a metastatic derivative PC3 line 6. Plectin levels robustly increased in the more aggressive line. Consistent with our finding, this study reflected alterations of biological processes relating to cell growth and proliferation, morphology, nucleic acid metabolism, cell assembly, and organization and cell death 6.
Given the lethality of metastatic disease, a better understanding of the molecular pathway regulating advanced prostate cancer growth and metastasis is critical. In this study, we discovered a critical requirement of plectin in prostate cancer cell migration, invasion, and metastatic colonization. In human prostate cancer, plectin levels increase when compared to normal adjacent tissue. Conversely, plectin knock-down mitigates growth and invasion potential of prostate cancer cells both in vitro and in vivo. Metastasis is also dramatically reduced by plectin knock-down, revealing that plectin is necessary for the tumor growth and metastatic colonization in prostate cancer. These findings suggest disruption of the cytoskeletal signaling axis, namely through plectin modulation, as a powerful tool to impair prostate cancer growth and reduce metastatic potential.
Supplementary Material
Significance.
The presence of metastatic prostate cancer is currently incurable and lethal. Our findings identify plectin as an essential regulator of growth and metastasis of prostate cancer.
Acknowledgements
T.S. is supported by Canary Foundation, National Institutes of Health/National Cancer Institute (NCI) R37CA240822, R01CA244281 and R03CA230819. TS was also supported by the U.S. Army Medical Research Acquisition Activity through the Congressionally Directed Medical Research Program (CDMRP) Award No. W81XWH1810323. M.A.R. is supported by the U.S. Army Medical Research Acquisition Activity, through the CDMRP Award No. W81XWH1810141. Research reported in this publication was supported in part by the National Institutes of Health under award number S10 OD023518-01A1 for the Celigo S Imaging Cytometer (200-BFFL-S). Opinions, interpretation, conclusions and recommendations are those of the authors and not necessarily endorsed by the US Army and the funding agencies.
Financial support: T.S. is supported by the Canary Foundation, the National Institutes of Health/National Cancer Institute (NCI) R37CA240822, R01CA244281 and R03CA230819. M.A.R. is supported by the CDMRP through Award No. W81XWH1810141.
Footnotes
Competing Interests: The authors declare no competing interests.
References
- 1.Andra K, Lassmann H, Bittner R, Shorny S, Fassler R, Propst F et al. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev 1997; 11: 3143–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bausch D, Thomas S, Mino-Kenudson M, Fernández-del CC, Bauer TW, Williams M et al. Plectin-1 as a Novel Biomarker for Pancreatic Cancer. Clinical Cancer Research 2011; 17: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekelman JE, Rumble RB, Chen RC, Pisansky TM, Finelli A, Feifer A et al. Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol 2018: Jco1800606. [DOI] [PubMed] [Google Scholar]
- 5.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 2000; 31: 578–583. [DOI] [PubMed] [Google Scholar]
- 6.Burch TC, Watson MT, Nyalwidhe JO. Variable metastatic potentials correlate with differential plectin and vimentin expression in syngeneic androgen independent prostate cancer cells. PLoS One 2013; 8: e65005–e65005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgstaller G, Gregor M, Winter L, Wiche G. Keeping the vimentin network under control: cell-matrix adhesion-associated plectin 1f affects cell shape and polarity of fibroblasts. Mol Biol Cell 2010; 21: 3362–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damber JE, Aus G. Prostate cancer. Lancet 2008; 371: 1710–1721. [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. New England Journal of Medicine 2019; 381: 2506–2518. [DOI] [PubMed] [Google Scholar]
- 11.DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010; 29: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drake CG. Visceral metastases and prostate cancer treatment: ‘die hard,’ ‘tough neighborhoods,’ or ‘evil humors’? Oncology (Williston Park) 2014; 28: 974–980. [PMC free article] [PubMed] [Google Scholar]
- 13.Evans CP, Higano CS, Keane T, Andriole G, Saad F, Iversen P et al. The PREVAIL Study: Primary Outcomes by Site and Extent of Baseline Disease for Enzalutamide-treated Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer. Eur Urol 2016; 70: 675–683. [DOI] [PubMed] [Google Scholar]
