Abstract
Angiotensin II (AngII) is a key mediator of the renin-angiotensin system and plays an important role in the regulation of cardiac electrophysiology by affecting various cardiac ion currents, including transient outward potassium current, Ito. AngII receptors and molecular components of Ito, Kv4.2 and Kv4.3 channels, have been linked to caveolae structures. However, their functional interaction and the importance of such proximity within 50- to 100-nm caveolar nanodomains remain unknown. To address this, we studied the mechanisms of Ito regulation by AngII in atrial myocytes of wild-type (WT) and cardiac-specific caveolin-3 (Cav3) conditional knockout (Cav3KO) mice. We showed that in WT atrial myocytes, a short-term (2 h) treatment with AngII (5 µM) significantly reduced Ito density. This effect was prevented 1) by a 30-min pretreatment with a selective antagonist of AngII receptor 1 (Ang1R) losartan (2 µM) or 2) by a selective inhibition of protein kinase C (PKC) by BIM1 (10 µM). The effect of AngII on Ito was completely abolished in Cav3-KO mice, with no change in a baseline Ito current density. In WT atria, Ang1Rs co-localized with Cav3, and the expression of Ang1Rs was significantly decreased in Cav3KO in comparison with WT mice, whereas no change in Kv4.2 and Kv4.3 protein expression was observed. Overall, our findings demonstrate that Cav3 is involved in the regulation of Ang1R expression and is required for the modulation of Ito by AngII in mouse atrial myocytes.
NEW & NOTEWORTHY Angiotensin II receptor 1 is associated with caveolae and caveolar scaffolding protein caveolin-3 in mouse atrial myocytes that is required for the regulation of Ito by angiotensin II. Downregulation of caveolae/caveolin-3 disrupts this regulation and may be implicated in pathophysiological atrial remodeling.
Keywords: angiotensin II, angiotensin II receptor 1, atrial cardiomyocyte, caveolin 3, transient outward potassium current (Ito)
INTRODUCTION
The renin-angiotensin system plays an important role in the maintenance of cardiovascular homeostasis, and dysfunction of the renin-angiotensin system could lead to the development of various pathologies, including cardiac hypertrophy, atrial fibrillation, hypertension, and heart failure (1–4). Abnormal function of the renin-angiotensin system has been associated with the occurrence of atrial and ventricular arrhythmias in experimental animal models (5, 6). Angiotensin II (AngII), an octapeptide, is the principal component of the renin-angiotensin system and is involved in vasoconstriction, increase of blood pressure, and regulation of cardiac action potential and contractility. In cardiac myocytes, AngII binds specific G protein-coupled receptors, angiotensin type 1 receptor (Ang1R), and angiotensin type 2 receptor (Ang2R). Ang1Rs are widely expressed in different cells and tissues, and mediate most of the AngII effects, both physiological and pathological (7). There is increasing evidence that AngII, through the activation of Ang1Rs, can modulate a variety of ionic currents in cardiomyocytes, including the transient outward potassium current (Ito). In HEK293 cells cotransfected with Kv4.3 (one of the channels responsible for Ito) and Ang1R proteins, 1-h incubation with AngII has been shown to significantly decrease IKv4.3 (8). Similarly, in mouse ventricular myocytes, 2-h incubation with AngII led to a significant reduction of Ito density (9). In a mouse model of hypertrophy induced by 3-wk treatment with AngII, atrial myocytes have demonstrated a reduced Ito density (10). Downstream signaling of Ang1R activation includes activation of protein kinase C (PKC) (11). Subsequently, it has been shown that activation of PKC reduced Ito in rat ventricular myocytes (12).
It was previously reported that Kv4.2 and Kv4.3 channels that are responsible for Ito can be associated with caveolae structures in cardiomyocytes (13, 14). Caveolae are small (50–100 nm) omega-shaped invaginations of the plasma membrane enriched by cholesterol and sphingolipids (15). They serve as signaling hubs localizing a number of different ion channels, G protein-coupled receptors, and signaling molecules. Caveolin-3 (Cav3) is one of the key components of caveolae required for the formation of their structure in cardiomyocytes (16, 17). Several studies demonstrated that a subpopulation of Kv4.2/4.3 channels is localized in caveolae/lipid raft structures in rat ventricular myocytes where the channels are associated with Cav3 and form a supramolecular complex with protein kinase A (13, 14). Recently, we have linked the long QT syndrome associated with mutations of Cav3, namely, LQT9 Cav3-F97C and Cav3-S141R, with a reduction in Ito current density when cotransfected with Kv4.2 and Kv4.3 channels in HEK293 cells (18). Moreover, it has been shown that Ang1Rs coimmunoprecipitate with Cav3 in mouse ventricular myocytes and thus may be localized in caveolae as well (19). However, the importance of such proximity of AngR1s and Kv4.2/Kv4.3 channels within caveolae nanodomains and their functional interaction remains unknown.
In the present study, we hypothesized that the association of AngR1s and Kv4.2/Kv4.3 channels within caveolae nanodomains plays a key role in the AngII-mediated regulation of Ito in healthy mouse atrial myocytes. We found a reduction of Ito after short-term (2-h) incubation with AngII and that AngII-dependent regulation Ito involves the activation of Ang1Rs mediated by PKC. Surprisingly, the inhibitory effect of AngII on Ito was completely abolished in Cav3 knockout mice, with no change in a baseline Ito current density. Immunofluorescent and immunoblotting studies showed colocalization of Cav3 and Ang1R, and deletion of Cav3 significantly reduced the expression of Ang1Rs. These findings demonstrate that Cav3 might be involved in the regulation of Ang1R expression and is required for modulation of Ito by AngII in mouse atrial myocytes. Downregulation of caveolae/caveolin-3 observed in various pathological conditions (20, 21) may disrupt AngII modulation of Ito and thus contribute to pathophysiological atrial remodeling and arrhythmogenesis.
METHODS
Isolation of Mouse Atrial Myocytes
All methods and protocols used in this study were approved by the animal care and use committee of the University of Wisconsin-Madison following the Guidelines for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH; Publication No. 85-23, revised 1996). All animals used in this study received humane care in compliance with Guide for the Care and Use of Laboratory Animals.
Adult (6- to 10-wk-old) wild-type mice (WT; C57BL6, N = 17) and conditional cardiac-specific Cav3 knockout mice (Cav3KO, N = 9) of both sexes were used. Cav3KO mice were provided by Dr. Timothy Kamp and generated as previously described (22). Briefly, Cav3KO mice were generated by crossing a mouse line with an loxP-flanked exon 2 of Cav3 with a tamoxifen-inducible Cre recombinase protein fused to two mutant estrogen-receptor ligand-binding domains (MerCreMer) under the control of the α-myosin heavy chain promoter (23) mouse producing a tamoxifen-inducible, cardiac-specific Cav3KO mouse (22, 24). Tamoxifen was delivered in the form of tamoxifen-laced food, at 0.5 mg tamoxifen/1 g pulverized chow, reconstituted with 1 mL H2O/1 g chow, reformed, and dried for 24–48 h. Mice remained on the diet for 14 days and euthanized 14 days post tamoxifen treatment. Mice were injected with 100 units of heparin and anesthetized with isoflurane (induced at 3%–5% and maintained at 1%–3%) and loss of pain reflex was checked to assure appropriate level of anesthesia. Hearts were extracted, cannulated, and perfused with Tyrode’s solution (37°C, 2–4 min). Tyrode’s solution for isolation contained (in mM): 140 NaCl, 5.4 KCl, 1.2 KH2PO4, 1 MgCl2, 1.8 CaCl2, 5 HEPES, 5.5 dextrose, pH = 7.4 (NaOH). Atria were separated, cut into eight small pieces and then rinsed in “low Ca2+/Mg2+-free” solution for 4 min. “Low Ca2+/Mg2+-free” solution contained the following (in mM): 140 NaCl, 5.4 KCl, 0.07 CaCl2, 50 taurine, 5 HEPES, 18.5 dextrose, 1 mg/mL bovine serum albumin (BSA), pH = 6.9 (NaOH). Atrial pieces were transferred to “low Ca2+/Mg2+-free” solution containing 1.9 mg/mL collagenase from Clostridium histolyticum (type V, Sigma-Aldrich, St. Louis, MO) for 15 min (37°C). Then, the pieces were additionally cut and resuspended in modified KB solution containing the following (in mM): 100 KC5H8NO4, 10 potassium aspartate, 25 KCl, 10 KH2PO4, 2 MgSO4, 20 taurine, 5 creatine, 0.5 EGTA, 5 HEPES, 20 dextrose, 1 mg/mL BSA, pH = 7.4 (KOH). Isolated single atrial myocytes were recalcified to a final 1 mM Ca2+ concentration and kept in modified KB solution.
