Abstract
Oocyte maturation failure observed in assisted reproduction technology (ART) cycles can limit the number of quality oocytes obtained and present a pronounced barrier for some patients. The potential exists to use unmatured oocytes for ART through in vitro maturation. Understanding the molecular basis of oocyte maturation failure is pertinent to minimizing this loss of oocytes and considerations of whether such oocytes can be used safely for ART. We identified shared transcriptome abnormalities for rhesus monkey and human failed-to-mature (FTM) oocytes relative to healthy matured MII stage oocytes. We discovered that, although the number of shared affected genes was comparatively small, FTM oocytes in both species shared effects for several pathways and functions, including predicted activation of oxidative phosphorylation (OxPhos) with additional effects on mitochondrial function, lipid metabolism, transcription, nucleotide excision repair, endoplasmic reticulum stress, unfolded protein response, and cell viability. RICTOR emerged as a prominent upstream regulator with predicted inhibition across all analyses. Alterations in KDM5A, MTOR, MTORC1, INSR, CAB39L, and STK11 activities were implicated along with RICTOR in modulating mitochondrial activity and OxPhos. Defects in cell cycle progression were not a prominent feature of FTM oocytes. These results identify a common set of transcriptome abnormalities associated with oocyte maturation failure. While our results do not demonstrate causality, they indicate that fundamental aspects of cellular function are abnormal in FTM oocytes and raise significant concerns about the potential risks of using FTM oocytes for ART.
Keywords: meiosis, mitochondria, oocyte maturation, oxidative phosphorylation, transcriptome
INTRODUCTION
Infertility is estimated to affect, on average, 1 out of the 10 women of reproductive age, leading to an increased demand for assisted reproductive technologies (ART) worldwide (1, 2). There are a variety of factors that can cause infertility and ∼15%–30% of infertility cases are due to unexplained factors (3). Recent research has suggested defects in oocyte maturation currently known as the “oocyte factor” as a possible cause for unexplained female infertility (4). Cytoplasmic maturation arrest can happen in a number of different ways including oocytes can arrest at the germinal vesicle (GV) stage, oocytes can arrest at the first meiotic division [metaphase I (MI) stage], or oocytes can fail to activate after insemination even after reaching the metaphase II (MII) stage. While complete maturation failure at the germinal vesicle stage of all oocytes during ART is extremely rare, on average, 20% of oocytes collected during in vitro fertilization (IVF) are arrested at the GV or MI stage (5, 6). Combined, oocyte maturation failure can either lead to severe infertility or reduce efficiency with ART, creating a significant need for understanding the mechanisms of oocyte maturation failure.
Our recent transcriptome analysis of rhesus monkey oocytes that failed-to-mature (FTM) after a standard hormonal stimulation protocol and an ovulatory stimulus (7) identified numerous differentially expressed genes compared with MII oocytes. Ingenuity Pathway Analysis (IPA) implicated DNA methylation (reduced activity) and mitochondrial function (increased) as prominent changes associated with maturation failure. The mitochondrial effect was associated with increased expression of nuclear genes encoding mitochondrial proteins, particularly components of complex I of the electron transport chain (7).
Our results with the rhesus monkey study indicated that the typical maturation-associated modulations of expression of mRNAs associated with mitochondrial function, oxidative metabolism, and DNA methylation are inhibited in oocytes that fail to mature, such that FTM oocytes may be fundamentally compromised and of uncertain value for ART. The effects on mitochondria function are especially notable for potential negative impact on oocyte quality. Oocyte mitochondria are more spherical than elongated, have underdeveloped cristae, slower ATP production, and suppressed mtDNA replication when compared with somatic cell mitochondria (8–10). There is a substantial decline in nuclear-encoded mitochondrial protein mRNAs during oocyte maturation, and a transition in mitochondria activity state that is essential for early embryo viability. Although too little mitochondrial activity is associated with infertility (11), a precise balance between processes generating reactive oxygen species (ROS) and those decomposing ROS is also critical for normal embryo development. Excessive mitochondrial activity leading to excess production of ROS can be highly detrimental to embryos because they have greatly reduced expression of antioxidant enzymes during early developmental stages (12, 13).
The above observations in the rhesus monkey prompted us to ask whether oocyte maturation failure in humans is associated with disturbances in the same pathways, processes, and functions. We compared transcriptomes of human in vivo matured and FTM oocytes and performed applied IPA to resulting differentially expressed genes (DEGs) and then assessed overlap with the rhesus monkey results. We observed many DEGs in human FTM oocytes, with a small number of these being shared with the rhesus monkey. Most importantly, the two species shared effects on many pathways and functions and associated upstream regulators, including increased expression of nuclear-encoded mitochondrial protein mRNAs and predicted effects on mitochondrial function and oxidative phosphorylation. These results reveal a commonality of shared disturbances in the regulation of essential cellular functions in FTM oocytes. And although it is unclear whether such abnormalities cause maturation arrest, such shared abnormalities indicate fundamental cellular defects that call into question the suitability of such oocytes for ART approaches.
MATERIALS AND METHODS
Rhesus Samples and RNA-Seq Processing
Female adult rhesus macaques aged 6–12 yr old were housed at the California National Primate Center in accordance with the Institutional Animal Use and Care Administrative Advisory Committee at the University of California, Davis ethical guidelines (14). For the collection of oocytes, rhesus macaques underwent a controlled ovarian stimulation protocol that included twice daily intramuscular injections of 37.5 IU of recombinant human FSH (La Jolla Discount Pharmacy, La Jolla, CA), within 4 days of the start of their menses, for a total of 7 days. Then on day 8, n = 10 animals were administered an intramuscular injection of 1,000 IU human chorionic gonadotropin (hCG; La Jolla Discount Pharmacy, La Jolla, CA). The day after hCG injection, metaphase II (MII) and failed-to-mature (FTM) oocytes were collected by ultrasound guided needle aspiration, as described (7). GV/FTM oocytes were classified by either having an expanded cumulus and may or may not have undergone GV break down (GVBD), but no polar body was evident, or they displayed a tight ring of cumulus cells around them at recovery and had undergone GVBD. Oocytes were collected during the breeding season to avoid detrimental effects of summer collection (14).
Rhesus RNA-Seq libraries were previously generated using Ovation RNA-Seq System v2 using RiboSPIATM Technology (NuGen, San Carlos, CA) and were sequenced on an Illumina HiSeq 4000 platform (Illumina, San Diego, CA) as published (7). The two sample types in this study were FTM (GSE112533) and MII stage (GSE112534). Four samples from the GEO data sets were excluded from the analysis for having low read counts or having been generated with a different library kit (Supplemental Table S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.13456382). Raw sequencing data in fastq format were downloaded for processing. Initial quality metrics were conducted using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/), which identified aberrant nucleotide distribution in the first 13 base pairs of reads. Trimming of adapters and low-quality reads was conducted with Fastp (15). Trimming settings were minimum quality threshold of 20, minimum length of 20, removal of low complexity reads, and hard trim of 13 base pairs from start of reads. The rhesus genome (Mmul build v10, genome annotation relase-10) (16) was indexed, and mRNA abundance quantified with Kallisto (17), using standard settings. mRNA abundance was imported into DESeq2 (18), and transcript abundances were calculated for individual genes using Ensembl identifiers converted with biomartR (19). Genes with an fragments per kilobase million (FPKM) above 1 in at least one sample were included for differential expression calculation. Differential expression calculations were conducted comparing FTM|MII, where genes with an adjusted P value (q value via Benjamini-Hochberg method) below 0.05 were considered statistically significant, positive values in Log2FoldChange indicate higher expression levels in FTM samples.
Ovarian Stimulation Protocol in Women
Samples for human expression array analysis were collected as described (20). Ovarian stimulation followed standard procedures where women were given daily injections of either highly purified urinary hMG (Menopur, Ferring, Spain) or follitropin alpha (Gonal, Merck-Serono, Spain) as described (21). A GnRH antagonist (0.25 mg of Cetrorelix acetate, Cetrotide, Merck Serono, Spain) was administered daily starting on day 6 of stimulation for pituitary suppression (22). Final oocyte maturation was triggered with 0.2 mg of the GnRHa triptorelin (Decapeptyl, Ipsen Pharma, Spain) when three or more follicles of >18 mm of diameter were detected on the ovaries. Oocyte retrieval was performed 36 h later via ultrasound-guided transvaginal follicular aspiration. Ethical approval for the study was obtained by the Ethics Committee for Clinical Research (CEIm) and all women included in the study gave written informed consent.
Study Population of Women
The eight fertile women who donated oocytes for the microarray analysis were, on average 26.1 ± 4.0 yr old (range 21–31 yr), with a mean antral follicle count (AFC) of 25.5 ± 8.6 follicles (range 16–31). Oocytes were classified according to their maturation stage: GV–FTM oocytes and MII oocytes. All women had a BMI < 30 kg/m2, normal karyotype, and no known systemic or reproductive conditions.
