Summary
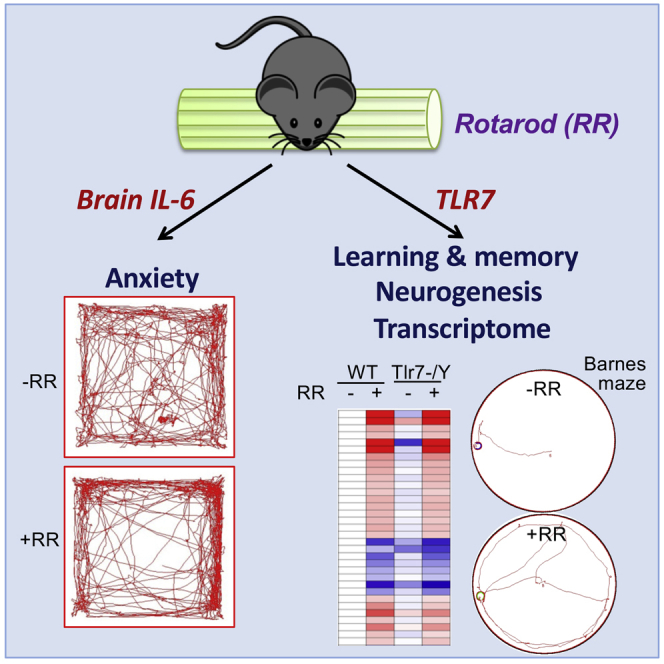
Voluntary exercise is well known to benefit brain performance. In contrast, forced exercise induces inflammation-related stress responses and may cause psychiatric disorders. Here, we unexpectedly found that rotarod testing, a frequently applied assay for evaluating rodent motor coordination, induces anxiety and alters spatial learning/memory performance of mice. Rotarod testing upregulated genes involved in the unfolded protein response and stress responses and downregulated genes associated with neurogenesis and neuronal differentiation. It impacts two downstream pathways. The first is the IL-6-dependent pathway, which mediates rotarod-induced anxiety. The second is the Toll-like receptor 7 (TLR7)-dependent pathway, which is involved in the effect of rotarod exercise on gene expression and its impact on contextual learning and memory of mice. Thus, although rotarod exercise does not induce systemic inflammation, it influences innate immunity-related responses in the brain, controls gene expression and, consequently, regulates anxiety and contextual learning and memory.
Subject areas: Molecular Neuroscience, Immunology, Transcriptomics
Graphical abstract
Highlights
-
•
Rotarod training at 5 or 10 weeks of age induces anxious behavior in an open field
-
•
Rotarod training upregulates IL-6 expression in the brain and results in anxiety
-
•
Rotarod training alters performances of test mice in spatial learning and memory
-
•
TLR7 controls the rotarod-impacted transcriptomic profiles and contextual memory
Molecular Neuroscience; Immunology; Transcriptomics
Introduction
Exercise is known to influence brain functions in human and rodents (Delezie and Handschin, 2018; Pedersen, 2019). Voluntary exercise benefits neurogenesis and enhances the expression of neurotrophic factors to improve learning and memory performance (Bettio et al., 2019; Cooper et al., 2018; Hamilton and Rhodes, 2015; Pedersen, 2019; Rendeiro and Rhodes, 2018; Ryan and Nolan, 2016; Voss et al., 2019). However, for forced exercise, it is more complex because despite the beneficial effects provided by the muscular and cardiovascular systems, forced exercise (such as treadmills for rodents) also can induce stress responses (Contarteze et al., 2008; Svensson et al., 2016) and systemic inflammation (Cook et al., 2013; Nambot et al., 2020). Stress triggers the unfolded protein response of the ER(Gold et al., 2013; Hotamisligil, 2010), influences neural plasticity (Duman and Li, 2012; Pittenger and Duman, 2008) and neurogenesis (De Miguel et al., 2019), and it decreases dendritic spine density and shortens the dendritic branches of neurons (De Miguel et al., 2019). Consequently, forced exercise impairs spatial memory (de Quervain et al., 2017) and results in anxiety (Gold et al., 2015). Thus, the stress condition caused by forced exercise damages the brain function, although the detailed mechanism remained unclear.
Toll-like receptors (TLR) detect both exogenic pathogenic pattern molecules and endogenous damage signals to trigger innate immune responses. Consequently, in addition to removing pathogens, TLR activation also fine-tunes organ development and morphogenesis (Akira and Sato, 2003; Chen et al., 2019; Kumar et al., 2009; Liu et al., 2014; Sommer and Bäckhed, 2013). For instance, activation of TLR3 or TLR4 by systemic administration of poly(I:C) or lipopolysaccharide at prenatal or neonatal stages, respectively, results in anxiety and other behavioral defects similar to the features of neurodevelopmental disorders (Bilbo et al., 2018; Chen et al., 2019; Khandaker et al., 2015; Knuesel et al., 2014). TLRs are also involved in sensing gut microbiota to modulate homeostasis and development of host immune and gastrointestinal systems, as well as influencing metabolism, development and brain function (Burgueño and Abreu, 2020; Caputi and Giron, 2018; Lin et al., 2019; Sommer and Bäckhed, 2013; Spiljar et al., 2017; Yiu et al., 2017). Deletion of TLRs also alters mouse behaviors (Chen et al., 2019; Hung et al., 2018a; Liu et al., 2013), strengthening evidence for the roles of TLRs in regulating brain function.
In this study, we report that rotarod testing, a popular exercise assay for assessing the motor coordination and balance of rodents, induces anxiety and alters spatial learning/memory of mice. Using genetically modified mice, we demonstrate that rotarod training uses IL-6 and TLR7 pathways to regulate neurogenesis and transcription and thereby controls various mouse behaviors. Our study dissects mechanistic details of how innate immune molecules control behaviors and brain plasticity, and it also suggests that rotarod testing can interfere with other brain functions, such as emotion and learning/memory.
