Abstract
Background:
We performed a meta-analysis to determine whether a consistent relationship exists between the use of angiotensin converting enzyme inhibitors (ACEIs) and the risk of lung cancer. Accordingly, we summarized and reviewed previously published quantitative studies.
Methods:
Eligible studies with reference lists published before June 1st, 2019 were obtained from searching several databases. Random effects’ models were used to summarize the overall estimate of the multivariate adjusted odds ratios (ORs) with 95% confidence intervals (CIs)
Results:
Thirteen observational studies involving 458,686 ACEI users were included in the analysis, Overall, pooled risk ratios indicate that ACEIs use was not a risk factor for lung cancer (RR 0.982, 95% C.I. 0.873 – 1.104; P = .76). There was significant heterogeneity between the studies (Q = 52.54; P < .001; I2 = 86.07). There was no significant association between ACEIs use and lung cancer in studies with over five years of ACEIs exposure (RR 0.95, 95% C.I. 0.75 – 1.20; P = .70); and ≤ 5years of exposure to ACEIs (RR 0.98, 95% C.I. 0.83 – 1.15; P = .77). There were no statistically significant differences in the pooled risk ratio obtained according to the study design (Q = 0.65; P = .723) and the comparator regimen (Q = 3.37; P = .19).
Conclusions:
The use of ACEIs was not associated with an increased risk of lung cancer. Nevertheless, well-designed observational studies with different ethnic populations are still needed to evaluate the long-term (over 10 years) association between ACEIs use and lung cancer.
Keywords: angiotensin converting enzyme inhibitors, association, lung cancer, meta-analysis, risk
1. Introduction
Angiotensin converting enzyme inhibitors (ACEIs) are crucial in the management of high blood pressure, other cardiovascular diseases and chronic renal failure.[1] ACEIs lowers the synthesis of angiotensin II (the active peptide of the renin-angiotensin system (RAS) that regulates blood pressure) decreasing its ability to bind to angiotensin receptor.[1] Therefore, its central mechanism of action operates around inhibiting the RAS.[1,2] Beyond its cardiovascular effects, the RAS plays a role in carcinogenesis through stimulating angiogenesis, inflammation and tissue proliferation.[3] Also, angiotensin receptors have been found to be upregulated in many cancer tissues (particularly breast, pancreatic and lung cancers) as well as increases the production of vascular endothelial growth factors.[4] Based on these observations, it has been postulated that pharmacotherapeutic RAS modulation, may play a role on the occurrence of specific malignancies.
A potential role for RAS in lung carcinogenesis was hypothesized by studies conducted over three decades ago which showed that individuals with lung malignancies had lower concentrations of circulating angiotensin converting enzyme (ACE).[5–7] A study conducted among 141 individuals newly-diagnosed with a primary pulmonary tumor showed that they had substantially lower serum ACE concentrations compared with healthy control subjects.[5] Other studies reported similar findings of low serum ACE levels in patients with lung cancer as compared to patients with other pulmonary diseases or carcinomas.[6,7] Furthermore, other studies found that ACE activity rises in individuals with bronchial carcinoma after radiation therapy or chemotherapy[4,6,8,9] as well as in patients in clinical remission.[4,6,8,9] Prochazka et al reported a significant decrease in ACE activity in primary human lung cancers tissues compared to normal lung tissue.[10] Moreover, studies on gene polymorphisms reported that the distribution of genotypes of endothelial nitric oxide synthase gene was substantially different in individuals with lung cancer compared to the control population.[11,12] Therefore, a decrease in serum ACE in patients with lung cancer is likely reflective of increased tumor burden, leading to reduced pulmonary epithelial cells which are the primary source for circulating ACE, as well as diminished ACE production by the lung cancer cells.[6] Taken together, these results suggest that plasma ACE activity may serve as an effective biomarker for cancer patients with poor prognosis as well as an indicator of responders to therapeutic intervention.[4,6]
The effects of ACEIs on cancer risk remains controversial; previous studies have suggested increased, decreased, or no association of ACEIs and angiotensin receptor blockers with cancer.[13–15] Although, a recent meta-analysis reported that angiotensin receptor blockers may be associated with decreased risk of lung cancer[16]; studies specifically assessing the effects of ACEIs and lung cancer risk have conflicting and limited evidence.[17–19] For example, Azoulay and colleagues, found an increased risk of lung cancer associated with the use of ACEIs but not angiotensin receptor blockers.[17] Hallas and colleagues, however, found weak evidence of an association between lung cancer risk and at least five years use of ACEIs.[18] However, a recent cohort study reported a 14% relative risk increase in lung cancer incidence in patients receiving ACEIs.[19] We conducted a meta-analysis to summarize the observed association between ACEIs and lung cancer across studies, thus dramatically increasing the power to detect and evaluate this association. In addition, we conducted subgroup analysis to evaluate whether study design, duration of ACEIs exposure or comparator medication moderated the observed association. Although a previous meta-analysis has examined the association between angiotensin receptor blockers use and the risk of lung cancer, to the best of our knowledge, this is the first meta-analysis specifically evaluating the link between ACEIs use and lung cancer.