Whole Cell Patch Clamp Electrophysiology
Electrophysiological experiments were carried out in the whole cell configuration of the patch-clamp technique at room temperature using the Axopatch 200B amplifier (Axon Instruments, Foster City, CA) with pCLAMP 10.7 software. Recording pipettes were pulled from thin-walled borosilicate glass capillaries (World Precision Instruments, Inc., Sarasota, FL) with pipette resistance of 3–5 MΩ. The recordings were filtered at 2 kHz and digitized at 20 kHz. Ito currents were recorded in a voltage clamp mode of the patch-clamp technique. All solutions and buffers are indicated in mM/l. The Ito external bath solution contained 90 NaCl, 50 TEA-Cl, 4 KCl, 1 MgCl2, 1 CaCl2, 2 CoCl2, 11 dextrose, 10 HEPES, and pH 7.4 (NaOH). Intracellular pipette solution contained 80 K-aspartate, 42 KCl, 10 KH2PO4, 5 Mg-ATP, 3 phosphocreatine, 5 EGTA, and 5 HEPES, pH 7.2 (KOH); 50 mM TEA-Cl was added to bath extracellular solution to inhibit IK,slow1. To avoid contamination with Iss, the Ito current amplitudes were measured from steady-state level to the current peak. Whole cell currents were recorded from a holding potential −70 mV with 400-ms test pulses from −40 to 60 mV, in 10 mV increments, with short prepulse −40 mV with 15 ms to inactivate INa. AngII (Sigma) and Losartan (Tocris) were dissolved in the extracellular solution. BIM 1 (bisindolylmaleimide 1; Cayman) was dissolved in the internal solution. Data were analyzed using Microcal Origin software (Origin Lab Corporation Northampton, MA).
Immunofluorescence Confocal Microscopy
Immunolabeling was performed on atrial myocytes using a rabbit polyclonal antibody to Cav3 (ab2912; Abcam) and a mouse monoclonal antibody to Ang1R (SC515884; Santa Cruz Biotechnology). Atrial myocytes were fixed with ice-cold methanol for 10 min. After being washed with PBS containing 0.1% Tween (PBS-T) (2 × 10 min), cells were incubated with blocking solution (2% BSA and 2% goat serum in PBS-T) for 2 h in 15-mm tissue culture dishes with gentle agitation at room temperature to block nonspecific binding sites. Subsequently, cells were incubated overnight with respective primary antibodies in blocking solution at 4°C. Antibody dilution for primary antibodies was 1:500 for the polyclonal anti-Cav3 and 1:100 for the monoclonal anti-Ang1R. Excess primary antibody was washed off with PBS-T (5 ×10 min). Cells were then incubated for 1.5 h with Alexa conjugated secondary antibodies (2 mg/mL, Invitrogen) diluted in 1:800 blocking solution. Highly cross-absorbed Alexa 568 goat anti-rabbit IgG (H + L) (Cat. No. A-11011, Invitrogen) and Alexa 488 goat anti-mouse IgG (H + L) (A-11001, Invitrogen) were used for immunostaining of atrial myocytes. Cells were then washed with blocking solution (3 × 2 h). After the final wash, cells were mounted using ProLong Diamond Antifade Mountant (Thermo Fisher Scientific). To determine nonspecific immunolabeling by the secondary antibody, control experiments were performed with only secondary antibody. These experiments demonstrated minimal fluorescence signal (data not shown). Myocytes were imaged with an SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) using ×63 oil objective and filters. Excitation and emission were set at 488/525 ± 20 nm for Alexa 488 and 561/525 ± 20 nm for Alexa 568. A sequencing pattern and 128-line averaging was applied during imaging. The analysis of protein colocalization was performed by scoring Mander’s coefficients using the Jacob plugin in ImageJ (25). The thresholds for each color were determined using an automatic threshold algorithm of ImageJ.
Immunoblotting
Immunoblotting was performed on adult mouse atrial homogenates prepared from either WT or Cav3KO mice (N = 5–9 mice/group, –three to four technical replicates per blot). Homogenates were solubilized using ice-cold CHAPS lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% CHAPS, 1× protease inhibitor cocktail, Bimake). The lysate was centrifuged at 13.2 g for 10 min to remove insoluble debris. Protein content was estimated in the soluble supernatant via BCA (Thermo Fisher Scientific), and 5–10 μg of each sample (equally loaded) was analyzed by SDS-PAGE (Tris-HCl 4–20% gradient gels, BioRad). Gels were transferred to PVDF membranes via the Powerblotter system (Invitrogen) set for mixed range. Membranes were blocked for 1 h at room temperature with 3% BSA in PBS and incubated overnight (∼16 h) with primary antibody (1–5 μg/mL) at 4°C. Corresponding Alexa Fluor 488 fluorescent secondary antibodies were used to evaluate protein expression. Antibodies targeting Cav3 (Abcam), GAPDH (Millipore), Kv4.2 (NeuroMab, UC Davis, CA), Kv4.3 (Alomone Labs), Ang1R (Santa Cruz Biotechnology), and Ang2R (Alomone Labs) were used. Protein expression was quantified relative to GAPDH using ImageJ.
Coimmunoprecipitation
Using the aforementioned antibodies, coimmunoprecipitation was performed targeting Cav3, GAPDH, Kv4.2, Kv4.3, Ang1R, and mouse IgG (Sigma-Aldrich) as a negative control using lysate from mouse atria (n = 3 biological replicates). Protein complexes were analyzed via Western blot using the aforementioned immunoblotting methods.
Statistical Analysis
Data are reported as means ± SE. Determinations of statistical significance were performed using a Student t test for comparisons of two means. Statistical significance was determined by a value of P < 0.05. Statistical analysis was performed using Origin 7.5 (OriginLab Corporation, Northampton, MA).
