Highlights
-
•
This study examined the resting-state functional network connectivity underlying eating disorder symptoms in a large sample of healthy young adults (n = 693).
-
•
Individuals with higher levels of eating disorder symptoms displayed weaker intra-network connectivity of the executive control network and basal ganglia network, as well as weaker inter-network connectivity in the three examined networks (i.e., the executive control network, basal ganglia network, and default mode network).
-
•
The findings suggest that these neural circuits may play a key role in symptoms of disordered eating in healthy adults. They further reveal that the less efficient information exchange within and between intrinsic networks associated with self-referential thinking, inhibitory control, and reward sensitivity are strongly related to eating disorder symptoms.
Abbreviations: EDDS, Eating disorder diagnosis scale; AN, Anorexia nervosa; BN, Bulimia nervosa; BED, Binge-eating disorder; SD, Standard deviation; BMI, Body mass index; rs-fMRI, Resting-state functional magnetic resonance imaging; RSN, Resting-state network; TPN, Task-positive network; TNN, Task-negative network; FNC, Functional network connectivity; ROI, Region of interest; FC, Functional connectivity; FD, Framewise displacement; BOLD, Blood oxygen level-dependent; DVARS, Temporal Derivative of root mean square VARiance over voxelS; VAN, Ventral attention network; SMN, Sensorimotor network; SN, Salience network; ASN, Anterior salience network; PSN, Posterior salience network; ECN, Executive control network; LECN, Left executive control network; RECN, Right executive control network; DMN, Default mode network; BGN, Basal ganglia network; PCUN, Precuneus network; VSN, Visuospatial network; MFG, Middle frontal gyrus; SFG, Superior frontal gyrus; AG, Angular gyrus; IFG, Inferior frontal gyrus; PCC, Posterior cingulate cortex; PHG, Parahippocampal gyrus; MOG, Middle occipital gyrus; TMS, Transcranial magnetic stimulation; tDCS, Transcranial direct current stimulation
Keywords: Eating disorder symptoms, Resting-state network, Functional network connectivity, Inhibitory control, Reward sensitivity, Self-referential thinking
Abstract
Previous neuroimaging research of eating disorders such as anorexia nervosa and bulimia nervosa has mainly focused on clinical patients, indicating the crucial role of intrinsic connectivity networks involved in aberrant behavioral control (i.e., executive control network), reward reactivity (i.e., basal ganglia network), and excessive self-focused and body-focused ruminations (i.e., default mode network) in the onset and maintenance of eating disturbances. However, examinations of large-scale resting-state networks that support the role of cognitive control, reward sensitivity, and self-directed thinking in disordered eating have rarely involved non-clinical samples from the general population. This study, involving a total of 693 healthy young adults (68.69% females; mean age, 18.37 years), investigated these issues by using pre-defined functional regions of interest from the executive control network, basal ganglia network, default mode network, and a seed-based region of interest-to-region of interest approach. After statistically controlling for differences in age, sex, body mass index, and head motion, we observed significant associations of higher levels of eating disorder symptoms, especially bulimia-type eating (i.e., binge eating and a combination of binge eating and compensatory behaviors, such as purging via self-induced vomiting or laxative use, and compulsive exercise), with weaker intra-network and inter-network functional synchrony. These results remained significant after excluding underweight, overweight, and obese participants. These findings suggest that these neural circuits may play a key role in the symptoms of disordered eating in healthy adults. They further reveal that the less efficient information exchange within and between intrinsic networks associated with self-referential thinking, inhibitory control, and reward sensitivity are strongly related to eating disorder symptoms.
1. Introduction
Eating disorders and associated disordered eating behaviors are relatively common serious mental health problems that considerably affect physical health and psychosocial behavior (Sparti et al., 2019, Treasure et al., 2020). In particular, eating disorders have been associated with alterations and impairments in executive functions and reward sensitivity, as well as in self-referential thinking (Domakonda et al., 2019, Park et al., 2018, Steward et al., 2018).
The functionality of the brain networks/circuits underlying maladaptive eating is of interest to those seeking a clearer understanding of neurobiological mechanisms involved in eating disorders (for reviews, see Monteleone et al., 2018, Steward et al., 2018). Several intrinsic connectivity networks that may drive disordered eating and perpetuate eating disorders, such as the default mode network (DMN) (Boehm et al., 2014, Stopyra et al., 2019), executive control network (ECN) (Boehm et al., 2014, Park et al., 2018), reward network (i.e., basal ganglia network [BGN]) (Monteleone et al., 2018, Steward et al., 2018), ventral attention network (VAN) (Domakonda et al., 2019), and sensorimotor network (SMN) (McFadden et al., 2014) have been identified. Recent studies have sought to further reveal how these neural networks contribute to abnormal eating (e.g., Domakonda et al., 2019, McFadden et al., 2014). It has been suggested that the involvement of the DMN, ECN, and BGN represents the crucial neural substrates that could explain the roles of self-referential processing (e.g., records of bodily sensation and self), executive functioning (e.g., inhibitory control), and reward processing (e.g., reward sensitivity) in the development and maintenance of eating disorders (Esposito et al., 2018, Kessler et al., 2016, Monteleone et al., 2018). However, the neurocircuitry underlying symptoms of disordered eating before the development of severe psychiatric disorders (anorexia nervosa [AN]) are not yet sufficiently defined. Regarding non-clinical populations, the functional networks that support the roles of cognitive control, self-relevant mentalizing and interoception, and reward valuation in disordered eating have been understudied. Behavioral data of healthy subjects have suggested that individuals with more eating disorder symptoms are prone to having more negative self-evaluations and altered behavioral control and reward sensitivity (Brooks et al., 2012, Eneva et al., 2017, Goldschmidt et al., 2018, Williams and Levinson, 2020). Importantly, several major brain systems, such as the dopamine mesolimbic circuit that is related to reward sensitivity and the frontal cognitive networks sustaining dietary self-control, have been shown to possibly influence maladaptive eating in non-clinical conditions (Bartholdy et al., 2019, Park et al., 2018, Vainik et al., 2019). Although previous neuroimaging research of eating disorders has mainly focused on clinical patients, some changes in their brain structure may be related to states of dehydration or malnutrition; therefore, the precise eating disorder pathophysiological mechanisms are still difficult to clarify (King et al., 2018, Monteleone et al., 2018). Because early symptomatic behavior is predictive of the later development of clinical disorders (Kotler et al., 2001), and because understanding the relationship between eating-related psychopathology and particular neural circuits may provide a foundation for developing more targeted and effective prevention strategies, it is of critical importance to determine the functionally connected brain networks related to disordered eating in the general population.
Resting-state networks (RSN) are defined by strong temporal coherence; therefore, it is believed that the brain regions in each of these networks are functionally connected and serve a common purpose (van den Heuvel and Hulshoff Pol, 2010). The ECN is a major task-positive network (TPN) related to disordered eating that comprises a medial-frontal system that includes the anterior cingulate cortex and para-cingulate cortex, which underlie executive functioning such as working memory, goal-oriented cognition, and impulse control (Barkhof et al., 2014, Smith et al., 2009). Additionally, the BGN (e.g., caudate, putamen) and several relevant circuits (e.g., the frontostriatal circuits) have key roles in the reward-based eating drive and reward sensitivity (Donnelly et al., 2018, Frank, 2013, McFadden et al., 2014, Monteleone et al., 2018). Conversely, the DMN is a task-negative network (TNN) that supports self-referential thinking, memory encoding and retrieval, and social reasoning (Greicius et al., 2003). The DMN comprises the medial prefrontal cortex, posterior cingulate cortex, precuneus, hippocampus, and inferior parietal cortex (Raichle et al., 2001). In healthy individuals, activation/activity in these networks (i.e., between TPN and TNN) is typically negatively correlated (i.e., anti-correlated) during cognitive tasks and at rest. The strength of anti-correlation increases over the course of the developmental span from childhood to early adulthood. This is critical in light of recent evidence that has suggested that normal brain development is related to the magnitude of negatively correlated or “push–pull” activity among major cerebral networks (Chai et al., 2014, Fox et al., 2005, Uddin et al., 2009).
Resting-state functional magnetic resonance imaging (rs-fMRI) reflects intrinsic interactions between functionally connected (i.e., temporally correlated) brain regions of interest (ROI), thereby allowing the investigation of functional brain organization not confounded by the task type or stimulus (Raichle et al., 2001). Previous rs-fMRI studies have examined altered connectivity patterns of severe eating disorders (AN, bulimia nervosa [BN]) in clinical patients and reported dysfunctions in the DMN involved in self-focused and body-focused thoughts and in the ECN responsible for cognitive control (Boehm et al., 2014, Cowdrey et al., 2014, Gaudio et al., 2016, Stopyra et al., 2019). By focusing on the functional architecture between large-scale RSN through more broadly functional network connectivity (FNC) analyses, researchers have further explored the cross-network interactions that may be related to eating disorders (Boehm et al., 2016, Domakonda et al., 2019, Uniacke et al., 2019). Uniacke et al. (2019) demonstrated reduced connectivity between the ECN and salience network (SN) among AN patients relative to healthy controls. However, Boehm et al. (2016) did not find this significant group difference in cross-network connectivity (i.e., ECN-SN, ECN-DMN).
