ABSTRACT
Clostridium butyricum is a butyrate-producing human gut symbiont that has been safely used as a probiotic for decades. C. butyricum strains have been investigated for potential protective or ameliorative effects in a wide range of human diseases, including gut-acquired infection, intestinal injury, irritable bowel syndrome, inflammatory bowel disease, neurodegenerative disease, metabolic disease, and colorectal cancer. In this review we summarize the studies on C. butyricum supplementation with special attention to proposed mechanisms for the associated health benefits and the supporting experimental evidence. These mechanisms center on molecular signals (especially butyrate) as well as immunological signals in the digestive system that cascade well beyond the gut to the liver, adipose tissue, brain, and more. The safety of probiotic C. butyricum strains appears well-established. We identify areas where additional human randomized controlled trials would provide valuable further data related to the strains’ utility as an intervention.
KEYWORDS: Clostridium butyricum, short chain fatty acid, butyrate, immunity, intestinal barrier, inflammation, irritable bowel syndrome, metabolic disease, cancer, neurodegeneration
Clostridium butyricum, a butyrogenic gut symbiont
C. butyricum, a butyrate-producing, spore-forming anaerobic bacterium, is found in a wide variety of environments, including soil, cultured milk products, and vegetables. It is also present in the human gut: it is detected in 10–20% of the adult human population and is often one of the earliest colonizers in infants.1,2 In the human gut, where it is considered a ‘symbiont3ʹ (living together with the host), C. butyricum has a fermentative lifestyle and can consume undigested dietary fibers and generate short-chain fatty acids (SCFAs), specifically butyrate and acetate. Butyrate is one of the dominant fermentation end-products and is produced by C. butyricum via the butyrate kinase (buk) pathway.4 SCFAs produced by microbial organisms in the colon are known to have myriad and important effects on host health, including modulating intestinal immune homeostasis, improving gastrointestinal barrier function, and alleviating inflammation.5 As such, butyrate-producing organisms like C. butyricum have become attractive candidates to test for beneficial effects in a host. Genomic analyses are increasingly identifying novel bacterial strains with health-promoting potential that are distinct from classic probiotics (Lactobacilli and Bifidobacteria).
C. butyricum is a species that encompasses various known strains, some of which have genes equipping them to produce toxins.6 However, genomic analyses confirm that other strains do not have these genes nor other markers of pathogenesis potential, and that these nonpathogenic strains have excellent potential to benefit host health through several mechanisms. Certain strains of C. butyricum have been used as a probiotic7 for decades. Strain MIYAIRI 588 (or MIYARI 588; CBM 588), first isolated from the feces of a healthy human by Dr. Chikaji Miyairi in 1933, and later from soil in 1963, is a commercially-available, over-the-counter probiotic widely used in Japan, Korea, and China for the treatment of (antimicrobial-associated) diarrhea.8,9 Strain CBM 588 is also authorized under the regulation of the European Parliament and of the Council as a novel food ingredient.10 Its widespread use is enabled by its safe, nonpathogenic and nontoxic profile: studies have shown that it is sensitive to antibiotics, devoid of pathogenic markers, and lacks clostridial toxin genes.11
Dysbiosis in the gut microbiome has been implicated in multiple diseases, including gastrointestinal, neurological, and metabolic disorders, as well as cancer. Across many of these disorders there is evidence to suggest that C. butyricum is a promising therapeutic candidate. Although the precise mechanisms explaining these health benefits are not fully elucidated, current evidence suggests that in vivo, localized production of SCFAs is important for conferring health benefits. There are numerous pathways by which acetate and butyrate impact immune homeostasis and the physiology of the gut barrier. It is also suggested that C. butyricum may modulate the composition of the gut microbiome, possibly increasing certain beneficial bacterial taxa such as Lactobacillus and Bifidobacterium.12–15
Here we review studies pertaining to C. butyricum supplementation and host health, with an emphasis on the mechanisms of action of the probiotic. We have divided the review into two broad sections: (1) conserved interactions between C. butyricum and host physiological and immunological pathways, and (2) health outcomes in humans and in murine models of human disease.
C. butyricum supplementation: mechanisms of action and target pathways
The following sections detail well-supported mechanisms by which C. butyricum impacts host health with implications in multiple disease states: namely, effects of C. butyricum on gut microbial composition, gut barrier function, and immunological and inflammatory pathways. The interaction between C. butyricum and the Akt signaling pathway – a multifaceted regulator relevant to cancer, diabetes, and cardiovascular and neurological diseases – is also discussed.
C. butyricum and modulation of the gut microbiome
Multiple murine disease models have been employed to examine the effect of C. butyricum on gut microbiome composition, using a variety of techniques (Table 1). PCR amplification of the V3, V4, or V5 regions of the 16S rRNA gene from fecal samples, and subsequent Illumina deep sequencing, is the most common technique, used across 10 of the 16 studies detailed in Table 1. Three studies profile gastrointestinal microbiota using PCR-DGGE (denaturing gradient gel electrophoresis), three employ a culture-based approach, and four use qPCR to quantify either specific taxa or specific functions
Table 1.
The effect of C. butyricum supplementation on gut microbiome composition across murine models of disease
| Disease | Study | Probiotic | Dose (daily) | Treatment Duration | Study size | Treatment groups | Community analysis method | Community analysis general metrics | Community analysis specific taxa |
|---|---|---|---|---|---|---|---|---|---|
| Kong et al. 2011 | C. butyricum | 104 CFU (low-dose); 106 CFU (medium); 108 CFU (high) | 14 days | n = 10/group | vehicle control, low-dose, medium-dose, high-dose |
culture-based | Bifidobacterium spp. increased in medium/high-dose groups; Clostridium perfringens below detection in all C. butyricum groups (vs. above in control) | ||
| Long et al. 2018 | C. butyricum Sx-01; L. salivarius C-1-3 | 4x108 CFU (L. salivarius); 4 × 108 CFU (C. butyricum); 2 × 108 CFU each (combination) | 14 days | n = 20/group | vehicle control, L. salivarius (LS), C. butyricum (CB), combination |
16S rRNA PCR (V3-V4) and Illumina sequencing | increase in Bray-Curtis dissimilarity index between C. butyricum group and other 3 groups | LEfSe (Linear discriminant analysis effect size) shows Erysipelatoclostridium and Coriobacteriaceae, Coriobacteriales and Coriobacteria responsible for differences in C. butyricum-treated groups | |
| antibiotic – associated diarrhea (AAD) | Ling et al. 2015 | C. butyricum CGMCC 0313.1; B. infantis CGMCC 0313–2 | 1.2x109 CFU (C. butyricum); 2 × 1010 CFU (B. infantis); 4 × 107 CFU each (combination) | 5 days; 15 days | n = 10/group | healthy control, AAD, AAD+saline, AAD+C. butyricum, AAD+B. infantis, AAD+combination |
PCR-DGGE; qPCR on select taxa | DGGE profiles different for AAD vs. healthy; C. butyricum and combination treatments increase diversity of DGGE profiles and revert total bacterial counts to normal after 15 days | C. butyricum restores Bacteroides/Prevotella, Clostridium clusters XI and I, F. prausnitzii, Bifidobacterium and Lactobacillus, and decreases Enterococcus group |
| AAD | Hagihara et al. 2018 | CBM588 | 3.4x108 CFU/kg | 4 days | n = 5/group | healthy control, Clindamycin (AAD), AAD+C. butyricum, AAD+combination |
16S rRNA PCR (V3-V4) and Illumina sequencing | alpha-diversity not different between clindamycin and combination groups (both decreased); alpha-diversity not different between control and C. butyricum groups (both stable) | Bifidobacterium, Coprococcus, and Bacteroides increased in the combination group |
| AAD | Hagihara et al. 2019 | CBM588 | 3.4x108 CFU/kg | 4 days | n = 5/group | healthy control, clindamycin (AAD), AAD+C. butyricum, AAD+combination |
16s rRNA PCR (V3-V4) and Illumina sequencing | C. butyricum increases Actinobacteria (mainly Bifidobacterium) post day 10; PICRUSt predicts similar carbohydrate, lipid and amino acid pathways; combination shows increase in starch/sucrose metabolism and steroid hormone biosynthesis vs. clindamycin | |
| AAD | Hagihara et al. 2019 | CBM588 | 3.4x108 CFU/kg | 4 days | n = 5/group | healthy control, clindamycin (AAD), AAD+C. butyricum, AAD+combination |
16S rRNA PCR (V3-V4) and Illumina sequencing | alpha-diversity not different between clindamycin and combination groups, nor between control and C. butyricum groups; PCoA (weighted Unifrac distance) shows no change in community composition between C. butyricum and control group; clindamycin group separate from the control group; combination separates from the clindamycin group at day 4 | combination shows increase in Bifidobacterium, Lactobacillus and Lactococcus vs. clindamycin |
| ulcerative colitis (UC) | Zhang et al. 2009 | C. butyricum CGMCC0313.1 | 4.6x108 CFU (C. butyricum); 0.2 g (mesalamine); 0.1 mmol (sodium butyrate) | 21 days | n = 10/group (UC); n = 8 (healthy control) | healthy control, UC, UC+C. butyricum, UC+mesalamine, UC+sodium butyrate |
culture-based | model shows decrease in Bifidobacterium and Acidobacterium, and increase in Colibacter, Fusobacterium and Clostridium; C. butyricum, mesalamine and sodium butyrate reverse both trends | |
| Type 1 diabetes | Jia et al. 2017 | C. butyricum CGMCC0313.1 | 2.5 × 108 CFU/kg | 42 weeks | n = 28 (NOD); n = 25 (NOD+CB) | NOD, NOD+C. butyricum |
16s rRNA PCR (V4) and Illumina sequencing; qPCR (buk, but) | PCoA shows change in community structure in C. butyricum group | C. butyricum shows increase in buk and but genes and decreased Bacteroidetes, and increased Firmicutes, Clostridia and Clostridiales; other changes in specific taxa |
| Type 2 diabetes | Jia et al. 2017 | C. butyricum CGMCC0313.1 | 2.5 × 108 CFU/kg | 5 weeks (db/db); 13 weeks (HFD-induced) | n = 8/group (db/db); n = 8–12/group (HFD) | db/db+ vehicle, db/db+C. butyricum; healthy control, HFD, HFD+C. butyricum, HFD+sodium butyrate |
16s rRNA PCR (V4) and Illumina sequencing; qPCR (buk, but) | PCoA shows change in community structure in C. butyricum group and increase in diversity (Shannon) | C. butyricum shows increase in buk and but genes, increase in Bacteroidetes, Clostridia and Clostridiales, and decrease in Firmicutes; other changes in specific taxa |
| Acute pancreatitis (AP) and severe AP (SAP) | Pan et al. 2019 | CBM588; C. butyricum CGMCC0313.1 | 9.6x108 CFU/kg (MIYAIRI); 5.7 × 109 CFU/kg (CB0313.1); 9.6 × 108 CFU/kg (low-dose CB0313.1) | 21 days (pre AP or SAP induction) | n = 7/group | healthy control, AP, AP+MIYAIRI 588, AP+CB0313.1; Healthy control, SAP, SAP+MIYARI588, SAP+CB0313.1, SAP+low-dose CB0313.1 |
16s rRNA PCR (V4) and Illumina sequencing | PCA shows change in community structure in C. butyricum group | C. butyricum shows decrease in Desulfovibrionaceae and increase Akkermansia muciniphila, Clostridiaceae and Lactobacillaceae |
| acute liver injury (ALI) | Lui et al. 2017 | C. butyricum | 5x108 CFU | 5 days (pre ALI) | n = 10 (normal); n = 28 (ALI); n = 30 (CB) | healthy control, ALI, ALI+C. butyricum |
16s rRNA PCR (V4-V5) and Illumina sequencing | increase in diversity (Shannon) in C. butyricum vs. model group | C. butyricum shows increase in Clostridiales, Lactobacillales and Firmicutes |
| vascular dementia (VaD) | Lui et al. 2015 | C. butyricum WZMC1016 (CGMCC 9831) | 106 CFU (low); 107 CFU (medium); 108 CFU (high) | 6 weeks (post rUCCAO) | n = 12 (VaD and sham groups); n = 15 (CB) | sham control, VaD, VaD+CB low-dose, VaD+CB medium-dose, VaD+CB high-dose |
PCR-DGGE | model decreased diversity vs. sham; C. butyricum (high-dose) increased diversity; DGGE profile cluster analysis shows sham and medium/high doses of C. butyricum cluster together, and away from model and low-dose | |
| cerebral ischemia/reperfusion (I/R) injury in diabetic mouse | Sun et al. 2016 | C. butyricum WZMC1016 (CGMCC 9831) | 108 CFU | 6 weeks (24 h post I/R injury) | n = 12/group | sham control, I/R, I/R + C. butyricum |
PCR-DGGE; qPCR on select taxa | DGGE profiles show decreased diversity in model vs. sham; C. butyricum restores diversity | model shows decrease in Bacteroides/Prevotella, Clostridium cluster XIVab, F. prausnitzii, Bifidobacterium, and Lactobacillus, and increase in Clostridium cluster XI, Clostridium cluster I, Enterobacteriaceae and Enterococcus; C. butyricum reverses both trends |
| colorectal cancer (CRC) | Chen et al. 2020 | C. butyricum ATCC 19398 | 2x109 CFU 3x/week | 12 weeks | n = 10/group | basal diet, HFD, HFD+C. butyricum |
16S rRNA PCR (V3-V4) and Illumina sequencing | model shows decrease in diversity (Shannon/Simpson) vs. basal diet; no effect of C. butyricum on diversity and PCoA cannot separate C. butyricum from model group | model shows increase in Firmicutes/Bacteroidetes ratio vs basal diet; LEfSe links Ruminococcaceae and Eubacterium with C. butyricum treatment |
| Colitis-associated colorectal cancer (CAC) | Liu et al. 2020 | C. butyricum | 2x108 CFU 3x/week | 78 days | n = 10/group | healthy control, CRC, CRC+C. butyricum |
16S rRNA PCR (V3-V4) and Illumina sequencing | no differences among groups in diversity metrics (Chao1, Shannon, Simpson); PCoA shows no separation among groups | C. butyricum shows decrease in Firmicutes/Bacteroides ratio; other changes in specific taxa |
| neonatal development | Miao et al. 2018 | C. butyricum CGMCC0313.1 | 5x108 CFU | 20 days (lactation, maternal mice); 7 days (post-weaning, offspring) | n = 10/group | control, maternal intervention, offspring intervention, maternal and offspring intervention |
culture-based | maternal intervention shows decrease in Enterococcus, Enterobacter, and increase in Lactobacillus, Bifidobacterium in the offspring vs. control; offspring intervention shows increase in Lactobacillus and Bifidobacterium vs. control |
Culture and qPCR-based measurements support increases in specific taxa in response to C. butyricum supplementation, especially Bifidobacterium spp. and Lactobacillus spp.13,16,17 Additionally, culturing and qPCR consistently support a decrease in Enterococcus and/or Enterobacteriaceae.13,18,19 Interestingly, two studies20,21 demonstrate via qPCR an increase in butyrate producing genes in the collected stool microbiota (both butyrate kinase (buk) and butyryl-CoA:acetate CoA-transferase (but)). As the C. butyricum genome contains only buk, the observed increase implies a concomitant increase in other butyrate-producing microorganisms.
