Abstract
COVID-19 infection may lead to acute respiratory distress syndrome (CARDS) where severe gas exchange derangements may be associated, at least in the early stages, only with minor pulmonary infiltrates. This may suggest that the shunt associated to the gasless lung parenchyma is not sufficient to explain CARDS hypoxemia. We designed an algorithm (VentriQlar), based on the same conceptual grounds described by J.B. West in 1969. We set 498 ventilation–perfusion (VA/Q) compartments and, after calculating their blood composition (PO2, PCO2, and pH), we randomly chose 106 combinations of five parameters controlling a bimodal distribution of blood flow. The solutions were accepted if the predicted PaO2 and PaCO2 were within 10% of the patient’s values. We assumed that the shunt fraction equaled the fraction of non-aerated lung tissue at the CT quantitative analysis. Five critically-ill patients later deceased were studied. The PaO2/FiO2 was 91.1 ± 18.6 mmHg and PaCO2 69.0 ± 16.1 mmHg. Cardiac output was 9.58 ± 0.99 L/min. The fraction of non-aerated tissue was 0.33 ± 0.06. The model showed that a large fraction of the blood flow was likely distributed in regions with very low VA/Q (Qmean = 0.06 ± 0.02) and a smaller fraction in regions with moderately high VA/Q. Overall LogSD, Q was 1.66 ± 0.14, suggestive of high VA/Q inequality. Our data suggest that shunt alone cannot completely account for the observed hypoxemia and a significant VA/Q inequality must be present in COVID-19. The high cardiac output and the extensive microthrombosis later found in the autopsy further support the hypothesis of a pathological perfusion of non/poorly ventilated lung tissue.
NEW & NOTEWORTHY Hypothesizing that the non-aerated lung fraction as evaluated by the quantitative analysis of the lung computed tomography (CT) equals shunt (VA/Q = 0), we used a computational approach to estimate the magnitude of the ventilation–perfusion inequality in severe COVID-19. The results show that a severe hyperperfusion of poorly ventilated lung region is likely the cause of the observed hypoxemia. The extensive microthrombosis or abnormal vasodilation of the pulmonary circulation may represent the pathophysiological mechanism of such VA/Q distribution.
Keywords: COVID-19, gas exchange, lung physiology, mechanical ventilation, ventilation-perfusion
INTRODUCTION
The severe cases of COVID-19–associated pneumonia almost invariably present with a disease that can be classified as acute respiratory distress syndrome (ARDS), entirely fulfilling the Berlin definition criteria: bilateral infiltrates at the chest X-ray and severe hypoxemia defined as an arterial partial pressure of oxygen to inspired oxygen fraction ratio (Pao2/FiO2) < 300 mmHg (1). Classically, in ARDS, right-to-left shunt represents the main cause of hypoxemia (2): part of the mixed venous blood enters the pulmonary circulation and perfuses areas of the lung that are not ventilated due to consolidation or atelectasis.
In March 2020, at the beginning of the pandemics, observing lung computed tomography (CT) scans and arterial blood gas analysis of patients affected by COVID-19, we noticed that the profound hypoxemia was associated with relatively mild parenchymal infiltrates. This anatomo-functional dissociation led us to define the COVID-19–related ARDS (CARDS) as “atypical”, raising a hot debate in the intensive care community (3).
In this regard, evidence is growing about the central role of the vasculature in the pathogenesis of CARDS. The extensive presence of pulmonary micro and macro thrombosis (4–6), neonagiogenesis with vessel sprouting (7), and abnormal perfusion of collapsed lung regions (8, 9) have been shown in multiple studies. This raises the possibility that the right-to-left shunt due to the presence of gasless tissue in the lung is not sufficient to explain the severe hypoxemia observed in CARDS.
In 1969, J.B. West (10) developed a complex computer-based algorithm to model the effects of the VA/Q inequality on gas exchange. Fifty years later, in the context of COVID-19 pandemics, we started from the same concept to design a computational algorithm to estimate the magnitude of the VA/Q inequality that must be present to justify the severity of gas exchange derangement observed in this new disease.
METHODS
Study Population
The study population was retrospectively enrolled from the mixed medical-surgical Intensive Care Unit of ASST Papa Giovanni XXIII Hospital, Bergamo, Italy, during COVID-19 outbreak between March 1st and April 1st, 2020. Patients were ventilated in volume-controlled mode with inspired oxygen fraction (FiO2) and positive end-expiratory pressure (PEEP) set according to the clinical judgement of the attending physician. A pulmonary artery catheter (PAC) was in place for clinical reasons. Only patients with a complete set of recorded variables for gas exchange, hemodynamics, and lung mechanics on the same day, as well as a chest CT scan within 2 days were enrolled. The ethics committee of Papa Giovanni XXIII Hospital approved the use of patient data for scientific research.
Quantitative CT Scan Analysis
A CT scan of the whole lung was acquired. Each lung slice was manually segmented and whole lung quantitative analysis was performed. The quantitative analysis considers two compartments: tissue, with a density close to water (0 hounsfield units, HU), and gas, with a density of −1000 HU (11). For each voxel:
The voxel gas volume and voxel tissue mass were multiplied by the total number of voxels to obtain the total tissue mass and the total gas volume.
Lung tissue was classified according to its gas/tissue content: non-aerated tissue has a CT number between −100 and +100 HU. As an assumption, in our model we considered right-to-left shunt (from here on simply referred to as “shunt”) the fraction of non-aerated tissue + 0.005 (to account for the anatomical shunt), calculated as follows:
This compartment represents the fraction of lung tissue where shunt occurs (VA/Q = 0). The assumption is that the perfusion of 1 g of tissue is the same throughout the lung parenchyma regardless of its association with gas (i.e. the perfusion per gram of well-aerated tissue is equal to the perfusion per gram of non-aerated tissue). It is important to note that this assumption may be an overestimate, as it implies an absence of hypoxic vasoconstriction (12).
Computational Model
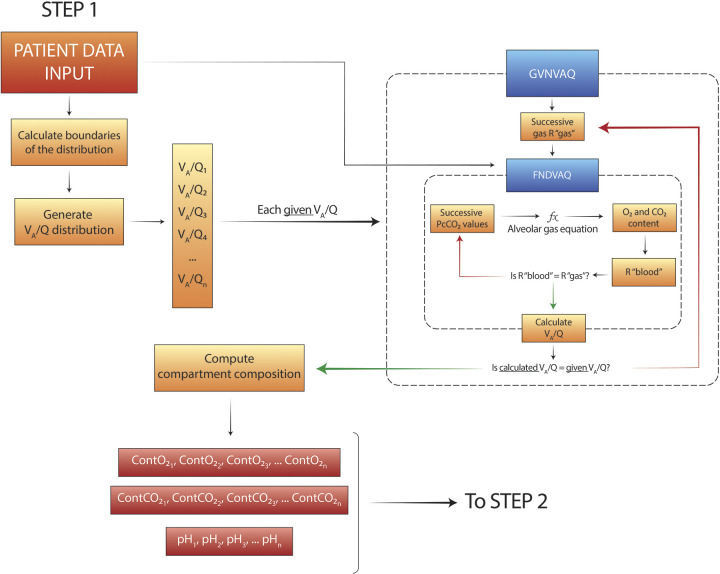
To estimate and quantify the degree of VA/Q inequality in severe CARDS, we designed a computational model that we called VentriQlar. The program is conceptually divided in two sections. In the first part (Fig. 1), the aim is to calculate the blood composition returning from the predefined VA/Q compartments. The core functions (“FNDVAQ” and “GVNVAQ”) were named after West (10), as they conceptually perform the same calculations as in his original algorithm, called LOGNOR. However, the original code was not available. Consequently, the solutions taken to solve the programming problems may differ.
