Abstract
This large, nationwide, population-based, seroepidemiological study provides evidence of the effectiveness of physical distancing (>1.5 m) and indoor group size reductions in reducing severe acute respiratory syndrome coronavirus 2 infection. Additionally, young adults may play an important role in viral spread, contrary to children up until age 12 years with whom close contact is permitted.
Clinical Trials Registration
NTR8473.
Keywords: COVID-19 pandemic, SARS-CoV-2 seroprevalence, social distancing, transmission, the Netherlands
The coronavirus disease 2019 (COVID-19) pandemic is an unprecedented global crisis. Stringent measures to suppress the spread of its causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), have been implemented to reduce incidence of disease and prevent health systems from becoming overwhelmed. Assessment of the impact of social-distancing measures is vital for informing public health decisions, particularly since the worldwide availability of vaccines is still very limited in this phase.
In the Netherlands, the first case of COVID-19 was reported on 27 February 2020. Key governmental interventions implemented since mid-March 2020 included keeping physical distance (≥1.5 m) from adults for those aged >12 years, whereas close contact between children aged <18 years was permitted; closing schools, restaurants/bars/cafés, cultural institutions, and sport facilities; working from home if possible; prohibiting contact professions; closing nursing homes to visitors; and reducing group sizes to a maximum of 3 visitors at home as well as 3 persons from different households outside and prohibiting all events and gatherings, except for weddings, funerals, religious gatherings (maximum of 30 persons), legally required meetings, and work-related meetings necessary for continuation of daily activities (maximum of 100 persons). In May, daycare and primary schools were reopened and contact professions were allowed to resume. Measures were further relaxed from June until the end of summer, while adhering to physical distancing measures and obligation of wearing a nonmedical mask in public transportation.
Seroprevalence of antibodies against SARS-CoV-2, acquired from validated laboratory assays and well-designed population-based studies, provides an important indicator of cumulative infection [1, 2]. In combining seroprevalence with questionnaire data, the current nationwide population-based study (PIENTER-Corona [PICO]) [3], performed after the first epidemic wave in the Netherlands in June 2020, enabled us to identify risk factors for infection to support assessment of the impact of globally applied social distancing measures.
METHODS
Randomly selected participants of all age groups from the first PICO serosurvey in April 2020 [3, 4], who were initially sampled from the PIENTER3 serosurvey cohort established in 2016/2017 [4], were invited for the current study in June 2020, and 2317 enrolled (of 4926 invited). To enhance countrywide geographical coverage and given the low anticipated seroprevalence, the study sample from April 2020 was supplemented with an additional sample of 4496 (of 26 854) randomly selected persons from the population registry, resulting in a total cohort of 6813 participants in the current study (combined response rate, 21.4%; for further details on sampling, see Supplementary Materials, pp. 3–4)). Participants were requested to collect a fingerstick blood sample in a microtainer (SARSTEDT) and return it by mail. A (online) questionnaire was completed on potential SARS-CoV-2 exposure (number and age group of nonhousehold close contacts [<1.5 m] the day before filling out the questionnaire, attendance of indoor meetings with >20 persons, nursing home visits, working from home the previous week, profession, close contact [voluntary] work with patients/clients and children, and household size) and sociodemographic characteristics (sex, age, ethnic background, religion, educational level, and postal codes were used to determine geographical sites).
Quantitative measures of serum immunoglobulin G (IgG) antibodies against SARS-CoV-2 spike-S1 antigen were derived via a validated immunoassay [5] (see Supplementary Materials, pp. 6–7 for further details on the assay). Based on low anticipated seroprevalence [3], we aimed for a specificity of 99.9% to keep false-positive rates to a minimum. Mixture model analyses (using a validation panel as prior distribution) showed that such specificity could be obtained (at a cutoff for seropositivity of 0.04 log (Arbitratry Units [AU])/mL) with associated sensitivity of 94.3% (95% confidence interval [CI], 90.6–96.7) (Supplementary Materials, pp. 6–16). Applying this cutoff, all seroprevalence estimates (and 95% CIs) for the general Dutch population took into account the survey design, included weighting factors to match the distribution of the general Dutch population (based on sex, age, ethnic background, and degree of urbanization; Supplementary Materials, p. 6), and were controlled for test characteristics subsequently [6, 7]. Smooth age-specific seroprevalence was modeled with B-splines (second degree, 3 percentile-placed internal knots, following lowest Akaike’s information criteria [AIC]).