While several studies indicate no effect of C. butyricum supplementation on metrics of community composition (diversity indices, clustering in PCA/PCoA analysis),14,22,23 others show a pronounced effect on diversity and/or a distinct community composition post supplementation.12,15,20,21,24,25 Some of these differences might be methodological, for example PCR-DGGE data from models of antibiotic-associated diarrhea indicate increased diversity following C. butyricum supplementation while, in similar models, illumina 16S rRNA gene sequencing data do not. Profiles obtained with DGGE are limited in both their ability to detect lower abundance organisms and to provide higher taxonomic resolution. Even with 16S rRNA gene sequencing, however, community-level shifts in diversity should not be given undue importance as a wide number of factors are known to contribute including animal genotype, litter, housing, diet, and vendor.26–28 Additionally, the different C. butyricum strains tested, the different animal models employed, as well as the generally small number of animals used in most studies, should lead researchers to interpret observed C. butyricum-driven changes in community composition with caution.
C. butyricum strengthens the gut barrier
The intestinal barrier performs a critical balancing act: maintaining the tolerance of gut microbiota and absorption of nutrients on the one hand, and defense against pathogen invasion on the other. It acts as a selective barrier, preventing the permeation of toxins and antigens, while facilitating the absorption of nutrients, electrolytes, and water. Structurally, the intestinal barrier consists of three layers: the mucus layer, the epithelium, and the lamina propria (Figure 1).30,31
Figure 1.
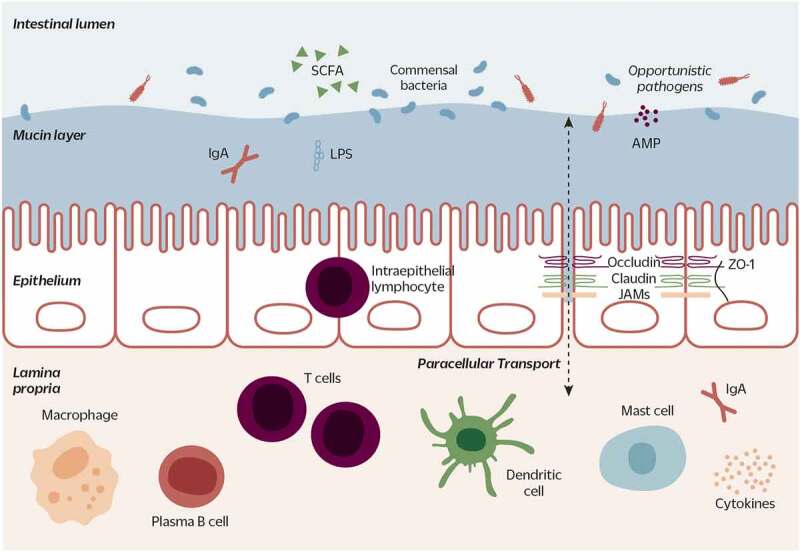
The intestinal barrier separates the intestinal lumen (where microorganisms reside) from host tissues. The gut barrier is composed of three layers: the outermost mucus layer acts as a physical barrier against invading microorganisms; AMPs and antibodies (e.g. IgA) form a chemical barrier. Subsequent to the mucosal layer is the epithelium: a monolayer of epithelial cells mechanically linked by TJ proteins (e.g. claudin, occludin, JAMS, ZO-1). TJs regulate paracellular transport, selectively allowing the passage of small molecules. The epithelium also harbors the mucus-secreting goblet cells (not shown), hormone-secreting enteroendocrine cells (not shown), and several other immune cell types (e.g. intraepithelial lymphocytes). Finally, the lamina propria is a layer of connective tissue beneath the epithelium. It contains the majority of immune cells: innate immune cells such as macrophages, dendritic cells, and mast cells, and adaptive immune cells, such as T cells and antibody-producing plasma B cells. The epithelial and immune cells produce cytokines, or signaling molecules, to communicate across the layers of the intestinal barrier. (Figure adapted from König et al. 201629)
AMP: antimicrobial peptide; TJ: tight junction; JAMs: Junctional adhesion molecules, ZO-1: zonulin-1
The mucus layer represents the primary physical barrier against pathogen invasion. This gel-like layer varies in thickness along the length of the GI tract and is primarily composed of glycoproteins (i.e mucins) secreted by goblet cells in the epithelium.32 Mucin 2 (MUC2) is the most abundant and well studied secreted gastrointestinal mucin. Knocking out MUC2 in mice can cause the development of spontaneous colitis and increases the risk of colorectal cancer, highlighting the importance of this barrier for immune homeostasis and overall health.33,34 Introducing C. butyricum has been shown to protect properties of the mucosal layer in two different mouse models. Administration of C. butyricum Sx-01 significantly increased colonic mucosal thickness in a murine study of probiotic supplementation for general intestinal health,25 while administration of CBM 588 increased mucin production (measured as increased expression of MUC2) and significantly decreased epithelial damage in the colonic tissue of mice with clindamycin-induced antibiotic-associated diarrhea.15 The effect of C. butyricum on mucosal health may be explained by its capacity for butyrate production. Indeed, butyrate has been shown to increase mucin production by goblet cells in vitro by increasing expression of the MUC genes35,36 (Figure 2).
Figure 2.
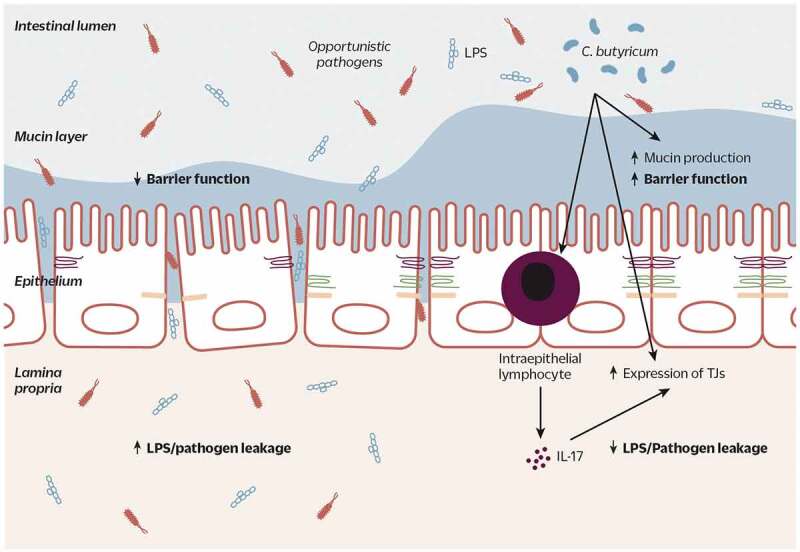
C. butyricum strains improve gut barrier function by increasing the thickness of the mucosal layer and increasing expression of TJ proteins. Increased expression of TJ proteins may also result from C. butyricum’s stimulation of IL-17 production from intraepithelial T cells. This improved barrier results in decreased permeation of LPS and pathogenic bacteria into host tissue and blood
TJ: tight junction; IL-17: interleukin-17; LPS: lipopolysaccharide
Subjacent to the mucosal layer is the epithelial monolayer (Figure 1). Here, tight junctions (TJs) bind epithelial cells together and regulate paracellular transport.37 Lowered expression of TJ proteins (occludin, claudin, JAMs, ZO-1) increases gut permeability. Increased permeability, in turn, allows bacteria or bacterial components such as lipopolysaccharides (LPS) and other antigens to leak into the lamina propria and incite an inflammatory response. The importance of TJs is seen in occludin knock-out mice, where histopathological abnormalities including chronic inflammation and hyperplasia of the gastric epithelium are observed.30 As well as influencing mucin production directly, C. butyricum has been shown to strengthen barrier function by influencing TJ protein expression across several different disease models, likely hinting at a conserved mechanism of action behind these organisms’ efficacy (Figure 2). In a mouse model of antibiotic-associated diarrhea, treatment with CBM 588 increased expression of TJ proteins occludin, claudin-4, and ZO-1.12,15 Similarly, in a mouse model of severe acute pancreatitis (SAP), two C. butyricum strains (CBM 588 and CGMCC0313.1) restored expression of TJ proteins ZO-1, ZO-2, and occludin.12,38 Following treatment with C. butyricum, mice with SAP also had decreased pancreatic levels of E. coli and Enterococcus, providing further indication of improved barrier function.12,38 In a mouse model of traumatic brain injury, C. butyricum supplementation again induced the recovery of occludin and ZO-1 expression in the brain and colonic tissue of mice.12,38 As in the SAP murine model, improved barrier function was further highlighted by decreased serum levels of D-lactate, a microbial byproduct and marker of a ‘leaky gut’. Finally, in a high-fat diet (HFD)-induced obesity mouse model, C. butyricum CGMCC0313.1 supplementation again increased expression of claudin-1 and occludin (measured at both the protein and mRNA level) as well as decreased serum LPS.39 The replicability in the above described studies strongly supports a role for C. butyricum in gut barrier integrity.
As with increased mucin secretion, C. butyricum’s protective effect on the intestinal epithelium may be attributed to butyrate production (Figure 2). Multiple studies note increases in fecal butyrate concentrations following C. butyricum supplementation and/or the proliferation of other butyrate-producing bacteria.12,15,20,21,24,39 Animal studies have shown beneficial effects of direct supplementation with sodium butyrate against increased intestinal permeability,40,41 and several in vitro studies have identified the signaling pathways involved in butyrate regulation of TJ protein expression.42,43 Alternatively or in parallel, immunomodulation of the versatile cytokine interleukin-17 (IL-17) by C. butyricum may contribute to these effects. Hagihara and colleagues describe an enhanced barrier integrity effect following probiotic administration of C. butyricum that also resulted in an increased production of IL-17 by γδ T cells, a specific subset of intraepithelial T cells that act as a part of the first line of defense, in the colonic lamina propria (Figure 2).15 Although often perceived as pro-inflammatory, IL-17 secreted locally by γδ T cells serves to protect and repair the gut barrier by ensuring expression of TJ proteins.44 Finally, Hagihara and colleagues have also reported that the protective effect of C. butyricum on the intestinal barrier was associated with host metabolic alterations that lead to attenuation of antibiotic-induced gut inflammation.15,45 C. butyricum promoted the production of anti-inflammatory lipid metabolites such as palmitoleic acids, prostaglandin metabolites, and specialized pro-resolving mediators in mouse colonic tissues. Such lipid metabolites, especially protectin D1,46 contribute to the promotion of anti-inflammatory IL-10 secreting T-cells in the colon. Therefore, C. butyricum-mediated modulation of host immunological and metabolic pathways supports yet another protective mechanism of C. butyricum in intestinal inflammation.
C. butyricum modulates immune function and inflammation
Box 1: Immune homeostasis and the gut microbiome
As described above, the gut barrier separates the host from the trillions of microorganisms that inhabit the gut lumen. To provide an effective defense, the gut barrier harbors a multitude of immune cells and represents the largest immune organ in the human body.47 The intestinal epithelium monitors the gut microbiota via pathogen recognition receptors (PRRs) capable of recognizing pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS). PRRs distinguish the pathogens from non-pathogens and mediate immune response against the former. The inflammatory mediators released by the epithelium upon detection of pathogens trigger the maturation of antigen presenting cells (APCs) such as dendritic cells. Mature APCs then produce signals to activate the differentiation and expansion of appropriate T cell types. Additionally, microbial components and metabolites of both pathogens and commensals can stimulate the release of host-derived antimicrobial peptides (AMP) or secreted immunoglobulin A (IgA) that provide an additional chemical barrier between the luminal microorganisms and the host (Figure 1). Therefore, changes in bacterial composition in the gut can shift the immune system, with effects often extending beyond the gut.48
Immune homeostasis – the balance between pro – and anti-inflammatory profiles – is maintained through the proper regulation of immune cells and cytokines. Under homeostasis, the types of immune cells and the cytokines they produce maintain a balance between pro – and anti-inflammatory profiles. Inappropriate activation of the immune response can be self-deleterious, and is an underlying problem of many modern inflammatory diseases: immune disorders such as allergies and autoimmune diseases; inflammatory bowel disease; metabolic diseases such as diabetes and hepatitis; and neurodegenerative diseases. All of these inflammatory diseases have been associated with changes in the composition of gut microbiota. Thus, there currently exists a rationale for probiotic bacteria, such as C. butyricum, playing a protective role against infectious gut-acquired pathogens, intestinal injury, and inflammatory conditions by modulation of the host immune system.16,19,48
T cells are a part of the adaptive immune system and are major contributors to shaping host immune profiles.49 Differentiation of naive T cells to effector T cells is controlled by distinct sets of cytokines and transcription factors, typically secreted by innate immune cells including APCs. For example, dendritic cell production of IL-6 or IL-23 induces expansion of T helper (Th)17 cells, while the production of TGF-β in the absence of IL-6 or IL-23 leads to the expansion of regulatory T cells (Tregs). Effector T cells are grouped depending on the profile of the cytokines they produce and the effect of each cytokine. Th1 and Th17 responses, driven by interferon-gamma (IFN-γ) and interleukin (IL)-17, respectively, typically dominate during inflammatory disease. IFN-γ and IL-17 mediated inflammations – involving the killing of infected cells and engulfing of invading pathogens – often lead to tissue damage and exacerbation of inflammation.16,50 On the other hand, Th2 response suppresses Th1 and Th17 response but leads to B cell-mediated antibody production, an important step for the body to prepare immune responses to future infections but also a contributor to allergic diseases when improperly controlled. Tregs are a special group of effector T cells that oppose the inflammatory Th responses and therefore are anti-inflammatory. The Treg response restrains the inflammatory response by the production of IL-10, an anti-inflammatory cytokine that suppresses the Th1, Th2, and Th17-mediated inflammation.51 Restoring the Treg response and maintaining a balance with pro-inflammatory responses may be a critical part of combating inflammatory diseases.