Figure 1.
Schematic representation of the programming structure of the first part of algorithm, named VentriQlar. Here the composition of the blood leaving the 498 ventilation-perfusion (VA/Q) compartments is calculated. PcCO2, compartment PCO2.
VentriQlar requires the following input data: cardiac output, FiO2, hemoglobin concentration, base excess (BE), mixed venous PO2 (PvO2), mixed venous PCO2 (PvCO2), and shunt. Since PvCO2 was not available, its value was calculated assuming a metabolic respiratory quotient (R) of 0.85.
Here we summarize the key steps of the program (for further details please consult the Supplemental Material) (Supplemental Fig. S1: all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.13061075.v1):
A log-spaced distribution of 498 VA/Q (+ VA/Q = 0 and VA/Q = ∞) compartments is generated: for each compartment, the composition of the venous blood, FiO2, and VA/Q are the only determinants of the gas exchange (13).
- To calculate the composition of the blood returning from each compartment a nested, two-step iteration is required:
- GVNVAQ: progressive values of respiratory quotient (“R gas”) are chosen (log distributed from 0.01 to 100).
- FNDVAQ: progressive values of compartment PCO2 (PcCO2) are chosen and, together with the input “R gas” and patient’s FiO2, the compartment PO2 is calculated through the alveolar gas equation:
- From that, compartment content of O2 and CO2 and pH can be calculated. O2 contents can be calculated with the classic formula:
while CO2 content was calculated with Douglas’ equation: - These values allow the “R blood” to be derived. “R gas” and “R blood” must necessarily be equal in a compartment, but this is not guaranteed in the calculations, given the non-linearity of the functions governing O2 and CO2 content. If the “R blood” is sufficiently close to the given “R gas” the assigned PCO2 is accepted and FNDVAQ iteration stopped. Otherwise it is allowed to proceed to the next PCO2 value. For each iteration where “R blood = R gas” the resulting VA/Q of the compartment is calculated.
where CvCO2 is the mixed venous content of CO2 and CcCO2 is the compartment content of CO2. The constant 8.63 is a conversion constant to account for the different conditions in which gases and blood contents are expressed (standard temperature & pressure dry – STPD and body temperature & pressure saturated – BTPS).
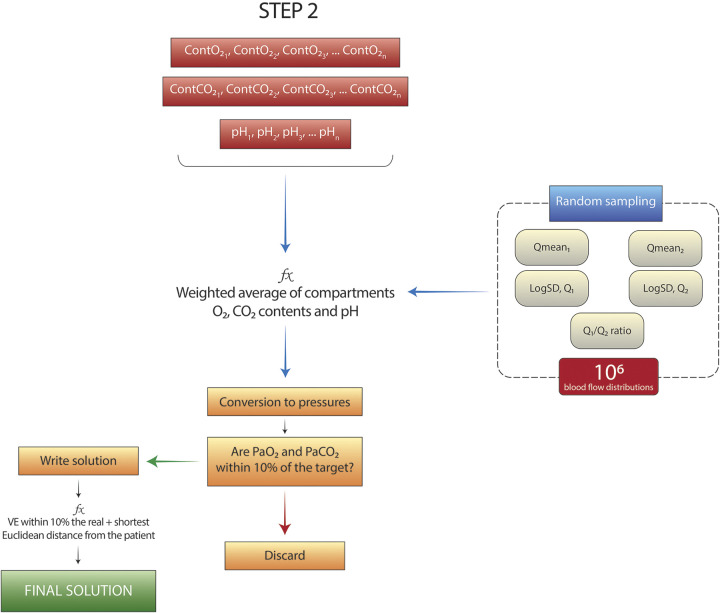
At the end of this procedure, each of the 498 VA/Q compartments is associated with a value of O2 content, CO2 content, and pH. The next step (Fig. 2) is needed to estimate which fraction of cardiac output reaching each one of these compartments is required to approximate the arterial gas composition of the real subject. To do so, we hypothesized a bimodal distribution of the perfusion through the compartments, regulated by 5 parameters: two means (Qmean1, Qmean2), the associated standard deviations (SD, Q1, SD, Q2), and the ratio of blood flow between the two means (Q1/Q2 ratio) (15). The possible values for Qmean1 and Qmean2 range between the extremes of the VA/Q distribution (∼10−2 to ∼102, slightly different for each patient). SD, Q1, SD, Q2 between 0.3 and 2, and the Q1/Q2 ratio between 0% and 100%. One million random combinations of the 5 parameters are then generated.
Figure 2.
Schematic representation of the programming structure of the second part of the program. One million random combinations of the 5 parameters of the bimodal distributions are extracted and, for each one, the blood composition in the left atrium is calculated. Solutions close enough to the subject’s target values are included in the solution space. PaO2, arterial partial pressure of O2; PaCO2, arterial partial pressure of CO2.
For each random combination of parameters:
A weighted average of O2 and CO2 contents and hydrogen ions (to derive the related pH) is calculated to obtain the resulting composition of blood in the left atrium. The conversion from contents to pressures is performed according to standard equations (14, 16, 17). The multiplication between the blood flow through each compartment and its VA/Q ratio gives the alveolar ventilation (VA) distribution. The sum of the VA for each compartment results in the total VA.
If the calculated PaO2, PaCO2, and minute ventilation are within 10% of the real values of the patient (target values), the global R value is ≤ 1, the solution is added to the solutions space, otherwise discarded. The effect of pH, base excess, and PCO2 on the hemoglobin dissociation curve are modeled with Zander’s correction (18).
The solution we chose to display was the one with the smallest Euclidean distance from the patient PaO2 and PaCO2.
VentriQlar has been designed in Julia (19), a modern, open source, compiled, high performance language. For further details, please consult the Supplemental Materials.