2. Methods
2.1. Data source and search strategy
This systematic review and meta-analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) framework, and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.[20,21] The study protocol was based on a PICO search framework: adult population exposed to ACEIs medications, compared with no drug or non-ACEI antihypertensive drugs, was evaluated for risk of carcinoma of the lungs. Studies published from 1 January 1980 to 31 May 2019 without any language or date restrictions were identified through electronic searches of six databases; PubMed, Embase, SCOPUS, Web of Science, Cochrane Database of Systematic Reviews, and Cumulative Index to Nursing and Allied Health Literature. We added to this search evaluating reference lists of relevant articles (including studies as well as narrative and systematic reviews) and by backward and forward citation searching of all included studies using the Science Citation Index, the “Related Articles” feature on PubMed and Google scholar.
The electronic search combined terms related to “lung cancer”, OR “lung carcinoma” OR “lung neoplasm” in various combinations as well as “angiotensin converting enzyme inhibitors”, “ACE inhibitors”, “ACEIs” and with the names of individual medications. Details of the search strategy for the databases are as shown (Supplementary Table S1). When the results of a particular study were reported in more than one publication, only the most recent and complete data were included in the meta-analysis.
2.2. Inclusion and exclusion criteria
Eligible studies included case-control or cohort studies as well as randomized controlled trials comparing exposure to ACEIs versus no drug or non-ACEI antihypertensive drugs and lung cancer risk; have follow-up of at least 1 year; enrolled at least 100 patients; and be able to report the outcome of interest – lung cancer. We excluded the following papers from the review: case reports or case series, duplicate publications, letters, reviews, or editorials. Also, studies with insufficient published data for estimation of the risk ratio (RR) and confidence interval (CI) were excluded if attempt to reach the corresponding author for additional information was not successful. Finally, we excluded studies assessing cancer incidence among individuals with ACEI exposures compared with general population-level expected rates, to lower the risk of confounding by indication.
2.3. Data extraction and quality assessments
Two investigators (SA and MA) independently performed the data extraction and quality assessment, any differences between the investigators were resolved by a third reviewer (AA). Full texts of the articles selected were retrieved for further review and the reviewers extracted all study data independently. The researchers extracted information on the following parameters: first author, year of publication, country, sample size, study design, study period, length of follow-up / exposure to ACEIs (for cohort/case control studies), comparator reference group, estimated effect size, and whether adjustment for covariates were performed or not. Unadjusted or when available adjusted effect size data i.e., risk ratios (RR), odds ratios [OR], and hazard ratios [HR], as well as their 95% confidence interval (95% CI) for lung cancer in ACEIs users versus non-ACEIs users were extracted from every study. For papers that reported data on incidence rates, the RR and 95% CI were estimated.[22]
Two authors completed the quality assessment independently. The Newcastle–Ottawa Scale (NOS) was used to evaluate the methodological quality, which scored studies by the selection of the study groups, the comparability of the groups, and the ascertainment of the outcome of interest.[23] The studies were assessed by an overall quality score – with scores ranging from 0 to 9 with higher scores indicating higher quality. We considered studies with a NOS score of seven or more to be high-quality studies.
2.4. Statistical analysis
Pooled overall and subgroups risk ratios for lung cancer among ACEIs users compared with non-ACEIs users were estimated by pooling unadjusted and adjusted effect estimates reported by individual studies. Hazard ratios and odds ratios were assumed to approximate the same measure of RR value since the absolute risk of lung cancer is low as was done in previous meta-analyses.[24,25] The heterogeneity of the data was quantified by the Q statistic in combination with the I2 statistic. Heterogeneity among studies was considered significant when P < .1 for the Q statistic or when the I2 > 50%.[26] All meta-analyses were performed using the random-effects model.
Subgroup analysis was performed for duration of exposure, smoking status, study design and reference comparator groups. Duration of exposure was dichotomized as ≤5 versus > 5 years. Data for exposure duration were based on the reported mean duration of exposure for studies reporting direct measures, and on mean study follow-up as a surrogate measure otherwise. All subgroup analyses were calculated using a random- effects model.
Publication bias was estimated visually by funnel plots for outcomes with more than 10 studies. Egger's regression test was applied to assess symmetry of the funnel plot and a two-sided P < .05 was considered as significant. Comprehensive Meta-Analysis software version 2.2 (Biostat Inc, USA), was used to perform the meta-analysis. and the meta-regression.