Results
Rotarod training induces anxious behaviors
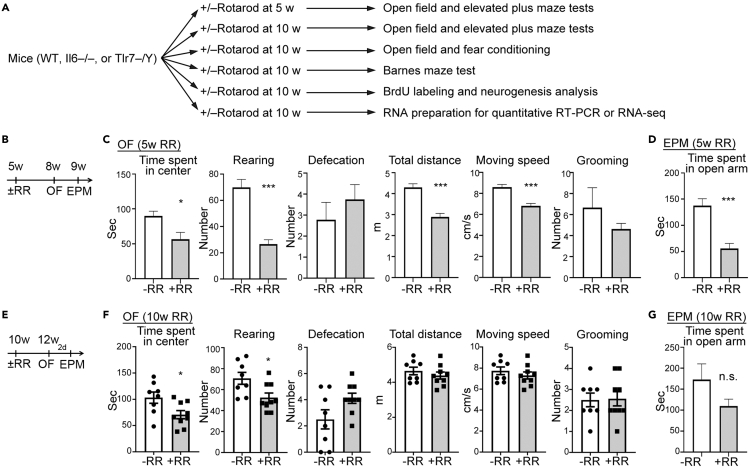
To characterize the behavioral features of mice, the same mice were usually subjected to a series of behavioral paradigms. We unexpectedly found that accelerating rotarod test likely influenced the results of behavioral assays following the rotarod test. It seems that the accelerating rotarod test with three trials per day for three consecutive days can change brain activity and function and therefore alter the performance of mice in other behavioral tests. To investigate this possibility, we subjected naive mice to a rotarod test on three consecutive days at the ages of 5 or 10 weeks (Figure 1A). Data on animal usage for each experiment are available in Table S1. Mouse performance in rotarod testing is summarized in Figure S1. After rotarod training, various behavioral tests, immunostaining, quantitative RT-PCR and transcriptomic analyses were applied to establish if and how rotarod training influences brain function and plasticity (Figure 1A). First, we characterized mice subjected to rotarod training at the age of 5 weeks (Figure 1B). Compared with mock control mice, 5-week-old mice that underwent rotarod training spent less time in the central area and exhibited a reduced number of rearing events and lower overall moving distance and speed in the open field assay (Figure 1C). Mice treated with rotarod training at 5 weeks old also spent less time exploring the open arm of an elevated plus maze (Figure 1D). These results suggest that rotarod treatment at the age of 5 weeks impairs both horizontal and vertical movements and induces anxious behavior in mice at the age of 8–9 weeks. For mice subjected to the rotarod test at the age of 10 weeks (Figure 1E), they still spent less time in the central area of the open field and presented fewer rearing events (Figure 1F). However, although rotarod training at the age of 10 weeks tended to reduce the time spent exploring the open arm of an elevated plus maze, the difference relative to control was not significant (Figure 1G). Thus, compared to juvenile mice, adult mice were relatively resistant to rotarod treatment, but still exhibited abnormal behaviors in an open field.
Figure 1.
Rotarod training at 5 or 10 weeks of age induces anxiety in adult mice
(A) Outline of our experimental design.
(B) Arrangement of behavioral paradigms for (C and D). A rotarod test (RR) was carried out on mice at 5 weeks (w) of age, followed by open field (OF) and elevated plus maze (EPM) as indicated.
(C) The results of open field test on mice with rotarod training at 5 weeks of age.
(D) The results of elevated plus maze test of mice with rotarod training at 5 weeks of age (n = 9 for WT–RR, n = 8 for WT + RR).
(E) Arrangement of behavioral paradigms for (F)-(G). Mice were subjected to rotarod training at 10 weeks of age.
(F) The results of open field test on mice with rotarod training at 10 weeks of age.
(G) The results of elevated plus maze test on mice with rotarod training at 10 weeks of age (n = 8 for WT–RR mice, n = 9 for WT + RR mice).
Data represent mean plus SEM. Two-tailed Mann-Whitney U test (B-C). ∗p < 0.05; ∗∗∗p < 0.001.
Note, for our 5-week-old mice cohort, some of the mice tended to jump away from the moving rod at day 3 of rotarod training, consequently shortening the time they stayed on the rod (Figure S1A). Ten-week-old mice exhibited normal motor learning ability to stay on the rotarod longer when they received more training trials (Figure S1B). It is unclear why some of the 5-week-old mice tended to jump away from the rotarod. It may be due to hyperactivity at the adolescence stage or hypersensitivity to the duress of rotarod testing. Since 10-week-old mice behaved much more stably than 5-week-old mice on the rotarod, we used 10-week-old mice in the following experiments.
IL-6 is involved in rotarod-induced anxiety
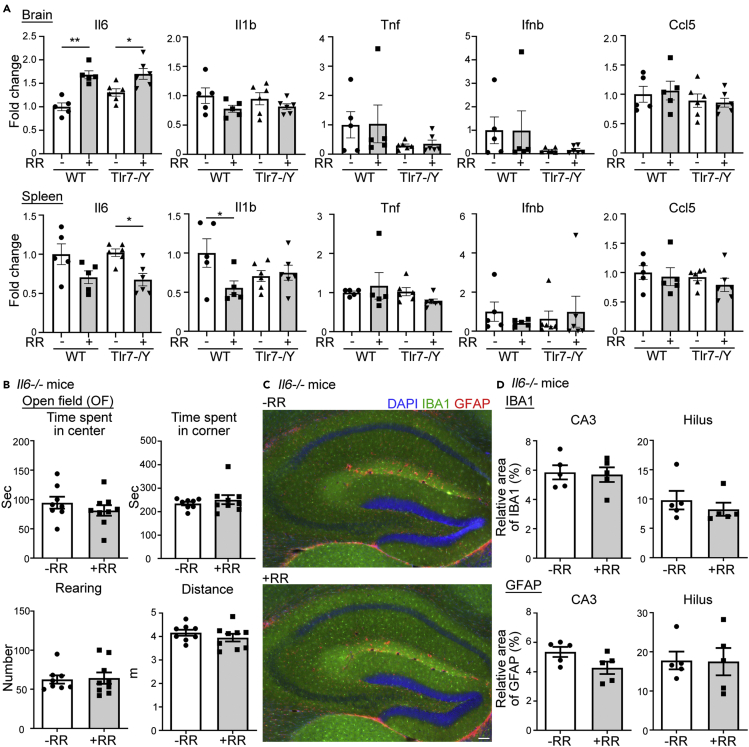
To further confirm and characterize rotarod-induced anxiety in adult mice, we used quantitative RT-PCR (Table S2) to analyze cytokine expression in cortices, hippocampi and spleens 3 hr after undergoing rotarod treatment at the age of 10 weeks. In this report, we always mixed cortical and hippocampus tissues to represent RNA samples prepared from brains. Among the five cytokines we examined (i.e., IL-6, IL-1β, TNFα, IFNβ and CCL5), we found that mRNA levels of Il6 were increased in brains of adult WT mice that undertook rotarod training (Figure 2A, upper) but not in their spleens (Figure 2A, lower). In fact, mRNA levels of both Il6 and Il1β tended to be reduced in the spleen of adult WT mice following rotarod training (Figure 2A, lower). Thus, rotarod training specifically induced Il6 expression in brains among examined cytokines. Although Il6 levels in the brain were increased, the population of glial cells was not altered by rotarod training (Figure S2, both microglia and astrocytes). Expression of various Tlr genes in both brains and spleens was also not influenced by rotarod training (Figure S3). These results suggest that although the rotarod assay induces expression of IL-6 in cortices and hippocampi, the effect of rotarod testing on inflammation was not global. Rotarod training did not induce noticeable inflammation in peripheral tissues.
Figure 2.
Rotarod training at 10 week of age induces anxiety through IL-6
(A) Rotarod training at 10 weeks of age differentially regulates cytokine expression in brain tissue (cortices and hippocampi) and spleens. Quantitative PCR was performed to measure relative cytokine expression levels in WT and Tlr7–/y mice (n = 5 for WT, n = 6 for Tlr7–/y).