Risk factors for seropositivity were identified using random-effects logistic regression, taking into account municipality as a unit of clustering. In the main analysis, all participants without missing data for the tested determinants were included (n = 6331). Odds ratios (ORs) and 95% CIs were derived from univariable analyses, and 2-way interactions with age were tested for significance. Variables with an overall P < .15 were tested in multivariable analysis in which stepwise-backward selection was applied, yielding a final model that included only independent risk factors (based on lowest AIC). Sensitivity analyses were performed applying forward selection and by testing models without the variable “being religious” (n = 6487), as it comprised the most missing values, without “educational level” (n = 6339) and without nonhousehold contact data (n = 6338), the latter 2 to test potential associations with profession.
Analyses were performed using Stan v.2.21 (mixture modeling) and SAS v.9.4 (SAS Institute Inc). The Medical Research Ethics Committees United (MEC-U) approved the study, and all participants provided written informed consent.
RESULTS
Median inclusion date was 14 June 2020 (range, 9 June–24 Augustus; 90% were enrolled by 22 June) (Note: Sociodemographic characteristics available from nonresponders were compared with those of responders and are shown in Supplementary Materials, pp. 4–5.). The cohort comprised 55% women, and regions were equally represented following population size (Supplementary Materials, pp. 5–7). Half of the participants reported to have had ≥2 nonhousehold close contacts the day before filling out the questionnaire. Since the start of the epidemic, one quarter had attended an indoor meeting with >20 persons, and 8% had visited a nursing home. Among those aged 18–66 years, 36% (voluntarily) worked in close contact with clients/patients, 18% were healthcare workers, and 40% had been (partly) working from home the previous week.
After the first epidemic wave, overall seroprevalence in the Dutch population was 4.5% (95% CI, 3.8–5.2). No statistically significant differences were observed between sexes or ethnic backgrounds. Estimates were low (0%–2%) in children aged 1–12 years, high (9%) in young adults in their early twenties, and 4%–7% in individuals aged ≥35 years (Figure 1A). Low urbanized areas were hit hardest, predominantly in the southeast (up to 16%) (Supplementary Materials, pp. 17).
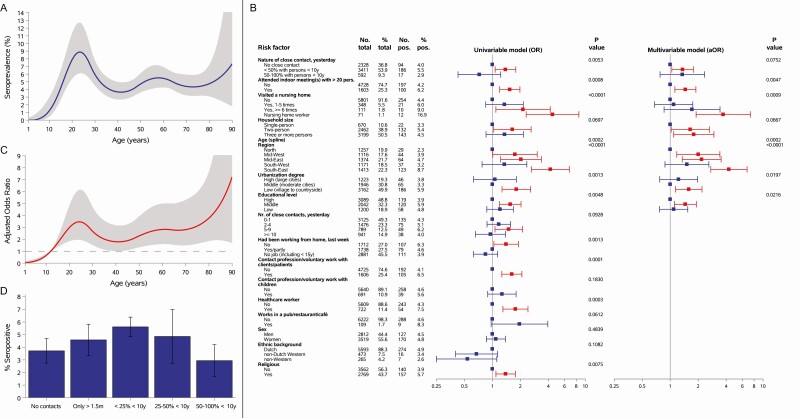
Figure 1.