- 14.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–992. [DOI] [PubMed] [Google Scholar]
- 15.Gatto L, Lilley KS. MSnbase-an R/Bioconductor package for isobaric tagged mass spectrometry data visualization, processing and quantitation. Bioinformatics 2012; 28: 288–289. [DOI] [PubMed] [Google Scholar]
- 16.Graff JN, Gordon MJ, Beer TM. Safety and effectiveness of enzalutamide in men with metastatic, castration-resistant prostate cancer. Expert Opin Pharmacother 2015; 16: 749–754. [DOI] [PubMed] [Google Scholar]
- 17.Hsu EC, Rice MA, Bermudez A, Marques FJG, Aslan M, Liu S et al. Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc Natl Acad Sci U S A 2020; 117: 2032–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Yao JL, Zhang L, Bourne PA, Quinn AM, di Sant’Agnese PA et al. Differential expression of interleukin-8 and its receptors in the neuroendocrine and non-neuroendocrine compartments of prostate cancer. Am J Pathol 2005; 166: 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin Has Chaperone-like Activity Similar to That of Small Heat Shock Proteins. Journal of Biological Chemistry 1999; 274: 6875–6881. [DOI] [PubMed] [Google Scholar]
- 20.Katada K, Tomonaga T, Satoh M, Matsushita K, Tonoike Y, Kodera Y et al. Plectin promotes migration and invasion of cancer cells and is a novel prognostic marker for head and neck squamous cell carcinoma. Journal of Proteomics 2012; 75: 1803–1815. [DOI] [PubMed] [Google Scholar]
- 21.Kim N, Han JY, Roh GS, Kim HJ, Kang SS, Cho GJ et al. Nuclear clusterin is associated with neuronal apoptosis in the developing rat brain upon ethanol exposure. Alcohol Clin Exp Res 2012; 36: 72–82. [DOI] [PubMed] [Google Scholar]
- 22.Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 2018; 554: 378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koster J, van Wilpe S, Kuikman I, Litjens SHM, Sonnenberg A. Role of binding of plectin to the integrin beta4 subunit in the assembly of hemidesmosomes. Mol Biol Cell 2004; 15: 1211–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. Journal of Clinical Oncology 2018; 36: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol 2006; 16: 376–383. [DOI] [PubMed] [Google Scholar]
- 26.Mi H, Muruganujan A, Huang X, Ebert D, Mills C, Guo X et al. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nature Protocols 2019; 14: 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro P, Trevisan-Herraz M, Bonzon-Kulichenko E, Nunez E, Martinez-Acedo P, Perez-Hernandez D et al. General statistical framework for quantitative proteomics by stable isotope labeling. J Proteome Res 2014; 13: 1234–1247. [DOI] [PubMed] [Google Scholar]
- 28.Osmanagic-Myers S, Wiche G. Plectin-RACK1 (receptor for activated C kinase 1) scaffolding: a novel mechanism to regulate protein kinase C activity. J Biol Chem 2004; 279: 18701–18710. [DOI] [PubMed] [Google Scholar]
- 29.Osmanagic-Myers S, Gregor M, Walko G, Burgstaller G, Reipert S, Wiche G. Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol 2006; 174: 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osmanagic-Myers S, Rus S, Wolfram M, Brunner D, Goldmann WH, Bonakdar N et al. Plectin reinforces vascular integrity by mediating crosstalk between the vimentin and the actin networks. Journal of Cell Science 2015; 128: 4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng M, Deng J, Zhou S, Tao T, Su Q, Yang X et al. The role of Clusterin in cancer metastasis. Cancer Manag Res 2019; 11: 2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porporato PE, Payen VL, Baselet B, Sonveaux P. Metabolic changes associated with tumor metastasis, part 2: Mitochondria, lipid and amino acid metabolism. Cell Mol Life Sci 2016; 73: 1349–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raymond AC, Gao B, Girard L, Minna JD, Gomika Udugamasooriya D. Unbiased peptoid combinatorial cell screen identifies plectin protein as a potential biomarker for lung cancer stem cells. Scientific Reports 2019; 9: 14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice MA, Hsu EC, Aslan M, Ghoochani A, Su A, Stoyanova T. Loss of Notch1 activity inhibits prostate cancer growth and metastasis and sensitizes prostate cancer cells to anti-androgen therapies. Molecular Cancer Therapeutics 2019: molcanther.0804.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rice MA, Malhotra SV, Stoyanova T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front Oncol 2019; 9: 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol 2010; 28: 1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–160. [DOI] [PubMed] [Google Scholar]
