Abstract
Preeclampsia is characterized by increases in blood pressure and proteinuria in late pregnancy, and neurological symptoms can appear in the form of headaches, blurred vision, cerebral edema, and, in the most severe cases, seizures (eclampsia). The causes for these cerebral manifestations remain unknown, so the use of animal models that mimic preeclampsia is essential to understanding its pathogenesis. The Dahl salt-sensitive (Dahl SS/jr) rat model develops spontaneous preeclampsia superimposed on chronic hypertension; therefore, we hypothesized that the Dahl SS/jr rat would display cerebrovascular features similar to those seen in human preeclampsia. Furthermore, we predicted that this model would allow for the identification of mechanisms underlying these changes. The pregnant Dahl SS/jr rat displayed increased cerebral edema and blood-brain barrier disruption despite tighter control of cerebral blood flow autoregulation and vascular smooth muscle myogenic tone. Analysis of cerebral endothelial cell morphology revealed increased opening of tight junctions, basement membrane dissolution, and vesicle formation. RNAseq analysis identified that genes related to endothelial cell tight junctions and blood-brain barrier integrity were differentially expressed in cerebral vessels from pregnant Dahl SS/jr compared with healthy pregnant Sprague Dawley rats. Overall, our data reveal new insights into mechanisms involved in the cerebrovascular dysfunction of preeclampsia.
NEW & NOTEWORTHY This study uses the Dahl SS/jr rat as a preclinical model of spontaneous superimposed preeclampsia to demonstrate uncoupling of cerebral vascular permeability and blood-brain barrier disruption from cerebral blood flow autoregulatory dysfunction and myogenic tone. Additionally, the data presented in this study lay the foundational framework on which future experiments assessing specific transcellular transport components such as individual transporter protein expression and components of the vesicular transport system (caveolae) can be built to help reveal a potential direct mechanistic insight into the causes of cerebrovascular complications during preeclamptic pregnancies.
Keywords: autoregulation, cerebral blood flow, electron microscopy, fluorescent dextran, myogenic tone
INTRODUCTION
Preeclampsia (PE) is a multiorgan, pregnancy-specific syndrome that accounts for up to 8% of all pregnancy complications, making it one of the leading causes of maternal and fetal morbidity and mortality worldwide (1). Multiple risk factors including hypertension, diabetes, and obesity increase a woman’s risk of developing PE, and the relative risk of developing PE in women who have established chronic hypertension is nearly eightfold higher than that of the general population (2, 3). Symptoms typically begin late in the second trimester of pregnancy (after 20 wk of gestation) and often include hypertension (>140/90 mmHg) with one or more of the following characteristics: proteinuria (>300 mg/day), thrombocytopenia (platelet count <100,000), impaired liver function, elevated serum creatinine (>1.1 mg/dL), edema, and disturbances of the central nervous system (headache, blurred vision, cortical blindness, nausea, drowsiness, and, in severe cases, eclampsia) (4). A preeclamptic pregnancy not only immediately affects the mother and fetus but also has significant long-term consequences including increased risks of maternal cardiovascular disease (CVD) and stroke later in life as well as increased risk of CVD in the child (5, 6).
The brain is one of the most highly perfused organs of the body, utilizing 15–20% of the total cardiac output because of its high metabolic demand and constant need for blood flow and supply (7). The protective nature of the skull limits the expansion of tissue (brain) and fluid, and as a result the cerebral circulation and blood-brain barrier (BBB) must function properly (7). Healthy pregnancies substantially change the maternal cardiovascular system, largely by increasing cardiac output and inducing systemic vasodilation, which yields a drop in blood pressure despite a 40–50% increase in plasma volume (8). Therefore, the cerebral circulation must adapt to maintain constant blood flow and tolerate these changes in maternal blood volume and blood pressure (9, 10). Previous animal studies in normal pregnancy have shown that cerebral blood flow (CBF) autoregulation curves are shifted at both the upper and lower limits to higher pressures in Sprague Dawley (SD) rats (11, 12). Studies have also shown increased BBB permeability at pressures > 180 mmHg while tight junction protein expression remains unchanged, suggesting that paracellular permeability may not be increased during normal pregnancy (13). Furthermore, studies have shown that normal pregnancy does not affect hydraulic conductivity and transvascular filtration (water movement through the vascular wall in response to hydrostatic pressure) (13). In contrast, studies using experimental models of PE have shown increased BBB permeability and cerebral edema and suggest impaired cerebral vascular myogenic activity and CBF autoregulation (13, 14). However, the mechanisms underlying these changes in preeclamptic pregnancies remain unclear.
The Dahl salt-sensitive rat (Dahl SS/jr) is a model of spontaneous superimposed PE, exhibiting increased blood pressure and proteinuria during pregnancy, which results in reduced litters, increased fetal resorption, as well as intrauterine growth restriction (15, 16). In the present study, the Dahl SS/jr model was used to elucidate the cerebrovascular changes occurring in a rat model of spontaneous superimposed PE. We hypothesized that the pregnant Dahl SS/jr rat would exhibit cerebral vascular smooth muscle and endothelial cell dysfunction. In addition, a whole transcriptome approach (RNA sequencing) was used to identify potential genetic mediators of cerebrovascular dysfunction in the Dahl SS/jr rat.
MATERIALS AND METHODS
Animals
Female Dahl SS/jr rats and spontaneously hypertensive rats (SHR) were obtained from the colonies maintained by Dr. Michael Garrett at The University of Mississippi Medical Center (UMMC). Sprague Dawley (SD) rats were purchased from Harlan Laboratories (Indianapolis, IN). SD rats, used as the controls, are well-characterized models of normal pregnancy, and the SHR is a genetic model of hypertension that does not develop a preeclamptic phenotype during pregnancy (15). All rats (total study sample size n = 132) were housed under a 12:12-h light-dark cycle at 23°C and maintained on 0.3% NaCl rodent chow (Teklad 7034) and water ad libitum. Conventional (type 3) cages were provided with Sani-Chip bedding (7090 Teklad Laboratory Grade) and enriched with a paper straw as nesting material. Timed breeding was performed with one or two female rats from each strain with one male rat of the same strain per cage between 16 and 17 wk of age, and gestational day (G)1 of pregnancy was defined by the detection of sperm histologically from a vaginal swab. Randomization was performed based on litter and proteinuria measurements, and investigators were not blinded during experiments but were blinded, however, for all analysis. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, approved by The UMMC Institutional Animal Care and Use Committee (protocol 1344A “New animal models to investigate hypertensive complications of pregnancy,” approved on 24 September 2015, amended on 6 November 2017, and renewed as protocol 1344B on 1 June 2018), and reported according to ARRIVE guidelines. Sample sizes for each protocol were based on our prior experience using these procedures in virgin and pregnant rats (14, 17, 18).
Blood Pressure and Proteinuria
Pregnant Dahl SS/jr and SD rats were placed in metabolic cages for 24-h urine collection at baseline (before breeding) and G19 of pregnancy. Urine collections for age-matched virgin littermate control rats for each strain were also performed at the same time points. Urinary protein excretion was measured via Bradford assay (Bio-Rad). Mean arterial blood pressure measurements were obtained on G20 via abdominal aorta catheter while the rat was under 1–2% isoflurane anesthesia as previously described (19).
Brain Water Content
Rats were placed under isoflurane anesthesia, and brains were isolated from pregnant (G20) and age-matched virgin Dahl SS/jr rats and separated into anterior (rostral to middle cerebral artery) and posterior cerebrum. Regions were weighed separately (wet weight), heated at 60°C for 72 h, and reweighed (dry weight) to determine the dry-to-wet weight ratio as a measurement of brain water content as previously described (14).
Cerebral Vascular Permeability
Integrity of the middle cerebral artery to maintain intraluminal pressure as a surrogate measure of vascular permeability was assessed from middle cerebral arteries isolated from G19–20 pregnant and age-matched virgin Dahl SS/jr rats. Isolated arteries (1 per rat) were placed in a vessel chamber (Living Systems), cannulated to tapered glass pipettes, and secured with silk ligatures. Physiological salt solution (PSS) consisting of 7.598 g of NaCl, 0.298 g of KCl, 0.296 g of MgSO4, 0.336 g of NaHCO3, 0.264 g of CaCl2, 2.384 g of HEPES, 0.160 g of KH2PO4, 1.080 g of glucose, and 0.012 g of EDTA in 1 L of ddH2O was placed in the bath, and the vessels were servo-controlled at 75 mmHg for a 30-min stabilization period. Then, baseline measurements of intraluminal diameter and length were recorded. The servo-controller was turned off, drops in intraluminal pressure were recorded, and pictures were taken every 5 min for 1 h. At the end of the experiment the PSS bath was replaced with 40 mM KCl + PSS (3.156 g NaCl, 5.964 g KCl, 0.296 g MgSO4, 0.336 g NaHCO3, 0.264 g CaCl2, 2.384 g HEPES, 0.160 g KH2PO4, 1.080 g glucose, and 0.012 g EDTA in 1 L ddH2O), and vessel contraction was recorded to ensure vessel viability.
Cerebral Blood Flow Autoregulation
Cerebral blood flow was assessed on G18–20 in pregnant Dahl SS/jr rats and SHR and in age-matched virgin littermates. Rats were anesthetized with Inactin (50 mg/kg ip) and ketamine (30 mg/kg im), intubated, and ventilated to control arterial carbon dioxide levels. Briefly, catheters were placed in the femoral artery and vein to monitor blood pressure and to administer phenylephrine, respectively. A closed cranial window was created by thinning the bone overlying the parietal cortex with a dental drill and securing the head in a stereotactic apparatus. Laser Doppler flow probes were positioned above two closed cranial windows 3–4 mm lateral and 2 mm distal to bregma, through which perfusion was recorded in real time. Baseline flow was recorded for 10 min after stabilization. To assess relative changes in CBF in response to increases in blood pressure, an infusion of phenylephrine (starting with 0.5 μg/min for 30 min) was administered, and the rate of infusion increased in a stepwise fashion, resulting in 10-mmHg blood pressure increments until reaching a final blood pressure of 200 mmHg.