RESULTS
Effects of Angiotensin II on Ito Currents
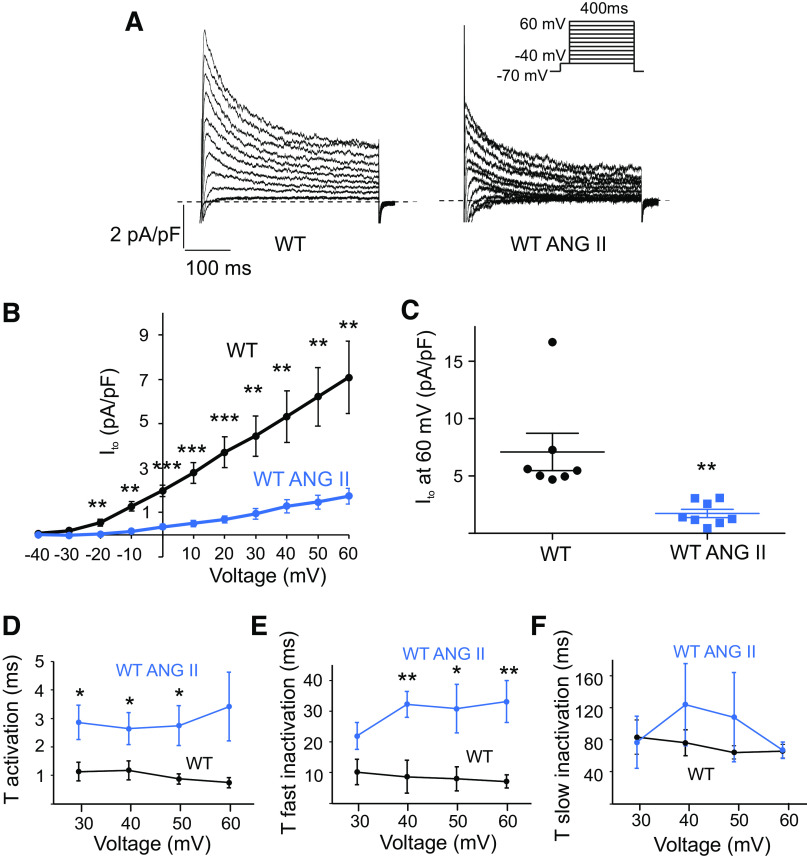
Freshly isolated mouse atrial myocytes were treated with 5 µM AngII for 2 h (9). As shown in Fig. 1, AngII significantly decreased Ito current amplitudes in WT cells at voltages from −20 to 60 mV. The peak of Ito normalized to cell capacitance was significantly smaller in AngII-treated cells (1.7 ± 0.4 pA pF−1 at 60 mV, n = 8 cells, P < 0.01) than in untreated cells (7.1 ± 1.6 pA pF−1 at 60 mV, n = 7 cells, Fig. 1, A–C). The kinetics of voltage-dependent activation and inactivation contribute to the determination of peak current of Ito. We analyzed the effect of AngII on activation and inactivation of Ito in WT cells. The activation of Ito was analyzed by fitting the rising phase of current traces to a single exponential fit at test voltages from 40 to 60 mV. As shown in Fig. 1D, the activation time constants for WT-AngII were significantly greater than those for WT, from 30 to 50 mV. The fast (τfast inactivation; Fig. 1E) and slow inactivation (τslow inactivation; Fig. 1F) time constants were determined for Ito by fitting the decaying phase of the current traces to a double exponential function at test voltages from 30 to 60 mV. The τfast inactivation time constants were significantly greater for WT-AngII than for WT from 40 to 60 mV (Fig. 1E). The slow inactivation (τslow, Fig. 1F) time constants were not different between WT-AngII and WT. These data demonstrate that AngII results in both slower activation and fast inactivation kinetics of Ito relative to untreated WT. We also measured Iss currents as a current component from baseline values to the values at the end of test pulses, where Ito is inactivated. Amplitudes of Iss were not significantly changed by AngII treatment (4.1 ± 1.2 pA pF−1 at 60 mV, n = 8 cells) compared with untreated WT cells (4.1 ± 1.1 pA pF−1 at 60 mV, n = 7 cells). It has been previously shown that in a mouse model of cardiac hypertrophy induced by a 3-wk treatment with AngII, left atrial myocyte capacitance was significantly increased (10). In our experiments, 2-h treatment with AngII did not change cell capacitance (60.8 ± 4.1 pF, n = 5 cells in WT mice vs. 54.5 ± 8.9, n = 6 cells in WT with AngII mice).
Figure 1.
AngII reduces Ito density in WT mouse atrial myocytes. A: representative current traces illustrating Ito currents at baseline and after 2-h incubation with AngII (5 µM). Whole cell currents were recorded using the protocol indicated in the inset. B: mean current-voltage (I-V) relationships of the peak Ito density in untreated WT cells and WT cells treated with AngII (n = 7–8 cells). ***P < 0.001, **P < 0.01. C: dot-and-whisker plots showing peak Ito at 60 mV for WT and WT with AngII cells (n = 7–8 cells). **P < 0.01. D: average τ activation for Ito from WT and WT-AngII cells as a function of test voltage (means ± SE, n = 7–8 cells, **P < 0.01, *P < 0.05). E and F: average τfast inactivation and τslow inactivation for Ito from WT and WT-AngII cells as a function of test voltage (means ± SE, n = 7–8 cells, **P < 0.01, *P < 0.05). AngII, angiotensin II, Ito, transient outward potassium current; WT, wild-type.
Effects of AngII on Ito are Mediated by AngII Receptor 1 (Ang1R)
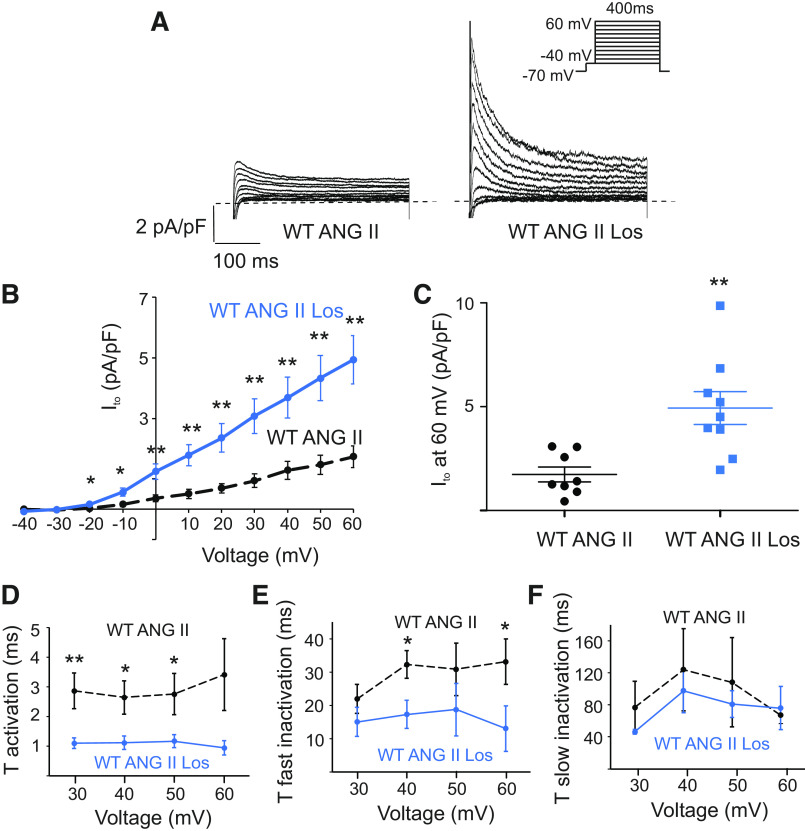
In the heart, AngII binds two types of angiotensin receptors, namely, AngII receptor 1 (Ang1R) and AngII receptor 2 (Ang2R), with Ang1Rs mediating most of the AngII effects, both physiological and pathological (7). To determine if Ang1Rs are involved in Ito regulation, we treated atrial myocytes with 2 µM losartan, a selective antagonist of Ang1Rs, for 30 min prior to the treatment with AngII. Figure 2 shows that losartan prevented the reduction of Ito by AngII. Peaks of Ito currents in myocytes treated with losartan and Ang II were significantly larger (4.9 ± 0.8 pA pF−1 at 60 mV, n = 9 cells, P < 0.01) than those in cells treated with AngII only (1.7 ± 0.4 pA pF−1 at 60 mV, n = 8 cells Fig. 2, A–C). Ito peak currents were similar to those measured in untreated WT myocytes (7.1 ± 1.6 pA pF−1 at 60 mV in WT at baseline, n = 7 cells vs 4.9 ± 0.8 pA pF − 1 at 60 mV in WT with AngII and losartan, n = 9 cells, P = 0.2). Analysis of activation and inactivation properties of Ito revealed a difference between WT-AngII with and without losartan for activation and fast inactivation. As shown in Fig. 2D, the activation constants for WT-AngII with losartan were significantly smaller than those for WT-AngII at 30 to 50 mV. The τfast inactivation time constants were significantly smaller for WT-AngII with losartan than for WT-AngII at 40 and 60 mV (Fig. 2E). The slow inactivation (τslow, Fig. 2F) time constants were not different between WT-AngII with losartan and WT-AngII without losartan. Losartan not only increased Ito peak currents of WT-AngII but also resulted in faster activation and fast inactivation kinetics of Ito relative to WT-AngII. These findings demonstrate that the AngII-mediated reduction in Ito occurs through the activation of Ang1Rs in mouse atrial myocytes.
Figure 2.
Incubation with Ang1R-specific antagonist losartan (Los) prevents downregulation of Ito by AngII. A: representative traces illustrating Ito currents treated with 5 µM AngII-only (2 h) and after the pretreatment with 2 µM Losartan (2.5 h; WT AngII Losartan). Whole cell currents were recorded using the protocol indicated in the inset. B: mean I-V relationships of peak Ito density in WT cells treated with AngII-only (the same as in Fig. 1B) and WT cells treated with AngII and Los (n = 8–9 cells). **P < 0.01, *P < 0.05. C: dot-and-whisker plots showing peak Ito at 60 mV for WT cells treated with AngII-only (the same as in Fig. 1C) and WT cells treated with AngII and Los cells (n = 8–9 cells). **P < 0.01. D: average τ activation for Ito from WT cells treated with AngII only (the same as in Fig. 1D) and WT cells treated with AngII and Los as a function of test voltage (means ± SE, n = 8–9 cells, **P < 0.01, *P < 0.05). E and F: average τfast inactivation and τslow inactivation for Ito from cells treated with AngII only (the same as in Fig. 1, E and F) and WT cells treated with AngII and Los as a function of test voltage (means ± SE, n = 8–9 cells, *P < 0.05). Ang1R, angiotensin type 1 receptor; AngII, angiotensin II; Ito, transient outward potassium current; WT, wild-type.
