Abstract
The escalating social and economic burden of an aging world population has placed aging research at center stage. The hallmarks of aging comprise diverse molecular mechanisms and cellular systems that are interrelated and act in concert to drive the aging process. Here, through the lens of telomere biology, we examine how telomere dysfunction may amplify or drive molecular biological processes underlying each hallmark of aging and contribute to the development of age-related diseases such as neurodegeneration and cancer. The intimate link of telomeres to aging hallmarks informs preventive and therapeutic interventions designed to attenuate aging itself and reduce the incidence of age-associated diseases.
DePinho eTOC blurb
Telomere attrition and disfunction is both a cause and consequence of cellular and molecular aging and age-related disease. Mounting data revel how telomeres are entwined with other aging hallmarks and may be therapeutic targets for aging and age-related disease.
INTRODUCTION
Over the last century, advances in public health and medicine have fueled a dramatic rise in life expectancy worldwide. On the current trajectory, 2.1 billion individuals will be older than age 60 by 2050 (United Nations, 2017). This demographic milestone will be accompanied by major increases in age-associated diseases, such as Alzheimer’s disease, cardiovascular disease, and cancer (Melzer et al., 2020), which essentially double in incidence every 5 years after age 60. In the absence of new medical and wellness paradigms, the world will experience an unsustainable burden of chronic disease that already extracts a significant social and economic toll. The link between aging and such diseases has motivated fundamental investigations into the mechanisms of aging and strategies to attenuate its impact.
Aging is a progressive degenerative state accompanied by tissue stem cell depletion, tissue inflammation, matrix alterations, cellular senescence, and metabolic dysfunction (Lopez-Otin et al., 2013). These cellular and tissue changes reflect underlying molecular aberrations in mitochondria, proteostasis, intercellular communication, nutrient sensing, epigenetics, and DNA repair which leads to genomic instability and damage including telomere dysfunction (Lopez-Otin et al., 2013). An ever-greater understanding of the diverse molecular mechanisms of aging points to the telomere as an instigator or amplifier of the molecular circuitry driving the aging process and its associated diseases.
Telomeres are composed of repetitive nucleotide sequences that form a ‘cap structure’ which functions to maintain the integrity of chromosomes. Human telomere maintenance-associated gene defects are linked to germline and somatic degenerative diseases such as dyskeratosis congenita, idiopathic pulmonary fibrosis, ulcerative colitis and others (Calado and Young, 2009). Mice engineered with null or inducible telomerase alleles (Blasco et al., 1997; Jaskelioff et al., 2011) helped researchers to forge a link between telomere dysfunction and aging itself (Blasco et al., 1997; Lee et al., 1998; Rudolph et al., 1999), progeric syndromes (Chang et al., 2004), and chronic inflammatory and degenerative conditions (Chakravarti D., 2020; Rudolph et al., 2000). In mice, reactivation of endogenous telomerase reverses premature aging in mice with telomere dysfunction (Jaskelioff et al., 2011). Both killifish (Harel et al., 2015) and zebrafish (Carneiro et al., 2016) serve as models for telomere biology studies; their telomere length is similar to that of humans, while their telomere dysfunction phenotypes mirror those in murine models.
This review provides an overview of the history and current state of telomere research, highlights mechanistic connections between telomere dysfunction and aging hallmarks, and examines the seemingly pervasive roles of telomeres and telomerase in driving hallmarks of aging, progeria syndromes, and common age-associated diseases such as neurodegeneration and cancer. We describe how the intimate link of telomeres and aging mechanisms informs the development of anti-aging and disease preventive strategies.
HISTORY OF TELOMERES AND TELOMERASE
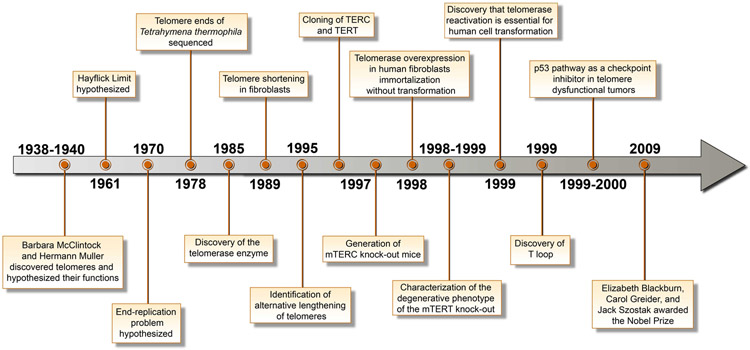
The concept of telomeres was born in the 1930s, when McClintock and Muller inferred the existence of a unique structure at the ends of chromosomes in Zea mays and Drosophila melanogaster and hypothesized that it was critical for prevention of chromosome end fusion (Creighton and McClintock, 1931; Muller, 1938). Muller coined the term ‘telomere’ from the Greek ‘telos’ meaning ‘end’ and ‘meros’ meaning ‘part,’ hence ‘end-part’ (Figure 1). In 1961, Hayflick and Moorhead demonstrated that human fetal cells possessed finite replicative potential of 50 to 60 doublings, subsequently dubbed the ‘Hayflick limit’ or replicative senescence (Hayflick, 1965; Hayflick and Moorhead, 1961). In the early 1970s, Olovnikov and Watson introduced the ‘end replication problem’ by observing the asymmetry in linear DNA replication and predicting that each cell division would incur a loss of chromosomal DNA from the termini of the lagging strand due to removal of the terminal RNA primer, thereby leading to progressive chromosomal shortening (Olovnikov, 1973; Watson, 1972 ).
Figure 1.
Timeline of discoveries in the human telomere field.
It was not until 1978 when Blackburn and Gall sequenced the rDNA of the ciliated protozoan Tetrahymena thermophila, revealing termini composed of tandem repeats of hexanucleotide sequences of 5′-CCCCAA-3′ and 3′-TTGGGG-5′ on the complementary strand (Blackburn and Gall, 1978). In 1985, Blackburn and Greider uncovered a novel enzymatic activity capable of adding DNA repeat sequences to chromosome ends and extending telomere length, which is now known as telomerase (Greider and Blackburn, 1985). In 1989, the RNA component was cloned from T. thermophila (Greider and Blackburn, 1989). In 1990, this journey of discovery culminated in evidence that telomere attrition occurs in parallel with the replicative lifespan of human primary cells in culture, establishing that short telomeres trigger the Hayflick limit (Harley et al., 1990). The role of telomere attrition and damage in inducing a permanent cell cycle arrest was further demonstrated using human fibroblasts overexpressing a mutant form of TRF2 (TRF2ΔBΔM) through activation of DNA damage check-points (d'Adda di Fagagna et al., 2003)
The next major breakthrough occurred in 1996, with the demonstration that telomerase was a ribonucleoprotein that acted on a 3′ overhang (Lingner et al., 1997). This was followed by the cloning of human telomerase reverse transcriptase (hTERT) (Harrington et al., 1997) and the telomerase RNA component (hTERC) (Feng et al., 1995). Consequently, the first TERC knockout mouse was generated in 1997 (Blasco et al., 1997). In the ensuing years, mice null for TERC and TERT, alone or in combination with progeria or cancer-relevant alleles, established that telomere dysfunction can drive premature aging, cancer, and various degenerative diseases. These mouse models proved that intact telomeres maintain genome stability, tissue stem cell reserves, organ system homeostasis, and normal lifespan (Chang et al., 2004; Jaskelioff et al., 2011; Rudolph et al., 1999; Sahin et al., 2011; Wong et al., 2003). In 1998, a capstone study showed that enforced hTERT expression endows unlimited replicative potential to primary human cells including fibroblasts, retinal pigment epithelial cells, and vascular endothelial cells (Bodnar et al., 1998). Notably, these cell cultures maintained a normal karyotype and showed no malignant properties.
Historically, while telomerase was known to be consistently upregulated in cancer, its role in cellular transformation was documented in 1999 when enforced TERT expression, together with classical oncogenes, promoted full malignant transformation of primary human cells (Hahn et al., 1999). Contemporaneously, genetic models of cancer established that the status of p53-dependent DNA damage response dictated whether short telomeres promote or suppress cancer in vivo (Chin et al., 1999; Greenberg et al., 1999). In 2000, further analysis of late-generation mTERC−/−;p53+/− mice (Artandi et al., 2000) revealed a humanized tumor spectrum of epithelial cancers possessing chromosomal rearrangements and nonreciprocal translocations typical of human cancer genomes. Thus, mice engineered to experience telomere-based crisis in the context of deactivated DNA damage signaling (p53 deficiency) illuminated a major mechanism driving the preponderance of epithelial cancers in aged humans and explained why such cancers develop radically altered cytogenetic profiles.
The TERC and TERT knockout mouse models authenticated the role of telomeres in aging and identified a core signaling pathway driving the aging process. First, these models established that telomere dysfunction accelerates signs and symptoms of aging characterized by shortened life expectancy, an aged appearance, declining tissue stem cell reserves, organ atrophy, and diminished capacity to cope with stress, injury, and regenerative demands (Lee et al., 1998; Rudolph et al., 1999). Second, these models highlighted the essentiality of telomere dysfunction in progeroid syndromes and Parkinson’s disease (Chang et al., 2004; Lee et al., 1998; Rudolph et al., 1999; Wong et al., 2003). Third, unbiased transcriptomic analyses across diverse tissues of the late-generation TERC−/− mouse revealed the p53-PGC pathway of aging, integrating three previously separate theories of aging—genotoxic stress (telomere dysfunction), oxidative damage, and mitochondrial decline (Sahin et al., 2011). Finally, an inducible TERT mouse model demonstrated that reactivation of endogenous telomerase reverses advanced premature aging in mice (Jaskelioff et al., 2011). Furthermore, adenoviral delivery of telomerase in aged mice improves cardiac function following acute myocardial infarction, enhances muscle coordination and kidney and liver function, reduces insulin resistance and subcutaneous fat depletion, increases bone mineral density, and extends life expectancy without causing an increase in cancer incidence (Hong and Yun, 2019). Together, this collective body of work across decades and diverse model systems has defined the molecular biology of telomeres and its role in health and disease.