Cerebrovascular Myogenic Tone
Middle cerebral arteries were isolated from pregnant and virgin Dahl SS/jr and SHR rats on G20 and transferred to a vessel chamber (Living Systems) as previously described by our laboratory (17). The artery was cannulated with tapered glass pipettes and secured with silk ligatures. Vessel diameter and wall thickness were assessed with a microscope, a CoolSNAP camera, and Nikon Imaging Software (NIS Elements v5.20.00). Luminal pressure was servo-controlled at 75 mmHg at 37°C and equilibrated for 1 h. After equilibration, intraluminal pressure was decreased to 25 mmHg, followed by stepwise increases in 25-mmHg increments to 150 mmHg. The protocol was performed under active (7.598 g NaCl, 0.298 g KCl, 0.296 g MgSO4, 0.336 g NaHCO3, 0.264 g CaCl2, 2.384 g HEPES, 0.160 g KH2PO4, 1.080 g glucose, and 0.012 g EDTA in 1 L ddH2O) and passive (7.598 g NaCl, 0.298 g KCl, 0.296 g MgSO4, 0.336 g NaHCO3, 2.384 g HEPES, 0.160 g KH2PO4, 1.080 g glucose, 0.012 g EDTA, and 0.760 g EGTA in 1 L ddH2O) conditions. Vessel diameter measurements were taken at each pressure increment and used to calculate vessel tone according to the following formula: [(Diameterpassive − Diameteractive)/Diameterpassive] × 100.
Fluorescent Texas Red Dextran Extravasation
Pregnant (G20) and age-matched virgin Dahl SS/jr rats were perfused via the femoral vein with 5 mg/kg (500 μL) of 3 kDa Texas Red dextran (Invitrogen) or saline as control. This was allowed to circulate for 1 h before a second 500-μL injection of 5 mg/kg of 500 kDa FITC (Sigma). After a 3-min circulation period, brains were collected and immediately transferred to 4% paraformaldehyde solution for 24 h. Brains were washed in PBS for 3 h before transfer to a 10% cytoprotection solution (16.28 g NaH2PO4, 4.28 g NaOH, 100 g sucrose in 1 L ddH2O). After 24 h, brains were transferred to a 30% cytoprotection solution (16.28 g NaH2PO4, 4.28 g NaOH, 300 g sucrose in 1 L ddH2O) for storage at 4°C until the end of the study. Brains were then frozen in Tissue-Plus Optimal Cutting Temperature (OCT) compound (Fisher), and 5-μm cryostat sections from the posterior cerebral cortex were used for confocal image analysis (102–266 total images for pregnant Dahl SS/jr, 75–238 total images for virgin Dahl SS/jr rat) with Nikon Imaging Software (NIS Elements v5.20.00). The investigator was blinded to experimental groups during analysis. Vessel boundaries were established by using FITC images to mark regions of interest (ROIs). Extra- and intravascular ROIs were calculated, and Texas Red mean intensity values were determined for each image. Average saline extra- and intravascular values were subtracted from average extra- and intravascular values for each rat, and a ratio of extravascular-to-intravascular Texas Red mean intensity values was determined. After filtering to remove values less than those in saline-infused control rats, an average of 99 vessels per pregnant Dahl SS/jr rat and 44 vessels per virgin Dahl SS/jr rat were used to determine the percentage of Texas Red extravasation.
Electron Microscopy
Pregnant (G20) and age-matched virgin Dahl SS/jr and SD rats under isoflurane anesthesia were perfused at a rate of 12 mL/min until blood free with cold heparinized PBS (0.1 mL heparin/mL PBS), followed by a 60-mL perfusion of a 1.0% paraformaldehyde-2.0% glutaraldehyde solution. Brains were collected and stored in fresh 1% paraformaldehyde-2.0% glutaraldehyde solution until processed for fixation. One-centimeter-square blocks of posterior cerebral cortices were fixed in 10% formaldehyde overnight, followed by postfixation for 1 h in 2% osmium tetroxide. A series of dehydration steps occurred before tissues were embedded in Epon resin. One hundred-nanometer sections were stained with 2% uranyl acetate and calcinated lead citrate and used to acquire transmission electron microscopic images (average 4 or 5 capillaries/rat/strain) with a Leo 912 transmission electron microscope. The following parameters of brain capillaries were assessed: basement membrane status (% dissolved), basement membrane thickness, vesicle formation, tight junction status (% open), luminal membrane protrusions, and vessel diameter. Basement membrane status determined by a percentage of basement membrane area as image resolution/appearance was either clearly defined (intact) or blurred (dissolved). Tight junction status was determined as Boolean data, 0 being open and 1 being intact. Individual luminal membrane protrusions were counted if any portion of the luminal membrane was interrupted. Blinded to experimental groups, image analysis was performed with Nikon Imaging Software (NIS Elements v5.20.00).
RNA Sequencing
Brains were isolated from pregnant (G20) and age-matched virgin Dahl SS/jr and SD rats, and the cerebrovascular unit consisting of the anterior, posterior, and middle cerebral arteries and circle of Willis was dissected from cerebral tissue for RNA isolation. Total RNA was isolated with the automated KingFisher Flex nucleic acid system along with the KingFisher Pure RNA Kit. RNA was evaluated for quantity (Nanodrop One and Qubit Fluorimeter) and quality with Qiagen QIAxcel Advanced System [minimum concentration and fidelity (i.e., 18S and 28S bands)]. Libraries were developed with the TruSeq mRNA Stranded Library Prep Kit (Set-A) and a Perkin Elmer Zephyr G3 NGS workstation, evaluated by Qubit Fluorometer (Invitrogen), and assessed for quality and size with Qiagen QIAxcel Advanced System. Individually indexed samples were pooled into single multiplexed libraries (n = 10 pooled samples/library) and sequenced with the NextSeq 500 High Output Kit (300 cycles, paired end 100 bp) on the Illumina NextSeq 500 platform. Sequenced reads were assessed for quality and trimmed with the Illumina BaseSpace Cloud Computing Platform. Resultant reads were aligned to the rat reference genome [Rnor_6.0] with the STAR aligner (version 2.6.0a) (20). Subsequent counts were enumerated to the gene level with the featureCounts program in the subread software package (version 1.6.2) (21). Differentially expressed genes were statistically determined with a linear model consisting of four different contrasts: “GroupDP vs. GroupSDP” for pregnant strain differences, “GroupDP vs. GroupDV” for pregnancy-induced changes in Dahl SS/jr, “GroupSDP vs. GroupSDV” for pregnancy induced changes in SD, and “GroupDV vs. GroupSDV” for virgin strain differences. Empirical Bayes variance moderation was used for correction, and gene expression differences between groups were considered to be statistically significant with a P value < 0.05. Gene set enrichment testing for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and functional annotations was conducted with Bioconductor packages: topGO, biomaRt, and edgeR (22–25).
Statistical Analysis
All data are presented as means ± SE. Statistical analyses were performed by two-way analysis of variance (ANOVA) followed by Tukey post hoc analysis for multiple comparisons (among strains). Unpaired t tests were performed for experiments that included only pregnancy differences within one strain. For myogenic tone experiments, linear regression lines were generated and the slopes compared between each group with a one-way ANOVA with Newman–Keuls post hoc analysis. Vascular permeability and CBF experiments were analyzed by performing two-way ANOVA with Sidak post hoc analysis. Tight junction status for electron microscope images was analyzed by Fisher’s exact test of independence. Means were considered statistically significant if P < 0.05.
RESULTS
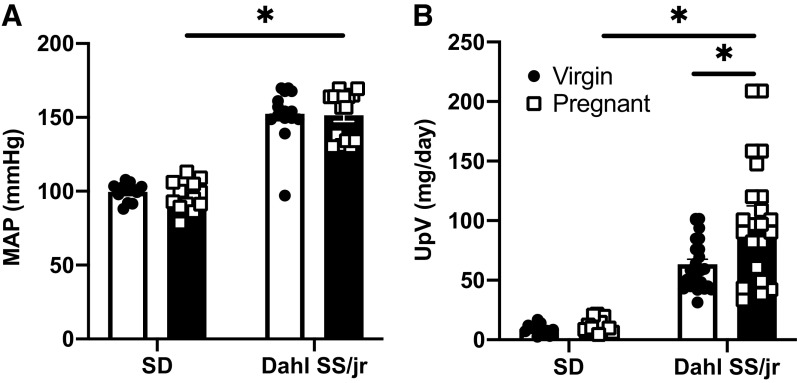
To confirm the previously observed PE phenotype in the Dahl SS/jr rat, we measured blood pressure (Fig. 1A) and urinary protein excretion (Fig. 1B) in a subgroup of rats. Mean arterial pressure (MAP) was significantly greater on G20 of pregnancy in Dahl SS/jr rats (n = 15) compared with SD rats (n = 13) (151.4 ± 4.2 vs. 96.7 ± 4.6 mmHg, P < 0.0001), and the physiological drop in MAP observed in healthy pregnancy was absent in the Dahl SS/jr rats compared with virgin littermates (152.4 ± 4.6 mmHg, P = 0.9976, n = 15 rats), consistent with our previously published work (15). Urinary protein excretion was increased during late pregnancy in the Dahl SS/jr rat (n = 22) compared with SD rats (n = 13) (101.8 ± 10.7 vs. 12.2 ± 1.5 mg/day, P < 0.0001) and with virgin Dahl SS/jr rats (n = 25) (63.4 ± 4.2 mg/day, P = 0.0004).
Figure 1.
Confirmation of preeclamptic phenotype in Dahl SS/jr rats. Mean arterial pressure (MAP, A) [Dahl SS/jr Pregnant n = 15 rats, Dahl SS/jr Virgin n = 15 rats, Sprague Dawley (SD) Pregnant n = 13 rats, SD Virgin n = 12 rats] and urinary protein excretion (UpV, B) (Dahl SS/jr Pregnant n = 22 rats, Dahl SS/jr Virgin n = 25 rats, SD Pregnant n = 13 rats, SD Virgin n = 12 rats) are increased in the Dahl SS/jr rat. *P < 0.05.