(B) Deletion of Il6 mitigates rotarod-induced anxiety in an open field (n = 8 for Il6–/––RR mice, n = 9 for Il6−/−+RR mice).
(C) Rotarod training does not alter the population of glial cells in hippocampi (n = 5 for ± RR). Representative images of GFAP (red) and IBA1 (green) dual staining are shown.
(D) Quantification of IBA1 and GFAP immunoreactivities. Signal coverage (area) percentage of the CA3 and hilus regions of hippocampi was determined.
Data represent mean +/− SEM and the results of individual samples are shown. Two-tailed Mann-Whitney U test (B and D); two-way ANOVA with Bonferroni's multiple comparisons test (A). ∗, p < 0.05; ∗∗, p < 0.01. Scale bar: (C) 100 μm.
To evaluate the role of IL-6 in rotarod-induced anxiety, we subjected Il6 knockout mice to rotarod training. Unlike adult WT mice, Il6−/− mice pretrained with a rotarod assay did not spend more time in corners of an open field test and rearing behavior was not affected (Figure 2B). Numbers of glial cells were also unaltered (Figures 2C and 2D, information on antibodies is available in Table S3).
Taken together, these results suggest that IL-6 is critical for rotarod-induced anxiety in an open field test, and that glial cells are not involved in the effect of rotarod training on adult mice.
TLR7 is not involved in rotarod-induced anxiety
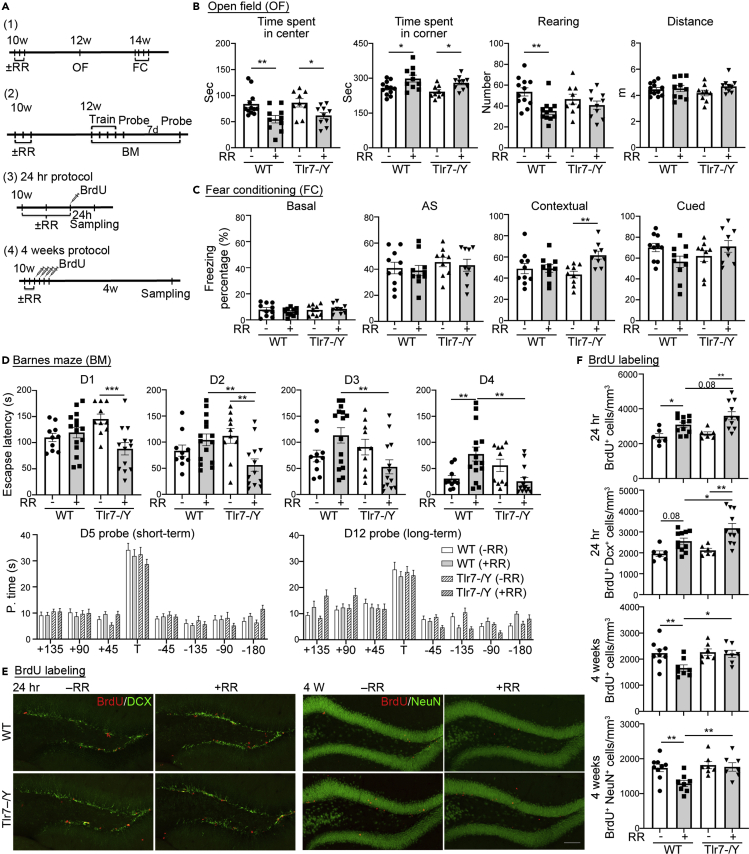
Next, we tested Tlr7 knockout mice for rotarod-induced stress responses because our previous studies have shown that TLR7 activation in neurons induces IL-6 expression to control neuronal morphology (Liu et al., 2013) and that Tlr7 knockout mice (i.e. Tlr7–/y mice) are less anxious and perform normally in rotarod test (Hung et al., 2018a) Since Il6 is critical for rotarod-induced anxiety (Figure 1D), we investigated if TLR7 is involved in that response. Consistent with our previous study, Tlr7 deletion did not influence the results of rotarod testing (Figure S1D). Similar to WT mice, Il6 RNA levels were still upregulated in Tlr7–/y mouse cortices and hippocampi after rotarod training, although reduced Il6 expression was found in the spleen of Tlr7–/y mice (Figure 2A). This result suggests that TLR7 is unlikely involved in rotarod-induced Il6 expression. In the open field, Tlr7–/y mice subjected to rotarod training (Tlr7–/y + RR) also spent less time in the central area and longer in the corners, which is a similar outcome to rotarod-trained WT mice (WT + RR) (Figures 3A (1) and 3B). However, in contrast to WT mice, rotarod training did not reduce the number of rearing events of Tlr7–/y mice in the open field (Figure 3B, Tlr7–/y–RR vs. Tlr7–/y + RR). Thus, Tlr7 deletion influences some but not all rotarod-induced behavioral alterations in the open field.
Figure 3.
Tlr7 deletion alters rotarod-induced behavioral patterns and neurogenesis
(A) Experimental arrangements for mice with rotarod training at 10 weeks of age. (1) for (B) and (C); (2) for (D); (3) and (4) for (E) and (F).
(B) The effect of rotarod training (RR) on open field (OF) responses. Rotarod training reduced the time spent in the center of an open field for both WT and Tlr7–/y mice, but not the number of rearing events of Tlr7–/y mice (n = 12 for WT–RR, n = 10 for WT + RR, n = 9 for Tlr7–/y–RR, n = 10 for Tlr7–/y + RR).
(C) The effect of RR on fear conditioning. Rotarod training solely enhanced contextual memory of Tlr7–/y mice (n = 10 for WT ± RR, n = 9 for Tlr7–/y ± RR). AS, right after stimulation (representing pain sensation).
(D) In Barnes maze, RR differentially impacted escape latency in WT and Tlr7–/y mice but did not affect short- (D5) or long-term (D12) memory (n = 10 for WT–RR, n = 15 for WT + RR, n = 10 for Tlr7–/y–RR, n = 13 for Tlr7–/y + RR).
(E) Representative images of BrdU labeling.
(F) The results of BrdU labeling. For short-term 24-hr BrdU labeling, RR enhanced neurogenesis in both WT and Tlr7–/y mice (n = 6 for WT–RR, n = 11 for WT + RR, n = 6 for Tlr7–/y–RR, n = 11 for Tlr7–/y + RR). For long-term 4-week BrdU labeling, RR reduced neural differentiation only in WT mice but not in Tlr7–/y mice (n = 9 for WT–RR, n = 8 for WT + RR, n = 7 for Tlr7–/y–RR, n = 7 for Tlr7–/y + RR).
Data represent mean +/− SEM and the results of individual samples are shown. two-way ANOVA with Bonferroni's multiple comparisons test (B–D and F). ∗, p < 0.05; ∗∗, p < 0.01. Scale bar: (E) 100 μm.
