Abstract
Background
As preterm infants’ neurodevelopment is shaped by NICU-related factors during their hospitalization, it is essential to evaluate which interventions are more beneficial for their neurodevelopment at this specific time. The objective of this systematic review and meta-analysis was to evaluate the effectiveness of interventions initiated during NICU hospitalization on preterm infants’ early neurodevelopment during their hospitalization and up to two weeks corrected age (CA).
Methods
This systematic review referred to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] guidelines and was registered in PROSPERO (CRD42017047072). We searched CINAHL, MEDLINE, PubMed, EMBASE (OVID), Cochrane Systematic Reviews, CENTRAL, and Web of Science from 2002 to February 2020 and included randomized controlled/clinical trials conducted with preterm infants born between 24 and 366/7 weeks of gestation. All types of interventions instigated during NICU hospitalization were included. Two independent reviewers performed the study selection, data extraction, assessment of risks of bias and quality of evidence.
Results
Findings of 12 studies involving 901 preterm infants were synthesized. We combined three studies in a meta-analysis showing that compared to standard care, the NIDCAP intervention is effective in improving preterm infants’ neurobehavioral and neurological development at two weeks CA. We also combined two other studies in a meta-analysis indicating that parental participation did not significantly improve preterm infants’ neurobehavioral development during NICU hospitalization. For all other interventions (i.e., developmental care, sensory stimulation, music and physical therapy), the synthesis of results shows that compared to standard care or other types of comparators, the effectiveness was either controversial or partially effective.
Conclusions
The overall quality of evidence was rated low to very low. Future studies are needed to identify interventions that are the most effective in promoting preterm infants’ early neurodevelopment during NICU hospitalization or close to term age. Interventions should be appropriately designed to allow comparison with previous studies and a combination of different instruments could provide a more global assessment of preterm infants’ neurodevelopment and thus allow for comparisons across studies.
Trial registration
Prospero CRD42017047072.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-021-02559-6.
Keywords: Neurodevelopment, Preterm infants, Systematic review, Interventions, NICU, Meta-analysis
Background
Rationale
Over the last decades, the increasing survival rates of preterm infants admitted in the Neonatal Intensive Care Unit [NICU] have emphasized the importance of considering their neurodevelopmental outcomes [1]. Preterm infants are more likely to have significant short- and long-term neurodevelopmental impairments, since their brains go through a critical period of development and maturation between 24 and 40 weeks of gestation occurring mainly during their NICU hospitalization [2, 3]. Significant neurodevelopmental disabilities associated with neurosensory, motor, behavioral and cognitive outcomes may then arise shortly after preterm infants’ birth and last beyond school age and adolescence [4] given that neurocognitive impairments in infants born preterm are still reported in adulthood [5].
Many factors in the NICU environment may be congruent or incongruent with the preterm infants’ developing brain and consequently influence their neurodevelopmental outcomes. Environmental stimulation (i.e., light, noise) [2, 6–8], social interactions with parents [7, 9] and caregiving experience [2] are among influential environmental NICU factors. Interventions targeting these elements during NICU hospitalization should therefore foster preterm infants’ early neurodevelopment.
Different interventions initiated during NICU hospitalization targeting some of these influential NICU factors with the objective of improving preterm infants’ neurodevelopment have been examined in previous systematic reviews. These interventions consist of developmental care a wide-ranging group of interventions to minimize preterm infants’ NICU environmental stress [10–12], including Neonatal Individualized Developmental Care Assessment Program [NIDCAP], which is an individualized approach based on observing preterm infants’ behaviors to guide caregiving activities provided by professionals and infants’ family [13], noise reduction management, [14] in addition to interventions with parental involvement (i.e. NIDCAP, kangaroo care and developmental care) [15] and skin-to-skin contact [16]. The effectiveness of these interventions has been evaluated on the long-term neurodevelopment of preterm infants at 12 months or 24 months of age, providing important evidence. Yet, measuring the effects of interventions implemented during hospitalization only at one year of age and older raises concerns about confounding factors that may have occurred between NICU discharge and the timing of the assessments and that could have influenced the infants’ long-term neurodevelopment along with interventions.
Neurodevelopmental assessments of preterm infants during NICU hospitalization are currently performed to predict long-term neurodevelopmental outcomes and plan early interventions [17–19]. A systematic review confirmed that neurobehavioral assessments of infants’ general movements (GMs) referring to the most common and complex spontaneous movements patterns [20] and infants’ posture and movements with the Test of Infant motor Performance (TIMP) [21] done before term corrected age [CA] are the most significant predictors of long-term neurodevelopment [18]. Nonetheless, to our knowledge, no systematic review has evaluated the effectiveness of interventions on the preterm infants’ short-term neurodevelopment, that is, during NICU hospitalization or close to term CA. As preterm infants’ neurodevelopment is shaped by NICU-related factors as soon as they are hospitalized, it is imperative to explore which interventions are most beneficial for their neurodevelopment at this specific time encompassing a decisive period for their brain development and maturation.
Objective
The primary objective of this systematic review and meta-analysis was to evaluate the effectiveness of interventions initiated during NICU hospitalization and delivered by healthcare professionals and/or parents on preterm infants’ early neurodevelopment during their NICU hospitalization and by two weeks CA.
Methods
Protocol and registration
This systematic review protocol was published [22] and registered in the International Prospective Register of Systematic Reviews [PROSPERO] (CRD42017047072). The systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] guidelines [23].
The published protocol [22] was modified for the search strategy, as we did not manually search journals. Journals focusing on neonatology, neonates and/or neurodevelopment were already indexed, thus articles published in these journals were captured by our searches in various databases. It was initially planned to only include studies measuring preterm infants’ neurodevelopment during NICU hospitalization, but we also considered studies assessing this outcome at infants’ two weeks CA since it was sufficiently close to NICU hospitalization. We included as well studies where preterm infants in study samples had an intraventricular hemorrhage [IVH] greater than grade II [24–26] and various brain injuries (i.e., IVH, cerebellar hemorrhage and periventricular leukomalacia) [27], which differed from the original protocol. These four studies were included as their samples were representative of the neonatal population and the infants’ baseline characteristics and physiologic stability were similar to those allocated to the experimental and control groups, suggesting that they were not significantly different because of IVH or brain injuries. We also included interventions with different components when they were specifically described as being provided together as a complete intervention.
Eligibility criteria of the selected studies
We included randomized controlled/clinical trials [RCTs] and one pilot RCT conducted with preterm infants born between 24 and 366/7 weeks of gestation. Interventions were instigated during NICU hospitalization and were delivered by healthcare professionals or parents, or both and all types of interventions were included. The studies included were written in English or French and were published in the past 18 years, from 2002 to February 2020. All types of comparator groups, such as non-exposed control group or a group exposed to different interventions, were included in this systematic review. Studies reporting preterm infants’ neurodevelopment as an outcome evaluated with a standardized instrument, scale or test were also considered.
Search strategy and information sources
An expert librarian was consulted to conduct the search in the following electronic databases from 2002 to February 2020: CINAHL, MEDLINE, Pubmed, EMBASE [OVID], Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials [CENTRAL] and Web of Science (see Additional File 1, Table S1 for an example of a search strategy in MEDLINE). The Scopus database was also reviewed to search for trials in conference proceedings and ProQuest for theses and dissertations. We also looked for ongoing trials at clinicaltrials.gov, clinicaltrialsregister.eu, the World Health Organization International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/), the International Standard Randomized.
Controlled Trial Number (ISRCTN) registry and the Australian New Zealand Clinical Trials Registry to identify trials that were underway. We also searched three grey literature websites: http://www.opengrey.eu/, http://opengrey.org/ and www.greylit.org. We manually checked the references of included studies.
Study selection
All references for the studies selected for this review were managed in EndNote© X9. After removing duplicates, the screening was conducted by separate reviewers (MA, GDF, AL). The reviewers screened the remaining studies for eligibility by reviewing study titles and abstracts, then the full-text reports (GDF, ER, MHP) to evaluate their appropriateness to be included in the systematic review. For these steps, agreement was reached between two reviewers and disagreement was resolved by consensus with a third reviewer (MA).
Data extraction
Data extraction was performed independently by two authors (ER, MHP) using a data extraction form and pilot tested with two studies [28]. The extracted data were compared for all included studies, and disagreement was solved with a third reviewer (GDF). The extracted information for each study was described in the protocol [22]. All the extracted data were recorded in Review Manager (RevMan) [computer program] (version 5.1 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The data were double-checked by two reviewers (GDF, AL) before conducting the analysis, to avoid errors.