C. butyricum restores immune homeostasis by promoting Treg responses
The Treg response suppresses activation of inflammatory responses driven by effector T cells such as Th1, Th2 and Th17 (See Box 1 for background on immune homeostasis). The balance between Tregs and pro-inflammatory effector T cells in the intestine is increasingly recognized as regulated by the gut microbiome.52 Murine disease models have shown that administration of C. butyricum promotes intestinal immune tolerance by increasing the abundance of Tregs, as evidenced by the accumulation of induced Tregs especially in the mesenteric lymph node,21,50,53 along with the decreased proportion of Th1 and Th17 cells in the disease target organs such as the pancreas,21,50,53 spleen,21,50,53 liver,24,54 brain, and intestines.21,50,53 In a murine model of colitis, depletion of Tregs partially abolished the preventive effect of CBM 588.48 The mechanism behind the increased Treg population in groups administered C. butyricum has been attributed to increased production of TGF-β, a cytokine that induces Treg differentiation16,50,55,56 (Figure 3). Several studies have pointed to colonic dendritic cells as the source of C. butyricum-induced TGF-β.57,58 Dendritic cells, as the major antigen presenting cells (APCs), translate the external stimuli to differentiate naive T cells and modulate the adaptive immune response. CBM 588 has been shown to activate Toll-like Receptor 2 (TLR2)-dependent TGF-β expression in colonic dendritic cells, and thus promote Treg differentiation.57 As a result, mice administered C. butyricum were protected from chemical-induced colitis via a dendritic TGF-β and Treg dependent mechanism. C. butyricum also suppressed the maturation of dendritic cells and the emergence of an inflammatory phenotype. In a chemical-induced irritable bowel syndrome (IBS) mouse model, C. butyricum decreased the proportion of dendritic cells expressing T cell immunoglobulin and mucin domain 3 (TIM3).58 TIM3-expressing dendritic cells are positively associated with pro-inflammatory cytokine secretion. Therefore, C. butyricum is postulated to promote an anti-inflammatory Treg response by increasing TGF-β secretion, possibly by modulating dendritic cell signaling. Alternatively, butyrate produced by C. butyricum may directly facilitate the differentiation of Tregs via histone deacetylase (HDAC) inhibition,59,60 rather than modulating dendritic TGF-β57
Figure 3.
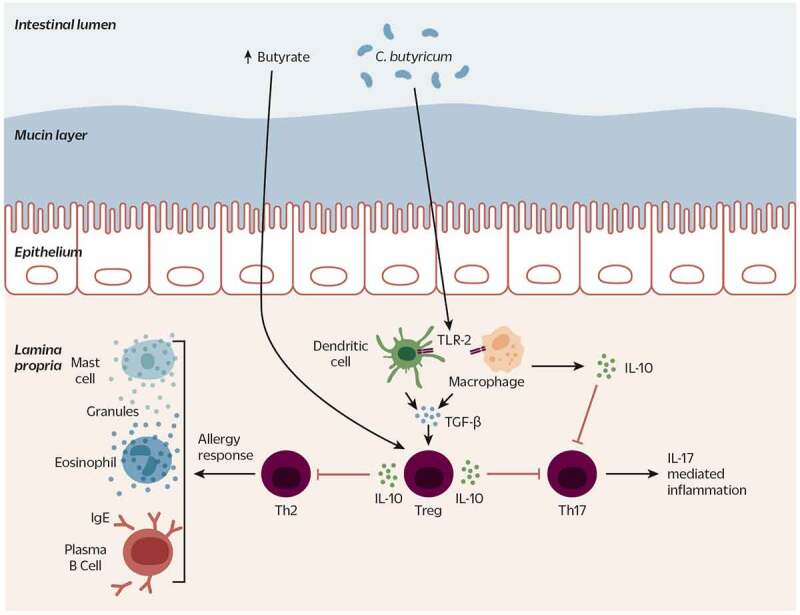
C. butyricum strains modulate the immune system to present a tolerant and anti-inflammatory signature. Possibly via butyrate signaling and/or an LTA-activated TLR2-dependent pathway, C. butyricum stimulates TGF-β and IL-10 secretion from APCs (dendritic cells and macrophages), Tregs and intestinal epithelial cells. Increased levels of butyrate, TGF-β, and IL-10 contribute to Treg differentiation. An increased population of Tregs and IL-10 inhibit differentiation of Th17 cells that induce IL-17-mediated inflammation, and Th2 cells that induce an allergic response from eosinophils, mast cells, and plasma B cells. Dotted lines indicate indirect effect
LTA: Lipoteichoic acid; TLR2: Toll-like receptor 2; TGF-β transforming growth factor beta; APC: antigen presenting cell; IL-10: interleukin-10; Treg: regulatory T cell; Th17: T helper 17; Th2: T helper 2
An anti-inflammatory cytokine, IL-10, suppresses the effector T cell response in a variety of ways. First, IL-10 signals to APCs to produce TGF-β and therefore triggers Treg differentiation.61 IL-10 amplifies the suppressive actions of Tregs by inducing further IL-10 production. Additionally, IL-10 suppresses pro-inflammatory cytokine production in various APCs and T cells by inhibiting nuclear factor-kappa B (NF-κB).62 The anti-inflammatory effect of C. butyricum and its role in promoting Treg response is tied to the increased level of IL-1016,48,50,55,56 (Figure 3). In a murine colitis model, depletion of macrophage-produced IL-10 diminished the protective effect of CBM 588, confirming its indispensable role.48 C. butyricum increases IL-10 levels by activating TLR2 in colonic macrophages. Specifically, CBM 588 lipoteichoic acid (LTA) has been shown to modulate TLR2 signaling and suppress pathogen-induced inflammation and apoptosis in vitro.63,64 Thus, C. butyricum promotes an anti-inflammatory Treg response by induction of IL-10 and TGF-β, possibly through activation of a TLR2-dependent pathway (Figure 3).
C. butyricum has been shown to suppress Th1 and Th17 mediated inflammations, presumably via the increased Treg response (Figure 3). Uncontrolled Th1 and Th17 responses are the culprits behind autoimmune and other inflammatory diseases.65,66 Treatment with C. butyricum in several inflammatory disease models decreased levels of the pro-inflammatory cytokines of Th1 and Th17 cells. Rats with oxazolone-induced colitis receiving either C. butyricum CGMCC0313.1 or sodium butyrate displayed improved pathologies in the colon compared to the no treatment group, including significantly decreased serum levels of IL-23, suggesting a possible anti-inflammatory mechanism behind the improved colitis.16 In a model of food allergy-induced diarrhea, treatment with C. butyricum CGMCC0313.1 prior to milk protein challenge significantly reduced the incidence and severity of diarrhea, and concomitantly decreased serum levels of IL-17.50 The beneficial effects of C. butyricum extended beyond the gastrointestinal tract; C. butyricum CGMCC0313.1 treatment improved pancreatic damage and decreased the Th1 and Th17 cells in the pancreas in a model of type 1 diabetes.21 Similarly, in a model of nonalcoholic fatty liver disease (NAFLD), CBM 588 alleviated liver damage while decreasing levels of IFN-γ and IL-17 in the liver and the ileum.54
Allergic diseases are driven by improper activation of the Th2 response.13,67 Th2 cytokines induce B cells to produce immunoglobulin E (IgE) (i.e., antibodies) and activate allergy-responsive immune cells (eosinophils, basophils, and mast cells). Because of its role in inducing memory and repair, the Th2 response is typically considered anti-inflammatory. However, in the context of allergic disease overproduction of Th2 cells can trigger inappropriate immune responses that can be damaging to the host. C. butyricum administration has been shown to alleviate allergic responses, again possibly by the modulation of Tregs (Figure 3). In a mouse model of asthma, C. butyricum CGMCC0313.1 reduced airway hyperresponsiveness and lung inflammation by suppressing the Th2 response and increasing anti-inflammatory IL-10.68 In asthmatic lungs, mast cells infiltrate the airway and degranulate, releasing inflammatory molecules and proteases that result in tissue swelling.69 In lungs from mice administered C. butyricum, the number of infiltrating immune cells, level of Th2 cytokines (IL-4 and IL-5), markers of mast cell degranulation, and levels of IgE were all decreased. Similarly, in a food allergy mouse model, C. butyricum CGMCC0313.1 treatment resulted in attenuation of allergic reaction, along with an increased measure of forkhead box P3 (Foxp3), an indication of increased Treg.50 Pre – or post-treatment with C. butyricum reduced the severity of diarrhea and anaphylaxis, as well as circulating mast cell proteases, IgE, and Th2 cytokines IL-4, IL-5, and IL-13. Increased abundance of Tregs in the intestine (mesenteric lymph node) and the associated cytokines IL-10 and TGF-β in the serum were noted. Thus, C. butyricum attenuates the Th2 response-driven allergic reaction and the associated inflammation by mediating the Th2/Treg balance.
In summary, C. butyricum plays an immunomodulatory role in the intestinal epithelium, by mediating tolerogenic APC signaling to promote the Treg response in the presence of pathogenic or pro-inflammatory stimuli. Such modulation results in suppression of pro-inflammatory effector T cell responses, such as Th1, Th2, or Th17. An alternative, non-mutually exclusive explanation for these effects may be direct suppression of Th response by IL-10 production from the intestinal epithelial cells or APCs (Figure 3).
Activation of Akt pathway by C. butyricum
Akt, also called Protein Kinase B (PKB), is a serine/threonine-specific protein kinase that is involved in multiple cellular processes such as cell survival, proliferation, apoptosis, and metabolism. The Akt signaling pathway is highly regulated, and its dysregulation is linked to neurodegenerative and metabolic diseases, as well as cancer.
Cellular apoptosis, or programmed cell death, underlies many complications of diseases, especially neurodegenerative disorders. Activation of Akt releases phosphorylated Akt (pAkt) to translocate to the membrane, where it influences downstream factors to promote cell survival or inhibit apoptosis. C. butyricum administration prevents unwanted neuronal apoptosis and brain damage via activation of the Akt pathway (Figure 4a).19,38,70 For example, C. butyricum supplementation restored levels of cerebral pAkt in murine models of diabetic ischemia/reperfusion,19 vascular dementia,70 and traumatic brain injury,38 alongside improvements in cognitive impairment and/or histopathological evidence of ameliorated neuronal apoptosis and brain damage. Upstream molecules such as brain-derived neurotrophic factor (BDNF)19,70,71 and glucagon-like peptide-1 (GLP-1) were also increased.38,71 Downstream of Akt phosphorylation, the mitochondria mediates apoptosis via several pathways, one of which is regulated by B cell lymphoma 2 (Bcl-2) and Bcl-2 associated x protein (Bax).72 In a model of vascular dementia, C. butyricum CGMCC 9831 treatment contributed to preventing apoptosis through an Akt-dependent mechanism as evidenced by increased concentrations of the anti-apoptotic Bcl-2 and decreased the pro-apoptotic Bax.70 Furthermore, caspase-3, a protease activated by Bax and a central mediator of cell death, was suppressed as a result of CGMCC 9831 treatment in a mouse model of cerebral ischemia/reperfusion injury.73
Figure 4.
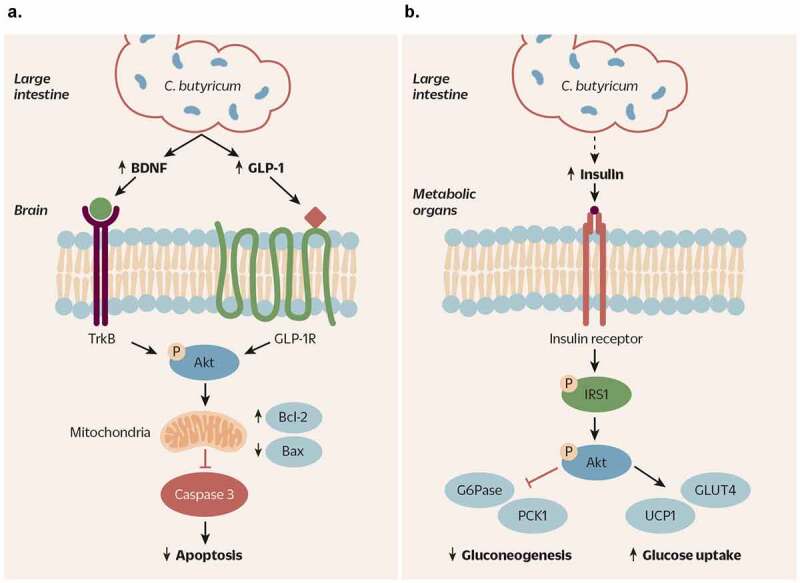
Signal transductions of C. butyricum-activated Akt pathways and their impacts in neurodegenerative disorders and metabolic diseases. A) Treatment of various neurodegenerative animal models with C. butyricum activates the Akt pathway in the brain via increasing BDNF and GLP-1. BDNF binding to its receptor, TrkB, or GLP-1 binding to GLP-1 R in the brain activates Akt via phosphorylation. Downstream of phosphorylated Akt, elevation of Bcl2 and downregulation of Bax in the mitochondria leads to inhibition of caspase-3-mediated apoptosis. As a result, subjects treated with C. butyricum are protected from neuronal death and damage. B) C. butyricum treatment of diabetic models, presumably via increased GLP-1 signaling and insulin sensitivity, results in increased phosphorylation of IRS-1 and the downstream activation of Akt in metabolic organs such as the liver, adipose tissue, and skeletal muscle. Activation of Akt pathway suppresses gluconeogenesis by increasing expression of G6Pase and PCK1, and induces glucose uptake by upregulation of GLUT4 and UCP1. Therefore, C. butyricum treatment of diabetic individuals may be effective in improvement of insulin sensitivity and glucose homeostasis via activation of Akt pathway
BDNF: brain derived neurotrophic factor; GLP-1: glucagon-like peptide 1; TrkB: tropomyosin receptor kinase B; GLP-1 R: glucagon-like peptide 1 receptor; Bcl2: B cell lymphoma 2; Bax: Bcl2 associated x protein; IRS-1: insulin receptor substrate 1; G6Pase: glucose-6-phosphatase; PCK1: phosphoenolpyruvate carboxykinase 1; GLUT4: glucose transporter type 4; UCP1: uncoupling protein 1
GLP-1 is an upstream ligand of the Akt pathway, participating in, among other things, gut-brain communication.74 C. butyricum supplementation increased GLP-1 secretion from the L cells within the intestinal epithelium, putatively due to increased butyrate production triggering the activation of G-protein-coupled receptors 41 and 43 (GPR41 and GPR43).20,71,75 Therefore, modulation of GLP-1 signaling may be a mechanism by which C. butyricum CGMCC 9831 in the gut exerts anti-apoptotic neuroprotection in the model of traumatic brain injury19,38,70 (Figure 4a). Similarly, in a model of chronic stress-induced depression, C. butyricum CGMCC 9830 administration reversed depression-like behavior, increased levels of hippocampal BDNF, and also increased intestinal levels of GLP-1.71 Thus, C. butyricum-mediated anti-apoptotic effects may be due to increased SCFA, increased GLP-1 signaling, and the consequent activation of PI3K/Akt pathway (Figure 4a).