Rotarod training differentially regulates contextual learning and memory in WT and Tlr7–/y mice
We then investigated if rotarod training influences the outcomes of fear conditioning and Barnes maze assays in WT and Tlr7–/y mice (Figure 3A (1)-(2)). The fear conditioning assay tested both contextual and cued fear memory. For WT mice, we did not observe an effect of rotarod training on contextual or cued fear memory. However, we did find that the performance of Tlr7–/y mice in contextual fear memory was enhanced after rotarod treatment (Figure 3C).
For the Barnes maze test, mice underwent four training trials to find an escape hole every day for four consecutive training days (D1-D4), and were then subjected to a short-term memory test at D5 and a long-term memory test at D12. We found that the rotarod treatment impaired the learning performance of WT mice, as the WT + RR group took much longer to find the escape hole at D4 (Figure 3D, upper). The learning performance of the Tlr7–/y–RR group was similar to that of the WT–RR group (Figure 3D, upper, D4). Consistent with their better performance in contextual fear memory, Tlr7–/y + RR mice took less time to find the escape hole than non-rotarod-trained counterparts (i.e. Tlr7–/y–RR) at D1 and D2 or the WT + RR group from D2 to D4 (Figure 3D, upper). Performances of these four groups in both short-term and long-term memory tests were similar to each other (Figure 3D, lower).
Together, the results of our fear conditioning and Barnes maze tests demonstrate that rotarod pretreatment may impair the spatial learning ability of WT mice but can enhance the spatial learning performance of Tlr7–/y mice, indicating that Tlr7 deletion alters the response of mice to rotarod training, especially with regard to spatial learning.
Rotarod training differentially enhances neurogenesis and neural differentiation in WT and Tlr7–/y mice
Next, we investigated the effect of rotarod training on neurogenesis and differentiation of the hippocampal dentate gyrus (Figure 3A (3) and (4)). After the last day of rotarod training, we intraperitoneally injected bromodeoxyuridine (BrdU) into mice to label newborn neurons. Twenty-four hours after BrdU treatment, we observed that numbers of BrdU + cells were increased in both WT and Tlr7–/y mice with rotarod training (Figures 3E and 3F). The majority of those BrdU + cells were also doublecortin (DCX)-positive (Figures 3E and 3F), confirming that BrdU labeled newly generated neurons. Thus, neurogenesis was enhanced by rotarod training in both WT and Tlr7–/y mice. However, when we performed BrdU staining 4 weeks later (Figure 3A (4)), we found that the WT + RR group presented reduced numbers of BrdU + cells and BrdU + NeuN + cells compared with the other three groups (Figures 3E and 3F). Since NeuN labels differentiated neurons (Gusel'nikova and Korzhevskiy, 2015), these results suggest that although rotarod training promoted neurogenesis in WT mice, the newborn neurons could not differentiate and integrate into the brain circuitry. In contrast, numbers of both BrdU + cells and BrdU + NeuN + cells of the Tlr7–/y + RR group were comparable to those of the WT–RR and Tlr7–/y–RR groups (Figures 3E and 3F). Together, these data suggest that Tlr7 deletion neutralizes the negative effect of rotarod training on neural differentiation.
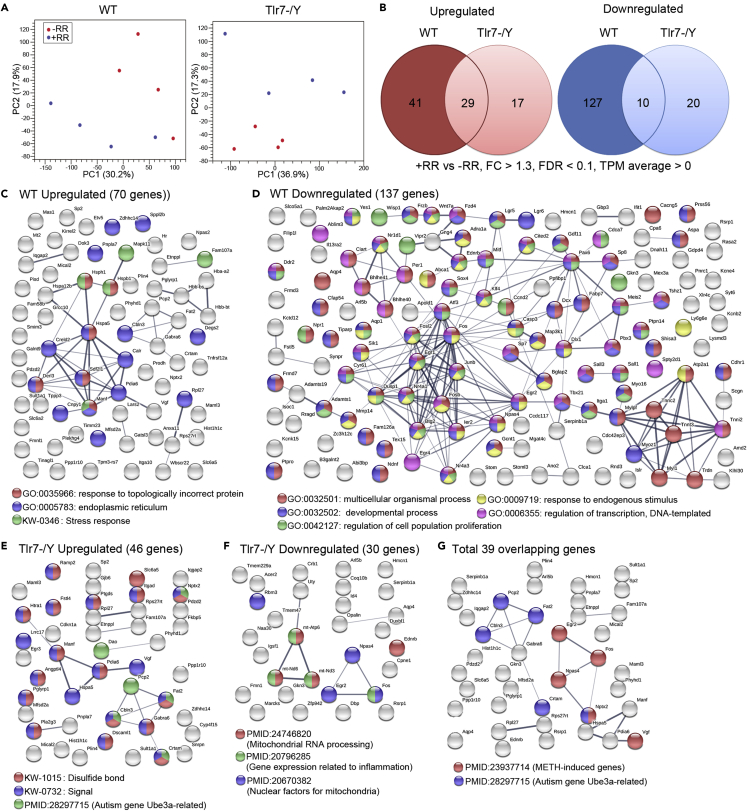
Rotarod training differentially alters the transcriptomic profiles of WT and Tlr7–/y mice
To characterize the molecular features of rotarod-induced stress and the effect of Tlr7 knockout, we analyzed the transcriptomic profiles of the WT–RR, WT + RR, Tlr7–/y–RR and Tlr7–/y + RR groups. Four mice were used for each group. Three hours after undergoing rotarod training or mock control, total RNA was isolated from mouse cortices and hippocampi and analyzed using RNA-seq. Principle component analysis (PCA) indicated that rotarod training indeed elicits differential gene expression in WT and Tlr7–/y mouse brains (Figure 4A). Based on criteria of fold-change > 1.3, a false discovery rate <0.1 and average transcripts per million (TPM) > 0 (in each group), we identified 70 upregulated and 137 downregulated genes in WT brains (Figure 4B, Table S4). For Tlr7–/y mice, 46 and 30 up- and downregulated genes were influenced by rotarod training, respectively (Figure 4B, Table S4). Only 29 upregulated and 10 downregulated genes overlapped between the WT and Tlr7–/y groups (Figure 4B, Table S5). These data suggest that Tlr7 knockout alters gene expression caused by rotarod training.
Figure 4.
Transcriptomic profiles and networks are differentially altered by rotarod training in WT and Tlr7–/y mice
(A) Principal component analysis of RNA-seq data from WT and Tlr7–/y mouse brains with/without rotarod training.
(B) Venn diagram showing overlap of upregulated (left) and downregulated (right) genes between WT and Tlr7–/y mice following rotarod training. Gene lists are provided in Tables S1 and S2. FC, fold-change; FDR, false discovery rate; TPM, transcripts per million.