3. Results
3.1. Description of selected studies
Our search retrieved 1112 studies. This number became 512 after duplicate publications were removed. Screening of the titles of the studies identified 42 studies for full-text assessment. A total of 13 studies (four case control and eight cohort studies as well as one randomized control trial) were carried out between 1980 and 2015 were included in the systematic review and meta-analysis.[17–19,27–36] The flow chart of study selection for the systematic review is shown in Figure 1. The studies provided data on 458,686 ACEI users and 656,119 non-ACEIs antihypertensive users or controls. The range of follow-up period of the studies was 2.0 to 9.6 years. Most of the studies included in the review were high quality studies with a NOS score of 7 to 9. The characteristics of studies included in the systematic review are summarized in Table 1.
Figure 1.

Flow chart of study selection for the systematic review.
Table 1.
Characteristics of studies included in the systematic review.
| s/no | Author, (year) | Countries | Study period | Study design | Cases / Control [Exposed/unexposed] | Follow-up (years) | Reference group | Adjustments | Study quality |
| 1 | ∗Pahor, (1996) | USA | 1988–1992 | Cohort | 124/428 | 2 | BB | No | 7 |
| 2 | Jick, (1997) | UK | 1990–1995 | Case-Control | 85/422 | 4 | BB | No | 7 |
| 3 | Lever, (1998) | Scotland | 1980–1995 | Cohort | 1559/3648 | 6.6 | BB/CB/D | Yes | 6 |
| 4 | Rosenberg, (1998) | USA | 1983–1996 | Case/Control | 994/6492 | 3.8 | Hospital controls | No | 7 |
| 5 | Assimes, (2008) | Canada | 1980–2003 | Case/Control | 1507/15,070 | 3.6 | D | No | 7 |
| 6 | ONTARGET, (2008) | Multi-center | 2004–2004 | RCT | 8576/ 8542 | 4.7 | ARB | Yes | 9 |
| 7 | van der Knaap, (2008) | Netherlands | 1991–2004 | Cohort | 138/7541 | 9.6 | Healthy controls | Yes | 8 |
| 8 | Pasternak, (2011) | Denmark | 1998–2006 | Cohort | 209,692/107,466 | 2.1 | ARB | Yes | 8 |
| 9 | Azoulay, (2012) | UK | 1995–2010 | Cohort | 4200/39,668 | 6.4 | BB/D | Yes | 8 |
| 10 | Bhaskaran (2012) | UK | 1995–2010 | Cohort | 2144/360,679 | 4.6 | ARB | Yes | 8 |
| 11 | Hallas, (2012) | Denmark | 2000–2005 | Case-Control | 16,343/65281 | 2.7 | Healthy controls | Yes | 7 |
| 12 | ∗Chiang, (2014) | Taiwan | 2000–2009 | Cohort | 4971/ 24,855 | 2.36 | BB/CB/D | Yes | 6 |
| 13 | Hicks, (2018) | UK | 1995–2015 | Cohort | 208,353/16,027 | 6.4 | ARB | Yes | 8 |
3.2. ACEIs use and lung cancer
Figure 2 summarizes the meta-analysis on the association between ACEIs use and lung cancer. Overall, pooled risk ratios indicate that ACEIs use was not associated with an increased risk of lung cancer among individuals who received the medication (RR 0.982, 95% C.I. 0.873 – 1.104; P = .76). There was significant heterogeneity between the studies (Q = 52.54; P < .001; I2 = 86.07).
Figure 2.
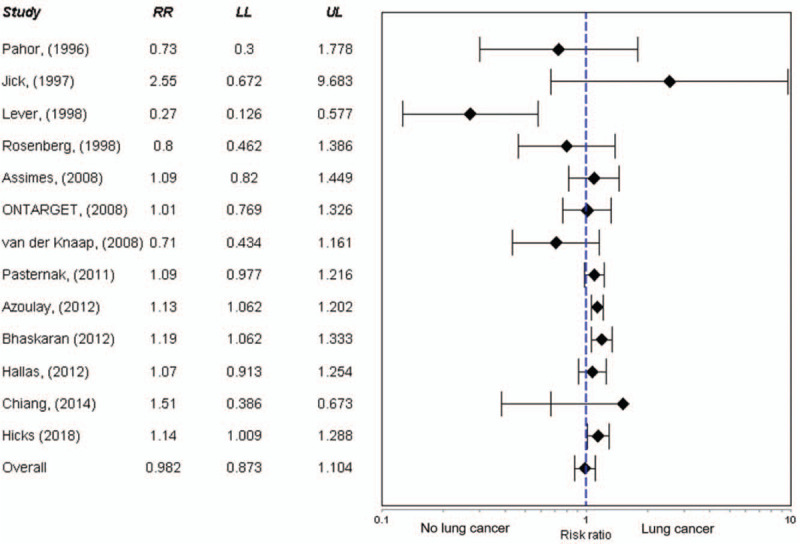
Forest plot of the meta-analysis on the association between ACEIs use and lung cancer.