SHELTERTIN AND THE TELOMERASE COMPLEX
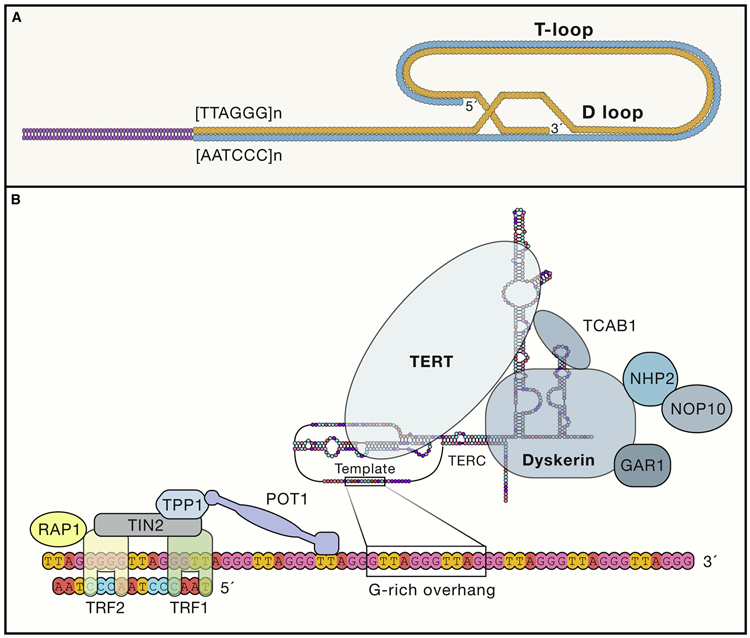
Telomeres maintain chromosomal integrity, which is essential for sustaining health-span and propagation of the species. The evolutionarily conserved function of telomere end protection spans from lower multicellular organisms such as T. thermophila to higher-order organisms including Homo sapiens (Roake and Artandi, 2020). Structurally, telomeres consist of tandem repeat sequences of TTAGGG measuring from several to tens of kilobases and terminating at the 3′ end in a single-stranded 75-to-300–nucleotide overhang enriched in guanine nucleotides (Figure 2B). Seminal work by Griffith and de Lange established that the overhang folds back on itself to form a lariat-like structure named the T loop (Figure 2A) (Griffith et al., 1999).
Figure 2. The vertebrate telomere/telomerase complex.
(A) Structure of the D-loop and T-loop at the telomeric end.
(B) Structure of tetomerase. NOP10, nucleolar protein family A, member 3; NHP2, nucleolar protein family A, member 2; TIN2, TERF1-interacting nuclear factor 2; TPP1, telomere protection protein 1; TRF1, telomeric repeat binding factor 1; TRF2, telomeric repeat binding factor 2, POT1, protection of telomeres 1; RAP1, TERF2-interacting protein; TCAB1, telomerase Cajal body protein 1; GAR1, nucleolar protein family A. member 1.
The telomere is cloaked in specialized proteins dubbed the shelterin complex, a multimer composed of six protein subunits: TRF1, TRF2, TPP1, POT1, TIN2, and RAP1(de Lange, 2018). This higher-order structure of telomeres functions to inhibit DNA damage signaling from the telomere ends, prevent DNA repair programs from fusing ends via recombination or classical/alternative non-homologous end joining, and regulate the access and activity of telomerase at the termini. Correspondingly, mutations in the aforementioned components can disrupt the shelterin-telomere complex, causing end fusions and premature senescence (van Steensel et al., 1998). Similarly, overexpression of TRF1 or deregulation of POT1 impairs telomerase binding to telomere ends, causing telomere shortening (Loayza and De Lange, 2003; Smogorzewska et al., 2000). Conversely, loss of TRF1 also leads to the formation of common fragile sites in telomeric DNA due to its inability to recruit the Bloom (BLM) helicase and therefore BLM’s execution of robust double-strand break repair during replication (Yang et al., 2020).
In normal tissues, telomerase expression is abundant in germ cells (Lee et al., 1998) and present in undifferentiated stem and progenitor cells of the skin (Lee et al., 1998; Rudolph et al., 1999), intestine (Schepers et al., 2011), hematopoietic system (Colla et al., 2015), hair bulge (Sarin et al., 2005), and testes (Rudolph et al., 1999 ). Differentiated cells such as keratinocytes, fibroblasts, skeletal muscle myocytes, neurons, cardiac myocytes, and spermatids show modest or undetectable TERT levels (Artandi and DePinho, 2010; Pech et al., 2015).
TERC is related to small Cajal body RNAs (scaRNAs) and small nucleolar RNAs (snoRNAs). While such RNAs are encoded within introns of other genes, TERC is a prototypical gene with its own promoter (Feng et al., 1995). TERC RNA is transcribed in a precursor format with a 5′ methylguanosine cap and a poly (A) tail. These precursors are de-adenylated by the disease-associated poly (A)-specific ribonuclease (PARN), which promotes TERC maturation and accumulation (Roake and Artandi, 2020). TERC contains an H/ACA domain consisting of a three-nucleotide sequence, termed the CAB box, that is essential for binding to telomerase Cajal body protein 1 (TCAB1). TCAB1 is required for both catalytic activity and trafficking of telomerase to Cajal bodies, which aids in trafficking telomerase to telomere ends .Telomerase associates with TERC through its PK/T and CR4/5 domains. Multiple proteins are essential for proper functioning of the holoenzyme, including dyskerin, NHP2, NOP10, and GAR1, which form core components along with other proteins.
In summary, the structure of telomeres, coupled with the highly regulated activity and recruitment of telomerase, ensures proper telomere maintenance in normal cells. At the same time, each of these features is vulnerable to mutations and dysregulation, leading to familial and sporadic diseases. Nevertheless, several questions remain regarding how the telomerase complex senses and is recruited to the shortest telomeres and the specific order in which the different components are assembled. Uncertainty also surrounds how these processes may be differentially regulated between normal and evolving neoplastic cells. Most importantly, a deeper understanding of the regulation of telomerase expression and function is needed to define the contributions of telomerase to normal aging as well as inherited and somatic degenerative disease pathogenesis in humans.
CROSSTALK BETWEEN DYSFUNCTIONAL TELOMERES AND THE HALLMARKS OF CELLULAR AGING
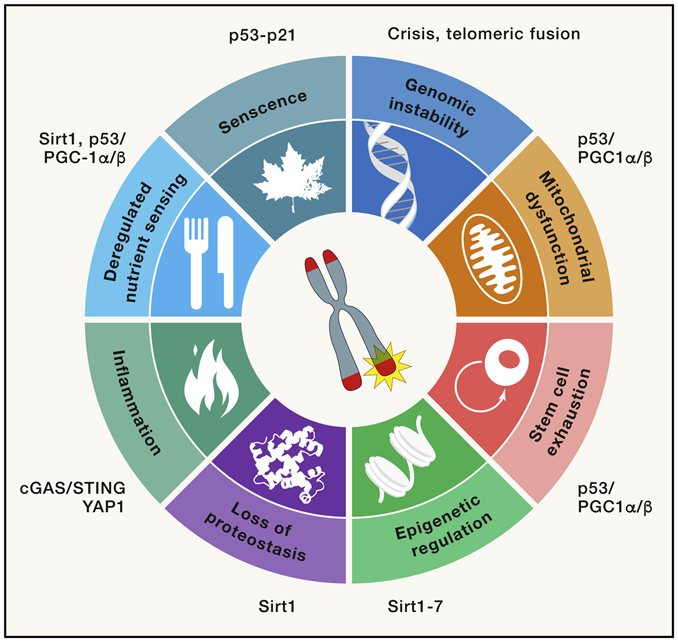
Limiting telomere reserves serve as a roadblock to cellular immortalization, but the loss of telomere function coincides with both age-related decline in fitness and cancer-inducing genome instability. Indeed, telomere dysfunction has been described as one of the nine cellular and molecular hallmarks of aging (Lopez-Otin et al., 2013). In this section, we detail the role of telomeres in processes of aging with an emphasis of how telomeres interrelate with the other hallmarks of aging, serving to drive or amplify these mechanisms (Figure 3). Additionally, we summarize the role of genetic model systems in revealing the interconnectedness of telomeres with other mechanisms and pathways driving aging as well as premature aging syndromes. The following subsections outline how telomere dysfunction links to the mechanisms underlying each hallmark of aging.
Figure 3. Relevance of telomere dysfunction to cellular aging hallmarks.
Telomere dysfunction can drive the hallmarks of cellular aging.
Cellular senescence.
Senescent cells accumulate in aging tissues and contribute to aging and age-related diseases via various mechanisms. The following are the two most well-studied ones: the first involves impeding the replicative potential of tissue stem cells (another hallmark of aging: stem cell exhaustion) immune cells (so-called immunosenescence) and stromal cells; the second stems from disrupting organ function through release of pro-inflammatory factors including but not limited to interleukin 6 (IL-6) and tumor necrosis factor alpha (TNFα) (Coppe et al., 2010) by senescent cells. With respect to the latter, the Hayflick limit triggers senescence and proliferative arrest via activation of the p53-p19ARF and p16Ink4a-Rb signaling pathways (Shay et al., 1991). Whether and how telomere dysfunction activates the senescence program in low-proliferative stroma tissues during normal aging requires further investigation, although it is tempting to speculate that reactive oxygen species (ROS)-induced damage of telomeres leads to TIF generation and induction of senescence.
Stem cell exhaustion.
Tissue stem cell exhaustion is a cardinal feature of aging. In late generation TERT- or TERC-null mice, progressive telomere erosion leads to p53-dependent apoptotic elimination of tissue stem cells, which contributes to organ atrophy, particularly in highly proliferative tissues with high rates of self-renewal including the skin, intestine, testis, regenerating injured liver, and blood (Blasco et al., 1997; Chin et al., 1999; Colla et al., 2015; Jaskelioff et al., 2011; Lee et al., 1998; Rudolph et al., 1999; Rudolph et al., 2000; Sahin et al., 2011). Beyond telomere maintenance, TERT may also impact stem cell biology via non-classical functions of TERT involving activation of the WNT pathway, a major regulator of stem cell homeostasis. Specifically, in mouse cells, TERT can physically interact with BRG1 and serve as a co-factor in the β-catenin complex, resulting in upregulation of WNT network genes (Park et al., 2009).
Genomic instability.
Genomic instability, another hallmark of aging, can lead to stem cell exhaustion and provoke inflammation. Telomere dysfunction can fuel chromosomal instability, as evidenced by cytogenetic analysis of late-generation TERC-null cells and tissues; these analyses showed amplifications, deletions, translocations, and anaphase bridge formation in tumors including colorectal cancers (detailed below) (Artandi et al., 2000; Blasco et al., 1997; O'Hagan et al., 2002; Rudolph et al., 1999; Rudolph et al., 2001). Mechanistically, eroded or uncapped telomeres undergo end-to-end fusion, forming dicentric chromosomes and resulting in breakage-fusion-bridge (BFB) cycles, aneuploidy and tetraploidization, translocations, amplifications, which create genomic instability through kataegis (localized hypermutations) and chromothripsis (clustered chromosomal rearrangements) during mitosis (Maciejowski and de Lange, 2017). Moreover, the loss of p53 enables cells to survive these DNA double-strand breakage events to produce aberrant chromosomal imbalances and nonreciprocal translocations that drive cancer initiation (see “TELOMERES AND TELOMERASE IN AGE-RELATED DISEASES AND CANCER”). These chromosomal abnormalities have been documented in nonmalignant aged stem cell compartments where the mutational burden strongly correlates with increasing age in human tissues, including colonic crypts and the hematopoietic system (Calado et al., 2012; Hsieh et al., 2013).
Mitochondrial dysfunction.