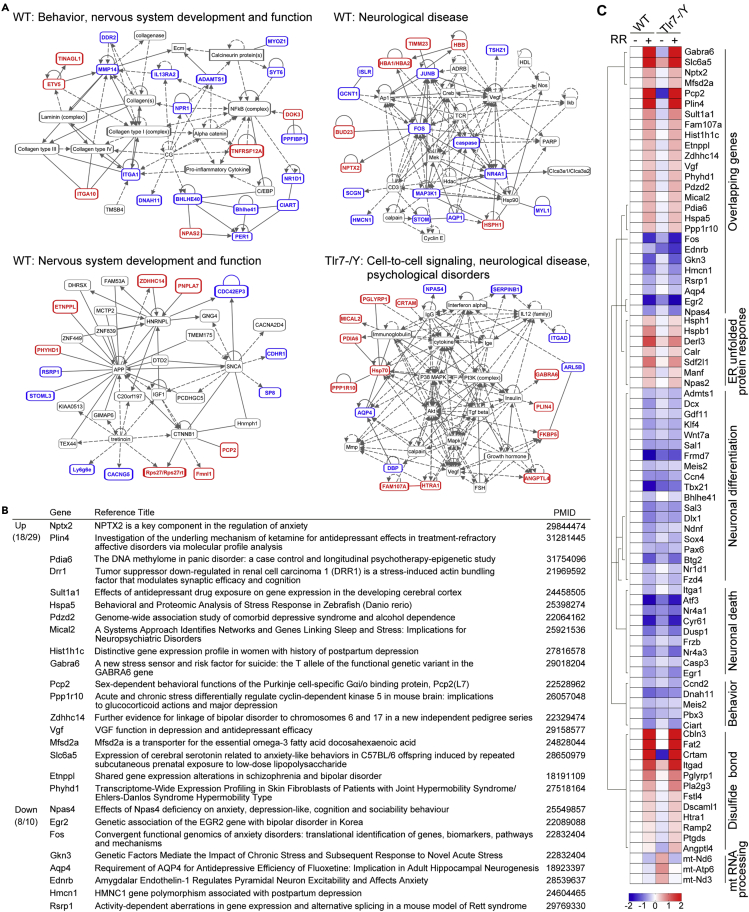
(C–G) Protein interaction networks analyzed by STRING. Node color represents an association with the different networks indicated at the bottom. Nodes with multiple colors indicate associations with multiple networks.
We employed STRING to analyze the differentially expressed genes (DEG). In WT mice, “response to topologically incorrect protein”, “ER” and “stress response” were three notable gene ontology (GO) terms for upregulated DEG (Figure 4C), suggesting that rotarod training induced a stress response even at molecular levels. For downregulated DEG in WT mice, GO terms were highly relevant to development, cell proliferation, response to endogenous stimulation, and transcriptional regulation (Figure 4D), consistent with the effect of rotarod training on altering neural differentiation and neurogenesis (Figures 3E and 3F). This outcome further indicates that rotarod training impairs responses to stimulation and transcriptional regulation and that it likely results in defective neurogenesis and differentiation in WT brains. For Tlr7–/y mice, STRING analysis did not reveal any notable GO terms, except that many upregulated DEG contained a signal peptide or a disulfide bond (Figures 4E and 4F). For the combined 39 overlapping DEG shared by WT and Tlr7–/y mice, no particular GO terms were apparent (Figure 4G).
Next, we used Ingenuity Pathway Analysis (IPA) to characterize the identified DEG. We found that the DEG of WT mice were associated with several protein networks, with three of them controlling behavior, nervous system development and function, and neurological disorders (Figure 5A), echoing the phenotype of WT mice that underwent the rotarod treatment. For Tlr7–/y mice, only one network was apparently relevant to behavioral features, i.e., that related to cell-to-cell signaling and neurological and psychological diseases (Figure 5A). Together, these analyses suggest that rotarod treatment elicits differential gene expression in WT and Tlr7–/y mice, but Tlr7–/y mice seem to be less sensitive to the impacts of rotarod training.
Figure 5.
Genes regulated by rotarod training are highly correlated with neurological functions and diseases
(A) Ingenuity Pathway Analysis (IPA) of genes regulated by rotarod training in WT and Tlr7–/y mouse brains. Both upregulated (red) and downregulated (blue) genes were combined for IPA. The functions of each network are indicated. Black font depicts genes unaffected by rotarod training but that interact with identified differentially expressed genes (DEG).
(B) Overlapping DEG are highly relevant to anxiety. Eighteen and eight genes out of a total of 29 upregulated and 10 downregulated genes, respectively, are associated with anxiety. The related manuscripts and their PMIDs are indicated.
(C) Heatmap of DEG. Color intensity represents relative TPM levels (log2 values) normalized to the WT–RR group. Gene groupings are also indicated. The color-coded bar represents the Z score.
Overlapping DEG are relevant to anxiety
Since we found that rotarod training induces anxiety in both WT and Tlr7–/y mice, we expected that the overlapping DEG would have some relevance to anxiolytic behaviors. However, neither STRING nor IPA analyses presented an association of overlapping DEG with anxiety. Apart from Il6, we anticipated that more genes relevant to anxiety are regulated in both WT and Tlr7–/y mice. Therefore, we manually annotated the overlapping DEG by searching PubMed (https://www.ncbi.nlm.nih.gov). Of the 39 overlapping DEG, 26 (66%) of them have been associated with anxiety, depression or other mental disorders in patient studies and/or animal models (Figure 5B). Thus, together with IL-6, these 26 overlapping DEG appear to control expression of anxiety to rotarod training.
Tlr7 deletion diminishes the negative effect of rotarod training on expression of genes related to memory and neurogenesis
We then investigated how Tlr7 deletion benefits spatial learning and memory in mice. We speculated that genes less sensitive or even resistant to rotarod treatment in Tlr7–/y mice would more likely be relevant to the better learning performance of the Tlr7–/y + RR group. We used heatmaps to compare gene expression among the four groups of mice. We observed very similar expression levels of the 39 overlapping DEG in the WT + RR and Tlr7–/y + RR groups (Figure 5C). For genes belonging to WT-specific GO, such as ER unfolded protein response, neuronal differentiation, cell death and behaviors, the WT + RR and Tlr7–/y + RR groups still presented the same expression tendency (either up- or down-regulation) (Figure 5C). Those genes were not present in the list of Tlr7–/y DEG because their expression was already reduced in the Tlr7–/y–RR group. The effect of rotarod treatment was therefore diminished when we compared the Tlr7–/y–RR and Tlr7–/y + RR groups. Nevertheless, this heatmap comparison allowed us to observe that expressions of some genes, such as Tbx21, Frmd7 and others, were indeed less sensitive to rotarod training in Tlr7–/y mice (Figure 5C).