Data items
The primary outcome was preterm infants’ neurodevelopment assessed by a standardized instrument or scale during NICU hospitalization or at term-corrected age. For this systematic review, we considered neurobehavioral, neuromotor, neuromuscular and neurological development evaluation measured by the following instruments: preterm infants’ neurobehavioral development (Assessment of Preterm Infant Behavior – APIB; NICU Neonatal Neurobehavioral Scale – NNNS; Neonatal Neurobehavioral Examination – NNE), neuromotor development (Infant Neurological International Battery – INFANIB; Test of Infant Motor Performance – TIMP), neuromuscular development (New Ballard Score and Dubowitz examination) and neurological development (Prechtl Neurological Examination of the Full-term Newborn). Two of these instruments included subscales; six in the APIB: autonomic, motor, state, interaction-attentional, and self-regulation systems, in addition to the assessment of the degree of examiner facilitation necessary to support the infant’s reorganization when disorganized, and 13 in the NNNS: orientation, habituation, hypertonicity, hypotonicity, excitability, arousal, lethargy, nonoptimal reflexes, asymmetric reflexes, stress, self-regulation, quality of movement and handling. We considered measurements performed before NICU discharge and no later than two weeks after term CA. For measurements performed before term age, we considered the closest measure to discharge.
Assessment of risk of bias of individual studies
The Cochrane risk of bias [28] assessment tool was used to determine the studies’ risk of bias, classified as low, high, or unclear risk of bias. The Cochrane tool considers sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting. The risk of bias was assessed independently by three reviewers (GDF, ER, MHP). In the case of disagreement, consensus was reached by consulting two other reviewers (MA, AL).
Summary measures
Statistical analyses were conducted with the Review Manager (RevMan) 5.1 software, using a random-effect model with a 95% confidence interval [CI]. Different instruments were used to measure neurodevelopment so to maintain homogenous data, we chose to treat each instrument separately. For continuous outcomes, mean differences [MD] were calculated from extracted means and standard deviations. For dichotomous outcomes (frequency and percentage), relative risk [RR] was reported.
Synthesis of results
If two or more studies reported the same outcome with the same instrument, a meta-analysis was conducted with RevMan 5.1 using a random-effect model and inverse variance, as suggested for studies with heterogeneity [PRISMA], with a 95% CI. Heterogeneity was evaluated using the Chi-squared test with a significance level of 0.1 and the I2 using the classification suggested by PRISMA-P: 0 to 40%, not important heterogeneity; 30 to 60%, moderate heterogeneity; 50 to 90%, substantial heterogeneity; and 75,100%, considerable heterogeneity [29]. The unit of analysis in our systematic review consisted of preterm infants receiving an intervention or a control comparator during NICU hospitalization.
Missing data
One author was contacted to obtain data [30]. As the author was not able to provide the data to allow for imputation, the study was excluded from the meta-analysis.
Quality of evidence
The quality of evidence was assessed for each outcome using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines [31] by three authors (MA, GDF, AL). The GRADE system rates the quality of evidence as high, moderate, low or very low in five areas: risk of bias, inconsistency, indirectness, imprecision and publication bias. We downgraded each area by one or two points based on judgment criteria. For risk of bias, we did not downgrade if most risk of bias judgments were rated as “low” and downgraded by one point if the majority were rated “unclear” or “high.” For inconsistency, we did not downgrade if heterogeneity was considered as not important (< 40%), and we downgraded by one point if there was moderate or substantial heterogeneity among the studies (40 to 75%). For indirectness of evidence, we did not downgrade for any outcome. For imprecision, we downgraded by one point if the total number of participants was lower than 400 for the assessed outcome and downgraded by two points if the number of participants was lower than 150. The summary of findings table was generated using the GRADE profiler Guideline Development Tool software and the GRADE criteria (2015, McMaster University and Evidence Prime Inc.).
Results
Study selection
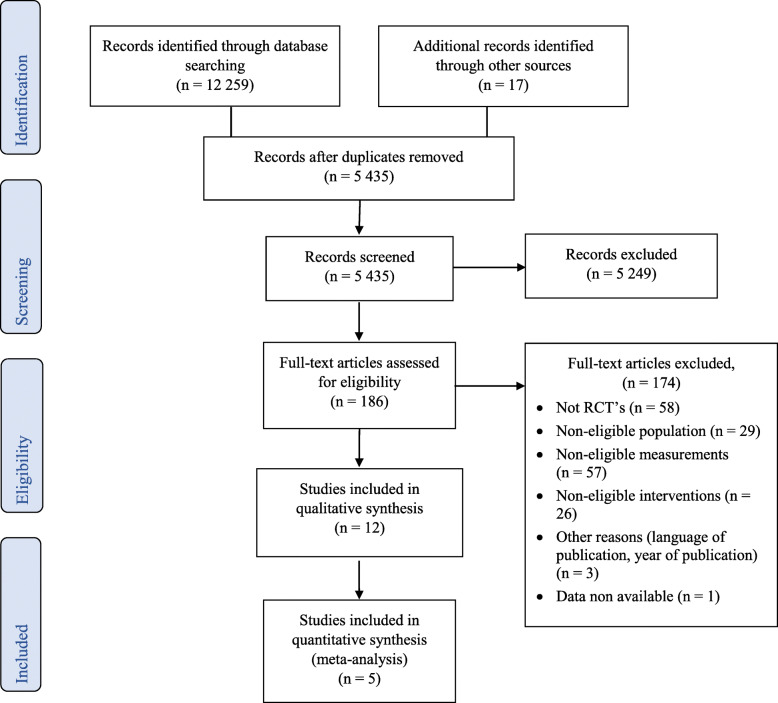
The study selection process is illustrated by the PRISMA Study Flow Diagram (see Fig. 1). Of the 12,259 screened studies, 186 were assessed for eligibility in the final selection. A total of 174 full-text articles were excluded for different reasons: a) 58 articles were not randomized controlled trials; b) 29 articles included exclusively preterm infants with brain abnormalities; c) 57 articles did not measure neurodevelopment or did not measure it with a standardized instrument; d) 26 articles were not eligible interventions; e) three articles were excluded for other reasons (language of publications other than French and English or year of publication before 2002); and f) data was not available for one article. Finally, 12 studies met the inclusion criteria and were included in this systematic review [24–27, 32–39]. Five studies were included in meta-analysis [24, 26, 32, 34, 38] for infants’ neurodevelopment. All the other studies were not suitable for meta-analysis because the nature of the intervention or the instrument used to measure neurodevelopment was different, precluding data pooling [25, 27, 33, 35–37, 39].
Fig. 1.
PRISMA Flow diagram
Characteristics of included studies
The main characteristics of the included studies are summarized in Table 1. The 12 studies published between 2002 and February 2020 included 933 preterm infants. Five studies were conducted in the United States [24, 27, 32, 34, 36], one in the Netherlands [25], two in India [33, 35], one in Thailand [26], one in Taiwan [38], and two in Iran [37, 39]. Eleven studies were RCTs [24–27, 32–35, 37–39] and one was a pilot RCT with published results [36].
Table 1.