Comparative research of the link between disordered eating and functional brain network organization in healthy subjects among the general population is lacking (for a review, see Donofry et al., 2020). Shapiro et al. (2019) recently investigated associations among FNC and disinhibited eating behaviors (i.e., overeating) of healthy children 4 to 6 years of age; their study showed that stronger within-BGN connectivity was correlated with more overeating. Significantly inverse correlations were further observed between overeating and cross-network synchrony (i.e., BGN-DMN, ECN-BGN), suggesting that neurobiological alterations in brain connectivity may prepare individuals for overeating via reward reactivity and inhibitory deficits that promote greater enjoyment and consumption of food (Shapiro et al., 2019). However, the sample size of that study was quite small (n = 18), and the authors did not elucidate the potential anti-correlation or positive correlation between TPN and DMN or its relation to disordered eating. Therefore, more research is warranted to reveal the essential contributions of FNC (especially the DMN, ECN, and BGN) to individual differences in disordered eating and how these networks interact (e.g., information exchange) to influence eating behaviors. Investigating connectivity within and between these circuits would deepen our understanding of the underlying neurobiological processes before clinical eating disturbances become prevalent.
This study further expanded the work in this area by examining the associations between eating disorder symptoms and the strength of functional connectivity (FC) within and between RSN in a large sample of healthy young adults (n = 693). Because of the previous clinical observations suggesting that eating disorders are related to greater functional deficits in self-reflective cognition, executive functions, and reward, it was hypothesized that higher levels of disordered eating would be related to weaker FC within the DMN, ECN, and BGN. Based on previous findings of more disordered eating linked with lesser inter-network interactions (i.e., BGN-DMN, ECN-BGN) in healthy subjects, it was further hypothesized that higher levels of disordered eating would be related to weaker cross-network FC.
2. Materials and methods
2.1. Participants
Participants selected for this study were enrolled in the Behavioral Brain Research Project of Chinese Personality. A total of 693 healthy undergraduate students (68.69% females; mean age, 18.37 years, standard deviation [SD], 0.87; 89.61% right-hand-dominant) were recruited from Chongqing (south of China). Participants fulfilled the following inclusion criteria, which were assessed via self-report, for enrollment in this study: age 17 to 25 years; absence of neurological or psychiatric disorders; no use of psychoactive medications and no other chronic diseases (binary variables indicating the presence or absence of particular diseases or disorders); high school degree or higher education; and Chinese as the primary language. Measurements were performed from September to December 2019. All participants volunteered to participate in the study without coercion and signed a written informed consent form after full written and verbal explanations of this research prior to enrollment. Participants received nominal financial compensation for their time. The research protocol was reviewed for compliance with the standards for the ethical treatment of human participants and approved by the Ethical Committee for Scientific Research at the university with which the authors are affiliated.
2.2. Measures
Demographic characteristics. Age, sex (coded as 1 for men and 2 for women), highest education level, and self-reported handedness data were collected. Body mass index (BMI) was measured using a medical body composition analyzer (M515; seca, Hamburg, Germany). BMI categories were defined according to the BMI categories for Chinese young adults (i.e., underweight [BMI < 18.5], normal weight [BMI, 18.5–23.9], overweight [BMI, 24.0–27.9], and obese [BMI ≥ 28]) reported by the Chinese Bureau of Disease Control and Prevention.
Eating disorder symptoms. The Eating Disorder Diagnosis Scale (EDDS) (Stice et al., 2000) is a 22-item self-report screen based on the Diagnostic and Statistical Manual of Mental Disorders (fourth edition) eating disorder criteria. A symptom composite calculated by summing 18 standardized EDDS items, excluding height, weight, birth control pill use, and missed menstrual periods, is internally consistent, stable, and has excellent concordance with other self-report measures of disordered eating and diagnoses based on structured interviews (Stice et al., 2000). Higher composite scores reflect more frequent symptomatic behaviors. Principal components analyses of Chinese samples of each sex found that all items loaded on one a priori factor, suggesting that the EDDS composite has a gender-equivalent univariate structure comprising all 18 items (Jackson and Chen, 2008). Validity support for the EDDS used for Chinese samples has been found for adolescent and adult samples from Chongqing (Jackson and Chen, 2010, Luo et al., 2020) and different regions of mainland China (Chen and Jackson, 2008, Jackson and Chen, 2007). Because the EDDS can be used to diagnose AN, BN, and binge-eating disorder (BED) through a scoring algorithm (for details, see Stice et al., 2000), it was further used to assess specific symptomatic behaviors distinguished by an appropriate scale (AN, 4 standardized items; BN, 9 standardized items; and BED, 13 standardized items). We reverse-scored the negatively worded items (i.e., items 15, 16, 17, 18 for BED) and then summed the ratings of the items corresponding to each eating disorder symptom. Higher total scores indicated more symptoms of AN, BN, and BED. In this study, Cronbach’s alpha for the EDDS symptom composite was 0.91, and the three specific symptom composites had adequate internal consistency (α = 0.74, 0.80, and 0.78 for AN, BN, and BED, respectively). This scale was also used to assess specific disordered eating behaviors and cognitive patterns (body image concerns: 4 standardized items, α = 0.90; loss of control eating: 10 standardized items, α = 0.94; a combination of binge eating with compensatory behaviors [i.e., purging via self-induced vomiting or laxative use, restrictive eating, and compulsive exercise]: 4 standardized items, α = 0.76).
2.3. Image acquisition and preprocessing
MRI was performed using a 3-T Trio scanner (Siemens Prisma, Erlangen, Germany). Data were obtained during an 8-minute scan using rs-fMRI and a gradient echo planar imaging sequence (parameters: repetition time [TR], 2000 ms; echo time [TE], 30 ms; slices, 62; slice thickness, 2 mm; field of view [FOV], 224 × 224 mm2; flip angle, 90°; resolution matrix, 112 × 112; voxel size, 2 × 2 × 2 mm3; phase encoding direction, PC ≫ AC). Each section contained 240 volumes. High-resolution T1-weighted structural images were acquired for coregistration purposes (parameters: TR, 2530 ms; TE, 2.98 ms; FOV, 256 × 256 mm2; flip angle, 7°; base resolution, 256 × 256; slice per slab, 192; slice oversampling, 33.3%; voxel size, 0.5 × 0.5 × 1 mm3; phase encoding direction, AC ≫ PC).
Initial preprocessing of the functional images was performed using Data Processing & Analysis of Brain Imaging software (DPABI_V2.3; http://restfmri.net/forum/DPABI), which is based on Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm/). The first 10 images were discarded for steady-state longitudinal magnetization. The remaining images were corrected for temporal shifts between slices and realigned to the middle volume. Using the EPI templates in SPM8, each image volume was spatially normalized to the Montreal Neurological Institute 152-brain template using a resolution voxel size of 3 × 3 × 3 mm3. Subsequently, several spurious covariates and their temporal derivatives were removed from the data by linear regression analysis to minimize the impact of cardiac effects and respiratory effects, including white matter, cerebrospinal fluid, and Friston-24 parameters of head motion (Friston et al., 1996, Hallquist et al., 2013). To address head motion concerns, data scrubbing was further implemented. The bad time points were regarded as regressors defined as volumes with framewise displacement (FD) power > 0.5 mm as well as the two succeeding volumes and one preceding volume to reduce the spillover effect of head motion (Power et al., 2012). Linear and quadratic trends were also included as regressors because the blood oxygen level-dependent (BOLD) signal exhibits low frequency drifts. Spatial smoothing was applied with a 4-mm full-width at half-maximum (FWHM) Gaussian kernel; then, linear trends were removed. Finally, bandpass temporal filtering (0.01 < f < 0.08) was used to remove the effects of very-low-frequency drifts and high-frequency noises.
During scanning, each participant was instructed to close the eyes, relax, and stay awake; only participants who were confirmed to have not fallen asleep were included. Foam pads and earplugs were used to minimize head movement and scanning noise. No participants performed head motion between volumes in any direction > 3 mm or rotation in any axis > 3° during scanning. The mean FD values did not exceed 0.50; therefore, no participants were excluded during preprocessing.