3.3. ACEIs use and lung cancer according to duration of exposure
Figure 3 summarizes the meta-analysis on the association between ACEIs use and lung cancer according to duration of exposure to ACEIs. Overall, four studies reported outcomes on participants who were exposed to ACEIs over five years. There was no significant association between ACEIs use and lung cancer in studies with over five years of ACEIs exposure (RR 0.95, 95% C.I. 0.75 – 1.20; P = .70). Also, no significant association was detected in the nine studies with ≤ 5years of exposure to ACEIs (RR 0.98, 95% C.I. 0.83 – 1.15; P = .77). In addition, the interaction test evaluating the difference between the stratified subgroups was not statistically significant (P = .83). There was significant heterogeneity in the studies belonging to both groups (P = .0001, I2 = 82.3%; P < .001, I2 = 76.7%%, for > 5 years and ≤5 years duration studies, respectively)
Figure 3.

Forest plot of the meta-analysis on the association between ACEIs use and lung cancer according to duration of exposure to ACEIs.
3.4. ACEIs use and lung cancer: subgroup analysis
The study also assessed the pooled risk ratios of lung cancer in individuals exposed to ACEIs stratified by study design (Table 2). There were no statistically significant differences in the pooled risk ratio obtained from case control studies (RR 1.06, 95% C.I. 0.82 – 1.37; P = .66), cohort studies (RR 0.94, 95% C.I. 0.81 – 1.10; P = 0.12) and randomized controlled trials (RR 1.01, 95% C.I. 0.65 – 1.56; P = .55). Also, the interaction test evaluating the difference between these stratified subgroups was not statistically significant (Q = 0.65; P = .723).
Table 2.
Pooled risk ratios of lung cancer in individuals exposed to ACEIs stratified by subgroups.
| Subgroups | RR (95% CI) | Q (P -value) | Number of studies | Heterogeneity I2 (P -value) |
| Study design | 0.65 (.72) | |||
| Case-Control | 1.06 (0.82–1.37) | 4 | (0).44 | |
| Cohort | 0.94 (0.81–1.10) | 8 | (85.8%) <.001 | |
| RCT | 1.01 (0.65–1.56) | 1 | (0) 1.00 | |
| Comparator regimen | 3.37 (.19) | |||
| ARB | 1.11 (0.89–1.39) | 4 | (0) .60 | |
| BB/CCB/D | 0.82 (0.64–1.05) | 6 | (88.9%) <.001 | |
| Controls | 0.91 (0.66–1.25) | 3 | (36.9) .21 | |
| Duration of ACEI use | 0.05 (.83) | |||
| ≤5 years | 0.95 (0.75–1.20) | 9 | (76.7%) .001 | |
| >5 – 10 years | 0.98 (0.83–1.15) | 4 | (82.3%) .001 |
Furthermore, we also assessed the pooled risk ratios of lung cancer in individuals exposed to ACEIs stratified by the comparator regimen (Table 2). There were no statistically significant differences in the pooled risk ratio obtained from individuals whose comparator regimen was an angiotensin receptor blocker (RR 1.11; 95% C.I. 0.89 – 1.39), a β-blocker/calcium channel blocker/diuretics (RR 0.82; 95% C.I. 0.64 – 1.05) and those who were healthy/hospital controls (RR 0.91; 95% C. I. 0.66 – 1.25). The interaction test evaluating the difference between these stratified subgroups was not statistically significant (Q = 3.37; P = .19).
In studies where adjustments for smoking were not made in the study population, the risk of lung cancer among ACEIs users was (RR 0.85, 95% C.I. 0.72 – 1.01; P = .06), while in studies where adjustment for smoking status were made, the risk was (RR 1.14, 95% C.I. 0.96 – 1.35; P = .13). The interaction test evaluating the difference between these stratified subgroups was statistically significant (Q = 5.85; P = .02). In studies conducted predominantly among European population (N = 8), the risk of lung cancer among ACEIs users was (RR 1.09, 95% C.I. 0.99 – 1.20; P = .07), while in studies conducted predominantly in North American population (N = 3), the risk was (RR 0.98, 95% C.I. 0.74 – 1.29; P = .87). Also, the interaction test evaluating the difference between these stratified subgroups was not statistically significant (Q = 0.54; P = .46). One study was a multicenter study involving 40 countries, while there was also one study in predominantly Asian population.
3.5. Meta-regression and publication bias
A meta-regression analysis was performed using the random-effects model to evaluate the relationship between exposure to ACEIs, risk for lung cancer with duration of exposure as a continuous moderator. We found a very small trend of a reduction in the risk of lung cancer with a higher duration of treatment with ACEIs (β coefficient for slope = -00027; 95% CI -0.13 to 0.12); however, this trend was not statistically significant (Q = 2.29; P = .13).
