Abstract
Rose oxide (RO) is a monoterpene found in rose oil fragrances. This monoterpene has been reported to possess anti-inflammatory activity, however, little is known regarding its pharmacological activity. The present study was carried out to evaluate its antidepressant action and possible mechanisms of action. Analysis of ADMET pharmacokinetic properties (absorption, distribution, metabolism, excretion and toxicity) of rose oxide was performed by computational prediction analysis. Behavioral tests were performed to assess the interaction between rose oxide and the central nervous system and antidepressant effect that includes: forced swim test (FST), tail suspension test (TST), open field test (OFT) and rota-rod test. The results of pharmacokinetic and toxicological properties indicate that rose oxide could be used orally, since it has good intestinal absorption as well as pharmacological and toxicological properties that can be similar to pharmacological compounds (regular hepatic metabolism and low toxicity). Treatment with 50 mg/kg of rose oxide was able to decrease the immobility time of animals not affected by FST and TST and was not able to alter the motor activity of the OFT and rota-rod test, suggesting modulation and antidepressant activity. Docking data suggest that rose oxide can bind to receptors in the serotonergic pathway. The results described here suggest that rose oxide has antidepressant activity, modulating the serotonergic pathway.
Keywords: Depression, Oxide rose, Serotonergic pathway, ADMET
Depression; Oxide Rose; serotonergic pathway, ADMET.
1. Introduction
Depressive disorders affect 4.4% of the global population and have an estimated lifetime prevalence of 6.2% among adolescents and up to 19% among adults, making mental health conditions worldwide a major health problem (Dunn et al., 2020; König et al., 2020). It is estimated that the absence of treatment for depression has an impact on productivity attributed to more than 12billion days of loss of productivity (equivalent to more than 50 million years of work), at an estimated cost of US$925 billion (Chisholm et al., 2016). Depression is a psychiatric disorder characterized primarily by depressed mood or anecdotal observations for at least two weeks, accompanied by sleep and food deregulation, psychomotor changes, guilt and, in more severe cases, suicidal thoughts. In addition, it is associated with an increased risk of all-cause mortality and a reduction in life expectancy, thus representing high cost for health services (Chisholm et al., 2016; Laursen et al., 2016).
Despite advances in the treatment of depression, the medications used promote several common adverse effects like nausea, sexual dysfunction, headache, insomnia, daytime drowsiness, restlessness and weight loss (Alexopoulos, 2019; Kraus et al., 2019; Paul et al., 2019). The occurrence of these adverse effects may lead to withdrawal of pharmacological treatment (Huang et al., 2018). In addition, a significant number of patients do not respond satisfactorily to treatments. In view of these limitations, it is necessary to use complementary drugs for the treatment of depression as well as the search for new pharmacological alternatives (Chisholm et al., 2016).
Rose Oxide (RO) is an organic compound of the class of monoterpenes. It is a fragrance found in roses such as rose, geranium, damask rose, Laggera spp., Eucalyptus citriodora and Dracocephalum heterophyllum (Babu et al., 2002; Batish et al., 2006; Kuiate et al., 2002; Singh et al., 2008). RO can also be produced through the photo oxygenation of citronellol (Alsters et al., 2010; Xiao et al., 2017). Due to its commercial application, many studies report the use of rose oxide in the production of aromas and reactions of drug production (Cavazzana et al., 2018; Gevorgyan et al., 2020; Guo et al., 2020; Xiao et al., 2017). Besides having a delicate, sweet and mild aroma, the rose essential oil has been described as a compound that has anti-inflammatory, calming, relaxing and antioxidant effects (Guo et al., 2020; Nonato et al., 2012). The anti-inflammatory activity of rose oxide was associated with its ability to inhibit IL-1β production (Nonato et al., 2012).
Given the evidence of its anti-inflammatory, relaxing and calming effects, a study of the antidepressant action of rose oxide was systematized and investigated. Thus, the present study aims to evaluate the antidepressant effect of RO in vivo models, comparing with in silico docking models as well as assessing its kinetic and toxicological characteristics (ADMET).
2. Material and methods
2.1. Animals
Mice (Mus musculus), Swiss adults males, weighing between 25 and 30 g were used. The animals were housed in acrylic cages (six animals/cage) and kept at 22 ± 2 °C with water and feed ad libitum (commercial rodent stock diet - Purina, Campinas, Brazil), 40–50% humidity, and light/dark cycle 12/12h (7:00 a.m. to 7:00 p.m.). The animals were obtained from the Central animal facility and kept in the sectoral laboratory of LAPNEX, both of the Federal University of Piauí and After the experimental procedures, the animals underwent euthanasia with intraperitoneal (ip) injection of overdose of sodium pentobarbital, (150 mg/kg, ip) associated with 10 mg/kg lidocaine. The study was elaborated in compliance with the ethical guidelines of animal experimentation, according to Law n° 11.794–2008. The experiments were also approved by the Animal Experimentation Ethics Committee of the Federal University of Piaui (CEEA/UFPI Nº449/18). Animals were randomly divided into groups (n = 6/group) before the studies and were acclimatized to the laboratory conditions for at least 2h before testing and were used only once throughout the experiments. To carry out the experiment, all researchers were blinded to the treatment of each animal individually.
2.2. Drugs
(+) Rose oxide (RO) - enantiomers dextrorotatory, was purchased from Fluka (Steinheim, Germany), with 99.0% of purity.
2.3. Ligand docking, binding site and ADMET analysis (absorption, distribution, metabolism, excretion and toxicity) in silicon
The molecular docking of the rose oxide was performed using the SWISSTARGET PREDICTION and SWISSDOCK tools. The target structures of GABA receptor, Glutamate receptor, Serotonin receptor and Albumin were evaluated using the RCSB PDB data repository (Table 1) and later carried out using the CHIMERA 1.12 software. The docking structures were viewed by means of the PyMol application.
Table 1.
GABA receptor, Glutamate receptor, Serotonin receptor and Albumin used like target for Rose Oxide docking in in silico models.
| PDB code | Chain | Active Site Residue | Organism | |
|---|---|---|---|---|
| Albumin | 4F5S | A | Try-134 | bos taurus |
| Try-212 | bos taurus | |||
| Serotonin receptors | 6BQG | A | Asp-134 | human being |
| Glutamate receptors | 5KZN | A | Mg2+ | human being |
| GABA B receptors | 4F11 | A | Gly-424 | human being |
PreADMET and SwissADME tools were used for the prediction of pharmacokinetic parameters (ADMET) as well as toxicological parameters. Concerning metabolic parameters, the following criteria were evaluated: if the substances undergo phase 1 metabolism, if they inhibited some CYP and which CYP were inhibited considering that mainly CYP3A4 and CYP2C9 are mostly involved in pharmacological interactions involving metabolism. The mathematical models used in silico of these programs prospect results associated with mutagenicity according to the Ames Test, however, a more detailed analysis may be required to predict its carcinogenic potential. The prediction of mutagenicity is performed based on several strains of the bacterium Salmonella typhimurium (TA98, TA100 and TA1535). The variable being tested is the mutagen's ability to cause a reversion to growth on a histidine-free medium (B N Ames et al., 1975). Regarding interpretation of these results, it was considered positive when reverse mutation was observed in one or more bacteria evaluated, negative when no reverse mutation was observed. When a false positive was observed, that is, no reverse mutation was seen in any of the bacterial strains and even the software program classified as mutagenic, the result was not considered.