2.4. ROI definition
The ROI were obtained from a freely available atlas of regions defined by correlated activation patterns (http://findlab.stanford.edu/functional_ROIs.html). This atlas includes 90 ROI grouped into several networks: the dorsal and ventral DMN; the left and right ECN, BGN, and SMN; the anterior and posterior SN (ASN, PSN); auditory network; higher visual network; language network; primary visual network; visuospatial network; and precuneus network (for details, see Shirer et al., 2012). The left and right ECN, ventral DMN, and BGN ROI were applied in the current analyses (Fig. 1).
Fig. 1.
Illustration of the ROIs within the (A) ECN, (B) DMN, and (C) BGN. A total of 23 major ROI of the three networks are shown for a better presentation of the connections (i.e., edges) between these core nodes in each network without showing the left and right crus I of the ECN, the right lobule IX of the DMN, and the pons of the BGN. Images are displayed according to neurological convention; therefore, the right hemisphere corresponds to the right side in axial displays. Abbreviations: ROI, region of interest; OFG, orbitofrontal gyrus; SFG, superior frontal gyrus; MFG, middle frontal gyrus; CAU, caudate; THA, thalamus; MTG, middle temporal gyrus; AG, angular gyrus; PHG, parahippocampal gyrus; PCC, posterior cingulate cortex; PCUN, precuneus; MOG, middle occipital gyrus; IFG, inferior frontal gyrus; PUT, putamen; L, left; R, right.
2.5. Functional network connectivity analyses
FNC analyses to assess network connectivity in relation to eating disorder symptoms were performed using a seed-based ROI-to-ROI approach implemented in the CONN toolbox (version 17.f; https://www.nitrc.org/projects/conn). A detailed description of this methodology has been published in previous reports of RSN (Domakonda et al., 2019, Shapiro et al., 2019). To produce first-level correlation maps for each participant, the average BOLD time series for all voxels in each seed ROI was extracted and Fisher z-transformed. Then, pairwise bivariate correlation coefficients (i.e., Pearson’s correlation) were calculated using the time courses for each of the ROI, producing a symmetrical 27 × 27 correlation matrix (i.e., ROI-to-ROI connectivity matrix). At the second level, the resultant correlation coefficients were further correlated with EDDS scores using multiple regression analyses to test the relationship between FNC and eating disorder symptoms. Results were corrected for age, sex, BMI, and head motion (i.e., FD) and held at a threshold based on a false discovery rate of p < 0.05. Group-level estimates of ROI-to-ROI connectivity have shown a high degree of reliability (Whitfield-Gabrieli and Nieto-Castanon, 2012). Therefore, mean connectivity strength values (Z-scores) were calculated at the group level.
2.6. Exploratory analyses
Association between network connectivity and specific eating-related categories. After the aforementioned FNC analyses provided significant connectivity, the mean within-network connectivity and mean cross-network connectivity were calculated by averaging ROI-to-ROI pairwise correlations in/across each network (Domakonda et al., 2019). Pearson’s correlations of mean network connectivity with specific eating disorder diagnostic categories (AN, BN, and BED) and specific disordered eating behaviors/cognitions (i.e., body image concerns, loss of control eating, and a combination of binge eating with compensatory behaviors) were further assessed while correcting for age, sex, BMI, and FD using partial correlation. The significance levels were set at p < 0.05 (two-tailed). Statistical analyses were performed using R (Kim, 2015, Rcore, 2016).
Sex differences in network connectivity associated with eating disorder symptoms. Potential sex differences in FNC associated with EDDS scores were further assessed using an analysis of covariance model (i.e., comparing regressions of the male and female groups) implemented in the CONN toolbox. Statistical significance was set at p < 0.05 with false discovery rate correction. Age, BMI, and FD were defined as covariates of no interest.
Potential confounding effects. Because the current data showed significant correlations between BMI and EDDS scores, and because BMI was associated with resting-state functional synchrony within the DMN and ECN (Kullmann et al., 2012, Park et al., 2018), the associations between eating disorder symptoms and FNC were further assessed after excluding those participants who were underweight (BMI < 18.5), overweight (BMI, 24.0–27.9), and obese (BMI ≥ 28). The covariates and statistical thresholds were the same as those mentioned in the preceding section (Functional network connectivity analyses).
Association between other network connectivity and eating disorder symptoms. Given that other RSN (e.g., the SN; Boehm et al., 2016) and corresponding regions (e.g., the precuneus; Song et al., 2019) have also been linked to eating-related psychopathology, we further explored the associations between EDDS scores and FC within and between the ASN (7 ROI) and precuneus network (PCUN; 4 ROI). To assess whether the significant FNC patterns (i.e., ECN, BGN, DMN) inversely associated with eating disorder symptoms in the current data were specific, an unrelated network, the visuospatial network (VSN; 11 ROI), was selected as a control and EDDS scores were correlated with the FC of the VSN. The covariates and statistical thresholds were the same as those mentioned in the functional network connectivity analyses section above.
2.7. Sensitivity analyses
Sensitivity analyses using other definitions of RSN were carried out to assess the sensitivity of the current ROI (i.e., ECN, BGN, and DMN). Specifically, the ROI for the frontoparietal network (13 ROI), limbic network (5 ROI), and default network (24 ROI) were defined based on the well-established Yeo network (i.e., 100 parcellations of seven networks; https://github.com/ThomasYeoLab/CBIG/tree/master/stable_projects/brain_parcellation/Schaefer2018_LocalGlobal) and were 5-mm-diameter spherical regions centered at peak coordinates (converted to Montreal Neurosciences Institute space). The analyses to test network connectivity in relation to EDDS scores were the same as those mentioned in the Functional network connectivity analyses section.
3. Results
3.1. Demographic information and behavioral results
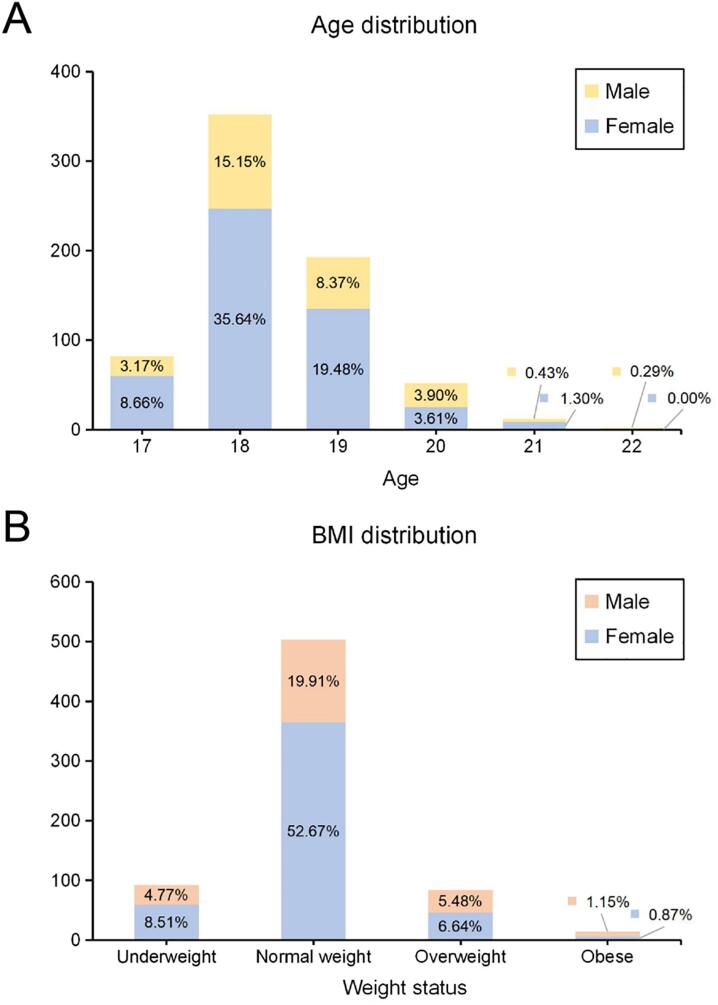
The mean age of this sample (n = 693; females, 476 [68.69%]; males, 217 [31.31%]) was 18.37 years (SD, 0.87; range, 17–22 years). The mean BMI was 21.20 (SD, 2.71; range, 14.25–34.37). Regarding weight, 13.28% (n = 92) of the participants were underweight, 72.58% (n = 503) were normal weight (BMI, 18.5–23.9), 12.12% (n = 84) were overweight (BMI, 24.0–27.9, and 2.02% (n = 14) were obese. The distributions of age and BMI are shown in Fig. 2.
Fig. 2.
Distributions of (A) age (n = 693; males, 217 [31.31%]; females, 476 [68.69%]) and (B) BMI (underweight, 92 [13.28%]; normal weight, 503 [72.58%]; overweight, 84 [12.12%]; and obese, 14 [2.02%]).