The physiological roles of the Akt pathway are not limited to cellular apoptosis. Also upstream of Akt is insulin receptor substrate 1 (IRS-1), an adaptor protein crucial in insulin signaling. Dysregulation of IRS proteins and the subsequent failure to activate the Akt pathway can lead to insulin resistance. In a diabetes mouse model, C. butyricum CGMCC0313.1 or sodium butyrate supplementation increased levels of GLP-1 in the intestine and in the serum.20 GLP-1 is an insulinotropic hormone (i.e., stimulates insulin secretion), and thus the serum insulin level was increased as compared to untreated mice. Accordingly, the IRS-1/Akt pathway was activated, and improved insulin signaling was evidenced by improvements in glucose homeostasis and adipose tissue metabolism, and alleviated pancreatic inflammation. The observed benefits are driven by decreased gluconeogenesis within the liver––through downregulation of glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase 1 (PCK1)––concomitant with increased glucose uptake by insulin targeted tissues, mediated by glucose transporter type 4 (GLUT4) and uncoupling protein 1 (UCP1) (Figure 4b). Therefore, C. butyricum, presumably via butyrate, may reduce insulin resistance through GLP-1-induced activation of the IRS-1/Akt pathway.
Benefits of C. butyricum supplementation across disease endpoints
The mechanisms described above show potential for beneficial effects of C. butyricum. From a host perspective, what matters is the ability of probiotic strains to actually confer a measurable health benefit. The following sections detail evidence for the host health effects of C. butyricum strains – first in animal models of human disease, and then in human clinical trials. Taken together, these studies demonstrate specific health benefits of C. butyricum supplementation in a variety of immune-linked diseases. The safety of C. butyricum is also borne out in these animal and human studies.
Evidence from murine models of disease
C. butyricum and gastrointestinal health
Several studies in animal models have demonstrated C. butyricum’s ability to reduce the incidence of antibiotic-associated diarrhea (AAD) and antibiotic-induced gut dysbiosis.8,14,18,76 In an antibiotic-associated diarrhea mouse model, administration of a probiotic mix containing C. butyricum and Bifidobacterium infantis for 15 days restored colonic mucosal architecture and fecal microbial diversity, and decreased systemic inflammation.18 Similarly, C. butyricum alone effectively prevented colonic damage when administered in combination with antibiotics.14,15
Studies have also identified C. butyricum as a preventative intervention for gut pathogen infection. Enterohemorrhagic Escherichia coli (EHEC) O157:H7 causes diarrhea and hemorrhagic colitis in humans. Takahashi et al. demonstrated that EHEC O157:H7 growth, toxin production, and adhesion to epithelial cells is inhibited in vitro by CBM 588.77,78 Prophylactic C. butyricum administration to germ-free mice prior to EHEC O157:H7 infection prevented death completely, while administration post infection increased the survival rate from 0% in non-treated controls to 50%.77 C. butyricum may also play a preventative role in Clostridium difficile infection. C. difficile is a common pathogen responsible for a large proportion of AAD cases, and in a human study C. butyricum administration was associated with decreased fecal levels of C. difficile as well as the toxin it produces.79 More recently, Woo and colleagues demonstrated that in vitro co-culture of C. difficile with CBM 588 decreased the pathogen’s toxicity in a dose-dependent manner.80 Oka et al. used a non-lethal rat model of C. difficile infection to show a reduced incidence of diarrhea with CBM 588 supplementation.81
C. butyricum supplementation has also demonstrated a positive effect across several murine models of colitis, ulcerative colitis (UC), and irritable bowel syndrome (IBS).16,48,56,58,82,83 CBM 588 culture supernatant (termed “C. butyricum derivative”) reduced mucosal damage and diarrhea in a dextran sulfate sodium (DSS)-induced colitis model in rats.82 CBM 588 probiotic supplementation prevented DSS-induced colitis in mice, an effect mediated by modulation of IL-10.48 Zhao and colleagues also found protective effects in a 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced mouse model of IBS: C. butyricum supplementation reduced intestinal visceral hypersensitivity and mucosal inflammation.58 C. butyricum supplementation has also been investigated in three types of gastric ulcer models: alcohol-induced, restraint cold stress, or pyloric ligation; supplementation decreased gastric mucosal injury alongside improvements in oxidative stress and inflammatory status.84
C. butyricum in the management of metabolic diseases
Metabolic diseases including diabetes (type 1 and type 2) and liver diseases are considered systemic conditions, as they arise from and continue to affect dysregulated inter-organ crosstalk. Even though C. butyricum and other gut microbes are physically located in the gut lumen, their indirect effects extend from gut barrier integrity to immune modulation and endocrine regulation, consequently influencing metabolic signals throughout the body (Figure 5). In this section, C. butyricum’s potential in improving energy metabolism in the pancreas, adipose tissue, and liver is explored in animal models of human metabolic diseases.
Figure 5.
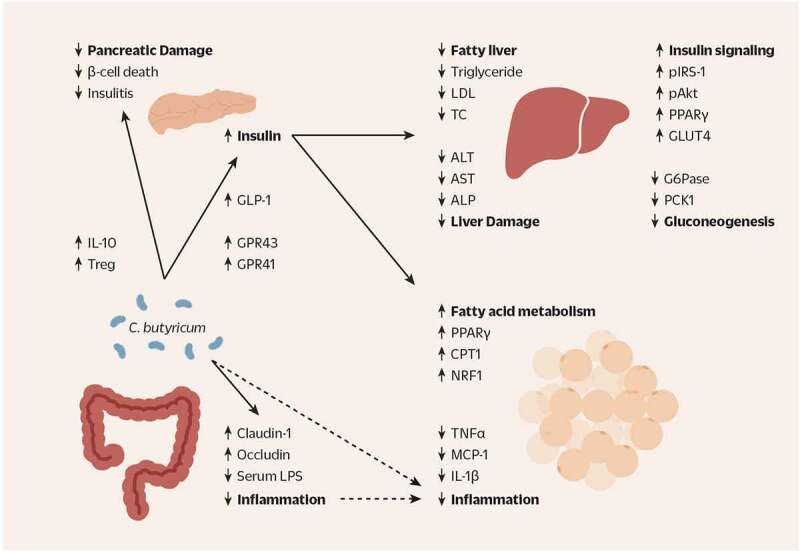
The proposed beneficial effects of C. butyricum strains in the gut, pancreas, liver, and adipose tissue of individuals with metabolic disease. C. butyricum increases the concentration of SCFA as well as the abundance of butyrate-producing bacteria in the gut. The increased secretion of GLP-1 in the colon and the bloodstream can be attributed to the increased SCFA receptor signaling via GPR41 and GPR43. GLP-1 has pleiotropic effects across many organs in reducing appetite, slowing gastrointestinal motility, decreasing gluconeogenesis and increasing glucose uptake, and most importantly, increasing secretion of insulin from the pancreas. As such, in the liver C. butyricum increases insulin signaling (pIRS-1, pAkt, PPAR-γ, GLUT4) and decreases gluconeogenesis (G6Pase and PCK1). Serum markers of hepatic lipid storage such as triglyceride, LDL, and TC are decreased, indicating improved lipid metabolism. Concurrently, the markers of hepatic damage (ALT, AST and ALP) are decreased as well. In the adipose tissue, C. butyricum upregulates genes involved in mitochondrial fatty acid oxidation, indicating enhanced fatty acid metabolism: PPAR-γ, CPT1α, and NRF2. Finally, pro-inflammatory cytokines TNF-α, IL-1β, and MCP1 are decreased in the adipose tissue. Such anti-inflammatory effect may be associated with the direct effect of C. butyricum-produced metabolites such as butyrate, indirect effects of butyrate such as increased GLP-1 signaling and insulin secretion, and/or the decreased gut inflammation due to healthier gut epithelium evidenced by increased tight junction proteins claudin-1 and occludin and increased levels of serum LPS. Additionally, the population of Tregs and the level of anti-inflammatory cytokine IL-10 are increased due to C. butyricum, contributing to the reduced inflammation in the other organs, allowing proper insulin secretion and energy metabolism
SCFA: short chain fatty acid; GPR41/43: G-protein-coupled receptor 41/43; GLP-1: glucagon-like peptide 1; pIRS-1: phosphorylated insulin receptor substrate 1; pAkt: phosphorylated Akt; PPAR-γ: peroxisome proliferator-activated receptor-gamma; GLUT4: glucose transporter type 4; g6pase: glucose-6 phosphatase; PCK1: phosphoenolpyruvate carboxykinase 1; LDL: low density lipoprotein; TC: total cholesterol; ALT: alanine transaminase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; CPT1α: carnitine palmitoyltransferase-1α; NRF2: nuclear factor erythroid 2-related factor 2; TNF-α: tumor necrosis factor alpha; IL-1β: interleukin-1 beta; MCP1: monocyte chemoattractant protein 1; LPS: lipopolysaccharide; Treg: regulatory T cell; IL-10: interleukin 10
Obesity and type 2 diabetes
C. butyricum has potential anti-diabetic effects as murine type 2 diabetes studies have reported that C. butyricum supplementation ameliorates abnormalities in host metabolism and microbial butyrate production, which is shown to be deficient in humans with this condition.85 Jia et al. examined both leptin receptor-deficient (lepdb/db) and high-fat diet (HFD) + streptozotocin induced diabetic mice and Shang et al. examined HFD-induced diabetic mice.20,39 Both studies noted C. butyricum supplementation reduced weight gain and fat accumulation, and improved glucose tolerance and insulin sensitivity. Parameters of improved metabolism included increased serum insulin and decreased fructosamine and G6Pase supporting glucose metabolism, and increased respiratory exchange ratio (RER) indicating better fat oxidation.20,39 Authors invoke promotion of mitochondrial function as a part of the metabolism-boosting effect of C. butyricum, supported by increased markers of mitochondrial metabolism in the adipose tissue, as well as the activation of the insulin-mediated hepatic peroxisome proliferator-activated receptor gamma (PPAR-γ).20,39 In addition, both studies showed decreased systemic and localized inflammation in the adipose tissue, pancreas, and/or colon with C. butyricum. Jia and colleagues also noted a C. butyricum-induced decrease in hepatic and pancreatic damage, and Shang et al. additionally saw improved gut barrier function post supplementation. Finally, C. butyricum supplementation across both studies lead to improvements in the HFD-induced reduction of fecal SCFAs, and Jia and colleagues further measured increased expression of microbial butyrate-production genes, as well as increased mRNA levels of host colonic GPR41 and GPR43 with treatment.
Type 1 diabetes
Type 1 diabetes involves autoimmune damage to the pancreatic β-cells (responsible for insulin production and secretion), leading to insulin deficiency. Both dysregulation of the gut immune system and decreased gut butyrate production are implicated in the pathology of type 1 diabetes.86,87 Thus, Jia and colleagues investigated the possible effects of C. butyricum in the development and progression of type 1 diabetes.21 C. butyricum supplementation of non-obese diabetic (NOD) mice both delayed the onset and reduced the incidence of diabetes.21 As detailed earlier in the mechanisms section, this study demonstrated the role of C. butyricum supplementation in the modulation of Treg development and migration as the basis of improving insulitis, the main culprit in T1D. C. butyricum induced and propagated more Tregs from the gut, and suppressed pro-inflammatory IFNγ and IL-1 cytokines in the pancreas. Thus, it is hypothesized that the improvements noted may stem from an increased Treg response and immune tolerance, decreasing the destructive autoimmunity in the pancreas. In addition, C. butyricum supplementation again increased expression of microbial genes involved in butyrate production, and trended toward increased fecal butyrate levels. Interestingly, direct oral administration of sodium butyrate led to an earlier onset of T1D compared to control mice. The efficiency of orally administered butyrate in increasing colonic butyrate levels is unresolved and researchers in this study did not see an increase in fecal butyrate levels with oral sodium butyrate supplementation.