Characteristics of included studies in systematic review
| Author, year Country |
Sample and participants (size, WGA and/or birthweight) |
Study Design |
Intervention Description | Intervention (who delivered, duration, dose, frequency) |
Comparator group | Outcome measurement | Timing of assessment | Main Findings |
|---|---|---|---|---|---|---|---|---|
|
Als, 2003 [24] USA |
N = 92 < 28 WGA < 1250 g BW |
RCT 2 parallel groups from 3 different centers. |
Developmental Care - NIDCAP emphasizes the behavioral individuality of each infant. It seeks to diminish the infant’s experiences of stress and to enhance the infant’s strengths. | Delivered daily (7 days per wk) by two professionals (a NICU nurse and a developmental professional). | Standard care - some degree of shielding of incubators, sound containment, use of breast milk, and referral to occupational and physical therapy and to community early intervention. Kangaroo care (skin-to-skin holding) was used in one study center. |
Neurobehavioral: Assessment of Preterm Infant’s Behavior (APIB). Measurements: Autonomic (respiration, digestion, color), motor (tone, movement, postures), state organization (range, robustness, transition patterns), attention and self-regulation. |
At 2 weeks’ CA Infant’s behaviors were recorded every 2 min for approximately 1 h during a medical, nursing, or parent caregiving activity. |
Infants of the experimental group had enhanced neuro-behavioral development (for all APIB system scores) compared to infants of the comparator group. |
|
Als, 2004 [32] USA |
N = 30 28 to 33 WGA BW between 5 and 95% |
RCT 2 parallel groups. |
Developmental care - NIDCAP Developmental care emphasizes the behavioral individuality of each infant. Each infant is seen as an active participant in all care. |
Initiated within 72 h of admission and continued to the age of 2 weeks CA. Delivered daily (7 days/wk) by two certified NIDCAP professionals, psychologist, and infant developmentalist. |
Standard care - shielding incubators, early use of dressing in T-shirts, and side and foot rolls; liberal provision of pacifiers; and inconsistent nurse-dependent encouragement of skin-to-skin holding and breastfeeding. |
Neurobehavioral: APIB. Neurological: Precthl Neurological Examination of the Full-term Newborn. |
At 2 weeks’ CA. | Infants of the experimental group had enhanced neurobehavioral and neurological development (for all APIB system scores and Precthl variables) compared to infants of the comparator group. |
|
Kanagasabai, 2013 [33] India |
N = 50 28 to 36 WGA 1000-2000 g BW |
RCT 2 parallel groups. |
Sensory Stmulation Multisensory - ATVV stimulation program: Auditory - Soft lullaby between (30–40 dB) for 3 min using a miniature speaker and an mp3 player; Tactile - Gentle stroking massage for 3 min in a sequence of chest, upper limbs and lower limbs in supine position; Visual - Black and white visual stimulation card hung at a distance of 8–10 in. from the neonate for 3 min; Vestibular - Gentle horizontal and vertical rocking for 3 min. |
Initiated when infants reached 33 weeks of GA and within 48 h of birth for infants born at 33–36 wk. Delivered five days per wk. × 12 min daily and until discharge from the hospital. |
Usual care: Kangaroo mother care and exclusive breastfeeding. |
Neuromotor development: Infant Neurological International Battery (INFANIB) Measurements: Spasticity, head and trunk, vestibular function, legs, French angles (scarf sign, heel to ear, popliteal angle, abductor’s angle). |
At term age (between 38 to 40 weeks GA). | Infants in the experimental group had significantly improved neuromotor development compared to infants of the comparator group. |
|
Madlinger-Lewis, 2014 [27] USA |
N = 92 ≤ 32 WGA |
RCT 2 parallel groups. |
Developmental Care - Alternative positioning: Structured blanket (Dandle Roo) made of stretchable cotton with adjustable straps for the upper extremities, a pouch for the lower extremities, and a head boundary designed to hold the legs in a weight-bearing, flexed position, while allowing for movement with recoil back to flexion. | Delivered continuously by nurses and parents whenever in bed and not being held or fed until discharge. | Traditional positioning consisted of any positioning devices or adaptations without the use Dandle Roo (swaddling, use of blankets and cloth rolls). |
Neurobehavioral: NICU Network Neurobehavioral Scale (NNNS). Measurements: habituation (not evaluated), orientation, tolerance of handling, quality of movement, self regulation, non-optimal reflexes, stress signs, arousal, hypertonia, hypotonia, asymmetry, excitability, and lethargy. |
Between 35 to 40 weeks PMA. | Infants in the experimental group showed significantly less asymmetry of reflex and motor responses than those in the comparator group. No significant effect was found between groups for the other NNNS subscales. |
|
Maguire, 2008 [25] Netherlands |
N = 192 <32 WGA |
RCT 2 parallel groups. |
Developmental care – Positioning and incubators covers reduction of light and sound through use of incubator covers and using positioning aids to optimize physiologic stability and motor development and promote nesting. |
Delivered continuously within 48 h of life until discharge. | Usual care consisted of no covers or nesting. | Neurological: Precthl Neurological Assessment of the Full-Term Newborn. | At 40 weeks PMA. | No significant effect between the groups was found for infants’ neurological development. |
|
McAnulty, 2009 [34] USA |
N = 107 < 29 weeks WGA < 1250 g BW |
RCT 2 parallel groups. |
Developmental Care – NIDCAP |
Initiated upon NICU admission and continued to the age of 2 weeks CA. Delivered daily (7 days per wk) by two certified NIDCAP professionals, an nurse and a psychologist. |
Control group received standard NICU care. |
Neurobehavioral: APIB. Neurological: Precthl Neurological Assessment of the Full-Term Newborn. |
Weekly observations throughout hospital stay to 2 weeks CA. | Infants of the experimental group had enhanced neurobehavioral development (for 5 APIB scores) and a trend towards better performance on the neurological assessement (Precthl) compared to infants of the comparator group. |
|
Nakwa, 2017 [35] India |
N = 36 33 WGA (mean) |
RCT 2 parallel groups. |
Music: Lullaby (30 to 40 dB during 30 min. Preterm infants received the developmental program as a standard of care. |
Delivered three times a week × 30 min for a total of 3 weeks. | Developmental program (as a standard of care) including a tactile, visual, auditory and vistubural stimulation. |
Neuromotor development: Test of Infant Motor Performance (TIMP) (posture and movements). Neurological devlopment: INFANIB. |
First and last day of intervention during hospitalization. | Infants in the experimental group had significantly improved neuromotor and neurological development compared to infants of the comparator group. |
|
Namprom, 2018 [26] Thailand |
N = 50 28 to 32 WGA < 2500 g BW |
RCT 2 parallel groups. |
Parental participation program (maternal): Three key components: 1- Psychosocial support for mothers to participate in their infant’s care 2-Parent education: teaching the content of developmental care practices for preterm infants 3-Therapeutic developmental interventions pertaining to performance of care practices. |
Delivered by a nurse and mothers. Activities were four 1-h teaching sessions and 4 1-h practice sessions over 4 weeks. | Standard care (no description mentioned). |
Neurobehavioral development: Neonatal Neurobehavioral Examination (NNE). Measurements: Tone and motor patterns, primitive reflexes, and behavioral responses. |
At day 14 and 28 after infants’ birth. | Infants in the experimental group had significantly better neuro-behavioral development (tone and motor pattern, behavioral responses and total score) compared to infants of the comparator group. |
|
Smith, 2014 [36] USA |
N = 20 < 30 WGA < 1000 g BW |
Pilot RCT 2 parallel groups. |
Sensory Stimulation – Tactile (Relaxation (M technique) Structured touch of 8 distinctive patterns, stroking preterm infant’s back on each side of the spine (with the pads of the third and fourth fingers of both hands). |
Initiated when the infant reached 30 weeks of GA. Delivered by a nurse or a another research team member. A 7 min of the M technique, 6 times per week for a total of 5 weeks. | Standard care in the two groups (family-centered care and neurodevelopmental strategies including parental presence, skin-to-skin contact, adequate positioning, environment supporting sleep). |
Neurobehavioral development: NNNS Habituation (not evaluated in study), orientation, tolerance of handling, quality of movement, self-regulation, nonoptimal reflexes, stress signs, arousal, hypertonia, hypotonia, asymmetry, excitability, and lethargy. |
At 35 weeks PMA at the end of the 5 weeks of intervention. | No significant difference between groups was found for any of the NNNS subscales. |
|
Valizadeh, 2017 [37] Iran |
N = 76 25 to 30 WGA 1000 g to 2000 g BW |
RCT, 4 parallel groups Containement group, combination group, hydrotherapy group, physical activity group. |
Three interventions 1. Hydrotherapy group Preterm infant placed in water. Head, neck and pelvis supported (Sweeney and Vignochi method). 2. Physical activity group: Extension and flexion: wrist, elbow, shoulder, ankle, knee and hip joints (Moyer-Mileur protocol). 3. Combination group Hydrotherapy and physical activity. |
Initiated at 32 weeks GA. Delivered by a nurse. For each 3 interventions delivered 10 min daily (30 min or 1 h before feeding) for 14 days. |
Containment group: Preterm infants placed in a fetal position (lateral) with one hand on the preterm infant’s head (at the top) and one hand over the trunk and hip area, |
Neuromotor development: TIMP. Neuromuscular development: New Ballard Score and 2 items from Dubowitz examination. Measurements: New Ballard Score: Posture, Arm Recoil, Popliteal Angle, Scarf Sign, Heel to Ear, Square window. Items from Dubowitz examination: Leg recoil and ankle dorsiflexion. |
At 34 weeks PMA (post-intervention). | No significant effect between groups was found for’ neuromotor and neuromuscu-lar development. Infants of the physical therapy and hydrotherapy groups had significantly better leg recoil from Dubowitz examination compared to infants of the comparator group. |
|
Yu, [38] 2019 Taiwan |
N = 76 32 to 36 WGA < 1500 g BW |
RCT, 2 parallel groups. | Parental Participation Program: interventions on parental participation, feeding, massage, activities, parental support, home transition. |
Delivered by physical therapists, parents and nurses. During the NICU stay 5 sessions of interventions of one hour. |
Standard care: 5 interventions in the NICU (according to the synactive theory of development) and 7 phone calls after discharge. |
Neurobehavioral development: NNE (Chinese version). |
Around 40 weeks PMA (between 38 to 44 weeks). |
Infants in the experimental group had significantly better neuro-behavioral development (tone and motor patterns and total score) than infants of the comparator group. |
|
Zeraati, [39] 2018 Iran |
N = 80 32 to 36 WGA |
RCT, 2 parallel groups. |
Sensory Stimulation - Multisensory Auditory stimulation (lullaby, 30 to 40 dB for 3 min), tactile stimulation (3 min massage), visual stimulation (3 min black an white card), vestibular stimulation (gentle rockin for 3 min). |
Initiated 48 h after birth. Delivered 12 min/session and 5 sessions per week until NICU discharge. | Standard care. |
Neuromuscular development: New Ballard Score. |
At discharge. | Infants of the experimental group had significantly better neuromuscu-lar development after the intervention compared to infants of the comparator group. |
More than half the studies (n = 7) included preterm infants born younger than or at 32 weeks of gestational age [24–27, 34, 36, 37]. Five studies included infants born at a gestational age higher than 32 weeks [32, 33, 35, 38, 39].