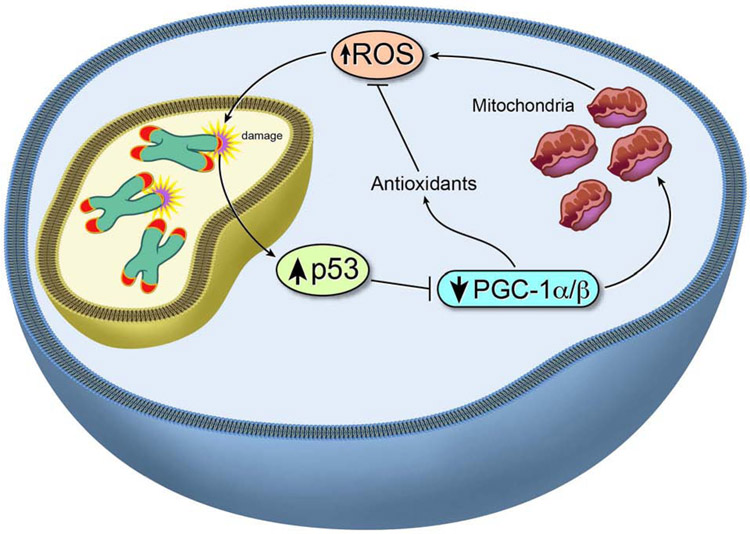
Telomeres and mitochondria are intimately linked (Sahin et al., 2011). In aging, mitochondrial function declines, leading to diminished energy (ATP) production as well as increased intracellular ROS. Diminished energy production causes overall frailty, while increased ROS causes cellular damage, including formation of 8-oxoguanine base lesions in DNA (note, telomeres are guanine-rich). Direct evidence of a role for mitochondrial decline in driving processes of aging derives from mice harboring mutations in the mitochondrial polymerase POLG. These mutant mice show reduced mitochondrial abundance and abnormal mitochondrial morphology (e.g., fragmented cristae, disrupted external membrane) (Trifunovic et al., 2004) and exhibit premature aging (e.g., alopecia, kyphosis, reduced body weight, reduced subcutaneous fat, reduced bone mineral density consistent with osteoporosis, anemia, and cardiomyopathy). Notably, the premature aging phenotypes associated with mitochondrial dysfunction mirror those of telomerase-deficient mice, p53-hyperactivated mice (Tyner et al., 2002), and PGC1α/β-null mice (Lai et al., 2008). Given the centrality of mitochondria in aging, the overlapping phenotypes of TERC-, PGC1α/β-, and POLG-null mice enabled us to link telomeres, mitochondria, and oxidative defense mechanisms in driving aging. Indeed, TERC-null mice were documented (Sahin et al., 2011) to have impaired mitochondrial function and diminished oxidative defense. Furthermore, transcriptomic analysis of diverse tissues in the late-generation TERC−/− mouse revealed prominent representation of p53 and PGC1α/β target genes, enabling us to identify a common pathway integrating three competing theories of aging—accumulating genotoxic stress, declining mitochondrial function, and increasing oxidative damage. Specifically, telomere dysfunction activates p53, which in turn represses PGC1α and PGC1β expression (Sahin et al., 2011) (Figure 4). Diminished PGC1α/β expression, in turn, results in decreased mitochondrial biogenesis and function and reduces expression of genes governing oxidative defense. This signaling circuit of telomere→p53→PGC1α/β→mitochondria leads to escalating ROS levels and further ROS-mediated 8-hydroxydeoxyguanosine modification of guanosine bases at telomeres (Figure 4). This axis creates a feed-forward loop linking telomere dysfunction, mitochondria, and oxidative stress pathways, resulting in accelerated aging.
Figure 4. Telomere dysfunction drives mitochondrial defects.
Telomere dysfunction compromises mitochondrial function and oxidative defense, increasing ROS and creating a feed-forward loop involving p53/PGC-1α/β signaling.
Epigenetic dysregulation.
Age-dependent changes in the epigenetic landscape include increased local methylation and decreased global methylation, increased H4K16 acetylation, H3K4 trimethylation, and H4K20 trimethylation, and decreased H3K9 monomethylation and H3K27 trimethylation (Barnes et al., 2019). Direct telomere and epigenetic interaction is reflected in the regulation of sirtuins, a family of seven (SIRT1-7) NAD+ dependent deacetylases regulating life- and health-span (Amano and Sahin, 2019). In mammals, SIRT1 deacetylates several aging-relevant transcription factors including p53, FOXO, PGC1α, and NF-κB, which regulate crucial molecular pathways related to stress resistance, metabolism, and oxidative defense (Houtkooper et al., 2012) The interplay between telomere dysfunction and SIRT (both SIRT1 and SIRT6) is evidenced by telomere dysfunction-induced p53-mediated repression of all seven sirtuins (Amano et al., 2019). Moreover, telomere dysfunction-mediated p53 activation represses expression of PGC1α/β, which in turn regulates expression of mitochondrial sirtuins 3, 4, and 5. In addition, p53 activation results in the transcriptional upregulation of microRNAs (miR-34a-5p, miR-26a-5p, and miR-145-5p) that suppress translation of non-mitochondrial sirtuins 1, 2, 6, and 7 (Amano et al., 2019). Thus, telomere-regulated epigenetic networks are relevant to aging regulators.
Loss of proteostasis.
Telomere dysfunction and resultant p53-mediated repression of SIRT1 expression is also relevant to the aging hallmark of altered proteostasis. Loss of proteostasis stems from a decline in the activity of the chaperone network that is responsible for proper protein-folding capacity. Mutant mice deficient in the carboxy terminus of HSP70-interacting protein (CHIP), a co-chaperone of the heat-shock family, exhibit accelerated aging phenotypes. In mammalian cells, SIRT1-mediated deacetylation of HSF-1 potentiates transcriptional activation of heat-shock genes such as HSP70. Thus, it is plausible that telomere dysfunction-induced repression of SIRT1 and resultant decreased HSP70 levels impair protein homeostasis and heat shock responses during stress (Westerheide et al., 2009). Protein folding is essential to maintaining neuronal homeostasis; post-mitotic long-lived neurons are extremely vulnerable to misfolded proteins, as they are unable to reduce misfolded protein load through cell division (Muchowski and Wacker, 2005). Deregulated protein folding occurs in several neurological diseases of aging, such as Alzheimer’s disease and Parkinson’s disease, which exhibit misfolded β-amyloid peptide and α-synuclein, respectively. The accumulation leads to gradual neuronal death and cognitive impairment. Recent studies have shown that overexpression of HSP70 is neuroprotective in Drosophila and in mouse and rat models of both Alzheimer’s disease and Parkinson’s disease (Auluck et al., 2002; Kakimura et al., 2002; Klucken et al., 2004).
Altered nutrient sensing.
Deregulated nutrient sensing in aging relates to a highly conserved network consisting of IGF-1 and mTOR-S6 signaling pathways and members of the FOXO and AMPK families of proteins. These pathways reciprocally regulate each other to maintain metabolic homeostasis. AMPK is a central sensor of nutrients that modulates mTOR signaling and transcriptionally activates FOXO transcription factors and SIRT1 (Saxton and Sabatini, 2017). SIRT1 can in turn activate FOXO and PGC1α, which are essential for mitochondrial biogenesis (Brunet et al., 2004; Rodgers et al., 2005). Thus, the capacity of telomere dysfunction to impact metabolism stems from its activation of p53 and repression of PGC1α and SIRT1. Indeed, telomere-dysfunctional mice fail to maintain plasma glucose levels under fasting conditions due to defects in gluconeogenesis regulated by p53-mediated repression of PGC1α/β and its downstream effectors GLC-6-P and PEPCK (Sahin et al., 2011). Enforced expression of mTERT or PGC1α or genetic ablation of p53 elevates expression of PGC1α/β, GLC-6-P and PEPCK and restores gluconeogenesis (Sahin et al., 2011). Telomere dysfunction-induced mitochondrial impairment increases the dependency of tissues on glucose metabolism (Missios et al., 2014). Increases in glycolysis versus mitochondrial oxidative metabolism may also lead to changes in NAD/NADH pools, further impairing sirtuin activity. Additionally, DNA damage signaling and PARP activation, as a consequence of telomere dysfunction, reduce NAD pools and impair sirtuin function. PARP and sirtuins compete for the common pool of NAD+, such that inhibition of PARP or genetic ablation leads to increased sirtuin function in brown adipose tissue and muscle, as observed in PARP1 knockout mice (Bai et al., 2011).
Inflammation.
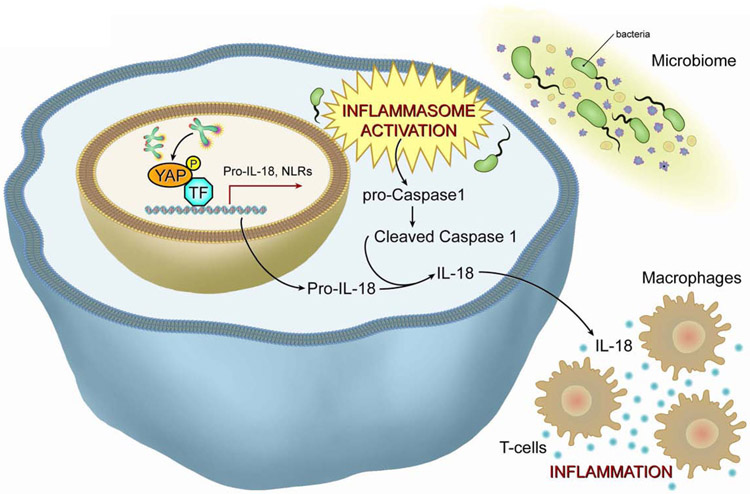
Aging processes encompass inflammatory signaling from genomically damaged or senescent cells. Telomere dysfunction can initiate and maintain inflammation on several levels. First, telomere dysfunction provokes cellular senescence, which stimulates production and secretion of interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNFα), among other inflammatory factors (Coppe et al., 2010). Second, telomere dysfunction results in the production of extrachromosomal fragments that can precipitate autophagic cell death viaactivation of the cGAS/STING pathway (Nassour et al., 2019). In the context of cancer specifically, a recent study demonstrated, targeting telomeres with a new drug named 6-thio-2′-deoxyguanosine (6-thio-dG) induces generation of TIFs and release of extrachromosomal DNA into the tumor microenvironment of the cancer cells. The uptake of this DNA by dendritic cells triggers the intracellular cGAS/STING pathway and initiates an inflammatory cascade via IRF3-TBK1–mediated upregulation of Type I interferons leading to enhanced cross-priming activity and recruitment of IFNγ producing CD8+ T cells (Mender et al., 2020). Recent studies have forged a direct link between telomere dysfunction and tissue inflammation (Figure 5) (Chakravarti D., 2020). Specifically, telomere dysfunction activates ATM/cABL-induced phosphorylation of YAP1, resulting in its nuclear translocation. Nuclear YAP1 upregulates inflammation and inflammasome genes such as pro-IL18, NLRC5, NLRP1b, and NLRP6. Together with gut microbiome activation of the inflammasome axis, the resultant activated caspase-1 cleaves pro-IL-18 into its mature IL-18 form, whose secretion recruits and activates T cells and initiates inflammation. The treatment of telomere-dysfunctional mice with a YAP1 inhibitor, caspase-1 inhibitor, or antibiotics in vivo, or the reactivation of telomerase in the intestinal compartment, leads to a reduction in the cleavage of procaspase-1 to caspase-1 and in the production of mature IL-18, consequently reducing tissue inflammation. This study (Chakravarti D., 2020) established a direct link between telomere dysfunction and inflammation that can partially explain the age-related increase in inflammatory response seen in the aged population. This work also highlights the cooperation between cell-intrinsic telomere dysfunction–driven molecular pathways and the microbiome in driving the inflammatory response.