Liver disease
The potential benefits of C. butyricum have also been investigated in liver metabolic dysfunction. Nonalcoholic fatty liver disease (NAFLD) is common in obese and insulin resistant individuals, especially those with type 2 diabetes. Studies of C. butyricum supplementation in murine models of NAFLD reported improved hepatic lipid metabolism and immunoregulation. Seo and colleagues investigated the effect of CBM 588 supplementation in a rat model of HFD-induced NAFLD and found C. butyricum treatment helped maintain metabolic parameters including body weight, fat mass, and insulin resistance at normal levels while also protecting from liver injury and dysregulation of lipid metabolism.88 Hepatic accumulation of lipid droplets decreased, alongside decreased hepatic levels of cholesterol, free fatty acids, and phospholipids. Supporting these observations, hepatic gene expression analysis reflected a decrease in triglyceride synthesis and an increase in excretion of excess lipids via the conversion of cholesterol to bile acids. In another study, mice treated with C. butyricum B1 exhibited improved symptoms of HFD-induced steatohepatitis, and their liver and intestines showed an anti-inflammatory signature.54 The authors reported increased levels of butyrate but not other SCFAs in the liver and feces of C. butyricum-treated mice, and showed in vitro that butyrate effectively induced anti-inflammatory T cell differentiation. Therefore, C. butyricum modulates the dysregulation of lipid metabolism and immune response against a prolonged high-fat diet, possibly via butyrate production, resulting in protection from fatty liver disease.
Acute liver injury is one of the main forms of liver disease, caused by oxidative stress and inflammation. Liu and colleagues examined the hepatoprotective effects of C. butyricum CGMCC 8808 in a mouse model of acute liver injury and found prophylactic administration of the probiotic increased the survival rate compared to control mice.24 C. butyricum administered prior to the insult also attenuated liver injury, as revealed by histological analyses and by decreased serum markers of liver damage. The hepatoprotective ability of C. butyricum was partially attributed to its anti-oxidation and anti-inflammatory effects: administration of C. butyricum prior to the event increased the activities of anti-oxidative enzymes (superoxide dismutase and catalase) and the oxidative stress-sensor nuclear factor erythroid 2-related factor 2, and improved inflammatory tone.
C. butyricum in neuroprotection
The microbiota-gut-brain axis is a developing area of research that posits microbial influence on the bi-directional communication between the central and enteric nervous systems.89 Gastrointestinal bacteria have the ability to alter the endocrine and neurotransmitter signaling, as well as incite immune reactions in response to inflammatory neurological conditions. Therefore, probiotics have been suggested as a potential intervention for various neurological disorders. C. butyricum engages in cross-functional communication between systems by influencing several immune and metabolic pathways, deterring the progression of detrimental neuroinflammatory reactions.
C. butyricum reduces neurodegeneration via anti-apoptotic pathway
As described in a separate mechanisms section above, C. butyricum activates an anti-apoptotic Akt pathway. Treatment with C. butyricum has been shown to alleviate a number of neurodegenerative disease conditions in murine models, and the effect has been correlated with increased Akt pathway activation and reduced apoptosis. In a diabetic mouse model with induced cerebral ischemia-reperfusion injury, treatment with C. butyricum CGMCC 9831 was able to not only lower blood glucose levels, but also reverse histopathological damage to the hippocampal neurons, and improve learning and memory deficits.19 Similarly, treatment with CGMCX 9831 in a mouse model of induced vascular dementia improved histopathological damage in the hippocampus and consequently spatial learning ability;70 in a model of traumatic brain injury (TBI), the treatment improved cognitive, fine motor, and sensory functions as well as markers of neurodegeneration.38 Some of these observed benefits were paired with increased butyrate levels in the brain.70,73 Across all studies, C. butyricum activated anti-apoptotic pathways in the brain and reduced neurodegeneration.
C. butyricum prevents blood-brain-barrier dysfunction
In a model of TBI, cerebral edema – an accumulation of extracellular fluid in the brain – was accompanied by neuronal injury and blood-brain-barrier (BBB) dysfunction.38 The BBB is maintained by endothelial cells and tight junction proteins to prevent nonselective permeation of the blood into the CNS, a function comparable to that of the gut epithelial barrier. Remarkably, C. butyricum demonstrated the ability to prevent brain endothelial barrier dysfunction, evidenced by decreased brain water content and the restoration of normal levels of tight junction protein expression. Interestingly, disrupted intestinal barrier function is often a complication of TBI, and indeed C. butyricum treatment maintained pre-TBI induction levels of tight junction proteins in the colon and suppressed colonic inflammation. These results suggest a connection between intestinal and brain barrier improvement by C. butyricum, although further investigation is necessary. One such connection may be provided by C. butyricum’s ability to stimulate the secretion of GLP-1. GLP-1 is neuroprotective in models of brain injury and protects the BBB, potentially via modulation of tight junctions.90–92 Indeed, C. butyricum treatment in the TBI model also increased the expression of the GLP-1 receptor in both the brain and the gut,38 supporting the notion that C. butyricum mediates barrier function via GLP-1 signaling.
C. butyricum suppresses neuroinflammatory response
Multiple sclerosis is a chronic neurological autoimmune disease of the central nervous system. In multiple sclerosis, the immune system attacks the sheath around nerve fibers, called myelin. Additionally, inflammatory immune cells infiltrate and cause encephalitis, i.e. inflammation in the brain. In a mouse model of multiple sclerosis, treatment with C. butyricum significantly improved neuropathological inflammation in the lumbar spinal cord, evidenced by a decreased percentage of lymphocyte infiltration and myelin loss when compared to controls.53 This alleviation of disease severity was also supported by a lowered incidence of external disability in the C. butyricum-treated group.
C. butyricum against tumorigenesis
Disrupted epithelial homeostasis, inflammation, and tumorigenesis have a well-established connection.93,94 In addition, the gut microbiome is critical in the establishment and development of immune homeostasis,95 and therefore is implicated in cancer development, progression, and treatment (extensively reviewed elsewhere96-98). In this section, we summarize the beneficial effects of C. butyricum in animal cancer models.
Colorectal Cancer
Colorectal cancer (CRC) is the third most diagnosed cancer across both genders, and is commonly malignant.22,99 Multiple lines of evidence heavily implicate the gut microbiome in CRC.100–102 High fiber diets have been shown to decrease the risk of CRC, hinting at the gut microbiome-CRC connection.103,104 Further, fecal microbiome profiling of CRC patients shows a decrease in butyrate-producing Roseburia and Lachnospiraceae when compared to healthy individuals, as well as a significant decrease in genes for microbial butyrate production.105 On the other hand, certain opportunistic pathogens (e.g. Enterococcus, Escherichia, Streptococcus) are associated with an increased risk of CRC in humans105 and murine mechanistic studies have shown that Enterotoxigenic Bacteroides fragilis, adherent-invasive Escherichia coli strain NC101, and Fusobacterium nucleatum can induce colonic tumorigenesis and promote colitis-associated CRC via pro-inflammatory pathways.106–109 Moreover, several bacterial metabolites have been implicated in the initiation and/or progression of CRC.100
C. butyricum has shown anti-tumorigenic properties in vivo across four separate mouse models of CRC. In a 1,2-dimethylhydrazine dihydrochloride (DMH)-induced CRC murine model, researchers concluded that oral treatment with C. butyricum restored the DMH-induced weight decline, reduced tumor incidence, and decreased tumor size.110 Two separate studies of colitis-associated CRC (CAC) showed decreased tumorigenesis and increased survival with C. butyricum supplementation.23,111 Finally, in a HFD-induced Apcmin/+ mouse model of CRC, C. butyricum supplementation reversed the HFD-induced weight gain, and again, decreased intestinal tumor size and burden.22,111
Across these studies, several mechanisms behind decreased tumorigenesis are proposed (Figure 6a). C. butyricum induced an anti-inflammatory immune signature and improved gut barrier function, both in vivo and in vitro. Liu et al. saw inhibition of NF-κB signaling and a decrease in pro-inflammatory cytokines in vivo with C. butyricum supplementation.23 Chen et al. showed a reversal of the DMH-induced skew of Th2 and Th17 responses in vivo, and decreased expression of TLR4, NF-κB and IL-22 both in vitro and in vivo.110 While known for its role in tissue repair and regeneration, IL-22 expression in tumor environments can be detrimental due to its cell proliferation effect.112 C. butyricum cultured medium has been previously shown to significantly reduce TLR4 expression, an effect attributed to the metabolite butyrate.113 Finally, Xiao and colleagues showed reduced colonic inflammation (measured by myeloperoxidase activity) and improved gut barrier function (in histological parameters in vivo and increased transepithelial resistance and decreased permeability in vitro).111
Figure 6.
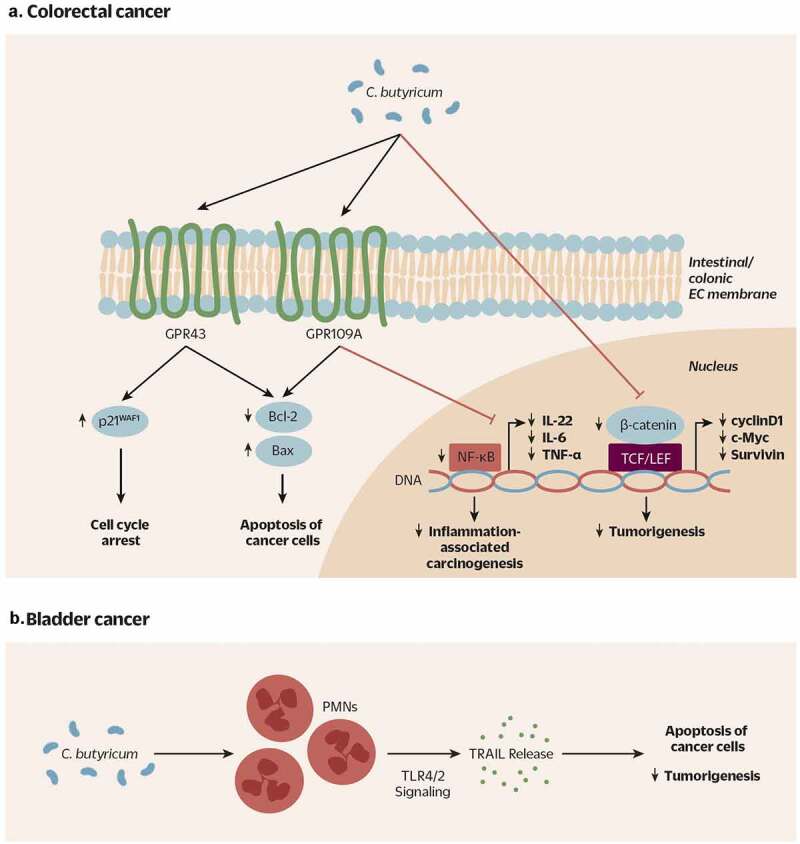
Proposed mechanism of action of beneficial C. butyricum strains across animal models of A) colorectal cancer (CRC) and B) bladder cancer. A) C. butyricum increases expression of SCFA receptors GPR43 and GPR109A in colonic and intestinal epithfiguelial cells. Activation of GPR43 eventually leads to an increased expression of p21WAF1 and cell cycle arrest in cancer cells. Again likely via activation of GPR43 and GPR109A, C. butyricum triggers a decrease in anti-apoptotic proteins Bcl-2, and an increase in pro-apoptotic protein Bax, resulting in the apoptosis of cancer cells. Moreover, C. butyricum inhibits NF-κB signaling (TLR4-MyD88-NF-κB) and decreases certain proinflammatory factors (IL-22, IL-6, TNF-ɑ), potentially leading to a decrease in inflammation-associated carcinogenesis. Finally, C. butyricum may also act to decrease CRC development by inhibiting the Wnt signaling pathway (β-catenin, cyclinD1, c-Myc, Survivin etc.). Cross-talk among these pathways is likely, but not yet fully elucidated. B) C. butyricum treatment of PMNs stimulates their release of TRAIL, a cytokine that specifically induces apoptosis in tumor cells
CRC: colorectal cancer; SCFA: short chain fatty acids; GPR43/109A: G-protein-coupled receptor 43/109A; EC: epithelial cell; Bcl2: B cell lymphoma 2; Bax: Bcl2 associated x protein; TLR4: Toll-like receptor 4; MyD88: Myeloid differentiation primary response 88; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; IL-22: interleukin-22; IL-6: interleukin-6; TNF-ɑ: tumor necrosis factor alpha; PMN: polymorphonuclear leukocyte; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand
Additionally, C. butyricum suppressed tumorigenesis in vitro and in vivo by decreasing cancer cell proliferation, triggering cell cycle arrest and increasing apoptosis.22,23,110,111 Two studies showed that treatment of CRC cells in vitro with C. butyricum-conditioned media significantly inhibited cell proliferation and increased cell apoptosis.22,110 This effect was driven by an increased expression of p21,WAF1114 a cell cycle inhibitor, dependent on the activation of SCFA receptor GPR43.22,99 In vivo, C. butyricum suppressed the Wnt pathway,115,116 a signaling cascade essential for cell proliferation and tissue homeostasis.22 Additionally, another study reported an increased proportion of apoptotic cells in the colonic tissues of C. butyricum treated CAC mice, supported by decreased Bcl-2 (an anti-apoptotic protein) and increased Bax (a pro-apoptotic protein) levels.23
Bladder Cancer
Another common form of cancer affects the bladder. Approximately 70% of newly diagnosed bladder cancers are non-muscle invasive (superficial), for which the standard of care treatment is Bacillus Calmette-Guérin (BCG) intravesical therapy.117 This form of immunotherapy involves intravesical injection of a “vaccine” derived from Mycobacterium bovis. However, BGC can have serious side effects, including sepsis. This has motivated a search for other bacteria with anticancer effects, which could act as a safer replacement for Mycobacterium bovis. Given the anticancer properties of C. butyricum outlined above, Shinnoh and colleagues investigated its efficacy as a novel intravesical therapy against superficial bladder cancer.118 Previous work in this area has demonstrated that post BCG treatment, polymorphonuclear neutrophils (PMNs) migrate to the bladder and release tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a cytokine that specifically induces apoptosis in tumor cells.119–121 Shinnoh et al. find that, similar to the BCG mechanism outlined above, in vitro treatment of PMNs with CBM 588 enhanced their release of TRAIL (through TLR2/4 signaling pathways) and that the released TRAIL stimulated apoptosis of human cancer cells (Figure 6b). The induction of TRAIL release from PMNs was unique to BCG and C. butyricum, and did not occur with other strains or anticancer compounds (Lactobacillus, Krestin, and Lentinan). Researchers further confirmed these effects in vivo: the anti-cancer effects of intra-tumor injection of C. butyricum and BCG were compared in C3H/HeN mice inoculated with murine bladder cancer cells. C. butyricum proved to be more effective than BCG, nearly completely inhibiting tumor growth rather than partially suppressing it.118 Thus, C. butyricum was found to suppress the growth of bladder cancer cells both in vivo and in vitro, and may represent a promising new intravesical intervention against superficial bladder cancer.