The 12 studies include a variety of interventions, including NIDCAP [24, 32, 34], positioning and incubators covers [25], alternative positioning [27], sensory stimulation interventions considering tactile stimulation [36] and multisensory stimulation [33, 35], parental participation programs [26, 38], music [39] and physical activity and/or hydrotherapy [37].
Four studies did not specify who performed the intervention [25, 33, 35, 39]. For three studies, the intervention was delivered by two certified NIDCAP professionals [24, 32, 34]. The other interventions were either performed by a nurse [37], mothers with guidance from nurses [26], nurses and/or parents when they were at the bedside [27], the principal investigator (a nurse) or a trained research team member [36], or physical therapists, parents and nurses [38]. The majority of the studies (n = 8) comprehensively described their control group (i.e., standard care or comparator group) [24, 25, 27, 32, 33, 35–38], and three that described their control group as standard care did not provide specifics [26, 34, 39].
The shortest intervention duration was 14 consecutive days [37], whereas the longest was five weeks, with the intervention performed six days per week [36]. In some studies, the intervention was performed at a distinctive dose and frequency during NICU hospitalization [26, 33, 35–39] (see Table 1 for characteristics of studies). Four interventions were carried out for short periods of time ranging from five to 15 min [33, 36, 37, 39], 30 min [35] or one hour [38], whereas the others were almost always regularly integrated into care, with no details about frequency or dose [24, 25, 27, 32, 34].
The studies measured neurodevelopment using different scales, such as the APIB [24, 32, 34], the Prechtl Neurological Examination of the Full-term Newborn [25, 32, 34], the INFANIB [33, 35], the NNNS [27, 36] the TIMP [35, 37], the NNE [26, 38], the New Ballard Score [37, 39] and items from the Dubowitz examination [37]. Four studies used more than one scale [32, 34, 35, 37]. Eight studies included preterm infants whose neurodevelopment was measured during NICU hospitalization or at discharge [25–27, 33–39] and three studies measured the primary outcome at two weeks CA [24, 32, 34]. Three studies did their measurements at the end of the intervention [35–37], while one study performed their last measurement 28 days after the infants’ birth rather than at the end [26].
Risk of bias of included studies
The risk of bias assessment graph for the included studies is presented in Additional File 3 – Figure S1 (details for each included study); a summarization figure in Fig. 2 and a summary table in Additional File 2 – Table S2 are also presented. Six of the 12 studies reported the random-sequence generation appropriately [25, 27, 33, 36, 38, 39], while the process used was not clearly indicated in the other studies. Only two studies adequately describe their allocation concealment method [32, 36]. The risk of bias from the blinding of personnel was high in three studies [25, 34, 38] due to the nature of the intervention, and it was unclear for all the other studies because of insufficient information. Blinding of outcome assessment was adequately performed in 10 studies except for one rated high-risk [33] and one unclear for the study not addressing this outcome [35]. All the studies were judged low for incomplete outcome data except for two: one did not provide reasons for missing data [32] and one had an imbalance numbers in groups [34]. Selective reporting was unclear in all studies and this risk of bias was high in three studies because not all prespecified outcomes were reported [33], or a difference was noticed between the protocol registered and the publication [25, 37]. We considered six studies to be free from other sources of bias [25, 27, 35–37, 39], whereas two studies were judged high since an important risk of bias was associated with threats of study validity [24, 34] and four had unclear risk of bias for insufficient rationale or evidence provided [26, 32, 33, 38] (see Additional File 3 – Table S2 for a detailed explanation).
Fig. 2.
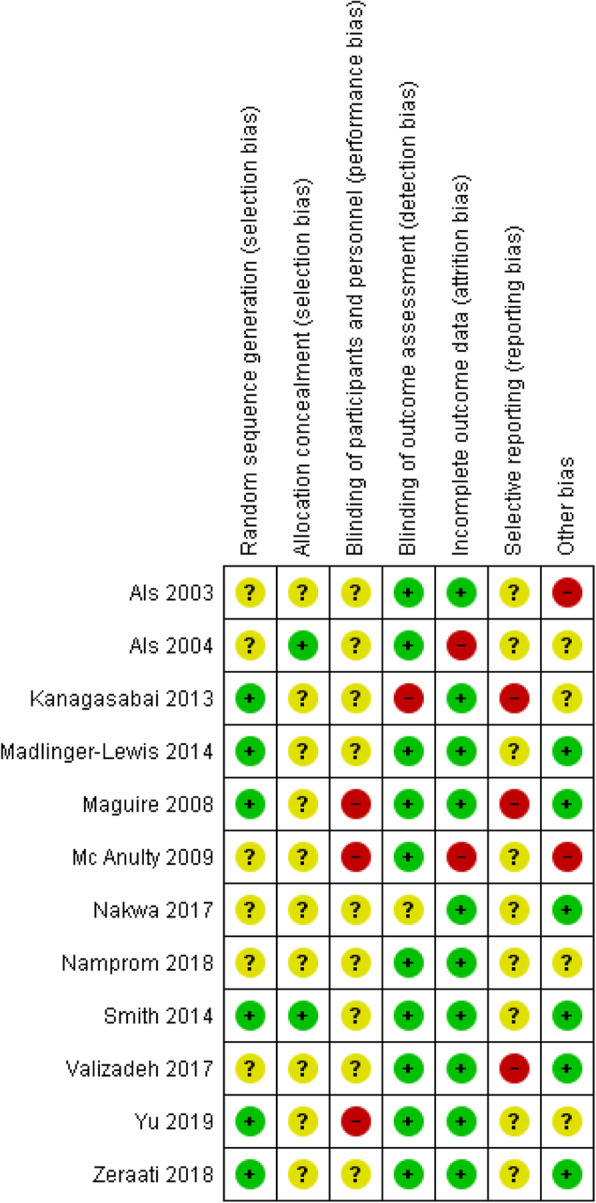
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study
Risk of bias across studies
As no more than ten studies were included in the meta-analysis, funnel plot asymmetry was not tested, as the power of this test would be too low to distinguish an asymmetry indicating a publication bias [28]. However, we performed different strategies to decrease potential reporting bias, including a comprehensive search by an expert librarian using nine different databases, an online search of several trial registries to identify relevant published trials and contacting authors by email to obtain missing data.