Figure 5. Telomere dysfunction drives tissue inflammation.
Telomere dysfunction drives tissue inflammation through activation of the ATM/cABL/YAP1 axis and driving secretion of mature IL18 to recruit and potentiate T cells and macrophages.
ATM, Ataxia telangiectasia mutated; cABL, Abelson murine leukemia viral oncogene homolog 1; YAP1, Yes-associated protein1; IL 18, interleukin 18.
In summary, numerous studies link telomere dynamics to pathways and biological processes underlying all hallmarks of aging. Moreover, many of these stress responses create feedback loops that further damage telomeres, amplifying and accelerating degenerative aging phenotypes. This interconnectedness of telomeres virtually all the hallmarks of aging serves to both initiate and escalate the aging process.
TELOMERES AND TELOMERASE IN AGE-RELATED DISEASES AND CANCER
How telomeres contribute to aging and age-related diseases came to light through the study of mice and individuals harboring germline alterations in telomere maintenance genes. Murine and human telomeres show differences in length and regulation. The laboratory mouse, Mus musculus, possesses telomeres up to ten times longer than those of humans (30-150 kb in mice versus 10-15 kb in humans) (Kipling and Cooke, 1990 ). Moreover, murine somatic cells exhibit high levels of telomerase activity relative to the low activity found in human somatic cells (Kipling, 1997; Wright et al., 1996). These species differences enabled elucidation of the function of telomeres and telomerase in genome stability, aging, and cancer through the engineering and characterization of mice with shorter, human-like telomere lengths. Specifically, in mice null for TERT or TERC, successive intergenerational crosses progressively shorten telomeres, culminating in telomere dysfunction (end-to-end fusions) by generation 3 (G3) (Artandi et al., 2000; Blasco et al., 1997). This telomere dysfunction coincides with widespread tissue stem cell depletion, progressive tissue atrophy, germ cell depletion, reduced fecundity, impaired adaptive immunity, decreased memory, delayed wound healing, diminished stress responses, increased hair graying and alopecia, diminished cardiac function, weakened skeletal frame, increased cancer incidence, and overall frailty (Allsopp et al., 2003; Artandi et al., 2000; Chin et al., 1999; Colla et al., 2015; Jaskelioff et al., 2011; Leri et al., 2003; Pignolo et al., 2008; Rudolph et al., 1999; Sahin et al., 2011).
This section summarizes mounting evidence linking telomere functionality to aging processes such as frailty as well as age-related diseases including cardiovascular diseases (atherosclerosis, vascular dementia, coronary artery disease), metabolic disease (type II diabetes), neurological disease (Parkinson’s disease) and cancer.
Telomere dysfunction and telomerase in age-related diseases.
Numerous studies have implicated telomere dysfunction in age-related diseases. First, in high-proliferative tissues such as the skin, gastrointestinal tract, and hematopoietic system, low levels of telomerase in progenitor cell compartments and continual tissue renewal cause progressive telomere attrition over decades. This attrition ultimately triggers DNA damage responses such as cell cycle arrest, apoptosis, impaired differentiation, and/or senescence. Second, low-proliferative tissues such as heart, brain, and liver could experience ROS-induced damage of telomere sequences, causing attrition and uncapping over time. Specifically, murine and human studies show that oxidative stress itself accelerates telomere attrition in vascular endothelium (Gonzalez-Guardia et al., 2014), skeletal muscle (Ludlow et al., 2014), cardiomyocytes (Puente et al., 2014), and innate and adaptive immune cells (Vaziri et al., 1993) (Kaneko et al., 1996). Additionally, while telomere shortening per se generates telomere dysfunction-induced foci (TIFs), ROS-induced guanine modification also produces TIFs, reflecting uncapping from shelterin disengagement (Anderson et al., 2019). This is evident from pioneering studies from the de Lange laboratory demonstrating that perturbations in shelterin components, such as dominant-negative mutant forms of TRF2, can induce TIFs without changes in telomere length (van Steensel et al., 1998).
The entwined processes of senescence and inflammation may be particularly relevant to the telomere-aging connection. These pleiotropic actions can drive atherosclerosis, type II diabetes, osteoarthritis, and Parkinson’s and Alzheimer’s diseases. Specifically, a recent study highlighted the role of senescent cells in driving Alzheimer’s disease (Bussian et al., 2018). In this study, mice engineered with mutant Tau (MAPTP301SPS19) that develop neurofibrillary tangles and Alzheimer’s-like symptoms show preservation of cognitive function upon genetic or pharmacological removal of p16INK4a-expressing senescent astrocytes and microglia (Bussian et al., 2018). The significance of this study lies in the finding that accumulation of senescent cells precedes formation of the neurofibrillary tangles, suggesting that senescent cells may influence initiation of tangle formation. Similarly, removal of senescent cells from the BubR1 progeroid mouse model leads to extended health-span (Baker et al., 2011). These studies catalyzed development of ‘senolytic’ agents capable of clearing senescent cells in humans (Ellison-Hughes, 2020). The senescence-inflammation axis may also be active in patients with end-stage heart failure, cardiac hypertrophy, and coronary artery disease (Birks et al., 2008).
Telomere dysfunction in inflammatory diseases.
That telomere dysfunction can drive inflammatory diseases in human aging is evident from the study of telomeropathy. Telomeropathy results from germline defects of telomere maintenance genes including TERT, TERC, DKC, PARN, RTEL1, TINF2, and POT1 as reviewed elsewhere (Opresko and Shay, 2017). On the cellular level, telomeropathy is characterized by (i) hematopoietic stem cell depletion leading to bone marrow failure, (ii) immunosenescence of lymphocytes and (iii) intestinal stem cell loss resulting in intestinal villous atrophy associated with crypt cell apoptosis, villous blunting, basal plasmacytosis, and intraepithelial lymphocytosis (Jonassaint et al., 2013). On the tissue level, telomeropathy shows increased incidence of idiopathic pulmonary fibrosis, liver cirrhosis, and kidney diseases (Armanios and Blackburn, 2012), all of which are relate to increased inflammation. Given that high ROS levels are associated with telomere shortening and are postulated as drivers of tissue inflammation, even in the absence of genetic mutations in telomere-maintenance genes, telomere shortening and damage could be instrumental in driving various inflammatory diseases in older individuals without classical germline mutations affecting telomeres (Wiemann et al., 2002) . These conditions could stem from dysfunctional telomeres in a few cells of an affected tissue leading to a local increase in inflammatory cytokines and driving further tissue damage and telomere shortening. Such inflammatory diseases include inflammatory bowel disease (Risques et al., 2008; Risques et al., 2011), pancreatitis (van Heek et al., 2002), non-alcoholic fatty liver disease (Laish et al., 2016), chronic obstructive pulmonary disease (Birch et al., 2016), and liver cirrhosis as a consequence of chronic liver disease (Wiemann et al., 2002) among others. In such conditions, telomere dysfunction may serve to amplify and cooperate with the primary drivers of disease.
Mitochondrial dysfunction and associated increased ROS are linked to aging, and specifically to senescence and inflammation. The maintenance of mitochondrial activity has been a prime therapeutic objective as murine studies have shown beneficial effects of PGC1α overexpression on various aging-relevant biological processes including metabolism and ROS control(Dillon et al., 2012). Uncontrolled ROS are also known to impact gluconeogenesis, fatty acid metabolism, and β-oxidation, which can drive age-related metabolic diseases including diabetes, cancer (see “Telomere dysfunction, telomerase and cancer”), inflammatory conditions, sarcopenia, and neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and amyotrophic lateral sclerosis, as well as overall frailty.
Telomere dysfunction, telomerase and cancer.
Cancer is a disease of aging, with 1 in 2 men and 1 in 3 women in the US receiving a cancer diagnosis by age 80 (National Cancer Institute). Moreover, epithelial cancers are the predominant cancer types of the aged; these cancers are typically aneuploid and possess many chromosome structural alterations. Curiously, as mice age, the cancer spectrum consists primarily of hematopoietic (lymphoma) and mesenchymal (sarcoma) cancers, and few epithelial cancers (Brayton et al., 2012). Also, these murine malignancies exhibit minimal chromosome structural aberrations. These cross-species differences enabled elucidation of a key mechanism driving the preponderance of epithelial cancers in aged humans, as well as the basis of genomic instability in these cancers. Specifically, by engineering telomerase-deficient mice possessing shorter human-like telomeres, we demonstrated that (Artandi et al., 2000) telomere dysfunction, in the setting of p53 deficiency, enables cells in crisis to survive chromosomal breakage events fueling amplification, deletion, and translocation of cancer-relevant genes (Figure 5). Strikingly, these TERC/p53-null mice not only show increased cancer initiation but also a humanized tumor spectrum possessing complex karyotypes. These mouse genetic experiments, coupled with subsequent genomic evidence of telomere dysfunction in early-stage human cancers (Ding et al., 2012; Fernandes et al., 2020; Rudolph et al., 2001), established that telomere dysfunction is a major mechanism driving epithelial carcinogenesis in the aged. Stated differently, the absence of this mutational mechanism in the mouse (long telomeres and promiscuous telomerase expression) protects mice from developing epithelial cancers.
These murine models revealed the complex role of telomeres in the evolution of cancer, specifically, how telomere dysfunction enhances tumor initiation while restricting full malignant progression (Chin et al., 1999; Rudolph et al., 2001). For example, in the APCmin TERC-null mouse model, telomere dysfunction initially increases adenoma frequency yet ultimately constrains progression to macroadenomas and increases survival (Rudolph et al., 2001). This constrained malignant progression relates prominently to the activation of p53 in aspiring cancer cells experiencing telomere dysfunction. Inactivation of the p53 tumor suppressor is one of the most frequent events in human epithelial cancers (Brosh and Rotter, 2009) and the status of p53 can dictate whether telomere dysfunction enhances or suppresses cancer development. Specifically, late-generation TERC−/− mice null for p53 show an increase in cancer incidence and decreased survival (Artandi et al., 2000; Chin et al., 1999), whereas late-generation TERC−/− mice null for Ink4a/Arf show a significant reduction in tumor incidence and increased survival (Greenberg et al., 1999). Notably, even though Ink4a/Arf-null mice lack p19ARF, which signals p53 during oncogenic activation, a functional p53-dependent DNA damage response is retained in these mice (e.g., apoptosis) (Kamijo et al., 1997). Thus, the role of telomeres in tumor initiation depends on the integrity of p53-mediated DNA damage signaling and associated cellular checkpoint responses.
