Abstract
PURPOSE
Pediatric neuro-oncology resources are mostly unknown in Chile. We report the human and material resources available in Chilean hospitals providing pediatric neuro-oncology services.
METHODS
A cross-sectional survey was distributed to 17 hospitals providing pediatric neuro-oncology services (Programa Infantil Nacional de Drogas Antineoplásicas [PINDA] hospitals, 11; private, 6).
RESULTS
Response rate was 71% (PINDA, 8; private, 4). Pediatric neuro-oncology services were mainly provided within general hospitals (67%). Registries for pediatric CNS tumors and chemotherapy-related toxicities were available in 100% and 67% of hospitals, respectively. CNS tumors were treated by pediatric oncologists in 92% of hospitals; none were formally trained in neuro-oncology. The most used treatment protocols were the national PINDA protocols. All WHO essential medicines for childhood cancer were available in more than 80% of the hospitals except for gemcitabine, oxaliplatin, paclitaxel, and procarbazine. The median number of pediatric neurosurgeons per hospital was two (range, 2-6). General neuroradiologists were available in 83% of the centers. Pathology specimens were sent to neuropathologists (58%), adult pathologists (25%), and pediatric pathologists (17%). Intensity-modulated radiotherapy, conformal radiotherapy, and cobalt radiotherapy were used by 67%, 58%, and 42% of hospitals, respectively. Only one private hospital performed autologous hematopoietic cell transplant for children with CNS tumors.
CONCLUSION
A wide range of up-to-date treatment modalities are available for children with CNS tumors. Our survey highlights future directions to improve the pediatric neuro-oncology services available in Chile such as the expansion of multidisciplinary clinics, palliative care services, long-term cancer survivorship programs, dedicated clinical research support teams, establishing standardized mechanism for sending pathologic specimen for second opinion to international specialized centers, and establishing specialized neuro-oncology training program.
INTRODUCTION
In the last decade, Chile achieved significant accomplishments in various child health indices. The under-five mortality rate was reduced to seven per 1,000 live births in 2017.1 The under-five mortality rate inversely correlates with childhood cancer incidence rates.2,3 Cancer is the second most common cause of death among children of age 5-14 years in Chile.4 Between 2007 and 2011, the 5-year overall survival rate of children with CNS tumors was 60%.4 Moreover, the burden of pediatric tumors in Chile is increasing secondary to the continuous decrease in infant and childhood mortality secondary to malnutrition and infection.5-9 Addressing the burden of pediatric CNS tumors is dependent on the availability of numerous pediatric subspecialties, ancillary staff, and costly infrastructure resources.5
CONTEXT
Key Objective
What are the available pediatric neuro-oncology services in Chile?
Knowledge Generated