Synthesis of results
Developmental care vs. Standard care
NIDCAP. Neurobehavioral development
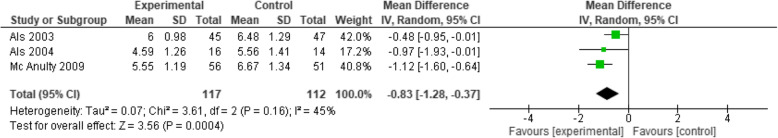
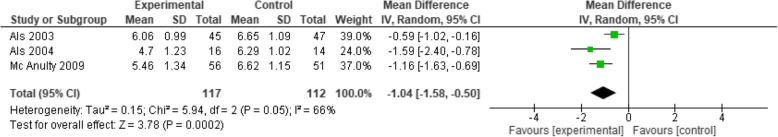
Three studies [24, 32, 34] that included a total of 229 participants (treatment: n = 117, control: n = 112) investigated the effects of NIDCAP compared to standard care using the APIB scale. Compared to standard care, the effect of NIDCAP was found to significantly improve preterm infants’ autonomic system (MD -0.83; 95% CI − 1.28 to − 0.37; I2 = 45%; p = 0.0004) (see Fig. 3), motor system (MD -1.04; 95% CI − 1.58 to − 0.50; I2 = 66%; p = 0.0002) (see Fig. 4), state system (MD -0.74; 95% CI − 1.06 to − 0.42; I2 = 0%; p < 0.00001) (Additional File 4 – Figure S2), interaction-attentional system (MD -0.48; 95% CI − 0.85 to − 0.11; I2 = 0%; p = 0.01) (Additional File 5 – Figure S3), and self-regulatory system (MD -0.84; 95% CI − 1.17 to − 0.51; I2 = 9%; p < 0.00001) (Additional File 6 – Figure S4). The effect of NIDCAP also significantly improved the examiner facilitation subscale (MD -1.02; 95% CI − 1.44 to − 0.60; I2 = 0%; p < 0.00001) (Additional File 7 – Figure S5).
Fig. 3.
NIDCAP vs. Standard care for the neurobehavioral development (autonomic system - APIB)
Fig. 4.
NIDCAP vs. Standard care for the neurobehavioral development (motor system - APIB)
NIDCAP. Neurological development
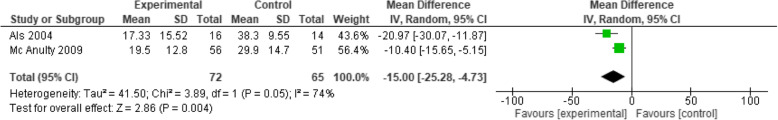
Two studies [32, 34] totalling 137 participants (treatment: n = 72, control: n = 65) investigated the effects of NIDCAP compared to standard care using the Prechtl Neurological Examination of the Full-term Newborn. The NIDCAP was found to significantly improve preterm infants’ neurological development (MD -15.00; 95% CI − 25.28 to − 4.73; I2 = 74%; p = 0.004) (see Fig. 5).
Fig. 5.
NIDCAP vs. Standard care for neurological development (Prechtl)
Alternative positioning. Neurobehavioral development
In one study [27], the effect of positioning was evaluated using the NNNS and the preterm infants in the treatment group showed significantly less asymmetry than those in the control group. Only one significant effect was reported for the asymmetry subscale (MD 0.88; 95% CI 0.45–1.31; p < 0.0001), while no significant effect was found for the other NNNS subscales (i.e., attention, handling, quality of movement, regulation, nonoptimal reflexes, stress abstinence, arousal, hypotonicity, hypertonicity, excitability and lethargy).
Positioning and incubator covers. Neurological development
Only one study [25] with 148 participants (treatment: n = 76, control: n = 72) investigated the effects of incubator covers and positioning compared to standard care on preterm infants using the Prechtl Neurological Examination of the Full-term Newborn (normal vs. abnormal). No significant effect between groups was found (RR 0.93; 95% CI 0.70 to 1.22; p = 0.58).
Parental participation intervention vs. Standard care
Neurobehavioral development
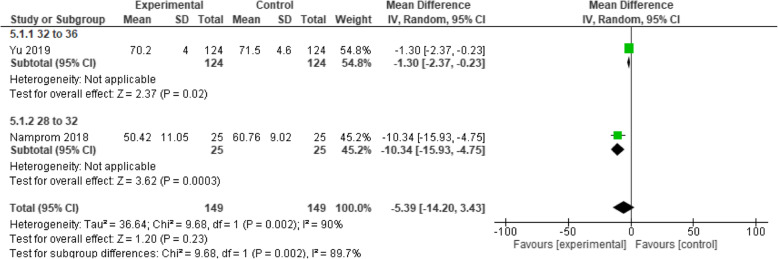
Two studies [26, 38] that included 294 participants (treatment: n = 145, control: n = 149) investigated the effects of a parental participation program compared to standard care on preterm infants using the NNE. Compared to standard care, the program was not found to significantly improve neurobehavioral development (MD 5.39; 95% CI − 3.43 to 14.20; I2 = 90%; p = 0.23) (see Fig. 6).
Fig. 6.
Parental participation programs vs. Standard care forn eurobehavioral development (NNE)
Sensory stimulation vs. Standard care
Tactile. Neurobehavioral development
One study [36] that included 18 participants (treatment: n = 9, control: n = 9) investigated the effects of a tactile intervention using the NNNS. No significant difference between groups was found for any of the 12 subscales (i.e., attention, handling, quality of movement, regulation, nonoptimal reflexes, asymmetric reflexes, stress abstinence, arousal, hypotonicity, hypertonicity, excitability and lethargy).
Multisensory. Neuromotor development
Only one study [33] that included 50 participants (treatment: n = 25, control: n = 25) investigated the effects of a multisensory stimulation intervention compared to standard care, assessed with the INFANIB. The multisensory stimulation was significantly in favour of the experimental group (MD 3.08; 95% CI 1.33–4.83; p = 0.0005).
Multisensory. Neuromuscular Development
One study [39] with 80 participants (treatment: n = 40; control: n = 40) evaluated the effects of a multisensory intervention compared to standard care using the New Ballard score. Both groups showed significant improvement before and after, but infants of the experimental group had significantly higher neuromuscular development after the intervention compared to infants of the comparator group (MD 5.60; 95% CI 4.65–6.55; p < 0.00001).
Music vs. Developmental care
Neuromotor and neuromotor development
In one study [35] that included 36 participants (treatment: n = 18, control: n = 18), the effect of music compared to developmental care was evaluated using the TIMPS and the INFANIB. Significant effects of music were reported for infants’ neuromotor development measured with the TIMPS (MD 0.39; 95% CI 0.08–0.70; p = 0.01) and the INFANIB (MD 1.89; 95% CI 0.42–3.36; p = 0.01) compared the control group.
Physical activity and/or hydrotherapy vs. Containment
Neuromotor and neuromuscular development
One study [37] of 38 preterm infants (treatment: n = 19, control: n = 19) investigated the effects on neuromotor and neuromuscular development of three different interventions – physical activity, hydrotherapy and a combination of physical therapy and hydrotherapy – compared to containment, using the TIMP, the New Ballard score and items from the Dubowitz examination. For all interventions, the ANOVA effects were not significant: physical therapy (mean: 50.21) vs. containment (mean: 51.57); hydrotherapy (mean 48.05) vs. containment (mean 51.57); or physical therapy combined with hydrotherapy (mean: 52.00) vs. containment (mean: 51.57): p = 0.11. For the neuromuscular development, no significant findings were found for the New Ballard score (p > 0.05) while for the two items of the Dubowitz, ankle dorsiflexion was not significantly different between groups, but leg recoil was significantly better for the physical therapy and hydrotherapy groups (p = 0.04).
Quality of evidence
The overall quality of evidence was considered low to very low. The summary findings table is presented by outcome (see Additional File 8 – Table S3). For the comparison between NIDCAP and standard care, the overall quality of evidence was rated low to very low for the autonomic system, motor system, state system, interaction-attention system, self-regulatory systems and examiner facilitation (neurobehavioral development), and very low for neurological development. For the comparison between parental participation program and standard care, the quality of evidence was rated very low. The main reasons for downgrading scores were high risk of bias, high heterogeneity between studies and small sample sizes. For the other comparisons including only one study, the summary of findings table is reported for each outcome (see Additional File 8 – Table S3).