Recent single-cell DNA sequencing of human cancers has uncovered that many cancers evolve as a consequence of a single punctuated genomic event (Gao et al., 2016; Navin et al., 2011) where bursts of copy number alterations and mutations dominate all clones. While the mechanisms driving this punctuated evolution or ‘episodic instability’ are under active investigation, mouse and human evidence clearly implicates telomere-based crisis and subsequent telomerase reactivation as a mechanism shaping cancer genomes and driving epithelial carcinogenesis in the aged. While this episodic instability model is consistent with human genomic data (Sottoriva et al., 2015) on evolving cancers, we acknowledge that the “Vogelstein mechanism” (Fearon and Vogelstein, 1990)—the stepwise accumulation of driver mutations—may also be at work here, and that the two models are not mutually exclusive
Additional evidence supporting a telomere-cancer link includes age-dependent telomere shortening in colonic mucosa of aged individuals and shorter telomeres in cancers than in adjacent nonmalignant tissues (Hastie et al., 1990). Additionally, human tumor tissues show elevated TERT and TERC expression compared to matched normal tissues (Meyerson et al., 1997), and malignant transformation of cultured human primary cells requires enforced TERT expression, indicating that telomere maintenance is essential for full malignant transformation (Hahn et al., 1999). Finally, cancer-prone mice engineered with an inducible TERT allele first experience telomere-based crisis followed by subsequent telomerase reactivation. This crisis-reactivation sequence generates more aggressive tumors, establishing that crisis enables acquisition of genomic events that endow a more aggressive malignant phenotype (Ding et al., 2012; Hu et al., 2012). Similarly, transient induction of telomere uncapping in telomerase wildtype mice (by mutant TRF2 protein and disruption of the shelterin complex) increases initiation and progression of hepatocellular carcinoma in carcinogen-treated mice (Begus-Nahrmann et al., 2012). In contrast, telomere shortening in TERC-knockout mice increases tumor initiation but fails to induce tumor progression in a model of carcinogen-induced hepatocellular carcinoma (Farazi et al., 2003). Thus, multiple lines of evidence substantiate the role of telomere-based crisis and telomerase reactivation in cancer initiation and progression, respectively.
Telomere- and telomerase-dependent mechanisms of carcinogenesis.
As noted, loss of telomere function leads to end-to-end fusion. During mitosis, these dicentric chromosomes generate anaphase bridges with subsequent chromosome breakage leading to the BFB cycle first proposed by Barbara McClintock (Creighton and McClintock, 1931). The surviving BFB daughter cells accumulate cancer-relevant chromosomal alterations during crisis and ultimately activate telomerase to dampen DNA damage signaling and quell rampant chromosomal instability, enabling full malignant progression. A TERT-inducible model of prostate cancer has experimentally validated this cancer genome evolution concept. In this cancer model (Ding et al., 2012), critically short telomeres result in increased cancer cell apoptosis and decreased proliferation, producing smaller and less aggressive tumors compared to telomere-proficient controls. In these chromosomally unstable high-grade prostate intraepithelial neoplasias, experimental reactivation of telomerase results in malignant progression including the acquisition of new phenotypes such as bone metastasis. In contrast, telomere intact controls (which did not experience crisis followed by telomerase reactivation) exhibited only local invasion (Ding et al., 2012). Moreover, genomic analysis of the tumors experiencing crisis showed additional pro-metastasis mutations (e.g., SMAD4 deletion and additional perturbations to the TGFβ pathway). Thus, telomere-based crisis drives chromosomal instability to initiate cancer, and subsequent telomerase reactivation enables full malignant progression.
Large-scale sequencing studies show that TERT promoter mutations are the most frequent noncoding mutations in human cancer (Fredriksson et al., 2014; Icgc Tcga Pan-Cancer Analysis of Whole Genomes Consortium, 2020; Rheinbay et al., 2020); such mutations are highly enriched in cancers originating in tissues with relatively low rates of self-renewal (Killela et al., 2013). For example, melanoma shows highly recurrent TERT promoter mutations, with an average 4-fold increase in TERT expression (Horn et al., 2013), and primary glioblastoma shows TERT promoter mutations in over 80% of cases (Killela et al., 2013). The most common recurrent single-nucleotide mutations in the TERT promoter occur at G228A and G250A, which generate de novo ETS consensus binding motifs to recruit GAPB, and potentially other ETS transcription factors, to elevate TERT expression (Bell et al., 2015). Additional mechanisms driving increased TERT expression in cancer also result from crisis-induced chromosomal alterations (Artandi et al., 2000), which produce focal amplifications of the TERT locus in hepatocellular carcinoma (Totoki et al., 2014). Finally, gene therapy studies have shown that CRISPR-mediated correction of the TERT promoter to wildtype sequences significantly prolongs the survival of glioma-bearing mice (Li et al., 2020).
In addition to the genomic mechanisms driving TERT upregulation, oncogenic signaling pathways can enhance TERT gene transcription. c-MYC can bind Myc binding elements in the TERT promoter to induce TERT transcription and increase telomerase activity in primary human fibroblasts, although enforced TERT expression alone was an insufficient substitute for c-MYC in promoting transformation (Greenberg et al., 1999; Wang et al., 1998). Similarly, activated WNT signaling cooperates with KLF4 to activate TERT transcription (Hoffmeyer et al., 2012). While key developmental and cancer-related pathways can activate TERT, there is also evidence that TERT can in turn activate these pathways in cis. Activation of telomerase in TERT-overexpression mouse models showed proliferation of quiescent hair follicle stem cells and kidney podocytes can occur independent of telomere length, TERC function or TERT reverse transcriptase function (Flores et al., 2005; Sarin et al., 2005). Mechanistic studies revealed that TERT directly binds TCF elements and enhances MYC and WNT transcriptional programs (Choi et al., 2008; Park et al., 2009)—pathways known to govern stem cell homeostasis and drive cancer (Dang, 2012; Reya and Clevers, 2005). In addition, TERT interacts with the NF-κB complex and binds IL-6 and TNFα promoter elements, increasing cell proliferation and resistance to cell death (Ghosh et al., 2012). The increased cell proliferation and resistance to cell death upon ectopic TERT and TERC expression are attenuated through repression of p65. Functionally, G1 TERC-null mice were more resistant to endotoxic shock than their littermate controls, with more than 50% of mice surviving lipopolysaccharide-induced shock compared to 25% of controls, which suggests that telomerase regulates the NF-κB inflammatory response independently of telomere length. Together, these studies point to the interactions of oncogenic signaling molecules and TERT in the regulation of cancer-relevant circuits. Further investigation is needed to assess the role of these circuits in human cancer.
Mutations in and/or altered expression of shelterin proteins have been detected in cancer, although their precise roles in cancer initiation and progression are not yet determined. In early-stage chronic lymphocytic leukemia, telomere dysfunction is linked to disease initiation and end-to-end fusions (Chang, 2013). Accordingly, in this disease, there is reduced expression of shelterin proteins including TRF1, RAP1, and POT1as well as TIN2 and TPP1. POT1 is the most mutated shelterin-related protein in human cancers, with somatic mutations present in 5% of chronic lymphocytic leukemia cases. Somatic mutations in POT1 or RAP1 can occur in familial glioma, familial melanoma, Li-Fraumeni–like syndrome, parathyroid adenoma, cardiac angiosarcomas, mantle cell lymphoma, and hepatocellular carcinoma (Fernandes et al., 2020). Both TRF1 and TRF2 are upregulated in various tumor types, including cancers of the lung, breast, colon, stomach, and kidney – suggestive of a supportive role for shelterin proteins in human cancer.
Alternative mechanisms of telomere maintenance in cancer.
While telomerase is the most common telomere maintenance mechanism in cancer, cancer cells also utilize a telomerase-independent recombination-mediated mechanism, termed alternative lengthening of telomeres (ALT) (Henson et al., 2002). ALT occurs in 5% to 15% of human cancers, particularly in osteosarcoma and glioblastoma, and is typically associated with poor prognosis (Heaphy et al., 2011). ALT lengthens telomeres using DNA homology–directed repair mechanisms in which telomeric DNA templates are copied from sister chromatids or non-homologous chromosomes. Essential factors for ALT include MRE11, RAD50, NBS1, FEN1, MUS81, and FANCD2 (Cesare and Reddel, 2010). Interestingly, ALT-positive cells are commonly defective in their ability to sense cytosolic DNA, and studies have demonstrated that extrachromosomal telomere repeats (ECTR) elicit an interferon response through the cGAS-STING cytosolic DNA–sensing pathway (Chen et al., 2017). Thus, cancer cells employing ALT have additional anti-proliferative barriers to overcome, such as senescence induction and innate immune surveillance due to the constant production of ECTRs. Interestingly, although ALT is an important factor that drives tumorigenesis in the absence of telomerase reactivation, it is less efficient in driving aggressive malignancy and metastasis. Evidence derives from a study where mouse mTerc−/− Ink4a/Arf−/− fibroblasts which were passaged extensively and showed development of ALT. While ALT-positive cells maintained telomeres, they were unable to form lung metastases; however, upon mTERC transduction, telomerase-positive cancer cells acquired metastatic potential, establishing that ALT-positive and telomerase-positive cancers are not biologically equivalent (Chang et al., 2003).
The ALT pathway can also serve as a resistance mechanism for telomerase inhibitor therapies. In a lymphoma-prone ATM-mutant mouse model engineered with a tamoxifen-controlled TERT allele (TERT-ER) (Hu et al., 2012), extinction of telomerase activity resulted in re-entry of lymphoma cells into telomere-based crisis. While telomerase extinction cured two-thirds of mice, the remaining mice developed recurrent tumors possessing hallmark features of ALT (Hu et al., 2012). Interestingly, these recurrent tumors acquired amplifications and deletions in genes involved in homologous recombination that are known to play essential roles in ALT, as well as amplification of the PGc1α locus, which may signify ongoing mitochondrial stress in ALT-positive cells (Hu et al., 2012). These mechanistic insights inform potential regimens combining telomerase inhibitors with agents targeting homologous recombination or mitochondrial mechanisms. With respect to the latter, ALT-positive cancer cells, which show diminished mitochondrial function and associated high intracellular ROS, are particularly susceptible to agents that target antioxidant proteins.