The prediction of the carcinogenic potential of compounds in rodents (Rodent Carcinogenicity) were performed based on data from the National Toxicology Program (NTP) and FDA (Food and Drug Administration). The results were expressed as: + carcinogenic and - non - carcinogenic.
In order to evaluate the toxicity criteria in marine organisms, the following parameters were considered: algal toxicity - toxic <1 mg/L and non-toxic > 1 mg/L; toxicity in Daphnia sp - toxic <0.22 μg/mL and non-toxic > 0.22 μg/mL; toxicity in Medaka fish - very toxic <1 mg/L, toxic between 1-10 mg/L, harmful between 10-100 mg/L and non-toxic > 100 mg/L (Guilhermino et al., 2000; Ramaiah et al., 2020).
2.4. Behavioral tests
2.4.1. Forced swim test (FST)
In this test, the animals were divided into 3 groups: group 1 (negative control-NC or vehicle - 0.1 mL/10g), group 2 (GT), and this group was divided into four subgroups according to the RO dose: 12.5; 25; 50 and 100 mg/kg. RO was administered orally to all animals of group 2, Group 3 or positive control (PC) received 20 mg/kg to fluoxetine orally.
FST was performed according to the protocol established by Porsolt et al. (1977). Each mouse was placed in a plastic cylinder 10 cm in diameter and 24 cm in height containing 19 cm of water at 25°C ± 1 °C. The immobility time of the animal was defined as the absence of active movements in the time period of 5 min (Porsolt et al., 1977).
2.4.2. Tail suspension test (TST)
For this protocol, the animals were divided similarly to the previous protocol, where group 1 received the vehicle (NC - 0.1 mL/10g po), group 2 (GT) received different doses of RO (12,5, 25, 50 and 100 mg/Kg po) and group 3 (FLX-20 mg/kg of fluoxetine orally). The mice were suspended 50 cm above the ground by an adhesive tape and the immobility time recorded during a period 5 min. The animals were isolated acoustically and visually. The mice were considered immobile only when they hung passively and were completely motionless (Thierry et al., 1986).
2.4.3. Open field test (OFT)
To perform the open field test the animals were divided into three groups, group 1 (NC-0.1 mL/10g p.o), group 2 receiving different doses of RO 50 mg/kg, all orally and group 3 (FLX - 20 mg/kg fluoxetine orally). This model follows the method proposed by Hall (1936). Each mouse was placed in the center of the cage where the number of quadrants crossed in a period of 5 min was recorded (Hall, 1936).
2.4.4. Rota rod test
In order to perform the route test, 3 groups were used: (NC-0.1 mL/10g po), group 2 that received only one dose used in previous tests (50 mg/kg) orally and group 3 (FLX - 20 mg/g fluoxetine orally). The Rota Rod (Model RR - 2002, Insight equipment) consisted of a 2.5 cm diameter bar, subdivided into four compartments by 25cm diameter disks, rotating at 12 revolutions per min. The animals were selected 24 h previously by eliminating those mice which did not remain on the bar for three consecutive periods of 60 s.
The rotating bar evaluated motor coordination and possible muscle relaxation. The animals were placed one at a time, with all four legs resting on a rotating bar for 3 min. All animals underwent a pre-test on the rotating bar to get accustomed to the equipment conditions prior to performing the experiments. The permanency time on the rotating bar in seconds as well as the number of falls off of the bar were recorded.
2.4.5. Evaluation of the antidepressant mechanism of action of rose oxide in FST. Involvement of the serotonergic system and 5-HT 1A receptors
To evaluate the mechanism of action of rose oxide, the participation of serotonergic receptors was verified. In view of this, two protocols were performed both using the forced swim test model. In the first protocol, animals of different groups were treated with sub-effective doses of RO (12.5 mg/kg po) and fluoxetine (10 mg/kg po) both orally 60 min before the forced swim test. In another group, the two sub-doses were also associated orally. For this protocol, positive and negative control groups were also used according to previous protocols. To investigate specifically the participation of 5-HT 1A receptors in the antidepressant effect of RO, animals were pretreated with WAY100635 (0.1 mg/kg, sc, selective 5-HT 1A receptor antagonist) and after 30 min of administration, the animals received rose oxide at the dose of 50 mg/kg (mean effective dose). After 60 min the animals were submitted to FST as previously described.
2.5. Statistical analysis
Data were analyzed using One-Way ANOVA followed by Tukey's post-test. A significance level of 5% was considered for all tests corresponding to a confidence level of 95%. Results were expressed as means and standard deviation. The results were considered positive when a statistically significant increase (p < 0.05) was observed.
3. Results
3.1. Analysis of ADMET (absorption, distribution, metabolism, excretion and toxicity)
Values obtained from in silico analysis of RO related to pharmacokinetic properties can be seen in Table 2. Predicted values for RO related to LogP, LogS, AIH and Caco2 as well as other parameters such as plasma protein binding, metabolism and excretion of RO are also observed. Table 3 shows the toxicity of Rose Oxide in in silico test and Table 4 shows the physico-chemical parameters of RO based on Lipinsk rule.
Table 2.
Analysis of absorption, distribution, metabolism and excretion of Rose Oxide in in silico test.
| Prediction of absorption | Rose Oxide |
|---|---|
| SKlogP_value | 2.84935 |
| SKlogS_pure | -2.34974 |
| Intestinal Absorption in Human – IAH (%) | 100% |
| CACO2 (nm/seg) |
38.1376 |
| Prediction of distribuition |
Rose Oxide |
| SKlogD_value | 2.84935 |
| Hematoencephalic Barrier Penetration (BHE) in vivo | 2.40358 |
| Binding to Plasma Proteins | 100.000 |
| Inhibition Pgp | No |
| MDCK (mandin darby canine kidney) – Cell permeability in vitro |
100% |
| Prediction of metabolism and excretion |
Rose Oxide |
| CYP_2C19 inhibition | Inhibitor |
| CYP_2C9 inhibition | Inhibitor |
| CYP_2D6 inhibition | No |
| CYP_2D6 substrate | Weakly |
| CYP_3A4 inhibition | No |
| CYP_3A4 substrate | Weakly |
Table 3.
Analysis of Toxicity of Rose Oxide in in silico test.
| Prediction of toxicity | Rose Oxide |
|---|---|
| seaweed (mg/mL) | 0.03486 |
| AMES test | Mutagenic |
| Carcinogenicity in mice | Positive |
| Carcinogenicity in rats | Negative |
| Inhibition of hERG | Medium risk |
| Medaka (mg/mL) | 0.33846 |
| Daphnia (mg/mL) | 0.58637 |
| Minnow (mg/mL) | 0.13390 |
| TA100-10RLI | Negative |
| TA100-NA | Positive |
| TA535-10RLI | Negative |
Table 4.