RESULTS
Study Population
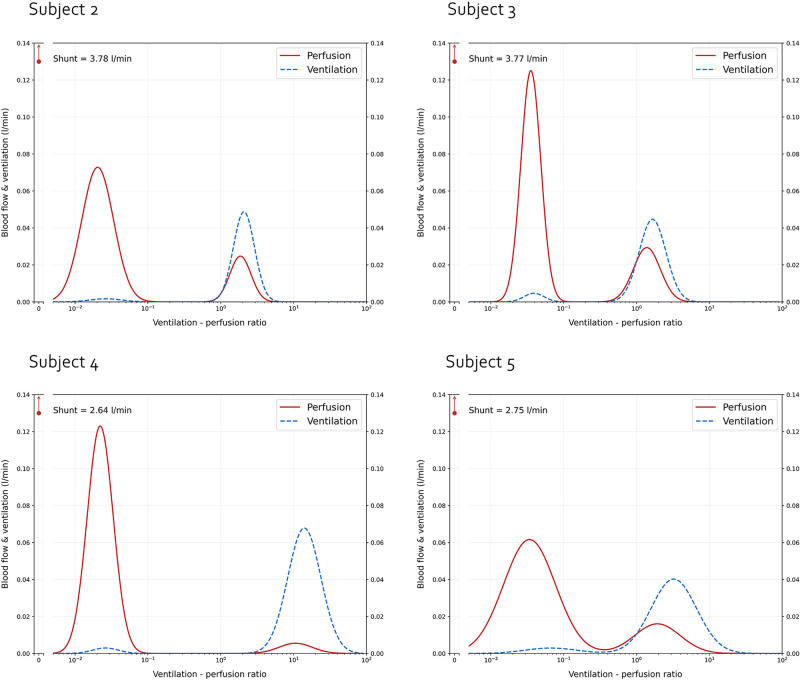
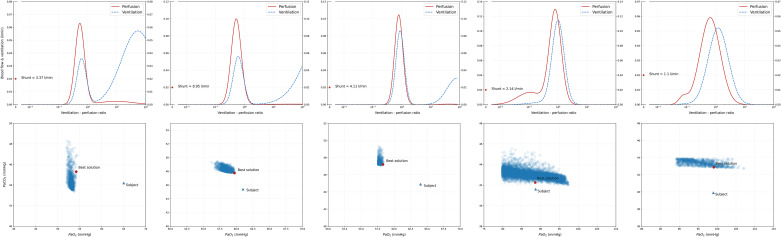
The clinical characteristics of the five patients who met the inclusion criteria are displayed in Table 1 In summary, the subjects showed a high clinical severity of the disease: respiratory system compliance was low and gas exchange was severely abnormal, with extremely high values of AaDO2 and Paco2. Thanks to the highly positive BE, pH was within normal limits. Cardiac output was remarkably elevated in all subjects and signs of systemic disease were present in subjects 2 and 3, with elevated hepatic transaminases and low platelet count. D-dimers (1 missing value) were elevated. The lung weight was on average elevated. Interestingly, the fraction of non-aerated tissue was smaller than the calculated venous admixture except in subject 1, where the opposite occurred. Interestingly, subject 1 was the only one that, during the measurements, was receiving inhaled nitric oxide (iNO).
Table 1.
Clinical characteristics of the 5 subjects studied
| Subjects |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Means ± SD | |
| Anthropometrics | ||||||
| Age (years) | 43 | 31 | 31 | 65 | 72 | 48.4 ± 19.2 |
| Sex | F | M | M | M | M | |
| Body mass index (kg/m2) | 20.8 | 30.8 | 29 | 23 | 25 | 26.2 ± 4.6 |
| Respiratory mechanics | ||||||
| Tidal volume (mL) | 400 | 300 | 300 | 460 | 440 | 380.0 ± 76.6 |
| Respiratory rate (bpm) | 26 | 30 | 29 | 24 | 24 | 26.6 ± 2.8 |
| Peak pressure (cmH2O) | 30 | 28 | 28 | 20 | 30 | 27.2 ± 4.15 |
| Plateau pressure (cmH2O) | 26 | 26 | 25 | 18 | 28 | 24.6 ± 3.85 |
| PEEP (cmH2O) | 14 | 12 | 10 | 8 | 5 | 9.8 ± 3.4 |
| Driving pressure (cmH2O) | 12 | 14 | 15 | 10 | 23 | 14.8 ± 5.0 |
| Respiratory system compliance (mL/cmH2O) | 33.3 | 21.4 | 20.0 | 46.0 | 19.1 | 28.0 ± 11.6 |
| Gas exchange | ||||||
| PaO2 (mmHg) | 105.0 | 62.0 | 65.3 | 64.0 | 62.5 | 71.8 ± 18.8 |
| FiO2 | 0.90 | 0.85 | 0.80 | 0.80 | 0.60 | 0.79 ± 0.11 |
| PaO2/FiO2 (mmHg) | 117 | 72.9 | 81.6 | 80.0 | 104.2 | 91.1 ± 18.6 |
| AaDO2 (mmHg) | 452.9 | 451.1 | 423.6 | 457.0 | 267.3 | 410.3 ± 81.0 |
| SaO2 | 98.0 | 89.1 | 91.6 | 92.7 | 88.7 | 92.0 ± 3.8 |
| pH | 7.39 | 7.31 | 7.36 | 7.43 | 7.29 | 7.36 ± 0.06 |
| PaCO2 (mmHg) | 71.0 | 79.0 | 69.5 | 42.0 | 83.3 | 69.0 ± 16.1 |
| EtCO2 (mmHg) | 53 | 61 | 66 | 29 | 63 | 54.4 ± 15.0 |
| Base excess (mEq/L) | 15.2 | 10.4 | 10.7 | 3.2 | 9.7 | 9.8 ± 4.3 |
| PvO2 (mmHg) | 36.4 | 39.7 | 41.1 | 34.2 | 41.3 | 38.6 ± 3.1 |
| SvO2 | 69.0 | 69.0 | 74.1 | 68.0 | 70.0 | 70.0 ± 2.3 |
| PvCO2 (mmHg) | 80.1 | 85.7 | 76.6 | 45.7 | 90.8 | 75.9 ± 17.7 |
| QVA/QT | 0.29 | 0.50 | 0.47 | 0.43 | 0.46 | 0.43 ± 0.08 |
| Hemodynamics | ||||||
| Temperature (°C) | 36.8 | 37.0 | 38.1 | 36.0 | 36.0 | 36.8 ± 0.87 |
| Cardiac output (L/min) | 8.06 | 9.49 | 10.40 | 9.45 | 10.55 | 9.58 ± 0.99 |
| VO2 (mL/min) | 326 | 271 | 306 | 288 | 319 | 302.0 ± 22.7 |
| VCO2 (mL/min) | 277 | 230 | 260 | 244 | 272 | 256.7 ± 19.3 |
| Laboratory | ||||||
| Hemoglobin (g/dL) | 9.5 | 10.0 | 11.7 | 8.6 | 11.4 | 10.2 ± 1.3 |
| White blood count (109/L) | 18.4 | 5.0 | 30.6 | 15.8 | 15.6 | 17.1 ± 9.1 |
| ALT (U/L) | 34 | 198 | 342 | 15 | 60 | 129.8 ± 138.7 |
| AST (U/L) | 74 | 81 | 86 | 15 | 21 | 55.4 ± 34.5 |
| Bilirubin (mg/dL) | 0.5 | 1.1 | 1.1 | 0.4 | 0.5 | 0.72 ± 0.35 |
| Platelets (109/L) | 365 | 49 | 79 | 188 | 261 | 188.4 ± 140.2 |
| Fibrinogen (g/dL) | 68 | 134 | 365 | 525 | 273.0 ± 210.1 | |
| D-dimer (ng/mL) | 1060 | 900 | 3339 | 918 | 1554 ± 1191 | |
| Procalcitonin (µg/L) | 0.54 | 0.21 | 0.11 | 0.19 | 0.12 | 0.24 ± 0.17 |
| CT scan variables | ||||||
| Lung weight (g) | 2064 | 1496 | 937 | 1914 | 1401 | 1563 ± 447 |
| Gas volume (mL) | 1637 | 525 | 482 | 3007 | 1215 | 1373 ± 1034 |
| Non-aerated tissue fraction | 0.40 | 0.39 | 0.36 | 0.27 | 0.26 | 0.33 ± 0.06 |
AaDO2, alveolar-arterial PO2 difference; PEEP, positive end-expiratory pressure; PaO2, arterial partial pressure of O2; PaCO2, arterial partial pressure of CO2; FiO2, inspired O2 fraction; EtCO2, end-tidal CO2; PvO2, mixed venous O2 content; PvCO2,mixed venous CO2 content; SaO2, arterial oxygen saturation; SvO2, mixed venous oxygen saturation; VO2, oxygen consumption, VCO2, CO2 production.