OUTLOOK FOR TELOMERASE THERAPEUTICS AND FUTURE PERSPECTIVES
The link between telomere dysfunction and the hallmarks of aging, the incidence of age-associated diseases, and the development of inherited and acquired degenerative conditions has catalyzed interest in telomerase restoration therapy as a potential antiaging strategy (Figure 6). The optimal application of such therapy would likely be in the form of transient telomerase induction to enable restoration of telomere reserves and healing, while avoiding the potential for fueling cancer that might come from constitutive telomerase activation. Indeed, in preclinical mouse models with long telomeres, constitutive TERT expression increased lifespan by 40%, although these mice also harbored extra copies of p53, p16, and ARF (Tomas-Loba et al., 2008), which enhanced their cancer-resistance. It remains to be determined whether the increased lifespan and disease-modifying activities of enforced TERT expression relate to TERT’s actions on telomeres or to its activation of WNT, which may enhance stem cell reserves.
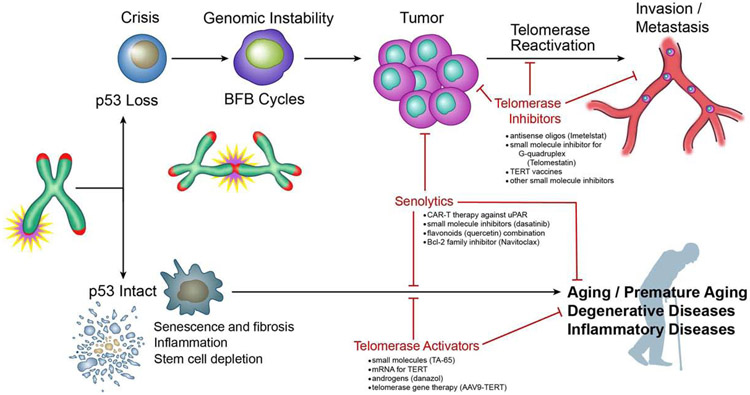
Figure 6. Telomere/telomerase in aging, cancer and potential therapy.
Telomere shortening leads to senescence, fibrosis, inflammation and stem cell depletion in the presence of a functional p53 checkpoint. These processes lead to aging and other degenerative and inflammatory diseases. Telomerase activators and senolytics can inhibit these processes and inhibit aging and age related diseases. Shortened telomeres also lead to telomeric fusion and cycles of break-fusion-bridges. In the absence of a p53 checkpoint, these events lead to tumorigenesis. Further activation of telomerase leads to the progression to invasion and metastasis. Telomerase inhibitors and senolytics inhibit processes that can thwart tumor progression, invasion and metastasis.
Advancing knowledge of the molecular networks regulated by telomerase and the rejuvenation of prematurely aged mice with telomerase activation (Jaskelioff et al., 2011) has fueled interest in the development of agents that activate TERT expression for antiaging therapy. Several small molecules have been identified that appear to activate TERT (Figure 6), including TA-65 (Harley et al., 2011) and histone deacetylase inhibitors (Won et al., 2004), although a clear understanding of their mechanisms of action is lacking. TA-65 (cyclastragenol) is a natural compound isolated from various plant species in the genus of Astragalus. Ongoing human trials of TA-65 have reported improved macular function (Dow and Harley, 2016), reduced high-density lipoprotein levels, and reduced levels of the inflammation markers Creactive protein and TNFα (Fernandez et al., 2018). In addition, hormonal agents such as danazol (an antiestrogenic and antiprogestogenic) and 5α-dihydrotestosterone (an androgen), which can increase telomerase levels, are being tested in patients with telomeropathies (Calado et al., 2009; Townsley et al., 2016). Finally, the recent development of agents targeting TERC stability may offer new therapeutic options for telomeropathies. Specifically, PAPD5 degrades TERC by 3′ oligoadenylation, priming these transcripts for destruction by the RNA exosome. As mentioned earlier, PARN, involved in the de-adenylation and maturation of TERC, is mutated in several diseases including dyskeratosis congenita. A PAPD5 small molecule inhibitor increased telomere length in induced pluripotent stem cells from dyskeratosis congenita patients and was well tolerated in mice over an extended period of time (Nagpal et al., 2020).
A particularly intriguing application of pulsatile telomerase activation therapy would be the treatment of progeroid diseases such as Werner and Bloom syndromes. This concept emerges from the astonishing lack of degenerative phenotypes in mice null for genes connected to inherited degenerative conditions in humans including (i) ataxia-telangiectasia, which results from mutational inactivation of ATM; (ii) Bloom syndrome, caused by a mutation in BLM of the RecQ helicase family, and (iii) Duchenne muscular dystrophy, which arises from mutation of dystrophin, DMD. Strikingly, when each of these alleles was crossed to the TERC-knockout mouse, the salient phenotypes of these syndromes surfaced as telomeres reached shorter, more human-like lengths (typically at G2-G3) (Chang et al., 2004; Du et al., 2004; Sacco et al., 2010; Wong et al., 2003). For example, WRN/TERC-null mice recapitulated the hallmark features of Werner syndrome including kyphosis, pathological long bone fractures, alopecia and hair graying, defects in stem c ell and immune compartments, senile cataracts, hematopoietic deficiencies such as pancytopenia and unbalanced response to immunogens, and metabolic deficiencies including insulin resistance (Chang et al., 2004).
As noted, telomere-intact mouse models with mutations in WRN or BLM genes do not exhibit any degenerative phenotypes, providing a rationale for telomerase activation therapy to delay or reduce the intensity of symptoms and extend life expectancy. Along similar lines, in patients with germline mutations affecting telomere maintenance (e.g., DKC), telomerase activation therapy could alleviate progressive symptoms such as anemia, pulmonary fibrosis, and gastrointestinal dysfunction. Another group of diseases with limited treatment options, in which telomerase activation may play a beneficial role, is chronic inflammatory diseases such as liver cirrhosis, pancreatitis, and ulcerative colitis (Figure 6). Telomere dysfunction at disease onset can drive tissue inflammation, which in turn can accelerate telomere shortening, creating a feed-forward loop that ultimately leads to disease recurrence and even cancer brought about by genomic instability, p53 loss, and telomerase reactivation. Here again, telomerase activation at very early stages of disease prior to entry into telomere-based crisis could prevent disease flares and carcinogenesis. Telomerase activation may also be useful in treating neurodegenerative diseases given the strong improvement in brain health in mice following genetic induction of telomerase (Ding et al., 2012; Jaskelioff et al., 2011). In addition to telomerase activation, it may also be possible to ameliorate telomere dysfunction–induced organ degeneration and mortality by impairing the function of checkpoints that mediate the tissue-disruptive effects of dysfunctional telomeres. Along these lines, it was shown that the selective inhibition of p21-dependent cell cycle arrest (Choudhury et al., 2007) or Puma-mediated apoptosis (Sperka et al., 2011) could prolong the maintenance of tissue integrity and lifespan in telomere-dysfunctional mice without increasing cancer development. Also, deletion of Exo1 increases tissue maintenance and lifespan in telomere-dysfunctional mice by inhibiting the formation of chromosomal fusions and “BFB “ cycles that drive the induction of DNA damage and the activation of p53-checkpoints in telomere-dysfunctional mice (Schaetzlein et al., 2007).
Contrary to the potential applications of telomerase activation in antiaging therapy, the increased telomerase activity observed in most cancers has led to the development of antitelomerase therapeutics. Several strategies to target TERT in cancer have been engineered, including antisense oligos, vaccines, and small molecule inhibitors (Ruden and Puri, 2013) (Figure 6), but no antitelomerase agents have reached randomized phase III trials. This limited efficacy may be attributable to the time required for telomeres to shorten to a length that can induce tumor shrinkage. In addition, alternative strategies to inhibit telomerase could generate a more meaningful impact in the clinic. To start, cancers with intact p53 would be more suitable for telomerase inhibition owing to their functional checkpoint machinery, which would trigger senescence. This strategy still requires caution, as preclinical animal studies have demonstrated that TERT inhibition can lead to activation of the ALT pathway in lymphoma (Hu et al., 2012). Thus, combined telomerase and ALT pathway–suppressing drugs (Flynn et al., 2015) could minimize emergence of resistance. In the subset of cancers that are ALT-positive, targeting relevant immune circuits may also enhance response rates and outcomes. That is, due to the persistent production of cytosolic DNA in ALT-positive cancer cells (Chen et al., 2017), it is tempting to speculate that these tumors may be more responsive to immunotherapy because cytosolic DNA upregulates interferon signaling via activation of cGAS-STING. In preclinical studies, the nucleoside analog 6-thio-dG, which incorporates into newly synthesized telomeres to cause telomeric DNA damage, can render cancers that are resistant to anti-PD-L1 treatment susceptible to immune checkpoint therapy (Mender et al., 2020). To summarize, antitelomerase therapy remains a viable anti-cancer strategy based on its potential role in the inhibition of advanced malignancies, although the genotypic and biological context will be important to determine along with the specific combination therapies for synergistic activity.
In closing, the pursuit of the fundamental knowledge of chromosome structure and cell biology has illuminated mechanisms central to many major human diseases and aging itself. The telomere field has been an exemplary model of basic science and multidisciplinary convergence, revealing the role of telomeres and telomerase in the hallmarks of aging and the pathogenesis of cancer. Many knowledge gaps remain, such as elucidating the mechanisms governing telomerase expression and activity, the non-canonical functions of TERT, and the interplay between telomere dysfunction and pathological processes such as inflammatory, fibrotic, and degenerative diseases. Regardless of whether telomere dysfunction initiates the disease or is only a participant, telomeres clearly play an integral pathogenetic role in human disease. Such an elemental role encourages the development and rigorous testing of telomerase activators for the treatment of aging and age-associated diseases as well as the assessment of efficient telomerase inhibitors for the treatment of advanced cancers. The hallmarks delineated here outline a framework to stimulate further study of the role of telomeres and telomerases, which may help confront the lethal disease ultimately suffered by all—aging.
Acknowledgements
We regret being unable to cite the work of many of our colleagues due to limitations in space. We are grateful to Steven E. Artandi, Karl-Lenhard Rudolph, Sandy Chang, and members of the DePinho lab for helpful suggestions. We would like to thank Denise J. Spring and Amy L. Ninetto for meticulously editing the document as well as Editing Services, Research Medical Library at MD Anderson Cancer Center. We would also like to acknowledge David Aten for his help with the illustrations and the Department of Medical Illustration at MD Anderson Cancer Center. D.C. was supported by the CPRIT Research Training Program (RP140106 & 170067) and by pilot funding through the DDC-NIDDK award P30CA16672. K.A.L. is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers TL1TR003169 and UL1TR003167. This work was also supported by NIH R01 CA084628 (to R.A.D.).