Oocyte Collection and RNA Extraction
MII oocytes were vitrified on the day of retrieval and were warmed following standard procedures (Cryotop, Kitazato, BioPharma Co., Ltd, Japan). After warming, MII oocytes were placed in an incubator for 2 h at 37°C and 6% CO2 to allow the assembly of the metaphase plate. Failed-to-mature human oocytes were obtained at the GV stage upon aspiration following the same controlled ovarian stimulation protocol and were immediately processed. Zonae pellucidae were removed with Pronase (Roche Diagnostics, Spain), and oocytes lysed individually in 45 µL of a Proteinase K-based lysis buffer (20 mM DTT, 0.5% SDS, 1 μg/μL proteinase K, 10 mM Tris-HCl, pH 7.4), incubated at 65°C for 15 min, and stored at −80°C. Total RNA extraction and amplification were performed with the Pico-Profiling protocol modified for single oocyte use (20, 23, 24).
Microarray Preparation, Hybridization, and Raw Data Processing
Expression array analysis was performed as described by Barragan et al. (20). Libraries were prepared by Whole Transcriptome Amplification following manufacturer’s instructions (WTA2, Sigma-Aldrich, Spain). Briefly, SYBRGreen (Sigma-Aldrich, Spain) was added to the amplification reaction, performed in a CFX real-time machine (Bio-Rad, CA) to monitor amplification yield. Amplification was stopped when the SYBRGreen signal reached a plateau, and cDNA was purified and quantified on a Nanodrop ND-1000 spectrophotometer (Thermo Fischer, MA). This was followed by an additional round of amplification starting from 10 ng of cDNA; 0.8 µg of cDNA were cleaved with DNAse I and biotinylated by terminal transferase (GeneChip Mapping 250K Nsp assay kit; Affymetrix, CA). Biotin-labeled cDNA was then hybridized on an Affymetrix GeneChip Human Transcriptome Array 2.0 (HTA 2.0). Each array was hybridized, washed, and scanned according to the manufacturer’s protocol. The washing and staining steps were performed in a GeneChip Fluidics Station 450 and the scanning in a GeneChip Scanner 3000. The scanned images were transformed into intensities by GeneChip Command Console Software (AGCC, Affymetrix), and the downstream analysis performed with the Transcriptome Analysis Console v. 4.0.1.
Microarray Analysis
Statistical analysis of the microarray data was performed using the Transcriptome Analysis Console (TAC; v. 4.0.1) (Affymetrix) applying one-way between-subject ANOVA (unpaired) and false discovery rate (FDR) based on Benjamini–Hochberg step-up below 0.05 to define significant results. Microarray data for mature MII oocytes and nonmatured GV oocytes were deposited at GEO under GSE152525. Because only four GV/FTM oocytes were available, we used four good MII oocytes that had similar demographic characteristics of the GV/FTM donors, and approximately the same age and antral follicle count (AFC) as the GV/FTM, for this analysis. The samples used in this analysis were GSM4617232, GSM4617233, GSM4617234, and GSM4617235 for GV/FTMs and GSM4617240, GSM4617241, GSM4617244, and GSM4617246 for in vivo matured MII.
Public Human RNA Sequencing Data
We accessed two publicly available RNA-Seq data sets of human oocytes (25, 26). These data sets included human oocytes from IVF patients that underwent hormonal stimulation. Because all patients received an hCG stimulus, GV oocytes from these collections corresponded to FTM oocytes, as the GV oocyte failed to undergo meiotic maturation after the in vivo ovulatory stimulus. GVs and MII oocytes from Hendrickson’s study were pooled according to their developmental stage before RNA extraction, libraries were prepared with TotalScript RNA-Seq kit (Epicentre) and sequenced on a HiSeq 2000 with 2× 100 bp reads (25). Reyes et al. (26) study took single GV and MII oocyte and extracted RNA with PicoPure kit, with libraries prepared with Clontech Smarter Ultra-Low RNA kit followed by sequencing on a HiSeq 2500 with 100 bp reads. The analysis pipeline of processing human sequencing data was the same utilized for the rhesus samples for each study. To identify the overall differential expression of the GV/FTM versus MII oocytes in human RNA-Seq data, the R package metaRNASeq (27) was used to implement a P value combination between studies via the Fisher combination method. In short, Fisher’s combination method assumes that gene counts follow a negative binomial distribution within each included study. For each gene, the null hypothesis was tested; that each gene is not differentially expressed. Whereupon the Fisher’s exact test is applied to calculate gene- and study-wise P values. In a review of different library preparation kits used in RNA-Seq (28), there are differences in captured features. With these differences, it is necessary to consider the true representation of a given transcriptome. While one can assume that comparing studies with different underlying methods is invalid, by leveraging the metaRNAseq calculation, greater rigor and statistical power are achieved while overcoming potential effects of interstudy variables. For example, a gene could be calculated as a non-DEG in both study A and study B, albeit close to the level of significance, but with the application of Fisher’s combination method, this gene can emerge as a DEG due to increased power.
Comparison of Human and Rhesus DEGs
To compare across data sets (human metaRNASeq, human microarray, rhesus sequencing), it was first essential to unify identifiers for genomic features. Therefore, each data set of resultant DEG calculations was submitted to IPA to convert respective gene IDs (Ensembl and Affy probes) to gene symbol. After data retrieval from IPA, there remained gene symbols mapping to multiple Ensembl/Affy IDs. In these cases, a tiered approach was taken per multimapping gene symbols: 1) removal of genes present on nonchromosomal scaffolds/contigs and 2) if all genes displayed the same DEG calculation, use that calculation for said gene symbol. This method resulted in no duplicate genes for each data set. These resultant filtered gene lists were used to compare across studies. To find a unified human DEG list, the human metaRNAseq and microarray data sets were compared, and genes with the same direction of change between GV/FTM and MII were identified. Human (Supplemental Table S2) and rhesus (Supplemental Table S3) gene lists were then compared and established human specific (Supplemental Table S4), rhesus specific (Supplemental Table S5), and human/rhesus shared (Supplemental Table S6).
To assess potential impact of isoform switching or differential splicing between FTM and MII for both rhesus and human, the Kallisto abundance output for the RNAseq data sets for each species was imported into the R package IsoformSwitchAnalyzeR (29–32). Genes and isoforms were limited to those with one FPKM present in at least one sample. Differential isoform detection was conducted with the IsoformSwitchTestDEXSeq function, comparing FTM and MII stages. Significant differences were set at a threshold of an adjusted P value < 0.05.
Ingenuity Pathway Analysis
Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis). QIAGEN Ingenuity Pathway Analysis (IPA; Hilden, Germany) is a unique program that provides a breadth of analysis options compared with other programs (77). Results derived from the IPA are based on a Knowledge Base that contains more than 7 million research findings. Additionally, IPA result categories are much more specific, and the directions of changes for pathways and functions can be determined. Lastly, IPA can reveal affected upstream regulators, an output that is relevant to identifying and pursuing testable hypotheses to determine underlying mechanisms.
Each of the above three gene lists was processed independently through IPA, using canonical pathway (CP), disease and functions (DF), and upstream regulator (UR) analysis tools. Significant effects were revealed for each of the analyses (P < 0.05), indicating overrepresentation of molecules in the DEG lists. If a given entry had sufficient information, then IPA reports directionally (activation/increase or inhibition/decrease), expressed as a z-score (|z| > 1.96 is considered significant). All resultant IPA data were further processed in R to identify common and unique features. The reported results for UR analysis excluded exogenous chemicals, drugs, and toxins, focusing on biologically expressed molecules. The reported results from DF analysis excluded diseases, thereby focusing on “biological functions.” Additionally, to simplify presentation of biological function data in graphical form, functionally similar IPA-defined biological functions were assigned to aggregate functions.
To gain further mechanistic insight into affected pathways and functions, we leveraged the IPA Knowledge Base to develop expanded networks connecting affected URs to other genes positioned further upstream in the regulatory cascade or downstream. We first confirmed that a candidate UR was expressed in the data set and whether that UR was itself differentially regulated. The IPA Knowledge Base was used to identify comembers of complexes containing the affected URs. We then determined whether essential complex comembers were also expressed in the data set. A network was then expanded by including downstream DEGs and their relationships to particular functions and then by using the IPA custom pathway utility to identify other upstream regulators. This expansion was repeated as needed to expand the network further upstream in successive increments, and additional upstream network components that were also DEGs were noted. This way, it was possible to identify groups of DEGs that act collectively to regulate cellular functions.
Information about the different studies, DEG lists, and IPA results are provided in Supplemental data (https://doi.org/10.6084/m9.figshare.13456382).
RESULTS
We previously observed that a small portion of rhesus macaque oocytes failed to undergo meiotic maturation in vivo [classified as failed-to-mature (FTM) oocytes] following an ovulatory stimulus (7). IPA revealed aberrant regulation of several pathways in FTM oocytes, most notably disturbances in the regulation of nuclear genes encoding mitochondrial proteins. Our goal here was to determine whether human FTM oocytes display similar features in their transcriptomes.