Physico-chemical properties of Rose Oxide in in silico test.
| Physico-chemical properties | Rose Oxide |
|---|---|
| Formula | C10H20O2 |
| Log P | 2.84935 |
| Molecular mass (MM) | 172,6 g.mol−1 |
| Number of Hydrogen bond acceptors | 2 |
| Number of Hydrogen Bonding Donors | 1 |
| Polar Surface Area (ASP) | 18.45 Å2 |
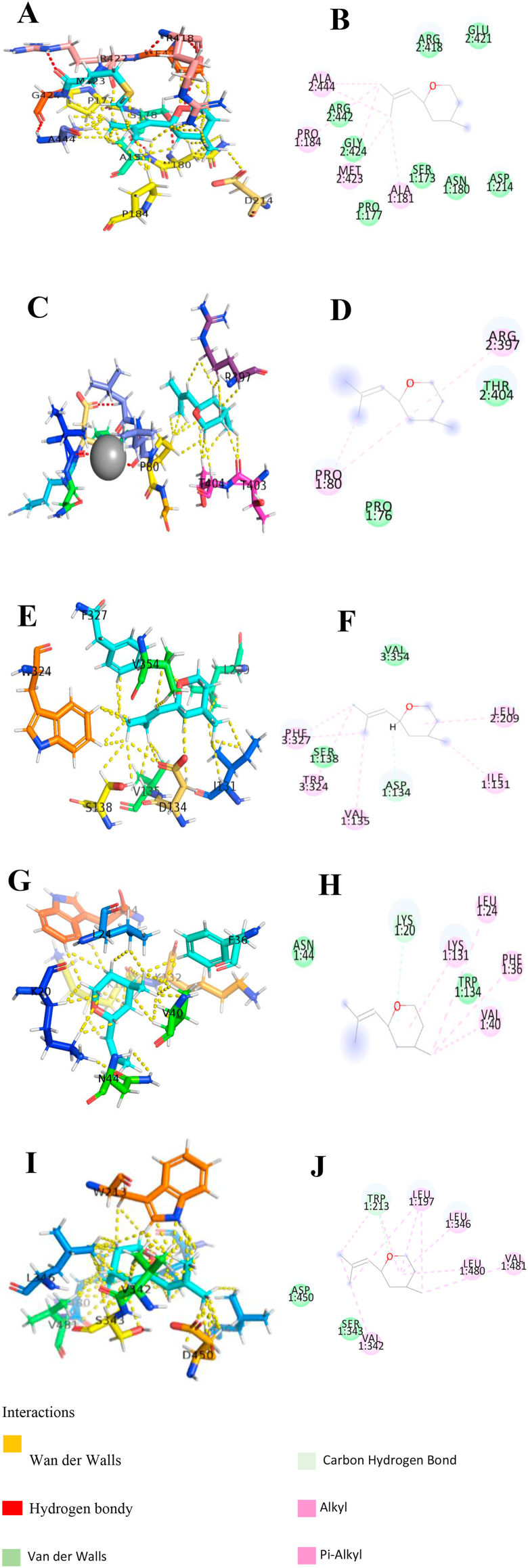
According to the data obtained (Figure 1) in the study of binding targets for the RO, molecular docking analyses were performed for different cell membrane receptors: GABA receptor, Glutamate receptor and Serotonin receptor, and Albumin transport protein. During docking studies, several dockage points with favorable binding energy for each analyzed structure were found.
Figure 1.
Rose Oxide molecular docking and molecular interaction with GABA receptor, Glutamate receptor, Serotonin receptor and Albumin. (A) Rose Oxide alignment wich GABA receptor pocket; (B) molecular interaction of Rose Oxide with GABA active pocket fragments; (C) Rose Oxide alignment wich Glutamate receptor pocket; (D) molecular interaction of Rose Oxide with Glutamate receptor active pocket fragments; (E) Rose Oxide alignment wich Serotonin receptor pocket; (F) molecular interaction of Rose Oxide with Serotonin receptor active pocket fragments; (G) Rose Oxide alignment wich Albumin pocket (Try-134 active residue); (H) molecular interaction of Rose Oxide with Albumin active pocket fragments (Try-134 active residue); (I) Rose Oxide alignment wich Albumin pocket (Try-212 active residue); (J) molecular interaction of Rose Oxide with Albumin active pocket fragments (Try-212 active residue). The dashed lines in yellow evidence the hydrophobic interactions; the green dashed lines the interactions by hydrogen bonds.
According to Figure 1 and Table 5, the interaction of the rose oxide with GABA receptors occurs through the van der Waals interactions of the compound with some amino acids with an estimated binding ΔG at -22.80 KJ/mol for binding with GABA receptors. Regarding glutamate receptors, the interactions occur with estimated binding ΔG at - 23.60 KJ/mol for glutamate binding. The compound also has docking points on serotonin receptors with estimated binding ΔG at -23.50 KJ/mol. Docking analysis was also performed on albumin, where an estimated binding ΔG at -22.72 KJ/mol was observed. Considering the receptors evaluated in the molecular docking studies, the participation of these receptor was evaluated in vivo models of depression and compared with the in silico model.
Table 5.
Active sites of Rose Oxide anchorage docking in in silico with GABA receptor, Glutamate receptor, Serotonin receptor and Albumin.
| Protein/Enzymatic receptor | Active Site Marker | Full Fitness (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|---|
| Albumin | Try-134 | -4003,54 | -6,81 |
| Try-212 | -4002,10 | -6,49 | |
| Serotonin receptors | Asp-134 | -2318,14 | -6,43 |
| Glutamate receptors | Mg2+ | -2501,66 | -6,46 |
| GABA B receptors | Gly-424 | -2300,40 | -6,29 |
3.2. Pharmacological analysis of the antidepressant action of RO
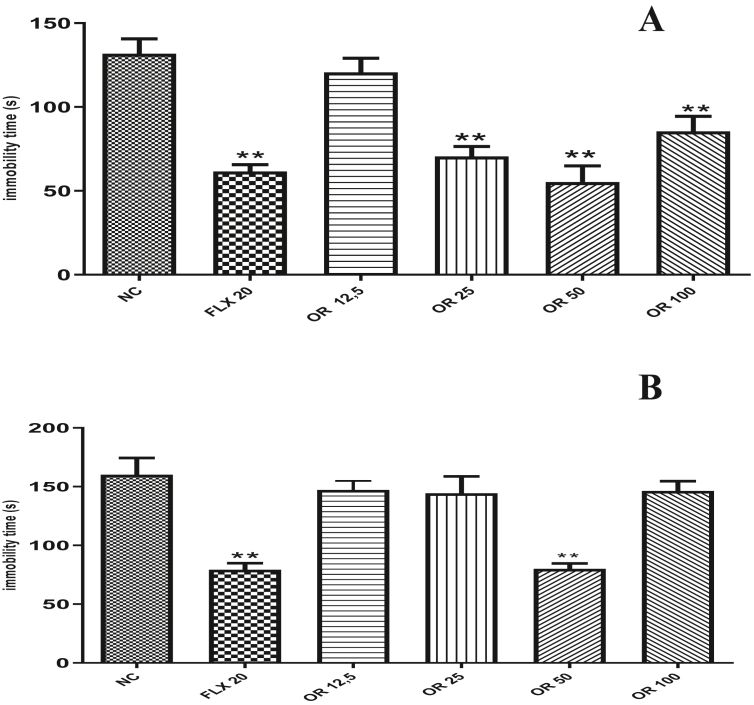
Rose oxide at the doses of 25, 50 and 100 mg/kg (70.5 ± 5.97; 55.25 ± 9.69; 85.5 ± 8.91) altered the immobility time of the animals submitted to FST when compared to the negative control group that received the vehicle or negative control (131.8 ± 8.81). However, the dose of 12.5 mg/kg of rose oxide did not reduce the immobility time. A reduction in the immobility time was observed in the animals treated with the positive control Fluoxetine 20 mg/kg (Figure 2A), (61.6 ± 4.05).
Figure 2.
Pharmacological analysis of different doses of Rose Oxide about the time of immobility animal in Forced Swim Test (A) and Tail Suspension Test (B) in mice. ∗∗ = p < 0,01 when compared to negative control (NC); FLX (Fluoxetine 20 mg/Kg v.o.); RO 12,5 (Rose Oxide 12,5 mg/Kg, v.o.); RO 25 (Rose Oxide 25 mg/Kg, v.o.); RO 50 (Rose Oxide 50 mg/Kg, v.o.) and RO 100 (Rose Oxide 100 mg/Kg, v.o.).