C. butyricum in immune checkpoint blockade therapy
Immune checkpoint blockade (ICB) is a revolutionary cancer therapy that has been under extensive investigations in the past 20 years. ICB utilizes the immune checkpoint proteins of T cells that are designated to eliminate cancer cells. When ICB inhibits negative immune checkpoint proteins such as programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4), T cells can continuously kill the cancers without a “brake.”
Microbiome differences have been proposed to directly affect the effectiveness of ICB.122,123 Exposure to antibiotics prior to receiving ICB therapy is especially detrimental to the outcome.124 Recently, first-in-human clinical trials showed that fecal microbiota transplantation (FMT) improved the efficacies of anti-PD-1 immunotherapy in patients with anti-PD-1-refractory metastatic melanoma, while also reprogramming the gut microbiome.125,126 As C. butyricum supplementation has been repeatedly shown to improve symptoms related to microbial dysbiosis, Tomita et al. retrospectively analyzed the survival of non-small lung cancer patients who received ICB therapy, with or without CBM 588 within 6 months prior to and concurrently with ICB.127 Taking CBM 588 significantly improved the progression-free survival (PFS) and overall survival (OS) in patients that have been treated with ICB compared to no CBM 588, even in the population that had been exposed to antibiotics prior to ICB. While this retrospective study did not investigate the effect of C. butyricum on the gut microbial composition or the patient’s immune profile, several studies as presented in this review support that the underlying mechanism may be due to the promotion of certain beneficial taxa such as Bifidobacterium, strengthening of the intestinal barrier, and modulation of the immune system. An on-going Phase I clinical trial (NCT03829111) is aimed to test the effect of CBM 588 in kidney cancer patients being treated with a combination of CTLA-4 and PD-1 inhibitors. Stool microbial composition and systemic immunological shifts are to be assessed along with the efficacy of the ICB drugs. More human intervention trials confirming the C. butyricum-improved efficacies of ICB therapies and investigating the mechanism behind the benefits will open a novel avenue of overcoming non-responsiveness to cancer immunotherapies with probiotics administration.
C. butyricum in human clinical trials
As detailed above, numerous studies in murine models have shown protective or ameliorative effects of C. butyricum across a range of disease models. In humans, we could find seven additional papers examining C. butyricum’s potential as a probiotic in clinical trials of gastrointestinal, psychiatric, and metabolic disorders (Table 2).
Table 2.
The effect of C. butyricum supplementation on human health and gut microbiome composition across human clinical trials
| Study | Probiotic | Dose (daily) | Duration of treatment | Disease | Study size | Study design | Primary end-point(s) | Community analysis method | Community analysis primary result | Other analyses |
|---|---|---|---|---|---|---|---|---|---|---|
| Seki et al. 2003 | CBM588 | 1–4 × 107 CFU | 3 days; 6 days | Antibiotic – associated diarrhea (AAD) in children | n = 27 (antibiotics); n = 38 (3 day C. butyricum); n = 45 (6 day C. butyricum) | randomized, open label | decreased incidence of diarrhea in both C. butyricum treatment arms vs. antibiotic-only | culture-based | treatments prevented antibiotic-induced reduction in total anerobe counts and levels of Bifidobacterium spp., Eubacterium spp., Lactobacillus spp. | |
| Imase et al. 2008 | CBM588 | 6x107 (low); 1.2x108 CFU (high) |
1 week | Antibiotic – associated diarrhea (AAD) | n = 7 (antibiotics); n = 7 (low-dose) n = 5 (high-dose C. butyricum) |
randomized, open label | decreased incidence of diarrhea in both C. butyricum treatment arms vs. antibiotic-only | culture-based | treatments prevented antibiotic-induced reduction in total anerobe counts and levels of Bifidobacterium spp., Lactobacillus spp. | Treatment decreased the detection rate of C. difficile toxin A |
| Sun et al. 2018 | C. butyricum | 5.67x107 CFU | 4 weeks | IBS-D | n = 95 (placebo); n = 105 (C. butyricum) | multi-center, randomized, double-blind, placebo- controlled |
reduction of IBS symptom severity scale (IBS-SSS) in treatment group vs. placebo | 16 s rRNA PCR and Illumina sequencing; metagenomic (shotgun) Illumina | PCoA shows groups diverge after treatment; 45 OTUs changed from baseline to week 4 between arms (e.g. Clostridium sensu stricto reduced in treatment group); several KEGG pathways distinguish arms post treatment | improvement in change of overall IBS-QOL score and stool frequency in treatment vs. placebo arm; no difference in change of Bristol stool scale between arms; |
| Yasueda et al. 2016 | CBM588 | CFU not specified; 3 tablets 3x/day | 24 months (post hospital discharge) | pouchitis | n = 8 (placebo); n = 9 (C. butyricum) | randomized, placebo – controlled | 1/9 subjects in the treatment arm and 4/8 subjects in the placebo arm developed pouchitis (NS) | T-RFLP | obligate anaerobes increase in treatment arm group (confounded by unknowns); Clostridium coccoides group increased in the placebo group; Escherichia subgroup decreased in the treatment group | serum CRP levels decreased with treatment; safe and well-tolerated |
| Miyaoka et al. 2018 | CBM588 | CFU not specified; 60 mg/day | 8 weeks | treatment-resistant major depressive disorder (TRD) | n = 20 (control); n = 20 (C. butyricum) | randomized; open label | treatment improved depression (reduced median HAMD-17, BDI, and BAI scores) | safe and well tolerated | ||
| Xia et al. 2018 | C. butyricum CGMCC0313.1 and B. infantis CGMCC0313-2 | >4.5 x107 CFU (C. butyricum) and >4.5x106 CFU (B. infantis) | 3 months | minimal hepatic encephalopathy (MHE) | n = 37 (control); n = 30 (C. butyricum & B. infantis) | randomized; open label | treatment improved the results of the psychometric tests for MHE (NCT-A and DST) | taxa-specific qPCR | total bacterial number similar between arms; treatment increased Clostridium cluster I and Bifidobacterium, and decreased Enterococcus and Enterobacteriaceae | mean venous ammonia level, and serum LPS, D-lactate and DAO decreased with treatment |
| Perraudeau & McMurdie et al. 2020 | WBF-11: Akkermansia muciniphila, Anaerobutyricum hallii, Clostridium beijerinckii, Clostridium Butyricum CBUT, Bifidobacterium infantis); WBF-10: excludes A. muciniphila and A. hallii |
1.2x109 AFU (A. muciniphila); 9.0 × 108 AFU (A. hallii); 1.6 × 1010 AFU (C. beijerinckii); 3.3 × 109 AFU (C. butyricum); 2.0 × 109 AFU (B. infantis) | 12 weeks | type 2 diabetes | n = 26 (placebo); n = 27 (WBF-10); n = 23 (WBF-11) | multi-center, randomized, double-blind, placebo – controlled | treatment improved total glucose AUC | 16S rRNA (V4) PCR and Illumina sequencing | no change in alpha-diversity (Shannon) or beta-diversity (weighted UniFrac distance) among arms or timepoints; no separation of arms in PCoA analysis | both formulations safe and well tolerated; no change in CRP and other inflammatory markers for either formulation vs. placebo |
Two of these clinical studies pertain to antibiotic-associated diarrhea (AAD), both in children8 and in adult patients with gastro-duodenal ulcers undergoing Helicobacter pylori eradication therapy.128 The primary endpoint, diarrhea incidence, decreased across both open-label trials: Seki et al. reported a decrease from 59% in the antibiotics-only arm, to 5% in children receiving C. butyricum for three days, and to 9% in those that received treatment for all six days.8 Imase et al. similarly saw a decrease from 43% in the antibiotics-only group to 14% in low dose and 0% in high dose C. butyricum supplementation groups; the H. pylori eradication rate was unaffected by the supplementation.128 Similarly, Sun et al. reported improved diarrhea symptoms (IBS symptom severity scale) and quality of life ((IBS-QOL scores) in a double-blind, placebo-controlled study of subjects with diarrhea-predominant IBS receiving C. butyricum daily for 4 weeks, compared to the placebo group.129 Finally, C. butyricum has also been investigated in humans for the management of pouchitis. Total proctocolectomy with ileal pouch anal anastomosis (IPAA) is the standard surgical procedure for patients with UC, whereby surgeons create a pouch to replace the damaged colon and rectum. However, the pouch can become inflamed and damaged in a complication known as pouchitis.130 Yasueda et al. found that commercially available CBM 588 tablets reduced the incidence of pouchitis in UC patients from 50% in the placebo group to 11% in the C. butyricum-treated group.83 Due to a small sample size (9 treatment, 8 placebo patients), this decrease was non-significant.
C. butyricum has also been investigated in humans as a treatment for psychological disorders: treatment-resistant major depressive disorder (TRD) and minimal hepatic encephalopathy (MHE). Miyaoka et al. found that subjects with TRD receiving C. butyricum in addition to SSRI (selective serotonin reuptake inhibitors) antidepressants131 reported significantly lower median scores across several indices compared to the control group.131 Along these lines, Xia et al. reported that a probiotic multi-strain formulation featuring C. butyricum CGMCC0313.1 and B. infantis CGMCC0313-2 improved cognition (measured via the number connection test A and digit symbol test) in subjects with minimal hepatic encephalopathy (MHE),132 a complication of liver cirrhosis that leads to mild cognitive and motor impairment.133
Finally, Perraudeau & McMurdie et al. investigated C. butyricum strain CBUT as part of a 5-strain and 3-strain consortium in a double-blind, placebo-controlled study of glycemic control in subjects with type 2 diabetes.134 Researchers concluded that the 5-strain consortium containing CBUT alongside strains of Akkermansia muciniphila, Anaerobutyricum hallii, Clostridium beijerinckii, and Bifidobacterium infantis caused a significant improvement in total glucose AUC0-180min relative to placebo following 12 weeks administration, as well as nominally-significant improvements in incremental glucose AUC0-180min and hemoglobin A1c. Although CBUT was present in both formulation arms and therefore it could not be determined whether it was a necessary and direct contributor to the observed improvements, butyrate production is among the hypothesized mechanisms.
Across these studies, a similar dose of the chosen strains of C. butyricum (~ 107 CFU/g) administered orally was well tolerated and deemed safe, and had a positive impact across the primary endpoints of each study (Table 2). However, of the seven studies, only three were placebo-controlled and only two were double-blind; hence, interpretations of benefit must be made with caution. Additional double-blind and placebo-controlled clinical studies are needed across indications to resolve and expand upon these findings.
While these clinical studies have shown safety and efficacy of C. butyricum in various human diseases, the mechanism behind improvements in clinical endpoints remains unconfirmed. Mirroring results from animal studies, culture-based and taxa-specific qPCR analyses show C. butyricum administration in humans restoring total fecal anaerobe counts after antibiotic treatment, as well as increasing levels of certain beneficial species (Bifidobacterium, Lactobacillus) and decreasing Enterococcus/Enterobacteriaceae.8,128,132 Sequencing of the 16S rRNA gene has not shown a change in overall gut microbiota diversity. Sun et al. via PCoA detected a compositional shift after 4 weeks of administration, whereas Perraudeau & McMurdie et al. did not detect a separation of study arms in their PCoA analysis.129,134 Among the putative mechanisms, butyrate is often regarded as causal to the effects of C. butyricum supplementation. We previously described studies in murine models suggesting that C. butyricum can not only directly produce butyrate, it can also stimulate additional butyrate-producing taxa.20,21,24 Yet among human clinical trials, only one study measured fecal butyrate levels after supplementation, finding only weak trends of increase that were not statistically significant.134 Mechanistic insight can also be derived via a suite of secondary inflammatory endpoints. Three clinical studies measured changes in serum markers of inflammation and gut barrier function following C. butyricum administration: Yasueda et al. found a reduction in CRP,83,132 and Xia et al. found decreased D-lactate, LPS, and diamine oxidase83,132 Perraudeau & McMurdie et al. did not detect a significant change in inflammatory markers (CRP, IL-6, IL-10, TNF-α, or TGF-β). Future clinical research should move toward evaluating mechanistic hypotheses: using high-resolution methods to monitor changes in gut microbiota, probing for changes in various fecal and serum metabolites, and including markers of host immune response, barrier function, and metabolism.
Conclusions and Perspective
The gut microbiota and its effect on host health is increasingly relevant and supported by an ever growing number of investigations in model systems and proof-of-concept clinical trials. As summarized above, strains of C. butyricum appear to enhance the gut’s ability to reduce microbial and immunological perturbations induced by antibiotics, pathogens, or other factors, making them interventions of potential importance in a number of health conditions. For humans, probiotic C. butyricum strains are promising in the management of gastrointestinal symptoms, psychological conditions, and metabolic disease. According to the available literature, C. butyricum strains have shown beneficial effects in a wide range of animal models of human disease, yet their beneficial effects in humans for these same diseases remains underexplored in clinical trials. Although authors across preclinical and clinical studies suggest butyrate as causal to the effects of C. butyricum supplementation, direct evidence of this still remains elusive.134 Mechanisms and effects on host physiological pathways also appear to differ depending on the disease state or model. For example, C. butyricum supplementation has been shown to increase apoptosis in cancer models, leading to suppression of tumorigenesis; however, in models of neurodegenerative diseases, the effect on the same apoptosis-related proteins (Bcl-1, Bax) is reversed, leading to decreased apoptosis and consequently decreased neuronal death. Another largely unresolved effect of C. butyricum treatment is the extent to which it shifts the overall diversity or composition of the gut microbiota. Currently available studies have used vastly different metrics to assess gut microbial composition, and hence the effect of a specific C. butyricum strain is difficult to define. Most studies suggest shifts in specific taxa following supplementation, but the mechanism behind this modulation is unknown.