In the funnel plot analysis (Fig. 4), there was absence of asymmetry suggesting that there is no evidence of publication bias. However, Duval and Tweedie's trim and fill indicate that there might be one missing study to the right of the mean of the funnel plot. Egger's regression test (Egger test P = .05); and Begg and Mazumdar's test for rank correlation (P = .10) both did not reveal any evidence of publication bias.
Figure 4.

Funnel plot analysis of the meta-analysis on the association between ACEIs use and lung cancer.
4. Discussion
In this study, we found that the use of ACEIs was not significantly associated with a risk of lung cancer. Also, no significant relationships were evident between ACEIs use and the risk of lung cancer when the data was stratified according to duration of exposure, study design or comparator regimen. Also, we found that there was no significant trend between exposure to ACEI and the risk for lung cancer with duration of exposure as a continuous moderator. Given the importance of ACEIs in the management of individuals with cardiovascular and renal diseases, our study provides important information to further strengthen the evidence for the safety of ACEIs use.
Our finding of no significant association between ACEIs use and the risk of lung cancer affirms the findings of previous meta-analyses of randomized controlled trials which did not report any evidence of an association between the exposure to ACEIs/ angiotensin-receptor blockers and any cancer risk, as well as no link between ACEI use and lung cancer based on RCTs.[13] Our findings further affirm the safety of ACEIs especially with respect to its association with the risk of developing lung cancer. Furthermore, when the risk ratios were stratified according to the comparator regimens, there was an 11% increase in risk and an 18% reduction in risk of lung cancer when ACEI use was compared to ARBs and other hypertensive medications (β-blockers, calcium blockers and diuretics), respectively. However, these changes in risk were not statistically significant. This suggest that receiving any of the comparator regimens might modify the relationship between ACEI use and lung cancer and this deserves further studies.
Furthermore, we did not find any significant association in the trend in risk of lung cancer with duration of treatment with ACEIs. A recent cohort study suggested that the use of ACEIs for less than five years, 5 to 10 years and over 10 years were associated with no risk, 22% increased risk and 33% increased risk for lung cancer, respectively.[19] Therefore, there is a need for further long-term follow up of individuals on treatment with ACEIs for over a decade to better characterize its cancer (and lung cancer) risk.
Earlier studies have suggested that the possibility of an association between exposure to ACEIs and the development of lung cancer is biologically-plausible.[37–39] This is because both angiotensin I and angiotensin converting enzyme contributes to the metabolism of bradykinin in the lungs.[40] Therefore, with consistent inhibition of the angiotensin converting enzyme and its pathway, bradykinin accumulates in the lung tissues.[39–41] Moreover, in several malignant tissues including pulmonary tumors, studies have found increased activity of bradykinin receptors, and bradykinin has been reported to directly stimulate the growth of pulmonary cancers through amplification of the proliferation of vascular endothelial growth factors as well as indirectly through increasing vascular permeability by activating matrix metallopeptidases which play a role in angiogenesis and organogenesis.[39–42]
The findings of this meta-analysis are in contrast with these observations. The potential mechanism to explain the findings of this study is that ACEIs vary in their absorption, potency and tissue affinity.[43,44] Older generation ACEIs such as captopril, and enalapril have been found to have low-tissue affinity whereas newer generation ACEIs such as ramipril, perindopril and quinapril have been shown to be lipophilic with a high-tissue affinity for the ACE.[43,44] Previous experimental studies have shown that exposure to high-affinity ACEIs such as quinapril or perindopril reportedly promotes angiogenesis in some individuals with malignancy mediated by the bradykinin B(2) receptor pathway or by the stimulation of the vascular endothelial growth factors.[4,45,46] However, other studies have shown that exposure to low-tissue affinity ACEIs such as captopril inhibited tumor angiogenesis and growth, induced apoptosis and reduced metastasis.[47,48] Furthermore, other studies have shown that ACEIs which activates peroxisome proliferator-activated receptor-gamma induce apoptosis of malignant cells lowering lung cancer growth and lymph node metastasis.[49,50] Therefore, differences in the type ACEIs administered to participants might have contributed to the risk of lung cancer reported by some previous studies.
The study has a number of strengths and limitations. A major strength of this study is that despite the serious concerns regarding the use of ACEI and the risk of lung cancer, this is the first attempt to systematically evaluate and quantify this risk. Secondly, we were able to quantify this risk in a large group of patients exposed to ACEIs compared with non-ACEIs exposed persons. Third, we found that the use of ACEI was not associated with an increased risk of lung cancer.