Treatment of animals with RO at doses of 12,5, 25 and 100 mg/kg was not able to alter the immobility time (147,16 ± 7.45; 144.33 ± 14.34; 145 ± 10.10) when compared to the negative control group (160.20 ± 14.22) in the tail suspension test (Figure 2B). However, the dose of 50 mg/kg RO reduced the immobility time of the animals (81.4 ± 5.41). The positive control used in the test, Fluoxetine 20 mg/kg, reduced the immobility time of treated animals (79 ± 5.56).
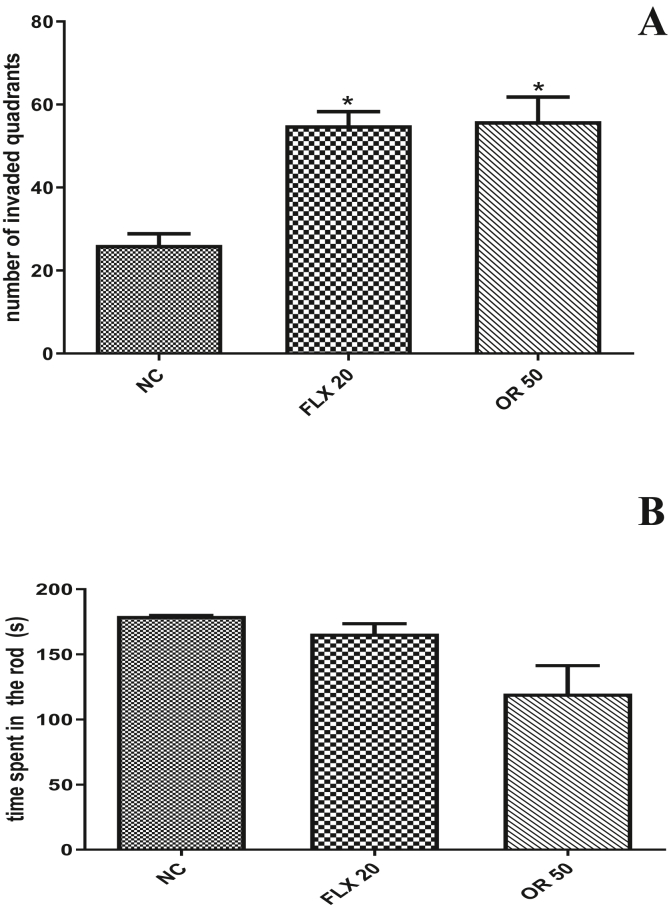
In the open field-test none of the treatments produced alterations in the exploratory activity of the animals when compared control group (55 ± 3.29). Animals treated with rose oxide at the dose (50 mg/kg po) (56 ± 5.82) which were submitted to Rota rod test demonstrated no in change the permanency time of the animals on the rotating bar when compared to the negative control group. The positive control Fluoxetine 20 mg/kg, was not able to change the time of permanence of the animals in the rotating bar when compared to the negative control group (Figure 3A and B).
Figure 3.
Effect of different doses of Rose Oxide on the open field test (A) and rota-rod test (B) in mice. ∗ = p < 0,01 when compared to negative control (NC); NC (Negative Control); FLX (Fluoxetine 20 mg/Kg v.o.) and RO 50 (Rose Oxide 50 mg/Kg, v.o.).
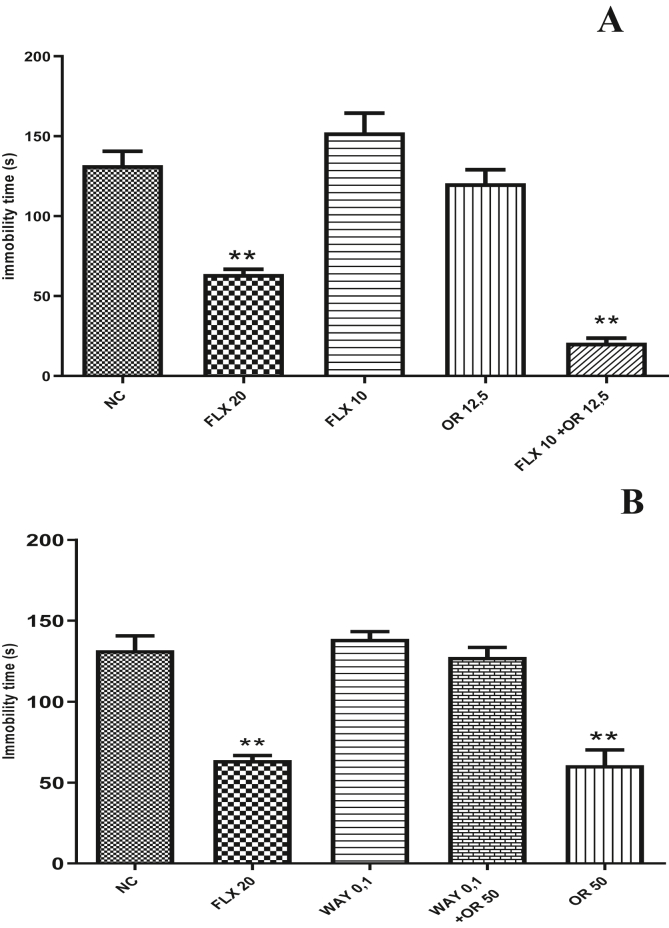
In the evaluation of the action mechanism of the RO seeking to verify the participation of the serotonin pathway, it was observed that treatment with sub effective dose of RO (12,5 mg/Kg, v.o.) and fluoxetine (10 mg/Kg, v.o.) did not reduce the immobility time of the animals (120 ± 8.48 and 152 ± 12.08 respectively). However, when associated with the two substances (RO and fluoxetine) at the same doses, synergism was observed with decreased immobility time of the animals (20 ± 2.88) compared to the negative control group that received the vehicle (131 ± 8.31). In the evaluation of the mechanism of action of rose oxide, it could be observe that the pre-treatment with WAY100635 at the dose of 0.1 mg/kg s.c reversed the effect of the RO which was viewed by the increasing immobility time of treated animals (Figure 4A and B).
Figure 4.
Analysis of the mechanism of antidepressant action of rose oxide. In A: Effects of treatment of rose oxide at subclinical dose, in the time of immobility in forced swim test. In B: Effects of treatment of rose oxide and WAY100635 0,1 mg/kg i.p., in the time of immobility under forced swim test. ∗∗ = p < 0,01 when compared to negative control (NC); NC (Negative Control); FLX (Fluoxetine 20 mg/Kg v.o.); RO 12,5 (Rose Oxide 12,5 mg/Kg, v.o.); WAY 0,1 (WAY100635 0,1 mg/kg i.p.); RO 50 (Rose Oxide 50 mg/Kg, v.o.); RO 100 (Rose Oxide 100 mg/Kg, v.o.).
4. Discussion
In the present study, the prediction and distribution of performance indices or their ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) properties were investigated. These aspects are important in the pharmacokinetics of a potential drug candidate molecule (Saurav et al., 2014). In addition, data search methods were used in investigating the interaction between substances with specific receptors. Besides, the antidepressant effect of RO in experimental models of depression was evaluated.