We employed four in vivo matured (MII) oocytes and four failed-to-mature (FTM)–germinal vesicle (GV) stage oocytes, individually, from a total of eight women who were recruited from an oocyte donation program for microarray analysis. All human samples met the hybridization control standards and showed a normal distribution of probe set expression values and signal frequencies after normalization. In addition, we accessed publicly available data sets of human oocyte RNA-sequencing (25, 26) and compared data of GV–FTM oocytes to MII oocytes from women undergoing fertility treatments. We then took the overlap of the two human gene lists and identified 1,303 mRNAs significantly different between human FTM and MII oocytes. For rhesus monkeys’ samples, we had 7 MII stage oocyte and 10 FTM oocyte samples from a previous study (7) and identified 412 mRNAs that were significantly different in the FTM oocytes compared with in vivo matured rhesus MII oocytes. These rhesus monkey FTM oocytes were at different stages of meiotic progression, but all were meiotically incompetent, having failed to respond appropriately to an ovulatory stimulus in vivo.
DEGs for each of the four included studies were identified (Supplemental Table S2) and displayed on volcano plots to illustrate the relationship between significance and Log2 fold-change (Supplemental Fig. S1). The 1,303 DEGs seen between human FTM and MII oocytes (Supplemental Table S2) included 646 more abundantly expressed in FTM oocytes and 657 more abundantly expressed in MII oocytes. In the monkey, we observed slightly more genes showing increased mRNA expression in the FTM oocytes than reduced expression (412 total, 255 up, and 157 down) (Supplemental Table S3). Among the most highly affected human-specific DEGs were those encoding RNA polymerase II subunits 1 and G (Supplemental Table S4). Many mitochondrial-associated genes were also differentially expressed. Of the 1,303 DEGs from the human study and the 412 DEGs from the rhesus monkey study (7), we observed 1,232 human-specific (Supplemental Table S4), 168 rhesus-specific (Supplemental Table S5), and only 65 shared concordant DEGs (Supplemental Table S6). The shared DEGs included those that encoded mitochondrial proteins (increased), metabolic genes, and zygotic arrest genes (Supplemental Table S6). The expression of a different form of DNA methyltransferase was reduced in the two species. No significant differences in isoform switching or splicing were detected for the RNAseq data sets analyzed for either species.
We note that at the whole transcriptome level, neither rhesus monkey nor human FTM oocytes displayed a greater degree of within-group variance than either GV or MII stage oocytes for the four included data sets used in this paper (Principal Component Analysis plot; Supplemental Fig. S2) (7, 25, 26). Consequently, we interpret the limited number of shared DEGs to indicate that there are many species-specific DEGs and a comparatively limited set of shared DEGs associated with oocyte maturation failure. However, because these shared DEGs could be part of more broadly affected shared differences in pathways and functions (i.e., DEGs might not overlap but their associated pathways and functions could), we focused our further analysis on using IPA to examine pathways and biofunctions associated with species-specific and shared DEGs.
Ingenuity Pathway Analysis of Species-Specific DEGs Observed between Human FTM and MII Oocytes (“Human-Specific DEGs”)
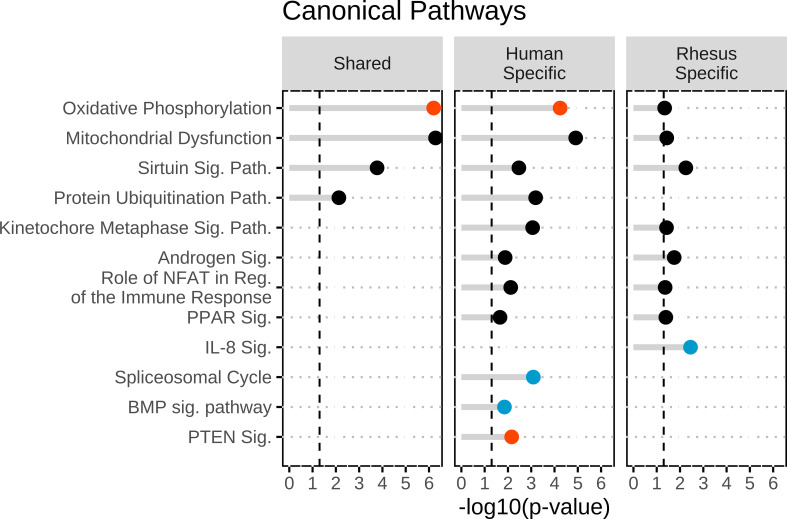
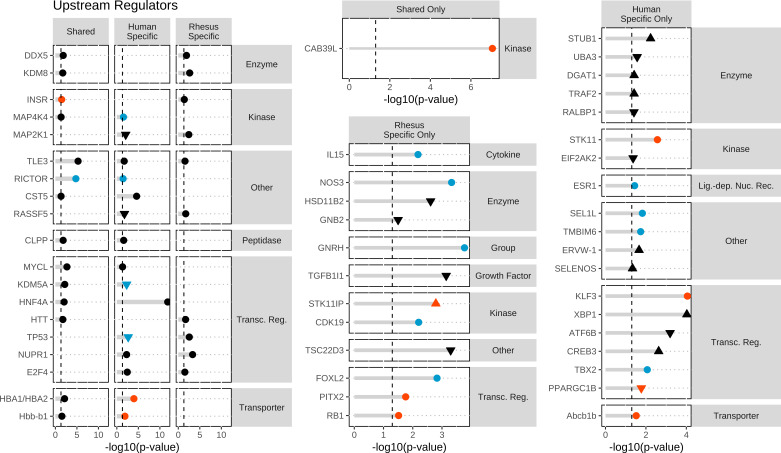
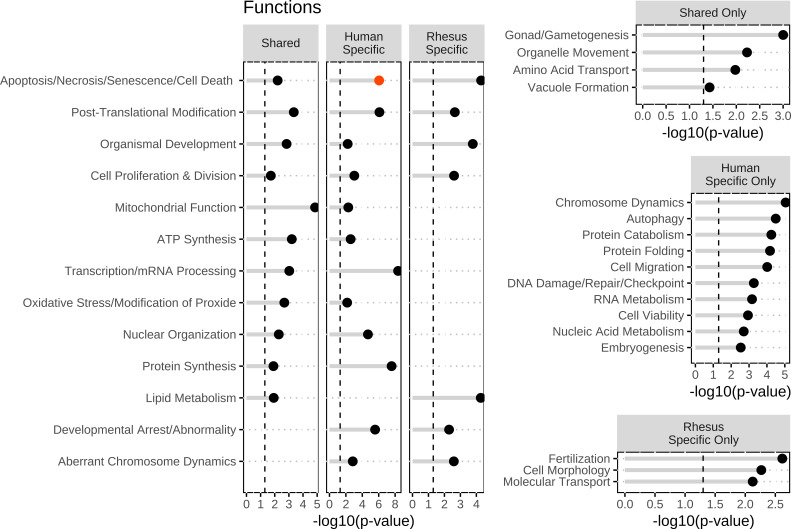
To identify affected functions corresponding to human-specific DEGs, we first examined pathways, upstream regulators, and biological functions associated with the human-specific set of DEGs (Figs. 1, 2, and 3). IPA revealed 98 affected canonical pathways, 122 affected upstream regulators, and 34 affected aggregate biofunctions. For the CP analysis, OxPhos, PTEN signaling, and Semaphorin Neuronal Repulsive Signaling Pathway displayed significant z-scores indicating activation and BMP signaling and Spliceosomal Cycle displayed inhibition (Supplemental Table S7; Fig. 1). Additional significant effects were also observed on mitochondrial dysfunction, nucleotide excision repair, assembly of RNA polymerase II complex, protein ubiquitination, endoplasmic reticulum (ER) stress, G2/M DNA checkpoint regulation, and estrogen receptor signaling, among others (Supplemental Table S7; Fig. 1). For UR analysis, there was significant inhibition or activation predicted for 6 and 8 URs, respectively, including most prominently RICTOR, TBX2, TP53, and ABCB1B. Additional significant effects (but no z-score) for HNF4A, as well as 2 URs with increased (XBP1, CREB3) or decreased (KDM5A, RASSF5) expression were shown (Supplemental Table S8; Fig. 2); these four affected URs have functions related to endoplasmic reticulum stress, chromatin binding, and growth and differentiation. Increased activation states were predicted for 4 URs. Two oocyte-expressed miRNAs (miR-376a-5p and miR-210-3p) and two mature miRNAs (let-7 and mir-210) were found to be significant, although none had significant z-scores. The most prominent affected IPA functions included two with predicted increased activity (protein synthesis, quantity of gonad), and many additional significantly affected functions, including mRNA splicing and processing and protein ubiquitination (slightly negative z-scores) (Supplemental Table S9), and numerous functions related to chromosome dynamics, protein folding, embryogenesis and developmental arrest, nucleic acid metabolism, autophagy, and DNA repair (Fig. 3). Notably, affected functions also included several entries related to gonad function, oogenesis, and ovarian folliculogenesis (Supplemental Table S9).
Figure 1.