Increased Brain Water Content and Vascular Permeability during Pregnancy in the Dahl SS/jr Rat
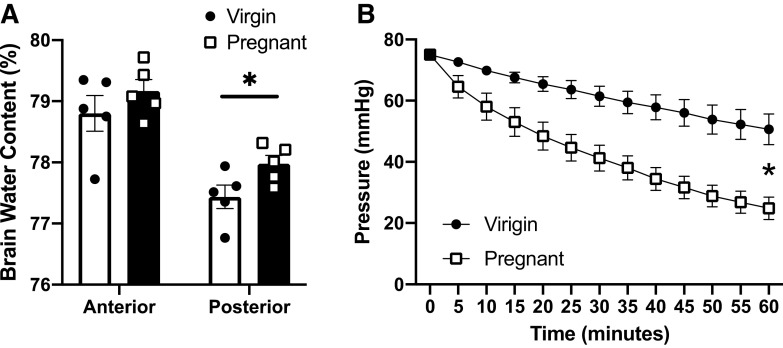
Changes in brain water content and the integrity of the middle cerebral artery to maintain intraluminal pressure as an indicator of vascular permeability were evaluated during pregnancy in the Dahl SS/jr rat. In these initial studies, comparisons were made between pregnant and virgin Dahl SS/jr rats. Pregnant Dahl SS/jr rats had increased posterior brain water content as measured by dry-to-wet weight ratio compared with virgin littermates (78.0 ± 0.1 vs. 77.4 ± 0.2, P = 0.0255, n = 5) (Fig. 2A). No significant differences were observed in the anterior brain. Cannulated middle cerebral arteries from pregnant Dahl SS/jr rats were more permeable to fluid, as indicated by a significantly greater drop in intravascular pressure over time compared with arteries from virgin littermate controls (Δ = 50.2 ± 3.7 vs. 24.4 ± 5.0 mmHg, respectively, P = 0.0133, n = 5 rats/group) (Fig. 2B).
Figure 2.
Changes in brain water content and cerebrovascular permeability. Posterior brain water content (A) and ex vivo vascular permeability (B) are increased in pregnant Dahl SS/jr rats compared with virgin littermates. *P < 0.05; n = 5 rats/group.
Blood-Brain Barrier Dysfunction is Observed during Pregnancy in the Dahl SS/jr Rat
To test BBB function in the Dahl SS/jr rat, extravasation of a 3 kDa dextran was examined by measuring ratio of extra- to intravascular Texas Red mean intensity values. Texas Red intensity is clearly observed at higher levels within the vessels from Dahl SS/jr virgin rats compared with pregnant Dahl SS/jr rats, which demonstrate higher fluorescence outside the vessels. Specifically, Pregnant Dahl SS/jr rats (n = 6) displayed a significant increase in Texas Red extravasation compared with Dahl SS/jr virgin rats (n = 8) (31.6 ± 4.9% vs. 14.3 ± 1.9%, P = 0.0148) (Fig. 3).
Figure 3.
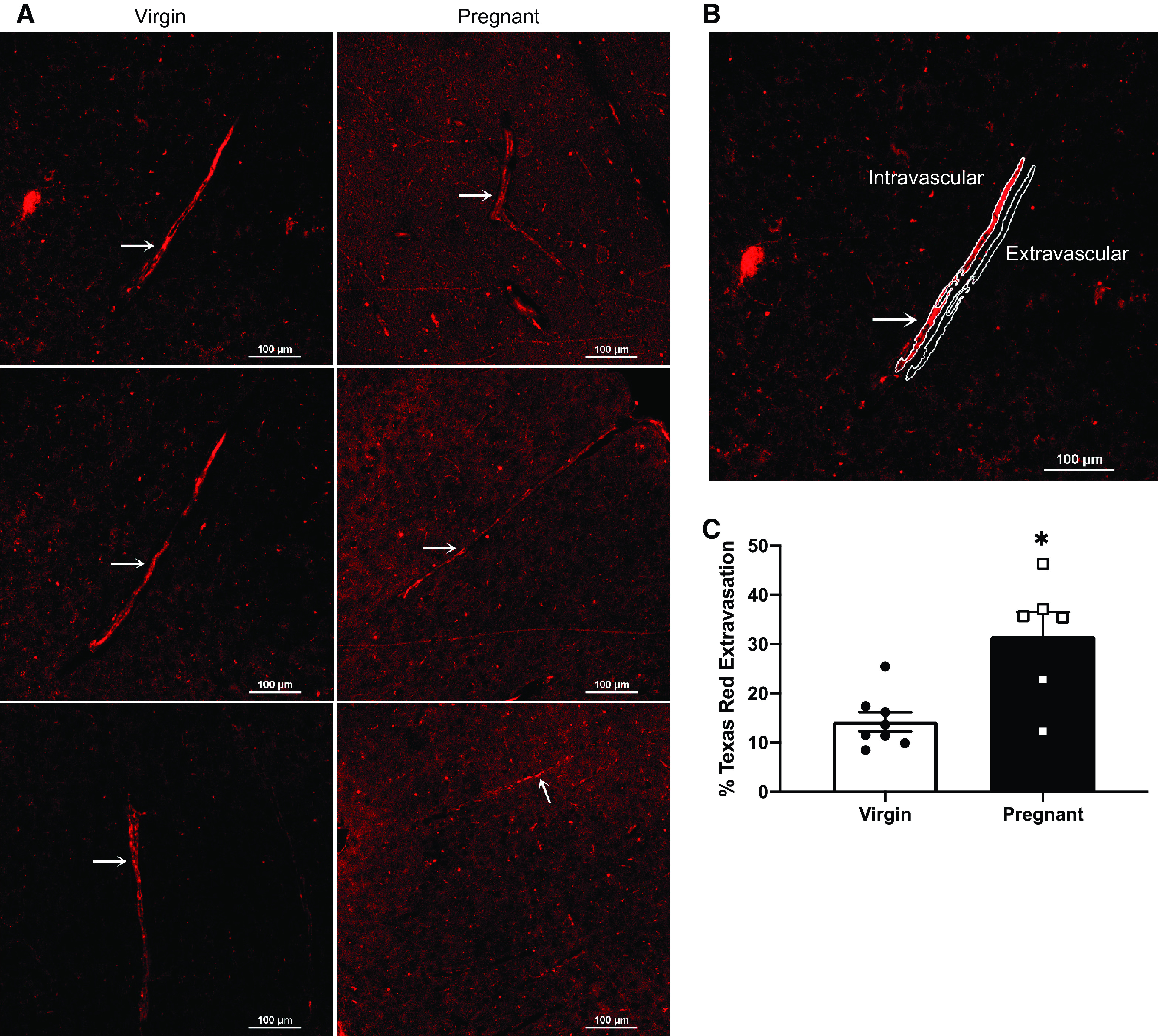
Cerebrovascular extravasation of Texas Red dextran: representative images (A and B) and quantification (C) of pregnant Dahl SS/jr rats displaying an increased extravasation of 3 kDa Texas Red dextran compared with virgin littermates. Arrow denotes the vessel analyzed in the representative images. *P < 0.05; virgin n = 6 rats, pregnant n = 8 rats.
Cerebral Blood Flow Autoregulation and Myogenic Tone Are Enhanced in Dahl SS/jr Rats during Pregnancy
Because brain water content was increased in the Dahl SS/jr rats during pregnancy, increased vascular permeability and BBB dysfunction were evaluated to test the hypothesis that reduced CBF autoregulation and myogenic tone would contribute to the observed changes. Contrary to this hypothesis, CBF autoregulation, measured via the relationship between percent change in CBF and MAP, in both Dahl SS/jr and SHR virgin rats displayed a greater increase in CBF at a given pressure compared with same-strain pregnant rats (Fig. 4), with significant interactions between pregnancy status and MAP in the Dahl SS/jr rats (P < 0.0001). Post hoc analysis of pressures within same-strain rats showed that CBF was significantly higher in the Dahl SS/jr virgin rats (n = 9) between 180 and 220 mmHg (P < 0.05) compared with pregnant Dahl SS/jr rats (n = 8), whereas CBF was only significantly higher (P < 0.05) in the SHR virgin rats (n = 8) at 200 and 210 mmHg compared with pregnant SHR rats (n = 8).
Figure 4.
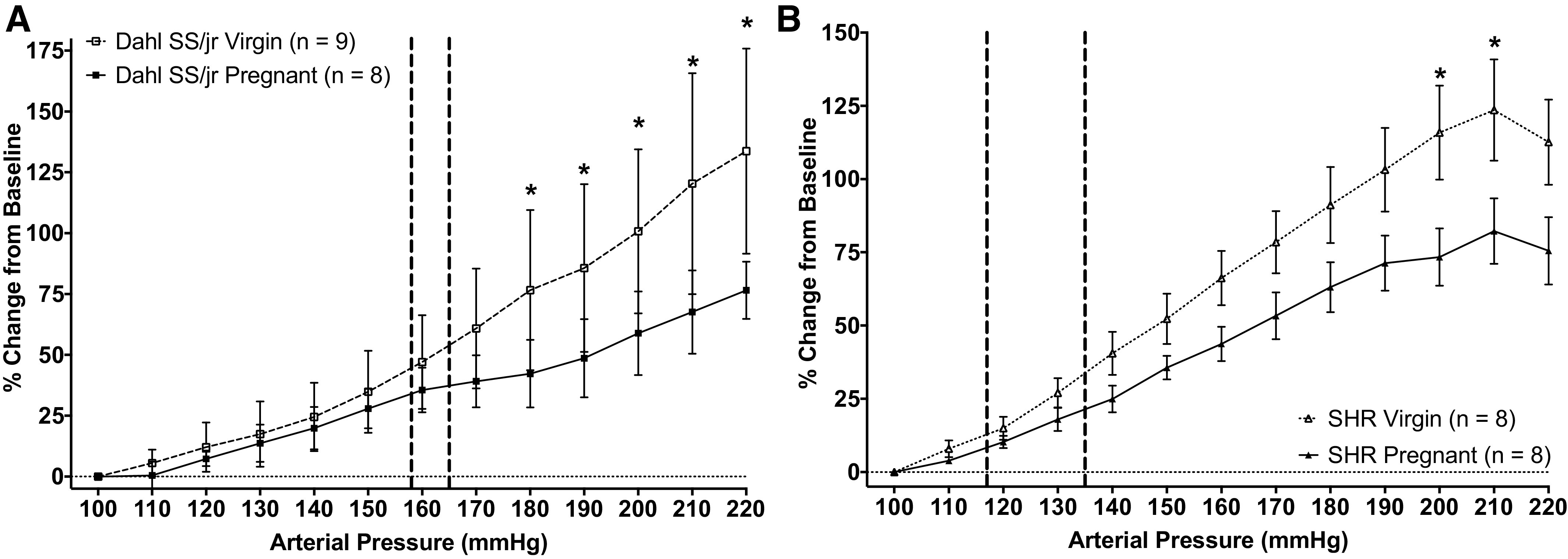
Changes in cerebral blood flow (CBF) autoregulation. CBF is enhanced in pregnant Dahl SS/jr rats (A) and spontaneously hypertensive rats (SHR, B) compared with virgin littermates. Vertical dashed lines represent ambulatory blood pressures. *P < 0.05; Dahl SS/jr Pregnant n = 8 rats, Dahl SS/jr Virgin n = 9 rats, SHR Pregnant n = 8 rats, SHR Virgin n = 8 rats.