Chile has a population-based pediatric cancer registry since 2006. Up-to-date treatment modalities, national treatment protocols, and essential medicines for childhood CNS tumors are readily available. Pediatric neurosurgeons, photon therapy facilities, up-to-date imaging modalities, and neuropathologists are available in the metropolitan centers.
Relevance
Our survey highlights a few future directions to help the constant progress of pediatric neuro-oncology services. Pediatric neuro-oncology services' centralization may increase the patient load and enhance the specialized treating centers' experience. Expanding the existing multidisciplinary approach to integrate other related ancillary services such as palliative care, psychiatry, long-term cancer survivorship clinics, and child life programs may provide more comprehensive care. Collaboration between international and local hospitals may help in clinical research implementation, second-opinion pathologic evaluation, and molecular genetic studies. Chile may provide a regional partner for future global pediatric neuro-oncology initiatives serving Latin America.
In 1998, the Chilean government established the National Pediatric Program for Antineoplastic Agents Programa Infantil Nacional de Drogas Antineoplásicas (PINDA) to fund universal medical care coverage for children with cancer.7 Children younger than 15 years of age comprise approximately 20% of the population of Chile.10 Approximately 85% of these children receive their medical care through public health services (PINDA hospitals).4 Eleven PINDA and six private hospitals offer treatment for children with CNS tumors throughout the country (Fig 1). Between 2007 and 2011, CNS tumors' incidence rate was 20 cases per million among children younger than 15 years of age, ranging between 60 and 80 patients annually, making it the most prevalent malignancy after leukemia.4 Pilocytic astrocytoma followed by embryonal CNS tumors such as medulloblastoma were the most common CNS tumors among children < 15 years of age.4 Uniform treatment protocols are provided for children with CNS tumors attending PINDA hospitals.9
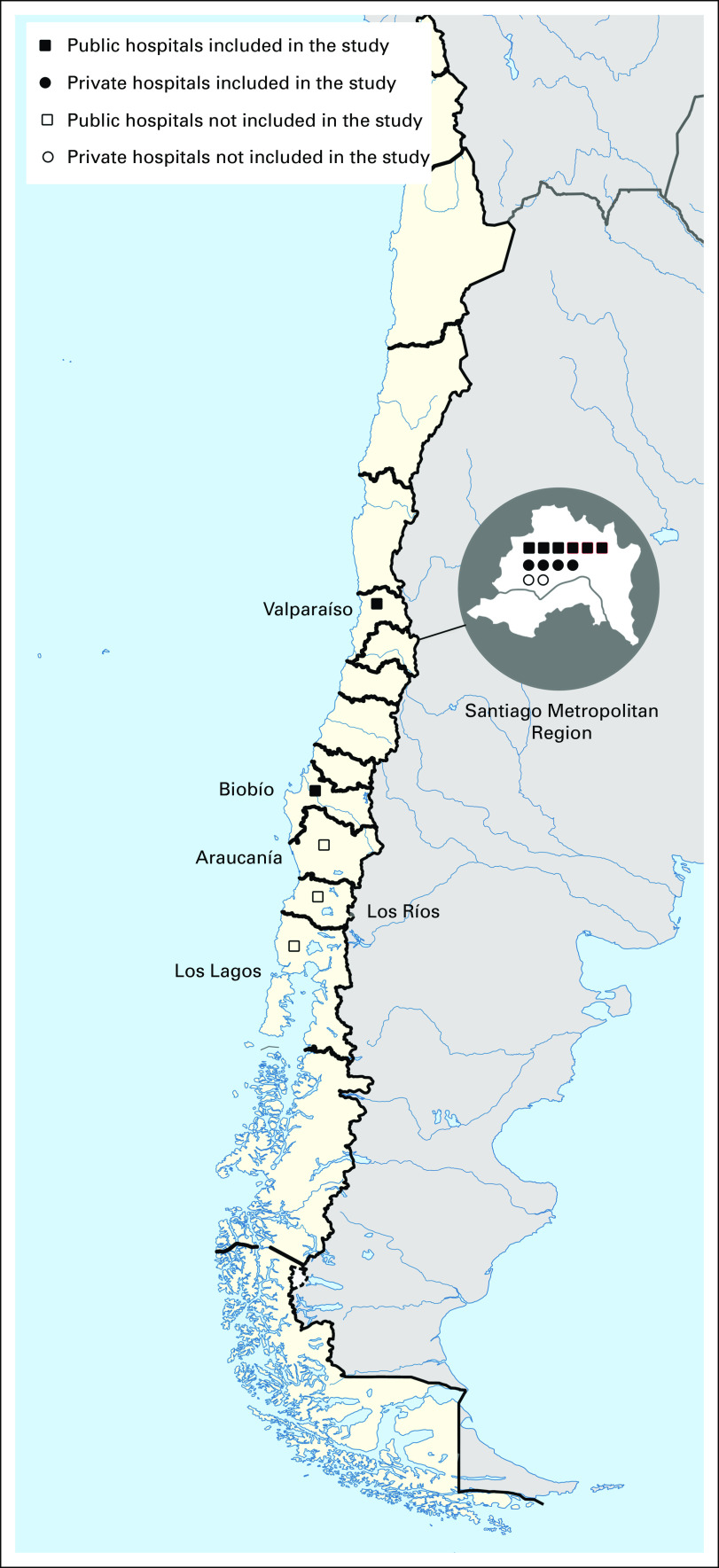
FIG 1.
The map of Chile demonstrating the location of all hospitals providing pediatric neuro-oncology services. Map attribution: Chile location map Wikimedia Commons CC BY 1.2 version, map was modified by the authors. Source: https://commons.wikimedia.org/wiki/File:Chile_location_map.svg.
The objective of our survey was to give a snapshot of the available pediatric neuro-oncology services in Chile. Moreover, we aimed to identify the strengths and weaknesses of the available services to help guide the pediatric neuro-oncology services' continuous progress in Chile.
METHODS
A cross-sectional survey was sent via email to 17 oncologists representing 17 hospitals offering pediatric neuro-oncology services in Chile. Dr. Ximena Espinoza identified the 17 oncologists. The questionnaire was designed via the Google Forms platform. The survey questions were written in Spanish (an English translation is available in Table 1). The questions were reviewed by six authors (M.H.A-.A, A.M.L.M., A.L., J.F., A.C., and D.S.O.). The study was approved by the Institutional Review Board at the Nationwide Children's Hospital.