Top canonical pathways identified within the shared DEGs, human-specific DEGs, and rhesus-specific DEGs groups. Canonical pathways were identified in ingenuity pathway analysis and then were graphed in R. Significance was set at a −log10(P value) of 1.3 or greater as indicated by the vertical dashed lines and all entries were found to be significant. The red dots indicate activation of the canonical pathway with a z > 1.96. The blue dots indicated inhibition of the canonical pathway with a z < −1.96. The black dots indicate no significant z-score to determine directionally of the canonical pathways. DEGs, differentially expressed genes.
Figure 2.
Top upstream regulators identified within all three groups (shared, human specific, and rhesus specific), in at least two groups, human-specific only or rhesus-specific DEGs groups. Upstream regulators (URs) were identified in ingenuity pathway analysis and then graphed in R. URs were classified according to functions. Significance was set at a −log10(P value) of 1.3 or greater as indicated by the vertical dashed lines and all entries were found to be significant. The red dots indicate activation of the UR with a z > 1.96. The blue dots indicated inhibition of the UR with a z < −1.96. The black dots indicate no significant z-score to determine directionally of the URs. Solid symbols denote URs for which mRNA expression was detected in the RNAseq data, and open symbols denote URs for which mRNA expression was not detected. DEGs, differentially expressed genes.
Figure 3.
Top biological functions identified within all three groups (shared, human specific, and rhesus specific), in at least two groups, human-specific only, rhesus-specific, and shared-specific DEGs groups. Biological functions were identified in ingenuity pathway analysis and then were graphed in R. Significance was set at a −log10(P value) of 1.3 or greater as indicated by the vertical dashed lines and all entries were found to be significant. The red dots indicate activation of the functions with a z > 1.96. The dots indicate inhibition of the functions with a z < −1.96. The black dots indicate no significant z-score to determine directionally of the functions. DEGs, differentially expressed genes.
Ingenuity Pathway Analysis of Species-Specific DEGs Observed between Rhesus Monkey FTM and MII Oocytes (“Rhesus-Specific DEGs”)
Next, we applied IPA to the rhesus monkey-specific DEGs. IPA revealed 27 affected CPs, 215 affected URs, and 19 affected aggregate biofunctions. For CP analysis (Supplemental Table S10), OxPhos was significant (no activation score) as above with the human-specific DEGs. Significant effects were again seen for mitochondrial dysfunction, IL-8 signaling (inhibited), and protein kinase A signaling (Supplemental Table S10; Fig. 1). For UR analysis, we saw predicted activation for STK11IP and RB1 and inhibition of GNRH and FOXL2 (Supplemental Table S11; Fig. 2). One UR had increased in expression and four were reduced in expression (Supplemental Table S11). These affected URs contribute to steroid and hormone biogenesis (HSD11B2) and signaling and inflammatory response (GNB2, TGFB1I1, TSC22D3, and STK11IP), among other functions. Two oocyte-expressed miRNAs (miR-199a-5p and miR-26a-5p) and two mature miRNAs (mir-143 and mir-19) were found to be significant within the rhesus-specific DEGs, but none with significant z-scores. The most prominent affected biofunctions were related to cell viability, transcription, molecular transport, cell contact, protein catabolism, and fertilization (Supplemental Table S12; Fig. 3).
Overlap of IPA Results for Species-Specific and Shared DEGs
Assessing the degree of overlap between human and rhesus monkey DEGs associated with oocyte maturation failure is complicated by the possibility that while DEG lists may have limited overlap, there may nevertheless be commonality in affected pathways and functions. Applying IPA to identify and understand such commonalities is a powerful means of addressing this possibility. We therefore used IPA to assess shared impacts of species-specific DEGs, shared DEGs, and the complete DEG lists for the two species.
Examining the overlap between the IPA results obtained for the two species-specific DEG sets highlighted eight CPs, including OxPhos, mitochondrial dysfunction, protein ubiquitination, kinetochore metaphase signaling, and androgen signaling (Supplemental Tables S7 and S10; Fig. 1). Overlap in the IPA UR results for species-specific DEGs indicated 6 relevant URs, including three transcription regulators (Supplemental Tables S8 and S11; Fig. 2). In addition, we identified seven common aggregate biofunctions (Supplemental Tables S9 and S12; Fig. 3) that included effects on apoptosis, posttranslational modification, organismal development, cell proliferation, lipid metabolism, developmental arrest, and aberrant chromosome dynamics (Supplemental Tables S9 and S12; Fig. 3). Thus, there was a significant amount of overlap in the pathways and processes associated with the species-specific DEGs.
IPA of the 65 shared DEGs revealed effects on OxPhos (predicted activation, z = 2.449), mitochondrial dysfunction, sirtuin signaling, and protein ubiquitination pathway (Supplemental Table S13; Fig. 1). UR analysis for the shared DEGs revealed effects on 33 URs, including one with predicted inhibition (RICTOR) and two with predicted activation (CAB39L and INSR). These URs regulate numerous genes encoding mitochondrial proteins, mRNAs for which have increased abundance in FTM oocytes of both species. Additional strong effects (no significant z-score) included HNF4A, with the largest number of affected downstream target DEGs (Supplemental Table S14). Additional URs predicted to be significantly affected are related to metabolism (HNF4A, PPARGC1A, and PRKN). No miRNAs were identified as significant URs for the shared human and rhesus DEGs. Among the 77 affected IPA functions (Supplemental Table S15), top entries included numerous results related to assembly of respiratory chain complex I. Additional aggregate biofunction categories included mitochondrial function, mitochondrial dysfunction, lipid metabolism, posttranslational modification, protein synthesis, nuclear organization, DNA repair/breakage of chromosomes, and cell migration (Supplemental Table S15; Fig. 3). Consistent with the mitochondrial effects, the abundances of mRNAs encoding mitochondrial proteins were increased in FTM oocytes of both species. Although the specific affected genes in this category differed in part, there were seven shared between the two species. The observations for the shared genes overlap significantly with the results revealed for overlap for the two species-specific DEG sets (Figs. 1–3).
Next, we compared IPA results for all human DEGs (Supplemental Table S2) to all rhesus monkey DEGs (Supplemental Table S3) to further assess common effects associated with FTM oocytes and taking advantage of attendant increased statistical power. FTM oocytes for both human and monkey displayed predicted activation states for OxPhos and significant P values for NER pathway (significantly activated in rhesus monkey) and mitochondrial dysfunction (Supplemental Table S16). Human and rhesus shared 4 activated URs (CAB39L, INSR, HBA1/HBA2, and Hbb-b1) and 2 inhibited (RICTOR and KDM5A) related to OxPhos and mitochondrial function. There were an additional 23 URs populated in both data sets (Supplemental Table S17), and these were associated with overlapping aggregate biofunctions (n = 17), including DNA damage/repair, ATP synthesis, mRNA processing, and posttranscriptional modification (Supplemental Table S18). These results confirm observation above for shared and species-specific DEGs.
In our previous rhesus monkey study, we used RNA-Seq data from GV, FTM, and MIIs to determine how the FTM gene expression profiles changed in relation to normal maturation. We did this by classifying DEGs into categories A (FTMs look similar to normal MIIs), B (FTMs showed partial modulation toward the MII phenotype), C (FTMs looked similar GVs), and F/G (FTMs showed aberrant profiles when compared with normal maturation) [Ruebel et al. (7)]. We identified which shared DEGs overlapped with the DEGs from the rhesus monkey DEG categories A, B, C, and F/G (data not shown). The largest amount of overlap was seen for categories B and C (13.8% and 16.9% of interspecies overlapping DEGs, respectively). We note that both B and C categories from the earlier rhesus monkey study were associated with significant predicted effects on mitochondrial dysfunction and OxPhos and predicted effects on RICTOR, KDM5A, and HNF4A activity. Additional effects were seen in the previous rhesus monkey study on protein ubiquitination (category B) and sirtuin signaling (category C). Both B and C overlapping IPA results were also predictive of effects on the activities of RICTOR (z = −3.0 and 3.13, respectively), KDM5A (z = 2.45, −2.24), and HNF4A (P = 1.70, 2.58). We observed little overlap with categories A and F/G.
To further explore the relationship between the transcriptome differences observed for FTM oocytes and normal meiotic maturation progression, we divided the shared FTM versus MII DEGs into two groups FTM > MII (n = 48) and MII > FTM (n = 17) and then compared these to lists of mRNAs in three different stability classes defined based on rhesus monkey GV and MII stage oocyte mRNA abundances (rhesus was used due to unavailability of normal human GV data). The three stability classes were highly degraded during maturation (GV > MII), moderately degraded (GV MII, and recognizing the background of massive maternal mRNA degradation during maturation), and stable (MII > GV). Importantly, of the 17 MII > FTM mRNAs, 14 are in the moderately degraded class (GV > MII), and one (SLC36A1) was highly degraded. Of the 48 FTM > MII DEGs, 25 are categorized as highly degraded and 23 as moderately degraded.