Model Testing
To assess the performance of VentriQlar in retrieving a reasonable set of VA/Q distribution parameters (Fig. 3), we tested the model with a set of typical physiological input values (figure caption) to target a PaO2 of 90 mmHg and PaCO2 of 40 mmHg. As shown, the distribution with the smallest Euclidean distance closely followed the classical VA/Q distribution obtained by Multiple Inert Gas Elimination Technique (MIGET) in the healthy subject (20). The associated derived variables are presented in Table 2, whereas the PaO2-PaCO2 plot of the solutions space is shown in Fig. 4. In Supplemental Fig. S1, we show the distribution of Qmean1 and Qmean2 for the whole solution space. As shown, the program selected Qmean1 and Qmean2 mostly between 0.6 and 1.2, well within physiological limits.
Figure 3.
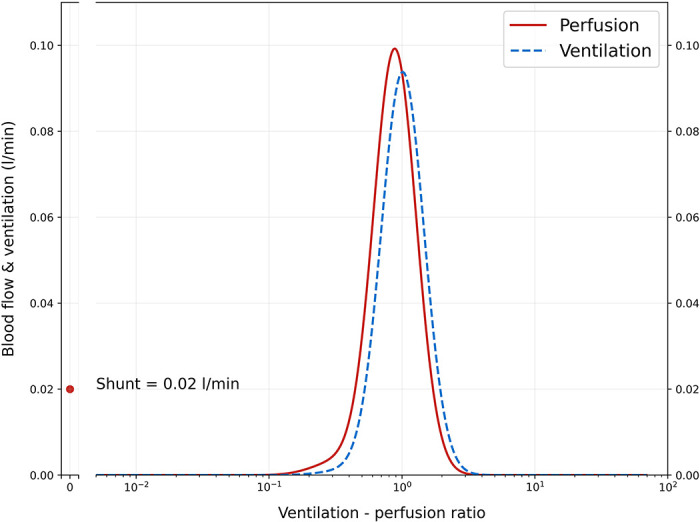
Graphical representation of the ventilation–perfusion (VA/Q) distribution of the solution with the shortest Euclidean distance from the target (PaO2 = 90 mmHg, PaCO2 = 40 mmHg). On the x-axis a log-space of 500 VA/Q compartments (498 + shunt and dead space) is represented, whereas on the y-axis, the relative amount of blood flow and alveolar ventilation for each compartment is shown. The parameters used to recover this distribution were: QT = 5 L/min, FiO2 = 0.21, shunt = 0.5%, hemoglobin = 14 g/dL, BE = 0 mEq/L, PvO2 = 40 mmHg, PvCO2 = 45 mmHg. Despite the assumption of a bimodal distribution, Qmean1 and Qmean2 were close enough on a logarithmic scale that the resulting distribution was practically unimodal, as expected from a healthy subject. Of note, the shunt compartment is visually larger than the traditional MIGET data acquired on healthy subjects. This is due to the use of 500 compartments instead of the traditional 50: through each one of them flows a comparatively smaller blood flow, resulting in a visually smaller scale on the y-axis.
Table 2.
Variables derived from the solution with the smallest Euclidean distance in the healthy, ideal subject
| Subject |
|
|---|---|
| Healthy Subject | |
| Gas exchange | |
| Global VA/Q | 0.83 |
| PaO2 (mmHg) | 90.1 |
| SaO2 (%) | 96.9 |
| pH | 7.40 |
| PaCO2 (mmHg) | 40.1 |
| Alveolar ventilation (L/min) | 4.18 |
| VA/Q distribution parameters | |
| Qmean1 | 0.88 |
| Qmean2 | 0.32 |
| SD, Q1 | 0.37 |
| SD, Q2 | 0.43 |
| Q1/Q2 ratio | 0.03 |
VA/Q, ventilation-perfusion; PaO2, arterial partial pressure of O2; PaCO2, arterial partial pressure of CO2; SaO2, arterial O2 saturation; SD, standard deviation.
Figure 4.
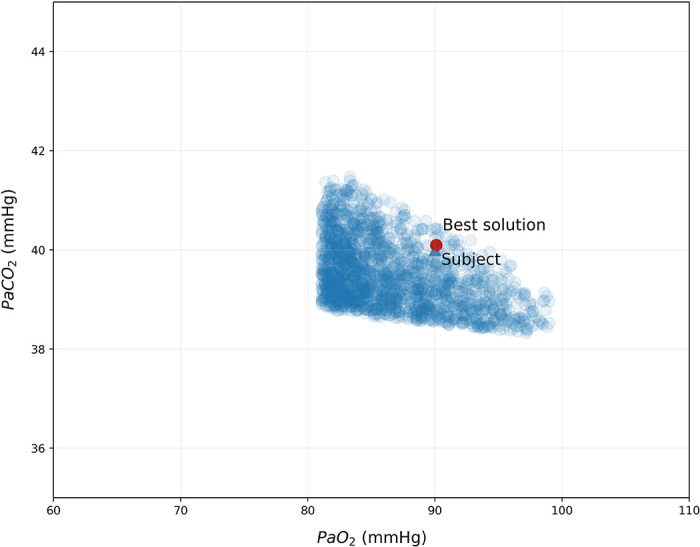
Solution space for an ideal healthy subject. Each one of the single data points (●) represents one of the 106 solutions that fell within the boundaries we considered acceptable.
Subject Prediction
In Fig. 5 we show the predicted VA/Q distribution for each subject. Multiple considerations can be drawn from these results. Interestingly, we found no solutions for subject 1. Of note, subject 1 was also the only subject with a non-aerated tissue fraction greater than venous admixture and the only patient treated with iNO. Second, the recovered distributions share remarkably similar features: upon inspection, the distribution is strongly bimodal, with a consistent proportion of the blood flow distributed in regions with low VA/Q. In all 4 patients for which a solution space was found, the second mode was centered on a compartment population with moderately high VA/Q, but not higher than 10. In Table 3 this finding is confirmed by an overall low VA/Q ratio given by the combination between an elevated cardiac output and low alveolar ventilation. Table 4 presents the calculated distribution moments (quantitative descriptors of the distribution shape) for each subject. As expected, the simulation run with the parameters of a physiological distribution returned moments within normal range. Conversely, in COVID-19 patients, the calculated moments were severely abnormal, particularly Qmean and LogSD, Q. Of note, whereas LogSD, Q represents a true standard deviation only in presence of a unimodal distribution, in our case it still retains the benefit of conveying, in a single number, the magnitude of ventilation-perfusion inequality.
Figure 5.
Graphical representation of the ventilation-perfusion (VA/Q) distribution of the solution with the shortest Euclidean distance from the target for each of the 4 patients for whom VentriQlar found a solution. As shown, in all cases, the recovered distribution was remarkably bimodal, with a large fraction of the blood flow distributed in regions with low or very low VA/Q, whereas a smaller fraction in regions with moderately increased VA/Q.