Inhibition of Protein Kinase C Prevents the Downregulation of Ito by AngII
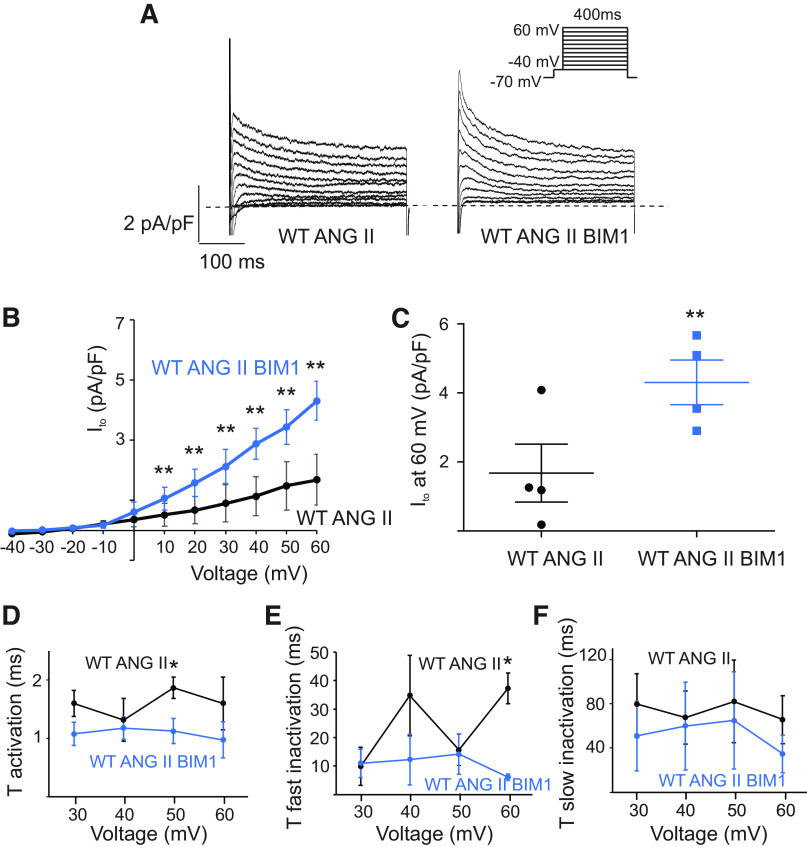
Ang1Rs mediate the activation of phospholipase C, which catalyzes the hydrolysis of the membrane phospholipid, phosphatidylinositol biphosphate, producing diacylglycerol (DAG), a well-known activator of PKC (7). Scholz et al. (12) have shown that selective activation of PKCα by PMA (Phorbol 12-myristate 13-acetate) significantly reduced Ito in rat ventricular myocytes, highlighting a key role of PKC in AngII-mediated regulation of Ito. Moreover, inhibition of PKC by a selective blocker bisindolylmaleimide 1 (BIM 1) prevented the reduction of the fast sodium current INa in atrial myocytes chronically (three weeks) treated with AngII (10). Accordingly, we hypothesized that an elevated PKC activity may underlie the reduction of Ito induced by AngII in atrial myocytes. We tested the effect of PKC inhibition on Ito by dialyzing the AngII-treated myocytes with BIM 1 (10 µM) (26). Representative traces, I-V curves, and summary plots shown in Fig. 3, A–C, demonstrate that BIM 1 potently reversed the reduction of Ito density in the myocytes treated with AngII (1.7 ± 0.8 pA pF−1 at 60 mV before BIM 1, n = 4 cells, vs 4.3 ± 0.7 pA pF − 1 at 60 mV after BIM 1, n = 4 cells, P < 0.01). Then we analyzed time constants of activation and inactivation of Ito for WT-AngII before and after BIM1. As shown in Fig. 3, D and E, BIM1 results in significantly faster activation of Ito for WT AngII at 50 mV and accelerated a fast component of inactivation of Ito for WT-AngII at 60 mV. Analysis of the slow inactivation time constant did not reveal the difference between groups (Fig. 3F). These results confirm that AngII reduces Ito by stimulating PKC activity in mouse atrial myocytes.
Figure 3.
Inhibition of protein kinase C (PKC) prevents downregulation of Ito by AngII. A: representative Ito recordings in AngII-treated atrial myocytes following dialysis with PKC inhibitor BIM1 (10 µM) and in myocytes treated with AngII-only. BIM1 was dialyzed into the myocytes via patch-clamp pipette for 5 min before recording. Whole cell currents were recorded using the protocol indicated in the inset. B: summary of Ito I-V curves for AngII and AngII with BIM1 dialysis (n = 4 cells). ***P < 0.001, **P < 0.01. C: dot-and-whisker plots showing peak Ito at 60 mV for AngII and AngII with BIM1 dialysis (n = 4 cells). **P < 0.01. D: average τ activation for Ito from AngII and AngII with BIM1 dialysis as a function of test voltage (means ± SE, n = 4 cells, *P < 0.05). E and F: average τfast inactivation and τslow inactivation for Ito from AngII and AngII with BIM1 dialysis as a function of test voltage (means ± SE, n = 4 cells, *P < 0.05). AngII, angiotensin II; Ito, transient outward potassium current.
Caveolin-3 is required for the Reduction of Ito by AngII
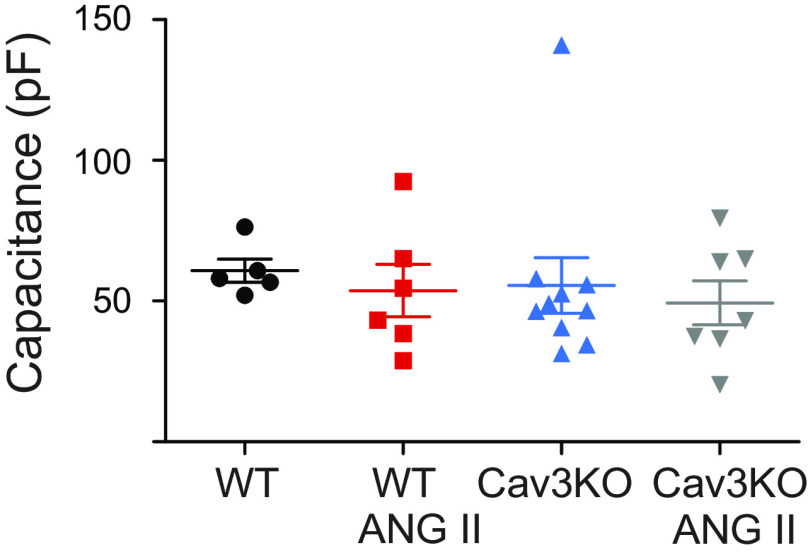
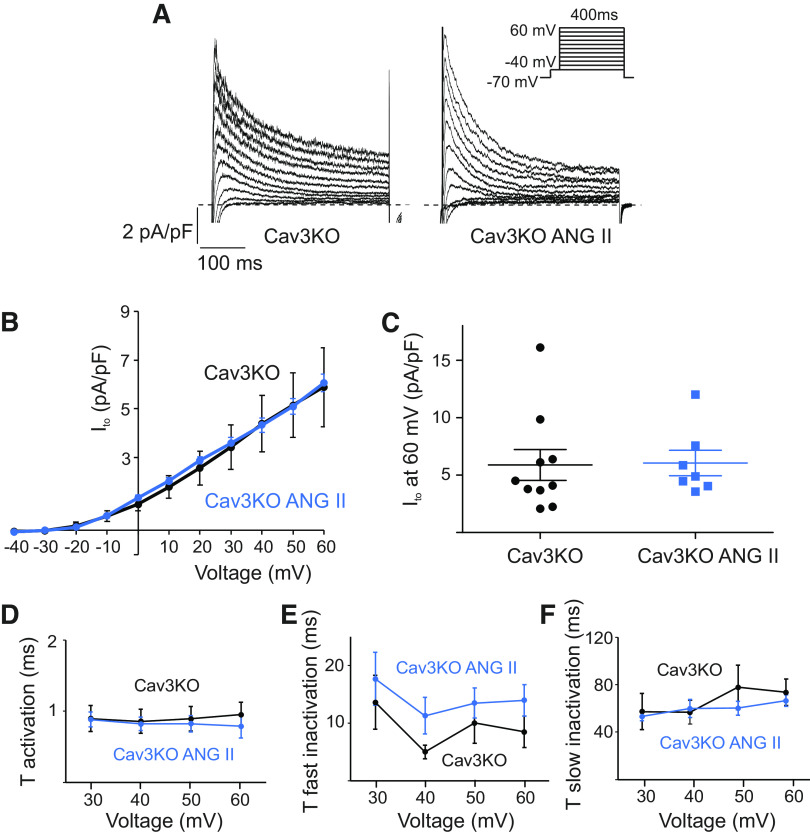
To determine the role of Cav3 in Ito regulation by AngII, we used cardiac-specific tamoxifen-induced Cav3KO mice (22, 24). Mean capacitance of atrial myocytes was not different between WT and Cav3KO groups, indicating the absence of cell hypertrophy in KO mice (Fig. 4). Treatment with AngII also did not affect the mean cell capacitance in Cav3KO (55.5 ± 9.9 pF in Cav3KO, n = 10 cells, vs 49.4 ± 7.8 in Cav3KO with AngII, n = 7 cells). At baseline, the Ito current densities measured at 60 mV in WT and Cav3KO were not different (7.1 ± 1.6 pA pF−1 at 60 mV in WT, n = 7 cells, vs 5.9 ± 1.3 pA pF−1 at 60 mV in Cav3KO, n = 10 cells, P = 0.6). Interestingly, AngII did not