Dosage and consistency of effects across strains have yet to be resolved: human clinical studies across diseases tend to examine only a single dose of a single strain. Optimized dosing could increase beneficial effects; similarly, with a more in-depth understanding of strain-level differences and a more diverse strain culture collection, disease-specific, or perhaps tailored strain selection may broaden the benefits of supplementation.
The preceding review of the literature indicates that the full potential of C. butyricum to improve human health has not yet been fully elucidated. While the human trials completed to date are consistent with the larger body of preclinical data for this species, fundamental differences in the human and murine microbiota, as well as in the anatomy and physiology of the gastrointestinal tract, pose inevitable limitations in fully recapitulating the biology of the human gut-microbiome interaction. Further human studies designed around mechanistic hypotheses are needed to ascertain the true clinical utility of specific C. butyricum strains. Disease areas of great interest include metabolic diseases, due to their prevalence across the globe and the need for new clinical interventions with lower risk profiles; and psychological disorders, given their complex nature and the extent to which these disorders affect individuals’ quality of life.
As we look toward the future of health benefits conferred by C. butyricum and other live microbes, studies need to be conducted in a more strategic manner with more advanced tools than in previous decades of probiotic research. Creating new microbiota-focused products that convey health benefits requires, at minimum, (1) the use of genomic analysis for the selection of novel strains and initial safety assessments, and (2) rigorous, double-blind, placebo-controlled human clinical trials. Additionally, refining the technology essential to in situ measurements will enable a more complete understanding of complex microbiome-host interactions – for example, new ingestible sensor technologies that can profile in situ microbiomes135 and perhaps spatially resolve SCFA profiles in the gut in real time.136 Since microbial metabolites may be key players in the host health benefits conferred by microbes, increased serum and fecal metabolomic profiling of human clinical subjects may provide further mechanistic insight into the effects of these strains in host physiology and pathophysiology. By employing advanced technologies and co-ordinated approaches to discovering and testing novel strains, scientists in the field will successfully bridge the gap between rodent and human studies and make more rapid progress in clarifying the relationships between live microorganisms, host signaling pathways, and health outcomes.
Funding Statement
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure statement
All authors are employees and stock/stock option shareholders of Pendulum Therapeutics, Inc (formerly known as ‘Whole Biome Inc.’). OK owns stock in GlySens, Inc, has stock options in ViaCyte, Inc, and is a consultant to NuSirt BioPharma, Circius, and NanoPrecision Medical.
References
- 1.Finegold SM, Sutter VL, Mathisen GE.. Normal indigenous intestinal flora. In: Hentges D, editor. Human intestinal microflora in health and disease. New York: Academic Press; 1983. p. 3–28. [Google Scholar]
- 2.Mountzouris KC, McCartney AL, Gibson GR. Intestinal microflora of human infants and current trends for its nutritional modulation. Br J Nutr. 2002;87:405–420. doi: 10.1079/BJNBJN2002563. [DOI] [PubMed] [Google Scholar]
- 3.Tipton L, Darcy JL, Hynson NA. A Developing Symbiosis: enabling Cross-Talk Between Ecologists and Microbiome Scientists. Front Microbiol. 2019;10:292. doi: 10.3389/fmicb.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo P, Zhang K, Ma X, He P. Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol. 2020;11:24. doi: 10.1186/s40104-019-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Cassir N, Benamar S, La Scola B. Clostridium butyricum: from beneficial to a new emerging pathogen. Clin Microbiol Infect. 2016;22:37–45. doi: 10.1016/j.cmi.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. [DOI] [PubMed] [Google Scholar]
- 8.Seki H, Shiohara M, Matsumura T, Miyagawa N, Tanaka M, Komiyama A, Kurata S. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatr Int. 2003;45:86–90. doi: 10.1046/j.1442-200X.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 9.MIYARISAN PHARMACEUTICAL CO., LTD . Clostridium butyricum MIYAIRI strain [Internet]. Clostridium butyricum MIYAIRI strain [cited 2020. September 1]; Available from: http://www.miyarisan.com/english_index.htm
- 10.The European Commission . Commission Implementing Decision of 11 December 2014 authorising the placing on the market of Clostridium butyricum (CBM 588) as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (notified under document C(2014) 9345). OJEU. 2014;57:153. [Google Scholar]
- 11.Isa K, Oka K, Beauchamp N, Sato M, Wada K, Ohtani K, Nakanishi S, McCartney E, Tanaka M, Shimizu T, et al. Safety assessment of the Clostridium butyricum MIYAIRI 588® probiotic strain including evaluation of antimicrobial sensitivity and presence of Clostridium toxin genes in vitro and teratogenicity in vivo. Hum Exp Toxicol. 2016;35:818–832. doi: 10.1177/0960327115607372. [DOI] [PubMed] [Google Scholar]
- 12.Pan -L-L, Niu W, Fang X, Liang W, Li H, Chen W, Zhang H, Bhatia M, Sun J. Clostridium butyricum Strains Suppress Experimental Acute Pancreatitis by Maintaining Intestinal Homeostasis. Mol Nutr Food Res. 2019:e1801419. doi: 10.1002/mnfr.201801419. [DOI] [PubMed] [Google Scholar]
- 13.Miao R-X, Zhu -X-X, Wan C-M, Wang Z-L, Wen Y, Li -Y-Y. Effect of Clostridium butyricum supplementation on the development of intestinal flora and the immune system of neonatal mice. Exp Ther Med. 2018;15:1081–1086. doi: 10.3892/etm.2017.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagihara M, Yamashita R, Matsumoto A, Mori T, Kuroki Y, Kudo H, Oka K, Takahashi M, Nonogaki T, Yamagishi Y, et al. The impact of Clostridium butyricum MIYAIRI 588 on the murine gut microbiome and colonic tissue. Anaerobe. 2018;54:8–18. doi: 10.1016/j.anaerobe.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Hagihara M, Kuroki Y, Ariyoshi T, Higashi S, Fukuda K, Yamashita R, Matsumoto A, Mori T, Mimura K, Yamaguchi N, et al. Clostridium butyricum Modulates the Microbiome to Protect Intestinal Barrier Function in Mice with Antibiotic-Induced Dysbiosis. iScience. 2020;23:100772. doi: 10.1016/j.isci.2019.100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H-Q. Therapeutic effects ofClostridium butyricum on experimental colitis induced by oxazolone in rats. World J Gastroenterol. 2009;15:1821. doi: 10.3748/wjg.15.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong Q, He G-Q, Jia J-L, Zhu Q-L, Ruan H. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr Microbiol. 2011;62:512–517. doi: 10.1007/s00284-010-9737-8. [DOI] [PubMed] [Google Scholar]
- 18.Ling Z, Liu X, Cheng Y, Luo Y, Yuan L, Li L, Xiang C. Clostridium butyricum combined with Bifidobacterium infantis probiotic mixture restores fecal microbiota and attenuates systemic inflammation in mice with antibiotic-associated diarrhea. Biomed Res Int. 2015;2015:582048. doi: 10.1155/2015/582048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, Jin J, Pang M, Zhang H, Yu J, et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 20.Jia L, Li D, Feng N, Shamoon M, Sun Z, Ding L, Zhang H, Chen W, Sun J, Chen YQ. Anti-diabetic Effects of Clostridium butyricum CGMCC0313.1 through Promoting the Growth of Gut Butyrate-producing Bacteria in Type 2 Diabetic Mice. Sci Rep. 2017;7:7046. doi: 10.1038/s41598-017-07335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia L, Shan K, Pan -L-L, Feng N, Lv Z, Sun Y, Li J, Wu C, Zhang H, Chen W, et al. Clostridium butyricum CGMCC0313.1 Protects against Autoimmune Diabetes by Modulating Intestinal Immune Homeostasis and Inducing Pancreatic Regulatory T Cells. Front Immunol. 2017;8:1345. doi: 10.3389/fimmu.2017.01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D, Jin D, Huang S, Wu J, Xu M, Liu T, Dong W, Liu X, Wang S, Zhong W, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020;469:456–467. doi: 10.1016/j.canlet.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Xie W, Wan X, Deng T. Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice. Int Immunopharmacol. 2020;88:106862. doi: 10.1016/j.intimp.2020.106862. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Fu Y, Zhang H, Wang J, Zhu J, Wang Y, Guo Y, Wang G, Xu T, Chu M, et al. The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice. Food Funct. 2017;8:4042–4052. doi: 10.1039/C7FO00355B. [DOI] [PubMed] [Google Scholar]
- 25.Long M, Yang S, Li P, Song X, Pan J, He J, Zhang Y, Wu R. Combined Use of C. butyricum Sx-01 and L. salivarius C-1-3 Improves Intestinal Health and Reduces the Amount of Lipids in Serum via Modulation of Gut Microbiota in Mice. Nutrients. 2018;10:810. doi: 10.3390/nu10070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velazquez EM, Nguyen H, Heasley KT, Saechao CH, Gil LM, Rogers AWL, Miller BM, Rolston MR, Lopez CA, Litvak Y, et al. Endogenous Enterobacteriaceae underlie variation in susceptibility to Salmonella infection. Nat Microbiol. 2019;4:1057–1064. doi: 10.1038/s41564-019-0407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson SJ, Lemire P, Maughan H, Goethel A, Turpin W, Bedrani L, Guttman DS, Croitoru K, Girardin SE, Philpott DJ. Comparison of Co-housing and Littermate Methods for Microbiota Standardization in Mouse Models. Cell Rep. 2019;27:1910–1919.e2. doi: 10.1016/j.celrep.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Ericsson AC, Davis JW, Spollen W, Bivens N, Givan S, Hagan CE, McIntosh M, Franklin CL. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PLoS ONE. 2015;10:e0116704. doi: 10.1371/journal.pone.0116704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer R-J. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad R, Sorrell MF, Batra SK, Dhawan P, Singh AB. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol. 2017;10:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunyady B, Mezey E, Palkovits M. Gastrointestinal immunology: cell types in the lamina propria–a morphological review. Acta Physiol Hung. 2000;87:305–328. [PubMed] [Google Scholar]
- 32.Corfield AP. The Interaction of the Gut Microbiota with the Mucus Barrier in Health and Disease in Human. Microorganisms. 2018;6:78. doi: 10.3390/microorganisms6030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 35.Gaudier E, Jarry A, Blottière HM, De Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1168–74. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 36.Burger-van Paassen N, Vincent A, Puiman PJ, Van Der Sluis M, Bouma J, Boehm G, Van Goudoever JB, Van Seuningen I, Renes IB. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 37.Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:416–422. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Sun J, Du J, Wang F, Fang R, Yu C, Xiong J, Chen W, Lu Z, Liu J. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil. 2018;30:e13260. doi: 10.1111/nmo.13260. [DOI] [PubMed] [Google Scholar]
- 39.Shang H, Sun J, Chen YQ. Clostridium butyricum CGMCC0313.1 modulates lipid profile, insulin resistance and colon homeostasis in obese mice. PLoS ONE. 2016;11:e0154373. doi: 10.1371/journal.pone.0154373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng W, Wu Y, Chen G, Fu S, Li B, Huang B, Wang D, Wang W, Liu J. Sodium Butyrate Attenuates Diarrhea in Weaned Piglets and Promotes Tight Junction Protein Expression in Colon in a GPR109A-Dependent Manner. Cell Physiol Biochem. 2018;47:1617–1629. doi: 10.1159/000490981. [DOI] [PubMed] [Google Scholar]
- 41.Chen G, Ran X, Li B, Li Y, He D, Huang B, Fu S, Liu J, Wang W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50:103. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727–738. doi: 10.1016/j.immuni.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ariyoshi T, Hagihara M, Eguchi S, Fukuda A, Iwasaki K, Oka K, Takahashi M, Yamagishi Y, Mikamo H. Clostridium butyricum MIYAIRI 588-Induced Protectin D1 Has an Anti-inflammatory Effect on Antibiotic-Induced Intestinal Disorder. Front Microbiol. 2020;11:587725. doi: 10.3389/fmicb.2020.587725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gobbetti T, Dalli J, Colas RA, Federici Canova D, Aursnes M, Bonnet D, Alric L, Vergnolle N, Deraison C, Hansen TV, et al. Protectin D1n-3 DPA and resolvin D5n-3 DPA are effectors of intestinal protection. Proc Natl Acad Sci USA. 2017;114:3963–3968. doi: 10.1073/pnas.1617290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janeway Jr CA, Travers P, Walport M, Shlomchik MJ. The mucosal immune system. In: Immunobiology: the Immune System in Health and Disease 5th. United Kingdom, Garland Science; 2001. [Google Scholar]
- 48.Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Janeway Jr CA, Travers P, Walport M, Shlomchik MJ. The production of armed effector T cells. In: Immunobiology: the Immune System in Health and Disease 5th. United Kingdom, Garland Science; 2001. [Google Scholar]
- 50.Zhang J, Su H, Li Q, Wu H, Liu M, Huang J, Zeng M, Zheng Y, Sun X. Oral administration of Clostridium butyricum CGMCC0313-1 inhibits β-lactoglobulin-induced intestinal anaphylaxis in a mouse model of food allergy. Gut Pathog. 2017;9:11. doi: 10.1186/s13099-017-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivanov II, Frutos R De L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen H, Ma X, Liu Y, Ma L, Chen Z, Lin X, Si L, Ma X, Chen X. Gut Microbiota Interventions With Clostridium butyricum and Norfloxacin Modulate Immune Response in Experimental Autoimmune Encephalomyelitis Mice. Front Immunol. 2019;10:1662. doi: 10.3389/fimmu.2019.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou D, Pan Q, Liu X-L, Yang R-X, Chen Y-W, Liu C, Fan J-G. Clostridium butyricum B1 alleviates high-fat diet-induced steatohepatitis in mice via enterohepatic immunoregulation. J Gastroenterol Hepatol. 2017;32:1640–1648. doi: 10.1111/jgh.13742. [DOI] [PubMed] [Google Scholar]