Table 3.
Variables derived from the solutions with the smallest Euclidean distance in the severe COVID-19 patients
| Subject |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Gas exchange | |||||
| Global VA/Q | 0.33 | 0.28 | 0.74 | 0.51 | |
| PaO2 (mmHg) | 65.7 | 70.0 | 66.5 | 63.2 | |
| SaO2 (%) | 90.1 | 92.6 | 92.9 | 88.6 | |
| pH | 7.29 | 7.34 | 7.40 | 7.28 | |
| PaCO2 (mmHg) | 82.2 | 74.4 | 45.1 | 85.0 | |
| Alveolar ventilation (L/min) | 3.16 | 2.95 | 7.00 | 5.43 | |
| VA/Q distribution parameters | |||||
| Qmean1 | 0.02 | 0.04 | 0.02 | 0.03 | |
| Qmean2 | 1.86 | 1.39 | 10.67 | 1.93 | |
| SD, Q1 | 0.51 | 0.32 | 0.41 | 0.82 | |
| SD, Q2 | 0.36 | 0.43 | 0.53 | 0.72 | |
| Q1/Q2 ratio | 0.34 | 0.24 | 0.05 | 0.26 | |
VA/Q, ventilation-perfusion; PaO2, arterial partial pressure of O2; PaCO2, arterial partial pressure of CO2; SaO2, arterial O2 saturation; SD, standard deviation.
Table 4.
Ventilation–perfusion distribution parameters of the best solution recovered from VentriQlar
| Subjects |
|||||||
|---|---|---|---|---|---|---|---|
| Healthy Subject | 1 | 2 | 3 | 4 | 5 | Means ± SD | |
| Qmean | 0.85 | 0.05 | 0.09 | 0.03 | 0.07 | 0.06 ± 0.02 | |
| VAmean | 1.00 | 1.68 | 1.27 | 11.43 | 2.42 | 4.20 ± 4.19 | |
| LogSD, Q | 0.41 | 1.81 | 1.6 | 1.46 | 1.75 | 1.66 ± 0.14 | |
| LogSD, VA | 0.39 | 1.01 | 1.05 | 1.24 | 1.26 | 1.14 ± 0.11 | |
| Qskew | 0.33 | 2.04 | 1.69 | −2.22 | −1.82 | −2.01 ± 0.14 | |
| VAskeaw | 0.23 | 1.54 | 1.43 | 1.94 | 1.54 | 1.61 ± 0.19 | |
Q, perfusion; VA, ventilation.
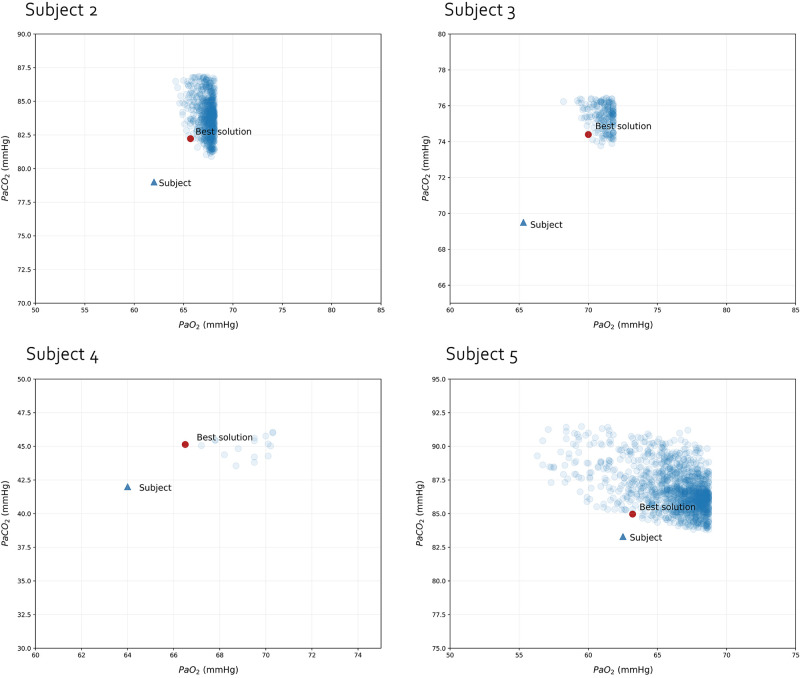
Supplemental Fig. S2 shows the Qmean1 and Qmean2 of the whole solution space for each patient: not only the solution with the shortest Euclidean distance shows a strongly bimodal distribution, but out of 106 combinations tried, the ones that fulfilled our criteria had a Qmean1 below 0.05 and Qmean2 above 1 but mostly below 20. In Fig. 6 we present the whole PaO2–PaCO2 solution space for each subject. Interestingly, the relationship between the “best solution” and the target values is similar: the patient tended to be slightly more hypoxemic and hypocapnic compared to the solutions found. Finally, in Supplemental Fig. S3, all 106 solutions for subject 1 are displayed. None of them fulfilled the criteria we imposed. As show, all solutions had consistently lower predicted PaO2.
Figure 6.
Solution space for the 4 COVID-19, critically-ill subjects for which VentriQlar found at least one solution. Each one of the single data points represents one of the solutions that fell within the boundaries we considered acceptable.
Performance
The overall calculation time per patient averaged 24.0 ± 16.9 min. To calculate the composition of the 499 compartments the software required 10.4 ± 4.0 min. After that, each random choice of distribution parameters and atrium composition calculation required 1022 ± 303 µs.
DISCUSSION
In a pivotal study, Dantzker et al. (2) showed that the VA/Q distribution in ARDS is essentially dominated by a large fraction of shunt, without further major derangements. High VA/Q regions may indeed be present, and may worsen when higher PEEP is applied. In this theoretical computational study, on 5 severe COVID-19 patients, we found that gas exchange impairment can be explained only if an extremely severe degree of VA/Q inequality is present. Indeed, a consistent finding across the patients studied was the presence, together with a large shunt fraction as in ARDS, of a large fraction of cardiac output in lung compartments with very low VA/Q ratio. Remarkably, all 4 patients for which VentriQlar found a solution, despite very different input data, displayed a very similar, strongly bimodal distribution: not only the VA/Q distribution was highly skewed toward the low VA/Q domain, but it was also highly mismatched, as reflected by the extremely high values of LogSD, Q. Regions of moderately high VA/Q were represented, but received only a modest proportion of the cardiac output. To justify these findings, we suspect that the shunt associated to the gasless lung parenchyma is not sufficient to explain the severe gas exchange derangements of our subjects.