Expanded Network Analysis Using IPA
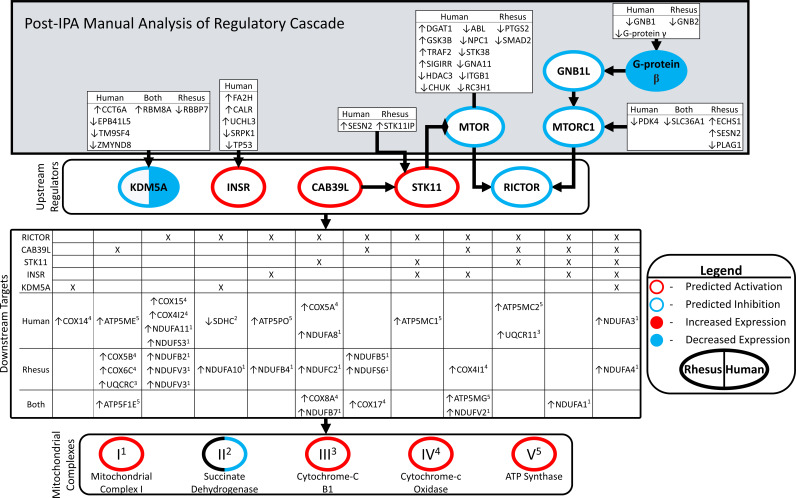
We performed an expanded network analysis to gain further insight into the mechanisms regulating OxPhos. This analysis revealed additional upstream and downstream network components including additional DEGs associated with regulating OxPhos (Fig. 4). The DEGs within the OxPhos pathway were annotated according to the species in which they were affected, the direction of expression difference in FTM oocytes, and in which complex of the electron of the transport chain they are contained. We identified four significantly affected URs (KDM5A, INSR, CAB39L, STK11, and RICTOR) that have downstream targets in the OxPhos pathway. We identified additional molecules upstream of these URs. These additional upstream molecules included other affected URs, such as MTOR, MTORC2, GNB1, and G-Protein beta, as well as other DEGs (white boxes in Fig. 4) that were seen in the human, rhesus, or both data sets. These results show that different upstream genes may be modulated in the two species but ultimately lead to a convergent effect on a cellular function such as OxPhos in both species. In Fig. 4, we show this effect reoccurring at multiple levels of the regulatory cascade.
Figure 4.
Linking upstream regulators and oxidative phosphorylation. Network diagram connecting upstream regulators, their upstream effectors, downstream targets in oxidative phosphorylation, and membership in mitochondrial complexes. Figure consists of four hierarchical blocked areas. “Upstream regulators” (KDM5A, INSR, CAB39L, and RICTOR) were significant in both human and rhesus, with significant z-scores. The overlap of their downstream targets, and the membership in either human/rhesus DEG lists “downstream targets.” Superscripts adjacent to DEG targets denote membership in mitochondrial complexes 1–4. “Post-IPA Manual Analysis of Regulatory Cascade”: IPA queried for molecules upstream of “upstream regulators” present in human/rhesus DEG lists. Border colors denote predicted significant z-scores: red = activated in FTM compared with MII, blue = inhibited, black = not significant z-score. Filled colors denote significant changes in expression from DEG analysis: red = upregulated in FTM compared with MII, blue = downregulated. Coloring on left relates to rhesus, right to human. Arrows preceding gene symbols denote direction of change in FTM compared with MII. DEGs, differentially expressed genes; FTM, failed-to-mature; MII, metaphase II; IPA, ingenuity pathway analysis.
DISCUSSION
This study revealed for the first-time commonality between human and rhesus monkey transcriptome alterations in FTM oocytes compared with in vivo matured MII oocytes. The most striking similarities include overexpression of nuclear-encoded mitochondrial protein mRNAs and associated predicted alterations in oxidative phosphorylation. This central feature of FTM oocytes emanates from an extensive network of affected DEGs, some shared, others species-specific, but all being linked to upstream regulators having predicted increases or decreases in activity in both species and directing alterations in activities at all five mitochondrial electron transport chain complexes. In addition to this central effect on mitochondrial function and oxidative phosphorylation, other shared effects were seen for major cellular processes including lipid metabolism, transcription, DNA repair, and cell viability. RICTOR and HNFA emerged as key predicted prominent affected upstream regulators comparing both species-specific and shared DEG sets. These results thus identify a common set of molecular alterations indicative of cellular alterations in FTM oocytes.
The question arises whether such maternal mRNA transcriptome alterations linked to mitochondrial function or other cellular functions are causative or symptomatic of oocyte maturation failure. Aberrant transcriptional production, accumulation, and regulation of maternal mRNAs in FTM oocytes could deprive the maturing oocyte of essential proteins at crucial times during maturation or drive aberrantly high activities that impact cell physiology and thus play a causal role in the arrest. Distinct cohorts of maternal mRNAs are regulated with different temporal patterns (33–35), and this involves a complex system of RNA binding proteins, siRNAs, translational regulators, RNA editing, and mediators of mRNA decay (36–43). Because of this, dysregulation of the maternal mRNA population would lead to a complex and disastrous loss of coordination between these mRNA cohorts, leading to chaotic disruptions in diverse cellular processes. Alternatively, aberrant control of the oocyte transcriptome could merely be a symptom of maturation failure, with a specific disruption of mRNA translation-coupled degradation normally linked to progression through meiosis and impacting specific functions (33). One way to distinguish between the possibilities is to assess the temporal patterns of aberrant mRNA regulation in FTM oocytes. In our previous study of FTM monkey oocytes, we observed mRNAs that displayed normal, partial, or complete inhibition of the maturation-associated change in abundance, and some mRNAs that displayed completely aberrant regulation (7). Principal component analysis of the monkey GV, MII, and FTM oocyte transcriptomes, indicated that FTM samples were variably distributed at an intermediate position between MII and GV stage oocytes or were more similar to MII than GV stage. This indicated that the transcriptome changes in FTM oocytes are not easily explained by a simple meiotic arrest that either stalls or delays normal mRNA regulation, that is, “freezing” the FTM oocytes at a discrete point in the normal maturation progression. Instead, our analysis indicates that the transcriptome alterations reflect a more complex disruption in mRNA regulation. That analysis in the rhesus monkey accomplished what cannot be easily done in the human, given typical IVF cycles precluding the collection of GV stage oocytes not subjected to an in vivo ovulatory stimulus. However, comparisons of the human FTM oocyte DEGs to the patterns of regulation seen in the monkey revealed a similarly complex mode of transcriptome disruption, most notably shared effects in category C genes (those with little change from GV stage) impacting mitochondrial function and oxidative phosphorylation, fundamental, and heritable features of cell phenotype with the potential to impact later development. Additionally, defects in cell cycle progression were not a prominent feature of FTM oocytes. Interestingly, the majority of the shared MII > FTM DEGs were in the moderately degraded class and four were in the stable class, and conversely, 25 of the 48 FTM > MII DEGs were in the highly degraded class. These changes may reflect a diminishment in the normal large-scale temporal degradation of maternal mRNAs in the FTM oocytes, which would tend to increase the apparent abundance of highly degraded mRNAs (seen as FTM > MII) and reduce the apparent abundances of more stable mRNAs (seen as MII > FTM) relative to the overall mRNA background population. Collectively, these observations are consistent with widespread aberrations in the regulation of mRNAs in a manner not apparently associated with a simple meiotic blockade. Thus, the disruptions in maternal mRNA handling appear to be more complex. The affected pathways and processes could negatively impact oocyte physiology, as well as impacting later embryo quality. Additionally, some of the DEGs observed for FTM oocytes encode key transcription regulators that could be essential for correct gene regulation after fertilization. As a result, the safety of applying in vitro maturation to human FTM oocytes to acquire matured oocytes for use in ART bears further examination.
We are unable to ascertain from these data whether shared mRNAs displayed reduced initial levels of accumulation before maturation. The human data sets do not include normal GV stage oocytes, and an insufficient number of rhesus monkey GV stage oocytes were analyzed to allow a statistically valid assessment of whether a population of GV stage oocytes displays disruptions in gene expression predictive of later maturation failure. However, we note a number of the upstream regulators identified by IPA are transcription regulators. This raises the possibility that some of the aberrations seen in the FTM oocyte transcriptome emerge from altered gene transcription and mRNA accumulation.
Many nuclear-encoded mitochondrial protein mRNAs decline in abundance during maturation but fail to do so in the FTM oocytes (7). Such mRNAs could also be expressed more highly before maturation. The profound effects observed for mitochondrial function associated with increased expression of nuclear genes encoding mitochondrial proteins, particularly components of complex I, suggest that correct down-modulation of electron transport chain is a key feature of high-quality oocytes. This modulation likely reduces the propensity for production of ROS (produced primarily at complex I) in healthy oocytes, likely an adaptive response to avoid damaging the oocyte and early-stage embryos, which have reduced abilities to eliminate ROS compared with somatic cells (13). A large portion of these nuclear-encoded mitochondria proteins are NDUF proteins that can be found in complex I of the electron transport chain, as well as biological functions related to the respiratory chain, were found in both species. Complex I is important for the conversion of NADH to NAD and to the release of hydrogen ions. This release of hydrogen ions can further lead to increased free radicals, which advances to ROS production and is detrimental to oocyte quality and early embryo development. During oogenesis, the mitochondria in the oocyte are classified as “underdeveloped” because they are small and spherical, have underdeveloped cristae, and lower ATP production (8–10). If mitochondrial activity remains aberrantly high in FTM oocytes, this could lead to increased OxPhos and possible elevated ROS levels. Additional studies would be needed to assess mitochondria function of these FTM oocytes.