The mean EDDS score was 0.003 (SD, 11.21; range, −11.01 to 64.08). Females had significantly higher EDDS scores (means: males, −2.78 ± 9.27; females, 1.27 ± 11.78; t = −4.88; p < 0.001) than males. No sex differences were observed in FD (t = 0.97; p = 0.33) and BMI (means: males, 21.49 ± 3.10; females, 21.06 ± 2.51; t = 1.78; p = 0.08). EDDS scores were positively associated with age (r = 0.07; p = 0.051) and BMI (r = 0.28; p < 0.001).
3.2. Functional network connectivity analyses
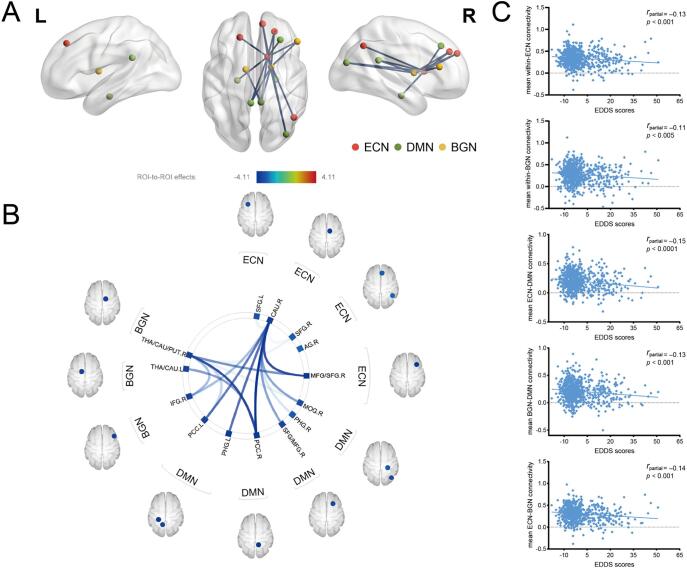
Within-network functional connectivity. After accounting for age, sex, BMI, and FD as covariates, EDDS scores were inversely associated with the connectivity of the ECN (five ROI-to-ROI pairs: right caudate-right middle frontal gyrus [MFG]/superior frontal gyrus [SFG]; right caudate-right angular gyrus; right caudate-right SFG; right caudate-left SFG; and right MFG/SFG-right caudate) and the BGN (two ROI-to-ROI pairs: right thalamus/caudate/putamen-right inferior frontal gyrus [IFG] and right IFG-right thalamus/caudate/putamen; false discovery rate seed-level corrected p < 0.05, two-sided) (Table 1 and Fig. 3).
Table 1.
Functional connectivity within the ECN and within the BGN: Negative correlations with EDDS scores.a.
| Functional network connectivity | t-values | p-values b | Connectivity value (Z-scores) c |
|---|---|---|---|
| RECN (right caudate) - RECN (right MFG/SFG) | −3.70 | 0.003 | 0.39 (±0.20) |
| RECN (right caudate) - RECN (right AG) | −2.43 | 0.036 | 0.29 (±0.19) |
| RECN (right caudate) - RECN (right SFG) | −2.51 | 0.032 | 0.32 (±0.19) |
| RECN (right caudate) - LECN (left SFG) | −2.58 | 0.029 | 0.32 (±0.20) |
| RECN (right MFG/SFG) - RECN (right caudate) | −3.70 | 0.006 | 0.39 (±0.20) |
| BGN (right thalamus/caudate/putamen) - BGN (right IFG) | −3.00 | 0.024 | 0.28 (±0.20) |
| BGN (right IFG) - BGN (right thalamus/caudate/putamen) | −3.00 | 0.037 | 0.28 (±0.20) |
aOnly significant results are reported (n = 693).
bFalse discovery rate (FDR) seed-level corrected p < 0.05, two-sided.
cValues are mean ± standard deviation (SD).
Abbreviations: EDDS, Eating Disorder Diagnosis Scale; LECN, left executive control network; RECN, right executive control network; BGN, basal ganglia network; MFG, middle frontal gyrus; SFG, superior frontal gyrus; AG, angular gyrus; IFG, inferior frontal gyrus.
Fig. 3.
Resting-state FNC of eating disorder symptoms (n = 693). (A) Visual depiction of the intra-network connectivity and inter-network connectivity inversely associated with EDDS scores (FDR seed-level corrected p < 0.05, two-sided). (B) Connectivity patterns related to EDDS scores, as evidenced in this “connectome ring.” The color bar represents t values, with darker color indicating stronger significant associations. (C) Scatter plots depicting the correlations between EDDS scores and mean network connectivity strength. See Table 1, Table 2 for associated statistics. Abbreviations: ECN, executive control network; DMN, default mode network; BGN, basal ganglia network; ROI, region of interest; THA, thalamus; CAU, caudate; PUT, putamen; IFG, inferior frontal gyrus; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; SFG, superior frontal gyrus; MFG, middle frontal gyrus; MOG, middle occipital gyrus; AG, angular gyrus; EDDS, Eating Disorder Diagnosis Scale; FNC, functional network connectivity; FDR, false discovery rate; L, left; R, right.
Between-network functional connectivity. Using the same aforementioned covariates and statistical thresholds, results revealed significant patterns of between-network FC that were inversely correlated with eating disorder symptoms (i.e., the composite EDDS score). The DMN demonstrated over-connectivity with the BGN (especially the right thalamus/caudate/putamen) and the right ECN (RECN; i.e., the right caudate) (Table 2, Fig. 3). Specifically, higher EDDS scores were correlated with lesser connectivity between the ECN (ROI: right caudate) and DMN (ROI: left and right posterior cingulate cortex [PCC], left and right parahippocampal gyrus, right middle occipital gyrus [MOG], and right SFG/MFG) (Table 2a). Higher EDDS scores were associated with lesser connectivity between the BGN (ROI: left thalamus/caudate and right thalamus/caudate/putamen) and DMN (ROI: left and right PCC, and right MOG) (Table 2b). Higher EDDS scores were associated with lesser connectivity between the ECN (ROI: right MFG/SFG, right caudate, and right SFG) and BGN (ROI: right thalamus/caudate/putamen and right IFG) (Table 2c). Within-network and between-network correlation matrices are shown in Fig. S1.
Table 2.
Functional connectivity among the ECN, DMN, and BGN: Negative correlations with EDDS scores. a
| Functional network connectivity | t-values | p-valuesb | Connectivity value (Z-scores)c |
|---|---|---|---|
| (a)Between ECN and DMN | |||
| RECN (right caudate) - DMN (left PCC) | −3.42 | 0.004 | 0.17 (±0.19) |
| RECN (right caudate) - DMN (left PHG) | −3.50 | 0.004 | 0.17 (±0.20) |
| RECN (right caudate) - DMN (right PCC) | −4.11 | 0.001 | 0.17 (±0.19) |
| RECN (right caudate) - DMN (right SFG/MFG) | −3.17 | 0.008 | 0.34 (±0.19) |
| RECN (right caudate) - DMN (right PHG) | −2.68 | 0.025 | 0.15 (±0.18) |
| RECN (right caudate) - DMN (right MOG) | −3.14 | 0.008 | 0.17 (±0.19) |
| DMN (left PCC) - RECN (right caudate) | −3.42 | 0.017 | 0.17 (±0.19) |
| DMN (left PHG) - RECN (right caudate) | −3.50 | 0.013 | 0.17 (±0.20) |
| DMN (right PCC) - RECN (right caudate) | −4.11 | 0.001 | 0.17 (±0.19) |
| DMN (right SFG/MFG) - RECN (right caudate) | −3.17 | 0.042 | 0.34 (±0.19) |
| DMN (right MOG) - RECN (right caudate) | −3.14 | 0.045 | 0.17(±0.19) |
| (b)Between BGN and DMN | |||
| BGN (right thalamus/caudate/putamen) - DMN (left PCC) | −2.80 | 0.034 | 0.20 (±0.19) |
| BGN (right thalamus/caudate/putamen) - DMN (right PCC) | −3.70 | 0.006 | 0.21 (±0.19) |
| BGN (right thalamus/caudate/putamen) - DMN (right MOG) | −2.57 | 0.049 | 0.17 (±0.19) |
| BGN (left thalamus/caudate) - DMN (right PCC) | −3.21 | 0.036 | 0.23 (±0.19) |
| DMN (right PCC) - BGN (right thalamus/caudate/putamen) | −3.70 | 0.003 | 0.21 (±0.19) |
| DMN (right PCC) - BGN (left thalamus/caudate) | −3.21 | 0.012 | 0.23 (±0.19) |
| (c)Between ECN and BGN | |||
| RECN (right MFG/SFG) - BGN (right thalamus/caudate/putamen) | −3.27 | 0.015 | 0.35 (±0.21) |
| RECN (right caudate) - BGN (right IFG) | −3.00 | 0.010 | 0.27 (±0.20) |
| BGN (right thalamus/caudate/putamen) - RECN (right MFG/SFG) | −3.27 | 0.015 | 0.35 (±0.21) |
| BGN (right thalamus/caudate/putamen) - RECN (right SFG) | −2.54 | 0.049 | 0.28 (±0.20) |
| BGN (right IFG) - RECN (right caudate) | −3.00 | 0.037 | 0.27 (±0.20) |
aOnly significant results are reported (n = 693).
bFalse discovery rate (FDR) seed-level corrected p < 0.05, two-sided.
cValues are mean ± standard deviation (SD).