Likewise, middle cerebral artery myogenic tone, calculated by using active and passive internal diameters, was enhanced in the pregnant Dahl SS/jr rats (n = 7) compared with virgin littermates (n = 10) (Fig. 5A), with the slope of the pregnant Dahl SS/jr rats (0.09 ± 0.01, r2 = 0.4715) being significantly different (P = 0.0077) from the virgin Dahl SS/jr rats (0.03 ± 0.02, r2 = 0.0255). SHR rats (n = 5/group) showed no significant difference in slopes between pregnant and virgin littermates (0.05 ± 0.02, r2 = 0.08749 vs. 0.07 ± 0.03, r2 = 0.1084, P = 0.6845). These data, combined with the CBF autoregulation findings above, suggest that increased myogenic tone may be responsible for improved CBF control during pregnancy in these hypertensive models. Baseline measurements consisting of media:lumen, wall:lumen, and media thickness were not statistically different between experimental groups under active conditions at 75 mmHg (Figure 5, B–D).
Figure 5.
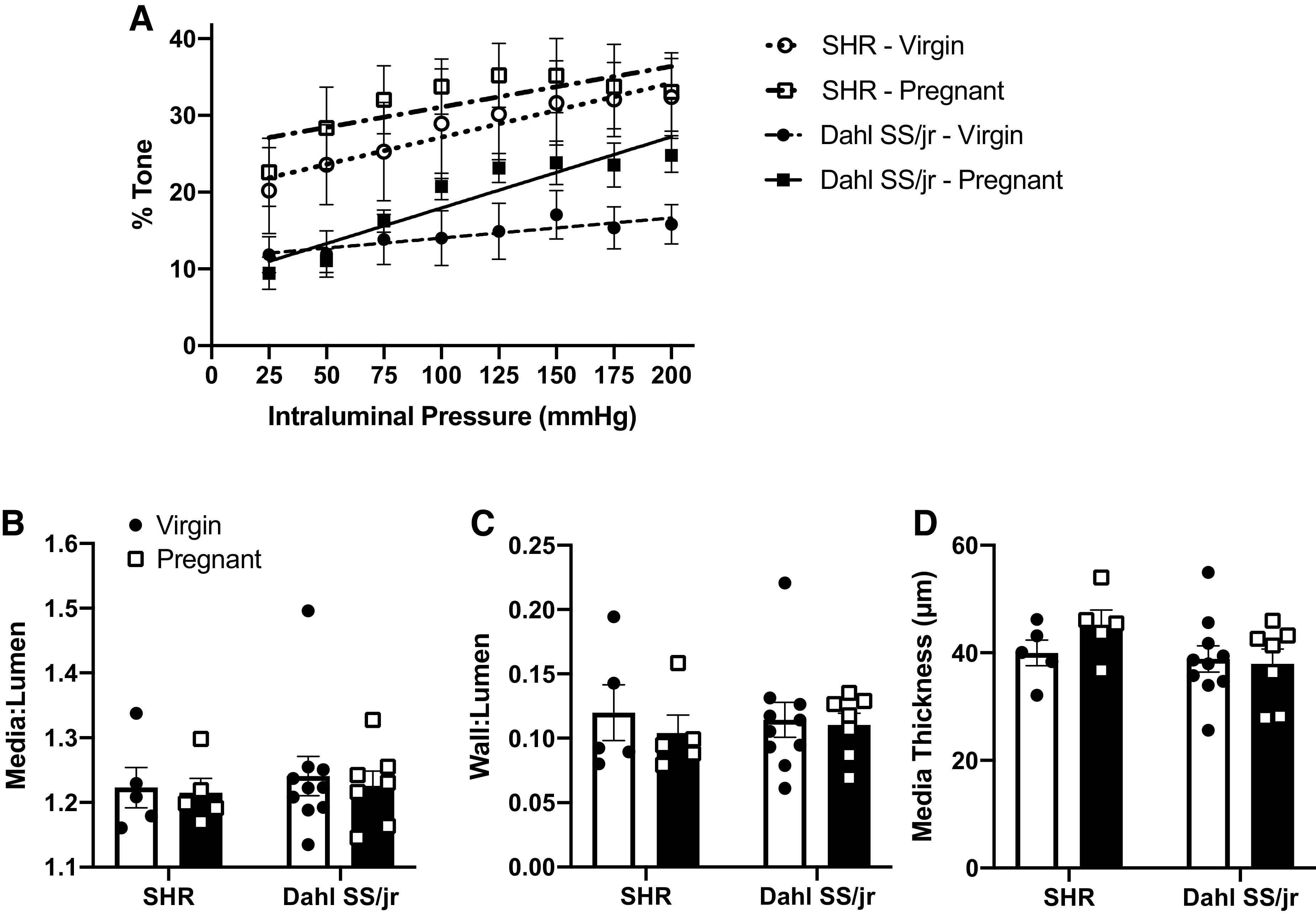
Changes in middle cerebral artery myogenic tone. Middle cerebral artery myogenic tone (A) is enhanced during pregnancy in Dahl SS/jr rats compared with virgin littermates, whereas no differences in vessel properties were observed (B–D). P < 0.05; spontaneously hypertensive rat (SHR) Virgin n =5 rats, SHR Pregnant n = 5 rats, Dahl SS/jr Virgin n = 10 rats, Dahl SS/jr Pregnant n = 7 rats).
Brain Capillary Endothelial Cell Morphology Is Disrupted in the Dahl SS/jr Rat during Pregnancy
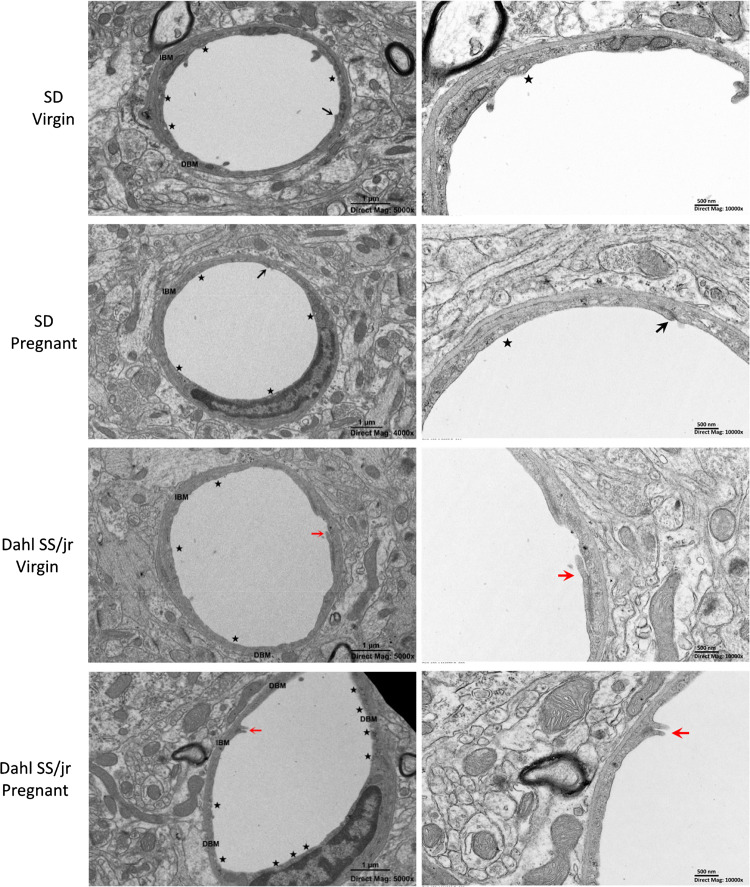
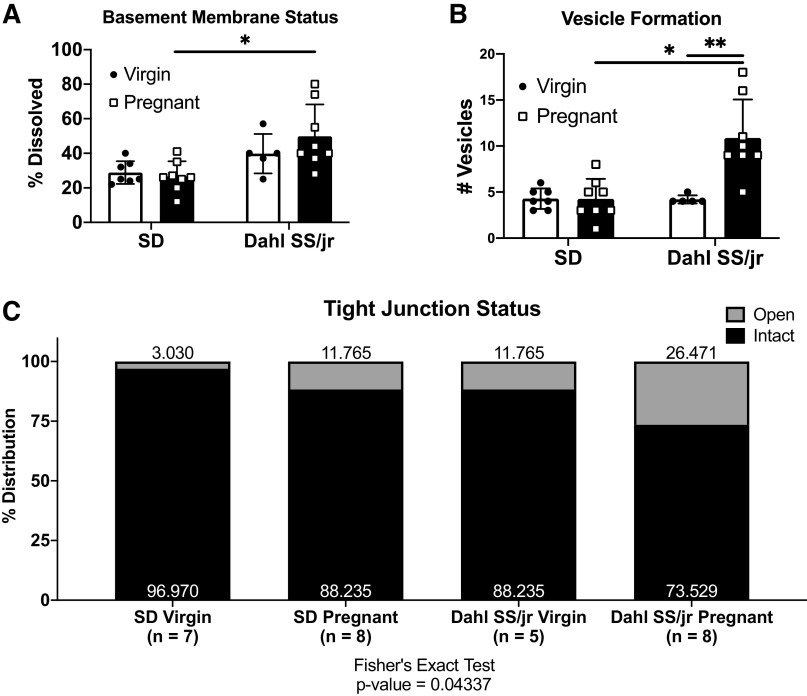
Because impaired autoregulation and myogenic tone were not responsible for the observed increases in brain water content, vascular permeability, and BBB dysfunction, the impact of endothelial disruption was investigated. Pregnant Dahl SS/jr rats (n = 8) showed a significant difference in basement membrane dissolution (49.8 ± 6.5% vs. 26.5 ± 3.1%, P = 0.0052) and endothelial cell vesicle formation (11 ± 1 vs. 4 ± 1, P = 0.0002) compared with pregnant SD rats (n = 8) (Fig. 6 and Fig. 7). Significant differences were observed in the percentage of open tight junctions among all groups (SD Virgin = 3.03%, SD Pregnant = 11.77%, Dahl SS/jr Virgin = 11.77%, Dahl SS/jr Pregnant = 26.47%, Fisher’s exact test, P = 0.04337). No significant differences in basement membrane thickness, luminal membrane protrusions, or vessel diameter were observed (Table 1).