RICTOR activity was predicted to be inhibited in FTM oocytes across both species-specific DEG sets and for the shared DEG set, and in animal models, it has been associated with proper ovarian function, with a possible role in oocyte physiology. RICTOR acts with mTOR in the mTORC2 complex to regulate cell growth and survival in response to hormonal stimulation, including insulin, growth factors, and hormones signaling through the PI3 Kinase, promoting metabolism, cytoskeletal rearrangement, and cell survival (44). mTORC2 function is sensitive to HIPPO pathway signaling (45), which in turn, is sensitive to p38/MAPK signaling (46), which is also altered in both human and monkey FTM oocytes. Reduced mTORC2 activity could impede AKT and mTORC1 signaling, thereby compromising downstream metabolic regulation. Mice with oocyte-specific depletion of RICTOR display compromised fertility and attribute to deficient prosurvival signaling (47). Interestingly, reduced mTOR signaling can enhance viability when ER stress response is activated (48).
In that sense, we also observed effects on signaling pathways and functions related to endoplasmic reticulum (ER) stress and unfolded protein response genes in the FTM oocytes of both species. The ER stress mediator XBP1 was increased in expression in the human but not monkey FTM oocytes. Previous studies found a connection between ER stress and oocyte maturation, and ER stress inhibitors can improve oocyte maturation and reduce apoptosis (49). ER stress may serve as a sensitive global sensor of different forms of stress in oocytes and embryos (50, 51), and its interaction with the RICTOR/mTOR pathway might represent a determinant for a correct oocyte maturation.
We also observed predicted activation of the regulator serine/threonine kinase 11 (STK11, a.k.a. LKB1) in human and STK11-interacting protein (a.k.a. LIP) in rhesus monkey FTM oocytes. STK11 has diverse roles in the cell, regulating metabolism via AMP kinase, as well as regulating energy homeostasis, RhoA, PTEN, mTOR, SMAD4, and TGFβ signaling with effects on other processes such as nutrient sensing, cell polarity, apoptosis, gap junction communication, cell proliferation and mitosis, and many other functions (52–60). STK11 activity can also impact mitochondrial membrane potential (61). Additionally, CAB39 interacts with STRADA–STK11 complex to anchor LKB1 in the cytoplasm and stimulate its activity (62); another affected UR, CAB39L interacts with STK11 in a similar manner (62). With these diverse actions, STK11 and STK11IP can work through the mTOR pathway to affect OxPhos but may also affect oocyte maturation through other mechanisms, for example, via effects on gap junction communication (63) and cell cycle progression. Interestingly, STK11 deficiency in mice disrupts normal control of follicular maturation, leading to premature ovarian failure due to accelerated recruitment of primordial follicles that then fail to mature and be ovulated (64). Our results indicate an additional possible role for STK11 in the oocyte, with increased activity possibly impacting female fertility by interfering with oocyte maturation.
Our expanded network analysis applied to the two species provides new insight into evolutionary conservation of mechanisms controlling oocyte maturation. IPA initially identified a common set of upstream regulators (KDM5A, RICTOR, INSR, CAB39L, and STK11) controlling OxPhos, a key target of dysregulation in the FTM oocytes. By expanding this network using the IPA custom pathway utility, we identified several additional shared regulators upstream of those initial URs, and yet additional DEGs functioning upstream of those regulators. Strikingly, we found that the uppermost tier of DEGs displayed only partial overlap between the two species. This indicates that differences in the upstream regulatory cascades exist in the two species, even though they ultimately affect a shared biological function (OxPhos). This raises the interesting possibility that groups of mRNAs related to one another by encoding proteins in an extended network may be regulated in the oocyte as a functional unit. The species difference that we observed at the upper tiers in the expanded network suggests possible divergence in the cis-regulatory elements responsible for regulating specific mRNA stability, translation, and degradation in the oocyte. Further research is needed to better understand how coordinated posttranscriptional mRNA regulation is achieved in oocytes and how groups of coregulated target mRNAs can diverge between species.
We also observed effects on the estrogen signaling pathway. Disruption of estrogen receptors, specially ESR2, leads to failure of ovulation and altered oocyte maturation (65). The estradiol–estrogen receptor system is important for maintaining oocyte meiotic arrest (66). We observed a significant increase in steroid receptor RNA activator 1 (SRA1) in the human FTM oocytes compared with normal MII oocytes. The UR analyses also identified estrogen-related receptor alpha (ESRRA) to be significantly activated in the FTM oocytes (z = 2.18). Recent studies showed that ESRRA can regulate transcription of genes related to mitochondrial function, autophagy, and metabolism (67, 68).
The above results reveal significant defects in the normal progression of changes related to cytoplasmic maturation in these FTM oocytes of both species. Effects related to cell viability, DNA repair, chromosome, and nuclear organization indicate additional aberrant control of nuclear maturation events as well. One of the most striking genes affected with a high potential to compromise genome function is KDM5A, which is predicted to have reduced activity in the shared DEG list. Although histone methylation is important for oocyte transcriptional silencing (69, 70), KDM5A (a.k.a. RBBP2, Jumonji, a Histone H3K4 demethylase) has emerged recently as a key factor required for the control of embryonic genome activation by restricting histone methylation to specific sites (71–73). Histone demethylases also support enhanced nuclear reprogramming for the development of cloned embryos (74). Additionally, Drosophila KDM5 plays a key role in preparing the oocyte genome for functions during late prophase 1 (75). H3K4 demethylation can also impact spindle formation and spindle checkpoint (76). The reduced activity predicted for KDM5A, combined with the deficient expression of DNMTs, indicates that insufficient epigenetic modification is associated with oocyte maturation failure.
Overall, it is clear that aberrant mRNA expression in FTM oocytes likely impacts many key cellular processes and functions. Our results do not reveal which of these effects are causal or which are consequences of maturation failure. However, our results provide new insight into oocyte maturation pathways and suggest specific molecular networks for further mechanistic study. In particular, our results indicate that the correct regulation of nuclear-encoded mitochondrial genes and attendant mitochondrial activity is an important part of oocyte development and that deficiencies in this regulation are a hallmark of oocyte maturation failure. We note that the regulation of these mitochondrial genes and mitochondrial activity is complex and that overall outcome of that regulation likely reflects the sum of multiple impinging signals impacting the oocyte. Genetic, epigenetic, nutritional, and other factors may thus constitute key upstream factors that lead to the defects observed here. We further note that oocyte maturation failure does not impact entire cohorts of oocytes from many females. This suggests heterogeneity among the follicular population with respect to success of the oocytes to undergo appropriate epigenetic changes. We believe this discovery that down-modulation of nuclear-encoded mitochondrial genes is associated with high oocyte quality and oocyte maturation competence provides an important new focus for mechanistic enquiry, for integrating diverse data relating maternal health to fertility, and for identifying therapeutic interventions to enhance fertility.
GRANTS
This work was supported in part by intramural funding from Clinica EUGIN and from Michigan State University and Michigan State University AgBioResearch. Previously published RNAseq data for rhesus monkey oocytes were generated under awards from the National Institutes of Health Office of Research Infrastructure Programs Division of Comparative Medicine [OD012221 to K.E.L., OD011107/RR00169 (California National Primate Research Center) and OD010967/RR025880 to C.A.V.]. Research training for P. Z. Schall and M. L. Ruebel was provided in part by a training grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under the Award Number T32HD087166.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L.R., R.V., and K.E.L. conceived and designed research; C.A.V. and K.E.L. performed experiments; M.L.R., F.Z., P.Z.S., M.B., C.A.V., and R.V. analyzed data; M.L.R., F.Z., P.Z.S., M.B., C.A.V., R.V., and K.E.L. interpreted results of experiments; M.L.R., F.Z., P.Z.S., M.B., and C.A.V. prepared figures; M.L.R., F.Z., P.Z.S., M.B., C.A.V., and K.E.L. drafted manuscript; M.L.R., F.Z., P.Z.S., M.B., C.A.V., R.V., and K.E.L. edited and revised manuscript; M.L.R., F.Z., P.Z.S., M.B., C.A.V., R.V., and K.E.L. approved final version of manuscript.