TABLE 1.
Survey Questions
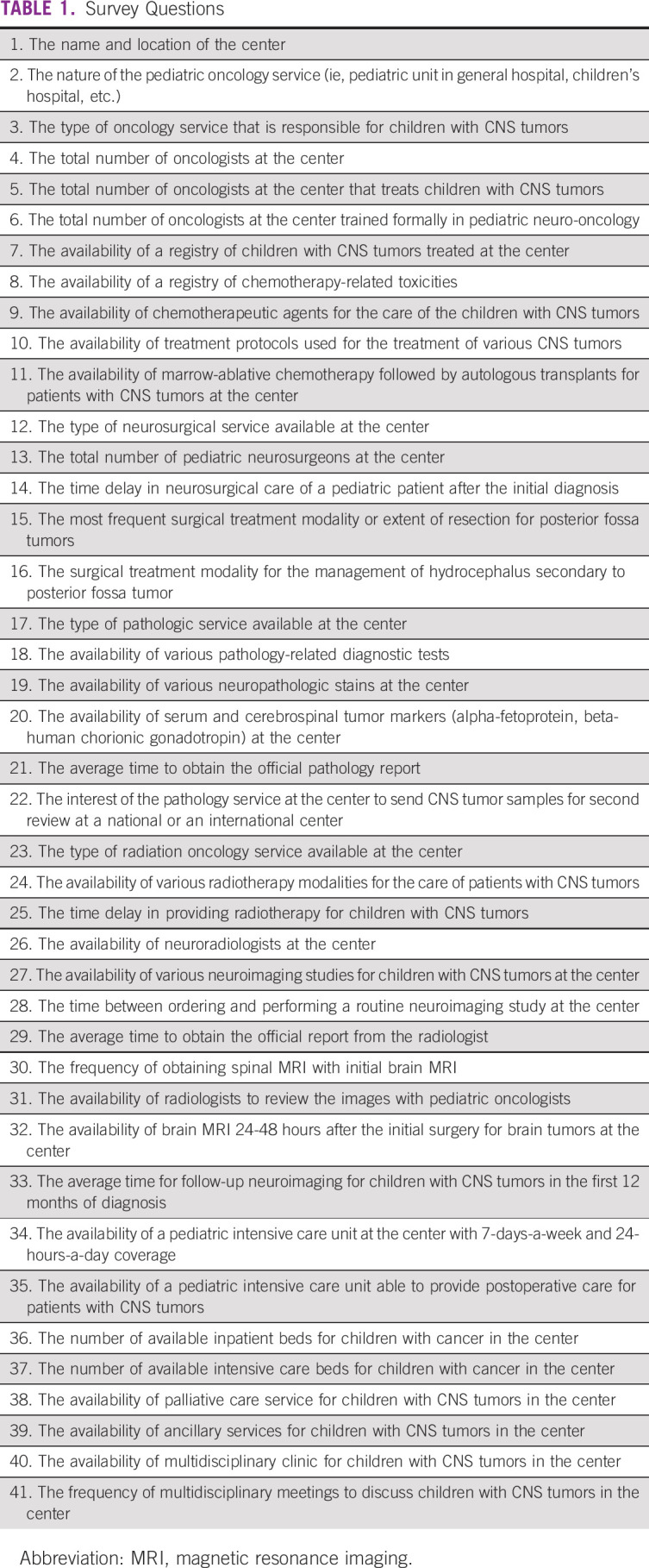
Data were collected from February 2018 to July 2018. Reminders were sent to all eligible participants over the same earlier-mentioned period. Participation in the survey was optional, and no personal identifying information was collected. The Nationwide Children's Hospital Institutional Review Board waived the need to obtain signed consent to participate in the study. Study personnel used standard measures to protect confidentiality. All data were stored online via a password-protected form in Google Forms and a password-protected Microsoft Excel sheet.
RESULTS
Eight of the 11 PINDA and four of the six private hospitals completed the survey (71% response rate). Eleven of the 12 hospitals were located in the metropolitan areas of three regions: Santiago Metropolitan, Viña del Mar Valparaiso, and Biobío (Fig 1). The pediatric services were provided in three public children's hospitals, one private children's hospital, and a pediatric department within a general hospital in eight of the 12 participating hospitals.
The median number of general pediatric oncology beds was 12 (range, 4-17). The median number of available general pediatric intensive care beds in the participating hospitals was 12 (range, 2-26). All 12 hospitals had 24-hour continuous coverage in their pediatric intensive care unit. Eleven hospitals (92%) had intensive care units able to manage postoperative children with CNS tumors. Registries for children with CNS tumors and toxicities associated with chemotherapeutic agents were available at 100% and 67% of the hospitals, respectively.
Pediatric Neuro-Oncology
General pediatric oncologists treated children with CNS tumors in 92% of the hospitals. None of the pediatric oncologists were formally trained in pediatric neuro-oncology. The median number of pediatric oncologists per hospital was 5.5 (range, 1-12). The median number of pediatric oncologists who treated children with CNS tumors was 5.5 (range, 1-8).
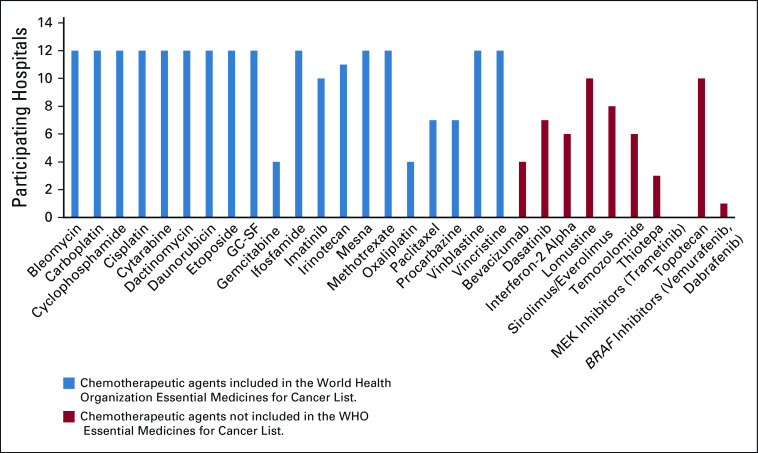
We identified 20 chemotherapeutic agents relevant for pediatric neuro-oncology from the WHO Essential Medicines for Cancer List (Fig 2).11 All 20 chemotherapeutic agents were available in all the 12 hospitals except for the following agents (the percentage of hospitals that provide the essential chemotherapy is within brackets for each agent): gemcitabine (33%), oxaliplatin (33%), paclitaxel (58%), and procarbazine (58%). Nonessential medications such as thiotepa (25%), mitogen-activated protein kinase kinase (MEK) inhibitors (0%), mechanistic target of rapamycin inhibitors (67%), temozolomide (50%), and BRAF inhibitors (8%) were not readily available in the participating hospitals.
FIG 2.
The availability of various chemotherapeutic agents in the participating hospitals. GC-SF, granulocyte colony-stimulating factor; MEK, mitogen-activated protein kinase kinase.