affect baseline Ito current amplitudes in Cav3KO atrial cells (Fig. 5). The peak of Ito normalized to cell capacitance was not different in AngII-treated Cav3KO cells either (6.1 ± 1.1 pA pF−1 at 60 mV, n = 7 cells, vs 5.9 ± 1.3 pA pF−1 at 60 mV in untreated Cav3KO cells, n = 10 cells, P = 0.9, Fig. 5, A–C). The time constants of activation (Fig. 5D) and inactivation (Fig. 5, E and F) were not different between Cav3KO and Cav3KO-AngII. Taken together, these data show that although Ito is unchanged in Cav3KO atria at baseline, Cav3 is required for the regulation of Ito by AngII.
Figure 4.
Cell capacitance measured from wild-type (WT) and conditional knockout (Cav3KO) atrial myocytes with/without 2-h incubation with angiotensin II (AngII; 5 µM; n = 5–10 cells).
Figure 5.
AngII does not affect Ito density in Cav3KO myocytes. A: representative current traces illustrating Ito currents at baseline and after 2-h incubation with AngII (5 µM) in Cav3KO atrial myocytes. Whole cell currents were recorded using the protocol indicated in the inset. B: mean I-V relationships of peak Ito density in Cav3KO cells at baseline and after treatment with AngII (n = 7–10 cells). C: dot-and-whisker plots showing peak Ito at 60 mV for Cav3KO and Cav3KO with AngII cells (n = 7–10 cells). D: average τ activation for Ito from Cav3KO and Cav3KO-AngII cells as a function of test voltage (means ± SE, n = 7–10 cells). E and F: average τfast inactivation and τslow inactivation for Ito from Cav3KO and Cav3KO-AngII cells as a function of test voltage (means ± SE, n = 7–10 cells). AngII, angiotensin II; Cav3KO, conditional knockout; Ito, transient outward potassium current.
Ang1R and Cav3 are Colocalized in Atrial Myocytes
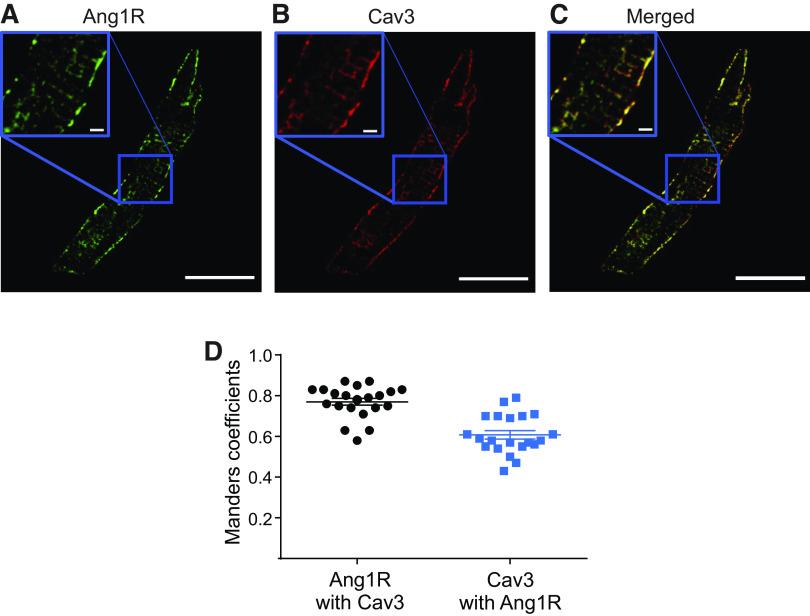
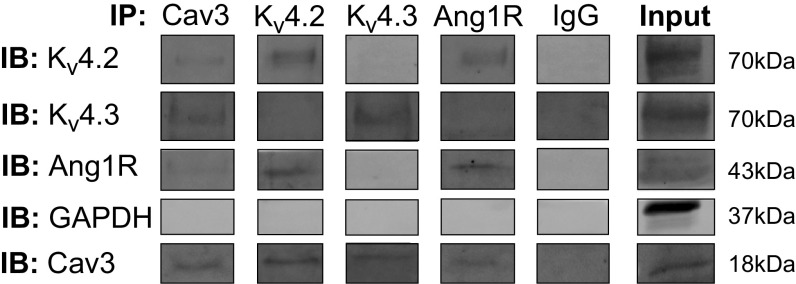
As our patch-clamp experiments revealed that AngII regulates Ito through activation of Ang1Rs and Cav3 is required for AngII-mediated regulation of Ito, we performed immunofluorescent staining to determine whether Cav3 impacts membrane localization of Ang1Rs. WT atrial myocytes were immunolabeled for Cav3 and Ang1Rs. Immunolabeling with Cav3 showed regions of colocalization with Ang1Rs having a punctate expression pattern in the surface membrane that likely represent caveolae structures (Fig. 6, A–C). Analysis of Mander’s coefficients for Ang1Rs and Cav3 showed a high degree of protein colocalization (0.77 ± 0.02 A.U. for Ang1Rs and 0.61 ± 0.02 A.U. for Cav3, Fig. 6D; n = 21 cells from N = 3 mice). Direct association between Ang1Rs and Cav3 has been confirmed in immunoprecipitation experiments, as shown in Fig. 7 Moreover, we found that in addition to Ang1Rs, both Kv4.2 and Kv4.3 coimmunoprecipitated with Cav3 (Fig. 7). Interestingly, when the lysate was subjected to immunoprecipitation with anti-Ang1R and anti-Kv4.2, the following Western blot analysis showed that Cav3, Ang1R, and Kv4.2 (but not Kv4.3) are closely associated with each other that may indicate the presence of macromolecular complex between these proteins. This was different for Kv4.3 which was found to be associated only with Cav3 but not with Ang1R or Kv4.2 (Fig. 7).
Figure 6.
Ang1R showed regions of high co-localization with Cav3 in WT atrial myocytes. Representative images of atrial cells immunolabeled with, A: anti-Ang1R (green), B: anti-Cav3 (red) antibodies, and C: merged colors, scale is 20 µm. Inlets show magnified areas from (A–C), outlined by blue square, scale is 2 µm. D: Mander’s coefficient analysis of colocalization for Ang1R and Cav3 (n = 21 cells from N = 3 mice). Ang1R, angiotensin type 1 receptor; WT, wild-type.
Figure 7.