However, the study has a number of limitations. First, all except one, of the included studies were analytical studies, therefore any causal inference between the use of ACEIs and lung cancer risk needs to be interpreted with caution. Secondly, because majority of the studies included in the meta-analysis were either case-control or cohort studies, they are prone to bias from both residual confounding and confounding by indication.[51] Third, in some of the studies included in the analysis, the effect size estimates were not adjusted for important confounders such as smoking status – this could have contributed to the conflicting findings of previous studies. We have added a sub-group analysis in our results according to whether adjustments were made for smoking status in the primary studies. Fourth, the type and dose of ACEIs administered might have contributed to the differences in lung cancer risk reported by previous studies. Only a few of the studies included in the review mentioned the type, and none of them gave the doses of ACEIs administered. Given that clinicians are at liberty to prescribe any type of medication they feel will be beneficial to their patients as well as adjust the dose as necessary; and disaggregation of the risk according to the type/dose of ACEI given were not available in the included studies, we were unable to analyze lung cancer risk according to the type of ACEIs used. Similarly, none of the studies analyzed or reported risks according to gender. Fifth, we did not include individual patient data in the meta-analysis, data availability bias is thus a potential concern and bias in the misclassification of the outcomes of the studies included in the analysis cannot be completely ruled-out. Sixth, we did not perform a subgroup analysis on statin use. A previous study has suggested that statin use for over 6 months was associated with a 55% risk reduction for lung cancer,[52] and another study suggested that statin use moderated the risk of several types of cancers.[53] These limitations need to be considered in the design of future studies. Finally, although there is no evidence for publication bias, this cannot be completely ruled out. Despite these limitations, our findings are very crucial for clinical care and policy.
In conclusion, use of ACEIs was not associated with an increased risk of lung cancer. The findings of this study should reassure treating physicians and patients about the safety of ACEIs. Also, this finding is reassuring considering the widespread use and beneficial effects of ACEIs for several renal and cardiovascular conditions. However, there is a need for further longitudinal studies to evaluate the long-term (over 10 years) association between ACEIs use and lung cancer. Also, future studies should evaluate the moderation effects of co-administration of statins, ACEIs use and lung cancer risk, and adequate adjustments needs to be made for known confounders.
Author contributions
Conceptualization: Mohammed Batais, Turky Almigbal.
Data curation: Mohammed Batais, Turky Almigbal.
Formal analysis: Turky Almigbal, Mashhor Alhantoushi.
Investigation: Mohammed Batais, Turky Almigbal, Mashhor Alhantoushi.
Methodology: Mashhor Alhantoushi, Abdulaziz Alodhayani, Abdullah Alkhushail, Sultan Khalid Al Dalbhi.
Project administration: Khalid Alotaibi, Abdullah Alkhushail, Sultan Khalid Al Dalbhi.
Resources: Khalid Alotaibi, Saad Alsaad, Abdullah Alkhushail.
Supervision: Abdulaziz Alodhayani.
Validation: Khalid Alotaibi, Saad Alsaad.
Visualization: Saad Alsaad, Abdulrahman Altheaby, Yasser Alghamdi.
Writing – original draft: Abdulrahman Altheaby, Yasser Alghamdi.
Writing – review & editing: Abdulaziz Alodhayani, Abdulrahman Altheaby, Sultan Khalid Al Dalbhi, Yasser Alghamdi.
Supplementary Material
Footnotes
Abbreviations: ACE = Angiotensin Converting Enzyme, ACEI = Angiotensin Converting Enzyme Inhibitor, CIs = 95% Confidence Intervals, HR = Hazard Ratio, MOOSE = Meta-analysis Of Observational Studies in Epidemiology guideline, NOS = Newcastle–Ottawa Scale, OR = Odds Ratio, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis framework, RAS = Renin-Angiotensin System, RR = Risk Ratio.
How to cite this article: Batais M, Almigbal T, Alotaibi K, Alodhayani A, Alkhushail A, Altheaby A, Alhantoushi M, Alsaad S, Dalbhi SA, Alghamdi Y. Angiotensin converting enzyme inhibitors and risk of lung cancer: A systematic review and meta-analysis. Medicine. 2021;100:17(e25714).
The authors have no conflicts of interest to disclose.
the ethics approval was not obtained because this research is a systematic review and meta-analysis and does not include human subjects, human data or tissue, or animals.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
Assessed risk of group of cancers including lung cancers; ARB = angiotensin receptor blocke, BB = beta blockers, CB = calcium channel blockers, D = diuretics.
ARB = angiotensin receptor blocker, BB = beta blockers, CB = calcium channel blockers, D = diuretics.