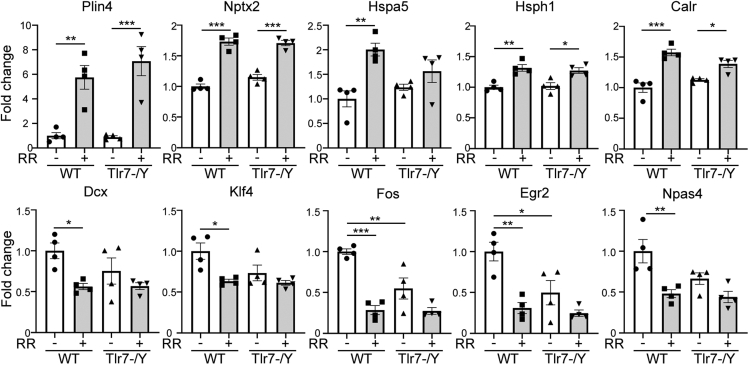
To verify the results of our RNA-seq analysis, we analyzed via quantitative RT-PCR the expression levels of a total of ten genes, including Plin4, Nptx2, Hspa5, Hsph1, Calr, Dcx, Klf4, Fos, Egr2, and Npas4. PLIN4, NPTX2, HSPA5, FOS, EGR2, and NPAS4 are all relevant to anxiety (Figure 5B). HSPA5, HSPH1 and CALR also participate in the ER unfolded protein response (Figure 5C). DCX and KLF4 are involved in neuronal differentiation (Figure 5C). It is also well known that FOS, EGR2, and NPAS4 contribute to neuronal activation. The results of quantitative RT-PCR were generally consistent with the results of RNA-seq (Figure 6), confirming the reliability of our RNA-seq analysis.
Figure 6.
Quantitative RT-PCR verifies differential expression of DEGs identified from RNA-seq
A total of ten DEGs were selected from the heatmap shown in Figure 5C for quantitative RT-PCR. Data represent mean ± SEM. The results of individual animals are also shown. The sequences of primers and the numbers of Universal probes for PCR are available in Table S6. two-way ANOVA with Bonferroni's multiple comparisons test showed that rotarod treatment altered expression of all ten examined genes compared with mock control. The results of post-testing on significant differences are indicated. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001. Sample size N = 4.
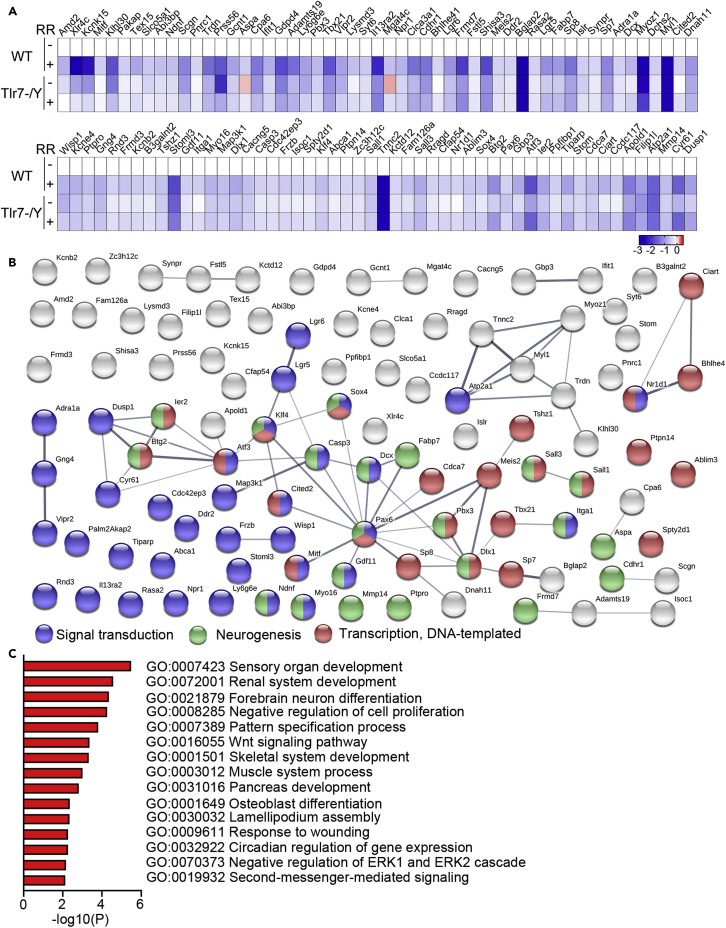
We further applied the same heatmap analysis to the total of 137 downregulated DEG in WT mice and found that 105 of them presented smaller changes in the Tlr7–/y + RR group relative to the WT + RR group, i.e., the ratio of normalized Tlr7–/y + RR expression to normalized WT + RR expression was greater than 1 (Figure 7A; Table S6). Based on STRING and Metascape analyses, these 105 rotarod-resistant genes were highly relevant to signal transduction, neurogenesis, neuron differentiation, development and transcription (Figures 7B and 7C). More specifically, thirteen of these rotarod-resistant genes have been found to play roles in learning and memory (Table S7, red font), ten are relevant to neurogenesis (Table S7, green font), and thirty-nine are involved in neuronal and/or brain functions (Table S7, brown font). These results indicate that Tlr7 deletion exerts a protective effect to regulate the gene expression controlled by rotarod training. Altered expression of these rotarod-resistant genes in Tlr7–/y mice may regulate the activity of other DEG in the network (Figure 7B) to influence learning and memory of mice.
Figure 7.
Tlr7 deletion influences the expression of DEG regulated by rotarod training
(A) Heatmaps of downregulated DEG. The genes listed here have expression ratios of Tlr7–/y + RR to WT + RR greater than 1 (Table S3). Relative expression levels (log2) were normalized to WT–RR. The color-coded bar represents the Z score.
(B) STRING analysis of the protein networks of selected genes (the same gene set in A). Node color represents an association with the different networks indicated at the bottom.
(C) Gene ontology analysis of selected genes assessed using Metascape.
Discussion
In the current report, we show that rotarod training, a regular test for motor coordination and balance, are actually able to induce anxiety and alter spatial learning/memory of mice via at least two different mechanisms. One is an IL-6-dependent pathway, which is involved in rotarod-induced anxiety. The other is via TLR7-regulated gene expression, which is critical for spatial learning/memory. Our study strengthens the role of neuronal innate immunity in controlling emotion and cognitive function and also unexpectedly reveals an impact of rotarod training on brain function. Since rotarod training influences anxiety and spatial learning/memory, caution should be taken when rotarod test and other behavioral assays are being arranged to analyze the same animals. These results also imply that exercise training might not always be positive for cognitive and emotional responses. Stressful duress in exercise training may result in negative effects. Although it is unclear if stressful exercise training also results in negative effects in human, we advocate carefully designing training programs to avoid potential negative effects.