Pediatric Neurosurgery
Five hospitals (42%) had an in-house pediatric neurosurgery service (PINDA, 2; private hospitals, 3). Four hospitals (33%) used pediatric neurosurgery services from other hospitals. Three hospitals (25%) used the in-house general adult neurosurgery service. The median number of pediatric neurosurgeons per hospital, where pediatric neurosurgery service was available, was two (range, 2-6). The waiting interval between the neurosurgical intervention and the initial diagnosis was ≤ 2 weeks in eleven hospitals (92%). In ten hospitals (83%), achieving gross total resection of a posterior fossa tumor was the most pursued surgical intervention. Hydrocephalus secondary to posterior fossa tumor was managed primarily by external ventricular shunt in five hospitals (42%), ventriculoperitoneal shunt in four hospitals (33%), and endoscopic third ventriculocisternostomy in three hospitals (25%).
Neuropathology
Seven hospitals (58%) provided neuropathology service (PINDA, 5; private hospitals, 2). Three hospitals (25%) used general adult pathology. Two hospitals (17%) used general pediatric pathology services for their patients.
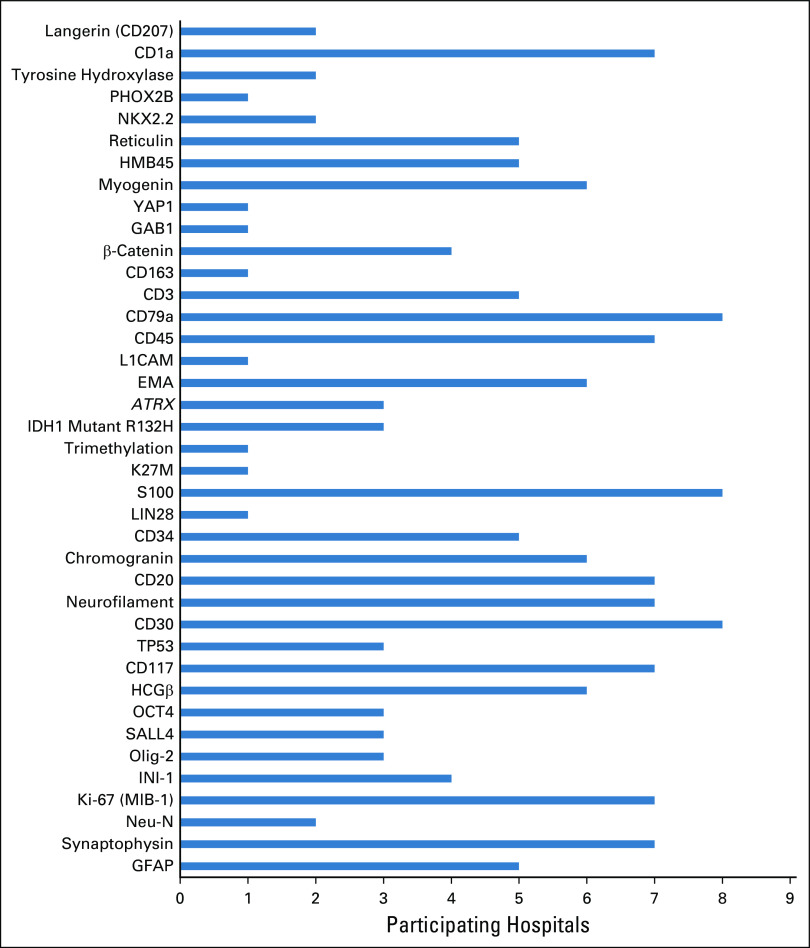
Hematoxylin and eosin stains were available at all hospitals. BRAF V600E mutation studies were available in four hospitals (33%). Fluorescence in situ hybridization was available in three hospitals (25%). Electron microscopy was available in one hospital (8%). DNA methylation microarray for 850,000 CpG sites or 450,000 CpG studies were not available. Serum and cerebrospinal beta-human chorionic gonadotropin and alpha-fetoprotein for the diagnosis of CNS germ cell tumors were available in eleven hospitals (92%). Figure 3 demonstrates the special stains available in the participating hospitals.
FIG 3.
The histopathologic stains available in the participating hospitals. ATRX, α-thalassemia/mental-retardation-syndrome-X-linked gene; EMA, Epithelial membrane antigen; GFAP, glial fibrillary acidic protein; HCGβ, beta-human chorionic gonadotropin.
On average, the official pathology report was available in 1 week in four hospitals and in 2 weeks in another four hospitals and needed more than 3 weeks in four PINDA hospitals. Five hospitals sent pathology slides for second-opinion review to medical centers outside the country. St Jude Children's Research Hospital was the most common destination for a second-opinion pathologic examination.
Pediatric Radiotherapy
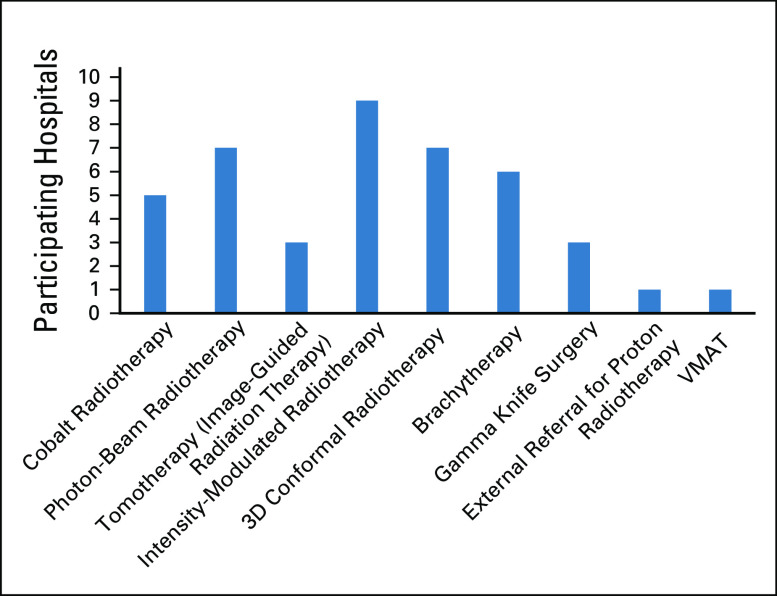
Six hospitals (50%) used outside pediatric radiotherapy services. Six hospitals (50%) used adult radiotherapy services for their patients. The waiting time to initiate radiotherapy was ≤ 2 weeks, 2 weeks, and 2-4 weeks in three hospitals (25%), five hospitals (42%), and four hospitals (33%), respectively. Figure 4 lists the available radiotherapy modalities in Chile.