Figure 6.
Representative electron microscopic images of pregnant Dahl SS/jr rats displaying an increased basement membrane dissolution, vesicle formation, and tight junction opening compared with virgin littermates and pregnant Sprague Dawley (SD) rats. Star, vesicles, black arrow, tight junction; red arrow, open tight junction. DBM, dissolved basement membrane; IBM, intact basement membrane.
Figure 7.
Pregnant Dahl SS/jr rats display increased basement membrane dissolution (A), vesicle formation (B), and tight junction opening (C) compared with virgin littermates and pregnant Sprague Dawley (SD) rats. *P < 0.05 vs. SD, **P < 0.05 vs. virgin. SD Virgin n = 7 rats, SD Pregnant n = 8 rats, Dahl SS/jr Virgin n = 5 rats, Dahl SS/jr Pregnant n = 8 rats.
Table 1.
Brain capillary endothelial cell electron microscopy measurements
| n |
P Value |
||||
|---|---|---|---|---|---|
| Strain | Pregnancy | Interaction | |||
| Basement membrane thickness, µm | |||||
| SD | |||||
| Virgin | 0.063 ± 0.004 | 7 | 0.1010 | 0.5074 | 0.6862 |
| Pregnant | 0.064 ± 0.003 | 8 | |||
| Dahl SS/jr | |||||
| Virgin | 0.068 ± 0.003 | 5 | |||
| Pregnant | 0.071 ± 0.003 | 8 | |||
| Luminal membrane protrusions | |||||
| SD | |||||
| Virgin | 1.714 ± 0.286 | 7 | 0.2549 | 0.9540 | 0.2549 |
| Pregnant | 1.375 ± 0.263 | 8 | |||
| Dahl SS/jr | |||||
| Virgin | 1.000 ± 0.000 | 5 | |||
| Pregnant | 1.375 ± 0.375 | 8 | |||
| Vessel diameter, µm | |||||
| SD | |||||
| Virgin | 4.649 ± 0.167 | 7 | 0.2253 | 0.1087 | 0.1841 |
| Pregnant | 4.653 ± 0.196 | 8 | |||
| Dahl SS/jr | |||||
| Virgin | 4.604 ± 0.294 | 5 | |||
| Pregnant | 5.598 ± 0.537 | 8 | |||
Values are means ± SE for n rats. SD, Sprague Dawley. P values determined by 2-way ANOVA.
Genes and Pathways Associated with Vascular Changes during Pregnancy in the Dahl SS/jr Rat
An unbiased hypothesis-generating approach was used to identify potential mechanisms associated with observed differences in cerebrovascular changes during pregnancy in Dahl SS/jr rats compared with healthy SD control rats. Transcriptome analysis using RNAseq of the major cerebrovascular unit from pregnant animals, consisting of the anterior, posterior, and middle cerebral arteries and circle of Willis, showed that a total of 719 genes were significantly differentially expressed in the pregnant Dahl SS/jr rat (n = 5) compared with the healthy pregnant SD rat (n = 5), whereas 3,131 genes were found to be differentially expressed between virgin Dahl SS/jr (n = 5) and virgin SD (n = 8) rats. These findings underscore the baseline differences between these rat strains and highlight the overlap of changes that are due to pregnancy adaptations (Fig. 8A). Table 2 lists differentially expressed genes within each strain due to pregnancy alone. Figure 8B displays the distribution of significantly up- and downregulated differentially expressed genes for each comparison among all groups. KEGG pathway analysis revealed several significantly differentially regulated genes expressed in both pregnant rats and virgins related to pathways including endocytosis, cell adhesion molecules, and vascular smooth muscle contraction (Tables 3 and 4). Table 5 shows the top most significantly differentially expressed genes during pregnancy (Dahl SS/jr pregnant vs. SD pregnant) with fold changes greater or less than ±1.8. Other significant differentially expressed genes (fold changes) of interest included major BBB transporter genes: abcb1 (mdr1, p-gp) (1.02), slc16a2 (mct8) (0.587), lrp-1 (0.387), rage (ager) (−0.533), and slc2a1 (glut1) (−0.508). Additionally, tight junction protein genes cldn3 and cldn5 were decreased in the arteries from the Dahl SS/jr rats (−2.02 and −0.769 log fold change, respectively).
Figure 8.

Overlap of differentially expressed genes in the cerebrovascular unit in Dahl SS/jr and Sprague Dawley (SD) rats due to pregnancy (A) and distribution of up- and downregulated differentially expressed genes between groups (B). n = 5 rats/strain.
Table 2.
Genes for SD and Dahl SS/jr pregnancies
| Gene | logFC | Adjusted P Value |
|---|---|---|
| SD Pregnancy | ||
| Cdkl1 | −0.8962406 | 0.00680444 |
| Pdlim2 | 0.44146147 | 0.04608951 |
| Tap1* | 0.59038407 | 0.03756805 |
| Samd11 | 0.60378457 | 0.02186238 |
| Fkbp11* | 0.63468701 | 0.02516835 |
| Ly6e | 0.66234635 | 0.01396057 |
| Slfn2 | 0.95180533 | 0.02186238 |
| RT1-S3* | 0.98278498 | 0.00237037 |
| Socs2* | 1.00325982 | 0.00017996 |
| RT1-T24-1 | 1.22106782 | 0.00680444 |
| RT1-T24-3 | 1.7572042 | 0.00022617 |
| Dahl SS/jr pregnancy | ||
| Scube3 | −0.9731015 | 0.00693662 |
| Klf15 | −0.5066084 | 0.02639122 |
| Parp8 | 0.36229895 | 0.01457577 |
| Heyl | 0.59042466 | 0.0179671 |
| Tap1* | 0.63051538 | 0.01457577 |
| Fkbp11* | 0.63925989 | 0.02876405 |
| RT1-S3* | 0.80082125 | 0.01789672 |
| Hmmr | 0.8205076 | 0.01457577 |
| Socs2* | 0.92046537 | 0.00080664 |
| Arhgef39 | 0.95970582 | 0.04156359 |
| Anln | 1.03900273 | 0.01817405 |
| Kif11 | 1.06432146 | 0.01457577 |
| Iqgap3 | 1.1585707 | 0.01789672 |
| Kif2c | 1.16152371 | 0.0230094 |
| Ndc80 | 1.18114803 | 0.01378637 |
| Top2a | 1.2027674 | 0.00667538 |
| Ccna2 | 1.24048533 | 0.01457577 |
| Cenpf | 1.24775794 | 0.00850524 |
| Nusap1 | 1.2503174 | 0.01457577 |
| Ect2 | 1.26051221 | 0.01457577 |
| Prc1 | 1.29675035 | 0.01117947 |
| Cdkn3 | 1.30928181 | 0.01806758 |
| Cdk1 | 1.3372125 | 0.00693662 |
| Knstrn | 1.36805279 | 0.01457577 |
| Kif20a | 1.37235091 | 0.00693662 |
| Ckap2 | 1.39072037 | 0.00850524 |
| Gbp5 | 1.44822273 | 0.00693662 |
| Diaph3 | 1.50826234 | 0.00059864 |
| Troap | 1.52288883 | 0.00764954 |
| Nek2 | 1.61950243 | 0.01457577 |
| Pimreg | 1.62428645 | 0.01457577 |
| Rrm2 | 1.69041658 | 0.00080664 |
| Pbk | 1.79995165 | 0.01457577 |
| Trpm8 | 1.87421714 | 0.00693662 |
FC, fold change; SD Sprague Dawley. *Shared between strains.
Table 3.