Abbreviations: EDDS, Eating Disorder Diagnosis Scale; RECN, right executive control network; LECN, left executive control network; DMN, default mode network; BGN, basal ganglia network; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; SFG, superior frontal gyrus; MFG, middle frontal gyrus; MOG, middle occipital gyrus; IFG, inferior frontal gyrus.
In addition, the significant inverse associations of FNC with EDDS scores were maintained when BMI was not included as a covariate (i.e., not controlling for BMI), with t/p ranging from −3.99/0.002 to −2.43/0.045 (Fig. S4A). These significant within and between FNC results (i.e., ECN, ECN-DMN, BGN-DMN) were also found at p < 0.05 with a false discovery rate analysis-level correction (two-sided), with t/p ranging from −4.11/0.016 to −3.42/0.046 (Fig. S4B). Given that the majority of participants had a low EDDS score, we further defined outliers as composite EDDS values exceeding ± 3SD the mean (Alleva et al., 2021, Field, 2009) and excluded the corresponding participants (i.e., eleven highly symptomatic individuals). Repeated analysis revealed that the original FNC patterns (ECN, BGN, and DMN) remained significant after correcting for age, sex, BMI, and FD, with t/p ranging from −3.77/0.003 to −2.32/0.043 (n = 682; Fig. S7A); in addition, similar FNC patterns were found after correcting for the normality problems of FD (i.e., a log transformation), with t/p ranging from −3.75/0.003 to −2.31/0.044 (Fig. S7B). Fig. S8 further shows the significant FNC patterns obtained after excluding subjects with > 50% (n = 2), >30% (n = 7), and > 10% (n = 45) of the time points being scrubbed (scrubbing procedure for motion outliers); furthermore, the mean motion (i.e., FD and temporal Derivative of root mean square VARiance over voxelS [DVARS]; Parkes et al., 2018, Power et al., 2012, Power et al., 2013) of this sample (n = 639; subjects with > 10% volumes being scrubbed were excluded) was 0.05/1.10 (SD, 0.02/0.17) and there were no significant association between the EDDS scores and FD (r = 0.06; p = 0.14) or DVARS (r = 0.08; p = 0.053).
3.3. Exploratory analyses
Association between network connectivity and specific eating-related categories. Partial correlation analyses showed that AN symptom severity was inversely associated with mean ECN-DMN connectivity (p < 0.05). BN and BED symptom severity levels were inversely correlated with mean within-network connectivity of the ECN and BGN, mean ECN-DMN connectivity, mean BGN-DMN connectivity, and mean ECN-BGN connectivity (p < 0.05). Regarding specific disordered eating behaviors/cognitions, body image concern was found to be inversely correlated with mean ECN-DMN and BGN-DMN connectivity, and loss of control eating was inversely correlated with all mean within-network and between-network connectivity (p < 0.05) (Table S1).
Sex differences in network connectivity associated with eating disorder symptoms. Significant sex differences were observed for within-DMN connectivity (two ROI-to-ROI pairs: right PCC-right MOG and right MOG-right PCC) and eating disorder symptoms when controlling for age, BMI, and FD (contrast: female group > male group; t = −3.39; false discovery rate seed-level corrected p < 0.05 and p = 0.02 [two-sided], respectively) (see Fig. S2).
Potential confounding effects. After excluding participants who were underweight, overweight, and obese, the mean age of the new sample (n = 503; females, 365 [72.56%]; males, 138 [27.44%]) was 18.38 years (SD, 0.89; range, 17–22 years). The mean BMI was 20.94 (SD, 1.40; range, 18.50–23.88). The mean EDDS score was 0.14 (SD, 11.12; range, −11.01 to 44.78). Furthermore, after correcting for age, sex, BMI, and FD, the significant patterns of intra-network connectivity and inter-network connectivity inversely associated with EDDS scores were almost maintained (false discovery rate seed-level corrected p < 0.05, two-sided). Similarly, the DMN demonstrated over-connectivity with the BGN and RECN (Table S2 and Fig. S3). These results remained significant (n = 544; females, 389 [71.51%]; males, 155 [28.49%]) when using the international BMI criteria (i.e., underweight [BMI < 18.5, n = 92], healthy weight [18.5 ≤ BMI < 25, n = 544], overweight [25 ≤ BMI < 30, n = 51], and obese [BMI ≥ 30, n = 6]), with t/p ranging from −3.89/0.002 to −2.50/0.047 (Fig. S4C).
Association between other network connectivity and eating disorder symptoms. Using the same aforementioned covariates and statistical thresholds, there were no significant associations between the EDDS scores and the FNC within and between the ASN and PCUN (11 × 11 connectivity matrix), with p ranging from 0.794 to 0.937. The original significant FNC patterns (i.e., ECN, BGN, and DMN) were maintained when examining the five networks (i.e., ECN, BGN, DMN, ASN, and PCUN; 38 × 38 connectivity matrix), with t/p ranging from −4.11/0.002 to −2.51/0.048 (Fig. S5A). Furthermore, EDDS scores were found to be positively associated with within-VSN (i.e., 11 × 11 connectivity matrix) connectivity (i.e., two ROI-to-ROI pairs: right lobule VIII-left lobule VIII and left lobule VIII -right lobule VIII; t = 2.89, p = 0.040) (Fig. S5B). The original FNC patterns (i.e., ECN, BGN, and DMN) remained significant when examining the four networks (i.e., ECN, BGN, DMN, and VSN; 38 × 38 connectivity matrix), with t/p ranging from −4.11/0.002 to −2.51/0.048 (Fig. S5C). The significant connectivity patterns of the four networks without controlling for BMI are shown in Fig. S5D.
3.4. Sensitivity analyses
The analyses exploring the sensitivity and specificity of our original ROI are presented in Fig. S6. The results showed that the EDDS scores were inversely associated with the connectivity of the frontoparietal and default networks, as well as with the frontoparietal-default network connectivity (false discovery rate seed-level corrected p < 0.05, two-sided).
4. Discussion
This study investigated the underlying neurocircuitry symptoms of disordered eating, which represents an important first step in addressing the role of these large-scale RSN in eating disorder symptoms among healthy adults. We observed that individuals with higher levels of disordered eating displayed weaker intra-network FC of the ECN and BGN and weaker inter-network FC in the three examined networks. Importantly, age, sex, BMI, and head motion were each statistically controlled for, enabling greater confidence that these results cannot be attributed to any of these other factors (especially because there were sex differences in EDDS scores in our sample and BMI was positively related to EDDS scores). These findings also remained significant even after excluding participants who were underweight, overweight, and obese. The significant FNC patterns (ECN, BGN, and DMN) remained when including other networks (ASN, PCUN, and VSN) that were either related or unrelated to eating-related psychopathology. Additionally, no similar FNC patterns were identified in these other networks, suggesting the specificity of our findings. Moreover, the qualitatively similar network-level results provided added confidence in the robustness of our findings.
4.1. Major findings
The finding that higher levels of disordered eating (as indicated by higher EDDS scores) were associated with weaker resting FC within the ECN is consistent with the results of previous studies linking intra-network synchrony of the ECN to eating disorder symptoms in both clinical and non-clinical samples (Bartholdy et al., 2019, Boehm et al., 2014, Park et al., 2018, Stopyra et al., 2019). The ECN is responsible for a set of cognitive functions, including inhibition, memory, and attention, and it regulates eating behaviors; furthermore, altered brain activation in the ECN has been shown to be linked to errant behaviors of food intake, which are significant features of eating disorders (Barkhof et al., 2014, Siep et al., 2012, Val-Laillet et al., 2015, Walsh, 2011). It is highly plausible that the less efficient information exchange between ECN regions (as reflected by lesser FC) relates to lower dietary self-control, thus promoting more disordered eating (e.g., uncontrolled eating) (Vainik et al., 2019).