References
- [1].George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 2010;10:745–59. [DOI] [PubMed] [Google Scholar]
- [2].Ager EI, Neo J, Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis 2008;29:1675–84. [DOI] [PubMed] [Google Scholar]
- [3].Rosenthal T, Gavras I. Angiotensin inhibition and malignancies: a review. J Hum Hypertens 2009;23:623–35. [DOI] [PubMed] [Google Scholar]
- [4].Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci Transl Med 2017;9:eaan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rømer FK. Angiotensin-converting enzyme and its association with outcome in lung cancer. Br J Cancer 1981;43:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gallagher PE, Cook K, Soto-Pantoja D, et al. Angiotensin peptides and lung cancer. Curr Cancer Drug Targets 2011;11:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tan WSD, Liao W, Zhou S, et al. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol 2018;40:09–17. [DOI] [PubMed] [Google Scholar]
- [8].Roulston JE, Galloway PJ, Douglas G. Plasma angiotensin-converting enzyme activity in patients with bronchial carcinoma. Br J Dis Chest 1986;80:229–34. [DOI] [PubMed] [Google Scholar]
- [9].Nussinovitch N, Peleg E, Yaron A, et al. Angiotensin converting enzyme in bleomycin-treated patients. Int J Clin Pharmacol Ther Toxicol 1988;26:310–3. [PubMed] [Google Scholar]
- [10].Procházka J, Krepela E, Sedo A, et al. Aminopeptidases and angiotensin I-converting enzyme activities in primary human lung tumors and lung parenchyma. Neoplasma 1991;38:501–8. [PubMed] [Google Scholar]
- [11].Peddireddy V, Badabagni SP, Gundimeda SD, et al. Association of eNOS and ACE gene polymorphisms and plasma nitric oxide with risk of non-small cell lung cancer in South India. Clin Respir J 2018;12:207–17. [DOI] [PubMed] [Google Scholar]
- [12].Cheon KT, Choi KH, Lee HB, et al. Gene polymorphisms of endothelial nitric oxide synthase and angiotensin-converting enzyme in patients with lung cancer. Lung 2000;178:351–60. [DOI] [PubMed] [Google Scholar]
- [13].Bangalore S, Kumar S, Kjeldsen SE, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol 2011;12:65–82. [DOI] [PubMed] [Google Scholar]
- [14].Yoon C, Yang HS, Jeon I, et al. Use of angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers and cancer risk: a meta-analysis of observational studies. CMAJ 2011;183:E1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Datzmann T, Fuchs S, Andree D, et al. Systematic review and meta-analysis of randomised controlled clinical trial evidence refutes relationship between pharmacotherapy with angiotensin-receptor blockers and an increased risk of cancer. Eur J Intern Med 2019;64:01–9. [DOI] [PubMed] [Google Scholar]
- [16].Zhang W, Liang Z, Li J, et al. Angiotensin receptor blockers use and the risk of lung cancer: a meta-analysis. J Renin Angiotensin Aldosterone Syst 2015;16:768–73. [DOI] [PubMed] [Google Scholar]
- [17].Azoulay L, Assimes TL, Yin H, et al. Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS One 2012;7:e50893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hallas J, Christensen R, Andersen M, et al. Long term use of drugs affecting the renin-angiotensin system and the risk of cancer: a population-based case-control study. Br J Clin Pharmacol 2012;74:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hicks BM, Filion KB, Yin H, et al. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018;363:k4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moher D, Liberati A, Tetzlaff J, et al. Systematic reviews and meta-analyses CHECK LIST: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology a proposal for reporting. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [22].Relative Risk Calculator. Relative risk calculator [Internet]. 2019 https://www.medcalc.org/calc/relative_risk.php [Accessed August 3, 2019]. [Google Scholar]
- [23]. Wells GA, Shea B, O’Connell D, et al The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses, 2012. Available from: http://wwwohrica/programs/clinical_epidemiology/oxfordasp. (Accessed July 10, 2019) [Google Scholar]
- [24].Guha N, Merletti F, Steenland NK, et al. Lung cancer risk in painters: a meta-analysis. Environ Health Perspect 2010;118:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rotshild V, Azoulay L, Zarifeh M, et al. The risk for lung cancer incidence with calcium channel blockers: a systematic review and meta-analysis of observational studies. Drug Saf 2018;41:555–64. [DOI] [PubMed] [Google Scholar]
- [26].Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. Wiley, 1st ed.Hoboken:2009. [Google Scholar]
- [27].Lever AF, Hole DJ, Gillis CR, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet 1998;352:179–84. [DOI] [PubMed] [Google Scholar]