FIG 4.
The radiotherapy modalities available in the participating hospitals. 3D, three dimensional; VMAT, volumetric-modulated arc radiotherapy.
Neuroradiology
Ten hospitals (83%) had an in-house neuroradiologist. Routine neuroimaging was performed within 1 day of request in four hospitals (33%), within 1 week in five hospitals (42%), within 1-2 weeks in one hospital (8%), and within 2-3 months in one hospital (8%). One hospital reported that urgent neuroimaging was performed within 1-3 days, whereas routine imaging was completed within 1 month of the request. The official neuroimaging report was available within 1 day in four hospitals (33%), within 1 week in another four hospitals (33%), within 2 weeks in one hospital (8%), and within 1 month in three hospitals (25%).
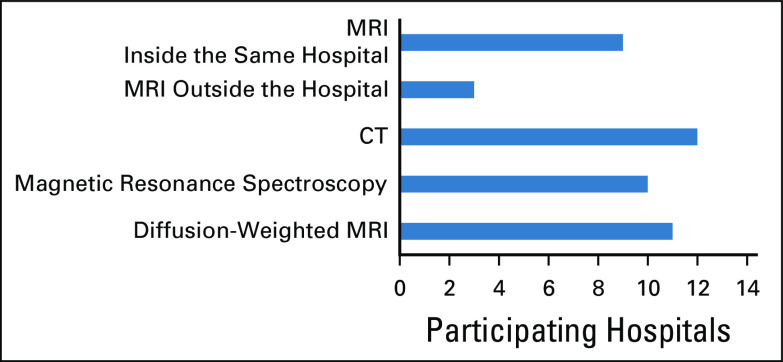
In ten hospitals (83%), neuroradiologists were available in-person for study revision. Brain magnetic resonance imaging was available in eight hospitals (67%) in the initial 48 hours after the initial tumor surgical resection. The average time for neuroimaging follow-up studies was every three months during the first 12 months of follow-up in nine hospitals (75%), every 4 months in one hospital (8%), and every 6 months in two hospitals (17%). Spine magnetic resonance imaging was always obtained with the initial diagnostic brain imaging for brain tumors in seven hospitals (58%), whereas it was obtained sometimes in three hospitals (25%) and rarely in two hospitals (17%). Figure 5 demonstrates the neuroimaging modalities available in the participating hospitals.
FIG 5.
The neuroimaging modalities available in the participating hospitals. CT, computed tomography; MRI, magnetic resonance imaging.
Ancillary Services
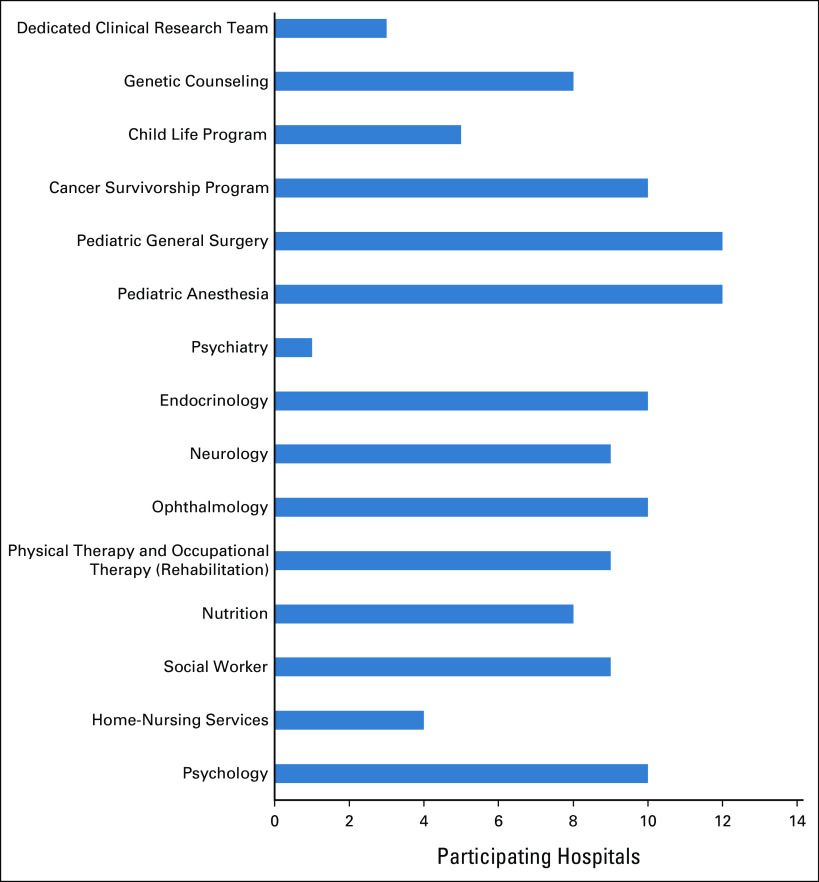
Palliative care services were available in five hospitals (50%). In the remaining hospitals, palliative care was provided by the pediatric oncologists. Multidisciplinary clinics were available in seven hospitals (58%). Multidisciplinary meetings to discuss children with CNS tumors occurred every month in nine hospitals (75%), once a week in one hospital (8%), and biweekly in another one hospital (8%), and multidisciplinary meetings were not established in one hospital (8%). Figure 6 provides a summary of the available ancillary services at the participating hospitals.