We also observed an inverse relation between EDDS scores (as well as BN and BED scores) and FC within the BGN, which is inconsistent with the results of a recent RSN study involving healthy subjects that reported that BGN connectivity is positively related to disinhibited eating behaviors (Shapiro et al., 2019). The authors did not conclude that greater interactions between BGN regions contribute to more overeating through stronger reward reactivity or reward-based eating drive, perhaps because their small sample size of young children limited the generalizability of findings. Additionally, these findings varied depending on the groups studied (i.e., children or adults) and the means of assessing disordered eating (i.e., test paradigms or self-report measures). Nonetheless, there is evidence that patients with AN or BN experience altered reward sensitivity related to reward circuitry (Kessler et al., 2016, Monteleone et al., 2018, Steward et al., 2018). It appears plausible to suggest that diminished involvement of BGN-mediated reward processing (e.g., reward sensitivity) manifesting as decreased synchrony within the BGN may facilitate symptomatic behaviors. Because there is little research of the relation of BGN and eating behaviors of healthy subjects (i.e., network-level hypotheses), this finding should be interpreted with caution.
The current result indicating that adults with more disordered eating demonstrated weaker communication between the reward and response inhibition-related networks (as reflected by lesser cross-network FC) is in accordance with the results of a previous study that confirmed an inverse association of overeating with BGN-ECN connectivity (Shapiro et al., 2019), suggesting that inter-network neural markers may play a key role in the context of symptoms of disordered eating in healthy adults. Clinically, individuals with AN may compensate for dysfunctional reward processing by using exaggerated cognitive control (e.g., dietary restraint); in contrast, those with BN may have deficient cognitive control, thus increasing instability and erratic responses to appetite stimuli (e.g., binge eating) (Friederich et al., 2013, King et al., 2016, Monteleone et al., 2018, Smith and Robbins, 2013). Because extremes of eating behaviors are considered to be linked to an altered balance of reward and inhibitory processing (for a review, see Wierenga et al., 2014), our findings may suggest the decreased ability to maintain balance between reward reactivity and dietary self-control in adults with highly disordered eating habits. This result warrants further investigation.
Our data further revealed that higher EDDS scores were related to weaker positively correlated spontaneous fMRI signals between specific TPN and TNN, such that the greater the positive correlation strength between the ECN/BGN and DMN, the lower the EDDS scores; however, near-zero correlations between the two networks were associated with the highest EDDS scores. Behavioral findings have suggested aberrant inhibitory control, altered sensitivity to reward, and negative self-reflective cognition in individuals with highly disordered eating habits (Brooks et al., 2012, Eneva et al., 2017, Goldschmidt et al., 2018, Williams and Levinson, 2020). Therefore, the weakened (or near-zero) ECN/BGN-DMN synchrony in adults with greater severity of eating disorder symptoms could reflect that dysfunctional inhibitory control/reward sensitivity might interfere with the appropriate expression of TNN (i.e., DMN) at rest, or that excessive self-focused and body-focused thought processes might interfere with the appropriate suppression of TPN (i.e., ECN, BGN) at rest. Future studies should further investigate these interesting possibilities by including response inhibition tasks and using dynamic causal modeling analyses that can determine the intrinsic effective connectivity between RSN. Notably, suboptimal, developmentally immature, and clinically aberrant patterns of cognitive performance tend to be associated with decreased anti-correlations or even positive correlations between TPN and DMN (Chai et al., 2014). This does not mean that less negative connectivity or positive connectivity between these networks is necessarily directly associated with psychopathology (Killgore et al., 2017); however, there is evidence that they tend to be correlated with excessive self-referential thoughts and concerns about body shape/weight, which may serve as potential risk factors for eating disorders (Domakonda et al., 2019). At the node level, the finding that DMN regions exhibited over-connectivity with the right caudate suggests that, as the central hub of densely interconnected BGN, the right caudate has a particularly important role in the information exchange with the DMN related to maladaptive eating. These results remained significant even without controlling for BMI, suggesting that the relationships between FNC and eating disorder symptoms in healthy individuals may be independent of whether BMI is controlled or not, thereby indicating high ecological validity of our findings.
In particular, adults with more frequent bulimia-type eating (i.e., BN, BED) and loss of control eating displayed weaker intra-network FC of the ECN and BGN and weaker inter-network FC in the three examined networks. This may further support the notion that abnormalities in reward network/circuitry are associated with diminished self-regulatory capacity and subsequently manifest as an inability to sufficiently curb eating behavior in individuals with bulimia-type eating (Berner and Marsh, 2014, Berridge et al., 2010). This study may expand the previous findings showing that neural alterations in the prefrontal cortex, parietal lobe, and insula are related to body image distortion in patients with AN (for a review, see Gaudio and Quattrocchi, 2012) by demonstrating that more body shape/weight concerns are linked to less pronounced inter-network interactions in healthy individuals. Additionally, we observed significant sex differences in the relationship between EDDS scores and within-DMN connectivity, suggesting that lesser synchrony of the DMN was related to fewer eating disorder symptoms in males but not in females. The DMN has been linked to cognitive experiences that occur spontaneously at rest (Buckner et al., 2008) as well as excessive body shape/weight concerns in adolescents with abnormal eating (Domakonda et al., 2019). Therefore, diminished engagement of DMN-mediated, self-focused, and body-focused thoughts may play a key role in reducing disordered eating in males relative to females. Such interpretation is consistent with our findings that males had significantly lower EDDS scores than females. Due to the potential impact of BMI on resting-state FC within the DMN and ECN (Kullmann et al., 2012, Park et al., 2018), it is important that the similar network-level results remained significant after excluding participants who were underweight, overweight, and obese.
4.2. Practical implications
Currently, cognitive training methods (e.g., attentional bias modification, response inhibition) and neuromodulation strategies (e.g., transcranial magnetic stimulation [TMS], transcranial direct current stimulation [tDCS], and neurofeedback) are two novel and promising approaches to reducing weight and improving unhealthy diets (Forcano et al., 2018). Despite the increasing amount of neurobiological data regarding reward circuits/mechanisms in clinical conditions, no therapeutic intervention devoted to the management of eating disorders has been specifically focused on reward (Monteleone et al., 2018). Therefore, future cognitive and neuromodulation strategies focused on ameliorating sensitivity to reward may lead to more effective treatments for AN. Although numerous studies have revealed that poor inhibitory control leads to more unhealthy eating behaviors (for a review, see Dohle et al., 2018), intervention measures targeting a combination of executive functions (e.g., updating, inhibiting, and shifting) are thought to be more effective than those focused on one function because the different facets of executive functions may facilitate different aspects of self-regulation (Hofmann et al., 2012). Together with the findings of the present study, prevention programs aiming to decrease disordered eating could further integrate self-related and body-related factors into specific prevention activities.
5. Limitations, future directions, and conclusions
One limitation of this study was that our sample had a fairly restricted age range (i.e., early adulthood) and had below-average EDDS scores, which may affect the generalizability of our results. The significant associations between the EDDS scores and RSN connectivity were not driven by a few highly symptomatic individuals; nevertheless, future studies are needed to replicate these findings in an independent sample including participants with a wider range of ages and eating disorder symptoms. Additionally, because our results are purely correlational, no causal/directional claims are warranted. Therefore, future studies, especially longitudinal investigations, are necessary to ascertain whether individuals with more unhealthy eating behaviors subsequently develop lesser FNC (especially the ECN, BGN, and DMN), or whether weaker network-level functional synchrony promotes errant food intake behaviors. At present, it appears most plausible to expect that such interactions would be bidirectional and mutually reinforcing. The relationship between eating disorder symptoms and neural circuitry may be further explored using graph theory metrics such as “network density” and “average path length,” which could help elucidate both common and unique neural network properties that may underlie potential differences in eating-related psychopathology (e.g., Geisler et al., 2016, Wang et al., 2019). Furthermore, because there does not appear to be one-to-one mapping between brain regions and psychological processes (Anderson, 2014), other interpretations of our results may be possible. Additional research is necessary to confirm that the associations we have observed between eating disorder symptoms and FNC are best explained by self-focused thoughts, executive functions, and reward sensitivity, as we have suggested.
In conclusion, our findings suggest that the involvement of the ECN, BGN, and DMN represents the crucial neural substrates that could explain the potential interactions of dysfunctional inhibitory control and reward sensitivity with negative self-referential thinking in abnormal eating. Future research should focus on the behavioral and cognitive consequences for these FNC alterations to determine whether these neuroimaging findings could guide the development of targeted and circuit-level prevention and intervention aimed at decreasing maladaptive eating behaviors.