Differentially expressed genes in enriched pathways associated with vascular change during pregnancy
| Genes | logFC | Adjusted P Value |
|---|---|---|
| Endocytosis (rno04144) | ||
| RT1-T24-1 | −1.8954104 | 0.00016123 |
| RT1-CE5 | −0.9521472 | 0.00065068 |
| RT1-T24-3 | −1.5051583 | 0.00069523 |
| Washc5 | 0.41562476 | 0.00124156 |
| Igf2r | 0.73143706 | 0.00807887 |
| Ap2s1 | −0.4407505 | 0.01069065 |
| Gbf1 | 0.36912001 | 0.01732892 |
| Egfr* | 0.52666122 | 0.01836792 |
| Ap2b1 | 0.55158071 | 0.02562886 |
| Tfrc* | 0.41008398 | 0.02729657 |
| Vps35* | 0.24749648 | 0.03275091 |
| Vps28* | −0.2918485 | 0.03732335 |
| Rab31 | 0.2307909 | 0.04102501 |
| Stam2 | 0.60099759 | 0.04125232 |
| Ubb* | −0.4214775 | 0.04191601 |
| Arrb1* | 0.2979953 | 0.04304641 |
| Vps26b* | 0.20272241 | 0.04646427 |
| Cell adhesion molecule (rno04514) | ||
| RT1-T24-1* | −1.8954104 | 0.00016123 |
| RT1-CE5* | −0.9521472 | 0.00065068 |
| RT1-T24-3* | −1.5051583 | 0.00069523 |
| RT1-Bb* | 1.34228528 | 0.00260124 |
| Cldn5* | −0.7695119 | 0.00636124 |
| Glg1* | 0.8652644 | 0.0084503 |
| Itga8* | 0.93041203 | 0.02562886 |
| RT1-Ba* | 0.83828736 | 0.02636128 |
| Itga9* | 0.88436668 | 0.02651378 |
| F11r* | −0.4457938 | 0.03561501 |
| Itgam* | 0.53418603 | 0.04984969 |
| Vascular smooth muscle contraction (rno04270) | ||
| Pla2g12a | −0.5066628 | 0.00528555 |
| Gna11 | 0.47734613 | 0.00911017 |
| Ramp2 | −0.4783029 | 0.01543466 |
| Edn1* | −1.0713506 | 0.01925476 |
| Pla2g2d | 1.7306706 | 0.02330807 |
| Arhgef1* | −0.3051994 | 0.02332887 |
| Adora2b | −0.6417687 | 0.02562886 |
| Rock1 | 0.5090851 | 0.03524969 |
| Scart1 | −2.4974685 | 0.03916115 |
| Pla2g4b* | −0.4119394 | 0.03942963 |
| Avpr1a | 0.88118128 | 0.04108323 |
| Itpr1 | 0.6847391 | 0.04908477 |
FC, fold change. *Unique to pregnancy.
Table 4.
Differentially expressed genes in enriched pathways associated with vascular change in virgins
| Genes | logFC | Adjusted P Value |
|---|---|---|
| Endocytosis (rno04144) | ||
| Igf2r | 1.07214645 | 5.32E−05 |
| Washc5 | 0.42014909 | 0.00014335 |
| RT1-CE5 | −0.841826 | 0.00031699 |
| Smurf1* | 0.44776377 | 0.00069412 |
| Ldlr* | 1.1064348 | 0.00072605 |
| Stam2 | 0.82533814 | 0.00089458 |
| Egfr | 0.57757056 | 0.00167132 |
| Ldlrap1* | 0.90885664 | 0.00278776 |
| RT1-A1* | 0.70125264 | 0.00288714 |
| Ap2s1 | −0.393635 | 0.00410154 |
| Tgfbr1* | 0.67025551 | 0.00554016 |
| Ap2b1 | 0.53355777 | 0.00560196 |
| RT1-CE3* | −0.5435886 | 0.00575491 |
| RT1-T24-1 | −1.1653139 | 0.00694437 |
| Rab35* | 0.21198805 | 0.00696039 |
| Smurf2* | 0.46101918 | 0.00697639 |
| Gbf1 | 0.33163525 | 0.00720083 |
| Rab11fip2* | 0.71295699 | 0.00785494 |
| Wipf1* | 0.48207921 | 0.00907999 |
| Traf6* | 0.8578216 | 0.01076451 |
| Rab11fip3* | 0.17748121 | 0.01221519 |
| Smad3* | 0.31325773 | 0.01286929 |
| Wipf2* | 0.35069081 | 0.01288555 |
| Asap1* | 0.43457572 | 0.0130679 |
| Arf6* | 0.2988177 | 0.01461624 |
| Prkci* | 0.28963422 | 0.01474284 |
| Cbl* | 0.39212626 | 0.01629271 |
| Arfgef1* | 0.27299589 | 0.0167549 |
| Nedd4* | 0.35925428 | 0.0170128 |
| Grk5* | 0.54775389 | 0.01726178 |
| LOC108348108* | 1.03960189 | 0.01799993 |
| Pard6g* | 0.63268633 | 0.01881493 |
| Capza1* | 0.30906037 | 0.01921984 |
| Pml* | 0.36056246 | 0.02162068 |
| Pdcd6ip* | 0.32893428 | 0.02288074 |
| Smad2* | 0.22089215 | 0.02387647 |
| RT1-T24-3 | −0.914242 | 0.02418818 |
| Psd2* | −1.3746896 | 0.02453949 |
| Pdgfra* | 0.60733811 | 0.02541231 |
| Pard3* | 0.31460809 | 0.02556487 |
| Wipf3* | −0.7897819 | 0.02558284 |
| Asap3* | 0.49968836 | 0.0265341 |
| Dnm2* | 0.19314159 | 0.02729332 |
| Rab22a* | 0.2367043 | 0.02733381 |
| Tgfbr2* | 0.40298856 | 0.02879358 |
| RT1-CE10* | −0.4724288 | 0.02969642 |
| Snx32* | −0.4076472 | 0.0320064 |
| Eps15* | 0.37163035 | 0.0327039 |
| Eea1* | 0.62630041 | 0.03356776 |
| Rab31 | 0.17984003 | 0.03364551 |
| Vps4b* | 0.16900347 | 0.03530268 |
| Prkcz* | −0.956167 | 0.0355857 |
| RT1-M2* | −1.8747475 | 0.0360575 |
| Cblb* | 0.25553999 | 0.03612664 |
| Dab2* | 0.55641378 | 0.03612664 |
| Chmp4c* | 0.49154453 | 3.67E−02 |
| Cltc* | 0.19873296 | 0.04052095 |
| Spart* | 0.25246102 | 0.04239346 |
| Acap3* | −0.254748 | 0.04322249 |
| Hgs* | 0.13271725 | 0.04411995 |
| AC120486.2* | −4.4930317 | 0.04477856 |
| Vps45* | −0.2499854 | 0.04657633 |
| Vascular smooth muscle contraction (rno04270) | ||
| Gna11 | 0.5012163 | 0.00086507 |
| Pla2g2d | 1.90915563 | 0.00129087 |
| Itpr1 | 0.96854715 | 0.00145957 |
| Gna13* | 1.08756049 | 0.00291747 |
| Pla2g12a | −0.4179401 | 0.00336318 |
| Agtr1b* | 2.25301692 | 0.00476156 |
| Itpr2* | 0.51245391 | 0.00564378 |
| Gnaq* | 0.77507753 | 0.00784604 |
| Pla2g3* | −2.2148901 | 0.00793775 |
| Adcy6* | 0.5111389 | 8.01E−03 |
| Scart1 | −1.9656073 | 0.00800761 |
| Rock2* | 0.52748866 | 0.00922219 |
| Raf1* | 0.30020404 | 0.0093178 |
| Kcnma1* | 0.91501588 | 0.01035623 |
| Adora2b | −0.4702114 | 0.01457666 |
| Arhgef12* | 0.66483327 | 0.01812456 |
| Rock1 | 0.43707243 | 0.02019393 |
| Adcy9* | 0.24093617 | 0.02335909 |
| Ppp1r12b* | 0.81537514 | 0.02397435 |
| Mrvi1* | 0.6702386 | 0.03028809 |
| Adcy5* | 0.4943762 | 0.03437972 |
| Avpr1a | 0.67446088 | 0.03635684 |
| Cald1* | 0.64812289 | 0.03723502 |
| Agt* | −1.3116587 | 0.03934063 |
| Gna12* | 0.18664668 | 0.0423656 |
| Ramp2 | −0.2922992 | 0.04252544 |
| Mylk* | 0.78313792 | 4.94E−02 |
FC, fold change. *Unique to virgins.
Table 5.
Top significantly differentially expressed genes during pregnancy
| Gene | logFC | Adjusted P Value |
|---|---|---|
| Grlf1-ps1 | 4.752594 | 0.000245 |
| LOC103694308 | 3.755922 | 0.006734 |
| RT1-S2 | 2.747548 | 0.037323 |
| LOC108353208 | 2.724812 | 0.003721 |
| Fbln7 | 2.388976 | 4.43E−05 |
| LOC103690108 | 2.30313 | 6.28E−08 |
| LOC100364335 | 2.173615 | 6.28E−08 |
| Igf2bp3 | 2.12632 | 0.030392 |
| Ect2 | 2.096913 | 0.000245 |
| Parpbp | 2.018892 | 0.006624 |
| LOC100361025 | 1.92775 | 0.026434 |
| LOC103693415 | 1.904107 | 0.001495 |
| Slc7a9 | 1.899214 | 0.041334 |
| LOC103694069 | 1.897402 | 0.006648 |
| F2rl1 | 1.841341 | 0.011384 |
| LOC103690936 | −5.3591 | 3.62E−05 |
| LOC108348337 | −5.12155 | 0.000172 |
| LOC108352132 | −4.75313 | 0.00013 |
| LOC685989 | −3.83943 | 0.000695 |
| LOC108353254 | −3.60863 | 0.000169 |
| LOC100362023 | −3.31602 | 0.012162 |
| LOC688649 | −3.24293 | 0.001366 |
| RT1-CE13 | −3.18168 | 0.001745 |
| Samd7 | −3.13073 | 0.010723 |
| LOC100359752 | −2.88319 | 0.041727 |
| LOC679730 | −2.80779 | 0.013578 |
| Hpd | −2.74876 | 0.027498 |
| Scart1 | −2.49747 | 0.039161 |
| LOC363301 | −2.43818 | 0.001875 |
| LOC108348488 | −2.35286 | 0.010691 |
| LOC108353207 | −2.27875 | 0.00013 |
| LOC102551606 | −2.27423 | 0.022837 |
| LOC691722 | −2.21256 | 0.032441 |
| Gal3st2 | −2.2091 | 0.017604 |
| LOC103690234 | −2.15383 | 0.032453 |
| Lrrc31 | −2.13026 | 0.004583 |
| Col9a1 | −2.10059 | 0.006476 |
| LOC501317 | −2.09289 | 0.040345 |
| Kmo | −2.0879 | 0.000245 |
| Kcns3 | −2.08471 | 0.025629 |
| Dbp | −2.06492 | 0.01883 |
| LOC102550991 | −2.01649 | 0.000722 |
| Krt12 | −1.96446 | 0.003441 |
| Ly6g5b | −1.9472 | 0.000432 |
| RT1-T24-1 | −1.89541 | 0.000161 |
| Insrr | −1.8605 | 0.004583 |
| LOC103691190 | −1.81915 | 0.037269 |
FC, fold change.