- [28].Bhaskaran K, Douglas I, Evans S, et al. Angiotensin receptor blockers and risk of cancer: cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ 2012;344:e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chiang YY, Chen KB, Tsai TH, et al. Lowered cancer risk with ACE inhibitors/ARBs: a population-based cohort study. J Clin Hypertens (Greenwich) 2014;16:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pasternak B, Svanström H, Callréus T, et al. Use of angiotensin receptor blockers and the risk of cancer. Circulation 2011;123:1729–36. [DOI] [PubMed] [Google Scholar]
- [31].Jick H, Jick S, Derby LE, et al. Calcium-channel blockers and risk of cancer. Lancet 1997;349:525–8. [DOI] [PubMed] [Google Scholar]
- [32].Sipahi I, Debanne SM, Rowland DY, et al. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol 2010;11:627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].van der Knaap R, Siemes C, Coebergh JW, et al. Renin-angiotensin system inhibitors, angiotensin I-converting enzyme gene insertion/deletion polymorphism, and cancer: the Rotterdam study. Cancer 2008;112:748–57. [DOI] [PubMed] [Google Scholar]
- [34].Rosenberg L, Rao RS, Palmer JR, et al. Calcium channel blockers and the risk of cancer. JAMA 1998;279:1000–4. [DOI] [PubMed] [Google Scholar]
- [35].Assimes TL, Elstein E, Langleben A, et al. Long-term use of antihypertensive drugs and risk of cancer. Pharmacoepidemiol Drug Saf 2008;17:1039–49. [DOI] [PubMed] [Google Scholar]
- [36].Pahor M, Guralnik JM, Salive ME, et al. Do calcium channel blockers increase the risk of cancer? Am J Hypertens 1996;9:695–9. [DOI] [PubMed] [Google Scholar]
- [37].Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 2003;24:261–71. [DOI] [PubMed] [Google Scholar]
- [38].Trifilieff A, Da Silva A, Gies JP. Kinins and respiratory tract diseases. Eur Respir J 1993;6:576–87. [PubMed] [Google Scholar]
- [39].Sethi T, Rozengurt E. Multiple neuropeptides stimulate clonal growth of small cell lung cancer: effects of bradykinin, vasopressin, cholecystokinin, galanin, and neurotensin. Cancer Res 1991;51:3621–3. [PubMed] [Google Scholar]
- [40].Golias Ch, Charalabopoulos A, Stagikas D, et al. The kinin system-bradykinin: biological effects and clinical implications. multiple role of the kinin system-bradykinin. Hippokratia 2007;11:124–8. [PMC free article] [PubMed] [Google Scholar]
- [41].Ishihara K, Hayash I, Yamashina S, et al. A potential role of bradykinin in angiogenesis and growth of S-180 mouse tumors. Jpn J Pharmacol 2001;87:318–26. [DOI] [PubMed] [Google Scholar]
- [42].Catarata MJ, Ribeiro R, Oliveira MJ, et al. Renin-angiotensin system in lung tumor and microenvironment interactions. Cancers (Basel) 2020;12:1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rachow T, Schiffl H, Lang SM. Risk of lung cancer and renin-angiotensin blockade: a concise review. J Cancer Res Clin Oncol 2021;147:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lazar HL, Bao Y, Rivers S, et al. High tissue affinity angiotensin-converting enzyme inhibitors improve endothelial function and reduce infarct size. Ann Thorac Surg 2001;72:548–53. [DOI] [PubMed] [Google Scholar]
- [45].Fabre JE, Rivard A, Magner M, et al. Tissue inhibition of angiotensin-converting enzyme activity stimulates angiogenesis in vivo. Circulation 1999;99:3043–9. [DOI] [PubMed] [Google Scholar]
- [46].Silvestre JS, Bergaya S, Tamarat R, et al. Proangiogenic effect of angiotensin-converting enzyme inhibition is mediated by the bradykinin B(2) receptor pathway. Circ Res 2001;89:678–83. [DOI] [PubMed] [Google Scholar]
- [47].Volpert OV, Ward WF, Lingen MW, et al. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J Clin Invest 1996;98:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Attoub S, Gaben AM, Al-Salam S, et al. Captopril as a potential inhibitor of lung tumor growth and metastasis. Ann N Y Acad Sci 2008;1138:65–72. [DOI] [PubMed] [Google Scholar]
- [49].Jackson L, Wahli W, Michalik L, et al. Potential role for peroxisome proliferator activated receptor (PPAR) in preventing colon cancer. Gut 2003;52:1317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang M, Zou P, Bai M, et al. [Apoptosis of human lung cancer cells induced by activated peroxisome proliferator-activated receptor-gamma and its mechanism]. Zhonghua Yi Xue Za Zhi 2003;83:1169–72. [PubMed] [Google Scholar]
- [51].Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA 2016;316:1818–9. [DOI] [PubMed] [Google Scholar]
- [52].Khurana V, Bejjanki HR, Caldito G, et al. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest 2007;131:1282–8. [DOI] [PubMed] [Google Scholar]
- [53].Undela K, Shah CS, Mothe RK. Statin use and risk of cancer: an overview of meta-analyses. World J Meta-Anal 2017;5:41–53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