The Role of Microthrombosis
Pulmonary micro and macrothrombosis are emerging as a key characteristic of COVID-19–related ARDS (4, 6, 7). Gas exchange and VA/Q distribution in pulmonary embolism has been extensively studied, but the findings are less obvious than one might expect. Dantzker and Bower (21), in 1979, surprisingly show that high VA/Q and dead space were not the core findings of pulmonary embolism. On the contrary, VA/Q inequality associated to hypoxemia is relevant. The reasons put forward by the authors were either a redistribution of blood flow, which is forced to overperfuse otherwise normally aerated units (thereby lowering their VA/Q) and/or the interstitial edema that stems from the high vascular pressure in a lung with embolized units. Furthermore, some authors have also shown that bronchoconstriction of the regions adjacent to the embolized regions may be concurrent causes of the hypoxemia observed in pulmonary embolism (22, 23). Bratel et al. (24) instead found that embolism led to dead space regions, in a more traditional pathophysiological view of pulmonary embolism. To unify these apparently contrasting views, Delcroix and co-workers (25) have shown in dogs that although relatively larger emboli (1000 µm) preferentially lead to high VA/Q areas, smaller emboli (100 µm) actually cause shunt and low VA/Q areas. Consistently with recent reports of severe perfusion defects (26), all our patients died in the ICU and autopsy revealed in all cases extensive thrombosis of intraseptal capillaries, hence possibly justifying the prevalence of low VA/Q and shunt as opposed to high VA/Q and dead space. Of note, relatively high VA/Q ratios (> 1), present in our patients, are calculated as dead space by Riley and Cournard’s (27) tricompartimental model, thus our results are not necessarily in contrast with reports of increased dead space in CARDS.
The Role of Cardiac Output
It is worth noting that the VA/Q is a ratio, and regions with a “normal” or “high” VA/Q may suddenly become low VA/Q regions if a cardiac output increase is not followed by an associated increase in alveolar ventilation. A key characteristic of our subjects was the very high cardiac output associated with low alveolar ventilation as a consequence of the lung protective ventilatory strategy used. It is therefore possible that the very low Qmean could be the result of relatively overperfused regions whose VA/Q is further worsened by the high cardiac output (28, 29). Additionally, regions that would have exhibited a VA/Q > 100 (dead space) in the presence of a normal cardiac output, actually showed only a moderately increased VA/Q with a high cardiac output. The reason for such cardiac output in COVID-19 patients is not known. Our patients, except for subject 4, were severely hypercapnic. In 1998, Pfeiffer et al. (30) showed that the permissive hypercapnia in ARDS led to a shift of the VA/Q distribution toward lower values. Hypoventilation significantly increased cardiac output and high PvCO2 (which leads to dysregulation of perfusion) were proposed by the authors as causative mechanisms. In COVID-19, a reduction of the transit time due to a pathological splanchnic vasodilation (31) may be a concurrent mechanism for the observed hyperdynamic state.
Further Possible Mechanisms
An interesting aspect of our work is that the deviation between the modeled and the target values appears to be systematic: the subjects consistently showed slightly lower PaO2 and lower PaCO2. For this reason, a fraction of the blood flow that the simulation attributed to low VA/Q might, hypothetically, represent shunt. We arbitrarily decided to impose as shunt the tissue mass with a CT density between −100 and +100 HU as, in these regions, tissue represents at least 90% of the voxel volume. In the poorly aerated compartment (−200 to −500 HU) gas represents more than 20% of the voxel, making it reasonable to think that at least some degree of gas exchange happens during the tidal breath. Indeed, Hedenstierna et al. (32) elegantly showed that atelectasis, as they appear at the CT scan, and shunt are highly correlated (R2 = 0.93).
To admit a higher shunt fraction than the fraction of non-aerated tissue, it is possible to hypothesize the existence of other causes of intrapulmonary shunt not directly related to the gasless tissue. In a recent report, Reynolds et al. (33, 34) demonstrated the presence of microbubbles at the Contrast Enhanced transcranial Doppler of the middle cerebral artery of mechanically ventilated, COVID-19 patients. This may be related to the presence of pathological vascular dilations or arterio-venous anastomosis (31, 35). Vascular growth in the form of intussusceptive neoangiogenesis has also been detected histologically (7). Clearly, shunt related to these causes would remain undetected by our model. To test what extent our results were influenced by the assumption that the fraction of non-aerated tissue equals shunt, we run again VentriQlar increasing and decreasing shunt by 20% of the original value. We could not find solutions when shunt was decreased. Instead, as shown in Supplemental Fig. S4, when shunt was increased, the solutions were similar to the ones originally found and possibly closer to the target. Therefore, we cannot exclude that, at least in part, what VentriQlar classified as regions with extremely low VA/Q may actually represent shunt (VA/Q = 0). One should also keep in mind that setting shunt to 100% of the non-aerated tissue fraction is already an overestimation, as it implies a complete absence of hypoxic vasoconstriction. These results further support the idea of a pathological hyperperfusion of the non/poorly aerated tissue, but the way to solve this conundrum is only through a proper measurement of the VA/Q distribution.
Limitations and Methodology Clarifications
Our results must be interpreted in light of evident limitations, mainly the retrospective nature of the study and the limited number of patients, the assumption on the overall metabolic respiratory quotient, the strict bimodality of the VA/Q distribution, and the classification of the non-aerated tissue fraction as shunt, which is certainly an oversimplification of the reality. Moreover, the absence of a measurement of VCO2 forced us to rely on assumptions also for the calculation of the anatomical dead space. Nevertheless, the VA/Q distribution is described on a logarithmic scale, making the results very stable against errors of measurement and assumptions. It must also be noted that these patients were in an extremely severe clinical condition, with serious derangements in gas exchange, lung mechanics, and acid-base balance. An important point must be noted about our methodology: we assumed a bimodal VA/Q distribution, but this does not automatically imply that the recovered distributions were actually bimodal. Indeed, as the astute reader will notice, the recovered distribution, when physiological targets and parameters are used, is essentially unimodal. This apparently contradictory result can be understood if one considers the logarithmic distribution of the VA/Q. Indeed, when the Qmean1 and Qmean2 are close enough on a logarithmic scale (e.g., 0.6–0.8) the two peaks will merge one into the other, effectively resembling a unimodal distribution. Therefore, assuming a bimodal distribution does not automatically imply that a functionally bimodal distribution is found.
Subject 1 represented a clear challenge for the model as we could not find any solution within the boundaries we imposed. Supplemental Fig. S3 shows that, whereas PaCO2 could be modeled within 10% of the target value, PaO2 was always consistently lower. Interestingly, subject 1 was also the only one in whom iNO was in use and with a venous admixture lower than the non-aerated tissue fraction. This implies that the degree of shunt we imposed on the simulation was higher than the one actually present: it is therefore reasonable to think that iNO therapy in this patient was particularly effective, diverting the blood flow away from the gasless tissue to well-ventilated regions (36). Overall, these considerations highlight the most important limitation of our calculations: the model is able to provide useful insights into the patient’s gas exchange until the basic assumption that shunt = non-aerated tissue fraction holds true. In the presence of strong hypoxic vasoconstriction or, conversely, loss of it, the link between the two variables is broken. Finally, we also tried to progressively decrease the shunt in subject 1. The first results appeared when shunt was set to 28% (initial value = 40%). It is by now clear that this method, given the reasonable but still theoretical assumptions on which it relies, should not be meant as an alternative to the MIGET.