Coimmunoprecipitation Western blots showing degree of association between Cav3, Ang1R, and Kv4.2 and Kv4.3 channels in mouse atrial myocardium (N = 3 mice). IB indicates immunoblot; IP, immunoprecipitation. Individual bands are composed from the original captures as shown in Supplemental Fig. S1. at https://doi.org/10.6084/m9.figshare.13515347.v1. The rearrangements are shown by white spaces between the individual bands. Ang1R, angiotensin type 1 receptor.
Cav3KO Mice Show Decreased Ang1R, But Not Ang2R, Kv4.2, or Kv4.3 Expression
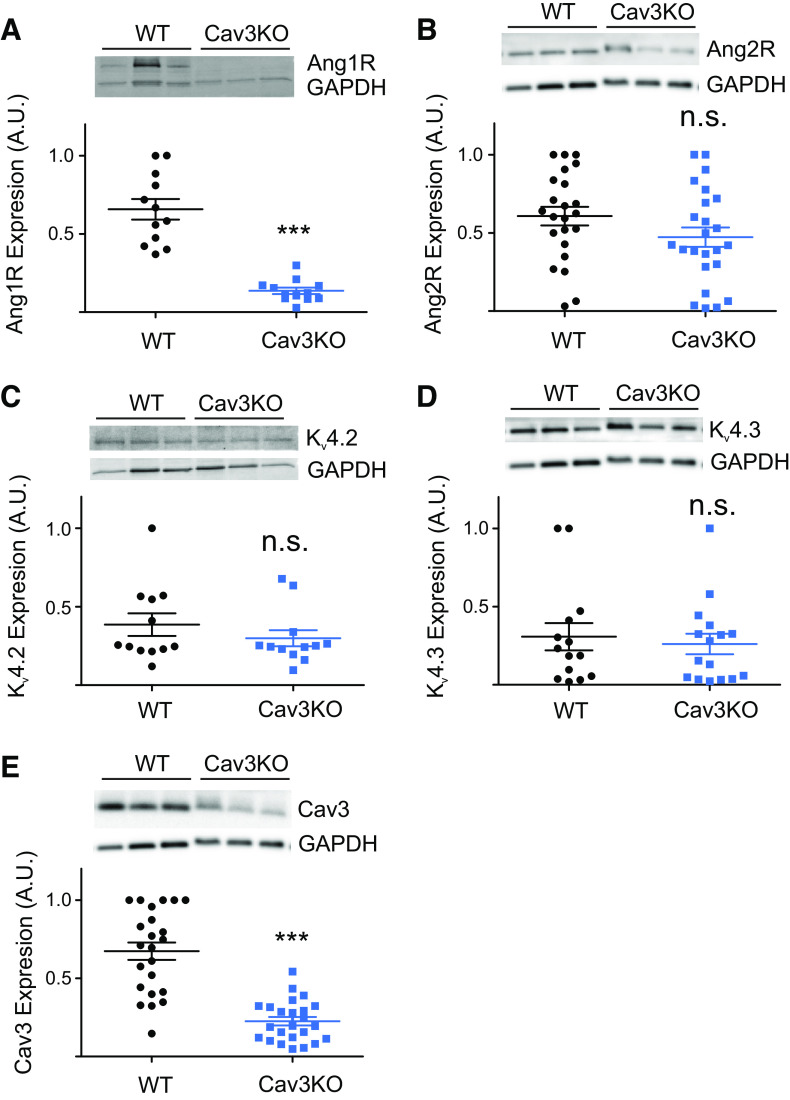
The lack of AngII effect on Cav3KO atrial myocytes may be linked to the changes in ion channels responsible for Ito (Kv4.2 and Kv4.3) or AngII receptors (Ang1R or Ang2R) expression. As shown in Fig. 8, immunoblot analysis demonstrated that the protein expression of the Kv4.2 (Fig. 8C; N = 6 mice/group; Cav3KO 0.71 ± 0.12 vs WT 0.91 ± 0.17) and Kv4.3 (Fig. 8D; N = 6 mice/group; Cav3KO 0.26 ± 0.07 vs WT 0.31 ± 0.09) was unchanged between WT and Cav3KO groups. It confirms our patch-clamp data showing no significant difference for Ito between mouse groups. In contrast, protein expression of Ang1R (Fig. 8A; N = 6 mice/group; Cav3KO 0.21 ± 0.03 vs WT 0.99 ± 0.1; P < 0.001) was significantly decreased in Cav3KO mice compared with WT mice. At the same time, the expression of Ang2R (Fig. 8B; N = 5–6 mice/group; Cav3KO 0.47 ± 0.06 vs WT 0.61 ± 0.06) was unchanged between mouse groups. We then performed a confirmatory immunoblot to show that Cav3 was significantly decreased in Cav3KO mice (Fig. 8E; N = 9 mice/group; Cav3KO 0.23 ± 0.03 vs WT 0.67 ± 0.06; P < 0.001). These results show that Cav3 is involved in the regulation of Ito by AngII and the presence of Cav3 is required for the expression of Ang1Rs.
Figure 8.
Atrial tissue from Cav3KO mice showed decreased protein expression of Ang1Rs (A), no change in Ang2R (B), Kv4.2 (C), and Kv4.3 (D). Reduced Cav3 protein expression was confirmed in Cav3KO hearts (E). ***P < 0.001; n.s. indicates not significant. Data are presented as means ± SE; N = 5–9 mice/group, three to four technical replicates per blot. Values are shown as normalized to the maximal expression level within each blot. Ang1R, angiotensin type 1 receptor; Ang2R, angiotensin type 2 receptor; Cav3KO, conditional knockout.
DISCUSSION
In the present study, we demonstrated that Ang1Rs are associated with caveolae structures and involved in the regulation of Ito via activation of PKC in mouse atrial myocytes. Our results are consistent with previous findings observed in mouse ventricular myocytes. Kim et al. (9) have shown that a short-term treatment of ventricular myocytes with AngII leads to Ito reduction. Moreover, in the model of hypertrophy, when increased levels of AngII in mouse atrial myocytes were reached by using implanted osmotic minipumps, the reduction of Ito density was also observed (10). Effects of AngII through the activation of Ang1Rs have been demonstrated on various currents in different cell types. Doronin et al. (8) showed the inhibitory effect of AngII on IKv4.3 in HEK293 cells transiently transfected Ang1Rs. Moreover, a selective inhibition of Ang1Rs by valsartan prevented the stimulatory effects of AngII on IKs in guinea pig atrial myocytes (27).
Similar to our findings, findings of several other studies showed that downstream Ang1R signaling involves the activation of PKC. Jansen et al. (10) have demonstrated that dialysis of BIM 1, a selective PKC antagonist, had a stimulatory effect on INa reduced by AngII in mouse left atrial myocytes. Similarly, a stimulatory effect of AngII on IKs was abolished by another specific PKC inhibitor Bis I, as well as a nonspecific PKC inhibitor H-7 in guinea pig atrial myocytes (27). A stimulatory effect of AngII on ICaL was shown to involve the activation of Ang1Rs and PKC in cat ventricular myocytes (28). These data demonstrate a significant role of AngII signaling in electrical remodeling in cardiomyocytes from different species. Our study is of interest, as it shows effects of AngII signaling on Ito in healthy mouse atrial myocytes for the first time.
Importantly, for the first time, we linked the regulation of Ito by AngII to caveolae structures. It was recently shown that in rat and human ventricular myocytes, there are two populations of Ito channels with regard to their localization in caveolae: one population of Kv4.2/Kv4.3 channels has been described as caveolar and interacts with Cav3, and another one was associated with noncaveolar lipid rafts (13, 14). The caveolar population of Kv4.x channels was found to be selectively regulated by α1-adrenoreceptors (14). In contrast, noncaveolae population of Kv4.x channels was associated with Ca2+/calmodulin-dependent protein kinase II (CaMKII) to form a functional macromolecular complex (13). It was also reported that in canine ventricular myocytes, Kv4.3 channels coimmunoprecipitated with Ang1Rs (8), which may be critical for the channel’s regulation by AngII. To note, the role of Cav3 for regulation of ionic currents has been shown for not only Ito but also ICaL. Balijepalli et al. (29) have revealed that localization of Cav1.2 channels in caveolae is necessary for the regulation of these channels by ß2-adrenoreceptors.