A, The weighted smooth age-specific severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seroprevalence with 95% confidence envelope in the general population of the Netherlands after the first epidemic wave. B, The risk factor analyses for SARS-CoV-2 seropositivity. Number and percent of total participants per potential risk factor category are provided as well as the number and percent of seropositive participants and P values. Forest plots are shown of crude odds ratios (ORs) for univariable analyses and aORs for the multivariable analysis and depicted by squares and 95% confidence intervals (95% CIs) with lines. Those in red are significantly associated with seropositivity, and those in blue are nonsignificant. Time window of attending indoor meetings with >20 persons and visiting a nursing home was from the beginning of the epidemic in the Netherlands (27 February 2020) until the day of completing a questionnaire or until closure (for visitors) of nursing homes (20 March 2020), respectively. Nature and number of nonhousehold close contacts yesterday and working from home last week were related to the day or week, respectively, before the questionnaire was completed. Receiver operating characteristic curve analysis of the multivariable model yielded an area under the curve (as a measure of goodness of fit) of 0.72. C, The aOR with 95% confidence envelope for age (which was included with a flexible [spline] function) derived from the multivariable model, with 12 years as reference category. D, The percentage of SARS-CoV-2 seropositive participants (and 95% CIs) by number and nature of nonhousehold close contact the day before the questionnaire was completed. Nature of nonhousehold close contact was defined as the proportion of nonhousehold close contacts with children aged <10 years of the total number of nonhousehold close contacts. Abbreviation: aOR, adjusted odds ratio.
All potential risk factors for seropositivity tested in univariable analyses are shown in Figure 1B (see Supplementary Materials, pp. 18–20 for additional details). Close contact (voluntary) work with children and work with clients/patients was not associated with seropositivity. Social distancing–related risk factors in the multivariable model (Figure 1B, C, and Supplementary Materials, pp. 18–19) included nonhousehold close contacts with ≥50% persons aged ≥10 years, but not close contact with ≥50% children aged <10 years compared with no contacts (see also Figure 1D); attending indoor meetings with >20 persons; working in a nursing home (rather than visiting); increased household size; and age, with low adjusted odds in children aged ≤12 years, with greater than 2.5 times higher odds in adults aged 18–30 and ≥50 years compared with those aged 12 years (Figure 1C). Notably, total number of nonhousehold close contacts did not remain in the final model after including the variable nature of close contact. Sensitivity analyses yielded similar results (Supplementary Materials, p. 21).
DISCUSSION
Here, we provide evidence from a large population-based study on the effectiveness of physical distancing (>1.5 m) as well as indoor group size reductions on SARS-CoV-2 infection. These data suggest that close contact with children up until age 12 years may pose little risk for infection.
Our results on physical distancing are in line with the few previous reports mostly derived from healthcare settings and households [8]. Seroprevalence rates were low in children aged ≤12 years despite close contact and similar to observations from other European countries with comparable nationwide estimates [1, 9]. Interestingly, the likelihood of infection among persons in close contact with children was not statistically significantly increased, most likely indicating a low contribution in transmission, as suggested previously [10–13]. In contrast, particularly young adults who engage in relatively more social interaction as opposed to older age groups [14] and often living in larger (student) households in the Netherlands, most probably played an increased role. Applying physical distancing measures within households may not always be feasible; however, stressing its relevance in outbreak management could help to reduce (ongoing) transmission. Further, as observed in other countries [15], these data underline the increased risk of infection among nursing home workers. Hence, while working with the most vulnerable, this requires specific attention.
Our study has strengths and limitations. A strength is that our study provides a large population sample that covers a full age range from young to old, combining a sound indicator of prior infection, that is, seropositivity, with extensive questionnaire data. Also, samples could be classified accurately since antibodies were measured with a highly specific and sensitive immunoassay. A limitation includes the relatively low response rate, which may have introduced potential selection bias, for example, participation of relatively more health-conscious individuals who possibly adhere to social distancing measures, healthcare workers, and persons from Dutch descent; however, we expect little effect on our main outcome. Further, some variables might be proxies of risk of viral exposure, for example, on contacts, thus associations should be interpreted with care as they may not reflect causal effects.