FIG 6.
The available support and ancillary services in the participating hospitals.
DISCUSSION
A high-quality population-based cancer registry covers < 20% of Chile's population.12 Cancer registries provide cancer prevalence in particular regions and communities, incidence rates, change in cancer behavior, and survival outcomes.5 In 2006, aiming to offer comprehensive epidemiologic data, all PINDA and private hospital registries were integrated into a population-based cancer registry, the Registro Nacional de Cáncer Infantil.
Children with CNS tumors were mainly treated in a pediatric department within general hospitals in Chile. In the United States, children with solid tumors, including brain tumors, had higher survival rates and fewer relapses and complication rates when treated in specialized cancer centers compared with community hospitals.13 This may be attributed to the availability of highly specialized multidisciplinary teams and advanced technologies at specialized centers. The centralization of pediatric neuro-oncology care may provide a way to increase the patient load, which may improve the care by expanding the dedicated treating center's experience and help justify the investment in newer technologies.5,14 However, this may prove geographically challenging in a country such as Chile.
The availability of and access to essential chemotherapeutic agents are required to improve the outcomes of children with cancer.11,15 WHO-Health Action International identified the 80% threshold to define essential medicine's optimal availability. A recent survey found that Chile has an ideal availability of essential medicines for childhood cancer (95% or greater proportional availability) on the basis of the WHO-Health Action International methodology.16,17 In our survey, we identified 20 agents relevant to pediatric neuro-oncology from the WHO Essential Medicines for Cancer List. All were ideally available except for four chemotherapeutic agents (gemcitabine, oxaliplatin, paclitaxel, and procarbazine). Gemcitabine, oxaliplatin, paclitaxel, and procarbazine are not commonly used in standard protocols for children with CNS tumors. Moreover, they are not included in the national PINDA protocols, which may explain the less-than-ideal availability of these chemotherapies in Chile. Temozolomide is commonly used for patients with high-grade gliomas. The national PINDA protocol for children with high-grade gliomas recommends against the utilization of temozolomide for children and limits it for adults (the national PINDA protocol for children with high-grade gliomas can be provided upon request from the corresponding author). This may explain the limited availability of temozolomide in the participating hospitals. None of the national PINDA protocols include MEK and BRAF inhibitors. This may explain the low availability of these agents in the participating hospitals. This may be attributed to the newer medications' cost-effectiveness and the exclusion of these medications from standard pediatric neuro-oncology treatment protocols. Moreover, molecular studies are not readily available for patients in Chile, restricting the ability to use targeted inhibitors.
The implementation of defined standardized national pediatric tumors’ protocols improves the survival outcomes, reduces discrepancies in disease classification, and improves the administration of quality measures.6,7,18 The national PINDA protocols were the most used treatment protocols for children with CNS tumors younger than 15 years of age in Chile. Most Chilean hospitals do not have a dedicated clinical research support team (Fig 6). The treating oncologists do the clinical research data collection and follow patients treated on the PINDA protocols in most programs. Even in the three centers where such clinical research team is available, the clinical research work is mainly done by the treating physicians and the team is limited to few research coordinators or nurses. This provides an opportunity for future collaboration between international research centers and Chilean hospitals to train and support dedicated clinical research teams.
Pediatric neuro-oncology requires a specialized knowledge base and skill set to manage children with CNS tumors. Moreover, novel diagnostic strategies, therapeutics, and prognostic factors are being discovered rapidly. Currently, 2-year formal training in pediatric hematology and oncology is available at four hospitals (PINDA, 3; private, 1). There is no structured formal training for pediatric neuro-oncology in Chile. Pediatric oncologists interested in pediatric neuro-oncology need to pursue such specialized training outside the country. Establishing a national pediatric neuro-oncology fellowship or specialized training in a tertiary referral center may provide enhanced training opportunities to meet the needs of the rapidly changing field. Collaborations between the local centers and international cooperative groups such as the Latin American Brain Tumor Board based in the Nationwide Children's Hospital and the Global Alliance for Pediatric Neuro-Oncology established by the St Jude Children's Research Hospital may facilitate the creation of a structured pediatric neuro-oncology education program that meets the needs of Chile.19
The complex nature of pediatric CNS tumors requires extensive input from highly specialized medical professionals of various disciplines.5,20 Therefore, multidisciplinary cancer care has become the standard of care for pediatric cancers in multiple high-income countries.21 Multidisciplinary meetings to discuss children with CNS tumors occurred at least once monthly in all participating hospitals except for one hospital. Moreover, multidisciplinary clinics were available in seven hospitals. Multiple Chilean pediatric oncologists attend the Latin American Brain Tumor Board weekly teleconference to discuss challenging cases.22 The multidisciplinary approach may help integrate more ancillary services and enrich the management discussion. Expanding the telemedicine-based collaboration between global highly specialized centers and the Chilean hospitals may represent a promising avenue to facilitate long-distance consultation, provide continuous educational opportunities, and improve management plans.22-24
Pediatric neurosurgery is available among half of the participating hospitals. Evidence suggests that pediatric neurosurgery services' centralization is associated with higher tumor resection rates, lower complication rates, lower ventricular shunt failure, and lower post–brain tumor surgery mortality rates.25-27 Therefore, designating regional hospitals for providing pediatric neurosurgery services may help increase the patient volume and expand the pediatric neurosurgeons' expertise in the field of CNS tumors.