Footnotes
Declaration of Interests
R.A.D. is co-Founder, Director and Advisor of Tvardi Therapeutics; co-Founder and Advisor of Asylia Therapeutics; co-Founder and Advisor of Nirogy Therapeutics; and co-Founder and Advisor of Stellanova Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Allsopp RC, Morin GB, Horner JW, DePinho R, Harley CB, and Weissman IL (2003). Effect of TERT over-expression on the long-term transplantation capacity of hematopoietic stem cells. Nat Med 9, 369–371. [DOI] [PubMed] [Google Scholar]
- Amano H, Chaudhury A, Rodriguez-Aguayo C, Lu L, Akhanov V, Catic A, Popov YV, Verdin E, Johnson H, Stossi F, et al. (2019). Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell Metab 29, 1274–1290 e1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano H, and Sahin E (2019). Telomeres and sirtuins: at the end we meet again. Mol Cell Oncol 6, e1632613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D, et al. (2019). Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, and Blackburn EH (2012). The telomere syndromes. Nat Rev Genet 13, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, and DePinho RA (2000). Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645. [DOI] [PubMed] [Google Scholar]
- Artandi SE, and DePinho RA (2010). Telomeres and telomerase in cancer. Carcinogenesis 31, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, and Bonini NM (2002). Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295, 865–868. [DOI] [PubMed] [Google Scholar]
- Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, et al. (2011). PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, and van Deursen JM (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes RP, Fouquerel E, and Opresko PL (2019). The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev 177, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begus-Nahrmann Y, Hartmann D, Kraus J, Eshraghi P, Scheffold A, Grieb M, Rasche V, Schirmacher P, Lee HW, Kestler HA, et al. (2012). Transient telomere dysfunction induces chromosomal instability and promotes carcinogenesis. J Clin Invest 122, 2283–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RJ, Rube HT, Kreig A, Mancini A, Fouse SD, Nagarajan RP, Choi S, Hong C, He D, Pekmezci M et al. (2015). Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 348, 1036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch J, Victorelli S, Rahmatika D, Anderson RK, Jiwa K, Moisey E, Ward C, Fisher AJ, De Soyza A, and Passos JF (2016). Telomere Dysfunction and Senescence-associated Pathways in Bronchiectasis. Am J Respir Crit Care Med 193, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks EJ, Latif N, Enesa K, Folkvang T, Luong le A, Sarathchandra P, Khan M, Ovaa H, Terracciano CM, Barton PJ, et al. (2008). Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res 79, 472–480. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, and Gall JG (1978). A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol 120, 33–53. [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, and Greider CW (1997). Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, and Wright WE (1998). Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352. [DOI] [PubMed] [Google Scholar]
- Brayton CF, Treuting PM, and Ward JM (2012). Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol 49, 85–105. [DOI] [PubMed] [Google Scholar]
- Brosh R, and Rotter V (2009). When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 9, 701–713. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015. [DOI] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, and Baker DJ (2018). Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Cooper JN, Padilla-Nash HM, Sloand EM, Wu CO, Scheinberg P, Ried T, and Young NS (2012). Short telomeres result in chromosomal instability in hematopoietic cells and precede malignant evolution in human aplastic anemia. Leukemia 26, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, and Young NS (2009). Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 114, 2236–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, and Young NS (2009). Telomere diseases. N Engl J Med 361, 2353–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro MC, de Castro IP, and Ferreira MG (2016). Telomeres in aging and disease: lessons from zebrafish. Dis Model Mech 9, 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare AJ, and Reddel RR (2010). Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet 11, 319–330. [DOI] [PubMed] [Google Scholar]
- Chakravarti D, H.B., Mao X, Rashid A, Li J, Liao w., Whitley EM, Dey P, Hou P, LaBella KA, Chang A, Wang G, Spring DJ, Deng P, Zhao D, Liang X, Lan Z, Lin Y, Sarkar S, Terranova C, Deribe YL, Blutt SE, Okhuysen P, Zhang J, Sanchez V, Nielsen ON, Dupont A, Younes M, Patel KR, Shroyer N, Rai K, Estes M, Alan W, Bertuch A, DePinho RA (2020). Telomere dysfunction activates YAP1 to drive tissue inflammation. Nature Communications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S (2013). Cancer chromosomes going to POT1. Nat Genet 45, 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Khoo CM, Naylor ML, Maser RS, and DePinho RA (2003). Telomere-based crisis: functional differences between telomerase activation and ALT in tumor progression. Genes Dev 17, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, Lombard D, Pathak S, Guarente L, and DePinho RA (2004). Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet 36, 877–882. [DOI] [PubMed] [Google Scholar]
- Chen C, Gu P, Wu J, Chen X, Niu S, Sun H, Wu L, Li N, Peng J, Shi S, et al. (2017). Structural insights into POT1-TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat Commun 8, 14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, and DePinho RA (1999). p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538. [DOI] [PubMed] [Google Scholar]
- Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N, et al. (2008). TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet 4, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, Buer J, et al. (2007). Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet 39, 99–105. [DOI] [PubMed] [Google Scholar]
- Colla S, Ong DS, Ogoti Y, Marchesini M, Mistry NA, Clise-Dwyer K, Ang SA, Storti P, Viale A, Giuliani N, et al. (2015). Telomere dysfunction drives aberrant hematopoietic differentiation and myelodysplastic syndrome. Cancer Cell 27, 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, and Campisi J (2010). The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton HB, and McClintock B (1931). A Correlation of Cytological and Genetical Crossing-Over in Zea Mays. Proc Natl Acad Sci U S A 17, 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, and Jackson SP (2003). A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198. [DOI] [PubMed] [Google Scholar]
- Dang CV (2012). MYC on the path to cancer. Cell 149, 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T (2018). Shelterin-Mediated Telomere Protection. Annu Rev Genet 52, 223–247. [DOI] [PubMed] [Google Scholar]
- Dillon LM, Williams SL, Hida A, Peacock JD, Prolla TA, Lincoln J, and Moraes CT (2012). Increased mitochondrial biogenesis in muscle improves aging phenotypes in the mtDNA mutator mouse. Hum Mol Genet 21, 2288–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Wu CJ, Jaskelioff M, Ivanova E, Kost-Alimova M, Protopopov A, Chu GC, Wang G, Lu X, Labrot ES, et al. (2012). Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell 148, 896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow CT, and Harley CB (2016). Evaluation of an oral telomerase activator for early age-related macular degeneration - a pilot study. Clin Ophthalmol 10, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Shen J, Kugan N, Furth EE, Lombard DB, Cheung C, Pak S, Luo G, Pignolo RJ, DePinho RA, et al. (2004). Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol Cell Biol 24, 8437–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Hughes GM (2020). First evidence that senolytics are effective at decreasing senescent cells in humans. EBioMedicine 56, 102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazi PA, Glickman J, Jiang S, Yu A, Rudolph KL, and DePinho RA (2003). Differential impact of telomere dysfunction on initiation and progression of hepatocellular carcinoma. Cancer Res 63, 5021–5027. [PubMed] [Google Scholar]
- Fearon ER, and Vogelstein B (1990). A genetic model for colorectal tumorigenesis. Cell 61, 759–767. [DOI] [PubMed] [Google Scholar]
- Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al. (1995). The RNA component of human telomerase. Science 269, 1236–1241. [DOI] [PubMed] [Google Scholar]
- Fernandes SG, Dsouza R, Pandya G, Kirtonia A, Tergaonkar V, Lee SY, Garg M, and Khattar E (2020). Role of Telomeres and Telomeric Proteins in Human Malignancies and Their Therapeutic Potential. Cancers (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez ML, Thomas MS, Lemos BS, DiMarco DM, Missimer A, Melough M, Chun OK, Murillo AG, Alyousef HM, and Medina-Vera I (2018). TA-65, A Telomerase Activator improves Cardiovascular Markers in Patients with Metabolic Syndrome. Curr Pharm Des 24, 1905–1911. [DOI] [PubMed] [Google Scholar]
- Flores I, Cayuela ML, and Blasco MA (2005). Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309, 1253–1256. [DOI] [PubMed] [Google Scholar]
- Flynn RL, Cox KE, Jeitany M, Wakimoto H, Bryll AR, Ganem NJ, Bersani F, Pineda JR, Suva ML, Benes CH, et al. (2015). Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 347, 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson NJ, Ny L, Nilsson JA, and Larsson E (2014). Systematic analysis of noncoding somatic mutations and gene expression alterations across 14 tumor types. Nat Genet 46, 1258–1263. [DOI] [PubMed] [Google Scholar]
- Gao R, Davis A, McDonald TO, Sei E, Shi X, Wang Y, Tsai PC, Casasent A, Waters J, Zhang H, et al. (2016). Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat Genet 48, 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, et al. (2012). Telomerase directly regulates NF-kappaB-dependent transcription. Nat Cell Biol 14, 1270–1281. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Guardia L, Yubero-Serrano EM, Rangel-Zuniga O, Marin C, Camargo A, Perez-Martinez P, Delgado-Lista J, Gomez-Delgado F, Garcia-Rios A, Tinahones FJ, et al. (2014). Influence of endothelial dysfunction on telomere length in subjects with metabolic syndrome: LIPGENE study. Age (Dordr) 36, 9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RA, Chin L, Femino A, Lee KH, Gottlieb GJ, Singer RH, Greider CW, and DePinho RA (1999). Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell 97, 515–525. [DOI] [PubMed] [Google Scholar]
- Greider CW, and Blackburn EH (1985). Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405–413. [DOI] [PubMed] [Google Scholar]
- Greider CW, and Blackburn EH (1989). A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337, 331–337. [DOI] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, and de Lange T (1999). Mammalian telomeres end in a large duplex loop. Cell 97, 503–514. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, and Weinberg RA (1999). Creation of human tumour cells with defined genetic elements. Nature 400, 464–468. [DOI] [PubMed] [Google Scholar]
- Harel I, Benayoun BA, Machado B, Singh PP, Hu CK, Pech MF, Valenzano DR, Zhang E, Sharp SC, Artandi SE, et al. (2015). A platform for rapid exploration of aging and diseases in a naturally short-lived vertebrate. Cell 160, 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, and Greider CW (1990). Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460. [DOI] [PubMed] [Google Scholar]
- Harley CB, Liu W, Blasco M, Vera E, Andrews WH, Briggs LA, and Raffaele JM (2011). A natural product telomerase activator as part of a health maintenance program. Rejuvenation Res 14, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, and Robinson MO (1997). A mammalian telomerase-associated protein. Science 275, 973–977. [DOI] [PubMed] [Google Scholar]
- Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, and Allshire RC (1990). Telomere reduction in human colorectal carcinoma and with ageing. Nature 346, 866–868. [DOI] [PubMed] [Google Scholar]
- Hayflick L (1965). The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res 37, 614–636. [DOI] [PubMed] [Google Scholar]
- Hayflick L, and Moorhead PS (1961). The serial cultivation of human diploid cell strains. Exp Cell Res 25, 585–621. [DOI] [PubMed] [Google Scholar]
- Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S, et al. (2011). Altered telomeres in tumors with ATRX and DAXX mutations. Science 333, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson JD, Neumann AA, Yeager TR, and Reddel RR (2002). Alternative lengthening of telomeres in mammalian cells. Oncogene 21, 598–610. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, and Kemler R (2012). Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science 336, 1549–1554. [DOI] [PubMed] [Google Scholar]