The lipid solubility was verified from the partition coefficient (Log P) that interferes directly with the passage of compounds from the extracellular medium to the intracellular medium through the lipid bilayer. This can also be a good indicator of the presence of a barrier such as the blood-brain barrier (Golani and Golani, 2015). The recommended LogP values for drugs acting on the CNS are between 1 and 4 (Eigenmann et al., 2016). In this analysis, the RO had good liposolubility (Log P = 2.84), indicating good penetration into the CNS, a fundamental criterion that favors its anti-depressant effect. Another important fact that contributes to this property is the molecular mass of the RO that according to our analysis was 176.2 g.mol−1. Another important analysis to reinforce this property is the value of logS. According to the literature, the value of logS should be between -1 to -5 for most drugs. Values within this range reflect the relationship between the polarity required for adequate aqueous solubility and sufficient hydrophobicity that allows passage through the membrane. In our results, the RO was compatible with this property, further highlighting its penetration capacity in the central nervous system, thus justifying its pharmacological properties.
Considering specifically its penetration capacity through the hematoencephalic Barrier, RO was highly approved in relation to this parameter. According to the literature, values >2 are indicative of good penetration through the barrier. The RO exhibited a value of 2.4 demonstrating its penetration capacity through the barrier. It is important to remember that this parameter is fundamental to consider the antidepressant effect of this compound, besides being liposoluble is not enough to guarantee penetration through the barrier considering that it displays a differentiated structure and the presence of efflux transporters (P- Pgp glycoprotein) hindering the penetration of substances justifying its analysis in ADMET parameters. In view of this, the RO showed to be very promising in vivo analysis contrast to in silico analysis, where it prove not to be substrate for Pgp (Ajay et al., 1999; Cho et al., 2017).
Regarding the absorption of the compounds, the software program evaluated the permeability in epithelial cells, Caco2 and MDCK cells. Caco2 cells originate from human colon (large intestine) adenocarcinoma and have the ability to differentiate into enterocytes. MDCK (Madin-Darby canine kidney) cells originate from the canine kidney and undergo differentiation in columnar epithelial cells with semipermeable membranes. These properties should be present in a drug molecule candidate with antidepressant effects because of its oral use.
According to the literature, values from 0 to 20% for poorly absorbed molecules are considered for the evaluation of permeability and intestinal absorption. From 20 to 70% for molecules with moderately absorbed molecules and from 70 to 100% for well absorbed molecules (Al-Heibshy et al., 2020; Kamiya et al., 2020; Yee, 1997). Our results in silico for the rose oxide predict a percentage of human intestinal absorption (AIH%) of 100%, which demonstrates that the substance is able to be completely absorbed by the intestine. In an analysis performed considering Caco2 cells to verify the parameter intestinal absorption, we observed a medium absorption capacity for the rose oxide. For this analysis, values of high permeability (>70 nm/sec), medium permeability (4–70 nm/sec) and low permeability (<4 nm/sec) are considered (Kovačević et al., 2014; Miteva et al., 2006; Trifunović et al., 2016; Vistoli et al., 2008; Yazdanian et al., 1998). For this analysis, rose oxide demonstrated medium permeability through the intestinal epithelium corroborating with previous analysis. However, considering MDK cells, the in silico analysis demonstrated values of 100% for rose oxide permeability highlighting its tissue penetration capacity.
Plasma protein binding is another very important pharmacokinetic property in drug design. It has a central role in the transport of drugs in the human organism, affecting in a significant manner the distribution and excretion, and consequently the pharmacological and toxicological effects of drugs. According to the results obtained in this study, rose oxide strongly binded to albumin, based on the respective albumin binding values above >90%. This value may indicate a longer half-life for rose oxide, thus favoring a longer effect of the molecule, which may be useful according to its observed effects (Xiang et al., 2020; Yuan et al., 2020).
Complementing the evaluation of the in silico ADMET parameters, our results show inhibitory activity of cytochrome P 450 (CYP) enzymes for RO. The substance showed inhibitory activity for CYP2C19 and CYP2C9 enzymes. This type of inhibition may result in an increase in the plasma concentrations of some drugs and may lead to adverse results (Ghose et al., 1998; Oprea, 2000; Sekhar et al., 2014; Teague et al., 1999). However, in contrast to other isoforms (including CYP3A4), this activity is not observed. We note that some of these isoforms in which the RO does not inhibit, are responsible for the metabolism of several drugs such as antiarrhythmics, adrenoceptor antagonists and antidepressants (Oprea, 2000). This interaction profile may be favorable considering aspects related to the safety of the rose oxide against the use of other substances.
Finalizing the aspects related to the kinetic characteristics of the rose oxide, our results evaluated the molecule according to the Lipinski rule that establishes that a molecule must present values for at least 4 of the following parameters. The molecule should be well absorbed must have Log P ≤ 5 (be able to solubilize in water and cross cell membranes), molecular weight ≤500 Da, maximum 10 acceptor groups of hydrogen and a maximum of 5 hydrogen donors and some extenders as number of rotational connections (nRot≤10) and polar surface area (ASP ≤140Å2) (Lipinski, 2004). Lipinski's rule evaluates whether a particular compound has physicochemical properties suitable to be bioavailable orally (Lipinski, 2004; Mignani et al., 2016). Rose oxide is a promising molecule, because, it meets all criteria of this rule thus exhibiting favorable characteristics of a good drug candidate. All kinetic and physico-chemical data are in Table 1 and 3.
In silico toxicological studies (Table 2) are essential to understand mechanisms of interaction between toxic agents and important targets, in order to allow a rational planning of drugs with low affinity for these targets. Regarding the RO's toxicity evaluation, this substance was positive for the Ames test, a simple method used in evaluating the mutagenicity of a compound, which compares with strains of Salmonella typhimurium bacteria that carry mutations in genes involved with histidine synthesis (Araki et al., 2004; Prival and Zeiger, 1998). The test in pre ADMET also predicted toxicity to TA100, a strain of Salmonella typhimurium which is frequently used in the Ames test (Ames et al., 1972).
In the case of fish toxicity prediction, RO does not present a toxicity effect to these species of fish analyzed by the in silico method (Ankley and Villeneuve, 2006; Maki, 1979; Padilla et al., 2009). However, this model predicted that the substance showed carcinogenicity, the ability to cause cancer in mice when exposed to RO. The acute toxicity tests for rodents, fish, Daphnia, and the algae growth inhibition test were carried out based on physicochemical and ecotoxicological properties according to the data obtained and based on the Directive 92/32/EEC of the European legislation on chemical substances (Weyers et al., 2000). Therefore, based on the results obtained, this compound has the potential to cause long-term adverse effects in the aquatic environment (solubility <1mg/L). RO presents a medium risk for inhibition of the hERG gene, which delays the rectified voltage potassium channel into the heart (IKr) involved in cardiac repolarization.
In order to verify the in vivo effect of the RO, studies were performed on animal models of depression (FST and TST). In these models, treatment with rose oxide at the doses of 25, 50 and 100 mg/kg were able to alter the immobility time of the animals through the FST test (Figure 3). In the tail suspension test, a similar response at a dose of 50 mg/kg weight was observed. There were no changes with increasing dose to 100 mg/kg (Figure 3B). Although both TST and FST are similar in their constructs and objectives, they are different in terms of biological substrates underlying the observed behavior (Freitas et al., 2010). TST was proposed to have greater pharmacological sensitivity when compared to FST (Can et al., 2012). Thus considering the dose of 50mg/kg of weight as the best dose promoting the antidepressant effect of RO (Figure 2).