REFERENCES
- 1.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982-2010: data from the National Survey of Family Growth. Natl Health Stat Report 11: 1–18, 2013. [PubMed] [Google Scholar]
- 2.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 9: e1001356, 2012. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Practice Committee of the American Society for Reproductive M. Effectiveness and treatment for unexplained infertility. Fertil Steril 86: S111–114, 2006. doi: 10.1016/j.fertnstert.2006.07.1475. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande PS, Gupta AS. Causes and prevalence of factors causing infertility in a public health facility. J Hum Reprod Sci 12: 287–293., 2019. doi: 10.4103/jhrs.JHRS_140_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avrech OM, Goldman GA, Rufas O, Stein A, Amit S, Yoles I, Pinkas H, Fisch B. Treatment variables in relation to oocyte maturation: lessons from a clinical micromanipulation-assisted in vitro fertilization program. J Assist Reprod Genet 14: 337–342, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZQ, Ming TX, Nielsen HI. Maturation arrest of human oocytes at germinal vesicle stage. J Hum Reprod Sci 3: 153–157, 2010. doi: 10.4103/0974-1208.74161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruebel ML, Schall PZ, Midic U, Vincent KA, Goheen B, VandeVoort CA, Latham KE. Transcriptome analysis of rhesus monkey failed-to-mature oocytes: deficiencies in transcriptional regulation and cytoplasmic maturation of the oocyte mRNA population. Mol Hum Reprod 24: 478–494, 2018. doi: 10.1093/molehr/gay032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babayev E, Seli E. Oocyte mitochondrial function and reproduction. Curr Opin Obstet Gynecol 27: 175–181, 2015. doi: 10.1097/GCO.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction 128: 269–280, 2004. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- 10.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 11: 797–813, 2011. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Cecchino GN, Seli E, Alves da Motta EL, Garcia-Velasco JA. The role of mitochondrial activity in female fertility and assisted reproductive technologies: overview and current insights. Reprod Biomed Online 36: 686–697, 2018. doi: 10.1016/j.rbmo.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Hansen JM. Oxidative stress as a mechanism of teratogenesis. Birth Defects Res C Embryo Today 78: 293–307, 2006. doi: 10.1002/bdrc.20085. [DOI] [PubMed] [Google Scholar]
- 13.Leese HJ, Guerif F, Allgar V, Brison DR, Lundin K, Sturmey RG. Biological optimization, the Goldilocks principle, and how much is lagom in the preimplantation embryo. Mol Reprod Dev 83: 748–754, 2016. doi: 10.1002/mrd.22684. [DOI] [PubMed] [Google Scholar]
- 14.VandeVoort CA, Mtango NR, Midic U, Latham KE. Disruptions in follicle cell functions in the ovaries of rhesus monkeys during summer. Physiol Genomics 47: 102–112, 2015. doi: 10.1152/physiolgenomics.00092.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34: i884–i890, 2018. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howe KL, Contreras-Moreira B, De Silva N, Maslen G, Akanni W, Allen J, et al. Ensembl genomes 2020-enabling non-vertebrate genomic research. Nucleic Acids Res 48: D689–D695, 2020. doi: 10.1093/nar/gkz890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34: 525–527, 2016. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 18.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinsella RJ, Kahari A, Haider S, Zamora J, Proctor G, Spudich G, Almeida-King J, Staines D, Derwent P, Kerhornou A, Kersey P, Flicek P. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database (Oxford) 2011: bar030, 2011. doi: 10.1093/database/bar030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barragan M, Pons J, Ferrer-Vaquer A, Cornet-Bartolome D, Schweitzer A, Hubbard J, Auer H, Rodolosse A, Vassena R. The transcriptome of human oocytes is related to age and ovarian reserve. Mol Hum Reprod 23: 535–548, 2017. doi: 10.1093/molehr/gax033. [DOI] [PubMed] [Google Scholar]
- 21.Blazquez A, Guillen JJ, Colome C, Coll O, Vassena R, Vernaeve V. Empty follicle syndrome prevalence and management in oocyte donors. Hum Reprod 29: 2221–2227, 2014. doi: 10.1093/humrep/deu203. [DOI] [PubMed] [Google Scholar]
- 22.Olivennes F, Fanchin R, Bouchard P, Taieb J, Selva J, Frydman R. Scheduled administration of a gonadotrophin-releasing hormone antagonist (Cetrorelix) on day 8 of in-vitro fertilization cycles: a pilot study. Hum Reprod 10: 1382–1386, 1995. [PubMed] [Google Scholar]
- 23.Gonzalez-Roca E, Garcia-Albeniz X, Rodriguez-Mulero S, Gomis RR, Kornacker K, Auer H. Accurate expression profiling of very small cell populations. PLoS One 5: e14418, 2010. doi: 10.1371/journal.pone.0014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vassena R, Boue S, Gonzalez-Roca E, Aran B, Auer H, Veiga A, Izpisua Belmonte JC. Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development. Development 138: 3699–3709, 2011. doi: 10.1242/dev.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendrickson PG, Dorais JA, Grow EJ, Whiddon JL, Lim JW, Wike CL, Weaver BD, Pflueger C, Emery BR, Wilcox AL, Nix DA, Peterson CM, Tapscott SJ, Carrell DT, Cairns BR. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat Genet 49: 925–934, 2017. doi: 10.1038/ng.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes JM, Silva E, Chitwood JL, Schoolcraft WB, Krisher RL, Ross PJ. Differing molecular response of young and advanced maternal age human oocytes to IVM. Hum Reprod 32: 2199–2208, 2017. doi: 10.1093/humrep/dex284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rau A, Marot G, Jaffrezic F. Differential meta-analysis of RNA-seq data from multiple studies. BMC Bioinformatics 15: 91, 2014. doi: 10.1186/1471-2105-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y, Milon B, Ott S, Zhao X, Sadzewicz L, Shetty A, Boger ET, Tallon LJ, Morell RJ, Mahurkar A, Hertzano R. A comparative analysis of library prep approaches for sequencing low input translatome samples. BMC Genomics 19: 696, 2018. doi: 10.1186/s12864-018-5066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders S, Reyes A, Huber W. Detecting differential usage of exons from RNA-seq data. Genome Res 22: 2008–2017, 2012. doi: 10.1101/gr.133744.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4: 1521, 2015. doi: 10.12688/f1000research.7563.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vitting-Seerup K, Sandelin A. The landscape of isoform switches in human cancers. Mol Cancer Res 15: 1206–1220, 2017. doi: 10.1158/1541-7786.MCR-16-0459. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Melton C, Suh N, Oh JS, Horner K, Xie F, Sette C, Blelloch R, Conti M. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev 25: 755–766, 2011. doi: 10.1101/gad.2028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mtango NR, Potireddy S, Latham KE. Oocyte quality and maternal control of development. Int Rev Cell Mol Biol 268: 223–290, 2008. doi: 10.1016/S1937-6448(08)00807-1. [DOI] [PubMed] [Google Scholar]
- 35.Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol 302: 104–117, 2007. doi: 10.1016/j.ydbio.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brachova P, Alvarez NS, Hong X, Gunewardena S, Vincent KA, Latham KE, Christenson LK. Inosine RNA modifications are enriched at the codon wobble position in mouse oocytes and eggs dagger. Biol Reprod 101: 938–949, 2019. doi: 10.1093/biolre/ioz130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma J, Flemr M, Stein P, Berninger P, Malik R, Zavolan M, Svoboda P, Schultz RM. MicroRNA activity is suppressed in mouse oocytes. Curr Biol 20: 265–270, 2010. doi: 10.1016/j.cub.2009.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rong Y, Ji SY, Zhu YZ, Wu YW, Shen L, Fan HY. ZAR1 and ZAR2 are required for oocyte meiotic maturation by regulating the maternal transcriptome and mRNA translational activation. Nucleic Acids Res 47: 11387–11402, 2019. doi: 10.1093/nar/gkz863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein P, Rozhkov NV, Li F, Cárdenas FL, Davydenko O, Davydenk O, Vandivier LE, Gregory BD, Hannon GJ, Schultz RM. Essential role for endogenous siRNAs during meiosis in mouse oocytes. PLoS Genet 11: e1005013, 2015. doi: 10.1371/journal.pgen.1005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svoboda P, Franke V, Schultz RM. Sculpting the transcriptome during the oocyte-to-embryo transition in mouse. Curr Top Dev Biol 113: 305–349, 2015. doi: 10.1016/bs.ctdb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Yu C, Ji SY, Sha QQ, Dang Y, Zhou JJ, Zhang YL, Liu Y, Wang ZW, Hu B, Sun QY, Sun SC, Tang F, Fan HY. BTG4 is a meiotic cell cycle-coupled maternal-zygotic-transition licensing factor in oocytes. Nat Struct Mol Biol 23: 387–394, 2016. doi: 10.1038/nsmb.3204. [DOI] [PubMed] [Google Scholar]