- Hong J, and Yun CO (2019). Telomere Gene Therapy: Polarizing Therapeutic Goals for Treatment of Various Diseases. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al. (2013). TERT promoter mutations in familial and sporadic melanoma. Science 339, 959–961. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, and Auwerx J (2012). Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Van Den Berg D, Kang H, Hsieh CL, and Lieber MR (2013). Large chromosome deletions, duplications, and gene conversion events accumulate with age in normal human colon crypts. Aging Cell 12, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Hwang SS, Liesa M, Gan B, Sahin E, Jaskelioff M, Ding Z, Ying H, Boutin AT, Zhang H, et al. (2012). Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell 148, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icgc Tcga Pan-Cancer Analysis of Whole Genomes Consortium (2020). Pan-cancer analysis of whole genomes. Nature 578, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, et al. (2011). Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassaint NL, Guo N, Califano JA, Montgomery EA, and Armanios M (2013). The gastrointestinal manifestations of telomere-mediated disease. Aging Cell 12, 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimura J, Kitamura Y, Takata K, Umeki M, Suzuki S, Shibagaki K, Taniguchi T, Nomura Y, Gebicke-Haerter PJ, Smith MA, et al. (2002). Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J 16, 601–603. [DOI] [PubMed] [Google Scholar]
- Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, Grosveld G, and Sherr CJ (1997). Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Tahara S, and Matsuo M (1996). Non-linear accumulation of 8-hydroxy-2'-deoxyguanosine, a marker of oxidized DNA damage, during aging. Mutat Res 316, 277–285. [DOI] [PubMed] [Google Scholar]
- Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr., Friedman AH, Friedman H, Gallia GL, Giovanella BC, et al. (2013). TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A 110, 6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipling D (1997). Telomere structure and telomerase expression during mouse development and tumorigenesis. Eur J Cancer 33, 792–800. [DOI] [PubMed] [Google Scholar]
- Kipling D, and Cooke HJ (1990). Hypervariable ultra-long telomeres in mice. Nature 347, 400–402. [DOI] [PubMed] [Google Scholar]
- Klucken J, Shin Y, Masliah E, Hyman BT, and McLean PJ (2004). Hsp70 Reduces alpha-Synuclein Aggregation and Toxicity. J Biol Chem 279, 25497–25502. [DOI] [PubMed] [Google Scholar]
- Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, and Kelly DP (2008). Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev 22, 1948–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laish I, Mannasse-Green B, Hadary R, Biron-Shental T, Konikoff FM, Amiel A, and Kitay-Cohen Y (2016). Telomere Dysfunction in Nonalcoholic Fatty Liver Disease and Cryptogenic Cirrhosis. Cytogenet Genome Res 150, 93–99. [DOI] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW 2nd, Greider CW, and DePinho RA (1998). Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574. [DOI] [PubMed] [Google Scholar]
- Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Nadal-Ginard B, Kajstura J, Anversa P, and Blasco MA (2003). Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J 22, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qian X, Wang B, Xia Y, Zheng Y, Du L, Xu D, Xing D, DePinho RA, and Lu Z (2020). Programmable base editing of mutated TERT promoter inhibits brain tumour growth. Nat Cell Biol 22, 282–288. [DOI] [PubMed] [Google Scholar]
- Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, and Cech TR (1997). Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276, 561–567. [DOI] [PubMed] [Google Scholar]
- Loayza D, and De Lange T (2003). POT1 as a terminal transducer of TRF1 telomere length control. Nature 423, 1013–1018. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, and Kroemer G (2013). The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Spangenburg EE, Chin ER, Cheng WH, and Roth SM (2014). Telomeres shorten in response to oxidative stress in mouse skeletal muscle fibers. J Gerontol A Biol Sci Med Sci 69, 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejowski J, and de Lange T (2017). Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol 18, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Pilling LC, and Ferrucci L (2020). The genetics of human ageing. Nat Rev Genet 21, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mender I, Zhang A, Ren Z, Han C, Deng Y, Siteni S, Li H, Zhu J, Vemula A, Shay JW, et al. (2020). Telomere Stress Potentiates STING-Dependent Anti-tumor Immunity. Cancer Cell 38, 400–411e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, et al. (1997). hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785–795. [DOI] [PubMed] [Google Scholar]
- Missios P, Zhou Y, Guachalla LM, von Figura G, Wegner A, Chakkarappan SR, Binz T, Gompf A, Hartleben G, Burkhalter MD, et al. (2014). Glucose substitution prolongs maintenance of energy homeostasis and lifespan of telomere dysfunctional mice. Nat Commun 5, 4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, and Wacker JL (2005). Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 6, 11–22. [DOI] [PubMed] [Google Scholar]
- Muller H (1938). The remaking of chromosomes. . [Google Scholar]
- Nagpal N, Wang J, Zeng J, Lo E, Moon DH, Luk K, Braun RO, Burroughs LM, Keel SB, Reilly C, et al. (2020). Small-Molecule PAPD5 Inhibitors Restore Telomerase Activity in Patient Stem Cells. Cell Stem Cell 26, 896–909 e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassour J, Radford R, Correia A, Fuste JM, Schoell B, Jauch A, Shaw RJ, and Karlseder J (2019). Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 565, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. Cancer Stat Facts.
- Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, Cook K, Stepansky A, Levy D, Esposito D, et al. (2011). Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan RC, Chang S, Maser RS, Mohan R, Artandi SE, Chin L, and DePinho RA (2002). Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell 2, 149–155. [DOI] [PubMed] [Google Scholar]
- Olovnikov AM (1973). A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 41, 181–190. [DOI] [PubMed] [Google Scholar]
- Opresko PL, and Shay JW (2017). Telomere-associated aging disorders. Ageing Res Rev 33, 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al. (2009). Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech MF, Garbuzov A, Hasegawa K, Sukhwani M, Zhang RJ, Benayoun BA, Brockman SA, Lin S, Brunet A, Orwig KE, et al. (2015). High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev 29, 2420–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignolo RJ, Suda RK, McMillan EA, Shen J, Lee SH, Choi Y, Wright AC, and Johnson FB (2008). Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell 7, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, et al. (2014). The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, and Clevers H (2005). Wnt signalling in stem cells and cancer. Nature 434, 843–850. [DOI] [PubMed] [Google Scholar]
- Rheinbay E, Nielsen MM, Abascal F, Wala JA, Shapira O, Tiao G, Hornshoj H, Hess JM, Juul RI, Lin Z, et al. (2020). Analyses of non-coding somatic drivers in 2,658 cancer whole genomes. Nature 578, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risques RA, Lai LA, Brentnall TA, Li L, Feng Z, Gallaher J, Mandelson MT, Potter JD, Bronner MP, and Rabinovitch PS (2008). Ulcerative colitis is a disease of accelerated colon aging: evidence from telomere attrition and DNA damage. Gastroenterology 135, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risques RA, Lai LA, Himmetoglu C, Ebaee A, Li L, Feng Z, Bronner MP, Al-Lahham B, Kowdley KV, Lindor KD, et al. (2011). Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Res 71, 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roake CM, and Artandi SE (2020). Regulation of human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol 21, 384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, and Puigserver P (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434, 113–118. [DOI] [PubMed] [Google Scholar]
- Ruden M, and Puri N (2013). Novel anticancer therapeutics targeting telomerase. Cancer Treat Rev 39, 444–456. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, and DePinho RA (1999). Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Millard M, Schreiber-Agus N, and DePinho RA (2000). Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 287, 1253–1258. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Millard M, Bosenberg MW, and DePinho RA (2001). Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet 28, 155–159. [DOI] [PubMed] [Google Scholar]
- Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, et al. (2010). Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 143, 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, et al. (2011). Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, and Artandi SE (2005). Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436, 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, and Sabatini DM (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell 169, 361–371. [DOI] [PubMed] [Google Scholar]
- Schaetzlein S, Kodandaramireddy NR, Ju Z, Lechel A, Stepczynska A, Lilli DR, Clark AB, Rudolph C, Kuhnel F, Wei K et al. (2007). Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell 130, 863–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers AG, Vries R, van den Born M, van de Wetering M, and Clevers H (2011). Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J 30, 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Pereira-Smith OM, and Wright WE (1991). A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res 196, 33–39. [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, and de Lange T (2000). Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 20, 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, Marjoram P, Siegmund K, Press MF, Shibata D et al. (2015). A Big Bang model of human colorectal tumor growth. Nat Genet 47, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperka T, Song Z, Morita Y, Nalapareddy K, Guachalla LM, Lechel A, Begus-Nahrmann Y, Burkhalter MD, Mach M, Schlaudraff F et al. (2011). Puma and p21 represent cooperating checkpoints limiting self-renewal and chromosomal instability of somatic stem cells in response to telomere dysfunction. Nat Cell Biol 14, 73–79. [DOI] [PubMed] [Google Scholar]
- Tomas-Loba A, Flores I, Fernandez-Marcos PJ, Cayuela ML, Maraver A, Tejera A, Borras C, Matheu A, Klatt P, Flores JM, et al. (2008). Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 135, 609–622. [DOI] [PubMed] [Google Scholar]
- Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, Tsuji S, Donehower LA, Slagle BL, Nakamura H et al. (2014). Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet 46, 1267–1273. [DOI] [PubMed] [Google Scholar]
- Townsley DM, Dumitriu B, Liu D, Biancotto A, Weinstein B, Chen C, Hardy N, Mihalek AD, Lingala S, Kim YJ, et al. (2016). Danazol Treatment for Telomere Diseases. N Engl J Med 374, 1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R et al. (2004). Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C et al. (2002). p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45–53. [DOI] [PubMed] [Google Scholar]
- United Nations (2017). World population aging.
- van Heek NT, Meeker AK, Kern SE, Yeo CJ, Lillemoe KD, Cameron JL, Offerhaus GJ, Hicks JL, Wilentz RE, Goggins MG, et al. (2002). Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol 161, 1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B, Smogorzewska A, and de Lange T (1998). TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, and Harley CB (1993). Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet 52, 661–667. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie LY, Allan S, Beach D, and Hannon GJ (1998). Myc activates telomerase. Genes Dev 12, 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD (1972). Origin of concatemeric T7 DNA. Nat New Biol 239, 197–201. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM Jr., Sistonen L, and Morimoto RI (2009). Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323, 1063–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP, et al. (2002). Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J 16, 935–942. [DOI] [PubMed] [Google Scholar]
- Won J, Chang S, Oh S, and Kim TK (2004). Small-molecule-based identification of dynamic assembly of E2F-pocket protein-histone deacetylase complex for telomerase regulation in human cells. Proc Natl Acad Sci U S A 101, 11328–11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, and DePinho RA (2003). Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421, 643–648. [DOI] [PubMed] [Google Scholar]
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, and Shay JW (1996). Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 18, 173–179. [DOI] [PubMed] [Google Scholar]
- Yang Z, Takai KK, Lovejoy CA, and de Lange T (2020). Break-induced replication promotes fragile telomere formation. Genes Dev. [DOI] [PMC free article] [PubMed] [Google Scholar]