To evaluate the action of rose oxide on motor activity, we used the Open Field Test (OFT). The results showed that the groups treated with RO and fluoxetine did not change in the number of squares invaded by the animals in the test when compared to the vehicle group. These results demonstrate that rose oxide does not alter the motor activity of the animals. Behaviors such as exploring the surrounding environment is a natural rodent behavior. The test may be useful for evaluating depressant effects on the locomotor system and its absence in the test may rule out such an effect (Xie et al., 2012).
In rodents, reduced exploratory behavior in an unfamiliar environment reflects a "refractory loss of interest" is related to anhedonia, which is the loss of the ability to feel pleasure, characteristic of severely depressive states, because strange things are usually seen as rewarding in rodents (Uttl et al., 2018). We also determined using the most effective dose of RO (50 mg/kg), the substance's ability to cause a myorelaxant effect, motor incoordination and balancing through the Rota-Rod test. Treatment at the dose of 50 mg/kg was not able to change the length of stay of the animals to stay on the rotating rod when compared to the negative control group, demonstrating a similar result to that of fluoxetine (Figure 3).
In order to clarify the antidepressant mechanisms of the RO, two protocols were performed. Initially we performed the molecular docking analysis and then compared with an in vivo model of mechanism evaluation. With regards to docking analysis, our results evidenced potential binding sites of ROs with serotonergic, gabaergic and glutamatergic receptors. The "PDBs" of the target proteins and receptors as well as active sites of the Rose Oxide anchorage are shown in Figure 1 as Tables 4 and 5.
These results are important because the serotonergic pathway is considered to be one of the most important of the therapeutic effects of antidepressant drugs. Substances such as fluoxetine, sertraline and others act by increasing the levels of serotonin in the central nervous system and this increase is responsible for the changes that lead to decreased manifestations of depression. Not only increasing serotonin levels is important, but the interaction of this neurotransmitter with its different receptors. Among the most important receptors are 5HT1A receptors.
Studies show that there is a decrease in these receptors in the hippocampus of individuals with depressive disorders, as well as in animals submitted to chronic stress and elevated glucocorticoid levels. On the other hand, treatment with antidepressant drugs promotes increased sensitivity of these receptors in the limbic system in post-synaptic structures and this increase in sensitivity of receptors would be directly related to the manifestation of the antidepressant effect of these substances (De Deurwaerdère and Di Giovanni, 2017; Petrunich-Rutherford et al., 2018). Thus, it is important to evaluate the interaction between RO and the serotonergic system and specifically the 5HT1A receptors. This evaluation was carried out in two steps.
We initially administered a sub-effective dose of RO (12.5 mg/kg) associated with a sub-effective dose of fluoxetine (10 mg/kg) both orally. After 1 h of administration, we can see a synergism between the doses through a significant decrease in the immobility time of the animals in the FST (Figure 4 A).
Fluoxetine is an antidepressant drug that when applied at the clinical dose promotes an increase in serotonin levels due to the inhibition of the reuptake of this neurotransmitter into the synaptic cleft. This blockage causes an increase in serotonin, but at a sub-effective dose this would not be enough to generate FST effect (Dafsari and Jessen, 2020; Han et al., 2019; Jeong et al., 2019; Melo et al., 2015; Nakai et al., 2017). In association with the RO and considering that this substance could also interfere with serotonergic neurotransmission, we can thus justify the effect observed in the association of the two substances. This protocol is not sufficient to indicate the possible receptor in which the RO may be interacting. Faced with this need and considering the importance of the 5HT1A receptor in the pathophysiology of depression, we investigated the role of this receptor in the effects of RO.
For this, the results of the present study demonstrated that pre-treatment with WAY100635 (a 5HT1A receptor antagonist) administered prior to RO at 50mg/kg dose, caused a reversal of the antidepressant effect of terpene on FST, thus demonstrating that RO may exerts its effect due to its interaction with these specific serotonin receptors (Figure 4 B). Thus, we can conclude that the RO has favorable kinetic characteristics to be a good candidate for an antidepressant drug and displays a good safety profile in the in silico analysis. In addition, the RO showed antidepressant effect in vivo acting probably through the serotonergic pathway that involves interaction with 5HT1A receptors.
Declarations
Author contribution statement
Wcleubianne Matias Nascimento Maia; Francisco Das Chagas Pereira de Andrade; Livia Alves Filgueiras: Performed the experiments; Analyzed and interpreted the data.
Anderson Nogueira Mendes; Amanda Fonseca Costa Assunção; Nicolas Davidson Sérvulo Rodrigues: Performed the experiments; Analyzed and interpreted the data.
Rosemarie Brandim Marques; Antônio Luiz Martins Maia Filho; Luciano Da Silva Lopes: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Damião Pergentino de Sousa: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Federal University of Piaui, CAPES, CNPq and FAPEPI (FAPEPI/MCTIC/CNPq Nº 008/2018 – PRONEM).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ajay, Bemis G.W., Murcko M.A. Designing libraries with CNS activity. J. Med. Chem. 1999;42(24):4942–4951. doi: 10.1021/jm990017w. [DOI] [PubMed] [Google Scholar]
- Al-Heibshy F.N.S., Başaran E., Arslan R., Öztürk N., Erol K., Demirel M. Physicochemical characterization and pharmacokinetic evaluation of rosuvastatin calcium incorporated solid lipid nanoparticles. Int. J. Pharm. 2020;578:119106. doi: 10.1016/j.ijpharm.2020.119106. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G.S. Mechanisms and treatment of late-life depression. Transl. Psychiatry. 2019;9(1):188. doi: 10.1038/s41398-019-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsters P.L., Jary W., Nardello-Rataj V., Aubry J.-M. “Dark” singlet oxygenation of β-citronellol: a key step in the manufacture of rose oxide. Org. Process Res. Dev. 2010;14(1):259–262. [Google Scholar]
- Ames B.N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Ames Bruce N., Gurney E.G., Miller J.A., Bartsch H. Carcinogens as frameshift mutagens: metabolites and derivatives of 2-acetylaminofluorene and other aromatic amine carcinogens. Proc. Natl. Acad. Sci. U. S. A. 1972;69(11):3128–3132. doi: 10.1073/pnas.69.11.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley G.T., Villeneuve D.L. The fathead minnow in aquatic toxicology: past, present and future. Aquat. Toxicol. (Amst.) 2006;78(1):91–102. doi: 10.1016/j.aquatox.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Araki A., Kamigaito N., Sasaki T., Matsushima T. Mutagenicity of carbon tetrachloride and chloroform in Salmonella typhimurium TA98, TA100, TA1535, and TA1537, and Escherichia coli WP2uvrA/pKM101 and WP2/pKM101, using a gas exposure method. Environ. Mol. Mutagen. 2004;43(2):128–133. doi: 10.1002/em.20005. [DOI] [PubMed] [Google Scholar]