- 55.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 56.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kashiwagi I, Morita R, Schichita T, Komai K, Saeki K, Matsumoto M, Takeda K, Nomura M, Hayashi A, Kanai T, et al. Smad2 and Smad3 Inversely Regulate TGF-β Autoinduction in Clostridium butyricum-Activated Dendritic Cells. Immunity. 2015;43:65–79. doi: 10.1016/j.immuni.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Zhao Q, Yang W-R, Wang X-H, Li G-Q, Xu L-Q, Cui X, Liu Y, Zuo X-L. Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells. World J Gastroenterol. 2019;25:5469–5482. doi: 10.3748/wjg.v25.i36.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arpaia N, Campbell C, Fan X, Dikiy S, Van Der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 61.O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, Qi L, Mei L, Wu Z, Wang H. C. butyricum lipoteichoic acid inhibits the inflammatory response and apoptosis in HT-29 cells induced by S. aureus lipoteichoic acid. Int J Biol Macromol. 2016;88:81–87. doi: 10.1016/j.ijbiomac.2016.03.054. [DOI] [PubMed] [Google Scholar]
- 64.Gao Q, Qi L, Wu T, Wang J. Clostridium butyricum activates TLR2-mediated MyD88-independent signaling pathway in HT-29 cells. Mol Cell Biochem. 2012;361:31–37. doi: 10.1007/s11010-011-1084-y. [DOI] [PubMed] [Google Scholar]
- 65.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berger A. Th1 and Th2 responses: what are they? BMJ. 2000;321:424. doi: 10.1136/bmj.321.7258.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juan Z, Zhao-Ling S, Ming-Hua Z, Chun W, Hai-Xia W, Meng-Yun L, Jian-Qiong H, Yue-Jie Z, Xin S. Oral administration of Clostridium butyricum CGMCC0313-1 reduces ovalbumin-induced allergic airway inflammation in mice. Respirology. 2017;22:898–904. [DOI] [PubMed] [Google Scholar]
- 69.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: a Multi-Functional Master Cell. Front Immunol. 2015;6:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, Zhang H, Jin J, Chen W, Pang M, et al. Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed Res Int. 2015;2015:412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun J, Wang F, Hu X, Yang C, Xu H, Yao Y, Liu J. Clostridium butyricum Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior in Mice via the Gut-Brain Axis. J Agric Food Chem. 2018;66:8415–8421. [DOI] [PubMed] [Google Scholar]
- 72.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun J, Ling Z, Wang F, Chen W, Li H, Jin J, Zhang H, Pang M, Yu J, Liu J. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett. 2016;613:30–35. doi: 10.1016/j.neulet.2015.12.047. [DOI] [PubMed] [Google Scholar]
- 74.Rupprecht LE, Mietlicki-Baase EG, Zimmer DJ, McGrath LE, Olivos DR, Hayes MR, Hindbrain GLP-1. receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. Am J Physiol Endocrinol Metab. 2013;305:E751–9. doi: 10.1152/ajpendo.00367.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J-M, Sun Y-S, Zhao L-Q, Chen -T-T, Fan M-N, Jiao H-C, Zhao J-P, Wang X-J, Li F-C, Li H-F, et al. SCFAs-Induced GLP-1 Secretion Links the Regulation of Gut Microbiome on Hepatic Lipogenesis in Chickens. Front Microbiol. 2019;10:2176. doi: 10.3389/fmicb.2019.02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taguchi N, Abe F, Mikami Y, Arai T. [Prevention of experimental antibiotic-associated diarrhea by Clostridium butyricum]. Nihon Saikingaku Zasshi. 1988;43:829–835. doi: 10.3412/jsb.43.829. [DOI] [PubMed] [Google Scholar]
- 77.Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Komatsu A, Kamiya S. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157: h7infection in mice. FEMS Immunol Med Microbiol. 2004;41:219–226. doi: 10.1016/j.femsim.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Sakazaki R, Kamiya S. [Antagonistic interaction between Clostridium butyricum and enterohemorrhagic Escherichia coli O157:H7]. Kansenshogaku Zasshi. 1999;73:7–14. doi: 10.11150/kansenshogakuzasshi1970.73.7. [DOI] [PubMed] [Google Scholar]
- 79.Kuroiwa T, Iwanaga M, Kobari K, Higashionna A, Kinjyo F, Saito A. [Preventive effect of Clostridium butyricum M588 against the proliferation of Clostridium difficile during antimicrobial therapy]. Kansenshogaku Zasshi. 1990;64:1425–1432. doi: 10.11150/kansenshogakuzasshi1970.64.1425. [DOI] [PubMed] [Google Scholar]
- 80.Woo TDH, Oka K, Takahashi M, Hojo F, Osaki T, Hanawa T, Kurata S, Yonezawa H, Kamiya S. Inhibition of the cytotoxic effect of Clostridium difficile in vitro by Clostridium butyricum MIYAIRI 588 strain. J Med Microbiol. 2011;60:1617–1625. doi: 10.1099/jmm.0.033423-0. [DOI] [PubMed] [Google Scholar]
- 81.Oka K, Osaki T, Hanawa T, Kurata S, Sugiyama E, Takahashi M, Tanaka M, Taguchi H, Kamiya S. Establishment of an Endogenous Clostridium difficile Rat Infection Model and Evaluation of the Effects of Clostridium butyricum MIYAIRI 588 Probiotic Strain. Front Microbiol. 2018;9:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Araki Y, Andoh A, Takizawa J, Takizawa W, Fujiyama Y. Clostridium butyricum, a probiotic derivative, suppresses dextran sulfate sodium-induced experimental colitis in rats. Int J Mol Med. 2004;13:577–580. [PubMed] [Google Scholar]
- 83.Yasueda A, Mizushima T, Nezu R, Sumi R, Tanaka M, Nishimura J, Kai Y, Hirota M, Osawa H, Nakajima K, et al. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg Today. 2016;46:939–949. doi: 10.1007/s00595-015-1261-9. [DOI] [PubMed] [Google Scholar]
- 84.Wang F-Y, Liu J-M, Luo -H-H, Liu A-H JY. Potential protective effects of Clostridium butyricum on experimental gastric ulcers in mice. World J Gastroenterol. 2015;21:8340–8351. doi: 10.3748/wjg.v21.i27.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 86.Hansen CHF, Krych L, Buschard K, Metzdorff SB, Nellemann C, Hansen LH, Nielsen DS, Frøkiær H, Skov S, Hansen AK. A maternal gluten-free diet reduces inflammation and diabetes incidence in the offspring of NOD mice. Diabetes. 2014;63:2821–2832. doi: 10.2337/db13-1612. [DOI] [PubMed] [Google Scholar]
- 87.De Goffau MC, Luopajärvi K, Knip M, Ilonen J, Ruohtula T, Härkönen T, Orivuori L, Hakala S, Welling GW, Harmsen HJ, et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. 2013;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seo M, Inoue I, Tanaka M, Matsuda N, Nakano T, Awata T, Katayama S, Alpers DH, Komoda T. Clostridium butyricum MIYAIRI 588 improves high-fat diet-induced non-alcoholic fatty liver disease in rats. Dig Dis Sci. 2013;58:3534–3544. doi: 10.1007/s10620-013-2879-3. [DOI] [PubMed] [Google Scholar]
- 89.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fukuda S, Nakagawa S, Tatsumi R, Morofuji Y, Takeshita T, Hayashi K, Tanaka K, Matsuo T, Niwa M. Glucagon-Like Peptide-1 Strengthens the Barrier Integrity in Primary Cultures of Rat Brain Endothelial Cells Under Basal and Hyperglycemia Conditions. J Mol Neurosci. 2016;59:211–219. [DOI] [PubMed] [Google Scholar]
- 92.Shan Y, Tan S, Lin Y, Liao S, Zhang B, Chen X, Wang J, Deng Z, Zeng Q, Zhang L, et al. The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J Neuroinflammation. 2019;16:242. doi: 10.1186/s12974-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 94.Wang J-L, Chang C-H, Lin J-W, Wu L-C, Chuang L-M, Lai M-S. Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case-control study in patients with Type 2 diabetes mellitus. Int J Cancer. 2014;135:956–967. doi: 10.1002/ijc.28738. [DOI] [PubMed] [Google Scholar]
- 95.Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017;46:562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, Kroemer G. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7:271. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 98.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. doi: 10.3389/fimmu.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 101.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sánchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, Gómez-Millán J, Queipo-Ortuño MI. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers (Basel). 2020;12:1406. doi: 10.3390/cancers12061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aune D, Chan DSM, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang J, Yu J. The association of diet, gut microbiota and colorectal cancer: what we eat may imply what we get. Protein Cell. 2018;9:474–487. doi: 10.1007/s13238-018-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. Isme J. 2012;6:320–329. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu S, Rhee K-J, Albesiano E, Rabizadeh S, Wu X, Yen H-R, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan T-J, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tilg H, Adolph TE, Gerner RR, Moschen AR. The intestinal microbiota in colorectal cancer. Cancer Cell. 2018;33:954–964. doi: 10.1016/j.ccell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 110.Chen Z-F, Ai L-Y, Wang J-L, Ren -L-L, Yu Y-N, Xu J, Chen H-Y, Yu J, Li M, Qin W-X, et al. Probiotics Clostridium butyricum and Bacillus subtilis ameliorate intestinal tumorigenesis. Future Microbiol. 2015;10:1433–1445. doi: 10.2217/fmb.15.66. [DOI] [PubMed] [Google Scholar]
- 111.Xiao Y, Dai X, Li K, Gui G, Liu J, Yang H. Clostridium butyricum partially regulates the development of colitis-associated cancer through miR-200c. Cell Mol Biol (Noisy-le-grand). 2017;63:59–66. doi: 10.14715/cmb/2017.63.4.10. [DOI] [PubMed] [Google Scholar]
- 112.Hernandez P, Gronke K, Diefenbach A. A catch-22: interleukin-22 and cancer. Eur J Immunol. 2018;48:15–31. doi: 10.1002/eji.201747183. [DOI] [PubMed] [Google Scholar]
- 113.Isono A, Katsuno T, Sato T, Nakagawa T, Kato Y, Sato N, Seo G, Suzuki Y, Saito Y. Clostridium butyricum TO-A culture supernatant downregulates TLR4 in human colonic epithelial cells. Dig Dis Sci. 2007;52:2963–2971. doi: 10.1007/s10620-006-9593-3. [DOI] [PubMed] [Google Scholar]
- 114.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 115.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 116.Cheng X, Xu X, Chen D, Zhao F, Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473–481. doi: 10.1016/j.biopha.2018.11.082. [DOI] [PubMed] [Google Scholar]
- 117.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 118.Shinnoh M, Horinaka M, Yasuda T, Yoshikawa S, Morita M, Yamada T, Miki T, Sakai T. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int J Oncol. 2013;42:903–911. doi: 10.3892/ijo.2013.1790. [DOI] [PubMed] [Google Scholar]
- 119.Ludwig AT, Moore JM, Luo Y, Chen X, Saltsgaver NA, O’Donnell MA, Griffith TS. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette-Guérin-induced antitumor activity. Cancer Res. 2004;64:3386–3390. doi: 10.1158/0008-5472.CAN-04-0374. [DOI] [PubMed] [Google Scholar]
- 120.Kemp TJ, Ludwig AT, Earel JK, Moore JM, Vanoosten RL, Moses B, Leidal K, Nauseef WM, Griffith TS. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 2005;106:3474–3482. doi: 10.1182/blood-2005-03-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 122.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CPM, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 124.Wilson BE, Routy B, Nagrial A, Chin VT. The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies. Cancer Immunol Immunother. 2020;69:343–354. doi: 10.1007/s00262-019-02453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 126.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin J-M, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, Jodai T, Akaike K, Ishizuka S, Saeki S, et al. Association of Probiotic Clostridium butyricum Therapy with Survival and Response to Immune Checkpoint Blockade in Patients with Lung Cancer. Cancer Immunol Res. 2020;8:1236–1242. doi: 10.1158/2326-6066.CIR-20-0051. [DOI] [PubMed] [Google Scholar]
- 128.Imase K, Takahashi M, Tanaka A, Tokunaga K, Sugano H, Tanaka M, Ishida H, Kamiya S, Takahashi S. Efficacy of Clostridium butyricum preparation concomitantly with Helicobacter pylori eradication therapy in relation to changes in the intestinal microbiota. Microbiol Immunol. 2008;52:156–161. doi: 10.1111/j.1348-0421.2008.00026.x. [DOI] [PubMed] [Google Scholar]
- 129.Sun -Y-Y, Li M, Li -Y-Y, Li L-X, Zhai W-Z, Wang P, Yang -X-X, Gu X, Song L-J, Li Z, et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci Rep. 2018;8:2964. doi: 10.1038/s41598-018-21241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dalal RL, Shen B, Schwartz DA. Management of pouchitis and other common complications of the pouch. Inflamm Bowel Dis. 2018;24:989–996. doi: 10.1093/ibd/izy020. [DOI] [PubMed] [Google Scholar]
- 131.Miyaoka T, Kanayama M, Wake R, Hashioka S, Hayashida M, Nagahama M, Okazaki S, Yamashita S, Miura S, Miki H, et al. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: a Prospective Open-Label Trial. Clin Neuropharmacol. 2018;41:151–155. doi: 10.1097/WNF.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 132.Xia X, Chen J, Xia J, Wang B, Liu H, Yang L, Wang Y, Ling Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J Int Med Res. 2018;46:3596–3604. doi: 10.1177/0300060518776064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Acharya C, Bajaj JS. Current management of hepatic encephalopathy. Am J Gastroenterol. 2018;113:1600–1612. doi: 10.1038/s41395-018-0179-4. [DOI] [PubMed] [Google Scholar]
- 134.Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, Eid J, Gines J, Iyer M, Justice N, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care. 2020;8:e001319. doi: 10.1136/bmjdrc-2020-001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rezaei Nejad H, Oliveira BCM, Sadeqi A, Dehkharghani A, Kondova I, Langermans JAM, Guasto JS, Tzipori S, Widmer G, Sonkusale SR. Ingestible osmotic pill for in vivo sampling of gut microbiomes. Adv Intell Syst. 2019;1:1900053. doi: 10.1002/aisy.201900053. [DOI] [Google Scholar]
- 136.Kalantar-Zadeh K, Ha N, Ou JZ, Berean KJ. Ingestible Sensors. ACS Sens. 2017;2:468–483. doi: 10.1021/acssensors.7b00045. [DOI] [PubMed] [Google Scholar]