DISCUSSION
Preeclampsia remains a disorder in which the mechanistic insights remain elusive. Neurological complications associated with PE are particularly challenging to assess because of the limitation of obtaining measurements from pregnant women, thus presenting a large knowledge gap in this field of research. Here, as previously observed, the Dahl SS/jr model of superimposed PE mimics the maternal syndrome of human PE with increased blood pressure, proteinuria, cerebral edema, and BBB disruption (15). Surprisingly, CBF autoregulation and vascular smooth muscle myogenic tone were enhanced during pregnancy in this model of chronic hypertension, suggesting that these mechanisms do not contribute to the edema and BBB disruption in pregnant Dahl SS/jr rats. Analysis of the cerebral endothelial cell morphology displayed opening of tight junctions, increases in basement membrane dissolution, and increased vesicle formation, all of which could contribute to the observed increases of cerebrovascular permeability. Furthermore, transcriptome analysis revealed several pathways that could mechanistically contribute to these observed changes during pregnancy, including genes related to tight junctions, the BBB, and transport across the BBB.
The Dahl SS/jr rat provides a setting in which a clear and consistent preeclamptic phenotype is observed that develops spontaneously without the presence of surgical, dietary, or pharmacological interventions (15). The present study compared Dahl SS/jr rats to healthy pregnant SD rats as well as another spontaneous hypertensive nonpreeclamptic model (SHR) to assess several cerebrovascular characteristics in a setting of spontaneous superimposed PE. Previous studies using different preclinical models of PE have assessed cerebrovascular changes; however, the exact mechanism remains unclear (9, 12, 14, 17, 18, 26, 27).Our findings that the Dahl SS/jr rat exhibited increases in water content in the posterior brain compared with normal healthy SD pregnancy are consistent with other studies in rodent models of preeclampsia and the preeclampsia patient. For example, previous studies reported that pregnant Dahl SS/jr rats fed a low- or high-salt diet had impaired myogenic reactivity in the posterior cerebral arteries (28). Studies also showed that the posterior circulation (occipito-parietal lobe) is more often affected than other regions in patients with severe preeclampsia and women who previously experienced preeclampsia (29, 30). Therefore, our findings are in agreement with previous work by Cipolla et al. (12), who found that the posterior cerebral cortex may be more susceptible to the edema associated with preeclampsia/eclampsia. Additionally, we also observed significant increases in vascular permeability in the pregnant Dahl SS/jr rat compared with virgin control rats. These findings mimic those observed in human PE, namely, vasogenic edema (31–33). Previous studies used Evans blue extravasation to demonstrate increased BBB permeability during placental ischemia (14), and our preliminary data suggested increased Evans blue leakage in the brain of the Dahl SS/jr rat during pregnancy (34). Here, using a low-molecular-mass fluorescent dextran, 3 kDa Texas Red, we showed that there was greater permeability in the pregnant Dahl SS/jr rats compared with virgin littermates.
This increased permeability and edema in other models of PE was proposed to result from a loss in myogenic tone and CBF autoregulatory control, leading to disruption of the BBB (9, 18, 27, 33, 35, 36). Interestingly, our data showed that CBF autoregulation in the Dahl SS/jr rat was enhanced during preeclamptic pregnancy, a finding further supported by an increase in middle cerebral artery myogenic tone. In contrast, Aukes et al. (28) showed that young (7 wk old) female Dahl SS/jr rats fed a high-salt (8% NaCl) diet to induce hypertension displayed decreases in myogenic activity and hypertensive remodeling in the posterior cerebral artery during pregnancy. However, they found that these changes were primarily due to pregnancy itself rather than increases in blood pressure (28). The discrepancies between these studies are likely the result of different study designs (i.e., different age, diet, and cerebral arteries studied), given that the same strain (Dahl SS/jr) rat was used. Nonetheless, the results presented in this study suggest that the pregnant Dahl SS/jr rat exhibits an intact cerebral vascular smooth muscle, thus revealing a setting in which cerebral edema occurs independently of changes in autoregulatory function and myogenic tone. However, there are limitations to the methods that we used in these studies. First, the brains were cut along the middle cerebral artery, which means that some tissue sections within the posterior cerebrum were supplied by the middle cerebral artery. We are unable to pinpoint whether the increases in brain water content occurred closest to the occipital or parietal lobe or throughout the posterior cerebrum. Additionally, in these studies we focused solely on middle cerebral artery myogenic tone based on our previous findings in the reduced uterine perfusion pressure model of placental ischemia (17). Future studies will examine changes specifically in posterior cerebral arteries.
Our data, coupled with the idea of widespread multiorgan system endothelial dysfunction and its role in PE, led us to assess how BBB permeability is specifically related to cerebral endothelial cell disruption (37–42). Assessment of brain capillary endothelial cell morphology using electron microscope images revealed increases in basement membrane dissolution and tight junction opening, further supporting the disruption of the cerebral capillary endothelial cell layers in the pregnant Dahl SS/jr rat. Of interest was the finding of increased endothelial cell vesicle formation, suggesting that the increased BBB permeability may be due to changes in transcellular transport processes in addition to the paracellular transport pathway (43).
RNAseq analysis from the cerebrovascular network of the pregnant Dahl SS/jr compared with healthy control pregnancy (SD) yielded a moderate, but significant number of differentially expressed genes. Several of these genes mapped to pathways related to endocytosis, cell adhesion molecules, and vascular smooth muscle contraction. The endocytic pathway plays a critical role in the BBB in that it functions to facilitate transport of nutrients across the barrier via fluid-phase, adsorptive, and receptor-mediated endocytosis (44). This pathway is of particular interest as a recent study showed that maternal extracellular vesicles formed via endocytosis may play a role in the progression of PE (45). In that regard, the same inflammatory maternal circulating factors that lead to inflammation and endothelial dysfunction in the periphery may play a significant role in disruption of the BBB. Cell adhesion molecules (CAMs) have been used as markers of endothelial dysfunction and vascular inflammation in the brain (46). Pathway analysis revealed several CAMs to be differentially expressed in the pregnant Dahl SS/jr, including a decrease in F11r, a cell adhesion protein expressed within tight junctions of endothelial cells, and a decrease in Cldn5, whose major role is the organization of endothelial cell tight junctions and a main contributor to the tightness of the tight junction seal (47, 48). Although the present CBF autoregulation and myogenic experiments support an intact vascular smooth muscle, several differentially expressed genes may have contributed to the pathway of vascular smooth muscle contraction. Vascular smooth muscle gene Ramp2 was significantly decreased from the cerebrovascular unit of the pregnant Dahl SS/jr, and loss of Ramp2 has been linked to increased paracellular leakage due to a decrease in tight junction, adherence junction, and basement membrane molecules (49). Studies assessing arginine vasopressin have shown increased cerebral edema, and an upregulation of Avpr1a, arginine vasopressin receptor 1a, is associated with traumatic brain injury (50). Another study reported that blockade of the Avpr1a decreases BBB breakdown (51). Together, these studies suggest how the significantly elevated levels of Avpr1a gene in the pregnant Dahl SS/jr may contribute to increased BBB permeability. These genes along with the BBB transport and tight junction genes suggest that the cause of the permeability changes observed are more directly related to that of a dysfunctional cerebral endothelial cell rather than vascular smooth muscle.
One limitation of our study is that we used the entire circle of Willis and associated vessels and did not differentiate changes in gene expression within specific cell types. Nevertheless, our findings suggest that endothelial cells are more vulnerable to damage compared with disruptions in smooth muscle function in the Dahl SS/jr superimposed PE model. Future studies will also evaluate changes in gene expression within endothelial cells and smooth muscle cells isolated from these different rat models, and changes in gene expression in other components of the neurovascular unit will also be assessed.
In summary, this study provides further support of the Dahl SS/jr rat as a preclinical model of spontaneous superimposed PE and describes uncoupling of cerebral vascular permeability and BBB disruption from CBF autoregulatory dysfunction and myogenic tone. Additionally, the data presented in this study lay the foundational framework on which future experiments assessing specific transcellular transport components such as individual transporter protein expression and components of the vesicular transport system (caveolae) can be built to help reveal potential direct mechanistic insight into the causes of cerebrovascular complications during preeclamptic pregnancies.
GRANTS
K.J.M. was supported by NIH Grant 5T32 HL-105324-10 and American Heart Association Grant 20PRE352000021. D.M.M. was supported by the UMMC Medical Student Research Program (MSRP). Work was supported by NIH Grants R01 HL-136684 to M.J.R., 1R01 HL-137673 to M.R.G., and R01 HL-134711 to J.M.S. The work performed through the UMMC Molecular and Genomics Facility is supported, in part, by funds from the NIGMS, including Mississippi INBRE (P20 GM-103476), Obesity, Cardiorenal and Metabolic Diseases-COBRE (P20 GM-104357), and Mississippi Center of Excellence in Perinatal Research (MS-CEPR)-COBRE (P20 GM-121334).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.J.M. and J.M.S. conceived and designed research; K.J.M., D.M.M., and J.P.W. performed experiments; K.J.M., D.M.M., and K.C.S. analyzed data; K.J.M., K.C.S., and J.M.S. interpreted results of experiments; K.J.M. prepared figures; K.J.M. drafted manuscript; K.J.M., D.M.M., K.C.S., J.P.W., M.J.R., M.R.G., and J.M.S. edited and revised manuscript; K.J.M., D.M.M., K.C.S., J.P.W., M.J.R., M.R.G., and J.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Glenn Hoskins, Subhi T. Younes, Josh Jefferson, Ashley Johnson, John Aaron Howell, Dr. Gene Lee Bidwell, and Dr. Jeremy Duncan for technical assistance. The authors also acknowledge Dr. Marilyn J. Cipolla for valuable input.