Five PINDA-affiliated pediatric radiotherapy centers are located in the cities of Santiago, Concepcion, Valdivia, Temuco, and Viña del Mar Valparaiso. Moreover, there are four private pediatric radiotherapy centers in Santiago. The waiting time to receive radiotherapy was within 4 weeks from the request in most centers. Forty-two percent of the hospitals reported using cobalt-based radiotherapy for some of their patients. However, the majority of hospitals (75%) reported switching to intensity-modulated radiotherapy with the aim to stop using cobalt-based radiotherapy.
Various up-to-date neuroimaging modalities and in-house neuroradiologists were available in the majority of the participating hospitals. Delays in performing routine imaging studies and providing official reports in a timely manner were reported in a few hospitals. This warrants future studies to explore the reasons behind these delays and how to improve it.
In our survey, seven hospitals had an available neuropathologist. Moreover, histopathologic staining is centralized in one of the PINDA hospitals, which explains the significant gaps in the availability of multiple essential histopathologic stains for pediatric CNS tumors in most of the participating hospitals (Fig 3). Genetic counseling was available at eight hospitals. However, PINDA hospitals do not provide diagnostic molecular genetic studies. The lack of access to diagnostic molecular studies may affect the design and the implementation of newer treatment protocols. This may provide an opportunity for future collaboration between international centers and local hospitals in Chile, especially if diagnostic molecular genomic studies start to become more cost-effective.
The majority of the hospitals reported the availability of pediatric surgery, pediatric anesthesia, neurology, ophthalmology, endocrinology, nutrition, and psychology services (Fig 6). Rehabilitation services were available at nine centers, and the other three centers referred to outside private centers. Genetic counseling, psychiatry, home-nursing services, and child life programs were not readily available, which provides an opportunity to improve these services in the future.
The study has multiple limitations that may influence the interpretation of its results. The cross-sectional design limits our ability to examine any causality or association relationships. We achieved a 71% response rate and represented the hospitals in three regions of the six regions that have hospitals providing pediatric neuro-oncology services. Therefore, our results are not generalizable to all the hospitals providing pediatric neuro-oncology services. Additionally, pediatric oncologists filled all surveys, which may lead to sampling bias as other allied medical professionals were not represented in the study.
The survey provides a snapshot of the pediatric neuro-oncology services available in Chile. Chile has a population-based pediatric cancer registry since 2006, providing essential data for guiding cancer-related health policies. A wide range of up-to-date treatment modalities, national treatment protocols, and WHO essential medicines for childhood cancer are available for pediatric CNS tumors. Moreover, the availability of standardized national protocols may improve the application of quality measures, reporting treatment events, and morbidity and mortality outcomes. Our survey highlights a few future directions that may help the constant progress of pediatric neuro-oncology services in Chile. For example, the centralization of pediatric neuro-oncology services may increase the patient load and improve the care provided by expanding specialized treating centers' experience. Providing opportunities for formal pediatric neuro-oncology training may be beneficial. Expanding the existing multidisciplinary approach to integrate other related ancillary services such as palliative care, psychiatry, long-term cancer survivorship clinics, and child life programs may provide more comprehensive care. Collaboration between international and local hospitals may help in the fields of clinical research, second-opinion pathologic evaluation, and molecular genetic studies. Finally, expanding the telemedicine-based partnership may facilitate long-distance consultation and provide continuous educational opportunities.
ACKNOWLEDGMENT
The authors thank The Division of Hematology, Oncology, Blood and Marrow Transplants at Nationwide Children's Hospital for assistance with article processing charges.
PRIOR PRESENTATION
Presented at the 2019 American Society of Pediatric Hematology/Oncology Annual Meeting. New Orleans, LA, May 2019. Pediatr Blood Cancer, 66:e27713. doi:10.1002/pbc.27713. Poster presentation at the 19th International Symposium on Pediatric Neuro-Oncology in Karuizawa, Nagano, Japan, December 13-16, 2020.