Although our study suggests that rotarod-dependent regulation is highly relevant to IL-6 and TLR7, two important innate immune regulators, neither astrocytes nor microglial cells were activated by rotarod training. Thus, the effect of IL-6 and TLR7 may primarily act on neurons. Indeed, previous findings have indicated that neurons express various cytokines, including IL-6 (Hung et al., 2018a; Liu et al., 2013; Wu et al., 2016) and different TLRs, such as TLR3, TLR7, and TLR8 (Chen et al., 2017; Hung et al., 2018b; Liu et al., 2015). It is know that IL-6 regulates neuronal morphology (Liu et al., 2013) and anxious behaviors (Lazaridou et al., 2018; O'Donovan et al., 2010). TLR3, TLR7 and TLR8 all cell-autonomously fine-tune neuronal morphology, including axonal and dendritic growth and/or dendritic spine formation (Chen et al., 2017; Hung et al., 2018b; Liu et al., 2013). Rotarod training likely controls expression and/or activity of IL-6 and TLR7, thereby influencing anxiety and spatial learning/memory of mice.
Note, the impact of rotarod training and TLR7 on different spatial memory tests varied. For WT mice, rotarod pretraining did not affect the spatial memory in contextual fear conditioning and Barnes maze assays, but it did slow down the learning process in Barnes maze. However, upon Tlr7 knockout, rotarod pretreatment enhanced contextual fear memory and facilitated spatial learning in Barnes maze. Thus, Tlr7 knockout appears to neutralize or even reverse the negative effect of rotarod training on spatial learning/memory. Our transcriptomic analyses further indicate that the beneficial effect of rotarod training on learning and memory in Tlr7–/y mice likely arises through altered expression of genes controlling neurogenesis and mouse behaviors.
In our experiment, the mice performed rotarod pretraining at least two weeks before undertaking other behavioral assays. Thus, the effect of rotarod training on anxiety and spatial learning/memory is long-lasting. Many genes involved in the ER-related unfolding protein response were upregulated 3 hr after rotarod training, echoing our observation that rotarod exercise does indeed induce a stress response, at least in the cortex and hippocampus. Based on our transcriptomic profiling, we noticed that several transcriptional regulators were impacted by rotarod training, suggesting that altered regulation of gene expression is likely involved in the long-lasting effect of forced rotarod exercise. Since our data indicate an involvement of TLR7 and given that our previous study demonstrated that TLR7 activation in neurons can evoke the MYD88-dependent pathway to induce C-FOS expression (Liu et al., 2013), TLR7 likely contributes to the transcriptional regulation induced by rotarod exercise. Moreover, TLR7 can recognize endogenous miRNA (Lehmann et al., 2012; Liu et al., 2015). Therefore, since miRNA can be transported via exosomes to activate TLR7 (Lehmann et al., 2012; Liu et al., 2015), it would be very intriguing to explore if miRNAs/exosomes represent critical mediators of how forced rotarod exercise controls TLR7 activation and consequently influence transcriptional and behavioral changes.
For typical forced exercise, treadmill is a popular model for rodents (Contarteze et al., 2008; Svensson et al., 2016). It is a very stressful treatment that induces global inflammation in both brain and peripheral tissues (Cook et al., 2013; Nambot et al., 2020; Svensson et al., 2016). In this report, we found that rotarod testing also influence Il-6 expression in cortices and hippocampi. The rotarod test is a much milder task compared to treadmill, as we found that it did not trigger cytokine expression in mouse spleen. However, the duress of being placed on the moving rod is sufficient to increase Il6 mRNA levels in the cortex and hippocampus and it has a long-term impact on mouse behaviors. Thus, a rotarod test can serve as a relatively specific model to investigate the effect of forced exercise-induced stress on brains.
Limitations of the study
First, although we have demonstrated the involvement of IL-6 and TLR7 in rotarod-regulated anxiety and spatial learning/memory, we are still unsure how rotarod training modulates gene expression, including of Il-6. The signal pathway operating downstream of rotarod training remains unclear. Second, we have focused on the effect of rotarod training on the cortex and hippocampus because these two brain regions are highly relevant to anxiety and spatial learning/memory. Since it is unclear if any specific region accounts for the effect of rotarod training, we pooled cortex and hippocampus together for analysis. We have identified and validated several DEGs from the mixed hippocampus and cortex samples in this report. It would be illuminating to further explore more specific brain regions, such as dorsal or ventral hippocampi and somatosensory, cingulate or motor cortices, and also to investigate the role of other brain regions. Third, we found that rotarod training at 5 weeks old had a stronger effect on anxiety and locomotion compared to training at 10 weeks old. Thus, rotarod training at 5 weeks could likely serve as a model to investigate how early life experiences influence emotion and cognitive activity in adults, warranting further investigation. Finally, we used male mice in the current report. We do not know whether female mice are also sensitive or even more susceptible to rotarod training. Given that the prevalence of anxiety disorders is much higher for women than men (https://www.texashealth.org/Health-and-Wellness/Behavioral-Health/How-Anxiety-Affects-Men-and-Women-Differently), it would be intriguing to compare the responses of female and male mice to rotarod training. Thus, sex-biased effects need to be considered in future studies.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Yi-Ping Hsueh (yph@gate.sinica.edu.tw).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement of Academia Sinica.
Data and code availability
The accession number for the RNA-seq reported in this paper is NCBI BioProject: PRJNA702827.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
We thank the Genomics Core, the Bioinformatics Core and the Animal Facility of the Institute of Molecular Biology, Academia Sinica, for excellent technical assistance, and Dr. John O'Brien for English editing. This work was supported by grants from Academia Sinica (AS-IA-106-L04) and the Ministry of Science and Technology (MOST 108-2311-B-001-008-MY3) to Y.-P. Hsueh.
Author contributions
Conceptualization, Y.-F.H., and Y.-P.H.; methodology and investigation, Y.-F.H.; writing, Y.-F.H., and Y.-P.H.; funding acquisition, Y.- P.H.; supervision and project administration, Y.-P.H.
Declaration of interests
The authors declare no competing interests.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102384.