CRediT authorship contribution statement
Ximei Chen: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Xiao Gao: Conceptualization, Writing - original draft, Writing - review & editing. Jingmin Qin: Formal analysis, Investigation, Methodology, Visualization. Chuan Wang: Methodology, Validation, Visualization. Mingyue Xiao: Project administration, Resources, Supervision. Yun Tian: Methodology, Validation, Visualization. Yi-jun Luo: Conceptualization, Writing - original draft. Jiang Qiu: Funding acquisition, Project administration, Resources, Supervision. Tingyong Feng: Funding acquisition, Project administration, Resources, Supervision. Qinghua He: Funding acquisition, Project administration, Resources, Supervision. Xu Lei: Funding acquisition, Project administration, Resources, Supervision. Hong Chen: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing - review & editing.
Acknowledgments
Acknowledgements
This study was funded by National Natural Science Foundation of China (No. 31771237) and the Fundamental Research Funds for the Central Universities (No. SWU1709106). We would like to thank all the members of the Self and Health Research Lab, Southwest University for their invaluable help in data acquisition. We would like to thank Li He from Southwest University for kindly sharing part of the code used for data analysis and Feng Zhou from University of Electronic Science and Technology of China for his statistical advice. We would also like to thank the Editage (www.editage.cn) for English language editing.
Disclosures
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102671.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alleva J.M., Medoch M.M., Priestley K., Philippi J.L., Hamaekers J., Salvino E.N., Humblet S., Custers M. “I appreciate your body, because…” does promoting positive body image to a friend affect one’s own positive body image? Body Image. 2021;36:134–138. doi: 10.1016/j.bodyim.2020.11.002. [DOI] [PubMed] [Google Scholar]
- Anderson M.L. MIT Press; Cambridge, MA: 2014. After Phrenology: Neural Reuse and the Interactive Brain. [Google Scholar]
- Barkhof F., Haller S., Rombouts S.A.R.B. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014;272:29–49. doi: 10.1148/radiol.14132388. [DOI] [PubMed] [Google Scholar]
- Bartholdy S., O’Daly O.G., Campbell I.C., Banaschewski T., Barker G., Bokde A.L.W., Bromberg U., Büchel C., Quinlan E.B., Desrivières S., Flor H., Frouin V., Garavan H., Gowland P., Heinz A., Ittermann B., Martinot J.-L., Paillère Martinot M.-L., Nees F., Orfanos D.P., Poustka L., Hohmann S., Fröhner J.H., Smolka M.N., Walter H., Whelan R., Schumann G., Schmidt U., Rapp M., Artiges E., Schneider S., Bach C., Paus T., Barbot A., Gareth B., Bokde A., Vetter N., Büchel C., Cattrell A., Constant P., Gowland P., Crombag H., Czech K., Dalley J., Decideur B., Spranger T., Ripley T., Heym N., Flor H., Sommer W., Fuchs B., Gallinat J., Spanagel R., Kaviani M., Heinrichs B., Andreas H., Subramaniam N., Jia T., Ihlenfeld A., Ireland J., Ittermann B., Conrod P., Banaschewski T., Jones J., Klaassen A., Lalanne C., Lanzerath D., Lawrence C., Lemaitre H., Desrivieres S., Mallik C., Karl M., Mar A., Martinez-Medina L., Jean-Luc M., Mennigen E., Mesquita de Carvahlo F., Schwartz Y., Bruehl R., Müller K., Nees F., Nymberg C., Lathrop M., Trevor R., Pausova Z., Jani P., Biondo F., Jean-Baptiste P., Hohmann S., Poustka L., Millenet S., Michael S., Fröhner J., Struve M., Steve W., Hübner T., Bromberg U., Aydin S., Rogers J., Romanowski A., Schmäl C., Schmidt D., Ripke S., Arroyo M., Schubert F., Pena-Oliver Y., Fauth-Bühler M., Mignon X., Whelan R., Speiser C., Fadai T., Dai S., Ströhle A., Paillere M.-L., Strache N., Theobald D., Jurk S., Vulser H., Miranda R., Yacubian J., Frouin V., Genauck A., Parchetka C., Gemmeke I., Kruschwitz J., Weiß K., Walter H., Feng J., Papadopoulos D., Filippi I., Ing A., Ruggeri B., Xu B., Macare C., Chu C., Hanratty E., Burke Quinlan E., Robert G., Schumann G., Yu T., Ziesch V., Stedman A. Neural correlates of failed inhibitory control as an early marker of disordered eating in adolescents. Biol. Psychiatry. 2019;85:956–965. doi: 10.1016/j.biopsych.2019.01.027. [DOI] [PubMed] [Google Scholar]
- Berner L.A., Marsh R. Frontostriatal circuits and the development of bulimia nervosa. Front. Behav. Neurosci. 2014;8:395. doi: 10.3389/fnbeh.2014.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Ho C.-Y., Richard J.M., DiFeliceantonio A.G. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm I., Geisler D., King J.A., Ritschel F., Seidel M., Deza Araujo Y., Petermann J., Lohmeier H., Weiss J., Walter M., Roessner V., Ehrlich S. Increased resting state functional connectivity in the fronto-parietal and default mode network in anorexia nervosa. Front. Behav. Neurosci. 2014;8:346. doi: 10.3389/fnbeh.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm I., Geisler D., Tam F., King J.A., Ritschel F., Seidel M., Bernardoni F., Murr J., Goschke T., Calhoun V.D., Roessner V., Ehrlich S. Partially restored resting-state functional connectivity in women recovered from anorexia nervosa. J. Psychiatry Neurosci. 2016;41:377–385. doi: 10.1503/jpn.150259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S.J., Rask-Andersen M., Benedict C., Schiöth H.B. A debate on current eating disorder diagnoses in light of neurobiological findings: is it time for a spectrum model? BMC Psychiatry. 2012;12:76. doi: 10.1186/1471-244X-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Ofen N., Gabrieli J.D.E., Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cognit. Neurosci. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Jackson T. Prevalence and sociodemographic correlates of eating disorder endorsements among adolescents and young adults from China. Eur. Eating Disorders Rev. 2008:375–385. doi: 10.1002/erv.837. [DOI] [PubMed] [Google Scholar]
- Cowdrey F.A., Filippini N., Park R.J., Smith S.M., McCabe C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum. Brain Mapp. 2014;35:483–491. doi: 10.1002/hbm.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohle S., Diel K., Hofmann W. Executive functions and the self-regulation of eating behavior: a review. Appetite. 2018;124:4–9. doi: 10.1016/j.appet.2017.05.041. [DOI] [PubMed] [Google Scholar]
- Domakonda M.J., He X., Lee S., Cyr M., Marsh R. Increased functional connectivity between ventral attention and default mode networks in adolescents with bulimia nervosa. J. Am. Acad. Child Adolesc. Psychiatry. 2019;58:232–241. doi: 10.1016/j.jaac.2018.09.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly B., Touyz S., Hay P., Burton A., Russell J., Caterson I. Neuroimaging in bulimia nervosa and binge eating disorder: a systematic review. J. Eating Disorders. 2018;6:3. doi: 10.1186/s40337-018-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donofry S.D., Stillman C.M., Erickson K.I. A review of the relationship between eating behavior, obesity and functional brain network organization. Social Cogn. Affective Neurosci. 2020;15:1157–1181. doi: 10.1093/scan/nsz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eneva K.T., Murray S., O’Garro-Moore J., Yiu A., Alloy L.B., Avena N.M., Chen E.Y. Reward and punishment sensitivity and disordered eating behaviors in men and women. J. Eating Disorders. 2017;5:6. doi: 10.1186/s40337-017-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R., Cieri F., di Giannantonio M., Tartaro A. The role of body image and self-perception in anorexia nervosa: the neuroimaging perspective. J. Neuropsychol. 2018;12:41–52. doi: 10.1111/jnp.12106. [DOI] [PubMed] [Google Scholar]
- Field A. third ed. Sage Publications; 2009. Discopering Statistics using SPSS. [Google Scholar]
- Forcano L., Mata F., de la Torre R., Verdejo-Garcia A. Cognitive and neuromodulation strategies for unhealthy eating and obesity: systematic review and discussion of neurocognitive mechanisms. Neurosci. Biobehav. Rev. 2018;87:161–191. doi: 10.1016/j.neubiorev.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G.K.W. Altered brain reward circuits in eating disorders: chicken or egg? Curr. Psychiatry Rep. 2013;15:396. doi: 10.1007/s11920-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich H.-C., Wu M., Simon J.J., Herzog W. Neurocircuit function in eating disorders. Int. J. Eat. Disord. 2013;46:425–432. doi: 10.1002/eat.22099. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S.J., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gaudio S., Quattrocchi C.C. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci. Biobehav. Rev. 2012;36:1839–1847. doi: 10.1016/j.neubiorev.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Gaudio S., Wiemerslage L., Brooks S.J., Schiöth H.B. A systematic review of resting-state functional-MRI studies in anorexia nervosa: evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neurosci. Biobehav. Rev. 2016;71:578–589. doi: 10.1016/j.neubiorev.2016.09.032. [DOI] [PubMed] [Google Scholar]