CLINICAL IMPLICATIONS
Understanding the root mechanism of gas exchange derangement in CARDS is not only a semantic question: whereas shunt due to atelectasis may benefit from the use of higher PEEP and recruitment maneuvers, VA/Q inequality is more responsive to an increased FiO2 (37). In this theoretical study we showed that the gas exchange derangements of severe COVID-19 patients are not to be attributable only to shunt due to gasless tissue as in all-cause ARDS, of which, in the Appendix, we present example calculations performed with the same methodology. It ultimately becomes of paramount importance to understand the nature of the low VA/Q regions in COVID-19: if a pathological overperfusion of the diseased lung as a consequence of the capillary thrombosis is the cause, any intervention aimed to further inflate the lung is likely not the soundest treatment. Reports suggest that recruitability in COVID-19 is widely variable (38, 39). Therefore, the oxygenation improvement observed at high PEEP is likely due to blood flow redistribution and to a lesser extent to the recruitment of previously collapsed units, a positive effect that must be carefully evaluated against the risk of overinflation. The brief and temporary response to the prone position (40, 41) and the positive response to almitrine administration (42) both support the hypothesis of a dysregulation of the lung perfusion. Of note, Herrmann and co-workers (43) have elegantly shown that a severe vessel tone dysregulation may indeed lead to such gas exchange abnormalities.
Fifty years after the first comprehensive computational model on VA/Q inequality, VentriQlar showed that this type of complex simulations can still help today to characterize and, most importantly, quantify the derangements observed in a new disease and to ultimately help in understanding its pathophysiology.
SUPPLEMENTAL DATA
Supplemental Figs. 1−4 are available at https://doi.org/10.6084/m9.figshare.13061075.v1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHORS CONTRIBUTIONS
M.B., M.Q., and L. Gattinoni conceived and designed research; M.B., F.R., S.G., I.S., A.M., A.S., L.L., and M.M.P. performed experiments; M.B., P.H., K.M., and L. Gattinoni analyzed data; M.B., F.R., S.G., I.S., P.H., K.M., M.Q., L. Gattinoni, M.C., A.M., A.S., L.L., and M.M.P. interpreted results of experiments; M.B. prepared figures; M.B., L. Giosa, L. Gattinoni, M.C., A.G., and L.D. drafted manuscript; M.B., M.Q., L. Giosa, L. Gattinoni, M.C., A.G., and L.D. edited and revised manuscript; M.B., F.R., S.G., I.S., P.H., K.M., M.Q., L. Giosa, L. Gattinoni, M.C., A.G., L.D., A.M., A.S., L.L., and M.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sartorius AG, Göttingen, Germany for an unrestricted grant for lung injury-related research toward the Department of Anesthesiology of Göttingen University Medical Center.
APPENDIX
In this appendix we present the results we obtained by running VentriQlar on data from 7 patients suffering from non-COVID-19 ARDS. From our historical ARDS dataset, we extracted patients who had all the variables needed to perform the calculations.
For two patients we could not find solutions. For the other 5 subjects, Table 5 and Fig. 7 summarize the findings. Overall, the non-COVID 19 ARDS population showed less hyperperfusion of poorly ventilated lung regions and the VA/Q inequality was predominantly due to higher VA/Q regions. An interesting finding comes from the PaO2 – PaCO2 plots, which show that the solutions for non-COVID-19 patients fell more frequently to the left of the target values, in contrast to what happened in COVID-19 patients, where the solutions tended to fall to the right. The most likely explanation is that, in all-cause ARDS, hypoxic vasoconstriction is effective and the assumption that shunt = non-aerated tissue fraction tends to overestimate true shunt. Conversely, in COVID-19, hypoxic vasoconstriction fails/capillary are massively enlarged/arterio-venous anastomosis are present and the assumption tends to underestimate true shunt. This comparison, however, should be taken with care, given the absence of a careful match of the two, rather small, populations on anthropometrics and lung features.
Table 5.
Gas exchange and ventilation–perfusion distribution variables of the best solution recovered from VentriQla on a non-COVID-19 ARDS population
| Subjects |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Means ± SD | |
| PaO2 | 54.2 | 59.7 | 58.3 | 88.6 | 99.1 | 72.0 ± 18.3 |
| SaO2 | 84.9 | 91.0 | 89.2 | 96.3 | 98.3 | 91.9 ± 4.8 |
| pH | 7.32 | 7.42 | 7.38 | 7.35 | 7.53 | 7.42 ± 0.07 |
| PaCO2 | 45.3 | 47.7 | 47.2 | 42.3 | 42.9 | 45.1 ± 2.18 |
| Qmean | 0.74 | 0.54 | 0.81 | 0.52 | 0.59 | 0.63 ± 0.11 |
| VA,mean | 13.77 | 3.94 | 2.90 | 0.94 | 1.20 | 4.55 ± 4.74 |
| LogSD, Q | 1.07 | 0.60 | 0.49 | 1.03 | 0.88 | 0.81 ± 0.23 |
| LogSD, VA | 1.77 | 2.18 | 1.85 | 0.58 | 0.81 | 1.44 ± 0.63 |
PaO2, arterial partial pressure of O2; PaCO2, arterial partial pressure of CO2; SaO2, arterial O2 saturation; Q, perfusion; VA, ventilation; SD, standard deviation.
Figure 7.
Graphical representation of the ventilation–perfusion (VA/Q) distribution of the solution with shortest Euclidean distance from the target for each of the 5, non-COVID-19 patients for whom VentriQlar found a solution (top) and the corresponding solution space (bottom). The patients are presented in the same sequence of Table 5.
REFERENCES
- 1.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA 307: 2526–2533, 2012. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Dantzker DR, Brook CJ, Dehart P, Lynch JP, Weg JG. Ventilation-perfusion distributions in the adult respiratory distress syndrome. Am Rev Respir Dis 120: 1039–1052, 1979. doi: 10.1164/arrd.1979.120.5.1039. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 201: 1299–1300, 2020. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 20: 1135–1140, 2020. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F,Jeanpierre E, Rauch A, Labreuche J, Susen S, . Lille ICU Haemostasis COVID-19 Group. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation 142: 184–186, 2020. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 6.Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schroder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Puschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020: M20–2003, 2020. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383: 120–128, 2020. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang M, Som A, Mendoza DP, Flores EJ, Reid N, Carey D, Li MD, Witkin A, Rodriguez-Lopez JM, Shepard JO, Little BP. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 20: 1365–1366, 2020. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santamarina MG, Boisier D, Contreras R, Baque M, Volpacchio M, Beddings I. COVID-19: a hypothesis regarding the ventilation-perfusion mismatch. Crit Care 24: 395, 2020. doi: 10.1186/s13054-020-03125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West JB. Ventilation-perfusion inequality and overall gas exchange in computer models of the lung. Respir Physiol 7: 88–110, 1969. doi: 10.1016/0034-5687(69)90071-1. [DOI] [PubMed] [Google Scholar]
- 11.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 164: 1701–1711, 2001. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 12.Cressoni M, Caironi P, Polli F, Carlesso E, Chiumello D, Cadringher P, Quintel M, Ranieri VM, Bugedo G, Gattinoni L. Anatomical and functional intrapulmonary shunt in acute respiratory distress syndrome. Crit Care Med 36: 669–675, 2008. doi: 10.1097/01.CCM.0000300276.12074.E1. [DOI] [PubMed] [Google Scholar]