Discussion
Summary of evidence
To our knowledge, this is the first systematic review examining the effectiveness of interventions on preterm infants’ neurodevelopment during NICU hospitalization or close to term CA. Our review synthesized the findings of 12 studies that included 901 preterm infants. In our systematic review, we combined the studies which had the same/similar interventions. Combining studies for a meta-analysis was only possible for NIDCAP and programs involving parents. For all other studies included, those were not combined for analysis and results were only described individually in a narrative form. We combined three studies [24, 32, 34] in a meta-analysis showing the positive effects of the NIDCAP intervention compared to standard care on preterm infants’ neurobehavioral and neurological development at two weeks CA. We also combined two other studies [26, 38] in a meta-analysis indicating that, compared to standard care, parental participation interventions do not improve preterm infants’ neurobehavioral development during NICU hospitalization.
For all other interventions, the synthesis shows that compared to standard care or other type of comparators, the effectiveness was either controversial or partial, as significant findings were only reported for one or some subscales of the instruments used to assess the preterm infants’ neurodevelopment. Overall, multisensory stimulation interventions were found to improve neuromuscular development [39] and neurological development [33]. In addition, music was reported to improve neuromotor and neurological development [38]. Conversely, a tactile stimulation intervention along with physical activity and/or hydrotherapy was not found to improve neurobehavioral [36] or neuromotor and neuromuscular development [37]. Developmental care interventions were not found to improve neurobehavioral [27] or neurological development [25]. It should be noted that these findings are only based on single studies with distinctive differences in the nature and components of the interventions, along with the instruments used to assess neurodevelopment.
Quality of evidence
The overall quality of evidence of the studies included in this systematic review is low to very low, which may be attributed to many high to unclear risk of bias, heterogeneity and small sample sizes. For the allocation risk of bias, all studies except two [32, 36] were judged unclear, as insufficient details were provided. Also, the risk of bias associated with blinding of personnel and participants was either unclear or high in all studies, whereas the selective reporting bias was rated as unclear or high in all studies. Among the 12 included studies, only three included more than 100 participants [25, 34, 38], suggesting that the majority of the included studies were underpowered to detect effects.
Although the meta-analysis indicated that NIDCAP developmental care intervention improved the neurobehavioral and neurological development of preterm infants, these results are based on low to very low evidence that may specifically be explained by unclear to high risk of biases and the small sample size of the combined studies (n = 229). It is also interesting to note in the meta-analysis that only three APIB subscales (i.e., autonomic, motor and examiner facilitation) and the Prechtl Neurological Examination of the Full-term Newborn had moderate to considerable statistical heterogeneity (I2 from 45 to 74%), which could be attributed to methodological variability arising from potential differences among the evaluators assessing these outcomes [28]. For the parental participation programs, even though the meta-analysis did not support these interventions as favouring the infants’ neurodevelopment, the quality of evidence was very low and may, in this case, be attributed to significant clinical heterogeneity (I2 = 90%), possibly resulting from differences in the components of the interventions and the gestational age of preterm infants targeted in these studies [28].
Comparisons with previous studies
Previous systematic reviews and meta-analysis were conducted with NIDCAP and developmental care interventions to assess the effectiveness of these interventions on the mid- and long-term neurodevelopmental outcomes of preterm infants [10, 11, 13, 40]. For NIDCAP, the systematic review and meta-analysis concluded that significant findings favoring the NIDCAP were only found at 9 months of age but not at 4-, 12-, 18- or 24-months CA. Other recent systematic reviews reported that developmental care interventions favored long-term neurodevelopmental outcomes in preterm infants with significant effects for cognitive, mental, psychomotor and language development up to 18 months of age and IQ at the age of 5 [10, 11]. Our systematic review adds to the efforts of these systematic reviews by bringing in new evidence and reinforcing that the NIDCAP favors preterm infants’ early neurodevelopment at a time that is crucial for brain development and maturation [2, 3].
Implications for clinical practice
It is imperative to know which interventions during NICU hospitalization promote optimal preterm infants’ early and long-term neurodevelopmental outcomes. Based on our meta-analysis, the NIDCAP favored preterm infants’ neurobehavioral and neurological development at two weeks CA, and even though the quality of evidence was low to very low, it could be recommended in clinical practice. As recent guidelines recommendations arising from systematic reviews suggest, the NIDCAP is recommended as a support to neuroprotective developmental practice care in NICUs [41]. The NIDCAP requires extensive training and time investment to maintain knowledge and expertise [42]. Although it may not be readily accessible for all NICUs, principles guiding developmental care, as NIDCAP, should still be encouraged in neonatal care as DC interventions are recommended to promote short- and long-term neurodevelopmental outcomes in preterm infants [6].
Parental participation programs were not found to improve preterm infants’ neurobehavioral development and had very low quality of evidence, which may be explained by the different intervention components and the gestational age of preterm infants included in the studies. The diverse role that parents may play in the early NICU participation programs could perhaps have accounted for the high heterogeneity between studies. Still, family-centered care in the NICU is a central developmental care intervention, and interventions fostering parental participation in infant care are recommended for clinical practice [43]. Parental presence, educational sessions and active parental participation in preterm infants’ care have been recently been reported to improve both infant and parental outcomes [44] and should therefore be promoted in NICUs. A summary of the meta-analysis main findings and clinical implications is presented in Figure S6 – see Additional file 9.
Implications for research
First, a clear conceptual definition of preterm infants’ neurodevelopment should be formulated to guide the choice of interventions as well as the measurement tools used in experimental studies. Future studies could test the different interventions included in this systematic review to build on evidence for those interventions. In addition, analysis according to gender difference could also contribute to knowledge development about the effectiveness of the interventions.
Different instruments were used in our systematic review to evaluate the effectiveness of interventions on preterm infants’ neurodevelopment during NICU hospitalization. Although these instruments all have standardized administration approaches and scoring [45], they measure slightly different aspects of neurodevelopment (i.e., the APIB assesses neurobehavioral development, the Precthl assesses neurological development and the TIMP assesses neuromotor development), limiting comparisons between included studies. Including a combination of different instruments [32, 35, 37, 39] could provide a more global assessment of preterm infants’ neurodevelopment and thus allow for comparisons across studies.
One systematic review on developmental care [10] recommended combining electroencephalography (EEG) to a measure of neurobehavioral development for assessing the effectiveness of interventions with preterm infants. Recent advances support the idea that brain activity and oxygenation during the neonatal period play a crucial role in the preservation and development of brain connections, and thus in infants’ brain functioning and growth [46, 47], and can predict their neurodevelopmental status in early childhood [48, 49]. Therefore, combined with other standardized instruments, EEG as a measure of infants’ neurodevelopment could be used in future studies. Still, EEG as a measure of neurodevelopment needs more research in order to be more objective and therefore more comparable across studies.
For research purposes, a systematic review of neonatal assessments supports the use of the APIB and the NNNS, which have adequate psychometrics qualities [19]. The choice of instruments to measure preterm infants’ neurodevelopment during NICU hospitalization could also be based on its predictive validity for long-term neurodevelopment. The NNNS has predictive validity, as it correlates with the Bayley cognitive scores at 12 or 24 months of age [50]. Of the instruments used in the studies included in our systematic review, the TIMP is the neuromotor assessment with the best predictive validity for long-term neurodevelopmental outcomes [18].
Limitations
Our review differed from the published protocol with respect to the inclusion criteria and term age: we included neurodevelopment measurements done soon after discharge at two weeks CA. We only included French and English literature, and missing data precluded us from including one study in the meta-analysis for the NICDAP intervention. These differences were clearly reported in the manuscript.
Moreover, a description of the standard of care was provided in 8 of the 12 studies [24, 27, 32, 33, 35–38], but the description was very different among the studies and may reflect the evolution of care in neonatology, since our systematic review included studies conducted 16 years apart. Likewise, the standard of care may have differed based on the NICU design of the included studies, since in recent decades neonatal units have modified their unit configuration to single-family rooms, as this is the recommended NICU design [51, 52]. The ability to operationalize interventions between the different NICU designs could have played a role and other environmental conditions not readily evident may account for some variability. Standard care and interventions conditions in experimental studies should be reported as per the guidelines. Together, these aspects limit the conclusions of our systematic review, and future studies evaluating the effectiveness of interventions are recommended.