- Babu K.G.D., Singh B., Joshi V.P., Singh V. Essential oil composition of Damask rose (Rosa damascena Mill.) distilled under different pressures and temperatures. Flavour Fragrance J. 2002;17(2):136–140. [Google Scholar]
- Batish D.R., Singh H.P., Setia N., Kaur S., Kohli R.K. Chemical composition and inhibitory activity of essential oil from decaying leaves of Eucalyptus citriodora. Zeitschrift Fur Naturforschung. C, Journal of Biosciences. 2006;61(1–2):52–56. doi: 10.1515/znc-2006-1-210. [DOI] [PubMed] [Google Scholar]
- Can A., Dao D.T., Terrillion C.E., Piantadosi S.C., Bhat S., Gould T.D. The tail suspension test. JoVE: JoVE. 2012;59 doi: 10.3791/3769. e3769–e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana A., Poletti S.C., Guducu C., Larsson M., Hummel T. Electro-olfactogram responses before and after aversive olfactory conditioning in humans. Neuroscience. 2018;373:199–206. doi: 10.1016/j.neuroscience.2018.01.025. [DOI] [PubMed] [Google Scholar]
- Chisholm D., Sweeny K., Sheehan P., Rasmussen B., Smit F., Cuijpers P., Saxena S. Scaling-up treatment of depression and anxiety: a global return on investment analysis. The Lancet Psychiatry. 2016;3(5):415–424. doi: 10.1016/S2215-0366(16)30024-4. [DOI] [PubMed] [Google Scholar]
- Cho C.-F., Wolfe J.M., Fadzen C.M., Calligaris D., Hornburg K., Chiocca E.A., Agar N.Y.R., Pentelute B.L., Lawler S.E. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017;8(1):15623. doi: 10.1038/ncomms15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafsari F.S., Jessen F. Depression—an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl. Psychiatry. 2020;10(1):160. doi: 10.1038/s41398-020-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdère P., Di Giovanni G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: therapeutic implications. Prog. Neurobiol. 2017;151:175–236. doi: 10.1016/j.pneurobio.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Dunn E.C., Wang M.-J., Perlis R.H. The Harvard University Center on the Developing Child (Dr. Dunn) and by National Institute of Mental Health grant Numbers: K01MH102403 (Dr. Dunn) Elsevier; 2020. Chapter 1 - a summary of recent updates on the genetic determinants of Depression∗∗Supported; pp. 1–27. R0 (R. S. B. T.-M. D. D. McIntyre. [Google Scholar]
- Eigenmann D.E., Dürig C., Jähne E.A., Smieško M., Culot M., Gosselet F., Cecchelli R., Helms H.C.C., Brodin B., Wimmer L., Mihovilovic M.D., Hamburger M., Oufir M. In vitro blood–brain barrier permeability predictions for GABAA receptor modulating piperine analogs. Eur. J. Pharm. Biopharm. 2016;103:118–126. doi: 10.1016/j.ejpb.2016.03.029. [DOI] [PubMed] [Google Scholar]
- Freitas A.E., Budni J., Lobato K.R., Binfaré R.W., Machado D.G., Jacinto J., Veronezi P.O., Pizzolatti M.G., Rodrigues A.L.S. Antidepressant-like action of the ethanolic extract from Tabebuia avellanedae in mice: evidence for the involvement of the monoaminergic system. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(2):335–343. doi: 10.1016/j.pnpbp.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Gevorgyan A., Hopmann K.H., Bayer A. Exploration of new biomass-derived solvents: application to carboxylation reactions. ChemSusChem. 2020;13(8):2080–2088. doi: 10.1002/cssc.201903224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose A.K., Viswanadhan V.N., Wendoloski J.J. Prediction of hydrophobic (lipophilic) properties of small organic molecules using fragmental methods: an analysis of ALOGP and CLOGP methods. J. Phys. Chem. 1998;102(21):3762–3772. [Google Scholar]
- Golani M., Golani I. On a simulation approach to drug blood brain barrier permeation: neural net ensemble based. IAENG Int. J. Comput. Sci. 2015;42:31–40. [Google Scholar]
- Guilhermino L., Diamantino T., Carolina Silva M., Soares A.M.V.M. Acute toxicity test with Daphnia magna: an alternative to mammals in the prescreening of chemical toxicity? Ecotoxicol. Environ. Saf. 2000;46(3):357–362. doi: 10.1006/eesa.2000.1916. [DOI] [PubMed] [Google Scholar]
- Guo C., Zhang J., Liu C., Bian Y., Shan Q. Extracting rose essential oil from rose slag with ionic liquid. Biomass Convers. Bioref. 2020:1–8. [Google Scholar]
- Hall C.S. Emotional behavior in the rat. III. The relationship between emotionality and ambulatory activity. J. Comp. Psychol. 1936;22(3):345–352. [Google Scholar]
- Han W., Li J., Pelkey K.A., Pandey S., Chen X., Wang Y.-X., Wu K., Ge L., Li T., Castellano D., Liu C., Wu L.-G., Petralia R.S., Lynch J.W., McBain C.J., Lu W. Shisa7 is a GABAA receptor auxiliary subunit controlling benzodiazepine actions. Science. 2019;366(6462):246 LP–250. doi: 10.1126/science.aax5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-L., Lim S.-L., Lu K.-H., Sheen L.-Y. Antidepressant-like effects of Gan-Mai-Dazao-Tang via monoamine regulatory pathways on forced swimming test in rats. J. Tradit. Complem. Med. 2018;8(1):53–59. doi: 10.1016/j.jtcme.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S., Yang D., Beyene A.G., Del Bonis-O’Donnell J.T., Gest A.M.M., Navarro N., Sun X., Landry M.P. High-throughput evolution of near-infrared serotonin nanosensors. Sci. Adv. 2019;5(12) doi: 10.1126/sciadv.aay3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y., Takaku H., Yamada R., Akase C., Abe Y., Sekiguchi Y., Murayama N., Shimizu M., Kitajima M., Shono F., Funatsu K., Yamazaki H. Determination and prediction of permeability across intestinal epithelial cell monolayer of a diverse range of industrial chemicals/drugs for estimation of oral absorption as a putative marker of hepatotoxicity. Toxicology Reports. 2020;7:149–154. doi: 10.1016/j.toxrep.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König H., König H.-H., Konnopka A. The excess costs of depression: a systematic review and meta-analysis. Epidemiol. Psychiatr. Sci. 2020;29:e30. doi: 10.1017/S2045796019000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovačević S.Z., Jevrić L.R., Podunavac Kuzmanović S.O., Lončar E.S. Prediction of in-silico ADME properties of 1,2-O-isopropylidene aldohexose derivatives. Iran. J. Pharm. Res. (IJPR) 2014;13(3):899–907. [PMC free article] [PubMed] [Google Scholar]
- Kraus C., Kadriu B., Lanzenberger R., Zarate C.A., Jr., Kasper S. Prognosis and improved outcomes in major depression: a review. Transl. Psychiatry. 2019;9(1):127. doi: 10.1038/s41398-019-0460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiate J.-R., Bessière J.-M., Amvam Zollo P.-H. Composition of the essential oils from three Laggera Spp. from Cameroon. Flavour Fragrance J. 2002;17(2):105–108. [Google Scholar]
- Laursen T.M., Musliner K.L., Benros M.E., Vestergaard M., Munk-Olsen T. Mortality and life expectancy in persons with severe unipolar depression. J. Affect. Disord. 2016;193:203–207. doi: 10.1016/j.jad.2015.12.067. [DOI] [PubMed] [Google Scholar]
- Lipinski C.A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Maki A.W. Correlations between Daphnia magna and fathead minnow (Pimephales promelas) chronic toxicity values for several classes of test substances. J. Fish. Res. Board Can. 1979;36(4):411–421. [Google Scholar]