Supplemental information
References
- Akira S., Sato S. Toll-like receptors and their signaling mechanisms. Scand. J. Infect. Dis. 2003;35:555–562. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- Bettio L., Thacker J.S., Hutton C., Christie B.R. Modulation of synaptic plasticity by exercise. Int. Rev. Neurobiol. 2019;147:295–322. doi: 10.1016/bs.irn.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Bilbo S.D., Block C.L., Bolton J.L., Hanamsagar R., Tran P.K. Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 2018;299:241–251. doi: 10.1016/j.expneurol.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgueño J.F., Abreu M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:263–278. doi: 10.1038/s41575-019-0261-4. [DOI] [PubMed] [Google Scholar]
- Caputi V., Giron M.C. Microbiome-gut-brain Axis and toll-like receptors in Parkinson's disease. Int. J. Mol. Sci. 2018;19:1689. doi: 10.3390/ijms19061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., Liu H.Y., Hsueh Y.P. TLR3 downregulates expression of schizophrenia gene Disc1 via MYD88 to control neuronal morphology. EMBO Rep. 2017;18:169–183. doi: 10.15252/embr.201642586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., Shih Y.C., Hung Y.F., Hsueh Y.P. Beyond defense: regulation of neuronal morphogenesis and brain functions via Toll-like receptors. J. Biomed. Sci. 2019;26:90. doi: 10.1186/s12929-019-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarteze R.V., Manchado Fde B., Gobatto C.A., De Mello M.A. Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2008;151:415–422. doi: 10.1016/j.cbpa.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cook M.D., Martin S.A., Williams C., Whitlock K., Wallig M.A., Pence B.D., Woods J.A. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav. Immun. 2013;33:46–56. doi: 10.1016/j.bbi.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Moon H.Y., van Praag H. On the run for hippocampal plasticity. Cold Spring Harb. Perspect. Med. 2018;8:a029736. doi: 10.1101/cshperspect.a029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel Z., Haditsch U., Palmer T.D., Azpiroz A., Sapolsky R.M. Adult-generated neurons born during chronic social stress are uniquely adapted to respond to subsequent chronic social stress. Mol. Psychiatry. 2019;24:1178–1188. doi: 10.1038/s41380-017-0013-1. [DOI] [PubMed] [Google Scholar]
- de Quervain D., Schwabe L., Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat. Rev. Neurosci. 2017;18:7–19. doi: 10.1038/nrn.2016.155. [DOI] [PubMed] [Google Scholar]
- Delezie J., Handschin C. Endocrine crosstalk between skeletal muscle and the brain. Front. Neurol. 2018;9:698. doi: 10.3389/fneur.2018.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:2475–2484. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P.W., Licinio J., Pavlatou M.G. Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-γ systems. Mol. Psychiatry. 2013;18:154–165. doi: 10.1038/mp.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P.W., Machado-Vieira R., Pavlatou M.G. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plast. 2015;2015:581976. doi: 10.1155/2015/581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusel'nikova V.V., Korzhevskiy D.E. NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Nat. 2015;7:42–47. [PMC free article] [PubMed] [Google Scholar]
- Hamilton G.F., Rhodes J.S. Exercise regulation of cognitive function and neuroplasticity in the healthy and diseased brain. Prog. Mol. Biol. Transl Sci. 2015;135:381–406. doi: 10.1016/bs.pmbts.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y.F., Chen C.Y., Li W.C., Wang T.F., Hsueh Y.P. Tlr7 deletion alters expression profiles of genes related to neural function and regulates mouse behaviors and contextual memory. Brain Behav. Immun. 2018;72:101–113. doi: 10.1016/j.bbi.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Hung Y.F., Chen C.Y., Shih Y.C., Liu H.Y., Huang C.M., Hsueh Y.P. Endosomal TLR3, TLR7, and TLR8 control neuronal morphology through different transcriptional programs. J. Cell Biol. 2018;217:2727–2742. doi: 10.1083/jcb.201712113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Cousins L., Deakin J., Lennox B.R., Yolken R., Jones P.B. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2:258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I., Chicha L., Britschgi M., Schobel S.A., Bodmer M., Hellings J.A., Toovey S., Prinssen E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Lazaridou A., Martel M.O., Cahalan C.M., Cornelius M.C., Franceschelli O., Campbell C.M., Haythornthwaite J.A., Smith M., Riley J., Edwards R.R. The impact of anxiety and catastrophizing on interleukin-6 responses to acute painful stress. J. Pain Res. 2018;11:637–647. doi: 10.2147/JPR.S147735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S.M., Kruger C., Park B., Derkow K., Rosenberger K., Baumgart J., Trimbuch T., Eom G., Hinz M., Kaul D. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- Lin C., Zhao S., Zhu Y., Fan Z., Wang J., Zhang B., Chen Y. Microbiota-gut-brain axis and toll-like receptors in Alzheimer's disease. Comput. Struct. Biotechnol. J. 2019;17:1309–1317. doi: 10.1016/j.csbj.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.Y., Chen C.Y., Hsueh Y.P. Innate immune responses regulate morphogenesis and degeneration: roles of Toll-like receptors and Sarm1 in neurons. Neurosci. Bull. 2014;30:645–654. doi: 10.1007/s12264-014-1445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.Y., Hong Y.F., Huang C.M., Chen C.Y., Huang T.N., Hsueh Y.P. TLR7 negatively regulates dendrite outgrowth through the Myd88-c-Fos-IL-6 pathway. J. Neurosci. 2013;33:11479–11493. doi: 10.1523/JNEUROSCI.5566-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.Y., Huang C.M., Hung Y.F., Hsueh Y.P. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp. Neurol. 2015;269:202–212. doi: 10.1016/j.expneurol.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Nambot S., Faivre L., Mirzaa G., Thevenon J., Bruel A.L., Mosca-Boidron A.L., Masurel-Paulet A., Goldenberg A., Le Meur N., Charollais A. De novo TBR1 variants cause a neurocognitive phenotype with ID and autistic traits: report of 25 new individuals and review of the literature. Eur. J. Hum. Genet. 2020;28:770–782. doi: 10.1038/s41431-020-0571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A., Hughes B.M., Slavich G.M., Lynch L., Cronin M.T., O'Farrelly C., Malone K.M. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav. Immun. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019;15:383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- Pittenger C., Duman R.S. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Rendeiro C., Rhodes J.S. A new perspective of the hippocampus in the origin of exercise-brain interactions. Brain Struct. Funct. 2018;223:2527–2545. doi: 10.1007/s00429-018-1665-6. [DOI] [PubMed] [Google Scholar]
- Ryan S.M., Nolan Y.M. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: can exercise compensate? Neurosci. Biobehav Rev. 2016;61:121–131. doi: 10.1016/j.neubiorev.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Sommer F., Bäckhed F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Spiljar M., Merkler D., Trajkovski M. The immune system bridges the gut microbiota with systemic energy homeostasis: focus on tlrs, mucosal barrier, and SCFAs. Front. Immunol. 2017;8:1353. doi: 10.3389/fimmu.2017.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M., Rosvall P., Boza-Serrano A., Andersson E., Lexell J., Deierborg T. Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol. Stress. 2016;5:8–18. doi: 10.1016/j.ynstr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Soto C., Yoo S., Sodoma M., Vivar C., van Praag H. Exercise and hippocampal memory systems. Trends Cogn. Sci. 2019;23:318–333. doi: 10.1016/j.tics.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.J., Liu H.Y., Huang T.N., Hsueh Y.P. AIM 2 inflammasomes regulate neuronal morphology and influence anxiety and memory in mice. Sci. Rep. 2016;6:32405. doi: 10.1038/srep32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu J.H., Dorweiler B., Woo C.W. Interaction between gut microbiota and toll-like receptor: from immunity to metabolism. J. Mol. Med. (Berl) 2017;95:13–20. doi: 10.1007/s00109-016-1474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the RNA-seq reported in this paper is NCBI BioProject: PRJNA702827.