Conclusions
Future studies are needed to identify the interventions that are the most effective in promoting preterm infants’ neurodevelopment during NICU hospitalization or close to term age. Without a clear definition of neurodevelopment, interventions should be appropriately designed to allow comparison with previous studies and instruments should be combined to measure different aspects of infants’ neurodevelopment. NICU hospitalization is a critical period for the brain development of preterm infants, and all experiences encountered can significantly shape their neurodevelopment, so it is imperative to identify which interventions in the NICU optimize short-term health outcomes in preterm infants.
Supplementary Information
Additional file 1: Table S1. Search strategy in MEDLINE – table presenting the search strategy with keywords and results.
Additional file 2: Table S2. Bias summary of included tudies – table presenting the judgement of the Cochrane’s risk of bias tool for each individual study.
Additional file 3: Figure S1. Risk of bias summary: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Additional file 4: Figure S2. NIDCAP vs. Standard care for the neurobehavioral development (state system - APIB) – figure presenting a meta-analysis.
Additional file 5: Figure S3. NIDCAP vs. Standard care for the neurobehavioral development (attention-interaction system - APIB) - figure presenting a meta-analysis.
Additional file 6: Figure S4. NIDCAP vs. Standard care for the neurobehavioral development (self-regulation system - APIB) - figure presenting a meta-analysis.
Additional file 7: Figure S5. NIDCAP vs. Standard care for the n eurobehavioral development (examiner facilitation - APIB) - figure presenting a meta-analysis.
Additional file 8: Table S3. Quality of evidence of studies included in meta-analysis – table presenting the quality of evidence according to the GRADE tool for each intervention and outcomes.
Additional file 9: Figure S6. Summary of meta-analysis main findings and clinical implications.
Acknowledgements
The authors would like to thank the following people for their valuable implication: Assia Mourid, librarian at the Faculty of Nursing of the Université de Montréal, and Josianne Dupuis, librarian, for their help in designing and performing the search strategy.
Abbreviations
- BW
Birthweight
- APIB
Assessment of Preterm Infants’ Behavior
- CA
corrected age
- CI
Confidence Interval
- EEG
Electroencephalogram
- GRADE
Grading of Recommendations Assessment, Development and Evaluation working group methodology
- INFANIB
Infant Neurological International Battery
- IVH
Intraventricular Hemorrhage
- NIDCAP
Newborn Individualized Developmental Care and Assessment Program
- NICU
Neonatal Intensive Care Unit
- NNE
Neonatal Neurobehavioral Examination
- NNNS
NICU Network Neurobehavioral Scale
- PRISMA
Preferred Reporting Items for Systematic review and Meta-Analysis
- RCT
Randomized Controlled Trial
- MD
Mean Difference
- TIMP
Test of Infant Motor Performance
- WGA
Weeks Gestational Age
Authors’ contributions
MA conceptualized and designed the systematic review, developed the study protocol and review, interpreted the results and wrote the manuscript. GDF and AL participated in the conceptualization and design of the systematic review, the development of the study review, the evaluation of articles for eligibility and the interpretation of the results and they drafted sections of the manuscript. NF and RS participated in the conceptualization of the systematic review and provided a critical review of the systematic review manuscript. ER and MP evaluated articles for eligibility, extracted relevant data, drafted sections of the manuscript and revised the manuscript for final submission. All authors read and approved the manuscript.
Funding
Thank you to the Quebec Network on Nursing Intervention Research (RRISIQ) for funding allowing data collection, analysis, and data interpretation.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2014;35(6):394–407. doi: 10.1097/01.DBP.0000452240.39511.d4. [DOI] [PubMed] [Google Scholar]
- 2.Pickler RH, McGrath JM, Reyna BA, McCain N, Lewis M, Cone S, et al. A model of neurodevelopmental risk and protection for preterm infants. J Perinat Neonatal Nurs. 2010;24(4):356–365. doi: 10.1097/JPN.0b013e3181fb1e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers EE, Hintz SR. Early neurodevelopmental outcomes of extremely preterm infants. Semin Perinatol. 2016;40(8):497–509. doi: 10.1053/j.semperi.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Eryigit Madzwamuse S, Baumann N, Jaekel J, Bartmann P, Wolke D. Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. J Child Psychol Psychiatry. 2015;56(8):857–864. doi: 10.1111/jcpp.12358. [DOI] [PubMed] [Google Scholar]
- 6.Aita M, Snider L. The art of developmental care in the NICU: a concept analysis. J Adv Nurs. 2003;41(3):223–232. doi: 10.1046/j.1365-2648.2003.02526.x. [DOI] [PubMed] [Google Scholar]
- 7.Santos J, Pearce SE, Stroustrup A. Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr. 2015;27(2):254–260. doi: 10.1097/MOP.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong T, Gonzalez F, Mu DZ. An overview of risk factors for poor neurodevelopmental outcome associated with prematurity. World J Pediatr. 2012;8(4):293–300. doi: 10.1007/s12519-012-0372-2. [DOI] [PubMed] [Google Scholar]
- 9.Milgrom J, Newnham C, Anderson PJ, Doyle LW, Gemmill AW, Lee K, et al. Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatr Res. 2010;67(3):330–335. doi: 10.1203/PDR.0b013e3181cb8e2f. [DOI] [PubMed] [Google Scholar]
- 10.Burke S. Systematic review of developmental care interventions in the neonatal intensive care unit since 2006. J Child Health Care. 2018;22(2):269–286. doi: 10.1177/1367493517753085. [DOI] [PubMed] [Google Scholar]
- 11.Soleimani F, Azari N, Ghiasvand H, Shahrokhi A, Rahmani N, Fatollahierad S. Do NICU developmental care improve cognitive and motor outcomes for preterm infants? A systematic review and meta-analysis. BMC Pediatr. 2020;20(1):67. doi: 10.1186/s12887-020-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev. 2006 Apr 19;(2):CD001814. [DOI] [PMC free article] [PubMed]
- 13.Ohlsson A, Jacobs SE. NIDCAP: a systematic review and meta-analyses of randomized controlled trials. Pediatrics. 2013;131(3):e881–e893. doi: 10.1542/peds.2012-2121. [DOI] [PubMed] [Google Scholar]
- 14.Almadhoob A, Ohlsson A. Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants. Cochrane Database Syst Rev. 2015;1:Cd010333. doi: 10.1002/14651858.CD010333.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Vanderveen JA, Bassler D, Robertson CM, Kirpalani H. Early interventions involving parents to improve neurodevelopmental outcomes of premature infants: a meta-analysis. J Perinatol. 2009;29(5):343–351. doi: 10.1038/jp.2008.229. [DOI] [PubMed] [Google Scholar]
- 16.Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016 Aug 23;2016(8):CD002771 [DOI] [PMC free article] [PubMed]
- 17.Olsen JE, Allinson LG, Doyle LW, Brown NC, Lee KJ, Eeles AL, et al. Preterm and term-equivalent age general movements and 1-year neurodevelopmental outcomes for infants born before 30 weeks' gestation. Dev Med Child Neurol. 2018;60(1):47–53. doi: 10.1111/dmcn.13558. [DOI] [PubMed] [Google Scholar]
- 18.Craciunoiu O, Holsti L. A systematic review of the predictive validity of neurobehavioral assessments during the preterm period. Phys Occup Ther Pediatr. 2017;37(3):292–307. doi: 10.1080/01942638.2016.1185501. [DOI] [PubMed] [Google Scholar]
- 19.Noble Y, Boyd R. Neonatal assessments for the preterm infant up to 4 months corrected age: a systematic review. Dev Med Child Neurol. 2012;54(2):129–139. doi: 10.1111/j.1469-8749.2010.03903.x. [DOI] [PubMed] [Google Scholar]
- 20.Prechtl HF. General movement assessment as a method of developmental neurology: new paradigms and their consequences. The 1999 Ronnie MacKeith lecture. Dev Med Child Neurol. 2001;43(12):836–842. doi: 10.1017/S0012162201001529. [DOI] [PubMed] [Google Scholar]
- 21.Campbell SK, Hedeker D. Validity of the test of infant motor performance for discriminating among infants with varying risk for poor motor outcome. J Pediatr. 2001;139(4):546–551. doi: 10.1067/mpd.2001.117581. [DOI] [PubMed] [Google Scholar]