In conclusion, these results underscore the effectiveness of social distancing–related measures to reduce SARS-CoV-2 transmission in an era of limited availability of vaccines. Additionally, our data suggest a diminished role of young children in viral spread that, combined with a proactive testing policy, might justify keeping primary schools open, while young adults may seem to play a more considerable role.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. E. R. A. V. wrote the manuscript. F. R. M. vK., H. E. dM., E. R. A. V., L. M., R. S. vB., G. dH., and J. A. B. conceived the study. E. R. A. V. conducted the epidemiological data analyses. M. vB. performed the mixture modeling and supervised the epidemiological analyses together with H. E. dM. and H. B.; R. S. vB. and G. dH. supervised the laboratory analyses. E. R. A. V., L. M., C. C. E. vH., and G. dH. processed the data. F. R. M. vK. was principal investigator of the study. E. R. A. V. and F. R. M. vK. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the interpretation of data and read, edited, and approved the final manuscript.
Acknowledgments. We gratefully acknowledge the participants of the study. This study would not have been possible without the contribution of colleagues from the National Institute of Public Health and Environment (RIVM), more specifically the Department of Immunology of Infectious Diseases and Vaccines (related to logistics and laboratory analyses) and the Department of Epidemiology and Surveillance (EPI; related to logistics and methodological and statistical insights). We also thank Maarten Schipper for performing the sampling and Susan van den Hof (EPI Department head) for reviewing the manuscript. The study protocol was approved by the Medical Research Ethics Committees United (MEC-U), the Netherlands, and conformed to the principles embodied in the Declaration of Helsinki. Our data are accessible to researchers upon reasonable request for data sharing to the corresponding author.
Disclaimer. The views expressed are those of the authors and not necessarily those of Ministry of Health, Welfare and Sports (VWS) or the RIVM. The funder played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by VWS, the Netherlands.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rostami A, Sepidarkish M, Leeflang M, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect 2021; 19:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koopmans M, Haagmans B. Assessing the extent of SARS-CoV-2 circulation through serological studies. Nat Med 2020; 26:1171–2. [DOI] [PubMed] [Google Scholar]
- 3. Vos ERA, den Hartog G, Schepp RM, et al. Nationwide seroprevalence of SARS-CoV-2 and identification of risk factors in the general population of the Netherlands during the first epidemic wave. J Epidemiol Community Health 2020; 75:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verberk JDM, Vos RA, Mollema L, et al. Third national biobank for population-based seroprevalence studies in the Netherlands, including the Caribbean Netherlands. BMC Infect Dis 2019; 19:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. den Hartog G, Schepp RM, Kuijer M, et al. SARS-CoV-2–specific antibody detection for seroepidemiology: a multiplex analysis approach accounting for accurate seroprevalence. J Infect Dis 2020; 222:1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am J Epidemiol 1978; 107:71–6. [DOI] [PubMed] [Google Scholar]
- 7. Lang Z, Reiczigel J. Confidence limits for prevalence of disease adjusted for estimated sensitivity and specificity. Prev Vet Med 2014; 113:13–22. [DOI] [PubMed] [Google Scholar]
- 8. Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ; COVID-19 Systematic Urgent Review Group Effort Study Authors . Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020; 395:1973–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM; CMMID COVID-19 Working Group . Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med 2020; 26:1205–11. [DOI] [PubMed] [Google Scholar]
- 11. van der Hoek W, Backer JA, Bodewes R, et al. The role of children in the transmission of SARS-CoV-2. Ned Tijdschr Geneeskd 2020; 164:D5140. [PubMed] [Google Scholar]
- 12. Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr 2020; 175:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ludvigsson JF. Children are unlikely to be the main drivers of the COVID-19 pandemic–A systematic review. Acta Paediatr 2020; 109:1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Backer JA, Mollema L, Vos ERA, et al. The impact of physical distancing measures against COVID-19 transmission on contacts and mixing patterns in the Netherlands: repeated cross-sectional surveys in 2016/2017, April 2020 and June 2020. Euro Surveill 2021; 26:pii=2000994. doi: 10.2807/1560-7917.ES.2021.26.8.2000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 2020; 5:e475–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.