AUTHOR CONTRIBUTIONS
Conception and design: Mohammad H. Abu-Arja, Alvaro Lassaletta, Ibrahim Qaddoumi, Jonathan L. Finlay, Adrián Cáceres, Diana S. Osorio
Provision of study materials or patients: Nicolás Rojas del Río, Miguel Valero, Veronica Perez, Felipe Espinoza, Juan Tordecilla, Katherine Kopp, Mauricio Reyes, Ximena Espinoza
Collection and assembly of data: Mohammad H. Abu-Arja, Nicolás Rojas del Río, Rosa Moreno, Miguel Valero, Veronica Perez, Felipe Espinoza, Eduardo Fernandez, José Santander, Juan Tordecilla, Veronica Oyarce, Katherine Kopp, Mauricio Reyes
Data analysis and interpretation: Mohammad H. Abu-Arja, Andres Morales La Madrid, Scott L. Coven, Ute Bartels, Jonathan L. Finlay, Diana S. Osorio
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.World Health Organization : Chile Profile. https://www.who.int/countries/chl/en/ [Google Scholar]
- 2.Wang H, Liddell CA, Coates MM, et al. : Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:957-979, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al. : Toward the cure of all children with cancer through collaborative efforts: Pediatric oncology as a global challenge. J Clin Oncol 33:3065-3073, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilean Ministerio de Salud. Vigilancia Epidemiologica de Cancer : Registro Nacional de Cáncer Infantil (RENCI) Quinquenio 2007-2011. http://www.ipsuss.cl/ipsuss/site/artic/20180117/asocfile/20180117150429/informe_renci_2007_2011registro_nacional_c__ncer_infantildepto_epidemiolog__aminsal2018.pdf [Google Scholar]
- 5.Chan MH, Boop F, Qaddoumi I: Challenges and opportunities to advance pediatric neuro-oncology care in the developing world. Childs Nerv Syst 31:1227-1237, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Quintana J, Advis P, Becker A, et al. : Acute myelogenous leukemia in Chile PINDA protocols 87 and 92 results. Leukemia 19:2143-2146, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Joannon P, Becker A, Kabalan P, et al. : Results of therapy for Wilms tumor and other malignant kidney tumors: A report from the Chilean Pediatric National Cancer Program (PINDA). J Pediatr Hematol Oncol 38:372-377, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Conter V, Valsecchi MG, Silvestri D, et al. : Pulses of vincristine and dexamethasone in addition to intensive chemotherapy for children with intermediate-risk acute lymphoblastic leukaemia: A multicentre randomised trial. Lancet 369:123-131, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Rivera GK, Quintana J, Villarroel M, et al. : Transfer of complex frontline anticancer therapy to a developing country: The St Jude osteosarcoma experience in Chile. Pediatr Blood Cancer 50:1143-1146, 2008 [DOI] [PubMed] [Google Scholar]
- 10.National Institute of Statistics (Chile) : Estimaciones y proyecciones de la población de Chile 1992-2050. http://www.censo2017.cl/descargas/proyecciones/sintesis-estimaciones-y-proyecciones-de-la-poblacion-chile-1992-2050.pdf [Google Scholar]
- 11.Robertson J, Barr R, Shulman LN, et al. : Essential medicines for cancer: WHO recommendations and national priorities. Bull World Health Organ 94:735-742, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piñeros M, Abriata MG, Mery L, et al. : Cancer registration for cancer control in Latin America: A status and progress report. Rev Panam Salud Publica 41:e2, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer S, Meadows AT, Pastore G, et al. : Influence of place of treatment on diagnosis, treatment, and survival in three pediatric solid tumors. J Clin Oncol 2:917-923, 1984 [DOI] [PubMed] [Google Scholar]
- 14.Qaddoumi I: Centralized services and large patient volumes are clinical necessities for a better outcome in pediatric brain tumors. Childs Nerv Syst 32:591-592, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unguru Y, Bernhardt MB, Berg SL, et al. : Chemotherapy and supportive care agents as essential medicines for children with cancer. JAMA Pediatr 173:477-484, 2019 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization : Medium-Term Strategic Plan 2008–2013. Geneva, 2008. https://apps.who.int/gb/ebwha/pdf_files/WHA64/A64_7-en.pdf [Google Scholar]
- 17.Cohen P, Friedrich P, Lam C, et al. : Global access to essential medicines for childhood cancer: A cross-sectional survey. J Glob Oncol 4:1-11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elzomor H, Taha H, Nour R, et al. : A multidisciplinary approach to improving the care and outcomes of patients with retinoblastoma at a pediatric cancer hospital in Egypt. Ophthalmic Genet 38:345-351, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Moreira DC, Rajagopal R, Navarro-Martin Del Campo RM, et al. : Bridging the gap in access to care for children with CNS tumors worldwide. JCO Glob Oncol 6:583-584, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Baki MS, Hanzlik E, Kieran MW: Multidisciplinary pediatric brain tumor clinics: The key to successful treatment? CNS Oncol 4:147-155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics : Standards for pediatric cancer centers. Pediatrics 134:410–414, 2014 [Google Scholar]
- 22.Abu Arja MH, Stanek JR, Morales La Madrid AE, et al. : The Latin American Brain Tumor Board teleconference: Results of a web-based survey to evaluate participant experience utilizing this resource. Childs Nerv Syst 35:257-265, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Hazin R, Qaddoumi I: Teleoncology: Current and future applications for improving cancer care globally. Lancet Oncol 11:204-210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amayiri N, Swaidan M, Abuirmeileh N, et al. : Video-teleconferencing in pediatric neuro-oncology: Ten years of experience. J Glob Oncol 4:1-7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry JG, Hall MA, Sharma V, et al. : A multi-institutional, 5-year analysis of initial and multiple ventricular shunt revisions in children. Neurosurgery 62:445-453, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Smith ER, Butler WE, Barker FG, II: Craniotomy for resection of pediatric brain tumors in the United States, 1988 to 2000: Effects of provider caseloads and progressive centralization and specialization of care. Neurosurgery 54:553-563, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Albright AL, Sposto R, Holmes E, et al. : Correlation of neurosurgical subspecialization with outcomes in children with malignant brain tumors. Neurosurgery 47:879-885, 2000 [DOI] [PubMed] [Google Scholar]