- Geisler D., Borchardt V., Lord A.R., Boehm I., Ritschel F., Zwipp J., Clas S., King J.A., Wolff-Stephan S., Roessner V., Walter M., Ehrlich S. Abnormal functional global and local brain connectivity in female patients with anorexia nervosa. J. Psychiatry Neurosci. 2016;41:6–15. doi: 10.1503/jpn.140310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt A.B., O'Brien S., Lavender J.M., Pearson C.M., Le Grange D., Hunter S.J. Executive functioning in a racially diverse sample of children who are overweight and at risk for eating disorders. Appetite. 2018;124:43–49. doi: 10.1016/j.appet.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. 2003;100:253. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W., Schmeichel B.J., Baddeley A.D. Executive functions and self-regulation. Trends Cogn. Sci. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Jackson T., Chen H. Identifying the eating disorder symptomatic in China: the role of sociocultural factors and culturally defined appearance concerns. J. Psychosom. Res. 2007;62:241–249. doi: 10.1016/j.jpsychores.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Jackson T., Chen H. Predicting changes in eating disorder symptoms among adolescents in China: an 18-month prospective study. J. Clin. Child Adolescent Psychol. 2008;37:874–885. doi: 10.1080/15374410802359841. [DOI] [PubMed] [Google Scholar]
- Jackson T., Chen H. Sociocultural experiences of bulimic and non-bulimic adolescents in a school-based Chinese sample. J. Abnorm. Child Psychol. 2010;38:69–76. doi: 10.1007/s10802-009-9350-0. [DOI] [PubMed] [Google Scholar]
- Kessler R.M., Hutson P.H., Herman B.K., Potenza M.N. The neurobiological basis of binge-eating disorder. Neurosci. Biobehav. Rev. 2016;63:223–238. doi: 10.1016/j.neubiorev.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Smith R., Olson E.A., Weber M., Rauch S.L., Nickerson L.D. Emotional intelligence is associated with connectivity within and between resting state networks. Social Cogn. Affective Neurosci. 2017;12:1624–1636. doi: 10.1093/scan/nsx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. ppcor: an R package for a fast calculation to semi-partial correlation coefficients. Commun. Statistical Appl. Methods. 2015;22:665. doi: 10.5351/CSAM.2015.22.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.A., Geisler D., Bernardoni F., Ritschel F., Böhm I., Seidel M., Mennigen E., Ripke S., Smolka M.N., Roessner V., Ehrlich S. Altered neural efficiency of decision making during temporal reward discounting in anorexia nervosa. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55:972–979. doi: 10.1016/j.jaac.2016.08.005. [DOI] [PubMed] [Google Scholar]
- King J.A., Frank G.K.W., Thompson P.M., Ehrlich S. Structural neuroimaging of anorexia nervosa: Future directions in the quest for mechanisms underlying dynamic alterations. Biol. Psychiatry. 2018;83:224–234. doi: 10.1016/j.biopsych.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler L.A., Cohen P., Davies M., Pine D.S., Walsh B.T. Longitudinal relationships between childhood, adolescent, and adult eating disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:1434–1440. doi: 10.1097/00004583-200112000-00014. [DOI] [PubMed] [Google Scholar]
- Kullmann S., Heni M., Veit R., Ketterer C., Schick F., Häring H.-U., Fritsche A., Preissl H. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum. Brain Mapp. 2012;33:1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.-J., Jackson T., Niu G.-F., Chen H. Effects of gender and appearance comparisons on associations between media-based appearance pressure and disordered eating: testing a moderated mediation model. Sex Roles. 2020;82:293–305. [Google Scholar]
- McFadden K.L., Tregellas J.R., Shott M.E., Frank G.K. Reduced salience and default mode network activity in women with anorexia nervosa. J. Psychiatry Neurosci. 2014;39:178–188. doi: 10.1503/jpn.130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone A.M., Castellini G., Volpe U., Ricca V., Lelli L., Monteleone P., Maj M. Neuroendocrinology and brain imaging of reward in eating disorders: a possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;80:132–142. doi: 10.1016/j.pnpbp.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Park B.-Y., Moon T., Park H. Dynamic functional connectivity analysis reveals improved association between brain networks and eating behaviors compared to static analysis. Behav. Brain Res. 2018;337:114–121. doi: 10.1016/j.bbr.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Parkes L., Fulcher B., Yücel M., Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage. 2018;171:415–436. doi: 10.1016/j.neuroimage.2017.12.073. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. NeuroImage. 2013;76:439–441. doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98:676. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rcore T. R Foundation for Statistical Computing; Vienna, Austria: 2016. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Shapiro A.L.B., Johnson S.L., Sutton B., Legget K.T., Dabelea D., Tregellas J.R. Eating in the absence of hunger in young children is related to brain reward network hyperactivity and reduced functional connectivity in executive control networks. Pediatric Obesity. 2019;14 doi: 10.1111/ijpo.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N., Roefs A., Roebroeck A., Havermans R., Bonte M., Jansen A. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. NeuroImage. 2012;60:213–220. doi: 10.1016/j.neuroimage.2011.12.067. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. 2009;106:13040. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.G., Robbins T.W. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol. Psychiatry. 2013;73:804–810. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Song S., Zhang Y., Qiu J., Li X., Ma K., Chen S., Chen H. Brain structures associated with eating behaviors in normal-weight young females. Neuropsychologia. 2019;133 doi: 10.1016/j.neuropsychologia.2019.107171. [DOI] [PubMed] [Google Scholar]
- Sparti C., Santomauro D., Cruwys T., Burgess P., Harris M. Disordered eating among Australian adolescents: prevalence, functioning, and help received. Int. J. Eat. Disord. 2019;52:246–254. doi: 10.1002/eat.23032. [DOI] [PubMed] [Google Scholar]
- Steward T., Menchon J.M., Jiménez-Murcia S., Soriano-Mas C., Fernandez-Aranda F. Neural network alterations across eating disorders: a narrative review of fMRI studies. Curr. Neuropharmacol. 2018;16:1150–1163. doi: 10.2174/1570159X15666171017111532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Telch C.F., Rizvi S.L. Development and validation of the eating disorder diagnostic scale: a brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol. Assess. 2000;12:123–131. doi: 10.1037//1040-3590.12.2.123. [DOI] [PubMed] [Google Scholar]
- Stopyra M.A., Simon J.J., Skunde M., Walther S., Bendszus M., Herzog W., Friederich H.-C. Altered functional connectivity in binge eating disorder and bulimia nervosa: a resting-state fMRI study. Brain Behav. 2019;9 doi: 10.1002/brb3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure J., Duarte T.A., Schmidt U. Eating disorders. Lancet. 2020;395:899–911. doi: 10.1016/S0140-6736(20)30059-3. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Clare Kelly A.M., Biswal B.B., Xavier Castellanos F., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniacke B., Wang Y., Biezonski D., Sussman T., Lee S., Posner J., Steinglass J. Resting-state connectivity within and across neural circuits in anorexia nervosa. Brain Behav. 2019 doi: 10.1002/brb3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainik U., García-García I., Dagher A. Uncontrolled eating: a unifying heritable trait linked with obesity, overeating, personality and the brain. Eur. J. Neurosci. 2019;50:2430–2445. doi: 10.1111/ejn.14352. [DOI] [PubMed] [Google Scholar]
- Val-Laillet D., Aarts E., Weber B., Ferrari M., Quaresima V., Stoeckel L.E., Alonso-Alonso M., Audette M., Malbert C.H., Stice E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage: Clinical. 2015;8:1–31. doi: 10.1016/j.nicl.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Walsh B.T. The importance of eating behavior in eating disorders. Physiol. Behav. 2011;104:525–529. doi: 10.1016/j.physbeh.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Bi K., An J., Li M., Li K., Kong Q.-M., Li X.-N., Lu Q., Si T.-M. Abnormal structural brain network and hemisphere-specific changes in bulimia nervosa. Transl. Psychiatry. 2019;9:206. doi: 10.1038/s41398-019-0543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wierenga C.E., Ely A., Bischoff-Grethe A., Bailer U.F., Simmons A.N., Kaye W.H. Are extremes of consumption in eating disorders related to an altered balance between reward and inhibition? Front. Behav. Neurosci. 2014;8:410. doi: 10.3389/fnbeh.2014.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.M., Levinson C.A. Negative beliefs about the self prospectively predict eating disorder severity among undergraduate women. Eat. Behav. 2020;37 doi: 10.1016/j.eatbeh.2020.101384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.