REFERENCES
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 33: 130–137, 2009. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 348: g2301, 2014. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control 8: 7–12, 2015. doi: 10.2147/IBPC.S50641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 10: 466–480, 2014. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokslag A, van Weissenbruch M, Mol BW, de Groot CJ. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev 102: 47–50, 2016. doi: 10.1016/j.earlhumdev.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 28: 1–19, 2013. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 7.Cipolla MJ. The Cerebral Circulation. San Rafael, CA: Morgan & Claypool Publishers, 2009. [PubMed] [Google Scholar]
- 8.Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr 27: 89–94, 2016. doi: 10.5830/CVJA-2016-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipolla MJ. The adaptation of the cerebral circulation to pregnancy: mechanisms and consequences. J Cereb Blood Flow Metab 33: 465–478, 2013. doi: 10.1038/jcbfm.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson AC, Cipolla MJ. The cerebral circulation during pregnancy: adapting to preserve normalcy. Physiology 30: 139–147, 2015. doi: 10.1152/physiol.00048.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman AC, Cipolla MJ, Chan SL. Effect of pregnancy and nitric oxide on the myogenic vasodilation of posterior cerebral arteries and the lower limit of cerebral blood flow autoregulation. Reprod Sci 20: 1046–1054, 2013. doi: 10.1177/1933719112473661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipolla MJ, Bishop N, Chan SL. Effect of pregnancy on autoregulation of cerebral blood flow in anterior versus posterior cerebrum. Hypertension 60: 705–711, 2012. doi: 10.1161/HYPERTENSIONAHA.112.198952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreurs MP, Houston EM, May V, Cipolla MJ. The adaptation of the blood‐brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy. FASEB J 26: 355–362, 2012. doi: 10.1096/fj.11-191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warrington JP, Drummond HA, Granger JP, Ryan MJ. Placental ischemia-induced increases in brain water content and cerebrovascular permeability: role of TNF-α. Am J Physiol Regul Integr Comp Physiol 309: R1425–R1431, 2015. doi: 10.1152/ajpregu.00372.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R62–R70, 2015. doi: 10.1152/ajpregu.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takushima S, Nishi Y, Nonoshita A, Mifune H, Hirata R, Tanaka E, Doi R, Hori D, Kamura T, Ushijima K. Changes in the nitric oxide-soluble guanylate cyclase system and natriuretic peptide receptor system in placentas of pregnant Dahl salt-sensitive rats. J Obstet Gynaecol Res 41: 540–550, 2015. doi: 10.1111/jog.12602. [DOI] [PubMed] [Google Scholar]
- 17.Ryan MJ, Gilbert EL, Glover PH, George EM, Masterson CW, McLemore GR Jr, LaMarca B, Granger JP, Drummond HA. Placental ischemia impairs middle cerebral artery myogenic responses in the pregnant rat. Hypertension 58: 1126–1131, 2011. doi: 10.1161/HYPERTENSIONAHA.111.181453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warrington JP, Fan F, Murphy SR, Roman RJ, Drummond HA, Granger JP, Ryan MJ. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol Rep 2: e12134, 2014. doi: 10.14814/phy2.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terstappen F, Clarke SM, Joles JA, Ross CA, Garrett MR, Minnion M, Feelisch M, van Goor H, Sasser JM, Lely AT. Sodium thiosulfate in the pregnant Dahl salt-sensitive rat, a model of preeclampsia. Biomolecules 10: 302, 2020. doi: 10.3390/biom10020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 22.Alexa A, Rahnenfuhrer J. topGO: Enrichment analysis for Gene Ontology. R package version 2.22.0. Vienna: R Foundation for Statistical Computing, 2010. [Google Scholar]
- 23.Durinck S, Moreau Y, Kasprzyk A, Davis S, De Moor B, Brazma A, Huber W. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 21: 3439–3440, 2005. doi: 10.1093/bioinformatics/bti525. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40: 4288–4297, 2012. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipolla MJ, Sweet JG, Chan SL. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J Appl Physiol (1985) 110: 329–339, 2011. doi: 10.1152/japplphysiol.01159.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Euser AG, Cipolla MJ. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension 49: 334–340, 2007. doi: 10.1161/01.HYP.0000255791.54655.29. [DOI] [PubMed] [Google Scholar]
- 28.Aukes AM, Vitullo L, Zeeman GG, Cipolla MJ. Pregnancy prevents hypertensive remodeling and decreases myogenic reactivity in posterior cerebral arteries from Dahl salt-sensitive rats: a role in eclampsia? Am J Physiol Heart Circ Physiol 292: H1071–H1076, 2007. doi: 10.1152/ajpheart.00980.2006. [DOI] [PubMed] [Google Scholar]
- 29.Raman MR, Tosakulwong N, Zuk SM, Senjem ML, White WM, Fields JA, Mielke MM, Lesnick TG, Bailey KR, Jack CR Jr, Miller VM, Garovic VD, Kantarci K. Influence of preeclampsia and late-life hypertension on MRI measures of cortical atrophy. J Hypertens 35: 2479–2485, 2017. doi: 10.1097/HJH.0000000000001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topuz S, Kalelioğlu I, Iyibozkurt AC, Akhan S, Has R, Tunaci M, Ibrahimoğlu L. Cranial imaging spectrum in hypertensive disease of pregnancy. Clin Exp Obstet Gynecol 35: 194–197, 2008. [PubMed] [Google Scholar]
- 31.Ay H, Buonanno FS, Schaefer PW, Le DA, Wang B, Gonzalez RG, Koroshetz WJ. Posterior leukoencephalopathy without severe hypertension: utility of diffusion-weighted MRI. Neurology 51: 1369–1376, 1998. doi: 10.1212/wnl.51.5.1369. [DOI] [PubMed] [Google Scholar]
- 32.Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, Lamarca B, Martin JN. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol 208: 468.e1–468.e6, 2013. doi: 10.1016/j.ajog.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Younes ST, Ryan MJ. Pathophysiology of cerebral vascular dysfunction in pregnancy-induced hypertension. Curr Hypertens Rep 21: 52, 2019. doi: 10.1007/s11906-019-0961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda KJ, Warrington JP, Duncan J, Granger JP, Garrett MR, Ryan MJ, Sasser JM. Cerebral edema and blood brain barrier dysfunction in the Dahl S rat model of superimposed preeclampsia ( Abstract). FASEB J 31: lb857, 2017. [Google Scholar]
- 35.Shekhar S, Liu R, Travis OK, Roman RJ, Fan F. Cerebral autoregulation in hypertension and ischemic stroke: a mini review. J Pharm Sci Exp Pharmacol 2017: 21–27, 2017. doi: 10.29199/PSEP.101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smeda JS, Payne GW. Alterations in autoregulatory and myogenic function in the cerebrovasculature of Dahl salt-sensitive rats. Stroke 34: 1484–1490, 2003. doi: 10.1161/01.STR.0000073842.18224.AA. [DOI] [PubMed] [Google Scholar]
- 37.Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol 232: R27–R44, 2017. doi: 10.1530/JOE-16-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550, 2008. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 39.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension 38: 718–722, 2001. doi: 10.1161/01.HYP.38.3.718. [DOI] [PubMed] [Google Scholar]
- 40.Lamarca B. Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol 64: 309–320, 2012. [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien M, Baczyk D, Kingdom JC. Endothelial dysfunction in severe preeclampsia is mediated by soluble factors, rather than extracellular vesicles. Sci Rep 7: 5887, 2017. doi: 10.1038/s41598-017-06178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Med 16: 5–15, 1998. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 43.Mayhan WG, Arrick DM. The Blood-Brain Barrier in Health and Disease. San Rafael, CA: Morgan & Claypool Life Sciences, 2016. doi: 10.4199/C00148ED1V01Y201612ISP072. [DOI] [Google Scholar]
- 44.Smith MW, Gumbleton M. Endocytosis at the blood-brain barrier: from basic understanding to drug delivery strategies. J Drug Target 14: 191–214, 2006. doi: 10.1080/10611860600650086. [DOI] [PubMed] [Google Scholar]
- 45.Gilani SI, Weissgerber TL, Garovic VD, Jayachandran M. Preeclampsia and extracellular vesicles. Curr Hypertens Rep 18: 68, 2016. doi: 10.1007/s11906-016-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis 107: 41–56, 2017. doi: 10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azari BM, Marmur JD, Salifu MO, Ehrlich YH, Kornecki E, Babinska A. Transcription and translation of human F11R gene are required for an initial step of atherogenesis induced by inflammatory cytokines. J Transl Med 9: 98, 2011. doi: 10.1186/1479-5876-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma SC, Li Q, Peng JY, Zhouwen JL, Diao JF, Niu JX, Wang X, Guan XD, Jia W, Jiang WG. Claudin-5 regulates blood-brain barrier permeability by modifying brain microvascular endothelial cell proliferation, migration, and adhesion to prevent lung cancer metastasis. CNS Neurosci Ther 23: 947–960, 2017. doi: 10.1111/cns.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ichikawa-Shindo Y, Sakurai T, Kamiyoshi A, Kawate H, Iinuma N, Yoshizawa T, Koyama T, Fukuchi J, Iimuro S, Moriyama N, Kawakami H, Murata T, Kangawa K, Nagai R, Shindo T. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J Clin Invest 118: 29–39, 2008. doi: 10.1172/JCI33022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2: 492–516, 2011. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viñuela-Berni V, Gómez-González B, Quintanar-Stephano A. Blockade of arginine vasopressin receptors prevents blood-brain barrier breakdown in experimental autoimmune encephalomyelitis. Sci Rep 10: 467, 2020. doi: 10.1038/s41598-019-57134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]