Interestingly, although Cav3 is required for AngII regulation of Ito, loss of Cav3 did not change the amplitude of baseline Ito in atrial myocytes. This is different from the effect of Cav3 downregulation found on basal ICa,L in mouse ventricular myocytes with global Cav3KO (30), where the deletion of Cav3 may affect the channel’s basal phosphorylation and thus decrease the current (31). At the same time, as we recently demonstrated (18), Cav3 mutations, that were associated with the long QT syndrome, namely, LQT9 Cav3-F97C and Cav3-S141R, result in significant downregulation of Ito current density in HEK293 cells. Therefore, the effect of Cav3 on Ito channels may be more complex and distinct from that of caveolae and caveolar components, as in our experiments, Cav3 was downregulated in Cav3KO mice. Whether inhibition of Cav3 function in the presence of intact caveolae structures affects Ito channels, as it was found for L-type Ca2+ channels (31) remains unknown and would require additional studies.
Knockout of Cav3 significantly decreased the expression of Ang1Rs (Fig. 7). Though it may explain the loss of AngII regulation of Ito, the mechanisms underlying Ang1R downregulation are unknown and may involve receptor’s expression, trafficking, and/or protein stability on the membrane (17, 32, 33). It was shown that Cav3 may act as a chaperone for the Ang1Rs, allowing the receptor to traffic through the exocytic pathway and to localize at the cell membrane in transfection systems (33). This interaction seems to be mediated by the caveolin scaffolding domain and is required to prevent the mislocalization of Ang1Rs to lipid bodies or Golgi, which results in aberrant maturation and surface expression of Ang1Rs. Similarly, the expression of Ang1Rs has been shown to be significantly downregulated in rat atrial myocytes, where caveolae were disrupted via cholesterol depletion by 1-h incubation with 10 mM methyl-β-cyclodextrin (34).
Loss of AngII regulation of Ito (and likely other ion currents) may be implicated in pathophysiological atrial remodeling associated with Cav3/caveolae downregulation as seen in various pathophysiological conditions including atrial fibrillation (21), hypertension (20), and heart failure (35, 36). The renin-angiotensin system is critically involved in the physiological regulation of blood pressure and volume homeostasis. Circulating AngII, as well as AngII released locally in the myocardium in response to stretch (7, 37), modulates the heart’s performance to attenuate mechanical overload of heart chambers and synchronize cardiac output with venous return, arterial blood supply, and humoral length. These include changes in action potential duration and rate-dependent action potential duration adaptation that are lost during atrial fibrillation (38). Loss of Ito regulation by AngII can also contribute to action potential shortening observed in the remodeled atria (38).
Recent studies indicate that elevated blood pressure and an associated increase in cardiomyocyte stretch lead to disruption of caveolae structures and downregulation of Cav3 protein expression (20) which can provide a mechanistic foundation for atrial remodeling and arrhythmogenesis. A similar caveolae-mediated disruption of AngII regulation may be also applied to other channels. Indeed, overexpression of Cav3 in mice attenuates pressure overload-induced cardiac hypertrophy, via the inhibition of T-type Ca2+ current modulated by PKCα (19) or via the increase in the expression of natriuretic peptides (39). It is interesting to note that Cav3OE resulted in the increased expression of Kv4.3 but not Kv4.2 in mouse ventricular myocytes (40). In contrast, mice with global Cav3KO develop a progressive, mild-to-moderate cardiomyopathic phenotype (41). Bryant et al. (30) confirmed these findings and linked them to the disruption of ventricular t-tubule structure and decrease in t-tubular ICa,L density. In our studies, we used mice with conditional, cardiac-specific Cav3KO that did not demonstrate any structural abnormalities and hemodynamic changes (22, 24). However, as it was recently reported, these mice with conditional, cardiac-specific Cav3KO demonstrate an increased risk of ventricular arrhythmogenesis and significant prolongation of action potential duration associated with upregulation of late INa and decrease in ICa,L, Ito, and Iss current densities (24). Such changes over time may thus result in cardiomyopathic phenotype as observed in mice with global Cav3KO (30, 41). Overall, these findings highlight cardioprotective functions of caveolae structures and thus may open completely new avenues for more effective therapeutic approaches targeted on caveolae and associated signaling pathways to protect atrial myocardium from damage and ameliorate atrial arrhythmias.
GRANTS
This work was supported by NIH Grant R01HL141214, American Heart Association Grant 16SDG29120011, the Wisconsin Partnership Program 4140 (to A.V.G.), and NIH predoctoral training Grant T32GM008688 (to D.T).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.T., and A.V.G. conceived and designed research; L.T., D.T., K.R.K., R.Y.M., and E.L. performed experiments; L.T., D.T., K.R.K., R.Y.M., and E.L. analyzed data; L.T., D.T., K.R.K., R.Y.M., E.L., and A.V.G. interpreted results of experiments; L.T., D.T., and R.Y.M. prepared figures; L.T., and A.V.G. drafted manuscript; L.T., D.T., K.R.K., R.Y.M., and A.V.G. edited and revised manuscript; L.T., D.T., K.R.K., R.Y.M., E.L., and A.V.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Timothy J. Kamp (University of Wisconsin-Madison) for sharing Cav3KO mice and for critical reading and valuable comments on the manuscript.
REFERENCES
- 1.Higaki J, Aoki M, Morishita R, Kida I, Taniyama Y, Tomita N, Yamamoto K, Moriguchi A, Kaneda Y, Ogihara T. In vivo evidence of the importance of cardiac angiotensin-converting enzyme in the pathogenesis of cardiac hypertrophy. Arterioscler Thromb Vasc Biol 20: 428–434, 2000. doi: 10.1161/01.ATV.20.2.428. [DOI] [PubMed] [Google Scholar]
- 2.Mascolo A, Urbanek K, De Angelis A, Sessa M, Scavone C, Berrino L, Rosano GMC, Capuano A, Rossi F. Angiotensin II and angiotensin 1-7: which is their role in atrial fibrillation? Heart Fail Rev 25: 367–380, 2020. doi: 10.1007/s10741-019-09837-7. [DOI] [PubMed] [Google Scholar]
- 3.Opie LH, Sack MN. Enhanced angiotensin II activity in heart failure: reevaluation of the counterregulatory hypothesis of receptor subtypes. Circ Res 88: 654–658, 2001. doi: 10.1161/hh0701.089175. [DOI] [PubMed] [Google Scholar]
- 4.Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci USA 97: 931–936, 2000. doi: 10.1073/pnas.97.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada K, Komuro I, Hayashi D, Sugaya T, Murakami K, Yazaki Y. Angiotensin II type 1a receptor is involved in the occurrence of reperfusion arrhythmias. Circulation 97: 315–317, 1998. doi: 10.1161/01.CIR.97.4.315. [DOI] [PubMed] [Google Scholar]
- 6.Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol 41: 2197–2204, 2003. doi: 10.1016/S0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system – focusing on the vascular system. Peptides 32: 2141–2150, 2011. doi: 10.1016/j.peptides.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Doronin SV, Potapova IA, Lu Z, Cohen IS. Angiotensin receptor type 1 forms a complex with the transient outward potassium channel Kv4.3 and regulates its gating properties and intracellular localization. J Biol Chem 279: 48231–48237, 2004. doi: 10.1074/jbc.M405789200. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Gao J, Cohen IS, Mathias RT. Angiotensin II type 1 receptor-mediated electrical remodeling in mouse cardiac myocytes. PLoS One 10: e0138711, 2015. e0138711. doi: 10.1371/journal.pone.0138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen HJ, Mackasey M, Moghtadaei M, Belke DD, Egom EE, Tuomi JM, Rafferty SA, Kirkby AW, Rose RA. Distinct patterns of atrial electrical and structural remodeling in angiotensin II mediated atrial fibrillation. J Mol Cell Cardiol 124: 12–25, 2018. doi: 10.1016/j.yjmcc.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Rogers TB, Lokuta AJ. Angiotensin II signal transduction pathways in the cardiovascular system. Trends Cardiovasc Med 4: 110–116, 1994. doi: 10.1016/1050-1738(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 12.Scholz EP, Welke F, Joss N, Seyler C, Zhang W, Scherer D, Völkers M, Bloehs R, Thomas D, Katus HA, Karle CA, Zitron E. Central role of PKCα in isoenzyme-selective regulation of cardiac transient outward current Ito and Kv4.3 channels. J Mol Cell Cardiol 51: 722–729, 2011. doi: 10.1016/j.yjmcc.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Alday A, Ahyayauch H, Fernández-López V, Echeazarra L, Urrutia J, Casis O, Gallego M. CaMKII modulates the cardiac transient outward K. Cell Physiol Biochem 54: 27–39, 2020. [DOI] [PubMed] [Google Scholar]