- 13.Rahn H, Fenn WO. A Graphical Analysis of the Respiratory Gas Exchange; the Ob2-COb2 Diagram. Washington, DC: American Physiological Society, 1955, p. 6–38. [Google Scholar]
- 14.Kelman GR. Computer program for the production of O2-CO2 diagrams. Respir Physiol 4: 260–269, 1968. doi: 10.1016/0034-5687(68)90057-1. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins SR, Wagner PD. The multiple inert gas elimination technique (MIGET). In: Methods in Physiology. New York, NY: Springer US, 2017, p. 1 doi: 10.1007/978-1-4939-7441-2. [DOI] [Google Scholar]
- 16.Douglas AR, Jones NL, Reed JW. Calculation of whole blood CO2 content. J Appl Physiol (1985) 65: 473–477, 1988. doi: 10.1152/jappl.1988.65.1.473. [DOI] [PubMed] [Google Scholar]
- 17.Zander R, Lang W. Base excess and strong ion difference: clinical limitations related to inaccuracy. Anesthesiology 100: 459–460, 2004. doi: 10.1097/00000542-200402000-00053. [DOI] [PubMed] [Google Scholar]
- 18.Lang W, Zander R. The accuracy of calculated base excess in blood. Clin Chem Lab Med 40: 404–410, 2002. doi: 10.1515/CCLM.2002.065. [DOI] [PubMed] [Google Scholar]
- 19.Bezanson J, Edelman A, Karpinski S, Shah VB. Julia: a fresh approach to numerical computing. SIAM Rev 59: 65–98, 2017. doi: 10.1137/141000671. [DOI] [Google Scholar]
- 20.Wagner PD, Laravuso RB, Uhl RR, West JB. Continuous distributions of ventilation-perfusion ratios in normal subjects breathing air and 100 per cent O2. J Clin Invest 54: 54–68, 1974. doi: 10.1172/JCI107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dantzker DR, Bower JS. Mechanisms of gas exchange abnormality in patients with chronic obliterative pulmonary vascular disease. J Clin Invest 64: 1050–1055, 1979. doi: 10.1172/JCI109542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy SE, Simmons DH. Redistribution of alveolar ventilation following pulmonary thromboembolism in the dog. J Appl Physiol 36: 60–68, 1974. doi: 10.1152/jappl.1974.36.1.60. [DOI] [PubMed] [Google Scholar]
- 23.Sasahara AA, Cannilla JE, Morse RL, Sidd JJ, Tremblay GM. Clinical and physiologic studies in pulmonary thromboembolism. Am J Cardiol 20: 10–20, 1967. doi: 10.1016/0002-9149(67)90105-1. [DOI] [PubMed] [Google Scholar]
- 24.Bratel T, Lagerstrand L, Brodin LA, Nowak J, Randmaa I. Ventilation-perfusion relationships in pulmonary arterial hypertension: effect of intravenous and inhaled prostacyclin treatment. Respir Physiol Neurobiol 158: 59–69, 2007. doi: 10.1016/j.resp.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Delcroix M, Melot C, Vachiery JL, Lejeune P, Leeman M, Vanderhoeft P, Naeije R. Effects of embolus size on hemodynamics and gas exchange in canine embolic pulmonary hypertension. J Appl Physiol (1985) 69: 2254–2261, 1990. doi: 10.1152/jappl.1990.69.6.2254. [DOI] [PubMed] [Google Scholar]
- 26.Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, Ledot S, Morgan C, Passariello M, Price S, Singh S, Thakuria L, Trenfield S, Trimlett R, Weaver C, Wort SJ, Xu T, Padley SPG, Devaraj A, Desai SR. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med 202: 690–699, 2020.doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasques F, Sanderson B, Formenti F, Shankar-Hari M, Camporota L. Physiological dead space ventilation, disease severity and outcome in ventilated patients with hypoxaemic respiratory failure due to coronavirus disease 2019. Intensive Care Med 46: 2092–2093, 2020. doi: 10.1007/s00134-020-06197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton GH, Seed WA, Vernon P. Observations on the mechanism of hypoxaemia in acute minor pulmonary embolism. Br Med J (Clin Res Ed) 289: 276–279, 1984. doi: 10.1136/bmj.289.6440.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manier G, Castaing Y. Influence of cardiac output on oxygen exchange in acute pulmonary embolism. Am Rev Respir Dis 145: 130–136, 1992. doi: 10.1164/ajrccm/145.1.130. [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer B, Hachenberg T, Feyerherd F, Wendt M. Ventilation-perfusion distribution with volume-reduced, pressure-limited ventilation with permissive hypercapnia. Anasthesiol Intensivmed Notfallmed Schmerzther 33: 367–372, 1998. doi: 10.1055/s-2007-994265. [DOI] [PubMed] [Google Scholar]
- 31.Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int 40: 2110–2116, 2020. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedenstierna G, Tokics L, Strandberg A, Lundquist H, Brismar B. Correlation of gas exchange impairment to development of atelectasis during anaesthesia and muscle paralysis. Acta Anaesthesiol Scand 30: 183–191, 1986. doi: 10.1111/j.1399-6576.1986.tb02393.x. [DOI] [PubMed] [Google Scholar]
- 33.DuBrock HM, Krowka MJ. Bubble trouble in COVID-19. Am J Respir Crit Care Med 202: 926–928, 2020. doi: 10.1164/rccm.202008-3096ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynolds AS, Lee AG, Renz J, DeSantis K, Liang J, Powell CA, Ventetuolo CE, Poor HD. Pulmonary vascular dilatation detected by automated transcranial doppler in COVID-19 pneumonia. Am J Respir Crit Care Med 202: 1037–1039, 2020. doi: 10.1164/rccm.202006-2219LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez FP, Martinez-Palli G, Barbera JA, Roca J, Navasa M, Rodriguez-Roisin R. Gas exchange mechanism of orthodeoxia in hepatopulmonary syndrome. Hepatology 40: 660–666, 2004. doi: 10.1002/hep.20358. [DOI] [PubMed] [Google Scholar]
- 36.Dunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LRG, Mewburn JD, Parlow JL, Archer SL. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest 151: 181–192, 2017. doi: 10.1016/j.chest.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenfant C, Okubo T. Distribution function of pulmonary blood flow and ventilation-perfusion ratio in man. J Appl Physiol 24: 668–677, 1968. doi: 10.1152/jappl.1968.24.5.668. [DOI] [PubMed] [Google Scholar]
- 38.Pan C, Chen L, Lu C, Zhang W, Xia JA, Sklar MC, Du B, Brochard L, Qiu H. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med 201: 1294–1297, 2020. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roesthuis L, van den Berg M, van der Hoeven H. Advanced respiratory monitoring in COVID-19 patients: use less PEEP! Crit Care 24: 230, 2020. doi: 10.1186/s13054-020-02953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppo A, Bellani G, Winterton D, Di Pierro M, Soria A, Faverio P, Cairo M, Mori S, Messinesi G, Contro E, Bonfanti P, Benini A, Valsecchi MG, Antolini L, Foti G. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med 8: 765–774, 2020. doi: 10.1016/S2213-2600(20)30268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elharrar X, Trigui Y, Dols A-M, Touchon F, Martinez S, Prud’homme E, Papazian L. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA 323: 2336, 2020. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Losser MR, Lapoix C, Delannoy M, Champigneulle B, Payen D. Almitrine as a non-ventilatory strategy to improve intrapulmonary shunt in COVID-19 patients. Anaesth Crit Care Pain Med 39: 467–469, 2020. doi: 10.1016/j.accpm.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrmann J, Mori V, Bates JHT, Suki B. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun 11: 4883, 2020. doi: 10.1038/s41467-020-18672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]