- Melo A., Kokras N., Dalla C., Ferreira C., Ventura-Silva A.P., Sousa N., Pêgo J.M. The positive effect on ketamine as a priming adjuvant in antidepressant treatment. Transl. Psychiatry. 2015;5(5) doi: 10.1038/tp.2015.66. e573–e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignani S., Huber S., Tomás H., Rodrigues J., Majoral J.-P. Why and how have drug discovery strategies in pharma changed? What are the new mindsets? Drug Discov. Today. 2016;21(2):239–249. doi: 10.1016/j.drudis.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Miteva M.A., Violas S., Montes M., Gomez D., Tuffery P., Villoutreix B.O. FAF-Drugs: free ADME/tox filtering of compound collections. Nucleic Acids Res. 2006;34:W738–W744. doi: 10.1093/nar/gkl065. (Web Server issue), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai N., Nagano M., Saitow F., Watanabe Y., Kawamura Y., Kawamoto A., Tamada K., Mizuma H., Onoe H., Watanabe Y., Monai H., Hirase H., Nakatani J., Inagaki H., Kawada T., Miyazaki T., Watanabe M., Sato Y., Okabe S. Serotonin rebalances cortical tuning and behavior linked to autism symptoms in 15q11-13 CNV mice. Sci. Adv. 2017;3(6) doi: 10.1126/sciadv.1603001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonato F.R., Santana D.G., de Melo F.M., dos Santos G.G.L., Brustolim D., Camargo E.A., de Sousa D.P., Soares M.B.P., Villarreal C.F. Anti-inflammatory properties of rose oxide. Int. Immunopharm. 2012;14(4):779–784. doi: 10.1016/j.intimp.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Oprea T.I. Property distribution of drug-related chemical databases. J. Comput. Aided Mol. Des. 2000;14(3):251–264. doi: 10.1023/a:1008130001697. [DOI] [PubMed] [Google Scholar]
- Padilla S., Cowden J., Hinton D.E., Yuen B., Law S., Kullman S.W., Johnson R., Hardman R.C., Flynn K., Au D.W.T. 2009. Use of Medaka in Toxicity Testing. Current Protocols in Toxicology, Chapter 1, Unit1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R., Andlauer T.F.M., Czamara D., Hoehn D., Lucae S., Pütz B., Lewis C.M., Uher R., Müller-Myhsok B., Ising M., Sämann P.G. Treatment response classes in major depressive disorder identified by model-based clustering and validated by clinical prediction models. Transl. Psychiatry. 2019;9(1):187. doi: 10.1038/s41398-019-0524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrunich-Rutherford M.L., Garcia F., Battaglia G. 5-HT(1A) receptor-mediated activation of neuroendocrine responses and multiple protein kinase pathways in the peripubertal rat hypothalamus. Neuropharmacology. 2018;139:173–181. doi: 10.1016/j.neuropharm.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prival M.J., Zeiger E. Chemicals mutagenic in Salmonella typhimurium strain TA1535 but not in TA100. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998;412(3):251–260. doi: 10.1016/s1383-5718(97)00196-4. [DOI] [PubMed] [Google Scholar]
- Ramaiah M.J., Karthikeyan D., Mathavan S., Yamajala R.B.R.D., Ramachandran S., Vasavi P.J., Chandana N.V. Synthesis, in vitro and structural aspects of benzothiazole analogs as anti-oxidants and potential neuroprotective agents. Environ. Toxicol. Pharmacol. 2020;79:103415. doi: 10.1016/j.etap.2020.103415. [DOI] [PubMed] [Google Scholar]
- Saurav K., Zhang W., Saha S., Zhang H., Li S., Zhang Q., Wu Z., Zhang G., Zhu Y., Verma G. In silico molecular docking, preclinical evaluation of spiroindimicins A-D, lynamicin A and D isolated from deep marine sea derived Streptomyces sp. SCSIO 03032. Interdiscipl. Sci. Comput. Life Sci. 2014;6(3):187–196. doi: 10.1007/s12539-013-0200-y. [DOI] [PubMed] [Google Scholar]
- Sekhar K.C., Syed R., Golla M., Kumar M.V.,J., Yellapu N.K., Chippada A.R., Chamarthi N.R. Novel heteroaryl phosphonicdiamides PTPs inhibitors as anti-hyperglycemic agents. Daru: J. Facul. Pharm. 2014;22(1):76. doi: 10.1186/s40199-014-0076-3. Tehran University of Medical Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Kaul V.K., Megeji N.W., Singh V., Ahuja P.S. Essential oil composition of three accessions of Dracocephalum heterophyllum Benth. cultivated at Palampur, India. Nat. Prod. Res. 2008;22(11):927–936. doi: 10.1080/14786410701642847. [DOI] [PubMed] [Google Scholar]
- Teague S.J., Davis A.M., Leeson P.D., Oprea T. The design of leadlike combinatorial libraries. Angew. Chem. Int. Ed. 1999;38(24):3743–3748. doi: 10.1002/(SICI)1521-3773(19991216)38:24<3743::AID-ANIE3743>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Thierry B., Stéru L., Simon P., Porsolt R.D. The tail suspension test: ethical considerations. Psychopharmacology. 1986;90(2):284–285. doi: 10.1007/BF00181261. [DOI] [PubMed] [Google Scholar]
- Trifunović J., Borčić V., Vukmirović S., Kon S.G., Mikov M. Retention data of bile acids and their oxo derivatives in characterization of pharmacokinetic properties and in silico ADME modeling. Eur. J. Pharmaceut. Sci. 2016;92:194–202. doi: 10.1016/j.ejps.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Uttl L., Szczurowska E., Hájková K., Horsley R.R., Štefková K., Hložek T., Šíchová K., Balíková M., Kuchař M., Micale V., Páleníček T. Behavioral and pharmacokinetic profile of indole-derived synthetic cannabinoids JWH-073 and JWH-210 as compared to the phytocannabinoid Δ(9)-THC in rats. Front. Neurosci. 2018;12:703. doi: 10.3389/fnins.2018.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistoli G., Pedretti A., Testa B. Assessing drug-likeness - what are we missing? Drug Discov. Today. 2008;13(7–8):285–294. doi: 10.1016/j.drudis.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Weyers A., Sokull-Klüttgen B., Baraibar-Fentanes J., Vollmer G. Acute toxicity data: a comprehensive comparison of results of fish, daphnia, and algae tests with new substances notified in the European Union. Environ. Toxicol. Chem. 2000;19(7):1931–1933. [Google Scholar]
- Xiang Y., Liu S., Yang J., Wang Z., Zhang H., Gui C. Investigation of the interactions between flavonoids and human organic anion transporting polypeptide 1B1 using fluorescent substrate and 3D-QSAR analysis. Biochim. Biophys. Acta Biomembr. 2020;1862(5):183210. doi: 10.1016/j.bbamem.2020.183210. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Li J., Niu Y., Liu Q., Liu J. Verification of key odorants in rose oil by gas chromatography-olfactometry/aroma extract dilution analysis, odour activity value and aroma recombination. Nat. Prod. Res. 2017;31(19):2294–2302. doi: 10.1080/14786419.2017.1303693. [DOI] [PubMed] [Google Scholar]
- Xie Y., Wang Y., Zhang T., Ren G., Yang Z. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J. Biomed. Sci. 2012;19(1):14. doi: 10.1186/1423-0127-19-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanian M., Glynn S.L., Wright J.L., Hawi A. Correlating partitioning and caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharmaceut. Res. 1998;15(9):1490–1494. doi: 10.1023/a:1011930411574. [DOI] [PubMed] [Google Scholar]
- Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man--fact or myth. Pharmaceut. Res. 1997;14(6):763–766. doi: 10.1023/a:1012102522787. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Chang S., Zhang Z., Li Z., Li S., Xie P., Yau W.-P., Lin H., Cai W., Zhang Y., Xiang X. A novel strategy for prediction of human plasma protein binding using machine learning techniques. Chemometr. Intell. Lab. Syst. 2020;199:103962. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.