- 14.Alday A, Urrutia J, Gallego M, Casis O. α1-adrenoceptors regulate only the caveolae-located subpopulation of cardiac K(V)4 channels. Channels (Austin) 4: 168–178, 2010. doi: 10.4161/chan.4.3.11479. [DOI] [PubMed] [Google Scholar]
- 15.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 271: 15160–15165, 1996. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 16.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol 98: 149–160, 2008. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busija AR, Patel HH, Insel PA. Caveolins and cavins in the trafficking, maturation, and degradation of caveolae: implications for cell physiology. Am J Physiol Cell Physiol 312: C459–C477, 2017. doi: 10.1152/ajpcell.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyan L, Foell JD, Vincent KP, Woon MT, Mesquitta WT, Lang D, Best JM, Ackerman MJ, McCulloch AD, Glukhov AV, Balijepalli RC, Kamp TJ. Long QT syndrome caveolin-3 mutations differentially modulate K v 4 and Ca v 1.2 channels to contribute to action potential prolongation. J Physiol 597: 1531–1551, 2019. doi: 10.1113/JP276014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markandeya YS, Phelan LJ, Woon MT, Keefe AM, Reynolds CR, August BK, Hacker TA, Roth DM, Patel HH, Balijepalli RC. Caveolin-3 overexpression attenuates cardiac hypertrophy via inhibition of T-type Ca2+ current modulated by protein kinase calpha in cardiomyocytes. J Biol Chem 290: 22085–22100, 2015. doi: 10.1074/jbc.M115.674945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egorov YV, Lang D, Tyan L, Turner D, Lim E, Piro ZD, Hernandez JJ, Lodin R, Wang R, Schmuck EG, Raval AN, Ralphe CJ, Kamp TJ, Rosenshtraukh LV, Glukhov AV. Caveolae-mediated activation of mechanosensitive chloride channels in pulmonary veins triggers atrial arrhythmogenesis. J Am Heart Assoc 8: e012748, 2019. doi: 10.1161/JAHA.119.012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly SN, Liu X, Carnicer R, Recalde A, Muszkiewicz A, Jayaram R, Carena MC, Wijesurendra R, Stefanini M, Surdo NC, Lomas O, Ratnatunga C, Sayeed R, Krasopoulos G, Rajakumar T, Bueno-Orovio A, Verheule S, Fulga TA, Rodriguez B, Schotten U, Casadei B. Up-regulation of miR-31 in human atrial fibrillation begets the arrhythmia by depleting dystrophin and neuronal nitric oxide synthase. Sci Transl Med 8: 340ra374, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright PT, Bhogal NK, Diakonov I, Pannell LMK, Perera RK, Bork NI, Schobesberger S, Lucarelli C, Faggian G, Alvarez-Laviada A, Zaccolo M, Kamp TJ, Balijepalli RC, Lyon AR, Harding SE, Nikolaev VO, Gorelik J. Cardiomyocyte membrane structure and cAMP compartmentation produce anatomical variation in β. Cell Rep 23: 459–469, 2018. doi: 10.1016/j.celrep.2018.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89: 20–25, 2001. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 24.Markandeya YS, Feng L, Ramchandran V, Vaidyanathan R, Best J, Lea Ml Keefe A, Kalscheur MM, O’Hara T, Eckhardt LL, Makielski JC, Trayanova NA, Balijepalli RC, Kamp TJ. Cardiac-specific deletion of caveolin-3 delays repolarization and increases susceptibility to ventricular arrhythmia. Circulation 132: 132, Suppl 3, 2015. [Google Scholar]
- 25.Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232 2006. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 26.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781, 1991. doi: 10.1016/S0021-9258(18)98476-0. [DOI] [PubMed] [Google Scholar]
- 27.Zankov DP, Omatsu-Kanbe M, Isono T, Toyoda F, Ding WG, Matsuura H, Horie M. Angiotensin II potentiates the slow component of delayed rectifier K+ current via the AT1 receptor in guinea pig atrial myocytes. Circulation 113: 1278–1286, 2006. doi: 10.1161/CIRCULATIONAHA.104.530592. [DOI] [PubMed] [Google Scholar]
- 28.Aiello EA, Cingolani HE. Angiotensin II stimulates cardiac L-type Ca(2+) current by a Ca(2+)- and protein kinase C-dependent mechanism. Am J Physiol Heart Circ Physiol 280: H1528–1536, 2001. doi: 10.1152/ajpheart.2001.280.4.H1528. [DOI] [PubMed] [Google Scholar]
- 29.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A 103: 7500–7505, 2006. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryant SM, Kong CHT, Watson JJ, Gadeberg HC, Roth DM, Patel HH, Cannell MB, James AF, Orchard CH. Caveolin-3 KO disrupts t-tubule structure and decreases t-tubular ICa density in mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 315: H1101–H1111, 2018. doi: 10.1152/ajpheart.00209.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryant S, Kimura TE, Kong CH, Watson JJ, Chase A, Suleiman MS, James AF, Orchard CH. Stimulation of ICa by basal PKA activity is facilitated by caveolin-3 in cardiac ventricular myocytes. J Mol Cell Cardiol 68: 47–55, 2014. doi: 10.1016/j.yjmcc.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ushio-Fukai M, Alexander RW. Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension 48: 797–803, 2006. doi: 10.1161/01.HYP.0000242907.70697.5d. [DOI] [PubMed] [Google Scholar]
- 33.Wyse BD, Prior IA, Qian H, Morrow IC, Nixon S, Muncke C, Kurzchalia TV, Thomas WG, Parton RG, Hancock JF. Caveolin interacts with the angiotensin II type 1 receptor during exocytic transport but not at the plasma membrane. J Biol Chem 278: 23738–23746, 2003. doi: 10.1074/jbc.M212892200. [DOI] [PubMed] [Google Scholar]
- 34.Oh YB, Gao S, Lim JM, Kim HT, Park BH, Kim SH. Caveolae are essential for angiotensin II type 1 receptor-mediated ANP secretion. Peptides 32: 1422–1430, 2011. doi: 10.1016/j.peptides.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Piech A, Massart PE, Dessy C, Feron O, Havaux X, Morel N, Vanoverschelde JL, Donckier J, Balligand JL. Decreased expression of myocardial eNOS and caveolin in dogs with hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 282: H219–231, 2002. doi: 10.1152/ajpheart.2002.282.1.H219. [DOI] [PubMed] [Google Scholar]
- 36.Ratajczak P, Damy T, Heymes C, Oliviéro P, Marotte F, Robidel E, Sercombe R, Boczkowski J, Rappaport L, Samuel JL. Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovasc Res 57: 358–369, 2003. doi: 10.1016/S0008-6363(02)00660-0. [DOI] [PubMed] [Google Scholar]
- 37.Bader M, Ganten D. Update on tissue renin-angiotensin systems. J Mol Med (Berl) 86: 615–621, 2008. doi: 10.1007/s00109-008-0336-0. [DOI] [PubMed] [Google Scholar]
- 38.Brundel BJ, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, Grandjean JG, Van Gilst WH, Crijns HJ. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation 103: 684–690, 2001. doi: 10.1161/01.CIR.103.5.684. [DOI] [PubMed] [Google Scholar]
- 39.Horikawa YT, Panneerselvam M, Kawaraguchi Y, Tsutsumi YM, Ali SS, Balijepalli RC, Murray F, Head BP, Niesman IR, Rieg T, Vallon V, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J Am Coll Cardiol 57: 2273–2283, 2011. doi: 10.1016/j.jacc.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schilling JM, Horikawa YT, Zemljic-Harpf AE, Vincent KP, Tyan L, Yu JK, McCulloch AD, Balijepalli RC, Patel HH, Roth DM. Electrophysiology and metabolism of caveolin-3-overexpressing mice. Basic Res Cardiol 111: 28, 2016. doi: 10.1007/s00395-016-0542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem 277: 38988–38997, 2002. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]