- 42.Zhang JM, Hou WB, Du JW, Zong M, Zheng KL, Wang WJ, Wang JQ, Zhang H, Mu YS, Yin Z, Ding CM, Sun QY, Liu ZH, Kong QR. Argonaute 2 is a key regulator of maternal mRNA degradation in mouse early embryos. Cell Death Discov 6: 133, 2020. doi: 10.1038/s41420-020-00368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao LW, Zhu YZ, Chen H, Wu YW, Pi SB, Chen L, Shen L, Fan HY. PABPN1L mediates cytoplasmic mRNA decay as a placeholder during the maternal-to-zygotic transition. EMBO Rep 21: e49956, 2020. doi: 10.15252/embr.201949956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients 9: 1176, 2017. doi: 10.3390/nu9111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Artinian N, Cloninger C, Holmes B, Benavides-Serrato A, Bashir T, Gera J. Phosphorylation of the hippo pathway component AMOTL2 by the mTORC2 kinase promotes YAP signaling, resulting in enhanced glioblastoma growth and invasiveness. J Biol Chem 290: 19387–19401, 2015. doi: 10.1074/jbc.M115.656587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang D, Li X, Sun L, Huang P, Ying H, Wang H, Wu J, Song H. Regulation of Hippo signalling by p38 signalling. J Mol Cell Biol 8: 328–337, 2016. doi: 10.1093/jmcb/mjw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z, Kang X, Wang L, Dong H, Wang C, Xiong Z, Zhao W, Jia C, Lin J, Zhang W, Yuan W, Zhong M, Du H, Bai X. Rictor/mTORC2 pathway in oocytes regulates folliculogenesis, and its inactivation causes premature ovarian failure. J Biol Chem 290: 6387–6396, 2015. doi: 10.1074/jbc.M114.605261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapuy O, Vinod PK, Banhegyi G. mTOR inhibition increases cell viability via autophagy induction during endoplasmic reticulum stress—an experimental and modeling study. FEBS Open Bio 4: 704–713, 2014. doi: 10.1016/j.fob.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin T, Lee JE, Kang JW, Shin HY, Lee JB, Jin DI. Endoplasmic reticulum (ER) stress and unfolded protein response (UPR) in mammalian oocyte maturation and preimplantation embryo development. Int J Mol Sci 20: 409, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Latham KE. Endoplasmic reticulum stress signaling in mammalian oocytes and embryos: life in balance. Int Rev Cell Mol Biol 316: 227–265, 2015. doi: 10.1016/bs.ircmb.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latham KE. Stress signaling in mammalian oocytes and embryos: a basis for intervention and improvement of outcomes. Cell Tissue Res 363: 159–167, 2016. doi: 10.1007/s00441-015-2124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116: 457–466, 2004. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 53.Forcet C, Etienne-Manneville S, Gaude H, Fournier L, Debilly S, Salmi M, Baas A, Olschwang S, Clevers H, Billaud M. Functional analysis of Peutz-Jeghers mutations reveals that the LKB1 C-terminal region exerts a crucial role in regulating both the AMPK pathway and the cell polarity. Hum Mol Genet 14: 1283–1292, 2005. doi: 10.1093/hmg/ddi139. [DOI] [PubMed] [Google Scholar]
- 54.Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, Fletcher-Sananikone E, Colla S, Wang YA, Chin L, Depinho RA. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 468: 701–704, 2010. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, Tzatsos A, Ozsolak F, Milos P, Ferrari F, Park PJ, Shirihai OS, Scadden DT, Bardeesy N. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 468: 659–663, 2010. [Erratum in Nature 476: 114, 2011]. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karuman P, Gozani O, Odze RD, Zhou XC, Zhu H, Shaw R, Brien TP, Bozzuto CD, Ooi D, Cantley LC, Yuan J. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell 7: 1307–1319, 2001. doi: 10.1016/S1097-2765(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 57.Mehenni H, Lin-Marq N, Buchet-Poyau K, Reymond A, Collart MA, Picard D, Antonarakis SE. LKB1 interacts with and phosphorylates PTEN: a functional link between two proteins involved in cancer predisposing syndromes. Hum Mol Genet 14: 2209–2219, 2005. doi: 10.1093/hmg/ddi225. [DOI] [PubMed] [Google Scholar]
- 58.Moren A, Raja E, Heldin CH, Moustakas A. Negative regulation of TGFbeta signaling by the kinase LKB1 and the scaffolding protein LIP1. J Biol Chem 286: 341–353, 2011. doi: 10.1074/jbc.M110.190660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanchez-Garrido J, Shenoy AR. Regulation and repurposing of nutrient sensing and autophagy in innate immunity. Autophagy: 1–21, 2020. doi: 10.1080/15548627.2020.1783119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu X, Jin D, Durgan J, Hall A. LKB1 controls human bronchial epithelial morphogenesis through p114RhoGEF-dependent RhoA activation. Mol Cell Biol 33: 2671–2682, 2013. doi: 10.1128/MCB.00154-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468: 653–658, 2010. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J 22: 5102–5114, 2003. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ozcan C, Battaglia E, Young R, Suzuki G. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J Am Heart Assoc 4: e001733, 2015. doi: 10.1161/JAHA.114.001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang ZZ, Hu MW, Ma XS, Schatten H, Fan HY, Wang ZB, Sun QY. LKB1 acts as a critical gatekeeper of ovarian primordial follicle pool. Oncotarget 7: 5738–5753, 2016. doi: 10.18632/oncotarget.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakravarthi VP, Ghosh S, Roy R, Dai E, Pathak D, Rumi MAK. Transcriptome datasets of gonadotropin-induced ESR2-regulated genes in rat oocytes. Data Brief 27: 104786, 2019. doi: 10.1016/j.dib.2019.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu W, Xin Q, Wang X, Wang S, Wang H, Zhang W, Yang Y, Zhang Y, Zhang Z, Wang C, Xu Y, Duan E, Xia G. Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals. Cell Death Dis 8: e2662, 2017. doi: 10.1038/cddis.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ranhotra HS. The orphan estrogen-related receptor alpha and metabolic regulation: new frontiers. J Recept Signal Transduct Res 35: 565–568, 2015. doi: 10.3109/10799893.2015.1024853. [DOI] [PubMed] [Google Scholar]
- 68.Tripathi M, Yen PM, Singh BK. Estrogen-related receptor alpha: an under-appreciated potential target for the treatment of metabolic diseases. Int J Mol Sci 21: 1645, 2020. doi: 10.3390/ijms21051645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dumdie JN, Cho K, Ramaiah M, Skarbrevik D, Mora-Castilla S, Stumpo DJ, Lykke-Andersen J, Laurent LC, Blackshear PJ, Wilkinson MF, Cook-Andersen H. Chromatin modification and global transcriptional silencing in the oocyte mediated by the mRNA decay activator ZFP36L2. Dev Cell 44: 392–402, 2018. doi: 10.1016/j.devcel.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang B, Zheng H, Huang B, Li W, Xiang Y, Peng X, Ming J, Wu X, Zhang Y, Xu Q, Liu W, Kou X, Zhao Y, He W, Li C, Chen B, Li Y, Wang Q, Ma J, Yin Q, Kee K, Meng A, Gao S, Xu F, Na J, Xie W. Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature 537: 553–557, 2016. doi: 10.1038/nature19361. [DOI] [PubMed] [Google Scholar]
- 71.Dahl JA, Jung I, Aanes H, Greggains GD, Manaf A, Lerdrup M, Li G, Kuan S, Li B, Lee AY, Preissl S, Jermstad I, Haugen MH, Suganthan R, Bjoras M, Hansen K, Dalen KT, Fedorcsak P, Ren B, Klungland A. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature 537: 548–552, 2016. doi: 10.1038/nature19360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sankar A, Kooistra SM, Gonzalez JM, Ohlsson C, Poutanen M, Helin K. Maternal expression of the histone demethylase Kdm4a is crucial for pre-implantation development. Development 144: 3264–3277, 2017. doi: 10.1242/dev.155473. [DOI] [PubMed] [Google Scholar]
- 73.Shao GB, Chen JC, Zhang LP, Huang P, Lu HY, Jin J, Gong AH, Sang JR. Dynamic patterns of histone H3 lysine 4 methyltransferases and demethylases during mouse preimplantation development. In Vitro Cell Dev Biol Animal 50: 603–613, 2014. doi: 10.1007/s11626-014-9741-6. [DOI] [PubMed] [Google Scholar]
- 74.Liu Z, Cai Y, Wang Y, Nie Y, Zhang C, Xu Y, Zhang X, Lu Y, Wang Z, Poo M, Sun Q. Cloning of macaque monkeys by somatic cell nuclear transfer. Cell 172: 881–887, 2018. doi: 10.1016/j.cell.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 75.Navarro-Costa P, McCarthy A, Prudencio P, Greer C, Guilgur LG, Becker JD, Secombe J, Rangan P, Martinho RG. Early programming of the oocyte epigenome temporally controls late prophase I transcription and chromatin remodelling. Nat Commun 7: 12331, 2016. doi: 10.1038/ncomms12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schibler A, Koutelou E, Tomida J, Wilson-Pham M, Wang L, Lu Y, Cabrera AP, Chosed RJ, Li W, Li B, Shi X, Wood RD, Dent SY. Histone H3K4 methylation regulates deactivation of the spindle assembly checkpoint through direct binding of Mad2. Genes Dev 30: 1187–1197, 2016. doi: 10.1101/gad.278887.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kramer A, Green J, Pollard J Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30: 523–530, 2014. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]