- 39.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307: 1098–1101. [DOI] [PubMed] [Google Scholar]
- 40.Scaltriti M, Santamaria A, Paciucci R, Bettuzzi S. Intracellular Clusterin Induces G2/M Phase Arrest and Cell Death in PC-3 Prostate Cancer Cells. Cancer Research 2004; 64: 6174–6182. [DOI] [PubMed] [Google Scholar]
- 41.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al. Fiji: an open-source platform for biological-image analysis. Nature Methods 2012; 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ševčík J, Urbániková Lu, Košt’an J, Janda L, Wiche G. Actin-binding domain of mouse plectin. European Journal of Biochemistry 2004; 271: 1873–1884. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro B, Tocci P, Haase G, Gavert N, Ben-Ze’ev A. Clusterin, a gene enriched in intestinal stem cells, is required for L1-mediated colon cancer metastasis. Oncotarget 2015; 6: 34389–34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin SJ, Smith JA, Rezniczek GA, Pan S, Chen R, Brentnall TA et al. Unexpected gain of function for the scaffolding protein plectin due to mislocalization in pancreatic cancer. Proceedings of the National Academy of Sciences 2013; 110: 19414–19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 46.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH et al. Placebo-Controlled Phase III Trial of Immunologic Therapy with Sipuleucel-T (APC8015) in Patients with Metastatic, Asymptomatic Hormone Refractory Prostate Cancer. Journal of Clinical Oncology 2006; 24: 3089–3094. [DOI] [PubMed] [Google Scholar]
- 47.Song J-G, Kostan J, Drepper F, Knapp B, de Almeida Ribeiro E Jr., Konarev Petr V et al. Structural Insights into Ca2+-Calmodulin Regulation of Plectin 1a-Integrin β4 Interaction in Hemidesmosomes. Structure 2015; 23: 558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoyanova T, Riedinger M, Lin S, Faltermeier CM, Smith BA, Zhang KX et al. Activation of Notch1 synergizes with multiple pathways in promoting castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2016; 113: E6457–E6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strmiska V, Michalek P, Eckschlager T, Stiborova M, Adam V, Krizkova S et al. Prostate cancer-specific hallmarks of amino acids metabolism: Towards a paradigm of precision medicine. Biochim Biophys Acta Rev Cancer 2019; 1871: 248–258. [DOI] [PubMed] [Google Scholar]
- 50.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences 2005; 102: 15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutoh Yoneyama M, Hatakeyama S, Habuchi T, Inoue T, Nakamura T, Funyu T et al. Vimentin intermediate filament and plectin provide a scaffold for invadopodia, facilitating cancer cell invasion and extravasation for metastasis. Eur J Cell Biol 2014; 93: 157–169. [DOI] [PubMed] [Google Scholar]
- 52.Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods 2016; 13: 731–740. [DOI] [PubMed] [Google Scholar]
- 53.Wang C, Jin G, Jin H, Wang N, Luo Q, Zhang Y et al. Clusterin facilitates metastasis by EIF3I/Akt/MMP13 signaling in hepatocellular carcinoma. Oncotarget 2015; 6: 2903–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Place RF, Huang V, Wang X, Noonan EJ, Magyar CE et al. Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Res 2010; 70: 10182–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci 1998; 111 ( Pt 17): 2477–2486. [DOI] [PubMed] [Google Scholar]
- 56.Wiche G, Winter L. Plectin isoforms as organizers of intermediate filament cytoarchitecture. Bioarchitecture 2011; 1: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winter L, Wiche G. The many faces of plectin and plectinopathies: pathology and mechanisms. Acta Neuropathol 2013; 125: 77–93. [DOI] [PubMed] [Google Scholar]
- 58.Zhou W, Gui M, Zhu M, Long Z, Huang L, Zhou J et al. Nicotinamide N-methyltransferase is overexpressed in prostate cancer and correlates with prolonged progression-free and overall survival times. Oncol Lett 2014; 8: 1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Y, Chen P, Gao Y, Ta N, Zhang Y, Cai J et al. MEG3 Activated by Vitamin D Inhibits Colorectal Cancer Cells Proliferation and Migration via Regulating Clusterin. EBioMedicine 2018; 30: 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.