- 22.Aita M, Stremler R, Feeley N, Lavallée A, De Clifford-Faugère G. Effectiveness of interventions during NICU hospitalization on the neurodevelopment of preterm infants: a systematic review protocol. Syst Rev. 2017;6(1):225. doi: 10.1186/s13643-017-0613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Als H, Gilkerson L, Duffy FH, McAnulty GB, Buehler DM, Vandenberg K, et al. A three-center, randomized, controlled trial of individualized developmental care for very low birth weight preterm infants: medical, neurodevelopmental, parenting, and caregiving effects. J Dev Behav Pediatr. 2003;24(6):399–408. doi: 10.1097/00004703-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Maguire CM, Veen S, Sprij AJ, Le Cessie S, Wit JM, Walther FJ. Effects of basic developmental care on neonatal morbidity, neuromotor development, and growth at term age of infants who were born at <32 weeks. Pediatrics. 2008;121(2):e239–e245. doi: 10.1542/peds.2007-1189. [DOI] [PubMed] [Google Scholar]
- 26.Namprom N, Picheansathian W, Jintrawet U, Chotibang J. The effect of maternal participation in preterm's care and improved short-term growth and neurodevelopment outcomes. J Neonatal Nurs. 2018;24(4):196–202. doi: 10.1016/j.jnn.2018.03.003. [DOI] [Google Scholar]
- 27.Madlinger-Lewis L, Reynolds L, Zarem C, Crapnell T, Inder T, Pineda R. The effects of alternative positioning on preterm infants in the neonatal intensive care unit: a randomized clinical trial. Res Dev Disabil. 2014;35(2):490–497. doi: 10.1016/j.ridd.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Second edition. Hoboken, NJ: Wiley-Blackwell; 2019. [Google Scholar]
- 29.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 30.Maguire CM, Walther FJ, Sprij AJ, Le Cessie S, Wit JM, Veen S. Effects of individualized developmental care in a randomized trial of preterm infants <32 weeks. Pediatrics. 2009;124(4):1021–1030. doi: 10.1542/peds.2008-1881. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, et al. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 33.Kanagasabai PS, Mohan D, Lewis LE, Kamath A, Rao BK. Effect of multisensory stimulation on neuromotor development in preterm infants. Indian J Pediatr. 2013;80(6):460–464. doi: 10.1007/s12098-012-0945-z. [DOI] [PubMed] [Google Scholar]
- 34.McAnulty G, Duffy FH, Butler S, Parad R, Ringer S, Zurakowski D, et al. Individualized developmental care for a large sample of very preterm infants: health, neurobehaviour and neurophysiology. Acta Paediatr. 2009;98(12):1920–1926. doi: 10.1111/j.1651-2227.2009.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakhwa PK, Malawade M, Shrikhande DY, Shrikhande S, Rokade P. Efficacy of music therapy in improvement of neuromotor development in preterm infants. Romanian J Phys Ther. 2017;23(39):5–11. [Google Scholar]
- 36.Smith JR, McGrath J, Brotto M, Inder T. A randomized-controlled trial pilot study examining the neurodevelopmental effects of a 5-week M technique intervention on very preterm infants. Adv Neonatal Care. 2014;14(3):187–200. doi: 10.1097/ANC.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valizadeh L, Sanaeefar M, Hosseini MB, Asgari Jafarabadi M, Shamili A. Effect of early physical activity programs on motor performance and neuromuscular development in infants born preterm: a randomized clinical trial. J Caring Sci. 2017;6(1):67–79. doi: 10.15171/jcs.2017.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu YT, Huang WC, Hsieh WS, Chang JH, Lin CH, Hsieh S, Lu L, Yao NJ, Fan PC, Lee CL, Tu YK, Jeng SF. Family-Centered Care Enhanced Neonatal Neurophysiological Function in Preterm Infants: Randomized Controlled Trial. Phys Ther. 2019;99(12):1690–1702. [DOI] [PubMed]
- 39.Zeraati H, Nasimi F, Rezaeian A, Shahinfar J, Ghorban ZM. Effect of multi-sensory stimulation on neuromuscular development of premature infants: a randomized clinical trial. Iran J Child Neurol. 2018;12(3):32–39. [PMC free article] [PubMed] [Google Scholar]
- 40.Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev. 2009;2:CD001814. doi: 10.1002/14651858.CD001814.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffiths N, Spence K, Loughran-Fowlds A, Westrup B. Individualised developmental care for babies and parents in the NICU: evidence-based best practice guideline recommendations. Early Hum Dev. 2019;139:104840. doi: 10.1016/j.earlhumdev.2019.104840. [DOI] [PubMed] [Google Scholar]
- 42.Kenner C, Lott JW. Comprehensive neonatal nursing care 5th ed. St. Louis, Mo: Saunders Elsevier; 2013. [Google Scholar]
- 43.Lavallée A, De Clifford-Faugère G, Garcia C, Fernandez Oviedo AN, Héon M, Aita M. PART 2: practice and research recommendations for quality developmental care in the NICU. J Neonatal Nurs. 2019;25(4):160–165. doi: 10.1016/j.jnn.2019.03.008. [DOI] [Google Scholar]
- 44.O'Brien K, Robson K, Bracht M, Cruz M, Lui K, Alvaro R, et al. Effectiveness of family integrated care in neonatal intensive care units on infant and parent outcomes: a multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc Health. 2018;2(4):245–254. doi: 10.1016/S2352-4642(18)30039-7. [DOI] [PubMed] [Google Scholar]
- 45.Spittle AJ, Doyle LW, Boyd RN. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev Med Child Neurol. 2008;50(4):254–266. doi: 10.1111/j.1469-8749.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- 46.Dix LM, van Bel F, Lemmers PM. Monitoring cerebral oxygenation in neonates: an update. Front Pediatr. 2017;5:46. doi: 10.3389/fped.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benders MJ, Palmu K, Menache C, Borradori-Tolsa C, Lazeyras F, Sizonenko S, et al. Early brain activity relates to subsequent brain growth in premature infants. Cereb Cortex. 2015;25(9):3014–3024. doi: 10.1093/cercor/bhu097. [DOI] [PubMed] [Google Scholar]
- 48.Song J, Xu F, Wang L, Gao L, Guo J, Xia L, et al. Early amplitude-integrated electroencephalography predicts brain injury and neurological outcome in very preterm infants. Sci Rep. 2015;5:13810. doi: 10.1038/srep13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verhagen EA, Van Braeckel KN, van der Veere CN, Groen H, Dijk PH, Hulzebos CV, et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev Med Child Neurol. 2015;57(5):449–455. doi: 10.1111/dmcn.12622. [DOI] [PubMed] [Google Scholar]
- 50.El-Dib M, Massaro AN, Glass P, Aly H. Neurobehavioral assessment as a predictor of neurodevelopmental outcome in preterm infants. J Perinatol. 2012;32(4):299–303. doi: 10.1038/jp.2011.100. [DOI] [PubMed] [Google Scholar]
- 51.Kuhn P, Sizun J, Casper C. Recommendations on the environment for hospitalised newborn infants from the French neonatal society: rationale, methods and first recommendation on neonatal intensive care unit design. Acta Paediatr. 2018;107(11):1860–1866. doi: 10.1111/apa.14501. [DOI] [PubMed] [Google Scholar]
- 52.O'Callaghan N, Dee A, Philip RK. Evidence-based design for neonatal units: a systematic review. Matern Health Neonatol Perinatol. 2019;5:6. doi: 10.1186/s40748-019-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Search strategy in MEDLINE – table presenting the search strategy with keywords and results.
Additional file 2: Table S2. Bias summary of included tudies – table presenting the judgement of the Cochrane’s risk of bias tool for each individual study.
Additional file 3: Figure S1. Risk of bias summary: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Additional file 4: Figure S2. NIDCAP vs. Standard care for the neurobehavioral development (state system - APIB) – figure presenting a meta-analysis.
Additional file 5: Figure S3. NIDCAP vs. Standard care for the neurobehavioral development (attention-interaction system - APIB) - figure presenting a meta-analysis.
Additional file 6: Figure S4. NIDCAP vs. Standard care for the neurobehavioral development (self-regulation system - APIB) - figure presenting a meta-analysis.
Additional file 7: Figure S5. NIDCAP vs. Standard care for the n eurobehavioral development (examiner facilitation - APIB) - figure presenting a meta-analysis.
Additional file 8: Table S3. Quality of evidence of studies included in meta-analysis – table presenting the quality of evidence according to the GRADE tool for each intervention and outcomes.
Additional file 9: Figure S6. Summary of meta-analysis main findings and clinical implications.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.